|
|
|
- Bruno Allen
- 6 years ago
- Views:
Transcription
1 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert s law. 2. State Beer s law. 3. State Lambert-Beer law. 4. State the major process involved in AAS. 5. Name the types of detectors used for IR spectrometry. 6. Define spectroscopy. 7. Name the different types of spectrophotometers. 8. What are the light sources used for AAS? 9. Give any two applications of flame emission spectrometry. 10. Specify the classification of IR region of spectrum. 11. Name the instruments used in IR spectrometry. 12. Name few IR radiation sources. 13. Give the advantages of grating monochromators 14. Give 4 different techniques used for sampling of solids. 15. Name two different types of IR spectrometers 16. Give the advantages and disadvantages of Fourier transform IR spectrometers. 17. Specify the major design requirements of monochromators. 18. Name the different mountings used in grating monochromators. 19. Name the factors on which the radiant power received by a detector depends. 20. State the principle of operation of flame emission spectrometry. Unit-I Part B 1. Explain flame emission photometer with its instrumentation. 2. Explain mono-chromators and detectors. 3. Explain about photo multiplier tube. 4. Explain Fourier transform infra-red spectrometers. 5. Explain different radiation source in absorption spectrophotometry. 6. Explain the working principle of various radiation sources and detectors in IR Spectrophotometers. With a neat diagram, explain the instrumentation setup of Atomic absorption spectroscopy (MAY 2013) 7. With a neat sketch, explain the instrumentation setup and working principle of IR spectrometer. (May 2013) 8. Draw and Explain the schematic diagram of a typical double beam spectrophotometer.
2 (Nov 2013) 9. What are non-dispersive spectrophotometers? Explain in detail the FTIR spectrometer. Also specify the advantages of the FTIR spectrophotometer. (Nov 2013) 10. State and derive Beer s law from basic principles. Discuss the limitations of it. 1. Define chromatography. UNIT II CHROMATOGRAPHY PART-A 2. What are the different types of gas chromatography?. 3. Define retention time in a chromatography 4. What are the different parts of gas chromatography? 5. Give the selection criteria for carrier gas. 6. Write short notes about gas chromatographic column. 7. What is pyrolysis in gas chromatography? 8. List some detectors in gas chromatography. 9. Give the principle of Gas-Solid chromatography. 10. Give the principle of Gas-Liquid chromatography. 11. What are the advantages of gas chromatography? 12. Write the features of thermal conductivity detector. 13. On what factor does the choice of detector will depend on liquid chromatography? 14. What are the limitations of bulk property detector? 15. Write the limitations of gas chromatography 16. What are the different types of liquid chromatography? 17. Name the different types of pumps used for mobile-phase delivery system in liquid chromatography 18. What are the different types of columns used in liquid chromatography. 19. Write short notes on thin layer chromatography (TLC) 20. What are the typical column applications in liquid solid chromatography? PART-B
3 1. Draw the schematic diagram of a gas chromatography and explain the different parts in Gas chromatography.(may 2013), (Nov 2013) 2. Describe a gas liquid chromatography with a schematic diagram. Explain how it works. 3. Give a typical chromatogram. Discuss some applications of gas liquid chromatography. 4. Explain in detail the following detectors used in Gas Chromatography. a).thermal conductivity detector b).flame ionization detector 5. With a neat schematic diagram discuss the separation principle of HPLC. (May 2013) 6. What are the requirements of HPLC pumping system and enumerate the application of HPLC. 7. Give in detail the classification of chromatography. Explain liquid chromatography. Chromatography 8. Explain various detectors used in liquid chromatography. 9. Explain about Refractive index detector and electrochemical detector 10. Explain with neat diagram about flame photometric detector, photo ionization detector and electron capture detector. UNIT III INDUSTRIAL GAS ANALYSER AND POLLUTION MONITORING INSTRUMENTS Part A 1. Define Thermal Conductivity of a gas? 2. Why thermistors are used in thermal conductivity analyzer as a heat sensing elements?. 3. What are the applications of thermal conductivity gas analyzer? 4. How is nitrogen-di-oxide prepared by chemiluminescence? 5. What are the advantages of Hydrogen Sulfide analyzer?. 6. What is the use of gold films in H 2 S analyzer? 7. Where are the electrochemical sensors used? 8. What is the principle of H2S analyzer? 9. What is the principle of CO analyzer? 10. What is the use of protective filter in Industrial analyzer? 11. What is the use of stream drying equipment? 12. What is the need of bypass pumping devices? 13. What are the applications of oxygen analyzer? 14. What are the sources of error in oxygen analyzer?. 15. What is the principle of thermal conductivity analyzer?.16. Explain the different analysis methods of Nitrogen Oxide? 17. What are the applications of Electrochemical and Infrared sensors? 18. Explain the detection methods of Carbon Monoxide Analyzer? 19. What is necessity of converting nitric oxide to nitrogen-di-oxide? 20. Explain the calibration methods used in NO2 analyzer? Unit-III Part B 1. With a neat sketch, explain the construction and working principle of parametric oxygen analyzer. (May 2013)
4 2. With a block diagram, explain the method of measuring carbon monoxide using Nondispersive Infrared Analyzer. (Nov 2013) Explain how the NO 2 analyzer is used. (May 2013) Explain how the H 2 S analyzer is used. 5. Explain how the dust and smoke analyzer is used. (May 2013) 6. Explain how the IR analyzer is used. 7. With a suitable diagram explain the construction and working principle of thermal conductivity analyzer. (Nov 2013) 8. What are the sources of air pollution and explain in detail? 9. What are the type s gas analyzers? Explain anyone with example. 10. Discuss the method of analysis based on ionization of gases. UNIT IV ph METER AND DISSOLVED COMPONENT ANALYZER Part A 1. What are the three types of membrane sensor? 2. How measurements are done in ion selective electrodes? 3. Define conductivity of electrolyte. 4. Give the methods of measuring conductance?. 5. Why temperature compensation necessary in conductivity measurement? 6. Give the methods of measurements of Oxygen? 7. Give the working principle of electrical conductivity meter? 8. Give the application of Silica analyzer? 9. What are the two measurements made in Silica analyzer? 10. What is chemical blank measurement? 11. What is the use of blank in silica analyzer? 12. Give the application of Sodium analyzer? 13. Why ammonia gas is added to the sample in Sodium analyzer? 14. How PH of a solution is measured & gives the Nernst equation? 15. Give the different types of electrodes used for PH measurements? 16. Give the characteristics of glass electrode? 17. Give the design criteria of PH meter? 18. Give the general classification of PH cell? 19. Define humidity &Dew point? 20. How humidity or moisture is measured &give the types of it? PART-B 1. Describe how a conductivity cell is used. Give the applications of conductivity measurement of a liquid. Explain how the oxygen analyzer is used. 2. Describe the construction of a ph electrode. Draw the electronic circuit diagram for measuring ph of a liquid and explain its working.
5 3. Discuss how ph values are measured. Explain the role of calomel electrodes in this measurement. 4. Briefly explain about dissolved oxygen analyzer. 5. Discuss in detail the principle, characteristics of electrodes used in ph meters. 6. Explain about sodium analyzer in detail. 7. Explain different types of ion selective electrodes. 8. Describe a method of measuring dissolved oxygen content in the boiler feed water. Why dissolved oxygen content is monitored in feed water? Also specify the cause of dissolved oxygen content in feed water. 9. Explain the working principles of biosensors with a conceptual diagram. Also specify the various parameters to be measured using Biosensor. 10 Briefly explain about dissolved oxygen analyzer. UNIT V ELECTRO MAGNETIC RESONANCE AND MICROSCOPIC TECHNIQUES 1. Define state of resonance. PART-A 2. Define Chemical shift. 3. Brief the principle of scanning electron microscope. 4. Write down the principle of Mass Spectrometry. 5. What is Nuclear magnetic resonance? 6. Write the principle of Electron Spin Resonance (ESR) spectrometer. 7. What are the types of NMR spectrometer. 8. What are the different types of mass spectrometer? 9. Give some applications of NMR spectroscopy. 10. What is Spin-Spin coupling? 11. What are the different types of mass spectrometer? 12. Write the components of electron spectroscopy. 13. What are the types of detectors in mass spectrometer? 14. What are the different components of a mass spectrometer? 15. Define time of flight. 16. Define mass spectrum. 17. What are the factors that influence chemical shift? 18. Write the components of electron spectroscopy. 19. What is the advantage of ESR Spectroscopy? 20. Write the limitation of NMR spectroscopy. PART-B
6 1. Draw the block diagram of an NMR spectrometer. Explain the function of each part and explain how it is used to obtain NMR spectra. How are these spectra useful? (May 2013) 2. What are Mass spectrometers? Describe magnetic deflection mass spectrometers. (May 2013) 3. Explain time of flight and quadrupole mass spectrometers. 4. Describe the working of double beam mass spectrometer and give its applications. 5. Explain the instrumentation and applications of Scanning Electron Microscope (SEM) and Transmission Electron Microscope (TEM). 6. Describe how various samples are analyzed using NMR spectrometer with neat diagram. 7. Explain the construction and working principle of Electron Spin Resonance (ESR) spectrometer with neat diagram. 8. Explain the construction and working of Radio frequency spectrometer with neat diagram. 9. Explain the various components of a mass spectrometer. 10. Explain in detail the construction and working principle of single focusing mass spectrophotometers. (Nov 2013) 11. In NMR spectroscopy mention the advantages of using a magnet with as great a field strength as possible. Also explain the difference between a continuous wave and fourier transform NMR. 12. With neat sketch explain the construction and working principle of mass spectrometer. 13. What are the basic components of electron Spectroscopy? Also explain the working principle of Electron spectroscopy with a neat block diagram. (Nov 2013)
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur
 VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
EI2302 Analytical instruments Dept. of EIE
 EI2302-ANALYTICAL INSTRUMENTS V SEMESTER (EIE & ICE) III YEAR UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. What is Spectrophotometer? Spectrophotometer are dispersive analyzers where a prism is used
EI2302-ANALYTICAL INSTRUMENTS V SEMESTER (EIE & ICE) III YEAR UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. What is Spectrophotometer? Spectrophotometer are dispersive analyzers where a prism is used
(DCHE21) ASSIGNMENT - 1 M.Sc. (Second) DEGREE EXAMINATION, MAY 2019 Second Year CHEMISTRY Analytical Chemistry MAXIMUM : 30 MARKS ANSWER ALL QUESTIONS
 ASSIGNMENT - 1 Analytical Chemistry (DCHE21) Q1) State and explain Beer s Law mention its limitations. Q2) Write the basic instrumentation, principle and applications of nephelometry. Q3) Define Fluorescence
ASSIGNMENT - 1 Analytical Chemistry (DCHE21) Q1) State and explain Beer s Law mention its limitations. Q2) Write the basic instrumentation, principle and applications of nephelometry. Q3) Define Fluorescence
Process Analyzer and Sampling Systems
 Process Analyzer and Sampling Systems Course Price 3050 Course Description Analytical instruments used for online chemical analysis of process streams or plant environments are generally called Process
Process Analyzer and Sampling Systems Course Price 3050 Course Description Analytical instruments used for online chemical analysis of process streams or plant environments are generally called Process
Questions on Instrumental Methods of Analysis
 Questions on Instrumental Methods of Analysis 1. Which one of the following techniques can be used for the detection in a liquid chromatograph? a. Ultraviolet absorbance or refractive index measurement.
Questions on Instrumental Methods of Analysis 1. Which one of the following techniques can be used for the detection in a liquid chromatograph? a. Ultraviolet absorbance or refractive index measurement.
ii) Describe the various types of detectors used in IR spectroscopy.
 (DCHE 21) ASSIGNMENT - 1, DEC - 2014. PAPER - V : ANALYTICAL 1) Describe a method for the determination of manganese in complex matrices. 2) Describe the instrumentation and working principles of IR. 3)
(DCHE 21) ASSIGNMENT - 1, DEC - 2014. PAPER - V : ANALYTICAL 1) Describe a method for the determination of manganese in complex matrices. 2) Describe the instrumentation and working principles of IR. 3)
VI SEMESTER ANALYTICAL INSTRUMENTS(EI 1352)
 VI SEMESTER ANALYTICAL INSTRUMENTS(EI 1352) Two marks Questiions & Answers UNIT I 1. State Bouguer s law. The mathematical statement which states that the radiant power absorbed is proportional to the
VI SEMESTER ANALYTICAL INSTRUMENTS(EI 1352) Two marks Questiions & Answers UNIT I 1. State Bouguer s law. The mathematical statement which states that the radiant power absorbed is proportional to the
: INSTRUMENTATION AND PROCESS CONTROL COURSE CODE : 6071 COURSE CATEGORY : A PERIODS/ WEEK : 5 PERIODS/ SEMESTER : 75 CREDIT : 5 TIME SCHEDULE
 COURSE TITLE : INSTRUMENTATION AND PROCESS CONTROL COURSE CODE : 6071 COURSE CATEGORY : A PERIODS/ WEEK : 5 PERIODS/ SEMESTER : 75 CREDIT : 5 TIME SCHEDULE MODULE TOPIC PERIODS 1 Measuring Instruments
COURSE TITLE : INSTRUMENTATION AND PROCESS CONTROL COURSE CODE : 6071 COURSE CATEGORY : A PERIODS/ WEEK : 5 PERIODS/ SEMESTER : 75 CREDIT : 5 TIME SCHEDULE MODULE TOPIC PERIODS 1 Measuring Instruments
UNIT 4 Part 2 Measurements of Environmental Air Pollution Parameters j
 UNIT 4 Part 2 Measurements of Environmental Air Pollution Parameters j 1 Environmental Air Pollution The addition of any harmful material (having harmful effects on our lives) to our atmosphere is called
UNIT 4 Part 2 Measurements of Environmental Air Pollution Parameters j 1 Environmental Air Pollution The addition of any harmful material (having harmful effects on our lives) to our atmosphere is called
Gaseous CEMS technology
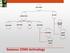 GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
Cork Institute of Technology. Summer 2005 Instrumental Analysis (Time: 3 Hours) Section A
 Cork Institute of Technology Higher Certificate in Science in Applied Biology Award (National Certificate in Science in Applied Biology Award) Answer FIVE questions; answer Section A, TWO questions from
Cork Institute of Technology Higher Certificate in Science in Applied Biology Award (National Certificate in Science in Applied Biology Award) Answer FIVE questions; answer Section A, TWO questions from
Chromatography & instrumentation in Organic Chemistry
 Chromatography & instrumentation in Organic Chemistry What is Chromatography? Chromatography is a technique for separating mixtures into their components in order to analyze, identify, purify, and/or quantify
Chromatography & instrumentation in Organic Chemistry What is Chromatography? Chromatography is a technique for separating mixtures into their components in order to analyze, identify, purify, and/or quantify
Gas chromatography. Advantages of GC. Disadvantages of GC
 Advantages of GC Gas chromatography Fast analysis, typically minutes Effi cient, providing high resolution Sensitive, easily detecting ppm and often ppb Nondestructive, making possible on - line coupling;
Advantages of GC Gas chromatography Fast analysis, typically minutes Effi cient, providing high resolution Sensitive, easily detecting ppm and often ppb Nondestructive, making possible on - line coupling;
COURSE DELIVERY PLAN - THEORY Page 1 of 6
 COURSE DELIVERY PLAN - THEORY Page 1 of 6 Department of Applied Chemistry B.Tech: Chemical Engineering Regulation: 2013 Sub. Code / Sub. Name : CH6501 / Instrumental Methods of Analysis Unit: I LP: Sub
COURSE DELIVERY PLAN - THEORY Page 1 of 6 Department of Applied Chemistry B.Tech: Chemical Engineering Regulation: 2013 Sub. Code / Sub. Name : CH6501 / Instrumental Methods of Analysis Unit: I LP: Sub
Spectroscopy. a laboratory method of analyzing matter using electromagnetic radiation.
 Spectroscopy a laboratory method of analyzing matter using electromagnetic radiation. Mass Spectrometry Determines the relative abundance of the different isotopes of an element Used to determine the average
Spectroscopy a laboratory method of analyzing matter using electromagnetic radiation. Mass Spectrometry Determines the relative abundance of the different isotopes of an element Used to determine the average
Spectroscopy. a laboratory method of analyzing matter using electromagnetic radiation
 Spectroscopy a laboratory method of analyzing matter using electromagnetic radiation The electromagnetic spectrum Radiation Scale of Absorption involves: Example of spectroscopy Gamma rays pm Nuclear reactions
Spectroscopy a laboratory method of analyzing matter using electromagnetic radiation The electromagnetic spectrum Radiation Scale of Absorption involves: Example of spectroscopy Gamma rays pm Nuclear reactions
Chemistry 524--Final Exam--Keiderling Dec. 12, pm SES
 Chemistry 524--Final Exam--Keiderling Dec. 12, 2002 --4-8 pm -- 238 SES Please answer all questions in the answer book provided. Calculators, rulers, pens and pencils are permitted plus one 8.5 x 11 sheet
Chemistry 524--Final Exam--Keiderling Dec. 12, 2002 --4-8 pm -- 238 SES Please answer all questions in the answer book provided. Calculators, rulers, pens and pencils are permitted plus one 8.5 x 11 sheet
Research Equipment in the UCA Chemistry Department
 Research Equipment in the UCA Chemistry Department Varian 220 Atomic Absorption Spectrometer Atomic Absorption spectroscopy is used to measure the amount of a metal ion present in a solution. It is particularly
Research Equipment in the UCA Chemistry Department Varian 220 Atomic Absorption Spectrometer Atomic Absorption spectroscopy is used to measure the amount of a metal ion present in a solution. It is particularly
Analytical techniques: Environmental samples. Lecture 2 Universidade do Algarve
 Analytical techniques: Environmental samples Lecture 2 Universidade do Algarve Terms, definitions & applications Difference between technique and method: Analytical technique: Fundamental scientific application
Analytical techniques: Environmental samples Lecture 2 Universidade do Algarve Terms, definitions & applications Difference between technique and method: Analytical technique: Fundamental scientific application
CH0302 Process Instrumentation. Lecture 9 Composition Analysis
 CH0302 Process Instrumentation Lecture 9 Composition Analysis Department of Chemical Engineering School of Bioengineering SRM University Kattankulathur 603203 4/3/15 Chemical Engineering 1 Industrial significance
CH0302 Process Instrumentation Lecture 9 Composition Analysis Department of Chemical Engineering School of Bioengineering SRM University Kattankulathur 603203 4/3/15 Chemical Engineering 1 Industrial significance
3 - Atomic Absorption Spectroscopy
 3 - Atomic Absorption Spectroscopy Introduction Atomic-absorption (AA) spectroscopy uses the absorption of light to measure the concentration of gas-phase atoms. Since samples are usually liquids or solids,
3 - Atomic Absorption Spectroscopy Introduction Atomic-absorption (AA) spectroscopy uses the absorption of light to measure the concentration of gas-phase atoms. Since samples are usually liquids or solids,
LAB007 Industrial Analytical Chemistry & Process Analyzer System
 LAB007 Industrial Analytical Chemistry & Process Analyzer System H.H. Sheikh Sultan Tower (0) Floor Corniche Street Abu Dhabi U.A.E www.ictd.ae ictd@ictd.ae Course Introduction: Analytical instruments
LAB007 Industrial Analytical Chemistry & Process Analyzer System H.H. Sheikh Sultan Tower (0) Floor Corniche Street Abu Dhabi U.A.E www.ictd.ae ictd@ictd.ae Course Introduction: Analytical instruments
Ondansetron Hydrochloride Tablets
 Ondansetron Hydrochloride Tablets Dissolution Perform the test with 1 tablet of Ondansetron Hydrochloride Tablets at 50 revolutions per minute according to the Paddle method, using 900 ml of water
Ondansetron Hydrochloride Tablets Dissolution Perform the test with 1 tablet of Ondansetron Hydrochloride Tablets at 50 revolutions per minute according to the Paddle method, using 900 ml of water
ASSIGNMENT - 1, DEC M.Sc. (FINAL) SECOND YEAR DEGREE CHEMISTRY. Maximum : 20 MARKS Answer ALL questions.
 (DCHE 21) ASSIGNMENT - 1, DEC-2013. PAPER- V : ANALYTICAL 1. How can you determine PK values of an acid base indicator? Explain with an example. 2. Explain the principle of Nephelometry. Write few applications
(DCHE 21) ASSIGNMENT - 1, DEC-2013. PAPER- V : ANALYTICAL 1. How can you determine PK values of an acid base indicator? Explain with an example. 2. Explain the principle of Nephelometry. Write few applications
Ch 313 FINAL EXAM OUTLINE Spring 2010
 Ch 313 FINAL EXAM OUTLINE Spring 2010 NOTE: Use this outline at your own risk sometimes a topic is omitted that you are still responsible for. It is meant to be a study aid and is not meant to be a replacement
Ch 313 FINAL EXAM OUTLINE Spring 2010 NOTE: Use this outline at your own risk sometimes a topic is omitted that you are still responsible for. It is meant to be a study aid and is not meant to be a replacement
Atmospheric Analysis Gases. Sampling and analysis of gaseous compounds
 Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Chemistry Instrumental Analysis Lecture 15. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 15 IR Instruments Types of Instrumentation Dispersive Spectrophotometers (gratings) Fourier transform spectrometers (interferometer) Single beam Double beam
Chemistry 4631 Instrumental Analysis Lecture 15 IR Instruments Types of Instrumentation Dispersive Spectrophotometers (gratings) Fourier transform spectrometers (interferometer) Single beam Double beam
2401 Gas (liquid) Chromatography
 2401 Gas (liquid) Chromatography Chromatography Scheme Gas chromatography - specifically gas-liquid chromatography - involves a sample being vaporized and injected onto the head of the chromatographic
2401 Gas (liquid) Chromatography Chromatography Scheme Gas chromatography - specifically gas-liquid chromatography - involves a sample being vaporized and injected onto the head of the chromatographic
Introduction. Types of Testing: On-line v. Off-line
 Introduction There are many useful analytical tools, such as photometric analysis, electrometric analysis, chromatography, mass spectrometry, thermal conductivity, and various physical property measurements
Introduction There are many useful analytical tools, such as photometric analysis, electrometric analysis, chromatography, mass spectrometry, thermal conductivity, and various physical property measurements
2101 Atomic Spectroscopy
 2101 Atomic Spectroscopy Atomic identification Atomic spectroscopy refers to the absorption and emission of ultraviolet to visible light by atoms and monoatomic ions. It is best used to analyze metals.
2101 Atomic Spectroscopy Atomic identification Atomic spectroscopy refers to the absorption and emission of ultraviolet to visible light by atoms and monoatomic ions. It is best used to analyze metals.
(DCHE 21) M.Sc. (Final) DEGREE EXAMINATION, DECEMBER Second Year. Chemistry. Paper V ANALYTICAL CHEMISTRY. PART A (4 10 = 40 marks)
 (DCHE 21) M.Sc. (Final) DEGREE EXAMINATION, DECEMBER 2010. Second Year Chemistry Paper V ANALYTICAL CHEMISTRY Time : Three hours Maximum : 100 marks PART A (4 10 = 40 marks) Answer any FOUR questions.
(DCHE 21) M.Sc. (Final) DEGREE EXAMINATION, DECEMBER 2010. Second Year Chemistry Paper V ANALYTICAL CHEMISTRY Time : Three hours Maximum : 100 marks PART A (4 10 = 40 marks) Answer any FOUR questions.
Luminescence transitions. Fluorescence spectroscopy
 Luminescence transitions Fluorescence spectroscopy Advantages: High sensitivity (single molecule detection!) Measuring increment in signal against a dark (zero) background Emission is proportional to excitation
Luminescence transitions Fluorescence spectroscopy Advantages: High sensitivity (single molecule detection!) Measuring increment in signal against a dark (zero) background Emission is proportional to excitation
AN INTRODUCTION TO ATOMIC SPECTROSCOPY
 AN INTRODUCTION TO ATOMIC SPECTROSCOPY Atomic spectroscopy deals with the absorption, emission, or fluorescence by atom or elementary ions. Two regions of the spectrum yield atomic information- the UV-visible
AN INTRODUCTION TO ATOMIC SPECTROSCOPY Atomic spectroscopy deals with the absorption, emission, or fluorescence by atom or elementary ions. Two regions of the spectrum yield atomic information- the UV-visible
25 Instruments for Optical Spectrometry
 25 Instruments for Optical Spectrometry 25A INSTRUMENT COMPONENTS (1) source of radiant energy (2) wavelength selector (3) sample container (4) detector (5) signal processor and readout (a) (b) (c) Fig.
25 Instruments for Optical Spectrometry 25A INSTRUMENT COMPONENTS (1) source of radiant energy (2) wavelength selector (3) sample container (4) detector (5) signal processor and readout (a) (b) (c) Fig.
Introduction to Pharmaceutical Chemical Analysis
 Introduction to Pharmaceutical Chemical Analysis Hansen, Steen ISBN-13: 9780470661222 Table of Contents Preface xv 1 Introduction to Pharmaceutical Analysis 1 1.1 Applications and Definitions 1 1.2 The
Introduction to Pharmaceutical Chemical Analysis Hansen, Steen ISBN-13: 9780470661222 Table of Contents Preface xv 1 Introduction to Pharmaceutical Analysis 1 1.1 Applications and Definitions 1 1.2 The
M.Sc. (Final) DEGREE EXAMINATION, DECEMBER Second Year Chemistry Paper V ANALYTICAL CHEMISTRY. PART A (4 10 = 40 marks)
 (DCHE 21) M.Sc. (Final) DEGREE EXAMINATION, DECEMBER 2009. Second Year Chemistry Paper V ANALYTICAL CHEMISTRY Time : Three hours Maximum : 100 marks PART A (4 10 = 40 marks) Answer any FOUR questions.
(DCHE 21) M.Sc. (Final) DEGREE EXAMINATION, DECEMBER 2009. Second Year Chemistry Paper V ANALYTICAL CHEMISTRY Time : Three hours Maximum : 100 marks PART A (4 10 = 40 marks) Answer any FOUR questions.
Analytical Topics to Consider in preparation for the MFAT/GRE
 Analytical Topics to Consider in preparation for the MFAT/GRE 1. Solutions and Measurement: (CHEM 222) Describe the steps in a chemical analysis Know the meaning and use of the following solution concentrations.
Analytical Topics to Consider in preparation for the MFAT/GRE 1. Solutions and Measurement: (CHEM 222) Describe the steps in a chemical analysis Know the meaning and use of the following solution concentrations.
Spectrophotometry. Introduction
 Spectrophotometry Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. The basic principle
Spectrophotometry Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. The basic principle
a. An emission line as close as possible to the analyte resonance line
 Practice Problem Set 5 Atomic Emission Spectroscopy 10-1 What is an internal standard and why is it used? An internal standard is a substance added to samples, blank, and standards. The ratio of the signal
Practice Problem Set 5 Atomic Emission Spectroscopy 10-1 What is an internal standard and why is it used? An internal standard is a substance added to samples, blank, and standards. The ratio of the signal
AL108 Process Analyzers & Sampling Systems
 AL108 Process Analyzers & Sampling Systems AL108 Rev.002 CMCT COURSE OUTLINE Page 1 of 8 Training Description: Analytical instruments used for online chemical analysis of process streams or plant environments
AL108 Process Analyzers & Sampling Systems AL108 Rev.002 CMCT COURSE OUTLINE Page 1 of 8 Training Description: Analytical instruments used for online chemical analysis of process streams or plant environments
White Paper. Overview: NDIR Definition:
 Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
Spectroscopy and Chromatography
 Spectroscopy and Chromatography Introduction Visible light is one very small part of the electromagnetic spectrum. The different properties of the various types of radiation depend upon their wavelength.
Spectroscopy and Chromatography Introduction Visible light is one very small part of the electromagnetic spectrum. The different properties of the various types of radiation depend upon their wavelength.
3) In CE separation is based on what two properties of the solutes? (3 pts)
 Final Exam Chem 311 Fall 2002 December 16 Name 1) (3 pts) In GC separation is based on the following two properties of the solutes a) polarity and size b) vapor pressure and molecular weight c) vapor pressure
Final Exam Chem 311 Fall 2002 December 16 Name 1) (3 pts) In GC separation is based on the following two properties of the solutes a) polarity and size b) vapor pressure and molecular weight c) vapor pressure
Chem 321 Name Answer Key D. Miller
 1. For a reversed-phase chromatography experiment, it is noted that the retention time of an analyte decreases as the percent of acetonitrile (CH 3 CN) increases in a CH 3 CN/H 2 O mobile phase. Explain
1. For a reversed-phase chromatography experiment, it is noted that the retention time of an analyte decreases as the percent of acetonitrile (CH 3 CN) increases in a CH 3 CN/H 2 O mobile phase. Explain
Course Details. Analytical Techniques Based on Optical Spectroscopy. Course Details. Textbook. SCCH 211: Analytical Chemistry I
 SCCH 211: Analytical Chemistry I Analytical Techniques Based on Optical Spectroscopy Course Details September 22 October 10 September 22 November 7 November 17 December 1 Topic Period Introduction to Spectrometric
SCCH 211: Analytical Chemistry I Analytical Techniques Based on Optical Spectroscopy Course Details September 22 October 10 September 22 November 7 November 17 December 1 Topic Period Introduction to Spectrometric
Instrumental Analysis
 Chem 454 Name: Instrumental Analysis Exam I February 5, 1999 80 possible points 1] 5 points Which of the following samples would be suitable for analysis by a calibration curve technique using a potentiometric
Chem 454 Name: Instrumental Analysis Exam I February 5, 1999 80 possible points 1] 5 points Which of the following samples would be suitable for analysis by a calibration curve technique using a potentiometric
Sampling for Gases and Vapors
 Sampling for Gases and Vapors EOH 466B Evaluating the Occupational Environment Spring 2008 Gases and Vapors Gases: don't exist in liquid state at STP e.g.: nitrous oxides, ozone, carbon monoxide Vapors:
Sampling for Gases and Vapors EOH 466B Evaluating the Occupational Environment Spring 2008 Gases and Vapors Gases: don't exist in liquid state at STP e.g.: nitrous oxides, ozone, carbon monoxide Vapors:
Design and Development of a Smartphone Based Visible Spectrophotometer for Analytical Applications
 Design and Development of a Smartphone Based Visible Spectrophotometer for Analytical Applications Bedanta Kr. Deka, D. Thakuria, H. Bora and S. Banerjee # Department of Physicis, B. Borooah College, Ulubari,
Design and Development of a Smartphone Based Visible Spectrophotometer for Analytical Applications Bedanta Kr. Deka, D. Thakuria, H. Bora and S. Banerjee # Department of Physicis, B. Borooah College, Ulubari,
R O Y G B V. Spin States. Outer Shell Electrons. Molecular Rotations. Inner Shell Electrons. Molecular Vibrations. Nuclear Transitions
 Spin States Molecular Rotations Molecular Vibrations Outer Shell Electrons Inner Shell Electrons Nuclear Transitions NMR EPR Microwave Absorption Spectroscopy Infrared Absorption Spectroscopy UV-vis Absorption,
Spin States Molecular Rotations Molecular Vibrations Outer Shell Electrons Inner Shell Electrons Nuclear Transitions NMR EPR Microwave Absorption Spectroscopy Infrared Absorption Spectroscopy UV-vis Absorption,
COMMON PAPER (FIRST SEMESTER) (ALL BRANCHES) I/II M.PHARMACY ADVANCED INSTRUMENTAL METHODS OF ANALYSIS (THEORY)
 COMMON PAPER (FIRST SEMESTER) (ALL BRANCHES) MPH 101 (T) I/II M.PHARMACY ADVANCED INSTRUMENTAL METHODS OF ANALYSIS (THEORY) Unit : 1 : UV.VISIBLE SPECTROSCOPY : Brief review of electromagnetic spectrum
COMMON PAPER (FIRST SEMESTER) (ALL BRANCHES) MPH 101 (T) I/II M.PHARMACY ADVANCED INSTRUMENTAL METHODS OF ANALYSIS (THEORY) Unit : 1 : UV.VISIBLE SPECTROSCOPY : Brief review of electromagnetic spectrum
Fourier Transform Infrared. Spectrometry
 Fourier Transform Infrared. Spectrometry Second Editio n PETER R. GRIFFITH S JAMES A. de HASETH PREFACE x v CHAPTER 1 INTRODUCTION TO VIBRATIONAL SPECTROSCOPY 1 1.1. Introduction 1 1.2. Molecular Vibrations
Fourier Transform Infrared. Spectrometry Second Editio n PETER R. GRIFFITH S JAMES A. de HASETH PREFACE x v CHAPTER 1 INTRODUCTION TO VIBRATIONAL SPECTROSCOPY 1 1.1. Introduction 1 1.2. Molecular Vibrations
FACULTY OF PHARMACY. M. Pharmacy I Semester (Suppl.) Examination, November 2015 (Common To All) Subject: Pharmaceutical Analytical Techniques
 M. Pharmacy I Semester (Suppl.) Examination, November 2015 (Common To All) Subject: Pharmaceutical Analytical Techniques Code No. 6001 / S Note: Answer any Five questions. All questions carry equal marks.
M. Pharmacy I Semester (Suppl.) Examination, November 2015 (Common To All) Subject: Pharmaceutical Analytical Techniques Code No. 6001 / S Note: Answer any Five questions. All questions carry equal marks.
FLAME PHOTOMETRY AIM INTRODUCTION
 FLAME PHOTOMETRY AIM INTRODUCTION Atomic spectroscopy is based on the absorption, emission or fluorescence process of light by atoms or elementary ions. Information for atomic scale is obtained in two
FLAME PHOTOMETRY AIM INTRODUCTION Atomic spectroscopy is based on the absorption, emission or fluorescence process of light by atoms or elementary ions. Information for atomic scale is obtained in two
Unit title: Atomic and Nuclear Physics for Spectroscopic Applications
 Unit title: Atomic and Nuclear Physics for Spectroscopic Applications Unit code: Y/601/0417 QCF level: 4 Credit value: 15 Aim This unit provides an understanding of the underlying atomic and nuclear physics
Unit title: Atomic and Nuclear Physics for Spectroscopic Applications Unit code: Y/601/0417 QCF level: 4 Credit value: 15 Aim This unit provides an understanding of the underlying atomic and nuclear physics
高等食品分析 (Advanced Food Analysis) I. SPECTROSCOPIC METHODS *Instrumental methods: 1. Spectroscopic methods (spectroscopy): a) Electromagnetic radiation
 *Instrumental methods: 1. Spectroscopic methods (spectroscopy): a) Electromagnetic radiation (EMR): γ-ray emission X-Ray absorption, emission, fluorescence and diffraction Vacuum ultraviolet (UV) absorption
*Instrumental methods: 1. Spectroscopic methods (spectroscopy): a) Electromagnetic radiation (EMR): γ-ray emission X-Ray absorption, emission, fluorescence and diffraction Vacuum ultraviolet (UV) absorption
PRINCIPLES AND APPLICATION OF CHROMATOGRAPHY. Dr. P. Jayachandra Reddy Mpharm PhD Principal & professor KTPC
 PRINCIPLES AND APPLICATION OF CHROMATOGRAPHY Dr. P. Jayachandra Reddy Mpharm PhD Principal & professor KTPC CHROMATOGRAPHY Laboratory technique for the Separation of mixtures Chroma -"color" and graphein
PRINCIPLES AND APPLICATION OF CHROMATOGRAPHY Dr. P. Jayachandra Reddy Mpharm PhD Principal & professor KTPC CHROMATOGRAPHY Laboratory technique for the Separation of mixtures Chroma -"color" and graphein
Module 2. Measurement Systems. Version 2 EE IIT, Kharagpur 1
 Module 2 Measurement Systems Version 2 EE IIT, Kharagpur 1 Lesson 8 Measurement of Level, Humidity and ph Version 2 EE IIT, Kharagpur 2 Instructional Objectives At the end of this lesson, the student will
Module 2 Measurement Systems Version 2 EE IIT, Kharagpur 1 Lesson 8 Measurement of Level, Humidity and ph Version 2 EE IIT, Kharagpur 2 Instructional Objectives At the end of this lesson, the student will
Mini review for final
 Mini review for final NB: This does not cover all material! (very) Rough outline Units, Conversions, Powers of 10, Prefixes Errors: Sig Figs, Error propagation Statistics: Normal distribution, t-test,
Mini review for final NB: This does not cover all material! (very) Rough outline Units, Conversions, Powers of 10, Prefixes Errors: Sig Figs, Error propagation Statistics: Normal distribution, t-test,
VALLIAMMAI ENGINEERING COLLEGE DEPARTMENT OF MECHANICAL ENGINEERING ME (CAD/CAM) QUESTION BANK
 VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF MECHANICAL ENGINEERING ME (CAD/CAM) QUESTION BANK III SEMESTER CC 7007 METROLOGY AND NON DESTRUCTIVE TESTING Regulation 2013
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF MECHANICAL ENGINEERING ME (CAD/CAM) QUESTION BANK III SEMESTER CC 7007 METROLOGY AND NON DESTRUCTIVE TESTING Regulation 2013
Contents. Foreword by Darrell H. Reneker
 Table of Foreword by Darrell H. Reneker Preface page xi xiii 1 Introduction 1 1.1 How big is a nanometer? 1 1.2 What is nanotechnology? 1 1.3 Historical development of nanotechnology 2 1.4 Classification
Table of Foreword by Darrell H. Reneker Preface page xi xiii 1 Introduction 1 1.1 How big is a nanometer? 1 1.2 What is nanotechnology? 1 1.3 Historical development of nanotechnology 2 1.4 Classification
PAPER No. 12: ORGANIC SPECTROSCOPY MODULE No. 4: Basic principles and Instrumentation for IR spectroscopy
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy Module 4: Basic principles and Instrumentation for IR spectroscopy CHE_P12_M4_e-Text TABLE OF CONTENTS
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy Module 4: Basic principles and Instrumentation for IR spectroscopy CHE_P12_M4_e-Text TABLE OF CONTENTS
Supplementary information for Organically doped palladium: a highly efficient catalyst for electroreduction of CO 2 to methanol
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2015 Supplementary information for rganically doped palladium: a highly efficient catalyst for
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2015 Supplementary information for rganically doped palladium: a highly efficient catalyst for
Ultraviolet-Visible and Infrared Spectrophotometry
 Ultraviolet-Visible and Infrared Spectrophotometry Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451
Ultraviolet-Visible and Infrared Spectrophotometry Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451
MAE 214 FUEL CELL FUNDAMENTALS & TECHNOLOGY FC ANALYSES TECHNIQUES
 MAE 214 FUEL CELL FUNDAMENTALS & TECHNOLOGY Fuel Cell Analyses Methods NFCRC DR. JACK BROUWER MAE 214 Lecture #11 Spring, 2005 FC ANALYSES TECHNIQUES Potential Sweep Methods Linear Sweep Voltammetry (I-V)
MAE 214 FUEL CELL FUNDAMENTALS & TECHNOLOGY Fuel Cell Analyses Methods NFCRC DR. JACK BROUWER MAE 214 Lecture #11 Spring, 2005 FC ANALYSES TECHNIQUES Potential Sweep Methods Linear Sweep Voltammetry (I-V)
4) Discuss the limitation of Floorimetry and Phosphorimetry. 5) Describe the instrumentation and principle of IR spectroscopy. Write its limitations.
 (DCHE 21) ASSIGNMENT - 1, DEC - 2016. PAPER - V : ANALYTICAL 1) Write a note on photometric titrations. 2) Determine PK values of an acid-base indicator. 3) Compare and contrast Nephelometry and Turbidimetry.
(DCHE 21) ASSIGNMENT - 1, DEC - 2016. PAPER - V : ANALYTICAL 1) Write a note on photometric titrations. 2) Determine PK values of an acid-base indicator. 3) Compare and contrast Nephelometry and Turbidimetry.
2001 Spectrometers. Instrument Machinery. Movies from this presentation can be access at
 2001 Spectrometers Instrument Machinery Movies from this presentation can be access at http://www.shsu.edu/~chm_tgc/sounds/sound.html Chp20: 1 Optical Instruments Instrument Components Components of various
2001 Spectrometers Instrument Machinery Movies from this presentation can be access at http://www.shsu.edu/~chm_tgc/sounds/sound.html Chp20: 1 Optical Instruments Instrument Components Components of various
Gas Chromatography. Chromatography Laboratory Course. Dr. Christian Jungnickel Chromatography Course GC September 2005
 Gas Chromatography Chromatography Laboratory Course The laboratory course experiments General Aim: Gain general experience using a GC Constant Injection technique Temperature variations Qualitative and
Gas Chromatography Chromatography Laboratory Course The laboratory course experiments General Aim: Gain general experience using a GC Constant Injection technique Temperature variations Qualitative and
Sub-category: Physics and Principles of Measurement Topic: Monitoring anesthetic gases and vapours Date: January 15-17, 2016 Language: English
 Course n : Course 3 Title: RESPIRATORY PHYSIOLOGY, PHYSICS AND PATHOLOGY IN RELATION TO ANAESTHESIA AND INTENSIVE CARE Sub-category: Physics and Principles of Measurement Topic: Monitoring anesthetic gases
Course n : Course 3 Title: RESPIRATORY PHYSIOLOGY, PHYSICS AND PATHOLOGY IN RELATION TO ANAESTHESIA AND INTENSIVE CARE Sub-category: Physics and Principles of Measurement Topic: Monitoring anesthetic gases
The Claviature of Gas Analysis
 The Claviature of Gas Analysis Mass spectrometric gas analysis for quality assurance of gases and gas mixtures High purity and special gases are becoming increasingly important in the development of modern
The Claviature of Gas Analysis Mass spectrometric gas analysis for quality assurance of gases and gas mixtures High purity and special gases are becoming increasingly important in the development of modern
Reference literature. (See: CHEM 2470 notes, Module 8 Textbook 6th ed., Chapters )
 September 17, 2018 Reference literature (See: CHEM 2470 notes, Module 8 Textbook 6th ed., Chapters 13-14 ) Reference.: https://slideplayer.com/slide/8354408/ Spectroscopy Usual Wavelength Type of Quantum
September 17, 2018 Reference literature (See: CHEM 2470 notes, Module 8 Textbook 6th ed., Chapters 13-14 ) Reference.: https://slideplayer.com/slide/8354408/ Spectroscopy Usual Wavelength Type of Quantum
Advanced Pharmaceutical Analysis
 Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
Fourier Transform Infrared Spectrometry Prelab Last modified: June 17, 2014
 Fourier Transform Infrared Spectrometry Prelab Recommended reading: AirUCI Lab Manual: Environmental Chemistry Text: FTIR Lab Pages: 6, 7, 13 16 on Light Absorption Pages: 175 177, 184, 185 on Molecular
Fourier Transform Infrared Spectrometry Prelab Recommended reading: AirUCI Lab Manual: Environmental Chemistry Text: FTIR Lab Pages: 6, 7, 13 16 on Light Absorption Pages: 175 177, 184, 185 on Molecular
Atomization. In Flame Emission
 FLAME SPECTROSCOPY The concentration of an element in a solution is determined by measuring the absorption, emission or fluorescence of electromagnetic by its monatomic particles in gaseous state in the
FLAME SPECTROSCOPY The concentration of an element in a solution is determined by measuring the absorption, emission or fluorescence of electromagnetic by its monatomic particles in gaseous state in the
Chemistry 325 Instrumental Methods of Analysis March 13, Final Exam. Name
 Final Exam Name Instructions: This exam is worth 100 points. Some questions allow a choice as to which parts are answered. Only answer the number of parts requested. 1. (32 points) Circle the best answer
Final Exam Name Instructions: This exam is worth 100 points. Some questions allow a choice as to which parts are answered. Only answer the number of parts requested. 1. (32 points) Circle the best answer
Siddharth Institute of Engineering & Technology
 SIDDHARTH INSTITUTE OF ENGINEERING & TECHNOLOGY :: PUTTUR (AUTONOMOUS) (Approved by AICTE, New Delhi & Affiliated to JNTUA, Anantapuramu) (Accredited by NBA & Accredited by NAAC with A Grade) (An ISO 9001:2008
SIDDHARTH INSTITUTE OF ENGINEERING & TECHNOLOGY :: PUTTUR (AUTONOMOUS) (Approved by AICTE, New Delhi & Affiliated to JNTUA, Anantapuramu) (Accredited by NBA & Accredited by NAAC with A Grade) (An ISO 9001:2008
Technical know-how in thermal analysis measurement
 Technical articles Technical know-how in thermal analysis measurement Evolved gas analysis by thermogravimetry-differential thermal analysis-mass spectrometry (TG-DTA-MS) technique Kazuko Motomura* and
Technical articles Technical know-how in thermal analysis measurement Evolved gas analysis by thermogravimetry-differential thermal analysis-mass spectrometry (TG-DTA-MS) technique Kazuko Motomura* and
Atomic Emission Spectroscopy
 Atomic Emission Spectroscopy Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451 Saudi Arabia Building:
Atomic Emission Spectroscopy Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451 Saudi Arabia Building:
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth
 FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
EMISSION SPECTROSCOPY
 IFM The Department of Physics, Chemistry and Biology LAB 57 EMISSION SPECTROSCOPY NAME PERSONAL NUMBER DATE APPROVED I. OBJECTIVES - Understand the principle of atomic emission spectra. - Know how to acquire
IFM The Department of Physics, Chemistry and Biology LAB 57 EMISSION SPECTROSCOPY NAME PERSONAL NUMBER DATE APPROVED I. OBJECTIVES - Understand the principle of atomic emission spectra. - Know how to acquire
III B.Tech. II Semester Regular Examinations, April/May INSTRUMENTATION & CONTROL SYSTEMS (Mechanical Engineering) Time: 3 Hours Max Marks: 75
 R10 Set No: 1 1. (a) Distinguish between accuracy and Precision. Which of these is more desirable during the act of measurement and why? (b) Discuss the necessity and importance of dynamic performance
R10 Set No: 1 1. (a) Distinguish between accuracy and Precision. Which of these is more desirable during the act of measurement and why? (b) Discuss the necessity and importance of dynamic performance
Chapter 13 An Introduction to Ultraviolet/Visible Molecular Absorption Spectrometry
 Chapter 13 An Introduction to Ultraviolet/Visible Molecular Absorption Spectrometry 13A Measurement Of Transmittance and Absorbance Absorption measurements based upon ultraviolet and visible radiation
Chapter 13 An Introduction to Ultraviolet/Visible Molecular Absorption Spectrometry 13A Measurement Of Transmittance and Absorbance Absorption measurements based upon ultraviolet and visible radiation
ADVANCED ANALYTICAL LABORATORY
 An Information Brochure on ADVANCED ANALYTICAL LABORATORY ANDHRA UNIVERSITY Visakhapatnam - 530 003 Andhra Pradesh, India Sponsored by DEPARTMENT OF SCIENCE & TECHNOLOGY Government of India New Delhi 110016
An Information Brochure on ADVANCED ANALYTICAL LABORATORY ANDHRA UNIVERSITY Visakhapatnam - 530 003 Andhra Pradesh, India Sponsored by DEPARTMENT OF SCIENCE & TECHNOLOGY Government of India New Delhi 110016
CHEM*3440. Photon Energy Units. Spectrum of Electromagnetic Radiation. Chemical Instrumentation. Spectroscopic Experimental Concept.
 Spectrum of Electromagnetic Radiation Electromagnetic radiation is light. Different energy light interacts with different motions in molecules. CHEM*344 Chemical Instrumentation Topic 7 Spectrometry Radiofrequency
Spectrum of Electromagnetic Radiation Electromagnetic radiation is light. Different energy light interacts with different motions in molecules. CHEM*344 Chemical Instrumentation Topic 7 Spectrometry Radiofrequency
Instrumental Chemical Analysis
 L1 Page1 Instrumental Chemical Analysis Dr. Ahmad Najjar Philadelphia University Faculty of Pharmacy Department of Pharmaceutical Sciences 2 nd semester, 2016/2017 L1 Page2 Course Syllabus Course title:
L1 Page1 Instrumental Chemical Analysis Dr. Ahmad Najjar Philadelphia University Faculty of Pharmacy Department of Pharmaceutical Sciences 2 nd semester, 2016/2017 L1 Page2 Course Syllabus Course title:
UNIT 3 CHEMISTRY. Fundamental Principles in Chemistry
 UNIT 3 CHEMISTRY NOTE: This list has been compiled based on the topics covered in the 2016 Master Class program. Once all of the 2017 Chemistry program materials have been finalised, this summary will
UNIT 3 CHEMISTRY NOTE: This list has been compiled based on the topics covered in the 2016 Master Class program. Once all of the 2017 Chemistry program materials have been finalised, this summary will
photo-mineralization of 2-propanol under visible light irradiation
 Electronic Supplementary Information for WO 3 modified titanate network film: highly efficient photo-mineralization of 2-propanol under visible light irradiation Experimental Preparation of STN, and WO
Electronic Supplementary Information for WO 3 modified titanate network film: highly efficient photo-mineralization of 2-propanol under visible light irradiation Experimental Preparation of STN, and WO
Chapter 31 Gas Chromatography. Carrier Gas System
 Chapter 31 Gas Chromatography GAS-LIQUID CHROMATOGRAPHY In gas chromatography, the components of a vaporized sample are fractionated as a consequence of being partitioned between a mobile gaseous phase
Chapter 31 Gas Chromatography GAS-LIQUID CHROMATOGRAPHY In gas chromatography, the components of a vaporized sample are fractionated as a consequence of being partitioned between a mobile gaseous phase
EE 5344 Introduction to MEMS CHAPTER 7 Biochemical Sensors. Biochemical Microsensors
 I. Basic Considerations & Definitions 1. Definitions: EE 5344 Introduction to MEMS CHAPTER 7 Biochemical Sensors Chemical/ Biological quantity Biochemical Microsensors Electrical Signal Ex: Chemical species
I. Basic Considerations & Definitions 1. Definitions: EE 5344 Introduction to MEMS CHAPTER 7 Biochemical Sensors Chemical/ Biological quantity Biochemical Microsensors Electrical Signal Ex: Chemical species
VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR
 AP-157 VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR Grant M. Plummer, Ph.D. Peter Zemek, Ph.D. Special Projects Manager Applications Manager Enthalpy Analytical,
AP-157 VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR Grant M. Plummer, Ph.D. Peter Zemek, Ph.D. Special Projects Manager Applications Manager Enthalpy Analytical,
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY
 Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY Chapter 23. Gas Chromatography What did they eat in the year 1,000? GC of Cholesterol and other lipids extracted from
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY Chapter 23. Gas Chromatography What did they eat in the year 1,000? GC of Cholesterol and other lipids extracted from
SCANNING-PROBE TECHNIQUES OR APPARATUS; APPLICATIONS OF SCANNING-PROBE TECHNIQUES, e.g. SCANNING PROBE MICROSCOPY [SPM]
![SCANNING-PROBE TECHNIQUES OR APPARATUS; APPLICATIONS OF SCANNING-PROBE TECHNIQUES, e.g. SCANNING PROBE MICROSCOPY [SPM] SCANNING-PROBE TECHNIQUES OR APPARATUS; APPLICATIONS OF SCANNING-PROBE TECHNIQUES, e.g. SCANNING PROBE MICROSCOPY [SPM]](/thumbs/81/83165631.jpg) G01Q SCANNING-PROBE TECHNIQUES OR APPARATUS; APPLICATIONS OF SCANNING-PROBE TECHNIQUES, e.g. SCANNING PROBE MICROSCOPY [SPM] Scanning probes, i.e. devices having at least a tip of nanometre sized dimensions
G01Q SCANNING-PROBE TECHNIQUES OR APPARATUS; APPLICATIONS OF SCANNING-PROBE TECHNIQUES, e.g. SCANNING PROBE MICROSCOPY [SPM] Scanning probes, i.e. devices having at least a tip of nanometre sized dimensions
PHYSICAL AND CHEMICAL PROPERTIES OF ATMOSPHERIC PRESSURE PLASMA POLYMER FILMS
 PHYSICAL AND CHEMICAL PROPERTIES OF ATMOSPHERIC PRESSURE PLASMA POLYMER FILMS O. Goossens, D. Vangeneugden, S. Paulussen and E. Dekempeneer VITO Flemish Institute for Technological Research, Boeretang
PHYSICAL AND CHEMICAL PROPERTIES OF ATMOSPHERIC PRESSURE PLASMA POLYMER FILMS O. Goossens, D. Vangeneugden, S. Paulussen and E. Dekempeneer VITO Flemish Institute for Technological Research, Boeretang
MASS ANALYSER. Mass analysers - separate the ions according to their mass-to-charge ratio. sample. Vacuum pumps
 ION ANALYZERS MASS ANALYSER sample Vacuum pumps Mass analysers - separate the ions according to their mass-to-charge ratio MASS ANALYSER Separate the ions according to their mass-to-charge ratio in space
ION ANALYZERS MASS ANALYSER sample Vacuum pumps Mass analysers - separate the ions according to their mass-to-charge ratio MASS ANALYSER Separate the ions according to their mass-to-charge ratio in space
Glove Box / BRAUN MB 150B-G
 Glove Box / BRAUN MB 150B-G Glove box: This box is always filled with N 2 or Ar gas without moisture, which can be perform for the measurement and/or synthetic experiments of air- and/or moisturesensitive
Glove Box / BRAUN MB 150B-G Glove box: This box is always filled with N 2 or Ar gas without moisture, which can be perform for the measurement and/or synthetic experiments of air- and/or moisturesensitive
Course: M.Sc (Chemistry) Analytical Chemistry Unit: III
 Course: M.Sc (Chemistry) Analytical Chemistry Unit: III Syllabus: Principle of spectrophotometry Types of spectrophotometer Applications - Dissociation constants of an indicator simultaneous spectrophotometric
Course: M.Sc (Chemistry) Analytical Chemistry Unit: III Syllabus: Principle of spectrophotometry Types of spectrophotometer Applications - Dissociation constants of an indicator simultaneous spectrophotometric
Spectroscopy: Introduction. Required reading Chapter 18 (pages ) Chapter 20 (pages )
 Spectroscopy: Introduction Required reading Chapter 18 (pages 378-397) Chapter 20 (pages 424-449) Spectrophotometry is any procedure that uses light to measure chemical concentrations Properties of Light
Spectroscopy: Introduction Required reading Chapter 18 (pages 378-397) Chapter 20 (pages 424-449) Spectrophotometry is any procedure that uses light to measure chemical concentrations Properties of Light
Analytical methods. Vladimíra Kvasnicová
 Analytical methods Vladimíra Kvasnicová Laboratory analysis analyte = compound of interest 1. Qualitative analysis ~ WHAT IS IT? = determining the nature of a pure unknown compound(s) in a mixture 2.
Analytical methods Vladimíra Kvasnicová Laboratory analysis analyte = compound of interest 1. Qualitative analysis ~ WHAT IS IT? = determining the nature of a pure unknown compound(s) in a mixture 2.
1. Cyclic voltammetry involves the measurement of a diffusion controlled at an electrode in which the is controlled. (4 points)
 Chem 454 First Exam Feb. 20, 2002 1. Cyclic voltammetry involves the measurement of a diffusion controlled at an electrode in which the is controlled. (4 points) 2. (5 points) A. Sketch a cyclic voltammogram
Chem 454 First Exam Feb. 20, 2002 1. Cyclic voltammetry involves the measurement of a diffusion controlled at an electrode in which the is controlled. (4 points) 2. (5 points) A. Sketch a cyclic voltammogram
RS DYNAMICS ECOPROBE 5. Portable IR/PID Gas Analyzer PID. PID and IR Analyzers
 RS DYNAMICS ECOPROBE 5 Portable IR/PID Gas Analyzer PID + IR PID and IR Analyzers General ECOPROBE 5 has two autonomous analyzers in one case. The combination of analyzers provides a set of data designed
RS DYNAMICS ECOPROBE 5 Portable IR/PID Gas Analyzer PID + IR PID and IR Analyzers General ECOPROBE 5 has two autonomous analyzers in one case. The combination of analyzers provides a set of data designed
1901 Application of Spectrophotometry
 1901 Application of Spectrophotometry Chemical Analysis Problem: 1 Application of Spectroscopy Organic Compounds Organic compounds with single bonds absorb in the UV region because electrons from single
1901 Application of Spectrophotometry Chemical Analysis Problem: 1 Application of Spectroscopy Organic Compounds Organic compounds with single bonds absorb in the UV region because electrons from single
