White Paper. Overview: NDIR Definition:
|
|
|
- Ami Spencer
- 5 years ago
- Views:
Transcription
1 Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06 NDIR Overview.docx Created by: R. Schrader Last Revision Date: 14-Jul-09 Overview: Bridge Exhaust Gas Analyzers use two measurement methods for exhaust gas analysis: NDIR for CO, CO2, and Hydrocarbons, and Chemical Sensors for O2 and NO. These technologies are approved for use in exhaust gas analyzers by all Federal, International, and State/Provincial agencies as below. As they are the standard methods used to measure exhaust gas constituents, some discussion of their nature and effectiveness is warranted. NDIR Definition: NDIR stands for Non-Dispersive InfraRed. This technology is used to measure the concentration of a gas (actually, the number of molecules in the optical path) by determining how much infrared energy is absorbed at a select wavelength band that corresponds to a resonant mode spectrum of the molecule being analyzed. For example, the CO2 molecule has a strong resonance at a frequency relating to the 4.26 micron electromagnetic wavelength near infrared. So CO2 will strongly absorb infrared energy at this select wavelength. The more CO2 molecules there are in the optical path the more energy gets absorbed although this is a highly non-linear function. The gas analyzer determines how much energy is absorbed (the infrared absorption) at a specific molecular resonance of a particular gas, and then relates this absorption to gas concentration the characteristic instrument curve for the gas being analyzed. This technique is very specific to the particular resonance of each gas molecule being measured, so it works well to measure certain gases in mixes of other gases providing that appropriate absorption bands unique to each gas can be isolated. In general, it is the technique of choice for measuring such common gases as CO (Carbon Monoxide), CO2 (Carbon Dioxide), SO2 (Sulfur Dioxide), NO2 (Nitrous Oxide Anesthetic Gas) and a variety of Hydrocarbons (generally the Alkane group looking at 1 of 5
2 the H-C stretch resonance) but other hydrocarbons are readily measurable as well for example fluorinated hydrocarbon anaesthetic agents. This technology is cost effective, robust, stable, well proven, and by far the most popular method for measuring the gases above with 100,000 s of installations operating worldwide, from CEM to Anesthesia Machines, to Exhaust Gas Analyzers, and a variety of industrial applications. EPA Method Compliance: The NDIR gas measurement technology is generally recognized as one of the technologies of choice for analyzing emission gases. The NDIR method is specified as an appropriate measurement technology by the EPA for several gases in official EPA- Defined test methods given below: EPA Method 3A: NDIR for measuring CO2 emissions from Stationary Sources EPA Method 6C: NDIR for measuring SO2 emissions from Stationary Sources EPA Method 10: NDIR for measuring CO emissions from Stationary Sources EPA Method 21: NDIR for measuring VOC Leaks EPA Method 25B: NDIR for measuring VOCs. In addition, NDUV (same approach as NDIR, but at an Ultra-Violet wavelength) is an EPA specified measurement method in: EPA Method 7B: NDUV for measuring NOx from Stationary Sources. Mobile Emissions Source Test Compliance: All Domestic and International Federal and State mobile emission programs (e. g. BAR- 74, BAR-80, BAR-84, BAR-90, BAR-97, ASM, IM-240, FTP, etc) specify NDIR as the gas measurement technology of choice for measuring CO, CO2, HC (measuring gasoline vapor, C4 C10 as Hexane) in the exhaust streams of internal combustion engines. In addition, NDUV is recognized as one of the measurement technologies of choice for NOx emission measurement from the same sources. NDIR has also been the analytical technology of choice for the measurement of C1 C20 (Methane and above) in a variety of applications, from wasteful site emission measurements to VOC elimination or recovery installations. 2 of 5
3 NDIR Instrument Utility and Costs: NDIR-based analytical equipment is available in a variety of styles, from hand-held portables to bench-top commercial and laboratory-grade analyzers to complete CEM installations. This equipment is relatively low cost, robust, stable, and easy to operate lending itself to a variety of commercial applications. It does not require frequent recalibration to remain accurate, and has demonstrated stability and longevity with many instruments providing decades of use in the field. Comparison of NDIR to other analytic technologies: NDIR Vs FTIR: The main difference between NDIR and FTIR (or the equivalent continuous spectral analysis technology) is that NDIR measured electromagnetic energy in a particular bandpass range generally determined by an interference flatter in the equipment and chosen to align with the infrared absorption spectrum of a specific gas molecule. FTIR (or the equivalent) examines the electromagnetic absorption spectrum continuously across a range of wavelengths. Thus, it is more versatile but less specific to a particular gas. In general, FTIR technology is used in a comparative, batch process manner in which the equipment is first characterized using a variety of calibration gases, and then the sample gas is run and compared to the calibration gas signature and energy absorption magnitude. From this data, the gas concentration for specific gases with unique spectral signatures may be made. Correlation between NDIR and FTIR should be high, although they should generally be verified by actual gas measurement using both approaches. FTIR or other continuous spectrum analytical technologies are costly, complex, require significant operator expertise, and are generally considered to be laboratory class instrumentation. They have yet to find much application in high volume commercialgrade field equipment. NDIR Vs FID: Both NDIR and FID (Flame Ionization Detection) are analytical technologies of choice for the measurement of HC compounds. The difference between NDIR and FID is that NDIR makes use of the infrared frequency resonance absorption characteristics of HC molecules for measurement (generally, the H-C stretch resonance, although other 3 of 5
4 resonance s may be used as the HC molecule becomes more complex), while the FID approach strips the hydrogen from the HC molecule and then ionizes the remaining Carbon atom into an emissive state. The Carbon atom emission frequency intensity is then measured, and a relationship made between the energy measured and the concentration of Carbon ions made (from the characteristic instrument curve and subsequent calibration with a known gas and concentration). Using this technique, the equivalent HC concentration may be determined in the sample gas. It is important to realize that both NDIR and FID are largely non-specific in that FID disassembles the HC molecules in the gas stream before analysis, and NDIR generally looks at molecular resonance s which are commonly found in a variety of HC molecules. In either case, the resulting HC gas concentration of the HC gases in the measurement stream determined by either technique is reported as equivalent concentration of a specific HC gas usually (but not always) the gas used to calibrate the instrument. FID may be used to measure any HC compound as the technique isolates the Carbon atom from the molecule and then measures its emissive energy. The resulting response is strictly a function of the concentration of Carbon atoms in the measured gas. NDIR is more molecule specific, in that each HC molecular structure has its own IR absorption signature and magnitude so some molecules produce more IR absorption in a particular wavelength band than others. However, both the NDIR absorption and Carbon atom emissive relationships are generally tied to the number of Carbon atoms in the molecule so NDIR and FID analytical technologies have similar responses for many HC molecules. Correlation between NDIR and FID are very gas-composition specific, and should generally be verified by actual gas measurement of representative sample gas using both approaches. NDIR Vs ElectroChemical Sensors: NDIR, being non-destructive in nature, is applicable to the measurement of partially or fully reacted gas molecules. Some gases are either too small or tightly bound to exhibit significant resonant absorption spectra at practical measurement wavelengths, and do not lend themselves to NDIR or even NDUV analytic techniques. However, many of these gases are chemically reactive, and so do lend themselves to measurement with gas-specific electro-chemical sensors. These sensors generally produce an electrical analog of the gas concentration, and are both zerostable and linear. They tend to vary in gas sensitivity with time and temperature, however, and require thermal compensation and relatively frequent span calibration to correct for temperature 4 of 5
5 or time related span drift. However, this is one of the technologies of choice for Oxygen, NO, CO (Concentrations below 1.0%), SO2, Ammonia, Chlorine, and other reactive gases. They are commonly used for ambient atmosphere toxic gas detection products where low level detection and zero stability are of paramount importance and toxic gases may exist in such low concentrations as to make NDIR methods impractical. This technology is not usable for fully reacted gases (CO2 or HC), Inert Gases (N2, He, etc), or for high concentrations. The chemical sensors used are based on measuring a chemical reaction in a gas stream where the reactions are limited by the amount of gas diffusing into the electrolyte. They become highly non-linear and unstable with high gas concentrations, and are more suitable for highly diluted gas streams flue gas or ambient air applications. They are generally unsuitable for concentrated gas streams such as internal combustion exhaust. In general, the most common use of the ElectroChemical sensor method is for oxygen analysis where it is by far the dominant method, with millions of commercial implementations. The sensors are generally low cost gas analysis solutions, with analyzers selling for a few hundred dollars (toxic gas detectors) to several thousand dollars for bench top or commercial industrial implementations. NDIR Vs Gas Chromatography (GC): Gas Chromatography (GC) is a method by which a gas mixture is first separated into specific species, and then quantified using a general method. Many GC systems use thermal conductivity methods to determine the mass flow of the effluent from a separation column in which smaller molecules move faster and therefore are expelled earlier. The advantage of such a system is that it is gas-specific and provides a unique output for each molecule in the test gas mixture. However, it is a batch-process analytical technique rather than a time-continuous method, requires significant operator expertise to use, and is generally considered time-unstable - requiring the equipment to first be calibrated on a known gas standard and then measurements made. NDIR Vs Mass Spectroscopy (MS): Mass Spectroscopy (MS) is a method by which the test gas is first ionized and then propelled through a magnetic or electrical field. The trajectory of the gas molecules is thus a function of the molecule mass and the ionization charge reaction to the applied field. However, rather like GC above, it is generally a batch-process analytical technique rather than a time-continuous method, requires significant operator expertise to use, and is generally considered time-unstable - requiring the equipment to first be calibrated on a known gas standard and then measurements made. 5 of 5
Atmospheric Analysis Gases. Sampling and analysis of gaseous compounds
 Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
The Claviature of Gas Analysis
 The Claviature of Gas Analysis Mass spectrometric gas analysis for quality assurance of gases and gas mixtures High purity and special gases are becoming increasingly important in the development of modern
The Claviature of Gas Analysis Mass spectrometric gas analysis for quality assurance of gases and gas mixtures High purity and special gases are becoming increasingly important in the development of modern
Gaseous CEMS technology
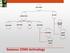 GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur
 VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
AUTOMATED ONLINE IDENTIFICATION AND MONITORING OF IMPURITIES IN GASES
 JPACSM 127 AUTOMATED ONLINE IDENTIFICATION AND MONITORING OF IMPURITIES IN GASES Trace Analytical Inc. Menlo Park, CA ABSTRACT GC based gas analyzers with Reduction Gas Detector (RGD) and Flame Ionization
JPACSM 127 AUTOMATED ONLINE IDENTIFICATION AND MONITORING OF IMPURITIES IN GASES Trace Analytical Inc. Menlo Park, CA ABSTRACT GC based gas analyzers with Reduction Gas Detector (RGD) and Flame Ionization
 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
ISA QATAR : 90 th Seminar
 ANALYTICAL INSTRUMENTATION & MAINTENANCE SYSTEMS (AIMS) ISA QATAR : 90 th Seminar UTILIZATION OF LASERS IN THE FIELD OF GAS ANALYZERS Presenter: ZAHEER JUDDY Electromagnetic Spectrum Electromagnetic Spectrum
ANALYTICAL INSTRUMENTATION & MAINTENANCE SYSTEMS (AIMS) ISA QATAR : 90 th Seminar UTILIZATION OF LASERS IN THE FIELD OF GAS ANALYZERS Presenter: ZAHEER JUDDY Electromagnetic Spectrum Electromagnetic Spectrum
TOUR OF SOIL CHEMISTRY LABS (09/22/07)
 Department of Crop and Soil Sciences Commemorating Our Centennial, 1907 2007 TOUR OF SOIL CHEMISTRY LABS (09/22/07) WELCOME TO THE TOUR OF SOIL CHEMISTRY LABS Instrument: Lachat QuikChem FIA + 8000 Analyzer
Department of Crop and Soil Sciences Commemorating Our Centennial, 1907 2007 TOUR OF SOIL CHEMISTRY LABS (09/22/07) WELCOME TO THE TOUR OF SOIL CHEMISTRY LABS Instrument: Lachat QuikChem FIA + 8000 Analyzer
Far UV Absorbance Detector
 Far UV Absorbance Detector Theory Most organic and inorganic species absorb strongly in the far UV. Notable exceptions are the inert gases, helium and nitrogen which absorb very weakly in this region.
Far UV Absorbance Detector Theory Most organic and inorganic species absorb strongly in the far UV. Notable exceptions are the inert gases, helium and nitrogen which absorb very weakly in this region.
Process Analyzer and Sampling Systems
 Process Analyzer and Sampling Systems Course Price 3050 Course Description Analytical instruments used for online chemical analysis of process streams or plant environments are generally called Process
Process Analyzer and Sampling Systems Course Price 3050 Course Description Analytical instruments used for online chemical analysis of process streams or plant environments are generally called Process
Measurement Technique and its Application to Trace Components of Atmospheric Gas
 F e a t u r e A r t i c l e Feature Article Measurement Technique and its Application to Trace Components of Atmospheric Gas Junji Kato From early on, HORIBA has been developing and adopting various measurement
F e a t u r e A r t i c l e Feature Article Measurement Technique and its Application to Trace Components of Atmospheric Gas Junji Kato From early on, HORIBA has been developing and adopting various measurement
C (s) + O 2 (g) CO 2 (g) S (s) + O 2 (g) SO 2 (g)
 Combustion The rapid combination of oxygen with a substance. A major type of chemical reaction. When elemental carbon or carbon-containing compounds burn in air, oxygen combines with the carbon to form
Combustion The rapid combination of oxygen with a substance. A major type of chemical reaction. When elemental carbon or carbon-containing compounds burn in air, oxygen combines with the carbon to form
Atomic Absorption Spectroscopy (AAS)
 Atomic Absorption Spectroscopy (AAS) Alex Miller ABC s of Electrochemistry 3/8/2012 Contents What is Atomic Absorption Spectroscopy? Basic Anatomy of an AAS system Theory of Operation Practical Operation
Atomic Absorption Spectroscopy (AAS) Alex Miller ABC s of Electrochemistry 3/8/2012 Contents What is Atomic Absorption Spectroscopy? Basic Anatomy of an AAS system Theory of Operation Practical Operation
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY
 Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY Chapter 23. Gas Chromatography What did they eat in the year 1,000? GC of Cholesterol and other lipids extracted from
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY Chapter 23. Gas Chromatography What did they eat in the year 1,000? GC of Cholesterol and other lipids extracted from
Sub-category: Physics and Principles of Measurement Topic: Monitoring anesthetic gases and vapours Date: January 15-17, 2016 Language: English
 Course n : Course 3 Title: RESPIRATORY PHYSIOLOGY, PHYSICS AND PATHOLOGY IN RELATION TO ANAESTHESIA AND INTENSIVE CARE Sub-category: Physics and Principles of Measurement Topic: Monitoring anesthetic gases
Course n : Course 3 Title: RESPIRATORY PHYSIOLOGY, PHYSICS AND PATHOLOGY IN RELATION TO ANAESTHESIA AND INTENSIVE CARE Sub-category: Physics and Principles of Measurement Topic: Monitoring anesthetic gases
Monitoring Flammable Vapors and Gases in Industrial Processes
 Flammability Hazards Industrial fires and explosions happen more frequently than most people think. They cause downtime, property damage, injury and sometimes death. These fires and explosions result from
Flammability Hazards Industrial fires and explosions happen more frequently than most people think. They cause downtime, property damage, injury and sometimes death. These fires and explosions result from
Thermo Scientific Electrochemistry Products
 Comparing Performance of DO Methods The world leader in serving science in Water and Seawaters Thermo Scientific Electrochemistry Products Dissolved Oxygen Measurement Oxygen is essential for most of the
Comparing Performance of DO Methods The world leader in serving science in Water and Seawaters Thermo Scientific Electrochemistry Products Dissolved Oxygen Measurement Oxygen is essential for most of the
Chromatography & instrumentation in Organic Chemistry
 Chromatography & instrumentation in Organic Chemistry What is Chromatography? Chromatography is a technique for separating mixtures into their components in order to analyze, identify, purify, and/or quantify
Chromatography & instrumentation in Organic Chemistry What is Chromatography? Chromatography is a technique for separating mixtures into their components in order to analyze, identify, purify, and/or quantify
AASS, Örebro University
 Mobile Robotics Achim J. and Lilienthal Olfaction Lab, AASS, Örebro University 1 Contents 1. "Classical" Electronic Nose 2. Further Gas Sensing Technologies for Mobile Robots 3. (Signal Processing in Electronic
Mobile Robotics Achim J. and Lilienthal Olfaction Lab, AASS, Örebro University 1 Contents 1. "Classical" Electronic Nose 2. Further Gas Sensing Technologies for Mobile Robots 3. (Signal Processing in Electronic
Analytical techniques: Environmental samples. Lecture 2 Universidade do Algarve
 Analytical techniques: Environmental samples Lecture 2 Universidade do Algarve Terms, definitions & applications Difference between technique and method: Analytical technique: Fundamental scientific application
Analytical techniques: Environmental samples Lecture 2 Universidade do Algarve Terms, definitions & applications Difference between technique and method: Analytical technique: Fundamental scientific application
MASS and INFRA RED SPECTROSCOPY
 MASS and INFRA RED SPECTRSCPY Mass Spectroscopy The mass spectrometer was looked at in Unit 1. It was noted there that compounds produce fragmentation patterns when passes through a mass spectrometer.
MASS and INFRA RED SPECTRSCPY Mass Spectroscopy The mass spectrometer was looked at in Unit 1. It was noted there that compounds produce fragmentation patterns when passes through a mass spectrometer.
UV Hound Series. Dependable High Quality Air Monitoring. Accurate Readings Within Seconds. Portable UVDOAS Multi-gas Analyzers. Multi-Gas Capability
 UV Hound Series Portable UVDOAS Multi-gas Analyzers Multi-Gas Capability Dependable High Quality Air Monitoring Individual VOCs PPB Sensitivity Non-Contact Optical Measurement Automated Reporting Configurable
UV Hound Series Portable UVDOAS Multi-gas Analyzers Multi-Gas Capability Dependable High Quality Air Monitoring Individual VOCs PPB Sensitivity Non-Contact Optical Measurement Automated Reporting Configurable
Training Coarse ECOPROBE 5 CHEMISTRY RS DYNAMICS
 Training Coarse ECOPROBE 5 CHEMISTRY Contents Ecoprobe analytical principles Photoionization Infrared spectroscopy Oxygen measurement Pollutants Types and responses Head space New application of Ecoprobe
Training Coarse ECOPROBE 5 CHEMISTRY Contents Ecoprobe analytical principles Photoionization Infrared spectroscopy Oxygen measurement Pollutants Types and responses Head space New application of Ecoprobe
Understanding catalytic LEL combustible gas sensor performance
 : Understanding catalytic LEL combustible gas sensor performance These four conditions are frequently diagrammed as the "Fire Tetrahedron". If any side of the tetrahedron is missing, incomplete or insubstantial;
: Understanding catalytic LEL combustible gas sensor performance These four conditions are frequently diagrammed as the "Fire Tetrahedron". If any side of the tetrahedron is missing, incomplete or insubstantial;
Questions, Myths and Misconceptions about Using Photoionization Detectors
 Questions, Myths and Misconceptions about Using Photoionization Detectors Solvent, fuel and other VOC vapours are pervasively common in many workplace environments. Increased awareness of the toxicity
Questions, Myths and Misconceptions about Using Photoionization Detectors Solvent, fuel and other VOC vapours are pervasively common in many workplace environments. Increased awareness of the toxicity
Gas Chromatography. Chromatography Laboratory Course. Dr. Christian Jungnickel Chromatography Course GC September 2005
 Gas Chromatography Chromatography Laboratory Course The laboratory course experiments General Aim: Gain general experience using a GC Constant Injection technique Temperature variations Qualitative and
Gas Chromatography Chromatography Laboratory Course The laboratory course experiments General Aim: Gain general experience using a GC Constant Injection technique Temperature variations Qualitative and
FOURIER TRANSFORM INFRARED SPECTROSCOPY (FTIR)
 I(k)= 0[I(Δd) I( )]cos(2πkδd)dδd FOURIER TRANSFORM INFRARED SPECTROSCOPY (FTIR) Colorado SESHA, 2018 George Evans, CIH ESH-POC Carrie Wyse, ESH-POC Shames Stevens, SERF Chief Engineer What is FTIR spectroscopy?
I(k)= 0[I(Δd) I( )]cos(2πkδd)dδd FOURIER TRANSFORM INFRARED SPECTROSCOPY (FTIR) Colorado SESHA, 2018 George Evans, CIH ESH-POC Carrie Wyse, ESH-POC Shames Stevens, SERF Chief Engineer What is FTIR spectroscopy?
Chapter 12 Mass Spectrometry and Infrared Spectroscopy
 Organic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 12 Mass Spectrometry and Infrared Spectroscopy Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice
Organic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 12 Mass Spectrometry and Infrared Spectroscopy Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice
Chlorine and Hydrogen Chloride Monitoring Utilizing Ion Mobility Spectroscopy (IMS)
 Chlorine and Hydrogen Chloride Monitoring Utilizing Ion Mobility Spectroscopy (IMS) Tad Bacon Jim Taylor Kurt Webber VP Engineering Sr. Chemical Engineer VP Sales Molecular Analytics Molecular Analytics
Chlorine and Hydrogen Chloride Monitoring Utilizing Ion Mobility Spectroscopy (IMS) Tad Bacon Jim Taylor Kurt Webber VP Engineering Sr. Chemical Engineer VP Sales Molecular Analytics Molecular Analytics
CHEM 221 Instrumental Analysis FINAL EXAM May 10, 2016
 CHEM 221 Instrumental Analysis FINAL EXAM May 10, 2016 Name: INSTRUCTIONS: Read through the entire exam before you begin. Answer all of the questions. For questions involving calculations, show all of
CHEM 221 Instrumental Analysis FINAL EXAM May 10, 2016 Name: INSTRUCTIONS: Read through the entire exam before you begin. Answer all of the questions. For questions involving calculations, show all of
Cracking. 191 minutes. 186 marks. Page 1 of 27
 3.1.6.2 Cracking 191 minutes 186 marks Page 1 of 27 Q1. (a) Gas oil (diesel), kerosine (paraffin), mineral oil (lubricating oil) and petrol (gasoline) are four of the five fractions obtained by the fractional
3.1.6.2 Cracking 191 minutes 186 marks Page 1 of 27 Q1. (a) Gas oil (diesel), kerosine (paraffin), mineral oil (lubricating oil) and petrol (gasoline) are four of the five fractions obtained by the fractional
S is referred to as sour. The sulfide loading lends an unpleasant odor and makes the water corrosive. Additionally, the presence of H 2
 Application Summary Analytes: S (hydrogen sulfide), (ammonia) Detection Technology: OMA-300 Process Analyzer Process Stream: stripped sour water Introduction In the context of petrochemical refining and
Application Summary Analytes: S (hydrogen sulfide), (ammonia) Detection Technology: OMA-300 Process Analyzer Process Stream: stripped sour water Introduction In the context of petrochemical refining and
CH0302 Process Instrumentation. Lecture 9 Composition Analysis
 CH0302 Process Instrumentation Lecture 9 Composition Analysis Department of Chemical Engineering School of Bioengineering SRM University Kattankulathur 603203 4/3/15 Chemical Engineering 1 Industrial significance
CH0302 Process Instrumentation Lecture 9 Composition Analysis Department of Chemical Engineering School of Bioengineering SRM University Kattankulathur 603203 4/3/15 Chemical Engineering 1 Industrial significance
Physics of capnography
 Strona 1 z 7 Physics of capnography Bhavani Shankar Kodali MD There are five Physical methods 1. Infra-red Spectrography 2. Molecular Correlation Spectrography 3. Raman Spectrography 4. Mass Spectrography
Strona 1 z 7 Physics of capnography Bhavani Shankar Kodali MD There are five Physical methods 1. Infra-red Spectrography 2. Molecular Correlation Spectrography 3. Raman Spectrography 4. Mass Spectrography
RS DYNAMICS ECOPROBE 5. Portable IR/PID Gas Analyzer PID. PID and IR Analyzers
 RS DYNAMICS ECOPROBE 5 Portable IR/PID Gas Analyzer PID + IR PID and IR Analyzers General ECOPROBE 5 has two autonomous analyzers in one case. The combination of analyzers provides a set of data designed
RS DYNAMICS ECOPROBE 5 Portable IR/PID Gas Analyzer PID + IR PID and IR Analyzers General ECOPROBE 5 has two autonomous analyzers in one case. The combination of analyzers provides a set of data designed
Gas Sensors and Solar Water Splitting. Yang Xu
 Gas Sensors and Solar Water Splitting Yang Xu 11/16/14 Seite 1 Gas Sensor 11/16/14 Seite 2 What are sensors? American National Standards Institute (ANSI) Definition: a device which provides a usable output
Gas Sensors and Solar Water Splitting Yang Xu 11/16/14 Seite 1 Gas Sensor 11/16/14 Seite 2 What are sensors? American National Standards Institute (ANSI) Definition: a device which provides a usable output
Methane contains atoms of two elements, combined chemically. Methane is a mixture of two different elements.
 Q1.Methane (CH 4) is used as a fuel. (a) The displayed structure of methane is: Draw a ring around a part of the displayed structure that represents a covalent bond. (b) Why is methane a compound? Tick
Q1.Methane (CH 4) is used as a fuel. (a) The displayed structure of methane is: Draw a ring around a part of the displayed structure that represents a covalent bond. (b) Why is methane a compound? Tick
Dual Function Gas Analyzer For Industrial Monitoring Requirements. Requirements
 Dual Function Gas Analyzer For Industrial Monitoring Requirements Bill Pearman Ph.D Senior Scientist October 16, 2014 IPEC 2014 1 Discussion Points Quick background on IMACC and FTIR Needs based assessment
Dual Function Gas Analyzer For Industrial Monitoring Requirements Bill Pearman Ph.D Senior Scientist October 16, 2014 IPEC 2014 1 Discussion Points Quick background on IMACC and FTIR Needs based assessment
Chapter 31 Gas Chromatography. Carrier Gas System
 Chapter 31 Gas Chromatography GAS-LIQUID CHROMATOGRAPHY In gas chromatography, the components of a vaporized sample are fractionated as a consequence of being partitioned between a mobile gaseous phase
Chapter 31 Gas Chromatography GAS-LIQUID CHROMATOGRAPHY In gas chromatography, the components of a vaporized sample are fractionated as a consequence of being partitioned between a mobile gaseous phase
VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR
 AP-157 VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR Grant M. Plummer, Ph.D. Peter Zemek, Ph.D. Special Projects Manager Applications Manager Enthalpy Analytical,
AP-157 VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR Grant M. Plummer, Ph.D. Peter Zemek, Ph.D. Special Projects Manager Applications Manager Enthalpy Analytical,
Choosing the best detection technologies for measuring combustible gas and VOC vapors
 AP 11: Choosing the best detection technologies for measuring combustible gas and VOC vapors gas an oxygen sensor responds to is oxygen. Electrochemical sensors designed to measure a particular gas may
AP 11: Choosing the best detection technologies for measuring combustible gas and VOC vapors gas an oxygen sensor responds to is oxygen. Electrochemical sensors designed to measure a particular gas may
Introduction to Gas Phase FTIR Spectroscopy
 Introduction to Gas Phase FTIR Spectroscopy Introduction to FTIR spectroscopy FTIR stands for Fourier transform infrared, the preferred method of infrared spectroscopy. In infrared (IR) spectroscopy, radiation
Introduction to Gas Phase FTIR Spectroscopy Introduction to FTIR spectroscopy FTIR stands for Fourier transform infrared, the preferred method of infrared spectroscopy. In infrared (IR) spectroscopy, radiation
Q1. Which one of the following is least likely to occur in the reaction between methane and chlorine?
 Q1. Which one of the following is least likely to occur in the reaction between methane and chlorine? A B C D C 4 + Cl C 3 + Cl C 3 + Cl C 3 Cl + C 3 + Cl 2 C 3 Cl + Cl C 3 Cl + Cl C 2 Cl + Cl (Total 1
Q1. Which one of the following is least likely to occur in the reaction between methane and chlorine? A B C D C 4 + Cl C 3 + Cl C 3 + Cl C 3 Cl + C 3 + Cl 2 C 3 Cl + Cl C 3 Cl + Cl C 2 Cl + Cl (Total 1
Introduction to Pharmaceutical Chemical Analysis
 Introduction to Pharmaceutical Chemical Analysis Hansen, Steen ISBN-13: 9780470661222 Table of Contents Preface xv 1 Introduction to Pharmaceutical Analysis 1 1.1 Applications and Definitions 1 1.2 The
Introduction to Pharmaceutical Chemical Analysis Hansen, Steen ISBN-13: 9780470661222 Table of Contents Preface xv 1 Introduction to Pharmaceutical Analysis 1 1.1 Applications and Definitions 1 1.2 The
New advances in folded pathlength technology for Process Tunable Diode Laser Absorption Spectrometers (TDLAS)
 New advances in folded pathlength technology for Process Tunable Diode Laser Absorption Spectrometers (TDLAS) Jean-Nicolas Adami, PhD Head of Strategic Product Group Gas Analytics Mettler-Toledo GmbH,
New advances in folded pathlength technology for Process Tunable Diode Laser Absorption Spectrometers (TDLAS) Jean-Nicolas Adami, PhD Head of Strategic Product Group Gas Analytics Mettler-Toledo GmbH,
Questions on Instrumental Methods of Analysis
 Questions on Instrumental Methods of Analysis 1. Which one of the following techniques can be used for the detection in a liquid chromatograph? a. Ultraviolet absorbance or refractive index measurement.
Questions on Instrumental Methods of Analysis 1. Which one of the following techniques can be used for the detection in a liquid chromatograph? a. Ultraviolet absorbance or refractive index measurement.
Chemical Oxidation Oxidizing agents
 Chemical Oxidation CENG 4710 Environmental Control Chemical oxidation is used to detoxify waste by adding an oxidizing agent to chemically transform waste compounds. It is capable of destroying a wide
Chemical Oxidation CENG 4710 Environmental Control Chemical oxidation is used to detoxify waste by adding an oxidizing agent to chemically transform waste compounds. It is capable of destroying a wide
Chapter 27: Gas Chromatography
 Chapter 27: Gas Chromatography Gas Chromatography Mobile phase (carrier gas): gas (He, N 2, H 2 ) - do not interact with analytes - only transport the analyte through the column Analyte: volatile liquid
Chapter 27: Gas Chromatography Gas Chromatography Mobile phase (carrier gas): gas (He, N 2, H 2 ) - do not interact with analytes - only transport the analyte through the column Analyte: volatile liquid
UV3000. Accurate, precise, and portable ambient gas point analyzer
 UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
SULFUR RECOVERY ANALYZERS; UV Diode-Array Fiber- Optics Technology
 SULFUR RECOVERY ANALYZERS; UV Diode-Array Fiber- Optics Technology Yoav Barshad Applied Analytics, Inc. 29 Domino Drive Concord, Massachusetts, 01742 KEYWORDS Sulfur recovery, H2S, SO2, tail gas, UV, Spectroscopy,
SULFUR RECOVERY ANALYZERS; UV Diode-Array Fiber- Optics Technology Yoav Barshad Applied Analytics, Inc. 29 Domino Drive Concord, Massachusetts, 01742 KEYWORDS Sulfur recovery, H2S, SO2, tail gas, UV, Spectroscopy,
Achim J. Lilienthal Erik Schaffernicht Achim J. Lilienthal. AASS, Örebro University
 Achim J. Lilienthal Erik Schaffernicht Mobile Achim J. Achim J. Lilienthal Room T1222 Robotics and Lilienthal Olfaction Room T1211 Lab, Erik Schaffernicht achim.lilienthal@oru.se AASS, Örebro University
Achim J. Lilienthal Erik Schaffernicht Mobile Achim J. Achim J. Lilienthal Room T1222 Robotics and Lilienthal Olfaction Room T1211 Lab, Erik Schaffernicht achim.lilienthal@oru.se AASS, Örebro University
Sampling for Gases and Vapors
 Sampling for Gases and Vapors EOH 466B Evaluating the Occupational Environment Spring 2008 Gases and Vapors Gases: don't exist in liquid state at STP e.g.: nitrous oxides, ozone, carbon monoxide Vapors:
Sampling for Gases and Vapors EOH 466B Evaluating the Occupational Environment Spring 2008 Gases and Vapors Gases: don't exist in liquid state at STP e.g.: nitrous oxides, ozone, carbon monoxide Vapors:
FLAME PHOTOMETRY AIM INTRODUCTION
 FLAME PHOTOMETRY AIM INTRODUCTION Atomic spectroscopy is based on the absorption, emission or fluorescence process of light by atoms or elementary ions. Information for atomic scale is obtained in two
FLAME PHOTOMETRY AIM INTRODUCTION Atomic spectroscopy is based on the absorption, emission or fluorescence process of light by atoms or elementary ions. Information for atomic scale is obtained in two
Ozone: Earth s shield from UV radiation
 Outline Ozone: Earth s shield from UV radiation Review electromagnetic radiation absorptivity by selective gases temperature vs. height in atmosphere Ozone production and destruction natural balance anthropogenic
Outline Ozone: Earth s shield from UV radiation Review electromagnetic radiation absorptivity by selective gases temperature vs. height in atmosphere Ozone production and destruction natural balance anthropogenic
2401 Gas (liquid) Chromatography
 2401 Gas (liquid) Chromatography Chromatography Scheme Gas chromatography - specifically gas-liquid chromatography - involves a sample being vaporized and injected onto the head of the chromatographic
2401 Gas (liquid) Chromatography Chromatography Scheme Gas chromatography - specifically gas-liquid chromatography - involves a sample being vaporized and injected onto the head of the chromatographic
PRINCIPLE OF ICP- AES
 INTRODUCTION Non- flame atomic emission techniques, which use electrothermal means to atomize and excite the analyte, include inductively coupled plasma and arc spark. It has been 30 years since Inductively
INTRODUCTION Non- flame atomic emission techniques, which use electrothermal means to atomize and excite the analyte, include inductively coupled plasma and arc spark. It has been 30 years since Inductively
Balancing chemical reaction equations (stoichiometry)
 Balancing chemical reaction equations (stoichiometry) This worksheet and all related files are licensed under the Creative Commons Attribution License, version 1.0. To view a copy of this license, visit
Balancing chemical reaction equations (stoichiometry) This worksheet and all related files are licensed under the Creative Commons Attribution License, version 1.0. To view a copy of this license, visit
Infra Red Spectroscopy
 CH 2252 Instrumental Methods of Analysis Unit I Infra Red Spectroscopy M. Subramanian Assistant Professor Department of Chemical Engineering Sri Sivasubramaniya Nadar College of Engineering Kalavakkam
CH 2252 Instrumental Methods of Analysis Unit I Infra Red Spectroscopy M. Subramanian Assistant Professor Department of Chemical Engineering Sri Sivasubramaniya Nadar College of Engineering Kalavakkam
3 Use of Mass Spectra to Obtain Structural Information
 3 Use of Mass Spectra to Obtain Structural Information 1 Mass Spectrometry One of the most sensitive and versatile analytical tools More sensitive than other spectroscopic methods (e.g. IR spectroscopy)
3 Use of Mass Spectra to Obtain Structural Information 1 Mass Spectrometry One of the most sensitive and versatile analytical tools More sensitive than other spectroscopic methods (e.g. IR spectroscopy)
Permanent Gas Analysis by Gas Chromatography Vacuum Ultraviolet Spectroscopy
 Application Note Permanent Gas Analysis by Gas Chromatography Vacuum Ultraviolet Spectroscopy Permanent Gas Analysis by Gas Chromatography Vacuum Ultraviolet Spectroscopy Introduction Gas Chromatography
Application Note Permanent Gas Analysis by Gas Chromatography Vacuum Ultraviolet Spectroscopy Permanent Gas Analysis by Gas Chromatography Vacuum Ultraviolet Spectroscopy Introduction Gas Chromatography
The Nature of Light. We have a dual model
 Light and Atoms Properties of Light We can come to understand the composition of distant bodies by analyzing the light they emit This analysis can tell us about the composition as well as the temperature
Light and Atoms Properties of Light We can come to understand the composition of distant bodies by analyzing the light they emit This analysis can tell us about the composition as well as the temperature
Chapter 3: Source Measurement Techniques
 Chapter 3 Source Measurement Techniques Measurement Methods Method 18, Measurement of Gaseous Organic Compound Emissions by Gas Chromatography Method 25, Determination of Total Gaseous Non-Methane Organic
Chapter 3 Source Measurement Techniques Measurement Methods Method 18, Measurement of Gaseous Organic Compound Emissions by Gas Chromatography Method 25, Determination of Total Gaseous Non-Methane Organic
GCSE OCR Revision Chemistry. GCSE OCR Revision Chemistry. GCSE OCR Revision Chemistry. Bonding. GCSE OCR Revision Chemistry
 Particle Model and Atomic Structure The following symbols describe two different substances. Deduce all the information you can from these symbols. 13 C 12 6 6 C 1 Particle Model and Atomic Structure The
Particle Model and Atomic Structure The following symbols describe two different substances. Deduce all the information you can from these symbols. 13 C 12 6 6 C 1 Particle Model and Atomic Structure The
AN INTRODUCTION TO ATOMIC SPECTROSCOPY
 AN INTRODUCTION TO ATOMIC SPECTROSCOPY Atomic spectroscopy deals with the absorption, emission, or fluorescence by atom or elementary ions. Two regions of the spectrum yield atomic information- the UV-visible
AN INTRODUCTION TO ATOMIC SPECTROSCOPY Atomic spectroscopy deals with the absorption, emission, or fluorescence by atom or elementary ions. Two regions of the spectrum yield atomic information- the UV-visible
Spectroscopy and Chromatography
 Spectroscopy and Chromatography Introduction Visible light is one very small part of the electromagnetic spectrum. The different properties of the various types of radiation depend upon their wavelength.
Spectroscopy and Chromatography Introduction Visible light is one very small part of the electromagnetic spectrum. The different properties of the various types of radiation depend upon their wavelength.
ATOMIC SPECROSCOPY (AS)
 ATOMIC ABSORPTION ANALYTICAL CHEMISTRY ATOMIC SPECROSCOPY (AS) Atomic Absorption Spectroscopy 1- Flame Atomic Absorption Spectreoscopy (FAAS) 2- Electrothermal ( Flame-less ) Atomic Absorption Spectroscopy
ATOMIC ABSORPTION ANALYTICAL CHEMISTRY ATOMIC SPECROSCOPY (AS) Atomic Absorption Spectroscopy 1- Flame Atomic Absorption Spectreoscopy (FAAS) 2- Electrothermal ( Flame-less ) Atomic Absorption Spectroscopy
Validation Study of FTIR-Based Emissions Measurements at a Municipal Waste Combustor
 13th North American Waste to Energy Conference May 23-25, 2005, Orlando, Florida USA NAWTEC13-3158 Validation Study of FTIR-Based Emissions Measurements at a Municipal Waste Combustor G. J. Aldina Jeffrey
13th North American Waste to Energy Conference May 23-25, 2005, Orlando, Florida USA NAWTEC13-3158 Validation Study of FTIR-Based Emissions Measurements at a Municipal Waste Combustor G. J. Aldina Jeffrey
THE APPLICATION OF PROCESS MASS SPECTROMETRY TO FUMED SILICA PRODUCTION
 JPACSM 5 Process Analytical Chemistry THE APPLICATION OF PROCESS MASS SPECTROMETRY TO FUMED SILICA PRODUCTION Peter Traynor and Steven Trimuar Thermo Electron Corporation Houston, TX Robert G. Wright Thermo
JPACSM 5 Process Analytical Chemistry THE APPLICATION OF PROCESS MASS SPECTROMETRY TO FUMED SILICA PRODUCTION Peter Traynor and Steven Trimuar Thermo Electron Corporation Houston, TX Robert G. Wright Thermo
AQA Chemistry (Combined Science) Specification Checklists. Name: Teacher:
 AQA Chemistry (Combined Science) Specification Checklists Name: Teacher: Paper 1-4.1 Atomic structure and the periodic table 4.1.1 A simple model of the atom, symbols, relative atomic mass, electronic
AQA Chemistry (Combined Science) Specification Checklists Name: Teacher: Paper 1-4.1 Atomic structure and the periodic table 4.1.1 A simple model of the atom, symbols, relative atomic mass, electronic
Alternative Monitoring Technologies: A Brief Overview
 Alternative Monitoring Technologies: A Brief Overview a technical solution to meet every need Cemtek Environmental Inc. 3041 S. Orange Ave. Santa Ana, CA 92707 800-400-0200 www.cemteks.com 1 What s New?
Alternative Monitoring Technologies: A Brief Overview a technical solution to meet every need Cemtek Environmental Inc. 3041 S. Orange Ave. Santa Ana, CA 92707 800-400-0200 www.cemteks.com 1 What s New?
LAB007 Industrial Analytical Chemistry & Process Analyzer System
 LAB007 Industrial Analytical Chemistry & Process Analyzer System H.H. Sheikh Sultan Tower (0) Floor Corniche Street Abu Dhabi U.A.E www.ictd.ae ictd@ictd.ae Course Introduction: Analytical instruments
LAB007 Industrial Analytical Chemistry & Process Analyzer System H.H. Sheikh Sultan Tower (0) Floor Corniche Street Abu Dhabi U.A.E www.ictd.ae ictd@ictd.ae Course Introduction: Analytical instruments
Advanced Pharmaceutical Analysis
 Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
Monomer Analysis. Analysis by Gas Chromatography WASSON - ECE INSTRUMENTATION. Engineered Solutions, Guaranteed Results.
 Monomer Analysis Analysis by Gas Chromatography Engineered Solutions, Guaranteed Results. WASSON - ECE INSTRUMENTATION Polymer Grade Monomer Analysis Monomer Analysis Impurities in feedstocks can adversely
Monomer Analysis Analysis by Gas Chromatography Engineered Solutions, Guaranteed Results. WASSON - ECE INSTRUMENTATION Polymer Grade Monomer Analysis Monomer Analysis Impurities in feedstocks can adversely
M06/4/CHEMI/HP3/ENG/TZ0/XX CHEMISTRY HIGHER LEVEL PAPER 3. Candidate session number 0 0. Friday 19 May 2006 (morning) 1 hour 15 minutes
 IB CHEMISTRY HIGHER LEVEL PAPER 3 DIPLOMA PROGRAMME PROGRAMME DU DIPLÔME DU BI PROGRAMA DEL DIPLOMA DEL BI Friday 19 May 2006 (morning) 1 hour 15 minutes M06/4/CHEMI/HP3/ENG/TZ0/XX 22066103 Candidate session
IB CHEMISTRY HIGHER LEVEL PAPER 3 DIPLOMA PROGRAMME PROGRAMME DU DIPLÔME DU BI PROGRAMA DEL DIPLOMA DEL BI Friday 19 May 2006 (morning) 1 hour 15 minutes M06/4/CHEMI/HP3/ENG/TZ0/XX 22066103 Candidate session
4.1.1 A simple model of the atom, symbols, relative atomic mass, electronic charge and isotopes. Unit 1 Unit 2 Unit 3. C2.1.1a Structure and bonding
 Summary of changes This resource outlines the main changes that have been made to the assessment and subject content from our previous GCSE Chemistry (4402) to the new specification (8462). Our new specifications
Summary of changes This resource outlines the main changes that have been made to the assessment and subject content from our previous GCSE Chemistry (4402) to the new specification (8462). Our new specifications
10/27/10. Chapter 27. Injector typically 50 C hotter than oven
 Sample and solvent are vaporized onto the head of a column Vaporized solvent and solute are carried through the column by an inert gas (mobile phase) The mobile phase does not interact with compounds of
Sample and solvent are vaporized onto the head of a column Vaporized solvent and solute are carried through the column by an inert gas (mobile phase) The mobile phase does not interact with compounds of
Application Note S/SL
 Analysis of Ultra low Sulfur Compounds in Natural Gas and Gaseous Fuels by Gas Chromatography and Chemiluminescence according to ASTM D Ultra low detection limits Excellent Sensitivity, Repeatability &
Analysis of Ultra low Sulfur Compounds in Natural Gas and Gaseous Fuels by Gas Chromatography and Chemiluminescence according to ASTM D Ultra low detection limits Excellent Sensitivity, Repeatability &
Class III Wastewater Laboratory Analyst Examination Study Guide 2008
 Class III Wastewater Laboratory Analyst Examination Study Guide 2008 Specific laboratory analyses included on Class III Exam: All Information and tests from Class I and Class II Plus the following - Biological
Class III Wastewater Laboratory Analyst Examination Study Guide 2008 Specific laboratory analyses included on Class III Exam: All Information and tests from Class I and Class II Plus the following - Biological
APES Chapter 2 Science, Matter, Energy, and Systems
 Name: Date: Period: APES Chapter 2 Science, Matter, Energy, and Systems Lesson 1: What Do Scientists Do? Concept 2-1 Scientists collect data and develop theories, models, and laws about how nature works.
Name: Date: Period: APES Chapter 2 Science, Matter, Energy, and Systems Lesson 1: What Do Scientists Do? Concept 2-1 Scientists collect data and develop theories, models, and laws about how nature works.
Unit 11 Instrumentation. Mass, Infrared and NMR Spectroscopy
 Unit 11 Instrumentation Mass, Infrared and NMR Spectroscopy Spectroscopic identification of organic compounds Qualitative analysis: presence but not quantity (i.e. PEDs) Quantitative analysis: quantity
Unit 11 Instrumentation Mass, Infrared and NMR Spectroscopy Spectroscopic identification of organic compounds Qualitative analysis: presence but not quantity (i.e. PEDs) Quantitative analysis: quantity
FTIR CEM Performance Specification Modification Considerations
 FTIR CEM Performance Specification Modification Considerations EPA/ICAC Emissions Measurements Roundtable Meeting September 17, 2013 Peter G. Zemek MKS Instruments Marty Spartz Prism Analytical MultiGas
FTIR CEM Performance Specification Modification Considerations EPA/ICAC Emissions Measurements Roundtable Meeting September 17, 2013 Peter G. Zemek MKS Instruments Marty Spartz Prism Analytical MultiGas
NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25)
 Gas Chromatography NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25) The Non-Methane Organic Compounds (NMOC) Analyzer is a gas chromatograph configured for analyzing gaseous samples for total organic
Gas Chromatography NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25) The Non-Methane Organic Compounds (NMOC) Analyzer is a gas chromatograph configured for analyzing gaseous samples for total organic
Chapter 5 Light and Matter: Reading Messages from the Cosmos. What is light? Properties of Waves. Waves. The Electromagnetic Spectrum
 Chapter 5 Light and Matter: Reading Messages from the Cosmos What is light? Light is a form of radiant energy Light can act either like a wave or like a particle (photon) Spectrum of the Sun 1 2 Waves
Chapter 5 Light and Matter: Reading Messages from the Cosmos What is light? Light is a form of radiant energy Light can act either like a wave or like a particle (photon) Spectrum of the Sun 1 2 Waves
Skoog/Holler/Crouch Chapter 26 Principles of Instrumental Analysis, 6th ed. CHAPTER 26
 Skoog/Holler/Crouch Chapter 26 Principles of Instrumental Analysis, 6th ed. Instructor s Manual CHAPTE 26 26-1. (a) Elution is a process in which species are washed through a chromatographic column by
Skoog/Holler/Crouch Chapter 26 Principles of Instrumental Analysis, 6th ed. Instructor s Manual CHAPTE 26 26-1. (a) Elution is a process in which species are washed through a chromatographic column by
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth
 FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
7a. Structure Elucidation: IR and 13 C-NMR Spectroscopies (text , , 12.10)
 2009, Department of Chemistry, The University of Western Ontario 7a.1 7a. Structure Elucidation: IR and 13 C-NMR Spectroscopies (text 11.1 11.5, 12.1 12.5, 12.10) A. Electromagnetic Radiation Energy is
2009, Department of Chemistry, The University of Western Ontario 7a.1 7a. Structure Elucidation: IR and 13 C-NMR Spectroscopies (text 11.1 11.5, 12.1 12.5, 12.10) A. Electromagnetic Radiation Energy is
Describe how the inter-conversion of solids, liquids and gases are achieved and recall names used for these inter-conversions
 Understand the arrangements, movements and energy of the particle in each of the 3 states of matter : solid, liquid and gas Describe how the inter-conversion of solids, liquids and gases are achieved and
Understand the arrangements, movements and energy of the particle in each of the 3 states of matter : solid, liquid and gas Describe how the inter-conversion of solids, liquids and gases are achieved and
CHEMISTRY & YOU What is the difference between the oxygen you breathe and the oxygen in ozone in the atmosphere?
 CHEMISTRY & YOU What is the difference between the oxygen you breathe and the oxygen in ozone in the atmosphere? Our atmosphere contains two different molecules that are both made of oxygen atoms. The
CHEMISTRY & YOU What is the difference between the oxygen you breathe and the oxygen in ozone in the atmosphere? Our atmosphere contains two different molecules that are both made of oxygen atoms. The
ISO 8245 INTERNATIONAL STANDARD. Water quality Guidelines for the determination of total organic carbon (TOC) and dissolved organic carbon (DOC)
 INTERNATIONAL STANDARD ISO 8245 Second edition 1999-03-01 Water quality Guidelines for the determination of total organic carbon (TOC) and dissolved organic carbon (DOC) Qualité de l'eau Lignes directrices
INTERNATIONAL STANDARD ISO 8245 Second edition 1999-03-01 Water quality Guidelines for the determination of total organic carbon (TOC) and dissolved organic carbon (DOC) Qualité de l'eau Lignes directrices
chemical sensing linear devices chemical sensing - linear devices 1
 chemical sensing linear devices 20090403 16722 mws@cmu.edu chemical sensing - linear devices 1 chemical sensing introduction to chemical sensing and sensors vapor detection techniques (mostly chemistry)
chemical sensing linear devices 20090403 16722 mws@cmu.edu chemical sensing - linear devices 1 chemical sensing introduction to chemical sensing and sensors vapor detection techniques (mostly chemistry)
Robustness of the QAL2 calibration (EN 14181) Uncertainty on the results given by a calibrated AMS
 Robustness of the 2 calibration (EN 14181) Uncertainty on the results given by a calibrated Jean.Poulleau*¹, Cécile.Raventos 1, Philippe.Fayolle², Emmanuel.Fiani 3 ¹ INERIS, Parc technologique ALATA BP
Robustness of the 2 calibration (EN 14181) Uncertainty on the results given by a calibrated Jean.Poulleau*¹, Cécile.Raventos 1, Philippe.Fayolle², Emmanuel.Fiani 3 ¹ INERIS, Parc technologique ALATA BP
Chapter 4 Ultraviolet and visible spectroscopy Molecular Spectrophotometry
 Chapter 4 Ultraviolet and visible spectroscopy Molecular Spectrophotometry Properties of light Electromagnetic radiation and electromagnetic spectrum Absorption of light Beer s law Limitation of Beer s
Chapter 4 Ultraviolet and visible spectroscopy Molecular Spectrophotometry Properties of light Electromagnetic radiation and electromagnetic spectrum Absorption of light Beer s law Limitation of Beer s
Combined Science: Trilogy
 Co-teaching GCSE Chemistry and GCSE Combined Science: Trilogy This high level co-teaching guide will help you plan your route through the course. You ll be able to see what common themes and topics span
Co-teaching GCSE Chemistry and GCSE Combined Science: Trilogy This high level co-teaching guide will help you plan your route through the course. You ll be able to see what common themes and topics span
MARIYA INTERNATIONAL SCHOOL. Work sheet III. Term I. Level 8 Chemistry [MCQ] Name: CHEMICAL REACTIONS & SULFUR
![MARIYA INTERNATIONAL SCHOOL. Work sheet III. Term I. Level 8 Chemistry [MCQ] Name: CHEMICAL REACTIONS & SULFUR MARIYA INTERNATIONAL SCHOOL. Work sheet III. Term I. Level 8 Chemistry [MCQ] Name: CHEMICAL REACTIONS & SULFUR](/thumbs/95/123248955.jpg) MARIYA INTERNATIONAL SCHOOL Work sheet III Term I Level 8 Chemistry [MCQ] Name: CHEMICAL REACTIONS & SULFUR 1. A steel works and a chemical works are built near to a city. The limestone buildings in the
MARIYA INTERNATIONAL SCHOOL Work sheet III Term I Level 8 Chemistry [MCQ] Name: CHEMICAL REACTIONS & SULFUR 1. A steel works and a chemical works are built near to a city. The limestone buildings in the
CHEM Chapter 12 Infrared and Mass Spec (homework). Stafford. S18
 Exhibit 12-4 The following question(s) refer to the mass spectrum shown below. 1. Refer to Exhibit 12-4. This compound contains C, H, and one other atom. Identify the other atom from the mass spectrum
Exhibit 12-4 The following question(s) refer to the mass spectrum shown below. 1. Refer to Exhibit 12-4. This compound contains C, H, and one other atom. Identify the other atom from the mass spectrum
Chromatographic Methods of Analysis Section: 5 Gas Chromatography (GC) Prof. Tarek A. Fayed
 Chromatographic Methods of Analysis Section: 5 Gas Chromatography (GC) Prof. Tarek A. Fayed Gas Chromatography (GC) In gas chromatography, the sample is vaporized and injected onto the head of a chromatographic
Chromatographic Methods of Analysis Section: 5 Gas Chromatography (GC) Prof. Tarek A. Fayed Gas Chromatography (GC) In gas chromatography, the sample is vaporized and injected onto the head of a chromatographic
Subject Overview Curriculum pathway
 Subject Overview Curriculum pathway Course Summary Course: A Level Chemistry Overall Summary Unit / Module Exam / Controlled % of course UMS allocation Marks available UMS / RAW mark grade boundaries from
Subject Overview Curriculum pathway Course Summary Course: A Level Chemistry Overall Summary Unit / Module Exam / Controlled % of course UMS allocation Marks available UMS / RAW mark grade boundaries from
Low cost, rapid and in situ accurate quantification of chloramines and ammonia
 Low cost, rapid and in situ accurate quantification of chloramines and ammonia National Environmental Monitoring Conference 2018 Merwan Benhabib, PhD VP Engineering Chlorine + Ammonia Rate of formation
Low cost, rapid and in situ accurate quantification of chloramines and ammonia National Environmental Monitoring Conference 2018 Merwan Benhabib, PhD VP Engineering Chlorine + Ammonia Rate of formation
Reprinted from February Hydrocarbon
 February2012 When speed matters Loek van Eijck, Yokogawa, The Netherlands, questions whether rapid analysis of gases and liquids can be better achieved through use of a gas chromatograph or near infrared
February2012 When speed matters Loek van Eijck, Yokogawa, The Netherlands, questions whether rapid analysis of gases and liquids can be better achieved through use of a gas chromatograph or near infrared
Chromatography. Gas Chromatography
 Chromatography Chromatography is essentially the separation of a mixture into its component parts for qualitative and quantitative analysis. The basis of separation is the partitioning of the analyte mixture
Chromatography Chromatography is essentially the separation of a mixture into its component parts for qualitative and quantitative analysis. The basis of separation is the partitioning of the analyte mixture
