CHEMICAL KINETICS. 2. RATE OF RE ACTION : The change in concentration of either reactant or product per unit time. 2NH 3. d [NH 3 ] dt.
|
|
|
- Blanche Harrell
- 6 years ago
- Views:
Transcription
1 . CL ASSIFICATION OF RE ACTIONS : [IN TERMS OF R ATES] (i) There re cerin recions which re oo slow e.g. rusing of iron, wehering of rocs. ( i i) Insnneous recions i.e. oo fs e.g. Deonion of explosives, cid-bse neurlizion, (iii) precipiion of AgCl by NCl nd AgNO 3. Neiher oo fs nor oo slow e.g. combinion of H nd Cl in presence of ligh, hydrolysis of ehyl cee clysed by cid, decomposiion of zomehne. RATE OF RE ACTION : The chnge in concenrion of eiher recn or produc per uni ime. Formul : v ± dc dc chnge in concenrion in smll inervl. [ ] sign is used when we refer o recn concenrion. [+] sign is used when we refer o produc concenrion. Exmple : N + 3H NH 3 (i) Re of formion of mmoni + (ii) Re of disppernce of nirogen (iii) Re of disppernce of hydrogen CHEMICAL KINETICS d [NH 3 ] d [N ] d [H ] Re of recion + d [NH 3 ] d [N ] 3 d [H ] Thus, Re of recion d [N ] d [NH 3 ] or re of formion of mmoni Twice he re of disppernce of nirogen i.e. d [NH 3 ] 3 d [H ] 3. AVERAGE VELOCITY OF REACTION : Chnge in he concenrion of recns or producs per uni ime is clled verge recion velociy. If c is he chnge in he concenrion of recns nd produc in ime, hen Averge velociy ± c or Averge velociy ( ) Chnge in he concenrion of recns Time (+ ) Chnge in he concenrion of producs Time
2 Concenrion Produc Recn Uni of verge velociy Time Uni of concenrion Uni of ime grm mole lire Second grm mole lire second 4. INSTANTANEOUS RATE OF THE RE ACTION : The re of recion deermined specified concenrion or specified ime is clled insnneous re. The insnneous re of he recion cn be deermined by mesuring concenrion of recn or produc insn of ime nd ploing concenrion versus ime. The insnneous re ny ime is deermined by he slope of he ngen poin on he ime-concenrion curve corresponding o he specified ime. The slope of he ngen poin is he limiing vlue of c Lim dc In erms of he concenrion of recn, he re of he recion dc. The sign indices h he concenrion of recn decreses wih ime. In erms of he concenrion of produc, he re of he recion + dc. The +sign indices h he concenrion of produc increses wih ime. In he recion if ime he concenrion of produc is x nd c. ime +, he concenrion becomes x + dx hen he recion re dx. For exmple he re of recion : N + 3H NH 3 in erms of he concenrions of N, H nd NH 3 cn be expressed s : Concenrion B Time d[n ], 3 d[h ], + d[nh 3 ] E x. Wh should be () he re of disppernce of B nd (b) he re of formion of C, if he re of disppernce of A for he recion A + B C is mole/lire/second priculr emperure? () Re of disppernce of A Re of disppernce of B mole/lire/second (b) Re of disppernce of A Re of formion of C Re of formion of C Re of disppernce of A mole/lire/second
3 Ex. Sol. Ex.3 Sol. A gseous recion : A(g) + B(g) C(g), Show decrese in pressure from mm o mm in minues. The re of ppernce of C is - [A] mm/min [B] 4 mm/min [C] mm/min [D] mm/min. Ans. [B] Suppose p is he pressure of C fer min. Fll in pressure of A p ; Fll in pressure of B p Tol fll in pressure (p + p) p p mm Pressure of C p 4 mm Re of ppernce of C 4/ 4 mm/min The erm dx in he re expression refers o he - [A] concenrion of he recns [B] increse in concenrion of he recns [C] insnneous re of he recion [D] verge re of he recion Ans. [C] I is expression for insnneous re Ex.4 Which of he following expression cn be used o describe he insnneous re of he recion? Sol. A + B A B [A] d [A] [B] d [A] d [A B] d [A] d [B] [C] [D]. The insnneous re of he recion cn be expressed by ny of he following expressions Ans. [A] d [A] or d [B] or d [A B] Ex.5 Which of he following will rec he highes re? Sol. [A] mol of A nd mol of B in L vessel [B] mol of A nd mol of B in L vessel [C] 3 mol of A nd 3 mol of B in 3 L vessel [D] All would rec he sme re Since ll hve sme conc. of recns, ll would rec sme re. 5. FACTORS AFFECTING THE RATE OF RE ACTION : (i) Ans. [D] Concenrion : Lw of mss cion enuncies h greer is he conc. of he recns, he more rpidly he recion proceeds. ( i i ) Pressure (Gseous recion) : On incresing he pressure, volume decreses nd conc. increses nd hence he re increses. ( i i i ) Temperure : I is generlly observed h rise in emperure increses he recion re. ( i v ) Nure of he recns : The re depends upon specific bonds involved nd hence on he nure of recns. g s ( v ) Sur fce re of he recn s : In heerogeneous recions, more powdered is he form of recns, more is he velociy. [s more cive cenres re provided] ( v i ) Clys : Affecs he re immensely. E x. 6 For he recion : 4NH 3 (g) + 5O (g) 4NO(g) + 6H O(g) Given : d [NO] mol L s Clcule : (i) re of disppernce of mmoni (ii) re of formion of wer
4 Sol. From he eqn. i is cler h Re 4 d[nh 3 ] 4 d [NO] 6 d[ho] Thus : 4 d[nh 3 ] 4 d [N O ] or d[nh 3 ] d [NO] mol L s Also 4 d [NO] 6 d[ho] E x. 7 3 d [NO] d[ho] d[ho] d[ho] mol L s The following recion ws sudied in closed vessel. N O 5 (g) 4NO (g) + O (g) I ws found h concenrion of NO increses by 4. mol L in five seconds, clcule () he re of recion (b) he re of chnge of concenrion N O 5. () d[no ] Re 4 Bu d [NO ] 4. mol L 8 3 mol L 5 sec s Re of recion mol L s 3 mol L s (b) Re of chnge of conc. of N O 5 d[n O 5 ] Re of formion of NO [d[n O 5 ]] mol L s E x. 8 The re of chnge in concenrion of R in he recion, P + Q R + 3S, ws repored s. mol L sec. Clcule he recion re s well s re of chnge of concenrion of P, Q nd S. d[p] d[q] d[r] d[s] Re of recion 3 d[r]. moll s d[p] d[r]. mol L s d[q] d[r].5 moll s d[s] 3 d[r] 3.5 moll s Re of recion d[c].5 mol L s
5 6. SPECIFIC RE ACTION RATE : Applying lw of mss cion o he recion : m A + m B n C + n D m m Re [A] [B] m or v [A] [B] This equion is nown s re lw. Where is he proporionliy consn nd is clled (i) Velociy consn or (ii) Velociy coefficien or (iii) Specific recion re. On puing [A] [B], where hve : v m Hence specific recion re is he re of he recion when he concenrion of ech recn is en s uni. Uni of Specific Recion Re m m v [A]. [B] conc. ime m + m [conc.] [ (m + m )] mole [conc.] [ime] or lire [ (m + m )]. [second ] 7. DISTINCTION BETWEEN UNIT OF R ATE AND R ATE CONSTANT : Re of recion : Is unis re lwys mole lire ime. 8. RATE LAW : Re consn : Is uni depends upon he order of recion. ( ) I my lso no depend upon he concenrion of ech recn or produc of he recion. Suppose, ma + nb Produc m R [A] [B] n Ex.9 ( b ) Re of chemicl recion is direcly proporionl o he produc of he concenrion of recns rise o he power of heir soichiomeric coffecien. ( c ) The re lw represens he experimenlly observed re of recion which depends upon he slowes sep of he recion. ( d ) Re lw cnno be deduce from he equion for given recion. I cn be find by experimens only. (e) The re lw my no ber simple relionship of he soichiomeric equion. In he recion, A + B 6C + D, if he iniil re d [A] vlue of d [B]? [A] 8.5 M sec [B].5 M sec is.6 M sec, wh will be he [C] 5. M sec [D] 7.5 M sec Ans. [C] From he recion i is eviden h when mole of A is recing, moles of B mus rec. Hence he decrese in he concenrion of B mus be wice h of A d [B] d [A].6 5. M sec
6 E x. The dimensions of re consn of second order recion involves : [A] ime nd concenrion [B] neiher ime nor concenrion [C] ime only [D] concenrion only Ans. [A] Re Sol. [A] E x. Sol. mol L s (mol L ) s mol L (mol L ) s The re consn of recion hs sme unis s he re of recion. The recion is of [A] zero order [B] firs order [C] second order [D] none of hese Ans. [A] For zero order recion, r [A]. Thus he unis of re he sme s h of re of recion. E x. The re consn of n h order recion hs unis : [A] lire n mol n sec [C] n mol [B] mol n lire n sec n lire sec [D] mol n lire n sec Ans. [D] For n n h order recion : re [conc.] n re [conc.] n Unis of mol L s (mol L ) n mol n L n s E x. 3 On which of he following fcors, he re consn does no depend? [A] Temperure [B] Concenrion [C] Presence of clys [D] Nure of recns Ans. [B] Re consn is independen of he conc. of he recns. 9. ORDER OF REACTION : The sum of he power of he concenrion erms on which he re of recion cully depends s observed experimenlly is clled he order of he recion. For exmple, Order of recion x + y Thus, he order of recion my lso be defined s he sum of he exponens (powers) o which he concenrion erms in he re lw equion re rised in order o express he observed re of he recion. Thus, recion is sid o be of he firs order if is re is given by he expression of he ype, r C A Second order if he re is given by he expression of he ype, N o e : r C A or r C A C B hird order if he re is given by he expression of he ype r 3 C 3 A or r 3 C A C B or r 3 C A C B or 3 C A C B C C nd so on For zero order recion, he re equion is wrien s R. I is o be noed h he order of recion is essenilly n experimenl quniy. Order my be zero, frc ionl, i neger or neg ive. Exmple : R e cion Experimenl re equion o r d e r H + Cl HCl v zero H + Br HBr v [H ] [Br ] / one nd hlf H + I HI v [H ] [I ] wo
7 E x. 4 Exmples of frcionl order recion Recion : CO(g) + Cl (g) COCl (g) v [CO] [Cl ] /, order.5 Recion : COCl (g) CO(g) + Cl (g) v [COCl ] 3/, order.5 For he chemicl recion, 4HBr + O H O + Br Re [HBr] [O ] Wh is he probble mechnism of he recion? HBr + O HOOBr (slow) HOOBr + HBr HOBr HOBr + HBr H O + [Br ] (fs) (fs) E x. 5 Niric oxide (NO) recs wih oxygen o produce nirogen dioxide : NO (g) + O (g) NO (g) Wh is he prediced re lw, if he mechnism is :- NO (g) + O (g) NO 3 (g) (fs) NO 3 (g) + NO (g) From he slow sep, Re [NO 3 ] [NO] From fs sep : Equilibrium consn (K) NO (g) [NO 3 ] [NO][O ] (slow)...(i)...(ii) Subsiuing he vlue of [NO 3 ] from equion (ii) ino equion (i), we ge Re '[NO] [O ]. MOLECULARITY OF A REACTION : Moleculriy is defined s he number of molecules, oms, or rdicls h mus collide simulneously in order for he recion o e plce. I is lwys whole number nd cnno be negive. In he elemenry processes : Priciping species Molec ulr i y One species pricipes... unimoleculr, Two species pricipes... bimoleculr, Three species pricipes... rimoleculr, 3 Exmple : N O 4 NO... unimoleculr H + I HI... bimoleculr FeCl 3 + SnCl FeCl + SnCl 4... rimoleculr Noe : If he recion es plce in wo or more seps hen he overll moleculriy of he recion is moniored by he slow or re deermining sep.
8 . DIFFERENCE BETWEEN MOLECULARITY AND ORDER OF REACTION : Moleculriy Order of Recion. Moleculriy cn neiher be zero nor frcionl. Order of recion cn be zero, frcionl or ineger. I is he number of molecules of recns. I is sum of power rised or he re expression. concenrion erms ing pr in elemenry sep of recion. 3. I cn no hve negive vlue. 3. Order of recion my hve negive vlue. 4. Moleculriy is heoreicl propery. 4. Order is n experimenl propery. 5. Moleculriy concerns wih mechnism. 5. Order concerns wih ineic (re lw). E x. R e cion Re lw O r d e r CH 3 CHO CH 4 + CO Re [CH 3 CHO] 3/.5 NH 3 N + 3 H Re [NH 3 ] Hl H + I Re [HI], i.e. Re Order my chnge wih chnge in experimenl condiions while moleculriy cn'. E x. isomerizion HC CH 3 This recion follows firs order ineics high pressure nd nd order ineics low pressure of cyclopropne.. PSEUDO UNIMOLECULAR REACTION : E x. 6 Consider he recion : CH 3 COOC H 5 + H O + H CH 3 COOH + C H 5 OH Since wer is presen in lrge excess, is concenrion hrdly chnges during he course of he recion. And s such re depends only on he concenrion of eser. The order is one bu he moleculriy is wo. Such recions re clled pseudo unimoleculr rec ion. For chemicl recion, A producs, he re of recion doubles when he concenrion of A is incresed by 4 imes. The order of recion is [A] 4 [B] [C] / [D] Ans. [C] A n s. r [A] n... (i) ; r [4A] n... (ii) E x. 7 Dividing (ii) by (i) r r 4A A n or n or n or n / For hypoheicl recion A + B producs, he re lw is, r [B] [A], he order of recion is : [A] [B] [C] [D] 3 Ans. [B] + E x. 8 The slowes sep of priculr recion is found o be X + Y XY The order of he recion is [A] [B] 3 [C] 3.5 [D].5 Ans. [D] Sol. r [X ] / [Y ] Order.5 +.5
9 E x. 9 The re of cerin hypoheicl recion A + B + C producs, is given by r da [A] / [B] /3 [C] /4 The order of recion is given by [A] [B] / [C] [D] 3/ Ans. [D] Sol. Order of recion ZERO ORDER REACTION : Recion whose re is no ffeced by concenrion sid o be of zero order recion. Exmple : ( i ) Recion beween Aceone nd Bromine (ii) ( A ) Uni of Re Consn : Dissociion of HI on gold surfce mol L sec Uni of re of recion Uni of re consn. ( B ) Re Consn of Zero Order Recion : x The re of recion is independen of he concenrion of he recion subsnce. ( C ) Deerminion of Hlf life Period of Zero Order Recion : A ½ ; x / or / The hlf life period is direcly proporionl o he iniil concenrion of he recns. E x. The re equion of recion is [A] / [B] / [C]. Wh should be he order of he recion? Sol. n + Order of he recion is zero. Grphicl represenion x Slope dx Exmple : Phoochemicl recions, lie H + Cl HCl, re zero order recion. Decomposiion of NH 3 on plinum surfce is lso zero order recion.
10 4. FIRST ORDER REACTION : When he re of recion depends only on one concenrion erm of recn. A firs order recion is one whose re vries s firs power of he concenrion of he recn, i.e. he re increses s number of imes s he concenrion of recn is incresed. Le us, consider unimoleculr firs order recion represened by he generl equion, A Produc A A x x The iniil concenrion of A is mole L nd is concenrion fer ime is ( x) mole L. This mens during he ime inervl, x mole L of A hs reced. The re of recion ny ime is given by he following firs order ineics. or d( x) ( x) d(x) ( x) or dx ( x) where is he re consn of he recion. dx x This is differenil re equion nd cn be solved by inegrion. dx x or ln ( x). + C...() where C is inegrion consn. The consn C cn be evlued by pplying he iniil condiion of he recion i.e. when, x. Puing hese in equion (), we ge C ln Puing he vlue of C in equion (), we ge ln ( x). ln or ln x.33 x...().33 ( x ) Also, ( ) ( x ).33 nd ( ) R R where ( x ) is concenrion ime nd ( x ) is concenrion fer ime nd R is re ime nd R is re ime. If [A] nd [A] be he concenrions of recn zero ime nd ime respecively, hen Eq. () my be pu s ln [A] [A] Also, [A] [A] e
11 This is he inegred re expression for firs order recion. As, ln ln ln ( x) Also, ( x) e x ( e ) x ( x) ().33 Degree of dissociion x ( e ) Uni of Re consn A ( x) O Slope.33 OA A The differenil re expression for n h order recion is s follows : dx ( x) n dx (concenrion) or (conc.) n ime n n ( x) (concenrion) ime If concenrion be expressed in mole L nd ime in minues, hen (mole L ) n min For zero order recion, n nd hence, mole L min For firs order recion, n nd hence, (mole L ) min min For second order recion, n nd hence, (mole L ) min mole L min The re consn of firs order recion hs only ime in is uni. I hs no concenrion erm in he uni. This mens he numericl vlue of for firs order recion is independen of he uni in which concenrion is expressed. If concenrion uni is chnged, he numericl vlue of for firs order recion will no chnge. However, i would chnge wih chnge in ime. Sy, is 6. 3 min hen i my lso be wrien s 4 s, i.e., numericl vlue of will decrese 6 imes if ime uni is chnged from hour o minue or from minue o second. Hlf - ime or hlf - life period of firs order recion : The hlf - ime of recion is defined s he ime required o reduce he concenrion of he recn o hlf of is iniil vlue. I is denoed by he symbol /. Thus, When x, / Puing hese vlues in Eq. (), we ge.33 /.33 /.33 /.33 (.33) / (3)
12 Since is consn for given recion given emperure nd he expression lcs ny concenrion erm so from Eq. (3) i is eviden h hlf-ime 75 of firs order recion is consn independen of 5 iniil concenrion of recn. This mens if we sr wih 4 moles L of recn recing by firs order 5 ineics, hen fer minues i is reduced o moles L. Th is, fer minues from he sr of recion he concenrion of he recn will be moles L (min) fer 4 minues from he sr of recion, he concenrion is mole L. Afer 6 minues from he C(% ) sr of recion, he concenrion of he recn will be reduced o.5 mol L. In oher words, if during minues 5% of he recion complees, hen in 4 minues 75%, in 6 minues 85.5% of he recion nd so on, will complee s shown in he figure bove. Thus, frcion lef fer n hlf-lives Concenrion lef fer n hlf-lives, [A] [A] I is lso o be noed h Eq. (3) helps o clcule / or. n n A generl expression for / is s follows. Hlf - life of n h order recion Le us find ou / for n h order recion where n. d[a] d[a] n [A] n [A] n [ A ] / d[a] [A] n [ A ] [A ] [A] n d[a] n / [ A ] n A n [A] n n [A ] n [A ] [A ] / n [A ] n / n n / n [ n ] ( n) A n n / (n )[A] n n n / / (order n ) Therefore, for n h order recion, he hlf-life is inversely reled o he iniil concenrion rised o he power of (n ). / n where n order of recion. Exmple : ( i ) All rdiocive recions (ii) A Produc (iii) NO N + O (iv) Cl O 7 Cl + 7O ( A ) Uni of re consn of firs order recion K (sec) n ( B ) Veloci y cons n for fir s order rec ion.33 ( x) where ime, iniil concenrion ( x) concenrion fer ime K Re consn.33 ( x)
13 ( C ) Grphicl Represenion Grph beween v/s ( x) is srigh line.33 Slope /( x) E x. A firs order recion ges 9% compleed in 4 minue. Find ou he hlf-life period of he recion. Sol. Suppose h he iniil concenrion of recn () 4 minues 9% of he recion ge compleed in 4 minues. Therefore, x 9.33 x minues.693 ½ minues E x. Prove wih he help of he following d h hydrolysis of H O is firs order recion. Iniil concenrion in he recion 5.. Time, 3 (in minues) V For firs order recion,.33 x ; Here, 5 minues minues minues Consn vlue of shows h hydrolysis of H O in queous medium is firs order recion.
14 E x. 3 The re consn for n isomerizion recion, A B, is.5 3 min. If he iniil concenrion of A is M, clcule he re of recion fer hr. As,.33 E x , for firs order recion ( x) ( x) ( x).867 The re fer 6 minues ( x) The re of firs order recion is.8 mol L min. nd.6 mol L 4 min. fer sr of recion. Find he hlf-life of recion. As, re [A].8 [A].6 [A] [A].8 4 [A].6 3 For firs order recion : E x [A ] [A] when (4 ) min min / 48.3 min.44 Rdiocive decy of n omic nucleus is firs order recion. Hlf-life period of rdium [ 88 R 6 ] is 59 yers. Find ou is decy consn ½ / SECOND ORDER REACTION Y A + A Produc A + B Produc A A ( x) ( x) x A ( x ) ( x ) x A ( x ) ( x ) x As per re lw, dx [A]n [A] [A][B] dx ( x) ( re consn for second order recion) Also, ( x) ( x) or ( ) ( x ) ( x ) Where ( x ) nd ( - x ) re he concenrion of he recn A ime nd respecively. If recn A nd B hve differen concenrions nd b, hen.33 ( b) b( x) (b x)
15 when >> b hen ( b) ( x) Equion reduces o.33 b x '.33 b b x This is n exmple of pseudo firs order recion. Equion for second order recion cn be rewrien s ( x) + Grphicl Represenion (equion for firs order ineics) x A (i) Slope (ii) Inercep, OA In generl for n h order recion, E x. 6 n n n (n ) ( x) In generl for n h n /(n) (n ) () /(n) n (n ) /(n) ( n) order recion, n (n ) Iniil concenrions of boh he recns of second order recion re equl nd 6% of he recion ges compleed in 3 seconds. How much ime will be en in % compleion of he recion? Sol. x ( x) Suppose, Now, for % compleion 3.6 (.6) x ( x) (.) (.) second
16 E x. 7 Sol. A second order recion requires 7 minues o chnge he concenrion of recns from.8 M o. M. How much ime will i require o become.4 M. For second order recion when, ( x). x.( x) (.) ( x) (.4) From he equion () nd () (.).4.8 (.4) minues 6. THIRD ORDER REACTION :...()...() A recion is sid o be of hird order if is re is deermined by he vriion of hree concenrion erms. When he concenrion of ll he hree recns is sme or hree molecules of he sme recn re involved, he re expression is given s ( i ) NO + O NO ( i i ) A + B + C Produc Re cons n of h ird order rec ion x( x). ( x) 3 3 Hlf life period / Thus, hlf life is inversely proporionl o he squre of iniil concenrion. nh order recion : A Produc n n ( x) n n [n, n order] / n. n n (n ) Side or concuren recion : B A Consecuive recion : ; ln C [A] [A] ( + ) ; [B] [C] B C : mx n : [B] mx [A] A 7. THRESHOLD ENERGY AND ACTIVATION ENERGY : For recion o e plce he recing molecules mus colloid ogeher, bu only hose collisions, in which colliding molecules possess cerin minimum energy is clled hreshold energy (E T ).
17 Acivion energy (E ) : The exr energy needed for he recn molecules o be ble o rec chemiclly is nown s Acivion energy. E E T Threshold energy E' E Acivion energy of forwrd recion E' civion energy of bcwrd recion P Poenil energy of recns P Poenil energy of producs 8. EFFECT OF CATALYST : Energy E T Recns P Produc Recion co-ordines A clys is subsnce, which increses he re of recion wihou iself being consumed he end of he recion, nd he phenomenon is clled clysis. There re some clyss which decrese he re of recion nd such clyss re clled negive clys. Obviously, he clys ccelering he re will be posiive clys. However, he erm posiive is seldom used nd clys iself implies posiive clys. Clyss re generlly foreign subsnces bu someimes one of he produc formed my c s clys nd such clys is clled "uo clys" nd he phenomenon is clled uo clysis. Therml decomposiion of KClO 3 is found o be ccelered by he presence of MnO. Here, MnO (foreign subsnce) c s clys KClO 3 + [MnO ] KCl + 3O + [MnO ] MnO cn be received in he sme composiion nd mss he end of he recion. In he permngne irion of oxlic cid in he presence of bench H SO 4 (cid medium), i is found h here is slow dischrge of he colour of permngne soluion in he beginning bu fer someime he dischrge of he colour becomes fser. This is due o he formion of MnSO 4 during he recion which cs s clys for he sme recion. Thus, MnSO 4 is n "uo clys" for his recion. This is n exmple of uo clys. P KMnO 4 + 4H SO 4 + 5H C O K SO 4 + 8H O + CO Generl Chrcerisic of Clys A clys does no iniie he recion, i simply fsens i. Only smll moun of clys cn clyse he recion. A clys does no ler he posiion of equilibrium i.e. mgniude of equilibrium consn nd hence G. I simply lowers he ime needed o in equilibrium. This men if reversible recion in bsence of clys complees o go o he exen of 75% ill inmen of equilibrium, nd his se of equilibrium is ined in minues hen in presence of clys lso he recion will go o 75% of compleion before he inmen of equilibrium bu he ime needed for his will be less hn minues. A clys drives he recion hrough differen roue for which energy brrier is of shores heigh nd Hence, E is of lower mgniude. Th is, he funcion of he clys is o lower down he civion energy. P.E. E E' E Energy of civion in bsence of clys. H R Recns E' Energy of civion in presence of clys. H P Producs Recion Coordine E E' Lowering of civion energy by clys.
18 If nd c be he re consn of recion given emperure T, nd E nd E' re he civion energies of he recion in bsence nd presence of clys, respecively, he c Ae Ae E ' / RT E / RT c (E E ' ) / RT Ae c Since E, E' is +ve, so c >. The rio gives he number of imes he re of recion will increse by he use of clys given emperure nd his depends upon E E'. Greer he vlue of E E', more number of imes c is greer hn. The re of recion in he presence of clys ny emperure T my be mde equl o he re of recion in bsence of clys bu we will hve o rise he emperure. Le, his emperure be T, hen E x. 8 e e E ' / RT E / RT E ' E or T T A hydrogenion recion is crried ou 5 K. If he sme recion is crried ou in presence of clys he sme re, he emperure required is 4K. Clcule he civion energy of he recion if he clys lowers he civion energy brrier by 4 J/mol. Le, E nd E' be he energy of civion in bsence nd presence of clys for hydrogenion recion, s Ae E / RT E / R 5 Ae (In bsence of clys) E ' / R 4 Ae (In presence of clys) Given, r r ; Hence e e E / R 5 E ' / R 4 E E ' R 5 R 4 or E E (As E E' 4) E J/mol 9. DETERMINATION OF ORDER OF RE ACTION : Inegrion Mehod In his mehod, vlue of is deermined by puing vlues of iniil concenrion of recns nd chnge in concenrion wih ime in ineic equion of firs, second nd hird order recions. The equion by which consn vlue of is obined is clled order of h recion..33 x x ( x) (For firs order recion) (For second order recion) E x. 9 3 x( x) ( x) (For hird order recion) For recion, A B, i hs been found h he order of he recion is zero wih respec o A. Which of he following expression correcly describes he recion? [A].33 [A] [A] d [A] [A], d[a] [B] [A] [A] [C] ½.693 [D] ½ [A] Ans. [B] Inegring from o [A] [A]
19 Grphicl Mehod If srigh line is obined on drwing grph beween ( x) nd ime hen i is firs order recion. If srigh line is obined on drwing grph beween ( x) nd dx, hen i is second order recion. E x. 3 Which of he following grphs is for second order recion? [A] Re [B] Re [C] Re [D] Re [A] [A] [A] [A] Ans. [C] For second order recion re vs [A] is srigh line wih slope equl o re [A] E x. 3 If srigh line is obined on drwing grph beween ( x) 3 nd dx, hen i is hird order recion. Hlf-life Mehod Relion beween hlf-life period of recion nd iniil concenrion is s follows : / n For firs order recion (Hlf life ) For second order recion (Hlf life /) For hird order recion (Hlf life / ) For firs order recion,.75 is 386 seconds. Therefore, he specific re consn is [A] s [B] 3 s [C] s [D] 4 s Ans. [B] s.5 ; E x. 3 ½ of firs order recions is given by.693 [A].693 3/4 ( ½ ).693 E x. 3 3 [B] s ; s, 3/4 would be equl o [C] s [D].94 Ans. [C] The ½ of firs order recion is found o be minues. The percenge of he recn lef fer 36 seconds is : [A].5 [B] 5 [C] 5 [D] 7.5 Ans. [A] 36 seconds 6 min 3 hlf-lives / 5 Oswld Isolion Mehod / 5 /.5 This mehod is used o find ou he order of complex recions. If na, nb nd nc molecules of subsnce A, B nd C, respecively, re presen in recion, hen na + nb + nc will be he order of recion. When B nd C re in excess, he order of recion will be na. When A nd B re in excess, he order of recion will be nc. When A nd C re in excess, he order of recion will be nb. E x. 3 4 When he iniil concenrion of recion ws doubled, is hlf life become hlf, Wh should be he order of he recion? Re lw for produc of recion is s follows : Re [A] n nd [] n ; n n.5.5 n
20 . TEMPER ATURE EFFECT : The re of recion is dependen on emperure. This is expressed in erms of emperure coefficien which is rio of wo re consns differing by emperure of. Generlly he emperure seleced re 98K nd 38K. I is mhemiclly expressed s, re consn 38K Temperure coefficien re consn 98K T he vlue of emperure coefficien for mos of he rec ions lie s be ween o 3.. ARRHENIUS EQUATION : Arrhenius derived mhemicl expression o give quniive relionship beween re consn nd emperure. The expression is E /RT A.e (Here, A frequency fcor; E civion energy ; R gs consn nd T emperure). If! nd re re consns emperure T nd T hen E T T.33 R T T E x. 3 5 Ehylene oxide is decomposed ino CH 4 nd CO. Re consn for his recion my be described by 4.5 he equion (s ) 4.34 T (i) Wh will be he energy of civion of his recion? (ii) Wh will be he vlue of 67 K? (iii) A wh emperure will is hlf-life period be 5.6 minues? (i) We now, A E.33RT...(i) Given, (s ) T 4...(ii) Compring Eqs. (i) nd (ii), we ge E.33R.5 4 (ii) E E J/mol Subsiuing he vlue of T (67 K) in Eq. (ii), (s ) s.693 (iii) / sec sec T 4 T K
21 E x. 3 6 The re consn of forwrd recion recion increses by 6% when he emperure of he recion is incresed from 3 o 3 K, wheres equilibrium consn increses by %. Clcule he civion energy for he forwrd s well s bcwrd recion. E (f ) T T According o Arrhenius equion,.33r T T If 3 K hen 3 K, E x ( f ) E ( f ) E (.6) J/mol J/mol H T T According o vn' Hoff equion,.33r T T If 3, +.. H H (.) J/mol 4869 J mol 4.87 J/mol Thus, recion is endohermic. For such recion, H E (f) E (b) E (b) E (f) H J/mol J mol Vlue of re consn for firs order recion 5 K is.6 5 second, wheres 6 K, i is second. Find ou he civion energy of he recion. E x. 3 8 E.33 R T T E.49 5 E E E An exohermic recion, X Y, hs n civion energy 3 J mol. If energy chnge (E) during he recion is J, hen he civion energy for he reverse recion is [A] 3J [B] J [C] 5 J [D] J Ans. [C] E E E (f) ; 3 E (b) ; E (b) 5 J (b) E x. 3 9 An endohermic recion, A B, hs n civion energy s x J mol of A. If energy chnge of he recion is y J, he civion energy of reverse recion is [A] x [B] x y [C] x + y [D] y x Ans. [B] E E (f) E ; y x E ; (b) (b) E x. 4 Which of he following relions is correc? E (b) x y [A] A e E/RT [B] ln ln A E RT [C] ln A ln E RT E [D] ln A ln RT Ans. [C] Ae E/RT E ln ln A RT E or ln A ln RT
22 E x. 4 Which of he following expression give he effec of emperure on he re consn? S o l. [A] ln A RT ln E ln [B] ln ln A E /RT [C] AE /RT [D] None of hese Ans. [B] The effec of emperure on re consn is quniively given by Arrhenius equion Ae E/RT or ln ln A E /RT E x. 4 The plo of vs T helps o clcule [A] Energy of civion [C] Order of he recion [B] Re consn of he recion [D] Energy of civions s well s he frequency fcor Ans. [D] Sol. According o Arrhenius equion : A E.33. T Plo of vs. T is srigh line E x. 4 3 Slope E.33 R Inercep A The progress of he recion given below, consider he recion given below, /T CH 3 COOC H 5 + H O H CH 3 COOH + C H 5 OH he recion cn be followed by mesuring he concenrion of cid (HCl cid used s clys plus ceic cid formed during he recion) by mens of lli irion. Clcule he volume of lli (NOH) needed for he end poin h will increse wih ime. CH 3 COOC H 5 + H O H CH 3 COOH + C H 5 OH A excess A x x x A If V, V nd V re he volumes of NOH soluion needed for he end poin of irion of he recion mixure zero ime, ime nd infiniy, i.e. fer compleion of he recion he condiion being chieved by heing he recion mixure for some ime, hen V [cid clys] V [cid clys] + x V [cid clys] + V V x V V (since concenrion of HCl cid cing s clys will remin consn). The bove recion which is of firs order ( cully pseudo unimoleculr) will, herefore, obey following equion..33 V V V V
23 E x. 4 4 E x. 4 5 H O (q.) H O + O A A x x x Since H O cs s reducing gen owrds KMnO 4, so concenrions of H O vrious ime inervls my be deermined by he irion of he recion mixure gins sndrd KMnO 4 soluion. The ire vlue will go on decresing wih ime. If V nd V be he ire vlues zero ime nd ny ime hen V nd V x The bove recion being firs order, is re consn my be expressed s.33 V V The recion menioned below is firs order w.r.. sucrose nd zero order w.r.. wer, since wer is in lrge exce ss s compred o sucrose. Th is, i is n exmple of pseudo unimoleculr rec ion. Sucrose, glucose nd frucose ll re opiclly cive subsnces. Therefore, he progress of he recion cn be followed by mesuring ngle of roions of he recion mixure vrious ime inervls. During he recion, ngle of roion goes on decresing nd fer someime here is reversl of he direcion of roion, i.e. from dexro o levo nd Hence, he recion is clled "inversion of cne sugr" or inversion of sucrose. H C H O + H O C 6 H O 6 + C 6 H O 6 d-sucrose d-glucose -Frucose Iniilly Excess Afer ime x Consn x x A infiniy Consn Angle of opicl roion is mesured by mens of n insrumen clled polrimeer. Opicl roion is mhemiclly expressed s, where R obs.c. [] D lengh of he polrimeer ube C concenrion of es soluion [] D specific roion For given smple nd polrimeer, nd [] D re consn. R obs C, or R obs C, If r, r nd r be he observed ngle of roions of he smple zero ime, ime nd infiniy respecively, nd, nd 3 be proporione in erms of sucrose, glucose nd frucose, respecively. Then, r r ( x) + x + 3 x r + 3 From hese equions i cn be shown h r r x r r So, he expression for he re consn of his recion in erms of he opicl roionl d my be pu s.33 r r r r
24 E x. 4 6 N O 5 4NO + O A P A P x 4x x A P ½ P The progress of he recion cn be followed by mesuring he pressure of he gseous mixure in closed vessel, i.e. consn volume. The expression for he re consn in erms of pressure d will be s given below. E x P P, where P P x If ol pressure fer ny ime nd is given, hen i is possible o find P nd x nd hence, my be clculed. Consider firs order recion, A B + C Assume h A, B nd C re gses. The given d is Time T Pril pressure of A P P And we hve o find he re consn of he recion. Since A is gs ssuming i o be idel, we cn se h P A [A] RT [From PV nrt] A, P [A] RT nd, P [A] RT. Thus, he rio of he concenrion of A wo differen ime inervls is equl o he rio of is pril pressure hose sme ime inervls. [A] [A] P P ln P P E x. 4 8 A B + C Time Tol pressure of A + B + C P P Find. In his cse, we re given ol pressure of he sysem hese ime inervls. The ol pressure obviously includes he pressure of A, B nd C. A, he sysem would only hve A. Therefore, he ol pressure would be he iniil pressure of A. P is he iniil pressure of A. A ime, le us ssume moles of A will decompose o give B nd C becuse of which is pressure is reduced by n moun x while h of B nd C is incresed by x ech. Th is : A B + C Iniil P A ime P x x x Tol pressure ime P + x P x P P Now he pressure of A ime would be P x P (P P ) P P ln [A] P ln [A] (P P )
25 E x. 4 9 Sol. For he given following firs order recion, A B + C Time T Tol pressure of A + B + C P P 3 Clcule. Here mens h he recion is complee. Now, we hve A B + C A P A (P x) x x A P P P P 3 P P 3 A ime, P + x P P 3 + x P x P P3 P x P 3 (P P 3 ) P P 3 ln [A] P 3 / ln [A] (P P ) 3. PARALLEL REACTIONS : P3 ln (P P ) 3 These re recions in which recion subsnces do no follow priculr ph o give priculr se of producs. I follows one or more phs o give differen producs, e.g. B A The recn A follows wo differen phs o form B nd C s shown below : C A C B Conc. Re d[a ] [A] + [A] [A] [As, ( + ) ] % yield of B
26 E x. 5 A follows prllel ph, firs order recion giving B nd C s B 5A C If iniil concenrion of A is.5 M, clcule he concenrion of C fer 5 hours of recion. [Given :.5 5 s, 5 6 s ].33 [A] [A] ( s ) [A] [A].744 M [A] decomposed [A] [A] M Frcion of C formed [A] ( ) decomped M (5 moles of A re used o give moles of C) 3. SEQUENTIAL REACTIONS : These re recions which proceed from recns o produc hrough one or more inermedie sges, e.g. A B C Grphicl represenion d[a ] d[b] [A]...() [A] [B]...() Conc. A B C d[c] [B]...(3) Inegring Eq. (), we ge [A] [A] e Now, we shll inegre Eq. () nd find he concenrion of B reled o ime. d[b] [A] [B] Subsiuing [A] s [A] e d[b] + [B] [A] d[b] + [B] [A] e...(4) Inegrion of he bove equion is no possible s we re no ble o sepre he wo vribles, [B] nd. Therefore, we muliply Eq. (4) by inegring fcor e, on boh he sides of he equion. d[b] [B] e [A] e ( ) We cn see h he lef hnd side of he equion is differenil of [B] e. d ([B]e ) [A] e ( )
27 ( d ([B]e ) ) [A] e Inegring wihin he limis o. ( ) d ([B]e ) [A] e [A] ( ) ( [B]e e ) [B]e ( ) e [A] ( ) [A] [B] e e ( ) ( ) [A] [B] e e...(5) Now, in order o find [C], subsiue Eq. (5) in Eq. (3), we ge d[c] [A] [e e ] [A] d[c] On inegring, we ge e e [A] d[c] [e e ] ( ) [A] e e [C] ( ) e e [C] [A ] ( ) e e [C] [A ] [A] [C] [ ( e )( e )] B mx nd mx : We cn lso emp o find he ime when [B] becomes mximum. For his, we differenie Eq. (5) nd find d[b] nd eque i o zero. d[b] [A] ( ) e ( ) e ( ) e e e, ing of boh he sides mx ln Subsiuing Eq. (6) in Eq. (5)...(6) B mx [A] /
28 4. HYPOTHESIS OF STE A DY STATE : There re mny recions which involve muli seps nd he inermedies (one or more) do no pper in he overll equion, e.g. NO + O NO Seps involved in he bove recion re NO + O NO 3 NO + NO 3 NO NO + O NO In he bove seps, NO 3 is n inermedie species nd do no pper in he overll blnced equion. Usully hese inermedies re very recive nd do no ccumule o ny significn exen during he recion. For hypoheicl recion, A B The recion cn proceeds in he following seps : A I I B The concenrion of he inermedie [I] is much less hn he recn [A] s well s he produc [B]. Accordingly he formion of inermedie will sr zero, rises o mximum, nd hen fll bc o zero. If he concenrion of inermedie remins smll during he recion, hen he curves of recns, inermedie nd produc versus will be given s below : From he plo, i is cler h he slope of he curve for inermedie much less hn hose for recns A nd producs B. I is, herefore A Conc. B good pproximion o e d[i], for ech recion inermedie. T h is is sedy se (s ionr y se) pproxim ion. 5. RADIOACTIVITY : All rdiocive decy follows firs order ineics nd his is where he similriy ends. This will be explined ler in he chper. We hve mesured he re of recion in chemicl ineics bsed on he re of chnge of concenrion of recns or producs. Bu his procedure will no wor for clculing he re of rdiocive recion. This is becuse mos of he ime he rdiocive subsnce is solid. Therefore, is concenrion would be consn wih ime (ssuming i o be pure nd h he produc does no remin wih he recns). Therefore, he re of rdiocive recions is mesured by clculing he re of chnge of number of nuclei of he rdiocive subsnce. For rdiocive decy A B, he re of recion is clculed s dn A N A where decy consn of recion. N A number of nuclei of he rdiocive subsnce he ime when re is clculed. As you cn see, he bove re lw is very much similr o he re lw of firs order chemicl recion, bu ll oher similriies ceses here. For exmple unlie chemicl recion he decy consn () does no depend on emperure. Arrhenius equion is no vlid for rdiocive decy. dn A N A
29 Inegring he differenil re lw, we ge N dn N A N A where N N N number of nuclei of A, N number of nuclei of A, decy consn The expression cn be rerrnged o give N N e...() This sugges h he number of nuclei of rdiocive subsnce A ny insn of ime cn be clculed, if we now he number of nuclei, is decy consn nd he ime. Hlf-Life Jus lie firs order recion, he hlf-life of rdiocive decy is given by /.693 [Noe : Le us sr wih nuclei. If he hlf-life is 5 minues, hen he end of firs 5 minues, number of nuclei would be 5. Now, wh would be he number of nuclei fer nex 5 minues? Will i be.5 or or 3? We cn clerly see h i cnno be.5 nd if i is or 3 hen i cnno be clled s hlf-life. This dilemm cn be overcome by undersnding h ll formul reling o ineics re only vlid when he smple size is very lrge nd in such lrge smple size, smll difference of.5 will be insignificn. The fc h rdiocive decy follows he exponenil lw implies h his phenomenon is sisicl in nure. Every nucleus in smple of rdionuclide hs cerin probbiliy of decying, bu here is no wy o now in dvnce which nuclei will cully decy in priculr ime spn. If he smple is lrge enough, i.e. if mny nuclei re presen - he cul frcion of i h decys in cerin ime spn will be very close o he probbiliy for ny individul nucleus o decy. To sy h, cerin rdioisoope hs hlf-life of 5 hr. signifies h every nucleus of his isoope hs 5 percen chnce of decying in every 5 hr. period. This does no men probbiliy of percen decying is hr. A nucleus does no hve memory, nd is decy probbiliy per uni ime is consn unil i cully does decy. A hlf-life of 5 hr. implies 75 probbiliy of decy in hr., which increses o 87.5% in 5 hr., o 93.75% in hr., nd so on, becuse in every 5 hr. The probbiliy of decy is 5 percen. Averge Life Time Averge life ime is defined s he life ime of single isoled nucleus. Le us imgine, single nucleus which decys in second. Assuming second ime inervl o be very smll, he re of chnge of nuclei dn would be / (becuse dn nd ). We cn lso see h since N, for single isoled dn nucleus N,. Therefore, in his presen cse,. dn Now, le us ssume, he sme nucleus decys in seconds, we cn see h, i.e. is equl o ½. You will lso noice h in he s cse he nucleus survived for second nd in he second cse i survived for seconds. Therefore, he life ime of single isoled nucleus is. v
30 Aciviy : Aciviy is he re of decy of rdiocive elemen. I is represened s 'A' nd is equl o N. By no dn mens should civiy be confused wih re of chnge of rdiocive nuclei represened by. This dn is becuse ls bou he overll chnge in he number of nuclei in given insn of ime while civiy only ls bou h chnge which is decy. For exmple, if you go o mre wih Rs. 5 in your poce nd you spend Rs. in 5 minues hen your re of chnge of money in he wlle is Rs. 4/min nd in fc he re of spending he money is lso Rs. 4/min. Here, you cn see boh re sme. Bu if while spending Rs. in 5 minues, somebody eeps Rs. in your wlle, hen he re of chnge of money in your wlle would become Rs..5 /min while he re of spending he money is Rs. 4/min. This implies h s long s he rdiocive subsnce is only decying he re of chnge of nuclei nd civiy re sme nd Eq.() in erms civiy of rdiocive subsnce cn be wrien s A A e. Bu if he rdiocive subsnce is lso being produced, hen dn re of producion civiy (of course i's differen mer h re of producion my or my no be consn). Specific Aciviy I is defined s per uni mss of he smple. Le, rdiocive smple weighing w g hve decy consn w. The number of nuclei in he w g would be N M, where M moleculr weigh of he rdiocive subsnce nd N Avogdro's number. w N M N Specific civiy w M I should be remembered h if rdiocive smple is pure nd he produc does no remin wih recn, hen specific civiy is consn. Unis of Aciviy The uni of rdiociviy of subsnce is mesured s he re which i chnges ino dugher nucleus. I hs been derived on he scle of disinegrion of rdium. Le us consider, g of rdium (omic mss 6 nd / 6 yrs) undergoes decy, hen Re of decy of rdium Number of nuclei of R in g curie ( Ci 3.7 dps) Ruherford ( Rd 6 dps) The SI uni of civiy is dps or Becquerel dps 3.7 becquerel E x. 5 Po decys wih emission of -pricle o 6 Pb wih hlf-life period of 38.4 dys. If g of Po is plced in seled ube, how much helium will be ccumuled in 69. dys? Express he nswer in cm 3 STP. 6 Po Po + 4 He 84 8 Amoun of Po lef fer 69. dys cn be clculed by pplying 84 N N (/) n n / / N /.7 g
31 Amoun of polonium disinegred g Moles of polonium in.98 g.98 Moles of helium oms formed.98 Volume of helium colleced CARBON DATING : cm 3 The cosmic ry generes neurons in he mosphere which bombrds he nucleus of mospheric nirogen o form rdiocive 4 C hence 4 C in he mosphere hs been remining consn over housnds of yers. In living merils, he rio of 4 C o C remins relively consn. When he issue in n niml or pln dies, ssimilion of rdiocive 4 C cesed o coninue. Therefore, in he ded issue he rio of 4 C o C would decrese depending on he ge of he issue. 4 N + 7 n 4 6 C + p 4 C N + e A smple of ded issue is burn o give crbon dioxide nd he crbon dioxide is nlysed for he rio of 4 C o C. From his d, ge of ded issue (pln or niml) cn be deermined..33 N Age () N 4.33 / ( C) Age.693 N N N rio of 4 C/ C in living pln N rio of 4 C/ C in he wood.33 / Age.693 A Originl civiy A Finl civiy A A Also, N N n where n / 7. ROCK DATING : I is bsed on he ineics of rdiocive decy. I is ssumed h no led ws originlly presen in he smple nd whole of i cme from urnium. Iniil no. of mole (N ) [U] + [Pb] Finl no. of mole (N) [U] N [U] [Pb] N [U].33 N N.33 [Pb] [U] Also, [Pb] [U] + [Pb] [U] () n n /
32 E x. 5 A smple of urnium minerl ws found o conin 6 Pb nd 38 U in he rio of.8 :. Esime he ge of he minerl (hlf-life of 38 U is yers) Pb 38 U.33 / 6 Pb U Rio by mss 6 Pb : 38 U.8 : Rio by moles 6 Pb : 38 U.8 : ( +.9) yers STA BILIT Y OF NUCLEI WITH RESPECT TO NEUTRON - PROTON R ATIO : If number of neurons is ploed gins he number of proons, he sble nuclei lie wihin well-defined region clled zone of sbiliy. All he nuclei flling ouside his zone re invribly rdiocive nd unsble in nure. Nuclei h fll bove he sbiliy zone hs n excess of neurons while hose lying below hve more proons. These nuclei in sbiliy by ming djusmen in n/p rio When (n/p) rio is higher hn h required for sbiliy : Number of proons (p) Such nuclei hve endency o emi -rys (rnsforming neuron ino proon). n p n p : 5 36 n p + e (-pricle) 4 U 4 N + e Kr Rb + e 5 37 Number of neurons (n) Unsble region Zone of sbiliy Unsble region n/p When (n/p) rio is lower hn h required for sbiliy : Such nuclei hve endency o increse n/p rio by doping ny of he following hree wys. By emission of n -pricle (nurl rdiociviy) U He (-pricle) 9 9 n P By emission of posiron n p By K-elecron cpure e n 5 6 P emission is usully observed in nurl rdiocive isoopes while emission of posiron or K-elecron cpure is observed in rificil rdiocive isoopes. The unsble nuclei coninue o emi or -pricle unil sble nucleus comes ino exisence.
33 9. NUCLEAR FISSION : I is nucler recion in which hevy nucleus splis ino ligher nuclei of comprble msses wih relese of lrge moun of energy by bombrdmen wih suible sub-omic pricles, i.e B + 36Kr + 3n n + 35 U Xe + 38Sr + n Cs + 37Rb + n If he neurons from ech nucler fission re bsorbed by oher 9 U 35 nuclei, hese nuclei spli nd relese even more neurons. Thus, chin recion cn occur. A nucler chin recion is self susining series of nucler fissions cused by he previous neurons relesed from he previous nucler recions. 35 n + 9U B + n 9U + n 9U 35 36Kr 93 + n 9U 35 n 35 9U n 35 9U n 35 9U There should be criicl moun of he fissionble meril o minin fission chin. This in urn requires, minimum criicl mss of he fissionble meril. I is he smll mss of he fissionble meril in which chin recion cn be susined. If mss is lrger hn criicl mss (supercriicl mss), hen he number of nuclei h spli, muliplies rpidly. An omic bomb is deoned wih smll moun of chemicl explosive h push ogeher wo or more msses of fissionble meril o ge supercriicl mss. A nucler fission recor is device h permis conrolled chin nucler fissions. Conrol rods mde of elemens such s boron nd cdmium, bsorb ddiionl neurons nd cn herefore, slow he chin recions. 3. NUCLEAR FUSION : I is nucler recion in which wo ligher nuclei re fused ogeher o form hevier nuclei. To chieve his, colliding nuclei mus posses enough ineic energy o overcome he iniil force of repulsion beween he posiively chrged core. A very high emperure of he order of 6 o 7 K, he nuclei my hve he sufficien energy o overcome he repulsive forces nd fuse. Such recions re herefore lso nown s hermonucler recions. H H He n 7.8MeV 3 4 H H He 4.9 MeV 4 H H He. MeV 3 4 Li H He 7.7 MeV The energy of fusion process is due o mss defec (convered ino binding energy). The high emperure required o iniie such recion my be ined iniilly hrough fission process. Hydrogen bomb is bsed on he principle of fusion recions. Energy relesed is so enormous h i is bou imes h of omic bomb. In hydrogen bomb, mixure of deuerium oxide (D O) nd riium oxide (T O) is enclosed in spce surrounding n ordinry omic bomb. The emperure produced by he explosion of he omic bomb iniies he fusion recion beween H nd 3 H relesing huge moun of energy. I is believed h he high emperure of srs including he sun is due o fusion recions. E. Sl Peer in 953, proposed proon-proon chin recion. H H H e H H He 3 He H He e H He e 4.7MeV 4
34 MEMORY TIPS. Expression for re consns for recion of differen orders. Type of recion Inegred re Uni of re Hlf-life 3/4 life equion consn period p e r i o d Zero order recion d[a] [A] Concenrion -- Differeniion form dx.33 Firs order recion ( x) ime.693 ime K b( x) Second order ( b) (b x) recion Differenil form dx ( x) Mole lire ime x ( x) Third order recion 3 ( x) 3 Lire mole ime Differenil form dx ( x). Some ypicl liner plos for recions of differen orders : 3 ( i ) r Zero order ( ) r Firs order ( ) r Second order ( ) r Third order 3 ( ) ( i i ) ( x) Zero order ( x) Firs order ( x) Second order ( x) Third order (iii) / Zero order ( ) / Firs order ( ) / Second order ( ) / Third order 3. Amoun lef fer n hlf-lives [A ] n Tol ime No. of hlf-lives
35 4. Exponenil form of expression for re consn for recion of s order : [A] [A] e or C C e 5. [A] n 6. Arrhenius equion for effec of emperure on re consn, Ae E /RT on R Also, d ln dt E RT E.33RT If nd re re consns emperure T nd T, hen E.33R T T T T 7. Exmples of recions of s order nd heir formul for re consns (i) N O 5 4NO + O ;.33 V V V where V volume of O gs colleced infinie ime V volume of O gs colleced ime (ii) NH 4 NO H O + N ;.33 V V V where V nd V re volumes of N gs colleced fer infiniy ime nd fer ime respecively. (iii) H O H O + O ;.33 V V D where V D nd V re he volumes of KMnO 4 soluion used for iring definie volume of he recion mixure D nd ime respecively. (iv) CH 3 COOC H 5 + H O CH 3 COOH + C H 5 OH.33 V V V V D where V D, V nd V re he volume of NOH soluion used for irion mixure zero ime, fer ime nd fer infiniy respecively. (v) C H O + H O H C 6 H O 6 + C 6 H O 6 Glucose Frucose.33 (r r ) (r r ) where r, r nd r re he polrimeric reding zero ime, fer ime nd fer infiniy respecively.
36 8. Re lw equion for recions involving prllel recion B (9%) A C (%) Re d[a ] [] [A] + [A] [ + ] [A] 9. Degree of dissociion ny ime ( e )
37 E x. SOLVED OBJECTIVE PROBLEMS Find he civion energy [J/mol] for he recion, A(g) + B(g) C(g) + D(g). From he plo given below : C+D A + B Recion course [A] [B] 6 [C] 4 [D] 8 E 4 6 J mol Hence he nswer is [B]. E x. In n endohermic recion, H represens he enhlpy of recion in J/mol, he minimum vlue for he energy of civion will be. (A) less hn H (B) zero (C) more hn H (D) equl o H H E > H E x. 3 Progress of recion Hence, (C) is he correc nswer. Consider he following firs order compeing recions A B, C D, he rio of, if only 5% of A hve been reced wheres 5% of C hs been reced, clcule he rio of (A).45 (B).46 (C).6 (D) for 5% (A) reced.33 5 for 5% (C) reced.49.3 Since Hence, (A) is he correc nswer.
38 E x. 4 E x. 5 For recion A B d [B] C. If he recions re of s order hen is equl o [A] [B] [B] + [A] [C] [A] [B] [D] [A] + [B] Re of increse in [B] [A] Similrly re of decrese in [B] [B] Thus, d [B] [A] [B] Hence he nswer is [C] The hlf life period / is independen of iniil concenrion of recn when he order of recion is [A] Negive [B] [C] [D] Frcionl ½ of recion of n order n is reled o iniil concenrion by he expression ½ n C (Here, n order of recion) E x. 6 E x. 7 E x. 8 for n, ½ is independen of concenrion erm. Hence he nswer is [C]. For firs order recion, A B, he re of recion [A]. M is. mol L min. The hlf-life period for he recion is (A) 4 sec (B) sec (C) sec (D) 8 sec r [A] r [A] min sec Hence, (A) is he correc nswer. A clys lowers he civion energy of recion from 3 J mol o 5 J mol. The emperure which he unclysed recion will hve he sme re s h of he clysed 7 C is (A) 3 C (B) 37 C (C) 37 C (D) +3 C E ' T E T 5 3 T T 6 K 37 C Hence, (B) is he correc nswer. SO Cl SO + Cl, is he firs order gs recion wih. 5 sec 7 C. The percenge of SO Cl decomposed on heing for 5 minues is (A).8 (B).8 (C) 8. (D) ( x) ( x).33 Hence, x.33 ( x).68 x.936 x.936 x % Hence, (D) is he correc nswer.
The order of reaction is defined as the number of atoms or molecules whose concentration change during the chemical reaction.
 www.hechemisryguru.com Re Lw Expression Order of Recion The order of recion is defined s he number of oms or molecules whose concenrion chnge during he chemicl recion. Or The ol number of molecules or
www.hechemisryguru.com Re Lw Expression Order of Recion The order of recion is defined s he number of oms or molecules whose concenrion chnge during he chemicl recion. Or The ol number of molecules or
For the reaction, R P, the is given by,
 Dr JADU SAMUEL CHEMICAL KINETICS Inroducion Chemicl ineics is brnch of physicl chemisry, which dels wih he sudy of he re of chemicl recions nd he vrious fcors ffecing i Such sudies lso enble us o elucide
Dr JADU SAMUEL CHEMICAL KINETICS Inroducion Chemicl ineics is brnch of physicl chemisry, which dels wih he sudy of he re of chemicl recions nd he vrious fcors ffecing i Such sudies lso enble us o elucide
e t dt e t dt = lim e t dt T (1 e T ) = 1
 Improper Inegrls There re wo ypes of improper inegrls - hose wih infinie limis of inegrion, nd hose wih inegrnds h pproch some poin wihin he limis of inegrion. Firs we will consider inegrls wih infinie
Improper Inegrls There re wo ypes of improper inegrls - hose wih infinie limis of inegrion, nd hose wih inegrnds h pproch some poin wihin he limis of inegrion. Firs we will consider inegrls wih infinie
0 for t < 0 1 for t > 0
 8.0 Sep nd del funcions Auhor: Jeremy Orloff The uni Sep Funcion We define he uni sep funcion by u() = 0 for < 0 for > 0 I is clled he uni sep funcion becuse i kes uni sep = 0. I is someimes clled he Heviside
8.0 Sep nd del funcions Auhor: Jeremy Orloff The uni Sep Funcion We define he uni sep funcion by u() = 0 for < 0 for > 0 I is clled he uni sep funcion becuse i kes uni sep = 0. I is someimes clled he Heviside
Motion. Part 2: Constant Acceleration. Acceleration. October Lab Physics. Ms. Levine 1. Acceleration. Acceleration. Units for Acceleration.
 Moion Accelerion Pr : Consn Accelerion Accelerion Accelerion Accelerion is he re of chnge of velociy. = v - vo = Δv Δ ccelerion = = v - vo chnge of velociy elpsed ime Accelerion is vecor, lhough in one-dimensionl
Moion Accelerion Pr : Consn Accelerion Accelerion Accelerion Accelerion is he re of chnge of velociy. = v - vo = Δv Δ ccelerion = = v - vo chnge of velociy elpsed ime Accelerion is vecor, lhough in one-dimensionl
4.8 Improper Integrals
 4.8 Improper Inegrls Well you ve mde i hrough ll he inegrion echniques. Congrs! Unforunely for us, we sill need o cover one more inegrl. They re clled Improper Inegrls. A his poin, we ve only del wih inegrls
4.8 Improper Inegrls Well you ve mde i hrough ll he inegrion echniques. Congrs! Unforunely for us, we sill need o cover one more inegrl. They re clled Improper Inegrls. A his poin, we ve only del wih inegrls
5.1-The Initial-Value Problems For Ordinary Differential Equations
 5.-The Iniil-Vlue Problems For Ordinry Differenil Equions Consider solving iniil-vlue problems for ordinry differenil equions: (*) y f, y, b, y. If we know he generl soluion y of he ordinry differenil
5.-The Iniil-Vlue Problems For Ordinry Differenil Equions Consider solving iniil-vlue problems for ordinry differenil equions: (*) y f, y, b, y. If we know he generl soluion y of he ordinry differenil
MATH 124 AND 125 FINAL EXAM REVIEW PACKET (Revised spring 2008)
 MATH 14 AND 15 FINAL EXAM REVIEW PACKET (Revised spring 8) The following quesions cn be used s review for Mh 14/ 15 These quesions re no cul smples of quesions h will pper on he finl em, bu hey will provide
MATH 14 AND 15 FINAL EXAM REVIEW PACKET (Revised spring 8) The following quesions cn be used s review for Mh 14/ 15 These quesions re no cul smples of quesions h will pper on he finl em, bu hey will provide
Physics 2A HW #3 Solutions
 Chper 3 Focus on Conceps: 3, 4, 6, 9 Problems: 9, 9, 3, 41, 66, 7, 75, 77 Phsics A HW #3 Soluions Focus On Conceps 3-3 (c) The ccelerion due o grvi is he sme for boh blls, despie he fc h he hve differen
Chper 3 Focus on Conceps: 3, 4, 6, 9 Problems: 9, 9, 3, 41, 66, 7, 75, 77 Phsics A HW #3 Soluions Focus On Conceps 3-3 (c) The ccelerion due o grvi is he sme for boh blls, despie he fc h he hve differen
f t f a f x dx By Lin McMullin f x dx= f b f a. 2
 Accumulion: Thoughs On () By Lin McMullin f f f d = + The gols of he AP* Clculus progrm include he semen, Sudens should undersnd he definie inegrl s he ne ccumulion of chnge. 1 The Topicl Ouline includes
Accumulion: Thoughs On () By Lin McMullin f f f d = + The gols of he AP* Clculus progrm include he semen, Sudens should undersnd he definie inegrl s he ne ccumulion of chnge. 1 The Topicl Ouline includes
Average & instantaneous velocity and acceleration Motion with constant acceleration
 Physics 7: Lecure Reminders Discussion nd Lb secions sr meeing ne week Fill ou Pink dd/drop form if you need o swich o differen secion h is FULL. Do i TODAY. Homework Ch. : 5, 7,, 3,, nd 6 Ch.: 6,, 3 Submission
Physics 7: Lecure Reminders Discussion nd Lb secions sr meeing ne week Fill ou Pink dd/drop form if you need o swich o differen secion h is FULL. Do i TODAY. Homework Ch. : 5, 7,, 3,, nd 6 Ch.: 6,, 3 Submission
September 20 Homework Solutions
 College of Engineering nd Compuer Science Mechnicl Engineering Deprmen Mechnicl Engineering A Seminr in Engineering Anlysis Fll 7 Number 66 Insrucor: Lrry Creo Sepember Homework Soluions Find he specrum
College of Engineering nd Compuer Science Mechnicl Engineering Deprmen Mechnicl Engineering A Seminr in Engineering Anlysis Fll 7 Number 66 Insrucor: Lrry Creo Sepember Homework Soluions Find he specrum
(b) 10 yr. (b) 13 m. 1.6 m s, m s m s (c) 13.1 s. 32. (a) 20.0 s (b) No, the minimum distance to stop = 1.00 km. 1.
 Answers o Een Numbered Problems Chper. () 7 m s, 6 m s (b) 8 5 yr 4.. m ih 6. () 5. m s (b).5 m s (c).5 m s (d) 3.33 m s (e) 8. ().3 min (b) 64 mi..3 h. ().3 s (b) 3 m 4..8 mi wes of he flgpole 6. (b)
Answers o Een Numbered Problems Chper. () 7 m s, 6 m s (b) 8 5 yr 4.. m ih 6. () 5. m s (b).5 m s (c).5 m s (d) 3.33 m s (e) 8. ().3 min (b) 64 mi..3 h. ().3 s (b) 3 m 4..8 mi wes of he flgpole 6. (b)
1.0 Electrical Systems
 . Elecricl Sysems The ypes of dynmicl sysems we will e sudying cn e modeled in erms of lgeric equions, differenil equions, or inegrl equions. We will egin y looking fmilir mhemicl models of idel resisors,
. Elecricl Sysems The ypes of dynmicl sysems we will e sudying cn e modeled in erms of lgeric equions, differenil equions, or inegrl equions. We will egin y looking fmilir mhemicl models of idel resisors,
Magnetostatics Bar Magnet. Magnetostatics Oersted s Experiment
 Mgneosics Br Mgne As fr bck s 4500 yers go, he Chinese discovered h cerin ypes of iron ore could rc ech oher nd cerin mels. Iron filings "mp" of br mgne s field Crefully suspended slivers of his mel were
Mgneosics Br Mgne As fr bck s 4500 yers go, he Chinese discovered h cerin ypes of iron ore could rc ech oher nd cerin mels. Iron filings "mp" of br mgne s field Crefully suspended slivers of his mel were
Contraction Mapping Principle Approach to Differential Equations
 epl Journl of Science echnology 0 (009) 49-53 Conrcion pping Principle pproch o Differenil Equions Bishnu P. Dhungn Deprmen of hemics, hendr Rn Cmpus ribhuvn Universiy, Khmu epl bsrc Using n eension of
epl Journl of Science echnology 0 (009) 49-53 Conrcion pping Principle pproch o Differenil Equions Bishnu P. Dhungn Deprmen of hemics, hendr Rn Cmpus ribhuvn Universiy, Khmu epl bsrc Using n eension of
PHYSICS 1210 Exam 1 University of Wyoming 14 February points
 PHYSICS 1210 Em 1 Uniersiy of Wyoming 14 Februry 2013 150 poins This es is open-noe nd closed-book. Clculors re permied bu compuers re no. No collborion, consulion, or communicion wih oher people (oher
PHYSICS 1210 Em 1 Uniersiy of Wyoming 14 Februry 2013 150 poins This es is open-noe nd closed-book. Clculors re permied bu compuers re no. No collborion, consulion, or communicion wih oher people (oher
Motion in a Straight Line
 Moion in Srigh Line. Preei reched he mero sion nd found h he esclor ws no working. She wlked up he sionry esclor in ime. On oher dys, if she remins sionry on he moing esclor, hen he esclor kes her up in
Moion in Srigh Line. Preei reched he mero sion nd found h he esclor ws no working. She wlked up he sionry esclor in ime. On oher dys, if she remins sionry on he moing esclor, hen he esclor kes her up in
A Kalman filtering simulation
 A Klmn filering simulion The performnce of Klmn filering hs been esed on he bsis of wo differen dynmicl models, ssuming eiher moion wih consn elociy or wih consn ccelerion. The former is epeced o beer
A Klmn filering simulion The performnce of Klmn filering hs been esed on he bsis of wo differen dynmicl models, ssuming eiher moion wih consn elociy or wih consn ccelerion. The former is epeced o beer
S Radio transmission and network access Exercise 1-2
 S-7.330 Rdio rnsmission nd nework ccess Exercise 1 - P1 In four-symbol digil sysem wih eqully probble symbols he pulses in he figure re used in rnsmission over AWGN-chnnel. s () s () s () s () 1 3 4 )
S-7.330 Rdio rnsmission nd nework ccess Exercise 1 - P1 In four-symbol digil sysem wih eqully probble symbols he pulses in he figure re used in rnsmission over AWGN-chnnel. s () s () s () s () 1 3 4 )
K The slowest step in a mechanism has this
 CM 6 Generl Chemisry II Nme SLUTINS Exm, Spring 009 Dr. Seel. (0 pins) Selec he nswer frm he clumn n he righ h bes mches ech descripin frm he clumn n he lef. Ech nswer cn be used, ms, nly nce. E G This
CM 6 Generl Chemisry II Nme SLUTINS Exm, Spring 009 Dr. Seel. (0 pins) Selec he nswer frm he clumn n he righ h bes mches ech descripin frm he clumn n he lef. Ech nswer cn be used, ms, nly nce. E G This
ENGR 1990 Engineering Mathematics The Integral of a Function as a Function
 ENGR 1990 Engineering Mhemics The Inegrl of Funcion s Funcion Previously, we lerned how o esime he inegrl of funcion f( ) over some inervl y dding he res of finie se of rpezoids h represen he re under
ENGR 1990 Engineering Mhemics The Inegrl of Funcion s Funcion Previously, we lerned how o esime he inegrl of funcion f( ) over some inervl y dding he res of finie se of rpezoids h represen he re under
CHEMICAL KINETICS: 1. Rate Order Rate law Rate constant Half-life Temperature Dependence
 CHEMICL KINETICS: Rae Order Rae law Rae consan Half-life Temperaure Dependence Chemical Reacions Kineics Chemical ineics is he sudy of ime dependence of he change in he concenraion of reacans and producs.
CHEMICL KINETICS: Rae Order Rae law Rae consan Half-life Temperaure Dependence Chemical Reacions Kineics Chemical ineics is he sudy of ime dependence of he change in he concenraion of reacans and producs.
The solution is often represented as a vector: 2xI + 4X2 + 2X3 + 4X4 + 2X5 = 4 2xI + 4X2 + 3X3 + 3X4 + 3X5 = 4. 3xI + 6X2 + 6X3 + 3X4 + 6X5 = 6.
 [~ o o :- o o ill] i 1. Mrices, Vecors, nd Guss-Jordn Eliminion 1 x y = = - z= The soluion is ofen represened s vecor: n his exmple, he process of eliminion works very smoohly. We cn elimine ll enries
[~ o o :- o o ill] i 1. Mrices, Vecors, nd Guss-Jordn Eliminion 1 x y = = - z= The soluion is ofen represened s vecor: n his exmple, he process of eliminion works very smoohly. We cn elimine ll enries
A 1.3 m 2.5 m 2.8 m. x = m m = 8400 m. y = 4900 m 3200 m = 1700 m
 PHYS : Soluions o Chper 3 Home Work. SSM REASONING The displcemen is ecor drwn from he iniil posiion o he finl posiion. The mgniude of he displcemen is he shores disnce beween he posiions. Noe h i is onl
PHYS : Soluions o Chper 3 Home Work. SSM REASONING The displcemen is ecor drwn from he iniil posiion o he finl posiion. The mgniude of he displcemen is he shores disnce beween he posiions. Noe h i is onl
Solutions to Problems from Chapter 2
 Soluions o Problems rom Chper Problem. The signls u() :5sgn(), u () :5sgn(), nd u h () :5sgn() re ploed respecively in Figures.,b,c. Noe h u h () :5sgn() :5; 8 including, bu u () :5sgn() is undeined..5
Soluions o Problems rom Chper Problem. The signls u() :5sgn(), u () :5sgn(), nd u h () :5sgn() re ploed respecively in Figures.,b,c. Noe h u h () :5sgn() :5; 8 including, bu u () :5sgn() is undeined..5
Chapter Direct Method of Interpolation
 Chper 5. Direc Mehod of Inerpolion Afer reding his chper, you should be ble o:. pply he direc mehod of inerpolion,. sole problems using he direc mehod of inerpolion, nd. use he direc mehod inerpolns o
Chper 5. Direc Mehod of Inerpolion Afer reding his chper, you should be ble o:. pply he direc mehod of inerpolion,. sole problems using he direc mehod of inerpolion, nd. use he direc mehod inerpolns o
Version 001 test-1 swinney (57010) 1. is constant at m/s.
 Version 001 es-1 swinne (57010) 1 This prin-ou should hve 20 quesions. Muliple-choice quesions m coninue on he nex column or pge find ll choices before nswering. CubeUniVec1x76 001 10.0 poins Acubeis1.4fee
Version 001 es-1 swinne (57010) 1 This prin-ou should hve 20 quesions. Muliple-choice quesions m coninue on he nex column or pge find ll choices before nswering. CubeUniVec1x76 001 10.0 poins Acubeis1.4fee
Minimum Squared Error
 Minimum Squred Error LDF: Minimum Squred-Error Procedures Ide: conver o esier nd eer undersood prolem Percepron y i > for ll smples y i solve sysem of liner inequliies MSE procedure y i = i for ll smples
Minimum Squred Error LDF: Minimum Squred-Error Procedures Ide: conver o esier nd eer undersood prolem Percepron y i > for ll smples y i solve sysem of liner inequliies MSE procedure y i = i for ll smples
Neural assembly binding in linguistic representation
 Neurl ssembly binding in linguisic represenion Frnk vn der Velde & Mrc de Kmps Cogniive Psychology Uni, Universiy of Leiden, Wssenrseweg 52, 2333 AK Leiden, The Neherlnds, vdvelde@fsw.leidenuniv.nl Absrc.
Neurl ssembly binding in linguisic represenion Frnk vn der Velde & Mrc de Kmps Cogniive Psychology Uni, Universiy of Leiden, Wssenrseweg 52, 2333 AK Leiden, The Neherlnds, vdvelde@fsw.leidenuniv.nl Absrc.
Minimum Squared Error
 Minimum Squred Error LDF: Minimum Squred-Error Procedures Ide: conver o esier nd eer undersood prolem Percepron y i > 0 for ll smples y i solve sysem of liner inequliies MSE procedure y i i for ll smples
Minimum Squred Error LDF: Minimum Squred-Error Procedures Ide: conver o esier nd eer undersood prolem Percepron y i > 0 for ll smples y i solve sysem of liner inequliies MSE procedure y i i for ll smples
CHEMICAL KINETICS
 CHEMICAL KINETICS Long Answer Questions: 1. Explin the following terms with suitble exmples ) Averge rte of Rection b) Slow nd Fst Rections c) Order of Rection d) Moleculrity of Rection e) Activtion Energy
CHEMICAL KINETICS Long Answer Questions: 1. Explin the following terms with suitble exmples ) Averge rte of Rection b) Slow nd Fst Rections c) Order of Rection d) Moleculrity of Rection e) Activtion Energy
RESPONSE UNDER A GENERAL PERIODIC FORCE. When the external force F(t) is periodic with periodτ = 2π
 RESPONSE UNDER A GENERAL PERIODIC FORCE When he exernl force F() is periodic wih periodτ / ω,i cn be expnded in Fourier series F( ) o α ω α b ω () where τ F( ) ω d, τ,,,... () nd b τ F( ) ω d, τ,,... (3)
RESPONSE UNDER A GENERAL PERIODIC FORCE When he exernl force F() is periodic wih periodτ / ω,i cn be expnded in Fourier series F( ) o α ω α b ω () where τ F( ) ω d, τ,,,... () nd b τ F( ) ω d, τ,,... (3)
Chapter 15: Phenomena. Chapter 15 Chemical Kinetics. Reaction Rates. Reaction Rates R P. Reaction Rates. Rate Laws
 Chaper 5: Phenomena Phenomena: The reacion (aq) + B(aq) C(aq) was sudied a wo differen emperaures (98 K and 35 K). For each emperaure he reacion was sared by puing differen concenraions of he 3 species
Chaper 5: Phenomena Phenomena: The reacion (aq) + B(aq) C(aq) was sudied a wo differen emperaures (98 K and 35 K). For each emperaure he reacion was sared by puing differen concenraions of he 3 species
Mathematics 805 Final Examination Answers
 . 5 poins Se he Weiersrss M-es. Mhemics 85 Finl Eminion Answers Answer: Suppose h A R, nd f n : A R. Suppose furher h f n M n for ll A, nd h Mn converges. Then f n converges uniformly on A.. 5 poins Se
. 5 poins Se he Weiersrss M-es. Mhemics 85 Finl Eminion Answers Answer: Suppose h A R, nd f n : A R. Suppose furher h f n M n for ll A, nd h Mn converges. Then f n converges uniformly on A.. 5 poins Se
INTEGRALS. Exercise 1. Let f : [a, b] R be bounded, and let P and Q be partitions of [a, b]. Prove that if P Q then U(P ) U(Q) and L(P ) L(Q).
![INTEGRALS. Exercise 1. Let f : [a, b] R be bounded, and let P and Q be partitions of [a, b]. Prove that if P Q then U(P ) U(Q) and L(P ) L(Q). INTEGRALS. Exercise 1. Let f : [a, b] R be bounded, and let P and Q be partitions of [a, b]. Prove that if P Q then U(P ) U(Q) and L(P ) L(Q).](/thumbs/78/77677920.jpg) INTEGRALS JOHN QUIGG Eercise. Le f : [, b] R be bounded, nd le P nd Q be priions of [, b]. Prove h if P Q hen U(P ) U(Q) nd L(P ) L(Q). Soluion: Le P = {,..., n }. Since Q is obined from P by dding finiely
INTEGRALS JOHN QUIGG Eercise. Le f : [, b] R be bounded, nd le P nd Q be priions of [, b]. Prove h if P Q hen U(P ) U(Q) nd L(P ) L(Q). Soluion: Le P = {,..., n }. Since Q is obined from P by dding finiely
( ) ( ) ( ) ( ) ( ) ( y )
 8. Lengh of Plne Curve The mos fmous heorem in ll of mhemics is he Pyhgoren Theorem. I s formulion s he disnce formul is used o find he lenghs of line segmens in he coordine plne. In his secion you ll
8. Lengh of Plne Curve The mos fmous heorem in ll of mhemics is he Pyhgoren Theorem. I s formulion s he disnce formul is used o find he lenghs of line segmens in he coordine plne. In his secion you ll
Properties of Logarithms. Solving Exponential and Logarithmic Equations. Properties of Logarithms. Properties of Logarithms. ( x)
 Properies of Logrihms Solving Eponenil nd Logrihmic Equions Properies of Logrihms Produc Rule ( ) log mn = log m + log n ( ) log = log + log Properies of Logrihms Quoien Rule log m = logm logn n log7 =
Properies of Logrihms Solving Eponenil nd Logrihmic Equions Properies of Logrihms Produc Rule ( ) log mn = log m + log n ( ) log = log + log Properies of Logrihms Quoien Rule log m = logm logn n log7 =
1. Consider a PSA initially at rest in the beginning of the left-hand end of a long ISS corridor. Assume xo = 0 on the left end of the ISS corridor.
 In Eercise 1, use sndrd recngulr Cresin coordine sysem. Le ime be represened long he horizonl is. Assume ll ccelerions nd decelerions re consn. 1. Consider PSA iniilly res in he beginning of he lef-hnd
In Eercise 1, use sndrd recngulr Cresin coordine sysem. Le ime be represened long he horizonl is. Assume ll ccelerions nd decelerions re consn. 1. Consider PSA iniilly res in he beginning of he lef-hnd
Chapter 2. Motion along a straight line. 9/9/2015 Physics 218
 Chper Moion long srigh line 9/9/05 Physics 8 Gols for Chper How o describe srigh line moion in erms of displcemen nd erge elociy. The mening of insnneous elociy nd speed. Aerge elociy/insnneous elociy
Chper Moion long srigh line 9/9/05 Physics 8 Gols for Chper How o describe srigh line moion in erms of displcemen nd erge elociy. The mening of insnneous elociy nd speed. Aerge elociy/insnneous elociy
2D Motion WS. A horizontally launched projectile s initial vertical velocity is zero. Solve the following problems with this information.
 Nme D Moion WS The equions of moion h rele o projeciles were discussed in he Projecile Moion Anlsis Acii. ou found h projecile moes wih consn eloci in he horizonl direcion nd consn ccelerion in he ericl
Nme D Moion WS The equions of moion h rele o projeciles were discussed in he Projecile Moion Anlsis Acii. ou found h projecile moes wih consn eloci in he horizonl direcion nd consn ccelerion in he ericl
EXERCISE - 01 CHECK YOUR GRASP
 UNIT # 09 PARABOLA, ELLIPSE & HYPERBOLA PARABOLA EXERCISE - 0 CHECK YOUR GRASP. Hin : Disnce beween direcri nd focus is 5. Given (, be one end of focl chord hen oher end be, lengh of focl chord 6. Focus
UNIT # 09 PARABOLA, ELLIPSE & HYPERBOLA PARABOLA EXERCISE - 0 CHECK YOUR GRASP. Hin : Disnce beween direcri nd focus is 5. Given (, be one end of focl chord hen oher end be, lengh of focl chord 6. Focus
MTH 146 Class 11 Notes
 8.- Are of Surfce of Revoluion MTH 6 Clss Noes Suppose we wish o revolve curve C round n is nd find he surfce re of he resuling solid. Suppose f( ) is nonnegive funcion wih coninuous firs derivive on he
8.- Are of Surfce of Revoluion MTH 6 Clss Noes Suppose we wish o revolve curve C round n is nd find he surfce re of he resuling solid. Suppose f( ) is nonnegive funcion wih coninuous firs derivive on he
REAL ANALYSIS I HOMEWORK 3. Chapter 1
 REAL ANALYSIS I HOMEWORK 3 CİHAN BAHRAN The quesions re from Sein nd Shkrchi s e. Chper 1 18. Prove he following sserion: Every mesurble funcion is he limi.e. of sequence of coninuous funcions. We firs
REAL ANALYSIS I HOMEWORK 3 CİHAN BAHRAN The quesions re from Sein nd Shkrchi s e. Chper 1 18. Prove he following sserion: Every mesurble funcion is he limi.e. of sequence of coninuous funcions. We firs
4. CHEMICAL KINETICS
 4. CHEMICAL KINETICS Synopsis: The study of rtes of chemicl rections mechnisms nd fctors ffecting rtes of rections is clled chemicl kinetics. Spontneous chemicl rection mens, the rection which occurs on
4. CHEMICAL KINETICS Synopsis: The study of rtes of chemicl rections mechnisms nd fctors ffecting rtes of rections is clled chemicl kinetics. Spontneous chemicl rection mens, the rection which occurs on
Probability, Estimators, and Stationarity
 Chper Probbiliy, Esimors, nd Sionriy Consider signl genered by dynmicl process, R, R. Considering s funcion of ime, we re opering in he ime domin. A fundmenl wy o chrcerize he dynmics using he ime domin
Chper Probbiliy, Esimors, nd Sionriy Consider signl genered by dynmicl process, R, R. Considering s funcion of ime, we re opering in he ime domin. A fundmenl wy o chrcerize he dynmics using he ime domin
Think of the Relationship Between Time and Space Again
 Repor nd Opinion, 1(3),009 hp://wwwsciencepubne sciencepub@gmilcom Think of he Relionship Beween Time nd Spce Agin Yng F-cheng Compny of Ruid Cenre in Xinjing 15 Hongxing Sree, Klmyi, Xingjing 834000,
Repor nd Opinion, 1(3),009 hp://wwwsciencepubne sciencepub@gmilcom Think of he Relionship Beween Time nd Spce Agin Yng F-cheng Compny of Ruid Cenre in Xinjing 15 Hongxing Sree, Klmyi, Xingjing 834000,
Chapter 14 Homework Answers
 4. Suden responses will vary. (a) combusion of gasoline (b) cooking an egg in boiling waer (c) curing of cemen Chaper 4 Homework Answers 4. A collision beween only wo molecules is much more probable han
4. Suden responses will vary. (a) combusion of gasoline (b) cooking an egg in boiling waer (c) curing of cemen Chaper 4 Homework Answers 4. A collision beween only wo molecules is much more probable han
A LIMIT-POINT CRITERION FOR A SECOND-ORDER LINEAR DIFFERENTIAL OPERATOR IAN KNOWLES
 A LIMIT-POINT CRITERION FOR A SECOND-ORDER LINEAR DIFFERENTIAL OPERATOR j IAN KNOWLES 1. Inroducion Consider he forml differenil operor T defined by el, (1) where he funcion q{) is rel-vlued nd loclly
A LIMIT-POINT CRITERION FOR A SECOND-ORDER LINEAR DIFFERENTIAL OPERATOR j IAN KNOWLES 1. Inroducion Consider he forml differenil operor T defined by el, (1) where he funcion q{) is rel-vlued nd loclly
IX.1.1 The Laplace Transform Definition 700. IX.1.2 Properties 701. IX.1.3 Examples 702. IX.1.4 Solution of IVP for ODEs 704
 Chper IX The Inegrl Trnform Mehod IX. The plce Trnform November 4, 7 699 IX. THE APACE TRANSFORM IX.. The plce Trnform Definiion 7 IX.. Properie 7 IX..3 Emple 7 IX..4 Soluion of IVP for ODE 74 IX..5 Soluion
Chper IX The Inegrl Trnform Mehod IX. The plce Trnform November 4, 7 699 IX. THE APACE TRANSFORM IX.. The plce Trnform Definiion 7 IX.. Properie 7 IX..3 Emple 7 IX..4 Soluion of IVP for ODE 74 IX..5 Soluion
IX.1.1 The Laplace Transform Definition 700. IX.1.2 Properties 701. IX.1.3 Examples 702. IX.1.4 Solution of IVP for ODEs 704
 Chper IX The Inegrl Trnform Mehod IX. The plce Trnform November 6, 8 699 IX. THE APACE TRANSFORM IX.. The plce Trnform Definiion 7 IX.. Properie 7 IX..3 Emple 7 IX..4 Soluion of IVP for ODE 74 IX..5 Soluion
Chper IX The Inegrl Trnform Mehod IX. The plce Trnform November 6, 8 699 IX. THE APACE TRANSFORM IX.. The plce Trnform Definiion 7 IX.. Properie 7 IX..3 Emple 7 IX..4 Soluion of IVP for ODE 74 IX..5 Soluion
An integral having either an infinite limit of integration or an unbounded integrand is called improper. Here are two examples.
 Improper Inegrls To his poin we hve only considered inegrls f(x) wih he is of inegrion nd b finie nd he inegrnd f(x) bounded (nd in fc coninuous excep possibly for finiely mny jump disconinuiies) An inegrl
Improper Inegrls To his poin we hve only considered inegrls f(x) wih he is of inegrion nd b finie nd he inegrnd f(x) bounded (nd in fc coninuous excep possibly for finiely mny jump disconinuiies) An inegrl
22.615, MHD Theory of Fusion Systems Prof. Freidberg Lecture 9: The High Beta Tokamak
 .65, MHD Theory of Fusion Sysems Prof. Freidberg Lecure 9: The High e Tokmk Summry of he Properies of n Ohmic Tokmk. Advnges:. good euilibrium (smll shif) b. good sbiliy ( ) c. good confinemen ( τ nr )
.65, MHD Theory of Fusion Sysems Prof. Freidberg Lecure 9: The High e Tokmk Summry of he Properies of n Ohmic Tokmk. Advnges:. good euilibrium (smll shif) b. good sbiliy ( ) c. good confinemen ( τ nr )
Phys 110. Answers to even numbered problems on Midterm Map
 Phys Answers o een numbered problems on Miderm Mp. REASONING The word per indices rio, so.35 mm per dy mens.35 mm/d, which is o be epressed s re in f/cenury. These unis differ from he gien unis in boh
Phys Answers o een numbered problems on Miderm Mp. REASONING The word per indices rio, so.35 mm per dy mens.35 mm/d, which is o be epressed s re in f/cenury. These unis differ from he gien unis in boh
A new model for limit order book dynamics
 Anewmodelforlimiorderbookdynmics JeffreyR.Russell UniversiyofChicgo,GrdueSchoolofBusiness TejinKim UniversiyofChicgo,DeprmenofSisics Absrc:Thispperproposesnewmodelforlimiorderbookdynmics.Thelimiorderbookconsiss
Anewmodelforlimiorderbookdynmics JeffreyR.Russell UniversiyofChicgo,GrdueSchoolofBusiness TejinKim UniversiyofChicgo,DeprmenofSisics Absrc:Thispperproposesnewmodelforlimiorderbookdynmics.Thelimiorderbookconsiss
Honours Introductory Maths Course 2011 Integration, Differential and Difference Equations
 Honours Inroducory Mhs Course 0 Inegrion, Differenil nd Difference Equions Reding: Ching Chper 4 Noe: These noes do no fully cover he meril in Ching, u re men o supplemen your reding in Ching. Thus fr
Honours Inroducory Mhs Course 0 Inegrion, Differenil nd Difference Equions Reding: Ching Chper 4 Noe: These noes do no fully cover he meril in Ching, u re men o supplemen your reding in Ching. Thus fr
FM Applications of Integration 1.Centroid of Area
 FM Applicions of Inegrion.Cenroid of Are The cenroid of ody is is geomeric cenre. For n ojec mde of uniform meril, he cenroid coincides wih he poin which he ody cn e suppored in perfecly lnced se ie, is
FM Applicions of Inegrion.Cenroid of Are The cenroid of ody is is geomeric cenre. For n ojec mde of uniform meril, he cenroid coincides wih he poin which he ody cn e suppored in perfecly lnced se ie, is
Chapter 13 Homework Answers
 Chaper 3 Homework Answers 3.. The answer is c, doubling he [C] o while keeping he [A] o and [B] o consan. 3.2. a. Since he graph is no linear, here is no way o deermine he reacion order by inspecion. A
Chaper 3 Homework Answers 3.. The answer is c, doubling he [C] o while keeping he [A] o and [B] o consan. 3.2. a. Since he graph is no linear, here is no way o deermine he reacion order by inspecion. A
T-Match: Matching Techniques For Driving Yagi-Uda Antennas: T-Match. 2a s. Z in. (Sections 9.5 & 9.7 of Balanis)
 3/0/018 _mch.doc Pge 1 of 6 T-Mch: Mching Techniques For Driving Ygi-Ud Anenns: T-Mch (Secions 9.5 & 9.7 of Blnis) l s l / l / in The T-Mch is shun-mching echnique h cn be used o feed he driven elemen
3/0/018 _mch.doc Pge 1 of 6 T-Mch: Mching Techniques For Driving Ygi-Ud Anenns: T-Mch (Secions 9.5 & 9.7 of Blnis) l s l / l / in The T-Mch is shun-mching echnique h cn be used o feed he driven elemen
Chapter 2: Evaluative Feedback
 Chper 2: Evluive Feedbck Evluing cions vs. insrucing by giving correc cions Pure evluive feedbck depends olly on he cion ken. Pure insrucive feedbck depends no ll on he cion ken. Supervised lerning is
Chper 2: Evluive Feedbck Evluing cions vs. insrucing by giving correc cions Pure evluive feedbck depends olly on he cion ken. Pure insrucive feedbck depends no ll on he cion ken. Supervised lerning is
d 1 = c 1 b 2 - b 1 c 2 d 2 = c 1 b 3 - b 1 c 3
 and d = c b - b c c d = c b - b c c This process is coninued unil he nh row has been compleed. The complee array of coefficiens is riangular. Noe ha in developing he array an enire row may be divided or
and d = c b - b c c d = c b - b c c This process is coninued unil he nh row has been compleed. The complee array of coefficiens is riangular. Noe ha in developing he array an enire row may be divided or
6. Gas dynamics. Ideal gases Speed of infinitesimal disturbances in still gas
 6. Gs dynmics Dr. Gergely Krisóf De. of Fluid echnics, BE Februry, 009. Seed of infiniesiml disurbnces in sill gs dv d, dv d, Coninuiy: ( dv)( ) dv omenum r r heorem: ( ( dv) ) d 3443 4 q m dv d dv llievi
6. Gs dynmics Dr. Gergely Krisóf De. of Fluid echnics, BE Februry, 009. Seed of infiniesiml disurbnces in sill gs dv d, dv d, Coninuiy: ( dv)( ) dv omenum r r heorem: ( ( dv) ) d 3443 4 q m dv d dv llievi
A Time Truncated Improved Group Sampling Plans for Rayleigh and Log - Logistic Distributions
 ISSNOnline : 39-8753 ISSN Prin : 347-67 An ISO 397: 7 Cerified Orgnizion Vol. 5, Issue 5, My 6 A Time Trunced Improved Group Smpling Plns for Ryleigh nd og - ogisic Disribuions P.Kvipriy, A.R. Sudmni Rmswmy
ISSNOnline : 39-8753 ISSN Prin : 347-67 An ISO 397: 7 Cerified Orgnizion Vol. 5, Issue 5, My 6 A Time Trunced Improved Group Smpling Plns for Ryleigh nd og - ogisic Disribuions P.Kvipriy, A.R. Sudmni Rmswmy
Question Details Int Vocab 1 [ ] Question Details Int Vocab 2 [ ]
![Question Details Int Vocab 1 [ ] Question Details Int Vocab 2 [ ] Question Details Int Vocab 1 [ ] Question Details Int Vocab 2 [ ]](/thumbs/92/109283538.jpg) /3/5 Assignmen Previewer 3 Bsic: Definie Inegrls (67795) Due: Wed Apr 5 5 9: AM MDT Quesion 3 5 6 7 8 9 3 5 6 7 8 9 3 5 6 Insrucions Red ody's Noes nd Lerning Gols. Quesion Deils In Vocb [37897] The chnge
/3/5 Assignmen Previewer 3 Bsic: Definie Inegrls (67795) Due: Wed Apr 5 5 9: AM MDT Quesion 3 5 6 7 8 9 3 5 6 7 8 9 3 5 6 Insrucions Red ody's Noes nd Lerning Gols. Quesion Deils In Vocb [37897] The chnge
graph of unit step function t
 .5 Piecewie coninuou forcing funcion...e.g. urning he forcing on nd off. The following Lplce rnform meril i ueful in yem where we urn forcing funcion on nd off, nd when we hve righ hnd ide "forcing funcion"
.5 Piecewie coninuou forcing funcion...e.g. urning he forcing on nd off. The following Lplce rnform meril i ueful in yem where we urn forcing funcion on nd off, nd when we hve righ hnd ide "forcing funcion"
EXISTENCE AND UNIQUENESS OF SOLUTIONS FOR A SECOND-ORDER ITERATIVE BOUNDARY-VALUE PROBLEM
 Elecronic Journl of Differenil Equions, Vol. 208 (208), No. 50, pp. 6. ISSN: 072-669. URL: hp://ejde.mh.xse.edu or hp://ejde.mh.un.edu EXISTENCE AND UNIQUENESS OF SOLUTIONS FOR A SECOND-ORDER ITERATIVE
Elecronic Journl of Differenil Equions, Vol. 208 (208), No. 50, pp. 6. ISSN: 072-669. URL: hp://ejde.mh.xse.edu or hp://ejde.mh.un.edu EXISTENCE AND UNIQUENESS OF SOLUTIONS FOR A SECOND-ORDER ITERATIVE
3. Renewal Limit Theorems
 Virul Lborories > 14. Renewl Processes > 1 2 3 3. Renewl Limi Theorems In he inroducion o renewl processes, we noed h he rrivl ime process nd he couning process re inverses, in sens The rrivl ime process
Virul Lborories > 14. Renewl Processes > 1 2 3 3. Renewl Limi Theorems In he inroducion o renewl processes, we noed h he rrivl ime process nd he couning process re inverses, in sens The rrivl ime process
CBSE 2014 ANNUAL EXAMINATION ALL INDIA
 CBSE ANNUAL EXAMINATION ALL INDIA SET Wih Complee Eplnions M Mrks : SECTION A Q If R = {(, y) : + y = 8} is relion on N, wrie he rnge of R Sol Since + y = 8 h implies, y = (8 ) R = {(, ), (, ), (6, )}
CBSE ANNUAL EXAMINATION ALL INDIA SET Wih Complee Eplnions M Mrks : SECTION A Q If R = {(, y) : + y = 8} is relion on N, wrie he rnge of R Sol Since + y = 8 h implies, y = (8 ) R = {(, ), (, ), (6, )}
Procedia Computer Science
 Procedi Compuer Science 00 (0) 000 000 Procedi Compuer Science www.elsevier.com/loce/procedi The Third Informion Sysems Inernionl Conference The Exisence of Polynomil Soluion of he Nonliner Dynmicl Sysems
Procedi Compuer Science 00 (0) 000 000 Procedi Compuer Science www.elsevier.com/loce/procedi The Third Informion Sysems Inernionl Conference The Exisence of Polynomil Soluion of he Nonliner Dynmicl Sysems
CHAPTER 2 KINEMATICS IN ONE DIMENSION ANSWERS TO FOCUS ON CONCEPTS QUESTIONS
 Physics h Ediion Cunell Johnson Young Sdler Soluions Mnul Soluions Mnul, Answer keys, Insrucor's Resource Mnul for ll chpers re included. Compleed downlod links: hps://esbnkre.com/downlod/physics-h-ediion-soluions-mnulcunell-johnson-young-sdler/
Physics h Ediion Cunell Johnson Young Sdler Soluions Mnul Soluions Mnul, Answer keys, Insrucor's Resource Mnul for ll chpers re included. Compleed downlod links: hps://esbnkre.com/downlod/physics-h-ediion-soluions-mnulcunell-johnson-young-sdler/
LAPLACE TRANSFORMS. 1. Basic transforms
 LAPLACE TRANSFORMS. Bic rnform In hi coure, Lplce Trnform will be inroduced nd heir properie exmined; ble of common rnform will be buil up; nd rnform will be ued o olve ome dierenil equion by rnforming
LAPLACE TRANSFORMS. Bic rnform In hi coure, Lplce Trnform will be inroduced nd heir properie exmined; ble of common rnform will be buil up; nd rnform will be ued o olve ome dierenil equion by rnforming
Some Inequalities variations on a common theme Lecture I, UL 2007
 Some Inequliies vriions on common heme Lecure I, UL 2007 Finbrr Hollnd, Deprmen of Mhemics, Universiy College Cork, fhollnd@uccie; July 2, 2007 Three Problems Problem Assume i, b i, c i, i =, 2, 3 re rel
Some Inequliies vriions on common heme Lecure I, UL 2007 Finbrr Hollnd, Deprmen of Mhemics, Universiy College Cork, fhollnd@uccie; July 2, 2007 Three Problems Problem Assume i, b i, c i, i =, 2, 3 re rel
1 jordan.mcd Eigenvalue-eigenvector approach to solving first order ODEs. -- Jordan normal (canonical) form. Instructor: Nam Sun Wang
 jordnmcd Eigenvlue-eigenvecor pproch o solving firs order ODEs -- ordn norml (cnonicl) form Insrucor: Nm Sun Wng Consider he following se of coupled firs order ODEs d d x x 5 x x d d x d d x x x 5 x x
jordnmcd Eigenvlue-eigenvecor pproch o solving firs order ODEs -- ordn norml (cnonicl) form Insrucor: Nm Sun Wng Consider he following se of coupled firs order ODEs d d x x 5 x x d d x d d x x x 5 x x
ME 141. Engineering Mechanics
 ME 141 Engineeing Mechnics Lecue 13: Kinemics of igid bodies hmd Shhedi Shkil Lecue, ep. of Mechnicl Engg, UET E-mil: sshkil@me.bue.c.bd, shkil6791@gmil.com Websie: eche.bue.c.bd/sshkil Couesy: Veco Mechnics
ME 141 Engineeing Mechnics Lecue 13: Kinemics of igid bodies hmd Shhedi Shkil Lecue, ep. of Mechnicl Engg, UET E-mil: sshkil@me.bue.c.bd, shkil6791@gmil.com Websie: eche.bue.c.bd/sshkil Couesy: Veco Mechnics
Suggested Problem Solutions Associated with Homework #5
 Suggesed Problem Soluions Associaed wih Homework #5 431 (a) 8 Si has proons and neurons (b) 85 3 Rb has 3 proons and 48 neurons (c) 5 Tl 81 has 81 proons and neurons 43 IDENTIFY and SET UP: The ex calculaes
Suggesed Problem Soluions Associaed wih Homework #5 431 (a) 8 Si has proons and neurons (b) 85 3 Rb has 3 proons and 48 neurons (c) 5 Tl 81 has 81 proons and neurons 43 IDENTIFY and SET UP: The ex calculaes
AP Chemistry--Chapter 12: Chemical Kinetics
 AP Chemisry--Chaper 12: Chemical Kineics I. Reacion Raes A. The area of chemisry ha deals wih reacion raes, or how fas a reacion occurs, is called chemical kineics. B. The rae of reacion depends on he
AP Chemisry--Chaper 12: Chemical Kineics I. Reacion Raes A. The area of chemisry ha deals wih reacion raes, or how fas a reacion occurs, is called chemical kineics. B. The rae of reacion depends on he
Thermal neutron self-shielding factor in foils: a universal curve
 Proceedings of he Inernionl Conference on Reserch Recor Uilizion, Sfey, Decommissioning, Fuel nd Wse Mngemen (Snigo, Chile, -4 Nov.3) Pper IAEA-CN-/, IAEA Proceedings Series, Vienn, 5 Therml neuron self-shielding
Proceedings of he Inernionl Conference on Reserch Recor Uilizion, Sfey, Decommissioning, Fuel nd Wse Mngemen (Snigo, Chile, -4 Nov.3) Pper IAEA-CN-/, IAEA Proceedings Series, Vienn, 5 Therml neuron self-shielding
An object moving with speed v around a point at distance r, has an angular velocity. m/s m
 Roion The mosphere roes wih he erh n moions wihin he mosphere clerly follow cure phs (cyclones, nicyclones, hurricnes, ornoes ec.) We nee o epress roion quniiely. For soli objec or ny mss h oes no isor
Roion The mosphere roes wih he erh n moions wihin he mosphere clerly follow cure phs (cyclones, nicyclones, hurricnes, ornoes ec.) We nee o epress roion quniiely. For soli objec or ny mss h oes no isor
Physics 101 Lecture 4 Motion in 2D and 3D
 Phsics 11 Lecure 4 Moion in D nd 3D Dr. Ali ÖVGÜN EMU Phsics Deprmen www.ogun.com Vecor nd is componens The componens re he legs of he righ ringle whose hpoenuse is A A A A A n ( θ ) A Acos( θ) A A A nd
Phsics 11 Lecure 4 Moion in D nd 3D Dr. Ali ÖVGÜN EMU Phsics Deprmen www.ogun.com Vecor nd is componens The componens re he legs of he righ ringle whose hpoenuse is A A A A A n ( θ ) A Acos( θ) A A A nd
Chapter 2 PROBLEM SOLUTIONS
 Chper PROBLEM SOLUTIONS. We ssume h you re pproximely m ll nd h he nere impulse rels uniform speed. The elpsed ime is hen Δ x m Δ = m s s. s.3 Disnces reled beween pirs of ciies re ( ) Δx = Δ = 8. km h.5
Chper PROBLEM SOLUTIONS. We ssume h you re pproximely m ll nd h he nere impulse rels uniform speed. The elpsed ime is hen Δ x m Δ = m s s. s.3 Disnces reled beween pirs of ciies re ( ) Δx = Δ = 8. km h.5
Forms of Energy. Mass = Energy. Page 1. SPH4U: Introduction to Work. Work & Energy. Particle Physics:
 SPH4U: Inroducion o ork ork & Energy ork & Energy Discussion Definiion Do Produc ork of consn force ork/kineic energy heore ork of uliple consn forces Coens One of he os iporn conceps in physics Alernive
SPH4U: Inroducion o ork ork & Energy ork & Energy Discussion Definiion Do Produc ork of consn force ork/kineic energy heore ork of uliple consn forces Coens One of he os iporn conceps in physics Alernive
Physics Worksheet Lesson 4: Linear Motion Section: Name:
 Physics Workshee Lesson 4: Liner Moion Secion: Nme: 1. Relie Moion:. All moion is. b. is n rbirry coorine sysem wih reference o which he posiion or moion of somehing is escribe or physicl lws re formule.
Physics Workshee Lesson 4: Liner Moion Secion: Nme: 1. Relie Moion:. All moion is. b. is n rbirry coorine sysem wih reference o which he posiion or moion of somehing is escribe or physicl lws re formule.
How to Prove the Riemann Hypothesis Author: Fayez Fok Al Adeh.
 How o Prove he Riemnn Hohesis Auhor: Fez Fok Al Adeh. Presiden of he Srin Cosmologicl Socie P.O.Bo,387,Dmscus,Sri Tels:963--77679,735 Emil:hf@scs-ne.org Commens: 3 ges Subj-Clss: Funcionl nlsis, comle
How o Prove he Riemnn Hohesis Auhor: Fez Fok Al Adeh. Presiden of he Srin Cosmologicl Socie P.O.Bo,387,Dmscus,Sri Tels:963--77679,735 Emil:hf@scs-ne.org Commens: 3 ges Subj-Clss: Funcionl nlsis, comle
CHAPTER 11 PARAMETRIC EQUATIONS AND POLAR COORDINATES
 CHAPTER PARAMETRIC EQUATIONS AND POLAR COORDINATES. PARAMETRIZATIONS OF PLANE CURVES., 9, _ _ Ê.,, Ê or, Ÿ. 5, 7, _ _.,, Ÿ Ÿ Ê Ê 5 Ê ( 5) Ê ˆ Ê 6 Ê ( 5) 7 Ê Ê, Ÿ Ÿ $ 5. cos, sin, Ÿ Ÿ 6. cos ( ), sin (
CHAPTER PARAMETRIC EQUATIONS AND POLAR COORDINATES. PARAMETRIZATIONS OF PLANE CURVES., 9, _ _ Ê.,, Ê or, Ÿ. 5, 7, _ _.,, Ÿ Ÿ Ê Ê 5 Ê ( 5) Ê ˆ Ê 6 Ê ( 5) 7 Ê Ê, Ÿ Ÿ $ 5. cos, sin, Ÿ Ÿ 6. cos ( ), sin (
Collision Detection and Bouncing
 Collision Deecion nd Bouncing Collisions re Hndled in Two Prs. Deecing he collision Mike Biley mj@cs.oregonse.edu. Hndling he physics of he collision collision-ouncing.ppx If You re Lucky, You Cn Deec
Collision Deecion nd Bouncing Collisions re Hndled in Two Prs. Deecing he collision Mike Biley mj@cs.oregonse.edu. Hndling he physics of he collision collision-ouncing.ppx If You re Lucky, You Cn Deec
Tax Audit and Vertical Externalities
 T Audi nd Vericl Eernliies Hidey Ko Misuyoshi Yngihr Ngoy Keizi Universiy Ngoy Universiy 1. Inroducion The vericl fiscl eernliies rise when he differen levels of governmens, such s he federl nd se governmens,
T Audi nd Vericl Eernliies Hidey Ko Misuyoshi Yngihr Ngoy Keizi Universiy Ngoy Universiy 1. Inroducion The vericl fiscl eernliies rise when he differen levels of governmens, such s he federl nd se governmens,
SOME USEFUL MATHEMATICS
 SOME USEFU MAHEMAICS SOME USEFU MAHEMAICS I is esy o mesure n preic he behvior of n elecricl circui h conins only c volges n currens. However, mos useful elecricl signls h crry informion vry wih ime. Since
SOME USEFU MAHEMAICS SOME USEFU MAHEMAICS I is esy o mesure n preic he behvior of n elecricl circui h conins only c volges n currens. However, mos useful elecricl signls h crry informion vry wih ime. Since
Math 333 Problem Set #2 Solution 14 February 2003
 Mah 333 Problem Se #2 Soluion 14 February 2003 A1. Solve he iniial value problem dy dx = x2 + e 3x ; 2y 4 y(0) = 1. Soluion: This is separable; we wrie 2y 4 dy = x 2 + e x dx and inegrae o ge The iniial
Mah 333 Problem Se #2 Soluion 14 February 2003 A1. Solve he iniial value problem dy dx = x2 + e 3x ; 2y 4 y(0) = 1. Soluion: This is separable; we wrie 2y 4 dy = x 2 + e x dx and inegrae o ge The iniial
Name: Per: L o s A l t o s H i g h S c h o o l. Physics Unit 1 Workbook. 1D Kinematics. Mr. Randall Room 705
 Nme: Per: L o s A l o s H i g h S c h o o l Physics Uni 1 Workbook 1D Kinemics Mr. Rndll Room 705 Adm.Rndll@ml.ne www.laphysics.com Uni 1 - Objecies Te: Physics 6 h Ediion Cunel & Johnson The objecies
Nme: Per: L o s A l o s H i g h S c h o o l Physics Uni 1 Workbook 1D Kinemics Mr. Rndll Room 705 Adm.Rndll@ml.ne www.laphysics.com Uni 1 - Objecies Te: Physics 6 h Ediion Cunel & Johnson The objecies
P441 Analytical Mechanics - I. Coupled Oscillators. c Alex R. Dzierba
 Lecure 3 Mondy - Deceber 5, 005 Wrien or ls upded: Deceber 3, 005 P44 Anlyicl Mechnics - I oupled Oscillors c Alex R. Dzierb oupled oscillors - rix echnique In Figure we show n exple of wo coupled oscillors,
Lecure 3 Mondy - Deceber 5, 005 Wrien or ls upded: Deceber 3, 005 P44 Anlyicl Mechnics - I oupled Oscillors c Alex R. Dzierb oupled oscillors - rix echnique In Figure we show n exple of wo coupled oscillors,
Rates of chemical reactions
 Rtes of chemicl rections Mesuring rtes of chemicl rections Experimentl mesuring progress of the rection Monitoring pressure in the rection involving gses 2 NO( g) 4 NO ( g) + O ( g) 2 5 2 2 n(1 α) 2αn
Rtes of chemicl rections Mesuring rtes of chemicl rections Experimentl mesuring progress of the rection Monitoring pressure in the rection involving gses 2 NO( g) 4 NO ( g) + O ( g) 2 5 2 2 n(1 α) 2αn
Estimating the population parameter, r, q and K based on surplus production model. Wang, Chien-Hsiung
 SCTB15 Working Pper ALB 7 Esiming he populion prmeer, r, q nd K bsed on surplus producion model Wng, Chien-Hsiung Biologicl nd Fishery Division Insiue of Ocenogrphy Nionl Tiwn Universiy Tipei, Tiwn Tile:
SCTB15 Working Pper ALB 7 Esiming he populion prmeer, r, q nd K bsed on surplus producion model Wng, Chien-Hsiung Biologicl nd Fishery Division Insiue of Ocenogrphy Nionl Tiwn Universiy Tipei, Tiwn Tile:
Some Basic Information about M-S-D Systems
 Some Basic Informaion abou M-S-D Sysems 1 Inroducion We wan o give some summary of he facs concerning unforced (homogeneous) and forced (non-homogeneous) models for linear oscillaors governed by second-order,
Some Basic Informaion abou M-S-D Sysems 1 Inroducion We wan o give some summary of he facs concerning unforced (homogeneous) and forced (non-homogeneous) models for linear oscillaors governed by second-order,
Chapter 2. First Order Scalar Equations
 Chaper. Firs Order Scalar Equaions We sar our sudy of differenial equaions in he same way he pioneers in his field did. We show paricular echniques o solve paricular ypes of firs order differenial equaions.
Chaper. Firs Order Scalar Equaions We sar our sudy of differenial equaions in he same way he pioneers in his field did. We show paricular echniques o solve paricular ypes of firs order differenial equaions.
MAT 266 Calculus for Engineers II Notes on Chapter 6 Professor: John Quigg Semester: spring 2017
 MAT 66 Clculus for Engineers II Noes on Chper 6 Professor: John Quigg Semeser: spring 7 Secion 6.: Inegrion by prs The Produc Rule is d d f()g() = f()g () + f ()g() Tking indefinie inegrls gives [f()g
MAT 66 Clculus for Engineers II Noes on Chper 6 Professor: John Quigg Semeser: spring 7 Secion 6.: Inegrion by prs The Produc Rule is d d f()g() = f()g () + f ()g() Tking indefinie inegrls gives [f()g
Module 2 F c i k c s la l w a s o s f dif di fusi s o i n
 Module Fick s laws of diffusion Fick s laws of diffusion and hin film soluion Adolf Fick (1855) proposed: d J α d d d J (mole/m s) flu (m /s) diffusion coefficien and (mole/m 3 ) concenraion of ions, aoms
Module Fick s laws of diffusion Fick s laws of diffusion and hin film soluion Adolf Fick (1855) proposed: d J α d d d J (mole/m s) flu (m /s) diffusion coefficien and (mole/m 3 ) concenraion of ions, aoms
ECE Microwave Engineering. Fall Prof. David R. Jackson Dept. of ECE. Notes 10. Waveguides Part 7: Transverse Equivalent Network (TEN)
 EE 537-635 Microwve Engineering Fll 7 Prof. Dvid R. Jcson Dep. of EE Noes Wveguides Pr 7: Trnsverse Equivlen Newor (N) Wveguide Trnsmission Line Model Our gol is o come up wih rnsmission line model for
EE 537-635 Microwve Engineering Fll 7 Prof. Dvid R. Jcson Dep. of EE Noes Wveguides Pr 7: Trnsverse Equivlen Newor (N) Wveguide Trnsmission Line Model Our gol is o come up wih rnsmission line model for
 Rel Gses 1. Gses (N, CO ) which don t obey gs lws or gs eqution P=RT t ll pressure nd tempertures re clled rel gses.. Rel gses obey gs lws t extremely low pressure nd high temperture. Rel gses devited
Rel Gses 1. Gses (N, CO ) which don t obey gs lws or gs eqution P=RT t ll pressure nd tempertures re clled rel gses.. Rel gses obey gs lws t extremely low pressure nd high temperture. Rel gses devited
22.615, MHD Theory of Fusion Systems Prof. Freidberg Lecture 10: The High Beta Tokamak Con d and the High Flux Conserving Tokamak.
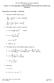 .615, MHD Theory of Fusion Sysems Prof. Freidberg Lecure 1: The High Be Tokmk Con d nd he High Flux Conserving Tokmk Proeries of he High Tokmk 1. Evlue he MHD sfey fcor: ψ B * ( ) 1 3 ρ 1+ ν ρ ρ cosθ *
.615, MHD Theory of Fusion Sysems Prof. Freidberg Lecure 1: The High Be Tokmk Con d nd he High Flux Conserving Tokmk Proeries of he High Tokmk 1. Evlue he MHD sfey fcor: ψ B * ( ) 1 3 ρ 1+ ν ρ ρ cosθ *
Solutions to Assignment 1
 MA 2326 Differenial Equaions Insrucor: Peronela Radu Friday, February 8, 203 Soluions o Assignmen. Find he general soluions of he following ODEs: (a) 2 x = an x Soluion: I is a separable equaion as we
MA 2326 Differenial Equaions Insrucor: Peronela Radu Friday, February 8, 203 Soluions o Assignmen. Find he general soluions of he following ODEs: (a) 2 x = an x Soluion: I is a separable equaion as we
