Course Notes: Thermodynamics and the Nernst-Planck Eqn. Reading: Johnston and Wu, chapts 2, 3, 5; Hille, chapts 10, 13, 14.
|
|
|
- Hilary Holt
- 6 years ago
- Views:
Transcription
1 Course Notes: Thermodynamcs and the Nernst-Planck Eqn. Readng: Johnston and Wu, chapts 2, 3, 5; Hlle, chapts 10, 13, 14. In these lectures, the nature of on flux n free soluton and n dffuse membranes s dscussed, usng deas from thermodynamcs. Ths theory gnores the specfc propertes of on channels, but s mportant as a general background for more specfc theores that are consdered later. Most mportant, the thermodynamc theores provde boundary condtons for all models of on flux n bologcal membranes. Frst law of thermodynamcs The startng pont for ths dscusson s the frst and second laws of thermodynamcs. These laws are concerned wth functons of state of systems. A system s smply whatever collecton of objects s of nterest. For ths course, the systems wll generally consst of a membrane and the solutons boundng the two sdes of the membrane, as n Fg. 1. The mportant consttuents of the system are the membrane and the onc solutes n the solutons. Systems have varous parameters, ncludng pressures, temperatures, concentratons of solutes, etc. These are generally dvded nto extensve parameters, such as volume or the total quantty of a solute n the system, whch depend on the sze of the system and ntensve parameters, such as concentraton and pressure whch do not depend on the sze of the system. Functons of state are thermodynamc quanttes that are unquely defned by the extensve and ntensve parameters of the system. That s, all solutons lke the one n Fg. 1 wll have the same thermodynamc state functons f ther temperatures, volumes, solute concentratons, etc. are the same. An example of a thermodynamc functon of state s the nternal energy U. The frst law of thermodynamcs provdes an ndrect defnton of U by statng the condtons under whch U can be changed: Frst law: the nternal energy U of a system s a functon of state that s changed only by heat flow or work done on the system: U = U2 U1 = q+ w (1) When the system goes from state 1 to state 2 ts nternal energy changes by an amount equal to the heat q that flows nto the system plus the work w done on the system by ts surroundngs. Notce that U has unts of Joules, or smlar unts of energy. The frst law s essentally a statement of conservaton of energy. 140 mm Na mm Cl- 10 mm Na + 10 mm Cl- Fgure 1: example of a system consstng of a membrane (fuzzy lne) separatng two NaCl solutons
2 2 As an example, suppose that an deal gas s compressed from volume V 1 to volume V 2. In ths stuaton the work done on the gas s gven by V2 w = PdV V1 (2) PdV s the pressure-volume work done by the gas when t expands by a volume change dv aganst a pressure P. The mnus sgn makes ths the work done on the gas durng such an expanson and the ntegral computes the total work gong from one volume to another. The change n U of the gas durng the compresson from V 1 to V 2 s the sum of the work n Eqn. 2 and whatever heat s allowed to flow. Suppose that no heat s allowed to flow nto or out of the gas durng the compresson (a system that does not exchange heat wth ts envronment s called adabatc). In ths case, the pressure and volume of an deal monoatomc gas follow the rule PV γ = c, where c s a constant and γ 5/3. Usng ths rule, the work done n compressng the gas s and U V 2 c w V dv c 1 1 = = (3) γ γ 1 γ 1 γ 1 V V V1 = w n ths case, snce q=0. As another example, suppose the gas s placed on a heat reservor at temperature T durng the compresson and that heat flows between the gas and the reservor n such a way as to mantan the temperature of the gas constant at T. Now, the nternal energy of an deal gas turns out to depend only on ts temperature, so n ths case U = 0 snce the temperature of the gas does not change. Usng the frst law, we can conclude that the heat flow nto the gas s the negatve of the compresson work done on the gas, q = -w so that V n q w PdV V dv n V2 = = = = ln (4) V V1 where the deal gas law PV=n was used. V V1 Queston 1: An engne runs n a cycle; each tme t goes around the cycle once, t absorbs heat q 1 from one reservor and delvers heat q 2 to a second reservor; t also delvers work w to ts envronment and ends up n exactly the same thermodynamc state as at the begnnng of the cycle. What does the frst law tell you about q 1, q 2, and w? Second law of thermodynamcs The second law provdes a rule that descrbes the drecton of change n a system n the absence of external forces. We know from experence, for example, that heat flows from warm objects to cold objects, that objects fall downward n a gravty feld, that gas expands from a pressure nto a vacuum, and that solutes dffuse from regons of hgh concentraton nto regons of low concentraton. The second law s a rule whch captures these facts n a remarkably concse way. 1
3 3 Flows n thermodynamc systems are drven by forces; flows and forces occur n conjugate pars. That s, heat flow s drven by dfferences n temperature, volume flow by dfferences n pressure, charge flow by dfferences n electrcal potental, and mass flow by dfferences n concentraton. In complex systems, there may be cross-couplng between forces and non-conjugate flows, but ths subtlety wll be gnored for the tme beng. Essental to the second law s the dea of a reversble flow. A flow s reversble when t s drven by an nfntesmal force,.e. a force whch s so close to zero that a small change n the magntude of the force at the approprate place can reverse the drecton of the flow. In real systems, flows are almost always rreversble, for example the flow of electrc current through a lamp whch occurs across a substantal electrcal potental dfference or the flow of heat nto an ce cube from a glass of warm water, whch occurs between a substantal temperature dfference. The second law of thermodynamcs deals wth heat flow and defnes a new state functon, the entropy. Second law: the entropy S of a system s a state functon whch changes wth heat flow as S = S2 S1 = 2 1 dq T (5) by a reversble process. For an rreversble process, the entropy change s greater than the ntegral above. The lmts n the ntegral n Eqn. 5 mean that the quantty dq/t should be summated over the path that the system takes to get from state 1 to state 2. Exactly how the lmts are wrtten wll depend on the problem. The pont s that entropy s the accumulaton of dq/t, heat flow nto the system dvded by temperature, by a reversble process. Note that for adabatc systems, S 0 wth equalty only for reversble changes. Thus n an adabatc system any naturally occurrng rreversble process must occur n the drecton whch ncreases the entropy of the system. The fact that the entropy of an adabatc system can only ncrease sets a drecton for all natural processes. As an example of ths, consder the case of an rreversble heat flow q between two reservors at temperatures T 1 and T 2, as shown n Fg. 2A. In order to compute the entropy change assocated wth ths flow, t s shown as an equvalent process n Fg. 2B consstng of two reversble flows. The entropy change assocated wth the heat flow can then be computed as A q T 1 T 2 B T 1 T 2 q q Fgure 2: A. Heat q flows by an rreversble process between temperatures T 1 and T 2. B. Same flow, but by two reversble processes. T 1 T 2
4 4 q q S = + T T 1 2 (6) The frst term s the entropy change of the T 1 part of the system and the second term s the entropy change of the T 2 part. Now the system n Fg. 2A s adabatc, n that heat flows only nternally, so the second law says that S>0. Therefore, f q>0, then 1 1 S = q > 0 T1 > T2 (6a) T T 2 1 In other words, the second law says that heat flows from a hgher temperature to a lower one n an rreversble process. Note that the assumpton q>0 s not necessary; f we had assumed q<0 then the same concluson would be reached, except now T 2 >T 1. Queston 2: An alternate statement of the second law s that net work cannot be done by an engne whch only draws heat from a sngle reservor. To see that the second law mples ths statement, consder an engne whch runs n a cycle. Each tme around the cycle t draws heat q from a reservor at temperature T and delvers work w to the envronment. There are no other heat flows. The engne s cyclc, meanng that ts state s exactly the same at the end of a cycle as at the begnnng (n partcular, S and U are the same at the begnnng and end of each cycle). Show that ths engne s consstent wth the frst law, but volates the second law. Does the engne of Queston 1 necessarly volate the second law (Hnt: suppose the heat exchanges n queston 1 occur reversbly)? Gbbs Free Energy The analyss of Fg. 2 shows how the second law can be appled to heat flows. For systems consstng of onc solutons, t s dffcult to make a smlar analyss, because the heat flows assocated wth onc movements n soluton are hard to compute. Ths problem can be smplfed by usng a dfferent state functon, called the Gbbs free energy G, whch s defned as G = U + PV TS (7) Here, P s pressure and V s volume. The Gbbs free energy allows an alternatve statement of the second law whch s more useful for our purposes. Consder a small change n G whch can be defned by dfferentatng Eqn. 7: dg = du + PdV + VdP TdS SdT (8) Rearrangng ths equaton and usng the fact that du=q+w (frst law) and that ds q/t(second law) gves dg VdP + SdT = q + w + PdV TdS w + PdV (9)
5 5 Now n onc solutons, the pressure and temperature are usually constant, so dp=dt=0 and Eqn. 9 can be wrtten as dg w + PdV = w (10) TP, ' The notaton dg T,P means the change n free energy n a system n whch temperature and pressure are constant. PdV s the pressure-volume work done by the system on ts envronment,.e. the work done by expanson or contracton of the system. w s the sum of varous knds of work, one component of whch s PdV, the pressure-volume work done by the envronment on the system. Thus, w+pdv s the net work done by the envronment on the system, exclusve of pressure-volume work. Ths s denoted w n Eqn. 10. Eqn. 10 s an alternate statement of the second law of thermodynamcs whch says that, for a system at constant temperature and pressure, the change n Gbbs free energy n any change of state s less than or equal to the non-pv work done on the system by the envronment. Equalty holds only for reversble changes. Typcally n membrane transport problems w =0, and the second law says that the Gbbs free energy must decrease or stay constant n any spontaneously occurrng state change. Electrochemcal potental G s an extensve parameter of a system,.e. t ncreases lnearly wth the sze of the system. Because t s nconvenent to keep track of system sze n most calculatons, the electrochemcal potental µ s used nstead. µ s the contrbuton of one mole of the th consttuent of the system to the system s free energy, ts molar free energy. If n s the number of moles of the th consttuent n the system, then or equvalently, G n = µ. G = n µ (11) The electrochemcal potental s the drve for flux of substances across a dffuson barrer. Consder the stuaton dagrammed n Fg. 3. A soluton s separated nto two phases by a membrane. The electrochemcal potentals of the solute n the two phases are µ 1 and µ 2. Suppose that a small amount of solute dn moves from sde 1 to sde 2. Usng Eqns. 10 and 11, t must be the case that dg = µ 1dn + µ 2dn 0 (12) µ 1 dn µ 2 Fgure 3: dn moles of solute moves through the membrane between electrochemcal potentals µ 1 and µ 2. because the process occurs at fxed temperature and pressure and there s no external work. That s, the change n free energy of the whole system (dg) s the free energy lost on sde 1 (-µ 1 dn) plus the free energy ganed on sde 2 (µ 2 dn). If dn>0 as drawn, then Eqn. 12 mples that µ 2 µ 1. Thus transport of solutes occurs from regons of hgher electrochemcal potental to regons of lower
6 6 potental. Ths s an alternatve statement of the second law whch s convenent for membrane problems. Note n partcular that f µ 2 =µ 1 then dg=0 for any flux through the membrane; that s, there s no way to decrease G by transport of solute through the membrane. Ths condton s equlbrum. As we wll see below, there s no net flux through the membrane of a solute that s at equlbrum. In order to use the electrochemcal potental, t s necessary to dscover how t vares wth mportant system parameters; n the case of membrane transport, mportant parameters are the on concentraton and the electrcal potental (pressure can also be ncluded for cases where osmotc flows are mportant, but these are neglgble n neurons). The approprate expresson s Eqn µ = µ + ln C + z FV + (13) The subscrpt dentfes the partcular on to whch ths equatons refers; there s one such equaton for each solute. The thrd term on the rght hand sde, z FV s the contrbuton of electrcal potental. z s the charge on the on (e.g. +1 for Na +, -1 for Cl -, +2 for Ca ++ ); F s the number of Coulombs of charge n a mole of unt charges (9.65x10 4 coul/mole), and V s the electrcal potental (NOTE the change n notaton, V s voltage, not volume, from here on). The product z FV s the work requred to brng a mole of ons wth charge z from 0 potental to potental V. Consstent wth the defntons of G as the non-pressure-volume work done on the system (Eqns 9 and 10), ths s the electrcal contrbuton, per mole, to G. The second term on the rght hand sde of Eqn. 13, lnc, s the contrbuton of the on s concentraton C. R s the gas constant (8.315 Joule/ mole) and T s the temperature. It s not possble to gve a smple dervaton of ths term. Ultmately, t depends on the emprcal behavor of solutons, as expressed by phenomena lke osmotc pressures. A dervaton of ths type s gven by Katchalsky and Curran (1965, pp ). For the present, the form of ths term wll be accepted as an assumpton. Later, t wll be shown to be consstent wth the results of other, qute dfferent, approaches. Ths term s expressed n terms of concentraton C ; n many cases, especally for more concentrated solutons, ths term s naccurate, whch has led to the development of an emprcally corrected concentraton, called actvty. For the purposes of ths course, however, concentraton wll be used. The frst term on the rght hand sde of Eqn. 13, µ 0 s the electrochemcal potental of the on at unty concentraton and zero electrcal potental. It contans the contrbutons of all factors other than concentraton and electrcal potental to the electrochemcal potental of the on. Ths ncludes effects such as nteracton between the on and the solvent, the effects of pressure, and other such effects. Queston 3: When there s a flux of solute across the membrane n the stuaton dagrammed n Fg. 3, the concentraton of solute wll decrease on sde 1 and ncrease on sde 2. From Eqn 13, ths should produce a change n µ n the solutons. Such a change was not consdered n the analyss leadng to Eqn. 12. That s, the full dfferental dg should nclude terms lke n 1 dµ 1. By usng Eqn. 13 to compute dµ, show that, even when such terms are consdered, the result n Eqn. 12 s correct, as long as the flux dn s small.
7 7 Equlbrum The dscusson of Fg. 3 and Eqn. 12 showed that transport through membranes s drven by electrochemcal potental dfferences. Equlbrum occurs when there are no electrochemcal potental dfferences. In ths stuaton, there s no force drvng transport n the system, and no flux should be observed. Fg. 4 shows a membrane wth the relevant parameters dentfed. An on exsts at concentratons C 1 and C 2 on the two sdes of a membrane. There s also an electrcal potental dfference V = V 2 - V 1 across the membrane. As a result of these dfferences, there could be a dfference n the electrochemcal potental of the on across the membrane. However, t s also possble that the potental due to the concentraton dfference could be equal and opposte to the electrcal potental, producng no dfference n electrochemcal potental,.e. an equlbrum. The Nernst equaton expresses the condtons under whch ths s true. C 1, V 1 C 2, V 2 Fgure 4: a membrane separatng two solutons. The concentraton of an on dffers n the two solutons and there s a dfference n electrcal potental between the solutons. gves: Drectly wrtng the condton for equalty of electrochemcal potental across the membrane µ + lnc + z FV = µ + ln C + z FV (14) Note the assumpton that µ 0 s the same n both solutons. Ths should be true f the solutons dffer only n on concentraton and electrcal potentals. Cancelng common terms and rearrangng Eqn. 14 gves the Nernst equaton: C1 V2 V1 = E = ln (15) zf C That s, when the electrcal potental dfference s equal to the value E, gven by the functon of concentraton on the rght-hand sde, then the electrochemcal potental of the on s the same n the two solutons and the on s at equlbrum. The value E s called the equlbrum potental of the on. Speakng loosely, the equlbrum of Eqn. 15 can be consdered to descrbe the condton n whch the electrcal force pushng the on one way through the membrane s just balanced by an equal and opposte concentraton force pushng the other way. Queston 4: An mportant condton for many analyses of membrane systems s charge electroneutralty, whch means that the net charge n a soluton s zero. That s, the total concentraton of anonc charge s equal to the total concentraton of catonc charge: all catons zc 2 = za (16) all anons
8 8 Of course, n order to have a membrane potental, there must be some charge separaton across the membrane; thus, f the membrane potental s negatve, then there must be a net negatve charge nsde the cell and a net postve charge outsde the cell. Consder a sphercal cell of radus 10 µm wth a membrane potental of 70 mv. The cell s flled wth a 140 mm soluton of KCl. How large s the charge mbalance relatve to the total concentraton of on nsde the cell? Do ths problem by assumng a membrane capactance of 1 µfd/cm 2 and compute the charge on the membrane capactance necessary to produce the 70 mv potental. You should conclude that Eqn. 16 s a very good approxmaton. Queston 5: For a typcal mammalan cell, the on concentratons are somethng lke those gven n the table at rght. Compute the equlbrum potental for each on. If the membrane potental s 60 mv, whch ons are at equlbrum? For the ons that are not at equlbrum tell whch drecton (nto the cell or out of the cell) they wll flow. That s, on whch sde of the membrane s ther electrochemcal potental lower? Queston 6: Argue that the equlbrum dscussed n connecton wth Fg. 4 s stable. That s, suppose that the membrane potental V s slghtly smaller or larger than the equlbrum potental E for on. The on wll not be at equlbrum and there wll be a net flux of the on through the membrane. Argue that the flux wll carry charge n such a drecton as to brng the on back to equlbrum. Is ths result related n any way to the rule that G 0? Queston 7: Usually the onc consttuents of real cells are not at equlbrum across the cell membrane. However, the Donnan equlbrum s an approxmaton for the membrane potentals of certan cells. The stuaton s dagrammed n Fg. 5. A membrane permeable to both potassum and chlorde separates the solutons ndcated. The concentratons of potassum and chlorde outsde the cell are fxed at 10 mm. The concentratons nsde the cell are adjusted by transmembrane fluxes untl both potassum and chlorde are at equlbrum across the membrane. N n represents the concentraton of fxed negatve charges nsde the cell. These charges are mpermeable to the membrane and ther concentraton cannot change. By usng the Nernst equaton to express the equlbrum potentals for potassum and chlorde and by assumng that charge electroneutralty (Eqn. 16) holds n both solutons, compute K n, Cl n, and V=V n -V out, the transmembrane potental, n terms of the external concentratons and N n. (Note the stuaton analyzed here s not realstc for a membrane system; n partcular there s a large osmotc pressure dfference on nsde cell outsde cell Na + 20 mm 120 mm K mm 4 mm Cl - 7 mm 140 mm Ca mm 1.5 mm K out =10 mm Cl out =10 mm K n =? Cl n =? N n =50 mm between the two solutons whch would lead to substantal water flow through the membranes; see the next queston.) V Fgure 5: A membrane separates two solutons. Potassum and chlorde are allowed to come to a Donnan equlbrum.
9 9 Queston 7.5: The effects of pressure-volume effects can be added to the electrochemcal potental by addng a term VP to the r.h.s. of Eqn. 13, where V s the partal molar volume of the th consttuent of the system and P s the pressure appled to the soluton. The partal molar volume s a constant equal to the change n volume of the soluton when a mole of the th solute s added. Thus VP can be consdered as the work requred to add a mole of the th solute aganst a pressure P. Consder a cell contanng an aqueous soluton of a sngle non-onc solute (so that z =0). The solute has concentratons C out outsde and C n nsde the cell. Show that the solute s not at equlbrum f C out C n, unless there s a dfference n pressure between the nsde and outsde of the cell. Wrte an expresson for the equlbrum pressure dfference n terms of the concentratons (ths should remnd you of the development of Eqn. 15). The osmotc pressure of a solute s usually wrtten as Π =lnc ; justfy ths termnalogy. Osmotc pressure dfferences usually lead to water flux through membranes, because f the solutes are out of equlbrum, then so s the water. Queston 8: Suppose that an aqueous soluton of NaCl has an nterface wth a solvent (ol) whch does not mx wth water. What s the equlbrum dstrbuton of Na and Cl between the two phases? Assume that, at equlbrum, the electrochemcal potentals of Na and Cl are the same n the two phases, that charge electroneutralty (Eqn. 16) holds n both phases, and that the concentratons of Na and Cl n the aqueous phase are fxed at 100 mm. Assume also that µ Na 0 (water) µ Na 0 (ol) and that µ Cl 0 (water) µ Cl 0 (ol), to account for dfferent solute/solvent nteractons n the two phases. Is there a potental dfference between the two phases at ther nterface? If so, what s ts value? The potental dfference that develops n ths stuaton s called a juncton potental. Such potentals should exst at the surfaces of the membrane models to be consdered below, but they wll be gnored, n order to focus on the propertes of the dffuson regmes nsde the membrane. Real membranes have addtonal potentals at ther surfaces, due to fxed charges on the membrane lpds (dscussed brefly by Hlle, p ). These potentals wll also be gnored. Queston 9: The on concentraton gradents n the table of Queston 5 are mantaned by actve transport. One such transport system s Na-K-ATPase, whch moves 3 Na ons out of the cell and 2 K ons nto the cell, usng the energy suppled by hydrolyss of an ATP molecule to ADP. Compute the work requred to transport 3 moles of Na and 2 moles of K under the condtons of Queston 5. You should fnd that substantal postve work s requred, meanng that the free energy of the Na and K ons ncreases when such transport occurs. In order to make the free energy of the total system decrease durng actve transport, there must be a large decrease n free energy of the ATP molecule when t s hydrolyzed to ADP and phosphate. Compare the free energy ncrease of the ons wth the free energy release of ATP hydrolys ( 60 kj/mole under cellular condtons). Nernst-Planck Equaton The goal of membrane modelng s usually computng fluxes of ons n non-equlbrum stuatons. Ths requres development of models that relate flows to forces n onc solutons. In the followng, two approaches to ths problem wll be taken. The frst depends on models of dffuson and of on transport drven by electrc felds. The second wll use the electrochemcal potental dscussed above as the potental feld drvng the flux. Consder frst the stuaton of an onc soluton of unform concentraton wth an mposed electrc feld gven by dv. The feld wll produce a force on a charge q equal to qdv. The
10 10 charge q carred by a mole of ons s gven by z F, so the force on a mole of ons due to the electrc feld s z F dv. In an aqueous soluton, the nteractons of solute and solvent molecules result n transport processes beng lmted largely by the equvalent of frctonal forces; there are no elastc forces restranng an on n a lqud soluton (.e. no lttle sprngs restrctng an on to certan postons) and the frctonal forces turn out to be larger than nertal (f=ma) forces. Thus when an on s acted on by an electrc feld, t tends to move wth a drft velocty that s proportonal to the force provded by the feld. Ths assumpton s motvated by the usual behavor of frcton, n whch the force needed to overcome frcton s proportonal to the velocty. The moblty u of an on s the rato between the drft velocty and the appled force. That s, drft = u ( force / mole)= uzf dv (17) velocty where u has unts (m/s)/(n/mole). In some texts, moblty s defned as the electrcal moblty, the rato of drft velocty to the quantty z I dv/. The flux J of the on s the number of moles of on passng through a unt area per second and s gven by J =C. x(drft velocty). Thus the on flux drven by an electrc feld s J = u C z F dv (18) law: Net flux can also be produced n soluton by concentraton gradents, as descrbed by Fck s J D dc = (19) Fck s law can be derved from a consderaton of the effects of random thermal moton of partcles n a concentraton gradent. The net flux n soluton s then the sum of Eqns. 18 and 19. Usually the expresson s smplfed by notng that u =D (Ensten relatonshp, see Feynman, pp for a dervaton). The result s the Nernst-Planck equaton. J u dc CzF dv uc d C zf dv = + = ln + (20) Another way to approach the Nernst-Planck equaton s to assume that the spatal gradent of the electrochemcal potental s the force that drves ons n soluton, that s force = -dµ /. Ths assumpton s justfed by the general relatonshp between force and work (energy), where the latter s the ntegral of the former through dstance. Wth the same defntons for moblty and flux, Eqn. 20 follows drectly from dfferentatng Eqn. 13. Thus, the Nernst-Planck equaton can be derved from ether the electrochemcal potental of equlbrum thermodynamcs or from propertes of dffuson and electrostatcs.
11 11 In order to model on transport through a cell membrane, a set of dfferental equatons lke Eqn. 20 has to be solved, one for each on. Addtonal constrants, such as charge electroneutralty, steady state, or some model for the electrcal potental are usually added. Because the term C dv/ makes the equatons non-lnear, they cannot be solved n general n closed form. Implct solutons have been obtaned, but these are dffcult to use n practce. Thus the Nernst-Planck equatons are usually solved for specal cases or usng approxmatons for dealzed stuatons. Electrcal equvalent crcut An mportant nsght nto on transport across a dffuson barrer comes from ntegratng the Nernst-Planck equaton for the stuaton shown n Fg. 6. The concentraton C(x) of an on s sketched along wth the electrcal potental profle V(x) n a membrane separatng two solutons, representng the outsde and nsde of a cell. Of course, there are other ons present, but we consder only ths one for the present. The concentraton and electrcal potental profles n Fg. 6 are smplfed n that no transtons are shown between soluton and membrane, at the outsde edges of the membrane. Such transtons exst n real membranes (Questons 8 and 11), but are gnored here. They do not affect the man results of the analyss below. Eqn. 20 expresses the chemcal flux of the on n moles/m 2 s. Because current-voltage relatonshps are of nterest, Eqn. 20 s converted to electrcal current densty by multplyng by z F, the charge per mole. Flux J s postve for net flow n the postve x drecton, as ndcated by the arrow n Fg. 6. However, electrcal current densty I s defned as postve n the opposte drecton, n order to be consstent wth the usual conventon n electrcal crcut theory, n whch current s postve when t flows from the postve sde of the voltage arrow ( V n Fg. 6). Ths s also the conventon n membrane physology, where the membrane potental s the potental nsde the cell mnus the potental outsde and current s postve n the outward drecton. The Nernst-Planck equaton n terms of current densty, wth the reversed sgn conventon, s I J V 0 d C(x) V(x) Fgure 6: A membrane separates two solutons. The concentraton C(x) of an on and the electrcal potental V(x) are shown. x nsde I z Fu C d C zf dv = ln + (21) Assume that the membrane system s n steady state. Steady state means that all parameters of the system are constant n tme, that s dc/dt = dv/dt =... = 0. Of course, ths s an dealzaton because, f there s a net flux of on through the membrane, then the concentraton must be decreasng on one sde and ncreasng on the other. That effect wll be gnored by assumng that the solutons boundng the membrane are large enough that the concentratons do not change over the perod of observaton, or by assumng that other mechansms such as actve transport mantan the concentratons. We also gnore the small electrochemcal potental gradents n soluton that are necessary to move ons to the surface of the membrane.
12 The steady-state assumpton mples that flux J and the current densty I are constant n the membrae, not functons of x. To see ths, consder Fg. 7 whch shows the flux at two ponts x and x+ n the membrane. The total amount of on n a unt area between x and x+ s C (x) and the tme rate of change of ths amount s the dfference between the flux nto ths regon and the flux out. 12 ( C ) = J( x) J( x+ ) (22) t J (x) C (x) J (x+) Dvdng through by and takng the lmt as goes to zero, x x+ C t J = (23) x Fgure 7: Relatonshp of fluxes and concentraton at two ponts n the membrane. Now n the steady state, C / t=0 so that J / x=0 also; thus n the steady state, the flux, and the current densty I are constant, ndependent of x. Now Eqn. 21 can be rearranged and ntegrated through the membrane as follows: I d 2 2 z F uc dlnc = + zf d d dv (24) Current densty I has been taken out of the ntegral on the left-hand sde because of the steady state assumpton. The ntegrals on the rght hand sde can be evaluated, gvng I d z F uc C ( d) = ln + Vd ( ) V( 0) (25) zf C ( 0) whch can be wrtten n the form IR= V E (26) where R s the ntegral on the left hand sde of Eqn. 25, V s the transmembrane potental (V(d)- V(0)), and E s the equlbrum potental for the on (Eqn. 15). Eqn. 26 s just a statement of Ohm s law for electrcal crcuts; t shows that the Nernst- Planck equaton s equvalent to the followng electrcal model for current flow through a membrane:
13 13 V outsde + nsde Fgure 8: electrcal crcut equvalent of Eqn. 26 R E I The model of Eqn. 26 and Fg. 8 separates on permeaton nto two parts: the drvng force represented by V-E and the resstance of the membrane represented by R. The drvng force s the dfference between the electrcal potental across the membrane and electrcal equvalent of the concentraton gradent, as represented by the equlbrum potental. Thus the drvng force s zero when the on s at equlbrum (Eqn. 15). The membrane resstance s generally a complex expresson whch depends on the detals of the conductance mechansm n the membrane. Note that R s a nonlnear element, the resstance of whch vares wth membrane potental and concentraton. The model of Fg. 8 s the bass for most models of current flow through membranes. The dffuson potental Eqn. 21 can be ntegrated n a dfferent way, agan for the stuaton n Fg. 6; ths ntegraton wll yeld useful nformaton about current-voltage relatonshps and membrane potentals n two specal cases. Note that [ ] = + d Ce z e dc FV / zfv / C zf dv (27) so that Eqn. 21 can be rewrtten as follows I z Fu dc C zf = + zfu d Ce dv zfv = [ ] Integratng Eqn. 28 through the membrane gves, d d (28) / e zfv / zfv d I e / zfu Ce zfv / = [ ] (29) 0 0 The current densty has been taken out of the ntegral because of the steady state assumpton. The rght hand sde can be evaluated, gvng an expresson for the current-voltage relatonshp for the on. zf V/ [ ] I z Fu C ( d ) e C ( 0) = d zfv e / 0 (30)
14 14 As n Eqn. 25, there remans one ntegral that cannot be evaluated, n ths case nvolvng the membrane potental. In one specal case, shown n Fg. 9, a useful result can be obtaned wthout evaluatng the ntegral n the denomnator of Eqn. 30. Suppose that there are only two ons A and B permeable through the membrane and suppose that z A =z B. If the system s n steady state, then the membrane potental must be constant n tme, meanng that there can be no net current flow through the membrane: I A + I = 0 (31) B Substtutng Eqn. 30 for I A and I B n Eqn. 31 gves outsde B(x) V 0 d A(x) V(x) Fgure 9: A membrane separates two solutons. The membrane s permeable only to ons A and B. The concentraton profles of the ons and the electrcal potental are shown. x nsde [ ] + [ ] = zfu Ad e zaf V/ A zfu Bd e zbf V/ ( ) ( 0) ( ) B ( 0) A A d B B d zafv/ zbfv/ e e (32) Because z A =z B, the ntegrals n the denomnator are the same. Because the value of the ntegral s non-zero for all fnte V, the ntegrals can be cancelled. Wth that and wth some rearrangement, the followng expresson relatng the transmembrane potental to the on concentratons results: V = zf uaa( 0) + ubb( 0) ln = u A( d) + u B( d) A B zf u A ln u A + u B + u B A out B out A n B n (33) where z=z A =z B and t has been assumed that the concentratons of A and B at the edges of the membrane (x=0 and x=d) are equal to the concentratons n free soluton, as drawn n Fg. 9 (but see Queston 11). Eqn. 33 s commonly used to determne the relatve moblty (or permeablty, see Queston 12) of two equal-valence ons through a membrane. The steady-state membrane potental n Eqn. 33 s a dffuson potental. It arses through the acton of the steady state assumpton, Eqn. 31. Consder the stuaton n Fg. 9. The concentraton gradents of the two ons through the membrane wll drve fluxes I A and I B. If these are not equal and opposte, then there wll be net charge transport through the membrane, whch wll produce a membrane potental. The sgn of the membrane potental wll depend on the drectons of the currents and on whch current s larger. In the stuaton of Fg. 9, suppose that A and B are catons. I A wll be negatve (net flow to the rght, usng the conventon of Fg. 6) and I B wll be postve. Suppose I A s larger n magntude than I B. Then the potental wll be postve snce net charge s flowng nto the cell. The postve potental wll ncrease I B and decrease I A ; the potental wll contnue to ncrease untl the steady state of zero charge transfer s reached. Ths s the characterstc of dffuson potentals, whch are the potentals needed to acheve a steady state of zero net charge transfer.
15 15 Queston 10: Consder what happens when the relatve moblty u A /u B ncreases to nfnty,.e. the membrane becomes sem-permeable to A only because u B goes to 0. Show that, n ths case, V approaches E A the equlbrum potental of A. What does Eqn. 30 predct about I A and I B n ths lmt? You should conclude that the net flux of both A and B go to zero n ths case, but for very dfferent reasons. Make sure you understand the dfference. Queston 11: Usually Eqn. 33 s expressed n terms of membrane permeabltes P A and P B nstead of mobltes u A and u B. The relatonshp between these two s gven by u P = β (34) d where β s the partton coeffcent, whch gves the relatve solublty of the on n the membrane vs n soluton. That s, A(0) = β A A out, see Fg. 10. Explan why A(0) and A out mght be dfferent outsde (see Queston 8). Repeat the dervaton of Eqn. 33 to show how the partton coeffcent enters nto the problem. Queston 12: Startng from Eqn. 33, as modfed n queston 11, derve an expresson for relatve permeablty P A /P B of two equal-valence ons (z A =z B ) and explan how t could be determned from expermental data. A out A(0) 0 d A(d) x A n nsde Fgure 10: Showng the effect of the partton coeffcent at the membrane surface on the concentraton profle. Queston 13: Another dffuson potental stuaton arses n the case of a sngle salt soluton whch s placed at dfferent concentratons on the two sdes of a membrane (Fg. 11). From charge electroneutralty, the concentratons of the two ons A(x) and C(x) must be equal everywhere. Use Eqn. 20 and the steady state assumpton to derve an expresson for the dffuson potental that arses n ths case. Assume that z A =-1 and z C =1. The result should have a dfferent form than Eqn. 33. Make sure you understand how these two stuatons are dfferent. Explan qualtatvely why a dffuson potental arses n ths case,.e. why a potental dfference s needed to acheve a steady state. outsde A(x)=C(x) V nsde V(x) 0 d x Fgure 11: A membrane separates two solutons contanng only one anon A and one caton C. The concentratons A(x) and C(x) are equal everywhere by charge electroneutralty. The constant-feld equaton Frequently t s assumed that the membrane potental s a lnear functon of dstance through the membrane (as drawn n Fgs. 6, 9 and 11). Whle ths can be shown to be true n one specal case (see Queston 14), t s at best an approxmaton n most cases. Nevertheless, t provdes a
16 16 useful approxmaton for many membrane currents. Wth the assumpton that V(x)= Vx/d for x=[0,d], the ntegral n the denomnator of Eqn. 30 can be evaluated, gvng the constant-feld equaton: I 2 zf V/ [ 0 ] ( zf ) u V C d e ( ) C ( ) = z F V d e / (35) 1 Fgure 12. Current-voltage plots for sodum and potassum usng the constant-feld theory. E K Normalzed current, arbtrary unts V, mv Fg. 12 shows a plot of constantfeld currents I K and I Na aganst membrane potental V, for the on concentratons lsted n Queston 5. Note that the currents go to zero at the equlbrum potentals, as expected. The currentvoltage curves are nonlnear; ths nonlnearty s called rectfcaton. The sodum current s larger for nward currents (negatve), called nward rectfcaton and the potassum current s the opposte, outward rectfcaton. The orgn of the rectfcaton n ths case s the dfference n ntracellular and extracellular concentratons. Essentally, the outward current for V>E s suppled by the ntracellular concentraton and vce versa. Thus the current wll be outward rectfyng (lke potassum) f the on concentraton s hgher nsde than outsde the cell. To further llustrate the rectfcaton behavor of these curves, consder the behavor of Eqn. 35 n the lmt as V becomes very large and postve or very large and negatve. The relevant lmts are V I const. C ( d) V and V I const. C ( 0 ) V (36) >> ( ) << ( ) The currents are asymptotcally lnear, wth a slope proportonal to the concentraton from whch the current flows. These asymptotc lnes are plotted as dashed lnes n Fg. 11 for the sodum current. Rectfcaton n membrane currents comes from two sources. One s rectfcaton due to channel conductance propertes. The rectfcaton n Fg. 12 s of ths type. The second s rectfcaton due to channel gatng, whch wll be dscussed later n the course. I Na E Na I K
17 17 The constant-feld equaton can be used to derve an expresson for a dffuson potental whch s smlar to Eqn. 33. Consder the stuaton drawn n Fg. 13 n whch there are three ons, sodum, potassum, and chlorde. The concentraton gradents are arranged n a fashon smlar to those n a real cell, except that the chlorde concentraton nsde real cells s much smaller because of negatvely charged macromolecules n cells. Consstent wth charge electroneutralty, the chlorde concentraton s equal to the sum of the sodum and potassum concentratons everywhere; ths assumes that there are no other ons present. In steady state, to gve zero net charge transfer through the membrane, outsde Cl(x) Na(x) K(x) V nsde 0 d x Fgure 13: A membrane separates two solutons contanng Na, K, and Cl at the concentratons shown. By charge electroneutralty, Cl(x)=Na(x)+K(x) (see also Queston 14). IK + INa + ICl = 0 (37) Substtutng Eqn. 35 for the three currents n Eqn. 37 and rearrangng gves the Goldman-Hodgkn- Katz equaton: V = F PK K out + PNaNaout + PCl n ln (38) PK + P Na + PCl K n Na n Cl out The propertes of ths equaton are smlar to those of Eqn. 33. Unlke Eqn. 33, Eqn. 38 depends on the constant-feld assumpton. Whle ths assumpton can be shown to be vald for the specal case of Fg. 13 (see Queston 14), t s certanly not true n general. Nevertheless, Eqn. 38 turns out to predct the behavor of data n many cases and serves as a useful approxmaton for membrane potentals. Queston 14: For the specal case dagrammed n Fg. 13, the potental n the membrane s lnear. To see ths, consder the frst form of the Nernst-Planck equaton n Eqn. 20. Form the sums all catons and anons J u zj u and (39) all catons and anons Usng these sums, the steady state assumpton, and the electroneutralty condton (Eqn. 16) you should be able to show two results: dn dv f N = C then = ( const) and = ( const) 2 z C all ons all ons (40) For the specal case of Fg. 13, you should be able to conclude from Eqn. 40 that N=(const) n the membrane and dv/=(const) n the membrane.
18 18 Queston 15: For the specal case of Fg. 13, show that the concentraton of potassum n the membrane s gven by K() 0 ( e e )+ K() d 1 e Kx ( ) = F V/ 1 e ( ) F Vx / d F V / F Vx / d To do ths, start wth the NP equaton for potassum and assume that dv/ = V/d, where d s the thckness of the membrane. As part of ths development, you should derve the constant-feld flux equaton (lke Eqn. 35, except for flux J K ). Alternatvely, you can start wth Eqn. 35 and the constant feld equaton and solve the NP equaton for K. Smlar equatons can be derved for sodum and chlorde. Nature of the cellular steady state In the models consdered above, the means by whch concentratons gradents are set up and mantaned was gnored. Of course, n a real cell, there must be actve transport mechansms to mantan the ons out of equlbrum. A varety of mechansms have been descrbed (see Läuger, 1991 for a complete descrpton). The most common mechansms n neurons nclude Na-K-ATPase, whch transports sodum and potassum aganst ther electrochemcal potental gradents (Na out of the cell, K nto the cell) usng ATP hydrolyss as the energy source (Queston 9); Ca-ATPase, whch does the same for calcum; and the Na-Ca exchanger, whch transports calcum out of the cell usng the energy n the sodum electrochemcal potental. In the presence of actve transport, the nature of the steady state equatons used above (Eqns. 31 and 37) s dfferent. For each on n the system there must be both an actve transport I A and a passve transport I P. The passve transport s descrbed by the flux equatons developed above (.e. Eqns. 30 and 35). For smlar models of actve transport, see Läuger (1991). In the steady state, the on s concentratons must be constant, so that the net flux of on through the membrane must be 0, I A + I P = 0. If ths equaton holds for every on n the system, then there can be no net flux of any on through the membrane and the net charge transfer through the membrane s guaranteed to be zero. Lookng at the system ths way, Eqns. 31 and 37 do not capture the true nature of the steady state. Apparently the true steady state n a cell s a more complex stuaton than has been consdered n dervng the tradtonal dffuson-potental models above (Eqns. 33 and 38). A natural queston s why these models apparently work for data from real cells, gven the naccuracy n the assumptons that underle them. One specal case n whch actve transport can be ncluded n the membrane-potental model occurs when only sodum and potassum are permeable through the membrane by passve transport. Ther concentratons are mantaned by actve transport through Na- K-ATPase. A characterstc of ths enzyme s that 3 Na ons are transported for each 2 K ons. The steady state equatons then become:
19 19 I A Na I K A P Na + I = 0 + I = 0 K P (41a) (41b) I A Na = ri K A (41c) The frst two equatons express the steady state condton for sodum and potassum concentraton and the thrd equaton s the transport rato for the ATPase (r=3/2). No equaton s needed to guarantee that dv/dt=0, because the frst two equatons guarantee that no net charge s transferred n ths system (assumng that no other ons are permeable). The three equatons together mply that I Na P + ri K P = 0. Usng ths as the steady state condton and substtutng Eqn. 30 for the sodum and potassum currents gves the followng equaton for the dffuson potental (the Mullns-Noda eqn.): V = F una Naout + ruk Kout ln (42) u Na + ru K Na n K n In ths case, the actve transport only changes the apparent relatve permeablty of potassum and sodum! In a real cell, the actual steady state wll nvolve a complex set of condtons lke Eqns. 41. The steady-state wll be the smultaneous soluton of ths set of equatons. The passve currents wll be represented by models lke Eqn. 35 and the actve currents wll be represented by smlar equatons that capture the membrane potental dependency of the actve transport. Queston 16: Eqn. 42 s the steady-state dffuson potental n the presence of actve transport. If the actve transport s completely blocked pharmacologcally, then the assumptons of Eqns. 31 and 33 become accurate. That s, the concentratons wll be constant (approxmately, they wll actually change slowly) and membrane potental wll equal the dffuson potental modeled by those equatons; n partcular, there wll be no actve fluxes, so the only on fluxes wll be passve. Comparng Eqns. 33 and 42 shows that the dffuson potental n the absence of actve transport s dfferent than the potental n the presence of actve transport. Ths change s expected from the fact the that Na-K-ATPase transports net charge through the membrane (3 Na n one drecton for every 2 K n the other). Ths s called an electrogenc actve transport process. Wrte an equaton for the dfference V A - V P, where V A s the membrane potental n the presence of actve transport and V P s the dffuson potental wth the actve transport blocked. The value of ths dfference should depend on the relatve permeablty of the ons, u Na /u K. What s the maxmum potental dfference that could result from an actve transport rato r=3/2? Argue that no change n potental should occur (n the short-term, before concentratons change) f a non-electrogenc actve transport s blocked. Queston 17: More nsght nto the effects of actve transport can be ganed by consderng a cell n whch there are several ons whch are transported both actvely and passvely. In the steady state, an equaton lke Eqn. 41a or 41b holds for each transported on n the system. Usng Eqn. 30 as the model for passve current flow of the on, show that the dffuson potental n ths case can be wrtten as
20 20 A C ( outsde) I V = ln zf C ( nsde) const f( V) C( nsde) (43) where f I ( V) s a functon of membrane potental related to the denomnator of Eqn. 30. If there are n ons n the system, Eqn. 43 must be true for each of them. Explan how ths can be so; that s, specfy a set of equatons and unknowns that could lead to a unque soluton for ths problem. Notce that V E I as I A 0, that s the membrane potental becomes equal to the on s equlbrum potental f the on s not actvely transported. Explan what ths means (Hnt: what happens to the on s concentraton rato f t s not transported?). References: The followng sources were used n preparng these notes. Feynman, R.P., Leghton, R.B., and Sands, M. Lectures on Physcs, Volume 1. Addson-Wesley, Readng MA (1963). Fredman, M.H. Prncples and Models of Bologcal Transport. Sprnger-Verlag, Berln (1986). Katchalsky, A. and Curran, P.F. Nonequlbrum Thermodynamcs n Bophyscs. Harvard Unv. Press, Cambrdge (1965). Läuger, P. Electrogenc Ion Pumps. Snauer Assoc., Sunderland, MA (1991).
Open Systems: Chemical Potential and Partial Molar Quantities Chemical Potential
 Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
...Thermodynamics. If Clausius Clapeyron fails. l T (v 2 v 1 ) = 0/0 Second order phase transition ( S, v = 0)
 If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
Solution Thermodynamics
 Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
Introduction to Vapor/Liquid Equilibrium, part 2. Raoult s Law:
 CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
Temperature. Chapter Heat Engine
 Chapter 3 Temperature In prevous chapters of these notes we ntroduced the Prncple of Maxmum ntropy as a technque for estmatng probablty dstrbutons consstent wth constrants. In Chapter 9 we dscussed the
Chapter 3 Temperature In prevous chapters of these notes we ntroduced the Prncple of Maxmum ntropy as a technque for estmatng probablty dstrbutons consstent wth constrants. In Chapter 9 we dscussed the
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM
 ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
Week3, Chapter 4. Position and Displacement. Motion in Two Dimensions. Instantaneous Velocity. Average Velocity
 Week3, Chapter 4 Moton n Two Dmensons Lecture Quz A partcle confned to moton along the x axs moves wth constant acceleraton from x =.0 m to x = 8.0 m durng a 1-s tme nterval. The velocty of the partcle
Week3, Chapter 4 Moton n Two Dmensons Lecture Quz A partcle confned to moton along the x axs moves wth constant acceleraton from x =.0 m to x = 8.0 m durng a 1-s tme nterval. The velocty of the partcle
Thermodynamics General
 Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
Thermodynamics Second Law Entropy
 Thermodynamcs Second Law Entropy Lana Sherdan De Anza College May 8, 2018 Last tme the Boltzmann dstrbuton (dstrbuton of energes) the Maxwell-Boltzmann dstrbuton (dstrbuton of speeds) the Second Law of
Thermodynamcs Second Law Entropy Lana Sherdan De Anza College May 8, 2018 Last tme the Boltzmann dstrbuton (dstrbuton of energes) the Maxwell-Boltzmann dstrbuton (dstrbuton of speeds) the Second Law of
9 Derivation of Rate Equations from Single-Cell Conductance (Hodgkin-Huxley-like) Equations
 Physcs 171/271 - Chapter 9R -Davd Klenfeld - Fall 2005 9 Dervaton of Rate Equatons from Sngle-Cell Conductance (Hodgkn-Huxley-lke) Equatons We consder a network of many neurons, each of whch obeys a set
Physcs 171/271 - Chapter 9R -Davd Klenfeld - Fall 2005 9 Dervaton of Rate Equatons from Sngle-Cell Conductance (Hodgkn-Huxley-lke) Equatons We consder a network of many neurons, each of whch obeys a set
Supplementary Notes for Chapter 9 Mixture Thermodynamics
 Supplementary Notes for Chapter 9 Mxture Thermodynamcs Key ponts Nne major topcs of Chapter 9 are revewed below: 1. Notaton and operatonal equatons for mxtures 2. PVTN EOSs for mxtures 3. General effects
Supplementary Notes for Chapter 9 Mxture Thermodynamcs Key ponts Nne major topcs of Chapter 9 are revewed below: 1. Notaton and operatonal equatons for mxtures 2. PVTN EOSs for mxtures 3. General effects
1 Derivation of Rate Equations from Single-Cell Conductance (Hodgkin-Huxley-like) Equations
 Physcs 171/271 -Davd Klenfeld - Fall 2005 (revsed Wnter 2011) 1 Dervaton of Rate Equatons from Sngle-Cell Conductance (Hodgkn-Huxley-lke) Equatons We consder a network of many neurons, each of whch obeys
Physcs 171/271 -Davd Klenfeld - Fall 2005 (revsed Wnter 2011) 1 Dervaton of Rate Equatons from Sngle-Cell Conductance (Hodgkn-Huxley-lke) Equatons We consder a network of many neurons, each of whch obeys
8 Derivation of Network Rate Equations from Single- Cell Conductance Equations
 Physcs 178/278 - Davd Klenfeld - Wnter 2015 8 Dervaton of Network Rate Equatons from Sngle- Cell Conductance Equatons We consder a network of many neurons, each of whch obeys a set of conductancebased,
Physcs 178/278 - Davd Klenfeld - Wnter 2015 8 Dervaton of Network Rate Equatons from Sngle- Cell Conductance Equatons We consder a network of many neurons, each of whch obeys a set of conductancebased,
4.2 Chemical Driving Force
 4.2. CHEMICL DRIVING FORCE 103 4.2 Chemcal Drvng Force second effect of a chemcal concentraton gradent on dffuson s to change the nature of the drvng force. Ths s because dffuson changes the bondng n a
4.2. CHEMICL DRIVING FORCE 103 4.2 Chemcal Drvng Force second effect of a chemcal concentraton gradent on dffuson s to change the nature of the drvng force. Ths s because dffuson changes the bondng n a
Chemical Equilibrium. Chapter 6 Spontaneity of Reactive Mixtures (gases) Taking into account there are many types of work that a sysem can perform
 Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
8 Derivation of Network Rate Equations from Single- Cell Conductance Equations
 Physcs 178/278 - Davd Klenfeld - Wnter 2019 8 Dervaton of Network Rate Equatons from Sngle- Cell Conductance Equatons Our goal to derve the form of the abstract quanttes n rate equatons, such as synaptc
Physcs 178/278 - Davd Klenfeld - Wnter 2019 8 Dervaton of Network Rate Equatons from Sngle- Cell Conductance Equatons Our goal to derve the form of the abstract quanttes n rate equatons, such as synaptc
Electrostatic Potential from Transmembrane Currents
 Electrostatc Potental from Transmembrane Currents Let s assume that the current densty j(r, t) s ohmc;.e., lnearly proportonal to the electrc feld E(r, t): j = σ c (r)e (1) wth conductvty σ c = σ c (r).
Electrostatc Potental from Transmembrane Currents Let s assume that the current densty j(r, t) s ohmc;.e., lnearly proportonal to the electrc feld E(r, t): j = σ c (r)e (1) wth conductvty σ c = σ c (r).
A particle in a state of uniform motion remain in that state of motion unless acted upon by external force.
 The fundamental prncples of classcal mechancs were lad down by Galleo and Newton n the 16th and 17th centures. In 1686, Newton wrote the Prncpa where he gave us three laws of moton, one law of gravty,
The fundamental prncples of classcal mechancs were lad down by Galleo and Newton n the 16th and 17th centures. In 1686, Newton wrote the Prncpa where he gave us three laws of moton, one law of gravty,
Physics 5153 Classical Mechanics. Principle of Virtual Work-1
 P. Guterrez 1 Introducton Physcs 5153 Classcal Mechancs Prncple of Vrtual Work The frst varatonal prncple we encounter n mechancs s the prncple of vrtual work. It establshes the equlbrum condton of a mechancal
P. Guterrez 1 Introducton Physcs 5153 Classcal Mechancs Prncple of Vrtual Work The frst varatonal prncple we encounter n mechancs s the prncple of vrtual work. It establshes the equlbrum condton of a mechancal
Lecture Note 3. Eshelby s Inclusion II
 ME340B Elastcty of Mcroscopc Structures Stanford Unversty Wnter 004 Lecture Note 3. Eshelby s Incluson II Chrs Wenberger and We Ca c All rghts reserved January 6, 004 Contents 1 Incluson energy n an nfnte
ME340B Elastcty of Mcroscopc Structures Stanford Unversty Wnter 004 Lecture Note 3. Eshelby s Incluson II Chrs Wenberger and We Ca c All rghts reserved January 6, 004 Contents 1 Incluson energy n an nfnte
Physics 5153 Classical Mechanics. D Alembert s Principle and The Lagrangian-1
 P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
and Statistical Mechanics Material Properties
 Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
STATISTICAL MECHANICS
 STATISTICAL MECHANICS Thermal Energy Recall that KE can always be separated nto 2 terms: KE system = 1 2 M 2 total v CM KE nternal Rgd-body rotaton and elastc / sound waves Use smplfyng assumptons KE of
STATISTICAL MECHANICS Thermal Energy Recall that KE can always be separated nto 2 terms: KE system = 1 2 M 2 total v CM KE nternal Rgd-body rotaton and elastc / sound waves Use smplfyng assumptons KE of
Osmotic pressure and protein binding
 Osmotc pressure and proten bndng Igor R. Kuznetsov, KochLab Symposum talk 5/15/09 Today we take a closer look at one of the soluton thermodynamcs key ponts from Steve s presentaton. Here t s: d[ln(k off
Osmotc pressure and proten bndng Igor R. Kuznetsov, KochLab Symposum talk 5/15/09 Today we take a closer look at one of the soluton thermodynamcs key ponts from Steve s presentaton. Here t s: d[ln(k off
Physics 181. Particle Systems
 Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
Review of Classical Thermodynamics
 Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
Physics 53. Rotational Motion 3. Sir, I have found you an argument, but I am not obliged to find you an understanding.
 Physcs 53 Rotatonal Moton 3 Sr, I have found you an argument, but I am not oblged to fnd you an understandng. Samuel Johnson Angular momentum Wth respect to rotatonal moton of a body, moment of nerta plays
Physcs 53 Rotatonal Moton 3 Sr, I have found you an argument, but I am not oblged to fnd you an understandng. Samuel Johnson Angular momentum Wth respect to rotatonal moton of a body, moment of nerta plays
( ) 1/ 2. ( P SO2 )( P O2 ) 1/ 2.
 Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
CHEMICAL REACTIONS AND DIFFUSION
 CHEMICAL REACTIONS AND DIFFUSION A.K.A. NETWORK THERMODYNAMICS BACKGROUND Classcal thermodynamcs descrbes equlbrum states. Non-equlbrum thermodynamcs descrbes steady states. Network thermodynamcs descrbes
CHEMICAL REACTIONS AND DIFFUSION A.K.A. NETWORK THERMODYNAMICS BACKGROUND Classcal thermodynamcs descrbes equlbrum states. Non-equlbrum thermodynamcs descrbes steady states. Network thermodynamcs descrbes
Structure and Drive Paul A. Jensen Copyright July 20, 2003
 Structure and Drve Paul A. Jensen Copyrght July 20, 2003 A system s made up of several operatons wth flow passng between them. The structure of the system descrbes the flow paths from nputs to outputs.
Structure and Drve Paul A. Jensen Copyrght July 20, 2003 A system s made up of several operatons wth flow passng between them. The structure of the system descrbes the flow paths from nputs to outputs.
PES 1120 Spring 2014, Spendier Lecture 6/Page 1
 PES 110 Sprng 014, Spender Lecture 6/Page 1 Lecture today: Chapter 1) Electrc feld due to charge dstrbutons -> charged rod -> charged rng We ntroduced the electrc feld, E. I defned t as an nvsble aura
PES 110 Sprng 014, Spender Lecture 6/Page 1 Lecture today: Chapter 1) Electrc feld due to charge dstrbutons -> charged rod -> charged rng We ntroduced the electrc feld, E. I defned t as an nvsble aura
Electrical double layer: revisit based on boundary conditions
 Electrcal double layer: revst based on boundary condtons Jong U. Km Department of Electrcal and Computer Engneerng, Texas A&M Unversty College Staton, TX 77843-318, USA Abstract The electrcal double layer
Electrcal double layer: revst based on boundary condtons Jong U. Km Department of Electrcal and Computer Engneerng, Texas A&M Unversty College Staton, TX 77843-318, USA Abstract The electrcal double layer
EPR Paradox and the Physical Meaning of an Experiment in Quantum Mechanics. Vesselin C. Noninski
 EPR Paradox and the Physcal Meanng of an Experment n Quantum Mechancs Vesseln C Nonnsk vesselnnonnsk@verzonnet Abstract It s shown that there s one purely determnstc outcome when measurement s made on
EPR Paradox and the Physcal Meanng of an Experment n Quantum Mechancs Vesseln C Nonnsk vesselnnonnsk@verzonnet Abstract It s shown that there s one purely determnstc outcome when measurement s made on
NAME and Section No.
 Chemstry 391 Fall 2007 Exam I KEY (Monday September 17) 1. (25 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). Defne the terms: open system, closed system and solated system
Chemstry 391 Fall 2007 Exam I KEY (Monday September 17) 1. (25 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). Defne the terms: open system, closed system and solated system
Lecture 7: Boltzmann distribution & Thermodynamics of mixing
 Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
Introduction to Statistical Methods
 Introducton to Statstcal Methods Physcs 4362, Lecture #3 hermodynamcs Classcal Statstcal Knetc heory Classcal hermodynamcs Macroscopc approach General propertes of the system Macroscopc varables 1 hermodynamc
Introducton to Statstcal Methods Physcs 4362, Lecture #3 hermodynamcs Classcal Statstcal Knetc heory Classcal hermodynamcs Macroscopc approach General propertes of the system Macroscopc varables 1 hermodynamc
3.1 Expectation of Functions of Several Random Variables. )' be a k-dimensional discrete or continuous random vector, with joint PMF p (, E X E X1 E X
 Statstcs 1: Probablty Theory II 37 3 EPECTATION OF SEVERAL RANDOM VARIABLES As n Probablty Theory I, the nterest n most stuatons les not on the actual dstrbuton of a random vector, but rather on a number
Statstcs 1: Probablty Theory II 37 3 EPECTATION OF SEVERAL RANDOM VARIABLES As n Probablty Theory I, the nterest n most stuatons les not on the actual dstrbuton of a random vector, but rather on a number
First Law: A body at rest remains at rest, a body in motion continues to move at constant velocity, unless acted upon by an external force.
 Secton 1. Dynamcs (Newton s Laws of Moton) Two approaches: 1) Gven all the forces actng on a body, predct the subsequent (changes n) moton. 2) Gven the (changes n) moton of a body, nfer what forces act
Secton 1. Dynamcs (Newton s Laws of Moton) Two approaches: 1) Gven all the forces actng on a body, predct the subsequent (changes n) moton. 2) Gven the (changes n) moton of a body, nfer what forces act
A quote of the week (or camel of the week): There is no expedience to which a man will not go to avoid the labor of thinking. Thomas A.
 A quote of the week (or camel of the week): here s no expedence to whch a man wll not go to avod the labor of thnkng. homas A. Edson Hess law. Algorthm S Select a reacton, possbly contanng specfc compounds
A quote of the week (or camel of the week): here s no expedence to whch a man wll not go to avod the labor of thnkng. homas A. Edson Hess law. Algorthm S Select a reacton, possbly contanng specfc compounds
Lectures - Week 4 Matrix norms, Conditioning, Vector Spaces, Linear Independence, Spanning sets and Basis, Null space and Range of a Matrix
 Lectures - Week 4 Matrx norms, Condtonng, Vector Spaces, Lnear Independence, Spannng sets and Bass, Null space and Range of a Matrx Matrx Norms Now we turn to assocatng a number to each matrx. We could
Lectures - Week 4 Matrx norms, Condtonng, Vector Spaces, Lnear Independence, Spannng sets and Bass, Null space and Range of a Matrx Matrx Norms Now we turn to assocatng a number to each matrx. We could
1. Mean-Field Theory. 2. Bjerrum length
 1. Mean-Feld Theory Contnuum models lke the Posson-Nernst-Planck equatons are mean-feld approxmatons whch descrbe how dscrete ons are affected by the mean concentratons c and potental φ. Each on mgrates
1. Mean-Feld Theory Contnuum models lke the Posson-Nernst-Planck equatons are mean-feld approxmatons whch descrbe how dscrete ons are affected by the mean concentratons c and potental φ. Each on mgrates
Numerical Heat and Mass Transfer
 Master degree n Mechancal Engneerng Numercal Heat and Mass Transfer 06-Fnte-Dfference Method (One-dmensonal, steady state heat conducton) Fausto Arpno f.arpno@uncas.t Introducton Why we use models and
Master degree n Mechancal Engneerng Numercal Heat and Mass Transfer 06-Fnte-Dfference Method (One-dmensonal, steady state heat conducton) Fausto Arpno f.arpno@uncas.t Introducton Why we use models and
Module 1 : The equation of continuity. Lecture 1: Equation of Continuity
 1 Module 1 : The equaton of contnuty Lecture 1: Equaton of Contnuty 2 Advanced Heat and Mass Transfer: Modules 1. THE EQUATION OF CONTINUITY : Lectures 1-6 () () () (v) (v) Overall Mass Balance Momentum
1 Module 1 : The equaton of contnuty Lecture 1: Equaton of Contnuty 2 Advanced Heat and Mass Transfer: Modules 1. THE EQUATION OF CONTINUITY : Lectures 1-6 () () () (v) (v) Overall Mass Balance Momentum
Lecture. Polymer Thermodynamics 0331 L Chemical Potential
 Prof. Dr. rer. nat. habl. S. Enders Faculty III for Process Scence Insttute of Chemcal Engneerng Department of Thermodynamcs Lecture Polymer Thermodynamcs 033 L 337 3. Chemcal Potental Polymer Thermodynamcs
Prof. Dr. rer. nat. habl. S. Enders Faculty III for Process Scence Insttute of Chemcal Engneerng Department of Thermodynamcs Lecture Polymer Thermodynamcs 033 L 337 3. Chemcal Potental Polymer Thermodynamcs
Energy, Entropy, and Availability Balances Phase Equilibria. Nonideal Thermodynamic Property Models. Selecting an Appropriate Model
 Lecture 4. Thermodynamcs [Ch. 2] Energy, Entropy, and Avalablty Balances Phase Equlbra - Fugactes and actvty coeffcents -K-values Nondeal Thermodynamc Property Models - P-v-T equaton-of-state models -
Lecture 4. Thermodynamcs [Ch. 2] Energy, Entropy, and Avalablty Balances Phase Equlbra - Fugactes and actvty coeffcents -K-values Nondeal Thermodynamc Property Models - P-v-T equaton-of-state models -
Physics 240: Worksheet 30 Name:
 (1) One mole of an deal monatomc gas doubles ts temperature and doubles ts volume. What s the change n entropy of the gas? () 1 kg of ce at 0 0 C melts to become water at 0 0 C. What s the change n entropy
(1) One mole of an deal monatomc gas doubles ts temperature and doubles ts volume. What s the change n entropy of the gas? () 1 kg of ce at 0 0 C melts to become water at 0 0 C. What s the change n entropy
PHYS 705: Classical Mechanics. Newtonian Mechanics
 1 PHYS 705: Classcal Mechancs Newtonan Mechancs Quck Revew of Newtonan Mechancs Basc Descrpton: -An dealzed pont partcle or a system of pont partcles n an nertal reference frame [Rgd bodes (ch. 5 later)]
1 PHYS 705: Classcal Mechancs Newtonan Mechancs Quck Revew of Newtonan Mechancs Basc Descrpton: -An dealzed pont partcle or a system of pont partcles n an nertal reference frame [Rgd bodes (ch. 5 later)]
Kernel Methods and SVMs Extension
 Kernel Methods and SVMs Extenson The purpose of ths document s to revew materal covered n Machne Learnng 1 Supervsed Learnng regardng support vector machnes (SVMs). Ths document also provdes a general
Kernel Methods and SVMs Extenson The purpose of ths document s to revew materal covered n Machne Learnng 1 Supervsed Learnng regardng support vector machnes (SVMs). Ths document also provdes a general
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2015
 Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Chapter 2. Electrode/electrolyte interface: ----Structure and properties
 Chapter 2 Electrode/electrolyte nterface: ----Structure and propertes Electrochemcal reactons are nterfacal reactons, the structure and propertes of electrode / electrolytc soluton nterface greatly nfluences
Chapter 2 Electrode/electrolyte nterface: ----Structure and propertes Electrochemcal reactons are nterfacal reactons, the structure and propertes of electrode / electrolytc soluton nterface greatly nfluences
π e ax2 dx = x 2 e ax2 dx or x 3 e ax2 dx = 1 x 4 e ax2 dx = 3 π 8a 5/2 (a) We are considering the Maxwell velocity distribution function: 2πτ/m
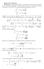 Homework Solutons Problem In solvng ths problem, we wll need to calculate some moments of the Gaussan dstrbuton. The brute-force method s to ntegrate by parts but there s a nce trck. The followng ntegrals
Homework Solutons Problem In solvng ths problem, we wll need to calculate some moments of the Gaussan dstrbuton. The brute-force method s to ntegrate by parts but there s a nce trck. The followng ntegrals
EXAM I Comparative Animal Physiology ZOO 424 Fall 2002
 EXAM I Comparatve Anmal Physology ZOO 424 Fall 2002 V Eq = RT X o. ln( [ zf [ X ) RT p K[K o pna[na o pcl[cl V = m ln F pk[k pna[na pcl[cl o I = g(v m V eq. ) Q = C m V m Drvng Force = V m V eq. Ionc Speces
EXAM I Comparatve Anmal Physology ZOO 424 Fall 2002 V Eq = RT X o. ln( [ zf [ X ) RT p K[K o pna[na o pcl[cl V = m ln F pk[k pna[na pcl[cl o I = g(v m V eq. ) Q = C m V m Drvng Force = V m V eq. Ionc Speces
Electrochemical Equilibrium Electromotive Force
 CHM465/865, 24-3, Lecture 5-7, 2 th Sep., 24 lectrochemcal qulbrum lectromotve Force Relaton between chemcal and electrc drvng forces lectrochemcal system at constant T and p: consder Gbbs free energy
CHM465/865, 24-3, Lecture 5-7, 2 th Sep., 24 lectrochemcal qulbrum lectromotve Force Relaton between chemcal and electrc drvng forces lectrochemcal system at constant T and p: consder Gbbs free energy
Math1110 (Spring 2009) Prelim 3 - Solutions
 Math 1110 (Sprng 2009) Solutons to Prelm 3 (04/21/2009) 1 Queston 1. (16 ponts) Short answer. Math1110 (Sprng 2009) Prelm 3 - Solutons x a 1 (a) (4 ponts) Please evaluate lm, where a and b are postve numbers.
Math 1110 (Sprng 2009) Solutons to Prelm 3 (04/21/2009) 1 Queston 1. (16 ponts) Short answer. Math1110 (Sprng 2009) Prelm 3 - Solutons x a 1 (a) (4 ponts) Please evaluate lm, where a and b are postve numbers.
Normally, in one phase reservoir simulation we would deal with one of the following fluid systems:
 TPG4160 Reservor Smulaton 2017 page 1 of 9 ONE-DIMENSIONAL, ONE-PHASE RESERVOIR SIMULATION Flud systems The term sngle phase apples to any system wth only one phase present n the reservor In some cases
TPG4160 Reservor Smulaton 2017 page 1 of 9 ONE-DIMENSIONAL, ONE-PHASE RESERVOIR SIMULATION Flud systems The term sngle phase apples to any system wth only one phase present n the reservor In some cases
Outline. Unit Eight Calculations with Entropy. The Second Law. Second Law Notes. Uses of Entropy. Entropy is a Property.
 Unt Eght Calculatons wth Entropy Mechancal Engneerng 370 Thermodynamcs Larry Caretto October 6, 010 Outlne Quz Seven Solutons Second law revew Goals for unt eght Usng entropy to calculate the maxmum work
Unt Eght Calculatons wth Entropy Mechancal Engneerng 370 Thermodynamcs Larry Caretto October 6, 010 Outlne Quz Seven Solutons Second law revew Goals for unt eght Usng entropy to calculate the maxmum work
Force = F Piston area = A
 CHAPTER III Ths chapter s an mportant transton between the propertes o pure substances and the most mportant chapter whch s: the rst law o thermodynamcs In ths chapter, we wll ntroduce the notons o heat,
CHAPTER III Ths chapter s an mportant transton between the propertes o pure substances and the most mportant chapter whch s: the rst law o thermodynamcs In ths chapter, we wll ntroduce the notons o heat,
CinChE Problem-Solving Strategy Chapter 4 Development of a Mathematical Model. formulation. procedure
 nhe roblem-solvng Strategy hapter 4 Transformaton rocess onceptual Model formulaton procedure Mathematcal Model The mathematcal model s an abstracton that represents the engneerng phenomena occurrng n
nhe roblem-solvng Strategy hapter 4 Transformaton rocess onceptual Model formulaton procedure Mathematcal Model The mathematcal model s an abstracton that represents the engneerng phenomena occurrng n
Inductance Calculation for Conductors of Arbitrary Shape
 CRYO/02/028 Aprl 5, 2002 Inductance Calculaton for Conductors of Arbtrary Shape L. Bottura Dstrbuton: Internal Summary In ths note we descrbe a method for the numercal calculaton of nductances among conductors
CRYO/02/028 Aprl 5, 2002 Inductance Calculaton for Conductors of Arbtrary Shape L. Bottura Dstrbuton: Internal Summary In ths note we descrbe a method for the numercal calculaton of nductances among conductors
Limited Dependent Variables
 Lmted Dependent Varables. What f the left-hand sde varable s not a contnuous thng spread from mnus nfnty to plus nfnty? That s, gven a model = f (, β, ε, where a. s bounded below at zero, such as wages
Lmted Dependent Varables. What f the left-hand sde varable s not a contnuous thng spread from mnus nfnty to plus nfnty? That s, gven a model = f (, β, ε, where a. s bounded below at zero, such as wages
CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE
 CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE Analytcal soluton s usually not possble when exctaton vares arbtrarly wth tme or f the system s nonlnear. Such problems can be solved by numercal tmesteppng
CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE Analytcal soluton s usually not possble when exctaton vares arbtrarly wth tme or f the system s nonlnear. Such problems can be solved by numercal tmesteppng
Introduction to circuit analysis. Classification of Materials
 Introducton to crcut analyss OUTLINE Electrcal quanttes Charge Current Voltage Power The deal basc crcut element Sgn conventons Current versus voltage (I-V) graph Readng: 1.2, 1.3,1.6 Lecture 2, Slde 1
Introducton to crcut analyss OUTLINE Electrcal quanttes Charge Current Voltage Power The deal basc crcut element Sgn conventons Current versus voltage (I-V) graph Readng: 1.2, 1.3,1.6 Lecture 2, Slde 1
Frequency dependence of the permittivity
 Frequency dependence of the permttvty February 7, 016 In materals, the delectrc constant and permeablty are actually frequency dependent. Ths does not affect our results for sngle frequency modes, but
Frequency dependence of the permttvty February 7, 016 In materals, the delectrc constant and permeablty are actually frequency dependent. Ths does not affect our results for sngle frequency modes, but
Physics 4B. A positive value is obtained, so the current is counterclockwise around the circuit.
 Physcs 4B Solutons to Chapter 7 HW Chapter 7: Questons:, 8, 0 Problems:,,, 45, 48,,, 7, 9 Queston 7- (a) no (b) yes (c) all te Queston 7-8 0 μc Queston 7-0, c;, a;, d; 4, b Problem 7- (a) Let be the current
Physcs 4B Solutons to Chapter 7 HW Chapter 7: Questons:, 8, 0 Problems:,,, 45, 48,,, 7, 9 Queston 7- (a) no (b) yes (c) all te Queston 7-8 0 μc Queston 7-0, c;, a;, d; 4, b Problem 7- (a) Let be the current
Moments of Inertia. and reminds us of the analogous equation for linear momentum p= mv, which is of the form. The kinetic energy of the body is.
 Moments of Inerta Suppose a body s movng on a crcular path wth constant speed Let s consder two quanttes: the body s angular momentum L about the center of the crcle, and ts knetc energy T How are these
Moments of Inerta Suppose a body s movng on a crcular path wth constant speed Let s consder two quanttes: the body s angular momentum L about the center of the crcle, and ts knetc energy T How are these
Week 9 Chapter 10 Section 1-5
 Week 9 Chapter 10 Secton 1-5 Rotaton Rgd Object A rgd object s one that s nondeformable The relatve locatons of all partcles makng up the object reman constant All real objects are deformable to some extent,
Week 9 Chapter 10 Secton 1-5 Rotaton Rgd Object A rgd object s one that s nondeformable The relatve locatons of all partcles makng up the object reman constant All real objects are deformable to some extent,
Chapter 6 Electrical Systems and Electromechanical Systems
 ME 43 Systems Dynamcs & Control Chapter 6: Electrcal Systems and Electromechancal Systems Chapter 6 Electrcal Systems and Electromechancal Systems 6. INTODUCTION A. Bazoune The majorty of engneerng systems
ME 43 Systems Dynamcs & Control Chapter 6: Electrcal Systems and Electromechancal Systems Chapter 6 Electrcal Systems and Electromechancal Systems 6. INTODUCTION A. Bazoune The majorty of engneerng systems
CHAPTER 14 GENERAL PERTURBATION THEORY
 CHAPTER 4 GENERAL PERTURBATION THEORY 4 Introducton A partcle n orbt around a pont mass or a sphercally symmetrc mass dstrbuton s movng n a gravtatonal potental of the form GM / r In ths potental t moves
CHAPTER 4 GENERAL PERTURBATION THEORY 4 Introducton A partcle n orbt around a pont mass or a sphercally symmetrc mass dstrbuton s movng n a gravtatonal potental of the form GM / r In ths potental t moves
Econ107 Applied Econometrics Topic 3: Classical Model (Studenmund, Chapter 4)
 I. Classcal Assumptons Econ7 Appled Econometrcs Topc 3: Classcal Model (Studenmund, Chapter 4) We have defned OLS and studed some algebrac propertes of OLS. In ths topc we wll study statstcal propertes
I. Classcal Assumptons Econ7 Appled Econometrcs Topc 3: Classcal Model (Studenmund, Chapter 4) We have defned OLS and studed some algebrac propertes of OLS. In ths topc we wll study statstcal propertes
The Feynman path integral
 The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
Entropy generation in a chemical reaction
 Entropy generaton n a chemcal reacton E Mranda Área de Cencas Exactas COICET CCT Mendoza 5500 Mendoza, rgentna and Departamento de Físca Unversdad aconal de San Lus 5700 San Lus, rgentna bstract: Entropy
Entropy generaton n a chemcal reacton E Mranda Área de Cencas Exactas COICET CCT Mendoza 5500 Mendoza, rgentna and Departamento de Físca Unversdad aconal de San Lus 5700 San Lus, rgentna bstract: Entropy
Physics 207 Lecture 6
 Physcs 207 Lecture 6 Agenda: Physcs 207, Lecture 6, Sept. 25 Chapter 4 Frames of reference Chapter 5 ewton s Law Mass Inerta s (contact and non-contact) Frcton (a external force that opposes moton) Free
Physcs 207 Lecture 6 Agenda: Physcs 207, Lecture 6, Sept. 25 Chapter 4 Frames of reference Chapter 5 ewton s Law Mass Inerta s (contact and non-contact) Frcton (a external force that opposes moton) Free
Problem Set #6 solution, Chem 340, Fall 2013 Due Friday, Oct 11, 2013 Please show all work for credit
 Problem Set #6 soluton, Chem 340, Fall 2013 Due Frday, Oct 11, 2013 Please show all work for credt To hand n: Atkns Chap 3 Exercses: 3.3(b), 3.8(b), 3.13(b), 3.15(b) Problems: 3.1, 3.12, 3.36, 3.43 Engel
Problem Set #6 soluton, Chem 340, Fall 2013 Due Frday, Oct 11, 2013 Please show all work for credt To hand n: Atkns Chap 3 Exercses: 3.3(b), 3.8(b), 3.13(b), 3.15(b) Problems: 3.1, 3.12, 3.36, 3.43 Engel
G4023 Mid-Term Exam #1 Solutions
 Exam1Solutons.nb 1 G03 Md-Term Exam #1 Solutons 1-Oct-0, 1:10 p.m to :5 p.m n 1 Pupn Ths exam s open-book, open-notes. You may also use prnt-outs of the homework solutons and a calculator. 1 (30 ponts,
Exam1Solutons.nb 1 G03 Md-Term Exam #1 Solutons 1-Oct-0, 1:10 p.m to :5 p.m n 1 Pupn Ths exam s open-book, open-notes. You may also use prnt-outs of the homework solutons and a calculator. 1 (30 ponts,
NUMERICAL DIFFERENTIATION
 NUMERICAL DIFFERENTIATION 1 Introducton Dfferentaton s a method to compute the rate at whch a dependent output y changes wth respect to the change n the ndependent nput x. Ths rate of change s called the
NUMERICAL DIFFERENTIATION 1 Introducton Dfferentaton s a method to compute the rate at whch a dependent output y changes wth respect to the change n the ndependent nput x. Ths rate of change s called the
8.592J: Solutions for Assignment 7 Spring 2005
 8.59J: Solutons for Assgnment 7 Sprng 5 Problem 1 (a) A flament of length l can be created by addton of a monomer to one of length l 1 (at rate a) or removal of a monomer from a flament of length l + 1
8.59J: Solutons for Assgnment 7 Sprng 5 Problem 1 (a) A flament of length l can be created by addton of a monomer to one of length l 1 (at rate a) or removal of a monomer from a flament of length l + 1
NAME and Section No. it is found that 0.6 mol of O
 NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
Transfer Functions. Convenient representation of a linear, dynamic model. A transfer function (TF) relates one input and one output: ( ) system
 Transfer Functons Convenent representaton of a lnear, dynamc model. A transfer functon (TF) relates one nput and one output: x t X s y t system Y s The followng termnology s used: x y nput output forcng
Transfer Functons Convenent representaton of a lnear, dynamc model. A transfer functon (TF) relates one nput and one output: x t X s y t system Y s The followng termnology s used: x y nput output forcng
Global Sensitivity. Tuesday 20 th February, 2018
 Global Senstvty Tuesday 2 th February, 28 ) Local Senstvty Most senstvty analyses [] are based on local estmates of senstvty, typcally by expandng the response n a Taylor seres about some specfc values
Global Senstvty Tuesday 2 th February, 28 ) Local Senstvty Most senstvty analyses [] are based on local estmates of senstvty, typcally by expandng the response n a Taylor seres about some specfc values
Electrochemistry Thermodynamics
 CHEM 51 Analytcal Electrochemstry Chapter Oct 5, 016 Electrochemstry Thermodynamcs Bo Zhang Department of Chemstry Unversty of Washngton Seattle, WA 98195 Former SEAC presdent Andy Ewng sellng T-shrts
CHEM 51 Analytcal Electrochemstry Chapter Oct 5, 016 Electrochemstry Thermodynamcs Bo Zhang Department of Chemstry Unversty of Washngton Seattle, WA 98195 Former SEAC presdent Andy Ewng sellng T-shrts
One-sided finite-difference approximations suitable for use with Richardson extrapolation
 Journal of Computatonal Physcs 219 (2006) 13 20 Short note One-sded fnte-dfference approxmatons sutable for use wth Rchardson extrapolaton Kumar Rahul, S.N. Bhattacharyya * Department of Mechancal Engneerng,
Journal of Computatonal Physcs 219 (2006) 13 20 Short note One-sded fnte-dfference approxmatons sutable for use wth Rchardson extrapolaton Kumar Rahul, S.N. Bhattacharyya * Department of Mechancal Engneerng,
3. Be able to derive the chemical equilibrium constants from statistical mechanics.
 Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
Physics 2A Chapters 6 - Work & Energy Fall 2017
 Physcs A Chapters 6 - Work & Energy Fall 017 These notes are eght pages. A quck summary: The work-energy theorem s a combnaton o Chap and Chap 4 equatons. Work s dened as the product o the orce actng on
Physcs A Chapters 6 - Work & Energy Fall 017 These notes are eght pages. A quck summary: The work-energy theorem s a combnaton o Chap and Chap 4 equatons. Work s dened as the product o the orce actng on
DC Circuits. Crossing the emf in this direction +ΔV
 DC Crcuts Delverng a steady flow of electrc charge to a crcut requres an emf devce such as a battery, solar cell or electrc generator for example. mf stands for electromotve force, but an emf devce transforms
DC Crcuts Delverng a steady flow of electrc charge to a crcut requres an emf devce such as a battery, solar cell or electrc generator for example. mf stands for electromotve force, but an emf devce transforms
Physics 607 Exam 1. ( ) = 1, Γ( z +1) = zγ( z) x n e x2 dx = 1. e x2
 Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
Psychology 282 Lecture #24 Outline Regression Diagnostics: Outliers
 Psychology 282 Lecture #24 Outlne Regresson Dagnostcs: Outlers In an earler lecture we studed the statstcal assumptons underlyng the regresson model, ncludng the followng ponts: Formal statement of assumptons.
Psychology 282 Lecture #24 Outlne Regresson Dagnostcs: Outlers In an earler lecture we studed the statstcal assumptons underlyng the regresson model, ncludng the followng ponts: Formal statement of assumptons.
Physics 114 Exam 2 Fall 2014 Solutions. Name:
 Physcs 114 Exam Fall 014 Name: For gradng purposes (do not wrte here): Queston 1. 1... 3. 3. Problem Answer each of the followng questons. Ponts for each queston are ndcated n red. Unless otherwse ndcated,
Physcs 114 Exam Fall 014 Name: For gradng purposes (do not wrte here): Queston 1. 1... 3. 3. Problem Answer each of the followng questons. Ponts for each queston are ndcated n red. Unless otherwse ndcated,
Inner Product. Euclidean Space. Orthonormal Basis. Orthogonal
 Inner Product Defnton 1 () A Eucldean space s a fnte-dmensonal vector space over the reals R, wth an nner product,. Defnton 2 (Inner Product) An nner product, on a real vector space X s a symmetrc, blnear,
Inner Product Defnton 1 () A Eucldean space s a fnte-dmensonal vector space over the reals R, wth an nner product,. Defnton 2 (Inner Product) An nner product, on a real vector space X s a symmetrc, blnear,
From Biot-Savart Law to Divergence of B (1)
 From Bot-Savart Law to Dvergence of B (1) Let s prove that Bot-Savart gves us B (r ) = 0 for an arbtrary current densty. Frst take the dvergence of both sdes of Bot-Savart. The dervatve s wth respect to
From Bot-Savart Law to Dvergence of B (1) Let s prove that Bot-Savart gves us B (r ) = 0 for an arbtrary current densty. Frst take the dvergence of both sdes of Bot-Savart. The dervatve s wth respect to
#64. ΔS for Isothermal Mixing of Ideal Gases
 #64 Carnot Heat Engne ΔS for Isothermal Mxng of Ideal Gases ds = S dt + S T V V S = P V T T V PV = nrt, P T ds = v T = nr V dv V nr V V = nrln V V = - nrln V V ΔS ΔS ΔS for Isothermal Mxng for Ideal Gases
#64 Carnot Heat Engne ΔS for Isothermal Mxng of Ideal Gases ds = S dt + S T V V S = P V T T V PV = nrt, P T ds = v T = nr V dv V nr V V = nrln V V = - nrln V V ΔS ΔS ΔS for Isothermal Mxng for Ideal Gases
CSci 6974 and ECSE 6966 Math. Tech. for Vision, Graphics and Robotics Lecture 21, April 17, 2006 Estimating A Plane Homography
 CSc 6974 and ECSE 6966 Math. Tech. for Vson, Graphcs and Robotcs Lecture 21, Aprl 17, 2006 Estmatng A Plane Homography Overvew We contnue wth a dscusson of the major ssues, usng estmaton of plane projectve
CSc 6974 and ECSE 6966 Math. Tech. for Vson, Graphcs and Robotcs Lecture 21, Aprl 17, 2006 Estmatng A Plane Homography Overvew We contnue wth a dscusson of the major ssues, usng estmaton of plane projectve
ANSWERS. Problem 1. and the moment generating function (mgf) by. defined for any real t. Use this to show that E( U) var( U)
 Econ 413 Exam 13 H ANSWERS Settet er nndelt 9 deloppgaver, A,B,C, som alle anbefales å telle lkt for å gøre det ltt lettere å stå. Svar er gtt . Unfortunately, there s a prntng error n the hnt of
Econ 413 Exam 13 H ANSWERS Settet er nndelt 9 deloppgaver, A,B,C, som alle anbefales å telle lkt for å gøre det ltt lettere å stå. Svar er gtt . Unfortunately, there s a prntng error n the hnt of
Thermodynamics and statistical mechanics in materials modelling II
 Course MP3 Lecture 8/11/006 (JAE) Course MP3 Lecture 8/11/006 Thermodynamcs and statstcal mechancs n materals modellng II A bref résumé of the physcal concepts used n materals modellng Dr James Ellott.1
Course MP3 Lecture 8/11/006 (JAE) Course MP3 Lecture 8/11/006 Thermodynamcs and statstcal mechancs n materals modellng II A bref résumé of the physcal concepts used n materals modellng Dr James Ellott.1
College of Computer & Information Science Fall 2009 Northeastern University 20 October 2009
 College of Computer & Informaton Scence Fall 2009 Northeastern Unversty 20 October 2009 CS7880: Algorthmc Power Tools Scrbe: Jan Wen and Laura Poplawsk Lecture Outlne: Prmal-dual schema Network Desgn:
College of Computer & Informaton Scence Fall 2009 Northeastern Unversty 20 October 2009 CS7880: Algorthmc Power Tools Scrbe: Jan Wen and Laura Poplawsk Lecture Outlne: Prmal-dual schema Network Desgn:
Difference Equations
 Dfference Equatons c Jan Vrbk 1 Bascs Suppose a sequence of numbers, say a 0,a 1,a,a 3,... s defned by a certan general relatonshp between, say, three consecutve values of the sequence, e.g. a + +3a +1
Dfference Equatons c Jan Vrbk 1 Bascs Suppose a sequence of numbers, say a 0,a 1,a,a 3,... s defned by a certan general relatonshp between, say, three consecutve values of the sequence, e.g. a + +3a +1
Spin-rotation coupling of the angularly accelerated rigid body
 Spn-rotaton couplng of the angularly accelerated rgd body Loua Hassan Elzen Basher Khartoum, Sudan. Postal code:11123 E-mal: louaelzen@gmal.com November 1, 2017 All Rghts Reserved. Abstract Ths paper s
Spn-rotaton couplng of the angularly accelerated rgd body Loua Hassan Elzen Basher Khartoum, Sudan. Postal code:11123 E-mal: louaelzen@gmal.com November 1, 2017 All Rghts Reserved. Abstract Ths paper s
χ x B E (c) Figure 2.1.1: (a) a material particle in a body, (b) a place in space, (c) a configuration of the body
 Secton.. Moton.. The Materal Body and Moton hyscal materals n the real world are modeled usng an abstract mathematcal entty called a body. Ths body conssts of an nfnte number of materal partcles. Shown
Secton.. Moton.. The Materal Body and Moton hyscal materals n the real world are modeled usng an abstract mathematcal entty called a body. Ths body conssts of an nfnte number of materal partcles. Shown
Physics 207: Lecture 20. Today s Agenda Homework for Monday
 Physcs 207: Lecture 20 Today s Agenda Homework for Monday Recap: Systems of Partcles Center of mass Velocty and acceleraton of the center of mass Dynamcs of the center of mass Lnear Momentum Example problems
Physcs 207: Lecture 20 Today s Agenda Homework for Monday Recap: Systems of Partcles Center of mass Velocty and acceleraton of the center of mass Dynamcs of the center of mass Lnear Momentum Example problems
Week 8: Chapter 9. Linear Momentum. Newton Law and Momentum. Linear Momentum, cont. Conservation of Linear Momentum. Conservation of Momentum, 2
 Lnear omentum Week 8: Chapter 9 Lnear omentum and Collsons The lnear momentum of a partcle, or an object that can be modeled as a partcle, of mass m movng wth a velocty v s defned to be the product of
Lnear omentum Week 8: Chapter 9 Lnear omentum and Collsons The lnear momentum of a partcle, or an object that can be modeled as a partcle, of mass m movng wth a velocty v s defned to be the product of
Lecture 12. Transport in Membranes (2)
 Lecture 12. Transport n embranes (2) odule Flow Patterns - Perfect mxng - Countercurrent flow - Cocurrent flow - Crossflow embrane Cascades External ass-transfer Resstances Concentraton Polarzaton and
Lecture 12. Transport n embranes (2) odule Flow Patterns - Perfect mxng - Countercurrent flow - Cocurrent flow - Crossflow embrane Cascades External ass-transfer Resstances Concentraton Polarzaton and
