å Q d = 0 T dq =0 ò T reversible processes 1
|
|
|
- Christian Norton
- 6 years ago
- Views:
Transcription
1 δ reeribe ree d
2 Caiu Inequaity fr an Irreeribe Cye eeribe Cye fr a
3 CaiuInequaity fr a yemed f a reeribe andan irreeribe re irre re Entry Definitin and Cange DEFINE A POPEY S S re irre S S S ENOPY reeribe irreeribe d irre irre d d irre d d reeribe internay reeribe re
4 taentry Cange,S,fr an iatedytem Fr a Fr an reeribe ytem, irreeribe ytem, S S > 4
5 Cmetey eeribe Pre - Sytem and urrunding returned t te rigina tate Internay eeribe - Sytem returned t te rigina tate. Externay eeribe - Surrunding returned t te rigina tate. 5
6 Heat tranfer ar a temerature differene i an irreeribe re. b/e f team i ndened at 65 C by -4a biing at 6 C. m S S S 4 a 4 a 4a team 4a m 45.4 J/e 9. J/g m ISOLAED SYSEM team 4 a team m S m 4 a fg 6 ( ) g/e ( ) ( ) 6.86 g/e ( ) 4a team S 65 C 6.86 g/e fg team C g/e 45.4 J/g J/g J/g 7.44 J/g Ke Ke Ke 6
7 Heat tranfer ar a temerature differene i a reeribe ( an a imibe) re. b/e f team i ndened at 6 C by -4a biing at 6 C. Fr ideaized eat tranfer at a ntant 6 C m S S S 4a 4a 4a team 4a m 57.7 J/e 9.J/g m ISOLAED SYSEM team 4a team m 4a S m fg 6 ( ) g/e ( ) ( ) g/e ( ) 4a team S 6 C g/e fg team C g/e 57.7 J/g J/g J/g 7.87 J/g K e K e K e 7
8 emerature Entry Prerty Diagram ds fr ntant, ds ntant S W in ut net HS L S in ut ( )S H L 8
9 emerature Entry Prerty Diagram Water 9
10 Entry Cange f an Idea Ga Law Send d δq Law Firt δw du δq d d d d du Fr an ideaga: d du d d du d d d d d d d d Fr an idea ga, d d d d - d d d d d d d d du d Subtituting int d d d du d d du d u
11 Oxygen at.8 ubi meter/g and 5 C i mreed in a itn yinder t. ubi meter/g and 87 C. Wat i te entry ange f te xygen? 87 C 5 C.m /g.8 m /g J.69 g K J.5 g K 7.5 K K 5 C.59 C J g K.m /g.8 m /g 6-6
12 Steam at C and Pa ndene in a iter ed radiatr wit bt te inet and exit ae ed t a temerature f 8 C.Determine te entry ange f te P 8 V x f.849 m g 7.58J/g.9 V f fg C f x m g, C.45 m g fg g Ced, ed team radiatr m S S S V S S S 8 C m C Pa Vnt. m.849 m /g ( ) ( ) J K.85 g 6-
13 Ientri Adiabati Pre IdeaGa Ientri, Adiabati ntant ntant integrating d _ d d d d d d d d d, ga an idea fr d d W du Adiabati re Law Firt W du ( ) ( ) ( ) ( ) i a ntant entry ntant adiabati re, ubtitute frm ntant
14 U W d ubitiuting, d ubitiuting, d d fr d d d dw du du dw d d ds dv d du d u d d Firt Law du d d Send Law Bundary Wr u rerty definitin rerty definitin, i an exat differentia d d d d w Exame: water umed frm ia t ia ( ) ( ia 5 ia) 44 f/i w 6.4b/ft bf 6 ft - bf w ft 4.6, (ft f fuid) bm 6.4 bm ft ft ft bf BU w BU/b b 778 ft b m Exame: water umed frm Pa t C w w.86 ( ) ( ) w.4 m g Pa, f J/g 4 m
15 Exanin Pre Atua Wr Ientri Wr w w atua ientri a 5
16 g/e f team exand in an 9 % effiient turbine frm 8 MPa, 5 C t Pa. Determine te exit temerature and wer f te turbine. Pt ' C C 8 MPa Pa Pa W ( W.9 ' ' f J/g W W x x ( ) W atura reereibe i e tan aturatin fg ' ) g fg g Pa 69.9 C 9. J/g tw ae Pwer m W g/e 9.J/g 57 KW
17 Wat i te wr dne by g/e f nitrgen exanded frm 9 Pa, 5 C t Pa at an 85% effiieny. 9 Pa 5 C i i i i W W O 7.4 K Watua.85 W atua atua ( 5 7)(.58) ientri.85 g/e J O ( K 7.4 K) i Pa 7
18 Cmrein Pre Ientri Wr Atua Wr W ientri W atua a 8
19 @ Pa, V Pt i. ubi meter/e f -4a i mreed at 8% effiieny frm aturated ar at Pa t Ma. Wat i te diarge temerature and wr? g g g J/g 5 6./ entry rati J/g.6m /g g/e K C i a) b) 6.97 ( )/ J/g by interatin i ( O i W m.8 C by interatin ( ) ) /.8 W.8 g/e i ( ) W.65 J/e.65 KW MPa Pa 9 6-
20 Air i mreed frm 5 ia, 6 F t ia at 85% effiieny. Wat i te atua diarge temerature? i ia.857 i 5 i 5.9 i 6.9 ( i ) ( ) F O 654. η 85% 5 ia, 6 F
21 W Exanin Pre Steady Fw Energy Equatin H ntant atua ientri a V V atua V V a ientri
22 AI ABLE - abe A-, A-E a) ariabe u eifi ( ) ( ) d d d eat fr u, and. d Setin.7, Setin age 6.., abe bae age F, C b) de te,, (tabe (tabe r r ) ) auatin wit ariabe Setin 6.7., age 56 fr an ientri re ( tabe (tabe r ) ) r
23 temerature, K, K, , ( ) K ntant aue, 9 ariabe eifi eat, r Air underge an adiabati, ientri exanin frm 9 Ka, K t Pa. r r r r r ( ) , idea Figure abe A 7 r ga 56 aue, K K K Figure 4-4 abe A -7 Uing Idea Ga Law r r.78 m /g 56.66m /g, aw gie a gd 9 Pa Pa m /g Pa r r r m /g.86. 9Pa (amt n differene. Idea ga ume etimate)
The Second Law implies:
 e Send Law ilie: ) Heat Engine η W in H H L H L H, H H ) Ablute eerature H H L L Sale, L L W ) Fr a yle H H L L H 4) Fr an Ideal Ga Cyle H H L L L δ reerible ree d Claiu Inequality δ eerible Cyle fr a
e Send Law ilie: ) Heat Engine η W in H H L H L H, H H ) Ablute eerature H H L L Sale, L L W ) Fr a yle H H L L H 4) Fr an Ideal Ga Cyle H H L L L δ reerible ree d Claiu Inequality δ eerible Cyle fr a
SIMPLE RANKINE CYCLE. 3 expander. boiler. pump. condenser 1 W Q. cycle cycle. net. shaft
 SIMPLE RANKINE CYCLE um boiler exander condener Steady Flow, Oen Sytem - region ace Steady Flow Energy Equation for Procee m (u Pum Proce,, Boiler Proce,, V ρg) 0, 0, Exanion Proce,, 0, Condener Proce,,
SIMPLE RANKINE CYCLE um boiler exander condener Steady Flow, Oen Sytem - region ace Steady Flow Energy Equation for Procee m (u Pum Proce,, Boiler Proce,, V ρg) 0, 0, Exanion Proce,, 0, Condener Proce,,
6-5. H 2 O 200 kpa 200 C Q. Entropy Changes of Pure Substances
 Canges f ure Substances 6-0C Yes, because an ternally reversible, adiabatic prcess vlves n irreversibilities r eat transfer. 6- e radiatr f a steam eatg system is itially filled wit supereated steam. e
Canges f ure Substances 6-0C Yes, because an ternally reversible, adiabatic prcess vlves n irreversibilities r eat transfer. 6- e radiatr f a steam eatg system is itially filled wit supereated steam. e
NEWCOMEN ATMOSPHERIC ENGINE
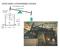 NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m F 7 stroke 5 sia steam 8 50 F water NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m 5 sia steam 8 F 50 F water 7
NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m F 7 stroke 5 sia steam 8 50 F water NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m 5 sia steam 8 F 50 F water 7
P=1 atm. vapor. liquid
 P1 atm ar liqid Q Gien and P Ser Heat Regin i, P < P sat > sat Cmressed Liqid Regin i, P > P sat < sat SEAM PRESUE AND EMPERAURE ABLES Satrated Liqid Line Satrated Var Line s s g g g g ree ables emeratre
P1 atm ar liqid Q Gien and P Ser Heat Regin i, P < P sat > sat Cmressed Liqid Regin i, P > P sat < sat SEAM PRESUE AND EMPERAURE ABLES Satrated Liqid Line Satrated Var Line s s g g g g ree ables emeratre
KNOWN: Air undergoes a polytropic process in a piston-cylinder assembly. The work is known.
 PROBLEM.7 A hown in Fig. P.7, 0 ft of air at T = 00 o R, 00 lbf/in. undergoe a polytropic expanion to a final preure of 5.4 lbf/in. The proce follow pv. = contant. The work i W = 94.4 Btu. Auming ideal
PROBLEM.7 A hown in Fig. P.7, 0 ft of air at T = 00 o R, 00 lbf/in. undergoe a polytropic expanion to a final preure of 5.4 lbf/in. The proce follow pv. = contant. The work i W = 94.4 Btu. Auming ideal
Thermodynamics EAS 204 Spring 2004 Class Month Day Chapter Topic Reading Due 1 January 12 M Introduction 2 14 W Chapter 1 Concepts Chapter 1 19 M MLK
 Thermdynamics EAS 204 Spring 2004 Class Mnth Day Chapter Tpic Reading Due 1 January 12 M Intrductin 2 14 W Chapter 1 Cncepts Chapter 1 19 M MLK Hliday n class 3 21 W Chapter 2 Prperties Chapter 2 PS1 4
Thermdynamics EAS 204 Spring 2004 Class Mnth Day Chapter Tpic Reading Due 1 January 12 M Intrductin 2 14 W Chapter 1 Cncepts Chapter 1 19 M MLK Hliday n class 3 21 W Chapter 2 Prperties Chapter 2 PS1 4
= T. (kj/k) (kj/k) 0 (kj/k) int rev. Chapter 6 SUMMARY
 Capter 6 SUMMARY e second la of termodynamics leads to te definition of a ne property called entropy ic is a quantitative measure of microscopic disorder for a system. e definition of entropy is based
Capter 6 SUMMARY e second la of termodynamics leads to te definition of a ne property called entropy ic is a quantitative measure of microscopic disorder for a system. e definition of entropy is based
EVAPORATION. Robert evaporator. Balance equations Material balance (total) Component balance. Heat balance
 EAPORATION Roert eorator Balance equation Material alance (total) S S=Solution =aor =Steam K=Steam condenate S Comonent alance S S Heat alance S S v Merkel lot can e ued for otaining entaly data Heat ower
EAPORATION Roert eorator Balance equation Material alance (total) S S=Solution =aor =Steam K=Steam condenate S Comonent alance S S Heat alance S S v Merkel lot can e ued for otaining entaly data Heat ower
EF 152 Exam #3, Fall, 2012 Page 1 of 6
 EF 5 Exam #3, Fall, 0 Page of 6 Name: Setion: Guidelines: ssume 3 signifiant figures for all given numbers. Sow all of your work no work, no redit Write your final answer in te box provided - inlude units
EF 5 Exam #3, Fall, 0 Page of 6 Name: Setion: Guidelines: ssume 3 signifiant figures for all given numbers. Sow all of your work no work, no redit Write your final answer in te box provided - inlude units
Entropy and the Second Law of Thermodynamics
 Entropy and the Second Law of Thermodynamics Reading Problems 7-1 7-3 7-88, 7-131, 7-135 7-6 7-10 8-24, 8-44, 8-46, 8-60, 8-73, 8-99, 8-128, 8-132, 8-1 8-10, 8-13 8-135, 8-148, 8-152, 8-166, 8-168, 8-189
Entropy and the Second Law of Thermodynamics Reading Problems 7-1 7-3 7-88, 7-131, 7-135 7-6 7-10 8-24, 8-44, 8-46, 8-60, 8-73, 8-99, 8-128, 8-132, 8-1 8-10, 8-13 8-135, 8-148, 8-152, 8-166, 8-168, 8-189
Instructions: Show all work for complete credit. Work in symbols first, plugging in numbers and performing calculations last. / 26.
 CM ROSE-HULMAN INSTITUTE OF TECHNOLOGY Name Circle sectin: 01 [4 th Lui] 02 [5 th Lui] 03 [4 th Thm] 04 [5 th Thm] 05 [4 th Mech] ME301 Applicatins f Thermdynamics Exam 1 Sep 29, 2017 Rules: Clsed bk/ntes
CM ROSE-HULMAN INSTITUTE OF TECHNOLOGY Name Circle sectin: 01 [4 th Lui] 02 [5 th Lui] 03 [4 th Thm] 04 [5 th Thm] 05 [4 th Mech] ME301 Applicatins f Thermdynamics Exam 1 Sep 29, 2017 Rules: Clsed bk/ntes
Longitudinal Dispersion
 Updated: 3 Otber 017 Print verin Leture #10 (River & Stream, nt) Chapra, L14 (nt.) David A. Rekhw CEE 577 #10 1 Lngitudinal Diperin Frm Fiher et al., 1979 m/ m -1 E U B 0 011 HU. * Width (m) Where the
Updated: 3 Otber 017 Print verin Leture #10 (River & Stream, nt) Chapra, L14 (nt.) David A. Rekhw CEE 577 #10 1 Lngitudinal Diperin Frm Fiher et al., 1979 m/ m -1 E U B 0 011 HU. * Width (m) Where the
Previous lecture. Today lecture
 Previous lecture ds relations (derive from steady energy balance) Gibb s equations Entropy change in liquid and solid Equations of & v, & P, and P & for steady isentropic process of ideal gas Isentropic
Previous lecture ds relations (derive from steady energy balance) Gibb s equations Entropy change in liquid and solid Equations of & v, & P, and P & for steady isentropic process of ideal gas Isentropic
Thermodynamics I Chapter 6 Entropy
 hermodynamics I hater 6 Entroy Mohsin Mohd ies Fakulti Keuruteraan Mekanikal, Uniersiti eknologi Malaysia Entroy (Motiation) he referred direction imlied by the nd Law can be better understood and quantified
hermodynamics I hater 6 Entroy Mohsin Mohd ies Fakulti Keuruteraan Mekanikal, Uniersiti eknologi Malaysia Entroy (Motiation) he referred direction imlied by the nd Law can be better understood and quantified
K E L LY T H O M P S O N
 K E L LY T H O M P S O N S E A O LO G Y C R E ATO R, F O U N D E R, A N D PA R T N E R K e l l y T h o m p s o n i s t h e c r e a t o r, f o u n d e r, a n d p a r t n e r o f S e a o l o g y, a n e x
K E L LY T H O M P S O N S E A O LO G Y C R E ATO R, F O U N D E R, A N D PA R T N E R K e l l y T h o m p s o n i s t h e c r e a t o r, f o u n d e r, a n d p a r t n e r o f S e a o l o g y, a n e x
P a g e 5 1 of R e p o r t P B 4 / 0 9
 P a g e 5 1 of R e p o r t P B 4 / 0 9 J A R T a l s o c o n c l u d e d t h a t a l t h o u g h t h e i n t e n t o f N e l s o n s r e h a b i l i t a t i o n p l a n i s t o e n h a n c e c o n n e
P a g e 5 1 of R e p o r t P B 4 / 0 9 J A R T a l s o c o n c l u d e d t h a t a l t h o u g h t h e i n t e n t o f N e l s o n s r e h a b i l i t a t i o n p l a n i s t o e n h a n c e c o n n e
7-84. Chapter 7 External Forced Convection
 Chapter 7 External Frced Cnvectin 7-99 Wind i blwing ver the rf f a hue. The rate f heat tranfer thrugh the rf and the ct f thi heat l fr -h perid are t be deterined. Auptin Steady perating cnditin exit.
Chapter 7 External Frced Cnvectin 7-99 Wind i blwing ver the rf f a hue. The rate f heat tranfer thrugh the rf and the ct f thi heat l fr -h perid are t be deterined. Auptin Steady perating cnditin exit.
Kelvin Planck Statement of the Second Law. Clausius Statement of the Second Law
 Kelv Planck Statement of te Second aw It is imossible to construct an enge wic, oeratg a cycle, will roduce no oter effect tan te extraction of eat from a sgle reservoir and te erformance of an equivalent
Kelv Planck Statement of te Second aw It is imossible to construct an enge wic, oeratg a cycle, will roduce no oter effect tan te extraction of eat from a sgle reservoir and te erformance of an equivalent
Developing Transfer Functions from Heat & Material Balances
 Colorado Sool of Mine CHEN43 Stirred ank Heater Develoing ranfer untion from Heat & Material Balane Examle ranfer untion Stirred ank Heater,,, A,,,,, We will develo te tranfer funtion for a tirred tank
Colorado Sool of Mine CHEN43 Stirred ank Heater Develoing ranfer untion from Heat & Material Balane Examle ranfer untion Stirred ank Heater,,, A,,,,, We will develo te tranfer funtion for a tirred tank
c Dr. Md. Zahurul Haq (BUET) Thermodynamic Processes & Efficiency ME 6101 (2017) 2 / 25 T145 = Q + W cv + i h 2 = h (V2 1 V 2 2)
 Thermodynamic Processes & Isentropic Efficiency Dr. Md. Zahurul Haq Professor Department of Mechanical Engineering Bangladesh University of Engineering & Technology (BUET Dhaka-1000, Bangladesh zahurul@me.buet.ac.bd
Thermodynamic Processes & Isentropic Efficiency Dr. Md. Zahurul Haq Professor Department of Mechanical Engineering Bangladesh University of Engineering & Technology (BUET Dhaka-1000, Bangladesh zahurul@me.buet.ac.bd
FP2 Mark Schemes from old P4, P5, P6 and FP1, FP2, FP3 papers (back to June 2002)
 FP Mark Schemes from old P, P5, P6 and FP, FP, FP papers (back to June 00) Please note that the following pages contain mark schemes for questions from past papers. The standard of the mark schemes is
FP Mark Schemes from old P, P5, P6 and FP, FP, FP papers (back to June 00) Please note that the following pages contain mark schemes for questions from past papers. The standard of the mark schemes is
Thermodynamics Lecture Series
 Termodynamics Lecture Series Ideal Ranke Cycle Te Practical Cycle Applied Sciences Education Researc Group (ASERG) Faculty of Applied Sciences Universiti Teknologi MARA email: drjjlanita@otmail.com ttp://www5.uitm.edu.my/faculties/fsg/drjj1.tml
Termodynamics Lecture Series Ideal Ranke Cycle Te Practical Cycle Applied Sciences Education Researc Group (ASERG) Faculty of Applied Sciences Universiti Teknologi MARA email: drjjlanita@otmail.com ttp://www5.uitm.edu.my/faculties/fsg/drjj1.tml
ATMOS Lecture 7. The First Law and Its Consequences Pressure-Volume Work Internal Energy Heat Capacity Special Cases of the First Law
 TMOS 5130 Lecture 7 The First Law and Its Consequences Pressure-Volume Work Internal Energy Heat Caacity Secial Cases of the First Law Pressure-Volume Work Exanding Volume Pressure δw = f & dx δw = F ds
TMOS 5130 Lecture 7 The First Law and Its Consequences Pressure-Volume Work Internal Energy Heat Caacity Secial Cases of the First Law Pressure-Volume Work Exanding Volume Pressure δw = f & dx δw = F ds
**YOU ARE NOT ALLOWED TO TAKE SPARE COPIES OF THIS EXAM FROM THE TESTING ROOM**
 EM 233, Fall 2017 Midterm #2 Ian R. Gould MPLETE TIS SETIN : Up to TW PINTS will be removed for incorrect/missing information! PRINTED FIRST PRINTED LAST Person on your LEFT (or Empty or Aisle) Person
EM 233, Fall 2017 Midterm #2 Ian R. Gould MPLETE TIS SETIN : Up to TW PINTS will be removed for incorrect/missing information! PRINTED FIRST PRINTED LAST Person on your LEFT (or Empty or Aisle) Person
REVIEW SHEET 1 SOLUTIONS ( ) ( ) ( ) x 2 ( ) t + 2. t x +1. ( x 2 + x +1 + x 2 # x ) 2 +1 x ( 1 +1 x +1 x #1 x ) = 2 2 = 1
 REVIEW SHEET SOLUTIONS Limit Concepts and Problems + + + e sin t + t t + + + + + e sin t + t t e cos t + + t + + + + + + + + + + + + + t + + t + t t t + + + + + + + + + + + + + + + + t + + a b c - d DNE
REVIEW SHEET SOLUTIONS Limit Concepts and Problems + + + e sin t + t t + + + + + e sin t + t t e cos t + + t + + + + + + + + + + + + + t + + t + t t t + + + + + + + + + + + + + + + + t + + a b c - d DNE
P a g e 3 6 of R e p o r t P B 4 / 0 9
 P a g e 3 6 of R e p o r t P B 4 / 0 9 p r o t e c t h um a n h e a l t h a n d p r o p e r t y fr om t h e d a n g e rs i n h e r e n t i n m i n i n g o p e r a t i o n s s u c h a s a q u a r r y. J
P a g e 3 6 of R e p o r t P B 4 / 0 9 p r o t e c t h um a n h e a l t h a n d p r o p e r t y fr om t h e d a n g e rs i n h e r e n t i n m i n i n g o p e r a t i o n s s u c h a s a q u a r r y. J
ADARSHA H G. EDUSAT PROGRAMME 15 Turbomachines (Unit 3) Axial flow compressors and pumps
 EDSA PROGRAMME 5 urbmacines (nit 3) Axial flw cmressrs and ums Axial flw cmressrs and ums are wer absrbing turbmacines. ese macines absrb external wer and tereby increase te entaly f te flwing fluid. Axial
EDSA PROGRAMME 5 urbmacines (nit 3) Axial flw cmressrs and ums Axial flw cmressrs and ums are wer absrbing turbmacines. ese macines absrb external wer and tereby increase te entaly f te flwing fluid. Axial
"NEET / AIIMS " SOLUTION (6) Avail Video Lectures of Experienced Faculty.
 07 NEET EXAMINATION SOLUTION (6) Avail Vide Lectures f Exerienced Faculty Page Sl. The lean exressin which satisfies the utut f this lgic gate is C = A., Whichindicates fr AND gate. We can see, utut C
07 NEET EXAMINATION SOLUTION (6) Avail Vide Lectures f Exerienced Faculty Page Sl. The lean exressin which satisfies the utut f this lgic gate is C = A., Whichindicates fr AND gate. We can see, utut C
T098. c Dr. Md. Zahurul Haq (BUET) First Law of Thermodynamics ME 201 (2012) 2 / 26
 Conservation of Energy for a Closed System Dr. Md. Zahurul Haq Professor Department of Mechanical Engineering Bangladesh University of Engineering & Technology (BUET Dhaka-, Bangladesh zahurul@me.buet.ac.bd
Conservation of Energy for a Closed System Dr. Md. Zahurul Haq Professor Department of Mechanical Engineering Bangladesh University of Engineering & Technology (BUET Dhaka-, Bangladesh zahurul@me.buet.ac.bd
3. Construct a perpendicular at point B, then bisect the right angle that is formed. 45 o
 Unit 1, Tpic 1 1. pint, line, and plane. angle bisectr cnstructin 3. Cnstruct a perpendicular at pint B, then bisect the right angle that is frmed. B 45 4. Draw a line thrugh pint H, then cpy the angle
Unit 1, Tpic 1 1. pint, line, and plane. angle bisectr cnstructin 3. Cnstruct a perpendicular at pint B, then bisect the right angle that is frmed. B 45 4. Draw a line thrugh pint H, then cpy the angle
CHBE320 LECTURE V LAPLACE TRANSFORM AND TRANSFER FUNCTION. Professor Dae Ryook Yang
 CHBE3 ECTURE V APACE TRANSFORM AND TRANSFER FUNCTION Profeor Dae Ryook Yang Spring 8 Dept. of Chemical and Biological Engineering 5- Road Map of the ecture V aplace Tranform and Tranfer function Definition
CHBE3 ECTURE V APACE TRANSFORM AND TRANSFER FUNCTION Profeor Dae Ryook Yang Spring 8 Dept. of Chemical and Biological Engineering 5- Road Map of the ecture V aplace Tranform and Tranfer function Definition
Chapter 7. Entropy: A Measure of Disorder
 Chapter 7 Entropy: A Measure of Disorder Entropy and the Clausius Inequality The second law of thermodynamics leads to the definition of a new property called entropy, a quantitative measure of microscopic
Chapter 7 Entropy: A Measure of Disorder Entropy and the Clausius Inequality The second law of thermodynamics leads to the definition of a new property called entropy, a quantitative measure of microscopic
Semester Exam Review Answers. 3. Construct a perpendicular at point B, then bisect the right angle that is formed. 45 o
 Unit 1, Tpic 1 1. pint, line, and plane 2. angle bisectr cnstructin 3. Cnstruct a perpendicular at pint B, then bisect the right angle that is frmed. B 45 4. Draw a line thrugh pint H, then cpy the angle
Unit 1, Tpic 1 1. pint, line, and plane 2. angle bisectr cnstructin 3. Cnstruct a perpendicular at pint B, then bisect the right angle that is frmed. B 45 4. Draw a line thrugh pint H, then cpy the angle
Section A 01. (12 M) (s 2 s 3 ) = 313 s 2 = s 1, h 3 = h 4 (s 1 s 3 ) = kj/kgk. = kj/kgk. 313 (s 3 s 4f ) = ln
 0. (a) Sol: Section A A refrigerator macine uses R- as te working fluid. Te temperature of R- in te evaporator coil is 5C, and te gas leaves te compressor as dry saturated at a temperature of 40C. Te mean
0. (a) Sol: Section A A refrigerator macine uses R- as te working fluid. Te temperature of R- in te evaporator coil is 5C, and te gas leaves te compressor as dry saturated at a temperature of 40C. Te mean
Chapter 5: The First Law of Thermodynamics: Closed Systems
 Chapter 5: The First Law of Thermodynamics: Closed Systems The first law of thermodynamics can be simply stated as follows: during an interaction between a system and its surroundings, the amount of energy
Chapter 5: The First Law of Thermodynamics: Closed Systems The first law of thermodynamics can be simply stated as follows: during an interaction between a system and its surroundings, the amount of energy
Chapter 10. Closed-Loop Control Systems
 hapter 0 loed-loop ontrol Sytem ontrol Diagram of a Typical ontrol Loop Actuator Sytem F F 2 T T 2 ontroller T Senor Sytem T TT omponent and Signal of a Typical ontrol Loop F F 2 T Air 3-5 pig 4-20 ma
hapter 0 loed-loop ontrol Sytem ontrol Diagram of a Typical ontrol Loop Actuator Sytem F F 2 T T 2 ontroller T Senor Sytem T TT omponent and Signal of a Typical ontrol Loop F F 2 T Air 3-5 pig 4-20 ma
Physics 212. Lecture 12. Today's Concept: Magnetic Force on moving charges. Physics 212 Lecture 12, Slide 1
 Physics 1 Lecture 1 Tday's Cncept: Magnetic Frce n mving charges F qv Physics 1 Lecture 1, Slide 1 Music Wh is the Artist? A) The Meters ) The Neville rthers C) Trmbne Shrty D) Michael Franti E) Radiatrs
Physics 1 Lecture 1 Tday's Cncept: Magnetic Frce n mving charges F qv Physics 1 Lecture 1, Slide 1 Music Wh is the Artist? A) The Meters ) The Neville rthers C) Trmbne Shrty D) Michael Franti E) Radiatrs
Chapter 8 Sections 8.4 through 8.6 Internal Flow: Heat Transfer Correlations. In fully-developed region. Neglect axial conduction
 Chapter 8 Sectin 8.4 thrugh 8.6 Internal Flw: Heat Tranfer Crrelatin T v cu p cp ( rt) k r T T k x r r r r r x In fully-develped regin Neglect axial cnductin u ( rt) r x r r r r r x T v T T T T T u r x
Chapter 8 Sectin 8.4 thrugh 8.6 Internal Flw: Heat Tranfer Crrelatin T v cu p cp ( rt) k r T T k x r r r r r x In fully-develped regin Neglect axial cnductin u ( rt) r x r r r r r x T v T T T T T u r x
ECE-320: Linear Control Systems Homework 1. 1) For the following transfer functions, determine both the impulse response and the unit step response.
 Due: Mnday Marh 4, 6 at the beginning f la ECE-: Linear Cntrl Sytem Hmewrk ) Fr the fllwing tranfer funtin, determine bth the imule rene and the unit te rene. Srambled Anwer: H ( ) H ( ) ( )( ) ( )( )
Due: Mnday Marh 4, 6 at the beginning f la ECE-: Linear Cntrl Sytem Hmewrk ) Fr the fllwing tranfer funtin, determine bth the imule rene and the unit te rene. Srambled Anwer: H ( ) H ( ) ( )( ) ( )( )
Outline. Heat Exchangers. Heat Exchangers. Compact Heat Exchangers. Compact Heat Exchangers II. Heat Exchangers April 18, ME 375 Heat Transfer 1
 Hat Exangr April 8, 007 Hat Exangr Larry artt Manial Engrg 375 Hat ranfr April 8, 007 Outl Bai ida f at xangr Ovrall at tranfr ffiint Lg-man tmpratur diffrn mtd Efftivn NU mtd ratial nidratin Hat Exangr
Hat Exangr April 8, 007 Hat Exangr Larry artt Manial Engrg 375 Hat ranfr April 8, 007 Outl Bai ida f at xangr Ovrall at tranfr ffiint Lg-man tmpratur diffrn mtd Efftivn NU mtd ratial nidratin Hat Exangr
NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor
 NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor T o T T o T F o, Q o F T m,q m T m T m T mo Aumption: 1. Homogeneou Sytem 2. Single Reaction 3. Steady State Two type of problem: 1. Given deired
NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor T o T T o T F o, Q o F T m,q m T m T m T mo Aumption: 1. Homogeneou Sytem 2. Single Reaction 3. Steady State Two type of problem: 1. Given deired
8-4 P 2. = 12 kw. AIR T = const. Therefore, Q &
 8-4 8-4 Air i compreed teadily by a compreor. e air temperature i mataed contant by eat rejection to te urroundg. e rate o entropy cange o air i to be determed. Aumption i i a teady-low proce ce tere i
8-4 8-4 Air i compreed teadily by a compreor. e air temperature i mataed contant by eat rejection to te urroundg. e rate o entropy cange o air i to be determed. Aumption i i a teady-low proce ce tere i
FP2 Mark Schemes from old P4, P5, P6 and FP1, FP2, FP3 papers (back to June 2002)
 FP Mark Schemes from old P, P5, P6 and FP, FP, FP papers (back to June 00) Please note that the following pages contain mark schemes for questions from past papers. The standard of the mark schemes is
FP Mark Schemes from old P, P5, P6 and FP, FP, FP papers (back to June 00) Please note that the following pages contain mark schemes for questions from past papers. The standard of the mark schemes is
Chapter 8. Root Locus Techniques
 Chapter 8 Rt Lcu Technique Intrductin Sytem perfrmance and tability dt determined dby cled-lp l ple Typical cled-lp feedback cntrl ytem G Open-lp TF KG H Zer -, - Ple 0, -, -4 K 4 Lcatin f ple eaily fund
Chapter 8 Rt Lcu Technique Intrductin Sytem perfrmance and tability dt determined dby cled-lp l ple Typical cled-lp feedback cntrl ytem G Open-lp TF KG H Zer -, - Ple 0, -, -4 K 4 Lcatin f ple eaily fund
CHE302 LECTURE V LAPLACE TRANSFORM AND TRANSFER FUNCTION. Professor Dae Ryook Yang
 CHE3 ECTURE V APACE TRANSFORM AND TRANSFER FUNCTION Profeor Dae Ryook Yang Fall Dept. of Chemical and Biological Engineering Korea Univerity CHE3 Proce Dynamic and Control Korea Univerity 5- SOUTION OF
CHE3 ECTURE V APACE TRANSFORM AND TRANSFER FUNCTION Profeor Dae Ryook Yang Fall Dept. of Chemical and Biological Engineering Korea Univerity CHE3 Proce Dynamic and Control Korea Univerity 5- SOUTION OF
(1)5. Which of the following equations is always valid for a fixed mass system undergoing an irreversible or reversible process:
 Last Name First Name ME 300 Engineering Thermodynamics Exam #2 Spring 2008 March 28, 2008 Form A Note : (i) (ii) (iii) (iv) Closed book, closed notes; one 8.5 x 11 sheet allowed. 60 points total; 60 minutes;
Last Name First Name ME 300 Engineering Thermodynamics Exam #2 Spring 2008 March 28, 2008 Form A Note : (i) (ii) (iii) (iv) Closed book, closed notes; one 8.5 x 11 sheet allowed. 60 points total; 60 minutes;
Parts Manual. EPIC II Critical Care Bed REF 2031
 EPIC II Critical Care Bed REF 2031 Parts Manual For parts or technical assistance call: USA: 1-800-327-0770 2013/05 B.0 2031-109-006 REV B www.stryker.com Table of Contents English Product Labels... 4
EPIC II Critical Care Bed REF 2031 Parts Manual For parts or technical assistance call: USA: 1-800-327-0770 2013/05 B.0 2031-109-006 REV B www.stryker.com Table of Contents English Product Labels... 4
Consequences of Second Law of Thermodynamics. Entropy. Clausius Inequity
 onsequences of Second Law of hermodynamics Dr. Md. Zahurul Haq Professor Department of Mechanical Engineering Bangladesh University of Engineering & echnology BUE Dhaka-000, Bangladesh zahurul@me.buet.ac.bd
onsequences of Second Law of hermodynamics Dr. Md. Zahurul Haq Professor Department of Mechanical Engineering Bangladesh University of Engineering & echnology BUE Dhaka-000, Bangladesh zahurul@me.buet.ac.bd
Exclusive Technology Feature. Eliminate The Guesswork When Selecting Primary Switch V DD Capacitors. ISSUE: May 2011
 Excluive Technlgy Feature Eliminate The Guewrk When Selecting Primary Switch DD aacitr by Ed Wenzel, STMicrelectrnic, Schaumburg, ll. SSUE: May 2011 A rimary witch, ued fr ff-line alicatin, ften cntain
Excluive Technlgy Feature Eliminate The Guewrk When Selecting Primary Switch DD aacitr by Ed Wenzel, STMicrelectrnic, Schaumburg, ll. SSUE: May 2011 A rimary witch, ued fr ff-line alicatin, ften cntain
2-18. (a) For mercury, (b) For water,
 -8-5 CD EES Bt a gage and a manmeter are attaced t a gas t measure its pressure. Fr a specified reading f gage pressure, te difference between te fluid levels f te tw arms f te manmeter is t be determined
-8-5 CD EES Bt a gage and a manmeter are attaced t a gas t measure its pressure. Fr a specified reading f gage pressure, te difference between te fluid levels f te tw arms f te manmeter is t be determined
Physics 41 Chapter 22 HW
 Pysis 41 apter 22 H 1. eat ine performs 200 J of work in ea yle and as an effiieny of 30.0%. For ea yle, ow mu energy is (a) taken in and (b) expelled as eat? = = 200 J (1) e = 1 0.300 = = (2) From (2),
Pysis 41 apter 22 H 1. eat ine performs 200 J of work in ea yle and as an effiieny of 30.0%. For ea yle, ow mu energy is (a) taken in and (b) expelled as eat? = = 200 J (1) e = 1 0.300 = = (2) From (2),
π (sinπ ) + cos π + C = C = 0 C = 1
 National Mu Alpha Theta Diff EQ page Mississippi, 00 Differential Equations Topic Test: Complete Solutions Note: For each problem, where there is no choice (e), assume (e) none of the above.. State the
National Mu Alpha Theta Diff EQ page Mississippi, 00 Differential Equations Topic Test: Complete Solutions Note: For each problem, where there is no choice (e), assume (e) none of the above.. State the
Department of Mechanical Engineering ME 322 Mechanical Engineering Thermodynamics. Calculation of Entropy Changes. Lecture 19
 Department of Mecanical Engineering ME Mecanical Engineering ermodynamics Calculation of Entropy Canges Lecture 9 e Gibbs Equations How are entropy alues calculated? Clausius found tat, dq dq m re re ds
Department of Mecanical Engineering ME Mecanical Engineering ermodynamics Calculation of Entropy Canges Lecture 9 e Gibbs Equations How are entropy alues calculated? Clausius found tat, dq dq m re re ds
Exercises for lectures 20 Digital Control
 Exercie for lecture 0 Digital Control Micael Šebek Automatic control 06-4- Sampling: and z relationip for complex pole Continuou ignal Laplace tranform wit pole Dicrete ignal z-tranform, t y( t) e in t,
Exercie for lecture 0 Digital Control Micael Šebek Automatic control 06-4- Sampling: and z relationip for complex pole Continuou ignal Laplace tranform wit pole Dicrete ignal z-tranform, t y( t) e in t,
Lecture 38: Vapor-compression refrigeration systems
 ME 200 Termodynamics I Lecture 38: Vapor-compression refrigeration systems Yong Li Sangai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Cuan Road Sangai, 200240, P. R. Cina Email
ME 200 Termodynamics I Lecture 38: Vapor-compression refrigeration systems Yong Li Sangai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Cuan Road Sangai, 200240, P. R. Cina Email
Outline. Property diagrams involving entropy. Heat transfer for internally reversible process
 Outline roperty diagrams involving entropy What is entropy? T-ds relations Entropy change of substances ure substances (near wet dome) Solids and liquids Ideal gases roperty diagrams involving entropy
Outline roperty diagrams involving entropy What is entropy? T-ds relations Entropy change of substances ure substances (near wet dome) Solids and liquids Ideal gases roperty diagrams involving entropy
Department of Civil Engineering & Applied Mechanics McGill University, Montreal, Quebec Canada
 Department f Ciil ngeerg Applied Mechanics McGill Uniersity, Mntreal, Quebec Canada CI 90 THRMODYNAMICS HAT TRANSFR Assignment #4 SOLUTIONS. A 68-kg man whse aerage bdy temperature is 9 C drks L f cld
Department f Ciil ngeerg Applied Mechanics McGill Uniersity, Mntreal, Quebec Canada CI 90 THRMODYNAMICS HAT TRANSFR Assignment #4 SOLUTIONS. A 68-kg man whse aerage bdy temperature is 9 C drks L f cld
1) What is the reflected angle 3 measured WITH RESPECT TO THE BOUNDRY as shown? a) 0 b) 11 c) 16 d) 50 e) 42
 Light in ne medium (n =.) enunters a bundary t a send medium (with n =. 8) where part f the light is transmitted int the send media and part is refleted bak int the first media. The inident angle is =
Light in ne medium (n =.) enunters a bundary t a send medium (with n =. 8) where part f the light is transmitted int the send media and part is refleted bak int the first media. The inident angle is =
( ) Given: In a constant pressure combustor. C4H10 and theoretical air burns at P1 = 0.2 MPa, T1 = 600K. Products exit at P2 = 0.
 (SP 9) N-butane (C4H1) i burned with 85 percent theoretical air, and the product of combution, an equilibrium mixture containing only O, CO, CO, H, HO, N, and NO, exit from a combution chamber at K,. MPa.
(SP 9) N-butane (C4H1) i burned with 85 percent theoretical air, and the product of combution, an equilibrium mixture containing only O, CO, CO, H, HO, N, and NO, exit from a combution chamber at K,. MPa.
Thermodynamics II. Week 9
 hermodynamics II Week 9 Example Oxygen gas in a piston cylinder at 300K, 00 kpa with volume o. m 3 is compressed in a reversible adiabatic process to a final temperature of 700K. Find the final pressure
hermodynamics II Week 9 Example Oxygen gas in a piston cylinder at 300K, 00 kpa with volume o. m 3 is compressed in a reversible adiabatic process to a final temperature of 700K. Find the final pressure
Consequences of Second Law of Thermodynamics. Entropy. Clausius Inequity
 onsequences of Second Law of hermodynamics Dr. Md. Zahurul Haq Professor Department of Mechanical Engineering Bangladesh University of Engineering & echnology BUE Dhaka-000, Bangladesh zahurul@me.buet.ac.bd
onsequences of Second Law of hermodynamics Dr. Md. Zahurul Haq Professor Department of Mechanical Engineering Bangladesh University of Engineering & echnology BUE Dhaka-000, Bangladesh zahurul@me.buet.ac.bd
T Turbine 8. Boiler fwh fwh I Condenser 4 3 P II P I P III. (a) From the steam tables (Tables A-4, A-5, and A-6), = = 10 MPa. = 0.
 - - A team poer plant operate on an ideal regenerative anke cycle it to open feedater eater. e poer put of te poer plant and te termal efficiency of te cycle are to be determed. Aumption Steady operatg
- - A team poer plant operate on an ideal regenerative anke cycle it to open feedater eater. e poer put of te poer plant and te termal efficiency of te cycle are to be determed. Aumption Steady operatg
Where F1 is the force and dl1 is the infinitesimal displacement, but F1 = p1a1
 In order to force the fluid to flow across the boundary of the system against a pressure p1, work is done on the boundary of the system. The amount of work done is dw = - F1.dl1, Where F1 is the force
In order to force the fluid to flow across the boundary of the system against a pressure p1, work is done on the boundary of the system. The amount of work done is dw = - F1.dl1, Where F1 is the force
Lecture 10 Adiabatic Processes
 ASME231 Atmsheri hermdynamis NC A& State U Deartment f Physis Dr. Yuh-Lang Lin htt://meslab.rg ylin@nat.edu Leture 10 Adiabati Presses (Se.3.5 f Hess) [Classial equatin editr: 0 dq ] Definitin: If a thermdynami
ASME231 Atmsheri hermdynamis NC A& State U Deartment f Physis Dr. Yuh-Lang Lin htt://meslab.rg ylin@nat.edu Leture 10 Adiabati Presses (Se.3.5 f Hess) [Classial equatin editr: 0 dq ] Definitin: If a thermdynami
( )( ) 7 MPa q in = = 10 kpa q out. 1 h. = s. Thus, and = 38.9% (b) (c) The rate of heat rejection to the cooling water and its temperature rise are
 . A team poer plant operate on a imple ideal Ranke cycle beteen te peciied preure limit. e termal eiciency o te cycle, te ma lo rate o te team, and te temperature rie o te coolg ater are to be determed.
. A team poer plant operate on a imple ideal Ranke cycle beteen te peciied preure limit. e termal eiciency o te cycle, te ma lo rate o te team, and te temperature rie o te coolg ater are to be determed.
AEROSPACE ENGINEERING GATE 2018 ANSWERS & EXPLANATIONS
 AEROSPACE ENGINEERING GATE 08 ANSWERS & EXPLANATIONS NOTE : Use the same questin aer that is uladed n the fllwing link fr the rret sequene f questins htt://gateathshala.m/resures.html is the first institute
AEROSPACE ENGINEERING GATE 08 ANSWERS & EXPLANATIONS NOTE : Use the same questin aer that is uladed n the fllwing link fr the rret sequene f questins htt://gateathshala.m/resures.html is the first institute
Lecture 27: Entropy and Information Prof. WAN, Xin
 General Pysis I Leture 27: Entropy and Information Prof. WAN, Xin xinwan@zju.edu.n ttp://zimp.zju.edu.n/~xinwan/ 1st & 2nd Laws of ermodynamis e 1st law speifies tat we annot get more energy out of a yli
General Pysis I Leture 27: Entropy and Information Prof. WAN, Xin xinwan@zju.edu.n ttp://zimp.zju.edu.n/~xinwan/ 1st & 2nd Laws of ermodynamis e 1st law speifies tat we annot get more energy out of a yli
Examiner: Dr. Mohamed Elsharnoby Time: 180 min. Attempt all the following questions Solve the following five questions, and assume any missing data
 Benha University Cllege f Engineering at Banha Department f Mechanical Eng. First Year Mechanical Subject : Fluid Mechanics M111 Date:4/5/016 Questins Fr Final Crrective Examinatin Examiner: Dr. Mhamed
Benha University Cllege f Engineering at Banha Department f Mechanical Eng. First Year Mechanical Subject : Fluid Mechanics M111 Date:4/5/016 Questins Fr Final Crrective Examinatin Examiner: Dr. Mhamed
c Dr. Md. Zahurul Haq (BUET) Entropy ME 203 (2017) 2 / 27 T037
 onsequences of Second Law of hermodynamics Dr. Md. Zahurul Haq Professor Department of Mechanical Engineering Bangladesh University of Engineering & echnology BUE Dhaka-000, Bangladesh zahurul@me.buet.ac.bd
onsequences of Second Law of hermodynamics Dr. Md. Zahurul Haq Professor Department of Mechanical Engineering Bangladesh University of Engineering & echnology BUE Dhaka-000, Bangladesh zahurul@me.buet.ac.bd
Coupled Inductors and Transformers
 Cupled nductrs and Transfrmers Self-nductance When current i flws thrugh the cil, a magnetic flux is prduced arund it. d d di di v= = = dt di dt dt nductance: = d di This inductance is cmmnly called self-inductance,
Cupled nductrs and Transfrmers Self-nductance When current i flws thrugh the cil, a magnetic flux is prduced arund it. d d di di v= = = dt di dt dt nductance: = d di This inductance is cmmnly called self-inductance,
Digital Filter Specifications. Digital Filter Specifications. Digital Filter Design. Digital Filter Specifications. Digital Filter Specifications
 Digital Filter Deign Objetive - Determinatin f a realiable tranfer funtin G() arximating a given frequeny rene eifiatin i an imrtant te in the develment f a digital filter If an IIR filter i deired, G()
Digital Filter Deign Objetive - Determinatin f a realiable tranfer funtin G() arximating a given frequeny rene eifiatin i an imrtant te in the develment f a digital filter If an IIR filter i deired, G()
4F-5 : Performance of an Ideal Gas Cycle 10 pts
 4F-5 : Perfrmance f an Cycle 0 pts An ideal gas, initially at 0 C and 00 kpa, underges an internally reversible, cyclic prcess in a clsed system. The gas is first cmpressed adiabatically t 500 kpa, then
4F-5 : Perfrmance f an Cycle 0 pts An ideal gas, initially at 0 C and 00 kpa, underges an internally reversible, cyclic prcess in a clsed system. The gas is first cmpressed adiabatically t 500 kpa, then
Special Topic: Binary Vapor Cycles
 0- Special opic: Bary Vapor ycle 0- Bary poer cycle i a cycle ic i actually a combation o to cycle; one te ig temperature region, and te oter te lo temperature region. It purpoe i to creae termal eiciency.
0- Special opic: Bary Vapor ycle 0- Bary poer cycle i a cycle ic i actually a combation o to cycle; one te ig temperature region, and te oter te lo temperature region. It purpoe i to creae termal eiciency.
Linear System Fundamentals
 Linear Sytem Fundamental MEM 355 Performance Enhancement of Dynamical Sytem Harry G. Kwatny Department of Mechanical Engineering & Mechanic Drexel Univerity Content Sytem Repreentation Stability Concept
Linear Sytem Fundamental MEM 355 Performance Enhancement of Dynamical Sytem Harry G. Kwatny Department of Mechanical Engineering & Mechanic Drexel Univerity Content Sytem Repreentation Stability Concept
1 = The rate at which the entropy of the high temperature reservoir changes, according to the definition of the entropy, is
 8-7 8-9 A reverible eat um wit eciied reervoir temerature i conidered. e entroy cange o two reervoir i to be calculated and it i to be determed i ti eat um atiie te creae entroy rcile. Aumtion e eat um
8-7 8-9 A reverible eat um wit eciied reervoir temerature i conidered. e entroy cange o two reervoir i to be calculated and it i to be determed i ti eat um atiie te creae entroy rcile. Aumtion e eat um
Read each question and its parts carefully before starting. Show all your work. Give units with your answers (where appropriate). 1 / 3 F.
 ECSE 10 NAME: Quiz 18 May 011 ID: Read each question and its parts carefully before starting. Show all your work. Give units with your answers (where appropriate). 1. Consider the circuit diagram below.
ECSE 10 NAME: Quiz 18 May 011 ID: Read each question and its parts carefully before starting. Show all your work. Give units with your answers (where appropriate). 1. Consider the circuit diagram below.
Bernoulli s equation may be developed as a special form of the momentum or energy equation.
 BERNOULLI S EQUATION Bernoulli equation may be developed a a pecial form of the momentum or energy equation. Here, we will develop it a pecial cae of momentum equation. Conider a teady incompreible flow
BERNOULLI S EQUATION Bernoulli equation may be developed a a pecial form of the momentum or energy equation. Here, we will develop it a pecial cae of momentum equation. Conider a teady incompreible flow
Course: MECH-341 Thermodynamics II Semester: Fall 2006
 FINAL EXAM Date: Thursday, December 21, 2006, 9 am 12 am Examiner: Prof. E. Timofeev Associate Examiner: Prof. D. Frost READ CAREFULLY BEFORE YOU PROCEED: Course: MECH-341 Thermodynamics II Semester: Fall
FINAL EXAM Date: Thursday, December 21, 2006, 9 am 12 am Examiner: Prof. E. Timofeev Associate Examiner: Prof. D. Frost READ CAREFULLY BEFORE YOU PROCEED: Course: MECH-341 Thermodynamics II Semester: Fall
Chapter 9 Compressible Flow 667
 Chapter 9 Cmpreible Flw 667 9.57 Air flw frm a tank thrugh a nzzle int the tandard atmphere, a in Fig. P9.57. A nrmal hck tand in the exit f the nzzle, a hwn. Etimate (a) the tank preure; and (b) the ma
Chapter 9 Cmpreible Flw 667 9.57 Air flw frm a tank thrugh a nzzle int the tandard atmphere, a in Fig. P9.57. A nrmal hck tand in the exit f the nzzle, a hwn. Etimate (a) the tank preure; and (b) the ma
Three charges, all with a charge of 10 C are situated as shown (each grid line is separated by 1 meter).
 Three charges, all with a charge f 0 are situated as shwn (each grid line is separated by meter). ) What is the net wrk needed t assemble this charge distributin? a) +0.5 J b) +0.8 J c) 0 J d) -0.8 J e)
Three charges, all with a charge f 0 are situated as shwn (each grid line is separated by meter). ) What is the net wrk needed t assemble this charge distributin? a) +0.5 J b) +0.8 J c) 0 J d) -0.8 J e)
**YOU ARE NOT ALLOWED TO TAKE SPARE COPIES OF THIS EXAM FROM THE TESTING ROOM**
 EM 24, Spring 2017 Midterm #2 Ian R. Gould MPLETE TIS SETIN : Up to TW PINTS will be removed for incorrect/missing information! PRINTED FIRST Answer Key Person on your LEFT (or Empty or Aisle) Person on
EM 24, Spring 2017 Midterm #2 Ian R. Gould MPLETE TIS SETIN : Up to TW PINTS will be removed for incorrect/missing information! PRINTED FIRST Answer Key Person on your LEFT (or Empty or Aisle) Person on
= h. Geometrically this quantity represents the slope of the secant line connecting the points
 Section 3.7: Rates of Cange in te Natural and Social Sciences Recall: Average rate of cange: y y y ) ) ), ere Geometrically tis quantity represents te slope of te secant line connecting te points, f (
Section 3.7: Rates of Cange in te Natural and Social Sciences Recall: Average rate of cange: y y y ) ) ), ere Geometrically tis quantity represents te slope of te secant line connecting te points, f (
Phys102 First Major-122 Zero Version Coordinator: Sunaidi Wednesday, March 06, 2013 Page: 1
 Crdinatr: Sunaidi Wednesday, March 06, 2013 Page: 1 Q1. An 8.00 m lng wire with a mass f 10.0 g is under a tensin f 25.0 N. A transverse wave fr which the wavelength is 0.100 m, and the amplitude is 3.70
Crdinatr: Sunaidi Wednesday, March 06, 2013 Page: 1 Q1. An 8.00 m lng wire with a mass f 10.0 g is under a tensin f 25.0 N. A transverse wave fr which the wavelength is 0.100 m, and the amplitude is 3.70
2b m 1b: Sat liq C, h = kj/kg tot 3a: 1 MPa, s = s 3 -> h 3a = kj/kg, T 3b
 .6 A upercritical team power plant ha a high preure of 0 Ma and an exit condener temperature of 50 C. he maximum temperature in the boiler i 000 C and the turbine exhaut i aturated vapor here i one open
.6 A upercritical team power plant ha a high preure of 0 Ma and an exit condener temperature of 50 C. he maximum temperature in the boiler i 000 C and the turbine exhaut i aturated vapor here i one open
Chapter 3. Electric Flux Density, Gauss s Law and Divergence
 Chapter 3. Electric Flu Denity, Gau aw and Diergence Hayt; 9/7/009; 3-1 3.1 Electric Flu Denity Faraday Eperiment Cncentric phere filled with dielectric material. + i gien t the inner phere. - i induced
Chapter 3. Electric Flu Denity, Gau aw and Diergence Hayt; 9/7/009; 3-1 3.1 Electric Flu Denity Faraday Eperiment Cncentric phere filled with dielectric material. + i gien t the inner phere. - i induced
ELABORATING AND ANALYSING THE REAL BALANCE OF HEAT FOR THE STEAM GENERATOR RGL10/D-D
 Annals f te Unversty f Petrşan, Mecancal Engneerng, 10 (2008), 155-160 155 ELABORATING AND ANALYSING THE REAL BALANCE OF HEAT FOR THE STEAM GENERATOR RGL10/D-D DAN CODRUŢ PETRILEAN 1 Abstract: Te real
Annals f te Unversty f Petrşan, Mecancal Engneerng, 10 (2008), 155-160 155 ELABORATING AND ANALYSING THE REAL BALANCE OF HEAT FOR THE STEAM GENERATOR RGL10/D-D DAN CODRUŢ PETRILEAN 1 Abstract: Te real
Copyright 1967, by the author(s). All rights reserved.
 Copyright 1967, by the author(). All right reerved. Permiion to make digital or hard copie of all or part of thi work for peronal or claroom ue i granted without fee provided that copie are not made or
Copyright 1967, by the author(). All right reerved. Permiion to make digital or hard copie of all or part of thi work for peronal or claroom ue i granted without fee provided that copie are not made or
INDUCTANCE Self Inductance
 DUCTCE 3. Sef nductance Cnsider the circuit shwn in the Figure. S R When the switch is csed the current, and s the magnetic fied, thrugh the circuit increases frm zer t a specific vaue. The increasing
DUCTCE 3. Sef nductance Cnsider the circuit shwn in the Figure. S R When the switch is csed the current, and s the magnetic fied, thrugh the circuit increases frm zer t a specific vaue. The increasing
Engineering Thermodynamics. Chapter 6. Entropy: a measure of Disorder 6.1 Introduction
 Engineering hermodynamics AAi Chapter 6 Entropy: a measure of Disorder 6. Introduction he second law of thermodynamics leads to the definition of a new property called entropy, a quantitative measure of
Engineering hermodynamics AAi Chapter 6 Entropy: a measure of Disorder 6. Introduction he second law of thermodynamics leads to the definition of a new property called entropy, a quantitative measure of
Calculus I, Fall Solutions to Review Problems II
 Calculus I, Fall 202 - Solutions to Review Problems II. Find te following limits. tan a. lim 0. sin 2 b. lim 0 sin 3. tan( + π/4) c. lim 0. cos 2 d. lim 0. a. From tan = sin, we ave cos tan = sin cos =
Calculus I, Fall 202 - Solutions to Review Problems II. Find te following limits. tan a. lim 0. sin 2 b. lim 0 sin 3. tan( + π/4) c. lim 0. cos 2 d. lim 0. a. From tan = sin, we ave cos tan = sin cos =
Control Systems Engineering ( Chapter 7. Steady-State Errors ) Prof. Kwang-Chun Ho Tel: Fax:
 Control Sytem Engineering ( Chapter 7. Steady-State Error Prof. Kwang-Chun Ho kwangho@hanung.ac.kr Tel: 0-760-453 Fax:0-760-4435 Introduction In thi leon, you will learn the following : How to find the
Control Sytem Engineering ( Chapter 7. Steady-State Error Prof. Kwang-Chun Ho kwangho@hanung.ac.kr Tel: 0-760-453 Fax:0-760-4435 Introduction In thi leon, you will learn the following : How to find the
Exergy and the Dead State
 EXERGY The energy content of the universe is constant, just as its mass content is. Yet at times of crisis we are bombarded with speeches and articles on how to conserve energy. As engineers, we know that
EXERGY The energy content of the universe is constant, just as its mass content is. Yet at times of crisis we are bombarded with speeches and articles on how to conserve energy. As engineers, we know that
I N A C O M P L E X W O R L D
 IS L A M I C E C O N O M I C S I N A C O M P L E X W O R L D E x p l o r a t i o n s i n A g-b eanste d S i m u l a t i o n S a m i A l-s u w a i l e m 1 4 2 9 H 2 0 0 8 I s l a m i c D e v e l o p m e
IS L A M I C E C O N O M I C S I N A C O M P L E X W O R L D E x p l o r a t i o n s i n A g-b eanste d S i m u l a t i o n S a m i A l-s u w a i l e m 1 4 2 9 H 2 0 0 8 I s l a m i c D e v e l o p m e
Entropy and the Second Law of Thermodynamics
 Entropy and the Second Law of hermodynamics Reading Problems 6-, 6-2, 6-7, 6-8, 6-6-8, 6-87, 7-7-0, 7-2, 7-3 7-39, 7-46, 7-6, 7-89, 7-, 7-22, 7-24, 7-30, 7-55, 7-58 Why do we need another law in thermodynamics?
Entropy and the Second Law of hermodynamics Reading Problems 6-, 6-2, 6-7, 6-8, 6-6-8, 6-87, 7-7-0, 7-2, 7-3 7-39, 7-46, 7-6, 7-89, 7-, 7-22, 7-24, 7-30, 7-55, 7-58 Why do we need another law in thermodynamics?
Lecture 4. The First Law of Thermodynamics
 Lecture 4. The First Law f Thermdynamics THERMODYNAMICS: Basic Cncepts Thermdynamics: (frm the Greek therme, meaning "heat" and, dynamis, meaning "pwer") is the study f energy cnversin between heat and
Lecture 4. The First Law f Thermdynamics THERMODYNAMICS: Basic Cncepts Thermdynamics: (frm the Greek therme, meaning "heat" and, dynamis, meaning "pwer") is the study f energy cnversin between heat and
COMPLETE THIS SECTION : Up to TWO POINTS will be removed for incorrect/missing information!
 M 234, Spring 2019 Midterm #1 Ian R. Gould MPLETE TIS SETIN : Up to TW PINTS will be removed for incorrect/missing information! PRINTED FIRST NAME Answer Key Person on your LEFT (or Empty or Aisle) Person
M 234, Spring 2019 Midterm #1 Ian R. Gould MPLETE TIS SETIN : Up to TW PINTS will be removed for incorrect/missing information! PRINTED FIRST NAME Answer Key Person on your LEFT (or Empty or Aisle) Person
Lecture two. January 17, 2019
 Lecture two January 17, 2019 We will learn how to solve rst-order linear equations in this lecture. Example 1. 1) Find all solutions satisfy the equation u x (x, y) = 0. 2) Find the solution if we know
Lecture two January 17, 2019 We will learn how to solve rst-order linear equations in this lecture. Example 1. 1) Find all solutions satisfy the equation u x (x, y) = 0. 2) Find the solution if we know
The First Law of Thermodynamics. By: Yidnekachew Messele
 The First Law of Thermodynamics By: Yidnekachew Messele It is the law that relates the various forms of energies for system of different types. It is simply the expression of the conservation of energy
The First Law of Thermodynamics By: Yidnekachew Messele It is the law that relates the various forms of energies for system of different types. It is simply the expression of the conservation of energy
I M P O R T A N T S A F E T Y I N S T R U C T I O N S W h e n u s i n g t h i s e l e c t r o n i c d e v i c e, b a s i c p r e c a u t i o n s s h o
 I M P O R T A N T S A F E T Y I N S T R U C T I O N S W h e n u s i n g t h i s e l e c t r o n i c d e v i c e, b a s i c p r e c a u t i o n s s h o u l d a l w a y s b e t a k e n, i n c l u d f o l
I M P O R T A N T S A F E T Y I N S T R U C T I O N S W h e n u s i n g t h i s e l e c t r o n i c d e v i c e, b a s i c p r e c a u t i o n s s h o u l d a l w a y s b e t a k e n, i n c l u d f o l
