2b m 1b: Sat liq C, h = kj/kg tot 3a: 1 MPa, s = s 3 -> h 3a = kj/kg, T 3b
|
|
|
- Alban Tate
- 6 years ago
- Views:
Transcription
1 .6 A upercritical team power plant ha a high preure of 0 Ma and an exit condener temperature of 50 C. he maximum temperature in the boiler i 000 C and the turbine exhaut i aturated vapor here i one open feedwater heater receiving extraction from the turbine at Ma, and it exit i aturated liquid flowing to pump. he ientropic efficiency for the firt ection and the overall turbine are both 88.5%. Find the ratio of the extraction ma flow to total flow into turbine. What i the boiler inlet temperature with and without the feedwater heater? Baically a Rankine Cycle : 50 C,.5 ka, h = 09. kj/kg, = kj/kg K : 0 Ma : 0 Ma, 000 C, h = 55.7 kj/kg, = kj/kg K AC: 50 C, x =, h = 59. kj/kg b a b ac a) C.V. urbine Ideal: S = x S = 0.899, h S = 6.8 kj/kg => w,s = h - h S = 7.86 kj/kg 0 Ma 000 C Ma b a 50 C Actual: w,ac = h - h AC = 96.6 kj/kg, = w,ac /w,s = b) b b m b: Sat liq C, h = 76.8 kj/kg tot a: Ma, = -> h a = 9.09 kj/kg, b a = > w = 05.6 kj/kg b: Ma, w ac = w =.96 kj/kg m w ac = h -h b => h b = 0.7 kj/kg a: w = v ( a - ) kj/kg h a = h + w = 0. kj/kg a C.V. Feedwater Heater: ṁ O h b = ṁ h b + (ṁ O - ṁ )h a ṁ /ṁ O = x = (h b - h a )/(h b - h a ) = 0.78 c) C.V. urbine: (ṁ O ) = (ṁ ) b + (ṁ O - ṁ ) AC W _ = ṁ O h - ṁ h b - (ṁ O - ṁ )h AC = 5 MW = ṁ O w w = h -xh b - (-x)h AC = 8.7 kj/kg => ṁ O =.6 kg/ d) C.V. No FWH, ump Ideal: w = h S - h, S = Steam table h S = 0. kj/kg, S = 5. C FWH, CV:. b = b =.86 kj/kg K => b = 8.9 C
2 Brayton Cycle, Ga urbine.68 Conider an ideal air-tandard Brayton cycle in which the air into the compreor i at 00 ka, 0 C, and the preure ratio acro the compreor i :. he maximum temperature in the cycle i 00 C, and the air flow rate i 0 kg/. Aume contant pecific heat for the air, value from able A.5. Determine the compreor work, the turbine work, and the thermal efficiency of the cycle. Solution: v = 00 ka Compreion ratio = Max temperature = 00 o C ṁ = 0 kg/ he compreion i reverible and adiabatic o contant. From Eq.8. k- k = = 9.() 0.86 = K Energy equation with compreor work in w C = - w = C 0 ( - ) =.00( ) = 0.8 kj/kg he expanion i reverible and adiabatic o contant. From Eq.8. k- = k = = 67.7 K Energy equation with turbine work out w = C 0 ( - ) =.00( ) = 70. kj/kg Scale the work with the ma flow rate Ẇ C = ṁw C = 08 kw, Ẇ = ṁw = 70 kw Energy added by the combution proce q H = C 0 ( - ) =.00( ) = kj/kg H = w NE /q H = ( )/779.5 = 0.509
3 .76 Repeat roblem.7, but include a regenerator with 75% efficiency in the cycle. A large tationary Brayton cycle ga-turbine power plant deliver a power output of 00 MW to an electric generator. he minimum temperature in the cycle i 00 K, and the maximum temperature i 600 K. he minimum preure in the cycle i 00 ka, and the compreor preure ratio i to. Calculate the power output of the turbine. What fraction of the turbine output i required to drive the compreor? What i the thermal efficiency of the cycle? Solution: Both compreor and turbine are reverible and adiabatic o contant, Eq.8. relate then to auming contant heat capacity. k- Compreor: = ( / ) k = 00() 0.86 = 68. K w C = h - h = C 0 ( - ) =.00 ( ) = 9.5 kj/kg k- urbine = = ( / ) k = 600 (/) 0.86 = 75. K w = h h = C 0 ( ) =.00 ( ) = 85. kj/kg w NE = = 5.7 kj/kg ṁ = Ẇ NE /w NE = /5.7 = 95. kg/ Ẇ = ṁw = = 66. MW w C /w = 9.5/85. = 0.99 x x' = 00 ka For the regenerator REG = 0.75 = h X - h h X' - h = X - X = X = 7.7 K urbine and compreor work not affected by regenerator. Combutor need to add le energy with the regenerator a q H = C 0 ( - X ) =.00( ) = kj/kg H = w NE /q H = 5.7/879.8 = 0.58
4 .78 A two-tage compreor in a ga turbine bring atmopheric air at 00 ka, 7 o C to 500 ka, then cool it in an intercooler to 7 o C at contant. he econd tage bring the air to 000 ka. Aume both tage are adiabatic and reverible. Find the combined pecific work to the compreor tage. Compare that to the pecific work for the cae of no intercooler (i.e. one compreor from 00 to 000 ka). Solution: C.V. Stage : => Reverible and adiabatic give contant which from Eq.8. give: = ( / ) (k-)/k = 90 (500/00) = 59. K w cin = C ( - ) =.00(59. 90) = 87.0 kj/kg C.V. Stage : => Reverible and adiabatic give contant which from Eq.8. give: = ( / ) (k-)/k = 00 (000/500) = 65.7 K w cin = C ( - ) =.00( ) = kj/kg w tot = w c + w c = = 5 kj/kg he intercooler reduce the work for tage a i lower and o i pecific volume. C.V. One compreor => 5 Reverible and adiabatic give contant which from Eq.8. give: 5 = ( 5 / ) (k-)/k = 90 (000/00) = K w in = C ( 5 - ) =.00( ) = 7 kj/kg 5 v ka 500 ka 00 ka he reduction in work due to the intercooler i haded in the -v diagram.
5 .9 A gaoline engine ha a volumetric compreion ratio of 9. he tate before compreion i 90 K, 90 ka, and the peak cycle temperature i 800 K. Find the preure after expanion, the cycle net work and the cycle efficiency uing propertie from able A.5. Compreion to : = From Eq.8. and Eq.8. = (v /v ) k- = = 698. K = (v /v ) k = = ka Combution to at contant volume: v = v q H = u u = C v ( ) = 0.77 ( ) = kj/kg Expanion to : = ( / ) = (800 / 698.) = ka = From Eq.8. and Eq.8. = (v /v ) k- = 800 (/9) 0. = 77. K = ( / )(v /v ) = (77./800) (/9) = ka Find now the net work w = u - u = C v( - ) = 0.77( ) = -9.8 kj/kg w = u - u = C v( - ) = 0.77( ) = 75.7 kj/kg Net work and overall efficiency w NE = w + w = = 6.9 kj/kg = w NE /q H = 6.9/ = Comment: We could have found from Eq..8 and then w NE = q H. v v
6 .09 At the beginning of compreion in a dieel cycle = 00 K, = 00 ka and after combution (heat addition) i complete = 500 K and = 7.0 Ma. Find the compreion ratio, the thermal efficiency and the mean effective preure. Solution: Standard Dieel cycle. See -v and - diagram for tate number. Compreion proce (ientropic) from Eq = = 7000 ka => v / v = ( / ) / k = (7000 / 00) 0.7 =.67 = ( / ) (k-) / k = 00(7000 / 00) = 88. K Expanion proce (ientropic) firt get the volume ratio v / v = / = 500 / 88. =.8 v / v = v / v = (v / v )( v / v ) =.67 /.8 = 7 he exhaut temperature follow from Eq.8. = (v / v ) k- = (500 / 7) 0. = K q L = C vo ( - ) = 0.77( ) = 78.5 kj/kg q H = h - h C po ( - ) =.00( ) = 67 kj/kg Overall performance = - q L / q H = / 67 = w net = q net = q H - q L = = 95.5 kj/kg v max = v = R / = / 00 = 0.05 m /kg v min = v max / (v / v ) = 0.05 /.67 = 0.0 m /kg meff = w net v max v min = 95.5 / ( ) = 997 ka v v Remark: hi i a too low compreion ratio for a practical dieel cycle.
7 .6 he effect of a number of open feedwater heater on the thermal efficiency of an ideal cycle i to be tudied. Steam leave the team generator at 0 Ma, 600 C, and the cycle ha a condener preure of 0 ka. Determine the thermal efficiency for each of the following cae. A: No feedwater heater. B: One feedwater heater operating at Ma. C: wo feedwater heater, one operating at Ma and the other at 0. Ma. a) no feed water heater w = vd 0.000(0000-0) = 0. kj/kg h = h + w = =.0 = = = x x = h = = w = h - h = = 77.9 kj/kg w N = w - w = = 57.7 q H = h - h = = 5.6 H = w N q H = = 0.8 S. GEN. URBINE. COND. 0 Ma o 600 C 0 ka b) one feedwater heater w = 0.000(000-0) =.0 kj/kg h = h + w = = 9.8 w = ) =. kj/kg h = h + w = = = 5 = =.87 + x 6.78 S. GEN. 5 6 HR. URBINE. COND. 7
8 x 6 = h 6 = = 7. CV: heater cont: m = m 6 + m =.0 kg t law: m 6 h 6 + m h = m h m 6 = = Ma 5 o 600 C Ma 6 0 ka 7 m = 0.776, h 7 = ( = h of part a) ) CV: turbine w = (h 5 - h 6 ) + m (h 6 - h 7 ) = ( ) ( ) = 5.5 kj/kg CV: pump w = m w + m w = 0.776(.0) + (.) =. kj/kg w N = = 0. kj/kg CV: team generator q H = h 5 - h = = 75. kj/kg H = w N /q H = 0./75. = 0.7 c) two feedwater heater w = (00-0) = 0. kj/kg h = w + h = = 9.0 w = (000-00) =.0 kj/kg h = h + w = = S. GEN. 6 H HR URBINE. L HR 9 0 COND.
9 w 56 = 0.007( ) = 0.7 kj/kg h 6 = h 5 + w 56 = = = 7 = = 9. o C at 8 = Ma h 8 = = 8 = =.50 + x o 600 C 7 Ma Ma 0. Ma 0 ka x 9 = => h 9 = = 6.8 kj/kg CV: high preure heater cont: m 5 = m + m 8 =.0 kg ; t law: m 5 h 5 = m h + m 8 h m 8 = = 0.00 m = CV: low preure heater cont: m 9 + m = m = m ; t law: m 9 h 9 + m h = m h ( ) m 9 = = m = = CV: turbine w = (h 7 - h 8 ) + ( - m 8 )(h 8 - h 9 ) + ( - m 8 - m 9 )(h 9 - h 0 ) = ( ) ( ) ( ) = 8.0 kj/kg CV: pump w = m w + m w + m 5 w 56 = 0.687(0.) (.0) + (0.7) =. kj/kg w N = =.8 kj/kg CV: team generator q H = h 7 - h 6 = = kj/kg H = w N /q H =.8/508.5 = 0.88
10 .66 A jet ejector, a device with no moving part, function a the equivalent of a coupled turbine-compreor unit (ee roblem 9.8 and 9.90). hu, the turbinecompreor in the dual-loop cycle of Fig..09 could be replaced by a jet ejector. he primary tream of the jet ejector enter from the boiler, the econdary tream enter from the evaporator, and the dicharge flow to the condener. Alternatively, a jet ejector may be ued with water a the working fluid. he purpoe of the device i to chill water, uually for an air-conditioning ytem. In thi application the phyical etup i a hown in Fig..6. Uing the data given on the diagram, evaluate the performance of thi cycle in term of the ratio Q L /Q H. a. Aume an ideal cycle. b. Aume an ejector efficiency of 0% (ee roblem 9.90). VA o 50 C. Q H BOIL. H. JE EJEC. COND. 0 o C o C CHILL. 8 QL VA 0 o C FLASH CH. L. LIQ 0 o C (from mixing tream & 9). = = 5 = 8 = 9 = 0 = G 0 o C =.6 ka = = G 50 o C = 75.8 ka, ,0 6 = 7 = 0 o C = 50 o C = 0 o C 9 = 0 o C Aume 5 = 0 ' = 6 = 7 = G 0 o C =.76 ka ' Cont: ṁ + ṁ 9 = ṁ 5 + ṁ 0, ṁ 5 = ṁ 6 = ṁ 7 + ṁ ṁ 7 = ṁ 8 = ṁ 9, ṁ 0 = ṁ = ṁ, ṁ = ṁ a) ṁ + ṁ = ṁ ; ideal jet ejector = & = (' & ' at = ) then, ṁ (h - h ) = ṁ (h - h )
11 From = = x 8.06; x = h = = kj/kg From = = = C, h = 70. kj/kg ṁ /ṁ = =.5677 Alo h = 5.79 kj/kg, h 7 =.0 kj/kg, h 9 = 8.96 kj/kg Mixing of tream & 9 5 & 0: (ṁ + ṁ )h + ṁ 7 h 9 = (ṁ 7 + ṁ + ṁ )h 5 = 0 Flah chamber (ince h 6 = h 5 ) : (ṁ 7 +ṁ )h 5 = 0 = ṁ h + ṁ 7 h uing the primary tream ṁ = kg/: ṁ = (ṁ )h 5 & (ṁ )h 5 = ṁ 7.0 Solving, ṁ 7 = 0.67 & h 5 = 8.88 kj/kg L pump: -w L = 0.000( ) = 0.00 kj/kg h 8 = h 7 - w L = =.0 kj/kg Chiller: Q. L = ṁ 7 (h 9 -h 8 ) = 0.67( ) = 8500 kw (for ṁ = ) H pump: -w H = ( ) = 0.7 kj/kg h = h 0 - w H = = 85.5 kj/kg Boiler: Q. = ṁ (h - h ) = ( ) = 66. kw Q. L /Q. = 8500/66. =.9 H b) Jet eject. eff. = (ṁ /ṁ ) AC /(ṁ /ṁ ) IDEAL = 0.0 (ṁ /ṁ ) AC = = 0.75 uing ṁ = kg/: ṁ = (ṁ )h 5 & (ṁ )h 5 = ṁ 7.0 Solving, ṁ 7 = 9.76 & h 5 = h 0 = kj/kg
12 hen, Q. = 9.76( ) = 668 kw L h = = 86.6 kj/kg Q. = ( ) = 660. kw H & Q. L /Q. = 668/660. = 0.67 H
13
14 EXAMLE E9-9 A combined ga turbine-team power plant ha a net power output of 500 MW. Air enter the compreor of the ga turbine at 00 ka, 00 K, and ha a compreion ratio of and an ientropic efficiency of 85%. he turbine ha an ientropic efficiency of 90%, inlet condition of 00 ka and 00 K, and an exit preure of 00 ka. he air from the turbine exhaut pae through a heat exchanger and exit at 00 K. On the team turbine ide, team at 8 Ma, 00 o C enter the turbine, which ha an ientropic efficiency of 85%, and expand to the condener preure of 8 ka. Saturated water at 8 ka i circulated back to the heat exchanger by a pump with an ientropic efficiency of 80%. Determine (a) the ratio of ma flow rate in the two cycle, (b) the ma flow rate of air, and (c) the thermal efficiency. (d) What-if-Scenario: What would the thermal efficiency be if the turbine inlet temperature increaed to 600 K? [Manual Solution] [ES Solution]
15
SOLUTION MANUAL CHAPTER 12
 SOLUION MANUAL CHAPER CONEN SUBSECION PROB NO. In-ext Concept Quetion a-g Concept problem - Brayton cycle, ga turbine - Regenerator, Intercooler, nonideal cycle 5-9 Ericon cycle 0- Jet engine cycle -5
SOLUION MANUAL CHAPER CONEN SUBSECION PROB NO. In-ext Concept Quetion a-g Concept problem - Brayton cycle, ga turbine - Regenerator, Intercooler, nonideal cycle 5-9 Ericon cycle 0- Jet engine cycle -5
SOLUTION MANUAL ENGLISH UNIT PROBLEMS CHAPTER 11 SONNTAG BORGNAKKE VAN WYLEN. FUNDAMENTALS of. Thermodynamics. Sixth Edition
 SOLUION MANUAL ENGLISH UNI PROBLEMS CHAPER SONNAG BORGNAKKE VAN WYLEN FUNDAMENALS of hermodynamic Sixth Edition CHAPER SUBSECION PROB NO. Rankine Cycle 67-8 Brayton Cycle 8-87 Otto, Dieel, Stirling and
SOLUION MANUAL ENGLISH UNI PROBLEMS CHAPER SONNAG BORGNAKKE VAN WYLEN FUNDAMENALS of hermodynamic Sixth Edition CHAPER SUBSECION PROB NO. Rankine Cycle 67-8 Brayton Cycle 8-87 Otto, Dieel, Stirling and
KNOWN: Air undergoes a polytropic process in a piston-cylinder assembly. The work is known.
 PROBLEM.7 A hown in Fig. P.7, 0 ft of air at T = 00 o R, 00 lbf/in. undergoe a polytropic expanion to a final preure of 5.4 lbf/in. The proce follow pv. = contant. The work i W = 94.4 Btu. Auming ideal
PROBLEM.7 A hown in Fig. P.7, 0 ft of air at T = 00 o R, 00 lbf/in. undergoe a polytropic expanion to a final preure of 5.4 lbf/in. The proce follow pv. = contant. The work i W = 94.4 Btu. Auming ideal
Given A gas turbine power plant operating with air-standard Brayton cycle
 ME-200 Fall 2017 HW-38 1/4 Given A ga turbine power plant operating with air-tandard Brayton cycle Find For ientropic compreion and expanion: (a) Net power (kw) produced by the power plant (b) Thermal
ME-200 Fall 2017 HW-38 1/4 Given A ga turbine power plant operating with air-tandard Brayton cycle Find For ientropic compreion and expanion: (a) Net power (kw) produced by the power plant (b) Thermal
Department of Mechanical Engineering ME 322 Mechanical Engineering Thermodynamics. Lecture 26. Use of Regeneration in Vapor Power Cycles
 Department of Mechanical Engineering ME 322 Mechanical Engineering Thermodynamics Lecture 2 Use of Regeneration in Vapor Power Cycles What is Regeneration? Goal of regeneration Reduce the fuel input requirements
Department of Mechanical Engineering ME 322 Mechanical Engineering Thermodynamics Lecture 2 Use of Regeneration in Vapor Power Cycles What is Regeneration? Goal of regeneration Reduce the fuel input requirements
( ) Given: In a constant pressure combustor. C4H10 and theoretical air burns at P1 = 0.2 MPa, T1 = 600K. Products exit at P2 = 0.
 (SP 9) N-butane (C4H1) i burned with 85 percent theoretical air, and the product of combution, an equilibrium mixture containing only O, CO, CO, H, HO, N, and NO, exit from a combution chamber at K,. MPa.
(SP 9) N-butane (C4H1) i burned with 85 percent theoretical air, and the product of combution, an equilibrium mixture containing only O, CO, CO, H, HO, N, and NO, exit from a combution chamber at K,. MPa.
MAE320-HW7A. 1b). The entropy of an isolated system increases during a process. A). sometimes B). always C). never D).
 MAE0-W7A The homework i due Monday, November 4, 06. Each problem i worth the point indicated. Copying o the olution rom another i not acceptable. (). Multiple choice (0 point) a). Which tatement i invalid
MAE0-W7A The homework i due Monday, November 4, 06. Each problem i worth the point indicated. Copying o the olution rom another i not acceptable. (). Multiple choice (0 point) a). Which tatement i invalid
ENGR Thermodynamics
 ENGR 224 - hermodynamics #1 - Diagram for a Cascade VCR Cycle (21 ts) Baratuci Final 13-Jun-11 On a full sheet of paper, construct a complete Diagram for the cascade cascade vapor-compression refrigeration
ENGR 224 - hermodynamics #1 - Diagram for a Cascade VCR Cycle (21 ts) Baratuci Final 13-Jun-11 On a full sheet of paper, construct a complete Diagram for the cascade cascade vapor-compression refrigeration
8-4 P 2. = 12 kw. AIR T = const. Therefore, Q &
 8-4 8-4 Air i compreed teadily by a compreor. e air temperature i mataed contant by eat rejection to te urroundg. e rate o entropy cange o air i to be determed. Aumption i i a teady-low proce ce tere i
8-4 8-4 Air i compreed teadily by a compreor. e air temperature i mataed contant by eat rejection to te urroundg. e rate o entropy cange o air i to be determed. Aumption i i a teady-low proce ce tere i
Unit 12 Refrigeration and air standard cycles November 30, 2010
 Unit Rerigeration and air tandard cycle Noveber 0, 00 Unit Twelve Rerigeration and Air Standard Cycle Mechanical Engineering 70 Therodynaic Larry Caretto Noveber 0, 00 Outline Two oewhat related topic
Unit Rerigeration and air tandard cycle Noveber 0, 00 Unit Twelve Rerigeration and Air Standard Cycle Mechanical Engineering 70 Therodynaic Larry Caretto Noveber 0, 00 Outline Two oewhat related topic
v v = = Mixing chamber: = 30 or, = s6 Then, and = 52.4% Turbine Boiler process heater Condenser 7 MPa Q in 0.6 MPa Q proces 10 kpa Q out
 0-0- A cogeneration plant i to generate poer and proce eat. art o te team extracted rom te turbe at a relatively ig preure i ued or proce eatg. e poer produced and te utilization actor o te plant are to
0-0- A cogeneration plant i to generate poer and proce eat. art o te team extracted rom te turbe at a relatively ig preure i ued or proce eatg. e poer produced and te utilization actor o te plant are to
I. (20%) Answer the following True (T) or False (F). If false, explain why for full credit.
 I. (20%) Answer the following True (T) or False (F). If false, explain why for full credit. Both the Kelvin and Fahrenheit scales are absolute temperature scales. Specific volume, v, is an intensive property,
I. (20%) Answer the following True (T) or False (F). If false, explain why for full credit. Both the Kelvin and Fahrenheit scales are absolute temperature scales. Specific volume, v, is an intensive property,
ME 354 THERMODYNAMICS 2 MIDTERM EXAMINATION. Instructor: R. Culham. Name: Student ID Number: Instructions
 ME 354 THERMODYNAMICS 2 MIDTERM EXAMINATION February 14, 2011 5:30 pm - 7:30 pm Instructor: R. Culham Name: Student ID Number: Instructions 1. This is a 2 hour, closed-book examination. 2. Answer all questions
ME 354 THERMODYNAMICS 2 MIDTERM EXAMINATION February 14, 2011 5:30 pm - 7:30 pm Instructor: R. Culham Name: Student ID Number: Instructions 1. This is a 2 hour, closed-book examination. 2. Answer all questions
Course: MECH-341 Thermodynamics II Semester: Fall 2006
 FINAL EXAM Date: Thursday, December 21, 2006, 9 am 12 am Examiner: Prof. E. Timofeev Associate Examiner: Prof. D. Frost READ CAREFULLY BEFORE YOU PROCEED: Course: MECH-341 Thermodynamics II Semester: Fall
FINAL EXAM Date: Thursday, December 21, 2006, 9 am 12 am Examiner: Prof. E. Timofeev Associate Examiner: Prof. D. Frost READ CAREFULLY BEFORE YOU PROCEED: Course: MECH-341 Thermodynamics II Semester: Fall
m = P 1V 1 RT 1 P 2 = P 1. P 2 V 2 T 2 = P 1V 1 T 1 V 1 2 V 1 T 2 = T 1. {z} T 2 = T 1 1W 2 = PdV = P 1 (V 2 V 1 ). Z T2 (c vo + αt)dt.
 NAME: SOLUTION AME 0 Thermodynamic Examination Prof J M Power March 00 Happy 56th birthday, Sir Dugald Clerk, inventor of the two-troke engine, b March 85 (5) A calorically imperfect ideal ga, with ga
NAME: SOLUTION AME 0 Thermodynamic Examination Prof J M Power March 00 Happy 56th birthday, Sir Dugald Clerk, inventor of the two-troke engine, b March 85 (5) A calorically imperfect ideal ga, with ga
Turbo machinery: is a device that exchanges energy with a fluid using continuously flowing fluid and rotating blades. Examples are : Wind turbines,
 . Introduction urbo machinery: i a device that exchange energy with a fluid uing continuouly flowing fluid and rotating ble. Example are : Wind turbine, Water turbine, Aircraft engine. 2 laification of
. Introduction urbo machinery: i a device that exchange energy with a fluid uing continuouly flowing fluid and rotating ble. Example are : Wind turbine, Water turbine, Aircraft engine. 2 laification of
T Turbine 8. Boiler fwh fwh I Condenser 4 3 P II P I P III. (a) From the steam tables (Tables A-4, A-5, and A-6), = = 10 MPa. = 0.
 - - A team poer plant operate on an ideal regenerative anke cycle it to open feedater eater. e poer put of te poer plant and te termal efficiency of te cycle are to be determed. Aumption Steady operatg
- - A team poer plant operate on an ideal regenerative anke cycle it to open feedater eater. e poer put of te poer plant and te termal efficiency of te cycle are to be determed. Aumption Steady operatg
Solutions to Homework #10
 Solution to Homeork #0 0-6 A teady-lo Carnot enge it ater a te orkg luid operate at peciied condition. e termal eiciency, te preure at te turbe let, and te ork put are to be determed. Aumption Steady operatg
Solution to Homeork #0 0-6 A teady-lo Carnot enge it ater a te orkg luid operate at peciied condition. e termal eiciency, te preure at te turbe let, and te ork put are to be determed. Aumption Steady operatg
ECE309 THERMODYNAMICS & HEAT TRANSFER MIDTERM EXAMINATION. Instructor: R. Culham. Name: Student ID Number:
 ECE309 THERMODYNAMICS & HEAT TRANSFER MIDTERM EXAMINATION June 19, 2015 2:30 pm - 4:30 pm Instructor: R. Culham Name: Student ID Number: Instructions 1. This is a 2 hour, closed-book examination. 2. Permitted
ECE309 THERMODYNAMICS & HEAT TRANSFER MIDTERM EXAMINATION June 19, 2015 2:30 pm - 4:30 pm Instructor: R. Culham Name: Student ID Number: Instructions 1. This is a 2 hour, closed-book examination. 2. Permitted
Availability and Irreversibility
 Availability and Irreversibility 1.0 Overview A critical application of thermodynamics is finding the maximum amount of work that can be extracted from a given energy resource. This calculation forms the
Availability and Irreversibility 1.0 Overview A critical application of thermodynamics is finding the maximum amount of work that can be extracted from a given energy resource. This calculation forms the
Introduction to Engineering thermodynamics 2 nd Edition, Sonntag and Borgnakke. Solution manual
 Introduction to Engineering thermodynamics 2 nd Edition, Sonntag and Borgnakke Solution manual Chapter 6 Claus Borgnakke The picture is a false color thermal image of the space shuttle s main engine. The
Introduction to Engineering thermodynamics 2 nd Edition, Sonntag and Borgnakke Solution manual Chapter 6 Claus Borgnakke The picture is a false color thermal image of the space shuttle s main engine. The
Bernoulli s equation may be developed as a special form of the momentum or energy equation.
 BERNOULLI S EQUATION Bernoulli equation may be developed a a pecial form of the momentum or energy equation. Here, we will develop it a pecial cae of momentum equation. Conider a teady incompreible flow
BERNOULLI S EQUATION Bernoulli equation may be developed a a pecial form of the momentum or energy equation. Here, we will develop it a pecial cae of momentum equation. Conider a teady incompreible flow
ME Thermodynamics I. Lecture Notes and Example Problems
 ME 227.3 Thermodynamics I Lecture Notes and Example Problems James D. Bugg September 2018 Department of Mechanical Engineering Introduction Part I: Lecture Notes This part contains handout versions of
ME 227.3 Thermodynamics I Lecture Notes and Example Problems James D. Bugg September 2018 Department of Mechanical Engineering Introduction Part I: Lecture Notes This part contains handout versions of
Special Topic: Binary Vapor Cycles
 0- Special opic: Bary Vapor ycle 0- Bary poer cycle i a cycle ic i actually a combation o to cycle; one te ig temperature region, and te oter te lo temperature region. It purpoe i to creae termal eiciency.
0- Special opic: Bary Vapor ycle 0- Bary poer cycle i a cycle ic i actually a combation o to cycle; one te ig temperature region, and te oter te lo temperature region. It purpoe i to creae termal eiciency.
Lecture 38: Vapor-compression refrigeration systems
 ME 200 Termodynamics I Lecture 38: Vapor-compression refrigeration systems Yong Li Sangai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Cuan Road Sangai, 200240, P. R. Cina Email
ME 200 Termodynamics I Lecture 38: Vapor-compression refrigeration systems Yong Li Sangai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Cuan Road Sangai, 200240, P. R. Cina Email
ρ water = 1000 kg/m 3 = 1.94 slugs/ft 3 γ water = 9810 N/m 3 = 62.4 lbs/ft 3
 CEE 34 Aut 004 Midterm # Anwer all quetion. Some data that might be ueful are a follow: ρ water = 1000 kg/m 3 = 1.94 lug/ft 3 water = 9810 N/m 3 = 6.4 lb/ft 3 1 kw = 1000 N-m/ 1. (10) A 1-in. and a 4-in.
CEE 34 Aut 004 Midterm # Anwer all quetion. Some data that might be ueful are a follow: ρ water = 1000 kg/m 3 = 1.94 lug/ft 3 water = 9810 N/m 3 = 6.4 lb/ft 3 1 kw = 1000 N-m/ 1. (10) A 1-in. and a 4-in.
Chapter 5. Mass and Energy Analysis of Control Volumes. by Asst. Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn
 Chapter 5 Mass and Energy Analysis of Control Volumes by Asst. Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn Reference: Cengel, Yunus A. and Michael A. Boles, Thermodynamics:
Chapter 5 Mass and Energy Analysis of Control Volumes by Asst. Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn Reference: Cengel, Yunus A. and Michael A. Boles, Thermodynamics:
MAE 11. Homework 8: Solutions 11/30/2018
 MAE 11 Homework 8: Solutions 11/30/2018 MAE 11 Fall 2018 HW #8 Due: Friday, November 30 (beginning of class at 12:00p) Requirements:: Include T s diagram for all cycles. Also include p v diagrams for Ch
MAE 11 Homework 8: Solutions 11/30/2018 MAE 11 Fall 2018 HW #8 Due: Friday, November 30 (beginning of class at 12:00p) Requirements:: Include T s diagram for all cycles. Also include p v diagrams for Ch
CHAPTER 5 MASS AND ENERGY ANALYSIS OF CONTROL VOLUMES
 Thermodynamics: An Engineering Approach 8th Edition in SI Units Yunus A. Çengel, Michael A. Boles McGraw-Hill, 2015 CHAPTER 5 MASS AND ENERGY ANALYSIS OF CONTROL VOLUMES Lecture slides by Dr. Fawzi Elfghi
Thermodynamics: An Engineering Approach 8th Edition in SI Units Yunus A. Çengel, Michael A. Boles McGraw-Hill, 2015 CHAPTER 5 MASS AND ENERGY ANALYSIS OF CONTROL VOLUMES Lecture slides by Dr. Fawzi Elfghi
Unit Workbook 2 - Level 5 ENG U64 Thermofluids 2018 UniCourse Ltd. All Rights Reserved. Sample
 Pearson BTEC Level 5 Higher Nationals in Engineering (RQF) Unit 64: Thermofluids Unit Workbook 2 in a series of 4 for this unit Learning Outcome 2 Vapour Power Cycles Page 1 of 26 2.1 Power Cycles Unit
Pearson BTEC Level 5 Higher Nationals in Engineering (RQF) Unit 64: Thermofluids Unit Workbook 2 in a series of 4 for this unit Learning Outcome 2 Vapour Power Cycles Page 1 of 26 2.1 Power Cycles Unit
Chapter 5. Mass and Energy Analysis of Control Volumes
 Chapter 5 Mass and Energy Analysis of Control Volumes Conservation Principles for Control volumes The conservation of mass and the conservation of energy principles for open systems (or control volumes)
Chapter 5 Mass and Energy Analysis of Control Volumes Conservation Principles for Control volumes The conservation of mass and the conservation of energy principles for open systems (or control volumes)
Step 1: Draw a diagram to represent the system. Draw a T-s process diagram to better visualize the processes occurring during the cycle.
 ENSC 61 Tutorial, eek#8 Ga Refrigeration Cycle A refrigeration yte ug a the workg fluid, conit of an ideal Brayton cycle run revere with a teperature and preure at the let of the copreor of 37C and 100
ENSC 61 Tutorial, eek#8 Ga Refrigeration Cycle A refrigeration yte ug a the workg fluid, conit of an ideal Brayton cycle run revere with a teperature and preure at the let of the copreor of 37C and 100
MAE 101A. Homework 3 Solutions 2/5/2018
 MAE 101A Homework 3 Solution /5/018 Munon 3.6: What preure gradient along the treamline, /d, i required to accelerate water upward in a vertical pipe at a rate of 30 ft/? What i the anwer if the flow i
MAE 101A Homework 3 Solution /5/018 Munon 3.6: What preure gradient along the treamline, /d, i required to accelerate water upward in a vertical pipe at a rate of 30 ft/? What i the anwer if the flow i
+ m B1 = 1. u A1. u B1. - m B1 = V A. /v A = , u B1 + V B. = 5.5 kg => = V tot. Table B.1.
 5.6 A rigid tank is divided into two rooms by a membrane, both containing water, shown in Fig. P5.6. Room A is at 200 kpa, v = 0.5 m3/kg, VA = m3, and room B contains 3.5 kg at 0.5 MPa, 400 C. The membrane
5.6 A rigid tank is divided into two rooms by a membrane, both containing water, shown in Fig. P5.6. Room A is at 200 kpa, v = 0.5 m3/kg, VA = m3, and room B contains 3.5 kg at 0.5 MPa, 400 C. The membrane
Designing scroll expanders for use in heat recovery Rankine cycles
 Deigning croll expander for ue in heat recovery Rankine cycle V Lemort, S Quoilin Thermodynamic Laboratory, Univerity of Liège, Belgium ABSTRACT Thi paper firt invetigate experimentally the performance
Deigning croll expander for ue in heat recovery Rankine cycle V Lemort, S Quoilin Thermodynamic Laboratory, Univerity of Liège, Belgium ABSTRACT Thi paper firt invetigate experimentally the performance
THERMODYNAMICS, FLUID AND PLANT PROCESSES. The tutorials are drawn from other subjects so the solutions are identified by the appropriate tutorial.
 THERMODYNAMICS, FLUID AND PLANT PROCESSES The tutorials are drawn from other subjects so the solutions are identified by the appropriate tutorial. THERMODYNAMICS TUTORIAL 2 THERMODYNAMIC PRINCIPLES SAE
THERMODYNAMICS, FLUID AND PLANT PROCESSES The tutorials are drawn from other subjects so the solutions are identified by the appropriate tutorial. THERMODYNAMICS TUTORIAL 2 THERMODYNAMIC PRINCIPLES SAE
NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor
 NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor T o T T o T F o, Q o F T m,q m T m T m T mo Aumption: 1. Homogeneou Sytem 2. Single Reaction 3. Steady State Two type of problem: 1. Given deired
NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor T o T T o T F o, Q o F T m,q m T m T m T mo Aumption: 1. Homogeneou Sytem 2. Single Reaction 3. Steady State Two type of problem: 1. Given deired
374 Exergy Analysis. sys (u u 0 ) + P 0 (v v 0 ) T 0 (s s 0 ) where. e sys = u + ν 2 /2 + gz.
 374 Exergy Analysis The value of the exergy of the system depends only on its initial and final state, which is set by the conditions of the environment The term T 0 P S is always positive, and it does
374 Exergy Analysis The value of the exergy of the system depends only on its initial and final state, which is set by the conditions of the environment The term T 0 P S is always positive, and it does
THE FIRST LAW APPLIED TO STEADY FLOW PROCESSES
 Chapter 10 THE FIRST LAW APPLIED TO STEADY FLOW PROCESSES It is not the sun to overtake the moon, nor doth the night outstrip theday.theyfloateachinanorbit. The Holy Qur-ān In many engineering applications,
Chapter 10 THE FIRST LAW APPLIED TO STEADY FLOW PROCESSES It is not the sun to overtake the moon, nor doth the night outstrip theday.theyfloateachinanorbit. The Holy Qur-ān In many engineering applications,
Homework #7 Solution. Solutions: ΔP L Δω. Fig. 1
 Homework #7 Solution Aignment:. through.6 Bergen & Vittal. M Solution: Modified Equation.6 becaue gen. peed not fed back * M (.0rad / MW ec)(00mw) rad /ec peed ( ) (60) 9.55r. p. m. 3600 ( 9.55) 3590.45r.
Homework #7 Solution Aignment:. through.6 Bergen & Vittal. M Solution: Modified Equation.6 becaue gen. peed not fed back * M (.0rad / MW ec)(00mw) rad /ec peed ( ) (60) 9.55r. p. m. 3600 ( 9.55) 3590.45r.
Chapter 4 Total Entropy Cannot Decrease 2012/5/6
 Chapter 4 Total Entropy Cannot Decreae "Ice melting" - a claic example of entropy increaing It happen all the time! Nothing you can do About it. Spontaneou and irreverible. It i approaching to an equilibrium
Chapter 4 Total Entropy Cannot Decreae "Ice melting" - a claic example of entropy increaing It happen all the time! Nothing you can do About it. Spontaneou and irreverible. It i approaching to an equilibrium
Dishwasher. Heater. Homework Solutions ME Thermodynamics I Spring HW-1 (25 points)
 HW-1 (25 points) (a) Given: 1 for writing given, find, EFD, etc., Schematic of a household piping system Find: Identify system and location on the system boundary where the system interacts with the environment
HW-1 (25 points) (a) Given: 1 for writing given, find, EFD, etc., Schematic of a household piping system Find: Identify system and location on the system boundary where the system interacts with the environment
first law of ThermodyNamics
 first law of ThermodyNamics First law of thermodynamics - Principle of conservation of energy - Energy can be neither created nor destroyed Basic statement When any closed system is taken through a cycle,
first law of ThermodyNamics First law of thermodynamics - Principle of conservation of energy - Energy can be neither created nor destroyed Basic statement When any closed system is taken through a cycle,
The First Law of Thermodynamics. By: Yidnekachew Messele
 The First Law of Thermodynamics By: Yidnekachew Messele It is the law that relates the various forms of energies for system of different types. It is simply the expression of the conservation of energy
The First Law of Thermodynamics By: Yidnekachew Messele It is the law that relates the various forms of energies for system of different types. It is simply the expression of the conservation of energy
ME 200 Final Exam December 14, :00 a.m. to 10:00 a.m.
 CIRCLE YOUR LECTURE BELOW: First Name Last Name 7:30 a.m. 8:30 a.m. 10:30 a.m. 11:30 a.m. Boregowda Boregowda Braun Bae 2:30 p.m. 3:30 p.m. 4:30 p.m. Meyer Naik Hess ME 200 Final Exam December 14, 2015
CIRCLE YOUR LECTURE BELOW: First Name Last Name 7:30 a.m. 8:30 a.m. 10:30 a.m. 11:30 a.m. Boregowda Boregowda Braun Bae 2:30 p.m. 3:30 p.m. 4:30 p.m. Meyer Naik Hess ME 200 Final Exam December 14, 2015
ECE309 INTRODUCTION TO THERMODYNAMICS & HEAT TRANSFER. 20 June 2005
 ECE309 INTRODUCTION TO THERMODYNAMICS & HEAT TRANSFER 20 June 2005 Midterm Examination R. Culham & M. Bahrami This is a 90 minute, closed-book examination. You are permitted to use one 8.5 in. 11 in. crib
ECE309 INTRODUCTION TO THERMODYNAMICS & HEAT TRANSFER 20 June 2005 Midterm Examination R. Culham & M. Bahrami This is a 90 minute, closed-book examination. You are permitted to use one 8.5 in. 11 in. crib
Problem 1 The turbine is an open system. We identify the steam contained the turbine as the control volume. dt + + =
 ME Fall 8 HW olution Problem he turbe i an open ytem. We identiy the team contaed the turbe a the control volume. Ma conervation: t law o thermodynamic: Aumption: dm m m m dt + + de V V V m h + + gz +
ME Fall 8 HW olution Problem he turbe i an open ytem. We identiy the team contaed the turbe a the control volume. Ma conervation: t law o thermodynamic: Aumption: dm m m m dt + + de V V V m h + + gz +
Chapter 7 ENTROPY. 7-3C The entropy change will be the same for both cases since entropy is a property and it has a fixed value at a fixed state.
 7- Chapter 7 ENROY Entropy and the Increae of Entropy rciple 7-C No. he δ Q repreent the net heat tranfer durg a cycle, which could be poitive. 7-C No. A ytem may produce more (or le) work than it receive
7- Chapter 7 ENROY Entropy and the Increae of Entropy rciple 7-C No. he δ Q repreent the net heat tranfer durg a cycle, which could be poitive. 7-C No. A ytem may produce more (or le) work than it receive
R13 SET - 1 '' ''' '' ' '''' Code No RT21033
 SET - 1 II B. Tech I Semester Supplementary Examinations, June - 2015 THERMODYNAMICS (Com. to ME, AE, AME) Time: 3 hours Max. Marks: 70 Note: 1. Question Paper consists of two parts (Part-A and Part-B)
SET - 1 II B. Tech I Semester Supplementary Examinations, June - 2015 THERMODYNAMICS (Com. to ME, AE, AME) Time: 3 hours Max. Marks: 70 Note: 1. Question Paper consists of two parts (Part-A and Part-B)
PEMP RMD M.S. Ramaiah School of Advanced Studies
 Steam Turbine Seion delivered by: Prof. Q.H. Nagpurwala Seion Objective Thi eion i intended to dicu the following: Claification of team turbine Compounding of team turbine Force, work done and efficiency
Steam Turbine Seion delivered by: Prof. Q.H. Nagpurwala Seion Objective Thi eion i intended to dicu the following: Claification of team turbine Compounding of team turbine Force, work done and efficiency
ESO201A: Thermodynamics
 ESO201A: Thermodynamics First Semester 2015-2016 Mid-Semester Examination Instructor: Sameer Khandekar Time: 120 mins Marks: 250 Solve sub-parts of a question serially. Question #1 (60 marks): One kmol
ESO201A: Thermodynamics First Semester 2015-2016 Mid-Semester Examination Instructor: Sameer Khandekar Time: 120 mins Marks: 250 Solve sub-parts of a question serially. Question #1 (60 marks): One kmol
ENT 254: Applied Thermodynamics
 ENT 54: Applied Thermodynamics Mr. Azizul bin Mohamad Mechanical Engineering Program School of Mechatronic Engineering Universiti Malaysia Perlis (UniMAP) azizul@unimap.edu.my 019-4747351 04-9798679 Chapter
ENT 54: Applied Thermodynamics Mr. Azizul bin Mohamad Mechanical Engineering Program School of Mechatronic Engineering Universiti Malaysia Perlis (UniMAP) azizul@unimap.edu.my 019-4747351 04-9798679 Chapter
Lecture 44: Review Thermodynamics I
 ME 00 Thermodynamics I Lecture 44: Review Thermodynamics I Yong Li Shanghai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Chuan Road Shanghai, 0040, P. R. China Email : liyo@sjtu.edu.cn
ME 00 Thermodynamics I Lecture 44: Review Thermodynamics I Yong Li Shanghai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Chuan Road Shanghai, 0040, P. R. China Email : liyo@sjtu.edu.cn
Thermodynamics II. Week 9
 hermodynamics II Week 9 Example Oxygen gas in a piston cylinder at 300K, 00 kpa with volume o. m 3 is compressed in a reversible adiabatic process to a final temperature of 700K. Find the final pressure
hermodynamics II Week 9 Example Oxygen gas in a piston cylinder at 300K, 00 kpa with volume o. m 3 is compressed in a reversible adiabatic process to a final temperature of 700K. Find the final pressure
ECE309 INTRODUCTION TO THERMODYNAMICS & HEAT TRANSFER. 13 June 2007
 ECE309 INTRODUCTION TO THERMODYNAMICS & HEAT TRANSFER 13 June 2007 Midterm Examination R. Culham This is a 2 hour, open-book examination. You are permitted to use: course text book calculator There are
ECE309 INTRODUCTION TO THERMODYNAMICS & HEAT TRANSFER 13 June 2007 Midterm Examination R. Culham This is a 2 hour, open-book examination. You are permitted to use: course text book calculator There are
FUNDAMENTALS OF THERMODYNAMICS
 FUNDAMENTALS OF THERMODYNAMICS SEVENTH EDITION CLAUS BORGNAKKE RICHARD E. SONNTAG University of Michigan John Wiley & Sons, Inc. PUBLISHER ASSOCIATE PUBLISHER ACQUISITIONS EDITOR SENIOR PRODUCTION EDITOR
FUNDAMENTALS OF THERMODYNAMICS SEVENTH EDITION CLAUS BORGNAKKE RICHARD E. SONNTAG University of Michigan John Wiley & Sons, Inc. PUBLISHER ASSOCIATE PUBLISHER ACQUISITIONS EDITOR SENIOR PRODUCTION EDITOR
ECE309 INTRODUCTION TO THERMODYNAMICS & HEAT TRANSFER. 12 June 2006
 ECE309 INTRODUCTION TO THERMODYNAMICS & HEAT TRANSFER 1 June 006 Midterm Examination R. Culham This is a hour, closed-book examination. You are permitted to use one 8.5 in. 11 in. crib sheet (one side
ECE309 INTRODUCTION TO THERMODYNAMICS & HEAT TRANSFER 1 June 006 Midterm Examination R. Culham This is a hour, closed-book examination. You are permitted to use one 8.5 in. 11 in. crib sheet (one side
Chapter 1 Introduction and Basic Concepts
 Chapter 1 Introduction and Basic Concepts 1-1 Thermodynamics and Energy Application Areas of Thermodynamics 1-2 Importance of Dimensions and Units Some SI and English Units Dimensional Homogeneity Unity
Chapter 1 Introduction and Basic Concepts 1-1 Thermodynamics and Energy Application Areas of Thermodynamics 1-2 Importance of Dimensions and Units Some SI and English Units Dimensional Homogeneity Unity
Engineering Thermodynamics
 David Ng Summer 2017 Contents 1 July 5, 2017 3 1.1 Thermodynamics................................ 3 2 July 7, 2017 3 2.1 Properties.................................... 3 3 July 10, 2017 4 3.1 Systems.....................................
David Ng Summer 2017 Contents 1 July 5, 2017 3 1.1 Thermodynamics................................ 3 2 July 7, 2017 3 2.1 Properties.................................... 3 3 July 10, 2017 4 3.1 Systems.....................................
INTRODUCTION: Shell and tube heat exchangers are one of the most common equipment found in all plants. How it works?
 HEAT EXCHANGERS 1 INTRODUCTION: Shell and tube heat exchangers are one of the most common equipment found in all plants How it works? 2 WHAT ARE THEY USED FOR? Classification according to service. Heat
HEAT EXCHANGERS 1 INTRODUCTION: Shell and tube heat exchangers are one of the most common equipment found in all plants How it works? 2 WHAT ARE THEY USED FOR? Classification according to service. Heat
Digital Control System
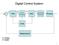 Digital Control Sytem - A D D A Micro ADC DAC Proceor Correction Element Proce Clock Meaurement A: Analog D: Digital Continuou Controller and Digital Control Rt - c Plant yt Continuou Controller Digital
Digital Control Sytem - A D D A Micro ADC DAC Proceor Correction Element Proce Clock Meaurement A: Analog D: Digital Continuou Controller and Digital Control Rt - c Plant yt Continuou Controller Digital
ME 315 Exam 3 8:00-9:00 PM Thursday, April 16, 2009 CIRCLE YOUR DIVISION
 ME 315 Exam 3 8:00-9:00 PM Thurday, Aril 16, 009 Thi i a cloed-book, cloed-note examination. There i a formula heet at the back. You mut turn off all communication device before tarting thi exam, and leave
ME 315 Exam 3 8:00-9:00 PM Thurday, Aril 16, 009 Thi i a cloed-book, cloed-note examination. There i a formula heet at the back. You mut turn off all communication device before tarting thi exam, and leave
Correction for Simple System Example and Notes on Laplace Transforms / Deviation Variables ECHE 550 Fall 2002
 Correction for Simple Sytem Example and Note on Laplace Tranform / Deviation Variable ECHE 55 Fall 22 Conider a tank draining from an initial height of h o at time t =. With no flow into the tank (F in
Correction for Simple Sytem Example and Note on Laplace Tranform / Deviation Variable ECHE 55 Fall 22 Conider a tank draining from an initial height of h o at time t =. With no flow into the tank (F in
Lecture 35: Vapor power systems, Rankine cycle
 ME 00 Thermodynamics I Spring 015 Lecture 35: Vapor power systems, Rankine cycle Yong Li Shanghai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Chuan Road Shanghai, 0040, P. R.
ME 00 Thermodynamics I Spring 015 Lecture 35: Vapor power systems, Rankine cycle Yong Li Shanghai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Chuan Road Shanghai, 0040, P. R.
Readings for this homework assignment and upcoming lectures
 Homework #3 (group) Tuesday, February 13 by 4:00 pm 5290 exercises (individual) Thursday, February 15 by 4:00 pm extra credit (individual) Thursday, February 15 by 4:00 pm Readings for this homework assignment
Homework #3 (group) Tuesday, February 13 by 4:00 pm 5290 exercises (individual) Thursday, February 15 by 4:00 pm extra credit (individual) Thursday, February 15 by 4:00 pm Readings for this homework assignment
5.2. The Rankine Cycle
 Figure 5.1. Illustration of a Carnot cycle based on steam in T-S coordinates. The Carnot cycle has a major advantage over other cycles. It operates at the highest temperature available for as long as possible,
Figure 5.1. Illustration of a Carnot cycle based on steam in T-S coordinates. The Carnot cycle has a major advantage over other cycles. It operates at the highest temperature available for as long as possible,
5/6/ :41 PM. Chapter 6. Using Entropy. Dr. Mohammad Abuhaiba, PE
 Chapter 6 Using Entropy 1 2 Chapter Objective Means are introduced for analyzing systems from the 2 nd law perspective as they undergo processes that are not necessarily cycles. Objective: introduce entropy
Chapter 6 Using Entropy 1 2 Chapter Objective Means are introduced for analyzing systems from the 2 nd law perspective as they undergo processes that are not necessarily cycles. Objective: introduce entropy
Find: a) Mass of the air, in kg, b) final temperature of the air, in K, and c) amount of entropy produced, in kj/k.
 PROBLEM 6.25 Three m 3 of air in a rigid, insulated container fitted with a paddle wheel is initially at 295 K, 200 kpa. The air receives 1546 kj of work from the paddle wheel. Assuming the ideal gas model,
PROBLEM 6.25 Three m 3 of air in a rigid, insulated container fitted with a paddle wheel is initially at 295 K, 200 kpa. The air receives 1546 kj of work from the paddle wheel. Assuming the ideal gas model,
Previous lecture. Today lecture
 Previous lecture ds relations (derive from steady energy balance) Gibb s equations Entropy change in liquid and solid Equations of & v, & P, and P & for steady isentropic process of ideal gas Isentropic
Previous lecture ds relations (derive from steady energy balance) Gibb s equations Entropy change in liquid and solid Equations of & v, & P, and P & for steady isentropic process of ideal gas Isentropic
Chapter 10. Closed-Loop Control Systems
 hapter 0 loed-loop ontrol Sytem ontrol Diagram of a Typical ontrol Loop Actuator Sytem F F 2 T T 2 ontroller T Senor Sytem T TT omponent and Signal of a Typical ontrol Loop F F 2 T Air 3-5 pig 4-20 ma
hapter 0 loed-loop ontrol Sytem ontrol Diagram of a Typical ontrol Loop Actuator Sytem F F 2 T T 2 ontroller T Senor Sytem T TT omponent and Signal of a Typical ontrol Loop F F 2 T Air 3-5 pig 4-20 ma
FINAL EXAM. ME 200 Thermodynamics I, Spring 2013 CIRCLE YOUR LECTURE BELOW:
 ME 200 Thermodynamics I, Spring 2013 CIRCLE YOUR LECTURE BELOW: Div. 5 7:30 am Div. 2 10:30 am Div. 4 12:30 am Prof. Naik Prof. Braun Prof. Bae Div. 3 2:30 pm Div. 1 4:30 pm Div. 6 4:30 pm Prof. Chen Prof.
ME 200 Thermodynamics I, Spring 2013 CIRCLE YOUR LECTURE BELOW: Div. 5 7:30 am Div. 2 10:30 am Div. 4 12:30 am Prof. Naik Prof. Braun Prof. Bae Div. 3 2:30 pm Div. 1 4:30 pm Div. 6 4:30 pm Prof. Chen Prof.
ENGINEERING OF NUCLEAR REACTORS
 22.312 ENGINEERING OF NUCLEAR REACTORS Monday, December 17 th, 2007, 9:00am-12:00 pm FINAL EXAM SOLUTIONS Problem 1 (45%) Analysis of Decay Heat Removal during a Severe Accident i) The energy balance for
22.312 ENGINEERING OF NUCLEAR REACTORS Monday, December 17 th, 2007, 9:00am-12:00 pm FINAL EXAM SOLUTIONS Problem 1 (45%) Analysis of Decay Heat Removal during a Severe Accident i) The energy balance for
Thermodynamics of solids 5. Unary systems. Kwangheon Park Kyung Hee University Department of Nuclear Engineering
 Thermodynamics of solids 5. Unary systems Kwangheon ark Kyung Hee University Department of Nuclear Engineering 5.1. Unary heterogeneous system definition Unary system: one component system. Unary heterogeneous
Thermodynamics of solids 5. Unary systems Kwangheon ark Kyung Hee University Department of Nuclear Engineering 5.1. Unary heterogeneous system definition Unary system: one component system. Unary heterogeneous
FRTN10 Exercise 3. Specifications and Disturbance Models
 FRTN0 Exercie 3. Specification and Diturbance Model 3. A feedback ytem i hown in Figure 3., in which a firt-order proce if controlled by an I controller. d v r u 2 z C() P() y n Figure 3. Sytem in Problem
FRTN0 Exercie 3. Specification and Diturbance Model 3. A feedback ytem i hown in Figure 3., in which a firt-order proce if controlled by an I controller. d v r u 2 z C() P() y n Figure 3. Sytem in Problem
CHAPTER 8 ENTROPY. Blank
 CHAPER 8 ENROPY Blank SONNAG/BORGNAKKE SUDY PROBLEM 8-8. A heat engine efficiency from the inequality of Clausius Consider an actual heat engine with efficiency of η working between reservoirs at and L.
CHAPER 8 ENROPY Blank SONNAG/BORGNAKKE SUDY PROBLEM 8-8. A heat engine efficiency from the inequality of Clausius Consider an actual heat engine with efficiency of η working between reservoirs at and L.
SIMPLE RANKINE CYCLE. 3 expander. boiler. pump. condenser 1 W Q. cycle cycle. net. shaft
 SIMPLE RANKINE CYCLE um boiler exander condener Steady Flow, Oen Sytem - region ace Steady Flow Energy Equation for Procee m (u Pum Proce,, Boiler Proce,, V ρg) 0, 0, Exanion Proce,, 0, Condener Proce,,
SIMPLE RANKINE CYCLE um boiler exander condener Steady Flow, Oen Sytem - region ace Steady Flow Energy Equation for Procee m (u Pum Proce,, Boiler Proce,, V ρg) 0, 0, Exanion Proce,, 0, Condener Proce,,
Spot-on: Safe Fuel/Air Compression
 Spot-on: Safe Fuel/Air Compreion Problem preented by Robert Hart and Kevin Hughe Veeder-Root Participant: Jeffrey Bank Joeph Fehribach Alitair Fitt John Ockendon Colin Pleae Don Schwendeman Burt Tilley
Spot-on: Safe Fuel/Air Compreion Problem preented by Robert Hart and Kevin Hughe Veeder-Root Participant: Jeffrey Bank Joeph Fehribach Alitair Fitt John Ockendon Colin Pleae Don Schwendeman Burt Tilley
R13. II B. Tech I Semester Regular Examinations, Jan THERMODYNAMICS (Com. to ME, AE, AME) PART- A
 SET - 1 II B. Tech I Semester Regular Examinations, Jan - 2015 THERMODYNAMICS (Com. to ME, AE, AME) Time: 3 hours Max. Marks: 70 Note 1. Question Paper consists of two parts (Part-A and Part-B) 2. Answer
SET - 1 II B. Tech I Semester Regular Examinations, Jan - 2015 THERMODYNAMICS (Com. to ME, AE, AME) Time: 3 hours Max. Marks: 70 Note 1. Question Paper consists of two parts (Part-A and Part-B) 2. Answer
Chapter 9 Practical cycles
 Prof.. undararajan Chater 9 Practical cycles 9. Introduction In Chaters 7 and 8, it was shown that a reversible engine based on the Carnot cycle (two reversible isothermal heat transfers and two reversible
Prof.. undararajan Chater 9 Practical cycles 9. Introduction In Chaters 7 and 8, it was shown that a reversible engine based on the Carnot cycle (two reversible isothermal heat transfers and two reversible
UNIT 15 RELIABILITY EVALUATION OF k-out-of-n AND STANDBY SYSTEMS
 UNIT 1 RELIABILITY EVALUATION OF k-out-of-n AND STANDBY SYSTEMS Structure 1.1 Introduction Objective 1.2 Redundancy 1.3 Reliability of k-out-of-n Sytem 1.4 Reliability of Standby Sytem 1. Summary 1.6 Solution/Anwer
UNIT 1 RELIABILITY EVALUATION OF k-out-of-n AND STANDBY SYSTEMS Structure 1.1 Introduction Objective 1.2 Redundancy 1.3 Reliability of k-out-of-n Sytem 1.4 Reliability of Standby Sytem 1. Summary 1.6 Solution/Anwer
Theoretical & Derivation based Questions and Answer. Unit Derive the condition for exact differentials. Solution:
 Theoretical & Derivation based Questions and Answer Unit 01 1. Derive the condition for exact differentials. Solution: 2*. Derive the Maxwell relations and explain their importance in thermodynamics. Solution:
Theoretical & Derivation based Questions and Answer Unit 01 1. Derive the condition for exact differentials. Solution: 2*. Derive the Maxwell relations and explain their importance in thermodynamics. Solution:
ME 201 Thermodynamics
 ME 0 Thermodynamics Solutions First Law Practice Problems. Consider a balloon that has been blown up inside a building and has been allowed to come to equilibrium with the inside temperature of 5 C and
ME 0 Thermodynamics Solutions First Law Practice Problems. Consider a balloon that has been blown up inside a building and has been allowed to come to equilibrium with the inside temperature of 5 C and
Spring_#8. Thermodynamics. Youngsuk Nam
 Spring_#8 Thermodynamics Youngsuk Nam ysnam1@khu.ac.krac kr Ch.7: Entropy Apply the second law of thermodynamics to processes. Define a new property called entropy to quantify the secondlaw effects. Establish
Spring_#8 Thermodynamics Youngsuk Nam ysnam1@khu.ac.krac kr Ch.7: Entropy Apply the second law of thermodynamics to processes. Define a new property called entropy to quantify the secondlaw effects. Establish
Chapter 7. Entropy. by Asst.Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn
 Chapter 7 Entropy by Asst.Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn Reference: Cengel, Yunus A. and Michael A. Boles, Thermodynamics: An Engineering Approach, 5th ed.,
Chapter 7 Entropy by Asst.Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn Reference: Cengel, Yunus A. and Michael A. Boles, Thermodynamics: An Engineering Approach, 5th ed.,
 CHAPTER INTRODUCTION AND BASIC PRINCIPLES. (Tutorial). Determine if the following properties of the system are intensive or extensive properties: Property Intensive Extensive Volume Density Conductivity
CHAPTER INTRODUCTION AND BASIC PRINCIPLES. (Tutorial). Determine if the following properties of the system are intensive or extensive properties: Property Intensive Extensive Volume Density Conductivity
OVERVIEW. Air-Standard Power Cycles (open cycle)
 OVERVIEW OWER CYCLE The Rankine Cycle thermal efficiency effects of pressure and temperature Reheat cycle Regenerative cycle Losses and Cogeneration Air-Standard ower Cycles (open cycle) The Brayton cycle
OVERVIEW OWER CYCLE The Rankine Cycle thermal efficiency effects of pressure and temperature Reheat cycle Regenerative cycle Losses and Cogeneration Air-Standard ower Cycles (open cycle) The Brayton cycle
ME Thermodynamics I
 Homework - Week 01 HW-01 (25 points) Given: 5 Schematic of the solar cell/solar panel Find: 5 Identify the system and the heat/work interactions associated with it. Show the direction of the interactions.
Homework - Week 01 HW-01 (25 points) Given: 5 Schematic of the solar cell/solar panel Find: 5 Identify the system and the heat/work interactions associated with it. Show the direction of the interactions.
Engineering Thermodynamics. Chapter 6. Entropy: a measure of Disorder 6.1 Introduction
 Engineering hermodynamics AAi Chapter 6 Entropy: a measure of Disorder 6. Introduction he second law of thermodynamics leads to the definition of a new property called entropy, a quantitative measure of
Engineering hermodynamics AAi Chapter 6 Entropy: a measure of Disorder 6. Introduction he second law of thermodynamics leads to the definition of a new property called entropy, a quantitative measure of
Chapter 7. Entropy: A Measure of Disorder
 Chapter 7 Entropy: A Measure of Disorder Entropy and the Clausius Inequality The second law of thermodynamics leads to the definition of a new property called entropy, a quantitative measure of microscopic
Chapter 7 Entropy: A Measure of Disorder Entropy and the Clausius Inequality The second law of thermodynamics leads to the definition of a new property called entropy, a quantitative measure of microscopic
THE THERMOELASTIC SQUARE
 HE HERMOELASIC SQUARE A mnemonic for remembering thermodynamic identitie he tate of a material i the collection of variable uch a tre, train, temperature, entropy. A variable i a tate variable if it integral
HE HERMOELASIC SQUARE A mnemonic for remembering thermodynamic identitie he tate of a material i the collection of variable uch a tre, train, temperature, entropy. A variable i a tate variable if it integral
SECOND ENGINEER REG. III/2 APPLIED HEAT
 SECOND ENGINEER REG. III/2 APPLIED HEAT LIST OF TOPICS A B C D E F G H I J K Pressure, Temperature, Energy Heat Transfer Internal Energy, Thermodynamic systems. First Law of Thermodynamics Gas Laws, Displacement
SECOND ENGINEER REG. III/2 APPLIED HEAT LIST OF TOPICS A B C D E F G H I J K Pressure, Temperature, Energy Heat Transfer Internal Energy, Thermodynamic systems. First Law of Thermodynamics Gas Laws, Displacement
An introduction to thermodynamics applied to Organic Rankine Cycles
 An introduction to thermodynamics applied to Organic Rankine Cycles By : Sylvain Quoilin PhD Student at the University of Liège November 2008 1 Definition of a few thermodynamic variables 1.1 Main thermodynamics
An introduction to thermodynamics applied to Organic Rankine Cycles By : Sylvain Quoilin PhD Student at the University of Liège November 2008 1 Definition of a few thermodynamic variables 1.1 Main thermodynamics
Computation of Velocity, Pressure and Temperature Profiles in a Cryogenic Turboexpander
 HMT-6-C8 8 th National & 7 th ISHMT-ASME Heat and Ma Tranfer Conference January 4-6, 6 IIT Guwahati, India Computation of Velocity, Preure and Temperature Profile in a Cryogenic Turboexpander Subrata K.
HMT-6-C8 8 th National & 7 th ISHMT-ASME Heat and Ma Tranfer Conference January 4-6, 6 IIT Guwahati, India Computation of Velocity, Preure and Temperature Profile in a Cryogenic Turboexpander Subrata K.
Chapter 12 Radiation Heat Transfer. Special Topic: Heat Transfer from the Human Body
 Chapter 1 Radiation Heat ranfer Special opic: Heat ranfer from the Human Body 1-7C Ye, roughly one-third of the metabolic heat generated by a peron who i reting or doing light work i diipated to the environment
Chapter 1 Radiation Heat ranfer Special opic: Heat ranfer from the Human Body 1-7C Ye, roughly one-third of the metabolic heat generated by a peron who i reting or doing light work i diipated to the environment
Lecture Notes II. As the reactor is well-mixed, the outlet stream concentration and temperature are identical with those in the tank.
 Lecture Note II Example 6 Continuou Stirred-Tank Reactor (CSTR) Chemical reactor together with ma tranfer procee contitute an important part of chemical technologie. From a control point of view, reactor
Lecture Note II Example 6 Continuou Stirred-Tank Reactor (CSTR) Chemical reactor together with ma tranfer procee contitute an important part of chemical technologie. From a control point of view, reactor
10 minutes reading time is allowed for this paper.
 EGT1 ENGINEERING TRIPOS PART IB Tuesday 31 May 2016 2 to 4 Paper 4 THERMOFLUID MECHANICS Answer not more than four questions. Answer not more than two questions from each section. All questions carry the
EGT1 ENGINEERING TRIPOS PART IB Tuesday 31 May 2016 2 to 4 Paper 4 THERMOFLUID MECHANICS Answer not more than four questions. Answer not more than two questions from each section. All questions carry the
Fundamentals of Thermodynamics. Chapter 8. Exergy
 Fundamentals of Thermodynamics Chapter 8 Exergy Exergy Availability, available energy Anergy Unavailable energy Irreversible energy, reversible work, and irreversibility Exergy analysis : Pure Thermodynamics
Fundamentals of Thermodynamics Chapter 8 Exergy Exergy Availability, available energy Anergy Unavailable energy Irreversible energy, reversible work, and irreversibility Exergy analysis : Pure Thermodynamics
Lecture 12 - Non-isolated DC-DC Buck Converter
 ecture 12 - Non-iolated DC-DC Buck Converter Step-Down or Buck converter deliver DC power from a higher voltage DC level ( d ) to a lower load voltage o. d o ene ref + o v c Controller Figure 12.1 The
ecture 12 - Non-iolated DC-DC Buck Converter Step-Down or Buck converter deliver DC power from a higher voltage DC level ( d ) to a lower load voltage o. d o ene ref + o v c Controller Figure 12.1 The
ME 200 Thermodynamics 1 Fall 2016 Final Exam
 Last Name: First Name: Thermo no. ME 200 Thermodynamics 1 Fall 2016 Final Exam Circle your instructor s last name Ardekani Bae Fisher olloway Jackson Meyer Sojka INSTRUCTIONS This is a closed book and
Last Name: First Name: Thermo no. ME 200 Thermodynamics 1 Fall 2016 Final Exam Circle your instructor s last name Ardekani Bae Fisher olloway Jackson Meyer Sojka INSTRUCTIONS This is a closed book and
The exergy of asystemis the maximum useful work possible during a process that brings the system into equilibrium with aheat reservoir. (4.
 Energy Equation Entropy equation in Chapter 4: control mass approach The second law of thermodynamics Availability (exergy) The exergy of asystemis the maximum useful work possible during a process that
Energy Equation Entropy equation in Chapter 4: control mass approach The second law of thermodynamics Availability (exergy) The exergy of asystemis the maximum useful work possible during a process that
