NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor
|
|
|
- Roxanne Poole
- 5 years ago
- Views:
Transcription
1 NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor T o T T o T F o, Q o F T m,q m T m T m T mo Aumption: 1. Homogeneou Sytem 2. Single Reaction 3. Steady State Two type of problem: 1. Given deired production rate, converion and kinetic and other parameter, determine the required reactor ize, heat duty and temperature profile. 2. Given reactor ize, kinetic, etc., determine the compoition of the exit tream. Let u conider a ingle reaction υ =1 A = 0 (1) with the rate given by r = k 10 e E 1 / RT Π α C k 20 e E 2 / RT Π β C (2) =1 = 1 with υ M / A x υ A C = C A T o P Ao (3) 1 + ε A TP o The ma balance in the reactor for pecie can be written a: df dv = υ r (4) v = 0 F = F o (4a) 1
2 or F Ao d dv =( v A) r =R A (4 ) V = 0 = 0 (4 a) The energy balance baed on (a) negligible change in potential and kinetic energy and (b) no work other than flow work i d dv =1 F H +Ý q v = 0 (5) V = 0 F H 1 = F o H o (5a) Baed on further aumption of (c) ideal mixture and (d) ideal gae one get: =1 F C ~ p dt dv =1 H ~ df dv + q ś v = 0 (6a) Uing the idea of (e) mean pecific heat which are contant and (f) contant heat of reaction, one get dt (Qρ)C p dv + (ΔH r )r + q ś v = 0 (6) Qρ = Ý m tot i the ma flow rate which i contant q Ý J v m 3 i the rate of heat addition per unit reactor volume The implet contitutive relationhip for the rate of heat exchange i: q ś v = Ua v (T m T) (7) a m 2 v m 3 - area for heat tranfer per unit reactor volume The equation to be olved imultaneouly are: F ao d dv + υ Ar = 0 (A) 2
3 QρC pm ś dt 647 q v 48 dv (ΔH ) + U (T T ) r r av m = 0 (B) mq m ρ m C pm ś 647 dt q v 48 m dv U (T T) av m = 0 (C) V = 0; = 0; T = T o,, (T m = T mo for cocurrent flow V = V; (T m = T mo for countercurrent flow) (D) and G du dz + dp dz + F = 0 (E) G = ρu - ma velocity P = preure z = V A - axial ditance u = Q A - velocity A cro ectional reactor area F frictional loe Equation (E) i the momentum balance. However thi equation i uually olved eparately and a mean preure i elected for evaluation of ga concentration in eq (3). For gae the ue of ma fraction, w, and extent per unit ma, ξ '' i recommended. (See lecture 1). The equation can then be written a: '' dξ G dz = r (8) G dt dz = β'' '' r + q v (9) z = 0 ξ '' = 0, T = T o (10) 3
4 β '' = ΔH r C p ; q '' v = q v C p (11) where the rate i expreed by: r = k 10 e E 1 / RT Π = 1 «α m 1 + υ tot ξ F o C o α =1 T o P 1 TP o 1 + υ M avo ξ =1 υ k 20 e E 2 / RT Π =1 β m Ý 1 +υ bot F o C o ξ '' β T o P 1 TP o 1+ ( y)m avo ξ "" v (12) M avo - average molecular weight at feed condition Ý m tot = GA ma flow rate «m tot F o = M w o w o ma faction of in the feed. For liquid one can write dξ dτ = r (13) dt dτ = β r + q v (14) τ = 0 ; ξ = 0 ; T = T o (15) β = ΔH r ρc p ; q v = q v '' = Q v ρc p ρ (16) where the rate i given by r = k 10 e E 1 / RT Π ( C o + υ ξ) α = 1 k 20 e E 2 / RT Π ( C o + υ ξ) β = 1 (17) 4
5 τ = z u = V Q - reidence time along the reactor. From eq (8) and (9) or (13) & (14) we can alway get the following relationhip between temperature and extent or T = T o + β '' ξ '' + 1 G q '' vdz z o (18a) τ T = T o + β ξ + q dτ (18b) v o '' For adiabatic operation (q v = 0, q v = 0 ) thi yield the equation of the adiabatic line, i.e extent and temperature atify the relationhip below at any and every point of the reactor T = T o + β '' ξ '' T = T o + β ξ (19a) (19b) The maximum fractional adiabatic temperature rie i given by the Prater number ut like in the cae of a CSTR. ( = β = ΔH r )C Ao (20) T o ( υ A )T o ρc p ΔT ad max Baic type of problem 1. The temperature in the reactor i precribed a. T(z) = T o iothermal reactor. Integrate (8) or (13) and find extent along the reactor. From eq. (9) or (14) find the heat addition/removal requirement along the reactor and the overall heat duty for the reactor. b. T(z) pecified. Integrate (8) or (13) find ξ (z). Ue ξ (z) and T(z) in eq (9)or (14) to get q v (z) 2. The heat addition (removal) rate i precribed a) Adiabatic operation. T = T o + β '' ξ '' or T = T o + βξ. Subtitute into eq (8) or (13) and integrate 5
6 b) Heat duty i precribed. q v '' (z) or q v (z) precribed. Simultaneouly integrate (8) or (9) or ubtitute z T = T o + β '' ξ '' + 1 G q '' vdz into (8) and integrate. o 3. Rate of heat addition (removal) controlled by another equation q ś v = Ua v (T T m ) a) T m = cont. Integrate eq (8) and (9) or eq (13) and (14) imultaneouly. Thi i the cae when reactor tube are immered in boiling medium or condening medium. b) T m determined with T and ξ by equation (A) to (E). ( ) dt G m m dz = mκ ' m T m T κ m = Ua v m C pm G m = Q mρ m A m Note: With cocurrent cooling a PFR can be kept iothermal with countercurrent cooling it cannot in the cae of n-th order reaction. Prove that for an exercie. There i alway a unique teady tate in a PFR. Main problem with PFR i: hot pot formation parametric enitivity and temperature runaway. Claical example of temperature runaway preented by Bilou & Amundon (AIChE J., 2, 117 (1956). PFR cooled from the wall t contant T m = T wall. 6
7 T T m = A hot pot i formed due to a very mall change in wall temperature. The ytem how extreme parameter enitivity. τ Reaction runaway i the phenomenon when a mall change in feed concentration, temperature, flow rate or in coolant temperature trigger a dramatic change in he temperature profile and lead to runaway reaction and exploion. Exact criteria for runaway are difficult to develop. Approximate criteria are given on the encloed graph.. Example 1 A reverible firt order reaction (conidered earlier in a CSTR) i now to be per formed in a PFR. A R (liquid phae) k 1 = 5x10 8 e 12,500 / RT (min 1 ) k 2 = 3.4x10 21 e 32,500 / RT( min 1 ) o ΔH r =20,000 cal/ mol ΔG 298 ρc p 2,000 (cal / lit o C) C Ao = 2(mol / lit) =2,500 cal / mol 7
8 If the feed rate i Q = 100 (lit/min) and the PFR ize i V = 1,500 (lit): a) find final converion in an iothermal reactor operated at 0, 10, 20, 100 C b) determine converion in an adiabatic reactor if the feed i at i) 0 C, ii) 20 C, c) if the maximum permiible temperature i 80 C determine the optimal temperature profile along the reactor neceary to maximize exit converion. d) If the deired converion i 85% find the minimum reactor volume and the deired heat removal rate along the reactor. Permiible temperature range i 0 to 100 C. Solution a) For an iothermal reactor only the ma balance ha to be olved τ = V dx = C A Q Ao o r o A r A = k 1 C A k 2 C R = C Ao [ k 1 (1 ) k 2 ] r A = k 1 C Ao 1 (1e ) in ce k 2 = k 1 e K = k 1 1 e e (r A ) = k C 1 Ao (x x Ae ) e = K Ae 1 + K = k 1 k 1 + k 2 (r A ) = (k 1 + k 2 )C Ao (e ) x 1 A dx τ = A 1 x = ln Ae k 1 + k 2 o e k 1 + k 2 e Solve for converion 1 exp(k 1 (1 + 1 K )τ ) = e 1 exp k 1 τ e τ = 1500 =15 min 100 ( ) 8
9 We get the following reult: T K k 1 x ae x a Same a equilibrium converion The reactor pace time i o large that above 50 C practically equilibrium converion i obtained. a) The adiabatic operating line i T = T o + β A C Ao β A = ΔH r A = 20,000 ρc p 2,000 =10 lit o C mol C Ao = 2 mol lit T = T o + 20 Subtitute thi relationhip into the ma balance and integrate: dx C A Ao dτ =( k 1 + k 2 ) C Ao ( e ) = k 1 C Ao ( k 1 + k 2 ) C Ao τ = 0 = 0 k 1 = k 10 e E 1 / RT ad = k 10 e E 1 / R(T o + 20 ) k 2 = k 20 e E 2 / R(T o +20 ) e = K 1 + K = k 1 k 1 + k 2 Thu integrate numerically d dτ = k 10e E 1 / RT(T o + 20 x ) A k 10 e E1 / R( To+20 ) + k20 e E 2 / R(T o +20 ) τ = 0 = 0 9
10 12, 500 d dτ = 5x (T e o +20 x ) A 5x10 8 e τ = 0 ; = 0 Deired reult i obtained at τ = , (T o +20 ) + 3.4x10 21 e Alternatively we could olve by trial and error the following integral: We find: τ = 15 = o 32, (T o +20 dx 5x10 8 e 12, (T o + 20 x ) 5x10 8 e 12, (T o +20 x ) + 3.4x10 21 e 32,500 i) T o = 0 C = 273 K = 0.78 ΔT adiabatic = 15.7K = 16K T = 289 K ii) T o = 20 C = 293 K = 0.94 = e ΔT adiabatic = 18.8 = 19K T = 292K 1.987(T o +20 x ) x c) In order to maximize converion at given pace time we hould follow the line of maximum rate. T m = ( E 2 E 1 / R) ln k 20E 2 k 10 E 1 + ln 1 = 10, 065 x ln A 1 Since maximum permiible temperature i 80 C (353 K) we have to preheat the feed to 33 K, cool the reactor and keep it iothermal a 353 K until the locu of maximum rate i reached and then run along the locu of maximum rate. The interection of the iothermal line T = 353 K and the T m line determine up to which point the reactor ha to be run iothermally. T = 353 = T m = 10,065 x ln A 1 10, x30.51 exp 353 = = , x exp
11 τ = 1 k 1 + k 2 dx 1 = (e ) (k 1 + k 2 ) ln e e o At 80 C (353 K) from the table given earlier τ = (1+ 1 ln 0.35 ) = 0.017(min) The iothermal operation hould occur in the very entry ection of he reactor. After that the T m line hould be followed. d dτ = 5x108 e 12, T m (1 ) 3.42x10 21 e 32, T m 10,065 T m = x ln A 1 τ = = Deired reult at τ = 15 =0.988 T exit = 288K Really one hould preheat only to adiabatic line. Adiabatic line hould end at T = 353 K, = Hence, the fluid mut be preheated up to T o = T β A C Ao = x0.119 = 350K The graphical repreentation of part (a-c) ha the following form: e a. Iothermal. Solid line are operating line for τ = 15 min T 11
12 e b. Adiabatic. Adiabatic line with τ = 15 T T m e T max c. Operating along the locu of maximum rate d) Permiible temperature range i 0 C to 100 C. We want minimum reactor ize for = Preheat to 100 C, run along the locu of maximum rate τ = = 0.85 o dx 5x10 8 e 12, T m 5x10 8 e 12, T m + 3.4x10 21 e 32, T m 10,065 with T m = x ln A 1 τ = 1.8min Thu with Q = 100 lit/min we need only V = 160 liter The deired temperature profile along the reactor i preented in the encloed graph. The heat removal per unit volume i x 12
13 q ś Q = ρc p(t o T) + (ΔH r )C Ao = 2, 000(100 T) + (20, 000x2) Thi curve i alo preented in the figure. The total heat denity i: q Ý = 2,000(100 70) + 40,000x0.85 Q tot = 1.56x10 5 (cal / lit) With Q = 100 lit/min q Ý tot = 1.56x10 7 (cal / min) For comparion, if cooling failed and reactor ran adiabatically with T o = 100 C one would get ad = 0.068,T exit =126 o C The adiabatic temperature profile i hown alo on the encloed figure. Extenion to Multiple Reaction υ i =1 A = 0 i =1,2,...R (1) or R df dv + υ ir i = 0 = 1,2,...R (2) R υ i i =1 i=1 d X Ý i dv + d X Ý i dv + r i = 0 ( H ) df dv R υ r = 0 i i i=1 + q Ý v = 0 (3) (2a) V = 0 ; F = F o ( Ý X i = Ý X i o ) ; H = H o With the uual aumption made about the energy balance (ee the lecture on CSTR) one get: 13
14 F o =1 C ~ p dt dv + R i =1 ( ΔH r Ti ) r i + q Ý v = 0 (4) The equation to be olved for a et of multiple reaction are: d X Ý i dv + r i = 0 i =1,2...R (A) ρc p Q dt R dv + ( ΔH r i )r i + q Ý v = 0 i=1 V = 0 ; X Ý i = X Ý io T = T o ρq = cont (B) r i = k i10 e E 1i / RT Π α C i k i 20 e E 2i RT Π =1 = 1 C β i (C) with 1+ ρt C = C o o ρ o T 1+ R υ Ý i X i i =1 F o R υ Ý i X i i =1 =1 F bot o (D) The contitutive relationhip for Ý q v i: Ý q v = U av (T m T) a) T m = cont b) T m i governed by another D.E. mρ m Q m C pm dt m dv Ý q v = 0 (E) V=0 T m = T mo (cocurrent flow) V = V T m = T mo (countercurrent flow) 14
15 Problem Conider the reaction introduced in he lat lecture A R R=k 1 C A -k 2 C R (mol/lit ) k 1 = exp 7 83,700 RT x10 3 ( -1 ) k 2 exp , ( -1 ) RT ΔH r =80,000 (J / mol) C ~ p = 40(J / mol K) Activation energie given in oule. 1. The above reaction occur in liquid phae! Permiible temperature range of operation i 300 <T < 900 K. Feed condition: Q o = 100 (lit/) ; T o = 300 K ; C Ao = 1(mol/lit) You have a V = 100 liter PFR. How would you operate thi reactor if the only obective i to maximize the production rate of R. a) What i maximum F R. b) What are final and ΔT. c) What i the profile of heat addition or removal for every 10% of reactor volume. d) What i the overall heat duty for the reactor and any heat exchanger preceding it. e) Sketch your ytem. 2. The above reaction occur in ga phae. The ga feed ate i Q o =100(lit / ) at T o = 300K, P o = 24.6 atm The feed i 50%A, 50% inert. Permiible temperature range i 250< T < 900 K. Preure i contant in the reactor. Gae tart to condene below 250 K. Deired converion i 85%. 15
16 a) What reactor volume i needed if you operate along the locu of maximum rate? b) What i the ditribution of heat duty along the reactor? c) What i the production rate of R? 3. For the above problem what would F R and be if you had a reactor (PFR) of V = 100 liter available? 4. Suppoe that the reactor can only be operated adiabatically and the deired converion i 85%. Minimize the required reactor ize. a) What reactor type do you recommend? b) What feed temperature would you ue? c) What i the heat duty? 16
NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor. F j. T mo Assumptions:
 NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flw Reactr T T T T F j, Q F j T m,q m T m T m T m Aumptin: 1. Hmgeneu Sytem 2. Single Reactin 3. Steady State Tw type f prblem: 1. Given deired prductin rate,
NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flw Reactr T T T T F j, Q F j T m,q m T m T m T m Aumptin: 1. Hmgeneu Sytem 2. Single Reactin 3. Steady State Tw type f prblem: 1. Given deired prductin rate,
Bernoulli s equation may be developed as a special form of the momentum or energy equation.
 BERNOULLI S EQUATION Bernoulli equation may be developed a a pecial form of the momentum or energy equation. Here, we will develop it a pecial cae of momentum equation. Conider a teady incompreible flow
BERNOULLI S EQUATION Bernoulli equation may be developed a a pecial form of the momentum or energy equation. Here, we will develop it a pecial cae of momentum equation. Conider a teady incompreible flow
Feedback Control Systems (FCS)
 Feedback Control Sytem (FCS) Lecture19-20 Routh-Herwitz Stability Criterion Dr. Imtiaz Huain email: imtiaz.huain@faculty.muet.edu.pk URL :http://imtiazhuainkalwar.weebly.com/ Stability of Higher Order
Feedback Control Sytem (FCS) Lecture19-20 Routh-Herwitz Stability Criterion Dr. Imtiaz Huain email: imtiaz.huain@faculty.muet.edu.pk URL :http://imtiazhuainkalwar.weebly.com/ Stability of Higher Order
1 Routh Array: 15 points
 EE C28 / ME34 Problem Set 3 Solution Fall 2 Routh Array: 5 point Conider the ytem below, with D() k(+), w(t), G() +2, and H y() 2 ++2 2(+). Find the cloed loop tranfer function Y () R(), and range of k
EE C28 / ME34 Problem Set 3 Solution Fall 2 Routh Array: 5 point Conider the ytem below, with D() k(+), w(t), G() +2, and H y() 2 ++2 2(+). Find the cloed loop tranfer function Y () R(), and range of k
MAE 101A. Homework 3 Solutions 2/5/2018
 MAE 101A Homework 3 Solution /5/018 Munon 3.6: What preure gradient along the treamline, /d, i required to accelerate water upward in a vertical pipe at a rate of 30 ft/? What i the anwer if the flow i
MAE 101A Homework 3 Solution /5/018 Munon 3.6: What preure gradient along the treamline, /d, i required to accelerate water upward in a vertical pipe at a rate of 30 ft/? What i the anwer if the flow i
External Flow: Flow over Bluff Objects (Cylinders, Spheres, Packed Beds) and Impinging Jets
 External Flow: Flow over Bluff Object (Cylinder, Sphere, Packed Bed) and Impinging Jet he Cylinder in Cro Flow - Condition depend on pecial feature of boundary layer development, including onet at a tagnation
External Flow: Flow over Bluff Object (Cylinder, Sphere, Packed Bed) and Impinging Jet he Cylinder in Cro Flow - Condition depend on pecial feature of boundary layer development, including onet at a tagnation
Lecture Notes II. As the reactor is well-mixed, the outlet stream concentration and temperature are identical with those in the tank.
 Lecture Note II Example 6 Continuou Stirred-Tank Reactor (CSTR) Chemical reactor together with ma tranfer procee contitute an important part of chemical technologie. From a control point of view, reactor
Lecture Note II Example 6 Continuou Stirred-Tank Reactor (CSTR) Chemical reactor together with ma tranfer procee contitute an important part of chemical technologie. From a control point of view, reactor
Q.1. x A =0.8, ε A =δ A *y A = 0.8*5=4 (because feed contains 80 mol% A, y A = 0.8, δ A =((6-1)/1)=5) k= 0.3 hr -1. So, θ = hr Q.
 Q.1 k [ 1 ln(1 x)] x x =.8, ε =δ *y =.8*5=4 (becaue feed contain 8 mol%, y =.8, δ =((6-1)/1)=5) k=. hr -1 So, θ = 16.157 hr Q.2 Q.2 Continue (c) V PFR
Q.1 k [ 1 ln(1 x)] x x =.8, ε =δ *y =.8*5=4 (becaue feed contain 8 mol%, y =.8, δ =((6-1)/1)=5) k=. hr -1 So, θ = 16.157 hr Q.2 Q.2 Continue (c) V PFR
ME 375 FINAL EXAM Wednesday, May 6, 2009
 ME 375 FINAL EXAM Wedneday, May 6, 9 Diviion Meckl :3 / Adam :3 (circle one) Name_ Intruction () Thi i a cloed book examination, but you are allowed three ingle-ided 8.5 crib heet. A calculator i NOT allowed.
ME 375 FINAL EXAM Wedneday, May 6, 9 Diviion Meckl :3 / Adam :3 (circle one) Name_ Intruction () Thi i a cloed book examination, but you are allowed three ingle-ided 8.5 crib heet. A calculator i NOT allowed.
J.P. Holman: 3.09) T sur := Use table 3-1 to determine the shape factor for this problem. 4π r S := T sphere := 30K r 1. S = m k := 1.
 .P. Holman:.09) T ur : 0 Ue table - to determine the hape factor for thi problem. D :.m r : 0.5m π r S : T phere : 0 r D S 7.0 m :.7 m Ue eq. - to calculate the heat lo. q : S T phere T ur q 57.70 .P.
.P. Holman:.09) T ur : 0 Ue table - to determine the hape factor for thi problem. D :.m r : 0.5m π r S : T phere : 0 r D S 7.0 m :.7 m Ue eq. - to calculate the heat lo. q : S T phere T ur q 57.70 .P.
Digital Control System
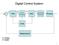 Digital Control Sytem - A D D A Micro ADC DAC Proceor Correction Element Proce Clock Meaurement A: Analog D: Digital Continuou Controller and Digital Control Rt - c Plant yt Continuou Controller Digital
Digital Control Sytem - A D D A Micro ADC DAC Proceor Correction Element Proce Clock Meaurement A: Analog D: Digital Continuou Controller and Digital Control Rt - c Plant yt Continuou Controller Digital
Laplace Transformation
 Univerity of Technology Electromechanical Department Energy Branch Advance Mathematic Laplace Tranformation nd Cla Lecture 6 Page of 7 Laplace Tranformation Definition Suppoe that f(t) i a piecewie continuou
Univerity of Technology Electromechanical Department Energy Branch Advance Mathematic Laplace Tranformation nd Cla Lecture 6 Page of 7 Laplace Tranformation Definition Suppoe that f(t) i a piecewie continuou
MODERN CONTROL SYSTEMS
 MODERN CONTROL SYSTEMS Lecture 1 Root Locu Emam Fathy Department of Electrical and Control Engineering email: emfmz@aat.edu http://www.aat.edu/cv.php?dip_unit=346&er=68525 1 Introduction What i root locu?
MODERN CONTROL SYSTEMS Lecture 1 Root Locu Emam Fathy Department of Electrical and Control Engineering email: emfmz@aat.edu http://www.aat.edu/cv.php?dip_unit=346&er=68525 1 Introduction What i root locu?
Modeling of Transport and Reaction in a Catalytic Bed Using a Catalyst Particle Model.
 Excerpt from the Proceeding of the COMSOL Conference 2010 Boton Modeling of Tranport and Reaction in a Catalytic Bed Uing a Catalyt Particle Model. F. Allain *,1, A.G. Dixon 1 1 Worceter Polytechnic Intitute
Excerpt from the Proceeding of the COMSOL Conference 2010 Boton Modeling of Tranport and Reaction in a Catalytic Bed Uing a Catalyt Particle Model. F. Allain *,1, A.G. Dixon 1 1 Worceter Polytechnic Intitute
KNOWN: Air undergoes a polytropic process in a piston-cylinder assembly. The work is known.
 PROBLEM.7 A hown in Fig. P.7, 0 ft of air at T = 00 o R, 00 lbf/in. undergoe a polytropic expanion to a final preure of 5.4 lbf/in. The proce follow pv. = contant. The work i W = 94.4 Btu. Auming ideal
PROBLEM.7 A hown in Fig. P.7, 0 ft of air at T = 00 o R, 00 lbf/in. undergoe a polytropic expanion to a final preure of 5.4 lbf/in. The proce follow pv. = contant. The work i W = 94.4 Btu. Auming ideal
AOS 104 Fundamentals of Air and Water Pollution
 AOS 104 Fundamental of Air and Water Pollution Dr. Jeffrey Lew lew@atmo.ucla.edu AIM: jklew888 MS1961 310-825-3023 1 Grade Homework 150 pt 2 Mierm 300 pt Final Exam Total 550 pt 1000 pt 2 Homework There
AOS 104 Fundamental of Air and Water Pollution Dr. Jeffrey Lew lew@atmo.ucla.edu AIM: jklew888 MS1961 310-825-3023 1 Grade Homework 150 pt 2 Mierm 300 pt Final Exam Total 550 pt 1000 pt 2 Homework There
Lecture 13. Thermodynamic Potentials (Ch. 5)
 Lecture 13. hermodynamic Potential (Ch. 5) So far we have been uing the total internal energy U and ometime the enthalpy H to characterize variou macrocopic ytem. hee function are called the thermodynamic
Lecture 13. hermodynamic Potential (Ch. 5) So far we have been uing the total internal energy U and ometime the enthalpy H to characterize variou macrocopic ytem. hee function are called the thermodynamic
Lecture 4. Chapter 11 Nise. Controller Design via Frequency Response. G. Hovland 2004
 METR4200 Advanced Control Lecture 4 Chapter Nie Controller Deign via Frequency Repone G. Hovland 2004 Deign Goal Tranient repone via imple gain adjutment Cacade compenator to improve teady-tate error Cacade
METR4200 Advanced Control Lecture 4 Chapter Nie Controller Deign via Frequency Repone G. Hovland 2004 Deign Goal Tranient repone via imple gain adjutment Cacade compenator to improve teady-tate error Cacade
Nonlinear Single-Particle Dynamics in High Energy Accelerators
 Nonlinear Single-Particle Dynamic in High Energy Accelerator Part 6: Canonical Perturbation Theory Nonlinear Single-Particle Dynamic in High Energy Accelerator Thi coure conit of eight lecture: 1. Introduction
Nonlinear Single-Particle Dynamic in High Energy Accelerator Part 6: Canonical Perturbation Theory Nonlinear Single-Particle Dynamic in High Energy Accelerator Thi coure conit of eight lecture: 1. Introduction
Function and Impulse Response
 Tranfer Function and Impule Repone Solution of Selected Unolved Example. Tranfer Function Q.8 Solution : The -domain network i hown in the Fig... Applying VL to the two loop, R R R I () I () L I () L V()
Tranfer Function and Impule Repone Solution of Selected Unolved Example. Tranfer Function Q.8 Solution : The -domain network i hown in the Fig... Applying VL to the two loop, R R R I () I () L I () L V()
Chemical Reaction Engineering
 Lecture 21 Chemical Reaction Engineering (CRE) is the field that studies the rates and mechanisms of chemical reactions and the design of the reactors in which they take place. Web Lecture 21 Class Lecture
Lecture 21 Chemical Reaction Engineering (CRE) is the field that studies the rates and mechanisms of chemical reactions and the design of the reactors in which they take place. Web Lecture 21 Class Lecture
Entropy Minimization in Design of Extractive Distillation System with Internal Heat Exchangers
 Entropy Minimization in Deign of Extractive Ditillation Sytem with Internal Heat Exchanger Diego F. Mendoza, Carlo.M. Riaco * Group of Proce Sytem Engineering, Department of Chemical and Environmental
Entropy Minimization in Deign of Extractive Ditillation Sytem with Internal Heat Exchanger Diego F. Mendoza, Carlo.M. Riaco * Group of Proce Sytem Engineering, Department of Chemical and Environmental
Lecture 4 Topic 3: General linear models (GLMs), the fundamentals of the analysis of variance (ANOVA), and completely randomized designs (CRDs)
 Lecture 4 Topic 3: General linear model (GLM), the fundamental of the analyi of variance (ANOVA), and completely randomized deign (CRD) The general linear model One population: An obervation i explained
Lecture 4 Topic 3: General linear model (GLM), the fundamental of the analyi of variance (ANOVA), and completely randomized deign (CRD) The general linear model One population: An obervation i explained
( ) Given: In a constant pressure combustor. C4H10 and theoretical air burns at P1 = 0.2 MPa, T1 = 600K. Products exit at P2 = 0.
 (SP 9) N-butane (C4H1) i burned with 85 percent theoretical air, and the product of combution, an equilibrium mixture containing only O, CO, CO, H, HO, N, and NO, exit from a combution chamber at K,. MPa.
(SP 9) N-butane (C4H1) i burned with 85 percent theoretical air, and the product of combution, an equilibrium mixture containing only O, CO, CO, H, HO, N, and NO, exit from a combution chamber at K,. MPa.
Lecture 21. The Lovasz splitting-off lemma Topics in Combinatorial Optimization April 29th, 2004
 18.997 Topic in Combinatorial Optimization April 29th, 2004 Lecture 21 Lecturer: Michel X. Goeman Scribe: Mohammad Mahdian 1 The Lovaz plitting-off lemma Lovaz plitting-off lemma tate the following. Theorem
18.997 Topic in Combinatorial Optimization April 29th, 2004 Lecture 21 Lecturer: Michel X. Goeman Scribe: Mohammad Mahdian 1 The Lovaz plitting-off lemma Lovaz plitting-off lemma tate the following. Theorem
On the Isentropic Forchheimer s Sound Waves Propagation in a Cylindrical Tube Filled with a Porous Media
 5th WSEAS Int. Conf. on FLUID MECHANICS (FLUIDS') Acapulco, Mexico, January 5-7, On the Ientropic Forchheimer Sound Wave Propagation in a Cylindrical Tube Filled with a Porou Media H. M. Dwairi Civil Engineering
5th WSEAS Int. Conf. on FLUID MECHANICS (FLUIDS') Acapulco, Mexico, January 5-7, On the Ientropic Forchheimer Sound Wave Propagation in a Cylindrical Tube Filled with a Porou Media H. M. Dwairi Civil Engineering
MODULE 4: ABSORPTION
 MODULE 4: ABSORPTION LECTURE NO. 3 4.4. Deign of packed tower baed on overall ma tranfer coefficient * From overall ma tranfer equation, N K ( y y ) one can write for packed tower a N A K y (y-y*) Then,
MODULE 4: ABSORPTION LECTURE NO. 3 4.4. Deign of packed tower baed on overall ma tranfer coefficient * From overall ma tranfer equation, N K ( y y ) one can write for packed tower a N A K y (y-y*) Then,
ρ water = 1000 kg/m 3 = 1.94 slugs/ft 3 γ water = 9810 N/m 3 = 62.4 lbs/ft 3
 CEE 34 Aut 004 Midterm # Anwer all quetion. Some data that might be ueful are a follow: ρ water = 1000 kg/m 3 = 1.94 lug/ft 3 water = 9810 N/m 3 = 6.4 lb/ft 3 1 kw = 1000 N-m/ 1. (10) A 1-in. and a 4-in.
CEE 34 Aut 004 Midterm # Anwer all quetion. Some data that might be ueful are a follow: ρ water = 1000 kg/m 3 = 1.94 lug/ft 3 water = 9810 N/m 3 = 6.4 lb/ft 3 1 kw = 1000 N-m/ 1. (10) A 1-in. and a 4-in.
Math 273 Solutions to Review Problems for Exam 1
 Math 7 Solution to Review Problem for Exam True or Fale? Circle ONE anwer for each Hint: For effective tudy, explain why if true and give a counterexample if fale (a) T or F : If a b and b c, then a c
Math 7 Solution to Review Problem for Exam True or Fale? Circle ONE anwer for each Hint: For effective tudy, explain why if true and give a counterexample if fale (a) T or F : If a b and b c, then a c
Fermi Distribution Function. n(e) T = 0 T > 0 E F
 LECTURE 3 Maxwell{Boltzmann, Fermi, and Boe Statitic Suppoe we have a ga of N identical point particle in a box ofvolume V. When we ay \ga", we mean that the particle are not interacting with one another.
LECTURE 3 Maxwell{Boltzmann, Fermi, and Boe Statitic Suppoe we have a ga of N identical point particle in a box ofvolume V. When we ay \ga", we mean that the particle are not interacting with one another.
Correction for Simple System Example and Notes on Laplace Transforms / Deviation Variables ECHE 550 Fall 2002
 Correction for Simple Sytem Example and Note on Laplace Tranform / Deviation Variable ECHE 55 Fall 22 Conider a tank draining from an initial height of h o at time t =. With no flow into the tank (F in
Correction for Simple Sytem Example and Note on Laplace Tranform / Deviation Variable ECHE 55 Fall 22 Conider a tank draining from an initial height of h o at time t =. With no flow into the tank (F in
Bogoliubov Transformation in Classical Mechanics
 Bogoliubov Tranformation in Claical Mechanic Canonical Tranformation Suppoe we have a et of complex canonical variable, {a j }, and would like to conider another et of variable, {b }, b b ({a j }). How
Bogoliubov Tranformation in Claical Mechanic Canonical Tranformation Suppoe we have a et of complex canonical variable, {a j }, and would like to conider another et of variable, {b }, b b ({a j }). How
ME 322 Worksheet Winter 2007 Introduction to Compressible Flow
 ME 3 Workheet Winter 007 Introduction to Compreible Flow 1. A two-liter cylindrical tank, 10 cm in diameter, ha a piton that fit perfectly. The piton doe not leak, and there i no friction between the piton
ME 3 Workheet Winter 007 Introduction to Compreible Flow 1. A two-liter cylindrical tank, 10 cm in diameter, ha a piton that fit perfectly. The piton doe not leak, and there i no friction between the piton
CHAPTER 4 DESIGN OF STATE FEEDBACK CONTROLLERS AND STATE OBSERVERS USING REDUCED ORDER MODEL
 98 CHAPTER DESIGN OF STATE FEEDBACK CONTROLLERS AND STATE OBSERVERS USING REDUCED ORDER MODEL INTRODUCTION The deign of ytem uing tate pace model for the deign i called a modern control deign and it i
98 CHAPTER DESIGN OF STATE FEEDBACK CONTROLLERS AND STATE OBSERVERS USING REDUCED ORDER MODEL INTRODUCTION The deign of ytem uing tate pace model for the deign i called a modern control deign and it i
Chapter 10. Closed-Loop Control Systems
 hapter 0 loed-loop ontrol Sytem ontrol Diagram of a Typical ontrol Loop Actuator Sytem F F 2 T T 2 ontroller T Senor Sytem T TT omponent and Signal of a Typical ontrol Loop F F 2 T Air 3-5 pig 4-20 ma
hapter 0 loed-loop ontrol Sytem ontrol Diagram of a Typical ontrol Loop Actuator Sytem F F 2 T T 2 ontroller T Senor Sytem T TT omponent and Signal of a Typical ontrol Loop F F 2 T Air 3-5 pig 4-20 ma
Gain and Phase Margins Based Delay Dependent Stability Analysis of Two- Area LFC System with Communication Delays
 Gain and Phae Margin Baed Delay Dependent Stability Analyi of Two- Area LFC Sytem with Communication Delay Şahin Sönmez and Saffet Ayaun Department of Electrical Engineering, Niğde Ömer Halidemir Univerity,
Gain and Phae Margin Baed Delay Dependent Stability Analyi of Two- Area LFC Sytem with Communication Delay Şahin Sönmez and Saffet Ayaun Department of Electrical Engineering, Niğde Ömer Halidemir Univerity,
7.2 INVERSE TRANSFORMS AND TRANSFORMS OF DERIVATIVES 281
 72 INVERSE TRANSFORMS AND TRANSFORMS OF DERIVATIVES 28 and i 2 Show how Euler formula (page 33) can then be ued to deduce the reult a ( a) 2 b 2 {e at co bt} {e at in bt} b ( a) 2 b 2 5 Under what condition
72 INVERSE TRANSFORMS AND TRANSFORMS OF DERIVATIVES 28 and i 2 Show how Euler formula (page 33) can then be ued to deduce the reult a ( a) 2 b 2 {e at co bt} {e at in bt} b ( a) 2 b 2 5 Under what condition
Solutions to exercises week 45 FYS2160
 Solution to exercie week 45 FYS2160 Kritian Bjørke, Knut Oddvar Høie Vadla November 29, 2017 Schroeder 5.23 a) Writing Φ = U T S µn in term of infiniteimal change of the quantitie involved: dφ = du T ds
Solution to exercie week 45 FYS2160 Kritian Bjørke, Knut Oddvar Høie Vadla November 29, 2017 Schroeder 5.23 a) Writing Φ = U T S µn in term of infiniteimal change of the quantitie involved: dφ = du T ds
Isentropic Sound Waves Propagation in a Tube Filled with a Porous Media
 INTERNATIONAL JOURNAL OF ECHANICS Ientropic Sound Wave Propagation in a Tube Filled with a Porou edia H.. Duwairi Abtract A rigid frame, cylindrical capillary theory of ound propagation in porou media
INTERNATIONAL JOURNAL OF ECHANICS Ientropic Sound Wave Propagation in a Tube Filled with a Porou edia H.. Duwairi Abtract A rigid frame, cylindrical capillary theory of ound propagation in porou media
CHAPTER 8 OBSERVER BASED REDUCED ORDER CONTROLLER DESIGN FOR LARGE SCALE LINEAR DISCRETE-TIME CONTROL SYSTEMS
 CHAPTER 8 OBSERVER BASED REDUCED ORDER CONTROLLER DESIGN FOR LARGE SCALE LINEAR DISCRETE-TIME CONTROL SYSTEMS 8.1 INTRODUCTION 8.2 REDUCED ORDER MODEL DESIGN FOR LINEAR DISCRETE-TIME CONTROL SYSTEMS 8.3
CHAPTER 8 OBSERVER BASED REDUCED ORDER CONTROLLER DESIGN FOR LARGE SCALE LINEAR DISCRETE-TIME CONTROL SYSTEMS 8.1 INTRODUCTION 8.2 REDUCED ORDER MODEL DESIGN FOR LINEAR DISCRETE-TIME CONTROL SYSTEMS 8.3
Linear Motion, Speed & Velocity
 Add Important Linear Motion, Speed & Velocity Page: 136 Linear Motion, Speed & Velocity NGSS Standard: N/A MA Curriculum Framework (006): 1.1, 1. AP Phyic 1 Learning Objective: 3.A.1.1, 3.A.1.3 Knowledge/Undertanding
Add Important Linear Motion, Speed & Velocity Page: 136 Linear Motion, Speed & Velocity NGSS Standard: N/A MA Curriculum Framework (006): 1.1, 1. AP Phyic 1 Learning Objective: 3.A.1.1, 3.A.1.3 Knowledge/Undertanding
Blackbody radiation. Main radiation laws. Sun as an energy source. Solar spectrum and solar constant.
 Lecture 3. lackbody radiation. Main radiation law. Sun a an energy ource. Solar pectrum and olar contant. Objective:. Concept of a blackbody, thermodynamical equilibrium, and local thermodynamical equilibrium..
Lecture 3. lackbody radiation. Main radiation law. Sun a an energy ource. Solar pectrum and olar contant. Objective:. Concept of a blackbody, thermodynamical equilibrium, and local thermodynamical equilibrium..
UNIT 15 RELIABILITY EVALUATION OF k-out-of-n AND STANDBY SYSTEMS
 UNIT 1 RELIABILITY EVALUATION OF k-out-of-n AND STANDBY SYSTEMS Structure 1.1 Introduction Objective 1.2 Redundancy 1.3 Reliability of k-out-of-n Sytem 1.4 Reliability of Standby Sytem 1. Summary 1.6 Solution/Anwer
UNIT 1 RELIABILITY EVALUATION OF k-out-of-n AND STANDBY SYSTEMS Structure 1.1 Introduction Objective 1.2 Redundancy 1.3 Reliability of k-out-of-n Sytem 1.4 Reliability of Standby Sytem 1. Summary 1.6 Solution/Anwer
FLOW REACTORS FOR HOMOGENOUS REACTION: PERFORMANCE EQUATIONS AND APPLICATIONS
 FLOW REACTORS FOR HOMOGENOUS REACTION: PERFORMANCE EQUATIONS AND APPLICATIONS At the end of this week s lecture, students should be able to: Develop and apply the performance equation for plug flow reactors.
FLOW REACTORS FOR HOMOGENOUS REACTION: PERFORMANCE EQUATIONS AND APPLICATIONS At the end of this week s lecture, students should be able to: Develop and apply the performance equation for plug flow reactors.
A First Course on Kinetics and Reaction Engineering. Class 20 on Unit 19
 A First Course on Kinetics and Reaction Engineering Class 20 on Unit 19 Part I - Chemical Reactions Part II - Chemical Reaction Kinetics Where We re Going Part III - Chemical Reaction Engineering A. Ideal
A First Course on Kinetics and Reaction Engineering Class 20 on Unit 19 Part I - Chemical Reactions Part II - Chemical Reaction Kinetics Where We re Going Part III - Chemical Reaction Engineering A. Ideal
Control Systems Analysis and Design by the Root-Locus Method
 6 Control Sytem Analyi and Deign by the Root-Locu Method 6 1 INTRODUCTION The baic characteritic of the tranient repone of a cloed-loop ytem i cloely related to the location of the cloed-loop pole. If
6 Control Sytem Analyi and Deign by the Root-Locu Method 6 1 INTRODUCTION The baic characteritic of the tranient repone of a cloed-loop ytem i cloely related to the location of the cloed-loop pole. If
Finite Element Truss Problem
 6. rue Uing FEA Finite Element ru Problem We tarted thi erie of lecture looking at tru problem. We limited the dicuion to tatically determinate tructure and olved for the force in element and reaction
6. rue Uing FEA Finite Element ru Problem We tarted thi erie of lecture looking at tru problem. We limited the dicuion to tatically determinate tructure and olved for the force in element and reaction
Midterm II. ChE 142 April 11, (Closed Book and notes, two 8.5 x11 sheet of notes is allowed) Printed Name
 ChE 142 pril 11, 25 Midterm II (Closed Book and notes, two 8.5 x11 sheet of notes is allowed) Printed Name KEY By signing this sheet, you agree to adhere to the U.C. Berkeley Honor Code Signed Name_ KEY
ChE 142 pril 11, 25 Midterm II (Closed Book and notes, two 8.5 x11 sheet of notes is allowed) Printed Name KEY By signing this sheet, you agree to adhere to the U.C. Berkeley Honor Code Signed Name_ KEY
NCAAPMT Calculus Challenge Challenge #3 Due: October 26, 2011
 NCAAPMT Calculu Challenge 011 01 Challenge #3 Due: October 6, 011 A Model of Traffic Flow Everyone ha at ome time been on a multi-lane highway and encountered road contruction that required the traffic
NCAAPMT Calculu Challenge 011 01 Challenge #3 Due: October 6, 011 A Model of Traffic Flow Everyone ha at ome time been on a multi-lane highway and encountered road contruction that required the traffic
/University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2009
 Lecture 0 /6/09 /Univerity of Wahington Department of Chemitry Chemitry 453 Winter Quarter 009. Wave Function and Molecule Can quantum mechanic explain the tructure of molecule by determining wave function
Lecture 0 /6/09 /Univerity of Wahington Department of Chemitry Chemitry 453 Winter Quarter 009. Wave Function and Molecule Can quantum mechanic explain the tructure of molecule by determining wave function
Copyright 1967, by the author(s). All rights reserved.
 Copyright 1967, by the author(). All right reerved. Permiion to make digital or hard copie of all or part of thi work for peronal or claroom ue i granted without fee provided that copie are not made or
Copyright 1967, by the author(). All right reerved. Permiion to make digital or hard copie of all or part of thi work for peronal or claroom ue i granted without fee provided that copie are not made or
Root Locus Contents. Root locus, sketching algorithm. Root locus, examples. Root locus, proofs. Root locus, control examples
 Root Locu Content Root locu, ketching algorithm Root locu, example Root locu, proof Root locu, control example Root locu, influence of zero and pole Root locu, lead lag controller deign 9 Spring ME45 -
Root Locu Content Root locu, ketching algorithm Root locu, example Root locu, proof Root locu, control example Root locu, influence of zero and pole Root locu, lead lag controller deign 9 Spring ME45 -
1. Basic introduction to electromagnetic field. wave properties and particulate properties.
 Lecture Baic Radiometric Quantitie. The Beer-Bouguer-Lambert law. Concept of extinction cattering plu aborption and emiion. Schwarzchild equation. Objective:. Baic introduction to electromagnetic field:
Lecture Baic Radiometric Quantitie. The Beer-Bouguer-Lambert law. Concept of extinction cattering plu aborption and emiion. Schwarzchild equation. Objective:. Baic introduction to electromagnetic field:
two equations that govern the motion of the fluid through some medium, like a pipe. These two equations are the
 Fluid and Fluid Mechanic Fluid in motion Dynamic Equation of Continuity After having worked on fluid at ret we turn to a moving fluid To decribe a moving fluid we develop two equation that govern the motion
Fluid and Fluid Mechanic Fluid in motion Dynamic Equation of Continuity After having worked on fluid at ret we turn to a moving fluid To decribe a moving fluid we develop two equation that govern the motion
Module 4: Time Response of discrete time systems Lecture Note 1
 Digital Control Module 4 Lecture Module 4: ime Repone of dicrete time ytem Lecture Note ime Repone of dicrete time ytem Abolute tability i a baic requirement of all control ytem. Apart from that, good
Digital Control Module 4 Lecture Module 4: ime Repone of dicrete time ytem Lecture Note ime Repone of dicrete time ytem Abolute tability i a baic requirement of all control ytem. Apart from that, good
Calculation of the temperature of boundary layer beside wall with time-dependent heat transfer coefficient
 Ŕ periodica polytechnica Mechanical Engineering 54/1 21 15 2 doi: 1.3311/pp.me.21-1.3 web: http:// www.pp.bme.hu/ me c Periodica Polytechnica 21 RESERCH RTICLE Calculation of the temperature of boundary
Ŕ periodica polytechnica Mechanical Engineering 54/1 21 15 2 doi: 1.3311/pp.me.21-1.3 web: http:// www.pp.bme.hu/ me c Periodica Polytechnica 21 RESERCH RTICLE Calculation of the temperature of boundary
Green-Kubo formulas with symmetrized correlation functions for quantum systems in steady states: the shear viscosity of a fluid in a steady shear flow
 Green-Kubo formula with ymmetrized correlation function for quantum ytem in teady tate: the hear vicoity of a fluid in a teady hear flow Hirohi Matuoa Department of Phyic, Illinoi State Univerity, Normal,
Green-Kubo formula with ymmetrized correlation function for quantum ytem in teady tate: the hear vicoity of a fluid in a teady hear flow Hirohi Matuoa Department of Phyic, Illinoi State Univerity, Normal,
Chapter 7. Root Locus Analysis
 Chapter 7 Root Locu Analyi jw + KGH ( ) GH ( ) - K 0 z O 4 p 2 p 3 p Root Locu Analyi The root of the cloed-loop characteritic equation define the ytem characteritic repone. Their location in the complex
Chapter 7 Root Locu Analyi jw + KGH ( ) GH ( ) - K 0 z O 4 p 2 p 3 p Root Locu Analyi The root of the cloed-loop characteritic equation define the ytem characteritic repone. Their location in the complex
Chapter 7: 17, 20, 24, 25, 32, 35, 37, 40, 47, 66 and 79.
 hapter 7: 17, 0,, 5,, 5, 7, 0, 7, 66 and 79. 77 A power tranitor mounted on the wall diipate 0.18 W. he urface temperature of the tranitor i to be determined. Aumption 1 Steady operating condition exit.
hapter 7: 17, 0,, 5,, 5, 7, 0, 7, 66 and 79. 77 A power tranitor mounted on the wall diipate 0.18 W. he urface temperature of the tranitor i to be determined. Aumption 1 Steady operating condition exit.
CE 329, Fall 2015 Second Mid-Term Exam
 CE 39, Fall 15 Second Mid-erm Exam You may only use pencils, pens and erasers while taking this exam. You may NO use a calculator. You may not leave the room for any reason if you do, you must first turn
CE 39, Fall 15 Second Mid-erm Exam You may only use pencils, pens and erasers while taking this exam. You may NO use a calculator. You may not leave the room for any reason if you do, you must first turn
Mass Transfer (Stoffaustausch) Fall Semester 2014
 Ma Tranfer (Stoffautauch) Fall Semeter 4 Tet 5 Noember 4 Name: Legi-Nr.: Tet Duration: 45 minute Permitted material: NOT permitted: calculator copy of Culer book Diffuion ( nd or rd edition) printout of
Ma Tranfer (Stoffautauch) Fall Semeter 4 Tet 5 Noember 4 Name: Legi-Nr.: Tet Duration: 45 minute Permitted material: NOT permitted: calculator copy of Culer book Diffuion ( nd or rd edition) printout of
SERIES COMPENSATION: VOLTAGE COMPENSATION USING DVR (Lectures 41-48)
 Chapter 5 SERIES COMPENSATION: VOLTAGE COMPENSATION USING DVR (Lecture 41-48) 5.1 Introduction Power ytem hould enure good quality of electric power upply, which mean voltage and current waveform hould
Chapter 5 SERIES COMPENSATION: VOLTAGE COMPENSATION USING DVR (Lecture 41-48) 5.1 Introduction Power ytem hould enure good quality of electric power upply, which mean voltage and current waveform hould
Thermodynamics revisited
 Thermodynamics revisited How can I do an energy balance for a reactor system? 1 st law of thermodynamics (differential form): de de = = dq dq--dw dw Energy: de = du + de kin + de pot + de other du = Work:
Thermodynamics revisited How can I do an energy balance for a reactor system? 1 st law of thermodynamics (differential form): de de = = dq dq--dw dw Energy: de = du + de kin + de pot + de other du = Work:
Chemical Reaction Engineering
 Lecture 19 Chemical Reaction Engineering (CRE) is the field that studies the rates and mechanisms of chemical reactions and the design of the reactors in which they take place. oday s lecture Gas Phase
Lecture 19 Chemical Reaction Engineering (CRE) is the field that studies the rates and mechanisms of chemical reactions and the design of the reactors in which they take place. oday s lecture Gas Phase
THERMODYNAMICS OF REACTING SYSTEMS
 ChE 505 Chapter N THERMODYNAMICS OF REACTING SYSTEMS. Introduction Thi chapter explain the baic of thermodynamic calculation for reacting ytem. Reaction toichiometry, heat and entropy of reaction a well
ChE 505 Chapter N THERMODYNAMICS OF REACTING SYSTEMS. Introduction Thi chapter explain the baic of thermodynamic calculation for reacting ytem. Reaction toichiometry, heat and entropy of reaction a well
Jump condition at the boundary between a porous catalyst and a homogeneous fluid
 From the SelectedWork of Francico J. Valde-Parada 2005 Jump condition at the boundary between a porou catalyt and a homogeneou fluid Francico J. Valde-Parada J. Alberto Ochoa-Tapia Available at: http://work.bepre.com/francico_j_valde_parada/12/
From the SelectedWork of Francico J. Valde-Parada 2005 Jump condition at the boundary between a porou catalyt and a homogeneou fluid Francico J. Valde-Parada J. Alberto Ochoa-Tapia Available at: http://work.bepre.com/francico_j_valde_parada/12/
ME 375 FINAL EXAM SOLUTIONS Friday December 17, 2004
 ME 375 FINAL EXAM SOLUTIONS Friday December 7, 004 Diviion Adam 0:30 / Yao :30 (circle one) Name Intruction () Thi i a cloed book eamination, but you are allowed three 8.5 crib heet. () You have two hour
ME 375 FINAL EXAM SOLUTIONS Friday December 7, 004 Diviion Adam 0:30 / Yao :30 (circle one) Name Intruction () Thi i a cloed book eamination, but you are allowed three 8.5 crib heet. () You have two hour
Cake ltration analysis the eect of the relationship between the pore liquid pressure and the cake compressive stress
 Chemical Engineering Science 56 (21) 5361 5369 www.elevier.com/locate/ce Cake ltration analyi the eect of the relationhip between the pore liquid preure and the cake compreive tre C. Tien, S. K. Teoh,
Chemical Engineering Science 56 (21) 5361 5369 www.elevier.com/locate/ce Cake ltration analyi the eect of the relationhip between the pore liquid preure and the cake compreive tre C. Tien, S. K. Teoh,
Euler-Bernoulli Beams
 Euler-Bernoulli Beam The Euler-Bernoulli beam theory wa etablihed around 750 with contribution from Leonard Euler and Daniel Bernoulli. Bernoulli provided an expreion for the train energy in beam bending,
Euler-Bernoulli Beam The Euler-Bernoulli beam theory wa etablihed around 750 with contribution from Leonard Euler and Daniel Bernoulli. Bernoulli provided an expreion for the train energy in beam bending,
Chapter 1 Basic Description of Laser Diode Dynamics by Spatially Averaged Rate Equations: Conditions of Validity
 Chapter 1 Baic Decription of Laer Diode Dynamic by Spatially Averaged Rate Equation: Condition of Validity A laer diode i a device in which an electric current input i converted to an output of photon.
Chapter 1 Baic Decription of Laer Diode Dynamic by Spatially Averaged Rate Equation: Condition of Validity A laer diode i a device in which an electric current input i converted to an output of photon.
Molecular Dynamics Simulations of Nonequilibrium Effects Associated with Thermally Activated Exothermic Reactions
 Original Paper orma, 5, 9 7, Molecular Dynamic Simulation of Nonequilibrium Effect ociated with Thermally ctivated Exothermic Reaction Jerzy GORECKI and Joanna Natalia GORECK Intitute of Phyical Chemitry,
Original Paper orma, 5, 9 7, Molecular Dynamic Simulation of Nonequilibrium Effect ociated with Thermally ctivated Exothermic Reaction Jerzy GORECKI and Joanna Natalia GORECK Intitute of Phyical Chemitry,
SOLUTION MANUAL CHAPTER 12
 SOLUION MANUAL CHAPER CONEN SUBSECION PROB NO. In-ext Concept Quetion a-g Concept problem - Brayton cycle, ga turbine - Regenerator, Intercooler, nonideal cycle 5-9 Ericon cycle 0- Jet engine cycle -5
SOLUION MANUAL CHAPER CONEN SUBSECION PROB NO. In-ext Concept Quetion a-g Concept problem - Brayton cycle, ga turbine - Regenerator, Intercooler, nonideal cycle 5-9 Ericon cycle 0- Jet engine cycle -5
Module 1: Learning objectives
 Heat and Ma Tranfer Module 1: Learning objective Overview: Although much of the material of thi module will be dicued in greater detail, the objective of thi module i to give you a reaonable overview of
Heat and Ma Tranfer Module 1: Learning objective Overview: Although much of the material of thi module will be dicued in greater detail, the objective of thi module i to give you a reaonable overview of
EE Control Systems LECTURE 6
 Copyright FL Lewi 999 All right reerved EE - Control Sytem LECTURE 6 Updated: Sunday, February, 999 BLOCK DIAGRAM AND MASON'S FORMULA A linear time-invariant (LTI) ytem can be repreented in many way, including:
Copyright FL Lewi 999 All right reerved EE - Control Sytem LECTURE 6 Updated: Sunday, February, 999 BLOCK DIAGRAM AND MASON'S FORMULA A linear time-invariant (LTI) ytem can be repreented in many way, including:
Lecture 3 Basic radiometric quantities.
 Lecture 3 Baic radiometric quantitie. The Beer-Bouguer-Lambert law. Concept of extinction cattering plu aborption and emiion. Schwarzchild equation.. Baic introduction to electromagnetic field: Definition,
Lecture 3 Baic radiometric quantitie. The Beer-Bouguer-Lambert law. Concept of extinction cattering plu aborption and emiion. Schwarzchild equation.. Baic introduction to electromagnetic field: Definition,
NAME (pinyin/italian)... MATRICULATION NUMBER... SIGNATURE
 POLITONG SHANGHAI BASIC AUTOMATIC CONTROL June Academic Year / Exam grade NAME (pinyin/italian)... MATRICULATION NUMBER... SIGNATURE Ue only thee page (including the bac) for anwer. Do not ue additional
POLITONG SHANGHAI BASIC AUTOMATIC CONTROL June Academic Year / Exam grade NAME (pinyin/italian)... MATRICULATION NUMBER... SIGNATURE Ue only thee page (including the bac) for anwer. Do not ue additional
ChE 344 Winter 2013 Final Exam + Solution. Open Course Textbook Only Closed everything else (i.e., Notes, In-Class Problems and Home Problems
 ChE 344 Winter 03 Final Exam + Solution Thursday, May, 03 Open Course Textbook Only Closed everything else (i.e., Notes, In-Class Problems and Home Problems Name Honor Code (Please sign in the space provided
ChE 344 Winter 03 Final Exam + Solution Thursday, May, 03 Open Course Textbook Only Closed everything else (i.e., Notes, In-Class Problems and Home Problems Name Honor Code (Please sign in the space provided
MATHEMATICAL MODELING OF INDUCTION MOTORS
 37 CHAPTER 3 MATHEMATICAL MODELING OF INDUCTION MOTORS To tart with, a well-known technique called the SVPWM technique i dicued a thi form the bai of the mathematical modeling of IM. Furthermore, the d
37 CHAPTER 3 MATHEMATICAL MODELING OF INDUCTION MOTORS To tart with, a well-known technique called the SVPWM technique i dicued a thi form the bai of the mathematical modeling of IM. Furthermore, the d
ROOT LOCUS. Poles and Zeros
 Automatic Control Sytem, 343 Deartment of Mechatronic Engineering, German Jordanian Univerity ROOT LOCUS The Root Locu i the ath of the root of the characteritic equation traced out in the - lane a a ytem
Automatic Control Sytem, 343 Deartment of Mechatronic Engineering, German Jordanian Univerity ROOT LOCUS The Root Locu i the ath of the root of the characteritic equation traced out in the - lane a a ytem
AMS 212B Perturbation Methods Lecture 20 Part 1 Copyright by Hongyun Wang, UCSC. is the kinematic viscosity and ˆp = p ρ 0
 Lecture Part 1 Copyright by Hongyun Wang, UCSC Prandtl boundary layer Navier-Stoke equation: Conervation of ma: ρ t + ( ρ u) = Balance of momentum: u ρ t + u = p+ µδ u + ( λ + µ ) u where µ i the firt
Lecture Part 1 Copyright by Hongyun Wang, UCSC Prandtl boundary layer Navier-Stoke equation: Conervation of ma: ρ t + ( ρ u) = Balance of momentum: u ρ t + u = p+ µδ u + ( λ + µ ) u where µ i the firt
2.7 Aerosols and coagulation
 1 Note on 1.63 Advanced Environmental Fluid Mechanic Intructor: C. C. Mei, 1 ccmei@mit.edu, 1 617 53 994 December 1,.7 Aerool and coagulation [Ref]: Preent, Kinetic Theory of Gae Fuch, Mechanic of Aerool
1 Note on 1.63 Advanced Environmental Fluid Mechanic Intructor: C. C. Mei, 1 ccmei@mit.edu, 1 617 53 994 December 1,.7 Aerool and coagulation [Ref]: Preent, Kinetic Theory of Gae Fuch, Mechanic of Aerool
List coloring hypergraphs
 Lit coloring hypergraph Penny Haxell Jacque Vertraete Department of Combinatoric and Optimization Univerity of Waterloo Waterloo, Ontario, Canada pehaxell@uwaterloo.ca Department of Mathematic Univerity
Lit coloring hypergraph Penny Haxell Jacque Vertraete Department of Combinatoric and Optimization Univerity of Waterloo Waterloo, Ontario, Canada pehaxell@uwaterloo.ca Department of Mathematic Univerity
TRIPLE SOLUTIONS FOR THE ONE-DIMENSIONAL
 GLASNIK MATEMATIČKI Vol. 38583, 73 84 TRIPLE SOLUTIONS FOR THE ONE-DIMENSIONAL p-laplacian Haihen Lü, Donal O Regan and Ravi P. Agarwal Academy of Mathematic and Sytem Science, Beijing, China, National
GLASNIK MATEMATIČKI Vol. 38583, 73 84 TRIPLE SOLUTIONS FOR THE ONE-DIMENSIONAL p-laplacian Haihen Lü, Donal O Regan and Ravi P. Agarwal Academy of Mathematic and Sytem Science, Beijing, China, National
ME2142/ME2142E Feedback Control Systems
 Root Locu Analyi Root Locu Analyi Conider the cloed-loop ytem R + E - G C B H The tranient repone, and tability, of the cloed-loop ytem i determined by the value of the root of the characteritic equation
Root Locu Analyi Root Locu Analyi Conider the cloed-loop ytem R + E - G C B H The tranient repone, and tability, of the cloed-loop ytem i determined by the value of the root of the characteritic equation
2b m 1b: Sat liq C, h = kj/kg tot 3a: 1 MPa, s = s 3 -> h 3a = kj/kg, T 3b
 .6 A upercritical team power plant ha a high preure of 0 Ma and an exit condener temperature of 50 C. he maximum temperature in the boiler i 000 C and the turbine exhaut i aturated vapor here i one open
.6 A upercritical team power plant ha a high preure of 0 Ma and an exit condener temperature of 50 C. he maximum temperature in the boiler i 000 C and the turbine exhaut i aturated vapor here i one open
Computation of Velocity, Pressure and Temperature Profiles in a Cryogenic Turboexpander
 HMT-6-C8 8 th National & 7 th ISHMT-ASME Heat and Ma Tranfer Conference January 4-6, 6 IIT Guwahati, India Computation of Velocity, Preure and Temperature Profile in a Cryogenic Turboexpander Subrata K.
HMT-6-C8 8 th National & 7 th ISHMT-ASME Heat and Ma Tranfer Conference January 4-6, 6 IIT Guwahati, India Computation of Velocity, Preure and Temperature Profile in a Cryogenic Turboexpander Subrata K.
THEORETICAL CONSIDERATIONS AT CYLINDRICAL DRAWING AND FLANGING OUTSIDE OF EDGE ON THE DEFORMATION STATES
 THEOETICAL CONSIDEATIONS AT CYLINDICAL DAWING AND FLANGING OUTSIDE OF EDGE ON THE DEFOMATION STATES Lucian V. Severin 1, Dorin Grădinaru, Traian Lucian Severin 3 1,,3 Stefan cel Mare Univerity of Suceava,
THEOETICAL CONSIDEATIONS AT CYLINDICAL DAWING AND FLANGING OUTSIDE OF EDGE ON THE DEFOMATION STATES Lucian V. Severin 1, Dorin Grădinaru, Traian Lucian Severin 3 1,,3 Stefan cel Mare Univerity of Suceava,
Introduction to Laplace Transform Techniques in Circuit Analysis
 Unit 6 Introduction to Laplace Tranform Technique in Circuit Analyi In thi unit we conider the application of Laplace Tranform to circuit analyi. A relevant dicuion of the one-ided Laplace tranform i found
Unit 6 Introduction to Laplace Tranform Technique in Circuit Analyi In thi unit we conider the application of Laplace Tranform to circuit analyi. A relevant dicuion of the one-ided Laplace tranform i found
At the end of this lesson, the students should be able to understand:
 Intructional Objective: At the end of thi leon, the tudent hould be able to undertand: Baic failure mechanim of riveted joint. Concept of deign of a riveted joint. 1. Strength of riveted joint: Strength
Intructional Objective: At the end of thi leon, the tudent hould be able to undertand: Baic failure mechanim of riveted joint. Concept of deign of a riveted joint. 1. Strength of riveted joint: Strength
THE PARAMETERIZATION OF ALL TWO-DEGREES-OF-FREEDOM SEMISTRONGLY STABILIZING CONTROLLERS. Tatsuya Hoshikawa, Kou Yamada and Yuko Tatsumi
 International Journal of Innovative Computing, Information Control ICIC International c 206 ISSN 349-498 Volume 2, Number 2, April 206 pp. 357 370 THE PARAMETERIZATION OF ALL TWO-DEGREES-OF-FREEDOM SEMISTRONGLY
International Journal of Innovative Computing, Information Control ICIC International c 206 ISSN 349-498 Volume 2, Number 2, April 206 pp. 357 370 THE PARAMETERIZATION OF ALL TWO-DEGREES-OF-FREEDOM SEMISTRONGLY
CONTROL SYSTEMS. Chapter 5 : Root Locus Diagram. GATE Objective & Numerical Type Solutions. The transfer function of a closed loop system is
 CONTROL SYSTEMS Chapter 5 : Root Locu Diagram GATE Objective & Numerical Type Solution Quetion 1 [Work Book] [GATE EC 199 IISc-Bangalore : Mark] The tranfer function of a cloed loop ytem i T () where i
CONTROL SYSTEMS Chapter 5 : Root Locu Diagram GATE Objective & Numerical Type Solution Quetion 1 [Work Book] [GATE EC 199 IISc-Bangalore : Mark] The tranfer function of a cloed loop ytem i T () where i
Section Induction motor drives
 Section 5.1 - nduction motor drive Electric Drive Sytem 5.1.1. ntroduction he AC induction motor i by far the mot widely ued motor in the indutry. raditionally, it ha been ued in contant and lowly variable-peed
Section 5.1 - nduction motor drive Electric Drive Sytem 5.1.1. ntroduction he AC induction motor i by far the mot widely ued motor in the indutry. raditionally, it ha been ued in contant and lowly variable-peed
Sampling and the Discrete Fourier Transform
 Sampling and the Dicrete Fourier Tranform Sampling Method Sampling i mot commonly done with two device, the ample-and-hold (S/H) and the analog-to-digital-converter (ADC) The S/H acquire a CT ignal at
Sampling and the Dicrete Fourier Tranform Sampling Method Sampling i mot commonly done with two device, the ample-and-hold (S/H) and the analog-to-digital-converter (ADC) The S/H acquire a CT ignal at
Solving Differential Equations by the Laplace Transform and by Numerical Methods
 36CH_PHCalter_TechMath_95099 3//007 :8 PM Page Solving Differential Equation by the Laplace Tranform and by Numerical Method OBJECTIVES When you have completed thi chapter, you hould be able to: Find the
36CH_PHCalter_TechMath_95099 3//007 :8 PM Page Solving Differential Equation by the Laplace Tranform and by Numerical Method OBJECTIVES When you have completed thi chapter, you hould be able to: Find the
EE Control Systems LECTURE 14
 Updated: Tueday, March 3, 999 EE 434 - Control Sytem LECTURE 4 Copyright FL Lewi 999 All right reerved ROOT LOCUS DESIGN TECHNIQUE Suppoe the cloed-loop tranfer function depend on a deign parameter k We
Updated: Tueday, March 3, 999 EE 434 - Control Sytem LECTURE 4 Copyright FL Lewi 999 All right reerved ROOT LOCUS DESIGN TECHNIQUE Suppoe the cloed-loop tranfer function depend on a deign parameter k We
CHE 404 Chemical Reaction Engineering. Chapter 8 Steady-State Nonisothermal Reactor Design
 Textbook: Elements of Chemical Reaction Engineering, 4 th Edition 1 CHE 404 Chemical Reaction Engineering Chapter 8 Steady-State Nonisothermal Reactor Design Contents 2 PART 1. Steady-State Energy Balance
Textbook: Elements of Chemical Reaction Engineering, 4 th Edition 1 CHE 404 Chemical Reaction Engineering Chapter 8 Steady-State Nonisothermal Reactor Design Contents 2 PART 1. Steady-State Energy Balance
Singular perturbation theory
 Singular perturbation theory Marc R. Rouel June 21, 2004 1 Introduction When we apply the teady-tate approximation (SSA) in chemical kinetic, we typically argue that ome of the intermediate are highly
Singular perturbation theory Marc R. Rouel June 21, 2004 1 Introduction When we apply the teady-tate approximation (SSA) in chemical kinetic, we typically argue that ome of the intermediate are highly
THE THERMOELASTIC SQUARE
 HE HERMOELASIC SQUARE A mnemonic for remembering thermodynamic identitie he tate of a material i the collection of variable uch a tre, train, temperature, entropy. A variable i a tate variable if it integral
HE HERMOELASIC SQUARE A mnemonic for remembering thermodynamic identitie he tate of a material i the collection of variable uch a tre, train, temperature, entropy. A variable i a tate variable if it integral
696 Fu Jing-Li et al Vol. 12 form in generalized coordinate Q ffiq dt = 0 ( = 1; ;n): (3) For nonholonomic ytem, ffiq are not independent of
 Vol 12 No 7, July 2003 cfl 2003 Chin. Phy. Soc. 1009-1963/2003/12(07)/0695-05 Chinee Phyic and IOP Publihing Ltd Lie ymmetrie and conerved quantitie of controllable nonholonomic dynamical ytem Fu Jing-Li(ΛΠ±)
Vol 12 No 7, July 2003 cfl 2003 Chin. Phy. Soc. 1009-1963/2003/12(07)/0695-05 Chinee Phyic and IOP Publihing Ltd Lie ymmetrie and conerved quantitie of controllable nonholonomic dynamical ytem Fu Jing-Li(ΛΠ±)
PRESSURE WORK EFFECTS IN UNSTEADY CONVECTIVELY DRIVEN FLOW ALONG A VERTICAL PLATE
 Proceeding of 3ICCHMT 3 rd International Conference on Computational Heat and Ma Tranfer May 6 3, 3, Banff, CANADA Paper Number 87 PRESSURE WORK EFFECTS IN UNSTEADY CONVECTIVELY DRIVEN FLOW ALONG A VERTICAL
Proceeding of 3ICCHMT 3 rd International Conference on Computational Heat and Ma Tranfer May 6 3, 3, Banff, CANADA Paper Number 87 PRESSURE WORK EFFECTS IN UNSTEADY CONVECTIVELY DRIVEN FLOW ALONG A VERTICAL
