Kelvin Planck Statement of the Second Law. Clausius Statement of the Second Law
|
|
|
- Lynette Mosley
- 6 years ago
- Views:
Transcription
1 Kelv Planck Statement of te Second aw It is imossible to construct an enge wic, oeratg a cycle, will roduce no oter effect tan te extraction of eat from a sgle reservoir and te erformance of an equivalent amount of work. Clausius Statement of te Second aw It is imossible to ave a system oeratg a cycle wic transfers eat from a cooler to a otter body witout work beg done on te system by te surroundgs Reversible eat Enge Reversible Refrigerator
2 Actual eat Enge
3 Actual Refrigeration Mace
4 Carnot Power Cycle Reversible constant temerature rocess Reversible adiabatic, rocess exansion, rocess, rocess eat transfer, Efficiency Desired Effect Required Inut cycle ork
5 Carnot Refrigeration Cycle Reversible constant temerature eat transfer, rocess, rocess Reversible adiabatic exansion, rocess rocess Coefficien t of Performance CP CP refrigerator eat um Desired Effect Required Inut ork out ork
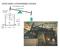
6 NECMEN AMSPERIC ENGINE Ford Museum,Detroit, 760, strokes/m
7 NECMEN AMSPERIC ENGINE Ford Museum, 760 strokes/m atmoseric ressure F 7 stroke 5 sia steam 8 50 F water
8 NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m 5 sia steam 8 F 7 stroke 50 F water A πr F A F d Power Power π 6 (.7 sia.8 sia) 9 lbs ( 7/) ft ft lb 5,68 stroke 765,000 ft lb/m,000 /ft lb/m strokes m 6 9 lbs 5,68 ft lb/ stroke 5 sia ( F) ft lb 765,000 m P or 7.5 kw 50 F (.8 sia) v m V v 6 /stroke strokes ft / strokes ft /6.6 m lb. lb/m
9 NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m 5 sia steam F A F d m 8 F A πr π 6 (.7 sia.8 sia) 9 lbs ( 7/) ft ft lb Power 5,68 stroke 765,000 ft lb/m Power,000 /ft lb/m 7 stroke strokes m 50 F water 6 9 lbs 5,68 ft lb/ stroke ft lb 765,000 m P or 7.5 kw V 6 /stroke strokes ft strokes /6.6. lb/m v ft / m lb g 5 sia ( F) v05.5 BU/lb 80 F (.507 sia) l9.08 sia l m F 9.08 BU/lbm,759. BU/lbm 765,000 ftlb/m 778 ftlb/bu ( BU/lb). lb/m 50.5 BU/lb 9. BU/m CYCE CYCE CARN l 9.BU/m,759. BU/lbm 6.% 80 0.% 60 +
10
11 Carnot Prciles. No enge oeratg between two eat reservoirs, eac avg a fixed temerature, can be more efficient tan a reversible enge oeratg between te same reservoirs. actual Carnot. All reversible enges oeratg between two eat reservoirs, eac avg its own fixed temerature, ave te same efficiency.. e efficiency of any reversible enge oeratg between two reservoirs is deendent of te nature of te workg fluid and deends only on te temerature of te reservoirs.. An absolute temerature scale can be defed a manner deendent of te termometric material.
12 FIGURE 5-7 Proof of te first Carnot rcile. Coyrigt e McGraw-ill Comanies, Inc. Permission required for reroduction or dislay. 5-5
13 function(, ) from enge scematics by identity, function(, ) f(, substitutg, ) f(, ) f(, ) f(, ) f(, ) ermodynamic emerature Scale f(, ) tis equation can be satisfied only if, l and l l l A reversibleenge (or a real enge corrected to reversibe)can be used to measure temerature difference. Second aw eat Enge ermodynamic emerature Scale
14 SECND A and ut and s s.66 i i.6 i substituti ng.66 Scale Range.66 Scale Range for, is meaasured, - absolute temeratures. emerature scales can be setu for any arbitrarily sleected scale 0 ot and Scale Range of degrees between ice and steam. ScaleRange, en a reversible enge (or a real enge correctable to reversible ) is run between ice and steam temeratures wit a constant eat s s i out i i i s For ice as Scale 0 00 i Celsius K For : : Celsius Scale Scale less 80 i 9.68 K.66 Fareneigt Fareneigt 0 Scale Range Fareneig t Scale Range tan ice as Scale K R
15 Carnot Cycle Performance ork CP ork CP ork - CP are, and Carnot efficiency e scale, temerature termodynamic absolute te Usg out CYCE REVERSIBE CARN EA PUMP CYCE REVERSIBE CARN REFRIGERA R CYCE REVERSIBE CARN ENGINE
16 alf te work of an enge oeratg between 800 C and 0 C is used to ower a refrigeration mace absorbg eat at C and rejectg 6,000 kj/r at C ow muc eat is sulied to te enge? CP eat um eat um eat enge out CP ea ea,556 out out tum l tum kj/r out eat um 6,000 kj/r l 89. kj/kg 780 K eat enge C eat um eat enge eat um C 0 C C 6,000 kj/kg 5-6
17 .00 kg steam executes te followg cycle. e absolute ig temerature is twice te absolute low temerature and te net work outut is 5 kj. eat is rejected durg a ase cange from a vaor to a liquid. at is te rejection temerature? l out l l l 5 kj.5 l 5 kj 50 kj.5 out m 5 kj out 5 kj 7. kj/kg m.00 fg 7. fg ( ) ( ) 5. C fg.00 kg steam S
18 00 K 00 K wo Carnot enges oerate series at te same efficiency. e ig temerature enge receives eat at 00 K and te low temerature enge rejects eat at 00. at is te temerature between te enges? l ( 00 ) 00 ( 00 00) K.5 5-
19 Sce cycle l oneof l 0 may be deendent of l te caracteristics at, of a termodynamocroerty. l In First aw, cycle ( d ) 0 lead to te defitionof termodynamic roerty E + energy as a
20 Ideal Gas Carnot Cycle roerty reversible cycle like a beaves tis d 0 P ln R ln R n d Note R ln P R ln R ln P P or P P constant v for P R ln R ln P R ln R ln l net n n n n n net rocess, rocess Reversible adiabatic exansion,,rocess rocess Reversible constant temerature eat transfer,
21 An engeer roosed an attemted to imrove te efficiency of a ower cycle by transferrg eat from te available ig temerature source to am alternate iger temerature source usg a eat um. at do you tk of tis suggestion?, enge CP eat[um enge enge eatum Enge enge eat um, eat Pum eat um were enge enge eat um eat um tere is no net work ga wit reversible maces and tere would be a net loss wit real maces.,
NEWCOMEN ATMOSPHERIC ENGINE
 NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m F 7 stroke 5 sia steam 8 50 F water NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m 5 sia steam 8 F 50 F water 7
NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m F 7 stroke 5 sia steam 8 50 F water NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m 5 sia steam 8 F 50 F water 7
SIMPLE RANKINE CYCLE. 3 expander. boiler. pump. condenser 1 W Q. cycle cycle. net. shaft
 SIMPLE RANKINE CYCLE um boiler exander condener Steady Flow, Oen Sytem - region ace Steady Flow Energy Equation for Procee m (u Pum Proce,, Boiler Proce,, V ρg) 0, 0, Exanion Proce,, 0, Condener Proce,,
SIMPLE RANKINE CYCLE um boiler exander condener Steady Flow, Oen Sytem - region ace Steady Flow Energy Equation for Procee m (u Pum Proce,, Boiler Proce,, V ρg) 0, 0, Exanion Proce,, 0, Condener Proce,,
(b) The heat transfer can be determined from an energy balance on the system
 8-5 Heat is transferred to a iston-cylinder device wit a set of stos. e work done, te eat transfer, te exergy destroyed, and te second-law efficiency are to be deterined. Assutions e device is stationary
8-5 Heat is transferred to a iston-cylinder device wit a set of stos. e work done, te eat transfer, te exergy destroyed, and te second-law efficiency are to be deterined. Assutions e device is stationary
Lecture 10: Carnot theorem
 ecture 0: Carnot teorem Feb 7, 005 Equivalence of Kelvin and Clausius formulations ast time we learned tat te Second aw can be formulated in two ways. e Kelvin formulation: No process is possible wose
ecture 0: Carnot teorem Feb 7, 005 Equivalence of Kelvin and Clausius formulations ast time we learned tat te Second aw can be formulated in two ways. e Kelvin formulation: No process is possible wose
Thermodynamics Lecture Series
 Termodynamics Lecture Series Ideal Ranke Cycle Te Practical Cycle Applied Sciences Education Researc Group (ASERG) Faculty of Applied Sciences Universiti Teknologi MARA email: drjjlanita@otmail.com ttp://www5.uitm.edu.my/faculties/fsg/drjj1.tml
Termodynamics Lecture Series Ideal Ranke Cycle Te Practical Cycle Applied Sciences Education Researc Group (ASERG) Faculty of Applied Sciences Universiti Teknologi MARA email: drjjlanita@otmail.com ttp://www5.uitm.edu.my/faculties/fsg/drjj1.tml
300 kpa 77 C. (d) If we neglect kinetic energy in the calculation of energy transport by mass
 6-6- Air flows steadily a ie at a secified state. The diameter of the ie, the rate of flow energy, and the rate of energy transort by mass are to be determed. Also, the error oled the determation of energy
6-6- Air flows steadily a ie at a secified state. The diameter of the ie, the rate of flow energy, and the rate of energy transort by mass are to be determed. Also, the error oled the determation of energy
Chapters 19 & 20 Heat and the First Law of Thermodynamics
 Capters 19 & 20 Heat and te First Law of Termodynamics Te Zerot Law of Termodynamics Te First Law of Termodynamics Termal Processes Te Second Law of Termodynamics Heat Engines and te Carnot Cycle Refrigerators,
Capters 19 & 20 Heat and te First Law of Termodynamics Te Zerot Law of Termodynamics Te First Law of Termodynamics Termal Processes Te Second Law of Termodynamics Heat Engines and te Carnot Cycle Refrigerators,
the first derivative with respect to time is obtained by carefully applying the chain rule ( surf init ) T Tinit
 .005 ermal Fluids Engineering I Fall`08 roblem Set 8 Solutions roblem ( ( a e -D eat equation is α t x d erfc( u du π x, 4αt te first derivative wit respect to time is obtained by carefully applying te
.005 ermal Fluids Engineering I Fall`08 roblem Set 8 Solutions roblem ( ( a e -D eat equation is α t x d erfc( u du π x, 4αt te first derivative wit respect to time is obtained by carefully applying te
Chapter 9 Practical cycles
 Prof.. undararajan Chater 9 Practical cycles 9. Introduction In Chaters 7 and 8, it was shown that a reversible engine based on the Carnot cycle (two reversible isothermal heat transfers and two reversible
Prof.. undararajan Chater 9 Practical cycles 9. Introduction In Chaters 7 and 8, it was shown that a reversible engine based on the Carnot cycle (two reversible isothermal heat transfers and two reversible
General Physics I. New Lecture 27: Carnot Cycle, The 2nd Law, Entropy and Information. Prof. WAN, Xin
 General Pysics I New Lecture 27: Carnot Cycle, e 2nd Law, Entropy and Information Prof. AN, Xin xinwan@zju.edu.cn ttp://zimp.zju.edu.cn/~xinwan/ Carnot s Engine Efficiency of a Carnot Engine isotermal
General Pysics I New Lecture 27: Carnot Cycle, e 2nd Law, Entropy and Information Prof. AN, Xin xinwan@zju.edu.cn ttp://zimp.zju.edu.cn/~xinwan/ Carnot s Engine Efficiency of a Carnot Engine isotermal
Lecture 13. Heat Engines. Thermodynamic processes and entropy Thermodynamic cycles Extracting work from heat
 Lecture 3 Heat Engines hermodynamic rocesses and entroy hermodynamic cycles Extracting work from heat - How do we define engine efficiency? - Carnot cycle: the best ossible efficiency Reading for this
Lecture 3 Heat Engines hermodynamic rocesses and entroy hermodynamic cycles Extracting work from heat - How do we define engine efficiency? - Carnot cycle: the best ossible efficiency Reading for this
Lecture 13 Heat Engines
 Lecture 3 Heat Engines hermodynamic rocesses and entroy hermodynamic cycles Extracting work from heat - How do we define engine efficiency? - Carnot cycle: the best ossible efficiency Reading for this
Lecture 3 Heat Engines hermodynamic rocesses and entroy hermodynamic cycles Extracting work from heat - How do we define engine efficiency? - Carnot cycle: the best ossible efficiency Reading for this
Notes on the function gsw_enthalpy_first_derivatives_ct_exact(sa,ct,p)
 Notes on gsw_entaly_first_derivatives_c_exact 1 Notes on te function gsw_entaly_first_derivatives_c_exact(c) is function gsw_entaly_first_derivatives_c_exact(c) evaluates two of te first order artial derivatives
Notes on gsw_entaly_first_derivatives_c_exact 1 Notes on te function gsw_entaly_first_derivatives_c_exact(c) is function gsw_entaly_first_derivatives_c_exact(c) evaluates two of te first order artial derivatives
The average velocity of water in the tube and the Reynolds number are Hot R-134a
 hater 0:, 8, 4, 47, 50, 5, 55, 7, 75, 77, 8 and 85. 0- Refrigerant-4a is cooled by water a double-ie heat exchanger. he overall heat transfer coefficient is to be determed. Assumtions he thermal resistance
hater 0:, 8, 4, 47, 50, 5, 55, 7, 75, 77, 8 and 85. 0- Refrigerant-4a is cooled by water a double-ie heat exchanger. he overall heat transfer coefficient is to be determed. Assumtions he thermal resistance
8-4 P 2. = 12 kw. AIR T = const. Therefore, Q &
 8-4 8-4 Air i compreed teadily by a compreor. e air temperature i mataed contant by eat rejection to te urroundg. e rate o entropy cange o air i to be determed. Aumption i i a teady-low proce ce tere i
8-4 8-4 Air i compreed teadily by a compreor. e air temperature i mataed contant by eat rejection to te urroundg. e rate o entropy cange o air i to be determed. Aumption i i a teady-low proce ce tere i
Chapter 20: Exercises: 3, 7, 11, 22, 28, 34 EOC: 40, 43, 46, 58
 Chater 0: Exercises:, 7,,, 8, 4 EOC: 40, 4, 46, 8 E: A gasoline engine takes in.80 0 4 and delivers 800 of work er cycle. The heat is obtained by burning gasoline with a heat of combustion of 4.60 0 4.
Chater 0: Exercises:, 7,,, 8, 4 EOC: 40, 4, 46, 8 E: A gasoline engine takes in.80 0 4 and delivers 800 of work er cycle. The heat is obtained by burning gasoline with a heat of combustion of 4.60 0 4.
Physics 207 Lecture 23
 ysics 07 Lecture ysics 07, Lecture 8, Dec. Agenda:. Finis, Start. Ideal gas at te molecular level, Internal Energy Molar Specific Heat ( = m c = n ) Ideal Molar Heat apacity (and U int = + W) onstant :
ysics 07 Lecture ysics 07, Lecture 8, Dec. Agenda:. Finis, Start. Ideal gas at te molecular level, Internal Energy Molar Specific Heat ( = m c = n ) Ideal Molar Heat apacity (and U int = + W) onstant :
6-5. H 2 O 200 kpa 200 C Q. Entropy Changes of Pure Substances
 Canges f ure Substances 6-0C Yes, because an ternally reversible, adiabatic prcess vlves n irreversibilities r eat transfer. 6- e radiatr f a steam eatg system is itially filled wit supereated steam. e
Canges f ure Substances 6-0C Yes, because an ternally reversible, adiabatic prcess vlves n irreversibilities r eat transfer. 6- e radiatr f a steam eatg system is itially filled wit supereated steam. e
Then the amount of water that flows through the pipe during a differential time interval dt is (1) 4
 5-98 Review Problems 5-45 A tank oen to te atmosere is itially filled wit. e tank discarges to te atmosere troug a long ie connected to a valve. e itial discarge velocity from te tank and te time required
5-98 Review Problems 5-45 A tank oen to te atmosere is itially filled wit. e tank discarges to te atmosere troug a long ie connected to a valve. e itial discarge velocity from te tank and te time required
CHEE 221: Chemical Processes and Systems
 CH 221: Chemical Processes and Systems Module 4. nergy Balances without Reaction Part a: Introduction to nergy Balances (Felder & Rousseau Ch 7.0 7.4 nergy and nergy Balances: very chemical rocess volves
CH 221: Chemical Processes and Systems Module 4. nergy Balances without Reaction Part a: Introduction to nergy Balances (Felder & Rousseau Ch 7.0 7.4 nergy and nergy Balances: very chemical rocess volves
The Second Law of Thermodynamics. (Second Law of Thermodynamics)
 he Second aw of hermodynamics For the free exansion, we have >. It is an irreversible rocess in a closed system. For the reversible isothermal rocess, for the gas > for exansion and < for comression. owever,
he Second aw of hermodynamics For the free exansion, we have >. It is an irreversible rocess in a closed system. For the reversible isothermal rocess, for the gas > for exansion and < for comression. owever,
Compressor 1. Evaporator. Condenser. Expansion valve. CHE 323, October 8, Chemical Engineering Thermodynamics. Tutorial problem 5.
 CHE 33, October 8, 014. Cemical Engineering Termodynamics. Tutorial problem 5. In a simple compression refrigeration process, an adiabatic reversible compressor is used to compress propane, used as a refrigerant.
CHE 33, October 8, 014. Cemical Engineering Termodynamics. Tutorial problem 5. In a simple compression refrigeration process, an adiabatic reversible compressor is used to compress propane, used as a refrigerant.
1 = The rate at which the entropy of the high temperature reservoir changes, according to the definition of the entropy, is
 8-7 8-9 A reverible eat um wit eciied reervoir temerature i conidered. e entroy cange o two reervoir i to be calculated and it i to be determed i ti eat um atiie te creae entroy rcile. Aumtion e eat um
8-7 8-9 A reverible eat um wit eciied reervoir temerature i conidered. e entroy cange o two reervoir i to be calculated and it i to be determed i ti eat um atiie te creae entroy rcile. Aumtion e eat um
Carnot Factor of a Vapour Power Cycle with Regenerative Extraction
 Journal of Modern Pysics, 2017, 8, 1795-1808 ttp://www.scirp.org/journal/jmp ISSN Online: 2153-120X ISSN Print: 2153-1196 arnot Factor of a Vapour Power ycle wit Regenerative Extraction Duparquet Alain
Journal of Modern Pysics, 2017, 8, 1795-1808 ttp://www.scirp.org/journal/jmp ISSN Online: 2153-120X ISSN Print: 2153-1196 arnot Factor of a Vapour Power ycle wit Regenerative Extraction Duparquet Alain
Thermodynamics Lecture Series
 Thermodynamics Lecture Series Second Law uality of Energy Applied Sciences Education Research Group (ASERG) Faculty of Applied Sciences Universiti Teknologi MARA email: drjjlanita@hotmail.com http://www.uitm.edu.my/faculties/fsg/drjj.html
Thermodynamics Lecture Series Second Law uality of Energy Applied Sciences Education Research Group (ASERG) Faculty of Applied Sciences Universiti Teknologi MARA email: drjjlanita@hotmail.com http://www.uitm.edu.my/faculties/fsg/drjj.html
First Name Last Name CIRCLE YOUR LECTURE BELOW: Div. 1 10:30 am Div. 2 2:30 pm Div. 3 4:30 pm Prof. Gore Prof. Udupa Prof. Chen
 CIRCLE YOUR LECURE BELOW: Div. 1 10:30 am Div. :30 m Div. 3 4:30 m Prof. Gore Prof. Udua Prof. Chen EXAM # 3 INSRUCIONS 1. his is a closed book examination. All needed roerty tables are rovided.. Do not
CIRCLE YOUR LECURE BELOW: Div. 1 10:30 am Div. :30 m Div. 3 4:30 m Prof. Gore Prof. Udua Prof. Chen EXAM # 3 INSRUCIONS 1. his is a closed book examination. All needed roerty tables are rovided.. Do not
Thermodynamics [ENGR 251] [Lyes KADEM 2007]
![Thermodynamics [ENGR 251] [Lyes KADEM 2007] Thermodynamics [ENGR 251] [Lyes KADEM 2007]](/thumbs/79/79128760.jpg) CHAPTER V The first law of thermodynamics is a representation of the conservation of energy. It is a necessary, but not a sufficient, condition for a process to occur. Indeed, no restriction is imposed
CHAPTER V The first law of thermodynamics is a representation of the conservation of energy. It is a necessary, but not a sufficient, condition for a process to occur. Indeed, no restriction is imposed
The isentropic exponent in plasmas
 Te isentroic exonent in lasmas Burm K.T.A.L.; Goedeer W.J.; cram D.C. Publised in: Pysics of Plasmas DOI: 10.1063/1.873535 Publised: 01/01/1999 Document Version Publiser s PDF also known as Version of
Te isentroic exonent in lasmas Burm K.T.A.L.; Goedeer W.J.; cram D.C. Publised in: Pysics of Plasmas DOI: 10.1063/1.873535 Publised: 01/01/1999 Document Version Publiser s PDF also known as Version of
Basic thermodynamics. heat to the high temperature reservoir.
 Consider a heat engine that is operating in a cyclic process takes heat (QH) from a high temperature reservoir & converts completely into work (W), violating the Kelvin Planck statement. Let the work W,
Consider a heat engine that is operating in a cyclic process takes heat (QH) from a high temperature reservoir & converts completely into work (W), violating the Kelvin Planck statement. Let the work W,
At 1atm = 101,325Pa, one mole of gas at 20 0 C = 293K has volume V = 2.40 x10-2 m 3 = 24 litres
 Ideal Gases and eat Enges he oeration, and theoretial effiieny, of ombustion driven iston enges (e.g. diesel or etrol fuelled) an be analysed by onsiderg harges to ressure, temerature and volume of the
Ideal Gases and eat Enges he oeration, and theoretial effiieny, of ombustion driven iston enges (e.g. diesel or etrol fuelled) an be analysed by onsiderg harges to ressure, temerature and volume of the
Numerical Differentiation
 Numerical Differentiation Finite Difference Formulas for te first derivative (Using Taylor Expansion tecnique) (section 8.3.) Suppose tat f() = g() is a function of te variable, and tat as 0 te function
Numerical Differentiation Finite Difference Formulas for te first derivative (Using Taylor Expansion tecnique) (section 8.3.) Suppose tat f() = g() is a function of te variable, and tat as 0 te function
 Two mark questions and answers UNIT II SECOND LAW 1. Define Clausius statement. It is impossible for a self-acting machine working in a cyclic process, to transfer heat from a body at lower temperature
Two mark questions and answers UNIT II SECOND LAW 1. Define Clausius statement. It is impossible for a self-acting machine working in a cyclic process, to transfer heat from a body at lower temperature
SOLUTION: Consider the system to be the refrigerator (shown in the following schematic), which operates over a cycle in normal operation.
 Soln_21 An ordinary household refrigerator operating in steady state receives electrical work while discharging net energy by heat transfer to its surroundings (e.g., the kitchen). a. Is this a violation
Soln_21 An ordinary household refrigerator operating in steady state receives electrical work while discharging net energy by heat transfer to its surroundings (e.g., the kitchen). a. Is this a violation
Department of Mechanical Engineering ME 322 Mechanical Engineering Thermodynamics. Calculation of Entropy Changes. Lecture 19
 Department of Mecanical Engineering ME Mecanical Engineering ermodynamics Calculation of Entropy Canges Lecture 9 e Gibbs Equations How are entropy alues calculated? Clausius found tat, dq dq m re re ds
Department of Mecanical Engineering ME Mecanical Engineering ermodynamics Calculation of Entropy Canges Lecture 9 e Gibbs Equations How are entropy alues calculated? Clausius found tat, dq dq m re re ds
Physics 2A (Fall 2012) Chapters 11:Using Energy and 12: Thermal Properties of Matter
 Physics 2A (Fall 2012) Chaters 11:Using Energy and 12: Thermal Proerties of Matter "Kee in mind that neither success nor failure is ever final." Roger Ward Babson Our greatest glory is not in never failing,
Physics 2A (Fall 2012) Chaters 11:Using Energy and 12: Thermal Proerties of Matter "Kee in mind that neither success nor failure is ever final." Roger Ward Babson Our greatest glory is not in never failing,
Actual exergy intake to perform the same task
 CHAPER : PRINCIPLES OF ENERGY CONSERVAION INRODUCION Energy conservation rinciles are based on thermodynamics If we look into the simle and most direct statement of the first law of thermodynamics, we
CHAPER : PRINCIPLES OF ENERGY CONSERVAION INRODUCION Energy conservation rinciles are based on thermodynamics If we look into the simle and most direct statement of the first law of thermodynamics, we
EF 152 Exam #3, Fall, 2012 Page 1 of 6
 EF 5 Exam #3, Fall, 0 Page of 6 Name: Setion: Guidelines: ssume 3 signifiant figures for all given numbers. Sow all of your work no work, no redit Write your final answer in te box provided - inlude units
EF 5 Exam #3, Fall, 0 Page of 6 Name: Setion: Guidelines: ssume 3 signifiant figures for all given numbers. Sow all of your work no work, no redit Write your final answer in te box provided - inlude units
A short note on Reitlinger thermodynamic cycles
 short note on Reitlinger thermodynamic cycles melia arolina Saravigna eartment of lied Science and echnology, Politecnico di orino, orino, Italy bstract: It is well known that arnot cycle is the thermodynamic
short note on Reitlinger thermodynamic cycles melia arolina Saravigna eartment of lied Science and echnology, Politecnico di orino, orino, Italy bstract: It is well known that arnot cycle is the thermodynamic
Spring_#7. Thermodynamics. Youngsuk Nam.
 Spring_#7 Thermodynamics Youngsuk Nam ysnam1@khu.ac.kr You can t connect the dots looking forward; you can only connect them looking backwards. So you have to trust that the dots will somehow connect in
Spring_#7 Thermodynamics Youngsuk Nam ysnam1@khu.ac.kr You can t connect the dots looking forward; you can only connect them looking backwards. So you have to trust that the dots will somehow connect in
NODIA AND COMPANY. GATE SOLVED PAPER Mechanical Engineering Thermodynamics. Copyright By NODIA & COMPANY
 No art of this ublication may be reroduced or distributed in any form or any means, electronic, mechanical, hotocoying, or otherwise without the rior ermission of the author. GAE SOLVED PAPER Mechanical
No art of this ublication may be reroduced or distributed in any form or any means, electronic, mechanical, hotocoying, or otherwise without the rior ermission of the author. GAE SOLVED PAPER Mechanical
Quotes. Review - First Law. Review - First Law. Review - First Law. Review - First Law. Thermodynamics Lecture Series
 8//005 herodynaics ecture Series Entropy uantifyg Energy Degradation Applied Sciences Education Research Group (ASERG) Faculty of Applied Sciences Universiti eknologi MARA eail: drjjlanita@hotail.co http://www3.uit.edu.y/staff/drjj/
8//005 herodynaics ecture Series Entropy uantifyg Energy Degradation Applied Sciences Education Research Group (ASERG) Faculty of Applied Sciences Universiti eknologi MARA eail: drjjlanita@hotail.co http://www3.uit.edu.y/staff/drjj/
PHYSICAL PROCESSES IN ANISOTROPIC THERMOELEMENT AND THEIR FEATURES
 J. Nano- Electron. Pys. (009) No3, P. 43-5 009 SumDU (Sumy State University) PACS numbers: 7.5.Jf, 7.0.Pa, 85.80. b PHYSICAL PROCESSES IN ANISOROPIC HERMOELEMEN AND HEIR FEAURES V.M. Ìàtyega, O.G. Danalakiy
J. Nano- Electron. Pys. (009) No3, P. 43-5 009 SumDU (Sumy State University) PACS numbers: 7.5.Jf, 7.0.Pa, 85.80. b PHYSICAL PROCESSES IN ANISOROPIC HERMOELEMEN AND HEIR FEAURES V.M. Ìàtyega, O.G. Danalakiy
Reminder: Exam 3 Friday, July 6. The Compton Effect. General Physics (PHY 2140) Lecture questions. Show your work for credit.
 General Pysics (PHY 2140) Lecture 15 Modern Pysics Cater 27 1. Quantum Pysics Te Comton Effect Potons and EM Waves Wave Proerties of Particles Wave Functions Te Uncertainty Princile Reminder: Exam 3 Friday,
General Pysics (PHY 2140) Lecture 15 Modern Pysics Cater 27 1. Quantum Pysics Te Comton Effect Potons and EM Waves Wave Proerties of Particles Wave Functions Te Uncertainty Princile Reminder: Exam 3 Friday,
Chapter 2 Solutions. 2.1 (D) Pressure and temperature are dependent during phase change and independent when in a single phase.
 Chater Solutions.1 (D) Pressure and temerature are deendent during hase change and indeendent when in a single hase.. (B) Sublimation is the direct conversion of a solid to a gas. To observe this rocess,
Chater Solutions.1 (D) Pressure and temerature are deendent during hase change and indeendent when in a single hase.. (B) Sublimation is the direct conversion of a solid to a gas. To observe this rocess,
FINITE TIME THERMODYNAMIC MODELING AND ANALYSIS FOR AN IRREVERSIBLE ATKINSON CYCLE. By Yanlin GE, Lingen CHEN, and Fengrui SUN
 FINIE IME HERMODYNAMIC MODELING AND ANALYSIS FOR AN IRREVERSIBLE AKINSON CYCLE By Yanlin GE, Lingen CHEN, and Fengrui SUN Performance of an air-standard Atkinson cycle is analyzed by using finite-time
FINIE IME HERMODYNAMIC MODELING AND ANALYSIS FOR AN IRREVERSIBLE AKINSON CYCLE By Yanlin GE, Lingen CHEN, and Fengrui SUN Performance of an air-standard Atkinson cycle is analyzed by using finite-time
Engineering Services Examination - UPSC MECHANICAL ENGINEERING. Topic-wise Conventional Papers I & II (Last 30 Years Questions)
 Engineering Services Examination - UPSC MECHANICAL ENGINEERING Toic-wise Conventional Paers I & II (Last 0 Years Questions) Dedicated to Mechanical Engineers and Engineering Services asirants. 04 by Engineers
Engineering Services Examination - UPSC MECHANICAL ENGINEERING Toic-wise Conventional Paers I & II (Last 0 Years Questions) Dedicated to Mechanical Engineers and Engineering Services asirants. 04 by Engineers
Physics 231 Lecture 35
 ysis 1 Leture 5 Main points of last leture: Heat engines and effiieny: eng e 1 Carnot yle and Carnot engine. eng e 1 is in Kelvin. Refrigerators CO eng Ideal refrigerator CO rev reversible Entropy ΔS Computation
ysis 1 Leture 5 Main points of last leture: Heat engines and effiieny: eng e 1 Carnot yle and Carnot engine. eng e 1 is in Kelvin. Refrigerators CO eng Ideal refrigerator CO rev reversible Entropy ΔS Computation
Chapter 1 Fundamentals
 Chater Fundamentals. Overview of Thermodynamics Industrial Revolution brought in large scale automation of many tedious tasks which were earlier being erformed through manual or animal labour. Inventors
Chater Fundamentals. Overview of Thermodynamics Industrial Revolution brought in large scale automation of many tedious tasks which were earlier being erformed through manual or animal labour. Inventors
BME-A PREVIOUS YEAR QUESTIONS
 BME-A PREVIOUS YEAR QUESTIONS CREDITS CHANGE ACCHA HAI TEAM UNIT-1 Introduction: Introduction to Thermodynamics, Concepts of systems, control volume, state, properties, equilibrium, quasi-static process,
BME-A PREVIOUS YEAR QUESTIONS CREDITS CHANGE ACCHA HAI TEAM UNIT-1 Introduction: Introduction to Thermodynamics, Concepts of systems, control volume, state, properties, equilibrium, quasi-static process,
V. The Second Law of Thermodynamics. V. The Second Law of Thermodynamics
 P V. he Second aw of hermodynamics E. he Carnot Cycle. he reversible heat engine (a piston cylinder device) that operates on a cycle between heat reservoirs at and, as shown below on a P-v diagram, is
P V. he Second aw of hermodynamics E. he Carnot Cycle. he reversible heat engine (a piston cylinder device) that operates on a cycle between heat reservoirs at and, as shown below on a P-v diagram, is
1 Power is transferred through a machine as shown. power input P I machine. power output P O. power loss P L. What is the efficiency of the machine?
 1 1 Power is transferred troug a macine as sown. power input P I macine power output P O power loss P L Wat is te efficiency of te macine? P I P L P P P O + P L I O P L P O P I 2 ir in a bicycle pump is
1 1 Power is transferred troug a macine as sown. power input P I macine power output P O power loss P L Wat is te efficiency of te macine? P I P L P P P O + P L I O P L P O P I 2 ir in a bicycle pump is
Analysis: The speed of the proton is much less than light speed, so we can use the
 Section 1.3: Wave Proerties of Classical Particles Tutorial 1 Practice, age 634 1. Given: 1.8! 10 "5 kg # m/s; 6.63! 10 "34 J #s Analysis: Use te de Broglie relation, λ. Solution:! 6.63 " 10#34 kg $ m
Section 1.3: Wave Proerties of Classical Particles Tutorial 1 Practice, age 634 1. Given: 1.8! 10 "5 kg # m/s; 6.63! 10 "34 J #s Analysis: Use te de Broglie relation, λ. Solution:! 6.63 " 10#34 kg $ m
PV/T = k or PV = kt Describe the difference between an ideal gas and a real gas.
 10.1 Thermodynamics 10.2 Processes 10 10.3 The second law of thermodynamics and entroy 10.1 Thermodynamics From the combined gas laws, we determined that: P/T = k or P = kt 10.1.1 State the equation of
10.1 Thermodynamics 10.2 Processes 10 10.3 The second law of thermodynamics and entroy 10.1 Thermodynamics From the combined gas laws, we determined that: P/T = k or P = kt 10.1.1 State the equation of
Department of Mechanical Engineering ME 322 Mechanical Engineering Thermodynamics. Introduction to 2 nd Law and Entropy.
 Department of Mechanical Engineering ME 322 Mechanical Engineering hermodynamics Introduction to 2 nd aw and Entropy ecture 18 Example Consider an adiabatic compressor steadily moving R125, P2 2 430 psia
Department of Mechanical Engineering ME 322 Mechanical Engineering hermodynamics Introduction to 2 nd aw and Entropy ecture 18 Example Consider an adiabatic compressor steadily moving R125, P2 2 430 psia
Example problems. Chapter 2: Temperature, heat, and the 1 st law of Thermodynamic. Homework: 2, 3, 4, 5, 6, 10, 15, 19, 21 (pages )
 Examle roblems Chater : emerature, heat, and the 1 st law of hermodynamic Homework:,, 4, 5, 6, 1, 15, 19, 1 (ages 5-51) . (Page 5) wo constant-volume gas thermometers are assembled, one with nitrogen and
Examle roblems Chater : emerature, heat, and the 1 st law of hermodynamic Homework:,, 4, 5, 6, 1, 15, 19, 1 (ages 5-51) . (Page 5) wo constant-volume gas thermometers are assembled, one with nitrogen and
Work and Energy. Introduction. Work. PHY energy - J. Hedberg
 Work and Energy PHY 207 - energy - J. Hedberg - 2017 1. Introduction 2. Work 3. Kinetic Energy 4. Potential Energy 5. Conservation of Mecanical Energy 6. Ex: Te Loop te Loop 7. Conservative and Non-conservative
Work and Energy PHY 207 - energy - J. Hedberg - 2017 1. Introduction 2. Work 3. Kinetic Energy 4. Potential Energy 5. Conservation of Mecanical Energy 6. Ex: Te Loop te Loop 7. Conservative and Non-conservative
(British) (SI) British Metric L T [V] = L T. [a] = 2 [F] = F = 2 T
![(British) (SI) British Metric L T [V] = L T. [a] = 2 [F] = F = 2 T (British) (SI) British Metric L T [V] = L T. [a] = 2 [F] = F = 2 T](/thumbs/77/76088169.jpg) Hydraulics ecture # CWR 40 age () ecture # Outline: Review of terminology in fluid mechanics: Energy or work Hydraulic head Bernoulli s aw, Conductivity (examle) ransient & turbulent Friction head loss
Hydraulics ecture # CWR 40 age () ecture # Outline: Review of terminology in fluid mechanics: Energy or work Hydraulic head Bernoulli s aw, Conductivity (examle) ransient & turbulent Friction head loss
F = U TS G = H TS. Availability, Irreversibility S K Mondal s Chapter 5. max. actual. univ. Ns =
 Availability, Irreversibility S K Mondal s Chater 5 I = W W 0 max ( S) = Δ univ actual 7. Irreversibility rate = I = rate of energy degradation = rate of energy loss (Wlost) = 0 S gen for all rocesses
Availability, Irreversibility S K Mondal s Chater 5 I = W W 0 max ( S) = Δ univ actual 7. Irreversibility rate = I = rate of energy degradation = rate of energy loss (Wlost) = 0 S gen for all rocesses
Classical Approach to 2 nd Law for CM
 Classical Approach to 2 nd aw for CM Start with observations about the ability to build devices (thermodynamic cycles) Clausius Statement of 2 nd aw concerns cycles that cause heat transfer from low temperature
Classical Approach to 2 nd aw for CM Start with observations about the ability to build devices (thermodynamic cycles) Clausius Statement of 2 nd aw concerns cycles that cause heat transfer from low temperature
Lecture 38: Vapor-compression refrigeration systems
 ME 200 Termodynamics I Lecture 38: Vapor-compression refrigeration systems Yong Li Sangai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Cuan Road Sangai, 200240, P. R. Cina Email
ME 200 Termodynamics I Lecture 38: Vapor-compression refrigeration systems Yong Li Sangai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Cuan Road Sangai, 200240, P. R. Cina Email
Combining functions: algebraic methods
 Combining functions: algebraic metods Functions can be added, subtracted, multiplied, divided, and raised to a power, just like numbers or algebra expressions. If f(x) = x 2 and g(x) = x + 2, clearly f(x)
Combining functions: algebraic metods Functions can be added, subtracted, multiplied, divided, and raised to a power, just like numbers or algebra expressions. If f(x) = x 2 and g(x) = x + 2, clearly f(x)
Developing Transfer Functions from Heat & Material Balances
 Colorado Sool of Mine CHEN43 Stirred ank Heater Develoing ranfer untion from Heat & Material Balane Examle ranfer untion Stirred ank Heater,,, A,,,,, We will develo te tranfer funtion for a tirred tank
Colorado Sool of Mine CHEN43 Stirred ank Heater Develoing ranfer untion from Heat & Material Balane Examle ranfer untion Stirred ank Heater,,, A,,,,, We will develo te tranfer funtion for a tirred tank
Chapter-6: Entropy. 1 Clausius Inequality. 2 Entropy - A Property
 hater-6: Entroy When the first law of thermodynamics was stated, the existence of roerty, the internal energy, was found. imilarly, econd law also leads to definition of another roerty, known as entroy.
hater-6: Entroy When the first law of thermodynamics was stated, the existence of roerty, the internal energy, was found. imilarly, econd law also leads to definition of another roerty, known as entroy.
02. Equilibrium Thermodynamics II: Engines
 University of Rhode Island DigitalCommons@URI Equilibrium Statistical Physics Physics Course Materials 205 02. Equilibrium Thermodynamics II: Engines Gerhard Müller University of Rhode Island, gmuller@uri.edu
University of Rhode Island DigitalCommons@URI Equilibrium Statistical Physics Physics Course Materials 205 02. Equilibrium Thermodynamics II: Engines Gerhard Müller University of Rhode Island, gmuller@uri.edu
Chapter 5. The Second Law of Thermodynamics (continued)
 hapter 5 he Second Law of hermodynamics (continued) Second Law of hermodynamics Alternative statements of the second law, lausius Statement of the Second Law It is impossible for any system to operate
hapter 5 he Second Law of hermodynamics (continued) Second Law of hermodynamics Alternative statements of the second law, lausius Statement of the Second Law It is impossible for any system to operate
CHAPTER 8 ENTROPY. Blank
 CHAPER 8 ENROPY Blank SONNAG/BORGNAKKE SUDY PROBLEM 8-8. A heat engine efficiency from the inequality of Clausius Consider an actual heat engine with efficiency of η working between reservoirs at and L.
CHAPER 8 ENROPY Blank SONNAG/BORGNAKKE SUDY PROBLEM 8-8. A heat engine efficiency from the inequality of Clausius Consider an actual heat engine with efficiency of η working between reservoirs at and L.
1. Which one of the following expressions is not equal to all the others? 1 C. 1 D. 25x. 2. Simplify this expression as much as possible.
 004 Algebra Pretest answers and scoring Part A. Multiple coice questions. Directions: Circle te letter ( A, B, C, D, or E ) net to te correct answer. points eac, no partial credit. Wic one of te following
004 Algebra Pretest answers and scoring Part A. Multiple coice questions. Directions: Circle te letter ( A, B, C, D, or E ) net to te correct answer. points eac, no partial credit. Wic one of te following
Chapter 4 Optimal Design
 4- Capter 4 Optimal Design e optimum design of termoelectric devices (termoelectric generator and cooler) in conjunction wit eat sins was developed using dimensional analysis. ew dimensionless groups were
4- Capter 4 Optimal Design e optimum design of termoelectric devices (termoelectric generator and cooler) in conjunction wit eat sins was developed using dimensional analysis. ew dimensionless groups were
The Foundations of Chemistry 1
 Te Foundations of Cemistry 1 1-1 (a) Biocemistry is te study of te cemistry of living tings. (b) Analytical cemistry studies te quantitative and qualitative composition analysis of substances. (c) Geocemistry
Te Foundations of Cemistry 1 1-1 (a) Biocemistry is te study of te cemistry of living tings. (b) Analytical cemistry studies te quantitative and qualitative composition analysis of substances. (c) Geocemistry
4. Energy balances Partly based on Chapter 4 of the De Nevers textbook.
 Lecture Notes CHE 31 Fluid Mechanics (Fall 010) 4 Energy balances Partly based on Chater 4 of the De Nevers textbook Energy fluid mechanics As for any quantity, we can set u an energy balance for a secific
Lecture Notes CHE 31 Fluid Mechanics (Fall 010) 4 Energy balances Partly based on Chater 4 of the De Nevers textbook Energy fluid mechanics As for any quantity, we can set u an energy balance for a secific
I. (20%) Answer the following True (T) or False (F). If false, explain why for full credit.
 I. (20%) Answer the following True (T) or False (F). If false, explain why for full credit. Both the Kelvin and Fahrenheit scales are absolute temperature scales. Specific volume, v, is an intensive property,
I. (20%) Answer the following True (T) or False (F). If false, explain why for full credit. Both the Kelvin and Fahrenheit scales are absolute temperature scales. Specific volume, v, is an intensive property,
Where F1 is the force and dl1 is the infinitesimal displacement, but F1 = p1a1
 In order to force the fluid to flow across the boundary of the system against a pressure p1, work is done on the boundary of the system. The amount of work done is dw = - F1.dl1, Where F1 is the force
In order to force the fluid to flow across the boundary of the system against a pressure p1, work is done on the boundary of the system. The amount of work done is dw = - F1.dl1, Where F1 is the force
Chapter 3 Thermoelectric Coolers
 3- Capter 3 ermoelectric Coolers Contents Capter 3 ermoelectric Coolers... 3- Contents... 3-3. deal Equations... 3-3. Maximum Parameters... 3-7 3.3 Normalized Parameters... 3-8 Example 3. ermoelectric
3- Capter 3 ermoelectric Coolers Contents Capter 3 ermoelectric Coolers... 3- Contents... 3-3. deal Equations... 3-3. Maximum Parameters... 3-7 3.3 Normalized Parameters... 3-8 Example 3. ermoelectric
2.8 The Derivative as a Function
 .8 Te Derivative as a Function Typically, we can find te derivative of a function f at many points of its domain: Definition. Suppose tat f is a function wic is differentiable at every point of an open
.8 Te Derivative as a Function Typically, we can find te derivative of a function f at many points of its domain: Definition. Suppose tat f is a function wic is differentiable at every point of an open
3 Minority carrier profiles (the hyperbolic functions) Consider a
 Microelectronic Devices and Circuits October 9, 013 - Homework #3 Due Nov 9, 013 1 Te pn junction Consider an abrupt Si pn + junction tat as 10 15 acceptors cm -3 on te p-side and 10 19 donors on te n-side.
Microelectronic Devices and Circuits October 9, 013 - Homework #3 Due Nov 9, 013 1 Te pn junction Consider an abrupt Si pn + junction tat as 10 15 acceptors cm -3 on te p-side and 10 19 donors on te n-side.
Exergy and the Dead State
 EXERGY The energy content of the universe is constant, just as its mass content is. Yet at times of crisis we are bombarded with speeches and articles on how to conserve energy. As engineers, we know that
EXERGY The energy content of the universe is constant, just as its mass content is. Yet at times of crisis we are bombarded with speeches and articles on how to conserve energy. As engineers, we know that
Chapter 7. Entropy. by Asst.Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn
 Chapter 7 Entropy by Asst.Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn Reference: Cengel, Yunus A. and Michael A. Boles, Thermodynamics: An Engineering Approach, 5th ed.,
Chapter 7 Entropy by Asst.Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn Reference: Cengel, Yunus A. and Michael A. Boles, Thermodynamics: An Engineering Approach, 5th ed.,
Fluids and Buoyancy. 1. What will happen to the scale reading as the mass is lowered?
 Fluids and Buoyancy. Wat will appen to te scale reading as te mass is lowered? M Using rcimedes Principle: any body fully or partially submerged in a fluid is buoyed up by a force equal to te weigt of
Fluids and Buoyancy. Wat will appen to te scale reading as te mass is lowered? M Using rcimedes Principle: any body fully or partially submerged in a fluid is buoyed up by a force equal to te weigt of
= T. (kj/k) (kj/k) 0 (kj/k) int rev. Chapter 6 SUMMARY
 Capter 6 SUMMARY e second la of termodynamics leads to te definition of a ne property called entropy ic is a quantitative measure of microscopic disorder for a system. e definition of entropy is based
Capter 6 SUMMARY e second la of termodynamics leads to te definition of a ne property called entropy ic is a quantitative measure of microscopic disorder for a system. e definition of entropy is based
Lecture XVII. Abstract We introduce the concept of directional derivative of a scalar function and discuss its relation with the gradient operator.
 Lecture XVII Abstract We introduce te concept of directional derivative of a scalar function and discuss its relation wit te gradient operator. Directional derivative and gradient Te directional derivative
Lecture XVII Abstract We introduce te concept of directional derivative of a scalar function and discuss its relation wit te gradient operator. Directional derivative and gradient Te directional derivative
MAT 145. Type of Calculator Used TI-89 Titanium 100 points Score 100 possible points
 MAT 15 Test #2 Name Solution Guide Type of Calculator Used TI-89 Titanium 100 points Score 100 possible points Use te grap of a function sown ere as you respond to questions 1 to 8. 1. lim f (x) 0 2. lim
MAT 15 Test #2 Name Solution Guide Type of Calculator Used TI-89 Titanium 100 points Score 100 possible points Use te grap of a function sown ere as you respond to questions 1 to 8. 1. lim f (x) 0 2. lim
Unsteady State Simulation of Vapor Compression Heat Pump Systems With Modular Analysis (This paper will not be presented.)
 Purdue University Purdue e-pubs nternational Refrigeration and ir Conditioning Conference Scool of Mecanical Engineering 0 Unsteady State Simulation of Vapor Compression Heat Pump Systems Wit Modular nalysis
Purdue University Purdue e-pubs nternational Refrigeration and ir Conditioning Conference Scool of Mecanical Engineering 0 Unsteady State Simulation of Vapor Compression Heat Pump Systems Wit Modular nalysis
5/6/ :41 PM. Chapter 6. Using Entropy. Dr. Mohammad Abuhaiba, PE
 Chapter 6 Using Entropy 1 2 Chapter Objective Means are introduced for analyzing systems from the 2 nd law perspective as they undergo processes that are not necessarily cycles. Objective: introduce entropy
Chapter 6 Using Entropy 1 2 Chapter Objective Means are introduced for analyzing systems from the 2 nd law perspective as they undergo processes that are not necessarily cycles. Objective: introduce entropy
Maths for Computer Graphics
 Trigonometry Is concerned wit te analysis of triangles. Degrees and radians Te degree (or sexagesimal unit of measure derives from defining one complete rotation as 360. Eac degree divides into 60 minutes,
Trigonometry Is concerned wit te analysis of triangles. Degrees and radians Te degree (or sexagesimal unit of measure derives from defining one complete rotation as 360. Eac degree divides into 60 minutes,
5.1 We will begin this section with the definition of a rational expression. We
 Basic Properties and Reducing to Lowest Terms 5.1 We will begin tis section wit te definition of a rational epression. We will ten state te two basic properties associated wit rational epressions and go
Basic Properties and Reducing to Lowest Terms 5.1 We will begin tis section wit te definition of a rational epression. We will ten state te two basic properties associated wit rational epressions and go
f self = 1/T self (b) With revolution, rotaton period T rot in second and the frequency Ω rot are T yr T yr + T day T rot = T self > f self
 Problem : Units : Q-a Mathematically exress the relationshi between the different units of the hysical variables: i) Temerature: ) Fahrenheit and Celsius; 2) Fahrenheit and Kelvin ii) Length: ) foot and
Problem : Units : Q-a Mathematically exress the relationshi between the different units of the hysical variables: i) Temerature: ) Fahrenheit and Celsius; 2) Fahrenheit and Kelvin ii) Length: ) foot and
MAE 11. Homework 8: Solutions 11/30/2018
 MAE 11 Homework 8: Solutions 11/30/2018 MAE 11 Fall 2018 HW #8 Due: Friday, November 30 (beginning of class at 12:00p) Requirements:: Include T s diagram for all cycles. Also include p v diagrams for Ch
MAE 11 Homework 8: Solutions 11/30/2018 MAE 11 Fall 2018 HW #8 Due: Friday, November 30 (beginning of class at 12:00p) Requirements:: Include T s diagram for all cycles. Also include p v diagrams for Ch
Chemistry 420/523 Chemical Thermodynamics (Spring ) Examination 1
 Chemistry 420/523 Chemical hermodynamics (Sring 2001-02) Examination 1 1 Boyle temerature is defined as the temerature at which the comression factor Z m /(R ) of a gas is exactly equal to 1 For a gas
Chemistry 420/523 Chemical hermodynamics (Sring 2001-02) Examination 1 1 Boyle temerature is defined as the temerature at which the comression factor Z m /(R ) of a gas is exactly equal to 1 For a gas
Solutions to Homework #10
 Solution to Homeork #0 0-6 A teady-lo Carnot enge it ater a te orkg luid operate at peciied condition. e termal eiciency, te preure at te turbe let, and te ork put are to be determed. Aumption Steady operatg
Solution to Homeork #0 0-6 A teady-lo Carnot enge it ater a te orkg luid operate at peciied condition. e termal eiciency, te preure at te turbe let, and te ork put are to be determed. Aumption Steady operatg
This follows from the Clausius inequality as a consequence of the second law of thermodynamics. Therefore. (for reversible process only) (22.
 Entropy Clausius inequality can be used to analyze the cyclic process in a quantitative manner. The second law became a law of wider applicability when Clausius introduced the property called entropy.
Entropy Clausius inequality can be used to analyze the cyclic process in a quantitative manner. The second law became a law of wider applicability when Clausius introduced the property called entropy.
SECOND LAW OF THERMODYNAMICS
 SECOND LAW OF THERMODYNAMICS 2 ND Law of Thermodynamics Puts a limitation on the conversion of some forms of energy Determines the scope of an energy conversion and if an energy conversion is possible
SECOND LAW OF THERMODYNAMICS 2 ND Law of Thermodynamics Puts a limitation on the conversion of some forms of energy Determines the scope of an energy conversion and if an energy conversion is possible
Phy 231 Sp 02 Homework #6 Page 1 of 4
 Py 231 Sp 02 Homework #6 Page 1 of 4 6-1A. Te force sown in te force-time diagram at te rigt versus time acts on a 2 kg mass. Wat is te impulse of te force on te mass from 0 to 5 sec? (a) 9 N-s (b) 6 N-s
Py 231 Sp 02 Homework #6 Page 1 of 4 6-1A. Te force sown in te force-time diagram at te rigt versus time acts on a 2 kg mass. Wat is te impulse of te force on te mass from 0 to 5 sec? (a) 9 N-s (b) 6 N-s
SECOND LAW OF THERMODYNAMICS
 SECOND LAW OF THERMODYNAMICS 2 ND Law of Thermodynamics Puts a limitation on the conversion of some forms of energy Determines the scope of an energy conversion and if an energy conversion is possible
SECOND LAW OF THERMODYNAMICS 2 ND Law of Thermodynamics Puts a limitation on the conversion of some forms of energy Determines the scope of an energy conversion and if an energy conversion is possible
ENERGY TRANSFER BY WORK: Electrical Work: When N Coulombs of electrical charge move through a potential difference V
 Weight, W = mg Where m=mass, g=gravitational acceleration ENERGY TRANSFER BY WOR: Sign convention: Work done on a system = (+) Work done by a system = (-) Density, ρ = m V kg m 3 Where m=mass, V =Volume
Weight, W = mg Where m=mass, g=gravitational acceleration ENERGY TRANSFER BY WOR: Sign convention: Work done on a system = (+) Work done by a system = (-) Density, ρ = m V kg m 3 Where m=mass, V =Volume
ME 201 Thermodynamics
 ME 0 Thermodynamics Solutions First Law Practice Problems. Consider a balloon that has been blown up inside a building and has been allowed to come to equilibrium with the inside temperature of 5 C and
ME 0 Thermodynamics Solutions First Law Practice Problems. Consider a balloon that has been blown up inside a building and has been allowed to come to equilibrium with the inside temperature of 5 C and
JJMIE Jordan Journal of Mechanical and Industrial Engineering
 JJMIE Jordan Journal of Mechanical and Industrial Engineering Volume, Number, Jun. 8 ISSN 995-6665 Pages 7-75 Efficiency of Atkinson Engine at Maximum Power Density using emerature Deendent Secific Heats
JJMIE Jordan Journal of Mechanical and Industrial Engineering Volume, Number, Jun. 8 ISSN 995-6665 Pages 7-75 Efficiency of Atkinson Engine at Maximum Power Density using emerature Deendent Secific Heats
EVAPORATION. Robert evaporator. Balance equations Material balance (total) Component balance. Heat balance
 EAPORATION Roert eorator Balance equation Material alance (total) S S=Solution =aor =Steam K=Steam condenate S Comonent alance S S Heat alance S S v Merkel lot can e ued for otaining entaly data Heat ower
EAPORATION Roert eorator Balance equation Material alance (total) S S=Solution =aor =Steam K=Steam condenate S Comonent alance S S Heat alance S S v Merkel lot can e ued for otaining entaly data Heat ower
kg m kg kg m =1 slope
 (5) Win loa Wen structure blocks te flow of win, te win's kinetic energy is converte into otential energy of ressure, wic causes a win loaing. ensity an velocity of air te angle of incience of te win 3
(5) Win loa Wen structure blocks te flow of win, te win's kinetic energy is converte into otential energy of ressure, wic causes a win loaing. ensity an velocity of air te angle of incience of te win 3
3. Semiconductor heterostructures and nanostructures
 3. Semiconductor eterostructures and nanostructures We discussed before ow te periodicity of a crystal results in te formation of bands. or a 1D crystal, we obtained: a (x) x In 3D, te crystal lattices
3. Semiconductor eterostructures and nanostructures We discussed before ow te periodicity of a crystal results in te formation of bands. or a 1D crystal, we obtained: a (x) x In 3D, te crystal lattices
Unit code: H/ QCF level: 5 Credit value: 15 OUTCOME 1 - THERMODYNAMIC SYSTEMS TUTORIAL 2
 Unit 43: Plant and Process Princiles Unit code: H/60 44 QCF level: 5 Credit value: 5 OUCOME - HERMODYNAMIC SYSEMS UORIAL Understand thermodynamic systems as alied to lant engineering rocesses hermodynamic
Unit 43: Plant and Process Princiles Unit code: H/60 44 QCF level: 5 Credit value: 5 OUCOME - HERMODYNAMIC SYSEMS UORIAL Understand thermodynamic systems as alied to lant engineering rocesses hermodynamic
