At 1atm = 101,325Pa, one mole of gas at 20 0 C = 293K has volume V = 2.40 x10-2 m 3 = 24 litres
|
|
|
- Willis Tyrone Oliver
- 6 years ago
- Views:
Transcription
1 Ideal Gases and eat Enges he oeration, and theoretial effiieny, of ombustion driven iston enges (e.g. diesel or etrol fuelled) an be analysed by onsiderg harges to ressure, temerature and volume of the gaseous omonents. his roeeds by aountg for the energy hanges the gas as a result of heat alied and work done ombed with the ideal gas equation, whih relates the hysial roerties of the gas. Ideal Gas Equation n ressure asals (a) olume / ubi metres Number of moles of gas emerature sales 00 o is equivalent to o F. R olar gas onstant 8. Jmol - K - emerature /Kelv F An ideal gas assumes a large number of ot artiles ollidg elastially. It neglets any short-range termoleular fores resultg from reulsion or attration due to moleular harges, and the fat that moleules have a fite volume i.e. are not fitely small! his means a real gas is not fitely omressible whereas an ideal gas has no suh limits. At atm = 0,5a, one mole of gas at 0 0 = 9K has volume =.0 x0 - m = litres he Kelv temerature sale (or absolute sale) is roortional to the mean keti energy of moleules. Ideal Gas Equation ratial units n atm litres K n / atm.87 / litres Seial ases of the ideal gas equation: Boyles s Law. At onstant temerature, gas ressure is versely roortional to volume. onstant harles Law. At onstant ressure, gas volume is roortional to temerature. / atm o / 7 n / atm.87 / litres o / 7 onstant / K U -0 o F = -0 o Internal energy of n moles of gas mol = 6.0 x 0 moleules. So energy of a moleule is Boltzmann's onstant u nkb k R/ JK B Number of degrees of freedom of moleular motion (e.g = for x,y,z translation) o 0 o 500 / litres Fahrenheit is a temerature sale, where o F is the freezg ot of water and o F is the boilg ot of water, defed at sea level at standard atmosheri ressure (0,5a). It was roosed 7 by Daniel Gabriel Fahrenheit. 0 o F orresonded to the lowest temerature he ould ool bre (salt water) and 00 o F was the average human body temerature (7 o ). A more oular sale is the elsius sale, with 0 o and 00 o reresentg the freezg and boilg ots of water at standard atmosheri ressure. Anders elsius 70-7 Daniel Fahrenheit illiam homson (Lord Kelv) Robert Boyle Jaques harles 76-8 hysis toi handout - Ideal gases & heat enges Dr Andrew Frenh. AGE
2 eat, work and ternal energy of an ideal gas x dx A F onsider a ylder of gas beg omressed by a fore F. he work done on the gas by the fore is: d Fdx he ressure atg uon the gas is: F A and the volume hange is: d Adx d d A A d d If heat d is sulied to the gas then the First Law of hermodynamis (that Energy a losed system is onserved) means the ternal energy hange is du d d d du d First Law he ternal energy for n moles of an ideal gas is U onstant volume roess (isohori) d d d d d 0 d du d he Ideal Gas Equation is U U du onstant volume heat aaity for n moles d i.e. no work done on gas First Law d So for a onstant volume hange, the heat aaity is a onstant for an ideal gas. R onstant volume seifi heat aaity. is the molar volume /kg Gas /gmol - Aetylene 6.0 Air Ammonia 7.0 Argon 9.98 Benzene 78. Butane 58. arbon dioxide.0 arbon onoxide 8.0 hlore Ethyl Alohol 6.07 Fluore elium.00 ydrogen hloride ydrogen Sulhide Kryton 8.80 ethane 6.0 Natural Gas 9.00 Nitrogen 8.0 Neon 0.79 Oxygen.9988 Ozone roane.097 Sulhur dioxide 6.06 onstant ressure roess (isobari) d d d d U du Se d onstant d d d du d d d d onstant ressure heat aaity for n moles of ideal gas Ideal Gas Equation First Law ayer Relationshi Exerimentally it is very hard to mata a onstant volume as heat is added, so onstant volume heat aaity is diffiult to measure diretly. owever, onstant ressure heat aaity is muh easier to measure, as one an allow volumes to hange order to mata equilibrium with the ambient ressure. otal amount of heat sulied to m kg of gas is therefore: m oluene 9. Xenon.0 ater vaour 8.0 he ayer relationshi is therefore very useful workg out the onstant volume heat aaity from the onstant ressure heat aaity. htt:// hysis toi handout - Ideal gases & heat enges Dr Andrew Frenh. AGE
3 eat sulied and work done for an isobari roess d d he ayer Relationshi and m n n n R( ) 0 d n n n R 0 Se is onstant R R R R Adiabati (or isentroi) roess i.e. no heat added. ork done is the sole ause of hanges ternal energy U d 0 ln ln ln U du d( ) du d d du d du d d d d d d d d d onstant d k 0 0 k onstant his defes an adiabati hange onstant temerature (isothermal) roess du 0 onstant d d d d d 0 ln 0 ork done on gas for an adiabati hange d d d d First Law Usg the Ideal Gas Equation eat sulied to gas ork done on gas d d d d So work done by gas on the surroundgs is: ln hysis toi handout - Ideal gases & heat enges Dr Andrew Frenh. AGE
4 eat Enges arnot yle U ositions to Isothermal exansion of an ideal gas at the hot reservoir temerature. Se gas temerature, and therefore ternal energy is onstant, the work done by the gas on the surroundgs must exatly equate to the heat absorbed by the gas. ositions to Isentroi (i.e. adiabati or no heat added or lost ) exansion of the gas. he work done by the gas on the surroundgs is owered by the loss of ternal energy of the gas as it ools from the temerature of the hot reservoir to the temerature of the old reservoir. ositions to Isothermal omression of the gas. In order for the temerature, and hene the ternal energy, to rema onstant, the heat lost by the gas to the old reservoir must equate to the work done on it by the surroundgs. ositions to Isentroi omression of the gas, heatg it from the temerature of the old reservoir to the temerature of the old reservoir. onstant ln 0 Assume we have n moles of ideal gas, none of whih are lost the roess. Inut arameters are: ot reservoir temerature (Kelv) old reservoir temerature (Kelv) Ideal gas equation olume of gas at osition the yle olume of gas at osition the yle hese two volumes are derived from the other uts: olume of gas at osition the yle olume of gas at osition the yle ayer relation onstant ork done by the ideal gas on the surroundgs Between ositions and ln, ln Between ositions and Between ositions and out ln, ln Between ositions and,, (,) o omlete the yle, d out, ork done by the gas on the surroundgs is the area enlosed by the yle, Niolas Léonard Sadi arnot (796-8), hysis toi handout - Ideal gases & heat enges Dr Andrew Frenh. AGE
5 arnot enge ont... otal work done is: d,,,, ln ln ln ln ln ln ln ln Defe heat enge effiieny as the ratio of ork done by the gas to the heat ut ln ln he arnot Enge effiieny deends only on the reservoir temeratures. Se the temerature range annot go beyond the range of reservoir temeratures, the arnot yle reresents the most effiient way of extratg work given an amount of heat ut.* Any other roess would ouy less area the S, diagram. ln he effiieny of a arnot heat enge an be more simly derived by onsideration of Entroy S. his is a measure of disorder a substane. he Seond Law of hermodynamis states for any hange, the total amount of Entroy the Universe must rease. d If heat is added a reversible roess: ds For the arnot yle, the isentroi stages have no heat hange hene they are at onstant Entroy. (Note this alies to the ideal gas, the surroundgs will hange entroy due to the exhange of work with the ideal gas). e annot reate entroy the yle for the gas, as the yle returns to the origal state (,, ), and Entroy is a salar funtion of state (i.e. like otential energy the ath does not matter). he (S,) urve for the yle is therefore a retangle. In the isothermal stages, temerature is a onstant, so both ases out S ln From the First Law of hermodynamis du d d du ds d Over the whole yle the ternal energy doesn t hange, so the work done by the gas is: d ds S ln ln ln *here is a better justifiation on the next age! hysis toi handout - Ideal gases & heat enges Dr Andrew Frenh. AGE 5
6 General notes regardg the Seond Law of hermodynamis, and the maximum ossible effiieny of heat enges It is ossible to bound the effiieny of any heat enge usg (i) the law of onservation of energy and (ii) the Seond Law of hermodynamis. hat the uer bound of effiieny equates with the effiieny of the arnot enge is erhas an even stronger justifiation of the statement that a arnot enge is the most effiient sheme ossible, if deed it ould be ratially realized. Any heat enge is essentially flow of heat from a hot reservoir to a older one. By a reservoir we mean a thermal mass that is so large that it will not hange temerature when the heat we assoiate with our enge is taken from or added to it. he differene heat taken from the hot reservoir, and the heat transferred to the old reservoir, is the maximum ossible work done by the enge. his must be true to satisfy the law of energy onservation, or the First Law of hermodynamis. ot reservoir First Law: out he Seond Law of hermodynamis states that for every hange there an never be an overall derease Entroy. For our idealized system, this means the loss of entroy of the hot reservoir must at least be omensated for by the ga entroy of the old reservoir. eat Enge out old reservoir S S out 0 out 0 Stotal S S S total Entroy hanges of hot and old reservoirs Seond Law: Notes on reversibility A reversible heat enge is one whih is assumed to oerate at thermodynami equilibrium at all times. he ideal gas equations, and assoiated relationshis, hold and there are no losses due to frition et. In other words, the differential form of the First Law holds at all times; i.e. where hanges du ternal energy are fully aounted for by heat hange d and work done d = -d. his means that there is no net ternal energy and deed entroy hange over the omlete yle. his means the ideal yle ould be run reverse without breakg the Seond Law, se a zero net entroy hange is ermitted. out ombg with the First Law exression: out 0 out Defe enge effiieny: 0 So se the arnot enge has effiieny this is as effiient as thermodynamis allows, so the arnot yle is (one examle*) of the most effiient heat enge ossible. *A Brayton Enge (adiabati omression, isobari heatg, adiabati exansion, isobari oolg) has a similar theoretial effiieny as a arnot yle. hysis toi handout - Ideal gases & heat enges Dr Andrew Frenh. AGE 6
7 eat Enges - Retangular, yle Assume we have n moles of ideal gas, none of whih are lost the roess. Inut arameters are: ressure at osition the yle ressure at osition the yle, (, ) (, ),, (, ) Gas temerature at osition the yle olume of gas at osition, the yle olume of gas at osition, the yle, (, ) Note arnot effiieny for this heat enge would be % 87 7 Between ositions and n n, ( ), d R S, n n ln Between ositions and n,, 0 d eat ut to gas ork done by gas eat outut from gas ork done by gas S, n n ln Net heat ut,, n ( ) n ( ) Between ositions and n, ( ), d S, n n ln Between ositions and n n ( ) ( ) ( ) ( ) R ( ) ( ) n,, 0 d hysis toi handout - Ideal gases & heat enges Dr Andrew Frenh. AGE 7 eat outut from gas ork done by gas eat ut to gas ork done by gas S, n n ln Net work done by gas,,,, ( - ) - ( - ) - - Enge effiieny - - ( ) ( ) - - R R R
8 eat Enges he Otto yle he Otto yle is the basis of sark-ignition iston enge, whih is essentially how a tyial etrol driven enge oerates. ositions 0-: Air is drawn to iston/ylder arrangement at onstant ressure. roess Adiabati (isentroi) omression of the air via a iston. Between ositions and Between ositions and n 0, S 0,,, R n,, 0 d S, n n ln -ve se work is beg done on the gas eat outut via exhaust o omlete the yle roess onstant-volume heat transfer to the workg gas from an external soure while the iston is at maximum omression. his roess is tended to reresent the ignition of the fuel-air mixture and the subsequent raid burng. roess Adiabati (isentroi) exansion (ower stroke). roess onstant-volume roess whih heat is rejeted from the air while the iston is at maximum exansion. roess 0 Air is released to the atmoshere at onstant ressure. Assume we have n moles of ideal gas, none of whih are lost the roess. Inut arameters are: ressure of gas at osition the yle ressure of gas at osition the yle olume of gas at osition the yle olume of gas at osition the yle emerature of gas at osition the yle R R R Between ositions and n,, 0 d S, n n ln Between ositions and 0, S 0,,, eat ut to gas via sark ignition ork done by gas (, ) ork done by the gas on the surroundgs is the area enlosed by the yle d (, ) n, n out, Nikolaus Otto (8-89) (, ) out (, ) hysis toi handout - Ideal gases & heat enges Dr Andrew Frenh. AGE 8
9 Otto enge ont... otal work done is: d,, r r r r r r r r r r Defe the omression ratio r Defe heat enge effiieny as the ratio of ork done by the gas to the heat ut r r r r So the Otto Enge effiieny deends only on the omression ratio, and the ratio of seifi heats (, ) ork done by the gas on the surroundgs is the area enlosed by the yle d (, ) (, ) out (, ) From the revious age: n n R R r R R R hysis toi handout - Ideal gases & heat enges Dr Andrew Frenh. AGE 9
10 eat Enges he Diesel yle he Diesel yle is the basis of a diesel enge, whih is ubiquitous transort aliations. Unlike etrol-driven enges, diesel variants are more suited to heavy mahery. hey an be found owerg most shis as well as truks, buses and ars. ositions 0-: Air is drawn to iston/ylder arrangement at onstant ressure. roess Adiabati (isentroi) omression of the air via a iston. roess onstant-ressure (isobari) heat transfer to the workg gas from an external soure while the iston is at maximum omression. his roess is tended to reresent the ignition of the fuel-air mixture and the subsequent raid burng. his is different from the Otto yle, whih is onstant volume (isohori) heatg durg this stage. In the Diesel yle, the heat generated from air omression is suffiient to ignite trodued fuel vaours. In the Otto yle a sark lug is used stead to ignite the fuel. roess Adiabati (isentroi) exansion (ower stroke). roess onstant-volume roess whih heat is rejeted from the air while the iston is at maximum exansion. roess 0 Air is released to the atmoshere at onstant ressure. Assume we have n moles of ideal gas, none of whih are lost the roess. Inut arameters are: ressure of gas at osition the yle olume of gas at osition the yle olume of gas at osition the yle olume of gas at osition the yle emerature of gas at osition the yle R R R Between ositions and Between ositions and n R eat outut, n via exhaust, 0 d S, n n ln, -ve se work is beg done, 0, S, 0 on the gas Between ositions and n,, Between ositions and d S, n nln 0, S 0,,, eat ut to gas durg ombustion ork done by gas (, ) (, ) o omlete the yle ork done by the gas on the surroundgs is the area enlosed by the yle n, n out, d Rudolf Diesel (858-9) out (, ) (, ) hysis toi handout - Ideal gases & heat enges Dr Andrew Frenh. AGE 0
11 Diesel enge ont... otal work done is: d,,, r s r r r r s s s r r r s r s r r r r s r s s r ( ) ( ) r s r s r s ( ) r s r s Defe the omression ratios r s r s Defe heat enge effiieny as the ratio of ork done by the gas to the heat ut r s r s ( ) r s From the revious age: r r( s ) r s r s r s r s (, ) (, ) ork done by the gas on the surroundgs is the area enlosed by the yle d (, ) out (, ) n R n R R r s s r s he Diesel enge is tyially more effiient than a etrol (Otto) enge se the former works on the basis of self ignition due to high omression. his knokg is undesirable for etrol enges, so a lower r value is required. hysis toi handout - Ideal gases & heat enges Dr Andrew Frenh. AGE
12 omarg Otto and Diesel heat enges r s R (, ) r s R R ork done by the gas on the surroundgs is the area enlosed by the yle d (, ) (, ) out (, ) (, ) (, ) s r s r diesel otto Diesel r( s ) r s r s Otto r r r ork done by the gas on the surroundgs is the area enlosed by the yle d (, ) out (, ) hysis toi handout - Ideal gases & heat enges Dr Andrew Frenh. AGE
Subject: Modeling of Thermal Rocket Engines; Nozzle flow; Control of mass flow. p c. Thrust Chamber mixing and combustion
 16.50 Leture 6 Subjet: Modeling of Thermal Roket Engines; Nozzle flow; Control of mass flow Though onetually simle, a roket engine is in fat hysially a very omlex devie and diffiult to reresent quantitatively
16.50 Leture 6 Subjet: Modeling of Thermal Roket Engines; Nozzle flow; Control of mass flow Though onetually simle, a roket engine is in fat hysially a very omlex devie and diffiult to reresent quantitatively
Lecture 13. Heat Engines. Thermodynamic processes and entropy Thermodynamic cycles Extracting work from heat
 Lecture 3 Heat Engines hermodynamic rocesses and entroy hermodynamic cycles Extracting work from heat - How do we define engine efficiency? - Carnot cycle: the best ossible efficiency Reading for this
Lecture 3 Heat Engines hermodynamic rocesses and entroy hermodynamic cycles Extracting work from heat - How do we define engine efficiency? - Carnot cycle: the best ossible efficiency Reading for this
Lecture 13 Heat Engines
 Lecture 3 Heat Engines hermodynamic rocesses and entroy hermodynamic cycles Extracting work from heat - How do we define engine efficiency? - Carnot cycle: the best ossible efficiency Reading for this
Lecture 3 Heat Engines hermodynamic rocesses and entroy hermodynamic cycles Extracting work from heat - How do we define engine efficiency? - Carnot cycle: the best ossible efficiency Reading for this
Research Article Substance Independence of Efficiency of a Class of Heat Engines Undergoing Two Isothermal Processes
 hermodynamis olume 0, Artile ID 6797, 5 pages doi:0.55/0/6797 Researh Artile Substane Independene of Effiieny of a Class of Heat Engines Undergoing wo Isothermal roesses Y. Haseli Department of Mehanial
hermodynamis olume 0, Artile ID 6797, 5 pages doi:0.55/0/6797 Researh Artile Substane Independene of Effiieny of a Class of Heat Engines Undergoing wo Isothermal roesses Y. Haseli Department of Mehanial
Review of classical thermodynamics
 Review of lassial thermodynamis Fundamental Laws, roperties and roesses () First Law - Energy Balane hermodynami funtions of state Internal energy, heat and work ypes of paths (isobari, isohori, isothermal,
Review of lassial thermodynamis Fundamental Laws, roperties and roesses () First Law - Energy Balane hermodynami funtions of state Internal energy, heat and work ypes of paths (isobari, isohori, isothermal,
Chapter 20: Exercises: 3, 7, 11, 22, 28, 34 EOC: 40, 43, 46, 58
 Chater 0: Exercises:, 7,,, 8, 4 EOC: 40, 4, 46, 8 E: A gasoline engine takes in.80 0 4 and delivers 800 of work er cycle. The heat is obtained by burning gasoline with a heat of combustion of 4.60 0 4.
Chater 0: Exercises:, 7,,, 8, 4 EOC: 40, 4, 46, 8 E: A gasoline engine takes in.80 0 4 and delivers 800 of work er cycle. The heat is obtained by burning gasoline with a heat of combustion of 4.60 0 4.
SOLVED QUESTIONS 1 / 2. in a closed container at equilibrium. What would be the effect of addition of CaCO 3 on the equilibrium concentration of CO 2?
 SOLVED QUESTIONS Multile Choie Questions. and are the veloity onstants of forward and bakward reations. The equilibrium onstant k of the reation is (A) (B) (C) (D). Whih of the following reations will
SOLVED QUESTIONS Multile Choie Questions. and are the veloity onstants of forward and bakward reations. The equilibrium onstant k of the reation is (A) (B) (C) (D). Whih of the following reations will
Kelvin Planck Statement of the Second Law. Clausius Statement of the Second Law
 Kelv Planck Statement of te Second aw It is imossible to construct an enge wic, oeratg a cycle, will roduce no oter effect tan te extraction of eat from a sgle reservoir and te erformance of an equivalent
Kelv Planck Statement of te Second aw It is imossible to construct an enge wic, oeratg a cycle, will roduce no oter effect tan te extraction of eat from a sgle reservoir and te erformance of an equivalent
Anelastic MHD Equations : Theory
 Anelasti MHD Equations : Theory Abhishek Kr. Srivastava Armagh Observatory Northern Ireland Different zones of the Sun Comlex magneti field is the key behind all tyes of solar ativity and formation of
Anelasti MHD Equations : Theory Abhishek Kr. Srivastava Armagh Observatory Northern Ireland Different zones of the Sun Comlex magneti field is the key behind all tyes of solar ativity and formation of
Chapter 3. Volumetric Properties of Pure Fluids
 Chapter 3. olumetri roperties of ure Fluids Introdution hermodynami properties (U, H and thus Q, W) are alulated from data data are important for sizing vessels and pipelines Subjets behavior of pure fluids
Chapter 3. olumetri roperties of ure Fluids Introdution hermodynami properties (U, H and thus Q, W) are alulated from data data are important for sizing vessels and pipelines Subjets behavior of pure fluids
THE FIRST LAW OF THERMODYNAMICS
 THE FIRST LA OF THERMODYNAMIS 9 9 (a) IDENTIFY and SET UP: The ressure is constant and the volume increases (b) = d Figure 9 Since is constant, = d = ( ) The -diagram is sketched in Figure 9 The roblem
THE FIRST LA OF THERMODYNAMIS 9 9 (a) IDENTIFY and SET UP: The ressure is constant and the volume increases (b) = d Figure 9 Since is constant, = d = ( ) The -diagram is sketched in Figure 9 The roblem
PHYS1001 PHYSICS 1 REGULAR Module 2 Thermal Physics Chapter 17 First Law of Thermodynamics
 PHYS1001 PHYSICS 1 REGULAR Module Thermal Physics Chater 17 First Law of Thermodynamics References: 17.1 to 17.9 Examles: 17.1 to 17.7 Checklist Thermodynamic system collection of objects and fields. If
PHYS1001 PHYSICS 1 REGULAR Module Thermal Physics Chater 17 First Law of Thermodynamics References: 17.1 to 17.9 Examles: 17.1 to 17.7 Checklist Thermodynamic system collection of objects and fields. If
The Second Law: The Machinery
 The Second Law: The Machinery Chater 5 of Atkins: The Second Law: The Concets Sections 3.7-3.9 8th Ed, 3.3 9th Ed; 3.4 10 Ed.; 3E 11th Ed. Combining First and Second Laws Proerties of the Internal Energy
The Second Law: The Machinery Chater 5 of Atkins: The Second Law: The Concets Sections 3.7-3.9 8th Ed, 3.3 9th Ed; 3.4 10 Ed.; 3E 11th Ed. Combining First and Second Laws Proerties of the Internal Energy
PV/T = k or PV = kt Describe the difference between an ideal gas and a real gas.
 10.1 Thermodynamics 10.2 Processes 10 10.3 The second law of thermodynamics and entroy 10.1 Thermodynamics From the combined gas laws, we determined that: P/T = k or P = kt 10.1.1 State the equation of
10.1 Thermodynamics 10.2 Processes 10 10.3 The second law of thermodynamics and entroy 10.1 Thermodynamics From the combined gas laws, we determined that: P/T = k or P = kt 10.1.1 State the equation of
Chemistry 420/523 Chemical Thermodynamics (Spring ) Examination 1
 Chemistry 420/523 Chemical hermodynamics (Sring 2001-02) Examination 1 1 Boyle temerature is defined as the temerature at which the comression factor Z m /(R ) of a gas is exactly equal to 1 For a gas
Chemistry 420/523 Chemical hermodynamics (Sring 2001-02) Examination 1 1 Boyle temerature is defined as the temerature at which the comression factor Z m /(R ) of a gas is exactly equal to 1 For a gas
Thermodynamica 1. college 5 boek hoofdstuk 3. Bendiks Jan Boersma Wiebren de Jong Thijs J.H. Vlugt Theo Woudstra. Process and Energy Department
 hermodynamia Bendiks Jan Boersma Wiebren de Jong hijs J.H. Vlugt heo Woudstra Proess and Energy Deartment February 9, 20 ollege 5 boek hoofdstuk 3 summary leture 4 roerties of matter -V- surfae isobar,
hermodynamia Bendiks Jan Boersma Wiebren de Jong hijs J.H. Vlugt heo Woudstra Proess and Energy Deartment February 9, 20 ollege 5 boek hoofdstuk 3 summary leture 4 roerties of matter -V- surfae isobar,
Influence of Real-Fluid Properties in Modeling Decompression Wave Interacting with Ductile Fracture Propagation
 Oil & Gas Siene and ehnology Rev. IFP, Vol. 6 (5), No. 4,. 7-79 Coyright 5, Institut français du étrole Influene of Real-Fluid Proerties in Modeling Deomression Wave Interating with Dutile Frature Proagation
Oil & Gas Siene and ehnology Rev. IFP, Vol. 6 (5), No. 4,. 7-79 Coyright 5, Institut français du étrole Influene of Real-Fluid Proerties in Modeling Deomression Wave Interating with Dutile Frature Proagation
CHAPTER 16. Basic Concepts. Basic Concepts. The Equilibrium Constant. Reaction Quotient & Equilibrium Constant. Chemical Equilibrium
 Proerties of an Equilibrium System CHAPTER 6 Chemial Equilibrium Equilibrium systems are DYNAMIC (in onstant motion) REVERSIBLE an be aroahed from either diretion Pink to blue Co(H O) 6 Cl ---> > Co(H
Proerties of an Equilibrium System CHAPTER 6 Chemial Equilibrium Equilibrium systems are DYNAMIC (in onstant motion) REVERSIBLE an be aroahed from either diretion Pink to blue Co(H O) 6 Cl ---> > Co(H
Phase transition. Asaf Pe er Background
 Phase transition Asaf Pe er 1 November 18, 2013 1. Background A hase is a region of sace, throughout which all hysical roerties (density, magnetization, etc.) of a material (or thermodynamic system) are
Phase transition Asaf Pe er 1 November 18, 2013 1. Background A hase is a region of sace, throughout which all hysical roerties (density, magnetization, etc.) of a material (or thermodynamic system) are
University of Washington Department of Chemistry Chemistry 452/456 Summer Quarter 2011
 Homework Assignment #4: Due at 500 pm Monday 8 July,. University of Washington Department of Chemistry Chemistry 45/456 Summer Quarter 0 ) o a very good approximation, ammonia obeys the Bertholet equation
Homework Assignment #4: Due at 500 pm Monday 8 July,. University of Washington Department of Chemistry Chemistry 45/456 Summer Quarter 0 ) o a very good approximation, ammonia obeys the Bertholet equation
02. Equilibrium Thermodynamics II: Engines
 University of Rhode Island DigitalCommons@URI Equilibrium Statistical Physics Physics Course Materials 205 02. Equilibrium Thermodynamics II: Engines Gerhard Müller University of Rhode Island, gmuller@uri.edu
University of Rhode Island DigitalCommons@URI Equilibrium Statistical Physics Physics Course Materials 205 02. Equilibrium Thermodynamics II: Engines Gerhard Müller University of Rhode Island, gmuller@uri.edu
THERMODYNAMICS. Prepared by Sibaprasad Maity Asst. Prof. in Chemistry For any queries contact at
 HERMODYNAMIS reared by Sibarasad Maity Asst. rof. in hemistry For any queries contact at 943445393 he word thermo-dynamic, used first by illiam homson (later Lord Kelvin), has Greek origin, and is translated
HERMODYNAMIS reared by Sibarasad Maity Asst. rof. in hemistry For any queries contact at 943445393 he word thermo-dynamic, used first by illiam homson (later Lord Kelvin), has Greek origin, and is translated
Risk Analysis in Water Quality Problems. Souza, Raimundo 1 Chagas, Patrícia 2 1,2 Departamento de Engenharia Hidráulica e Ambiental
 Risk Analysis in Water Quality Problems. Downloaded from aselibrary.org by Uf - Universidade Federal Do Ceara on 1/29/14. Coyright ASCE. For ersonal use only; all rights reserved. Souza, Raimundo 1 Chagas,
Risk Analysis in Water Quality Problems. Downloaded from aselibrary.org by Uf - Universidade Federal Do Ceara on 1/29/14. Coyright ASCE. For ersonal use only; all rights reserved. Souza, Raimundo 1 Chagas,
Ideal Gas Law. September 2, 2014
 Ideal Gas Law Setember 2, 2014 Thermodynamics deals with internal transformations of the energy of a system and exchanges of energy between that system and its environment. A thermodynamic system refers
Ideal Gas Law Setember 2, 2014 Thermodynamics deals with internal transformations of the energy of a system and exchanges of energy between that system and its environment. A thermodynamic system refers
Chapter-6: Entropy. 1 Clausius Inequality. 2 Entropy - A Property
 hater-6: Entroy When the first law of thermodynamics was stated, the existence of roerty, the internal energy, was found. imilarly, econd law also leads to definition of another roerty, known as entroy.
hater-6: Entroy When the first law of thermodynamics was stated, the existence of roerty, the internal energy, was found. imilarly, econd law also leads to definition of another roerty, known as entroy.
Chapter 8 Thermodynamic Relations
 Chapter 8 Thermodynami Relations 8.1 Types of Thermodynami roperties The thermodynami state of a system an be haraterized by its properties that an be lassified as measured, fundamental, or deried properties.
Chapter 8 Thermodynami Relations 8.1 Types of Thermodynami roperties The thermodynami state of a system an be haraterized by its properties that an be lassified as measured, fundamental, or deried properties.
Test bank chapter (14)
 Test bank hater (14) Choose the most orret answer 1. Whih is the orret equilibrium onstant exression for the following reation? Fe 2 O 3 (s) + 3H 2 (g) 2Fe(s) + 3H 2 O(g) a) K = [Fe 2 O 3 ] [H 2 ] 3 /[Fe]
Test bank hater (14) Choose the most orret answer 1. Whih is the orret equilibrium onstant exression for the following reation? Fe 2 O 3 (s) + 3H 2 (g) 2Fe(s) + 3H 2 O(g) a) K = [Fe 2 O 3 ] [H 2 ] 3 /[Fe]
General Equilibrium. What happens to cause a reaction to come to equilibrium?
 General Equilibrium Chemial Equilibrium Most hemial reations that are enountered are reversible. In other words, they go fairly easily in either the forward or reverse diretions. The thing to remember
General Equilibrium Chemial Equilibrium Most hemial reations that are enountered are reversible. In other words, they go fairly easily in either the forward or reverse diretions. The thing to remember
Internal Energy in terms of Properties
 Lecture #3 Internal Energy in terms of roerties Internal energy is a state function. A change in the state of the system causes a change in its roerties. So, we exress the change in internal energy in
Lecture #3 Internal Energy in terms of roerties Internal energy is a state function. A change in the state of the system causes a change in its roerties. So, we exress the change in internal energy in
dn i where we have used the Gibbs equation for the Gibbs energy and the definition of chemical potential
 Chem 467 Sulement to Lectures 33 Phase Equilibrium Chemical Potential Revisited We introduced the chemical otential as the conjugate variable to amount. Briefly reviewing, the total Gibbs energy of a system
Chem 467 Sulement to Lectures 33 Phase Equilibrium Chemical Potential Revisited We introduced the chemical otential as the conjugate variable to amount. Briefly reviewing, the total Gibbs energy of a system
Unit code: H/ QCF level: 5 Credit value: 15 OUTCOME 1 - THERMODYNAMIC SYSTEMS TUTORIAL 2
 Unit 43: Plant and Process Princiles Unit code: H/60 44 QCF level: 5 Credit value: 5 OUCOME - HERMODYNAMIC SYSEMS UORIAL Understand thermodynamic systems as alied to lant engineering rocesses hermodynamic
Unit 43: Plant and Process Princiles Unit code: H/60 44 QCF level: 5 Credit value: 5 OUCOME - HERMODYNAMIC SYSEMS UORIAL Understand thermodynamic systems as alied to lant engineering rocesses hermodynamic
δq T = nr ln(v B/V A )
 hysical Chemistry 007 Homework assignment, solutions roblem 1: An ideal gas undergoes the following reversible, cyclic rocess It first exands isothermally from state A to state B It is then comressed adiabatically
hysical Chemistry 007 Homework assignment, solutions roblem 1: An ideal gas undergoes the following reversible, cyclic rocess It first exands isothermally from state A to state B It is then comressed adiabatically
The Second Law of Thermodynamics. (Second Law of Thermodynamics)
 he Second aw of hermodynamics For the free exansion, we have >. It is an irreversible rocess in a closed system. For the reversible isothermal rocess, for the gas > for exansion and < for comression. owever,
he Second aw of hermodynamics For the free exansion, we have >. It is an irreversible rocess in a closed system. For the reversible isothermal rocess, for the gas > for exansion and < for comression. owever,
CHEE 221: Chemical Processes and Systems
 CH 221: Chemical Processes and Systems Module 4. nergy Balances without Reaction Part a: Introduction to nergy Balances (Felder & Rousseau Ch 7.0 7.4 nergy and nergy Balances: very chemical rocess volves
CH 221: Chemical Processes and Systems Module 4. nergy Balances without Reaction Part a: Introduction to nergy Balances (Felder & Rousseau Ch 7.0 7.4 nergy and nergy Balances: very chemical rocess volves
FINITE TIME THERMODYNAMIC MODELING AND ANALYSIS FOR AN IRREVERSIBLE ATKINSON CYCLE. By Yanlin GE, Lingen CHEN, and Fengrui SUN
 FINIE IME HERMODYNAMIC MODELING AND ANALYSIS FOR AN IRREVERSIBLE AKINSON CYCLE By Yanlin GE, Lingen CHEN, and Fengrui SUN Performance of an air-standard Atkinson cycle is analyzed by using finite-time
FINIE IME HERMODYNAMIC MODELING AND ANALYSIS FOR AN IRREVERSIBLE AKINSON CYCLE By Yanlin GE, Lingen CHEN, and Fengrui SUN Performance of an air-standard Atkinson cycle is analyzed by using finite-time
Simple Cyclic loading model based on Modified Cam Clay
 Simle li loading model based on Modified am la Imlemented in RISP main rogram version 00. and higher B Amir Rahim, The RISP onsortium Ltd Introdution This reort resents a simle soil model whih rovides
Simle li loading model based on Modified am la Imlemented in RISP main rogram version 00. and higher B Amir Rahim, The RISP onsortium Ltd Introdution This reort resents a simle soil model whih rovides
Downloaded on T02:40:49Z
 Title Author(s) Primary wave energy onversions of osillating water olumns Sheng, Wanan; Alorn, Raymond; Lewis, Anthony Publiation date 213-9 Original itation Tye of ubliation Link to ublisher's version
Title Author(s) Primary wave energy onversions of osillating water olumns Sheng, Wanan; Alorn, Raymond; Lewis, Anthony Publiation date 213-9 Original itation Tye of ubliation Link to ublisher's version
HEAT, WORK, AND THE FIRST LAW OF THERMODYNAMICS
 HET, ORK, ND THE FIRST L OF THERMODYNMIS 8 EXERISES Section 8. The First Law of Thermodynamics 5. INTERPRET e identify the system as the water in the insulated container. The roblem involves calculating
HET, ORK, ND THE FIRST L OF THERMODYNMIS 8 EXERISES Section 8. The First Law of Thermodynamics 5. INTERPRET e identify the system as the water in the insulated container. The roblem involves calculating
EF 152 Exam #3, Spring 2016 Page 1 of 6
 EF 5 Exam #3, Spring 06 Page of 6 Name: Setion: Instrutions Do not open te exam until instruted to do so. Do not leave if tere is less tan 5 minutes to go in te exam. Wen time is alled, immediately stop
EF 5 Exam #3, Spring 06 Page of 6 Name: Setion: Instrutions Do not open te exam until instruted to do so. Do not leave if tere is less tan 5 minutes to go in te exam. Wen time is alled, immediately stop
NEWCOMEN ATMOSPHERIC ENGINE
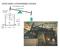 NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m F 7 stroke 5 sia steam 8 50 F water NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m 5 sia steam 8 F 50 F water 7
NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m F 7 stroke 5 sia steam 8 50 F water NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m 5 sia steam 8 F 50 F water 7
4. Energy balances Partly based on Chapter 4 of the De Nevers textbook.
 Lecture Notes CHE 31 Fluid Mechanics (Fall 010) 4 Energy balances Partly based on Chater 4 of the De Nevers textbook Energy fluid mechanics As for any quantity, we can set u an energy balance for a secific
Lecture Notes CHE 31 Fluid Mechanics (Fall 010) 4 Energy balances Partly based on Chater 4 of the De Nevers textbook Energy fluid mechanics As for any quantity, we can set u an energy balance for a secific
Panos Kouvelis Olin School of Business Washington University
 Quality-Based Cometition, Profitability, and Variable Costs Chester Chambers Co Shool of Business Dallas, TX 7575 hamber@mailosmuedu -768-35 Panos Kouvelis Olin Shool of Business Washington University
Quality-Based Cometition, Profitability, and Variable Costs Chester Chambers Co Shool of Business Dallas, TX 7575 hamber@mailosmuedu -768-35 Panos Kouvelis Olin Shool of Business Washington University
Computation of Thermodynamic Cycle for Novel Detonation Aircraft Engine
 Comutation of Thermodynami Cyle for Novel Detonation Airraft Enine Cleoatra F. Cuiumita COMOTI Romanian Researh and Develoment Institute for Gas Turbines Senior Researher 0 D Iuliu Maniu Bd., setor 6,
Comutation of Thermodynami Cyle for Novel Detonation Airraft Enine Cleoatra F. Cuiumita COMOTI Romanian Researh and Develoment Institute for Gas Turbines Senior Researher 0 D Iuliu Maniu Bd., setor 6,
Physics 2A (Fall 2012) Chapters 11:Using Energy and 12: Thermal Properties of Matter
 Physics 2A (Fall 2012) Chaters 11:Using Energy and 12: Thermal Proerties of Matter "Kee in mind that neither success nor failure is ever final." Roger Ward Babson Our greatest glory is not in never failing,
Physics 2A (Fall 2012) Chaters 11:Using Energy and 12: Thermal Proerties of Matter "Kee in mind that neither success nor failure is ever final." Roger Ward Babson Our greatest glory is not in never failing,
EE451/551: Digital Control. Relationship Between s and z Planes. The Relationship Between s and z Planes 11/10/2011
 /0/0 EE45/55: Digital Control Chater 6: Digital Control System Design he Relationshi Between s and Planes As noted reviously: s j e e e e r s j where r e and If an analog system has oles at: s n jn a jd
/0/0 EE45/55: Digital Control Chater 6: Digital Control System Design he Relationshi Between s and Planes As noted reviously: s j e e e e r s j where r e and If an analog system has oles at: s n jn a jd
The Procedure of Finding the Stress-Energy. Tensor and Equations of Vector Field of Any Form
 Advaned Studies in Theoretial Phsis Vol. 8, 14, no. 18, 771-779 HIKARI Ltd, www.m-hikari.om htt://d.doi.org/1.1988/ast.14.4711 The Proedure of Finding the Stress-Energ Tensor and Equations of Vetor Field
Advaned Studies in Theoretial Phsis Vol. 8, 14, no. 18, 771-779 HIKARI Ltd, www.m-hikari.om htt://d.doi.org/1.1988/ast.14.4711 The Proedure of Finding the Stress-Energ Tensor and Equations of Vetor Field
Investigating the Performance of a Hydraulic Power Take-Off
 Investigating the Performane of a Hydrauli Power Take-Off A.R. Plummer and M. Shlotter Centre for Power Transmission and Control, University of Bath, Bath, BA 7AY, UK E-mail: A.R.Plummer@bath.a.uk E-mail:
Investigating the Performane of a Hydrauli Power Take-Off A.R. Plummer and M. Shlotter Centre for Power Transmission and Control, University of Bath, Bath, BA 7AY, UK E-mail: A.R.Plummer@bath.a.uk E-mail:
Proportional-Integral-Derivative PID Controls
 Proortional-Integral-Derivative PID Controls Dr M.J. Willis Det. of Chemial and Proess Engineering University of Newastle e-mail: mark.willis@nl.a.uk Written: 7 th November, 998 Udated: 6 th Otober, 999
Proortional-Integral-Derivative PID Controls Dr M.J. Willis Det. of Chemial and Proess Engineering University of Newastle e-mail: mark.willis@nl.a.uk Written: 7 th November, 998 Udated: 6 th Otober, 999
Chapter 1 Fundamentals
 Chater Fundamentals. Overview of Thermodynamics Industrial Revolution brought in large scale automation of many tedious tasks which were earlier being erformed through manual or animal labour. Inventors
Chater Fundamentals. Overview of Thermodynamics Industrial Revolution brought in large scale automation of many tedious tasks which were earlier being erformed through manual or animal labour. Inventors
I have not proofread these notes; so please watch out for typos, anything misleading or just plain wrong.
 hermodynamics I have not roofread these notes; so lease watch out for tyos, anything misleading or just lain wrong. Please read ages 227 246 in Chater 8 of Kittel and Kroemer and ay attention to the first
hermodynamics I have not roofread these notes; so lease watch out for tyos, anything misleading or just lain wrong. Please read ages 227 246 in Chater 8 of Kittel and Kroemer and ay attention to the first
CONSTRUCTION OF MIXED SAMPLING PLAN WITH DOUBLE SAMPLING PLAN AS ATTRIBUTE PLAN INDEXED THROUGH (MAPD, MAAOQ) AND (MAPD, AOQL)
 Global J. of Arts & Mgmt., 0: () Researh Paer: Samath kumar et al., 0: P.07- CONSTRUCTION OF MIXED SAMPLING PLAN WITH DOUBLE SAMPLING PLAN AS ATTRIBUTE PLAN INDEXED THROUGH (MAPD, MAAOQ) AND (MAPD, AOQL)
Global J. of Arts & Mgmt., 0: () Researh Paer: Samath kumar et al., 0: P.07- CONSTRUCTION OF MIXED SAMPLING PLAN WITH DOUBLE SAMPLING PLAN AS ATTRIBUTE PLAN INDEXED THROUGH (MAPD, MAAOQ) AND (MAPD, AOQL)
Physics 41 Chapter 22 HW
 Pysis 41 apter 22 H 1. eat ine performs 200 J of work in ea yle and as an effiieny of 30.0%. For ea yle, ow mu energy is (a) taken in and (b) expelled as eat? = = 200 J (1) e = 1 0.300 = = (2) From (2),
Pysis 41 apter 22 H 1. eat ine performs 200 J of work in ea yle and as an effiieny of 30.0%. For ea yle, ow mu energy is (a) taken in and (b) expelled as eat? = = 200 J (1) e = 1 0.300 = = (2) From (2),
REAL GASES. (B) pv. (D) pv. 3. The compressibility factor of a gas is less than unity at STP. Therefore, molar volume (V m.
 SINGLE ORRET ANSWER REAL GASES 1. A real gas is suosed to obey the gas equation ( b) = at STP. If one mole of a gas occuies 5dm 3 volume at STP, then its comressibility factor is (b=.586 L mol 1F) (A)
SINGLE ORRET ANSWER REAL GASES 1. A real gas is suosed to obey the gas equation ( b) = at STP. If one mole of a gas occuies 5dm 3 volume at STP, then its comressibility factor is (b=.586 L mol 1F) (A)
Physics 231 Lecture 35
 ysis 1 Leture 5 Main points of last leture: Heat engines and effiieny: eng e 1 Carnot yle and Carnot engine. eng e 1 is in Kelvin. Refrigerators CO eng Ideal refrigerator CO rev reversible Entropy ΔS Computation
ysis 1 Leture 5 Main points of last leture: Heat engines and effiieny: eng e 1 Carnot yle and Carnot engine. eng e 1 is in Kelvin. Refrigerators CO eng Ideal refrigerator CO rev reversible Entropy ΔS Computation
Reversibility. Processes in nature are always irreversible: far from equilibrium
 Reversibility Processes in nature are always irreversible: far from equilibrium Reversible process: idealized process infinitely close to thermodynamic equilibrium (quasi-equilibrium) Necessary conditions
Reversibility Processes in nature are always irreversible: far from equilibrium Reversible process: idealized process infinitely close to thermodynamic equilibrium (quasi-equilibrium) Necessary conditions
Performance of an irreversible Diesel cycle under variable stroke length and compression ratio
 Marsland Press Journal of Amerian Siene 00;6():58-6 Performane of an irreversible iesel yle under variable stroke length and ompression ratio epartment of Agriulture Mahine Mehanis, Shahrekord University,
Marsland Press Journal of Amerian Siene 00;6():58-6 Performane of an irreversible iesel yle under variable stroke length and ompression ratio epartment of Agriulture Mahine Mehanis, Shahrekord University,
NODIA AND COMPANY. GATE SOLVED PAPER Mechanical Engineering Thermodynamics. Copyright By NODIA & COMPANY
 No art of this ublication may be reroduced or distributed in any form or any means, electronic, mechanical, hotocoying, or otherwise without the rior ermission of the author. GAE SOLVED PAPER Mechanical
No art of this ublication may be reroduced or distributed in any form or any means, electronic, mechanical, hotocoying, or otherwise without the rior ermission of the author. GAE SOLVED PAPER Mechanical
Solutions of burnt-bridge models for molecular motor transport
 PHYSICAL REVIEW E 75, 0390 2007 Solutions of burnt-bridge models for moleular motor transort Alexander Yu. Morozov, Ekaterina Pronina, and Anatoly B. Kolomeisky Deartment of Chemistry, Rie University,
PHYSICAL REVIEW E 75, 0390 2007 Solutions of burnt-bridge models for moleular motor transort Alexander Yu. Morozov, Ekaterina Pronina, and Anatoly B. Kolomeisky Deartment of Chemistry, Rie University,
Maximum entropy generation rate in a heat exchanger at constant inlet parameters
 Maximum entroy generation rate in a heat exhanger at onstant inlet arameters Rafał LASKOWSKI, Maiej JAWORSKI Online: htt://www.jmee.tu.koszalin.l/download_artile/jmee_7 7986.df Cite this artile as: Laskowski
Maximum entroy generation rate in a heat exhanger at onstant inlet arameters Rafał LASKOWSKI, Maiej JAWORSKI Online: htt://www.jmee.tu.koszalin.l/download_artile/jmee_7 7986.df Cite this artile as: Laskowski
COMPENDIUM OF EQUATIONS Unified Engineering Thermodynamics
 COMPENDIUM OF EQUAIONS Unified Engineering hermodynamics Note: It is with some reseration that I suly this comendium of equations. One of the common itfalls for engineering students is that they sole roblems
COMPENDIUM OF EQUAIONS Unified Engineering hermodynamics Note: It is with some reseration that I suly this comendium of equations. One of the common itfalls for engineering students is that they sole roblems
Theory of the Integer and Fractional Quantum Hall Effects
 Theory of the Integer and Frational Quantum all Effets Shosuke SASAKI Center for Advaned igh Magneti Field Siene, Graduate Shool of Siene, Osaka University, - Mahikaneyama, Toyonaka, Osaka 56-, Jaan Prefae
Theory of the Integer and Frational Quantum all Effets Shosuke SASAKI Center for Advaned igh Magneti Field Siene, Graduate Shool of Siene, Osaka University, - Mahikaneyama, Toyonaka, Osaka 56-, Jaan Prefae
Investigation of electrons interaction in a superconductor
 Investigation of eletrons interation in a suerondutor Iogann Tolbatov Physis and Engineering Deartment, Kuban State University, Krasnodar, Russia (talbot108@mailru) Investigation of eletrons interation
Investigation of eletrons interation in a suerondutor Iogann Tolbatov Physis and Engineering Deartment, Kuban State University, Krasnodar, Russia (talbot108@mailru) Investigation of eletrons interation
F = U TS G = H TS. Availability, Irreversibility S K Mondal s Chapter 5. max. actual. univ. Ns =
 Availability, Irreversibility S K Mondal s Chater 5 I = W W 0 max ( S) = Δ univ actual 7. Irreversibility rate = I = rate of energy degradation = rate of energy loss (Wlost) = 0 S gen for all rocesses
Availability, Irreversibility S K Mondal s Chater 5 I = W W 0 max ( S) = Δ univ actual 7. Irreversibility rate = I = rate of energy degradation = rate of energy loss (Wlost) = 0 S gen for all rocesses
GEF2200 vår 2017 Løsningsforslag sett 1
 GEF2200 vår 2017 Løsningsforslag sett 1 A.1.T R is the universal gas constant, with value 8.3143JK 1 mol 1. R is the gas constant for a secic gas, given by R R M (1) where M is the molecular weight of
GEF2200 vår 2017 Løsningsforslag sett 1 A.1.T R is the universal gas constant, with value 8.3143JK 1 mol 1. R is the gas constant for a secic gas, given by R R M (1) where M is the molecular weight of
Application of Drucker-Prager Plasticity Model for Stress- Strain Modeling of FRP Confined Concrete Columns
 Available online at www.sienediret.om Proedia Engineering 14 (211) 687 694 The Twelfth East Asia-Paifi Conferene on Strutural Engineering and Constrution Aliation of Druker-Prager Plastiity Model for Stress-
Available online at www.sienediret.om Proedia Engineering 14 (211) 687 694 The Twelfth East Asia-Paifi Conferene on Strutural Engineering and Constrution Aliation of Druker-Prager Plastiity Model for Stress-
Actual exergy intake to perform the same task
 CHAPER : PRINCIPLES OF ENERGY CONSERVAION INRODUCION Energy conservation rinciles are based on thermodynamics If we look into the simle and most direct statement of the first law of thermodynamics, we
CHAPER : PRINCIPLES OF ENERGY CONSERVAION INRODUCION Energy conservation rinciles are based on thermodynamics If we look into the simle and most direct statement of the first law of thermodynamics, we
MODULE 2: DIFFUSION LECTURE NO. 2
 PTEL Chemical Mass Transfer Oeration MODULE : DIFFUSIO LECTURE O.. STEDY STTE MOLECULR DIFFUSIO I FLUIDS UDER STGT D LMIR FLOW CODITIOS.. Steady state diffusion through a constant area Steady state diffusion
PTEL Chemical Mass Transfer Oeration MODULE : DIFFUSIO LECTURE O.. STEDY STTE MOLECULR DIFFUSIO I FLUIDS UDER STGT D LMIR FLOW CODITIOS.. Steady state diffusion through a constant area Steady state diffusion
Example problems. Chapter 3: The Kinetic Theory of Gases. Homework: 13, 18, 20, 23, 25, 27 (p )
 Examle roblems Chater : he Kinetic heory o Gases Homework:, 8,,, 5, 7 (. 5-5) 9. An automobile tire has a volume o.64 x m and contains air at a gauge ressure (above atmosheric ressure) o 65 kpa when the
Examle roblems Chater : he Kinetic heory o Gases Homework:, 8,,, 5, 7 (. 5-5) 9. An automobile tire has a volume o.64 x m and contains air at a gauge ressure (above atmosheric ressure) o 65 kpa when the
A special reference frame is the center of mass or zero momentum system frame. It is very useful when discussing high energy particle reactions.
 High nergy Partile Physis A seial referene frame is the enter of mass or zero momentum system frame. It is very useful when disussing high energy artile reations. We onsider a ollision between two artiles
High nergy Partile Physis A seial referene frame is the enter of mass or zero momentum system frame. It is very useful when disussing high energy artile reations. We onsider a ollision between two artiles
Lecture 8, the outline
 Lecture, the outline loose end: Debye theory of solids more remarks on the first order hase transition. Bose Einstein condensation as a first order hase transition 4He as Bose Einstein liquid Lecturer:
Lecture, the outline loose end: Debye theory of solids more remarks on the first order hase transition. Bose Einstein condensation as a first order hase transition 4He as Bose Einstein liquid Lecturer:
A short note on Reitlinger thermodynamic cycles
 short note on Reitlinger thermodynamic cycles melia arolina Saravigna eartment of lied Science and echnology, Politecnico di orino, orino, Italy bstract: It is well known that arnot cycle is the thermodynamic
short note on Reitlinger thermodynamic cycles melia arolina Saravigna eartment of lied Science and echnology, Politecnico di orino, orino, Italy bstract: It is well known that arnot cycle is the thermodynamic
Chapter 20. Heat Engines, Entropy and the Second Law of Thermodynamics. Dr. Armen Kocharian
 Chapter 20 Heat Engines, Entropy and the Second Law of Thermodynamics Dr. Armen Kocharian First Law of Thermodynamics Review Review: The first law states that a change in internal energy in a system can
Chapter 20 Heat Engines, Entropy and the Second Law of Thermodynamics Dr. Armen Kocharian First Law of Thermodynamics Review Review: The first law states that a change in internal energy in a system can
not to be republished NCERT STATES OF MATTER UNIT 5 INTRODUCTION
 32 CHEMISRY UNI 5 SAES OF MAER After studying this unit you will be able to exlain the existence of different states of matter in terms of balance between intermolecular forces and thermal energy of articles;
32 CHEMISRY UNI 5 SAES OF MAER After studying this unit you will be able to exlain the existence of different states of matter in terms of balance between intermolecular forces and thermal energy of articles;
ATMOS Lecture 7. The First Law and Its Consequences Pressure-Volume Work Internal Energy Heat Capacity Special Cases of the First Law
 TMOS 5130 Lecture 7 The First Law and Its Consequences Pressure-Volume Work Internal Energy Heat Caacity Secial Cases of the First Law Pressure-Volume Work Exanding Volume Pressure δw = f & dx δw = F ds
TMOS 5130 Lecture 7 The First Law and Its Consequences Pressure-Volume Work Internal Energy Heat Caacity Secial Cases of the First Law Pressure-Volume Work Exanding Volume Pressure δw = f & dx δw = F ds
MODENA. Deliverable 1.5. Comprehensive kinetics model. Andreas Daiss, BASF (D) Peter Deglmann, BASF (D) Volker Settels, BASF (D)
 Delivery date: Feb 5, 05 MODEN uthors: ndreas Daiss BSF Peter Deglmann Deliverable.5 Comrehensive inetis model WP s leader: UNTS Sabrina Pril, UNTS () BSF Voler Settels BSF Prinial investigator: ndreas
Delivery date: Feb 5, 05 MODEN uthors: ndreas Daiss BSF Peter Deglmann Deliverable.5 Comrehensive inetis model WP s leader: UNTS Sabrina Pril, UNTS () BSF Voler Settels BSF Prinial investigator: ndreas
= 0, no work has been added
 hater Practice Problems (P1) Enery Balance: Eqn 7 d u z u z u z m U m m Q + + + + + + & + + & lets lets E + S (P) closed system, no mass flow m & m& 0, valve stuck revent any steam from o m& 0, no chane
hater Practice Problems (P1) Enery Balance: Eqn 7 d u z u z u z m U m m Q + + + + + + & + + & lets lets E + S (P) closed system, no mass flow m & m& 0, valve stuck revent any steam from o m& 0, no chane
Speed Distribution at CONSTANT Temperature is given by the Maxwell Boltzmann Speed Distribution
 Temperature ~ Average KE of each particle Particles have different speeds Gas Particles are in constant RANDOM motion Average KE of each particle is: 3/2 kt Pressure is due to momentum transfer Speed Distribution
Temperature ~ Average KE of each particle Particles have different speeds Gas Particles are in constant RANDOM motion Average KE of each particle is: 3/2 kt Pressure is due to momentum transfer Speed Distribution
DEB-oriented Modelling and Control of Coal-Fired Power Plant
 Prerints of the 9th World Congress he International Federation of Automati Control Cae own, South Afria. August 4-9, 4 DEB-oriented Modelling and Control of Coal-Fired Power Plant Li Sun*. Junyi Dong*.
Prerints of the 9th World Congress he International Federation of Automati Control Cae own, South Afria. August 4-9, 4 DEB-oriented Modelling and Control of Coal-Fired Power Plant Li Sun*. Junyi Dong*.
Chapter 9 Practical cycles
 Prof.. undararajan Chater 9 Practical cycles 9. Introduction In Chaters 7 and 8, it was shown that a reversible engine based on the Carnot cycle (two reversible isothermal heat transfers and two reversible
Prof.. undararajan Chater 9 Practical cycles 9. Introduction In Chaters 7 and 8, it was shown that a reversible engine based on the Carnot cycle (two reversible isothermal heat transfers and two reversible
FUGACITY. It is simply a measure of molar Gibbs energy of a real gas.
 FUGACITY It is simly a measure of molar Gibbs energy of a real gas. Modifying the simle equation for the chemical otential of an ideal gas by introducing the concet of a fugacity (f). The fugacity is an
FUGACITY It is simly a measure of molar Gibbs energy of a real gas. Modifying the simle equation for the chemical otential of an ideal gas by introducing the concet of a fugacity (f). The fugacity is an
PERFORMANCE OF MILLER CYCLE WITH HEAT TRANSFER, FRICTION AND VARIABLE SPECIFIC HEATS OF WORKING FLUID
 PERFORMANCE OF MILLER CYCLE WITH HEAT TRANSFER, FRICTION AND VARIABLE SPECIFIC HEATS OF WORKING FLUID Lingen CHEN, Yanlin GE, Fengrui SUN POSTGRADUATE SCHOOL, NAVAL UNIVERSITY OF ENGINEERING, Wuhan, P.
PERFORMANCE OF MILLER CYCLE WITH HEAT TRANSFER, FRICTION AND VARIABLE SPECIFIC HEATS OF WORKING FLUID Lingen CHEN, Yanlin GE, Fengrui SUN POSTGRADUATE SCHOOL, NAVAL UNIVERSITY OF ENGINEERING, Wuhan, P.
gradp -the fluid is inviscid -the fluid is barotropic -the mass forces form a potential field
 J. Szantyr Leture No. 5 Bernoulli equation Bernoulli equation exresses, under ertain assumtions, the riniles of momentum onservation and energy onservation of the fluid. Assumtions: -the flow is stationary
J. Szantyr Leture No. 5 Bernoulli equation Bernoulli equation exresses, under ertain assumtions, the riniles of momentum onservation and energy onservation of the fluid. Assumtions: -the flow is stationary
SUBSONIC GAS-PARTICLE TWO-PHASE FLOW IN PIPES
 Proeedings of the 006 WSEAS/IASME International Conferene on Fluid Mehanis, Miami, Florida, USA, January 8-0, 006 (95-00) SUBSONIC GAS-PARTICLE TWO-PHASE FLOW IN PIPES Mofreh H. Hamed Mehanial Power Engineering
Proeedings of the 006 WSEAS/IASME International Conferene on Fluid Mehanis, Miami, Florida, USA, January 8-0, 006 (95-00) SUBSONIC GAS-PARTICLE TWO-PHASE FLOW IN PIPES Mofreh H. Hamed Mehanial Power Engineering
Fast, Approximately Optimal Solutions for Single and Dynamic MRFs
 Fast, Aroximately Otimal Solutions for Single and Dynami MRFs Nikos Komodakis, Georgios Tziritas University of Crete, Comuter Siene Deartment {komod,tziritas}@sd.uo.gr Nikos Paragios MAS, Eole Centrale
Fast, Aroximately Otimal Solutions for Single and Dynami MRFs Nikos Komodakis, Georgios Tziritas University of Crete, Comuter Siene Deartment {komod,tziritas}@sd.uo.gr Nikos Paragios MAS, Eole Centrale
Heat Machines (Chapters 18.6, 19)
 eat Machines (hapters 8.6, 9) eat machines eat engines eat pumps The Second Law of thermodynamics Entropy Ideal heat engines arnot cycle Other cycles: Brayton, Otto, Diesel eat Machines Description The
eat Machines (hapters 8.6, 9) eat machines eat engines eat pumps The Second Law of thermodynamics Entropy Ideal heat engines arnot cycle Other cycles: Brayton, Otto, Diesel eat Machines Description The
, given by. , I y. and I z. , are self adjoint, meaning that the adjoint of the operator is equal to the operator. This follows as A.
 Further relaxation 6. ntrodution As resented so far the theory is aable of rediting the rate of transitions between energy levels i.e. it is onerned with oulations. The theory is thus erfetly aetable for
Further relaxation 6. ntrodution As resented so far the theory is aable of rediting the rate of transitions between energy levels i.e. it is onerned with oulations. The theory is thus erfetly aetable for
REVERSIBLE NON-FLOW PROCESS CONSTANT VOLUME PROCESS (ISOCHORIC PROCESS) In a constant volume process, he working substance is contained in a rigid
 REVERSIBLE NON-FLOW PROCESS CONSTANT VOLUME PROCESS (ISOCHORIC PROCESS) I a ostat olume roess, he workig substae is otaied i a rigid essel, hee the boudaries of the system are immoable, so work aot be
REVERSIBLE NON-FLOW PROCESS CONSTANT VOLUME PROCESS (ISOCHORIC PROCESS) I a ostat olume roess, he workig substae is otaied i a rigid essel, hee the boudaries of the system are immoable, so work aot be
Non-Equilibrium Thermodynamics for Engineers
 Non-Equilibrium Thermodynamics for Engineers How do we find the otimal rocess unit? Signe Kjelstru, Chair of Engineering Thermodynamics Deartment of Process and Energy TU Delft ecture no. 7 Why is the
Non-Equilibrium Thermodynamics for Engineers How do we find the otimal rocess unit? Signe Kjelstru, Chair of Engineering Thermodynamics Deartment of Process and Energy TU Delft ecture no. 7 Why is the
HEAT ENGINES AND REFRIGERATORS
 EA ENGINES AND REFRIGERAORS 9 onceptual uestions 9.. s =. (a) < 0, > 0. ork is done by the system; the area under the curve is positive. s (b) > 0, < 0. ork is done on the system to compress it to a smaller
EA ENGINES AND REFRIGERAORS 9 onceptual uestions 9.. s =. (a) < 0, > 0. ork is done by the system; the area under the curve is positive. s (b) > 0, < 0. ork is done on the system to compress it to a smaller
VIBRATIONAL ENERGY SCAVENGING WITH SI TECHNOLOGY ELECTROMAGNETIC INERTIAL MICROGENERATORS
 Stresa, Italy, 6-8 Aril 6 VIBRATIONAL ENERGY SCAVENGING WITH SI TECHNOLOGY ELECTROMAGNETIC INERTIAL MICROGENERATORS C. Serre 1, A. Pérez-Rodríguez 1, N. Fondevilla 1, J.R. Morante 1, J. Montserrat, and
Stresa, Italy, 6-8 Aril 6 VIBRATIONAL ENERGY SCAVENGING WITH SI TECHNOLOGY ELECTROMAGNETIC INERTIAL MICROGENERATORS C. Serre 1, A. Pérez-Rodríguez 1, N. Fondevilla 1, J.R. Morante 1, J. Montserrat, and
Equilibrium Thermodynamics
 Part I Equilibrium hermodynamics 1 Molecular hermodynamics Perhas the most basic equation in atmosheric thermodynamics is the ideal gas law = rr where is ressure, r is the air density, is temerature, and
Part I Equilibrium hermodynamics 1 Molecular hermodynamics Perhas the most basic equation in atmosheric thermodynamics is the ideal gas law = rr where is ressure, r is the air density, is temerature, and
SYNTHESIS AND OPTIMIZATION OF EPICYCLIC-TYPE AUTOMATIC TRANSMISSIONS BASED ON NOMOGRAPHS
 Al-Qadisiya Journal For Engineering Sienes Vol. 4 o. 3 Year 0 SYTHESIS AD OPTIMIZATIO OF EPICYCLIC-TYPE AUTOMATIC TASMISSIOS BASED O OMOGAPHS E. L. Esmail K. H. Salih Leturer Assistant Leturer Deartment
Al-Qadisiya Journal For Engineering Sienes Vol. 4 o. 3 Year 0 SYTHESIS AD OPTIMIZATIO OF EPICYCLIC-TYPE AUTOMATIC TASMISSIOS BASED O OMOGAPHS E. L. Esmail K. H. Salih Leturer Assistant Leturer Deartment
Lecture 27: Entropy and Information Prof. WAN, Xin
 General Pysis I Leture 27: Entropy and Information Prof. WAN, Xin xinwan@zju.edu.n ttp://zimp.zju.edu.n/~xinwan/ 1st & 2nd Laws of ermodynamis e 1st law speifies tat we annot get more energy out of a yli
General Pysis I Leture 27: Entropy and Information Prof. WAN, Xin xinwan@zju.edu.n ttp://zimp.zju.edu.n/~xinwan/ 1st & 2nd Laws of ermodynamis e 1st law speifies tat we annot get more energy out of a yli
3. THE SOLUTION OF TRANSFORMATION PARAMETERS
 Deartment of Geosatial Siene. HE SOLUION OF RANSFORMAION PARAMEERS Coordinate transformations, as used in ratie, are models desribing the assumed mathematial relationshis between oints in two retangular
Deartment of Geosatial Siene. HE SOLUION OF RANSFORMAION PARAMEERS Coordinate transformations, as used in ratie, are models desribing the assumed mathematial relationshis between oints in two retangular
Flow Characteristics of High-Pressure Hydrogen Gas in the Critical Nozzle
 Flow Charateristis of High-Pressure Hydrogen Gas in the Critial Nozzle Shigeru MATSUO *1, Soihiro KOYAMA *2, Junji NAGAO *3 and Toshiaki SETOGUCHI *4 *1 Saga University, Det. of Mehanial Engineering, 1
Flow Charateristis of High-Pressure Hydrogen Gas in the Critial Nozzle Shigeru MATSUO *1, Soihiro KOYAMA *2, Junji NAGAO *3 and Toshiaki SETOGUCHI *4 *1 Saga University, Det. of Mehanial Engineering, 1
PHYS 1101 Practice problem set 6, Chapter 19: 7, 12, 19, 30, 37, 44, 53, 61, 69
 PYS 0 Practice problem set 6, hapter 9: 7,, 9, 0, 7, 44,, 6, 69 9.7. Solve: (a) he heat extracted from the cold reservoir is calculated as follows: (b) he heat exhausted to the hot reservoir is K 4.0 00
PYS 0 Practice problem set 6, hapter 9: 7,, 9, 0, 7, 44,, 6, 69 9.7. Solve: (a) he heat extracted from the cold reservoir is calculated as follows: (b) he heat exhausted to the hot reservoir is K 4.0 00
INCOME AND SUBSTITUTION EFFECTS
 3076-C5.df 3/12/04 10:56 AM Page 121 121 Chater 5 INCOME AND SUBSTITUTION EFFECTS In this hater we will use the utility-maimization model to study how the quantity of a good that an individual hooses is
3076-C5.df 3/12/04 10:56 AM Page 121 121 Chater 5 INCOME AND SUBSTITUTION EFFECTS In this hater we will use the utility-maimization model to study how the quantity of a good that an individual hooses is
1 atm = 1.01x10 Pa = 760 Torr = 14.7 lb / in
 Last class we began discussion of ressure in fluids, with ressure defined as, F = ; units N 1 Pa = 1 m 2 There are a number of other ressure units in common use having the following equivalence, 5 2 1
Last class we began discussion of ressure in fluids, with ressure defined as, F = ; units N 1 Pa = 1 m 2 There are a number of other ressure units in common use having the following equivalence, 5 2 1
/ p) TA,. Returning to the
 Toic2610 Proerties; Equilibrium and Frozen A given closed system having Gibbs energy G at temerature T, ressure, molecular comosition (organisation ) and affinity for sontaneous change A is described by
Toic2610 Proerties; Equilibrium and Frozen A given closed system having Gibbs energy G at temerature T, ressure, molecular comosition (organisation ) and affinity for sontaneous change A is described by
THERMODYNAMICS. Contents
 THERMODYAMICS EREST YEUG ERESTYALUMI@GMAIL.COM Contents Part 1. otes and Solutions for Thermal Physics by Ralh Baierlein 1 1. Background 1 1.1. Heating and Temerature 1 1.2. Some dilute gas relationshis
THERMODYAMICS EREST YEUG ERESTYALUMI@GMAIL.COM Contents Part 1. otes and Solutions for Thermal Physics by Ralh Baierlein 1 1. Background 1 1.1. Heating and Temerature 1 1.2. Some dilute gas relationshis
