University of Washington Department of Chemistry Chemistry 452/456 Summer Quarter 2011
|
|
|
- Augustine Reed
- 5 years ago
- Views:
Transcription
1 Homework Assignment #4: Due at 500 pm Monday 8 July,. University of Washington Department of Chemistry Chemistry 45/456 Summer Quarter 0 ) o a very good approximation, ammonia obeys the Bertholet equation of state, whih reads: 9 nr V = nr + 8 where and are onstants and are alled the ritial pressure and temperature, respetively. For ammonia = and =.5atm. a) Suppose we have 500 grams of ammonia under a pressure of =3.04 atm and at =33. Calulate the volume of ammonia aording to the Bertholet equation of state and ompare to the result predited by the ideal gas law. nr 9 nr g Latm mol V = + 6 = 8 7gmol 3.04atm 9 500g ( 0.08Latm mol )( ) gmol.5atm 33 = 56.6L+ 0.67L 8.45 = 5.4L he ideal gas result is the first term V = 56.6L b) Assuming ammonia obeys the Bertholet equation of state obtain V expressions for the oeffiient of thermal expansion β = and the V V isothermal ompressibility κ =. Evaluate β and κ for 500 V grams of ammonia at =3.04 atm and at =33.
2 V nr 9 nr nr = + 6 = 8 ( 9.4mol)( 0.08Latm mol )( 33 ) ( 3.04atm) = = 84.4Latm V κ = = ( 84.4Latm ) = 0.336atm V 5.4L 3 V nr 9 nr nr 54 nr = + 6 = nr 8 nr ( 9.4mol)( 0.08Latm mol ) = + = atm 3 7 ( 9.4mol)( 0.08Latm mol ) atm L L.98 = 0.794L L = 0.830L = V β = = ( 0.830L ) = V 5.4L U H ) Using your results from part b, alulate and for 500 grams V of ammonia at atm and =33. U β = = ( 33) 3.04atm 0.3atm = V κ 0.336atm H = ( β + ) V = ( ( 33)( ) + )( 5.4L) = 6.6L ) Wind turbines are now reognized as useful devies for power generation. Wind turbines of various types are wide-spread in the U.S. and Europe and are bound to beome more ommon in the future. a) Calulate the power of wind moving through a irular area 6 meters in 3 diameter if the density of air at sea level is ρ =.3kgm and the wind veloity 0 ms -. 6m mv Svv Sv kgm ms 3 3 = = ρ = ρ = ( 0.5)(.3 )( 3.4) ( 0 ) ( 0.5)(.3kgm )( 3.4)( 3969m )( 8000m s ) 6.3 Js wind = = 3
3 b) Consider an ideal wind turbine with a rotor diameter of 6m. What is the power generated by this turbine if the wind moves at 0 ms - into the turbine, exerts no drags on the rotor blades, et., and exits the turbine at ms -. What is the effiieny of this turbine? turbine = m ( v v) = ρsv( v v) = ρs ( v+ v)( v v) 3 6m = ( 0.5)(.3kgm )( 3.4) ( ms + 0ms )( 400m s 00m s ) 3 7 = 0.5.3kgm m 30ms 300m s = 3.45 Js 7 turbine 3.45 Js ε = = = Js wind ) he U.S. onsumes 0,680,000 barrels of rude oil per day. here are 59 L in a barrel of oil. Crude oil has an average density of 900 kg m -3 and an average heat of ombustion H omb =4.47x 4 kj/kg. Suppose we burn on average 90% of the oil that we onsume per day. How many wind turbines operating at the effiieny in part b would we require to replae the energy we obtain by burning oil? m 0, 680, 000brlday 59Lbrl 0.00m L 900kgm =.96 kgday oil oil, total oil, burn 9 9 = ( 0.90)(.53 ) =.38 = 4.47 kjkg.96 kgday day / 86400s =.53 kjs kjs kjs 4 wind = 3.45 kjs 9 oil, burn.38 kjs 4 = = 4 turbines kjs wind Note: he United States is the world s largest produer of wind power with a total national wind power apaity of 35,000 MW=3.5x 7 kjs -. his is about.5% of our present energy output from oil. 3) Suppose a protein denatures from a strutured form N to an unstrutured form D at a melting temperature m =343 and at a pressure of m =atm. Suppose under these onditions the heat of denaturation is q m =638 kjmol -. Furthermore, when a protein 3 denatures it dereases in volume i.e. V = VD VN = 70m mol. a) Calulate H, U, S, w and G for the denaturation of this protein at m =343k and m =atm.
4 H = qm = 638kJmol H 638kJmol S = = =.86kJ mol 343 w = V = atm Lmol.kJL atm = 0.007kJmol U = qm V 638kJmol G = 0 b) When the protein denatures its heat apaity inreases. Assume the heat apaity D N hange is given by C C C 8.37kJ = = mol. Calulate H, S, and G for the protein at =3 and = atm. Assume C is onstant between =343 and =3. Is the N form of the protein more stable or less stable than the D form under these onditions. Explain. H 3 = H C = 638kJmol kJ mol = 36.8kJmol 3 S ( 3 ) = S ( 343 ) + C ln =.86kJ mol + ( 8.37kJ mol ) ln =.0kJ mol m 343 G = H S = 36.8kJmol 3.0kJ mol = 48.7kJmol G>0 so N form is more stable. ) Suppose the pressure on the protein solution is inreased to =00atm at m =343. Calulate Dg for the onversion of N to D at =00atm and m=343. Whih form of the protein is more stable under these onditions? Explain. Assume V is a onstant in the pressure range under onsideration. Set up the yle: N N m = atm m = 343 = 00atm = 343 D D hen: G = 343, = atm = 0 = G + G = 343, = 00atm + G ( m m ) N ( m ) D G ( 343, 00atm) G G V ( 00atm atm) V ( atm 00atm) m = = = D N = N + D = V V atm 00atm = 0.07L 999atm = 69.9Latm JL atm = 7.06kJmol ( D N) G<0 so the D form is favored beause it has a smaller volume. 4) Many biologial maromoleules undergo a transition alled denaturation. Denaturation is a proess whereby a strutured, biologial ative moleule, alled the native form, unfolds or beomes unstrutured and biologially inative. he equilibrium is
5 unfolds native denatured folds For the enzyme hymotrypsin at ph= the enthalpy hange assoiated with denaturation 0 is H = 48kJ mol 0 and the entropy hange is S =.3kJ mol a) Calulate the Gibbs energy hange for the denaturation of hymotrypsin at ph= and =303. Assume the enthalpy and entropy are temperature independent between 98 and 303. G den = H den S den = 48 3 Jmol 303 = 48kJmol 400kJmol = 8.0kJmol ( 30J mol ) b) Calulate the equilibrium onstant for the denaturation of hymotrypsin at ph and = = exp G den R = exp 8000Jmol = 7.86 ( 8.3J mol 4 )303 ) Based on your answer for parts a and b, is hymotrypsin struturally stable at ph and =303. fd = so at 303 fd fn. he protein is struturally stable. f N d) he melting temperature is defined as the temperature at whih the equilibrium onstant for denaturation =. Assuming that the enthalpy of denaturation is temperature independent, alulate the melting temperature of hymotrypsin at ph. G = H S so 0 = Hden m Sden. herefore den den den m = H den S den = 48 3 Jmol 30J mol = 37 5) he tehnology to build small mirosopi-sized motors (e.g. moleular motors) exists. In priniple, if a mirosopi motor an be built, whose ation is based on flutuations in the environment, the limit imposed on the effiieny of an engine by the Seond Law an be irumvented! Or an it? Consider the following mirosopi engine, held at onstant temperature:
6 Assume the motor is so small that a gas moleule (random motion a flutuation) hits the paddle wheel ausing it to turn lokwise or ounterlokwise. When the paddle turns the axle turns lifting a one ell organism whih is tethered to the axle in some way. Now beause the motion of gas moleules is random, as many moleules hit the paddle ausing it to turn lokwise, as hit it in the opposite diretion ausing it to turn ounterlokwise, so the ell is randomly raised up and down with no net work aomplished. But it is proposed that a rathet, held down with a spring against a gear, an prevent ounterlokwise motions of the paddle. hen the ell will be raised by lokwise motions of the paddle. Net work is done (mass of the ell times the hange in gravitational potential) and beause the fore ausing the work originates in flutuations, the effiieny of this engine is not governed by Seond Law limits. a) Will the motor work? Hint: onsider and disuss the proposed ation of the rathet in terms of random motions imposed by flutuations. he engine does not work. he reason it does not work is that the temperature is assumed uniform over this tiny mahine. herefore the same number of moleules that bombard a unit area of the paddle wheel per unit time also bombard the rathet per unit time. Insofar as the rathet and the wheel are both tiny, they are both perturbed by moleular ollisions. he rathet annot work as advertised unless there is some way to abate the moleular ollisions that fall upon it. b) If the motor will not work as shown, an you think of a simple hange that might make it work? One way to abate moleular ollisions against the rathet would be to plae it in an enlosure muh older than the area around the wheel. But then we would have reated a temperature gradient, and this mahine would then just operate as any other mahine. We have to provide fuel to pump heat out of the enlosure around the rathet, so the mahine requires fuel and will have a finite effiieny.
7 6) A sample onsisting of.50 moles of an ideal gas at 98 is expanded from an initial volume of.0l to a final volume of 50.0L. Calulate G and A for: a) an isothermal reversible path; b) an isothermal expansion against a onstant external pressure of bar. Explain why G and A do not differ. 7) In the last two problem sets we have alulated H and S at =00 for the 3 reation of nitrogen rwith hydrogen to form ammonia: N( g) + H( g) NH3( g). Using the standard heats of formation at =98 and the heat apaities at onstant pressure as a funtion of temperature, alulate G for this reation at =500 and alulate the equilibrium onstant at =500. Based on your alulation, does the formation of ammonia beome more or less favorable as temperature is inreased? Explain. 00 f f 98 ( 00 ) ( 98 ) H = H + C d 3 from HW : C ( ) = C d d = 3.( ) + ( ) ( ) J mol =
8 ( 3.( 0) 5.44 (.6 ).965 ( 9.85 )) = + = Jmol = 40Jmol f f 98 Jmol H 00 = H 98 + C d = 46.kJmol 4.0kJmol = 50.kJmol ( 500 ) ( 98 ) From last hom ework : 500 S S d = S 500 S d 99J 3.ln 9 98 C S 98 = S NH S N S H = 9.8J 95.8J 96J = 99.0J = + + = = 99J J =09.4J J 3 (,500 ) (,500 ) (,500 ) G NH = H NH S NH rxn 3 rxn 3 rxn 3 = 50.kJ J = 4.58kJ (,98 ) (,98 ) (,980 ) G NH = H NH S NH rxn 3 rxn 3 rxn 3 = 46.kJ 98 99J =6.6kJ herefore the reation is thermodynamially less favored at higher temperature. ( J mol ) S H f f,9 C J mol (kj mol - ) NH3 ( g ) N ( g ) H ( g ) ) he van t Hoff equation relates the temperature dependene of the equilibrium onstant to the enthalpy for a reation : 0 Hrxn dln = d R 0 0
9 For the reation: CuO( s) Cu ( s) + O ( g ), the following data are available for =98: CuO(s) Cu(s) O (g) H ( f kjmol ) -57 G kjmol -30 C f ( J mol ) a. Integrate the van t Hoff equation given above assuming H rxn is independent of temperature. Calulate for the reation at = Hrxn ln = R d d Hrxn d H rxn ln ( 0) ln ( 98) = R = R 98 3 G = G O + G Cu G CuO = 30 Jmol f f f 3 ( 30 Jmol ) G ln ( 98) = = =05 R ( 8.3J mol )( 98 ) 0 H rxn ln ( 0) = ln ( 98) R 3 ( 57 Jmol ) =05 8.3J mol 0 98 = = 7.89 b. Assume H rxn is temperature-dependent and given by: Hrxn = Hrxn ( 0) + C ( 0) Integrate the van t Hoff equation assuming this temperature dependene for H and alulate. How is the equilibrium affeted assuming rxn H rxn is temperature dependent?
10 H ln ( 0) = ln ( 98) R 0 98 C 0 C ( 98 ) + ln + R 98 R 0 98 ( 6.4)( 98) 6.4 = 7.89 (.39) = = he equilibrium onstant is diminished by the temperature dependene of the enthalpy.
General Equilibrium. What happens to cause a reaction to come to equilibrium?
 General Equilibrium Chemial Equilibrium Most hemial reations that are enountered are reversible. In other words, they go fairly easily in either the forward or reverse diretions. The thing to remember
General Equilibrium Chemial Equilibrium Most hemial reations that are enountered are reversible. In other words, they go fairly easily in either the forward or reverse diretions. The thing to remember
( )( )( )( )( ) University of Washington Department of Chemistry Chemistry 452/456 Summer Quarter 2010
 Hoework Assignent 4: Due at 5 p.. 7/9/ Text Probles: 6.4, 6.9, 6, 6.3 University o Washington Departent o Cheistry Cheistry 45/456 Suer Quarter P6.9) Jaes Watt once observed that a hard-working horse can
Hoework Assignent 4: Due at 5 p.. 7/9/ Text Probles: 6.4, 6.9, 6, 6.3 University o Washington Departent o Cheistry Cheistry 45/456 Suer Quarter P6.9) Jaes Watt once observed that a hard-working horse can
University of Washington Department of Chemistry Chemistry 452/456 Summer Quarter 2011
 Homework Assignment #: Due at 500 pm Wednesday July 6. University of Washington Department of Chemistry Chemistry 45/456 Summer Quarter 0 ) he respiratory system uses oxygen to degrade glucose to carbon
Homework Assignment #: Due at 500 pm Wednesday July 6. University of Washington Department of Chemistry Chemistry 45/456 Summer Quarter 0 ) he respiratory system uses oxygen to degrade glucose to carbon
Chapter 8 Thermodynamic Relations
 Chapter 8 Thermodynami Relations 8.1 Types of Thermodynami roperties The thermodynami state of a system an be haraterized by its properties that an be lassified as measured, fundamental, or deried properties.
Chapter 8 Thermodynami Relations 8.1 Types of Thermodynami roperties The thermodynami state of a system an be haraterized by its properties that an be lassified as measured, fundamental, or deried properties.
Review of classical thermodynamics
 Review of lassial thermodynamis Fundamental Laws, roperties and roesses () First Law - Energy Balane hermodynami funtions of state Internal energy, heat and work ypes of paths (isobari, isohori, isothermal,
Review of lassial thermodynamis Fundamental Laws, roperties and roesses () First Law - Energy Balane hermodynami funtions of state Internal energy, heat and work ypes of paths (isobari, isohori, isothermal,
JF Physical Chemistry JF CH 1101: Introduction to Physical Chemistry.
 JF Physial Chemistry 010-011. JF CH 1101: Introdution to Physial Chemistry. Dr Mike Lyons. Shool of Chemistry Trinity College Dublin. melyons@td.ie A ompendium of past examination questions set on Physial
JF Physial Chemistry 010-011. JF CH 1101: Introdution to Physial Chemistry. Dr Mike Lyons. Shool of Chemistry Trinity College Dublin. melyons@td.ie A ompendium of past examination questions set on Physial
Answer: Easiest way to determine equilibrium concentrations is to set up a table as follows: 2 SO 2 + O 2 2 SO 3 initial conc change
 Problem #1 6 mol of SO and 4 mol of O are plaed into a 1 L flask at temperature, T. The equilibrium onentration of SO is found to be 4 mol/l. Determine K. SO (g) + O (g) SO (g) K = [SO ] / [SO ] [O ] Answer:
Problem #1 6 mol of SO and 4 mol of O are plaed into a 1 L flask at temperature, T. The equilibrium onentration of SO is found to be 4 mol/l. Determine K. SO (g) + O (g) SO (g) K = [SO ] / [SO ] [O ] Answer:
Mean Activity Coefficients of Peroxodisulfates in Saturated Solutions of the Conversion System 2NH 4. H 2 O at 20 C and 30 C
 Mean Ativity Coeffiients of Peroxodisulfates in Saturated Solutions of the Conversion System NH 4 Na S O 8 H O at 0 C and 0 C Jan Balej Heřmanova 5, 170 00 Prague 7, Czeh Republi balejan@seznam.z Abstrat:
Mean Ativity Coeffiients of Peroxodisulfates in Saturated Solutions of the Conversion System NH 4 Na S O 8 H O at 0 C and 0 C Jan Balej Heřmanova 5, 170 00 Prague 7, Czeh Republi balejan@seznam.z Abstrat:
Chapter 14. The Concept of Equilibrium and the Equilibrium Constant. We have for the most part depicted reactions as going one way.
 Chapter 14 The Conept of Equilibrium and the Equilibrium Constant In hapter 1 we dealt with Physial Equilibrium Physial Changes HO 2 (l) HO 2 (g) In hapter 14 we will learn about Chemial Equilibrium. We
Chapter 14 The Conept of Equilibrium and the Equilibrium Constant In hapter 1 we dealt with Physial Equilibrium Physial Changes HO 2 (l) HO 2 (g) In hapter 14 we will learn about Chemial Equilibrium. We
Homework Problem Set 8 Solutions
 Chemistry 360 Dr. Jean M. Standard Homework roblem Set 8 Solutions. Starting from G = H S, derive the fundamental equation for G. o begin, we take the differential of G, dg = dh d( S) = dh ds Sd. Next,
Chemistry 360 Dr. Jean M. Standard Homework roblem Set 8 Solutions. Starting from G = H S, derive the fundamental equation for G. o begin, we take the differential of G, dg = dh d( S) = dh ds Sd. Next,
Chapter 15 Chemical Equilibrium
 Chapter 5 Chemial Equilibrium 5. The Conept of Equilibrium Figure: 3. from Chemistry by MMurray & Fey Figure 3.(a) NO 4( g) NO( g) olorless brown we start with reatant, N O 4, so the solution is olorless
Chapter 5 Chemial Equilibrium 5. The Conept of Equilibrium Figure: 3. from Chemistry by MMurray & Fey Figure 3.(a) NO 4( g) NO( g) olorless brown we start with reatant, N O 4, so the solution is olorless
Chapter 15: Chemical Equilibrium
 Chapter 5: Chemial Equilibrium ahoot!. At eq, the rate of the forward reation is the rate of the reverse reation. equal to, slower than, faster than, the reverse of. Selet the statement that BEST desribes
Chapter 5: Chemial Equilibrium ahoot!. At eq, the rate of the forward reation is the rate of the reverse reation. equal to, slower than, faster than, the reverse of. Selet the statement that BEST desribes
Chemistry 452 July 23, Enter answers in a Blue Book Examination
 Chemistry 45 July 3, 014 Enter answers in a Blue Book Examination Midterm Useful Constants: 1 Newton=1 N= 1 kg m s 1 Joule=1J=1 N m=1 kg m /s 1 Pascal=1Pa=1N m 1atm=10135 Pa 1 bar=10 5 Pa 1L=0.001m 3 Universal
Chemistry 45 July 3, 014 Enter answers in a Blue Book Examination Midterm Useful Constants: 1 Newton=1 N= 1 kg m s 1 Joule=1J=1 N m=1 kg m /s 1 Pascal=1Pa=1N m 1atm=10135 Pa 1 bar=10 5 Pa 1L=0.001m 3 Universal
Mass Transfer (Stoffaustausch) Fall 2012
 Mass Transfer (Stoffaustaush) Fall Examination 9. Januar Name: Legi-Nr.: Edition Diffusion by E. L. Cussler: none nd rd Test Duration: minutes The following materials are not permitted at your table and
Mass Transfer (Stoffaustaush) Fall Examination 9. Januar Name: Legi-Nr.: Edition Diffusion by E. L. Cussler: none nd rd Test Duration: minutes The following materials are not permitted at your table and
THEORETICAL PROBLEM No. 3 WHY ARE STARS SO LARGE?
 THEORETICAL PROBLEM No. 3 WHY ARE STARS SO LARGE? The stars are spheres of hot gas. Most of them shine beause they are fusing hydrogen into helium in their entral parts. In this problem we use onepts of
THEORETICAL PROBLEM No. 3 WHY ARE STARS SO LARGE? The stars are spheres of hot gas. Most of them shine beause they are fusing hydrogen into helium in their entral parts. In this problem we use onepts of
Introduction to Exergoeconomic and Exergoenvironmental Analyses
 Tehnishe Universität Berlin Introdution to Exergoeonomi and Exergoenvironmental Analyses George Tsatsaronis The Summer Course on Exergy and its Appliation for Better Environment Oshawa, Canada April, 30
Tehnishe Universität Berlin Introdution to Exergoeonomi and Exergoenvironmental Analyses George Tsatsaronis The Summer Course on Exergy and its Appliation for Better Environment Oshawa, Canada April, 30
Subject: Introduction to Component Matching and Off-Design Operation % % ( (1) R T % (
 16.50 Leture 0 Subjet: Introdution to Component Mathing and Off-Design Operation At this point it is well to reflet on whih of the many parameters we have introdued (like M, τ, τ t, ϑ t, f, et.) are free
16.50 Leture 0 Subjet: Introdution to Component Mathing and Off-Design Operation At this point it is well to reflet on whih of the many parameters we have introdued (like M, τ, τ t, ϑ t, f, et.) are free
Solutions to Exercises: Chapter 7
 Solutions to Exerises: Chapter 7 7.1 The heat of vaporization of hexane is 30.8 kj. mol -1. The boiling point of hexane at a pressure of 1.00 atm is 68.9 C. What will the boiling point be at a pressure
Solutions to Exerises: Chapter 7 7.1 The heat of vaporization of hexane is 30.8 kj. mol -1. The boiling point of hexane at a pressure of 1.00 atm is 68.9 C. What will the boiling point be at a pressure
EF 152 Exam #3, Spring 2016 Page 1 of 6
 EF 5 Exam #3, Spring 06 Page of 6 Name: Setion: Instrutions Do not open te exam until instruted to do so. Do not leave if tere is less tan 5 minutes to go in te exam. Wen time is alled, immediately stop
EF 5 Exam #3, Spring 06 Page of 6 Name: Setion: Instrutions Do not open te exam until instruted to do so. Do not leave if tere is less tan 5 minutes to go in te exam. Wen time is alled, immediately stop
Physics 231 Lecture 35
 ysis 1 Leture 5 Main points of last leture: Heat engines and effiieny: eng e 1 Carnot yle and Carnot engine. eng e 1 is in Kelvin. Refrigerators CO eng Ideal refrigerator CO rev reversible Entropy ΔS Computation
ysis 1 Leture 5 Main points of last leture: Heat engines and effiieny: eng e 1 Carnot yle and Carnot engine. eng e 1 is in Kelvin. Refrigerators CO eng Ideal refrigerator CO rev reversible Entropy ΔS Computation
2 How far? Equilibrium Answers
 How far? Equilibrium Answers ratie: pages 37 39 1 Answer is D. Only a hange in temperature harges the value of the equilibrium onstant. Answer is D. [B] /[A] so [B] [A] or [B] [A] 1/ 3 Answer is B. Amounts
How far? Equilibrium Answers ratie: pages 37 39 1 Answer is D. Only a hange in temperature harges the value of the equilibrium onstant. Answer is D. [B] /[A] so [B] [A] or [B] [A] 1/ 3 Answer is B. Amounts
Chapter 15 Equilibrium. Reversible Reactions & Equilibrium. Reversible Reactions & Equilibrium. Reversible Reactions & Equilibrium 2/3/2014
 Amount of reatant/produt //01 quilibrium in Chemial Reations Lets look bak at our hypothetial reation from the kinetis hapter. A + B C Chapter 15 quilibrium [A] Why doesn t the onentration of A ever go
Amount of reatant/produt //01 quilibrium in Chemial Reations Lets look bak at our hypothetial reation from the kinetis hapter. A + B C Chapter 15 quilibrium [A] Why doesn t the onentration of A ever go
Sample Teaching Sequence (Hong Kong Secondary 4 6 Chemistry)
 Revised (1 Sept 009 Sample Teahing Suene (Hong Kong Seondary 4 6 Chemistry Topi: Chemial Equilibrium Teahing Suene Content 1.1 Reversible reations Examples of reversible reation; forward reation; reverse
Revised (1 Sept 009 Sample Teahing Suene (Hong Kong Seondary 4 6 Chemistry Topi: Chemial Equilibrium Teahing Suene Content 1.1 Reversible reations Examples of reversible reation; forward reation; reverse
Physical Laws, Absolutes, Relative Absolutes and Relativistic Time Phenomena
 Page 1 of 10 Physial Laws, Absolutes, Relative Absolutes and Relativisti Time Phenomena Antonio Ruggeri modexp@iafria.om Sine in the field of knowledge we deal with absolutes, there are absolute laws that
Page 1 of 10 Physial Laws, Absolutes, Relative Absolutes and Relativisti Time Phenomena Antonio Ruggeri modexp@iafria.om Sine in the field of knowledge we deal with absolutes, there are absolute laws that
PHYSICS 212 FINAL EXAM 21 March 2003
 PHYSIS INAL EXAM Marh 00 Eam is losed book, losed notes. Use only the provided formula sheet. Write all work and answers in eam booklets. The baks of pages will not be graded unless you so ruest on the
PHYSIS INAL EXAM Marh 00 Eam is losed book, losed notes. Use only the provided formula sheet. Write all work and answers in eam booklets. The baks of pages will not be graded unless you so ruest on the
Chapter 13, Chemical Equilibrium
 Chapter 13, Chemial Equilibrium You may have gotten the impression that when 2 reatants mix, the ensuing rxn goes to ompletion. In other words, reatants are onverted ompletely to produts. We will now learn
Chapter 13, Chemial Equilibrium You may have gotten the impression that when 2 reatants mix, the ensuing rxn goes to ompletion. In other words, reatants are onverted ompletely to produts. We will now learn
Line Radiative Transfer
 http://www.v.nrao.edu/ourse/astr534/ineradxfer.html ine Radiative Transfer Einstein Coeffiients We used armor's equation to estimate the spontaneous emission oeffiients A U for À reombination lines. A
http://www.v.nrao.edu/ourse/astr534/ineradxfer.html ine Radiative Transfer Einstein Coeffiients We used armor's equation to estimate the spontaneous emission oeffiients A U for À reombination lines. A
Chapter 15 Equilibrium. Reversible Reactions & Equilibrium. Reversible Reactions & Equilibrium. Reversible Reactions & Equilibrium 5/27/2014
 Amount of reatant/produt 5/7/01 quilibrium in Chemial Reations Lets look bak at our hypothetial reation from the kinetis hapter. A + B C Chapter 15 quilibrium [A] Why doesn t the onentration of A ever
Amount of reatant/produt 5/7/01 quilibrium in Chemial Reations Lets look bak at our hypothetial reation from the kinetis hapter. A + B C Chapter 15 quilibrium [A] Why doesn t the onentration of A ever
Problem Set. Assigned October 18, 2013 Due Friday, October 25, Please show all work for credit
 Problem Set Assigned October 18, 2013 Due Friday, October 25, 2013 Please show all work for credit To hand in: Thermodynamic Relations 1. The shells of marine organisms contain CaCO 3 largely in the crystalline
Problem Set Assigned October 18, 2013 Due Friday, October 25, 2013 Please show all work for credit To hand in: Thermodynamic Relations 1. The shells of marine organisms contain CaCO 3 largely in the crystalline
The gravitational phenomena without the curved spacetime
 The gravitational phenomena without the urved spaetime Mirosław J. Kubiak Abstrat: In this paper was presented a desription of the gravitational phenomena in the new medium, different than the urved spaetime,
The gravitational phenomena without the urved spaetime Mirosław J. Kubiak Abstrat: In this paper was presented a desription of the gravitational phenomena in the new medium, different than the urved spaetime,
INFLUENCE OF OPERATING AND CONSTRUCTION PARAMETERS ON THE BEHAVIOR OF HYDRAULIC CYLINDER SUBJECTED TO JERKY MOTION
 Proeedings of ICFDP 8: 8 th International Congress of Fluid Dynamis & Propulsion Deember 14-17, 006, Sharm El-Shiekh, Sinai, Egypt ICFDP8-EG-154 INFLUENCE OF OPERATING AND CONSTRUCTION PARAMETERS ON THE
Proeedings of ICFDP 8: 8 th International Congress of Fluid Dynamis & Propulsion Deember 14-17, 006, Sharm El-Shiekh, Sinai, Egypt ICFDP8-EG-154 INFLUENCE OF OPERATING AND CONSTRUCTION PARAMETERS ON THE
KINETIC THEORY OF GASES
 Kineti Theory of Gases 10 KINETIC THEORY OF GASES As you have studied in the previous lessons, at standard temperature and pressure, matter exists in three states solid, liquid and gas. These are omposed
Kineti Theory of Gases 10 KINETIC THEORY OF GASES As you have studied in the previous lessons, at standard temperature and pressure, matter exists in three states solid, liquid and gas. These are omposed
In this problem, we are given the following quantities: We want to find: Equations and basic calculations:
 .1 It takes. million tons of oal per year to a 1000-W power plant that operates at a apaity fator of 70%. If the heating value of the oal is 1,000 /lb, alulate the plant s effiieny and the heat rate. In
.1 It takes. million tons of oal per year to a 1000-W power plant that operates at a apaity fator of 70%. If the heating value of the oal is 1,000 /lb, alulate the plant s effiieny and the heat rate. In
Equations: q trans = 2 mkt h 2. , Q = q N, Q = qn N! , < P > = kt P = , C v = < E > V 2. e 1 e h /kt vib = h k = h k, rot = h2.
 Constants: R = 8.314 J mol -1 K -1 = 0.08206 L atm mol -1 K -1 k B = 0.697 cm -1 /K = 1.38 x 10-23 J/K 1 a.m.u. = 1.672 x 10-27 kg 1 atm = 1.0133 x 10 5 Nm -2 = 760 Torr h = 6.626 x 10-34 Js For H 2 O
Constants: R = 8.314 J mol -1 K -1 = 0.08206 L atm mol -1 K -1 k B = 0.697 cm -1 /K = 1.38 x 10-23 J/K 1 a.m.u. = 1.672 x 10-27 kg 1 atm = 1.0133 x 10 5 Nm -2 = 760 Torr h = 6.626 x 10-34 Js For H 2 O
Experiment 03: Work and Energy
 MASSACHUSETTS INSTITUTE OF TECHNOLOGY Physis Department Physis 8.01 Purpose of the Experiment: Experiment 03: Work and Energy In this experiment you allow a art to roll down an inlined ramp and run into
MASSACHUSETTS INSTITUTE OF TECHNOLOGY Physis Department Physis 8.01 Purpose of the Experiment: Experiment 03: Work and Energy In this experiment you allow a art to roll down an inlined ramp and run into
University of Washington Department of Chemistry Chemistry 452/456 Summer Quarter 2008
 University of Washington Department of Chemistry Chemistry 45/456 Summer Quarter 008 Midterm Examination Key July 5 008 Blue books are required. Answer only the number of questions requested. Chose wisely!
University of Washington Department of Chemistry Chemistry 45/456 Summer Quarter 008 Midterm Examination Key July 5 008 Blue books are required. Answer only the number of questions requested. Chose wisely!
POROUS CARBON PARTICLE COMBUSTION IN AIR
 MCS 7 Chia Laguna, Cagliari, Sardinia, taly, 11-15, 11 POOUS CABON PATCLE COMBUSTON N A V. M. Gremyahkin grema@ipmnet.ru nstitute for Problems in Mehanis, AS, Mosow, ussia Abstrat Theoretial investigation
MCS 7 Chia Laguna, Cagliari, Sardinia, taly, 11-15, 11 POOUS CABON PATCLE COMBUSTON N A V. M. Gremyahkin grema@ipmnet.ru nstitute for Problems in Mehanis, AS, Mosow, ussia Abstrat Theoretial investigation
= (-22) = +2kJ /mol
 Lecture 8: Thermodynamics & Protein Stability Assigned reading in Campbell: Chapter 4.4-4.6 Key Terms: DG = -RT lnk eq = DH - TDS Transition Curve, Melting Curve, Tm DH calculation DS calculation van der
Lecture 8: Thermodynamics & Protein Stability Assigned reading in Campbell: Chapter 4.4-4.6 Key Terms: DG = -RT lnk eq = DH - TDS Transition Curve, Melting Curve, Tm DH calculation DS calculation van der
CHAPTER 16. Basic Concepts. Basic Concepts. The Equilibrium Constant. Reaction Quotient & Equilibrium Constant. Chemical Equilibrium
 Proerties of an Equilibrium System CHAPTER 6 Chemial Equilibrium Equilibrium systems are DYNAMIC (in onstant motion) REVERSIBLE an be aroahed from either diretion Pink to blue Co(H O) 6 Cl ---> > Co(H
Proerties of an Equilibrium System CHAPTER 6 Chemial Equilibrium Equilibrium systems are DYNAMIC (in onstant motion) REVERSIBLE an be aroahed from either diretion Pink to blue Co(H O) 6 Cl ---> > Co(H
EF 152 Exam #3, Fall, 2012 Page 1 of 6
 EF 5 Exam #3, Fall, 0 Page of 6 Name: Setion: Guidelines: ssume 3 signifiant figures for all given numbers. Sow all of your work no work, no redit Write your final answer in te box provided - inlude units
EF 5 Exam #3, Fall, 0 Page of 6 Name: Setion: Guidelines: ssume 3 signifiant figures for all given numbers. Sow all of your work no work, no redit Write your final answer in te box provided - inlude units
MC Practice F2 Solubility Equilibrium, Ksp Name
 MC Pratie F Solubility Equilibrium, Ksp Name This is pratie - Do NOT heat yourself of finding out what you are apable of doing. Be sure you follow the testing onditions outlined below. DO NOT USE A CALCULATOR.
MC Pratie F Solubility Equilibrium, Ksp Name This is pratie - Do NOT heat yourself of finding out what you are apable of doing. Be sure you follow the testing onditions outlined below. DO NOT USE A CALCULATOR.
REVIEW QUESTIONS Chapter 15
 hemistry 10 ANSWER EY REVIEW QUESTIONS hapter 15 1. A mixture of 0.10 mol of NO, 0.050 mol of H and 0.10 mol of HO is plaed in a 1.0-L flask and allowed to reah equilibrium as shown below: NO (g) + H (g)
hemistry 10 ANSWER EY REVIEW QUESTIONS hapter 15 1. A mixture of 0.10 mol of NO, 0.050 mol of H and 0.10 mol of HO is plaed in a 1.0-L flask and allowed to reah equilibrium as shown below: NO (g) + H (g)
Improvements in the Modeling of the Self-ignition of Tetrafluoroethylene
 Exerpt from the Proeedings of the OMSOL onferene 010 Paris Improvements in the Modeling of the Self-ignition of Tetrafluoroethylene M. Bekmann-Kluge 1 *,. errero 1, V. Shröder 1, A. Aikalin and J. Steinbah
Exerpt from the Proeedings of the OMSOL onferene 010 Paris Improvements in the Modeling of the Self-ignition of Tetrafluoroethylene M. Bekmann-Kluge 1 *,. errero 1, V. Shröder 1, A. Aikalin and J. Steinbah
Final Exam: know your section, bring your ID!
 Chapter 15: Equilibrium Part 1 Read: BLB 15.1 3 HW: BLB 15:13,14, 21 Supplemental 15:1 4 Know: Chemial Equilibrium Catalysts Equilibrium Constant Equilibrium onstant expression Homogeneous/Heterogeneous
Chapter 15: Equilibrium Part 1 Read: BLB 15.1 3 HW: BLB 15:13,14, 21 Supplemental 15:1 4 Know: Chemial Equilibrium Catalysts Equilibrium Constant Equilibrium onstant expression Homogeneous/Heterogeneous
Physics 41 Chapter 22 HW
 Pysis 41 apter 22 H 1. eat ine performs 200 J of work in ea yle and as an effiieny of 30.0%. For ea yle, ow mu energy is (a) taken in and (b) expelled as eat? = = 200 J (1) e = 1 0.300 = = (2) From (2),
Pysis 41 apter 22 H 1. eat ine performs 200 J of work in ea yle and as an effiieny of 30.0%. For ea yle, ow mu energy is (a) taken in and (b) expelled as eat? = = 200 J (1) e = 1 0.300 = = (2) From (2),
Millennium Relativity Acceleration Composition. The Relativistic Relationship between Acceleration and Uniform Motion
 Millennium Relativity Aeleration Composition he Relativisti Relationship between Aeleration and niform Motion Copyright 003 Joseph A. Rybzyk Abstrat he relativisti priniples developed throughout the six
Millennium Relativity Aeleration Composition he Relativisti Relationship between Aeleration and niform Motion Copyright 003 Joseph A. Rybzyk Abstrat he relativisti priniples developed throughout the six
Relativistic Dynamics
 Chapter 7 Relativisti Dynamis 7.1 General Priniples of Dynamis 7.2 Relativisti Ation As stated in Setion A.2, all of dynamis is derived from the priniple of least ation. Thus it is our hore to find a suitable
Chapter 7 Relativisti Dynamis 7.1 General Priniples of Dynamis 7.2 Relativisti Ation As stated in Setion A.2, all of dynamis is derived from the priniple of least ation. Thus it is our hore to find a suitable
Pure Component Phase Diagram. Definitions. Definitions (cont.) Class 17 Non-Ideal Gases
 Class 17 Non-Ideal Gases Definitions Critial emperature, ressure Vapor Gas Van der Waals EOS Other Equations of State Compressibility Fator riniple of Corresponding States Kay s Rule Water hase Change
Class 17 Non-Ideal Gases Definitions Critial emperature, ressure Vapor Gas Van der Waals EOS Other Equations of State Compressibility Fator riniple of Corresponding States Kay s Rule Water hase Change
There are five problems on the exam. Do all of the problems. Show your work
 CHM 3400 Fundamentals of Physical Chemistry Second Hour Exam March 8, 2017 There are five problems on the exam. Do all of the problems. Show your work R = 0.08206 L atm/mole K N A = 6.022 x 10 23 R = 0.08314
CHM 3400 Fundamentals of Physical Chemistry Second Hour Exam March 8, 2017 There are five problems on the exam. Do all of the problems. Show your work R = 0.08206 L atm/mole K N A = 6.022 x 10 23 R = 0.08314
Homework Set 4. gas B open end
 Homework Set 4 (1). A steady-state Arnold ell is used to determine the diffusivity of toluene (speies A) in air (speies B) at 298 K and 1 atm. If the diffusivity is DAB = 0.0844 m 2 /s = 8.44 x 10-6 m
Homework Set 4 (1). A steady-state Arnold ell is used to determine the diffusivity of toluene (speies A) in air (speies B) at 298 K and 1 atm. If the diffusivity is DAB = 0.0844 m 2 /s = 8.44 x 10-6 m
Wavetech, LLC. Ultrafast Pulses and GVD. John O Hara Created: Dec. 6, 2013
 Ultrafast Pulses and GVD John O Hara Created: De. 6, 3 Introdution This doument overs the basi onepts of group veloity dispersion (GVD) and ultrafast pulse propagation in an optial fiber. Neessarily, it
Ultrafast Pulses and GVD John O Hara Created: De. 6, 3 Introdution This doument overs the basi onepts of group veloity dispersion (GVD) and ultrafast pulse propagation in an optial fiber. Neessarily, it
Ph1c Analytic Quiz 2 Solution
 Ph1 Analyti Quiz 2 olution Chefung Chan, pring 2007 Problem 1 (6 points total) A small loop of width w and height h falls with veloity v, under the influene of gravity, into a uniform magneti field B between
Ph1 Analyti Quiz 2 olution Chefung Chan, pring 2007 Problem 1 (6 points total) A small loop of width w and height h falls with veloity v, under the influene of gravity, into a uniform magneti field B between
Heat exchangers: Heat exchanger types:
 Heat exhangers: he proess of heat exhange between two fluids that are at different temperatures and separated by a solid wall ours in many engineering appliations. he devie used to implement this exhange
Heat exhangers: he proess of heat exhange between two fluids that are at different temperatures and separated by a solid wall ours in many engineering appliations. he devie used to implement this exhange
University of Washington Department of Chemistry Chemistry 452 Summer Quarter 2014
 Lecture 0 7/6/ ERD: 5. DeVoe:.3.,.3.3 University of Washington Department of Chemistry Chemistry 5 Summer Quarter 0 A. Work and the Second Law of Thermodynamics: Efficiency of eat Engines One of the most
Lecture 0 7/6/ ERD: 5. DeVoe:.3.,.3.3 University of Washington Department of Chemistry Chemistry 5 Summer Quarter 0 A. Work and the Second Law of Thermodynamics: Efficiency of eat Engines One of the most
The Hanging Chain. John McCuan. January 19, 2006
 The Hanging Chain John MCuan January 19, 2006 1 Introdution We onsider a hain of length L attahed to two points (a, u a and (b, u b in the plane. It is assumed that the hain hangs in the plane under a
The Hanging Chain John MCuan January 19, 2006 1 Introdution We onsider a hain of length L attahed to two points (a, u a and (b, u b in the plane. It is assumed that the hain hangs in the plane under a
Test bank chapter (14)
 Test bank hater (14) Choose the most orret answer 1. Whih is the orret equilibrium onstant exression for the following reation? Fe 2 O 3 (s) + 3H 2 (g) 2Fe(s) + 3H 2 O(g) a) K = [Fe 2 O 3 ] [H 2 ] 3 /[Fe]
Test bank hater (14) Choose the most orret answer 1. Whih is the orret equilibrium onstant exression for the following reation? Fe 2 O 3 (s) + 3H 2 (g) 2Fe(s) + 3H 2 O(g) a) K = [Fe 2 O 3 ] [H 2 ] 3 /[Fe]
Acoustic Waves in a Duct
 Aousti Waves in a Dut 1 One-Dimensional Waves The one-dimensional wave approximation is valid when the wavelength λ is muh larger than the diameter of the dut D, λ D. The aousti pressure disturbane p is
Aousti Waves in a Dut 1 One-Dimensional Waves The one-dimensional wave approximation is valid when the wavelength λ is muh larger than the diameter of the dut D, λ D. The aousti pressure disturbane p is
BINARY RANKINE CYCLE OPTIMIZATION Golub, M., Koscak-Kolin, S., Kurevija, T.
 BINARY RANKINE CYCLE OPTIMIZATION Golub, M., Kosak-Kolin, S., Kurevija, T. Faulty of Mining, Geology and Petroleum Engineering Department of Petroleum Engineering Pierottijeva 6, Zagreb 0 000, Croatia
BINARY RANKINE CYCLE OPTIMIZATION Golub, M., Kosak-Kolin, S., Kurevija, T. Faulty of Mining, Geology and Petroleum Engineering Department of Petroleum Engineering Pierottijeva 6, Zagreb 0 000, Croatia
3 Tidal systems modelling: ASMITA model
 3 Tidal systems modelling: ASMITA model 3.1 Introdution For many pratial appliations, simulation and predition of oastal behaviour (morphologial development of shorefae, beahes and dunes) at a ertain level
3 Tidal systems modelling: ASMITA model 3.1 Introdution For many pratial appliations, simulation and predition of oastal behaviour (morphologial development of shorefae, beahes and dunes) at a ertain level
III. SURFACE PROPERTIES III.A. SURFACE TENSION SURFACE PROPERTIES
 III. SURFACE PROPERTIES III.A. SURFACE TENSION GOAL: To investigate the influene of the solution onentration and/or the kind of the solute on the surfae tension INTRODUCTION Liquids tend to adopt shapes
III. SURFACE PROPERTIES III.A. SURFACE TENSION GOAL: To investigate the influene of the solution onentration and/or the kind of the solute on the surfae tension INTRODUCTION Liquids tend to adopt shapes
SOLVED QUESTIONS 1 / 2. in a closed container at equilibrium. What would be the effect of addition of CaCO 3 on the equilibrium concentration of CO 2?
 SOLVED QUESTIONS Multile Choie Questions. and are the veloity onstants of forward and bakward reations. The equilibrium onstant k of the reation is (A) (B) (C) (D). Whih of the following reations will
SOLVED QUESTIONS Multile Choie Questions. and are the veloity onstants of forward and bakward reations. The equilibrium onstant k of the reation is (A) (B) (C) (D). Whih of the following reations will
Analysis of discretization in the direct simulation Monte Carlo
 PHYSICS OF FLUIDS VOLUME 1, UMBER 1 OCTOBER Analysis of disretization in the diret simulation Monte Carlo iolas G. Hadjionstantinou a) Department of Mehanial Engineering, Massahusetts Institute of Tehnology,
PHYSICS OF FLUIDS VOLUME 1, UMBER 1 OCTOBER Analysis of disretization in the diret simulation Monte Carlo iolas G. Hadjionstantinou a) Department of Mehanial Engineering, Massahusetts Institute of Tehnology,
On the Quantum Theory of Radiation.
 Physikalishe Zeitshrift, Band 18, Seite 121-128 1917) On the Quantum Theory of Radiation. Albert Einstein The formal similarity between the hromati distribution urve for thermal radiation and the Maxwell
Physikalishe Zeitshrift, Band 18, Seite 121-128 1917) On the Quantum Theory of Radiation. Albert Einstein The formal similarity between the hromati distribution urve for thermal radiation and the Maxwell
Possibility of Refuse Derived Fuel Fire Inception by Spontaneous Ignition
 Possibility of Refuse Derived Fuel Fire Ineption by Spontaneous Ignition Lijing Gao 1, Takashi Tsuruda 1, Takeshi Suzuki 1, Yoshio Ogawa 1, Chihong Liao 1 and Yuko Saso 1 1 National Researh Institute of
Possibility of Refuse Derived Fuel Fire Ineption by Spontaneous Ignition Lijing Gao 1, Takashi Tsuruda 1, Takeshi Suzuki 1, Yoshio Ogawa 1, Chihong Liao 1 and Yuko Saso 1 1 National Researh Institute of
Lecture 27: Entropy and Information Prof. WAN, Xin
 General Pysis I Leture 27: Entropy and Information Prof. WAN, Xin xinwan@zju.edu.n ttp://zimp.zju.edu.n/~xinwan/ 1st & 2nd Laws of ermodynamis e 1st law speifies tat we annot get more energy out of a yli
General Pysis I Leture 27: Entropy and Information Prof. WAN, Xin xinwan@zju.edu.n ttp://zimp.zju.edu.n/~xinwan/ 1st & 2nd Laws of ermodynamis e 1st law speifies tat we annot get more energy out of a yli
Determination of the Aerodynamic Characteristics of Flying Vehicles Using Method Large Eddy Simulation with Software ANSYS
 Automation, Control and Intelligent Systems 15; 3(6): 118-13 Published online Deember, 15 (http://www.sienepublishinggroup.om//ais) doi: 1.11648/.ais.1536.14 ISSN: 38-5583 (Print); ISSN: 38-5591 (Online)
Automation, Control and Intelligent Systems 15; 3(6): 118-13 Published online Deember, 15 (http://www.sienepublishinggroup.om//ais) doi: 1.11648/.ais.1536.14 ISSN: 38-5583 (Print); ISSN: 38-5591 (Online)
Name... Class... Date...
 Energy transfers Speifiation referenes: Maths skills 1a, 1b, 2a, 2h, 3a, 3b, 3, 3d Aims In this worksheet you will learn how to alulate kineti energy, gravitational, and elasti potential energy. You will
Energy transfers Speifiation referenes: Maths skills 1a, 1b, 2a, 2h, 3a, 3b, 3, 3d Aims In this worksheet you will learn how to alulate kineti energy, gravitational, and elasti potential energy. You will
Green s function for the wave equation
 Green s funtion for the wave equation Non-relativisti ase January 2019 1 The wave equations In the Lorentz Gauge, the wave equations for the potentials are (Notes 1 eqns 43 and 44): 1 2 A 2 2 2 A = µ 0
Green s funtion for the wave equation Non-relativisti ase January 2019 1 The wave equations In the Lorentz Gauge, the wave equations for the potentials are (Notes 1 eqns 43 and 44): 1 2 A 2 2 2 A = µ 0
Energy is the capacity to do work
 1 of 10 After completing this chapter, you should, at a minimum, be able to do the following. This information can be found in my lecture notes for this and other chapters and also in your text. Correctly
1 of 10 After completing this chapter, you should, at a minimum, be able to do the following. This information can be found in my lecture notes for this and other chapters and also in your text. Correctly
Effect of magnetization process on levitation force between a superconducting. disk and a permanent magnet
 Effet of magnetization proess on levitation fore between a superonduting disk and a permanent magnet L. Liu, Y. Hou, C.Y. He, Z.X. Gao Department of Physis, State Key Laboratory for Artifiial Mirostruture
Effet of magnetization proess on levitation fore between a superonduting disk and a permanent magnet L. Liu, Y. Hou, C.Y. He, Z.X. Gao Department of Physis, State Key Laboratory for Artifiial Mirostruture
Chapter 3. Volumetric Properties of Pure Fluids
 Chapter 3. olumetri roperties of ure Fluids Introdution hermodynami properties (U, H and thus Q, W) are alulated from data data are important for sizing vessels and pipelines Subjets behavior of pure fluids
Chapter 3. olumetri roperties of ure Fluids Introdution hermodynami properties (U, H and thus Q, W) are alulated from data data are important for sizing vessels and pipelines Subjets behavior of pure fluids
STUDY OF INHERENT FREQUENCY OF HELMHOLTZ RESONATOR
 005 WJTA Amerian Waterjet Conferene August -3, 005! Houston, Texas Paper 6B-4 STUDY OF INHERENT FREQUENCY OF HELMHOLT RESONATOR Gong Weili An Liqian Cui Longlian Xie Guixin Shool of Mehanis, Arhiteture
005 WJTA Amerian Waterjet Conferene August -3, 005! Houston, Texas Paper 6B-4 STUDY OF INHERENT FREQUENCY OF HELMHOLT RESONATOR Gong Weili An Liqian Cui Longlian Xie Guixin Shool of Mehanis, Arhiteture
23.1 Tuning controllers, in the large view Quoting from Section 16.7:
 Lesson 23. Tuning a real ontroller - modeling, proess identifiation, fine tuning 23.0 Context We have learned to view proesses as dynami systems, taking are to identify their input, intermediate, and output
Lesson 23. Tuning a real ontroller - modeling, proess identifiation, fine tuning 23.0 Context We have learned to view proesses as dynami systems, taking are to identify their input, intermediate, and output
Chapter Outline The Relativity of Time and Time Dilation The Relativistic Addition of Velocities Relativistic Energy and E= mc 2
 Chapter 9 Relativeity Chapter Outline 9-1 The Postulate t of Speial Relativity it 9- The Relativity of Time and Time Dilation 9-3 The Relativity of Length and Length Contration 9-4 The Relativisti Addition
Chapter 9 Relativeity Chapter Outline 9-1 The Postulate t of Speial Relativity it 9- The Relativity of Time and Time Dilation 9-3 The Relativity of Length and Length Contration 9-4 The Relativisti Addition
Longitudinal Static Stability
 ongitudinal Stati Stability Some definitions C m M V S pithing moment without dimensions (so without influene of ρ, V and S) it is a shape parameter whih varies with the angle of attak. Note the hord in
ongitudinal Stati Stability Some definitions C m M V S pithing moment without dimensions (so without influene of ρ, V and S) it is a shape parameter whih varies with the angle of attak. Note the hord in
IMPACT MODELLING OF THE COEFFICIENT OF RESTITUTION OF POTATOES BASED ON THE KELVIN- VOIGHT PAIR
 Bulletin of the Transilvania University of Braşov Series II: Forestry Wood Industry Agriultural Food Engineering Vol. 9 (58) No. - 06 IMPACT MODELLING OF THE COEFFICIENT OF RESTITUTION OF POTATOES BASED
Bulletin of the Transilvania University of Braşov Series II: Forestry Wood Industry Agriultural Food Engineering Vol. 9 (58) No. - 06 IMPACT MODELLING OF THE COEFFICIENT OF RESTITUTION OF POTATOES BASED
Lecture 13 Bragg-Williams Theory
 Leture 13 Bragg-Williams Theory As noted in Chapter 11, an alternative mean-field approah is to derive a free energy, F, in terms of our order parameter,m, and then minimize F with respet to m. We begin
Leture 13 Bragg-Williams Theory As noted in Chapter 11, an alternative mean-field approah is to derive a free energy, F, in terms of our order parameter,m, and then minimize F with respet to m. We begin
Introduction to Quantum Chemistry
 Chem. 140B Dr. J.A. Mak Introdution to Quantum Chemistry Without Quantum Mehanis, how would you explain: Periodi trends in properties of the elements Struture of ompounds e.g. Tetrahedral arbon in ethane,
Chem. 140B Dr. J.A. Mak Introdution to Quantum Chemistry Without Quantum Mehanis, how would you explain: Periodi trends in properties of the elements Struture of ompounds e.g. Tetrahedral arbon in ethane,
MODELLING THE POSTPEAK STRESS DISPLACEMENT RELATIONSHIP OF CONCRETE IN UNIAXIAL COMPRESSION
 VIII International Conferene on Frature Mehanis of Conrete and Conrete Strutures FraMCoS-8 J.G.M. Van Mier, G. Ruiz, C. Andrade, R.C. Yu and X.X. Zhang Eds) MODELLING THE POSTPEAK STRESS DISPLACEMENT RELATIONSHIP
VIII International Conferene on Frature Mehanis of Conrete and Conrete Strutures FraMCoS-8 J.G.M. Van Mier, G. Ruiz, C. Andrade, R.C. Yu and X.X. Zhang Eds) MODELLING THE POSTPEAK STRESS DISPLACEMENT RELATIONSHIP
MultiPhysics Analysis of Trapped Field in Multi-Layer YBCO Plates
 Exerpt from the Proeedings of the COMSOL Conferene 9 Boston MultiPhysis Analysis of Trapped Field in Multi-Layer YBCO Plates Philippe. Masson Advaned Magnet Lab *7 Main Street, Bldg. #4, Palm Bay, Fl-95,
Exerpt from the Proeedings of the COMSOL Conferene 9 Boston MultiPhysis Analysis of Trapped Field in Multi-Layer YBCO Plates Philippe. Masson Advaned Magnet Lab *7 Main Street, Bldg. #4, Palm Bay, Fl-95,
Microeconomic Theory I Assignment #7 - Answer key
 Miroeonomi Theory I Assignment #7 - Answer key. [Menu priing in monopoly] Consider the example on seond-degree prie disrimination (see slides 9-93). To failitate your alulations, assume H = 5, L =, and
Miroeonomi Theory I Assignment #7 - Answer key. [Menu priing in monopoly] Consider the example on seond-degree prie disrimination (see slides 9-93). To failitate your alulations, assume H = 5, L =, and
Name Solutions to Test 1 September 23, 2016
 Name Solutions to Test 1 September 3, 016 This test onsists of three parts. Please note that in parts II and III, you an skip one question of those offered. Possibly useful formulas: F qequb x xvt E Evpx
Name Solutions to Test 1 September 3, 016 This test onsists of three parts. Please note that in parts II and III, you an skip one question of those offered. Possibly useful formulas: F qequb x xvt E Evpx
What s free about Gibbs free energy?
 What s free about Gibbs free energy? The change in free energy for a process equals the maximum work that can be done by the system on the surroundings in a spontaneous process occurring at constant temperature
What s free about Gibbs free energy? The change in free energy for a process equals the maximum work that can be done by the system on the surroundings in a spontaneous process occurring at constant temperature
Homework 01. Phase Changes and Solutions
 HW01 - Phase Changes and Solu!ons! This is a preview of the published version of the quiz Started: Jan 16 at 1:pm Quiz Instruc!ons Homework 01 Phase Changes and Solutions Question 1 Given that you have
HW01 - Phase Changes and Solu!ons! This is a preview of the published version of the quiz Started: Jan 16 at 1:pm Quiz Instruc!ons Homework 01 Phase Changes and Solutions Question 1 Given that you have
CHAPTERS 8-12 BOOKLET-3
 CHEMISTRY XI CHAPTERS 8-1 BKLET- Contents: Page No. Chapter 8 Chemial Equilibrium 181-199 Chapter 9 Redox Reations 00-19 Chapter 10 s & p Blok Elements part 1 0-49 Chapter 11 s & p Blok Elements part 50-77
CHEMISTRY XI CHAPTERS 8-1 BKLET- Contents: Page No. Chapter 8 Chemial Equilibrium 181-199 Chapter 9 Redox Reations 00-19 Chapter 10 s & p Blok Elements part 1 0-49 Chapter 11 s & p Blok Elements part 50-77
Determination of the reaction order
 5/7/07 A quote of the wee (or amel of the wee): Apply yourself. Get all the eduation you an, but then... do something. Don't just stand there, mae it happen. Lee Iaoa Physial Chemistry GTM/5 reation order
5/7/07 A quote of the wee (or amel of the wee): Apply yourself. Get all the eduation you an, but then... do something. Don't just stand there, mae it happen. Lee Iaoa Physial Chemistry GTM/5 reation order
Homework Week 8 G = H T S. Given that G = H T S, using the first and second laws we can write,
 Statistical Molecular hermodynamics University of Minnesota Homework Week 8 1. By comparing the formal derivative of G with the derivative obtained taking account of the first and second laws, use Maxwell
Statistical Molecular hermodynamics University of Minnesota Homework Week 8 1. By comparing the formal derivative of G with the derivative obtained taking account of the first and second laws, use Maxwell
Nuclear Shell Structure Evolution Theory
 Nulear Shell Struture Evolution Theory Zhengda Wang (1) Xiaobin Wang () Xiaodong Zhang () Xiaohun Wang () (1) Institute of Modern physis Chinese Aademy of SienesLan Zhou P. R. China 70000 () Seagate Tehnology
Nulear Shell Struture Evolution Theory Zhengda Wang (1) Xiaobin Wang () Xiaodong Zhang () Xiaohun Wang () (1) Institute of Modern physis Chinese Aademy of SienesLan Zhou P. R. China 70000 () Seagate Tehnology
Chemistry (Physical chemistry) Lecture 10.
 Chemistry (Physial hemistry) Leture 0. EPM, semester II by Wojieh Chrzanowsi, PhD, DS Wyłady współfinansowane ze środów Unii Europejsiej w ramah EFS, UDA-POKL 04.0.02.-00-37/-00 Absolwent Wydziału Chemiznego
Chemistry (Physial hemistry) Leture 0. EPM, semester II by Wojieh Chrzanowsi, PhD, DS Wyłady współfinansowane ze środów Unii Europejsiej w ramah EFS, UDA-POKL 04.0.02.-00-37/-00 Absolwent Wydziału Chemiznego
Numerical Tests of Nucleation Theories for the Ising Models. Abstract
 to be submitted to Physial Review E Numerial Tests of Nuleation Theories for the Ising Models Seunghwa Ryu 1 and Wei Cai 2 1 Department of Physis, Stanford University, Stanford, California 94305 2 Department
to be submitted to Physial Review E Numerial Tests of Nuleation Theories for the Ising Models Seunghwa Ryu 1 and Wei Cai 2 1 Department of Physis, Stanford University, Stanford, California 94305 2 Department
COPYRIGHTED MATERIAL. Gravimetrics. Key Terms. Key Concepts. Words that can be used as topics in essays: accuracy atomic theory
 8684-X Ch02.F 2/9/01 7:49 AM Page 23 Gravimetris Key Terms Words that an be used as topis in essays: auray atomi theory density empirial formula extensive property frational rystallization heterogeneous
8684-X Ch02.F 2/9/01 7:49 AM Page 23 Gravimetris Key Terms Words that an be used as topis in essays: auray atomi theory density empirial formula extensive property frational rystallization heterogeneous
Chemistry 1A, Fall 2009 Midterm Exam #3 November 10, 2009 (90 min, closed book)
 Chemistry 1A, Fall 2009 Midterm Exam #3 November 10, 2009 (90 min, closed book) Name: SID: GSI Name: The test consists of 7 short answer questions and 18 multiple choice questions. Put your written answers
Chemistry 1A, Fall 2009 Midterm Exam #3 November 10, 2009 (90 min, closed book) Name: SID: GSI Name: The test consists of 7 short answer questions and 18 multiple choice questions. Put your written answers
Directional Coupler. 4-port Network
 Diretional Coupler 4-port Network 3 4 A diretional oupler is a 4-port network exhibiting: All ports mathed on the referene load (i.e. S =S =S 33 =S 44 =0) Two pair of ports unoupled (i.e. the orresponding
Diretional Coupler 4-port Network 3 4 A diretional oupler is a 4-port network exhibiting: All ports mathed on the referene load (i.e. S =S =S 33 =S 44 =0) Two pair of ports unoupled (i.e. the orresponding
PHY 108: Optical Physics. Solution to Midterm Test
 PHY 108: Optial Physis Solution to Midterm Test TA: Xun Jia 1 May 14, 2008 1 Email: jiaxun@physis.ula.edu Spring 2008 Physis 108 Xun Jia (May 14, 2008) Problem #1 For a two mirror resonant avity, the resonane
PHY 108: Optial Physis Solution to Midterm Test TA: Xun Jia 1 May 14, 2008 1 Email: jiaxun@physis.ula.edu Spring 2008 Physis 108 Xun Jia (May 14, 2008) Problem #1 For a two mirror resonant avity, the resonane
22.54 Neutron Interactions and Applications (Spring 2004) Chapter 6 (2/24/04) Energy Transfer Kernel F(E E')
 22.54 Neutron Interations and Appliations (Spring 2004) Chapter 6 (2/24/04) Energy Transfer Kernel F(E E') Referenes -- J. R. Lamarsh, Introdution to Nulear Reator Theory (Addison-Wesley, Reading, 1966),
22.54 Neutron Interations and Appliations (Spring 2004) Chapter 6 (2/24/04) Energy Transfer Kernel F(E E') Referenes -- J. R. Lamarsh, Introdution to Nulear Reator Theory (Addison-Wesley, Reading, 1966),
2. The Energy Principle in Open Channel Flows
 . The Energy Priniple in Open Channel Flows. Basi Energy Equation In the one-dimensional analysis of steady open-hannel flow, the energy equation in the form of Bernoulli equation is used. Aording to this
. The Energy Priniple in Open Channel Flows. Basi Energy Equation In the one-dimensional analysis of steady open-hannel flow, the energy equation in the form of Bernoulli equation is used. Aording to this
reduction kj/mol
 1. Glucose is oxidized to water and CO 2 as a result of glycolysis and the TCA cycle. The net heat of reaction for the oxidation is -2870 kj/mol. a) How much energy is required to produce glucose from
1. Glucose is oxidized to water and CO 2 as a result of glycolysis and the TCA cycle. The net heat of reaction for the oxidation is -2870 kj/mol. a) How much energy is required to produce glucose from
Physics 30 Lesson 32 x-rays and the Compton Effect
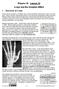 I. Disovery of x-rays Physis 30 Lesson 32 x-rays and the Compton ffet During all the researh on athode rays, several sientists missed their hane at some glory. Hertz narrowly missed disovering x-rays during
I. Disovery of x-rays Physis 30 Lesson 32 x-rays and the Compton ffet During all the researh on athode rays, several sientists missed their hane at some glory. Hertz narrowly missed disovering x-rays during
Answers to Coursebook questions Chapter J2
 Answers to Courseook questions Chapter J 1 a Partiles are produed in ollisions one example out of many is: a ollision of an eletron with a positron in a synhrotron. If we produe a pair of a partile and
Answers to Courseook questions Chapter J 1 a Partiles are produed in ollisions one example out of many is: a ollision of an eletron with a positron in a synhrotron. If we produe a pair of a partile and
IV Transport Phenomena. Lecture 21: Solids and Concentrated Solutions
 IV Transport Phenomena Leture 21: Solids and Conentrated Solutions MIT Student (and MZB) Marh 28, 2011 1 Transport in Solids 1.1 Diffusion The general model of hemial reations an also be used for thermally
IV Transport Phenomena Leture 21: Solids and Conentrated Solutions MIT Student (and MZB) Marh 28, 2011 1 Transport in Solids 1.1 Diffusion The general model of hemial reations an also be used for thermally
