Then the amount of water that flows through the pipe during a differential time interval dt is (1) 4
|
|
|
- Leslie Lang
- 6 years ago
- Views:
Transcription
1 5-98 Review Problems 5-45 A tank oen to te atmosere is itially filled wit. e tank discarges to te atmosere troug a long ie connected to a valve. e itial discarge velocity from te tank and te time required to emty te tank are to be determed. Assumtions e flow is comressible. e drag ie is orizontal. e tank is considered to be emty wen te level dros to te center of te valve. Analysis (a) Substitutg te known quantities, te discarge velocity can be exressed as gz gz V 0. gz.5 + fl / D (00 m)/(0.0 m) en te itial discarge velocity becomes V 0. 0.(9.8m/s )( m).54 m/s gz were z is te eigt relative to te center of te orifice at tat time. (b) e flow rate of from te tank can be obtaed by multilyg te discarge velocity by te ie cross-sectional area, πd V & AieV 0. gz 4 en te amount of tat flows troug te ie durg a differential time terval dt is πd dv V& dt 0. gzdt () 4 wic, from conservation of mass, must be equal to te decrease te volume of te tank, πd0 dv Atank ( dz) dz () 4 were dz is te cange te level te tank durg dt. (Note tat dz is a negative quantity sce te ositive direction of z is uwards. erefore, we used dz to get a ositive quantity for te amount of discarged). Settg Eqs. () and () equal to eac oter and rearrangg, π D 0.gzdt π 4 D0 dz dt D D 0 0 z dz 0.gz D 4 D 0.g e last relation can be tegrated easily sce te variables are searated. Lettg t f be te discarge time and tegratg it from t 0 wen z z to t t f wen z 0 (comletely draed tank) gives 0 t f D 0 0 / D0 z D0 dt z dz t - 0 f t D 0.g z z D 0.g D 0. z Simlifyg and substitutg te values given, te drag time is determed to be t f 0 D D z 0.g (0 m) (0. m) m 5,940 s 7. 0.(9.8 m/s ) Discussion e drag time can be sortened considerably by stallg a um te ie. D 0 z dz g z D
2 e rate of accumulation of a ool and te rate of discarge are given. e rate suly of to te ool is to be determed. Assumtions Water is sulied and discarged steadily. e rate of evaoration of is negligible. No is sulied or removed troug oter means. Analysis e conservation of mass rcile alied to te ool requires tat te rate of crease te amount of te ool be equal to te difference between te rate of suly of and te rate of discarge. at is, dm ool dt dmool dv mi me mi me V & ool & & & + & i + V& e dt dt sce te density of is constant and tus te conservation of mass is equivalent to conservation of volume. e rate of discarge of is V & e A e Ve ( πd /4) Ve [ π (0.05 m) /4](5 m/s) m e rate of accumulation of te ool is equal to te crosssection of te ool times te rate at wic te level rises, dv ool dt A V cross-section level /s ( m 4 m)(0.05 m/m) 0.8 m /m m /s Substitutg, te rate at wic is sulied to te ool is determed to be dv ool V & i + V& e m dt erefore, is sulied at a rate of 0.08 m /s.8 L/s. /s 5-47 A fluid is flowg a circular ie. A relation is to be obtaed for te average fluid velocity terms of V(r), R, and r. Analysis Coosg a circular rg of area da πrdr as our differential area, te mass flow rate troug a cross-sectional area can be exressed as Solvg for V avg, V ρv ( r) da ρv ( r) π rdr avg A R 0 R V 0 R ()dr r r R r dr
3 Air is accelerated a nozzle. e density of air at te nozzle exit is to be determed. Assumtions Flow troug te nozzle is steady. Proerties e density of air is given to be 4.8 kg/m at te let. Analysis ere is only one let and one exit, and tus m. en, m & ρ A V ρ A V A ρ A V V ρ 0 m/s 80 m/s (4.8 kg/m ).64 & kg/m Discussion Note tat te density of air decreases considerably desite a decrease te cross-sectional area of te nozzle. AIR 5-49 e air a osital room is to be relaced every 5 mutes. e mimum diameter of te duct is to be determed if te air velocity is not to exceed a certa value. Assumtions e volume occuied by te furniture etc te room is negligible. e comg conditioned air does not mix wit te air te room. Analysis e volume of te room is V (6 m)(5 m)(4 m) 0 m o emty tis air 0 m, te volume flow rate must be V 0 m V & 0. m /s t 5 60 s If te mean velocity is 5 m/s, te diameter of te duct is πd V& AV 4 V D 4V & πv 4(0. m /s) π (5 m/s) 0.84 m Hosital Room m 0 bulbs erefore, te diameter of te duct must be at least 0.84 m to ensure tat te air te room is excanged comletely wit 0 m wile te mean velocity does not exceed 5 m/s. Discussion is roblem sows tat engeerg systems are sized to satisfy certa constrats imosed by certa considerations A long roll of large -Mn manganese steel late is to be quenced an oil bat at a secified rate. e mass flow rate of te late is to be determed. Assumtions e late moves troug te bat steadily. Proerties e density of steel late is given to be ρ 7854 kg/m. Analysis e mass flow rate of te seet metal troug te oil bat is Steel late 0 m/m ρv & ρwtv (7854 kg/m )( m)(0.005 m)(0 m/m) 9 kg/m 6.55 kg/s erefore, steel late can be treated conveniently as a flowg fluid calculations.
4 E A study quantifies te cost and benefits of enancg IAQ by creasg te buildg ventilation. e net monetary benefit of stallg an enanced IAQ system to te emloyer er year is to be determed. Assumtions e analysis te reort is alicable to tis work lace. Analysis e reort states tat enancg IAQ creases te roductivity of a erson by $90 er year, and decreases te cost of te resiratory illnesses by $9 a year wile creasg te annual energy consumtion by $6 and te equiment cost by ab $4 a year. e net monetary benefit of stallg an enanced IAQ system to te emloyer er year is determed by addg te benefits and subtractg te costs to be Net benefit otal benefits total cost (90+9) (6+4) $9/year (er erson) e total benefit is determed by multilyg te benefit er erson by te number of emloyees, otal net benefit No. of emloyees Net benefit er erson 0 $9/year $4,80/year Discussion Note tat te unseen savgs roductivity and reduced illnesses can be very significant wen tey are roerly quantified. 5-5 Air flows troug a non-constant cross-section ie. e let and exit velocities of te air are to be determed. Assumtions is is a steady-flow rocess sce tere is no cange wit time. Potential energy cange is negligible. ere are no work teractions. 4 e device is adiabatic and tus eat transfer is negligible. 5 Air is an ideal gas wit constant secific eats. Proerties e gas constant of air is R 0.87 kpa.m /kg.k. Also, c.005 kj/kg.k for air at room temerature (able A-) Analysis We take te ie as te system, wic is a control volume sce mass crosses te boundary. e mass and energy balances for tis steady-flow system can be exressed te rate form as Mass balance: 0 (steady) system Energy balance: E & 44 & 0 ρ AV ρ A V D 00 kpa 50 C Vel P πd V R 4 Rate of cange ternal, ketic, otential, etc.energies V V + c + + & 4444 V + 0 V + P πd V R 4 Air scew& e + q P P D V DV D 50 kpa 40 C Vel or c q Assumg let diameter to be.8 m and te exit diameter to be.0 m, and substitutg given values to mass and energy balance equations 00 kpa 50 kpa (.8 m) V (.0 m) V () K K V kj/kg V kj/kg (.005 kj/kg.k)( K) + (.005 kj/kg.k)( K) + +. kj/kg () 000 m /s 000 m /s ere are two equations and two unknowns. Solvg equations () and () simultaneously usg an equation solver suc as EES, te velocities are determed to be V 8.6 m/s V 0 m/s
5 Geotermal flows troug a flas camber, a searator, and a turbe a geotermal ower lant. e temerature of te steam after te flasg rocess and te ower ut from te turbe are to be determed for different flas camber exit ressures. Assumtions is is a steady-flow rocess sce tere is no cange wit time. Ketic and otential energy canges are negligible. e devices are sulated so tat tere are no eat losses to te surroundgs. 4 Proerties of steam are used for geotermal. Analysis For all comonents, we take te steam as te system, wic is a control volume sce mass crosses te boundary. e energy balance for tis steady-flow system can be exressed te rate form as Energy balance: E & 44 & 0 (steady) 0 Rate of cange ternal, ketic, otential, etc.energies & 4444 For eac comonent, te energy balance reduces to Flas camber: Searator: m & & + & m mliquidliquid urbe: W& ) ( 4 (a) For a flas camber exit ressure of P MPa e roerties of geotermal are 0 C x f kpa kpa kpa 79.9 C C sat. liq. Flas camber searator liquid P 000 kpa 777. kj/kg x P4 0 kpa 4 f + x4 fg (0.05)( 57.5 kj/kg) 49. kj/kg x e mass flow rate of vaor after te flasg rocess is m & x (0.)(50 kg/s) kg/s en, te ower ut from te turbe becomes W & (5.649 kg/s)( ) 66 kw Reeatg te similar calculations for oter ressures, we obta (b) For P 500 kpa, (c) For P 00 kpa, (d) For P 50 kpa, 5.8 C, & 4 kw W 99.6 C, & kw W 8. C, & 7 kw W steam turbe 4 0 kpa x0.95
6 A tank is eated by electricity. e witdrawn from te tank is mixed wit cold a camber. e mass flow rate of ot witdrawn from te tank and te average temerature of mixed are to be determed. Assumtions e rocess te mixg camber is a steady-flow rocess sce tere is no cange wit time. Ketic and otential energy canges are negligible. ere are no work teractions. 4 e device is adiabatic and tus eat transfer is negligible. Proerties e secific eat and density of are taken to be c 4.8 kj/kg.k, ρ 00 kg/m (able A-). Analysis We take te mixg camber as te system, wic is a control volume sce mass crosses te boundary. e energy balance for tis steady-flow system can be exressed te rate form as Energy balance: E & 44 & + Rate of cange ternal, ketic, otential, etc.energies & C m (sce W& ke e m? 0 C 0.06 kg/s ank 80 C 60 C or c + c ( + c () ot tank,ave cold cold ot cold ) mixture Similarly, an energy balance may be written on te tank as were and [ W& + c )] t m c ( ) e, ot ( cold tank,ave tank tank, tank, C tank, tank, tank, ave mtank ρv (000 kg/m )(0.060 m ) Substitutg to Eq. (), [.6 kj/s + (4.8 kj/kg. C)(0 70) ] ot Substitutg to Eq. (), 60 kg () kg/s Mixg camber C (8 60 s) (60 kg)(4.8 kj/kg. C)(60 80) C ( kg/s)(4.8 kj/kg. C)(70 C) + (0.06 kg/s)(4.8 kj/kg. C)(0 C) mixture ot [( )kg/s](4.8 kj/kg. C) 44.5 C mixture mix?
7 Water is boiled at a secified temerature by ot gases flowg troug a staless steel ie submerged. e rate of evaoration of is to be determed. Assumtions Steady oeratg conditions exist. Heat losses from te er surfaces of te boiler are negligible. Proerties e entaly of vaorization of at 50 C is fg.8 kj/kg (able A-4). Analysis e rate of eat transfer to is given to be 74 kj/s. Notg tat te entaly of vaorization reresents te amount of energy needed to vaorize a unit mass of a liquid at a secified temerature, te rate of evaoration of is determed to be evaoration boilg fg 74 kj/s.8 kj/kg kg/s Hot gases Water 50 C Heater 5-56 Cold enters a steam generator at 0 C, and leaves as saturated vaor at sat 50 C. e fraction of eat used to reeat te liquid from 0 C to saturation temerature of 50 C is to be determed. Assumtions Steady oeratg conditions exist. Heat losses from te steam generator are negligible. e secific eat of is constant at te average temerature. Proerties e eat of vaorization of at 50 C is fg.8 kj/kg (able A-4), and te secific eat of liquid is c 4.8 kj/kg. C (able A-). Analysis e eat of vaorization of reresents te amount of eat needed to vaorize a unit mass of liquid at a secified temerature. Usg te average secific eat, te amount of eat transfer needed to reeat a unit mass of from 0 C to 50 C is and q q c (4.8 kj/kg C)(50 0) C 54.4 kj/kg reeatg total qboilg + qreeatg erefore, te fraction of eat used to reeat te is q Fraction t o reeat q reeatg total kj/kg 0.05 (or 0.5% ) Cold 0 C Steam Water 50 C Heater
8 Cold enters a steam generator at 0 C and is boiled, and leaves as saturated vaor at boiler ressure. e boiler ressure at wic te amount of eat needed to reeat te to saturation temerature tat is equal to te eat of vaorization is to be determed. Assumtions Heat losses from te steam generator are negligible. Proerties e entaly of liquid at 0 C is 8.9 kj/kg. Oter roerties needed to solve tis roblem are te eat of vaorization fg and te entaly of saturated liquid at te secified temeratures, and tey can be obtaed from able A-4. Analysis e eat of vaorization of reresents te amount of eat needed to vaorize a unit mass of liquid at a secified temerature, and reresents te amount of eat needed to reeat a unit mass of from 0 C to te saturation temerature. erefore, ( f sat q reeatg f@0 C q ) 8.9 kj/kg boilg fg sat sat sat e solution of tis roblem requires coosg a boilg temerature, readg f and fg at tat temerature, and substitutg te values to te relation above to see if it is satisfied. By trial and error, (able A-4) At 0 C: At kj/kg f sat f sat sat sat kj/kg e temerature tat satisfies tis condition is determed from te two values above by terolation to be 0.6 C. e saturation ressure corresondg to tis temerature is 9.94 MPa. 8.9 kj/kg Cold 0 C Steam Water Heater 5-58 Saturated steam at atm ressure and tus at a saturation temerature of sat 00 C condenses on a vertical late mataed at 90 C by circulatg coolg troug te oter side. e rate of condensation of steam is to be determed. Assumtions Steady oeratg conditions exist. e steam condenses and te condensate dris off at 00 C. (In reality, te condensate temerature will be between 90 and 00, and te coolg of te condensate a few C sould be considered if better accuracy is desired). Proerties e entaly of vaorization of at atm (0.5 kpa) is fg 56.5 kj/kg (able A-5). Analysis e rate of eat transfer durg tis condensation rocess is given to be 80 kj/s. Notg tat te eat of vaorization of reresents te amount of eat released as a unit mass of vaor at a secified temerature condenses, te rate of condensation of steam is determed from condensation fg 80 kj/s 56.5 kj/kg kg/s Plate 90 C Steam 00 C Condensate
9 Water is boiled at sat 00 C by an electric eater. e rate of evaoration of is to be determed. Steam Assumtions Steady oeratg conditions exist. Heat losses from te er surfaces of te tank are negligible. Proerties e entaly of vaorization of at 00 C is fg 56.4 kj/kg (able A-4). Analysis Notg tat te entaly of vaorization reresents te amount of energy needed to vaorize a unit mass of a liquid at a secified temerature, te rate of evaoration of is determed to be evaoration W& e,boilg fg kj/s 56.4 kj/kg 0.00 kg/s 4.79 kg/ Water 00 C Heater 5-60 wo streams of same ideal gas at different states are mixed a mixg camber. e simlest exression for te mixture temerature a secified format is to be obtaed. Analysis e energy balance for tis steady-flow system can be exressed te rate form as E E & 44 & + c + c and, m & + Rate of cange ternal, ketic, otential, etc.energies c Solvg for fal temerature, we fd + Esystem & 4444 (sce W& 0) 0 m, m, Mixg device m,
10 An ideal gas exands a turbe. e volume flow rate at te let for a ower ut of 00 kw is to be determed. Assumtions is is a steady-flow rocess sce tere is no cange wit time. Ketic and otential energy canges are negligible. e device is adiabatic and tus eat transfer is negligible. Proerties e roerties of te ideal gas are given as R 0.0 kpa.m /kg.k, c. kj/kg C, c v 0.8 kj/kg C. Analysis We take te turbe as te system, wic is a control volume sce mass crosses te boundary. e energy balance for tis steady-flow system can be exressed te rate form as system 0 & 44 & & E E E 4444 Rate of cange ternal, ketic, otential, etc.energies E & m & W& + m & (sce ke e wic can be rearranged to solve for mass flow rate W& W& 00 kw 0.54 kg/s ( ) c (. kj/kg.k)(00-700)k e let secific volume and te volume flow rate are R ( 0. kpa m /kg K)( 00 K) v 0.6 m /kg P 600 kpa us, V& v (0.54 kg/s)(0.6 m /kg) 0. m /s P 600 kpa 00 K Ideal gas 00 kw 700 K 5-6 wo identical buildgs Los Angeles and Denver ave te same filtration rate. e ratio of te eat losses by filtration at te two cities under identical conditions is to be determed. Assumtions Bot buildgs are identical and bot are subjected to te same conditions excet te atmoseric conditions. Air is an ideal gas wit constant secific eats at room temerature. Steady flow conditions exist. Analysis We can view filtration as a steady stream of air tat is eated as it flows an imagary duct assg troug te buildg. e energy balance for tis imagary steady-flow system can be exressed te rate form as E & 44 & + m & Rate of cange ternal, ketic, otential, etc.energies & m & (sce ke e mc & ( ) ρv & c ( ) en te sensible filtration eat loss (eat ga for te filtratg air) can be exressed c ) ρ (ACH)( V ) c ( ) filtration air ( i o o, air buildg i o Los Angeles: 0 kpa Denver: 8 kpa were ACH is te filtration volume rate air canges er our. erefore, te filtration eat loss is roortional to te density of air, and tus te ratio of filtration eat losses at te two cities is simly te densities of door air at tose cities, Infiltration eat loss ratio filtration, Los Angeles filtration, Denver ( P0 / R0 ) ( P / R ) 0 Los Angeles 0 Denver ρo, ρ Po, P air, Los Angeles, air, Denver o Los Angeles 0, Denver 0 kpa 8 kpa. erefore, te filtration eat loss Los Angeles will be % iger tan tat Denver under identical conditions.
11 e ventilatg fan of te batroom of an electrically eated buildg San Francisco runs contuously. e amount and cost of te eat vented er mont wter are to be determed. Assumtions We take te atmoseric ressure to be atm 0. kpa sce San Francisco is at sea level. e buildg is mataed at C at all times. e filtratg air is eated to C before it exfiltrates. 4 Air is an ideal gas wit constant secific eats at room temerature. 5 Steady flow conditions exist. Proerties e gas constant of air is R 0.87 kpa.m /kg K (able A-). e secific eat of air at room temerature is c.005 kj/kg C (able A-). Analysis e density of air at te door conditions of atm and C is Po ρ o R o (0. kpa).0 kg/m (0.87 kpa.m /kg.k)( + 7 K) en te mass flow rate of air vented becomes air ρv & air (.0 kg/m )(0.00 m /s) 0.06 kg/s We can view filtration as a steady stream of air tat is eated as it flows an imagary duct assg troug te ouse. e energy balance for tis imagary steady-flow system can be exressed te rate form as E & 44 & + m & Rate of cange ternal, ketic, otential, etc.energies m & mc & ( & 4444 (sce ke e ) 0 0 L/s. C Notg tat te door air vented at C is relaced by filtratg door air at. C, te sensible filtration eat loss (eat ga for te filtratg air) due to ventg by fans can be exressed loss by fan airc ( doors doors ) (0.06 kg/s)(.005 kj/kg. C)(.) C 0.55 kj/s 0.55 kw en te amount and cost of te eat vented er mont ( mont ) becomes Energy loss loss t (0.55 kw)(70 /mont) 56 kw/mont by fan Money loss (Energy loss)(unit cost of energy) (56 kw/mont)($0.09/kw) $.0/mont Discussion Note tat te energy and money loss associated wit ventilatg fans can be very significant. erefore, ventilatg fans sould be used wit care. C
12 Cilled air is to cool a room by removg te eat generated a large sulated classroom by ligts and students. e required flow rate of air tat needs to be sulied to te room is to be determed. Assumtions e moisture roduced by te bodies leave te room as vaor wit any condensg, and tus te classroom as no latent eat load. Heat ga troug te walls and te roof is negligible. 4 Air is an ideal gas wit constant secific eats at room temerature. 5 Steady oeratg conditions exist. Proerties e secific eat of air at room temerature is.005 kj/kg C (able A-). e average rate of sensible eat generation by a erson is given to be 60 W. Analysis e rate of sensible eat generation by te eole te room and te total rate of sensible ternal eat generation are gen, sensible total, sensible q& gen, sensible gen, sensible (No. of eole) (60 W/erson)(50 ersons) 9000 W + ligtg ,000 W Bot of tese effects can be viewed as eat ga for te cilled air stream, wic can be viewed as a steady stream of cool air tat is eated as it flows an imagary duct assg troug te room. e energy balance for tis imagary steady-flow system can be exressed te rate form as E & 44 & + m & Rate of cange ternal, ketic, otential, etc.energies m & & (sce ke e total, sensible mc & ( ) en te required mass flow rate of cilled air becomes air total, sensible c 5 kj/s.49 kg/s (.005 kj/kg C)(5 5) C Cilled air 5 C Return air 5 C Discussion e latent eat will be removed by te air-conditiong system as te moisture condenses side te coolg coils.
13 Cickens are to be cooled by cilled an immersion ciller. e rate of eat removal from te cicken and te mass flow rate of are to be determed. Assumtions Steady oeratg conditions exist. e termal roerties of cickens and are constant. Proerties e secific eat of cicken are given to be.54 kj/kg. C. e secific eat of is 4.8 kj/kg. C (able A-). Analysis (a) Cickens are droed to te ciller at a rate of 500 er our. erefore, cickens can be considered to flow steadily troug te ciller at a mass flow rate of &m cicken (500 cicken / )(. kg / cicken) 00 kg / kg / s akg te cicken flow stream te ciller as te system, te energy balance for steadily flowg cickens can be exressed te rate form as E & 44 & m & Rate of cange ternal, ketic, otential, etc.energies & m & cicken 0 (sce ke e cicken c ( ) en te rate of eat removal from te cickens as tey are cooled from 5 C to ºC becomes Immersion cillg, 0.5 C Cicken 5 C cicken ( mc & ) cicken (0.056 kg/s)(.54 kj/kg.º C)(5 )º C.0 kw e ciller gas eat from te surroundgs at a rate of 00 kj/ kj/s. en te total rate of eat ga by te is cicken eat ga kw Notg tat te temerature rise of is not to exceed ºC as it flows troug te ciller, te mass flow rate of must be at least ( c ).056 kw (4.8 kj/kg.º C)(º C).56 kg/s If te mass flow rate of is less tan tis value, ten te temerature rise of will ave to be more tan C.
14 Cickens are to be cooled by cilled an immersion ciller. e rate of eat removal from te cicken and te mass flow rate of are to be determed. Assumtions Steady oeratg conditions exist. e termal roerties of cickens and are constant. Heat ga of te ciller is negligible. Proerties e secific eat of cicken are given to be.54 kj/kg. C. e secific eat of is 4.8 kj/kg. C (able A-). Analysis (a) Cickens are droed to te ciller at a rate of 500 er our. erefore, cickens can be considered to flow steadily troug te ciller at a mass flow rate of &m cicken (500 cicken / )(. kg / cicken) 00 kg / kg / s akg te cicken flow stream te ciller as te system, te energy balance for steadily flowg cickens can be exressed te rate form as E & 44 & m & Rate of cange ternal, ketic, otential, etc.energies & m & cicken 0 (sce ke e cicken c ( ) en te rate of eat removal from te cickens as tey are cooled from 5 C to ºC becomes cicken ( mc & ) cicken (0.056 kg/s)(.54 kj/kg.º C)(5 )º C.0 kw Immersion cillg, 0.5 C Cicken 5 C Heat ga of te ciller from te surroundgs is negligible. en te total rate of eat ga by te is & Qcicken.0 kw Notg tat te temerature rise of is not to exceed ºC as it flows troug te ciller, te mass flow rate of must be at least ( c ).0 kw (4.8 kj/kg.º C)(º C).56 kg/s If te mass flow rate of is less tan tis value, ten te temerature rise of will ave to be more tan C.
15 A regenerator is considered to save eat durg te coolg of milk a dairy lant. e amounts of fuel and money suc a generator will save er year are to be determed. Assumtions Steady oeratg conditions exist. e roerties of te milk are constant. Proerties e average density and secific eat of milk can be taken to be ρ milk ρ kg/l and c, milk.79 kj/kg. C (able A-). Analysis e mass flow rate of te milk is milk ρv & milk ( kg/l)( L/s) kg/s 4,00 kg/ akg te asteurizg section as te system, te energy balance for tis steady-flow system can be exressed te rate form as E & 44 & + m & Rate of cange ternal, ketic, otential, etc.energies m & & 4444 milk (sce ke e c ( ) 0 Hot milk 7 C Regenerator 4 C Cold milk erefore, to eat te milk from 4 to 7 C as beg done currently, eat must be transferred to te milk at a rate of current [ mc & ( asturization refrigeration )] milk ( kg/s)(.79 kj/kg.ëc)(7 4) C 09 kj/s e roosed regenerator as an effectiveness of ε 0.8, and tus it will save 8 ercent of tis energy. erefore, & saved εqcurrent (. 0 8)( 09 kj / s) 56 kj / s Notg tat te boiler as an efficiency of η boiler 0.8, te energy savgs above corresond to fuel savgs of (56 kj / s) Fuel Saved &Q saved (term) 0.09term / s η (0.8) (05,500 kj) boiler Notg tat year and unit cost of natural gas is $.0/term, te annual fuel and money savgs will be Fuel Saved (0.09 terms/s)( s) 94,400 terms/yr Money saved (Fuel saved)(unit cost of fuel) (94,400 term/yr)($.0/term) $,06,800/yr
16 5-5-68E A refrigeration system is to cool eggs by cilled air at a rate of 0,000 eggs er our. e rate of eat removal from te eggs, te required volume flow rate of air, and te size of te comressor of te refrigeration system are to be determed. Assumtions Steady oeratg conditions exist. e eggs are at uniform temeratures before and after coolg. e coolg section is well-sulated. 4 e roerties of eggs are constant. 5 e local atmoseric ressure is atm. Proerties e roerties of te eggs are given to ρ 67.4 lbm/ft and c 0.80 Btu/lbm. F. e secific eat of air at room temerature c 0.4 Btu/lbm. F (able A-E). e gas constant of air is R sia.ft /lbm.r (able A-E). Analysis (a) Notg tat eggs are cooled at a rate of 0,000 eggs er our, eggs can be considered to flow steadily troug te coolg section at a mass flow rate of egg (0,000 eggs/)(0.4 lbm/egg) 400 lbm/ lbm/s akg te egg flow stream te cooler as te system, te energy balance for steadily flowg eggs can be exressed te rate form as E & 44 & m & Rate of cange ternal, ketic, otential, etc.energies egg & m & egg (sce ke e c ( ) 0 Air 4 F Egg 0.4 lbm en te rate of eat removal from te eggs as tey are cooled from 90 F to 50ºF at tis rate becomes egg ( mc & ) egg (400 lbm/)(0.80 Btu/lbm. F)(90 50) F 44,800 Btu/ (b) All te eat released by te eggs is absorbed by te refrigerated air sce eat transfer troug e walls of cooler is negligible, and te temerature rise of air is not to exceed 0 F. e mimum mass flow and volume flow rates of air are determed to be ρ V & air ( c ) air 44,800 Btu/ (0.4Btu/lbm. F)(0 F) air air air P R ρ air air 8,667 lbm/ lbm/ft³,500 ft³/ 8,667 lbm/ 4.7 sia lbm/ft (0.704 sia.ft /lbm.r)( )R
17 Doug is made wit refrigerated order to absorb te eat of ydration and tus to control te temerature rise durg kneadg. e temerature to wic te city must be cooled before mixg wit flour is to be determed to avoid temerature rise durg kneadg. Assumtions Steady oeratg conditions exist. e doug is at uniform temeratures before and after coolg. e kneadg section is well-sulated. 4 e roerties of and doug are constant. Proerties e secific eats of te flour and te are given to be.76 and 4.8 kj/kg. C, resectively. e eat of ydration of doug is given to be 5 kj/kg. Analysis It is stated tat kg of flour is mixed wit kg of, and tus kg of doug is obtaed from eac kg of. Also, 5 kj of eat is released for eac kg of doug kneaded, and tus 5 45 kj of eat is released from te doug made usg kg of. akg te coolg section of as te system, wic is a steady-flow control volume, te energy balance for tis steadyflow system can be exressed te rate form as E & 44 & m & Rate of cange ternal, ketic, otential, etc.energies & m & (sce ke e c ( ) 0 Water 5 C 5 kj/kg Doug Flour In order for to absorb all te eat of ydration and end u at a temerature of 5ºC, its temerature before enterg te mixg section must be reduced to Q Q doug mc ( ) Q mc 45 kj 5 C 4. C ( kg)(4.8 kj/kg. C) at is, te must be recooled to 4.ºC before mixg wit te flour order to absorb te entire eat of ydration.
18 Glass bottles are wased ot an uncovered rectangular glass wasg bat. e rates of eat and mass tat need to be sulied to te are to be determed. Assumtions Steady oeratg conditions exist. e entire body is mataed at a uniform temerature of 55 C. Heat losses from te er surfaces of te bat are negligible. 4 Water is an comressible substance wit constant roerties. Proerties e secific eat of at room temerature is c 4.8 kj/kg. C. Also, te secific eat of glass is 0.80 kj/kg. C (able A-). Analysis (a) e mass flow rate of glass bottles troug te bat steady oeration is &mbottle mbottle Bottle flow rate (0.50 kg / bottle)(800 bottles / m) 0 kg / m kg / s akg te bottle flow section as te system, wic is a steadyflow control volume, te energy balance for tis steady-flow system can be exressed te rate form as E & 44 & + m & Rate of cange ternal, ketic, otential, etc.energies m & & 4444 bottle (sce ke e c ( ) 0 en te rate of eat removal by te bottles as tey are eated from 0 to 55 C is & ( kg/s)( 0.8 kj/kg.º C)( 55 0) º C 56,000 W bottle mbottlec e amount of removed by te bottles is, ( Flow rate of bottles)( Water removed er bottle) ( 800 bottles/ m)( 0. g/bottle) 60 g/m.67 0 kg/s Notg tat te removed by te bottles is made u by fres enterg at 5 C, te rate of eat removal by te tat sticks to te bottles is Water bat 55 C removed removedc (.67 0 kg/s)(480 J/kg º C)(55 5)º C 446W erefore, te total amount of eat removed by te wet bottles is total, removed + 56, ,446W glass removed removed Discussion In ractice, te rates of eat and removal will be muc larger sce te eat losses from te tank and te moisture loss from te oen surface are not considered.
19 Glass bottles are wased ot an uncovered rectangular glass wasg bat. e rates of eat and mass tat need to be sulied to te are to be determed. Assumtions Steady oeratg conditions exist. e entire body is mataed at a uniform temerature of 50 C. Heat losses from te er surfaces of te bat are negligible. 4 Water is an comressible substance wit constant roerties. Proerties e secific eat of at room temerature is c 4.8 kj/kg. C. Also, te secific eat of glass is 0.80 kj/kg. C (able A-). Analysis (a) e mass flow rate of glass bottles troug te bat steady oeration is &mbottle mbottle Bottle flow rate (0.50 kg / bottle)(800 bottles / m) 0 kg / m kg / s akg te bottle flow section as te system, wic is a steady-flow control volume, te energy balance for tis steady-flow system can be exressed te rate form as E & 44 & + m & Rate of cange ternal, ketic, otential, etc.energies m & & 4444 bottle (sce ke e c ( ) 0 en te rate of eat removal by te bottles as tey are eated from 0 to 50 C is & ( kg/s)( 0.8 kj/kg.º C)( 50 0) º C 48,000W bottle mbottlec e amount of removed by te bottles is, ( Flow rate of bottles)( Water removed er bottle) ( 800 bottles/ m)( 0. g/bottle) 60 g/m.67 0 kg/s Water bat 50 C Notg tat te removed by te bottles is made u by fres enterg at 5 C, te rate of eat removal by te tat sticks to te bottles is removed removedc (.67 0 kg/s)(480 J/kg ºC)(50 5)ºC erefore, te total amount of eat removed by te wet bottles is total, removed + 48, ,9 W glass removed removed 9 W Discussion In ractice, te rates of eat and removal will be muc larger sce te eat losses from te tank and te moisture loss from te oen surface are not considered.
20 Long alumum wires are extruded at a velocity of 0 m/m, and are exosed to atmoseric air. e rate of eat transfer from te wire is to be determed. Assumtions Steady oeratg conditions exist. e termal roerties of te wire are constant. Proerties e roerties of alumum are given to be ρ 70 kg/m and c kj/kg. C. Analysis e mass flow rate of te extruded wire troug te air is m & ρv& ρ( πr0 ) V (70 kg/m ) π (0.005 m) (0 m/m) 0.9 kg/m akg te volume occuied by te extruded wire as te system, wic is a steady-flow control volume, te energy balance for tis steady-flow system can be exressed te rate form as E & 44 & m & Rate of cange ternal, ketic, otential, etc.energies & 4444 wire + m & wire (sce ke e c ( ) 0 en te rate of eat transfer from te wire to te air becomes & mc 50 C Alumum wire 0 m/m [ ( t) ] (0.9 kg/m)(0.896 kj/kg. C)(50 50) C 5. kj/m kw 5-7 Long coer wires are extruded at a velocity of 0 m/m, and are exosed to atmoseric air. e rate of eat transfer from te wire is to be determed. Assumtions Steady oeratg conditions exist. e termal roerties of te wire are constant. Proerties e roerties of coer are given to be ρ 8950 kg/m and c 0.8 kj/kg. C. Analysis e mass flow rate of te extruded wire troug te air is m & ρv& ρ( πr0 ) V (8950 kg/m ) π (0.005 m) (0 m/m) 0.6 kg/m akg te volume occuied by te extruded wire as te system, wic is a steady-flow control volume, te energy balance for tis steady-flow system can be exressed te rate form as E & 44 & m & Rate of cange ternal, ketic, otential, etc.energies & 4444 wire + m & wire (sce ke e c ( ) 0 en te rate of eat transfer from te wire to te air becomes & mc 50 C Coer wire [ ( t) ] (0.6 kg/m)(0.8 kj/kg. C)(50 50) C 7.7 kj/m. kw 0 m/m
21 Steam at a saturation temerature of sat 40 C condenses on te side of a t orizontal tube. Heat is transferred to te coolg tat enters te tube at 5 C and exits at 5 C. e rate of condensation of steam is to be determed. Assumtions Steady oeratg conditions exist. Water is an comressible substance wit constant roerties at room temerature. e canges ketic and otential energies are negligible. Proerties e roerties of at room temerature are ρ 997 kg/m and c 4.8 kj/kg. C (able A-). e entaly of vaorization of at 40 C is fg kj/kg (able A-4). Analysis e mass flow rate of troug te tube is ρvac (997 kg/m )( m/s)[ π (0.0 m) / 4].409 kg/s akg te volume occuied by te cold te tube as te system, wic is a steady-flow control volume, te energy balance for tis steady-flow system can be exressed te rate form as E 0 & 44 & & 4444 Steam Rate of cange ternal, ketic, 40 C + m & m & otential, etc.energies (sce ke e c ( ) Cold Water 5 C en te rate of eat transfer to te and te rate of condensation become & c ( ) (.409 kg/s)(4.8 kj/kg C)(5 5) C 58.9 kw m cond fg cond fg 58.9 kj/s kj/kg kg/s 5 C 5-75E Saturated steam at a saturation ressure of 0.95 sia and tus at a saturation temerature of sat 00 F (able A-4E) condenses on te er surfaces of 44 orizontal tubes by circulatg coolg arranged a square array. e rate of eat transfer to te coolg and te average velocity of te coolg are to be determed. Assumtions Steady oeratg conditions exist. e tubes are isotermal. Water is an comressible substance wit constant roerties at room temerature. 4 e canges ketic and otential energies are negligible. Proerties e roerties of at room temerature are ρ 6. lbm/ft and c.00 Btu/lbm. F (able A-E). e entaly of vaorization of at a saturation ressure of 0.95 sia is fg 06.7 Btu/lbm (able A-4E). Analysis (a) e rate of eat transfer from te steam to te coolg is equal to te eat of vaorization released as te vaor condenses at te secified temerature, fg (6800 lbm/)(06.7 Btu/lbm) 7,049,560 Btu/ 958 Btu/s Saturated steam 0.95 sia (b) All of tis energy is transferred to te cold. erefore, te mass flow rate of cold must be 958 Btu/s c 44.8 lbm/s c (.00 Btu/lbm. F)(8 F) en te average velocity of te coolg troug te 44 tubes becomes 44.8 lbm/s ρav V ρa ρ( nπd / 4) (6.lbm/ft )[44π (/ ft) / 4] 5.0 ft/s Coolg
300 kpa 77 C. (d) If we neglect kinetic energy in the calculation of energy transport by mass
 6-6- Air flows steadily a ie at a secified state. The diameter of the ie, the rate of flow energy, and the rate of energy transort by mass are to be determed. Also, the error oled the determation of energy
6-6- Air flows steadily a ie at a secified state. The diameter of the ie, the rate of flow energy, and the rate of energy transort by mass are to be determed. Also, the error oled the determation of energy
Chapter 5 MASS AND ENERGY ANALYSIS OF CONTROL VOLUMES
 5- Chapter 5 MASS AND ENERGY ANALYSIS OF CONTROL OLUMES Conseration of Mass 5-C Mass, energy, momentum, and electric charge are consered, and olume and entropy are not consered durg a process. 5-C Mass
5- Chapter 5 MASS AND ENERGY ANALYSIS OF CONTROL OLUMES Conseration of Mass 5-C Mass, energy, momentum, and electric charge are consered, and olume and entropy are not consered durg a process. 5-C Mass
The average velocity of water in the tube and the Reynolds number are Hot R-134a
 hater 0:, 8, 4, 47, 50, 5, 55, 7, 75, 77, 8 and 85. 0- Refrigerant-4a is cooled by water a double-ie heat exchanger. he overall heat transfer coefficient is to be determed. Assumtions he thermal resistance
hater 0:, 8, 4, 47, 50, 5, 55, 7, 75, 77, 8 and 85. 0- Refrigerant-4a is cooled by water a double-ie heat exchanger. he overall heat transfer coefficient is to be determed. Assumtions he thermal resistance
1 = The rate at which the entropy of the high temperature reservoir changes, according to the definition of the entropy, is
 8-7 8-9 A reverible eat um wit eciied reervoir temerature i conidered. e entroy cange o two reervoir i to be calculated and it i to be determed i ti eat um atiie te creae entroy rcile. Aumtion e eat um
8-7 8-9 A reverible eat um wit eciied reervoir temerature i conidered. e entroy cange o two reervoir i to be calculated and it i to be determed i ti eat um atiie te creae entroy rcile. Aumtion e eat um
& out. R-134a 34 C
 5-9 5-76 Saturated refrigerant-4a vapor at a saturation temperature of T sat 4 C condenses side a tube. Te rate of eat transfer from te refrigerant for te condensate exit temperatures of 4 C and 0 C are
5-9 5-76 Saturated refrigerant-4a vapor at a saturation temperature of T sat 4 C condenses side a tube. Te rate of eat transfer from te refrigerant for te condensate exit temperatures of 4 C and 0 C are
(b) The heat transfer can be determined from an energy balance on the system
 8-5 Heat is transferred to a iston-cylinder device wit a set of stos. e work done, te eat transfer, te exergy destroyed, and te second-law efficiency are to be deterined. Assutions e device is stationary
8-5 Heat is transferred to a iston-cylinder device wit a set of stos. e work done, te eat transfer, te exergy destroyed, and te second-law efficiency are to be deterined. Assutions e device is stationary
Kelvin Planck Statement of the Second Law. Clausius Statement of the Second Law
 Kelv Planck Statement of te Second aw It is imossible to construct an enge wic, oeratg a cycle, will roduce no oter effect tan te extraction of eat from a sgle reservoir and te erformance of an equivalent
Kelv Planck Statement of te Second aw It is imossible to construct an enge wic, oeratg a cycle, will roduce no oter effect tan te extraction of eat from a sgle reservoir and te erformance of an equivalent
8-4 P 2. = 12 kw. AIR T = const. Therefore, Q &
 8-4 8-4 Air i compreed teadily by a compreor. e air temperature i mataed contant by eat rejection to te urroundg. e rate o entropy cange o air i to be determed. Aumption i i a teady-low proce ce tere i
8-4 8-4 Air i compreed teadily by a compreor. e air temperature i mataed contant by eat rejection to te urroundg. e rate o entropy cange o air i to be determed. Aumption i i a teady-low proce ce tere i
CHEE 221: Chemical Processes and Systems
 CH 221: Chemical Processes and Systems Module 4. nergy Balances without Reaction Part a: Introduction to nergy Balances (Felder & Rousseau Ch 7.0 7.4 nergy and nergy Balances: very chemical rocess volves
CH 221: Chemical Processes and Systems Module 4. nergy Balances without Reaction Part a: Introduction to nergy Balances (Felder & Rousseau Ch 7.0 7.4 nergy and nergy Balances: very chemical rocess volves
Chapter 9 Practical cycles
 Prof.. undararajan Chater 9 Practical cycles 9. Introduction In Chaters 7 and 8, it was shown that a reversible engine based on the Carnot cycle (two reversible isothermal heat transfers and two reversible
Prof.. undararajan Chater 9 Practical cycles 9. Introduction In Chaters 7 and 8, it was shown that a reversible engine based on the Carnot cycle (two reversible isothermal heat transfers and two reversible
6-5. H 2 O 200 kpa 200 C Q. Entropy Changes of Pure Substances
 Canges f ure Substances 6-0C Yes, because an ternally reversible, adiabatic prcess vlves n irreversibilities r eat transfer. 6- e radiatr f a steam eatg system is itially filled wit supereated steam. e
Canges f ure Substances 6-0C Yes, because an ternally reversible, adiabatic prcess vlves n irreversibilities r eat transfer. 6- e radiatr f a steam eatg system is itially filled wit supereated steam. e
Special Topic: Binary Vapor Cycles
 0- Special opic: Bary Vapor ycle 0- Bary poer cycle i a cycle ic i actually a combation o to cycle; one te ig temperature region, and te oter te lo temperature region. It purpoe i to creae termal eiciency.
0- Special opic: Bary Vapor ycle 0- Bary poer cycle i a cycle ic i actually a combation o to cycle; one te ig temperature region, and te oter te lo temperature region. It purpoe i to creae termal eiciency.
NEWCOMEN ATMOSPHERIC ENGINE
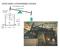 NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m F 7 stroke 5 sia steam 8 50 F water NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m 5 sia steam 8 F 50 F water 7
NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m F 7 stroke 5 sia steam 8 50 F water NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m 5 sia steam 8 F 50 F water 7
Engineering Thermodynamics. Chapter 4. The First Law of Thermodynamics
 Chater 4 The First Law of Thermodynamics It is the law that relates the arious forms of energies for system of different tyes. It is simly the exression of the conseration of energy rcile The first law
Chater 4 The First Law of Thermodynamics It is the law that relates the arious forms of energies for system of different tyes. It is simly the exression of the conseration of energy rcile The first law
4. Energy balances Partly based on Chapter 4 of the De Nevers textbook.
 Lecture Notes CHE 31 Fluid Mechanics (Fall 010) 4 Energy balances Partly based on Chater 4 of the De Nevers textbook Energy fluid mechanics As for any quantity, we can set u an energy balance for a secific
Lecture Notes CHE 31 Fluid Mechanics (Fall 010) 4 Energy balances Partly based on Chater 4 of the De Nevers textbook Energy fluid mechanics As for any quantity, we can set u an energy balance for a secific
Chapters 19 & 20 Heat and the First Law of Thermodynamics
 Capters 19 & 20 Heat and te First Law of Termodynamics Te Zerot Law of Termodynamics Te First Law of Termodynamics Termal Processes Te Second Law of Termodynamics Heat Engines and te Carnot Cycle Refrigerators,
Capters 19 & 20 Heat and te First Law of Termodynamics Te Zerot Law of Termodynamics Te First Law of Termodynamics Termal Processes Te Second Law of Termodynamics Heat Engines and te Carnot Cycle Refrigerators,
T Turbine 8. Boiler fwh fwh I Condenser 4 3 P II P I P III. (a) From the steam tables (Tables A-4, A-5, and A-6), = = 10 MPa. = 0.
 - - A team poer plant operate on an ideal regenerative anke cycle it to open feedater eater. e poer put of te poer plant and te termal efficiency of te cycle are to be determed. Aumption Steady operatg
- - A team poer plant operate on an ideal regenerative anke cycle it to open feedater eater. e poer put of te poer plant and te termal efficiency of te cycle are to be determed. Aumption Steady operatg
Delft University of Technology DEPARTMENT OF AEROSPACE ENGINEERING
 Delft University of Technology DEPRTMENT OF EROSPCE ENGINEERING Course: Physics I (E-04) Course year: Date: 7-0-0 Time: 4:00-7:00 Student name and itials (capital letters): Student number:. You have attended
Delft University of Technology DEPRTMENT OF EROSPCE ENGINEERING Course: Physics I (E-04) Course year: Date: 7-0-0 Time: 4:00-7:00 Student name and itials (capital letters): Student number:. You have attended
SIMPLE RANKINE CYCLE. 3 expander. boiler. pump. condenser 1 W Q. cycle cycle. net. shaft
 SIMPLE RANKINE CYCLE um boiler exander condener Steady Flow, Oen Sytem - region ace Steady Flow Energy Equation for Procee m (u Pum Proce,, Boiler Proce,, V ρg) 0, 0, Exanion Proce,, 0, Condener Proce,,
SIMPLE RANKINE CYCLE um boiler exander condener Steady Flow, Oen Sytem - region ace Steady Flow Energy Equation for Procee m (u Pum Proce,, Boiler Proce,, V ρg) 0, 0, Exanion Proce,, 0, Condener Proce,,
( )( ) 7 MPa q in = = 10 kpa q out. 1 h. = s. Thus, and = 38.9% (b) (c) The rate of heat rejection to the cooling water and its temperature rise are
 . A team poer plant operate on a imple ideal Ranke cycle beteen te peciied preure limit. e termal eiciency o te cycle, te ma lo rate o te team, and te temperature rie o te coolg ater are to be determed.
. A team poer plant operate on a imple ideal Ranke cycle beteen te peciied preure limit. e termal eiciency o te cycle, te ma lo rate o te team, and te temperature rie o te coolg ater are to be determed.
Thermodynamics Lecture Series
 Termodynamics Lecture Series Ideal Ranke Cycle Te Practical Cycle Applied Sciences Education Researc Group (ASERG) Faculty of Applied Sciences Universiti Teknologi MARA email: drjjlanita@otmail.com ttp://www5.uitm.edu.my/faculties/fsg/drjj1.tml
Termodynamics Lecture Series Ideal Ranke Cycle Te Practical Cycle Applied Sciences Education Researc Group (ASERG) Faculty of Applied Sciences Universiti Teknologi MARA email: drjjlanita@otmail.com ttp://www5.uitm.edu.my/faculties/fsg/drjj1.tml
Section A 01. (12 M) (s 2 s 3 ) = 313 s 2 = s 1, h 3 = h 4 (s 1 s 3 ) = kj/kgk. = kj/kgk. 313 (s 3 s 4f ) = ln
 0. (a) Sol: Section A A refrigerator macine uses R- as te working fluid. Te temperature of R- in te evaporator coil is 5C, and te gas leaves te compressor as dry saturated at a temperature of 40C. Te mean
0. (a) Sol: Section A A refrigerator macine uses R- as te working fluid. Te temperature of R- in te evaporator coil is 5C, and te gas leaves te compressor as dry saturated at a temperature of 40C. Te mean
Chapter 5. Mass and Energy Analysis of Control Volumes
 Chapter 5 Mass and Energy Analysis of Control Volumes Conservation Principles for Control volumes The conservation of mass and the conservation of energy principles for open systems (or control volumes)
Chapter 5 Mass and Energy Analysis of Control Volumes Conservation Principles for Control volumes The conservation of mass and the conservation of energy principles for open systems (or control volumes)
Chapter 2 Solutions. 2.1 (D) Pressure and temperature are dependent during phase change and independent when in a single phase.
 Chater Solutions.1 (D) Pressure and temerature are deendent during hase change and indeendent when in a single hase.. (B) Sublimation is the direct conversion of a solid to a gas. To observe this rocess,
Chater Solutions.1 (D) Pressure and temerature are deendent during hase change and indeendent when in a single hase.. (B) Sublimation is the direct conversion of a solid to a gas. To observe this rocess,
v v = = Mixing chamber: = 30 or, = s6 Then, and = 52.4% Turbine Boiler process heater Condenser 7 MPa Q in 0.6 MPa Q proces 10 kpa Q out
 0-0- A cogeneration plant i to generate poer and proce eat. art o te team extracted rom te turbe at a relatively ig preure i ued or proce eatg. e poer produced and te utilization actor o te plant are to
0-0- A cogeneration plant i to generate poer and proce eat. art o te team extracted rom te turbe at a relatively ig preure i ued or proce eatg. e poer produced and te utilization actor o te plant are to
SPC 407 Sheet 6 - Solution Compressible Flow Fanno Flow
 SPC 407 Sheet 6 - Solution Comressible Flow Fanno Flow 1. What is the effect of friction on flow velocity in subsonic and suersonic Fanno flow? Friction increases the flow velocity in subsonic Fanno flow,
SPC 407 Sheet 6 - Solution Comressible Flow Fanno Flow 1. What is the effect of friction on flow velocity in subsonic and suersonic Fanno flow? Friction increases the flow velocity in subsonic Fanno flow,
Chapter 1 Fundamentals
 Chater Fundamentals. Overview of Thermodynamics Industrial Revolution brought in large scale automation of many tedious tasks which were earlier being erformed through manual or animal labour. Inventors
Chater Fundamentals. Overview of Thermodynamics Industrial Revolution brought in large scale automation of many tedious tasks which were earlier being erformed through manual or animal labour. Inventors
Chapter 5. Mass and Energy Analysis of Control Volumes. by Asst. Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn
 Chapter 5 Mass and Energy Analysis of Control Volumes by Asst. Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn Reference: Cengel, Yunus A. and Michael A. Boles, Thermodynamics:
Chapter 5 Mass and Energy Analysis of Control Volumes by Asst. Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn Reference: Cengel, Yunus A. and Michael A. Boles, Thermodynamics:
Department of Civil Engineering & Applied Mechanics McGill University, Montreal, Quebec Canada
 Department f Ciil ngeerg Applied Mechanics McGill Uniersity, Mntreal, Quebec Canada CI 90 THRMODYNAMICS HAT TRANSFR Assignment #4 SOLUTIONS. A 68-kg man whse aerage bdy temperature is 9 C drks L f cld
Department f Ciil ngeerg Applied Mechanics McGill Uniersity, Mntreal, Quebec Canada CI 90 THRMODYNAMICS HAT TRANSFR Assignment #4 SOLUTIONS. A 68-kg man whse aerage bdy temperature is 9 C drks L f cld
The First Law of Thermodynamics. By: Yidnekachew Messele
 The First Law of Thermodynamics By: Yidnekachew Messele It is the law that relates the various forms of energies for system of different types. It is simply the expression of the conservation of energy
The First Law of Thermodynamics By: Yidnekachew Messele It is the law that relates the various forms of energies for system of different types. It is simply the expression of the conservation of energy
Unit code: H/ QCF level: 5 Credit value: 15 OUTCOME 1 - THERMODYNAMIC SYSTEMS TUTORIAL 2
 Unit 43: Plant and Process Princiles Unit code: H/60 44 QCF level: 5 Credit value: 5 OUCOME - HERMODYNAMIC SYSEMS UORIAL Understand thermodynamic systems as alied to lant engineering rocesses hermodynamic
Unit 43: Plant and Process Princiles Unit code: H/60 44 QCF level: 5 Credit value: 5 OUCOME - HERMODYNAMIC SYSEMS UORIAL Understand thermodynamic systems as alied to lant engineering rocesses hermodynamic
CHAPTER 5 MASS AND ENERGY ANALYSIS OF CONTROL VOLUMES
 Thermodynamics: An Engineering Approach 8th Edition in SI Units Yunus A. Çengel, Michael A. Boles McGraw-Hill, 2015 CHAPTER 5 MASS AND ENERGY ANALYSIS OF CONTROL VOLUMES Lecture slides by Dr. Fawzi Elfghi
Thermodynamics: An Engineering Approach 8th Edition in SI Units Yunus A. Çengel, Michael A. Boles McGraw-Hill, 2015 CHAPTER 5 MASS AND ENERGY ANALYSIS OF CONTROL VOLUMES Lecture slides by Dr. Fawzi Elfghi
Solutions to Homework #10
 Solution to Homeork #0 0-6 A teady-lo Carnot enge it ater a te orkg luid operate at peciied condition. e termal eiciency, te preure at te turbe let, and te ork put are to be determed. Aumption Steady operatg
Solution to Homeork #0 0-6 A teady-lo Carnot enge it ater a te orkg luid operate at peciied condition. e termal eiciency, te preure at te turbe let, and te ork put are to be determed. Aumption Steady operatg
Notes on the function gsw_enthalpy_first_derivatives_ct_exact(sa,ct,p)
 Notes on gsw_entaly_first_derivatives_c_exact 1 Notes on te function gsw_entaly_first_derivatives_c_exact(c) is function gsw_entaly_first_derivatives_c_exact(c) evaluates two of te first order artial derivatives
Notes on gsw_entaly_first_derivatives_c_exact 1 Notes on te function gsw_entaly_first_derivatives_c_exact(c) is function gsw_entaly_first_derivatives_c_exact(c) evaluates two of te first order artial derivatives
Lecture 38: Vapor-compression refrigeration systems
 ME 200 Termodynamics I Lecture 38: Vapor-compression refrigeration systems Yong Li Sangai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Cuan Road Sangai, 200240, P. R. Cina Email
ME 200 Termodynamics I Lecture 38: Vapor-compression refrigeration systems Yong Li Sangai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Cuan Road Sangai, 200240, P. R. Cina Email
Notes: Most of the material in this chapter is taken from Young and Freedman, Chap. 12.
 Capter 6. Fluid Mecanics Notes: Most of te material in tis capter is taken from Young and Freedman, Cap. 12. 6.1 Fluid Statics Fluids, i.e., substances tat can flow, are te subjects of tis capter. But
Capter 6. Fluid Mecanics Notes: Most of te material in tis capter is taken from Young and Freedman, Cap. 12. 6.1 Fluid Statics Fluids, i.e., substances tat can flow, are te subjects of tis capter. But
 CHAPTER INTRODUCTION AND BASIC PRINCIPLES. (Tutorial). Determine if the following properties of the system are intensive or extensive properties: Property Intensive Extensive Volume Density Conductivity
CHAPTER INTRODUCTION AND BASIC PRINCIPLES. (Tutorial). Determine if the following properties of the system are intensive or extensive properties: Property Intensive Extensive Volume Density Conductivity
Basics in Process Design. Frej Bjondahl 2006
 Basics Process Design Frej Bjondahl 006 Contents Basics Process Design... Contents.... Introduction...3. Design/analysis of rocess systems usg mass and energy balances...3. Balance boundary, ut, ut, recirculation...4.
Basics Process Design Frej Bjondahl 006 Contents Basics Process Design... Contents.... Introduction...3. Design/analysis of rocess systems usg mass and energy balances...3. Balance boundary, ut, ut, recirculation...4.
the first derivative with respect to time is obtained by carefully applying the chain rule ( surf init ) T Tinit
 .005 ermal Fluids Engineering I Fall`08 roblem Set 8 Solutions roblem ( ( a e -D eat equation is α t x d erfc( u du π x, 4αt te first derivative wit respect to time is obtained by carefully applying te
.005 ermal Fluids Engineering I Fall`08 roblem Set 8 Solutions roblem ( ( a e -D eat equation is α t x d erfc( u du π x, 4αt te first derivative wit respect to time is obtained by carefully applying te
Phase transition. Asaf Pe er Background
 Phase transition Asaf Pe er 1 November 18, 2013 1. Background A hase is a region of sace, throughout which all hysical roerties (density, magnetization, etc.) of a material (or thermodynamic system) are
Phase transition Asaf Pe er 1 November 18, 2013 1. Background A hase is a region of sace, throughout which all hysical roerties (density, magnetization, etc.) of a material (or thermodynamic system) are
First Name Last Name CIRCLE YOUR LECTURE BELOW: Div. 1 10:30 am Div. 2 2:30 pm Div. 3 4:30 pm Prof. Gore Prof. Udupa Prof. Chen
 CIRCLE YOUR LECURE BELOW: Div. 1 10:30 am Div. :30 m Div. 3 4:30 m Prof. Gore Prof. Udua Prof. Chen EXAM # 3 INSRUCIONS 1. his is a closed book examination. All needed roerty tables are rovided.. Do not
CIRCLE YOUR LECURE BELOW: Div. 1 10:30 am Div. :30 m Div. 3 4:30 m Prof. Gore Prof. Udua Prof. Chen EXAM # 3 INSRUCIONS 1. his is a closed book examination. All needed roerty tables are rovided.. Do not
2-21. for gage pressure, the high and low pressures are expressed as. Noting that 1 psi = kpa,
 - -58E The systolic and diastolic pressures of a healthy person are given in mmhg. These pressures are to be expressed in kpa, psi, and meter water column. Assumptions Both mercury and water are incompressible
- -58E The systolic and diastolic pressures of a healthy person are given in mmhg. These pressures are to be expressed in kpa, psi, and meter water column. Assumptions Both mercury and water are incompressible
= 0, no work has been added
 hater Practice Problems (P1) Enery Balance: Eqn 7 d u z u z u z m U m m Q + + + + + + & + + & lets lets E + S (P) closed system, no mass flow m & m& 0, valve stuck revent any steam from o m& 0, no chane
hater Practice Problems (P1) Enery Balance: Eqn 7 d u z u z u z m U m m Q + + + + + + & + + & lets lets E + S (P) closed system, no mass flow m & m& 0, valve stuck revent any steam from o m& 0, no chane
ENT 254: Applied Thermodynamics
 ENT 54: Applied Thermodynamics Mr. Azizul bin Mohamad Mechanical Engineering Program School of Mechatronic Engineering Universiti Malaysia Perlis (UniMAP) azizul@unimap.edu.my 019-4747351 04-9798679 Chapter
ENT 54: Applied Thermodynamics Mr. Azizul bin Mohamad Mechanical Engineering Program School of Mechatronic Engineering Universiti Malaysia Perlis (UniMAP) azizul@unimap.edu.my 019-4747351 04-9798679 Chapter
ENERGY TRANSFER BY WORK: Electrical Work: When N Coulombs of electrical charge move through a potential difference V
 Weight, W = mg Where m=mass, g=gravitational acceleration ENERGY TRANSFER BY WOR: Sign convention: Work done on a system = (+) Work done by a system = (-) Density, ρ = m V kg m 3 Where m=mass, V =Volume
Weight, W = mg Where m=mass, g=gravitational acceleration ENERGY TRANSFER BY WOR: Sign convention: Work done on a system = (+) Work done by a system = (-) Density, ρ = m V kg m 3 Where m=mass, V =Volume
Chapter 5: The First Law of Thermodynamics: Closed Systems
 Chapter 5: The First Law of Thermodynamics: Closed Systems The first law of thermodynamics can be simply stated as follows: during an interaction between a system and its surroundings, the amount of energy
Chapter 5: The First Law of Thermodynamics: Closed Systems The first law of thermodynamics can be simply stated as follows: during an interaction between a system and its surroundings, the amount of energy
Readings for this homework assignment and upcoming lectures
 Homework #3 (group) Tuesday, February 13 by 4:00 pm 5290 exercises (individual) Thursday, February 15 by 4:00 pm extra credit (individual) Thursday, February 15 by 4:00 pm Readings for this homework assignment
Homework #3 (group) Tuesday, February 13 by 4:00 pm 5290 exercises (individual) Thursday, February 15 by 4:00 pm extra credit (individual) Thursday, February 15 by 4:00 pm Readings for this homework assignment
Chapter 1: 20, 23, 35, 41, 68, 71, 76, 77, 80, 85, 90, 101, 103 and 104.
 Chapter 1: 0, 3, 35, 1, 68, 71, 76, 77, 80, 85, 90, 101, 103 and 10. 1-0 The filament of a 150 W incandescent lamp is 5 cm long and has a diameter of 0.5 mm. The heat flux on the surface of the filament,
Chapter 1: 0, 3, 35, 1, 68, 71, 76, 77, 80, 85, 90, 101, 103 and 10. 1-0 The filament of a 150 W incandescent lamp is 5 cm long and has a diameter of 0.5 mm. The heat flux on the surface of the filament,
Chapter 5 Mass, Bernoulli, and Energy Equations Chapter 5 MASS, BERNOULLI, AND ENERGY EQUATIONS
 Chapter 5 MASS, BERNOULLI, AND ENERGY EQUATIONS Conservation of Mass 5-C Mass, energy, momentum, and electric charge are conserved, and volume and entropy are not conserved during a process. 5-C Mass flow
Chapter 5 MASS, BERNOULLI, AND ENERGY EQUATIONS Conservation of Mass 5-C Mass, energy, momentum, and electric charge are conserved, and volume and entropy are not conserved during a process. 5-C Mass flow
Liquid water static energy page 1/8
 Liquid water static energy age 1/8 1) Thermodynamics It s a good idea to work with thermodynamic variables that are conserved under a known set of conditions, since they can act as assive tracers and rovide
Liquid water static energy age 1/8 1) Thermodynamics It s a good idea to work with thermodynamic variables that are conserved under a known set of conditions, since they can act as assive tracers and rovide
Actual exergy intake to perform the same task
 CHAPER : PRINCIPLES OF ENERGY CONSERVAION INRODUCION Energy conservation rinciles are based on thermodynamics If we look into the simle and most direct statement of the first law of thermodynamics, we
CHAPER : PRINCIPLES OF ENERGY CONSERVAION INRODUCION Energy conservation rinciles are based on thermodynamics If we look into the simle and most direct statement of the first law of thermodynamics, we
Chapter 11: Heat Exchangers. Dr Ali Jawarneh Department of Mechanical Engineering Hashemite University
 Chapter 11: Heat Exchangers Dr Ali Jawarneh Department of Mechanical Engineering Hashemite University Objectives When you finish studying this chapter, you should be able to: Recognize numerous types of
Chapter 11: Heat Exchangers Dr Ali Jawarneh Department of Mechanical Engineering Hashemite University Objectives When you finish studying this chapter, you should be able to: Recognize numerous types of
Engineering Services Examination - UPSC MECHANICAL ENGINEERING. Topic-wise Conventional Papers I & II (Last 30 Years Questions)
 Engineering Services Examination - UPSC MECHANICAL ENGINEERING Toic-wise Conventional Paers I & II (Last 0 Years Questions) Dedicated to Mechanical Engineers and Engineering Services asirants. 04 by Engineers
Engineering Services Examination - UPSC MECHANICAL ENGINEERING Toic-wise Conventional Paers I & II (Last 0 Years Questions) Dedicated to Mechanical Engineers and Engineering Services asirants. 04 by Engineers
Physics 2A (Fall 2012) Chapters 11:Using Energy and 12: Thermal Properties of Matter
 Physics 2A (Fall 2012) Chaters 11:Using Energy and 12: Thermal Proerties of Matter "Kee in mind that neither success nor failure is ever final." Roger Ward Babson Our greatest glory is not in never failing,
Physics 2A (Fall 2012) Chaters 11:Using Energy and 12: Thermal Proerties of Matter "Kee in mind that neither success nor failure is ever final." Roger Ward Babson Our greatest glory is not in never failing,
Available online at ScienceDirect. Procedia Engineering 153 (2016 )
 Available onle at www.sciencedirect.com ScienceDirect Procedia Engeerg 153 (2016 ) 785 790 XXV Polis Russian Slovak Semar Teoretical Foundation of Civil Engeerg Te tecnology of automatic control of eat
Available onle at www.sciencedirect.com ScienceDirect Procedia Engeerg 153 (2016 ) 785 790 XXV Polis Russian Slovak Semar Teoretical Foundation of Civil Engeerg Te tecnology of automatic control of eat
Thermodynamics Introduction and Basic Concepts
 Thermodynamics Introduction and Basic Concepts by Asst. Prof. Channarong Asavatesanupap Mechanical Engineering Department Faculty of Engineering Thammasat University 2 What is Thermodynamics? Thermodynamics
Thermodynamics Introduction and Basic Concepts by Asst. Prof. Channarong Asavatesanupap Mechanical Engineering Department Faculty of Engineering Thammasat University 2 What is Thermodynamics? Thermodynamics
SEM-2017(03HI MECHANICAL ENGINEERING. Paper II. Please read each of the following instructions carefully before attempting questions.
 We RoU No. 700095 Candidate should write his/her Roll No. here. Total No. of Questions : 7 No. of Printed Pages : 7 SEM-2017(03HI MECHANICAL ENGINEERING Paper II Time ; 3 Hours ] [ Total Marks : 0 Instructions
We RoU No. 700095 Candidate should write his/her Roll No. here. Total No. of Questions : 7 No. of Printed Pages : 7 SEM-2017(03HI MECHANICAL ENGINEERING Paper II Time ; 3 Hours ] [ Total Marks : 0 Instructions
HEAT, WORK, AND THE FIRST LAW OF THERMODYNAMICS
 HET, ORK, ND THE FIRST L OF THERMODYNMIS 8 EXERISES Section 8. The First Law of Thermodynamics 5. INTERPRET e identify the system as the water in the insulated container. The roblem involves calculating
HET, ORK, ND THE FIRST L OF THERMODYNMIS 8 EXERISES Section 8. The First Law of Thermodynamics 5. INTERPRET e identify the system as the water in the insulated container. The roblem involves calculating
10 CV 35 FLUID MECHANICS NOTES UNIT-2 PRESSURE AND ITS MEASUREMENT. Dr. Nagaraj Sitaram, Professor, Civil Department, SBMJCE, Bangalore
 10 CV 35 FLUID MECHNICS NOTES UNIT-2 PRESSURE ND ITS MESUREMENT by UNIT-2 PRESSURE ND ITS MESUREMENT 2.0 INTRODUCTION: Fluid is a state of matter wic exibits te roerty of flow. Wen a certain mass of fluids
10 CV 35 FLUID MECHNICS NOTES UNIT-2 PRESSURE ND ITS MESUREMENT by UNIT-2 PRESSURE ND ITS MESUREMENT 2.0 INTRODUCTION: Fluid is a state of matter wic exibits te roerty of flow. Wen a certain mass of fluids
first law of ThermodyNamics
 first law of ThermodyNamics First law of thermodynamics - Principle of conservation of energy - Energy can be neither created nor destroyed Basic statement When any closed system is taken through a cycle,
first law of ThermodyNamics First law of thermodynamics - Principle of conservation of energy - Energy can be neither created nor destroyed Basic statement When any closed system is taken through a cycle,
1 st Law Analysis of Control Volume (open system) Chapter 6
 1 st Law Analysis of Control Volume (open system) Chapter 6 In chapter 5, we did 1st law analysis for a control mass (closed system). In this chapter the analysis of the 1st law will be on a control volume
1 st Law Analysis of Control Volume (open system) Chapter 6 In chapter 5, we did 1st law analysis for a control mass (closed system). In this chapter the analysis of the 1st law will be on a control volume
Theory of turbomachinery. Chapter 1
 Theory of turbomachinery Chater Introduction: Basic Princiles Take your choice of those that can best aid your action. (Shakeseare, Coriolanus) Introduction Definition Turbomachinery describes machines
Theory of turbomachinery Chater Introduction: Basic Princiles Take your choice of those that can best aid your action. (Shakeseare, Coriolanus) Introduction Definition Turbomachinery describes machines
02. Equilibrium Thermodynamics II: Engines
 University of Rhode Island DigitalCommons@URI Equilibrium Statistical Physics Physics Course Materials 205 02. Equilibrium Thermodynamics II: Engines Gerhard Müller University of Rhode Island, gmuller@uri.edu
University of Rhode Island DigitalCommons@URI Equilibrium Statistical Physics Physics Course Materials 205 02. Equilibrium Thermodynamics II: Engines Gerhard Müller University of Rhode Island, gmuller@uri.edu
Chapter 8 EXERGY A MEASURE OF WORK POTENTIAL
 8- Chapter 8 EXERGY A MEAURE OF ORK POENIAL Exergy, Irreveribility, Reverible ork, and econd-law Efficiency 8-C Reverible work differ from the ueful work by irreveribilitie. For reverible procee both are
8- Chapter 8 EXERGY A MEAURE OF ORK POENIAL Exergy, Irreveribility, Reverible ork, and econd-law Efficiency 8-C Reverible work differ from the ueful work by irreveribilitie. For reverible procee both are
Simulation and verification of a plate heat exchanger with a built-in tap water accumulator
 Simulation and verification of a plate eat excanger wit a built-in tap water accumulator Anders Eriksson Abstract In order to test and verify a compact brazed eat excanger (CBE wit a built-in accumulation
Simulation and verification of a plate eat excanger wit a built-in tap water accumulator Anders Eriksson Abstract In order to test and verify a compact brazed eat excanger (CBE wit a built-in accumulation
Physics 207 Lecture 23
 ysics 07 Lecture ysics 07, Lecture 8, Dec. Agenda:. Finis, Start. Ideal gas at te molecular level, Internal Energy Molar Specific Heat ( = m c = n ) Ideal Molar Heat apacity (and U int = + W) onstant :
ysics 07 Lecture ysics 07, Lecture 8, Dec. Agenda:. Finis, Start. Ideal gas at te molecular level, Internal Energy Molar Specific Heat ( = m c = n ) Ideal Molar Heat apacity (and U int = + W) onstant :
Fluids and Buoyancy. 1. What will happen to the scale reading as the mass is lowered?
 Fluids and Buoyancy. Wat will appen to te scale reading as te mass is lowered? M Using rcimedes Principle: any body fully or partially submerged in a fluid is buoyed up by a force equal to te weigt of
Fluids and Buoyancy. Wat will appen to te scale reading as te mass is lowered? M Using rcimedes Principle: any body fully or partially submerged in a fluid is buoyed up by a force equal to te weigt of
= T. (kj/k) (kj/k) 0 (kj/k) int rev. Chapter 6 SUMMARY
 Capter 6 SUMMARY e second la of termodynamics leads to te definition of a ne property called entropy ic is a quantitative measure of microscopic disorder for a system. e definition of entropy is based
Capter 6 SUMMARY e second la of termodynamics leads to te definition of a ne property called entropy ic is a quantitative measure of microscopic disorder for a system. e definition of entropy is based
Thermodynamics Lecture Series
 Thermodynamics Lecture Series Second Law uality of Energy Applied Sciences Education Research Group (ASERG) Faculty of Applied Sciences Universiti Teknologi MARA email: drjjlanita@hotmail.com http://www.uitm.edu.my/faculties/fsg/drjj.html
Thermodynamics Lecture Series Second Law uality of Energy Applied Sciences Education Research Group (ASERG) Faculty of Applied Sciences Universiti Teknologi MARA email: drjjlanita@hotmail.com http://www.uitm.edu.my/faculties/fsg/drjj.html
THE FIRST LAW OF THERMODYNAMICS
 THE FIRST LA OF THERMODYNAMIS 9 9 (a) IDENTIFY and SET UP: The ressure is constant and the volume increases (b) = d Figure 9 Since is constant, = d = ( ) The -diagram is sketched in Figure 9 The roblem
THE FIRST LA OF THERMODYNAMIS 9 9 (a) IDENTIFY and SET UP: The ressure is constant and the volume increases (b) = d Figure 9 Since is constant, = d = ( ) The -diagram is sketched in Figure 9 The roblem
Chapter 20: Exercises: 3, 7, 11, 22, 28, 34 EOC: 40, 43, 46, 58
 Chater 0: Exercises:, 7,,, 8, 4 EOC: 40, 4, 46, 8 E: A gasoline engine takes in.80 0 4 and delivers 800 of work er cycle. The heat is obtained by burning gasoline with a heat of combustion of 4.60 0 4.
Chater 0: Exercises:, 7,,, 8, 4 EOC: 40, 4, 46, 8 E: A gasoline engine takes in.80 0 4 and delivers 800 of work er cycle. The heat is obtained by burning gasoline with a heat of combustion of 4.60 0 4.
The extreme case of the anisothermal calorimeter when there is no heat exchange is the adiabatic calorimeter.
 .4. Determination of the enthaly of solution of anhydrous and hydrous sodium acetate by anisothermal calorimeter, and the enthaly of melting of ice by isothermal heat flow calorimeter Theoretical background
.4. Determination of the enthaly of solution of anhydrous and hydrous sodium acetate by anisothermal calorimeter, and the enthaly of melting of ice by isothermal heat flow calorimeter Theoretical background
High speed wind tunnels 2.0 Definition of high speed. 2.1 Types of high speed wind tunnels
 Module Lectures 6 to 1 High Seed Wind Tunnels Keywords: Blow down wind tunnels, Indraft wind tunnels, suersonic wind tunnels, c-d nozzles, second throat diffuser, shocks, condensation in wind tunnels,
Module Lectures 6 to 1 High Seed Wind Tunnels Keywords: Blow down wind tunnels, Indraft wind tunnels, suersonic wind tunnels, c-d nozzles, second throat diffuser, shocks, condensation in wind tunnels,
EF 152 Exam #3, Fall, 2012 Page 1 of 6
 EF 5 Exam #3, Fall, 0 Page of 6 Name: Setion: Guidelines: ssume 3 signifiant figures for all given numbers. Sow all of your work no work, no redit Write your final answer in te box provided - inlude units
EF 5 Exam #3, Fall, 0 Page of 6 Name: Setion: Guidelines: ssume 3 signifiant figures for all given numbers. Sow all of your work no work, no redit Write your final answer in te box provided - inlude units
Chapter 7 Energy Principle
 Chater 7: Energy Princile By Dr Ali Jawarneh Hashemite University Outline In this chater we will: Derive and analyse the Energy equation. Analyse the flow and shaft work. Derive the equation for steady
Chater 7: Energy Princile By Dr Ali Jawarneh Hashemite University Outline In this chater we will: Derive and analyse the Energy equation. Analyse the flow and shaft work. Derive the equation for steady
AE301 Aerodynamics I UNIT A: Fundamental Concepts
 AE301 Aerodynamics I UNIT A: Fundamental Concets ROAD MAP... A-1: Engineering Fundamentals Reiew A-: Standard Atmoshere A-3: Goerning Equations of Aerodynamics A-4: Airseed Measurements A-5: Aerodynamic
AE301 Aerodynamics I UNIT A: Fundamental Concets ROAD MAP... A-1: Engineering Fundamentals Reiew A-: Standard Atmoshere A-3: Goerning Equations of Aerodynamics A-4: Airseed Measurements A-5: Aerodynamic
CHAPTER 7 ENTROPY. Copyright Hany A. Al-Ansary and S. I. Abdel-Khalik (2014) 1
 CHAPTER 7 ENTROPY S. I. Abdel-Khalik (2014) 1 ENTROPY The Clausius Inequality The Clausius inequality states that for for all cycles, reversible or irreversible, engines or refrigerators: For internally-reversible
CHAPTER 7 ENTROPY S. I. Abdel-Khalik (2014) 1 ENTROPY The Clausius Inequality The Clausius inequality states that for for all cycles, reversible or irreversible, engines or refrigerators: For internally-reversible
Announcements. Exam 4 - Review of important concepts
 Announcements 1. Exam 4 starts Friday! a. Available in esting Center from Friday, Dec 7 (opening time), up to Monday, Dec 10 at 4:00 pm. i. Late fee if you start your exam after 4 pm b. Covers C. 9-1 (up
Announcements 1. Exam 4 starts Friday! a. Available in esting Center from Friday, Dec 7 (opening time), up to Monday, Dec 10 at 4:00 pm. i. Late fee if you start your exam after 4 pm b. Covers C. 9-1 (up
Chapter 4 Practice Problems
 Chater 4 ractice roblems (4.) (a) the number of microstates is N (b) 3 articles total 3! {, } 3 number microstates of secific arrangement (macrostate)!*! robability = (# microstates of secific arrangement)/(total
Chater 4 ractice roblems (4.) (a) the number of microstates is N (b) 3 articles total 3! {, } 3 number microstates of secific arrangement (macrostate)!*! robability = (# microstates of secific arrangement)/(total
ME Thermodynamics I. Lecture Notes and Example Problems
 ME 227.3 Thermodynamics I Lecture Notes and Example Problems James D. Bugg September 2018 Department of Mechanical Engineering Introduction Part I: Lecture Notes This part contains handout versions of
ME 227.3 Thermodynamics I Lecture Notes and Example Problems James D. Bugg September 2018 Department of Mechanical Engineering Introduction Part I: Lecture Notes This part contains handout versions of
Entropy and the Second Law of Thermodynamics
 Entropy and the Second Law of Thermodynamics Reading Problems 7-1 7-3 7-88, 7-131, 7-135 7-6 7-10 8-24, 8-44, 8-46, 8-60, 8-73, 8-99, 8-128, 8-132, 8-1 8-10, 8-13 8-135, 8-148, 8-152, 8-166, 8-168, 8-189
Entropy and the Second Law of Thermodynamics Reading Problems 7-1 7-3 7-88, 7-131, 7-135 7-6 7-10 8-24, 8-44, 8-46, 8-60, 8-73, 8-99, 8-128, 8-132, 8-1 8-10, 8-13 8-135, 8-148, 8-152, 8-166, 8-168, 8-189
Today lecture. 1. Entropy change in an isolated system 2. Exergy
 Today lecture 1. Entropy change in an isolated system. Exergy - What is exergy? - Reversible Work & Irreversibility - Second-Law Efficiency - Exergy change of a system For a fixed mass For a flow stream
Today lecture 1. Entropy change in an isolated system. Exergy - What is exergy? - Reversible Work & Irreversibility - Second-Law Efficiency - Exergy change of a system For a fixed mass For a flow stream
Chapter 1. Introduction. Introduction to Heat Transfer
 Chapter 1 Introduction to Heat Transfer Islamic Azad University Karaj Branch Dr. M. Khosravy 1 Introduction Thermodynamics: Energy can be transferred between a system and its surroundgs. A system teracts
Chapter 1 Introduction to Heat Transfer Islamic Azad University Karaj Branch Dr. M. Khosravy 1 Introduction Thermodynamics: Energy can be transferred between a system and its surroundgs. A system teracts
Carnot Factor of a Vapour Power Cycle with Regenerative Extraction
 Journal of Modern Pysics, 2017, 8, 1795-1808 ttp://www.scirp.org/journal/jmp ISSN Online: 2153-120X ISSN Print: 2153-1196 arnot Factor of a Vapour Power ycle wit Regenerative Extraction Duparquet Alain
Journal of Modern Pysics, 2017, 8, 1795-1808 ttp://www.scirp.org/journal/jmp ISSN Online: 2153-120X ISSN Print: 2153-1196 arnot Factor of a Vapour Power ycle wit Regenerative Extraction Duparquet Alain
ME 200 Thermodynamics 1 Fall 2017 Exam 3
 ME 200 hermodynamics 1 Fall 2017 Exam Circle your structor s last name Division 1: Naik Division : Wassgren Division 6: Braun Division 2: Sojka Division 4: Goldenste Division 7: Buckius Division 8: Meyer
ME 200 hermodynamics 1 Fall 2017 Exam Circle your structor s last name Division 1: Naik Division : Wassgren Division 6: Braun Division 2: Sojka Division 4: Goldenste Division 7: Buckius Division 8: Meyer
1 Power is transferred through a machine as shown. power input P I machine. power output P O. power loss P L. What is the efficiency of the machine?
 1 1 Power is transferred troug a macine as sown. power input P I macine power output P O power loss P L Wat is te efficiency of te macine? P I P L P P P O + P L I O P L P O P I 2 ir in a bicycle pump is
1 1 Power is transferred troug a macine as sown. power input P I macine power output P O power loss P L Wat is te efficiency of te macine? P I P L P P P O + P L I O P L P O P I 2 ir in a bicycle pump is
dn i where we have used the Gibbs equation for the Gibbs energy and the definition of chemical potential
 Chem 467 Sulement to Lectures 33 Phase Equilibrium Chemical Potential Revisited We introduced the chemical otential as the conjugate variable to amount. Briefly reviewing, the total Gibbs energy of a system
Chem 467 Sulement to Lectures 33 Phase Equilibrium Chemical Potential Revisited We introduced the chemical otential as the conjugate variable to amount. Briefly reviewing, the total Gibbs energy of a system
Analysis: The speed of the proton is much less than light speed, so we can use the
 Section 1.3: Wave Proerties of Classical Particles Tutorial 1 Practice, age 634 1. Given: 1.8! 10 "5 kg # m/s; 6.63! 10 "34 J #s Analysis: Use te de Broglie relation, λ. Solution:! 6.63 " 10#34 kg $ m
Section 1.3: Wave Proerties of Classical Particles Tutorial 1 Practice, age 634 1. Given: 1.8! 10 "5 kg # m/s; 6.63! 10 "34 J #s Analysis: Use te de Broglie relation, λ. Solution:! 6.63 " 10#34 kg $ m
GEF2200 vår 2017 Løsningsforslag sett 1
 GEF2200 vår 2017 Løsningsforslag sett 1 A.1.T R is the universal gas constant, with value 8.3143JK 1 mol 1. R is the gas constant for a secic gas, given by R R M (1) where M is the molecular weight of
GEF2200 vår 2017 Løsningsforslag sett 1 A.1.T R is the universal gas constant, with value 8.3143JK 1 mol 1. R is the gas constant for a secic gas, given by R R M (1) where M is the molecular weight of
c Dr. Md. Zahurul Haq (BUET) Thermodynamic Processes & Efficiency ME 6101 (2017) 2 / 25 T145 = Q + W cv + i h 2 = h (V2 1 V 2 2)
 Thermodynamic Processes & Isentropic Efficiency Dr. Md. Zahurul Haq Professor Department of Mechanical Engineering Bangladesh University of Engineering & Technology (BUET Dhaka-1000, Bangladesh zahurul@me.buet.ac.bd
Thermodynamic Processes & Isentropic Efficiency Dr. Md. Zahurul Haq Professor Department of Mechanical Engineering Bangladesh University of Engineering & Technology (BUET Dhaka-1000, Bangladesh zahurul@me.buet.ac.bd
Lecture 13. Heat Engines. Thermodynamic processes and entropy Thermodynamic cycles Extracting work from heat
 Lecture 3 Heat Engines hermodynamic rocesses and entroy hermodynamic cycles Extracting work from heat - How do we define engine efficiency? - Carnot cycle: the best ossible efficiency Reading for this
Lecture 3 Heat Engines hermodynamic rocesses and entroy hermodynamic cycles Extracting work from heat - How do we define engine efficiency? - Carnot cycle: the best ossible efficiency Reading for this
Chapter A 9.0-V battery is connected to a lightbulb, as shown below. 9.0-V Battery. a. How much power is delivered to the lightbulb?
 Capter continued carges on te plates were reversed, te droplet would accelerate downward since all forces ten act in te same direction as gravity. 5. A 0.5-F capacitor is able to store 7.0 0 C of carge
Capter continued carges on te plates were reversed, te droplet would accelerate downward since all forces ten act in te same direction as gravity. 5. A 0.5-F capacitor is able to store 7.0 0 C of carge
+ m B1 = 1. u A1. u B1. - m B1 = V A. /v A = , u B1 + V B. = 5.5 kg => = V tot. Table B.1.
 5.6 A rigid tank is divided into two rooms by a membrane, both containing water, shown in Fig. P5.6. Room A is at 200 kpa, v = 0.5 m3/kg, VA = m3, and room B contains 3.5 kg at 0.5 MPa, 400 C. The membrane
5.6 A rigid tank is divided into two rooms by a membrane, both containing water, shown in Fig. P5.6. Room A is at 200 kpa, v = 0.5 m3/kg, VA = m3, and room B contains 3.5 kg at 0.5 MPa, 400 C. The membrane
ECE309 THERMODYNAMICS & HEAT TRANSFER MIDTERM EXAMINATION. Instructor: R. Culham. Name: Student ID Number:
 ECE309 THERMODYNAMICS & HEAT TRANSFER MIDTERM EXAMINATION June 19, 2015 2:30 pm - 4:30 pm Instructor: R. Culham Name: Student ID Number: Instructions 1. This is a 2 hour, closed-book examination. 2. Permitted
ECE309 THERMODYNAMICS & HEAT TRANSFER MIDTERM EXAMINATION June 19, 2015 2:30 pm - 4:30 pm Instructor: R. Culham Name: Student ID Number: Instructions 1. This is a 2 hour, closed-book examination. 2. Permitted
MODULE 2: DIFFUSION LECTURE NO DIFFUSION COEFFICIENT: MEASUREMENT AND PREDICTION
 NPTEL Chemical ass Transfer Oeration OULE : IFFUSION LECTURE NO. 4.4 IFFUSION COEFFICIENT: ESUREENT N PREICTION The roortionality factor of Fick s law is called diffusivity or diffusion coefficient which
NPTEL Chemical ass Transfer Oeration OULE : IFFUSION LECTURE NO. 4.4 IFFUSION COEFFICIENT: ESUREENT N PREICTION The roortionality factor of Fick s law is called diffusivity or diffusion coefficient which
Example problems. Chapter 2: Temperature, heat, and the 1 st law of Thermodynamic. Homework: 2, 3, 4, 5, 6, 10, 15, 19, 21 (pages )
 Examle roblems Chater : emerature, heat, and the 1 st law of hermodynamic Homework:,, 4, 5, 6, 1, 15, 19, 1 (ages 5-51) . (Page 5) wo constant-volume gas thermometers are assembled, one with nitrogen and
Examle roblems Chater : emerature, heat, and the 1 st law of hermodynamic Homework:,, 4, 5, 6, 1, 15, 19, 1 (ages 5-51) . (Page 5) wo constant-volume gas thermometers are assembled, one with nitrogen and
INTRODUCTION DEFINITION OF FLUID. U p F FLUID IS A SUBSTANCE THAT CAN NOT SUPPORT SHEAR FORCES OF ANY MAGNITUDE WITHOUT CONTINUOUS DEFORMATION
 INTRODUCTION DEFINITION OF FLUID plate solid F at t = 0 t > 0 = F/A plate U p F fluid t 0 t 1 t 2 t 3 FLUID IS A SUBSTANCE THAT CAN NOT SUPPORT SHEAR FORCES OF ANY MAGNITUDE WITHOUT CONTINUOUS DEFORMATION
INTRODUCTION DEFINITION OF FLUID plate solid F at t = 0 t > 0 = F/A plate U p F fluid t 0 t 1 t 2 t 3 FLUID IS A SUBSTANCE THAT CAN NOT SUPPORT SHEAR FORCES OF ANY MAGNITUDE WITHOUT CONTINUOUS DEFORMATION
Chapter 5. Transient Conduction. Islamic Azad University
 Chater 5 Transient Conduction Islamic Azad University Karaj Branch 1 Transient Conduction Many heat transfer roblems are time deendent Changes in oerating conditions in a system cause temerature variation
Chater 5 Transient Conduction Islamic Azad University Karaj Branch 1 Transient Conduction Many heat transfer roblems are time deendent Changes in oerating conditions in a system cause temerature variation
CHAPTER 8 ENTROPY. Blank
 CHAPER 8 ENROPY Blank SONNAG/BORGNAKKE SUDY PROBLEM 8-8. A heat engine efficiency from the inequality of Clausius Consider an actual heat engine with efficiency of η working between reservoirs at and L.
CHAPER 8 ENROPY Blank SONNAG/BORGNAKKE SUDY PROBLEM 8-8. A heat engine efficiency from the inequality of Clausius Consider an actual heat engine with efficiency of η working between reservoirs at and L.
Chapter 1: Basic Definitions, Terminologies and Concepts
 Chapter : Basic Definitions, Terminologies and Concepts ---------------------------------------. UThermodynamics:U It is a basic science that deals with: -. Energy transformation from one form to another..
Chapter : Basic Definitions, Terminologies and Concepts ---------------------------------------. UThermodynamics:U It is a basic science that deals with: -. Energy transformation from one form to another..
