Supplementary material: Orbitofrontal and striatal circuits dynamically encode the shift. between goal-directed and habitual actions
|
|
|
- Neal Pierce
- 5 years ago
- Views:
Transcription
1 Supplementary material: Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions Christina M. Gremel 1 & Rui M. Costa 1,2 1 Laboratory for Integrative Neuroscience, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, USA 2 Champalimaud Neuroscience Programme, Champalimaud Institute for the Unknown, Lisbon, Portugal
2 Gremel et al., supplementary material 2 Supplementary Figure S1. Lever-press related behaviors under RI and RR schedule training. (a-d) Average lever presses (a), rewards earned (b), reward rate (c), and head entries by C57BL/6J
3 Gremel et al., supplementary material 3 mice (n = 10) during concurrent RI and RR schedule training. (e, f) Average lever presses (e) and head entries (f) performed in previously RI and RR training contexts during outcome revaluation testing. (g, h) Example of the micro-structure of lever-press related behaviors in a mouse on the last day of RI (g) and RR (h) schedule training. Represented are executed lever-presses (red ticks) and head entries (blue ticks), as well as reinforcer deliveries (black triangles) across time within each schedule, with a probability of reinforcement set at p = 0.1; or 10%). Inserts within each panel show a representative distribution of behaviors across time leading up to a reinforcer delivery. As previously reported (DeRusso et al., 2011), there is no evidence of RI-schedule induced scalloped responding as seen for fixed-interval schedules of reinforcement. (i, j) Distribution of inter-reward intervals on the last day of schedule training in the RI (i) and (j) RR contexts, with similar distributions (unpaired t-tests t 206 < 0.66, p > 0.05). Error bars indicate s.e.m. * = Repeated measures ANOVA, Bonferroni corrected p < See Supplementary Methods for statistical analysis details. Statistical Analysis (a) Lever presses repeated measures ANOVA: Interaction: [F(8, 160) = 3.21, p <0.01], Training Day: [F(8, 160) = 47.58, p < 0.001], Training Schedule: [F(1, 160) = 12.15, p < 0.01] (b) Rewards earned repeated measures ANOVA: Interaction: [F(8, 160) = 0037, p > 0.05], Training day: [F(8,160) = 49.42, p < 0.001], Schedule: [F(1,160) = 0.34, p > 0.05] (c) Reward rate/min repeated measures ANOVA: Interaction: [F(8, 160) = 0.23, p > 0.05], Training day: [F(8,160) = 9.72, p < 0.001], Schedule: [F(1,160) = 1.86, p > 0.05] (d) Head entries repeated measures ANOVA: Interaction: [F(8, 160) = 0.37, p > 0.05], Training Day: [F(8,160) = 2.4, p < 0.05], Schedule: [F(1,160) = 0.82 p > 0.05] (e) Lever presses Repeated measures ANOVA Interaction (Schedule x Revaluation state: [F(1,19) = 4.51, p < 0.05] (VRI vs. DVRI: Bonferroni corrected p > 0.05; VRR vs. DVRR: Bonferroni corrected p > 0.05), Schedule [F(1,19) = 1.12, p < 0.05]; Revaluation State [F(1,19) = 17.07, p < 0.001] (f) Head entries Repeated measures ANOVA Interaction (Schedule x Revaluation state: [F(1,19) = 0.66, p > 0.05] Schedule [F(1,19) =.17, p > 0.05]; Revaluation State [F(1,19) = 6.19, p < 0.05]
4 Gremel et al., supplementary material 4 Supplementary Figure S2. Histological analyses of DMS, DLS, and OFC lesions and projections. (a) Schematic adapted from Paxinos and Franklin mouse atlas of DMS and DLS NMDA-induced lesions, with lesion inclusion area in black, and a representative lesion in grey. (b) Schematic of OFC ibotenic acid-induced lesions, with lesion inclusion area in black, and a representative lesion in grey.
5 Gremel et al., supplementary material 5 Supplementary Figure S3. Effect of dorsal medial and dorsal lateral striatum lesions on within-subject shifting between goal-directed and habitual actions. (a) Example schematic of within-subject training and outcome revaluation testing. Mice were trained to press the same lever for the same reinforcer under RI and RR training schedules. Using sensory-specific satiation, mice were given a outcome revaluation test where responding in previously RI and RR trained contexts was probed in valued and devalued states. (b-d) Average lever-presses performed (b), rewards earned (c), and head entries made (d) by Sham (n = 9), DMS (n = 5), and DLS (n = 7) lesioned across CRF and RI and RR schedule training. (e) Average consumption of pellets and sucrose
6 Gremel et al., supplementary material 6 (counterbalanced across earned reinforcer and home-cage reward) during the outcome revaluation test for Sham and DMS or DLS lesioned mice. (f-h) Average lever-presses (f), head-entries (g), and normalized (test day) head entries (h) performed in valued and devalued states in previously RI and RR schedule trained contexts in Sham, DMS, and DLS lesioned mice. Error bars indicate s.e.m. * = Repeated measures ANOVA, Bonferroni corrected p < See Supplementary Methods for statistical analysis details. Statistical Analysis (b) Lever-presses RI schedule Repeated measures ANOVA: Interaction: [F(16, 128) = 0.34, p > 0.05], Training day: [F(8, 128) = 29.12, p < ], Lesion group: [F(2, 128) = 0.58, p > 0.05] RR schedule Repeated measures ANOVA: Interaction: [F(16, 128) = 1.27, p > 0.05], Training day: [F(8, 128) = 31.56, p < ], Lesion group: [F(2, 128) = 0.93, p > 0.05] (c) Rewards earned RI schedule Repeated measures ANOVA: Interaction: [F(16, 128) = 0.67, p > 0.05], Training day: [F(8, 128) = 26.06, p < ], Lesion group: [F(2, 128) = 2.78, p > 0.05] RR schedule Repeated measures ANOVA: Interaction: [F(16, 128) = 0.89, p > 0.05], Training day: [F(8, 128) = 39.95, p < ], Lesion group: [F(2, 128) = 2.51, p > 0.05] (d) Reward rate/min RI schedule Repeated measures ANOVA: Interaction: [F(16, 128) = 2.03, p < 0.05], Training day: [F(8, 128) = 9.248, p < ] (Day 2 of CRF training CTLRI vs. DLSRI Bonferroni corrected p < 0.001, Day 3 of CRF training CtlRI vs. DLSRI or DMSRI Bonferroni corrected p < 0.001), Lesion group: [F(2, 128) = 5.404, p < 0.05] RR schedule Repeated measures ANOVA: Interaction: [F(16, 128) = 0.54, p > 0.05], Training day: [F(8, 128) = 8.94, p < ], Lesion group: [F(2, 128) = 2.37, p > 0.05] (e) Head Entries RI schedule Repeated measures ANOVA: Interaction: [F(16, 128) = 0.71, p > 0.05], Training day: [F(8, 128) = 1.55, p > 0.05], Lesion group: [F(2, 128) = 0.17, p > 0.05] RR schedule Repeated measures ANOVA: Interaction: [F(16, 128) = 0.69, p > 0.05], Training day: [F(8, 128) = 1.17, p > 0.05], Lesion group: [F(2, 128) = 0.31, p > 0.05] (f) Consumption: Interaction: [F(2, 34) = 0.50, p > 0.05], Outcome: [F(2, 34) = 2.4, p > 0.05], Lesion group: [F(1, 34) = 0.05, p > 0.05] (g) Lever Presses Repeated measures ANOVA: Sham mice: Interaction: [F(1, 14) = 2.55, p > 0.05], Schedule: [F(1, 14) = 0.52, p > 0.05], Revaluation state: [F(1, 14) = 16.18, p < 0.05] DMS lesioned mice: Interaction: [F(1, 11) = 0.26, p > 0.05], Schedule: [F(1, 11) = 1.35, p > 0.05], Revaluation state: [F(1, 11) = 2.156, p > 0.05]
7 Gremel et al., supplementary material 7 DLS lesioned mice: Interaction: [F(1, 12) =.21, p > 0.05], Schedule: [F(1, 12) = 0.28, p > 0.05], Revaluation state: [F(1, 12) = 6.97, p < 0.05] (h) Head Entries Repeated measures ANOVA: Sham mice: Interaction: [F(1, 14) = 0.22, p > 0.05], Schedule: [F(1, 14) = 0.15, p > 0.05], Revaluation state: [F(1, 14) = 7.54, p < 0.05] DMS lesioned mice: Interaction: [F(1, 11) = 0.26, p > 0.05], Schedule: [F(1, 11) 5.91, p < 0.05], Revaluation state: [F(1, 11) =0.515, p > 0.05] DLS lesioned mice: Interaction: [F(1, 12) = 1.07, p > 0.05], Schedule: [F(1, 12) = 0.02, p > 0.05], Revaluation state: [F(1, 12) = 3.374, p > 0.05] (i) Normalized Head Entries Repeated measures ANOVA: Sham mice: Interaction: [F(1, 14) = 0.72, p > 0.05], Schedule: [F(1, 14) = 5, p = 0.05], Revaluation state: [F(1, 14) = 7.47, p < 0.05] DMS lesioned mice: Interaction: [F(1, 11) = 0.00, p > 0.05], Schedule: [F(1, 11) = 0.43, p > 0.05], Revaluation state: [F(1, 11) =0.35, p > 0.05] DLS lesioned mice: Interaction: [F(1, 12) = 2.63, p > 0.05], Schedule: [F(1, 12) = 0.00, p > 0.05], Revaluation state: [F(1, 12) = 9.25, p < 0.05]
8 Gremel et al., supplementary material 8
9 Gremel et al., supplementary material 9 Supplementary Figure S4. Effect of OFC lesions on shifting between goal-directed and habitual actions. (a) Example schematic of within-subject training and outcome revaluation testing. Mice were trained to press the same lever for the same reinforcer under RI and RR training schedules. Using sensory-specific satiation, mice were given a outcome revaluation test where responding in previously RI and RR trained contexts was probed in valued and devalued states. (be) Average lever-presses performed (b), head entries made (c), rewards earned (d), and reward rate (e) by Sham (n =7) and OFC-lesioned (n = 5) mice across CRF and RI and RR schedule training in the within-subject procedure. (f) Average lever presses and (g) head-entries and normalized (test day) head entries performed in previously RI and RR trained contexts across valued and devalued states by Sham and OFC lesioned mice. (h) Average consumption of pellets and sucrose between Sham and OFC lesioned mice during outcome revaluation testing. (i, p) Example schematic of RI only (i) or RR only (p) schedule training and outcome revaluation testing. Mice were trained to press the lever under an RI or RR schedule, and during the subsequent outcome revaluation test responding was probed in valued and devalued states. (j-l, and q-s) Average response rate and lever-presses performed (j, q), head-entries made (k, r), and rewards earned (l, s), under RI (j-l) and RR (q-s) schedules of reinforcement by Sham (RI n = 10, RR n = 8) and OFC-lesioned (RI n = 10, RR n = 11) mice. (m-o and t-v) Average normalized (test day) lever presses and lever presses performed (m, t), normalized (test day) head entries and head entries performed (n, s), and average consumption of pellets and sucrose (o, v) by Sham and OFC lesioned mice in previously RI or RR trained contexts across valued and devalued days. Error bars indicate s.e.m. * = Repeated measures, one-way, two- ANOVA, Bonferroni corrected p < See Supplementary Methods for statistical analysis details. Statistical Analysis b) Lever presses Repeated measures ANOVA RI schedule: Interaction: [F(8, 56) = 0.47, p > 0.05], Training day: [F(8, 56) = 10.14, p < ], Lesion group: [F(1, 7) = 0.04, p > 0.05]
10 Gremel et al., supplementary material 10 RR schedule Repeated measures ANOVA: Interaction: [F(8, 56) = 1.89, p = 0.08] (post hoc analyses revealed Day 5, 6 Bonferroni corrected ps < 0.05, Training day: [F(8, 56) = , p < ]), Lesion group: [F(1, 7) = 7.80, p < 0.05] c) Head entries Repeated measures ANOVA RI schedule: Interaction: [F(8, 56) = 0.67, p > 0.05], Training day: [F(8, 56) = 26.06, p < ], Lesion group: [F(1,7) = 2.78, p > 0.05] RR schedule Repeated measures ANOVA: Interaction: [F(8, 56) = 0.50, p > 0.05], RR Training day: [F(8, 56) = 1.98 p = 0.06], RR Lesion group: [F(1, 7) = 0.40, p > 0.05] d) Rewards earned Repeated measures ANOVA RI schedule: Interaction: [F(8, 56) = 1.67, p > 0.05], Training day: [F(8, 156) = 28.87, p < ], Lesion group: [F(1, 7) = 0.15, p > 0.05] RR schedule: Interaction: [F(8, 56) = 0.65, p > 0.05], Training day: [F(8, 56) = 9.81, p < ], Lesion group: [F(1, 7) = 1.82 p > 0.05] e) Reward rate/min Repeated measures ANOVA RI schedule: Interaction: [F(8, 56) = 0.48, p > 0.05], Training day: [F(8, 56) = 3.92, p < 0.01], Lesion group: [F(1, 7) = 0.09, p > 0.05] RR schedule: Interaction: [F(8, 56) = 0.25, p > 0.05], Training day: [F(8, 56) = 7.85, p < ], Lesion group: [F(1, 7) = 0.21, p > 0.05] f) Lever presses Repeated measures ANOVA Sham Interaction: [F(1,12) = 3.9, p > 0.05], Schedule: [F(1, 12) = 0.25, p > 0.05], Revaluation state: [F(1,12) = 4.19, p = 0.09] OFC lesion Interaction: [F(1,8) = 0.43, p > 0.05], Schedule: [F(1, 8) = 0.47, p > 0.05], Revaluation state: [F(1,8) = 0.25, p > 0.05] g) Head entries Repeated measures ANOVA; Sham Interaction: [F(1,12) = 0.16, p > 0.05], Schedule: [F(1, 12) = 1.3, p > 0.05], Revaluation state: [F(1,12) = 0.0, p > 0.05] OFC lesion Interaction: [F(1,8) = 0.07, p > 0.05], Schedule: [F(1, 8) = 0.17, p > 0.05], Revaluation state: [F(1,8) = 0.23, p > 0.05] h) Consumption; Interaction: [F(1, 22) = 0.03, p > 0.05], outcome: [F(1, 22 = 20.6, p < 0.001], Lesion group: [F(1, 22) = 1.45, p > 0.05] RI schedule Repeated measures ANOVA j) Lever presses/min: Interaction: [F(8, 144) = 0.55, p > 0.05], Training day: [F(8, 144) = 13.84, p < ], Lesion group: [F(1, 29) = 0.15, p > 0.05]. Lever presses: Interaction: [F(8, 144) = 0.97, p > 0.05], Training day: [F(8, 144) = 13.34, p < ], Lesion group: [F(1, 29) = 1.08, p > 0.05] k) Head entries RI schedule: Interaction: [F(8, 144) = 0.19, p > 0.05], Training day: [F(8, 144) = 21.68, p < ], Lesion group: [F(1, 29) = 0.10, p > 0.05] l) Rewards earned RI schedule: Interaction: [F(8, 144) = 0.19, p > 0.05], Training day: [F(8, 144) = 21.68, p < ], Lesion group: [F(1, 29) = 0.10, p > 0.05] m) RI schedule Revaluation test Repeated measures ANOVA
11 Gremel et al., supplementary material 11 Normalized Lever presses RI schedule: Interaction [F(1,18) = 0.06 p > 0.05], Revaluation state [F(1,18) =0.07, p > 0.05], Lesion Group [F(1,18) = 0.00, p > 0.05]. Lever presses RI schedule: Interaction [F(1,18) = 1.42 p > 0.05], Revaluation state [F(1,18) =1.29, p > 0.05], Lesion group [F(1,18) = 0.14, p > 0.05] n) Head entries RI schedule: Interaction [F(1,36) = 0.004, p > 0.05], Day [F(1,36) =0.92, p > 0.05], Lesion group [F(1,36) = 0.13, p > 0.05]. Normalized (test day) Head entries RI schedule: Interaction [F(1,18) = 1.16, p > 0.05], Revaluation state [F(1,18) =0.27, p > 0.05], Lesion group [F(1,18) = 0.00, p > 0.05] o) Consumption RI schedule; Interaction: [F(1, 38) = 0.30, p > 0.05], outcome: [F(1, 38 = 21.64, p < 0.001], Lesion group: [F(1, 38) = 0.28, p > 0.05] q) RR schedule only Repeated measures ANOVA Lever presses/min: [F(8, 136) = 2.37, p = 0.02], Training day [F(8,136 ) =17.95 p < 0.001], Lesion group [F(1,17) = 4.90, p=0.04]. Lever presses RR: Interaction [F(8,136) = 0.96, p > 0.05], Training day [F(8,136) =31.73, p < 0.001], Lesion group [F(1,17) = 0.32, p < 0.001] r) Head entries RR schedule: Interaction [F(8,136) = 0.63, p > 0.05], Training day [F(8,136) =4.05, p < 0.001], Lesion group [F(1,17) = 2.70, p> 0.05] s) Rewards earned RR schedule: Interaction [F(8,136) = 0.29, p > 0.05], Training day [F(8,136) =1.14, p < 0.001], Lesion group [F(1,17) = 0.85, p< 0.05] t) RR schedule only Revaluation test Normalized (test day) Lever presses (RR schedule): Interaction [F(1,17) = 3.61, p =0.06] (Sham V vs. DV Bonferroni corrected p < 0.05; Wilcoxon W = -23, p = 0.06), Revaluation state [F(1,17) =3.08 p = 0.09], Lesion group [F(1,17) = 3.4, p = 0.08]. Lever presses (RR schedule): Interaction [F(1,17) = 1.77, p > 0.05], Revaluation state [F(1,17) =5.01, p < 0.05], Lesion group [F(1,17) = 2.81, p > 0.05] u) Normalized (test day) Head entries RR schedule: Interaction [F(1,17) = 0.52 p >0.05], Revaluation state [F(1,17) =0.00, p >0.05], Lesion group [F(1,17) = 0.00, p > 0.05]. Head entries RR schedule: Interaction [F(1,17) = 1.18 p =0.06], Revaluation state [F(1,17) =0.02, p < 0.02], Lesion group [F(1,17) = 0.07, p > 0.05]. v) Consumption; Interaction: [F(1, 28) = 0.13, p > 0.05], outcome: [F(1, 28 = 31.55, p < 0.001], Lesion group: [F(1, 28) = 0.12, p > 0.05]
12 Gremel et al., supplementary material 12 Supplementary Figure S5. In vivo simultaneous recordings of OFC, DMS and DLS during learning and execution of goal-directed actions and habits. (a) Average lever presses, (b) rewards earned, and (c) head entries across each of the six days of RI and RR schedule training in mice used in the in vivo recording experiments using the within-schedule design. (d) Average lever
13 Gremel et al., supplementary material 13 presses, (e) head entries, and (f) normalized (test day) head entries made during devaluation testing (valued and devalued test days) in the previous RI and RR training contexts. (g) Example of waveform, cluster separation from noise on the basis of principle component analyses, and interspike interval histogram for a single unit recorded in OFC. (h) Verified electrode placements in OFC and dorsal striatum (black squares represent electrode placement in the OFC, while blue and red squares represent electrode location in the DMS and DLS respectively). (i-k) Histogram showing the distribution of time for the beginning of significant firing rate modulation relative to lever-pressing for up-modulated and down-modulated DMS (i), DLS (j), and OFC (k). Error bars indicate s.e.m. See Supplementary Methods for statistical analysis details. Statistical analysis a) Lever presses: [F(8,112) = 0.76, p > 0.05], Day [F(8,112) =25.6, p < 0.001], Schedule [F(1,112) = 0.05, p< 0.05] b) Rewards earned: [F(7,98) = 0.94, p > 0.05], Day [F(7,98) =1.6, p >0.05], Schedule [F(1,98) = 2.08, p > 0.05] c) Head entries: [F(8,112) = 0.76, p > 0.05], Day [F(8,112) =25.6, p < 0.001], Schedule [F(1,112) = 0.05, p< 0.05] d) Lever presses: [F(1,16) = 2.43, p > 0.05], Day [F(1,16) =.31, p >0.05], Schedule [F(1,28) = 0.01, p > 0.05] e) Head entries: [F(1,16) = 0.31, p > 0.05], Day [F(1,16) =0.03, p >0.05], Schedule [F(1,16) = 0.56, p > 0.05] f) Normalized (test day) Head entries: [F(1,16) = 1.77, p > 0.05], Day [F(1,16) =0.76, p >0.05], Schedule [F(1,16) = 0.00, p > 0.05]
14 Gremel et al., supplementary material 14 Supplementary Figure S6. Scatter-plots of baseline frequency in RI (x-axis) versus RR (y-axis) contexts across training for all recorded units within DMS (a), DLS (f), and OFC (k). Summary bar graphs showing average baseline frequency of all units recorded in RI and RR contexts across training in DMS (b), DLS (g), and OFC (l). Absolute modulation rate for all lever-press related neurons in DMS (c), DLS (h), and OFC (m). Absolute modulation rate for Both lever-press related neurons in DMS (d), DLS (i), and OFC (n). Absolute modulation rate for Specific lever-press
15 Gremel et al., supplementary material 15 related neurons in DMS (e), DLS (j), and OFC (o). Error bars indicate s.e.m. * = Repeated measures ANOVA, Bonferroni corrected p < Statistical analysis b) DMS mean baseline firing rate: paired t-test (t 82 = 0.18, p > 0.05). c) DMS Both neuron mean modulation rate: repeated measures ANOVA (Schedule x Training Day) [F(1,30) = 0.004, p > 0.05]. Schedule [F(1,30) = 0.14, p >0.05], Training Day [F(1,30) = 5.37, p < 0.05]. d) DMS Specific neuron mean modulation rate: 2-way ANOVA (Schedule x Training Day) [F(1,25) = 3.66, p = 0.06]. Schedule [F(1,25) = 1.17, p >0.05], Training Day [F(1,25) = 0.000, p > 0.05]. f) DLS mean baseline firing rate: paired t-test (t 89 = 0.46, p > 0.05). g) DLS Both neuron mean modulation rate: repeated measures ANOVA (Schedule x Training Day) [F(1,26) = 0.26, p > 0.05]. Schedule [F(1,26) = 0.62, p >0.05], Training Day [F(1,26) = 0.47, p > 0.05]. h) DLS Specific neuron mean modulation rate: 2-way ANOVA (Schedule x Training Day) [F(1,39) = 0.42, p >0.05]. Schedule [F(1,39) = 0.43, p >0.05], Training Day [F(1,39) = 0.67, p > 0.05]. j) OFC mean baseline firing rate: paired t-test (t 309 = 0.71, p > 0.05). k) OFC Both neuron mean modulation rate: repeated measures ANOVA (Schedule x Training Day) [F(1,37) = 2.25, p > 0.05]. Schedule [F(1,37) = 1.63, p >0.05], Training Day [F(1,37) = 1.15, p > 0.05]. l) OFC Specific neuron mean modulation rate: 2-way ANOVA (Schedule x Training Day) [F(1,70) = 0.38, p >0.05]. Schedule [F(1,70) = 0.61, p >0.05], Training Day [F(1,70) = 5.36, p < 0.05].
16 Gremel et al., supplementary material 16 Supplementary Figure S7. Modulation direction of lever-press related neurons. Summary plots showing the percentage of neurons recorded (DMS n = 134; DLS n = 173; OFC n = 470) across training) in both RI and RR contexts that were up or down-modulated across training and on Valued and Devalued days during outcome revaluation testing under RI schedule for DMS (a), DLS (b), and OFC (c), and under RR schedules of reinforcement for DMS (d), DLS (e), and OFC (f). Error bars indicate s.e.m. Statistical analysis a) DMS direction of lever-press related activity for RI vs. RR context: Up modulated Chi square = 3.53, p >0.05. b) DLS direction of lever-press related activity for RI vs. RR context: Up modulated Chi square = 7.47, p = 0.06
17 Gremel et al., supplementary material 17 c) OFC direction of lever-press related activity for RI vs. RR context: Up modulated Chi square = 0.77, p > d) DMS direction of lever-press related activity for RI vs. RR context: Down modulated Chi square = 12.82, p < 0.05 e) DLS direction of lever-press related activity for RI vs. RR context: Down modulated Chi square = 4.69, p > 0.05 f) OFC direction of lever-press related activity for RI vs. RR context: Down modulated Chi square = 16.29, p < 0.05 g) DMS mean modulation rate: 2-way ANOVA (Schedule x Training Day) [F(1,89) = 0.89, p > 0.05]. Schedule [F(1,74) = 1.89, p > 0.05], Training Day [F(1,89) = 3.87, p = 0.052]. h) DLS mean modulation rate: 2-way ANOVA (Schedule x Training Day) [F(1,95) = 0.24, p > 0.05]. Schedule [F(1,95) = 0.00, p > 0.05], Training Day [F(1,95) = 1.37, p > 0.05]. i) OFC mean modulation rate: 2-way ANOVA (Schedule x Training Day) [F(1,148) = 1.88, p > 0.05]. Schedule [F(1,148) = 1.29, p > 0.05], Training Day [F(1,148) = 0.00, p > 0.05].
18 Gremel et al., supplementary material 18 Supplementary Figure S8. Lever-press related activity during outcome revaluation testing. Scatter-plot showing baseline firing rate of recorded neurons in RI (x-axis) and RR (y-axis) training contexts in DMS (a), DLS (e), and OFC (i). Averaged baseline firing rate for RI and RR training contexts for DMS (b), DLS (f), and OFC (j). Percentage of recorded neurons per mouse showing lever-press related changes in activity in RI and RR training contexts across Valued and Devalued states of outcome revaluation testing in DMS (c), DLS (g), and OFC (k). The percentage of leverpress related neurons that modulated firing rate during lever-press behavior in only one previous
19 Gremel et al., supplementary material 19 schedule trained context (Specific) or modulated firing rate during lever-press behavior in both previously schedule trained contexts (Both) for DMS (d), DLS (h), and OFC (l). Error bars indicate s.e.m. * = Chi square analyses p < Statitistical analysis b) DMS mean baseline firing rate: paired t-test (t 82 = 0.18, p > 0.05). c) DMS %lever-press related activity for RI vs. RR context: Chi square = 8.89, p < 0.05 d) DMS %lever-press neurons for Both vs. Specific for RI vs. RR context: Chi square = 21.59, p < f) DLS mean baseline firing rate: paired t-test (t 83 = 0.51, p > 0.05). g) DLS %lever-press related activity for RI vs. RR context: Chi square = 0.50, p >0.05 h) DLS %lever-press neurons for Both vs. Specific for RI vs. RR context: Chi square = 4.66, p < 0.05 j) OFC mean baseline firing rate: paired t-test (t 181 = 0.50, p > 0.05). k) OFC %lever-press related activity for RI vs. RR context: Chi square = 0.46, p >0.05 l) OFC %lever-press neurons for Both vs. Specific for RI vs. RR context: Chi square = 17.26, p < 0.001
20 Gremel et al., supplementary material 20 Supplementary Figure S9. Context specific lever-press related neural activity modulation. Average modulation rate for neurons that modulated firing rate only in RI or RR training contexts (Specific neurons) in the devalued state for DMS (a), DLS (b), and OFC (c). Scatter-plots of the devaluation index for the RI training context (x-axis) versus the modulation rate of Specific neurons in the RI training context (y-axis) in the Devalued state during outcome revaluation testing for DMS (d), DLS (e), and OFC (f). Scatter-plots of the devaluation index for the RR training context (x-axis) versus the modulation rate of Specific neurons in the RR training context (y-axis) in the Devalued
21 Gremel et al., supplementary material 21 state during outcome revaluation testing for DMS (g), DLS (h), and OFC (i). Error bars indicate s.e.m. r = Pearson correlation analyses. Statistical analysis a) DMS mean Specific neuron modulation rate for RI and RR contexts: unpaired t-test (t 11 = 0.32, p > 0.05). b) DLS mean Specific neuron modulation rate for RI and RR contexts: unpaired t-test (t 12 = 0.60, p > 0.05). c) OFC mean Specific neuron modulation rate for RI and RR contexts: unpaired t-test (t 33 = 0.94, p > 0.05).
22 Gremel et al., supplementary material 22 Supplementary Figure S10. Valued to devalued state-induced change in neural modulation. (a, b, c) For each mouse (n = 6), the z-score of the net modulation for DMS (a), DLS (b), and OFC (c) in the Valued and Devalued states in the RR context. (d, e, f) For each mouse, the z-score of the net modulation for DMS (a), DLS (b), and OFC (c) One mouse was removed from each DMS and DLS in RI and RR contexts due to the lack of significant modulation in recorded neurons during one or the other valuation states.
23 Gremel et al., supplementary material 23 Supplementary Figure S11. Chemicogenetic inhibition of OFC projection neurons via hm4d i receptor activation during outcome revaluation testing. (a,b) Fluorescence images showing representative spread of AAV2/9.CamKII.HI.GFP-Cre expression (green) (a) and AAV9.hSyn-DIOhM4D i -mcherry (red) expression (b) following OFC injection (100 nl/each/per side) (*note- each injection contained an additional 100 nl of AAV2/9.DIO.ChR2-YFP, for a total injection volume of
24 Gremel et al., supplementary material nl). Of note, hm4di receptor expression is going to be limited to cells that also express crerecombinase. Note the lesser degree of DREADD expression, further limiting any effect of activation to OFC projection neurons (red) (scale bar (mm) is in lower right corner) (c, d) An electrode array was implanted at the injection site, and the effect of hm4d i receptor agonist clozapine-n-oxide (CNO) systemic administration (1 mg/kg) (10ml/kg) on neural activity was examined in awakebehaving mice in a separate trial. OFC neural activity was recorded for 30 min prior to CNO administration, and the effect of CNO on OFC activity was examined for ~1 h and 45 min in mice expressing hm4d receptors in the OFC. CNO led to a decrease neural activity within 1 h, as shown in examples (c) and (d) from 2 different mice. Given the temporal limitations associated with this method and with extracellular recordings, we cannot say whether recorded neurons expressed hm4d i receptors. However, we did see a significant reduction in mean firing rate (t-test pre CNO to post CNO treatment ps < 0.05) in ~60% percent of recorded units suggesting that at a circuit level the firing rate of OFC neurons was reduced. (e) Effect of CNO on lever presses during outcome revaluation testing for Ctl mice previously co-injected with a GFP virus and the conditional virus expressing DREADD receptors (resulting in no expression of DREADD receptors), and hm4d i mice co-injected with the Cre virus and the conditional virus expressing DREADD receptors. During outcome revaluation testing, on valued and devalued days, all mice were given a 1 h pretreatment of CNO (1 mg/kg) (10ml/kg), and the effect on lever-press behavior in previously RI and RR trained contexts was examined. (F) Schematic of spread area in OFC following Cre or GFP virus injections (green), as well as spread of DREADD expression (red). Error bars indicate s.e.m. Statistical analysis e) Lever presses, repeated measures ANOVA: Ctl interaction (Schedule x Revaluation State) [F(1, 20) = 2.041, p > 0.05], Revaluation State [F1,20) = 11.37, p < 0.001], Schedule [1, 20] = 0.64, p > 0.05]; intra-ofc hm4d i interaction (Schedule x Revaluation State) [F(1, 18) = 0.15, p > 0.05], Revaluation State [F1,18) = 0.02, p > 0.05], Schedule [1, 18] = 0.00, p > 0.05]
25 Gremel et al., supplementary material 25
26 Gremel et al., supplementary material 26 Supplementary Figure S12. Optogenetic activation of OFC during outcome revaluation testing. (a-d) Fluorescence images showing spread of AAV2/9.CamKII.ChR2-YFP.SV40 expression (green) following (a) OFC injection (300 nl) (Stanford-Deisseroth Lab) to (b) dorsal striatum (CPu), (c) basolateral amygdala (BLA), and (d) substantia nigra (SN) and ventral tegmental area (VTA) (scale bar (mm) is in lower right or left corners). (e) Schematic of spread area in OFC following virus injections (green), as well site of bilateral stimulation (solid black dot = left hemisphere, open black dot = right hemisphere. An electrode array was lowered into the injection site in a subset of mice to examine the effect of photostimulation on OFC neural activity. (f) In an anesthetized mouse, photostimulation with 5 msec light pulse at a frequency of 10 Hz reliably evoked a ~1:1 ratio of spike to light pulse. Note that the waveforms did not change between stimulation and non-stimulation. (g) In an awake behaving mice, 5 msec light pulse at 10 Hz did not evoke a clear 1:1 spike to light pulse ratio, but (h) did lead to an overall increase in firing rate. (i, j) Lever presses (i) and normalized (test day) lever presses (j) during outcome revaluation testing. On valued and devalued days, following pre-feeding mice were lightly anesthetized and optical fibers were connected to bilateral indwelling optical fiber ferrules. After 15 min, mice were place into the operant chambers, and lever pressing in the absence of photostimulation (5 min) and in the presence of photostimulation with 473 nm wavelength light, < 5 mw, at 10 hz, 5 ms pulse (5 min) was examined. (k) Effect of photostimulation on the revaluation index for each mouse in the RI and RR training contexts. Photostimulation had no effect on revaluation indices in the RI context (paired t-test: t 5 = 1.62, p > 0.17), but did drastically affect revaluation indices in the RR context (biasing devalued conditions towards valuation, paired t-test: t 5 = 4.35, p < 0.02, the only animal not affected was an animal that did not show devaluation without light). Error bars indicate s.e.m. * = Repeated measures ANOVA, Bonferroni corrected p < Statistical analysis i) Lever presses, repeated measures ANOVA: No photostimulation interaction (Schedule x Revaluation state) [F(1, 10) = 2.17, p > 0.05], Day [F1,10) = 2.58, p > 0.05], Schedule [1, 10] = 0.95,
27 Gremel et al., supplementary material 27 p > 0.05]; 10 hz 473 nm wavelength light interaction (Schedule x Revaluation state) [F(1, 10) = 1.74, p > 0.05], Day [F1,10) = 0.19, p > 0.05], Schedule [1, 10] = 3.2, p > 0.05] j) Normalized (test day) Lever presses, repeated measures ANOVA: No photostimulation interaction (Schedule x Revaluation state) [F(1, 10) = 8.10, p < 0.05], Day [F1,10) = 1.75, p > 0.05], Schedule [1, 10] = 0.00, p > 0.05]; 10 hz 473 nm wavelength light interaction (Schedule x Revaluation state) [F(1, 10) = 3.68, p = 0.08], Day [F1,10) = 0.28, p > 0.05], Schedule [1, 10] = 6.67, p < 0.05]
Nature Neuroscience: doi: /nn Supplementary Figure 1. Amygdaloid complex and evoked synaptic currents recorded in CeM amygdala neurons.
 Supplementary Figure 1 Amygdaloid complex and evoked synaptic currents recorded in CeM amygdala neurons. (a) Left: Schematic representation (modified from: Allen Brain Atlas) of coronal sections containing
Supplementary Figure 1 Amygdaloid complex and evoked synaptic currents recorded in CeM amygdala neurons. (a) Left: Schematic representation (modified from: Allen Brain Atlas) of coronal sections containing
Frequency (Hz) Amplitude (pa) D1 WT D1 KO D2 WT D2 KO D1 WT D1 KO D2 WT D2 KO
 A D1 MSNs B D2 MSNs C Frequency (Hz) 4 3 2 1 D Amplitude (pa) 5 4 3 2 1 D1 D1 D2 D2 D1 D1 D2 D2 Supplemental Figure 1. B deletion did not alter GABA-mIPSCs in D1 or D2 MSNs. (A,B) Representative recording
A D1 MSNs B D2 MSNs C Frequency (Hz) 4 3 2 1 D Amplitude (pa) 5 4 3 2 1 D1 D1 D2 D2 D1 D1 D2 D2 Supplemental Figure 1. B deletion did not alter GABA-mIPSCs in D1 or D2 MSNs. (A,B) Representative recording
Every animal is represented by a blue circle. Correlation was measured by Spearman s rank correlation coefficient (ρ).
 Supplementary Figure 1 Correlations between tone and context freezing by animal in each of the four groups in experiment 1. Every animal is represented by a blue circle. Correlation was measured by Spearman
Supplementary Figure 1 Correlations between tone and context freezing by animal in each of the four groups in experiment 1. Every animal is represented by a blue circle. Correlation was measured by Spearman
Nature Methods: doi: /nmeth Supplementary Figure 1. In vitro screening of recombinant R-CaMP2 variants.
 Supplementary Figure 1 In vitro screening of recombinant R-CaMP2 variants. Baseline fluorescence compared to R-CaMP1.07 at nominally zero calcium plotted versus dynamic range ( F/F) for 150 recombinant
Supplementary Figure 1 In vitro screening of recombinant R-CaMP2 variants. Baseline fluorescence compared to R-CaMP1.07 at nominally zero calcium plotted versus dynamic range ( F/F) for 150 recombinant
Consider the following spike trains from two different neurons N1 and N2:
 About synchrony and oscillations So far, our discussions have assumed that we are either observing a single neuron at a, or that neurons fire independent of each other. This assumption may be correct in
About synchrony and oscillations So far, our discussions have assumed that we are either observing a single neuron at a, or that neurons fire independent of each other. This assumption may be correct in
Supplementary Figure 1
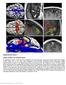 Supplementary Figure 1 Human cerebral vein characterization a, Superficial venous structures were identified and reconstructed using post-contrast brain MRI images (n=50). Scale bar, 3 cm. b-c, Vein diameters
Supplementary Figure 1 Human cerebral vein characterization a, Superficial venous structures were identified and reconstructed using post-contrast brain MRI images (n=50). Scale bar, 3 cm. b-c, Vein diameters
Neuronal Dynamics: Computational Neuroscience of Single Neurons
 Week 5 part 3a :Three definitions of rate code Neuronal Dynamics: Computational Neuroscience of Single Neurons Week 5 Variability and Noise: The question of the neural code Wulfram Gerstner EPFL, Lausanne,
Week 5 part 3a :Three definitions of rate code Neuronal Dynamics: Computational Neuroscience of Single Neurons Week 5 Variability and Noise: The question of the neural code Wulfram Gerstner EPFL, Lausanne,
Nature Neuroscience: doi: /nn Supplementary Figure 1. Expression of GCaMP6s in LGN and their axons in V1.
 Supplementary Figure 1 Expression of GCaMP6s in LGN and their axons in V1. (a, b) Coronal section of LGN and V1 expressing GCaMP6s. a Coronal slice (bregma -2.3 mm) including thalamus confirmed that GCaMP6s
Supplementary Figure 1 Expression of GCaMP6s in LGN and their axons in V1. (a, b) Coronal section of LGN and V1 expressing GCaMP6s. a Coronal slice (bregma -2.3 mm) including thalamus confirmed that GCaMP6s
Nature Neuroscience: doi: /nn.2283
 Supplemental Material for NN-A2678-T Phase-to-rate transformations encode touch in cortical neurons of a scanning sensorimotor system by John Curtis and David Kleinfeld Figure S. Overall distribution of
Supplemental Material for NN-A2678-T Phase-to-rate transformations encode touch in cortical neurons of a scanning sensorimotor system by John Curtis and David Kleinfeld Figure S. Overall distribution of
Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03
 Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03 Roger W. Sperry The problem of central nervous reorganization after nerve regeneration and muscle transposition. R.W. Sperry. Quart. Rev. Biol.
Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03 Roger W. Sperry The problem of central nervous reorganization after nerve regeneration and muscle transposition. R.W. Sperry. Quart. Rev. Biol.
The homogeneous Poisson process
 The homogeneous Poisson process during very short time interval Δt there is a fixed probability of an event (spike) occurring independent of what happened previously if r is the rate of the Poisson process,
The homogeneous Poisson process during very short time interval Δt there is a fixed probability of an event (spike) occurring independent of what happened previously if r is the rate of the Poisson process,
CJ LI LRRK2 MOUSE COMPARISON STUDY. Phenotyping Data Results
 CJ LI MOUSE COMPARISON STUDY Phenotyping Data Results NOTE FOR USE This comparison study was run as an opportunistic look at the phenotype of multiple CJ Li-generated mouse lines. The primary purpose of
CJ LI MOUSE COMPARISON STUDY Phenotyping Data Results NOTE FOR USE This comparison study was run as an opportunistic look at the phenotype of multiple CJ Li-generated mouse lines. The primary purpose of
Math in systems neuroscience. Quan Wen
 Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
+ + ( + ) = Linear recurrent networks. Simpler, much more amenable to analytic treatment E.g. by choosing
 Linear recurrent networks Simpler, much more amenable to analytic treatment E.g. by choosing + ( + ) = Firing rates can be negative Approximates dynamics around fixed point Approximation often reasonable
Linear recurrent networks Simpler, much more amenable to analytic treatment E.g. by choosing + ( + ) = Firing rates can be negative Approximates dynamics around fixed point Approximation often reasonable
Singapore Institute for Neurotechnology & Memory Network Programme, National University of Singapore, Singapore
 SUPPLEMENTARY INFORMATION: Gene expression links functional networks across cortex and striatum Kevin M Anderson 1, Fenna M Krienen 2, Eun Young Choi 3, Jenna M Reinen 1, B T Thomas Yeo 4,5, Avram J Holmes
SUPPLEMENTARY INFORMATION: Gene expression links functional networks across cortex and striatum Kevin M Anderson 1, Fenna M Krienen 2, Eun Young Choi 3, Jenna M Reinen 1, B T Thomas Yeo 4,5, Avram J Holmes
Reporting Checklist for Nature Neuroscience
 Corresponding Author: Manuscript Number: Manuscript Type: Geoffrey Schoenbaum NNA771T Article Reporting Checklist for Nature Neuroscience # Main Figures: # Supplementary Figures: # Supplementary s: # Supplementary
Corresponding Author: Manuscript Number: Manuscript Type: Geoffrey Schoenbaum NNA771T Article Reporting Checklist for Nature Neuroscience # Main Figures: # Supplementary Figures: # Supplementary s: # Supplementary
Microsystems for Neuroscience and Medicine. Lecture 9
 1 Microsystems for Neuroscience and Medicine Lecture 9 2 Neural Microsystems Neurons - Structure and behaviour Measuring neural activity Interfacing with neurons Medical applications - DBS, Retinal Implants
1 Microsystems for Neuroscience and Medicine Lecture 9 2 Neural Microsystems Neurons - Structure and behaviour Measuring neural activity Interfacing with neurons Medical applications - DBS, Retinal Implants
Announcements: Test4: Wednesday on: week4 material CH5 CH6 & NIA CAPE Evaluations please do them for me!! ask questions...discuss listen learn.
 Announcements: Test4: Wednesday on: week4 material CH5 CH6 & NIA CAPE Evaluations please do them for me!! ask questions...discuss listen learn. The Chemical Senses: Olfaction Mary ET Boyle, Ph.D. Department
Announcements: Test4: Wednesday on: week4 material CH5 CH6 & NIA CAPE Evaluations please do them for me!! ask questions...discuss listen learn. The Chemical Senses: Olfaction Mary ET Boyle, Ph.D. Department
Supplementary Figure 1. Structural MRIs.
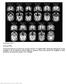 Supplementary Figure 1 Structural MRIs. Coronal and transverse sections of pre-electrode insertion T1 weighted MRIs, illustrating radiologically normal amygdala in the 10 patients for which ierps are presented.
Supplementary Figure 1 Structural MRIs. Coronal and transverse sections of pre-electrode insertion T1 weighted MRIs, illustrating radiologically normal amygdala in the 10 patients for which ierps are presented.
The Bayesian Brain. Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester. May 11, 2017
 The Bayesian Brain Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester May 11, 2017 Bayesian Brain How do neurons represent the states of the world? How do neurons represent
The Bayesian Brain Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester May 11, 2017 Bayesian Brain How do neurons represent the states of the world? How do neurons represent
Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1
 Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1 F/F ;mir-17~92 F/F (Pkd1-miR-17~92KO) mice. (A) Q-PCR
Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1 F/F ;mir-17~92 F/F (Pkd1-miR-17~92KO) mice. (A) Q-PCR
An Introductory Course in Computational Neuroscience
 An Introductory Course in Computational Neuroscience Contents Series Foreword Acknowledgments Preface 1 Preliminary Material 1.1. Introduction 1.1.1 The Cell, the Circuit, and the Brain 1.1.2 Physics of
An Introductory Course in Computational Neuroscience Contents Series Foreword Acknowledgments Preface 1 Preliminary Material 1.1. Introduction 1.1.1 The Cell, the Circuit, and the Brain 1.1.2 Physics of
Model neurons!!poisson neurons!
 Model neurons!!poisson neurons! Suggested reading:! Chapter 1.4 in Dayan, P. & Abbott, L., heoretical Neuroscience, MI Press, 2001.! Model neurons: Poisson neurons! Contents: Probability of a spike sequence
Model neurons!!poisson neurons! Suggested reading:! Chapter 1.4 in Dayan, P. & Abbott, L., heoretical Neuroscience, MI Press, 2001.! Model neurons: Poisson neurons! Contents: Probability of a spike sequence
Temporal context calibrates interval timing
 Temporal context calibrates interval timing, Mehrdad Jazayeri & Michael N. Shadlen Helen Hay Whitney Foundation HHMI, NPRC, Department of Physiology and Biophysics, University of Washington, Seattle, Washington
Temporal context calibrates interval timing, Mehrdad Jazayeri & Michael N. Shadlen Helen Hay Whitney Foundation HHMI, NPRC, Department of Physiology and Biophysics, University of Washington, Seattle, Washington
SUPPLEMENTARY INFORMATION
 Figure S1 Multiplicative scaling of the granule cell input-output relation is not dependent on input rate. Input-output relations with short-term depression (STD) from Fig. 1d after normalizing by the
Figure S1 Multiplicative scaling of the granule cell input-output relation is not dependent on input rate. Input-output relations with short-term depression (STD) from Fig. 1d after normalizing by the
Summary of part I: prediction and RL
 Summary of part I: prediction and RL Prediction is important for action selection The problem: prediction of future reward The algorithm: temporal difference learning Neural implementation: dopamine dependent
Summary of part I: prediction and RL Prediction is important for action selection The problem: prediction of future reward The algorithm: temporal difference learning Neural implementation: dopamine dependent
Lecture 11 : Simple Neuron Models. Dr Eileen Nugent
 Lecture 11 : Simple Neuron Models Dr Eileen Nugent Reading List Nelson, Biological Physics, Chapter 12 Phillips, PBoC, Chapter 17 Gerstner, Neuronal Dynamics: from single neurons to networks and models
Lecture 11 : Simple Neuron Models Dr Eileen Nugent Reading List Nelson, Biological Physics, Chapter 12 Phillips, PBoC, Chapter 17 Gerstner, Neuronal Dynamics: from single neurons to networks and models
Collective Dynamics in Human and Monkey Sensorimotor Cortex: Predicting Single Neuron Spikes
 Collective Dynamics in Human and Monkey Sensorimotor Cortex: Predicting Single Neuron Spikes Supplementary Information Wilson Truccolo 1,2,5, Leigh R. Hochberg 2-6 and John P. Donoghue 4,1,2 1 Department
Collective Dynamics in Human and Monkey Sensorimotor Cortex: Predicting Single Neuron Spikes Supplementary Information Wilson Truccolo 1,2,5, Leigh R. Hochberg 2-6 and John P. Donoghue 4,1,2 1 Department
Regulation of interneuron excitability by gap junction coupling with principal cells
 Regulation of interneuron excitability by gap junction coupling with principal cells Pierre F Apostolides 1,2 & Laurence O Trussell 2 Electrical coupling of inhibitory interneurons can synchronize activity
Regulation of interneuron excitability by gap junction coupling with principal cells Pierre F Apostolides 1,2 & Laurence O Trussell 2 Electrical coupling of inhibitory interneurons can synchronize activity
High-dimensional geometry of cortical population activity. Marius Pachitariu University College London
 High-dimensional geometry of cortical population activity Marius Pachitariu University College London Part I: introduction to the brave new world of large-scale neuroscience Part II: large-scale data preprocessing
High-dimensional geometry of cortical population activity Marius Pachitariu University College London Part I: introduction to the brave new world of large-scale neuroscience Part II: large-scale data preprocessing
Principal component analysis of neuronal ensemble activity reveals multidimensional somatosensory representations
 Journal of Neuroscience Methods 94 (1999) 121 140 www.elsevier.com/locate/jneumeth Principal component analysis of neuronal ensemble activity reveals multidimensional somatosensory representations John
Journal of Neuroscience Methods 94 (1999) 121 140 www.elsevier.com/locate/jneumeth Principal component analysis of neuronal ensemble activity reveals multidimensional somatosensory representations John
Internally generated preactivation of single neurons in human medial frontal cortex predicts volition
 Internally generated preactivation of single neurons in human medial frontal cortex predicts volition Itzhak Fried, Roy Mukamel, Gabriel Kreiman List of supplementary material Supplementary Tables (2)
Internally generated preactivation of single neurons in human medial frontal cortex predicts volition Itzhak Fried, Roy Mukamel, Gabriel Kreiman List of supplementary material Supplementary Tables (2)
The idiosyncratic nature of confidence
 SUPPLEMENTARY INFORMATION Articles DOI: 10.1038/s41562-017-0215-1 In the format provided by the authors and unedited. The idiosyncratic nature of confidence 1,2 Joaquin Navajas *, Chandni Hindocha 1,3,
SUPPLEMENTARY INFORMATION Articles DOI: 10.1038/s41562-017-0215-1 In the format provided by the authors and unedited. The idiosyncratic nature of confidence 1,2 Joaquin Navajas *, Chandni Hindocha 1,3,
Animal learning theory
 Animal learning theory Based on [Sutton and Barto, 1990, Dayan and Abbott, 2001] Bert Kappen [Sutton and Barto, 1990] Classical conditioning: - A conditioned stimulus (CS) and unconditioned stimulus (US)
Animal learning theory Based on [Sutton and Barto, 1990, Dayan and Abbott, 2001] Bert Kappen [Sutton and Barto, 1990] Classical conditioning: - A conditioned stimulus (CS) and unconditioned stimulus (US)
This script will produce a series of pulses of amplitude 40 na, duration 1ms, recurring every 50 ms.
 9.16 Problem Set #4 In the final problem set you will combine the pieces of knowledge gained in the previous assignments to build a full-blown model of a plastic synapse. You will investigate the effects
9.16 Problem Set #4 In the final problem set you will combine the pieces of knowledge gained in the previous assignments to build a full-blown model of a plastic synapse. You will investigate the effects
An algorithm for detecting oscillatory behavior in discretized data: the damped-oscillator oscillator detector
 An algorithm for detecting oscillatory behavior in discretized data: the damped-oscillator oscillator detector David Hsu, Murielle Hsu, He Huang and Erwin B. Montgomery, Jr Department of Neurology University
An algorithm for detecting oscillatory behavior in discretized data: the damped-oscillator oscillator detector David Hsu, Murielle Hsu, He Huang and Erwin B. Montgomery, Jr Department of Neurology University
Decision-making and Weber s law: a neurophysiological model
 European Journal of Neuroscience, Vol. 24, pp. 901 916, 2006 doi:10.1111/j.14-9568.2006.04940.x Decision-making and Weber s law: a neurophysiological model Gustavo Deco 1 and Edmund T. Rolls 2 1 Institucio
European Journal of Neuroscience, Vol. 24, pp. 901 916, 2006 doi:10.1111/j.14-9568.2006.04940.x Decision-making and Weber s law: a neurophysiological model Gustavo Deco 1 and Edmund T. Rolls 2 1 Institucio
Supplementary Figure 1. Characterization of the single-photon quantum light source based on spontaneous parametric down-conversion (SPDC).
 .2 Classical light source.8 g (2) ().6.4.2 EMCCD SPAD 2 3.2.4.6.8..2.4.6.8.2 Mean number of photon pairs per pump pulse 4 5 6 7 8 9 2 3 4 Supplementary Figure. Characterization of the single-photon quantum
.2 Classical light source.8 g (2) ().6.4.2 EMCCD SPAD 2 3.2.4.6.8..2.4.6.8.2 Mean number of photon pairs per pump pulse 4 5 6 7 8 9 2 3 4 Supplementary Figure. Characterization of the single-photon quantum
Contents. Acknowledgments. xix
 Table of Preface Acknowledgments page xv xix 1 Introduction 1 The Role of the Computer in Data Analysis 1 Statistics: Descriptive and Inferential 2 Variables and Constants 3 The Measurement of Variables
Table of Preface Acknowledgments page xv xix 1 Introduction 1 The Role of the Computer in Data Analysis 1 Statistics: Descriptive and Inferential 2 Variables and Constants 3 The Measurement of Variables
Reinforcement Learning. Odelia Schwartz 2016
 Reinforcement Learning Odelia Schwartz 2016 Forms of learning? Forms of learning Unsupervised learning Supervised learning Reinforcement learning Forms of learning Unsupervised learning Supervised learning
Reinforcement Learning Odelia Schwartz 2016 Forms of learning? Forms of learning Unsupervised learning Supervised learning Reinforcement learning Forms of learning Unsupervised learning Supervised learning
Waithe et al Supplementary Figures
 Waithe et al Supplementary Figures Supplementary Figure 1 Expression and properties of WT and W391A mutant YFP- Ca V 2.2. A Immunoblot using Ca V 2.2 Ab for untransfected cells (UT, lane 1), YFP-Ca V 2.2
Waithe et al Supplementary Figures Supplementary Figure 1 Expression and properties of WT and W391A mutant YFP- Ca V 2.2. A Immunoblot using Ca V 2.2 Ab for untransfected cells (UT, lane 1), YFP-Ca V 2.2
Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae. BioNB4910 Cornell University.
 Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae BioNB4910 Cornell University Goals 1) Observe the behavioral effects of remotely activating different populations
Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae BioNB4910 Cornell University Goals 1) Observe the behavioral effects of remotely activating different populations
Supplementary Figure 1
 Supplementry Figure (nesthetized) (wke) Normlized mplitude.5 Pek width (ms).6.4.2 4 2 2 x 3 Wveform slope Normlized mplitude.5 Pek width (ms).6.4.2 x 3 3 2 Wveform slope c (nesthetized) d (wke) Normlized
Supplementry Figure (nesthetized) (wke) Normlized mplitude.5 Pek width (ms).6.4.2 4 2 2 x 3 Wveform slope Normlized mplitude.5 Pek width (ms).6.4.2 x 3 3 2 Wveform slope c (nesthetized) d (wke) Normlized
Introduction and Descriptive Statistics p. 1 Introduction to Statistics p. 3 Statistics, Science, and Observations p. 5 Populations and Samples p.
 Preface p. xi Introduction and Descriptive Statistics p. 1 Introduction to Statistics p. 3 Statistics, Science, and Observations p. 5 Populations and Samples p. 6 The Scientific Method and the Design of
Preface p. xi Introduction and Descriptive Statistics p. 1 Introduction to Statistics p. 3 Statistics, Science, and Observations p. 5 Populations and Samples p. 6 The Scientific Method and the Design of
Synaptic dynamics. John D. Murray. Synaptic currents. Simple model of the synaptic gating variable. First-order kinetics
 Synaptic dynamics John D. Murray A dynamical model for synaptic gating variables is presented. We use this to study the saturation of synaptic gating at high firing rate. Shunting inhibition and the voltage
Synaptic dynamics John D. Murray A dynamical model for synaptic gating variables is presented. We use this to study the saturation of synaptic gating at high firing rate. Shunting inhibition and the voltage
Figure 1: Graphene release, transfer and stacking processes. The graphene stacking began with CVD
 Supplementary figure 1 Graphene Growth and Transfer Graphene PMMA FeCl 3 DI water Copper foil CVD growth Back side etch PMMA coating Copper etch in 0.25M FeCl 3 DI water rinse 1 st transfer DI water 1:10
Supplementary figure 1 Graphene Growth and Transfer Graphene PMMA FeCl 3 DI water Copper foil CVD growth Back side etch PMMA coating Copper etch in 0.25M FeCl 3 DI water rinse 1 st transfer DI water 1:10
Single-Trial Neural Correlates. of Arm Movement Preparation. Neuron, Volume 71. Supplemental Information
 Neuron, Volume 71 Supplemental Information Single-Trial Neural Correlates of Arm Movement Preparation Afsheen Afshar, Gopal Santhanam, Byron M. Yu, Stephen I. Ryu, Maneesh Sahani, and K rishna V. Shenoy
Neuron, Volume 71 Supplemental Information Single-Trial Neural Correlates of Arm Movement Preparation Afsheen Afshar, Gopal Santhanam, Byron M. Yu, Stephen I. Ryu, Maneesh Sahani, and K rishna V. Shenoy
Nature Neuroscience: doi: /nn.2717
 Supplementary Fig. 1. Dendrite length is not secondary to body length. Dendrite growth proceeds independently of the rate of body growth and decreases in rate in adults. n 20 on dendrite measurement, n
Supplementary Fig. 1. Dendrite length is not secondary to body length. Dendrite growth proceeds independently of the rate of body growth and decreases in rate in adults. n 20 on dendrite measurement, n
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature09714 Supplementary Figure 1 The mouse olfactory system and summary of our results. a, Schematic of the mouse olfactory system (sagittal view). See text for more details. The red and
doi:10.1038/nature09714 Supplementary Figure 1 The mouse olfactory system and summary of our results. a, Schematic of the mouse olfactory system (sagittal view). See text for more details. The red and
Supplementary Figure 1
 Supplementary Figure 1 Single-cell RNA sequencing reveals unique sets of neuropeptides, transcription factors and receptors in specific types of sympathetic neurons (a) Dissection of paravertebral SGs
Supplementary Figure 1 Single-cell RNA sequencing reveals unique sets of neuropeptides, transcription factors and receptors in specific types of sympathetic neurons (a) Dissection of paravertebral SGs
The Spike Response Model: A Framework to Predict Neuronal Spike Trains
 The Spike Response Model: A Framework to Predict Neuronal Spike Trains Renaud Jolivet, Timothy J. Lewis 2, and Wulfram Gerstner Laboratory of Computational Neuroscience, Swiss Federal Institute of Technology
The Spike Response Model: A Framework to Predict Neuronal Spike Trains Renaud Jolivet, Timothy J. Lewis 2, and Wulfram Gerstner Laboratory of Computational Neuroscience, Swiss Federal Institute of Technology
Turning a research question into a statistical question.
 Turning a research question into a statistical question. IGINAL QUESTION: Concept Concept Concept ABOUT ONE CONCEPT ABOUT RELATIONSHIPS BETWEEN CONCEPTS TYPE OF QUESTION: DESCRIBE what s going on? DECIDE
Turning a research question into a statistical question. IGINAL QUESTION: Concept Concept Concept ABOUT ONE CONCEPT ABOUT RELATIONSHIPS BETWEEN CONCEPTS TYPE OF QUESTION: DESCRIBE what s going on? DECIDE
Fitting a Stochastic Neural Network Model to Real Data
 Fitting a Stochastic Neural Network Model to Real Data Christophe Pouzat, Ludmila Brochini, Pierre Hodara and Guilherme Ost MAP5 Univ. Paris-Descartes and CNRS Neuromat, USP christophe.pouzat@parisdescartes.fr
Fitting a Stochastic Neural Network Model to Real Data Christophe Pouzat, Ludmila Brochini, Pierre Hodara and Guilherme Ost MAP5 Univ. Paris-Descartes and CNRS Neuromat, USP christophe.pouzat@parisdescartes.fr
Biological Modeling of Neural Networks:
 Week 14 Dynamics and Plasticity 14.1 Reservoir computing - Review:Random Networks - Computing with rich dynamics Biological Modeling of Neural Networks: 14.2 Random Networks - stationary state - chaos
Week 14 Dynamics and Plasticity 14.1 Reservoir computing - Review:Random Networks - Computing with rich dynamics Biological Modeling of Neural Networks: 14.2 Random Networks - stationary state - chaos
What is it? Where is it? How strong is it? Perceived quantity. Intensity Coding in Sensory Systems. What must sensory systems encode?
 Sensory Neurophysiology Neural response Intensity Coding in Sensory Systems Behavioral Neuroscience Psychophysics Percept What must sensory systems encode? What is it? Where is it? How strong is it? Perceived
Sensory Neurophysiology Neural response Intensity Coding in Sensory Systems Behavioral Neuroscience Psychophysics Percept What must sensory systems encode? What is it? Where is it? How strong is it? Perceived
Reinforcement learning
 einforcement learning How to learn to make decisions in sequential problems (like: chess, a maze) Why is this difficult? Temporal credit assignment Prediction can help Further reading For modeling: Chapter
einforcement learning How to learn to make decisions in sequential problems (like: chess, a maze) Why is this difficult? Temporal credit assignment Prediction can help Further reading For modeling: Chapter
Finding informative neurons in the brain using Multi-Scale Relevance
 Finding informative neurons in the brain using Multi-Scale Relevance Ryan John Cubero,,3, Matteo Marsili, and Yasser Roudi arxiv:.3v [q-bio.nc] Feb Kavli Institute for Systems Neuroscience and Centre for
Finding informative neurons in the brain using Multi-Scale Relevance Ryan John Cubero,,3, Matteo Marsili, and Yasser Roudi arxiv:.3v [q-bio.nc] Feb Kavli Institute for Systems Neuroscience and Centre for
SUPPLEMENTARY INFORMATION
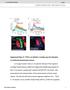 Supplemental Figure S1. WISL is not elicited by courtship song and is dependent on chordotonal mechanosensory neurons. (a) Average locomotor velocity vs. time plots for wild-type CS flies exposed to recordings
Supplemental Figure S1. WISL is not elicited by courtship song and is dependent on chordotonal mechanosensory neurons. (a) Average locomotor velocity vs. time plots for wild-type CS flies exposed to recordings
Membrane equation. VCl. dv dt + V = V Na G Na + V K G K + V Cl G Cl. G total. C m. G total = G Na + G K + G Cl
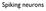 Spiking neurons Membrane equation V GNa GK GCl Cm VNa VK VCl dv dt + V = V Na G Na + V K G K + V Cl G Cl G total G total = G Na + G K + G Cl = C m G total Membrane with synaptic inputs V Gleak GNa GK
Spiking neurons Membrane equation V GNa GK GCl Cm VNa VK VCl dv dt + V = V Na G Na + V K G K + V Cl G Cl G total G total = G Na + G K + G Cl = C m G total Membrane with synaptic inputs V Gleak GNa GK
Nature Neuroscience: doi: /nn Supplementary Figure 1. Localization of responses
 Supplementary Figure 1 Localization of responses a. For each subject, we classified neural activity using an electrode s response to a localizer task (see Experimental Procedures). Auditory (green), indicates
Supplementary Figure 1 Localization of responses a. For each subject, we classified neural activity using an electrode s response to a localizer task (see Experimental Procedures). Auditory (green), indicates
Nature Protocols: doi: /nprot Supplementary Figure 1
 Supplementary Figure 1 Photographs of the 3D-MTC device and the confocal fluorescence microscopy. I: The system consists of a Leica SP8-Confocal microscope (with an option of STED), a confocal PC, a 3D-MTC
Supplementary Figure 1 Photographs of the 3D-MTC device and the confocal fluorescence microscopy. I: The system consists of a Leica SP8-Confocal microscope (with an option of STED), a confocal PC, a 3D-MTC
Limulus. The Neural Code. Response of Visual Neurons 9/21/2011
 Crab cam (Barlow et al., 2001) self inhibition recurrent inhibition lateral inhibition - L16. Neural processing in Linear Systems: Temporal and Spatial Filtering C. D. Hopkins Sept. 21, 2011 The Neural
Crab cam (Barlow et al., 2001) self inhibition recurrent inhibition lateral inhibition - L16. Neural processing in Linear Systems: Temporal and Spatial Filtering C. D. Hopkins Sept. 21, 2011 The Neural
Temporal whitening by power-law adaptation in neocortical neurons
 Temporal whitening by power-law adaptation in neocortical neurons Christian Pozzorini, Richard Naud, Skander Mensi and Wulfram Gerstner School of Computer and Communication Sciences and School of Life
Temporal whitening by power-law adaptation in neocortical neurons Christian Pozzorini, Richard Naud, Skander Mensi and Wulfram Gerstner School of Computer and Communication Sciences and School of Life
Inventory of Supplemental Information
 Neuron, Volume 71 Supplemental Information Hippocampal Time Cells Bridge the Gap in Memory for Discontiguous Events Christopher J. MacDonald, Kyle Q. Lepage, Uri T. Eden, and Howard Eichenbaum Inventory
Neuron, Volume 71 Supplemental Information Hippocampal Time Cells Bridge the Gap in Memory for Discontiguous Events Christopher J. MacDonald, Kyle Q. Lepage, Uri T. Eden, and Howard Eichenbaum Inventory
How to read a burst duration code
 Neurocomputing 58 60 (2004) 1 6 www.elsevier.com/locate/neucom How to read a burst duration code Adam Kepecs a;, John Lisman b a Cold Spring Harbor Laboratory, Marks Building, 1 Bungtown Road, Cold Spring
Neurocomputing 58 60 (2004) 1 6 www.elsevier.com/locate/neucom How to read a burst duration code Adam Kepecs a;, John Lisman b a Cold Spring Harbor Laboratory, Marks Building, 1 Bungtown Road, Cold Spring
Biosciences in the 21st century
 Biosciences in the 21st century Lecture 1: Neurons, Synapses, and Signaling Dr. Michael Burger Outline: 1. Why neuroscience? 2. The neuron 3. Action potentials 4. Synapses 5. Organization of the nervous
Biosciences in the 21st century Lecture 1: Neurons, Synapses, and Signaling Dr. Michael Burger Outline: 1. Why neuroscience? 2. The neuron 3. Action potentials 4. Synapses 5. Organization of the nervous
Nature Neuroscience: doi: /nn.2662
 Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests
 Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests J. Benda, M. Bethge, M. Hennig, K. Pawelzik & A.V.M. Herz February, 7 Abstract Spike-frequency adaptation is a common feature of
Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests J. Benda, M. Bethge, M. Hennig, K. Pawelzik & A.V.M. Herz February, 7 Abstract Spike-frequency adaptation is a common feature of
#A offered. #B offered. Firing rate. Value of X
 15 12 #A offered 9 6 3 2 1 0 0 1 2 3 4 5 6 8 10 15 20 25 30 35 40 45 #B offered Figure S1. The x-axis and y-axis represent, respectively, the quantities of juices B and A offered in any given trial, and
15 12 #A offered 9 6 3 2 1 0 0 1 2 3 4 5 6 8 10 15 20 25 30 35 40 45 #B offered Figure S1. The x-axis and y-axis represent, respectively, the quantities of juices B and A offered in any given trial, and
Introduction to Statistics with GraphPad Prism 7
 Introduction to Statistics with GraphPad Prism 7 Outline of the course Power analysis with G*Power Basic structure of a GraphPad Prism project Analysis of qualitative data Chi-square test Analysis of quantitative
Introduction to Statistics with GraphPad Prism 7 Outline of the course Power analysis with G*Power Basic structure of a GraphPad Prism project Analysis of qualitative data Chi-square test Analysis of quantitative
DETAILED CONTENTS PART I INTRODUCTION AND DESCRIPTIVE STATISTICS. 1. Introduction to Statistics
 DETAILED CONTENTS About the Author Preface to the Instructor To the Student How to Use SPSS With This Book PART I INTRODUCTION AND DESCRIPTIVE STATISTICS 1. Introduction to Statistics 1.1 Descriptive and
DETAILED CONTENTS About the Author Preface to the Instructor To the Student How to Use SPSS With This Book PART I INTRODUCTION AND DESCRIPTIVE STATISTICS 1. Introduction to Statistics 1.1 Descriptive and
Subthreshold cross-correlations between cortical neurons: Areference model with static synapses
 Neurocomputing 65 66 (25) 685 69 www.elsevier.com/locate/neucom Subthreshold cross-correlations between cortical neurons: Areference model with static synapses Ofer Melamed a,b, Gilad Silberberg b, Henry
Neurocomputing 65 66 (25) 685 69 www.elsevier.com/locate/neucom Subthreshold cross-correlations between cortical neurons: Areference model with static synapses Ofer Melamed a,b, Gilad Silberberg b, Henry
Introduction to Statistical Analysis using IBM SPSS Statistics (v24)
 to Statistical Analysis using IBM SPSS Statistics (v24) to Statistical Analysis Using IBM SPSS Statistics is a two day instructor-led classroom course that provides an application-oriented introduction
to Statistical Analysis using IBM SPSS Statistics (v24) to Statistical Analysis Using IBM SPSS Statistics is a two day instructor-led classroom course that provides an application-oriented introduction
Neural Encoding: Firing Rates and Spike Statistics
 Neural Encoding: Firing Rates and Spike Statistics Dayan and Abbott (21) Chapter 1 Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Background: Dirac δ Function Dirac δ function has the following
Neural Encoding: Firing Rates and Spike Statistics Dayan and Abbott (21) Chapter 1 Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Background: Dirac δ Function Dirac δ function has the following
How Behavioral Constraints May Determine Optimal Sensory Representations
 How Behavioral Constraints May Determine Optimal Sensory Representations by Salinas (2006) CPSC 644 Presented by Yoonsuck Choe Motivation Neural response is typically characterized in terms of a tuning
How Behavioral Constraints May Determine Optimal Sensory Representations by Salinas (2006) CPSC 644 Presented by Yoonsuck Choe Motivation Neural response is typically characterized in terms of a tuning
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11419 Supplementary Figure 1 Schematic representation of innate immune signaling pathways induced by intracellular Salmonella in cultured macrophages. a, During the infection Salmonella
doi:10.1038/nature11419 Supplementary Figure 1 Schematic representation of innate immune signaling pathways induced by intracellular Salmonella in cultured macrophages. a, During the infection Salmonella
Will Penny. 21st April The Macroscopic Brain. Will Penny. Cortical Unit. Spectral Responses. Macroscopic Models. Steady-State Responses
 The The 21st April 2011 Jansen and Rit (1995), building on the work of Lopes Da Sliva and others, developed a biologically inspired model of EEG activity. It was originally developed to explain alpha activity
The The 21st April 2011 Jansen and Rit (1995), building on the work of Lopes Da Sliva and others, developed a biologically inspired model of EEG activity. It was originally developed to explain alpha activity
Neuroscience Introduction
 Neuroscience Introduction The brain As humans, we can identify galaxies light years away, we can study particles smaller than an atom. But we still haven t unlocked the mystery of the three pounds of matter
Neuroscience Introduction The brain As humans, we can identify galaxies light years away, we can study particles smaller than an atom. But we still haven t unlocked the mystery of the three pounds of matter
Parallel processing of visual space by neighboring neurons in mouse visual cortex
 Parallel processing of visual space by neighboring neurons in mouse visual cortex Spencer Lavere Smith, Michael Hausser To cite this version: Spencer Lavere Smith, Michael Hausser. Parallel processing
Parallel processing of visual space by neighboring neurons in mouse visual cortex Spencer Lavere Smith, Michael Hausser To cite this version: Spencer Lavere Smith, Michael Hausser. Parallel processing
Simultaneous intracellular chloride and ph measurements using a GFPbased
 nature methods Simultaneous intracellular chloride and ph measurements using a GFPbased sensor Daniele Arosio, Fernanda Ricci, Laura Marchetti, Roberta Gualdani, Lorenzo Albertazzi & Fabio Beltram Supplementary
nature methods Simultaneous intracellular chloride and ph measurements using a GFPbased sensor Daniele Arosio, Fernanda Ricci, Laura Marchetti, Roberta Gualdani, Lorenzo Albertazzi & Fabio Beltram Supplementary
Supplementary Figure 1 Analysis of beige fat and cells and characteristics of exosome release, related to Figure 1
 Supplementary Figure 1 Analysis of beige fat and cells and characteristics of exosome release, related to Figure 1 (a) Fold-change in UCP-1 mrna abundance in white adipocytes upon β-adrenergic stimulation
Supplementary Figure 1 Analysis of beige fat and cells and characteristics of exosome release, related to Figure 1 (a) Fold-change in UCP-1 mrna abundance in white adipocytes upon β-adrenergic stimulation
A Multivariate Time-Frequency Based Phase Synchrony Measure for Quantifying Functional Connectivity in the Brain
 A Multivariate Time-Frequency Based Phase Synchrony Measure for Quantifying Functional Connectivity in the Brain Dr. Ali Yener Mutlu Department of Electrical and Electronics Engineering, Izmir Katip Celebi
A Multivariate Time-Frequency Based Phase Synchrony Measure for Quantifying Functional Connectivity in the Brain Dr. Ali Yener Mutlu Department of Electrical and Electronics Engineering, Izmir Katip Celebi
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. Sarcomere length-dependence of total fluorescence intensity in a relaxed muscle fibre containing BSR-RLC. a) Fluorescence intensity (I) relative to the value
Supplementary Figures Supplementary Figure 1. Sarcomere length-dependence of total fluorescence intensity in a relaxed muscle fibre containing BSR-RLC. a) Fluorescence intensity (I) relative to the value
When do Correlations Increase with Firing Rates? Abstract. Author Summary. Andrea K. Barreiro 1* and Cheng Ly 2
 When do Correlations Increase with Firing Rates? Andrea K. Barreiro 1* and Cheng Ly 2 1 Department of Mathematics, Southern Methodist University, Dallas, TX 75275 U.S.A. 2 Department of Statistical Sciences
When do Correlations Increase with Firing Rates? Andrea K. Barreiro 1* and Cheng Ly 2 1 Department of Mathematics, Southern Methodist University, Dallas, TX 75275 U.S.A. 2 Department of Statistical Sciences
Frequency Adaptation and Bursting
 BioE332A Lab 3, 2010 1 Lab 3 January 5, 2010 Frequency Adaptation and Bursting In the last lab, we explored spiking due to sodium channels. In this lab, we explore adaptation and bursting due to potassium
BioE332A Lab 3, 2010 1 Lab 3 January 5, 2010 Frequency Adaptation and Bursting In the last lab, we explored spiking due to sodium channels. In this lab, we explore adaptation and bursting due to potassium
LAB 3 INSTRUCTIONS SIMPLE LINEAR REGRESSION
 LAB 3 INSTRUCTIONS SIMPLE LINEAR REGRESSION In this lab you will first learn how to display the relationship between two quantitative variables with a scatterplot and also how to measure the strength of
LAB 3 INSTRUCTIONS SIMPLE LINEAR REGRESSION In this lab you will first learn how to display the relationship between two quantitative variables with a scatterplot and also how to measure the strength of
9 Generation of Action Potential Hodgkin-Huxley Model
 9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 12, W.W. Lytton, Hodgkin-Huxley Model) 9.1 Passive and active membrane models In the previous lecture we have considered a passive
9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 12, W.W. Lytton, Hodgkin-Huxley Model) 9.1 Passive and active membrane models In the previous lecture we have considered a passive
Single-Compartment Neural Models
 Single-Compartment Neural Models BENG/BGGN 260 Neurodynamics University of California, San Diego Week 2 BENG/BGGN 260 Neurodynamics (UCSD) Single-Compartment Neural Models Week 2 1 / 18 Reading Materials
Single-Compartment Neural Models BENG/BGGN 260 Neurodynamics University of California, San Diego Week 2 BENG/BGGN 260 Neurodynamics (UCSD) Single-Compartment Neural Models Week 2 1 / 18 Reading Materials
Introduction and summary of the chapters
 Introduction and summary of the chapters 1. Electroreception Electroreception is the ability of animal species to detect weak electric fields. It is mediated by a sensory system that occurs in some aquatic
Introduction and summary of the chapters 1. Electroreception Electroreception is the ability of animal species to detect weak electric fields. It is mediated by a sensory system that occurs in some aquatic
Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons
 Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons Dileep George a,b Friedrich T. Sommer b a Dept. of Electrical Engineering, Stanford University 350 Serra Mall, Stanford,
Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons Dileep George a,b Friedrich T. Sommer b a Dept. of Electrical Engineering, Stanford University 350 Serra Mall, Stanford,
encoding and estimation bottleneck and limits to visual fidelity
 Retina Light Optic Nerve photoreceptors encoding and estimation bottleneck and limits to visual fidelity interneurons ganglion cells light The Neural Coding Problem s(t) {t i } Central goals for today:
Retina Light Optic Nerve photoreceptors encoding and estimation bottleneck and limits to visual fidelity interneurons ganglion cells light The Neural Coding Problem s(t) {t i } Central goals for today:
Jan 16: The Visual System
 Geometry of Neuroscience Matilde Marcolli & Doris Tsao Jan 16: The Visual System References for this lecture 1977 Hubel, D. H., Wiesel, T. N., Ferrier lecture 2010 Freiwald, W., Tsao, DY. Functional compartmentalization
Geometry of Neuroscience Matilde Marcolli & Doris Tsao Jan 16: The Visual System References for this lecture 1977 Hubel, D. H., Wiesel, T. N., Ferrier lecture 2010 Freiwald, W., Tsao, DY. Functional compartmentalization
AT2 Neuromodeling: Problem set #3 SPIKE TRAINS
 AT2 Neuromodeling: Problem set #3 SPIKE TRAINS Younesse Kaddar PROBLEM 1: Poisson spike trains Link of the ipython notebook for the code Brain neuron emit spikes seemingly randomly: we will aim to model
AT2 Neuromodeling: Problem set #3 SPIKE TRAINS Younesse Kaddar PROBLEM 1: Poisson spike trains Link of the ipython notebook for the code Brain neuron emit spikes seemingly randomly: we will aim to model
Computation of linear acceleration through an internal model. in the macaque cerebellum
 Computation of linear acceleration through an internal model in the macaque cerebellum Jean Laurens, Hui Meng and Dora E. Angelaki Supplementary Materials Legends of Supplementary Movies 1 and 2 Supplementary
Computation of linear acceleration through an internal model in the macaque cerebellum Jean Laurens, Hui Meng and Dora E. Angelaki Supplementary Materials Legends of Supplementary Movies 1 and 2 Supplementary
Probing Real Sensory Worlds of Receivers with Unsupervised Clustering
 with Unsupervised Clustering Michael Pfeiffer 1,2 *, Manfred Hartbauer 3, Alexander B. Lang 3, Wolfgang Maass 1, Heinrich Römer 3 1 Institute for Theoretical Computer Science, TU Graz, Graz, Austria, 2
with Unsupervised Clustering Michael Pfeiffer 1,2 *, Manfred Hartbauer 3, Alexander B. Lang 3, Wolfgang Maass 1, Heinrich Römer 3 1 Institute for Theoretical Computer Science, TU Graz, Graz, Austria, 2
We observe the model neuron s response to constant input current, studying the dependence of:
 BioE332A Lab 2, 21 1 Lab 2 December 23, 29 A Spiking Neuron Like biological neurons, the model neuron we characterize in this lab has a repertoire of (model) ion-channel populations. Here we focus on the
BioE332A Lab 2, 21 1 Lab 2 December 23, 29 A Spiking Neuron Like biological neurons, the model neuron we characterize in this lab has a repertoire of (model) ion-channel populations. Here we focus on the
When is an Integrate-and-fire Neuron like a Poisson Neuron?
 When is an Integrate-and-fire Neuron like a Poisson Neuron? Charles F. Stevens Salk Institute MNL/S La Jolla, CA 92037 cfs@salk.edu Anthony Zador Salk Institute MNL/S La Jolla, CA 92037 zador@salk.edu
When is an Integrate-and-fire Neuron like a Poisson Neuron? Charles F. Stevens Salk Institute MNL/S La Jolla, CA 92037 cfs@salk.edu Anthony Zador Salk Institute MNL/S La Jolla, CA 92037 zador@salk.edu
Food delivered. Food obtained S 3
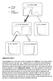 Press lever Enter magazine * S 0 Initial state S 1 Food delivered * S 2 No reward S 2 No reward S 3 Food obtained Supplementary Figure 1 Value propagation in tree search, after 50 steps of learning the
Press lever Enter magazine * S 0 Initial state S 1 Food delivered * S 2 No reward S 2 No reward S 3 Food obtained Supplementary Figure 1 Value propagation in tree search, after 50 steps of learning the
The functional organization of the visual cortex in primates
 The functional organization of the visual cortex in primates Dominated by LGN M-cell input Drosal stream for motion perception & spatial localization V5 LIP/7a V2 V4 IT Ventral stream for object recognition
The functional organization of the visual cortex in primates Dominated by LGN M-cell input Drosal stream for motion perception & spatial localization V5 LIP/7a V2 V4 IT Ventral stream for object recognition
Biomedical Instrumentation
 ELEC ENG 4BD4: Biomedical Instrumentation Lecture 5 Bioelectricity 1. INTRODUCTION TO BIOELECTRICITY AND EXCITABLE CELLS Historical perspective: Bioelectricity first discovered by Luigi Galvani in 1780s
ELEC ENG 4BD4: Biomedical Instrumentation Lecture 5 Bioelectricity 1. INTRODUCTION TO BIOELECTRICITY AND EXCITABLE CELLS Historical perspective: Bioelectricity first discovered by Luigi Galvani in 1780s
