Constraints on Subduction Zone Fluid Compositions Based on Mineral Solubility and Dielectric Constant of H 2 O
|
|
|
- Rosalyn Shields
- 5 years ago
- Views:
Transcription
1 Running Title: Subduction Zone Fluid Constraints Constraints on Subduction Zone Fluid Compositions Based on Mineral Solubility and Dielectric Constant of H 2 O Jeffrey B. Creamer * Department of Earth Science University of California, Santa Barbara Frank J. Spera Department of Earth Science, University of California, Santa Barbara Institute for Crustal Studies, University of California, Santa Barbara * Corresponding author: jcreamer@umail.ucsb.edu 1
2 Abstract A method is developed to estimate the solute content of aqueous fluids in subduction zones combining the Born solvation model with laboratory solubility data including a model for the temperature-pressure variation of the relative permittivity (dielectric constant) of H 2 O. A molecular model for the dielectric constant of H 2 O for densities to 1400 kg/m 3 is calibrated using existing measurements, the dipole moment and water molecule diameter. Computed values are reported for the P-T range 0.5 to 4.5 GPa and 400 to 1400 o C. Solubility relations for SiO 2 -H 2 O and Mg 2 SiO 4 -CaMgSi 2 O 6 - H 2 O are mapped onto hot and cold subduction zone models. At depths relevant to magma genesis, the solubility of common phases at equivalent positions within hot and cold slabs can differ by <1 to >3 orders of magnitude. This impacts consideration of trace element redistribution via metasomatic fluid migration in the mantle wedge and the composition of subduction zone primary melts. 2
3 Introduction Aqueous fluids are ubiquitous in subduction zones and are critical for both magma generation and mantle metasomatism and hence the geochemical evolution of the Earth s upper mantle. Fluids transferred from the slab to the mantle wedge traverse strong lateral temperature and bulk composition gradients and are thus expected to carry a wide diversity and concentration of solutes (i.e. Kessel et al 2005). There are several approaches to constrain fluid solute content in subduction zones: analysis of fluid inclusions from exhumed slabs (i.e. Scambelluri and Philippot 2001); inverse modeling of arc basalts to constrain compositions of source components (including slab-derived fluids), and experimental solubility studies on simple (i.e. SiO 2 ; Manning 1994) or multicomponent (i.e. H 2 O-CO 2 -basalt; Holloway 1971) systems. Existing mineral solubility studies cover an incomplete patchwork of temperature, pressure and composition variations encountered in subduction zones. Thus, some basis for interpolation and extrapolation of mineral solubility is desired. The goal here is to assess the solute content of supercritical subduction zone fluids to conditions of pressures, P in range GPa and temperatures, T from o C relevant to hydrous phase dehydration and magma generation. The approach used in this study is the establishment of an empirical solubility scaling law based on identity of the solid phase, temperature T, and the relative permittivity or dielectric constant, ε(ρ,t), of water, the predominant species in subduction zone fluids Dielectric Constant Model 3
4 A fundamental parameter governing mineral solubility in a dipolar fluid is its dielectric constant. Fluids of high dielectric constant are good solvents because the dielectric fluid reduces the electrostatic forces holding the crystal together. At 20 o C and 1 bar the dielectric constant of water is 83 whereas at 500 C and 0.5 GPa, ε 19 (Heger et al 1980). Although increasing temperature decreases the solvent abilities of H 2 O, pressure has just the opposite effect. Using the results of this study, at 1000 C ε increases by a factor of three as pressure increases from 0.5 to 4.5 GPa. A useful functional form to scale mineral solubility based on the law of mass action and the Born solvation model is: [1] log m = A + 1 # T B+ C & % ( $ "( p,t) ' where m is the molality of the solute, T is temperature, ε is the dielectric constant and A, B, and C are empirically determined constants derived from mineral solubilities. Model equations for fluid dielectric constants are typically expressed as functions of fluid temperature and density rather than pressure. Since mineral solubility studies often report fluid pressure rather than density, conversion using an equation of state (EOS) for water is required. Pitzer and Sterner (1994) present an EOS for water valid to 10 GPa and 10,000 K. A calibrated model for the dielectric constant of water based on experimental data has previously been generated by Franck et al (1990) using existing experimental dielectric constant data (Heger et al 1980; Deul 1984). However, the expression utilized used by Franck et al (1990) reaches a numerical instability at water densities >1250 kg/m 3, restricting its geochemical application. As an alternative, a model for the dielectric constant of a generic fluid developed by Tani et al (1983) and modified by Valisko et al (2001) is not subject to numerical instabilities at high pressures 4
5 encountered in subduction zones, and is calibrated here for water following the procedure of Franck et al (see Fig. 1). Experimental data for the dielectric constant at subcritical conditions (<374 o C) are omitted from consideration. Molecular Dynamics simulations of the dielectric constant at high pressure and temperature (Wasserman et al 1995) are also omitted because they fail to conform to the behavior predicted by the Valisko et al 2001 model, even under conditions where they overlap with experimental data. This suggests that the rigid molecule potential used in the MD simulations of Wasserman et al is an inadequate representation of the orientational disorder of the water dipole in the pressure-temperature range relevant to subduction metasomatism. The molecular model used to predict the dielectric constant of water described by Valsiko et al (2001) approximates a generic dipolar fluid as a collection of spherically symmetric molecules with an imbedded dipole moment. The dielectric constant is related to the properties of the fluid according to + % 9I [2] "(p,t) = n 2 1+ f (n) y + g(n) y 2 + f (n)' & 16# $h(n) (. - * y 4, 2 0 ) / where y = 4πρµ 2 /9kT, and k is the Boltzmann constant, T is temperature (K), ρ = N/V is the number-density of water molecules, N is the number of water molecules in volume V, µ is the magnitude of the water dipole moment and I=(17π 2 /9)*( ρ* ρ* 2 )/( ρ* ρ* 2 ) where ρ* = ρσ 3 and σ is the molecular diameter. The refractive index functions f(n), g(n), and h(n) are defined f(n) = (n 2 +2)/(2n 2 +1), g(n) = (n 2 +2) 4 /(2n+1) 3 and h(n)= (2n 2-1) (n 2 +2) 4 /(2n 2 +1) 4 respectively and are functions of temperature and fluid density (Schiebener et al 1990). Fluid temperature is input from experimental data, and fluid mass-density is calculated from experimental temperature and pressure using the EOS from Pitzer and Sterner The 5
6 diameter of the water molecule (σ) and magnitude of the dipole moment (µ) are independent parameters, and were chosen to minimize residuals for experimental data for the dielectric constant of water. Here, σ=2.3 x m and µ=7.19 x Coloumbmeters were chosen to optimize experimental data. We note that the model equation developed by Tani et al 1983, which does not incorporate the fluid refractive index but is otherwise identical to the equation of Valsiko et al 2001, does not represent experimental data well. In distinction, by allowing for the density and temperature dependence of the index of refraction of water the fit is significantly improved and allows extrapolation. Interpolated and extrapolated values for the dielectric constant of water in the range 400 to 1400 o C and 0.5 to 4.5 GPa are reported in Table 1 and serve as provisional values until further laboratory measurements are made Solubility Scaling Five different mineral solubility datasets were evaluated to establish the utility of Eq. [1] (Fig. 2). These data are limited to solubility in pure H 2 O and one to three coexisting crystalline phases. Despite the significant range in experimental pressure (0.005 to 2.3 GPa), temperature (100 to 1000 o C), solute content (dilute to >12 wt % total solute), and a diversity of bulk compositions, a consistent relationship between log(m) and 1/T. [B+C/ε] exists for all observed conditions. The extrapolation of these scaling relationships should be limited to the stability field of the mineral species and by the second critical endpoint of the mineral-water systems (i.e. Keppler, 1996; Kessel et al. 2005). For example, the solubility of quartz is extended to regions of P-T space where coesite is the stable polymorph. We are not aware of experimental coesite solubility data. 6
7 Application to Subduction Zones The solubility scaling relationships for simple mineral-water systems can be applied to thermal models for subduction zones to gain insight into the range of solute concentrations both within an individual subduction zone and between different (i.e. hot and cold ) subduction zones. Because of extreme lateral gradients in temperature at fixed depth in and around a subducting slab, very strong mineral solubility gradients are anticipated. This expectation is indeed correct and, to our knowledge, has not been addressed previously. The thermal models analyzed here were developed by van Keken et al (2002) to include temperature and strain-rate dependent mantle viscosity, and are calibrated to approximate natural subduction zones based on observed or inferred parameters such as convergence rate, slab geometry, and forearc heatflow. The Cascadia and NE Japan subduction zone models were selected since they approximate global extremes for minimum and maximum penetration of isotherms into the mantle. Solubilities for the quartz and forsterite-diopside systems within the upper 10 km of subducting lithosphere and adjoining 10 km of mantle wedge are portrayed in Figure 3. Due to steep lateral thermal gradients, there are strong solubility gradients along hypothesized transport paths for slab-derived fluids migrating into the mantle wedge. As an extreme case, at a fixed depth of 100 km the solubility of rutile is 1000 times greater at the slab-mantle interface than at a position 3 km within the cold subducting lithosphere. Diopside-forsterite solubility is 10 to 100 times greater in hot than cold subduction zones at most equivalent positions within the slab for most depths < 100km. For example, the molality (moles of solute/kg of fluid) of diopside-forsterite within the 10 km lateral 7
8 section of slab at 80 km depth ranges from -4.3 and -2 log units for the cold slab and -2.2 to -1.8 for the hot slab Discussion Available mineral solubility data relevant to the deep lithosphere, the mantle wedge and adjacent descending slabs cover temperature-pressure-composition space in a patchwork manner. Methods of extrapolating available data based on theoretical or semitheoretical formulations are therefore useful in assessing in the solute content of subduction zone fluids. The realization that Born theory correlates the solubility of oxide and silicate geomaterials at diverse state conditions, and from dilute solutions to solutions with >12 wt% solute, lends credence to the conclusion that very large solubility gradients exist near the region of strong temperature contrast near the slab-wedge interface. There are no state conditions where the relationship between solute content of the fluid (log m) and the inverses of temperature (T) and dielectric constant (ε) is not linear and strongly correlated (fig. 2), so extrapolation using the solubility scaling relationships explored here (eq. [1]) is reasonable. The electrostatic approach to mineral solubility has the advantage of bypassing a traditional pitfall experienced using solubility models based on the free energy of solvation (i.e. Zhang and Frantz 2000) in that the model is not dependant upon the speciation of dissolved material (Walther and Schott 1988). With the eventual goal of treating real, complex fluids, a model in which solubility can be expressed independent of aqueous speciation will be particularly advantageous. The approach to mineral solubility outlined here could be applied to complex fluids. There has been a recent proliferation of 8
9 experimental mineral solubility studies with fluids in the system H-O-C-N-S-Cl-F. Mixing rules for the dielectric constant of complex fluids are well established (i.e. Looyenga 1965), but the major inhibition is the lack of knowledge of the dielectric constant of volatile species other than H 2 O. Further experimental measurement or Molecular Dynamics simulations using flexible molecule dipolar potentials for mixed fluids for determination of the relative permittivity at elevated temperatures and pressures should improve upon the reliability of the molecular model for dielectric constant for water presented here. While the estimated absolute value of the dielectric constant at a given P,T condition would be subject to change with the availability of further experimental data, the magnitude of the contrast in mineral solubility across the slab-wedge interface is so great that predicted solubility contrasts between hot and cold subduction zones are extremely unlikely to disappear. If the solubility variability for simple mineral-water systems for both inter- and intra-slab scenarios is indeed even grossly representative of real systems, there are important implications. For example, the volumetric proportion of slab-derived refractory material that ends up in parental arc basalts should vary strongly as a function of the slab temperature conditions Conclusion The method developed here to estimate mineral solubility as a function of temperature and pressure using the Born solvation model calibrated from experimental solubility data and a molecular model for the relative permittivity of dipolar H 2 O yields surprisingly well-correlated values for a wide range of experimental conditions (pressure, 9
10 temperature, and solute content). The H 2 O molecular model suggests that the dielectric constant of water varies between ~ 4 and 50, in the range 0.5 to 4.5 GPa and 400 to 1400 o C. Solubility relations suggest differences in the solute-content of slab fluids between hot and cold subduction zones varies by a factor of <10 to >100 within the slab at 100 km depth. The accuracy of this analogy to more realistic phase assemblages and mixed fluids in subduction environments is uncertain, but study of more complex fluids and mineral assemblages is a promising avenue to pursue in the future Acknowledgements The authors wish to thank Peter van Keken for his consultations with thermal modeling. Support from the NSF (NSF/ITR ) is gratefully acknowledged
11 Figure 1. The dielectric constant of water using the model of Valisko et al 2001 extrapolated as a function of fluid pressure along isotherms, superimposed on experimental data. Data from 400 o C are depicted as diamonds, data from 500 o C as squares and data from 550 o C as triangles. Higher temperature experimental measurements for ε do not currently exist. 11
12 o C GPa Table 1. Dielectric constant of water at selected P,T conditions based on the model developed as part of this study. 12
13 Figure 2. Calibration of solubility scaling laws. Experimentally determined solubilities are used to calculate constants in the model equation log(m) = A + 1/T [B+ C/ε]. Experimental Pressure (P) and Temperature (T) are labeled for each experimental set. a: Quartz (Manning 1994), b: Corundum (Walther 1997), c: Rutile (Audetát and Keppler 2005), d: Diopside-Forsterite (Macris and Manning 2005), and e: Paragonite-Quartz- Albite (Woodland and Walther 1987). Note that Diopside solubility is slightly incongruent since a minor amount of forsterite was precipitated. 13
14 Figure 3. The top 10 km section of subducting slab and adjacent 10 km section of mantle wedge, between depths of 50 and 125 km, are considered in this figure (a). Within this section of the subduction zone, temperature fields modeled by van Keken et al 2002 for hot and cold subduction zones are displayed (b). The solubility, log (m), scaling relationships developed in this study for quartz (c) and diopside (d) are superimposed on these sections. Solubility data come from Manning 1994 and Macris and Manning 2005, respectively. 14
15 References Audetát, A. and Keppler, H. (2005). Solubility of rutile in subduction zone fluids, as determined by experiments in the hydrothermal diamond anvil cell. Earth and Planetary Sci. Letters, 232, Dandurand, J. and Schott, J. (1992). Prediction of Ion Association In Mixed-Crustal Fluids. J. Phys. Chem., 96, Deul, R. (1984) Dielektrizitatskonstante und Dichte von Wasser-Benzol-Mischungen bis 450 o C und 3000 bar, Thesis, Inst. Of Phys. Chem., Univ. Karlsruhe. Franck, E., Rosenzweig, S., and Christoforakos, M. (1990). Calculation of the Dielectric Constant of Water to 1000 o C and Very High Pressures. Ber. Bunsenges. Phys. Chem., 94, Heger, K., Uematsu, M., amd Franck, E.U. (1980) The Static Dielectric Constant of Water at High Pressures and Temperatures to 500 MPa and 550 o C. Ber. Bunsenges. Phys. Chem., 84, Holloway, J.R. (1971). Composition of Fluid Phase Solutes in a Basalt-H 2 O-CO 2 System. Geol. Soc. Am., 82, Kessel, R., Ulmer, P., Pettke, T., Schimdt, M.W., and Thompson, A.B., (2005). The water-basalt system at 4 to 6 GPa: Phase relations and second critical endpoint in a K-free ecolgite at 700 to 1400 o C. Earth and Planetary Science Letters, 237, Looyenga, H. (1965) Dielectric constants of heterogeneous mixtures. Physica, 31, 3, Manning, C.E. (1994). The solubility of quartz in H 2 O in the lower crust and upper mantle. Geochim et Cosmochim. Acta., 58, 22, Macris, C.A. and Manning, C.E. (2005) The Solubility of Diopside in Water at 10 to 15 kbar and 650 to 900 C, EOS Trans. AGU, 86(52), Fall Meet. Suppl., Abstract V31C Patey, G.N., Levesque, D., and Weis, J.J. (1979) Integral equation approximations for fluids of hard spheres with dipoles and quardupoles. Molecular Phys., 38, 5, Pitzer, K. S. and Sterner, S.M. (1994) Equations of state valid continuously from zero to extreme pressures for H 2 O and CO 2. J. Chem. Phys., 101, 4, Scambelluri, M. and Philippot, P. (2001). Deep fluids in subduction zones. Lithos, 55, 15
16 Schiebener, P., Straub, J., Levelt-Sengers, J.M.H., and Gallagher, J.S. (1990) Refractive index of water and steam as function of wavelength, temperature, and density. J. Phys. Chem. Ref. Data., 19, 3, Tani, A., Henderson, D., and Barker, J.A. (1983) Application of perturbation theory to the calculation of the dielectric constant of a dipolar hard sphere fluid. Molecular Phys., 48, 4, Valiskó, M., Boda, D., Liszi, J., and Szalai, I. (2001). Relative perimittivity of dipolar liquids and their mixtures. Comparison of theory and experiment. Phys. Chem. Chem. Phys., 3, Van Keken, P.E., Kiefer, B., and Peacock, S.M. (2002). High-resolution models of subduction zones: Implications for mineral dehydration reactions and the transport of water into the deep mantle. Geochem. Geophys. Geosys. 3, 10, /2001GC Walther, J.V. (1997) Experimental determination and interpretation of the solubility of corundum in H 2 O between 350 and 600 o C from 0.5 to 2.2 kbar. Geochim. Et Cosmochim. Acta, 61, 23, Walther, J. and Schott, J. (1988). The dielectric constant approach to speciation and ion paring at high temperature and pressure. Nature, 322, 14, Wasserman, E., Wood, B., and Brodholt, J. (1995). The static dielectric constant of water at pressures up to 20 kbar and temperatures to 1273K: Experiments, simulations, and empirical equations. Geochimica et Cosmochimica Acta, 59, 1, 1-6. Woodland, A.B. and Walther, J.V. (1987). Experimental determination of the solubility of the assemblage paragonite, albite, quartz in supercritical water. Geochim. Et Cosmochim. Acta, 51, Xie, Z. and Walther, J.V. (1992). Quartz solubilities in NaCl solutions with and without wollastonite at elevated temperatures and pressures. Geochim. Cosmochim. Acta., 57, Zhang, Y. and Frantz, J.D. (2000) Enstatite-forsterite-water equilibria at elevated temperatures and pressures. Am. Mineralogist, 85,
GSA Data Repository
 GSA Data Repository 218145 Parolari et al., 218, A balancing act of crust creation and destruction along the western Mexican convergent margin: Geology, https://doi.org/1.113/g39972.1. 218145_Tables DR1-DR4.xls
GSA Data Repository 218145 Parolari et al., 218, A balancing act of crust creation and destruction along the western Mexican convergent margin: Geology, https://doi.org/1.113/g39972.1. 218145_Tables DR1-DR4.xls
Fluids, melts, and supercriticality in the MSH system and element transport in subduction zones
 cosmic rays Fluids, s, and supercriticality in the MSH system and element transport in subduction zones 10 Be volcanic front N, O 10 Be ocean water + CO 2 tracing petrologic and geotectonic processes (trace)
cosmic rays Fluids, s, and supercriticality in the MSH system and element transport in subduction zones 10 Be volcanic front N, O 10 Be ocean water + CO 2 tracing petrologic and geotectonic processes (trace)
Description of Supplementary Files
 Description of Supplementary Files File Name: Supplementary Information Description: Supplementary Figures, Supplementary Tables and Supplementary References File Name: Peer Review File Supplementary figure
Description of Supplementary Files File Name: Supplementary Information Description: Supplementary Figures, Supplementary Tables and Supplementary References File Name: Peer Review File Supplementary figure
IAPWS Certified Research Need - ICRN
 IAPWS Certified Research Need - ICRN ICRN 21 Thermophysical Properties Associated with Ultra-supercritical Coal-fired Steam Generators The IAPWS Working Groups Physical Chemistry of Aqueous Systems and
IAPWS Certified Research Need - ICRN ICRN 21 Thermophysical Properties Associated with Ultra-supercritical Coal-fired Steam Generators The IAPWS Working Groups Physical Chemistry of Aqueous Systems and
Constitution of Magmas. Magmas. Gas Law. Composition. Atomic Structure of Magma. Structural Model. PV = nrt H 2 O + O -2 = 2(OH) -
 Constitution of Magmas Magmas Best, Ch. 8 Hot molten rock T = 700-1200 degrees C Composed of ions or complexes Phase Homogeneous Separable part of the system With an interface Composition Most components
Constitution of Magmas Magmas Best, Ch. 8 Hot molten rock T = 700-1200 degrees C Composed of ions or complexes Phase Homogeneous Separable part of the system With an interface Composition Most components
COH FLUIDS AT UPPER-MANTLE CONDITIONS: AN EXPERIMENTAL STUDY ON VOLATILE SPECIATION AND MINERAL SOLUBILITY IN THE MS + COH SYSTEM
 COH FLUIDS AT UPPER-MANTLE CONDITIONS: AN EXPERIMENTAL STUDY ON VOLATILE SPECIATION AND MINERAL SOLUBILITY IN THE MS + COH SYSTEM CARLA TIRABOSCHI Dipartimento di Scienze della Terra Ardito Desio, Università
COH FLUIDS AT UPPER-MANTLE CONDITIONS: AN EXPERIMENTAL STUDY ON VOLATILE SPECIATION AND MINERAL SOLUBILITY IN THE MS + COH SYSTEM CARLA TIRABOSCHI Dipartimento di Scienze della Terra Ardito Desio, Università
Phase Equilibrium. Phase Rule. Phase Diagram
 Phase Equilibrium Phase Rule Phase Diagram Makaopuhi Lava Lake Magma samples recovered from various depths beneath solid crust From Wright and Okamura, (1977) USGS Prof. Paper, 1004. Makaopuhi Lava Lake
Phase Equilibrium Phase Rule Phase Diagram Makaopuhi Lava Lake Magma samples recovered from various depths beneath solid crust From Wright and Okamura, (1977) USGS Prof. Paper, 1004. Makaopuhi Lava Lake
Mineral Stability and Phase Diagrams Introduction
 1 of 10 10/10/2002 2:50 PM Prof. Stephen A. Nelson Geology 211 Tulane University Mineralogy and Phase Diagrams Introduction This document last updated on 10-Oct-2002 As we discussed previously, there are
1 of 10 10/10/2002 2:50 PM Prof. Stephen A. Nelson Geology 211 Tulane University Mineralogy and Phase Diagrams Introduction This document last updated on 10-Oct-2002 As we discussed previously, there are
Geochemical monitoring of the response ofgeothermal reservoirs to production load examples from Krafla, Iceland
 International Geothermal Conference, Reykjavík, Sept. 23 Session #7 Geochemical monitoring of the response ofgeothermal reservoirs to production load examples from Krafla, Iceland Stefán Arnórsson 1 and
International Geothermal Conference, Reykjavík, Sept. 23 Session #7 Geochemical monitoring of the response ofgeothermal reservoirs to production load examples from Krafla, Iceland Stefán Arnórsson 1 and
GSA DATA REPOSITORY
 GSA DATA REPOSITORY 2013019 Supplemental information for The Solidus of Alkaline Carbonatite in the Deep Mantle Konstantin D. Litasov, Anton Shatskiy, Eiji Ohtani, and Gregory M. Yaxley EXPERIMENTAL METHODS
GSA DATA REPOSITORY 2013019 Supplemental information for The Solidus of Alkaline Carbonatite in the Deep Mantle Konstantin D. Litasov, Anton Shatskiy, Eiji Ohtani, and Gregory M. Yaxley EXPERIMENTAL METHODS
Received 18 July 2013; revised 31 October 2013; accepted 18 November 2013; published 19 December 2013.
 JOURNAL OF GEOPHYSICAL RESEARCH: SOLID EARTH, VOL. 118, 6076 6085, doi:10.1002/2013jb010537, 2013 Structure and equilibria among silicate species in aqueous fluids in the upper mantle: Experimental SiO
JOURNAL OF GEOPHYSICAL RESEARCH: SOLID EARTH, VOL. 118, 6076 6085, doi:10.1002/2013jb010537, 2013 Structure and equilibria among silicate species in aqueous fluids in the upper mantle: Experimental SiO
Thermodynamics of mantle systems
 Thermodynamics of mantle systems Paula Smith*, Laura Baker Hebert, Paul Asimow, Mike Gurnis *a.k.a. Antoshechkina psmith@gps.caltech.edu Why use thermodynamics? Alternatives are simple forcing functions,
Thermodynamics of mantle systems Paula Smith*, Laura Baker Hebert, Paul Asimow, Mike Gurnis *a.k.a. Antoshechkina psmith@gps.caltech.edu Why use thermodynamics? Alternatives are simple forcing functions,
Dynamic processes in the mantle wedge
 Mantle Processes and Geodynamics Dynamic processes in the mantle wedge Ikuko Wada Woods Hole Oceanographic Institution Something Amazing: Despite the apparent complexity of the mantle wedge dynamics, the
Mantle Processes and Geodynamics Dynamic processes in the mantle wedge Ikuko Wada Woods Hole Oceanographic Institution Something Amazing: Despite the apparent complexity of the mantle wedge dynamics, the
Received February 1, 2017; in final form, March 24, 2017
 ISSN 0869-5911, Petrology, 017, Vol. 5, No. 5, pp. 449 457. Pleiades Publishing, Ltd., 017. Published in Russian in Petrologiya, 017, Vol. 5, No. 5, pp. 45 460. H O Activity in Albite Melts at Deep Crustal
ISSN 0869-5911, Petrology, 017, Vol. 5, No. 5, pp. 449 457. Pleiades Publishing, Ltd., 017. Published in Russian in Petrologiya, 017, Vol. 5, No. 5, pp. 45 460. H O Activity in Albite Melts at Deep Crustal
high and ultrahigh pressures.
 Stability of hydrous phases in serpentinites to high and ultrahigh pressures. Study of fluid and multiphase solid inclusions in high, very high pressure rocks and metamorphic veins The crust to mantle
Stability of hydrous phases in serpentinites to high and ultrahigh pressures. Study of fluid and multiphase solid inclusions in high, very high pressure rocks and metamorphic veins The crust to mantle
At the beginning. Matter + antimatter. Matter has the advantage. baryons quarks, leptons, electrons, photons (no protons or neutrons)
 At the beginning Matter + antimatter Matter has the advantage baryons quarks, leptons, electrons, photons (no protons or neutrons) Hadrons protons, neutrons Hydrogen, helium (:0 H:He) Origin of the Universe
At the beginning Matter + antimatter Matter has the advantage baryons quarks, leptons, electrons, photons (no protons or neutrons) Hadrons protons, neutrons Hydrogen, helium (:0 H:He) Origin of the Universe
Geoffrey Abers. Water and volcanism. Imaging. Lessons from Central America. Oceanic. Terrestrial
 Imaging Circum-Pacific Subduction Zones with Earthquakes: Fluid Pathways and the Origins of Volcanic Arcs Geoffrey Abers Oceanic Terrestrial Water and volcanism Imaging Lessons from Central America Wet
Imaging Circum-Pacific Subduction Zones with Earthquakes: Fluid Pathways and the Origins of Volcanic Arcs Geoffrey Abers Oceanic Terrestrial Water and volcanism Imaging Lessons from Central America Wet
Chapter 7 Metamorphism, Metamorphic Rocks, and Hydrothermal Rocks
 Chapter 7 Metamorphism, Metamorphic Rocks, and Hydrothermal Rocks Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Metamorphism What happens to rocks that are
Chapter 7 Metamorphism, Metamorphic Rocks, and Hydrothermal Rocks Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Metamorphism What happens to rocks that are
TWO COMPONENT (BINARY) PHASE DIAGRAMS. Experimental Determination of 2-Component Phase Diagrams
 Page 1 of 12 EENS 211 Earth Materials Tulane University Prof. Stephen A. Nelson TWO COMPONENT (BINARY) PHASE DIAGRAMS This document last updated on 08-Oct-2003 Experimental Determination of 2-Component
Page 1 of 12 EENS 211 Earth Materials Tulane University Prof. Stephen A. Nelson TWO COMPONENT (BINARY) PHASE DIAGRAMS This document last updated on 08-Oct-2003 Experimental Determination of 2-Component
Primary spinel + chlorite inclusions in mantle garnet formed at ultrahigh-pressure
 2017 European Association of Geochemistry Primary spinel + chlorite inclusions in mantle garnet formed at ultrahigh-pressure M. Campione 1*, S. Tumiati 2, N. Malaspina 1 Abstract doi: 10.7185/geochemlet.1730
2017 European Association of Geochemistry Primary spinel + chlorite inclusions in mantle garnet formed at ultrahigh-pressure M. Campione 1*, S. Tumiati 2, N. Malaspina 1 Abstract doi: 10.7185/geochemlet.1730
Chapter 21: Metamorphism. Fresh basalt and weathered basalt
 Chapter 21: Metamorphism Fresh basalt and weathered basalt Chapter 21: Metamorphism The IUGS-SCMR proposed this definition: Metamorphism is a subsolidus process leading to changes in mineralogy and/or
Chapter 21: Metamorphism Fresh basalt and weathered basalt Chapter 21: Metamorphism The IUGS-SCMR proposed this definition: Metamorphism is a subsolidus process leading to changes in mineralogy and/or
Activity coefficient and polymerization of aqueous silica at 800 C, 12 kbar, from solubility measurements on SiO 2 -buffering mineral assemblages
 Contrib Mineral Petrol (2003) 146: 135 143 DOI 10.1007/s00410-003-0483-9 ORIGINAL PAPER Robert C. Newton Æ Craig E. Manning Activity coefficient and polymerization of aqueous silica at 800 C, 12 kbar,
Contrib Mineral Petrol (2003) 146: 135 143 DOI 10.1007/s00410-003-0483-9 ORIGINAL PAPER Robert C. Newton Æ Craig E. Manning Activity coefficient and polymerization of aqueous silica at 800 C, 12 kbar,
Solubility of enstatite forsterite in H 2 O at deep crust/upper mantle conditions: 4 to 15 kbar and 700 to 900 C
 Pergamon PII S0016-7037(02)00998-5 Geochimica et Cosmochimica Acta, Vol. 66, No. 23, pp. 4165 4176, 2002 Copyright 2002 Elsevier Science Ltd Printed in the USA. All rights reserved 0016-7037/02 $22.00.00
Pergamon PII S0016-7037(02)00998-5 Geochimica et Cosmochimica Acta, Vol. 66, No. 23, pp. 4165 4176, 2002 Copyright 2002 Elsevier Science Ltd Printed in the USA. All rights reserved 0016-7037/02 $22.00.00
Magma Formation and Behavior
 Magma Formation and Behavior Questions What causes mantle rock to melt, resulting in magma formation? Why is magma formation restricted to specific plate tectonic settings? Why are mafic (basaltic) magmas
Magma Formation and Behavior Questions What causes mantle rock to melt, resulting in magma formation? Why is magma formation restricted to specific plate tectonic settings? Why are mafic (basaltic) magmas
BIBLIOGRAPHIC REFERENCE
 BIBLIOGRAPHIC REFERENCE Chambefort, I; Bignall, G. 2013. Preliminary stable isotope study on the Lahendong geothermal system, Indonesia, GNS Science Report 2013/14. 9p. I. Chambefort, GNS Science, Wairakei
BIBLIOGRAPHIC REFERENCE Chambefort, I; Bignall, G. 2013. Preliminary stable isotope study on the Lahendong geothermal system, Indonesia, GNS Science Report 2013/14. 9p. I. Chambefort, GNS Science, Wairakei
N = N 0 e -λt D* = N 0 -N D* = N 0 (1-e -λt ) or N(e λt -1) where N is number of parent atoms at time t, N 0
 N = N 0 e -λt D* = N 0 -N D* = N 0 (1-e -λt ) or N(e λt -1) where N is number of parent atoms at time t, N 0 is initial number of parents, D* is number of radiogenic daughter atoms, and λ is the decay
N = N 0 e -λt D* = N 0 -N D* = N 0 (1-e -λt ) or N(e λt -1) where N is number of parent atoms at time t, N 0 is initial number of parents, D* is number of radiogenic daughter atoms, and λ is the decay
Examples of Invariant Points and Bundles of Reactions. Start with the Phase Rule (P + F = C + 2) The phase rule is P + F = C + 2.
 1 Method of Schreinemaker--A Geometric Approach to Constructing Phase Diagrams Dexter Perkins, University of North Dakota & Dave Mogk, Montana State University C:\Courses\320\fall2007\in class\2000-schreinemakers
1 Method of Schreinemaker--A Geometric Approach to Constructing Phase Diagrams Dexter Perkins, University of North Dakota & Dave Mogk, Montana State University C:\Courses\320\fall2007\in class\2000-schreinemakers
XPermission granted.
 Author Ni Sun Title In situ Investigation of Brucite and Aqueous Fluids in the MgO-H 2 0 System at Elevated Pressure and Temperature submitted in partial fulfillment of the requirements for the degree
Author Ni Sun Title In situ Investigation of Brucite and Aqueous Fluids in the MgO-H 2 0 System at Elevated Pressure and Temperature submitted in partial fulfillment of the requirements for the degree
Fluids and trace element transport in subduction zones
 1 Manuscript 5716, 1 st revision 2 3 Fluids and trace element transport in subduction zones 4 5 HANS KEPPLER 6 7 8 Bayerisches Geoinstitut, Universität Bayreuth, 95440 Bayreuth, Germany hans.keppler@uni-bayreuth.de
1 Manuscript 5716, 1 st revision 2 3 Fluids and trace element transport in subduction zones 4 5 HANS KEPPLER 6 7 8 Bayerisches Geoinstitut, Universität Bayreuth, 95440 Bayreuth, Germany hans.keppler@uni-bayreuth.de
The influence of short wavelength variations in viscosity on subduction dynamics
 1 Introduction Deformation within the earth, driven by mantle convection due primarily to cooling and subduction of oceanic lithosphere, is expressed at every length scale in various geophysical observations.
1 Introduction Deformation within the earth, driven by mantle convection due primarily to cooling and subduction of oceanic lithosphere, is expressed at every length scale in various geophysical observations.
Modeling Viscosity of Multicomponent Electrolyte Solutions 1
 International Journal of Thermophysics, Vol. 19, No. 2, 1998 Modeling Viscosity of Multicomponent Electrolyte Solutions 1 M. M. Lencka, 2 A. Anderko, 2,3 S. J. Sanders, 2 and R. D. Young 2 A comprehensive
International Journal of Thermophysics, Vol. 19, No. 2, 1998 Modeling Viscosity of Multicomponent Electrolyte Solutions 1 M. M. Lencka, 2 A. Anderko, 2,3 S. J. Sanders, 2 and R. D. Young 2 A comprehensive
2-C Eutectic Systems
 Phase Equilibrium 2-C Eutectic Systems Example: Diopside - Anorthite No solid solution * For system at this T, X, phase(s) plot where? Fig. 6.11. Isobaric T-X phase diagram at atmospheric pressure. After
Phase Equilibrium 2-C Eutectic Systems Example: Diopside - Anorthite No solid solution * For system at this T, X, phase(s) plot where? Fig. 6.11. Isobaric T-X phase diagram at atmospheric pressure. After
Theoretical mineralogy, density, seismic wavespeeds and H 2
 The Cascadia Subduction Zone and Related Subduction Systems 133 Theoretical mineralogy, density, seismic wavespeeds and H 2 O content of the Cascadia subduction zone, with implications for intermediate-depth
The Cascadia Subduction Zone and Related Subduction Systems 133 Theoretical mineralogy, density, seismic wavespeeds and H 2 O content of the Cascadia subduction zone, with implications for intermediate-depth
Interpreting Phase Diagrams
 Interpreting Phase Diagrams Understanding chemical reactions requires that we know something about how materials behave as the temperature and pressure change. For a single component (like quartz or ice)
Interpreting Phase Diagrams Understanding chemical reactions requires that we know something about how materials behave as the temperature and pressure change. For a single component (like quartz or ice)
Chapter 18: Granitoid Rocks. Chapter 18: Granitoid Rocks. Melting of crustal materials at high pressure
 Melting of crustal materials at high pressure Melting in the crust: the traditional low pressure view to be applied to HP CaO P 2 O 5 Zircon from a HP granite HP-HT garnets from Massif Central (Vielzeuf
Melting of crustal materials at high pressure Melting in the crust: the traditional low pressure view to be applied to HP CaO P 2 O 5 Zircon from a HP granite HP-HT garnets from Massif Central (Vielzeuf
Metasomatism Model. Metasomatism. Fluid Buffers. Volatile Species. C-O-H-S System. Speciation in C-O-H-S fluids
 Metasomatism Model Metasomatism Reading: Winter, Chapter 30 Obvious in rocks with contrasting mineral layers Related to unequal partitioning of elements between solid phases and fluids Model uses ion-exchange
Metasomatism Model Metasomatism Reading: Winter, Chapter 30 Obvious in rocks with contrasting mineral layers Related to unequal partitioning of elements between solid phases and fluids Model uses ion-exchange
Geodynamics of MARGINS
 Geodynamics of MARGINS Scott King (Virginia Tech), Peter van Keken (Univ. Michigan), Mike Gurnis (Caltech), Marc Spiegelman (Lamont), Shijie Zhong (Univ. Colorado), Magali Billen (U.C. Davis) contact:
Geodynamics of MARGINS Scott King (Virginia Tech), Peter van Keken (Univ. Michigan), Mike Gurnis (Caltech), Marc Spiegelman (Lamont), Shijie Zhong (Univ. Colorado), Magali Billen (U.C. Davis) contact:
T-X Diagrams C:\Courses\320\fall2007\in class\5000-t-x Exercise.wpd; September 25, 2003 (11:45am)
 1 T-X Diagrams C:\Courses\320\fall2007\in class\5000-t-x Exercise.wpd; September 25, 2003 (11:45am) T-X diagrams are most often used for describing metamorphism of carbonate-rich rocks (marbles or marls)
1 T-X Diagrams C:\Courses\320\fall2007\in class\5000-t-x Exercise.wpd; September 25, 2003 (11:45am) T-X diagrams are most often used for describing metamorphism of carbonate-rich rocks (marbles or marls)
GEOL 414/514 ACTIVITY COEFFICIENTS OF DISSOLVED SPECIES
 GEOL 414/514 ACTIVITY COEFFICIENTS OF DISSOLVED SPECIES Chapter 4 LANGMUIR ACTIVITY & ACTIVITY COEFFICIENTS Earlier we studied common ion effect on decreasing the solubility CaCO 3 Ca +2 + CO 3 Add Ca
GEOL 414/514 ACTIVITY COEFFICIENTS OF DISSOLVED SPECIES Chapter 4 LANGMUIR ACTIVITY & ACTIVITY COEFFICIENTS Earlier we studied common ion effect on decreasing the solubility CaCO 3 Ca +2 + CO 3 Add Ca
Anderson RN, Uyeda S, Miyashiro A. (1976) Geophysical and geochemical constraints
 REFERENCES RELEVANT TO PERIDOTITE PARTIAL MELTING IN THE MANTLE WEDGE Anderson RN, Uyeda S, Miyashiro A. (1976) Geophysical and geochemical constraints at converging plate boundaries - I: Dehydration in
REFERENCES RELEVANT TO PERIDOTITE PARTIAL MELTING IN THE MANTLE WEDGE Anderson RN, Uyeda S, Miyashiro A. (1976) Geophysical and geochemical constraints at converging plate boundaries - I: Dehydration in
Prediction of Calcite Scaling at the Oguni Geothermal Field, Japan: Chemical Modeling Approach
 Todaka et Prediction of Calcite Scaling at the Oguni Geothermal Field, Japan: Chemical Modeling Approach Norifumi Yoshiyuki Hideo and Nobuyuki Electric Power Development Co., Ltd. 6-15-1, Ginza, Chuo-ku,
Todaka et Prediction of Calcite Scaling at the Oguni Geothermal Field, Japan: Chemical Modeling Approach Norifumi Yoshiyuki Hideo and Nobuyuki Electric Power Development Co., Ltd. 6-15-1, Ginza, Chuo-ku,
A thermodynamic model for the system SiO 2 H 2 O near the upper critical end point based on quartz solubility experiments at C and 5 20 kbar
 Available online at www.sciencedirect.com Geochimica et Cosmochimica Acta 86 (2012) 196 213 www.elsevier.com/locate/gca A thermodynamic model for the system SiO 2 H 2 O near the upper critical end point
Available online at www.sciencedirect.com Geochimica et Cosmochimica Acta 86 (2012) 196 213 www.elsevier.com/locate/gca A thermodynamic model for the system SiO 2 H 2 O near the upper critical end point
Hydration of Olivine and. Earth s Deep Water Cycle
 Hydration of Olivine and Earth s Deep Water Cycle IASPEI October 5, 2005 Olivine Hydration and Earth s Deep Water Cycle J. R. Smyth, (University of Colorado) Dan Frost and Fabrizio Nestola (Bayerisches
Hydration of Olivine and Earth s Deep Water Cycle IASPEI October 5, 2005 Olivine Hydration and Earth s Deep Water Cycle J. R. Smyth, (University of Colorado) Dan Frost and Fabrizio Nestola (Bayerisches
Subduction zones 3 arc magmatism
 5. 3 Subduction zones 3 arc magmatism Where can we observe magmatic/volcanic activities along subduction zones? Characteristics of arc magmatism (vs. mid-ocean ridge/intraplate magmatism) Structure of
5. 3 Subduction zones 3 arc magmatism Where can we observe magmatic/volcanic activities along subduction zones? Characteristics of arc magmatism (vs. mid-ocean ridge/intraplate magmatism) Structure of
The Effect of H 2 O on the 410-km Seismic Discontinuity
 The Effect of H 2 O on the 410-km Seismic Discontinuity B.J. Wood Science Paper (1995) Presented by HuajianYao Background Seismic discontinuities at 410 km and 660 km ------ important jumps in mantle density
The Effect of H 2 O on the 410-km Seismic Discontinuity B.J. Wood Science Paper (1995) Presented by HuajianYao Background Seismic discontinuities at 410 km and 660 km ------ important jumps in mantle density
SUPPLEMENTARY INFORMATION
 GSA Data Repository 080 Schorn et al., 08, Thermal buffering in the orogenic crust: Geology, https://doi.org/0.30/g4046.. SUPPLEMENTARY INFORMATION 3 PHASE DIAGRAM MODELING 4 5 6 7 8 9 0 3 4 Phase diagrams
GSA Data Repository 080 Schorn et al., 08, Thermal buffering in the orogenic crust: Geology, https://doi.org/0.30/g4046.. SUPPLEMENTARY INFORMATION 3 PHASE DIAGRAM MODELING 4 5 6 7 8 9 0 3 4 Phase diagrams
Lecture 2: Causes of metamorphism
 Lecture 2: Causes of metamorphism Metamorphism refers to a suite of processes that change the mineralogy, composition and texture of pre-existing materials this is a broad definition and certain industrial
Lecture 2: Causes of metamorphism Metamorphism refers to a suite of processes that change the mineralogy, composition and texture of pre-existing materials this is a broad definition and certain industrial
Thermodynamic behaviour of mixtures containing CO 2. A molecular simulation study
 Thermodynamic behaviour of mixtures containing. A molecular simulation study V. Lachet, C. Nieto-Draghi, B. Creton (IFPEN) Å. Ervik, G. Skaugen, Ø. Wilhelmsen, M. Hammer (SINTEF) Introduction quality issues
Thermodynamic behaviour of mixtures containing. A molecular simulation study V. Lachet, C. Nieto-Draghi, B. Creton (IFPEN) Å. Ervik, G. Skaugen, Ø. Wilhelmsen, M. Hammer (SINTEF) Introduction quality issues
Subsolidus and melting experiments of a K-rich basaltic composition to 27 GPa: Implication for the behavior of potassium in the mantle
 American Mineralogist, Volume 84, pages 357 361, 1999 Subsolidus and melting experiments of a K-rich basaltic composition to 27 GPa: Implication for the behavior of potassium in the mantle WUYI WANG* AND
American Mineralogist, Volume 84, pages 357 361, 1999 Subsolidus and melting experiments of a K-rich basaltic composition to 27 GPa: Implication for the behavior of potassium in the mantle WUYI WANG* AND
CHAPTER 9: INTRODUCTION TO THERMODYNAMICS. Sarah Lambart
 CHAPTER 9: INTRODUCTION TO THERMODYNAMICS Sarah Lambart RECAP CHAP. 8: SILICATE MINERALOGY Orthosilicate: islands olivine: solid solution, ie physical properties vary between 2 endmembers: Forsterite (Mg
CHAPTER 9: INTRODUCTION TO THERMODYNAMICS Sarah Lambart RECAP CHAP. 8: SILICATE MINERALOGY Orthosilicate: islands olivine: solid solution, ie physical properties vary between 2 endmembers: Forsterite (Mg
Lecture 12 COMPLEX MELTING MODELS. (see books by Shaw, Trace Elements in Magmas (2006) and Zou, Quantitative Geochemistry (2007))
 Lecture 12 COMPLEX MELTING MODELS (see books by Shaw, Trace Elements in Magmas (2006) and Zou, Quantitative Geochemistry (2007)) Thus far we have considered two end-member melting models, batch melting
Lecture 12 COMPLEX MELTING MODELS (see books by Shaw, Trace Elements in Magmas (2006) and Zou, Quantitative Geochemistry (2007)) Thus far we have considered two end-member melting models, batch melting
V. B. NAUMOV 1, V. A. KOVALENKER 2 and V. L. RUSINOV 2
 CHEMICAL COMPOSITION, TRACE ELEMENTS, AND VOLATILE COMPONENTS OF MELTS: EVIDENCE FROM INCLUSIONS IN THE MINERALS OF NEOVOLCANITES FROM THE CENTRAL AND EASTERN SLOVAKIA V. B. NAUMOV 1, V. A. KOVALENKER
CHEMICAL COMPOSITION, TRACE ELEMENTS, AND VOLATILE COMPONENTS OF MELTS: EVIDENCE FROM INCLUSIONS IN THE MINERALS OF NEOVOLCANITES FROM THE CENTRAL AND EASTERN SLOVAKIA V. B. NAUMOV 1, V. A. KOVALENKER
ZIRCON SOLUBILITY IN ALKALINE AQUEOUS FLUIDS AT UPPER CRUSTAL CONDITIONS
 ZIRCON SOLUBILITY IN ALKALINE AQUEOUS FLUIDS AT UPPER CRUSTAL CONDITIONS Ayers J.C. 1, Zhang L. 1,2, Luo Y. 1,3, Peters T. 1 1. Dept. of Earth & Environmental Sciences, Vanderbilt University 2. Division
ZIRCON SOLUBILITY IN ALKALINE AQUEOUS FLUIDS AT UPPER CRUSTAL CONDITIONS Ayers J.C. 1, Zhang L. 1,2, Luo Y. 1,3, Peters T. 1 1. Dept. of Earth & Environmental Sciences, Vanderbilt University 2. Division
The International Association for the Properties of Water and Steam
 The International Association for the Properties of Water and Steam Gaithersburg, Maryland, USA September 2001 Guideline on the Use of Fundamental Physical Constants and Basic Constants of Water 2001 International
The International Association for the Properties of Water and Steam Gaithersburg, Maryland, USA September 2001 Guideline on the Use of Fundamental Physical Constants and Basic Constants of Water 2001 International
Geology 633 Metamorphism and Lithosphere Evolution. Thermodynamic calculation of mineral reactions I: Reactions involving pure phases
 Geology 633 Metamorphism and Lithosphere Evolution Thermodynamic calculation of mineral reactions I: Reactions involving pure phases The formulation for the free energy change of any reaction involving
Geology 633 Metamorphism and Lithosphere Evolution Thermodynamic calculation of mineral reactions I: Reactions involving pure phases The formulation for the free energy change of any reaction involving
Prediction of Nitrogen Solubility in Pure Water and Aqueous NaCl Solutions up to High Temperature, Pressure, and Ionic Strength
 Journal of Solution Chemistry, Vol. 30, No. 6, 2001 Prediction of Nitrogen Solubility in Pure Water and Aqueous NaCl Solutions up to High Temperature, Pressure, and Ionic Strength Rui Sun, 1 Wenxuan Hu,
Journal of Solution Chemistry, Vol. 30, No. 6, 2001 Prediction of Nitrogen Solubility in Pure Water and Aqueous NaCl Solutions up to High Temperature, Pressure, and Ionic Strength Rui Sun, 1 Wenxuan Hu,
CURRICULUM VITAE. Andreas Audétat. Born: June 7, 1970 in Bern, Switzerland Nationality: Swiss
 CURRICULUM VITAE Andreas Audétat Born: June 7, 1970 in Bern, Switzerland Nationality: Swiss Research Interests formation of magmatic-hydrothermal ore deposits melt inclusions, fluid inclusions experimental
CURRICULUM VITAE Andreas Audétat Born: June 7, 1970 in Bern, Switzerland Nationality: Swiss Research Interests formation of magmatic-hydrothermal ore deposits melt inclusions, fluid inclusions experimental
 Title Super- and subcritical hydration of Thermodynamics of hydration Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2000), 8109 Issue Date 2000-05-08 URL http://hdl.handle.net/2433/50350
Title Super- and subcritical hydration of Thermodynamics of hydration Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2000), 8109 Issue Date 2000-05-08 URL http://hdl.handle.net/2433/50350
Liquids and Solids Chapter 10
 Liquids and Solids Chapter 10 Nov 15 9:56 AM Types of Solids Crystalline solids: Solids with highly regular arrangement of their components Amorphous solids: Solids with considerable disorder in their
Liquids and Solids Chapter 10 Nov 15 9:56 AM Types of Solids Crystalline solids: Solids with highly regular arrangement of their components Amorphous solids: Solids with considerable disorder in their
Petrology. Petrology: the study of rocks, especially aspects such as physical, chemical, spatial and chronoligic. Associated fields include:
 Petrology Petrology: the study of rocks, especially aspects such as physical, chemical, spatial and chronoligic. Associated fields include: Petrography: study of description and classification of rocks
Petrology Petrology: the study of rocks, especially aspects such as physical, chemical, spatial and chronoligic. Associated fields include: Petrography: study of description and classification of rocks
Effect of tectonic setting on chemistry of mantle-derived melts
 Effect of tectonic setting on chemistry of mantle-derived melts Lherzolite Basalt Factors controlling magma composition Composition of the source Partial melting process Fractional crystallization Crustal
Effect of tectonic setting on chemistry of mantle-derived melts Lherzolite Basalt Factors controlling magma composition Composition of the source Partial melting process Fractional crystallization Crustal
Predicting Mineral Transformations in Wet Supercritical CO 2 : The Critical Role of Water
 Predicting Mineral Transformations in Wet Supercritical CO 2 : The Critical Role of Water Andrew R. Felmy Eugene S. Ilton Andre Anderko Kevin M. Rosso Ja Hun Kwak Jian Zhi Hu 1 Outline Background Importance
Predicting Mineral Transformations in Wet Supercritical CO 2 : The Critical Role of Water Andrew R. Felmy Eugene S. Ilton Andre Anderko Kevin M. Rosso Ja Hun Kwak Jian Zhi Hu 1 Outline Background Importance
Mechanisms of metamorphism and metasomatism on the local mineral scale : The role of dissolution-reprecipitation during mineral re-equilibration
 Chapter 5 Mechanisms of metamorphism and metasomatism on the local mineral scale : The role of dissolution-reprecipitation during mineral re-equilibration Andrew Putnis & Håkon Austrheim Equilibration
Chapter 5 Mechanisms of metamorphism and metasomatism on the local mineral scale : The role of dissolution-reprecipitation during mineral re-equilibration Andrew Putnis & Håkon Austrheim Equilibration
Effect of water on the spinel-postspinel transformation in Mg 2 SiO 4
 Effect of water on the spinel-postspinel transformation in Mg 2 SiO 4 * Pressures for spinel postspinel phase boundary has been subject of debate - XRD measurements indicates that the transition pressure
Effect of water on the spinel-postspinel transformation in Mg 2 SiO 4 * Pressures for spinel postspinel phase boundary has been subject of debate - XRD measurements indicates that the transition pressure
C = 3: Ternary Systems: Example 1: Ternary Eutectic
 Phase Equilibrium C = 3: Ternary Systems: Example 1: Ternary Eutectic Adding components, becomes increasingly difficult to depict 1-C: P - T diagrams easy 2-C: isobaric T-X, isothermal P-X 3-C:?? Still
Phase Equilibrium C = 3: Ternary Systems: Example 1: Ternary Eutectic Adding components, becomes increasingly difficult to depict 1-C: P - T diagrams easy 2-C: isobaric T-X, isothermal P-X 3-C:?? Still
WATER IN THE EARTH S MANTLE:
 WATER IN THE EARTH S MANTLE: NATHALIE BOLFAN-CASANOVA LABORATOIRE MAGMAS ET VOLCANS => Heterogeneous distribution of water in the mantle > 10000 pmm wt H2O ~ 10000 pmm wt H2O ~ 1000 pmm wt H2O On what
WATER IN THE EARTH S MANTLE: NATHALIE BOLFAN-CASANOVA LABORATOIRE MAGMAS ET VOLCANS => Heterogeneous distribution of water in the mantle > 10000 pmm wt H2O ~ 10000 pmm wt H2O ~ 1000 pmm wt H2O On what
DISSOLUTION, TRANSPORT AND PRECIPITATION OF SILICA IN GEOTHERMAL SYSTEM
 DISSOLUTION, TRANSPORT AND PRECIPITATION OF SILICA IN GEOTHERMAL SYSTEM Naoto Takeno 1, Tsuneo Ishido 1, and John W. Pritchett 1 Geological Survey of Japan, Tsukuba, Ibaraki 305-8567, Japan Maxwell Technologies
DISSOLUTION, TRANSPORT AND PRECIPITATION OF SILICA IN GEOTHERMAL SYSTEM Naoto Takeno 1, Tsuneo Ishido 1, and John W. Pritchett 1 Geological Survey of Japan, Tsukuba, Ibaraki 305-8567, Japan Maxwell Technologies
Chapter 4 Rocks & Igneous Rocks
 Chapter 4 Rocks & Igneous Rocks Rock Definition A naturally occurring consolidated mixture of one or more minerals e.g, marble, granite, sandstone, limestone Rock Definition Must naturally occur in nature,
Chapter 4 Rocks & Igneous Rocks Rock Definition A naturally occurring consolidated mixture of one or more minerals e.g, marble, granite, sandstone, limestone Rock Definition Must naturally occur in nature,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11294 Review of previous works on deep-liquid properties The major parameters controlling the buoyancy of deep-mantle melts are (i) the changes in atomic packing
SUPPLEMENTARY INFORMATION doi:10.1038/nature11294 Review of previous works on deep-liquid properties The major parameters controlling the buoyancy of deep-mantle melts are (i) the changes in atomic packing
Supplementary information. Jadeite in Chelyabinsk meteorite and the nature of an impact event on its parent. body
 Supplementary information Jadeite in Chelyabinsk meteorite and the nature of an impact event on its parent body Shin Ozawa 1*, Masaaki Miyahara 1, 2, Eiji Ohtani 1, 3, Olga N. Koroleva 4, Yoshinori Ito
Supplementary information Jadeite in Chelyabinsk meteorite and the nature of an impact event on its parent body Shin Ozawa 1*, Masaaki Miyahara 1, 2, Eiji Ohtani 1, 3, Olga N. Koroleva 4, Yoshinori Ito
1 Potassic adakite magmas and where they come from: a mystery solved?
 1 Potassic adakite magmas and where they come from: a mystery solved? 2 3 John Clemens Kingston University (London) Long Xiao China University of Geosciences (Wuhan) 4 Adakites are volcanic and intrusive
1 Potassic adakite magmas and where they come from: a mystery solved? 2 3 John Clemens Kingston University (London) Long Xiao China University of Geosciences (Wuhan) 4 Adakites are volcanic and intrusive
FORMATION OF GRANITE PEGMATITES IN THE LIGHT OF MELT AND FLUID INCLUSION STUDIES AND NEW AND OLD EXPERIMENTAL WORK
 1 Rainer Thomas 1, Ilya Veksler 1 FORMATION OF GRANITE PEGMATITES IN THE LIGHT OF MELT AND FLUID INCLUSION STUDIES AND NEW AND OLD EXPERIMENTAL WORK The origin of pegmatites, especially those which are
1 Rainer Thomas 1, Ilya Veksler 1 FORMATION OF GRANITE PEGMATITES IN THE LIGHT OF MELT AND FLUID INCLUSION STUDIES AND NEW AND OLD EXPERIMENTAL WORK The origin of pegmatites, especially those which are
What P-T conditions do rocks experience inside a subduction zone?
 What P-T conditions do rocks experience inside a subduction zone? This exercise allows students to examine the pressure-temperature conditions predicted by thermal modeling in a scientific study funded
What P-T conditions do rocks experience inside a subduction zone? This exercise allows students to examine the pressure-temperature conditions predicted by thermal modeling in a scientific study funded
Notes for Use of the Cpx-Plag-Ol Thermobar Workbook Last Updated:
 Notes for Use of the Cpx-Plag-Ol Thermobar Workbook Last Updated: 7-22-05 Cpx-Plag-Ol Thermobar is an Excel workbook that can be used to calculate crystallization pressures and temperatures for clinopyroxene-
Notes for Use of the Cpx-Plag-Ol Thermobar Workbook Last Updated: 7-22-05 Cpx-Plag-Ol Thermobar is an Excel workbook that can be used to calculate crystallization pressures and temperatures for clinopyroxene-
The change in free energy on transferring an ion from a medium of low dielectric constantε1 to one of high dielectric constant ε2:
 The Born Energy of an Ion The free energy density of an electric field E arising from a charge is ½(ε 0 ε E 2 ) per unit volume Integrating the energy density of an ion over all of space = Born energy:
The Born Energy of an Ion The free energy density of an electric field E arising from a charge is ½(ε 0 ε E 2 ) per unit volume Integrating the energy density of an ion over all of space = Born energy:
Post-perovskite 1. Galley Proofs
 16 September 2005 21:39 YB061180.tex McGraw Hill YB of Science & Technology Keystroked: 29/08/2005 Initial MS Page Sequence Stamp: 02350 Article Title: Post-perovskite Article ID: YB061180 1st Classification
16 September 2005 21:39 YB061180.tex McGraw Hill YB of Science & Technology Keystroked: 29/08/2005 Initial MS Page Sequence Stamp: 02350 Article Title: Post-perovskite Article ID: YB061180 1st Classification
Molecular simulation of adsorption from dilute solutions
 Vol. 52 No. 3/2005 685 689 on-line at: www.actabp.pl Molecular simulation of adsorption from dilute solutions Werner Billes Rupert Tscheliessnig and Johann Fischer Institut für Verfahrens- und Energietechnik
Vol. 52 No. 3/2005 685 689 on-line at: www.actabp.pl Molecular simulation of adsorption from dilute solutions Werner Billes Rupert Tscheliessnig and Johann Fischer Institut für Verfahrens- und Energietechnik
Partial melting of mantle peridotite
 Partial melting of mantle peridotite 1100 1200 1300 1400 1500 (TºC) Depth (km) 50 100 150 Plag lherzolite (ol-opx-cpx-pl) Spinel lherzolite (Ol-opx-cpx-sp) Garnet lherzolite (Ol-opx-cpx-gar) Graphite Diamond
Partial melting of mantle peridotite 1100 1200 1300 1400 1500 (TºC) Depth (km) 50 100 150 Plag lherzolite (ol-opx-cpx-pl) Spinel lherzolite (Ol-opx-cpx-sp) Garnet lherzolite (Ol-opx-cpx-gar) Graphite Diamond
The Gibbs Phase Rule F = 2 + C - P
 The Gibbs Phase Rule The phase rule allows one to determine the number of degrees of freedom (F) or variance of a chemical system. This is useful for interpreting phase diagrams. F = 2 + C - P Where F
The Gibbs Phase Rule The phase rule allows one to determine the number of degrees of freedom (F) or variance of a chemical system. This is useful for interpreting phase diagrams. F = 2 + C - P Where F
Structure of the Earth and the Origin of Magmas
 Page 1 of 12 EENS 2120 Petrology Tulane University Prof. Stephen A. Nelson Structure of the Earth and the Origin of Magmas This document last updated on 23-Jan-2015 Magmas do not form everywhere beneath
Page 1 of 12 EENS 2120 Petrology Tulane University Prof. Stephen A. Nelson Structure of the Earth and the Origin of Magmas This document last updated on 23-Jan-2015 Magmas do not form everywhere beneath
predictive mineral discovery*cooperative Research Centre A legacy for mineral exploration science Mineral Systems Q3 Fluid reservoirs
 Mineral Systems Q3 Fluid reservoirs 1 Key Parameter Mineral System Exploration is reflected in scale-dependent translation A. Gradient in hydraulic potential B. Permeability C. Solubility sensitivity to
Mineral Systems Q3 Fluid reservoirs 1 Key Parameter Mineral System Exploration is reflected in scale-dependent translation A. Gradient in hydraulic potential B. Permeability C. Solubility sensitivity to
Earth s Interior and Geophysical Properties. Chapter 13
 Earth s Interior and Geophysical Properties Chapter 13 Introduction Can we just go there? Deep interior of the Earth must be studied indirectly Direct access only to crustal rocks and upper mantle fragments
Earth s Interior and Geophysical Properties Chapter 13 Introduction Can we just go there? Deep interior of the Earth must be studied indirectly Direct access only to crustal rocks and upper mantle fragments
Chapter 2. Dielectric Theories
 Chapter Dielectric Theories . Dielectric Theories 1.1. Introduction Measurements of dielectric properties of materials is very important because it provide vital information regarding the material characteristics,
Chapter Dielectric Theories . Dielectric Theories 1.1. Introduction Measurements of dielectric properties of materials is very important because it provide vital information regarding the material characteristics,
554 Chapter 10 Liquids and Solids
 554 Chapter 10 Liquids and Solids above 7376 kpa, CO 2 is a supercritical fluid, with properties of both gas and liquid. Like a gas, it penetrates deep into the coffee beans; like a liquid, it effectively
554 Chapter 10 Liquids and Solids above 7376 kpa, CO 2 is a supercritical fluid, with properties of both gas and liquid. Like a gas, it penetrates deep into the coffee beans; like a liquid, it effectively
Lecture 13. Hydrothermal Circulation
 Lecture 13. Hydrothermal Circulation The discovery of hot springs on the ocean floor during the 1970s was one of the most exciting events in the history of oceanography. Although hydrothermal activity
Lecture 13. Hydrothermal Circulation The discovery of hot springs on the ocean floor during the 1970s was one of the most exciting events in the history of oceanography. Although hydrothermal activity
Whole Mantle Convection
 Whole Mantle Convection Overview 1. Evidence for whole mantle convection 2. Model of whole mantle convection reconciling geophysical and geochemical data Transition Zone Water Filter Model 3. Evidence
Whole Mantle Convection Overview 1. Evidence for whole mantle convection 2. Model of whole mantle convection reconciling geophysical and geochemical data Transition Zone Water Filter Model 3. Evidence
Magma Formation and Behavior
 Magma Formation and Behavior Introduction: The study of body waves as they pass through Earth's interior provides strong evidence that the Earth's mantle is composed almost entirely of solid ultramafic
Magma Formation and Behavior Introduction: The study of body waves as they pass through Earth's interior provides strong evidence that the Earth's mantle is composed almost entirely of solid ultramafic
NOTICE CONCERNING COPYRIGHT RESTRICTIONS
 NOTICE CONCERNING COPYRIGHT RESTRICTIONS This document may contain copyrighted materials. These materials have been made available for use in research, teaching, and private study, but may not be used
NOTICE CONCERNING COPYRIGHT RESTRICTIONS This document may contain copyrighted materials. These materials have been made available for use in research, teaching, and private study, but may not be used
Dissolution and precipitation during flow in porous media
 1/25 Class project for GEOS 692: Transport processes and physical properties of rocks Dissolution and precipitation during flow in porous media Gry Andrup-Henriksen Fall 2006 1 2/25 Outline Introduction
1/25 Class project for GEOS 692: Transport processes and physical properties of rocks Dissolution and precipitation during flow in porous media Gry Andrup-Henriksen Fall 2006 1 2/25 Outline Introduction
Chapter 13. Characteristics of a Solution. Example of A Homogenous Mixtures. Solutions
 Chapter 13 Solutions Characteristics of a Solution A solution is a homogeneous mixture A solution is composed of a: Solute: the substance in lesser amount Solvent: the substance in greater amount Two liquid
Chapter 13 Solutions Characteristics of a Solution A solution is a homogeneous mixture A solution is composed of a: Solute: the substance in lesser amount Solvent: the substance in greater amount Two liquid
T-X Diagrams Answers C:\Courses\320\fall2007\in class\5000-t-x ExerciseAnswers.wpd; September 25, 2003 (11:45am) Problems
 1 T-X Diagrams Answers C:\Courses\320\fall2007\in class\5000-t-x ExerciseAnswers.wpd; September 25, 2003 (11:45am) Problems Problem 1. Look at Figure 10. One reaction (that plots as a horizontal line)
1 T-X Diagrams Answers C:\Courses\320\fall2007\in class\5000-t-x ExerciseAnswers.wpd; September 25, 2003 (11:45am) Problems Problem 1. Look at Figure 10. One reaction (that plots as a horizontal line)
What can noble gases really say about mantle. 2) Extent of mantle degassing
 What can noble gases really say about mantle convection and the deep Earth volatile cycles? 1) Constraints on mass flow 1) Constraints on mass flow 2) Extent of mantle degassing Outline: -Noble gas geochemistry
What can noble gases really say about mantle convection and the deep Earth volatile cycles? 1) Constraints on mass flow 1) Constraints on mass flow 2) Extent of mantle degassing Outline: -Noble gas geochemistry
Thermodynamics and structural properties of the dipolar Yukawa fluid. Citation Journal Of Chemical Physics, 1999, v. 111 n. 1, p.
 Title Thermodynamics and structural properties of the dipolar Yukawa fluid Author(s) Szalai, I; Henderson, D; Boda, D; Chan, KY Citation Journal Of Chemical Physics, 1999, v. 111 n. 1, p. 337-344 Issued
Title Thermodynamics and structural properties of the dipolar Yukawa fluid Author(s) Szalai, I; Henderson, D; Boda, D; Chan, KY Citation Journal Of Chemical Physics, 1999, v. 111 n. 1, p. 337-344 Issued
Lecture 14: A brief review
 Lecture 14: A brief review A few updates for the remainder of the course Report for the lab on pelite metamorphism - Lab 3 Needs to be handed in before Tuesday the 14 th of March at 17:00. My most important
Lecture 14: A brief review A few updates for the remainder of the course Report for the lab on pelite metamorphism - Lab 3 Needs to be handed in before Tuesday the 14 th of March at 17:00. My most important
Slow cooling of the lowermost oceanic crust at the fastspreading
 GSA Data Repository 2016030 Slow cooling of the lowermost oceanic crust at the fastspreading East Pacific Rise Kathrin Faak and Kathryn M. Gillis Model parameters and input conditions for the diffusion
GSA Data Repository 2016030 Slow cooling of the lowermost oceanic crust at the fastspreading East Pacific Rise Kathrin Faak and Kathryn M. Gillis Model parameters and input conditions for the diffusion
Magmatic Processes at Subduction Zones
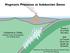 Magmatic Processes at Subduction Zones Katherine A. Kelley Graduate School of Oceanography Univ. of Rhode Island Thanks to Terry Plank Erik Hauri GVP: Liz Cottrell Simon Carn Jennifer Jay Ed Venzke Subduction
Magmatic Processes at Subduction Zones Katherine A. Kelley Graduate School of Oceanography Univ. of Rhode Island Thanks to Terry Plank Erik Hauri GVP: Liz Cottrell Simon Carn Jennifer Jay Ed Venzke Subduction
Three-dimensional numerical simulations of thermo-chemical multiphase convection in Earth s mantle Takashi Nakagawa a, Paul J.
 Three-dimensional numerical simulations of thermo-chemical multiphase convection in Earth s mantle Takashi Nakagawa a, Paul J. Tackley b a Department of Earth and Planetary Sciences, University of Tokyo,
Three-dimensional numerical simulations of thermo-chemical multiphase convection in Earth s mantle Takashi Nakagawa a, Paul J. Tackley b a Department of Earth and Planetary Sciences, University of Tokyo,
Graduate School of Science and Engineering, Kagoshima University
 20 +- -,+ +. / +- Geochemical Interpretation on Hydrothermal Formation Mechanism of the Thermal Water in the Iwodani Region and the Vicinity of the Hayashida Region of the Kirishima Volcanic Area Shun-ichi
20 +- -,+ +. / +- Geochemical Interpretation on Hydrothermal Formation Mechanism of the Thermal Water in the Iwodani Region and the Vicinity of the Hayashida Region of the Kirishima Volcanic Area Shun-ichi
A. One component system (c = 1)
 A. One component system (c = 1) Example: SiO 2 system. Since all phases in this system have the same composition, there are no compositional variables to consider. Phase equilibria can be shown completely
A. One component system (c = 1) Example: SiO 2 system. Since all phases in this system have the same composition, there are no compositional variables to consider. Phase equilibria can be shown completely
Water contents and hydrogen isotopes in nominally anhydrous minerals from UHP metamorphic rocks in the Dabie-Sulu orogenic belt
 Progress SPECIAL TOPIC Tectonics of Continental Collision Orogens December 2013 Vol.58 No.35: 4384 4389 doi: 10.1007/s11434-013-6069-7 Water contents and hydrogen isotopes in nominally anhydrous minerals
Progress SPECIAL TOPIC Tectonics of Continental Collision Orogens December 2013 Vol.58 No.35: 4384 4389 doi: 10.1007/s11434-013-6069-7 Water contents and hydrogen isotopes in nominally anhydrous minerals
