TWO COMPONENT (BINARY) PHASE DIAGRAMS. Experimental Determination of 2-Component Phase Diagrams
|
|
|
- Randall Kennedy
- 5 years ago
- Views:
Transcription
1 Page 1 of 12 EENS 211 Earth Materials Tulane University Prof. Stephen A. Nelson TWO COMPONENT (BINARY) PHASE DIAGRAMS This document last updated on 08-Oct-2003 Experimental Determination of 2-Component Phase Diagrams As an example, we're going to look at how one might go about determining the stability of a mixture of 2 mineral phases, A and B. To perform these experiments we start with pure minerals A and B and then make mixtures in varying proportions. Each one of these mixtures, plus the pure A and pure B represent different compositions. In this case, we are only going to look at how the stability varies as with temperature and composition, holding Pressure constant at 1 atmosphere. This type of experiment can be done in a furnace with controlled temperature. Pressure does not have to be controlled because the phase relations will be determined at atmospheric pressure. The various compositions are placed in a capsule that will not react with any of the phases produced. Usually this would be Platinum. Each mixture is then placed in the furnace, the temperature is raised to some point and held at that temperature long enough for equilibrium between all of the phases to occur. The capsules are then quickly removed from the furnace and quenched rapidly. The rapid decrease in temperature that occurs during quenching helps to assure that no further reactions take place and the phase assemblage that was present at the higher temperature is preserved at room temperature. During quenching, any liquid that may have been present at high temperature is found to be glass. After quenching, each capsule is opened and the phases present are determined using a microscope. In the example shown, we use different symbols to represent the mineral phase assemblages present for each composition run. In this set of experiments 6 different assemblages are found, and are plotted on a diagram with Temperature of the experiment plotted on the vertical axis, and composition in terms of %A or %B plotted on the horizontal axis. Note that pure A plots at 100% A which corresponds to 0% B, and pure B plots at 100%B, which corresponds to 0% A. Note also that no experiments were run at temperatures higher than those where the first complete liquid appeared for each composition.
2 Page 2 of 12 Once the points are plotted, we can then draw best-fit curves or lines between the data points to determine the Temperature - Composition stability fields for the various phase assemblages. These curves/lines are shown here, and the stability fields for each phase assemblage are labeled. the resulting phase diagram is called a binary eutectic diagram. Not all binary melting diagrams look like this, but this is the simplest and the type that we will discuss first. TWO COMPONENT EUTECTIC SYSTEMS Figure 1 shows the simplest of two component phase diagrams. The components are A and B, and the possible phases are pure crystals of A, pure crystals of B, and liquid with compositions ranging between pure A and pure B. Compositions are plotted across the bottom of the diagram. Note that composition can be expressed as either a percentage of A or a percentage of B, since the total percentage must add up to 100. (Compositions might also be expressed as mole fraction of A or B, in which case the total must add up to 1). Temperature or pressure is plotted on the vertical axis. For the case shown, we consider pressure to be constant, and therefore have plotted temperature on the vertical axis. The curves separating the fields of A + Liquid from Liquid and B + Liquid from Liquid are termed liquidus curves. The horizontal line separating the fields of A + Liquid and B + Liquid from A + B all solid, is termed the solidus. The point, E, where the liquidus curves and solidus intersect, is termed the eutectic point. At the eutectic point in this two component system, all three phases, that is Liquid, crystals of A and crystals of B, all exist in equilibrium. Note that the eutectic is the only point on the diagram where this is true. Since we looking at a system at constant pressure, the phase rule in this case is F = C +1 - P.
3 Page 3 of 12 The eutectic point is therefore an invariant point. If we change the composition of the liquid or the temperature, the number of phases will be reduced to 2. If the system contains only pure A, then the system is a one component system and phase A melts at only one temperature, the melting temperature of pure A, T ma. If the system contains only pure B, then it is a one component system and B melts only at the melting temperature of pure B, T mb. For all compositions between pure A and pure B, the melting temperature is drastically reduced, and melting begins at the eutectic temperature T E. Note that for all compositions between A and B the melting also occurs over a range of temperatures between the solidus and the liquidus. This is true for all compositions except one, that of the eutectic. The eutectic composition melts at only one temperature, T E. We will now consider the crystallization of a liquid with composition X in Figure 1. First, however, we must state the following rule, which must always be obeyed: Rule 1 - In equilibrium crystallization or melting in a closed system, the final composition of the system will be identical to the initial composition of the system. Therefore, according to rule 1, composition X, which is made up of a mixture of 80% A and 20% B, will have, as its final crystalline product a mixture of 80% crystals of A and 20% crystals of B. Composition X will be all liquid above the temperature T1, because it will lie in the field of all Liquid. If the temperature is lowered to T 1, at T 1 crystals of A begin to form. Further lowering of the temperature causes more crystals of A to form. As a result, the liquid composition must become more enriched in B as more crystals of A form out of the liquid. Thus, with lowering of temperature, the liquid composition will change from point 1 to point 2 to point 3 to point E as the temperature is lowered from T 1 to T 2 to T 3 to T E respectively. At all temperatures between T 1 and T E, two phases will be present in the system; liquid and crystals of A. At the eutectic temperature, T E, crystals of B will begin to form, and three phases will coexist; crystals of A, crystals of B, and liquid. The temperature must remain at T E until one of the phases disappears. Thus when the liquid crystallizes completely, only pure solid A and pure solid B will remain and mixture of these two solid phases will be in the proportions of the original mixture, that is 80% A and 20% B. The crystallization history of composition X can be written in abbreviated form as follows: T > T 1 -- all liquid T 1 - T E -- liquid + A at T E -- liquid + A + B T < T E -- A + B all solid
4 Page 4 of 12 If we were to stop the crystallization process at any point during crystallization and observe how much of each phase is present we can use the following example to determine what we would see. For example, at a temperature T 2 the amount of crystals of A and liquid (the only two phases present at this temperature) could be determined by measuring the distances a and b on figure 1. The percentages would then be given by the lever rule: % crystals of A = b/(a + b) x 100 % liquid = a/(a + b) x 100 Note that since the amount of crystals must increase with falling temperature the proportional distance between the vertical line which marks the initial composition and the liquidus increases as temperature falls. Thus the distance used to calculate the amount of solid is always measured toward the liquid side of the initial composition. At the temperature T 3, note that more crystals must have formed since the proportional distance d/(c+d) is greater than the proportional distance b/(a+b). Thus at T 3 the lever rule gives: % crystals of A = d/(d + c) x 100 % liquid = c/(c + d) x 100 At T 3, note that the composition of the liquid is given at point 3, i.e. 53% A, the composition of the solid is pure A, and the composition of the system is still 80% A and 20% B. Make sure you understand the difference between composition of the phases and the amount or percentages of the phases. The melting process is exactly the reverse of the crystallization process. That is if we started with composition X at some temperature below T E the first liquid would form at T E. The temperature would remain constant at T E until all of the crystals of B were melted. The liquid composition would then change along the liquidus curve from E to point 1 as temperature increased until the temperature T 1 was reached. Above T 1 the system would contain only liquid with a composition of 80% A and 20% B. The melting process in abbreviated form is listed below: T < T E -- all solid A + B at T E -- Liquid + A + B T E - T 1 -- Liquid + A T > T 1 -- all Liquid
5 Page 5 of 12 INCONGRUENT MELTING Definition of terms: Liquidus - The line separating the field of all liquid from that of liquid plus crystals. Solidus - The line separating the field of all solid from that of liquid plus crystals. Eutectic point - the point on a phase diagram where the maximum number of allowable phases are in equilibrium. When this point is reached, the temperature must remain constant until one of the phases disappears. A eutectic is an invariant point. Peritectic point - The point on a phase diagram where a reaction takes place between a previously precipitated phase and the liquid to produce a new solid phase. When this point is reached, the temperature must remain constant until the reaction has run to completion. A peritectic is also an invariant point. Intermediate compound - A phase that has a composition intermediate between two other phases. Congruent melting - melting wherein a phase melts to a liquid with the same composition as the solid. Incongruent melting - melting wherein a phase melts to a liquid with a composition different from the solid and produces a solid of different composition to the original solid. For the case of incongruent melting, we will use the system forsterite (Mg 2 SiO 4 ) - silica (SiO 2 ), which has an intermediate compound, enstatite (MgSiO 3 ). This system is a prime example of the phenomena of incongruent melting in rocks, and therefore gives insights into many aspects of mineral formation. A simplified version of the system forsterite - silica with its intermediate compound enstatite is shown in Figure 2. The crystallization histories for compositions X, Y, and Z will be documented in the following discussion. Each of these compositions behaves in a slightly different manner
6 Page 6 of 12 Crystallization of Composition X Composition X is a mixture of 13 wt. % SiO 2 and 87 wt. % Mg 2 SiO 4. Because this composition falls between the compositions of pure forsterite and pure enstatite, it must end its crystallization history containing only crystals of forsterite and enstatite. i.e. no quartz will occur in the final crystalline mixture. If a mixture such as composition X is taken to a temperature above its liquidus (i.e. above 1800 o C in Figure 2) it will be in an all liquid state. We now trace the cooling history of composition X. As a liquid of composition X is cooled, nothing will happen until the temperature is equal to the liquidus temperature at 1800 o. At this point crystals of forsterite (Fo) begin to precipitate out of the liquid. As the temperature is further lowered, the composition of the liquid will change along the liquidus toward the peritectic (P), and the crystals forming from the liquid will always be pure Fo until P is reached. At the temperature of the peritectic, about 1580 o, note that three phases must be in equilibrium, Fo, liquid, and enstatite (En). At this point some of the crystals of Fo react with the liquid to produce crystals of En. The reaction that takes place can be written as follows: Mg 2 SiO 4 + SiO 2 = 2MgSiO 3 Fo + liq = 2En After all of the liquid is consumed by this reaction, only crystals of Fo and En will remain. The proportions of Fo and En in the final crystalline product can be found by applying the lever rule. %Fo crystals = [d/(c + d)] x 100 %En crystals = [c/(c + d)] x 100
7 Page 7 of 12 At any intermediate stage in the process, such as at 1700 o the proportion of all phases present (Fo and liquid in this case) can similarly be found by applying the lever rule. at 1700 o C %Fo crystals = [b/(a + b)] x 100 %liquid = [a/(a + b)] x 100 Note that melting of composition X is exactly the reverse of crystallization. Mixture X will begin to melt at the peritectic temperature. At this point En will melt to crystals of Fo plus liquid (incongruent melting). As soon as all of the En crystals have been consumed by this reaction, the temperature can be increased until it reaches 1800 o at which point all of the Fo crystals will have been consumed and the only phase left will be liquid with a composition of the starting material. Crystallization of Composition Y Composition Y is equivalent to pure En. Thus only En may appear in the final crystalline product if perfect equilibrium is maintained. If composition Y is cooled from an all liquid state it first begins to crystallize at about 1650 o. At 1650 o crystals of Fo will begin to precipitate from the liquid. This will continue with further cooling until the temperature of the peritectic is reached. In this interval, the composition of the liquid must become more enriched in SiO 2 and will thus change along the liquidus until it has the composition of the peritectic, P. At the peritectic temperature (1580 o ) all of the remaining liquid will react with all of the previously precipitated Fo to produce crystals of En. The temperature will remain constant until this reaction has gone to completion, after which the only phase present will be pure En. Thus, it can be seen that enstatite melts incongruently. If pure enstatite is heated to a temperature of 1580 o it melts to Fo plus liquid. Crystallization of Composition Z Since composition Z lies between En and SiO 2, it must end up with crystals of En and Qz (Quartz). If such a composition were cooled from some high temperature where it is in the all liquid state, it would remain all liquid until it reached the liquidus temperature at about 1600 o. At this temperature crystals of Fo would begin to precipitate and the composition of the liquid would begin to change along the liquidus toward the peritectic, P. At P, all of the Fo previously precipitated would react with the liquid to produce crystals of En. After this reaction has run to completion, and all of the previously precipitated Fo is consumed, there would still remain some liquid. With decreasing temperature, more crystals of En would form, and the liquid composition would change along the liquidus toward the eutectic, E. At E crystals of Qz would begin to form, the temperature would remain constant until all of the liquid was used up, leaving crystals of Qz and En as the final solid. Note that because composition Z lies very close to the composition of pure En, the final crystalline product would consist mostly of En with a very small amount of Qz. For all compositions between P and 100% SiO 2 the system would behave in an identical fashion to the simple Eutectic system discussed previously.
8 Page 8 of 12 Fractional Crystallization in the System Up to this point we have always been discussing the case of equilibrium crystallization. That is all solids remain in contact with the liquid until any reaction that takes place has run to completion. As is often the case in natural systems crystals can somehow become separated from the system so that they will not react at reaction points such as P. This is the case of fractional crystallization. Under fractional crystallization conditions the cooling and crystallization histories will be drastically different. In particular, the rule that the final composition must equal the initial composition will not be followed. As an example of this phenomena we will examine the fractional crystallization of composition X. Furthermore, we will look at the case of perfect fractional crystallization. During perfect fractional crystallization of composition X all of the Fo that is precipitated will be somehow removed from the system. (In nature this can occur by crystals sinking to the bottom of the liquid due to the fact that crystals generally tend to be more dense than liquids.) Note that if only some of the crystals are removed from the liquid we will have a case intermediate between perfect fractional crystallization and equilibrium crystallization. Cooling a liquid of composition X to the liquidus at 1800 o will cause Fo to precipitate as before. With further cooling the liquid composition will change along the liquidus and more Fo will be precipitated. In this case, however, all of the Fo will be removed from the system as it crystallizes. Since the Fo is no longer present, the composition of the system will have the composition of the liquid (the Fo removed can no longer contribute to the composition of the system). Therefore, when the temperature reaches the peritectic temperature, 1580 o, there will be no Fo available to react with the liquid, and the liquid (and system) will have a composition, P. Thus the liquid will now precipitate crystals of En and continue cooling to the eutectic, E, where crystals of Qz will form. The final crystalline product will consist of Qz and En. Compare this case with the previously discussed case of equilibrium crystallization of composition X. Note that under equilibrium conditions the final crystalline product of composition X contained crystals of Fo and En, while in the fractional crystallization case the final product contains En and Qz. Thus fractional crystallization has allowed an originally Fo rich composition to produce an SiO 2 rich liquid and Qz appears in the final crystalline product. If you go back and look at simple eutectic systems, or look at fractional crystallization of composition Z in the more complex system, you should be able to see that fractional crystallization will have no effect on the phases produced in the final crystalline product, but will only change the proportions of the phases produced. Fractional crystallization is only effective in producing a different final phase assemblage if there is a reaction relationship of one of the phases to the liquid. SOLID SOLUTION SYSTEMS In the systems we've discussed so far, all of the mineral or solid phases have been pure phases, that is they have one and only one possible composition. This is not usually the case in nature, since substitution of one element for another often occurs due to the fact that some elements behave in a chemically similar fashion to other elements. When such substitutions occur, the phase can have a range of possible compositions, depending on the amount of substitution that takes place. Such solids that can have various amounts of elemental substitution are called solid solutions. A good example of a solid solution mineral is the mineral olivine. The general chemical formula for olivine is (Mg,Fe) 2 SiO 4. Since Mg +2 and Fe +2 are about the same size and have the same charge, they may substitute for one another in the crystal structure of
9 Page 9 of 12 olivine. Thus olivine may have a composition anywhere between the pure Mg end member, forsterite (Mg 2 SiO 4 ), and the pure Fe end member, fayalite (Fe 2 SiO 4 ). When all compositions between two end members are possible, the solid solution is said to be a complete solid solution. Another good example of a complete solid solution is displayed in the plagioclase feldspars. In this case the solid solution is between the end members albite (NaAlSi 3 O 8 ) and anorthite (CaAl 2 Si 2 O 8 ). In order to maintain charge balance we cannot simply substitute Na + for Ca +2, so this solid solution is what is called a coupled solid solution. In this case Na + Si +4 is substituted for Ca +2 Al +3 in the plagioclase structure to produce intermediate compositions of plagioclase. Because the elements that substitute are not exactly the same size (they are similar in size) the amount of substitution is dependent on temperature and pressure and the solid solutions behave in a somewhat orderly fashion as illustrated below. Since plagioclase is one of the most common minerals in the earth's crust, we will discuss the phase diagram for the plagioclase system. The phase relations in the plagioclase system are shown in Figure 3 at constant pressure equal to that of the atmosphere (atmospheric pressure is 1 bar). In Figure 3 the upper curve is called the liquidus and the lower curve is called the solidus. At temperatures above the liquidus everything is liquid, below the solidus everything is solid (crystals of plagioclase solid solution). At temperatures between the solidus and liquidus crystals of plagioclase solid solution coexist in equilibrium with liquid. Pure albite melts (or crystallizes) at 1118 o C, and pure anorthite melts (or crystallizes) at 1500 o C. Note that any composition of plagioclase between the two end members melts or crystallizes over a range of temperatures unlike the pure end members which have only one melting point. Thus we can read from the diagram that a solid solution containing 50% albite and 50% anorthite (Ab 50 An 50 ) begins to melt at 1220 o, point F, and the melting is complete at 1410 o, point A. Inversely, if a melt of composition Ab 50 An 50 is cooled it will begin to crystallize at 1410 o and will be completely crystalline at 1220 o. We will now trace the crystallization history of composition X, which is Ab 50 An 50.
10 Page 10 of 12 Composition X is completely liquid above the liquidus (above 1410 o ). Cooling to the liquidus at point A results in the crystallization of a small amount of plagioclase solid solution. The composition of this plagioclase can be found by drawing an isotherm (line of constant temperature, a horizontal line in this diagram) through the temperature 1410 o. Where this isotherm intersects the solidus (at point B), the composition of the solid can be found by drawing a vertical line to the base of the diagram. Thus it is seen that the first crystals precipitated from composition X will have the composition Ab 10 An 90. Note that in this diagram crystals that are in equilibrium with liquid will always be enriched in anorthite component relative to the liquid. As crystallization continues with lowering of temperature the composition of the plagioclase will change along the solidus, continually reacting with the liquid to produce crystals more enriched in the Ab component. Meanwhile, the composition of the liquid will change along the liquidus, thus also becoming more enriched in the Ab component. At a temperature of 1395 o the liquid composition will be at point C, while the solid composition will be at point D. Crystallization proceeds until a temperature of about 1220 o, at which point the last remaining liquid will have a composition at E, and the solid will have a composition equal to the original starting composition at point F. At this point all of the liquid will be consumed and the final crystalline product will have the composition Ab 50 An 50. During crystallization the proportion of the solid continually increases while that of the liquid continually decreases. Thus as the composition of the liquid becomes more sodic, approaching E, its volume steadily decreases. Thus it can be seen that the amount of liquid in equilibrium with the solid of composition F will be extremely small. If at any point during the crystallization we wish to determine the amount of solid and liquid, we can apply the lever rule. As an example, we will determine the proportions of liquid and solid in the system at a temperature of 1395 o. At this point, we measure the distances oc, od, and CD. The percentages of liquid and solid are then given as follows: % solid (with composition D) = [x/(x + y)] x 100 % liquid (with composition C) = [y/(x + y)] x 100 The foregoing discussion assumes that equilibrium is maintained throughout the course of crystallization. This means that with falling temperature and continuing crystallization, the earlier-formed, more calcic crystals must react continuously with the liquid to produce homogeneous crystals that will become continuously more enriched in the sodic component. If this equilibrium cannot be maintained, then fractional crystallization will take place. We will distinguish between three contrasting conditions. 1. In equilibrium crystallization, the crystals remain suspended in the melt, and cooling and crystallization are slow enough to allow continuous, complete reaction between crystals and melt. The early formed crystals will, on cooling, react with the melt continuously and thereby gradually change their composition along the solidus from B to F, while simultaneously the liquid changes from A to E. In such circumstances the crystals will not change composition beyond F, and the end product is a homogeneous mixed crystal (solid solution) having the same composition as the initial melt. 2. Assume that the crystals are continuously removed from the melt, by sinking or some natural filtering process. Reaction of crystals with the melt is prevented, and the composition of the liquid will continue to change along the liquidus curve toward the
11 Page 11 of 12 sodic feldspar component. The only limit to this change of composition of the liquid is the composition of the pure Na feldspar, but the relative amount of very sodic liquid would be very small. As the liquid phase changed composition with continuing removal of crystals, the successively formed crystals would become continuously more sodic; the final product would be pure albite, but it would constitute a very small proportion of the initial amount. 3. If the crystals remain suspended in the liquid, but relatively rapid crystallization does not allow complete reaction between crystals and liquid, the effect will be somewhat different. In effect, failure to react completely partially removes the already formed crystals from the system. The melt becomes increasingly more sodic, and earlier formed more calcic crystals serve as nuclei on which increasingly more sodic feldspar crystallizes. The resulting crystal contain zones of differing composition; the inner zones being more calcic, and the outer zones more sodic. The bulk (average) composition of the zoned crystal is that of the initial system, but the range of composition between the inner and outer zones might theoretically be as large as from B to pure Ab in the example shown for composition X. EXSOLUTION Many minerals that show complete solid solution at higher temperatures do not show such solid solution at lower temperatures. When this is the case, the phenomenon of exsolution occurs. Since solid solutions are really one mineral phase dissolved in another mineral phase to form a single mineral phase, exsolution implies that one or the other of the mineral phases in the solution must "exsolve" or come out of solution with the other mineral phase. Figure 4 illustrates a phase diagram (much simplified) of the alkali feldspar system which exhibits such exsolution behavior at low temperatures. At high temperatures the diagram shows that albite (Ab) or NaAlSi 3 O 8 and orthoclase (Or) or KAlSi 3 O 8 form a complete solid solution series. This solid solution series is different from the plagioclase solid solution series only in that it has a minimum composition in the middle rather than at the composition of one of the pure end members. At temperatures just below the solidus, alkali feldspar solid solutions are stable. At lower temperatures, along the curve labeled "solvus" the solid solution is no longer stable.
12 Page 12 of 12 In this case the exsolution phenomena occurs below the solidus and so is a "sub-solidus" reaction. In order to see what happens during exsolution we will examine what happens to a composition labeled X in Figure 4. We will start at a temperature of 750 o in the region where alkali feldspar solid solutions are stable. At 750 o the composition of the alkali feldspar solid solution is 70% orthoclase and 30% albite (Or 70 Ab 30 ). This solid solution remains stable with lowering of temperature until the temperature of the solvus is obtained at point A (a temperature of about 590 o ). At this temperature the solid solution is no longer stable and begins to exsolve. The composition of coexisting exsolved phases can be found by drawing an isotherm until it intersects the solvus. Such an isotherm at 590 o shows that at this temperature a solid solution having the composition of point B (Or 32 Ab 68 ) coexists with an alkali feldspar solid solution with the composition of point A (Or 70 Ab 30 ). With further lowering of temperature further exsolution occurs. At a temperature of 300 o our original composition X has exsolved into two alkali feldspar solid solutions, one with the composition of point C and one with a composition of point D. To find the relative proportions or percentages of each of the solid solutions, the lever rule can once again be applied. For example at 300 o for composition X the percentage of the albite-rich solid solution is [z/(z+y)] x 100, while that of the orthoclase-rich solid solution is [y/(z+y)] x 100. With further lowering of temperature all of the albite and orthoclase in the two solid solutions could exsolve completely to produce a pure albite phase and a pure orthoclase phase. Such complete exsolution does occur in nature, but only if the temperature is lowered very slowly. Complete exsolution is only common in metamorphic rocks. More often, especially in granitic rocks, the two exsolved phases do not separate as individual crystals, but occur as intergrown crystals with exsolution lamellae of one crystal occurring within the other crystal. In the alkali feldspars containing such exsolution lamellae the result is to produce a texture called perthitic or perthite. Perthite on a microscopic scale is illustrated on page 539, figure of your mineralogy text (Klein and Hurlbut). Return to EENS 211 Home Page
1 - C Systems. The system H 2 O. Heat an ice at 1 atm from-5 to 120 o C. Heat vs. Temperature
 1 - C Systems The system H 2 O Heat an ice at 1 atm from-5 to 120 o C Heat vs. Temperature Fig. 6.7. After Bridgman (1911) Proc. Amer. Acad. Arts and Sci., 5, 441-513; (1936) J. Chem. Phys., 3, 597-605;
1 - C Systems The system H 2 O Heat an ice at 1 atm from-5 to 120 o C Heat vs. Temperature Fig. 6.7. After Bridgman (1911) Proc. Amer. Acad. Arts and Sci., 5, 441-513; (1936) J. Chem. Phys., 3, 597-605;
Interpreting Phase Diagrams
 Interpreting Phase Diagrams Understanding chemical reactions requires that we know something about how materials behave as the temperature and pressure change. For a single component (like quartz or ice)
Interpreting Phase Diagrams Understanding chemical reactions requires that we know something about how materials behave as the temperature and pressure change. For a single component (like quartz or ice)
2-C Eutectic Systems
 Phase Equilibrium 2-C Eutectic Systems Example: Diopside - Anorthite No solid solution * For system at this T, X, phase(s) plot where? Fig. 6.11. Isobaric T-X phase diagram at atmospheric pressure. After
Phase Equilibrium 2-C Eutectic Systems Example: Diopside - Anorthite No solid solution * For system at this T, X, phase(s) plot where? Fig. 6.11. Isobaric T-X phase diagram at atmospheric pressure. After
Phase Equilibrium. Phase Rule. Phase Diagram
 Phase Equilibrium Phase Rule Phase Diagram Makaopuhi Lava Lake Magma samples recovered from various depths beneath solid crust From Wright and Okamura, (1977) USGS Prof. Paper, 1004. Makaopuhi Lava Lake
Phase Equilibrium Phase Rule Phase Diagram Makaopuhi Lava Lake Magma samples recovered from various depths beneath solid crust From Wright and Okamura, (1977) USGS Prof. Paper, 1004. Makaopuhi Lava Lake
A. One component system (c = 1)
 A. One component system (c = 1) Example: SiO 2 system. Since all phases in this system have the same composition, there are no compositional variables to consider. Phase equilibria can be shown completely
A. One component system (c = 1) Example: SiO 2 system. Since all phases in this system have the same composition, there are no compositional variables to consider. Phase equilibria can be shown completely
Feldspars. Structure. The feldspars are by far the most abundant group of minerals and are found in igneous, metamorphic and many sedimentary rocks.
 Feldspars The feldspars are by far the most abundant group of minerals and are found in igneous, metamorphic and many sedimentary rocks. Structure Felsdpars are framework silicates where each silica tetrahedra
Feldspars The feldspars are by far the most abundant group of minerals and are found in igneous, metamorphic and many sedimentary rocks. Structure Felsdpars are framework silicates where each silica tetrahedra
Mineral Stability and Phase Diagrams Introduction
 1 of 10 10/10/2002 2:50 PM Prof. Stephen A. Nelson Geology 211 Tulane University Mineralogy and Phase Diagrams Introduction This document last updated on 10-Oct-2002 As we discussed previously, there are
1 of 10 10/10/2002 2:50 PM Prof. Stephen A. Nelson Geology 211 Tulane University Mineralogy and Phase Diagrams Introduction This document last updated on 10-Oct-2002 As we discussed previously, there are
Introductory Statement:
 The use of visualization and sketches of thin sections to encourage a better understanding of phase diagrams: Binary and ternary phase diagram exercises Jennifer M. Wenner Drew S. Coleman Introductory
The use of visualization and sketches of thin sections to encourage a better understanding of phase diagrams: Binary and ternary phase diagram exercises Jennifer M. Wenner Drew S. Coleman Introductory
Exam I. 1. (10 points) Give the following optical properties for the minerals listed below.
 GLY306 Petrology Exam I 1. (10 points) Give the following optical properties for the minerals listed below. Color/pleochroism Extinction angle Cleavage angle Twining Refractive index Hornblende Plagioclase
GLY306 Petrology Exam I 1. (10 points) Give the following optical properties for the minerals listed below. Color/pleochroism Extinction angle Cleavage angle Twining Refractive index Hornblende Plagioclase
Chapter 6: The Phase Rule and One and Two-Component Systems aka Phase Equilibria
 Chapter 6: The Phase Rule and One and Two-Component Systems aka Phase Equilibria Makaopuhi Lava Lake Magma samples recovered from various depths beneath solid crust From Wright and Okamura, (1977) USGS
Chapter 6: The Phase Rule and One and Two-Component Systems aka Phase Equilibria Makaopuhi Lava Lake Magma samples recovered from various depths beneath solid crust From Wright and Okamura, (1977) USGS
Hirsch - Binary phase diagrams problems KEY
 Hirsch - Binary phase diagrams problems KEY Name Question 1. 1A. Using the phase rule, determine the degrees of freedom: a. at point e F = 2-3 + 1 = 0 b. within the field A + B F = 2-2 + 1 = 1 c. within
Hirsch - Binary phase diagrams problems KEY Name Question 1. 1A. Using the phase rule, determine the degrees of freedom: a. at point e F = 2-3 + 1 = 0 b. within the field A + B F = 2-2 + 1 = 1 c. within
C = 3: Ternary Systems: Example 1: Ternary Eutectic
 Phase Equilibrium C = 3: Ternary Systems: Example 1: Ternary Eutectic Adding components, becomes increasingly difficult to depict 1-C: P - T diagrams easy 2-C: isobaric T-X, isothermal P-X 3-C:?? Still
Phase Equilibrium C = 3: Ternary Systems: Example 1: Ternary Eutectic Adding components, becomes increasingly difficult to depict 1-C: P - T diagrams easy 2-C: isobaric T-X, isothermal P-X 3-C:?? Still
DIFFERENTIATION OF MAGMAS BY FRACTIONAL CRYSTALLIZATION THE M&M MAGMA CHAMBER
 Geol 2312 Igneous and Metamorphic Petrology Spring 2009 Name DIFFERENTIATION OF MAGMAS BY FRACTIONAL CRYSTALLIZATION THE M&M MAGMA CHAMBER Objective: This exercise is intended to improve understanding
Geol 2312 Igneous and Metamorphic Petrology Spring 2009 Name DIFFERENTIATION OF MAGMAS BY FRACTIONAL CRYSTALLIZATION THE M&M MAGMA CHAMBER Objective: This exercise is intended to improve understanding
Environments of Mineral Formation. Stability Diagrams
 Environments of Mineral Formation Unary, Binary, and Ternary Mineral Stability Diagrams Minerals of differing composition (or polymorphs of the same mineral) that coexist at a set of pressure (P) temperature
Environments of Mineral Formation Unary, Binary, and Ternary Mineral Stability Diagrams Minerals of differing composition (or polymorphs of the same mineral) that coexist at a set of pressure (P) temperature
Phase transitions and exsolution phenomena in pyroxenes
 Phase transitions and exsolution phenomena in pyroxenes Cleavage in the pyroxenes 001 100 010 110 110 Optical micrograph showing two cleavages at 90 o Exsolution lamellae in pyroxenes Because exsolution
Phase transitions and exsolution phenomena in pyroxenes Cleavage in the pyroxenes 001 100 010 110 110 Optical micrograph showing two cleavages at 90 o Exsolution lamellae in pyroxenes Because exsolution
Textures of Igneous Rocks
 Page 1 of 6 EENS 212 Prof. Stephen A. Nelson Petrology Tulane University This document last updated on 12-Feb-2004 Introduction to Igneous Rocks An igneous rock is any crystalline or glassy rock that forms
Page 1 of 6 EENS 212 Prof. Stephen A. Nelson Petrology Tulane University This document last updated on 12-Feb-2004 Introduction to Igneous Rocks An igneous rock is any crystalline or glassy rock that forms
Pyroxenes (Mg, Fe 2+ ) 2 Si 2 O 6 (monoclinic) and. MgSiO 3 FeSiO 3 (orthorhombic) Structure (Figure 2 of handout)
 Pyroxenes (Mg, Fe 2+ ) 2 Si 2 O 6 (monoclinic) and 20 MgSiO 3 FeSiO 3 (orthorhombic) Structure (Figure 2 of handout) Chain silicate eg Diopside Mg and Fe ions link SiO 3 chains The chain runs up and down
Pyroxenes (Mg, Fe 2+ ) 2 Si 2 O 6 (monoclinic) and 20 MgSiO 3 FeSiO 3 (orthorhombic) Structure (Figure 2 of handout) Chain silicate eg Diopside Mg and Fe ions link SiO 3 chains The chain runs up and down
Common non-silicate planetary minerals
 Common non-silicate planetary minerals Many of the non-silicate minerals are simple oxides. Corundum Al2O3 Al2+3 O3-2 Rutile Ti2O3 Ti2+3 O3-2 Ilmenite FeTiO3 Fe+3Ti+3O3-2 Hematite Fe2O3 Fe2+3 O3-2 Families
Common non-silicate planetary minerals Many of the non-silicate minerals are simple oxides. Corundum Al2O3 Al2+3 O3-2 Rutile Ti2O3 Ti2+3 O3-2 Ilmenite FeTiO3 Fe+3Ti+3O3-2 Hematite Fe2O3 Fe2+3 O3-2 Families
Triangular Diagrams. 1. On the diagram above, plot the following (mostly mineral) compositions: silicon quartz corundum kyanite
 Triangular Diagrams Note: For this exercise, everything is plotted using mole%. Sometimes it is done with wt% but that just adds unnecessary complications. 1. On the diagram above, plot the following (mostly
Triangular Diagrams Note: For this exercise, everything is plotted using mole%. Sometimes it is done with wt% but that just adds unnecessary complications. 1. On the diagram above, plot the following (mostly
A Rock is a solid aggregate of minerals.
 Quartz A Rock is a solid aggregate of minerals. Orthoclase Feldspar Plagioclase Feldspar Biotite Four different minerals are obvious in this piece of Granite. The average automobile contains: Minerals
Quartz A Rock is a solid aggregate of minerals. Orthoclase Feldspar Plagioclase Feldspar Biotite Four different minerals are obvious in this piece of Granite. The average automobile contains: Minerals
T-X Diagrams C:\Courses\320\fall2007\in class\5000-t-x Exercise.wpd; September 25, 2003 (11:45am)
 1 T-X Diagrams C:\Courses\320\fall2007\in class\5000-t-x Exercise.wpd; September 25, 2003 (11:45am) T-X diagrams are most often used for describing metamorphism of carbonate-rich rocks (marbles or marls)
1 T-X Diagrams C:\Courses\320\fall2007\in class\5000-t-x Exercise.wpd; September 25, 2003 (11:45am) T-X diagrams are most often used for describing metamorphism of carbonate-rich rocks (marbles or marls)
T-X Diagrams Answers C:\Courses\320\fall2007\in class\5000-t-x ExerciseAnswers.wpd; September 25, 2003 (11:45am) Problems
 1 T-X Diagrams Answers C:\Courses\320\fall2007\in class\5000-t-x ExerciseAnswers.wpd; September 25, 2003 (11:45am) Problems Problem 1. Look at Figure 10. One reaction (that plots as a horizontal line)
1 T-X Diagrams Answers C:\Courses\320\fall2007\in class\5000-t-x ExerciseAnswers.wpd; September 25, 2003 (11:45am) Problems Problem 1. Look at Figure 10. One reaction (that plots as a horizontal line)
12 Chemistry (Mg,Fe) 2 SiO 4 Olivine is forms what is called an isomorphous solid solution series that ranges between two end members: Forsterite Mg
 11 Olivine Structure Olivine is a common green or brown rock forming minerals which consists of a solid-solution series between Forsterite (Fo) and Fayalite (Fa). It is an orthorhombic orthosilicate with
11 Olivine Structure Olivine is a common green or brown rock forming minerals which consists of a solid-solution series between Forsterite (Fo) and Fayalite (Fa). It is an orthorhombic orthosilicate with
GEOL 2312 Igneous and Metamorphic Petrology Spring 2016 Score / 58. Midterm 1 Chapters 1-10
 GEOL 2312 Igneous and Metamorphic Petrology Name KEY Spring 2016 Score / 58 Midterm 1 Chapters 1-10 1) Name two things that petrologists want to know about magmas (1 pt) Formation, source, composition,
GEOL 2312 Igneous and Metamorphic Petrology Name KEY Spring 2016 Score / 58 Midterm 1 Chapters 1-10 1) Name two things that petrologists want to know about magmas (1 pt) Formation, source, composition,
The Gibbs Phase Rule F = 2 + C - P
 The Gibbs Phase Rule The phase rule allows one to determine the number of degrees of freedom (F) or variance of a chemical system. This is useful for interpreting phase diagrams. F = 2 + C - P Where F
The Gibbs Phase Rule The phase rule allows one to determine the number of degrees of freedom (F) or variance of a chemical system. This is useful for interpreting phase diagrams. F = 2 + C - P Where F
CERAMIC GLAZING as an IGNEOUS PROCESS
 GEOL 640: Geology through Global Arts and Artifacts CERAMIC GLAZING as an IGNEOUS PROCESS GLAZE COMPONENTS A glaze is a waterproof silica glass on the surface of a ceramic pot, and was first produced by
GEOL 640: Geology through Global Arts and Artifacts CERAMIC GLAZING as an IGNEOUS PROCESS GLAZE COMPONENTS A glaze is a waterproof silica glass on the surface of a ceramic pot, and was first produced by
EPSC 233. Compositional variation in minerals. Recommended reading: PERKINS, p. 286, 41 (Box 2-4).
 EPSC 233 Compositional variation in minerals Recommended reading: PERKINS, p. 286, 41 (Box 2-4). Some minerals are nearly pure elements. These are grouped under the category of native elements. This includes
EPSC 233 Compositional variation in minerals Recommended reading: PERKINS, p. 286, 41 (Box 2-4). Some minerals are nearly pure elements. These are grouped under the category of native elements. This includes
Biaxial Minerals This document last updated on 27-Oct-2014
 1 of 18 10/27/2014 1:10 PM EENS 2110 Tulane University Biaxial Minerals Mineralogy Prof. Stephen A. Nelson This document last updated on 27-Oct-2014 All minerals that crystallize in the orthorhombic, monoclinic,
1 of 18 10/27/2014 1:10 PM EENS 2110 Tulane University Biaxial Minerals Mineralogy Prof. Stephen A. Nelson This document last updated on 27-Oct-2014 All minerals that crystallize in the orthorhombic, monoclinic,
Lecture 36. Igneous geochemistry
 Lecture 36 Igneous geochemistry Reading - White Chapter 7 Today 1. Overview 2. solid-melt distribution coefficients Igneous geochemistry The chemistry of igneous systems provides clues to a number of important
Lecture 36 Igneous geochemistry Reading - White Chapter 7 Today 1. Overview 2. solid-melt distribution coefficients Igneous geochemistry The chemistry of igneous systems provides clues to a number of important
Imagine the first rock and the cycles that it has been through.
 A rock is a naturally formed, consolidated material usually composed of grains of one or more minerals The rock cycle shows how one type of rocky material gets transformed into another The Rock Cycle Representation
A rock is a naturally formed, consolidated material usually composed of grains of one or more minerals The rock cycle shows how one type of rocky material gets transformed into another The Rock Cycle Representation
Basalt-Atmosphere Interactions on Venus - The Rocks Perspective. Allan Treiman Susanne Schwenzer LPI
 Basalt-Atmosphere Interactions on Venus - The Rocks Perspective Allan Treiman Susanne Schwenzer LPI Plan of Talk Hoped to have more results - sorry! So, a selection of research problems on Venus rock-atmosphere
Basalt-Atmosphere Interactions on Venus - The Rocks Perspective Allan Treiman Susanne Schwenzer LPI Plan of Talk Hoped to have more results - sorry! So, a selection of research problems on Venus rock-atmosphere
Metamorphic Petrology GLY 262 Lecture 3: An introduction to metamorphism (II)
 Metamorphic Petrology GLY 262 Lecture 3: An introduction to metamorphism (II) Metamorphic processes Metamorphism is very complex and involves a large number of chemical and physical processes occurring
Metamorphic Petrology GLY 262 Lecture 3: An introduction to metamorphism (II) Metamorphic processes Metamorphism is very complex and involves a large number of chemical and physical processes occurring
Partial melting of mantle peridotite
 Partial melting of mantle peridotite 1100 1200 1300 1400 1500 (TºC) Depth (km) 50 100 150 Plag lherzolite (ol-opx-cpx-pl) Spinel lherzolite (Ol-opx-cpx-sp) Garnet lherzolite (Ol-opx-cpx-gar) Graphite Diamond
Partial melting of mantle peridotite 1100 1200 1300 1400 1500 (TºC) Depth (km) 50 100 150 Plag lherzolite (ol-opx-cpx-pl) Spinel lherzolite (Ol-opx-cpx-sp) Garnet lherzolite (Ol-opx-cpx-gar) Graphite Diamond
Lab 4 - Identification of Igneous Rocks
 Lab 4 - Identification of Igneous Rocks Page - Introduction A rock is a substance made up of one or more different minerals. Thus an essential part of rock identification is the ability to correctly recognize
Lab 4 - Identification of Igneous Rocks Page - Introduction A rock is a substance made up of one or more different minerals. Thus an essential part of rock identification is the ability to correctly recognize
3.012 PS 6 THERMODYANMICS SOLUTIONS Issued: Fall 2005 Due:
 3.012 PS 6 THERMODYANMICS SOLUTIONS 3.012 Issued: 11.28.05 Fall 2005 Due: THERMODYNAMICS 1. Building a binary phase diagram. Given below are data for a binary system of two materials A and B. The two components
3.012 PS 6 THERMODYANMICS SOLUTIONS 3.012 Issued: 11.28.05 Fall 2005 Due: THERMODYNAMICS 1. Building a binary phase diagram. Given below are data for a binary system of two materials A and B. The two components
IGNEOUS ROCKS. SECTION 5.1 What are igneous rocks?
 Date Period Name IGNEOUS ROCKS SECTION.1 What are igneous rocks? In your textbook, read about the nature of igneous rocks. Use each of the terms below just once to complete the following statements. basaltic
Date Period Name IGNEOUS ROCKS SECTION.1 What are igneous rocks? In your textbook, read about the nature of igneous rocks. Use each of the terms below just once to complete the following statements. basaltic
amphibole PART 3 Pyroxene: augite CHAIN SILICATES
 amphibole PART 3 Pyroxene: augite CHAIN SILICATES CHAIN SILICATES = INOSILICATES inos = chains Basic structural group: Si 2 O 6 (each tetrahedra shared two corners) Simple or double chains linked by cations
amphibole PART 3 Pyroxene: augite CHAIN SILICATES CHAIN SILICATES = INOSILICATES inos = chains Basic structural group: Si 2 O 6 (each tetrahedra shared two corners) Simple or double chains linked by cations
2.2 Acid mine drainage
 PROBLEM SOLVING 2. CHEMICAL REACTIONS AND EQUILIBRIA 1 2.2 Acid mine drainage Problem 2.2 The weathering of iron sulphide minerals produces acidified water, leading to major environmental problems from
PROBLEM SOLVING 2. CHEMICAL REACTIONS AND EQUILIBRIA 1 2.2 Acid mine drainage Problem 2.2 The weathering of iron sulphide minerals produces acidified water, leading to major environmental problems from
Chapter 4 Up from the Inferno: Magma and Igneous Rocks
 Chapter 4 Up from the Inferno: Magma and Igneous Rocks Up from the Inferno: Magma and Igneous Rocks Updated by: Rick Oches, Professor of Geology & Environmental Sciences Bentley University Waltham, Massachusetts
Chapter 4 Up from the Inferno: Magma and Igneous Rocks Up from the Inferno: Magma and Igneous Rocks Updated by: Rick Oches, Professor of Geology & Environmental Sciences Bentley University Waltham, Massachusetts
Differentiation of Magmas By Fractional Crystallization Modified from Karl Wirth, rev. July 2011
 M&M s Magma Chamber 1 Differentiation of Magmas By Fractional Crystallization Modified from Karl Wirth, rev. July 2011 Objective The objective of this exercise is to gain first-hand knowledge of the process
M&M s Magma Chamber 1 Differentiation of Magmas By Fractional Crystallization Modified from Karl Wirth, rev. July 2011 Objective The objective of this exercise is to gain first-hand knowledge of the process
Lab 3 - Identification of Igneous Rocks
 Lab 3 - Identification of Igneous Rocks Page - 1 Introduction A rock is a substance made up of one or more different minerals. Thus an essential part of rock identification is the ability to correctly
Lab 3 - Identification of Igneous Rocks Page - 1 Introduction A rock is a substance made up of one or more different minerals. Thus an essential part of rock identification is the ability to correctly
Cloud Condensation Chemistry. in Low-Mass Objects. Katharina Lodders Washington University, St. Louis
 Cloud Condensation Chemistry in Low-Mass Objects Katharina Lodders Washington University, St. Louis For a recent review on this topic, see Lodders & Fegley 2006, Astrophysics Update 2, Springer, p. 1 ff.
Cloud Condensation Chemistry in Low-Mass Objects Katharina Lodders Washington University, St. Louis For a recent review on this topic, see Lodders & Fegley 2006, Astrophysics Update 2, Springer, p. 1 ff.
UNIVERSITY OF EDINBURGH. College of Science and Engineering School of GeoSciences. Earth Materials UO4824 DEGREE EXAMINATION (MOCK) xxxxxxxxxxxxxxxxx
 UNIVERSITY OF EDINBURGH College of Science and Engineering School of GeoSciences Earth Materials UO4824 DEGREE EXAMINATION (MOCK) xxxxxxxxxxxxxxxxxxxx xxxxxxxxxxxxxxxxxxxxxx Chairman: External Examiners:
UNIVERSITY OF EDINBURGH College of Science and Engineering School of GeoSciences Earth Materials UO4824 DEGREE EXAMINATION (MOCK) xxxxxxxxxxxxxxxxxxxx xxxxxxxxxxxxxxxxxxxxxx Chairman: External Examiners:
Chapter 4 8/27/2013. Igneous Rocks. and Intrusive Igneous Activity. Introduction. The Properties and Behavior of Magma and Lava
 Introduction Chapter 4 Igneous rocks form by the cooling of magma (or lava). Large parts of the continents and all the oceanic crust are composed of. and Intrusive Igneous Activity The Properties and Behavior
Introduction Chapter 4 Igneous rocks form by the cooling of magma (or lava). Large parts of the continents and all the oceanic crust are composed of. and Intrusive Igneous Activity The Properties and Behavior
Textural Terms in Igneous Petrology
 Textural Terms in Igneous Petrology Adcumulate - Cumulus crystals continue to grow and displace the intercumulus liquid. Example: Opx adcumulate texture with minor interstitial chromite and plagioclase
Textural Terms in Igneous Petrology Adcumulate - Cumulus crystals continue to grow and displace the intercumulus liquid. Example: Opx adcumulate texture with minor interstitial chromite and plagioclase
Name Class Date STUDY GUIDE FOR CONTENT MASTERY
 Igneous Rocks What are igneous rocks? In your textbook, read about the nature of igneous rocks. Use each of the terms below just once to complete the following statements. extrusive igneous rock intrusive
Igneous Rocks What are igneous rocks? In your textbook, read about the nature of igneous rocks. Use each of the terms below just once to complete the following statements. extrusive igneous rock intrusive
GLY 155 Introduction to Physical Geology, W. Altermann
 Earth Materials Systematic subdivision of magmatic rocks Subdivision of magmatic rocks according to their mineral components: Content of quartz SiO 2 ( free quartz presence) Quartz with conchoidal breakage
Earth Materials Systematic subdivision of magmatic rocks Subdivision of magmatic rocks according to their mineral components: Content of quartz SiO 2 ( free quartz presence) Quartz with conchoidal breakage
CHAPTER 9: INTRODUCTION TO THERMODYNAMICS. Sarah Lambart
 CHAPTER 9: INTRODUCTION TO THERMODYNAMICS Sarah Lambart RECAP CHAP. 8: SILICATE MINERALOGY Orthosilicate: islands olivine: solid solution, ie physical properties vary between 2 endmembers: Forsterite (Mg
CHAPTER 9: INTRODUCTION TO THERMODYNAMICS Sarah Lambart RECAP CHAP. 8: SILICATE MINERALOGY Orthosilicate: islands olivine: solid solution, ie physical properties vary between 2 endmembers: Forsterite (Mg
The Nature of Igneous Rocks
 The Nature of Igneous Rocks Form from Magma Hot, partially molten mixture of solid liquid and gas Mineral crystals form in the magma making a crystal slush Gases - H 2 O, CO 2, etc. - are dissolved in
The Nature of Igneous Rocks Form from Magma Hot, partially molten mixture of solid liquid and gas Mineral crystals form in the magma making a crystal slush Gases - H 2 O, CO 2, etc. - are dissolved in
WORKING WITH ELECTRON MICROPROBE DATA FROM A HIGH PRESSURE EXPERIMENT CALCULATING MINERAL FORMULAS, UNIT CELL CONTENT, AND GEOTHERMOMETRY
 WORKING WITH ELECTRON MICROPROBE DATA FROM A HIGH PRESSURE EXPERIMENT CALCULATING MINERAL FORMULAS, UNIT CELL CONTENT, AND GEOTHERMOMETRY Brandon E. Schwab Department of Geology Humboldt State University
WORKING WITH ELECTRON MICROPROBE DATA FROM A HIGH PRESSURE EXPERIMENT CALCULATING MINERAL FORMULAS, UNIT CELL CONTENT, AND GEOTHERMOMETRY Brandon E. Schwab Department of Geology Humboldt State University
Differentiation of Magmas By Fractional Crystallization
 Wirth Magmatic Differentiation Using M&M s 1 HANDOUT Differentiation of Magmas By Fractional Crystallization Objective The objective of this exercise is to gain first-hand knowledge of the process of magmatic
Wirth Magmatic Differentiation Using M&M s 1 HANDOUT Differentiation of Magmas By Fractional Crystallization Objective The objective of this exercise is to gain first-hand knowledge of the process of magmatic
Geology 212 Petrology Prof. Stephen A. Nelson. Thermodynamics and Metamorphism. Equilibrium and Thermodynamics
 Geology 212 Petrology Prof. Stephen A. Nelson This document last updated on 02-Apr-2002 Thermodynamics and Metamorphism Equilibrium and Thermodynamics Although the stability relationships between various
Geology 212 Petrology Prof. Stephen A. Nelson This document last updated on 02-Apr-2002 Thermodynamics and Metamorphism Equilibrium and Thermodynamics Although the stability relationships between various
Earth Materials II Review Optical Mineralogy and Igneous Minerals
 Earth Materials II Review Optical Mineralogy and Igneous Minerals Refractive Index and Angle of Refraction Refractive Index(R. I. ) = velocity of light in a vacuum velocity of light in a medium The refractive
Earth Materials II Review Optical Mineralogy and Igneous Minerals Refractive Index and Angle of Refraction Refractive Index(R. I. ) = velocity of light in a vacuum velocity of light in a medium The refractive
Engineering Geology ECIV 2204
 Engineering Geology ECIV 2204 Instructor : Dr. Jehad Hamad 2017-2016 Chapter (3) Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth
Engineering Geology ECIV 2204 Instructor : Dr. Jehad Hamad 2017-2016 Chapter (3) Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth
Silicates. The most common group of minerals forming the silicate Earth
 Silicates The most common group of minerals forming the silicate Earth 25% of all minerals (~1000) 40% of rock forming minerals 90% of earth s crust i.e those minerals you are likely to find ~100 of earth
Silicates The most common group of minerals forming the silicate Earth 25% of all minerals (~1000) 40% of rock forming minerals 90% of earth s crust i.e those minerals you are likely to find ~100 of earth
Classification of Igneous Rocks
 Classification of Igneous Rocks Textures: Glassy- no crystals formed Aphanitic- crystals too small to see by eye Phaneritic- can see the constituent minerals Fine grained- < 1 mm diameter Medium grained-
Classification of Igneous Rocks Textures: Glassy- no crystals formed Aphanitic- crystals too small to see by eye Phaneritic- can see the constituent minerals Fine grained- < 1 mm diameter Medium grained-
Earth Science 11: Minerals
 lname: Date: Earth Science 11: Minerals Purpose: Text Pages: I can identify and classify minerals using their physical and chemical properties 90-111 *This is recommended reading! Matter and Atoms (5.1)
lname: Date: Earth Science 11: Minerals Purpose: Text Pages: I can identify and classify minerals using their physical and chemical properties 90-111 *This is recommended reading! Matter and Atoms (5.1)
Earth Science 232 Petrography
 Earth Science 232 Petrography Course notes by Shaun Frape and Alec Blyth Winter 2002 1 Petrology - Introduction Some Definitions Petra Greek for rock Logos Greek for disclosure or explanation Petrology
Earth Science 232 Petrography Course notes by Shaun Frape and Alec Blyth Winter 2002 1 Petrology - Introduction Some Definitions Petra Greek for rock Logos Greek for disclosure or explanation Petrology
Lecture 6 - Igneous Rocks and Volcanoes
 Lecture 6 - Igneous Rocks and Volcanoes Learning objectives Understand and be able to predict where and why magma will be forming at different tectonic settings Understand the factors controlling magma
Lecture 6 - Igneous Rocks and Volcanoes Learning objectives Understand and be able to predict where and why magma will be forming at different tectonic settings Understand the factors controlling magma
Geos 306, Mineralogy Final Exam, Dec 12, pts
 Name: Geos 306, Mineralogy Final Exam, Dec 12, 2014 200 pts 1. (9 pts) What are the 4 most abundant elements found in the Earth and what are their atomic abundances? Create a reasonable hypothetical charge-balanced
Name: Geos 306, Mineralogy Final Exam, Dec 12, 2014 200 pts 1. (9 pts) What are the 4 most abundant elements found in the Earth and what are their atomic abundances? Create a reasonable hypothetical charge-balanced
Topic 5 : Crystal chemistry
 GEOL360 LECTURE NOTES: T5 : CRYSTAL CHEMISTRY 1/15 GEOL360 Topic 5 : Crystal chemistry 5.1 Introduction what is a crystal? A crystal is a homogeneous, solid body of a chemical element, compound, or isomorphous
GEOL360 LECTURE NOTES: T5 : CRYSTAL CHEMISTRY 1/15 GEOL360 Topic 5 : Crystal chemistry 5.1 Introduction what is a crystal? A crystal is a homogeneous, solid body of a chemical element, compound, or isomorphous
Block: Igneous Rocks. From this list, select the terms which answer the following questions.
 Geology 12 Name: Mix and Match: Igneous Rocks Refer to the following list. Block: porphyritic volatiles mafic glassy magma mixing concordant discontinuous reaction series igneous vesicular partial melting
Geology 12 Name: Mix and Match: Igneous Rocks Refer to the following list. Block: porphyritic volatiles mafic glassy magma mixing concordant discontinuous reaction series igneous vesicular partial melting
Name Class Date. In your textbook, read about the nature of igneous rocks. Use each of the terms below just once to complete the following statements.
 CHAPTER 5 Igneous Rocks SECTION 5.1 What are igneous rocks? In your textbook, read about the nature of igneous rocks. Use each of the terms below just once to complete the following statements. basaltic
CHAPTER 5 Igneous Rocks SECTION 5.1 What are igneous rocks? In your textbook, read about the nature of igneous rocks. Use each of the terms below just once to complete the following statements. basaltic
Rocks Rock- A group of minerals, glass, mineroid bound together in some way.
 Rocks Rock- A group of minerals, glass, mineroid bound together in some way. All rocks fit into one of three categories: Igneous- formed by the cooling and hardening of hot molten rock Sedimentary- formed
Rocks Rock- A group of minerals, glass, mineroid bound together in some way. All rocks fit into one of three categories: Igneous- formed by the cooling and hardening of hot molten rock Sedimentary- formed
Earth and Planetary Materials
 Earth and Planetary Materials Spring 2013 Lecture 4 2013.01.16 Example Beryl Be 3 Al 2 (SiO 3 ) 6 Goshenite Aquamarine Emerald Heliodor Red beryl Morganite pure Fe 2+ & Fe 3+ Cr 3+ Fe 3+ Mn 3+ Mn 2+ Rules
Earth and Planetary Materials Spring 2013 Lecture 4 2013.01.16 Example Beryl Be 3 Al 2 (SiO 3 ) 6 Goshenite Aquamarine Emerald Heliodor Red beryl Morganite pure Fe 2+ & Fe 3+ Cr 3+ Fe 3+ Mn 3+ Mn 2+ Rules
GY 302: Crystallography & Mineralogy
 UNIVERSITY OF SOUTH ALABAMA GY 302: Crystallography & Mineralogy Lecture 26: Class VIII-Silicates Tektosilicates part 2: Feldspars Last Time Class VIII Minerals (Tektosilicates) 1. Quartz Group Tektosilicate
UNIVERSITY OF SOUTH ALABAMA GY 302: Crystallography & Mineralogy Lecture 26: Class VIII-Silicates Tektosilicates part 2: Feldspars Last Time Class VIII Minerals (Tektosilicates) 1. Quartz Group Tektosilicate
THE ORDER OF CRYSTALLIZATION IN IGNEOUS ROCKS
 THE ORDER OF CRYSTALLIZATION IN IGNEOUS ROCKS N. L. BOWEN Massachusetts Institute of Technology The order of crystallization in igneous rocks is necessarily determined from the end product, the solid rock.
THE ORDER OF CRYSTALLIZATION IN IGNEOUS ROCKS N. L. BOWEN Massachusetts Institute of Technology The order of crystallization in igneous rocks is necessarily determined from the end product, the solid rock.
Pyroxene, amphibole, and feldspar
 Pyroxene, amphibole, and feldspar Sometimes, common minerals in igneous and metamorphic rocks are hard to distinguish, unless you know the tricks. Colored mineral such as pyroxene and amphibole can be
Pyroxene, amphibole, and feldspar Sometimes, common minerals in igneous and metamorphic rocks are hard to distinguish, unless you know the tricks. Colored mineral such as pyroxene and amphibole can be
ANALYSIS OF GEOLOGIC MATERIALS USING RIETVELD QUANTIATIVE X-RAY DIFFRACTION
 Copyright JCPDS - International Centre for Diffraction Data 2003, Advances in X-ray Analysis, Volume 46. 204 ANALYSIS OF GEOLOGIC MATERIALS USING RIETVELD QUANTIATIVE X-RAY DIFFRACTION Robin M. Gonzalez,
Copyright JCPDS - International Centre for Diffraction Data 2003, Advances in X-ray Analysis, Volume 46. 204 ANALYSIS OF GEOLOGIC MATERIALS USING RIETVELD QUANTIATIVE X-RAY DIFFRACTION Robin M. Gonzalez,
Chapter 4 Rocks & Igneous Rocks
 Chapter 4 Rocks & Igneous Rocks Rock Definition A naturally occurring consolidated mixture of one or more minerals e.g, marble, granite, sandstone, limestone Rock Definition Must naturally occur in nature,
Chapter 4 Rocks & Igneous Rocks Rock Definition A naturally occurring consolidated mixture of one or more minerals e.g, marble, granite, sandstone, limestone Rock Definition Must naturally occur in nature,
Rocks: Materials of the Solid Earth
 1 Rocks: Materials of the Solid Earth Presentation modified from: Instructor Resource Center on CD-ROM, Foundations of Earth Science,, 4 th Edition, Lutgens/Tarbuck, Rock Cycle Igneous Rocks Today 2 Rock
1 Rocks: Materials of the Solid Earth Presentation modified from: Instructor Resource Center on CD-ROM, Foundations of Earth Science,, 4 th Edition, Lutgens/Tarbuck, Rock Cycle Igneous Rocks Today 2 Rock
Phase Equilibria C:\a-StudioClassroom\minex20.doc; July 7, 2005
 1 Phase Equilibria C:\a-StudioClassroom\minex20.doc; July 7, 2005 S/mole V/mole E/mole J/mol-K cc/mol J/mol grossular 255.5 125.3-6656700 quartz 41.46 22.688-910700 anorthite 199.3 100.79-4243040 wollastonite
1 Phase Equilibria C:\a-StudioClassroom\minex20.doc; July 7, 2005 S/mole V/mole E/mole J/mol-K cc/mol J/mol grossular 255.5 125.3-6656700 quartz 41.46 22.688-910700 anorthite 199.3 100.79-4243040 wollastonite
Chapter 4 Minerals Sec. 4.1 What is a Mineral?
 Chapter 4 Minerals Sec. 4.1 What is a Mineral? Minerals Earth s crust is composed of about 3000 minerals. Besides forming rocks and shaping Earth s surface, some minerals have helped to develop civilization.
Chapter 4 Minerals Sec. 4.1 What is a Mineral? Minerals Earth s crust is composed of about 3000 minerals. Besides forming rocks and shaping Earth s surface, some minerals have helped to develop civilization.
Lorence G. Collins. July 9, 1997
 1 ISSN 1526-5757 20. FAILURE OF THE EXSOLUTION SILICA-PUMP MODEL FOR THE ORIGIN OF MYRMEKITE: EXAMINATION OF K-FELDSPAR CRYSTALS IN THE SHARPNERS POND TONALITE, MASSACHUSETTS, USA Introduction Lorence
1 ISSN 1526-5757 20. FAILURE OF THE EXSOLUTION SILICA-PUMP MODEL FOR THE ORIGIN OF MYRMEKITE: EXAMINATION OF K-FELDSPAR CRYSTALS IN THE SHARPNERS POND TONALITE, MASSACHUSETTS, USA Introduction Lorence
Fluids, melts, and supercriticality in the MSH system and element transport in subduction zones
 cosmic rays Fluids, s, and supercriticality in the MSH system and element transport in subduction zones 10 Be volcanic front N, O 10 Be ocean water + CO 2 tracing petrologic and geotectonic processes (trace)
cosmic rays Fluids, s, and supercriticality in the MSH system and element transport in subduction zones 10 Be volcanic front N, O 10 Be ocean water + CO 2 tracing petrologic and geotectonic processes (trace)
LAB 9: ULTRAMAFIC ROCKS, CUMULATES AND MELT SOURCES
 Geology 316 (Petrology) (03/26/2012) Name LAB 9: ULTRAMAFIC ROCKS, CUMULATES AND MELT SOURCES INTRODUCTION Ultramafic rocks are igneous rocks containing less than 10% felsic minerals (quartz + feldspars
Geology 316 (Petrology) (03/26/2012) Name LAB 9: ULTRAMAFIC ROCKS, CUMULATES AND MELT SOURCES INTRODUCTION Ultramafic rocks are igneous rocks containing less than 10% felsic minerals (quartz + feldspars
Quartz. ! Naturally occurring - formed by nature. ! Solid - not liquid or gas. Liquid water is not a mineral
 GEOL 110 - Minerals, Igneous Rocks Minerals Diamond Azurite Quartz Why Study Minerals?! Rocks = aggregates of minerals! Importance to Society?! Importance to Geology? 5 part definition, must satisfy all
GEOL 110 - Minerals, Igneous Rocks Minerals Diamond Azurite Quartz Why Study Minerals?! Rocks = aggregates of minerals! Importance to Society?! Importance to Geology? 5 part definition, must satisfy all
Constitution of Magmas. Magmas. Gas Law. Composition. Atomic Structure of Magma. Structural Model. PV = nrt H 2 O + O -2 = 2(OH) -
 Constitution of Magmas Magmas Best, Ch. 8 Hot molten rock T = 700-1200 degrees C Composed of ions or complexes Phase Homogeneous Separable part of the system With an interface Composition Most components
Constitution of Magmas Magmas Best, Ch. 8 Hot molten rock T = 700-1200 degrees C Composed of ions or complexes Phase Homogeneous Separable part of the system With an interface Composition Most components
N = N 0 e -λt D* = N 0 -N D* = N 0 (1-e -λt ) or N(e λt -1) where N is number of parent atoms at time t, N 0
 N = N 0 e -λt D* = N 0 -N D* = N 0 (1-e -λt ) or N(e λt -1) where N is number of parent atoms at time t, N 0 is initial number of parents, D* is number of radiogenic daughter atoms, and λ is the decay
N = N 0 e -λt D* = N 0 -N D* = N 0 (1-e -λt ) or N(e λt -1) where N is number of parent atoms at time t, N 0 is initial number of parents, D* is number of radiogenic daughter atoms, and λ is the decay
GY303 Igneous & Metamorphic Petrology. Lecture 7: Magma Sources and Tectonic Environments
 GY303 Igneous & Metamorphic Petrology Lecture 7: Magma Sources and Tectonic Environments Factors controlling Magma production Source rock composition Amount of fluids, especially H 2 O Pressure (Depth)
GY303 Igneous & Metamorphic Petrology Lecture 7: Magma Sources and Tectonic Environments Factors controlling Magma production Source rock composition Amount of fluids, especially H 2 O Pressure (Depth)
Exsolution and Ordering in Solid Solutions
 Exsolution and Ordering in Solid Solutions 1.1 Introduction to solid solutions A solid solution is a single phase which exists over a range of chemical compositions. Some materials are able to tolerate
Exsolution and Ordering in Solid Solutions 1.1 Introduction to solid solutions A solid solution is a single phase which exists over a range of chemical compositions. Some materials are able to tolerate
Name Class Date STUDY GUIDE FOR CONTENT MASTERY
 Igneous Rocks What are igneous rocks? In your textbook, read about the nature of igneous rocks. Use each of the terms below just once to complete the following statements. extrusive igneous rock intrusive
Igneous Rocks What are igneous rocks? In your textbook, read about the nature of igneous rocks. Use each of the terms below just once to complete the following statements. extrusive igneous rock intrusive
About Earth Materials
 Grotzinger Jordan Understanding Earth Sixth Edition Chapter 3: EARTH MATERIALS Minerals and Rocks 2011 by W. H. Freeman and Company About Earth Materials All Earth materials are composed of atoms bound
Grotzinger Jordan Understanding Earth Sixth Edition Chapter 3: EARTH MATERIALS Minerals and Rocks 2011 by W. H. Freeman and Company About Earth Materials All Earth materials are composed of atoms bound
LAB 2: SILICATE MINERALS
 GEOLOGY 640: Geology through Global Arts and Artifacts LAB 2: SILICATE MINERALS FRAMEWORK SILICATES The framework silicates quartz and feldspar are the most common minerals in Earth s crust. Quartz (SiO
GEOLOGY 640: Geology through Global Arts and Artifacts LAB 2: SILICATE MINERALS FRAMEWORK SILICATES The framework silicates quartz and feldspar are the most common minerals in Earth s crust. Quartz (SiO
2. REPLACEMENT OF PRIMARY PLAGIOCLASE BY SECONDARY K-FELDSPAR AND MYRMEKITE
 1 ISSN 1526-5757 2. REPLACEMENT OF PRIMARY PLAGIOCLASE BY SECONDARY K-FELDSPAR AND MYRMEKITE Lorence G. Collins email: lorencec@sysmatrix.net November 21, 1996; revised February 17, 1997 The following
1 ISSN 1526-5757 2. REPLACEMENT OF PRIMARY PLAGIOCLASE BY SECONDARY K-FELDSPAR AND MYRMEKITE Lorence G. Collins email: lorencec@sysmatrix.net November 21, 1996; revised February 17, 1997 The following
SUPPLEMENTARY INFORMATION
 GSA Data Repository 080 Schorn et al., 08, Thermal buffering in the orogenic crust: Geology, https://doi.org/0.30/g4046.. SUPPLEMENTARY INFORMATION 3 PHASE DIAGRAM MODELING 4 5 6 7 8 9 0 3 4 Phase diagrams
GSA Data Repository 080 Schorn et al., 08, Thermal buffering in the orogenic crust: Geology, https://doi.org/0.30/g4046.. SUPPLEMENTARY INFORMATION 3 PHASE DIAGRAM MODELING 4 5 6 7 8 9 0 3 4 Phase diagrams
LAB 3: COMMON MINERALS IN SEDIMENTARY ROCKS, Part 1
 EESC 2100: Mineralogy LAB 3: COMMON MINERALS IN SEDIMENTARY ROCKS, Part 1 Learning Objectives: Students will be able to identify minerals that occur commonly in sandstones (quartz and feldspars), both
EESC 2100: Mineralogy LAB 3: COMMON MINERALS IN SEDIMENTARY ROCKS, Part 1 Learning Objectives: Students will be able to identify minerals that occur commonly in sandstones (quartz and feldspars), both
Earth Science 11: Earth Materials: Rock Cycle
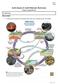 Name: Date: Earth Science 11: Earth Materials: Rock Cycle Chapter 2, pages 44 to 46 2.1: Rock Cycle What is a Rock? A solid mass of mineral or mineral-like matter that occurs naturally as part of our planet
Name: Date: Earth Science 11: Earth Materials: Rock Cycle Chapter 2, pages 44 to 46 2.1: Rock Cycle What is a Rock? A solid mass of mineral or mineral-like matter that occurs naturally as part of our planet
Chapter: Earth Materials
 Table of Contents Chapter: Earth Materials Section 1: Minerals Section 2: Igneous Rocks Section 3: Sedimentary Rocks Section 4: Metamorphic Rocks and the Rock Cycle 1 Minerals Common Elements Composition
Table of Contents Chapter: Earth Materials Section 1: Minerals Section 2: Igneous Rocks Section 3: Sedimentary Rocks Section 4: Metamorphic Rocks and the Rock Cycle 1 Minerals Common Elements Composition
JOURNAL OF GEOLOGY THE. August-September 1922 THE BEHAVIOR OF INCLUSIONS IN IGNEOUS MAGMAS
 VOLUME XXX SUPPLEMENT TO NUMBER 6 THE JOURNAL OF GEOLOGY August-September 1922 SUPPLEMENT THE BEHAVIOR OF INCLUSIONS IN IGNEOUS MAGMAS Geophysical Laboratory, Carnegie Institution of Washington INTRODUCTION
VOLUME XXX SUPPLEMENT TO NUMBER 6 THE JOURNAL OF GEOLOGY August-September 1922 SUPPLEMENT THE BEHAVIOR OF INCLUSIONS IN IGNEOUS MAGMAS Geophysical Laboratory, Carnegie Institution of Washington INTRODUCTION
Igneous Rock Classification, Processes and Identification Physical Geology GEOL 100
 Igneous Rock Classification, Processes and Identification Physical Geology GEOL 100 Ray Rector - Instructor Major Concepts 1) Igneous rocks form directly from the crystallization of a magma or lava 2)
Igneous Rock Classification, Processes and Identification Physical Geology GEOL 100 Ray Rector - Instructor Major Concepts 1) Igneous rocks form directly from the crystallization of a magma or lava 2)
Plate tectonics, rock cycle
 Dikes, Antarctica Rock Cycle Plate tectonics, rock cycle The Rock Cycle A rock is a naturally formed, consolidated material usually composed of grains of one or more minerals The rock cycle shows how one
Dikes, Antarctica Rock Cycle Plate tectonics, rock cycle The Rock Cycle A rock is a naturally formed, consolidated material usually composed of grains of one or more minerals The rock cycle shows how one
LAB 6: COMMON MINERALS IN IGNEOUS ROCKS
 GEOLOGY 17.01: Mineralogy LAB 6: COMMON MINERALS IN IGNEOUS ROCKS Part 2: Minerals in Gabbroic Rocks Learning Objectives: Students will be able to identify the most common silicate minerals in gabbroic
GEOLOGY 17.01: Mineralogy LAB 6: COMMON MINERALS IN IGNEOUS ROCKS Part 2: Minerals in Gabbroic Rocks Learning Objectives: Students will be able to identify the most common silicate minerals in gabbroic
CHAPTER 4. Crystal Structure
 CHAPTER 4 Crystal Structure We can assume minerals to be made of orderly packing of atoms or rather ions or molecules. Many mineral properties like symmetry, density etc are dependent on how the atoms
CHAPTER 4 Crystal Structure We can assume minerals to be made of orderly packing of atoms or rather ions or molecules. Many mineral properties like symmetry, density etc are dependent on how the atoms
Igneous Rocks. Sedimentary Rocks. Metamorphic Rocks
 Name: Date: Igneous Rocks Igneous rocks form from the solidification of magma either below (intrusive igneous rocks) or above (extrusive igneous rocks) the Earth s surface. For example, the igneous rock
Name: Date: Igneous Rocks Igneous rocks form from the solidification of magma either below (intrusive igneous rocks) or above (extrusive igneous rocks) the Earth s surface. For example, the igneous rock
Chapter 3: Igneous Rocks 3.2 IGNEOUS ROCK ORIGIN
 Chapter 3: Igneous Rocks Adapted by Lyndsay R. Hauber & Michael B. Cuggy (2018) University of Saskatchewan from Deline B, Harris R & Tefend K. (2015) "Laboratory Manual for Introductory Geology". First
Chapter 3: Igneous Rocks Adapted by Lyndsay R. Hauber & Michael B. Cuggy (2018) University of Saskatchewan from Deline B, Harris R & Tefend K. (2015) "Laboratory Manual for Introductory Geology". First
Phase Diagrams and Chemographic Diagrams C:\Courses\320\fall2005\inclass, etc\57-projections.wpd; October 9, 2003 (6:09pm)
 1 Phase Diagrams and Chemographic Diagrams C:\Courses\320\fall2005\inclass, etc\57-projections.wpd; October 9, 2003 (6:09pm) Recall the phase rule: C + 2 = P + F. At a point on a phase diagram where two
1 Phase Diagrams and Chemographic Diagrams C:\Courses\320\fall2005\inclass, etc\57-projections.wpd; October 9, 2003 (6:09pm) Recall the phase rule: C + 2 = P + F. At a point on a phase diagram where two
GSA DATA REPOSITORY
 GSA DATA REPOSITORY 2013019 Supplemental information for The Solidus of Alkaline Carbonatite in the Deep Mantle Konstantin D. Litasov, Anton Shatskiy, Eiji Ohtani, and Gregory M. Yaxley EXPERIMENTAL METHODS
GSA DATA REPOSITORY 2013019 Supplemental information for The Solidus of Alkaline Carbonatite in the Deep Mantle Konstantin D. Litasov, Anton Shatskiy, Eiji Ohtani, and Gregory M. Yaxley EXPERIMENTAL METHODS
Structure of Earth Materials
 12.108 Structure of Earth Materials I. Lecture 1: Minerals and Symmetry Operations Definition of a mineral A mineral is a naturally occurring homogeneous solid usually formed by inorganic processes. It
12.108 Structure of Earth Materials I. Lecture 1: Minerals and Symmetry Operations Definition of a mineral A mineral is a naturally occurring homogeneous solid usually formed by inorganic processes. It
EAS 4550: Geochemistry Problem Set 4 Solutions Due Sept. 27, 2017
 1. Interaction parameters for the enstatite diopside solid solution have been determined as follows: WH-En = 34.0 kj/mol, WH-Di = 24.74 kj/mol (assume WV and WS are 0). (a) Use the asymmetric solution
1. Interaction parameters for the enstatite diopside solid solution have been determined as follows: WH-En = 34.0 kj/mol, WH-Di = 24.74 kj/mol (assume WV and WS are 0). (a) Use the asymmetric solution
