The International Association for the Properties of Water and Steam
|
|
|
- Philip Hodges
- 5 years ago
- Views:
Transcription
1 The International Association for the Properties of Water and Steam Gaithersburg, Maryland, USA September 2001 Guideline on the Use of Fundamental Physical Constants and Basic Constants of Water 2001 International Association for the Properties of Water and Steam Publication in whole or in part is allowed in all countries provided that attribution is given to the International Association for the Properties of Water and Steam President (in 2001): Dr. Bert Rukes Siemens AG Power Generation P.O. Box 3220 D Erlangen, Germany Executive Secretary: Dr. R. B. Dooley Electric Power Research Institute 1300 West W.T. Harris Blvd. Charlotte, North Carolina 28262, USA This guideline contains 7 pages, including this cover page. This guideline was first authorized by the International Association for the Properties of Water and Steam (IAPWS) at its meeting in Gaithersburg, Maryland, United States of America, 9-14 September 2001, for issue by its Secretariat. The members of IAPWS are Argentina and Brazil, Britain and Ireland, Canada, the Czech Republic, Denmark, France, Germany, Greece, Italy, Japan, Russia, and the United States of America. This Guideline is reviewed annually in or near September and revised as necessary. Date of this revision July Previous revision was July Further information about this guideline and other documents issued by IAPWS can be obtained from the Executive Secretary of IAPWS, or on the IAPWS Website at
2 2 Contents 1 Introduction 3 2 Physical Fundamentals Fundamental Physical Constants Temperature Scale 3 3 Molecular Properties Isotopic Composition Relative Molar Mass of Ordinary Water Substance Relative Molar Mass of Heavy Water Dipole Moment Polarizability 4 4 Reference Thermophysical Quantities Critical Constants Triple Point Density Maximum Reference Liquid Viscosity 5 5 References 6
3 3 1 Introduction The purpose of this Guideline is to set forth a consistent set of recommendations for best practice in the use of various fundamental quantities in IAPWS Releases and Guidelines. Additionally, this Guideline is available for others who may wish to make their scientific or engineering work as consistent as possible with IAPWS and other standards. Section 2 contains recommendations for use of fundamental physical constants. Section 3 contains recommendations for molecular-level properties of water. Section 4 contains recommendations for thermophysical properties at unique physical states and states that are widely used as calibration standards. The recommendations in this Guideline are primarily for ordinary water, but some recommendations for heavy water are included. The background information for most of these recommendations is given in Reference [1]. 2 Physical Fundamentals 2.1 Fundamental Physical Constants The international body CODATA determines accepted values of fundamental physical constants (such as the molar gas constant, Boltzmann s constant, and the unit electronic charge) at periodic intervals. IAPWS recommends that scientific work use the most recent CODATA values of these constants available at the time the work is performed. As of this writing, the most recent CODATA adjustment of values for physical constants is their 2002 adjustment [2]. The CODATA values are also available on the World Wide Web at: Temperature Scale IAPWS recommends that scientific work use the most recent representation of the temperature scale as adopted by the International Committee for Weights and Measures (CIPM). As of this writing, the current standard is the International Temperature Scale of 1990 (ITS-90) [3]. Where possible, especially for precise measurements, data should be converted to the current temperature scale before being used to produce correlations, etc. Equations for converting temperatures to ITS-90 from its predecessor, the International Practical Temperature Scale of 1968 (IPTS-68) are given by Rusby [4, 5]. Conversion to IPTS-68 from its predecessor, IPTS-48, is described by Bedford and Kirby [6]. 3 Molecular Properties 3.1 Isotopic Composition We define the isotopic composition of ordinary water substance to be that of Vienna Standard Mean Ocean Water (VSMOW), which is documented in [7]. The isotopic composition of VSMOW is as follows [8]: the amount of 2 H is (5) atom percent of the total hydrogen, and the amounts of the total oxygen as 17 O and 18 O are (9)
4 4 and (5) atom percent, respectively. The numbers in parentheses reflect the uncertainties in the last digit. There may be situations (for example, in analysis of specific experiments) in which the isotopic composition is known to be different from that of VSMOW. In such cases, it would be appropriate to use that known composition in calculations such as conversion of data between mass-based and molar units. 3.2 Relative Molar Mass of Ordinary Water Substance The relative molar mass used in calculations (with the exception, mentioned above, of cases in which the isotopic composition is known to be different) should be that of VSMOW. This is computed by combining the isotopic composition of VSMOW with the accepted values of the masses of each isotope. Performing this calculation with the 2003 atomic mass evaluation [9] yields a relative molar mass of , with an uncertainty of no greater than two in the last digit. 3.3 Relative Molar Mass of Heavy Water We define heavy water as water whose hydrogen content is pure 2 H and whose oxygen has the isotopic composition of VSMOW [10]. The computation then proceeds in the same manner as for ordinary water, yielding a relative molar mass of , with an uncertainty of no greater than two in the last digit. 3.4 Dipole Moment For purposes of making a standard definition, we consider the dipole moment of an isolated, nonrotating water molecule in its ground vibrational state. This is different from the equilibrium dipole moment sometimes discussed in the literature; the equilibrium moment is a hypothetical value which would be obtained in the absence of any vibration, ground state or otherwise. We accept the results of Shostak et al. [11] who measured a dipole moment of (9) debyes for H 2 O. They also measured the dipole moment for the HDO molecule (where D is deuterium, 2 H) in the ground state; this was (5) debyes. In order to get an effective dipole moment value for ordinary water substance, one should average the moments of H 2 O and HDO according to the isotopic composition of the hydrogen in VSMOW. In this case, the amount of 2 H is sufficiently small that the average is indistinguishable from that for H 2 O. Therefore, for the dipole moment of the isolated water molecule in its ground vibrational state, we adopt the value (9) debyes. In SI units, this is (3) C m. 3.5 Polarizability The dipole polarizability of water is not a single number but rather a tensor. Because the anisotropy of water s polarizability is relatively small, it is a fair approximation for many purposes to ignore the anisotropy and use only the mean polarizability, which is one-third of the trace of this tensor. We consider only the mean polarizability here. It is common practice to tabulate not the polarizability α itself, but rather the combination α/4πε 0 (where
5 5 ε 0 is the permittivity of free space), which has dimensions of volume. We adopt that convention here; if α itself is required, the value of ε 0 adopted by CODATA (see section 2) should be used in the conversion. The polarizability is also frequency-dependent; we consider here the high-frequency (electronic) value and the low-frequency (static) limit. The latter includes not only the response of the electrons to an applied field but also a vibrational contribution from the response of the atoms in the molecule. For the mean electronic polarizability, we adopt the value 1.457(3) m 3, which Russell and Spackman [12] extracted from refractive index data. For the vibrational contribution to the total polarizability in the low-frequency limit, we adopt the estimate of Bishop and Cheung [13], which is m 3. The uncertainty in the vibrational contribution is probably on the order of ten percent [13]. For the mean total dipole polarizability (in the static limit), which is the sum of the electronic and vibrational effects, we therefore adopt a value of 1.494(7) m 3. 4 Reference Thermophysical Quantities 4.1 Critical Constants IAPWS standards exist for water s critical temperature, pressure, and density [14]. These values (T c = K, p c = MPa, ρ c = 322 kg/m 3 ) should be uniformly used. For heavy water, values (T c = K, p c = MPa, ρ c = 356 kg/m 3 ) are given in the same document. Where it is necessary to consider uncertainties in the values, those listed in the IAPWS release should be used. 4.2 Triple Point The triple-point temperature of ordinary water is used to define the base SI unit of temperature, the kelvin, and is K. For the triple-point pressure, we recommend the value measured by Guildner et al. [15], which is ( ± 0.010) Pa. 4.3 Density Maximum Liquid water at atmospheric pressure ( MPa) has a maximum density at a temperature slightly below 4 C. This point is often recommended as a reference standard for precise density measurement. The CCM (Consultative Committee for Mass and Related Quantities) of the CIPM (Comité International des Poids et Mesures) has adopted a value for this maximum density (for the isotopic composition of VSMOW) as a part of its standard table for temperatures from 0 C to 40 C [16]. This value is ( ± ) kg/m Reference Liquid Viscosity The dynamic viscosity of liquid water at kpa and K is an important calibration standard. The International Organization for Standardization (ISO) [17] has accepted the value of mpa s for this reference value, with an estimated uncertainty of 0.17 %.
6 6 5 References [1] Harvey, A.H., Use of Fundamental Constants in IAPWS Work, Report to IAPWS Working Group on Thermophysical Properties of Water and Steam, September [2] Mohr, P.J. and Taylor, B.N., CODATA Recommended Values of the Fundamental Physical Constants: 2002, Rev. Mod. Phys., 77, (2005). [3] Preston-Thomas, H., The International Temperature Scale of 1990 (ITS-90), Metrologia, 27, 3-10 (1990). [4] Rusby, R.L., The conversion of thermal reference values to the ITS-90, J. Chem. Thermodyn., 23, (1991). [5] Rusby, R.L., Hudson, R.P. and Durieux, M., Revised Values for (t 90 t 68 ) from 630 C to 1064 C, Metrologia, 31, (1994). [6] Bedford, R.E. and Kirby, C.G.M., Notes on the Application of the International Practical Temperature Scale of 1968, Metrologia, 5, (1969). [7] Gonfiantini, R., Standards for stable isotope measurements in natural compounds, Nature, 271, (1978). [8] National Institute of Standards and Technology, Report of Investigation, Reference Materials 8535, 8536, 8537, NIST Standard Reference Materials Program, Gaithersburg, MD (2005 revision of 1992 original report). [9] Audi, G., Wapstra, A.H. and Thibault, C., The AME2003 atomic mass evaluation (II). Tables, graphs and references, Nucl. Phys. A, 729, (2003). [10] Kell, G.S., Effects of Isotopic Composition, Temperature, Pressure, and Dissolved Gases on the Density of Liquid Water, J. Phys. Chem. Ref. Data, 6, (1977). [11] Shostak, S.L., Ebenstein, W.L. and Muenter, J.S., The dipole moment of water. I. Dipole moments and hyperfine properties of H 2 O and HDO in the ground and excited vibrational states, J. Chem. Phys., 94, (1991). [12] Russell, A.J. and Spackman, M.A., Vibrational averaging of electrical properties. Development of a routine theoretical method for polyatomic molecules, Mol. Phys., 84, (1995). [13] Bishop, D.M. and Cheung, L.M., Vibrational Contributions to Molecular Dipole Polarizabilities, J. Phys. Chem. Ref. Data, 11, (1982). [14] IAPWS, Release on the Values of Temperature, Pressure and Density of Ordinary and Heavy Water Substances at Their Respective Critical Points, in Physical Chemistry of Aqueous Systems, Proc. 12th ICPWS, Orlando, Florida, 1994, H.J. White, Jr., J.V. Sengers, D.B. Neumann, and J.C. Bellows, ed. Begell House, New York (1995). Up-to-date versions of IAPWS releases can be obtained from the Executive Secretary of IAPWS, Dr. R.B. Dooley, Electric Power Research Institute, 1300 West W.T. Harris Blvd., Charlotte, NC 28262, USA and from
7 7 [15] Guildner, L.A., Johnson, D.P. and Jones, F.E., Vapor Pressure of Water at its Triple Point, J. Res. NBS, 80A, (1976). [16] Tanaka, M., Girard, G., Davis, R., Peuto, A. and Bignell, N., Recommended table for the density of water between 0 C and 40 C based on recent experimental reports, Metrologia, 38, (2001). [17] ISO, Viscosity of Water, International Organization for Standardization, ISO/TR 3666 (1998).
The International Association for the Properties of Water and Steam
 IAPWS G7-04 The International Association for the Properties of Water and Steam Kyoto, Japan September 2004 Guideline on the Henry s Constant and Vapor-Liquid Distribution Constant for Gases in H 2 O and
IAPWS G7-04 The International Association for the Properties of Water and Steam Kyoto, Japan September 2004 Guideline on the Henry s Constant and Vapor-Liquid Distribution Constant for Gases in H 2 O and
The International Association for the Properties of Water and Steam. Kyoto, Japan September 2004
 IAPWS AN2-04(2016) The International Association for the Properties of Water and Steam Kyoto, Japan September 2004 Advisory Note No. 2 Roles of Various IAPWS Documents Concerning the Thermodynamic Properties
IAPWS AN2-04(2016) The International Association for the Properties of Water and Steam Kyoto, Japan September 2004 Advisory Note No. 2 Roles of Various IAPWS Documents Concerning the Thermodynamic Properties
The International Association for the Properties of Water and Steam. Revised Release on Surface Tension of Ordinary Water Substance
 IAPWS R1-76(2014) The International Association for the Properties of Water and Steam Moscow, Russia June 2014 Revised Release on Surface Tension of Ordinary Water Substance 2014 International Association
IAPWS R1-76(2014) The International Association for the Properties of Water and Steam Moscow, Russia June 2014 Revised Release on Surface Tension of Ordinary Water Substance 2014 International Association
The International Association for the Properties of Water and Steam
 IAPWS R14-08(011) The International Association for the Properties of Water and Steam Plzeň, Czech Republic September 011 Revised Release on the Pressure along the Melting and Sublimation Curves of Ordinary
IAPWS R14-08(011) The International Association for the Properties of Water and Steam Plzeň, Czech Republic September 011 Revised Release on the Pressure along the Melting and Sublimation Curves of Ordinary
The International Association for the Properties of Water and Steam
 IAPWS R-8 The International Association for the Properties of Water and Steam Berlin, Germany September 8 Release on the IAPWS Formulation 8 for the Viscosity of Ordinary Water Substance 8 International
IAPWS R-8 The International Association for the Properties of Water and Steam Berlin, Germany September 8 Release on the IAPWS Formulation 8 for the Viscosity of Ordinary Water Substance 8 International
IAPWS Certified Research Need - ICRN
 IAPWS Certified Research Need - ICRN ICRN 21 Thermophysical Properties Associated with Ultra-supercritical Coal-fired Steam Generators The IAPWS Working Groups Physical Chemistry of Aqueous Systems and
IAPWS Certified Research Need - ICRN ICRN 21 Thermophysical Properties Associated with Ultra-supercritical Coal-fired Steam Generators The IAPWS Working Groups Physical Chemistry of Aqueous Systems and
The International Association for the Properties of Water and Steam
 he International Association for the Properties of Water and Steam Kyoto, Japan September 2004 Reised Supplementary Release on Backward Equations for the Functions (p,h), (p,h) and (p,s), (p,s) for Region
he International Association for the Properties of Water and Steam Kyoto, Japan September 2004 Reised Supplementary Release on Backward Equations for the Functions (p,h), (p,h) and (p,s), (p,s) for Region
Report on the Final Results of ASHRAE RP-1767 Transport Properties of Moist Air
 Report on the Final Results of ASHRAE RP-767 Transport Properties of Moist Air S. Herrmann, H.-J. Kretzschmar (Zittau/Görlitz University of Applied Sciences, Zittau, Germany), V.C. Aute (University of
Report on the Final Results of ASHRAE RP-767 Transport Properties of Moist Air S. Herrmann, H.-J. Kretzschmar (Zittau/Görlitz University of Applied Sciences, Zittau, Germany), V.C. Aute (University of
The International Association for the Properties of Water and Steam
 IAPWS SR3-03(2014) he International Association for the Properties of Water and Steam Moscow, Russia June 2014 Reised Supplementary Release on Backward Equations for the Functions (h), (h) and (s), (s)
IAPWS SR3-03(2014) he International Association for the Properties of Water and Steam Moscow, Russia June 2014 Reised Supplementary Release on Backward Equations for the Functions (h), (h) and (s), (s)
The International Association for the Properties of Water and Steam
 he International Association for the Properties of Water and Steam Vejle, Denmark August 200 Supplementary Release on Backward Equations for the Functions (p,h), (p,h) and (p,s), (p,s) for Region of the
he International Association for the Properties of Water and Steam Vejle, Denmark August 200 Supplementary Release on Backward Equations for the Functions (p,h), (p,h) and (p,s), (p,s) for Region of the
2-412 PHYSICAL AND CHEMICAL DATA
 2-412 PHYSICAL AND CHEMICAL DATA TABLE 2-304 Saturated Solid/Vapor Water* Volume, Enthalpy, Entropy, Temp., Pressure, ft 3 /lb Btu/lb Btu/(lb)( F) F lb/in 2 abs. Solid Vapor Solid Vapor Solid Vapor 160
2-412 PHYSICAL AND CHEMICAL DATA TABLE 2-304 Saturated Solid/Vapor Water* Volume, Enthalpy, Entropy, Temp., Pressure, ft 3 /lb Btu/lb Btu/(lb)( F) F lb/in 2 abs. Solid Vapor Solid Vapor Solid Vapor 160
International Temperature Scale of 1990 From Wikipedia, the free encyclopedia
 International Temperature Scale of 1990 From Wikipedia, the free encyclopedia The International Temperature Scale of 1990 (ITS-90) published by the Consultative Committee for Thermometry (CCT) of the International
International Temperature Scale of 1990 From Wikipedia, the free encyclopedia The International Temperature Scale of 1990 (ITS-90) published by the Consultative Committee for Thermometry (CCT) of the International
ITTC Recommended Procedures
 7.5- Page 1 of 45 FRESH WATER AND SEAWATER PROPERTIES... 2 1. INTRODUCTION... 2 2. FRESH WATER PROPERTIES... 2 2.1 Uncertainty estimates for fresh water properties... 6 2.1.1 Example uncertainty calculation
7.5- Page 1 of 45 FRESH WATER AND SEAWATER PROPERTIES... 2 1. INTRODUCTION... 2 2. FRESH WATER PROPERTIES... 2 2.1 Uncertainty estimates for fresh water properties... 6 2.1.1 Example uncertainty calculation
MASS DETERMINATION OF SILICON SPHERES USED FOR THE AVOGADRO PROJECT
 MASS DETERMINATION OF SILICON SPHERES USED FOR THE AVOGADRO PROJECT Michael Borys, Michael Gläser, Michael Mecke Physikalisch-Technische Bundesanstalt (PTB), Germany ABSTRACT Spheres made of a silicon
MASS DETERMINATION OF SILICON SPHERES USED FOR THE AVOGADRO PROJECT Michael Borys, Michael Gläser, Michael Mecke Physikalisch-Technische Bundesanstalt (PTB), Germany ABSTRACT Spheres made of a silicon
IAPWS Certified Research Need ICRN. Thermophysical Properties of Supercooled Water
 ICRN 30 IAPWS Certified Research Need ICRN Thermophysical Properties of Supercooled Water The IAPWS Working Group Thermophysical Properties of Water and Steam and the IAPWS Subcommittee on Seawater have
ICRN 30 IAPWS Certified Research Need ICRN Thermophysical Properties of Supercooled Water The IAPWS Working Group Thermophysical Properties of Water and Steam and the IAPWS Subcommittee on Seawater have
Chapter 10. Thermal Physics
 Chapter 10 Thermal Physics Thermal Physics Thermal physics is the study of Temperature Heat How these affect matter Thermal Physics, cont Descriptions require definitions of temperature, heat and internal
Chapter 10 Thermal Physics Thermal Physics Thermal physics is the study of Temperature Heat How these affect matter Thermal Physics, cont Descriptions require definitions of temperature, heat and internal
Guest Forum. ph Measurement using the Harned Cell. The Screening Committee Lecture for the First Dr. Masao Horiba s Award
 Guest Forum The Screening Committee Lecture for the First Dr. Masao Horiba s Award ph Measurement using the Harned Cell -ph Values Acceptable for the International Society- Susumu Nakamura National Institute
Guest Forum The Screening Committee Lecture for the First Dr. Masao Horiba s Award ph Measurement using the Harned Cell -ph Values Acceptable for the International Society- Susumu Nakamura National Institute
Viscosity of H 2 O in the Critical Region*
 P R E P R I N T ICPWS XV Berlin, September 8 11, 008 Viscosity of H O in the Critical Region* Jan V. Sengers a,b, Richard A. Perkins c, Marcia L. Huber c, and Daniel G. Friend c a Institute for Physical
P R E P R I N T ICPWS XV Berlin, September 8 11, 008 Viscosity of H O in the Critical Region* Jan V. Sengers a,b, Richard A. Perkins c, Marcia L. Huber c, and Daniel G. Friend c a Institute for Physical
Revising the International System (SI) of Units of Measurement. Dr Barry IngIis President, International Committee for Weights and Measures
 Revising the International System (SI) of Units of Measurement Dr Barry IngIis President, International Committee for Weights and Measures Symposium on International Trend of Metrology Metrology for Industrial
Revising the International System (SI) of Units of Measurement Dr Barry IngIis President, International Committee for Weights and Measures Symposium on International Trend of Metrology Metrology for Industrial
VSMOW Triple Point of Water Cells: Borosilicate versus Fused- Quartz
 VSMOW Triple Point of Water Cells: Borosilicate versus Fused- Quartz M. Zhao 1,3 and G. F. Strouse 2 1 Fluke Corporation, Hart Scientific Division, American Fork, Utah 84003, U.S.A. 2 National Institute
VSMOW Triple Point of Water Cells: Borosilicate versus Fused- Quartz M. Zhao 1,3 and G. F. Strouse 2 1 Fluke Corporation, Hart Scientific Division, American Fork, Utah 84003, U.S.A. 2 National Institute
Thermodynamic Properties for Real Moist Air, Dry Air, Steam, Water, and Ice (ASHRAE RP-1485)
 Zittau/Görlitz University of Applied Sciences Department of Technical Thermodynamics www.thermodynamics-zittau.de Thermodynamic Properties for Real Moist Air, Dry Air, Steam, Water, and Ice (ASHRAE RP-1485)
Zittau/Görlitz University of Applied Sciences Department of Technical Thermodynamics www.thermodynamics-zittau.de Thermodynamic Properties for Real Moist Air, Dry Air, Steam, Water, and Ice (ASHRAE RP-1485)
CCEM 2017 Report CODATA Task Group for Fundamental Constants
 CCEM/17-18 CCEM 2017 Report CODATA Task Group for Fundamental Constants Barry Wood BIPM Feb 22, 2017 Outline CODATA TGFC TGFC s Role in SI Redefinition The special LSA Data for the Planck, Avogadro & Boltzmann
CCEM/17-18 CCEM 2017 Report CODATA Task Group for Fundamental Constants Barry Wood BIPM Feb 22, 2017 Outline CODATA TGFC TGFC s Role in SI Redefinition The special LSA Data for the Planck, Avogadro & Boltzmann
The International Temperature Scale of 1990
 The International Temperature Scale of 199 metrologia Springer-Verlag 199 This copy incorporates textual corrections detailed in Metrologia 27, 17 (199) The International Temperature Scale of 199 (ITS-9)
The International Temperature Scale of 199 metrologia Springer-Verlag 199 This copy incorporates textual corrections detailed in Metrologia 27, 17 (199) The International Temperature Scale of 199 (ITS-9)
Mise en pratique for the definition of the ampere and other electric units in the SI
 Mise en pratique for the definition of the ampere and other electric units in the SI Consultative Committee for Electricity and Magnetism 1. Introduction The purpose of this Mise en pratique, prepared
Mise en pratique for the definition of the ampere and other electric units in the SI Consultative Committee for Electricity and Magnetism 1. Introduction The purpose of this Mise en pratique, prepared
Mutually consistent thermodynamic potentials for fluid water, ice and seawater: a new standard for oceanography
 Ocean Sci., 4, 275 291, 2008 Author(s) 2008. This work is distributed under the Creative Commons Attribution 3.0 License. Ocean Science Mutually consistent thermodynamic potentials for fluid water, ice
Ocean Sci., 4, 275 291, 2008 Author(s) 2008. This work is distributed under the Creative Commons Attribution 3.0 License. Ocean Science Mutually consistent thermodynamic potentials for fluid water, ice
DETERMINATION OF THE POTENTIAL ENERGY SURFACES OF REFRIGERANT MIXTURES AND THEIR GAS TRANSPORT COEFFICIENTS
 THERMAL SCIENCE: Year 07, Vo., No. 6B, pp. 85-858 85 DETERMINATION OF THE POTENTIAL ENERGY SURFACES OF REFRIGERANT MIXTURES AND THEIR GAS TRANSPORT COEFFICIENTS Introduction by Bo SONG, Xiaopo WANG *,
THERMAL SCIENCE: Year 07, Vo., No. 6B, pp. 85-858 85 DETERMINATION OF THE POTENTIAL ENERGY SURFACES OF REFRIGERANT MIXTURES AND THEIR GAS TRANSPORT COEFFICIENTS Introduction by Bo SONG, Xiaopo WANG *,
Henry s Law Constants of Methane and Acid Gases at Pressures above the Saturation Line of Water
 Henry s Law Constants of Methane and Acid Gases at Pressures above the Saturation Line of Water Josef Sedlbauer and Vladimir Majer 2* Department of Chemistry, Technical University of Liberec, 46 7 Liberec,
Henry s Law Constants of Methane and Acid Gases at Pressures above the Saturation Line of Water Josef Sedlbauer and Vladimir Majer 2* Department of Chemistry, Technical University of Liberec, 46 7 Liberec,
The General Conference on Weights and Measures (CGPM), at its 24th meeting,
 On the possible future revision of the International System of Units, the SI Resolution 1 The General Conference on Weights and Measures (CGPM), at its 24th meeting, considering the international consensus
On the possible future revision of the International System of Units, the SI Resolution 1 The General Conference on Weights and Measures (CGPM), at its 24th meeting, considering the international consensus
Thermodynamic behaviour of mixtures containing CO 2. A molecular simulation study
 Thermodynamic behaviour of mixtures containing. A molecular simulation study V. Lachet, C. Nieto-Draghi, B. Creton (IFPEN) Å. Ervik, G. Skaugen, Ø. Wilhelmsen, M. Hammer (SINTEF) Introduction quality issues
Thermodynamic behaviour of mixtures containing. A molecular simulation study V. Lachet, C. Nieto-Draghi, B. Creton (IFPEN) Å. Ervik, G. Skaugen, Ø. Wilhelmsen, M. Hammer (SINTEF) Introduction quality issues
DATA THAT YOU MAY USE UNITS Conventional Volume ml or cm 3 = cm 3 or 10-3 dm 3 Liter (L) = dm 3 Pressure atm = 760 torr = Pa CONSTANTS
 DATA THAT YOU MAY USE UNITS Conventional S.I. Volume ml or cm 3 = cm 3 or 0-3 dm 3 Liter (L) = dm 3 Pressure atm = 760 torr =.03 0 5 Pa torr = 33.3 Pa Temperature C 0 C = 73.5 K PV L-atm =.03 0 5 dm 3
DATA THAT YOU MAY USE UNITS Conventional S.I. Volume ml or cm 3 = cm 3 or 0-3 dm 3 Liter (L) = dm 3 Pressure atm = 760 torr =.03 0 5 Pa torr = 33.3 Pa Temperature C 0 C = 73.5 K PV L-atm =.03 0 5 dm 3
NIST CERTIFICATION OF ITS-90 FIXED-POINT CELLS FROM K TO K: METHODS AND UNCERTAINTIES
 NIST CERTIFICATION OF ITS-90 FIXED-POINT CELLS FROM 83.8058 K TO 1234.93 K: METHODS AND UNCERTAINTIES Gregory F. Strouse National Institute of Standards and Technology, Gaithersburg, Maryland, USA ABSTRACT
NIST CERTIFICATION OF ITS-90 FIXED-POINT CELLS FROM 83.8058 K TO 1234.93 K: METHODS AND UNCERTAINTIES Gregory F. Strouse National Institute of Standards and Technology, Gaithersburg, Maryland, USA ABSTRACT
INTERNATIONAL COMPARISON OF WATER TRIPLE POINT CELLS LEADING TO A MORE PRECISE DEFINITION OF THE KELVIN
 INTERNATIONAL COMPARISON OF WATER TRIPLE POINT CELLS LEADING TO A MORE PRECISE DEFINITION OF THE KELVIN Michael Stock and Stéphane Solve Bureau International des Poids et Mesures (BIPM) Pavillon de Breteuil,
INTERNATIONAL COMPARISON OF WATER TRIPLE POINT CELLS LEADING TO A MORE PRECISE DEFINITION OF THE KELVIN Michael Stock and Stéphane Solve Bureau International des Poids et Mesures (BIPM) Pavillon de Breteuil,
Edexcel Chemistry A-level
 Edexcel Chemistry A-level Topic 5 - Formulae, Equations and Amounts of Substance Flashcards What is the symbol for amount of substance? What is the symbol for amount of substance? n What is the unit used
Edexcel Chemistry A-level Topic 5 - Formulae, Equations and Amounts of Substance Flashcards What is the symbol for amount of substance? What is the symbol for amount of substance? n What is the unit used
STEAMEST: A Software Tool for Estimation of Physical Properties of Water and Steam
 226 JOURNAL OF SOFTWARE, VOL. 4, NO. 3, MAY 2009 STEAMEST: A Software Tool for Estimation of Physical Properties of Water and Steam Muhammad Faheem Department of Chemical Engineering, University of Engineering
226 JOURNAL OF SOFTWARE, VOL. 4, NO. 3, MAY 2009 STEAMEST: A Software Tool for Estimation of Physical Properties of Water and Steam Muhammad Faheem Department of Chemical Engineering, University of Engineering
Gases. Properties of Gases Kinetic Molecular Theory of Gases Pressure Boyle s and Charles Law The Ideal Gas Law Gas reactions Partial pressures.
 Gases Properties of Gases Kinetic Molecular Theory of Gases Pressure Boyle s and Charles Law The Ideal Gas Law Gas reactions Partial pressures Gases Properties of Gases All elements will form a gas at
Gases Properties of Gases Kinetic Molecular Theory of Gases Pressure Boyle s and Charles Law The Ideal Gas Law Gas reactions Partial pressures Gases Properties of Gases All elements will form a gas at
2. CURRENT DEFINITION OF THE TEMPERATURE UNIT
 METROLOGY AND MEASUREMENT SYSTEMS VOL. XV, NUMBER 2 (2008) LESZEK LIPIŃSKI, ANNA SZMYRKA-GRZEBYK Institute of Low Temperature and Structure Research Polish Academy of Sciences, Wrocław e-mail: A. Szmyrka@int.pan.wroc.pl
METROLOGY AND MEASUREMENT SYSTEMS VOL. XV, NUMBER 2 (2008) LESZEK LIPIŃSKI, ANNA SZMYRKA-GRZEBYK Institute of Low Temperature and Structure Research Polish Academy of Sciences, Wrocław e-mail: A. Szmyrka@int.pan.wroc.pl
Ingvar Lindgren Symposium: New Trends in Atomic Physics II 2 3 June 2006 The Quantum SI: A possible new International System of Units
 Ingvar Lindgren Symposium: New Trends in Atomic Physics II 2 3 June 2006 The Quantum SI: A possible new International System of Units Peter Mohr National Institute of Standards and Technology Gaithersburg,
Ingvar Lindgren Symposium: New Trends in Atomic Physics II 2 3 June 2006 The Quantum SI: A possible new International System of Units Peter Mohr National Institute of Standards and Technology Gaithersburg,
SI base units. SI : Système International d'unités (International System of Units)
 2 Units SI base units SI : Système International d'unités (International System of Units) Unite name (symbol) Definition established mass kilogram (kg) The mass of the International Prototype of the Kilogram
2 Units SI base units SI : Système International d'unités (International System of Units) Unite name (symbol) Definition established mass kilogram (kg) The mass of the International Prototype of the Kilogram
Calculation methods for the physical properties of air used in the calibration of microphones
 Downloaded from orbit.dtu.dk on: Jun 15, 018 Calculation methods for the physical properties of air used in the calibration of microphones Rasmussen, Knud Publication date: 1997 Document Version Publisher's
Downloaded from orbit.dtu.dk on: Jun 15, 018 Calculation methods for the physical properties of air used in the calibration of microphones Rasmussen, Knud Publication date: 1997 Document Version Publisher's
Introduction. (hqp://www.bipm.org/en/measurement- units/new- si/)
 OUT US WORLDWIDE METROLOGY INTERNATIONAL EQUIVALENCE the future revision of the SI MEASUREMENT UNITS Introduction SERVICES PUBLICATIONS (hqp://www.bipm.org/en/measurement- units/new- si/) Future revision
OUT US WORLDWIDE METROLOGY INTERNATIONAL EQUIVALENCE the future revision of the SI MEASUREMENT UNITS Introduction SERVICES PUBLICATIONS (hqp://www.bipm.org/en/measurement- units/new- si/) Future revision
Explicit-unit formulations of proposed redefinitions of SI base units
 Explicit-unit formulations of proposed redefinitions of SI base units B. P. Leonard The University of Akron, Akron, Ohio, USA 1849 Brookfield Drive, Akron, OH 44313, USA phone: (330) 867-7152 e-mail: bpleona@uakron.edu
Explicit-unit formulations of proposed redefinitions of SI base units B. P. Leonard The University of Akron, Akron, Ohio, USA 1849 Brookfield Drive, Akron, OH 44313, USA phone: (330) 867-7152 e-mail: bpleona@uakron.edu
14 The IDEAL GAS LAW. and KINETIC THEORY Molecular Mass, The Mole, and Avogadro s Number. Atomic Masses
 14 The IDEAL GAS LAW and KINETIC THEORY 14.1 Molecular Mass, The Mole, and Avogadro s Number Atomic Masses The SI Unit of mass: An atomic mass unit is de ned as a unit of mass equal to 1/12 of the mass
14 The IDEAL GAS LAW and KINETIC THEORY 14.1 Molecular Mass, The Mole, and Avogadro s Number Atomic Masses The SI Unit of mass: An atomic mass unit is de ned as a unit of mass equal to 1/12 of the mass
Comparison of the air kerma standards for 137 Cs and 60 Co gamma-ray beams between the IAEA and the NIST. Ronaldo Minniti 1 and Ladislav Czap 2
 Comparison of the air kerma standards for 137 Cs and 60 Co gamma-ray beams between the IAEA and the NIST Ronaldo Minniti 1 and Ladislav Czap 2 1 National Institute of Standards and Technology (NIST), Gaithersburg,
Comparison of the air kerma standards for 137 Cs and 60 Co gamma-ray beams between the IAEA and the NIST Ronaldo Minniti 1 and Ladislav Czap 2 1 National Institute of Standards and Technology (NIST), Gaithersburg,
Rapid Measurements of Thermodynamic Properties for Alternative Refrigerants with Vibrating-Tube
 Rapid Measurements of Thermodynamic Properties for Alternative Refrigerants with Vibrating-Tube Densimeter Fifteenth Symposium on Thermophysical Properties Y. Kano : speaker M. Hasumoto Y. Kayukawa K.
Rapid Measurements of Thermodynamic Properties for Alternative Refrigerants with Vibrating-Tube Densimeter Fifteenth Symposium on Thermophysical Properties Y. Kano : speaker M. Hasumoto Y. Kayukawa K.
MCGILL UNIVERSITY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEM 120 MONDAY MARCH 16, :30PM 8:30PM VERSION NUMBER: 1
 MCGILL UNIVERSITY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEM 120 MONDAY MARCH 16, 2009 6:30PM 8:30PM VERSION NUMBER: 1 Instructions: BEFORE YOU BEGIN: Enter your student number and name on the computer
MCGILL UNIVERSITY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEM 120 MONDAY MARCH 16, 2009 6:30PM 8:30PM VERSION NUMBER: 1 Instructions: BEFORE YOU BEGIN: Enter your student number and name on the computer
Part I. Temperature Measurements in the Range from 0.1 K to 300 K
 Part I Temperature Measurements in the Range from 0.1 K to 300 K Introduction Part I describes modem methods for measuring temperatures lower than O C based on the use of substances that are gaseous at
Part I Temperature Measurements in the Range from 0.1 K to 300 K Introduction Part I describes modem methods for measuring temperatures lower than O C based on the use of substances that are gaseous at
PHASE CHEMISTRY AND COLLIGATIVE PROPERTIES
 PHASE CHEMISTRY AND COLLIGATIVE PROPERTIES Phase Diagrams Solutions Solution Concentrations Colligative Properties Brown et al., Chapter 10, 385 394, Chapter 11, 423-437 CHEM120 Lecture Series Two : 2013/01
PHASE CHEMISTRY AND COLLIGATIVE PROPERTIES Phase Diagrams Solutions Solution Concentrations Colligative Properties Brown et al., Chapter 10, 385 394, Chapter 11, 423-437 CHEM120 Lecture Series Two : 2013/01
Since the coefficients are only determined up to a multiplicative constant, set c 1 1 and solve for the coefficients: c 1 1 c c 3 1
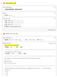 In[1]:= dipole moment of S4 Input interpretation: sulfur tetrafluoride dipole moment Result:.632 D (debyes) Unit conversions: 2.18 1-18 pc m (picocoulomb meters) 2.18 1-21 nc m (nanocoulomb meters) 2.18
In[1]:= dipole moment of S4 Input interpretation: sulfur tetrafluoride dipole moment Result:.632 D (debyes) Unit conversions: 2.18 1-18 pc m (picocoulomb meters) 2.18 1-21 nc m (nanocoulomb meters) 2.18
Essentials of expressing measurement uncertainty
 Essentials of expressing measurement uncertainty This is a brief summary of the method of evaluating and expressing uncertainty in measurement adopted widely by U.S. industry, companies in other countries,
Essentials of expressing measurement uncertainty This is a brief summary of the method of evaluating and expressing uncertainty in measurement adopted widely by U.S. industry, companies in other countries,
MISE EN PRATIQUE OF THE REALIZATION OF THE KELVIN
 MISE EN PRATIQUE OF THE REALIZATION OF THE KELVIN Adopted by the Consultative Committee for Thermometry (CCT) in XXX 1. Scope The text of and supplementary information on the International Temperature
MISE EN PRATIQUE OF THE REALIZATION OF THE KELVIN Adopted by the Consultative Committee for Thermometry (CCT) in XXX 1. Scope The text of and supplementary information on the International Temperature
Standard physical units
 Standard physical units SI base units (SI = Système International d Unités) Basic quantity SI base unit Name Symbol Length metre m Mass kilogram kg Time second s Electric current ampere A Temperature (thermodynamic
Standard physical units SI base units (SI = Système International d Unités) Basic quantity SI base unit Name Symbol Length metre m Mass kilogram kg Time second s Electric current ampere A Temperature (thermodynamic
Density dependence of dielectric permittivity of water and estimation of the electric field for the breakdown inception
 Journal of Physics: Conference Series PAPER OPEN ACCESS Density dependence of dielectric permittivity of water and estimation of the electric field for the breakdown inception To cite this article: D I
Journal of Physics: Conference Series PAPER OPEN ACCESS Density dependence of dielectric permittivity of water and estimation of the electric field for the breakdown inception To cite this article: D I
WATT BALANCES AND THE FUTURE OF THE KILOGRAM
 WATT BALANCES AND THE FUTURE OF THE KILOGRAM Michael Stock Bureau International des Poids et Mesures (BIPM) Pavillon de Breteuil, 92312 Sèvres CEDEX, France Tel.: +33 1 4507 7070 Fax: +33 1 4534 2021 email:
WATT BALANCES AND THE FUTURE OF THE KILOGRAM Michael Stock Bureau International des Poids et Mesures (BIPM) Pavillon de Breteuil, 92312 Sèvres CEDEX, France Tel.: +33 1 4507 7070 Fax: +33 1 4534 2021 email:
Ch. 9 Liquids and Solids
 Intermolecular Forces I. A note about gases, liquids and gases. A. Gases: very disordered, particles move fast and are far apart. B. Liquid: disordered, particles are close together but can still move.
Intermolecular Forces I. A note about gases, liquids and gases. A. Gases: very disordered, particles move fast and are far apart. B. Liquid: disordered, particles are close together but can still move.
How to define the base units of the revised SI from seven constants with fixed numerical values
 Rapport BIPM-018/0 Bureau International des Poids et Mesures How to define the base units of the revised SI from seven constants with fixed numerical values Richard Davis *See Reference 7 February 018
Rapport BIPM-018/0 Bureau International des Poids et Mesures How to define the base units of the revised SI from seven constants with fixed numerical values Richard Davis *See Reference 7 February 018
L = 6.02 x mol Determine the number of particles and the amount of substance (in moles)
 1.1 The Mole 1.1.1 - Apply the mole concept to substances A mole is the name given to a certain quantity. It represents 6.02 x 10 23 particles. This number is also known as Avogadro's constant, symbolised
1.1 The Mole 1.1.1 - Apply the mole concept to substances A mole is the name given to a certain quantity. It represents 6.02 x 10 23 particles. This number is also known as Avogadro's constant, symbolised
Alternative set of defining constants for redefinition of four SI units
 Alternative set of defining constants for redefinition of four SI units V V Khruschov 1,2 1 Centre for Gravitation and Fundamental Metrology, VNIIMS, 119361 Moscow, Russia 2 National Research Centre Kurchatov
Alternative set of defining constants for redefinition of four SI units V V Khruschov 1,2 1 Centre for Gravitation and Fundamental Metrology, VNIIMS, 119361 Moscow, Russia 2 National Research Centre Kurchatov
Quantities, Units, Symbols and Nomenclature used in NCEA Chemistry Level 3 and Scholarship Examination Papers
 1 Quantities, Units, Symbols and Nomenclature used in NCEA Chemistry Level 3 and Scholarship Examination Papers NCEA Chemistry examinations will use the following information, which has been based on International
1 Quantities, Units, Symbols and Nomenclature used in NCEA Chemistry Level 3 and Scholarship Examination Papers NCEA Chemistry examinations will use the following information, which has been based on International
water Plays dominant role in radiation All three phases emit and absorb in longwave radiation
 4.,4. water Plays dominant role in radiation All three phases emit and absorb in longwave radiation Some shortwave (solar) radiation is absorbed by all phases of water Principal role in the shortwave radiation
4.,4. water Plays dominant role in radiation All three phases emit and absorb in longwave radiation Some shortwave (solar) radiation is absorbed by all phases of water Principal role in the shortwave radiation
The Equilibrium Law. Calculating Equilibrium Constants. then (at constant temperature) [C] c. [D] d = a constant, ( K c )
![The Equilibrium Law. Calculating Equilibrium Constants. then (at constant temperature) [C] c. [D] d = a constant, ( K c ) The Equilibrium Law. Calculating Equilibrium Constants. then (at constant temperature) [C] c. [D] d = a constant, ( K c )](/thumbs/79/79140820.jpg) Chemical Equilibrium 1 The Equilibrium Law States If the concentrations of all the substances present at equilibrium are raised to the power of the number of moles they appear in the equation, the product
Chemical Equilibrium 1 The Equilibrium Law States If the concentrations of all the substances present at equilibrium are raised to the power of the number of moles they appear in the equation, the product
Fall Possibly Useful Information: 1 atm = lb/in 2 = kpa. 1 atm = 101,325 N/m 2 = 760 mmhg. 1 atm = 101,325 Pa = 1.
 Chemistry 122 (Tyvoll) Fall 2005 PRACTICE EXAMINATION I Possibly Useful Information: 1 atm = 14.70 lb/in 2 = 101.325 kpa 1 atm = 101,325 N/m 2 = 760 mmg 1 atm = 101,325 Pa = 1.01325 bar 1 atm = 1013.25
Chemistry 122 (Tyvoll) Fall 2005 PRACTICE EXAMINATION I Possibly Useful Information: 1 atm = 14.70 lb/in 2 = 101.325 kpa 1 atm = 101,325 N/m 2 = 760 mmg 1 atm = 101,325 Pa = 1.01325 bar 1 atm = 1013.25
Chapter 11. Liquids and Intermolecular Forces
 Chapter 11 Liquids and Intermolecular Forces States of Matter The three states of matter are 1) Solid Definite shape Definite volume 2) Liquid Indefinite shape Definite volume 3) Gas Indefinite shape Indefinite
Chapter 11 Liquids and Intermolecular Forces States of Matter The three states of matter are 1) Solid Definite shape Definite volume 2) Liquid Indefinite shape Definite volume 3) Gas Indefinite shape Indefinite
Summary of Gas Laws V T. Boyle s Law (T and n constant) Charles Law (p and n constant) Combined Gas Law (n constant) 1 =
 Summary of Gas Laws Boyle s Law (T and n constant) p 1 V 1 = p 2 V 2 Charles Law (p and n constant) V 1 = T 1 V T 2 2 Combined Gas Law (n constant) pv 1 T 1 1 = pv 2 T 2 2 1 Ideal Gas Equation pv = nrt
Summary of Gas Laws Boyle s Law (T and n constant) p 1 V 1 = p 2 V 2 Charles Law (p and n constant) V 1 = T 1 V T 2 2 Combined Gas Law (n constant) pv 1 T 1 1 = pv 2 T 2 2 1 Ideal Gas Equation pv = nrt
Chem Midterm 3 April 23, 2009
 Chem. 101 - Midterm 3 April 3, 009 Name All work must be shown on the exam for partial credit. Points will be taken off for incorrect or no units and for the incorrect number of significant figures. Only
Chem. 101 - Midterm 3 April 3, 009 Name All work must be shown on the exam for partial credit. Points will be taken off for incorrect or no units and for the incorrect number of significant figures. Only
10/12/10. Chapter 16. A Macroscopic Description of Matter. Chapter 16. A Macroscopic Description of Matter. State Variables.
 Chapter 16. A Macroscopic Description of Matter Macroscopic systems are characterized as being either solid, liquid, or gas. These are called the phases of matter, and in this chapter we ll be interested
Chapter 16. A Macroscopic Description of Matter Macroscopic systems are characterized as being either solid, liquid, or gas. These are called the phases of matter, and in this chapter we ll be interested
Final report Comparison Identifier: APMP.SIM.M.P-K1c Other designation: NIST-NPLI/Pressure/2
 Final report Comparison Identifier: APMP.SIM.M.P-K1c Other designation: NIST-NPLI/Pressure/2 Bilateral comparison between NIST (USA) and NPLI (India) in the pneumatic pressure region 0.4 MPa to 4.0 MPa
Final report Comparison Identifier: APMP.SIM.M.P-K1c Other designation: NIST-NPLI/Pressure/2 Bilateral comparison between NIST (USA) and NPLI (India) in the pneumatic pressure region 0.4 MPa to 4.0 MPa
Conceptual Chemistry West Linn High School
 Conceptual Chemistry West Linn High School Unit I: Branches of Science, Metric, Sci. Method, Density Unit II: Matter, Change, Chemical Formulas and Equations Ch. 1, 6 Unit III: Periodic Table and Atomic
Conceptual Chemistry West Linn High School Unit I: Branches of Science, Metric, Sci. Method, Density Unit II: Matter, Change, Chemical Formulas and Equations Ch. 1, 6 Unit III: Periodic Table and Atomic
Solutions and Intermolecular Forces
 Solutions and Intermolecular Forces REVIEW Chemical Bonds Three basic types of bonds: Ionic Electrostatic attraction between ions Covalent Sharing of electrons Metallic Metal atoms bonded to several other
Solutions and Intermolecular Forces REVIEW Chemical Bonds Three basic types of bonds: Ionic Electrostatic attraction between ions Covalent Sharing of electrons Metallic Metal atoms bonded to several other
S I. A concise summary of the International System of Units, SI
 Concise summary of the SI 1 S I A concise summary of the International System of Units, SI Metrology is the science of measurement and its application. Metrology includes all theoretical and practical
Concise summary of the SI 1 S I A concise summary of the International System of Units, SI Metrology is the science of measurement and its application. Metrology includes all theoretical and practical
Properties of Gases. Properties of Gases. Pressure. Three phases of matter. Definite shape and volume. solid. Definite volume, shape of container
 Properties of Gases Properties of Gases Three phases of matter solid Definite shape and volume liquid Definite volume, shape of container gas Shape and volume of container Properties of Gases A gas is
Properties of Gases Properties of Gases Three phases of matter solid Definite shape and volume liquid Definite volume, shape of container gas Shape and volume of container Properties of Gases A gas is
CHE-201. I n t r o d u c t i o n t o Chemical E n g i n e e r i n g. I N S T R U CTOR: D r. N a b e e l S a l i m A b o - Ghander.
 I n t r o d u c t i o n t o Chemical E n g i n e e r i n g CHE-201 I N S T R U CTOR: D r. N a b e e l S a l i m A b o - Ghander C h a p t e r 3 Processes and Process Variables Introduction What is a process?
I n t r o d u c t i o n t o Chemical E n g i n e e r i n g CHE-201 I N S T R U CTOR: D r. N a b e e l S a l i m A b o - Ghander C h a p t e r 3 Processes and Process Variables Introduction What is a process?
INTRODUCTORY CHEMISTRY FOR WATER QUALITY TECHNOLOGY I. Chemistry 11 and Principles of Mathematics 12 is strongly recommended.
 CHEMISTRY 115 INTRODUCTORY CHEMISTRY FOR WATER QUALITY TECHNOLOGY I Prerequisites: Format: Chemistry 11 and Principles of Mathematics 12 is strongly recommended. 4 hours lecture + 3 hours lab per week
CHEMISTRY 115 INTRODUCTORY CHEMISTRY FOR WATER QUALITY TECHNOLOGY I Prerequisites: Format: Chemistry 11 and Principles of Mathematics 12 is strongly recommended. 4 hours lecture + 3 hours lab per week
Environmental Isotopes in Hydrology. Woocay substituting for Walton
 Environmental Isotopes in Hydrology Oct 7, 2010 1 What is an Isotope? An element is defined by the number of protons (Z) in the nucleus The number of neutrons (N) defines the isotope(s) of that element
Environmental Isotopes in Hydrology Oct 7, 2010 1 What is an Isotope? An element is defined by the number of protons (Z) in the nucleus The number of neutrons (N) defines the isotope(s) of that element
A new definition of the SI for the new century
 A new definition of the SI for the new century to celebrate the PTB s first 3 42 1 years = 1111101 years Presentation from Ian Mills, President of the CCU We have known for more than 50 years that the
A new definition of the SI for the new century to celebrate the PTB s first 3 42 1 years = 1111101 years Presentation from Ian Mills, President of the CCU We have known for more than 50 years that the
INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 80000-9 First edition 2009-04-01 Quantities and units Part 9: Physical chemistry and molecular physics Grandeurs et unités Partie 9: Chimie physique et physique moléculaire Reference
INTERNATIONAL STANDARD ISO 80000-9 First edition 2009-04-01 Quantities and units Part 9: Physical chemistry and molecular physics Grandeurs et unités Partie 9: Chimie physique et physique moléculaire Reference
Fig Note the three different types of systems based on the type of boundary between system and surroundings.
 CHAPTER 1 LECTURE NOTES System, Surroundings, and States Fig. 1.4 Note the three different types of systems based on the type of boundary between system and surroundings. Intensive and Extensive Properties
CHAPTER 1 LECTURE NOTES System, Surroundings, and States Fig. 1.4 Note the three different types of systems based on the type of boundary between system and surroundings. Intensive and Extensive Properties
Chemistry 111 Syllabus
 Chemistry 111 Syllabus Chapter 1: Chemistry: The Science of Change The Study of Chemistry Chemistry You May Already Know The Scientific Method Classification of Matter Pure Substances States of Matter
Chemistry 111 Syllabus Chapter 1: Chemistry: The Science of Change The Study of Chemistry Chemistry You May Already Know The Scientific Method Classification of Matter Pure Substances States of Matter
RECOMMENDATION 1 (CI-2002): Revision of the practical realization of the definition of the metre
 194 91st Meeting of the CIPM RECOMMENDATION 1 (CI-2002): Revision of the practical realization of the definition of the metre The International Committee for Weights and Measures, recalling that in 1983
194 91st Meeting of the CIPM RECOMMENDATION 1 (CI-2002): Revision of the practical realization of the definition of the metre The International Committee for Weights and Measures, recalling that in 1983
Implementation of MRA NMIs, BIPM,RMO-TCs Improvement of Global Measurement Standards NMIs, BIPM
 CCM Progress Report for 2003-2007 Mass and Related Quantities Implementation of MRA NMIs, BIPM,RMO-TCs Improvement of Global Measurement Standards NMIs, BIPM. Structure & functions 2. Progress in CIPM-KC
CCM Progress Report for 2003-2007 Mass and Related Quantities Implementation of MRA NMIs, BIPM,RMO-TCs Improvement of Global Measurement Standards NMIs, BIPM. Structure & functions 2. Progress in CIPM-KC
Lecture Presentation. Chapter 11. Liquids and Intermolecular Forces. John D. Bookstaver St. Charles Community College Cottleville, MO
 Lecture Presentation Chapter 11 Liquids and Intermolecular Forces John D. Bookstaver St. Charles Community College Cottleville, MO Properties of Gases, Liquids, and Solids State Volume Shape of State Density
Lecture Presentation Chapter 11 Liquids and Intermolecular Forces John D. Bookstaver St. Charles Community College Cottleville, MO Properties of Gases, Liquids, and Solids State Volume Shape of State Density
Introduction. Chemistry the science of matter and the changes it can undergo.
 Introduction Chemistry the science of matter and the changes it can undergo. Physical Chemistry concerned with the physical principles that underlie chemistry. Seeks to account for the properties of matter
Introduction Chemistry the science of matter and the changes it can undergo. Physical Chemistry concerned with the physical principles that underlie chemistry. Seeks to account for the properties of matter
European Association of National Metrology Institutes
 European Association of National Metrology Institutes EURAMET GUIDELINES ON TEMPERATURE: Extrapolation of SPRT calibrations below the triple point of argon, 83.8058 K, and traceability in baths of liquid
European Association of National Metrology Institutes EURAMET GUIDELINES ON TEMPERATURE: Extrapolation of SPRT calibrations below the triple point of argon, 83.8058 K, and traceability in baths of liquid
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 A.P. Chemistry Practice Test: Ch. 10 - Liquids and Solids Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Crystalline solids. A) exist only
A.P. Chemistry Practice Test: Ch. 10 - Liquids and Solids Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Crystalline solids. A) exist only
SIM SIM.M.D-K3. provide. SIM.M.M-K5 for. A set of. Institute LACOMET LATU INTI. Country Costa Rica Uruguay Argentina Chile México Canada Brazil
 FINAL REPORT SIM COMPARISON IN VOLUME OF WEIGHTS SIM.M.D-K3 Kornblit, F 1 ; Leiblich, J. 1 ; Becerra, L.O. ; Peña, L.M. ; Luján, L. ; Díaz,J.C. ; Centeno, L.M. ; Ramos, O 3 ; Rodríguez, S. 3 ; Santo, C.
FINAL REPORT SIM COMPARISON IN VOLUME OF WEIGHTS SIM.M.D-K3 Kornblit, F 1 ; Leiblich, J. 1 ; Becerra, L.O. ; Peña, L.M. ; Luján, L. ; Díaz,J.C. ; Centeno, L.M. ; Ramos, O 3 ; Rodríguez, S. 3 ; Santo, C.
PX-III Chem 1411 Chaps 11 & 12 Ebbing
 PX-III Chem 1411 Chaps 11 & 12 Ebbing 1. What is the name for the following phase change? I 2 (s) I 2 (g) A) melting B) condensation C) sublimation D) freezing E) vaporization 2. Which of the following
PX-III Chem 1411 Chaps 11 & 12 Ebbing 1. What is the name for the following phase change? I 2 (s) I 2 (g) A) melting B) condensation C) sublimation D) freezing E) vaporization 2. Which of the following
exothermic reaction and that ΔH c will therefore be a negative value. Heat change, q = mcδt q = m(h 2
 Worked solutions hapter 5 Exercises 1 B If the temperature drops, the process must be endothermic. Δ for endothermic reactions is always positive. 2 B All exothermic reactions give out heat. While there
Worked solutions hapter 5 Exercises 1 B If the temperature drops, the process must be endothermic. Δ for endothermic reactions is always positive. 2 B All exothermic reactions give out heat. While there
PLEASE DO NOT MARK ON THE EXAM. ALL ANSWERS SHOULD BE INDICATED ON THE ANSWER SHEET. c) SeF 4
 Chem 130 EXAM 4 Fall 99 PLEASE DO NOT MARK ON THE EXAM. ALL ANSWERS SHOULD BE INDICATED ON THE ANSWER SHEET QUESTIONS 1-5 MAY HAVE MORE THAN ONE POSSIBLE ANSWER CIRCLE ALL CORRECT RESPONSES TO EACH QUESTION
Chem 130 EXAM 4 Fall 99 PLEASE DO NOT MARK ON THE EXAM. ALL ANSWERS SHOULD BE INDICATED ON THE ANSWER SHEET QUESTIONS 1-5 MAY HAVE MORE THAN ONE POSSIBLE ANSWER CIRCLE ALL CORRECT RESPONSES TO EACH QUESTION
Chapter 1 IB Chemistry Warm Ups Stoichiometry. Mrs. Hilliard
 Chapter 1 IB Chemistry Warm Ups Stoichiometry Mrs. Hilliard Vocabulary 1. Atomic theory 2. Kelvin 3. Mole 4. Relative abundance 5. Molar Mass 6. Empirical formula 7. Molecular formula 8. Stoichiometry
Chapter 1 IB Chemistry Warm Ups Stoichiometry Mrs. Hilliard Vocabulary 1. Atomic theory 2. Kelvin 3. Mole 4. Relative abundance 5. Molar Mass 6. Empirical formula 7. Molecular formula 8. Stoichiometry
LECTURE 4 - Units Used in Measurements
 LECTURE 4 - Units Used in Measurements Note: Slide numbers refer to the PowerPoint presentation which accompanies the lecture. Units, slide 1 here Introduction Geochemical measurements may be expressed
LECTURE 4 - Units Used in Measurements Note: Slide numbers refer to the PowerPoint presentation which accompanies the lecture. Units, slide 1 here Introduction Geochemical measurements may be expressed
MEASUREMENTS OF ISOBARIC HEAT CAPACITY OF LIQUID PENTAFLUOROETHANE (R125)
 MEASUREMENTS OF ISOBARIC HEAT CAPACITY OF LIQUID PENTAFLUOROETHANE (R15) Xiang ZHAO, Shigehiro MATSUEDA, and Haruki SATO Faculty of Science and Technology Keio University 3-14-1, Hiyoshi, Kohoku-ku Yokohama
MEASUREMENTS OF ISOBARIC HEAT CAPACITY OF LIQUID PENTAFLUOROETHANE (R15) Xiang ZHAO, Shigehiro MATSUEDA, and Haruki SATO Faculty of Science and Technology Keio University 3-14-1, Hiyoshi, Kohoku-ku Yokohama
Physics 213. Practice Final Exam Spring The next two questions pertain to the following situation:
 The next two questions pertain to the following situation: Consider the following two systems: A: three interacting harmonic oscillators with total energy 6ε. B: two interacting harmonic oscillators, with
The next two questions pertain to the following situation: Consider the following two systems: A: three interacting harmonic oscillators with total energy 6ε. B: two interacting harmonic oscillators, with
Temperature Thermal Expansion Ideal Gas Law Kinetic Theory Heat Heat Transfer Phase Changes Specific Heat Calorimetry Heat Engines
 Temperature Thermal Expansion Ideal Gas Law Kinetic Theory Heat Heat Transfer Phase Changes Specific Heat Calorimetry Heat Engines Zeroeth Law Two systems individually in thermal equilibrium with a third
Temperature Thermal Expansion Ideal Gas Law Kinetic Theory Heat Heat Transfer Phase Changes Specific Heat Calorimetry Heat Engines Zeroeth Law Two systems individually in thermal equilibrium with a third
On the possible future revision of the SI
 Bureau International des Poids et Mesures On the possible future revision of the SI Martin Milton (Director) and Michael Stock (Director of Electricity and Mass) BIPM 2 nd July 2013 Bureau International
Bureau International des Poids et Mesures On the possible future revision of the SI Martin Milton (Director) and Michael Stock (Director of Electricity and Mass) BIPM 2 nd July 2013 Bureau International
INSTRUCTIONS: CHEM Exam I. September 13, 1994 Lab Section
 CHEM 1314.05 Exam I John I. Gelder September 13, 1994 Name TA's Name Lab Section Please sign your name below to give permission to post, by the last 4 digits of your student I.D. number, your course scores
CHEM 1314.05 Exam I John I. Gelder September 13, 1994 Name TA's Name Lab Section Please sign your name below to give permission to post, by the last 4 digits of your student I.D. number, your course scores
ME 262A - Physical Gas Dynamics 1996 Final Exam: Open Book Portion. h = 6.62 x J s Energy conversion factor: 1 calorie = 4.
 Name: ME 262A - Physical Gas Dynamics 1996 Final Exam: Open Book Portion Useful data and information: k = 1.38 x 10-23 J/K h = 6.62 x 10-34 J s Energy conversion factor: 1 calorie = 4.2 J 1. (40 points)
Name: ME 262A - Physical Gas Dynamics 1996 Final Exam: Open Book Portion Useful data and information: k = 1.38 x 10-23 J/K h = 6.62 x 10-34 J s Energy conversion factor: 1 calorie = 4.2 J 1. (40 points)
Chapter 14. The Ideal Gas Law and Kinetic Theory
 Chapter 14 The Ideal Gas Law and Kinetic Theory 14.1 Molecular Mass, the Mole, and Avogadro s Number To facilitate comparison of the mass of one atom with another, a mass scale know as the atomic mass
Chapter 14 The Ideal Gas Law and Kinetic Theory 14.1 Molecular Mass, the Mole, and Avogadro s Number To facilitate comparison of the mass of one atom with another, a mass scale know as the atomic mass
Questions 1 13 cover material from Exam 1
 Questions 1 13 cover material from Exam 1 1. Which intermolecular forces are present in H Te(l)? A. dispersion only C. dispersion, dipole-dipole, and hydrogen bonding B. dispersion and dipole-dipole D.
Questions 1 13 cover material from Exam 1 1. Which intermolecular forces are present in H Te(l)? A. dispersion only C. dispersion, dipole-dipole, and hydrogen bonding B. dispersion and dipole-dipole D.
Mole: base unit for an amount of substance A mole contains Avogadro s number (N A ) of particles (atoms, molecules, ions, formula units )
 Mole: base unit for an amount of substance A mole contains Avogadro s number (N A ) of particles (atoms, molecules, ions, formula units ) N A 6.0 10 mol -1 1 mol substance contains N A Molar mass (g/mol)
Mole: base unit for an amount of substance A mole contains Avogadro s number (N A ) of particles (atoms, molecules, ions, formula units ) N A 6.0 10 mol -1 1 mol substance contains N A Molar mass (g/mol)
Requirements of a reference measurement procedure and how they relate to a certified reference material for ctni that is fit for purpose
 Requirements of a reference measurement procedure and how they relate to a certified reference material for ctni that is fit for purpose David M. Bunk, Ph.D. National Institute of Standards and Technology
Requirements of a reference measurement procedure and how they relate to a certified reference material for ctni that is fit for purpose David M. Bunk, Ph.D. National Institute of Standards and Technology
CHAPTER 16 A MACROSCOPIC DESCRIPTION OF MATTER
 CHAPTER 16 A MACROSCOPIC DESCRIPTION OF MATTER This brief chapter provides an introduction to thermodynamics. The goal is to use phenomenological descriptions of the microscopic details of matter in order
CHAPTER 16 A MACROSCOPIC DESCRIPTION OF MATTER This brief chapter provides an introduction to thermodynamics. The goal is to use phenomenological descriptions of the microscopic details of matter in order
