!!Unsaturated Hydrocarbons. !!Properties of Alkanes
|
|
|
- Thomasina Davis
- 5 years ago
- Views:
Transcription
1 !Organic Chemistry!Why are there so many organic compounds? (three special properties of carbon)!what is a hydrocarbon?!what are the four classifications of hydrocarbons and how do they differ?!know the names of the first 10 hydrocarbons and how to write the molecular and structural formula.!know the bond angle of a H-C-H bond in an alkane.!valence!what is the valence of an atom, and how is it related to valence electrons?!know the rules of valence for an element and how to determine the valence.!be able to write the structural formula for a molecule using the rules of valence.!isomers!what is the definition of isomer?!know what a straight chain alkane vs. a branched alkane is.!know that the prefix n- is used to signify a "!Free rotation about the C-C bond straight chain. "!BP increases as size (# of C) increases LRSVDS CH110 Molec. Geometry 2 LRSVDS CH110 Molec. Geometry 1!!Unsaturated Hydrocarbons "!What is the definition of saturated and unsaturated? "!Which hydrocarbons contain multiple bonds? Single bonds? "!In which hydrocarbons is the rotation about the C-C bond restricted? "!What does a -ene suffix indicate? An -yne suffix?!!isomers "!What is a structural isomer? "!What is a geometric isomer? "!Which types of hydrocarbons do not have geometric isomers? Which do? Why? "!Give an example of each kind of isomer!!properties of Alkanes "!Flexible
2 !!Reactivity of alkanes "!Relatively inert; takes E to initiate reactions "!Combustion reaction; basis of use as fuels, gives off a lot of energy.!!combustion of hydrocarbons "!Complete combustion (hydrocarbon + xs O 2! CO 2 + H 2 O) "!Incomplete combustion (xs hydrocarbon + O 2! CO + H 2 O; basis of production of lethal CO gas when hydrocarbons burned in a closed space)!!functional Groups (NOT ether, amide, or ester) "!Definition of a functional group "!Know the name of each functional group "!Know the structure of each functional group "!Know how to identify them within a molecule. LRSVDS CH110 Molec. Geometry 3!!Non-Carbonyl Functional Groups "!Alcohol; R-OH where R can t be H, polar, water soluble. "!Amines; R 3 N, weak bases, important physiologically "!Ether; R-O-R where R can t be H, polar, unreactive, used as solvents!!carbonyl Functional Groups "!Aldehyde; RCOH, unreactive, used as solvents "!Ketone; RCOR, unreactive, used as solvents "!Carboxylic Acids; RCOOH, weak acids, sour taste, product of air oxidation of alcohols (acetic acid = vinegar) "!Esters; RCOOR, sweet smelling "!Amides; RCONR, weak bases, essential parts of RNA and DNA LRSVDS CH110 Molec. Geometry 4
3 Chapter 9 part 1: Molecular Geometry Read:!BLB " HW:!BLB 9.16, 17, 22, 23, 25, 27" # Packet 9:1 7" Know: " VSEPR theory" # electron pair (electron domain) geometry" # molecular geometry" molecules with more than one central atom" $molecules with multiple bonds" Which Skill Check Test Bonus Deadline is Approaching?? #! What do Structural Formulas tell us? # What do Lewis structures tell us? # Does Shape influence reactivity? # Predict the shape of the molecule by counting: 1." 2." When is EXAM 2??? LRSVDS CH110 Molec. Geometry 5 LRSVDS CH110 Molec. Geometry 6
4 What Determines the Shape of a Molecule?! Electron pairs each other.! Where should the electron pairs be located in the molecule? Electron Domains! Electron pairs " Electron domains! One lone pair = electron domain! One bond (single, double or triple bond) = electron domain. Valence Shell Electron Pair Repulsion Theory (VSEPR) LRSVDS CH110 Molec. Geometry 7 How many electron domains around A? A.! 3 B.! 4 C.! 5 D.! 6 LRSVDS CH110 Molec. Geometry 8
5 Electron-Domain Geometries Electron-Domain Geometries! Count the number of in the Lewis structure.! The geometry corresponds to that number of electron domains. LRSVDS CH110 Molec. Geometry 9 LRSVDS CH110 Molec. Geometry 10
6 Molecular Geometries! Is the electron-domain geometry always the shape of the molecule? Molecular Geometries Some electron domains have more than one molecular geometry.! What is the molecular geometry defined by? LRSVDS CH110 Molec. Geometry 11 LRSVDS CH110 Molec. Geometry 12
7 Linear Electron Domain Trigonal Planar Electron Domain! In this domain, there is only one molecular geometry: linear.! If there are only two atoms in the molecule, what does the geometry HAVE to be?! Two molecular geometries: $!Trigonal planar, if all the electron domains are. $!Bent, if one of the domains is a. LRSVDS CH110 Molec. Geometry 13 LRSVDS CH110 Molec. Geometry 14
8 Lone Pairs and Bond Angles Multiple Bonds and Bond Angles! Nonbonding pairs are physically larger than bonding pairs.! How does this affect neighboring electron pairs?! Double/triple bonds: $!greater electron density on one side of the central atom $!bond angles involving multiple bonds are, and angles on other side of the molecule are. LRSVDS CH110 Molec. Geometry 15 LRSVDS CH110 Molec. Geometry 16
9 Tetrahedral Electron Domain Trigonal Bipyramidal Electron Domain! Three molecular geometries: $!Tetrahedral, if all are pairs $!Trigonal pyramidal if one is a $!Bent if there are two Lone pairs prefer to be equatorial LRSVDS CH110 Molec. Geometry 17 LRSVDS CH110 Molec. Geometry 18
10 Trigonal Bipyramidal Electron Domain Octahedral Electron Domain Four distinct molecular geometries : $!Trigonal bipyramidal $!Seesaw $!T-shaped $!Linear LRSVDS CH110 Molec. Geometry 19! All positions are equivalent! Two lone pairs go on sides of the molecule! Three molecular geometries: $!Octahedral $!Square pyramidal $!Square planar LRSVDS CH110 Molec. Geometry 20
11 How to determine the shape of a molecule 1." Draw the Lewis structure of the molecule. 2." Determine the electron-pair geometry (EPG: count electron domains: multiple bonds count as one domain). 3." Focus on bonded-electron pairs ONLY to determine the molecular geometry (MG) Examples: COCl 2 PCl 5 BrF 3 Bond Angles Octahedral 90 Tetrahedral Trigonal Planar 120 Linear 180 Determine the approximate bond angles indicated Angle #1 Angle #2 1 LP 2 LP A B C D LRSVDS CH110 Molec. Geometry 21 LRSVDS CH110 Molec. Geometry 22
12 Larger Molecules # Describe geometry around a particular atom Number of electron domains Molecular Shapes: Summary Number of bonded atoms Molecular geometry Example 2 2 linear CO trigonal planar BF bent O tetrahedral CH trigonal pyramidal NH bent H 2 O 5 5 trigonal PCl 5 bipyramidal 5 4 seesaw SF T-shaped BrF linear XeF octahedral SF square pyramidal BrF square planar XeF 4 Geometry around atom LRSVDS CH110 Molec. Geometry 23 LRSVDS CH110 Molec. Geometry 24
13 Practice Problems Practice Problem! Which of the following molecules has four electron domains about the central atom?! The molecular geometry of which molecule is square planar? A.! CO 2 A.! CCl 4 B.! XeF 4 B.! SO 3 C.! PH 3 C.! CHCl 3 D.! XeF 2 D.! SeF 4 LRSVDS CH110 Molec. Geometry 25 LRSVDS CH110 Molec. Geometry 26
14 Practice Problems! The molecular geometry of TeF 3 - is best described as: A.! Trigonal pyramidal B.! Trigonal planar C.! See-saw D.! T-shaped LRSVDS CH110 Molec. Geometry 27
Lewis structures show the number and type of bonds between atoms in a molecule or polyatomic ion.
 VSEPR & Geometry Lewis structures show the number and type of bonds between atoms in a molecule or polyatomic ion. Lewis structures are not intended to show the 3-dimensional structure (i.e. shape or geometry)
VSEPR & Geometry Lewis structures show the number and type of bonds between atoms in a molecule or polyatomic ion. Lewis structures are not intended to show the 3-dimensional structure (i.e. shape or geometry)
Molecular Geometry. Valence Shell Electron Pair. What Determines the Shape of a Molecule? Repulsion Theory (VSEPR) Localized Electron Model
 Molecular Geometry Learn Shapes you will Because the physical and chemical properties of compounds are tied to their structures, the importance of molecular geometry can not be overstated. Localized Electron
Molecular Geometry Learn Shapes you will Because the physical and chemical properties of compounds are tied to their structures, the importance of molecular geometry can not be overstated. Localized Electron
Valence Shell Electron Pair repulsion
 Molecular Geometry Valence Shell Electron Pair repulsion The valence shell electron pair repulsion model (VSEPR model) assumes that electron pairs repel one another. (VSEPR) model gives helps determine
Molecular Geometry Valence Shell Electron Pair repulsion The valence shell electron pair repulsion model (VSEPR model) assumes that electron pairs repel one another. (VSEPR) model gives helps determine
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline
 Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
At the end of this lesson, students should be able to :
 At the end of this lesson, students should be able to : (a) Explain Valence Shell Electron Pair Repulsion theory (VSEPR) (b) Draw the basic molecular shapes: linear, planar, tetrahedral, and octahedral.
At the end of this lesson, students should be able to : (a) Explain Valence Shell Electron Pair Repulsion theory (VSEPR) (b) Draw the basic molecular shapes: linear, planar, tetrahedral, and octahedral.
Structures, Shapes and Polarity. of Molecules. Level 2 recap: - Polar and non polar bonds - Lewis diagrams - Lone pairs - Shapes - Polarity
 Structures, Shapes and Polarity Level 2 recap: - Polar and non polar bonds - Lewis diagrams - Lone pairs - Shapes - Polarity of Molecules Do now: Brainstorm what you know/remember about these L2 concepts
Structures, Shapes and Polarity Level 2 recap: - Polar and non polar bonds - Lewis diagrams - Lone pairs - Shapes - Polarity of Molecules Do now: Brainstorm what you know/remember about these L2 concepts
Lewis Structure. Lewis Structures & VSEPR. Octet & Duet Rules. Steps for drawing Lewis Structures
 Lewis Structure Lewis Structures & VSEPR Lewis Structures shows how the are arranged among the atoms of a molecule There are rules for Lewis Structures that are based on the formation of a Atoms want to
Lewis Structure Lewis Structures & VSEPR Lewis Structures shows how the are arranged among the atoms of a molecule There are rules for Lewis Structures that are based on the formation of a Atoms want to
VSEPR. Ch10. Valence Shell Electron Pair Repulsion theory allows you to predict molecular shape. Lewis Dot theory extended to 3 dimensions.
 Ch10 VSEPR Valence Shell Electron Pair Repulsion theory allows you to predict molecular shape. Lewis Dot theory extended to 3 dimensions. version 1.5 Nick DeMello, PhD. 2007-2016 Valence Shell Electron
Ch10 VSEPR Valence Shell Electron Pair Repulsion theory allows you to predict molecular shape. Lewis Dot theory extended to 3 dimensions. version 1.5 Nick DeMello, PhD. 2007-2016 Valence Shell Electron
Molecular Geometry and Chemical Bonding Theory
 Molecular Geometry and Chemical Bonding Theory The Valence -Shell Electron -Pair Repulsion (VSEPR) Model predicts the shapes of the molecules and ions by assuming that the valence shell electron pairs
Molecular Geometry and Chemical Bonding Theory The Valence -Shell Electron -Pair Repulsion (VSEPR) Model predicts the shapes of the molecules and ions by assuming that the valence shell electron pairs
Fill in the chart below to determine the valence electrons of elements 3-10
 Chemistry 11 Atomic Theory IV Name: Date: Block: 1. Lewis Diagrams 2. VSEPR Lewis Diagrams Lewis diagrams show the bonding between atoms of a molecule. Only the outermost electrons of an atom (called electrons)
Chemistry 11 Atomic Theory IV Name: Date: Block: 1. Lewis Diagrams 2. VSEPR Lewis Diagrams Lewis diagrams show the bonding between atoms of a molecule. Only the outermost electrons of an atom (called electrons)
Chapter 9 Molecular Geometries. and Bonding Theories
 Chapter 9 Molecular Geometries and Bonding Theories Coverage of Chapter 9 9.1 All 9.2 All 9.3 All 9.4 All 9.5 Omit Hybridization Involving d Orbitals 9.6 All 9.7 and 9.8 Omit ALL MOLECULAR SHAPES The shape
Chapter 9 Molecular Geometries and Bonding Theories Coverage of Chapter 9 9.1 All 9.2 All 9.3 All 9.4 All 9.5 Omit Hybridization Involving d Orbitals 9.6 All 9.7 and 9.8 Omit ALL MOLECULAR SHAPES The shape
Chapter 9 Molecular Geometry. Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory
 Chapter 9 Molecular Geometry Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Sulfanilamide Lewis Structures and the Real 3D-Shape of Molecules Lewis Theory of Molecular Shape and Polarity
Chapter 9 Molecular Geometry Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Sulfanilamide Lewis Structures and the Real 3D-Shape of Molecules Lewis Theory of Molecular Shape and Polarity
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals 1 Chemical Bonding II Molecular Geometry (10.1) Dipole Moments (10.2) Valence Bond Theory (10.3) Hybridization of Atomic Orbitals
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals 1 Chemical Bonding II Molecular Geometry (10.1) Dipole Moments (10.2) Valence Bond Theory (10.3) Hybridization of Atomic Orbitals
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. How to get the book of
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. How to get the book of
2011, Robert Ayton. All rights reserved.
 Chemical Bonding Outline 1. Lewis Dot Structures 2. Bonds 3. Formal Charges 4. VSEPR (Molecular Geometry and Hybridzation) 5. Common Resonance Structures and Dimerization Review 1. Lewis Dot Structures
Chemical Bonding Outline 1. Lewis Dot Structures 2. Bonds 3. Formal Charges 4. VSEPR (Molecular Geometry and Hybridzation) 5. Common Resonance Structures and Dimerization Review 1. Lewis Dot Structures
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
CHEMISTRY 110 EXAM 2 Feb 25, 2013 FORM A
 EMISTRY 110 EXAM 2 Feb 25, 2013 FORM A 1. ow many valence electrons and lone pairs are in the structure of the ammonium ion? # valence electrons # lone pairs A. 8 0 B. 10 1. 8 1 D. 10 2 E. 12 3 2. Which
EMISTRY 110 EXAM 2 Feb 25, 2013 FORM A 1. ow many valence electrons and lone pairs are in the structure of the ammonium ion? # valence electrons # lone pairs A. 8 0 B. 10 1. 8 1 D. 10 2 E. 12 3 2. Which
VSEPR. Valence Shell Electron Pair Repulsion Theory
 VSEPR Valence Shell Electron Pair Repulsion Theory Vocabulary: domain = any electron pair or bond (single, double or triple) is considered one domain. bonding pair = shared pair = any electron pair that
VSEPR Valence Shell Electron Pair Repulsion Theory Vocabulary: domain = any electron pair or bond (single, double or triple) is considered one domain. bonding pair = shared pair = any electron pair that
EXPERIMENT #13 Lewis Structures and Molecular Geometry
 OBJECTIVES: EXPERIMENT #13 s and Draw Lewis structures of atoms, ions, and molecules Build models of linear, trigonal planar tetrahedral, trigonal bipyramidal, and octahedral arrangements of electron pairs
OBJECTIVES: EXPERIMENT #13 s and Draw Lewis structures of atoms, ions, and molecules Build models of linear, trigonal planar tetrahedral, trigonal bipyramidal, and octahedral arrangements of electron pairs
Chemistry and the material world Lecture 3
 Chemistry and the material world 123.102 Lecture 3 Electronic bookkeeping we need a way of finding out in which proportions two or more atoms make up a molecule is it CH 3 or CH 4 or CH 5? counting valence
Chemistry and the material world 123.102 Lecture 3 Electronic bookkeeping we need a way of finding out in which proportions two or more atoms make up a molecule is it CH 3 or CH 4 or CH 5? counting valence
Chapter 25 Organic and Biological Chemistry
 Chapter 25 Organic and Biological Chemistry Organic Chemistry The chemistry of carbon compounds. Carbon has the ability to form long chains. Without this property, large biomolecules such as proteins,
Chapter 25 Organic and Biological Chemistry Organic Chemistry The chemistry of carbon compounds. Carbon has the ability to form long chains. Without this property, large biomolecules such as proteins,
8.3 Bonding Theories > Chapter 8 Covalent Bonding. 8.3 Bonding Theories. 8.1 Molecular Compounds 8.2 The Nature of Covalent Bonding
 Chapter 8 Covalent Bonding 8.1 Molecular Compounds 8.2 The Nature of Covalent Bonding 8.3 Bonding Theories 8.4 Polar Bonds and Molecules 1 Molecular Shape What information does a structural formula give
Chapter 8 Covalent Bonding 8.1 Molecular Compounds 8.2 The Nature of Covalent Bonding 8.3 Bonding Theories 8.4 Polar Bonds and Molecules 1 Molecular Shape What information does a structural formula give
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Linear Trigonal 180 o planar 120 o Tetrahedral 109.5 o Trigonal Bipyramidal 120 and 90 o Octahedral 90 o linear Linear
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Linear Trigonal 180 o planar 120 o Tetrahedral 109.5 o Trigonal Bipyramidal 120 and 90 o Octahedral 90 o linear Linear
Chemical Bonding. Types of Bonds. Ionic Bonding. Resonance Structures. Molecular Geometries. VSEPR Basic Shapes 3-D Notation Hybridization (Lab)
 Chemical Bonding Types of Bonds Ionic Bonding Lewis Structures Covalent Bonding Resonance Structures Octet Rule Polar Molecules Molecular Geometries VSEPR Basic Shapes 3-D Notation Hybridization (Lab)
Chemical Bonding Types of Bonds Ionic Bonding Lewis Structures Covalent Bonding Resonance Structures Octet Rule Polar Molecules Molecular Geometries VSEPR Basic Shapes 3-D Notation Hybridization (Lab)
10-1. The Shapes of Molecules, chapter 10
 10-1 The Shapes of Molecules, chapter 10 The Shapes of Molecules; Goals 10.1 Depicting Molecules and Ions with Lewis Structures 10.2 Valence-Shell Electron-Pair Repulsion (VSEPR) Theory 10.3 Molecular
10-1 The Shapes of Molecules, chapter 10 The Shapes of Molecules; Goals 10.1 Depicting Molecules and Ions with Lewis Structures 10.2 Valence-Shell Electron-Pair Repulsion (VSEPR) Theory 10.3 Molecular
General and Inorganic Chemistry I.
 General and Inorganic Chemistry I. Lecture 1 István Szalai Eötvös University István Szalai (Eötvös University) Lecture 1 1 / 29 Outline István Szalai (Eötvös University) Lecture 1 2 / 29 Lewis Formulas
General and Inorganic Chemistry I. Lecture 1 István Szalai Eötvös University István Szalai (Eötvös University) Lecture 1 1 / 29 Outline István Szalai (Eötvös University) Lecture 1 2 / 29 Lewis Formulas
Molecular shapes. Balls and sticks
 Molecular shapes Balls and sticks Learning objectives Apply VSEPR to predict electronic geometry and shapes of simple molecules Determine molecule shape from electronic geometry Distinguish between polar
Molecular shapes Balls and sticks Learning objectives Apply VSEPR to predict electronic geometry and shapes of simple molecules Determine molecule shape from electronic geometry Distinguish between polar
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Chang & Goldsby Modified by Dr. Juliet Hahn Copyright McGraw-Hill Education. All rights reserved. No reproduction
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Chang & Goldsby Modified by Dr. Juliet Hahn Copyright McGraw-Hill Education. All rights reserved. No reproduction
Shapes of Molecules. Lewis structures are useful but don t allow prediction of the shape of a molecule.
 Shapes of Molecules Lewis structures are useful but don t allow prediction of the shape of a molecule. H O H H O H Can use a simple theory based on electron repulsion to predict structure (for non-transition
Shapes of Molecules Lewis structures are useful but don t allow prediction of the shape of a molecule. H O H H O H Can use a simple theory based on electron repulsion to predict structure (for non-transition
Valence Shell Electron Pair Repulsion Model
 Valence Shell Electron Pair Repulsion Model Why? Molecules adopt a shape that minimizes their energy. In most cases simply considering the repulsive energy of electron pairs is sufficient to predict molecular
Valence Shell Electron Pair Repulsion Model Why? Molecules adopt a shape that minimizes their energy. In most cases simply considering the repulsive energy of electron pairs is sufficient to predict molecular
Covalent Compounds: Bonding Theories and Molecular Structure
 CHM 123 Chapter 8 Covalent Compounds: Bonding Theories and Molecular Structure 8.1 Molecular shapes and VSEPR theory VSEPR theory proposes that the geometric arrangement of terminal atoms, or groups of
CHM 123 Chapter 8 Covalent Compounds: Bonding Theories and Molecular Structure 8.1 Molecular shapes and VSEPR theory VSEPR theory proposes that the geometric arrangement of terminal atoms, or groups of
Chapters 2 & 25: Covalent bonds & Organic Chemistry
 hapters 2 & 25: ovalent bonds & Organic hemistry Read: BLB 2.6, 2.9; 25.1-25.4 (only nomenclature in Table 25.1, NOT reactions) W: BLB 2:43, 45, 69, 76, 77 BLB 25:11, 12, 25, 40a, c-f Packet Organic:1
hapters 2 & 25: ovalent bonds & Organic hemistry Read: BLB 2.6, 2.9; 25.1-25.4 (only nomenclature in Table 25.1, NOT reactions) W: BLB 2:43, 45, 69, 76, 77 BLB 25:11, 12, 25, 40a, c-f Packet Organic:1
Chapter 13: Phenomena
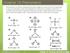 Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Molecular Geometry. Objectives N H H. The objectives of this laboratory are to:
 Objectives The objectives of this laboratory are to: Molecular Geometry Write Lewis structure representations of the bonding and valence electrons in molecules. Use the VSEPR model to predict the molecular
Objectives The objectives of this laboratory are to: Molecular Geometry Write Lewis structure representations of the bonding and valence electrons in molecules. Use the VSEPR model to predict the molecular
Name: Period: Date: What Is VSEPR? Now explore the Compare Two Structures link. Try changing the display to explore different combinations.
 Name: Period: Date: What Is VSEPR? Exploring The Valence Shell Electron Pair Repulsion (VSEPR) model. Go to the Purdue University website to explore VSEPR theory. http://www.chem.purdue.edu/gchelp/vsepr/structur2.html
Name: Period: Date: What Is VSEPR? Exploring The Valence Shell Electron Pair Repulsion (VSEPR) model. Go to the Purdue University website to explore VSEPR theory. http://www.chem.purdue.edu/gchelp/vsepr/structur2.html
Practice Worksheet for Lewis Structures (Mahaffy Ch )
 Practice Worksheet for Lewis Structures (Mahaffy Ch. 10.1 10.5 ) 1. Main concepts Lewis Structures a. Connectivity b. Bonds & Lone pairs c. Electron Geometry & Molecular Shape d. Resonance Structures Formal
Practice Worksheet for Lewis Structures (Mahaffy Ch. 10.1 10.5 ) 1. Main concepts Lewis Structures a. Connectivity b. Bonds & Lone pairs c. Electron Geometry & Molecular Shape d. Resonance Structures Formal
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding.
 Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Chapter 10. Geometry
 Chapter 10 Molec cular Geometry 1 CHAPTER OUTLINE Molecular Geometry Molecular Polarity VSEPR Model Summary of Molecular Shapes Hybridization Molecular Orbital Theory Bond Angles 2 MOLECULAR GEOMETRY Molecular
Chapter 10 Molec cular Geometry 1 CHAPTER OUTLINE Molecular Geometry Molecular Polarity VSEPR Model Summary of Molecular Shapes Hybridization Molecular Orbital Theory Bond Angles 2 MOLECULAR GEOMETRY Molecular
Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chapter 10. The Shapes of Molecules 10-1
 Chapter 10 The Shapes of Molecules 10-1 The Shapes of Molecules 10.1 Depicting Molecules and Ions with Lewis Structures 10.2 Valence-Shell Electron-Pair Repulsion (VSEPR) Theory and Molecular Shape 10.3
Chapter 10 The Shapes of Molecules 10-1 The Shapes of Molecules 10.1 Depicting Molecules and Ions with Lewis Structures 10.2 Valence-Shell Electron-Pair Repulsion (VSEPR) Theory and Molecular Shape 10.3
Lab 6. Use of VSEPR to Predict Molecular Structure and IR Spectroscopy to Identify an Unknown
 Lab 6. Use of VSEPR to Predict Molecular Structure and IR Spectroscopy to Identify an Unknown Prelab Assignment Before coming to lab: In addition to reading introduction of this lab handout, read and understand
Lab 6. Use of VSEPR to Predict Molecular Structure and IR Spectroscopy to Identify an Unknown Prelab Assignment Before coming to lab: In addition to reading introduction of this lab handout, read and understand
CHEMICAL BONDING. Chemical Bonds. Ionic Bonding. Lewis Symbols
 CHEMICAL BONDING Chemical Bonds Lewis Symbols Octet Rule whenever possible, valence electrons in covalent compounds distribute so that each main-group element is surrounded by 8 electrons (except hydrogen
CHEMICAL BONDING Chemical Bonds Lewis Symbols Octet Rule whenever possible, valence electrons in covalent compounds distribute so that each main-group element is surrounded by 8 electrons (except hydrogen
Chapter 9 The Shapes of Molecules Cocaine
 Chapter 9 The Shapes of Molecules 1 Cocaine 10.1 Depicting Molecules & Ions with Lewis Structures 2 Number of Covalent Bonds 3 The number of covalent bonds can be determined from the number of electrons
Chapter 9 The Shapes of Molecules 1 Cocaine 10.1 Depicting Molecules & Ions with Lewis Structures 2 Number of Covalent Bonds 3 The number of covalent bonds can be determined from the number of electrons
Subtopic 4.2 MOLECULAR SHAPE AND POLARITY
 Subtopic 4.2 MOLECULAR SHAPE AND POLARITY 1 LEARNING OUTCOMES (covalent bonding) 1. Draw the Lewis structure of covalent molecules (octet rule such as NH 3, CCl 4, H 2 O, CO 2, N 2 O 4, and exception to
Subtopic 4.2 MOLECULAR SHAPE AND POLARITY 1 LEARNING OUTCOMES (covalent bonding) 1. Draw the Lewis structure of covalent molecules (octet rule such as NH 3, CCl 4, H 2 O, CO 2, N 2 O 4, and exception to
CHEM1101 Worksheet 6: Lone Pairs and Molecular Geometry
 CHEM1101 Worksheet 6: Lone Pairs and Molecular Geometry Model 1: Oxidation numbers Oxidation numbers are a useful accountancy tool to help keep track of electrons in compounds and reactions. This is particularly
CHEM1101 Worksheet 6: Lone Pairs and Molecular Geometry Model 1: Oxidation numbers Oxidation numbers are a useful accountancy tool to help keep track of electrons in compounds and reactions. This is particularly
Molecular Geometry and intermolecular forces. Unit 4 Chapter 9 and 11.2
 1 Molecular Geometry and intermolecular forces Unit 4 Chapter 9 and 11.2 2 Unit 4.1 Chapter 9.1-9.3 3 Review of bonding Ionic compound (metal/nonmetal) creates a lattice Formula doesn t tell the exact
1 Molecular Geometry and intermolecular forces Unit 4 Chapter 9 and 11.2 2 Unit 4.1 Chapter 9.1-9.3 3 Review of bonding Ionic compound (metal/nonmetal) creates a lattice Formula doesn t tell the exact
N = 727 Mean = 68% Diff T-Test P-Value SI 223 (31%) 71% No SI 504 (69%) 66% Test 2 - Letter Grade Distribution by SI Attendance
 CHEM 200/202 Exam 2 N = 727 Mean = 68% Diff T-Test P-Value SI 223 (31%) 71% No SI 504 (69%) 66% 5%
CHEM 200/202 Exam 2 N = 727 Mean = 68% Diff T-Test P-Value SI 223 (31%) 71% No SI 504 (69%) 66% 5%
CHM151LL: VSEPR and Molecular Geometry Tables
 CHM151LL: VSEPR and Molecular Geometry Tables VSEPR Model VALENCE-SHELL ELECTRON-PAIR REPULSION (VSEPR) MODEL Lewis structures show the two-dimensional distribution of atoms and electrons. The molecular
CHM151LL: VSEPR and Molecular Geometry Tables VSEPR Model VALENCE-SHELL ELECTRON-PAIR REPULSION (VSEPR) MODEL Lewis structures show the two-dimensional distribution of atoms and electrons. The molecular
Chapter 10. VSEPR Model: Geometries
 Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Farthest apart
Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Farthest apart
Learning Organic Chemistry
 Objective 1 Represent organic molecules with chemical formulas, expanded formulas, Lewis structures, skeletal structures. Determine shape (VSEPR), bond polarity, and molecule polarity. Identify functional
Objective 1 Represent organic molecules with chemical formulas, expanded formulas, Lewis structures, skeletal structures. Determine shape (VSEPR), bond polarity, and molecule polarity. Identify functional
Chemistry 1A Spring 1998 Exam #4 KEY Chapters 9 & 10
 Chemistry 1A Spring 1998 Exam #4 KEY Chapters 9 & 10 For each of the following, write the word, words, or number in each blank that best completes each sentence. (2 points each) 1. A(n) molecular orbital
Chemistry 1A Spring 1998 Exam #4 KEY Chapters 9 & 10 For each of the following, write the word, words, or number in each blank that best completes each sentence. (2 points each) 1. A(n) molecular orbital
Chapter 10. VSEPR Model: Geometries
 Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Two bonds Two
Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Two bonds Two
Introduction to VSEPR Theory 1
 1 Class 8: Introduction to VSEPR Theory Sec 10.2 VSEPR Theory: The Five Basic Shapes Two Electron Groups: Linear Geometry Three Electron Groups: Trigonal Planar Geometry Four Electron Groups: Tetrahedral
1 Class 8: Introduction to VSEPR Theory Sec 10.2 VSEPR Theory: The Five Basic Shapes Two Electron Groups: Linear Geometry Three Electron Groups: Trigonal Planar Geometry Four Electron Groups: Tetrahedral
From 2-D to 3-D. Chapter 9 Molecular Geometry
 From 2-D to 3-D Chapter 9 Molecular Geometry 1 Moving on to Shapes A chemical formula tells us The identity of atoms The number of atoms The Lewis structure tells us The identity of atoms The number of
From 2-D to 3-D Chapter 9 Molecular Geometry 1 Moving on to Shapes A chemical formula tells us The identity of atoms The number of atoms The Lewis structure tells us The identity of atoms The number of
The Shapes of Molecules. Chemistry II
 The Shapes of Molecules Chemistry II Lewis Structures DEFINITIN: A structure of a molecule showing how the valence electrons are arranged. 1) nly the valence electrons appear in a Lewis structure. 2) The
The Shapes of Molecules Chemistry II Lewis Structures DEFINITIN: A structure of a molecule showing how the valence electrons are arranged. 1) nly the valence electrons appear in a Lewis structure. 2) The
Chapter 9. Molecular Geometry and Bonding Theories
 9.1 Molecular Shapes Read Sec. 9.1 and 9.2, then complete the Sample and Practice Exercises in these sections. Sample Exercise 9.1 (p. 347) Use the VSEPR model to predict the molecular geometries of a)
9.1 Molecular Shapes Read Sec. 9.1 and 9.2, then complete the Sample and Practice Exercises in these sections. Sample Exercise 9.1 (p. 347) Use the VSEPR model to predict the molecular geometries of a)
Experiment 15. The Valence Shell Electron Pair Repulsion (VSEPR) Theory of Directed Valency: An exercise
 Experiment 15 The Valence Shell Electron Pair Repulsion (VSEPR) Theory of Directed Valency: An exercise Attempts to understand and predict the shapes of molecules using either the valencebond theory or
Experiment 15 The Valence Shell Electron Pair Repulsion (VSEPR) Theory of Directed Valency: An exercise Attempts to understand and predict the shapes of molecules using either the valencebond theory or
Homework 08 VSEPR. The active ingredient in some oral anesthetics used in sore throat sprays. What is the molar mass of phenol?
 HW08 VSEPR This is a preview of the published version of the quiz Started: Oct 21 at 11:14am Quiz Instruc ons Homework 08 VSEPR Question 1 Consider the structural formula of phenol. The active ingredient
HW08 VSEPR This is a preview of the published version of the quiz Started: Oct 21 at 11:14am Quiz Instruc ons Homework 08 VSEPR Question 1 Consider the structural formula of phenol. The active ingredient
Valence-Shell Electron-Pair Repulsion Theory (VSEPR)
 ; Set 09- Molecular Geometry All course materials, including lectures, class notes, quizzes, exams, handouts, presentations, and other materials provided to students for this course are protected intellectual
; Set 09- Molecular Geometry All course materials, including lectures, class notes, quizzes, exams, handouts, presentations, and other materials provided to students for this course are protected intellectual
Ex. 1) F F bond in F = 0 < % covalent, no transfer of electrons
 #60 Notes Unit 8: Bonding Ch. Bonding I. Bond Character Bonds are usually combinations of ionic and covalent character. The electronegativity difference is used to determine a bond s character. Electronegativity
#60 Notes Unit 8: Bonding Ch. Bonding I. Bond Character Bonds are usually combinations of ionic and covalent character. The electronegativity difference is used to determine a bond s character. Electronegativity
Lewis Structure and Electron Dot Models
 Lewis Structure and Electron Dot Models The Lewis Structure is a method of displaying the electrons present in any given atom or compound. Steps: 1. Make a skeleton structure 2. Count all e- available
Lewis Structure and Electron Dot Models The Lewis Structure is a method of displaying the electrons present in any given atom or compound. Steps: 1. Make a skeleton structure 2. Count all e- available
Molecular Geometry and Bonding Theories. Molecular Shapes. Molecular Shapes. Chapter 9 Part 2 November 16 th, 2004
 Molecular Geometry and Bonding Theories Chapter 9 Part 2 November 16 th, 2004 8 Molecular Shapes When considering the geometry about the central atom, we consider all electrons (lone pairs and bonding
Molecular Geometry and Bonding Theories Chapter 9 Part 2 November 16 th, 2004 8 Molecular Shapes When considering the geometry about the central atom, we consider all electrons (lone pairs and bonding
Check Your Solution A comparison with the figures in Figure 4.31 on page 234 of the student textbook confirms the results.
 Predicting the Shape of a Molecule (Student textbook page 236) 11. What molecular shape is represented by each of the following VSEPR notations? a. AX 3 b. AX 5 E You need to assign a molecular shape that
Predicting the Shape of a Molecule (Student textbook page 236) 11. What molecular shape is represented by each of the following VSEPR notations? a. AX 3 b. AX 5 E You need to assign a molecular shape that
Lecture 17 - Covalent Bonding. Lecture 17 - VSEPR and Molecular Shape. Lecture 17 - Introduction. Lecture 17 - VSEPR and Molecular Shape
 Chem 103, Section F0F Unit VI - Compounds Part II: Covalent Compounds Lecture 17 Using the Valence-Shell Electron-Pair Repulsion (VSEPR) Theory to predict molecular shapes Molecular shape and polarity
Chem 103, Section F0F Unit VI - Compounds Part II: Covalent Compounds Lecture 17 Using the Valence-Shell Electron-Pair Repulsion (VSEPR) Theory to predict molecular shapes Molecular shape and polarity
Bonding and Molecular Structure - PART 1 - VSEPR
 Bonding and Molecular Structure - PART 1 - VSEPR Objectives: 1. Understand and become proficient at using VSEPR to predict the geometries of simple molecules and ions. 2. Become proficient at predicting
Bonding and Molecular Structure - PART 1 - VSEPR Objectives: 1. Understand and become proficient at using VSEPR to predict the geometries of simple molecules and ions. 2. Become proficient at predicting
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: and ybridization of Atomic rbitals Chapter 10 Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the
Chemical Bonding II: and ybridization of Atomic rbitals Chapter 10 Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the
LESSON 10. Glossary: Molecular Geometry. a quantitative measure of the degree of charge separation in a molecule. Dipole moment
 LESSON 10 Glossary: Molecular Geometry Dipole moment Electronegativity Molecular geometry Pi bond Polar covalent bond Sigma bond Valence-shell electronpair repulsion (VSEPR) model a quantitative measure
LESSON 10 Glossary: Molecular Geometry Dipole moment Electronegativity Molecular geometry Pi bond Polar covalent bond Sigma bond Valence-shell electronpair repulsion (VSEPR) model a quantitative measure
QuickTime and a TIFF (Uncompressed) decompressor are needed to see this picture. Organic Chemistry. QuickTime and a are needed to see this picture.
 QuickTime and a TIFF (Uncompressed) decompressor are needed to see this picture. Organic Chemistry QuickTime and a TIFF (Uncompressed) decompressor are needed to see this picture. Organic Chemistry Has
QuickTime and a TIFF (Uncompressed) decompressor are needed to see this picture. Organic Chemistry QuickTime and a TIFF (Uncompressed) decompressor are needed to see this picture. Organic Chemistry Has
MOLECULAR MODELS OBJECTIVES
 MOLECULAR MODELS OBJECTIVES 1. To learn to draw Lewis structures for common compounds 2. To identify electron pairs as bonding pairs or lone pairs 3. To use electron pair repulsion theory to predict electronic
MOLECULAR MODELS OBJECTIVES 1. To learn to draw Lewis structures for common compounds 2. To identify electron pairs as bonding pairs or lone pairs 3. To use electron pair repulsion theory to predict electronic
Chemistry 212 MOLECULAR STRUCTURES AND GEOMETRIES
 Chemistry 212 MOLECULAR STRUCTURES AND GEOMETRIES LEARNING OBJECTIVES To build models of selected molecules using VSEPR theory. To illustrate patterns of molecular shapes. BACKGROUND The shapes exhibited
Chemistry 212 MOLECULAR STRUCTURES AND GEOMETRIES LEARNING OBJECTIVES To build models of selected molecules using VSEPR theory. To illustrate patterns of molecular shapes. BACKGROUND The shapes exhibited
Illinois Central College CHEMISTRY 130 Laboratory Section: To predict the shapes of molecules based on their Lewis Structures.
 Exercise 12 Page 1 Illinois Central College CEMISTRY 130 Laboratory Section: Molecular Structure Name: Objectives To predict the shapes of molecules based on their Lewis Structures. Background The Valence
Exercise 12 Page 1 Illinois Central College CEMISTRY 130 Laboratory Section: Molecular Structure Name: Objectives To predict the shapes of molecules based on their Lewis Structures. Background The Valence
CHAPTER TEN MOLECULAR GEOMETRY MOLECULAR GEOMETRY V S E P R CHEMICAL BONDING II: MOLECULAR GEOMETRY AND HYBRIDIZATION OF ATOMIC ORBITALS
 CHAPTER TEN CHEMICAL BONDING II: AND HYBRIDIZATION O ATOMIC ORBITALS V S E P R VSEPR Theory In VSEPR theory, multiple bonds behave like a single electron pair Valence shell electron pair repulsion (VSEPR)
CHAPTER TEN CHEMICAL BONDING II: AND HYBRIDIZATION O ATOMIC ORBITALS V S E P R VSEPR Theory In VSEPR theory, multiple bonds behave like a single electron pair Valence shell electron pair repulsion (VSEPR)
CHEMISTRY 110 EXAM 2 Oct.
 EMISTRY 110 EXAM 2 ct. 15, 2012 FRM A 1. Which of the following is an incorrect formula for a neutral compound made from the given ions?! "#$%&'! #'%&'! (&)*+,#! -.!! aluminum oxide Al 2 3 /.!! magnesium
EMISTRY 110 EXAM 2 ct. 15, 2012 FRM A 1. Which of the following is an incorrect formula for a neutral compound made from the given ions?! "#$%&'! #'%&'! (&)*+,#! -.!! aluminum oxide Al 2 3 /.!! magnesium
Electron Geometry Hybrid Orbitals
 Molecular Shape and Hybridized Orbitals CH2000: Introduction to General Chemistry, Plymouth State University Introduction: In chemistry, the three dimensional shape of a molecule is as important as the
Molecular Shape and Hybridized Orbitals CH2000: Introduction to General Chemistry, Plymouth State University Introduction: In chemistry, the three dimensional shape of a molecule is as important as the
Molecular Structure. Valence Bond Theory Overlap of atomic orbitals is a covalent bond that joins atoms together to form a molecule
 Molecular Structure Topics 3-D structure shape (location of atoms in space) Molecular Geometry Valence Bond Theory Hybrid Orbitals Multiple Bonds VSEPR (Valence Shell Electron Pair Repulsion) Valence Bond
Molecular Structure Topics 3-D structure shape (location of atoms in space) Molecular Geometry Valence Bond Theory Hybrid Orbitals Multiple Bonds VSEPR (Valence Shell Electron Pair Repulsion) Valence Bond
Activity Formal Charge and VSEPR Theory for Expanded Octets
 Activity 201 7 Formal Charge and VSEPR Theory for Expanded Octets Directions: This Guided Learning Activity (GLA) goes over formal charge and the structures of molecules with expanded octets. Part A introduces
Activity 201 7 Formal Charge and VSEPR Theory for Expanded Octets Directions: This Guided Learning Activity (GLA) goes over formal charge and the structures of molecules with expanded octets. Part A introduces
PART 3 Chemical Bonds, Valence Bond Method, and Molecular Shapes. Reference: Chapter 9 10 in textbook
 PART 3 Chemical Bonds, Valence Bond Method, and Molecular Shapes Reference: Chapter 9 10 in textbook 1 Valence Electrons Valence ae Electron Define: the outer shell electrons Important for determination
PART 3 Chemical Bonds, Valence Bond Method, and Molecular Shapes Reference: Chapter 9 10 in textbook 1 Valence Electrons Valence ae Electron Define: the outer shell electrons Important for determination
CHAPTER 5: Bonding Theories - Explaining Molecular Geometry. Chapter Outline
 CHAPTER 5: Bonding Theories - Explaining Molecular Geometry Chapter Outline 5.1 Molecular Shape 5.2 Valence-Shell Electron-Pair Repulsion Theory (VSEPR) 5.3 Polar Bonds and Polar Molecules» What Makes
CHAPTER 5: Bonding Theories - Explaining Molecular Geometry Chapter Outline 5.1 Molecular Shape 5.2 Valence-Shell Electron-Pair Repulsion Theory (VSEPR) 5.3 Polar Bonds and Polar Molecules» What Makes
Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory
 10.1 Artificial Sweeteners: Fooled by Molecular Shape 425 10.2 VSEPR Theory: The Five Basic Shapes 426 10.3 VSEPR Theory: The Effect of Lone Pairs 430 10.4 VSEPR Theory: Predicting Molecular Geometries
10.1 Artificial Sweeteners: Fooled by Molecular Shape 425 10.2 VSEPR Theory: The Five Basic Shapes 426 10.3 VSEPR Theory: The Effect of Lone Pairs 430 10.4 VSEPR Theory: Predicting Molecular Geometries
video 14.4 isomers isomers Isomers have the molecular formula but are rearranged in a structure with different properties. Example: Both C 4 H 10
 video 14.4 isomers isomers Isomers have the molecular formula but are rearranged in a structure with different properties. Example: Both C 4 H 10 Butane Methylpropane 1 match the isomers drawing an isomer
video 14.4 isomers isomers Isomers have the molecular formula but are rearranged in a structure with different properties. Example: Both C 4 H 10 Butane Methylpropane 1 match the isomers drawing an isomer
Shapes of Molecules and Hybridization
 Shapes of Molecules and Hybridization A. Molecular Geometry Lewis structures provide us with the number and types of bonds around a central atom, as well as any NB electron pairs. They do not tell us the
Shapes of Molecules and Hybridization A. Molecular Geometry Lewis structures provide us with the number and types of bonds around a central atom, as well as any NB electron pairs. They do not tell us the
Lecture B2 VSEPR Theory
 Lecture B2 VSEPR Theory Covalent Bond Theories 1. VSEPR (valence shell electron pair repulsion model). A set of empirical rules for predicting a molecular geometry using, as input, a correct Lewis Dot
Lecture B2 VSEPR Theory Covalent Bond Theories 1. VSEPR (valence shell electron pair repulsion model). A set of empirical rules for predicting a molecular geometry using, as input, a correct Lewis Dot
Organic Chemistry. Introduction to Organic Chemistry 01/03/2018. Organic Chemistry
 Organic Chemistry Chemistry 30 Ms. Hayduk Introduction to Organic Chemistry https://www.youtube.com/watch?v=i9r1dmhh2m0 Organic Chemistry Study of compounds that contain carbon as the main element Relevant
Organic Chemistry Chemistry 30 Ms. Hayduk Introduction to Organic Chemistry https://www.youtube.com/watch?v=i9r1dmhh2m0 Organic Chemistry Study of compounds that contain carbon as the main element Relevant
Lewis Dot Structures for Methane, CH 4 The central C atom is bonded by single bonds (-) to 4 individual H atoms
 Chapter 10 (Hill/Petrucci/McCreary/Perry Bonding Theory and Molecular Structure This chapter deals with two additional approaches chemists use to describe chemical bonding: valence-shell electron pair
Chapter 10 (Hill/Petrucci/McCreary/Perry Bonding Theory and Molecular Structure This chapter deals with two additional approaches chemists use to describe chemical bonding: valence-shell electron pair
Alkanes, Alkenes and Alkynes
 Alkanes, Alkenes and Alkynes Hydrocarbons Hydrocarbons generally fall into 2 general groupings, aliphatic hydrocarbons and aromatic hydrocarbons. Aliphatic hydrocarbons contain chains and rings of hydrocarbons,
Alkanes, Alkenes and Alkynes Hydrocarbons Hydrocarbons generally fall into 2 general groupings, aliphatic hydrocarbons and aromatic hydrocarbons. Aliphatic hydrocarbons contain chains and rings of hydrocarbons,
CHEMISTRY 112 LECTURE EXAM II Material
 CHEMISTRY 112 LECTURE EXAM II Material Part I Chemical Bonding I Lewis Theory Chapter 9 pages 376-386 A. Drawing electron dot structures HOW TO: 1. Write e- dot structure for the individual atoms. 2. a)
CHEMISTRY 112 LECTURE EXAM II Material Part I Chemical Bonding I Lewis Theory Chapter 9 pages 376-386 A. Drawing electron dot structures HOW TO: 1. Write e- dot structure for the individual atoms. 2. a)
Honors Chemistry Unit 6 ( )
 Honors Chemistry Unit 6 (2017-2018) Lewis Dot Structures VSEPR Structures 1 We are learning to: 1. Represent compounds with Lewis structures. 2. Apply the VSEPR theory to determine the molecular geometry
Honors Chemistry Unit 6 (2017-2018) Lewis Dot Structures VSEPR Structures 1 We are learning to: 1. Represent compounds with Lewis structures. 2. Apply the VSEPR theory to determine the molecular geometry
Molecular Geometry. Dr. Williamson s Molecular Geometry Notes. VSEPR: Definition of Terms. Dr. V.M. Williamson Texas A & M University Student Version
 Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry. Dr. Williamson s Molecular Geometry Notes. VSEPR: Definition of Terms. VSEPR: Electronic Geometries VSEPR
 Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
ORGANIC - EGE 5E CH. 2 - COVALENT BONDING AND CHEMICAL REACTIVITY
 !! www.clutchprep.com CONCEPT: HYBRID ORBITAL THEORY The Aufbau Principle states that electrons fill orbitals in order of increasing energy. If carbon has only two unfilled orbitals, why does it like to
!! www.clutchprep.com CONCEPT: HYBRID ORBITAL THEORY The Aufbau Principle states that electrons fill orbitals in order of increasing energy. If carbon has only two unfilled orbitals, why does it like to
CHM2045 F13--Exam # MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 CHM2045 F13--Exam #2 2013.10.18 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) A valid Lewis structure of cannot be drawn without violating the
CHM2045 F13--Exam #2 2013.10.18 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) A valid Lewis structure of cannot be drawn without violating the
CHEM 110 Exam 2 - Practice Test 1 - Solutions
 CHEM 110 Exam 2 - Practice Test 1 - Solutions 1D 1 has a triple bond. 2 has a double bond. 3 and 4 have single bonds. The stronger the bond, the shorter the length. 2A A 1:1 ratio means there must be the
CHEM 110 Exam 2 - Practice Test 1 - Solutions 1D 1 has a triple bond. 2 has a double bond. 3 and 4 have single bonds. The stronger the bond, the shorter the length. 2A A 1:1 ratio means there must be the
Part A: There is a table attached to the end of this document. For each molecule or ion in the table, provide entries for columns I through IX.
 Chemistry 400 Discussion: VSEPR Theory and Molecular Shapes Instructions: Work through the exercises below. If you have access to molecular model kits, use them to build three dimensional models of the
Chemistry 400 Discussion: VSEPR Theory and Molecular Shapes Instructions: Work through the exercises below. If you have access to molecular model kits, use them to build three dimensional models of the
Chapter 25. Organic and Biological Chemistry. Organic and
 Chapter 25 Calculate grade: (Add exam1 - exam 4 scores)x1.5 Add 7 best quizzes (each quiz is worth 29) Gives you the # points you have so far. Final (worth 200 points) Grades: 800-4 750-3.5 700-3 650-2.5
Chapter 25 Calculate grade: (Add exam1 - exam 4 scores)x1.5 Add 7 best quizzes (each quiz is worth 29) Gives you the # points you have so far. Final (worth 200 points) Grades: 800-4 750-3.5 700-3 650-2.5
Electron Geometry Hybrid Orbitals
 Molecular Shape and Hybridized Orbitals CH2000: Introduction to General Chemistry, Plymouth State University, Fall 2014 Introduction: In chemistry, the three dimensional shape of a molecule is as important
Molecular Shape and Hybridized Orbitals CH2000: Introduction to General Chemistry, Plymouth State University, Fall 2014 Introduction: In chemistry, the three dimensional shape of a molecule is as important
Chapter 12 Structure and Shape
 Free Study Guide for Cracolice Peters Introductory Chemistry: An Active Learning Approach Second Edition www.brookscole.com/chemistry Chapter 12 Structure and Shape Chapter 12Assignment A: Lewis Diagrams
Free Study Guide for Cracolice Peters Introductory Chemistry: An Active Learning Approach Second Edition www.brookscole.com/chemistry Chapter 12 Structure and Shape Chapter 12Assignment A: Lewis Diagrams
Ch 13: Covalent Bonding
 Ch 13: Covalent Bonding Section 13: Valence-Shell Electron-Pair Repulsion 1. Recall the rules for drawing Lewis dot structures 2. Remember the special situations: - Resonance structures - ormal charges
Ch 13: Covalent Bonding Section 13: Valence-Shell Electron-Pair Repulsion 1. Recall the rules for drawing Lewis dot structures 2. Remember the special situations: - Resonance structures - ormal charges
Chapter 10 Shapes of Molecules. Dr. Sapna Gupta
 Chapter 10 Shapes of Molecules Dr. Sapna Gupta Shapes of Molecules - Importance All molecules have a 3D orientations; even the diatomic ones because atoms have a volume. In case of tri atomic or polyatomic
Chapter 10 Shapes of Molecules Dr. Sapna Gupta Shapes of Molecules - Importance All molecules have a 3D orientations; even the diatomic ones because atoms have a volume. In case of tri atomic or polyatomic
Would you expect SeF6 to be soluble in water? Yes No Explain your answer in terms of the shape and polarity of SeF6.
 COLLATED QUESTIONS Lewis structures and shapes (up to six electron pairs about the central atom for molecules and polyatomic ions, including those with multiple bonds), polarity of molecules. 2017:3 (c)
COLLATED QUESTIONS Lewis structures and shapes (up to six electron pairs about the central atom for molecules and polyatomic ions, including those with multiple bonds), polarity of molecules. 2017:3 (c)
Chemical Bonds. Chapter 6
 Chemical Bonds Chapter 6 1 Ch. 6 Chemical Bonding I. How and Why Atoms Bond A. Vocabulary B. Chemical Bonds - Basics C. Chemical Bonds Types D. Chemical Bonds Covalent E. Drawing Lewis Diagrams F. Bond
Chemical Bonds Chapter 6 1 Ch. 6 Chemical Bonding I. How and Why Atoms Bond A. Vocabulary B. Chemical Bonds - Basics C. Chemical Bonds Types D. Chemical Bonds Covalent E. Drawing Lewis Diagrams F. Bond
Chapter 7 Chemical Bonding and Molecular Structure
 Chapter 7 Chemical Bonding and Molecular Structure Three Types of Chemical Bonding (1) Ionic: formed by electron transfer (2) Covalent: formed by electron sharing (3) Metallic: attraction between metal
Chapter 7 Chemical Bonding and Molecular Structure Three Types of Chemical Bonding (1) Ionic: formed by electron transfer (2) Covalent: formed by electron sharing (3) Metallic: attraction between metal
