CHEMISTRY 110 EXAM 2 Feb 25, 2013 FORM A
|
|
|
- Jayson Parrish
- 6 years ago
- Views:
Transcription
1 EMISTRY 110 EXAM 2 Feb 25, 2013 FORM A 1. ow many valence electrons and lone pairs are in the structure of the ammonium ion? # valence electrons # lone pairs A. 8 0 B D E Which of the following best represents the molecular structure of OS? A. I B. II. III D. II and III are equally good representations. E. I and II are equally good representations.
2 3. What is the arrangement of electron domains about the central atom in formaldehyde, 2 O? A. Tetrahedral B. Trigonal planar. Square planar D. Trigonal bipyramidal E. Linear 4. Which of these molecules is/are non-polar? I II III IV A. I only B. II only. IV only D. I and IV E. II and III
3 5. What functional groups are present in the molecule below: A. alcohol, amine, aldehyde B. amine, aldehyde, ketone. amine, ketone, carboxylic acid D. alcohol, ketone, carboxylic acid E. ketone, carboxylic acid, ester 6. What is the correct molecular formula for the following molecule: A. 4 8 O 2 B O O 2 D O 2 E. None of the answers listed here are correct
4 7. Which one of the following organic molecules would not have dipole-dipole interactions as one of its intermolecular forces? O O N 2 A. B.. O O O D. E. 8. Which one of the following molecules is nonpolar? A. N 3 B. OF 2. 3 D. 2 O E. Be 2 Go on to the next page
5 9. What is the hybridization of As in the AsF 4 ion? A. sp B. sp 2. sp 3 D. sp 3 d E. sp 3 d The structure of citric acid is given below. What is the hybridization of the two labeled carbons?? 1 2 A. sp sp 2 B. sp 2 sp 3. sp 3 sp 2 D. sp 3 sp E. sp 2 sp 2
6 11. Arrange the following substances in order of increasing boiling point. i. ii. iii. A. i < ii < iii B. i < iii < ii. ii < iii < i D. iii < i < ii E. iii < ii < i 12. What is the percent carbon by mass in the compound below? A. 39.4% B. 42.2%. 51.7% D. 56.3% E % Go on to the next page
7 13. Select the electron-domain geometry and molecular geometry of chlorine in the perchlorate ion, O 4. Electron domain molecular geometry geometry A. trigonal planar tetrahedral B. trigonal planar trigonal planar. tetrahedral tetrahedral D. trigonal pyramidal trigonal pyramidal E. trigonal pyramidal trigonal planar 14. Which of the following molecules are isomers? A. I and II only B. II and III only. I and IV only D. I, II, and III E. All of them
8 15. As bond order increases, the strength of a covalent bond and bond length. As bond order increases strength length A. increases increases B. decreases decreases. increases decreases D. decreases increases E. increases remains constant 16. Which of the following have one or more atoms in the structure that have exceptions to the octet rule? BF 3 NF 3 SiF 4 XeF 4 A. BF 3 and NF 3 B. NF 3 and SiF 4. SiF 4 and XeF 4 D. BF 3 and XeF 4 E. NF 3 and XeF 4 Go on to the next page
9 17. Which of the following contains the largest Y-X-Y bond angle where X is the central atom of the molecule? A. BF 3 B. Si 4. N 3 D. 2 O E. They all have the same bond angle. 18. Which of the following molecules has a trigonal planar structure? 1. O SO O + 4. SO 3 A. 1 only B. 1 and 4. 1, 2 and 3 D. 1, 3 and 4 E. all 4
10 19. Rank the indicated bond angles in the molecule shown below in order of increasing bond angle. (Lone pairs are NOT explicitly shown.) 1 O 3 4 O O N 2 A. 1 < 4 < 3 < 2 B. 3 < 1 < = 1 < 2 < 3 D. 4 = 1 < 2 = 3 E. 3 < 4 < 1 < What is the total number of σ and π-bonds in the following molecule? N σ π A. 6 3 B D. 3 6 E. 3 9 Go on to the next page
11 21. Which of the following molecules will exhibit delocalized π- bonding? I. SO II. SO 2 III. SO 3 A. I only B. II only. III only D. I and II E. II and III 22. Which one of these molecule has the smallest % oxygen by mass? A. I B. II. III D. IV E. I and IV have the same and the smallest % oxygen
12 23. Draw Lewis structures for NO 2 +, NO 2 and NO 3. Based on the interpretation of the Lewis structures, put these in order of increasing bond order? A. NO + 2 < NO 2 = NO 3 B. NO + 2 < NO 3 < NO 2. NO 2 = NO 3 + < NO 2 D. NO 3 < NO 2 + < NO 2 E. NO 2 < NO + 2 < NO O 3 ow are the molecules below related to the molecule shown above? Are they a geometric isomer, a structural isomer or neither? O 3 O 3 O 3 3 I II III I II III A. neither structural geometric B. neither geometric structural. geometric geometric structural D. structural structural geometric E. geometric structural geometric Go on to the next page
13 25. Which of the following are pairs of resonance structures? A. I only B. II only. III only D. I and II E. II and III 26. What is the electron domain geometry of a molecule that only contains S and F and is 29.67% S by weight? A. Octahedral B. Trigonal planar. Linear D. Tetrahedral E. Trigonal bipyramidal
14 27. Which one of these correctly depicts hydrogen bonding in acetic acid ( 3 OO)? The dashed line ( ) represent the hydrogen bond. Go on to the last page
15 Br I Br I Boiling point i 174 ii. 219 iii The boiling points of the three molecules shown are given below their structures. Which of these statements are true? A. I only B. II only I. The first molecule (i) has the greatest dipole moment of the three. II. As the polarity increases the boiling point increases. III. As the London dispersion forces increase, the boiling point increases.. III only D. I and II E. I and III END OF EXAM
16 EMISTRY 110 EXAM 2 February 25, 2013 Answer Key FORM A 1. A 2. B 3. B 4. D 5. D 6. D 7. B 8. E 9. D A 12. D E D 17. A 18. B 19. E E 22. B 23. D 24. A E 27. B, D and E = full credit 28. E
CHEMISTRY 110 EXAM 2 Oct. 11, 2010 FORM A
 EMISTRY 110 EXAM 2 Oct. 11, 2010 FORM A --------------------------------------------------------------------------------- 1. What is the empirical formula that corresponds to a compound that contains only
EMISTRY 110 EXAM 2 Oct. 11, 2010 FORM A --------------------------------------------------------------------------------- 1. What is the empirical formula that corresponds to a compound that contains only
Which of the following is the most polar bond?
 !"#$%&'()*++,*#-.$*/* ct 14, 2013 FRM A 1. A neutral atom has the following electron configuration. What is the maximum number of covalent bonds this atom could form with hydrogen atoms? 3. Which of the
!"#$%&'()*++,*#-.$*/* ct 14, 2013 FRM A 1. A neutral atom has the following electron configuration. What is the maximum number of covalent bonds this atom could form with hydrogen atoms? 3. Which of the
CHEMISTRY 110 EXAM 2 Oct.
 EMISTRY 110 EXAM 2 ct. 15, 2012 FRM A 1. Which of the following is an incorrect formula for a neutral compound made from the given ions?! "#$%&'! #'%&'! (&)*+,#! -.!! aluminum oxide Al 2 3 /.!! magnesium
EMISTRY 110 EXAM 2 ct. 15, 2012 FRM A 1. Which of the following is an incorrect formula for a neutral compound made from the given ions?! "#$%&'! #'%&'! (&)*+,#! -.!! aluminum oxide Al 2 3 /.!! magnesium
Assignment 09 A. 2- The image below depicts a seesaw structure. Which of the following has such a structure?
 Assignment 09 A 1- Give the total number of electron domains, the number of bonding and nonbonding domains, and the molecular geometry, respectively, for the central atom of P 3. a) four electron domains,
Assignment 09 A 1- Give the total number of electron domains, the number of bonding and nonbonding domains, and the molecular geometry, respectively, for the central atom of P 3. a) four electron domains,
Valence Shell Electron Pair repulsion
 Molecular Geometry Valence Shell Electron Pair repulsion The valence shell electron pair repulsion model (VSEPR model) assumes that electron pairs repel one another. (VSEPR) model gives helps determine
Molecular Geometry Valence Shell Electron Pair repulsion The valence shell electron pair repulsion model (VSEPR model) assumes that electron pairs repel one another. (VSEPR) model gives helps determine
AP Chemistry- Practice Bonding Questions for Exam
 AP Chemistry- Practice Bonding Questions for Exam Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is a correct Lewis structure for
AP Chemistry- Practice Bonding Questions for Exam Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is a correct Lewis structure for
Form J. Test #4 Last Name First Name Zumdahl, Chapters 8 and 9 November 23, 2004
 Form J Chemistry 1441-023 Name (please print) Test #4 Last Name First Name Zumdahl, Chapters 8 and 9 November 23, 2004 Instructions: 1. This exam consists of 27 questions. 2. No scratch paper is allowed.
Form J Chemistry 1441-023 Name (please print) Test #4 Last Name First Name Zumdahl, Chapters 8 and 9 November 23, 2004 Instructions: 1. This exam consists of 27 questions. 2. No scratch paper is allowed.
Lewis Structures and Molecular Shapes
 Lewis Structures and Molecular Shapes Drawing Lewis Structures Determine from formula if ionic or covalent Count the electrons If ionic : add valence # to charge if (-), subtract if (+) - = 7+1 electrons;
Lewis Structures and Molecular Shapes Drawing Lewis Structures Determine from formula if ionic or covalent Count the electrons If ionic : add valence # to charge if (-), subtract if (+) - = 7+1 electrons;
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: and ybridization of Atomic rbitals Chapter 10 Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the
Chemical Bonding II: and ybridization of Atomic rbitals Chapter 10 Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the
Valence Bond Theory - Description
 Bonding and Molecular Structure - PART 2 - Valence Bond Theory and Hybridization 1. Understand and be able to describe the Valence Bond Theory description of covalent bond formation. 2. Understand and
Bonding and Molecular Structure - PART 2 - Valence Bond Theory and Hybridization 1. Understand and be able to describe the Valence Bond Theory description of covalent bond formation. 2. Understand and
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Take Home Exam Chem 1A Fall 2008 - Chapters 6 to 9: You may us any resource you wish accept people. On your honor, you may not ask another person for help. Show your work on every answer. Partial credit
Take Home Exam Chem 1A Fall 2008 - Chapters 6 to 9: You may us any resource you wish accept people. On your honor, you may not ask another person for help. Show your work on every answer. Partial credit
UNIVERSITY OF VICTORIA. CHEMISTRY 101 Mid-Term Test 2, November
 NAME Student No. SECTIN (circle one): A01 (Codding) A02 (Sirk) A03 (Briggs) Version A UNIVERSITY F VICTRIA CEMISTRY 101 Mid-Term Test 2, November 19 2010 Version A This test has two parts and 8 pages,
NAME Student No. SECTIN (circle one): A01 (Codding) A02 (Sirk) A03 (Briggs) Version A UNIVERSITY F VICTRIA CEMISTRY 101 Mid-Term Test 2, November 19 2010 Version A This test has two parts and 8 pages,
Name Unit Three MC Practice March 15, 2017
 Unit Three: Bonding & Molecular Geometry Name Unit Three MC Practice March 15, 2017 1. What is the hybridization of the oxygen atom in water? a) sp b) sp 2 c) sp 3 d) It is not hybridized 2. When a double
Unit Three: Bonding & Molecular Geometry Name Unit Three MC Practice March 15, 2017 1. What is the hybridization of the oxygen atom in water? a) sp b) sp 2 c) sp 3 d) It is not hybridized 2. When a double
Version 188 Exam 2 mccord (51600) 1
 Version 188 Exam 2 mccord (51600) 1 This print-out should have 35 questions. Multiple-choice questions may continue on the next column or page find all choices before answering. l I l l 001 3.0 points
Version 188 Exam 2 mccord (51600) 1 This print-out should have 35 questions. Multiple-choice questions may continue on the next column or page find all choices before answering. l I l l 001 3.0 points
Chapter 1: Structure and Bonding
 1. What is the ground-state electronic configuration of a carbon atom? A) 1s 2, 2s 2, 2p 5 B) 1s 2, 2s 2, 2p 2 C) 1s 2, 2s 2, 2p 6 D) 1s 2, 2s 2, 2p 4 2. What is the ground-state electronic configuration
1. What is the ground-state electronic configuration of a carbon atom? A) 1s 2, 2s 2, 2p 5 B) 1s 2, 2s 2, 2p 2 C) 1s 2, 2s 2, 2p 6 D) 1s 2, 2s 2, 2p 4 2. What is the ground-state electronic configuration
Molecular Geometry and Chemical Bonding Theory
 Molecular Geometry and Chemical Bonding Theory The Valence -Shell Electron -Pair Repulsion (VSEPR) Model predicts the shapes of the molecules and ions by assuming that the valence shell electron pairs
Molecular Geometry and Chemical Bonding Theory The Valence -Shell Electron -Pair Repulsion (VSEPR) Model predicts the shapes of the molecules and ions by assuming that the valence shell electron pairs
MOLECULAR ORBITAL DIAGRAM KEY
 365 MOLECULAR ORBITAL DIAGRAM KEY Draw molecular orbital diagrams for each of the following molecules or ions. Determine the bond order of each and use this to predict the stability of the bond. Determine
365 MOLECULAR ORBITAL DIAGRAM KEY Draw molecular orbital diagrams for each of the following molecules or ions. Determine the bond order of each and use this to predict the stability of the bond. Determine
Electron Geometry Hybrid Orbitals
 Molecular Shape and Hybridized Orbitals CH2000: Introduction to General Chemistry, Plymouth State University, Fall 2014 Introduction: In chemistry, the three dimensional shape of a molecule is as important
Molecular Shape and Hybridized Orbitals CH2000: Introduction to General Chemistry, Plymouth State University, Fall 2014 Introduction: In chemistry, the three dimensional shape of a molecule is as important
General and Inorganic Chemistry I.
 General and Inorganic Chemistry I. Lecture 1 István Szalai Eötvös University István Szalai (Eötvös University) Lecture 1 1 / 29 Outline István Szalai (Eötvös University) Lecture 1 2 / 29 Lewis Formulas
General and Inorganic Chemistry I. Lecture 1 István Szalai Eötvös University István Szalai (Eötvös University) Lecture 1 1 / 29 Outline István Szalai (Eötvös University) Lecture 1 2 / 29 Lewis Formulas
CHM2045 F13--Exam # MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 CHM2045 F13--Exam #2 2013.10.18 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) A valid Lewis structure of cannot be drawn without violating the
CHM2045 F13--Exam #2 2013.10.18 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) A valid Lewis structure of cannot be drawn without violating the
CHEM 110 Exam 2 - Practice Test 1 - Solutions
 CHEM 110 Exam 2 - Practice Test 1 - Solutions 1D 1 has a triple bond. 2 has a double bond. 3 and 4 have single bonds. The stronger the bond, the shorter the length. 2A A 1:1 ratio means there must be the
CHEM 110 Exam 2 - Practice Test 1 - Solutions 1D 1 has a triple bond. 2 has a double bond. 3 and 4 have single bonds. The stronger the bond, the shorter the length. 2A A 1:1 ratio means there must be the
Organic Chemistry. Review Information for Unit 1. VSEPR Hybrid Orbitals Polar Molecules
 rganic hemistry Review Information for Unit 1 VSEPR ybrid rbitals Polar Molecules VSEPR The valence shell electron pair repulsion model (VSEPR) can be used to predict the geometry around a particular atom
rganic hemistry Review Information for Unit 1 VSEPR ybrid rbitals Polar Molecules VSEPR The valence shell electron pair repulsion model (VSEPR) can be used to predict the geometry around a particular atom
Chapter 9 Molecular Geometry and Bonding Theories
 Chapter 9 Molecular Geometry and Bonding Theories molecular shapes the VSEPR model molecular shape and molecular polarity covalent bonding and orbital overlap hybrid orbitals multiple bonds 9.1 Molecular
Chapter 9 Molecular Geometry and Bonding Theories molecular shapes the VSEPR model molecular shape and molecular polarity covalent bonding and orbital overlap hybrid orbitals multiple bonds 9.1 Molecular
Chemical bonding is the combining of elements to form new substances.
 Name Covalent Bonding and Nomenclature: Unit Objective Study Guide Class Period Date Due 1. Define chemical bonding. What is chemical bonding? Chemical bonding is the combining of elements to form new
Name Covalent Bonding and Nomenclature: Unit Objective Study Guide Class Period Date Due 1. Define chemical bonding. What is chemical bonding? Chemical bonding is the combining of elements to form new
1. My answers for this Chemistry 102 exam should be graded with the answer sheet associated with: a. Form A b. Form B c. Form C d. Form D e.
 EMISTRY 10 Fall 010 our Exam Page 1 1. My answers for this hemistry 10 exam should be graded with the answer sheet associated with: a. Form A b. Form B c. Form d. Form D e. Form E. Which of the statements
EMISTRY 10 Fall 010 our Exam Page 1 1. My answers for this hemistry 10 exam should be graded with the answer sheet associated with: a. Form A b. Form B c. Form d. Form D e. Form E. Which of the statements
Review for Chapter 4: Structures and Properties of Substances
 Review for Chapter 4: Structures and Properties of Substances You are responsible for the following material: 1. Terms: You should be able to write definitions for the following terms. A complete definition
Review for Chapter 4: Structures and Properties of Substances You are responsible for the following material: 1. Terms: You should be able to write definitions for the following terms. A complete definition
SL Score. HL Score ! /30 ! /48. Practice Exam: Paper 1 Topic 4: Bonding. Name
 Name Practice Exam: Paper 1 Topic 4: Bonding SL SL Score! /30 HL Score! /48 1. What is the correct Lewis structure for hypochlorous acid, a compound containing chlorine, hydrogen and oxygen? A. B. C. D.
Name Practice Exam: Paper 1 Topic 4: Bonding SL SL Score! /30 HL Score! /48 1. What is the correct Lewis structure for hypochlorous acid, a compound containing chlorine, hydrogen and oxygen? A. B. C. D.
CHEMISTRY 101 Hour Exam III. Dr. D. DeCoste T.A (30 pts.) 16 (12 pts.) 17 (18 pts.) Total (60 pts)
 CHEMISTRY 101 Hour Exam III April 27, 2017 Dr. D. DeCoste Name Signature T.A. This exam contains 17 questions on 6 numbered pages. Check now to make sure you have a complete exam. You have one hour and
CHEMISTRY 101 Hour Exam III April 27, 2017 Dr. D. DeCoste Name Signature T.A. This exam contains 17 questions on 6 numbered pages. Check now to make sure you have a complete exam. You have one hour and
CHEMISTRY 102 Spring 2013 Hour Exam I Page 1. Which molecule(s) has/have tetrahedral shape and which molecule(s) is/are polar?
 Hour Exam I Page 1 1. Consider the following molecules: SiF 4, SeF 4, XeF 4 Which molecule(s) has/have tetrahedral shape and which molecule(s) is/are polar? a) SeF 4 has tetrahedral shape and XeF 4 is
Hour Exam I Page 1 1. Consider the following molecules: SiF 4, SeF 4, XeF 4 Which molecule(s) has/have tetrahedral shape and which molecule(s) is/are polar? a) SeF 4 has tetrahedral shape and XeF 4 is
Chemistry 1A Spring 1998 Exam #4 KEY Chapters 9 & 10
 Chemistry 1A Spring 1998 Exam #4 KEY Chapters 9 & 10 For each of the following, write the word, words, or number in each blank that best completes each sentence. (2 points each) 1. A(n) molecular orbital
Chemistry 1A Spring 1998 Exam #4 KEY Chapters 9 & 10 For each of the following, write the word, words, or number in each blank that best completes each sentence. (2 points each) 1. A(n) molecular orbital
CHEMISTRY 101 Hour Exam III. Dr. D. DeCoste T.A (30 pts.) 16 (12 pts.) 17 (18 pts.) Total (60 pts)
 CHEMISTRY 101 Hour Exam III April 27, 2017 Dr. D. DeCoste Name Signature T.A. This exam contains 17 questions on 6 numbered pages. Check now to make sure you have a complete exam. You have one hour and
CHEMISTRY 101 Hour Exam III April 27, 2017 Dr. D. DeCoste Name Signature T.A. This exam contains 17 questions on 6 numbered pages. Check now to make sure you have a complete exam. You have one hour and
Classes of Organic Compounds
 Unit 1 Functional Groups Depicting Structures of rganic ompounds Lewis Structures ondensed structural formulas Line angle drawings 3-dimensional structures Resonance Structures Acid-Base Reactions urved
Unit 1 Functional Groups Depicting Structures of rganic ompounds Lewis Structures ondensed structural formulas Line angle drawings 3-dimensional structures Resonance Structures Acid-Base Reactions urved
Bonding and IMF practice test MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name Bonding and IMF practice test MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) There are paired and unpaired electrons in the Lewis symbol
Exam Name Bonding and IMF practice test MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) There are paired and unpaired electrons in the Lewis symbol
LET S FIRST REVIEW IONIC BONDING
 COVALENT BONDING LET S FIRST REVIEW IONIC BONDING In an IONIC bond, electrons are lost or gained, resulting in the formation of IONS in ionic compounds. K F K F K F K F K F K F K + F _ The compound potassium
COVALENT BONDING LET S FIRST REVIEW IONIC BONDING In an IONIC bond, electrons are lost or gained, resulting in the formation of IONS in ionic compounds. K F K F K F K F K F K F K + F _ The compound potassium
Chem 121 Exam 4 Practice Exam
 Chem 121 Exam 4 Practice Exam 1. What is the correct electron configuration for bromine? b. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9 4s 2 4p 6 c. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5 d. 1s 2 2s 2 2p 6 3s 2 3p
Chem 121 Exam 4 Practice Exam 1. What is the correct electron configuration for bromine? b. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9 4s 2 4p 6 c. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5 d. 1s 2 2s 2 2p 6 3s 2 3p
Structures, Shapes and Polarity. of Molecules. Level 2 recap: - Polar and non polar bonds - Lewis diagrams - Lone pairs - Shapes - Polarity
 Structures, Shapes and Polarity Level 2 recap: - Polar and non polar bonds - Lewis diagrams - Lone pairs - Shapes - Polarity of Molecules Do now: Brainstorm what you know/remember about these L2 concepts
Structures, Shapes and Polarity Level 2 recap: - Polar and non polar bonds - Lewis diagrams - Lone pairs - Shapes - Polarity of Molecules Do now: Brainstorm what you know/remember about these L2 concepts
1. Which is the most electronegative atom in the compound below?
 1. Which is the most electronegative atom in the compound below? A) Carbon C) Oxygen B) Nitrogen D) Bromine 2. Which of the following correctly describes the electrons of a carbon atom in its ground state?
1. Which is the most electronegative atom in the compound below? A) Carbon C) Oxygen B) Nitrogen D) Bromine 2. Which of the following correctly describes the electrons of a carbon atom in its ground state?
Topic 5: Structure and Shape
 Topic 5: Structure and Shape Lewis structures Lewis structures are a means of determining stable electron arrangements in molecules. It considers the valence electrons of an atom only. A stable arrangement
Topic 5: Structure and Shape Lewis structures Lewis structures are a means of determining stable electron arrangements in molecules. It considers the valence electrons of an atom only. A stable arrangement
CHEMISTRY 121 AUTUMN 2009 CHAPTER 8 & 9 PROBLEMS
 Dr. Fus AU 2009 EM 121 EMISTRY 121 AUTUMN 2009 APTER 8 & 9 PROBLEMS All questions listed below are problems taken from old hemistry 121 exams given here at The Ohio State University. Read hapters 8 and
Dr. Fus AU 2009 EM 121 EMISTRY 121 AUTUMN 2009 APTER 8 & 9 PROBLEMS All questions listed below are problems taken from old hemistry 121 exams given here at The Ohio State University. Read hapters 8 and
Electron Geometry Hybrid Orbitals
 Molecular Shape and Hybridized Orbitals CH2000: Introduction to General Chemistry, Plymouth State University Introduction: In chemistry, the three dimensional shape of a molecule is as important as the
Molecular Shape and Hybridized Orbitals CH2000: Introduction to General Chemistry, Plymouth State University Introduction: In chemistry, the three dimensional shape of a molecule is as important as the
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) The F-B-F bond angle in the BF3 molecule is. A) 109.5e B) 120e C) 180e D) 90e E) 60e
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) The F-B-F bond angle in the BF3 molecule is. A) 109.5e B) 120e C) 180e D) 90e E) 60e
CHEMISTRY 102B Hour Exam III. Dr. D. DeCoste T.A. Show all of your work and provide complete answers to questions 16 and (45 pts.
 CHEMISTRY 102B Hour Exam III April 28, 2016 Dr. D. DeCoste Name Signature T.A. This exam contains 17 questions on 8 numbered pages. Check now to make sure you have a complete exam. You have one hour and
CHEMISTRY 102B Hour Exam III April 28, 2016 Dr. D. DeCoste Name Signature T.A. This exam contains 17 questions on 8 numbered pages. Check now to make sure you have a complete exam. You have one hour and
Bonding. Honors Chemistry 412 Chapter 6
 Bonding Honors Chemistry 412 Chapter 6 Chemical Bond Mutual attraction between the nuclei and valence electrons of different atoms that binds them together. Types of Bonds Ionic Bonds Force of attraction
Bonding Honors Chemistry 412 Chapter 6 Chemical Bond Mutual attraction between the nuclei and valence electrons of different atoms that binds them together. Types of Bonds Ionic Bonds Force of attraction
Group 1 Group 2 Group 3 Group 4 Group 5 Group 6 Group 7 Group 8. Na Mg Al Si P S Cl Ar
 CHM 111 Chapters 7 and 8 Worksheet and Study Guide Purpose: This is a guide for your as you work through the chapter. The major topics are provided so that you can write notes on each topic and work the
CHM 111 Chapters 7 and 8 Worksheet and Study Guide Purpose: This is a guide for your as you work through the chapter. The major topics are provided so that you can write notes on each topic and work the
Molecular Geometry and intermolecular forces. Unit 4 Chapter 9 and 11.2
 1 Molecular Geometry and intermolecular forces Unit 4 Chapter 9 and 11.2 2 Unit 4.1 Chapter 9.1-9.3 3 Review of bonding Ionic compound (metal/nonmetal) creates a lattice Formula doesn t tell the exact
1 Molecular Geometry and intermolecular forces Unit 4 Chapter 9 and 11.2 2 Unit 4.1 Chapter 9.1-9.3 3 Review of bonding Ionic compound (metal/nonmetal) creates a lattice Formula doesn t tell the exact
Chapter 9 Molecular Geometry. Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory
 Chapter 9 Molecular Geometry Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Sulfanilamide Lewis Structures and the Real 3D-Shape of Molecules Lewis Theory of Molecular Shape and Polarity
Chapter 9 Molecular Geometry Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Sulfanilamide Lewis Structures and the Real 3D-Shape of Molecules Lewis Theory of Molecular Shape and Polarity
Homework 08 VSEPR. The active ingredient in some oral anesthetics used in sore throat sprays. What is the molar mass of phenol?
 HW08 VSEPR This is a preview of the published version of the quiz Started: Oct 21 at 11:14am Quiz Instruc ons Homework 08 VSEPR Question 1 Consider the structural formula of phenol. The active ingredient
HW08 VSEPR This is a preview of the published version of the quiz Started: Oct 21 at 11:14am Quiz Instruc ons Homework 08 VSEPR Question 1 Consider the structural formula of phenol. The active ingredient
Bonding. Polar Vs. Nonpolar Covalent Bonds. Ionic or Covalent? Identifying Bond Types. Solutions + -
 Chemical Bond Mutual attraction between the nuclei and valence electrons of different atoms that binds them together. Bonding onors Chemistry 412 Chapter 6 Types of Bonds Ionic Bonds Force of attraction
Chemical Bond Mutual attraction between the nuclei and valence electrons of different atoms that binds them together. Bonding onors Chemistry 412 Chapter 6 Types of Bonds Ionic Bonds Force of attraction
Hybridization and Molecular Orbital (MO) Theory
 ybridization and Molecular Orbital (MO) Theory Chapter 10 istorical Models G.N.Lewis and I. Langmuir (~1920) laid out foundations Ionic species were formed by electron transfer Covalent molecules arise
ybridization and Molecular Orbital (MO) Theory Chapter 10 istorical Models G.N.Lewis and I. Langmuir (~1920) laid out foundations Ionic species were formed by electron transfer Covalent molecules arise
CHM2045 S13: Exam # MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 CHM2045 S13: Exam #2 2013.03.01 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) How would replacing one of benzene's C atoms and the H atom attached
CHM2045 S13: Exam #2 2013.03.01 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) How would replacing one of benzene's C atoms and the H atom attached
CHAPTER 8. Molecular Structure & Covalent Bonding Theories
 CAPTER 8 Molecular Structure & Covalent Bonding Theories 1 Chapter Goals 1. A Preview of the Chapter 2. Valence Shell Electron Pair Repulsion (VSEPR) Theory 3. Polar Molecules:The Influence of Molecular
CAPTER 8 Molecular Structure & Covalent Bonding Theories 1 Chapter Goals 1. A Preview of the Chapter 2. Valence Shell Electron Pair Repulsion (VSEPR) Theory 3. Polar Molecules:The Influence of Molecular
Lewis Structure. Lewis Structures & VSEPR. Octet & Duet Rules. Steps for drawing Lewis Structures
 Lewis Structure Lewis Structures & VSEPR Lewis Structures shows how the are arranged among the atoms of a molecule There are rules for Lewis Structures that are based on the formation of a Atoms want to
Lewis Structure Lewis Structures & VSEPR Lewis Structures shows how the are arranged among the atoms of a molecule There are rules for Lewis Structures that are based on the formation of a Atoms want to
Chemistry 12 Name Exam II Form A Section
 hemistry 12 Name Exam II Form A Section July 21, 2005 Student No. IMPORTANT: On the scantron (answer sheet), you MUST clearly fill your name, your student number, section number, and test form (white cover
hemistry 12 Name Exam II Form A Section July 21, 2005 Student No. IMPORTANT: On the scantron (answer sheet), you MUST clearly fill your name, your student number, section number, and test form (white cover
Chapter 10. VSEPR Model: Geometries
 Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Farthest apart
Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Farthest apart
CH 222 Chapter Seven Concept Guide
 CH 222 Chapter Seven Concept Guide 1. Lewis Structures Draw the Lewis Dot Structure for cyanide ion, CN -. 1 C at 4 electrons = 4 electrons 1 N at 5 electrons = 5 electrons -1 charge = + 1 electron Total
CH 222 Chapter Seven Concept Guide 1. Lewis Structures Draw the Lewis Dot Structure for cyanide ion, CN -. 1 C at 4 electrons = 4 electrons 1 N at 5 electrons = 5 electrons -1 charge = + 1 electron Total
Chapter 13: Phenomena
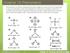 Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chapter 9 practice questions
 Class: Date: Chapter 9 practice questions Multiple Choice Identify the choice that best completes the statement or answers the question. 1. All of the following statements concerning valence bond (VB)
Class: Date: Chapter 9 practice questions Multiple Choice Identify the choice that best completes the statement or answers the question. 1. All of the following statements concerning valence bond (VB)
Chemistry 201. MW 12pm 1:15pm Examination #1 July 20 th Bronco ID. Question Score Possible Points. 1 (17pts) 2 (28pts) 3 (14pts) 4...
 Chemistry 201 MW 12pm 1:15pm Examination #1 July 20 th 2016 Name Bronco ID. Question Score Possible Points 1 (17pts) 2 (28pts) 3 (14pts) 4... (22pts) 5 (19pts). Total (100pts) 1. Read each question carefully.
Chemistry 201 MW 12pm 1:15pm Examination #1 July 20 th 2016 Name Bronco ID. Question Score Possible Points 1 (17pts) 2 (28pts) 3 (14pts) 4... (22pts) 5 (19pts). Total (100pts) 1. Read each question carefully.
Chapter 10. VSEPR Model: Geometries
 Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Two bonds Two
Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Two bonds Two
Molecular shape is determined by the number of bonds that form around individual atoms.
 Chapter 9 CH 180 Major Concepts: Molecular shape is determined by the number of bonds that form around individual atoms. Sublevels (s, p, d, & f) of separate atoms may overlap and result in hybrid orbitals
Chapter 9 CH 180 Major Concepts: Molecular shape is determined by the number of bonds that form around individual atoms. Sublevels (s, p, d, & f) of separate atoms may overlap and result in hybrid orbitals
Unit 6: Molecular Geometry
 Unit 6: Molecular Geometry Molecular Geometry [6-5] the polarity of each bond, along with the geometry of the molecule determines Molecular Polarity. To predict the geometries of more complicated molecules,
Unit 6: Molecular Geometry Molecular Geometry [6-5] the polarity of each bond, along with the geometry of the molecule determines Molecular Polarity. To predict the geometries of more complicated molecules,
Bent linear trigonal planar trigonal pyramidal Polar nonpolar nonpolar polar Sp 3 sp sp 2 sp 3
 Name period ap chemistry unit 3 worksheet 1. What are structural isomers? Draw two isomers of pentane. Compounds with the same formula but different structures. See in class 2. give the formula for each
Name period ap chemistry unit 3 worksheet 1. What are structural isomers? Draw two isomers of pentane. Compounds with the same formula but different structures. See in class 2. give the formula for each
CH 222 Sample Exam Exam I Name: Lab Section:
 222 Sample Exam Exam I Name: Lab Section: Part I: Multiple hoice Questions (100 Points) Use a scantron sheet for Part I. There is only one best answer for each question. 1. Which of the following statements
222 Sample Exam Exam I Name: Lab Section: Part I: Multiple hoice Questions (100 Points) Use a scantron sheet for Part I. There is only one best answer for each question. 1. Which of the following statements
Would you expect SeF6 to be soluble in water? Yes No Explain your answer in terms of the shape and polarity of SeF6.
 COLLATED QUESTIONS Lewis structures and shapes (up to six electron pairs about the central atom for molecules and polyatomic ions, including those with multiple bonds), polarity of molecules. 2017:3 (c)
COLLATED QUESTIONS Lewis structures and shapes (up to six electron pairs about the central atom for molecules and polyatomic ions, including those with multiple bonds), polarity of molecules. 2017:3 (c)
Fill in the chart below to determine the valence electrons of elements 3-10
 Chemistry 11 Atomic Theory IV Name: Date: Block: 1. Lewis Diagrams 2. VSEPR Lewis Diagrams Lewis diagrams show the bonding between atoms of a molecule. Only the outermost electrons of an atom (called electrons)
Chemistry 11 Atomic Theory IV Name: Date: Block: 1. Lewis Diagrams 2. VSEPR Lewis Diagrams Lewis diagrams show the bonding between atoms of a molecule. Only the outermost electrons of an atom (called electrons)
Name: Class: Date: 3. How many lone pairs of electrons are assigned to the carbon atom in carbon monoxide? a. 0 b. 1 c. 2 d. 3
 Class: Date: Midterm 3, Fall 2009 Record your name on the top of this exam and on the scantron form. Record the test ID letter in the top right box of the scantron form. Record all of your answers on the
Class: Date: Midterm 3, Fall 2009 Record your name on the top of this exam and on the scantron form. Record the test ID letter in the top right box of the scantron form. Record all of your answers on the
B. (i), (iii), and (v) C. (iv) D. (i), (ii), (iii), and (v) E. (i), (iii), (iv), and (v) Answer: B. SO 3, and NO 3 - both have 24 VE and have Lewis
 SCCH 161 Homework 3 1. Give the number of lone pairs around the central atom and the molecular geometry of CBr 4. Answer: Carbon has 4 valence electrons and bonds to four bromine atoms (each has 7 VE s).
SCCH 161 Homework 3 1. Give the number of lone pairs around the central atom and the molecular geometry of CBr 4. Answer: Carbon has 4 valence electrons and bonds to four bromine atoms (each has 7 VE s).
Examples: 1. Draw the possible resonance structures for the following covalent compounds: a. O3
 AP Chemistry Ms. Ye Name Date Block 1. Draw a Lewis structure for each of these molecules. Identify the molecular shape, hybridization, and bond angles. Determine the total number of valence electrons
AP Chemistry Ms. Ye Name Date Block 1. Draw a Lewis structure for each of these molecules. Identify the molecular shape, hybridization, and bond angles. Determine the total number of valence electrons
IB Chemistry 11 Kahoot! Review Q s Bonding
 IB Chemistry 11 Kahoot! Review Q s Bonding 1. What is the best description of the carbon-oxygen bond lengths in CO3 2-? A. One short and two long bonds B. One long and two short bonds C. Three bonds of
IB Chemistry 11 Kahoot! Review Q s Bonding 1. What is the best description of the carbon-oxygen bond lengths in CO3 2-? A. One short and two long bonds B. One long and two short bonds C. Three bonds of
CHEMICAL BONDING. Chemical Bonds. Ionic Bonding. Lewis Symbols
 CHEMICAL BONDING Chemical Bonds Lewis Symbols Octet Rule whenever possible, valence electrons in covalent compounds distribute so that each main-group element is surrounded by 8 electrons (except hydrogen
CHEMICAL BONDING Chemical Bonds Lewis Symbols Octet Rule whenever possible, valence electrons in covalent compounds distribute so that each main-group element is surrounded by 8 electrons (except hydrogen
1. Which is the most electronegative atom in the compound below? N H A) Carbon B) Nitrogen C) Oxygen D) Bromine Ans: C
 Chapter 1 1. Which is the most electronegative atom in the compound below? Br A) Carbon B) itrogen C) xygen D) Bromine 2. Which of the following correctly describes the electrons of a carbon atom in its
Chapter 1 1. Which is the most electronegative atom in the compound below? Br A) Carbon B) itrogen C) xygen D) Bromine 2. Which of the following correctly describes the electrons of a carbon atom in its
Chapter 6 Chemistry Review
 Chapter 6 Chemistry Review Multiple Choice Identify the choice that best completes the statement or answers the question. Put the LETTER of the correct answer in the blank. 1. The electrons involved in
Chapter 6 Chemistry Review Multiple Choice Identify the choice that best completes the statement or answers the question. Put the LETTER of the correct answer in the blank. 1. The electrons involved in
10-1. The Shapes of Molecules, chapter 10
 10-1 The Shapes of Molecules, chapter 10 The Shapes of Molecules; Goals 10.1 Depicting Molecules and Ions with Lewis Structures 10.2 Valence-Shell Electron-Pair Repulsion (VSEPR) Theory 10.3 Molecular
10-1 The Shapes of Molecules, chapter 10 The Shapes of Molecules; Goals 10.1 Depicting Molecules and Ions with Lewis Structures 10.2 Valence-Shell Electron-Pair Repulsion (VSEPR) Theory 10.3 Molecular
A REVIEW OF GENERAL CHEMISTRY: ELECTRONS, BONDS AND MOLECULAR PROPERTIES
 A REVIEW OF GENERAL CEMISTRY: ELECTRONS, BONDS AND MOLECULAR PROPERTIES A STUDENT SOULD BE ABLE TO: 1. Draw Lewis (electron dot and line) structural formulas for simple compounds and ions from molecular
A REVIEW OF GENERAL CEMISTRY: ELECTRONS, BONDS AND MOLECULAR PROPERTIES A STUDENT SOULD BE ABLE TO: 1. Draw Lewis (electron dot and line) structural formulas for simple compounds and ions from molecular
Adapted from CHM 130 Maricopa County, AZ Molecular Geometry and Lewis Dot Formulas Introduction
 Adapted from CHM 130 Maricopa County, AZ Molecular Geometry and Lewis Dot Formulas Introduction A chemical bond is an intramolecular (within the molecule) force holding two or more atoms together. Covalent
Adapted from CHM 130 Maricopa County, AZ Molecular Geometry and Lewis Dot Formulas Introduction A chemical bond is an intramolecular (within the molecule) force holding two or more atoms together. Covalent
Chapter 9 The Shapes of Molecules Cocaine
 Chapter 9 The Shapes of Molecules 1 Cocaine 10.1 Depicting Molecules & Ions with Lewis Structures 2 Number of Covalent Bonds 3 The number of covalent bonds can be determined from the number of electrons
Chapter 9 The Shapes of Molecules 1 Cocaine 10.1 Depicting Molecules & Ions with Lewis Structures 2 Number of Covalent Bonds 3 The number of covalent bonds can be determined from the number of electrons
McCord CH301 Exam 3 Fall 2016
 483 version last name first name signature McCord CH301 Exam 3 Fall 2016 49970 / 49975 Remember that the bubble sheet has the periodic table on the back. NOTE: Please keep your Exam copy intact (all pages
483 version last name first name signature McCord CH301 Exam 3 Fall 2016 49970 / 49975 Remember that the bubble sheet has the periodic table on the back. NOTE: Please keep your Exam copy intact (all pages
Chapter 7 Chemical Bonding and Molecular Structure
 Chapter 7 Chemical Bonding and Molecular Structure Three Types of Chemical Bonding (1) Ionic: formed by electron transfer (2) Covalent: formed by electron sharing (3) Metallic: attraction between metal
Chapter 7 Chemical Bonding and Molecular Structure Three Types of Chemical Bonding (1) Ionic: formed by electron transfer (2) Covalent: formed by electron sharing (3) Metallic: attraction between metal
Ch 6 Chemical Bonding
 Ch 6 Chemical Bonding What you should learn in this section (objectives): Define chemical bond Explain why most atoms form chemical bonds Describe ionic and covalent bonding Explain why most chemical bonding
Ch 6 Chemical Bonding What you should learn in this section (objectives): Define chemical bond Explain why most atoms form chemical bonds Describe ionic and covalent bonding Explain why most chemical bonding
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. How to get the book of
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. How to get the book of
Chapter 4. Molecular Structure and Orbitals
 Chapter 4 Molecular Structure and Orbitals Chapter 4 Table of Contents (4.1) (4.2) (4.3) (4.4) (4.5) (4.6) (4.7) Molecular structure: The VSEPR model Bond polarity and dipole moments Hybridization and
Chapter 4 Molecular Structure and Orbitals Chapter 4 Table of Contents (4.1) (4.2) (4.3) (4.4) (4.5) (4.6) (4.7) Molecular structure: The VSEPR model Bond polarity and dipole moments Hybridization and
CH1410 Practice Exam #3 (Katz) Sp2015
 H1410 Practice Eam #3 (Katz) p2015 ection 1 - Multiple hoice - Write the letter of the ET HIE in the space provided. 1. How many moles of H2 can be made from the complete reaction of 3.0 moles of l? Reaction:
H1410 Practice Eam #3 (Katz) p2015 ection 1 - Multiple hoice - Write the letter of the ET HIE in the space provided. 1. How many moles of H2 can be made from the complete reaction of 3.0 moles of l? Reaction:
CHAPTER TEN MOLECULAR GEOMETRY MOLECULAR GEOMETRY V S E P R CHEMICAL BONDING II: MOLECULAR GEOMETRY AND HYBRIDIZATION OF ATOMIC ORBITALS
 CHAPTER TEN CHEMICAL BONDING II: AND HYBRIDIZATION O ATOMIC ORBITALS V S E P R VSEPR Theory In VSEPR theory, multiple bonds behave like a single electron pair Valence shell electron pair repulsion (VSEPR)
CHAPTER TEN CHEMICAL BONDING II: AND HYBRIDIZATION O ATOMIC ORBITALS V S E P R VSEPR Theory In VSEPR theory, multiple bonds behave like a single electron pair Valence shell electron pair repulsion (VSEPR)
For more info visit Chemical bond is the attractive force which holds various constituents together in a molecule.
 Chemical bond:- Chemical bond is the attractive force which holds various constituents together in a molecule. There are three types of chemical bonds: Ionic Bond, Covalent Bond, Coordinate Bond. Octet
Chemical bond:- Chemical bond is the attractive force which holds various constituents together in a molecule. There are three types of chemical bonds: Ionic Bond, Covalent Bond, Coordinate Bond. Octet
Chapter 12 Structure and Shape
 Free Study Guide for Cracolice Peters Introductory Chemistry: An Active Learning Approach Second Edition www.brookscole.com/chemistry Chapter 12 Structure and Shape Chapter 12Assignment A: Lewis Diagrams
Free Study Guide for Cracolice Peters Introductory Chemistry: An Active Learning Approach Second Edition www.brookscole.com/chemistry Chapter 12 Structure and Shape Chapter 12Assignment A: Lewis Diagrams
Chem 1210 Final Spring points Dr. Luther Giddings
 Chem 1210 Final Spring 2002 150 points Dr. Luther Giddings Name Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam, until
Chem 1210 Final Spring 2002 150 points Dr. Luther Giddings Name Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam, until
Molecular Geometry & Polarity
 Molecular Geometry & Polarity Learn Shapes you will Because the physical and chemical properties of compounds are tied to their structures, the importance of molecular geometry can not be overstated. Localized
Molecular Geometry & Polarity Learn Shapes you will Because the physical and chemical properties of compounds are tied to their structures, the importance of molecular geometry can not be overstated. Localized
Atoms have the ability to do two things in order to become isoelectronic with a Noble Gas.
 CHEMICAL BONDING Atoms have the ability to do two things in order to become isoelectronic with a Noble Gas. 1.Electrons can be from one atom to another forming. Positive ions (cations) are formed when
CHEMICAL BONDING Atoms have the ability to do two things in order to become isoelectronic with a Noble Gas. 1.Electrons can be from one atom to another forming. Positive ions (cations) are formed when
Cannot be determined from this information. A. Incorrect. The electronegativity difference isn t great enough to be an ionic bond.
 AP Chemistry - Problem Drill 14: Chemical Bonding Theories No. 1 of 10 1. What type of bond is H O? (A) (B) (C) (D) (E) Ionic Non-polar Covalent Polar Covalent Metallic Cannot be determined from this information.
AP Chemistry - Problem Drill 14: Chemical Bonding Theories No. 1 of 10 1. What type of bond is H O? (A) (B) (C) (D) (E) Ionic Non-polar Covalent Polar Covalent Metallic Cannot be determined from this information.
2. Write the electron configuration notation and the electron dot notation for each: (a) Ni atom (b) Ni 2+ ion (c) Ni 3+ ion
 EXTRA HOMEWORK 2A 1. Predict whether each of the following types of matter will be bonded with ionic, covalent, or metallic bonds, and identify whether each will be composed of atoms, ions, or molcules
EXTRA HOMEWORK 2A 1. Predict whether each of the following types of matter will be bonded with ionic, covalent, or metallic bonds, and identify whether each will be composed of atoms, ions, or molcules
Chapter 10 Molecular Geometry and Chemical Bonding Theory. Copyright Cengage Learning. All rights reserved. 10 1
 Chapter 10 Molecular Geometry and Chemical Bonding Theory Copyright Cengage Learning. All rights reserved. 10 1 Molecular geometry is the general shape of a molecule, as determined by the relative positions
Chapter 10 Molecular Geometry and Chemical Bonding Theory Copyright Cengage Learning. All rights reserved. 10 1 Molecular geometry is the general shape of a molecule, as determined by the relative positions
REVIEW: VALENCE ELECTRONS CHEMICAL BONDS: LEWIS SYMBOLS: CHEMICAL BONDING. What are valence electrons?
 REVIEW: VALENCE ELECTRONS 13 CHEMICAL BONDING What are valence electrons? Which groups on the periodic table readily give up electrons? What group readily accepts electrons? CHEMICAL BONDS: What are chemical
REVIEW: VALENCE ELECTRONS 13 CHEMICAL BONDING What are valence electrons? Which groups on the periodic table readily give up electrons? What group readily accepts electrons? CHEMICAL BONDS: What are chemical
: O: (1) (2) (3) (4) Page 1 of 6 : : : : : : (8) H H
 Experiment #12 MOLECULAR MODELS An aspect of chemistry, which traditionally proves to be difficult to many students, is the visualization of compounds, ions, and molecules in three dimensional space. In
Experiment #12 MOLECULAR MODELS An aspect of chemistry, which traditionally proves to be difficult to many students, is the visualization of compounds, ions, and molecules in three dimensional space. In
1. There are paired and unpaired electrons in the Lewis symbol for a phosphorus atom. a. 4, 2 b. 2, 4 c. 2, 3 d. 4, 3 e. 0, 3
 Name: Score: 0 / 42 points (0%) [2 open ended questions not graded] C8&9Practice Multiple Choice Identify the choice that best completes the statement or answers the question. 1. There are paired and unpaired
Name: Score: 0 / 42 points (0%) [2 open ended questions not graded] C8&9Practice Multiple Choice Identify the choice that best completes the statement or answers the question. 1. There are paired and unpaired
Unit-3 Chemical Bonding Practice Exam
 Name: Class: _ Date: _ Unit-3 Chemical Bonding Practice Exam Multiple Choice - NO CALCULATORS, show your work and justify your answers. 1. The concentration of a red colored solution of cobalt ions needs
Name: Class: _ Date: _ Unit-3 Chemical Bonding Practice Exam Multiple Choice - NO CALCULATORS, show your work and justify your answers. 1. The concentration of a red colored solution of cobalt ions needs
AP Chemistry - Problem Drill 15: Lewis Structures and VSEPR Theory
 AP Chemistry - Problem Drill 15: Lewis Structures and VSEPR Theory No. 1 of 10 1. Which shape would have sp 3 hybridization? (A) Linear (B) Bent (C) Tetrahedron (D) Trigonal planar (E) Octahedron C. Correct.
AP Chemistry - Problem Drill 15: Lewis Structures and VSEPR Theory No. 1 of 10 1. Which shape would have sp 3 hybridization? (A) Linear (B) Bent (C) Tetrahedron (D) Trigonal planar (E) Octahedron C. Correct.
Covalent bonding does not involve electrostatic attraction between oppositely charged particles.
 SCH3U7 - Topic 4: Bonding Review SL Which of these bonding types would not be classified as strong? Metallic Covalent Ionic Dipole dipole The bond dissociation energy of NaCl is 411 kj mol -1, while that
SCH3U7 - Topic 4: Bonding Review SL Which of these bonding types would not be classified as strong? Metallic Covalent Ionic Dipole dipole The bond dissociation energy of NaCl is 411 kj mol -1, while that
Chapter 9. Molecular Geometries and Bonding Theories. Lecture Presentation. John D. Bookstaver St. Charles Community College Cottleville, MO
 Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
Check Your Solution A comparison with the figures in Figure 4.31 on page 234 of the student textbook confirms the results.
 Predicting the Shape of a Molecule (Student textbook page 236) 11. What molecular shape is represented by each of the following VSEPR notations? a. AX 3 b. AX 5 E You need to assign a molecular shape that
Predicting the Shape of a Molecule (Student textbook page 236) 11. What molecular shape is represented by each of the following VSEPR notations? a. AX 3 b. AX 5 E You need to assign a molecular shape that
Test bank for Chemistry The Central Science 10th Edition by Brown, LeMay, Bursten
 Test bank for Chemistry The Central Science 10th Edition by Brown, LeMay, Bursten Chapter 9, Molecular Geometry and Bonding Theories Multiple-Choice and Bimodal 1) For a molecule with the formula A) linear
Test bank for Chemistry The Central Science 10th Edition by Brown, LeMay, Bursten Chapter 9, Molecular Geometry and Bonding Theories Multiple-Choice and Bimodal 1) For a molecule with the formula A) linear
