1. Write date, topic and purpose in notebook. 2. Answer in your notebook: What are two signs that a chemical reaction is happening?
|
|
|
- Gladys Morton
- 6 years ago
- Views:
Transcription
1 Warm Up (5 min) Date: Monday, 1/4/16 Topic: Alka Seltzer Lab Purpose: To assess the Law of Conservation of Mass by designing an experiment to determine mass before and after a chemical reaction. 1. Write date, topic and purpose in notebook. 2. Answer in your notebook: What are two signs that a chemical reaction is happening? Nov 15 3:48 PM Quality Review Visitors from the Department of Education Looking for: What we do at IS5 Leadership/7 Habits Notebooks/Folders What you should do: If they talk to you, talk to them! Do what you normally do in class Tell them about what we are learning in class Be on your best behavior Jan 4 7:23 AM 1
2 Folder Prep Everyone must have a folder! Tests, labs, all self reflections completed Jan 4 8:00 AM Demonstration: What happens when the alka seltzer is placed in water? Write down observations. Be descriptive! Initial Observation: "Some matter disappears in a chemical change." Oct 29 7:26 AM 2
3 Research: The Law of Conservation of Mass (click link for BRAINPOP video) Oct 29 7:35 AM Important terms: Reactants > Chemical Reaction > Products Chemical Reaction: Reactants: substances that take part in and undergo changes during a reaction. (Chemical Change) the processes by which chemicals interact to form new chemicals with different compositions. Products: substances that form as the result of a chemical reaction. Oct 29 7:19 AM 3
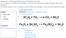
4 Problem: How does a chemical change affect the mass of the products (g) of an alka seltzer tablet and water reaction? IV: DV: chemical change mass (g) Oct 29 7:38 AM Background Information Alka Seltzer is a medical drug that works as a pain reliever and an antacid (antacids help neutralize stomach acidity e.g., heartburn). The pain reliever used is aspirin and the antacid used is baking soda (sodium bicarbonate, NaHCO 3 ). To take the tablets, you should fully dissolve them in a glass of water, where they famously undergo a chemical reaction that produces lots of carbon dioxide (CO 2 ) bubbles. As the tablets dissolve, the sodium bicarbonate splits apart to form bicarbonate (HCO 3 ). This reacts with hydrogen (H + ) from citric acid, another ingredient in the tablets. This chemical reaction forms water and carbon dioxide gas. 3HCO3 +3H+ 3H2O+3CO2 Oct 29 9:27 AM 4
5 Homework Finish IV/DV Finish hypothesis with research! Dec 24 7:50 PM Warm Up (5 min) Date: Tuesday, 1/5/2016 Topic: Alka Seltzer Lab Purpose: To assess the Law of Conservation of Mass by designing an experiment to determine mass before and after a chemical reaction. 1. Write date, topic and purpose in notebook. You may write Continued. 2. Take out your annotated background information. Begin writing your hypothesis Hypothesis: If we cause a chemical change using alka seltzer and water then the mass of the products will be (greater than, less than or equal to) the mass of the reactants because. Nov 15 3:48 PM 5
6 Hypothesis: If we cause a chemical change using alka seltzer and water then the mass of the products will be (greater than, less than or equal to) the mass of the reactants because. Nov 6 8:40 AM Warm Up (5 min) Date: Tuesday, 1/5/2016 Topic: Alka Seltzer Lab Purpose: To assess the Law of Conservation of Mass by designing an experiment to determine mass before and after a chemical reaction. 1. Write date, topic and purpose in notebook. You may write Continued. 2. Take out your hypothesis and review it with someone at your table. If we cause a chemical change using alkaseltzer and water then the mass of the products will be (greater than, less than or equal to) the mass of the reactants because. Nov 15 3:48 PM 6
7 Discussion Leader Share 3 4 hypothesis Make sure you are giving evidence in your "because" statement! Jan 4 5:27 PM Lab Demo Watch carefully! Nov 19 7:30 AM 7
8 At your table, write down your materials. Hint: see pictures. Materials: 1. Flask 2. Triple Beam Balance (TBB) 3. Alka-Seltzer 4. Water 5. Goggles 6. Balloons 7. stopwatch Oct 29 7:48 AM Warm Up (5 min) Date: Wednesday, 1/6/2016 Topic: Alka Seltzer Lab Purpose: To assess the Law of Conservation of Mass by designing an experiment to determine mass before and after a chemical reaction. 1. Write date, topic and purpose in notebook. You may write Continued. 2. Take out your procedures Nov 15 3:48 PM 8
9 With your group, write your procedure. Make sure that your procedure includes: finding the mass of the tools finding the mass of the tools and reactants finding the mass of the tools and products Multiple trials (3) Dec 24 8:01 PM A What step is this? B What step is this? With your group, write your procedure. C What step is this? E What step is this? D What step is this? Make sure that your procedure includes: finding the mass of the tools finding the mass of the tools and reactants finding the mass of the tools and products Multiple trials (3) Nov 15 4:30 PM 9
10 Procedure: 1. Wear appropriate safety gear (goggles). 2. Measure the mass of the flask and balloon. Record this in data table: Mass of tools. 3. Pour 100ml of water into the flask. 4. Calculate the entire mass of the flask, 100 ml of water, tablet and balloon. Record this in the data table: Mass of tools and Reactants. 5. Place the tablet into the balloon and carefully attach the balloon to the opening of the flask without dropping the tablet into the water. 6. Get the stopwatch ready. 7. When ready, drop tablet into the water. 8. When the reaction stops stop the stopwatch and record the time. Calculate the mass of the entire contraption. Record this in the data table: Mass of tools and Products. 9. Repeat steps 1 8 two more times. Nov 6 8:42 AM Looking at your procedure: 1. Underline all the data you must gather/record. 2. Create a data table that reflects the required data using the template. Mass of Tools (balloon and flask) Mass of Tools and Reactants Mass of Tools and Products Include a title Include 3 trials Nov 15 4:19 PM 10
11 Closing (5 min): Data Table Review Share group data table on ELMO Explain your labeling: what data are you collecting? Dec 24 8:09 PM Data: The effect of a chemical change on the mass of alka seltzer and water (g) (0 minutes) (3 minutes) Trial Mass of tools: flask & balloon Mass of tools & Reactants: flask with 100 ml of water & balloon with tablet Mass of tools & Products: flask with water & balloon with carbon dioxide gas Difference in Mass of tools Reactants and Products: Oct 29 9:04 AM 11
12 Homework Complete Procedure (if needed) Complete data table (if needed) Bring graph paper Nov 15 4:28 PM Warm Up (5 min) Date: Friday, 1/8/2016 Topic: Alka Seltzer Lab Purpose: To assess the Law of Conservation of Mass by designing an experiment to determine mass before and after a chemical reaction. 1. Write date, topic and purpose in notebook. 2. Take out procedure, data table, notebook/pencil and clear desk. Nov 15 3:48 PM 12
13 Data: The effect of a chemical reaction on mass (g) (0 minutes) (----- minutes) Trial Mass of tools: flask & balloon Mass of tools & Reactants: flask with 100 ml of water & balloon with tablet Mass of tools & Products: flask with water & balloon with carbon dioxide gas Difference in Mass of tools Reactants and Products: Oct 29 9:04 AM Procedure: Wear appropriate safety gear (goggles). 2. Measure the mass of the flask and balloon. Record this in data table: Mass of tools. 3. Pour 100ml of water into the flask. 4. Calculate the entire mass of the flask, 100 ml of water, tablet and balloon. Record this in the data table: Mass of tools and Reactants. 5. Place the tablet into the balloon and carefully attach the balloon to the opening of the flask without dropping the tablet into the water. 6. Get the stopwatch ready. 7. When ready, drop tablet into the water. 8. When the reaction stops stop the stopwatch and record the time. Calculate the mass of the entire contraption. Record this in the data table: Mass of tools and Products. 9. Repeat steps 1 8 two more times. Nov 6 8:42 AM 13
14 Data: The effect of a chemical reaction on mass (g) (0 minutes) (----- minutes) Trial Mass of tools: flask & balloon Mass of tools & Reactants: flask with 100 ml of water & balloon with tablet Mass of tools & Products: flask with water & balloon with carbon dioxide gas Difference in Mass of tools Reactants and Products: Oct 29 9:04 AM Clean Up! 1: Balloons in trash 2: Liquid in Ms. Brunner's sink, rinse flasks 3: TBB on back counter, water bottles on back counter 4: Goggles in drawer Nov 15 5:09 PM 14
15 Warm Up (5 min) Date: Friday, 1/8/16 Topic: Alka Seltzer Lab 4 Purpose: To assess the Law of Conservation of Mass by determining mass before and after a chemical reaction. 1. Write date, topic and purpose in notebook. 2. In our experiment, what were the reactants? What were the products? 3. Take out data and graph paper. Nov 15 3:48 PM Copy the data table in your notebook: Trial Number Mass of Reactants (g) Mass of Products (g) Jan 7 4:34 PM 15
16 To find the mass of the reactants and the mass of the products for each trial... Calculate the Mass of the JUST the Reactants: Mass of tools and Reactants (column 2) Mass of tools (column 1) = Mass of Reactants = Calculate the Mass of the JUST the Products: Mass of tools and Products (column 3) Mass of tools (column 1) = Mass of Products = Trial Number Mass of Reactants (g) Mass of Products (g) Nov 23 6:31 PM The Law of Conservation of Mass Mass of Reactants = Mass of Products Nov 23 6:40 PM 16
17 Line Graph 1 line for each trial Use a different shape/color for each Make a key Mass (grams) Trial 1 Trial 2 The effect of a chemical change on mass (g) Trial Trial 3 Oct 29 11:20 AM Warm Up (5 min) Date: Friday, 1/8/16 Topic: Alka Seltzer Lab 4 Purpose: To assess the Law of Conservation of Mass by determining mass before and after a chemical reaction. 1. Write date, topic and purpose in notebook. 2. Take out your graphs. Nov 15 3:48 PM 17
18 Line Graph 1 line for each trial Use a different shape/color for each Make a key Mass (grams) Mass of Reactants) (BEFORE) 0 minutes The effect of a chemical change on mass (g) Mass of Products (AFTER) 3 minutes Chemical Change Oct 29 11:20 AM Data Analysis Describe the trends in your data using words and numbers. For example: In our lab we noticed that the mass of the reactants was (less than, greater than, equal to) as the mass of the products. The mass of our reactants in trial one was. The mass of our products in trial one was etc... Describe what your data MEANS using observations and evidence. Use your background information about Law of Conservation of Mass to explain. This should all be about 5 6 sentences. Nov 16 4:39 PM 18
19 Conclusion: Restate (rewrite) your hypothesis. Was you hypothesis supported? Explain how you know using numbers and words. What does your data mean? Use evidence from research and data to explain your findings. Ask at least one further investigation question. This should be relevant to the lab! Maybe something about Law of Conservation of Mass, chemical reactions, changing our lab to test something new Nov 6 8:49 AM Nov 10 2:07 PM 19
20 Limitations (Sources of Error) What errors could have happened in your lab that would have affected your data? Ex: There was a hole in the side of our balloon, so the carbon dioxide gas was able to leak out. This could have lowered the mass because we are losing matter. You must have at least one and must explain how it connects to your data! Jan 5 5:13 PM WRITE THESE DOWN! Follow Up Questions: 1. What is the relationship between chemical reactions and mass? 2. What kind of change took place when the alka seltzer tablets were added to the water? (explain with evidence) Identify the Products and Reactants that were part of this chemical reaction. 3. Do you think matter can be created, destroyed, neither or both after this investigation? (explain with evidence) YOU MUST WRITE OUT ALL FOLLOW UP QUESTIONS IN YOUR LAB REPORTS!!!!!!!!!!!!!!!!!!!! Oct 29 9:52 AM 20
21 Closing: Continue working on conclusion, graph, etc... Completed Alka Seltzer Lab due Wednesday, January 13! FOLLOW THE RUBRIC!!!!!!!!!!! Nov 4 7:24 AM 726 Procedure: underline the materials in your procedure 1 Wear appropriate safety gear (goggles). A What step is this? 2 Measure the mass of the flask and balloon. Record this in data table: Mass of tools. C What step is this? 3 Pour 100ml of water into the flask. 4 Calculate the entire mass of the flask, 100 ml of water, tablet and balloon. Record this in the data table: Mass of tools and Reactants. 5 Place the tablet into the balloon and carefully attach the balloon to the opening of the flask without dropping the tablet into the water. 6 Get the stopwatch ready. 7 When ready, drop tablet into the water. 8 When the reaction stops stop the stopwatch and record the time. Calculate the mass of the entire contraption. Record this in the data table: Mass of tools and Products. 9 Repeat steps 1 8 two more times. E What step is this? B What step is this? D What step is this? Oct 29 7:30 AM 21
22 Data: (continued) Send one person from your group to write in your masses: Chemical Reactions and Conservation of Mass Class Data Group Mass of Reactants Mass of Products What do you notice??? WHY??? Oct 29 9:56 AM Homework: Read pages in your textbook. Answer the 3 Self Check questions. DUE FRIDAY! Nov 16 4:51 PM 22
Moles Lab Activity 1: PCU (Popcorn Counting Units)
 Moles Lab Activity 1: PCU (Popcorn Counting Units) Materials: A container of each of the following: Popcorn kernels Another type of beans A large unopened bag of popcorn Kernels Balance Safety goggles
Moles Lab Activity 1: PCU (Popcorn Counting Units) Materials: A container of each of the following: Popcorn kernels Another type of beans A large unopened bag of popcorn Kernels Balance Safety goggles
Bellevue College CHEM& 121 Experiment: Stoichiometric Analysis of an Antacid 1
 Experiment: Stoichiometric Analysis of an Antacid 1 Introduction In this lab, you will use the concept of stoichiometry to solve two sequential problems. First, you will try to determine the products of
Experiment: Stoichiometric Analysis of an Antacid 1 Introduction In this lab, you will use the concept of stoichiometry to solve two sequential problems. First, you will try to determine the products of
CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED)
 CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED) Overview: Students investigate chemical reactions using given substances. Students identify chemical reactions in their daily lives. Objectives: The student
CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED) Overview: Students investigate chemical reactions using given substances. Students identify chemical reactions in their daily lives. Objectives: The student
Student Notes. Chemical Reactions LINK
 LCPS Core Experience Chemical Reactions Student Notes OBJECTIVES Students will: investigate the relationship between reactants and products. investigate an exothermic reaction. investigate an endothermic
LCPS Core Experience Chemical Reactions Student Notes OBJECTIVES Students will: investigate the relationship between reactants and products. investigate an exothermic reaction. investigate an endothermic
Alka Seltzer Lab: Reaction rate - Factors that affect the rate of chemical reactions
 Alka Seltzer Lab: Reaction rate - Factors that affect the rate of chemical reactions Materials Lead Scribe Reporter/Questioner Clean-up WRITE ALL NOTES / DATA / OBSERVATIONS ON YOUR DATA SHEET The rate
Alka Seltzer Lab: Reaction rate - Factors that affect the rate of chemical reactions Materials Lead Scribe Reporter/Questioner Clean-up WRITE ALL NOTES / DATA / OBSERVATIONS ON YOUR DATA SHEET The rate
JHS Regents Chemistry Department Rates of Chemical Reactions Inquiry Investigation
 Name: Date: Partner: Period: JHS Regents Chemistry Department Rates of Chemical Reactions Inquiry Investigation Introduction: According to the collision theory, the rate of a reaction depends on the frequency
Name: Date: Partner: Period: JHS Regents Chemistry Department Rates of Chemical Reactions Inquiry Investigation Introduction: According to the collision theory, the rate of a reaction depends on the frequency
Chapter 6, Lesson 7: Energy Changes in Chemical Reactions
 Chapter 6, Lesson 7: Energy Changes in Chemical Reactions Key Concepts If two substances react and the temperature of the mixture decreases, the reaction is endothermic. If two substances react and the
Chapter 6, Lesson 7: Energy Changes in Chemical Reactions Key Concepts If two substances react and the temperature of the mixture decreases, the reaction is endothermic. If two substances react and the
Name Period Date. Lab 1: Mass of Ice Materials: beaker, ice and balance.
 Name Period Date Testing the Law of Conservation of MASS! Introduction: Does mass change in a chemical or physical reaction? In this series of experiments you will find the answer to this question. Lab
Name Period Date Testing the Law of Conservation of MASS! Introduction: Does mass change in a chemical or physical reaction? In this series of experiments you will find the answer to this question. Lab
Lab Activity 3: Factors Affecting Reaction Rate
 Chemistry 3202 Lab #3 factors affecting Reaction Rate Page 1 of 5 Lab Activity 3: Factors Affecting Reaction Rate Introduction Several factors influence how fast a reaction proceeds. In this activity,
Chemistry 3202 Lab #3 factors affecting Reaction Rate Page 1 of 5 Lab Activity 3: Factors Affecting Reaction Rate Introduction Several factors influence how fast a reaction proceeds. In this activity,
Limiting Reactants Lab
 Name: Teacher s Name: Class: Block: Date: Partners: Limiting Reactants Lab Purpose: Through experimentation, determine the limiting reactant and the percent yield in a chemical reaction that generates
Name: Teacher s Name: Class: Block: Date: Partners: Limiting Reactants Lab Purpose: Through experimentation, determine the limiting reactant and the percent yield in a chemical reaction that generates
Chapter 6, Lesson 9: Neutralizing Acids and Bases
 Chapter 6, Lesson 9: Neutralizing Acids and Bases Key Concepts ph is a measure of the concentration of H 3 O + ions in a solution. Adding an acid increases the concentration of H 3 O + ions in the solution.
Chapter 6, Lesson 9: Neutralizing Acids and Bases Key Concepts ph is a measure of the concentration of H 3 O + ions in a solution. Adding an acid increases the concentration of H 3 O + ions in the solution.
MONDAY (12/12) TUESDAY (12/13) WEDNESDAY (12/14) THURSDAY (12/15) FRIDAY (12/16) Making Acid Rain (a lab) Quiz
 Homework Activities Name: Date: Period: This week, we will be using our knowledge of acids and bases and studying how acids, specifically acid rain, affect our lives and our environment. We will also end
Homework Activities Name: Date: Period: This week, we will be using our knowledge of acids and bases and studying how acids, specifically acid rain, affect our lives and our environment. We will also end
Lesson 2. Color change
 Lesson 2 Color change T E A C H E R G U I D E Lesson summary Students meet marine chemist Sera Tuikabe, who is studying ocean acidification in the water surrounding the Republic of the Fiji Islands. Students
Lesson 2 Color change T E A C H E R G U I D E Lesson summary Students meet marine chemist Sera Tuikabe, who is studying ocean acidification in the water surrounding the Republic of the Fiji Islands. Students
INTRODUCTION. Seltzer relieves upset stomach, provides pain relief, breaks fevers, and reduces inflammation.
 Effervescence Laboratory Experiment Developed by: Alex Jannini, David Krause, Heather Malino and Kevin Sweeney, Rowan University, Department of Chemical Engineering Edited by: C. Stewart Slater and Mariano
Effervescence Laboratory Experiment Developed by: Alex Jannini, David Krause, Heather Malino and Kevin Sweeney, Rowan University, Department of Chemical Engineering Edited by: C. Stewart Slater and Mariano
Bags of Reactions Chemistry I Acc
 Introduction: Bags of Reactions Chemistry I Acc Plop, plop, fizz, fizz, oh, what a relief it is. claims the old TV ad for a popular antacid. Just what is in the tablet that is relieving the upset stomach?
Introduction: Bags of Reactions Chemistry I Acc Plop, plop, fizz, fizz, oh, what a relief it is. claims the old TV ad for a popular antacid. Just what is in the tablet that is relieving the upset stomach?
Safety: Safety goggles should be worn at all times. Students should hold the balloons on the test tubes tightly while the reaction takes place.
 LIMITING REAGENT LAB: THE REACTION BETWEEN VINEGAR AND BAKING SODA Goal: During this lab students will gain a quantitative understanding of limiting reagents. Safety: Safety goggles should be worn at all
LIMITING REAGENT LAB: THE REACTION BETWEEN VINEGAR AND BAKING SODA Goal: During this lab students will gain a quantitative understanding of limiting reagents. Safety: Safety goggles should be worn at all
CO 2. Lesson 1. Production of a gas
 Lesson 1 Production of a gas T E A C H E R G U I D E CO 2 Lesson summary Students meet volcanologist Victor Helguson, who is studying the gases released by volcanoes in Iceland. Students conduct a chemical
Lesson 1 Production of a gas T E A C H E R G U I D E CO 2 Lesson summary Students meet volcanologist Victor Helguson, who is studying the gases released by volcanoes in Iceland. Students conduct a chemical
5 th Grade Lesson Plan: Matter and Chemical Reactions
 5 th Grade Lesson Plan: Matter and Chemical Reactions Objective: Teach students that matter is neither created nor destroyed during a chemical reaction, rather, it is transformed. Identify evidence that
5 th Grade Lesson Plan: Matter and Chemical Reactions Objective: Teach students that matter is neither created nor destroyed during a chemical reaction, rather, it is transformed. Identify evidence that
Experiment 8 - Chemical Changes
 Experiment 8 - Chemical Changes When a chemical change occurs, the chemicals that you start with are changed into different chemicals. We know when this happens because the new chemicals have different
Experiment 8 - Chemical Changes When a chemical change occurs, the chemicals that you start with are changed into different chemicals. We know when this happens because the new chemicals have different
MiSP Chemical Reactions Lab Temperature L2. Plop, Plop, Fizz, Fizz... Temperature and Rate of Reaction Activity
 MiSP Chemical Reactions Lab Temperature L2 Name Date Plop, Plop, Fizz, Fizz... Temperature and Rate of Reaction Activity Introduction: Problem: Alka-Seltzer in water produces carbon dioxide. It is the
MiSP Chemical Reactions Lab Temperature L2 Name Date Plop, Plop, Fizz, Fizz... Temperature and Rate of Reaction Activity Introduction: Problem: Alka-Seltzer in water produces carbon dioxide. It is the
Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic
 Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic Key Concepts Carbon dioxide (CO 2 ) gas dissolved in water can cause water to become acidic. The acidity of water from dissolved CO 2 can
Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic Key Concepts Carbon dioxide (CO 2 ) gas dissolved in water can cause water to become acidic. The acidity of water from dissolved CO 2 can
Research Experiences for Teachers (RET) 2015 LESSON PLAN TEMPLATE
 LESSON PLAN TEMPLATE LESSON TOPIC: Molarity of a solution STANDARD(S) & INDICATOR(S): CCSS Mathematics: HSF-IF. Interpret functions that arise in applications in terms of the context. OBJECTIVE(S): Students
LESSON PLAN TEMPLATE LESSON TOPIC: Molarity of a solution STANDARD(S) & INDICATOR(S): CCSS Mathematics: HSF-IF. Interpret functions that arise in applications in terms of the context. OBJECTIVE(S): Students
Goal: During this lab students will gain a quantitative understanding of limiting reagents.
 LIMITING REAGENT LAB: THE REACTION BETWEEN VINEGAR AND BAKING SODA Goal: During this lab students will gain a quantitative understanding of limiting reagents. Safety: Safety goggles should be worn at all
LIMITING REAGENT LAB: THE REACTION BETWEEN VINEGAR AND BAKING SODA Goal: During this lab students will gain a quantitative understanding of limiting reagents. Safety: Safety goggles should be worn at all
MEASURING THE RATE OF PHOTOSYNTHESIS
 MEASURING THE RATE OF PHOTOSYNTHESIS QUESTION: What factors affect the rate of photosynthesis in plants? BACKGROUND KNOWLEDGE Leaf disks float, normally. When the air spaces in the leaf are filled with
MEASURING THE RATE OF PHOTOSYNTHESIS QUESTION: What factors affect the rate of photosynthesis in plants? BACKGROUND KNOWLEDGE Leaf disks float, normally. When the air spaces in the leaf are filled with
LAB 1B: THE SCIENTIFIC METHOD: MAKING OBSERVATIONS & DESIGNING EXPERIMENTS
 LAB 1B: THE SCIENTIFIC METHOD: MAKING OBSERVATIONS & DESIGNING EPERIMENTS PURPOSE: To practice the scientific method of observation, hypothesis, experimentation, & conclusion. SAFETY CONCERNS: Always wear
LAB 1B: THE SCIENTIFIC METHOD: MAKING OBSERVATIONS & DESIGNING EPERIMENTS PURPOSE: To practice the scientific method of observation, hypothesis, experimentation, & conclusion. SAFETY CONCERNS: Always wear
Chemical Reactions Investigation Two Data Record
 Chemical Reactions Investigation Two Data Record Name: Date: 1. During this Investigation, you will analyze how changing the amounts of the reactants in a chemical reaction affects the amount of the products
Chemical Reactions Investigation Two Data Record Name: Date: 1. During this Investigation, you will analyze how changing the amounts of the reactants in a chemical reaction affects the amount of the products
2/22/2019 NEW UNIT! Chemical Interactions. Atomic Basics #19
 NEW UNIT! Chemical Interactions Atomic Basics #19 1 Vocabulary: Matter: Anything that has mass and takes up space. Atom: the smallest particle of matter. Element: A pure substance made up of only one type
NEW UNIT! Chemical Interactions Atomic Basics #19 1 Vocabulary: Matter: Anything that has mass and takes up space. Atom: the smallest particle of matter. Element: A pure substance made up of only one type
Physical and Chemical Changes Or How Do You Know When You ve Made Something New?
 Introduction Or How Do You Know When You ve Made Something New? Remember that all matter has characteristic physical and chemical properties. Matter can also undergo physical and chemical changes. How
Introduction Or How Do You Know When You ve Made Something New? Remember that all matter has characteristic physical and chemical properties. Matter can also undergo physical and chemical changes. How
Photosynthesis Lab. (adapted from: Photosynthesis.pdf )
 Photosynthesis Lab (adapted from: http://media.collegeboard.com/digitalservices/pdf/ap/bio-manual/bio_lab5- Photosynthesis.pdf ) Background and PreLab: Photosynthesis fuels ecosystems and replenishes the
Photosynthesis Lab (adapted from: http://media.collegeboard.com/digitalservices/pdf/ap/bio-manual/bio_lab5- Photosynthesis.pdf ) Background and PreLab: Photosynthesis fuels ecosystems and replenishes the
ENTHALPY OF FORMATION OF MgO
 ENTHALPY OF FORMATION OF MgO ELECTRONIC LABORATORY NOTEBOOK (ELN) INSTRUCTIONS All work for this experiment must be recorded, attached, or answered in the ELN. Create a pre & inlab page in the Experiment
ENTHALPY OF FORMATION OF MgO ELECTRONIC LABORATORY NOTEBOOK (ELN) INSTRUCTIONS All work for this experiment must be recorded, attached, or answered in the ELN. Create a pre & inlab page in the Experiment
MiSP Chemical Reactions Lab Temperature L3. Plop, Plop, Fizz, Fizz... Temperature and Rate of Reaction Activity
 MiSP Chemical Reactions Lab Temperature L3 Name Date Plop, Plop, Fizz, Fizz... Temperature and Rate of Reaction Activity Introduction: Problem: Alka-Seltzer in water produces carbon dioxide. It is the
MiSP Chemical Reactions Lab Temperature L3 Name Date Plop, Plop, Fizz, Fizz... Temperature and Rate of Reaction Activity Introduction: Problem: Alka-Seltzer in water produces carbon dioxide. It is the
Lab 2-Investigating the Law of Conservation of Mass
 Name: Period: Lab 2-Investigating the Law of Conservation of Mass Objective: To corroborate the law of conservation of mass through laboratory experimentation. Background: When wood burns or water evaporates,
Name: Period: Lab 2-Investigating the Law of Conservation of Mass Objective: To corroborate the law of conservation of mass through laboratory experimentation. Background: When wood burns or water evaporates,
KEY. Chemistry Baseline Cornerstone Assessment: Part A. Experimental Design. Directions: Read the paragraph below and then respond to the questions.
 Chemistry Baseline Cornerstone Assessment: Part A. Experimental Design Directions: Read the paragraph below and then respond to the questions. Hydrogen peroxide is a liquid that breaks down and releases
Chemistry Baseline Cornerstone Assessment: Part A. Experimental Design Directions: Read the paragraph below and then respond to the questions. Hydrogen peroxide is a liquid that breaks down and releases
Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise.
 Experiment 10 Stoichiometry- Gravimetric Analysis Pre-lab Assignment Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose The purpose this experiment
Experiment 10 Stoichiometry- Gravimetric Analysis Pre-lab Assignment Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose The purpose this experiment
Lesson Plan Book-stacking Activity
 T o g o d i r e c t l y t o a l e s s o n, c l i c k o n e o f t h e f o l l o w i n g l i n k s : B o o k - s t a c k i n g A c t i v i t y B a l l o o n A c t i v i t y H y d r o g e n G a s L a b F
T o g o d i r e c t l y t o a l e s s o n, c l i c k o n e o f t h e f o l l o w i n g l i n k s : B o o k - s t a c k i n g A c t i v i t y B a l l o o n A c t i v i t y H y d r o g e n G a s L a b F
Post-Show. Chemistry. Periodic Table of the Elements. After the Show. Traveling Science Shows
 Traveling Science Shows Post-Show Chemistry After the Show We recently presented a Chemistry show at your school, and thought you and your students might like to continue investigating this topic. The
Traveling Science Shows Post-Show Chemistry After the Show We recently presented a Chemistry show at your school, and thought you and your students might like to continue investigating this topic. The
Name: Hour: Photosynthesis in Leaf Disks
 Name: Hour: Photosynthesis in Leaf Disks Safety Information: While the solutions may be handled without gloves and may be disposed of in the sink drains, goggles must be worn during the experiment. Background
Name: Hour: Photosynthesis in Leaf Disks Safety Information: While the solutions may be handled without gloves and may be disposed of in the sink drains, goggles must be worn during the experiment. Background
Lab #9 Stoichiometric Relationships & Theoretical Yield
 Stoichiometric Relationships & Theoretical Yield Name: Nov. 28, 2016 Background and Purpose: Carbonates when reacted with acids will produce carbon dioxide, water and an ionic salt. In this lab, we will
Stoichiometric Relationships & Theoretical Yield Name: Nov. 28, 2016 Background and Purpose: Carbonates when reacted with acids will produce carbon dioxide, water and an ionic salt. In this lab, we will
Chemistry. Baseline Cornerstone Assessment
 Chemistry Baseline Cornerstone Assessment The Cornerstone Assessments were developed with support through the VDOE Mathematics and Science Partnership Grant Program NCLB Title II, Part B program by high
Chemistry Baseline Cornerstone Assessment The Cornerstone Assessments were developed with support through the VDOE Mathematics and Science Partnership Grant Program NCLB Title II, Part B program by high
Chapter 6, Lesson 1: What is a Chemical Reaction?
 Chapter 6, Lesson 1: What is a Chemical Reaction? Key Concepts: A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. In a chemical reaction,
Chapter 6, Lesson 1: What is a Chemical Reaction? Key Concepts: A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. In a chemical reaction,
Name Period Date. Lab: Introduction to Stoichiometry
 Name Period Date Lab: Introduction to Stoichiometry Introduction: Reactants are not always present in the exact ratio required by a balanced chemical equation. In planning any cost-effective production
Name Period Date Lab: Introduction to Stoichiometry Introduction: Reactants are not always present in the exact ratio required by a balanced chemical equation. In planning any cost-effective production
Physical & Chemical PROPERTIES
 Name Test Date Hour Group Chem #4 Notebook Properties & Changes LEARNING TARGETS I can give examples of physical and chemical properties. I can give examples of physical and chemical changes. I can identify
Name Test Date Hour Group Chem #4 Notebook Properties & Changes LEARNING TARGETS I can give examples of physical and chemical properties. I can give examples of physical and chemical changes. I can identify
Friday, November 2, 2018 GLE/Standard: The fact that atoms are conserved, together
 Mrs. Chausse s Physical Science Gifted Lesson Plan: Unit 7 Chemical Reactions November 1 16, 2018 Thursday, November 1, 2018 Objective: SWBAT balance a chemical equation that satisfies the Law of Conservation
Mrs. Chausse s Physical Science Gifted Lesson Plan: Unit 7 Chemical Reactions November 1 16, 2018 Thursday, November 1, 2018 Objective: SWBAT balance a chemical equation that satisfies the Law of Conservation
Name Partner Lab Section M Tu W Th F Chemistry 130 Experiment 6: Titration and Analysis
 Name Partner Lab Section M Tu W Th F Chemistry 130 Experiment 6: Titration and Analysis Introduction A neutralization reaction is one in which an acid (proton donor) and a base (proton acceptor) react
Name Partner Lab Section M Tu W Th F Chemistry 130 Experiment 6: Titration and Analysis Introduction A neutralization reaction is one in which an acid (proton donor) and a base (proton acceptor) react
Chemical Reactions Investigation Two Data Record
 Chemical Reactions Investigation Two Data Record Name: Date: 1. During this Investigation, you will analyze how changing the amounts of the reactants in a chemical reaction affects the amount of the products
Chemical Reactions Investigation Two Data Record Name: Date: 1. During this Investigation, you will analyze how changing the amounts of the reactants in a chemical reaction affects the amount of the products
Physical Changes vs Chemical Changes Lab
 Physical Changes vs Chemical Changes Lab What was done? What can you observe? Is it a physical or chemical change and why? What else have you learned during the demonstration? #1) Crumpling Paper Notice
Physical Changes vs Chemical Changes Lab What was done? What can you observe? Is it a physical or chemical change and why? What else have you learned during the demonstration? #1) Crumpling Paper Notice
Objective: SWBAT identify a compound as either ionic, covalent or an acid after reviewing terminology at 85% accuracy.
 Mrs. Chausse s Chemistry Gifted Lesson Plan: Unit 11 Chemical Reactions January 4 18, 2018 Wednesday, January 3, 2018 Naming and writing formulas Objective: SWBAT identify a compound as either ionic, covalent
Mrs. Chausse s Chemistry Gifted Lesson Plan: Unit 11 Chemical Reactions January 4 18, 2018 Wednesday, January 3, 2018 Naming and writing formulas Objective: SWBAT identify a compound as either ionic, covalent
Maintaining Mass Created By: Allyn Short
 Maintaining Mass Created By: Allyn Short Focus on Inquiry The student will demonstrate that mass is conserved when substances undergo chemical and/or physical changes through experimentation and evaluation
Maintaining Mass Created By: Allyn Short Focus on Inquiry The student will demonstrate that mass is conserved when substances undergo chemical and/or physical changes through experimentation and evaluation
Activity Template. Drexel-SDP GK-12 ACTIVITY
 Activity Template Drexel-SDP GK-12 ACTIVITY Subject Area(s): Sound Associated Unit: None Associated Lesson: None Activity Title: Density and Pitch, is there a relationship? Grade Level: 8 (7-9) Activity
Activity Template Drexel-SDP GK-12 ACTIVITY Subject Area(s): Sound Associated Unit: None Associated Lesson: None Activity Title: Density and Pitch, is there a relationship? Grade Level: 8 (7-9) Activity
Experiment 17 It s A Gas and More!
 Energy Energy Experiment 17 It s A Gas and More! OUTCOMES After completing this lab activity, the student should be able to: explain a simple method for distinguishing carbon dioxide gas from oxygen gas.
Energy Energy Experiment 17 It s A Gas and More! OUTCOMES After completing this lab activity, the student should be able to: explain a simple method for distinguishing carbon dioxide gas from oxygen gas.
Photosynthesis. Introduction: Objectives:
 Photosynthesis Introduction: Photosynthesis is a process in which plants convert light energy (sunlight) into usable chemical energy (carbohydrates). Photosynthesis involves two simultaneous processes:
Photosynthesis Introduction: Photosynthesis is a process in which plants convert light energy (sunlight) into usable chemical energy (carbohydrates). Photosynthesis involves two simultaneous processes:
STEMscopedia: PHYSICAL AND CHEMICAL PROPERTIES AND CHANGES 8P1CD
 Reflect STEMscopedia: PHYSICAL AND CHEMICAL 8P1CD What do ice cream, root beer, and carbon dioxide gas have in common? Not only do these ingredients combine to make a good treat on a hot summer day, but
Reflect STEMscopedia: PHYSICAL AND CHEMICAL 8P1CD What do ice cream, root beer, and carbon dioxide gas have in common? Not only do these ingredients combine to make a good treat on a hot summer day, but
Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from
 Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from http://www.csun.edu/chemteach/activities/beginninganalysis.pdf ] In this lab, you will be observing physical and chemical properties
Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from http://www.csun.edu/chemteach/activities/beginninganalysis.pdf ] In this lab, you will be observing physical and chemical properties
Activity Sheet Chapter 6, Lesson 10 Carbon Dioxide Can Make a Solution Acidic
 Activity Sheet Chapter 6, Lesson 10 Carbon Dioxide Can Make a Solution Acidic Name Date DEMONSTRATION 1. Your teacher blew through a straw into a universal indicator solution until it changed color. Did
Activity Sheet Chapter 6, Lesson 10 Carbon Dioxide Can Make a Solution Acidic Name Date DEMONSTRATION 1. Your teacher blew through a straw into a universal indicator solution until it changed color. Did
Boiling Ice Lab. D) Materials A thermometer A beaker A stopwatch A hot plate Ice
 IP 644 Name: Date: Block: Boiling Ice Lab A) Introduction All matter can exist as a solid, liquid, or gas. The phase in which a substance exists depends on its temperature. The solid phase exists at a
IP 644 Name: Date: Block: Boiling Ice Lab A) Introduction All matter can exist as a solid, liquid, or gas. The phase in which a substance exists depends on its temperature. The solid phase exists at a
Chemistry 151 Last Updated Dec Lab 10: The Neutralizing Ability of an Antacid (Titrations, Pt II)
 Chemistry 151 Last Updated Dec. 2013 Lab 10: The Neutralizing Ability of an Antacid (Titrations, Pt II) Introduction The active ingredient of many antacids is a base that neutralizes excess stomach acid,
Chemistry 151 Last Updated Dec. 2013 Lab 10: The Neutralizing Ability of an Antacid (Titrations, Pt II) Introduction The active ingredient of many antacids is a base that neutralizes excess stomach acid,
Titration with an Acid and a Base
 Skills Practice Titration with an Acid and a Base Titration is a process in which you determine the concentration of a solution by measuring what volume of that solution is needed to react completely with
Skills Practice Titration with an Acid and a Base Titration is a process in which you determine the concentration of a solution by measuring what volume of that solution is needed to react completely with
PDFMAILER.COM Print and send PDF files as s with any application, ad-sponsored and free of charge Activity # 14.
 Activity # 14 Name Purpose Date Date due Activities 10c and 10d - Performing More Examples of Chemical Reactions To perform a number of different chemical reactions, determine what the reactants and products
Activity # 14 Name Purpose Date Date due Activities 10c and 10d - Performing More Examples of Chemical Reactions To perform a number of different chemical reactions, determine what the reactants and products
Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from
 Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from http://www.csun.edu/chemteach/activities/beginninganalysis.pdf ] In this lab, you will be observing physical and chemical properties
Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from http://www.csun.edu/chemteach/activities/beginninganalysis.pdf ] In this lab, you will be observing physical and chemical properties
Pencils or pens, calculator, Periodic Table with Polyatomic Ions Chart
 Lesson Plan Jerel Ray Perez Anderson High School Pre AP Chemistry 90 minutes Objective Students will identify oxidation reduction reactions based on the types of reactions that they are already familiar
Lesson Plan Jerel Ray Perez Anderson High School Pre AP Chemistry 90 minutes Objective Students will identify oxidation reduction reactions based on the types of reactions that they are already familiar
Chemistry. End of Year Cornerstone Assessment
 Chemistry End of Year Cornerstone Assessment The Cornerstone Assessments were developed with support through the VDOE Mathematics and Science Partnership Grant Program NCLB Title II, Part B program by
Chemistry End of Year Cornerstone Assessment The Cornerstone Assessments were developed with support through the VDOE Mathematics and Science Partnership Grant Program NCLB Title II, Part B program by
2. What type of bonding allows water to attract other water molecules? 3. What is the difference between solutions and mixtures?
 Biology Lab Name(s) Period: Date: Purpose: To investigate the properties of water, ph, and enzymes that biologically impact biological functions. Background Information: Water: Sometimes we call water
Biology Lab Name(s) Period: Date: Purpose: To investigate the properties of water, ph, and enzymes that biologically impact biological functions. Background Information: Water: Sometimes we call water
Precipitating Bubbles Introduction to the Scientific Method
 Precipitating Bubbles Introduction to the Scientific Method Florida Sunshine State Standards Benchmark: SC.H.1.3.4 The student knows that accurate record keeping, openness, and replication are essential
Precipitating Bubbles Introduction to the Scientific Method Florida Sunshine State Standards Benchmark: SC.H.1.3.4 The student knows that accurate record keeping, openness, and replication are essential
Acids and Bases. Figure 1
 DataQuest 9 Organisms are often very sensitive to the effect of s and s in their environment. They need to maintain a stable internal ph in order to survive even in the event of environmental changes.
DataQuest 9 Organisms are often very sensitive to the effect of s and s in their environment. They need to maintain a stable internal ph in order to survive even in the event of environmental changes.
Unit 2: Chemistry. Unit Overview:
 Unit 2: Chemistry Unit Overview: This unit will focus on basic chemistry and some of the major process of organic chemistry (dehydration synthesis, hydrolysis, and enzyme action) that help form carbon
Unit 2: Chemistry Unit Overview: This unit will focus on basic chemistry and some of the major process of organic chemistry (dehydration synthesis, hydrolysis, and enzyme action) that help form carbon
Experiment 7: Titration of an Antacid
 1 Experiment 7: Titration of an Antacid Objective: In this experiment, you will standardize a solution of base using the analytical technique known as titration. Using this standardized solution, you will
1 Experiment 7: Titration of an Antacid Objective: In this experiment, you will standardize a solution of base using the analytical technique known as titration. Using this standardized solution, you will
Experiment 8 Synthesis of Aspirin
 Experiment 8 Synthesis of Aspirin Aspirin is an effective analgesic (pain reliever), antipyretic (fever reducer) and anti-inflammatory agent and is one of the most widely used non-prescription drugs. The
Experiment 8 Synthesis of Aspirin Aspirin is an effective analgesic (pain reliever), antipyretic (fever reducer) and anti-inflammatory agent and is one of the most widely used non-prescription drugs. The
LAB: Photosynthesis in Leaf Disks
 Name Date Period LAB: Photosynthesis in Leaf Disks H O N O R S B I O L O G Y : U N I T 3 Introduction: Photosynthesis is a process in which plants convert light energy (sunlight) into usable chemical energy
Name Date Period LAB: Photosynthesis in Leaf Disks H O N O R S B I O L O G Y : U N I T 3 Introduction: Photosynthesis is a process in which plants convert light energy (sunlight) into usable chemical energy
Materials Per Class Per Bench. 50 ml beakers 6 1. Hole punch 6 1. Forceps 6 1. Timers or a clock with second hand 6 1
 Photosynthesis Materials Per Class Per Bench 1% solution of sodium bicarbonate (NaHCO 3 ) (by adding approximately 1g sodium bicarbonate to 100 ml DI water). Light sources, 60 watt bulb or higher 3 or
Photosynthesis Materials Per Class Per Bench 1% solution of sodium bicarbonate (NaHCO 3 ) (by adding approximately 1g sodium bicarbonate to 100 ml DI water). Light sources, 60 watt bulb or higher 3 or
Daily Assignments Calendar
 Daily Assignments Calendar I. Why is the Climate Changing? Macroscopic, symbolic, and atomic-molecular view of chemistry Obtain course materials, available at Turtle Creek Bookstore. Bring all to class
Daily Assignments Calendar I. Why is the Climate Changing? Macroscopic, symbolic, and atomic-molecular view of chemistry Obtain course materials, available at Turtle Creek Bookstore. Bring all to class
Are Chemical Reactions Closed Systems?
 Measuring a Chemical Reaction 1. Place about 100 ml of vinegar in a large plastic cup. Carefully weigh the cup AND the vinegar all together, and write down the total mass. (Don t worry about subtracting
Measuring a Chemical Reaction 1. Place about 100 ml of vinegar in a large plastic cup. Carefully weigh the cup AND the vinegar all together, and write down the total mass. (Don t worry about subtracting
Examples of Strong Acids: Strong Acid Formula Common Source Hydrochloric Acid HCl Stomach Acid
 ACIDS AND BASES: PH AND BUFFERS PURPOSE: To determine the ph of common acids and bases using a ph meter, ph paper, and red cabbage indicator. To test the effect of adding an acid or base to a buffer solution.
ACIDS AND BASES: PH AND BUFFERS PURPOSE: To determine the ph of common acids and bases using a ph meter, ph paper, and red cabbage indicator. To test the effect of adding an acid or base to a buffer solution.
Chemistry Foundations of Chemistry Test. This is due:
 Chemistry Foundations of Chemistry Test This is due: Directions: Answer the following questions on a separate sheet of paper (or on this paper if you have room), staple to this paper (if you used a separate
Chemistry Foundations of Chemistry Test This is due: Directions: Answer the following questions on a separate sheet of paper (or on this paper if you have room), staple to this paper (if you used a separate
Chem 2115 Experiment #7. Volumetric Analysis & Consumer Chemistry Standardization of an unknown solution, analysis of vinegar & antacid tablets
 Chem 2115 Experiment #7 Volumetric Analysis & Consumer Chemistry Standardization of an unknown solution, analysis of vinegar & antacid tablets OBJECTIVE: The goals of this experiment are to learn titration
Chem 2115 Experiment #7 Volumetric Analysis & Consumer Chemistry Standardization of an unknown solution, analysis of vinegar & antacid tablets OBJECTIVE: The goals of this experiment are to learn titration
Do Now May 1, Obj: Observe and describe neutralization reactions. Copy: Balance the neutralization reaction. KCl(aq) + H 2 O(l)
 Do Now May 1, 2017 Obj: Observe and describe neutralization reactions. Copy: Balance the neutralization reaction. HCl + KOH KCl(aq) + H 2 O(l) If I had 100 ml of a 0.01 M HCl solution, what is the ph of
Do Now May 1, 2017 Obj: Observe and describe neutralization reactions. Copy: Balance the neutralization reaction. HCl + KOH KCl(aq) + H 2 O(l) If I had 100 ml of a 0.01 M HCl solution, what is the ph of
Evaluation copy. Acids and Bases. computer OBJECTIVES MATERIALS
 Acids and Bases Computer 2 Organisms are often very sensitive to the effect of s and s in their environment. They need to maintain a stable internal ph in order to survive even in the event of environmental
Acids and Bases Computer 2 Organisms are often very sensitive to the effect of s and s in their environment. They need to maintain a stable internal ph in order to survive even in the event of environmental
Plop Plop, Fizz Fizz, Oh What A Relief It Is (Which Pain Reliever Works Fastest)
 Page 1 of 7 Plop Plop, Fizz Fizz, Oh What A Relief It Is (Which Pain Reliever Works Fastest) Learning Objectives: Study the dissolution rate (how quickly the compound dissolves) of common OTC (over the
Page 1 of 7 Plop Plop, Fizz Fizz, Oh What A Relief It Is (Which Pain Reliever Works Fastest) Learning Objectives: Study the dissolution rate (how quickly the compound dissolves) of common OTC (over the
Photosynthesis in Leaf Disks Teacher Preparation and Background Information
 AP Biology Name: Date: Photosynthesis in Leaf Disks Teacher Preparation and Background Information General Information: Solutions may be handled without gloves and may be disposed of in sink drains. In
AP Biology Name: Date: Photosynthesis in Leaf Disks Teacher Preparation and Background Information General Information: Solutions may be handled without gloves and may be disposed of in sink drains. In
Liquid X Lab. Number of Drops Before Spilling Trial 1 Trial 2 Trial 3. Write a conclusion: How do your results for Liquid X compare to water?
 Names Block Date BIG QUESTION: Liquid X Lab Station 1 Surface Tension, Cohesion, and Adhesion Water is cohesive, adhesive, and has surface tension. Does Liquid X have the same properties? 1. Use a pipette
Names Block Date BIG QUESTION: Liquid X Lab Station 1 Surface Tension, Cohesion, and Adhesion Water is cohesive, adhesive, and has surface tension. Does Liquid X have the same properties? 1. Use a pipette
2. Synthesis of Aspirin
 This is a two-part laboratory experiment. In part one, you will synthesize (make) the active ingredient in aspirin through a reaction involving a catalyst. The resulting product will then be purified through
This is a two-part laboratory experiment. In part one, you will synthesize (make) the active ingredient in aspirin through a reaction involving a catalyst. The resulting product will then be purified through
Experiment #10: Analysis of Antacids
 Experiment #10: Analysis of Antacids Purpose: In this experiment you will prepare one solution that is approximately 0.1 M NaOH. Then you will standardize this solution, which means that you will experimentally
Experiment #10: Analysis of Antacids Purpose: In this experiment you will prepare one solution that is approximately 0.1 M NaOH. Then you will standardize this solution, which means that you will experimentally
Just Keep. [ Wednesday. august 16,2017] swimming
![Just Keep. [ Wednesday. august 16,2017] swimming Just Keep. [ Wednesday. august 16,2017] swimming](/thumbs/82/85520852.jpg) [ Wednesday 1. Where is the safety shower located? 2. Name two ways you can ensure the safety of yourself and others in the lab. 3. Do you have any fears or worries associated with science? Just Keep 5
[ Wednesday 1. Where is the safety shower located? 2. Name two ways you can ensure the safety of yourself and others in the lab. 3. Do you have any fears or worries associated with science? Just Keep 5
Tuesday, February 3rd, 2015 Learning Target : I can make solutions and dilutions. Homework: n/a
 Tuesday, February 3rd, 2015 Learning Target : I can make solutions and dilutions. Homework: n/a As you enter... What is the definition and formula for molarity? (hint: check out your brochure) Big Idea:
Tuesday, February 3rd, 2015 Learning Target : I can make solutions and dilutions. Homework: n/a As you enter... What is the definition and formula for molarity? (hint: check out your brochure) Big Idea:
Experiment: Titration
 Experiment: Titration INTRODUCTION In this experiment you will be determining the volume of sodium hydroxide solution of known concentration required to neutralize a known mass of an unknown acid in solution.
Experiment: Titration INTRODUCTION In this experiment you will be determining the volume of sodium hydroxide solution of known concentration required to neutralize a known mass of an unknown acid in solution.
Alternative Reaction Pathways
 Section 1 Energy and Entropy: Alternative Reaction Pathways What Do You See? Learning Outcomes In this section you will Apply the engineering-design process to scientific and everyday situations. Generate
Section 1 Energy and Entropy: Alternative Reaction Pathways What Do You See? Learning Outcomes In this section you will Apply the engineering-design process to scientific and everyday situations. Generate
LEVEL ZERO VOICE CATALYST (10 minutes, individual work): 1. Counting atoms
 Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
Moles Lab Activity 2: Elements Copper
 Materials Sample of copper Balance Pre-1982 penny Moles Lab Activity 2: Elements Copper Procedure Take the necessary measurements, and record them with units. Show all your calculations, rounding your
Materials Sample of copper Balance Pre-1982 penny Moles Lab Activity 2: Elements Copper Procedure Take the necessary measurements, and record them with units. Show all your calculations, rounding your
8 Titration of Acids and bases
 8 Titration of Acids and bases Name: Date: Section: Objectives Reinforce acid-base chemistry principles from chapter 4 in Silberberg Standardize a sodium hydroxide solution Determine the molarity of an
8 Titration of Acids and bases Name: Date: Section: Objectives Reinforce acid-base chemistry principles from chapter 4 in Silberberg Standardize a sodium hydroxide solution Determine the molarity of an
Transformation of Matter: Physical and Chemical Changes
 Transformation of Matter: Physical and Chemical Changes What does it mean to transform? Transform: change in form, appearance, or makeup What kinds of things transform? How can it be transformed? How
Transformation of Matter: Physical and Chemical Changes What does it mean to transform? Transform: change in form, appearance, or makeup What kinds of things transform? How can it be transformed? How
Student Exploration: Chemical Changes
 Name: Date: Student Exploration: Chemical Changes Vocabulary: acid, base, catalyst, chemical change, coefficient, conservation of matter, decomposition, dissolve, double replacement, endothermic, exothermic,
Name: Date: Student Exploration: Chemical Changes Vocabulary: acid, base, catalyst, chemical change, coefficient, conservation of matter, decomposition, dissolve, double replacement, endothermic, exothermic,
Community in the Classroom Presentation Plan
 Community in the Classroom Presentation Plan Lesson Name How Can You Tell One Clear Gas From Another? Presenter(s) Michael Grass Grade Level 5th Standards Connection(s) Abstract: Many gases are clear and
Community in the Classroom Presentation Plan Lesson Name How Can You Tell One Clear Gas From Another? Presenter(s) Michael Grass Grade Level 5th Standards Connection(s) Abstract: Many gases are clear and
Do Now April 24, 2017
 Do Now April 24, 2017 Obj: Observe and describe neutralization reactions. Copy: Neutralization is when an acid and base react to product a salt and water. e.g. HCl + NaOH NaCl + H 2 O acid base salt water
Do Now April 24, 2017 Obj: Observe and describe neutralization reactions. Copy: Neutralization is when an acid and base react to product a salt and water. e.g. HCl + NaOH NaCl + H 2 O acid base salt water
Determination of the K a of a Weak Acid and the K b of a Weak Base from ph Measurements
 Experiment 6 Determination of the K a of a Weak Acid and the K b of a Weak Base from ph Measurements Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that
Experiment 6 Determination of the K a of a Weak Acid and the K b of a Weak Base from ph Measurements Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that
How Do I Make New Stuff From Old Stuff?
 How Do I Make New Stuff From Old Stuff? 7 th Grade Chemistry Unit Materials Jay Bingaman & Karen Ostrowski www.south7thscience.com Twitter: @South7thScience YouTube.com: South7thScience How Will I Be Graded?
How Do I Make New Stuff From Old Stuff? 7 th Grade Chemistry Unit Materials Jay Bingaman & Karen Ostrowski www.south7thscience.com Twitter: @South7thScience YouTube.com: South7thScience How Will I Be Graded?
Chemistry 3202 Lab 6 Hess s Law 1
 Chemistry 3202 Lab 6 Hess s Law 1 Lab 6 Hess's Law Introduction Chemical and physical changes are always accompanied by a change in energy. Energy changes may be observed by detecting heat flow between
Chemistry 3202 Lab 6 Hess s Law 1 Lab 6 Hess's Law Introduction Chemical and physical changes are always accompanied by a change in energy. Energy changes may be observed by detecting heat flow between
Chemical Names and Formulas
 Cool Chemistry Show Activity 3 Chemical Names and Formulas GOALS In this activity you will: Predict the charges of ions of some elements. Determine the formulas of ionic compounds. Write the conventional
Cool Chemistry Show Activity 3 Chemical Names and Formulas GOALS In this activity you will: Predict the charges of ions of some elements. Determine the formulas of ionic compounds. Write the conventional
CHAPTER 3: PART 2 8/9/2015. A chemical change (a chemical reaction) converts one substance into another.
 8/9/015 A chemical change (a chemical reaction) converts one substance into another. CHAPTER 3: PART Chemical Equations and Stoichiometry Chemical reactions involve: 1. Breaking bonds in the reactants.
8/9/015 A chemical change (a chemical reaction) converts one substance into another. CHAPTER 3: PART Chemical Equations and Stoichiometry Chemical reactions involve: 1. Breaking bonds in the reactants.
Pre-Lab Exercises Lab 3: Chemical Properties
 Pre-Lab Exercises Lab 3: Chemical Properties 1. How is a chemical property different from a physical property? Name Date Section 2. How is a chemical change different from a physical change? 3. Give two
Pre-Lab Exercises Lab 3: Chemical Properties 1. How is a chemical property different from a physical property? Name Date Section 2. How is a chemical change different from a physical change? 3. Give two
Bill Nye: Chemical Reactions
 Bill Nye: Chemical Reactions Name: Vocabulary chemical reaction compound element endothermic energy exothermic Use the word bank above to fill in the blanks. A(n) is matter that is composed of 2 or more
Bill Nye: Chemical Reactions Name: Vocabulary chemical reaction compound element endothermic energy exothermic Use the word bank above to fill in the blanks. A(n) is matter that is composed of 2 or more
Physical and Chemical Properties of Matter Lab
 Physical and Chemical Properties of Matter Lab Purpose To introduce the student to physical and chemical properties of matter and their use for the identification and separation of compounds. Each student
Physical and Chemical Properties of Matter Lab Purpose To introduce the student to physical and chemical properties of matter and their use for the identification and separation of compounds. Each student
