Name Period Date. Lab: Introduction to Stoichiometry
|
|
|
- Geoffrey Cole
- 6 years ago
- Views:
Transcription
1 Name Period Date Lab: Introduction to Stoichiometry Introduction: Reactants are not always present in the exact ratio required by a balanced chemical equation. In planning any cost-effective production process, it is necessary to recognize which components limit the amount of material that can be produced. This allows you to accurately predict how much product will be created and provide the correct amount of reactants to make the required amount of product. Materials: Vinegar Baking soda Erlenmeyer flasks, 125-mL, 5 Balloons, 12-in, 5 Funnel Spoons, 2 Tape measure marker Safety Alert: Baking soda is slightly toxic by ingestion. The dust can be irritating to respiratory system. Wear your Safety Goggles! Part A: Un-Chemistry Stoichiometry Model: Recipe for Making a Cake 2 cups of water 4 cups of flour 8 squares of chocolate 4 cups of sugar 8 ounces of butter 4 eggs Ingredients on Hand Lots of water 5 cups of flour 12 squares of chocolate 4 cups of sugar 16 ounces of butter 6 eggs 1. If you follow the recipe above, using only the ingredients you have on hand, how much of each ingredient will be left over after you have made one cake? Water Flour Chocolate Sugar Butter Eggs 2. Which ingredients did you have excess amounts of? 3. Which ingredients were used completely up when you made the cake? 4. Which ingredients limited, or kept you from making a second cake? 1
2 Part B: Chemistry Stoichiometry Preparation for Reactions: 1. Look carefully at the spreadsheet of assignments for the class. Each box you see your group s letter represents one reaction you must do. In Table 1, put a check mark in each box that represents one of the reactions you are responsible for doing. 2. Record the reactions you plan to do in Table 2. Write the amounts of vinegar and baking soda for each of your reactions. Using a marker, label your flasks with the reaction numbers (1-7). Performing the Reactions: 3. Before you start the first reaction, set your funnel upside down on your desk. Stretch one of your balloons across the opening of the funnel. Be sure it is secure. Pick up the funnel. Measure out the correct number of level spoonfuls of baking soda and pour them in the funnel. Shake the funnel a bit to be sure all the baking soda is down in the balloon. Take the balloon off the funnel and lay it on your desk. 4. Measure and pour the correct number of spoonfuls of vinegar in your flask. Without pouring the baking soda in the flask, stretch the end of the balloon over the neck of the flask. Once it is secure, shake the balloon over the flask, allowing the baking soda to fall into the vinegar. Swirl the flask so the vinegar and baking soda mix well. 5. Once the reaction is complete, measure the circumference of the largest part of the balloon using a measuring tape. Be sure to use the centimeter side of your measuring tape. Be careful not to press on the balloon while you are doing this. Record the circumference in Table 2. Repeat this process for each of the reactions you are assigned. After the Reactions: 6. Once all your reactions are done and all your measurements have been made, write your values in the class chart on the board. 7. Before you cleanup, sketch the mixture remaining in your reaction flasks (in Table 3), as they appear after the reaction is complete. Be sure to match up the reaction numbers to the ones used in Table 2. Focus on showing the amounts of the substances in the flask remaining (solid and liquid). You don t need to show the balloon size since we recorded the size of that already. 8. To cleanup, take the balloons off each of the flasks. Put these balloons back in their container for the next class. Wash out each of the flasks with a small amount of soapy water and rinse them well. Put everything back in your tub. 9. Fill in Table 4 with the average value for each reaction from the chart on the board. Data: Table 1: Your Group s Assignment Spoons of 1 Baking 3 Soda 5 Spoons of Vinegar Table 2: Your Group s Data Reaction Spoons of Vinegar Spoons of Baking Soda Circumference of Balloon 2
3 Table 3: Resulting Flask Diagrams: Reaction 1 Reaction 2 Reaction 3 Reaction 4 Reaction 5 Reaction 6 Reaction 7 Reaction 8 Table 4: Your Class Data Circumference of Balloon for various amounts of reactants Spoons of Vinegar Spoons of Baking Soda Questions: 1. How do you know there is a chemical reaction occurring? 2. What do you know about the states of the products of this reaction? 3. How does the size of the balloon relate to the amount of product made? 4. Vinegar is a dilute aqueous solution of acetic acid. a. What other compound is in the vinegar besides acetic acid? b. What is the formula for acetic acid? 3
4 5. Baking soda s chemical name is sodium hydrogen carbonate. What is the chemical formula for baking soda? 6. Do you know the identity of any of the products of this reaction? If so, list them. 7. From Table 4: Which combination of reactants created the most products? 8. From Table 4: Which combination of reactants created the least amount of products? 9. What do you notice about the sizes of the balloons in each column (vertical) in Table 4? 10. What do you notice about the sizes of the balloons in each row (horizontal) in Table 4? 11. Using the testing recorded in Table 4: What would you expect the circumference of the balloon to be if you used the following amounts: a. 5 spoons of vinegar and 2 spoons of baking soda b. 2 spoons of vinegar and 5 spoons of baking soda 12. Compare the balloon sizes in the first column (vertical) of Table 4. a. How are the amounts of reactants the same? b. How are the amounts of reactants different? c. Why did the balloons on flasks containing 5 spoons of baking soda not expand much more than those containing 1 spoons of baking soda? d. Explain the amount of change you see in the size of the balloons. 13. Compare the balloon sizes in the first row (horizontal) of Table 4. a. How are the amounts of reactants the same? b. How are the amounts of reactants different? c. What kept the balloons on flasks containing only 3 spoons of vinegar from expanding as much as those containing 9 spoons of vinegar? d. Explain the amount of change you see in the size of the balloons. 4
5 14. Look at the pattern across the 2 nd row in Table 4. a. Did the difference between the first two balloon sizes equal the difference between the last two balloon sizes? b. Was the change in size consistent across the row? c. Why did the change in balloon size change as more vinegar was added? d. What would you expect the circumferences for the balloons to be if you did a few more reactions in the 2 nd row using 15, 17 and 19 spoons of vinegar? e. Explain your answer in 14d. 5
STOICHIOMETRY AND THE CHEMICAL REACTION
 From Laboratory Manual for Guinn and Brewer s Essentials of General, Organic, and Biochemistry by Sara Selfe STOICHIOMETRY AND THE CHEMICAL REACTION You would be surprised at the number of chemical reactions
From Laboratory Manual for Guinn and Brewer s Essentials of General, Organic, and Biochemistry by Sara Selfe STOICHIOMETRY AND THE CHEMICAL REACTION You would be surprised at the number of chemical reactions
LEVEL ZERO VOICE CATALYST (10 minutes, individual work): 1. Counting atoms
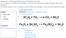 Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
How many molecules are in 0.25 moles of CH 4?
 Mass Moles- Particle Particles can be atoms, molecules, ions, etc. In one mole of particles, there are 6.02x10 23 particles These particles are so small and we need so many of them to be on a human scale,
Mass Moles- Particle Particles can be atoms, molecules, ions, etc. In one mole of particles, there are 6.02x10 23 particles These particles are so small and we need so many of them to be on a human scale,
Stoichiometry World of Chemistry: Chapter 9
 Stoichiometry World of Chemistry: Chapter 9 Chocolate Chip Cookies!! 1 cup butter 1/2 cup white sugar 1 cup packed brown sugar 1 teaspoon vanilla extract 2 eggs 2 1/2 cups all-purpose flour 1 teaspoon
Stoichiometry World of Chemistry: Chapter 9 Chocolate Chip Cookies!! 1 cup butter 1/2 cup white sugar 1 cup packed brown sugar 1 teaspoon vanilla extract 2 eggs 2 1/2 cups all-purpose flour 1 teaspoon
Student Notes. Chemical Reactions LINK
 LCPS Core Experience Chemical Reactions Student Notes OBJECTIVES Students will: investigate the relationship between reactants and products. investigate an exothermic reaction. investigate an endothermic
LCPS Core Experience Chemical Reactions Student Notes OBJECTIVES Students will: investigate the relationship between reactants and products. investigate an exothermic reaction. investigate an endothermic
Safety: Safety goggles should be worn at all times. Students should hold the balloons on the test tubes tightly while the reaction takes place.
 LIMITING REAGENT LAB: THE REACTION BETWEEN VINEGAR AND BAKING SODA Goal: During this lab students will gain a quantitative understanding of limiting reagents. Safety: Safety goggles should be worn at all
LIMITING REAGENT LAB: THE REACTION BETWEEN VINEGAR AND BAKING SODA Goal: During this lab students will gain a quantitative understanding of limiting reagents. Safety: Safety goggles should be worn at all
Exploring Acids & Bases
 Food Explorations Lab: Exploring Acids & Bases STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will use cabbage juice indicator to determine if two unknown samples are acids or
Food Explorations Lab: Exploring Acids & Bases STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will use cabbage juice indicator to determine if two unknown samples are acids or
Goal: During this lab students will gain a quantitative understanding of limiting reagents.
 LIMITING REAGENT LAB: THE REACTION BETWEEN VINEGAR AND BAKING SODA Goal: During this lab students will gain a quantitative understanding of limiting reagents. Safety: Safety goggles should be worn at all
LIMITING REAGENT LAB: THE REACTION BETWEEN VINEGAR AND BAKING SODA Goal: During this lab students will gain a quantitative understanding of limiting reagents. Safety: Safety goggles should be worn at all
Solution Chemistry: Making Solutions, Reactions, and Solubility
 1 Solution Chemistry: Making Solutions, Reactions, and Solubility ORGANIZATION Mode: laboratory, groups of 4 Grading: goggles, closed-toe shoes, appropriate attire Safety: lab report, individual, due at
1 Solution Chemistry: Making Solutions, Reactions, and Solubility ORGANIZATION Mode: laboratory, groups of 4 Grading: goggles, closed-toe shoes, appropriate attire Safety: lab report, individual, due at
Acids and Bases. How does ph affect biological solutions? Introduction. Prelab Preparation Review Section 2.3 on acids and bases in your textbook.
 Acids and Bases How does ph affect biological solutions? Learning Objectives To relate the ph scale to how acidic or basic a solution is. To explain how a buffer affects the ph of a solution. Process Objectives
Acids and Bases How does ph affect biological solutions? Learning Objectives To relate the ph scale to how acidic or basic a solution is. To explain how a buffer affects the ph of a solution. Process Objectives
Virtual Library Lesson: Oobleck, Gloop, and Glurch
 Oobleck, Gloop, and Glurch Lesson Overview Throughout this lesson, students will use inquiry skills to identify states of matter, describe physical properties, and modify the recipe to change physical
Oobleck, Gloop, and Glurch Lesson Overview Throughout this lesson, students will use inquiry skills to identify states of matter, describe physical properties, and modify the recipe to change physical
Experiment 20-Acid-Base Titration: Standardization of KOH and Determination of the Molarity and/or Percent Composition of an Acid Solution
 Experiment 20-Acid-Base Titration: Standardization of KOH and Determination of the Molarity and/or Percent Composition of an Acid Solution In this experiment, you will determine the molarity and percent
Experiment 20-Acid-Base Titration: Standardization of KOH and Determination of the Molarity and/or Percent Composition of an Acid Solution In this experiment, you will determine the molarity and percent
Lab- Properties of Acids and Bases. Name. PSI Chemistry
 Lab- Properties of Acids and Bases PSI Chemistry Name Introduction Acids and bases are useful reagents in the chemistry laboratory and play an important role in biology and nature. What are acids and bases?
Lab- Properties of Acids and Bases PSI Chemistry Name Introduction Acids and bases are useful reagents in the chemistry laboratory and play an important role in biology and nature. What are acids and bases?
Chemistry 143 Acid Base Titration Dr. Caddell. Titrating Acid
 Titrating Acid In this lab you will first determine the concentration of sodium hydroxide in a stock solution that you prepare. You will then use that stock sodium hydroxide solution to titrate a solution
Titrating Acid In this lab you will first determine the concentration of sodium hydroxide in a stock solution that you prepare. You will then use that stock sodium hydroxide solution to titrate a solution
Announcements. discussion tomorrow. tomorrow. 10/15 (Type I) and Wednesday, 10/17 (Type II) by 7:00pm. 1. Bring handout from Tuesday to
 Announcements 1. Bring handout from Tuesday to discussion tomorrow. 2. Lab write-up and text W due tomorrow. 3. Electronic omework due Monday, 10/15 (Type I) and Wednesday, 10/17 (Type II) by 7:00pm Stoichiometry
Announcements 1. Bring handout from Tuesday to discussion tomorrow. 2. Lab write-up and text W due tomorrow. 3. Electronic omework due Monday, 10/15 (Type I) and Wednesday, 10/17 (Type II) by 7:00pm Stoichiometry
Acid-Base Titration. M M V a
 Acid-Base Titration Pre-Lab Discussion In the chemistry laboratory, it is sometimes necessary to experimentally determine the concentration of an acid solution or a base solution. A procedure for making
Acid-Base Titration Pre-Lab Discussion In the chemistry laboratory, it is sometimes necessary to experimentally determine the concentration of an acid solution or a base solution. A procedure for making
Experiment 18 - Absorption Spectroscopy and Beer s Law: Analysis of Cu 2+
 Experiment 18 - Absorption Spectroscopy and Beer s Law: Analysis of Cu 2+ Many substances absorb light. When light is absorbed, electrons in the ground state are excited to higher energy levels. Colored
Experiment 18 - Absorption Spectroscopy and Beer s Law: Analysis of Cu 2+ Many substances absorb light. When light is absorbed, electrons in the ground state are excited to higher energy levels. Colored
STOICHIOMETRY. Engr. Yvonne Ligaya F. Musico 1
 STOICHIOMETRY Engr. Yvonne Ligaya F. Musico 1 Stoichiometry The study in chemistry dealing with calculations based on balanced chemical equations. The branch of chemistry dealing with mass relationships
STOICHIOMETRY Engr. Yvonne Ligaya F. Musico 1 Stoichiometry The study in chemistry dealing with calculations based on balanced chemical equations. The branch of chemistry dealing with mass relationships
EXPERIMENT. Stoichiometry of a Precipitation Reaction
 EXPERIMENT Stoichiometry of a Precipitation Reaction Hands-On Labs, Inc. Version 42-0201-00-02 Review the safety materials and wear goggles when working with chemicals. Read the entire exercise before
EXPERIMENT Stoichiometry of a Precipitation Reaction Hands-On Labs, Inc. Version 42-0201-00-02 Review the safety materials and wear goggles when working with chemicals. Read the entire exercise before
Acid-Base Titration Acetic Acid Content of Vinegar
 Acid-Base Titration Acetic Acid Content of Vinegar Prelab Assignment Read the entire lab. Write an objective and any hazards associated with this lab in your laboratory notebook. On a separate sheet of
Acid-Base Titration Acetic Acid Content of Vinegar Prelab Assignment Read the entire lab. Write an objective and any hazards associated with this lab in your laboratory notebook. On a separate sheet of
Lesson 2. Color change
 Lesson 2 Color change T E A C H E R G U I D E Lesson summary Students meet marine chemist Sera Tuikabe, who is studying ocean acidification in the water surrounding the Republic of the Fiji Islands. Students
Lesson 2 Color change T E A C H E R G U I D E Lesson summary Students meet marine chemist Sera Tuikabe, who is studying ocean acidification in the water surrounding the Republic of the Fiji Islands. Students
Stoichiometry Lab Report
 Stoichiometry Lab Report Touche G10 Variables Variable Dependent Controlled: Amount of NaHCO3 Theoretical Yield Vinegar Evaporation Manipulation We measure the remaining NaC 2 H 3 O 2 and compare it with
Stoichiometry Lab Report Touche G10 Variables Variable Dependent Controlled: Amount of NaHCO3 Theoretical Yield Vinegar Evaporation Manipulation We measure the remaining NaC 2 H 3 O 2 and compare it with
Molarity of Acetic Acid in Vinegar A Titration Experiment
 Molarity of Acetic Acid in Vinegar A Titration Experiment Introduction Vinegar is prepared commercially in two steps, both requiring microorganisms. The first step is the production of ethyl alcohol, C
Molarity of Acetic Acid in Vinegar A Titration Experiment Introduction Vinegar is prepared commercially in two steps, both requiring microorganisms. The first step is the production of ethyl alcohol, C
Physical and Chemical Properties and Changes Lab
 Name: Date: Period: Group Members Physical and Chemical Properties and Changes Lab Station 1 Color Station Instruction: Describe the color of the following substances. Substance Color 1. Sulfur 2. Ammonium
Name: Date: Period: Group Members Physical and Chemical Properties and Changes Lab Station 1 Color Station Instruction: Describe the color of the following substances. Substance Color 1. Sulfur 2. Ammonium
Bellevue College CHEM& 121 Experiment: Stoichiometric Analysis of an Antacid 1
 Experiment: Stoichiometric Analysis of an Antacid 1 Introduction In this lab, you will use the concept of stoichiometry to solve two sequential problems. First, you will try to determine the products of
Experiment: Stoichiometric Analysis of an Antacid 1 Introduction In this lab, you will use the concept of stoichiometry to solve two sequential problems. First, you will try to determine the products of
Chemistry 143 Experiment #11 Acid Base Titration Dr. Caddell. Titrating Acid
 Titrating Acid In this lab you will first determine the concentration of sodium hydroxide in a stock solution that you prepare. You will then use that stock sodium hydroxide solution to titrate a solution
Titrating Acid In this lab you will first determine the concentration of sodium hydroxide in a stock solution that you prepare. You will then use that stock sodium hydroxide solution to titrate a solution
Name Period Date. Lab 9: Analysis of Commercial Bleach
 Name Period Date Lab 9: Analysis of Commercial Bleach Introduction Many common products are effective because they contain oxidizing agents. Some products, which contain oxidizing agents, are bleaches,
Name Period Date Lab 9: Analysis of Commercial Bleach Introduction Many common products are effective because they contain oxidizing agents. Some products, which contain oxidizing agents, are bleaches,
7-A. Inquiry INVESTIGATION. 322 MHR Unit 3 Quantities in Chemical Reactions. Skill Check. Safety Precautions
 Inquiry INVESTIGATION 7-A Skill Check Initiating and Planning Performing and Recording Analyzing and Interpreting Communicating Safety Precautions Wear safety eyewear throughout this investigation. Wear
Inquiry INVESTIGATION 7-A Skill Check Initiating and Planning Performing and Recording Analyzing and Interpreting Communicating Safety Precautions Wear safety eyewear throughout this investigation. Wear
Ocean Acidification in a Cup Materials
 Ocean Acidification in a Cup Ocean acidification is a problem that humans will have to deal with as we release more and more carbon dioxide into the atmosphere. This activity demonstrates how water can
Ocean Acidification in a Cup Ocean acidification is a problem that humans will have to deal with as we release more and more carbon dioxide into the atmosphere. This activity demonstrates how water can
Chemistry 1B Experiment 11 49
 Chemistry 1B Experiment 11 49 11 Buffer Solutions Introduction Any solution that contains both a weak acid HA and its conjugate base A in significant amounts is a buffer solution. A buffer is a solution
Chemistry 1B Experiment 11 49 11 Buffer Solutions Introduction Any solution that contains both a weak acid HA and its conjugate base A in significant amounts is a buffer solution. A buffer is a solution
Lab 2-Investigating the Law of Conservation of Mass
 Name: Period: Lab 2-Investigating the Law of Conservation of Mass Objective: To corroborate the law of conservation of mass through laboratory experimentation. Background: When wood burns or water evaporates,
Name: Period: Lab 2-Investigating the Law of Conservation of Mass Objective: To corroborate the law of conservation of mass through laboratory experimentation. Background: When wood burns or water evaporates,
Modeling Conservation of Matter
 Modeling Conservation of Matter Imagine that you and two of your classmates want to make a strawberry banana smoothie. You lay out the ingredients: one banana, five strawberries and two scoops of ice cream.
Modeling Conservation of Matter Imagine that you and two of your classmates want to make a strawberry banana smoothie. You lay out the ingredients: one banana, five strawberries and two scoops of ice cream.
EXPERIMENT A7: VINEGAR TITRATION. Learning Outcomes. Introduction. Upon completion of this lab, the student will be able to:
 1 Learning Outcomes EXPERIMENT A7: VINEGAR TITRATION Upon completion of this lab, the student will be able to: 1) Prepare a solution of primary standard 2) Determine the molar concentration of a solution
1 Learning Outcomes EXPERIMENT A7: VINEGAR TITRATION Upon completion of this lab, the student will be able to: 1) Prepare a solution of primary standard 2) Determine the molar concentration of a solution
EXTRA CREDIT REMINDER
 EXTRA CREDIT REMINDER Due Tonight at Midnight (January 21 at 11:59 pm) via email kimberlyn.jackson@hcbe.net *** Kinesthetic: If you do not know how to use Prezi you may do a power point otherwise email
EXTRA CREDIT REMINDER Due Tonight at Midnight (January 21 at 11:59 pm) via email kimberlyn.jackson@hcbe.net *** Kinesthetic: If you do not know how to use Prezi you may do a power point otherwise email
Acid Rain Drops Keep Falling on My Head Investigating Acid Rain
 Investigating Acid Rain Carbonic acid is produced when carbon dioxide gas dissolves in rain droplets of unpolluted air: (1) CO 2(g) + H 2 O (l) H 2 CO 3(aq) CO 2 Nitrous acid and nitric acid result from
Investigating Acid Rain Carbonic acid is produced when carbon dioxide gas dissolves in rain droplets of unpolluted air: (1) CO 2(g) + H 2 O (l) H 2 CO 3(aq) CO 2 Nitrous acid and nitric acid result from
Pre-Lab Exercises Lab 3: Chemical Properties
 Pre-Lab Exercises Lab 3: Chemical Properties 1. How is a chemical property different from a physical property? Name Date Section 2. How is a chemical change different from a physical change? 3. Give two
Pre-Lab Exercises Lab 3: Chemical Properties 1. How is a chemical property different from a physical property? Name Date Section 2. How is a chemical change different from a physical change? 3. Give two
Moles Lab Activity 1: PCU (Popcorn Counting Units)
 Moles Lab Activity 1: PCU (Popcorn Counting Units) Materials: A container of each of the following: Popcorn kernels Another type of beans A large unopened bag of popcorn Kernels Balance Safety goggles
Moles Lab Activity 1: PCU (Popcorn Counting Units) Materials: A container of each of the following: Popcorn kernels Another type of beans A large unopened bag of popcorn Kernels Balance Safety goggles
Stoichiometry: Baking Soda and Vinegar Reactions Teacher Version
 Stoichiometry: Baking Soda and Vinegar Reactions Teacher Version In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household chemicals
Stoichiometry: Baking Soda and Vinegar Reactions Teacher Version In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household chemicals
TITRATION OF AN ACID WITH A BASE
 TITRATION OF AN ACID WITH A BASE 1 NOTE: You are required to view the podcast entitled Use of Burets for Titrations before coming to lab this week. To view the podcast, consisting of eight episodes, go
TITRATION OF AN ACID WITH A BASE 1 NOTE: You are required to view the podcast entitled Use of Burets for Titrations before coming to lab this week. To view the podcast, consisting of eight episodes, go
Are Chemical Reactions Closed Systems?
 Measuring a Chemical Reaction 1. Place about 100 ml of vinegar in a large plastic cup. Carefully weigh the cup AND the vinegar all together, and write down the total mass. (Don t worry about subtracting
Measuring a Chemical Reaction 1. Place about 100 ml of vinegar in a large plastic cup. Carefully weigh the cup AND the vinegar all together, and write down the total mass. (Don t worry about subtracting
Chemical Reactions Investigation Two Data Record
 Chemical Reactions Investigation Two Data Record Name: Date: 1. During this Investigation, you will analyze how changing the amounts of the reactants in a chemical reaction affects the amount of the products
Chemical Reactions Investigation Two Data Record Name: Date: 1. During this Investigation, you will analyze how changing the amounts of the reactants in a chemical reaction affects the amount of the products
THE LAWS LAB LAW OF CONSERVATION OF MASS, LAW OF DEFINITE PROPORTIONS, LAW OF MULTIPLE PROPORTIONS
 THE LAWS LAB LAW OF CONSERVATION OF MASS, LAW OF DEFINITE PROPORTIONS, LAW OF MULTIPLE PROPORTIONS PRELAB Please answer the following questions on a separate piece of paper using complete sentences. 1.
THE LAWS LAB LAW OF CONSERVATION OF MASS, LAW OF DEFINITE PROPORTIONS, LAW OF MULTIPLE PROPORTIONS PRELAB Please answer the following questions on a separate piece of paper using complete sentences. 1.
Learn to do quantitative titration reactions. Observe the mole ratios of several simple chemical reactions.
 CHAPTER 6 Stoichiometry of Reactions in Solution Objectives The objectives of this laboratory are to: Learn to do quantitative titration reactions. Observe the mole ratios of several simple chemical reactions.
CHAPTER 6 Stoichiometry of Reactions in Solution Objectives The objectives of this laboratory are to: Learn to do quantitative titration reactions. Observe the mole ratios of several simple chemical reactions.
Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic
 Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic Key Concepts Carbon dioxide (CO 2 ) gas dissolved in water can cause water to become acidic. The acidity of water from dissolved CO 2 can
Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic Key Concepts Carbon dioxide (CO 2 ) gas dissolved in water can cause water to become acidic. The acidity of water from dissolved CO 2 can
Unit 3.3 Test: Basic Chemistry
 Unit 3.3 Test: Basic Chemistry 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 1. A student placed 2 grams of baking soda in a balloon and
Unit 3.3 Test: Basic Chemistry 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 1. A student placed 2 grams of baking soda in a balloon and
Experiment #10: Analysis of Antacids
 Experiment #10: Analysis of Antacids Purpose: In this experiment you will prepare one solution that is approximately 0.1 M NaOH. Then you will standardize this solution, which means that you will experimentally
Experiment #10: Analysis of Antacids Purpose: In this experiment you will prepare one solution that is approximately 0.1 M NaOH. Then you will standardize this solution, which means that you will experimentally
5 th Grade Lesson Plan: Matter and Chemical Reactions
 5 th Grade Lesson Plan: Matter and Chemical Reactions Objective: Teach students that matter is neither created nor destroyed during a chemical reaction, rather, it is transformed. Identify evidence that
5 th Grade Lesson Plan: Matter and Chemical Reactions Objective: Teach students that matter is neither created nor destroyed during a chemical reaction, rather, it is transformed. Identify evidence that
UNIT 5: STOICHIOMETRY
 UNIT 5: STOICHIOMETRY Outline The Mole Molar Mass, Mass and atoms Molar Mass of Compounds Empirical Formula, Molecular Formula (Not Hydrates) Stoichiometry, Mole Ratios Limiting Reactants, Percent Yield
UNIT 5: STOICHIOMETRY Outline The Mole Molar Mass, Mass and atoms Molar Mass of Compounds Empirical Formula, Molecular Formula (Not Hydrates) Stoichiometry, Mole Ratios Limiting Reactants, Percent Yield
The Eight Solution Problem Exploring Reactions of Aqueous Ionic Compounds
 15 Exploring Reactions of Aqueous Ionic Compounds INTRODUCTION Your goal in this lab is to identify eight unknown solutions. You and your partner will first collect data by observing reactions between
15 Exploring Reactions of Aqueous Ionic Compounds INTRODUCTION Your goal in this lab is to identify eight unknown solutions. You and your partner will first collect data by observing reactions between
Titrations. Method for Titration. N Goalby chemrevise.org 1. Using the pipette
 Titrations Titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. They are often done with neutralisation reactions, but
Titrations Titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. They are often done with neutralisation reactions, but
What Do You Think? Investigate GOALS
 Activity 1 Chemical and Physical Changes GOALS In this activity you will: Learn to differentiate between chemical and physical changes. Make observations and cite evidence to identify changes as chemical
Activity 1 Chemical and Physical Changes GOALS In this activity you will: Learn to differentiate between chemical and physical changes. Make observations and cite evidence to identify changes as chemical
Acid / Base Titrations
 Acid / Base Titrations v051413_7pm Objectives: Determine the concentration of a base solution using an acid standard. Optional: Precipitate an ionic salt for percent yield determination using the standardized
Acid / Base Titrations v051413_7pm Objectives: Determine the concentration of a base solution using an acid standard. Optional: Precipitate an ionic salt for percent yield determination using the standardized
Experiment 8 - Chemical Changes
 Experiment 8 - Chemical Changes When a chemical change occurs, the chemicals that you start with are changed into different chemicals. We know when this happens because the new chemicals have different
Experiment 8 - Chemical Changes When a chemical change occurs, the chemicals that you start with are changed into different chemicals. We know when this happens because the new chemicals have different
Chemical Reactions Investigation Two Data Record
 Chemical Reactions Investigation Two Data Record Name: Date: 1. During this Investigation, you will analyze how changing the amounts of the reactants in a chemical reaction affects the amount of the products
Chemical Reactions Investigation Two Data Record Name: Date: 1. During this Investigation, you will analyze how changing the amounts of the reactants in a chemical reaction affects the amount of the products
Determining the Rate Law for a Chemical Reaction
 Determining the Rate Law for a Chemical Reaction Purpose: To determine the reaction orders, rate law, and rate constant for the reaction between persulfate ions, SO8 -, and iodide ions, I - Introduction
Determining the Rate Law for a Chemical Reaction Purpose: To determine the reaction orders, rate law, and rate constant for the reaction between persulfate ions, SO8 -, and iodide ions, I - Introduction
CO 2. Lesson 1. Production of a gas
 Lesson 1 Production of a gas T E A C H E R G U I D E CO 2 Lesson summary Students meet volcanologist Victor Helguson, who is studying the gases released by volcanoes in Iceland. Students conduct a chemical
Lesson 1 Production of a gas T E A C H E R G U I D E CO 2 Lesson summary Students meet volcanologist Victor Helguson, who is studying the gases released by volcanoes in Iceland. Students conduct a chemical
Experiment 2: Analysis of Commercial Bleach Solutions
 Experiment 2: Analysis of Commercial Bleach Solutions I. Introduction The ability of household bleach to remove stains is related to the amount of oxidizing agent in it. The oxidizing agent in bleach is
Experiment 2: Analysis of Commercial Bleach Solutions I. Introduction The ability of household bleach to remove stains is related to the amount of oxidizing agent in it. The oxidizing agent in bleach is
1. Write date, topic and purpose in notebook. 2. Answer in your notebook: What are two signs that a chemical reaction is happening?
 Warm Up (5 min) Date: Monday, 1/4/16 Topic: Alka Seltzer Lab Purpose: To assess the Law of Conservation of Mass by designing an experiment to determine mass before and after a chemical reaction. 1. Write
Warm Up (5 min) Date: Monday, 1/4/16 Topic: Alka Seltzer Lab Purpose: To assess the Law of Conservation of Mass by designing an experiment to determine mass before and after a chemical reaction. 1. Write
In this activity, you will observe and predict products for some simple
 Chemistry Not Chemistry My Type Not My Type Classifying Chemical Reactions In this activity, you will observe and predict products for some simple chemical reactions. You will classify the reactions as
Chemistry Not Chemistry My Type Not My Type Classifying Chemical Reactions In this activity, you will observe and predict products for some simple chemical reactions. You will classify the reactions as
Chapter 6, Lesson 9: Neutralizing Acids and Bases
 Chapter 6, Lesson 9: Neutralizing Acids and Bases Key Concepts ph is a measure of the concentration of H 3 O + ions in a solution. Adding an acid increases the concentration of H 3 O + ions in the solution.
Chapter 6, Lesson 9: Neutralizing Acids and Bases Key Concepts ph is a measure of the concentration of H 3 O + ions in a solution. Adding an acid increases the concentration of H 3 O + ions in the solution.
Upon completion of this lab, the student will be able to:
 1 Learning Outcomes EXPERIMENT 30A7: VINEGAR TITRATION Upon completion of this lab, the student will be able to: 1) Measure the amount of acetic acid in a solution of vinegar Introduction The molar concentration
1 Learning Outcomes EXPERIMENT 30A7: VINEGAR TITRATION Upon completion of this lab, the student will be able to: 1) Measure the amount of acetic acid in a solution of vinegar Introduction The molar concentration
Experiment 8 Synthesis of Aspirin
 Experiment 8 Synthesis of Aspirin Aspirin is an effective analgesic (pain reliever), antipyretic (fever reducer) and anti-inflammatory agent and is one of the most widely used non-prescription drugs. The
Experiment 8 Synthesis of Aspirin Aspirin is an effective analgesic (pain reliever), antipyretic (fever reducer) and anti-inflammatory agent and is one of the most widely used non-prescription drugs. The
Experiment 17. Synthesis of Aspirin. Introduction
 Experiment 17 Introduction Synthesis of Aspirin Aspirin (acetylsalicylic acid) is a synthetic organic derived from salicylic acid. Salicylic acid is a natural product found in the bark of the willow tree
Experiment 17 Introduction Synthesis of Aspirin Aspirin (acetylsalicylic acid) is a synthetic organic derived from salicylic acid. Salicylic acid is a natural product found in the bark of the willow tree
What Do You Think? Investigate GOALS
 Activity 2 More Chemical Changes GOALS In this activity you will: Observe several typical examples of evidence that a chemical change is occurring. Make generalizations about the combinations of materials
Activity 2 More Chemical Changes GOALS In this activity you will: Observe several typical examples of evidence that a chemical change is occurring. Make generalizations about the combinations of materials
UNIT 5: STOICHIOMETRY
 UNIT 5: STOICHIOMETRY Outline The Mole Molar Mass, Mass and atoms Molar Mass of Compounds Empirical Formula, Molecular Formula (Not Hydrates) Stoichiometry, Mole Ratios Limiting Reactants, Percent Yield
UNIT 5: STOICHIOMETRY Outline The Mole Molar Mass, Mass and atoms Molar Mass of Compounds Empirical Formula, Molecular Formula (Not Hydrates) Stoichiometry, Mole Ratios Limiting Reactants, Percent Yield
SNC2D CHEMISTRY 2/23/2013. CHEMICAL REACTIONS L Conservation of Mass (P ) Activity: Measuring Mass (Part 1) Activity: Measuring Mass (Part 1)
 SNC2D CHEMISTRY CHEMICAL REACTIONS L Conservation of Mass (P.176-177) Activity: Measuring Mass (Part 1) INSTRUCTIONS (CLOSED SYSTEM) A. Measure~ 30 ml of vinegar (acetic acid) into a small Erlenmeyer flask.
SNC2D CHEMISTRY CHEMICAL REACTIONS L Conservation of Mass (P.176-177) Activity: Measuring Mass (Part 1) INSTRUCTIONS (CLOSED SYSTEM) A. Measure~ 30 ml of vinegar (acetic acid) into a small Erlenmeyer flask.
Limiting Reactants Lab
 Name: Teacher s Name: Class: Block: Date: Partners: Limiting Reactants Lab Purpose: Through experimentation, determine the limiting reactant and the percent yield in a chemical reaction that generates
Name: Teacher s Name: Class: Block: Date: Partners: Limiting Reactants Lab Purpose: Through experimentation, determine the limiting reactant and the percent yield in a chemical reaction that generates
EXPERIMENT 20. Solutions INTRODUCTION
 EXPERIMENT 20 Solutions INTRODUCTION A solution is a homogeneous mixture. The solvent is the dissolving substance, while the solute is the dissolved substance. A saturated solution is one in which the
EXPERIMENT 20 Solutions INTRODUCTION A solution is a homogeneous mixture. The solvent is the dissolving substance, while the solute is the dissolved substance. A saturated solution is one in which the
Stoichiometry: Baking Soda and Vinegar Reactions Student Version
 Stoichiometry: Baking Soda and Vinegar Reactions Student Version In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household chemicals
Stoichiometry: Baking Soda and Vinegar Reactions Student Version In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household chemicals
Chemistry 1B Experiment 17 89
 Chemistry 1B Experiment 17 89 17 Thermodynamics of Borax Solubility Introduction In this experiment, you will determine the values of H and S for the reaction which occurs when borax (sodium tetraborate
Chemistry 1B Experiment 17 89 17 Thermodynamics of Borax Solubility Introduction In this experiment, you will determine the values of H and S for the reaction which occurs when borax (sodium tetraborate
Acid-Base Extraction
 Experiment: Acid-Base Extraction Background information on the theory of extraction is covered extensively online and will also be covered in your discussion The information here pertains specifically
Experiment: Acid-Base Extraction Background information on the theory of extraction is covered extensively online and will also be covered in your discussion The information here pertains specifically
CHEM 30A EXPERIMENT 8 & 9: ACID- BASE TITRATION. Learning Outcomes. Introduction. Upon completion of this lab, the student will be able to:
 1 Learning Outcomes CHEM 30A EXPERIMENT 8 & 9: ACID- BASE TITRATION Upon completion of this lab, the student will be able to: 1) Prepare a solution of primary standard 2) Determine the molar concentration
1 Learning Outcomes CHEM 30A EXPERIMENT 8 & 9: ACID- BASE TITRATION Upon completion of this lab, the student will be able to: 1) Prepare a solution of primary standard 2) Determine the molar concentration
Objective: Determine the general properties of ionic compounds and compare those properties to the properties of a covalent compound.
 LAB: PROPERTIES OF IONIC COMPOUNDS Name Introduction The goal of this lab is for you to discover some of the properties of ionic compounds. The physical properties of a substance such as flame color, crystal
LAB: PROPERTIES OF IONIC COMPOUNDS Name Introduction The goal of this lab is for you to discover some of the properties of ionic compounds. The physical properties of a substance such as flame color, crystal
Chemical Reaction Lab Bagged Chemical Reactions
 Learning Target: The student experiments and determines that the rates of reaction among atoms and molecules depend on the concentration, pressure, and temperature of the reactants and the presence or
Learning Target: The student experiments and determines that the rates of reaction among atoms and molecules depend on the concentration, pressure, and temperature of the reactants and the presence or
EXPERIMENT #8 Acid-Base I: Titration Techniques
 EXPERIMENT #8 Acid-Base I: Titration Techniques OBJECTIVES: Dispense a precise volume of a solution with a buret Titrate a known volume of acid solution with a standard solution of base Reach a proper
EXPERIMENT #8 Acid-Base I: Titration Techniques OBJECTIVES: Dispense a precise volume of a solution with a buret Titrate a known volume of acid solution with a standard solution of base Reach a proper
Chemical Reactions: The Copper Cycle
 1 Chemical Reactions: The Copper Cycle ORGANIZATION Mode: pairs assigned by instructor Grading: lab notes, lab performance and post-lab report Safety: Goggles, closed-toe shoes, lab coat, long pants/skirts
1 Chemical Reactions: The Copper Cycle ORGANIZATION Mode: pairs assigned by instructor Grading: lab notes, lab performance and post-lab report Safety: Goggles, closed-toe shoes, lab coat, long pants/skirts
Lesson Title: Elementary - CO2 vehicles Amount of time for this lesson = 90 minutes
 Lesson Title: Elementary - CO2 vehicles Amount of time for this lesson = 90 minutes 1. Standards and Safety and Materials: A. Standards - (Wyoming? NGSS? Number and write it out) Wyoming Standards SC4.1.10
Lesson Title: Elementary - CO2 vehicles Amount of time for this lesson = 90 minutes 1. Standards and Safety and Materials: A. Standards - (Wyoming? NGSS? Number and write it out) Wyoming Standards SC4.1.10
Experiment 20: Analysis of Vinegar. Materials:
 Experiment 20: Analysis of Vinegar Materials: graduated cylinder 6 M NaOH: Dilute Sodium Hydroxide 1000 ml Florence Flask & stopper KHC 8 H 4 O 4 : Potassium Hydrogen Phthalate (KHP) 125 ml Erlenmeyer
Experiment 20: Analysis of Vinegar Materials: graduated cylinder 6 M NaOH: Dilute Sodium Hydroxide 1000 ml Florence Flask & stopper KHC 8 H 4 O 4 : Potassium Hydrogen Phthalate (KHP) 125 ml Erlenmeyer
Kinetics of an Iodine Clock Reaction Lab_Student Copy
 Kinetics of an Iodine Clock Reaction Lab_Student Copy Purpose: Purpose: In this lab, you will find the reaction rate, rate law,, and observe the effects of a catalyst for the oxidation of iodide ions by
Kinetics of an Iodine Clock Reaction Lab_Student Copy Purpose: Purpose: In this lab, you will find the reaction rate, rate law,, and observe the effects of a catalyst for the oxidation of iodide ions by
Stoichiometry: Baking Soda and Vinegar Reactions Student Advanced Version
 Stoichiometry: Baking Soda and Vinegar Reactions Student Advanced Version In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household
Stoichiometry: Baking Soda and Vinegar Reactions Student Advanced Version In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household
Physical and Chemical Properties of Matter Lab
 Physical and Chemical Properties of Matter Lab Purpose To introduce the student to physical and chemical properties of matter and their use for the identification and separation of compounds. Each student
Physical and Chemical Properties of Matter Lab Purpose To introduce the student to physical and chemical properties of matter and their use for the identification and separation of compounds. Each student
Lab: Detecting ph of Commonly Used Acids and Bases
 Lab: Detecting ph of Commonly Used Acids and Bases FOR THE TEACHER Summary In this lab, students will use their knowledge of acids and bases to determine the acidity and basicity of every day items by
Lab: Detecting ph of Commonly Used Acids and Bases FOR THE TEACHER Summary In this lab, students will use their knowledge of acids and bases to determine the acidity and basicity of every day items by
Substances and Mixtures:Separating a Mixture into Its Components
 MiraCosta College Introductory Chemistry Laboratory Substances and Mixtures:Separating a Mixture into Its Components EXPERIMENTAL TASK To separate a mixture of calcium carbonate, iron and sodium chloride
MiraCosta College Introductory Chemistry Laboratory Substances and Mixtures:Separating a Mixture into Its Components EXPERIMENTAL TASK To separate a mixture of calcium carbonate, iron and sodium chloride
CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED)
 CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED) Overview: Students investigate chemical reactions using given substances. Students identify chemical reactions in their daily lives. Objectives: The student
CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED) Overview: Students investigate chemical reactions using given substances. Students identify chemical reactions in their daily lives. Objectives: The student
Chemical reactions are processes in which the atoms of one or more substances are rearranged to form different chemical compounds.
 Chapter 11 Chemical Reactions Notes Chemical Reactions: Chemical reactions are processes in which the atoms of one or more substances are rearranged to form different chemical compounds. How to tell if
Chapter 11 Chemical Reactions Notes Chemical Reactions: Chemical reactions are processes in which the atoms of one or more substances are rearranged to form different chemical compounds. How to tell if
Acid Base Titration Experiment ACID - BASE TITRATION LAB
 ACID - BASE TITRATION LAB MATERIALS and CHEMICALS Burette 50 ml Burette clamp Ring stand Stirring rod Plastic funnel Beakers (50 ml, 100 ml, 400 ml) Graduated cylinder (25 ml, 50 ml) 0.10 M NaOH 0.10 M
ACID - BASE TITRATION LAB MATERIALS and CHEMICALS Burette 50 ml Burette clamp Ring stand Stirring rod Plastic funnel Beakers (50 ml, 100 ml, 400 ml) Graduated cylinder (25 ml, 50 ml) 0.10 M NaOH 0.10 M
CHM101 Lab Stoichiometry Grading Rubric
 Spring 2017 Name CHM101 Lab Stoichiometry Grading Rubric Criteria Points possible Points earned Lab Report Q1 (work and units shown) 2 Q2 (work and units shown) 1.5 Q3 (work and units shown) 2.5 Q4 (reaction
Spring 2017 Name CHM101 Lab Stoichiometry Grading Rubric Criteria Points possible Points earned Lab Report Q1 (work and units shown) 2 Q2 (work and units shown) 1.5 Q3 (work and units shown) 2.5 Q4 (reaction
Recognizing Chemical and Physical Changes
 Chapter 2 Properties of Matter Investigation 2A Recognizing Chemical and Physical Changes Background Information Some chemical and physical changes are easy to recognize. Other changes may be easy to observe,
Chapter 2 Properties of Matter Investigation 2A Recognizing Chemical and Physical Changes Background Information Some chemical and physical changes are easy to recognize. Other changes may be easy to observe,
Name Period Date. Lab 1: Mass of Ice Materials: beaker, ice and balance.
 Name Period Date Testing the Law of Conservation of MASS! Introduction: Does mass change in a chemical or physical reaction? In this series of experiments you will find the answer to this question. Lab
Name Period Date Testing the Law of Conservation of MASS! Introduction: Does mass change in a chemical or physical reaction? In this series of experiments you will find the answer to this question. Lab
Most people think of volcanoes as destructive. The high temperature
 37 Volcanic Landforms M O D E L I N G Most people think of volcanoes as destructive. The high temperature of volcanic lava can burn almost everything in its path. Volcanoes also release large amounts of
37 Volcanic Landforms M O D E L I N G Most people think of volcanoes as destructive. The high temperature of volcanic lava can burn almost everything in its path. Volcanoes also release large amounts of
By All INdICATIONS (2 Hours)
 By All INdICATIONS (2 Hours) Addresses NGSS Level of Difficulty: 5 Grade Range: 6-8 OVERVIEW In this activity, students create an acid-base indicator using red cabbage extract. Students then use this indicator
By All INdICATIONS (2 Hours) Addresses NGSS Level of Difficulty: 5 Grade Range: 6-8 OVERVIEW In this activity, students create an acid-base indicator using red cabbage extract. Students then use this indicator
Experiment 17 It s A Gas and More!
 Energy Energy Experiment 17 It s A Gas and More! OUTCOMES After completing this lab activity, the student should be able to: explain a simple method for distinguishing carbon dioxide gas from oxygen gas.
Energy Energy Experiment 17 It s A Gas and More! OUTCOMES After completing this lab activity, the student should be able to: explain a simple method for distinguishing carbon dioxide gas from oxygen gas.
Solutions: Chemical or Physical Change?
 Section 1 Solutions: Chemical or Physical Change? What Do You See? Learning Outcomes In this section you will Learn to differentiate between chemical and physical changes. Make observations and cite evidence
Section 1 Solutions: Chemical or Physical Change? What Do You See? Learning Outcomes In this section you will Learn to differentiate between chemical and physical changes. Make observations and cite evidence
Post-Show. Chemistry. Periodic Table of the Elements. After the Show. Traveling Science Shows
 Traveling Science Shows Post-Show Chemistry After the Show We recently presented a Chemistry show at your school, and thought you and your students might like to continue investigating this topic. The
Traveling Science Shows Post-Show Chemistry After the Show We recently presented a Chemistry show at your school, and thought you and your students might like to continue investigating this topic. The
Identification of an Unknown Compound through Mass Correlations
 EXPERIMENT Identification of an Unknown Compound through Mass Correlations PURPOSE To carry out a series of decomposition reactions for five different unknown, and use stoichiometry in order to identify
EXPERIMENT Identification of an Unknown Compound through Mass Correlations PURPOSE To carry out a series of decomposition reactions for five different unknown, and use stoichiometry in order to identify
Chapter 6, Lesson 1: What is a Chemical Reaction?
 Chapter 6, Lesson 1: What is a Chemical Reaction? Key Concepts: A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. In a chemical reaction,
Chapter 6, Lesson 1: What is a Chemical Reaction? Key Concepts: A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. In a chemical reaction,
Activity Sheet Chapter 6, Lesson 10 Carbon Dioxide Can Make a Solution Acidic
 Activity Sheet Chapter 6, Lesson 10 Carbon Dioxide Can Make a Solution Acidic Name Date DEMONSTRATION 1. Your teacher blew through a straw into a universal indicator solution until it changed color. Did
Activity Sheet Chapter 6, Lesson 10 Carbon Dioxide Can Make a Solution Acidic Name Date DEMONSTRATION 1. Your teacher blew through a straw into a universal indicator solution until it changed color. Did
Introduction. Objectives
 Experiment: Acids, Bases, and Buffers * Introduction Many common household solutions contain acids and bases. Acid-base indicators, such as litmus and red cabbage juice, turn different colors in acidic
Experiment: Acids, Bases, and Buffers * Introduction Many common household solutions contain acids and bases. Acid-base indicators, such as litmus and red cabbage juice, turn different colors in acidic
Characteristics of Chemical Change
 Section 2 Characteristics of Chemical Change What Do You See? Learning Outcomes In this section you will Observe several typical examples of evidence that a chemical change is occurring. Make generalizations
Section 2 Characteristics of Chemical Change What Do You See? Learning Outcomes In this section you will Observe several typical examples of evidence that a chemical change is occurring. Make generalizations
Reactant A (g) Reactant B (ml) Product (ml)
 Pre-Test Key Name: Chemical Reactions Date: 1. The chart below shows the amount of gas that is produced when two reactants (a solid and a liquid) are combined. However, one of the boxes is missing information.
Pre-Test Key Name: Chemical Reactions Date: 1. The chart below shows the amount of gas that is produced when two reactants (a solid and a liquid) are combined. However, one of the boxes is missing information.
