Virtual Library Lesson: Oobleck, Gloop, and Glurch
|
|
|
- Beryl Bradford
- 6 years ago
- Views:
Transcription
1 Oobleck, Gloop, and Glurch Lesson Overview Throughout this lesson, students will use inquiry skills to identify states of matter, describe physical properties, and modify the recipe to change physical properties of a substance. Students will have the opportunity to work in groups, create a foreign substance by following a recipe, record qualitative and quantitative observations in a data table, and reflect on their experiences. Standards Addressed SC Compare physical properties of matter (including melting or boiling point, density, and color) to the chemical property of reactivity with a certain substance (including the ability to burn or to rust) Compare physical changes (including changes in size, shape, and state) to chemical changes that are the result of chemical reactions (including changes in color or temperature and formation of a precipitate or gas). SC P.2B.1 Analyze and interpret data to describe substances using physical properties (including state, boiling/melting point, density, conductivity, color, hardness, and magnetic properties) and chemical properties (the ability to burn or rust). 7.P.2B.4 Plan and conduct controlled scientific investigations to answer questions about how physical and chemical changes affect the properties of different substances. NGSS MS-PS 1-2 Analyze and interpret data on the properties of substances before and after the substances interact to determine if a chemical reaction has occurred. MS-PS 1-4 Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.
2 Disciplinary Literacy Best Practices Highlighting Think Pair Share Lesson Plan Time Required Two-Four 60-minute Class Periods Disciplinary Vocabulary : reactant, product, Law of Conservation of Matter, physical changes, chemical changes Materials Needed: Metric measuring spoons (1 set per student group) Hand lens (1 per student group) Safety goggles (1 per student) Liquid starch (60 ml per student group) Table salt (2 g per student group) Cornstarch (150 ml per student group) Water Elmer s white school glue (1 small bottle per group plus a large bottle to make the glue mixture) Borax powder (1 box per class) (this can be found in the laundry supply aisle as 20 Mule Team Borax) Clear 16-oz. plastic cups (7 per student group and 6 for materials distribution center) Paper towels (1 roll) Zipper-type freezer sandwich bag (4 per student group) Wax paper (about 1 m per student group) Ruler (1 per student group) Assessment: Completed Data Table
3 Teacher Advance Preparation: 1. Prepare a glue mixture by combining equal parts water with equal parts Elmer s white school glue so that each student group has 60 ml of the mixture. Be sure to use Elmer s white school glue, as it contains a polymer known as polyvinyl acetate that is at a specific dilution rate. 2. Prepare a borax solution by adding 20 ml of borax to a liter of water so that each student group has 90 ml. Some borax powder may settle to the bottom of the container, so be sure to stir the borax to dissolve as much of it as possible. 3. Prepare copies of the Oobleck, Goop, and Glurch student investigation pages for each student group. 4. Set up a materials distribution center so that students can easily collect the materials for this activity. Set out the borax solution, glue mixture, plain water, salt, liquid starch, and cornstarch in appropriately labeled 16-ounce plastic cups at the materials distribution center. Also set out the roll of paper towels, small bottles of glue, and the clear plastic cups for each group.
4 Engage o Students will be introduced to foreign substances and will be given the recipe to make the mystery substances. Students will review the data collection chart and procedures. They will recall the physical properties of matter and understand the properties they should include in the data tables. Students will use the highlighting strategy to understand the quantities needed for materials and the order of mixing for the individual recipes. Explore o SAFETY: Students must wear eye protection during this investigation. They should neither taste nor smell any of the materials. o Have students complete the Oobleck, Goop, and Glurch investigation pages. The recipe and data charts have been provided on their student sheet. o Students should read the questions they will be answering before they begin as well. Explain o Students will examine the physical properties of their new substances and the ingredients of each. They will have to make an inference as to which ingredient determines the properties (or combination of ingredients) in order to complete the second part of the lab. Extend o Ask students to describe how they changed their recipes and report their results. o Students will understand the concept of how a system is the whole picture and has input (ingredients) and output (the material) and changing the input into a system will change the output, or results. o Students should try to think of other things they know that operate as input/output systems.
5 Teacher Reflections and Biographical Information I tried to complete this lesson in two 60 minute class sessions. I should have extended it to three or even four days. However, I felt the crunch of time. Students in one of my classes did not actually make their new substance, they only made the prediction and was able to make the inferences of properties based on the ingredients. In retrospect, I would have taken the extra day or two and completed the lab in depth. So many standards are covered and the concept of physical properties is difficult for them to grasp. We discussed physical vs chemical properties. We were able to talk about mixtures vs compounds, and elements. It is wonderful that this inquiry is flexible in that you can cut it off or allow the time to explore. The students were actively engaged the whole time because the results were not what they expected. Lesson Author: DeDee Quinn is an 7 th grade science teacher at the Middle School of Pacolet in Spartanburg School District 3 in Spartanburg, S.C. She has 3 years teaching experience and currently teaches 6 th and 7 th grade science.
6 Name: Date: Materials: Set of metric measuring spoons Hand lens Safety goggles 30 ml liquid starch 30 ml glue mixture 1 table salt Oobleck, Goop, and Glurch Lab Investigation 75 g cornstarch 30 ml water 30 ml white glue 30 ml borax solution 7 plastic cups 3 zipper-type sandwich bags 1 m of wax paper Ruler Marker Procedures: SAFETY: Wear eye protection and do not taste or smell any of the materials. 1. At the materials distribution center, get 7 plastic cups. Label the plastic cups with the names starch, white school glue mixture, table salt, cornstarch, water, white school glue, and borax solution. Place a small sample of each material in its labeled plastic cup (see the necessary sample amounts in the materials list above). Be sure to wash and dry the measuring spoon with water and a clean paper towel after each use. 2. Observe the physical appearance of each sample of material. Construct a table to record your observations of the physical properties of the starch, white glue mixture, table salt, cornstarch, water, white glue, and borax solution. Include descriptions of what you see, hear, and feel. Solution/Mixture Starch White Glue Mixture Table Salt Cornstarch Water White School Glue Borax Solution Other Physical Properties Color Solid-liquid Particle size 3. Now you will observe how these materials interact with each other. Carefully follow the directions for each gunk recipe. Mix and test the Glurch recipe first. Then mix and test Oobleck, and finally mix and test Goop. Glurch 30 ml liquid starch 30 ml glue mixture 1 g table salt Mix the starch and table salt together in the sandwich bag. Knead the bag for 2-3 minutes until the substances are well mixed. Then add the white glue mixture, again kneading the bad until the substances are well mixed. When a lump forms and it is hard to knead, take the lump out of the bag and squeeze out any excess liquid into a waste container. Oobleck 75 g cornstarch 30 ml water Add the water to the sandwich bag. Next, slowly add the cornstarch a little at a time to the water. Knead the bag to mix the substances together, making sure all of the cornstarch is wet. You may need to add a little more or less cornstarch so that the oobleck has a consistency light enough so it will flow through your fingers but solid enough to squeeze.
7 Goop 30 ml white glue 30 ml borax solution Squeeze approximately 30 ml of white glue [not the glue mixture] into one corner of a zipper-type sandwich bag. Add 30 ml of borax solution. Seal the bag and then knead the contents until a new substance is formed. Make certain both substances are thoroughly mixed together. 4. Test each gunk recipe three times using the science methods listed below. Record your results in the table. Roll Test Roll your gunk into a snake on the wax paper and see how long a skinny snake you can make before it breaks. Measure the length to the nearest centimeter. Pancake Test Press your gunk into a pancake on the wax paper. Make the largest pancake you can, but you must be able to lift it off of the wax paper without it breaking apart. Measure the diameter of your pancake to the nearest centimeter. Bounce Test Roll your gunk into a ball and drop it from the height of your desk. Measure the distance, to the nearest centimeter, that it bounces back up from the floor. Stretch Test Roll your gunk into a ball and then pull it apart to see how far you can stretch it before it breaks. Measure the distance of the stretch to the nearest centimeter. Glurch Test Results Test 1 Test 2 Test 3 Average Roll Test Pancake Test Bounce Test Stretch Test Oobleck Test Results Test 1 Test 2 Test 3 Average Roll Test Pancake Test Bounce Test Stretch Test
8 Goop Test Results Roll Test Pancake Test Bounce Test Stretch Test Test 1 Test 2 Test 3 Average 5. Why do you test each recipe three times instead of just once? 6. On what test does Glurch do best? Oobleck? Goop? 7. How would you change the recipe for either Glurch, Oobleck, or Goop to make the results for one of the tests even better? Tell your teacher which substance (Glurch, Oobleck, or Goop) your group will modify. Record your new recipe below. Remember to change only one variable in your recipe. If you want to change more than one variable, make another recipe. Get the additional materials you need to test your recipe. Construct a table to report your test results. 8. Each new substance made from these recipes can be described as a system. Systems are made of parts. Pick one of the recipes and identify the parts that make up the system. Compare the parts of the system to the overall product. How are their physical properties different? 9. A system has inputs that produce outputs, and changing the input can alter the final output. Using this information on systems, explain how changing the input on one of the recipes would change the output. Compare this to changing the recipe for pizza, cookies, or sandwiches. How might the pizza, cookies, or sandwiches change?
Objective Students will gain an understanding of how the properties of a solid material can affect how it interacts with water.
 OOBLECK! (1 Hour) Addresses NGSS Level of Difficulty: 4 Grade Range: K-2 OVERVIEW Students will examine the behavior of different types of solids when they are dissolved in water and explain those behaviors
OOBLECK! (1 Hour) Addresses NGSS Level of Difficulty: 4 Grade Range: K-2 OVERVIEW Students will examine the behavior of different types of solids when they are dissolved in water and explain those behaviors
What is Oobleck? Can you use THE SCIENTIFIC METHOD AND your senses to solve the mystery of Oobleck? Problem
 What is Oobleck? Can you use THE SCIENTIFIC METHOD AND your senses to solve the mystery of Oobleck? Problem Three liquids are mixed together in a plastic bag. Using your senses (except for taste) can you
What is Oobleck? Can you use THE SCIENTIFIC METHOD AND your senses to solve the mystery of Oobleck? Problem Three liquids are mixed together in a plastic bag. Using your senses (except for taste) can you
Creative Classroom Lessons
 Matter, Matter Everywhere! Solids, Liquids and Gases Unit Creative Classroom Lessons Contents: 1. Solids, Liquids and gases student folder cover 2. Introduction Activity: Mystery Box 3. Solids, Liquids
Matter, Matter Everywhere! Solids, Liquids and Gases Unit Creative Classroom Lessons Contents: 1. Solids, Liquids and gases student folder cover 2. Introduction Activity: Mystery Box 3. Solids, Liquids
Section 1: Four States of Matter (pages 60-67)
 Name: Sci Number: Period: Parent Signature: My little book of: Word: Pg found States of matter Solid Four States of Matter: Section 1 definitions: (pg60) My definition Chp 3 Draw/paste examples of all
Name: Sci Number: Period: Parent Signature: My little book of: Word: Pg found States of matter Solid Four States of Matter: Section 1 definitions: (pg60) My definition Chp 3 Draw/paste examples of all
States of Matter: A Solid Lesson where Liquids Can be a Gas!
 TEACHER GUIDE STATES OF MATTER 60 Minute Physical Science Lesson Science- to- Go! Program Grades: 1-3 States of Matter: A Solid Lesson where Liquids Can be a Gas! Description Your classroom will be converted
TEACHER GUIDE STATES OF MATTER 60 Minute Physical Science Lesson Science- to- Go! Program Grades: 1-3 States of Matter: A Solid Lesson where Liquids Can be a Gas! Description Your classroom will be converted
CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED)
 CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED) Overview: Students investigate chemical reactions using given substances. Students identify chemical reactions in their daily lives. Objectives: The student
CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED) Overview: Students investigate chemical reactions using given substances. Students identify chemical reactions in their daily lives. Objectives: The student
Partnerships Implementing Engineering Education Worcester Polytechnic Institute Worcester Public Schools Supported by: National Science Foundation
 Introduction to Engineering: 1.A.III Chemical Engineering Grade Level 1 Sessions Seasonality Instructional Mode(s) Team Size MA Frameworks WPS Benchmarks Key Words Session I: What do chemical engineers
Introduction to Engineering: 1.A.III Chemical Engineering Grade Level 1 Sessions Seasonality Instructional Mode(s) Team Size MA Frameworks WPS Benchmarks Key Words Session I: What do chemical engineers
Chapter 5, Lesson 5 Using Dissolving to Identify an Unknown
 Chapter 5, Lesson 5 Using Dissolving to Identify an Unknown Key Concepts Different substances are made from different atoms, ions, or molecules, which interact with water in different ways. Since dissolving
Chapter 5, Lesson 5 Using Dissolving to Identify an Unknown Key Concepts Different substances are made from different atoms, ions, or molecules, which interact with water in different ways. Since dissolving
Chapter 6, Lesson 9: Neutralizing Acids and Bases
 Chapter 6, Lesson 9: Neutralizing Acids and Bases Key Concepts ph is a measure of the concentration of H 3 O + ions in a solution. Adding an acid increases the concentration of H 3 O + ions in the solution.
Chapter 6, Lesson 9: Neutralizing Acids and Bases Key Concepts ph is a measure of the concentration of H 3 O + ions in a solution. Adding an acid increases the concentration of H 3 O + ions in the solution.
Name Period Date. Lab: Introduction to Stoichiometry
 Name Period Date Lab: Introduction to Stoichiometry Introduction: Reactants are not always present in the exact ratio required by a balanced chemical equation. In planning any cost-effective production
Name Period Date Lab: Introduction to Stoichiometry Introduction: Reactants are not always present in the exact ratio required by a balanced chemical equation. In planning any cost-effective production
Oobleck: A Program about States of Matter Presented by the Sciencenter in Ithaca, NY. Program Overview
 Oobleck: A Program about States of Matter Presented by the Sciencenter in Ithaca, NY Program Overview Oobleck introduces students to states of matter and scientific observation. The program is designed
Oobleck: A Program about States of Matter Presented by the Sciencenter in Ithaca, NY Program Overview Oobleck introduces students to states of matter and scientific observation. The program is designed
Lesson 2. Color change
 Lesson 2 Color change T E A C H E R G U I D E Lesson summary Students meet marine chemist Sera Tuikabe, who is studying ocean acidification in the water surrounding the Republic of the Fiji Islands. Students
Lesson 2 Color change T E A C H E R G U I D E Lesson summary Students meet marine chemist Sera Tuikabe, who is studying ocean acidification in the water surrounding the Republic of the Fiji Islands. Students
Substances and Mixtures:Separating a Mixture into Its Components
 MiraCosta College Introductory Chemistry Laboratory Substances and Mixtures:Separating a Mixture into Its Components EXPERIMENTAL TASK To separate a mixture of calcium carbonate, iron and sodium chloride
MiraCosta College Introductory Chemistry Laboratory Substances and Mixtures:Separating a Mixture into Its Components EXPERIMENTAL TASK To separate a mixture of calcium carbonate, iron and sodium chloride
Castle Challenge Teacher Instructions (First Third Grade)
 Castle Challenge Teacher Instructions (First Third Grade) The set of experiments explores the concept of surface tension. Students are asked to help Prince Charming save his princess from the castle by
Castle Challenge Teacher Instructions (First Third Grade) The set of experiments explores the concept of surface tension. Students are asked to help Prince Charming save his princess from the castle by
Chapter 1, Lesson 3: The Ups and Downs of Thermometers
 Chapter 1, Lesson 3: The Ups and Downs of Thermometers Key Concepts The way a thermometer works is an example of heating and cooling a liquid. When heated, the molecules of the liquid in the thermometer
Chapter 1, Lesson 3: The Ups and Downs of Thermometers Key Concepts The way a thermometer works is an example of heating and cooling a liquid. When heated, the molecules of the liquid in the thermometer
Lesson 1 Matter and Its Properties
 Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 Math Skills 15 School to Home 16 Key Concept Builders
Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 Math Skills 15 School to Home 16 Key Concept Builders
Part I: How Dense Is It? Fundamental Question: What is matter, and how do we identify it?
 Part I: How Dense Is It? Fundamental Question: What is matter, and how do we identify it? Everything on Earth is made of matter. Matter is as simple as a single element or as complex as the entire planet.
Part I: How Dense Is It? Fundamental Question: What is matter, and how do we identify it? Everything on Earth is made of matter. Matter is as simple as a single element or as complex as the entire planet.
MiSP Weathering and Erosion Worksheet #1 L1
 MiSP Weathering and Erosion Worksheet #1 L1 Weathering and Erosion Worksheet#1L1 Name Date L 1, 2, 3 MASS WASTING - GLACIAL CREEPING Advance Preparation Making Glacial Ooze Recipe for each lab group: The
MiSP Weathering and Erosion Worksheet #1 L1 Weathering and Erosion Worksheet#1L1 Name Date L 1, 2, 3 MASS WASTING - GLACIAL CREEPING Advance Preparation Making Glacial Ooze Recipe for each lab group: The
Separating the Mixture
 Separating the Mixture 40- to 1 50-minute session ACTIVITY OVERVIEW I N V E S T 5 I O N I G AT Students perform their procedures written in Activity 3, A Plan to Separate the Mixture, to physically separate
Separating the Mixture 40- to 1 50-minute session ACTIVITY OVERVIEW I N V E S T 5 I O N I G AT Students perform their procedures written in Activity 3, A Plan to Separate the Mixture, to physically separate
Saturday Science Lesson Plan Fall 2008
 Saturday Science Lesson Plan Fall 2008 LEARNING OBJECTIVES STANDARDS 1.1.1 Observe, describe, draw, and sort objects carefully to learn about them. 1.2.6 Describe and compare objects in terms of number,
Saturday Science Lesson Plan Fall 2008 LEARNING OBJECTIVES STANDARDS 1.1.1 Observe, describe, draw, and sort objects carefully to learn about them. 1.2.6 Describe and compare objects in terms of number,
Science of Slime. Fig. 1 Structure of poly (vinyl alcohol)
 Name: Science of Slime Understanding the structure of a material and how it behaves is a large part of what chemists and materials scientists do for a living. Scientists and engineers cannot use new materials
Name: Science of Slime Understanding the structure of a material and how it behaves is a large part of what chemists and materials scientists do for a living. Scientists and engineers cannot use new materials
Chapter 5, Lesson 2 Surface Tension
 Chapter 5, Lesson 2 Surface Tension Key Concepts The attraction of molecules at the surface of a liquid is called surface tension. The polarity of water molecules can help explain why water has a strong
Chapter 5, Lesson 2 Surface Tension Key Concepts The attraction of molecules at the surface of a liquid is called surface tension. The polarity of water molecules can help explain why water has a strong
Bay Area Scientists in Schools Presentation Plan
 Bay Area Scientists in Schools Presentation Plan Lesson Name Green Polymers Presenter(s) The Sarpong Group Grade Level 5 Standards Connection(s) chemical reactions to make molecules Abstract: Polymers
Bay Area Scientists in Schools Presentation Plan Lesson Name Green Polymers Presenter(s) The Sarpong Group Grade Level 5 Standards Connection(s) chemical reactions to make molecules Abstract: Polymers
Chemical Reactions: The Copper Cycle
 1 Chemical Reactions: The Copper Cycle ORGANIZATION Mode: pairs assigned by instructor Grading: lab notes, lab performance and post-lab report Safety: Goggles, closed-toe shoes, lab coat, long pants/skirts
1 Chemical Reactions: The Copper Cycle ORGANIZATION Mode: pairs assigned by instructor Grading: lab notes, lab performance and post-lab report Safety: Goggles, closed-toe shoes, lab coat, long pants/skirts
Mystery Substance Laboratory Experiment
 Mystery Substance Laboratory Experiment Name: 5 th Grade PSI Science Score: / 5 Experiment Question: How effectively can you determine what a mystery substance is by testing its observable properties?
Mystery Substance Laboratory Experiment Name: 5 th Grade PSI Science Score: / 5 Experiment Question: How effectively can you determine what a mystery substance is by testing its observable properties?
Chapter 5, Lesson 1: Water is a Polar Molecule
 Chapter 5, Lesson 1: Water is a Polar Molecule Key Concepts The water molecule, as a whole, has 10 protons and 10 electrons, so it is neutral. In a water molecule, the oxygen atom and hydrogen atoms share
Chapter 5, Lesson 1: Water is a Polar Molecule Key Concepts The water molecule, as a whole, has 10 protons and 10 electrons, so it is neutral. In a water molecule, the oxygen atom and hydrogen atoms share
Density: The property that compares an object s mass to its volume. Mass is the measure of the amount of matter that makes up an object.
 Science Chapter 6: Matter Study Guide Lesson One: Properties of Matter A property is a characteristic of an object. You can identify properties of matter using your senses. Color, Size, Shape, Texture,
Science Chapter 6: Matter Study Guide Lesson One: Properties of Matter A property is a characteristic of an object. You can identify properties of matter using your senses. Color, Size, Shape, Texture,
Released Science Inquiry Task A Cool Investigation
 Date: Your Name: Released Science Inquiry Task A Cool Investigation 07 Grade Student Test Booklet Directions: Science You will be reading a story and analyzing the data provided to answer a set of questions.
Date: Your Name: Released Science Inquiry Task A Cool Investigation 07 Grade Student Test Booklet Directions: Science You will be reading a story and analyzing the data provided to answer a set of questions.
Mixtures. Part 2 Add 50 ml of water (one full syringe) to each cup. Stir and observe. Write your observations on the opposite page.
 Mixtures Part 1 Prepare three cups. Put 1 level spoon (5 ml) of each solid material in each cup. Observe the three solid materials. Fill in the property chart below. Color Texture Particle shape Particle
Mixtures Part 1 Prepare three cups. Put 1 level spoon (5 ml) of each solid material in each cup. Observe the three solid materials. Fill in the property chart below. Color Texture Particle shape Particle
Chemical Energy Conversions. Vanderbilt Student Volunteers for Science VINSE/VSVS Rural
 Chemical Energy Conversions Vanderbilt Student Volunteers for Science 2018-2019 VINSE/VSVS Rural Important!!! Please use this resource to reinforce your understanding of the lesson! Make sure you have
Chemical Energy Conversions Vanderbilt Student Volunteers for Science 2018-2019 VINSE/VSVS Rural Important!!! Please use this resource to reinforce your understanding of the lesson! Make sure you have
5 th GRADE PHYSICAL AND CHEMICAL TESTS
 5 th GRADE PHYSICAL AND CHEMICAL TESTS Summary: Students investigate 5 unknown white powders. They gather clues by observing the physical and chemical changes of the powders. At the end of the activity,
5 th GRADE PHYSICAL AND CHEMICAL TESTS Summary: Students investigate 5 unknown white powders. They gather clues by observing the physical and chemical changes of the powders. At the end of the activity,
Save time by mixing the two solutions below in advance of the activity. You could do this with the participants if you have plenty of time.
 CREEPY PUTTY Grades 3 5, 6 8 30 45 minutes DESIGN CHALLENGE Experiment with the properties of materials as you manipulate a Silly Putty-like material to have different degrees of viscoelasticity. Create
CREEPY PUTTY Grades 3 5, 6 8 30 45 minutes DESIGN CHALLENGE Experiment with the properties of materials as you manipulate a Silly Putty-like material to have different degrees of viscoelasticity. Create
Activity 6.5 From gas to liquid to solid
 Activity 6.5 This activity is an extension of Activity 6.4a in which ice is used to make a container cold. As in Activity 6.4a, this activity will work only with sufficient water vapor in the air. Here,
Activity 6.5 This activity is an extension of Activity 6.4a in which ice is used to make a container cold. As in Activity 6.4a, this activity will work only with sufficient water vapor in the air. Here,
Matter Lesson 2. Learning Goal 3: I can describe the differences between physical and chemical changes of matter.
 Matter Lesson 2 Learning Goal 2: I can describe the differences between intensive physical properties, extensive physical properties, and chemical properties of matter. Learning Goal 3: I can describe
Matter Lesson 2 Learning Goal 2: I can describe the differences between intensive physical properties, extensive physical properties, and chemical properties of matter. Learning Goal 3: I can describe
The ABCs of Chemistry
 Hands-On Science The ABCs of Chemistry Michael Margolin illustrated by Lloyd Birmingham WALCH EDUCATION Contents To the Teacher... v... vii... viii... xvi... 1... 9.... 17... 28... 38... 45.... 52... 62...
Hands-On Science The ABCs of Chemistry Michael Margolin illustrated by Lloyd Birmingham WALCH EDUCATION Contents To the Teacher... v... vii... viii... xvi... 1... 9.... 17... 28... 38... 45.... 52... 62...
Relative Solubility of Transition Elements
 Microscale Relative Solubility of Transition Elements The transition elements are found in periods 4, 5, and 6 between groups 2 and 13 of the periodic table. As the atomic number increases across a row
Microscale Relative Solubility of Transition Elements The transition elements are found in periods 4, 5, and 6 between groups 2 and 13 of the periodic table. As the atomic number increases across a row
Lesson 4. Temperature change
 Lesson 4 Temperature change T E A C H E R G U I D E Lesson summary Students meet scientist Jason Williams, an industrial chemist who designs the materials and processes for making solar cells. He explains
Lesson 4 Temperature change T E A C H E R G U I D E Lesson summary Students meet scientist Jason Williams, an industrial chemist who designs the materials and processes for making solar cells. He explains
Describing Matter Laboratory
 Describing Matter Laboratory Name: 5 th Grade PSI Science Score: / 5 Experiment Question: How is matter identified? What are the observable properties of matter? Hypothesis Starters: 1. Your eyes help
Describing Matter Laboratory Name: 5 th Grade PSI Science Score: / 5 Experiment Question: How is matter identified? What are the observable properties of matter? Hypothesis Starters: 1. Your eyes help
Identifying Solids 1-2 KEY CONCEPTS AND PROCESS SKILLS KEY VOCABULARY ACTIVITY OVERVIEW L A B O R ATO R Y A-69
 Identifying Solids 40- to 1-2 50-minute sessions ACTIVITY OVERVIEW 7 L A B O R ATO R Y Students conduct tests on the solids separated from the mixture to gain information about the physical and chemical
Identifying Solids 40- to 1-2 50-minute sessions ACTIVITY OVERVIEW 7 L A B O R ATO R Y Students conduct tests on the solids separated from the mixture to gain information about the physical and chemical
Bay Area Scientists in Schools Presentation Plan
 Bay Area Scientists in Schools Presentation Plan Lesson Name Presenter(s) Chemical Reactions Mercedes Taylor, Nick Settineri, Jessica Ziegler, Tyler Hurlburt, Parker Deal Grade Level 5 Standards Connection(s)
Bay Area Scientists in Schools Presentation Plan Lesson Name Presenter(s) Chemical Reactions Mercedes Taylor, Nick Settineri, Jessica Ziegler, Tyler Hurlburt, Parker Deal Grade Level 5 Standards Connection(s)
Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic
 Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic Key Concepts Carbon dioxide (CO 2 ) gas dissolved in water can cause water to become acidic. The acidity of water from dissolved CO 2 can
Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic Key Concepts Carbon dioxide (CO 2 ) gas dissolved in water can cause water to become acidic. The acidity of water from dissolved CO 2 can
What Do You Think? Investigate GOALS
 Activity 7 Polymers GOALS In this activity you will: Make a polymer-based material that has properties different from other states of matter that you have studied. Observe the material s properties and
Activity 7 Polymers GOALS In this activity you will: Make a polymer-based material that has properties different from other states of matter that you have studied. Observe the material s properties and
Western Carolina University. Chem 132 Lab 04 Introduction to Physical Changes and Chemical Reactions Introduction
 Chem 132 Lab 04 Introduction to Physical Changes and Chemical Reactions Introduction This lab serves as an introduction to physical changes. Physical changes involve a change in the form of matter without
Chem 132 Lab 04 Introduction to Physical Changes and Chemical Reactions Introduction This lab serves as an introduction to physical changes. Physical changes involve a change in the form of matter without
2/22/2019 NEW UNIT! Chemical Interactions. Atomic Basics #19
 NEW UNIT! Chemical Interactions Atomic Basics #19 1 Vocabulary: Matter: Anything that has mass and takes up space. Atom: the smallest particle of matter. Element: A pure substance made up of only one type
NEW UNIT! Chemical Interactions Atomic Basics #19 1 Vocabulary: Matter: Anything that has mass and takes up space. Atom: the smallest particle of matter. Element: A pure substance made up of only one type
Exploring Acids & Bases
 Food Explorations Lab: Exploring Acids & Bases STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will use cabbage juice indicator to determine if two unknown samples are acids or
Food Explorations Lab: Exploring Acids & Bases STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will use cabbage juice indicator to determine if two unknown samples are acids or
Table of Contents DSM II. Powders and Crystals (Grades 3 5) Place your order by calling us toll-free
 DSM II Powders and Crystals (Grades 3 5) Table of Contents Actual page size: 8.5" x 11" Philosophy and Structure Overview 1 Overview Chart 2 Materials List 3 Schedule of Activities 4 Preparing for the
DSM II Powders and Crystals (Grades 3 5) Table of Contents Actual page size: 8.5" x 11" Philosophy and Structure Overview 1 Overview Chart 2 Materials List 3 Schedule of Activities 4 Preparing for the
School of Rock (1 hour, Then 20 Minutes Each Day for the Following Five Days)
 School of Rock ( hour, Then 0 Minutes Each Day for the Following Five Days) Addresses NGSS Level of Difficulty: Grade Range: 6-8 OVERVIEW In this activity, students explore the effects of physical and
School of Rock ( hour, Then 0 Minutes Each Day for the Following Five Days) Addresses NGSS Level of Difficulty: Grade Range: 6-8 OVERVIEW In this activity, students explore the effects of physical and
6th Grade: Great Salt Lake is Salty
 Curriculum written by Megan Black in partnership with The Great Salt Lake Institute at Westminster College. 6th Grade: Great Salt Lake is Salty Lesson Description: In this lesson students will compare
Curriculum written by Megan Black in partnership with The Great Salt Lake Institute at Westminster College. 6th Grade: Great Salt Lake is Salty Lesson Description: In this lesson students will compare
Polymerization. Objectives: Vocabulary: Materials: Students will: Safety:
 Author: Carla Brathwaite Date Created: August 5, 2008 Subject: Chemistry Level: High school Standards: New York State www.emsc.nysed.gov/ciai/ Standard 1 Analysis = inquiry and design Standard 4 The physical
Author: Carla Brathwaite Date Created: August 5, 2008 Subject: Chemistry Level: High school Standards: New York State www.emsc.nysed.gov/ciai/ Standard 1 Analysis = inquiry and design Standard 4 The physical
Characteristics of Chemical Change
 Section 2 Characteristics of Chemical Change What Do You See? Learning Outcomes In this section you will Observe several typical examples of evidence that a chemical change is occurring. Make generalizations
Section 2 Characteristics of Chemical Change What Do You See? Learning Outcomes In this section you will Observe several typical examples of evidence that a chemical change is occurring. Make generalizations
Experiment 15: Exploring the World of Polymers
 1 Experiment 15: Exploring the World of Polymers bjective: In this experiment, you will explore a class of chemical compounds known as polymers. You will synthesize and modify polymers, test their properties
1 Experiment 15: Exploring the World of Polymers bjective: In this experiment, you will explore a class of chemical compounds known as polymers. You will synthesize and modify polymers, test their properties
Chemistry: classifying chemical and physical changes in various materials/substances
 Chemistry: classifying chemical and physical changes in various materials/substances Nikki Schilling, Ames, St. Paul Mn Based on original activity from Cool Chemistry Concoctions by Joe Rhatigan and Veronika
Chemistry: classifying chemical and physical changes in various materials/substances Nikki Schilling, Ames, St. Paul Mn Based on original activity from Cool Chemistry Concoctions by Joe Rhatigan and Veronika
Percentage of Acetic Acid in Vinegar
 Microscale Percentage of Acetic Acid in Vinegar When sweet apple cider is fermented in the absence of oxygen, the product is an acid, vinegar. Most commercial vinegars are made by fermentation, but some,
Microscale Percentage of Acetic Acid in Vinegar When sweet apple cider is fermented in the absence of oxygen, the product is an acid, vinegar. Most commercial vinegars are made by fermentation, but some,
What Do You Think? Investigate GOALS
 Activity 2 More Chemical Changes GOALS In this activity you will: Observe several typical examples of evidence that a chemical change is occurring. Make generalizations about the combinations of materials
Activity 2 More Chemical Changes GOALS In this activity you will: Observe several typical examples of evidence that a chemical change is occurring. Make generalizations about the combinations of materials
Fluids: How thick are liquids?
 Fluids: How thick are liquids? Student Advanced Version Introduction: Fluids are substances that can flow under an applied force. What are some examples of fluids? We often think of fluids as substances
Fluids: How thick are liquids? Student Advanced Version Introduction: Fluids are substances that can flow under an applied force. What are some examples of fluids? We often think of fluids as substances
Ocean Acidification in a Cup Materials
 Ocean Acidification in a Cup Ocean acidification is a problem that humans will have to deal with as we release more and more carbon dioxide into the atmosphere. This activity demonstrates how water can
Ocean Acidification in a Cup Ocean acidification is a problem that humans will have to deal with as we release more and more carbon dioxide into the atmosphere. This activity demonstrates how water can
Chapter 2, Lesson 1: Heat, Temperature, and Conduction
 Chapter 2, Lesson 1: Heat, Temperature, and Conduction Key Concepts Adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. Removing energy (cooling)
Chapter 2, Lesson 1: Heat, Temperature, and Conduction Key Concepts Adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. Removing energy (cooling)
Titration with an Acid and a Base
 Skills Practice Titration with an Acid and a Base Titration is a process in which you determine the concentration of a solution by measuring what volume of that solution is needed to react completely with
Skills Practice Titration with an Acid and a Base Titration is a process in which you determine the concentration of a solution by measuring what volume of that solution is needed to react completely with
Igneous Rocks. How Do Igneous Rocks Form? Liquid to Solid
 Igneous Rocks Answering the Big Question The activities in this lesson will help students answer the Big Question by modeling the result of different cooling rates of magma and lava and by learning how
Igneous Rocks Answering the Big Question The activities in this lesson will help students answer the Big Question by modeling the result of different cooling rates of magma and lava and by learning how
Topic Students devise an experiment to determine the types of bonds in three compounds.
 Types of Bonds Topic Students devise an experiment to determine the types of bonds in three compounds. Introduction Molecules are made of atoms that are held together by either ionic or covalent bonds.
Types of Bonds Topic Students devise an experiment to determine the types of bonds in three compounds. Introduction Molecules are made of atoms that are held together by either ionic or covalent bonds.
Physical Properties of Matter. Examples of Physical Properties. QUESTION: How could you find the volume of air in an "empty" room?
 QUESTION: How could you find the volume of air in an "empty" room? The volume of regularly shaped solids can be calculated from their dimensions. For example, the volume of a rectangular solid is the product
QUESTION: How could you find the volume of air in an "empty" room? The volume of regularly shaped solids can be calculated from their dimensions. For example, the volume of a rectangular solid is the product
The complete lesson plan for this topic is included below.
 Home Connection Parent Information: Magnets provide a simple way to explore force with children. The power of a magnet is somewhat like magic to them and requires exploration to understand. When forces
Home Connection Parent Information: Magnets provide a simple way to explore force with children. The power of a magnet is somewhat like magic to them and requires exploration to understand. When forces
Modeling Conservation of Matter
 Modeling Conservation of Matter Imagine that you and two of your classmates want to make a strawberry banana smoothie. You lay out the ingredients: one banana, five strawberries and two scoops of ice cream.
Modeling Conservation of Matter Imagine that you and two of your classmates want to make a strawberry banana smoothie. You lay out the ingredients: one banana, five strawberries and two scoops of ice cream.
By All INdICATIONS (2 Hours)
 By All INdICATIONS (2 Hours) Addresses NGSS Level of Difficulty: 5 Grade Range: 6-8 OVERVIEW In this activity, students create an acid-base indicator using red cabbage extract. Students then use this indicator
By All INdICATIONS (2 Hours) Addresses NGSS Level of Difficulty: 5 Grade Range: 6-8 OVERVIEW In this activity, students create an acid-base indicator using red cabbage extract. Students then use this indicator
Limiting Reactants Lab
 Name: Teacher s Name: Class: Block: Date: Partners: Limiting Reactants Lab Purpose: Through experimentation, determine the limiting reactant and the percent yield in a chemical reaction that generates
Name: Teacher s Name: Class: Block: Date: Partners: Limiting Reactants Lab Purpose: Through experimentation, determine the limiting reactant and the percent yield in a chemical reaction that generates
Activity Title: It s Either Very Hot or Very Cold Up There!
 Grades 3-5 Teacher Pages Activity Title: It s Either Very Hot or Very Cold Up There! Activity Objective(s): In this activity, and the follow-up activity next week, teams will design and conduct experiments
Grades 3-5 Teacher Pages Activity Title: It s Either Very Hot or Very Cold Up There! Activity Objective(s): In this activity, and the follow-up activity next week, teams will design and conduct experiments
Reactivity of Halide Ions
 Microscale Reactivity of Halide Ions The four halide salts used in this experiment are found in your body. Although sodium fluoride is poisonous, trace amounts seem to be beneficial to humans in the prevention
Microscale Reactivity of Halide Ions The four halide salts used in this experiment are found in your body. Although sodium fluoride is poisonous, trace amounts seem to be beneficial to humans in the prevention
2 Properties of Matter
 CHAPTER 2 2 Properties of Matter SECTION Matter KEY IDEAS As you read this section, keep these questions in mind: Why are color, volume, and density physical properties? Why are flammability and reactivity
CHAPTER 2 2 Properties of Matter SECTION Matter KEY IDEAS As you read this section, keep these questions in mind: Why are color, volume, and density physical properties? Why are flammability and reactivity
Oil and Natural Gas in Arkansas Fossil Fuel Resources from the Natural State
 NS.1.7.1 NS.1.6.4 PS.5.5.2 PS.5.6.5 ESS.8.5.7 Oil and Natural Gas in Arkansas Fossil Fuel Resources from the Natural State Middle School Lesson Plan Lesson 3 : Oil and Natural Gas Deposits Science Grades
NS.1.7.1 NS.1.6.4 PS.5.5.2 PS.5.6.5 ESS.8.5.7 Oil and Natural Gas in Arkansas Fossil Fuel Resources from the Natural State Middle School Lesson Plan Lesson 3 : Oil and Natural Gas Deposits Science Grades
Effective January 2008 All indicators in Standard / 11
 Scientific Inquiry 8-1 The student will demonstrate an understanding of technological design and scientific inquiry, including process skills, mathematical thinking, controlled investigative design and
Scientific Inquiry 8-1 The student will demonstrate an understanding of technological design and scientific inquiry, including process skills, mathematical thinking, controlled investigative design and
Moooooooving Science Fun with Butter!
 Moooooooving Science Fun with Butter! Elementary-Middle-High School (5-9) Materials Activity 1 8oz Ball plastic freezer container with lid (10) 1.5 quart heavy cream room temperature Cold water for rinsing
Moooooooving Science Fun with Butter! Elementary-Middle-High School (5-9) Materials Activity 1 8oz Ball plastic freezer container with lid (10) 1.5 quart heavy cream room temperature Cold water for rinsing
Matter has observable properties.
 KEY CONCEPT Matter has observable properties. BEFORE, you learned Matter has mass and volume Matter is made of atoms Matter exists in different states NOW, you will learn About physical and chemical properties
KEY CONCEPT Matter has observable properties. BEFORE, you learned Matter has mass and volume Matter is made of atoms Matter exists in different states NOW, you will learn About physical and chemical properties
Stoichiometry: Baking Soda and Vinegar Reactions Teacher Version
 Stoichiometry: Baking Soda and Vinegar Reactions Teacher Version In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household chemicals
Stoichiometry: Baking Soda and Vinegar Reactions Teacher Version In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household chemicals
Title: Does Size Matter?: A student investigation of the inverse relationship between molecule size and per-unit-volume concentrations.
 Title: Does Size Matter?: A student investigation of the inverse relationship between molecule size and per-unit-volume concentrations. Author: Eric Kolb Date: May 5, 2003 Background: When comparing two
Title: Does Size Matter?: A student investigation of the inverse relationship between molecule size and per-unit-volume concentrations. Author: Eric Kolb Date: May 5, 2003 Background: When comparing two
Post-Show. Chemistry. Periodic Table of the Elements. After the Show. Traveling Science Shows
 Traveling Science Shows Post-Show Chemistry After the Show We recently presented a Chemistry show at your school, and thought you and your students might like to continue investigating this topic. The
Traveling Science Shows Post-Show Chemistry After the Show We recently presented a Chemistry show at your school, and thought you and your students might like to continue investigating this topic. The
Science Grade 08 Unit 04 Exemplar Lesson 01: Formulas, Equations, and the Conservation of Mass
 Unit: 04 Lesson: 01 Suggested Duration: 15 days Grade 08 Unit 04 Exemplar Lesson 01: Formulas, Equations, and the Conservation of Mass This lesson is one approach to teaching the State Standards associated
Unit: 04 Lesson: 01 Suggested Duration: 15 days Grade 08 Unit 04 Exemplar Lesson 01: Formulas, Equations, and the Conservation of Mass This lesson is one approach to teaching the State Standards associated
LEVEL ZERO VOICE CATALYST (10 minutes, individual work): 1. Counting atoms
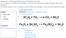 Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
The ability of a substance to be rolled. into wire The physical form of matter (solid, liquid, or gas)
 CHAPTER 2 2 Physical Properties SECTION The Properties of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What are physical properties of matter? What
CHAPTER 2 2 Physical Properties SECTION The Properties of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What are physical properties of matter? What
Physical and Chemical Changes Or How Do You Know When You ve Made Something New?
 Introduction Or How Do You Know When You ve Made Something New? Remember that all matter has characteristic physical and chemical properties. Matter can also undergo physical and chemical changes. How
Introduction Or How Do You Know When You ve Made Something New? Remember that all matter has characteristic physical and chemical properties. Matter can also undergo physical and chemical changes. How
A SLIPPERY SLIMY SUBSTANCE
 A SLIPPERY SLIMY SUBSTANCE PRE LAB DISCUSSION Scientists must use exact language to communicate their observations and ideas to other scientists. Many words have different meanings to different people.
A SLIPPERY SLIMY SUBSTANCE PRE LAB DISCUSSION Scientists must use exact language to communicate their observations and ideas to other scientists. Many words have different meanings to different people.
Unit 2: Chemistry. Unit Overview:
 Unit 2: Chemistry Unit Overview: This unit will focus on basic chemistry and some of the major process of organic chemistry (dehydration synthesis, hydrolysis, and enzyme action) that help form carbon
Unit 2: Chemistry Unit Overview: This unit will focus on basic chemistry and some of the major process of organic chemistry (dehydration synthesis, hydrolysis, and enzyme action) that help form carbon
Physical & Chemical PROPERTIES
 Name Test Date Hour Group Chem #4 Notebook Properties & Changes LEARNING TARGETS I can give examples of physical and chemical properties. I can give examples of physical and chemical changes. I can identify
Name Test Date Hour Group Chem #4 Notebook Properties & Changes LEARNING TARGETS I can give examples of physical and chemical properties. I can give examples of physical and chemical changes. I can identify
6.1- Chemical vs. Physical - Pre-Lab Questions
 6.1- Chemical vs. Physical - Pre-Lab Questions Name: Instructor: Date: Section/Group: 1. Using the procedures for each station provided as a guide, predict which properties you will be looking for in each
6.1- Chemical vs. Physical - Pre-Lab Questions Name: Instructor: Date: Section/Group: 1. Using the procedures for each station provided as a guide, predict which properties you will be looking for in each
Stoichiometry & Chemical Reactions
 Stoichiometry & Chemical Reactions Objectives: 1. Students will convert data from one unit of measure to another using dimensional analysis and stoichiometry. 2. Students will interpret data from data
Stoichiometry & Chemical Reactions Objectives: 1. Students will convert data from one unit of measure to another using dimensional analysis and stoichiometry. 2. Students will interpret data from data
Foundations of Chemistry
 Foundations of Chemistry Physical Properties Physical Properties As you read in Lesson 1, the arrangement of atoms determines whether matter is a substance or a mixture. The arrangement of atoms also determines
Foundations of Chemistry Physical Properties Physical Properties As you read in Lesson 1, the arrangement of atoms determines whether matter is a substance or a mixture. The arrangement of atoms also determines
Lesson Plan Book-stacking Activity
 T o g o d i r e c t l y t o a l e s s o n, c l i c k o n e o f t h e f o l l o w i n g l i n k s : B o o k - s t a c k i n g A c t i v i t y B a l l o o n A c t i v i t y H y d r o g e n G a s L a b F
T o g o d i r e c t l y t o a l e s s o n, c l i c k o n e o f t h e f o l l o w i n g l i n k s : B o o k - s t a c k i n g A c t i v i t y B a l l o o n A c t i v i t y H y d r o g e n G a s L a b F
A Visual Introduction to Ionic and Net Ionic Equations
 Overview This activity explores the phenomenon of chemical precipitation and asks students to construct an atomic level model of precipitation using ionic and net ionic equations. Initially, students run
Overview This activity explores the phenomenon of chemical precipitation and asks students to construct an atomic level model of precipitation using ionic and net ionic equations. Initially, students run
HOW DO PLANTS MEET THEIR NEEDS?
 Overview INSTRUCTIONS In this lesson students will germinate radish seeds and observe the root hairs on the root. Objectives On successful completion of this unit, students will be able to: germinate seeds;
Overview INSTRUCTIONS In this lesson students will germinate radish seeds and observe the root hairs on the root. Objectives On successful completion of this unit, students will be able to: germinate seeds;
From Which Planet is the Soil Sample From?
 Teacher From Which Planet is the Soil Sample From? NGSSS: SC.912.P.8.2: Differentiate between physical and chemical properties and physical and chemical changes of matter. Purpose of Lab/Activity: To separate
Teacher From Which Planet is the Soil Sample From? NGSSS: SC.912.P.8.2: Differentiate between physical and chemical properties and physical and chemical changes of matter. Purpose of Lab/Activity: To separate
Liquid X Lab. Number of Drops Before Spilling Trial 1 Trial 2 Trial 3. Write a conclusion: How do your results for Liquid X compare to water?
 Names Block Date BIG QUESTION: Liquid X Lab Station 1 Surface Tension, Cohesion, and Adhesion Water is cohesive, adhesive, and has surface tension. Does Liquid X have the same properties? 1. Use a pipette
Names Block Date BIG QUESTION: Liquid X Lab Station 1 Surface Tension, Cohesion, and Adhesion Water is cohesive, adhesive, and has surface tension. Does Liquid X have the same properties? 1. Use a pipette
Author(s): Tim Bumpus, Stephanie Sanders, Camilo Sarmiento, Daniel Urul. Standards: Next Generation Science Standards (www.nextgenscience.
 Unknown Powders Author(s): Tim Bumpus, Stephanie Sanders, Camilo Sarmiento, Daniel Urul Date Created: February, 2016 Subject: Chemistry Grade Level: Grades 3-6 Standards: Next Generation Science Standards
Unknown Powders Author(s): Tim Bumpus, Stephanie Sanders, Camilo Sarmiento, Daniel Urul Date Created: February, 2016 Subject: Chemistry Grade Level: Grades 3-6 Standards: Next Generation Science Standards
CLASSROOM KIT MAGNET EXPLORATION
 CLASSROOM KIT MAGNET EXPLORATION Page 1 1 Activity: What Do We Already Know? Teacher A simple, yet effective learning strategy, a K-W-L chart, is used to help Background: students clarify their ideas.
CLASSROOM KIT MAGNET EXPLORATION Page 1 1 Activity: What Do We Already Know? Teacher A simple, yet effective learning strategy, a K-W-L chart, is used to help Background: students clarify their ideas.
You be the Judge: Density
 1 2 You be the Judge: Density Objectives The students will: develop an understanding of density calculate density of objects collect, analyze, and interpret data solve equations collaborate with partners
1 2 You be the Judge: Density Objectives The students will: develop an understanding of density calculate density of objects collect, analyze, and interpret data solve equations collaborate with partners
Lesson Plan: Stearic Acid
 Lesson Plan: Stearic Acid Created by: In this lesson, students investigate how stearic acid undergoes a 2014 AACT Middle School phase change from solid to liquid and back from liquid to solid. Content
Lesson Plan: Stearic Acid Created by: In this lesson, students investigate how stearic acid undergoes a 2014 AACT Middle School phase change from solid to liquid and back from liquid to solid. Content
Chemistry Foundations of Chemistry Test. This is due:
 Chemistry Foundations of Chemistry Test This is due: Directions: Answer the following questions on a separate sheet of paper (or on this paper if you have room), staple to this paper (if you used a separate
Chemistry Foundations of Chemistry Test This is due: Directions: Answer the following questions on a separate sheet of paper (or on this paper if you have room), staple to this paper (if you used a separate
Bay Area Scientists in Schools Presentation Plan
 Bay Area Scientists in Schools Presentation Plan Lesson Name Presenter(s) The Water Cycle UC Berkeley PhD students Grade Level 1 Standards Connection(s) Earth Sciences, physics sciences CA Science Content
Bay Area Scientists in Schools Presentation Plan Lesson Name Presenter(s) The Water Cycle UC Berkeley PhD students Grade Level 1 Standards Connection(s) Earth Sciences, physics sciences CA Science Content
Name Date Period Molecular Nature of Water
 Name Date Period Molecular Nature of Water Purpose: To determine how water molecules react using molecular models and Lab demos. Materials: I cup of 12 water molecules (red & white), 1 Na (blue), 1 Cl
Name Date Period Molecular Nature of Water Purpose: To determine how water molecules react using molecular models and Lab demos. Materials: I cup of 12 water molecules (red & white), 1 Na (blue), 1 Cl
To understand concept of limiting reagents. To learn how to do a vacuum filtration. To understand the concept of recrystallization.
 E x p e r i m e n t Synthesis of Aspirin Experiment : http://genchemlab.wordpress.com/-aspirin/ objectives To synthesize aspirin. To understand concept of limiting reagents. To determine percent yield.
E x p e r i m e n t Synthesis of Aspirin Experiment : http://genchemlab.wordpress.com/-aspirin/ objectives To synthesize aspirin. To understand concept of limiting reagents. To determine percent yield.
Elements and Compounds
 Elements and Compounds Part I: Matter What is matter? Matter is everything that you can see, touch, taste, and feel. Anything that has mass and takes up space is matter. Mass measures how much matter is
Elements and Compounds Part I: Matter What is matter? Matter is everything that you can see, touch, taste, and feel. Anything that has mass and takes up space is matter. Mass measures how much matter is
THIRD GRADE OCEANS 1 WEEK LESSON PLANS AND ACTIVITIES
 THIRD GRADE OCEANS 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF THIRD GRADE WATER WEEK 1. PRE: Comparing the different components of the water cycle. LAB: Contrasting water with hydrogen
THIRD GRADE OCEANS 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF THIRD GRADE WATER WEEK 1. PRE: Comparing the different components of the water cycle. LAB: Contrasting water with hydrogen
