Table of Contents DSM II. Powders and Crystals (Grades 3 5) Place your order by calling us toll-free
|
|
|
- Harvey O’Connor’
- 6 years ago
- Views:
Transcription
1 DSM II Powders and Crystals (Grades 3 5) Table of Contents Actual page size: 8.5" x 11" Philosophy and Structure Overview 1 Overview Chart 2 Materials List 3 Schedule of Activities 4 Preparing for the Activities 5 Background Information Advance Preparation Materials Management Activity 1 7 What Is It? Activity 2 13 Use Your Senses! Activity 3 21 A Closer Look Activity 4 27 What s My Name? Activity 5 35 The Water Test Activity 6 43 The Vinegar Test Activity 7 49 The Iodine Test Activity 8 55 The BTB Test Activity 9 63 The Heat Test Activity Mystery Mixtures Activity Three New Substances ii Activity More Mystery Mixtures Assessment Activity 95 Glossary 99 References and Resources 100 Activity and Assessment Sheets 101
2 Overview This Delta Science Module introduces students to the techniques scientists use to study unknown substances, a process called chemical analysis. In Activity 1, students learn to describe and identify ordinary objects based on their properties. First, they describe the physical characteristics of classroom objects. Then they attempt to identify objects described by others. Finally, teams challenge one another to differentiate between similar objects based on their property descriptions. From this, students conclude that the best descriptions are those that are detailed and that focus on features peculiar to the object. In Activity 2, students are presented with six unidentified white substances (baking soda, citric acid, cornstarch, plaster of paris, salt, and sugar) and challenged to differentiate between them using their senses of sight, smell, hearing, and touch. In Activity 3, students use Pocketscopes, a kind of hand-held microscope, to make more detailed observations of the substances. They discover that three of the substances have a crystalline structure, and three are powders. Students learn the names of the six substances in Activity 4 after comparing them to six identified substances. In Activity 5, students observe how each substance interacts with water. By noting differences in the way each substance responds to water, students conclude that water tests can be used to differentiate between the substances. In Activity 6, students observe what happens when vinegar is added to each substance. They discover that vinegar fizzes when combined with baking soda evidence of a chemical reaction. From this, students conclude that vinegar can be used as an indicator for baking soda. In Activity 7, students observe what happens when iodine is added to each substance. They learn that iodine turns blue-black in the presence of starch. They note that other common materials, such as paper, also turn blue-black, indicating there is starch in them as well. In Activity 8, students test each substance with bromthymol blue (BTB), a ph indicator. Students are introduced to the concept of acids and bases, learn about the ph scale, and determine the relative ph of the six substances. Students begin Activity 9 by heating each substance over a candle and observing its response: Some melt, some burn, and some appear not to change at all. Students conclude that heat, too, can be used to help them distinguish between the substances. Students are challenged to identify four mystery mixtures in Activity 10. They test the mixtures, which are made from two or three of the substances, and compare their results with those obtained in previous activities. Students then use deductive reasoning to infer the ingredients in each mixture. Activity 11 introduces students to three new substances: baking powder, confectioners sugar, and flour. Students predict how these substances will respond to the same tests. They discover that two of these substances are actually mixtures, and the third, flour, is a relative of cornstarch derived from wheat. Finally, in Activity 12, students create their own mixtures from any three of the nine substances, then challenge their classmates to identify the ingredients in their mixture. Powders and Crystals 1
3 Materials List Qty Description 1 c aluminum foil 1 c baking powder, 10 oz 1 c baking soda, 1 lb 4 bottles, dropper, p/8 3 c BTB solution, 2 oz 16 c candles 1 c chart, Which Is Which? 1 c citric acid, 2 lb 16 clothespins 1 Color Sheet 1 c cornstarch, 1 lb 3 c cups, paper, soufflé, p/ cups, plastic, 1-oz 8 cups, plastic, 9-oz 1 c dots, adhesive, blue, p/96 1 c dots, adhesive, green, p/96 1 c dots, adhesive, orange, p/96 1 c dots, adhesive, red, p/96 1 c dots, adhesive, white, p/96 1 c dots, adhesive, yellow, p/96 1 c flour, baking, 2 lb 40 c index cards 2 c iodine, 2 oz 18 jars, with lids 3 c labels, p/100 8 magnifiers 16 pans, aluminum 1 c paper, construction, black, p/50 1 c plaster of paris, 2 lb Qty Description 16 Pocketscopes 1 c salt, 26 oz 1 slides, depression, p/40 18 spoons, plastic 1 c sticks, stirring, p/8 1 c sugar, confectioners, 1 lb 1 c sugar, granulated, 5 lb 2 c toothpicks, flat, p/750 8 trays, plastic 1 c vinegar, 16 oz 1 teacher s guide Teacher provided items 8 cans, metal, small 8 crayons 8 markers, felt-tip 8 matches, books newspaper paper towels 16 paper, plain 8 pencils 8 pens, ball-point 1 ruler, metric 32 safety goggles 8 scissors tape, transparent water, distilled water, tap = in separate box c = consumable item Powders and Crystals 3
4 Activity 3 A Closer Look Objectives Students use Pocketscopes to observe detailed differences in the structure of the mystery substances. The students use a Pocketscope to examine the mystery substances more closely learn the difference between powders and crystals differentiate between the substances based on the structure of their particles About 40 minutes Schedule For the class 2 jars *baking soda (blue dot) 2 jars * citric acid (green dot) 2 jars * cornstarch (orange dot) 1 cup, plastic, 1-oz 1 roll * paper towels 2 jars *plaster of paris (red dot) 2 jars *salt (white dot) 12 * spoons, plastic, color-coded 2 jars * sugar (yellow dot) * tape, transparent 1 toothpick, flat *provided by the teacher or from a previous activity Preparation 1. Make a copy of Activity Sheet 3 for each student. crystal particle powder Vocabulary Materials 2. Set up two distribution stations and place one set of six color-coded jars and spoons at each station. 3. You will need a pair of safety goggles, a toothpick, a 1-oz cup containing a very small amount of cornstarch, a depression slide, a Pocketscope, and a sheet of white paper for a class demonstration. For each student 1 Activity Sheet 3 1 pair * safety goggles For each team of four 1 * Color Sheet 6 * cups, plastic, 1-oz, color-coded 2 Pocketscopes 2 slides, depression 6 toothpicks, flat 1 tray, plastic 4. Each student will need a pair of safety goggles. Each team of four will need a tray, a Color Sheet, six color-coded cups, six toothpicks, two depression slides, two Pocketscopes, two sheets of white paper, three small pieces of transparent tape, several paper towels, and access to the substances at either distribution station. Activity 3 A Closer Look 21
5 Background Information In this activity, students use Pocketscopes to examine the structure of the particles that make up each of the six substances. They discover that three of the substances are in crystalline form composed of crystals and three are in powder form. A crystal is a transparent solid that has a regularly repeating internal arrangement of atoms or ions, resulting in a particular structure and shape. Of the crystals in this activity, the salt is the most regular in shape, appearing as tiny cubes of various sizes. The sugar and the citric acid are also composed of crystals, but are more irregular in shape, possibly because the conditions under which they form cause them to form imperfectly. The baking soda, plaster of paris, and cornstarch are not crystalline in form but are powders. A powder is a substance composed of finely ground or pulverized loose solid particles. Name Activity Sheet 3 A Closer Look Examine each substance with a Pocketscope. Then draw and describe it in the chart below. Substance (color code) blue green orange red white yellow What do the particles of this substance look like when viewed with the Pocketscope? Teaching Suggestions Distribute a copy of Activity Sheet 3 and a pair of safety goggles to each student. To each team of four distribute a tray, a Color Sheet, six color-coded cups, six toothpicks, two depression slides, two Pocketscopes, and several paper towels. Have students collect samples of each of the six substances from either distribution station. Demonstrate how to use a Pocketscope to examine the cornstarch, baking soda, and plaster of paris. 1 2 Additional Information Students need only a very small amount of each substance for this activity. Remind them to use the color-coded spoons to avoid cross-contaminating the substances. Put on a pair of safety goggles. Use a toothpick to place a very small amount of cornstarch in the well of a depression slide. Carefully insert the slide under the clips of the Pocketscope. Place your eye near the lens, point the Pocketscope toward the light, and slowly squeeze the frame together until the image is in focus. 22 Activity 3 A Closer Look
6 Next, demonstrate how to use the Pocketscope to examine the salt, sugar, and citric acid by dropping a few salt grains on to the adhesive side of a small piece of transparent tape and placing the tape over the viewing hole of the Pocketscope. Hold the Pocketscope up to the light as before. Tell students to put on their safety goggles and begin examining the substances with the Pocketscopes. Tell them to wipe their slides clean with a paper towel after each use and to record their observations on Activity Sheet 3. After students have had an opportunity to examine all six substances under a Pocketscope, have them place their materials on the tray and set it aside. Ask, Do the substances all look alike? Write the word particle on the board. Tell students that a particle is a tiny piece of something. Ask, How are the particles of these substances different from one another? Write the word crystal on the board. Tell students that a crystal is a solid form of a substance in which the atoms are arranged in repeating patterns. Because of this, crystals often have a distinctive shape characterized by regular, repeating surfaces. A substance composed of crystals is said to be crystalline in form. 3 Figure 3-1. Using a Pocketscope to view a powder. Circulate and help students focus the Pocketscopes. They should use a fresh piece of tape for each crystal they examine as well. Students should have noted that the substances do not all look alike. Some of the substances look like dust, even under magnification, while others look like tiny crystals of different shapes. Activity 3 A Closer Look 23
7 Ask, Which of the substances could we call crystals? The green-dot (citric acid), white-dot (salt), and yellow-dot (sugar) substances are composed of crystals. salt sugar citric acid Figure 3-2. Crystals of salt, sugar, and citric acid. Write the word powder on the board and explain that a powder is a substance that has been ground or pulverized into finely dispersed, loose, solid particles. The particles in a powder are not crystalline in form. Ask, Which of the substances could we call powders? Challenge students to help you write descriptions on the board that would allow them to tell the substances apart. If there are any disagreements over what to write, have students re-examine the substances and edit the descriptions until everyone agrees. Use the students own words for the descriptions. Ask, Are there any substances that you could not tell apart in Activity 2 that you now can after examining them with a Pocketscope? The blue-dot (baking soda), orange-dot (cornstarch), and red-dot (plaster of paris) substances are powders. For example, the blue-dot substance (baking soda) has tiny, somewhat transparent particles; the greendot substance (citric acid) has transparent, irregularly rounded crystals of different sizes; the orange-dot (cornstarch) and red-dot (plaster of paris) substances both have very fine, dustlike particles; the white-dot substance (salt) has cubeshaped crystals all about the same size; and the yellow-dot substance (sugar) has cubic crystals (two stuck together may appear rectangular) of various sizes. Answers may vary, but the most likely candidates are the substances with a crystalline form, such as the green-, white-, and yellow-dot substances (citric acid, salt, and sugar). 24 Activity 3 A Closer Look
8 Have students draw pictures of the salt, sugar, and citric acid crystals as seen with the Pocketscope. Challenge other students to try to tell which substance is which from Have students discard the toothpicks and the substances in their cups. Collect the trays, Color Sheets, cups, slides, and Pocketscopes. Wipe the cups and slides clean with a paper towel, and return all Reinforcement R Cleanup C the pictures. Have students add to or amend their drawings until other students can tell them apart. materials, including the jars and spoons, to the kit. Collect the activity sheets and save them for use in later activities. Have students wash their hands. Activity 3 A Closer Look 25
9 Connections Science Challenge After students have grown crystals as described in the first and second Science Extensions below, suggest that they experiment with different variables in crystal-growing contests. Variables include the strength of the solution, the temperature at which the crystals are grown, and the length of time they are allowed to grow. Who can grow the largest crystal? the tiniest? the greatest number? Science Extension Making rock candy is an enjoyable way for youngsters to investigate crystals. Have students tie a small, clean, rust-proof nail to a heavy string, then suspend the nail in a jar filled with a supersaturated sugar solution and leave the jar where it will not be disturbed. Have students examine the sugar crystals with a magnifier and identify their shape. (cubic or rectangular) When crystal growth has stopped, let students remove and eat the rock candy. Ask students to look through science textbooks and activity books to find other ways to grow crystals from a saturated solution of salt, sugar, or other material. For a unique method of growing sugar crystals in a gel, students can use A+ Projects in Chemistry by Janice VanCleave (John Wiley & Sons, 1993). Some mineral crystals are valued as precious or semiprecious gems. Encourage students to investigate various gems. If students investigate the six basic crystal forms in Science and Math below, they may want to identify examples of gems for each form. (Examples: cubic, diamond; hexagonal, ruby; tetragonal, zircon; orthorhombic, topaz; monoclinic, jade; and triclinic, moonstone) Science and the Arts Let each student draw a snowflake or a simple snow scene on dark blue construction paper with a brush dipped into a saturated salt solution (3 teaspoons of salt to 0.25 cup of water). Tell students to stir the solution with the brush before making each stroke. After the papers are left to dry in direct sunlight or in an oven preheated to 150 F and then turned off, the drawings will appear in white, shiny crystals on the dark background. Science and Math Your more advanced students might like to find out about the six basic geometric forms in which crystals are found: cubic, hexagonal, tetragonal, orthorhombic, monoclinic, and triclinic. In addition to researching the characteristics and names of the forms, students could build threedimensional models of them with oaktag or posterboard. Let students display their labeled models by suspending them from the classroom ceiling or from a cord stretched across the room above head level. Science, Technology, and Society In the early 1900s, radios had tuners that incorporated crystals. Have students research these early radios. Kits for making crystal radios are frequently available in hobby shops. Some students may want to build one and demonstrate it to the rest of the class. Suggest that students do library research to find out about magnifying lenses and how they are made. Ask students to share their findings in oral reports or bulletin board displays. Students also might like to read about early microscopes that used a drop of water as the lens. They may be able to find directions for making such a microscope. 26 Activity 3 A Closer Look
Virtual Library Lesson: Oobleck, Gloop, and Glurch
 Oobleck, Gloop, and Glurch Lesson Overview Throughout this lesson, students will use inquiry skills to identify states of matter, describe physical properties, and modify the recipe to change physical
Oobleck, Gloop, and Glurch Lesson Overview Throughout this lesson, students will use inquiry skills to identify states of matter, describe physical properties, and modify the recipe to change physical
Magnetism BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN
 activity 5 Magnetism BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN Grade 1 Quarter 1 Activity 5 SC.A.1.1.1 The student knows that objects can be described, classified, and compared by their composition
activity 5 Magnetism BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN Grade 1 Quarter 1 Activity 5 SC.A.1.1.1 The student knows that objects can be described, classified, and compared by their composition
5 th GRADE PHYSICAL AND CHEMICAL TESTS
 5 th GRADE PHYSICAL AND CHEMICAL TESTS Summary: Students investigate 5 unknown white powders. They gather clues by observing the physical and chemical changes of the powders. At the end of the activity,
5 th GRADE PHYSICAL AND CHEMICAL TESTS Summary: Students investigate 5 unknown white powders. They gather clues by observing the physical and chemical changes of the powders. At the end of the activity,
Describing Matter Laboratory
 Describing Matter Laboratory Name: 5 th Grade PSI Science Score: / 5 Experiment Question: How is matter identified? What are the observable properties of matter? Hypothesis Starters: 1. Your eyes help
Describing Matter Laboratory Name: 5 th Grade PSI Science Score: / 5 Experiment Question: How is matter identified? What are the observable properties of matter? Hypothesis Starters: 1. Your eyes help
Plant and Animal Populations
 Plant and Animal Populations T ABLE OF CONTENTS ABOUT DELTA SCIENCE MODULES Program Introduction................... iii Teacher s Guide..................... iv Delta Science Readers............... vi Equipment
Plant and Animal Populations T ABLE OF CONTENTS ABOUT DELTA SCIENCE MODULES Program Introduction................... iii Teacher s Guide..................... iv Delta Science Readers............... vi Equipment
Finding the Moon. DELTA SCIENCE READER Overview Before Reading Guide the Reading After Reading
 Finding the Moon T ABLE OF CONTENTS ABOUT DELTA SCIENCE MODULES Program Introduction................... iii Teacher s Guide..................... iv Delta Science Readers............... vi Equipment and
Finding the Moon T ABLE OF CONTENTS ABOUT DELTA SCIENCE MODULES Program Introduction................... iii Teacher s Guide..................... iv Delta Science Readers............... vi Equipment and
(Sessions I and II)* BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN FOR PERSONAL USE
 activities 19&20 What Do Plants Need? (Sessions I and II)* BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN Grade 1 Quarter 2 Activities 19 & 20 SC.A.1.1.1 The student knows that objects can be described,
activities 19&20 What Do Plants Need? (Sessions I and II)* BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN Grade 1 Quarter 2 Activities 19 & 20 SC.A.1.1.1 The student knows that objects can be described,
Chapter 6, Lesson 9: Neutralizing Acids and Bases
 Chapter 6, Lesson 9: Neutralizing Acids and Bases Key Concepts ph is a measure of the concentration of H 3 O + ions in a solution. Adding an acid increases the concentration of H 3 O + ions in the solution.
Chapter 6, Lesson 9: Neutralizing Acids and Bases Key Concepts ph is a measure of the concentration of H 3 O + ions in a solution. Adding an acid increases the concentration of H 3 O + ions in the solution.
CLASSROOM SCIENCE ACTIVITIES
 CLASSROOM SCIENCE E ACTIVITIES ITIES Instructional note Analyze and interpret data from fossils to provide evidence of the organisms and the environments in which they lived long ago. (3-LS4-1) 3 From
CLASSROOM SCIENCE E ACTIVITIES ITIES Instructional note Analyze and interpret data from fossils to provide evidence of the organisms and the environments in which they lived long ago. (3-LS4-1) 3 From
Saturday Science Lesson Plan Fall 2008
 Saturday Science Lesson Plan Fall 2008 LEARNING OBJECTIVES STANDARDS 1.1.1 Observe, describe, draw, and sort objects carefully to learn about them. 1.2.6 Describe and compare objects in terms of number,
Saturday Science Lesson Plan Fall 2008 LEARNING OBJECTIVES STANDARDS 1.1.1 Observe, describe, draw, and sort objects carefully to learn about them. 1.2.6 Describe and compare objects in terms of number,
Mystery Substance Laboratory Experiment
 Mystery Substance Laboratory Experiment Name: 5 th Grade PSI Science Score: / 5 Experiment Question: How effectively can you determine what a mystery substance is by testing its observable properties?
Mystery Substance Laboratory Experiment Name: 5 th Grade PSI Science Score: / 5 Experiment Question: How effectively can you determine what a mystery substance is by testing its observable properties?
Geology, Part 2: What Is the Mystery Rock?
 Geology, Part 2: What Is the Mystery Rock? You and your partner will be given 14 rocks to identify. Using the chart provided, follow the steps by observing your rocks closely and using the tools available.
Geology, Part 2: What Is the Mystery Rock? You and your partner will be given 14 rocks to identify. Using the chart provided, follow the steps by observing your rocks closely and using the tools available.
Activity 6.5 From gas to liquid to solid
 Activity 6.5 This activity is an extension of Activity 6.4a in which ice is used to make a container cold. As in Activity 6.4a, this activity will work only with sufficient water vapor in the air. Here,
Activity 6.5 This activity is an extension of Activity 6.4a in which ice is used to make a container cold. As in Activity 6.4a, this activity will work only with sufficient water vapor in the air. Here,
Chapter 5, Lesson 5 Using Dissolving to Identify an Unknown
 Chapter 5, Lesson 5 Using Dissolving to Identify an Unknown Key Concepts Different substances are made from different atoms, ions, or molecules, which interact with water in different ways. Since dissolving
Chapter 5, Lesson 5 Using Dissolving to Identify an Unknown Key Concepts Different substances are made from different atoms, ions, or molecules, which interact with water in different ways. Since dissolving
After you are finished, you can collect the salol, which should come off the plastic wrap easily. It can then be melted again and reused.
 IGNEOUS ROCK TEXTURES Teacher Information: Be sure to explain and use all standard laboratory safety practices and procedures. Be sure your students understand how to safely handle the chemicals and materials
IGNEOUS ROCK TEXTURES Teacher Information: Be sure to explain and use all standard laboratory safety practices and procedures. Be sure your students understand how to safely handle the chemicals and materials
Post-Show. Chemistry. Periodic Table of the Elements. After the Show. Traveling Science Shows
 Traveling Science Shows Post-Show Chemistry After the Show We recently presented a Chemistry show at your school, and thought you and your students might like to continue investigating this topic. The
Traveling Science Shows Post-Show Chemistry After the Show We recently presented a Chemistry show at your school, and thought you and your students might like to continue investigating this topic. The
INTRODUCTION TO LESSON CLUSTER 5
 INTRODUCTION TO LESSON CLUSTER 5 EXPLAINING DISSOLVING A. Lesson Cluster Goals and Lesson Objectives Goals Students should be able to explain dissolving of solids in liquids in terms of molecules. Lesson
INTRODUCTION TO LESSON CLUSTER 5 EXPLAINING DISSOLVING A. Lesson Cluster Goals and Lesson Objectives Goals Students should be able to explain dissolving of solids in liquids in terms of molecules. Lesson
Igneous Rocks. How Do Igneous Rocks Form? Liquid to Solid
 Igneous Rocks Answering the Big Question The activities in this lesson will help students answer the Big Question by modeling the result of different cooling rates of magma and lava and by learning how
Igneous Rocks Answering the Big Question The activities in this lesson will help students answer the Big Question by modeling the result of different cooling rates of magma and lava and by learning how
Lesson 2. Color change
 Lesson 2 Color change T E A C H E R G U I D E Lesson summary Students meet marine chemist Sera Tuikabe, who is studying ocean acidification in the water surrounding the Republic of the Fiji Islands. Students
Lesson 2 Color change T E A C H E R G U I D E Lesson summary Students meet marine chemist Sera Tuikabe, who is studying ocean acidification in the water surrounding the Republic of the Fiji Islands. Students
5th Grade. Slide 1 / 67. Slide 2 / 67. Slide 3 / 67. Matter and Its Interactions. Table of Contents: Matter and Its Interactions
 Slide 1 / 67 Slide 2 / 67 5th Grade Matter and Its Interactions 2015-11-02 www.njctl.org Table of Contents: Matter and Its Interactions Slide 3 / 67 Click on the topic to go to that section What Is Matter?
Slide 1 / 67 Slide 2 / 67 5th Grade Matter and Its Interactions 2015-11-02 www.njctl.org Table of Contents: Matter and Its Interactions Slide 3 / 67 Click on the topic to go to that section What Is Matter?
Separating the Mixture
 Separating the Mixture 40- to 1 50-minute session ACTIVITY OVERVIEW I N V E S T 5 I O N I G AT Students perform their procedures written in Activity 3, A Plan to Separate the Mixture, to physically separate
Separating the Mixture 40- to 1 50-minute session ACTIVITY OVERVIEW I N V E S T 5 I O N I G AT Students perform their procedures written in Activity 3, A Plan to Separate the Mixture, to physically separate
Sc1 Collecting & using evidence, making observations, evaluating & presenting results, making fair tests and comparisons, identifying patterns
 A collection of experiments in which pupils carefully study and analyse evidence to solve a crime. Could be used as an off-timetable activity for a Science Week etc. Curriculum Links: Sc1 Collecting &
A collection of experiments in which pupils carefully study and analyse evidence to solve a crime. Could be used as an off-timetable activity for a Science Week etc. Curriculum Links: Sc1 Collecting &
SUPPLY LIST. Science 500
 2017-18 SUPPLY LIST Science 500 Table of Contents UNIT 1: CELLS... 1 UNIT 2: PLANTS: LIFE CYCLES... 2 UNIT 3: ANIMALS: LIFE CYCLES... 3 UNIT 4: WEB OF LIFE... 3 UNIT 5: TRANSFORMATION OF ENERGY... 4 UNIT
2017-18 SUPPLY LIST Science 500 Table of Contents UNIT 1: CELLS... 1 UNIT 2: PLANTS: LIFE CYCLES... 2 UNIT 3: ANIMALS: LIFE CYCLES... 3 UNIT 4: WEB OF LIFE... 3 UNIT 5: TRANSFORMATION OF ENERGY... 4 UNIT
Erosion. erosion OBJECTIVES SCHEDULE PREPARATION VOCABULARY MATERIALS. For the class. The students. For each student. For each team of four
 activity 2 Erosion OBJECTIVES In this activity, students are introduced to the process of erosion. They use stream tables to demonstrate the relationship between moving water and erosion. The students
activity 2 Erosion OBJECTIVES In this activity, students are introduced to the process of erosion. They use stream tables to demonstrate the relationship between moving water and erosion. The students
States of Matter: A Solid Lesson where Liquids Can be a Gas!
 TEACHER GUIDE STATES OF MATTER 60 Minute Physical Science Lesson Science- to- Go! Program Grades: 1-3 States of Matter: A Solid Lesson where Liquids Can be a Gas! Description Your classroom will be converted
TEACHER GUIDE STATES OF MATTER 60 Minute Physical Science Lesson Science- to- Go! Program Grades: 1-3 States of Matter: A Solid Lesson where Liquids Can be a Gas! Description Your classroom will be converted
Exploring Acids & Bases
 Food Explorations Lab: Exploring Acids & Bases STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will use cabbage juice indicator to determine if two unknown samples are acids or
Food Explorations Lab: Exploring Acids & Bases STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will use cabbage juice indicator to determine if two unknown samples are acids or
SCIENCE 600 CA SUPPLY LIST
 2014-2015 Supply List SCIENCE 600 CA SUPPLY LIST 2014 GLYNLYON, INC. Table of Contents UNIT 1: PLANT SYSTEMS... 1 UNIT 2: WEATHER... 2 UNIT 3: PLANT AND ANIMAL BEHAVIOR... 2 UNIT 4: EARTH IN SPACE... 3
2014-2015 Supply List SCIENCE 600 CA SUPPLY LIST 2014 GLYNLYON, INC. Table of Contents UNIT 1: PLANT SYSTEMS... 1 UNIT 2: WEATHER... 2 UNIT 3: PLANT AND ANIMAL BEHAVIOR... 2 UNIT 4: EARTH IN SPACE... 3
Chemistry: classifying chemical and physical changes in various materials/substances
 Chemistry: classifying chemical and physical changes in various materials/substances Nikki Schilling, Ames, St. Paul Mn Based on original activity from Cool Chemistry Concoctions by Joe Rhatigan and Veronika
Chemistry: classifying chemical and physical changes in various materials/substances Nikki Schilling, Ames, St. Paul Mn Based on original activity from Cool Chemistry Concoctions by Joe Rhatigan and Veronika
Crystals! Table of Contents. Vocabulary 2. Word Search 6. What is a Crystal? 7. Atoms, Ions, Molecules. and the Unit Cell 13.
 Crystals! Table of Contents Vocabulary 2 Word Search 6 What is a Crystal? 7 Atoms, Ions, Molecules and the Unit Cell 13 Crystal Shapes 15 X-Ray Crystallography 17 Recipes for Making A Booklet for Elementary
Crystals! Table of Contents Vocabulary 2 Word Search 6 What is a Crystal? 7 Atoms, Ions, Molecules and the Unit Cell 13 Crystal Shapes 15 X-Ray Crystallography 17 Recipes for Making A Booklet for Elementary
How can you tell rocks on another planet apart?
 How can you tell rocks on another planet apart? Grade Range: K - 6 G.L.E Focus: 1.1.5 Time Budget: 1 hour WASL Vocabulary: Overview: Students learn that scientists send rovers to other planets to learn
How can you tell rocks on another planet apart? Grade Range: K - 6 G.L.E Focus: 1.1.5 Time Budget: 1 hour WASL Vocabulary: Overview: Students learn that scientists send rovers to other planets to learn
Weather Watching. DELTA SCIENCE READER Overview Before Reading Guide the Reading After Reading WEATHER WATCHING OVERVIEW
 Weather Watching T ABLE OF CONTENTS ABOUT DELTA SCIENCE MODULES Program Introduction................... iii Teacher s Guide..................... iv Delta Science Readers............... vi Equipment and
Weather Watching T ABLE OF CONTENTS ABOUT DELTA SCIENCE MODULES Program Introduction................... iii Teacher s Guide..................... iv Delta Science Readers............... vi Equipment and
Bay Area Scientists in Schools Presentation Plan
 Bay Area Scientists in Schools Presentation Plan Lesson Name Presenter(s) The Water Cycle UC Berkeley PhD students Grade Level 1 Standards Connection(s) Earth Sciences, physics sciences CA Science Content
Bay Area Scientists in Schools Presentation Plan Lesson Name Presenter(s) The Water Cycle UC Berkeley PhD students Grade Level 1 Standards Connection(s) Earth Sciences, physics sciences CA Science Content
Where do they come from?
 Exploring Meteorite Mysteries Lesson 5 Looking at Asteroids Objectives Students will: gather data by observing, measuring, and manipulating objects. record observations, analyze data and draw analogies.
Exploring Meteorite Mysteries Lesson 5 Looking at Asteroids Objectives Students will: gather data by observing, measuring, and manipulating objects. record observations, analyze data and draw analogies.
Chemical Energy Conversions. Vanderbilt Student Volunteers for Science VINSE/VSVS Rural
 Chemical Energy Conversions Vanderbilt Student Volunteers for Science 2018-2019 VINSE/VSVS Rural Important!!! Please use this resource to reinforce your understanding of the lesson! Make sure you have
Chemical Energy Conversions Vanderbilt Student Volunteers for Science 2018-2019 VINSE/VSVS Rural Important!!! Please use this resource to reinforce your understanding of the lesson! Make sure you have
Chemical Change. Day 1: 70 minutes; Day 2: minutes
 Chemical Change Lesson Concept When one substance interacts with another substance, a chemical change may occur. There are five indicators that a chemical change has occurred: gas production (bubbles),
Chemical Change Lesson Concept When one substance interacts with another substance, a chemical change may occur. There are five indicators that a chemical change has occurred: gas production (bubbles),
Introduction to the Microscope
 Title: Microscope Mania "Micro" (Greek!) refers to tiny, "scope" refers to view or look. Microscopes are tools used to enlarge images of small objects so they can be studied. The compound light microscope
Title: Microscope Mania "Micro" (Greek!) refers to tiny, "scope" refers to view or look. Microscopes are tools used to enlarge images of small objects so they can be studied. The compound light microscope
Mixtures. Part 2 Add 50 ml of water (one full syringe) to each cup. Stir and observe. Write your observations on the opposite page.
 Mixtures Part 1 Prepare three cups. Put 1 level spoon (5 ml) of each solid material in each cup. Observe the three solid materials. Fill in the property chart below. Color Texture Particle shape Particle
Mixtures Part 1 Prepare three cups. Put 1 level spoon (5 ml) of each solid material in each cup. Observe the three solid materials. Fill in the property chart below. Color Texture Particle shape Particle
Lesson 1 Matter and Its Properties
 Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 Math Skills 15 School to Home 16 Key Concept Builders
Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 Math Skills 15 School to Home 16 Key Concept Builders
Jell-O Rocks. Science Concept: Objectives: GLEs Addressed: Vocabulary: Materials: Most rocks are made of different substances.
 Science Concept: Most rocks are made of different substances. Objectives: The student will: describe a variety of rocks; observe and communicate about rocks; and take a photograph of a rock and describe
Science Concept: Most rocks are made of different substances. Objectives: The student will: describe a variety of rocks; observe and communicate about rocks; and take a photograph of a rock and describe
Making a Rock OBJECTIVES SCHEDULE VOCABULARY PREPARATION MATERIALS. For the class. The students. For each student. For each team of four
 activity 2 Making a Rock OBJECTIVES In this activity, students make their own rocks. In a later activity, students will dissect these rocks in an attempt to identify the minerals and other components that
activity 2 Making a Rock OBJECTIVES In this activity, students make their own rocks. In a later activity, students will dissect these rocks in an attempt to identify the minerals and other components that
LEVEL ZERO VOICE CATALYST (10 minutes, individual work): 1. Counting atoms
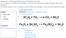 Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
T h e M yster y Pow der s L ab: Ph ysical an d Ch em ical Ch an ges
 T h e M yster y Pow der s L ab: Ph ysical an d Ch em ical Ch an ges Background Information: You have learned how to describe matter based on its physical and chemical properties. You have also learned
T h e M yster y Pow der s L ab: Ph ysical an d Ch em ical Ch an ges Background Information: You have learned how to describe matter based on its physical and chemical properties. You have also learned
Mixtures, Solutions, and Suspensions
 Purpose To explore how mixtures, solutions, and suspensions form by combining and then attempting to separate various materials. Process Skills Observe, measure, predict, collect data, interpret data,
Purpose To explore how mixtures, solutions, and suspensions form by combining and then attempting to separate various materials. Process Skills Observe, measure, predict, collect data, interpret data,
Mahopac Central School District Curriculum Introduction to Science 8
 Introduction to Science 8 A. The goal of science is to understand the natural world 1. As you make new observations and test new explanations your view of the natural world may change again and again 2.
Introduction to Science 8 A. The goal of science is to understand the natural world 1. As you make new observations and test new explanations your view of the natural world may change again and again 2.
6th Grade: Great Salt Lake is Salty
 Curriculum written by Megan Black in partnership with The Great Salt Lake Institute at Westminster College. 6th Grade: Great Salt Lake is Salty Lesson Description: In this lesson students will compare
Curriculum written by Megan Black in partnership with The Great Salt Lake Institute at Westminster College. 6th Grade: Great Salt Lake is Salty Lesson Description: In this lesson students will compare
The Basic Unit of Life Lab (Adapted from lab of same name) State Standard
 NAME: DATE: PERIOD: The Basic Unit of Life Lab (Adapted from lab of same name) State Standard 12.11.04 In this investigation, you will review the history of the microscope, practice the techniques for
NAME: DATE: PERIOD: The Basic Unit of Life Lab (Adapted from lab of same name) State Standard 12.11.04 In this investigation, you will review the history of the microscope, practice the techniques for
1 st Semester Exam Study Guide 1.) Which of the following is NOT a compound? Explain why. a. H2O b. O2
 1 st Semester Exam Study Guide 1.) Which of the following is NOT a compound? Explain why. a. H2O b. O2 2.) A chemist has discovered what she thinks is a new molecule. In order for it to be a molecule,
1 st Semester Exam Study Guide 1.) Which of the following is NOT a compound? Explain why. a. H2O b. O2 2.) A chemist has discovered what she thinks is a new molecule. In order for it to be a molecule,
SOIL SHAKE-UP (MODIFIED FOR ADEED)
 SOIL SHAKE-UP (MODIFIED FOR ADEED) Science Concept: Soil is made of many different parts, including small rocks. Objectives: The student will: describe the parts in a sample of soil; observe and communicate
SOIL SHAKE-UP (MODIFIED FOR ADEED) Science Concept: Soil is made of many different parts, including small rocks. Objectives: The student will: describe the parts in a sample of soil; observe and communicate
Creative Classroom Lessons
 Matter, Matter Everywhere! Solids, Liquids and Gases Unit Creative Classroom Lessons Contents: 1. Solids, Liquids and gases student folder cover 2. Introduction Activity: Mystery Box 3. Solids, Liquids
Matter, Matter Everywhere! Solids, Liquids and Gases Unit Creative Classroom Lessons Contents: 1. Solids, Liquids and gases student folder cover 2. Introduction Activity: Mystery Box 3. Solids, Liquids
Suggested Activities Processes that Shape the Earth: Weathering and Erosion
 Suggested Activities Processes that Shape the Earth: and Erosion From Harcourt Science Teacher Ed. Source (Grade Level) Title Pages Concept Harcourt Science (5) How Water Changes C4-5 Erosion Earth s Surface
Suggested Activities Processes that Shape the Earth: and Erosion From Harcourt Science Teacher Ed. Source (Grade Level) Title Pages Concept Harcourt Science (5) How Water Changes C4-5 Erosion Earth s Surface
You be the Judge: Density
 1 2 You be the Judge: Density Objectives The students will: develop an understanding of density calculate density of objects collect, analyze, and interpret data solve equations collaborate with partners
1 2 You be the Judge: Density Objectives The students will: develop an understanding of density calculate density of objects collect, analyze, and interpret data solve equations collaborate with partners
Supply List. Science 300. Released Glynlyon, Inc
 N Supply List Science 300 2012 Glynlyon, Inc Released 4-1-12 Table of Contents UNIT 1: YOU GROW AND CHANGE... 1 UNIT 2: PLANTS... 1 UNIT 3: ANIMALS: GROWTH AND CHANGE... 2 UNIT 4: YOU ARE WHAT YOU EAT...
N Supply List Science 300 2012 Glynlyon, Inc Released 4-1-12 Table of Contents UNIT 1: YOU GROW AND CHANGE... 1 UNIT 2: PLANTS... 1 UNIT 3: ANIMALS: GROWTH AND CHANGE... 2 UNIT 4: YOU ARE WHAT YOU EAT...
PREPARE FOR THE ACTIVITY. Activity Sheet Chapter 6, Lesson 8 ph and Color Change
 Activity Sheet Chapter 6, Lesson 8 ph and Color Change Name Date DEMONSTRATION 1. Your teacher poured green universal indicator into each of two cups. What does the change in color of the indicator solution
Activity Sheet Chapter 6, Lesson 8 ph and Color Change Name Date DEMONSTRATION 1. Your teacher poured green universal indicator into each of two cups. What does the change in color of the indicator solution
Physical and Chemical Changes Or How Do You Know When You ve Made Something New?
 Introduction Or How Do You Know When You ve Made Something New? Remember that all matter has characteristic physical and chemical properties. Matter can also undergo physical and chemical changes. How
Introduction Or How Do You Know When You ve Made Something New? Remember that all matter has characteristic physical and chemical properties. Matter can also undergo physical and chemical changes. How
EROSION RATES (1 Hour)
 EROSION RATES (1 Hour) Addresses NGSS Level of Difficulty: 2 Grade Range: 3-5 OVERVIEW In this activity, students will conduct simple investigations to collect data on erosion rates of different Earth
EROSION RATES (1 Hour) Addresses NGSS Level of Difficulty: 2 Grade Range: 3-5 OVERVIEW In this activity, students will conduct simple investigations to collect data on erosion rates of different Earth
ROCKS, FOSSILS AND SOILS SECTION 1: WHAT IS A ROCK? From Hands on Science by Linda Poore, 2003 Westminster College
 ROCKS, FOSSILS AND SOILS SECTION 1: WHAT IS A ROCK? From Hands on Science by Linda Poore, 2003 Westminster College STANDARDS: Students know how to compare the physical properties of different kinds of
ROCKS, FOSSILS AND SOILS SECTION 1: WHAT IS A ROCK? From Hands on Science by Linda Poore, 2003 Westminster College STANDARDS: Students know how to compare the physical properties of different kinds of
Atomic Structure BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN
 activity 5 Atomic Structure BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN Grade 5 Quarter 1 Activity 5 SC.A.2.2.1 The student knows that materials may be made of parts too small to be seen without magnification.
activity 5 Atomic Structure BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN Grade 5 Quarter 1 Activity 5 SC.A.2.2.1 The student knows that materials may be made of parts too small to be seen without magnification.
Fat: Who Says? Measuring Obesity by Bioelectrical Impedance Analysis
 Fat: Who Says? Measuring Obesity by Bioelectrical Impedance Analysis Circuitous Adventures Activity 3C Part 2 Objectives: Using simple electrical components, students will be able to: Identify a simple
Fat: Who Says? Measuring Obesity by Bioelectrical Impedance Analysis Circuitous Adventures Activity 3C Part 2 Objectives: Using simple electrical components, students will be able to: Identify a simple
Identifying Solids 1-2 KEY CONCEPTS AND PROCESS SKILLS KEY VOCABULARY ACTIVITY OVERVIEW L A B O R ATO R Y A-69
 Identifying Solids 40- to 1-2 50-minute sessions ACTIVITY OVERVIEW 7 L A B O R ATO R Y Students conduct tests on the solids separated from the mixture to gain information about the physical and chemical
Identifying Solids 40- to 1-2 50-minute sessions ACTIVITY OVERVIEW 7 L A B O R ATO R Y Students conduct tests on the solids separated from the mixture to gain information about the physical and chemical
Rainbow Ice. 3 small bowls or containers 3 small plastic spoons Science Journal Rainbow Ice (page 40) Word Cards Rainbow Ice (page 41)
 Physical Science Demonstration Materials clear, shoebox-size plastic container food coloring (red, blue, and yellow) water 3 clear, plastic cups (9 12 oz) 3 pipettes or eye droppers various types of salt
Physical Science Demonstration Materials clear, shoebox-size plastic container food coloring (red, blue, and yellow) water 3 clear, plastic cups (9 12 oz) 3 pipettes or eye droppers various types of salt
BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN. SC.F The student knows that living things are different but share similar structures.
 activities 38&39 Stomata and Transpiration (Sessions I and II) BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN Grade 5 Quarter 4 Activities 38 & 39 SC.F.1.2.3 The student knows that living things are
activities 38&39 Stomata and Transpiration (Sessions I and II) BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN Grade 5 Quarter 4 Activities 38 & 39 SC.F.1.2.3 The student knows that living things are
CLASSROOM KIT MAGNET EXPLORATION
 CLASSROOM KIT MAGNET EXPLORATION Page 1 1 Activity: What Do We Already Know? Teacher A simple, yet effective learning strategy, a K-W-L chart, is used to help Background: students clarify their ideas.
CLASSROOM KIT MAGNET EXPLORATION Page 1 1 Activity: What Do We Already Know? Teacher A simple, yet effective learning strategy, a K-W-L chart, is used to help Background: students clarify their ideas.
Lesson 1 Solids, Liquids, and Gases
 Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 School to Home 15 Key Concept Builders 16 Enrichment
Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 School to Home 15 Key Concept Builders 16 Enrichment
ELEMENTARY SCIENCE PROGRAM MATH, SCIENCE & TECHNOLOGY EDUCATION. A Collection of Learning Experiences on LOOKING AT LIQUIDS
 ELEMENTARY SCIENCE PROGRAM MATH, SCIENCE & TECHNOLOGY EDUCATION A Collection of Learning Experiences on LOOKING AT LIQUIDS CATTARAUGUS-ALLEGANY BOCES GRADES 5/6 TABLE OF CONTENTS Unit Overview... 2 Format
ELEMENTARY SCIENCE PROGRAM MATH, SCIENCE & TECHNOLOGY EDUCATION A Collection of Learning Experiences on LOOKING AT LIQUIDS CATTARAUGUS-ALLEGANY BOCES GRADES 5/6 TABLE OF CONTENTS Unit Overview... 2 Format
SUPPLY LIST. Earth Science
 2018-19 SUPPLY LIST Earth Science Table of Contents UNIT 1: ORIGIN OF THE EARTH... 1 UNIT 2: HISTORY OF THE EARTH... 1 UNIT 3: DYNAMIC STRUCTURE OF EARTH... 2 UNIT 4: FORCES AND FEATURES OF EARTH... 2
2018-19 SUPPLY LIST Earth Science Table of Contents UNIT 1: ORIGIN OF THE EARTH... 1 UNIT 2: HISTORY OF THE EARTH... 1 UNIT 3: DYNAMIC STRUCTURE OF EARTH... 2 UNIT 4: FORCES AND FEATURES OF EARTH... 2
Temperature Changes OBJECTIVES PREPARATION SCHEDULE MATERIALS. The students. For each student. For each team of two. For the class
 activity 3 Temperature Changes OBJECTIVES Students observe changes in air temperature and discover the role of the Sun in heating Earth. The students measure and record outdoor air temperature at three
activity 3 Temperature Changes OBJECTIVES Students observe changes in air temperature and discover the role of the Sun in heating Earth. The students measure and record outdoor air temperature at three
Physical & Chemical PROPERTIES
 Name Test Date Hour Group Chem #4 Notebook Properties & Changes LEARNING TARGETS I can give examples of physical and chemical properties. I can give examples of physical and chemical changes. I can identify
Name Test Date Hour Group Chem #4 Notebook Properties & Changes LEARNING TARGETS I can give examples of physical and chemical properties. I can give examples of physical and chemical changes. I can identify
Chapter 1, Lesson 3: The Ups and Downs of Thermometers
 Chapter 1, Lesson 3: The Ups and Downs of Thermometers Key Concepts The way a thermometer works is an example of heating and cooling a liquid. When heated, the molecules of the liquid in the thermometer
Chapter 1, Lesson 3: The Ups and Downs of Thermometers Key Concepts The way a thermometer works is an example of heating and cooling a liquid. When heated, the molecules of the liquid in the thermometer
Lesson 1 Substances and Mixtures
 Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 School to Home 15 Key Concept Builders 16 Enrichment
Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 School to Home 15 Key Concept Builders 16 Enrichment
BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN. SC.E The student understands the arrangement of planets in our Solar System.
 activity 12 Earth Orbits the Sun BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN Grade 4 Quarter 2 Activity 12 SC.E.1.2.4 The student knows that the planets differ in size, characteristics, and composition
activity 12 Earth Orbits the Sun BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN Grade 4 Quarter 2 Activity 12 SC.E.1.2.4 The student knows that the planets differ in size, characteristics, and composition
Po Kok Secondary School S.1 Integrated Science Chapter 1.1 Classwork What is Science? Class: S.1 ( ) Date: A. What is Science? P.
 Po Kok Secondary School S.1 Integrated Science Chapter 1.1 Classwork Name: What is Science? Class: S.1 ( ) Date: A. What is Science? P.3-4 The study of things and phenomena in nature and how they affect
Po Kok Secondary School S.1 Integrated Science Chapter 1.1 Classwork Name: What is Science? Class: S.1 ( ) Date: A. What is Science? P.3-4 The study of things and phenomena in nature and how they affect
2. Can you name any minerals? (Diamonds, quartz, salt, asbestos, sulfur, copper)
 MINERALS ACTIVITY TWO PARTS The purpose of this lab is to introduce the concept of minerals. In the first part, Students will discuss the properties of minerals and observe examples of minerals. In the
MINERALS ACTIVITY TWO PARTS The purpose of this lab is to introduce the concept of minerals. In the first part, Students will discuss the properties of minerals and observe examples of minerals. In the
Supply List. Earth Science. Released Glynlyon, Inc
 N Supply List Earth Science 2012 Glynlyon, Inc Released 4-1-12 Table of Contents UNIT 1: DYNAMIC STRUCTURE OF EARTH... 1 UNIT 2: FORCES AND FEATURES OF EARTH... 1 UNIT 3: FEATURES OF EARTH'S CRUST... 2
N Supply List Earth Science 2012 Glynlyon, Inc Released 4-1-12 Table of Contents UNIT 1: DYNAMIC STRUCTURE OF EARTH... 1 UNIT 2: FORCES AND FEATURES OF EARTH... 1 UNIT 3: FEATURES OF EARTH'S CRUST... 2
Literacy and Math Activities
 Literacy and Math Activities By April Larremore http://larremoreteachertips.blogspot.com/ Clipart by Thistlegirl Designs and DJ Inkers Suggested Read Alouds: Let s Go Rock Collecting by Roma Gans & Holly
Literacy and Math Activities By April Larremore http://larremoreteachertips.blogspot.com/ Clipart by Thistlegirl Designs and DJ Inkers Suggested Read Alouds: Let s Go Rock Collecting by Roma Gans & Holly
Elementary Science Investigations With the SmartMicroScope
 Elementary Science Investigations With the SmartMicroScope By SmartSchool Systems Copyright 2010 by SmartSchool Systems - All rights reserved Permission granted to reproduce the Student Worksheet pages
Elementary Science Investigations With the SmartMicroScope By SmartSchool Systems Copyright 2010 by SmartSchool Systems - All rights reserved Permission granted to reproduce the Student Worksheet pages
Science 150 SCHEDULE PLUS
 Science 150 SCHEDULE PLUS 150 Ages 12 16 Grades 7 11 Science Physical Science Schedule Plus By Sandy Hotz SCHEDULE PLUS Section Two Schedule and Notes Date: Day 1 1 Day 2 2 Day 3 3 Day 4 4 Day 5 5 Exploring
Science 150 SCHEDULE PLUS 150 Ages 12 16 Grades 7 11 Science Physical Science Schedule Plus By Sandy Hotz SCHEDULE PLUS Section Two Schedule and Notes Date: Day 1 1 Day 2 2 Day 3 3 Day 4 4 Day 5 5 Exploring
Weather Tanks. NC Standards 5.E.1, 5.P.2.1 Page 3. Grade 5 Earth Science, Physical Science. Activity Description & Estimated Class Time.
 Weather Tanks NC Standards 5.E.1, 5.P.2.1 Page 3 Grade 5 Earth Science, Physical Science Throughout the guide, teaching tips are in red. Activity Description & Estimated Class Time Objectives This activity
Weather Tanks NC Standards 5.E.1, 5.P.2.1 Page 3 Grade 5 Earth Science, Physical Science Throughout the guide, teaching tips are in red. Activity Description & Estimated Class Time Objectives This activity
Author(s): Tim Bumpus, Stephanie Sanders, Camilo Sarmiento, Daniel Urul. Standards: Next Generation Science Standards (www.nextgenscience.
 Unknown Powders Author(s): Tim Bumpus, Stephanie Sanders, Camilo Sarmiento, Daniel Urul Date Created: February, 2016 Subject: Chemistry Grade Level: Grades 3-6 Standards: Next Generation Science Standards
Unknown Powders Author(s): Tim Bumpus, Stephanie Sanders, Camilo Sarmiento, Daniel Urul Date Created: February, 2016 Subject: Chemistry Grade Level: Grades 3-6 Standards: Next Generation Science Standards
Background See background information on Student Sheet, Station 4, page 9.7.
 Exploring Meteorite Mysteries Lesson 9 Meteorite Sleuths! Objectives Students will: simulate techniques used by scientists. develop skills in acquiring data through the senses. observe, examine, record,
Exploring Meteorite Mysteries Lesson 9 Meteorite Sleuths! Objectives Students will: simulate techniques used by scientists. develop skills in acquiring data through the senses. observe, examine, record,
Southern Nevada Regional Professional Development Program. What happens when water changes to a solid?
 3-5 Physical Science What happens when water changes to a solid? Two large soft vials with caps One plastic 50 ml syringe Plastic ice tray Water Freezer compartment 1. Completely fill the ice tray and
3-5 Physical Science What happens when water changes to a solid? Two large soft vials with caps One plastic 50 ml syringe Plastic ice tray Water Freezer compartment 1. Completely fill the ice tray and
Science Supply List. Science Glynlyon, Inc.
 Science Supply List Science 300 2016 Glynlyon, Inc. Table of Contents UNIT 1: YOU GROW AND CHANGE... 1 UNIT 2: PLANTS... 2 UNIT 3: ANIMALS: GROWTH AND CHANGE... 3 UNIT 4: YOU ARE WHAT YOU EAT... 4 UNIT
Science Supply List Science 300 2016 Glynlyon, Inc. Table of Contents UNIT 1: YOU GROW AND CHANGE... 1 UNIT 2: PLANTS... 2 UNIT 3: ANIMALS: GROWTH AND CHANGE... 3 UNIT 4: YOU ARE WHAT YOU EAT... 4 UNIT
Observing Daphnia. Student Resources 1.4 Observing Daphnia, Pages 1 and Counting Daphnia Populations Inquiry Focus Observe
 Observing Daphnia Observing Daphnia, Page 1 30 minutes Pairs Observe the daphnia in your cup. List two ways you can tell the adults from the babies: 1 Babies are smaller. 2 Babies are brownish. How do
Observing Daphnia Observing Daphnia, Page 1 30 minutes Pairs Observe the daphnia in your cup. List two ways you can tell the adults from the babies: 1 Babies are smaller. 2 Babies are brownish. How do
5thscience physical (5thscience_physical)
 5thscience physical (5thscience_physical) Name: Date: 1. Which circuit would turn the light bulb on? A. B. C. D. 2. Which item would conduct electricity? A. a glass cup B. a chicken feather C. a plastic
5thscience physical (5thscience_physical) Name: Date: 1. Which circuit would turn the light bulb on? A. B. C. D. 2. Which item would conduct electricity? A. a glass cup B. a chicken feather C. a plastic
Science Grade 01 Unit 01 Exemplar Lesson 02: Observing and Recording Weather
 Unit: 01 Lesson: 02 Suggested Duration: 5 days Grade 01 Unit 01 Exemplar Lesson 02: Observing and Recording Weather This lesson is one approach to teaching the State Standards associated with this unit.
Unit: 01 Lesson: 02 Suggested Duration: 5 days Grade 01 Unit 01 Exemplar Lesson 02: Observing and Recording Weather This lesson is one approach to teaching the State Standards associated with this unit.
Objective Students will gain an understanding of how the properties of a solid material can affect how it interacts with water.
 OOBLECK! (1 Hour) Addresses NGSS Level of Difficulty: 4 Grade Range: K-2 OVERVIEW Students will examine the behavior of different types of solids when they are dissolved in water and explain those behaviors
OOBLECK! (1 Hour) Addresses NGSS Level of Difficulty: 4 Grade Range: K-2 OVERVIEW Students will examine the behavior of different types of solids when they are dissolved in water and explain those behaviors
Photosynthesis: How do plants get engery? Teacher Version
 Photosynthesis: How do plants get engery? Teacher Version In this lab, students explore the process of photosynthesis in spinach leaves. As oxygen is produced, the density of the leaves change and they
Photosynthesis: How do plants get engery? Teacher Version In this lab, students explore the process of photosynthesis in spinach leaves. As oxygen is produced, the density of the leaves change and they
The complete lesson plan for this topic is included below.
 Home Connection Parent Information: Magnets provide a simple way to explore force with children. The power of a magnet is somewhat like magic to them and requires exploration to understand. When forces
Home Connection Parent Information: Magnets provide a simple way to explore force with children. The power of a magnet is somewhat like magic to them and requires exploration to understand. When forces
Cool Chemistry. Middle School TEKS. Vocabulary
 Cool Chemistry Middle School TEKS Sixth Grade: 6.5A, 6.5B, 6.5C, 6.5D Seventh Grade: 7.6A, 7.6B, 7.6C Eighth Grade: 8.5A, 8.5B, 8.5C, 8.5E Vocabulary chemical change, chemical reaction, cirrhosis of the
Cool Chemistry Middle School TEKS Sixth Grade: 6.5A, 6.5B, 6.5C, 6.5D Seventh Grade: 7.6A, 7.6B, 7.6C Eighth Grade: 8.5A, 8.5B, 8.5C, 8.5E Vocabulary chemical change, chemical reaction, cirrhosis of the
Grade 5 Mixtures and Solutions Unit Template
 Delaware Science Coalition Grade 5 Mixtures and Solutions Unit Template Copyright 2008 Delaware Department of Education Copyright 2008 Delaware Department of Education 1 Preface: This unit has been created
Delaware Science Coalition Grade 5 Mixtures and Solutions Unit Template Copyright 2008 Delaware Department of Education Copyright 2008 Delaware Department of Education 1 Preface: This unit has been created
Are There Other Neighborhoods Like Our Own? Grades 5-8. Lesson 3: Searching for Signs of Life
 Are There Other Neighborhoods Like Our Own? Grades 5-8 Lesson 3: Searching for Signs of Life Lesson Abstract Students are presented with three soil samples representing simulated Martian soil. After visual
Are There Other Neighborhoods Like Our Own? Grades 5-8 Lesson 3: Searching for Signs of Life Lesson Abstract Students are presented with three soil samples representing simulated Martian soil. After visual
Soil and Erosion. Spring Lesson 5 - Grade 5. Lesson Description. Learning Objectives. Materials and Preparation
 Soil and Erosion Lesson Description In this lesson students learn about erosion. They learn about the four different kinds of erosion and do experiments that demonstrate each kind. They learn how erosion
Soil and Erosion Lesson Description In this lesson students learn about erosion. They learn about the four different kinds of erosion and do experiments that demonstrate each kind. They learn how erosion
Part I: How Dense Is It? Fundamental Question: What is matter, and how do we identify it?
 Part I: How Dense Is It? Fundamental Question: What is matter, and how do we identify it? Everything on Earth is made of matter. Matter is as simple as a single element or as complex as the entire planet.
Part I: How Dense Is It? Fundamental Question: What is matter, and how do we identify it? Everything on Earth is made of matter. Matter is as simple as a single element or as complex as the entire planet.
Partnerships Implementing Engineering Education Worcester Polytechnic Institute Worcester Public Schools Supported by: National Science Foundation
 Partnerships Implementing Engineering Education Chemical Engineering: K.L.1 Volcanoes Grade Level Sessions Seasonality Instructional Mode(s) Team Size WPS Benchmarks MA Frameworks Key Words K Part 1: 30
Partnerships Implementing Engineering Education Chemical Engineering: K.L.1 Volcanoes Grade Level Sessions Seasonality Instructional Mode(s) Team Size WPS Benchmarks MA Frameworks Key Words K Part 1: 30
Chromatography: Candy Coating and Marker Colors Student Version
 Chromatography: Candy Coating and Marker Colors Student Version In this lab you will separate a mixture of unknown composition using several common household items. You will then perform a more specific
Chromatography: Candy Coating and Marker Colors Student Version In this lab you will separate a mixture of unknown composition using several common household items. You will then perform a more specific
Science 350 Week 1 Module 1 Schedule
 Science 350 Week 1 Module 1 Schedule Date: Day 1 1 Day 2 2 Day 3 3 Day 4 4 Day 5 5 pp. 1 7 (top) pp. 7 13 ( Manipulating Units through pp. 13 19 ( More Complex through pp. 19 25 ( Making Measurements through
Science 350 Week 1 Module 1 Schedule Date: Day 1 1 Day 2 2 Day 3 3 Day 4 4 Day 5 5 pp. 1 7 (top) pp. 7 13 ( Manipulating Units through pp. 13 19 ( More Complex through pp. 19 25 ( Making Measurements through
Follow the instructions to determine if your sample is metamorphic, sedimentary or igneous rock.
 To gather some appreciation of our world, especially our rock world, we are going to gather an assortment of rocks from our campus. You will use a rock key to classify some of the samples we collect. Procedures:
To gather some appreciation of our world, especially our rock world, we are going to gather an assortment of rocks from our campus. You will use a rock key to classify some of the samples we collect. Procedures:
2/22/2019 NEW UNIT! Chemical Interactions. Atomic Basics #19
 NEW UNIT! Chemical Interactions Atomic Basics #19 1 Vocabulary: Matter: Anything that has mass and takes up space. Atom: the smallest particle of matter. Element: A pure substance made up of only one type
NEW UNIT! Chemical Interactions Atomic Basics #19 1 Vocabulary: Matter: Anything that has mass and takes up space. Atom: the smallest particle of matter. Element: A pure substance made up of only one type
Standards Alignment... 5 Safe Science... 9 Scientific Inquiry... 11
 Standards Alignment... 5 Safe Science... 9 Scientific Inquiry... 11 Properties of Matter Let Me Count the Ways... 15 A Bear Eggs-pedition...25 Color Match...33 Going Nuts...39 Soup-er Floaters and Sinkers...55
Standards Alignment... 5 Safe Science... 9 Scientific Inquiry... 11 Properties of Matter Let Me Count the Ways... 15 A Bear Eggs-pedition...25 Color Match...33 Going Nuts...39 Soup-er Floaters and Sinkers...55
Biology Activity: Science Process; Measurements; Tools; Data Presentation and Analysis Purpose Question Background
 Biology Activity: Science Process; Measurements; Tools; Data Presentation and Analysis Purpose: Review scientific practices, the use of measuring tools and microscopes, data collection, and the proper
Biology Activity: Science Process; Measurements; Tools; Data Presentation and Analysis Purpose: Review scientific practices, the use of measuring tools and microscopes, data collection, and the proper
School Year: 2009/2010 Developed for:
 Science Unit: Lesson 3: Weather and Seasons Rain and Rainbows School Year: 2009/2010 Developed for: Developed by: Grade level: Duration of lesson: Sir Guy Carleton and Sir Sandford Fleming Elementary Schools,
Science Unit: Lesson 3: Weather and Seasons Rain and Rainbows School Year: 2009/2010 Developed for: Developed by: Grade level: Duration of lesson: Sir Guy Carleton and Sir Sandford Fleming Elementary Schools,
