Student Notes. Chemical Reactions LINK
|
|
|
- Kristin Richard
- 5 years ago
- Views:
Transcription
1 LCPS Core Experience Chemical Reactions Student Notes OBJECTIVES Students will: investigate the relationship between reactants and products. investigate an exothermic reaction. investigate an endothermic reaction. LINK 1. How can you tell if an energy change has taken place in a chemical reaction? Background Chemical reactions usually have two or more chemicals called reactants that are different from one another. The reactants interact during the chemical reaction to form one or more products. These are substances that are different from the reactants and from each other. The reactants and products in a chemical reaction can be written as chemical formulas using letters to represent elements. All chemical reactions can be written as equations that are like sentences. The reactant and product formulas are like the words in a sentence. In an equation, the reactants and products are separated by an arrow ( ). When reading a chemical equation, the word yield is used for the arrow symbol ( ). The chemical equation is then read by saying that the interaction of the reactants yields the products. In a chemical reaction, the chemical bonds that hold the atoms of the reactants together are broken and reformed in a different arrangement to form the products. Breaking and reforming these bonds occurs when the reactants react with each other. The different arrangement of atoms can be seen by the different formulas that represent the reactants and products. When all of the reactants are consumed in a chemical reaction, they no longer exist in their original form. All the atoms originally found in the reactants can now be found in the products. Atoms and molecules react with each other in specified ratios in order for a chemical reaction to take place. The balanced chemical equation that represents each chemical reaction stipulates the relative numbers of reactants and products. In a chemical reaction every atom that is part of the reactants is part of the products. Page 1
2 LCPS Core Experience Background (continued) Chemical reactions are classified into two broad types: ones in which energy is released (exothermic) and ones in which energy is absorbed (endothermic). A clue that a chemical reaction has taken place is a transfer of energy. EXPERIMENT In this investigation, you will be investigating an endothermic reaction and an exothermic reaction. Lesson One: Measuring Energy Change in a Chemical Reaction Materials Per Lab Group for Lesson One 30 ml of hydrogen peroxide 1 weigh dish (3% solution) 1 triple beam balance 1 50 ml graduated cylinder 1 stopwatch 1 thermometer 1 pair of goggles ml Erlenmeyer flask 1 lab apron 2 g of yeast 1. Obtain the materials for Lesson One. You will be investigating the following chemical reaction. Yeast 2H2O2 2H2O + O2 Hydrogen Water Oxygen peroxide 2. Measure 30 ml of hydrogen peroxide using a 50 ml graduated cylinder. Pour the hydrogen peroxide into a 250 ml Erlenmeyer flask. 3. Put a thermometer into the flask and wait two minutes to allow the thermometer to reach the temperature of the hydrogen peroxide. During the time you are waiting, measure 2 g of yeast with the balance. 4. Record the starting temperature. One person should keep track of the time. Another person should keep track of the temperature. You will be measuring the temperature every 30 seconds. 5. Add the yeast to the beaker and immediately record the temperature in Table A, this is time zero, while gently stirring the contents of the flask. Continue to record the temperature in Table A every 30 seconds for 3 minutes. 6. Did the temperature increase or decrease? Page 2
3 LCPS Core Experience 7. Was energy taken in or released in the reaction? 8. Pour the mixture down the drain and rinse out the flask with water. Rinse the thermometer off. Lesson Two: Measuring Energy Change in a Chemical Reaction Materials Per Lab Group for Lesson Two 30 ml of vinegar (acetic acid) 1 triple beam balance 1 50 ml graduated cylinder 2 g of baking soda 1 125mL Erlenmeyer flask 1 stopwatch 1 thermometer 1 pair of goggles 1 weigh dish 1 lab apron 1. Obtain the materials for Lesson Two. You will be investigating the following chemical reaction. heat NaHCO3 + CH3COOH CH3COONa + H2O + CO2 Baking Soda Acetic Acid 2. Put a thermometer into the flask and wait two minutes to allow the thermometer to reach the temperature of the vinegar. During the time you are waiting, measure 2 g of baking soda with the balance. Refer to the procedural toolbox Using a Weigh Dish. 3. Record the starting temperature. Sodium Acetate Water Carbon Dioxide 4. Add the baking soda to the vinegar and immediately record the temperature, as time zero in Table A as you swirl the contents of the flask. Continue to record the temperature every 30 seconds as you observe the reaction for 3 minutes. 5. Did the temperature increase or decrease? 6. Was energy taken in or released in the reaction? Page 3
4 LCPS Core Experience 7. Pour the mixture down the drain and rinse out the flask with water. Rinse the thermometer. Table A: The Effect of a Chemical Reaction on the Temperature of a Solution Lesson One: Hydrogen Peroxide and Yeast Reaction Lesson Two: Vinegar and Baking Soda Reaction Time (min) Temperature ( 0 C) Temperature ( 0 C) Graph: Use the data from Table A to plot a graph of time vs. temperature for both solutions on the same graph paper. a. Use a different color for each set of data.. b. Determine which variable is the independent variable and which is the dependent variable. STOP AND DISCUSS Page 4
5 LCPS Core Experience LEARNING REVIEW List three things you learned about energy, endothermic and exothermic reactions. a. b. c. STOP AND DISCUSS EVALUATION 1. Which reaction was endothermic? Write the chemical equation. 2. Why did you select this reaction as the endothermic reaction? Page 5
6 LCPS Core Experience 3. Which reaction was exothermic? Write the chemical equation. 4. Why did you select this reaction as the exothermic reaction? Page 6
Chemical Reactions Investigation Two Data Record
 Chemical Reactions Investigation Two Data Record Name: Date: 1. During this Investigation, you will analyze how changing the amounts of the reactants in a chemical reaction affects the amount of the products
Chemical Reactions Investigation Two Data Record Name: Date: 1. During this Investigation, you will analyze how changing the amounts of the reactants in a chemical reaction affects the amount of the products
Goal: During this lab students will gain a quantitative understanding of limiting reagents.
 LIMITING REAGENT LAB: THE REACTION BETWEEN VINEGAR AND BAKING SODA Goal: During this lab students will gain a quantitative understanding of limiting reagents. Safety: Safety goggles should be worn at all
LIMITING REAGENT LAB: THE REACTION BETWEEN VINEGAR AND BAKING SODA Goal: During this lab students will gain a quantitative understanding of limiting reagents. Safety: Safety goggles should be worn at all
Chemical Reactions Investigation Two Data Record
 Chemical Reactions Investigation Two Data Record Name: Date: 1. During this Investigation, you will analyze how changing the amounts of the reactants in a chemical reaction affects the amount of the products
Chemical Reactions Investigation Two Data Record Name: Date: 1. During this Investigation, you will analyze how changing the amounts of the reactants in a chemical reaction affects the amount of the products
Chapter Introduction Lesson 1 Understanding Chemical Reactions Lesson 2 Types of Chemical Reactions Lesson 3 Energy Changes and Chemical Reactions
 Chapter Introduction Lesson 1 Understanding Chemical Reactions Lesson 2 Types of Chemical Reactions Lesson 3 Energy Changes and Chemical Reactions Chapter Wrap-Up Changes in Matter A physical change does
Chapter Introduction Lesson 1 Understanding Chemical Reactions Lesson 2 Types of Chemical Reactions Lesson 3 Energy Changes and Chemical Reactions Chapter Wrap-Up Changes in Matter A physical change does
Chapter 6, Lesson 7: Energy Changes in Chemical Reactions
 Chapter 6, Lesson 7: Energy Changes in Chemical Reactions Key Concepts If two substances react and the temperature of the mixture decreases, the reaction is endothermic. If two substances react and the
Chapter 6, Lesson 7: Energy Changes in Chemical Reactions Key Concepts If two substances react and the temperature of the mixture decreases, the reaction is endothermic. If two substances react and the
Friday, November 2, 2018 GLE/Standard: The fact that atoms are conserved, together
 Mrs. Chausse s Physical Science Gifted Lesson Plan: Unit 7 Chemical Reactions November 1 16, 2018 Thursday, November 1, 2018 Objective: SWBAT balance a chemical equation that satisfies the Law of Conservation
Mrs. Chausse s Physical Science Gifted Lesson Plan: Unit 7 Chemical Reactions November 1 16, 2018 Thursday, November 1, 2018 Objective: SWBAT balance a chemical equation that satisfies the Law of Conservation
Name: Block: Date: Student Notes. OBJECTIVE Students will investigate the relationship between temperature and the change of the state of matter.
 Name: Block: Date: LCPS Core Experience Heat Transfer Student Notes OBJECTIVE Students will investigate the relationship between temperature and the change of the state of matter. LINK 1. Particles in
Name: Block: Date: LCPS Core Experience Heat Transfer Student Notes OBJECTIVE Students will investigate the relationship between temperature and the change of the state of matter. LINK 1. Particles in
Section 1: What is a Chemical Reaction
 Section 1: What is a Chemical Reaction I can describe and give examples of physical and chemical changes. I can identify reactants and products. I can explain what happens to molecules in chemical reactions
Section 1: What is a Chemical Reaction I can describe and give examples of physical and chemical changes. I can identify reactants and products. I can explain what happens to molecules in chemical reactions
Experiment 17 It s A Gas and More!
 Energy Energy Experiment 17 It s A Gas and More! OUTCOMES After completing this lab activity, the student should be able to: explain a simple method for distinguishing carbon dioxide gas from oxygen gas.
Energy Energy Experiment 17 It s A Gas and More! OUTCOMES After completing this lab activity, the student should be able to: explain a simple method for distinguishing carbon dioxide gas from oxygen gas.
The Next Generation Science Standards (NGSS)
 The Next Generation Science Standards (NGSS) CHAPTER 6, LESSON 1: WHAT IS A CHEMICAL REACTION? MS-PS1-2. Analyze and interpret data on the properties of substances before and after the substances interact
The Next Generation Science Standards (NGSS) CHAPTER 6, LESSON 1: WHAT IS A CHEMICAL REACTION? MS-PS1-2. Analyze and interpret data on the properties of substances before and after the substances interact
Chemical Reactions. Chapter Test A. Multiple Choice. 1 Pearson Education, Inc., or its affiliates. All rights reserved.
 Name Date Class Chemical Reactions Chapter Test A Multiple Choice Write the letter of the correct answer on the line at the left. 1. Chemistry is a. a characteristic of a substance that can be observed
Name Date Class Chemical Reactions Chapter Test A Multiple Choice Write the letter of the correct answer on the line at the left. 1. Chemistry is a. a characteristic of a substance that can be observed
Lab Activity 3: Factors Affecting Reaction Rate
 Chemistry 3202 Lab #3 factors affecting Reaction Rate Page 1 of 5 Lab Activity 3: Factors Affecting Reaction Rate Introduction Several factors influence how fast a reaction proceeds. In this activity,
Chemistry 3202 Lab #3 factors affecting Reaction Rate Page 1 of 5 Lab Activity 3: Factors Affecting Reaction Rate Introduction Several factors influence how fast a reaction proceeds. In this activity,
Activity Sheet Transferring thermal energy by dissolving salts
 Student Name: Date: Activity Sheet Transferring thermal energy by dissolving salts 1) Define Thermal energy and temperature in the boxes below. Thermal Energy Temperature Practice Experiment: Aim: To practice
Student Name: Date: Activity Sheet Transferring thermal energy by dissolving salts 1) Define Thermal energy and temperature in the boxes below. Thermal Energy Temperature Practice Experiment: Aim: To practice
Student Exploration: Chemical Changes
 Name: Date: Student Exploration: Chemical Changes Vocabulary: acid, base, catalyst, chemical change, coefficient, conservation of matter, decomposition, dissolve, double replacement, endothermic, exothermic,
Name: Date: Student Exploration: Chemical Changes Vocabulary: acid, base, catalyst, chemical change, coefficient, conservation of matter, decomposition, dissolve, double replacement, endothermic, exothermic,
Limiting Reactants Lab
 Name: Teacher s Name: Class: Block: Date: Partners: Limiting Reactants Lab Purpose: Through experimentation, determine the limiting reactant and the percent yield in a chemical reaction that generates
Name: Teacher s Name: Class: Block: Date: Partners: Limiting Reactants Lab Purpose: Through experimentation, determine the limiting reactant and the percent yield in a chemical reaction that generates
Acids and Bases. How does ph affect biological solutions? Introduction. Prelab Preparation Review Section 2.3 on acids and bases in your textbook.
 Acids and Bases How does ph affect biological solutions? Learning Objectives To relate the ph scale to how acidic or basic a solution is. To explain how a buffer affects the ph of a solution. Process Objectives
Acids and Bases How does ph affect biological solutions? Learning Objectives To relate the ph scale to how acidic or basic a solution is. To explain how a buffer affects the ph of a solution. Process Objectives
1 The Nature of Chemical Reactions
 CHAPTER 7 1 The Nature of Chemical Reactions SECTION Chemical Reactions KEY IDEAS As you read this section, keep these questions in mind: What happens in a chemical reaction? What is the role of energy
CHAPTER 7 1 The Nature of Chemical Reactions SECTION Chemical Reactions KEY IDEAS As you read this section, keep these questions in mind: What happens in a chemical reaction? What is the role of energy
Pre-Lab Exercises Lab 3: Chemical Properties
 Pre-Lab Exercises Lab 3: Chemical Properties 1. How is a chemical property different from a physical property? Name Date Section 2. How is a chemical change different from a physical change? 3. Give two
Pre-Lab Exercises Lab 3: Chemical Properties 1. How is a chemical property different from a physical property? Name Date Section 2. How is a chemical change different from a physical change? 3. Give two
Moles Lab Activity 1: PCU (Popcorn Counting Units)
 Moles Lab Activity 1: PCU (Popcorn Counting Units) Materials: A container of each of the following: Popcorn kernels Another type of beans A large unopened bag of popcorn Kernels Balance Safety goggles
Moles Lab Activity 1: PCU (Popcorn Counting Units) Materials: A container of each of the following: Popcorn kernels Another type of beans A large unopened bag of popcorn Kernels Balance Safety goggles
Exploring Acids & Bases
 Food Explorations Lab: Exploring Acids & Bases STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will use cabbage juice indicator to determine if two unknown samples are acids or
Food Explorations Lab: Exploring Acids & Bases STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will use cabbage juice indicator to determine if two unknown samples are acids or
Chemistry 1B Experiment 17 89
 Chemistry 1B Experiment 17 89 17 Thermodynamics of Borax Solubility Introduction In this experiment, you will determine the values of H and S for the reaction which occurs when borax (sodium tetraborate
Chemistry 1B Experiment 17 89 17 Thermodynamics of Borax Solubility Introduction In this experiment, you will determine the values of H and S for the reaction which occurs when borax (sodium tetraborate
EXPERIMENT A7: VINEGAR TITRATION. Learning Outcomes. Introduction. Upon completion of this lab, the student will be able to:
 1 Learning Outcomes EXPERIMENT A7: VINEGAR TITRATION Upon completion of this lab, the student will be able to: 1) Prepare a solution of primary standard 2) Determine the molar concentration of a solution
1 Learning Outcomes EXPERIMENT A7: VINEGAR TITRATION Upon completion of this lab, the student will be able to: 1) Prepare a solution of primary standard 2) Determine the molar concentration of a solution
Safety: Safety goggles should be worn at all times. Students should hold the balloons on the test tubes tightly while the reaction takes place.
 LIMITING REAGENT LAB: THE REACTION BETWEEN VINEGAR AND BAKING SODA Goal: During this lab students will gain a quantitative understanding of limiting reagents. Safety: Safety goggles should be worn at all
LIMITING REAGENT LAB: THE REACTION BETWEEN VINEGAR AND BAKING SODA Goal: During this lab students will gain a quantitative understanding of limiting reagents. Safety: Safety goggles should be worn at all
Chapter 6, Lesson 1: What is a Chemical Reaction?
 Chapter 6, Lesson 1: What is a Chemical Reaction? Key Concepts: A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. In a chemical reaction,
Chapter 6, Lesson 1: What is a Chemical Reaction? Key Concepts: A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. In a chemical reaction,
Name: Block: Date: Student Notes
 Name: Block: Date: LCPS Core Experience Acids and Bases Student Notes OBJECTIVES Students will: recognize some acids and bases as common and familiar household chemicals. realize that acids and bases are
Name: Block: Date: LCPS Core Experience Acids and Bases Student Notes OBJECTIVES Students will: recognize some acids and bases as common and familiar household chemicals. realize that acids and bases are
Arrange the different varieties of crisps in order of increasing energy. The first one has been done for you. 1 Steak
 Calculating and explaining energy changes 1. Energy can be measured in kilojoules (kj) or in kilocalories (kcal). The table shows some information about different varieties of crisps. Variety of crisps
Calculating and explaining energy changes 1. Energy can be measured in kilojoules (kj) or in kilocalories (kcal). The table shows some information about different varieties of crisps. Variety of crisps
Bellevue College CHEM& 121 Experiment: Stoichiometric Analysis of an Antacid 1
 Experiment: Stoichiometric Analysis of an Antacid 1 Introduction In this lab, you will use the concept of stoichiometry to solve two sequential problems. First, you will try to determine the products of
Experiment: Stoichiometric Analysis of an Antacid 1 Introduction In this lab, you will use the concept of stoichiometry to solve two sequential problems. First, you will try to determine the products of
Chemical Reaction Lab Bagged Chemical Reactions
 Learning Target: The student experiments and determines that the rates of reaction among atoms and molecules depend on the concentration, pressure, and temperature of the reactants and the presence or
Learning Target: The student experiments and determines that the rates of reaction among atoms and molecules depend on the concentration, pressure, and temperature of the reactants and the presence or
Name Period Date. Lab 1: Mass of Ice Materials: beaker, ice and balance.
 Name Period Date Testing the Law of Conservation of MASS! Introduction: Does mass change in a chemical or physical reaction? In this series of experiments you will find the answer to this question. Lab
Name Period Date Testing the Law of Conservation of MASS! Introduction: Does mass change in a chemical or physical reaction? In this series of experiments you will find the answer to this question. Lab
Today is: Monday, October 17th
 Today is: Monday, October 17th Get out your homework! 10/17/2016 #motivationmonday (This is Week 11 Warm Ups!) 1. It s a new quarter, which means a fresh start! What can you do to stay motivated this quarter?
Today is: Monday, October 17th Get out your homework! 10/17/2016 #motivationmonday (This is Week 11 Warm Ups!) 1. It s a new quarter, which means a fresh start! What can you do to stay motivated this quarter?
Chapter 3 Cell Processes and Energy. Name: Teacher: Hour:
 Chapter 3 Cell Processes and Energy Name: Teacher: Hour: 1 Unit: Cell Processes and Energy Unit Completion Date: Vocabulary and Big Ideas Photosynthesis photosynthesis autotroph heterotroph chlorophyll
Chapter 3 Cell Processes and Energy Name: Teacher: Hour: 1 Unit: Cell Processes and Energy Unit Completion Date: Vocabulary and Big Ideas Photosynthesis photosynthesis autotroph heterotroph chlorophyll
3. Kira makes 93 greeting cards for a craft fair. She sells the cards in packs of 5. How many full packs of greeting cards does Kira make?
 Chapter 4 Test 1. 2. 3. Kira makes 93 greeting cards for a craft fair. She sells the cards in packs of 5. How many full packs of greeting cards does Kira make? packs Work Space: 5. A kennel is moving 160
Chapter 4 Test 1. 2. 3. Kira makes 93 greeting cards for a craft fair. She sells the cards in packs of 5. How many full packs of greeting cards does Kira make? packs Work Space: 5. A kennel is moving 160
Activity Sheet Chapter 6, Lesson 10 Carbon Dioxide Can Make a Solution Acidic
 Activity Sheet Chapter 6, Lesson 10 Carbon Dioxide Can Make a Solution Acidic Name Date DEMONSTRATION 1. Your teacher blew through a straw into a universal indicator solution until it changed color. Did
Activity Sheet Chapter 6, Lesson 10 Carbon Dioxide Can Make a Solution Acidic Name Date DEMONSTRATION 1. Your teacher blew through a straw into a universal indicator solution until it changed color. Did
Stoichiometry Lab Report
 Stoichiometry Lab Report Touche G10 Variables Variable Dependent Controlled: Amount of NaHCO3 Theoretical Yield Vinegar Evaporation Manipulation We measure the remaining NaC 2 H 3 O 2 and compare it with
Stoichiometry Lab Report Touche G10 Variables Variable Dependent Controlled: Amount of NaHCO3 Theoretical Yield Vinegar Evaporation Manipulation We measure the remaining NaC 2 H 3 O 2 and compare it with
Describing Chemical Reactions
 Describing Chemical Reactions This section explains how to show chemical reactions with symbols. It also states the principle of conservation of mass, and identifies three categories of chemical reactions.
Describing Chemical Reactions This section explains how to show chemical reactions with symbols. It also states the principle of conservation of mass, and identifies three categories of chemical reactions.
CALORIMETRY: Heat of Fusion of Ice
 Pre-Lab Discussion CALORIMETRY: Heat of Fusion of Ice When a chemical or physical change takes place, heat is either given off or absorbed That is, the change is either exothermic or endothermic It is
Pre-Lab Discussion CALORIMETRY: Heat of Fusion of Ice When a chemical or physical change takes place, heat is either given off or absorbed That is, the change is either exothermic or endothermic It is
Chapter 6 Classification of Matter
 Chapter 6 Classification of Matter Pure substances can be classified into two types elements cannot be broken down into other pure substances are made up of only one type of atom only 115 known elements
Chapter 6 Classification of Matter Pure substances can be classified into two types elements cannot be broken down into other pure substances are made up of only one type of atom only 115 known elements
Chemistry 143 Acid Base Titration Dr. Caddell. Titrating Acid
 Titrating Acid In this lab you will first determine the concentration of sodium hydroxide in a stock solution that you prepare. You will then use that stock sodium hydroxide solution to titrate a solution
Titrating Acid In this lab you will first determine the concentration of sodium hydroxide in a stock solution that you prepare. You will then use that stock sodium hydroxide solution to titrate a solution
Physical or Chemical Change?
 Chemical Formulas and Equations Physical or Chemical Change? Matter can change physically or chemically; a process that produces a chemical change is a chemical reaction. Chemical Formulas and Equations
Chemical Formulas and Equations Physical or Chemical Change? Matter can change physically or chemically; a process that produces a chemical change is a chemical reaction. Chemical Formulas and Equations
Chemistry CP Lab: Additivity of Heats of Reaction (Hess Law)
 Chemistry CP Lab: Additivity of Heats of Reaction (Hess Law) Name: Date: The formation or destruction of chemical bonds is always accompanied by an energy exchange between the reactant molecules and the
Chemistry CP Lab: Additivity of Heats of Reaction (Hess Law) Name: Date: The formation or destruction of chemical bonds is always accompanied by an energy exchange between the reactant molecules and the
Matter. Anything that has mass and takes up space. Solids Liquids Gases
 Introduction to Matter Matter Anything that has mass and takes up space Solids Liquids Gases Weight A measure of the gravitational force exerted on an object What is the difference between weight and mass?
Introduction to Matter Matter Anything that has mass and takes up space Solids Liquids Gases Weight A measure of the gravitational force exerted on an object What is the difference between weight and mass?
Titration of HCl with Sodium Hydroxide
 Titration of HCl with Sodium Hydroxide Lab Report for the Subject of Advanced Chemistry Anon Durongpisitkul, Karis Katekovit, Varun Saketharam,Thanon Thamvorapol, Chanon Anektanasup- January 28, 2017 1
Titration of HCl with Sodium Hydroxide Lab Report for the Subject of Advanced Chemistry Anon Durongpisitkul, Karis Katekovit, Varun Saketharam,Thanon Thamvorapol, Chanon Anektanasup- January 28, 2017 1
Physical and Chemical Changes Or How Do You Know When You ve Made Something New?
 Introduction Or How Do You Know When You ve Made Something New? Remember that all matter has characteristic physical and chemical properties. Matter can also undergo physical and chemical changes. How
Introduction Or How Do You Know When You ve Made Something New? Remember that all matter has characteristic physical and chemical properties. Matter can also undergo physical and chemical changes. How
Standards 8.5.c. I know chemical reactions usually liberate or absorbs heat.
 6 March 2013 Give an example of a physical change and a chemical change, and then describe how they are different from the other. Explain your answer in 2-3 sentences. Standards 8.5.c. I know chemical
6 March 2013 Give an example of a physical change and a chemical change, and then describe how they are different from the other. Explain your answer in 2-3 sentences. Standards 8.5.c. I know chemical
Bags of Reactions Chemistry I Acc
 Introduction: Bags of Reactions Chemistry I Acc Plop, plop, fizz, fizz, oh, what a relief it is. claims the old TV ad for a popular antacid. Just what is in the tablet that is relieving the upset stomach?
Introduction: Bags of Reactions Chemistry I Acc Plop, plop, fizz, fizz, oh, what a relief it is. claims the old TV ad for a popular antacid. Just what is in the tablet that is relieving the upset stomach?
CHEM 30A EXPERIMENT 8 & 9: ACID- BASE TITRATION. Learning Outcomes. Introduction. Upon completion of this lab, the student will be able to:
 1 Learning Outcomes CHEM 30A EXPERIMENT 8 & 9: ACID- BASE TITRATION Upon completion of this lab, the student will be able to: 1) Prepare a solution of primary standard 2) Determine the molar concentration
1 Learning Outcomes CHEM 30A EXPERIMENT 8 & 9: ACID- BASE TITRATION Upon completion of this lab, the student will be able to: 1) Prepare a solution of primary standard 2) Determine the molar concentration
SNC2D CHEMISTRY 2/23/2013. CHEMICAL REACTIONS L Conservation of Mass (P ) Activity: Measuring Mass (Part 1) Activity: Measuring Mass (Part 1)
 SNC2D CHEMISTRY CHEMICAL REACTIONS L Conservation of Mass (P.176-177) Activity: Measuring Mass (Part 1) INSTRUCTIONS (CLOSED SYSTEM) A. Measure~ 30 ml of vinegar (acetic acid) into a small Erlenmeyer flask.
SNC2D CHEMISTRY CHEMICAL REACTIONS L Conservation of Mass (P.176-177) Activity: Measuring Mass (Part 1) INSTRUCTIONS (CLOSED SYSTEM) A. Measure~ 30 ml of vinegar (acetic acid) into a small Erlenmeyer flask.
Sulfuric acid is hazardous: Safety glasses are REQUIRED during this experiment.
 CHEMICAL COMPOSITION Life exists on Earth because of the abundant presence of liquid water. While other planets have water, it may be primarily found as either a gas, as on Venus, or as a solid, such as
CHEMICAL COMPOSITION Life exists on Earth because of the abundant presence of liquid water. While other planets have water, it may be primarily found as either a gas, as on Venus, or as a solid, such as
Name Period Date. Lab: Introduction to Stoichiometry
 Name Period Date Lab: Introduction to Stoichiometry Introduction: Reactants are not always present in the exact ratio required by a balanced chemical equation. In planning any cost-effective production
Name Period Date Lab: Introduction to Stoichiometry Introduction: Reactants are not always present in the exact ratio required by a balanced chemical equation. In planning any cost-effective production
Physical & Chemical PROPERTIES
 Name Test Date Hour Group Chem #4 Notebook Properties & Changes LEARNING TARGETS I can give examples of physical and chemical properties. I can give examples of physical and chemical changes. I can identify
Name Test Date Hour Group Chem #4 Notebook Properties & Changes LEARNING TARGETS I can give examples of physical and chemical properties. I can give examples of physical and chemical changes. I can identify
Name SCS- Date. Experiment 5: Hydrogen Formation and Reaction with Oxygen
 Name SCS- Date Experiment 5: Hydrogen Formation and Reaction with Oxygen Aims: What are the energy changes associated with chemical bond formation and breakage? How are changes in potential energy related
Name SCS- Date Experiment 5: Hydrogen Formation and Reaction with Oxygen Aims: What are the energy changes associated with chemical bond formation and breakage? How are changes in potential energy related
AP Chemistry Unit 2 Test (Chapters 3 and 4)
 AP Chemistry Unit 2 Test (Chapters 3 and 4) NAME: 1. A student is assigned the task of determining the mass percent of silver in an alloy of copper and silver by dissolving a sample of the alloy in excess
AP Chemistry Unit 2 Test (Chapters 3 and 4) NAME: 1. A student is assigned the task of determining the mass percent of silver in an alloy of copper and silver by dissolving a sample of the alloy in excess
IODINE CLOCK REACTION KINETICS
 Name: Section Chemistry 104 Laboratory University of Massachusetts Boston IODINE CLOCK REACTION KINETICS PRELAB ASSIGNMENT Calculate the initial concentration of H 2 O 2 that exists immediately after mixing
Name: Section Chemistry 104 Laboratory University of Massachusetts Boston IODINE CLOCK REACTION KINETICS PRELAB ASSIGNMENT Calculate the initial concentration of H 2 O 2 that exists immediately after mixing
By: Michael Wild, Matt Huber, Jasmine Gilbert and Dr. Faith Yarberry
 Acid Chemistry By: Michael Wild, Matt Huber, Jasmine Gilbert and Dr. Faith Yarberry In this module the student will: Understand the concept of an Acid. Discover the differences between strong acids and
Acid Chemistry By: Michael Wild, Matt Huber, Jasmine Gilbert and Dr. Faith Yarberry In this module the student will: Understand the concept of an Acid. Discover the differences between strong acids and
6 th Grade Introduction to Chemistry
 Lesson 1 (Describing Matter) 6 th Grade Introduction to Chemistry Matter anything that has mass and takes up space All the stuff in the natural world is matter. Chapter 1: Introduction to Matter Chemistry
Lesson 1 (Describing Matter) 6 th Grade Introduction to Chemistry Matter anything that has mass and takes up space All the stuff in the natural world is matter. Chapter 1: Introduction to Matter Chemistry
Chemistry 143 Experiment #11 Acid Base Titration Dr. Caddell. Titrating Acid
 Titrating Acid In this lab you will first determine the concentration of sodium hydroxide in a stock solution that you prepare. You will then use that stock sodium hydroxide solution to titrate a solution
Titrating Acid In this lab you will first determine the concentration of sodium hydroxide in a stock solution that you prepare. You will then use that stock sodium hydroxide solution to titrate a solution
STOICHIOMETRY AND THE CHEMICAL REACTION
 From Laboratory Manual for Guinn and Brewer s Essentials of General, Organic, and Biochemistry by Sara Selfe STOICHIOMETRY AND THE CHEMICAL REACTION You would be surprised at the number of chemical reactions
From Laboratory Manual for Guinn and Brewer s Essentials of General, Organic, and Biochemistry by Sara Selfe STOICHIOMETRY AND THE CHEMICAL REACTION You would be surprised at the number of chemical reactions
Moles Lab Activity 2: Elements Copper
 Materials Sample of copper Balance Pre-1982 penny Moles Lab Activity 2: Elements Copper Procedure Take the necessary measurements, and record them with units. Show all your calculations, rounding your
Materials Sample of copper Balance Pre-1982 penny Moles Lab Activity 2: Elements Copper Procedure Take the necessary measurements, and record them with units. Show all your calculations, rounding your
The Chemistry Summary Sheet Chemistry is the scientific study of matter.
 The Chemistry Summary Sheet Chemistry is the scientific study of matter. ATOMIC STRUCTURE: -an atom is composed of three fundamental particles: proton, electron, neutron. -atom size mostly consists of
The Chemistry Summary Sheet Chemistry is the scientific study of matter. ATOMIC STRUCTURE: -an atom is composed of three fundamental particles: proton, electron, neutron. -atom size mostly consists of
Bay Area Scientists in Schools Presentation Plan
 Bay Area Scientists in Schools Presentation Plan Lesson Name Presenter(s) Chemical Reactions Mercedes Taylor, Nick Settineri, Jessica Ziegler, Tyler Hurlburt, Parker Deal Grade Level 5 Standards Connection(s)
Bay Area Scientists in Schools Presentation Plan Lesson Name Presenter(s) Chemical Reactions Mercedes Taylor, Nick Settineri, Jessica Ziegler, Tyler Hurlburt, Parker Deal Grade Level 5 Standards Connection(s)
Describing Chemical Reactions
 Name Date Class Chemical Reactions Guided Reading and Study Describing Chemical Reactions This section explains how to show chemical reactions with symbols. It also states the principle of conservation
Name Date Class Chemical Reactions Guided Reading and Study Describing Chemical Reactions This section explains how to show chemical reactions with symbols. It also states the principle of conservation
Section 1 Forming New Substances
 Section 1 Forming New Substances Key Concept During chemical reactions, atoms rearrange to form new substances that have different properties than the original substances had. What You Will Learn Four
Section 1 Forming New Substances Key Concept During chemical reactions, atoms rearrange to form new substances that have different properties than the original substances had. What You Will Learn Four
1. Write date, topic and purpose in notebook. 2. Answer in your notebook: What are two signs that a chemical reaction is happening?
 Warm Up (5 min) Date: Monday, 1/4/16 Topic: Alka Seltzer Lab Purpose: To assess the Law of Conservation of Mass by designing an experiment to determine mass before and after a chemical reaction. 1. Write
Warm Up (5 min) Date: Monday, 1/4/16 Topic: Alka Seltzer Lab Purpose: To assess the Law of Conservation of Mass by designing an experiment to determine mass before and after a chemical reaction. 1. Write
Matter & Energy: Temperature & Heat in Physical Processes
 Matter & Energy: Temperature & Heat in Physical Processes Objectives: 1) To observe changes in temperature and heat energy which occur during physical processes such as dissolving. 2) To become familiar
Matter & Energy: Temperature & Heat in Physical Processes Objectives: 1) To observe changes in temperature and heat energy which occur during physical processes such as dissolving. 2) To become familiar
THE LAWS LAB LAW OF CONSERVATION OF MASS, LAW OF DEFINITE PROPORTIONS, LAW OF MULTIPLE PROPORTIONS
 THE LAWS LAB LAW OF CONSERVATION OF MASS, LAW OF DEFINITE PROPORTIONS, LAW OF MULTIPLE PROPORTIONS PRELAB Please answer the following questions on a separate piece of paper using complete sentences. 1.
THE LAWS LAB LAW OF CONSERVATION OF MASS, LAW OF DEFINITE PROPORTIONS, LAW OF MULTIPLE PROPORTIONS PRELAB Please answer the following questions on a separate piece of paper using complete sentences. 1.
CHEMISTRY. Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A.
 CHEMISTRY Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A. CLEARLY SHOW THE METHOD USED AND THE STEPS INVOLVED IN ARRIVING AT YOUR ANSWERS. It is to your
CHEMISTRY Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A. CLEARLY SHOW THE METHOD USED AND THE STEPS INVOLVED IN ARRIVING AT YOUR ANSWERS. It is to your
8.1 Chemical Properties and Changes. chemical property chemical change dissolving
 8.1 Chemical Properties and Changes chemical property chemical change dissolving Ability to Change 8.1 Chemical Properties and Changes In a chemical change, the properties that give a substance its identity
8.1 Chemical Properties and Changes chemical property chemical change dissolving Ability to Change 8.1 Chemical Properties and Changes In a chemical change, the properties that give a substance its identity
CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED)
 CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED) Overview: Students investigate chemical reactions using given substances. Students identify chemical reactions in their daily lives. Objectives: The student
CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED) Overview: Students investigate chemical reactions using given substances. Students identify chemical reactions in their daily lives. Objectives: The student
Culinary Chemistry: Stoichiometry Made Simple
 Culinary Chemistry: Stoichiometry Made Simple Background Stoichiometry is based on the law of conservation of mass which states that the mass of the reactants in a chemical reaction equals the mass of
Culinary Chemistry: Stoichiometry Made Simple Background Stoichiometry is based on the law of conservation of mass which states that the mass of the reactants in a chemical reaction equals the mass of
Gas Laws. by Dawn Richardson PhD, Collin College, Preston Ridge Campus; revised by Jim Sizemore PhD, Collin College, Central Park Campus
 Gas Laws by Dawn Richardson PhD, Collin College, Preston Ridge Campus; revised by Jim Sizemore PhD, Collin College, Central Park Campus Introduction: A gas is a state of matter in which atoms or molecules
Gas Laws by Dawn Richardson PhD, Collin College, Preston Ridge Campus; revised by Jim Sizemore PhD, Collin College, Central Park Campus Introduction: A gas is a state of matter in which atoms or molecules
The Hand Warmer Design Challenge: Where Does the Heat Come From?
 The Hand Warmer Design Challenge: Where Does the Heat Come From? LSNED Learn Something New Every Day About Sharing and Contributions Interesting Facts Science In Your Mittens: The Chemistry Of Hand Warmers
The Hand Warmer Design Challenge: Where Does the Heat Come From? LSNED Learn Something New Every Day About Sharing and Contributions Interesting Facts Science In Your Mittens: The Chemistry Of Hand Warmers
Are Chemical Reactions Closed Systems?
 Measuring a Chemical Reaction 1. Place about 100 ml of vinegar in a large plastic cup. Carefully weigh the cup AND the vinegar all together, and write down the total mass. (Don t worry about subtracting
Measuring a Chemical Reaction 1. Place about 100 ml of vinegar in a large plastic cup. Carefully weigh the cup AND the vinegar all together, and write down the total mass. (Don t worry about subtracting
COPYRIGHT FOUNTAINHEAD PRESS
 Calorimetry: Heats of Solution Objective: Use calorimetric measurements to determine heats of solution of two ionic compounds. Materials: Solid ammonium nitrate (NH 4 NO 3 ) and anhydrous calcium chloride
Calorimetry: Heats of Solution Objective: Use calorimetric measurements to determine heats of solution of two ionic compounds. Materials: Solid ammonium nitrate (NH 4 NO 3 ) and anhydrous calcium chloride
Thermal Energy and Temperature Lab. Experiment Question: How can the difference between thermal energy and temperature be experimentally observed?
 Thermal Energy and Temperature Lab Name 7 th Grade PSI Grade / 20 Experiment Question: How can the difference between thermal energy and temperature be experimentally observed? Hypothesis Starters: 1.
Thermal Energy and Temperature Lab Name 7 th Grade PSI Grade / 20 Experiment Question: How can the difference between thermal energy and temperature be experimentally observed? Hypothesis Starters: 1.
Lab 2-Investigating the Law of Conservation of Mass
 Name: Period: Lab 2-Investigating the Law of Conservation of Mass Objective: To corroborate the law of conservation of mass through laboratory experimentation. Background: When wood burns or water evaporates,
Name: Period: Lab 2-Investigating the Law of Conservation of Mass Objective: To corroborate the law of conservation of mass through laboratory experimentation. Background: When wood burns or water evaporates,
Education is not the filling of a pail, but the lighting of a fire.
 Education is not the filling of a pail, but the lighting of a fire. William Butler Yeats I. Some reactions release energy to the surroundings when they occur, and others absorb energy in order to occur.
Education is not the filling of a pail, but the lighting of a fire. William Butler Yeats I. Some reactions release energy to the surroundings when they occur, and others absorb energy in order to occur.
Unit 2: Chemical Kinetics Chemistry 30
 Practice Questions Section 3.2 Factors Influencing Reaction Rate - Activation Energy 1. Answer the following questions based on the potential energy diagram shown here: a. Does the graph represent an endothermic
Practice Questions Section 3.2 Factors Influencing Reaction Rate - Activation Energy 1. Answer the following questions based on the potential energy diagram shown here: a. Does the graph represent an endothermic
Working with Solutions. (and why that s not always ideal)
 Page 1 of 13 Working with Solutions (and why that s not always ideal) Learning Objectives: Solutions are prepared by dissolving a solute into a solvent A solute is typically a solid, but may also be a
Page 1 of 13 Working with Solutions (and why that s not always ideal) Learning Objectives: Solutions are prepared by dissolving a solute into a solvent A solute is typically a solid, but may also be a
MiSP CHEMICAL REACTIONS, L3 Teacher Guide. Introduction
 MiSP CHEMICAL REACTIONS, L3 Teacher Guide Introduction This weeklong unit should be included with other chemistry content teaching and learning. It is designed to follow Intermediate Level Science Core
MiSP CHEMICAL REACTIONS, L3 Teacher Guide Introduction This weeklong unit should be included with other chemistry content teaching and learning. It is designed to follow Intermediate Level Science Core
Chemical Reactions Chapter 2 L book pages L44 - L73. examples?
 Name: Period: Chemical Reactions Chapter 2 L book pages L44 - L73 Vocabulary Word What is this? (definition) What are some examples? What does it look like? (draw a picture or diagram) Physical property
Name: Period: Chemical Reactions Chapter 2 L book pages L44 - L73 Vocabulary Word What is this? (definition) What are some examples? What does it look like? (draw a picture or diagram) Physical property
Chemical Reactions. Chemical Reaction occur when atoms collide. Reaction rates change from increasing or decreasing collisions
 Chemical Reactions Questions 1. What is a chemical reaction 2. What evidence suggests that a chemical reaction has taken place? 3. What factors can affect the rate of a chemical reaction (how fast the
Chemical Reactions Questions 1. What is a chemical reaction 2. What evidence suggests that a chemical reaction has taken place? 3. What factors can affect the rate of a chemical reaction (how fast the
CHM111 Lab Titration of Vinegar Grading Rubric
 Name Team Name CHM111 Lab Titration of Vinegar Grading Rubric Criteria Points possible Points earned Lab Performance Printed lab handout and rubric was brought to lab 3 Safety and proper waste disposal
Name Team Name CHM111 Lab Titration of Vinegar Grading Rubric Criteria Points possible Points earned Lab Performance Printed lab handout and rubric was brought to lab 3 Safety and proper waste disposal
Experiment 8 - Chemical Changes
 Experiment 8 - Chemical Changes When a chemical change occurs, the chemicals that you start with are changed into different chemicals. We know when this happens because the new chemicals have different
Experiment 8 - Chemical Changes When a chemical change occurs, the chemicals that you start with are changed into different chemicals. We know when this happens because the new chemicals have different
Signs of Chemical Reactions
 Signs of Chemical Reactions Tell the difference between changes which are PHYSICAL and those which involve a CHEMICAL reaction Doing six different experiments and observing the changes which
Signs of Chemical Reactions Tell the difference between changes which are PHYSICAL and those which involve a CHEMICAL reaction Doing six different experiments and observing the changes which
Apply the ideal gas law (PV = nrt) to experimentally determine the number of moles of carbon dioxide gas generated
 Teacher Information Ideal Gas Law Objectives Determine the number of moles of carbon dioxide gas generated during a reaction between hydrochloric acid and sodium bicarbonate. Through this investigation,
Teacher Information Ideal Gas Law Objectives Determine the number of moles of carbon dioxide gas generated during a reaction between hydrochloric acid and sodium bicarbonate. Through this investigation,
LEVEL ZERO VOICE CATALYST (10 minutes, individual work): 1. Counting atoms
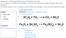 Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
Chapter 14 Study Questions Name: Sci Class:
 Chapter 14 Study Questions Name: Sci Class: Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Balancing a chemical equation so that the same
Chapter 14 Study Questions Name: Sci Class: Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Balancing a chemical equation so that the same
Experiment 20: Analysis of Vinegar. Materials:
 Experiment 20: Analysis of Vinegar Materials: graduated cylinder 6 M NaOH: Dilute Sodium Hydroxide 1000 ml Florence Flask & stopper KHC 8 H 4 O 4 : Potassium Hydrogen Phthalate (KHP) 125 ml Erlenmeyer
Experiment 20: Analysis of Vinegar Materials: graduated cylinder 6 M NaOH: Dilute Sodium Hydroxide 1000 ml Florence Flask & stopper KHC 8 H 4 O 4 : Potassium Hydrogen Phthalate (KHP) 125 ml Erlenmeyer
Matter is all around us everything is made of matter. Matter is anything that takes up space and has mass.
 Matter Study Guide Matter is all around us everything is made of matter. Matter is anything that takes up space and has mass. We can classify objects by their physical properties. Physical properties are
Matter Study Guide Matter is all around us everything is made of matter. Matter is anything that takes up space and has mass. We can classify objects by their physical properties. Physical properties are
Signs of Chemical Reactions
 Signs of Chemical Reactions Tell the difference between changes which are PHYSICAL and those which involve a CHEMICAL reaction Doing six different experiments and observing the changes which
Signs of Chemical Reactions Tell the difference between changes which are PHYSICAL and those which involve a CHEMICAL reaction Doing six different experiments and observing the changes which
Exp 09: Heat of Reaction
 Your job is to use a calorimeter to determine the heat of reaction for three different chemical reactions. Each of these reactions is an acid-base neutralization reaction. Before using your calorimeter
Your job is to use a calorimeter to determine the heat of reaction for three different chemical reactions. Each of these reactions is an acid-base neutralization reaction. Before using your calorimeter
Chemistry 3202 Lab 6 Hess s Law 1
 Chemistry 3202 Lab 6 Hess s Law 1 Lab 6 Hess's Law Introduction Chemical and physical changes are always accompanied by a change in energy. Energy changes may be observed by detecting heat flow between
Chemistry 3202 Lab 6 Hess s Law 1 Lab 6 Hess's Law Introduction Chemical and physical changes are always accompanied by a change in energy. Energy changes may be observed by detecting heat flow between
Every living and nonliving things is made up of matter. MATTER: anything that has mass & takes up space. What does all matter have in common?
 the basics Every living and nonliving things is made up of matter MATTER: anything that has mass & takes up space What does all matter have in common? Smallest unit of matter ALL matter is made of particles
the basics Every living and nonliving things is made up of matter MATTER: anything that has mass & takes up space What does all matter have in common? Smallest unit of matter ALL matter is made of particles
EXPERIMENT 9 ENTHALPY OF REACTION HESS S LAW
 EXPERIMENT 9 ENTHALPY OF REACTION HESS S LAW INTRODUCTION Chemical changes are generally accompanied by energy changes; energy is absorbed or evolved, usually as heat. Breaking chemical bonds in reactants
EXPERIMENT 9 ENTHALPY OF REACTION HESS S LAW INTRODUCTION Chemical changes are generally accompanied by energy changes; energy is absorbed or evolved, usually as heat. Breaking chemical bonds in reactants
Name: Block: Date: Student Notes
 Name: Block: Date: LCPS Core Experience Watersheds Student Notes OBJECTIVES Students will: investigate the difference between physical and chemical weathering identify the destination of the loss of mass
Name: Block: Date: LCPS Core Experience Watersheds Student Notes OBJECTIVES Students will: investigate the difference between physical and chemical weathering identify the destination of the loss of mass
Chemistry Midterm Review. Topics:
 Chemistry Midterm Review Unit 1: laboratory equipment and safety rules accuracy vs precision scientific method: observation, hypothesis. experimental design: independent vs dependent variables, control
Chemistry Midterm Review Unit 1: laboratory equipment and safety rules accuracy vs precision scientific method: observation, hypothesis. experimental design: independent vs dependent variables, control
composition of matter, and the changes that matter undergoes. Examples of Uses of Chemistry in Everyday Life
 Name Matter and Change: Unit Objective Study Guide Date Due Directions: Write your answers to the following questions in the space provided. For problem solving, all of the work leading up to the final
Name Matter and Change: Unit Objective Study Guide Date Due Directions: Write your answers to the following questions in the space provided. For problem solving, all of the work leading up to the final
EXPERIMENT A4: PRECIPITATION REACTION AND THE LIMITING REAGENT. Learning Outcomes. Introduction
 1 EXPERIMENT A4: PRECIPITATION REACTION AND THE LIMITING REAGENT Learning Outcomes Upon completion of this lab, the student will be able to: 1) Demonstrate the formation of a precipitate in a chemical
1 EXPERIMENT A4: PRECIPITATION REACTION AND THE LIMITING REAGENT Learning Outcomes Upon completion of this lab, the student will be able to: 1) Demonstrate the formation of a precipitate in a chemical
Chapter 2. Section 1
 Chapter 2 Section 1 Describing Matter Properties used to describe matter can be classified as extensive or intensive Extensive property - depends on the amount of matter in a sample. Ex. Mass and Volume
Chapter 2 Section 1 Describing Matter Properties used to describe matter can be classified as extensive or intensive Extensive property - depends on the amount of matter in a sample. Ex. Mass and Volume
High School Science Chemistry Unit 12 Exemplar Lesson 02: Heat Energy in Chemical Reactions
 Science Unit: 12 Lesson: 02 Suggested Duration: 7 days High School Science Unit 12 Exemplar Lesson 02: Heat Energy in Chemical Reactions This lesson is one approach to teaching the State Standards associated
Science Unit: 12 Lesson: 02 Suggested Duration: 7 days High School Science Unit 12 Exemplar Lesson 02: Heat Energy in Chemical Reactions This lesson is one approach to teaching the State Standards associated
