Safety: Safety goggles should be worn at all times. Students should hold the balloons on the test tubes tightly while the reaction takes place.
|
|
|
- Ralph Fitzgerald
- 6 years ago
- Views:
Transcription
1 LIMITING REAGENT LAB: THE REACTION BETWEEN VINEGAR AND BAKING SODA Goal: During this lab students will gain a quantitative understanding of limiting reagents. Safety: Safety goggles should be worn at all times. Students should hold the balloons on the test tubes tightly while the reaction takes place. Procedure 1. Students need to get together in teams of four students. 2. Students need to follow the directions below: Student A: Weigh the following six amounts of baking soda (sodium bicarbonate, NaHCO 3 ): 0.18 grams, 0.35 grams, 0.52 grams, 0.70 grams, 1.00 grams, and 1.70 grams. Student B: Student C: Student D: Label the balloons 1-6. Put the six different masses of baking soda into six balloons using a small plastic funnel. Make sure the baking soda goes to the bottom of the balloon. Using the graduated cylinder, accurately measure and transfer 10.0 ml vinegar (5% acetic acid, HC 2 H 3 O 2 ) into each of the 6 test tubes. Attach the filled balloons to the mouth of the test tubes. Make sure that the contents of the balloon and test tube are not mixed. 3. Before mixing the contents of the balloons and test tubes, make a prediction about which combination will produce the greatest amount of carbon dioxide gas. Prediction: 1
2 Observations Data Analysis 1. [1 mark] What was the independent variable in this lab? 2. [1 mark] What was the dependent variable in this lab? 3. [2 mark] What variables were controlled? 4. [1 mark] Does each balloon inflate to some degree? 5. [5 marks] On excel, make a graph of the mass of CO 2 vs. balloon number on excel. Make balloon number the independent variable. 6. Use your observations and the graph to compare the degree to which each balloon inflated. i) [1 mark] What is the pattern of inflation in test tubes 1-4? ii) [1 mark] Noting that in these first four test tubes the mass of bicarbonate increases, what effect does this have on the reaction? iii) [1 mark] What is the pattern of inflation in test tube 4-6? iv) [1 mark] Noting that in last three test tubes the mass of bicarbonate is still increasing, what effect does this have on the reaction? 2
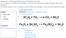
3 Post lab calculations: Answer the following questions and complete the table below. 1. [1 mark] Write the balanced molecular double displacement equation for the reaction that takes place during this lab. One of your products undergoes ad decomposition reaction. Highlight/circle the identity of the gas that inflated the balloons? 2. [1 mark] What is the true combining mole ratio of acetic acid to sodium bicarbonate for the reaction based on the balanced equation? 3. [3 marks] Find the number of moles of baking soda (NaHCO 3 ) used in each reaction: (NaHCO 3 = 84.0 g/mole). Record answers in data table. 4. [3 mark] Determine the mole ratio of sodium bicarbonate to acetic acid for each reaction. (There are 8.3 x 10-3 moles of acetic acid in 10.0 ml of acetic acid.) Record answers in data table. 5. [1 mark] How does the vinegar-bicarbonate mole ratio in the test tube #4 fit into the equation you wrote? What does this ratio mean? 6. [2 mark] Determine which reactants are limiting and which ones are in excess for all the test tubes. Write answers in the data table. 7. [1 mark] After the you have determined which chemical is the limiting reagent in each test tube reaction you can therefore determine how many moles of carbon dioxide were produced in each tube. Record your data in the table. 8. [3 marks] From the limiting reagent, find the mass of carbon dioxide produced. Show your work in the table below. 9. [3 marks] The mass of NaHCO3 increases in each of the test tube. Does this follow the inflation pattern shown in your graph? If not, how do you account for the difference? [Hint; discuss limiting reactants] 3
4 10. Consider the following reaction: Mg(s) + 2HCl(aq) MgCl 2 (aq) + H 2 (g) (a) What is the combining mole ratio of Mg to HCl? (b) Suppose this reaction is performed similarly to the acetic acid/baking soda reaction. A volume of HCl is put in the test tube and a mass of Mg metal is placed in the balloon. Hydrogen gas is produced and inflates the balloon. Which balloon will be the largest? Complete the following table: (There are mol of HCl in 10.0 ml of HCl; Mg = 24.3 g/mole). Test Tube # Mass of Mg, g Mg Volume of HCl, ml HCl Mole ratio Mg:HCl Excess Limiting
5 Test Tube # Mass of NaHCO 3 NaHCO 3 Volume of acid, ml acetic acid Mole ratio: NaHCO 3 : acetic acid Excess Limiting Maximum moles of CO 2 Maximum mass of CO g g g g g g
6
7 Exp. 15 Page 7
Goal: During this lab students will gain a quantitative understanding of limiting reagents.
 LIMITING REAGENT LAB: THE REACTION BETWEEN VINEGAR AND BAKING SODA Goal: During this lab students will gain a quantitative understanding of limiting reagents. Safety: Safety goggles should be worn at all
LIMITING REAGENT LAB: THE REACTION BETWEEN VINEGAR AND BAKING SODA Goal: During this lab students will gain a quantitative understanding of limiting reagents. Safety: Safety goggles should be worn at all
Limiting Reactants Lab
 Name: Teacher s Name: Class: Block: Date: Partners: Limiting Reactants Lab Purpose: Through experimentation, determine the limiting reactant and the percent yield in a chemical reaction that generates
Name: Teacher s Name: Class: Block: Date: Partners: Limiting Reactants Lab Purpose: Through experimentation, determine the limiting reactant and the percent yield in a chemical reaction that generates
Moles Lab Activity 1: PCU (Popcorn Counting Units)
 Moles Lab Activity 1: PCU (Popcorn Counting Units) Materials: A container of each of the following: Popcorn kernels Another type of beans A large unopened bag of popcorn Kernels Balance Safety goggles
Moles Lab Activity 1: PCU (Popcorn Counting Units) Materials: A container of each of the following: Popcorn kernels Another type of beans A large unopened bag of popcorn Kernels Balance Safety goggles
Chemical Reactions Investigation Two Data Record
 Chemical Reactions Investigation Two Data Record Name: Date: 1. During this Investigation, you will analyze how changing the amounts of the reactants in a chemical reaction affects the amount of the products
Chemical Reactions Investigation Two Data Record Name: Date: 1. During this Investigation, you will analyze how changing the amounts of the reactants in a chemical reaction affects the amount of the products
Are Chemical Reactions Closed Systems?
 Measuring a Chemical Reaction 1. Place about 100 ml of vinegar in a large plastic cup. Carefully weigh the cup AND the vinegar all together, and write down the total mass. (Don t worry about subtracting
Measuring a Chemical Reaction 1. Place about 100 ml of vinegar in a large plastic cup. Carefully weigh the cup AND the vinegar all together, and write down the total mass. (Don t worry about subtracting
Gas Laws. by Dawn Richardson PhD, Collin College, Preston Ridge Campus; revised by Jim Sizemore PhD, Collin College, Central Park Campus
 Gas Laws by Dawn Richardson PhD, Collin College, Preston Ridge Campus; revised by Jim Sizemore PhD, Collin College, Central Park Campus Introduction: A gas is a state of matter in which atoms or molecules
Gas Laws by Dawn Richardson PhD, Collin College, Preston Ridge Campus; revised by Jim Sizemore PhD, Collin College, Central Park Campus Introduction: A gas is a state of matter in which atoms or molecules
Chemical Reactions Investigation Two Data Record
 Chemical Reactions Investigation Two Data Record Name: Date: 1. During this Investigation, you will analyze how changing the amounts of the reactants in a chemical reaction affects the amount of the products
Chemical Reactions Investigation Two Data Record Name: Date: 1. During this Investigation, you will analyze how changing the amounts of the reactants in a chemical reaction affects the amount of the products
Student Notes. Chemical Reactions LINK
 LCPS Core Experience Chemical Reactions Student Notes OBJECTIVES Students will: investigate the relationship between reactants and products. investigate an exothermic reaction. investigate an endothermic
LCPS Core Experience Chemical Reactions Student Notes OBJECTIVES Students will: investigate the relationship between reactants and products. investigate an exothermic reaction. investigate an endothermic
Lab 2-Investigating the Law of Conservation of Mass
 Name: Period: Lab 2-Investigating the Law of Conservation of Mass Objective: To corroborate the law of conservation of mass through laboratory experimentation. Background: When wood burns or water evaporates,
Name: Period: Lab 2-Investigating the Law of Conservation of Mass Objective: To corroborate the law of conservation of mass through laboratory experimentation. Background: When wood burns or water evaporates,
Chemistry. End of Year Cornerstone Assessment
 Chemistry End of Year Cornerstone Assessment The Cornerstone Assessments were developed with support through the VDOE Mathematics and Science Partnership Grant Program NCLB Title II, Part B program by
Chemistry End of Year Cornerstone Assessment The Cornerstone Assessments were developed with support through the VDOE Mathematics and Science Partnership Grant Program NCLB Title II, Part B program by
Apply the ideal gas law (PV = nrt) to experimentally determine the number of moles of carbon dioxide gas generated
 Teacher Information Ideal Gas Law Objectives Determine the number of moles of carbon dioxide gas generated during a reaction between hydrochloric acid and sodium bicarbonate. Through this investigation,
Teacher Information Ideal Gas Law Objectives Determine the number of moles of carbon dioxide gas generated during a reaction between hydrochloric acid and sodium bicarbonate. Through this investigation,
Stoichiometry study of the relationships in a
 Note Taking Guide: Episode 801 Name Stoichiometry study of the relationships in a based on equations 2 Mg + O 2 2 MgO The in a give the for the involved in the. Ex. Problem: When elemental aluminum reacts
Note Taking Guide: Episode 801 Name Stoichiometry study of the relationships in a based on equations 2 Mg + O 2 2 MgO The in a give the for the involved in the. Ex. Problem: When elemental aluminum reacts
What Do You Think? Investigate GOALS
 Ideal Toy Activity 7 Moving Molecules GOALS In this activity you will: Determine the effect of molecular size on molecular motion. Predict quantities of gas produced in chemical reactions. What Do You
Ideal Toy Activity 7 Moving Molecules GOALS In this activity you will: Determine the effect of molecular size on molecular motion. Predict quantities of gas produced in chemical reactions. What Do You
Chemical Reaction Lab Bagged Chemical Reactions
 Learning Target: The student experiments and determines that the rates of reaction among atoms and molecules depend on the concentration, pressure, and temperature of the reactants and the presence or
Learning Target: The student experiments and determines that the rates of reaction among atoms and molecules depend on the concentration, pressure, and temperature of the reactants and the presence or
Stoichiometry: Baking Soda and Vinegar Reactions Student Version
 Stoichiometry: Baking Soda and Vinegar Reactions Student Version In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household chemicals
Stoichiometry: Baking Soda and Vinegar Reactions Student Version In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household chemicals
Stoichiometry Lab Report
 Stoichiometry Lab Report Touche G10 Variables Variable Dependent Controlled: Amount of NaHCO3 Theoretical Yield Vinegar Evaporation Manipulation We measure the remaining NaC 2 H 3 O 2 and compare it with
Stoichiometry Lab Report Touche G10 Variables Variable Dependent Controlled: Amount of NaHCO3 Theoretical Yield Vinegar Evaporation Manipulation We measure the remaining NaC 2 H 3 O 2 and compare it with
Stoichiometry: Baking Soda and Vinegar Reactions Student Advanced Version
 Stoichiometry: Baking Soda and Vinegar Reactions Student Advanced Version In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household
Stoichiometry: Baking Soda and Vinegar Reactions Student Advanced Version In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household
Bellevue College CHEM& 121 Experiment: Stoichiometric Analysis of an Antacid 1
 Experiment: Stoichiometric Analysis of an Antacid 1 Introduction In this lab, you will use the concept of stoichiometry to solve two sequential problems. First, you will try to determine the products of
Experiment: Stoichiometric Analysis of an Antacid 1 Introduction In this lab, you will use the concept of stoichiometry to solve two sequential problems. First, you will try to determine the products of
Acid Base Titration Experiment ACID - BASE TITRATION LAB
 ACID - BASE TITRATION LAB MATERIALS and CHEMICALS Burette 50 ml Burette clamp Ring stand Stirring rod Plastic funnel Beakers (50 ml, 100 ml, 400 ml) Graduated cylinder (25 ml, 50 ml) 0.10 M NaOH 0.10 M
ACID - BASE TITRATION LAB MATERIALS and CHEMICALS Burette 50 ml Burette clamp Ring stand Stirring rod Plastic funnel Beakers (50 ml, 100 ml, 400 ml) Graduated cylinder (25 ml, 50 ml) 0.10 M NaOH 0.10 M
JHS Regents Chemistry Department Rates of Chemical Reactions Inquiry Investigation
 Name: Date: Partner: Period: JHS Regents Chemistry Department Rates of Chemical Reactions Inquiry Investigation Introduction: According to the collision theory, the rate of a reaction depends on the frequency
Name: Date: Partner: Period: JHS Regents Chemistry Department Rates of Chemical Reactions Inquiry Investigation Introduction: According to the collision theory, the rate of a reaction depends on the frequency
Name Period Date. Lab: Introduction to Stoichiometry
 Name Period Date Lab: Introduction to Stoichiometry Introduction: Reactants are not always present in the exact ratio required by a balanced chemical equation. In planning any cost-effective production
Name Period Date Lab: Introduction to Stoichiometry Introduction: Reactants are not always present in the exact ratio required by a balanced chemical equation. In planning any cost-effective production
Stoichiometry Decomposition of Baking Soda For our soul is humbled down to the dust. Psalms 43:25
 Stoichiometry Decomposition of Baking Soda For our soul is humbled down to the dust. Psalms 43:25 Introduction A balanced chemical reaction shows products forming reactants such that the Law of Conservation
Stoichiometry Decomposition of Baking Soda For our soul is humbled down to the dust. Psalms 43:25 Introduction A balanced chemical reaction shows products forming reactants such that the Law of Conservation
CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED)
 CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED) Overview: Students investigate chemical reactions using given substances. Students identify chemical reactions in their daily lives. Objectives: The student
CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED) Overview: Students investigate chemical reactions using given substances. Students identify chemical reactions in their daily lives. Objectives: The student
Moles Lab Activity 2: Elements Copper
 Materials Sample of copper Balance Pre-1982 penny Moles Lab Activity 2: Elements Copper Procedure Take the necessary measurements, and record them with units. Show all your calculations, rounding your
Materials Sample of copper Balance Pre-1982 penny Moles Lab Activity 2: Elements Copper Procedure Take the necessary measurements, and record them with units. Show all your calculations, rounding your
Chapter 3 Stoichiometry
 Chapter 3 Sep 22 1:45 PM Average atomic mass: The weighted average of all isotopes of a specific element. Takes into consideration abundance of each isotope. (% x M 1 ) + (% x M 2 ) +... Sep 22 1:45 PM
Chapter 3 Sep 22 1:45 PM Average atomic mass: The weighted average of all isotopes of a specific element. Takes into consideration abundance of each isotope. (% x M 1 ) + (% x M 2 ) +... Sep 22 1:45 PM
MiSP Chemical Reactions Lab Temperature L2. Plop, Plop, Fizz, Fizz... Temperature and Rate of Reaction Activity
 MiSP Chemical Reactions Lab Temperature L2 Name Date Plop, Plop, Fizz, Fizz... Temperature and Rate of Reaction Activity Introduction: Problem: Alka-Seltzer in water produces carbon dioxide. It is the
MiSP Chemical Reactions Lab Temperature L2 Name Date Plop, Plop, Fizz, Fizz... Temperature and Rate of Reaction Activity Introduction: Problem: Alka-Seltzer in water produces carbon dioxide. It is the
Lab Activity 3: Factors Affecting Reaction Rate
 Chemistry 3202 Lab #3 factors affecting Reaction Rate Page 1 of 5 Lab Activity 3: Factors Affecting Reaction Rate Introduction Several factors influence how fast a reaction proceeds. In this activity,
Chemistry 3202 Lab #3 factors affecting Reaction Rate Page 1 of 5 Lab Activity 3: Factors Affecting Reaction Rate Introduction Several factors influence how fast a reaction proceeds. In this activity,
DETERMINING AND USING H
 DETERMINING AND USING H INTRODUCTION CHANGES IN CHEMISTRY Chemistry is the science that studies matter and the changes it undergoes. Changes are divided into two categories: physical and chemical. During
DETERMINING AND USING H INTRODUCTION CHANGES IN CHEMISTRY Chemistry is the science that studies matter and the changes it undergoes. Changes are divided into two categories: physical and chemical. During
Alternative Reaction Pathways
 Section 1 Energy and Entropy: Alternative Reaction Pathways What Do You See? Learning Outcomes In this section you will Apply the engineering-design process to scientific and everyday situations. Generate
Section 1 Energy and Entropy: Alternative Reaction Pathways What Do You See? Learning Outcomes In this section you will Apply the engineering-design process to scientific and everyday situations. Generate
Culinary Chemistry: Stoichiometry Made Simple
 Culinary Chemistry: Stoichiometry Made Simple Background Stoichiometry is based on the law of conservation of mass which states that the mass of the reactants in a chemical reaction equals the mass of
Culinary Chemistry: Stoichiometry Made Simple Background Stoichiometry is based on the law of conservation of mass which states that the mass of the reactants in a chemical reaction equals the mass of
Identification of an Unknown Compound through Mass Correlations
 EXPERIMENT Identification of an Unknown Compound through Mass Correlations PURPOSE To carry out a series of decomposition reactions for five different unknown, and use stoichiometry in order to identify
EXPERIMENT Identification of an Unknown Compound through Mass Correlations PURPOSE To carry out a series of decomposition reactions for five different unknown, and use stoichiometry in order to identify
VOCABULARY Define. 1. stoichiometry. 2. composition stoichiometry. 3. reaction stoichiometry. 4. unknown. 5. mole ratio
 CHAPTER 9 HOMEWORK 9-1 (pp. 275 279) Define. 1. stoichiometry 2. composition stoichiometry 3. reaction stoichiometry 4. unknown 5. mole ratio SKILL BUILDER On a separate sheet of paper, write five possible
CHAPTER 9 HOMEWORK 9-1 (pp. 275 279) Define. 1. stoichiometry 2. composition stoichiometry 3. reaction stoichiometry 4. unknown 5. mole ratio SKILL BUILDER On a separate sheet of paper, write five possible
AP Chemistry Unit 2 Test (Chapters 3 and 4)
 AP Chemistry Unit 2 Test (Chapters 3 and 4) NAME: 1. A student is assigned the task of determining the mass percent of silver in an alloy of copper and silver by dissolving a sample of the alloy in excess
AP Chemistry Unit 2 Test (Chapters 3 and 4) NAME: 1. A student is assigned the task of determining the mass percent of silver in an alloy of copper and silver by dissolving a sample of the alloy in excess
MiSP Chemical Reactions Lab Temperature L3. Plop, Plop, Fizz, Fizz... Temperature and Rate of Reaction Activity
 MiSP Chemical Reactions Lab Temperature L3 Name Date Plop, Plop, Fizz, Fizz... Temperature and Rate of Reaction Activity Introduction: Problem: Alka-Seltzer in water produces carbon dioxide. It is the
MiSP Chemical Reactions Lab Temperature L3 Name Date Plop, Plop, Fizz, Fizz... Temperature and Rate of Reaction Activity Introduction: Problem: Alka-Seltzer in water produces carbon dioxide. It is the
CHM111 Lab Titration of Vinegar Grading Rubric
 Name Team Name CHM111 Lab Titration of Vinegar Grading Rubric Criteria Points possible Points earned Lab Performance Printed lab handout and rubric was brought to lab 3 Safety and proper waste disposal
Name Team Name CHM111 Lab Titration of Vinegar Grading Rubric Criteria Points possible Points earned Lab Performance Printed lab handout and rubric was brought to lab 3 Safety and proper waste disposal
Lesson Title: Elementary - CO2 vehicles Amount of time for this lesson = 90 minutes
 Lesson Title: Elementary - CO2 vehicles Amount of time for this lesson = 90 minutes 1. Standards and Safety and Materials: A. Standards - (Wyoming? NGSS? Number and write it out) Wyoming Standards SC4.1.10
Lesson Title: Elementary - CO2 vehicles Amount of time for this lesson = 90 minutes 1. Standards and Safety and Materials: A. Standards - (Wyoming? NGSS? Number and write it out) Wyoming Standards SC4.1.10
Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise.
 Experiment 10 Stoichiometry- Gravimetric Analysis Pre-lab Assignment Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose The purpose this experiment
Experiment 10 Stoichiometry- Gravimetric Analysis Pre-lab Assignment Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose The purpose this experiment
Upon completion of this lab, the student will be able to:
 1 Learning Outcomes EXPERIMENT 30A5: MOLAR VOLUME OF A GAS Upon completion of this lab, the student will be able to: 1) Demonstrate a single replacement reaction. 2) Calculate the molar volume of a gas
1 Learning Outcomes EXPERIMENT 30A5: MOLAR VOLUME OF A GAS Upon completion of this lab, the student will be able to: 1) Demonstrate a single replacement reaction. 2) Calculate the molar volume of a gas
Burning a Hydrocarbon II
 Burning a Hydrocarbon II Name Lab Section Problem Statement: How are the masses of products limited by the amounts of reactants? I. Data Collection: A. Go to http://cheminfo.chem.ou.edu/~mra/home.html
Burning a Hydrocarbon II Name Lab Section Problem Statement: How are the masses of products limited by the amounts of reactants? I. Data Collection: A. Go to http://cheminfo.chem.ou.edu/~mra/home.html
2002 D Required 2001 D Required
 2002 D Required A student is asked to determine the molar enthalpy of neutralization, H neut, for the reaction represented above. The student combines equal volumes of 1.0 M HCl and 1.0 M NaOH in an open
2002 D Required A student is asked to determine the molar enthalpy of neutralization, H neut, for the reaction represented above. The student combines equal volumes of 1.0 M HCl and 1.0 M NaOH in an open
Stoichiometry ( ) ( )
 Stoichiometry Outline 1. Molar Calculations 2. Limiting Reactants 3. Empirical and Molecular Formula Calculations Review 1. Molar Calculations ( ) ( ) ( ) 6.02 x 10 23 particles (atoms or molecules) /
Stoichiometry Outline 1. Molar Calculations 2. Limiting Reactants 3. Empirical and Molecular Formula Calculations Review 1. Molar Calculations ( ) ( ) ( ) 6.02 x 10 23 particles (atoms or molecules) /
Stoichiometry. The quantitative study of reactants and products in a chemical reaction. Burlingame High School Chemistry
 Stoichiometry The quantitative study of reactants and products in a chemical reaction 1 Stoichiometry Whether the units given for reactants or products are moles, grams, liters (for gases), or some other
Stoichiometry The quantitative study of reactants and products in a chemical reaction 1 Stoichiometry Whether the units given for reactants or products are moles, grams, liters (for gases), or some other
Pre-Lab Exercises Lab 3: Chemical Properties
 Pre-Lab Exercises Lab 3: Chemical Properties 1. How is a chemical property different from a physical property? Name Date Section 2. How is a chemical change different from a physical change? 3. Give two
Pre-Lab Exercises Lab 3: Chemical Properties 1. How is a chemical property different from a physical property? Name Date Section 2. How is a chemical change different from a physical change? 3. Give two
Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic
 Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic Key Concepts Carbon dioxide (CO 2 ) gas dissolved in water can cause water to become acidic. The acidity of water from dissolved CO 2 can
Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic Key Concepts Carbon dioxide (CO 2 ) gas dissolved in water can cause water to become acidic. The acidity of water from dissolved CO 2 can
Exploring Acids & Bases
 Food Explorations Lab: Exploring Acids & Bases STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will use cabbage juice indicator to determine if two unknown samples are acids or
Food Explorations Lab: Exploring Acids & Bases STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will use cabbage juice indicator to determine if two unknown samples are acids or
Figure 1. Oxygen. (g) +... (g)... SO 3. The pressure of the reacting gases was increased.
 Q1. Figure 1 represents a reaction in the production of sulfuric acid. Figure 1 Oxygen Sulfur dioxide Sulfur trioxide (a) Complete and balance the equation for the reaction.... SO 2 (g) +... (g)... SO
Q1. Figure 1 represents a reaction in the production of sulfuric acid. Figure 1 Oxygen Sulfur dioxide Sulfur trioxide (a) Complete and balance the equation for the reaction.... SO 2 (g) +... (g)... SO
Chem. I Notes Ch. 11 STOICHIOMETRY NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 Chem. I Notes Ch. 11 STOICHIOMETRY NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. 11.1 notes 1 MOLE = 6.02 x 10 23 representative particles representative particles
Chem. I Notes Ch. 11 STOICHIOMETRY NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. 11.1 notes 1 MOLE = 6.02 x 10 23 representative particles representative particles
Unit VI Stoichiometry. Applying Mole Town to Reactions
 Unit VI Stoichiometry Applying Mole Town to Reactions Learning Goals I can apply mole town to reactions to determine the amount of product based on the amount of a reactant. I can apply mole town to reaction
Unit VI Stoichiometry Applying Mole Town to Reactions Learning Goals I can apply mole town to reactions to determine the amount of product based on the amount of a reactant. I can apply mole town to reaction
LEVEL ZERO VOICE CATALYST (10 minutes, individual work): 1. Counting atoms
 Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
Ch. 10 Notes STOICHIOMETRY NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 Ch. 10 Notes STOICHIOMETRY NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. 1 MOLE = 6.02 x 10 23 representative particles representative particles = ATOMS, IONS,
Ch. 10 Notes STOICHIOMETRY NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. 1 MOLE = 6.02 x 10 23 representative particles representative particles = ATOMS, IONS,
Chapter 6, Lesson 7: Energy Changes in Chemical Reactions
 Chapter 6, Lesson 7: Energy Changes in Chemical Reactions Key Concepts If two substances react and the temperature of the mixture decreases, the reaction is endothermic. If two substances react and the
Chapter 6, Lesson 7: Energy Changes in Chemical Reactions Key Concepts If two substances react and the temperature of the mixture decreases, the reaction is endothermic. If two substances react and the
Reactant A (g) Reactant B (ml) Product (ml)
 Pre-Test Key Name: Chemical Reactions Date: 1. The chart below shows the amount of gas that is produced when two reactants (a solid and a liquid) are combined. However, one of the boxes is missing information.
Pre-Test Key Name: Chemical Reactions Date: 1. The chart below shows the amount of gas that is produced when two reactants (a solid and a liquid) are combined. However, one of the boxes is missing information.
Stoichiometry: Baking Soda and Vinegar Reactions Teacher Version
 Stoichiometry: Baking Soda and Vinegar Reactions Teacher Version In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household chemicals
Stoichiometry: Baking Soda and Vinegar Reactions Teacher Version In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household chemicals
Lab #5 - Limiting Reagent
 Objective Chesapeake Campus Chemistry 111 Laboratory Lab #5 - Limiting Reagent Use stoichiometry to determine the limiting reactant. Calculate the theoretical yield. Calculate the percent yield of a reaction.
Objective Chesapeake Campus Chemistry 111 Laboratory Lab #5 - Limiting Reagent Use stoichiometry to determine the limiting reactant. Calculate the theoretical yield. Calculate the percent yield of a reaction.
Name: Period: Date: Skill 1: Determine the type of equation Evidence:
 Name: Period: Date: KIPP NYC College Prep General Chemistry CHEMISTRY LAB #12 Types of Reactions: Lab 60 MINUTES PRELAB Do Now! Consider the following reaction that produces NO 2. Skill 1: Determine the
Name: Period: Date: KIPP NYC College Prep General Chemistry CHEMISTRY LAB #12 Types of Reactions: Lab 60 MINUTES PRELAB Do Now! Consider the following reaction that produces NO 2. Skill 1: Determine the
Acid-Base Titration Lab
 Acid-Base Titration Lab Name Objectives: - To apply knowledge of molarity to properly dilute a concentrated base - To apply knowledge of solution stoichiometry in order to correctly determine the unknown
Acid-Base Titration Lab Name Objectives: - To apply knowledge of molarity to properly dilute a concentrated base - To apply knowledge of solution stoichiometry in order to correctly determine the unknown
Stoichiometry CHAPTER 12
 CHAPTER 12 Stoichiometry 12.1 Using Everyday Equations Stoichiometry is the calculation of quantities in chemical equations. * The balanced equation gives the ratios for the reactants and products. 3 eggs
CHAPTER 12 Stoichiometry 12.1 Using Everyday Equations Stoichiometry is the calculation of quantities in chemical equations. * The balanced equation gives the ratios for the reactants and products. 3 eggs
Proportional Relationships
 Stoichiometry Video Proportional Relationships 2 1/4 c. flour 1 tsp. baking soda 1 tsp. salt 1 c. butter 3/4 c. sugar 3/4 c. brown sugar 1 tsp vanilla extract 2 eggs 2 c. chocolate chips Makes 5 dozen
Stoichiometry Video Proportional Relationships 2 1/4 c. flour 1 tsp. baking soda 1 tsp. salt 1 c. butter 3/4 c. sugar 3/4 c. brown sugar 1 tsp vanilla extract 2 eggs 2 c. chocolate chips Makes 5 dozen
Experiment #7. Titration of Vinegar
 Experiment #7. Titration of Vinegar Goals 1. To determine the mass percent of acetic acid in a solution via titration. 2. To master the technique of titration. Introduction Vinegar is a common household
Experiment #7. Titration of Vinegar Goals 1. To determine the mass percent of acetic acid in a solution via titration. 2. To master the technique of titration. Introduction Vinegar is a common household
Acid / Base Titrations
 Acid / Base Titrations v051413_7pm Objectives: Determine the concentration of a base solution using an acid standard. Optional: Precipitate an ionic salt for percent yield determination using the standardized
Acid / Base Titrations v051413_7pm Objectives: Determine the concentration of a base solution using an acid standard. Optional: Precipitate an ionic salt for percent yield determination using the standardized
1. Write date, topic and purpose in notebook. 2. Answer in your notebook: What are two signs that a chemical reaction is happening?
 Warm Up (5 min) Date: Monday, 1/4/16 Topic: Alka Seltzer Lab Purpose: To assess the Law of Conservation of Mass by designing an experiment to determine mass before and after a chemical reaction. 1. Write
Warm Up (5 min) Date: Monday, 1/4/16 Topic: Alka Seltzer Lab Purpose: To assess the Law of Conservation of Mass by designing an experiment to determine mass before and after a chemical reaction. 1. Write
CO 2. Lesson 1. Production of a gas
 Lesson 1 Production of a gas T E A C H E R G U I D E CO 2 Lesson summary Students meet volcanologist Victor Helguson, who is studying the gases released by volcanoes in Iceland. Students conduct a chemical
Lesson 1 Production of a gas T E A C H E R G U I D E CO 2 Lesson summary Students meet volcanologist Victor Helguson, who is studying the gases released by volcanoes in Iceland. Students conduct a chemical
Name AP Chemistry / / Chapter 5 Collected AP Exam Free Response Questions Answers
 Name AP Chemistry / / Chapter 5 Collected AP Exam Free Response Questions 1980 2010 - Answers 1982 - #5 (a) From the standpoint of the kinetic-molecular theory, discuss briefly the properties of gas molecules
Name AP Chemistry / / Chapter 5 Collected AP Exam Free Response Questions 1980 2010 - Answers 1982 - #5 (a) From the standpoint of the kinetic-molecular theory, discuss briefly the properties of gas molecules
CHEM 30A EXPERIMENT 5: MOLAR VOLUME OF A GAS (MG + HCL) Learning Outcomes. Introduction. Upon completion of this lab, the student will be able to:
 1 CHEM 30A EXPERIMENT 5: MOLAR VOLUME OF A GAS (MG + HCL) Learning Outcomes Upon completion of this lab, the student will be able to: 1) Demonstrate a single replacement reaction. 2) Calculate the molar
1 CHEM 30A EXPERIMENT 5: MOLAR VOLUME OF A GAS (MG + HCL) Learning Outcomes Upon completion of this lab, the student will be able to: 1) Demonstrate a single replacement reaction. 2) Calculate the molar
Chemistry 143 Acid Base Titration Dr. Caddell. Titrating Acid
 Titrating Acid In this lab you will first determine the concentration of sodium hydroxide in a stock solution that you prepare. You will then use that stock sodium hydroxide solution to titrate a solution
Titrating Acid In this lab you will first determine the concentration of sodium hydroxide in a stock solution that you prepare. You will then use that stock sodium hydroxide solution to titrate a solution
Chemistry Semester One Exam Review
 Chemistry Semester One Exam Review Name: 1. Compare physical and chemical changes in matter. 2. State the law on conservation of mass. 3. On which type of mixture(s) does the Tyndall Effect scatter light?
Chemistry Semester One Exam Review Name: 1. Compare physical and chemical changes in matter. 2. State the law on conservation of mass. 3. On which type of mixture(s) does the Tyndall Effect scatter light?
Chemistry 3202 Lab 6 Hess s Law 1
 Chemistry 3202 Lab 6 Hess s Law 1 Lab 6 Hess's Law Introduction Chemical and physical changes are always accompanied by a change in energy. Energy changes may be observed by detecting heat flow between
Chemistry 3202 Lab 6 Hess s Law 1 Lab 6 Hess's Law Introduction Chemical and physical changes are always accompanied by a change in energy. Energy changes may be observed by detecting heat flow between
Name Period Date. Lab 9: Analysis of Commercial Bleach
 Name Period Date Lab 9: Analysis of Commercial Bleach Introduction Many common products are effective because they contain oxidizing agents. Some products, which contain oxidizing agents, are bleaches,
Name Period Date Lab 9: Analysis of Commercial Bleach Introduction Many common products are effective because they contain oxidizing agents. Some products, which contain oxidizing agents, are bleaches,
Lab #9 Stoichiometric Relationships & Theoretical Yield
 Stoichiometric Relationships & Theoretical Yield Name: Nov. 28, 2016 Background and Purpose: Carbonates when reacted with acids will produce carbon dioxide, water and an ionic salt. In this lab, we will
Stoichiometric Relationships & Theoretical Yield Name: Nov. 28, 2016 Background and Purpose: Carbonates when reacted with acids will produce carbon dioxide, water and an ionic salt. In this lab, we will
Chem 2115 Experiment #7. Volumetric Analysis & Consumer Chemistry Standardization of an unknown solution, analysis of vinegar & antacid tablets
 Chem 2115 Experiment #7 Volumetric Analysis & Consumer Chemistry Standardization of an unknown solution, analysis of vinegar & antacid tablets OBJECTIVE: The goals of this experiment are to learn titration
Chem 2115 Experiment #7 Volumetric Analysis & Consumer Chemistry Standardization of an unknown solution, analysis of vinegar & antacid tablets OBJECTIVE: The goals of this experiment are to learn titration
Heat of Combustion: Magnesium
 Heat of Combustion: Magnesium Experiment 21 In Experiment 17, you learned about the additivity of reaction heats as you confirmed Hess s Law. In this experiment, you will use this principle as you determine
Heat of Combustion: Magnesium Experiment 21 In Experiment 17, you learned about the additivity of reaction heats as you confirmed Hess s Law. In this experiment, you will use this principle as you determine
UNIT 6338: SAMPLE ACTIVITY 28 SAMPLE ASSESSMENT ACTIVITIES AND SCHEDULES PROTON TRANSFERS
 UNIT 6338: SAMPLE ACTIVITY 28 SAMPLE ASSESSMENT ACTIVITIES AND SCHEDULES 6.129 6338 CHEMISTRY LEVEL 2 PROTON TRANSFERS This activity assesses: Unit: 6338 Characterise the behaviour of weak and strong acids
UNIT 6338: SAMPLE ACTIVITY 28 SAMPLE ASSESSMENT ACTIVITIES AND SCHEDULES 6.129 6338 CHEMISTRY LEVEL 2 PROTON TRANSFERS This activity assesses: Unit: 6338 Characterise the behaviour of weak and strong acids
Ideal Gas & Gas Stoichiometry
 Ideal Gas & Gas Stoichiometry Avogadro s Law V a number of moles (n) V = constant x n Constant temperature Constant pressure V 1 /n 1 = V 2 /n 2 Ammonia burns in oxygen to form nitric oxide (NO) and water
Ideal Gas & Gas Stoichiometry Avogadro s Law V a number of moles (n) V = constant x n Constant temperature Constant pressure V 1 /n 1 = V 2 /n 2 Ammonia burns in oxygen to form nitric oxide (NO) and water
CHEMICAL CALCULATIONS - RELATING MASS AND ATOMS
 CHEMICAL CALCULATIONS - RELATING MASS AND ATOMS Chemical equations are written and balanced in terms of ATOMS and MOLECULES - While chemical equations are written in terms of ATOMS and MOLECULES, that's
CHEMICAL CALCULATIONS - RELATING MASS AND ATOMS Chemical equations are written and balanced in terms of ATOMS and MOLECULES - While chemical equations are written in terms of ATOMS and MOLECULES, that's
CHEMISTRY 202 Hour Exam I. Dr. D. DeCoste T.A.
 CHEMISTRY 202 Hour Exam I September 22, 2016 Dr. D. DeCoste Name Signature T.A. This exam contains 23 questions on 11 numbered pages. Check now to make sure you have a complete exam. You have two hours
CHEMISTRY 202 Hour Exam I September 22, 2016 Dr. D. DeCoste Name Signature T.A. This exam contains 23 questions on 11 numbered pages. Check now to make sure you have a complete exam. You have two hours
EXPERIMENT 23 Lab Report Guidelines
 EXPERIMENT 23 Listed below are some guidelines for completing the lab report for Experiment 23: For each part, follow the procedure outlined in the lab manual. Observe all safety rules, including wearing
EXPERIMENT 23 Listed below are some guidelines for completing the lab report for Experiment 23: For each part, follow the procedure outlined in the lab manual. Observe all safety rules, including wearing
Bags of Reactions Chemistry I Acc
 Introduction: Bags of Reactions Chemistry I Acc Plop, plop, fizz, fizz, oh, what a relief it is. claims the old TV ad for a popular antacid. Just what is in the tablet that is relieving the upset stomach?
Introduction: Bags of Reactions Chemistry I Acc Plop, plop, fizz, fizz, oh, what a relief it is. claims the old TV ad for a popular antacid. Just what is in the tablet that is relieving the upset stomach?
Chemistry 143 Experiment #11 Acid Base Titration Dr. Caddell. Titrating Acid
 Titrating Acid In this lab you will first determine the concentration of sodium hydroxide in a stock solution that you prepare. You will then use that stock sodium hydroxide solution to titrate a solution
Titrating Acid In this lab you will first determine the concentration of sodium hydroxide in a stock solution that you prepare. You will then use that stock sodium hydroxide solution to titrate a solution
o Test tube In this experiment, you ll be observing the signs of chemical reactions. These include the following:
 Experiment: Chemical Reactions & Chemical s Objective In this experiment, students perform a variety of chemical reactions. For each reaction, student identify the signs that a reaction has occurred, write
Experiment: Chemical Reactions & Chemical s Objective In this experiment, students perform a variety of chemical reactions. For each reaction, student identify the signs that a reaction has occurred, write
Hess' Law: Calorimetry
 Exercise 9 Page 1 Illinois Central College CHEMISTRY 130 Name: Hess' Law: Calorimetry Objectives The objectives of this experiment are to... - measure the heats of reaction for two chemical reactions.
Exercise 9 Page 1 Illinois Central College CHEMISTRY 130 Name: Hess' Law: Calorimetry Objectives The objectives of this experiment are to... - measure the heats of reaction for two chemical reactions.
Ch 3. Chemical Reactions and Reaction Stoichiometry
 Ch 3 Chemical Reactions and Reaction Stoichiometry { { Chemical Reactions involve the rearrangement of atoms. CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (g) Reactant Products s Law of Conservation of Mass
Ch 3 Chemical Reactions and Reaction Stoichiometry { { Chemical Reactions involve the rearrangement of atoms. CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (g) Reactant Products s Law of Conservation of Mass
KEY Chem 1A First Midterm Examination February 7, 2005 Professor David Chandler
 KEY Chem 1A First Midterm Examination February 7, 2005 Professor David Chandler 1 2 3 4 5 6 7 8 9 10 Extra Name: Signature: Section: GSI: Instructions As indicated, either fill in blank space with appropriate
KEY Chem 1A First Midterm Examination February 7, 2005 Professor David Chandler 1 2 3 4 5 6 7 8 9 10 Extra Name: Signature: Section: GSI: Instructions As indicated, either fill in blank space with appropriate
Name Class Date. Complete each of the following sentences by choosing the correct term from the word bank.
 Skills Worksheet Chapter Review USING KEY TERMS Complete each of the following sentences by choosing the correct term from the word bank. subscript exothermic reaction inhibitor synthesis reaction coefficient
Skills Worksheet Chapter Review USING KEY TERMS Complete each of the following sentences by choosing the correct term from the word bank. subscript exothermic reaction inhibitor synthesis reaction coefficient
High School Science Chemistry Unit 12 Exemplar Lesson 02: Heat Energy in Chemical Reactions
 Science Unit: 12 Lesson: 02 Suggested Duration: 7 days High School Science Unit 12 Exemplar Lesson 02: Heat Energy in Chemical Reactions This lesson is one approach to teaching the State Standards associated
Science Unit: 12 Lesson: 02 Suggested Duration: 7 days High School Science Unit 12 Exemplar Lesson 02: Heat Energy in Chemical Reactions This lesson is one approach to teaching the State Standards associated
Friday, November 2, 2018 GLE/Standard: The fact that atoms are conserved, together
 Mrs. Chausse s Physical Science Gifted Lesson Plan: Unit 7 Chemical Reactions November 1 16, 2018 Thursday, November 1, 2018 Objective: SWBAT balance a chemical equation that satisfies the Law of Conservation
Mrs. Chausse s Physical Science Gifted Lesson Plan: Unit 7 Chemical Reactions November 1 16, 2018 Thursday, November 1, 2018 Objective: SWBAT balance a chemical equation that satisfies the Law of Conservation
The Synthesis of Gun Cotton and TATP
 CHEM 121L General Chemistry Laboratory Revision 1.1 The Synthesis of Gun Cotton and TATP Learn about the Synthesis of "Energetic" Materials. Learn about the Safe Handling of Caustic Substances. In this
CHEM 121L General Chemistry Laboratory Revision 1.1 The Synthesis of Gun Cotton and TATP Learn about the Synthesis of "Energetic" Materials. Learn about the Safe Handling of Caustic Substances. In this
Chemistry CP Lab: Additivity of Heats of Reaction (Hess Law)
 Chemistry CP Lab: Additivity of Heats of Reaction (Hess Law) Name: Date: The formation or destruction of chemical bonds is always accompanied by an energy exchange between the reactant molecules and the
Chemistry CP Lab: Additivity of Heats of Reaction (Hess Law) Name: Date: The formation or destruction of chemical bonds is always accompanied by an energy exchange between the reactant molecules and the
INTRODUCTION. Seltzer relieves upset stomach, provides pain relief, breaks fevers, and reduces inflammation.
 Effervescence Laboratory Experiment Developed by: Alex Jannini, David Krause, Heather Malino and Kevin Sweeney, Rowan University, Department of Chemical Engineering Edited by: C. Stewart Slater and Mariano
Effervescence Laboratory Experiment Developed by: Alex Jannini, David Krause, Heather Malino and Kevin Sweeney, Rowan University, Department of Chemical Engineering Edited by: C. Stewart Slater and Mariano
EXPERIMENT A8: CALORIMETRY. Learning Outcomes. Introduction. Upon completion of this lab, the student will be able to:
 1 EXPERIMENT A8: CALORIMETRY Learning Outcomes Upon completion of this lab, the student will be able to: 1) Measure the heat of a reaction under constant pressure conditions. 2) Calculate the enthalpy
1 EXPERIMENT A8: CALORIMETRY Learning Outcomes Upon completion of this lab, the student will be able to: 1) Measure the heat of a reaction under constant pressure conditions. 2) Calculate the enthalpy
1. KINETICS. Kinetics answers
 1. KINETICS 1.1. Rate determining step 1.2. Calculating reaction rate 1.3. Measuring reaction rate in the lab 1.4. Determining the rate equation 1.5. Arrhenius and rate Kinetics answers 1.1. Rate determining
1. KINETICS 1.1. Rate determining step 1.2. Calculating reaction rate 1.3. Measuring reaction rate in the lab 1.4. Determining the rate equation 1.5. Arrhenius and rate Kinetics answers 1.1. Rate determining
Acid-Base Titration. M M V a
 Acid-Base Titration Pre-Lab Discussion In the chemistry laboratory, it is sometimes necessary to experimentally determine the concentration of an acid solution or a base solution. A procedure for making
Acid-Base Titration Pre-Lab Discussion In the chemistry laboratory, it is sometimes necessary to experimentally determine the concentration of an acid solution or a base solution. A procedure for making
Name: Hour: Photosynthesis in Leaf Disks
 Name: Hour: Photosynthesis in Leaf Disks Safety Information: While the solutions may be handled without gloves and may be disposed of in the sink drains, goggles must be worn during the experiment. Background
Name: Hour: Photosynthesis in Leaf Disks Safety Information: While the solutions may be handled without gloves and may be disposed of in the sink drains, goggles must be worn during the experiment. Background
The Great Gas Plot. Using Balloons and Graphs to Analyze Relationships
 Chemistry The Great Gas Plot Using Balloons and Graphs to Analyze Relationships MATERIALS AND RESOURCES EACH GROUP baking soda balance 8 balloons 8 clamps, balloon funnel, powder graduated cylinder, 1000
Chemistry The Great Gas Plot Using Balloons and Graphs to Analyze Relationships MATERIALS AND RESOURCES EACH GROUP baking soda balance 8 balloons 8 clamps, balloon funnel, powder graduated cylinder, 1000
Chem Lab Pretest ( a combo of last year s exam, Vanier s lab manual and a few new questions.)
 Chem Lab Pretest ( a combo of last year s exam, Vanier s lab manual and a few new questions.) Graduated beakers are used to measure volumes roughly; they are containers with a precision of ±5%. Graduated
Chem Lab Pretest ( a combo of last year s exam, Vanier s lab manual and a few new questions.) Graduated beakers are used to measure volumes roughly; they are containers with a precision of ±5%. Graduated
COEFFICIENTS. - Experimentally, we can usually determine the reactants and products of a reaction
 81 COEFFICIENTS - Experimentally, we can usually determine the reactants and products of a reaction - We can determine the proper ratios of reactants and products WITHOUT further experiments, using a process
81 COEFFICIENTS - Experimentally, we can usually determine the reactants and products of a reaction - We can determine the proper ratios of reactants and products WITHOUT further experiments, using a process
Chemical Reactions Lab PSI Chemistry
 Chemical Reactions Lab PSI Chemistry Name Purpose: Observe the different types of chemical reactions. Materials: - 6 test tubes - test tube rack - 0.3 M copper(ii)sulfate solution - 0.3 M sodium hydroxide
Chemical Reactions Lab PSI Chemistry Name Purpose: Observe the different types of chemical reactions. Materials: - 6 test tubes - test tube rack - 0.3 M copper(ii)sulfate solution - 0.3 M sodium hydroxide
Chapter 3 Test Bank. d. The decomposition of magnesium oxide produces 2.4 g of magnesium metal and 3.2 g of oxygen gas.
 1. Which of the following correctly provides evidence for the unit formula of magnesium oxide? a. The decomposition of magnesium oxide produces 1.2 g of magnesium metal and 1.6 g of oxygen gas. b. The
1. Which of the following correctly provides evidence for the unit formula of magnesium oxide? a. The decomposition of magnesium oxide produces 1.2 g of magnesium metal and 1.6 g of oxygen gas. b. The
Stoichiometry Ch. 11. I. Stoichiometric Calculations
 Stoichiometry Ch. 11 I. Stoichiometric Calculations Background on things you NEED to know how to do: 1. Name/write correct chemical formula 2. Write chemical equations 3. Balance chemical equations 4.
Stoichiometry Ch. 11 I. Stoichiometric Calculations Background on things you NEED to know how to do: 1. Name/write correct chemical formula 2. Write chemical equations 3. Balance chemical equations 4.
Percent yield Combustion analysis. General Chemistry I Dr. Stone Chapter 3 clicker 5
 Percent yield Combustion analysis General Chemistry I Dr. Stone Chapter 3 clicker 5 % Yield = Actual x 100% Theoretical Actual= what is made Theoretical = the amount that could be made from the limiting
Percent yield Combustion analysis General Chemistry I Dr. Stone Chapter 3 clicker 5 % Yield = Actual x 100% Theoretical Actual= what is made Theoretical = the amount that could be made from the limiting
Heat of Combustion: Magnesium. This equation can be obtained by combining equations (1), (2), and (3): (1) MgO(s) + 2 HCl(aq) MgCl 2 (aq) + H 2 O(l)
 Heat of Combustion: Magnesium Computer 19 In Experiment 18, you learned about the additivity of reaction heats as you confirmed Hess s Law. In this experiment, you will use this principle as you determine
Heat of Combustion: Magnesium Computer 19 In Experiment 18, you learned about the additivity of reaction heats as you confirmed Hess s Law. In this experiment, you will use this principle as you determine
