Invited Lecture. "Different Aspects of Electron Microscopy. Sardar Vallabhbhai National Institute of Technology, Surat. Deepak Rajput & S.K.
|
|
|
- Maude Bryan
- 5 years ago
- Views:
Transcription
1 Invited Lecture on "Different Aspects of Electron Microscopy at Sardar Vallabhbhai National Institute of Technology, Surat Deepak Rajput & S.K. Tiwary R&D and Product Development Essar Steel Limited Abstract The resolving power of any microscope depends upon the wavelength of the source of illumination, and smaller the wavelength higher is its resolving power. Light waves have wavelengths in between 4000 and 7000 Å that limit the resolution of conventional light microscopes to 0.2 and magnification to the order of 1000 times. The limitation of light microscope in terms of resolution and magnification leads to the need for better source of illumination than monochromatic beam of light. It is well known a fact that an electron has a wave like nature and its wavelength is given by de Broglie s equation ( = h/p; where h is plank s constant and p is momentum). A beam of highly accelerated electrons can be used as a source of illumination in microscopes to achieve very high level of resolutions and magnifications, and such microscopes are called as Electron Microscopes. Electrons are generated as a result of thermionic emission by applying high voltage to a thin hairpin filament (usually tungsten or lanthanum hexaboride). The generated beam of electrons is unstable in nature and thus requires a high level of vacuum for its stability. The attainment of vacuum for successful journey of electron beam from electron gun to specimen surface is done with the help of various kinds of pumps and is discussed in this paper. When a beam of high-energy electrons interacts with the specimen under study, various kinds of signals are generated as a result of elastic and inelastic scattering with the electrons of the specimen. The kinds of signals generated and the information they provide are discussed at length in this paper. Different features of electron microscopy are discussed in this paper to provide better understanding of the subject as a whole, but an emphasis is made more on Scanning Electron Microscopy. Typical applications of electron microscopy are also discussed in brief in this paper. 1
2 Introduction Microscopy is a technique that is utilized to produce images of details or structures too small to be seen by a normal human eye, using a microscope or any tool for magnification. The simplest microscope known to us is a light microscope that utilizes a beam of light for illuminating the sample under study. It works on the principle of refraction of light when it passes through a lens. The refracted light forms an image that is further magnified using another lens thus resulting in the formation of an enlarged image. The resolution and magnification of any microscope depend upon the wavelength of the source of illumination i.e. light in case of optical microscope and electrons in case of electron microscope. It is a well-known fact that shorter the wavelength of the illuminating source higher is the resolving power of the microscope. In case of optical microscope, a beam of light is used for illuminating the specimen under study and thus the resolution of the microscope depends upon the wavelength of the light used, which is between 4000 and 7000 Å. This high wavelength limits the resolution of light microscopes to around only 0.2. The resolving power of any microscope is defined as its ability to distinguish between two closely located points and the resolution is the distance between those two points. The resolution of an optical microscope is affected by the phenomenon known as Diffraction that limits its maximum resolution to a maximum of 0.2. The most important feature of a light microscope is its ease to use and the samples can be analyzed in air or water, and the images formed are in their natural color. But images obtained with the optical microscope are almost planar and examination of samples with rough or fractured surfaces will be almost impossible since the image will be in focus in some regions and out of focus in other regions. Rapidly moving electrons have a wave like character and can be used as the source of illumination in a microscope. The wavelength of moving electrons can be easily found by using de Broglie s equation ( = h/p; where h is plank s constant and p is momentum) and it depends upon the potential difference applied that makes the electrons move. Electron microscopes use high-energy electrons with energies of thousands of electron volts, which is far greater than that of visible light. Resolution as low as 2 nanometers has been achieved in case of Scanning Electron Microscopes (with magnifications of the order of 3,00,000 times) and 0.05 nanometers in case of modern Transmission Electron Microscopes (with magnifications of the order of 10,000,00 times). Magnifications in case of electron microscopes are limited to around 10,00,000 times, primarily because of spherical and chromatic aberrations. Few highly sophisticated modern electron microscopes have even measured lengths in achieving magnifications as high as 20 million times. An electron microscope is not only used to study details at higher magnifications but also to get various other information like elemental composition, crystallographic structure, etc. when coupled with different types of detectors. An attempt is made in 2
3 this paper to give a panoramic view of the existing features of electron microscopes and their utilities in gathering information for thorough investigation. A brief history of electron microscope The German physicist Ernst Ruska designed the first electron microscope and he was awarded the Nobel Prize in 1986 for its invention. He was aware that electrons possess a wave like character and so he planned to treat them in a fashion similar to light waves. Ruska was also aware of the fact that magnetic fields affect the motion of electrons and the same could be utilized to focus electrons as optical lenses do to light. After he researched a lot on these facts, he devised an electron microscope. Ruska was very much aware of the fact that magnification increases with the wavelength of the source of illumination and electrons have wavelengths shorter than that of light and thus using electrons could be used in place of light to get higher magnifications. In 1932 Ruska and his master, German physicist Max Knoll, constructed the first electron microscope that was capable of achieving magnifications to the order of 400 times. But the instrument was still not fit for practical purposes. Eli Franklin Burton and students at the University of Toronto, Canada built the first practical electron microscope in All the present day electron microscopes still work on the Ruska s prototype and his finding that magnification depends upon the wavelength of the source of illumination. The electron microscope is an integral part of many laboratories viz. metals, polymers, pharmaceutical, medical biopsy, forensics, etc. Principle When a beam of high-energy electrons falls on the specimen, there is interplay between the electrons of the beam and the electrons of the specimen, and during this interplay various kinds of scattering events take place. These scattering events are actually elastic and inelastic collisions between the beam electrons and the specimen electrons, which give out numerous signals. The elastic scattering conserves both momentum and energy and includes such scattering events as electron backscattering and diffraction. On the other hand, the inelastic scattering involves a transfer of energy between the incident electrons and the material with which it interacts, and results in the generation of secondary electrons and X-rays. The signals thus generated are detected by special detectors, attached at particular locations to the microscope, and translated to readable form. The signals produced include: Secondary electrons, Auger electrons, Transmitted electrons, Characteristic X-rays, Continuum X-rays 3
4 or Bremsstrahlung, Backscattered electrons and Cathodoluminescence. All these signals have different characteristics and are used to learn different features of the specimen under study, and there are different types of detectors that distinguish between these signals and acquire only specific signals e.g. Secondary electron detector, Backscattered electron detector, Energy Dispersive Spectrometer, etc. Types of electron microscopes Electron microscopes are divided into two major categories: Scanning Electron Microscope (SEM) Transmission Electron Microscope (TEM) Although both the types of microscopes share the common principle of using electron beam as a source of illumination, they differ in the type of signal used for the formation of image. A scanning electron microscope uses electrons reflected from the surface of specimen under study for the formation of image where as a transmission electron microscope uses electrons transmitted through the sample under study for the formation of image. The magnification achieved in case of scanning electron microscope is limited to around 3,00,000 times where as magnification as high as 20,00,000 times has been achieved in modern transmission electron microscopes. Maximum magnification that can be acquired depends upon many factors such as accelerating voltage, geometrical construction of the instrument, integral components of the microscope, etc. Other electron microscopes: Scanning Transmission Electron Microscope (STEM) Energy-filtered Transmission Electron Microscope (EFTEM) Reflection Electron Microscope (REM) Scanning Tunneling Microscope (STM) Scanning Transmission Electron Microscope is actually a specific kind of Transmission Electron Microscope where electrons transmit through the sample but are scanned across the specimen in a raster fashion as in a Scanning electron microscope. The principle of Reflection Electron Microscope (REM) is a combination of principles of light microscope and electron microscope. Though it uses electrons as the source of illumination, but unlike SEM and TEM where secondary/backscattered or transmitted electrons are used to form image, it uses reflected beam for the formation of image. REM is normally united with Reflection High Energy Electron Diffraction (RHEED) and Reflection High- Energy Loss Spectrum (REELS). Scanning Tunneling Microscope actually comes under the category 4
5 of Scanning Probe Microscope, which in itself is another type of microscopy technique, but it may be considered a type of electron microscope because ultimately it is electron that is responsible for the formation of image. A detailed description of these microscopes and their usefulness is discussed in this paper but an emphasis is given to Scanning Electron Microscope and Transmission Electron Microscope because of their popularity and prevalent use in numerous areas. Scanning Electron Microscope A scanning electron microscope (refer figure-3) uses a beam of high-energy electrons as a source of illumination to form an image. A very fine electron beam is focused on the surface of the specimen and scanned over it in a series of lines and frames called as raster (zigzag network). As a beam of electrons is highly unstable in normal conditions, it becomes very imperative to have a vacuum chamber to protect the electron beam from loosing its strength during its journey from electron gun towards the specimen. Hence, attainment of vacuum becomes a must before starting the generation of electrons and there must be a strong vacuum system to achieve that low level (around 10-8 torrs) of vacuum. Thus, vacuum system is the most vital division of a scanning electron microscope. The beauty of the instrument lies in the quality of the image that it forms. It is capable of forming three-dimensional images because of its large depth of focus that enables the investigators to understand the morphology of particle under study. Image-1 Image-2 The above images are of a radiolarian taken at same magnification under an optical microscope (Image-1) and a scanning electron microscope (Image-2). Image-2, taken on a scanning electron microscope, shows better visibility and sharpness of the image because of large depth of focus whereas Image-1, taken on an optical microscope, shows blurred image because of very small depth of focus. The three-dimensional appearance of an image depends on the depth of focus, and larger the depth of focus better is the image quality. 5
6 Vacuum system: The electron column (comprising the electron gun and the electromagnetic lenses) when united with the specimen chamber forms the body of the scanning electron microscope. To attain desired vacuum level in the body of the SEM, two pumps are employed in series: mechanical pump and diffusion pump or turbomolecular pump. A mechanical pump, usually a rotary pump (shown in figure-1), works under normal atmospheric pressure condition and lowers down the pressure of the column to an extent of 10-4 torrs, also it the working range of a diffusion pump. A diffusion pump (shown in figure-2) further takes the pressure down from 10-4 torrs to 10-8 torrs, which is the operational range of the scanning electron microscope. Figure-1 Figure-2 Mechanical Pumps: The mechanical pumps are operational in the range of ambient pressure to a maximum of 10-4 torrs. On the other hand, the diffusion pumps are operational only in the presence of the vacuum already created by the mechanical pumps as already mentioned, and has an operational range of to-10-4 torrs. The most common diffusion pump is the oil diffusion pump and the most common mechanical pump is the rotary vane pump. The rotary vane moves the chamber gas through the pump using a rotating assembly in the pumping chamber. Typically there are two or more rotating vanes that move the gas or fluid from inlet to outlet, refer figure-1. Rotary vane vacuum pumps are positive displacement pumps. Diffusion pumps: An oil diffusion pump is actually a stainless steel chamber containing a number of cone shaped jet assemblies. Typically there are 3 jet assemblies (refer figure-2) of diminishing sizes, with the largest at the bottom. There is a pool of specialized oil with low vapor pressure. The oil is heated to its boiling and the oil vapors go up the pump column and exit through the jet assemblies. The exit velocity of the vapor is so high (around 750 mph) the gas molecules present in the SEM specimen chamber are drawn into the vapor jet stream of the pump due to Bernoulli s effect. The gas molecules 6
7 are then removed from the base of the diffusion pump through a fore pump and the condensed oil begins a new cycle. Figure-3 Schematic view of a Scanning Electron Microscope 7
8 Generation of electrons and formation of beam: Electrons are generated as a result of thermionic emission when a high potential is applied to the electron gun (refer figure-4). The function of electron gun is not only to produce electrons but also to focus them into a beam. The electrons generated take the shape of a beam by selecting electromagnetic lenses that are present in the column of the microscope. Other lenses further control these electrons as they trek down the column of the microscope until they reach the surface of the specimen. The diameter of the electron beam (probe diameter) and the number of electrons (current) have a direct effect on quantity of signals that emerge out of the sample. Eventually, it s desirable to obtain a very stable, fine electron beam but containing a large current. The most commonly used type of electron gun is the conventional triode electron gun. This is made up of three components which are kept under vacuum in the gun chamber: 1) The filament or cathode that is held at a negative voltage relative to earth potential, 2) The Wehnelt or grid that is held at a few hundred volts relative to the cathode, 3) The anode that is positioned at the base of the gun chamber and held at earth potential. A very thin piece of wire bent in the shape of a hairpin is heated to around 2700 K by passing a current through it (the filament current) at which point electrons are thermionically emitted. A high negative voltage is applied to the filament and to the Wehnelt grid that is held at a slightly greater negative voltage to bias the gun. The strength of this voltage affects both the form of electrostatic field between the grid and the filament and also the number of electrons emitted from the filament for a given filament current applied. The form of the electrostatic field focuses the electrons to a crossover of diameter between the Wehnelt grid and the anode. The anode is held at ground potential which causes the electrons from the high negative voltage of the filament to be accelerated towards the anode. The potential or voltage difference between the filament and anode governs the velocity, energy and wavelength of the electrons and is called the accelerating voltage. A typical electron gun is shown below: Figure-4 8
9 Rastering: The raster/zigzag movement of the electron beam is achieved by means of controlling current in the scan coils. Scan coils are small coils or wire that carry variable current to regulate the movement of electron beam. Types of electron gun Tungsten hairpin electron gun: A tungsten filament is made from a very thin piece of wire bent in the shape of a hairpin. It is then heated to around 2700 K by passing a current through it (the filament current) at which point electrons are thermionically emitted. In order for electrons in the filament to escape from the material from which it is made, they require sufficient energy to overcome the workfunction energy E w of the material. The heat supplied by the filament heating current provides this energy. The crossover diameter for a tungsten filament is typically mm and, therefore, in order to achieve a probe diameter at the sample of a few nanometers, significant demagnification is required by the lenses. However the effect of this demagnification by the lenses is to reduce the number of electrons in the final probe. This is a particular problem when high-resolution images are required which require small probe diameters. However, the main advantage of a tungsten filament gun is the excellent current stability that is essential for accurate X-ray microanalysis. However its disadvantages are its limited lifetime and brightness. Lanthanum hexaboride (LaB 6 ) electron guns: The emitting component of the gun is made from a small piece of single-crystal LaB 6. One of the advantages of LaB 6 over tungsten is that it has a lower work function and therefore begins to emit electrons at a lower temperature. Since the lifetime of the filament is related to the temperature to which it is raised it has a longer lifetime than the tungsten filament. However, in order to prevent any contamination building up on the surface of the crystal, which will reduce emission, the gun area needs to be kept under a very good vacuum better than needed for a tungsten filament. Field emission electron guns: There are two types of field emission gun (FEG), cold field emission and thermally assisted field emission guns, both of which are very high brightness sources. The basic operation of the FEG is the extraction of electrons from a very fine single crystal tungsten tip, using the very high local electric field generated between the tip and the extraction anode. This extraction 9
10 anode is held at a voltage V 1 and it essentially regulates the emission current. The higher this extraction voltage, the greater the emission current. An additional anode is used to accelerate the electrons to the required accelerating voltage and is held at a voltage known as V 0. The tungsten tip is generally mounted onto a hairpin filament. The high electric field, concentrated at the tip of the source effectively reduces the potential barrier of the electrons in the material such that they are able to tunnel through this barrier and exit the material without the need to thermally activate them. These two anodes provide the electrostatic focusing similar to that found in the conventional thermionic gun arrangement. The electrons are focused to a point or crossover that is then subsequently demagnified by the lenses in the column onto the surface of the sample. Scan coils and Rastering: The deflection coils or scan coils in the SEM are situated above the objective lens in the column and are used to raster the electron beam over a defined area of the sample. In this way, it is possible to generate information from more than one location of the beam and hence forms the basis of imaging and mapping in the SEM. Other deflection coils that are commonly found in the SEM are the gun shift and tilt coils and the image shifting coils, which are used to align the gun and move the image respectively. The scanning is achieved by applying current to each of the horizontal and vertical scan coils, so that the beam is deflected to cover a two dimensional area on the sample. This scanning can either be digital or analogue. As a result of the interaction of the beam with the sample, a variety of signals are produced, which may be collected by appropriate detectors to produce information about the microstructure, topography and composition of the sample. Image formation: A common signal used for imaging in the SEM is the secondary electron signal. The raster of the electron beam on the sample is synchronous with that of the electron beam in the cathode ray tube (CRT) of the monitor. The signals that are emitted are detected and amplified and used to modulate the brightness of the electron beam in the CRT. In this way, variations in the signal emitted over the scanned area are displayed as variations in brightness on the CRT, and hence, the appropriate information can be displayed in image form. Since the area scanned on the CRT is much bigger than the area scanned on the sample, magnification is achieved. 10
11 Operation of the scanning coils: The scanning action of the beam is achieved by changing the amount of current through the scan coils so that the beam is moved either continuously (analogue) or discretely (digital) over the sample (refer figure-5). Current applied to the X scan coils moves the electron beam in the X direction, and the beam moves in the Y direction when current is applied to the Y scan coils. Analogue scanning means that the electron beam is continuously scanned over the sample, whereas digital scanning involves driving the beam to a particular location where it dwells before being driven to the next point. Since the Y direction always appears vertical on the viewing CRT, this is often referred to as the vertical and similarly, X direction as the horizontal. The dotted line on the surface of the sample, as seen in the figure below, is the retrace signal that does not appear on the CRT. Figure-5 Magnification: The magnification of an electron image is defined as the ratio of the width of the scan of the electron beam on the monitor to the width of the area scanned on the sample. The effect of changing this ratio is illustrated in the adjacent interactive diagram. An increase in magnification is achieved by reducing the width of the area scanned on the sample, since the width of the CRT is fixed. In this way magnifications of up to 3,00,000 can be achieved, provided all the conditions necessary for the best spatial resolution have been optimized. Above these magnifications, no greater detail is observed and greater magnification is often referred to as empty magnification. Theoretically, the spatial resolution of the SEM in secondary electron image mode is, finally, determined by the size of the scanning spot. This is influenced by electron optical parameters such as working distance, the electromagnetic lens settings and the electron gun brightness and also by electronic noise in the X and Y deflection currents. In practice there are a number of other factors that will influence the spatial resolution actually attainable from the scanning electron microscope in imaging mode. These include accelerating voltage, the state of the sample surface, detector position and set up and external 11
12 mechanical vibrations. Spatial resolutions of a nanometer may be obtained with all these conditions optimized. Working Distance: The working distance is defined as the distance between the lower pole piece of the objective lens and the plane at which electrons are focused. The strength of the objective lens essentially changes this distance. If you change the objective lens strength or the focus, the working distance as displayed in millimeters on the microscope monitor will change. In order to obtain sharp images, the sample height should be adjusted using the z drive of the stage until the image becomes in focus at the desired working distance. The probe can, therefore, be focused at different working distances to cope with different sample heights and different operating requirements. However, for X- ray microanalysis, there is a recommended working distance for optimum X-ray acquisition that is specific to the geometry of the detector mount on the SEM chamber. Electromagnetic lenses: In optical microscopes, the ability to focus light is achieved by using glass lenses. Electron microscopes use electrons as the source of illumination and the ability to focus electrons in the microscope is achieved using electron lenses. These may be either electrostatic or electromagnetic and different lenses may be found in different parts of the microscope. For example, the electrostatic lens at the top of the column in the gun area is responsible for focusing the electrons that are produced by the source down to a diameter referred to as the virtual crossover. The electromagnetic lenses found in the column are then used to demagnify this crossover in order to produce a much smaller diameter probe that is incident on the sample. The strength of an electromagnetic lens can be varied by adjusting the amount of current that passes through the windings around the central iron core of the magnet. There are two main electromagnetic lenses found in an SEM: the condenser and the objective. The condenser controls the number of electrons in the beam (the current) for a given objective aperture size, and the objective lens is responsible for focusing the electrons on to the sample. The construction of a magnetic lens: Figure-6 shows the basic construction of a conventional electromagnetic lens in cross section. The current that flows through the windings energizes the magnet. It is the magnitude of this current that determines the strength of the magnetic field in the lens. Electrons traveling down the column are affected by the field, and under certain conditions, their paths form helices. The focusing action of these probe-forming lenses is to converge the electrons by tightening the pitch of their helical paths. 12
13 Figure-6 Condenser lens: The main role of the condenser lens (refer figure-7) is to control the diameter of the electron beam and, for a given objective aperture size, it determines the number of electrons in the beam which are incident on the sample. Up to three condenser lenses are often found in SEMs. The control that adjusts the strength of the condenser lens differs from one microscope manufacturer to another and may be termed spot size, resolution or C1 strength. However the result of adjusting any one of these parameters is to change the magnitude of the current that flows through this lens. The greater the current, the greater is the lens strength. For an increase in lens strength, the beam diameter (at the crossover point of the electrons between the two set of lenses) will decrease. In this case the convergence angle to this crossover point will be greater and therefore, for a given objective aperture size, fewer electrons will actually reach the sample. This will result in a smaller beam current actually hitting the sample. Figure-7 13
14 Objective lens: The working distance is defined as the distance between the lower pole piece of the objective lens and the plane at which electrons are focused. The strength of the objective lens essentially changes this distance. If you change the objective lens strength or the focus, the working distance as displayed in millimeters on the microscope monitor will change. In order to obtain sharp images, the sample height should be adjusted using the z drive of the stage until the image becomes in focus at the desired working distance. The probe can, therefore, be focused at different working distances to cope with different sample heights and different operating requirements. There is generally an aperture located in the same plane as the objective lens. The diameter of this aperture may be varied. The objective aperture therefore controls the number of electrons that reach the sample. It also controls the final convergence angle of the electron beam onto the sample that hence determines the depth of field. Greater depths of field can be achieved with smaller convergence angles. A weak objective lens strength will give rise to a long working distance, whereas strong lens strength will give rise to a short working distance. Long working distances mean larger probe diameters on the sample, hence worsening the spatial resolution. However, such conditions will give a better depth of field. Short working distances should be used to obtain maximum spatial resolution. The diagram above allows you to interactively change the strength of the objective lens that essentially changes the point at which the electrons come to focus. In this diagram the surface of the sample is tied with the point at which the electrons are focused. If there is an EDX detector attached to the side of the SEM chamber, there exists a recommended working distance for X-ray microanalysis, depending on the angle that the detector makes with the horizontal. Each arrangement of detector on a particular SEM has a corresponding optimum working distance. Electron beam Specimen interaction: When the electrons of the beam interact with the specimen then it results in scattering events (elastic and inelastic scatterings). Elastic scattering (elastic collision) is an event where energy as well as momentum are conserved of the interacting electrons. Such events result in the emission of backscattered electrons and diffraction. On the other hand, inelastic scattering involves in transfer of energy between the incident electron beam and specimen and also change in momentum. Such scattering events result in generation of secondary signals such as x-rays and secondary electron emission. The interaction also results in the emission of Auger electrons, Cathodoluminescence and Bremsstrahlung. Interaction volume: The interaction of the electron beam with the sample is complex in that a whole host of interaction and scattering events are possible. Such interactions can be divided into two classes: inelastic, in which electrons are deflected through an angle of typically less than a degree, and elastic in which there is little or no energy transferred to the material. The Monte Carlo method is a 14
15 mathematical technique that attempts to model the shape of the interaction volume, by simulating a large number of electron trajectories through the solid. The overall shape of the interaction volume ultimately determines the shape of the individual production volumes for a variety of signals, such as characteristic X-ray production, Auger emission and secondary electron emission and, therefore, the spatial resolution attainable, using these signals. The shape and depth of the interaction volume that contains the distribution of trajectories is dependent on the beam energy, tilt, and atomic number of the material. The dominant scattering event (elastic or inelastic) will depend on the atomic number of the material and the energy of the incident electrons. As described above, elastic scattering will alter the direction of an electron trajectory, whereas inelastic scattering will result in loss of energy. If the dominant event is elastic, electrons will tend to scatter away from the incident beam direction, giving the interaction volume 'width'. On the other hand, if the dominant event is inelastic, electrons will be less deviated and penetrate into the sample along their original trajectories, but losing energy as they travel. Figure-8 shows the interaction volume in bulk silicon at 20kV for an untilted sample. Figure-8 Monte Carlo Simulation: On entering a sample, energetic incident electrons undergo a number of complex interactions, both inelastic and elastic, with the atoms of the sample. The resulting maze of electron trajectories can be modeled, using a form of Monte Carlo simulation. This type of simulation considers each electron in turn, and maps out its progress through the sample. A random number generator is used to give a realistic view of how physics and probability alter the trajectory at each point along its path. A number of parameters can be extracted from such a simulation including the backscatter coefficient (h), which is determined by counting the number of incident electrons that exit from the sample surface. A Monte Carlo simulation can also be used to determine the depth distribution of X-ray production within the sample. This essential parameter is required to calculate the various matrix effects, such as absorption and fluorescence, which occur as X-rays travel through the sample. Accurate determination of these matrix effects is required for sample composition to be determined from measured X-ray intensities. There are a number of Monte Carlo programs available to 15
16 simulate electron trajectories in a sample, each using a slightly different physical model. Regardless of the model used, the overall 'shape' of the interaction volume generated by the different simulations is very similar. A large number of trajectories ideally should be plotted in order to obtain a realistic shape of the interaction volume. X-rays production volume: The volume of material from which X-rays are produced is known as the X-ray production volume or X-ray generation volume, the size and dimensions of which depends on the X-ray line being excited. For example, in the case of lead, the sample volume producing the higher energy L series X-rays will be smaller and closer to the surface, than the volume where M series X-ray lines are generated. Types of Signals Figure-9 Secondary electrons: One of the most important and common signals used in the scanning electron microscope is the secondary electron signal. When an incident beam of electrons penetrates into the material, a number of scattering events, both inelastic and elastic can occur. One such event results in the emission of electrons whose energy distribution is typically less than 50eV, but strongly peaked around 10eV. These electrons are collectively known as secondary electrons. They are outer shell electrons that have been produced as a result of the inelastic scattering between the incident electrons and the sample. Since secondary electrons are such low energy, they are only able to escape from the near surface of the sample, where the spread of the incident beam is minimal. This allows images with high spatial resolution of the order of the incident beam diameter to be acquired. Changes in signal level due to secondary electron emission can arise from changes in both topography and composition of the material making it a particularly powerful imaging mode as a starting point for microanalysis. 16
17 Backscattered electrons: Another commonly used imaging signal in the scanning electron microscope is the backscattered electron signal. One of the many scattering events that occurs in the sample results in the backscattering of the incident beam electrons out of the sample. These electrons have either undergone large angle elastic scattering events in the material, enabling them to approach the surface with sufficient energy to escape or they have undergone a number of smaller angle scattering events after which they can eventually exit the sample. The backscattered electrons emerge with a range of energies, depending on the number and type of scattering events that they have undergone, prior to leaving the sample. The energy distribution varies smoothly extending from the primary energy, down to essentially zero. The backscattered fraction that escape is denoted by the backscatter coefficient, which is itself a function of the atomic number of the material. This dependence of the backscatter yield with atomic number forms the basis for differentiating between different phases, thus providing an ideal starting point for further microanalysis. The backscattered signal is also sensitive to local topography, crystallography, as well as the magnetic field structure of the material. An understanding of the elastic scattering which goes on inside the material is important, since it removes energy from the electron beam, which would otherwise have been used to produce X- rays or secondary electrons. Auger electrons: The mode by which energy is released when a transition takes place is governed by the Auger (a) and the fluorescence yield ( ). The fluorescence yield defines the probability of an X-ray being emitted in preference to an Auger electron: a + = 1 For any given shell, the probability that an Auger electron is emitted is greater than X-ray emission for light elements. If an inner K shell electron is ejected and an electron from the L shell fills this vacancy, releases energy, and ejects an Auger electron from the L shell, the Auger transition is termed a KLL transition. Measurement of the energy of the characteristic Auger electrons forms the basis of Auger spectroscopy. The energies of the Auger electron peaks allow all elements, with the exception of hydrogen and helium, to be identified since a minimum of three electrons are required for the emission process. Cathodoluminescence: Cathodoluminescence (CL) is a term that describes the process of the emission of electromagnetic radiation in the visible, ultraviolet and infrared regions of the spectrum when certain materials are bombarded with energetic electrons. These light emitting materials, which are generally insulators or semiconductors, have filled valence bands and empty conduction bands with band gaps specific to the material itself. When an incident electron inelastically scatters off the atom, 17
18 electrons in the filled valence band can be promoted to the conduction band, leaving a hole in the valence band. The band gap energies are, typically, between 2 and 5eV. Electron and hole pairs will recombine and release excess energy in the form of light or CL. The CL efficiency is determined by competition between radiative and non-radiative recombination events and, therefore, any imperfection in the crystal structure, whether chemical or structural, may alter the CL characteristics. The sensitivity to very small changes in chemistry also make it an ideal imaging technique for observing geological minerals, where, for example, small changes in rare earth composition lead to a difference in the cathodoluminescence. Scanning CL thus provides a technique for recording high spatial resolution images at different wavelengths and spectra, and is a versatile technique for structural and optical characterization particularly of semiconductors, in both thin epitaxial film and bulk form. Oxford Instruments Research Instruments Division supply detectors for CL imaging across a broad range of wavelengths and full spectroscopy systems, which allow spectra and monochromatic CL maps to be acquired for, for example, semiconductor applications. Characteristic X-rays: The electrons in an atom are arranged in particular energy levels or shells in the Bohr model. In X-ray microanalysis we refer to the shell or level closest to the nucleus as the K shell. Electrons fill this level first. The next closest level is the L shell and then the M and then N shell etc. Since the K shell is closest to the nucleus it requires the most energy to remove an electron from this shell. Therefore if a spectrum from an element contains a K, L and M line, the K will be the highest in energy i.e. furthest towards the right of the spectrum if the scale is defined in units of energy. When a high-energy electron interacts with an atom, it may result in the ejection of an electron from an inner atomic shell. This will leave the atom in an ionized or excited state, with a vacancy in this shell. The minimum energy required to remove an electron from a particular energy level is known as the critical ionization energy E c or X-ray absorption edge energy and therefore, for that transition to occur, the incident electron energy must be greater or equal to the critical energy. The critical energy has a specific value for any given energy level and is, typically, referred to as the K, L or M absorption edge. De-excitation can occur by an electron from an outer shell filling the vacancy. The change in energy or characteristic energy is determined by the electronic structure of the atom that is unique to the element. This characteristic energy can be released from the atom in two ways. One is the emission of an X-ray photon with a characteristic energy specific to that transition, and hence, to the element. The second way is by releasing so called Auger electrons. The detectors that read these characteristic x-rays are Energy Dispersive Spectrometer and Wavelength Dispersive Spectrometer and attached to the microscope for doing chemical analyses along with the image analyses. 18
19 Continuum X-ray emission or Bremsstrahlung: Continuum X-rays or bremsstrahlung (literally translated as braking radiation) are generated when electrons interact with matter. The emission of these X-ray photons is a result of the incident electrons decelerating in the field associated with the atom. This results in counts in the X-ray spectrum in channels between zero energy up to an energy that corresponds to the value of the incident electron beam energy. The most energetic X-ray seen in a spectrum, corresponds to an incident electron having lost its entire energy, as a result of the interaction with the field of the atom, and has an energy whose value is known as the Duane-Hunt limit. If the sample is not charging, the Duane-Hunt limit has the same value as the incident beam energy. Environment Scanning Electron Microscope: The ESEM was developed in the mid eighties. Its primary advantages lie in permitting the microscopist to vary the sample environment through a range of pressures, temperatures and gas compositions. The Environmental SEM retains all of the performance advantages of a conventional SEM, but removes the high vacuum constraint on the sample environment. Wet, oily, dirty, non-conductive samples may be examined in their natural state without modification or preparation. The ESEM offers high-resolution secondary electron imaging in a gaseous environment of practically any composition, at pressures as high as 50 torrs, and temperatures as high as 1500 C. Utilities of Environment Scanning Electron Microscope: Gas ionization in the sample chamber eliminates the charging artifacts, typically seen with nonconductive samples. So the specimens do not need to be coated with a conductive film. ESEM gets rid of the preparation process. The ESEM can image wet, dirty and oily samples. The contaminants do not damage or degrade the image quality. ESEM can acquire electron images from samples as hot as 1500ºC, because the Environmental Secondary Detector (ESD) is insensitive to heat. The detector is also insensitive to light. Light from the sample, for example incandescence from heated samples, cathodoluminescence and fluorescence do not interfere with imaging. ESEM eliminates the need for conductive coating, so delicate structure, which was often damaged during the sample preparation, can be imaged. ESEM can acquire x-ray data from insulating samples at high accelerating voltage. Eliminating the need for sample preparation, particularly the need for conductive coating, makes it possible to investigate specimen in dynamic processes, such as tension, compression, 19
20 deformation, crack propagation, adhesion, heating, cooling, freezing, melting, hydration, dehydration and sublimation. Field Emission Environment Scanning Electron Microscope: As the name suggests, this electron microscope is a type of Environment Scanning Electron Microscope which has Field Emission Gun for generation of electron beam for analysis. The ESEM-FEG retains the capabilities for conventional secondary and backscattered electron detection, and the field-emission source permits very high resolution imaging of coated or naturally conductive samples under normal highvacuum and high-voltage conditions. Various detectors like EDS, WDS, etc., can be attached to this microscope for elemental analysis at any spot in the specimen under study. Transmission Electron Microscope Transmission Electron Microscopes are akin to Scanning Electron Microscopes in principle except that the electrons transmitted through the specimen are used for the formation of image unlike SEMs where secondary or backscattered electrons are used for the formation of image. A brief history: The first practical transmission electron microscope was built by Albert Prebus and James Hillier at the University of Toronto in 1938 using concepts developed earlier by Max Knoll and Ernst Ruska in Working: TEM equipment is expensive, requiring both a high vacuum and a high voltage electron gun to enable the electrons to penetrate the sample. Extensive sample preparation is also required for this technique; samples undergo sectioning, polishing, and ion milling to reach the sub micron thickness necessary for the transmittance of electrons through the sample. Transmission Electron Microscopes are operated at kev to image thin samples. Elastic scattering is used in the formation of true electron-optical images by electrons transmitted through the sample. Diffraction contrast dominates low and medium magnification images. High-resolution images are based on phase contrast. Assessment of crystal defects is the main application of conventional TEM. The sample is illuminated by a broad (nearly) parallel beam and the imaging lenses at the other side of the sample form the image. Magnification has nothing to do with the size of the illuminated area, it is determined by the imaging lenses. The image is formed on an electroluminescent screen (or film or detector) in a fixed position in both modes of operation. In imaging mode, the image plane of the objective lens is projected to the screen. In diffraction mode, the focal plane of the same objective lens (containing a diffraction pattern) is projected to the same screen. 20
21 An additional class of these instruments is the electron cryomicroscope, which includes a specimen stage capable of maintaining the specimen at liquid nitrogen or liquid helium temperatures. This allows imaging specimens prepared in vitreous ice, the preferred preparation technique for imaging individual molecules or macromolecular assemblies. Basic construction of a Transmission Electron Microscope Imaging in TEM: The contrast in a TEM image is not like the contrast in a light microscope image. A crystalline material interacts with the electron beam mostly by diffraction rather than absorption, although the intensity of the transmitted beam is still affected by the volume and density of the material through which it passes. The intensity of the diffraction depends on the orientation of the planes of atoms in a crystal relative to the electron beam at certain angles the electron beam is diffracted strongly, sending electrons away from the axis of the incoming beam; while at other angles the beam is largely transmitted. Modern TEMs are often equipped with specimen holders that allow the user to tilt the specimen to a range of angles in order to obtain specific diffraction conditions, and apertures placed below the specimen allow the user to select electrons diffracted in a particular direction. A high contrast image can therefore be formed by blocking electrons deflected away from the optical axis of the microscope by placing the aperture to allow only unscattered electrons through. This produces a variation in the electron intensity that reveals information on the crystal structure, and can be viewed on a fluorescent screen, or recorded on photographic film or captured electronically. 21
22 This technique (known as Bright Field or Light Field) is particularly sensitive to extended crystal lattice defects in an otherwise ordered crystal. As the local distortion of the crystal around the defect changes the angle of the crystal plane, the intensity of the scattering will vary around the defect. As the image is formed by the distortion of the crystal planes around the defect, the contrast in these images does not normally coincide exactly with the defect, but is slightly to one side. It is also possible to produce an image from electrons deflected by a particular crystal plane. By either moving the aperture to the position of the deflected electrons, or tilting the electron beam so that the deflected electrons pass through the centered aperture, an image can be formed of only deflected electrons, known as a Dark Field image. In the most powerful diffraction contrast TEM instruments, crystal structure can also be investigated by High-resolution transmission electron microscopy (HRTEM), also known as phase contrast imaging as the images are formed due to differences in phase of electron waves scattered through a thin specimen. Resolution of the HRTEM is limited by spherical and chromatic aberration, but a new generation of aberration correctors has been able to overcome spherical aberration. Software correction of spherical aberration has allowed the production of images with sufficient resolution to show carbon atoms in diamond separated by only 0.89 Å units and atoms in silicon at 0.78 Å at magnifications of 50 million times. Improved resolution has also allowed the imaging of lighter atoms that scatter electrons less efficiently -- lithium atoms have been imaged in lithium battery materials. The ability to determine the positions of atoms within materials has made the HRTEM an indispensable tool for nanotechnologies research and development in many fields, including heterogeneous catalysis and the development of semiconductor devices for electronics and photonics. Energy Filtered Transmission Electron Microscope (EFTEM): Energy-filtered transmission electron microscopy (EFTEM) is a technique used in Transmission electron microscopy, in which only electrons of particular kinetic energies are used to form the image or diffraction pattern. The technique can be used to aid chemical analysis of the sample in conjunction with complementary techniques such as electron crystallography. Principle: If a very thin sample is illuminated with a beam of high-energy electrons, then a majority of the electrons will pass unhindered through the sample but some will interact with the sample, being scattered elastically or inelastically (phonon scattering, plasmon scattering or inner shell ionization). Inelastic scattering results in both a loss of energy and a change in momentum, which in the case of inner shell ionization is characteristic of the element in the sample. If the electron beam emerging from 22
23 the sample is passed through a magnetic prism, then the flight path of the electrons will vary depending on their energy. This technique is used to form spectra in Electron energy loss spectroscopy (EELS), but it is also possible to place an adjustable slit to allow only electrons with a certain range of energies through, and reform an image using these electrons on a detector. The energy slit can be adjusted so as to only allow electrons that have not lost energy to pass through to form the image. This prevents inelastic scattering from contributing to the image, and hence produces an enhanced contrast image. Adjusting the slit to only allow electrons that have lost a specific amount of energy can be used to obtain elementally sensitive images. As the ionization signal is often significantly smaller than the background signal, it is normally necessary to obtain more than one image at varying energies to remove the background effect. The simplest method is known as the jump ratio technique, where an image recorded using electrons at the energy of the maximum of the absorption peak caused by particular inner shell ionization is divided by an image recorded just before the ionization energy. It is often necessary to cross correlate the images to compensate for relative drift of the sample between the two images. Improved elemental maps can be obtained by taking a series of images, allowing quantitative analysis and improved accuracy of mapping where more than one element is involved. By taking a series of images, it is also possible to extract the EELS profile from particular features. Scanning Transmission Electron Microscope (STEM): STEM is a type of transmission electron microscope with a similar construction except that the beam focuses into a narrow spot that is scanned over the sample in a raster like a scanning electron microscope. The rastering of the beam across the sample makes these microscopes suitable for analysis techniques such as mapping by energy dispersive X-ray (EDX) spectroscopy, electron energy loss spectroscopy (EELS) and annular darkfield imaging (ADF). Applications of Electron Microscopes Scanning Electron Microscope Metal studies: Metal samples can be examined with the SEM to determine strength in different conditions such as cold and heat. Experts study the metal from the frame of a crashed airplane to determine if there was a flaw in the metal that caused the crash. Studies are also done on the metal before it is used in a plane, car, train, boat or anything that would require a strong metal for reasons of safety. By studying samples from the hull of the Titanic, scientists were able to discover that the metal had shattered because the cold water had caused it to become brittle. 23
24 Forensic studies: Police laboratories need SEM's to examine evidence. A SEM can be used to compare such things as metal fragments, paint, and inks. Hair and fibers can also be examined to prove a person's guilt or innocence. By comparing a sample found at the scene of a crime to a similar sample found belonging to a suspected criminal, detectives can closely determine if the samples are a match. Medical studies: A medical researcher can use a SEM to compare samples of healthy and unhealthy blood and tissue samples to determine what is causing an illness in a patient. They can also study the effect of medicine on a patient by observing the differences between the unhealthy patients and those patients given medicine. Scientific Research: There are many different areas in scientific research where the SEM can be used. Any scientist who needs to look at extremely small samples can use the SEM. For example, Biologists use the SEM to look at plant or animal cells and tissues, Chemists examine microscopic crystals, and Material Scientists view the structure of metals, ceramics, and plastics. These are only a few of the ways a SEM can be used for scientific research. SEM photographs taken at Essar Steel Limited (JEOL JSM 6380) (1) (2) Picture-1- It is a secondary electron image that shows complete cleavage fracture of an AISI 430 Ferritic Stainless Steel fractured by dipping a steel sample in liquid nitrogen at 187 C for 10 minutes. Picture-2- It is an image of iron pellet. It shows the uniform distribution of porosity in the core. 24
25 (3) (4) Picture-3 It is an image of fractured surface of an API grade microalloyed steel, fractured in Drop Weight Tear Test at 20 C. It shows dimple structure which is an indication of ductile fracture. Picture-4 It is a secondary electron image of the core of a roll used in finishing stand of a hot strip mill. The SEM photograph reveals the uniform distribution of graphite in the matrix of iron. Transmission Electron Microscope The TEM is used heavily in both material science/metallurgy and the biological sciences. In both cases the specimens must be very thin and able to withstand the high vacuum present inside the instrument. For biological specimens, the maximum specimen thickness is roughly 1 micrometer. To withstand the instrument vacuum, biological specimens are typically held at liquid nitrogen temperatures after embedding in vitreous ice, or fixated using a negative staining material such as uranyl acetate or by plastic embedding. Typical biological applications include tomographic reconstructions of small cells or thin sections of larger cells and 3-D reconstructions of individual molecules via Single Particle Reconstruction. In material science/metallurgy the specimens tend to be naturally resistant to vacuum, but must be prepared as a thin foil, or etched so some portion of the specimen is thin enough for the beam to penetrate. Preparation techniques to obtain an electron transparent region include ion beam milling and wedge polishing. The focused ion beam (FIB) is a relatively new technique to prepare thin samples for TEM examination from larger specimens. Because the FIB can be used to micro-machine samples very precisely, it is possible to mill very thin membranes from a specific area of a sample, such as a semiconductor or metal. The HRTEM technique (as discussed earlier) allows the direct observation of crystal structure and therefore has an advantage over other methods - e.g. there is no displacement between the location of a defect and the contrast variation caused in the image. However, it is not 25
26 always possible to interpret the lattice images directly in terms of sample structure or composition. This is because the image is sensitive to a number of factors (specimen thickness and orientation, objective lens defocus, spherical and chromatic aberration), and although quantitative interpretation of the contrast shown in lattice images is possible, it is inherently complicated and may require extensive simulation of the images. Computer modeling of these images has added a new layer of understanding to the study of crystalline materials. TEM photomicrographs: (1) (2) Picture-1 shows TEM photograph of a SiC-coated multi-wall carbon nano tube treated at 1350 C for 15 minutes Picture-2 shows the presence of paired-loops dislocations in a nickel based super alloy C263 Limitations of Electron Microscopes Electron microscopes are expensive to buy and maintain. As they are sensitive to vibration and external magnetic fields, suitable facilities are required to house microscopes aimed at achieving high resolutions. The samples have to be viewed in vacuum, as the molecules that make up air would scatter the electrons. Recent advances have allowed hydrated samples to be imaged using environmental scanning electron microscope. Scanning electron microscopes usually image conductive or semiconductive materials best. Non-conductive materials can be imaged by an environmental scanning electron microscope. A common preparation technique is to coat the sample with a several-nanometer 26
= 6 (1/ nm) So what is probability of finding electron tunneled into a barrier 3 ev high?
 STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
h p λ = mν Back to de Broglie and the electron as a wave you will learn more about this Equation in CHEM* 2060
 Back to de Broglie and the electron as a wave λ = mν h = h p you will learn more about this Equation in CHEM* 2060 We will soon see that the energies (speed for now if you like) of the electrons in the
Back to de Broglie and the electron as a wave λ = mν h = h p you will learn more about this Equation in CHEM* 2060 We will soon see that the energies (speed for now if you like) of the electrons in the
Basic structure of SEM
 Table of contents Basis structure of SEM SEM imaging modes Comparison of ordinary SEM and FESEM Electron behavior Electron matter interaction o Elastic interaction o Inelastic interaction o Interaction
Table of contents Basis structure of SEM SEM imaging modes Comparison of ordinary SEM and FESEM Electron behavior Electron matter interaction o Elastic interaction o Inelastic interaction o Interaction
Scanning Electron Microscopy
 Scanning Electron Microscopy Field emitting tip Grid 2kV 100kV Anode ZEISS SUPRA Variable Pressure FESEM Dr Heath Bagshaw CMA bagshawh@tcd.ie Why use an SEM? Fig 1. Examples of features resolvable using
Scanning Electron Microscopy Field emitting tip Grid 2kV 100kV Anode ZEISS SUPRA Variable Pressure FESEM Dr Heath Bagshaw CMA bagshawh@tcd.ie Why use an SEM? Fig 1. Examples of features resolvable using
Gaetano L Episcopo. Scanning Electron Microscopy Focus Ion Beam and. Pulsed Plasma Deposition
 Gaetano L Episcopo Scanning Electron Microscopy Focus Ion Beam and Pulsed Plasma Deposition Hystorical background Scientific discoveries 1897: J. Thomson discovers the electron. 1924: L. de Broglie propose
Gaetano L Episcopo Scanning Electron Microscopy Focus Ion Beam and Pulsed Plasma Deposition Hystorical background Scientific discoveries 1897: J. Thomson discovers the electron. 1924: L. de Broglie propose
Part II: Thin Film Characterization
 Part II: Thin Film Characterization General details of thin film characterization instruments 1. Introduction to Thin Film Characterization Techniques 2. Structural characterization: SEM, TEM, AFM, STM
Part II: Thin Film Characterization General details of thin film characterization instruments 1. Introduction to Thin Film Characterization Techniques 2. Structural characterization: SEM, TEM, AFM, STM
Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist
 12.141 Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist Massachusetts Institute of Technology Electron Microprobe Facility Department of Earth, Atmospheric and Planetary
12.141 Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist Massachusetts Institute of Technology Electron Microprobe Facility Department of Earth, Atmospheric and Planetary
AP5301/ Name the major parts of an optical microscope and state their functions.
 Review Problems on Optical Microscopy AP5301/8301-2015 1. Name the major parts of an optical microscope and state their functions. 2. Compare the focal lengths of two glass converging lenses, one with
Review Problems on Optical Microscopy AP5301/8301-2015 1. Name the major parts of an optical microscope and state their functions. 2. Compare the focal lengths of two glass converging lenses, one with
Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist
 12.141 Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist Massachusetts Institute of Technology Electron Microprobe Facility Department of Earth, Atmospheric and Planetary
12.141 Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist Massachusetts Institute of Technology Electron Microprobe Facility Department of Earth, Atmospheric and Planetary
Transmission Electron Microscopy
 L. Reimer H. Kohl Transmission Electron Microscopy Physics of Image Formation Fifth Edition el Springer Contents 1 Introduction... 1 1.1 Transmission Electron Microscopy... 1 1.1.1 Conventional Transmission
L. Reimer H. Kohl Transmission Electron Microscopy Physics of Image Formation Fifth Edition el Springer Contents 1 Introduction... 1 1.1 Transmission Electron Microscopy... 1 1.1.1 Conventional Transmission
Why microscopy?
 Electron Microscopy Why microscopy? http://www.cellsalive.com/howbig.htm 2 Microscopes are used as magnifying tools (although not exclusively as will see later on). The resolution of the human eye is limited
Electron Microscopy Why microscopy? http://www.cellsalive.com/howbig.htm 2 Microscopes are used as magnifying tools (although not exclusively as will see later on). The resolution of the human eye is limited
MT Electron microscopy Scanning electron microscopy and electron probe microanalysis
 MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
Chapter 9. Electron mean free path Microscopy principles of SEM, TEM, LEEM
 Chapter 9 Electron mean free path Microscopy principles of SEM, TEM, LEEM 9.1 Electron Mean Free Path 9. Scanning Electron Microscopy (SEM) -SEM design; Secondary electron imaging; Backscattered electron
Chapter 9 Electron mean free path Microscopy principles of SEM, TEM, LEEM 9.1 Electron Mean Free Path 9. Scanning Electron Microscopy (SEM) -SEM design; Secondary electron imaging; Backscattered electron
Praktikum zur. Materialanalytik
 Praktikum zur Materialanalytik Energy Dispersive X-ray Spectroscopy B513 Stand: 19.10.2016 Contents 1 Introduction... 2 2. Fundamental Physics and Notation... 3 2.1. Alignments of the microscope... 3 2.2.
Praktikum zur Materialanalytik Energy Dispersive X-ray Spectroscopy B513 Stand: 19.10.2016 Contents 1 Introduction... 2 2. Fundamental Physics and Notation... 3 2.1. Alignments of the microscope... 3 2.2.
object objective lens eyepiece lens
 Advancing Physics G495 June 2015 SET #1 ANSWERS Field and Particle Pictures Seeing with electrons The compound optical microscope Q1. Before attempting this question it may be helpful to review ray diagram
Advancing Physics G495 June 2015 SET #1 ANSWERS Field and Particle Pictures Seeing with electrons The compound optical microscope Q1. Before attempting this question it may be helpful to review ray diagram
Analytical Methods for Materials
 Analytical Methods for Materials Lesson 21 Electron Microscopy and X-ray Spectroscopy Suggested Reading Leng, Chapter 3, pp. 83-126; Chapter 4, pp. 127-160; Chapter 6, pp. 191-219 P.J. Goodhew, J. Humphreys
Analytical Methods for Materials Lesson 21 Electron Microscopy and X-ray Spectroscopy Suggested Reading Leng, Chapter 3, pp. 83-126; Chapter 4, pp. 127-160; Chapter 6, pp. 191-219 P.J. Goodhew, J. Humphreys
Modern Optical Spectroscopy
 Modern Optical Spectroscopy X-Ray Microanalysis Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Backscattered
Modern Optical Spectroscopy X-Ray Microanalysis Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Backscattered
CHARACTERIZATION of NANOMATERIALS KHP
 CHARACTERIZATION of NANOMATERIALS Overview of the most common nanocharacterization techniques MAIN CHARACTERIZATION TECHNIQUES: 1.Transmission Electron Microscope (TEM) 2. Scanning Electron Microscope
CHARACTERIZATION of NANOMATERIALS Overview of the most common nanocharacterization techniques MAIN CHARACTERIZATION TECHNIQUES: 1.Transmission Electron Microscope (TEM) 2. Scanning Electron Microscope
Chemical Analysis in TEM: XEDS, EELS and EFTEM. HRTEM PhD course Lecture 5
 Chemical Analysis in TEM: XEDS, EELS and EFTEM HRTEM PhD course Lecture 5 1 Part IV Subject Chapter Prio x-ray spectrometry 32 1 Spectra and mapping 33 2 Qualitative XEDS 34 1 Quantitative XEDS 35.1-35.4
Chemical Analysis in TEM: XEDS, EELS and EFTEM HRTEM PhD course Lecture 5 1 Part IV Subject Chapter Prio x-ray spectrometry 32 1 Spectra and mapping 33 2 Qualitative XEDS 34 1 Quantitative XEDS 35.1-35.4
Nano-Microscopy. Lecture 2. Scanning and Transmission Electron Microscopies: Principles. Pavel Zinin HIGP, University of Hawaii, Honolulu, USA
 GG 711: Advanced Techniques in Geophysics and Materials Science Nano-Microscopy. Lecture 2 Scanning and Transmission Electron Microscopies: Principles Pavel Zinin HIGP, University of Hawaii, Honolulu,
GG 711: Advanced Techniques in Geophysics and Materials Science Nano-Microscopy. Lecture 2 Scanning and Transmission Electron Microscopies: Principles Pavel Zinin HIGP, University of Hawaii, Honolulu,
Electron Microscopy I
 Characterization of Catalysts and Surfaces Characterization Techniques in Heterogeneous Catalysis Electron Microscopy I Introduction Properties of electrons Electron-matter interactions and their applications
Characterization of Catalysts and Surfaces Characterization Techniques in Heterogeneous Catalysis Electron Microscopy I Introduction Properties of electrons Electron-matter interactions and their applications
Electron Microprobe Analysis and Scanning Electron Microscopy
 Electron Microprobe Analysis and Scanning Electron Microscopy Electron microprobe analysis (EMPA) Analytical technique in which a beam of electrons is focused on a sample surface, producing X-rays from
Electron Microprobe Analysis and Scanning Electron Microscopy Electron microprobe analysis (EMPA) Analytical technique in which a beam of electrons is focused on a sample surface, producing X-rays from
tip conducting surface
 PhysicsAndMathsTutor.com 1 1. The diagram shows the tip of a scanning tunnelling microscope (STM) above a conducting surface. The tip is at a potential of 1.0 V relative to the surface. If the tip is sufficiently
PhysicsAndMathsTutor.com 1 1. The diagram shows the tip of a scanning tunnelling microscope (STM) above a conducting surface. The tip is at a potential of 1.0 V relative to the surface. If the tip is sufficiently
MSE 321 Structural Characterization
 Optical Microscope Plan Lenses In an "ideal" single-element lens system all planar wave fronts are focused to a point at distance f from the lens; therefore: Image near the optical axis will be in perfect
Optical Microscope Plan Lenses In an "ideal" single-element lens system all planar wave fronts are focused to a point at distance f from the lens; therefore: Image near the optical axis will be in perfect
Practical course in scanning electron microscopy
 Practical course in scanning electron microscopy Fortgeschrittenen Praktikum an der Technischen Universität München Wintersemester 2017/2018 Table of contents 1. Introduction 3 2. Formation of an electron
Practical course in scanning electron microscopy Fortgeschrittenen Praktikum an der Technischen Universität München Wintersemester 2017/2018 Table of contents 1. Introduction 3 2. Formation of an electron
MSE 321 Structural Characterization
 Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
4. Inelastic Scattering
 1 4. Inelastic Scattering Some inelastic scattering processes A vast range of inelastic scattering processes can occur during illumination of a specimen with a highenergy electron beam. In principle, many
1 4. Inelastic Scattering Some inelastic scattering processes A vast range of inelastic scattering processes can occur during illumination of a specimen with a highenergy electron beam. In principle, many
Imaging Methods: Scanning Force Microscopy (SFM / AFM)
 Imaging Methods: Scanning Force Microscopy (SFM / AFM) The atomic force microscope (AFM) probes the surface of a sample with a sharp tip, a couple of microns long and often less than 100 Å in diameter.
Imaging Methods: Scanning Force Microscopy (SFM / AFM) The atomic force microscope (AFM) probes the surface of a sample with a sharp tip, a couple of microns long and often less than 100 Å in diameter.
Electron probe microanalysis - Electron microprobe analysis EPMA (EMPA) What s EPMA all about? What can you learn?
 Electron probe microanalysis - Electron microprobe analysis EPMA (EMPA) What s EPMA all about? What can you learn? EPMA - what is it? Precise and accurate quantitative chemical analyses of micron-size
Electron probe microanalysis - Electron microprobe analysis EPMA (EMPA) What s EPMA all about? What can you learn? EPMA - what is it? Precise and accurate quantitative chemical analyses of micron-size
Chapter 10. Nanometrology. Oxford University Press All rights reserved.
 Chapter 10 Nanometrology Oxford University Press 2013. All rights reserved. 1 Introduction Nanometrology is the science of measurement at the nanoscale level. Figure illustrates where nanoscale stands
Chapter 10 Nanometrology Oxford University Press 2013. All rights reserved. 1 Introduction Nanometrology is the science of measurement at the nanoscale level. Figure illustrates where nanoscale stands
SEM stands for Scanning Electron Microscopy. The earliest known work describing
 1. HISTORY ABOUT SEM SEM stands for Scanning Electron Microscopy. The earliest known work describing the concept of a Scanning Electron Microscope was by M. Knoll (1935) who, along with other pioneers
1. HISTORY ABOUT SEM SEM stands for Scanning Electron Microscopy. The earliest known work describing the concept of a Scanning Electron Microscope was by M. Knoll (1935) who, along with other pioneers
SOLID STATE PHYSICS PHY F341. Dr. Manjuladevi.V Associate Professor Department of Physics BITS Pilani
 SOLID STATE PHYSICS PHY F341 Dr. Manjuladevi.V Associate Professor Department of Physics BITS Pilani 333031 manjula@bits-pilani.ac.in Characterization techniques SEM AFM STM BAM Outline What can we use
SOLID STATE PHYSICS PHY F341 Dr. Manjuladevi.V Associate Professor Department of Physics BITS Pilani 333031 manjula@bits-pilani.ac.in Characterization techniques SEM AFM STM BAM Outline What can we use
CHEM 681 Seminar Mingqi Zhao April 20, 1998 Room 2104, 4:00 p.m. High Resolution Transmission Electron Microscopy: theories and applications
 CHEM 681 Seminar Mingqi Zhao April 20, 1998 Room 2104, 4:00 p.m. High Resolution Transmission Electron Microscopy: theories and applications In materials science, people are always interested in viewing
CHEM 681 Seminar Mingqi Zhao April 20, 1998 Room 2104, 4:00 p.m. High Resolution Transmission Electron Microscopy: theories and applications In materials science, people are always interested in viewing
Part I Basics and Methods
 j1 Part I Basics and Methods In-situ Electron Microscopy: Applications in Physics, Chemistry and Materials Science, First Edition. Edited by Gerhard Dehm, James M. Howe, and Josef Zweck. Ó 2012 Wiley-VCH
j1 Part I Basics and Methods In-situ Electron Microscopy: Applications in Physics, Chemistry and Materials Science, First Edition. Edited by Gerhard Dehm, James M. Howe, and Josef Zweck. Ó 2012 Wiley-VCH
MSE 321 Structural Characterization
 Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
Everhart-Thornley detector
 SEI Detector Everhart-Thornley detector Microscope chamber wall Faraday cage Scintillator Electrons in Light pipe Photomultiplier Electrical signal out Screen Quartz window +200 V +10 kv Always contains
SEI Detector Everhart-Thornley detector Microscope chamber wall Faraday cage Scintillator Electrons in Light pipe Photomultiplier Electrical signal out Screen Quartz window +200 V +10 kv Always contains
M2 TP. Low-Energy Electron Diffraction (LEED)
 M2 TP Low-Energy Electron Diffraction (LEED) Guide for report preparation I. Introduction: Elastic scattering or diffraction of electrons is the standard technique in surface science for obtaining structural
M2 TP Low-Energy Electron Diffraction (LEED) Guide for report preparation I. Introduction: Elastic scattering or diffraction of electrons is the standard technique in surface science for obtaining structural
Weak-Beam Dark-Field Technique
 Basic Idea recall bright-field contrast of dislocations: specimen close to Bragg condition, s î 0 Weak-Beam Dark-Field Technique near the dislocation core, some planes curved to s = 0 ) strong Bragg reflection
Basic Idea recall bright-field contrast of dislocations: specimen close to Bragg condition, s î 0 Weak-Beam Dark-Field Technique near the dislocation core, some planes curved to s = 0 ) strong Bragg reflection
The illumination source: the electron beam
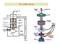 The SEM Column The illumination source: the electron beam The probe of the electron microscope is an electron beam with very high and stable energy (10-100 kev) in order to get images with high resolution.
The SEM Column The illumination source: the electron beam The probe of the electron microscope is an electron beam with very high and stable energy (10-100 kev) in order to get images with high resolution.
Practical 1P4 Energy Levels and Band Gaps
 Practical 1P4 Energy Levels and Band Gaps What you should learn from this practical Science This practical illustrates some of the points from the lecture course on Elementary Quantum Mechanics and Bonding
Practical 1P4 Energy Levels and Band Gaps What you should learn from this practical Science This practical illustrates some of the points from the lecture course on Elementary Quantum Mechanics and Bonding
MEMS Metrology. Prof. Tianhong Cui ME 8254
 MEMS Metrology Prof. Tianhong Cui ME 8254 What is metrology? Metrology It is the science of weights and measures Refers primarily to the measurements of length, weight, time, etc. Mensuration- A branch
MEMS Metrology Prof. Tianhong Cui ME 8254 What is metrology? Metrology It is the science of weights and measures Refers primarily to the measurements of length, weight, time, etc. Mensuration- A branch
Practical 1P4 Energy Levels and Band Gaps
 Practical 1P4 Energy Levels and Band Gaps What you should learn from this practical Science This practical illustrates some of the points from the lecture course on Elementary Quantum Mechanics and Bonding
Practical 1P4 Energy Levels and Band Gaps What you should learn from this practical Science This practical illustrates some of the points from the lecture course on Elementary Quantum Mechanics and Bonding
KMÜ 396 MATERIALS SCIENCE AND TECH. I PRESENTATION ELECTRON ENERGY LOSS SPECTROSCOPY (EELS) TUĞÇE SEZGİN
 KMÜ 396 MATERIALS SCIENCE AND TECH. I PRESENTATION ELECTRON ENERGY LOSS SPECTROSCOPY (EELS) TUĞÇE SEZGİN 20970725 HACETTEPE UNIVERSITY DEPARTMENT OF CHEMICAL ENGINEERING, SPRING 2011,APRIL,ANKARA CONTENTS
KMÜ 396 MATERIALS SCIENCE AND TECH. I PRESENTATION ELECTRON ENERGY LOSS SPECTROSCOPY (EELS) TUĞÇE SEZGİN 20970725 HACETTEPE UNIVERSITY DEPARTMENT OF CHEMICAL ENGINEERING, SPRING 2011,APRIL,ANKARA CONTENTS
Electron beam scanning
 Electron beam scanning The Electron beam scanning operates through an electro-optical system which has the task of deflecting the beam Synchronously with cathode ray tube which create the image, beam moves
Electron beam scanning The Electron beam scanning operates through an electro-optical system which has the task of deflecting the beam Synchronously with cathode ray tube which create the image, beam moves
Chapter Six: X-Rays. 6.1 Discovery of X-rays
 Chapter Six: X-Rays 6.1 Discovery of X-rays In late 1895, a German physicist, W. C. Roentgen was working with a cathode ray tube in his laboratory. He was working with tubes similar to our fluorescent
Chapter Six: X-Rays 6.1 Discovery of X-rays In late 1895, a German physicist, W. C. Roentgen was working with a cathode ray tube in his laboratory. He was working with tubes similar to our fluorescent
Generation of X-Rays in the SEM specimen
 Generation of X-Rays in the SEM specimen The electron beam generates X-ray photons in the beam-specimen interaction volume beneath the specimen surface. Some X-ray photons emerging from the specimen have
Generation of X-Rays in the SEM specimen The electron beam generates X-ray photons in the beam-specimen interaction volume beneath the specimen surface. Some X-ray photons emerging from the specimen have
Scanning Electron Microscopy & Ancillary Techniques
 Scanning Electron Microscopy & Ancillary Techniques By Pablo G. Caceres-Valencia The prototype of the first Stereoscan supplied by the Cambridge Instrument Company to the dupont Company, U.S.A. (1965)
Scanning Electron Microscopy & Ancillary Techniques By Pablo G. Caceres-Valencia The prototype of the first Stereoscan supplied by the Cambridge Instrument Company to the dupont Company, U.S.A. (1965)
Chapter 12. Nanometrology. Oxford University Press All rights reserved.
 Chapter 12 Nanometrology Introduction Nanometrology is the science of measurement at the nanoscale level. Figure illustrates where nanoscale stands in relation to a meter and sub divisions of meter. Nanometrology
Chapter 12 Nanometrology Introduction Nanometrology is the science of measurement at the nanoscale level. Figure illustrates where nanoscale stands in relation to a meter and sub divisions of meter. Nanometrology
X-RAY PRODUCTION. Prepared by:- EN KAMARUL AMIN BIN ABDULLAH
 X-RAY PRODUCTION Prepared by:- EN KAMARUL AMIN BIN ABDULLAH OBJECTIVES Discuss the process of x-ray being produced (conditions) Explain the principles of energy conversion in x-ray production (how energy
X-RAY PRODUCTION Prepared by:- EN KAMARUL AMIN BIN ABDULLAH OBJECTIVES Discuss the process of x-ray being produced (conditions) Explain the principles of energy conversion in x-ray production (how energy
SEM Doctoral Course MS-636. April 11-13, 2016
 Thomas LaGrange, Ph.D. Faculty Lecturer and Senior Staff Scientist Electron Sources, Optics and Detectors SEM Doctoral Course MS-636 April 11-13, 2016 Summary Electron propagation is only possible through
Thomas LaGrange, Ph.D. Faculty Lecturer and Senior Staff Scientist Electron Sources, Optics and Detectors SEM Doctoral Course MS-636 April 11-13, 2016 Summary Electron propagation is only possible through
Auger Electron Spectroscopy (AES)
 1. Introduction Auger Electron Spectroscopy (AES) Silvia Natividad, Gabriel Gonzalez and Arena Holguin Auger Electron Spectroscopy (Auger spectroscopy or AES) was developed in the late 1960's, deriving
1. Introduction Auger Electron Spectroscopy (AES) Silvia Natividad, Gabriel Gonzalez and Arena Holguin Auger Electron Spectroscopy (Auger spectroscopy or AES) was developed in the late 1960's, deriving
The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado
 Experiment 9 1 Introduction The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado During the late nineteenth century, a great deal of evidence accumulated indicating that radiation
Experiment 9 1 Introduction The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado During the late nineteenth century, a great deal of evidence accumulated indicating that radiation
EEE4106Z Radiation Interactions & Detection
 EEE4106Z Radiation Interactions & Detection 2. Radiation Detection Dr. Steve Peterson 5.14 RW James Department of Physics University of Cape Town steve.peterson@uct.ac.za May 06, 2015 EEE4106Z :: Radiation
EEE4106Z Radiation Interactions & Detection 2. Radiation Detection Dr. Steve Peterson 5.14 RW James Department of Physics University of Cape Town steve.peterson@uct.ac.za May 06, 2015 EEE4106Z :: Radiation
Chemistry Instrumental Analysis Lecture 19 Chapter 12. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 19 Chapter 12 There are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry X-ray Techniques include:
Chemistry 4631 Instrumental Analysis Lecture 19 Chapter 12 There are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry X-ray Techniques include:
CBE Science of Engineering Materials. Scanning Electron Microscopy (SEM)
 CBE 30361 Science of Engineering Materials Scanning Electron Microscopy (SEM) Scale of Structure Organization Units: micrometer = 10-6 m = 1µm nanometer= 10-9 m = 1nm Angstrom = 10-10 m = 1Å A hair is
CBE 30361 Science of Engineering Materials Scanning Electron Microscopy (SEM) Scale of Structure Organization Units: micrometer = 10-6 m = 1µm nanometer= 10-9 m = 1nm Angstrom = 10-10 m = 1Å A hair is
HOW TO APPROACH SCANNING ELECTRON MICROSCOPY AND ENERGY DISPERSIVE SPECTROSCOPY ANALYSIS. SCSAM Short Course Amir Avishai
 HOW TO APPROACH SCANNING ELECTRON MICROSCOPY AND ENERGY DISPERSIVE SPECTROSCOPY ANALYSIS SCSAM Short Course Amir Avishai RESEARCH QUESTIONS Sea Shell Cast Iron EDS+SE Fe Cr C Objective Ability to ask the
HOW TO APPROACH SCANNING ELECTRON MICROSCOPY AND ENERGY DISPERSIVE SPECTROSCOPY ANALYSIS SCSAM Short Course Amir Avishai RESEARCH QUESTIONS Sea Shell Cast Iron EDS+SE Fe Cr C Objective Ability to ask the
Chemistry Instrumental Analysis Lecture 34. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 34 From molecular to elemental analysis there are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry
Chemistry 4631 Instrumental Analysis Lecture 34 From molecular to elemental analysis there are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry
EDS User School. Principles of Electron Beam Microanalysis
 EDS User School Principles of Electron Beam Microanalysis Outline 1.) Beam-specimen interactions 2.) EDS spectra: Origin of Bremsstrahlung and characteristic peaks 3.) Moseley s law 4.) Characteristic
EDS User School Principles of Electron Beam Microanalysis Outline 1.) Beam-specimen interactions 2.) EDS spectra: Origin of Bremsstrahlung and characteristic peaks 3.) Moseley s law 4.) Characteristic
ELECTROMAGNETIC WAVES
 VISUAL PHYSICS ONLINE MODULE 7 NATURE OF LIGHT ELECTROMAGNETIC WAVES SPECTRA PRODUCED BY DISCHARGE TUBES CATHODE RAYS (electron beams) Streams of electrons (negatively charged particles) observed in vacuum
VISUAL PHYSICS ONLINE MODULE 7 NATURE OF LIGHT ELECTROMAGNETIC WAVES SPECTRA PRODUCED BY DISCHARGE TUBES CATHODE RAYS (electron beams) Streams of electrons (negatively charged particles) observed in vacuum
SEM Optics and Application to Current Research
 SEM Optics and Application to Current Research Azure Avery May 28, 2008 1 Introduction 1.1 History The optical microscope was invented in the early 17th century. Although revolutionary, the earliest microscopes
SEM Optics and Application to Current Research Azure Avery May 28, 2008 1 Introduction 1.1 History The optical microscope was invented in the early 17th century. Although revolutionary, the earliest microscopes
CHAPTER 3 The Experimental Basis of Quantum Theory
 CHAPTER 3 The Experimental Basis of Quantum Theory 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Discovery of the X Ray and the Electron Determination of Electron Charge Line Spectra Quantization As far as I can
CHAPTER 3 The Experimental Basis of Quantum Theory 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Discovery of the X Ray and the Electron Determination of Electron Charge Line Spectra Quantization As far as I can
1986 s Nobel Prize in Physics
 Revised version: 2017.12.19 1986 s Nobel Prize in Physics (Electron Microscope & STM) Huiwon Ahn Seoul National University Department of Physics & Astronomy, Korea Abstract The structure of matter or organisms
Revised version: 2017.12.19 1986 s Nobel Prize in Physics (Electron Microscope & STM) Huiwon Ahn Seoul National University Department of Physics & Astronomy, Korea Abstract The structure of matter or organisms
Basic physics Questions
 Chapter1 Basic physics Questions S. Ilyas 1. Which of the following statements regarding protons are correct? a. They have a negative charge b. They are equal to the number of electrons in a non-ionized
Chapter1 Basic physics Questions S. Ilyas 1. Which of the following statements regarding protons are correct? a. They have a negative charge b. They are equal to the number of electrons in a non-ionized
Ecole Franco-Roumaine : Magnétisme des systèmes nanoscopiques et structures hybrides - Brasov, Modern Analytical Microscopic Tools
 1. Introduction Solid Surfaces Analysis Group, Institute of Physics, Chemnitz University of Technology, Germany 2. Limitations of Conventional Optical Microscopy 3. Electron Microscopies Transmission Electron
1. Introduction Solid Surfaces Analysis Group, Institute of Physics, Chemnitz University of Technology, Germany 2. Limitations of Conventional Optical Microscopy 3. Electron Microscopies Transmission Electron
Chemistry 311: Instrumentation Analysis Topic 2: Atomic Spectroscopy. Chemistry 311: Instrumentation Analysis Topic 2: Atomic Spectroscopy
 Topic 2b: X-ray Fluorescence Spectrometry Text: Chapter 12 Rouessac (1 week) 4.0 X-ray Fluorescence Download, read and understand EPA method 6010C ICP-OES Winter 2009 Page 1 Atomic X-ray Spectrometry Fundamental
Topic 2b: X-ray Fluorescence Spectrometry Text: Chapter 12 Rouessac (1 week) 4.0 X-ray Fluorescence Download, read and understand EPA method 6010C ICP-OES Winter 2009 Page 1 Atomic X-ray Spectrometry Fundamental
Interactions with Matter
 Manetic Lenses Manetic fields can displace electrons Manetic field can be produced by passin an electrical current throuh coils of wire Manetic field strenth can be increased by usin a soft ferromanetic
Manetic Lenses Manetic fields can displace electrons Manetic field can be produced by passin an electrical current throuh coils of wire Manetic field strenth can be increased by usin a soft ferromanetic
X-RAY SPECTRA. Theory:
 12 Oct 18 X-ray.1 X-RAY SPECTRA In this experiment, a number of measurements involving x-rays will be made. The spectrum of x-rays emitted from a molybdenum target will be measured, and the experimental
12 Oct 18 X-ray.1 X-RAY SPECTRA In this experiment, a number of measurements involving x-rays will be made. The spectrum of x-rays emitted from a molybdenum target will be measured, and the experimental
April 10th-12th, 2017
 Thomas LaGrange, Ph.D. Faculty Lecturer and Senior Staff Scientist Introduction: Basics of Transmission Electron Microscopy (TEM) TEM Doctoral Course MS-637 April 10th-12th, 2017 Outline 1. What is microcopy?
Thomas LaGrange, Ph.D. Faculty Lecturer and Senior Staff Scientist Introduction: Basics of Transmission Electron Microscopy (TEM) TEM Doctoral Course MS-637 April 10th-12th, 2017 Outline 1. What is microcopy?
Secondary Ion Mass Spectroscopy (SIMS)
 Secondary Ion Mass Spectroscopy (SIMS) Analyzing Inorganic Solids * = under special conditions ** = semiconductors only + = limited number of elements or groups Analyzing Organic Solids * = under special
Secondary Ion Mass Spectroscopy (SIMS) Analyzing Inorganic Solids * = under special conditions ** = semiconductors only + = limited number of elements or groups Analyzing Organic Solids * = under special
Surface Sensitivity & Surface Specificity
 Surface Sensitivity & Surface Specificity The problems of sensitivity and detection limits are common to all forms of spectroscopy. In its simplest form, the question of sensitivity boils down to whether
Surface Sensitivity & Surface Specificity The problems of sensitivity and detection limits are common to all forms of spectroscopy. In its simplest form, the question of sensitivity boils down to whether
Low Vacuum Scanning Electron Microscopy and Microanalysis
 Low Vacuum Scanning Electron Microscopy and Microanalysis Principles and Practice of Variable Pressure/Environmental Scanning Electron Microscopy (VP-ESEM), Debbie J Stokes, John Wiley&Sons 2008 Several
Low Vacuum Scanning Electron Microscopy and Microanalysis Principles and Practice of Variable Pressure/Environmental Scanning Electron Microscopy (VP-ESEM), Debbie J Stokes, John Wiley&Sons 2008 Several
Chapter 10: Wave Properties of Particles
 Chapter 10: Wave Properties of Particles Particles such as electrons may demonstrate wave properties under certain conditions. The electron microscope uses these properties to produce magnified images
Chapter 10: Wave Properties of Particles Particles such as electrons may demonstrate wave properties under certain conditions. The electron microscope uses these properties to produce magnified images
Shell Atomic Model and Energy Levels
 Shell Atomic Model and Energy Levels (higher energy, deeper excitation) - Radio waves: Not absorbed and pass through tissue un-attenuated - Microwaves : Energies of Photos enough to cause molecular rotation
Shell Atomic Model and Energy Levels (higher energy, deeper excitation) - Radio waves: Not absorbed and pass through tissue un-attenuated - Microwaves : Energies of Photos enough to cause molecular rotation
An Introduction to Diffraction and Scattering. School of Chemistry The University of Sydney
 An Introduction to Diffraction and Scattering Brendan J. Kennedy School of Chemistry The University of Sydney 1) Strong forces 2) Weak forces Types of Forces 3) Electromagnetic forces 4) Gravity Types
An Introduction to Diffraction and Scattering Brendan J. Kennedy School of Chemistry The University of Sydney 1) Strong forces 2) Weak forces Types of Forces 3) Electromagnetic forces 4) Gravity Types
Sparks in Gases: Line Spectra
 Lecture 11 February 4, Chapter 3 The Particlelike Properties of Electromagnetic Radiation Sparks in Gases: Line Spectra This is one of the oldest tools available for the investigation of atoms and radiation.
Lecture 11 February 4, Chapter 3 The Particlelike Properties of Electromagnetic Radiation Sparks in Gases: Line Spectra This is one of the oldest tools available for the investigation of atoms and radiation.
6. Analytical Electron Microscopy
 Physical Principles of Electron Microscopy 6. Analytical Electron Microscopy Ray Egerton University of Alberta and National Institute of Nanotechnology Edmonton, Canada www.tem-eels.ca regerton@ualberta.ca
Physical Principles of Electron Microscopy 6. Analytical Electron Microscopy Ray Egerton University of Alberta and National Institute of Nanotechnology Edmonton, Canada www.tem-eels.ca regerton@ualberta.ca
1 P a g e h t t p s : / / w w w. c i e n o t e s. c o m / Physics (A-level)
 1 P a g e h t t p s : / / w w w. c i e n o t e s. c o m / Physics (A-level) Electromagnetic induction (Chapter 23): For a straight wire, the induced current or e.m.f. depends on: The magnitude of the magnetic
1 P a g e h t t p s : / / w w w. c i e n o t e s. c o m / Physics (A-level) Electromagnetic induction (Chapter 23): For a straight wire, the induced current or e.m.f. depends on: The magnitude of the magnetic
Information from Every Angle
 pplication Note Information from Every ngle Directional SE Detector for Next-Level Imaging Zinc oxide nanorods with surficial palladium particles imaged at 500 V in high vacuum. dding palladium increases
pplication Note Information from Every ngle Directional SE Detector for Next-Level Imaging Zinc oxide nanorods with surficial palladium particles imaged at 500 V in high vacuum. dding palladium increases
Introduction to Electron Microscopy Andres Kaech. Instrumentation
 Center for Microscopy and Image Analysis Introduction to Electron Microscopy Andres Kaech Instrumentation The types of electron microscopes Transmission electron microscope (TEM) Scanning electron microscope
Center for Microscopy and Image Analysis Introduction to Electron Microscopy Andres Kaech Instrumentation The types of electron microscopes Transmission electron microscope (TEM) Scanning electron microscope
MATERIAL SCIENCE AND TECHONOLOGY-1. Scanning Tunneling Microscope, STM Tunneling Electron Microscope, TEM HATİCE DOĞRUOĞLU
 MATERIAL SCIENCE AND TECHONOLOGY-1 Scanning Tunneling Microscope, STM Tunneling Electron Microscope, TEM HATİCE DOĞRUOĞLU Scanning Tunelling Microscope (STM) In 1981,Gerd Binnig and Heinrich Rohrer and
MATERIAL SCIENCE AND TECHONOLOGY-1 Scanning Tunneling Microscope, STM Tunneling Electron Microscope, TEM HATİCE DOĞRUOĞLU Scanning Tunelling Microscope (STM) In 1981,Gerd Binnig and Heinrich Rohrer and
Vacuum Pumps. Two general classes exist: Gas transfer physical removal of matter. Mechanical, diffusion, turbomolecular
 Vacuum Technology Vacuum Pumps Two general classes exist: Gas transfer physical removal of matter Mechanical, diffusion, turbomolecular Adsorption entrapment of matter Cryo, sublimation, ion Mechanical
Vacuum Technology Vacuum Pumps Two general classes exist: Gas transfer physical removal of matter Mechanical, diffusion, turbomolecular Adsorption entrapment of matter Cryo, sublimation, ion Mechanical
X-ray Interaction with Matter
 X-ray Interaction with Matter 10-526-197 Rhodes Module 2 Interaction with Matter kv & mas Peak kilovoltage (kvp) controls Quality, or penetrating power, Limited effects on quantity or number of photons
X-ray Interaction with Matter 10-526-197 Rhodes Module 2 Interaction with Matter kv & mas Peak kilovoltage (kvp) controls Quality, or penetrating power, Limited effects on quantity or number of photons
I live in this atom, with my other electron brothers
 Hello, my name is Electron, John Electron. I am going to tell you how my work is in an electron microscope. I live in this atom, with my other electron brothers The filament crowns the column of the electron
Hello, my name is Electron, John Electron. I am going to tell you how my work is in an electron microscope. I live in this atom, with my other electron brothers The filament crowns the column of the electron
School. Team Number. Optics
 School Team Number Optics Physical Optics (30%) Proceed to the laser shoot (40%) when your team number is called. 1. What are the four colors used in the CMYK color model? (2 points) 2. Muscae Volitantes
School Team Number Optics Physical Optics (30%) Proceed to the laser shoot (40%) when your team number is called. 1. What are the four colors used in the CMYK color model? (2 points) 2. Muscae Volitantes
Chapter 4 Scintillation Detectors
 Med Phys 4RA3, 4RB3/6R03 Radioisotopes and Radiation Methodology 4-1 4.1. Basic principle of the scintillator Chapter 4 Scintillation Detectors Scintillator Light sensor Ionizing radiation Light (visible,
Med Phys 4RA3, 4RB3/6R03 Radioisotopes and Radiation Methodology 4-1 4.1. Basic principle of the scintillator Chapter 4 Scintillation Detectors Scintillator Light sensor Ionizing radiation Light (visible,
Because light behaves like a wave, we can describe it in one of two ways by its wavelength or by its frequency.
 Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Chapter 37 Early Quantum Theory and Models of the Atom. Copyright 2009 Pearson Education, Inc.
 Chapter 37 Early Quantum Theory and Models of the Atom Planck s Quantum Hypothesis; Blackbody Radiation Photon Theory of Light and the Photoelectric Effect Energy, Mass, and Momentum of a Photon Compton
Chapter 37 Early Quantum Theory and Models of the Atom Planck s Quantum Hypothesis; Blackbody Radiation Photon Theory of Light and the Photoelectric Effect Energy, Mass, and Momentum of a Photon Compton
Auger Electron Spectroscopy (AES) Prof. Paul K. Chu
 Auger Electron Spectroscopy (AES) Prof. Paul K. Chu Auger Electron Spectroscopy Introduction Principles Instrumentation Qualitative analysis Quantitative analysis Depth profiling Mapping Examples The Auger
Auger Electron Spectroscopy (AES) Prof. Paul K. Chu Auger Electron Spectroscopy Introduction Principles Instrumentation Qualitative analysis Quantitative analysis Depth profiling Mapping Examples The Auger
INDIAN INSTITUTE OF TECHNOLOGY ROORKEE NPTEL NPTEL ONLINE CERTIFICATION COURSE. Biomedical Nanotechnology. Lec-05 Characterisation of Nanoparticles
 INDIAN INSTITUTE OF TECHNOLOGY ROORKEE NPTEL NPTEL ONLINE CERTIFICATION COURSE Biomedical Nanotechnology Lec-05 Characterisation of Nanoparticles Dr. P. Gopinath Department of Biotechnology Indian Institute
INDIAN INSTITUTE OF TECHNOLOGY ROORKEE NPTEL NPTEL ONLINE CERTIFICATION COURSE Biomedical Nanotechnology Lec-05 Characterisation of Nanoparticles Dr. P. Gopinath Department of Biotechnology Indian Institute
Discovered by German scientist Johann Hittorf in 1869 and in 1876 named by Eugen Goldstein.
 DO PHYSICS ONLINE CATHODE RAYS CATHODE RAYS (electron beams) Streams of electrons (negatively charged particles) observed in vacuum tubes - evacuated glass tubes that are equipped with at least two metal
DO PHYSICS ONLINE CATHODE RAYS CATHODE RAYS (electron beams) Streams of electrons (negatively charged particles) observed in vacuum tubes - evacuated glass tubes that are equipped with at least two metal
Methods of surface analysis
 Methods of surface analysis Nanomaterials characterisation I RNDr. Věra Vodičková, PhD. Surface of solid matter: last monoatomic layer + absorbed monolayer physical properties are effected (crystal lattice
Methods of surface analysis Nanomaterials characterisation I RNDr. Věra Vodičková, PhD. Surface of solid matter: last monoatomic layer + absorbed monolayer physical properties are effected (crystal lattice
MT Electron microscopy Scanning electron microscopy and electron probe microanalysis
 MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
High-Resolution. Transmission. Electron Microscopy
 Part 4 High-Resolution Transmission Electron Microscopy 186 Significance high-resolution transmission electron microscopy (HRTEM): resolve object details smaller than 1nm (10 9 m) image the interior of
Part 4 High-Resolution Transmission Electron Microscopy 186 Significance high-resolution transmission electron microscopy (HRTEM): resolve object details smaller than 1nm (10 9 m) image the interior of
QUANTUM PHYSICS. Limitation: This law holds well only for the short wavelength and not for the longer wavelength. Raleigh Jean s Law:
 Black body: A perfect black body is one which absorbs all the radiation of heat falling on it and emits all the radiation when heated in an isothermal enclosure. The heat radiation emitted by the black
Black body: A perfect black body is one which absorbs all the radiation of heat falling on it and emits all the radiation when heated in an isothermal enclosure. The heat radiation emitted by the black
Production of X-rays. Radiation Safety Training for Analytical X-Ray Devices Module 9
 Module 9 This module presents information on what X-rays are and how they are produced. Introduction Module 9, Page 2 X-rays are a type of electromagnetic radiation. Other types of electromagnetic radiation
Module 9 This module presents information on what X-rays are and how they are produced. Introduction Module 9, Page 2 X-rays are a type of electromagnetic radiation. Other types of electromagnetic radiation
Vibrational Spectroscopies. C-874 University of Delaware
 Vibrational Spectroscopies C-874 University of Delaware Vibrational Spectroscopies..everything that living things do can be understood in terms of the jigglings and wigglings of atoms.. R. P. Feymann Vibrational
Vibrational Spectroscopies C-874 University of Delaware Vibrational Spectroscopies..everything that living things do can be understood in terms of the jigglings and wigglings of atoms.. R. P. Feymann Vibrational
CHEM*3440. X-Ray Energies. Bremsstrahlung Radiation. X-ray Line Spectra. Chemical Instrumentation. X-Ray Spectroscopy. Topic 13
 X-Ray Energies very short wavelength radiation 0.1Å to 10 nm (100 Å) CHEM*3440 Chemical Instrumentation Topic 13 X-Ray Spectroscopy Visible - Ultraviolet (UV) - Vacuum UV (VUV) - Extreme UV (XUV) - Soft
X-Ray Energies very short wavelength radiation 0.1Å to 10 nm (100 Å) CHEM*3440 Chemical Instrumentation Topic 13 X-Ray Spectroscopy Visible - Ultraviolet (UV) - Vacuum UV (VUV) - Extreme UV (XUV) - Soft
sin" =1.22 # D "l =1.22 f# D I: In introduction to molecular electron microscopy - Imaging macromolecular assemblies
 I: In introduction to molecular electron microscopy - Imaging macromolecular assemblies Yifan Cheng Department of Biochemistry & Biophysics office: GH-S472D; email: ycheng@ucsf.edu 2/20/2015 - Introduction
I: In introduction to molecular electron microscopy - Imaging macromolecular assemblies Yifan Cheng Department of Biochemistry & Biophysics office: GH-S472D; email: ycheng@ucsf.edu 2/20/2015 - Introduction
Nova 600 NanoLab Dual beam Focused Ion Beam IITKanpur
 Nova 600 NanoLab Dual beam Focused Ion Beam system @ IITKanpur Dual Beam Nova 600 Nano Lab From FEI company (Dual Beam = SEM + FIB) SEM: The Electron Beam for SEM Field Emission Electron Gun Energy : 500
Nova 600 NanoLab Dual beam Focused Ion Beam system @ IITKanpur Dual Beam Nova 600 Nano Lab From FEI company (Dual Beam = SEM + FIB) SEM: The Electron Beam for SEM Field Emission Electron Gun Energy : 500
TEST BANK FOR PRESCOTTS MICROBIOLOGY 9TH EDITION BY WILLEY SHERWOOD WOOLVERTON
 TEST BANK FOR PRESCOTTS MICROBIOLOGY 9TH EDITION BY WILLEY SHERWOOD WOOLVERTON Link download full: https://testbankservice.com/download/test-bank-for-prescottsmicrobiology-9th-edition-by-willey-sherwood-woolverton/
TEST BANK FOR PRESCOTTS MICROBIOLOGY 9TH EDITION BY WILLEY SHERWOOD WOOLVERTON Link download full: https://testbankservice.com/download/test-bank-for-prescottsmicrobiology-9th-edition-by-willey-sherwood-woolverton/
