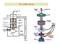
Basic structure of SEM
|
|
|
- Merry Jackson
- 6 years ago
- Views:
Transcription
1 Table of contents Basis structure of SEM SEM imaging modes Comparison of ordinary SEM and FESEM Electron behavior Electron matter interaction o Elastic interaction o Inelastic interaction o Interaction volume and its measurement Backscattered electrons and imaging Secondary electrons and imaging
2 Basic structure of SEM Various subsystem of SEM and their function is explained Two major component Electron column and Control console Electron Column Control Console Electron gun and 2 or more electron lenses to control the path of travelling electron in the vacuum column Computers, Monitors and knobs to control various imaging parameters
3 Basic structure of SEM Electron gun and lenses Electron gun generate electron and accelerated them to an energy level (0.1-30keV) Electron lenses focuses the beam to smaller spot size to produce sharp images
4 Basic structure of SEM Deflection system Deflection system consists of electromagnatic coils. Causes the beam to move in a series of discrete points in a rectangular region to scan complete specimen Excitation of coils also control the magnification In modern SEM, magnification is automatically compensated against working distance from sample
5 Basic structure of SEM Electron Detectors Detectors collect the signal generated from interaction of beam with specimen. Electronic of detectors convert the signal into digital images. Most often collected signal are SE (Secondary electrons) and BSE (Backscattered electrons) signal.
6 Basic structure of SEM Operator controls The very first control for generation of electron beam is Acceleration voltage and Emission current. Then condenser lens control amount of beam current available and minimum beam size Next is objective lens control to focus the beam at a smaller diameter spot. Finally viewing screen controls like brightness, contrast etc.
7 SEM imaging modes 4 parameters controls different imaging modes in SEM Electron probe size dia. (d p ) - Dia. of electron beam focused at the specimen Electron probe current (i p ) - Current impinging upon the specimen and generating various signals Electron probe convergence angle (α p ) - Half cone angle of electrons converging onto the specimen Electron beam accelerating voltage (V o ) kv
8 SEM imaging modes Mode I - Resolution Mode This mode is governed by the Electron probe size dia. (d p ) Resolution refers to the size of finest detail that can be observed To image the finest details d p must be comparable with or smaller than the feature itself. Resolution mode is only meaningful at high image magnifications (>10,000 X) Beam should contain sufficient current to exceed visibility threshold
9 SEM imaging modes Mode II High Current Mode This mode is governed by the Electron probe current (i p ) For the best image visibility and quality Large i p Unless a sufficient amount of current (required to overcome random noise) is there details cannot be seen even if the spot size is small enough. Noisy Perfect Blurred Smallest probe dia. 15 nm Minimum current 1 pa Smaller probe dia. 20 nm Adequate current - 5 pa Larger probe dia. 130 nm High current Mode 320 pa
10 SEM imaging modes Mode III Depth-of-Focus Mode This mode is governed by the probe convergence angle (α p ) For the best image quality α p as small as possible With low beam convergence angle, beam dia. changes only a little over a long vertical distance, so surface features at different heights will all appear to be in focus at the same time. α p = 15 mrad α p = 1 mrad
11 SEM imaging modes Mode III Low-Voltage Mode This mode is governed by the beam acceleration voltage At low accelerating voltage ( 5 kv), the beam interaction with the specimen is confined to the regions very close to the surface This provides an image rich in surface details compared to images at higher accelerating voltages. Sharp surface feature Poor surface feature Poor surface feature V 0 = 5 kev V 0 = 15 kev V 0 = 30 kev
12 Ordinary SEM Most of the older SEM uses Tungsten hairpin or thermionic Lanthanum Hexaboride (LaB 6 ) Electron sources These thermionic sources are resistively heated to very high temperature for the emission and release of electrons Advantage: Less expansive source, no special vacuum requirement Disadvantage: Low brightness, limited lifetime and large energy spread Tungsten hairpin (LaB 6 )
13 FE (Field emission) SEM Field emission is alternate method where potential barrier for electrons is lowered and narrowed by applying an negative potential at a very sharp wire tip (radius 100 nm) cathode. When the tip concentrated field reaches about 10 V/nm electrons emit FE sources provide enhanced performance, reliability and lifetime. Two class of field emitter (I) Cold field emitter (CFE) and (II) Schottky field emitters (SFE) and Thermal field emitters (TFE) CFE relies purely on the high applied field for electron emission independent of the tip temperature. TFE has the same property as CFE, but is operated at elevated temperature, helps to keep the tip clean, reducing noise and instability even in degraded vacuum condition. SFE guns are similar to other FE sources, but include a suppresser grid to eliminate unwanted thermionic emission from region outside of the tip. CFE SFE
14 Comparison of ordinary SEM and FESEM Comparison of various parameters for different electron sources at 20 kv Better performance of FE sources are evident from the comparison
15 Electrons Elementary particle carrying a negative charge Accelerated electron not only acts as a particle but acts as wave too. Rest mass of electron = m o = x kg Charge of electron = e = x C For an accelerating electron, wave length of moving electron (considering the relativistic effects) is given as, h ev 2m 0eV 1 2m0c 2 Where, h = Planck Constant, V= Applied Voltage c = Velocity of light in vacuum = x 10 8 m/s Above equation in useful in calculating the acceleration potential for a particular wave length of electron waves.
16 Electron wave Waves in electron beam can be coherent or incoherent. Coherent Waves of same wave lengths Incoherent Waves of different wave lengths Electron beam from a SEM electron source is a bundle of coherent electron waves, before hitting a specimen. After interacting with specimen, electron waves can form either coherent or incoherent beams
17 Electron matter Interaction Electron entering a material interacts as a negatively charged particle with electric fields of specimen atoms. The positive charge of the atom in strongly concentrated at the nucleus, whereas negatively charge electron atoms are much more dispersed in the a shell structure. Positively Charged nucleus Dispersed Negative electron charge These interactions are classified in to two different types. Elastic interactions: In this case no energy is transferred from electron to sample. As result electron leaving the sample still has the original energy. Inelastic interactions: The energy of the incident electron is transferred to the sample atoms. Hence, after the interaction electron energy is reduced.
18 Elastic Interaction Elastic interactions deflects the electron beam along new trajectory, causing them to spread laterally. A strong elastic scatter very near to the nucleus may result in beam electron leaving the specimen via back scattering, called Backscattered electrons (BSE). These electrons provide an important class of information for SEM imaging. Probability of elastic scattering Increases strongly with atomic number (as Z 2 ), as heavier atoms have much stronger positive charge at nucleus Decreases as electron energy increases (as 1/E 2 )
19 Inelastic Interaction Incident Electron - Secondary Electron Diffused Electron With the elastic scattering, beam electrons loose energy to specimen atoms in various ways. Such inelastic interactions gives rise to useful imaging signals such as Secondary Electrons (SE) and analytical signals such a X-rays. This energy loss happens gradually with distancee travelled inside the atom.
20 Interaction Volume Scattered Electron Beam Incident Electron Beam Interaction Volume Elastic and Inelastic scattering together distribute a single incident electron beam over a 3-Dimensional region inside the material. Region generated because of these phenomenon is called interaction volume. Size and shape of the interaction volume depends on the relative amount of elastic and inelastic scattering on electron beams. Direct measurement of interaction volume for materials with intermediate and high atomic number materials is not possible.
21 Interaction Volume - Simulation Monte Carlo simulation is highly useful tool to simulate the scattering of electron beam in a material. To construct a Monte Carlo electron trajectory simulation, the effects of elastic and inelastic scattering are calculated from appropriate models to determine scattering angles, distances between scattering sites ("step length"), and the rate of energy loss with distance traveled. Effect of different parameters like atomic number (Z), beam tilt angle, beam energy etc. can be studied using these simulations. An online version of this simulation can be found at following link
22 Electron Range A simple measure of interaction volume Interaction volume is a complex 3-dimensional region which depends on beam energy, material parameters, and tilt angle (complement of angle between the beam and surface plane). Despite this complexity, the interaction volume can be simply described with a single parameter called "electron range. The electron range is thus a simple value that is useful for rough comparisons and scaling various signal distributions. Within the electron range, the density of scattering events changes sharply with distance from the beam impact area. Analytical equations of the electron range are available in literatures.
23 Electron Range A simple measure of interaction volume Using the Monte Carlo depiction of the interaction volume, consider a hemisphere constructed with a radius whose origin is the entry point of the beam into the specimen and which contains a specified fraction of the electron trajectories, for example, 90%. Radius of this hemisphere is called electron range. R KO Electron Range (R KO ) A ( m) E 0.89 Z Where, A is the atomic weight (g/mole), Z is the atomic number, ρ is the density (g/cm 3 ) and E 0 is the beam energy (kev) For using this formula, the specimen is assumed to be flat, thick enough to be electron-opaque and of sufficiently large lateral extent that there are no edges or boundary within electron range. Tilt angle is 0 degree.
24 Backscattered Electrons - Imaging Backscattered electrons (BSE) are beam electrons whose trajectories have intercepted a surface usually, but not necessarily, the entrance surface and which thus escape the specimen. Backscattered electrons remove a significant amount of the total energy of the primary beam. Backscattering is quantified by the backscatter coefficient η which is defined as BSE ibse i B where η B is the number of beam electrons incident on the specimen and η BSE is the number of backscattered electrons (BSE). The backscatter coefficient can also be expressed in terms of currents, where i B refers to the beam current injected into the specimen and i BSE to the backscattered electron current passing out of the specimen. B
25 Backscattered Electrons - Imaging Atomic number dependence of BSE at E 0 =20keV Z Z Z Backscattering increases monotonically as Atomic number (Z) increases. Hence it forms the basis for atomic number contrast. The slope of η vs Z is initially steep but decreases with increase in Z. Hence atomic number contrast between adjacent pair of elements is strong at low atomic number and weak at high atomic number.
26 Backscattered Electrons - Imaging Beam energy dependence of BSE For beam energy 5-50 kev there is only a small change (<10%) in the backscatter coefficient (η) w.r.t. beam energy. At beam energies below 5 kev, η of light elements increases and those for heavy element decreases. ( Z, E) E m C where, m (0.9211/ Z ) and C ln Z (ln Z) (ln Z) 3
27 Backscattered Electrons - Imaging Title angle dependence of BSE Backscattering increases monotonically with tilt angle. This increase is the basis for topographic contrast in SEM, by which shape of object is recognized. At very high tilt angle, which correspond to grazing incidence, values of η tends towards unity. For different elements at high values of θ, η tend to converge for all elements
28 Backscattered Electrons - Imaging Angular distribution of BSE Normal beam incidence (0 tilt) Angular distribution of BSE is defined relative to normal of incident surface η at an angle φ is given by, ) cos ( n where, η n is the value measurement along normal vector n. Thus maximum number of BSE is emitted along the normal to surface As the detector is placed at an angle φ n away from surface normal, number of BSE decreases. At shallow angle just above the surface, there are virtually no BSE trajectories. Thus Angular distribution of BSE detector relative to surface specimen will have a strong influence on its collection efficiency.
29 Backscattered Electrons - Imaging Angular distribution of BSE Non-normal beam incidence For titled surface angular distribution becomes asymmetric. At higher tile angles (>45 ) distribution resembles a highly elongated ellipse, with the long axis at approx. same angle above the surface as the incident beam. Titling of specimen also results in asymmetry of interaction volume, this asymmetry also affects backscattering. For highly tilted surfaces, many of beam electrons skip off the first few atom layers and exit the specimen only after a few scattering events, so that the most are contained in a plane defined by the beam vector and the surface normal.
30 Backscattered Electrons - Imaging Energy distribution of BSE II I The energy distribution is a continuum extending from the incident beam energy to essentially no energy. Region I represents the high energy dump of BSEs that have lost less than 50% of E 0. For most target of intermediate and high atomic number, the majority of BSEs will be found in region I. Region II is the gradually decreasing tail of energy distribution represents the electron that travel progressively greater distances, loosing progressively more energy within the specimen prior to backscattering
31 Backscattered Electrons - Imaging Energy distribution of BSE BSE Energy distribution The energy distribution peak becomes more distinct for higher atomic number targets. Cumulative integrals calculated from the BSEs energy distributions show that BSEs retain at least 50% of the incident beam energy, with the fraction rising sharply for intermediate and high-atomicnumber targets. Low-atomic number scatterers produce far fewer high-energy BSEs.
32 Secondary Electrons - Imaging Secondary electrons (SE) are loosely bound outer shell electrons from the specimen atoms which receive sufficient kinetic energy during inelastic scattering of the beam electrons to be ejected from the atom and set into motion. Thus SE will propagate through the solid, and some will intersect the surface and escape. SE are defined purely on he basis of their KE, i.e. all emitted electron less than a particular energy (say 50eV) are considered as SE. The total SE coefficient δ is given by where η SE is the number of secondary electrons emitted from a sample bombarded by η B beam electrons and I designates the corresponding currents. SE B i i SE B
33 Secondary Electrons - Imaging δ generally rises as the beam energy is lowered, as shown in table below, from increase in energy from 5keV to 50keV, δ gradually decreases Secondary emission as a function of energy SE generated while the incident beam passes through specimen surface are designated as SE 1. The SE 1 signal is inherently is high-resolution signal that preserves both the lateral spatial resolution of the focused beam and the shallow sampling depth of the SE.
34 Secondary Electrons - Imaging SE are also generated by the BSE approaching the surface. These are designated as SE 2. SE 2 signal is a low-resolution signal. Compared to η, δ is relatively insensitive to atomic number. The emission on SE is very sensitive to the condition of the surface because the low KE severely limits the range of SEs. As the angle of tilt (θ) increases, δ increases.
35 Secondary Electrons - Imaging Upper crossover energy for various materials (Normal beam incidence) η + δ Total electron emission from sample (BSE + SE 2 ) Variation of η + δ with beam energy is shown. As energy decreases there comes a crossover point E 2, where η + δ reaches unity. With further decrease in energy, η + δ increases above unity, i.e. more electron are emitted from the surface then supplied by the beam. η + δ reaches a peak and then reduces on further decrease in energy, until the second crossover point E 1 is reached, and then continues to decreases. Values of E 1 are less than 1keV and is difficult to measure Values of E 2 can be as high as 5-20, as listed Knowledge E 2 point and η + δ behavior at low beam energy forms the basis for controlling charging in SEM imaging of insulators through careful choice of beam energy and specimen tilt.
36 Summary Basis structure of a SEM, construction and functioning of its important components are discussed. Different modes of imaging in the SEM is explained with the effect of different parameters on the quality of imaging in all modes. Difference between ordinary SEM and FESEM is discussed is brief With a short review of electron behavior and properties, interaction of electron beam with material inside the SEM is explained. Various imaging signals resulting from the interaction of beam and specimen are discussed. Effects of different signal parameters and their importance for best quality of imaging is explained in details
MT Electron microscopy Scanning electron microscopy and electron probe microanalysis
 MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
The illumination source: the electron beam
 The SEM Column The illumination source: the electron beam The probe of the electron microscope is an electron beam with very high and stable energy (10-100 kev) in order to get images with high resolution.
The SEM Column The illumination source: the electron beam The probe of the electron microscope is an electron beam with very high and stable energy (10-100 kev) in order to get images with high resolution.
Part II: Thin Film Characterization
 Part II: Thin Film Characterization General details of thin film characterization instruments 1. Introduction to Thin Film Characterization Techniques 2. Structural characterization: SEM, TEM, AFM, STM
Part II: Thin Film Characterization General details of thin film characterization instruments 1. Introduction to Thin Film Characterization Techniques 2. Structural characterization: SEM, TEM, AFM, STM
Scanning Electron Microscopy
 Scanning Electron Microscopy Field emitting tip Grid 2kV 100kV Anode ZEISS SUPRA Variable Pressure FESEM Dr Heath Bagshaw CMA bagshawh@tcd.ie Why use an SEM? Fig 1. Examples of features resolvable using
Scanning Electron Microscopy Field emitting tip Grid 2kV 100kV Anode ZEISS SUPRA Variable Pressure FESEM Dr Heath Bagshaw CMA bagshawh@tcd.ie Why use an SEM? Fig 1. Examples of features resolvable using
= 6 (1/ nm) So what is probability of finding electron tunneled into a barrier 3 ev high?
 STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
Electron beam scanning
 Electron beam scanning The Electron beam scanning operates through an electro-optical system which has the task of deflecting the beam Synchronously with cathode ray tube which create the image, beam moves
Electron beam scanning The Electron beam scanning operates through an electro-optical system which has the task of deflecting the beam Synchronously with cathode ray tube which create the image, beam moves
Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist
 12.141 Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist Massachusetts Institute of Technology Electron Microprobe Facility Department of Earth, Atmospheric and Planetary
12.141 Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist Massachusetts Institute of Technology Electron Microprobe Facility Department of Earth, Atmospheric and Planetary
Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist
 12.141 Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist Massachusetts Institute of Technology Electron Microprobe Facility Department of Earth, Atmospheric and Planetary
12.141 Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist Massachusetts Institute of Technology Electron Microprobe Facility Department of Earth, Atmospheric and Planetary
h p λ = mν Back to de Broglie and the electron as a wave you will learn more about this Equation in CHEM* 2060
 Back to de Broglie and the electron as a wave λ = mν h = h p you will learn more about this Equation in CHEM* 2060 We will soon see that the energies (speed for now if you like) of the electrons in the
Back to de Broglie and the electron as a wave λ = mν h = h p you will learn more about this Equation in CHEM* 2060 We will soon see that the energies (speed for now if you like) of the electrons in the
Part I Basics and Methods
 j1 Part I Basics and Methods In-situ Electron Microscopy: Applications in Physics, Chemistry and Materials Science, First Edition. Edited by Gerhard Dehm, James M. Howe, and Josef Zweck. Ó 2012 Wiley-VCH
j1 Part I Basics and Methods In-situ Electron Microscopy: Applications in Physics, Chemistry and Materials Science, First Edition. Edited by Gerhard Dehm, James M. Howe, and Josef Zweck. Ó 2012 Wiley-VCH
MSE 321 Structural Characterization
 Optical Microscope Plan Lenses In an "ideal" single-element lens system all planar wave fronts are focused to a point at distance f from the lens; therefore: Image near the optical axis will be in perfect
Optical Microscope Plan Lenses In an "ideal" single-element lens system all planar wave fronts are focused to a point at distance f from the lens; therefore: Image near the optical axis will be in perfect
Weak-Beam Dark-Field Technique
 Basic Idea recall bright-field contrast of dislocations: specimen close to Bragg condition, s î 0 Weak-Beam Dark-Field Technique near the dislocation core, some planes curved to s = 0 ) strong Bragg reflection
Basic Idea recall bright-field contrast of dislocations: specimen close to Bragg condition, s î 0 Weak-Beam Dark-Field Technique near the dislocation core, some planes curved to s = 0 ) strong Bragg reflection
Gaetano L Episcopo. Scanning Electron Microscopy Focus Ion Beam and. Pulsed Plasma Deposition
 Gaetano L Episcopo Scanning Electron Microscopy Focus Ion Beam and Pulsed Plasma Deposition Hystorical background Scientific discoveries 1897: J. Thomson discovers the electron. 1924: L. de Broglie propose
Gaetano L Episcopo Scanning Electron Microscopy Focus Ion Beam and Pulsed Plasma Deposition Hystorical background Scientific discoveries 1897: J. Thomson discovers the electron. 1924: L. de Broglie propose
object objective lens eyepiece lens
 Advancing Physics G495 June 2015 SET #1 ANSWERS Field and Particle Pictures Seeing with electrons The compound optical microscope Q1. Before attempting this question it may be helpful to review ray diagram
Advancing Physics G495 June 2015 SET #1 ANSWERS Field and Particle Pictures Seeing with electrons The compound optical microscope Q1. Before attempting this question it may be helpful to review ray diagram
Chapter 9. Electron mean free path Microscopy principles of SEM, TEM, LEEM
 Chapter 9 Electron mean free path Microscopy principles of SEM, TEM, LEEM 9.1 Electron Mean Free Path 9. Scanning Electron Microscopy (SEM) -SEM design; Secondary electron imaging; Backscattered electron
Chapter 9 Electron mean free path Microscopy principles of SEM, TEM, LEEM 9.1 Electron Mean Free Path 9. Scanning Electron Microscopy (SEM) -SEM design; Secondary electron imaging; Backscattered electron
Why microscopy?
 Electron Microscopy Why microscopy? http://www.cellsalive.com/howbig.htm 2 Microscopes are used as magnifying tools (although not exclusively as will see later on). The resolution of the human eye is limited
Electron Microscopy Why microscopy? http://www.cellsalive.com/howbig.htm 2 Microscopes are used as magnifying tools (although not exclusively as will see later on). The resolution of the human eye is limited
CBE Science of Engineering Materials. Scanning Electron Microscopy (SEM)
 CBE 30361 Science of Engineering Materials Scanning Electron Microscopy (SEM) Scale of Structure Organization Units: micrometer = 10-6 m = 1µm nanometer= 10-9 m = 1nm Angstrom = 10-10 m = 1Å A hair is
CBE 30361 Science of Engineering Materials Scanning Electron Microscopy (SEM) Scale of Structure Organization Units: micrometer = 10-6 m = 1µm nanometer= 10-9 m = 1nm Angstrom = 10-10 m = 1Å A hair is
Transmission Electron Microscopy
 L. Reimer H. Kohl Transmission Electron Microscopy Physics of Image Formation Fifth Edition el Springer Contents 1 Introduction... 1 1.1 Transmission Electron Microscopy... 1 1.1.1 Conventional Transmission
L. Reimer H. Kohl Transmission Electron Microscopy Physics of Image Formation Fifth Edition el Springer Contents 1 Introduction... 1 1.1 Transmission Electron Microscopy... 1 1.1.1 Conventional Transmission
5.8 Auger Electron Spectroscopy (AES)
 5.8 Auger Electron Spectroscopy (AES) 5.8.1 The Auger Process X-ray and high energy electron bombardment of atom can create core hole Core hole will eventually decay via either (i) photon emission (x-ray
5.8 Auger Electron Spectroscopy (AES) 5.8.1 The Auger Process X-ray and high energy electron bombardment of atom can create core hole Core hole will eventually decay via either (i) photon emission (x-ray
Modern Optical Spectroscopy
 Modern Optical Spectroscopy X-Ray Microanalysis Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Backscattered
Modern Optical Spectroscopy X-Ray Microanalysis Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Backscattered
Massachusetts Institute of Technology. Dr. Nilanjan Chatterjee
 Massachusetts Institute of Technology Dr. Nilanjan Chatterjee Electron Probe Micro-Analysis (EPMA) Imaging and micrometer-scale chemical compositional analysis of solids Signals produced in The Electron
Massachusetts Institute of Technology Dr. Nilanjan Chatterjee Electron Probe Micro-Analysis (EPMA) Imaging and micrometer-scale chemical compositional analysis of solids Signals produced in The Electron
Interactions with Matter
 Manetic Lenses Manetic fields can displace electrons Manetic field can be produced by passin an electrical current throuh coils of wire Manetic field strenth can be increased by usin a soft ferromanetic
Manetic Lenses Manetic fields can displace electrons Manetic field can be produced by passin an electrical current throuh coils of wire Manetic field strenth can be increased by usin a soft ferromanetic
M2 TP. Low-Energy Electron Diffraction (LEED)
 M2 TP Low-Energy Electron Diffraction (LEED) Guide for report preparation I. Introduction: Elastic scattering or diffraction of electrons is the standard technique in surface science for obtaining structural
M2 TP Low-Energy Electron Diffraction (LEED) Guide for report preparation I. Introduction: Elastic scattering or diffraction of electrons is the standard technique in surface science for obtaining structural
Electron probe microanalysis - Electron microprobe analysis EPMA (EMPA) What s EPMA all about? What can you learn?
 Electron probe microanalysis - Electron microprobe analysis EPMA (EMPA) What s EPMA all about? What can you learn? EPMA - what is it? Precise and accurate quantitative chemical analyses of micron-size
Electron probe microanalysis - Electron microprobe analysis EPMA (EMPA) What s EPMA all about? What can you learn? EPMA - what is it? Precise and accurate quantitative chemical analyses of micron-size
Electron Microprobe Analysis and Scanning Electron Microscopy
 Electron Microprobe Analysis and Scanning Electron Microscopy Electron microprobe analysis (EMPA) Analytical technique in which a beam of electrons is focused on a sample surface, producing X-rays from
Electron Microprobe Analysis and Scanning Electron Microscopy Electron microprobe analysis (EMPA) Analytical technique in which a beam of electrons is focused on a sample surface, producing X-rays from
Homework 2: Forces on Charged Particles
 Homework 2: Forces on Charged Particles 1. In the arrangement shown below, 2 C of positive charge is moved from plate S, which is at a potential of 250 V, to plate T, which is at a potential of 750 V.
Homework 2: Forces on Charged Particles 1. In the arrangement shown below, 2 C of positive charge is moved from plate S, which is at a potential of 250 V, to plate T, which is at a potential of 750 V.
Nova 600 NanoLab Dual beam Focused Ion Beam IITKanpur
 Nova 600 NanoLab Dual beam Focused Ion Beam system @ IITKanpur Dual Beam Nova 600 Nano Lab From FEI company (Dual Beam = SEM + FIB) SEM: The Electron Beam for SEM Field Emission Electron Gun Energy : 500
Nova 600 NanoLab Dual beam Focused Ion Beam system @ IITKanpur Dual Beam Nova 600 Nano Lab From FEI company (Dual Beam = SEM + FIB) SEM: The Electron Beam for SEM Field Emission Electron Gun Energy : 500
tip conducting surface
 PhysicsAndMathsTutor.com 1 1. The diagram shows the tip of a scanning tunnelling microscope (STM) above a conducting surface. The tip is at a potential of 1.0 V relative to the surface. If the tip is sufficiently
PhysicsAndMathsTutor.com 1 1. The diagram shows the tip of a scanning tunnelling microscope (STM) above a conducting surface. The tip is at a potential of 1.0 V relative to the surface. If the tip is sufficiently
SEM stands for Scanning Electron Microscopy. The earliest known work describing
 1. HISTORY ABOUT SEM SEM stands for Scanning Electron Microscopy. The earliest known work describing the concept of a Scanning Electron Microscope was by M. Knoll (1935) who, along with other pioneers
1. HISTORY ABOUT SEM SEM stands for Scanning Electron Microscopy. The earliest known work describing the concept of a Scanning Electron Microscope was by M. Knoll (1935) who, along with other pioneers
SEM Doctoral Course MS-636. April 11-13, 2016
 Thomas LaGrange, Ph.D. Faculty Lecturer and Senior Staff Scientist Electron Sources, Optics and Detectors SEM Doctoral Course MS-636 April 11-13, 2016 Summary Electron propagation is only possible through
Thomas LaGrange, Ph.D. Faculty Lecturer and Senior Staff Scientist Electron Sources, Optics and Detectors SEM Doctoral Course MS-636 April 11-13, 2016 Summary Electron propagation is only possible through
Invited Lecture. "Different Aspects of Electron Microscopy. Sardar Vallabhbhai National Institute of Technology, Surat. Deepak Rajput & S.K.
 Invited Lecture on "Different Aspects of Electron Microscopy at Sardar Vallabhbhai National Institute of Technology, Surat Deepak Rajput & S.K. Tiwary R&D and Product Development Essar Steel Limited Abstract
Invited Lecture on "Different Aspects of Electron Microscopy at Sardar Vallabhbhai National Institute of Technology, Surat Deepak Rajput & S.K. Tiwary R&D and Product Development Essar Steel Limited Abstract
Nano-Microscopy. Lecture 2. Scanning and Transmission Electron Microscopies: Principles. Pavel Zinin HIGP, University of Hawaii, Honolulu, USA
 GG 711: Advanced Techniques in Geophysics and Materials Science Nano-Microscopy. Lecture 2 Scanning and Transmission Electron Microscopies: Principles Pavel Zinin HIGP, University of Hawaii, Honolulu,
GG 711: Advanced Techniques in Geophysics and Materials Science Nano-Microscopy. Lecture 2 Scanning and Transmission Electron Microscopies: Principles Pavel Zinin HIGP, University of Hawaii, Honolulu,
The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado
 Experiment 9 1 Introduction The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado During the late nineteenth century, a great deal of evidence accumulated indicating that radiation
Experiment 9 1 Introduction The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado During the late nineteenth century, a great deal of evidence accumulated indicating that radiation
Analysis of Insulator Samples with AES
 Analysis of Insulator Samples with AES Kenichi Tsutsumi, Nobuyuki Ikeo, Akihiro Tanaka, and Toyohiko Tazawa SA Business Unit, JEL Ltd. Introduction Auger Electron Spectroscopy (AES) makes it possible to
Analysis of Insulator Samples with AES Kenichi Tsutsumi, Nobuyuki Ikeo, Akihiro Tanaka, and Toyohiko Tazawa SA Business Unit, JEL Ltd. Introduction Auger Electron Spectroscopy (AES) makes it possible to
Electron Microscopy I
 Characterization of Catalysts and Surfaces Characterization Techniques in Heterogeneous Catalysis Electron Microscopy I Introduction Properties of electrons Electron-matter interactions and their applications
Characterization of Catalysts and Surfaces Characterization Techniques in Heterogeneous Catalysis Electron Microscopy I Introduction Properties of electrons Electron-matter interactions and their applications
Structure of Surfaces
 Structure of Surfaces C Stepped surface Interference of two waves Bragg s law Path difference = AB+BC =2dsin ( =glancing angle) If, n =2dsin, constructive interference Ex) in a cubic lattice of unit cell
Structure of Surfaces C Stepped surface Interference of two waves Bragg s law Path difference = AB+BC =2dsin ( =glancing angle) If, n =2dsin, constructive interference Ex) in a cubic lattice of unit cell
Geology 777 Monte Carlo Exercise I
 Geology 777 Monte Carlo Exercise I Purpose The goal of this exercise is to get you to think like an electron... to start to think about where electrons from the stream of high energy electrons go when
Geology 777 Monte Carlo Exercise I Purpose The goal of this exercise is to get you to think like an electron... to start to think about where electrons from the stream of high energy electrons go when
Everhart-Thornley detector
 SEI Detector Everhart-Thornley detector Microscope chamber wall Faraday cage Scintillator Electrons in Light pipe Photomultiplier Electrical signal out Screen Quartz window +200 V +10 kv Always contains
SEI Detector Everhart-Thornley detector Microscope chamber wall Faraday cage Scintillator Electrons in Light pipe Photomultiplier Electrical signal out Screen Quartz window +200 V +10 kv Always contains
Auger Electron Spectroscopy (AES) Prof. Paul K. Chu
 Auger Electron Spectroscopy (AES) Prof. Paul K. Chu Auger Electron Spectroscopy Introduction Principles Instrumentation Qualitative analysis Quantitative analysis Depth profiling Mapping Examples The Auger
Auger Electron Spectroscopy (AES) Prof. Paul K. Chu Auger Electron Spectroscopy Introduction Principles Instrumentation Qualitative analysis Quantitative analysis Depth profiling Mapping Examples The Auger
X-RAY PRODUCTION. Prepared by:- EN KAMARUL AMIN BIN ABDULLAH
 X-RAY PRODUCTION Prepared by:- EN KAMARUL AMIN BIN ABDULLAH OBJECTIVES Discuss the process of x-ray being produced (conditions) Explain the principles of energy conversion in x-ray production (how energy
X-RAY PRODUCTION Prepared by:- EN KAMARUL AMIN BIN ABDULLAH OBJECTIVES Discuss the process of x-ray being produced (conditions) Explain the principles of energy conversion in x-ray production (how energy
PhysicsAndMathsTutor.com 1
 PhysicsAndMathsTutor.com 1 1. Millikan determined the charge on individual oil droplets using an arrangement as represented in the diagram. The plate voltage necessary to hold a charged droplet stationary
PhysicsAndMathsTutor.com 1 1. Millikan determined the charge on individual oil droplets using an arrangement as represented in the diagram. The plate voltage necessary to hold a charged droplet stationary
HOW TO APPROACH SCANNING ELECTRON MICROSCOPY AND ENERGY DISPERSIVE SPECTROSCOPY ANALYSIS. SCSAM Short Course Amir Avishai
 HOW TO APPROACH SCANNING ELECTRON MICROSCOPY AND ENERGY DISPERSIVE SPECTROSCOPY ANALYSIS SCSAM Short Course Amir Avishai RESEARCH QUESTIONS Sea Shell Cast Iron EDS+SE Fe Cr C Objective Ability to ask the
HOW TO APPROACH SCANNING ELECTRON MICROSCOPY AND ENERGY DISPERSIVE SPECTROSCOPY ANALYSIS SCSAM Short Course Amir Avishai RESEARCH QUESTIONS Sea Shell Cast Iron EDS+SE Fe Cr C Objective Ability to ask the
Questions/Answers. Chapter 1
 Questions/Answers Chapter 1 1.1 What are the advantages of the SEM over optical microscopy? Advantages: Higher resolution and greater depth of field and microchemical analysis Disadvantages: Expensive,
Questions/Answers Chapter 1 1.1 What are the advantages of the SEM over optical microscopy? Advantages: Higher resolution and greater depth of field and microchemical analysis Disadvantages: Expensive,
AP5301/ Name the major parts of an optical microscope and state their functions.
 Review Problems on Optical Microscopy AP5301/8301-2015 1. Name the major parts of an optical microscope and state their functions. 2. Compare the focal lengths of two glass converging lenses, one with
Review Problems on Optical Microscopy AP5301/8301-2015 1. Name the major parts of an optical microscope and state their functions. 2. Compare the focal lengths of two glass converging lenses, one with
Stellar Astrophysics: The Interaction of Light and Matter
 Stellar Astrophysics: The Interaction of Light and Matter The Photoelectric Effect Methods of electron emission Thermionic emission: Application of heat allows electrons to gain enough energy to escape
Stellar Astrophysics: The Interaction of Light and Matter The Photoelectric Effect Methods of electron emission Thermionic emission: Application of heat allows electrons to gain enough energy to escape
Interface (backside) & Extraction Lens
 Plasma Interface Interface (backside) & Extraction Lens Extraction Lens (-2000 volts) ION OPTICS Tip of the sampler cone is positioned to be in the region of maximum ionization Ions no longer under control
Plasma Interface Interface (backside) & Extraction Lens Extraction Lens (-2000 volts) ION OPTICS Tip of the sampler cone is positioned to be in the region of maximum ionization Ions no longer under control
Depth Distribution Functions of Secondary Electron Production and Emission
 Depth Distribution Functions of Secondary Electron Production and Emission Z.J. Ding*, Y.G. Li, R.G. Zeng, S.F. Mao, P. Zhang and Z.M. Zhang Hefei National Laboratory for Physical Sciences at Microscale
Depth Distribution Functions of Secondary Electron Production and Emission Z.J. Ding*, Y.G. Li, R.G. Zeng, S.F. Mao, P. Zhang and Z.M. Zhang Hefei National Laboratory for Physical Sciences at Microscale
EDS User School. Principles of Electron Beam Microanalysis
 EDS User School Principles of Electron Beam Microanalysis Outline 1.) Beam-specimen interactions 2.) EDS spectra: Origin of Bremsstrahlung and characteristic peaks 3.) Moseley s law 4.) Characteristic
EDS User School Principles of Electron Beam Microanalysis Outline 1.) Beam-specimen interactions 2.) EDS spectra: Origin of Bremsstrahlung and characteristic peaks 3.) Moseley s law 4.) Characteristic
SOLID STATE PHYSICS PHY F341. Dr. Manjuladevi.V Associate Professor Department of Physics BITS Pilani
 SOLID STATE PHYSICS PHY F341 Dr. Manjuladevi.V Associate Professor Department of Physics BITS Pilani 333031 manjula@bits-pilani.ac.in Characterization techniques SEM AFM STM BAM Outline What can we use
SOLID STATE PHYSICS PHY F341 Dr. Manjuladevi.V Associate Professor Department of Physics BITS Pilani 333031 manjula@bits-pilani.ac.in Characterization techniques SEM AFM STM BAM Outline What can we use
Measurement of Charge-to-Mass (e/m) Ratio for the Electron
 Measurement of Charge-to-Mass (e/m) Ratio for the Electron Experiment objectives: measure the ratio of the electron charge-to-mass ratio e/m by studying the electron trajectories in a uniform magnetic
Measurement of Charge-to-Mass (e/m) Ratio for the Electron Experiment objectives: measure the ratio of the electron charge-to-mass ratio e/m by studying the electron trajectories in a uniform magnetic
MSE 321 Structural Characterization
 Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
Simulation of the cathode surface damages in a HOPFED during ion bombardment
 Simulation of the cathode surface damages in a HOPFED during ion bombardment Hongping Zhao, Wei Lei, a Xiaobing Zhang, Xiaohua Li, and Qilong Wang Department of Electronic Engineering, Southeast University,
Simulation of the cathode surface damages in a HOPFED during ion bombardment Hongping Zhao, Wei Lei, a Xiaobing Zhang, Xiaohua Li, and Qilong Wang Department of Electronic Engineering, Southeast University,
Auger Electron Spectroscopy (AES)
 1. Introduction Auger Electron Spectroscopy (AES) Silvia Natividad, Gabriel Gonzalez and Arena Holguin Auger Electron Spectroscopy (Auger spectroscopy or AES) was developed in the late 1960's, deriving
1. Introduction Auger Electron Spectroscopy (AES) Silvia Natividad, Gabriel Gonzalez and Arena Holguin Auger Electron Spectroscopy (Auger spectroscopy or AES) was developed in the late 1960's, deriving
Imaging Methods: Scanning Force Microscopy (SFM / AFM)
 Imaging Methods: Scanning Force Microscopy (SFM / AFM) The atomic force microscope (AFM) probes the surface of a sample with a sharp tip, a couple of microns long and often less than 100 Å in diameter.
Imaging Methods: Scanning Force Microscopy (SFM / AFM) The atomic force microscope (AFM) probes the surface of a sample with a sharp tip, a couple of microns long and often less than 100 Å in diameter.
Chapter V: Interactions of neutrons with matter
 Chapter V: Interactions of neutrons with matter 1 Content of the chapter Introduction Interaction processes Interaction cross sections Moderation and neutrons path For more details see «Physique des Réacteurs
Chapter V: Interactions of neutrons with matter 1 Content of the chapter Introduction Interaction processes Interaction cross sections Moderation and neutrons path For more details see «Physique des Réacteurs
SEM Optics and Application to Current Research
 SEM Optics and Application to Current Research Azure Avery May 28, 2008 1 Introduction 1.1 History The optical microscope was invented in the early 17th century. Although revolutionary, the earliest microscopes
SEM Optics and Application to Current Research Azure Avery May 28, 2008 1 Introduction 1.1 History The optical microscope was invented in the early 17th century. Although revolutionary, the earliest microscopes
Chapter 10: Wave Properties of Particles
 Chapter 10: Wave Properties of Particles Particles such as electrons may demonstrate wave properties under certain conditions. The electron microscope uses these properties to produce magnified images
Chapter 10: Wave Properties of Particles Particles such as electrons may demonstrate wave properties under certain conditions. The electron microscope uses these properties to produce magnified images
High-Resolution. Transmission. Electron Microscopy
 Part 4 High-Resolution Transmission Electron Microscopy 186 Significance high-resolution transmission electron microscopy (HRTEM): resolve object details smaller than 1nm (10 9 m) image the interior of
Part 4 High-Resolution Transmission Electron Microscopy 186 Significance high-resolution transmission electron microscopy (HRTEM): resolve object details smaller than 1nm (10 9 m) image the interior of
Auger Electron Spectroscopy
 Auger Electron Spectroscopy Auger Electron Spectroscopy is an analytical technique that provides compositional information on the top few monolayers of material. Detect all elements above He Detection
Auger Electron Spectroscopy Auger Electron Spectroscopy is an analytical technique that provides compositional information on the top few monolayers of material. Detect all elements above He Detection
V 11: Electron Diffraction
 Martin-Luther-University Halle-Wittenberg Institute of Physics Advanced Practical Lab Course V 11: Electron Diffraction An electron beam conditioned by an electron optical system is diffracted by a polycrystalline,
Martin-Luther-University Halle-Wittenberg Institute of Physics Advanced Practical Lab Course V 11: Electron Diffraction An electron beam conditioned by an electron optical system is diffracted by a polycrystalline,
Practical course in scanning electron microscopy
 Practical course in scanning electron microscopy Fortgeschrittenen Praktikum an der Technischen Universität München Wintersemester 2017/2018 Table of contents 1. Introduction 3 2. Formation of an electron
Practical course in scanning electron microscopy Fortgeschrittenen Praktikum an der Technischen Universität München Wintersemester 2017/2018 Table of contents 1. Introduction 3 2. Formation of an electron
Scanning electron microscopy
 Scanning electron microscopy Fei Quanta Tabletop Hitachi Example: Tin soldier Pb M Sn L Secondary electrons Backscatter electrons EDS analysis Average composition Learning goals: Understanding the principle
Scanning electron microscopy Fei Quanta Tabletop Hitachi Example: Tin soldier Pb M Sn L Secondary electrons Backscatter electrons EDS analysis Average composition Learning goals: Understanding the principle
Analytical Methods for Materials
 Analytical Methods for Materials Lesson 21 Electron Microscopy and X-ray Spectroscopy Suggested Reading Leng, Chapter 3, pp. 83-126; Chapter 4, pp. 127-160; Chapter 6, pp. 191-219 P.J. Goodhew, J. Humphreys
Analytical Methods for Materials Lesson 21 Electron Microscopy and X-ray Spectroscopy Suggested Reading Leng, Chapter 3, pp. 83-126; Chapter 4, pp. 127-160; Chapter 6, pp. 191-219 P.J. Goodhew, J. Humphreys
MSE 321 Structural Characterization
 Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
CHARGED PARTICLE INTERACTIONS
 CHARGED PARTICLE INTERACTIONS Background Charged Particles Heavy charged particles Charged particles with Mass > m e α, proton, deuteron, heavy ion (e.g., C +, Fe + ), fission fragment, muon, etc. α is
CHARGED PARTICLE INTERACTIONS Background Charged Particles Heavy charged particles Charged particles with Mass > m e α, proton, deuteron, heavy ion (e.g., C +, Fe + ), fission fragment, muon, etc. α is
hν' Φ e - Gamma spectroscopy - Prelab questions 1. What characteristics distinguish x-rays from gamma rays? Is either more intrinsically dangerous?
 Gamma spectroscopy - Prelab questions 1. What characteristics distinguish x-rays from gamma rays? Is either more intrinsically dangerous? 2. Briefly discuss dead time in a detector. What factors are important
Gamma spectroscopy - Prelab questions 1. What characteristics distinguish x-rays from gamma rays? Is either more intrinsically dangerous? 2. Briefly discuss dead time in a detector. What factors are important
2 Give the compound nucleus resulting from 6-MeV protons bombarding a target of. my notes in the part 3 reading room or on the WEB.
 Lecture 15 Krane Enge Cohen Williams Reaction theories compound nucleus 11.10 13.7 13.1-3 direct reactions 11.11 13.11/12 ch 14 Admixed Wave functions residual interaction 5.1-4 Admixed Wave functions
Lecture 15 Krane Enge Cohen Williams Reaction theories compound nucleus 11.10 13.7 13.1-3 direct reactions 11.11 13.11/12 ch 14 Admixed Wave functions residual interaction 5.1-4 Admixed Wave functions
H2 Physics Set A Paper 3 H2 PHYSICS. Exam papers with worked solutions. (Selected from Top JC) SET A PAPER 3.
 H2 PHYSICS Exam papers with worked solutions (Selected from Top JC) SET A PAPER 3 Compiled by THE PHYSICS CAFE 1 P a g e Candidates answer on the Question Paper. No Additional Materials are required. READ
H2 PHYSICS Exam papers with worked solutions (Selected from Top JC) SET A PAPER 3 Compiled by THE PHYSICS CAFE 1 P a g e Candidates answer on the Question Paper. No Additional Materials are required. READ
Chapter 2 Problem Solutions
 Chapter Problem Solutions 1. If Planck's constant were smaller than it is, would quantum phenomena be more or less conspicuous than they are now? Planck s constant gives a measure of the energy at which
Chapter Problem Solutions 1. If Planck's constant were smaller than it is, would quantum phenomena be more or less conspicuous than they are now? Planck s constant gives a measure of the energy at which
Brown University PHYS 0060 Physics Department LAB B -190
 Physics Department LAB B -190 THE FORCE OF A MAGNETIC FIELD ON A MOVING ELECTRIC CHARGE DETERMINATION OF THE RATIO OF CHARGE TO MASS, e/m, FOR ELECTRONS References: H.D. Young, University Physics, Eleventh
Physics Department LAB B -190 THE FORCE OF A MAGNETIC FIELD ON A MOVING ELECTRIC CHARGE DETERMINATION OF THE RATIO OF CHARGE TO MASS, e/m, FOR ELECTRONS References: H.D. Young, University Physics, Eleventh
QUANTUM PHYSICS. Limitation: This law holds well only for the short wavelength and not for the longer wavelength. Raleigh Jean s Law:
 Black body: A perfect black body is one which absorbs all the radiation of heat falling on it and emits all the radiation when heated in an isothermal enclosure. The heat radiation emitted by the black
Black body: A perfect black body is one which absorbs all the radiation of heat falling on it and emits all the radiation when heated in an isothermal enclosure. The heat radiation emitted by the black
The Franck-Hertz Experiment David Ward The College of Charleston Phys 370/Experimental Physics Spring 1997
 The Franck-Hertz Experiment David Ward The College of Charleston Phys 370/Experimental Physics Spring 1997 Abstract One of the most important experiments supporting quantum theory is the Franck- Hertz
The Franck-Hertz Experiment David Ward The College of Charleston Phys 370/Experimental Physics Spring 1997 Abstract One of the most important experiments supporting quantum theory is the Franck- Hertz
Computations on Gabor lens having two different field distributions
 IOSR Journal of Applied Physics (IOSR-JAP) e-issn: 2278-4861.Volume 6, Issue 6 Ver. II (Nov.-Dec. 2014), PP 06-11 Computations on Gabor lens having two different field distributions Saif KamilShnain Department
IOSR Journal of Applied Physics (IOSR-JAP) e-issn: 2278-4861.Volume 6, Issue 6 Ver. II (Nov.-Dec. 2014), PP 06-11 Computations on Gabor lens having two different field distributions Saif KamilShnain Department
Crystal Structure and Electron Diffraction
 Crystal Structure and Electron Diffraction References: Kittel C.: Introduction to Solid State Physics, 8 th ed. Wiley 005 University of Michigan, PHY441-44 (Advanced Physics Laboratory Experiments, Electron
Crystal Structure and Electron Diffraction References: Kittel C.: Introduction to Solid State Physics, 8 th ed. Wiley 005 University of Michigan, PHY441-44 (Advanced Physics Laboratory Experiments, Electron
(1) CNR-Istituto LAMEL, Via Piero Gobetti n.101, Bologna, Italy (2) PASTIS-CNRSM SCpA, S.S.7 km 714.3, Brindisi, Italy
 Observations Microsc. Microanal. Microstruct. 499 Classification Physics Abstracts 61.16B Resolution of Semiconductor Multilayers using Backscattered Electrons in Scanning Electron Microscopy Donato Govoni(1),
Observations Microsc. Microanal. Microstruct. 499 Classification Physics Abstracts 61.16B Resolution of Semiconductor Multilayers using Backscattered Electrons in Scanning Electron Microscopy Donato Govoni(1),
Basic physics Questions
 Chapter1 Basic physics Questions S. Ilyas 1. Which of the following statements regarding protons are correct? a. They have a negative charge b. They are equal to the number of electrons in a non-ionized
Chapter1 Basic physics Questions S. Ilyas 1. Which of the following statements regarding protons are correct? a. They have a negative charge b. They are equal to the number of electrons in a non-ionized
Lecture 22 Ion Beam Techniques
 Lecture 22 Ion Beam Techniques Schroder: Chapter 11.3 1/44 Announcements Homework 6/6: Will be online on later today. Due Wednesday June 6th at 10:00am. I will return it at the final exam (14 th June).
Lecture 22 Ion Beam Techniques Schroder: Chapter 11.3 1/44 Announcements Homework 6/6: Will be online on later today. Due Wednesday June 6th at 10:00am. I will return it at the final exam (14 th June).
PhysicsAndMathsTutor.com 1
 PhysicsAndMathsTutor.com 1 Q1. (a) The diagram below shows a narrow beam of electrons produced by attracting electrons emitted from a filament wire to a metal plate which has a small hole in it. (i) Why
PhysicsAndMathsTutor.com 1 Q1. (a) The diagram below shows a narrow beam of electrons produced by attracting electrons emitted from a filament wire to a metal plate which has a small hole in it. (i) Why
MEASUREMENT OF THE CHARGE TO MASS RATIO (e/m e ) OF AN ELECTRON
 MEASUREMENT OF THE CHARGE TO MASS RATIO (e/m e ) OF AN ELECTRON Object This experiment will allow you to observe and understand the motion of a charged particle in a magnetic field and to measure the ratio
MEASUREMENT OF THE CHARGE TO MASS RATIO (e/m e ) OF AN ELECTRON Object This experiment will allow you to observe and understand the motion of a charged particle in a magnetic field and to measure the ratio
Neutron Interactions Part I. Rebecca M. Howell, Ph.D. Radiation Physics Y2.5321
 Neutron Interactions Part I Rebecca M. Howell, Ph.D. Radiation Physics rhowell@mdanderson.org Y2.5321 Why do we as Medical Physicists care about neutrons? Neutrons in Radiation Therapy Neutron Therapy
Neutron Interactions Part I Rebecca M. Howell, Ph.D. Radiation Physics rhowell@mdanderson.org Y2.5321 Why do we as Medical Physicists care about neutrons? Neutrons in Radiation Therapy Neutron Therapy
X-ray Spectroscopy. Danny Bennett and Maeve Madigan. October 12, 2015
 X-ray Spectroscopy Danny Bennett and Maeve Madigan October 12, 2015 Abstract Various X-ray spectra were obtained, and their properties were investigated. The characteristic peaks were identified for a
X-ray Spectroscopy Danny Bennett and Maeve Madigan October 12, 2015 Abstract Various X-ray spectra were obtained, and their properties were investigated. The characteristic peaks were identified for a
Lecture 5. X-ray Photoemission Spectroscopy (XPS)
 Lecture 5 X-ray Photoemission Spectroscopy (XPS) 5. Photoemission Spectroscopy (XPS) 5. Principles 5.2 Interpretation 5.3 Instrumentation 5.4 XPS vs UV Photoelectron Spectroscopy (UPS) 5.5 Auger Electron
Lecture 5 X-ray Photoemission Spectroscopy (XPS) 5. Photoemission Spectroscopy (XPS) 5. Principles 5.2 Interpretation 5.3 Instrumentation 5.4 XPS vs UV Photoelectron Spectroscopy (UPS) 5.5 Auger Electron
Physics Tutorial MF1 Magnetic Forces
 Physics Tutorial MF1 Magnetic Forces 1 Magnetic Forces The force F on a charge q moving with velocity v in a magnetic field is: F = qv The force F on a straight conductor of length L carrying a current
Physics Tutorial MF1 Magnetic Forces 1 Magnetic Forces The force F on a charge q moving with velocity v in a magnetic field is: F = qv The force F on a straight conductor of length L carrying a current
X-ray Interaction with Matter
 X-ray Interaction with Matter 10-526-197 Rhodes Module 2 Interaction with Matter kv & mas Peak kilovoltage (kvp) controls Quality, or penetrating power, Limited effects on quantity or number of photons
X-ray Interaction with Matter 10-526-197 Rhodes Module 2 Interaction with Matter kv & mas Peak kilovoltage (kvp) controls Quality, or penetrating power, Limited effects on quantity or number of photons
Electron Diffraction
 Electron iffraction o moving electrons display wave nature? To answer this question you will direct a beam of electrons through a thin layer of carbon and analyze the resulting pattern. Theory Louis de
Electron iffraction o moving electrons display wave nature? To answer this question you will direct a beam of electrons through a thin layer of carbon and analyze the resulting pattern. Theory Louis de
MT Electron microscopy Scanning electron microscopy and electron probe microanalysis
 MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
ICPMS Doherty Lecture 1
 ICPMS Doherty Lecture 1 Mass Spectrometry This material provides some background on how to measure isotope abundances by means of mass spectrometry. Mass spectrometers create and separate ionized atoms
ICPMS Doherty Lecture 1 Mass Spectrometry This material provides some background on how to measure isotope abundances by means of mass spectrometry. Mass spectrometers create and separate ionized atoms
Ecole Franco-Roumaine : Magnétisme des systèmes nanoscopiques et structures hybrides - Brasov, Modern Analytical Microscopic Tools
 1. Introduction Solid Surfaces Analysis Group, Institute of Physics, Chemnitz University of Technology, Germany 2. Limitations of Conventional Optical Microscopy 3. Electron Microscopies Transmission Electron
1. Introduction Solid Surfaces Analysis Group, Institute of Physics, Chemnitz University of Technology, Germany 2. Limitations of Conventional Optical Microscopy 3. Electron Microscopies Transmission Electron
Physics of Radiotherapy. Lecture II: Interaction of Ionizing Radiation With Matter
 Physics of Radiotherapy Lecture II: Interaction of Ionizing Radiation With Matter Charge Particle Interaction Energetic charged particles interact with matter by electrical forces and lose kinetic energy
Physics of Radiotherapy Lecture II: Interaction of Ionizing Radiation With Matter Charge Particle Interaction Energetic charged particles interact with matter by electrical forces and lose kinetic energy
Information from Every Angle
 pplication Note Information from Every ngle Directional SE Detector for Next-Level Imaging Zinc oxide nanorods with surficial palladium particles imaged at 500 V in high vacuum. dding palladium increases
pplication Note Information from Every ngle Directional SE Detector for Next-Level Imaging Zinc oxide nanorods with surficial palladium particles imaged at 500 V in high vacuum. dding palladium increases
Physics 100 PIXE F06
 Introduction: Ion Target Interaction Elastic Atomic Collisions Very low energies, typically below a few kev Surface composition and structure Ion Scattering spectrometry (ISS) Inelastic Atomic Collisions
Introduction: Ion Target Interaction Elastic Atomic Collisions Very low energies, typically below a few kev Surface composition and structure Ion Scattering spectrometry (ISS) Inelastic Atomic Collisions
Auger Electron Spectroscopy *
 OpenStax-CNX module: m43546 1 Auger Electron Spectroscopy * Amanda M. Goodman Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 1 Basic
OpenStax-CNX module: m43546 1 Auger Electron Spectroscopy * Amanda M. Goodman Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 1 Basic
Introduction to Electron Beam Lithography
 Introduction to Electron Beam Lithography Boštjan Berčič (bostjan.bercic@ijs.si), Jožef Štefan Institute, Jamova 39, 1000 Ljubljana, Slovenia 1. Introduction Electron Beam Lithography is a specialized
Introduction to Electron Beam Lithography Boštjan Berčič (bostjan.bercic@ijs.si), Jožef Štefan Institute, Jamova 39, 1000 Ljubljana, Slovenia 1. Introduction Electron Beam Lithography is a specialized
Dual Nature of Radiation and Matter-I
 Dual Nature of Radiation and Matter-I Physics Without Fear CONTENTS ELECTRON EMISSION PHOTOELECTRIC EFFECT; HERTZ S OBSERVATIONS HALLWACHS AND LENARD S OBSERVATIONS EXPERIMENTAL STUDY OF PHOTOELECTRIC
Dual Nature of Radiation and Matter-I Physics Without Fear CONTENTS ELECTRON EMISSION PHOTOELECTRIC EFFECT; HERTZ S OBSERVATIONS HALLWACHS AND LENARD S OBSERVATIONS EXPERIMENTAL STUDY OF PHOTOELECTRIC
Generation of X-Rays in the SEM specimen
 Generation of X-Rays in the SEM specimen The electron beam generates X-ray photons in the beam-specimen interaction volume beneath the specimen surface. Some X-ray photons emerging from the specimen have
Generation of X-Rays in the SEM specimen The electron beam generates X-ray photons in the beam-specimen interaction volume beneath the specimen surface. Some X-ray photons emerging from the specimen have
An Introduction to Diffraction and Scattering. School of Chemistry The University of Sydney
 An Introduction to Diffraction and Scattering Brendan J. Kennedy School of Chemistry The University of Sydney 1) Strong forces 2) Weak forces Types of Forces 3) Electromagnetic forces 4) Gravity Types
An Introduction to Diffraction and Scattering Brendan J. Kennedy School of Chemistry The University of Sydney 1) Strong forces 2) Weak forces Types of Forces 3) Electromagnetic forces 4) Gravity Types
I live in this atom, with my other electron brothers
 Hello, my name is Electron, John Electron. I am going to tell you how my work is in an electron microscope. I live in this atom, with my other electron brothers The filament crowns the column of the electron
Hello, my name is Electron, John Electron. I am going to tell you how my work is in an electron microscope. I live in this atom, with my other electron brothers The filament crowns the column of the electron
Revision Guide. Chapter 7 Quantum Behaviour
 Revision Guide Chapter 7 Quantum Behaviour Contents CONTENTS... 2 REVISION CHECKLIST... 3 REVISION NOTES... 4 QUANTUM BEHAVIOUR... 4 Random arrival of photons... 4 Photoelectric effect... 5 PHASE AN PHASORS...
Revision Guide Chapter 7 Quantum Behaviour Contents CONTENTS... 2 REVISION CHECKLIST... 3 REVISION NOTES... 4 QUANTUM BEHAVIOUR... 4 Random arrival of photons... 4 Photoelectric effect... 5 PHASE AN PHASORS...
Minicourse on Experimental techniques at the NSCL Fragment Separators
 Minicourse on Experimental techniques at the NSCL Fragment Separators Thomas Baumann National Superconducting Cyclotron Laboratory Michigan State University e-mail: baumann@nscl.msu.edu August 2, 2001
Minicourse on Experimental techniques at the NSCL Fragment Separators Thomas Baumann National Superconducting Cyclotron Laboratory Michigan State University e-mail: baumann@nscl.msu.edu August 2, 2001
How to distinguish EUV photons from out-of-band photons
 How to distinguish EUV photons from out-of-band photons Thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Physics Author: Student ID: Supervisors: 2 nd
How to distinguish EUV photons from out-of-band photons Thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Physics Author: Student ID: Supervisors: 2 nd
