Articles. On the H /2e Stoichiometry of the Respiratory Chain*
|
|
|
- Jemima Barker
- 5 years ago
- Views:
Transcription
1 2002 by The International Union of Biochemistry and Molecular Biology BIOCHEMISTRY AND MOLECULAR BIOLOGY EDUCATION Printed in U.S.A. Vol. 30, No. 6, pp , 2002 Articles On the H /2e Stoichiometry of the Respiratory Chain* Received for publication, February 12, 2002, and in revised form, July 1, 2002 Guadalupe Guerra, Federico Martínez, and Juan Pablo Pardo From the Departamento de Bioquímica, Facultad de Medicina, Universidad Nacional Autónoma de México, Apdo. Postal , México, D.F. and Departamento de Microbiología, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Apdo. Postal 4-870, México, D.F. To calculate the number of ATP molecules synthesized during oxidative phosphorylation, and to understand the yield and efficiency of this process, it is necessary to know the H /2e stoichiometry of the respiratory complexes, as well as the H /ATP ratio for the ATP synthase. However, in most biochemistry textbooks, this topic is treated poorly. For example, several books simply mention that mitochondrial respiratory complexes pump protons across the membrane, without any reference to the number of protons translocated per pair of electrons [1 4]. Stryer s textbook [5] mentions a 4H /2e, 2H /2e, 4H /2e stoichiometry for complex I, III, and IV, respectively, but most recent editions of the biochemistry textbooks of Nelson and Cox [6] and Voet et al. [7] cite a 4, 4, 2 stoichiometry [6, 7]. Several years ago Hinkle et al. [8] proposed a 4, 2, 4 stoichiometry for the effective pumping of protons across the membrane; interestingly, these values are identical to the number of charges moved across the inner mitochondrial membrane by respiratory complexes I, III, and IV. The present work describes several arguments in favor of the stoichiometry of 4, 2, 4 for complex I, III, and IV, respectively. Keywords: Mitochondrial respiratory chain, complex I, complex III, complex IV, H /2e stoichiometry, proton pumping, ATP synthesis. According to the chemiosmotic theory, mitochondrial complexes I, III, and IV couple the redox reaction to the translocation of protons across the membrane, generating a proton electrochemical gradient ( ) used for the synthesis of ATP. Both and the number of ATP molecules synthesized by the ATP synthase depend on the H /2e stoichiometry of the respiratory complexes. Four respiratory chain complexes are involved in the transfer of electrons from substrates to oxygen: complex I or NADH dehydrogenase [9], complex II or succinate dehydrogenase [10], complex III or cytochrome bc 1 [11], and complex IV or cytochrome oxidase [12]. Complexes I, III, and IV pump protons across the membrane whereas the synthesis of ATP occurs in complex V or ATP synthase [13, 14]. VECTORIAL AND CHEMICAL PROTONS To calculate the stoichiometric relationship between the H pumped and the transport of electrons it is important to recognize that, in addition to the vectorial protons (pumped protons), the chemical reaction contains scalar or chemical protons. This situation can be illustrated by detergent-solubilized complex III, catalyzing the reaction, QH 2 2cyt c ox 3 Q 2cyt c red 2H in the absence of a membrane. It can be seen that two * This work was supported in part by DGAPA Grant IN To whom correspondence should be addressed. Tel.: ; Fax: ; pardov@bq.unam.mx. This paper is available on line at scalar protons are released into the medium during the chemical reaction. As we will discuss later, the presence of these chemical protons leads to paradoxical conclusions. THE MITOCHONDRIAL COMPLEXES, THEIR REDOX REACTIONS, AND THE PUMPING OF PROTONS Complex I pumps 4H across the membrane per pair of electrons, resulting in the movement of four positive charges perpendicular to the plane of the membrane. In addition, the enzyme transfers 2H and 2e (two hydrogen atoms) to ubiquinone [6, 15]. The following equation describes the reaction catalyzed by complex I: NADH Q 5H N 3 NAD QH 2 4H P where N and P indicate the negative and positive side of the membrane. Complex III catalyzes the reduction of two molecules of cytochrome c by ubiquinol, as shown in the following equation: QH 2 2H N 2cyt c ox 3 Q 4H P 2cyt c red Two vectorial H are pumped from the matrix to the cytosolic side of the inner mitochondrial membrane, and two scalar protons are released in the intermembrane space of the mitochondrion [6, 16]. In addition, two positive charges migrate from the negative (N) 1 side to the positive (P) side of the membrane, corresponding to the movement of two electrons from the P side to the N side of the membrane [16]. It is worth noting that the number of H translocated
2 364 BAMBED, Vol. 30, No. 6, pp , 2002 FIG. 1.Scheme of the Na /K pump and complexes III and IV. For illustration purposes, only the source and destination of the protons involved in the reactions catalyzed by complexes III and IV are considered, such that the quinol and oxygen are included but not cytochrome c. In the case of mitochondrial complexes, the interior of the vesicle corresponds to the intermembrane space. from the matrix to the cytosol (2H ) is different from the number of protons that appears in the intermembrane space (4H ). Complex IV receives the electrons from four molecules of cytochrome c and reduces molecular oxygen, giving two molecules of H 2 O, as shown in the following equation: 4cyt c red 8H N O 2 3 2H 2 O 4cyt c ox 4H P Four vectorial H, corresponding to four positive charges migrating from the N to the P side of the membrane, are pumped into the intermembrane space [6, 17]. In addition, four electrons flow in the opposite direction to combine with molecular oxygen and with 4H coming from the mitochondrial matrix. This movement of electrons is equivalent to the flow of four positive charges from the N side to the P side of the membrane. If the movements of protons and electrons are summed up, a total of eight positive charges migrates toward the intermembrane space, four electrons are transferred to molecular oxygen, four protons are pumped across the membrane, and four scalar or chemical protons are consumed in the mitochondrial matrix during the formation of two molecules of H 2 O [17]. THE PROBLEM FOUND IN THE DEFINITION OF THE H /2e STOICHIOMETRY IN THE MITOCHONDRIAL RESPIRATORY COMPLEXES Helpful insights into the definition of the H /2e stoichiometry in the respiratory complexes can come from a consideration of the Na /K ATPase (Fig. 1). This pump catalyzes the following reaction: 3Na out 2K in ATP 3 3Na in 2K out ADP P i The classic stoichiometry of 3Na /ATP and 2K /ATP can be obtained by kinetic experiments in proteoliposomes, measuring the initial rates of ATP hydrolysis and Na and K transport [18]. It is important to mention that the same stoichiometry should be obtained if the measuring device is placed inside or outside the vesicle, tracking either the appearance of Na inside the vesicle or the disappearance of Na from the extravesicular space (Fig. 1). FIG. 2.Transport of H in mitochondria. The curved arrows represent the pathway followed by electrons in the respiratory chain, beginning with NADH and ending with H 2 O. These arrows have no mechanistic meaning, and they should not be associated with the actual movement of electrons across the membrane, a process occurring during the catalytic cycle of the complexes involved in the pumping of protons. The thick arrows show the pumping of H across the membrane catalyzed by the respiratory complexes and the ATP synthase. The number of positive charges translocated across the membrane per pair of electrons is shown in parentheses. In this way, the effective stoichiometry of H transport per pair of electrons is as follows: four for complex I, two for complex III, and four for complex IV. Three protons are required for the synthesis of one ATP in the mitochondrial matrix, and one proton is required for the transport of ATP, ADP, and P i (this figure was taken and modified from Ref. 8). This last situation does not occur in the case of complexes III and IV. The flux of positive charges from the N side of the inner membrane to the P side is 2 /2e and 4 /2e for complex III and IV, respectively [16, 17], and the same values are obtained whether the flow of positive charges is studied from the inside (mitochondrial matrix) or from the outside (intermembrane space). By contrast, when changes in ph are measured in the intermembrane space, complex III appears to transport 4H /2e, and complex IV appears to transport only 2H /2e, despite the fact that the redox change for the reaction catalyzed by complex IV is the larger one [6]. If now the ph change in the mitochondrial matrix is measured, a different result is obtained, 2H /2e for complex III and 4H /2e for complex IV. In other words, the stoichiometry of proton pumping depends on the position of the measuring device. The problem in the definition of the H /2e disappears when the number of positive charges flowing through each complex is determined. The values obtained (2 /2e for complex III and 4 /2e for complex IV) are the same whether we measure the flow from the mitochondrial ma-
3 TABLE I Thermodynamic characteristics of the reactions catalyzed by complexes I, III, and IV The actual G of the reaction was obtained with the following equation: G G RTln([Products]/[Reactants]), where R, T and G are the universal gas constant, the absolute temperature, and the change in standard free energy of the reaction, respectively. In addition, the value of G was obtained from E, using the well known relationship G mf E, where F is the Faraday constant, m the number of electrons participating in the reaction, and E the standard redox potential. n describes the number of vectorial protons pumped per pair of electrons. The G of the reactions catalyzed by complex I, III, and IV are as follows; 16.8, 8.1, and 27.7 kcal/mol [6]. The actual G were calculated using mitochondrial NADH/NAD [27] and Q red /Q ox [28] ratios of 1, and intracellular p O2 of kpa [29], and assuming a value of 1 for the cyt c red /cyt c ox ratio. Complex Reaction catalyzed E G n V kcal/mol H /2e I NADH Q H NAD QH III QH 2 2cyt c ox Q 2H 2cyt c red IV 2cyt c red 2H 1/2 O 2 H 2 O 2cyt c ox trix or the intermembrane space. For this reason, it seems logical to define the effective proton pumping in terms of the positive charges moving across the membrane, which in turn is related to the flow of protons and/or electrons across each complex [16, 17]. THE EFFECTIVE H /2e STOICHIOMETRY FOR COMPLEX I, III, AND IV IS 4, 2, AND 4, RESPECTIVELY As mentioned, the calculation of proton pumping stoichiometry for complex III and IV is made difficult by the presence of scalar protons. However, this problem can be circumvented when the positive charge moved by each complex is related to the total number of protons disappearing from the matrix and appearing in the intermembrane space. Fig. 2 shows that 10 positive charges flow from the N side of the internal mitochondrial membrane to the P side, whereas 10 protons disappear from the matrix and appear in the intermembrane space. Of the 10 positive charges flowing through the membrane, four are translocated by the NADH dehydrogenase, two by cytochrome bc 1 and four by cytochrome oxidase. On the other hand, of the 10 protons, complex I pumps four, whereas complex III and complex IV mechanistically pump two H apiece. The other two H that appear on the cytosolic side of the inner mitochondrial membrane are scalar, and they come from the reaction catalyzed by complex III. Also, the 2H that disappear from the mitochondrial matrix correspond to the chemical-scalar protons used in the formation of water by cytochrome oxidase. Next, we can correlate the flow of positive charges and the flow of protons across the membrane. One can see that four of the positive charges are related to the pumping of 4H by complex I (4H /2e ). The two vectorial H that complex III pumps are assigned to the two positive charges that the same complex moves across the membrane (2H /2e ). Finally, the two scalar H that disappear on the N side of the membrane and appear on the P side are assigned to cytochrome oxidase; in this way, the movement of positive charges (4 ) in cytochrome oxidase is the same as the movement of protons (4H ), giving a stoichiometry of 4H /2e for this complex. In summary, the effective H /2e stoichiometries for complex I, III, and IV are 4, 2, and 4, respectively. As pointed out before, these values also correspond to the number of charges moved across the inner mitochondrial membrane by respiratory complexes I, III, and IV (Fig. 2). THERMODYNAMIC CONSIDERATIONS There are several thermodynamic considerations that support a higher pumping of protons in complex I and IV. The change in redox potential for the reaction catalyzed by complex I is V, for that catalyzed by complex III it is V, and for the one catalyzed by complex IV it is V [6]. From these values one can calculate a standard free energy change of 16.8, 8.1, and 27.7 kcal/ mol, respectively. In addition, it has been reported that the membrane potential across the inner mitochondrial membrane is around 150 mv with a ph ranging from 0.5 to 1 unit [19, 20], resulting in a proton electrochemical gradient ( ) of 4.1 to 4.8 kcal/mol. It is well known that the pumping of protons across the membrane is limited by the Gof the reaction and the. If we assume a value of 4.4 kcal/mol for then we can calculate the maximum number of protons transported by each complex with the following equation [21]: n G/ equilibrium where n is the number of vectorial protons pumped by the respiratory complex, the proton electrochemical gradient, and G the change of free energy of the reaction. Table I summarizes the results of this analysis and shows that complex I can pump up to four protons, complex III can pump not more than two protons, and complex IV can pump up to six protons. These results agree with the stoichiometries of 4, 2, and 4 reported in 1991 by Hinkle et al. [8], where the term effective pumping of protons was recommend. According to this point of view, complex III and IV are working as if they were pumping two and four protons across the membrane, respectively. THE STOICHIOMETRY OF THE ATP SYNTHASE With regard to the ATP synthase, it has been shown that this enzyme can work with different stoichiometries, depending on the source of the enzyme and the environment in which the organism was grown [22]. Most biochemistry textbooks state that three H are required for the synthesis of one molecule of ATP in mitochondria. However, the structure of the F 0 sector of the ATP synthase [23], as well as experiments carried out with the ATP synthase of chloroplasts and cyanobacteria [24], suggest that the stoichiometry of this complex can be as high as 4H /ATP. Assuming a 3H /ATP stoichiometry for mitochondrial ATP synthase and the flow of one proton for the transport
4 366 BAMBED, Vol. 30, No. 6, pp , 2002 of ATP, ADP, and P i across the inner membrane, the synthesis of one ATP molecule consumes a total of 4H. When NADH is oxidized by the respiratory chain, 10 protons are pumped into the intermembrane space, and the maximum number of ATP molecules that can be synthesized is 2.5 (10/4 2.5). Also, for each FADH 2 that enters the respiratory chain, six protons are pumped, and the number of ATP molecules synthesized is 1.5 (6/4 1.5). When the stoichiometry of the ATP synthase is increased to 4H /ATP then the yield in ATP synthesis diminishes to 2 (10/5) and 1.2 (6/5) with NADH and FADH 2, respectively. THE OXIDATION OF GLUCOSE AND THE PRODUCTION OF ATP Fixing the stoichiometry of the ATP synthase at 3H / ATP, the complete oxidation of 1 mol of glucose will produce 29 mol of ATP. This value contrasts with the value of 36 mol of ATP described in many biochemistry textbooks. The previous calculation was based on the production of 8 mol of NADH and 2 mol of FADH 2 in the mitochondrial matrix, 2 mol of cytosolic NADH whose electrons are transferred to the respiratory chain via the glycerophosphate shuttle, 2 mol of cytosolic ATP produced by glycolysis, and 2 mol of GTP produced by the succinyl-coa synthetase in the mitochondrial matrix. Later, nucleoside diphosphokinase catalyzes the transformation of GTP into ATP. Here, it is important to consider that two protons are used to transport two molecules of ATP from the matrix to the cytosol, such that the total amount of ATP synthesized during the oxidative phosphorylation diminishes in 0.5. In addition, the 2H (0.5 ATP) used to transport two pyruvate molecules were taken into consideration. When cytosolic NADH is oxidized by the malate-aspartate shuttle then 4.5 mol of ATP are synthesized, because the glutamate/aspartate antiporter is coupled to the flow of one proton per glutamate [25] in such a way that the global yield would increase to 31 mol of ATP per mol of glucose oxidized. SUBSTRATE-LEVEL PHOSPHORYLATION AND OXIDATIVE PHOSPHORYLATION: WHICH ONE IS MORE EFFICIENT? To a first approximation, one may think that the efficiency of ATP synthesis is higher in mitochondrial oxidative phosphorylation than in glycolysis. However, this is not the case. To calculate the efficiency of the synthesis of ATP in each process, the following information is needed: 1) G o for the transformation of glucose into lactate and for the oxidation of pyruvate into CO 2 and H 2 O, and 2) G o of hydrolysis of ATP. The ratio of these two values determines the efficiency of the reaction under standard conditions. With regard to the first point, the transformation of 1 mol of glucose into 2 mol of lactate releases 47 kcal of free energy, whereas the complete oxidation of 2 mol of pyruvate into 6 mol of CO 2 and 4 mol of water releases 566 kcal. If we assume that 7.5 kcal of free energy are needed for the synthesis of 1 mol of ATP, then the efficiency of substrate-level phosphorylation is 32% (2 7.5/47) whereas the efficiency of oxidative phosphorylation is 33% (25 7.5/566), assuming 4H are used for the synthesis of ATP. This value is similar to the efficiency of glycolysis, but it is an upper limit, because up to 25% of the proton electrochemical gradient is dissipated by diffusion of H across the membrane [26], decreasing the oxidative phosphorylation efficiency. CONCLUSION Although the two stoichiometries (4,4,2 versus 4,2,4) result in the same number of protons translocated across the inner mitochondrial membrane (10 H ), it is important to define the capacity of each complex for effective proton pumping. The analysis of chemical reactions catalyzed by each complex, as well as thermodynamic considerations, suggest a stoichiometry of 4H /2e for complexes I and IV and 2H /2e for complex III. This means that complexes III and IV behave physiologically and thermodynamically as if they were pumping two and four protons, respectively. Furthermore, these values match the number of charges moved across the inner mitochondrial membrane by respiratory complexes III and IV. Acknowledgments We thank Dr. Carolyn Slayman from Yale University for critical reading of the manuscript. REFERENCES [1] J. Baynes, M. H. Dominiczak (1999) Medical Biochemistry, Mosby, Basildon, United Kingdom, pp [2] T. M. Devlin, Ed. (1997) Textbook of Biochemistry with Clinical Correlations, 4th ed., John Wiley & Sons, Inc., New York, pp [3] R. K Murray, D. K. Granner, P. A. Mayes, V. W. Rodwell (1999) Harper s Biochemistry, 25th ed., McGraw-Hill, Inc., New York, pp [4] C. K. Mathews, K. E. van Holde, K. G. Ahern (2000) Biochemistry, 3rd ed. Addison Wesley Longman, Inc., San Francisco, pp [5] L. Stryer (1995) Biochemistry, 4th ed., W. H. Freeman, New York, pp [6] D. L. Nelson, M. M. Cox (2000) Lehninger Principles of Biochemistry, 3rd ed., Worth Publishers, New York, pp [7] D. Voet, J. Voet, C. W. Pratt (1999) Fundamentals of Biochemistry, John Wiley & Sons, Inc., New York, pp [8] P. C. Hinkle, M. A. Kumar, A. Resetar, D. L. Harris (1991) Mechanistic stoichiometry of mitochondrial oxidative phosphorylation, Biochemistry 30, [9] N. Grigorieff (1999) Structure of the respiratory NADH:ubiquinone oxidoreductase, Curr. Opin. Struct. Biol. 9, [10] C. Hägerhäll (1997) Succinate:quinone oxidoreductases. Variations on a conserved theme, Biochim. Biophys. Acta 1320, [11] S. Iwata, J. W. Lee, K. Okada, J. K. Lee, M. Iwata, B. Rasmussen, T. A. Link, S. Ramaswamy, B. K. Jap (1998) Complete structure of the II-subunit bovine mitochondrial cytochrome bc 1 complex, Science 281, [12] T. Tsukihara, H. Aoyama, E. Yamashita, T. Tomizaki, H. Yamaguchi, K. Shinzawa-Itoh, R. Nakashima, R. Yaono, S. Yoshikawa (1996) The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 Å, Science 272, [13] J. Abrahams, A. Leslie, R. Lutter, J. Walker (1994) Structure at 2.8 Å resolution of F 1 -ATPase from bovine heart mitochondria, Nature 370, [14] V. K. Rastogi, M. E. Girvin (1999) Structural changes linked to proton translocation by subunit c of the ATP synthase, Nature 402, [15] P. L. Dutton, C. C. Moser, V. D. Sled, F. Daldal, T. Ohnishi (1998) A reductant-induced oxidation mechanism for Complex I, Biochim. Biophys. Acta 1364, [16] B. L. Trumpower (1990) The protonmotive Q cycle, J. Biol. Chem. 265, [17] R. B. Gennis (1998) How does cytochrome oxidase pump protons? Proc. Natl. Acad. Sci. U. S. A. 95, [18] P. J. Garrahan, I. M. Glynn (1967) The stoichiometry of the sodium pump, J. Physiol. 192, [19] S. Soboll (1995) Regulation of energy metabolism in liver, J. Bioenerg. Biomembr. 27, [20] J. J. Lemasters, E. Chacon, H. Ohata, I. S. Harper, A. Nieminen, S. A. Tesfai, B. Herman (1995) Measurement of electrical potential, ph, and free calcium ion concentration in mitochondria of living cells by laser scanning confocal microscopy, Methods Enzymol. 260, [21] W. A. Cramer, D. B. Knaff (1991) Energy Transduction in Biological Membranes. A Textbook of Bioenergetics, Springer-Verlag, New York, pp [22] J. J. Tomashek, W. S. Brusilow (2000) Stoichiometry of the energy
5 367 coupling by proton-translocating ATPases: a history of variability, J. Bioenerg. Biomembr. 32, [23] R. H. Fillingame, W. Jiang, O. Y. Dmitriev (2000) Coupling H transport to rotary catalysis in F-type ATP synthases: structure and organization of the transmembrane rotary motor, J. Exp. Biol. 203, [24] H. S. van Walraven, H. Strotmann, O. Schwarz, B. Rumberg (1996) The H /ATP coupling ratio of the ATP synthase from thiol-modulated chloroplasts and two cyanobacterial strains is four, FEBS Lett. 379, [25] K. F. LaNoue, A. J. Meijer, A. Brouwer (1974) Evidence for electrogenic aspartate transport in rat liver mitochondria, Arch. Biochem. Biophys. 161, [26] D. F. S. Rolfe, M. D. Brand (1996) Contribution of mitochondrial proton leak to skeletal muscle respiration and to standard metabolic rate, Am. J. Physiol. 271, C1380 C1389. [27] R. L. White, B. A. Wittenberg (2000) Mitochondrial NAD(P)H, ADP, oxidative phosphorylation, and contraction in isolated heart cells, Am. J. Physiol. Heart Circ. Physiol. 279, H1849 H1857. [28] B. M. Jorgensen, H. N. Rasmussen, U. F. Rasmussen (1985) Ubiquinone reduction pattern in pigeon heart mitochondria, Biochem. J. 229, [29] E. Gnaiger, R. Steinlechner-Maran, G. Méndez, T. Eberl, R. Margreiter (1995) Control of mitochondrial and cellular respiration by oxygen, J. Bioenerg. Biomembr. 27,
Electron Transport Chain (Respiratory Chain) - exercise - Vladimíra Kvasnicová
 Electron Transport Chain (Respiratory Chain) - exercise - Vladimíra Kvasnicová Respiratory chain (RCH) a) is found in all cells b) is located in a mitochondrion c) includes enzymes integrated in the inner
Electron Transport Chain (Respiratory Chain) - exercise - Vladimíra Kvasnicová Respiratory chain (RCH) a) is found in all cells b) is located in a mitochondrion c) includes enzymes integrated in the inner
20. Electron Transport and Oxidative Phosphorylation
 20. Electron Transport and Oxidative Phosphorylation 20.1 What Role Does Electron Transport Play in Metabolism? Electron transport - Role of oxygen in metabolism as final acceptor of electrons - In inner
20. Electron Transport and Oxidative Phosphorylation 20.1 What Role Does Electron Transport Play in Metabolism? Electron transport - Role of oxygen in metabolism as final acceptor of electrons - In inner
The Proton Motive Force. Overview. Compartmentalization 11/6/2015. Chapter 21 Stryer Short Course. ATP synthesis Shuttles
 The Proton Motive Force Chapter 21 Stryer Short Course Redox reactions Electron transport chain Proton gradient Overview ATP synthesis Shuttles Analogy: How does burning coal put flour in the grocery store?
The Proton Motive Force Chapter 21 Stryer Short Course Redox reactions Electron transport chain Proton gradient Overview ATP synthesis Shuttles Analogy: How does burning coal put flour in the grocery store?
MitoSeminar II: Some calculations in bioenergetics
 MitoSeminar II: Some calculations in bioenergetics MUDr. Jan Pláteník, PhD. Ústav lékařské biochemie 1.LF UK Helpful comments of Prof. MUDr. Jiří Kraml, DrSc., are acknowledged. 1 Respiratory chain and
MitoSeminar II: Some calculations in bioenergetics MUDr. Jan Pláteník, PhD. Ústav lékařské biochemie 1.LF UK Helpful comments of Prof. MUDr. Jiří Kraml, DrSc., are acknowledged. 1 Respiratory chain and
Cellular Respiration Stage 4: Electron Transport Chain
 Cellular Respiration Stage 4: Electron Transport Chain 2006-2007 Cellular respiration What s the point? The point is to make ATP! ATP 2006-2007 ATP accounting so far Glycolysis 2 ATP Kreb s cycle 2 ATP
Cellular Respiration Stage 4: Electron Transport Chain 2006-2007 Cellular respiration What s the point? The point is to make ATP! ATP 2006-2007 ATP accounting so far Glycolysis 2 ATP Kreb s cycle 2 ATP
Energy Transformation. Metabolism = total chemical reactions in cells.
 Energy Transformation Metabolism = total chemical reactions in cells. metabole = change Metabolism is concerned with managing the material and energy resources of the cell -Catabolism -Anabolism -Catabolism
Energy Transformation Metabolism = total chemical reactions in cells. metabole = change Metabolism is concerned with managing the material and energy resources of the cell -Catabolism -Anabolism -Catabolism
2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of October
 Name: Class: _ Date: _ 2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of 19-23 October Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which
Name: Class: _ Date: _ 2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of 19-23 October Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which
All organisms require a constant expenditure of energy to maintain the living state - "LIFE".
 CELLULAR RESPIRATION All organisms require a constant expenditure of energy to maintain the living state - "LIFE". Where does the energy come from and how is it made available for life? With rare exception,
CELLULAR RESPIRATION All organisms require a constant expenditure of energy to maintain the living state - "LIFE". Where does the energy come from and how is it made available for life? With rare exception,
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy
 Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
TCA Cycle. Voet Biochemistry 3e John Wiley & Sons, Inc.
 TCA Cycle Voet Biochemistry 3e Voet Biochemistry 3e The Electron Transport System (ETS) and Oxidative Phosphorylation (OxPhos) We have seen that glycolysis, the linking step, and TCA generate a large number
TCA Cycle Voet Biochemistry 3e Voet Biochemistry 3e The Electron Transport System (ETS) and Oxidative Phosphorylation (OxPhos) We have seen that glycolysis, the linking step, and TCA generate a large number
Lectures by Kathleen Fitzpatrick
 Chapter 10 Chemotrophic Energy Metabolism: Aerobic Respiration Lectures by Kathleen Fitzpatrick Simon Fraser University Figure 10-1 Figure 10-6 Conversion of pyruvate The conversion of pyruvate to acetyl
Chapter 10 Chemotrophic Energy Metabolism: Aerobic Respiration Lectures by Kathleen Fitzpatrick Simon Fraser University Figure 10-1 Figure 10-6 Conversion of pyruvate The conversion of pyruvate to acetyl
Cellular Respiration: Harvesting Chemical Energy. 9.1 Catabolic pathways yield energy by oxidizing organic fuels
 Cellular Respiration: Harvesting Chemical Energy 9.1 Catabolic pathways yield energy by oxidizing organic fuels 9.2 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate 9.3 The citric acid
Cellular Respiration: Harvesting Chemical Energy 9.1 Catabolic pathways yield energy by oxidizing organic fuels 9.2 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate 9.3 The citric acid
Metabolism. Fermentation vs. Respiration. End products of fermentations are waste products and not fully.
 Outline: Metabolism Part I: Fermentations Part II: Respiration Part III: Metabolic Diversity Learning objectives are: Learn about respiratory metabolism, ATP generation by respiration linked (oxidative)
Outline: Metabolism Part I: Fermentations Part II: Respiration Part III: Metabolic Diversity Learning objectives are: Learn about respiratory metabolism, ATP generation by respiration linked (oxidative)
Biophysics 490M Project
 Biophysics 490M Project Dan Han Department of Biochemistry Structure Exploration of aa 3 -type Cytochrome c Oxidase from Rhodobacter sphaeroides I. Introduction: All organisms need energy to live. They
Biophysics 490M Project Dan Han Department of Biochemistry Structure Exploration of aa 3 -type Cytochrome c Oxidase from Rhodobacter sphaeroides I. Introduction: All organisms need energy to live. They
Pathways that Harvest and Store Chemical Energy
 6 Pathways that Harvest and Store Chemical Energy Energy is stored in chemical bonds and can be released and transformed by metabolic pathways. Chemical energy available to do work is termed free energy
6 Pathways that Harvest and Store Chemical Energy Energy is stored in chemical bonds and can be released and transformed by metabolic pathways. Chemical energy available to do work is termed free energy
Biochemical bases for energy transformations. Biochemical bases for energy transformations. Nutrition 202 Animal Energetics R. D.
 Biochemical bases for energy transformations Biochemical bases for energy transformations Nutrition 202 Animal Energetics R. D. Sainz Lecture 02 Energy originally from radiant sun energy Captured in chemical
Biochemical bases for energy transformations Biochemical bases for energy transformations Nutrition 202 Animal Energetics R. D. Sainz Lecture 02 Energy originally from radiant sun energy Captured in chemical
Edexcel (B) Biology A-level
 Edexcel (B) Biology A-level Topic 5: Energy for Biological Processes Notes Aerobic Respiration Aerobic respiration as splitting of the respiratory substrate, to release carbon dioxide as a waste product
Edexcel (B) Biology A-level Topic 5: Energy for Biological Processes Notes Aerobic Respiration Aerobic respiration as splitting of the respiratory substrate, to release carbon dioxide as a waste product
Cellular respiration ATP. Cellular Respiration Stage 4: Electron Transport Chain. AP Biology. The point is to make ATP! What s the point?
 ellular respiration ellular Respiration Stage 4: Electron Transport hain What s the point? The point is to make! accounting so far Glycolysis 2 Kreb s cycle 2 Life takes a lot of energy to run, need to
ellular respiration ellular Respiration Stage 4: Electron Transport hain What s the point? The point is to make! accounting so far Glycolysis 2 Kreb s cycle 2 Life takes a lot of energy to run, need to
ETC/CHEMIOSIS. By: Leslie, Kelsey, Morgan
 ETC/CHEMIOSIS By: Leslie, Kelsey, Morgan WHY THIS IS IMPORTANT House Clip SO3E7 The Son of a Coma Guy- Time: 32:00 Patient was visiting his father who was in a vegetative state for 10 years, and his only
ETC/CHEMIOSIS By: Leslie, Kelsey, Morgan WHY THIS IS IMPORTANT House Clip SO3E7 The Son of a Coma Guy- Time: 32:00 Patient was visiting his father who was in a vegetative state for 10 years, and his only
AP Bio-Ms.Bell Unit#3 Cellular Energies Name
 AP Bio-Ms.Bell Unit#3 Cellular Energies Name 1. Base your answer to the following question on the image below. 7. Base your answer to the following question on Which of the following choices correctly
AP Bio-Ms.Bell Unit#3 Cellular Energies Name 1. Base your answer to the following question on the image below. 7. Base your answer to the following question on Which of the following choices correctly
ATP. Division Ave. High School AP Biology. Cellular Respiration Stage 4: Electron Transport Chain. Cellular respiration. The point is to make ATP!
 ellular Respiration Stage 4: Electron Transport hain 2006-2007 ellular respiration What s the point? The point is to make! 2006-2007 1 accounting so far Glycolysis 2 Kreb s cycle 2 Life takes a lot of
ellular Respiration Stage 4: Electron Transport hain 2006-2007 ellular respiration What s the point? The point is to make! 2006-2007 1 accounting so far Glycolysis 2 Kreb s cycle 2 Life takes a lot of
Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts)
 1 Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts) Objectives 1: Describe the overall action of the Krebs cycle in generating ATP, NADH and FADH 2 from acetyl-coa 2: Understand the generation
1 Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts) Objectives 1: Describe the overall action of the Krebs cycle in generating ATP, NADH and FADH 2 from acetyl-coa 2: Understand the generation
Bio102 Problems Photosynthesis
 Bio102 Problems Photosynthesis 1. Why is it advantageous for chloroplasts to have a very large (in surface area) thylakoid membrane contained within the inner membrane? A. This limits the amount of stroma
Bio102 Problems Photosynthesis 1. Why is it advantageous for chloroplasts to have a very large (in surface area) thylakoid membrane contained within the inner membrane? A. This limits the amount of stroma
BIOCHEMISTRY. František Vácha. JKU, Linz.
 BIOCHEMISTRY František Vácha http://www.prf.jcu.cz/~vacha/ JKU, Linz Recommended reading: D.L. Nelson, M.M. Cox Lehninger Principles of Biochemistry D.J. Voet, J.G. Voet, C.W. Pratt Principles of Biochemistry
BIOCHEMISTRY František Vácha http://www.prf.jcu.cz/~vacha/ JKU, Linz Recommended reading: D.L. Nelson, M.M. Cox Lehninger Principles of Biochemistry D.J. Voet, J.G. Voet, C.W. Pratt Principles of Biochemistry
Metabolism Review. A. Top 10
 A. Top 10 Metabolism Review 1. Energy production through chemiosmosis a. pumping of H+ ions onto one side of a membrane through protein pumps in an Electron Transport Chain (ETC) b. flow of H+ ions across
A. Top 10 Metabolism Review 1. Energy production through chemiosmosis a. pumping of H+ ions onto one side of a membrane through protein pumps in an Electron Transport Chain (ETC) b. flow of H+ ions across
C. Incorrect! Catalysts themselves are not altered or consumed during the reaction.
 Human Physiology - Problem Drill 04: Enzymes and Energy Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as needed, (3) Pick the answer,
Human Physiology - Problem Drill 04: Enzymes and Energy Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as needed, (3) Pick the answer,
Lecture 10. Proton Gradient-dependent ATP Synthesis. Oxidative. Photo-Phosphorylation
 Lecture 10 Proton Gradient-dependent ATP Synthesis Oxidative Phosphorylation Photo-Phosphorylation Model of the Electron Transport Chain (ETC) Glycerol-3-P Shuttle Outer Mitochondrial Membrane G3P DHAP
Lecture 10 Proton Gradient-dependent ATP Synthesis Oxidative Phosphorylation Photo-Phosphorylation Model of the Electron Transport Chain (ETC) Glycerol-3-P Shuttle Outer Mitochondrial Membrane G3P DHAP
Change to Office Hours this Friday and next Monday. Tomorrow (Abel): 8:30 10:30 am. Monday (Katrina): Cancelled (05/04)
 Change to Office Hours this Friday and next Monday Tomorrow (Abel): 8:30 10:30 am Monday (Katrina): Cancelled (05/04) Lecture 10 Proton Gradient-dependent ATP Synthesis Oxidative Phosphorylation Photo-Phosphorylation
Change to Office Hours this Friday and next Monday Tomorrow (Abel): 8:30 10:30 am Monday (Katrina): Cancelled (05/04) Lecture 10 Proton Gradient-dependent ATP Synthesis Oxidative Phosphorylation Photo-Phosphorylation
BCH 4054 Spring 2001 Chapter 21 Lecture Notes
 BCH 4054 Spring 2001 Chapter 21 Lecture Notes 1 Chapter 21 Electron Transport and Oxidative Phosphorylation 2 Overview Oxidation of NADH and CoQH 2 produced in TCA cycle by O 2 is very exergonic. Some
BCH 4054 Spring 2001 Chapter 21 Lecture Notes 1 Chapter 21 Electron Transport and Oxidative Phosphorylation 2 Overview Oxidation of NADH and CoQH 2 produced in TCA cycle by O 2 is very exergonic. Some
number Done by Corrected by Doctor Nafeth Abu Tarboush
 number 8 Done by Ali Yaghi Corrected by Mamoon Mohamad Alqtamin Doctor Nafeth Abu Tarboush 0 P a g e Oxidative phosphorylation Oxidative phosphorylation has 3 major aspects: 1. It involves flow of electrons
number 8 Done by Ali Yaghi Corrected by Mamoon Mohamad Alqtamin Doctor Nafeth Abu Tarboush 0 P a g e Oxidative phosphorylation Oxidative phosphorylation has 3 major aspects: 1. It involves flow of electrons
Giving you the energy you need!
 Giving you the energy you need! Use your dominant hand Open and close the pin (with your thumb and forefinger) as many times as you can for 20 seconds while holding the other fingers straight out! Repeat
Giving you the energy you need! Use your dominant hand Open and close the pin (with your thumb and forefinger) as many times as you can for 20 seconds while holding the other fingers straight out! Repeat
AP Biology Cellular Respiration
 AP Biology Cellular Respiration The bonds between H and C represents a shared pair of electrons These are high-energy electrons This represents chemical potential energy Hydro-carbons posses a lot of chemical
AP Biology Cellular Respiration The bonds between H and C represents a shared pair of electrons These are high-energy electrons This represents chemical potential energy Hydro-carbons posses a lot of chemical
Cellular Respiration. Mitochondria Rule! Mr. Kurt Kristensen
 Cellular Respiration Mitochondria Rule! Mr. Kurt Kristensen Harvard Biovisions Mitochondria Summer Session Week 1: Cellular Respiration Students should. 1) Understand the locations, and functions of the
Cellular Respiration Mitochondria Rule! Mr. Kurt Kristensen Harvard Biovisions Mitochondria Summer Session Week 1: Cellular Respiration Students should. 1) Understand the locations, and functions of the
Bis2A 5.6: Oxidative Phosphorylation and the Electron Transport Chain *
 OpenStax-CNX module: m59707 1 Bis2A 5.6: Oxidative Phosphorylation and the Electron Transport Chain * The BIS2A Team This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
OpenStax-CNX module: m59707 1 Bis2A 5.6: Oxidative Phosphorylation and the Electron Transport Chain * The BIS2A Team This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
Transporters and Membrane Motors Nov 15, 2007
 BtuB OM vitamin B12 transporter F O F 1 ATP synthase Human multiple drug resistance transporter P-glycoprotein Transporters and Membrane Motors Nov 15, 2007 Transport and membrane motors Concentrations
BtuB OM vitamin B12 transporter F O F 1 ATP synthase Human multiple drug resistance transporter P-glycoprotein Transporters and Membrane Motors Nov 15, 2007 Transport and membrane motors Concentrations
Biological Chemistry and Metabolic Pathways
 Biological Chemistry and Metabolic Pathways 1. Reaction a. Thermodynamics b. Kinetics 2. Enzyme a. Structure and Function b. Regulation of Activity c. Kinetics d. Inhibition 3. Metabolic Pathways a. REDOX
Biological Chemistry and Metabolic Pathways 1. Reaction a. Thermodynamics b. Kinetics 2. Enzyme a. Structure and Function b. Regulation of Activity c. Kinetics d. Inhibition 3. Metabolic Pathways a. REDOX
Be sure to understand:
 Learning Targets & Focus Questions for Unit 6: Bioenergetics Chapter 8: Thermodynamics Chapter 9: Cell Resp Focus Q Ch. 10: Photosynthesis Chapter 8 (141-150) 1. I can explain how living systems adhere
Learning Targets & Focus Questions for Unit 6: Bioenergetics Chapter 8: Thermodynamics Chapter 9: Cell Resp Focus Q Ch. 10: Photosynthesis Chapter 8 (141-150) 1. I can explain how living systems adhere
The Mitochondrion. Definition Structure, ultrastructure Functions
 The Mitochondrion Definition Structure, ultrastructure Functions Organelle definition Etymology of the name Carl Benda (1903): (mitos) thread; (khondrion) granule. Light microscopy identification First
The Mitochondrion Definition Structure, ultrastructure Functions Organelle definition Etymology of the name Carl Benda (1903): (mitos) thread; (khondrion) granule. Light microscopy identification First
Forms of stored energy in cells
 Forms of stored energy in cells Electrochemical gradients Covalent bonds (ATP) Reducing power (NADH) During photosynthesis, respiration and glycolysis these forms of energy are converted from one to another
Forms of stored energy in cells Electrochemical gradients Covalent bonds (ATP) Reducing power (NADH) During photosynthesis, respiration and glycolysis these forms of energy are converted from one to another
I. Flow of Energy in Living Things II. Laws of Thermodynamics & Free Energy III. Activation Energy IV. Enzymes V. Reaction Coupling VI.
 Chapter 6 Energy & Metabolism I. Flow of Energy in Living Things II. Laws of Thermodynamics & Free Energy III. Activation Energy IV. Enzymes V. Reaction Coupling VI. Metabolism I. Flow of Energy in Living
Chapter 6 Energy & Metabolism I. Flow of Energy in Living Things II. Laws of Thermodynamics & Free Energy III. Activation Energy IV. Enzymes V. Reaction Coupling VI. Metabolism I. Flow of Energy in Living
A thermodynamic principle for the coupled bioenergetic processes of ATP synthesis
 Pure & Appl. Chem., Vol. 7, No. 3, pp. 639-644, 1998. Printed in Great Britain. (8 1998 IUPAC A thermodynamic principle for the coupled bioenergetic processes of ATP synthesis Sunil Nath Department of
Pure & Appl. Chem., Vol. 7, No. 3, pp. 639-644, 1998. Printed in Great Britain. (8 1998 IUPAC A thermodynamic principle for the coupled bioenergetic processes of ATP synthesis Sunil Nath Department of
Photosynthesis and cellular respirations
 The Introduction of Biology Defining of life Basic chemistry, the chemistry of organic molecules Classification of living things History of cells and Cells structures and functions Photosynthesis and cellular
The Introduction of Biology Defining of life Basic chemistry, the chemistry of organic molecules Classification of living things History of cells and Cells structures and functions Photosynthesis and cellular
State state describe
 Warm-Up State the products of the light-dependent reaction of photosynthesis, state which product has chemical energy, and describe how that product is made. KREBS ETC FADH 2 Glucose Pyruvate H 2 O NADH
Warm-Up State the products of the light-dependent reaction of photosynthesis, state which product has chemical energy, and describe how that product is made. KREBS ETC FADH 2 Glucose Pyruvate H 2 O NADH
Chapter 2 Energy in Biology Demand and Use
 Chapter 2 Energy in Biology Demand and Use A coupled energy source is a prerequisite of sustained dynamics in thermodynamically open systems. Abstract From the point of view of energy management in biological
Chapter 2 Energy in Biology Demand and Use A coupled energy source is a prerequisite of sustained dynamics in thermodynamically open systems. Abstract From the point of view of energy management in biological
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 AP Exam Chapters 9 and 10; Photosynthesis and Respiration Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Carbon dioxide (CO2) is released
AP Exam Chapters 9 and 10; Photosynthesis and Respiration Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Carbon dioxide (CO2) is released
ACTIVE TRANSPORT AND GLUCOSE TRANSPORT. (Chapter 14 and 15, pp and pp )
 ACTIVE TRANSPORT AND GLUCOSE TRANSPORT (Chapter 14 and 15, pp 140-143 and pp 146-151) Overview Active transport is the movement of molecules across a cell membrane in the direction against their concentration
ACTIVE TRANSPORT AND GLUCOSE TRANSPORT (Chapter 14 and 15, pp 140-143 and pp 146-151) Overview Active transport is the movement of molecules across a cell membrane in the direction against their concentration
Center for Academic Services & Advising
 March 2, 2017 Biology I CSI Worksheet 6 1. List the four components of cellular respiration, where it occurs in the cell, and list major products consumed and produced in each step. i. Hint: Think about
March 2, 2017 Biology I CSI Worksheet 6 1. List the four components of cellular respiration, where it occurs in the cell, and list major products consumed and produced in each step. i. Hint: Think about
Cellular Respiration. The mechanism of creating cellular energy. Thursday, 11 October, 12
 Cellular Respiration The mechanism of creating cellular energy What do we know?? What do we know?? Grade 5 - Food --> Energy What do we know?? Grade 5 - Food --> Energy Grade 10 - glu. + O2 --> CO2 + H20
Cellular Respiration The mechanism of creating cellular energy What do we know?? What do we know?? Grade 5 - Food --> Energy What do we know?? Grade 5 - Food --> Energy Grade 10 - glu. + O2 --> CO2 + H20
BIOLOGY 111. CHAPTER 7: Vital Harvest: Deriving Energy From Food
 BIOLOGY 111 CHAPTER 7: Vital Harvest: Deriving Energy From Food Deriving Energy from Food: What is the best carbohydrate source (for energy) in our food? Glucose! Where is the energy stored in glucose?
BIOLOGY 111 CHAPTER 7: Vital Harvest: Deriving Energy From Food Deriving Energy from Food: What is the best carbohydrate source (for energy) in our food? Glucose! Where is the energy stored in glucose?
WHAT REGULATES RESPIRATION IN MITOCHONDRIA?
 Vol. 39, No. 2, May 1996 BIOCHEMISTRY and MOLECULAR BIOLOGY INTERNATIONAL Pages 415-4 ] 9 WHAT REGULATES RESPIRATION IN MITOCHONDRIA? Bernard Korzeniewski Institute of Molecular Biology, Jagiellonian University,
Vol. 39, No. 2, May 1996 BIOCHEMISTRY and MOLECULAR BIOLOGY INTERNATIONAL Pages 415-4 ] 9 WHAT REGULATES RESPIRATION IN MITOCHONDRIA? Bernard Korzeniewski Institute of Molecular Biology, Jagiellonian University,
Aerobic Cellular Respiration
 Aerobic Cellular Respiration Under aerobic conditions (oxygen gas is available), cells will undergo aerobic cellular respiration. The end products of aerobic cellular respiration are carbon dioxide gas,
Aerobic Cellular Respiration Under aerobic conditions (oxygen gas is available), cells will undergo aerobic cellular respiration. The end products of aerobic cellular respiration are carbon dioxide gas,
Energy and Cells. Appendix 1. The two primary energy transformations in plants are photosynthesis and respiration.
 Energy and Cells Appendix 1 Energy transformations play a key role in all physical and chemical processes that occur in plants. Energy by itself is insufficient to drive plant growth and development. Enzymes
Energy and Cells Appendix 1 Energy transformations play a key role in all physical and chemical processes that occur in plants. Energy by itself is insufficient to drive plant growth and development. Enzymes
Photosynthesis and Cellular Respiration Practice Test Name
 Photosynthesis and Cellular Respiration Practice Test Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which H+ has just passed through the
Photosynthesis and Cellular Respiration Practice Test Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which H+ has just passed through the
Kuntarti. PDF Created with deskpdf PDF Writer - Trial ::
 Kuntarti Principles of Bioenergetics Definitions the study of energy transformations in living organism (Albert L Lehninger) the study of the balance between energy intake in the form of food and energy
Kuntarti Principles of Bioenergetics Definitions the study of energy transformations in living organism (Albert L Lehninger) the study of the balance between energy intake in the form of food and energy
Outline. Metabolism: Energy and Enzymes. Forms of Energy. Chapter 6
 Metabolism: Energy and Enzymes Chapter 6 Forms of Energy Outline Laws of Thermodynamics Metabolic Reactions ATP Metabolic Pathways Energy of Activation Enzymes Photosynthesis Cellular Respiration 1 2 Forms
Metabolism: Energy and Enzymes Chapter 6 Forms of Energy Outline Laws of Thermodynamics Metabolic Reactions ATP Metabolic Pathways Energy of Activation Enzymes Photosynthesis Cellular Respiration 1 2 Forms
2.A.2- Capture and Storage of Free Energy
 2.A.2- Capture and Storage of Free Energy Big Idea 2: Biological systems utilize free energy and molecular building blocks to grow, to reproduce, and to maintain dynamic homeostasis. EU 2.A- Growth, reproduction
2.A.2- Capture and Storage of Free Energy Big Idea 2: Biological systems utilize free energy and molecular building blocks to grow, to reproduce, and to maintain dynamic homeostasis. EU 2.A- Growth, reproduction
Chapter Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow,
 Chapter 6 6.1 Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow, repair, reproduce, etc. 2. Kinetic energy is energy of motion;
Chapter 6 6.1 Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow, repair, reproduce, etc. 2. Kinetic energy is energy of motion;
Photosynthesis. Chapter 10. Active Lecture Questions for use with Classroom Response Systems Biology, Seventh Edition Neil Campbell and Jane Reece
 Chapter 10 Photosynthesis Active Lecture Questions for use with Classroom Response Systems Biology, Seventh Edition Neil Campbell and Jane Reece Edited by William Wischusen, Louisiana State University
Chapter 10 Photosynthesis Active Lecture Questions for use with Classroom Response Systems Biology, Seventh Edition Neil Campbell and Jane Reece Edited by William Wischusen, Louisiana State University
Photosynthesis and Cellular Respiration Note-taking Guide
 Photosynthesis and Cellular Respiration Note-taking Guide Preview to Photosynthesis glucose, reactions, light-dependent, Calvin cycle, thylakoid, photosystem II, oxygen, light-harvesting, two, chloroplasts,
Photosynthesis and Cellular Respiration Note-taking Guide Preview to Photosynthesis glucose, reactions, light-dependent, Calvin cycle, thylakoid, photosystem II, oxygen, light-harvesting, two, chloroplasts,
ΔG o' = ηf ΔΕ o' = (#e ( V mol) ΔΕ acceptor
 Reading: Sec. 19.1 Electron-Transfer Reactions in Mitochondria (listed subsections only) 19.1.1 Electrons are Funneled to Universal Electron Acceptors p. 692/709 19.1.2 Electrons Pass through a Series
Reading: Sec. 19.1 Electron-Transfer Reactions in Mitochondria (listed subsections only) 19.1.1 Electrons are Funneled to Universal Electron Acceptors p. 692/709 19.1.2 Electrons Pass through a Series
AHL Topic 8 IB Biology Miss Werba
 CELL RESPIRATION & PHOTOSYNTHESIS AHL Topic 8 IB Biology Miss Werba TOPIC 8 CELL RESPIRATION & PHOTOSYNTHESIS 8.1 CELL RESPIRATION 1. STATE that oxidation involves the loss of electrons from an element,
CELL RESPIRATION & PHOTOSYNTHESIS AHL Topic 8 IB Biology Miss Werba TOPIC 8 CELL RESPIRATION & PHOTOSYNTHESIS 8.1 CELL RESPIRATION 1. STATE that oxidation involves the loss of electrons from an element,
METABOLISM CHAPTER 04 BIO 211: ANATOMY & PHYSIOLOGY I. Dr. Lawrence G. Altman Some illustrations are courtesy of McGraw-Hill.
 BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. CELLULAR METABOLISM Dr. Lawrence G. Altman
BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. CELLULAR METABOLISM Dr. Lawrence G. Altman
CELL METABOLISM OVERVIEW Keep the big picture in mind as we discuss the particulars!
 BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 CELLULAR METABOLISM 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. Dr. Lawrence G. Altman
BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 CELLULAR METABOLISM 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. Dr. Lawrence G. Altman
REVIEW 3: METABOLISM UNIT RESPIRATION & PHOTOSYNTHESIS. A. Top 10 If you learned anything from this unit, you should have learned:
 Period Date REVIEW 3: METABOLISM UNIT RESPIRATION & PHOTOSYNTHESIS A. Top 10 If you learned anything from this unit, you should have learned: 1. Energy production through chemiosmosis a. pumping of H+
Period Date REVIEW 3: METABOLISM UNIT RESPIRATION & PHOTOSYNTHESIS A. Top 10 If you learned anything from this unit, you should have learned: 1. Energy production through chemiosmosis a. pumping of H+
Sara Khraim. Shaymaa Alnamos ... Dr. Nafeth
 10 Sara Khraim Shaymaa Alnamos... Dr. Nafeth *Requirement of oxidative phosphorylation: 1- Source and target for electrons(nadh+fadh2 >> O2). 2- Electron carriers. 3- Enzymes, like oxidoreductases and
10 Sara Khraim Shaymaa Alnamos... Dr. Nafeth *Requirement of oxidative phosphorylation: 1- Source and target for electrons(nadh+fadh2 >> O2). 2- Electron carriers. 3- Enzymes, like oxidoreductases and
MITOCW watch?v=0xajihttcns
 MITOCW watch?v=0xajihttcns The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
MITOCW watch?v=0xajihttcns The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
GR QUIZ WITH ANS KEY Cellular Processes. Part I: Multiple Choice. 1. In leaf cell, the synthesis of ATP occurs in which of the following?
 GR QUIZ WITH ANS KEY Cellular Processes Part I: Multiple Choice 1. In leaf cell, the synthesis of ATP occurs in which of the following? I. Ribosomes II. Mitochondria III. Chloroplasts A. I only B. II only
GR QUIZ WITH ANS KEY Cellular Processes Part I: Multiple Choice 1. In leaf cell, the synthesis of ATP occurs in which of the following? I. Ribosomes II. Mitochondria III. Chloroplasts A. I only B. II only
f) Adding an enzyme does not change the Gibbs free energy. It only increases the rate of the reaction by lowering the activation energy.
 Problem Set 2-Answer Key BILD1 SP16 1) How does an enzyme catalyze a chemical reaction? Define the terms and substrate and active site. An enzyme lowers the energy of activation so the reaction proceeds
Problem Set 2-Answer Key BILD1 SP16 1) How does an enzyme catalyze a chemical reaction? Define the terms and substrate and active site. An enzyme lowers the energy of activation so the reaction proceeds
STRUCTURES AND PROTON-PUMPING STRATEGIES
 Annu. Rev. Biophys. Biomol. Struct. 2001. 30:23 65 Copyright c 2001 by Annual Reviews. All rights reserved STRUCTURES AND PROTON-PUMPING STRATEGIES OF MITOCHONDRIAL RESPIRATORY ENZYMES Brian E. Schultz
Annu. Rev. Biophys. Biomol. Struct. 2001. 30:23 65 Copyright c 2001 by Annual Reviews. All rights reserved STRUCTURES AND PROTON-PUMPING STRATEGIES OF MITOCHONDRIAL RESPIRATORY ENZYMES Brian E. Schultz
Scale in the biological world
 Scale in the biological world 2 A cell seen by TEM 3 4 From living cells to atoms 5 Compartmentalisation in the cell: internal membranes and the cytosol 6 The Origin of mitochondria: The endosymbion hypothesis
Scale in the biological world 2 A cell seen by TEM 3 4 From living cells to atoms 5 Compartmentalisation in the cell: internal membranes and the cytosol 6 The Origin of mitochondria: The endosymbion hypothesis
Photosynthesis and Cellular Respiration Note-taking Guide
 Photosynthesis and Cellular Respiration Note-taking Guide Preview to Photosynthesis glucose, reectlons, light-dependent, Calvin cycle, thylakoid, oxygen, light-harvesting, two, chloroplasts, photosynthesis,
Photosynthesis and Cellular Respiration Note-taking Guide Preview to Photosynthesis glucose, reectlons, light-dependent, Calvin cycle, thylakoid, oxygen, light-harvesting, two, chloroplasts, photosynthesis,
Life Depends on Photosynthesis
 Photosynthesis Life Depends on Photosynthesis Most energy Comes from the Sun Life Depends on Photosynthesis Most energy Comes from the Sun Life Depends on Photosynthesis Most energy Comes from the Sun
Photosynthesis Life Depends on Photosynthesis Most energy Comes from the Sun Life Depends on Photosynthesis Most energy Comes from the Sun Life Depends on Photosynthesis Most energy Comes from the Sun
BBS2710 Microbial Physiology. Module 5 - Energy and Metabolism
 BBS2710 Microbial Physiology Module 5 - Energy and Metabolism Topics Energy production - an overview Fermentation Aerobic respiration Alternative approaches to respiration Photosynthesis Summary Introduction
BBS2710 Microbial Physiology Module 5 - Energy and Metabolism Topics Energy production - an overview Fermentation Aerobic respiration Alternative approaches to respiration Photosynthesis Summary Introduction
Division Ave. High School AP Biology
 Overview 10 reactions u convert () to pyruvate (3C) u produces: 4 & NADH u consumes: u net: & NADH C-C-C-C-C-C fructose-1,6bp P-C-C-C-C-C-C-P DHAP P-C-C-C G3P C-C-C-P H P i P i pyruvate C-C-C 4 4 NAD +
Overview 10 reactions u convert () to pyruvate (3C) u produces: 4 & NADH u consumes: u net: & NADH C-C-C-C-C-C fructose-1,6bp P-C-C-C-C-C-C-P DHAP P-C-C-C G3P C-C-C-P H P i P i pyruvate C-C-C 4 4 NAD +
Student Questions and Answers November 19, 2002
 Student Questions and Answers November 19, 2002 Q 1. Why is the D-glycerol phosphate shuttle used? The malate-aspartate shuttle is better, because there is no energy lost. If there is a problem with the
Student Questions and Answers November 19, 2002 Q 1. Why is the D-glycerol phosphate shuttle used? The malate-aspartate shuttle is better, because there is no energy lost. If there is a problem with the
Chapter 15 part 2. Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry. ATP 4- + H 2 O ADP 3- + P i + H +
 Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry ATP 4- + 2 ADP 3- + P i 2- + + Chapter 15 part 2 Dr. Ray 1 Energy flow in biological systems: Energy Transformations
Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry ATP 4- + 2 ADP 3- + P i 2- + + Chapter 15 part 2 Dr. Ray 1 Energy flow in biological systems: Energy Transformations
Bioenergetics. Code: ECTS Credits: 6. Degree Type Year Semester
 2017/2018 Bioenergetics Code: 100866 ECTS Credits: 6 Degree Type Year Semester 2500252 Biochemistry OB 3 1 Contact Name: Joan-Ramon Daban Email: JoanRamon.Daban@uab.cat Prerequisites Use of languages Principal
2017/2018 Bioenergetics Code: 100866 ECTS Credits: 6 Degree Type Year Semester 2500252 Biochemistry OB 3 1 Contact Name: Joan-Ramon Daban Email: JoanRamon.Daban@uab.cat Prerequisites Use of languages Principal
Metabolismo Biología de 12º
 DEPARTAMENTO DE CIENCIAS NATURALES Metabolismo Biología de 12º Nombre y Apellidos FOTOSÍNTESIS 1) Organisms that can exist with light as an energy source and an inorganic form of carbon and other raw materials
DEPARTAMENTO DE CIENCIAS NATURALES Metabolismo Biología de 12º Nombre y Apellidos FOTOSÍNTESIS 1) Organisms that can exist with light as an energy source and an inorganic form of carbon and other raw materials
Biochemical Pathways
 Biochemical Pathways Living organisms can be divided into two large groups according to the chemical form in which they obtain carbon from the environment. Autotrophs can use carbon dioxide from the atmosphere
Biochemical Pathways Living organisms can be divided into two large groups according to the chemical form in which they obtain carbon from the environment. Autotrophs can use carbon dioxide from the atmosphere
Cellular Energetics. Photosynthesis, Cellular Respiration and Fermentation
 Cellular Energetics Photosynthesis, Cellular Respiration and Fermentation TEKS B.4 Science concepts. The student knows that cells are the basic structures of all living things with specialized parts that
Cellular Energetics Photosynthesis, Cellular Respiration and Fermentation TEKS B.4 Science concepts. The student knows that cells are the basic structures of all living things with specialized parts that
A + B = C C + D = E E + F = A
 Photosynthesis - Plants obtain energy directly from the sun - Organisms that do this are autotrophs (make their own food from inorganic forms) - Photosynthesis is a series of chemical reactions where the
Photosynthesis - Plants obtain energy directly from the sun - Organisms that do this are autotrophs (make their own food from inorganic forms) - Photosynthesis is a series of chemical reactions where the
The Electron-Transfer Chain and Oxidative Phosphorylation
 The Electron-Transfer Chain and Oxidative Phosphorylation The Mitochondrion - Scene of the Action 1.1 The inner mitochondrial membrane compartmentalizes metabolic functions. Although mitochondria in different
The Electron-Transfer Chain and Oxidative Phosphorylation The Mitochondrion - Scene of the Action 1.1 The inner mitochondrial membrane compartmentalizes metabolic functions. Although mitochondria in different
Section A: The Principles of Energy Harvest
 CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL ENERGY Section A: The Principles of Energy Harvest 1. Cellular respiration and fermentation are catabolic, energy-yielding pathways 2. Cells recycle
CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL ENERGY Section A: The Principles of Energy Harvest 1. Cellular respiration and fermentation are catabolic, energy-yielding pathways 2. Cells recycle
Cellular Energy: Respiration. Goals: Anaerobic respiration
 Cellular Energy: Respiration Anaerobic respiration Goals: Define and describe the 3 sets of chemical reactions that comprise aerobic cellular respiration Describe the types of anaerobic respiration Compare
Cellular Energy: Respiration Anaerobic respiration Goals: Define and describe the 3 sets of chemical reactions that comprise aerobic cellular respiration Describe the types of anaerobic respiration Compare
BIOLOGY 10/11/2014. An Introduction to Metabolism. Outline. Overview: The Energy of Life
 8 An Introduction to Metabolism CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Outline I. Forms of Energy II. Laws of Thermodynamics III. Energy and metabolism IV. ATP V. Enzymes
8 An Introduction to Metabolism CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Outline I. Forms of Energy II. Laws of Thermodynamics III. Energy and metabolism IV. ATP V. Enzymes
Cell Energy Notes ATP THE ENDOSYMBIOTIC THEORY. CELL ENERGY Cells usable source of is called ATP stands for. Name Per
 Cell Energy Notes Name Per THE ENDOSYMBIOTIC THEORY The Endosymbiotic theory is the idea that a long time ago, engulfed other prokaryotic cells by. This resulted in the first First proposed by Explains
Cell Energy Notes Name Per THE ENDOSYMBIOTIC THEORY The Endosymbiotic theory is the idea that a long time ago, engulfed other prokaryotic cells by. This resulted in the first First proposed by Explains
Respiration and Photosynthesis
 Respiration and Photosynthesis Cellular Respiration Glycolysis The Krebs Cycle Electron Transport Chains Anabolic Pathway Photosynthesis Calvin Cycle Flow of Energy Energy is needed to support all forms
Respiration and Photosynthesis Cellular Respiration Glycolysis The Krebs Cycle Electron Transport Chains Anabolic Pathway Photosynthesis Calvin Cycle Flow of Energy Energy is needed to support all forms
Effect of enzyme deficiencies on oxidative phosphorylation: from isolated mitochondria to intact tissues. Theoretical studies.
 Effect of enzyme deficiencies on oxidative phosphorylation: from isolated mitochondria to intact tissues. Theoretical studies. Bernard Korzeniewski Institute of Molecular Biology and Biotechnology, Jagiellonian
Effect of enzyme deficiencies on oxidative phosphorylation: from isolated mitochondria to intact tissues. Theoretical studies. Bernard Korzeniewski Institute of Molecular Biology and Biotechnology, Jagiellonian
Cellular Energetics Review
 Cellular Energetics Review 1. What two molecules are formed when a phosphate is removed from ATP? 2. Describe how photosynthesis and cellular respiration are reverse processes. 3. What is the function
Cellular Energetics Review 1. What two molecules are formed when a phosphate is removed from ATP? 2. Describe how photosynthesis and cellular respiration are reverse processes. 3. What is the function
Metabolism Test D [50 marks]
![Metabolism Test D [50 marks] Metabolism Test D [50 marks]](/thumbs/95/123302288.jpg) Metabolism Test D [50 marks] 1. A cricket was placed in a respirometer at constant temperature for ten minutes. The soap bubble moved along the pipette. [Source: International Baccalaureate Organization
Metabolism Test D [50 marks] 1. A cricket was placed in a respirometer at constant temperature for ten minutes. The soap bubble moved along the pipette. [Source: International Baccalaureate Organization
Lecture 7 Cell Biolog y ٢٢٢ ١
 Lecture 7 ١ Mitochondria ٢ Mitochondria Mitochondria are the energy factories of the cells. The energy currency for the work that animals must do is the energy-rich molecule adenosine triphosphate (ATP).
Lecture 7 ١ Mitochondria ٢ Mitochondria Mitochondria are the energy factories of the cells. The energy currency for the work that animals must do is the energy-rich molecule adenosine triphosphate (ATP).
Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Outer Glycolysis mitochondrial membrane Glucose ATP
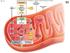 Fig. 7.5 uter Glycolysis mitochondrial membrane Glucose Intermembrane space xidation Mitochondrial matrix Acetyl-oA Krebs FAD e NAD + FAD Inner mitochondrial membrane e Electron e Transport hain hemiosmosis
Fig. 7.5 uter Glycolysis mitochondrial membrane Glucose Intermembrane space xidation Mitochondrial matrix Acetyl-oA Krebs FAD e NAD + FAD Inner mitochondrial membrane e Electron e Transport hain hemiosmosis
AP Biology Exam Review 5: Enzymes & Metabolism (Photosynthesis & Respiration)
 Name: Date: AP Biology Exam Review 5: Enzymes & Metabolism (Photosynthesis & Respiration) Helpful Videos and Animations: 1. Bozeman Biology: Photosynthesis and Respiration 2. Bozeman Biology: Photosynthesis
Name: Date: AP Biology Exam Review 5: Enzymes & Metabolism (Photosynthesis & Respiration) Helpful Videos and Animations: 1. Bozeman Biology: Photosynthesis and Respiration 2. Bozeman Biology: Photosynthesis
CP Biology Unit 5 Cell Energy Study Guide. Electron Carriers Electron Transport Chain Fermentation Glycolysis Krebs cycle Light-Dependent Reactions
 Name: KEY CP Biology Unit 5 Cell Energy Study Guide Vocabulary to know: ATP ADP Aerobic Anaerobic ATP Synthases Cellular Respiration Chlorophyll Chloroplast Electron Carriers Electron Transport Chain Fermentation
Name: KEY CP Biology Unit 5 Cell Energy Study Guide Vocabulary to know: ATP ADP Aerobic Anaerobic ATP Synthases Cellular Respiration Chlorophyll Chloroplast Electron Carriers Electron Transport Chain Fermentation
Cell and Molecular Biology
 Cell and Molecular Biology (3000719): academic year 2013 Content & Objective :Cell Chemistry and Biosynthesis 3 rd Edition, 1994, pp. 41-88. 4 th Edition, 2002, pp. 47-127. 5 th Edition, 2008, pp. 45-124.
Cell and Molecular Biology (3000719): academic year 2013 Content & Objective :Cell Chemistry and Biosynthesis 3 rd Edition, 1994, pp. 41-88. 4 th Edition, 2002, pp. 47-127. 5 th Edition, 2008, pp. 45-124.
6CO 2 + 6H 2 O C 6 H 12 O 6 + 6O 2. sun. Occurs in chloroplasts ATP. enzymes CO 2 O 2 H 2 O. sugars
 4.2 8.2 Overview Photosynthesis: of Photosynthesis An Overview Photosynthesis process by which plants make food using energy from the sun Plants are autotrophs that make their own source of chemical energy.
4.2 8.2 Overview Photosynthesis: of Photosynthesis An Overview Photosynthesis process by which plants make food using energy from the sun Plants are autotrophs that make their own source of chemical energy.
Oxidative Phosphorylation
 Paper : 04 Metabolism of carbohydrates Module : 15 Principal Investigator Paper Coordinator Content Reviewer Content Writer Dr.S.K.Khare,Professor IIT Delhi. Dr. Ramesh Kothari,Professor UGC-CAS Department
Paper : 04 Metabolism of carbohydrates Module : 15 Principal Investigator Paper Coordinator Content Reviewer Content Writer Dr.S.K.Khare,Professor IIT Delhi. Dr. Ramesh Kothari,Professor UGC-CAS Department
Review Questions - Lecture 5: Metabolism, Part 1
 Review Questions - Lecture 5: Metabolism, Part 1 Questions: 1. What is metabolism? 2. What does it mean to say that a cell has emergent properties? 3. Define metabolic pathway. 4. What is the difference
Review Questions - Lecture 5: Metabolism, Part 1 Questions: 1. What is metabolism? 2. What does it mean to say that a cell has emergent properties? 3. Define metabolic pathway. 4. What is the difference
Bioenergetics and high-energy compounds
 Bioenergetics and high-energy compounds Tomáš Kučera tomas.kucera@lfmotol.cuni.cz Department of Medical Chemistry and Clinical Biochemistry 2nd Faculty of Medicine, Charles University in Prague and Motol
Bioenergetics and high-energy compounds Tomáš Kučera tomas.kucera@lfmotol.cuni.cz Department of Medical Chemistry and Clinical Biochemistry 2nd Faculty of Medicine, Charles University in Prague and Motol
MITOCW watch?v=vykadbjib8a
 MITOCW watch?v=vykadbjib8a The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality, educational resources for free.
MITOCW watch?v=vykadbjib8a The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality, educational resources for free.
