Individual and population-level responses to ocean acidification
|
|
|
- Wendy Greene
- 5 years ago
- Views:
Transcription
1 Individual and population-level responses to ocean acidification Ben P. Harvey, Niall J. McKeown, Samuel P.S. Rastrick, Camilla Bertolini, Andy Foggo, Helen Graham, Jason M. Hall-Spencer, Marco Milazzo, Paul W. Shaw, Daniel P. Small, Pippa J. Moore Supplementary Information Measurements of seawater carbonate chemistry. Seawater ph, temperature, and salinity were measured at the three sites (Low ph, Control and Reference) on numerous occasions throughout the observations and experiments described in the main methods section. During the reciprocal transplants, measurements were taken twice daily in both the Low ph and Control sites, with measurements taken in the Reference site every 1-3 days. Total alkalinity (TA) was measured once weekly from all three sites. Measurements for all carbonate chemistry monitoring were taken as follows: ph NBS (Seven Easy ph InLab micro-electrode coupled to a Sevengo ph meter, Mettler-Toledo Ltd., Beaumont Leys, UK), temperature (digital thermometer, HH806AU, OMEGA Eng. Ltd., Manchester, UK), salinity (hand-held conductivity meter, TA 197 LFMulti350, WTW, Weilheim, Germany) and A T (Hanna HI 755 Alkalinity Checker, Leighton Buzzard, UK). In order to calculate the additional carbonate chemistry parameters, following Nisumaa et al. (ref 1 ), the dissolved inorganic carbon (DIC) was calculated using the software CO2SYS 2, with the measured ph NBS and A T as the input variables. Subsequently, the additional carbonate system parameters were calculated using the R package seacarb 3 using the calculated C T and measured A T. For both CO2SYS and seacarb, we used disassociation constants from Mehrbach et al. (ref 4 ), as adjusted by Dickson and Millero (ref 5 ), and KSO 4 using Dickson (ref 6 ) (Table S1). 1
2 Table S1. Seawater properties (Mean ± S.E.) at the three sites (Low ph, Control, Reference). ph T, temperature, salinity, and total alkalinity (A T ) are measured values. Seawater pco 2, dissolved inorganic carbon (DIC), bicarbonate (HCO 3 ), carbonate (CO 3 2 ), carbon dioxide (CO 2 ), saturation states for calcite (W calcite ) and aragonite (W aragonite ) are values calculated using the carbonate chemistry system analysis program CO2SYS 2 and the R package seacarb 3. Low ph Control Reference ph T 7.65 ± ± ± Temp ( C) ± ± ± Salinity (psu) ± ± ± 0.01 TA (µmol kg 1 ) ± ± ± 0.97 pco 2 (µatm) ± ± ± 4.05 DIC (µmol kg 1 ) ± ± ± 2.16 HCO 3 (µmol kg 1 ) ± ± ± 3.27 CO 3 2 (µmol kg 1 ) ± ± ± 1.33 CO 2 (µmol kg 1 ) ± ± ± 0.13 W calcite 2.50 ± ± ± 0.03 W aragonite 1.63 ± ± ± /6
3 Table S2. Descriptive statistics for the samples analysed by microsatellite (Locus A-D) and mtdna (COI) for the three sites (Low ph, Control, Reference) for the first temporal sample (1), the second temporal sample (2) and the two temporal samples together (Pooled). For microsatellites, observed (HO) and expected (HE) heterozygosity, allele numbers (NA) and p-values for tests of fit to Hardy-Weinberg equilibrium genotype expected proportions (phw). p-values in bold denotes values < For mtdna, haplotype (h) and nucleotide (p) diversities and associated standard deviations (SD). Microsatellite Low ph Control Reference 1 2 Pooled 1 2 Pooled 1 2 Pooled HO HE Locus A NA phw FIS HO HE Locus B NA phw FIS HO HE Locus C NA phw FIS HO HE Locus D NA phw FIS mtdna h (SD) COI (0.0777) (0.1133) (0.068) (0.0749) (0.0995) (0.061) (0.0855) (0.0971) (0.064) p (SD) (0.0052) (0.0026) (0.0042) (0.0047) (0.0043) (0.0044) (0.0034) (0.0042) (0.0036) 3/6
4 Table S3. Pairwise genetic differentiation (across loci) between sites (Low ph, Control and Reference) for a) temporal sample 1, b) temporal sample 2, and c) temporal samples 1 and 2 pooled. The lower triangular matrix of each square reports the unbiased F ST estimator 7, and the upper triangular matrix of each square reports p-value of the respective exact test of allele frequency homogeneity. a) Temporal sample 1 b) Temporal sample 2 Low ph Control Reference Low ph Control Reference Low ph p = 0.88 p = Low ph p = 0.28 p = 0.43 Control p = Control p = 0.33 Reference Reference c) Samples pooled Low ph Control Reference Low ph p = 0.08 p = Control p = Reference /6
5 Mitochondrial Markers. Pairwise tests of F ST demonstrated no significant differences between the sites (Low ph:control = , p = 0.80, Low ph:reference = , p = 0.39, Control:Reference = , p= 0.58) Therefore, the mtdna homogeneity among sites is concordant with the nuclear (microsatellite) pattern, supporting the observation that there is no significant breakdown in gene flow between sites. Table S4. Tukey HSD post-hoc test results (following the analysis of variance) for the effect of exposure to different pco 2 /ph conditions on the mean (± S.E.) oxygen consumption rate of H. trunculus. Pairwise differences in mean ṀO 2 (expressed as nmol O 2 h 1 mg 1 (WW) STPD) and associated p-values between individuals that were either (i) collected in the Control site and re-transplanted in the Control site (Control-Control), (ii) transplanted from the Control site to the Low ph site (Control-Low ph), (iii) re-transplanted within Low ph site (Low ph-low ph) and (iv) transplanted from Low ph into the Control Site (Low ph-control). Comparison 1 Comparison 2 Difference p-value Control:Control Control:Low ph Control:Control Low ph:control Control:Control Low ph:low ph Control:Low ph Low ph:low ph Low ph:control Control:Low ph Low ph:control Low ph:low ph /6
6 References 1. Nisumaa, A.-M. et al. EPOCA/EUR-OCEANS data compilation on the biological and biogeochemical responses to ocean acidification. Earth. Sys. Sci. Data 2, (2010). 2. Lewis, E. & Wallace, D.W.R. Program developed for CO 2 system calculations, ORNL/CDIAC (Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, Tennessee, U.S., 1998). 3. Lavigne, H. & Gattuso, J-P. Seacarb: seawater carbonate chemistry with R, R package version ). 4. Mehrbach, C., Culberson, C.H., Hawley, J.E. & Pytkowicz, R.M. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, (1973). 5. Dickson, A.G. & Millero, F.J. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res. Part I Oceanogr. Res. Pap. 34, (1987). 6. Dickson, A.G. Thermodynamics of the dissociation of boric acid in potassium chloride solutions from to K.. J. Chem. Eng. Data 35, (1990). 7. Weir, B. S. & Cockerham, C. C. Estimating F -statistics for the analysis of population structure. Evolution (1984). 6/6
Apical (Boron) Lateral (Boron) Growing edge (SNARF) Seawater ph T
 Supplemental Materials for: Coral calcifying fluid ph dictates response to ocean acidification Authors: M. Holcomb, A. A. Venn, E. Tambutté, S. Tambutté, D. Allemand, J. Trotter, M. McCulloch 1.4 1.2 1.0
Supplemental Materials for: Coral calcifying fluid ph dictates response to ocean acidification Authors: M. Holcomb, A. A. Venn, E. Tambutté, S. Tambutté, D. Allemand, J. Trotter, M. McCulloch 1.4 1.2 1.0
Strengthening seasonal marine CO 2 variations due to increasing atmospheric CO 2 - Supplementary material
 Strengthening seasonal marine CO 2 variations due to increasing atmospheric CO 2 - Supplementary material Peter Landschützer 1, Nicolas Gruber 2, Dorothee C. E. Bakker 3, Irene Stemmler 1, Katharina D.
Strengthening seasonal marine CO 2 variations due to increasing atmospheric CO 2 - Supplementary material Peter Landschützer 1, Nicolas Gruber 2, Dorothee C. E. Bakker 3, Irene Stemmler 1, Katharina D.
GSA DATA REPOSITORY Table DR1 displays the station locations and number of specimens employed in each
 GSA DATA REPOSITORY 2010022 Beer et al. Station Locations and Number of Specimens Table DR1 displays the station locations and number of specimens employed in each aliquot. A mean of 24 specimens were
GSA DATA REPOSITORY 2010022 Beer et al. Station Locations and Number of Specimens Table DR1 displays the station locations and number of specimens employed in each aliquot. A mean of 24 specimens were
Biogeochemistry of the Earth System QMS Lecture 5 Dr Zanna Chase 16 June 2015
 Biogeochemistry of the Earth System QMS512 2015 Lecture 5 Dr Zanna Chase 16 June 2015 Lecture 5: Inorganic carbon chemistry Outline Inorganic carbon speciation in seawater- conceptual overview Inorganic
Biogeochemistry of the Earth System QMS512 2015 Lecture 5 Dr Zanna Chase 16 June 2015 Lecture 5: Inorganic carbon chemistry Outline Inorganic carbon speciation in seawater- conceptual overview Inorganic
INTRODUCTION TO CO2 CHEMISTRY
 INTRODUCTION TO CO2 CHEMISTRY IN SEA WATER Andrew G. Dickson Scripps Institution of Oceanography, UC San Diego Mauna Loa Observatory, Hawaii Monthly Average Carbon Dioxide Concentration Data from Scripps
INTRODUCTION TO CO2 CHEMISTRY IN SEA WATER Andrew G. Dickson Scripps Institution of Oceanography, UC San Diego Mauna Loa Observatory, Hawaii Monthly Average Carbon Dioxide Concentration Data from Scripps
INTRODUCTION TO CO2 CHEMISTRY
 INTRODUCTION TO CO2 CHEMISTRY IN SEA WATER Andrew G. Dickson Scripps Institution of Oceanography, UC San Diego 410 Mauna Loa Observatory, Hawaii Monthly Average Carbon Dioxide Concentration Data from Scripps
INTRODUCTION TO CO2 CHEMISTRY IN SEA WATER Andrew G. Dickson Scripps Institution of Oceanography, UC San Diego 410 Mauna Loa Observatory, Hawaii Monthly Average Carbon Dioxide Concentration Data from Scripps
Chapter 12: Acids and Bases: Ocean Carbonate System James Murray 4/30/01 Univ. Washington
 Chapter 12: Acids and Bases: Ocean Carbonate System James Murray 4/30/01 Univ. Washington Last lecture was concerned with gas exchange and one example we looked at was the solubility of CO 2. Next we have
Chapter 12: Acids and Bases: Ocean Carbonate System James Murray 4/30/01 Univ. Washington Last lecture was concerned with gas exchange and one example we looked at was the solubility of CO 2. Next we have
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION DOI: 10.1038/NGEO1635 Extensive dissolution of live pteropods in the Southern Ocean Bednaršek N 1, 2, 3, Tarling GA 1 *, Bakker DCE 2, Fielding S 1, Jones EM 3, Venables HJ 1,
SUPPLEMENTARY INFORMATION DOI: 10.1038/NGEO1635 Extensive dissolution of live pteropods in the Southern Ocean Bednaršek N 1, 2, 3, Tarling GA 1 *, Bakker DCE 2, Fielding S 1, Jones EM 3, Venables HJ 1,
Table S1. Concentrations of dissolved inorganic nitrogen (DIN) and phosphate (DIP)
 Table S1. Concentrations of dissolved inorganic nitrogen (DIN) and phosphate (DIP) before and at the end of incubations at different nutrient conditions, and particulate organic nitrogen (PON) concentration
Table S1. Concentrations of dissolved inorganic nitrogen (DIN) and phosphate (DIP) before and at the end of incubations at different nutrient conditions, and particulate organic nitrogen (PON) concentration
Dissociation constants of carbonic acid in seawater as a function of salinity and temperature
 Marine Chemistry 100 (006) 80 94 www.elsevier.com/locate/marchem Dissociation constants of carbonic acid in seawater as a function of salinity and temperature Frank J. Millero *, Taylor B. Graham, Fen
Marine Chemistry 100 (006) 80 94 www.elsevier.com/locate/marchem Dissociation constants of carbonic acid in seawater as a function of salinity and temperature Frank J. Millero *, Taylor B. Graham, Fen
Experimental approaches of carbonate chemistry manipulation. in CO 2 pertubation studies. K. G. Schulz, U. Riebesell
 Experimental approaches of carbonate chemistry manipulation in CO 2 pertubation studies K. G. Schulz, U. Riebesell Monaco, 06.-09.10.2008 Atmospheric CO 2 variability and marine life increase to about
Experimental approaches of carbonate chemistry manipulation in CO 2 pertubation studies K. G. Schulz, U. Riebesell Monaco, 06.-09.10.2008 Atmospheric CO 2 variability and marine life increase to about
NOTES. The dissociation of hydrogen sulfide in seawater. E = E* - (RT/F)ln[H+], (1) potentiometric titrations of dilute NaHS solutions
![NOTES. The dissociation of hydrogen sulfide in seawater. E = E* - (RT/F)ln[H+], (1) potentiometric titrations of dilute NaHS solutions NOTES. The dissociation of hydrogen sulfide in seawater. E = E* - (RT/F)ln[H+], (1) potentiometric titrations of dilute NaHS solutions](/thumbs/87/96826488.jpg) NOTES Limnol. Oceanogr., 33(2), 1988, 269-274 0 1988, by the American Society of Limnology and Oceanography, Inc. The dissociation of hydrogen sulfide in seawater Abstract-The pk,* for the dissociation
NOTES Limnol. Oceanogr., 33(2), 1988, 269-274 0 1988, by the American Society of Limnology and Oceanography, Inc. The dissociation of hydrogen sulfide in seawater Abstract-The pk,* for the dissociation
MAR 510 Chemical Oceanography
 Carbonate Equilibrium -Key Concepts- Major buffer system influencing ph (master variable) Linked to geological, biological and climatological cycles Complex chemistry involving gaseous, dissolved, and
Carbonate Equilibrium -Key Concepts- Major buffer system influencing ph (master variable) Linked to geological, biological and climatological cycles Complex chemistry involving gaseous, dissolved, and
Carbon Dioxide, Alkalinity and ph
 Carbon Dioxide, Alkalinity and ph OCN 623 Chemical Oceanography 15 March 2018 Reading: Libes, Chapter 15, pp. 383 389 (Remainder of chapter will be used with the classes Global Carbon Dioxide and Biogenic
Carbon Dioxide, Alkalinity and ph OCN 623 Chemical Oceanography 15 March 2018 Reading: Libes, Chapter 15, pp. 383 389 (Remainder of chapter will be used with the classes Global Carbon Dioxide and Biogenic
Lecture 13: Population Structure. October 8, 2012
 Lecture 13: Population Structure October 8, 2012 Last Time Effective population size calculations Historical importance of drift: shifting balance or noise? Population structure Today Course feedback The
Lecture 13: Population Structure October 8, 2012 Last Time Effective population size calculations Historical importance of drift: shifting balance or noise? Population structure Today Course feedback The
INTRODUCTION TO CO2 MEASUREMENTS IN SEA WATER
 INTRODUCTION TO CO2 MEASUREMENTS IN SEA WATER Andrew G. Dickson Scripps Institution of Oceanography, UC San Diego METHODS FOR MEASURING THE CO 2 PARAMETERS IN SEA WATER USUAL PARAMETERS MEASURED Total
INTRODUCTION TO CO2 MEASUREMENTS IN SEA WATER Andrew G. Dickson Scripps Institution of Oceanography, UC San Diego METHODS FOR MEASURING THE CO 2 PARAMETERS IN SEA WATER USUAL PARAMETERS MEASURED Total
AEC 550 Conservation Genetics Lecture #2 Probability, Random mating, HW Expectations, & Genetic Diversity,
 AEC 550 Conservation Genetics Lecture #2 Probability, Random mating, HW Expectations, & Genetic Diversity, Today: Review Probability in Populatin Genetics Review basic statistics Population Definition
AEC 550 Conservation Genetics Lecture #2 Probability, Random mating, HW Expectations, & Genetic Diversity, Today: Review Probability in Populatin Genetics Review basic statistics Population Definition
SUPPORTING INFORMATION FOR. Spectrophotometric measurements of the carbonate ion concentration: aragonite
 SUPPORTING INFORMATION FOR Spectrophotometric measurements of the carbonate ion concentration: aragonite saturation states in the Mediterranean Sea and Atlantic Ocean Noelia M. Fajar, Maribel I. García-Ibáñez,,
SUPPORTING INFORMATION FOR Spectrophotometric measurements of the carbonate ion concentration: aragonite saturation states in the Mediterranean Sea and Atlantic Ocean Noelia M. Fajar, Maribel I. García-Ibáñez,,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Supplementary Information for Survival of mussels in extremely acidic waters on a submarine volcano Verena Tunnicliffe *, Kimberley T.A. Davies, David A. Butterfield, Robert W.
SUPPLEMENTARY INFORMATION Supplementary Information for Survival of mussels in extremely acidic waters on a submarine volcano Verena Tunnicliffe *, Kimberley T.A. Davies, David A. Butterfield, Robert W.
Supplementary Online Information
 Supplementary Online Information Revision : 1.5, June 10, 2005 Carbonate chemistry We computed [CO 2 3 ] and other carbonate system variables using the standard OCMIP carbonate chemistry routines. The
Supplementary Online Information Revision : 1.5, June 10, 2005 Carbonate chemistry We computed [CO 2 3 ] and other carbonate system variables using the standard OCMIP carbonate chemistry routines. The
Lecture 13: Variation Among Populations and Gene Flow. Oct 2, 2006
 Lecture 13: Variation Among Populations and Gene Flow Oct 2, 2006 Questions about exam? Last Time Variation within populations: genetic identity and spatial autocorrelation Today Variation among populations:
Lecture 13: Variation Among Populations and Gene Flow Oct 2, 2006 Questions about exam? Last Time Variation within populations: genetic identity and spatial autocorrelation Today Variation among populations:
Digestion in sea urchin larvae impaired under ocean acidification
 SUPPLEMENTARY INFORMATION DOI: 10.1038/NCLIMATE2028 Digestion in sea urchin larvae impaired under ocean acidification Supplementary information for: Digestion in sea urchin larvae impaired under ocean
SUPPLEMENTARY INFORMATION DOI: 10.1038/NCLIMATE2028 Digestion in sea urchin larvae impaired under ocean acidification Supplementary information for: Digestion in sea urchin larvae impaired under ocean
Ocean Acidification the other CO2 problem..
 Ocean Acidification the other CO2 problem.. Recall: Atm CO 2 already above recent planetary history CO 2 Today: What does this do to ocean water? Main Outline: 1. Chemistry. How does ocean absorb CO 2,
Ocean Acidification the other CO2 problem.. Recall: Atm CO 2 already above recent planetary history CO 2 Today: What does this do to ocean water? Main Outline: 1. Chemistry. How does ocean absorb CO 2,
I. Micro-nutrients in the oceans
 Impact of global change on ocean biogeochemical cycles (N, P, C and trace elements) Palma de Mallorca, 17 21 Oct 2011 I. Micro-nutrients in the oceans X. Antón Álvarez Salgado CSIC, Instituto de Investigacións
Impact of global change on ocean biogeochemical cycles (N, P, C and trace elements) Palma de Mallorca, 17 21 Oct 2011 I. Micro-nutrients in the oceans X. Antón Álvarez Salgado CSIC, Instituto de Investigacións
Population Structure
 Ch 4: Population Subdivision Population Structure v most natural populations exist across a landscape (or seascape) that is more or less divided into areas of suitable habitat v to the extent that populations
Ch 4: Population Subdivision Population Structure v most natural populations exist across a landscape (or seascape) that is more or less divided into areas of suitable habitat v to the extent that populations
Supplementary Figure 1. Observed Aragonite saturation variability and its drivers.
 Supplementary Figure 1. Observed Aragonite saturation variability and its drivers. Mean shift in aragonite saturation state from open ocean values, ΔΩ ocean-reef (left), due to freshwater fluxes, ΔΩ fresh
Supplementary Figure 1. Observed Aragonite saturation variability and its drivers. Mean shift in aragonite saturation state from open ocean values, ΔΩ ocean-reef (left), due to freshwater fluxes, ΔΩ fresh
1 Carbon - Motivation
 1 Carbon - Motivation Figure 1: Atmospheric pco 2 over the past 400 thousand years as recorded in the ice core from Vostok, Antarctica (Petit et al., 1999). Figure 2: Air-sea flux of CO 2 (mol m 2 yr 1
1 Carbon - Motivation Figure 1: Atmospheric pco 2 over the past 400 thousand years as recorded in the ice core from Vostok, Antarctica (Petit et al., 1999). Figure 2: Air-sea flux of CO 2 (mol m 2 yr 1
Ocean Acidification. The Basic Knowledge.
 Ocean Acidification The Basic Knowledge. Background Info Review ph measures the concentration of hydronium H 3 O + compared to hydroxide HO in a solution. Hydronium is a water molecule with a loosely bonded
Ocean Acidification The Basic Knowledge. Background Info Review ph measures the concentration of hydronium H 3 O + compared to hydroxide HO in a solution. Hydronium is a water molecule with a loosely bonded
Overview of CO 2 -induced Changes in Seawater Chemistry
 Overview of CO 2 -induced Changes in Seawater Chemistry Joan Kleypas & Chris Langdon I. Background II. Facts vs Hypotheses III. Future directions 10-Oct-00 9th Int Coral Reef Symp. 1 Effects of CO 2 on
Overview of CO 2 -induced Changes in Seawater Chemistry Joan Kleypas & Chris Langdon I. Background II. Facts vs Hypotheses III. Future directions 10-Oct-00 9th Int Coral Reef Symp. 1 Effects of CO 2 on
8. Genetic Diversity
 8. Genetic Diversity Many ways to measure the diversity of a population: For any measure of diversity, we expect an estimate to be: when only one kind of object is present; low when >1 kind of objects
8. Genetic Diversity Many ways to measure the diversity of a population: For any measure of diversity, we expect an estimate to be: when only one kind of object is present; low when >1 kind of objects
CO 2 perturbation experiments: similarities and differences between dissolved inorganic carbon and total alkalinity manipulations
 Biogeosciences, 6, 2145 215, 29 www.biogeosciences.net/6/2145/29/ Author(s) 29. This work is distributed under the Creative Commons Attribution. License. Biogeosciences CO 2 perturbation experiments: similarities
Biogeosciences, 6, 2145 215, 29 www.biogeosciences.net/6/2145/29/ Author(s) 29. This work is distributed under the Creative Commons Attribution. License. Biogeosciences CO 2 perturbation experiments: similarities
Emerson and Hedges, Chemical Oceanography Chapter IV page 1
 Emerson and Hedges, Chemical Oceanography Chapter IV page 1 CHAPTER IV: CARBONATE CHEMISTRY A. ACIDS AND BASES IN SEAWATER 1. The Important Acids and Bases in Seawater. The Alkalinity of Seawater B. CARBONATE
Emerson and Hedges, Chemical Oceanography Chapter IV page 1 CHAPTER IV: CARBONATE CHEMISTRY A. ACIDS AND BASES IN SEAWATER 1. The Important Acids and Bases in Seawater. The Alkalinity of Seawater B. CARBONATE
: 1.9 ppm y -1
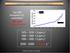 Atmospheric CO 2 Concentration Year 2006 Atmospheric CO 2 concentration: 381 ppm 35% above pre-industrial Atmoapheric [CO2] (ppmv) 4001850 1870 1890 1910 1930 1950 1970 1990 2010 380 360 340 320 300 280
Atmospheric CO 2 Concentration Year 2006 Atmospheric CO 2 concentration: 381 ppm 35% above pre-industrial Atmoapheric [CO2] (ppmv) 4001850 1870 1890 1910 1930 1950 1970 1990 2010 380 360 340 320 300 280
Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.)
 Ocean 421 Your Name Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.) 1. Due to the water molecule's (H 2 O) great abundance in
Ocean 421 Your Name Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.) 1. Due to the water molecule's (H 2 O) great abundance in
EPSS 15 Introduction to Oceanography Spring The Physical and Chemical Properties of Seawater
 EPSS 15 Introduction to Oceanography Spring 2017 The Physical and Chemical Properties of Seawater The focus of the Lab this week is seawater--its composition, physical and chemical properties. Seawater
EPSS 15 Introduction to Oceanography Spring 2017 The Physical and Chemical Properties of Seawater The focus of the Lab this week is seawater--its composition, physical and chemical properties. Seawater
Supplement of Simultaneous shifts in elemental stoichiometry and fatty acids of Emiliania huxleyi in response to environmental changes
 Supplement of Biogeosciences, 15, 1029 1045, 2018 https://doi.org/10.5194/bg-15-1029-2018-supplement Author(s) 2018. This work is distributed under the Creative Commons Attribution 3.0 License. Supplement
Supplement of Biogeosciences, 15, 1029 1045, 2018 https://doi.org/10.5194/bg-15-1029-2018-supplement Author(s) 2018. This work is distributed under the Creative Commons Attribution 3.0 License. Supplement
Continent-Ocean Interaction: Role of Weathering
 Institute of Astrophysics and Geophysics (Build. B5c) Room 0/13 email: Guy.Munhoven@ulg.ac.be Phone: 04-3669771 28th February 2018 Organisation of the Lecture 1 Carbon cycle processes time scales modelling:
Institute of Astrophysics and Geophysics (Build. B5c) Room 0/13 email: Guy.Munhoven@ulg.ac.be Phone: 04-3669771 28th February 2018 Organisation of the Lecture 1 Carbon cycle processes time scales modelling:
Package FinePop. October 26, 2018
 Type Package Title Fine-Scale Population Analysis Version 1.5.1 Date 2018-10-25 Package FinePop October 26, 2018 Author Reiichiro Nakamichi, Hirohisa Kishino, Shuichi Kitada Maintainer Reiichiro Nakamichi
Type Package Title Fine-Scale Population Analysis Version 1.5.1 Date 2018-10-25 Package FinePop October 26, 2018 Author Reiichiro Nakamichi, Hirohisa Kishino, Shuichi Kitada Maintainer Reiichiro Nakamichi
OCB Summer Workshop WHOI, July 16-19,
 Transformation and fluxes of carbon in a changing Arctic Ocean and it s impact on ocean acidification, the Atlantic view Leif G. Anderson Department t of Chemistry and Molecular l Biology University of
Transformation and fluxes of carbon in a changing Arctic Ocean and it s impact on ocean acidification, the Atlantic view Leif G. Anderson Department t of Chemistry and Molecular l Biology University of
19. Genetic Drift. The biological context. There are four basic consequences of genetic drift:
 9. Genetic Drift Genetic drift is the alteration of gene frequencies due to sampling variation from one generation to the next. It operates to some degree in all finite populations, but can be significant
9. Genetic Drift Genetic drift is the alteration of gene frequencies due to sampling variation from one generation to the next. It operates to some degree in all finite populations, but can be significant
Estimating the contribution of organic bases from microalgae to the titration alkalinity in coastal seawaters
 LIMNOLOGY and OCEANOGRAPHY: METHODS Limnol. Oceanogr.: Methods 5, 2007, 225 232 2007, by the American Society of Limnology and Oceanography, Inc. Estimating the contribution of organic bases from microalgae
LIMNOLOGY and OCEANOGRAPHY: METHODS Limnol. Oceanogr.: Methods 5, 2007, 225 232 2007, by the American Society of Limnology and Oceanography, Inc. Estimating the contribution of organic bases from microalgae
Satellite tools and approaches
 Satellite tools and approaches for OA research William M. Balch Bigelow Laboratory for Ocean Sciences E. Boothbay, ME 04544 With help from: J. Salisbury, D. Vandemark, B. Jönsson, S. Chakraborty,S Lohrenz,
Satellite tools and approaches for OA research William M. Balch Bigelow Laboratory for Ocean Sciences E. Boothbay, ME 04544 With help from: J. Salisbury, D. Vandemark, B. Jönsson, S. Chakraborty,S Lohrenz,
CO2 in atmosphere is influenced by pco2 of surface water (partial pressure of water is the CO2 (gas) that would be in equilibrium with water).
 EART 254, Lecture on April 6 & 11, 2011 Introduction (skipped most of this) Will look at C and N (maybe) cycles with respect to how they influence CO2 levels in the atmosphere. Ocean chemistry controls
EART 254, Lecture on April 6 & 11, 2011 Introduction (skipped most of this) Will look at C and N (maybe) cycles with respect to how they influence CO2 levels in the atmosphere. Ocean chemistry controls
Time-series observations in the Northern Indian Ocean V.V.S.S. Sarma National Institute of Oceanography Visakhapatnam, India
 The Second GEOSS Asia-Pacific Symposium, Tokyo, 14-16 th April 28 Time-series observations in the Northern Indian Ocean V.V.S.S. Sarma National Institute of Oceanography Visakhapatnam, India Seasonal variations
The Second GEOSS Asia-Pacific Symposium, Tokyo, 14-16 th April 28 Time-series observations in the Northern Indian Ocean V.V.S.S. Sarma National Institute of Oceanography Visakhapatnam, India Seasonal variations
Salinity distribution in the Oceans
 Salinity distribution in the Oceans Average practical salinity of open ocean waters 34.72 http://eps.mcgill.ca/~courses/c542/ 1/58 Salinity distribution in the Oceans Factors that control seawater salinity:
Salinity distribution in the Oceans Average practical salinity of open ocean waters 34.72 http://eps.mcgill.ca/~courses/c542/ 1/58 Salinity distribution in the Oceans Factors that control seawater salinity:
rotating at 1 rpm encompassing three acidification scenarios and 5 concentrations of the clay
 Respective publication: Passow, Uta, Christina L. De La Rocha, Caitlin Fairfield, Katrin Schmidt submitted to L&O in Dec 2012 : Aggregation as a function of pco 2 and mineral particles Methods Roller tanks
Respective publication: Passow, Uta, Christina L. De La Rocha, Caitlin Fairfield, Katrin Schmidt submitted to L&O in Dec 2012 : Aggregation as a function of pco 2 and mineral particles Methods Roller tanks
SW Density = kg/l at 20 o C (Pilson 1998)
 Composition of SW To Date We Have Covered: Descriptive Oceanography (Millero chapter 1) Special Properties of H 2 O (Millero chapter 4) Ion-Water & Ion-Ion Interactions (Millero chap 4) Continuing Coverage
Composition of SW To Date We Have Covered: Descriptive Oceanography (Millero chapter 1) Special Properties of H 2 O (Millero chapter 4) Ion-Water & Ion-Ion Interactions (Millero chap 4) Continuing Coverage
Solution chemistry of carbon dioxide in sea water
 Page 1 of 15 Solution chemistry of carbon dioxide in sea water 1. Introduction This chapter outlines the chemistry of carbon dioxide in sea water so as to provide a coherent background for the rest of
Page 1 of 15 Solution chemistry of carbon dioxide in sea water 1. Introduction This chapter outlines the chemistry of carbon dioxide in sea water so as to provide a coherent background for the rest of
Possible contribu.ons from the Solubility Data Project for arsenic and carbon dioxide environmental impacts mi.ga.on
 Possible contribu.ons from the Solubility Data Project for arsenic and carbon dioxide environmental impacts mi.ga.on M. Clara F. Magalhães a and Jus2n Salminen b a Department of Chemistry and CICECO, University
Possible contribu.ons from the Solubility Data Project for arsenic and carbon dioxide environmental impacts mi.ga.on M. Clara F. Magalhães a and Jus2n Salminen b a Department of Chemistry and CICECO, University
The Chemistry of Seawater. Unit 3
 The Chemistry of Seawater Unit 3 Water occurs naturally on earth in 3 phases: solid, liquid, or gas (liquid is most abundant) Water Phases Basic Chemistry Review What is an atom? Smallest particles of
The Chemistry of Seawater Unit 3 Water occurs naturally on earth in 3 phases: solid, liquid, or gas (liquid is most abundant) Water Phases Basic Chemistry Review What is an atom? Smallest particles of
Measurement of Seawater ph: A Theoretical and Analytical Investigation
 University of Miami Scholarly Repository Open Access Dissertations Electronic Theses and Dissertations 2012-12-05 Measurement of Seawater ph: A Theoretical and Analytical Investigation Jason F. Waters
University of Miami Scholarly Repository Open Access Dissertations Electronic Theses and Dissertations 2012-12-05 Measurement of Seawater ph: A Theoretical and Analytical Investigation Jason F. Waters
in seawater: Better fitting equations
 Notes 1307 Limnol. Oceanogr., 37(6), 1992, 1 307-1312 0 1992, by the American Society of Limnology and Oceanography, Inc. Oxygen solubility in seawater: Better fitting equations Abstract-We examined uncertainties
Notes 1307 Limnol. Oceanogr., 37(6), 1992, 1 307-1312 0 1992, by the American Society of Limnology and Oceanography, Inc. Oxygen solubility in seawater: Better fitting equations Abstract-We examined uncertainties
%Pluvial Input to the Ocean* Ocean Conc Range (nm) Major dissolved inorganic species in seawater yrs. Al
 Table 6.1 Estimated relative input of metals and metalloids to the ocean from the atmosphere, compared to other sources. Also listed are the range and average concentrations for open ocean waters, the
Table 6.1 Estimated relative input of metals and metalloids to the ocean from the atmosphere, compared to other sources. Also listed are the range and average concentrations for open ocean waters, the
Chemical Solution of Calcium Carbonate in Sea Water. Department of Oceanography, Oregon State University, Corvalis, Oregon 97331
 AM. ZOOLOCIST, 9:673-679 (1969). Chemical Solution of Calcium Carbonate in Sea Water RICARDO M. PYTKOWICZ Department of Oceanography, Oregon State University, Corvalis, Oregon 97331 SYNOPSIS. Sea water
AM. ZOOLOCIST, 9:673-679 (1969). Chemical Solution of Calcium Carbonate in Sea Water RICARDO M. PYTKOWICZ Department of Oceanography, Oregon State University, Corvalis, Oregon 97331 SYNOPSIS. Sea water
Statistical Methods and Software for Forensic Genetics. Lecture I.1: Basics
 Statistical Methods and Software for Forensic Genetics. Lecture I.1: Basics Thore Egeland (1),(2) (1) Norwegian University of Life Sciences, (2) Oslo University Hospital Workshop. Monterrey, Mexico, Nov
Statistical Methods and Software for Forensic Genetics. Lecture I.1: Basics Thore Egeland (1),(2) (1) Norwegian University of Life Sciences, (2) Oslo University Hospital Workshop. Monterrey, Mexico, Nov
Part 1: Seawater carbonate chemistry
 Part 1: Seawater carbonate chemistry 2 Approaches and tools to manipulate the carbonate chemistry Jean-Pierre Gattuso 1,2, Kunshan Gao 3, Kitack Lee 4, Björn Rost 5 and Kai G. Schulz 6 1 Laboratoire d
Part 1: Seawater carbonate chemistry 2 Approaches and tools to manipulate the carbonate chemistry Jean-Pierre Gattuso 1,2, Kunshan Gao 3, Kitack Lee 4, Björn Rost 5 and Kai G. Schulz 6 1 Laboratoire d
Permeable coral reef sediment dissolution driven by elevated pco 2 and pore water advection
 GEOPHYSICAL RESEARCH LETTERS, VOL. 40, 4876 4881, doi:10.1002/grl.50948, 2013 Permeable coral reef sediment dissolution driven by elevated pco 2 and pore water advection T. Cyronak, 1 I. R. Santos, 1 and
GEOPHYSICAL RESEARCH LETTERS, VOL. 40, 4876 4881, doi:10.1002/grl.50948, 2013 Permeable coral reef sediment dissolution driven by elevated pco 2 and pore water advection T. Cyronak, 1 I. R. Santos, 1 and
Seawater Chemistry Brooks/Cole, a division of Thomson Learning, Inc.
 Seawater Chemistry The ions of sodium and chloride in NaCl (table salt) are held together by ionic bonds, electrostatic attraction that exists between ions that have opposite charge. Sodium and chloride
Seawater Chemistry The ions of sodium and chloride in NaCl (table salt) are held together by ionic bonds, electrostatic attraction that exists between ions that have opposite charge. Sodium and chloride
Mid-Term #1 (125 points total)
 Ocean 520 Name: Chemical Oceanography 20 October 2009 Fall 2009 Points are in parentheses (show all your work) (use back if necessary) MidTerm #1 (125 points total) 1. Doney et al (2008) Ocean Acidification
Ocean 520 Name: Chemical Oceanography 20 October 2009 Fall 2009 Points are in parentheses (show all your work) (use back if necessary) MidTerm #1 (125 points total) 1. Doney et al (2008) Ocean Acidification
Hypothesis: What do you think will happen to the egg after it s left in Vinegar for 24 hours? Why?
 Ocean Acidification Lab The Naked Egg Name: Date: Prelab Questions: 1. What is vinegar s ph? 2. Is it acidic or basic? 3. Compounds make up vinegar? 4. What is the main component in egg shells? 5. Name
Ocean Acidification Lab The Naked Egg Name: Date: Prelab Questions: 1. What is vinegar s ph? 2. Is it acidic or basic? 3. Compounds make up vinegar? 4. What is the main component in egg shells? 5. Name
CO 2 mediation of adverse effects of seawater acidification in Calcidiscus leptoporus
 Article Volume 12, Number 5 5 May 2011 Q05001, doi:10.1029/2010gc003393 ISSN: 1525 2027 CO 2 mediation of adverse effects of seawater acidification in Calcidiscus leptoporus Gerald Langer ICTA, Autonomous
Article Volume 12, Number 5 5 May 2011 Q05001, doi:10.1029/2010gc003393 ISSN: 1525 2027 CO 2 mediation of adverse effects of seawater acidification in Calcidiscus leptoporus Gerald Langer ICTA, Autonomous
Distributions of dissolved inorganic carbon and total alkalinity in the Western Arctic Ocean
 Article Advances in Polar Science doi:10.3724/sp.j.1085.2011.00246 December 2011 Vol.22 No.4 246 252 Distributions of dissolved inorganic carbon and total alkalinity in the Western Arctic Ocean SUN Heng
Article Advances in Polar Science doi:10.3724/sp.j.1085.2011.00246 December 2011 Vol.22 No.4 246 252 Distributions of dissolved inorganic carbon and total alkalinity in the Western Arctic Ocean SUN Heng
Carbon is widely distributed in our
 C H A P T E R WATER SOFTENING Carbon is widely distributed in our ecosystem through five major spheres, namely the lithosphere, hydrosphere, atmosphere, pedosphere, and biosphere (see Table -). Sediments
C H A P T E R WATER SOFTENING Carbon is widely distributed in our ecosystem through five major spheres, namely the lithosphere, hydrosphere, atmosphere, pedosphere, and biosphere (see Table -). Sediments
Conservation Genetics. Outline
 Conservation Genetics The basis for an evolutionary conservation Outline Introduction to conservation genetics Genetic diversity and measurement Genetic consequences of small population size and extinction.
Conservation Genetics The basis for an evolutionary conservation Outline Introduction to conservation genetics Genetic diversity and measurement Genetic consequences of small population size and extinction.
Dissolution of olivine (potential, side effects) in simulated CO 2 removal experiments
 Peter Köhler 1 October 2015 Dissolution of olivine (potential, side effects) in simulated CO 2 removal experiments enhanced weathering, ocean alkalinization, ocean fertilization Peter Köhler, Judith Hauck
Peter Köhler 1 October 2015 Dissolution of olivine (potential, side effects) in simulated CO 2 removal experiments enhanced weathering, ocean alkalinization, ocean fertilization Peter Köhler, Judith Hauck
Seawater and Ocean Chemistry
 Seawater and Ocean Chemistry Seawater Chemistry Water Seawater Salts in seawater Water Composition Properties Water is a chemical compound (H 2 O) comprising two atoms of hydrogen and one atom of oxygen,
Seawater and Ocean Chemistry Seawater Chemistry Water Seawater Salts in seawater Water Composition Properties Water is a chemical compound (H 2 O) comprising two atoms of hydrogen and one atom of oxygen,
CO 2 and the carbonate system. 1/45
 CO 2 and the carbonate system http://eps.mcgill.ca/~courses/c542/ 1/45 The Atmospheric CO 2 -Climate Connection From: http://www.google.ca/imgres?imgurl=http:/ /www.tallbergfoundation.org/ From: Ruddiman,
CO 2 and the carbonate system http://eps.mcgill.ca/~courses/c542/ 1/45 The Atmospheric CO 2 -Climate Connection From: http://www.google.ca/imgres?imgurl=http:/ /www.tallbergfoundation.org/ From: Ruddiman,
organisms CaCO 3 + H 2 O + CO 2 shallow water
 Weathering and Reverse weathering Step I:Weathering of igneous rocks 1. Igneous rocks are mainly composed of Al, Si and O 2 with minor and varying quantities of Na, K, Ca and Mg composing pheldspar minerals
Weathering and Reverse weathering Step I:Weathering of igneous rocks 1. Igneous rocks are mainly composed of Al, Si and O 2 with minor and varying quantities of Na, K, Ca and Mg composing pheldspar minerals
Gas exchange of CO 2 and O 2 in partially ice-covered regions of the Arctic Ocean investigated using in situ sensors
 Gas exchange of CO 2 and O 2 in partially ice-covered regions of the Arctic Ocean investigated using in situ sensors F Islam 1, M DeGrandpre 1, C Beatty 1, R Krishfield 2 and J Toole 2 1 Department of
Gas exchange of CO 2 and O 2 in partially ice-covered regions of the Arctic Ocean investigated using in situ sensors F Islam 1, M DeGrandpre 1, C Beatty 1, R Krishfield 2 and J Toole 2 1 Department of
Workshop: Kinship analysis First lecture: Basics: Genetics, weight of evidence. I.1
 Workshop: Kinship analysis First lecture: Basics: Genetics, weight of evidence. I.1 Thore Egeland (1),(2), Klaas Slooten (3),(4) (1) Norwegian University of Life Sciences, (2) NIPH, (3) Netherlands Forensic
Workshop: Kinship analysis First lecture: Basics: Genetics, weight of evidence. I.1 Thore Egeland (1),(2), Klaas Slooten (3),(4) (1) Norwegian University of Life Sciences, (2) NIPH, (3) Netherlands Forensic
Calcium carbonate budget in the Atlantic Ocean based on water column inorganic carbon chemistry
 GLOBAL BIOGEOCHEMICAL CYCLES, VOL. 17, NO. 4, 1093, doi:10.1029/2002gb002001, 2003 Calcium carbonate budget in the Atlantic Ocean based on water column inorganic carbon chemistry S.-N. Chung, 1 K. Lee,
GLOBAL BIOGEOCHEMICAL CYCLES, VOL. 17, NO. 4, 1093, doi:10.1029/2002gb002001, 2003 Calcium carbonate budget in the Atlantic Ocean based on water column inorganic carbon chemistry S.-N. Chung, 1 K. Lee,
Lab 8 Dynamic Soil Systems I: Soil ph and Liming
 Lab 8 Dynamic Soil Systems I: Soil ph and Liming Objectives: To measure soil ph and observe conditions which change ph To distinguish between active acidity (soil solution ph) and exchangeable acidity
Lab 8 Dynamic Soil Systems I: Soil ph and Liming Objectives: To measure soil ph and observe conditions which change ph To distinguish between active acidity (soil solution ph) and exchangeable acidity
Neutral Theory of Molecular Evolution
 Neutral Theory of Molecular Evolution Kimura Nature (968) 7:64-66 King and Jukes Science (969) 64:788-798 (Non-Darwinian Evolution) Neutral Theory of Molecular Evolution Describes the source of variation
Neutral Theory of Molecular Evolution Kimura Nature (968) 7:64-66 King and Jukes Science (969) 64:788-798 (Non-Darwinian Evolution) Neutral Theory of Molecular Evolution Describes the source of variation
LECTURE # How does one test whether a population is in the HW equilibrium? (i) try the following example: Genotype Observed AA 50 Aa 0 aa 50
 LECTURE #10 A. The Hardy-Weinberg Equilibrium 1. From the definitions of p and q, and of p 2, 2pq, and q 2, an equilibrium is indicated (p + q) 2 = p 2 + 2pq + q 2 : if p and q remain constant, and if
LECTURE #10 A. The Hardy-Weinberg Equilibrium 1. From the definitions of p and q, and of p 2, 2pq, and q 2, an equilibrium is indicated (p + q) 2 = p 2 + 2pq + q 2 : if p and q remain constant, and if
Tracers. 1. Conservative tracers. 2. Non-conservative tracers. Temperature, salinity, SiO 2, Nd, 18 O. dissolved oxygen, phosphate, nitrate
 Tracers 1. Conservative tracers Temperature, salinity, SiO 2, Nd, 18 O 2. Non-conservative tracers dissolved oxygen, phosphate, nitrate Temperature itself is a tracer but other tracers (like oxygen isotopes)
Tracers 1. Conservative tracers Temperature, salinity, SiO 2, Nd, 18 O 2. Non-conservative tracers dissolved oxygen, phosphate, nitrate Temperature itself is a tracer but other tracers (like oxygen isotopes)
Chemistry 2000 Lecture 11: Chemical equilibrium
 Chemistry 2000 Lecture 11: Chemical equilibrium Marc R. Roussel February 4, 2019 Marc R. Roussel Chemical equilibrium February 4, 2019 1 / 27 Equilibrium and free energy Thermodynamic criterion for equilibrium
Chemistry 2000 Lecture 11: Chemical equilibrium Marc R. Roussel February 4, 2019 Marc R. Roussel Chemical equilibrium February 4, 2019 1 / 27 Equilibrium and free energy Thermodynamic criterion for equilibrium
[ ] Sparkling Water and the Carbon Cycle
![[ ] Sparkling Water and the Carbon Cycle [ ] Sparkling Water and the Carbon Cycle](/thumbs/87/96185022.jpg) Materials: Seltzer maker (SodaStream or similar) Some tasty cheese Tap water Glasses or cups for drinking ph paper Many people enjoy carbonated beverages, especially with food. The tiny bubbles and natural
Materials: Seltzer maker (SodaStream or similar) Some tasty cheese Tap water Glasses or cups for drinking ph paper Many people enjoy carbonated beverages, especially with food. The tiny bubbles and natural
Lecture 16 - Stable isotopes
 Lecture 16 - Stable isotopes 1. The fractionation of different isotopes of oxygen and their measurement in sediment cores has shown scientists that: (a) ice ages are common and lasted for hundreds of millions
Lecture 16 - Stable isotopes 1. The fractionation of different isotopes of oxygen and their measurement in sediment cores has shown scientists that: (a) ice ages are common and lasted for hundreds of millions
(4) Give an example of important reactions that are responsible for the composition of river water.
 Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
XI. the natural carbon cycle. with materials from J. Kasting (Penn State)
 XI. the natural carbon cycle with materials from J. Kasting (Penn State) outline properties of carbon the terrestrial biological cycle of carbon the ocean cycle of carbon carbon in the rock cycle overview
XI. the natural carbon cycle with materials from J. Kasting (Penn State) outline properties of carbon the terrestrial biological cycle of carbon the ocean cycle of carbon carbon in the rock cycle overview
"Enk AMHERST COLL MA F/0 S/10 SOL.UBILITIES OF DISSOLVED GASES IN SEA WATER. (U) NOV 80 B B BENSON,D KRAUSE A-0-A N00OOi-78-C-0198 W
 A-0-A110 675 UNCLASSIFIED AMHERST COLL MA F/0 S/10 SOL.UBILITIES OF DISSOLVED GASES IN SEA WATER. (U) NOV 80 B B BENSON,D KRAUSE N00OOi-78-C-0198 W "Enk SOLUBILITIES OF DISOLVED GASES IN SEA WATER. ofinal
A-0-A110 675 UNCLASSIFIED AMHERST COLL MA F/0 S/10 SOL.UBILITIES OF DISSOLVED GASES IN SEA WATER. (U) NOV 80 B B BENSON,D KRAUSE N00OOi-78-C-0198 W "Enk SOLUBILITIES OF DISOLVED GASES IN SEA WATER. ofinal
Long-term Climate Change. We are in a period of relative warmth right now but on the time scale of the Earth s history, the planet is cold.
 Long-term Climate Change We are in a period of relative warmth right now but on the time scale of the Earth s history, the planet is cold. Long-term Climate Change The Archean is thought to have been warmer,
Long-term Climate Change We are in a period of relative warmth right now but on the time scale of the Earth s history, the planet is cold. Long-term Climate Change The Archean is thought to have been warmer,
Introduction to Linkage Disequilibrium
 Introduction to September 10, 2014 Suppose we have two genes on a single chromosome gene A and gene B such that each gene has only two alleles Aalleles : A 1 and A 2 Balleles : B 1 and B 2 Suppose we have
Introduction to September 10, 2014 Suppose we have two genes on a single chromosome gene A and gene B such that each gene has only two alleles Aalleles : A 1 and A 2 Balleles : B 1 and B 2 Suppose we have
OCN 201. Chemistry & Physics of the Ocean. (but no need to panic) foot = 0.305m yard = 0.91m. Length. Area m 2 square feet ~0.09m2
 Chemistry & Physics of the Ocean OCN 201 (but no need to panic) Length m foot = 0.305m yard = 0.91m Area m 2 square feet ~0.09m2 Volume m 3 US pint ~ 0.47 L, L (liters) fl. oz. ~0.02 L Speed m/s mph Acceleration
Chemistry & Physics of the Ocean OCN 201 (but no need to panic) Length m foot = 0.305m yard = 0.91m Area m 2 square feet ~0.09m2 Volume m 3 US pint ~ 0.47 L, L (liters) fl. oz. ~0.02 L Speed m/s mph Acceleration
Ocean Temperatures. Atlantic Temp Section. Seasonal (Shallow) Thermocline. Better Atlantic Temp Section
 I. Fundamental Principles Physical Properties Conservation of Heat Why Does the Ocean Circulate? Waves & Tides Ocean Temperatures II. Ocean Circulation Conservation of Mass Forces & Balance of Forces Effects
I. Fundamental Principles Physical Properties Conservation of Heat Why Does the Ocean Circulate? Waves & Tides Ocean Temperatures II. Ocean Circulation Conservation of Mass Forces & Balance of Forces Effects
OCEAN ACIDIFICATION. Effects of an increasing atmospheric CO2 concentration on the ph of the Baltic Sea. Ville Berg Malmborg and Marcus Sjöstedt
 OCEAN ACIDIFICATION Effects of an increasing atmospheric CO2 concentration on the ph of the Baltic Sea Ville Berg Malmborg and Marcus Sjöstedt Department of Physics, Lund University Department of Physical
OCEAN ACIDIFICATION Effects of an increasing atmospheric CO2 concentration on the ph of the Baltic Sea Ville Berg Malmborg and Marcus Sjöstedt Department of Physics, Lund University Department of Physical
Nitrate and ph sensors for deployment in a global ocean observing array: simple sensors that operate for years without recalibration
 Nitrate and ph sensors for deployment in a global ocean observing array: simple sensors that operate for years without recalibration Ken Johnson Monterey Bay Aquarium Research Institute Moss Landing, CA
Nitrate and ph sensors for deployment in a global ocean observing array: simple sensors that operate for years without recalibration Ken Johnson Monterey Bay Aquarium Research Institute Moss Landing, CA
Chapter 6 Linkage Disequilibrium & Gene Mapping (Recombination)
 12/5/14 Chapter 6 Linkage Disequilibrium & Gene Mapping (Recombination) Linkage Disequilibrium Genealogical Interpretation of LD Association Mapping 1 Linkage and Recombination v linkage equilibrium ²
12/5/14 Chapter 6 Linkage Disequilibrium & Gene Mapping (Recombination) Linkage Disequilibrium Genealogical Interpretation of LD Association Mapping 1 Linkage and Recombination v linkage equilibrium ²
CLIMATE CHANGE EFFECTS ON SEAGRASSES, MACROALGAE AND THEIR ECOSYSTEMS: ELEVATED DIC, TEMPERATURE, OA AND THEIR INTERACTIONS
 CLIMATE CHANGE EFFECTS ON SEAGRASSES, MACROALGAE AND THEIR ECOSYSTEMS: ELEVATED DIC, TEMPERATURE, OA AND THEIR INTERACTIONS Marguerite S. Koch, George E. Bowes, Cliff Ross, XingHai Zhang Josh Filina, Kate
CLIMATE CHANGE EFFECTS ON SEAGRASSES, MACROALGAE AND THEIR ECOSYSTEMS: ELEVATED DIC, TEMPERATURE, OA AND THEIR INTERACTIONS Marguerite S. Koch, George E. Bowes, Cliff Ross, XingHai Zhang Josh Filina, Kate
Lect. 2: Chemical Water Quality
 The Islamic University of Gaza Faculty of Engineering Civil Engineering Department M.Sc. Water Resources Water Quality Management (ENGC 6304) Lect. 2: Chemical Water Quality ١ Chemical water quality parameters
The Islamic University of Gaza Faculty of Engineering Civil Engineering Department M.Sc. Water Resources Water Quality Management (ENGC 6304) Lect. 2: Chemical Water Quality ١ Chemical water quality parameters
Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions
 Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions OCN 623 Chemical Oceanography Reading: Libes, Chapters 15 and 16 Outline I. Deep sea sedimentation Detrital sediments
Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions OCN 623 Chemical Oceanography Reading: Libes, Chapters 15 and 16 Outline I. Deep sea sedimentation Detrital sediments
Salinity. See Appendix 1 of textbook x10 3 = See Appendix 1 of textbook
 Length Area Volume m m m foot = 0.305m yard = 0.91m square feet ~0.09m2 US pint ~ 0.47 L fl. oz. ~0.02 L Speed m/s mph Acceleration m/s mph/s Weight kg, gram pound ~0.45kg Temperature o o See Appendix
Length Area Volume m m m foot = 0.305m yard = 0.91m square feet ~0.09m2 US pint ~ 0.47 L fl. oz. ~0.02 L Speed m/s mph Acceleration m/s mph/s Weight kg, gram pound ~0.45kg Temperature o o See Appendix
Does the Iron Cycle Regulate Atmospheric CO2?
 Does the Iron Cycle Regulate Atmospheric CO2? Mick Follows, Dec 2005 http://ocean.mit.edu/~mick What regulates atmospheric CO2 on glacial-interglacial timescales? Role of ocean biology? Does the iron cycle
Does the Iron Cycle Regulate Atmospheric CO2? Mick Follows, Dec 2005 http://ocean.mit.edu/~mick What regulates atmospheric CO2 on glacial-interglacial timescales? Role of ocean biology? Does the iron cycle
Drift Inflates Variance among Populations. Geographic Population Structure. Variance among groups increases across generations (Buri 1956)
 Geographic Population Structure Alan R Rogers December 4, 205 / 26 Drift Inflates Variance among Populations 2 / 26 Variance among groups increases across generations Buri 956) 3 / 26 Gene flow migration)
Geographic Population Structure Alan R Rogers December 4, 205 / 26 Drift Inflates Variance among Populations 2 / 26 Variance among groups increases across generations Buri 956) 3 / 26 Gene flow migration)
Nutrients; Aerobic Carbon Production and Consumption
 Nutrients; Aerobic Carbon Production and Consumption OCN 623 Chemical Oceanography Reading: Libes, Chapters 8 and 9 Formation and respiration of organic matter DINutrients POM Primary Producers Autotrophs
Nutrients; Aerobic Carbon Production and Consumption OCN 623 Chemical Oceanography Reading: Libes, Chapters 8 and 9 Formation and respiration of organic matter DINutrients POM Primary Producers Autotrophs
Quantitative characters - exercises
 Quantitative characters - exercises 1. a) Calculate the genetic covariance between half sibs, expressed in the ij notation (Cockerham's notation), when up to loci are considered. b) Calculate the genetic
Quantitative characters - exercises 1. a) Calculate the genetic covariance between half sibs, expressed in the ij notation (Cockerham's notation), when up to loci are considered. b) Calculate the genetic
2 examples: Mg 2+ Rivers [Mg!! ]! (v r ) [Mg 2+ ] SW. **Answers with OR without vent effluent arrow are acceptable, since [Mg] in vent fluid is zero.
![2 examples: Mg 2+ Rivers [Mg!! ]! (v r ) [Mg 2+ ] SW. **Answers with OR without vent effluent arrow are acceptable, since [Mg] in vent fluid is zero. 2 examples: Mg 2+ Rivers [Mg!! ]! (v r ) [Mg 2+ ] SW. **Answers with OR without vent effluent arrow are acceptable, since [Mg] in vent fluid is zero.](/thumbs/91/107062387.jpg) OCN400 Winter 2015 PS2 - Key 1. You might think it is well known, but there is some debate about what controls the magnesium concentration in seawater. The main input is rivers (see Power Point Lecture
OCN400 Winter 2015 PS2 - Key 1. You might think it is well known, but there is some debate about what controls the magnesium concentration in seawater. The main input is rivers (see Power Point Lecture
The World Ocean. Pacific Ocean 181 x 10 6 km 2. Indian Ocean 74 x 10 6 km 2. Atlantic Ocean 106 x 10 6 km 2
 The World Ocean The ocean and adjacent seas cover 70.8% of the surface of Earth, an area of 361,254,000 km 2 Pacific Ocean 181 x 10 6 km 2 Indian Ocean 74 x 10 6 km 2 Atlantic Ocean 106 x 10 6 km 2 Oceanic
The World Ocean The ocean and adjacent seas cover 70.8% of the surface of Earth, an area of 361,254,000 km 2 Pacific Ocean 181 x 10 6 km 2 Indian Ocean 74 x 10 6 km 2 Atlantic Ocean 106 x 10 6 km 2 Oceanic
Biogeochemical Cycles
 s. 16 2553 Hydrologic cycle cycle Carbon cycle Contents 2 Did you know? 3 (bio) (chemical) (geo;, ) biogeochemical cycles Biogeochemistry = the study of the exchange or flux of materials between living
s. 16 2553 Hydrologic cycle cycle Carbon cycle Contents 2 Did you know? 3 (bio) (chemical) (geo;, ) biogeochemical cycles Biogeochemistry = the study of the exchange or flux of materials between living
