XI. the natural carbon cycle. with materials from J. Kasting (Penn State)
|
|
|
- Miles Bates
- 6 years ago
- Views:
Transcription
1 XI. the natural carbon cycle with materials from J. Kasting (Penn State)
2 outline properties of carbon the terrestrial biological cycle of carbon the ocean cycle of carbon carbon in the rock cycle overview of carbon stocks and exchanges timescales of exchange and renewal the silicate weathering thermostat
3 the carbon cycle flow of matter and energy between reservoirs (stocks and flows naturally in or near balance) linked to other biogeochemical and life cycles thru nutrient and energy flows linked to climate via greenhouse effect (and direct influence of vegetation on climate) to understand, first need to know something about carbon itself
4 organic and inorganic carbon C is cycled between reduced and oxidized forms by natural processes organic carbon inorganic carbon (reduced) (oxidized) CH 2 O CO 2 carbon dioxide H 2 CO 3 carbonic acid example: HCO 3 bicarbonate ion DIC Glucose -- C 6 H 12 O 6 CO = 3 carbonate ion CaCO 3 calcium carbonate example of transfer of oxidized to reduced C that also involves a flow of energy?
5 photosynthesis energy + CO 2 + H 2 O CH 2 O + O 2 energy + CO 2 + H 2 O CH 2 O + O 2 respiration and decay plants harness sun s energy to make chemical energy (carbohydrate) from inorganic CO 2 (& water)
6 photosynthesis energy + CO 2 + H 2 O CH 2 O + O 2 energy + CO 2 + H 2 O CH 2 O + O 2 respiration and decay photosynthesis requires an input of (light) energy, respiration and decay release stored chemical energy (on land, photosynthesis is roughly balanced by respiration and decay.)
7 coal biomass crude oil organic carbon (reduced) as biomass ages it takes on different forms
8 inorganic carbon (oxidized) limestone CO 2 DIC seashells coral
9 carbon can bond readily organic and carbon reversibly with H, O, N and also any oxygen, number nitrogen of (reduced additional form) associated with life! carbon atoms molecules with carbon and hydrogen (e.g., CH 4, ), inorganic provides carbon molecular limestone, sea shells, backbone for life. and CO 2 (oxidized form) can store and release energy via change in oxidation state
10 oxidation state oxidized intermediate reduced simplest example CO 2 CH 2 O CH 4 carbon oxidation state general category inorganic carbon hydrocarbon carbohydrate organic carbon oxygen is hungry for electrons and generally leaves bonded atoms depleted in electrons and therefore positively charged
11 oxidation state oxidized intermediate reduced simplest example CO 2 CH 2 O CH 4 carbon oxidation state general category inorganic carbon hydrocarbon carbohydrate organic carbon oxidized state most stable, so organic C will tend to move to this state over time
12 the terrestrial biological C-cycle photosynthesis CO 2 + H 2 O CH 2 O + O 2 respiration and decay on land, production of organic carbon by photosynthesis is largely balanced by respiration and decay -- respiration: used by both plants and animals to to produce energy for metabolism -- decay: consumption of dead organic matter by (aerobic or anaerobic) microorganisms
13 natural carbon stocks and flows on a global scale, we measure quantities of carbon in gigatons (Gt) 1 Gt = 1 billion metric tons 1 metric ton = 1,000 kilograms (so, 1 Gt = grams) Typically, we only count the weight of the carbon itself, i.e., for CH 2 O we neglect the weight of the H 2 O. So, we write these units as Gt(C) or GTC.
14 the terrestrial biological C-cycle photosynthesis plants 600 GTC atm. CO GTC respiration GTC/y consumers flows in GTC/year balanced? Plants respire all the time, offsetting much of new photosynthesis. The values here are for net photosynthesis which is offset by respiration by consumers on various timescales.
15 the terrestrial biological C-cycle photosynthesis plants 600 GTC atm. CO GTC soils 1,600 GTC respiration GTC/y death 30 consumers decay 30 not all new plant tissue survives- about as much new dead plant material enters soils as is lost from soils by decay (mostly oxidation)
16 seasonal var. in CO 2 season to season breathing of biosphere (~+/- 60 GTC/yr) leads to clear seasonal var. in amount of atmospheric CO 2
17 clicker question: the change in overall CO 2 amount from 1999 to 2000 and 2001 is due primarily to: a) more respiration b) less photosynthesis c) more decay d) burning of fossil fuels e) none of the above
18 monthly mean CO2
19 the terrestrial biological C-cycle output photosynthesis 60 GTC/yr atm. CO 2 input respiration & decay 60 GTC/yr CO 2 reservoir size: 700 GTC residence time: 700 GTC = ~11.7 yr 60 GTC/yr approx. lifetime of CO 2 molecule in atmosphere if there were no exchange with slowly moving deep ocean, sediments and rocks
20 the ocean C-cycle carbon uptake and release by the oceans: 1. exchange of CO 2 gas across air-sea interface 2. the biological pump
21 the ocean C-cycle atm. CO 2 recall phys. separation upper and deep ocean air-sea exchange ~100 m ~4 km surface ccean DIC deep ocean DIC biological pump C in sea water exists largely as DIC (dissolved inorganic carbon) biological activity transfers C from upper O. to deep O., leading to different [DIC] and pco 2 in upper and deep O.
22 the ocean s biological pump photosynthesis surface water CO 2 + H 2 O CH 2 O + O 2 sinking particles respiration CH 2 O + O 2 CO 2 + H 2 O deep water This pumps up the CO 2 of deep water
23 the ocean s biological pump transfer of CO 2 to the deep ocean: photosynthesis creates organic matter; this sinks to the deep ocean, where it decays back to CO 2 North Atlantic photosynthesis Pacific Ocean transfer of carbon deep water
24 the ocean s biological pump transfer of CO 2 to the deep ocean: deep water becomes enriched in CO 2 i.e. approx. residence time deep water from 14 C the carbon is recycled to the surface in ~1,000 years North Atlantic photosynthesis Pacific Ocean transfer of carbon deep water
25 recall ocean conveyor belt deep circulation dominated by a continuous circuit associated with formation of deep water in the N. Atlantic (i.e. NADW)
26 Dissolved Inorganic C (DIC), Pacific end of conveyor, must come up somewhere less more pls. see later note on molar units
27 atm. CO 2 pco 2 = 380 ppmv biological pump surface ocean DIC: 1,000 GTC pco 2 = 380 ppmv deep ocean DIC: 37,000 GTC pco ppmv deep water has a higher CO 2 partial pressure than does surface water The biological pump increases the [DIC] and pco 2 of the deep ocean. When deep water upwells to surface, some excess CO 2 escapes to atmosphere, but most is taken back up by the biological pump.
28 vigorous exchange atm. CO 2 90 GtC/yr pco 2 = 380 ppmv in equilibrium biological pump weaker exchange thru large reservoir 36 GTC/y surface ocean DIC: 1,000 GTC deep ocean DIC: 37,000 GTC pco 2 = 380 ppmv gradient maintained by pump pco ppmv deep water has a higher CO 2 partial pressure than does surface water Can say that the partitioning of C between surface and deep ocean by the biological pump helps to set the atmospheric concentration (in unperturbed C-cycle)
29 the ocean timescale(s) the surface ocean-to-atmosphere exchange is large with respect to the size of the atmospheric reservoir, but does not tug on the atmospheric concentration seasonally, because: unlike land, there is not a strong hemispheric or seasonal bias in biological production over the ocean (slide) it takes hundreds of years for all ocean water to contact the atmosphere due to slow overturning timescale of the conveyor (τ =???) most importantly, reactions between the CO 2 and DIC make it hard to change seawater pco 2 (a later lecture on the perturbed C-cycle..) ocean breathes on timescale of 5,000 year set by slow overturning and chemical reaction with sea sediments (a later lecture on Ice Age mysteries )
30 new production ocean vs. land June Dec.
31 the ocean timescale(s) the surface ocean-to-atmosphere exchange is large with respect to the size of the atmospheric reservoir, but does not tug on the atmospheric concentration seasonally, because: unlike land, there is not a strong hemispheric or seasonal bias in biological production over the ocean it takes hundreds of years for all ocean water to contact the atmosphere due to slow overturning timescale of the conveyor (~1000 yrs) most importantly, reactions between the CO 2 and DIC make it hard to change seawater pco 2 (see next lecture on the perturbed C-cycle..) ocean breathes on timescale of 5,000 year set by slow overturning and chemical reaction with sea sediments (see a later lecture on Ice Age mysteries )
32 natural carbon stocks and flows land: 600 GTC living 1500 GTC dead (soils and sediments) ocean: 1 GTC living 600 GTC dead (organic) 38,000 GTC inorganic atmosphere: 700 GTC inorganic (pre-industrial) rocks: 50,000,000 GTC inorganic + organic 5,000 GTC conc. organic (fossil fuel!) values are approximate
33 natural carbon stocks and flows ocean DIC: 38,000 DOC: 600 living C: 1 land living C: 500 dead C: metamorphism CaCO 3 + SiO 2 CaSiO 3 + CO 2 <0.1 GTC/yr atmosphere GTC/y weathering CaSiO 3 + CO 2 CaCO 3 + SiO 2 <0.1 GTC/yr sedimentary rocks CaCO 3 + OC: 50,000,000 fossil fuels: 500 stocks in GTC (gigatons C = g C) transfer fluxes in GTC/year
34 natural carbon stocks and flows ocean DIC: 38,000 DOC: 600 living C: 1 land living C: 500 dead C: metamorphism CaCO 3 + SiO 2 CaSiO 3 + CO 2 <0.1 GTC/yr atmosphere GTC/y weathering CaSiO 3 + CO 2 CaCO 3 + SiO 2 <0.1 GTC/yr sedimentary rocks CaCO 3 + OC: 50,000,000 fossil fuels: 5000 stocks in GTC (gigatons C = g C) transfer fluxes in GTC/year
35 natural carbon stocks and flows ocean DIC: 38,000 DOC: 600 living C: 1 90 metamorphism CaCO 3 + SiO 2 CaSiO 3 + CO 2 <0.1 GTC/yr atmosphere 700 land living C: 500 dead C: GTC/yr vigorous exchange weathering CaSiO 3 + CO 2 CaCO 3 + SiO 2 <0.1 GTC/yr sedimentary rocks CaCO 3 + OC: 50,000,000 fossil fuels: 5000 slow exchange stocks in GTC (gigatons C = g C) transfer fluxes in GTC/year
36 the long term rock C-cycle dominated by the weathering of Cacontaining silicate minerals and the subsequent use of Ca by organisms to build calcium carbonate shells the net result of the two reactions is the uptake of CO 2
37 silicate rock weathering: CaSiO H 2 O + 2 CO 2 Ca HCO SiO 2 + H 2 O carbonate formation: Ca HCO 3 - CaCO 3 + H 2 O + CO 2
38 silicate rock weathering: CaSiO H 2 O + 2 CO 2 Ca HCO SiO 2 + H 2 O carbonate formation: Ca HCO 3 - CaCO 3 + H 2 O + CO 2 net reaction: CaSiO 3 + CO 2 CaCO 3 + SiO 2
39 atmosphere the long term rock C-cycle CO 2 weathering CaSiO 3 + CO 2 CaCO 3 + SiO 2 WX ocean sediments
40 atmosphere the long term rock C-cycle CO 2 weathering CaSiO 3 + CO 2 CaCO 3 + SiO 2 WX dissolved CaCO 3 & SiO 2 ocean sediments
41 the long term rock C-cycle atmosphere CO 2 weathering CaSiO 3 + CO 2 CaCO 3 + SiO 2 WX dissolved CaCO 3 & SiO 2 solid CaCO 3 & SiO 2 shells ocean sediments
42 the long term rock C-cycle atmosphere CO 2 weathering CaSiO 3 + CO 2 CaCO 3 + SiO 2 WX dissolved CaCO 3 & SiO 2 solid CaCO 3 & SiO 2 shells ocean CaCO 3 & SiO 2 sediments subduction
43 the long term rock C-cycle atmosphere CO 2 weathering CaSiO 3 + CO 2 CaCO 3 + SiO 2 WX dissolved CaCO 3 & SiO 2 solid CaCO 3 & SiO 2 shells ocean CaCO 3 + SiO 2 CaSiO 2 + CO 2 metamorphism CaCO 3 & SiO 2 subduction sediments
44 long term rock inorganic C-cycle CaSiO 3 + CO 2 CaCO 3 + SiO 2
45 long term rock inorganic C-cycle ~0.1 GTC/yr CaSiO 3 + CO 2 CaCO 3 + SiO 2 ~0.1 GTC/yr
46 What controls silicate weathering rates? time temperature rainfall exposure of fresh rock surfaces vegetation (roots provide acid)
47 weathering feedback loop Atm. CO 2 Silicate weathering rates Surface Temperature weathering rates increase with increased temperature
48 weathering feedback loop Atm. CO 2 Silicate weathering rates Surface Temperature weathering reactions remove CO 2, and as CO 2 declines, planet temperatures go down
49 weathering feedback loop Atm. CO 2 Silicate weathering rates Surface Temperature this is a negative feedback loop and a key factor in helping to stabilize climate and CO 2 over long time scales (i.e., millions of years)
50 key points carbon cycle includes atmosphere, ocean, biosphere and lithosphere, but each has different time scale time scales depend on size of flux, and size of reservoirs the terrestrial biosphere is responsible for seasonal variations in CO 2 other reservoirs must be responsible for longer time scale changes in CO 2 as we will see, human activity and burning of fossil fuels connects the very long time scale of the rock cycle with the much shorter time scales of the atmosphere, ocean and biosphere
51 the long term rock C-cycle A small flux of organic carbon (0.05 Gt/yr) is also buried in sedimentary rocks, mostly on continental shelves. Over time, this small flux has accumulated to create a large reservoir: ~10,000,000 (or 1 x 10 7 ) Gton C. Concentrations of this buried organic carbon include coal, oil and gas--but most carbon is not concentrated. Organic carbon in sedimentary rocks is ultimately returned as CO 2 resulting from oxidation by exposure to O 2. This oxidation is also a weathering process.
52 the natural fate of fossil fuels weathering of rocks will ultimately allow the release of fossil fuels as CO 2 this would normally occur over 100s of millions of years humans simply speed up this process by burning fossil fuels (10s of years) as such, rapid burning of fossil fuels puts the carbon cycle out of balance
53 concept of the mole the last slide gave the sea water concentration of Dissolved Inorganic Carbon (DIC) in moles (i.e. as micromoles of DIC per kilogram of sea water) the number of moles is directly related to the number of molecules of a substance 1 mole is 6x10 23 molecules the molar mass of C is 12 ( formula weight ), so 1 mole of C weighs 12 g the molar mass of CO 2 is 44 ( formula weight ), so 1 mole of CO 2 weighs 44 g the molar mass of CO 3 = is 60 ( formula weight ), so 1 mole of CO 2 weighs 60 g the amount of C is the same in each..
54 learning goals be familiar w/ the special chemical attributes of C and how the stability of molecular C relates to its oxidation state be able to describe the photosynthesis/respiration reaction be able to describe the ambient Earth reservoirs of C, their relative sizes (or rank) and the sizes (or rank) of flows between them be able to determine a residence time from reservoir size and rate of in/output use this knowledge to determine which reservoirs exert the greatest influence on atmospheric CO 2 on brief (year to year) vs. very long (million year plus) timescales be able to describe the roles of silicate weathering and metamorphism and volcanism in the natural cycling of C be able to describe where sedimentary C and fossil fuel C come from
Global Carbon Cycle - I
 Global Carbon Cycle - I Reservoirs and Fluxes OCN 401 - Biogeochemical Systems 13 November 2012 Reading: Schlesinger, Chapter 11 Outline 1. Overview of global C cycle 2. Global C reservoirs 3. The contemporary
Global Carbon Cycle - I Reservoirs and Fluxes OCN 401 - Biogeochemical Systems 13 November 2012 Reading: Schlesinger, Chapter 11 Outline 1. Overview of global C cycle 2. Global C reservoirs 3. The contemporary
Global Carbon Cycle - I
 Global Carbon Cycle - I OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 11 1. Overview of global C cycle 2. Global C reservoirs Outline 3. The contemporary global C cycle 4. Fluxes and residence
Global Carbon Cycle - I OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 11 1. Overview of global C cycle 2. Global C reservoirs Outline 3. The contemporary global C cycle 4. Fluxes and residence
Global Carbon Cycle - I Systematics: Reservoirs and Fluxes
 OCN 401-10 Nov. 16, 2010 KCR Global Carbon Cycle - I Systematics: Reservoirs and Fluxes The Global carbon cycle Reservoirs: biomass on land in the oceans, atmosphere, soil and rocks, waters Processes:
OCN 401-10 Nov. 16, 2010 KCR Global Carbon Cycle - I Systematics: Reservoirs and Fluxes The Global carbon cycle Reservoirs: biomass on land in the oceans, atmosphere, soil and rocks, waters Processes:
This Week: Biogeochemical Cycles. Hydrologic Cycle Carbon Cycle
 This Week: Biogeochemical Cycles Hydrologic Cycle Carbon Cycle Announcements Reading: Chapters 4 (p. 74 81) and 8 Another Problem Set (Due next Tuesday) Exam 2: Friday Feb 29 My office hours today and
This Week: Biogeochemical Cycles Hydrologic Cycle Carbon Cycle Announcements Reading: Chapters 4 (p. 74 81) and 8 Another Problem Set (Due next Tuesday) Exam 2: Friday Feb 29 My office hours today and
Figure 65: Reservoir in a steady state condition where the input flux is equal to the output flux and the reservoir size remains constant.
 7. The carbon cycle 7.1. Box model of the carbon cycle Without the greenhouse effect, our planet would experience a permanent ice age and life as we know it would not be possible. The main contributors
7. The carbon cycle 7.1. Box model of the carbon cycle Without the greenhouse effect, our planet would experience a permanent ice age and life as we know it would not be possible. The main contributors
key to long-term sustainability is recycling..
 .. to support life over ~ 4 billion years, Earth must be sustainable system.. key to long-term sustainability is recycling.. Earth System how are key elements needed for life (C, N, P) recycled on the
.. to support life over ~ 4 billion years, Earth must be sustainable system.. key to long-term sustainability is recycling.. Earth System how are key elements needed for life (C, N, P) recycled on the
Part II: Past climates
 Part II: Past climates This week Solid Earth - excerpts of Ch 7 Carbon cycle - Ch 8 Early unexplainable things about the Earth Continental Drift (Alfred Wegener, 1920s) Ocean basins: trenches and midocean
Part II: Past climates This week Solid Earth - excerpts of Ch 7 Carbon cycle - Ch 8 Early unexplainable things about the Earth Continental Drift (Alfred Wegener, 1920s) Ocean basins: trenches and midocean
Long-term Climate Change. We are in a period of relative warmth right now but on the time scale of the Earth s history, the planet is cold.
 Long-term Climate Change We are in a period of relative warmth right now but on the time scale of the Earth s history, the planet is cold. Long-term Climate Change The Archean is thought to have been warmer,
Long-term Climate Change We are in a period of relative warmth right now but on the time scale of the Earth s history, the planet is cold. Long-term Climate Change The Archean is thought to have been warmer,
Carbon Cycle Activity
 David Faure, InThinking www.biology-inthinking.co.uk Carbon Cycle Activity IB Biology Different types of Carbon Carbon Dioxide Gas Dissolved Carbon dioxide Carbohydrates (e.g. glucose) Hydrogen carbonate
David Faure, InThinking www.biology-inthinking.co.uk Carbon Cycle Activity IB Biology Different types of Carbon Carbon Dioxide Gas Dissolved Carbon dioxide Carbohydrates (e.g. glucose) Hydrogen carbonate
The Biogeochemical Carbon Cycle: CO 2,the greenhouse effect, & climate feedbacks. Assigned Reading: Kump et al. (1999) The Earth System, Chap. 7.
 The Biogeochemical Carbon Cycle: CO 2,the greenhouse effect, & climate feedbacks Assigned Reading: Kump et al. (1999) The Earth System, Chap. 7. Overhead Transparencies Faint Faint Young Sun Paradox Young
The Biogeochemical Carbon Cycle: CO 2,the greenhouse effect, & climate feedbacks Assigned Reading: Kump et al. (1999) The Earth System, Chap. 7. Overhead Transparencies Faint Faint Young Sun Paradox Young
/ Past and Present Climate
 MIT OpenCourseWare http://ocw.mit.edu 12.842 / 12.301 Past and Present Climate Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. The Faint Young
MIT OpenCourseWare http://ocw.mit.edu 12.842 / 12.301 Past and Present Climate Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. The Faint Young
NUTRIENT CYCLES. Water Carbon Nitrogen
 NUTRIENT CYCLES Water Carbon Nitrogen NUTRIENT CYCLES Energy transfer through an ecosystem is ONE WAY Most energy is lost as heat Nutrients such as nitrogen, water and carbon are able to cycle through
NUTRIENT CYCLES Water Carbon Nitrogen NUTRIENT CYCLES Energy transfer through an ecosystem is ONE WAY Most energy is lost as heat Nutrients such as nitrogen, water and carbon are able to cycle through
Chapter 7: Environmental Systems and Ecosystem Ecology
 Chapter 7: Environmental Systems and Ecosystem Ecology Vocabulary words to know: Hypoxia Negative feedback Dynamic equilibrium Emergent properties Lithosphere Biosphere Gross primary production Nutrients
Chapter 7: Environmental Systems and Ecosystem Ecology Vocabulary words to know: Hypoxia Negative feedback Dynamic equilibrium Emergent properties Lithosphere Biosphere Gross primary production Nutrients
Carbon Cycling Internal
 Carbon Cycling Internal The 4 subcycles Atmosphere The Earth s Atmosphere The Earth has a radius of some 6400 km. Ninety-nine percent of the earth's atmosphere is contained within a layer approximately
Carbon Cycling Internal The 4 subcycles Atmosphere The Earth s Atmosphere The Earth has a radius of some 6400 km. Ninety-nine percent of the earth's atmosphere is contained within a layer approximately
Energy and Matter. Principles of Biology. Organisms interact with their environment, exchanging energy and matter. Topics Covered in this Module
 Principles of Biology contents 2 Energy and Matter Organisms interact with their environment, exchanging energy and matter. The Sun. Most ecosystems receive their energy from the Sun's radiation. NASA/European
Principles of Biology contents 2 Energy and Matter Organisms interact with their environment, exchanging energy and matter. The Sun. Most ecosystems receive their energy from the Sun's radiation. NASA/European
Evolution of Earth Environments Bio-Geo-Chemical Cycling
 Evolution of Earth Environments Bio-Geo-Chemical Cycling Evolution of the Earliest Atmospheres of Mars and Earth Volcanic Outgassing Evolving to Equilibrium Atmosphere To Atmosphere Lost to space (Abundant)
Evolution of Earth Environments Bio-Geo-Chemical Cycling Evolution of the Earliest Atmospheres of Mars and Earth Volcanic Outgassing Evolving to Equilibrium Atmosphere To Atmosphere Lost to space (Abundant)
 Carbon - I This figure from IPCC, 2001 illustrates the large variations in atmospheric CO 2 (a) Direct measurements of atmospheric CO 2 concentration, and O 2 from 1990 onwards. O 2 concentration is the
Carbon - I This figure from IPCC, 2001 illustrates the large variations in atmospheric CO 2 (a) Direct measurements of atmospheric CO 2 concentration, and O 2 from 1990 onwards. O 2 concentration is the
8. Carbon Cycle. Carbon ( 炭素 ) Family. Earth Watch: Antarctic lake hides bizarre ecosystem 無機탄소, 有機탄소
 arbon ( 炭素 ) Family 8. arbon ycle 지구시스템의이해읽기 : 탄소이야기 3 Earth Watch: Antarctic lake hides bizarre ecosystem Extremely alkaline waters with high dissolved 4 bacterial stromatolites 無機탄소, 有機탄소 Inorganic carbon:
arbon ( 炭素 ) Family 8. arbon ycle 지구시스템의이해읽기 : 탄소이야기 3 Earth Watch: Antarctic lake hides bizarre ecosystem Extremely alkaline waters with high dissolved 4 bacterial stromatolites 無機탄소, 有機탄소 Inorganic carbon:
The Global Carbon Cycle Recording the Evolution of Earth, from the origin of life to the industrialization of the planet
 The Global Carbon Cycle Recording the Evolution of Earth, from the origin of life to the industrialization of the planet Celebrating 5 years of world-leading collaborative and multidisciplinary research
The Global Carbon Cycle Recording the Evolution of Earth, from the origin of life to the industrialization of the planet Celebrating 5 years of world-leading collaborative and multidisciplinary research
DIAGRAM 1: Ocean Carbon Cycle DIAGRAM 2: Terrestrial Carbon Cycle
 DIAGRAM 1: Ocean Carbon Cycle DIAGRAM 2: Terrestrial Carbon Cycle DIAGRAM 3: Ocean Monthly CO 2 Flux Molecules of CO 2 enter the ocean by diffusing into the sea surface waters and dissolving a physio-chemical
DIAGRAM 1: Ocean Carbon Cycle DIAGRAM 2: Terrestrial Carbon Cycle DIAGRAM 3: Ocean Monthly CO 2 Flux Molecules of CO 2 enter the ocean by diffusing into the sea surface waters and dissolving a physio-chemical
Part II: Past climates
 Part II: Past climates This week Early unexplainable things about the Earth Solid Earth - excerpts of Ch 7 Continental Drift (Alfred Wegener, 1920s) Carbon cycle - Ch 8 Ocean basins: trenches and midocean
Part II: Past climates This week Early unexplainable things about the Earth Solid Earth - excerpts of Ch 7 Continental Drift (Alfred Wegener, 1920s) Carbon cycle - Ch 8 Ocean basins: trenches and midocean
Lecture 20. Origin of the atmosphere (Chap. 10) The carbon cycle and long-term climate (Chap. 8 of the textbook: p )
 Lecture 20 Origin of the atmosphere (Chap. 10) The carbon cycle and long-term climate (Chap. 8 of the textbook: p.158-170) end of last ice-age; begin civilization beginning of modern era of ice-ages asteroid
Lecture 20 Origin of the atmosphere (Chap. 10) The carbon cycle and long-term climate (Chap. 8 of the textbook: p.158-170) end of last ice-age; begin civilization beginning of modern era of ice-ages asteroid
BIOGEOCHEMICAL CYCLES
 BIOGEOCHEMICAL CYCLES BASICS Biogeochemical Cycle: The complete path a chemical takes through the four major components, or reservoirs, of Earth s system (atmosphere, lithosphere, hydrosphere and biosphere)
BIOGEOCHEMICAL CYCLES BASICS Biogeochemical Cycle: The complete path a chemical takes through the four major components, or reservoirs, of Earth s system (atmosphere, lithosphere, hydrosphere and biosphere)
Wednesday week 12. These ions move through the soil to streams and eventually to the ocean. In the ocean; CaCO 3 + H 2 O + CO 2 H 2 O + H 2 O
 Wednesday week 12 I. Control of CO 2 content of atmosphere by the ocean H 4 SiO 4 A. Consider a hypothetical planet with a crust made of single mineral (Wallastonite) CaSiO3. We could use the composition
Wednesday week 12 I. Control of CO 2 content of atmosphere by the ocean H 4 SiO 4 A. Consider a hypothetical planet with a crust made of single mineral (Wallastonite) CaSiO3. We could use the composition
Lecture 16 - Stable isotopes
 Lecture 16 - Stable isotopes 1. The fractionation of different isotopes of oxygen and their measurement in sediment cores has shown scientists that: (a) ice ages are common and lasted for hundreds of millions
Lecture 16 - Stable isotopes 1. The fractionation of different isotopes of oxygen and their measurement in sediment cores has shown scientists that: (a) ice ages are common and lasted for hundreds of millions
A bit of background on carbonates. CaCO 3 (solid)
 A bit of background on carbonates CaCO 3 (solid) Organisms need both carbon dioxide and carbonate Kleypas et al 2005 The two pumps put CO 2 into the deep ocean The long term record of climate change Or:
A bit of background on carbonates CaCO 3 (solid) Organisms need both carbon dioxide and carbonate Kleypas et al 2005 The two pumps put CO 2 into the deep ocean The long term record of climate change Or:
Lesson 3.1 Matter and the Environment. Water s abundance is a primary reason there is life on Earth.
 Lesson 3.1 Matter and the Environment Water s abundance is a primary reason there is life on Earth. Lesson 3.1 Matter and the Environment Atoms and Elements Atoms are the basic unit of matter. Nucleus:
Lesson 3.1 Matter and the Environment Water s abundance is a primary reason there is life on Earth. Lesson 3.1 Matter and the Environment Atoms and Elements Atoms are the basic unit of matter. Nucleus:
Lecture 3. - Global Sulfur, Nitrogen, Carbon Cycles - Short-term vs. Long-term carbon cycle - CO 2 & Temperature: Last 100,000+ years
 Lecture 3 - Global Sulfur, Nitrogen, Carbon Cycles - Short-term vs. Long-term carbon cycle - CO 2 & Temperature: Last 100,000+ years METR 113/ENVS 113 Spring Semester 2011 March 1, 2011 Suggested Reading
Lecture 3 - Global Sulfur, Nitrogen, Carbon Cycles - Short-term vs. Long-term carbon cycle - CO 2 & Temperature: Last 100,000+ years METR 113/ENVS 113 Spring Semester 2011 March 1, 2011 Suggested Reading
The Cycling of Matter. Day 1
 The Cycling of Matter Day 1 Objective I will learn the rock cycle is the series of processes in which rock changes from one form to another. I will learn in the water cycle, water condenses, precipitates
The Cycling of Matter Day 1 Objective I will learn the rock cycle is the series of processes in which rock changes from one form to another. I will learn in the water cycle, water condenses, precipitates
Continent-Ocean Interaction: Role of Weathering
 Institute of Astrophysics and Geophysics (Build. B5c) Room 0/13 email: Guy.Munhoven@ulg.ac.be Phone: 04-3669771 28th February 2018 Organisation of the Lecture 1 Carbon cycle processes time scales modelling:
Institute of Astrophysics and Geophysics (Build. B5c) Room 0/13 email: Guy.Munhoven@ulg.ac.be Phone: 04-3669771 28th February 2018 Organisation of the Lecture 1 Carbon cycle processes time scales modelling:
Carbon Dioxide, Alkalinity and ph
 Carbon Dioxide, Alkalinity and ph OCN 623 Chemical Oceanography 15 March 2018 Reading: Libes, Chapter 15, pp. 383 389 (Remainder of chapter will be used with the classes Global Carbon Dioxide and Biogenic
Carbon Dioxide, Alkalinity and ph OCN 623 Chemical Oceanography 15 March 2018 Reading: Libes, Chapter 15, pp. 383 389 (Remainder of chapter will be used with the classes Global Carbon Dioxide and Biogenic
SCOPE 35 Scales and Global Change (1988)
 1. Types and origins of marine sediments 2. Distribution of sediments: controls and patterns 3. Sedimentary diagenesis: (a) Sedimentary and organic matter burial (b) Aerobic and anaerobic decomposition
1. Types and origins of marine sediments 2. Distribution of sediments: controls and patterns 3. Sedimentary diagenesis: (a) Sedimentary and organic matter burial (b) Aerobic and anaerobic decomposition
(4) Give an example of important reactions that are responsible for the composition of river water.
 Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
CO2 in atmosphere is influenced by pco2 of surface water (partial pressure of water is the CO2 (gas) that would be in equilibrium with water).
 EART 254, Lecture on April 6 & 11, 2011 Introduction (skipped most of this) Will look at C and N (maybe) cycles with respect to how they influence CO2 levels in the atmosphere. Ocean chemistry controls
EART 254, Lecture on April 6 & 11, 2011 Introduction (skipped most of this) Will look at C and N (maybe) cycles with respect to how they influence CO2 levels in the atmosphere. Ocean chemistry controls
Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank. All are 3 points each.
 Part I Short Answer Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank. All are 3 points each. 1. A. ammonia D. HFCs B. CFCs E. NONE of these
Part I Short Answer Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank. All are 3 points each. 1. A. ammonia D. HFCs B. CFCs E. NONE of these
GEOL/ENVS 3520 Spring 2009 Hour Exam #2
 GEOL/ENVS 3520 Spring 2009 Hour Exam #2 Enter your name, the date, your ID number, and a made-up 4-digit code (for later recall and identification of your test results) on the separate test sheet. Carefully
GEOL/ENVS 3520 Spring 2009 Hour Exam #2 Enter your name, the date, your ID number, and a made-up 4-digit code (for later recall and identification of your test results) on the separate test sheet. Carefully
The Chemistry of Global Warming
 The Chemistry of Global Warming Venus Atmospheric pressure is 90x that of Earth 96% CO 2 and sulfuric acid clouds Average temperature = 450 C Expected temperature based on solar radiation and distance
The Chemistry of Global Warming Venus Atmospheric pressure is 90x that of Earth 96% CO 2 and sulfuric acid clouds Average temperature = 450 C Expected temperature based on solar radiation and distance
Water percolating through hot lava dissolves soluble minerals containing chlorine, bromine and sulphur compounds
 Figure 5 The sources of dissolved ions in sea water. Water falls as rain Compounds containing mainly calcium, magnesium, carbonate and silicate ions are leached from the soil Rivers carry ions in solution
Figure 5 The sources of dissolved ions in sea water. Water falls as rain Compounds containing mainly calcium, magnesium, carbonate and silicate ions are leached from the soil Rivers carry ions in solution
Chapter 03 Lecture Outline
 Chapter 03 Lecture Outline William P. Cunningham University of Minnesota Mary Ann Cunningham Vassar College Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1
Chapter 03 Lecture Outline William P. Cunningham University of Minnesota Mary Ann Cunningham Vassar College Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1
Chapter 5. The Biogeochemical Cycles. Botkin & Keller Environmental Science 5e
 Chapter 5 The Biogeochemical Cycles How Chemicals Cycle Biogeochemical Cycle The complete path a chemical takes through the four major components or reservoirs of Earth s systems 1. Atmosphere 2. Hydrosphere
Chapter 5 The Biogeochemical Cycles How Chemicals Cycle Biogeochemical Cycle The complete path a chemical takes through the four major components or reservoirs of Earth s systems 1. Atmosphere 2. Hydrosphere
[ ] Sparkling Water and the Carbon Cycle
![[ ] Sparkling Water and the Carbon Cycle [ ] Sparkling Water and the Carbon Cycle](/thumbs/87/96185022.jpg) Materials: Seltzer maker (SodaStream or similar) Some tasty cheese Tap water Glasses or cups for drinking ph paper Many people enjoy carbonated beverages, especially with food. The tiny bubbles and natural
Materials: Seltzer maker (SodaStream or similar) Some tasty cheese Tap water Glasses or cups for drinking ph paper Many people enjoy carbonated beverages, especially with food. The tiny bubbles and natural
Natural Climate Variability: Longer Term
 Natural Climate Variability: Longer Term Natural Climate Change Today: Natural Climate Change-2: Ice Ages, and Deep Time Geologic Time Scale background: Need a system for talking about unimaginable lengths
Natural Climate Variability: Longer Term Natural Climate Change Today: Natural Climate Change-2: Ice Ages, and Deep Time Geologic Time Scale background: Need a system for talking about unimaginable lengths
Cycles in the Phanerozoic
 Cycles in the Phanerozoic Evolutionary trends: extinctions, adaptive radiations, diversity over time Glaciations Sea level change Ocean chemistry Atmospheric CO 2 biosphere Mass extinctions in the..you
Cycles in the Phanerozoic Evolutionary trends: extinctions, adaptive radiations, diversity over time Glaciations Sea level change Ocean chemistry Atmospheric CO 2 biosphere Mass extinctions in the..you
Atmospheric Evolution: Earth s s Oxidation
 Earth s s Atmosphere Thematic Questions about the Atmosphere Observations of the Modern Atmosphere What is its structure and composition? What controls atmospheric dynamics? Information from the Rock Record
Earth s s Atmosphere Thematic Questions about the Atmosphere Observations of the Modern Atmosphere What is its structure and composition? What controls atmospheric dynamics? Information from the Rock Record
CHAPTER 5 WARM UPS. Mrs. Hilliard
 CHAPTER 5 WARM UPS Mrs. Hilliard CHAPTER 5 VOCABULARY 1. Photosynthesis 2. Cellular respiration 3. Producer 4. Consumer 5. Decomposer 6. Food chain 7. Food web 8. Trophic level 9. Carbon cycle 10. Nitrogen-fixing
CHAPTER 5 WARM UPS Mrs. Hilliard CHAPTER 5 VOCABULARY 1. Photosynthesis 2. Cellular respiration 3. Producer 4. Consumer 5. Decomposer 6. Food chain 7. Food web 8. Trophic level 9. Carbon cycle 10. Nitrogen-fixing
Ocean Acidification the other CO2 problem..
 Ocean Acidification the other CO2 problem.. Recall: Atm CO 2 already above recent planetary history CO 2 Today: What does this do to ocean water? Main Outline: 1. Chemistry. How does ocean absorb CO 2,
Ocean Acidification the other CO2 problem.. Recall: Atm CO 2 already above recent planetary history CO 2 Today: What does this do to ocean water? Main Outline: 1. Chemistry. How does ocean absorb CO 2,
1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and
 1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and climate change e) Oceanic water residence times 3.
1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and climate change e) Oceanic water residence times 3.
Chapter 6 The lithosphere and the hydrosphere
 Chapter 6 The lithosphere and the hydrosphere The lithosphere shell of the Earth, consists of crust and upper mantle contains minerals and rocks 1. Minerals solid, inorganic substances with defined composition
Chapter 6 The lithosphere and the hydrosphere The lithosphere shell of the Earth, consists of crust and upper mantle contains minerals and rocks 1. Minerals solid, inorganic substances with defined composition
Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.)
 Ocean 421 Your Name Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.) 1. Due to the water molecule's (H 2 O) great abundance in
Ocean 421 Your Name Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.) 1. Due to the water molecule's (H 2 O) great abundance in
What s up with Earth s Climate? David Archer Department of the Geophysical Sciences University of Chicago
 What s up with Earth s Climate? David Archer Department of the Geophysical Sciences University of Chicago This Sesson Began Sept. 29, 2014 Will begin again March 2015 Coursera.org Understanding the forecast
What s up with Earth s Climate? David Archer Department of the Geophysical Sciences University of Chicago This Sesson Began Sept. 29, 2014 Will begin again March 2015 Coursera.org Understanding the forecast
THE OCEAN CARBON CYCLE
 THE OCEAN CARBON CYCLE 21st February 2018 1 Box-model of the global ocean phosphorus, alkalinity, carbon 2 Pre-industrial model 3 Evolution during the industrial period 4 13 C isotopic evolution BOX-MODEL
THE OCEAN CARBON CYCLE 21st February 2018 1 Box-model of the global ocean phosphorus, alkalinity, carbon 2 Pre-industrial model 3 Evolution during the industrial period 4 13 C isotopic evolution BOX-MODEL
Fig. 3.2 on Page 101. Warming. Evidence for CO 2. History of Global Warming-2. Fig. 3.2 Page 101. Drilled cores from ocean floors
 Chemistry in Context: Chapter 3:The Chemistry of Global Warming Practice Problems: All Ch. 3 problems with the blue codes or answers on Page 521. Venus Atmospheric pressure is 90x that of Earth 96% CO
Chemistry in Context: Chapter 3:The Chemistry of Global Warming Practice Problems: All Ch. 3 problems with the blue codes or answers on Page 521. Venus Atmospheric pressure is 90x that of Earth 96% CO
Systems? Climate Systems. Earth Systems. Earth Interior Systems. Atmospheric/Biospheric Systems: Human Impact Hydrologic Cycle.
 Chapter 15 Climate Systems Systems? What is a system? Geologic phenomena are complex. All processes are related to, and interact with, other processes. So it is useful to think of geologic processes as
Chapter 15 Climate Systems Systems? What is a system? Geologic phenomena are complex. All processes are related to, and interact with, other processes. So it is useful to think of geologic processes as
Earth s Atmosphere. Atmospheric Composition 78% Nitrogen 21% Oxygen 1 % Argon, 0.03% Carbon dioxide, Water. Recall the Electro-Magnetic (EM) Spectrum
 Key Concepts: Lecture 11 Earth s Atmosphere and Greenhouse Effect Blackbody Radiation and Temperature Earth s Oceans Earth s Magnetic Field and Aurora The Green House Effect Temperature set by balancing
Key Concepts: Lecture 11 Earth s Atmosphere and Greenhouse Effect Blackbody Radiation and Temperature Earth s Oceans Earth s Magnetic Field and Aurora The Green House Effect Temperature set by balancing
Water Carbon Nitrogen. Nutrient Cycles
 Water Carbon Nitrogen Nutrient Cycles Nutrient Cycles Energy transfer through an ecosystem is ONE WAY Most energy is lost as heat Matter such as nitrogen, water and carbon are able to cycle through an
Water Carbon Nitrogen Nutrient Cycles Nutrient Cycles Energy transfer through an ecosystem is ONE WAY Most energy is lost as heat Matter such as nitrogen, water and carbon are able to cycle through an
Chapter 14: The Changing Climate
 Chapter 14: The Changing Climate Detecting Climate Change Natural Causes of Climate Change Anthropogenic Causes of Climate Change Possible Consequences of Global Warming Climate Change? -Paleo studies
Chapter 14: The Changing Climate Detecting Climate Change Natural Causes of Climate Change Anthropogenic Causes of Climate Change Possible Consequences of Global Warming Climate Change? -Paleo studies
Reminders: Week 14 Assessment closes tonight Watch for Week 15 Assessment (will close Wednesday, Dec. 13)
 Wednesday, December 6, 2017 The Pleistocene Glaciations, Continued (Chapter 14) Reminders: Week 14 Assessment closes tonight Watch for Week 15 Assessment (will close Wednesday, Dec. 13) Homework 5 due
Wednesday, December 6, 2017 The Pleistocene Glaciations, Continued (Chapter 14) Reminders: Week 14 Assessment closes tonight Watch for Week 15 Assessment (will close Wednesday, Dec. 13) Homework 5 due
Scholarship 2015 Earth and Space Science
 S 93104R Scholarship 2015 Earth and Space Science 2.00 p.m. Tuesday 1 December 2015 RESOURCE BOOKLET Refer to this booklet to answer the questions for Scholarship Earth and Space Science 93104. Check that
S 93104R Scholarship 2015 Earth and Space Science 2.00 p.m. Tuesday 1 December 2015 RESOURCE BOOKLET Refer to this booklet to answer the questions for Scholarship Earth and Space Science 93104. Check that
Physical Oceanography
 Physical Oceanography SECTION 15.1 The Oceans In your textbook, read about modern oceanography. For each item in Column A, write the letter of the matching item in Column B. Column A 1. German research
Physical Oceanography SECTION 15.1 The Oceans In your textbook, read about modern oceanography. For each item in Column A, write the letter of the matching item in Column B. Column A 1. German research
Problem Set #4 ANSWER KEY Fall 2009 Due: 9:30, Monday, Nov 30
 OCN 520 Problem Set #4 ANSWER KEY Fall 2009 Due: 9:30, Monday, Nov 30 1. Two-Box Ocean Model The B Flux Using a 2 box model like the one you have worked on in problem set #4 (question 1) assume the following
OCN 520 Problem Set #4 ANSWER KEY Fall 2009 Due: 9:30, Monday, Nov 30 1. Two-Box Ocean Model The B Flux Using a 2 box model like the one you have worked on in problem set #4 (question 1) assume the following
The Earth System Connections among the great spheres
 Why should we discuss the Earth System? The Earth System Connections among the great spheres Before we delve into the connection between geology, health, and forensics, we must gain an appreciation of
Why should we discuss the Earth System? The Earth System Connections among the great spheres Before we delve into the connection between geology, health, and forensics, we must gain an appreciation of
Physical Oceanography
 Physical Oceanography SECTION 15.1 The Oceans In your textbook, read about modern oceanography. For each item in Column A, write the letter of the matching item in Column B. e b c d a Column A 1. German
Physical Oceanography SECTION 15.1 The Oceans In your textbook, read about modern oceanography. For each item in Column A, write the letter of the matching item in Column B. e b c d a Column A 1. German
10/11/2010. Acceleration due to gravity, a. Bulk Properties Mass = 6 x kg Diameter = 12,756 km Density = 5515 kg/m 3 (mix of rock and iron)
 Acceleration due to gravity, a Bulk Properties Mass = 6 x 10 24 kg Diameter = 12,756 km Density = 5515 kg/m 3 (mix of rock and iron) Escape Velocity, v e Albedo Amount of sunlight reflected back into space
Acceleration due to gravity, a Bulk Properties Mass = 6 x 10 24 kg Diameter = 12,756 km Density = 5515 kg/m 3 (mix of rock and iron) Escape Velocity, v e Albedo Amount of sunlight reflected back into space
5 Stable and radioactive isotopes
 5 Stable and radioactive isotopes Outline 1 Stable isotopes Measuring stable isotopic abundances Equilibrium isotope effects Kinetic isotope effects Rayleigh distillation Isotopes: a mainstay of chemical
5 Stable and radioactive isotopes Outline 1 Stable isotopes Measuring stable isotopic abundances Equilibrium isotope effects Kinetic isotope effects Rayleigh distillation Isotopes: a mainstay of chemical
Geol. 656 Isotope Geochemistry
 THE ARBON YLE, ISOTOPES, AND LIMATE II THE LONG-TERM ARBON YLE On geologic times scales, the carbon cycle model must be augmented by 3 reservoirs, sedimentary carbonate, sedimentary organic carbon, and
THE ARBON YLE, ISOTOPES, AND LIMATE II THE LONG-TERM ARBON YLE On geologic times scales, the carbon cycle model must be augmented by 3 reservoirs, sedimentary carbonate, sedimentary organic carbon, and
1 General Introduction
 GENERAL INTRODUCTION 1 General Introduction 1.1 The Carbon Cycle Carbon dioxide (CO 2 ) present in our atmosphere absorbs the infrared (IR) radiation emitted by the Earth but is transparent to incoming
GENERAL INTRODUCTION 1 General Introduction 1.1 The Carbon Cycle Carbon dioxide (CO 2 ) present in our atmosphere absorbs the infrared (IR) radiation emitted by the Earth but is transparent to incoming
Integrated Science
 Carbon Cycle Story Name Assignment: Write a short story as a comic strip or slide show that depicts a single carbon atom in the carbon cycle. You can make it purely factual or have fun and turn it into
Carbon Cycle Story Name Assignment: Write a short story as a comic strip or slide show that depicts a single carbon atom in the carbon cycle. You can make it purely factual or have fun and turn it into
Biogeographic Processes
 Biogeographic Processes Energy and Matter Flow in Ecosystems Ecological Biogeography Ecological Succession Historical Biogeography Biogeographic Processes Biogeography examines the distribution of plants
Biogeographic Processes Energy and Matter Flow in Ecosystems Ecological Biogeography Ecological Succession Historical Biogeography Biogeographic Processes Biogeography examines the distribution of plants
Biogeochemical Cycles
 s. 16 2553 Hydrologic cycle cycle Carbon cycle Contents 2 Did you know? 3 (bio) (chemical) (geo;, ) biogeochemical cycles Biogeochemistry = the study of the exchange or flux of materials between living
s. 16 2553 Hydrologic cycle cycle Carbon cycle Contents 2 Did you know? 3 (bio) (chemical) (geo;, ) biogeochemical cycles Biogeochemistry = the study of the exchange or flux of materials between living
The Carbon Cycle and Energy Security
 The Carbon Cycle and Energy Security QU: Why is there no life on Mars? AIM: To explain the characteristics and understand the importance of different stores and fluxes within the carbon cycle. Which of
The Carbon Cycle and Energy Security QU: Why is there no life on Mars? AIM: To explain the characteristics and understand the importance of different stores and fluxes within the carbon cycle. Which of
Sedimentary Rocks. Rocks made of bits & pieces of other rocks.
 Sedimentary Rocks Rocks made of bits & pieces of other rocks. Sedimentary Rocks Igneous rocks are the most common rocks on Earth, but because most of them exist below the surface you might not have seen
Sedimentary Rocks Rocks made of bits & pieces of other rocks. Sedimentary Rocks Igneous rocks are the most common rocks on Earth, but because most of them exist below the surface you might not have seen
S Illustrate and explain how carbon, nitrogen, and oxygen are cycled through an ecosystem.
 Biogeochemical Cycles S2-1-01 Illustrate and explain how carbon, nitrogen, and oxygen are cycled through an ecosystem. Biogeochemical Cycles Let s take a closer look at the interactions between LIVING
Biogeochemical Cycles S2-1-01 Illustrate and explain how carbon, nitrogen, and oxygen are cycled through an ecosystem. Biogeochemical Cycles Let s take a closer look at the interactions between LIVING
Carbon Exchanges between the Continental Margins and the Open Ocean
 Carbon Exchanges between the Continental Margins and the Open Ocean Outline: 1. Introduction to problem 2. Example of how circulation can export carbon to open ocean 3. Example of how particle transport
Carbon Exchanges between the Continental Margins and the Open Ocean Outline: 1. Introduction to problem 2. Example of how circulation can export carbon to open ocean 3. Example of how particle transport
COMPUTER METHODS AND MODELING IN GEOLOGY THE GLOBAL PHOSPHORUS CYCLE
 COMPUTER METHODS AND MODELING IN GEOLOGY THE GLOBAL PHOSPHORUS CYCLE Phosphorous (P) is an essential nutrient for life. It is found in the RNA and DNA of all organisms, as well as in the adenosine triphosphate
COMPUTER METHODS AND MODELING IN GEOLOGY THE GLOBAL PHOSPHORUS CYCLE Phosphorous (P) is an essential nutrient for life. It is found in the RNA and DNA of all organisms, as well as in the adenosine triphosphate
Land Surface Sea Ice Land Ice. (from Our Changing Planet)
 Lecture 5: Land Surface and Cryosphere (Outline) Land Surface Sea Ice Land Ice (from Our Changing Planet) Earth s s Climate System Solar forcing Atmosphere Ocean Land Solid Earth Energy, Water, and Biochemistry
Lecture 5: Land Surface and Cryosphere (Outline) Land Surface Sea Ice Land Ice (from Our Changing Planet) Earth s s Climate System Solar forcing Atmosphere Ocean Land Solid Earth Energy, Water, and Biochemistry
Grades 9-12: Earth Sciences
 Grades 9-12: Earth Sciences Earth Sciences...1 Earth s Place in the Universe...1 Dynamic Earth Processes...2 Energy in the Earth System...2 Biogeochemical cycles...4 Structure and Composition of the Atmosphere...4
Grades 9-12: Earth Sciences Earth Sciences...1 Earth s Place in the Universe...1 Dynamic Earth Processes...2 Energy in the Earth System...2 Biogeochemical cycles...4 Structure and Composition of the Atmosphere...4
Semester 1: Unit 2: Energy Flow in Ecosystems
 Semester 1: Unit 2: Energy Flow in Ecosystems Obtaining and Using Energy What do you know? What is energy? What do you need energy for? How does your body use/store energy? Energy is the ability to do
Semester 1: Unit 2: Energy Flow in Ecosystems Obtaining and Using Energy What do you know? What is energy? What do you need energy for? How does your body use/store energy? Energy is the ability to do
Earth s Climate System. Surface Albedo. Climate Roles of Land Surface. Lecture 5: Land Surface and Cryosphere (Outline) Land Surface Sea Ice Land Ice
 Lecture 5: Land Surface and Cryosphere (Outline) Earth s Climate System Solar forcing Land Surface Sea Ice Land Ice Atmosphere Ocean Land Solid Earth Energy, Water, and Biochemistry Cycles (from Our Changing
Lecture 5: Land Surface and Cryosphere (Outline) Earth s Climate System Solar forcing Land Surface Sea Ice Land Ice Atmosphere Ocean Land Solid Earth Energy, Water, and Biochemistry Cycles (from Our Changing
Biology Test 2 BIO.2c-d: Metabolic Processes. For questions 1 16, choose the best answer. Indicate your answer on the Scantron and on the test.
 Name Block Date Biology Test 2 BIO.2c-d: Metabolic Processes For questions 1 16, choose the best answer. Indicate your answer on the Scantron and on the test. 1. Scientists hypothesize that oxygen began
Name Block Date Biology Test 2 BIO.2c-d: Metabolic Processes For questions 1 16, choose the best answer. Indicate your answer on the Scantron and on the test. 1. Scientists hypothesize that oxygen began
S= 95.02% S= 4.21% 35. S=radioactive 36 S=0.02% S= 0.75% 34 VI V IV III II I 0 -I -II SO 4 S 2 O 6 H 2 SO 3 HS 2 O 4- S 2 O 3
 SULFUR ISOTOPES 32 S= 95.02% 33 S= 0.75% 34 S= 4.21% 35 S=radioactive 36 S=0.02% S-H S-C S=C S-O S=O S-F S-Cl S-S VI V IV III II I 0 -I -II SO 4 2- S 2 O 6 2- H 2 SO 3 HS 2 O 4- S 2 O 3 2- S 2 F 2 S H
SULFUR ISOTOPES 32 S= 95.02% 33 S= 0.75% 34 S= 4.21% 35 S=radioactive 36 S=0.02% S-H S-C S=C S-O S=O S-F S-Cl S-S VI V IV III II I 0 -I -II SO 4 2- S 2 O 6 2- H 2 SO 3 HS 2 O 4- S 2 O 3 2- S 2 F 2 S H
Weather Vs. Climate. Weather Vs. Climate. Chapter 14
 Weather Vs. Climate Chapter 14 Weather: Conditions of the atmosphere at a particular time and place, for short periods of time (days). Climate: Long-term averages of weather (Averaged over 30 yrs). Weather
Weather Vs. Climate Chapter 14 Weather: Conditions of the atmosphere at a particular time and place, for short periods of time (days). Climate: Long-term averages of weather (Averaged over 30 yrs). Weather
FOSSIL FUELS, ENERGY, AND THE PERTURBED CARBON CYCLE
 FOSSIL FUELS, ENERGY, AND THE PERTURBED CARBON CYCLE 1. Introduction 2. Why are they called fossil fuels? 3. Burning buried sunshine 4. Perturbing the carbon cycle 5. Welcome to the Anthropocene A LOGICAL
FOSSIL FUELS, ENERGY, AND THE PERTURBED CARBON CYCLE 1. Introduction 2. Why are they called fossil fuels? 3. Burning buried sunshine 4. Perturbing the carbon cycle 5. Welcome to the Anthropocene A LOGICAL
10/4/2016. Matter, Energy, and Life
 DISCLAIMER: Principles and concepts on atomic structure, the Periodic Table, atoms, ions, ionic and covalent compounds, metals, and nonmetals will not be covered in this course. You are expected to know
DISCLAIMER: Principles and concepts on atomic structure, the Periodic Table, atoms, ions, ionic and covalent compounds, metals, and nonmetals will not be covered in this course. You are expected to know
PHOTOSYNTHESIS. Joseph Priestly 1772 experiment. SFSU Geography 316 Fall 2006 Dr. Barbara A. Holzman
 Nutrient Cycling I. A.Photosynthesis B. Respiration C. Production Primary productivity Gross Production Net Production II. Types of photosynthesis A. C3, B. C4, C. CAM D. Comparisons III. General Carbon
Nutrient Cycling I. A.Photosynthesis B. Respiration C. Production Primary productivity Gross Production Net Production II. Types of photosynthesis A. C3, B. C4, C. CAM D. Comparisons III. General Carbon
: 1.9 ppm y -1
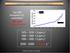 Atmospheric CO 2 Concentration Year 2006 Atmospheric CO 2 concentration: 381 ppm 35% above pre-industrial Atmoapheric [CO2] (ppmv) 4001850 1870 1890 1910 1930 1950 1970 1990 2010 380 360 340 320 300 280
Atmospheric CO 2 Concentration Year 2006 Atmospheric CO 2 concentration: 381 ppm 35% above pre-industrial Atmoapheric [CO2] (ppmv) 4001850 1870 1890 1910 1930 1950 1970 1990 2010 380 360 340 320 300 280
The Chemistry of Seawater. Unit 3
 The Chemistry of Seawater Unit 3 Water occurs naturally on earth in 3 phases: solid, liquid, or gas (liquid is most abundant) Water Phases Basic Chemistry Review What is an atom? Smallest particles of
The Chemistry of Seawater Unit 3 Water occurs naturally on earth in 3 phases: solid, liquid, or gas (liquid is most abundant) Water Phases Basic Chemistry Review What is an atom? Smallest particles of
OCN 201: Earth Structure
 OCN 201: Earth Structure Eric Heinen Eric H. De Carlo, Carlo: OCN 201, OCN Sp2010 201, Fall 2004 Early History of the Earth Rapid accretion of Earth and attendant dissipation of kinetic energy caused tremendous
OCN 201: Earth Structure Eric Heinen Eric H. De Carlo, Carlo: OCN 201, OCN Sp2010 201, Fall 2004 Early History of the Earth Rapid accretion of Earth and attendant dissipation of kinetic energy caused tremendous
2010 Pearson Education, Inc.
 Chapter 10 Planetary Atmospheres: Mars, Venus, Earth What is an atmosphere? An atmosphere is a (usually very thin) layer of gas that surrounds a world. How does the greenhouse effect warm a planet? No
Chapter 10 Planetary Atmospheres: Mars, Venus, Earth What is an atmosphere? An atmosphere is a (usually very thin) layer of gas that surrounds a world. How does the greenhouse effect warm a planet? No
Q1. Scientists study the atmosphere on planets and moons in the Solar System to understand how the Earth s atmosphere has changed.
 Q. Scientists study the atmosphere on planets and moons in the Solar System to understand how the Earth s atmosphere has changed. (a) Millions of years ago the Earth s atmosphere was probably just like
Q. Scientists study the atmosphere on planets and moons in the Solar System to understand how the Earth s atmosphere has changed. (a) Millions of years ago the Earth s atmosphere was probably just like
PHOTOSYNTHESIS PHOTOSYNTHESIS
 PHOTOSYNTHESIS PHOTOSYNTHESIS Life Processes are the basic functions performed by living organisms to maintain their life on this earth. Nutrition is the process by which the organisms can assimilate and
PHOTOSYNTHESIS PHOTOSYNTHESIS Life Processes are the basic functions performed by living organisms to maintain their life on this earth. Nutrition is the process by which the organisms can assimilate and
8. Climate changes Short-term regional variations
 8. Climate changes 8.1. Short-term regional variations By short-term climate changes, we refer here to changes occurring over years to decades. Over this timescale, climate is influenced by interactions
8. Climate changes 8.1. Short-term regional variations By short-term climate changes, we refer here to changes occurring over years to decades. Over this timescale, climate is influenced by interactions
Sedimentary Rocks. Rocks made of bits & pieces of other rocks.
 Sedimentary Rocks Rocks made of bits & pieces of other rocks. Sedimentary Rocks Igneous rocks are the most common rocks on Earth, but because most of them exist below the surface you might not have seen
Sedimentary Rocks Rocks made of bits & pieces of other rocks. Sedimentary Rocks Igneous rocks are the most common rocks on Earth, but because most of them exist below the surface you might not have seen
What is a system? What do the arrows in this diagram represent? What do the boxes represent? Why is it useful to study and understand systems?
 Systems What is a system? What do the arrows in this diagram represent? What do the boxes represent? Why is it useful to study and understand systems? evaporation River & Lake water rain Atmosphere Water
Systems What is a system? What do the arrows in this diagram represent? What do the boxes represent? Why is it useful to study and understand systems? evaporation River & Lake water rain Atmosphere Water
Website Lecture 3 The Physical Environment Part 1
 Website http://websites.rcc.edu/halama Lecture 3 The Physical Environment Part 1 1 Lectures 3 & 4 1. Biogeochemical Cycling 2. Solar Radiation 3. The Atmosphere 4. The Global Ocean 5. Weather and Climate
Website http://websites.rcc.edu/halama Lecture 3 The Physical Environment Part 1 1 Lectures 3 & 4 1. Biogeochemical Cycling 2. Solar Radiation 3. The Atmosphere 4. The Global Ocean 5. Weather and Climate
Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds. What is an atmosphere? About 10 km thick
 Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds What is an atmosphere? Sources of Gas Losses of Gas Thermal Escape Earth s Atmosphere About 10 km thick Consists mostly of molecular
Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds What is an atmosphere? Sources of Gas Losses of Gas Thermal Escape Earth s Atmosphere About 10 km thick Consists mostly of molecular
Cells the Building Blocks of Life. Cells the Building Blocks of Life. Cells the Building Blocks of Life. Cells the Building Blocks of Life
 1 What is gestation? 2 Give three ways in which alveoli are adapted for gas exchange. 3 Describe what happens to someone when they suffer from an asthma attack. 4 What is meant by the term antagonistic
1 What is gestation? 2 Give three ways in which alveoli are adapted for gas exchange. 3 Describe what happens to someone when they suffer from an asthma attack. 4 What is meant by the term antagonistic
Section 8. North American Biomes. What Do You See? Think About It. Investigate. Learning Outcomes
 Section 8 North American Biomes What Do You See? Learning Outcomes In this section, you will Define the major biomes of North America and identify your community s biome. Understand that organisms on land
Section 8 North American Biomes What Do You See? Learning Outcomes In this section, you will Define the major biomes of North America and identify your community s biome. Understand that organisms on land
The Earth System Connections among the great spheres
 The Earth System Connections among the great spheres Our Home Planet About 4.5 billion years old Only planet presently known to support life Has well-defined continents and ocean basins Very dynamic, both
The Earth System Connections among the great spheres Our Home Planet About 4.5 billion years old Only planet presently known to support life Has well-defined continents and ocean basins Very dynamic, both
HIGLEY UNIFIED SCHOOL DISTRICT INSTRUCTIONAL ALIGNMENT. Earth and Space Science Quarter 1. Earth and Space Science (Duration 1 Week)
 HIGLEY UNIFIED SCHOOL DISTRICT INSTRUCTIONAL ALIGNMENT Earth and Space Science Quarter 1 Earth and Space Science (Duration 1 Week) Big Idea: Essential Questions: 1. Describe how matter is classified by
HIGLEY UNIFIED SCHOOL DISTRICT INSTRUCTIONAL ALIGNMENT Earth and Space Science Quarter 1 Earth and Space Science (Duration 1 Week) Big Idea: Essential Questions: 1. Describe how matter is classified by
Global Biogeochemical Cycles and. II. Biological Metabolism
 Global Biogeochemical Cycles and Biological Metabolism I. Biogeochemistry & Biogeochemical Cycles A. Global cycles: nitrogen, water, carbon B. Carbon cycle through time II. Biological Metabolism A. Redox
Global Biogeochemical Cycles and Biological Metabolism I. Biogeochemistry & Biogeochemical Cycles A. Global cycles: nitrogen, water, carbon B. Carbon cycle through time II. Biological Metabolism A. Redox
