organisms CaCO 3 + H 2 O + CO 2 shallow water
|
|
|
- James Hines
- 5 years ago
- Views:
Transcription
1 Weathering and Reverse weathering Step I:Weathering of igneous rocks 1. Igneous rocks are mainly composed of Al, Si and O 2 with minor and varying quantities of Na, K, Ca and Mg composing pheldspar minerals 2. these minerals are weathered according to the following equations: CaAl 2 Si 2 O 8 Anorthite Ca 2+ 2KAlSi 3 O 8 + 9H 2 O + 6CO 2 2K + + 8SiO 2 (aq) + 3Al 2 Si 2 O 5 (OH) 4 Potassium feldspar 2Na + (Kaolinite) 2NaAlSi 3 O 8-6HCO 3 Sodium feldspar Igneous rock Rain water Seawater Detritus Step II: Equilibration in ocean 3Al 2 Si 2 O 5 (OH) 4 + 2K + + 2HCO 3-2K(AlSiO 4 )(OH 2 )O 2 (Si 2 O 4 ) + 5H 2 O + 2CO 2 (deep water) kaolinite + seawater illite Ca HCO 3 - organisms CaCO 3 + H 2 O + CO 2 shallow water 2HCl + 2HCO 3 - Volcanisms 3Cl - + 2H 2 O + 2CO 2
2 Step III: Metamorphosis of Clay KAlSi 3 O 8 Potassium pheldspar 2K(AlSiO 4 )(OH 2 )O 2 (Si 2 O 4 ) + Na + + Cl - + 8SiO 2 Heat NaAlSi 3 O 8 Sodium Pheldspar + HCl + 2SiO 2 + AlSi 2 O 5 (OH) Pressure KAl 2 (AlSi 3 O 10 )(OH) 2 Potassium Mica SiO 2 CLAY + interstitial water Granite + Volcanic gases + Quartz + Pyrophyllite: Step IV: left behind in Ocean Na + + Cl -
3 Characteristics of ph of seawater: Average ph of seawater 8.1±0.2 Buffering capacity of a separate liter of seawater is very limited, addition of only 3mmol of hydrochloric acid will lower the ph to less than 3 Marine system is globally buffered and resists any changes due to the addition of natural acids or bases. Three types of acids exist in seawater: 1. Oxiacids: H 2 CO 3 ; HCO ; H 3 BO 3 ; H 2 BO 3 2. Hydrated metallic ions that react with water (doubly or more charged cations) M(H 2 O) n+ x + H 2 O M(H 2 O) (n-1)+ x-1 + H 3 O + 3. Cations of very weak acidity (alkali and alkaline earth elements)
4 ph is therefore buffered by the action of the buffering system: carbonic acid bicarbonate carbonate And to a lesser extent, the action of the buffering system: Boric acid borate This explains the stability of the ph of seawater at 8.1 ± 0.2 (observation 1) The low buffering capacity of an isolated quantity of seawater is due to the fact that bicarbonate concentration of 1l of seawater is only 2.5x10-3 M; addition of 3 mmole of HCl would bring the ph lower than 6
5 How can we explain the contradiction between the limited buffering capacity of seawater and the very limited ph variability? Short term processes 1. Continuous mixing of seawater 2. Biogeochemical cycle of carbon Long term processes 1. Ion exchange between water and aluminosilicate minerals 3 Al 2 Si 2 O 5 (OH) 4(solid) + 4SiO 2 + 2K + + 2Ca 2+ +9H 2 O 2KCaAl 3 Si 5 O 16 (H 2 O) 6(s) + 6H + log k = 6log [H + ] 2log [K + ] - 2log [Ca + ] Buffering capacity of silicates is 2000 times the carbonate system buffering capacity Conclusion: 1. Carbonate system is a short term buffer, 2. In the absence of carbonate, boric acid/borate is the short term buffer 3. Silica is the long term buffering system 4. Ion exchange regulates the concentration of the major cations Na +, K +, Ca 2+ and Mg Silicate minerals control the concentration of dissolved silica in seawater
6 The iron paradox!! Dissolved iron concentration in seawater is higher than that expected from the dissolution of iron oxides! Seawater is an oxidizing environment as oxygen was added to the model ocean (0.027 mol) Most of the oxygen stays in the gaseous form and only 2x10-4 mol l -1 is soluble Dissolved oxygen assures an oxidizing potential expressed by the equation: E 0 = standard Redox potential R = constant = (8314 mv coulomb deg -1 mol -1 ) T = absolute temperature F = Faradays constant (96500 coulomb mol -1 ) RTF -1 ln10 = mv at 25 O C = mv at 0 O C pe = - log e - = E 0 /RTF -1 ln10
7 Calculation of pe The following equilibrium determines the pe of seawater or an ideal system: 0.5 O 2 + 2H + + 2e - H 2 O log k = log k = log (H 2 O) 0.5 log po 2 2log (H + ) 2log (e - ) = log (0.21) + 2 ph + 2 pe = (2 x 8.1) + 2 pe pe = 0.5 (25.5) = 12.5 Any increase of ph is accompanied with a decrease of pe po 2 has weak influence on the pe
8 pe is the master variable in any oxidation-reduction system Influence of pe on the ferric/ferrous system:
9 Concentration of dissolved iron (Fe 2+, Fe 3+ ) is controlled by the pe or the ph of the medium This does not appear true in the presence of solid iron oxides Three equilibria control the oxidation/reduction reactions of iron Fe(OOH) s + H + Fe(OH) 2 + log k = (1) Fe(OOH) 3 + 3H + Fe H 2 O log k = 41.0 (2) e - + Fe 3+ Fe 2+ log k = 13.0 (3) Sillén considered equation 1 as the most important as it gives concentration of Fe(OH) 2 + ions of M at ph of seawater 8.1 However dissolved iron concentration in seawater is higher than M Addition of equations 2&3 gives the concentration of ferrous iron Fe 2+ = M Fe(OOH) 3 + 3H + + e - Fe H 2 O log k = 17 It appears that the concentrations of Fe 2+ and Fe 2+ at equilibrium are lower than the measured iron concentration in seawater Most of the iron present in solution as colloidal iron and iron organic complexes particularly of humic acids
The Lithosphere. Definition
 10/14/2014 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2014 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
10/14/2014 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2014 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
Redox, ph, pe OUTLINE 9/12/17. Equilibrium? Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox
 Redox, ph, pe Equilibrium? OUTLINE Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox Reading: White p555-563 1 Question of the day? So what about the CO 2 system? CO
Redox, ph, pe Equilibrium? OUTLINE Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox Reading: White p555-563 1 Question of the day? So what about the CO 2 system? CO
Sedimentary Rocks and Processes
 Sedimentary Rocks and Processes Weathering Sedimentary Processes Breakdown of pre-existing rock by physical and chemical processes Transport Movement of sediments from environments of relatively high potential
Sedimentary Rocks and Processes Weathering Sedimentary Processes Breakdown of pre-existing rock by physical and chemical processes Transport Movement of sediments from environments of relatively high potential
Lecture 4 What Controls the Composition of Seawater
 Lecture 4 What Controls the Composition of Seawater Seawater is salty! Why? What controls the composition of seawater? Do Chemical Equilibrium reactions control the composition of the Ocean? What is meant
Lecture 4 What Controls the Composition of Seawater Seawater is salty! Why? What controls the composition of seawater? Do Chemical Equilibrium reactions control the composition of the Ocean? What is meant
(4) Give an example of important reactions that are responsible for the composition of river water.
 Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
2.2 Acid mine drainage
 PROBLEM SOLVING 2. CHEMICAL REACTIONS AND EQUILIBRIA 1 2.2 Acid mine drainage Problem 2.2 The weathering of iron sulphide minerals produces acidified water, leading to major environmental problems from
PROBLEM SOLVING 2. CHEMICAL REACTIONS AND EQUILIBRIA 1 2.2 Acid mine drainage Problem 2.2 The weathering of iron sulphide minerals produces acidified water, leading to major environmental problems from
Weathering and Erosion
 Weathering and Erosion Weathering the disintegration and decomposition of material at the surface Erosion the transportation of weathered material by water, wind, or ice Weathering Two kinds of weathering
Weathering and Erosion Weathering the disintegration and decomposition of material at the surface Erosion the transportation of weathered material by water, wind, or ice Weathering Two kinds of weathering
muscovite PART 4 SHEET SILICATES
 muscovite PART 4 SHEET SILICATES SHEET SILICATES = PHYLLOSILICATES Phyllon = leaf Large group of mineral including many common minerals: muscovite, biotite, serpentine, chlorite, talc, clay minerals Structure:
muscovite PART 4 SHEET SILICATES SHEET SILICATES = PHYLLOSILICATES Phyllon = leaf Large group of mineral including many common minerals: muscovite, biotite, serpentine, chlorite, talc, clay minerals Structure:
ph of natural waters
 ph of natural waters Na 2 CO 3 10H 2 O (natron) 2 Na + + CO 3 + 10H 2 O 4FeS 2 + 15O 2 + 14H 2 O 4 Fe(OH) 3 + 16H + + 8SO 4 4NaAlSi 3 O 8 + 11H 2 O 4Na + + 4OH - + Al 4 Si 4 O 10 (OH) 8 + 8Si(OH) 4 In
ph of natural waters Na 2 CO 3 10H 2 O (natron) 2 Na + + CO 3 + 10H 2 O 4FeS 2 + 15O 2 + 14H 2 O 4 Fe(OH) 3 + 16H + + 8SO 4 4NaAlSi 3 O 8 + 11H 2 O 4Na + + 4OH - + Al 4 Si 4 O 10 (OH) 8 + 8Si(OH) 4 In
Chapter 3: Acid Base Equilibria. HCl + KOH KCl + H 2 O acid + base salt + water
 Chapter 3: Acid Base Equilibria HCl + KOH KCl + H 2 O acid + base salt + water What is an acid? The Arrhenius concept proposed that acids are substances that produce hydrogen ions (H + ) in aqueous solutions.
Chapter 3: Acid Base Equilibria HCl + KOH KCl + H 2 O acid + base salt + water What is an acid? The Arrhenius concept proposed that acids are substances that produce hydrogen ions (H + ) in aqueous solutions.
predictive mineral discovery*cooperative Research Centre A legacy for mineral exploration science Mineral Systems Q3 Fluid reservoirs
 Mineral Systems Q3 Fluid reservoirs 1 Key Parameter Mineral System Exploration is reflected in scale-dependent translation A. Gradient in hydraulic potential B. Permeability C. Solubility sensitivity to
Mineral Systems Q3 Fluid reservoirs 1 Key Parameter Mineral System Exploration is reflected in scale-dependent translation A. Gradient in hydraulic potential B. Permeability C. Solubility sensitivity to
WEATHERING. Turning Rock to Sediment and Solutions 10/22/2012
 WEATHERING Turning Rock to Sediment and Solutions Igneous rocks form at high temperatures; at the Earth s surface they are chemically unstable and will begin to disintegrate and decompose in a process
WEATHERING Turning Rock to Sediment and Solutions Igneous rocks form at high temperatures; at the Earth s surface they are chemically unstable and will begin to disintegrate and decompose in a process
Acid Soil. Soil Acidity and ph
 Acid Soil Soil Acidity and ph ph ph = - log (H + ) H 2 O H + + OH - (H + ) x (OH - )= K w = 10-14 measures H + activity with an electrode (in the lab), solutions (in the field) reflects the acid intensity,
Acid Soil Soil Acidity and ph ph ph = - log (H + ) H 2 O H + + OH - (H + ) x (OH - )= K w = 10-14 measures H + activity with an electrode (in the lab), solutions (in the field) reflects the acid intensity,
Lecture 6. Physical Properties. Solid Phase. Particle Composition
 Lecture 6 Physical Properties Solid Phase Particle Composition 1 Questions What are tetrahedrons and octahedrons? How do silica tetrahedra bonds affect mineral weathering? Difference between primary and
Lecture 6 Physical Properties Solid Phase Particle Composition 1 Questions What are tetrahedrons and octahedrons? How do silica tetrahedra bonds affect mineral weathering? Difference between primary and
Lecture 16 Guest Lecturer this week. Prof. Greg Ravizza
 Lecture 16 Guest Lecturer this week. Prof. Greg Ravizza General Concepts for Natural Controls on Fresh Water Composition 1. Generalizations about freshwater compositions 2. Conservative/nonconservative
Lecture 16 Guest Lecturer this week. Prof. Greg Ravizza General Concepts for Natural Controls on Fresh Water Composition 1. Generalizations about freshwater compositions 2. Conservative/nonconservative
Groundwater chemistry
 Read: Ch. 3, sections 1, 2, 3, 5, 7, 9; Ch. 7, sections 2, 3 PART 14 Groundwater chemistry Introduction Matter present in water can be divided into three categories: (1) Suspended solids (finest among
Read: Ch. 3, sections 1, 2, 3, 5, 7, 9; Ch. 7, sections 2, 3 PART 14 Groundwater chemistry Introduction Matter present in water can be divided into three categories: (1) Suspended solids (finest among
CLASS EXERCISE 5.1 List processes occurring in soils that cause changes in the levels of ions.
 5 SIL CHEMISTRY 5.1 Introduction A knowledge of the chemical composition of a soil is less useful than a knowledge of its component minerals and organic materials. These dictate the reactions that occur
5 SIL CHEMISTRY 5.1 Introduction A knowledge of the chemical composition of a soil is less useful than a knowledge of its component minerals and organic materials. These dictate the reactions that occur
Weathering and Soils
 OCN 401-17 Aug. 29, 2016 KCR Weathering and Soils Biogeochemistry Chapter 4: The Lithosphere Introduction: the context Rock Weathering Soil Chemical Reactions Soil Development (see text) Weathering Rates
OCN 401-17 Aug. 29, 2016 KCR Weathering and Soils Biogeochemistry Chapter 4: The Lithosphere Introduction: the context Rock Weathering Soil Chemical Reactions Soil Development (see text) Weathering Rates
Wednesday week 12. These ions move through the soil to streams and eventually to the ocean. In the ocean; CaCO 3 + H 2 O + CO 2 H 2 O + H 2 O
 Wednesday week 12 I. Control of CO 2 content of atmosphere by the ocean H 4 SiO 4 A. Consider a hypothetical planet with a crust made of single mineral (Wallastonite) CaSiO3. We could use the composition
Wednesday week 12 I. Control of CO 2 content of atmosphere by the ocean H 4 SiO 4 A. Consider a hypothetical planet with a crust made of single mineral (Wallastonite) CaSiO3. We could use the composition
Learning Outcomes: At the end of this assignment, students will be able to:
 Chemical Equilibria & Sample Preparation Purpose: The purpose of this assignment is to predict how solute concentrations are controlled by chemical equilibria, understand the chemistry involved with sample
Chemical Equilibria & Sample Preparation Purpose: The purpose of this assignment is to predict how solute concentrations are controlled by chemical equilibria, understand the chemistry involved with sample
Continent-Ocean Interaction: Role of Weathering
 Institute of Astrophysics and Geophysics (Build. B5c) Room 0/13 email: Guy.Munhoven@ulg.ac.be Phone: 04-3669771 28th February 2018 Organisation of the Lecture 1 Carbon cycle processes time scales modelling:
Institute of Astrophysics and Geophysics (Build. B5c) Room 0/13 email: Guy.Munhoven@ulg.ac.be Phone: 04-3669771 28th February 2018 Organisation of the Lecture 1 Carbon cycle processes time scales modelling:
CEE 370 Environmental Engineering Principles. Equilibrium Chemistry
 Updated: 9 September 015 Print version CEE 370 Environmental Engineering Principles Lecture #6 Environmental Chemistry IV: Thermodynamics, Equilibria, Acids-bases I Reading: Mihelcic & Zimmerman, Chapter
Updated: 9 September 015 Print version CEE 370 Environmental Engineering Principles Lecture #6 Environmental Chemistry IV: Thermodynamics, Equilibria, Acids-bases I Reading: Mihelcic & Zimmerman, Chapter
Geology 560, Prof. Thomas Johnson, Fall 2003 Unit II: Chemical reactions: Guide Questions
 Geology 560, Prof. Thomas Johnson, Fall 2003 Unit II: Chemical reactions: Guide Questions Goals: Refresh your memory on the basics of chemical reactions Ponder the chaos that underlies chemical reactions;
Geology 560, Prof. Thomas Johnson, Fall 2003 Unit II: Chemical reactions: Guide Questions Goals: Refresh your memory on the basics of chemical reactions Ponder the chaos that underlies chemical reactions;
ph and Acid-Base reactions
 ph and Acid-Base reactions http://eps.mcgill.ca/~courses/c220/ Interaction of water with H + (H 3 O + ) Although it is a minor component of most aqueous solutions, the proton, hydrogen ion or H + concentration
ph and Acid-Base reactions http://eps.mcgill.ca/~courses/c220/ Interaction of water with H + (H 3 O + ) Although it is a minor component of most aqueous solutions, the proton, hydrogen ion or H + concentration
Mid-Term #1 (125 points total)
 Ocean 520 Name: Chemical Oceanography 20 October 2009 Fall 2009 Points are in parentheses (show all your work) (use back if necessary) MidTerm #1 (125 points total) 1. Doney et al (2008) Ocean Acidification
Ocean 520 Name: Chemical Oceanography 20 October 2009 Fall 2009 Points are in parentheses (show all your work) (use back if necessary) MidTerm #1 (125 points total) 1. Doney et al (2008) Ocean Acidification
Cation Exchange Capacity, CEC
 Cation Exchange Capacity, CEC The basic building blocks of clay minerals are: silicon atoms surrounded by four oxygen atoms (tetrahedra), and aluminium atoms surrounded by six hydroxide groups (dioctahedra),
Cation Exchange Capacity, CEC The basic building blocks of clay minerals are: silicon atoms surrounded by four oxygen atoms (tetrahedra), and aluminium atoms surrounded by six hydroxide groups (dioctahedra),
Sedimentary Geology. Strat and Sed, Ch. 1 1
 Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
Hydrological Cycle Rain and rivers OUTLINE
 Hydrological Cycle Rain and rivers The Hydrosphere Rain and rivers OUTLINE 1 Generalizations (non-political conservatism) Conservative (not affected) and Non-Conservative (affected) Ions Distinction: whether
Hydrological Cycle Rain and rivers The Hydrosphere Rain and rivers OUTLINE 1 Generalizations (non-political conservatism) Conservative (not affected) and Non-Conservative (affected) Ions Distinction: whether
CH 221 Chapter Four Part II Concept Guide
 CH 221 Chapter Four Part II Concept Guide 1. Solubility Why are some compounds soluble and others insoluble? In solid potassium permanganate, KMnO 4, the potassium ions, which have a charge of +1, are
CH 221 Chapter Four Part II Concept Guide 1. Solubility Why are some compounds soluble and others insoluble? In solid potassium permanganate, KMnO 4, the potassium ions, which have a charge of +1, are
WEATHERING. Weathering breakdown of rock materials Erosion transport of broken-down materials
 WEATHERING the interacting physical, chemical & biological processes that progressively alter the original lithologic character of rocks to produce secondary minerals (e.g. clays) & unconsolidated regolith
WEATHERING the interacting physical, chemical & biological processes that progressively alter the original lithologic character of rocks to produce secondary minerals (e.g. clays) & unconsolidated regolith
LAB 2: SILICATE MINERALS
 GEOLOGY 640: Geology through Global Arts and Artifacts LAB 2: SILICATE MINERALS FRAMEWORK SILICATES The framework silicates quartz and feldspar are the most common minerals in Earth s crust. Quartz (SiO
GEOLOGY 640: Geology through Global Arts and Artifacts LAB 2: SILICATE MINERALS FRAMEWORK SILICATES The framework silicates quartz and feldspar are the most common minerals in Earth s crust. Quartz (SiO
LAB 5: COMMON MINERALS IN IGNEOUS ROCKS
 EESC 2100: Mineralogy LAB 5: COMMON MINERALS IN IGNEOUS ROCKS Part 1: Minerals in Granitic Rocks Learning Objectives: Students will be able to identify the most common minerals in granitoids Students will
EESC 2100: Mineralogy LAB 5: COMMON MINERALS IN IGNEOUS ROCKS Part 1: Minerals in Granitic Rocks Learning Objectives: Students will be able to identify the most common minerals in granitoids Students will
Chapter 7: Anion and molecular retention
 I. Anions and molecules of importance in soils Anions of major importance to agricultural soils and soil chemistry are: H 2 PO - 4, HPO 2-4, SO 2-4, HCO - 3, NO - 3, Cl -, F - and OH -. Also, micronutrients
I. Anions and molecules of importance in soils Anions of major importance to agricultural soils and soil chemistry are: H 2 PO - 4, HPO 2-4, SO 2-4, HCO - 3, NO - 3, Cl -, F - and OH -. Also, micronutrients
Redox reactions.
 Redox reactions http://eps.mcgill.ca/~courses/c220/ Redox reactions In a similar way that acids and bases have been defined as proton donors and proton acceptors, reductants and oxidants are defined as
Redox reactions http://eps.mcgill.ca/~courses/c220/ Redox reactions In a similar way that acids and bases have been defined as proton donors and proton acceptors, reductants and oxidants are defined as
Ocean Chemical Dynamics. EAS 2200 Lecture 21
 Ocean Chemical Dynamics EAS 2200 Lecture 21 Summary of Water s H-bonding accounts for high heat capacity, latent heats of fusion and evaporation. important for heat transport, transfer to atmosphere, and
Ocean Chemical Dynamics EAS 2200 Lecture 21 Summary of Water s H-bonding accounts for high heat capacity, latent heats of fusion and evaporation. important for heat transport, transfer to atmosphere, and
Lect. 2: Chemical Water Quality
 The Islamic University of Gaza Faculty of Engineering Civil Engineering Department M.Sc. Water Resources Water Quality Management (ENGC 6304) Lect. 2: Chemical Water Quality ١ Chemical water quality parameters
The Islamic University of Gaza Faculty of Engineering Civil Engineering Department M.Sc. Water Resources Water Quality Management (ENGC 6304) Lect. 2: Chemical Water Quality ١ Chemical water quality parameters
Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions
 Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions OCN 623 Chemical Oceanography Reading: Libes, Chapters 15 and 16 Outline I. Deep sea sedimentation Detrital sediments
Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions OCN 623 Chemical Oceanography Reading: Libes, Chapters 15 and 16 Outline I. Deep sea sedimentation Detrital sediments
Weathering and Soils
 OCN 401-11 Oct. 13, 2011 KCR Weathering and Soils Biogeochemistry Chapter 4: The Lithosphere Introduction: the context Rock Weathering Soil Chemical Reactions Soil Development (see text) Weathering Rates
OCN 401-11 Oct. 13, 2011 KCR Weathering and Soils Biogeochemistry Chapter 4: The Lithosphere Introduction: the context Rock Weathering Soil Chemical Reactions Soil Development (see text) Weathering Rates
Salinity. See Appendix 1 of textbook x10 3 = See Appendix 1 of textbook
 Length Area Volume m m m foot = 0.305m yard = 0.91m square feet ~0.09m2 US pint ~ 0.47 L fl. oz. ~0.02 L Speed m/s mph Acceleration m/s mph/s Weight kg, gram pound ~0.45kg Temperature o o See Appendix
Length Area Volume m m m foot = 0.305m yard = 0.91m square feet ~0.09m2 US pint ~ 0.47 L fl. oz. ~0.02 L Speed m/s mph Acceleration m/s mph/s Weight kg, gram pound ~0.45kg Temperature o o See Appendix
1/31/2013. Weathering Includes Physical, Chemical, Biological processes. Weathering Mechanisms. Wind abrasion forming Ventifacts
 Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
Weathering Cycle Teacher Notes
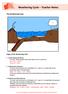 The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
Lecture 15: Adsorption; Soil Acidity
 Lecture 15: Adsorption; Soil Acidity Surface Complexation (Your textbook calls this adsorption ) Surface Complexation Both cations and anions can bind to sites on the external surfaces of soil minerals
Lecture 15: Adsorption; Soil Acidity Surface Complexation (Your textbook calls this adsorption ) Surface Complexation Both cations and anions can bind to sites on the external surfaces of soil minerals
Soil Colloidal Chemistry. Compiled and Edited by Dr. Syed Ismail, Marthwada Agril. University Parbhani,MS, India
 Soil Colloidal Chemistry Compiled and Edited by Dr. Syed Ismail, Marthwada Agril. University Parbhani,MS, India 1 The Colloidal Fraction Introduction What is a colloid? Why this is important in understanding
Soil Colloidal Chemistry Compiled and Edited by Dr. Syed Ismail, Marthwada Agril. University Parbhani,MS, India 1 The Colloidal Fraction Introduction What is a colloid? Why this is important in understanding
Effect of chemical composition to large scale CO 2 Injection in Morrow Sandstone, Farnsworth Hydrocarbon Field, Texas, USA
 Effect of chemical composition to large scale CO 2 Injection in Morrow Sandstone, Farnsworth Hydrocarbon Field, Texas, USA Bulbul Ahmmed Martin Appold Department of Geological Sciences University of Missouri-Columbia
Effect of chemical composition to large scale CO 2 Injection in Morrow Sandstone, Farnsworth Hydrocarbon Field, Texas, USA Bulbul Ahmmed Martin Appold Department of Geological Sciences University of Missouri-Columbia
Global Carbon Cycle - I
 Global Carbon Cycle - I OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 11 1. Overview of global C cycle 2. Global C reservoirs Outline 3. The contemporary global C cycle 4. Fluxes and residence
Global Carbon Cycle - I OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 11 1. Overview of global C cycle 2. Global C reservoirs Outline 3. The contemporary global C cycle 4. Fluxes and residence
EPSC 233. Compositional variation in minerals. Recommended reading: PERKINS, p. 286, 41 (Box 2-4).
 EPSC 233 Compositional variation in minerals Recommended reading: PERKINS, p. 286, 41 (Box 2-4). Some minerals are nearly pure elements. These are grouped under the category of native elements. This includes
EPSC 233 Compositional variation in minerals Recommended reading: PERKINS, p. 286, 41 (Box 2-4). Some minerals are nearly pure elements. These are grouped under the category of native elements. This includes
Search and Discovery Article #50999 (2014)** Posted August 18, Abstract
 Oil Degradation in the Gullfaks Field (Norway): How Hydrogeochemical Modeling can Help to Decipher Organic- Inorganic Interactions Controlling CO 2 Fate and Behavior* Wolfgang van Berk 1, Yunjiao Fu 2,
Oil Degradation in the Gullfaks Field (Norway): How Hydrogeochemical Modeling can Help to Decipher Organic- Inorganic Interactions Controlling CO 2 Fate and Behavior* Wolfgang van Berk 1, Yunjiao Fu 2,
Processes affecting continental shelves
 Marine Sediments Continental Shelves Processes affecting continental shelves 1. Glaciation 2. Sea-level change (±130 m during continental glaciation) 3. Waves and currents 4. Sedimentation 5. Carbonate
Marine Sediments Continental Shelves Processes affecting continental shelves 1. Glaciation 2. Sea-level change (±130 m during continental glaciation) 3. Waves and currents 4. Sedimentation 5. Carbonate
Equilibria in electrolytes solutions and water hardness
 Equilibria in electrolytes solutions and water hardness by Urszula LelekBorkowska Electrolytes When the substance dissolved in water or other solvent exhibits electrical conductivity it is called an electrolyte.
Equilibria in electrolytes solutions and water hardness by Urszula LelekBorkowska Electrolytes When the substance dissolved in water or other solvent exhibits electrical conductivity it is called an electrolyte.
Chemistry and the Earth. Martin F. Schmidt, Jr.
 Chemistry and the Earth Martin F. Schmidt, Jr. How were the elements made? How stars make elements. http://www.valdosta.edu/~cbarnbau/astro_ demos/stellar_evol/home_stellar.html Low mass - do #3 first,
Chemistry and the Earth Martin F. Schmidt, Jr. How were the elements made? How stars make elements. http://www.valdosta.edu/~cbarnbau/astro_ demos/stellar_evol/home_stellar.html Low mass - do #3 first,
Lecture 5. Introduction to Stable Isotopes
 Lecture 5 Introduction to Stable Isotopes Stable Isotope Geochemistry Primarily concerned with the isotope ratios of H, C, N, O, and S Si and B often included and new instrumentation has opened up others
Lecture 5 Introduction to Stable Isotopes Stable Isotope Geochemistry Primarily concerned with the isotope ratios of H, C, N, O, and S Si and B often included and new instrumentation has opened up others
SoilGen2 model: data requirements and model output
 SoilGen2 model: data requirements and model output 1. Essential plot data... 1 2. Essential soil data... 2 3. Precipitation data (for a typical year)... 2 4. Evapotranspiration and Air temperature data
SoilGen2 model: data requirements and model output 1. Essential plot data... 1 2. Essential soil data... 2 3. Precipitation data (for a typical year)... 2 4. Evapotranspiration and Air temperature data
Salinity. foot = 0.305m yard = 0.91m. Length. Area m 2 square feet ~0.09m2. Volume m 3 US pint ~ 0.47 L fl. oz. ~0.02 L.
 Length m foot = 0.305m yard = 0.91m Area m 2 square feet ~0.09m2 Volume m 3 US pint ~ 0.47 L, L (liters) fl. oz. ~0.02 L Speed m/s mph Acceleration m/s 2 mph/s Weight kg, gram pound ~0.45kg Temperature
Length m foot = 0.305m yard = 0.91m Area m 2 square feet ~0.09m2 Volume m 3 US pint ~ 0.47 L, L (liters) fl. oz. ~0.02 L Speed m/s mph Acceleration m/s 2 mph/s Weight kg, gram pound ~0.45kg Temperature
Copyright SOIL STRUCTURE and CLAY MINERALS
 SOIL STRUCTURE and CLAY MINERALS Soil Structure Structure of a soil may be defined as the mode of arrangement of soil grains relative to each other and the forces acting between them to hold them in their
SOIL STRUCTURE and CLAY MINERALS Soil Structure Structure of a soil may be defined as the mode of arrangement of soil grains relative to each other and the forces acting between them to hold them in their
About Earth Materials
 Grotzinger Jordan Understanding Earth Sixth Edition Chapter 3: EARTH MATERIALS Minerals and Rocks 2011 by W. H. Freeman and Company About Earth Materials All Earth materials are composed of atoms bound
Grotzinger Jordan Understanding Earth Sixth Edition Chapter 3: EARTH MATERIALS Minerals and Rocks 2011 by W. H. Freeman and Company About Earth Materials All Earth materials are composed of atoms bound
Ionic equation - shows the ions present - illustrates a reaction OR a process like dissolving (physical change)
 Chapter 4: Table 4.8 Soluble dissolves in (or other solvent, if specified) Insoluble doesn t dissolve in (or other solvent, if specified) NaCl, an ionic compound, dissolves in. Na + and Cl - ions are no
Chapter 4: Table 4.8 Soluble dissolves in (or other solvent, if specified) Insoluble doesn t dissolve in (or other solvent, if specified) NaCl, an ionic compound, dissolves in. Na + and Cl - ions are no
Overview. Rock weathering Functions of soil Soil forming factors Soil properties
 UN-FAO A. Healthy soils are the basis for healthy food production. B. A tablespoon of normal topsoil has more microorganisms than the entire human population on Earth. C. It can take up to 1,000 years
UN-FAO A. Healthy soils are the basis for healthy food production. B. A tablespoon of normal topsoil has more microorganisms than the entire human population on Earth. C. It can take up to 1,000 years
A Rock is a solid aggregate of minerals.
 Quartz A Rock is a solid aggregate of minerals. Orthoclase Feldspar Plagioclase Feldspar Biotite Four different minerals are obvious in this piece of Granite. The average automobile contains: Minerals
Quartz A Rock is a solid aggregate of minerals. Orthoclase Feldspar Plagioclase Feldspar Biotite Four different minerals are obvious in this piece of Granite. The average automobile contains: Minerals
INTRODUCTION TO CO2 CHEMISTRY
 INTRODUCTION TO CO2 CHEMISTRY IN SEA WATER Andrew G. Dickson Scripps Institution of Oceanography, UC San Diego Mauna Loa Observatory, Hawaii Monthly Average Carbon Dioxide Concentration Data from Scripps
INTRODUCTION TO CO2 CHEMISTRY IN SEA WATER Andrew G. Dickson Scripps Institution of Oceanography, UC San Diego Mauna Loa Observatory, Hawaii Monthly Average Carbon Dioxide Concentration Data from Scripps
Chapter 4 Reactions in Aqueous Solution
 Chapter 4 Reactions in Aqueous Solution Homework Chapter 4 11, 15, 21, 23, 27, 29, 35, 41, 45, 47, 51, 55, 57, 61, 63, 73, 75, 81, 85 1 2 Chapter Objectives Solution To understand the nature of ionic substances
Chapter 4 Reactions in Aqueous Solution Homework Chapter 4 11, 15, 21, 23, 27, 29, 35, 41, 45, 47, 51, 55, 57, 61, 63, 73, 75, 81, 85 1 2 Chapter Objectives Solution To understand the nature of ionic substances
A few more details on clays, Soil Colloids and their properties. What expandable clays do to surface area. Smectite. Kaolinite.
 A few more details on clays, Soil Colloids and their properties What expandable clays do to surface area Kaolinite Smectite Size 0.5-5 µm External surface 10-30 m 2 /g Internal surface - Size 0.1-1 µm
A few more details on clays, Soil Colloids and their properties What expandable clays do to surface area Kaolinite Smectite Size 0.5-5 µm External surface 10-30 m 2 /g Internal surface - Size 0.1-1 µm
Earth and Planetary Materials
 Earth and Planetary Materials Spring 2013 Lecture 4 2013.01.16 Example Beryl Be 3 Al 2 (SiO 3 ) 6 Goshenite Aquamarine Emerald Heliodor Red beryl Morganite pure Fe 2+ & Fe 3+ Cr 3+ Fe 3+ Mn 3+ Mn 2+ Rules
Earth and Planetary Materials Spring 2013 Lecture 4 2013.01.16 Example Beryl Be 3 Al 2 (SiO 3 ) 6 Goshenite Aquamarine Emerald Heliodor Red beryl Morganite pure Fe 2+ & Fe 3+ Cr 3+ Fe 3+ Mn 3+ Mn 2+ Rules
Hydrothermal Chemistry/ Reverse Weathering. Marine Chemistry Seminar
 Hydrothermal Chemistry/ Reverse Weathering Marine Chemistry Seminar 1974 Drever, The Sea Chapter 10:The Magnesium Problem 1979 Edmonds et al., Ridge Crest Hydrothermal Activity and the Balances of Major
Hydrothermal Chemistry/ Reverse Weathering Marine Chemistry Seminar 1974 Drever, The Sea Chapter 10:The Magnesium Problem 1979 Edmonds et al., Ridge Crest Hydrothermal Activity and the Balances of Major
LAB 3: COMMON MINERALS IN SEDIMENTARY ROCKS, Part 1
 EESC 2100: Mineralogy LAB 3: COMMON MINERALS IN SEDIMENTARY ROCKS, Part 1 Learning Objectives: Students will be able to identify minerals that occur commonly in sandstones (quartz and feldspars), both
EESC 2100: Mineralogy LAB 3: COMMON MINERALS IN SEDIMENTARY ROCKS, Part 1 Learning Objectives: Students will be able to identify minerals that occur commonly in sandstones (quartz and feldspars), both
2 EQUILIBRIUM 2.1 WHAT IS EQUILIBRIUM? 2.2 WHEN IS A SYSTEM AT EQUILIBRIUM? 2.3 THE EQUILIBRIUM CONSTANT
 2 EQUILIBRIUM 2.1 WHAT IS EQUILIBRIUM? In general terms equilibrium implies a situation that is unchanging or steady. This is generally achieved through a balance of opposing forces. In chemistry equilibrium
2 EQUILIBRIUM 2.1 WHAT IS EQUILIBRIUM? In general terms equilibrium implies a situation that is unchanging or steady. This is generally achieved through a balance of opposing forces. In chemistry equilibrium
Solutions to CHEM 301 Review Exercises
 Solutions to CHEM 301 Review Eercises naming 1. a) calcium phosphate b) chromium (III) oide c) chlorine dioide. a) NaOCl b) HgSO 4 significant figures 3. [H + ] 1.5 10 6 M has three significant figures,
Solutions to CHEM 301 Review Eercises naming 1. a) calcium phosphate b) chromium (III) oide c) chlorine dioide. a) NaOCl b) HgSO 4 significant figures 3. [H + ] 1.5 10 6 M has three significant figures,
Rock Weathering (West, Ch. 8)
 Geology 229 Engineering Geology Lecture 23 Rock Weathering (West, Ch. 8) Outline Introduction of weathering Mechanical weathering Chemical weathering Comparison of surface and subsurface conditions Subsurface
Geology 229 Engineering Geology Lecture 23 Rock Weathering (West, Ch. 8) Outline Introduction of weathering Mechanical weathering Chemical weathering Comparison of surface and subsurface conditions Subsurface
INTRODUCTION TO CO2 CHEMISTRY
 INTRODUCTION TO CO2 CHEMISTRY IN SEA WATER Andrew G. Dickson Scripps Institution of Oceanography, UC San Diego 410 Mauna Loa Observatory, Hawaii Monthly Average Carbon Dioxide Concentration Data from Scripps
INTRODUCTION TO CO2 CHEMISTRY IN SEA WATER Andrew G. Dickson Scripps Institution of Oceanography, UC San Diego 410 Mauna Loa Observatory, Hawaii Monthly Average Carbon Dioxide Concentration Data from Scripps
A horizon. Clay Basics. Clays: A horizon. Phyllosilicates phyllon = leaves. Clay formation. Zone were parent materials weather
 A horizon lay Basics Start focus on A horizon Zone were parent materials weather Original rocks and minerals break down into smaller and smaller pieces Eventually dissolve A horizon Zone were new materials
A horizon lay Basics Start focus on A horizon Zone were parent materials weather Original rocks and minerals break down into smaller and smaller pieces Eventually dissolve A horizon Zone were new materials
Classification of Igneous Rocks
 Classification of Igneous Rocks Textures: Glassy- no crystals formed Aphanitic- crystals too small to see by eye Phaneritic- can see the constituent minerals Fine grained- < 1 mm diameter Medium grained-
Classification of Igneous Rocks Textures: Glassy- no crystals formed Aphanitic- crystals too small to see by eye Phaneritic- can see the constituent minerals Fine grained- < 1 mm diameter Medium grained-
Chapter 12: Acids and Bases: Ocean Carbonate System James Murray 4/30/01 Univ. Washington
 Chapter 12: Acids and Bases: Ocean Carbonate System James Murray 4/30/01 Univ. Washington Last lecture was concerned with gas exchange and one example we looked at was the solubility of CO 2. Next we have
Chapter 12: Acids and Bases: Ocean Carbonate System James Murray 4/30/01 Univ. Washington Last lecture was concerned with gas exchange and one example we looked at was the solubility of CO 2. Next we have
Lecture series outline
 Water Quality 2 Lecture series outline Lecture 1a Water uses, water quality guidelines; physical, chemical and biological parameters Lecture 1b The origins of the constituents of water quality through
Water Quality 2 Lecture series outline Lecture 1a Water uses, water quality guidelines; physical, chemical and biological parameters Lecture 1b The origins of the constituents of water quality through
Soil ph: Review of Concepts
 Soils and Water, Spring 008 Soil ph: Review of Concepts Acid: substance that can donate a proton Base: substance that can accept a proton HA H A HA and A - are called conjugate acid-base pairs. The strength
Soils and Water, Spring 008 Soil ph: Review of Concepts Acid: substance that can donate a proton Base: substance that can accept a proton HA H A HA and A - are called conjugate acid-base pairs. The strength
Weathering, Soil, & Mass Movements. Chapter 5
 Weathering, Soil, & Mass Movements Chapter 5 5.1 Weathering Weathering: the breaking down and changing of rocks at or near the Earth s surface. Basic part of the rock cycle. 2 main types: 1. Mechanical
Weathering, Soil, & Mass Movements Chapter 5 5.1 Weathering Weathering: the breaking down and changing of rocks at or near the Earth s surface. Basic part of the rock cycle. 2 main types: 1. Mechanical
Chapter Test B. Chapter: Chemical Equilibrium. following equilibrium system? 2CO(g) O 2 (g) ^ 2CO 2 (g)
 Assessment Chapter Test B Chapter: Chemical Equilibrium PART I In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. What is
Assessment Chapter Test B Chapter: Chemical Equilibrium PART I In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. What is
Particles in aqueous environments
 Lecture 11 Particle-Aqueous Solute Interactions Today 1. Particle types and sizes 2. Particle charges 3. Particle-solute Interactions Next time Please continue to read Manahan Chapter 4. 1. Fresh-salt
Lecture 11 Particle-Aqueous Solute Interactions Today 1. Particle types and sizes 2. Particle charges 3. Particle-solute Interactions Next time Please continue to read Manahan Chapter 4. 1. Fresh-salt
GEOCHEMISTRY, GROUNDWATER AND POLLUTION,
 GEOCHEMISTRY, GROUNDWATER AND POLLUTION, 2 ND EDITION C.A.J. APPELO Hydrochemical Consultant, Amsterdam, the Netherlands D. POSTMA Environment & Resources DTU, Technical University of Denmark, Kgs. Lyngby,
GEOCHEMISTRY, GROUNDWATER AND POLLUTION, 2 ND EDITION C.A.J. APPELO Hydrochemical Consultant, Amsterdam, the Netherlands D. POSTMA Environment & Resources DTU, Technical University of Denmark, Kgs. Lyngby,
Standard Methods for the Examination of Water and Wastewater
 4500-CO 2 CARBON DIOXIDE*#(1) 4500-CO 2 A. Introduction 1. Occurrence and Significance Surface waters normally contain less than 10 mg free carbon dioxide (CO 2 ) per liter while some groundwaters may
4500-CO 2 CARBON DIOXIDE*#(1) 4500-CO 2 A. Introduction 1. Occurrence and Significance Surface waters normally contain less than 10 mg free carbon dioxide (CO 2 ) per liter while some groundwaters may
Chapter 6. Weathering, Erosion, and Soil
 Chapter 6 Weathering, Erosion, and Soil Introduction Rocks and minerals disintegrate and decompose by the processes of physical and chemical weathering. This breakdown occurs because the parent material
Chapter 6 Weathering, Erosion, and Soil Introduction Rocks and minerals disintegrate and decompose by the processes of physical and chemical weathering. This breakdown occurs because the parent material
Geology 560, Prof. Thomas Johnson, Fall 2009 Class Notes: Unit III Part 2. Weak Acids- Speciation
 Geology 560, Prof. Thomas Johnson, Fall 2009 Class Notes: Unit III Part 2. Weak Acids Speciation Reading: White 6.2; Walther Ch. 6 Sections on Acid/Base equilibria, buffers, and carbonate solubility We
Geology 560, Prof. Thomas Johnson, Fall 2009 Class Notes: Unit III Part 2. Weak Acids Speciation Reading: White 6.2; Walther Ch. 6 Sections on Acid/Base equilibria, buffers, and carbonate solubility We
Chapter 6 9/25/2012. Weathering, Erosion and Soils. Introduction. How Are Earth Materials Altered? Introduction. How Are Earth Materials Altered?
 Chapter 6 Introduction Rocks and minerals are disintegrated and decomposed by the processes of mechanical and chemical weathering. Weathering, Erosion and Soils This breakdown occurs because the parent
Chapter 6 Introduction Rocks and minerals are disintegrated and decomposed by the processes of mechanical and chemical weathering. Weathering, Erosion and Soils This breakdown occurs because the parent
) and/or dolomite (CaMg(CO 3
 Environmental Chemistry I Week 4 Alkalinity 1) Alkalinity Alkalinity is defined as the capacity of water to accept H + ions (protons). It can also be defined as the capacity of water to neutralize acids
Environmental Chemistry I Week 4 Alkalinity 1) Alkalinity Alkalinity is defined as the capacity of water to accept H + ions (protons). It can also be defined as the capacity of water to neutralize acids
MAR 510 Chemical Oceanography
 Carbonate Equilibrium -Key Concepts- Major buffer system influencing ph (master variable) Linked to geological, biological and climatological cycles Complex chemistry involving gaseous, dissolved, and
Carbonate Equilibrium -Key Concepts- Major buffer system influencing ph (master variable) Linked to geological, biological and climatological cycles Complex chemistry involving gaseous, dissolved, and
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Mechanisms
 Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes WEATHERING CHAPTER 7 Weathering
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes WEATHERING CHAPTER 7 Weathering
CO 2 and the carbonate system. 1/45
 CO 2 and the carbonate system http://eps.mcgill.ca/~courses/c542/ 1/45 The Atmospheric CO 2 -Climate Connection From: http://www.google.ca/imgres?imgurl=http:/ /www.tallbergfoundation.org/ From: Ruddiman,
CO 2 and the carbonate system http://eps.mcgill.ca/~courses/c542/ 1/45 The Atmospheric CO 2 -Climate Connection From: http://www.google.ca/imgres?imgurl=http:/ /www.tallbergfoundation.org/ From: Ruddiman,
Feldspars. Structure. The feldspars are by far the most abundant group of minerals and are found in igneous, metamorphic and many sedimentary rocks.
 Feldspars The feldspars are by far the most abundant group of minerals and are found in igneous, metamorphic and many sedimentary rocks. Structure Felsdpars are framework silicates where each silica tetrahedra
Feldspars The feldspars are by far the most abundant group of minerals and are found in igneous, metamorphic and many sedimentary rocks. Structure Felsdpars are framework silicates where each silica tetrahedra
Lab 8 Dynamic Soil Systems I: Soil ph and Liming
 Lab 8 Dynamic Soil Systems I: Soil ph and Liming Objectives: To measure soil ph and observe conditions which change ph To distinguish between active acidity (soil solution ph) and exchangeable acidity
Lab 8 Dynamic Soil Systems I: Soil ph and Liming Objectives: To measure soil ph and observe conditions which change ph To distinguish between active acidity (soil solution ph) and exchangeable acidity
Solubility Rules See also Table 4.1 in text and Appendix G in Lab Manual
 Ch 4 Chemical Reactions Ionic Theory of Solutions - Ionic substances produce freely moving ions when dissolved in water, and the ions carry electric current. (S. Arrhenius, 1884) - An electrolyte is a
Ch 4 Chemical Reactions Ionic Theory of Solutions - Ionic substances produce freely moving ions when dissolved in water, and the ions carry electric current. (S. Arrhenius, 1884) - An electrolyte is a
Formation of a salt (ionic compound): Neutralization reaction. molecular. Full ionic. Eliminate spect ions to yield net ionic
 Formation of a salt (ionic compound): Neutralization reaction molecular Full ionic Eliminate spect ions to yield net ionic Hydrolysis/ reaction with water Anions of Weak Acids Consider the weak acid HF
Formation of a salt (ionic compound): Neutralization reaction molecular Full ionic Eliminate spect ions to yield net ionic Hydrolysis/ reaction with water Anions of Weak Acids Consider the weak acid HF
V(STP) No.of mequivalents = n. Analytical chemistry
 Analytical chemistry 1-qualitative analysis. Is concerned with the identification of ions molecules elements and compounds present in sample 2- Quantitative analysis :- Is concerned with the Determination
Analytical chemistry 1-qualitative analysis. Is concerned with the identification of ions molecules elements and compounds present in sample 2- Quantitative analysis :- Is concerned with the Determination
The Lithosphere. Definition
 10/12/2015 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2015 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
10/12/2015 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2015 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
Effects of Weathering and Erosion on the Geochemistry Of Rocks And Soils
 2017 IJSRST Volume 3 Issue 6 Print ISSN: 2395-6011 Online ISSN: 2395-602X Themed Section: Science and Technology Effects of Weathering and Erosion on the Geochemistry Of Rocks And Soils Fagoyinbo Victor
2017 IJSRST Volume 3 Issue 6 Print ISSN: 2395-6011 Online ISSN: 2395-602X Themed Section: Science and Technology Effects of Weathering and Erosion on the Geochemistry Of Rocks And Soils Fagoyinbo Victor
Field Trips. Field Trips
 Field Trips Saturday field trips have been scheduled October 9, October 23 and December 4 Last all day (9:00 AM to 4:00 PM) Bus transportation provided from campus Joint with GG101 laboratory, GG101 Section
Field Trips Saturday field trips have been scheduled October 9, October 23 and December 4 Last all day (9:00 AM to 4:00 PM) Bus transportation provided from campus Joint with GG101 laboratory, GG101 Section
Environments of Mineral Formation. Stability Diagrams
 Environments of Mineral Formation Unary, Binary, and Ternary Mineral Stability Diagrams Minerals of differing composition (or polymorphs of the same mineral) that coexist at a set of pressure (P) temperature
Environments of Mineral Formation Unary, Binary, and Ternary Mineral Stability Diagrams Minerals of differing composition (or polymorphs of the same mineral) that coexist at a set of pressure (P) temperature
NaOH + HCl ---> NaCl + H 2 O
 EXERCISES, LESSON 2 INSTRUCTIONS. Write the word, words, symbols, or numbers that properly completes the statement in the space provided or mark the correct word/phrase from those given. After you complete
EXERCISES, LESSON 2 INSTRUCTIONS. Write the word, words, symbols, or numbers that properly completes the statement in the space provided or mark the correct word/phrase from those given. After you complete
Major Ions, Carbonate System, Alkalinity, ph (Kalff Chapters 13 and 14) There are 7 common ions in freshwater: (e.g.
 Major Ions, Carbonate System, Alkalinity, ph (Kalff Chapters 13 and 14) A. Major ions 1. Common Ions There are 7 common ions in freshwater: (e.g. Table 13-3, p207) cations: Ca +2, Mg +2, Na +1, K +1 anions:
Major Ions, Carbonate System, Alkalinity, ph (Kalff Chapters 13 and 14) A. Major ions 1. Common Ions There are 7 common ions in freshwater: (e.g. Table 13-3, p207) cations: Ca +2, Mg +2, Na +1, K +1 anions:
Stoichiometry: Chemical Calculations. Chemistry is concerned with the properties and the interchange of matter by reaction i.e. structure and change.
 Chemistry is concerned with the properties and the interchange of matter by reaction i.e. structure and change. In order to do this, we need to be able to talk about numbers of atoms. The key concept is
Chemistry is concerned with the properties and the interchange of matter by reaction i.e. structure and change. In order to do this, we need to be able to talk about numbers of atoms. The key concept is
4 Carbonates and CO2
 PhreeqcI Introductory Course (Exercises booklet, chapters 4 and 5) by Manuel Prieto Department of Geology, University of Oviedo, Spain mprieto@ @geol.uniovi.es http://wwwbrr.cr.usgs.gov/projects/gwc_coupled/phreeqci/
PhreeqcI Introductory Course (Exercises booklet, chapters 4 and 5) by Manuel Prieto Department of Geology, University of Oviedo, Spain mprieto@ @geol.uniovi.es http://wwwbrr.cr.usgs.gov/projects/gwc_coupled/phreeqci/
CHEMICAL GEOTHERMOMETERS FOR GEOTHERMAL EXPLORATION
 Presented at Short Course III on Exploration for Geothermal Resources, organized by UNU-GTP and KenGen, at Lake Naivasha, Kenya, October 4 - November 17, 008. GEOTHERMAL TRAINING PROGRAMME Kenya Electricity
Presented at Short Course III on Exploration for Geothermal Resources, organized by UNU-GTP and KenGen, at Lake Naivasha, Kenya, October 4 - November 17, 008. GEOTHERMAL TRAINING PROGRAMME Kenya Electricity
The Nucleus. Protons. Positive electrical charge The number of protons in the nucleus determines the atomic number
 Matter Atoms The smallest unit of an element that retain its properties Small nucleus surrounded by a cloud of electrons The nucleus contains protons and neutrons The Nucleus Protons Positive electrical
Matter Atoms The smallest unit of an element that retain its properties Small nucleus surrounded by a cloud of electrons The nucleus contains protons and neutrons The Nucleus Protons Positive electrical
