(4) Give an example of important reactions that are responsible for the composition of river water.
|
|
|
- Bridget Evans
- 5 years ago
- Views:
Transcription
1 Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The answer lies in the chemical behavior of dissolved constituents as they circulate through the hydrological cycle. Some are more soluble than others. (2) Would you expect rainwater to be more similar to seawater or river water (a) in its total salt content? (b) In the relative composition of dissolved elements in the solution? (a) Rain water will be more similar to river in it salt content (less total salts) (b) and to seawater in the relative composition of salts since most dissolved salts in rain water are from sea salt spray (3) Which of the following relative cation and anion abundances patterns apply to river water (corrected for cyclic salts) and which for seawater? (a) Na> Mg>Ca (b) Ca>Mg>Na (c) HCO 3 >SO 4 >Cl (d) Cl>SO 4 >HCO 3 B and C for River and A and D for seawater (4) Give an example of important reactions that are responsible for the composition of river water. Rock weathering: CaCO 3 + H 2 O + CO 2 == Ca HCO 3 2NaAlSi 3 O 8 + 2H 2 CO 3 + 8H 2 O == Al 2 Si 2 O 5 (OH) 4 +2Na + + 2HCO H 2 SiO 3 (5) If the only source of Na to seawater is weathering how many grams of rock must be weathered to supply the Na for 1 liter of seawater? Assume: ~11g of Na in 1 liter of seawater ~ 2.4% Na in continental rocks ~ 75% of the Na in rocks is leached during weathering reactions. Would weathering of the same weight of rock explain the of Zn, and Br in1 liter of seawater? The Zn, and Br rock contents are 0.007%, and % respectively and their concentrations in seawater 10 7 g/l and g/l. What may that imply? x X x 0.75 = 11; X=~ 600g We need to weather 600 g of rock! For Zn: The amount of Zn in 600 g rock is: x 600 = g; In 1 liter seawater we only have 10 7 g Zn, less than expected. This suggest that Zn does not readily go into solution For Br: The amount on 600 g rock is: x 600 = g; In 1 liter seawater we have g, this is more than in the 600g of rock implying an additional source for Br, e.g. volcanic source.
2 (6) It is sometimes said that weathering consumes acids and releases alkalinity into solution, whereas reverse weathering consumes alkalinity and releases acids. Can you interpret this with the help of a typical weathering equation? H + + CaCO 3 == Ca 2+ + HCO 3 Acid alkalinity 2NaAlSi 3 O 8 + 2H 2 CO 3 + 8H 2 O == Al 2 Si 2 O 5 (OH) 4 +2Na + + 2HCO H 2 SiO acid alkalinity (7) What is the reverse weathering hypothesis? What are some pros and cons for this theory when it was proposed? The major reason for this hypothesis is to resolve the Mg problem and suggest a sink for Mg (as well as a few other elements) it restores mass balance. In this hypothesis Mg precipitates as Mgsilicate minerals in seawater. The problem is that such minerals are not found in abundance and also lab experiments show that this reaction would not occur at ambient conditions in seawater. (8) The breakdown of Kfeldspar to form kaolinite clay is an important weathering reaction, particularly in humid climate soils. The reaction may be written: KAlSi 3 O 8 + H + + 9/2H 2 O(l) = 1/2 Al 2 Si 2 O 5 (OH) 4 + K + + 2H 4 SiO Kfeldspar kaolinite a). Calculate the equilibrium constant for this reaction given the following free energies of formation. Kfeldspar kj/mol H + 0 H 2 O(l) kj/mol kaolinite kj/mol K kj/mol H 4 SiO kj/mol Gr = {1/2(3785.9) (2 x )} ( (9/2 x ) = log K = Gr/ = 3.29 K = = 5.1 x 10 4 b) Assuming that soil moisture contains concentrations of [K + ] = 2 x 10 4 mol/l and [H 4 SiO ] = 2 x 10 4 mol/l, and behaves as an ideal solution (no activity corrections), at what ph would feldspar be in equilibrium with the kaolinite and, therefore not weather? K = K + + kaloinite 1/2 + H 4 SiO / H + Kfeldspar + H 2 O 9/2 = [K] x [H 4 SiO ] 2 / H H + = [K] x [H 4 SiO ] 2 /K; H + = (2 x 10 4 x (2 x 10 4 ) 2 /5.1 x 10 4 ) = 1.57 x 10 8 H + = 1.57 x 10 8 ph = log H + = 7.8
3 (9) Estimate the CO 2 consumed (neutralized) by silicate and carbonate weathering using the following simple model. Assume that on average silicate minerals produce one HCO 3 from each CO 2 consumed while releasing 2H 4 SiO 4 (e.g. silicate + CO 2 + H 2 O= HCO 3 + 2H 4 SiO ). Each CO 2 consumed by carbonate minerals produces two HCO 3. Below is a table with the limestone and silica derived HCO 3 as measured in rivers from various continents. Limestone HCO 3 was calculated from Ca 2+ + Mg 2+ SO 4 2 in rivers; Silica HCO 3 was calculated as ½ moles SiO 2 in rivers. Continent Limestone HCO 3 CO 2 consumed By limestone Silica HCO 3 CO 2 consumed By silicates Europe Asia North America Africa South America World Average * Unites are in mmol/liter Calculate the percent that is neutralized by silicate weathering from total weathering (for the world average). 0.11/0.53 = 0.20; 20% of the CO 2 consumed by weathering is from silicate weathering Why is the % CO 2 consumed by silicate or carbonate weathering so different in the different continents ranging? Different continents have different types of rocks exposed to weathering and different weatherability (how much rock is weathered and added to 1 liter of water)
4 (10) Following is a representation of global biogeochemical cycles from Garrels and Perry (Vol. 5 The Sea) all fluxes are in units of moles/year. Follow the Mg cycle describe who Mg moves from surface rocks through the cycle. What is the major sink of Mg in the ocean? The casual observer might surmise that McKenzie and Garrels could have obtained any result they wanted in their calculation of the fractions of riverine ions that can be removed by precipitating major authigenic phases such as pyrite, anhydrite, calcium carbonates, halite and opal. There are in fact at least three quantitative constraints that limit the possible outcome of their balance. river Which of the following is not a major constraint on Mackenzie and Garrels' model for chemical mass balance between rivers and oceans? 1. The authigenic mineral products formed must be realistic part of the marine sedimentary record. 2. The ocean is assumed to be at steady state, such that all introduced ions should be removed. 3. Authigenic mineral formation can be allowed to proceed until all ions of a given type are completely removed to form authigenic minerals. 4. The reactions for authigenic mineral formation must follow strict stoichiometries. 5. The ionic composition of rivers is assumed to have been the same as present day global average for the last 10 8 years x moles/year MgCO 3 and x moles/year MgSiO 3 are weathered from the rocks and thus x moles/year Mg(HCO 3 ) 2 is the river input to the ocean. This is deposited in the ocean as MgSiO 3 into the deep rock reservoir and eventually through metamorphism and uplift becomes part of the surface rocks. Note the deposition in the sediments through revered weathering reactions this has not been shown to be a significant sink (the model was published before hydrothermal vents were discovered).
5 (11) Which of the following aspects of the proposed model for the control of atmospheric O 2 concentrations over geologic time is/are not true? 1. Extensive weathering of organic matter relative to burial will result in O 2 uptake from the atmosphere during rock weathering 2. O 2 solubility in seawater may provide a feedback, during lower atmospheric oxygen less oxygen will be delivered to the deep ocean resulting in increased organic matter preservation and burial thus restoring atmospheric oxygen. 3. During periods with extensive pyrite burial less organic C would be buried 4. Extensive losses of sedimentary organic matter must occur concurrently with losses of sedimentary pyrite. 5. Substantial increases and decreases in atmospheric O 2 concentrations are possible on time scales of 500,000 years and can have major effects for living organisms. 6. A negative correlation between 13 C of carbonates and 34 S of sulfates is expected. (12) Evaporite sequences were used to constrain the possible range of change in major dissolved salts in seawater on geological time scales: Which of the following aspects in NOT true in accord with this model: 1. evaporite sequences suggest that Ca >> than SO 4 2. HCO 3 must be < 2 X Ca 3. NaCl always precipitates in seawater before KCl and MgSO 4 4. Ca in the ocean could not have been more than 3X its present value 5. Mg concentrations have possibly varied with time but did not exceed MgCO 3 saturation (13) Which of these sequences of events provide a negative feedback to increased/decreased atmospheric pco 2 that result from changes in spreading rate and which is a positive feedback? High spreading rate sea level rise reduction in continental area reduced weathering lower PP High spreading rate (SR) increased temperature increased weathering increased PP High SR sea level rise increased continental slope increased organic C burial at fast sed. rates Low SR sea level drops shelves exposed increased nutrient input from shelvesincreased PP The sequence in 1 and 4 provide a positive feedback while 2 and 3 provide a negative feedback.
Lecture 4 What Controls the Composition of Seawater
 Lecture 4 What Controls the Composition of Seawater Seawater is salty! Why? What controls the composition of seawater? Do Chemical Equilibrium reactions control the composition of the Ocean? What is meant
Lecture 4 What Controls the Composition of Seawater Seawater is salty! Why? What controls the composition of seawater? Do Chemical Equilibrium reactions control the composition of the Ocean? What is meant
Mid-Term #1 (125 points total)
 Ocean 520 Name: Chemical Oceanography 20 October 2009 Fall 2009 Points are in parentheses (show all your work) (use back if necessary) MidTerm #1 (125 points total) 1. Doney et al (2008) Ocean Acidification
Ocean 520 Name: Chemical Oceanography 20 October 2009 Fall 2009 Points are in parentheses (show all your work) (use back if necessary) MidTerm #1 (125 points total) 1. Doney et al (2008) Ocean Acidification
Sedimentary Rocks and Processes
 Sedimentary Rocks and Processes Weathering Sedimentary Processes Breakdown of pre-existing rock by physical and chemical processes Transport Movement of sediments from environments of relatively high potential
Sedimentary Rocks and Processes Weathering Sedimentary Processes Breakdown of pre-existing rock by physical and chemical processes Transport Movement of sediments from environments of relatively high potential
Where is all the water?
 Where is all the water? The distribution of water at the Earth's surface % of total Oceans 97.25 Ice caps and glaciers 2.05 Groundwater 0.68 Lakes 0.01 Soils 0.005 Atmosphere (as vapour) 0.001 Rivers 0.0001
Where is all the water? The distribution of water at the Earth's surface % of total Oceans 97.25 Ice caps and glaciers 2.05 Groundwater 0.68 Lakes 0.01 Soils 0.005 Atmosphere (as vapour) 0.001 Rivers 0.0001
1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and
 1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and climate change e) Oceanic water residence times 3.
1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and climate change e) Oceanic water residence times 3.
Global Carbon Cycle - I
 Global Carbon Cycle - I Reservoirs and Fluxes OCN 401 - Biogeochemical Systems 13 November 2012 Reading: Schlesinger, Chapter 11 Outline 1. Overview of global C cycle 2. Global C reservoirs 3. The contemporary
Global Carbon Cycle - I Reservoirs and Fluxes OCN 401 - Biogeochemical Systems 13 November 2012 Reading: Schlesinger, Chapter 11 Outline 1. Overview of global C cycle 2. Global C reservoirs 3. The contemporary
Lecture 6 - Determinants of Seawater Composition. Sets up electric dipole because O is more electronegative A o. Figure 3.
 12.742 - Marine Chemistry Fall 2004 Lecture 6 - Determinants of Seawater Composition Prof. Scott Doney What is seawater? Water Dissolved inorganic salts (major ions) Trace species, organics, colloids,
12.742 - Marine Chemistry Fall 2004 Lecture 6 - Determinants of Seawater Composition Prof. Scott Doney What is seawater? Water Dissolved inorganic salts (major ions) Trace species, organics, colloids,
Continent-Ocean Interaction: Role of Weathering
 Institute of Astrophysics and Geophysics (Build. B5c) Room 0/13 email: Guy.Munhoven@ulg.ac.be Phone: 04-3669771 28th February 2018 Organisation of the Lecture 1 Carbon cycle processes time scales modelling:
Institute of Astrophysics and Geophysics (Build. B5c) Room 0/13 email: Guy.Munhoven@ulg.ac.be Phone: 04-3669771 28th February 2018 Organisation of the Lecture 1 Carbon cycle processes time scales modelling:
Global Carbon Cycle - I
 Global Carbon Cycle - I OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 11 1. Overview of global C cycle 2. Global C reservoirs Outline 3. The contemporary global C cycle 4. Fluxes and residence
Global Carbon Cycle - I OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 11 1. Overview of global C cycle 2. Global C reservoirs Outline 3. The contemporary global C cycle 4. Fluxes and residence
Lecture 16 Guest Lecturer this week. Prof. Greg Ravizza
 Lecture 16 Guest Lecturer this week. Prof. Greg Ravizza General Concepts for Natural Controls on Fresh Water Composition 1. Generalizations about freshwater compositions 2. Conservative/nonconservative
Lecture 16 Guest Lecturer this week. Prof. Greg Ravizza General Concepts for Natural Controls on Fresh Water Composition 1. Generalizations about freshwater compositions 2. Conservative/nonconservative
Hydrological Cycle Rain and rivers OUTLINE
 Hydrological Cycle Rain and rivers The Hydrosphere Rain and rivers OUTLINE 1 Generalizations (non-political conservatism) Conservative (not affected) and Non-Conservative (affected) Ions Distinction: whether
Hydrological Cycle Rain and rivers The Hydrosphere Rain and rivers OUTLINE 1 Generalizations (non-political conservatism) Conservative (not affected) and Non-Conservative (affected) Ions Distinction: whether
/ Past and Present Climate
 MIT OpenCourseWare http://ocw.mit.edu 12.842 / 12.301 Past and Present Climate Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. The Faint Young
MIT OpenCourseWare http://ocw.mit.edu 12.842 / 12.301 Past and Present Climate Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. The Faint Young
organisms CaCO 3 + H 2 O + CO 2 shallow water
 Weathering and Reverse weathering Step I:Weathering of igneous rocks 1. Igneous rocks are mainly composed of Al, Si and O 2 with minor and varying quantities of Na, K, Ca and Mg composing pheldspar minerals
Weathering and Reverse weathering Step I:Weathering of igneous rocks 1. Igneous rocks are mainly composed of Al, Si and O 2 with minor and varying quantities of Na, K, Ca and Mg composing pheldspar minerals
Weathering Cycle Teacher Notes
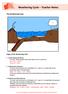 The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
Wednesday week 12. These ions move through the soil to streams and eventually to the ocean. In the ocean; CaCO 3 + H 2 O + CO 2 H 2 O + H 2 O
 Wednesday week 12 I. Control of CO 2 content of atmosphere by the ocean H 4 SiO 4 A. Consider a hypothetical planet with a crust made of single mineral (Wallastonite) CaSiO3. We could use the composition
Wednesday week 12 I. Control of CO 2 content of atmosphere by the ocean H 4 SiO 4 A. Consider a hypothetical planet with a crust made of single mineral (Wallastonite) CaSiO3. We could use the composition
Part 1. Ocean Composition & Circulation
 OCN 401 Biogeochemical Systems (10.19.17) (Schlesinger: Chapter 9) Part 1. Ocean Composition & Circulation 1. Introduction Lecture Outline 2. Ocean Circulation a) Global Patterns in T, S, ρ b) Thermohaline
OCN 401 Biogeochemical Systems (10.19.17) (Schlesinger: Chapter 9) Part 1. Ocean Composition & Circulation 1. Introduction Lecture Outline 2. Ocean Circulation a) Global Patterns in T, S, ρ b) Thermohaline
Long-term Climate Change. We are in a period of relative warmth right now but on the time scale of the Earth s history, the planet is cold.
 Long-term Climate Change We are in a period of relative warmth right now but on the time scale of the Earth s history, the planet is cold. Long-term Climate Change The Archean is thought to have been warmer,
Long-term Climate Change We are in a period of relative warmth right now but on the time scale of the Earth s history, the planet is cold. Long-term Climate Change The Archean is thought to have been warmer,
Redox, ph, pe OUTLINE 9/12/17. Equilibrium? Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox
 Redox, ph, pe Equilibrium? OUTLINE Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox Reading: White p555-563 1 Question of the day? So what about the CO 2 system? CO
Redox, ph, pe Equilibrium? OUTLINE Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox Reading: White p555-563 1 Question of the day? So what about the CO 2 system? CO
Water percolating through hot lava dissolves soluble minerals containing chlorine, bromine and sulphur compounds
 Figure 5 The sources of dissolved ions in sea water. Water falls as rain Compounds containing mainly calcium, magnesium, carbonate and silicate ions are leached from the soil Rivers carry ions in solution
Figure 5 The sources of dissolved ions in sea water. Water falls as rain Compounds containing mainly calcium, magnesium, carbonate and silicate ions are leached from the soil Rivers carry ions in solution
Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions
 Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions OCN 623 Chemical Oceanography Reading: Libes, Chapters 15 and 16 Outline I. Deep sea sedimentation Detrital sediments
Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions OCN 623 Chemical Oceanography Reading: Libes, Chapters 15 and 16 Outline I. Deep sea sedimentation Detrital sediments
Lect. 2: Chemical Water Quality
 The Islamic University of Gaza Faculty of Engineering Civil Engineering Department M.Sc. Water Resources Water Quality Management (ENGC 6304) Lect. 2: Chemical Water Quality ١ Chemical water quality parameters
The Islamic University of Gaza Faculty of Engineering Civil Engineering Department M.Sc. Water Resources Water Quality Management (ENGC 6304) Lect. 2: Chemical Water Quality ١ Chemical water quality parameters
The Biogeochemical Carbon Cycle: CO 2,the greenhouse effect, & climate feedbacks. Assigned Reading: Kump et al. (1999) The Earth System, Chap. 7.
 The Biogeochemical Carbon Cycle: CO 2,the greenhouse effect, & climate feedbacks Assigned Reading: Kump et al. (1999) The Earth System, Chap. 7. Overhead Transparencies Faint Faint Young Sun Paradox Young
The Biogeochemical Carbon Cycle: CO 2,the greenhouse effect, & climate feedbacks Assigned Reading: Kump et al. (1999) The Earth System, Chap. 7. Overhead Transparencies Faint Faint Young Sun Paradox Young
UNIT 4 SEDIMENTARY ROCKS
 UNIT 4 SEDIMENTARY ROCKS WHAT ARE SEDIMENTS Sediments are loose Earth materials (unconsolidated materials) such as sand which are transported by the action of water, wind, glacial ice and gravity. These
UNIT 4 SEDIMENTARY ROCKS WHAT ARE SEDIMENTS Sediments are loose Earth materials (unconsolidated materials) such as sand which are transported by the action of water, wind, glacial ice and gravity. These
XI. the natural carbon cycle. with materials from J. Kasting (Penn State)
 XI. the natural carbon cycle with materials from J. Kasting (Penn State) outline properties of carbon the terrestrial biological cycle of carbon the ocean cycle of carbon carbon in the rock cycle overview
XI. the natural carbon cycle with materials from J. Kasting (Penn State) outline properties of carbon the terrestrial biological cycle of carbon the ocean cycle of carbon carbon in the rock cycle overview
Processes affecting continental shelves
 Marine Sediments Continental Shelves Processes affecting continental shelves 1. Glaciation 2. Sea-level change (±130 m during continental glaciation) 3. Waves and currents 4. Sedimentation 5. Carbonate
Marine Sediments Continental Shelves Processes affecting continental shelves 1. Glaciation 2. Sea-level change (±130 m during continental glaciation) 3. Waves and currents 4. Sedimentation 5. Carbonate
predictive mineral discovery*cooperative Research Centre A legacy for mineral exploration science Mineral Systems Q3 Fluid reservoirs
 Mineral Systems Q3 Fluid reservoirs 1 Key Parameter Mineral System Exploration is reflected in scale-dependent translation A. Gradient in hydraulic potential B. Permeability C. Solubility sensitivity to
Mineral Systems Q3 Fluid reservoirs 1 Key Parameter Mineral System Exploration is reflected in scale-dependent translation A. Gradient in hydraulic potential B. Permeability C. Solubility sensitivity to
Ocean Chemical Dynamics. EAS 2200 Lecture 21
 Ocean Chemical Dynamics EAS 2200 Lecture 21 Summary of Water s H-bonding accounts for high heat capacity, latent heats of fusion and evaporation. important for heat transport, transfer to atmosphere, and
Ocean Chemical Dynamics EAS 2200 Lecture 21 Summary of Water s H-bonding accounts for high heat capacity, latent heats of fusion and evaporation. important for heat transport, transfer to atmosphere, and
Salinity. See Appendix 1 of textbook x10 3 = See Appendix 1 of textbook
 Length Area Volume m m m foot = 0.305m yard = 0.91m square feet ~0.09m2 US pint ~ 0.47 L fl. oz. ~0.02 L Speed m/s mph Acceleration m/s mph/s Weight kg, gram pound ~0.45kg Temperature o o See Appendix
Length Area Volume m m m foot = 0.305m yard = 0.91m square feet ~0.09m2 US pint ~ 0.47 L fl. oz. ~0.02 L Speed m/s mph Acceleration m/s mph/s Weight kg, gram pound ~0.45kg Temperature o o See Appendix
Global Carbon Cycle - I Systematics: Reservoirs and Fluxes
 OCN 401-10 Nov. 16, 2010 KCR Global Carbon Cycle - I Systematics: Reservoirs and Fluxes The Global carbon cycle Reservoirs: biomass on land in the oceans, atmosphere, soil and rocks, waters Processes:
OCN 401-10 Nov. 16, 2010 KCR Global Carbon Cycle - I Systematics: Reservoirs and Fluxes The Global carbon cycle Reservoirs: biomass on land in the oceans, atmosphere, soil and rocks, waters Processes:
Salinity. foot = 0.305m yard = 0.91m. Length. Area m 2 square feet ~0.09m2. Volume m 3 US pint ~ 0.47 L fl. oz. ~0.02 L.
 Length m foot = 0.305m yard = 0.91m Area m 2 square feet ~0.09m2 Volume m 3 US pint ~ 0.47 L, L (liters) fl. oz. ~0.02 L Speed m/s mph Acceleration m/s 2 mph/s Weight kg, gram pound ~0.45kg Temperature
Length m foot = 0.305m yard = 0.91m Area m 2 square feet ~0.09m2 Volume m 3 US pint ~ 0.47 L, L (liters) fl. oz. ~0.02 L Speed m/s mph Acceleration m/s 2 mph/s Weight kg, gram pound ~0.45kg Temperature
Quartz or opaline silica solubility
 Quartz or opaline silica solubility The simplest process that might regulate the concentration of an element in solution is equilibrium with respect to a solid phase containing the element as a major component.
Quartz or opaline silica solubility The simplest process that might regulate the concentration of an element in solution is equilibrium with respect to a solid phase containing the element as a major component.
EPSS 15 Introduction to Oceanography Spring The Physical and Chemical Properties of Seawater
 EPSS 15 Introduction to Oceanography Spring 2017 The Physical and Chemical Properties of Seawater The focus of the Lab this week is seawater--its composition, physical and chemical properties. Seawater
EPSS 15 Introduction to Oceanography Spring 2017 The Physical and Chemical Properties of Seawater The focus of the Lab this week is seawater--its composition, physical and chemical properties. Seawater
Lecture 15: Adsorption; Soil Acidity
 Lecture 15: Adsorption; Soil Acidity Surface Complexation (Your textbook calls this adsorption ) Surface Complexation Both cations and anions can bind to sites on the external surfaces of soil minerals
Lecture 15: Adsorption; Soil Acidity Surface Complexation (Your textbook calls this adsorption ) Surface Complexation Both cations and anions can bind to sites on the external surfaces of soil minerals
EPS 50 Lab 4: Sedimentary Rocks
 Name: EPS 50 Lab 4: Sedimentary Rocks Grotzinger and Jordan, Chapter 5 Introduction In this lab we will classify sedimentary rocks and investigate the relationship between environmental conditions and
Name: EPS 50 Lab 4: Sedimentary Rocks Grotzinger and Jordan, Chapter 5 Introduction In this lab we will classify sedimentary rocks and investigate the relationship between environmental conditions and
Hydrothermal Chemistry/ Reverse Weathering. Marine Chemistry Seminar
 Hydrothermal Chemistry/ Reverse Weathering Marine Chemistry Seminar 1974 Drever, The Sea Chapter 10:The Magnesium Problem 1979 Edmonds et al., Ridge Crest Hydrothermal Activity and the Balances of Major
Hydrothermal Chemistry/ Reverse Weathering Marine Chemistry Seminar 1974 Drever, The Sea Chapter 10:The Magnesium Problem 1979 Edmonds et al., Ridge Crest Hydrothermal Activity and the Balances of Major
1/31/2013. Weathering Includes Physical, Chemical, Biological processes. Weathering Mechanisms. Wind abrasion forming Ventifacts
 Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
Chapter 3: Acid Base Equilibria. HCl + KOH KCl + H 2 O acid + base salt + water
 Chapter 3: Acid Base Equilibria HCl + KOH KCl + H 2 O acid + base salt + water What is an acid? The Arrhenius concept proposed that acids are substances that produce hydrogen ions (H + ) in aqueous solutions.
Chapter 3: Acid Base Equilibria HCl + KOH KCl + H 2 O acid + base salt + water What is an acid? The Arrhenius concept proposed that acids are substances that produce hydrogen ions (H + ) in aqueous solutions.
SCOPE 35 Scales and Global Change (1988)
 1. Types and origins of marine sediments 2. Distribution of sediments: controls and patterns 3. Sedimentary diagenesis: (a) Sedimentary and organic matter burial (b) Aerobic and anaerobic decomposition
1. Types and origins of marine sediments 2. Distribution of sediments: controls and patterns 3. Sedimentary diagenesis: (a) Sedimentary and organic matter burial (b) Aerobic and anaerobic decomposition
Weathering: the disintegration, or breakdown of rock material
 Weathering: the disintegration, or breakdown of rock material Mechanical Weathering: no change in chemical composition--just disintegration into smaller pieces Chemical Weathering: breakdown as a result
Weathering: the disintegration, or breakdown of rock material Mechanical Weathering: no change in chemical composition--just disintegration into smaller pieces Chemical Weathering: breakdown as a result
S= 95.02% S= 4.21% 35. S=radioactive 36 S=0.02% S= 0.75% 34 VI V IV III II I 0 -I -II SO 4 S 2 O 6 H 2 SO 3 HS 2 O 4- S 2 O 3
 SULFUR ISOTOPES 32 S= 95.02% 33 S= 0.75% 34 S= 4.21% 35 S=radioactive 36 S=0.02% S-H S-C S=C S-O S=O S-F S-Cl S-S VI V IV III II I 0 -I -II SO 4 2- S 2 O 6 2- H 2 SO 3 HS 2 O 4- S 2 O 3 2- S 2 F 2 S H
SULFUR ISOTOPES 32 S= 95.02% 33 S= 0.75% 34 S= 4.21% 35 S=radioactive 36 S=0.02% S-H S-C S=C S-O S=O S-F S-Cl S-S VI V IV III II I 0 -I -II SO 4 2- S 2 O 6 2- H 2 SO 3 HS 2 O 4- S 2 O 3 2- S 2 F 2 S H
Weathering and Soils
 OCN 401-17 Aug. 29, 2016 KCR Weathering and Soils Biogeochemistry Chapter 4: The Lithosphere Introduction: the context Rock Weathering Soil Chemical Reactions Soil Development (see text) Weathering Rates
OCN 401-17 Aug. 29, 2016 KCR Weathering and Soils Biogeochemistry Chapter 4: The Lithosphere Introduction: the context Rock Weathering Soil Chemical Reactions Soil Development (see text) Weathering Rates
Geochemical Reservoirs and Transfer Processes
 Geochemical Reservoirs and Transfer Processes Ocn 623 Dr. Michael J. Mottl Dept. Of Oceanography Three Basic Questions 1. Why does Earth have oceans? 2. Why does Earth have dry land? 3. Why are the seas
Geochemical Reservoirs and Transfer Processes Ocn 623 Dr. Michael J. Mottl Dept. Of Oceanography Three Basic Questions 1. Why does Earth have oceans? 2. Why does Earth have dry land? 3. Why are the seas
Engineering Geology ECIV 2204
 Engineering Geology ECIV 2204 Instructor : Dr. Jehad Hamad 2017-2016 Chapter (6) : Sedimentary Rocks Chapter 6: Sedimentary Rocks Chapter 6: Sedimentary Rocks Origin and nature of sedimentary rocks: Sedimentary
Engineering Geology ECIV 2204 Instructor : Dr. Jehad Hamad 2017-2016 Chapter (6) : Sedimentary Rocks Chapter 6: Sedimentary Rocks Chapter 6: Sedimentary Rocks Origin and nature of sedimentary rocks: Sedimentary
The Chemistry of Seawater. Unit 3
 The Chemistry of Seawater Unit 3 Water occurs naturally on earth in 3 phases: solid, liquid, or gas (liquid is most abundant) Water Phases Basic Chemistry Review What is an atom? Smallest particles of
The Chemistry of Seawater Unit 3 Water occurs naturally on earth in 3 phases: solid, liquid, or gas (liquid is most abundant) Water Phases Basic Chemistry Review What is an atom? Smallest particles of
Salinity distribution in the Oceans
 Salinity distribution in the Oceans Average practical salinity of open ocean waters 34.72 http://eps.mcgill.ca/~courses/c542/ 1/58 Salinity distribution in the Oceans Factors that control seawater salinity:
Salinity distribution in the Oceans Average practical salinity of open ocean waters 34.72 http://eps.mcgill.ca/~courses/c542/ 1/58 Salinity distribution in the Oceans Factors that control seawater salinity:
Sedimentary Geology. Strat and Sed, Ch. 1 1
 Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
About Earth Materials
 Grotzinger Jordan Understanding Earth Sixth Edition Chapter 3: EARTH MATERIALS Minerals and Rocks 2011 by W. H. Freeman and Company About Earth Materials All Earth materials are composed of atoms bound
Grotzinger Jordan Understanding Earth Sixth Edition Chapter 3: EARTH MATERIALS Minerals and Rocks 2011 by W. H. Freeman and Company About Earth Materials All Earth materials are composed of atoms bound
Lecture 13 More Surface Reactions on Mineral Surfaces. & Intro to Soil Formation and Chemistry
 Lecture 13 More Surface Reactions on Mineral Surfaces & Intro to Soil Formation and Chemistry 3. charge transfer (e.g., ligand/donor sorption): Sorption involves a number of related processes that all
Lecture 13 More Surface Reactions on Mineral Surfaces & Intro to Soil Formation and Chemistry 3. charge transfer (e.g., ligand/donor sorption): Sorption involves a number of related processes that all
BIOGEOCHEMICAL CYCLES
 BIOGEOCHEMICAL CYCLES BASICS Biogeochemical Cycle: The complete path a chemical takes through the four major components, or reservoirs, of Earth s system (atmosphere, lithosphere, hydrosphere and biosphere)
BIOGEOCHEMICAL CYCLES BASICS Biogeochemical Cycle: The complete path a chemical takes through the four major components, or reservoirs, of Earth s system (atmosphere, lithosphere, hydrosphere and biosphere)
ph and Acid-Base reactions
 ph and Acid-Base reactions http://eps.mcgill.ca/~courses/c220/ Interaction of water with H + (H 3 O + ) Although it is a minor component of most aqueous solutions, the proton, hydrogen ion or H + concentration
ph and Acid-Base reactions http://eps.mcgill.ca/~courses/c220/ Interaction of water with H + (H 3 O + ) Although it is a minor component of most aqueous solutions, the proton, hydrogen ion or H + concentration
The Lithosphere. Definition
 10/14/2014 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2014 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
10/14/2014 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2014 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
CO2 in atmosphere is influenced by pco2 of surface water (partial pressure of water is the CO2 (gas) that would be in equilibrium with water).
 EART 254, Lecture on April 6 & 11, 2011 Introduction (skipped most of this) Will look at C and N (maybe) cycles with respect to how they influence CO2 levels in the atmosphere. Ocean chemistry controls
EART 254, Lecture on April 6 & 11, 2011 Introduction (skipped most of this) Will look at C and N (maybe) cycles with respect to how they influence CO2 levels in the atmosphere. Ocean chemistry controls
Major Ions, Carbonate System, Alkalinity, ph (Kalff Chapters 13 and 14) There are 7 common ions in freshwater: (e.g.
 Major Ions, Carbonate System, Alkalinity, ph (Kalff Chapters 13 and 14) A. Major ions 1. Common Ions There are 7 common ions in freshwater: (e.g. Table 13-3, p207) cations: Ca +2, Mg +2, Na +1, K +1 anions:
Major Ions, Carbonate System, Alkalinity, ph (Kalff Chapters 13 and 14) A. Major ions 1. Common Ions There are 7 common ions in freshwater: (e.g. Table 13-3, p207) cations: Ca +2, Mg +2, Na +1, K +1 anions:
Marine Sediments EPSS15 Spring 2017 Lab 4
 Marine Sediments EPSS15 Spring 2017 Lab 4 Why Sediments? Record of Earth s history - Tectonic plate movement - Past changes in climate - Ancient ocean circulation currents - Cataclysmic events 1 Classification
Marine Sediments EPSS15 Spring 2017 Lab 4 Why Sediments? Record of Earth s history - Tectonic plate movement - Past changes in climate - Ancient ocean circulation currents - Cataclysmic events 1 Classification
Chapter 6 9/25/2012. Weathering, Erosion and Soils. Introduction. How Are Earth Materials Altered? Introduction. How Are Earth Materials Altered?
 Chapter 6 Introduction Rocks and minerals are disintegrated and decomposed by the processes of mechanical and chemical weathering. Weathering, Erosion and Soils This breakdown occurs because the parent
Chapter 6 Introduction Rocks and minerals are disintegrated and decomposed by the processes of mechanical and chemical weathering. Weathering, Erosion and Soils This breakdown occurs because the parent
2.2 Acid mine drainage
 PROBLEM SOLVING 2. CHEMICAL REACTIONS AND EQUILIBRIA 1 2.2 Acid mine drainage Problem 2.2 The weathering of iron sulphide minerals produces acidified water, leading to major environmental problems from
PROBLEM SOLVING 2. CHEMICAL REACTIONS AND EQUILIBRIA 1 2.2 Acid mine drainage Problem 2.2 The weathering of iron sulphide minerals produces acidified water, leading to major environmental problems from
WEATHERING. Turning Rock to Sediment and Solutions 10/22/2012
 WEATHERING Turning Rock to Sediment and Solutions Igneous rocks form at high temperatures; at the Earth s surface they are chemically unstable and will begin to disintegrate and decompose in a process
WEATHERING Turning Rock to Sediment and Solutions Igneous rocks form at high temperatures; at the Earth s surface they are chemically unstable and will begin to disintegrate and decompose in a process
Lecture series outline
 Water Quality 2 Lecture series outline Lecture 1a Water uses, water quality guidelines; physical, chemical and biological parameters Lecture 1b The origins of the constituents of water quality through
Water Quality 2 Lecture series outline Lecture 1a Water uses, water quality guidelines; physical, chemical and biological parameters Lecture 1b The origins of the constituents of water quality through
Lecture 3 questions Temperature, Salinity, Density and Circulation
 Lecture 3 questions Temperature, Salinity, Density and Circulation (1) These are profiles of mean ocean temperature with depth at various locations in the ocean which in the following (a, b, c) figures
Lecture 3 questions Temperature, Salinity, Density and Circulation (1) These are profiles of mean ocean temperature with depth at various locations in the ocean which in the following (a, b, c) figures
Global phosphorus cycle
 Global phosphorus cycle OCN 623 Chemical Oceanography 11 April 2013 2013 Arisa Okazaki and Kathleen Ruttenberg Outline 1. Introduction on global phosphorus (P) cycle 2. Terrestrial environment 3. Atmospheric
Global phosphorus cycle OCN 623 Chemical Oceanography 11 April 2013 2013 Arisa Okazaki and Kathleen Ruttenberg Outline 1. Introduction on global phosphorus (P) cycle 2. Terrestrial environment 3. Atmospheric
Rocks and the Rock Cycle. Banded Iron Formation
 Rocks and the Rock Cycle Banded Iron Formation Rocks Big rocks into pebbles, Pebbles into sand. I really hold a million, million Rocks here in my hand. Florence Parry Heide How do rocks change? How are
Rocks and the Rock Cycle Banded Iron Formation Rocks Big rocks into pebbles, Pebbles into sand. I really hold a million, million Rocks here in my hand. Florence Parry Heide How do rocks change? How are
2 examples: Mg 2+ Rivers [Mg!! ]! (v r ) [Mg 2+ ] SW. **Answers with OR without vent effluent arrow are acceptable, since [Mg] in vent fluid is zero.
![2 examples: Mg 2+ Rivers [Mg!! ]! (v r ) [Mg 2+ ] SW. **Answers with OR without vent effluent arrow are acceptable, since [Mg] in vent fluid is zero. 2 examples: Mg 2+ Rivers [Mg!! ]! (v r ) [Mg 2+ ] SW. **Answers with OR without vent effluent arrow are acceptable, since [Mg] in vent fluid is zero.](/thumbs/91/107062387.jpg) OCN400 Winter 2015 PS2 - Key 1. You might think it is well known, but there is some debate about what controls the magnesium concentration in seawater. The main input is rivers (see Power Point Lecture
OCN400 Winter 2015 PS2 - Key 1. You might think it is well known, but there is some debate about what controls the magnesium concentration in seawater. The main input is rivers (see Power Point Lecture
Chapter 16. Solubility Equilibria 10/14/2010. Solubility Equilibria. Solubility Product (Constant), K sp. Solubility and the Solubility Product
 Solubility Equilibria These are associated with ionic solids dissolving in water to form aqueous solutions Chapter 16 Solubility Equilibria It is assumed that when an ionic compound dissolves in water,
Solubility Equilibria These are associated with ionic solids dissolving in water to form aqueous solutions Chapter 16 Solubility Equilibria It is assumed that when an ionic compound dissolves in water,
Chapter 3. Atoms and Minerals. Earth Materials
 Chapter 3 Atoms and Minerals Earth Materials Atoms and Elements: Isotopes and Ions A Review of Chemistry Atoms Atoms are composed of Protons, Neutrons and Electrons A proton has an electric charge of +1
Chapter 3 Atoms and Minerals Earth Materials Atoms and Elements: Isotopes and Ions A Review of Chemistry Atoms Atoms are composed of Protons, Neutrons and Electrons A proton has an electric charge of +1
This Week: Biogeochemical Cycles. Hydrologic Cycle Carbon Cycle
 This Week: Biogeochemical Cycles Hydrologic Cycle Carbon Cycle Announcements Reading: Chapters 4 (p. 74 81) and 8 Another Problem Set (Due next Tuesday) Exam 2: Friday Feb 29 My office hours today and
This Week: Biogeochemical Cycles Hydrologic Cycle Carbon Cycle Announcements Reading: Chapters 4 (p. 74 81) and 8 Another Problem Set (Due next Tuesday) Exam 2: Friday Feb 29 My office hours today and
Minerals. Atoms, Elements, and Chemical Bonding. Definition of a Mineral 2-1
 Minerals In order to define a what we mean by a mineral we must first make some definitions: 2-1 Most of the Earth s surface is composed of rocky material. An element is a substance which cannot be broken
Minerals In order to define a what we mean by a mineral we must first make some definitions: 2-1 Most of the Earth s surface is composed of rocky material. An element is a substance which cannot be broken
key to long-term sustainability is recycling..
 .. to support life over ~ 4 billion years, Earth must be sustainable system.. key to long-term sustainability is recycling.. Earth System how are key elements needed for life (C, N, P) recycled on the
.. to support life over ~ 4 billion years, Earth must be sustainable system.. key to long-term sustainability is recycling.. Earth System how are key elements needed for life (C, N, P) recycled on the
[ ] Sparkling Water and the Carbon Cycle
![[ ] Sparkling Water and the Carbon Cycle [ ] Sparkling Water and the Carbon Cycle](/thumbs/87/96185022.jpg) Materials: Seltzer maker (SodaStream or similar) Some tasty cheese Tap water Glasses or cups for drinking ph paper Many people enjoy carbonated beverages, especially with food. The tiny bubbles and natural
Materials: Seltzer maker (SodaStream or similar) Some tasty cheese Tap water Glasses or cups for drinking ph paper Many people enjoy carbonated beverages, especially with food. The tiny bubbles and natural
CLASS EXERCISE 5.1 List processes occurring in soils that cause changes in the levels of ions.
 5 SIL CHEMISTRY 5.1 Introduction A knowledge of the chemical composition of a soil is less useful than a knowledge of its component minerals and organic materials. These dictate the reactions that occur
5 SIL CHEMISTRY 5.1 Introduction A knowledge of the chemical composition of a soil is less useful than a knowledge of its component minerals and organic materials. These dictate the reactions that occur
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Mechanisms
 Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes WEATHERING CHAPTER 7 Weathering
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes WEATHERING CHAPTER 7 Weathering
Chapter 5: Weathering and Soils. Fig. 5.14
 Chapter 5: Weathering and Soils Fig. 5.14 OBJECTIVES Recognize that weathering breaks down minerals and rocks and occurs as a result of both mechanical and chemical processes. Explain the processes that
Chapter 5: Weathering and Soils Fig. 5.14 OBJECTIVES Recognize that weathering breaks down minerals and rocks and occurs as a result of both mechanical and chemical processes. Explain the processes that
Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.)
 Ocean 421 Your Name Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.) 1. Due to the water molecule's (H 2 O) great abundance in
Ocean 421 Your Name Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.) 1. Due to the water molecule's (H 2 O) great abundance in
WEATHERING. Weathering breakdown of rock materials Erosion transport of broken-down materials
 WEATHERING the interacting physical, chemical & biological processes that progressively alter the original lithologic character of rocks to produce secondary minerals (e.g. clays) & unconsolidated regolith
WEATHERING the interacting physical, chemical & biological processes that progressively alter the original lithologic character of rocks to produce secondary minerals (e.g. clays) & unconsolidated regolith
Soil ph: Review of Concepts
 Soils and Water, Spring 008 Soil ph: Review of Concepts Acid: substance that can donate a proton Base: substance that can accept a proton HA H A HA and A - are called conjugate acid-base pairs. The strength
Soils and Water, Spring 008 Soil ph: Review of Concepts Acid: substance that can donate a proton Base: substance that can accept a proton HA H A HA and A - are called conjugate acid-base pairs. The strength
Sci.tanta.edu.eg PALEOECOLOGY, GE 2218
 Sci.tanta.edu.eg PALEOECOLOGY, GE 2218 Lec. 4 1 Biosphere Lithosphere Community Hydrosphere Atmosphere 2 1 Temperature Temperature range in the ocean is approximately 2 to 40 º C. Coldest waters are found
Sci.tanta.edu.eg PALEOECOLOGY, GE 2218 Lec. 4 1 Biosphere Lithosphere Community Hydrosphere Atmosphere 2 1 Temperature Temperature range in the ocean is approximately 2 to 40 º C. Coldest waters are found
The Martian Sedimentary Mass: Constraints on its Composition, Age and Size. Scott McLennan Department of Geosciences, SUNY Stony Brook
 The Martian Sedimentary Mass: Constraints on its Composition, Age and Size Scott McLennan Department of Geosciences, SUNY Stony Brook Exploring Mars Habitability Lisbon 14 June, 2011 Martian Crustal Chemistry
The Martian Sedimentary Mass: Constraints on its Composition, Age and Size Scott McLennan Department of Geosciences, SUNY Stony Brook Exploring Mars Habitability Lisbon 14 June, 2011 Martian Crustal Chemistry
Chapter 3 Sedimentation of clay minerals
 Chapter 3 Sedimentation of clay minerals 3.1 Clay sedimentation on land 3.2 From land to sea 3.3 Clay sedimentation in the sea 1 3.1 Clay sedimentation on land Deserts Glaciers Rivers Lacustrine 2 University
Chapter 3 Sedimentation of clay minerals 3.1 Clay sedimentation on land 3.2 From land to sea 3.3 Clay sedimentation in the sea 1 3.1 Clay sedimentation on land Deserts Glaciers Rivers Lacustrine 2 University
Geology 560, Prof. Thomas Johnson, Fall 2003 Unit II: Chemical reactions: Guide Questions
 Geology 560, Prof. Thomas Johnson, Fall 2003 Unit II: Chemical reactions: Guide Questions Goals: Refresh your memory on the basics of chemical reactions Ponder the chaos that underlies chemical reactions;
Geology 560, Prof. Thomas Johnson, Fall 2003 Unit II: Chemical reactions: Guide Questions Goals: Refresh your memory on the basics of chemical reactions Ponder the chaos that underlies chemical reactions;
Solubility and Complex Ion Equilibria
 Solubility and Complex Ion Equilibria a mineral formed by marine organisms through biological precipitation CALCITE CaCO 3(s) Ca + (aq)+ CO 3 - (aq) K K sp [Ca + ][CO 3 - ].8 x 10-9 K sp solubility product
Solubility and Complex Ion Equilibria a mineral formed by marine organisms through biological precipitation CALCITE CaCO 3(s) Ca + (aq)+ CO 3 - (aq) K K sp [Ca + ][CO 3 - ].8 x 10-9 K sp solubility product
Weathering and Erosion
 Weathering and Erosion Weathering the disintegration and decomposition of material at the surface Erosion the transportation of weathered material by water, wind, or ice Weathering Two kinds of weathering
Weathering and Erosion Weathering the disintegration and decomposition of material at the surface Erosion the transportation of weathered material by water, wind, or ice Weathering Two kinds of weathering
Chapter 17: Solubility Equilibria
 Previous Chapter Table of Contents Next Chapter Chapter 17: Solubility Equilibria Sections 17.1-17.2: Solubility Equilibria and the K sp Table In this chapter, we consider the equilibrium associated with
Previous Chapter Table of Contents Next Chapter Chapter 17: Solubility Equilibria Sections 17.1-17.2: Solubility Equilibria and the K sp Table In this chapter, we consider the equilibrium associated with
Solubility and Complex Ion Equilibria
 CALCITE Solubility and Complex Ion Equilibria a mineral formed by marine organisms through biological precipitation CaCO (s) Ca + (aq)+ CO (aq) K K sp [Ca + ][CO ].8 x 10-9 K sp solubility product constant
CALCITE Solubility and Complex Ion Equilibria a mineral formed by marine organisms through biological precipitation CaCO (s) Ca + (aq)+ CO (aq) K K sp [Ca + ][CO ].8 x 10-9 K sp solubility product constant
Lecture 29: Soil Formation
 Lecture 29: Soil Formation Factors Controlling Soil Formation 1. Parent material: Soil precursor 2. Climate: Temperature and precipitation 3. Biota: Native vegetation, microbes, soil animals, humans 4.
Lecture 29: Soil Formation Factors Controlling Soil Formation 1. Parent material: Soil precursor 2. Climate: Temperature and precipitation 3. Biota: Native vegetation, microbes, soil animals, humans 4.
Minerals and Rocks Chapter 20
 Minerals and Rocks Chapter 20 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Minerals and Rocks Chapter 20 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Chapter 6. Weathering, Erosion, and Soil
 Chapter 6 Weathering, Erosion, and Soil Introduction Rocks and minerals disintegrate and decompose by the processes of physical and chemical weathering. This breakdown occurs because the parent material
Chapter 6 Weathering, Erosion, and Soil Introduction Rocks and minerals disintegrate and decompose by the processes of physical and chemical weathering. This breakdown occurs because the parent material
Chapter 5. The Biogeochemical Cycles. Botkin & Keller Environmental Science 5e
 Chapter 5 The Biogeochemical Cycles How Chemicals Cycle Biogeochemical Cycle The complete path a chemical takes through the four major components or reservoirs of Earth s systems 1. Atmosphere 2. Hydrosphere
Chapter 5 The Biogeochemical Cycles How Chemicals Cycle Biogeochemical Cycle The complete path a chemical takes through the four major components or reservoirs of Earth s systems 1. Atmosphere 2. Hydrosphere
Emily and Megan. Earth System Science. Elements of Earth by weight. Crust Elements, by weight. Minerals. Made of atoms Earth is mostly iron, by weight
 Emily and Megan Chapter 20 MINERALS AND ROCKS Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Emily and Megan Chapter 20 MINERALS AND ROCKS Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Weathering and Soils
 OCN 401-11 Oct. 13, 2011 KCR Weathering and Soils Biogeochemistry Chapter 4: The Lithosphere Introduction: the context Rock Weathering Soil Chemical Reactions Soil Development (see text) Weathering Rates
OCN 401-11 Oct. 13, 2011 KCR Weathering and Soils Biogeochemistry Chapter 4: The Lithosphere Introduction: the context Rock Weathering Soil Chemical Reactions Soil Development (see text) Weathering Rates
Crust Elements. Elements of Earth. Minerals. Crystals. Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air
 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Made of atoms Earth is mostly iron, by weight Elements of Earth Made of atoms
Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Made of atoms Earth is mostly iron, by weight Elements of Earth Made of atoms
Acid Soil. Soil Acidity and ph
 Acid Soil Soil Acidity and ph ph ph = - log (H + ) H 2 O H + + OH - (H + ) x (OH - )= K w = 10-14 measures H + activity with an electrode (in the lab), solutions (in the field) reflects the acid intensity,
Acid Soil Soil Acidity and ph ph ph = - log (H + ) H 2 O H + + OH - (H + ) x (OH - )= K w = 10-14 measures H + activity with an electrode (in the lab), solutions (in the field) reflects the acid intensity,
Question. What caused the recent explosive eruptions of hot ash and gas at Kilauea s Halema uma u crater:
 OCN 201 Deep Sea Sediments Question What caused the recent explosive eruptions of hot ash and gas at Kilauea s Halema uma u crater: A. The interaction of lava with seawater B. Drainage of the lava lake
OCN 201 Deep Sea Sediments Question What caused the recent explosive eruptions of hot ash and gas at Kilauea s Halema uma u crater: A. The interaction of lava with seawater B. Drainage of the lava lake
Wednesday, October 10 th
 Wednesday, October 10 th Page 13a (left side) / Place Lab on table Objective: We will describe the different types of weathering and erosion and identify evidence of each type. Warm-up: 1. What is weathering?
Wednesday, October 10 th Page 13a (left side) / Place Lab on table Objective: We will describe the different types of weathering and erosion and identify evidence of each type. Warm-up: 1. What is weathering?
Lab 8 Dynamic Soil Systems I: Soil ph and Liming
 Lab 8 Dynamic Soil Systems I: Soil ph and Liming Objectives: To measure soil ph and observe conditions which change ph To distinguish between active acidity (soil solution ph) and exchangeable acidity
Lab 8 Dynamic Soil Systems I: Soil ph and Liming Objectives: To measure soil ph and observe conditions which change ph To distinguish between active acidity (soil solution ph) and exchangeable acidity
Tailings and Mineral Carbonation: The Potential for Atmospheric CO 2 Sequestration
 Tailings and Mineral Carbonation: The Potential for Atmospheric CO 2 Sequestration H. Andrew Rollo Lorax Environmental Services Ltd. Heather. E. Jamieson Department of Geological Sciences and Geological
Tailings and Mineral Carbonation: The Potential for Atmospheric CO 2 Sequestration H. Andrew Rollo Lorax Environmental Services Ltd. Heather. E. Jamieson Department of Geological Sciences and Geological
SoilGen2 model: data requirements and model output
 SoilGen2 model: data requirements and model output 1. Essential plot data... 1 2. Essential soil data... 2 3. Precipitation data (for a typical year)... 2 4. Evapotranspiration and Air temperature data
SoilGen2 model: data requirements and model output 1. Essential plot data... 1 2. Essential soil data... 2 3. Precipitation data (for a typical year)... 2 4. Evapotranspiration and Air temperature data
Earth Science 11: Earth Materials, Sedimentary Rocks
 Name: Date: Earth Science 11: Earth Materials, Sedimentary Rocks Chapter 1, pages 56 to 66 2.4: Sedimentary Rocks Sedimentary Rock Formation All sedimentary rocks form through compaction and cementation
Name: Date: Earth Science 11: Earth Materials, Sedimentary Rocks Chapter 1, pages 56 to 66 2.4: Sedimentary Rocks Sedimentary Rock Formation All sedimentary rocks form through compaction and cementation
Module 9 Sedimentary Rocks
 Module 9 Sedimentary Rocks SEDIMENTARY ROCKS Rocks formed from material derived from preexisting rocks by surfacial processes followed by diagenesis There are two main classes of sedimentary rocks Clastic
Module 9 Sedimentary Rocks SEDIMENTARY ROCKS Rocks formed from material derived from preexisting rocks by surfacial processes followed by diagenesis There are two main classes of sedimentary rocks Clastic
Sedimentary Rocks, Stratigraphy, and Geologic Time
 Sedimentary Rocks, Stratigraphy, and Geologic Time A rock is any naturally formed, nonliving, coherent aggregate mass of solid matter that constitutes part of a planet, asteroid, moon, or other planetary
Sedimentary Rocks, Stratigraphy, and Geologic Time A rock is any naturally formed, nonliving, coherent aggregate mass of solid matter that constitutes part of a planet, asteroid, moon, or other planetary
Fall 2016 Due: 10:30 a.m., Tuesday, September 13
 Geol 330_634 Problem Set #1_Key Fall 2016 Due: 10:30 a.m., Tuesday, September 13 1. Why is the surface circulation in the central North Pacific dominated by a central gyre with clockwise circulation? (5)
Geol 330_634 Problem Set #1_Key Fall 2016 Due: 10:30 a.m., Tuesday, September 13 1. Why is the surface circulation in the central North Pacific dominated by a central gyre with clockwise circulation? (5)
Sedimentary Rocks. Rocks made of bits & pieces of other rocks.
 Sedimentary Rocks Rocks made of bits & pieces of other rocks. Sedimentary Rocks Igneous rocks are the most common rocks on Earth, but because most of them exist below the surface you might not have seen
Sedimentary Rocks Rocks made of bits & pieces of other rocks. Sedimentary Rocks Igneous rocks are the most common rocks on Earth, but because most of them exist below the surface you might not have seen
