Weathering and Soils
|
|
|
- Bryce Walters
- 6 years ago
- Views:
Transcription
1 OCN Oct. 13, 2011 KCR Weathering and Soils Biogeochemistry Chapter 4: The Lithosphere Introduction: the context Rock Weathering Soil Chemical Reactions Soil Development (see text) Weathering Rates & Denudation
2 Introduction: The Context Throughout Earth s history, the atmosphere has interacted with Earth s crust: -> rock weathering Basic Summary Equation (Siever 1974): Igneous rocks + acid volatiles = salty oceans + sedimentary rocks Progression of Weathering through Earth s history: Early Earth: acidic volcanic gases Later: atmospheric O 2, oxidation of reduced minerals Advent of land plants: metabolic CO 2, H 2 CO 3 Modern era: anthropogenic NO x, SO x, acid rain
3 The Global Cycle of Weathering Weathering of Silicate rocks Ions carried by Rivers to oceans 1 minerals at Earth surface exposed to acid forms of C, N, S derived from atmosphere products of weathering reactions are carried to the ocean via rivers weathering products accumulate as dissolved salts or sediments - 75% of rocks on Earth s surface today are sedimentary - once uplifted, these are subject to a renewed cycle of weathering Subduction carries sediments back into the deep earth -CO 2 released - 1 minerals re-formed at high T and P
4 The Global Cycle of Weathering Weathering of Silicate rocks Ions carried by Rivers to oceans Weathering and climate linked via CO 2 balance -CO 2 is released in volcanism -CO 2 is consumed in weathering -HCO 3- produced in weathering reactions is carried to the oceans via rivers - Biogenic carbonates [2HCO 3- + Ca 2+ = CaCO 3 + CO 2 + H 2 O] are buried in sediments The balance between weathering rate and seafloor spreading (subduction) exerts control on atmospheric P CO 2 As CO 2 is an important greenhouse gas, this cycle has important impacts on climate.
5 Why is weathering important? Climate regulation through CO 2 balance Weathering - Bioactivity Feedback (Biogeochemistry): Biological processes are affected by weathering Controls bioavailability of elements that have no gaseous form Impacts soil fertility, biological diversity, agricultural productivity Land plants and soil microbes affect rock weathering and soil development Critical part of global element cycles Global rate of rock weathering impacts atmospheric chemistry Global rate of delivery of weathering products to the sea
6 Table 4.1 Approximate Mean Composition of the Earth s Continental Crust a a Data from Wedepohl (1995). Nutrient elements that have no gaseous form.
7 Rock Weathering Exposure at Earth surface, by uplift or extrusion Mechanical weathering Fragmentation without chemical reaction Erosion (water, wind) Frost heaving Plant root fragmentation Catastrophic events (e.g. landslides) Important in extreme climate regimes Transport-limited systems develop thick soil layers Important factor in large yield of small, mountainous regions Creation of surface area Chemical weathering
8 Rock Weathering (cont d.) Chemical weathering Reaction of minerals in soils and rocks with acidic or oxidizing solutions Production of soluble elements that are available for plant uptake Chemical weathering rates: often depend upon rate of mechanical weathering depend upon mineral composition of rocks More labile minerals are attacked first, sometimes leaving more refractory ones un-weathered depend on climate
9 Weathering of 1 Silicate Minerals Increased weathering susceptibility Bowen Reaction Series Two classes of 1 silicate minerals: - ferromagnesian series - felsic series Based on the presence of Mg or Al in crystal structure Susceptibility to weathering is affected by: - crystal structure (isolated units more reactive than chains or sheets) - ratio of O:Si (lower ratio = more resistant) This weathering series is the reverse order in which these minerals are precipitated from cooling magma.
10 Chemical Weathering (cont d.) Two end-member soils produced: Saprolites: reduction of density due to weathering loss of some constituents, without collapse of initial rock volume (SE-US) Bauxites, Laterites: removal of constituents accompanied by collapse of soil profile, with apparent increase in concentration of remaining elements (e.g., Zr, Ti, Fe, Al, etc.) Climate: Chemical weathering reactions are most rapid at high T and high rainfall
11 Figure 4.2 Loss of silicon (SiO 2 ) in runoff is directly related to mean annual temperature and precipitation in various areas of the world. Modified from White and Blum (1995). Silica is a good indicator of chemical weathering. CaSiO 3 + 2CO 2 + H 2 O = Ca HCO 3- + SiO 2
12 Weathering by Carbonic Acid Dominant form of weathering Carbonic acid forms in soil solution H 2 O + CO 2 = H + + HCO 3- = H 2 CO 3 (4.2) Soil [H 2 CO 3 ] often exceeds equilibrium with atmospheric CO 2 (360 ppm, or 0.036%) Plant roots and microbes release [CO 2 ] to soil High CO 2 can extend to considerable depth in soil 36 m), leading to weathering of underlying rock High [CO 2 ] under snow pack implies significant weathering during winter
13 Biogeochemical Feedback Plant growth is greatest in warm and wet climates Warm humid climates maintain the highest soil [CO 2 ], and greatest weathering rates By maintaining high soil [CO 2 ] plants and associated organisms exert biotic control over the geochemical process of rock weathering on land
14 Figure 4.3 The relationship between the mean concentration of CO 2 in the soil pore space and the actual evapotranspiration of the site for various ecosystems of the world. From Brook et al. (1983). *Average soil [CO 2 ] varies as a function of evapotranspiration at a given site * Evapotranspiration scales with biological activity
15 Incongruent Dissolution Example: plagioclase -> kaolinite: 2NaAlSi 3 O 8 + 2H 2 CO 3 + 9H 2 O -> 2Na + + 2HCO H 4 SiO 4 + Al 2 Si 2 O 5 (OH) 4 Only some of the constituents of the 1 mineral are solubilized Results in formation of 2 minerals Removal of Na and Si Production of HCO 3- indicates this is carbonic acid weathering 2 mineral has lower Si:Al ratio Kaolinite can weather incongruently to form Gibbsite Al 2 Si 2 O 5 (OH) 4 + H 2 O -> 2H 4 SiO 4 + Al 2 O 3 3H 2 O
16 Congruent Dissolution Example: calcium carbonate -> constituent ions: CaCO 3 + H 2 CO 3 -> Ca HCO 3 - Other examples: olivine (FeMgSiO 4 ), pyrite (FeS 2 ) Mg 2+, H 4 SiO 4, SO 4 lost from soil to runoff waters Fe is retained as oxidized forms, e.g. Fe 2 O 3, often a microbially mediated reaction (Thiobacillus ferrooxidans) Acid mine drainage produced from weathering of pyrite: FeS 2 + 8H 2 O +15O 2 -> 2Fe 2 O H + + 8SO 4 2-
17 Organic Acids Released by soil organisms to soil solution Plant roots: acetic acid, citric acid Soil microbes: fulvic and humic acids, phenolic acids (tannins) Fungi: oxalic acid Affect weathering in two ways: Contribute to total acidity of soil These are chelators: any of a class of coordination or complex compounds consisting of a central metal atom attached to a large molecule, called a ligand, in a cyclic or ring structure.
18 Organic Acids (cont d.) Oxalic Acid: HOOC-COOH, or H 2 C 2 O 4 O=C C=O + Fe 2+ O=C C=O O - O - O O Fe When insoluble metals bind with chelators, they become mobile They can move lower in soil profile or be flushed out Their presence increases weathering rate by x Organic acids often dominate upper soil, carbonic acid more important at depth in soil profiles
19 Secondary Minerals Produced by weathering of 1 minerals May initiate as coatings on surfaces of 1 minerals Clays dominate 2 minerals in temperate forest soils Control structural and chemical properties of the soil Crystalline oxides and hydrous oxides of Fe and Al dominate tropical soils
20 Clay Minerals Layered aluminosilicates, < 2 µm in size Two types of layers Si layers Al layers (with other cations, e.g., Fe, Mg) Layers held together by shared O atoms Different clays are classified on the basis of the #, order, ratio of these layers Moderately weathered clays have 2:1 ratio of Si:Al, e.g. montmorillonite, illite More weathered clays have 1:1 ratio, e.g. kaolinite, reflecting a greater Si-loss.
21 Al2Si2O5(OH)4
22
23 K (illite), Mg (montmorillonite) ) Fig. 3. Basic layer structure of a 2:1 natural clay mineral (
24 Consequences of 2 Mineral Formation for Bioavailability Ions released from 1 minerals may not stay in solution if they are taken up into 2 minerals Mg 2+ often fixed in montmorillonite (temperate) K + often fixed in illite (temperate) NH 4+ often fixed in 2:1 clay minerals (can be >10% of total N in soils) Implications for N-limitation of soils Fe, Al insoluble as oxides (often microbially mediated) and hydroxides unless chelated Can sequester PO 3-4 Implications for P-limitation of soils
25 Tropical soils Crystalline oxides and hydrous oxides of Fe and Al Fe: goethite (FeOOH), hematite (Fe 2 O 3 ) Al: gibbsite [Al(OH) 3 ], boehmite High T and ppt cause rapid decomposition of plant debris, few chelators available, Fe and Al immobilized by 2 mineral formation 2 clays loose all Si, Ca, K to runoff Leave behind Fe and Al oxides and hydrous oxides (laterites, bauxites)
26 Phosphorus (P) Minerals Often a limiting nutrient Apatite is the only 1 mineral, weathers congruently: Ca 5 (PO 4 ) 3 (F,OH) + 4H 2 CO 3 -> 5Ca HPO HCO 3- + H 2 O Solubilized P is either taken up by biota or reprecipitated into 2 minerals Occluded in Fe and Al oxides / oxyhydroxides Biologically unavailable when occluded Bioavailability of P can be tied to abundance of organic acid chelators (fungi -> oxalate)
27 Figure 4.4 The solubility of P in soil solution as a function of ph. Precipitation with Al sets the upper limit on dissolved phosphate at low ph (bold line); precipitation with Ca sets a limit at high ph. Phosphorus is most available at a ph of about 7.0. Modified from Lindsay and Vlek (1977).
28 Figure 4.5 Changes in the forms of phosphorus found during soil development on sand dunes in New Zealand. Modified from Walker and Syers (1976).
29 Soil Chemical Reactions Release essential biochemical elements Bioavailability is controlled by K eq between soluble and insoluble forms Soil exchange reactions occur more rapidly than weathering reactions
30 Cation Exchange Reactions Clays have net negative charge, attract cations Internal/Structural (permanent): If Mg 2+ subs for Al 3+ Edges (reversible): Un-protonated OH - groups exposed on edges of clays (ph dependent) Soil organic matter (-OH, -OOH) has net negative charge, attracts cations (Ca 2+, K +, NH 4+ ) (ph-dep) Cation Exchange Capacity (CEC) = total negative charge in a soil (meq/100g) Cations are held and displace one another in sequence: Al 3+ > H + > Ca 2+ > Mg 2+ > K + > NH 4+ > Na +
31 Progression of Cation Exchange Reactions CEC increases during initial soil formation on newly exposed parent rock, then subsequently declines as soil ages CEC of temperate soils (2:1 clays dominate) >> CEC of tropical (1:1 clays dominate) soils Highly weathered tropical soils (laterites, bauxites) have no CEC
32 Soil Buffering CEC can buffer acidity of temperate soils Added H + are exchanged for cations on clays and organic matter In strongly acid tropical soils, which have little CEC, other reactions buffer acidity, involving Al Release of Al 3+ to soil solution results in increased soil acidity: 1) Al 3+ + H 2 O = Al(OH) 2+ + H + 2) Al(OH) 2+ + H 2 O = Al(OH) 2+ + H + 3) Al(OH) 2+ + H 2 O = Al(OH) 3 + H + reactions are reversible, so they buffer against H + additions
33 Anion Adsorption Capacity Tropical soils show variable charge depending on soil ph Under acid conditions they have a + charge, adsorb anions At high ph they develop CEC As ph increases from acid to alkaline, the soils pass through a zero point of charge (ZPC), where # of cation exchange sites = # anion exchange sites The ph of the ZPC is different for different soil compositions
34 Anion Adsorption Capacity Anion Adsorption Capacity (AAC) is greatest when Fe and Al oxyhydroxides are present and amorphous Anion adsorption follows the sequence: PO 3-4 > SO 2-4 > Cl - > NO - 3 Strong PO 4 3- adsorption explains why it frequently is limiting in tropical soils AAC is often modeled using Langmuir isotherms
35 Fig 4.8 The Langmuir adsorption isotherm is used to compare the affinity of soils for anions as a function of the concentration of the anion in solution. In this diagram, soil B has a lower affinity for phosphate than soil A; at equal concentrations of phosphate in solution, more P will be available in soil B. Conversely, if these soils are exposed to long-term additions of solutions with a given phosphate concentration, it will take longer for soil A to equilibrate with that solution (see Johnson and Cole 1980, Reuss and Johnson 1986).
36 Weathering Rates Need weathering rates to understand biogeochemistry of watersheds, global element cycling (primary source of many nutrients) Difficult to evaluate because processes occur slowly and are difficult to isolate Classical Approach: Infer rates from residuum in soil profile and/or losses in stream water Global weathering rates inferred from dissolved and suspended load of rivers
37 The Hubbard Brook Forest Several comparable watersheds Underlain by impermeable bedrock (no flow to groundwater) (Stream water loss) - (atm input) = (release from rocks) Weathering rate = (Ca stream ) - (Ca ppt ) / (Ca rx ) - (Ca soil ) Solution to this equation differs for different ions Biological uptake Formation of 2 minerals
38 Table 4.4 Calculation of the Rate of Primary Mineral Weathering, Using the Streamwater Losses and Mineral Concentrations of Cationic Elements a a Data from Johnson et al. (1968). Different elements show different weathering rates Slower rates imply secondary removal processes
39 Table 4.5 Inputs and Outputs of Elements from the Hubbard Brook a Nutrient elements are retained! a Data from Likens et al. (1981).
40 Chemical Weathering Rates (cont d.) Useful to look at Cl and Si Si source is bedrock, atmospheric inputs negligible: a good index of chemical weathering Cl content in rocks low, atmospheric input dominates: a good metric for evaluating hydrologic budget Losses of dissolved constituents during chemical weathering is defined as chemical denudation of the landscape
41 Total Denudation Mechanical Denudation (vs. chemical) less studied because products are not bioavailable 3 to 4 x > chemical denudation world wide Mean rate 1000 kg/ha/yr, 75% carried as suspended sediments in rivers Today s rates higher due to land use Importance increases with elevation Transports insoluble elements to the sea (Fe, Al, Si, P) Toxic metal (Cu, Zn) fluxes have increased due to human activities
42 Figure 4.14 Annual sediment flux from major drainage basins to the world s oceans. Data are millions of tons (10 12 g) per year, and arrows are drawn proportional to the flux. From Milliman and Meade (1983).
Weathering and Soils
 OCN 401-17 Aug. 29, 2016 KCR Weathering and Soils Biogeochemistry Chapter 4: The Lithosphere Introduction: the context Rock Weathering Soil Chemical Reactions Soil Development (see text) Weathering Rates
OCN 401-17 Aug. 29, 2016 KCR Weathering and Soils Biogeochemistry Chapter 4: The Lithosphere Introduction: the context Rock Weathering Soil Chemical Reactions Soil Development (see text) Weathering Rates
The Lithosphere. Definition
 10/14/2014 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2014 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
10/14/2014 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2014 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
Global Carbon Cycle - I
 Global Carbon Cycle - I OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 11 1. Overview of global C cycle 2. Global C reservoirs Outline 3. The contemporary global C cycle 4. Fluxes and residence
Global Carbon Cycle - I OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 11 1. Overview of global C cycle 2. Global C reservoirs Outline 3. The contemporary global C cycle 4. Fluxes and residence
The Lithosphere. Definition
 10/12/2015 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2015 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
10/12/2015 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2015 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
Global Carbon Cycle - I Systematics: Reservoirs and Fluxes
 OCN 401-10 Nov. 16, 2010 KCR Global Carbon Cycle - I Systematics: Reservoirs and Fluxes The Global carbon cycle Reservoirs: biomass on land in the oceans, atmosphere, soil and rocks, waters Processes:
OCN 401-10 Nov. 16, 2010 KCR Global Carbon Cycle - I Systematics: Reservoirs and Fluxes The Global carbon cycle Reservoirs: biomass on land in the oceans, atmosphere, soil and rocks, waters Processes:
Global Carbon Cycle - I
 Global Carbon Cycle - I Reservoirs and Fluxes OCN 401 - Biogeochemical Systems 13 November 2012 Reading: Schlesinger, Chapter 11 Outline 1. Overview of global C cycle 2. Global C reservoirs 3. The contemporary
Global Carbon Cycle - I Reservoirs and Fluxes OCN 401 - Biogeochemical Systems 13 November 2012 Reading: Schlesinger, Chapter 11 Outline 1. Overview of global C cycle 2. Global C reservoirs 3. The contemporary
(4) Give an example of important reactions that are responsible for the composition of river water.
 Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Mechanisms
 Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes WEATHERING CHAPTER 7 Weathering
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes WEATHERING CHAPTER 7 Weathering
Chapter 7: Anion and molecular retention
 I. Anions and molecules of importance in soils Anions of major importance to agricultural soils and soil chemistry are: H 2 PO - 4, HPO 2-4, SO 2-4, HCO - 3, NO - 3, Cl -, F - and OH -. Also, micronutrients
I. Anions and molecules of importance in soils Anions of major importance to agricultural soils and soil chemistry are: H 2 PO - 4, HPO 2-4, SO 2-4, HCO - 3, NO - 3, Cl -, F - and OH -. Also, micronutrients
Acid Soil. Soil Acidity and ph
 Acid Soil Soil Acidity and ph ph ph = - log (H + ) H 2 O H + + OH - (H + ) x (OH - )= K w = 10-14 measures H + activity with an electrode (in the lab), solutions (in the field) reflects the acid intensity,
Acid Soil Soil Acidity and ph ph ph = - log (H + ) H 2 O H + + OH - (H + ) x (OH - )= K w = 10-14 measures H + activity with an electrode (in the lab), solutions (in the field) reflects the acid intensity,
CLASS EXERCISE 5.1 List processes occurring in soils that cause changes in the levels of ions.
 5 SIL CHEMISTRY 5.1 Introduction A knowledge of the chemical composition of a soil is less useful than a knowledge of its component minerals and organic materials. These dictate the reactions that occur
5 SIL CHEMISTRY 5.1 Introduction A knowledge of the chemical composition of a soil is less useful than a knowledge of its component minerals and organic materials. These dictate the reactions that occur
Global phosphorus cycle
 Global phosphorus cycle OCN 623 Chemical Oceanography 11 April 2013 2013 Arisa Okazaki and Kathleen Ruttenberg Outline 1. Introduction on global phosphorus (P) cycle 2. Terrestrial environment 3. Atmospheric
Global phosphorus cycle OCN 623 Chemical Oceanography 11 April 2013 2013 Arisa Okazaki and Kathleen Ruttenberg Outline 1. Introduction on global phosphorus (P) cycle 2. Terrestrial environment 3. Atmospheric
organisms CaCO 3 + H 2 O + CO 2 shallow water
 Weathering and Reverse weathering Step I:Weathering of igneous rocks 1. Igneous rocks are mainly composed of Al, Si and O 2 with minor and varying quantities of Na, K, Ca and Mg composing pheldspar minerals
Weathering and Reverse weathering Step I:Weathering of igneous rocks 1. Igneous rocks are mainly composed of Al, Si and O 2 with minor and varying quantities of Na, K, Ca and Mg composing pheldspar minerals
Sedimentary Geology. Strat and Sed, Ch. 1 1
 Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
Hydrological Cycle Rain and rivers OUTLINE
 Hydrological Cycle Rain and rivers The Hydrosphere Rain and rivers OUTLINE 1 Generalizations (non-political conservatism) Conservative (not affected) and Non-Conservative (affected) Ions Distinction: whether
Hydrological Cycle Rain and rivers The Hydrosphere Rain and rivers OUTLINE 1 Generalizations (non-political conservatism) Conservative (not affected) and Non-Conservative (affected) Ions Distinction: whether
A few more details on clays, Soil Colloids and their properties. What expandable clays do to surface area. Smectite. Kaolinite.
 A few more details on clays, Soil Colloids and their properties What expandable clays do to surface area Kaolinite Smectite Size 0.5-5 µm External surface 10-30 m 2 /g Internal surface - Size 0.1-1 µm
A few more details on clays, Soil Colloids and their properties What expandable clays do to surface area Kaolinite Smectite Size 0.5-5 µm External surface 10-30 m 2 /g Internal surface - Size 0.1-1 µm
Lecture 4 What Controls the Composition of Seawater
 Lecture 4 What Controls the Composition of Seawater Seawater is salty! Why? What controls the composition of seawater? Do Chemical Equilibrium reactions control the composition of the Ocean? What is meant
Lecture 4 What Controls the Composition of Seawater Seawater is salty! Why? What controls the composition of seawater? Do Chemical Equilibrium reactions control the composition of the Ocean? What is meant
Lecture 13 More Surface Reactions on Mineral Surfaces. & Intro to Soil Formation and Chemistry
 Lecture 13 More Surface Reactions on Mineral Surfaces & Intro to Soil Formation and Chemistry 3. charge transfer (e.g., ligand/donor sorption): Sorption involves a number of related processes that all
Lecture 13 More Surface Reactions on Mineral Surfaces & Intro to Soil Formation and Chemistry 3. charge transfer (e.g., ligand/donor sorption): Sorption involves a number of related processes that all
WEATHERING. Turning Rock to Sediment and Solutions 10/22/2012
 WEATHERING Turning Rock to Sediment and Solutions Igneous rocks form at high temperatures; at the Earth s surface they are chemically unstable and will begin to disintegrate and decompose in a process
WEATHERING Turning Rock to Sediment and Solutions Igneous rocks form at high temperatures; at the Earth s surface they are chemically unstable and will begin to disintegrate and decompose in a process
Cation Exchange Capacity, CEC
 Cation Exchange Capacity, CEC The basic building blocks of clay minerals are: silicon atoms surrounded by four oxygen atoms (tetrahedra), and aluminium atoms surrounded by six hydroxide groups (dioctahedra),
Cation Exchange Capacity, CEC The basic building blocks of clay minerals are: silicon atoms surrounded by four oxygen atoms (tetrahedra), and aluminium atoms surrounded by six hydroxide groups (dioctahedra),
1/31/2013. Weathering Includes Physical, Chemical, Biological processes. Weathering Mechanisms. Wind abrasion forming Ventifacts
 Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
Soil Colloidal Chemistry. Compiled and Edited by Dr. Syed Ismail, Marthwada Agril. University Parbhani,MS, India
 Soil Colloidal Chemistry Compiled and Edited by Dr. Syed Ismail, Marthwada Agril. University Parbhani,MS, India 1 The Colloidal Fraction Introduction What is a colloid? Why this is important in understanding
Soil Colloidal Chemistry Compiled and Edited by Dr. Syed Ismail, Marthwada Agril. University Parbhani,MS, India 1 The Colloidal Fraction Introduction What is a colloid? Why this is important in understanding
1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and
 1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and climate change e) Oceanic water residence times 3.
1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and climate change e) Oceanic water residence times 3.
BIOGEOCHEMICAL CYCLES
 BIOGEOCHEMICAL CYCLES BASICS Biogeochemical Cycle: The complete path a chemical takes through the four major components, or reservoirs, of Earth s system (atmosphere, lithosphere, hydrosphere and biosphere)
BIOGEOCHEMICAL CYCLES BASICS Biogeochemical Cycle: The complete path a chemical takes through the four major components, or reservoirs, of Earth s system (atmosphere, lithosphere, hydrosphere and biosphere)
SCOPE 35 Scales and Global Change (1988)
 1. Types and origins of marine sediments 2. Distribution of sediments: controls and patterns 3. Sedimentary diagenesis: (a) Sedimentary and organic matter burial (b) Aerobic and anaerobic decomposition
1. Types and origins of marine sediments 2. Distribution of sediments: controls and patterns 3. Sedimentary diagenesis: (a) Sedimentary and organic matter burial (b) Aerobic and anaerobic decomposition
Igneous rocks + acid volatiles = sedimentary rocks + salty oceans
 The Lithosphere Weathering physical processes chemical processes biological processes weathering rates Soil development soil formation processes types of soils and vegetation soil properties physical chemical
The Lithosphere Weathering physical processes chemical processes biological processes weathering rates Soil development soil formation processes types of soils and vegetation soil properties physical chemical
Weathering & Soil. Chpt 6
 Weathering & Soil Chpt 6 Some important processes that break-down and transport solid material at the Earth s surface Weathering the physical breakdown and chemical decomposition of rock Mass wasting the
Weathering & Soil Chpt 6 Some important processes that break-down and transport solid material at the Earth s surface Weathering the physical breakdown and chemical decomposition of rock Mass wasting the
Adsorption of ions Ion exchange CEC& AEC Factors influencing ion
 Adsorption of ions Ion exchange CEC& AEC Factors influencing ion exchange- Significance. Adsorption of ions Ion adsorption and subsequent exchange are important processes that take place between soil colloidal
Adsorption of ions Ion exchange CEC& AEC Factors influencing ion exchange- Significance. Adsorption of ions Ion adsorption and subsequent exchange are important processes that take place between soil colloidal
Wednesday week 12. These ions move through the soil to streams and eventually to the ocean. In the ocean; CaCO 3 + H 2 O + CO 2 H 2 O + H 2 O
 Wednesday week 12 I. Control of CO 2 content of atmosphere by the ocean H 4 SiO 4 A. Consider a hypothetical planet with a crust made of single mineral (Wallastonite) CaSiO3. We could use the composition
Wednesday week 12 I. Control of CO 2 content of atmosphere by the ocean H 4 SiO 4 A. Consider a hypothetical planet with a crust made of single mineral (Wallastonite) CaSiO3. We could use the composition
WEATHERING. Weathering breakdown of rock materials Erosion transport of broken-down materials
 WEATHERING the interacting physical, chemical & biological processes that progressively alter the original lithologic character of rocks to produce secondary minerals (e.g. clays) & unconsolidated regolith
WEATHERING the interacting physical, chemical & biological processes that progressively alter the original lithologic character of rocks to produce secondary minerals (e.g. clays) & unconsolidated regolith
Sedimentology & Stratigraphy. Thanks to Rob Viens for slides
 Sedimentology & Stratigraphy Thanks to Rob Viens for slides Sedimentology The study of the processes that erode, transport and deposit sediments Sedimentary Petrology The study of the characteristics and
Sedimentology & Stratigraphy Thanks to Rob Viens for slides Sedimentology The study of the processes that erode, transport and deposit sediments Sedimentary Petrology The study of the characteristics and
Lecture 6. Physical Properties. Solid Phase. Particle Composition
 Lecture 6 Physical Properties Solid Phase Particle Composition 1 Questions What are tetrahedrons and octahedrons? How do silica tetrahedra bonds affect mineral weathering? Difference between primary and
Lecture 6 Physical Properties Solid Phase Particle Composition 1 Questions What are tetrahedrons and octahedrons? How do silica tetrahedra bonds affect mineral weathering? Difference between primary and
Chapter 6. Weathering, Erosion, and Soil
 Chapter 6 Weathering, Erosion, and Soil Introduction Rocks and minerals disintegrate and decompose by the processes of physical and chemical weathering. This breakdown occurs because the parent material
Chapter 6 Weathering, Erosion, and Soil Introduction Rocks and minerals disintegrate and decompose by the processes of physical and chemical weathering. This breakdown occurs because the parent material
Mechanical Weathering
 Weathering is the disintegration and decomposition of material at or near the surface. Erosion is the incorporation and transportation of material by a mobile agent, usually water, wind, or ice. Geologists
Weathering is the disintegration and decomposition of material at or near the surface. Erosion is the incorporation and transportation of material by a mobile agent, usually water, wind, or ice. Geologists
Lecture 16 Guest Lecturer this week. Prof. Greg Ravizza
 Lecture 16 Guest Lecturer this week. Prof. Greg Ravizza General Concepts for Natural Controls on Fresh Water Composition 1. Generalizations about freshwater compositions 2. Conservative/nonconservative
Lecture 16 Guest Lecturer this week. Prof. Greg Ravizza General Concepts for Natural Controls on Fresh Water Composition 1. Generalizations about freshwater compositions 2. Conservative/nonconservative
Earth: An Introduction to Physical Geology Weathering and Soil
 Chapter 6 Lecture Earth: An Introduction to Physical Geology Eleventh Edition Weathering and Soil Tarbuck and Lutgens Weathering Weathering involves the physical breakdown and chemical alteration of rock
Chapter 6 Lecture Earth: An Introduction to Physical Geology Eleventh Edition Weathering and Soil Tarbuck and Lutgens Weathering Weathering involves the physical breakdown and chemical alteration of rock
Chapter 6 9/25/2012. Weathering, Erosion and Soils. Introduction. How Are Earth Materials Altered? Introduction. How Are Earth Materials Altered?
 Chapter 6 Introduction Rocks and minerals are disintegrated and decomposed by the processes of mechanical and chemical weathering. Weathering, Erosion and Soils This breakdown occurs because the parent
Chapter 6 Introduction Rocks and minerals are disintegrated and decomposed by the processes of mechanical and chemical weathering. Weathering, Erosion and Soils This breakdown occurs because the parent
Soil ph: Review of Concepts
 Soils and Water, Spring 008 Soil ph: Review of Concepts Acid: substance that can donate a proton Base: substance that can accept a proton HA H A HA and A - are called conjugate acid-base pairs. The strength
Soils and Water, Spring 008 Soil ph: Review of Concepts Acid: substance that can donate a proton Base: substance that can accept a proton HA H A HA and A - are called conjugate acid-base pairs. The strength
Practice Questions for Lecture 5 Geology 1200
 Practice Questions for Lecture 5 Geology 1200 Use these questions to test your knowledge of Lecture5. The exams will be similar in format, except that they will deal with more than one chapter, and will
Practice Questions for Lecture 5 Geology 1200 Use these questions to test your knowledge of Lecture5. The exams will be similar in format, except that they will deal with more than one chapter, and will
Processes affecting continental shelves
 Marine Sediments Continental Shelves Processes affecting continental shelves 1. Glaciation 2. Sea-level change (±130 m during continental glaciation) 3. Waves and currents 4. Sedimentation 5. Carbonate
Marine Sediments Continental Shelves Processes affecting continental shelves 1. Glaciation 2. Sea-level change (±130 m during continental glaciation) 3. Waves and currents 4. Sedimentation 5. Carbonate
/ Past and Present Climate
 MIT OpenCourseWare http://ocw.mit.edu 12.842 / 12.301 Past and Present Climate Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. The Faint Young
MIT OpenCourseWare http://ocw.mit.edu 12.842 / 12.301 Past and Present Climate Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. The Faint Young
Sedimentary Rocks and Processes
 Sedimentary Rocks and Processes Weathering Sedimentary Processes Breakdown of pre-existing rock by physical and chemical processes Transport Movement of sediments from environments of relatively high potential
Sedimentary Rocks and Processes Weathering Sedimentary Processes Breakdown of pre-existing rock by physical and chemical processes Transport Movement of sediments from environments of relatively high potential
Lecture 15: Adsorption; Soil Acidity
 Lecture 15: Adsorption; Soil Acidity Surface Complexation (Your textbook calls this adsorption ) Surface Complexation Both cations and anions can bind to sites on the external surfaces of soil minerals
Lecture 15: Adsorption; Soil Acidity Surface Complexation (Your textbook calls this adsorption ) Surface Complexation Both cations and anions can bind to sites on the external surfaces of soil minerals
Feedback between nutrient availability, NPP and N release
 Feedback between nutrient availability, NPP and N release 1 Redfield ratios A typical plant = 45% C, 1.5% N, 0.2%P or C:N = 30 : 1 and C:P = 225 : 1 or C:N:P = 225 : 7.5 : 1 N:P = 7.5 : 1 Mobility of nutrients
Feedback between nutrient availability, NPP and N release 1 Redfield ratios A typical plant = 45% C, 1.5% N, 0.2%P or C:N = 30 : 1 and C:P = 225 : 1 or C:N:P = 225 : 7.5 : 1 N:P = 7.5 : 1 Mobility of nutrients
Where is all the water?
 Where is all the water? The distribution of water at the Earth's surface % of total Oceans 97.25 Ice caps and glaciers 2.05 Groundwater 0.68 Lakes 0.01 Soils 0.005 Atmosphere (as vapour) 0.001 Rivers 0.0001
Where is all the water? The distribution of water at the Earth's surface % of total Oceans 97.25 Ice caps and glaciers 2.05 Groundwater 0.68 Lakes 0.01 Soils 0.005 Atmosphere (as vapour) 0.001 Rivers 0.0001
Figure 65: Reservoir in a steady state condition where the input flux is equal to the output flux and the reservoir size remains constant.
 7. The carbon cycle 7.1. Box model of the carbon cycle Without the greenhouse effect, our planet would experience a permanent ice age and life as we know it would not be possible. The main contributors
7. The carbon cycle 7.1. Box model of the carbon cycle Without the greenhouse effect, our planet would experience a permanent ice age and life as we know it would not be possible. The main contributors
The Lithosphere. Definition
 10/23/2017 www.komar.de The Lithosphere Modified lecture of Dr. Ben Sullivan Definition io9.com tedquarters.net Lithos = rocky; Rocky sphere Skin of an apple Two basic types crust: oceanic, continental
10/23/2017 www.komar.de The Lithosphere Modified lecture of Dr. Ben Sullivan Definition io9.com tedquarters.net Lithos = rocky; Rocky sphere Skin of an apple Two basic types crust: oceanic, continental
Redox, ph, pe OUTLINE 9/12/17. Equilibrium? Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox
 Redox, ph, pe Equilibrium? OUTLINE Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox Reading: White p555-563 1 Question of the day? So what about the CO 2 system? CO
Redox, ph, pe Equilibrium? OUTLINE Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox Reading: White p555-563 1 Question of the day? So what about the CO 2 system? CO
Particles in aqueous environments
 Lecture 11 Particle-Aqueous Solute Interactions Today 1. Particle types and sizes 2. Particle charges 3. Particle-solute Interactions Next time Please continue to read Manahan Chapter 4. 1. Fresh-salt
Lecture 11 Particle-Aqueous Solute Interactions Today 1. Particle types and sizes 2. Particle charges 3. Particle-solute Interactions Next time Please continue to read Manahan Chapter 4. 1. Fresh-salt
Lecture 14: Cation Exchange and Surface Charging
 Lecture 14: Cation Exchange and Surface Charging Cation Exchange Cation Exchange Reactions Swapping of cations between hydrated clay interlayers and the soil solution Also occurs on organic matter functional
Lecture 14: Cation Exchange and Surface Charging Cation Exchange Cation Exchange Reactions Swapping of cations between hydrated clay interlayers and the soil solution Also occurs on organic matter functional
The Rock Cycle The Rock Cycle illustrates the origin of igneous, sedimentary and metamorphic rocks
 The Rock Cycle The Rock Cycle illustrates the origin of igneous, sedimentary and metamorphic rocks Igneous rocks form as molten magma or lava cools and solidifies. Magma is completely or partly molten
The Rock Cycle The Rock Cycle illustrates the origin of igneous, sedimentary and metamorphic rocks Igneous rocks form as molten magma or lava cools and solidifies. Magma is completely or partly molten
Overview. Rock weathering Functions of soil Soil forming factors Soil properties
 UN-FAO A. Healthy soils are the basis for healthy food production. B. A tablespoon of normal topsoil has more microorganisms than the entire human population on Earth. C. It can take up to 1,000 years
UN-FAO A. Healthy soils are the basis for healthy food production. B. A tablespoon of normal topsoil has more microorganisms than the entire human population on Earth. C. It can take up to 1,000 years
Earth systems the big idea guiding questions Chapter 1 & 2 Earth and Earth Systems review notes are in purple
 Earth systems the big idea guiding questions Chapter 1 & 2 Earth and Earth Systems review notes are in purple How can you describe Earth? What are the composition and the structure of the atmosphere? How
Earth systems the big idea guiding questions Chapter 1 & 2 Earth and Earth Systems review notes are in purple How can you describe Earth? What are the composition and the structure of the atmosphere? How
Rocks Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3
 Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification VI. Additional sub-classes
Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification VI. Additional sub-classes
Rocks Environmental Significance. Rocks Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3. Rocks Definition of a rock
 Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 Environmental Significance I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification
Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 Environmental Significance I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification
Lecture 29: Soil Formation
 Lecture 29: Soil Formation Factors Controlling Soil Formation 1. Parent material: Soil precursor 2. Climate: Temperature and precipitation 3. Biota: Native vegetation, microbes, soil animals, humans 4.
Lecture 29: Soil Formation Factors Controlling Soil Formation 1. Parent material: Soil precursor 2. Climate: Temperature and precipitation 3. Biota: Native vegetation, microbes, soil animals, humans 4.
Chemical Weathering and Soils
 Chemical Weathering and Soils Fresh rocks and minerals that once occupied the outermost position reached their present condition of decay through a complex of interacting physical, chemical, and biological
Chemical Weathering and Soils Fresh rocks and minerals that once occupied the outermost position reached their present condition of decay through a complex of interacting physical, chemical, and biological
Part II: Past climates
 Part II: Past climates This week Solid Earth - excerpts of Ch 7 Carbon cycle - Ch 8 Early unexplainable things about the Earth Continental Drift (Alfred Wegener, 1920s) Ocean basins: trenches and midocean
Part II: Past climates This week Solid Earth - excerpts of Ch 7 Carbon cycle - Ch 8 Early unexplainable things about the Earth Continental Drift (Alfred Wegener, 1920s) Ocean basins: trenches and midocean
Weathering Cycle Teacher Notes
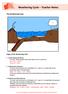 The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
XI. the natural carbon cycle. with materials from J. Kasting (Penn State)
 XI. the natural carbon cycle with materials from J. Kasting (Penn State) outline properties of carbon the terrestrial biological cycle of carbon the ocean cycle of carbon carbon in the rock cycle overview
XI. the natural carbon cycle with materials from J. Kasting (Penn State) outline properties of carbon the terrestrial biological cycle of carbon the ocean cycle of carbon carbon in the rock cycle overview
CHAPTER 3 WATER AND THE FITNESS OF THE ENVIRONMENT. Section B: The Dissociation of Water Molecules
 CHAPTER 3 WATER AND THE FITNESS OF THE ENVIRONMENT Section B: The Dissociation of Water Molecules 1. Organisms are sensitive to changes in ph 2. Acid precipitation threatens the fitness of the environment
CHAPTER 3 WATER AND THE FITNESS OF THE ENVIRONMENT Section B: The Dissociation of Water Molecules 1. Organisms are sensitive to changes in ph 2. Acid precipitation threatens the fitness of the environment
S= 95.02% S= 4.21% 35. S=radioactive 36 S=0.02% S= 0.75% 34 VI V IV III II I 0 -I -II SO 4 S 2 O 6 H 2 SO 3 HS 2 O 4- S 2 O 3
 SULFUR ISOTOPES 32 S= 95.02% 33 S= 0.75% 34 S= 4.21% 35 S=radioactive 36 S=0.02% S-H S-C S=C S-O S=O S-F S-Cl S-S VI V IV III II I 0 -I -II SO 4 2- S 2 O 6 2- H 2 SO 3 HS 2 O 4- S 2 O 3 2- S 2 F 2 S H
SULFUR ISOTOPES 32 S= 95.02% 33 S= 0.75% 34 S= 4.21% 35 S=radioactive 36 S=0.02% S-H S-C S=C S-O S=O S-F S-Cl S-S VI V IV III II I 0 -I -II SO 4 2- S 2 O 6 2- H 2 SO 3 HS 2 O 4- S 2 O 3 2- S 2 F 2 S H
 12 10 8 6 4 2 0 40-50 50-60 60-70 70-80 80-90 90-100 Fresh Water What we will cover The Hydrologic Cycle River systems Floods Groundwater Caves and Karst Topography Hot springs Distribution of water in
12 10 8 6 4 2 0 40-50 50-60 60-70 70-80 80-90 90-100 Fresh Water What we will cover The Hydrologic Cycle River systems Floods Groundwater Caves and Karst Topography Hot springs Distribution of water in
Chapter 4 Rocks & Igneous Rocks
 Chapter 4 Rocks & Igneous Rocks Rock Definition A naturally occurring consolidated mixture of one or more minerals e.g, marble, granite, sandstone, limestone Rock Definition Must naturally occur in nature,
Chapter 4 Rocks & Igneous Rocks Rock Definition A naturally occurring consolidated mixture of one or more minerals e.g, marble, granite, sandstone, limestone Rock Definition Must naturally occur in nature,
Surface Processes on the Earth. Rocks, Weathering, Erosion and Soil
 Surface Processes on the Earth Rocks, Weathering, Erosion and Soil ROCKS AND ROCK CYCLE Rock types Three main types of rock Igneous Metamorphic Sedimentary Igneous Form when magma or lava cools and hardens
Surface Processes on the Earth Rocks, Weathering, Erosion and Soil ROCKS AND ROCK CYCLE Rock types Three main types of rock Igneous Metamorphic Sedimentary Igneous Form when magma or lava cools and hardens
Essentials of Geology, 11e
 Essentials of Geology, 11e and s Chapter 5 Instructor Jennifer Barson Spokane Falls Community College Geology 101 Stanley Hatfield Southwestern Illinois College Jennifer Cole Northeastern University Earth
Essentials of Geology, 11e and s Chapter 5 Instructor Jennifer Barson Spokane Falls Community College Geology 101 Stanley Hatfield Southwestern Illinois College Jennifer Cole Northeastern University Earth
Why study Weathering?
 Why study Weathering? Weathering process of disintegrating solid rock & producing loose debris To understand geol process (like hydrologic systems) and how landscapes evolve (topo maps, landforms) Weathering
Why study Weathering? Weathering process of disintegrating solid rock & producing loose debris To understand geol process (like hydrologic systems) and how landscapes evolve (topo maps, landforms) Weathering
Copyright SOIL STRUCTURE and CLAY MINERALS
 SOIL STRUCTURE and CLAY MINERALS Soil Structure Structure of a soil may be defined as the mode of arrangement of soil grains relative to each other and the forces acting between them to hold them in their
SOIL STRUCTURE and CLAY MINERALS Soil Structure Structure of a soil may be defined as the mode of arrangement of soil grains relative to each other and the forces acting between them to hold them in their
Chapter 3: Acid Base Equilibria. HCl + KOH KCl + H 2 O acid + base salt + water
 Chapter 3: Acid Base Equilibria HCl + KOH KCl + H 2 O acid + base salt + water What is an acid? The Arrhenius concept proposed that acids are substances that produce hydrogen ions (H + ) in aqueous solutions.
Chapter 3: Acid Base Equilibria HCl + KOH KCl + H 2 O acid + base salt + water What is an acid? The Arrhenius concept proposed that acids are substances that produce hydrogen ions (H + ) in aqueous solutions.
Continent-Ocean Interaction: Role of Weathering
 Institute of Astrophysics and Geophysics (Build. B5c) Room 0/13 email: Guy.Munhoven@ulg.ac.be Phone: 04-3669771 28th February 2018 Organisation of the Lecture 1 Carbon cycle processes time scales modelling:
Institute of Astrophysics and Geophysics (Build. B5c) Room 0/13 email: Guy.Munhoven@ulg.ac.be Phone: 04-3669771 28th February 2018 Organisation of the Lecture 1 Carbon cycle processes time scales modelling:
Volume Composition of a Desirable Surface Soil
 Soil Chemistry Volume Composition of a Desirable Surface Soil 50% pore space 25% air 45 to 48% mineral matter 50% solid material 25% water 2 to 5% organic matter Soil Organic Matter Soil organic matter:
Soil Chemistry Volume Composition of a Desirable Surface Soil 50% pore space 25% air 45 to 48% mineral matter 50% solid material 25% water 2 to 5% organic matter Soil Organic Matter Soil organic matter:
Topic 6: Weathering, Erosion and Erosional-Deposition Systems (workbook p ) Workbook Chapter 4, 5 WEATHERING
 Topic 6: Weathering, Erosion and Erosional-Deposition Systems (workbook p. 95-125) Workbook Chapter 4, 5 THE BIG PICTURE: Weathering, erosion and deposition are processes that cause changes to rock material
Topic 6: Weathering, Erosion and Erosional-Deposition Systems (workbook p. 95-125) Workbook Chapter 4, 5 THE BIG PICTURE: Weathering, erosion and deposition are processes that cause changes to rock material
CO2 in atmosphere is influenced by pco2 of surface water (partial pressure of water is the CO2 (gas) that would be in equilibrium with water).
 EART 254, Lecture on April 6 & 11, 2011 Introduction (skipped most of this) Will look at C and N (maybe) cycles with respect to how they influence CO2 levels in the atmosphere. Ocean chemistry controls
EART 254, Lecture on April 6 & 11, 2011 Introduction (skipped most of this) Will look at C and N (maybe) cycles with respect to how they influence CO2 levels in the atmosphere. Ocean chemistry controls
key to long-term sustainability is recycling..
 .. to support life over ~ 4 billion years, Earth must be sustainable system.. key to long-term sustainability is recycling.. Earth System how are key elements needed for life (C, N, P) recycled on the
.. to support life over ~ 4 billion years, Earth must be sustainable system.. key to long-term sustainability is recycling.. Earth System how are key elements needed for life (C, N, P) recycled on the
Chapter 5: Weathering and Soils. Fig. 5.14
 Chapter 5: Weathering and Soils Fig. 5.14 OBJECTIVES Recognize that weathering breaks down minerals and rocks and occurs as a result of both mechanical and chemical processes. Explain the processes that
Chapter 5: Weathering and Soils Fig. 5.14 OBJECTIVES Recognize that weathering breaks down minerals and rocks and occurs as a result of both mechanical and chemical processes. Explain the processes that
SOIL: DEFINITION, FORMATION! & LAYERS"
 SOIL: DEFINITION, FORMATION & LAYERS" What Is Soil? soil - upper-most (relatively thin) layer of Earth s crust, which supports terrestrial plants, animals, & microorganisms basic natural resource that
SOIL: DEFINITION, FORMATION & LAYERS" What Is Soil? soil - upper-most (relatively thin) layer of Earth s crust, which supports terrestrial plants, animals, & microorganisms basic natural resource that
Limestone dissolved by naturally acidic rainwater. Weathering and Soils Lecture 5
 Last time Viscosity determines the ability of a melt to releases gasses coming out of solution Viscosity depends on silica content and TEMPERATURE Mafic (Basaltic) melts are extremely hot and have low
Last time Viscosity determines the ability of a melt to releases gasses coming out of solution Viscosity depends on silica content and TEMPERATURE Mafic (Basaltic) melts are extremely hot and have low
Be sure to show all calculations so that you can receive partial credit for your work!
 Agronomy 365T Exam 1 Spring 2004 Exam Score: Name TA Lab Hour Be sure to show all calculations so that you can receive partial credit for your work! 1) List 14 of the plant essential nutrient for plant
Agronomy 365T Exam 1 Spring 2004 Exam Score: Name TA Lab Hour Be sure to show all calculations so that you can receive partial credit for your work! 1) List 14 of the plant essential nutrient for plant
The Chemistry of Seawater. Unit 3
 The Chemistry of Seawater Unit 3 Water occurs naturally on earth in 3 phases: solid, liquid, or gas (liquid is most abundant) Water Phases Basic Chemistry Review What is an atom? Smallest particles of
The Chemistry of Seawater Unit 3 Water occurs naturally on earth in 3 phases: solid, liquid, or gas (liquid is most abundant) Water Phases Basic Chemistry Review What is an atom? Smallest particles of
Which process is represented by letter F? A) capillarity B) infiltration C) condensation D) vaporization
 1. Water's covalent bond is due to A) water's ability to stick to stick to other materials B) a slight negative charge of O and positive charge of H C) an uneven sharing of electrons D) both B and C 2.
1. Water's covalent bond is due to A) water's ability to stick to stick to other materials B) a slight negative charge of O and positive charge of H C) an uneven sharing of electrons D) both B and C 2.
Weathering The effects of the physical and chemical environment on the decomposition of rocks
 Weathering The effects of the physical and chemical environment on the decomposition of rocks - Igneous rocks form at high temperatures and the constituent minerals reflect the conditions of formation.
Weathering The effects of the physical and chemical environment on the decomposition of rocks - Igneous rocks form at high temperatures and the constituent minerals reflect the conditions of formation.
Microorganisms. Dissolved inorganics. Native vs. Introduced; Oligotrophic vs. Eutrophic Millions to billions per ml or g Complex consortia
 1 Microorganisms Native vs. Introduced; Oligotrophic vs. Eutrophic Millions to billions per ml or g Complex consortia Species makeup: f(t, O 2, ph, nutrients, etc.) Indicators & pathogens Dissolved inorganics
1 Microorganisms Native vs. Introduced; Oligotrophic vs. Eutrophic Millions to billions per ml or g Complex consortia Species makeup: f(t, O 2, ph, nutrients, etc.) Indicators & pathogens Dissolved inorganics
Lecture 3 Rocks and the Rock Cycle Dr. Shwan Omar
 Rocks A naturally occurring aggregate of one or more minerals (e.g., granite), or a body of non-crystalline material (e.g., obsidian glass), or of solid organic material (e.g., coal). Rock Cycle A sequence
Rocks A naturally occurring aggregate of one or more minerals (e.g., granite), or a body of non-crystalline material (e.g., obsidian glass), or of solid organic material (e.g., coal). Rock Cycle A sequence
AMD 101. Chemistry of Abandoned Mine Drainage. Bruce Golden WPCAMR
 AMD 101 Chemistry of Abandoned Mine Drainage Bruce Golden WPCAMR http://amrclearinghouse.org Western PA Coalition for Abandoned Mine Reclamation A helping hand to watershed groups grappling with the legacy
AMD 101 Chemistry of Abandoned Mine Drainage Bruce Golden WPCAMR http://amrclearinghouse.org Western PA Coalition for Abandoned Mine Reclamation A helping hand to watershed groups grappling with the legacy
Earth Science, 10e. Edward J. Tarbuck & Frederick K. Lutgens
 Earth Science, 10e Edward J. Tarbuck & Frederick K. Lutgens Weathering, Soil, and Mass Wasting Chapter 3 Earth Science, 10e Stan Hatfield and Ken Pinzke Southwestern Illinois College Earth's external processes
Earth Science, 10e Edward J. Tarbuck & Frederick K. Lutgens Weathering, Soil, and Mass Wasting Chapter 3 Earth Science, 10e Stan Hatfield and Ken Pinzke Southwestern Illinois College Earth's external processes
Nutrient Cycling in Land Vegetation and Soils
 Nutrient Cycling in Land Vegetation and Soils OCN 401 - Biogeochemical Systems 13 September 2012 Reading: Schlesinger, Chapter 6 Outline 1. The annual Intrasystem Nutrient Cycle 2. Mass balance of the
Nutrient Cycling in Land Vegetation and Soils OCN 401 - Biogeochemical Systems 13 September 2012 Reading: Schlesinger, Chapter 6 Outline 1. The annual Intrasystem Nutrient Cycle 2. Mass balance of the
Rocks and the Rock Cycle. Banded Iron Formation
 Rocks and the Rock Cycle Banded Iron Formation Rocks Big rocks into pebbles, Pebbles into sand. I really hold a million, million Rocks here in my hand. Florence Parry Heide How do rocks change? How are
Rocks and the Rock Cycle Banded Iron Formation Rocks Big rocks into pebbles, Pebbles into sand. I really hold a million, million Rocks here in my hand. Florence Parry Heide How do rocks change? How are
Chapter 5. The Biogeochemical Cycles. Botkin & Keller Environmental Science 5e
 Chapter 5 The Biogeochemical Cycles How Chemicals Cycle Biogeochemical Cycle The complete path a chemical takes through the four major components or reservoirs of Earth s systems 1. Atmosphere 2. Hydrosphere
Chapter 5 The Biogeochemical Cycles How Chemicals Cycle Biogeochemical Cycle The complete path a chemical takes through the four major components or reservoirs of Earth s systems 1. Atmosphere 2. Hydrosphere
Ecoregions Glossary. 7.8B: Changes To Texas Land Earth and Space
 Ecoregions Glossary Ecoregions The term ecoregions was developed by combining the terms ecology and region. Ecology is the study of the interrelationship of organisms and their environments. The term,
Ecoregions Glossary Ecoregions The term ecoregions was developed by combining the terms ecology and region. Ecology is the study of the interrelationship of organisms and their environments. The term,
A horizon. Clay Basics. Clays: A horizon. Phyllosilicates phyllon = leaves. Clay formation. Zone were parent materials weather
 A horizon lay Basics Start focus on A horizon Zone were parent materials weather Original rocks and minerals break down into smaller and smaller pieces Eventually dissolve A horizon Zone were new materials
A horizon lay Basics Start focus on A horizon Zone were parent materials weather Original rocks and minerals break down into smaller and smaller pieces Eventually dissolve A horizon Zone were new materials
List of Equipment, Tools, Supplies, and Facilities:
 Unit D: ph of Soil Lesson 2: Identifying ph Connection With Plant Growth Student Learning Objectives: Instruction in this lesson should result in the students achieving the following objectives: 1. Explain
Unit D: ph of Soil Lesson 2: Identifying ph Connection With Plant Growth Student Learning Objectives: Instruction in this lesson should result in the students achieving the following objectives: 1. Explain
Biogeochemical cycles
 Lecture -2: Biogeochemical cycles ENV 107: Introduction to Environmental Science Dr. A.K.M. Saiful Islam Case Study: Lake Washington The city of Seattle, USA lies between two major bodies of water- saltwater
Lecture -2: Biogeochemical cycles ENV 107: Introduction to Environmental Science Dr. A.K.M. Saiful Islam Case Study: Lake Washington The city of Seattle, USA lies between two major bodies of water- saltwater
Earth Systems Science Chapter 7. Earth Systems Science Chapter 7 11/11/2010. Seismology: study of earthquakes and related phenomena
 Earth Systems Science Chapter 7 I. Structure of the Earth II. Plate Tectonics The solid part of the earth system includes processes, just like the atmosphere and oceans. However, the time scales for processes
Earth Systems Science Chapter 7 I. Structure of the Earth II. Plate Tectonics The solid part of the earth system includes processes, just like the atmosphere and oceans. However, the time scales for processes
Weathering: the disintegration, or breakdown of rock material
 Weathering: the disintegration, or breakdown of rock material Mechanical Weathering: no change in chemical composition--just disintegration into smaller pieces Chemical Weathering: breakdown as a result
Weathering: the disintegration, or breakdown of rock material Mechanical Weathering: no change in chemical composition--just disintegration into smaller pieces Chemical Weathering: breakdown as a result
1. Let s quickly review some of the phosphorus fixation reactions in soils. 2. At low ph (acidic conditons below 6.0), phosphorus fixation occurs
 1 1. Let s quickly review some of the phosphorus fixation reactions in soils. 2. At low ph (acidic conditons below 6.0), phosphorus fixation occurs between phosphates and iron or aluminum in the soil solution
1 1. Let s quickly review some of the phosphorus fixation reactions in soils. 2. At low ph (acidic conditons below 6.0), phosphorus fixation occurs between phosphates and iron or aluminum in the soil solution
Only healthy soil can grow a nutrient dense food. You are what you eat!
 Understanding How Cation Nutrients & Soil Structure are Related By Michael Martin Meléndrez Only healthy soil can grow a nutrient dense food. You are what you eat! Soil Must be able to hold onto water,
Understanding How Cation Nutrients & Soil Structure are Related By Michael Martin Meléndrez Only healthy soil can grow a nutrient dense food. You are what you eat! Soil Must be able to hold onto water,
Pee Dee Explorer. Science Standards
 Science Standards About Pee Dee Explorer What does it mean when someone says they are from the "Pee Dee" of South Carolina? A place is bigger than its physical geography. A "sense of place" weaves together
Science Standards About Pee Dee Explorer What does it mean when someone says they are from the "Pee Dee" of South Carolina? A place is bigger than its physical geography. A "sense of place" weaves together
Page 1. Weathering & Erosion by Mass Wasting Pre-Test. Name:
 Weathering & Erosion by Mass Wasting Pre-Test 3048-1 - Page 1 Name: 1) As a particle of sediment in a stream breaks into several smaller pieces, the rate of weathering of the sediment will A) increase
Weathering & Erosion by Mass Wasting Pre-Test 3048-1 - Page 1 Name: 1) As a particle of sediment in a stream breaks into several smaller pieces, the rate of weathering of the sediment will A) increase
Engineering Geology ECIV 3302
 Engineering Geology ECIV 3302 Instructor : Dr. Jehad Hamad 2019-2018 Chapter (5) Weathering & Soil Chapter 5: Weathering, Soil, and Mass Wasting External processes include : (1) Weathering (2) Mass wasting
Engineering Geology ECIV 3302 Instructor : Dr. Jehad Hamad 2019-2018 Chapter (5) Weathering & Soil Chapter 5: Weathering, Soil, and Mass Wasting External processes include : (1) Weathering (2) Mass wasting
The Production of Sediment. Contents. Weathering. Chapters 1, 3
 The Production of Sediment Chapters 1, 3 Contents Weathering Physical, chemical, biogeochemical processes Rates Products Carbon cycle and global change Erosion/Soils Sediment Texture Weathering General
The Production of Sediment Chapters 1, 3 Contents Weathering Physical, chemical, biogeochemical processes Rates Products Carbon cycle and global change Erosion/Soils Sediment Texture Weathering General
