Wednesday week 12. These ions move through the soil to streams and eventually to the ocean. In the ocean; CaCO 3 + H 2 O + CO 2 H 2 O + H 2 O
|
|
|
- Jacob Atkinson
- 5 years ago
- Views:
Transcription
1 Wednesday week 12 I. Control of CO 2 content of atmosphere by the ocean H 4 SiO 4 A. Consider a hypothetical planet with a crust made of single mineral (Wallastonite) CaSiO3. We could use the composition of a feldspar just as well (fig.1) but using this mineral simplifies the exercise because its chemical composition is simpler than that of feldspars. Carbon dioxide in the atmosphere combines with water to produce weakly acidic rain. This acidic rain reacts with the wallastonite to produce a set of ions and a weak acid. 2CO 2 + 3H 2 O + CaSiO 3 Ca HCO 3 + These ions move through the soil to streams and eventually to the ocean. In the ocean; Ca HCO 3 CaCO 3 + H 2 O + CO 2 H 4 SiO 4 SiO 2. H 2 O + H 2 O These reactions release one carbon dioxide molecule back to the atmosphere and create two 1
2 insoluble compounds, opaline silica and calcium carbonate. The insoluble biological products; SiO 2 and CaCO 3 settle to the ocean floor and are moved by plate tectonics to subduction zones where they are carried deep into the Earth and heated converting them back into Wallastonite and releasing carbon dioxide thus ending the cycle and returning the second CO 2 molecule to the atmosphere and CaSiO 3 to the continents. SiO 2 + CaCO 3 CaSiO 3 + CO 2 B. How does this cycle control the CO 2 content of the atmosphere? 1) The rate of tectonic plate motions set the rate at which CO 2 is released from the Earth s interior to the atmosphere. If release from the earth s interior exceeds the rate at which CO 2 is removed from the ocean by the formation of calcium carbonate shells by oceanic biological processes then carbon dioxide will accumulate in the atmosphere and visa versa. 2
3 a) In the first case the planet, all else being the same, will become warmer (the greenhouse effect) and wetter (a warmer atmosphere causes greater evaporation, all else being equal). b) The carbon dioxide content of soils will rise causing an increase in the rate of weathering. c) An increase in moisture (rain) will, in areas where water is a limiting factor, cause increased vegetative growth. Root respiration by plants will cause an increase in the carbon dioxide concentration of soils. 2) Processes (a) through (c) will increase weathering rates sending more Ca ++ to the sea. This increased Ca ++ will increase the rate of formation of CaCO 3 shells by marine organisms. With time the CO 2 withdrawn from the sea by organisms will balance the increased CO 2 being added by volcanoes. 3
4 C. Exchange of carbon dioxide between the atmosphere and the ocean. 1. At a given temperature the exchange of carbon dioxide molecules between the atmosphere and the ocean reaches equilibrium. This equilibrium is controlled by the partial pressure (that pressure caused by the molecules of carbon dioxide alone) of carbon dioxide in the atmosphere and that at the surface of the sea. At these temperatures (all else being equal) the number of carbon dioxide molecules that escape from the sea surface is balanced by the number that enter the sea from the atmosphere. 2. If the temperature of the ocean were to rise (all else staying the same) then the kinetic energy of the carbon dioxide molecules in the seawater will rise and more carbon dioxide molecules will leave the ocean than would enter the ocean. This will continue until the partial pressure of 4
5 carbon dioxide in the atmosphere was increased to the point that it balanced that pressure at the sea surface. 3. If the ocean were to cool then the reverse of the above would happen. 4. Consequently carbon dioxide is more soluble in cold than in warm water. 5. When the oceans exchange ( breath ) carbon dioxide with the atmosphere they take it up ( inhale ) in the cold surface waters of high latitudes and release ( exhale ) from the warm surface waters of low latitudes. 6. Increasing the carbon dioxide concentration of the atmosphere will cause the ocean to take up (inhale) more carbon dioxide. Because the oceans surface layer mixes slowly with the deep ocean (hundreds of years) the increased carbon dioxide content of the surface ocean will be mixed very slowly into the large carbon reservoir of the deep ocean. The rate of our adding carbon 5
6 dioxide to the atmosphere is too fast for the deep ocean to be a significant reservoir. So the carbon dioxide content of the atmosphere will rise as the concentration in the shallow ocean rises. Alkalinity Both positive and negative ions are added to the sea from rivers. To keep the oceans charge neutral an adjustment occurs in the HCO 3 CO 3 system. If more positive than negative ions are added to the sea then HCO 3 changes to CO 3, CO 3 having two negative rather tan one negative charge. Alkalinity is defined as A HCO3 + 2CO3 The buffering capacity of the ocean is the total CO 3 concentration in the ocean. If carbon dioxide is added to the ocean there is a change from CO 3 to HCO 3. If enough carbon dioxide is added to the ocean then it could exceed the buffering capacity and the oceans acidity would increase. This is a real problem associated with adding carbon dioxide to the atmosphere independent of global warming. 6
7 The calcium carbonate deposits on the ocean floor are also a buffer for with added carbon dioxide the depth at which carbon dioxide dissolves (the carbonate compensation depth) will rise. If the buffering capacity of the ocean is exceeded and all the calcium carbonate on the ocean floor is dissolved as atmospheric concentrations of Carbon dioxide rise then the acidity of the ocean will rise. 7
Long-term Climate Change. We are in a period of relative warmth right now but on the time scale of the Earth s history, the planet is cold.
 Long-term Climate Change We are in a period of relative warmth right now but on the time scale of the Earth s history, the planet is cold. Long-term Climate Change The Archean is thought to have been warmer,
Long-term Climate Change We are in a period of relative warmth right now but on the time scale of the Earth s history, the planet is cold. Long-term Climate Change The Archean is thought to have been warmer,
Figure 65: Reservoir in a steady state condition where the input flux is equal to the output flux and the reservoir size remains constant.
 7. The carbon cycle 7.1. Box model of the carbon cycle Without the greenhouse effect, our planet would experience a permanent ice age and life as we know it would not be possible. The main contributors
7. The carbon cycle 7.1. Box model of the carbon cycle Without the greenhouse effect, our planet would experience a permanent ice age and life as we know it would not be possible. The main contributors
Carbon Cycling Internal
 Carbon Cycling Internal The 4 subcycles Atmosphere The Earth s Atmosphere The Earth has a radius of some 6400 km. Ninety-nine percent of the earth's atmosphere is contained within a layer approximately
Carbon Cycling Internal The 4 subcycles Atmosphere The Earth s Atmosphere The Earth has a radius of some 6400 km. Ninety-nine percent of the earth's atmosphere is contained within a layer approximately
DIAGRAM 1: Ocean Carbon Cycle DIAGRAM 2: Terrestrial Carbon Cycle
 DIAGRAM 1: Ocean Carbon Cycle DIAGRAM 2: Terrestrial Carbon Cycle DIAGRAM 3: Ocean Monthly CO 2 Flux Molecules of CO 2 enter the ocean by diffusing into the sea surface waters and dissolving a physio-chemical
DIAGRAM 1: Ocean Carbon Cycle DIAGRAM 2: Terrestrial Carbon Cycle DIAGRAM 3: Ocean Monthly CO 2 Flux Molecules of CO 2 enter the ocean by diffusing into the sea surface waters and dissolving a physio-chemical
/ Past and Present Climate
 MIT OpenCourseWare http://ocw.mit.edu 12.842 / 12.301 Past and Present Climate Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. The Faint Young
MIT OpenCourseWare http://ocw.mit.edu 12.842 / 12.301 Past and Present Climate Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. The Faint Young
Climate and Earth Systems
 OVERVIEW Climate and Earth Systems This activity focuses on the geological carbon cycle to foster student thinking on Earth systems and develop understanding of how models support and test scientific findings.
OVERVIEW Climate and Earth Systems This activity focuses on the geological carbon cycle to foster student thinking on Earth systems and develop understanding of how models support and test scientific findings.
Water percolating through hot lava dissolves soluble minerals containing chlorine, bromine and sulphur compounds
 Figure 5 The sources of dissolved ions in sea water. Water falls as rain Compounds containing mainly calcium, magnesium, carbonate and silicate ions are leached from the soil Rivers carry ions in solution
Figure 5 The sources of dissolved ions in sea water. Water falls as rain Compounds containing mainly calcium, magnesium, carbonate and silicate ions are leached from the soil Rivers carry ions in solution
[ ] Sparkling Water and the Carbon Cycle
![[ ] Sparkling Water and the Carbon Cycle [ ] Sparkling Water and the Carbon Cycle](/thumbs/87/96185022.jpg) Materials: Seltzer maker (SodaStream or similar) Some tasty cheese Tap water Glasses or cups for drinking ph paper Many people enjoy carbonated beverages, especially with food. The tiny bubbles and natural
Materials: Seltzer maker (SodaStream or similar) Some tasty cheese Tap water Glasses or cups for drinking ph paper Many people enjoy carbonated beverages, especially with food. The tiny bubbles and natural
BIOGEOCHEMICAL CYCLES
 BIOGEOCHEMICAL CYCLES BASICS Biogeochemical Cycle: The complete path a chemical takes through the four major components, or reservoirs, of Earth s system (atmosphere, lithosphere, hydrosphere and biosphere)
BIOGEOCHEMICAL CYCLES BASICS Biogeochemical Cycle: The complete path a chemical takes through the four major components, or reservoirs, of Earth s system (atmosphere, lithosphere, hydrosphere and biosphere)
Global Carbon Cycle - I
 Global Carbon Cycle - I OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 11 1. Overview of global C cycle 2. Global C reservoirs Outline 3. The contemporary global C cycle 4. Fluxes and residence
Global Carbon Cycle - I OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 11 1. Overview of global C cycle 2. Global C reservoirs Outline 3. The contemporary global C cycle 4. Fluxes and residence
Climate Regulation. - What stabilizes the climate - Greenhouse effect
 Climate Regulation - What stabilizes the climate - Greenhouse effect Last time! Processes that shaped Earth: Volcanism, tectonics! How we retain atmospheric molecules ( escape speed )! A magnetic field
Climate Regulation - What stabilizes the climate - Greenhouse effect Last time! Processes that shaped Earth: Volcanism, tectonics! How we retain atmospheric molecules ( escape speed )! A magnetic field
(4) Give an example of important reactions that are responsible for the composition of river water.
 Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
XI. the natural carbon cycle. with materials from J. Kasting (Penn State)
 XI. the natural carbon cycle with materials from J. Kasting (Penn State) outline properties of carbon the terrestrial biological cycle of carbon the ocean cycle of carbon carbon in the rock cycle overview
XI. the natural carbon cycle with materials from J. Kasting (Penn State) outline properties of carbon the terrestrial biological cycle of carbon the ocean cycle of carbon carbon in the rock cycle overview
6th Grade Science Sample Assessment Items S6E3c.
 Composition 6th Grade Science Sample Assessment Items Ocean water differs from freshwater in that it has. A. a lower temperature B. a higher temperature C. a higher concentration of silicon dioxide D.
Composition 6th Grade Science Sample Assessment Items Ocean water differs from freshwater in that it has. A. a lower temperature B. a higher temperature C. a higher concentration of silicon dioxide D.
Lecture 4 What Controls the Composition of Seawater
 Lecture 4 What Controls the Composition of Seawater Seawater is salty! Why? What controls the composition of seawater? Do Chemical Equilibrium reactions control the composition of the Ocean? What is meant
Lecture 4 What Controls the Composition of Seawater Seawater is salty! Why? What controls the composition of seawater? Do Chemical Equilibrium reactions control the composition of the Ocean? What is meant
Global Carbon Cycle - I Systematics: Reservoirs and Fluxes
 OCN 401-10 Nov. 16, 2010 KCR Global Carbon Cycle - I Systematics: Reservoirs and Fluxes The Global carbon cycle Reservoirs: biomass on land in the oceans, atmosphere, soil and rocks, waters Processes:
OCN 401-10 Nov. 16, 2010 KCR Global Carbon Cycle - I Systematics: Reservoirs and Fluxes The Global carbon cycle Reservoirs: biomass on land in the oceans, atmosphere, soil and rocks, waters Processes:
The Chemistry of Seawater. Unit 3
 The Chemistry of Seawater Unit 3 Water occurs naturally on earth in 3 phases: solid, liquid, or gas (liquid is most abundant) Water Phases Basic Chemistry Review What is an atom? Smallest particles of
The Chemistry of Seawater Unit 3 Water occurs naturally on earth in 3 phases: solid, liquid, or gas (liquid is most abundant) Water Phases Basic Chemistry Review What is an atom? Smallest particles of
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Mechanisms
 Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes WEATHERING CHAPTER 7 Weathering
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes WEATHERING CHAPTER 7 Weathering
1 Earth s Oceans. TAKE A LOOK 2. Identify What are the five main oceans?
 CHAPTER 13 1 Earth s Oceans SECTION Exploring the Oceans BEFORE YOU READ After you read this section, you should be able to answer these questions: What affects the salinity of ocean water? What affects
CHAPTER 13 1 Earth s Oceans SECTION Exploring the Oceans BEFORE YOU READ After you read this section, you should be able to answer these questions: What affects the salinity of ocean water? What affects
The Terrestrial Planets
 The Terrestrial Planets Large Bodies: Earth (1 R E, 1 M E ) Venus (0.95 R E, 0.82 M E ) Small Bodies: Mars (0.53 R E, 0.11 M E ) Mercury (0.38 R E, 0.055 M E ) Moon (0.27 R E, 0.012 M E ) The surfaces
The Terrestrial Planets Large Bodies: Earth (1 R E, 1 M E ) Venus (0.95 R E, 0.82 M E ) Small Bodies: Mars (0.53 R E, 0.11 M E ) Mercury (0.38 R E, 0.055 M E ) Moon (0.27 R E, 0.012 M E ) The surfaces
Global Carbon Cycle - I
 Global Carbon Cycle - I Reservoirs and Fluxes OCN 401 - Biogeochemical Systems 13 November 2012 Reading: Schlesinger, Chapter 11 Outline 1. Overview of global C cycle 2. Global C reservoirs 3. The contemporary
Global Carbon Cycle - I Reservoirs and Fluxes OCN 401 - Biogeochemical Systems 13 November 2012 Reading: Schlesinger, Chapter 11 Outline 1. Overview of global C cycle 2. Global C reservoirs 3. The contemporary
Physical Oceanography
 Physical Oceanography SECTION 15.1 The Oceans In your textbook, read about modern oceanography. For each item in Column A, write the letter of the matching item in Column B. e b c d a Column A 1. German
Physical Oceanography SECTION 15.1 The Oceans In your textbook, read about modern oceanography. For each item in Column A, write the letter of the matching item in Column B. e b c d a Column A 1. German
Physical Oceanography
 Physical Oceanography SECTION 15.1 The Oceans In your textbook, read about modern oceanography. For each item in Column A, write the letter of the matching item in Column B. Column A 1. German research
Physical Oceanography SECTION 15.1 The Oceans In your textbook, read about modern oceanography. For each item in Column A, write the letter of the matching item in Column B. Column A 1. German research
The performance expectation above was developed using the following elements from the NRC document A Framework for K-12 Science Education:
 MS-ESS2-1 Earth's Systems Students who demonstrate understanding can: MS-ESS2-1. Develop a model to describe the cycling of Earth's materials and the flow of energy that drives this process. [Clarification
MS-ESS2-1 Earth's Systems Students who demonstrate understanding can: MS-ESS2-1. Develop a model to describe the cycling of Earth's materials and the flow of energy that drives this process. [Clarification
Climate Change Lecture Notes
 Climate Change Lecture Notes (Topic 12A) page 1 Climate Change Lecture Notes Learning Outcomes for the Climate Change Unit 1. Students can list observations which suggest that the world is warming, and
Climate Change Lecture Notes (Topic 12A) page 1 Climate Change Lecture Notes Learning Outcomes for the Climate Change Unit 1. Students can list observations which suggest that the world is warming, and
EPSS 15 Introduction to Oceanography Spring The Physical and Chemical Properties of Seawater
 EPSS 15 Introduction to Oceanography Spring 2017 The Physical and Chemical Properties of Seawater The focus of the Lab this week is seawater--its composition, physical and chemical properties. Seawater
EPSS 15 Introduction to Oceanography Spring 2017 The Physical and Chemical Properties of Seawater The focus of the Lab this week is seawater--its composition, physical and chemical properties. Seawater
Q1. Scientists study the atmosphere on planets and moons in the Solar System to understand how the Earth s atmosphere has changed.
 Q. Scientists study the atmosphere on planets and moons in the Solar System to understand how the Earth s atmosphere has changed. (a) Millions of years ago the Earth s atmosphere was probably just like
Q. Scientists study the atmosphere on planets and moons in the Solar System to understand how the Earth s atmosphere has changed. (a) Millions of years ago the Earth s atmosphere was probably just like
Benchmark #: State Language: Student Friendly Language: The student models earth s cycles, constructive and
 Science, Grade: 7 Mastery Check Benchmark #: 7.4.1.2 4 State Language: Student Friendly Language: The student models earth s cycles, constructive and I will be able to describe weathering, erosion, and
Science, Grade: 7 Mastery Check Benchmark #: 7.4.1.2 4 State Language: Student Friendly Language: The student models earth s cycles, constructive and I will be able to describe weathering, erosion, and
Part II: Past climates
 Part II: Past climates This week Solid Earth - excerpts of Ch 7 Carbon cycle - Ch 8 Early unexplainable things about the Earth Continental Drift (Alfred Wegener, 1920s) Ocean basins: trenches and midocean
Part II: Past climates This week Solid Earth - excerpts of Ch 7 Carbon cycle - Ch 8 Early unexplainable things about the Earth Continental Drift (Alfred Wegener, 1920s) Ocean basins: trenches and midocean
How does Rock become Exposed to the Surface?
 Weathering How does Rock become Exposed to the Surface? Most rocks, like granite, form under earth s surface. The rocks uplift and eventually make their way to earth s surface. Conditions on the surface
Weathering How does Rock become Exposed to the Surface? Most rocks, like granite, form under earth s surface. The rocks uplift and eventually make their way to earth s surface. Conditions on the surface
Thermal / Solar. When air is warmed it... Rises. Solar Energy. Evaporation. Condensation Forms Clouds
 Thermal / Solar Light from the Sun is transformed into what type of energy when it hits Earth's surface? Rises When air is warmed it... Solar Energy Water moves through the water cycle using what type
Thermal / Solar Light from the Sun is transformed into what type of energy when it hits Earth's surface? Rises When air is warmed it... Solar Energy Water moves through the water cycle using what type
The Earth System Connections among the great spheres
 Why should we discuss the Earth System? The Earth System Connections among the great spheres Before we delve into the connection between geology, health, and forensics, we must gain an appreciation of
Why should we discuss the Earth System? The Earth System Connections among the great spheres Before we delve into the connection between geology, health, and forensics, we must gain an appreciation of
Lecture 20. Origin of the atmosphere (Chap. 10) The carbon cycle and long-term climate (Chap. 8 of the textbook: p )
 Lecture 20 Origin of the atmosphere (Chap. 10) The carbon cycle and long-term climate (Chap. 8 of the textbook: p.158-170) end of last ice-age; begin civilization beginning of modern era of ice-ages asteroid
Lecture 20 Origin of the atmosphere (Chap. 10) The carbon cycle and long-term climate (Chap. 8 of the textbook: p.158-170) end of last ice-age; begin civilization beginning of modern era of ice-ages asteroid
organisms CaCO 3 + H 2 O + CO 2 shallow water
 Weathering and Reverse weathering Step I:Weathering of igneous rocks 1. Igneous rocks are mainly composed of Al, Si and O 2 with minor and varying quantities of Na, K, Ca and Mg composing pheldspar minerals
Weathering and Reverse weathering Step I:Weathering of igneous rocks 1. Igneous rocks are mainly composed of Al, Si and O 2 with minor and varying quantities of Na, K, Ca and Mg composing pheldspar minerals
Cycles in the Phanerozoic
 Cycles in the Phanerozoic Evolutionary trends: extinctions, adaptive radiations, diversity over time Glaciations Sea level change Ocean chemistry Atmospheric CO 2 biosphere Mass extinctions in the..you
Cycles in the Phanerozoic Evolutionary trends: extinctions, adaptive radiations, diversity over time Glaciations Sea level change Ocean chemistry Atmospheric CO 2 biosphere Mass extinctions in the..you
Chemistry 8 Chapter 7 Review Kinetic Molecular Theory 1. Define Mass The amount of matter in a substance or object.
 Chemistry 8 Chapter 7 Review Kinetic Molecular Theory 1. Define Mass The amount of matter in a substance or object. 2. Define Volume The amount of space taken up by a substance or object. 3. What are the
Chemistry 8 Chapter 7 Review Kinetic Molecular Theory 1. Define Mass The amount of matter in a substance or object. 2. Define Volume The amount of space taken up by a substance or object. 3. What are the
Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions
 Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions OCN 623 Chemical Oceanography Reading: Libes, Chapters 15 and 16 Outline I. Deep sea sedimentation Detrital sediments
Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions OCN 623 Chemical Oceanography Reading: Libes, Chapters 15 and 16 Outline I. Deep sea sedimentation Detrital sediments
The Biogeochemical Carbon Cycle: CO 2,the greenhouse effect, & climate feedbacks. Assigned Reading: Kump et al. (1999) The Earth System, Chap. 7.
 The Biogeochemical Carbon Cycle: CO 2,the greenhouse effect, & climate feedbacks Assigned Reading: Kump et al. (1999) The Earth System, Chap. 7. Overhead Transparencies Faint Faint Young Sun Paradox Young
The Biogeochemical Carbon Cycle: CO 2,the greenhouse effect, & climate feedbacks Assigned Reading: Kump et al. (1999) The Earth System, Chap. 7. Overhead Transparencies Faint Faint Young Sun Paradox Young
Ocean Acidification the other CO2 problem..
 Ocean Acidification the other CO2 problem.. Recall: Atm CO 2 already above recent planetary history CO 2 Today: What does this do to ocean water? Main Outline: 1. Chemistry. How does ocean absorb CO 2,
Ocean Acidification the other CO2 problem.. Recall: Atm CO 2 already above recent planetary history CO 2 Today: What does this do to ocean water? Main Outline: 1. Chemistry. How does ocean absorb CO 2,
Chapter 1: Introduction to Earth Science
 Chapter 1: Introduction to Earth Science 1.1 What is Earth Science Earth science is the name for the group of sciences that deals with Earth and its neighbors in space. Includes: Geology Oceanography Meteorology
Chapter 1: Introduction to Earth Science 1.1 What is Earth Science Earth science is the name for the group of sciences that deals with Earth and its neighbors in space. Includes: Geology Oceanography Meteorology
key to long-term sustainability is recycling..
 .. to support life over ~ 4 billion years, Earth must be sustainable system.. key to long-term sustainability is recycling.. Earth System how are key elements needed for life (C, N, P) recycled on the
.. to support life over ~ 4 billion years, Earth must be sustainable system.. key to long-term sustainability is recycling.. Earth System how are key elements needed for life (C, N, P) recycled on the
1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and
 1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and climate change e) Oceanic water residence times 3.
1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and climate change e) Oceanic water residence times 3.
Our Planet Earth. I nteractions of Earth Systems
 CHAPTER 3 LESSON 2 Our Planet Earth I nteractions of Earth Systems Key Concepts How does the water cycle show interactions of Earth systems? How does weather show interactions of Earth systems? How does
CHAPTER 3 LESSON 2 Our Planet Earth I nteractions of Earth Systems Key Concepts How does the water cycle show interactions of Earth systems? How does weather show interactions of Earth systems? How does
Evolution of Earth Environments Bio-Geo-Chemical Cycling
 Evolution of Earth Environments Bio-Geo-Chemical Cycling Evolution of the Earliest Atmospheres of Mars and Earth Volcanic Outgassing Evolving to Equilibrium Atmosphere To Atmosphere Lost to space (Abundant)
Evolution of Earth Environments Bio-Geo-Chemical Cycling Evolution of the Earliest Atmospheres of Mars and Earth Volcanic Outgassing Evolving to Equilibrium Atmosphere To Atmosphere Lost to space (Abundant)
Earth s Dynamic Surface
 Earth s Dynamic Surface Key Concepts What is the difference between physical and chemical weathering? How do water, ice, and wind change Earth s surface? Changing Earth s Surface What do you think? Read
Earth s Dynamic Surface Key Concepts What is the difference between physical and chemical weathering? How do water, ice, and wind change Earth s surface? Changing Earth s Surface What do you think? Read
8 th Earth Science Chapter 4 Rocks Name Section 1 The Rock Cycle:
 8 th Earth Science Chapter 4 Rocks Name Section 1 The Rock Cycle: Most rock used for stone contains one or more common minerals, called rock-forming minerals, such as, feldspar,, or. When you look closely,
8 th Earth Science Chapter 4 Rocks Name Section 1 The Rock Cycle: Most rock used for stone contains one or more common minerals, called rock-forming minerals, such as, feldspar,, or. When you look closely,
Earth Systems Science Chapter 7. Earth Systems Science Chapter 7 11/11/2010. Seismology: study of earthquakes and related phenomena
 Earth Systems Science Chapter 7 I. Structure of the Earth II. Plate Tectonics The solid part of the earth system includes processes, just like the atmosphere and oceans. However, the time scales for processes
Earth Systems Science Chapter 7 I. Structure of the Earth II. Plate Tectonics The solid part of the earth system includes processes, just like the atmosphere and oceans. However, the time scales for processes
1. Oceans. Example 2. oxygen.
 1. Oceans a) Basic facts: There are five oceans on earth, making up about 72% of the planet s surface and holding 97% of the hydrosphere. Oceans supply the planet with most of its oxygen, play a vital
1. Oceans a) Basic facts: There are five oceans on earth, making up about 72% of the planet s surface and holding 97% of the hydrosphere. Oceans supply the planet with most of its oxygen, play a vital
2010 Pearson Education, Inc.
 Chapter 10 Planetary Atmospheres: Mars, Venus, Earth What is an atmosphere? An atmosphere is a (usually very thin) layer of gas that surrounds a world. How does the greenhouse effect warm a planet? No
Chapter 10 Planetary Atmospheres: Mars, Venus, Earth What is an atmosphere? An atmosphere is a (usually very thin) layer of gas that surrounds a world. How does the greenhouse effect warm a planet? No
THE CHANGING SURFACE OF THE EARTH
 THE CHANGING SURFACE OF THE EARTH Key words Drain geological agent weathering erosion Sediment deposition transport The landscape is a consequence of the action of two types of geological processes; internal
THE CHANGING SURFACE OF THE EARTH Key words Drain geological agent weathering erosion Sediment deposition transport The landscape is a consequence of the action of two types of geological processes; internal
Carbon Dioxide, Alkalinity and ph
 Carbon Dioxide, Alkalinity and ph OCN 623 Chemical Oceanography 15 March 2018 Reading: Libes, Chapter 15, pp. 383 389 (Remainder of chapter will be used with the classes Global Carbon Dioxide and Biogenic
Carbon Dioxide, Alkalinity and ph OCN 623 Chemical Oceanography 15 March 2018 Reading: Libes, Chapter 15, pp. 383 389 (Remainder of chapter will be used with the classes Global Carbon Dioxide and Biogenic
Marine Sediments EPSS15 Spring 2017 Lab 4
 Marine Sediments EPSS15 Spring 2017 Lab 4 Why Sediments? Record of Earth s history - Tectonic plate movement - Past changes in climate - Ancient ocean circulation currents - Cataclysmic events 1 Classification
Marine Sediments EPSS15 Spring 2017 Lab 4 Why Sediments? Record of Earth s history - Tectonic plate movement - Past changes in climate - Ancient ocean circulation currents - Cataclysmic events 1 Classification
25-Nov-14. The Structure of Earth s Interior. What unique features of Earth are important for life as we know it?
 What unique features of Earth are important for life as we know it? Surface liquid water Atmospheric oxygen These are obvious Less obvious are: Plate tectonics Climate stability The Structure of Earth
What unique features of Earth are important for life as we know it? Surface liquid water Atmospheric oxygen These are obvious Less obvious are: Plate tectonics Climate stability The Structure of Earth
UNIT 3 GEOLOGY VOCABULARY FLASHCARDS THESE KEY VOCABULARY WORDS AND PHRASES APPEAR ON THE UNIT 3 CBA
 UNIT 3 GEOLOGY VOCABULARY FLASHCARDS THESE KEY VOCABULARY WORDS AND PHRASES APPEAR ON THE UNIT 3 CBA A map that shows Earth s Topographic Map surface topography, which is Earth s shape and features Contour
UNIT 3 GEOLOGY VOCABULARY FLASHCARDS THESE KEY VOCABULARY WORDS AND PHRASES APPEAR ON THE UNIT 3 CBA A map that shows Earth s Topographic Map surface topography, which is Earth s shape and features Contour
Energy and Matter. Principles of Biology. Organisms interact with their environment, exchanging energy and matter. Topics Covered in this Module
 Principles of Biology contents 2 Energy and Matter Organisms interact with their environment, exchanging energy and matter. The Sun. Most ecosystems receive their energy from the Sun's radiation. NASA/European
Principles of Biology contents 2 Energy and Matter Organisms interact with their environment, exchanging energy and matter. The Sun. Most ecosystems receive their energy from the Sun's radiation. NASA/European
8.E.1.1 Notes.notebook. November 02, 2014
 Unit 2 Hydrosphere 8.E.1.1 Structure of the Hydrosphere Water is the only substance on Earth that occurs naturally as a solid, a liquid, and a gas. Water covers 71% of Earth's surface! 97% of water on
Unit 2 Hydrosphere 8.E.1.1 Structure of the Hydrosphere Water is the only substance on Earth that occurs naturally as a solid, a liquid, and a gas. Water covers 71% of Earth's surface! 97% of water on
I am getting absorbed by a leaf during photosynthesis to make sugar. Go to the Trees.
 I am getting absorbed by a leaf during photosynthesis to make sugar. Go to the Trees. I am getting absorbed by a leaf during photosynthesis to make sugar. Go to the Trees. I am getting absorbed by a leaf
I am getting absorbed by a leaf during photosynthesis to make sugar. Go to the Trees. I am getting absorbed by a leaf during photosynthesis to make sugar. Go to the Trees. I am getting absorbed by a leaf
Grades 9-12: Earth Sciences
 Grades 9-12: Earth Sciences Earth Sciences...1 Earth s Place in the Universe...1 Dynamic Earth Processes...2 Energy in the Earth System...2 Biogeochemical cycles...4 Structure and Composition of the Atmosphere...4
Grades 9-12: Earth Sciences Earth Sciences...1 Earth s Place in the Universe...1 Dynamic Earth Processes...2 Energy in the Earth System...2 Biogeochemical cycles...4 Structure and Composition of the Atmosphere...4
Reference pg and in Textbook
 Reference pg. 154-164 and 188-202 in Textbook Combustion Reactions During combustion (burning) of fossil fuels, collisions between the molecules of the fuel and oxygen result in the formation of new molecules.
Reference pg. 154-164 and 188-202 in Textbook Combustion Reactions During combustion (burning) of fossil fuels, collisions between the molecules of the fuel and oxygen result in the formation of new molecules.
Salt Water. Copyright 2012 LessonSnips
 Salt Water Humans need salt in their diet to achieve stable body chemistry. As Americans who frequently eat in fast food restaurants, our problem is ingesting more salt than needed rather than getting
Salt Water Humans need salt in their diet to achieve stable body chemistry. As Americans who frequently eat in fast food restaurants, our problem is ingesting more salt than needed rather than getting
The surface of the ocean floor is as varied as the land. The five major oceans, from largest to smallest, are
 11.1 Ocean Basins The surface of the ocean floor is as varied as the land. The five major oceans, from largest to smallest, are w the Pacific w the Atlantic w the Indian w the Southern w the Arctic The
11.1 Ocean Basins The surface of the ocean floor is as varied as the land. The five major oceans, from largest to smallest, are w the Pacific w the Atlantic w the Indian w the Southern w the Arctic The
Systems? Climate Systems. Earth Systems. Earth Interior Systems. Atmospheric/Biospheric Systems: Human Impact Hydrologic Cycle.
 Chapter 15 Climate Systems Systems? What is a system? Geologic phenomena are complex. All processes are related to, and interact with, other processes. So it is useful to think of geologic processes as
Chapter 15 Climate Systems Systems? What is a system? Geologic phenomena are complex. All processes are related to, and interact with, other processes. So it is useful to think of geologic processes as
ESS2.A: EARTH MATERIALS AND SYSTEMS
 such as plate tectonics (link to ESS2.B) and erosion, have destroyed or altered most of the very early rock record on Earth, other objects in the solar system, such as lunar rocks, asteroids, and meteorites,
such as plate tectonics (link to ESS2.B) and erosion, have destroyed or altered most of the very early rock record on Earth, other objects in the solar system, such as lunar rocks, asteroids, and meteorites,
Where is all the water?
 Where is all the water? The distribution of water at the Earth's surface % of total Oceans 97.25 Ice caps and glaciers 2.05 Groundwater 0.68 Lakes 0.01 Soils 0.005 Atmosphere (as vapour) 0.001 Rivers 0.0001
Where is all the water? The distribution of water at the Earth's surface % of total Oceans 97.25 Ice caps and glaciers 2.05 Groundwater 0.68 Lakes 0.01 Soils 0.005 Atmosphere (as vapour) 0.001 Rivers 0.0001
The Atmosphere of Earth
 The Atmosphere of Earth The probability of a storm can be predicted, but nothing can be done to stop or slow a storm. Understanding the atmosphere may help in predicting weather changes, but it is doubtful
The Atmosphere of Earth The probability of a storm can be predicted, but nothing can be done to stop or slow a storm. Understanding the atmosphere may help in predicting weather changes, but it is doubtful
Redox, ph, pe OUTLINE 9/12/17. Equilibrium? Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox
 Redox, ph, pe Equilibrium? OUTLINE Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox Reading: White p555-563 1 Question of the day? So what about the CO 2 system? CO
Redox, ph, pe Equilibrium? OUTLINE Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox Reading: White p555-563 1 Question of the day? So what about the CO 2 system? CO
UNIT 4 SEDIMENTARY ROCKS
 UNIT 4 SEDIMENTARY ROCKS WHAT ARE SEDIMENTS Sediments are loose Earth materials (unconsolidated materials) such as sand which are transported by the action of water, wind, glacial ice and gravity. These
UNIT 4 SEDIMENTARY ROCKS WHAT ARE SEDIMENTS Sediments are loose Earth materials (unconsolidated materials) such as sand which are transported by the action of water, wind, glacial ice and gravity. These
Components of the Climate System. Lecture 2: Earth s Climate System. Pop Quiz. Sub-components Global cycles What comes in What goes out
 Lecture 2: Earth s Climate System Components of the Climate System terrestrial radiation Atmosphere Ocean solar radiation Land Energy, Water, and Biogeochemistry Cycles Sub-components Global cycles What
Lecture 2: Earth s Climate System Components of the Climate System terrestrial radiation Atmosphere Ocean solar radiation Land Energy, Water, and Biogeochemistry Cycles Sub-components Global cycles What
Geosphere Final Exam Study Guide
 Geosphere Final Exam Study Guide Chapter 1 Intro to Earth Systems 1. Name and describe Earth s 4 major spheres Geosphere-- nonliving, mostly solid rock divided into crust, mantle, and core Atmosphere a
Geosphere Final Exam Study Guide Chapter 1 Intro to Earth Systems 1. Name and describe Earth s 4 major spheres Geosphere-- nonliving, mostly solid rock divided into crust, mantle, and core Atmosphere a
Lecture 2: Earth s Climate System
 Lecture 2: Earth s Climate System terrestrial radiation solar radiation Atmosphere Ocean Solid Earth Land Energy, Water, and Biogeochemistry Cycles Sub-components Global cycles What comes in What goes
Lecture 2: Earth s Climate System terrestrial radiation solar radiation Atmosphere Ocean Solid Earth Land Energy, Water, and Biogeochemistry Cycles Sub-components Global cycles What comes in What goes
C1.7 EARTH AND ITS ATMOSPHERE MarkScheme
 C.7 EARTH AND ITS ATMOSPHERE MarkScheme M. (a) (i) it = water vapour condensed accept temperature went below 00 C / boiling point of water allow cooled to fm liquid / water / rain do not accept evapated
C.7 EARTH AND ITS ATMOSPHERE MarkScheme M. (a) (i) it = water vapour condensed accept temperature went below 00 C / boiling point of water allow cooled to fm liquid / water / rain do not accept evapated
Weathering, Soil, & Mass Movements. Chapter 5
 Weathering, Soil, & Mass Movements Chapter 5 5.1 Weathering Weathering: the breaking down and changing of rocks at or near the Earth s surface. Basic part of the rock cycle. 2 main types: 1. Mechanical
Weathering, Soil, & Mass Movements Chapter 5 5.1 Weathering Weathering: the breaking down and changing of rocks at or near the Earth s surface. Basic part of the rock cycle. 2 main types: 1. Mechanical
Unit 3 Review Guide: Atmosphere
 Unit 3 Review Guide: Atmosphere Atmosphere: A thin layer of gases that forms a protective covering around the Earth. Photosynthesis: Process where plants take in carbon dioxide and release oxygen. Trace
Unit 3 Review Guide: Atmosphere Atmosphere: A thin layer of gases that forms a protective covering around the Earth. Photosynthesis: Process where plants take in carbon dioxide and release oxygen. Trace
8 th Grade Campus Assessment- NSMS Plate Tectonics
 1. A group of students were discussing plate tectonics in their science class. All of the following statements about the tectonic plates are incorrect EXCEPT: A. The Eurasian Plate consists of the Asian
1. A group of students were discussing plate tectonics in their science class. All of the following statements about the tectonic plates are incorrect EXCEPT: A. The Eurasian Plate consists of the Asian
The Role of Biology in the Climate System: Long Term Climate Regulation. Earth History, Gaia and Human Perturbations of Biological Systems
 The Role of Biology in the Climate System: Long Term Climate Regulation Earth History, Gaia and Human Perturbations of Biological Systems How would the Earth differ if Life had not evolved or expanded?
The Role of Biology in the Climate System: Long Term Climate Regulation Earth History, Gaia and Human Perturbations of Biological Systems How would the Earth differ if Life had not evolved or expanded?
Earth Science Institute II June 23, 2010 Day 3 Correlation of EarthComm Curriculum and HSCE s
 Earth Science Institute II June 23, 2010 Day 3 Correlation of EarthComm Curriculum and s EDG1 = Earth s Dynamic Geospheres: Chapter 1, Volcanoes EDG2 = Earth s Dynamic Geospheres: Chapter 2, Plate Tectonics
Earth Science Institute II June 23, 2010 Day 3 Correlation of EarthComm Curriculum and s EDG1 = Earth s Dynamic Geospheres: Chapter 1, Volcanoes EDG2 = Earth s Dynamic Geospheres: Chapter 2, Plate Tectonics
Earth s Structure and Natural Processes Practice Test
 Name: Earth s Structure and Natural Processes Practice Test Section: Directions: For each of the questions or incomplete statements below, choose the best of the answer choices given and write your answer
Name: Earth s Structure and Natural Processes Practice Test Section: Directions: For each of the questions or incomplete statements below, choose the best of the answer choices given and write your answer
Table of Contents. Chapter: Atmosphere. Section 1: Earth's Atmosphere. Section 2: Energy Transfer in the Atmosphere. Section 3: Air Movement
 Table of Contents Chapter: Atmosphere Section 1: Earth's Atmosphere Section 2: Energy Transfer in the Atmosphere Section 3: Air Movement Table of Contents Chapter: Atmosphere Section 2: Energy Transfer
Table of Contents Chapter: Atmosphere Section 1: Earth's Atmosphere Section 2: Energy Transfer in the Atmosphere Section 3: Air Movement Table of Contents Chapter: Atmosphere Section 2: Energy Transfer
Discusssion / Activity 1 Suggested Answers. INSPECTION COPY for schools only
 Earth Structure Discusssion / Activity 1 Suggested Answers 1. Clearly label the diagram to show the main layers of the Earth. 2. What is the lithosphere? The lithosphere is the crust, plus a thin part
Earth Structure Discusssion / Activity 1 Suggested Answers 1. Clearly label the diagram to show the main layers of the Earth. 2. What is the lithosphere? The lithosphere is the crust, plus a thin part
Chapter 10 Planetary Atmospheres Earth and the Other Terrestrial Worlds
 Chapter 10 Planetary Atmospheres Earth and the Other Terrestrial Worlds 10.1 Atmospheric Basics Our goals for learning: What is an atmosphere? How does the greenhouse effect warm a planet? Why do atmospheric
Chapter 10 Planetary Atmospheres Earth and the Other Terrestrial Worlds 10.1 Atmospheric Basics Our goals for learning: What is an atmosphere? How does the greenhouse effect warm a planet? Why do atmospheric
Weathering Cycle Teacher Notes
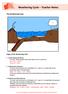 The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
Name: Period : Jaguar Review #10
 Name: Period : Earth & Space Sciences Benchmark B & C Jaguar Review #10 1. The most common element in living organisms is carbon. As new plants and animals grow, a great deal of carbon is required. Where
Name: Period : Earth & Space Sciences Benchmark B & C Jaguar Review #10 1. The most common element in living organisms is carbon. As new plants and animals grow, a great deal of carbon is required. Where
Igneous. Sedimentary Transformation by heat and pressure
 Melting, cooling and hardening Turns you into an Igneous Rock! Limestone A Sedimentary Rock Erosion, deposition and cementation Turns you into a Sedimentary Rock! Transformation by heat and pressure Turns
Melting, cooling and hardening Turns you into an Igneous Rock! Limestone A Sedimentary Rock Erosion, deposition and cementation Turns you into a Sedimentary Rock! Transformation by heat and pressure Turns
Core. Crust. Mesosphere. Asthenosphere. Mantle. Inner core. Lithosphere. Outer core
 Potter Name: Date: Hour: Score: /21 Learning Check 4.1 LT 4.1 Earth s Interior: I can draw and interpret models of the interior of the earth. Draw the following models (put the words in the right order)
Potter Name: Date: Hour: Score: /21 Learning Check 4.1 LT 4.1 Earth s Interior: I can draw and interpret models of the interior of the earth. Draw the following models (put the words in the right order)
Seawater Chemistry Brooks/Cole, a division of Thomson Learning, Inc.
 Seawater Chemistry The ions of sodium and chloride in NaCl (table salt) are held together by ionic bonds, electrostatic attraction that exists between ions that have opposite charge. Sodium and chloride
Seawater Chemistry The ions of sodium and chloride in NaCl (table salt) are held together by ionic bonds, electrostatic attraction that exists between ions that have opposite charge. Sodium and chloride
The Cosmic Perspective Planetary Atmospheres: Earth and the Other Terrestrial Worlds
 Chapter 10 Lecture The Cosmic Perspective Seventh Edition Planetary Atmospheres: Earth and the Other Terrestrial Worlds Planetary Atmospheres: Earth and the Other Terrestrial Worlds 10.1 Atmospheric Basics
Chapter 10 Lecture The Cosmic Perspective Seventh Edition Planetary Atmospheres: Earth and the Other Terrestrial Worlds Planetary Atmospheres: Earth and the Other Terrestrial Worlds 10.1 Atmospheric Basics
TAKE HOME EXAM 8R - Geology
 Name Period Date TAKE HOME EXAM 8R - Geology PART 1 - Multiple Choice 1. A volcanic cone made up of alternating layers of lava and rock particles is a cone. a. cinder b. lava c. shield d. composite 2.
Name Period Date TAKE HOME EXAM 8R - Geology PART 1 - Multiple Choice 1. A volcanic cone made up of alternating layers of lava and rock particles is a cone. a. cinder b. lava c. shield d. composite 2.
1/31/2013. Weathering Includes Physical, Chemical, Biological processes. Weathering Mechanisms. Wind abrasion forming Ventifacts
 Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
Chapter 14: The Changing Climate
 Chapter 14: The Changing Climate Detecting Climate Change Natural Causes of Climate Change Anthropogenic Causes of Climate Change Possible Consequences of Global Warming Climate Change? -Paleo studies
Chapter 14: The Changing Climate Detecting Climate Change Natural Causes of Climate Change Anthropogenic Causes of Climate Change Possible Consequences of Global Warming Climate Change? -Paleo studies
4.3 Climate (6.3.3) Explore this Phenomena. The same sun shines on the entire Earth. Explain why these two areas have such different climates.
 Explore this Phenomena The same sun shines on the entire Earth. 4.3 Climate (6.3.3) Explain why these two areas have such different climates. 89 6.3.3 Climate Develop and use a model to show how unequal
Explore this Phenomena The same sun shines on the entire Earth. 4.3 Climate (6.3.3) Explain why these two areas have such different climates. 89 6.3.3 Climate Develop and use a model to show how unequal
EARTH'S ATMOSPHERE. 1. The graph below shows the average concentration of ozone in Earth's atmosphere over Arizona during 4 months of the year.
 EARTH'S ATMOSPHERE 1. The graph below shows the average concentration of ozone in Earth's atmosphere over Arizona during 4 months of the year. Which layer of Earth's atmosphere contains the greatest concentration
EARTH'S ATMOSPHERE 1. The graph below shows the average concentration of ozone in Earth's atmosphere over Arizona during 4 months of the year. Which layer of Earth's atmosphere contains the greatest concentration
Sediment and Sedimentary rock
 Sediment and Sedimentary rock Sediment: An accumulation of loose mineral grains, such as boulders, pebbles, sand, silt or mud, which are not cemented together. Mechanical and chemical weathering produces
Sediment and Sedimentary rock Sediment: An accumulation of loose mineral grains, such as boulders, pebbles, sand, silt or mud, which are not cemented together. Mechanical and chemical weathering produces
Chapter 2 Earth s Interlocking Systems pg The Earth and Its Forces pg
 Chapter 2 Earth s Interlocking Systems pg. 24 55 2 1 The Earth and Its Forces pg. 27 33 Connecting to Your World and Internal Forces Shaping the Earth The Earth is unique in the solar system because it
Chapter 2 Earth s Interlocking Systems pg. 24 55 2 1 The Earth and Its Forces pg. 27 33 Connecting to Your World and Internal Forces Shaping the Earth The Earth is unique in the solar system because it
1. In the block diagram shown here, which is the oldest rock unit?
 Pre/Post GCI Name (print) 1. In the block diagram shown here, which is the oldest rock unit? 2. Referring to the same diagram as the previous question, which of the labeled rock units is the youngest?
Pre/Post GCI Name (print) 1. In the block diagram shown here, which is the oldest rock unit? 2. Referring to the same diagram as the previous question, which of the labeled rock units is the youngest?
Chapter 10 Planetary Atmospheres Earth and the Other Terrestrial Worlds
 Chapter 10 Planetary Atmospheres Earth and the Other Terrestrial Worlds What is an atmosphere? 10.1 Atmospheric Basics Our goals for learning:! What is an atmosphere?! How does the greenhouse effect warm
Chapter 10 Planetary Atmospheres Earth and the Other Terrestrial Worlds What is an atmosphere? 10.1 Atmospheric Basics Our goals for learning:! What is an atmosphere?! How does the greenhouse effect warm
Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds. What is an atmosphere? Earth s Atmosphere. Atmospheric Pressure
 Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds 10.1 Atmospheric Basics Our goals for learning What is an atmosphere? How does the greenhouse effect warm a planet? Why do atmospheric
Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds 10.1 Atmospheric Basics Our goals for learning What is an atmosphere? How does the greenhouse effect warm a planet? Why do atmospheric
Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds
 Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds 10.1 Atmospheric Basics Our goals for learning What is an atmosphere? How does the greenhouse effect warm a planet? Why do atmospheric
Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds 10.1 Atmospheric Basics Our goals for learning What is an atmosphere? How does the greenhouse effect warm a planet? Why do atmospheric
Chapter 2: Physical Geography
 Chapter 2: Physical Geography Pg. 39-68 Learning Goals for Chp2: q q q q q Explain how the Earth moves in space and why seasons change. Outline the factors that influence climate and recognize different
Chapter 2: Physical Geography Pg. 39-68 Learning Goals for Chp2: q q q q q Explain how the Earth moves in space and why seasons change. Outline the factors that influence climate and recognize different
Biochemistry Water and the Fitness of the Environment Concept: Acidic and Basic condi9ons affect living organisms
 Biochemistry Water and the Fitness of the Environment Concept: Acidic and Basic condi9ons affect living organisms Objec&ves: SWBAT calculate the ph of a substance and explain how it would change with the
Biochemistry Water and the Fitness of the Environment Concept: Acidic and Basic condi9ons affect living organisms Objec&ves: SWBAT calculate the ph of a substance and explain how it would change with the
The Global Carbon Cycle Recording the Evolution of Earth, from the origin of life to the industrialization of the planet
 The Global Carbon Cycle Recording the Evolution of Earth, from the origin of life to the industrialization of the planet Celebrating 5 years of world-leading collaborative and multidisciplinary research
The Global Carbon Cycle Recording the Evolution of Earth, from the origin of life to the industrialization of the planet Celebrating 5 years of world-leading collaborative and multidisciplinary research
