COMPARTMENTATJON AND FLUXES OF SUCROSE IN INTACT LEAF BLADES OF BARLEY
|
|
|
- Felix Ball
- 5 years ago
- Views:
Transcription
1 New Phytol. (1986) 103, COMPARTMENTATJON AND FLUXES OF SUCROSE IN INTACT LEAF BLADES OF BARLEY BY S. C. FARRAR AND J. F. FARRAR School of Plant Biology, University College of North Wales, Bangor, Gwynedd LL57 2UW, UK {Accepted 21 March 1986) SUMMARY Sucrose in barley leaf blades can be considered as being compartmented between two types of pool: transport (including mesophyll cytosol and apoplast, and vascular tissue) and vacuolar. The relative sizes of these pools, and their changes over a light/dark cycle, have been estimated using "C labelling of the intact leaf and washout of '"C from leaf discs. Both types of pool increase during the photoperiod, the proportional distribution between them remaining the same; roughly one-fifth of the sucrose is in the transport pool. The rate-constants describing translocation, and loading and unloading of the vacuole, show diel changes, and fluxes of sucrose across the tonoplast are likely to be critical in controlling fluxes of photosynthetically fixed carbon within and out of barley leaves. The unloading of sucrose from vacuoles is probably the site where control is exercised; this conclusion is supported by a simple simulation model. Key words; Sucrose, compartmentation, vacuoles, barley, translocation. INTRODUCTION Sucrose is synthesized in the cytosol of mesophyll cells (Giersch et al., 1980); it is also found in the apoplast (Madore & Webb, 1981; Huber & Moreland, 1980), vacuoles (Avigad, 1982; Gerhardt & Heldt, 1984; Fisher & Outlaw, 1979), and phloem (Giaquinta, 1980; Kursanov, 1984; Raven, 1977). The rapid appearance of ["CJsucrose in vacuoles during photosynthesis in i^coj has been shown in protoplasts of barley, wheat and spinach (Kaiser &Heber, 1984; Kaiser, Martinoia & Wiemken, 1982; Giersch et al., 1980). The dynamic relationships between sucrose in cytosol and vacuole are poorly understood. Procedures involving protoplasts are of limited use in understanding the behaviour of intact leaves where each mesophyll cell is under positive hydrostatic pressure, is in symplastic connection with other cells and rapidly exports a large proportion of the sucrose it manufactures. In particular, in intact leaves the quantitative importance of the vacuole in sucrose fluxes, and the rate of turnover of sucrose during light/dark cycles, are little explored. In those species characterized by the storage of starch during the photoperiod, such as spinach (Huber, 1981) beet (Fondy & Geiger, 1982) soybean (Silvius, Chatterton & Kremer, 1979; Kagawa & Wong, 1985) and Vicia faba (Outlaw, Fisher & Christy, 1975) the available evidence indicates that vacuolar sucrose is a small component of leaf carbohydrate metabolism, whether considered by amount or by fiux. Those species that characteristically store sucrose (rather than starch) during the photoperiod have been less explored, but the evidence suggests that here vacuolar storage of sucrose is of prime importance. Gordon & Johnson (1984) have used a simulation model to indicate how vacuolar sucrose storage might be integrated into carbon fiuxes in barley leaves, and data of Owera, Farrar & Whitbread (1983), Sicher, Kremer & Harris (1984) and Farrar & Farrar (1985a) X/86/ S03.00/ The New Phytologist
2 646 S. C. FARRAR AND J. F. FARRAR are consistent with a vacuolar pool of sucrose playing a large role in barley leaves. Limits on the size of this pool have also been suggested as a result of pulse-labelling and steady-state labelling of barley leaves with '''CO^, and a second type of pool, called transport sucrose (since it will probably include cytosolic, apoplastic and phloem pools of sucrose), was distinguished from it (Farrar & Farrar, 1985b) > t 70 o g 60 I 50 o 40 c (a) [ r/1 - \ ^ \siope = A 1 \ t{h) ^ b) tran sport ol Photosynthesis vacuolar pool doiosi ^" 1 ' F, tronslocation t (h) Fig. 1. An idealized semilogarithmic plot of translocatory "C efflux from a leaf (a), and the two-compartment model used to interpret it (b). The curve describing isotope efflux is Y = Ae^''' + Be'", where k and / are exponential coefficients (h"') (the inset shows how k can be obtained by replotting the shaded area of the main curve), and A and H are the initial percentage of isotope associated with eaeh compartment. The two-compartment model is a first-order model so that (for example) the translocatory Bux F = K^ Q^, where K,,, is a rate constant (b"') and Q^ a mass (g). A compartmental analysis is needed to relate the parameters in (a) and (b), except that K^ = k; half-times are given by (j = K"' (see Moorby & Jarman, 1975). When very long labelling times are used, B ~ 100 and then K.^, ~ I (K l) and K,^ (K l)-. This paper attempts to measure the sizes and rates of turnover of the transport and vacuolar pools of sucrose in mature leaves of the sucrose-storing species, barley, during a single light/dark cycle. Isotopic techniques are used: leaves are pulse-fed "COj before attaching them to Geiger-Muller tubes to obtain continuous traces for efflux of '"C from the leaves; leaves are fed "CO.^ until isotopic equilibrium is achieved and then either efflux curves are recorded for intact leaves, or discs cut from the leaves are placed in unlabelled sucrose and the washout of i*c monitored. Rate constants derived from these procedures are used in simple simulations to check their validity. The model adopted to relate "C fluxes to sucrose compartmentation (Fig. 1) is similar to that of Moorby & Jarman (1975) and Bell & Incoll (1982). It has been shown that, in barley, the first two phases of efflux of ^''C from leaf blades in the light are entirely attributable to loss of sucrose (Farrar & Farrar, 1985b). MATERIALS AND METHODS Plant growths and ^^CO^ feeding Plants of barley {Hordeum distichum (L.) Lam. cv. Maris Mink) were grown
3 Compartmentation of sucrose in barley leaves 647 as described previously (Farrar & Farrar, 1985b) and experiments were performed on 16-d-old plants at 19 C and 700/tmol m^^ g-i for 16 h d^^. All experiments were performed on fully expanded second leaf blades. Whole leaves, and bands of leaves 1 x 1 cm, were fed ^*CO,, at constant specific activity as described previously (Farrar & Farrar, 1985b). Bands about 7 cm from the leaf tip were fed by passing the leaves through slits in clear acrylic tubing of 1 cm internal diameter and then sealing with silicone rubber. The duration of feeding varied from 5 min to 72 h, and the combination of air flow rate, concentration of and rate of injection of carrier carbonate were adjusted to give a concentration of COj of about 350 ppm. Ejflux of ^*C from, and assay of, ^*C-fed leaves Continuous monitoring of ^*C remaining in intact leaves was carried out by attaching the leaf to a Geiger Muller tube as described previously (Owera et al., 1983). Loss of isotope was monitored through the normal light/dark cycles for up to 48 h following the feeding of '*CO.^. Frequently, many were fed in parallel. Only one was monitored, and from the others, the '"COg-fed areas were cut out and placed immediately into 5 cm^ 95 % ethanol at 80 C. This extract was analyzed for total sugars and "C activity, and sugar chromatography was performed, as described previously (Farrar & Farrar, 1985b). Monitoring of effective leaf mass with a /^-gauge was performed as described previously (Farrar & Farrar, 1985b). Where leaves were to be fed "CO^ until all non-structural carbohydrate was at constant specific activity, labelling began when the plants were 14 d old, at which stage leaf two was just fully expanded. The monitoring of subsequent loss of '*C was carried out from 16 d through at least 24 h of the normal light/dark cycle. Washout of ^"C from leaf discs Leaves which had been fed ^^COj for 48 h to achieve constant speciflc activity as described above were removed quickly from the feeding chamber. Leaf discs were then cut from the fed portion of each leaf and sets of four discs threaded onto lengths of fine steel wire for ease of handling. Fach set of discs was then transferred sequentially for 7 h through a series of sucrose solutions (3 cm^ of 25 mol m"''' sucrose and 0-1 mol m^^ CaSO^) so that ^*C sugars in the leaf discs could exchange with [^^CJsucrose in the medium. A photon fluence rate of 36 ju,mo\ m~'~ s~^ (near the light compensation point for these leaves) was provided by fluorescent tubes and temperature monitored continuously with copper constantan thermocouples. After the last transfer the leaf discs were removed from the wires and dropped into 5 cm''' 95 % ethanol at 80 C. Aliquots of the medium in each washout vial, and of extracts of leaf and leaf disc material, were assayed for '^*C activity; the ethanol extracts were also analyzed for total soluble sugars and the media chromatographed. The accumulated loss of ^*C from the discs was calculated as a percentage of the "C activity present in the leaf discs initially (total '""C washed out + "C remaining in ethanol soluble sugars extracted from discs at end of washout) and this was plotted semilogarithmically with time. Other leaves were fed simultaneously to constant specific activity and monitored continuously with Geiger Muller tubes, to provide parallel efflux curves. Numerical analysis Data are presented as the mean of three to five replicates +SEM. Both the
4 648 S. C. FARRAR AND J. F. FARRAR washout and efflux curves were fitted to double exponential curves using a maximum likelihood program, or to multiple exponential curves graphically. Straight lines were fitted by eye for efflux curves from pulse fed plants and by linear regression for efflux curves from plants fed to constant specific activity, and to washout data. Simulations of a first-order, open, two-compartment model (Fig. 1), like that of Moorby & Jarman (1975) and Owera et al. (1983), and where only one compartment can exchange materials with the environment, were performed on a DEC system 10 mainframe computer with an iteration time of 10~^h. Compartmental analysis was employed to calculate rate constants from exponential coefficients, using the method of Moorby & Jarman (1975). RESULTS Effect of duration of ^'^CO^ feeding on subsequent efflux kinetics Bands of leaves were fed ^""COj for 5, 15 or 30 min, or 48 h, and the efflux of ' 'C from the fed area then monitored continuously with a Geiger-Muller tube. To ensure that self-absorption of /i?-radiation by the leaf was not changing, the change in effective leaf mass was monitored continuously with a /S-gauge; it remained nearly constant at 18mgcm^^ throughout light/dark cycles. All the curves show at least two exponential phases of loss of isotope during the light period, the first representing loss from transport, and the second from vacuolar, sucrose. The kinetics of '''C efflux were different when '''COj feeding times were changed (Fig. 2). The percentage of the '^'*C lost in the first phase (the transport pool) decreased as the feeding time increased. The percentage ^*C in transport sucrose has been determined for many efflux curves; for 5 min feeds, % of the ^''C was lost from transport sucrose; for 15 and 30 min feeds, and %, and for feeds of over 48 h, only 4+ 1 % of ^*C was in transport sucrose. These differences are due to the long times taken for isotopic equilibrium to be reached in pools that turn over slowly; when "^^COg is fed for a short time, readily accessible pools with rapid turnover will acquire a disproportionately large share of the '''C. Pulse feeds of '^COj lasting 5 min thus provide efflux curves in which transport sucrose represents a large proportion of the efflux, and so are particularly suitable for examining this pool; conversely, leaves fed '''COj for long periods have high labelling of, and so are suitable for examining, vacuolar sucrose. During the dark period, an increased rate of '"C efflux could be seen, especially in those plants fed for short periods (F"ig. 2). This represents the mobilization of '^''C labelled starch, and is not considered further in this paper. Specific activity and abundance of sugars after ^'^CO,^ feeding The carbohydrates in leaves fed for long periods should all be at the same specific activity if feeding has been sufficiently prolonged. Following 50 h feeding of ^"COj, and at all times in the photoperiod, the specific activities of starch, low molecular weight fructan, sucrose, glucose and fructose were closely similar, showing that isotopic equilibrium had indeed been achieved. The efflux curves from these leaves fed to a constant specific activity show a smaller percentage '^'*C lostthan when leaves were fed for short pulses (50+ 10% as opposed to %); the percentage by weight and ^*C activity for starch were 38 and 39 % respectively. This implies that the largest percentage of the ^^C remaining in these leaves after loss of activity from sucrose is in the form of starch; the other soluble sugars and
5 Compartmentation of sucrose in barley leaves Time into photoperiod (h) Fig. 2. Efflux of '"C from intact leaf blades of barley previously fed '"COj at constant specific activity for the times, ranging from 5 min to 48 h, indicated by the curves. Continuous monitoring of tbe leaf blades was begun 6 h into the photoperiod and was continued through a normal light-dark cycle. The hatched bar on the x-axis indicates the dark period. fructans will appear to be lost during the 'sucrose phases', as they have high turnover rates; there can only be about 10% of the ^^C in protein. Changes in effiux from the transport pool during the photoperiod monitored after feeding ^^CO^for 5 min Bands of leaves were fed '''COg for 5 min at different times during the photoperiod. Each efflux curve showed two exponential phases of loss of isotope during the light period, and the percentage of '''C in transport sucrose decreased with time into the photoperiod. The rate constant for loss of isotope from the transport pool (the first phase of efflux) decreased through the photoperiod [Fig. 3(a)], from 0-82 to 0-31 h~\ and so the ti for turnover of transport sucrose {Q{) must therefore rise from about 0-9 h to 2 h during the photoperiod. The size of the transport pool of sucrose {Q^) may be estimated using the relationship Q^ = 0'i^io, where 0 is flux out of the pool - in this case the translocation rate at the appropriate times into the photoperiod (data from Farrar & Farrar, 1985b, Fig. 2) and i^^,,, is the rate constant for this loss, equal to K taken from Figure 3 (a). Transport pool size is estimated to have increased with time into photoperiod [Fig. 3(b)], from 298 to 2310 mg sucrose m~2 leaf (Table 1). Changes in effiux from the vacuolar pool during the photoperiod, monitored after feeding ^*CO^for 48 h When leaves were fed ^^COj for a minimum period of 48 h, and loss of this ^*C then monitored continuously during the light/dark cycle, two exponential phases of i*c loss were seen during the light period. For these curves, transport sucrose only represents an extremely small percentage of the "C in the fed area, because with the attainment of isotopic equilibrium a far larger proportion of ^*C is found in vacuolar sucrose. The exponential coefficient, /, for loss of "C from vacuolar sucrose in the light increased during the photoperiod and a linear regression of the relationship between / and time was highly significant [Fig. 4 (a)], implying that the half-time for turnover of vacuolar sucrose fell from 69 h at the start to 12 h at the end of the photoperiod. The rate constant for loss from vacuolar sucrose (calculated using the relationship in the legend to Fig. 1) also increased
6 650 S. C. FARRAR AND J. F. FARRAR Time into photoperiod (h) Fig. 3. (a) The change through the photoperiod in the exponential coefficient {k) for the initial slope of the efbux curves shown in Fig. 4. The fitted regression is A = <, where ( is time in h into the photoperiod. (b) The change through the photoperiod in the size of the transport pool of sucrose (Q,), estimated as 0/k where k is the exponential coefficient from Figure (5(a) and 0 the experimentally determined translocation rates (Farrar & Farrar, 1985a,b). The fitted regression is Qi = <, where ( is time in h into the photoperiod. Table 1. Sucrose compartmentation in second leaf blades of barley : assessment from efflux curves Method Time into photoperiod (h) 0 Efflux from intact leaves fed "CO., for 5 min Effiux from intact leaves fed "CO.; for 50 h 5 16 Qtotai (mg m-') 1 ransport Pool ' Qx (mg m-2) (j(h) %»c Mean concentration (mol m ^), of sucrose ~ Vacuolar Pool ' Q, (mg m--') h (h) % "C Q2 % Q total Mean concentration (mol m~'), of sucrose Notes; Qiotai- figures from Farrar & Farrar (1985b). Mean sucrose concentrations calculated using the relative volumes of vacuole, and cytoplasm plus wall, given for wheat leaves hy Altus & Canny (1985), at 53 & 12% of 180 g HjOm--' barley leaf blades. Q.^ calculated by 0u,tai " Oi- % '"C is percentage of total "C in leaf associated with the pool indicated.
7 Compartmentation of sucrose in barley leaves U-Ub a 0-06 D ^ (o) i 0-02 < Time into photoperiod Fig. 4. (a) The change through the photoperiod in the exponential coefficient (/) for the second slope of the efflux curves such as in Figure 6. The fitted regression is / = ( is time in h into the photoperiod. (b) The change through the photoperiod in the rate constant (K.^^ for loss of sucrose from the vacuole to the transport pool (Q^ to ),)- Values of K^^ were derived by compartmental analysis (Moorby & Jarman, 1975, and Fig. 1) using the changing values of the exponential coefficient (/) shown in Figure 4(a). (h) Table 2. Sucrose compartmentation in second leaf blades of barley: assessment by washout of isotope from leaves previously labelled with ^^CO^for 50 h Time into photoperiod (h) 16 sucrose) (mg m ^) Apoplast, (i (min) Apoplast, % "C washed out Symplast, ti (h) Symplast, % "C washed out Mean concentration of sucrose in transport pool (mol m"'') Q, (transport sucrose) (mg m~'^) Qj (vacuolar sucrose) (mg m"'^) OJ as % Ot^uu Vacuolar pool, ti (h) Vacuolar pool, % '''C washed out Mean concentration of sucrose in vacuolar pool (mol m~') Mean sucrose concentrations were calculated from relative volumes given for wheat leaves (Altus & Canny, 1985) and Qi^to, is from Farrar & Farrar (1985b). during the photoperiod [Fig. 4(b)]. Pool sizes calculated from these data are given in Table 1. Washout experiments with leaf discs Leaves were fed '''COa to a constant specific activity and discs were cut rapidly from them. These discs were allowed to exchange labelled "C sugars with external unlabelled [^^CJsucrose in low light, to obtain washout curves showing the amount of ^^C remaining in the discs with time. Where the efflux began 5 h into the photoperiod the washout curves showed three exponential phases of ^*C loss (Fig. 5). Lines were fitted by linear regression, and two log linear pbases subtracted sequentially to obtain the rate constant and percentage ^''C lost for each of the three phases. The percentage of "C lost in these
8 652 S. C. FARRAR AND J. F. FARRAR 00; 80.oL O 20 ok -^ 10 k 0-2 i 0-4 ' O 'i i k i--i s Duration ot washout ( h ) 1 1 Fig. 5. The washout of "C from leaf discs cut from leaf hlades of barley which had been fed "COj for 50 h and were then allowed to exchange ["C]sucrose with ['^CJsucrose in an external bathing medium. The three exponential components of three replicate curves were determined graphically; the means±se for the three curves are shown. 100 i.;;^:^ Time ot washout or ettiux ( h) Fig. 6. The comparison between the washout of "C activity from leaf discs exchanging ["C]sucrose with ['^CJsucrose into an external bathing medium ( ) and the efflux curves from leaf blades on intact plants ( ) starting at 0, 5 or 16 h, as indicated by the curves, into the photoperiod after feeding the second leaves of barley plants with "COj for 50h. phases, identified by half-time, were 11-5 for the rapid phase {ti~2 min), 8-7 for the second {ti~n min) and 79-8 for the slowest (<i~25 h) (Fig. 5; Table 2). The media were chromatographed following the washout; approximately 20% of the '^''C was in glucose and fructose and the remainder in sucrose. When this washout experiment was repeated, but starting the efflux at either 0 or 16 h into the photoperiod, it was found that the percentage of "C lost in each phase of efflux was similar at each time (Table 2), perhaps the only difference being a greater percentage in the second phase at 0 h into the photoperiod. The i-times may only be compared within experiments due to the temperature differences between experiments (Table 2). The washout curves that started at 0, 5 and 16 h into the photoperiod were compared with curves showing ^''C efflux from intact leaves fed ^^COg simultaneously with those used for the washout experiments. The amount of ^*C remaining in the leaf discs after 4 h efflux is substantially lower than that remaining in the intact leaf-71% as opposed to 85 % (Fig. 6), since the rapid phases of loss seen in the washout experiment are
9 Compartmentation of sucrose in barley leaves 653 (a) E / \ 16 6 Time into photoperiod {h} Fig. 7. Changes through the photoperiod in (a) the sucrose content and (b) the translocation rate of second leaf blades of barley as determined experimentally ( ) (Farrar & Farrar, 1985a, b) and as predicted by simulation where k (the exponential coefficient for the first phase of efflux) and / (the exponential coefficient for the second phase of efflux) remain constant through the photoperiod ( ), where k is decreased through the photoperiod whilst / remains constant ( ) and where / is increased through the photoperiod whilst k remains constant ( ) not seen in efflux from intact leaves; the slopes for "C loss from vacuolar sucrose are similar in both. Numerical simulation of sucrose pool sizes A program simulating the model of Figure 1 (b) was used to test the effects of the magnitude of rate constants on the diurnal changes in pool sizes of sucrose. When the exponential coefflcients k and / were held constant during the photoperiod, simulated total leaf sucrose and translocation were dissimilar to observed values (Fig. 7). Allowing k alone to decrease, in the manner noted above, during the photoperiod resulted in no improvement, but wben / was allowed to rise as noted above, then the simulations more closely approximated the observed values (Fig. 7). These analyses also allowed Q^ to be estimated: it rose throughout the photoperiod. DISCUSSION Sucrose in mature barley leaves must occur in at least two types of pool, vacuolar and transport (tbe latter comprising cytoplasm and apoplast of mesophyll cells, and the sieve element - companion cell complex). Fach of the techniques used is most suitable for estimating one or more features of these two types of pool, and the data they have provided is summarized in Tables 1 and 2. Three features are notable: a large proportion (about 80%) of sucrose is vacuolar; the flux through this vacuolar pool is about 40 % of the carbon fixed in photosynthesis; and the flux through the vacuole changes, relative to translocation, during the photoperiod. Fach of the methods used here indicated that about 80 % of the sucrose was in vacuolar, and the remainder in transport, pools (Table 1). This is within the limits suggested earlier for barley (Farrar & Farrar, 1985b), and is similar to the 65% of sucrose in barley leaves found to be vacuolar by Wagner, Keller & Wiemken (1983); they used protoplast and vacuolar isolation, during which processes some vacuolar sucrose may have been lost. In starcb-storing species, vacuolar sucrose in leaves has been reported to comprise 20 to 40 % (sugar beet; Geiger et al., 1983)
10 654 S. C. FARRAR AND J. F. FARRAR to 80% (spinach at the end of the light period; Gerhardt & Heldt, 1984; cultured tobacco cells; Delmer, 1979) of the total. The amount of sucrose in vacuoles can be re-expressed as a concentration, using the water content of barley leaf blades (180 g m"^) and the proportion of tissue volume occupied by vacuoles (53 %) given by Altus & Canny (1985) for wheat leaves; values range from 45 to 124 mol m~^ depending upon time in the photoperiod. The mean concentration in the transport pool, calculated similarly, ranges from 23 to 180 mol m~^. This is unlikely to represent cytosolic concentration, since autoradiographic evidence suggests that a large proportion of the '^^C ascribable to the transport pool is associated with veins (Farrar & Farrar, unpublished). If phloem, containing sucrose at 500 mol m"^, occupies 1 % of barley leaf volume, 300 mg m"^ sucrose would be within it. Separate estimates of sucrose in cytosol, phloem and apoplast are badly needed. The minimal mean concentration of 25 mol m~^ in the transport pool at the end of the dark period is similar to the cytosolic concentration estimated for the roots of barley (Farrar, 1985). Cytosolic sucrose concentrations of 5 to 13niolm~^ (barley leaf mesophyll; Kaiser et al., 1982; spinach protoplasts; Giersch et al., 1980; Gerhardt & Heldt, 1984), 24 mol m"^ (wheat protoplasts; Giersch et al., 1980), and 76 mol m"'' (sugar beet taproot; Saftner, Daie & Wyse, 1983) have been reported. Sucrose concentrations in vacuoles have been reported to vary from 514 mol m-^ in sugar beet taproot (Saftner et al., 1983) to 1 to 45 mol m~^, depending on the time in the photoperiod, in spinach (Gerhardt & Heldt, 1984), spanning the range reported here for barley leaves. Wagner et al. (1983) suggest that sucrose is unique among the soluble sugars of barley leaves as it occurs in the cytosol at a concentration equal to or greater than that in the vacuole; this is consonant with the failure to find evidence for an energyrequirement for sucrose transport at the tonoplast, reported by Kaiser & Heber (1984), and with our finding that the proportion of sucrose in the vacuolar pool does not change appreciably during a diel cycle in spite of considerable changes in concentration. Saftner et al. (1983) and Willenbrink & Doll (1979) give evidence for energized vacuolar loading of sucrose in the storage tissue of beet taproot, which also occurs in vacuoles from sugarcane suspension cultures (Komor, Thom & Maretzki, 1982). The question of energization of sucrose transport at the tonoplast thus seems open (Reinbold & Kaplan, 1984). The rise in the rate consant K^^ for loss of sucrose from the vacuole that occurs in these barley leaves, at a time when vacuolar concentrations are still increasing, may reflect an active efflux of sucrose across the tonoplast, but critical evidence at the appropriate time in the diel cycle is needed. The rates of turnover of the transport and vacuolar pools are not the same. The vacuolar pool has a half-time of 12 to 30 h over most of the photoperiod; since vacuoles contain a high proportion of the sucrose in the leaf, tbis implies that the tonoplast may have a major role in controlling fluxes of sugars. Indeed about 40 % of photosynthetically fixed carbon passes through the vacuole, as sucrose, before its eventual export from the leaf (calculated as net storage of sucrose in the leaf X 0-8, the proportion of sucrose that is vacuolar, plus rate of loss of sucrose from the vacuole, given by vacuolar pool size x /Cji)- Work with protoplasts has shown rapid appearance of photosynthetically fixed ^''C in the vacuoles of several species, including barley (Kaiser & Heber, 1984; Kaiser et al, 1982; Giersch et al, 1980) and therefore appears relevant to the situation in vivo. Our rate of vacuolar loading of sucrose can be re-expressed as 4-4 mmol sucrose g chlorophyll"^ h"^, about half of that obtained in vitro by Kaiser & Heber (1984).
11 Compartmentation of sucrose in barley leaves 655 The transport pool has a half time of 03 to O^85 h; this represents the rate of passage of sucrose through the mesophyll, from the cells in which it was synthesized to the veins and loading into and redistribution between veins (Altus & Canny, 1985) as well as eventual translocation out of the leaf. By contrast, the shorter half-times of 0^2 to 0^3 h for the phase of washout ascribable to the symplast will be due solely to leakage across the plasmalemma and out of leaf discs. That the half-time for this process is shorter than for translocation implies that the export of sucrose from mesophyll cells will not be a rate-limiting step m translocation. Washout allows the approximate estimate that apoplastic sugars can account for nearly half the transport sucrose, although this will include loss from cells damaged by cutting: a critical estimate of apoplastic sugars and their exchange across the plasmalemma in vivo is badly needed. The sizes of both the transport and the vacuolar pools of sucrose, and their rates of turnover, vary during the photoperiod. The transport sucrose pool increases from about 300 mg m"^ (measured by any of the three techniques used) to 860 to 2300 mg m"2 (low value from washout; high value from feeding "CO2 for 5 min). This parallels the increase in rate of translocation from, and the activity of sucrose phosphate synthetase in, these leaves during the photoperiod (Farrar & Farrar, 1985a,b). It is thus possible that the rate of translocation is dependent on the size of the transport sucrose pool, but the relative contributions to this pool of phloem and of mesophyll cytosol need to be known. The vacuolar pool similarly increases, with the proportioning of sucrose between the two pools staying roughly similar. The concentration in this pool increases by 79 mol m^^ Since the solute potential of these leaves is -Ml MPa, equivalent to 460 osmol m~^ (Farrar, unpubl.), this diel change in just one solute accounts for about 15 % of the total solutes in these leaves, and raises major problems of turgor regulation, and of the balances of solutes between cytosol, vacuole and apoplast, not experienced by starch-storing species. The rate constants describing fluxes between these pools also show changes with time, implying that the machinery responsible for membrane and phloem transport of sucrose is under control that varies during the photoperiod. Changes in the exponential coefficients describing effiux have previously been shown in wheat (Bell & Incoll, 1982) and Vicia faba (Pearson, 1974). Bell & Incoll (1982) suggested on this basis alone that phloem loading and transport was becoming limiting, perhaps due to saturation of membrane carriers, with a resultant fall in K. Our data shows clearly that this is not the case: K falls whilst the rate of translocation rises (Farrar & Farrar, 1985b) and so any change in K is independent of phloem loading, and in any case takes place during an increase in efflux from the vacuole, indicated by the rising value of K^^. The importance of varying rate constants is demonstrated by simulation models predicting translocation rate and pool sizes; only when rate constants are allowed to vary do the simulations approach observed values. These simulations also emphasize that compartmental analysis based on first order rate kinetics and unvarying rate constants will be inappropriate for precise work. Also, the simulations allow the relative sensitivity of the rate constants K^^, (translocation) and i^t^, (vacuolar unloading) to be explored; it is clear that vacuolar unloading is the more important of the two in allowing simulated to approach real values. This again emphasizes the importance of the tonoplast in the control of carbon fluxes in these leaves. At the start of the photoperiod, the concentration of sucrose in the vacuole is low and there is considerable accumulation of sucrose in it, at a rate that exceeds the rate of translocation (Farrar & Farrar, 1985b). Perhaps the vacuole has more
12 656 S. C. FARRAR AND J. F. FARRAR immediate access to cytosolically produced sucrose than the more distant sieve element-companion cell complex and so obtains a preferential share of sucrose, whether or not transport across the tonoplast is energized. At this stage, rate ot accumulation by the vacuole is greatly in excess of rate of loss from it. As the photoperiod proceeds, there is an increase in the rate of translocation and a fall in the rate of net accumulation of sucrose (Farrar & Farrar, 1985b). At least part of the fall in accumulation is due to the rise in the rate-constant for export across the tonoplast; this value needs to be high to account for the sucrose lost during the night, and it appears that it rises not just in response to darkness but during the latter part of the photoperiod. The control of this is not understood. Part of the fall in sucrose accumulation may be due to the relatively high sucrose (and possibly total solute) concentration in the vacuole making further vacuolar loading less probable than phloem loading. ACKNOWLEDGEMENTS We gratefully acknowledge the financial support of the A.F.R.C. REFERENCES ALTUS, D. P. & CANNY, M. J. (1985). Loading of assimilates in wheat leaves. II. The path from chloroplast to vein. Plant, Cell & Environment, 8, AviGAD, G. (1982). Sucrose and other disaccharides. In: Plant Carbohydrates I. Intracellular Carbohydrates (Ed. hy F. A. Loewus & W. Tanner), Encyclopedia of Plant Physiology, vol. 13A, pp Springer, Berlin. BELL, C. J. & INCOLL, L. D. (1982). Translocation from the flagleaf of winter wheat in the field. Journal of Experimental Botany, 33, DELMER, D. P. (1979), Dimethylsulfoxide as a potential tool for analysis of compartmentation in living plant cells. Plant Physiology, 64, FARRAR, J. F. (1985). Fluxes of carhon in roots of harley plants. New Phytologist, 99, FARRAR, S. C. & FARRAR, J. F. (1985a). Fluxes of carhon compounds in leaves and roots of barley plants. In: Regulation of Sources & Sinks in Crop Plants, Monograph 12 (Ed. hy B. Jeffcoat, A. F. Hawkins & A. Stead), pp British Plant Growth Regulator Group, Bristol. FARRAR, S. C. & FARRAR, J. F. (1985h). Carhon fluxes in leaf hlades of harley. New Phytologist, 100, FISHER, B. & OUTLAW, W. H. (1979). Sucrose compartmentation in the palisade parenchyma of Viciafaba L. Plant Physiology, 64, FoNDY, B. R. & GEIGER, D. R. (1982). Diurnal patterns of translocation and carhohydrate metabolism in source leaves of Beta vulgaris L. Plant Physiology, 70, GERHARDT, R. & HELDT, H. W. (1984). Measurement of suhcellular metabolite levels in leaves hy fractionation of freeze stopped material in non-aqueous media. Plant Physiology, 75, GEIGER, D. R., PLOEGER, B. J., Fox, T. C. & FONDY, B. R. (1983). Sources of sucrose translated from illuminated sugar heet source leaves. Plant Physiology, 72, GiAQUiNTA, R. T. (1980). Translocation of sucrose and oligosaccharides. In: Biochemistry of Plants, vol. 3 (Ed. hy J. Preiss), pp Academic Press, New York. GiERSCH, C, HEBER, U., KAISER, G., WALKER, D. A. & ROBINSON, S. P. (1980). Intracellular metaholite gradients and flow of carhon during photosynthesis of leaf protoplasts. Archives of Biochemistry and Biophysics, 205, GORDON, A. J. & JOHNSON, I. R. (1984). The control of photoassimilate supply from barley leaves during light and dark periods. In: Advances in Photosynthesis Research, vol. 4 (Ed. hy C. Syhesma), pp Junk, The Hague. HUBER, S. C. (1981). Inter- and intra-specific variation in photosynthetic formation of starch and sucrose. Zeitschrift fur Pfianzenphysiologie, 101, HUBER, S. C. & MORELAND, D. E. (1980). Efflux of sugars across the plasmalemma of mesophyll protoplasts. Plant Physiology, 65, KAGAWA, T. & WONG, J. H. H. (1985). Allocation and turnover of photosynthetically labelled ''COj in leaves of Glycine max L. Clark. Plant Physiology, 77, KAISER, G. & HEBER, U. (1984). Sucrose transport into vacuoles isolated from harley mesophyll protoplasts. Planta, 161,
13 Compartmentation of sucrose in barley leaves 657 KAISER, G., MARTINOIA, E. & WIEMKEN, A. (1982). Rapid appearance of photosynthetic products in tbe vacuoles isolated from barley mesophyll protoplasts by a new fast method. Zeitschrift fiir Pfianzenphysiologie, 107, KOMOR, E., THOM, M. & MARETZKI, A. (1982). Vacuoles from sugarcane suspension cultures Protonmotive potential difference. Plant Physiology, 69, KuRSANOV, A. L. (1984). Assimilate Transport in Plants. Elsevier, Amsterdam. MADORE, M. & WEBB, J. A. (1981). Leaf free space analysis and vein loading in Cucurbita pepo. Canadian Journal of Botany, 59, MOORBY, J. & JARMAN, P. D. (1975). Tbe use of compartmental analysis in tbe study of movement of carbon through leaves. Planta, 122, OUTLAW, W. H., FISHER, B. & CHRISTY, A. L. (1975). Compartmentation in Vicia faba leaves. 11. Kinetics of "C sucrose distribution among individual tissues following pulse labelling. Plant Physiology, 55, OWERA, S. A. P., FARRAR, J. F. & WHITBREAD, R. (1983). Translocation from leaves of barley infected with brown rust. New Phytologist, 94, PEARSON, C. J. (1974). Daily changes in carbon dioxide exchange and photosynthate translocation of leaves of Vicia faba. Planta, 119, RAVEN, J. A. (1977). H+ and Ca" in phloem and symplast: relation of relative immobility of the ions to the cytoplasmic nature of the transport paths. Nezo Phytologist, 79, REINHOLD, L. & KAPLAN, A. (1984). Membrane transport of sugars and aminoacids. Annual Review of Plant Physiology, 35, SAFTNER, R. A., DAIE, J. & WYSE, R. E. (1983). Sucrose uptake and compartmentation in sugar beet taproot tissue. Plant Physiology, 72, 1-6. SicHER, R. C, KREMER, D. F. & HARRIS, W. G. (1984). Diurnal carbohydrate metabolism of barley primary leaves. Plant Physiology, 76, SiLVius, J. E., CHATTERTON, N. J. & KREMER, D. F. (1979). Photosynthate partitioning in soybean leaves at two irradiance levels. Comparative responses of acclimated and unacclimated leaves. Plant Physiology, 64, WAGNER, W., KELLER, F. & WIEMKEN, A. (1983). Fructan metabolism in cereals: induction in leaves and compartmentation in protoplasts and vacuoles. Zietschrift fiir Pfianzenphysiologie, 112, WiLLENiiRiNK, J. & DOLL, S. (1979). Characteristics of the sucrose uptake system of vacuoles isolated from red beet tissues. Kinetics and specificity of two sucrose uptake systems. Planta, 147, ANP 103
14
Translocation 11/30/2010. Translocation is the transport of products of photosynthesis, mainly sugars, from mature leaves to areas of growth and
 Translocation Translocation is the transport of products of photosynthesis, mainly sugars, from mature leaves to areas of growth and storage. Phloem is the tissue through which translocation occurs. Sieve
Translocation Translocation is the transport of products of photosynthesis, mainly sugars, from mature leaves to areas of growth and storage. Phloem is the tissue through which translocation occurs. Sieve
The Dynamics of Carbon Supply from Leaves of Barley Plants Grown in Long or Short Days
 Journal of Experimental Botany, Vol. 33, No. 133, pp. 241-250, April 1982 The Dynamics of Carbon Supply from Leaves of Barley Plants Grown in Long or Short Days A. J. GORDON, G. J. A. RYLE, D. F. MITCHELL
Journal of Experimental Botany, Vol. 33, No. 133, pp. 241-250, April 1982 The Dynamics of Carbon Supply from Leaves of Barley Plants Grown in Long or Short Days A. J. GORDON, G. J. A. RYLE, D. F. MITCHELL
C MPETENC EN I C ES LECT EC UR U E R
 LECTURE 7: SUGAR TRANSPORT COMPETENCIES Students, after mastering the materials of Plant Physiology course, should be able to: 1. To explain the pathway of sugar transport in plants 2. To explain the mechanism
LECTURE 7: SUGAR TRANSPORT COMPETENCIES Students, after mastering the materials of Plant Physiology course, should be able to: 1. To explain the pathway of sugar transport in plants 2. To explain the mechanism
MODIFICATION OF RESPIRATION AND CARBOHYDRATE STATUS OF BARLEY ROOTS BY SELECTIVE PRUNING
 Phytol. i^9s6) 102, 5\3-52\ 513 MODIFICATION OF RESPIRATION AND CARBOHYDRATE STATUS OF BARLEY ROOTS BY SELECTIVE PRUNING BY J. F. FARRAR AND C. L. JONES School of Plant Biology, University College of North
Phytol. i^9s6) 102, 5\3-52\ 513 MODIFICATION OF RESPIRATION AND CARBOHYDRATE STATUS OF BARLEY ROOTS BY SELECTIVE PRUNING BY J. F. FARRAR AND C. L. JONES School of Plant Biology, University College of North
Chapter 36: Transport in Vascular Plants - Pathways for Survival
 Chapter 36: Transport in Vascular Plants - Pathways for Survival For vascular plants, the evolutionary journey onto land involved differentiation into roots and shoots Vascular tissue transports nutrients
Chapter 36: Transport in Vascular Plants - Pathways for Survival For vascular plants, the evolutionary journey onto land involved differentiation into roots and shoots Vascular tissue transports nutrients
The Relationship between Sucrose and Starch during 'Dark' Export from Leaves of Uniculm Barley
 Journal of Experimental Botany, Vol. 31, No. 122, pp. 845-850, June 1980 The Relationship between Sucrose and Starch during 'Dark' Export from Leaves of Uniculm Barley A. J. GORDON, G. J. A. RYLE, AND
Journal of Experimental Botany, Vol. 31, No. 122, pp. 845-850, June 1980 The Relationship between Sucrose and Starch during 'Dark' Export from Leaves of Uniculm Barley A. J. GORDON, G. J. A. RYLE, AND
NOTES: CH 36 - Transport in Plants
 NOTES: CH 36 - Transport in Plants Recall that transport across the cell membrane of plant cells occurs by: -diffusion -facilitated diffusion -osmosis (diffusion of water) -active transport (done by transport
NOTES: CH 36 - Transport in Plants Recall that transport across the cell membrane of plant cells occurs by: -diffusion -facilitated diffusion -osmosis (diffusion of water) -active transport (done by transport
Organs and leaf structure
 Organs and leaf structure Different types of tissues are arranged together to form organs. Structure: 2 parts (Petiole and Leaf Blade) Thin flat blade, large surface area Leaves contain all 3 types of
Organs and leaf structure Different types of tissues are arranged together to form organs. Structure: 2 parts (Petiole and Leaf Blade) Thin flat blade, large surface area Leaves contain all 3 types of
STUDIES IN THE PHYSIOLOGY OF LICHENS
 STUDIES IN THE PHYSIOLOGY OF LICHENS V. TRANSLOCATION FROM THE ALGAL LAYER TO THE MEDULLA IN PELTIGERA POLYDACTYLA BY D. C. SMITH AND E. A. DREW Department of Agriculture, University of Oxford {Received
STUDIES IN THE PHYSIOLOGY OF LICHENS V. TRANSLOCATION FROM THE ALGAL LAYER TO THE MEDULLA IN PELTIGERA POLYDACTYLA BY D. C. SMITH AND E. A. DREW Department of Agriculture, University of Oxford {Received
Please sit next to a partner. you are an A or a B
 Please sit next to a partner you are an A or a B Plants Transport in Vascular Plants Transport Overview Vascular tissue transports nutrients throughout a plant Such transport may occur over long distances
Please sit next to a partner you are an A or a B Plants Transport in Vascular Plants Transport Overview Vascular tissue transports nutrients throughout a plant Such transport may occur over long distances
Bio Factsheet. Transport in Plants. Number 342
 Number 342 Transport in Plants This Factsheet: Explains why plants need a transport system Describes what plants transport Describes the tissues which carry out transport Outlines the position of the xylem
Number 342 Transport in Plants This Factsheet: Explains why plants need a transport system Describes what plants transport Describes the tissues which carry out transport Outlines the position of the xylem
Chapter 36~ Transport in Plants
 Chapter 36~ Transport in Plants Structural Features Used for Resource Acquistion Roots and stems to do transport of resources Diffusion, active transport, and bulk flow Work in vascular plants to transport
Chapter 36~ Transport in Plants Structural Features Used for Resource Acquistion Roots and stems to do transport of resources Diffusion, active transport, and bulk flow Work in vascular plants to transport
Chapter 36. Transport in Vascular Plants
 Chapter 36 Transport in Vascular Plants Overview: Pathways for Survival For vascular plants The evolutionary journey onto land involved the differentiation of the plant body into roots and shoots Vascular
Chapter 36 Transport in Vascular Plants Overview: Pathways for Survival For vascular plants The evolutionary journey onto land involved the differentiation of the plant body into roots and shoots Vascular
Plant Structure and Function
 Plant Structure and Function A Meridian Biology AP Study Guide by John Ho and Tim Qi Plant Terms Growth: Growth Types Type Location Description Primary Primary Vertical growth (up-down), dominant direction
Plant Structure and Function A Meridian Biology AP Study Guide by John Ho and Tim Qi Plant Terms Growth: Growth Types Type Location Description Primary Primary Vertical growth (up-down), dominant direction
Respiration and Carbon Partitioning. Thomas G Chastain CROP 200 Crop Ecology and Morphology
 Respiration and Carbon Partitioning Thomas G Chastain CROP 200 Crop Ecology and Morphology Respiration Aerobic respiration is the controlled oxidation of reduced carbon substrates such as a carbohydrate
Respiration and Carbon Partitioning Thomas G Chastain CROP 200 Crop Ecology and Morphology Respiration Aerobic respiration is the controlled oxidation of reduced carbon substrates such as a carbohydrate
Transport of substances in plants
 Transport of substances in plants We have already looked at why many organisms need transport systems with special reference to surface area and volume. The larger the volume : surface area ratio, the
Transport of substances in plants We have already looked at why many organisms need transport systems with special reference to surface area and volume. The larger the volume : surface area ratio, the
Transport in Plants Notes AP Biology Mrs. Laux 3 levels of transport occur in plants: 1. Uptake of water and solutes by individual cells -for
 3 levels of transport occur in plants: 1. Uptake of water and solutes by individual cells -for photosynthesis and respiration -ex: absorption of H 2 O /minerals by root hairs 2. Short distance cell-to-cell
3 levels of transport occur in plants: 1. Uptake of water and solutes by individual cells -for photosynthesis and respiration -ex: absorption of H 2 O /minerals by root hairs 2. Short distance cell-to-cell
Plant Transport and Nutrition
 Plant Transport and Nutrition Chapter 36: Transport in Plants H 2 O & Minerals o Transport in xylem o Transpiration Evaporation, adhesion & cohesion Negative pressure. Sugars o Transport in phloem. o Bulk
Plant Transport and Nutrition Chapter 36: Transport in Plants H 2 O & Minerals o Transport in xylem o Transpiration Evaporation, adhesion & cohesion Negative pressure. Sugars o Transport in phloem. o Bulk
CBSE Quick Revision Notes (Class-11 Biology) CHAPTER-11 TRANSPORT IN PLANTS
 CBSE Quick Revision Notes (Class-11 Biology) CHAPTER-11 TRANSPORT IN PLANTS Plant transport various substance like gases, minerals, water, hormones, photosynthetes and organic solutes to short distance
CBSE Quick Revision Notes (Class-11 Biology) CHAPTER-11 TRANSPORT IN PLANTS Plant transport various substance like gases, minerals, water, hormones, photosynthetes and organic solutes to short distance
IB Bio: Plant Biology. Topic 9
 IB Bio: Plant Biology Topic 9 9.1: Transport in xylem How and why does water move up a plant? How do plants conserve water? 9.2: Transport in phloem How and why and where does food move in a plant? 9.3:
IB Bio: Plant Biology Topic 9 9.1: Transport in xylem How and why does water move up a plant? How do plants conserve water? 9.2: Transport in phloem How and why and where does food move in a plant? 9.3:
Tissues and organs PART 2
 Tissues and organs PART 2 The structure and function of the mesophytic leaf (a plant organ) The mesopyhtic leaf (lives in a moderately moist environment) contains 7 layers of tissue: 1. Upper epidermis
Tissues and organs PART 2 The structure and function of the mesophytic leaf (a plant organ) The mesopyhtic leaf (lives in a moderately moist environment) contains 7 layers of tissue: 1. Upper epidermis
Movement of water and solutes in plants Chapter 4 and 30
 Movement of water and solutes in plants Chapter 4 and 30 Molecular Movement Diffusion Molecules or ions moving in the opposite direction = movement against a diffusion gradient. Rates of diffusion are
Movement of water and solutes in plants Chapter 4 and 30 Molecular Movement Diffusion Molecules or ions moving in the opposite direction = movement against a diffusion gradient. Rates of diffusion are
Compartments and Transport. Three Major Pathways of Transport. Absorp+on of Water and Minerals by Root Cells. Bulk flow
 Plasmodesmata Channels connec+ng neighboring cells Cell membrane and cytosol are con+nuous from cell to cell Symplast Cytoplasmic con+nuum Apoplast Compartments and Transport Through plasmodesmata con+nuum
Plasmodesmata Channels connec+ng neighboring cells Cell membrane and cytosol are con+nuous from cell to cell Symplast Cytoplasmic con+nuum Apoplast Compartments and Transport Through plasmodesmata con+nuum
CHAPTER TRANSPORT
 CHAPTER 2 2.4 TRANSPORT Uptake of CO2 FOCUS: Uptake and transport of water and mineral salts Transport of organic substances Physical forces drive the transport of materials in plants over a range of distances
CHAPTER 2 2.4 TRANSPORT Uptake of CO2 FOCUS: Uptake and transport of water and mineral salts Transport of organic substances Physical forces drive the transport of materials in plants over a range of distances
PLANT PHYSIOLOGY. Az Agrármérnöki MSc szak tananyagfejlesztése TÁMOP /1/A
 PLANT PHYSIOLOGY Az Agrármérnöki MSc szak tananyagfejlesztése TÁMOP-4.1.2-08/1/A-2009-0010 Carbon reactions of the photosynthesis Photosynthetic activity and the environmental factors Overview 1. Carbon
PLANT PHYSIOLOGY Az Agrármérnöki MSc szak tananyagfejlesztése TÁMOP-4.1.2-08/1/A-2009-0010 Carbon reactions of the photosynthesis Photosynthetic activity and the environmental factors Overview 1. Carbon
Transport in Plants. Transport in plants. Transport across Membranes. Water potential 10/9/2016
 Transport in Plants Transport in plants How is a plant able to move water and nutrients from roots to the rest of the plant body? Especially tall trees? Sequoia can be over 300 feet tall! Transport across
Transport in Plants Transport in plants How is a plant able to move water and nutrients from roots to the rest of the plant body? Especially tall trees? Sequoia can be over 300 feet tall! Transport across
Chapter 10: PHOTOSYNTHESIS
 Chapter 10: PHOTOSYNTHESIS 1. Overview of Photosynthesis 2. Light Absorption 3. The Light Reactions 4. The Calvin Cycle 1. Overview of Photosynthesis Chapter Reading pp. 185-190, 206-207 What is Photosynthesis?
Chapter 10: PHOTOSYNTHESIS 1. Overview of Photosynthesis 2. Light Absorption 3. The Light Reactions 4. The Calvin Cycle 1. Overview of Photosynthesis Chapter Reading pp. 185-190, 206-207 What is Photosynthesis?
1/23/2011. Grapevine Anatomy & Physiology. What is Light? WSU Viticulture Certificate Program. Photosynthesis & Respiration.
 WSU Viticulture Certificate Program Grapevine Anatomy & Physiology & Respiration Markus Keller PHOTOS: Converts sunlight to chemical energy SYNTHESIS: Uses energy to convert inorganic compounds to organic
WSU Viticulture Certificate Program Grapevine Anatomy & Physiology & Respiration Markus Keller PHOTOS: Converts sunlight to chemical energy SYNTHESIS: Uses energy to convert inorganic compounds to organic
Light and Photosynthesis. Supplemental notes Lab 4 Horticultural Therapy
 Light and Photosynthesis Supplemental notes Lab 4 Horticultural Therapy Light The Electromagnetic Spectrum is a continuum of all electromagnetic waves arranged according to frequency and wavelength, the
Light and Photosynthesis Supplemental notes Lab 4 Horticultural Therapy Light The Electromagnetic Spectrum is a continuum of all electromagnetic waves arranged according to frequency and wavelength, the
Transport in Plants (Ch. 23.5)
 Transport in Plants (Ch. 23.5) Transport in plants H 2 O & minerals transport in xylem Transpiration Adhesion, cohesion & Evaporation Sugars transport in phloem bulk flow Gas exchange photosynthesis CO
Transport in Plants (Ch. 23.5) Transport in plants H 2 O & minerals transport in xylem Transpiration Adhesion, cohesion & Evaporation Sugars transport in phloem bulk flow Gas exchange photosynthesis CO
Resource Acquisition and Transport in Vascular Plants
 Chapter 36 Resource Acquisition and Transport in Vascular Plants PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley
Chapter 36 Resource Acquisition and Transport in Vascular Plants PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley
Chapter 30: Plant Nutrition & Transport
 Chapter 30: Plant Nutrition & Transport Carnivorous Plants Capture animals to supplement their nutrient intake Venus flytrap lures insects with sugary bait; closes on victim Cobra lily lures insects down
Chapter 30: Plant Nutrition & Transport Carnivorous Plants Capture animals to supplement their nutrient intake Venus flytrap lures insects with sugary bait; closes on victim Cobra lily lures insects down
STOMATAL RESPONSES TO LIGHT AND CARBON DIOXIDE IN THE HART'S-TONGUE FERN, PHYLLITIS SCOLOPENDRIUM NEWM.
 New PhytoL (1969) 68, 63-66.. STOMATAL RESPONSES TO LIGHT AND CARBON DIOXIDE IN THE HART'S-TONGUE FERN, PHYLLITIS SCOLOPENDRIUM NEWM. BY T. A. MANSFIELD AND C. M. WILLMER Department of Biological Sciences,
New PhytoL (1969) 68, 63-66.. STOMATAL RESPONSES TO LIGHT AND CARBON DIOXIDE IN THE HART'S-TONGUE FERN, PHYLLITIS SCOLOPENDRIUM NEWM. BY T. A. MANSFIELD AND C. M. WILLMER Department of Biological Sciences,
thebiotutor. AS Biology OCR. Unit F211: Cells, Exchange & Transport. Module 2.3 Transport in Plants. Answers.
 thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 2.3 Transport in Plants Answers Andy Todd 2013 1 1. (i) transports water (up plant); ACCEPT alternative wording for transport e.g.
thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 2.3 Transport in Plants Answers Andy Todd 2013 1 1. (i) transports water (up plant); ACCEPT alternative wording for transport e.g.
BY ROBERT T. FURBANK AND DAVID A. WALKER Research Institute for Photosynthesis, University of Sheffield, Sheffield SIO 2TN, UK
 New Phytol. (1986) 104, 207-213 2O7 CHLOROPHYLL A FLUORESCENCE AS A QUANTITATIVE PROBE OF PHOTOSYNTHESIS: EFFECTS OF CO^ CONCENTRATION DURING GAS TRANSIENTS ON CHLOROPHYLL FLUORESCENCE IN SPINACH LEAVES
New Phytol. (1986) 104, 207-213 2O7 CHLOROPHYLL A FLUORESCENCE AS A QUANTITATIVE PROBE OF PHOTOSYNTHESIS: EFFECTS OF CO^ CONCENTRATION DURING GAS TRANSIENTS ON CHLOROPHYLL FLUORESCENCE IN SPINACH LEAVES
AP Biology. Transport in plants. Chapter 36. Transport in Plants. Transport in plants. Transport in plants. Transport in plants. Transport in plants
 Chapter 36. Transport in Plants evaporation, adhesion & cohesion negative pressure evaporation, adhesion & cohesion negative pressure transport in phloem bulk flow Calvin cycle in leaves loads sucrose
Chapter 36. Transport in Plants evaporation, adhesion & cohesion negative pressure evaporation, adhesion & cohesion negative pressure transport in phloem bulk flow Calvin cycle in leaves loads sucrose
CHAPTER 32 TRANSPORT IN PLANTS OUTLINE OBJECTIVES
 CHAPTER 32 TRANSPORT IN PLANTS OUTLINE I. The traffic of water and solutes occurs on cellular, organ, and whole-plant levels: an overview of transport in plants A. Transport at the Cellular Level B. Short
CHAPTER 32 TRANSPORT IN PLANTS OUTLINE I. The traffic of water and solutes occurs on cellular, organ, and whole-plant levels: an overview of transport in plants A. Transport at the Cellular Level B. Short
Importance. The Reaction of Life : The conversion of the sun s energy into a form man and other living creatures can use.
 PLANT PROCESSES Photosynthesis Importance The Reaction of Life : The conversion of the sun s energy into a form man and other living creatures can use. Photo light Synthesis to put together 3 Important
PLANT PROCESSES Photosynthesis Importance The Reaction of Life : The conversion of the sun s energy into a form man and other living creatures can use. Photo light Synthesis to put together 3 Important
OCR (A) Biology A-level
 OCR (A) Biology A-level Topic 3.3: Transport in plants Notes Plants require a transport system to ensure that all the cells of a plant receive a sufficient amount of nutrients. This is achieved through
OCR (A) Biology A-level Topic 3.3: Transport in plants Notes Plants require a transport system to ensure that all the cells of a plant receive a sufficient amount of nutrients. This is achieved through
Ch. 36 Transport in Vascular Plants
 Ch. 36 Transport in Vascular Plants Feb 4 1:32 PM 1 Essential Question: How does a tall tree get the water from its roots to the top of the tree? Feb 4 1:38 PM 2 Shoot architecture and Light Capture: Phyllotaxy
Ch. 36 Transport in Vascular Plants Feb 4 1:32 PM 1 Essential Question: How does a tall tree get the water from its roots to the top of the tree? Feb 4 1:38 PM 2 Shoot architecture and Light Capture: Phyllotaxy
Page 1. Gross Anatomy of a typical plant (Angiosperm = Flowering Plant): Gross Anatomy of a typical plant (Angiosperm = Flowering Plant):
 Chapter 43: Plant Form and Function Gross Anatomy of a typical plant (Angiosperm = Flowering Plant): Root System Anchor plant Absorb water / nutrients Store surplus sugars Transport materials from / to
Chapter 43: Plant Form and Function Gross Anatomy of a typical plant (Angiosperm = Flowering Plant): Root System Anchor plant Absorb water / nutrients Store surplus sugars Transport materials from / to
Resource Acquisition and Transport in Vascular Plants
 Chapter 36 Resource Acquisition and Transport in Vascular Plants PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley
Chapter 36 Resource Acquisition and Transport in Vascular Plants PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley
Biology 1030 Winter 2009
 Meeting Tissue Needs II Chapter 36 (738-755) Chapter 37 (756-770) Cellular Currency Plants harvest solar energy Photosynthesis Produces sugars Proteins, nucleic acids, lipids? H 2 O CO 2 Plants cells still
Meeting Tissue Needs II Chapter 36 (738-755) Chapter 37 (756-770) Cellular Currency Plants harvest solar energy Photosynthesis Produces sugars Proteins, nucleic acids, lipids? H 2 O CO 2 Plants cells still
Answer Key. Vocabulary Practice. 1. guard cell 2. parenchyma cell 3. sclerenchyma cell 4. collenchyma cell 5. All are types of plant cells
 Answer Key Vocabulary Practice A. Choose the Right Word 1. guard cell 2. parenchyma cell 3. sclerenchyma cell 4. collenchyma cell 5. All are types of cells 6. meristem 7. ground tissue 8. dermal tissue
Answer Key Vocabulary Practice A. Choose the Right Word 1. guard cell 2. parenchyma cell 3. sclerenchyma cell 4. collenchyma cell 5. All are types of cells 6. meristem 7. ground tissue 8. dermal tissue
Supplementary Material. An analysis of the role of the ShSUT1 sucrose transporter in sugarcane using RNAi suppression
 10.1071/FP17073_AC CSIRO 2017 Supplementary Material: Functional Plant Biology, 2017, 44(8), 795 808. Supplementary Material An analysis of the role of the ShSUT1 sucrose transporter in sugarcane using
10.1071/FP17073_AC CSIRO 2017 Supplementary Material: Functional Plant Biology, 2017, 44(8), 795 808. Supplementary Material An analysis of the role of the ShSUT1 sucrose transporter in sugarcane using
Chapter 35~ Plant Structure and Growth
 Chapter 35~ Plant Structure and Growth Plant Organization Plant morphology is based on plant s evolutionary history Need to draw in nutrients from the ground and the air Plant Organs Root system = roots
Chapter 35~ Plant Structure and Growth Plant Organization Plant morphology is based on plant s evolutionary history Need to draw in nutrients from the ground and the air Plant Organs Root system = roots
VOCABULARY COMPTETENCIES. Students, after mastering the materials of Plant Physiology course, should be able to:
 1 VOCABULARY Forget not, exam includes ENGLISH WORDS 1. Involve 2. Bundle 3. Sheath 4. Subsequent 5. Ambient 6. Stick together 7. Determine 8. Evolution 9. Thrive 10. Allow COMPTETENCIES Students, after
1 VOCABULARY Forget not, exam includes ENGLISH WORDS 1. Involve 2. Bundle 3. Sheath 4. Subsequent 5. Ambient 6. Stick together 7. Determine 8. Evolution 9. Thrive 10. Allow COMPTETENCIES Students, after
CROSS SECTION OF A LEAF INTRODUCTION
 CROSS SECTION OF A LEAF INTRODUCTION The leaf is an organ in a plant consisting of many different tissues. The primary function of a leaf is to make (synthesize) food through a chemical reaction called.
CROSS SECTION OF A LEAF INTRODUCTION The leaf is an organ in a plant consisting of many different tissues. The primary function of a leaf is to make (synthesize) food through a chemical reaction called.
Chapter 23 Notes Roots Stems Leaves
 Chapter 23 Notes Roots Stems Leaves I. Specialized tissue in plants - effective way to ensure the plant s survival A. Seed plant structure 1. Roots - a. Absorbs water and dissolves nutrients b. anchors
Chapter 23 Notes Roots Stems Leaves I. Specialized tissue in plants - effective way to ensure the plant s survival A. Seed plant structure 1. Roots - a. Absorbs water and dissolves nutrients b. anchors
B2 Quick Revision Questions. B2 for AQA GCSE examination 2018 onwards
 B2 Quick Revision Questions Question 1 Which raw materials are used in photosynthesis and what are the products of the reaction? Answer 1 Carbon dioxide Water Glucose Oxygen Question 2 What type of reaction
B2 Quick Revision Questions Question 1 Which raw materials are used in photosynthesis and what are the products of the reaction? Answer 1 Carbon dioxide Water Glucose Oxygen Question 2 What type of reaction
AP Biology Chapter 36
 Chapter 36 Chapter 36 Transport in Plants 2006-2007 Transport in plants - Overview H2O & minerals transport in xylem transpiration evaporation, adhesion & cohesion negative pressure Sugars transport in
Chapter 36 Chapter 36 Transport in Plants 2006-2007 Transport in plants - Overview H2O & minerals transport in xylem transpiration evaporation, adhesion & cohesion negative pressure Sugars transport in
RuBP has 5 carbons and is regenerated in the Calvin cycle. In the Calvin cycle, carbon is conserved, ATP is used and NADPH is used.
 Carbon Reactions: CO 2 is fixed by Rubisco located in the stroma. The molecule that is carboxylated is RuBP. RuBP has 5 carbons and is regenerated in the Calvin cycle. In the Calvin cycle, carbon is conserved,
Carbon Reactions: CO 2 is fixed by Rubisco located in the stroma. The molecule that is carboxylated is RuBP. RuBP has 5 carbons and is regenerated in the Calvin cycle. In the Calvin cycle, carbon is conserved,
Forms strands that conduct water, minerals, and organic compounds. Much of the inside of nonwoody parts of plants. Includes roots, stems, and leaves
 Biology II Vascular plants have 3 tissue systems: Dermal Protective outer layer of plant Vascular Forms strands that conduct water, minerals, and organic compounds Ground Much of the inside of nonwoody
Biology II Vascular plants have 3 tissue systems: Dermal Protective outer layer of plant Vascular Forms strands that conduct water, minerals, and organic compounds Ground Much of the inside of nonwoody
Science Year 10 Unit 1 Biology
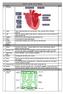 Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
cytosol stroma Photorespiration: Ribulose bisphosphate carboxylase/oxygenase (Rubisco) Ribulose bisphosphate carboxylase/oxygenase (Rubisco)
 Carbon Reactions: CO 2 is fixed by Rubisco located in the stroma. The molecule that is carboxylated is RuBP. RuBP has 5 carbons and is regenerated in the Calvin cycle. In the Calvin cycle, carbon is conserved,
Carbon Reactions: CO 2 is fixed by Rubisco located in the stroma. The molecule that is carboxylated is RuBP. RuBP has 5 carbons and is regenerated in the Calvin cycle. In the Calvin cycle, carbon is conserved,
PLANT SCIENCE. 9.2 Transport in Angiospermophytes
 PLANT SCIENCE 9.2 Transport in Angiospermophytes Support of terrestrial plants Support of terrestrial plants comes through: Thickened cellulose in cell walls Turgor pressure of cells Lignified xylem Xylem
PLANT SCIENCE 9.2 Transport in Angiospermophytes Support of terrestrial plants Support of terrestrial plants comes through: Thickened cellulose in cell walls Turgor pressure of cells Lignified xylem Xylem
Honors Biology I Ch 29 Plant Structure & Function
 3 Basic types of plant cells Honors Biology I Ch 29 Plant Structure & Function 1) Parenchyma cells- loosely packed or cells with a and thin, Involved in metabolic functions 2) Collenchyma cells- thicker
3 Basic types of plant cells Honors Biology I Ch 29 Plant Structure & Function 1) Parenchyma cells- loosely packed or cells with a and thin, Involved in metabolic functions 2) Collenchyma cells- thicker
Symplastic transport of Lucifer Yellow in mature leaf blades of barley: potential mesophyll-to-sieve-tube transfer
 New Phytol. (1992), 120, 191-196 Symplastic transport of Lucifer Yellow in mature leaf blades of barley: potential mesophyll-to-sieve-tube transfer BY JOHN FARRAR\ CHRIS VAN DER SCHOOT, PETER DRENT AND
New Phytol. (1992), 120, 191-196 Symplastic transport of Lucifer Yellow in mature leaf blades of barley: potential mesophyll-to-sieve-tube transfer BY JOHN FARRAR\ CHRIS VAN DER SCHOOT, PETER DRENT AND
Plant Anatomy: roots, stems and leaves
 Plant Anatomy: roots, stems and leaves The plant body has a hierarchy of organs, tissues and cells Plants, like animals, have organs composed of different tissues, which are composed of cells. Tissue is
Plant Anatomy: roots, stems and leaves The plant body has a hierarchy of organs, tissues and cells Plants, like animals, have organs composed of different tissues, which are composed of cells. Tissue is
-Each asexual organs. -Anchors the plant -Absorbs water and minerals -Stores sugars and starches
 Plants are made up of: -organs, tissues, and cells The three major plant organs are: -Roots, stems, and leaves -Each asexual organs Plants have a Root System beneath the ground that us a multicellular
Plants are made up of: -organs, tissues, and cells The three major plant organs are: -Roots, stems, and leaves -Each asexual organs Plants have a Root System beneath the ground that us a multicellular
DNA or RNA metabolism (1%) Signal transduction (2%) Development (2%) Other cellular processes (17%)
 Fig. 35-24 Other metabolism (18%) DNA or RNA metabolism (1%) Signal transduction (2%) Development (2%) Unknown (24%) Energy pathways (3%) Cell division and organization (3%) Transport (4%) Transcription
Fig. 35-24 Other metabolism (18%) DNA or RNA metabolism (1%) Signal transduction (2%) Development (2%) Unknown (24%) Energy pathways (3%) Cell division and organization (3%) Transport (4%) Transcription
Physiology of carrot growth and development
 Physiology of carrot growth and development Introduction Carrot (Daucus carota L. ssp. Sativus (Hoffm.) Schübl. & G. Martens) originates from the wild forms growing in Europe and southwestern Asia (Banga
Physiology of carrot growth and development Introduction Carrot (Daucus carota L. ssp. Sativus (Hoffm.) Schübl. & G. Martens) originates from the wild forms growing in Europe and southwestern Asia (Banga
Biology 2 Chapter 21 Review
 Biology 2 Chapter 21 Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is not a tissue system of vascular plants? a. vascular
Biology 2 Chapter 21 Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is not a tissue system of vascular plants? a. vascular
BioWash as an Adjuvant, Translocation Promoter, and Cationic Exchange Stimulator Overview of Processes within the Plant
 BioWash as an Adjuvant, Translocation Promoter, and Cationic Exchange Stimulator Overview of Processes within the Plant Photosynthesis is the primary driver of the plant. Through a series of complex steps,
BioWash as an Adjuvant, Translocation Promoter, and Cationic Exchange Stimulator Overview of Processes within the Plant Photosynthesis is the primary driver of the plant. Through a series of complex steps,
Introduction to Plant Transport
 Introduction to Plant Transport The algal ancestors of plants were completely immersed in water and dissolved minerals. The adaptation to land involved the differentiation of the plant body into roots,
Introduction to Plant Transport The algal ancestors of plants were completely immersed in water and dissolved minerals. The adaptation to land involved the differentiation of the plant body into roots,
13.2 The Vascular Plant Body (textbook p )
 13.2 The Vascular Plant Body (textbook p544 550) Learning Goal: Label and explain the anatomy of the Vascular Plant and it's Tissue Types Plants are classified into two main groups: and. Vascular plants
13.2 The Vascular Plant Body (textbook p544 550) Learning Goal: Label and explain the anatomy of the Vascular Plant and it's Tissue Types Plants are classified into two main groups: and. Vascular plants
2014 Pearson Education, Inc. 1
 1 CO 2 O 2 Light Sugar O 2 and minerals CO 2 2 Buds 42 29 21 34 13 26 5 18 10 31 23 8 15 28 16 2 24 Shoot apical meristem 7 3 20 1 mm 32 11 19 12 6 4 1 25 17 14 9 40 27 22 3 Cell wall Apoplastic route
1 CO 2 O 2 Light Sugar O 2 and minerals CO 2 2 Buds 42 29 21 34 13 26 5 18 10 31 23 8 15 28 16 2 24 Shoot apical meristem 7 3 20 1 mm 32 11 19 12 6 4 1 25 17 14 9 40 27 22 3 Cell wall Apoplastic route
BRAINSTORM ACTIVITY What do we depend on plants for?
 SBI3U1 BRAINSTORM ACTIVITY What do we depend on plants for? STOP! THINK! PAIR! SHARE! With your partner, brainstorm 5 significant uses of plants. Write them down. Now share your ideas with the rest of
SBI3U1 BRAINSTORM ACTIVITY What do we depend on plants for? STOP! THINK! PAIR! SHARE! With your partner, brainstorm 5 significant uses of plants. Write them down. Now share your ideas with the rest of
POTASSIUM IN PLANT GROWTH AND YIELD. by Ismail Cakmak Sabanci University Istanbul, Turkey
 POTASSIUM IN PLANT GROWTH AND YIELD by Ismail Cakmak Sabanci University Istanbul, Turkey Low K High K High K Low K Low K High K Low K High K Control K Deficiency Cakmak et al., 1994, J. Experimental Bot.
POTASSIUM IN PLANT GROWTH AND YIELD by Ismail Cakmak Sabanci University Istanbul, Turkey Low K High K High K Low K Low K High K Low K High K Control K Deficiency Cakmak et al., 1994, J. Experimental Bot.
 Question 1: What are the factors affecting the rate of diffusion? Diffusion is the passive movement of substances from a region of higher concentration to a region of lower concentration. Diffusion of
Question 1: What are the factors affecting the rate of diffusion? Diffusion is the passive movement of substances from a region of higher concentration to a region of lower concentration. Diffusion of
CHAPTER XI PHOTOSYNTHESIS. DMA: Chapter 11 Hartmann's 1
 CHAPTER XI PHOTOSYNTHESIS DMA: Chapter 11 Hartmann's 1 The nature of light The sun's energy travels through space to the earth as electromagnetic radiation waves at the speed of light, about 300,000 Km/s.
CHAPTER XI PHOTOSYNTHESIS DMA: Chapter 11 Hartmann's 1 The nature of light The sun's energy travels through space to the earth as electromagnetic radiation waves at the speed of light, about 300,000 Km/s.
AN ABSTRACT OF THE THESIS OF. Charles F. Forney for the degree of Doctor of Philosophy. in Horticulture presented on March 9, 1984
 AN ABSTRACT OF THE THESIS OF Charles F. Forney for the degree of Doctor of Philosophy in Horticulture presented on March 9, 1984 Title: Sugar Uptake, Fruit Growth and Carbon Partitioning in the Strawberry
AN ABSTRACT OF THE THESIS OF Charles F. Forney for the degree of Doctor of Philosophy in Horticulture presented on March 9, 1984 Title: Sugar Uptake, Fruit Growth and Carbon Partitioning in the Strawberry
The three principal organs of seed plants are roots, stems, and leaves.
 23 1 Specialized Tissues in Plants Seed Plant Structure The three principal organs of seed plants are roots, stems, and leaves. 1 of 34 23 1 Specialized Tissues in Plants Seed Plant Structure Roots: absorb
23 1 Specialized Tissues in Plants Seed Plant Structure The three principal organs of seed plants are roots, stems, and leaves. 1 of 34 23 1 Specialized Tissues in Plants Seed Plant Structure Roots: absorb
NOTES: CH 10, part 3 Calvin Cycle (10.3) & Alternative Mechanisms of C-Fixation (10.4)
 NOTES: CH 10, part 3 Calvin Cycle (10.3) & Alternative Mechanisms of C-Fixation (10.4) 10.3 - The Calvin cycle uses ATP and NADPH to convert CO 2 to sugar The Calvin cycle, like the citric acid cycle,
NOTES: CH 10, part 3 Calvin Cycle (10.3) & Alternative Mechanisms of C-Fixation (10.4) 10.3 - The Calvin cycle uses ATP and NADPH to convert CO 2 to sugar The Calvin cycle, like the citric acid cycle,
Transport in Vascular Plants
 Chapter 36 Transport in Vascular Plants PowerPoint Lectures for Biology, Seventh Edition Neil Campbell and Jane Reece Lectures by Chris Romero Vascular tissue Transports nutrients throughout a plant; such
Chapter 36 Transport in Vascular Plants PowerPoint Lectures for Biology, Seventh Edition Neil Campbell and Jane Reece Lectures by Chris Romero Vascular tissue Transports nutrients throughout a plant; such
Chapter C3: Multicellular Organisms Plants
 Chapter C3: Multicellular Organisms Plants Multicellular Organisms Multicellular organisms have specialized cells of many different types that allow them to grow to a larger size than single-celled organisms.
Chapter C3: Multicellular Organisms Plants Multicellular Organisms Multicellular organisms have specialized cells of many different types that allow them to grow to a larger size than single-celled organisms.
Chapter 4-2. Transpiration diffusion of water vapor
 Chapter 4-2 Transpiration diffusion of water vapor Transpiration diffusion of water vapor Diffusion is primary means of any further movement of the water out of the leaf. That is water movement is controlled
Chapter 4-2 Transpiration diffusion of water vapor Transpiration diffusion of water vapor Diffusion is primary means of any further movement of the water out of the leaf. That is water movement is controlled
Unit 1C Practice Exam (v.2: KEY)
 Unit 1C Practice Exam (v.2: KEY) 1. Which of the following statements concerning photosynthetic pigments (chlorophylls a and b, carotenes, and xanthophylls) is correct? (PT1-12) a. The R f values obtained
Unit 1C Practice Exam (v.2: KEY) 1. Which of the following statements concerning photosynthetic pigments (chlorophylls a and b, carotenes, and xanthophylls) is correct? (PT1-12) a. The R f values obtained
Plant Structure and Growth
 Plant Structure and Growth A. Flowering Plant Parts: The flowering plants or are the most diverse group of plants. They are divided into 2 classes and. Examples of monocots: Examples of dicots: The morphology
Plant Structure and Growth A. Flowering Plant Parts: The flowering plants or are the most diverse group of plants. They are divided into 2 classes and. Examples of monocots: Examples of dicots: The morphology
Plant Structure. Lab Exercise 24. Objectives. Introduction
 Lab Exercise Plant Structure Objectives - Be able to identify plant organs and give their functions. - Learn distinguishing characteristics between monocot and dicot plants. - Understand the anatomy of
Lab Exercise Plant Structure Objectives - Be able to identify plant organs and give their functions. - Learn distinguishing characteristics between monocot and dicot plants. - Understand the anatomy of
Lecture 9: Photosynthesis
 Lecture 9: Photosynthesis I. Characteristics of Light A. Light is composed of particles that travel as waves 1. Comprises a small part of the electromagnetic spectrum B. Radiation varies in wavelength
Lecture 9: Photosynthesis I. Characteristics of Light A. Light is composed of particles that travel as waves 1. Comprises a small part of the electromagnetic spectrum B. Radiation varies in wavelength
Plant Tissues and Organs. Topic 13 Plant Science Subtopics , ,
 Plant Tissues and Organs Topic 13 Plant Science Subtopics 13.1.2, 13.1.3, 13.1.4 Objectives: List and describe the major plant organs their structure and function List and describe the major types of plant
Plant Tissues and Organs Topic 13 Plant Science Subtopics 13.1.2, 13.1.3, 13.1.4 Objectives: List and describe the major plant organs their structure and function List and describe the major types of plant
Oxygen and Hydrogen in Plants
 Oxygen and Hydrogen in Plants Outline: Environmental factors Fractionation associated with uptake of water Metabolic Fractionation C3, CAM and C4 plants Environmental factors Regional Precipitation d 18
Oxygen and Hydrogen in Plants Outline: Environmental factors Fractionation associated with uptake of water Metabolic Fractionation C3, CAM and C4 plants Environmental factors Regional Precipitation d 18
Chapter 7: Photosynthesis
 Chapter 7: Photosynthesis Electromagnetic Spectrum Shortest wavelength Longest wavelength Gamma rays X-rays UV radiation Visible light Infrared radiation Microwaves Radio waves Photons Packets of light
Chapter 7: Photosynthesis Electromagnetic Spectrum Shortest wavelength Longest wavelength Gamma rays X-rays UV radiation Visible light Infrared radiation Microwaves Radio waves Photons Packets of light
Photosynthesis Revision 4
 Photosynthesis Revision 4 85 minutes 85 marks Page of 3 Q. Low light intensity is one factor that limits the yield of a crop. In Britain, many tomato growers use artificial lights to increase the yield
Photosynthesis Revision 4 85 minutes 85 marks Page of 3 Q. Low light intensity is one factor that limits the yield of a crop. In Britain, many tomato growers use artificial lights to increase the yield
PHOTOSYNTHESIS GR 11 LIFE SCIENCES
 PHOTOSYNTHESIS GR 11 LIFE SCIENCES Definition: Photosynthesis is the process where the energy of the sunlight is used by green plants (and some animals) to bond molecules together to form carbohydrates
PHOTOSYNTHESIS GR 11 LIFE SCIENCES Definition: Photosynthesis is the process where the energy of the sunlight is used by green plants (and some animals) to bond molecules together to form carbohydrates
1. What is the source of the oxygen released into the air as a product of photosynthesis? D. Both water and carbon dioxide (Total 1 mark)
 2.9 Photosynthesis Paper 1 Possible Mult Choice Questions 1. What is the source of the oxygen released into the air as a product of photosynthesis? A. Chlorophyll B. Carbon dioxide only C. Water only D.
2.9 Photosynthesis Paper 1 Possible Mult Choice Questions 1. What is the source of the oxygen released into the air as a product of photosynthesis? A. Chlorophyll B. Carbon dioxide only C. Water only D.
35 Transport in Plants
 Transport in Plants 35 Transport in Plants 35.1 How Do Plants Take Up Water and Solutes? 35.2 How Are Water and Minerals Transported in the Xylem? 35.3 How Do Stomata Control the Loss of Water and the
Transport in Plants 35 Transport in Plants 35.1 How Do Plants Take Up Water and Solutes? 35.2 How Are Water and Minerals Transported in the Xylem? 35.3 How Do Stomata Control the Loss of Water and the
Stomatal Movement and Sucrose Uptake by Guard Cell Protoplasts of Commelina benghalensis L.
 Plant Cell Physiol. 27(8): 1565-1570 JSPP 1986 Stomatal Movement and Sucrose Uptake by Guard Cell Protoplasts of Commelina benghalensis L. A. Ramachandra Reddy and V. S. Rama Das Department of Botany,
Plant Cell Physiol. 27(8): 1565-1570 JSPP 1986 Stomatal Movement and Sucrose Uptake by Guard Cell Protoplasts of Commelina benghalensis L. A. Ramachandra Reddy and V. S. Rama Das Department of Botany,
2018 Version. Photosynthesis Junior Science
 2018 Version Photosynthesis Junior Science 1 Plants fill the role of Producers in a community Plants are special because they have leaves and are able to produce their own food by the process of photosynthesis
2018 Version Photosynthesis Junior Science 1 Plants fill the role of Producers in a community Plants are special because they have leaves and are able to produce their own food by the process of photosynthesis
Chapter 8 PHOTOSYNTHESIS Chapter # Chapter Title PowerPoint Image Slideshow
 COLLEGE BIOLOGY PHYSICS Chapter 8 PHOTOSYNTHESIS Chapter # Chapter Title PowerPoint Image Slideshow Figure 8.0 Photosynthesis Figure 8.1 Earth s distribution of photosynthesis as seen via chlorophyll a
COLLEGE BIOLOGY PHYSICS Chapter 8 PHOTOSYNTHESIS Chapter # Chapter Title PowerPoint Image Slideshow Figure 8.0 Photosynthesis Figure 8.1 Earth s distribution of photosynthesis as seen via chlorophyll a
Crop Physiology. Plant/Crop Physiology. First
 Plant/Crop Physiology Plant physiology is a sub-discipline of botany concerned with the functioning of plants. Includes the study of all the internal activities of plants those chemical and physical processes
Plant/Crop Physiology Plant physiology is a sub-discipline of botany concerned with the functioning of plants. Includes the study of all the internal activities of plants those chemical and physical processes
Photosynthesis. Excitation of chlorophyll in a chloroplast
 Photosynthesis The process of photosynthesis begins with light-absorbing pigments in plant cells. A pigment molecule is able to absorb the energy from light only within a narrow range of wavelengths. In
Photosynthesis The process of photosynthesis begins with light-absorbing pigments in plant cells. A pigment molecule is able to absorb the energy from light only within a narrow range of wavelengths. In
Cell Review. 1. The diagram below represents levels of organization in living things.
 Cell Review 1. The diagram below represents levels of organization in living things. Which term would best represent X? 1) human 2) tissue 3) stomach 4) chloroplast 2. Which statement is not a part of
Cell Review 1. The diagram below represents levels of organization in living things. Which term would best represent X? 1) human 2) tissue 3) stomach 4) chloroplast 2. Which statement is not a part of
Chapter 29. Table of Contents. Section 1 Plant Cells and Tissues. Section 2 Roots. Section 3 Stems. Section 4 Leaves. Plant Structure and Function
 Plant Structure and Function Table of Contents Section 1 Plant Cells and Tissues Section 2 Roots Section 3 Stems Section 4 Leaves Section 1 Plant Cells and Tissues Objectives Describe the three basic types
Plant Structure and Function Table of Contents Section 1 Plant Cells and Tissues Section 2 Roots Section 3 Stems Section 4 Leaves Section 1 Plant Cells and Tissues Objectives Describe the three basic types
Save My Exams! The Home of Revision For more awesome GCSE and A level resources, visit us at Transport in plants
 Transport in plants Question Paper 1 Level A Level Subject Biology Exam Board OCR Topic Exchange and transport Sub-Topic Transport in plants Booklet Question Paper 1 Time Allowed: 75 minutes Score: / 62
Transport in plants Question Paper 1 Level A Level Subject Biology Exam Board OCR Topic Exchange and transport Sub-Topic Transport in plants Booklet Question Paper 1 Time Allowed: 75 minutes Score: / 62
Section A2: The Pathways of Photosynthesis
 CHAPTER 10 PHOTOSYNTHESIS Section A2: The Pathways of Photosynthesis 4. The Calvin cycle uses ATP and NADPH to convert CO2 to sugar: a closer look 5. Alternative mechanisms of carbon fixation have evolved
CHAPTER 10 PHOTOSYNTHESIS Section A2: The Pathways of Photosynthesis 4. The Calvin cycle uses ATP and NADPH to convert CO2 to sugar: a closer look 5. Alternative mechanisms of carbon fixation have evolved
Introduction to Plant Transport
 Introduction to Plant Transport The algal ancestors of plants were completely immersed in water and dissolved minerals. The adaptation to land involved the differentiation of the plant body into roots,
Introduction to Plant Transport The algal ancestors of plants were completely immersed in water and dissolved minerals. The adaptation to land involved the differentiation of the plant body into roots,
Plant Anatomy: roots, stems and leaves
 Plant Anatomy: roots, stems and leaves The plant body has a hierarchy of organs, tissues and cells Plants, like animals, have organs composed of different tissues, which are composed of cells. Tissue is
Plant Anatomy: roots, stems and leaves The plant body has a hierarchy of organs, tissues and cells Plants, like animals, have organs composed of different tissues, which are composed of cells. Tissue is
Photosynthesis. Dr. Bertolotti
 Photosynthesis Dr. Bertolotti Photosynthesis: Life from Light and Air How do plants and other organisms capture energy from the sun? What is ATP and why is it useful in cells? Plants are energy producers
Photosynthesis Dr. Bertolotti Photosynthesis: Life from Light and Air How do plants and other organisms capture energy from the sun? What is ATP and why is it useful in cells? Plants are energy producers
