Key Concepts, Methods and Machinery - lecture 2 -
|
|
|
- Harvey George
- 5 years ago
- Views:
Transcription
1 B. Roos V. A. Fok C. C. J. Roothaan W. Hetler Quantum Mechancs W. Paul J. Čížek P.A.M. Drac Key Concepts, Methods and Machnery - lecture - J. A. Pople M. S. Plesset N. Bohr E. Hückel E. Schrödnger and many other heroes W. Kohn J. C. Slater W. Hesenberg M. Born 1
2 I. On quantum mechancal state Sx postulates n QM The state of the system s descrbed by the wavefuncton = r,t, whch depends on the coordnates * of partcle r at tme t. Wavefuncton are n general complex functons of real varables, thus ( r,t) denotes the complex conjugate of ( ) II. ( r, t) ( x, t) dv = ( r, t) dv * P( r, t) = (probablstc nterpretaton) On operator representaton of mechancal quanttes The mechancal quanttes that descrbe the partcle (energy, momentum, angular momentum etc.) are represented by lnear operators actng on a wavefuncton The operator of the potental energy The total energy operator, Hamltonan: Hˆ = Tˆ + Vˆ The operator of the knetc energy Drac notaton: III. * ψ Aˆ φdτ ψ Aˆ φ Matrx element of the operator  On tme evoluton of the state The tme evoluton of the wave functon s gven by the equaton: ψ * φ d τ ψ φ Scalar product of two wavefunctons Hˆ ( r, t) = t ( r, t)
3 Sx postulates n QM IV. V. On nterpretaton of expermental measurements not dscussed here Spn angular momentum (n non-relatvstc formulaton of QM) Sˆ α = s( s + 1) S ˆ α = α z m s S ˆ β = z m s β ; ; α α β 1/ 1/ VI. where the spn magnetc quantum number m s = -s, -s+1,,s On the permutatonal symmetry ( 1,,...,,..., j,..., N ) = ( 1,,..., j,...,,..., N ) ( 1,,...,,..., j,..., N ) = ( 1,,..., j,...,,..., N ) Probablty densty of fndng two dentcal fermons n the same poston and wth the same spn coordnate equals to zero -fermons (electrons, ) non-nteger spn -bosons - nteger spn Ferm correlaton (Ferm hole) Paul excluson prncple 3
4 Quantum mechancs n Chemstry Let the molecular system under study contan atomc nucle (q nucle ), electrons (q electrons ) and possbly external felds. The key equaton n quantum mechancs s the nonrelatvstc Schrödnger equaton: Hˆ ( q, t) ( q, t) = t ( q, t) * The vector q collects the spatal and spn coordnates of all partcles (nucle and electrons) n the molecular system. * Postulate III. 4
5 The electronc Schrödnger equaton Hˆ ( q, t) ( q, t) = t ( q, t) Let the Hamltonan be tme-ndependent E tot ( q, t) = ( q) exp t ; Hˆ ( q) ( q) = E ( q) tot Born-Oppenhemer approxmaton Schrödnger equaton for statonary states ( q) ( ) ( ) q nucle q electrons 5
6 The electronc Schrödnger equaton ( q) ( ) ( ) ( q) q nucle q electrons ( Tˆ + E) ( q ) = E ( q ) Hˆ Hˆ nucle electrons = Tˆ nucle + ( q ) = E( q ) electrons nucle Hˆ electrons tot electrons Nuclear-moton Schrödnger equaton nucle electronc Schrödnger equaton The concept of potental energy hypersurfaces (of dmenson 3N-6): the energy of a molecule as a functon of ts geometry 6
7 Hˆ = The Hamltonan (spn-dependent terms not consdered) m Tˆnucle k k m Tˆ electrons e e Z r k k ˆ Ve n Coulombc potental k + < j ˆ e r Ve e j + k< l ˆ e Vn n Z r k kl Z l k,l nucle,j electrons Hˆ electrons = hˆ Ĥ electrons one electron + < j hˆ two electron Due to ths term analytcal soluton of SE s unkonwn Thus, the numercal soluton of the electronc Schrödnger equaton Hˆ electrons ( q ) = E( q ) electrons electrons through a favorte electronc-structure (quantum-chemcal, QC) method. QC methods are also devsed to optmze to the spatal confguraton of nucle to mnmze E - geometry optmzaton. 7
8 Hˆ electrons ( q ) = E( q ) electrons electrons Hˆ electrons = E f =1 hˆ one electron + hˆ, two electron j =, < j E 8
9 The Many Electron Wavefuncton A form for the electronc wavefuncton that satsfes the permutatonal antsymmetry (postulate VI) s the Slater determnant (SD) or a lnear combnaton of SDs. SD for two-electron system 3 SD = 1 ψ 1(1) α(1) ψ () α() 1 spatal component of one-electron wave functon (molecular orbtal, MO) ψ (1) α(1) = ψ () α() 1 spnorbtal χ (1) 1 χ () 1 χ (1) χ () spn component of one-electron wave functon SD for N-electron system SD 1 = N! χ (1) χ () χ ( N) χ (1) χ () χ ( N) χ (1) χ () N χ ( N) N N Symmetry and spn-adapted SD or lnear combnaton of SDs = confguraton state functon (CSF) ˆ S Sˆ z CSF CSF = S( S = M s + 1) CSF CSF & Hˆ electrons CSF = E CSF 9
10 Molecular orbtals, as a buldng elements n SD or CSF, are constructed from atomc orbtals: Bass set ψ = N j = 1 c a ϕa (Lnear combnaton of atomc orbtals, LCAO) Hydrogen-lke (one-electron) AOs are always of the form: ( θ, ϑ) R( r) Y ( θ ϑ) ϕ r =, where R(r) s the radal component that decays exponentally, lm wth ncreasng dstance from the nucleus e -ζr 10
11 The Many Electron Wavefuncton Snce t s mpossble to obtan analytc solutons n systems wth two or more electrons, the exponental behavor of the AOs Slater-type orbtals (STOs) were hence the frst to be used. They are characterzed by an exponental factor n the radal part. ϕ α ( r θ, ϑ) = P( r) e r Y ( θ, ϑ), lm STO Drawback: dffcultes assocate wth evaluatng ntegrals that appear n the soluton of electronc SE. contracted bass functon Lnear combnaton of several GTOs CGTO ϕ p = bapϕa a GTO prmtve Correcton ϕ α ( r, θ, ϑ) = P( r) e r Y ( θ, ϑ) or ϕ( α, l, m, n; x, y, z) = Ne lm αr (Gauss-type orbtal GTO) Drawback: qualtatvely ncorrect behavor at the nucleus and n the asymptotc lmt x l y m z n Segmented contracton scheme: each GTO contrbutes to exactly one CGTO General contracton scheme: each GTO can contrbute to more than one CGTO 11
12 double-, trple-, quadruple n-tuple zeta bass sets DZ, TZ, QZ More STO/GTO/CGTO functons descrbng one AO Balanced bass set - More art than scence Mnmal bass set - (one STO or GTO or CGTO for one core / valence AO) DZ DZP TZ TZP TZPD QZVPD Infnte bass set (deal but not realstc) } Not very flexble Dfferent types of STO/GTO/CGTO functons, e.g., polarzaton functons (P): e.g., for H atom add p functons for Fe atom add f functons dffuse functons (D) (wth small α n exp(-αr ) allowng to descrbe electron densty at larger dstances from nucleus. sutable for anons, soft, large molecules, Rydberg states.. - N electrons n MO t requres AOs orbtals Effectve core potental: f the core electrons (MOs, AOs) are replaced wth an approxmate pseudopotental 1
13 General strateges for solvng the electronc SE ( q electrons ) guess Hˆ electrons = E ( q electrons ) [ optmzed E ] optmzed ε Optmze and obtan E through a varaton guess electrons guess [ ] = E[ ] guess Hˆ guess guess ε(c) opt = opt Hˆ opt electrons ε[ ( c0, c1,..., cp )] ε ( c0, c1,.., cp ) = 0 c opt opt Optmze and obtan E through a perturbaton (0) Hˆ λ = Hˆ + λ ˆ Let λ be a perturbatonal parameter 0 λ 1 We seek the soluton n the form: E ( ) V (0) (1) () ( λ) = + λ + λ +... (0) (1) () ( λ) = E + λe + λ E +... Then, solvng Ĥ ( λ) ( λ) = E( λ) ( λ) c 13
14 Famly of standard Wave-Functon Theores (WFT) General overvew Welcome to the ZOO Sem-emprcal methods (MNDO, AM1, PM3, etc.) Computatonal Cost Ab nto methods Multconfguratonal HF (MCSCF, CASSCF) perturbatonal herarchy (CASPT, CASPT3) exctaton herarchy (MR-CISD, MR-CCSD) Hartree Fock (HF-SCF) Correlaton Energy (usually <1% of the total energy) perturbatonal herarchy (MP, MP3, MP4, ) exctaton herarchy (CIS, CISD, CISDT, ) (CCS, CCSD, CCSDT,...) Full CI Two contrbutons to correlaton energy : statc and dynamc correlaton 14
15 Hartree Fock (HF-SCF) method the Gate to the realm of WFT Equaton from page 8: [ ] = hˆ one electron + hˆ, two electron, j < j E spnorbtals f 1 Slater determnant E = { χχ j hˆ two electron χχ j χχ j hˆ two electron χ j } 1 χ hˆ one electron χ + χ one-electron ntegrals Condton: χ χ = δ MOs LCAO, j (and E mnmzed trough varatonal approach) j j two-electron Coulomb ntegrals Fock equaton Fˆχ = ε χ = Fˆ two-electron exchange ntegrals 1 hˆ ψ Jˆ Kˆ ψ = one electron + Jˆ Kˆ Fock operator = Fockan Fock matrx n the bass of AOs Workng Roothaan equaton: orbtal energy of j-th MO AO-overlap matrx { F( c) ε S} c = 0 Vector of LCAO coeffcents for j th MO { F ( c) ε 1} c = 0 In fact, F depends on c: see next page thus, equatons has to be solved teratvely -> self-consstent feld 15
16 F Matrx element of the Fock matrx n the bass of AOs explct form (for the restrcted Hartree-Fock method) nucle 1 ϕ Tˆ el ϕq ϕ p Vˆ e n ϕq + Prs ϕ pϕr Vˆ e e ϕqϕs ϕ k pϕr Vˆ, e e ϕsϕq k r, s pq = p AOs h pq densty matrx = occuped c c r s Ths s what s optmzed teratvely to get E mnmzed Program flow: Compute and store all overlap, one-electron and two electron ntegrals Choose a molecular geometry Guess ntal densty matrx P (0) Choose a bass set Construct and solve Roothaan equaton Replace P (n-1) wth P n Construct P from occuped MOs HF converged pq yes ( h F ) AO 1 E HF = Pqp pq + pq Is new P (n) smlar to P (n-1)? for restrcted Hartree-Fock method no 16
17 Hartree Fock (HF-SCF) method Computatonal Remarks Computatonal bottleneck the evaluaton of two-electron (four-center) ntegrals ϕ ˆ pϕr Ve e ϕqϕs Approxmatons of such ntegrals through Cholesky decomposton (CD) or Resoluton of Identty (RI-JK). Restrcted (closed-shell / open-shell HF) unrestrcted HF spn-symmetry broken α α α α { F ε S } c = 0 β β β β { F ε S } c = 0 β α β β α ( c, c ); F ( c c ) β F, S = S( S + 1) RHF RHF S S( S + 1) UHF UHF Spn contamnaton 17
18 Hartree Fock (HF-SCF) method Physcal Remarks Each electron experences the Coulombc repulson of other electrons through ther averaged feld (a mean feld) (the lack of dynamcal correlaton see later) Exchange nteracton among electrons wth the same spn projecton (Ferm correlaton) through the antsymmetrc nature of the Slater determnant. One Slater determnant (SD) = one electronc confguraton ( exact wave functon better expressed as a lnear combnaton of many confguratons - SDs). Only the ground-state wavefuncton and ts energy s solved by HF SCF. (HF not for excted states and ther energes) 18
19 ψ ψ +4 ψ +3 Exact non-realstc soluton wth Full Confguraton Interacton (FCI) n the nfnte bass set Hˆ electrons FCI = E exact FCI = Φ FCI C k SD, k k Correlaton energy: E corr = E exact - E HF Slater determnant ψ + H ˆ = FCI (f = 1 electrons FCI E exact FCI FCI ) ψ +1 kφ k Hˆ electrons k C C Φ = l l l E exact ψ Slater-Condon rules many ntegrals = 0 MOs ψ 1 also Brlloun theorem: Φ a HF SCF Ĥelectrons Φ = 0 19
20 Exact non-realstc soluton wth Full Confguraton Interacton (FCI) n the nfnte bass set n/ number of determnants N n 1 3 n Number of SD s: Exact soluton of electronc Schrödnger equaton (For n electrons n n orbtals)
21 Statc versus dynamcal correlaton? dynamcal Short range effects that arses as r 1 0 Dynamcal correlaton s related to the Coulomb hole. Statc ( non-dynamcal ) from confguratonal near-degeneraces or from defcences n Hartree-Fock orbtals He He Interacton energy E / µe h Hartree -Fock Bass set: 4s3pd Wdmark ANO Dynamcal correlaton CCSD r He-He /Å e.g., wth Φ1 Φ = C 1Φ1 + CΦ C = C =
22 QC also devsed for excted states and ther energes Hartree Fock (HF-SCF) perturbatonal herarchy (MP, MP3, MP4, ) exctaton herarchy (CIS, CISD, CISDT, ) (CCS, CCSD, CCSDT,...) Full CI Sngle-reference post-hf approaches (a porton of dynamcal correlaton ncluded) Møller-Plesset perturbaton theory of n-th order (MPn) Ĥ E ( λ) ( λ) = E( λ) ( λ) MP: Truncated CI methods occ a a ab ab = c0 Φ HF + c Φ + cj Φj +... CIS Coupled-cluster methods (CC) e Tˆ vrt a H (0) (1) () ( λ) = + λ + λ +... (0) (1) () ( λ) = E + λe + λ E +... Truncaton of perturbaton to second-order = E (0 ( λ ) = Hˆ + λvˆ ˆ ) from HF-SCF < j a< b CCSD(T) popular and often used as a golden standard method for sngle-reference systems (T) trple exctatons added as a perturbaton occ 1 [ 1+ ( Tˆ + Tˆ +..)] + ( Tˆ + Tˆ ) + Φ HF Φ HF =..] T ˆ = Tˆ T 1 ˆ e Φ = (1 + Tˆ + Tˆ +..) Φ CCD: HF (0) k HF E MP HF + (0) ( k ) k E0 E0 HF χ Vˆ χ vrt CC CISD = spnorbtals from HF-SCF Tˆ e Φ HF Cluster operator Tˆ = Tˆ + Tˆ ˆ + T
23 Formal scalng behavor of some sngle-reference QC methods N the number of bass functons 3
24 Multconfguratonal HF MCSCF (CASSCF / RASSCF) (a porton of statc correlaton ncluded) MCSCF = k C Φ k k ( C c) MCSCF, Φ k s a CSF arsng from selected exctatons wthn the actve space f all possble exctatons are allowed wthn the actve space -> FCI on a lmted set of orbtals CASSCF more general approach RASSCF (actve space dvded nto subspaces RAS1, RAS, RAS3 wthn RAS1&3 selected exctatons, wthn RAS - FCI Weyl s formula S + 1 n + 1 n + 1 N CSF = n + 1 N / S N / + S + 1 n= number of e - n the actve space N = number of orbtals n the actve space S = molecular spn state Current computatonal lmt for CASSCF actve space ~ 18-n-18 Modern approaches allowng to extent the actve spaces Densty-matrx renormalzaton group technology larger actve spaces wthn DMRG-CASSCF (e.g., 30-n-30) Example for S= N CSF 10-n-8: n-10: n-11: n-13: n-14: n-15:
25 Note on the selecton of an actve space Sometmes trval, sometmes more dffcult, sometmes mpossble B. Roos Selecton cannot be automatzed and depends on the partcular system /problem Chemcal nsght s mportant ngredent In choosng a proper actve space 5
26 Mult-reference wavefuncton approaches (a porton of statc and dynamc correlaton ncluded) CASPT PT on top of CASSCF Popular for spectroscopy RASPT PT on top of RASSCF DMRG-CASPT PT on top of DMRG-CASSCF MRCI(SD) CISD on top of CASSCF MRCC(SD) CCSD on top of CASSCF } Hgly Emergng method for complex electronc structure chemcal transformatons accurate but computatonally extremely demandng Very small molecules 6
27 Densty Functonal Theory - DFT The total energy s represented as a functonal of densty: E The realm of DFT methods bult on two fundamental theorems: 1 st Hohenberg-Kohn theorem: shows that electron densty of an arbtrary molecular system (n an electroncally nondegenerate ground state) n the absence of external electromagnetc felds determnes unambguously statc external potental nd Hohenberg-Kohn theorem: proves that the correct ground state electron densty mnmzes the energy E[ρ] [ ρ] = V [ ρ] + T[ ρ] + V [ ρ] = ρ( r) v ( r) dr + T[ ρ] V [ ρ] ne ee v ext ( r) = nucle k = 1 Z 1 ext + k r R k ee nucleus-electron attracton energy knetc energy of (nteractng) electrons electron-electron nteracton energy 7
28 Kohn-Sham Densty Functonal Theory (KS-DFT) [ ρ] = ρ( r) v( r dr + T[ ρ] V [ ρ] E ) + ee Coulomb electron-electron nteracton E [ ρ] = ρ( r) v ( r dr + T [ ρ] + J[ ρ] + ( T[ ρ] T [ ρ] ) + ( V [ ρ] J[ ρ] ) ext ) s s ee Knetc energy of non-nteractng electrons ( r' ) ( r) 1 ρ r ρ r' dr' dr [ ρ] ρ( r) v ( r dr + T [ ρ] + J[ ρ] + E [ ρ] E ) = ext s xc Exchange-Correlaton (XC) Energy 8
29 Workng Kohn-Sham Equaton The dea of consderng the determnantal WF of N non-nteractng electrons n N orbtals, the T s [ρ] s exactly gven as: T s N [ ρ] = = 1 χ χ m e Kohn-Sham spnorbtal & fulfllng condton: ρ = N = 1 χ χ Real electron densty Then, one-electron KS equaton: m e + v eff LCAO ansatz ( r) χ ( r) = ε χ ( r) wth: ( r' ) v (Fock-lke equatons) = vext ( r) + ρ dr' vxc( r) r r' eff + Roothaan-lke equatons Restrcted / Unrestrcted Kohn-Sham equatons - as n HF ρ = ρ α + ρ β Alpha-omega n KS-DFT exact form unknown 9
30 Most common of exchange-correlaton potentals Local densty approxmaton most popular way to do electronc structure calculatons n sold state physcs Generalzed gradent approxmaton (GGA) xc potentals are functonals of electron densty and ts frst spatal dervatves ( gradentcorrected LDA functonals) Meta-GGA approxmaton extenson of GGA. xc potentals are functonals of electron densty, ts frst and second spatal dervatves and knetc energy densty Hybrd exchange functonals a porton of exact exchange from HF theory s ncorporated nto xc potentals. Usually, GGA hybrd and GGA approach are combned. PBE, BP86 TPSS. TPSSH, B3LYP, PBE0. Hybrd exchange and hybrd correlaton (double-hybrd) functonals - essentally extenson of hybrd-gga, whch uses MP correcton to replace part of the sem-local GGA correlaton. BPLYP 30
31 Lmtatons of standard KS DFT methods Lack of long-range correlaton (dsperson) emprcal correctons ~1/R 6 B3LYP+D3 Incorrect long-range exchange behavor e.g. ncorrect energes of charge-transfer exctatons (exchange should decay asymptotcally as r 1-1 ; B3LYP : 0.r 1-1 ) CAM-B3LYP Lack of statc correlaton energy Generally lower senstvty of DFT to multreference character s dependent on the amount of HF exchange ncluded n the functonal Self-nteracton error SIE nterpreted as the nteracton of an electron wth tself. Whle the dagonal exchange terms K cancel exactly self-nteracton Coulomb terms J n HF, t s not vald for standard KS-DFT methods. Lack of systematc mprovablty!!!!! 31
32 Some fnal notes on solvng SE through WFT and DFT methods For a gven geometry wavefuncton optmzaton -> electronc energy E (sngle-pont calculaton) On the other hand: QC methods can be also used to optmze geometry algorthms allowng to evaluate (frst, second) dervatves of E wth respect to the nuclear coordnates and to search crucal ponts on the potental energy surface Mnma & frst-order statonary ponts (transton states) (geometry optmzaton) Thus now, n prncple, you are able to read the followng sentence: GGA-type PBE functonal n combnaton wth RI-J approxmaton and the DZP bass set was used for the geometry optmzaton, whle CASPT(10-n-8) approach combned wth a larger bass set (e.g. TZVP) was employed for the fnal sngle-pont energes. 3
33 APPENDIX 33
34 Propertes as dervatves of the energy - Bonus Consder a molecule n an external electrc feld ε. E de dε d E dε 1 ( ε ) = E( ε = 0) + ε + ε + ε = 0 ε = 0 Dpole moment (µ) Polarzablty (α) Frst hyperpolarzablty (ß) µ = α = β = de dε d E dε 3 d E 3 dε ε = 0 ε = 0 ε = 0 34
35 α de dε α α d E dε dε β d E dx dε α β 3 d E dε dε dε de dx d E dx dx 3 d E dx dx dx α 3 d E dx dε dε γ 4 d E dx dx dx dx j j j k k β l dpole moment; n a smlar way also multpole moments, electrc feld gradents, etc. polarzablty (frst) hyperpolarzablty forces on nucle harmonc force constants; harmonc vbratonal frequences cubc force constants; anharmonc correctons to dstances and rotatonal constants quartc force constants; anharmonc correctons to vbratonal frequences dpole dervatves; nfrared ntenstes polarzablty dervatves; Raman ntenstes 35
36 d E db db α d E di db α d E di di α d E db dj α d di α d ds de ds α E dj α E ds β β j β β β β magnetzablty nuclear magnetc sheldng tensor; relatve NMR shfts ndrect spn-spn couplng constants rotatonal g-tensor; rotatonal spectra n magnetc feld nuclear spn-rotaton tensor; fne structure n rotatonal spectra spn densty; hyperfne nteracton constants electronc g-tensor and many more... 36
37 Restrcted Hartree Fock (RHF) results for LF Hartree Fock Error r e / Å % ω e / cm % µ e / D % µ e / DÅ % D e / kj mol % For LF, the Hartree- Fock method s qute useful for calculatons around the equlbrum, although the bndng energy s too low by 6%. But the RHF model dssocates ncorrectly nto L + and F. E/E h D e LF ground state Comparson of restrcted Hartree-Fock results wth full CI Bass set: Ahlrchs pvdz r L-F /Å RHF(L + + F ) ROHF(L + F) FCI(L + F) 37
38 Post-Hartree-Fock for qualtatve or quanttatve reasons E/E h LF molecule State-averaged-CASSCF+nternallycontracted-MRCI results Bass set: Ahlrchs pvdz Why do we want to go beyond the Hartree-Fock descrpton? Frst, we may wsh to mprove the accuracy of the computed energy and other propertes. snglet excted state snglet ground state L + + F L + F Second, we are dealng wth a stuaton where the Hartree- Fock model s a very poor zeroth-order approxmaton of the wavefuncton. Near-degeneracy effects Ground-state equlbrum propertes r L-F /Å 38
39 Near-degeneracy problems of perturbaton theory LF ground state Closed-shell coupled-cluster RHF/CCSD(T) results Bass set: Ahlrchs pvdz MP E/E h MP4 The CCSD(T) trples correcton becomes very negatve for dstances > 3 Å. FCI The CCSD(T) method breaks down. The MP4(SDTQ) energy also shows ths behavor, but only at a much longer dstance (> 6 Å). B-CCD(T) r L-F /Å CCSD(T) 39
40 Multreference perturbaton theory appled to LF E/E h LF ground state (,)CASPT results (MOLPRO, g=4) Bass set: Ahlrchs pvdz RHF non-dynamcal correlaton (,)CASSCF dynamcal correlaton (,)CASPT FCI r L-F /Å 40
41 Unrestrcted UCCSD(T) coupled-cluster calculatons of the LF ground state The UCCSD(T) results compare favorably wth the full CI potental energy curve. The expectaton value <S > s zero for the (unprojected) UHF wavefuncton at dstances < 3 Å, but <S > 1.0 at larger dstances (> 3 Å). E/E h LF ground state Unrestrcted coupled-cluster UCCSD(T) results Bass set: Ahlrchs pvdz RHF In ths example, the spncontamnaton represents no real problem for the ground state energy. However, spn-contamnaton may make the UHF-based methods unsutable for the study of a varety of molecular propertes. UCCSD(T) FCI UHF r L-F /Å ROHF(L + F) 41
Introduction to Density Functional Theory. Jeremie Zaffran 2 nd year-msc. (Nanochemistry)
 Introducton to Densty Functonal Theory Jereme Zaffran nd year-msc. (anochemstry) A- Hartree appromatons Born- Oppenhemer appromaton H H H e The goal of computatonal chemstry H e??? Let s remnd H e T e
Introducton to Densty Functonal Theory Jereme Zaffran nd year-msc. (anochemstry) A- Hartree appromatons Born- Oppenhemer appromaton H H H e The goal of computatonal chemstry H e??? Let s remnd H e T e
Electronic Structure for Excited States (multiconfigurational methods) Spiridoula Matsika
 Electronc Structure for Excted States (multconfguratonal methods) Sprdoula Matska Excted Electronc States Theoretcal treatment of excted states s needed for: UV/Vs electronc spectroscopy Photochemstry
Electronc Structure for Excted States (multconfguratonal methods) Sprdoula Matska Excted Electronc States Theoretcal treatment of excted states s needed for: UV/Vs electronc spectroscopy Photochemstry
5. Response properties in ab initio schemes
 5. Response propertes n ab nto schemes A number of mportant physcal observables s expressed va dervatves of total energy (or free energy) E. Examples are: E R 2 E R a R b forces on the nucle; crtcal ponts
5. Response propertes n ab nto schemes A number of mportant physcal observables s expressed va dervatves of total energy (or free energy) E. Examples are: E R 2 E R a R b forces on the nucle; crtcal ponts
Probabilistic method to determine electron correlation energy
 Probablstc method to determne electron elaton energy T.R.S. Prasanna Department of Metallurgcal Engneerng and Materals Scence Indan Insttute of Technology, Bombay Mumba 400076 Inda A new method to determne
Probablstc method to determne electron elaton energy T.R.S. Prasanna Department of Metallurgcal Engneerng and Materals Scence Indan Insttute of Technology, Bombay Mumba 400076 Inda A new method to determne
where the sums are over the partcle labels. In general H = p2 2m + V s(r ) V j = V nt (jr, r j j) (5) where V s s the sngle-partcle potental and V nt
 Physcs 543 Quantum Mechancs II Fall 998 Hartree-Fock and the Self-consstent Feld Varatonal Methods In the dscusson of statonary perturbaton theory, I mentoned brey the dea of varatonal approxmaton schemes.
Physcs 543 Quantum Mechancs II Fall 998 Hartree-Fock and the Self-consstent Feld Varatonal Methods In the dscusson of statonary perturbaton theory, I mentoned brey the dea of varatonal approxmaton schemes.
The Feynman path integral
 The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
5.03, Inorganic Chemistry Prof. Daniel G. Nocera Lecture 2 May 11: Ligand Field Theory
 5.03, Inorganc Chemstry Prof. Danel G. Nocera Lecture May : Lgand Feld Theory The lgand feld problem s defned by the followng Hamltonan, h p Η = wth E n = KE = where = m m x y z h m Ze r hydrogen atom
5.03, Inorganc Chemstry Prof. Danel G. Nocera Lecture May : Lgand Feld Theory The lgand feld problem s defned by the followng Hamltonan, h p Η = wth E n = KE = where = m m x y z h m Ze r hydrogen atom
CI/CEPA. Introduction CI Size Consistency Derivation CEPA EPV Results Remarks
 CI/CEPA Introducton CI Sze Consstency Dervaton CEPA EPV Results Remarks 1 HY=EY H e Ψ e = E e Ψe Expanson of the Many-Electron Wave Functon Ψ HF Ψ e = Φ o + a a C Φ + ab ab C j Φ j +... Φo = A φ 1 φ 2...
CI/CEPA Introducton CI Sze Consstency Dervaton CEPA EPV Results Remarks 1 HY=EY H e Ψ e = E e Ψe Expanson of the Many-Electron Wave Functon Ψ HF Ψ e = Φ o + a a C Φ + ab ab C j Φ j +... Φo = A φ 1 φ 2...
ψ ij has the eigenvalue
 Moller Plesset Perturbaton Theory In Moller-Plesset (MP) perturbaton theory one taes the unperturbed Hamltonan for an atom or molecule as the sum of the one partcle Foc operators H F() where the egenfunctons
Moller Plesset Perturbaton Theory In Moller-Plesset (MP) perturbaton theory one taes the unperturbed Hamltonan for an atom or molecule as the sum of the one partcle Foc operators H F() where the egenfunctons
Advanced Quantum Mechanics
 Advanced Quantum Mechancs Rajdeep Sensarma! sensarma@theory.tfr.res.n ecture #9 QM of Relatvstc Partcles Recap of ast Class Scalar Felds and orentz nvarant actons Complex Scalar Feld and Charge conjugaton
Advanced Quantum Mechancs Rajdeep Sensarma! sensarma@theory.tfr.res.n ecture #9 QM of Relatvstc Partcles Recap of ast Class Scalar Felds and orentz nvarant actons Complex Scalar Feld and Charge conjugaton
Robert Eisberg Second edition CH 09 Multielectron atoms ground states and x-ray excitations
 Quantum Physcs 量 理 Robert Esberg Second edton CH 09 Multelectron atoms ground states and x-ray exctatons 9-01 By gong through the procedure ndcated n the text, develop the tme-ndependent Schroednger equaton
Quantum Physcs 量 理 Robert Esberg Second edton CH 09 Multelectron atoms ground states and x-ray exctatons 9-01 By gong through the procedure ndcated n the text, develop the tme-ndependent Schroednger equaton
Multi-electron atoms (11) 2010 update Extend the H-atom picture to more than 1 electron: H-atom sol'n use for N-elect., assume product wavefct.
 Mult-electron atoms (11) 2010 update Extend the H-atom pcture to more than 1 electron: VII 33 H-atom sol'n use for -elect., assume product wavefct. n ψ = φn l m where: ψ mult electron w/fct φ n l m one
Mult-electron atoms (11) 2010 update Extend the H-atom pcture to more than 1 electron: VII 33 H-atom sol'n use for -elect., assume product wavefct. n ψ = φn l m where: ψ mult electron w/fct φ n l m one
24. Atomic Spectra, Term Symbols and Hund s Rules
 Page of 4. Atomc Spectra, Term Symbols and Hund s Rules Date: 5 October 00 Suggested Readng: Chapters 8-8 to 8- of the text. Introducton Electron confguratons, at least n the forms used n general chemstry
Page of 4. Atomc Spectra, Term Symbols and Hund s Rules Date: 5 October 00 Suggested Readng: Chapters 8-8 to 8- of the text. Introducton Electron confguratons, at least n the forms used n general chemstry
Intermolecular force fields and how they can be determined
 Intermolecular force felds and how they can be determned Ad van der Avord Unversty of Njmegen Han-sur-Lesse, December 23 p.1 Equaton of state (Van der Waals) of non-deal gas ( p + a )( ) V 2 V b = kt repulson
Intermolecular force felds and how they can be determned Ad van der Avord Unversty of Njmegen Han-sur-Lesse, December 23 p.1 Equaton of state (Van der Waals) of non-deal gas ( p + a )( ) V 2 V b = kt repulson
Some Comments on Accelerating Convergence of Iterative Sequences Using Direct Inversion of the Iterative Subspace (DIIS)
 Some Comments on Acceleratng Convergence of Iteratve Sequences Usng Drect Inverson of the Iteratve Subspace (DIIS) C. Davd Sherrll School of Chemstry and Bochemstry Georga Insttute of Technology May 1998
Some Comments on Acceleratng Convergence of Iteratve Sequences Usng Drect Inverson of the Iteratve Subspace (DIIS) C. Davd Sherrll School of Chemstry and Bochemstry Georga Insttute of Technology May 1998
Molecular Dynamics and Density Functional Theory
 Molecular Dynamcs and Densty Functonal Theory What do we need? An account n pemfc cluster: Host name: pemfc.chem.sfu.ca I wll take care of that. Ths can be usually a common account for all of you but please
Molecular Dynamcs and Densty Functonal Theory What do we need? An account n pemfc cluster: Host name: pemfc.chem.sfu.ca I wll take care of that. Ths can be usually a common account for all of you but please
Lecture 14: Forces and Stresses
 The Nuts and Bolts of Frst-Prncples Smulaton Lecture 14: Forces and Stresses Durham, 6th-13th December 2001 CASTEP Developers Group wth support from the ESF ψ k Network Overvew of Lecture Why bother? Theoretcal
The Nuts and Bolts of Frst-Prncples Smulaton Lecture 14: Forces and Stresses Durham, 6th-13th December 2001 CASTEP Developers Group wth support from the ESF ψ k Network Overvew of Lecture Why bother? Theoretcal
Complex Atoms; The Exclusion Principle and the Periodic System
 Complex Atoms; The Excluson Prncple and the Perodc System In order to understand the electron dstrbutons n atoms, another prncple s needed. Ths s the Paul excluson prncple: No two electrons n an atom can
Complex Atoms; The Excluson Prncple and the Perodc System In order to understand the electron dstrbutons n atoms, another prncple s needed. Ths s the Paul excluson prncple: No two electrons n an atom can
5.76 Lecture #5 2/07/94 Page 1 of 10 pages. Lecture #5: Atoms: 1e and Alkali. centrifugal term ( +1)
 5.76 Lecture #5 /07/94 Page 1 of 10 pages 1e Atoms: H, H + e, L +, etc. coupled and uncoupled bass sets Lecture #5: Atoms: 1e and Alkal centrfugal term (+1) r radal Schrödnger Equaton spn-orbt l s r 3
5.76 Lecture #5 /07/94 Page 1 of 10 pages 1e Atoms: H, H + e, L +, etc. coupled and uncoupled bass sets Lecture #5: Atoms: 1e and Alkal centrfugal term (+1) r radal Schrödnger Equaton spn-orbt l s r 3
This chapter illustrates the idea that all properties of the homogeneous electron gas (HEG) can be calculated from electron density.
 1 Unform Electron Gas Ths chapter llustrates the dea that all propertes of the homogeneous electron gas (HEG) can be calculated from electron densty. Intutve Representaton of Densty Electron densty n s
1 Unform Electron Gas Ths chapter llustrates the dea that all propertes of the homogeneous electron gas (HEG) can be calculated from electron densty. Intutve Representaton of Densty Electron densty n s
SUPPLEMENTARY INFORMATION
 do: 0.08/nature09 I. Resonant absorpton of XUV pulses n Kr + usng the reduced densty matrx approach The quantum beats nvestgated n ths paper are the result of nterference between two exctaton paths of
do: 0.08/nature09 I. Resonant absorpton of XUV pulses n Kr + usng the reduced densty matrx approach The quantum beats nvestgated n ths paper are the result of nterference between two exctaton paths of
Chapter 7. Ab initio Theory
 Chapter 7. Ab nto Theory Ab nto: from the frst prnples. I. Roothaan-Hall approah Assumng Born-Oppenhemer approxmaton, the eletron Hamltonan: Hˆ Tˆ e + V en + V ee Z r a a a + > r Wavefunton Slater determnant:
Chapter 7. Ab nto Theory Ab nto: from the frst prnples. I. Roothaan-Hall approah Assumng Born-Oppenhemer approxmaton, the eletron Hamltonan: Hˆ Tˆ e + V en + V ee Z r a a a + > r Wavefunton Slater determnant:
Physics 181. Particle Systems
 Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
5.04, Principles of Inorganic Chemistry II MIT Department of Chemistry Lecture 32: Vibrational Spectroscopy and the IR
 5.0, Prncples of Inorganc Chemstry II MIT Department of Chemstry Lecture 3: Vbratonal Spectroscopy and the IR Vbratonal spectroscopy s confned to the 00-5000 cm - spectral regon. The absorpton of a photon
5.0, Prncples of Inorganc Chemstry II MIT Department of Chemstry Lecture 3: Vbratonal Spectroscopy and the IR Vbratonal spectroscopy s confned to the 00-5000 cm - spectral regon. The absorpton of a photon
A how to guide to second quantization method.
 Phys. 67 (Graduate Quantum Mechancs Sprng 2009 Prof. Pu K. Lam. Verson 3 (4/3/2009 A how to gude to second quantzaton method. -> Second quantzaton s a mathematcal notaton desgned to handle dentcal partcle
Phys. 67 (Graduate Quantum Mechancs Sprng 2009 Prof. Pu K. Lam. Verson 3 (4/3/2009 A how to gude to second quantzaton method. -> Second quantzaton s a mathematcal notaton desgned to handle dentcal partcle
Note on the Electron EDM
 Note on the Electron EDM W R Johnson October 25, 2002 Abstract Ths s a note on the setup of an electron EDM calculaton and Schff s Theorem 1 Basc Relatons The well-known relatvstc nteracton of the electron
Note on the Electron EDM W R Johnson October 25, 2002 Abstract Ths s a note on the setup of an electron EDM calculaton and Schff s Theorem 1 Basc Relatons The well-known relatvstc nteracton of the electron
Multiscale Modeling UCLA Prof. N. Ghoniem
 Interdscplnary graduate course Fall 003 Multscale Modelng UCLA Prof. N. Ghonem Introducton n Electronc Structure Calculatons Ncholas Kousss Department of Physcs Calforna State Unversty Northrdge The Fundamental
Interdscplnary graduate course Fall 003 Multscale Modelng UCLA Prof. N. Ghonem Introducton n Electronc Structure Calculatons Ncholas Kousss Department of Physcs Calforna State Unversty Northrdge The Fundamental
Computational Chemistry I
 Computational Chemistry I Text book Cramer: Essentials of Quantum Chemistry, Wiley (2 ed.) Chapter 3. Post Hartree-Fock methods (Cramer: chapter 7) There are many ways to improve the HF method. Most of
Computational Chemistry I Text book Cramer: Essentials of Quantum Chemistry, Wiley (2 ed.) Chapter 3. Post Hartree-Fock methods (Cramer: chapter 7) There are many ways to improve the HF method. Most of
Integrals and Invariants of Euler-Lagrange Equations
 Lecture 16 Integrals and Invarants of Euler-Lagrange Equatons ME 256 at the Indan Insttute of Scence, Bengaluru Varatonal Methods and Structural Optmzaton G. K. Ananthasuresh Professor, Mechancal Engneerng,
Lecture 16 Integrals and Invarants of Euler-Lagrange Equatons ME 256 at the Indan Insttute of Scence, Bengaluru Varatonal Methods and Structural Optmzaton G. K. Ananthasuresh Professor, Mechancal Engneerng,
The GW approximation in 90 minutes or so. F. Bruneval Service de Recherches de Métallurgie Physique CEA, DEN
 The GW approxmaton n 90 mnutes or so Servce de Recherches de Métallurge Physque CEA, DEN DFT tutoral, Lyon december 2012 Outlne I. Standard DFT suffers from the band gap problem II. Introducton of the
The GW approxmaton n 90 mnutes or so Servce de Recherches de Métallurge Physque CEA, DEN DFT tutoral, Lyon december 2012 Outlne I. Standard DFT suffers from the band gap problem II. Introducton of the
Solutions to Problems Fundamentals of Condensed Matter Physics
 Solutons to Problems Fundamentals of Condensed Matter Physcs Marvn L. Cohen Unversty of Calforna, Berkeley Steven G. Loue Unversty of Calforna, Berkeley c Cambrdge Unversty Press 016 1 Acknowledgement
Solutons to Problems Fundamentals of Condensed Matter Physcs Marvn L. Cohen Unversty of Calforna, Berkeley Steven G. Loue Unversty of Calforna, Berkeley c Cambrdge Unversty Press 016 1 Acknowledgement
Electron-Impact Double Ionization of the H 2
 I R A P 6(), Dec. 5, pp. 9- Electron-Impact Double Ionzaton of the H olecule Internatonal Scence Press ISSN: 9-59 Electron-Impact Double Ionzaton of the H olecule. S. PINDZOLA AND J. COLGAN Department
I R A P 6(), Dec. 5, pp. 9- Electron-Impact Double Ionzaton of the H olecule Internatonal Scence Press ISSN: 9-59 Electron-Impact Double Ionzaton of the H olecule. S. PINDZOLA AND J. COLGAN Department
Inductance Calculation for Conductors of Arbitrary Shape
 CRYO/02/028 Aprl 5, 2002 Inductance Calculaton for Conductors of Arbtrary Shape L. Bottura Dstrbuton: Internal Summary In ths note we descrbe a method for the numercal calculaton of nductances among conductors
CRYO/02/028 Aprl 5, 2002 Inductance Calculaton for Conductors of Arbtrary Shape L. Bottura Dstrbuton: Internal Summary In ths note we descrbe a method for the numercal calculaton of nductances among conductors
The Born-Oppenheimer Approximation
 5.76 Lecture otes /16/94 Page 1 The Born-Oppenhemer Approxmaton For atoms we use SCF to defne 1e eff orbtals. Get V for each e n feld of e s n all other occuped () r orbtals. ψ ( r) = φ 1( r1) φ( r ) sngle
5.76 Lecture otes /16/94 Page 1 The Born-Oppenhemer Approxmaton For atoms we use SCF to defne 1e eff orbtals. Get V for each e n feld of e s n all other occuped () r orbtals. ψ ( r) = φ 1( r1) φ( r ) sngle
12. The Hamilton-Jacobi Equation Michael Fowler
 1. The Hamlton-Jacob Equaton Mchael Fowler Back to Confguraton Space We ve establshed that the acton, regarded as a functon of ts coordnate endponts and tme, satsfes ( ) ( ) S q, t / t+ H qpt,, = 0, and
1. The Hamlton-Jacob Equaton Mchael Fowler Back to Confguraton Space We ve establshed that the acton, regarded as a functon of ts coordnate endponts and tme, satsfes ( ) ( ) S q, t / t+ H qpt,, = 0, and
Rate of Absorption and Stimulated Emission
 MIT Department of Chemstry 5.74, Sprng 005: Introductory Quantum Mechancs II Instructor: Professor Andre Tokmakoff p. 81 Rate of Absorpton and Stmulated Emsson The rate of absorpton nduced by the feld
MIT Department of Chemstry 5.74, Sprng 005: Introductory Quantum Mechancs II Instructor: Professor Andre Tokmakoff p. 81 Rate of Absorpton and Stmulated Emsson The rate of absorpton nduced by the feld
Non-interacting Spin-1/2 Particles in Non-commuting External Magnetic Fields
 EJTP 6, No. 0 009) 43 56 Electronc Journal of Theoretcal Physcs Non-nteractng Spn-1/ Partcles n Non-commutng External Magnetc Felds Kunle Adegoke Physcs Department, Obafem Awolowo Unversty, Ile-Ife, Ngera
EJTP 6, No. 0 009) 43 56 Electronc Journal of Theoretcal Physcs Non-nteractng Spn-1/ Partcles n Non-commutng External Magnetc Felds Kunle Adegoke Physcs Department, Obafem Awolowo Unversty, Ile-Ife, Ngera
A particle in a state of uniform motion remain in that state of motion unless acted upon by external force.
 The fundamental prncples of classcal mechancs were lad down by Galleo and Newton n the 16th and 17th centures. In 1686, Newton wrote the Prncpa where he gave us three laws of moton, one law of gravty,
The fundamental prncples of classcal mechancs were lad down by Galleo and Newton n the 16th and 17th centures. In 1686, Newton wrote the Prncpa where he gave us three laws of moton, one law of gravty,
THEOREMS OF QUANTUM MECHANICS
 THEOREMS OF QUANTUM MECHANICS In order to develop methods to treat many-electron systems (atoms & molecules), many of the theorems of quantum mechancs are useful. Useful Notaton The matrx element A mn
THEOREMS OF QUANTUM MECHANICS In order to develop methods to treat many-electron systems (atoms & molecules), many of the theorems of quantum mechancs are useful. Useful Notaton The matrx element A mn
Molecular magnetic properties
 Molecular magnetc propertes Trygve Helgaker Centre for Theoretcal and Computatonal Chemstry Department of Chemstry, Unversty of Oslo, Norway European Summer School n Quantum Chemstry (ESQC) 2011 Torre
Molecular magnetc propertes Trygve Helgaker Centre for Theoretcal and Computatonal Chemstry Department of Chemstry, Unversty of Oslo, Norway European Summer School n Quantum Chemstry (ESQC) 2011 Torre
Electron Correlation - Methods beyond Hartree-Fock
 Electron Correlation - Methods beyond Hartree-Fock how to approach chemical accuracy Alexander A. Auer Max-Planck-Institute for Chemical Energy Conversion, Mülheim September 4, 2014 MMER Summerschool 2014
Electron Correlation - Methods beyond Hartree-Fock how to approach chemical accuracy Alexander A. Auer Max-Planck-Institute for Chemical Energy Conversion, Mülheim September 4, 2014 MMER Summerschool 2014
Physics 5153 Classical Mechanics. D Alembert s Principle and The Lagrangian-1
 P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
Week 9 Chapter 10 Section 1-5
 Week 9 Chapter 10 Secton 1-5 Rotaton Rgd Object A rgd object s one that s nondeformable The relatve locatons of all partcles makng up the object reman constant All real objects are deformable to some extent,
Week 9 Chapter 10 Secton 1-5 Rotaton Rgd Object A rgd object s one that s nondeformable The relatve locatons of all partcles makng up the object reman constant All real objects are deformable to some extent,
Mathematical Preparations
 1 Introducton Mathematcal Preparatons The theory of relatvty was developed to explan experments whch studed the propagaton of electromagnetc radaton n movng coordnate systems. Wthn expermental error the
1 Introducton Mathematcal Preparatons The theory of relatvty was developed to explan experments whch studed the propagaton of electromagnetc radaton n movng coordnate systems. Wthn expermental error the
8. Superfluid to Mott-insulator transition
 8. Superflud to Mott-nsulator transton Overvew Optcal lattce potentals Soluton of the Schrödnger equaton for perodc potentals Band structure Bloch oscllaton of bosonc and fermonc atoms n optcal lattces
8. Superflud to Mott-nsulator transton Overvew Optcal lattce potentals Soluton of the Schrödnger equaton for perodc potentals Band structure Bloch oscllaton of bosonc and fermonc atoms n optcal lattces
International Journal of Pure and Applied Sciences and Technology
 Int. J. Pure Appl. Sc. Technol., 4() (03), pp. 5-30 Internatonal Journal of Pure and Appled Scences and Technology ISSN 9-607 Avalable onlne at www.jopaasat.n Research Paper Schrödnger State Space Matrx
Int. J. Pure Appl. Sc. Technol., 4() (03), pp. 5-30 Internatonal Journal of Pure and Appled Scences and Technology ISSN 9-607 Avalable onlne at www.jopaasat.n Research Paper Schrödnger State Space Matrx
Supplemental document
 Electronc Supplementary Materal (ESI) for Physcal Chemstry Chemcal Physcs. Ths journal s the Owner Socetes 01 Supplemental document Behnam Nkoobakht School of Chemstry, The Unversty of Sydney, Sydney,
Electronc Supplementary Materal (ESI) for Physcal Chemstry Chemcal Physcs. Ths journal s the Owner Socetes 01 Supplemental document Behnam Nkoobakht School of Chemstry, The Unversty of Sydney, Sydney,
Lecture 20: Noether s Theorem
 Lecture 20: Noether s Theorem In our revew of Newtonan Mechancs, we were remnded that some quanttes (energy, lnear momentum, and angular momentum) are conserved That s, they are constant f no external
Lecture 20: Noether s Theorem In our revew of Newtonan Mechancs, we were remnded that some quanttes (energy, lnear momentum, and angular momentum) are conserved That s, they are constant f no external
Tensor Analysis. For orthogonal curvilinear coordinates, ˆ ˆ (98) Expanding the derivative, we have, ˆ. h q. . h q h q
 For orthogonal curvlnear coordnates, eˆ grad a a= ( aˆ ˆ e). h q (98) Expandng the dervatve, we have, eˆ aˆ ˆ e a= ˆ ˆ a h e + q q 1 aˆ ˆ ˆ a e = ee ˆˆ ˆ + e. h q h q Now expandng eˆ / q (some of the detals
For orthogonal curvlnear coordnates, eˆ grad a a= ( aˆ ˆ e). h q (98) Expandng the dervatve, we have, eˆ aˆ ˆ e a= ˆ ˆ a h e + q q 1 aˆ ˆ ˆ a e = ee ˆˆ ˆ + e. h q h q Now expandng eˆ / q (some of the detals
Canonical transformations
 Canoncal transformatons November 23, 2014 Recall that we have defned a symplectc transformaton to be any lnear transformaton M A B leavng the symplectc form nvarant, Ω AB M A CM B DΩ CD Coordnate transformatons,
Canoncal transformatons November 23, 2014 Recall that we have defned a symplectc transformaton to be any lnear transformaton M A B leavng the symplectc form nvarant, Ω AB M A CM B DΩ CD Coordnate transformatons,
CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE
 CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE Analytcal soluton s usually not possble when exctaton vares arbtrarly wth tme or f the system s nonlnear. Such problems can be solved by numercal tmesteppng
CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE Analytcal soluton s usually not possble when exctaton vares arbtrarly wth tme or f the system s nonlnear. Such problems can be solved by numercal tmesteppng
Introduction to Vapor/Liquid Equilibrium, part 2. Raoult s Law:
 CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
Lecture 4. Macrostates and Microstates (Ch. 2 )
 Lecture 4. Macrostates and Mcrostates (Ch. ) The past three lectures: we have learned about thermal energy, how t s stored at the mcroscopc level, and how t can be transferred from one system to another.
Lecture 4. Macrostates and Mcrostates (Ch. ) The past three lectures: we have learned about thermal energy, how t s stored at the mcroscopc level, and how t can be transferred from one system to another.
Session 7. Spectroscopy and Thermochemistry. Session 7 Overview: Part A I. Prediction of Vibrational Frequencies (IR)
 Sesson 7 Spectroscopy and Thermochemstry CCCE 2008 1 Sesson 7 Overvew: Part A I. Predcton of Vbratonal Frequences (IR) II. Thermochemstry Part B III. Predcton of Electronc Transtons (UV-Vs) IV. NMR Predctons
Sesson 7 Spectroscopy and Thermochemstry CCCE 2008 1 Sesson 7 Overvew: Part A I. Predcton of Vbratonal Frequences (IR) II. Thermochemstry Part B III. Predcton of Electronc Transtons (UV-Vs) IV. NMR Predctons
) is the unite step-function, which signifies that the second term of the right-hand side of the
 Casmr nteracton of excted meda n electromagnetc felds Yury Sherkunov Introducton The long-range electrc dpole nteracton between an excted atom and a ground-state atom s consdered n ref. [1,] wth the help
Casmr nteracton of excted meda n electromagnetc felds Yury Sherkunov Introducton The long-range electrc dpole nteracton between an excted atom and a ground-state atom s consdered n ref. [1,] wth the help
Lecture 6/7 (February 10/12, 2014) DIRAC EQUATION. The non-relativistic Schrödinger equation was obtained by noting that the Hamiltonian 2
 P470 Lecture 6/7 (February 10/1, 014) DIRAC EQUATION The non-relatvstc Schrödnger equaton was obtaned by notng that the Hamltonan H = P (1) m can be transformed nto an operator form wth the substtutons
P470 Lecture 6/7 (February 10/1, 014) DIRAC EQUATION The non-relatvstc Schrödnger equaton was obtaned by notng that the Hamltonan H = P (1) m can be transformed nto an operator form wth the substtutons
XII. The Born-Oppenheimer Approximation
 X. The Born-Oppenhemer Approxmaton The Born- Oppenhemer (BO) approxmaton s probably the most fundamental approxmaton n chemstry. From a practcal pont of vew t wll allow us to treat the ectronc structure
X. The Born-Oppenhemer Approxmaton The Born- Oppenhemer (BO) approxmaton s probably the most fundamental approxmaton n chemstry. From a practcal pont of vew t wll allow us to treat the ectronc structure
Frequency dependence of the permittivity
 Frequency dependence of the permttvty February 7, 016 In materals, the delectrc constant and permeablty are actually frequency dependent. Ths does not affect our results for sngle frequency modes, but
Frequency dependence of the permttvty February 7, 016 In materals, the delectrc constant and permeablty are actually frequency dependent. Ths does not affect our results for sngle frequency modes, but
Density matrix. c α (t)φ α (q)
 Densty matrx Note: ths s supplementary materal. I strongly recommend that you read t for your own nterest. I beleve t wll help wth understandng the quantum ensembles, but t s not necessary to know t n
Densty matrx Note: ths s supplementary materal. I strongly recommend that you read t for your own nterest. I beleve t wll help wth understandng the quantum ensembles, but t s not necessary to know t n
Computational Methods. Chem 561
 Computational Methods Chem 561 Lecture Outline 1. Ab initio methods a) HF SCF b) Post-HF methods 2. Density Functional Theory 3. Semiempirical methods 4. Molecular Mechanics Computational Chemistry " Computational
Computational Methods Chem 561 Lecture Outline 1. Ab initio methods a) HF SCF b) Post-HF methods 2. Density Functional Theory 3. Semiempirical methods 4. Molecular Mechanics Computational Chemistry " Computational
Department of Chemistry Purdue University Garth J. Simpson
 Objectves: 1. Develop a smple conceptual 1D model for NLO effects. Extend to 3D and relate to computatonal chemcal calculatons of adabatc NLO polarzabltes. 2. Introduce Sum-Over-States (SOS) approaches
Objectves: 1. Develop a smple conceptual 1D model for NLO effects. Extend to 3D and relate to computatonal chemcal calculatons of adabatc NLO polarzabltes. 2. Introduce Sum-Over-States (SOS) approaches
Quantum Mechanics I Problem set No.1
 Quantum Mechancs I Problem set No.1 Septembe0, 2017 1 The Least Acton Prncple The acton reads S = d t L(q, q) (1) accordng to the least (extremal) acton prncple, the varaton of acton s zero 0 = δs = t
Quantum Mechancs I Problem set No.1 Septembe0, 2017 1 The Least Acton Prncple The acton reads S = d t L(q, q) (1) accordng to the least (extremal) acton prncple, the varaton of acton s zero 0 = δs = t
The non-negativity of probabilities and the collapse of state
 The non-negatvty of probabltes and the collapse of state Slobodan Prvanovć Insttute of Physcs, P.O. Box 57, 11080 Belgrade, Serba Abstract The dynamcal equaton, beng the combnaton of Schrödnger and Louvlle
The non-negatvty of probabltes and the collapse of state Slobodan Prvanovć Insttute of Physcs, P.O. Box 57, 11080 Belgrade, Serba Abstract The dynamcal equaton, beng the combnaton of Schrödnger and Louvlle
Thermodynamics General
 Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
Electronic Quantum Monte Carlo Calculations of Energies and Atomic Forces for Diatomic and Polyatomic Molecules
 RESERVE HIS SPACE Electronc Quantum Monte Carlo Calculatons of Energes and Atomc Forces for Datomc and Polyatomc Molecules Myung Won Lee 1, Massmo Mella 2, and Andrew M. Rappe 1,* 1 he Maknen heoretcal
RESERVE HIS SPACE Electronc Quantum Monte Carlo Calculatons of Energes and Atomc Forces for Datomc and Polyatomc Molecules Myung Won Lee 1, Massmo Mella 2, and Andrew M. Rappe 1,* 1 he Maknen heoretcal
Implicit Integration Henyey Method
 Implct Integraton Henyey Method In realstc stellar evoluton codes nstead of a drect ntegraton usng for example the Runge-Kutta method one employs an teratve mplct technque. Ths s because the structure
Implct Integraton Henyey Method In realstc stellar evoluton codes nstead of a drect ntegraton usng for example the Runge-Kutta method one employs an teratve mplct technque. Ths s because the structure
Snce h( q^; q) = hq ~ and h( p^ ; p) = hp, one can wrte ~ h hq hp = hq ~hp ~ (7) the uncertanty relaton for an arbtrary state. The states that mnmze t
 8.5: Many-body phenomena n condensed matter and atomc physcs Last moded: September, 003 Lecture. Squeezed States In ths lecture we shall contnue the dscusson of coherent states, focusng on ther propertes
8.5: Many-body phenomena n condensed matter and atomc physcs Last moded: September, 003 Lecture. Squeezed States In ths lecture we shall contnue the dscusson of coherent states, focusng on ther propertes
Lagrangian Field Theory
 Lagrangan Feld Theory Adam Lott PHY 391 Aprl 6, 017 1 Introducton Ths paper s a summary of Chapter of Mandl and Shaw s Quantum Feld Theory [1]. The frst thng to do s to fx the notaton. For the most part,
Lagrangan Feld Theory Adam Lott PHY 391 Aprl 6, 017 1 Introducton Ths paper s a summary of Chapter of Mandl and Shaw s Quantum Feld Theory [1]. The frst thng to do s to fx the notaton. For the most part,
Physics 141. Lecture 14. Frank L. H. Wolfs Department of Physics and Astronomy, University of Rochester, Lecture 14, Page 1
 Physcs 141. Lecture 14. Frank L. H. Wolfs Department of Physcs and Astronomy, Unversty of Rochester, Lecture 14, Page 1 Physcs 141. Lecture 14. Course Informaton: Lab report # 3. Exam # 2. Mult-Partcle
Physcs 141. Lecture 14. Frank L. H. Wolfs Department of Physcs and Astronomy, Unversty of Rochester, Lecture 14, Page 1 Physcs 141. Lecture 14. Course Informaton: Lab report # 3. Exam # 2. Mult-Partcle
Poisson brackets and canonical transformations
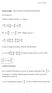 rof O B Wrght Mechancs Notes osson brackets and canoncal transformatons osson Brackets Consder an arbtrary functon f f ( qp t) df f f f q p q p t But q p p where ( qp ) pq q df f f f p q q p t In order
rof O B Wrght Mechancs Notes osson brackets and canoncal transformatons osson Brackets Consder an arbtrary functon f f ( qp t) df f f f q p q p t But q p p where ( qp ) pq q df f f f p q q p t In order
Numerical Heat and Mass Transfer
 Master degree n Mechancal Engneerng Numercal Heat and Mass Transfer 06-Fnte-Dfference Method (One-dmensonal, steady state heat conducton) Fausto Arpno f.arpno@uncas.t Introducton Why we use models and
Master degree n Mechancal Engneerng Numercal Heat and Mass Transfer 06-Fnte-Dfference Method (One-dmensonal, steady state heat conducton) Fausto Arpno f.arpno@uncas.t Introducton Why we use models and
Mechanics Physics 151
 Mechancs Physcs 5 Lecture 7 Specal Relatvty (Chapter 7) What We Dd Last Tme Worked on relatvstc knematcs Essental tool for epermental physcs Basc technques are easy: Defne all 4 vectors Calculate c-o-m
Mechancs Physcs 5 Lecture 7 Specal Relatvty (Chapter 7) What We Dd Last Tme Worked on relatvstc knematcs Essental tool for epermental physcs Basc technques are easy: Defne all 4 vectors Calculate c-o-m
Title: Radiative transitions and spectral broadening
 Lecture 6 Ttle: Radatve transtons and spectral broadenng Objectves The spectral lnes emtted by atomc vapors at moderate temperature and pressure show the wavelength spread around the central frequency.
Lecture 6 Ttle: Radatve transtons and spectral broadenng Objectves The spectral lnes emtted by atomc vapors at moderate temperature and pressure show the wavelength spread around the central frequency.
Efficient Optimal Control for a Unitary Operation under Dissipative Evolution
 Effcent Optmal Control for a Untary Operaton under Dsspatve Evoluton Mchael Goerz, Danel Rech, Chrstane P. Koch Unverstät Kassel March 20, 2014 DPG Frühjahrstagung 2014, Berln Sesson Q 43 Mchael Goerz
Effcent Optmal Control for a Untary Operaton under Dsspatve Evoluton Mchael Goerz, Danel Rech, Chrstane P. Koch Unverstät Kassel March 20, 2014 DPG Frühjahrstagung 2014, Berln Sesson Q 43 Mchael Goerz
Workshop: Approximating energies and wave functions Quantum aspects of physical chemistry
 Workshop: Approxmatng energes and wave functons Quantum aspects of physcal chemstry http://quantum.bu.edu/pltl/6/6.pdf Last updated Thursday, November 7, 25 7:9:5-5: Copyrght 25 Dan Dll (dan@bu.edu) Department
Workshop: Approxmatng energes and wave functons Quantum aspects of physcal chemstry http://quantum.bu.edu/pltl/6/6.pdf Last updated Thursday, November 7, 25 7:9:5-5: Copyrght 25 Dan Dll (dan@bu.edu) Department
PHYS 705: Classical Mechanics. Calculus of Variations II
 1 PHYS 705: Classcal Mechancs Calculus of Varatons II 2 Calculus of Varatons: Generalzaton (no constrant yet) Suppose now that F depends on several dependent varables : We need to fnd such that has a statonary
1 PHYS 705: Classcal Mechancs Calculus of Varatons II 2 Calculus of Varatons: Generalzaton (no constrant yet) Suppose now that F depends on several dependent varables : We need to fnd such that has a statonary
5. THE ADIABATIC APPROXIMATION
 5. THE ADIABATIC APPROXIMATION In quantum mechancs, the adabatc approxmaton refers to those solutons to the Schrödnger equaton that make use of a tme-scale separaton between fast and slow degrees of freedom,
5. THE ADIABATIC APPROXIMATION In quantum mechancs, the adabatc approxmaton refers to those solutons to the Schrödnger equaton that make use of a tme-scale separaton between fast and slow degrees of freedom,
CHE450G Final Exam. CP-109 December 11, :30-12:30 AM
 CH450G Fnal xam CP-09 December, 2006 0:30-2:30 AM Last name Frst Name Score [ /5] 00 = % () Construct a physcally realstc molecular orbtal dagram for CS. Draw all SALC s, molecular orbtals, and provde
CH450G Fnal xam CP-09 December, 2006 0:30-2:30 AM Last name Frst Name Score [ /5] 00 = % () Construct a physcally realstc molecular orbtal dagram for CS. Draw all SALC s, molecular orbtals, and provde
Physics 607 Exam 1. ( ) = 1, Γ( z +1) = zγ( z) x n e x2 dx = 1. e x2
 Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
Yukawa Potential and the Propagator Term
 PHY304 Partcle Physcs 4 Dr C N Booth Yukawa Potental an the Propagator Term Conser the electrostatc potental about a charge pont partcle Ths s gven by φ = 0, e whch has the soluton φ = Ths escrbes the
PHY304 Partcle Physcs 4 Dr C N Booth Yukawa Potental an the Propagator Term Conser the electrostatc potental about a charge pont partcle Ths s gven by φ = 0, e whch has the soluton φ = Ths escrbes the
Quantum Chemical Studies of Protein-Bound Chromophores, UV-Light Induced DNA Damages, and Lignin Formation
 omprehensve Summares of Uppsala Dssertatons from the Faculty of Scence and Technology 1010 Quantum hemcal Studes of Proten-Bound hromophores, UV-Lght Induced DA Damages, and Lgnn Formaton BY B DURBEEJ
omprehensve Summares of Uppsala Dssertatons from the Faculty of Scence and Technology 1010 Quantum hemcal Studes of Proten-Bound hromophores, UV-Lght Induced DA Damages, and Lgnn Formaton BY B DURBEEJ
Homework 4. 1 Electromagnetic surface waves (55 pts.) Nano Optics, Fall Semester 2015 Photonics Laboratory, ETH Zürich
 Homework 4 Contact: frmmerm@ethz.ch Due date: December 04, 015 Nano Optcs, Fall Semester 015 Photoncs Laboratory, ETH Zürch www.photoncs.ethz.ch The goal of ths problem set s to understand how surface
Homework 4 Contact: frmmerm@ethz.ch Due date: December 04, 015 Nano Optcs, Fall Semester 015 Photoncs Laboratory, ETH Zürch www.photoncs.ethz.ch The goal of ths problem set s to understand how surface
Integrals and Invariants of
 Lecture 16 Integrals and Invarants of Euler Lagrange Equatons NPTEL Course Varatonal Methods and Structural Optmzaton G. K. Ananthasuresh Professor, Mechancal Engneerng, Indan Insttute of Scence, Banagalore
Lecture 16 Integrals and Invarants of Euler Lagrange Equatons NPTEL Course Varatonal Methods and Structural Optmzaton G. K. Ananthasuresh Professor, Mechancal Engneerng, Indan Insttute of Scence, Banagalore
THE VIBRATIONS OF MOLECULES II THE CARBON DIOXIDE MOLECULE Student Instructions
 THE VIBRATIONS OF MOLECULES II THE CARBON DIOXIDE MOLECULE Student Instructons by George Hardgrove Chemstry Department St. Olaf College Northfeld, MN 55057 hardgrov@lars.acc.stolaf.edu Copyrght George
THE VIBRATIONS OF MOLECULES II THE CARBON DIOXIDE MOLECULE Student Instructons by George Hardgrove Chemstry Department St. Olaf College Northfeld, MN 55057 hardgrov@lars.acc.stolaf.edu Copyrght George
14 The Postulates of Quantum mechanics
 14 The Postulates of Quantum mechancs Postulate 1: The state of a system s descrbed completely n terms of a state vector Ψ(r, t), whch s quadratcally ntegrable. Postulate 2: To every physcally observable
14 The Postulates of Quantum mechancs Postulate 1: The state of a system s descrbed completely n terms of a state vector Ψ(r, t), whch s quadratcally ntegrable. Postulate 2: To every physcally observable
ECEN 5005 Crystals, Nanocrystals and Device Applications Class 19 Group Theory For Crystals
 ECEN 5005 Crystals, Nanocrystals and Devce Applcatons Class 9 Group Theory For Crystals Dee Dagram Radatve Transton Probablty Wgner-Ecart Theorem Selecton Rule Dee Dagram Expermentally determned energy
ECEN 5005 Crystals, Nanocrystals and Devce Applcatons Class 9 Group Theory For Crystals Dee Dagram Radatve Transton Probablty Wgner-Ecart Theorem Selecton Rule Dee Dagram Expermentally determned energy
Prof. Dr. I. Nasser Phys 630, T Aug-15 One_dimensional_Ising_Model
 EXACT OE-DIMESIOAL ISIG MODEL The one-dmensonal Isng model conssts of a chan of spns, each spn nteractng only wth ts two nearest neghbors. The smple Isng problem n one dmenson can be solved drectly n several
EXACT OE-DIMESIOAL ISIG MODEL The one-dmensonal Isng model conssts of a chan of spns, each spn nteractng only wth ts two nearest neghbors. The smple Isng problem n one dmenson can be solved drectly n several
Physics 53. Rotational Motion 3. Sir, I have found you an argument, but I am not obliged to find you an understanding.
 Physcs 53 Rotatonal Moton 3 Sr, I have found you an argument, but I am not oblged to fnd you an understandng. Samuel Johnson Angular momentum Wth respect to rotatonal moton of a body, moment of nerta plays
Physcs 53 Rotatonal Moton 3 Sr, I have found you an argument, but I am not oblged to fnd you an understandng. Samuel Johnson Angular momentum Wth respect to rotatonal moton of a body, moment of nerta plays
n α j x j = 0 j=1 has a nontrivial solution. Here A is the n k matrix whose jth column is the vector for all t j=0
 MODULE 2 Topcs: Lnear ndependence, bass and dmenson We have seen that f n a set of vectors one vector s a lnear combnaton of the remanng vectors n the set then the span of the set s unchanged f that vector
MODULE 2 Topcs: Lnear ndependence, bass and dmenson We have seen that f n a set of vectors one vector s a lnear combnaton of the remanng vectors n the set then the span of the set s unchanged f that vector
1 Derivation of Rate Equations from Single-Cell Conductance (Hodgkin-Huxley-like) Equations
 Physcs 171/271 -Davd Klenfeld - Fall 2005 (revsed Wnter 2011) 1 Dervaton of Rate Equatons from Sngle-Cell Conductance (Hodgkn-Huxley-lke) Equatons We consder a network of many neurons, each of whch obeys
Physcs 171/271 -Davd Klenfeld - Fall 2005 (revsed Wnter 2011) 1 Dervaton of Rate Equatons from Sngle-Cell Conductance (Hodgkn-Huxley-lke) Equatons We consder a network of many neurons, each of whch obeys
Turbulence. Lecture 21. Non-linear Dynamics. 30 s & 40 s Taylor s work on homogeneous turbulence Kolmogorov.
 Turbulence Lecture 1 Non-lnear Dynamcs Strong non-lnearty s a key feature of turbulence. 1. Unstable, chaotc behavor.. Strongly vortcal (vortex stretchng) 3 s & 4 s Taylor s work on homogeneous turbulence
Turbulence Lecture 1 Non-lnear Dynamcs Strong non-lnearty s a key feature of turbulence. 1. Unstable, chaotc behavor.. Strongly vortcal (vortex stretchng) 3 s & 4 s Taylor s work on homogeneous turbulence
Phys304 Quantum Physics II (2005) Quantum Mechanics Summary. 2. This kind of behaviour can be described in the mathematical language of vectors:
 MACQUARIE UNIVERSITY Department of Physcs Dvson of ICS Phys304 Quantum Physcs II (2005) Quantum Mechancs Summary The followng defntons and concepts set up the basc mathematcal language used n quantum mechancs,
MACQUARIE UNIVERSITY Department of Physcs Dvson of ICS Phys304 Quantum Physcs II (2005) Quantum Mechancs Summary The followng defntons and concepts set up the basc mathematcal language used n quantum mechancs,
Chapter 5. Solution of System of Linear Equations. Module No. 6. Solution of Inconsistent and Ill Conditioned Systems
 Numercal Analyss by Dr. Anta Pal Assstant Professor Department of Mathematcs Natonal Insttute of Technology Durgapur Durgapur-713209 emal: anta.bue@gmal.com 1 . Chapter 5 Soluton of System of Lnear Equatons
Numercal Analyss by Dr. Anta Pal Assstant Professor Department of Mathematcs Natonal Insttute of Technology Durgapur Durgapur-713209 emal: anta.bue@gmal.com 1 . Chapter 5 Soluton of System of Lnear Equatons
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM
 ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
NWChem: Planewave Density Functional Theory
 NWChem: Planewave Densty Functonal Theory Outlne Overvew of Plane-Wave Densty Functonal Module n NWChem NWPW capabltes Plane-Wave Bass Basc examples: Geometry optmzaton for S molecule Calculatons for damond
NWChem: Planewave Densty Functonal Theory Outlne Overvew of Plane-Wave Densty Functonal Module n NWChem NWPW capabltes Plane-Wave Bass Basc examples: Geometry optmzaton for S molecule Calculatons for damond
Chapter 13: Multiple Regression
 Chapter 13: Multple Regresson 13.1 Developng the multple-regresson Model The general model can be descrbed as: It smplfes for two ndependent varables: The sample ft parameter b 0, b 1, and b are used to
Chapter 13: Multple Regresson 13.1 Developng the multple-regresson Model The general model can be descrbed as: It smplfes for two ndependent varables: The sample ft parameter b 0, b 1, and b are used to
Lecture 5.8 Flux Vector Splitting
 Lecture 5.8 Flux Vector Splttng 1 Flux Vector Splttng The vector E n (5.7.) can be rewrtten as E = AU (5.8.1) (wth A as gven n (5.7.4) or (5.7.6) ) whenever, the equaton of state s of the separable form
Lecture 5.8 Flux Vector Splttng 1 Flux Vector Splttng The vector E n (5.7.) can be rewrtten as E = AU (5.8.1) (wth A as gven n (5.7.4) or (5.7.6) ) whenever, the equaton of state s of the separable form
Amplification and Relaxation of Electron Spin Polarization in Semiconductor Devices
 Amplfcaton and Relaxaton of Electron Spn Polarzaton n Semconductor Devces Yury V. Pershn and Vladmr Prvman Center for Quantum Devce Technology, Clarkson Unversty, Potsdam, New York 13699-570, USA Spn Relaxaton
Amplfcaton and Relaxaton of Electron Spn Polarzaton n Semconductor Devces Yury V. Pershn and Vladmr Prvman Center for Quantum Devce Technology, Clarkson Unversty, Potsdam, New York 13699-570, USA Spn Relaxaton
A critical re-assignment of the Rydberg states of iodomethane based on new polarization data J. Chem. Phys. 138, (2013); /1.
 Recent development of self-nteracton-free tme-dependent densty-functonal theory for nonperturbatve treatment of atomc and molecular multphoton processes n ntense laser felds Shh-I Chu Ctaton: The Journal
Recent development of self-nteracton-free tme-dependent densty-functonal theory for nonperturbatve treatment of atomc and molecular multphoton processes n ntense laser felds Shh-I Chu Ctaton: The Journal
Electron Correlation
 Electron Correlation Levels of QM Theory HΨ=EΨ Born-Oppenheimer approximation Nuclear equation: H n Ψ n =E n Ψ n Electronic equation: H e Ψ e =E e Ψ e Single determinant SCF Semi-empirical methods Correlation
Electron Correlation Levels of QM Theory HΨ=EΨ Born-Oppenheimer approximation Nuclear equation: H n Ψ n =E n Ψ n Electronic equation: H e Ψ e =E e Ψ e Single determinant SCF Semi-empirical methods Correlation
