Vacuum and Thin Film Technology
|
|
|
- Eugenia Cox
- 6 years ago
- Views:
Transcription
1 1 Instructions for the Laboratory Course Vacuum and Thin Film Technology (Vakuum- und Dünnschichttechnik) Part of the Course Advanced Laboratory Exercises (Praktikum für Fortgeschrittene) Graz University of Technology Institute of Solid State Physics Supervisor: Assist. Prof. Dr. Bettina Friedel (Office PH ,
2 2 1 Objectives of this Course The goal of this one-day course is the acquirement of basic skills in the use of vacuum equipment and the practice of different thin film deposition methods, leading towards applications in thin film electronics as one example. Computer-supported data acquisition (Labview) and analysis is trained. It is expected that the students familiarize themselves with the following topics before start of the experimental work: vacuum - definition, terms and theoretical basics - components and their function - applications thin films - definition and properties - vacuum-based deposition techniques - non-vacuum-based deposition techniques - methods for thickness determination of thin films - applications of thin films The fundamentals given below (Chapter 3) are for guideline only; the students are encouraged to gain further information from literature/ electronic resources. 2 Experimental Tasks a) Acquisition of pressure development data for the recipient depending on different starting conditions, filled either with (i) air or (ii) Ar (or CO 2 ). b) Determination of pumping speed and leakage rate of the system from pressure-timecharacteristics. c) Deposition of a transparent (!) metal film on glass via thermal evaporation method, thickness monitored by quartz microbalance. (Alternatively dip-coating deposition of transparent conducting oxide film; as soon as available.) d) Quantification of its transparency with a UV-VIS spectrophotometer. e) Spin-coating deposition of a photoactive polymer film. f) Measurement of its optical absorption via UV-VIS spectrophotometer and thickness estimation from a (provided) calibration series. g) Deposition of a reflective (!) metal film on glass via thermal evaporation method, thickness monitored by quartz microbalance. h) Electrical characterization of the derived thin film photodiode (dark IV, light IV). Further instructions are given on-site.
3 3 3 Fundamentals 3.1 Vacuum Fields, Units, Terms and Theory Vacuum is simply defined as reduced pressure. The vacuum achieved by common vacuum systems is generally far from being empty from matter. However, vacuum technology is an extremly important tool in many areas of physics. In condensed matter physics and materials science, vacuum systems are used in many surface processing steps. Without the vacuum, such processes as sputtering, evaporative metal deposition, ion beam implantation, and electron beam lithography would be impossible. A high vacuum is required in particle accelerators, from the cyclotrons used to create radionuclides in hospitals up to the gigantic high-energy physics colliders such as the LHC. Vacuum systems are also used in many precision physics applications where the scattering and forces induced by the atmosphere would introduce a major background to precise measurements. [1] The measures for vacuum are possibly the most versatile ones in physics. The most recent addition is the unit Pascal (1971) which is expressible in SI-base units as N kg 1Pa= 1 = m m s Nevertheless, plenty of other units are (still) used. The most popular ones and their equivalencies are found in table 1. Pascal Bar Technical atmosphere Standard atmosphere Torr Pound per square inch Pa bar at atm torr psi 1 Pa 1 N/m bar dyn/cm at kp/cm atm p Torr = 1 mm Hg psi lb F /in 2 Table 1: Commonly used pressure units and their equivalencies [3]. Vacuum is usually classified into different pressure ranges, as seen in the table 2. Table 2: Vacuum regimes and their pressure ranges [4]. The definition of these pressure ranges may vary, occasionally only low vacuum LV (e.g. used in industrial chip production lines, as transfer medium), high vacuum HV (e.g. in thin deposition) and ultrahigh vacuum UHV (e.g. essential for surface science) are distinguished, but the values can be taken as a rule of thumb.
4 4 As vacuum is described as reduced pressure it might be worth a thought, what actually defines pressure. For detailed theory the dedicated reader is referred to according literature [5], as for our practical applications a few basic concepts will do the job. According to the ideal gas law pressure can be expressed by p = nrt / V = Nk B T/V = ñk B T p is the absolute pressure of the gas n is the amount of substance T is the absolute temperature V is the volume R is the ideal gas constant N is the number of particles k B is the Boltzmann constant ñ is the particle density per volume. The law from Boyle and Mariotte shows that for a constant temperature and amount of substance the product of pressure p and volume V remains constant p 1 V 1 = p 2 V 2. According to Daltons law the total pressure p in a given volume is either the pressure of a pure gas or the sum of the partial pressures of different gaseous species (vapours and gases) in a mixture p total = p 1 + p 2 + : : : = Σp i (e.g. air, see composition in table 3). Table 3: Composition of air at normal pressure and partial pressures of the components [6]. A more figurative view of pressure is provided from the kinetic gas theory which basically characterizes pressure p by the average force F in Newton (or the rate of normal momentum transfer) exerted by gas molecules impacting on a surface of unit area A (in m 2 ). Therefore, pressure can be expressed as: p = F /A (Pa). Thereby some assumptions are made: 1. Gases are composed of a large number of particles that behave like hard, spherical objects in a state of constant, random motion. 2. These particles move in a straight line until they collide with another particle or the walls of the container. 3. These particles are much smaller than the distance between particles. Most of the volume of a gas is therefore empty space. 4. There is no force of attraction between gas particles or between the particles and the walls of the container. 5. Collisions between gas particles or collisions with the walls of the container are perfectly elastic. None of the energy of a gas particle is lost when it collides with another particle or with the walls of the container. 6. The average kinetic energy of a collection of gas particles depends on the temperature of the gas and nothing else. Figure 1: Random move of gas atoms in an enclosed volume (left), demonstration of one gas atom moving in x-direction in a cube of side length L (right). [7]
5 5 According to Newton, force F is the time rate of change of the momentum ᵽ (not to be mixed up with pressure p) F = dᵽ/dt = ma. The momentum change is equal to the momentum after collision mv minus the momentum before collision mv. Accordingly we get ᵽ = mv x (-mv x ) = 2mv x. As the collisions with the wall happen with a frequency f = v x /2L, we get for the force F in x- direction F = ᵽ/ t = ᵽ*f = 2mv x *(v x /2L) = mv x 2 /L and so consequently for the pressure p of a single atom/molecule in the box p = F/A = mv x 2 /V. Taking N atoms/molecules into account (via mean square speed) and all three dimensions we end up with p = Nmῡ 2 /3V. As the speed of the atoms in this model is purely dependent on the temperature with the kinetic energy given E kin = mv 2 /2 = 3/2* k B T, we finally get for the temperature dependent pressure derived from kinetic gas theory p = Nmῡ 2 /3V = N/3V * 2E kin = N k B T/V So, we are back to the ideal gas law (originally phenomenologically derived from thermodynamics). Another term which can be derived from kinetic gas theory and the Maxwell-Boltzmann velocity distribution is the mean free path λ of our gas particles, the distance they can travel before the next collision with eachother after a time τ. Naturally this would be a function of the density of particles ñ, the mean velocity ν and the size d of the gaseous particles λ = ντ = (1/ñπd 2 τ) *τ = 1/ñπd 2. But this is only the formula for one moving particle while the others are all fixed. With introduction of a factor 1/ 2 (which can be non-trivially derived from Maxwell-Boltzmann equation) also the movement of the other particles is accounted for and we use the ideal gas equation in the form p = ñk B T to get λ = kbt / 2πd 2 p. For air for example (for a rough estimation data are taken from nitrogen), the mean free path at room temperature is only 66 nm at normal atmosphere while being 6.7 m at 10-3 Pa [1]. Many interesting applications of vacuum technology depend on the mean free path being much larger than other dimensions in the system. For example, thermal evaporation deposition of metal films onto substrates is only efficient if the mean free path is significantly larger than the distance between the evaporator element and the substrate, often a distance on the order of 10 cm. The relation between the mean free path and the characteristic dimension d of the vacuum system (e.g. a tube diameter) results in a specific flow behaviour. The Knudsen number is a dimensionless measure therefore: K n = λ/d Figure 2: The three flow regimes of gases depending on the atoms/molecules mean free path. Accordingly, if K n <<1 (λ<<d), a gas molecule will encounter many collisions with other molecules during transport between two walls of a container, while when K n >1 (λ>d), the collisions between gas molecules can be neglected and the collisions with walls dominate (Figure 2). The regimes are called viscous/continous flow (K n <<1), Knudsen flow (K n <1) and molecular flow (K n >1).
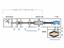
6 6 If there is a flow of gas, it has to be distinguished between dynamic and static pressure (i.e. static if gauge reading of the pressure is stationary, but dynamic, if it moves with the same velocity as the flow). Similarly, one speaks of a steady-state pressure, if the pressure at different locations in a vacuum system remains constant with time. So, if pressure is indeed the sum of forces from a bombardment of the recipients walls by the atoms/molecules of a gas and vacuum is reduced pressure, then the task of vacuum technology must be the reduction of the number of atoms/molecules from the system. This job is done by all sorts of pumps (see next chapter). The gas removal is quantified by three characteristics: the pumping speed, the gas throughput and the pumping capacity. The pumping speed S = dv/dt (or V/ t) refers to the volume flow rate of a pump at its inlet and is measured in volume per unit of time (e.g. L/s, m 3 /h). It might vary with the composition of the gas pumped. The throughput q PV refers to the pumping speed multiplied by the gas pressure at the inlet, Q = S p = (d/dt) pv (=(d/dt) mrt), measured in units of pressure volume/unit time (e.g. mbar L/s). Similar to electrical circuits also vacuum systems are characterized by a sort of conductance their ability to transport gas. This conductance C of a vacuum system depends on the geometry of the vacuum chamber, its surface, length and diameter of plumbing, valves bends and traps. Omnipresent loss mechanisms in vacuum systems are leaks, occurring at every kind of connection to the outsideworld. In those cases, throughput refers to the volume leak rate multiplied by the pressure at the vacuum side of the leak, so the leak throughput can be compared to the pump throughput. One last important feature, especially of interest in ultrahigh vacuum technology is the recovering time τ. It describes the time required for the adsorption of a monolayer of atoms or molecules on a formerly gas-free surface. Assuming that every particle sticks to the surface this is p τ = const 10-6 mbar s. A last remark: It is a common misunderstanding that vacuum pump sucks gas from a chamber. Until an atom/molecule, driven by random collisions, enters the pumping mechanism of a pump, it cannot be removed from the chamber. The pump does not reach out, grab a molecule from outside and suck it in. When gas molecules in one section of an enclosed volume are removed (e.g. at the entrance of the pump), molecules from the remaining volume will occasionally migrate to the lower pressure space, via their normal random flight, colliding and bouncing off walls and eachother. 3.2 Vacuum Generation Figure 3: General build of a high vacuum system and associated function. [2] Common high vacuum systems are built from a vacuum chamber (also called recipient) connected to a systems of pumps. Usually a roughing pump is used two ways: to create a pre-vacuum before the high vacuum pump is started and to carry away the exhaust gas from the high vacuum (or
7 7 secondary) pump. As it is handy to be able to open the vacuum chamber (e.g. for changing samples or modifications) without turning the pumps off, a range of valves are installed, serving to separate parts of the system, to evacuate or vent them. If for instance the recipient had been separated from the rest of the system and vented, it needs to be pre-evacuated again with the roughing pump, before opening the valve to the high vacuum side (which otherwise would cause heavy damage to certain high vacuum pumps), for that purpose these systems usually have a bypass between roughing pump and recipient. A schematic view of the common built of such a vacuum system is shown in Figure 3. Pumps can be divided into three functional types Positive displacement pump (capturing, compressing and expelling the gas molecules): Mechanical pumps Momentum transfer pump (giving the gas molecule a preferential direction): e.g. Diffusion pump, Turbo-molecular pump, Aspiration pump Adsorption or reaction pump (Capturing and keeping the gas molecules): e.g. Cryopump, Sorption pump, Ion pump, Evaporative getter pump, Absorption pump, Getter pump Positive displacement (mechanical) pumps are used for low to medium vacuum applications, the most common ones are the rotary vane pump and the diaphragm pump. Function of rotary vane pump (figuren 4 top): The rotary vane modules are immersed in an oil bath and driven by a motor with about rpm. The oil serves as coolant, lubricant and sealing. The pumping process works in 4 stages: intake, transfer, compression and exhaust (see Figure 4 bottom). Rotary vane pumps are available either singular (single stage) or two (or more) compression cycles (double stage) in series. Different to the diaphragm pump (described in the following), rotary vane pumps should not be used to pump continiously at high inlet-pressure (as gas transport pump), as gases and moisture might condensate in the compression process and lead to corrosion and damage of the pump. Figure 4: Built of a rotary vane pump (top) and the four stages of its function (bottom). [8][9]
8 8 Function of a diaphragm pump: A diapragm is lifted and lowered by a motor, thereby opening/closing inlet and outlet valve accordingly, to get gas in and exhaust gas out (see figure 5). These pumps are mostly used to support gas transport. Figure 5: Function of a diaphragm pump. [1] Momentum transfer pumps are used in medium to high and ultra high vacuum applications. The most commonly used ones are the oil-diffusion pump and the turbo molecular pump. Function of a oil-diffusion pump: The pump oil reservoir on the bottom of the pump is heated and creates an oil vapour which is ejected with high speed through nozzels on different levels of the pump. Thereby the oil vapour droplets/molecules (which are heavier and larger compared to general gas molecules) force the gas molecules by collisions downwards within the pump (see figure 6), producing a vertical pressure gradient with an enrichment of gas molecules at the bottom. At its exhaust it needs to be carried away by a backing pump. As the walls of the pump and the exhaust are cooled the oil vapour condensates and runs back into the reservoir. These pumps are advantageous because they hardly need any maintenance. Oppositely the danger of getting oil vapour accidentially into the apparatus is a drawback. Figure 6: Built and function of an oil-diffusion pump. [2][9]
9 9 Function of a turbomolecular pump: An arrangement of turbine blades is rotating at high speed (up to 60000rpm), another set of blades is installed stationary at the walls of the housing (stator). As the blades have a speed near molecular motion and particular angles they hit the gas molecules i.e. transfer momentum onto them to force them progressively down towards the exhaust. Also this pump needs a backing pump at the exhaust. The turbomolecular pump is a delicate system, the thin blades move with a high speed, any imbalance can cause heavy damage to the pump. Especially a large pressure gradient between the top and bottom of the pump causes a large amount of gas suddenly streaming into one end of the blade system, leading to an imbalance in the rotor system, which at that high speed transfers a lot of force onto the housing. For this reaon it is built extremely robust. Though the blades keep contained on those occassions they will be destroyed. Turbomolecular pumps are also not meant to pump at high pressure for a longer period, as the large amount of blade-gas collisions heats up the pump and might damage it. Figure 7: Built and function of a turbomolecular pump. [10][9] Adsorption or reaction pumps are used for high to ultrahigh vacuum applications. The biggest disadvantage of these pumps is that they enrich with captured gas molecules over time and accordingly require regeneration cycles. The most commonly used one is the cryopump. Function of a cryopump: A cryopump is a vacuum pump that traps gases and vapours by condensing them on a cold surface. They are only effective on selective gases, depending on the freezing and boiling points of the gas relative to the cryopump's temperature. In a very simple form they are used sometimes to block particular contaminents, for example in front of a diffusion pump to trap backstreaming oil. In this function, they are called a cryotrap or cold trap, even though the physical mechanism is the same as for a cryopump. Cryopumps are commonly cooled by liquid helium or liquid nitrogen, or stand-alone versions may include a built-in cryocooler. Over time, the surface eventually saturates with condensate and the pumping speed gradually drops to zero. It will hold the trapped gases as long as it remains cold, but it will not condense fresh gases from leaks or backstreaming until it is regenerated. Saturation happens very quickly in low vacuums, so cryopumps are usually only used in high or ultrahigh vacuum systems. Regeneration of a cryopump is the process of evaporating the trapped gases. This can be done at room temperature and pressure, or the process can be made more complete by exposure to vacuum and faster by elevated temperatures.
10 10 Figure 8: Built and function of a cryosorption pump. [11][12][2] The pressure ranges of most available pumps can be seen in figure 9. Figure 9: Pressure ranges of vacuum pumps. [13]
11 Vacuum Quantification Figure 10: Pressure ranges of common vacuum gauges. There is a wide range of instruments for pressure measurement in vacuum applications. None of these is capable of covering all vacuum ranges; accordingly on most vacuum systems combinations of two (or more) gauges are used. As all gauges can only deliver total pressures, partial pressures must be determined with mass spectrometer-based systems. Gauges are divided into two groups. The first group are the direct/absolute gauges, which measure pressure classically via the force-per-area (liquid and mechanical vacuometers). These methods are independent of the type of gaseous species, as they only depent on the number of particles (see theory part chapter 3.1). The second group are the indirect gauges, which determine the pressure from measuring a gas concentration - more precisely, a density dependent property (thermal conductivity, probability of ionization, electrical conductivity); these are dependent on the gas. They are usually calibrated on one specific sort of gas and need correction factors if other gases are involved. The function of vacuum gauges is based on one of the following mechanisms: Pressure exerted on a surface with respect to a reference (e.g. capacitance manometers) Thermal conductivity of system gases (e.g. thermocouple and Pirani gauge) Ionization and collecting of ions (e.g. hot and cold cathode ionization gauges) The most commonly used ones are the - mechanical-type capacitance diaphragm manometer Baratron (10 +5 to 10-6 mbar, accur. ±0.2-2%), - thermal conductivity-based Pirani gauge (operating 1000 to 10-4 mbar, accur. ±5%) and thermocouple gauge - hot cathode ionization Bayard-Alpert gauge (10-1 to 10-9 mbar, accur. ±1%) - cold cathode ionization Penning gauge (10-3 to mbar, accur. ±1%).
12 12 (a) (b) (c) Figure 11: Schematics of the five most common vacuum gauges. (a) Baratron, (b) Pirani, (c) Thermocouple, (d) Bayard-Alpert and (e) Penning gauge. [14][6] (d) (e) A capacitance manometer contains a thin metal diaphragm that is deflected when the pressure changes. The deflection is sensed electronically via a change in capacitance between the diaphragm and one or more fixed electrodes. One side of the diaphragm is maintained at a reference pressure, and the measured capacitance therefore allows an absolute pressure to be determined. Pirani gauges contain a metal wire that is heated by an electrical current. At the same time, collisions with the surrounding gas carry heat away from the wire and cool it, with the net effect being that the wire temperature settles at some equilibrium value. If the pressure is lowered, heat is carried away less effectively and the temperature of the wire increases, while an increase in pressure leads to more effective cooling and a decrease in the wire temperature. The temperature of the wire may therefore be used to measure the pressure. In practice this is achieved by monitoring the electrical resistance of the wire, which is temperature-dependent. Thermocouple gauges work in a very similar way to Pirani gauges, except that a thermocouple is used to measure the temperature of the wire directly, rather than inferring the temperature from a measurement of the resistance. The Bayard-Alpert gauge consists of a heated filament that emits electrons, an acceleration grid, and a thin wire detector. Electrons emitted from the filament are accelerated towards the grid, and ionise gas molecules along the way. The ions are collected at the detection wire, and the measured ion current is proportional to the gas pressure. This type of ionization gauge has the advantage that there is a linear dependence of the ion current on the gas pressure. Like any ionization gauge, correction factors need to be applied for different gases to account for differences in electron-impact ionization probability. The Penning gauge works on a similar principle to a hot cathode gauge, but the mechanism of ionization is somewhat different. There is no filament to produce electrons, simply a detection rod (the anode) and a cylindrical cathode, to which a high voltage (~4kV) is applied. Ionization is
13 13 initiated randomly by a cosmic ray or some other ionizing particle entering the gauge head (this occurs more frequently than you might think!). The electrons formed are accelerated towards the anode. A magnetic field causes them to follow spiral trajectories, increasing the path length through the gas, and therefore the chance of ionizing collisions. The ions are accelerated towards the cathode, where they are detected. More free electrons are emitted as the ions bombard the cathode, further increasing the signal. Eventually a steady state is reached, with the ion current being related to the background gas pressure. The relationship is not a linear one as in the case of a hot cathode gauge, and the pressure reading is only accurate to within around a factor of two. However, in its favour, the Penning gauge is more damage resistant than a hot cathode gauge. 3.4 Thin Films General Terms and Properties A thin film is a microscopically thin layer of material deposited on a carrier (substrate) as it is (normally) not capable of self-standing. The thickness of a thin film usually ranges from a couple of nanometers up to 1 micron. The material can be a metal, ceramic, semiconductor or organic and accordingly it can be conductive or dielectric (non-conductive), transparent, absorbing or reflective. A main characteristic of thin films is that the surfaces (and interfaces) have a considerable influence on its physical properties, oppositely to thick films (> 1micron) and bulk materials which show usually the bulk properties of a material. Traditional single crystal based technology is quite costly and complex, as it requires the growth of defect-free large single crystals of high purity material, to subsequently cut them into discs, followed by time consuming polishing processes to get electronic grade wafers. In recent years it gets more and more replaced by the introduction of thin film technology which brings a range of benefits for today s electronics: lower production costs and energy consumption - less material required - (partially) cheaper raw materials - lower technical effort - less logistics - few and shorter processes (no single crystal growth, no polishing) - lower process temperatures required flexibility in application and design - material can be deposited on almost every substrate - low film thickness enables thin and flexible bendable electronics Certainly also thin films have to meet specific requirements, to be adequate for applications in electronics and/or optics. Thereby the focus is on properties like - film uniformity - film quality: stress, adhesion, density, defects, stoichiometry, grain size, boundary property, orientation - electrical film properties: breakdown voltage, impurity/dopant level, spatial preferences 3.5 Thin Films Deposition Methods Vacuum-based The vacuum-based techniques can be sub-devided into two fields:
14 14 In physical vapour deposition (PVD) the film is formed by atoms directly transported from source to the substrate through a gas phase. Common methods with this type of deposition are Evaporation (thermal evaporation or e-beam evaporation) Sputtering (DC sputtering, DC Magnetron sputtering, RF sputtering) Reactive PVD During chemical vapour deposition (CVD) a film is formed by chemical reaction on the surface of substrate. The following methods use this type of deposition process. Low-Pressure CVD (LPCVD) Plasma-Enhanced CVD (PECVD) Atmosphere-Pressure CVD (APCVD) Metal-Organic CVD (MOCVD) Atomic Layer Deposition (ALD) As it would be beyond the scope of this script to introduce all above mentioned techniques, we focus on the most prominent PVD-based deposition techniques, which are thermal evaporation and DC diode sputtering. Below (figure 12) you see schematics of a typical thermal evaporation setup (a) and a sputter coater (b). A thermal evaporator consists of a vacuum chamber with a resistance-heated evaporation source on its bottom, a substrate holder in the top (heatable), which is mostly equipped with a shutter unit (for better deposition control) and a quartz crystal monitor for in-situ thickness determination (sometimes water-cooled). The evaporation sources come usually in forms of boats, crucibles (plus metal holder), boxes, baskets or wires, made from a high temperature stable metal (tungsten, molybdenum or tantalum) with an infinite number of different designs (see fig. 12c). The chamber is evacuated down to pressures of about 10-6 mbar (or below) and a voltage is applied to the source. The onset of evaporation is detectable by a frequency shift of the crystal monitor and a short raise of chamber pressure. After adjustment of an adequate evaporation rate, the shutter to the sample is opened and a layer deposited, controlled by the quartz crystal monitor. Materials which can be deposited with this method are metals, organics and low evaporation temperature inorganic compounds, as the achievable source temperature with the resistance heating is limited to about 1800 C. Higher temperatures can be reached with an e-beam evaporator (up to 3000 C) which enables a larger range of materials.[16] (a) (b)
15 15 Figure 12: Typical design of a thermal evaporator (a) and a DC sputter coater (b). Below the sources of a thermal evaporator (c) and of a sputter coater (d) are shown. [15] Also a DC sputter coater is built within a vacuum chamber, though it needs argon for operation, its pressure has to be low enough to enable the sputtered material to reach the substrate (mean free path, see Chapter 3.1). Here, the substrate holder (anode) is located on the bottom of the chamber, while the sputter target/source (cathode), is mounted at the top of the chamber. The target is basically a mountable metal plate with a coating (2 mm-14 mm thick) of the desired high purity material. A high voltage (~2-5 kv) is applied, generating an electric field between anode and cathode. Then a low pressure argon atmosphere is established within the chamber. Free electrons in the chamber are accelerated by the E-field and collide inelastically Ar atoms, causing ionization of Ar to Ar + with generation of a secondary electron. The generated Ar + ions are then accelerated towards the cathode target and impact, thereby blasting-off target atoms. These released target atoms then condensate on the substrate. As the ionized argon recombines occasionally with free electrons a glowing is visible (glow discharge plasma). Unfortunately this method qualifies only for metal deposition as otherwise an opposing electrical field is generated, hindering the process. [15] The table below concludes the characteristics of different vacuum-based methods (table 4). (c) (d) Table 4: List of specific characteristics of different vacuum-based thin film deposition methods. [16] Non-vacuum-based Compared to traditional vacuum based deposition methods, some of the non-vacuum deposition techniques are rather new - at least to electronic applications. However, some fields, like organic electronics, are unimaginable without them. The most common non-vacuum-based methods are: - Oxidation, - Electroplating - Spin coating - Dip coating - Doctor blading - Spray (pyrolysis) deposition
16 16 - Drop casting - Printing Though all these mentioned methods are used for thin film electronics, a detailed description of each one of them would be beyond the scope of this script. Therefore only short overviews of the most important ones: Oxidation This one is certainly not new and probably the best self-running thin film growth existing. Many metals (like aluminium) and semiconductors (like silicon) are subject to unavoidable oxidation. In Si-based transistors this is used as one part of the process, as the silicon dioxide layer generated on top of the silicon serves as the gate dielectric. Oxidation can be accelerated/triggered by use of oxidizing chemicals or an oxygen-plasma-etcher. Spin coating In this method the substrate to be coated is placed on the spinner chuck and fixed by a low vacuum. After a drop of material is deposited on the substrate, the spinner chuck is put on rotation with high speed (usual rpm). By centrifugal forces the liquid spreads and covers the substrate homogeneously (in the optimum case), the excess liquid flies off. After an liquid layer is established, the film begins to evaporate the solvent and the film solidifies. Though this appears to be quite simple, the film forming processes are complex and equilibrium based and so very sensitive to the substrate surface (morphology and energy), the solvent/s (viscosity, surface tension, boiling point, vapour pressure), the solute (surface energy, concentration), the environment (gas, temperature) and time (acceleration, speed). The thickness of a spin coated film for instance, can be altered by the concentration of the solution, the boiling point of the solvent, the spin speed and the temperature. Figure 13: Spin coater and schematic of the spin casting process. [17] Dip coating In the dip coating process a substrate is fixed at a vertical lifting stage. The substrate is dipped into the according solution of the material to be deposited and is subsequently withdrawn from the solution very slowly. The solid film is formed at the very end of the liquid meniscus a kind of drying zone (see figure 14). Oppositely to spin casting, which is the major process used currently in solution processible electronics research, dip coating is also applicable for larger substrates, thus of higher interest for industrial use. The disadvantage of dip coating is that the coating process takes quite long, depending on the evaporation rate (vapour pressure) of the solvent, and is consequently affected easily by air turbulence, vibration and shocks. Furthermore, the decreasing concentration during the coating process can be an obstacle, especially if the amount of dipping solution is not several magnitudes larger than the amount evaporated/deposited during the process.
17 17 The film forming properties themselves are affected by the same factors as in spin coating, apart from the spin-speed which is replaced by the retraction speed. Figure 14: Dip coating apparatus and film forming process. [18] spray coating Also this method is applicable on large areas. Basically a spray gun is used to deposit fine droplets of solution on a substrate. The challenge here is to achieve a homogeneous thin film. The method is mostly used for the deposition of transparent conductive oxides. Therefore the droplet size has to be small enough (to obtain optical transparency, avoid scattering grains ) and the solvent has to dry off as soon as the droplet hits the substrate. Accordingly the substrate is heated in most cases. Figure 15: Automated spray gun and schematic spray coating process. [19] printing Printing is so far the most promising approach to large area solution-processed optoelectronic devices on flexible substrates and seen as a serious alternative to conventional multistep lithographic methods. Therefore the printing techniques developed in the last 10 years are quite versatile. One differentiates between a large range of sub-techniques, e.g. screen printing, flexography, gravure, offset lithography and inkjet printing.
18 18 Figure 16: Printing techniques in solution processed electronics, examples: printed structures on PET foil (top left), inkjet printing droplet formation and printed circuit (top right), flat and rotary screen printing (middle right), gravure printing schematic and a resulting flexible OLED display. [20] 3.6 Thickness Determination of Thin Films non-optical e.g. - weighting - quartz monitor - imaging (AFM, SEM) - stylus profilometer optical e.g. - interference - transparency or reflectivity measurement Methods applied during this course are the quartz monitor and optical transparency and will be discussed in more detail in the following. The quartz monitor actually operates by measuring the shift of the resonance frequency of an oscillating quartz crystal plate, due to mass gain by deposition of a thin film. A relation between the resonance frequency shift f and this mass gain m was initially suggested by G. Sauerbrey (1959) [22]: The Sauerbrey equation is defined as (approximation for thin rigid films, f/f < 0.02, m F <<m q ): f = 2 mf A ρ µ q q = f ρ µ q 2 0 q m = C A SB m A f 0 Resonant frequency (Hz) (typically between 5 and 10 MHz) f Frequency change (Hz) m Mass change (g) A Piezoelectrically active crystal area (Area between electrodes, cm 2 ) ρ q Density of quartz (ρ q = g/cm 3 ) µ q Shear modulus of quartz for AT-cut crystal (µ q = 2.947x10 11 g/cm.s 2 ) C SB Sauerbrey constant or Sensitivity factor
19 19 The Sauerbrey constant depends on the quartz cut, for an AT-cut (see figure 17) 5MHz quartz 2 Hz cm crystal it is C SB =56.6 µ g this is then related to the film thickness via C m0 - Sauerbrey constant ρ F - film material density h F = film thickness m= ρ F Ah F Figure 17: Quartz monitor and crystal (left), schematic of the concept (right). [21] Optical measurements not only via interference methods - can be a useful tool for thickness determination. One simple way is by optical absorption. A calibration curve is needed, which is created by optical measurement This is easy if there are a couple (2-4) films of that material with known thicknesses, which can serve as a calibration curve. Because the law of Lambert-Beer says A= log I I α l = = αl = εlc (A ist he absorbance, I 0 is the initial incidence, I 1 the transmitted incidence, α the absorption constant and l the film thickness) As the density of absorbers of a thin film of the identical material (and treatment) stays (about) constant, this implies that the absorbance goes linear with the thickness of the film. So this can be used for extracting the thickness of an unknown film from an absorbance measurement by comparison to a calibrated thickness-absorbance series. 3.7 Photodiodes Figure 18: Schematic built of a thin film photodiode, a manufactured example and typical IV curves in the dark and under illumination. [23]
20 20 A photodiode is probably one of the simplest electrical devices which can be made using thin film technology. The schematic structure of such a device is shown in figure 18 (top left). A glass substrate is coated with a transparent electrode (anode), then a photoactive layer (in our case a blend of organic semiconductors) and finally a reflective back electrode (cathode). The result can look like the one shown in figure 18 (bottom left). To characterize such a photodiode, the easiest way is to measure its current-voltage behaviour, by contacting one electrode to the transparent anode and the other to the reflective cathode. IF the device works as it should, meaning: 1) it is not shortened, 2) there is a contact at all, 3) and in the optimum case a current is generated during illumination, THEN the achieved IV-curves should look similar to those in figure Literature, Electronic Resources [1] [2] Surface%20Engineering%20and%20Coatings/Surface%20Engineering%20and%20Coatings%20pdf/ Surface%20Engineering%20and%20Coatings%20pdf/L02a.pdf [3] [4] F. Weber, Dictionary of High Vacuum Science and Technology, American Elsevier Publishing Co., New York, 1968 [5] John F. O'Hanlon, A user's guide to vacuum technology, Wiley-Interscience; 3 edition (2003); Dorothy M. Hoffman,Bawa Singh,John H. Thomas, Handbook of vacuum science and technology, Academic Press; 1st edition (1997) [6] VacuumSystems.pdf [7] [8] Technical_Articles/Mechanical%20vacuum%20pumps.pdf [9] [10] [11] [12] [13] [14] [15] [16] %20Deposition-1.pdf [17] [18] _04_blaschke.pdf [19] DOI: / /19/6/019 DOI: /
21 21 [20] [21] [22] G. Sauerbrey, Z. Phys. 155 (1959) 206 [23] Scientific Talk, Dr B. Friedel, MRS Fall Meeting, Boston, 2008
Lecture 3 Vacuum Science and Technology
 Lecture 3 Vacuum Science and Technology Chapter 3 - Wolf and Tauber 1/56 Announcements Homework will be online from noon today. This is homework 1 of 4. 25 available marks (distributed as shown). This
Lecture 3 Vacuum Science and Technology Chapter 3 - Wolf and Tauber 1/56 Announcements Homework will be online from noon today. This is homework 1 of 4. 25 available marks (distributed as shown). This
DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD
 Chapter 4 DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD 4.1 INTRODUCTION Sputter deposition process is another old technique being used in modern semiconductor industries. Sputtering
Chapter 4 DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD 4.1 INTRODUCTION Sputter deposition process is another old technique being used in modern semiconductor industries. Sputtering
( KS A ) (1) , vapour, vapor (USA) , saturation vapour pressure. , standard reference conditions for gases. , degree of saturation
 ( KS A 3014-91 ) (1), standard reference conditions for gases 0, 101325 Pa (1 =760mmHg ), vacuum, low ( rough ) vacuum 100Pa, medium vacuum 100 01 Pa, high vacuum 01 10 5 Pa, ultra high vacuum ( UHV )
( KS A 3014-91 ) (1), standard reference conditions for gases 0, 101325 Pa (1 =760mmHg ), vacuum, low ( rough ) vacuum 100Pa, medium vacuum 100 01 Pa, high vacuum 01 10 5 Pa, ultra high vacuum ( UHV )
Vacuum Pumps. Two general classes exist: Gas transfer physical removal of matter. Mechanical, diffusion, turbomolecular
 Vacuum Technology Vacuum Pumps Two general classes exist: Gas transfer physical removal of matter Mechanical, diffusion, turbomolecular Adsorption entrapment of matter Cryo, sublimation, ion Mechanical
Vacuum Technology Vacuum Pumps Two general classes exist: Gas transfer physical removal of matter Mechanical, diffusion, turbomolecular Adsorption entrapment of matter Cryo, sublimation, ion Mechanical
Earlier Lecture. In the earlier lecture, we have seen non metallic sensors like Silicon diode, Cernox and Ruthenium Oxide.
 41 1 Earlier Lecture In the earlier lecture, we have seen non metallic sensors like Silicon diode, Cernox and Ruthenium Oxide. Silicon diodes have negligible i 2 R losses. Cernox RTDs offer high response
41 1 Earlier Lecture In the earlier lecture, we have seen non metallic sensors like Silicon diode, Cernox and Ruthenium Oxide. Silicon diodes have negligible i 2 R losses. Cernox RTDs offer high response
Previous Lecture. Electron beam lithoghraphy e - Electrons are generated in vacuum. Electron beams propagate in vacuum
 Previous Lecture Electron beam lithoghraphy e - Electrons are generated in vacuum Electron beams propagate in vacuum Lecture 6: Vacuum & plasmas Objectives From this vacuum lecture you will learn: What
Previous Lecture Electron beam lithoghraphy e - Electrons are generated in vacuum Electron beams propagate in vacuum Lecture 6: Vacuum & plasmas Objectives From this vacuum lecture you will learn: What
6.5 Optical-Coating-Deposition Technologies
 92 Chapter 6 6.5 Optical-Coating-Deposition Technologies The coating process takes place in an evaporation chamber with a fully controlled system for the specified requirements. Typical systems are depicted
92 Chapter 6 6.5 Optical-Coating-Deposition Technologies The coating process takes place in an evaporation chamber with a fully controlled system for the specified requirements. Typical systems are depicted
TMT4320 Nanomaterials November 10 th, Thin films by physical/chemical methods (From chapter 24 and 25)
 1 TMT4320 Nanomaterials November 10 th, 2015 Thin films by physical/chemical methods (From chapter 24 and 25) 2 Thin films by physical/chemical methods Vapor-phase growth (compared to liquid-phase growth)
1 TMT4320 Nanomaterials November 10 th, 2015 Thin films by physical/chemical methods (From chapter 24 and 25) 2 Thin films by physical/chemical methods Vapor-phase growth (compared to liquid-phase growth)
Generation of vacuum (pumps) and measurements
 Generation of vacuum (pumps) and measurements Generation of vacuum (pumps): ᴥ pumping systems general considerations on their use and match with physical quantities introduced for the dimensioning of vacuum
Generation of vacuum (pumps) and measurements Generation of vacuum (pumps): ᴥ pumping systems general considerations on their use and match with physical quantities introduced for the dimensioning of vacuum
Vacuum. Kai Schwarzwälder, Institut für Physik Universität Basel October 6 th 2006
 Physics,, Technology and Techniques of the Vacuum Kai Schwarzwälder, Institut für Physik Universität Basel October 6 th 2006 Outline Introduction and basics Defintion of Vacuum Vacuum A vacuum is a volume
Physics,, Technology and Techniques of the Vacuum Kai Schwarzwälder, Institut für Physik Universität Basel October 6 th 2006 Outline Introduction and basics Defintion of Vacuum Vacuum A vacuum is a volume
VACUUM PUMPING METHODS
 VACUUM PUMPING METHODS VACUUM PUMPS (METHODS) Positive Displacement Vacuum Gas Transfer Vacuum Kinetic Vacuum Entrapment Vacuum Adsorption Reciprocating Displacement Rotary Drag Fluid Entrainment Ion Transfer
VACUUM PUMPING METHODS VACUUM PUMPS (METHODS) Positive Displacement Vacuum Gas Transfer Vacuum Kinetic Vacuum Entrapment Vacuum Adsorption Reciprocating Displacement Rotary Drag Fluid Entrainment Ion Transfer
Section 5: Thin Film Deposition part 1 : sputtering and evaporation. Jaeger Chapter 6. EE143 Ali Javey
 Section 5: Thin Film Deposition part 1 : sputtering and evaporation Jaeger Chapter 6 Vacuum Basics 1. Units 1 atmosphere = 760 torr = 1.013x10 5 Pa 1 bar = 10 5 Pa = 750 torr 1 torr = 1 mm Hg 1 mtorr =
Section 5: Thin Film Deposition part 1 : sputtering and evaporation Jaeger Chapter 6 Vacuum Basics 1. Units 1 atmosphere = 760 torr = 1.013x10 5 Pa 1 bar = 10 5 Pa = 750 torr 1 torr = 1 mm Hg 1 mtorr =
Metal Deposition. Filament Evaporation E-beam Evaporation Sputter Deposition
 Metal Deposition Filament Evaporation E-beam Evaporation Sputter Deposition 1 Filament evaporation metals are raised to their melting point by resistive heating under vacuum metal pellets are placed on
Metal Deposition Filament Evaporation E-beam Evaporation Sputter Deposition 1 Filament evaporation metals are raised to their melting point by resistive heating under vacuum metal pellets are placed on
EE143 Fall 2016 Microfabrication Technologies. Lecture 6: Thin Film Deposition Reading: Jaeger Chapter 6
 EE143 Fall 2016 Microfabrication Technologies Lecture 6: Thin Film Deposition Reading: Jaeger Chapter 6 Prof. Ming C. Wu wu@eecs.berkeley.edu 511 Sutardja Dai Hall (SDH) 1 Vacuum Basics Units 1 atmosphere
EE143 Fall 2016 Microfabrication Technologies Lecture 6: Thin Film Deposition Reading: Jaeger Chapter 6 Prof. Ming C. Wu wu@eecs.berkeley.edu 511 Sutardja Dai Hall (SDH) 1 Vacuum Basics Units 1 atmosphere
Industrial Instrumentation Prof. A. Barua Department of Electrical Engineering Indian Institute of Technology - Kharagpur
 Industrial Instrumentation Prof. A. Barua Department of Electrical Engineering Indian Institute of Technology - Kharagpur Lecture - 19 Low Pressure Measurement Welcome to the lesson 19 of Industrial Instrumentation.
Industrial Instrumentation Prof. A. Barua Department of Electrical Engineering Indian Institute of Technology - Kharagpur Lecture - 19 Low Pressure Measurement Welcome to the lesson 19 of Industrial Instrumentation.
Generation of vacuum (pumps): Vacuum (pressure) measurements:
 Generation of vacuum (pumps) p and measurements Generation of vacuum (pumps): ᴥ pumping p systems general considerations on their use and match with physical quantities introduced for the dimensioning
Generation of vacuum (pumps) p and measurements Generation of vacuum (pumps): ᴥ pumping p systems general considerations on their use and match with physical quantities introduced for the dimensioning
Physical Vapor Deposition
 Physical Vapor Deposition EVAPORATION SPUTTERING Typically used for metallization of semiconductors. Both Evaporation & Sputtering are done in vacuum environments. Typically: y Evaporation Pressures are
Physical Vapor Deposition EVAPORATION SPUTTERING Typically used for metallization of semiconductors. Both Evaporation & Sputtering are done in vacuum environments. Typically: y Evaporation Pressures are
Lecture 4. Ultrahigh Vacuum Science and Technology
 Lecture 4 Ultrahigh Vacuum Science and Technology Why do we need UHV? 1 Atmosphere = 760 torr; 1 torr = 133 Pa; N ~ 2.5 10 19 molecules/cm 3 Hertz-Knudsen equation p ZW 1/ 2 ( 2mk T) At p = 10-6 Torr it
Lecture 4 Ultrahigh Vacuum Science and Technology Why do we need UHV? 1 Atmosphere = 760 torr; 1 torr = 133 Pa; N ~ 2.5 10 19 molecules/cm 3 Hertz-Knudsen equation p ZW 1/ 2 ( 2mk T) At p = 10-6 Torr it
Rough (low) vacuum : Medium vacuum : High vacuum (HV) : Ultrahigh vacuum (UHV) :
 4. UHV & Effects of Gas Pressure 4.1 What is Ultra-high Vacuum (UHV)? 4.2 Why is UHV required for Surface Studies? 4.3 Exercises - Effects of Gas Pressure Vacuum technology has advanced considerably over
4. UHV & Effects of Gas Pressure 4.1 What is Ultra-high Vacuum (UHV)? 4.2 Why is UHV required for Surface Studies? 4.3 Exercises - Effects of Gas Pressure Vacuum technology has advanced considerably over
UNIT 3. By: Ajay Kumar Gautam Asst. Prof. Dev Bhoomi Institute of Technology & Engineering, Dehradun
 UNIT 3 By: Ajay Kumar Gautam Asst. Prof. Dev Bhoomi Institute of Technology & Engineering, Dehradun 1 Syllabus Lithography: photolithography and pattern transfer, Optical and non optical lithography, electron,
UNIT 3 By: Ajay Kumar Gautam Asst. Prof. Dev Bhoomi Institute of Technology & Engineering, Dehradun 1 Syllabus Lithography: photolithography and pattern transfer, Optical and non optical lithography, electron,
Repetition: Physical Deposition Processes
 Repetition: Physical Deposition Processes PVD (Physical Vapour Deposition) Evaporation Sputtering Diode-system Triode-system Magnetron-system ("balanced/unbalanced") Ion beam-system Ionplating DC-glow-discharge
Repetition: Physical Deposition Processes PVD (Physical Vapour Deposition) Evaporation Sputtering Diode-system Triode-system Magnetron-system ("balanced/unbalanced") Ion beam-system Ionplating DC-glow-discharge
Repetition: Practical Aspects
 Repetition: Practical Aspects Reduction of the Cathode Dark Space! E x 0 Geometric limit of the extension of a sputter plant. Lowest distance between target and substrate V Cathode (Target/Source) - +
Repetition: Practical Aspects Reduction of the Cathode Dark Space! E x 0 Geometric limit of the extension of a sputter plant. Lowest distance between target and substrate V Cathode (Target/Source) - +
Ultra-High Vacuum Technology. Sputter Ion Pumps l/s
 Ultra-High Vacuum Technology 30-400 l/s 181.06.01 Excerpt from the Product Chapter C15 Edition November 2007 Contents General General..........................................................................
Ultra-High Vacuum Technology 30-400 l/s 181.06.01 Excerpt from the Product Chapter C15 Edition November 2007 Contents General General..........................................................................
Experimental techniques of Surface Science and how a theorist understands them
 Experimental techniques of Surface Science and how a theorist understands them Karsten Reuter Fritz-Haber-Institut der Max-Planck-Gesellschaft Faradayweg 4-6, D-14195 Berlin reuter@fhi-berlin.mpg.de 0.
Experimental techniques of Surface Science and how a theorist understands them Karsten Reuter Fritz-Haber-Institut der Max-Planck-Gesellschaft Faradayweg 4-6, D-14195 Berlin reuter@fhi-berlin.mpg.de 0.
Chemistry Instrumental Analysis Lecture 34. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 34 From molecular to elemental analysis there are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry
Chemistry 4631 Instrumental Analysis Lecture 34 From molecular to elemental analysis there are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry
ION Pumps for UHV Systems, Synchrotrons & Particle Accelerators. Mauro Audi, Academic, Government & Research Marketing Manager
 ION Pumps for UHV Systems, Synchrotrons & Particle Accelerators Mauro Audi, Academic, Government & Research Marketing Manager ION Pumps Agilent Technologies 1957-59 Varian Associates invents the first
ION Pumps for UHV Systems, Synchrotrons & Particle Accelerators Mauro Audi, Academic, Government & Research Marketing Manager ION Pumps Agilent Technologies 1957-59 Varian Associates invents the first
Table of Content. Mechanical Removing Techniques. Ultrasonic Machining (USM) Sputtering and Focused Ion Beam Milling (FIB)
 Table of Content Mechanical Removing Techniques Ultrasonic Machining (USM) Sputtering and Focused Ion Beam Milling (FIB) Ultrasonic Machining In ultrasonic machining (USM), also called ultrasonic grinding,
Table of Content Mechanical Removing Techniques Ultrasonic Machining (USM) Sputtering and Focused Ion Beam Milling (FIB) Ultrasonic Machining In ultrasonic machining (USM), also called ultrasonic grinding,
LECTURE 5 SUMMARY OF KEY IDEAS
 LECTURE 5 SUMMARY OF KEY IDEAS Etching is a processing step following lithography: it transfers a circuit image from the photoresist to materials form which devices are made or to hard masking or sacrificial
LECTURE 5 SUMMARY OF KEY IDEAS Etching is a processing step following lithography: it transfers a circuit image from the photoresist to materials form which devices are made or to hard masking or sacrificial
Vacuum. Residual pressure can thwart the best cryogenic design. Each gas molecule collision carries ~kt from the hot exterior to the cold interior.
 Vacuum Residual pressure can thwart the best cryogenic design Each gas molecule collision carries ~kt from the hot exterior to the cold interior. 1 millitorr = 3.5x10¹³/cm³ Gas atoms leaving the hot surfaces
Vacuum Residual pressure can thwart the best cryogenic design Each gas molecule collision carries ~kt from the hot exterior to the cold interior. 1 millitorr = 3.5x10¹³/cm³ Gas atoms leaving the hot surfaces
Keywords. 1=magnetron sputtering, 2= rotatable cathodes, 3=substrate temperature, 4=anode. Abstract
 Managing Anode Effects and Substrate Heating from Rotatable Sputter Targets. F. Papa*, V. Bellido-Gonzalez**, Alex Azzopardi**, Dr. Dermot Monaghan**, *Gencoa Technical & Business Support in US, Davis,
Managing Anode Effects and Substrate Heating from Rotatable Sputter Targets. F. Papa*, V. Bellido-Gonzalez**, Alex Azzopardi**, Dr. Dermot Monaghan**, *Gencoa Technical & Business Support in US, Davis,
THIN FLEXIBLE POLYMER SUBSTRATES COATED BY THICK FILMS IN ROLL-TO-ROLL VACUUM
 ARCOTRONICS INDUSTRIES SpA Via San Lorenzo, 19 40037 Sasso Marconi (BO) Italy Tel. (+39) 051939111 Fax (+39) 051840684 http://www.arcotronics.com THIN FLEXIBLE POLYMER SUBSTRATES COATED BY THICK FILMS
ARCOTRONICS INDUSTRIES SpA Via San Lorenzo, 19 40037 Sasso Marconi (BO) Italy Tel. (+39) 051939111 Fax (+39) 051840684 http://www.arcotronics.com THIN FLEXIBLE POLYMER SUBSTRATES COATED BY THICK FILMS
STATES OF MATTER STATES OF MATTER. The Four States of Matter 3/5/2015. Solid. Liquid Commonly found on Gas Earth Plasma
 Unit 10: States of Matter Lesson 10.1: States and Their Changes (Review) STATES OF MATTER The Four States of Matter Solid } Liquid Commonly found on Gas Earth Plasma STATES OF MATTER Based upon particle
Unit 10: States of Matter Lesson 10.1: States and Their Changes (Review) STATES OF MATTER The Four States of Matter Solid } Liquid Commonly found on Gas Earth Plasma STATES OF MATTER Based upon particle
STATES OF MATTER STATES OF MATTER. The Four States of Matter 3/5/2015
 The Four States of Matter Unit 10: States of Matter Lesson 10.1: States and Their Changes (Review) Solid } Liquid Commonly found on Gas Earth Plasma Based upon particle arrangement Based upon energy of
The Four States of Matter Unit 10: States of Matter Lesson 10.1: States and Their Changes (Review) Solid } Liquid Commonly found on Gas Earth Plasma Based upon particle arrangement Based upon energy of
To move a particle in a (straight) line over a large distance
 2.1 Introduction to Vacuum Technology 2.1.1 Importance of Vacuum Technology for Processing and Characterization Under partial vacuum conditions (pressures orders of magnitude below ambient atmospheric
2.1 Introduction to Vacuum Technology 2.1.1 Importance of Vacuum Technology for Processing and Characterization Under partial vacuum conditions (pressures orders of magnitude below ambient atmospheric
CHEMISTRY Matter and Change. Chapter 12: States of Matter
 CHEMISTRY Matter and Change Chapter 12: States of Matter CHAPTER 12 States of Matter Section 12.1 Section 12.2 Section 12.3 Section 12.4 Gases Forces of Attraction Liquids and Solids Phase Changes Click
CHEMISTRY Matter and Change Chapter 12: States of Matter CHAPTER 12 States of Matter Section 12.1 Section 12.2 Section 12.3 Section 12.4 Gases Forces of Attraction Liquids and Solids Phase Changes Click
PHYSICAL VAPOR DEPOSITION OF THIN FILMS
 PHYSICAL VAPOR DEPOSITION OF THIN FILMS JOHN E. MAHAN Colorado State University A Wiley-Interscience Publication JOHN WILEY & SONS, INC. New York Chichester Weinheim Brisbane Singapore Toronto CONTENTS
PHYSICAL VAPOR DEPOSITION OF THIN FILMS JOHN E. MAHAN Colorado State University A Wiley-Interscience Publication JOHN WILEY & SONS, INC. New York Chichester Weinheim Brisbane Singapore Toronto CONTENTS
Cryogenic Engineering Prof. M. D. Atrey Department of Mechanical Engineering Indian Institute of Technology, Bombay
 Cryogenic Engineering Prof. M. D. Atrey Department of Mechanical Engineering Indian Institute of Technology, Bombay Lecture No. # 41 Instrumentation in Cryogenics (Refer Slide Time: 00:31) So, welcome
Cryogenic Engineering Prof. M. D. Atrey Department of Mechanical Engineering Indian Institute of Technology, Bombay Lecture No. # 41 Instrumentation in Cryogenics (Refer Slide Time: 00:31) So, welcome
Speed Distribution at CONSTANT Temperature is given by the Maxwell Boltzmann Speed Distribution
 Temperature ~ Average KE of each particle Particles have different speeds Gas Particles are in constant RANDOM motion Average KE of each particle is: 3/2 kt Pressure is due to momentum transfer Speed Distribution
Temperature ~ Average KE of each particle Particles have different speeds Gas Particles are in constant RANDOM motion Average KE of each particle is: 3/2 kt Pressure is due to momentum transfer Speed Distribution
PhysicsAndMathsTutor.com 1
 PhysicsAndMathsTutor.com 1 1. Millikan determined the charge on individual oil droplets using an arrangement as represented in the diagram. The plate voltage necessary to hold a charged droplet stationary
PhysicsAndMathsTutor.com 1 1. Millikan determined the charge on individual oil droplets using an arrangement as represented in the diagram. The plate voltage necessary to hold a charged droplet stationary
Vacuum Technology and film growth. Diffusion Resistor
 Vacuum Technology and film growth Poly Gate pmos Polycrystaline Silicon Source Gate p-channel Metal-Oxide-Semiconductor (MOSFET) Drain polysilicon n-si ion-implanted Diffusion Resistor Poly Si Resistor
Vacuum Technology and film growth Poly Gate pmos Polycrystaline Silicon Source Gate p-channel Metal-Oxide-Semiconductor (MOSFET) Drain polysilicon n-si ion-implanted Diffusion Resistor Poly Si Resistor
The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado
 Experiment 9 1 Introduction The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado During the late nineteenth century, a great deal of evidence accumulated indicating that radiation
Experiment 9 1 Introduction The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado During the late nineteenth century, a great deal of evidence accumulated indicating that radiation
Angular Correlation Experiments
 Angular Correlation Experiments John M. LoSecco April 2, 2007 Angular Correlation Experiments J. LoSecco Notre Dame du Lac Nuclear Spin In atoms one can use the Zeeman Effect to determine the spin state.
Angular Correlation Experiments John M. LoSecco April 2, 2007 Angular Correlation Experiments J. LoSecco Notre Dame du Lac Nuclear Spin In atoms one can use the Zeeman Effect to determine the spin state.
Discovered by German scientist Johann Hittorf in 1869 and in 1876 named by Eugen Goldstein.
 DO PHYSICS ONLINE CATHODE RAYS CATHODE RAYS (electron beams) Streams of electrons (negatively charged particles) observed in vacuum tubes - evacuated glass tubes that are equipped with at least two metal
DO PHYSICS ONLINE CATHODE RAYS CATHODE RAYS (electron beams) Streams of electrons (negatively charged particles) observed in vacuum tubes - evacuated glass tubes that are equipped with at least two metal
Secondary ion mass spectrometry (SIMS)
 Secondary ion mass spectrometry (SIMS) ELEC-L3211 Postgraduate Course in Micro and Nanosciences Department of Micro and Nanosciences Personal motivation and experience on SIMS Offers the possibility to
Secondary ion mass spectrometry (SIMS) ELEC-L3211 Postgraduate Course in Micro and Nanosciences Department of Micro and Nanosciences Personal motivation and experience on SIMS Offers the possibility to
Vacuum I. G. Franchetti CAS - Bilbao. 30/5/2011 G. Franchetti 1
 Vacuum I G. Franchetti CAS - Bilbao 30/5/2011 G. Franchetti 1 Index Introduction to Vacuum Vacuum and the Beam Flow Regimes Creating Vacuum 30/5/2011 G. Franchetti 2 Vacuum in accelerators All beam dynamics
Vacuum I G. Franchetti CAS - Bilbao 30/5/2011 G. Franchetti 1 Index Introduction to Vacuum Vacuum and the Beam Flow Regimes Creating Vacuum 30/5/2011 G. Franchetti 2 Vacuum in accelerators All beam dynamics
Sputter Ion Pump (Ion Pump) By Biswajit
 Sputter Ion Pump (Ion Pump) By Biswajit 08-07-17 Sputter Ion Pump (Ion Pump) An ion pump is a type of vacuum pump capable of reaching pressures as low as 10 11 mbar under ideal conditions. An ion pump
Sputter Ion Pump (Ion Pump) By Biswajit 08-07-17 Sputter Ion Pump (Ion Pump) An ion pump is a type of vacuum pump capable of reaching pressures as low as 10 11 mbar under ideal conditions. An ion pump
- A spark is passed through the Argon in the presence of the RF field of the coil to initiate the plasma
 THE PLASMA Inductively Coupled Plasma Mass Spectrometry (ICP-MS) What is a Plasma? - The magnetic field created by a RF (radio frequency) coil produces a current within a stream of Argon (Ar) gas, which
THE PLASMA Inductively Coupled Plasma Mass Spectrometry (ICP-MS) What is a Plasma? - The magnetic field created by a RF (radio frequency) coil produces a current within a stream of Argon (Ar) gas, which
Introduction to Plasma
 What is a plasma? The fourth state of matter A partially ionized gas How is a plasma created? Energy must be added to a gas in the form of: Heat: Temperatures must be in excess of 4000 O C Radiation Electric
What is a plasma? The fourth state of matter A partially ionized gas How is a plasma created? Energy must be added to a gas in the form of: Heat: Temperatures must be in excess of 4000 O C Radiation Electric
Lecture 6 Plasmas. Chapters 10 &16 Wolf and Tauber. ECE611 / CHE611 Electronic Materials Processing Fall John Labram 1/68
 Lecture 6 Plasmas Chapters 10 &16 Wolf and Tauber 1/68 Announcements Homework: Homework will be returned to you on Thursday (12 th October). Solutions will be also posted online on Thursday (12 th October)
Lecture 6 Plasmas Chapters 10 &16 Wolf and Tauber 1/68 Announcements Homework: Homework will be returned to you on Thursday (12 th October). Solutions will be also posted online on Thursday (12 th October)
Plasma Deposition (Overview) Lecture 1
 Plasma Deposition (Overview) Lecture 1 Material Processes Plasma Processing Plasma-assisted Deposition Implantation Surface Modification Development of Plasma-based processing Microelectronics needs (fabrication
Plasma Deposition (Overview) Lecture 1 Material Processes Plasma Processing Plasma-assisted Deposition Implantation Surface Modification Development of Plasma-based processing Microelectronics needs (fabrication
Etching Issues - Anisotropy. Dry Etching. Dry Etching Overview. Etching Issues - Selectivity
 Etching Issues - Anisotropy Dry Etching Dr. Bruce K. Gale Fundamentals of Micromachining BIOEN 6421 EL EN 5221 and 6221 ME EN 5960 and 6960 Isotropic etchants etch at the same rate in every direction mask
Etching Issues - Anisotropy Dry Etching Dr. Bruce K. Gale Fundamentals of Micromachining BIOEN 6421 EL EN 5221 and 6221 ME EN 5960 and 6960 Isotropic etchants etch at the same rate in every direction mask
ELECTROMAGNETIC WAVES
 VISUAL PHYSICS ONLINE MODULE 7 NATURE OF LIGHT ELECTROMAGNETIC WAVES SPECTRA PRODUCED BY DISCHARGE TUBES CATHODE RAYS (electron beams) Streams of electrons (negatively charged particles) observed in vacuum
VISUAL PHYSICS ONLINE MODULE 7 NATURE OF LIGHT ELECTROMAGNETIC WAVES SPECTRA PRODUCED BY DISCHARGE TUBES CATHODE RAYS (electron beams) Streams of electrons (negatively charged particles) observed in vacuum
Modern Methods in Heterogeneous Catalysis Research: Preparation of Model Systems by Physical Methods
 Modern Methods in Heterogeneous Catalysis Research: Preparation of Model Systems by Physical Methods Methods for catalyst preparation Methods discussed in this lecture Physical vapour deposition - PLD
Modern Methods in Heterogeneous Catalysis Research: Preparation of Model Systems by Physical Methods Methods for catalyst preparation Methods discussed in this lecture Physical vapour deposition - PLD
Warning!! Chapter 5 Gases. Chapter Objectives. Chapter Objectives. Chapter Objectives. Air Pollution
 Warning!! Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 5 Gases These slides contains visual aids for learning BUT they are NOT the actual lecture notes! Failure to attend to lectures most
Warning!! Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 5 Gases These slides contains visual aids for learning BUT they are NOT the actual lecture notes! Failure to attend to lectures most
Name Date Class THE NATURE OF GASES
 13.1 THE NATURE OF GASES Section Review Objectives Describe the assumptions of the kinetic theory as it applies to gases Interpret gas pressure in terms of kinetic theory Define the relationship between
13.1 THE NATURE OF GASES Section Review Objectives Describe the assumptions of the kinetic theory as it applies to gases Interpret gas pressure in terms of kinetic theory Define the relationship between
Electrical Discharges Characterization of Planar Sputtering System
 International Journal of Recent Research and Review, Vol. V, March 213 ISSN 2277 8322 Electrical Discharges Characterization of Planar Sputtering System Bahaa T. Chaid 1, Nathera Abass Ali Al-Tememee 2,
International Journal of Recent Research and Review, Vol. V, March 213 ISSN 2277 8322 Electrical Discharges Characterization of Planar Sputtering System Bahaa T. Chaid 1, Nathera Abass Ali Al-Tememee 2,
Lecture 10. Vacuum Technology and Plasmas Reading: Chapter 10. ECE Dr. Alan Doolittle
 Lecture 10 Vacuum Technology and Plasmas Reading: Chapter 10 Vacuum Science and Plasmas In order to understand deposition techniques such as evaporation, sputtering,, plasma processing, chemical vapor
Lecture 10 Vacuum Technology and Plasmas Reading: Chapter 10 Vacuum Science and Plasmas In order to understand deposition techniques such as evaporation, sputtering,, plasma processing, chemical vapor
Vacuum II. G. Franchetti CAS - Bilbao. 30/5/2011 G. Franchetti 1
 Vacuum II G. Franchetti CAS Bilbao 30/5/2011 G. Franchetti 1 Index Creating Vacuum (continuation) Measuring Vacuum Partial Pressure Measurements 30/5/2011 G. Franchetti 2 Laminar flow Cold surface Diffusion
Vacuum II G. Franchetti CAS Bilbao 30/5/2011 G. Franchetti 1 Index Creating Vacuum (continuation) Measuring Vacuum Partial Pressure Measurements 30/5/2011 G. Franchetti 2 Laminar flow Cold surface Diffusion
A Gas Uniformly fills any container. Easily compressed. Mixes completely with any other gas. Exerts pressure on its surroundings.
 Chapter 5 Gases Chapter 5 A Gas Uniformly fills any container. Easily compressed. Mixes completely with any other gas. Exerts pressure on its surroundings. Copyright Cengage Learning. All rights reserved
Chapter 5 Gases Chapter 5 A Gas Uniformly fills any container. Easily compressed. Mixes completely with any other gas. Exerts pressure on its surroundings. Copyright Cengage Learning. All rights reserved
Application of the GD-Profiler 2 to the PV domain
 Application of the GD-Profiler 2 to the PV domain GD Profiler 2 RF GDOES permits to follow the distribution of the elements as function of depth. This is an ultra fast characterisation technique capable
Application of the GD-Profiler 2 to the PV domain GD Profiler 2 RF GDOES permits to follow the distribution of the elements as function of depth. This is an ultra fast characterisation technique capable
Solutions for Assignment-6
 Solutions for Assignment-6 Q1. What is the aim of thin film deposition? [1] (a) To maintain surface uniformity (b) To reduce the amount (or mass) of light absorbing materials (c) To decrease the weight
Solutions for Assignment-6 Q1. What is the aim of thin film deposition? [1] (a) To maintain surface uniformity (b) To reduce the amount (or mass) of light absorbing materials (c) To decrease the weight
k T m 8 B P m k T M T
 I. INTRODUCTION AND OBJECTIVE OF THE EXPERIENT The techniques for evaporation of chemicals in a vacuum are widely used for thin film deposition on rigid substrates, leading to multiple applications: production
I. INTRODUCTION AND OBJECTIVE OF THE EXPERIENT The techniques for evaporation of chemicals in a vacuum are widely used for thin film deposition on rigid substrates, leading to multiple applications: production
Secondary Ion Mass Spectroscopy (SIMS)
 Secondary Ion Mass Spectroscopy (SIMS) Analyzing Inorganic Solids * = under special conditions ** = semiconductors only + = limited number of elements or groups Analyzing Organic Solids * = under special
Secondary Ion Mass Spectroscopy (SIMS) Analyzing Inorganic Solids * = under special conditions ** = semiconductors only + = limited number of elements or groups Analyzing Organic Solids * = under special
Questions on Electric Fields
 Questions on Electric Fields 1. The diagram shows a positively charged oil drop held at rest between two parallel conducting plates A and B. Oil drop A B 2.50 cm The oil drop has a mass 9.79 x 10 15 kg.
Questions on Electric Fields 1. The diagram shows a positively charged oil drop held at rest between two parallel conducting plates A and B. Oil drop A B 2.50 cm The oil drop has a mass 9.79 x 10 15 kg.
ICPMS Doherty Lecture 1
 ICPMS Doherty Lecture 1 Mass Spectrometry This material provides some background on how to measure isotope abundances by means of mass spectrometry. Mass spectrometers create and separate ionized atoms
ICPMS Doherty Lecture 1 Mass Spectrometry This material provides some background on how to measure isotope abundances by means of mass spectrometry. Mass spectrometers create and separate ionized atoms
INTRODUCTION Strained Silicon Monochromator Magnesium Housing Windows for Monochromator Shutter and Collimator Fission Detector HOPG Monochromator
 Design for a Four-Blade Neutron Interferometer INTRODUCTION Strained Silicon Monochromator The neutron beam used for this interferometer is separated from the NIST reactor's main beam using a strained
Design for a Four-Blade Neutron Interferometer INTRODUCTION Strained Silicon Monochromator The neutron beam used for this interferometer is separated from the NIST reactor's main beam using a strained
Chemistry B11 Chapter 6 Gases, Liquids, and Solids
 Chapter 6 Gases, Liquids, and Solids States of matter: the physical state of matter depends on a balance between the kinetic energy of particles, which tends to keep them apart, and the attractive forces
Chapter 6 Gases, Liquids, and Solids States of matter: the physical state of matter depends on a balance between the kinetic energy of particles, which tends to keep them apart, and the attractive forces
Chapter: States of Matter
 Table of Contents Chapter: States of Matter Section 1: Matter Section 2: Changes of State Section 3: Behavior of Fluids 1 What is matter? Matter is anything that takes up space and has mass. Matter Matter
Table of Contents Chapter: States of Matter Section 1: Matter Section 2: Changes of State Section 3: Behavior of Fluids 1 What is matter? Matter is anything that takes up space and has mass. Matter Matter
CHAPTER 13. States of Matter. Kinetic = motion. Polar vs. Nonpolar. Gases. Hon Chem 13.notebook
 CHAPTER 13 States of Matter States that the tiny particles in all forms of matter are in constant motion. Kinetic = motion A gas is composed of particles, usually molecules or atoms, with negligible volume
CHAPTER 13 States of Matter States that the tiny particles in all forms of matter are in constant motion. Kinetic = motion A gas is composed of particles, usually molecules or atoms, with negligible volume
Understanding KMT using Gas Properties and States of Matter
 Understanding KMT using Gas Properties and States of Matter Learning Goals: Students will be able to describe matter in terms of particle motion. The description should include Diagrams to support the
Understanding KMT using Gas Properties and States of Matter Learning Goals: Students will be able to describe matter in terms of particle motion. The description should include Diagrams to support the
Chapter 13 - States of Matter. Section 13.1 The nature of Gases
 Chapter 13 - States of Matter Section 13.1 The nature of Gases Kinetic energy and gases Kinetic energy: the energy an object has because of its motion Kinetic theory: all matter is made if particles in
Chapter 13 - States of Matter Section 13.1 The nature of Gases Kinetic energy and gases Kinetic energy: the energy an object has because of its motion Kinetic theory: all matter is made if particles in
Technology for Micro- and Nanostructures Micro- and Nanotechnology
 Lecture 10: Deposition Technology for Micro- and Nanostructures Micro- and Nanotechnology Peter Unger mailto: peter.unger @ uni-ulm.de Institute of Optoelectronics University of Ulm http://www.uni-ulm.de/opto
Lecture 10: Deposition Technology for Micro- and Nanostructures Micro- and Nanotechnology Peter Unger mailto: peter.unger @ uni-ulm.de Institute of Optoelectronics University of Ulm http://www.uni-ulm.de/opto
ELEMENT2 High Resolution- ICP-MS INSTRUMENT OVERVIEW
 ELEMENT2 High Resolution- ICP-MS INSTRUMENT OVERVIEW Inductively Coupled Plasma Mass Spectrometry (ICP-MS) What is a Plasma? - The magnetic field created by a RF (radio frequency) coil produces
ELEMENT2 High Resolution- ICP-MS INSTRUMENT OVERVIEW Inductively Coupled Plasma Mass Spectrometry (ICP-MS) What is a Plasma? - The magnetic field created by a RF (radio frequency) coil produces
Chapter 7 Plasma Basic
 Chapter 7 Plasma Basic Hong Xiao, Ph. D. hxiao89@hotmail.com www2.austin.cc.tx.us/hongxiao/book.htm Hong Xiao, Ph. D. www2.austin.cc.tx.us/hongxiao/book.htm 1 Objectives List at least three IC processes
Chapter 7 Plasma Basic Hong Xiao, Ph. D. hxiao89@hotmail.com www2.austin.cc.tx.us/hongxiao/book.htm Hong Xiao, Ph. D. www2.austin.cc.tx.us/hongxiao/book.htm 1 Objectives List at least three IC processes
Ideal Gases. 247 minutes. 205 marks. theonlinephysicstutor.com. facebook.com/theonlinephysicstutor. Name: Class: Date: Time: Marks: Comments:
 Ideal Gases Name: Class: Date: Time: 247 minutes Marks: 205 marks Comments: Page 1 of 48 1 Which one of the graphs below shows the relationship between the internal energy of an ideal gas (y-axis) and
Ideal Gases Name: Class: Date: Time: 247 minutes Marks: 205 marks Comments: Page 1 of 48 1 Which one of the graphs below shows the relationship between the internal energy of an ideal gas (y-axis) and
Gaetano L Episcopo. Scanning Electron Microscopy Focus Ion Beam and. Pulsed Plasma Deposition
 Gaetano L Episcopo Scanning Electron Microscopy Focus Ion Beam and Pulsed Plasma Deposition Hystorical background Scientific discoveries 1897: J. Thomson discovers the electron. 1924: L. de Broglie propose
Gaetano L Episcopo Scanning Electron Microscopy Focus Ion Beam and Pulsed Plasma Deposition Hystorical background Scientific discoveries 1897: J. Thomson discovers the electron. 1924: L. de Broglie propose
CHAPTER 6: Etching. Chapter 6 1
 Chapter 6 1 CHAPTER 6: Etching Different etching processes are selected depending upon the particular material to be removed. As shown in Figure 6.1, wet chemical processes result in isotropic etching
Chapter 6 1 CHAPTER 6: Etching Different etching processes are selected depending upon the particular material to be removed. As shown in Figure 6.1, wet chemical processes result in isotropic etching
Mass Spectrometry in MCAL
 Mass Spectrometry in MCAL Two systems: GC-MS, LC-MS GC seperates small, volatile, non-polar material MS is detection devise (Agilent 320-MS TQ Mass Spectrometer) Full scan monitoring SIM single ion monitoring
Mass Spectrometry in MCAL Two systems: GC-MS, LC-MS GC seperates small, volatile, non-polar material MS is detection devise (Agilent 320-MS TQ Mass Spectrometer) Full scan monitoring SIM single ion monitoring
Institute for Electron Microscopy and Nanoanalysis Graz Centre for Electron Microscopy
 Institute for Electron Microscopy and Nanoanalysis Graz Centre for Electron Microscopy Micromechanics Ass.Prof. Priv.-Doz. DI Dr. Harald Plank a,b a Institute of Electron Microscopy and Nanoanalysis, Graz
Institute for Electron Microscopy and Nanoanalysis Graz Centre for Electron Microscopy Micromechanics Ass.Prof. Priv.-Doz. DI Dr. Harald Plank a,b a Institute of Electron Microscopy and Nanoanalysis, Graz
Matter and Thermal Energy
 Section States of Matter Can you identify the states of matter present in the photo shown? Kinetic Theory The kinetic theory is an explanation of how particles in matter behave. Kinetic Theory The three
Section States of Matter Can you identify the states of matter present in the photo shown? Kinetic Theory The kinetic theory is an explanation of how particles in matter behave. Kinetic Theory The three
Vacuum techniques (down to 1 K)
 Vacuum techniques (down to 1 K) For isolation (deep Knudsen regime) liquid helium dewar / inner vacuum jacket Leak testing at level 10-11 Pa m3/s (10-10 mbar l/s) liquid helium dewar & transfer syphon
Vacuum techniques (down to 1 K) For isolation (deep Knudsen regime) liquid helium dewar / inner vacuum jacket Leak testing at level 10-11 Pa m3/s (10-10 mbar l/s) liquid helium dewar & transfer syphon
Courtesy of ESS and TheRGA web pages part of a series of application and theory notes for public use which are provided free of charge by ESS.
 ESS The RGA freenotes Theory page 1 of 14 RGA Theory Notes Courtesy of ESS and TheRGA web pages part of a series of application and theory notes for public use which are provided free of charge by ESS.
ESS The RGA freenotes Theory page 1 of 14 RGA Theory Notes Courtesy of ESS and TheRGA web pages part of a series of application and theory notes for public use which are provided free of charge by ESS.
2101 Atomic Spectroscopy
 2101 Atomic Spectroscopy Atomic identification Atomic spectroscopy refers to the absorption and emission of ultraviolet to visible light by atoms and monoatomic ions. It is best used to analyze metals.
2101 Atomic Spectroscopy Atomic identification Atomic spectroscopy refers to the absorption and emission of ultraviolet to visible light by atoms and monoatomic ions. It is best used to analyze metals.
JARA FIT Ferienprakticum Nanoelektronik Experiment: Resonant tunneling in quantum structures
 JARA FIT Ferienprakticum Nanoelektronik 2013 Experiment: Resonant tunneling in quantum structures Dr. Mihail Ion Lepsa, Peter Grünberg Institut (PGI 9), Forschungszentrum Jülich GmbH 1. Introduction The
JARA FIT Ferienprakticum Nanoelektronik 2013 Experiment: Resonant tunneling in quantum structures Dr. Mihail Ion Lepsa, Peter Grünberg Institut (PGI 9), Forschungszentrum Jülich GmbH 1. Introduction The
Photoemission Spectroscopy
 FY13 Experimental Physics - Auger Electron Spectroscopy Photoemission Spectroscopy Supervisor: Per Morgen SDU, Institute of Physics Campusvej 55 DK - 5250 Odense S Ulrik Robenhagen,
FY13 Experimental Physics - Auger Electron Spectroscopy Photoemission Spectroscopy Supervisor: Per Morgen SDU, Institute of Physics Campusvej 55 DK - 5250 Odense S Ulrik Robenhagen,
CHEMISTRY NOTES Chapter 12. The Behavior of Gases
 Goals : To gain an understanding of : 1. The kinetic theory of matter. 2. Avogadro's hypothesis. 3. The behavior of gases and the gas laws. NOTES: CHEMISTRY NOTES Chapter 12 The Behavior of Gases The kinetic
Goals : To gain an understanding of : 1. The kinetic theory of matter. 2. Avogadro's hypothesis. 3. The behavior of gases and the gas laws. NOTES: CHEMISTRY NOTES Chapter 12 The Behavior of Gases The kinetic
Homework 2: Forces on Charged Particles
 Homework 2: Forces on Charged Particles 1. In the arrangement shown below, 2 C of positive charge is moved from plate S, which is at a potential of 250 V, to plate T, which is at a potential of 750 V.
Homework 2: Forces on Charged Particles 1. In the arrangement shown below, 2 C of positive charge is moved from plate S, which is at a potential of 250 V, to plate T, which is at a potential of 750 V.
Film Deposition Part 1
 1 Film Deposition Part 1 Chapter 11 : Semiconductor Manufacturing Technology by M. Quirk & J. Serda Spring Semester 2013 Saroj Kumar Patra Semidonductor Manufacturing Technology, Norwegian University of
1 Film Deposition Part 1 Chapter 11 : Semiconductor Manufacturing Technology by M. Quirk & J. Serda Spring Semester 2013 Saroj Kumar Patra Semidonductor Manufacturing Technology, Norwegian University of
E6 PROPERTIES OF GASES Flow-times, density, phase changes, solubility
 E6 PROPERTIES OF GASES Flow-times, density, phase changes, solubility Introduction Kinetic-Molecular Theory The kinetic energy of an object is dependent on its mass and its speed. The relationship, given
E6 PROPERTIES OF GASES Flow-times, density, phase changes, solubility Introduction Kinetic-Molecular Theory The kinetic energy of an object is dependent on its mass and its speed. The relationship, given
Although different gasses may differ widely in their chemical properties, they share many physical properties
 IV. Gases (text Chapter 9) A. Overview of Chapter 9 B. Properties of gases 1. Ideal gas law 2. Dalton s law of partial pressures, etc. C. Kinetic Theory 1. Particulate model of gases. 2. Temperature and
IV. Gases (text Chapter 9) A. Overview of Chapter 9 B. Properties of gases 1. Ideal gas law 2. Dalton s law of partial pressures, etc. C. Kinetic Theory 1. Particulate model of gases. 2. Temperature and
Gases. Pressure is formally defined as the force exerted on a surface per unit area:
 Gases Pressure is formally defined as the force exerted on a surface per unit area: Force is measure in Newtons Area is measured in m 2 and it refers to the Area the particle/object is touching (From the
Gases Pressure is formally defined as the force exerted on a surface per unit area: Force is measure in Newtons Area is measured in m 2 and it refers to the Area the particle/object is touching (From the
Ionization Techniques Part IV
 Ionization Techniques Part IV CU- Boulder CHEM 5181 Mass Spectrometry & Chromatography Presented by Prof. Jose L. Jimenez High Vacuum MS Interpretation Lectures Sample Inlet Ion Source Mass Analyzer Detector
Ionization Techniques Part IV CU- Boulder CHEM 5181 Mass Spectrometry & Chromatography Presented by Prof. Jose L. Jimenez High Vacuum MS Interpretation Lectures Sample Inlet Ion Source Mass Analyzer Detector
PRINCIPLES OF PLASMA DISCHARGES AND MATERIALS PROCESSING
 PRINCIPLES OF PLASMA DISCHARGES AND MATERIALS PROCESSING Second Edition MICHAEL A. LIEBERMAN ALLAN J, LICHTENBERG WILEY- INTERSCIENCE A JOHN WILEY & SONS, INC PUBLICATION CONTENTS PREFACE xrrii PREFACE
PRINCIPLES OF PLASMA DISCHARGES AND MATERIALS PROCESSING Second Edition MICHAEL A. LIEBERMAN ALLAN J, LICHTENBERG WILEY- INTERSCIENCE A JOHN WILEY & SONS, INC PUBLICATION CONTENTS PREFACE xrrii PREFACE
Statistical Physics. Problem Set 4: Kinetic Theory
 Statistical Physics xford hysics Second year physics course Dr A. A. Schekochihin and Prof. A. Boothroyd (with thanks to Prof. S. J. Blundell) Problem Set 4: Kinetic Theory PROBLEM SET 4: Collisions and
Statistical Physics xford hysics Second year physics course Dr A. A. Schekochihin and Prof. A. Boothroyd (with thanks to Prof. S. J. Blundell) Problem Set 4: Kinetic Theory PROBLEM SET 4: Collisions and
Lecture 1: Vapour Growth Techniques
 PH3EC2 Vapour Growth and Epitaxial Growth Lecturer: Dr. Shinoj V K Lecture 1: Vapour Growth Techniques 1.1 Vapour growth The growth of single crystal materials from the vapour phase. Deposition from the
PH3EC2 Vapour Growth and Epitaxial Growth Lecturer: Dr. Shinoj V K Lecture 1: Vapour Growth Techniques 1.1 Vapour growth The growth of single crystal materials from the vapour phase. Deposition from the
Chapter 7. Plasma Basics
 Chapter 7 Plasma Basics 2006/4/12 1 Objectives List at least three IC processes using plasma Name three important collisions in plasma Describe mean free path Explain how plasma enhance etch and CVD processes
Chapter 7 Plasma Basics 2006/4/12 1 Objectives List at least three IC processes using plasma Name three important collisions in plasma Describe mean free path Explain how plasma enhance etch and CVD processes
Mass Analyzers. Principles of the three most common types magnetic sector, quadrupole and time of flight - will be discussed herein.
 Mass Analyzers After the production of ions in ion sources, the next critical step in mass spectrometry is to separate these gas phase ions according to their mass-to-charge ratio (m/z). Ions are extracted
Mass Analyzers After the production of ions in ion sources, the next critical step in mass spectrometry is to separate these gas phase ions according to their mass-to-charge ratio (m/z). Ions are extracted
ETCHING Chapter 10. Mask. Photoresist
 ETCHING Chapter 10 Mask Light Deposited Substrate Photoresist Etch mask deposition Photoresist application Exposure Development Etching Resist removal Etching of thin films and sometimes the silicon substrate
ETCHING Chapter 10 Mask Light Deposited Substrate Photoresist Etch mask deposition Photoresist application Exposure Development Etching Resist removal Etching of thin films and sometimes the silicon substrate
Combinatorial RF Magnetron Sputtering for Rapid Materials Discovery: Methodology and Applications
 Combinatorial RF Magnetron Sputtering for Rapid Materials Discovery: Methodology and Applications Philip D. Rack,, Jason D. Fowlkes,, and Yuepeng Deng Department of Materials Science and Engineering University
Combinatorial RF Magnetron Sputtering for Rapid Materials Discovery: Methodology and Applications Philip D. Rack,, Jason D. Fowlkes,, and Yuepeng Deng Department of Materials Science and Engineering University
Chapter 4 Scintillation Detectors
 Med Phys 4RA3, 4RB3/6R03 Radioisotopes and Radiation Methodology 4-1 4.1. Basic principle of the scintillator Chapter 4 Scintillation Detectors Scintillator Light sensor Ionizing radiation Light (visible,
Med Phys 4RA3, 4RB3/6R03 Radioisotopes and Radiation Methodology 4-1 4.1. Basic principle of the scintillator Chapter 4 Scintillation Detectors Scintillator Light sensor Ionizing radiation Light (visible,
