General Principles of HPLC Method Development
|
|
|
- Roberta Robbins
- 6 years ago
- Views:
Transcription
1 General Principles of PLC Method Development Philip J. Koerner, Ph.D. Thailand September 213 Part 1. General Chromatographic Theory Part 2. The Stationary Phase: An verview of PLC Media Part 3. Role of the Mobile Phase in Selectivity
2 The Liquid Chromatographic Process 3 Column Chromatography: The Beginning of Liquid Chromatography Empty Column Adsorbent particles added Sample is loaded onto the top of the column Solvent is added to the top of the column Separation occurs as the bands move down the column 4 Mikhail Tsvet,
3 Basis of Chromatographic Separation Separation of compounds by PLC depends on the interaction of analyte molecules with the stationary phase and the mobile phase. Mobile Phase Stationary Phase 5 The Liquid Chromatographic Process Analytes Mobile Phase Stationary Phase 6
4 The Liquid Chromatographic Process Analytes Mobile Phase Stationary Phase 7 The Liquid Chromatographic Process Polar/Aqueous Mobile Phase N N N N N Benzo(a)pyrene Ethyl sulfate Non-Polar Stationary Phase (e.g. C18) 8
5 In any separation, almost never get a pure, single mode of separation. In RP, performance will be dictated by mixture of: 1. ydrophobic interactions 2. Polar interactions 3. Ionic interactions Mechanisms of Interaction In RP Chromatography Method Development = modulating stationary phase and mobile phase conditions to optimize these interactions and achieve a specific separation goal. Tapentadol C 3 N C3 9 C 3 C 3 ydrophobic Interactions ydrophobic C 3 3 C Weak, transient interactions between a non-polar stationary phase and the molecules C 3 C 3 ydrophobic & van Der Waals interactions 3C N C 3 Retention will be predicted by Log P values S i 3 C S i C S i 3 C S i 3 - C 3 S i S i S i S i S i S i S i S i S i S i S i 1
6 Polar Interactions C 3 3 C Polar Interactions between polar functions groups of analyte and residual silanols or polar groups on media C C 3 3 N 3 C 3 C ydrogen bonding, dipoledipole interactions Relatively weak and transient S i 3 C S i C S i 3 C S i 3 - C 3 S i S i S i S i S i S i S i S i S i S i S i 11 Ion-exchange Interactions C 3 3 C Interactions between ionizable functional groups on analyte and counter-charged moiety on stationary phase Ion-Exchange Ion-exchange Strong, slow interactions 3 C C 3 N + S i S i 3 C C C 3 3 C S i 3 C S i 3 - C 3 S i S i S i S i S i S i S i S i S i S i S i 12
7 Chromatographic Measurements 13 Chromatographic Measurements 14 k Asym N α
8 Void Volume Analytes which do not interact with the adsorbent elute from the column in a volume equal to the void volume in the column. The void volume of a column is the amount of mobile phase in the column between the adsorbent particles and in the pores of the porous adsorbent particles. Mobile phase occupies the space between the particles or the interstitial volume. Mobile phase fills the pores of the porous adsorbent particles. 15 Void Volume A compound which does not interact with the adsorbent at all elutes at what is termed the void volume or the solvent front. The time that it takes for non-retained components to elute is the void time or t. void volume solvent front t 16
9 Retention Factor (k ) The retention factor of the eluting compound is its elution volume (time) relative to the elution volume (time) of an unretained compound. The k value for a given analytes will be determined by its relative affinity for the stationary phase and mobile phase. Retention factor (k )= t R t t t 17 Retention Factor (k ) For any given analyte, the k value will be most readily modified by changing the % of strong mobile phase (e.g. methanol or ACN). Example: The Separation of Steroids: Column used: Phenomenex Luna C18(2) 15 x 4.6 mm 18
10 Retention Factor (k ): Effect of Changing % ACN 19 Peak Asymmetry (Asym) The asymmetry value (Asym) for a peak is a measure of the divergence of the peak from a perfect Gaussian peak shape. Peaks with asymmetry values > 1. are said to be tailing, those with asymmetry values < 1. are said to be fronting. Asymmetrical peaks can be attributed to a number of factors: (1) Strong secondary interactions (e.g. ionic interactions between basic compounds and residual silanols) (2) Sample overloading (3) Sample solvent incompatibility (4) Poor packing 2
11 Peak Tailing due to Secondary Interactions Classical peak tailing in reversed-phase methods is most commonly caused by strong ionic interactions between basic analytes and residual silanols on the surface of the silica. C 3 3 C Ion-Exchange 3C 3 C N + S i S i 3 C S i S i C 3-3 C C 3 C 3 S i S i S i S i S i S i S i S i S i S i S i 21 Peak Tailing due to Secondary Interactions Example: The Separation of Tricyclic Antidepressants: Column used: Kinetex 2.6µ XB-C18 5 x 2.1 mm Brand 2.7µ C18 5 x 2.1mm Amitriptyline (pka 9.4) Nortriptyline (pka 9.7) Protriptyline (pka 8.2) 22
12 DAD1 C, Sig=254,4 Ref=3,1 (DJ4611\DJGSK \GSK6.D) DAD1 C, Sig=254,4 Ref=3,1 (DJ4611\DJGSK \GSK8.D) DAD1 C, Sig=254,4 Ref=3,1 (DJ4611\DJGSK \GSK1.D) Peak Tailing due to Secondary Interactions Brand 2.7µ C18 5x2.1mm Kinetex 2.6µ XB-C18 5x2.1mm Sample 28 (alo C18-5x2, 2.7 um, Me:.1%... XIC of +MRM (8 pairs): 278.2/191.2 Max. Da 1.1e5 ID: Amitrip cps. from Sample 7 (TCA-Kin-XB-C18, 5x2, 2.6, Me Peak tailing Max. 1.2e5 cps Time, min Time, min XIC of +MRM (8 pairs): 264.2/191.1 Da ID: Protrip from Sample 28 (alo C18-5x2, 2.7 um, Me:.1%... XIC of +MRM (8 pairs): 264.2/191.1 Max. Da 1.1e5 ID: Protrip cps. from Sample 13 (Kinetex XB-C18-5x2, 2.6 um, Me... Max. 1.2e5 cps Time, min Time, min 23 Mobile phase: A =.1% Formic acid in water, B = 1% Methanol, Gradient: 15 to 6% B in 5 min, hold for 1 min Flow rate: 4 µl/min 1. Amoxapine 2. Imipramine 3. Desipramine 4. Protriptyline* 5. Amitriptyline 6. Nortriptyline* 7. Clomipramine 8. Norclomipramine Peak Tailing due to Sample verloading Strongly basic analytes are very sensitive to sample loading, and will display peak tailing effects as a function of increasing loading (µg on column): mau 2.5 µg on column; USP Tailing = pka 9.7 min mau 5 µg on column; USP Tailing = min mau 12 µg on column; USP Tailing = min
13 mau min Peak Fronting due to Sample verloading Neutral and acidic compounds will typical show peak fronting when the column is overloaded. Detector saturation! DAD1 A, Sig=24,1 Ref=off (MT42211\DJGSK \MUPIRCIN1.D) Fronting due to overload Peak Fronting due to Sample Solvent Effects Peak fronting can also be due to sample solvent effects: (1) Sample insolubility (2) Sample solvent is too strong: Sample in 5% Methanol 7.e4 6.5e4 6.e4 5.5e4 5.e4 4.5e4 ydromorphone Sample in 1% Methanol 2.2e5 2.e5 1.8e5 1.6e5 Breakthrough! 1.4e5 4.e4 3.5e4 3.e4 1.2e5 1.e5.24 Fronting 2.5e4 8.e4 2.e4 1.5e4 1.e4 5.. Morphine Norhydrocodone e4 4.e4 2.e
14 Selectivity (α) Selectivity is a measure of the difference in the interactions of two compounds with the stationary phase. Selectivity is a function of both the stationary phase and the mobile phase. α = k 2 /k 1 27 Selectivity (α) The choice of stationary phase will often have a dramatic effect on the selectivity of analytes. C 3 C 3 Estrone Estradiol m A U C18 Column α = m i n m A U 1 Phenyl Column α = m i n
15 Selectivity (α) But mobile phase is also a very powerful tool in modulating selectivity. Column: Gemini 5 µm C6-Phenyl 15 x 4.6mm Mobile phase: 2mM K2P4, p 2.5; % organic as noted Flow rate: 1. ml/min Detection: UV at 22nm 35% Methanol 1. Saccharin 2. p-ydroxybenzoic Acid 3. Sorbic Acid 4. Dehydroacetic Acid 5. Methylparaben 2% Acetonitrile 29 Column Efficiency (N) The amount of band (peak) broadening or dispersion that occurs in the column is measured by calculating the column efficiency (N) expressed as the number of theoretical plates in the column: tr W 1/2 Columns that cause a lot of peak broadening have few theoretical plates. Columns that produce very narrow peaks (little peak broadening) have a large number of theoretical plates. W 3 Peak Width (W)
16 Column Efficiency (N) is a Function of Particle Size Efficiency of a well-packed column will be a function of several factors, one of which will be particle size. As particle size decreases, efficiency will increase. In addition, back-pressure also increases as particle size decreases. 1 µm 5 µm 3 µm Core-Shell sub-2 µm 5, P/m 1, P/m 15, P/m 3, P/m 3, P/m Efficiency Back-Pressure 31 The Core-Shell Advantage Fully Porous Particles Columns packed with core-shell particles will deliver significantly higher efficiency (N) than columns packed with fully-porous particles of the same diameter.* Fully Porous Particles w 1/2 Injected Sample Band * Gritti et al., Journal of Chromatography A, 1217 (21) 1589 t R 6
17 The Core-Shell Advantage Core-Shell Particles Columns packed with core-shell particles will deliver significantly higher efficiency (N) than columns packed with fully-porous particles of the same diameter.* Kinetex Core-Shell Particles w 1/2 5 6 t R * Gritti et al., Journal of Chromatography A, 1217 (21) 1589 The Core-Shell Advantage Comparison Columns packed with core-shell particles will deliver significantly higher efficiency (N) than columns packed with fully-porous particles of the same diameter.* Fully Porous 3 µm C18; 15 x 4.6 mm N = 166,5 p/m Kinetex 2.6 µm C18; 15 x 4.6 mm N = 295,34 p/m 78% Increase in Efficiency! * Gritti et al., Journal of Chromatography A, 1217 (21)
18 The Core-Shell Advantage Efficiency vs. Diameter Columns packed with core-shell particles will deliver significantly higher efficiency (N) than columns packed with fully-porous particles of the same diameter.* 45 Efficiency( p/m) Fully Porous Core-Shell 5 3.5/ / Particle Diameter (µm) *Farcas et al., PLC 212 Conference The van Deemter Equation The three principle factors that cause band broadening and a decrease in column efficiency are described by the van Deemter equation: e e * Simplified version: = A d p + B/µ + C d e 2 µ Particle size Particle size Linear velocity (flow rate) Mass Transfer 36 Linear velocity (flow rate) Mobile phase viscosity
19 The Core-Shell Advantage A-term = A d p + B/u + C d e 2 u Eddy Diffusion Multi-path Effect w 1/2 t R 6 The Core-Shell Advantage B-term = A d p + B/u + C d e 2 u Longitudinal diffusion w 1/2 t R 6
20 The Core-Shell Advantage Fully Porous C-term = A d p + B/µ + C d e 2 µ Mass Transfer (Kinetics) Dispersion due to resistance to mass transfer A B 39 The Core-Shell Advantage Core-Shell C-term = A d p + B/µ + C d e 2 µ Dispersion due to resistance to mass transfer Mass Transfer (Kinetics) A B
21 The Core-Shell Advantage h vs. v Comparison = A d p + B/µ + C d e 2 µ Reduced plate height h x 1 mm Luna-C 18 (2) Eddy dispersion Longitudinal diffusion Solid-liquid mass transfer Reduced plate height h x 1 mm Kinetex-C 18 Eddy dispersion Longitudinal diffusion Solid-liquid mass transfer Reduced velocity ν Reduced velocity ν *Gritti and Guiochon, 212. LC-GC 3(7) Effect of Column Length on Efficiency For a given particle size, column efficiency will be directly proportional to the length of the column. owever, pressure and elution time will also be directly proportional to the column length. 5cm 1cm 15cm 25cm 5, Plates 8 min 5 Bar 1, Plates 16 min 1 Bar 15, Plates 24 min 15 Bar 25, Plates 4 min 25 Bar 42
22 Balancing Column Length and Particle Size Column Length (mm) Efficiency dp 5µm 25 25, 15 15, 1 1, 5 5, 43 Balancing Column Length and Particle Size Column Length (mm) Efficiency dp 5µm Efficiency dp 3µm 25 25, 37, , 22,5 1 1, 15, 5 5, 7,5 44
23 Balancing Column Length and Particle Size Column Length (mm) Efficiency dp 5µm Efficiency dp 3µm Efficiency sub-2µm / Core-shell 25 25, 37, , 22,5 45, 1 1, 15, 3, 5 5, 7,5 15, 45 Balancing Column Length and Particle Size Column Length (mm) Efficiency dp 5µm Efficiency dp 3µm Efficiency sub-2µm / Core-shell %Reduction in Analysis Time 25 25, 37, , 22,5 45, , 15, 3, 6 5 5, 7,5 15, 8 46 Use shorter columns packed with smaller particles to reduce analysis time while maintaining/improving efficiency!!
24 Effect of Flow Rate on Efficiency Effect of Flow Rate on Column Efficiency (1x4.6mm) 35 3 Core-Shell ~2 ml/min Core-Shell 2.6 Luna 3u N (Plates/column) µ ~1.5 ml/min Luna 5u 1 5 5µ ~1 ml/min Flow rate (ml/min) Quick Review The chromatographic measurements that we have discussed so far will all play a significant role in method development. 1. Retention factor (k ) retention of analyte relative to void t Controlled by modulating % strong mobile phase 2. Peak asymmetry peak shape (fronting, tailing, symmetrical) Result of secondary interactions (e.g. Ionic in RP mode) Sensitive to sample loading & sample solvent effects 3. Selectivity (α) difference in the k of two analytes Will be determined by mobile phase composition and nature of stationary phase 4. Efficiency (N) function of peak width and retention Determined by particle size, column length, flow rate Column packing will affect efficiency (vendor) 48
25 Any Questions? 49 Resolution: The Goal of Chromatography 5
26 Resolution: The Goal of Liquid Chromatography The goal and the purpose of liquid chromatography is to resolve the individual components of a sample from each other so that they may be identified and/or quantitated. 51 Resolution: The Goal of Liquid Chromatography Resolution is a measure of how well two peaks are separated from each other. It is calculated as the difference in retention time of two eluting peaks divided by the average width of the two peaks at the baseline. R (resolution) = RT B - RT A /.5 (width A + width B ) 52
27 Resolution: The Goal of Liquid Chromatography α (1) Retention time for the two peaks will be a function of retention factor (k ). k (2) The selectivity (α) will also affect the retention time values for the two peaks. (3) Peak width will be a function of column efficiency (N) and asymmetry (Asym). 53 N, Asym Resolution: The Goal of Liquid Chromatography The equation below allows us to calculate the relative importance of each of these three factors in overall resolution: Efficiency Retention factor Selectivity It is important to note that you (the analyst) have control over each of those factors through your choice of PLC column and running conditions: 1. Efficiency (N) Particle size/morphology and column length 2. Selectivity (α) Stationary phase and mobile phase 3. Retention factor (k ) Stationary phase and mobile phase 54
28 Resolution: The Relative Effectiveness of k, α, and N Most important determinant of resolution!! Constant increase in resolution Ineffective after k ~1 55 The Impact of Efficiency on Resolution Modulating column efficiency is a very effective way to optimize resolution. There is a strong, linear correlation between N and R s, but it is not a 1:1 ratio. Column efficiency is a flexible tool because we can easily modify it via changes in particle size and column length. Column Length (mm) Efficiency 5µm Efficiency 3µm Efficiency sub-2µm / Core-shell 25 25, 37,5 56 More Pressure Longer Run Time 15 15, 22,5 45, 1 1, 15, 3, 5 5, 7,5 15, More efficiency/resolution More Back-Pressure
29 The Impact of Efficiency on Resolution Relative Resolution Doubling column efficiency increases R s by a factor of 1.4x Column Efficiency (Plates) mau The Impact of Efficiency on Resolution µm 8, P/m min mau µm 15, P/m min 58
30 ptimizing Efficiency for Maximum Resolution 1. For method development, start with an intermediate column length, packed with the smallest particle size that system pressure limitations will allow. Conventional PLC 3µm 15x4.6mm or core-shell 1x4.6mm UPLC sub-2µm or core-shell particle Work at optimal flow rate for that particle size 2. Fine-tune for maximum productivity: Excessive resolution shorter column, increase flow rate Insufficient resolution longer column; modify flow rate to compensate for pressure 59 The Impact of Retention Factor on Resolution Retention factor is the most important, yet limited, factor in determining resolution. It is crucial to have a reasonable k value because analytes must be retained in order to separate them. The drawback is that at high k values, passive diffusion causes extensive band broadening and loss of performance. 6
31 ptimizing Retention Factor for Maximum Resolution 1. Adjust k value to be between 2 and 1 In RP, adjust % of organic (acetonitrile or methanol) Altering nature of stationary phase/media can modulate k as well 2. At k < 2, have sub-optimal resolution May also have interference from solvent, non-retained components 3. At k values > 1, band broadening due to diffusion limits resolution gain In RP, complex mixtures of polar and non-polar components will require gradient for optimal performance/run time balance Polar stationary phases can the total elution window of complex mixtures in isocratic mode 61 The Impact of Selectivity on Resolution Small changes in selectivity can have a dramatic effect on retention. This is one of the reason why the same stationary phases from different manufacturers can sometimes give very different results, and also why changes to mobile phase composition can alter the results so strongly. 62
32 Method Development Exercise 1: ptimization to Reduce Analysis Time and Increase Productivity 63 Mupirocin Impurity Profile Column: C8 25 x 4.6mm 5µm Mobile phase: Flow rate: Components: 7 / 3.1M Ammonium acetate / TF 1. ml/min 1-6 = Impurities A - G 7. Mupirocin Mupirocin 64
33 min Mupirocin: riginal Method DAD1 A, Sig=24,1 Ref=off (Z:\1\DATA\MT5211\DJGSK \MUPIRCIN1.D) mau R s 3/4 =.63; k = ~16 min Column: Luna 5µ C8(2) 25 x 4.6mm Mobile phase: 7/3.1M Ammonium acetate p 5.7/TF Flow rate: 1. ml/min 65 Step 1. Adjust k for better Resolution 66 Step 1. Reduce % organic to increase k : Increases R s Increases run time
34 Step 2. ptimize Efficiency and Length Column Length (mm) Efficiency dp 5µm Efficiency dp 3µm Efficiency sub-2µm / Core-shell %Reduction in Analysis Time 25 25, 37, , 22,5 45, , 15, 3, 6 5 5, 7,5 15, 8 67 Step 2. Switch to 15x4.6mm 3 µm media: Reduces analysis time Maintains efficiency Step 3. ptimize the Flow Rate Core-Shell 2.6 Luna 3u Luna 5u N (Plates/column) Flow rate (ml/min) Step 3. Increase flow rate to 1.5 ml/min: ptimizes efficiency for 3 µm
35 mau 8 VWD1 A, Wavelength=24 nm (JL5211\MUPI3.D) Mupirocin: Intermediate Method k = 9; R s 3/4 = min min Column: Luna 3µ C8(2) 15 x 4.6mm Mobile phase: 8/2.1M Ammonium acetate p 5.7/TF Flow rate: 1.5 ml/min 69 R s increased from.63 to 1.6 Run time increased from 16 to 2 minutes Step 4. Switch to Core-Shell Media Column Length (mm) Efficiency dp 5µm Efficiency dp 3µm Efficiency sub-2µm / Core-shell %Reduction in Analysis Time 25 25, 37, , 22,5 45, , 15, 3, 6 5 5, 7,5 15, 8 7 Step 4. Switch to 1x4.6mm Core-Shell media: Reduce analysis time Increase efficiency
36 Mupirocin: Final ptimized Method mau 25 VWD1 A, Wavelength=24 nm (JL5211\MUPI6.D) R s 3/4 = min min Column: Kinetex 2.6µ C8 1 x 4.6mm Mobile phase: 8/2.1M Ammonium acetate p 5.7:TF Flow rate: 1.5 ml/min (2mL/min if pressure allows) R s increased from 1.6 to 2.3 Run time decreased from 2 to 8 minutes 71 Mupirocin: Final ptimized Method Initial Method: m A U D AD 1 A, Sig= 24,1 R ef=o ff (Z:\1 \DAT A\MT 5 211\ DJG SK \MU PIR C IN 1.D) R s 3/4 = min m i n Final ptimized Method: VWD1 A, Wavelength=24 nm (JL5211\MUPI6.D) mau R s 3/4 = min min
37 Any Questions? 73 Part 1. General Chromatographic Theory Part 2. The Stationary Phase: An verview of PLC Media Part 3. Role of the Mobile Phase in Selectivity 74
38 Fully Porous Silica Polymerization Alkaline Tetraethoxysilane Silica Sol-Gel 99.9% of reactive surface area is internal 75 Fully Porous Silica Advantages: Ability to derivatize with numerous bonded phases igh mechanical strength Excellent efficiency ighly amenable to modulation of material characteristics (pore size, surface area, etc.) Disadvantages: Dissolution of silica at p > ~7.5 (may extend with bonded phase) ydrolysis of bonded phase at p <1.5 76
39 rganosilica ybrid Particle Conventional Silica Particle rganosilica ybrid Particle Siloxane Bridge Ethane linkage Dissolution at p > 7.5 Stable to p ~12 77 rganosilica ybrid Particle Advantages: Extended p range from 1-12 Performance and strength of conventional silica particle Unique selectivity Disadvantages: Fewer stationary phases available compared to conventional silica (e.g. cyano, amino) 78
40 Core-Shell Particle.35 µm Porous Shell 2.6 µm Core-Shell Particle 1.9 µm Solid Core 79 Core-Shell Particle Advantages: 3x the efficiency of 5 µm fullyporous media & 2x the efficiency of 3 µm media Pressures compatible with conventional PLC systems* Disadvantages: Pressure is still higher than 3 µm media More sensitive to system extracolumn volumes More sensitive to overload in some cases 8
41 RP Stationary Phase Classes Alkyl bonded phases (C18, C8, C4): Polar-embedded phases: Fusion Phenyl phases (Phenyl, PFP): Polar-endcapped phases: F F F F F ydro 81 Methylene Selectivity We use the methylene selectivity test to determine the ability of stationary phase to separate molecules based upon differences in their hydrophobic character. In general, very hydrophobic bonded phases (e.g. C18) will display higher levels of methylene selectivity than less hydrophobic phases. m A U 2 5 C m A U m i n 4 Phenyl m i n 82
42 Methylene Selectivity.2 C18 > C8 > C5 Phenyl > CN > Amino Slope of log k vs. # -C2- units Luna C18(2) Synergi ydro-rp Jupiter C18 Synergi Max-RP Luna C8(2) Luna C5 Luna Phe-ex Jupiter C4 Synergi Polar-RP Prodigy Phenyl Luna Cyano Luna Amino 83 Methylene Selectivity Columns: Mobile phase: Flow rate: Components: 5µm C18 15x4.6mm 5µm C8 15x4.6mm 5µm Phenyl 15x4.6mm 65:35 Acetonitrile:Water 1 ml/min Two steroids: 1. Testosterone 2. Methyltestosterone C 3 C 3 C 3 C 3 C 3 84 Testosterone Methyltestosterone
43 Phenyl Selectivity Columns: Dimensions: Mobile phase: Flow rate: C18 Phenyl 15 x 4.6 mm 75:25 Methanol:water 1 ml/min C 3 C 3 Components: 1. Estrone 2. Estradiol Estradiol Estrone 85 Phenyl Selectivity C 3 C 3 C18 m A U C18 Estradiol Estrone m in Phenyl m A U 1 Phenyl m in 86
44 Aqueous Stability of Embedded Phases Nucleic Acid Bases: Luna C18(2) Day 1 Polar-Endcapped C18 Day Day Day 6 Phase Collapse! min Aqueous Stability of Embedded Phases LC/MS/MS Analysis of ETG & ETS in Urine: Polar-Endcapped 2.5 µm C18 1x3.mm XIC of -MRM (6 pairs): 221.2/75. Da ID: ETG-1 from Sample 6 (P-2_yro-RP_1x4.6_4u_FR e5 9.5e4 9.e4 8.5e4 Max. 92. cps. 1mM Ammonium formate 8.e4 7.5e4 7.e4 6.5e4 ETG ETS 6.e4 1. Ethyl glucuronide 2. Ethyl sulfate Intensity, cps 5.5e4 5.e4 4.5e4 4.e4 3.5e4 3.e4 2.5e4 2.e4 1.5e4 1.e Time, min XIC of -MRM (6 pairs): 221.2/75. Da ID: ETG-1 from Sample 6 (P-2_yro-RP_1x4.6_4u_FR6... Max. 92. cps. 1.1e4 ETG 1.e4 9. ETG ETS Intensity, cps ETS Time, min 88
45 Part 1. General Chromatographic Theory Part 2. verview of PLC Media Part 3. Role of the Mobile Phase in Selectivity Solvents for RP Chromatography Mobile phase selection is much more challenging that stationary phase selection because the options are limitless. owever, in practical method development, we can dramatically narrow down the options to focus on those conditions which will give us the highest likelihood of success. Typical RP Solvents: Weak Solvent: Water/Buffer Strong Solvent: Acetonitrile (64) Methanol (34) Composite mixtures (1) TF (1) Frequency of use
46 Solvent Selectivity The elution strength of a given solvent is determined by its hydrophobicity (e.g. heptane would be stronger than hexane because it is more hydrophobic). The selectivity of a solvent, however, is determined by its polar characteristics (e.g. heptane and hexane would have the same solvent selectivity). Methanol is a strong proton donor and a strong proton acceptor in hydrogen bonding. Acetonitrile has a dipole moment but is only a very weak proton acceptor in hydrogen bonding. N C 3 δ δ + Tetrahyrofuran is able to accept a proton in hydrogen bonding but cannot donate a proton. Solvent Selectivity ptimum Separation of 4 Steroids in Different Solvents:
47 Solvent Screening for Isocratic Methods 1. Start at high % acetonitrile and work backwards until k is 2-1 (if possible) 8% ACN 4% ACN 25% ACN 21% ACN k = k ~.8 k ~ 6 k ~ 11 Solvent Screening for Isocratic Methods 2. Repeat with alternative solvent:
48 Buffer Selection for RP-PLC = Typical for LC/UV = Typical for LC/MS Effect of p on Base Silica Any silica-based RP material will have some residual silanols left after bonding and end-capping. These Si- groups can be deprotonated at values above p ~3.5. The deprotonated silanols are more likely to engage in ion-exchange with basic analytes, leading to peak tailing. p <3.5 p >3.5 Si Si Si Si Si Si Si Si Si Si Si Si Silanols protonated Less ion-exchange Less peak tailing Silanols deprotonated Increased ion-exchange Increased peak tailing
49 Effect of p on Analyte Ionization The primary mechanism of retention in RP chromatography is hydrophobic interaction. Ionizing compounds will cause them to behave as more polar species, and reduce their hydrophobic interaction with the stationary phase, leading to decreased retention. More hydrophobic More strongly retained Less hydrophobic Less strongly retained More hydrophobic More strongly retained Less hydrophobic Less strongly retained The ionization state of a molecule will be determined by the p of the mobile phase. Therefore, mobile phase p will dictate retention behavior of analytes with ionizable functional groups. Effect of p on Analyte Ionization Acidic Compounds: Basic Compounds: Retention Factor (k ) Retention Factor (k )
50 Effect of p on Analyte Ionization Alkaline + Acidic Alkyl Stationary Phase Aqueous Mobile Phase Effect of p on Analyte Ionization Alkaline + Acidic + Alkyl Stationary Phase Aqueous Mobile Phase
51 Effect of p on Analyte Retention Amitriptyline (pka 9.4) = (B)ase Toluene = (N)eutral Naproxen (pka 4.5) = (A)cid B A A B A B N N N Gradient Analysis The gradient slope is analogous to solvent strength in isocratic elution. Isocratic Solvent Strength: Increasing the solvent strength reduces analysis time but also reduces resolution. Decreasing the solvent strength increases resolution at the cost of increased analysis time. Solvent strength sometimes affects selectivity Gradient Slope: Increasing the gradient slope reduces analysis time but also reduces resolution. Decreasing the gradient slope increases the resolution at the cost of increased analysis time. Gradient slope sometimes affects selectivity The goal of gradient elution is to optimize resolution while minimizing analysis time.
52 Temperature in PLC Methods The use of temperature in PLC method development presents a challenge because it can have unpredictable effects on selectivity. The use of elevated temperatures will: 1. Reduce mobile phase viscosity and back-pressure. This can allow you to operate at higher flow rates, or to use longer columns/smaller particle sizes. 2. Reduce elution time. 3. Improve method reproducibility (as opposed to operating at room temperature). owever, it is impossible to determine if the use of elevated temperatures will help or hinder a specific separation. For complex separations, improvements in one portion of the chromatogram are almost always accompanied by decreases in another part of the same chromatogram. End of Section II Any Questions? 14
53 End of the PLC Method Development Portion
Reversed Phase Solvents
 Part 1. General Chromatographic Theory Part 2. verview of HPLC Media Part 3. The Role of the Mobile Phase in Selectivity Part 4. Column Care and Use Reversed Phase Solvents 2 Solvents for RP Chromatography
Part 1. General Chromatographic Theory Part 2. verview of HPLC Media Part 3. The Role of the Mobile Phase in Selectivity Part 4. Column Care and Use Reversed Phase Solvents 2 Solvents for RP Chromatography
Part 2. Overview of HPLC Media
 Part 1. General Chromatographic Theory Part 2. Overview of HPLC Media Part 3. The Role of the Mobile Phase in Selectivity Part 4. Column Care and Use 1 HPLC Particle Technology Core-Shell Particle Fully
Part 1. General Chromatographic Theory Part 2. Overview of HPLC Media Part 3. The Role of the Mobile Phase in Selectivity Part 4. Column Care and Use 1 HPLC Particle Technology Core-Shell Particle Fully
Chemistry Instrumental Analysis Lecture 28. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 28 High Performance Liquid Chromatography () Instrumentation Normal Phase Chromatography Normal Phase - a polar stationary phase with a less polar mobile phase.
Chemistry 4631 Instrumental Analysis Lecture 28 High Performance Liquid Chromatography () Instrumentation Normal Phase Chromatography Normal Phase - a polar stationary phase with a less polar mobile phase.
LC and LC/MS Column Selection Flow Chart
 LC and LC/MS Column Selection Flow Chart To use the column selection diagram below, simply follow the path for your analyte and mobile phase. At the far right, follow your final column selection to the
LC and LC/MS Column Selection Flow Chart To use the column selection diagram below, simply follow the path for your analyte and mobile phase. At the far right, follow your final column selection to the
penta-hilic UHPLC COLUMNS
 penta-hilic UHPLC COLUMNS penta-hilic Highly retentive, proprietary penta-hydroxy-ligand Excellent peak shape for polar compounds with a variety of functional groups: acids, bases, zwitterions strong and
penta-hilic UHPLC COLUMNS penta-hilic Highly retentive, proprietary penta-hydroxy-ligand Excellent peak shape for polar compounds with a variety of functional groups: acids, bases, zwitterions strong and
Choosing Columns and Conditions for the Best Peak Shape
 Choosing Columns and Conditions for the Best Peak Shape What is Good Peak Shape and Why is it Important? Good peak shape can be defined as a symmetrical or gaussian peak and poor peak shape can include
Choosing Columns and Conditions for the Best Peak Shape What is Good Peak Shape and Why is it Important? Good peak shape can be defined as a symmetrical or gaussian peak and poor peak shape can include
mau 200 ph min
 mau ph - 6 8 TAKE A LOOK INSIDE TWIN Technology TWIN (Two-In-One ) Technology is what gives Gei its superior performance edge. During the final stage of silica manufacturing, a unique silica-organic layer
mau ph - 6 8 TAKE A LOOK INSIDE TWIN Technology TWIN (Two-In-One ) Technology is what gives Gei its superior performance edge. During the final stage of silica manufacturing, a unique silica-organic layer
High Performance Liquid Chromatography
 High Performance Liquid Chromatography What is HPLC? It is a separation technique that involves: Injection of small volume of liquid sample Into a tube packed with a tiny particles (stationary phase).
High Performance Liquid Chromatography What is HPLC? It is a separation technique that involves: Injection of small volume of liquid sample Into a tube packed with a tiny particles (stationary phase).
HPLC Background Chem 250 F 2008 Page 1 of 24
 HPLC Background Chem 250 F 2008 Page 1 of 24 Outline: General and descriptive aspects of chromatographic retention and separation: phenomenological k, efficiency, selectivity. Quantitative description
HPLC Background Chem 250 F 2008 Page 1 of 24 Outline: General and descriptive aspects of chromatographic retention and separation: phenomenological k, efficiency, selectivity. Quantitative description
Chem 230, Fall, 2014 Homework Set # 3 Short Answer SOLUTIONS
 Chem 230, Fall, 2014 Homework Set # 3 Short Answer SOLUTIONS 1. List two advantages of temperature programming in GC. a) Allows separation of solutes with widely varying retention factors in a reasonable
Chem 230, Fall, 2014 Homework Set # 3 Short Answer SOLUTIONS 1. List two advantages of temperature programming in GC. a) Allows separation of solutes with widely varying retention factors in a reasonable
Too Polar for Reversed Phase What Do You Do?
 Too Polar for Reversed Phase What Do You Do? June 20, 2013 Mark Powell Columns and Consumables Technical Support Page 1 C8 or C18 Doesn t Always Do the Job Typical reversed phase conditions involve water/buffer
Too Polar for Reversed Phase What Do You Do? June 20, 2013 Mark Powell Columns and Consumables Technical Support Page 1 C8 or C18 Doesn t Always Do the Job Typical reversed phase conditions involve water/buffer
penta-hilic UHPLC COLUMNS
 penta-hilic UHPLC COLUMNS Highly retentive, proprietary penta-hydroxy-ligand penta-hilic Excellent peak shape for polar compounds with a variety of functional groups: acids, bases, zwitterions strong and
penta-hilic UHPLC COLUMNS Highly retentive, proprietary penta-hydroxy-ligand penta-hilic Excellent peak shape for polar compounds with a variety of functional groups: acids, bases, zwitterions strong and
Hypersil BDS Columns TG 01-05
 TG 0-0 Hypersil BDS Columns Introduction Hypersil BDS columns have gained a reputation over the years as one of the most robust, reproducible and reliable HPLC column brands available. This Technical Guide
TG 0-0 Hypersil BDS Columns Introduction Hypersil BDS columns have gained a reputation over the years as one of the most robust, reproducible and reliable HPLC column brands available. This Technical Guide
High Performance Liquid Chromatography
 Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
High Performance Liquid Chromatography
 Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 1 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 1 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
LC Technical Information
 LC Technical Information Method Transfer to Accucore.6 μm Columns Containing solid core particles, which are engineered to a diameter of.6μm and a very narrow particle size distribution; Accucore HPLC
LC Technical Information Method Transfer to Accucore.6 μm Columns Containing solid core particles, which are engineered to a diameter of.6μm and a very narrow particle size distribution; Accucore HPLC
Product Bulletin. ACE LC/MS and Rapid Analysis HPLC Columns. HPLC Columns
 Product Bulletin HPLC Columns ACE LC/MS and Rapid Analysis HPLC Columns 0 mm, 30 mm, 35 mm and 50 mm column lengths.0,., 3.0, 4.0 and 4.6 mm column diameters Configured for High Sample Throughput Specially
Product Bulletin HPLC Columns ACE LC/MS and Rapid Analysis HPLC Columns 0 mm, 30 mm, 35 mm and 50 mm column lengths.0,., 3.0, 4.0 and 4.6 mm column diameters Configured for High Sample Throughput Specially
William E. Barber, Ph.D. Applications Chemist October
 William E. Barber, Ph.D. Applications Chemist ctober 0 00 Secrets of Good Peak Shape in HPLC Time: :00 p.m. CET Telephone Number: + 44 0 76 088 Chairperson: John Vis The Secrets of Good Peak Shape in HPLC
William E. Barber, Ph.D. Applications Chemist ctober 0 00 Secrets of Good Peak Shape in HPLC Time: :00 p.m. CET Telephone Number: + 44 0 76 088 Chairperson: John Vis The Secrets of Good Peak Shape in HPLC
Your Reversed Phase Method
 PLC Columns for Your Reversed Phase Method Balanced Non-Polar & Polar Selectivity Strong Non-Polar & Polar Selectivity Strong Polar & Aromatic Selectivity Unique Isomeric & alogenated Selectivity Robust
PLC Columns for Your Reversed Phase Method Balanced Non-Polar & Polar Selectivity Strong Non-Polar & Polar Selectivity Strong Polar & Aromatic Selectivity Unique Isomeric & alogenated Selectivity Robust
Exceptional Selectivity of Agilent ZORBAX Eclipse Plus Phenyl-Hexyl Columns to Separate Estrogens
 Exceptional Selectivity of Agilent ZORBAX Eclipse Plus Phenyl-exyl Columns to Separate Estrogens Application Note Pharmaceutical, Environmental Authors John W. enderson Jr. and William J. Long Agilent
Exceptional Selectivity of Agilent ZORBAX Eclipse Plus Phenyl-exyl Columns to Separate Estrogens Application Note Pharmaceutical, Environmental Authors John W. enderson Jr. and William J. Long Agilent
RP C18 column with feature of a silanol group
 RP C8 column with feature of a silanol group lanol Activity Controlled C8 Column Sunrise ctacosyl C8) Sunrise ctadecyl C8) Sunrise ctadecyl-sac C8-SAC has an interaction of silanol groups ChromaNik Sunrise
RP C8 column with feature of a silanol group lanol Activity Controlled C8 Column Sunrise ctacosyl C8) Sunrise ctadecyl C8) Sunrise ctadecyl-sac C8-SAC has an interaction of silanol groups ChromaNik Sunrise
Packed Column for Ultra-Fast Reversed-Phase Liquid Chromatography, TSKgel Super-ODS. Table of Contents
 No. 089 SEPARATION REPORT Packed Column for Ultra-Fast Reversed-Phase Liquid Chromatography, TSKgel Super-ODS Table of Contents 1. Introduction 1 2. Column Specification 1 3. Features of Packing Materials
No. 089 SEPARATION REPORT Packed Column for Ultra-Fast Reversed-Phase Liquid Chromatography, TSKgel Super-ODS Table of Contents 1. Introduction 1 2. Column Specification 1 3. Features of Packing Materials
P R O D U C T B U L L E T I N. Fused-Core particle technology for hyper-fast and super-rugged HPLC columns
 P R O D U C T B U L L E T I N Fused-Core particle technology for hyper-fast and super-rugged HPLC columns HALO column packings are not made the typical way. Instead, the particles packed into HALO columns
P R O D U C T B U L L E T I N Fused-Core particle technology for hyper-fast and super-rugged HPLC columns HALO column packings are not made the typical way. Instead, the particles packed into HALO columns
ProntoSIL C18-EPS Reversed-Phase HPLC Columns
 ProntoSIL C8-EPS Reversed-Phase HPLC Columns Provides excellent separation of polar compounds Better peak shape for acids and bases Stabilized bonded phase for rugged, robust HPLC methods More retentive
ProntoSIL C8-EPS Reversed-Phase HPLC Columns Provides excellent separation of polar compounds Better peak shape for acids and bases Stabilized bonded phase for rugged, robust HPLC methods More retentive
Introduction to Chromatography
 Introduction to Chromatography Dr. Sana Mustafa Assistant Professor Department of Chemistry, Federal Urdu University of Arts, Science & Technology, Karachi. What is Chromatography? Derived from the Greek
Introduction to Chromatography Dr. Sana Mustafa Assistant Professor Department of Chemistry, Federal Urdu University of Arts, Science & Technology, Karachi. What is Chromatography? Derived from the Greek
UPLC Method Development and Validation
 UPLC Method Development and Validation Rev. 2 2008 Waters Corporation Challenges of Method Development Methods are developed throughout the drug development process Samples vary in complexity Redundancy
UPLC Method Development and Validation Rev. 2 2008 Waters Corporation Challenges of Method Development Methods are developed throughout the drug development process Samples vary in complexity Redundancy
The Secrets of Rapid HPLC Method Development. Choosing Columns for Rapid Method Development and Short Analysis Times
 The Secrets of Rapid HPLC Method Development Choosing Columns for Rapid Method Development and Short Analysis Times Rapid Analysis Is More Than Run Time It is developing a method to meet a goal and developing
The Secrets of Rapid HPLC Method Development Choosing Columns for Rapid Method Development and Short Analysis Times Rapid Analysis Is More Than Run Time It is developing a method to meet a goal and developing
Chromatographic Analysis
 Chromatographic Analysis Distribution of Analytes between Phases An analyte is in equilibrium between the two phases [S 1 ] [S 2 ] (in phase 1) (in phase 2) AS [S2 ] K 2 A S [S1 ] 1 AS, A 1 S Activity
Chromatographic Analysis Distribution of Analytes between Phases An analyte is in equilibrium between the two phases [S 1 ] [S 2 ] (in phase 1) (in phase 2) AS [S2 ] K 2 A S [S1 ] 1 AS, A 1 S Activity
Packings for HPLC. Packings for HPLC
 Summary of packings for HPLC In analytical HPLC, packings with particle sizes of 3 to 10 µm are preferred. For preparative separation tasks, also particles with diameters larger than 10 µm are applied.
Summary of packings for HPLC In analytical HPLC, packings with particle sizes of 3 to 10 µm are preferred. For preparative separation tasks, also particles with diameters larger than 10 µm are applied.
Open Column Chromatography, GC, TLC, and HPLC
 Open Column Chromatography, GC, TLC, and HPLC Murphy, B. (2017). Introduction to Chromatography: Lecture 1. Lecture presented at PHAR 423 Lecture in UIC College of Pharmacy, Chicago. USES OF CHROMATOGRAPHY
Open Column Chromatography, GC, TLC, and HPLC Murphy, B. (2017). Introduction to Chromatography: Lecture 1. Lecture presented at PHAR 423 Lecture in UIC College of Pharmacy, Chicago. USES OF CHROMATOGRAPHY
ACE SuperC18. ACE UHPLC and HPLC Columns. Ultra-Inert UHPLC and HPLC Columns with Extended ph Stability
 ACE SuperC8 TM Ultra-Inert UHPLC and HPLC Columns with Extended ph Stability Exploit selectivity at low, intermediate and high ph Use with LC/MS compatible buffers between ph.. High efficiency µm, µm,
ACE SuperC8 TM Ultra-Inert UHPLC and HPLC Columns with Extended ph Stability Exploit selectivity at low, intermediate and high ph Use with LC/MS compatible buffers between ph.. High efficiency µm, µm,
Biochemistry. Biochemical Techniques HPLC
 Description of Module Subject Name Paper Name 12 Module Name/Title 13 1. Objectives 1.1. To understand the basic concept and principle of 1.2. To understand the components and techniques of 1.3. To know
Description of Module Subject Name Paper Name 12 Module Name/Title 13 1. Objectives 1.1. To understand the basic concept and principle of 1.2. To understand the components and techniques of 1.3. To know
What is Chromatography?
 What is Chromatography? Chromatography is a physico-chemical process that belongs to fractionation methods same as distillation, crystallization or fractionated extraction. It is believed that the separation
What is Chromatography? Chromatography is a physico-chemical process that belongs to fractionation methods same as distillation, crystallization or fractionated extraction. It is believed that the separation
Introduction to Chromatographic Separations
 Introduction to Chromatographic Separations Analysis of complex samples usually involves previous separation prior to compound determination. Two main separation methods instrumentation are available:
Introduction to Chromatographic Separations Analysis of complex samples usually involves previous separation prior to compound determination. Two main separation methods instrumentation are available:
Introduction to Chromatographic Separations (Chapter 1) Many determinations involve separation followed by analysis chromatography electrophoresis
 Introduction to Chromatographic Separations (Chapter 1) Many determinations involve separation followed by analysis chromatography electrophoresis Chromatography: sample transported by mobile phase electrostatic
Introduction to Chromatographic Separations (Chapter 1) Many determinations involve separation followed by analysis chromatography electrophoresis Chromatography: sample transported by mobile phase electrostatic
New 5-micron HALO-5 columns based on Fused- Core particle technology boost the performance of HPLC. Compared to other HPLC columns, HALO-5 columns
 New 5-micron HALO-5 columns based on Fused- Core particle technology boost the performance of HPLC. Compared to other HPLC columns, HALO-5 columns have: the highest plate number versus any other 5-micron
New 5-micron HALO-5 columns based on Fused- Core particle technology boost the performance of HPLC. Compared to other HPLC columns, HALO-5 columns have: the highest plate number versus any other 5-micron
High Pressure/Performance Liquid Chromatography (HPLC)
 High Pressure/Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) is a form of column chromatography that pumps a sample mixture or analyte in a solvent (known as the
High Pressure/Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) is a form of column chromatography that pumps a sample mixture or analyte in a solvent (known as the
Chromatography. Gas Chromatography
 Chromatography Chromatography is essentially the separation of a mixture into its component parts for qualitative and quantitative analysis. The basis of separation is the partitioning of the analyte mixture
Chromatography Chromatography is essentially the separation of a mixture into its component parts for qualitative and quantitative analysis. The basis of separation is the partitioning of the analyte mixture
Shodex TM ODP2 HP series columns
 HPLC Columns Shodex TM ODP2 HP series columns Better retention of highly polar substances Technical notebook No. 6 Contents 1. Introduction 1-1. Specifications 1-2. Eluent Compatibility of ODP2 HP Series
HPLC Columns Shodex TM ODP2 HP series columns Better retention of highly polar substances Technical notebook No. 6 Contents 1. Introduction 1-1. Specifications 1-2. Eluent Compatibility of ODP2 HP Series
Method Development Kits
 Method Development Kits sales representative for ordering information or contact...77 Method Development Kits First column choice for Pharma Development USP L C Method Development Kits Maximize retention
Method Development Kits sales representative for ordering information or contact...77 Method Development Kits First column choice for Pharma Development USP L C Method Development Kits Maximize retention
Orosil HPLC Columns. OroSil HPLC columns are designed for the separation of polar, semi-polar, and nonpolar compounds at low to medium ph.
 Orosil OroSil HPLC columns are designed for the separation of polar, semi-polar, and nonpolar compounds at low to medium ph. Excellent organic base selectivity with very low asymmetry values Compatible
Orosil OroSil HPLC columns are designed for the separation of polar, semi-polar, and nonpolar compounds at low to medium ph. Excellent organic base selectivity with very low asymmetry values Compatible
Liquid Chromatography
 Liquid Chromatography 1. Introduction and Column Packing Material 2. Retention Mechanisms in Liquid Chromatography 3. Method Development 4. Column Preparation 5. General Instrumental aspects 6. Detectors
Liquid Chromatography 1. Introduction and Column Packing Material 2. Retention Mechanisms in Liquid Chromatography 3. Method Development 4. Column Preparation 5. General Instrumental aspects 6. Detectors
Instrumental Chemical Analysis
 L2 Page1 Instrumental Chemical Analysis Chromatography (General aspects of chromatography) Dr. Ahmad Najjar Philadelphia University Faculty of Pharmacy Department of Pharmaceutical Sciences 2 nd semester,
L2 Page1 Instrumental Chemical Analysis Chromatography (General aspects of chromatography) Dr. Ahmad Najjar Philadelphia University Faculty of Pharmacy Department of Pharmaceutical Sciences 2 nd semester,
State-of-the-art C18 HPLC Columns
 An HPLC GL Sciences Newest and Most Advanced ODS Phase-New For 00 State-of-the-art C HPLC s Improved Peak Shapes and Heights Enhancing Sensitivity High Resolution Fast Equilibration Compatible with 00%
An HPLC GL Sciences Newest and Most Advanced ODS Phase-New For 00 State-of-the-art C HPLC s Improved Peak Shapes and Heights Enhancing Sensitivity High Resolution Fast Equilibration Compatible with 00%
Put the. C18 aside. and take a look... Reasons. to try the. NEW Gemini C6-Phenyl
 Put the C18 aside and take a look... 6 Reasons to try the EW Gemini Reason #6 ALTERATE SELECTIVITY to your C18 Column Adding to your method development options, Gemini offers additional polar and aromatic
Put the C18 aside and take a look... 6 Reasons to try the EW Gemini Reason #6 ALTERATE SELECTIVITY to your C18 Column Adding to your method development options, Gemini offers additional polar and aromatic
3µm Fortis C18. Fortis 2.1µm. Increased Efficiency. Scalability and Sensitivity
 Fortis 2.1µm 2.1µm Fortis C18 (2) High Efficiency Robust Particles Operate to 15,000psi Fully Scalable to analytical and prep size Fortis 2.1µm particles are designed to provide characteristics, which
Fortis 2.1µm 2.1µm Fortis C18 (2) High Efficiency Robust Particles Operate to 15,000psi Fully Scalable to analytical and prep size Fortis 2.1µm particles are designed to provide characteristics, which
How proteins separate on reverse-phase HPLC
 1 Reverse Phase How proteins separate on reverse-phase HPLC RP chromatography separates proteins through the interaction of the hydrophobic foot of the protein with a nonpolar surface of the particle RP
1 Reverse Phase How proteins separate on reverse-phase HPLC RP chromatography separates proteins through the interaction of the hydrophobic foot of the protein with a nonpolar surface of the particle RP
Barry E. Boyes, Ph.D. Consumables and Accessories Business Unit May 10, 2000
 Barry E. Boyes, Ph.D. Consumables and Accessories Business Unit May 0, 000 HPLC Column Troubleshooting What Every HPLC User Should Know :00 a.m. EST Telephone Number: 86-650-06 Chair Person: Tim Spaeder
Barry E. Boyes, Ph.D. Consumables and Accessories Business Unit May 0, 000 HPLC Column Troubleshooting What Every HPLC User Should Know :00 a.m. EST Telephone Number: 86-650-06 Chair Person: Tim Spaeder
Silanol. Groups Liquid Chromatography
 Abstract umber: 0-8P Study of Secondary Interaction Based on Residual lanol Groups for Reversed-Phase Liquid Chromatography II orikazu agae Ph.D, Kouji Yamamoto, Chiaki Kadota Chromaik Technologies Inc.
Abstract umber: 0-8P Study of Secondary Interaction Based on Residual lanol Groups for Reversed-Phase Liquid Chromatography II orikazu agae Ph.D, Kouji Yamamoto, Chiaki Kadota Chromaik Technologies Inc.
LIQUID CHROMATOGRAPHY
 LIQUID CHROMATOGRAPHY RECENT TECHNIQUES HPLC High Performance Liquid Chromatography RRLC Rapid Resolution Liquid Chromatography UPLC Ultra Performance Liquid Chromatography UHPLC Ultra High Pressure Liquid
LIQUID CHROMATOGRAPHY RECENT TECHNIQUES HPLC High Performance Liquid Chromatography RRLC Rapid Resolution Liquid Chromatography UPLC Ultra Performance Liquid Chromatography UHPLC Ultra High Pressure Liquid
Thermo Scientific Accucore XL HPLC Columns. Technical Manual
 Thermo Scientific Accucore XL HPLC Columns Technical Manual Thermo Scientific Accucore XL HPLC Columns Based on Core Enhanced Technology using µm solid core particles, Accucore XL HPLC columns allow users
Thermo Scientific Accucore XL HPLC Columns Technical Manual Thermo Scientific Accucore XL HPLC Columns Based on Core Enhanced Technology using µm solid core particles, Accucore XL HPLC columns allow users
A Look at Agilent HPLC Columns Choices
 A Look at Agilent HPLC Columns Choices Brno & Prague, May 29-30 2018 Giorgio Ferlat IDO Product Specialist Chemistries and Consumable 1 Considerating Column Properties for Column Selection Silica Particle
A Look at Agilent HPLC Columns Choices Brno & Prague, May 29-30 2018 Giorgio Ferlat IDO Product Specialist Chemistries and Consumable 1 Considerating Column Properties for Column Selection Silica Particle
Luna 2.5 µm C18(2)-HST. Advantages of 2.5 µm for increasing the speed of analysis while maintaining high efficiency
 Luna 2.5 µm C18(2)-HST Advantages of 2.5 µm for increasing the speed of analysis while maintaining high efficiency Table of Contents Part 1 Theory 1.1 Abstract...3 1.2 Introduction...3 Part 2 Set Up 2.1
Luna 2.5 µm C18(2)-HST Advantages of 2.5 µm for increasing the speed of analysis while maintaining high efficiency Table of Contents Part 1 Theory 1.1 Abstract...3 1.2 Introduction...3 Part 2 Set Up 2.1
The Theory of HPLC. Ion Pair Chromatography
 The Theory of HPLC Ion Pair Chromatography i Wherever you see this symbol, it is important to access the on-line course as there is interactive material that cannot be fully shown in this reference manual.
The Theory of HPLC Ion Pair Chromatography i Wherever you see this symbol, it is important to access the on-line course as there is interactive material that cannot be fully shown in this reference manual.
Analytical Chemistry
 Analytical Chemistry Chromatographic Separations KAM021 2016 Dr. A. Jesorka, 6112, aldo@chalmers.se Introduction to Chromatographic Separations Theory of Separations -Chromatography Terms Summary: Chromatography
Analytical Chemistry Chromatographic Separations KAM021 2016 Dr. A. Jesorka, 6112, aldo@chalmers.se Introduction to Chromatographic Separations Theory of Separations -Chromatography Terms Summary: Chromatography
M > ACN > > THF
 Method Development in HPLC Dr. Amitha Hewavitharana School of Pharmacy University of Queensland Method Development in HPLC References: D A Skoog Principles of instrumental analysis, 3 rd Edition Chapters
Method Development in HPLC Dr. Amitha Hewavitharana School of Pharmacy University of Queensland Method Development in HPLC References: D A Skoog Principles of instrumental analysis, 3 rd Edition Chapters
Welcome to our E-Seminar: Choosing HPLC Columns for Faster Analysis Smaller and Faster
 Welcome to our E-Sear: Choosing HPLC Columns for Faster Analysis Smaller and Faster High Throughput/Fast LC Requires. Short columns 0 mm or shorter Small particle sizes. µm Rapid Resolution or new.8 µm
Welcome to our E-Sear: Choosing HPLC Columns for Faster Analysis Smaller and Faster High Throughput/Fast LC Requires. Short columns 0 mm or shorter Small particle sizes. µm Rapid Resolution or new.8 µm
Small Molecule Selectivity Sampler
 Small Molecule Selectivity Sampler HALO 90 Å C18, AQ-C18, BIPHENYL Fused-Core Particle Technology Why Choose Fused-Core Particle Technology? Fused-Core Out-Performs Totally Porous Particles. HALO particles
Small Molecule Selectivity Sampler HALO 90 Å C18, AQ-C18, BIPHENYL Fused-Core Particle Technology Why Choose Fused-Core Particle Technology? Fused-Core Out-Performs Totally Porous Particles. HALO particles
SELECTING HPLC COLUMNS: GOING UNDER THE HOOD. HPLC Column Selection Guide
 HPLC Column Selection Guide SELECTING HPLC COLUMNS: GOING UNDER THE HOOD INTRODUCTION With hundreds of stationary phases and columns commercially available in the market, it can be difficult to select
HPLC Column Selection Guide SELECTING HPLC COLUMNS: GOING UNDER THE HOOD INTRODUCTION With hundreds of stationary phases and columns commercially available in the market, it can be difficult to select
Overview Applications of NUCLEODUR HPLC Phases
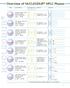 verview Applications of HPLC Phases Phase Specification Characteristics* Stability Structure A B C octadecyl phase, high density coating multi-endcapping 8% C USP L ph stability -, suitable for LC/MS (Si-
verview Applications of HPLC Phases Phase Specification Characteristics* Stability Structure A B C octadecyl phase, high density coating multi-endcapping 8% C USP L ph stability -, suitable for LC/MS (Si-
LEARNING OBJECTIVES CHEM 212: SEPARATION SCIENCE CHROMATOGRAPHY UNIT. Thomas Wenzel, Bates College. In-class Problem Set Extraction.
 LEARNING OBJECTIVES CHEM 212: SEPARATION SCIENCE CHROMATOGRAPHY UNIT Thomas Wenzel, Bates College In-class Problem Set Extraction Problem #1 1. Devise a scheme to be able to isolate organic acids, bases
LEARNING OBJECTIVES CHEM 212: SEPARATION SCIENCE CHROMATOGRAPHY UNIT Thomas Wenzel, Bates College In-class Problem Set Extraction Problem #1 1. Devise a scheme to be able to isolate organic acids, bases
1.7 m Fortis UHPLC Columns
 Strength in Technology 1.7 m Fortis Columns Ultra High Pressure Chromatography Improve your Performance 8 Chemistry Choices Increase Efficiency Increase Speed Improve Resolution Greater Sensitivity Lower
Strength in Technology 1.7 m Fortis Columns Ultra High Pressure Chromatography Improve your Performance 8 Chemistry Choices Increase Efficiency Increase Speed Improve Resolution Greater Sensitivity Lower
Improve Peak Shape and Productivity in HPLC Analysis of Pharmaceutical Compounds with Eclipse Plus C8 Columns Application
 Improve Peak Shape and Productivity in HPLC Analysis of Pharmaceutical Compounds with Eclipse Plus C8 Columns Application Pharmaceuticals Authors John W. Henderson Jr., Nona Martone, and Cliff Woodward
Improve Peak Shape and Productivity in HPLC Analysis of Pharmaceutical Compounds with Eclipse Plus C8 Columns Application Pharmaceuticals Authors John W. Henderson Jr., Nona Martone, and Cliff Woodward
HPLC Column Classification
 HPLC Column Classification Introduction 1978 USP XIX Fourth supplement : L1 designation for C18 column 1980 USP XX : 7 columns were classified -Classified HPLC column to 56 descriptions -More than 220
HPLC Column Classification Introduction 1978 USP XIX Fourth supplement : L1 designation for C18 column 1980 USP XX : 7 columns were classified -Classified HPLC column to 56 descriptions -More than 220
ACE Excel SuperC18. ACE UHPLC and HPLC Columns. Ultra-Inert UHPLC and HPLC Columns with Extended ph Stability
 ACE Excel SuperC8 TM Ultra-Inert UHPLC and HPLC s with Extended ph Stability Exploit selectivity at low, intermediate and high ph Use with LC/MS-compatible buffers between ph.. High efficiency µm, µm,
ACE Excel SuperC8 TM Ultra-Inert UHPLC and HPLC s with Extended ph Stability Exploit selectivity at low, intermediate and high ph Use with LC/MS-compatible buffers between ph.. High efficiency µm, µm,
Instrumental Analysis II Course Code: CH3109. Chromatographic &Thermal Methods of Analysis Part 1: General Introduction. Prof. Tarek A.
 Instrumental Analysis II Course Code: CH3109 Chromatographic &Thermal Methods of Analysis Part 1: General Introduction Prof. Tarek A. Fayed What is chemical analysis? Qualitative analysis (1) Chemical
Instrumental Analysis II Course Code: CH3109 Chromatographic &Thermal Methods of Analysis Part 1: General Introduction Prof. Tarek A. Fayed What is chemical analysis? Qualitative analysis (1) Chemical
Method Development in Solid Phase Extraction using Non-Polar ISOLUTE SPE Columns for the Extraction of Aqueous Samples
 Technical Note 101 Method Development in Solid Phase Extraction using Non-Polar ISOLUTE SPE Columns for the Extraction of Aqueous Samples This technical note includes by specific information on the extraction
Technical Note 101 Method Development in Solid Phase Extraction using Non-Polar ISOLUTE SPE Columns for the Extraction of Aqueous Samples This technical note includes by specific information on the extraction
Comparison of different aqueous mobile phase HPLC techniques
 June 009 ewsletter Pharmaceutical Analysis: What is Your Problem? SIELC Technologies, Inc., Prospect Heights, IL 0070 Pharmaceutical analysis involves liquid chromatography of various compounds, from active
June 009 ewsletter Pharmaceutical Analysis: What is Your Problem? SIELC Technologies, Inc., Prospect Heights, IL 0070 Pharmaceutical analysis involves liquid chromatography of various compounds, from active
Fast Separation of Vastly Different Compounds by Isocratic HPLC
 Fast Separation of Vastly Different Compounds by Isocratic HPLC Aimee N. Heyrman and Yury Zelechonok Resolution Systems, Inc. 590 E. 32nd St. Holland. MI, 49423 SIELC Technologies, 15 E. Palatine Rd, Suite
Fast Separation of Vastly Different Compounds by Isocratic HPLC Aimee N. Heyrman and Yury Zelechonok Resolution Systems, Inc. 590 E. 32nd St. Holland. MI, 49423 SIELC Technologies, 15 E. Palatine Rd, Suite
columns Acclaim Mixed-Mode WCX-1 for Separating Basic Molecules
 columns Acclaim Mixed-Mode WCX- for Separating Basic Molecules The Acclaim Mixed-Mode WCX- is a novel, high-efficiency, silica-based column specially designed for separating various basic analytes. This
columns Acclaim Mixed-Mode WCX- for Separating Basic Molecules The Acclaim Mixed-Mode WCX- is a novel, high-efficiency, silica-based column specially designed for separating various basic analytes. This
Novel LC/MS-Compatible Stationary Phase with Polar Selectivity
 Novel LC/MS-Compatible Stationary Phase with Polar Selectivity Carmen T. Santasania and David S. Bell Supelco 595 N. Harrison Rd., Bellefonte, PA 16823 T403168 GJU Introduction Stationary phases based
Novel LC/MS-Compatible Stationary Phase with Polar Selectivity Carmen T. Santasania and David S. Bell Supelco 595 N. Harrison Rd., Bellefonte, PA 16823 T403168 GJU Introduction Stationary phases based
8. Methods in Developing Mobile Phase Condition for C18 Column
 I. HPLC Columns Technical Information 8. Methods in Developing Mobile Phase Condition for C18 Column Introduction In reversed phase HPLC, octadecyl group bonded silica columns (C18, ODS) are the most widely
I. HPLC Columns Technical Information 8. Methods in Developing Mobile Phase Condition for C18 Column Introduction In reversed phase HPLC, octadecyl group bonded silica columns (C18, ODS) are the most widely
Chromatographic Separation
 What is? is the ability to separate molecules using partitioning characteristics of molecule to remain in a stationary phase versus a mobile phase. Once a molecule is separated from the mixture, it can
What is? is the ability to separate molecules using partitioning characteristics of molecule to remain in a stationary phase versus a mobile phase. Once a molecule is separated from the mixture, it can
ACE SuperC18. ACE UHPLC and HPLC Columns. Ultra-Inert UHPLC and HPLC Columns with Extended ph Stability
 ACE SuperC8 TM Ultra-Inert UHPLC and HPLC s with Extended ph Stability Exploit selectivity at low, intermediate and high ph Use with LC/MS compatible buffers between ph.. High efficiency µm, µm, µm and
ACE SuperC8 TM Ultra-Inert UHPLC and HPLC s with Extended ph Stability Exploit selectivity at low, intermediate and high ph Use with LC/MS compatible buffers between ph.. High efficiency µm, µm, µm and
Analysis - HPLC A.136. Primesep 5 µm columns B.136
 Primesep 5 µm columns Primesep columns feature double functionality of the bonding i.e : alkyl chain with anionic or cationic group, chelating group. This feature creates unique selectivities when using
Primesep 5 µm columns Primesep columns feature double functionality of the bonding i.e : alkyl chain with anionic or cationic group, chelating group. This feature creates unique selectivities when using
Optimisil HPLC & UPLC Column catalogue
 Optimisil HPLC & UPLC Column catalogue Analytical Hub Plot No: 37, P & T Colony, Vikrampuri, Secunderabad 500009 Tel: 040-64621313, Mobile: +91 7799931313 Website: www.analyticalhub.com email: contact@analyticalhub.com
Optimisil HPLC & UPLC Column catalogue Analytical Hub Plot No: 37, P & T Colony, Vikrampuri, Secunderabad 500009 Tel: 040-64621313, Mobile: +91 7799931313 Website: www.analyticalhub.com email: contact@analyticalhub.com
COMPARISON GUIDE. to C18 Reversed Phase HPLC Columns. Comparison Data on 60 Commonly Used C18 Phases. Stationary Phase Specifications
 COMPARISON GUIDE to C18 Reversed Phase HPLC Columns Comparison Data on 60 Commonly Used C18 Phases Stationary Phase Specifications Phases Compared According to Relative Hydrophobicity Phases Compared According
COMPARISON GUIDE to C18 Reversed Phase HPLC Columns Comparison Data on 60 Commonly Used C18 Phases Stationary Phase Specifications Phases Compared According to Relative Hydrophobicity Phases Compared According
Zirconia: the Ideal Substrate for Ion-Exchange LC and LC-MS
 Zirconia: the Ideal Substrate for Ion-Exchange LC and LC-MS EAS 2005 Bingwen Yan 1, Clayton V. McNeff 1, Richard A. Henry 2, and David S. Bell 2 1 ZirChrom Separations, Inc., 617 Pierce Street, Anoka,
Zirconia: the Ideal Substrate for Ion-Exchange LC and LC-MS EAS 2005 Bingwen Yan 1, Clayton V. McNeff 1, Richard A. Henry 2, and David S. Bell 2 1 ZirChrom Separations, Inc., 617 Pierce Street, Anoka,
Simultaneous estimation of amitryptyline and chlordiazepoxide by RP-HPLC method
 IJPAR Vol.3 Issue 4 Oct-Dec-2014 Journal Home page: ISSN: 2320-2831 Research article Open Access Simultaneous estimation of amitryptyline and chlordiazepoxide by RP-HPLC method Dr.R.Srinivasan*, K.Lurdhu
IJPAR Vol.3 Issue 4 Oct-Dec-2014 Journal Home page: ISSN: 2320-2831 Research article Open Access Simultaneous estimation of amitryptyline and chlordiazepoxide by RP-HPLC method Dr.R.Srinivasan*, K.Lurdhu
Fast Screening Methods for Steroids by HPLC with Agilent Poroshell 120 Columns
 Fast Screening Methods for Steroids by HPLC with Agilent Poroshell 2 Columns Application Note Pharma, BioPharma, and Clinical Research Author William Long Agilent Technologies, Inc. Introduction Steroids
Fast Screening Methods for Steroids by HPLC with Agilent Poroshell 2 Columns Application Note Pharma, BioPharma, and Clinical Research Author William Long Agilent Technologies, Inc. Introduction Steroids
CHROMATOGRAPHY. The term "chromatography" is derived from the original use of this method for separating yellow and green plant pigments.
 CHROMATOGRAPHY The term "chromatography" is derived from the original use of this method for separating yellow and green plant pigments. THEORY OF CHROMATOGRAPHY: Separation of two sample components in
CHROMATOGRAPHY The term "chromatography" is derived from the original use of this method for separating yellow and green plant pigments. THEORY OF CHROMATOGRAPHY: Separation of two sample components in
Partitioning. Separation is based on the analyte s relative solubility between two liquid phases or a liquid and solid.
 Chromatography Various techniques for the separation of complex mixtures that rely on the differential affinities of substances for a gas or liquid mobile medium and for a stationary adsorbing medium through
Chromatography Various techniques for the separation of complex mixtures that rely on the differential affinities of substances for a gas or liquid mobile medium and for a stationary adsorbing medium through
Kromasil. for your analytical HPLC. Kromasil. The way to peak performance in liquid chromatography
 Kromasil for your analytical HPLC Kromasil The way to peak performance in liquid chromatography Kromasil is known, worldwide, for its high performance and excellent total economy in preparative and industrial
Kromasil for your analytical HPLC Kromasil The way to peak performance in liquid chromatography Kromasil is known, worldwide, for its high performance and excellent total economy in preparative and industrial
Impact of Instrument Dispersion on Performance of HPLC Capillary Columns
 Impact of Instrument Dispersion on Performance of HPLC Capillary Columns Wendy Roe, Richard A. Henry, and Hillel Brandes Supelco, Div. of Sigma-Aldrich Bellefonte, PA 16823 USA T413131 sigma-aldrich.com/analytical
Impact of Instrument Dispersion on Performance of HPLC Capillary Columns Wendy Roe, Richard A. Henry, and Hillel Brandes Supelco, Div. of Sigma-Aldrich Bellefonte, PA 16823 USA T413131 sigma-aldrich.com/analytical
Acclaim Mixed-Mode WCX-1
 Acclaim Mixed-Mode WCX-1 Product Manual for the Acclaim Mixed-Mode WCX-1 Column Page 1 of 28 PRODUCT MANUAL for the Acclaim Mixed-Mode WCX-1 Columns 4.6 x 150 mm, P/N (068353) 4.6 x 250 mm, P/N (068352)
Acclaim Mixed-Mode WCX-1 Product Manual for the Acclaim Mixed-Mode WCX-1 Column Page 1 of 28 PRODUCT MANUAL for the Acclaim Mixed-Mode WCX-1 Columns 4.6 x 150 mm, P/N (068353) 4.6 x 250 mm, P/N (068352)
Theory and Instrumentation of GC. Chromatographic Parameters
 Theory and Instrumentation of GC Chromatographic Parameters i Wherever you see this symbol, it is important to access the on-line course as there is interactive material that cannot be fully shown in this
Theory and Instrumentation of GC Chromatographic Parameters i Wherever you see this symbol, it is important to access the on-line course as there is interactive material that cannot be fully shown in this
SEPARATIONS ESSENTIALS IN MODERN HPLC. 2University of Bucharest, Bucharest, Romania
 ESSENTIALS IN MODERN HPLC SEPARATIONS Serban C. Moldoveanu1, Victor David2 'R.J. Reynolds Tobacco Co., Winston-Salem, NC, USA 2University of Bucharest, Bucharest, Romania ELSEVIER AMSTERDAM BOSTON HEIDELBERG
ESSENTIALS IN MODERN HPLC SEPARATIONS Serban C. Moldoveanu1, Victor David2 'R.J. Reynolds Tobacco Co., Winston-Salem, NC, USA 2University of Bucharest, Bucharest, Romania ELSEVIER AMSTERDAM BOSTON HEIDELBERG
for Acclaim Mixed-Mode HILIC-1 Column
 for Acclaim Mixed-Mode HILIC-1 Column Product Manual for ACCLAIM Mixed-Mode HILIC-1 Page 1 of 17 Product Manual for ACCLAIM Mixed-Mode HILIC-1 Column 5µm, 4.6 x 250mm, P/N 066844 5µm, 4.6 x 150mm, P/N
for Acclaim Mixed-Mode HILIC-1 Column Product Manual for ACCLAIM Mixed-Mode HILIC-1 Page 1 of 17 Product Manual for ACCLAIM Mixed-Mode HILIC-1 Column 5µm, 4.6 x 250mm, P/N 066844 5µm, 4.6 x 150mm, P/N
ACE Ultra Inert Base Deactivated HPLC Columns
 ACE Ultra Inert Base Deactivated HPLC Columns Contents Page ACE HPLC Columns...- Independent Comparison of HPLC Columns #... Independent Comparison of HPLC Columns #... ACE 00Å HPLC Columns Specifications...-
ACE Ultra Inert Base Deactivated HPLC Columns Contents Page ACE HPLC Columns...- Independent Comparison of HPLC Columns #... Independent Comparison of HPLC Columns #... ACE 00Å HPLC Columns Specifications...-
for Acclaim Mixed-Mode WCX-1
 for Acclaim Mixed-Mode WCX- Product Manual for the Acclaim Mixed-Mode WCX- Column Page of 8 Product Manual for Acclaim Mixed-Mode WCX- Columns Acclaim Mixed-Mode WCX-, 3µm, Analytical column, 3.0 x 50mm
for Acclaim Mixed-Mode WCX- Product Manual for the Acclaim Mixed-Mode WCX- Column Page of 8 Product Manual for Acclaim Mixed-Mode WCX- Columns Acclaim Mixed-Mode WCX-, 3µm, Analytical column, 3.0 x 50mm
2. a) R N and L N so R L or L R 2.
 1. Use the formulae on the Some Key Equations and Definitions for Chromatography sheet. a) 0.74 (remember that w b = 1.70 x w ½ ) b) 5 c) 0.893 (α always refers to two adjacent peaks) d) 1.0x10 3 e) 0.1
1. Use the formulae on the Some Key Equations and Definitions for Chromatography sheet. a) 0.74 (remember that w b = 1.70 x w ½ ) b) 5 c) 0.893 (α always refers to two adjacent peaks) d) 1.0x10 3 e) 0.1
LC Column Troubleshooting. Do you have an equivalent column for my LC column?
 LC Column Troubleshooting Do you have an equivalent column for my LC column? LC columns from different suppliers may have very different retention properties even if the bonding is the same. Although there
LC Column Troubleshooting Do you have an equivalent column for my LC column? LC columns from different suppliers may have very different retention properties even if the bonding is the same. Although there
Phenyl-Hexyl. UHPLC Columns. Alternate, complementary selectivity to C18 and C8 bonded phases
 Phenyl-Hexyl UHPLC Columns Alternate, complementary selectivity to C8 and C8 bonded phases Particularly recommended for compounds containing aromatic groups Excellent bonded phase stability for durable,
Phenyl-Hexyl UHPLC Columns Alternate, complementary selectivity to C8 and C8 bonded phases Particularly recommended for compounds containing aromatic groups Excellent bonded phase stability for durable,
Minimizing Solvent Impact on Purification of Nitrogencontaining
 Minimizing Solvent Impact on Purification of Nitrogencontaining Compounds J. Liu and P. C. Rahn Biotage Discovery Chemistry Group US 1725 Discovery Drive Charlottesville, VA 22911 USA 1 Abstract This paper
Minimizing Solvent Impact on Purification of Nitrogencontaining Compounds J. Liu and P. C. Rahn Biotage Discovery Chemistry Group US 1725 Discovery Drive Charlottesville, VA 22911 USA 1 Abstract This paper
Trends in Method Development for HPLC and UHPLC... the movement toward using solid-core particles
 Trends in Method Development for HPLC and UHPLC... the movement toward using solid-core particles Richard A. Henry, Separation Technology Consultant On behalf of: Supelco/Sigma-Aldrich, Bellefonte, USA
Trends in Method Development for HPLC and UHPLC... the movement toward using solid-core particles Richard A. Henry, Separation Technology Consultant On behalf of: Supelco/Sigma-Aldrich, Bellefonte, USA
Alltech Alltima HP Introduction
 Alltech Introduction High-Stability, High-Purity, High-Performance, Low-Bleed Columns for Demanding Applications Better Peak Symmetry high-purity silica eliminates peak tailing problems Long Column Life
Alltech Introduction High-Stability, High-Purity, High-Performance, Low-Bleed Columns for Demanding Applications Better Peak Symmetry high-purity silica eliminates peak tailing problems Long Column Life
Comparison Guide to C18 Reversed Phase HPLC Columns
 Comparison Guide to C18 Reversed Phase HPLC Columns Comparison Data on Commonly Used C18 Phases Stationary Phase Specifications Phases Compared According to Relative Hydrophobicity Phases Compared According
Comparison Guide to C18 Reversed Phase HPLC Columns Comparison Data on Commonly Used C18 Phases Stationary Phase Specifications Phases Compared According to Relative Hydrophobicity Phases Compared According
CHEM340 Tutorial 4: Chromatography
 CHEM340 Tutorial 4: Chromatography 1. The data in the table below was obtained from a chromatogram obtained with a 10 cm liquid chromatography column. Under the conditions used, the compound uracil is
CHEM340 Tutorial 4: Chromatography 1. The data in the table below was obtained from a chromatogram obtained with a 10 cm liquid chromatography column. Under the conditions used, the compound uracil is
Hydrophilic Interaction Liquid Chromatography: Some Aspects of Solvent and Column Selectivity
 Hydrophilic Interaction Liquid Chromatography: Some Aspects of Solvent and Column Selectivity Monica Dolci, Thermo Fisher Scientific, Runcorn, Cheshire, UK Technical Note 20544 Key Words Hydrophilic, HILIC,
Hydrophilic Interaction Liquid Chromatography: Some Aspects of Solvent and Column Selectivity Monica Dolci, Thermo Fisher Scientific, Runcorn, Cheshire, UK Technical Note 20544 Key Words Hydrophilic, HILIC,
CHAPTER 6 GAS CHROMATOGRAPHY
 CHAPTER 6 GAS CHROMATOGRAPHY Expected Outcomes Explain the principles of gas chromatography Able to state the function of each components of GC instrumentation Able to state the applications of GC 6.1
CHAPTER 6 GAS CHROMATOGRAPHY Expected Outcomes Explain the principles of gas chromatography Able to state the function of each components of GC instrumentation Able to state the applications of GC 6.1
