Reversed phase HPLC Reversed Phase HPLC Application Gallery Appendices. Decades of Experience and Innovation in Chromatography
|
|
|
- Jeffry Lewis
- 5 years ago
- Views:
Transcription
1 Reversed phase HPLC Reversed Phase HPLC Application Gallery Appendices Decades of Experience and Innovation in Chromatography
2 Contents Reversed Phase HPLC Introduction Theoretical aspects of HPLC Demands on a high performance silica Stationary phases in Reversed Phase Chromatography Retention and selectivity Carbon content and polarity Steric (shape) selectivity ph value, stability and retention characteristics RP-HPLC of polar compounds Alternative RP selectivities The mobile phase in RP chromatography Polarity and miscibility of solvents Viscosity of solvents and solvent mixtures Additives (buffers and PIC reagents) Particle size, flow rate and separation efficiency Doʼs and donʼts Column care and maintenance HPLC troubleshooting Basic aspects of preparative HPLC References Application Gallery Drugs Biological and natural compounds Food analysis Environmental analysis Miscellaneous organic compounds Appendices Chromatogram index Glossary Unit conversion tables
3 Theoretical aspects of HPLC Reversed Phase HPLC Application Gallery Appendices The schematic chromatogram illustrates the most important parameters which characterise a separation. These parameters will be explained in the following paragraphs. Explanation of the most important parameters to characterise a separation: peak widths: w 1/2 = peak width at half height w = band width of the peak (intersection point of the inflectional tangents with the zero line) peak symmetry is measured at 10% of peak height symmetry parameters: A = peak front at 10% of peak height to peak maximum B = peak maximum to peak end at 10% of peak height retention times: t 0 = dead time of a column = retention time of an unretarded substance t R1, t R2.. = retention times of components 1, 2.. tʼr1, tʼr2.. = net retention times of components 1, 2.. Retention: In an elution chromatographic separation substances differ from each other only in their residence time in or at the stationary phase. From this the following definitions arise: The total retention time (t R1 or t R2 ) is the time, which is needed by a sample component to migrate from column inlet (sample injection) to the column end (detector). The dead time t 0 is the time required by an inert compound to migrate from column inlet to column end without any retardation by the stationary phase. Consequently, the dead time is identical with the residence time of the sample compound in the mobile phase. The net retention time (tʼr1 or tʼr2 ) is the difference between total retention time and dead time. That is the time the sample component remains in the stationary phase. t R1 = t R1 t 0 t R1 = t R1 t 0 The capacity factor kʼ is a measure of the position of a sample peak in the chromatogram. It is specific for a given substance. kʼ depends on the stationary phase, the mobile phase, the temperature, quality of the packing etc. k 1 = t R 1 t 0 t 0 k 2 = t R 2 t 0 t 0 2
4 Theoretical aspects of HPLC The relative retention α, also known as separation factor, is the ratio between two capacity factors, where the figure in the denominator is the reference compound. α = k 2 k 1 The relative retention describes the ability of a chromatographic system (stationary and mobile phase) to discriminate between two compounds. This is independent of column length, quality of packing, and flow velocity. It depends on the temperature and the properties of the mobile and stationary phases. Impurities in the mobile phase (e.g. water content) strongly influence the relative retention. Instead of the mobile phase volumetric flow rate F (ml/min) it is advantageous to use the linear velocity u (cm/sec). u = L t 0 The linear velocity is independent of the cross section of the column and proportional to the pressure drop in the column. The linear velocity can be calculated by means of the dead time, where L is the column length in cm and t 0 the dead time in sec. The permeability K of a column describes its transmittance for a mobile phase and characterises the hydraulic resistance. The permeability of a column depends on mobile phase, temperature, column length and pressure. A change in permeability indicates a change in the packing (e.g. swelling of ion exchangers, silica gel etc.). The number of theoretical plates n characterises the quality of a column packing and mass transfer phenomena. Large values for n qualify the column to separate complex sample mixtures. n = 16 ( t R 1 w )2 or n = ( t R 1 w 1/2 ) 2 The height equivalent of a theoretical plate h, HETP, is the length, in which the chromatographic equilibrium between mobile and stationary phase has been adjusted once. h = L n Since a large number of theoretical plates is desired, h should be as small as possible. There are, of course, no real plates in a chromatographic column, since the packing is homogeneous. The value of h is a criterion for the quality of a column. h values depend on the particle size, the flow velocity, the mobile phase viscosity and especially on the quality of a packing. For practical reasons, the peak symmetry is measured at 10% of peak height, where A is the distance from peak front to peak maximum and B is the distance from peak maximum to peak end. symmetry = B A Ideally symmetry should be 1, i. e. A = B. Values > 1 indicate peak tailing, whilst values < 1 characterise peak fronting. 3
5 Demands on a high performance silica Reversed Phase HPLC Application Gallery Appendices NUCLEODUR Ultrapure High Performance Silica for HPLC 4
6 High performance silica Purity For achieving an optimum resolution and symmetric peak shapes, a highly pure silica is required. It is well known, that metal ions, on the surface of silica largely are responsible for unwanted interactions with ionisable analytes, e. g. amines or phenolic compounds. The unique manufacturing process of NUCLEODUR provides a highly pure silica virtually free of metal impurities, and low acidic surface silanols. The following table shows the elementary analysis (metal ions), measured by AAS, of NUCLEODUR silica, 100 Å, 5 µm. Aluminium < 5 ppm Iron < 5 ppm Sodium < 5 ppm Calcium < 10 ppm Titanium < 1 ppm Zirconium < 1 ppm Arsenic < 0.5 ppm Mercury < 0.05 ppm Test for metal ions in NUCLEODUR MN Appl. No The ratio of the asymmetry factors of 2,3- dihydroxynaphthalene (2) and 2,7-dihydroxynaphthalene (1) is a measure for the metal ion content of the silica phase, because (2) can form complexes with metal ions, resulting in broad peaks for this compound on phases with high metal contaminations. Column: 125 x 4 mm NUCLEODUR C 8 Gravity, 5 µm Eluent: methanol 20 mm KH 2 PO 4, ph 7 (65:35, v/v) Temperature: 25 C Flow rate: 1 ml/min Detection: UV, 254 nm Peaks: 1. 2,7-Dihydroxynaphthalene 2. 2,3-Dihydroxynaphthalene As (2,3-DERT/2,7-DERT) < 1.1 5
7 Demands on a high performance silica Reversed Phase HPLC Application Gallery Appendices Physical stability The totally spherical, 100% synthetic NUCLEODUR silica gel exhibits an outstanding mechanical stability, even at high pressures, and elevated flow rates of the mobile phase. back pressure [bar] packing # Moreover, the material is re-usable. After several cycles of repeated packing, no significant changes in pressure drop can be observed. This is an important prerequisite for process-scale applications. Chemical stability Separation of theophylline and caffeine at ph 10 Column: 30 x 3 mm NUCLEODUR C 18 ec Mobile phase: MeOH aq. NH 3 (20:80, v/v), ph 10 Flow rate: 0.5 ml/min Temperature: 25 C Detection: UV, 254 nm The utmost purity of the base silica and the exceptional silane bonding chemistry minimises the risk of dissolution, or hydrolysis at ph extremes. The following chromatograms show the retention behaviour at ph values of 1.5 and 10.0 for NUCLEODUR C 18 endcapped. Separation of uracil, veratrol, tolu ene and ethylbenzene at ph 1.5 Column: 30 x 3 mm NUCLEODUR C 18 ec Mobile phase: ACN H 2 O (65:35, v/v), TFA, ph 1.5 Flow rate: 1.0 ml/min Temperature: 25 C Detection: UV, 254 nm Stability of NUCLEODUR at ph 1.5 over 1000 cycles (conditions see chromatogram) 6
8 High performance silica Loadability Loadability, probably the most important feature for preparative LC applications, is determined by pore size, pore volume and surface area of the packing. However, it can also be influenced by the molecular weight of the analytes. In the figure below the mass loading curve for acetophenone and butyrophenone on a NUCLEODUR C 18 ec column describes the correlation between the increase of column loading and the decrease of separation efficiency. Loading curve Column: 250 x 4.6 mm NUCLEODUR C 18 ec, mobile phase: acetonitrile H 2 O 80:20 (v/v), fl ow: 1.0 ml/min, temperature: 25 C, detection: UV, nm The elution of critical compounds such as basic drugs is possible without adsorption or peak deformation. The wide range of different particle sizes allow the use in analytical and preparative HPLC as well as for scale-up applications. Separation of phenols Column: 250 x 4 mm NUCLEODUR C 18 ec Mobile phase: methanol H 2 O, 60:40 (v/v) Flow: 1.0 ml/min Temperature: 30 C Detection: UV, 254 nm Inj. volume: 4 µl Peaks: 1. Pyrocatechol 2. Resorcinol 3. Phenol 4. Guaiacol 5. Veratrol Surface bonding chemistry The efficiency of a separation is controlled by particle size and selectivity of the stationary phase. NUCLEODUR is available in 8 different particle sizes (3, 5, 7, 10, 12, 16, 20, 30 and 50 µm) as plain silica and also with RP modifications. The exceptional surface coverage of monomerically bonded alkylsilanes combined with an exhaustive endcapping, results in a surface with lowest silanol activity. Physical data of NUCLEODUR C 18 ec Surface area (BET) 340 m 2 Pore size ~ 110 Å Pore volume 0.9 ml/g Carbon load 17.5% 7
9 Stationary phases in RP chromatography Reversed Phase HPLC Application Gallery Appendices Comprehensive range of stationary phases and columns for reversed phase HPLC 8
10 Reversed phase chromatography Strong points and benefits of RP chromatography Actually reversed phase HPLC is the most frequently used liquid chromatographic technique. In comparison with normal phase applications the RP chromatography is more stable and reproducible allows the use of various mobile phases and buffer additives can be easily controlled either isocratically or in gradient mode has numerous surface modifications with many different selectivities available. Basically RP phases can be characterised by physicochemical and chromatographic parameters such as: type of base silica specific surface size, volume and distribution of the pores applied surface chemistry in terms of silylation reagents, carbon content, bonded phase coverage, the level of residual silanols and subsequent endcapping procedures capacity factor (kʼ) separation factor (α) The figure below shows the basic silylation reaction with mono- or polyfunctional silanes which only leads to a surface coverage of approximately 50% due to steric reasons based on pore geometry. The use of small silanes in proprietary endcapping steps can decrease the number of unreacted silanol groups, a fundamental prerequisite for well base-deactivated silica surfaces. The R in the formula symbolises an alkyl chain either plain or containing functional groups. Corresponding to the bonding phase chemistry these packings are grouped to C 18, C 8, C 4, C 2, Phenyl (C 6 H 5 ), etc. C 18 phases, also named as ODS or octadecyl, are most commonly used in method development and routine analysis. Therefore it is comprehensible that most research work has been done in designing new selectivities and optimizing stability performance of C 18 stationary phases. The chart and table on the following pages exhibit a general overview of the available RP modifications: 9
11 Stationary phases in RP chromatography Reversed Phase HPLC Application Gallery Appendices For every separation the optimised RP phase high density octadecyl phases C 18 AB CN-RP cyano (nitrile) phase for RP applications C 18 HD increasing carbon content Protect I C 18 Gravity C 8 Gravity special RP phase with embedded polar group Sphinx RP C 8 HD C 18 Pyramid C 18 Nautilus high density octyl phases NUCLEODUR bifunctional RP phase octadecyl phase with hydrophilic endcapping octadecyl phase with embedded polar group NUCLEOSIL 10
12 RP phases Summary Phase Modification Structure NUCLEODUR phases high purity silica C 18 Gravity octadecyl phase -(CH 2 ) 17 -CH 3 base deactivated high density coating 18% C USP L1 NUCLEODUR (Si-O 2 ) n C 8 Gravity octyl phase -(CH 2 ) 7 -CH 3 base deactivated high density coating 11% C USP L7 NUCLEODUR (Si-O 2 ) n C 18 Pyramid octadecyl phase -(CH 2 ) 17 -CH 3 hydrophilic endcapping 14% C USP L1 NUCLEODUR (Si-O 2 ) n Sphinx RP bifunctional phase octadecyl / phenyl -(CH 2 ) 17 -CH 3 / -(CH 2 ) 3 -C 6 H 5 15% C USP L11 / L1 NUCLEODUR (Si-O 2 ) n C 18 ec octadecyl phase -(CH 2 ) 17 -CH 3 endcapped, medium density coating 17.5% C USP L1 NUCLEODUR (Si-O 2 ) n C 8 ec octyl phase -(CH 2 ) 7 -CH 3 endcapped, medium density coating 10.5% C USP L7 NUCLEODUR (Si-O 2 ) n 11
13 RP phases Summary Reversed Phase HPLC Application Gallery Appendices Phase Modification Structure CN-RP cyano (nitrile) phase -(CH 2 ) 3 -CN for RP separations 7% C USP L10 NUCLEOSIL phases standard silica C 18 HD C 8 HD octadecyl phase -(CH 2 ) 17 -CH 3 base deactivated, High Density modification 20% C USP L1 octyl phase -(CH 2 ) 7 -CH 3 base deactivated, High Density modification 13% C USP L7 C 18 Nautilus octadecyl phase -(CH 2 ) 17 -CH 3 embedded polar group for 100% aq. eluents Protect I C 18 AB 16% C USP L60 special RP phase, base deactivated protective polar group 11% C octadecyl phase -(CH 2 ) 17 -CH 3 cross-linked alkylsilanes polymer modification 25% C USP L1 NUCLEODUR NUCLEOSIL NUCLEOSIL NUCLEOSIL NUCLEOSIL NUCLEOSIL (Si-O 2 ) n (Si-O 2 ) n (Si-O 2 ) n (Si-O 2 ) n (Si-O 2 ) n (Si-O 2 ) n 12
14 RP phases Summary Phase Modification Structure C 18 C 8 ec C 8 octadecyl phase -(CH 2 ) 17 -CH 3 endcapped, medium density bonded alkylsilanes with noticeable silanol activity 15% C USP L1 octyl phase -(CH 2 ) 7 -CH 3 endcapped, medium density bonded alkylsilanes with noticeable silanol activity 9% C USP L7 octyl phase -(CH 2 ) 7 -CH 3 not endcapped 8.5% C USP L7 NUCLEOSIL NUCLEOSIL NUCLEOSIL (Si-O 2 ) n (Si-O 2 ) n (Si-O 2 ) n C 6 H 5 ec phenyl phase -(CH 2 ) 3 -C 6 H 5 endcapped, medium density bonded alkylsilanes with noticeable silanol activity 8% C USP L11 NUCLEOSIL (Si-O 2 ) n C 6 H 5 phenyl phase -(CH 2 ) 3 -C 6 H 5 not endcapped 8% C USP L11 NUCLEOSIL (Si-O 2 ) n C 4 butyl phase -(CH 2 ) 3 -CH 3 endcapped, medium density bonded alkylsilanes with noticeable silanol activity ~ 2% C USP L26 NUCLEOSIL (Si-O 2 ) n C 2 dimethyl phase -(CH 3 ) 2 3.5% C USP L16 NUCLEOSIL (Si-O 2 ) n 13
15 Retention and selectivity Reversed Phase HPLC Application Gallery Appendices Tailor-made HPLC solutions 14
16 Carbon content and polarity Carbon content and polarity High density coating with monofunctional alkylsilanes, followed by optimised endcapping, result in phases with a high carbon content and very low silanophilic activity. This allows tailing-free elution of polar compounds such as basic drugs. Examples for high density octadecyl phases are: NUCLEOSIL 100 C 18 HD (20% C) NUCLEODUR C 18 Gravity (18% C) NUCLEODUR C 18 ec (17.5% C) Examples for high density octyl phases are: NUCLEOSIL 100 C 8 HD (13% C) NUCLEODUR C 8 Gravity (11% C) NUCLEODUR 100 C 8 ec (10.5% C) Chromatographers now might wonder about the differences between C 8 and C 18 phases and the preferred range of application. Indeed there are no general guidelines which could make the choice easier but it will always be beneficial to add both phases to the existing pool of reversed phase columns. The following paragraphs outline some general selectivity patterns. The first chromatograms show the separation of compounds with different functional groups on NUCLEOSIL C 8 HD and C 18 HD. The main difference between both phases is the stronger hydrophobic selectivity of the octadecyl phase due to the longer alkyl chain, which unfortunately leads to a co-elution of the aromatic compound toluene and the polar basic lidocaine. Lidocaine itself can be eluted tailingfree on both phases, C 8 HD and C 18 HD. Separation of a test mixture on NUCLEOSIL C 8 HD and C 18 HD Columns: A) 250 x 4 mm NUCLEOSIL C 8 HD B) 250 x 4 mm NUCLEOSIL C 18 HD Eluent: MeOH 25 mm NaH 2 PO 4 ph 7.0 (65:35) Flow rate: 0.8 ml/min Temperature: 25 C Detection: UV, 254 nm Peaks: 1. Phenol (p-methylphenyl)-5-phenylhydantoin 3. Diethyl phthalate 4. Lidocaine 5. Toluene 6. Naphthalene A) B) 15
17 Retention and selectivity Reversed Phase HPLC Application Gallery Appendices The separation of various nonsteroidal anti-inflammatory drugs illustrates the differences in polarity between NUCLEODUR C 8 ec and C 18 ec and the resulting impact on efficiency. The octyl phase exhibits enhanced selectivity and excellent resolution for the polar compounds piroxicam and suprofen which co-elute on the C 18 ec column. However due to the longer alkyl chain the octadecyl phase shows a distinct hydrophobic selectivity that leads to baseline separation of the more nonpolar analytes carprofen and fenoprofen with superior peak shapes. Anti-inflammatory drugs Column: 250 x 4 mm NUCLEODUR C 8 ec / C 18 ec Eluent: acetonitrile water, 1% acetic acid (48 : 52, v/v) Flow rate: 1 ml/min Temperature: 25 C Detection: UV, 230 nm Injection volume: 10 µl Peaks: 1. Piroxicam 2. Suprofen 3. Ketoprofen 4. Carprofen 5. Fenoprofen 6. Diclofenac NUCLEODUR C 18 ec shows the higher CH 2 group selectivity compared to the octyl phase. The capacity factors kʼ for the non-polar compounds toluene and ethylbenzene are substantially higher on the octadecyl phase. Selectivity test acc. to Engelhardt Column: 250 x 4 mm NUCLEODUR C 8 ec / C 18 ec Eluent: methanol water (55 : 45, v/v) Flow rate: 1 ml/min Temperature: 40 C Detection: UV, 254 nm Peaks: 1. Uracil 2. Aniline 3. Phenol 4. 4-Ethylaniline 5. N,N-Dimethylaniline 6. Toluene 7. Ethylbenzene From the above examples one can deduce some principles: High density C 8 and C 18 phases allow tailing-free elution, also for very polar compounds Octyl phases (C 8 ) show superior polar selectivity Octadecyl phases (C 18 ) show superior hydrophobic selectivity Hydrophobic compounds show shorter retention times on C 8 phases 16
18 Steric selectivity Steric (shape) selectivity NUCLEOSIL 100 C 18 AB (25% C) This phase is an outstanding example for the cross-linked type of polymercoated octadecyl phase prepared with a trifunctional C 18 silane. It is a nonpolar, hydrophobic RP silica with increased stability to hydrolysis in Acidic as well as Basic media compared to conventional C 18 phases. Special features of this phase are a very high carbon content and a distinct steric selectivity as shown in the following chromatogram. While triphenylene (9) has a planar geometry, the aromatic rings of o-terphenyl (6) are twisted out of the plane. There are two effects worth to mention: 1. Due to steric hindrance the retention time of the non-planar o-terphenyl is significantly shorter in comparison to the flat triphenylene.. 2. On the polymeric NUCLEOSIL C 18 AB phase the separation factor achieved for the two aromatics is larger than on the standard C 18 column, which means that the polymer-coated ODS phase seems to exhibit stronger shape selectivity features, an advantage for molecules which cannot sufficiently be separated by hydrophobic or ionic interactions. Steric selectivity of NUCLEOSIL C 18 AB MN Appl. No Columns: a) 250 x 4 mm NUCLEOSIL C 18, b) 250 x 4 NUCLEOSIL C 18 AB; Eluent: MeOH H 2 O (80:20, v/v) Flow rate: 1 ml/min Temperature: 40 C Detection: UV, 254 nm Peaks: 1. Phenol 2. Diethyl phthalate 3. Naphthalene 4. Dibutyl phthalate 5. Butylbenzene 6. o-terphenyl 7. Anthracene 8. Pentylbenzene 9. Triphenylene
19 Retention and selectivity Reversed Phase HPLC Application Gallery Appendices ph value, stability, and retention characteristics Influence of the ph value on retention The option to work at an expanded ph range is often required in method development. Many nitrogen-containing compounds like basic drugs are protonated at acidic or neutral ph and exhibit poor retention on a standard C 18 phase. The retention behaviour can be improved by working at a higher ph, where the analyte is no longer protonated, but formally neutrally charged, as a rule at ph For acidic analytes it is exactly in inverse proportion, maximum retention can be attained at low ph. General correlation between retention and ph for basic and acidic compounds The figure below shows the extent of protonation of surface silanols and of two exemplary analytes at acidic and alkaline ph. Surface silanols at different ph values 18
20 ph value, stability and retention As mentioned above, enhanced ph stability of the stationary phase can be helpful for improving selectivity in method development. The two chromatograms below show the separation of 4 basic drugs under acid and basic conditions. At ph 2.5 the protonated analytes exhibit poor retention (early elution) and in addition an inadequate resolution for papaverine and noscapine, whilst the formally non ionised molecules can be baseline separated at alkaline ph due to the better retention pattern. Basic alkaloids Column: 125 x 4 mm NUCLEODUR C 18 Gravity, 5 µm Eluents: A) acetonitrile, B) 20 mm (NH 4 ) 2 HPO 4, ph 2.5 / 10.0 Gradient: 10 % A (1 min) 75 % A in 10 min Flow rate: 1.0 ml/min Temperature: 25 C, Detection: UV, 254 nm Peaks (injection volume 2 µl): 1. Lidocaine 2. Papaverine 3. Noscapine 4. Diphenhydramine Another example for the effect of ph on selectivity is the separation of the acid ketoprofen, the base lidocaine and of benzamide. Under acidic conditions the protonated lidocaine is eluted very fast due to lack of sufficiently strong hydrophobic interactions between analyte and C 18 chains, whereas the formally neutral ketoprofen is eluted after about 3 min. Contrary, at ph 10 a reversal of elution order is observed, with a notedly longer retention time for the basic lidocaine Influence of ph value on selectivity Column: 125 x 4 mm NUCLEODUR C 18 Gravity, 5 µm Eluents: A) acetonitrile 10 mm NH 4 HCO 2, ph 3.0 (50:50, v/v), B) acetonitrile 10 mm NH 4 HCO 3, ph 10.0 (50:50, v/v), fl ow rate: 1 ml/min, temperature: 30 C, detection: UV, 240 nm Peaks (injection volume 2 µl): 1. Lidocaine 2. Benzamide 3. Ketoprofen 19
21 Retention and selectivity Reversed Phase HPLC Application Gallery Appendices Enhanced ph stability One major disadvantage of silica stationary phases is the limited stability at strongly acidic or basic ph ranges. At acidic ph values, the siloxane bonds will be cleaved by hydrolysis: At alkaline ph values, dissolution of the silica will rapidly lead to a considerable loss in column performance: Therefore conventional RP phases should not be run with mobile phases at ph > 8 or ph < 2 for extended periods of time. The special surface bonding technology and the low content of trace elements of NUCLEODUR Gravity allow for use at an expanded ph range from ph 1 to 11. Stability of NUCLEODUR C 18 Gravity under acidic conditions Column: 125 x 4 mm NUCLEODUR C 18 Gravity, 5 µm; eluent: acetonitrile 1% TFA in water (50:50, v/v), ph 1.5, fl ow rate: 1.0 ml/min, temperature: 30 C, detection: UV, 230 nm, injection volume: 5 µl, Peaks: 1. pyridine, 2. toluene, 3. ethylbenzene Due to the extremely stable surface modifi cation, no cleavage of Si-O-Si bonding occurs, column deterioration is therefore successfully prevented. Stability of NUCLEODUR C 18 Gravity under alkaline conditions Column: 50 x 4.6 mm NUCLEODUR C 18 Gravity, 5 µm; eluent: methanol water ammonia (20:80:0.5, v/v/v), ph 11; fl ow rate: 1,3 ml /min, temperature: 30 C, detection: UV, 254 nm, injection volume: 2.0 µl; Peaks: 1. theophylline, 2. caffeine Gravity with its unique high density surface bonding technology withstands strongly alkaline mobile phase conditions even after 300 injections without loss of column effi ciency. 20
22 RP-HPLC of polar compounds RP-HPLC of polar compounds The efforts to neutralise unwanted activity of unreacted surface silanols often results in well base-deactivated phases with high carbon load, but a limited scope of selectivity beyond non-polar interactions. In particular polar compounds like carboxylic acids, drug metabolites, etc. show only weak retention on densely bonded reversed phase columns due to distinct hydrophobic properties but low polar selectivity. Very polar analytes require highly aqueous mobile phases for solubility and retention. Conventional reversed phase columns often display stability problems in eluent systems with high percentage of water (> 95%) as evidenced by a sudden decrease of retention time and overall poor reproducibility. This phenomenon is described as phase collapse caused by the mobile phase expelled from the pores due to the fact, that hydrophobic RP phases are incompletely wetted with the mobile phase 1). The non-polar alkyl chains lose their brush-type structure for maintaining the hydrophobic interactions between stationary phase and analyte. The result of this phase collapse is a drastic decrease of retention time and poor resolution. This phenomenon may occur very fast or after a while. The following figure shows the phase collapse and decrease in retention time for a conventional RP phase with 100% aqueous eluents. The following diagram shows, that special phases designed for highly aqueous eluents can successfully prevent this loss of retention and phase collapse: Phase collapse in aqueous media change of retention time with 100% aqueous eluents 21
23 Retention and selectivity Reversed Phase HPLC Application Gallery Appendices Different approaches have been made to increase column stability with highly aqueous mobile phase systems. The most promising concepts are or incorporating a polar group in the hydrophobic alkyl chain 2), example: NUCLEOSIL 100 C 18 Nautilus using hydrophilic endcapping procedures to improve the wettability of the RP modification, example: NUCLEODUR C 18 Pyramid Embedded polar group NUCLEOSIL C 18 Nautilus is a reversed phase C 18 column based on highly pure silica with thorough endcapping, which is totally stable if the mobile phase is 100% aqueous without any organic modifiers. This property is achieved by a covalently bonded C 18 silane with an embedded functional group, which is the driving force for the excellent water stability of the RP surface. Compared to standard C 18 phases where hydrophobic interactions between analyte and stationary phase predominate, NUCLEOSIL 100 C 18 Nautilus also shows polar interaction via hydrogen bonding activities and dipole-dipole forces. This can influence retention time (t R ) and improve selectivity (α) particularly for the separation of polar compounds. In spite of the polar character of the embedded functional group NUCLEOSIL 100 C 18 Nautilus exhibits sufficient hydrophobic properties and is very well suited for analysing basic compounds. In the selectivity test the strong base p-ethylaniline is eluted as a sharp signal with remarkably good peak shape. Selectivity test Column: 250 x 4 mm NUCLEOSIL C 18 Nautilus Eluent: methanol water (55:45, v/v) Flow rate: 0.8 ml/min Temperature: 40 C Detection: UV, 254 nm Peaks: 1. Uracil 2. Aniline 3. Phenol 4. p-ethylaniline 5. N,N-Dimethylaniline 6. Toluene 7. Ethylbenzene 22
24 RP-HPLC of polar compounds Hydrophilic endcapping NUCLEODUR C 18 Pyramid is a silica phase with hydrophilic endcapping, designed especially for use in eluent systems of up to 100% water. The figure below shows the retention behaviour of tartaric, acetic and maleic acid under purely aqueous conditions on the Pyramid in comparison with a conventionally bonded RP phase. NUCLEODUR C 18 Pyramid conventional RP column The polar surface derivatisation exhibits retention characteristics, which differentiate the Pyramid from conventional C 18 stationary phases. The following chromatogram shows the improved retention behaviour of very polar compounds such as organic acids, which are insufficiently retained on RP columns with predominantly hydrophobic surface properties. Separation of organic acids Column: 250 x 4 mm NUCLEODUR C 18 Pyramid, 5 µm; eluent: 0.2 % H 3 PO 4, fl ow rate: 0.7 ml/min; temperature: 25 C; detection: UV, 210 nm; injection volume: 2 µl Peaks: 1. Tartaric acid 2. Malic acid 3. Lactic acid 4. Succinic acid both columns 125 x 4 mm ID; 50 mm KH 2 PO 4 ph 2.5, 0.7 ml/min; 25 C; UV, 210 nm; injection volume 1 µl. Peaks: 1. tartaric acid, 2. acetic acid, 3. maleic acid It can be shown that the retention times for NUCLEODUR C 18 Pyramid remain nearly unchanged between initial injection and restart after the flow has been stopped for 12 hours, whilst the performance of the conventional RP column collapsed totally after the same period of time. The perceptible increase in polarity has no impact on the peak shapes of ionisable analytes. Even with the strongly basic compounds or the tricyclic antidepressant test mixture, no unwanted interactions or lack of base deactivation are observed. Tricyclic antidepressants Column: 125 x 4 mm NUCLEODUR C 18 Pyramid, 5 µm; eluent: MeOH 20 mm NH 4 H 2 PO 4 ph 6.95 (70:30, v/v), fl ow rate: 1 ml/min; temperature: 40 C; detection: UV, 254 nm; injection volume: 5 µl Peaks: 1. Protriptyline 2. Nortriptyline 3. Doxepin 4. Imipramine 5. Amitriptyline 23
25 Retention and selectivity Reversed Phase HPLC Application Gallery Appendices Alternative RP selectivities In reversed phase HPLC it is fairly common to start with C 18 or C 8 columns, whenever new methods have to be developed. However, superior polarity and selectivity properties often required for more sophisticated separations, are not always sufficiently provided by classical RP phases, which are usually characterised by a hydrophobic layer of monomeric or polymeric bonded alkylsilanes. One approach to improve the resolution of compounds poorly separated on nonpolar stationary phases, is to change bonded-phase functionality. Comparison of NUCLEODUR C 18 ec and CN-RP for a separation of coldmedicine ingredients Columns: A) 250 x 4 mm NUCLEODUR CN-RP B) 250 x 4 mm NUCLEODUR C 18 ec eluent: acetonitrile 100 mm sodium citrate ph 2.5 (15:85, v/v), fl ow rate: 1 ml/min temperature: 25 C, detection: UV, 270 nm, injection volume 10 µl Peaks: 1. Maleic acid 2. Norephedrine 3. Ephedrine 4. Acetaminophen 5. Chlorpheniramine 6. Brompheniramine Cyano (nitrile) phases For example, the fully endcapped and highly reproducible NUCLEODUR CN-RP phase has cyanopropyl groups on the surface able to generate a clearly recognisable different retention behaviour compared to purely alkyl-functionalised surface modifications. The polarity of the NUCLEODUR CN-RP phase can be classified as intermediate based on multiple retention mechanisms such as dipole-dipole, π-π, and also hydrophobic interactions 3). Therefore, this phase shows a distinct selectivity for polar organic compounds as well as for molecules containing π-electron systems (e.g. analytes with double bonds, tricyclic antidepressants) 4). 24
26 Alternative RP selectivities Short-chain bonded phases are sometimes suspected of revealing shortcomings in stability towards hydrolysis at low ph 5). The chromatogram below shows that even after 100 sample injections and four weeks storage at ph 1 (curve 2), neither a considerable shift in retention, nor a visible change in peak symmetry could be noticed (curve 1 = new column). Stability of NUCLEODUR CN-RP at ph 1 Column: NUCLEODUR CN- RP, 125 x 4 mm ID Eluent: acetonitrile water, 2 % TFA ph 1 (50:50, v/v) Flow rate: 1 ml/min Temperature: 25 C Detection: UV, 254 nm Injection volume: 5 µl Peaks: 1. Benzamide 2. Dimethyl phthalate 3. Phenetol 4. o-xylene 5. Biphenyl Bifunctional phases This approach combines the advantages of different separation modes in one phase. As an example, we present NUCLEODUR Sphinx RP, a dual-mode stationary phase with exceptional selectivity features generated by a wellbalanced ratio of covalently bonded octadecyl and phenyl groups. The combination of classical hydrophobic with π-π interactions (aromatic ring system) expands the scope of selectivity in comparison with conventional reversed phase packings. NUCLEODUR Sphinx RP is particularly suited for the separation of molecules containing aromatic and multiple bonds. For the separation of polar compounds, NUCLEODUR Sphinx RP can be especially recommended and can often outperform any customary C 18 phase. 25
27 Retention and selectivity Reversed Phase HPLC Application Gallery Appendices In addition, exhaustive endcapping steps minimise unwanted surface silanol activity and guarantee excellent peak shapes even for strongly basic analytes. Different from standard phenyl phases, NUCLEODUR Sphinx RP is not susceptible to hydrolysis and is also suited for LC/MS applications. Separation of flavonoids on 3 different NUCLEODUR phases Columns: 150 x 4.6 mm NUCLEODUR, A) C 8 Gravity, B) C 18 Gravity, C) Sphinx RP, all 5 µm Eluent: water methanol (40:60, v/v) Flow rate: 1 ml/min Temperature: 30 C Detection: UV, 270 nm Inj. volume: 3 µl Peaks: 1. Catechin 2. Rutin R 1 = R 3 = OH, R 2 = O-Rutinose 3. Fisetin R 1 = R 2 = OH, R 3 = H 4. Quercetin R 1 = R 2 = R 3 = OH 5. Kaempferol R 1 = H, R 2 = R 3 = OH 6. Isorhamnetin R 1 = OCH 3, R 2 = R 3 = OH 26
28 The mobile phase in RP chromatography Solvent Miscibility Viscosity Mobile Phase Additives 27
29 The mobile phase in RP chromatography Reversed Phase HPLC Application Gallery Appendices The mobile phase for reversed phase chromatography is a mixture of water and an organic solvent which is miscible with water. The time of elution of the substances to be separated depends to a large degree on the water content of the organic solvent and is therefore easily adjusted to a suitable value. Higher concentrations of organic solvent will cause shorter retention times. RP separations can be optimised by utilising the specific selectivity of organic solvents. Polarity and miscibility of solvents nonpolar Polarity index polar 0.0 Heptane 0.0 Hexane 0.0 Pentane 0.2 Cyclohexane 1.0 Trichloroethylene 1.6 Carbon tetrachloride 2.2 Di-i-propyl ether 2.4 Toluene 2.5 Xylene 2.5 Methyl t-butyl ether 2.7 Benzene 2.8 Diethyl ether 3.1 Dichloromethane 3.5 1,2-Dichloroethane 3.9 i-propanol 3.9 n-butanol 4.0 Butyl acetate 4.0 n-propanol 4.0 Tetrahydrofuran 4.1 Chloroform 4.4 Ethyl acetate 4.7 Methyl ethyl ketone 4.8 Dioxane 5.1 Acetone 5.1 Methanol 5.2 Ethanol 5.8 Acetonitrile 6.2 Acetic acid 6.4 Dimethylformamide 7.2 Dimethyl sulfoxide 9.0 Water Solvent 28
30 Miscibility of solvents miscible not miscible Solubility in water [% w/w] Heptane Hexane Pentane Cyclohexane Trichloroethylene Carbon tetrachloride Di-i-propyl ether Toluene Xylene Methyl t-butyl ether Benzene Diethyl ether Dichloromethane 1,2-Dichloroethane i-propanol n-butanol Butyl acetate n-propanol Tetrahydrofuran Chloroform Ethyl acetate Methyl ethyl ketone Dioxane Acetone Methanol Ethanol Acetonitrile Acetic acid N,N-Dimethylformamide Dimethyl sulfoxide
31 Viscosity of solvents Reversed Phase HPLC Application Gallery Appendices Viscosity of solvents and solvent mixtures The viscosity of the eluent should not be neglected. It is often not realised in practice, that eluents have very different viscosities (η) and that these differences in viscosity (with the same flow rate) cause changes in back pressure. Solvent Viscosity Acetic acid 1.26 Acetone 0.32 Acetonitrile 0.37 Benzene 0.65 n-butanol 2.98 Butyl acetate 0.73 Carbon tetrachloride 0.97 Chloroform 0.57 Cyclohexane ,2-Dichloroethane 0.79 Dichloromethane 0.44 Diethyl ether 0.32 Di-i-propyl ether 0.37 Dimethyl sulfoxide 2.00 N,N-Dimethylformamide 0.92 Dioxane 1.54 Ethanol 1.20 Ethyl acetate 0.45 Heptane 0.39 Hexane 0.33 Methanol 0.60 Methyl ethyl ketone 0.45 Methyl t-butyl ether 0.27 Pentane 0.23 i-propanol 2.30 n-propanol 2.27 Tetrahydrofuran 0.55 Toluene 0.59 Trichloroethylene 0.57 Water 1.00 Xylene 0.61 The back pressure changes according to the following equation: p 1 = η 1 η 2 p 2 The viscosity of a solvent mixture is often higher than that of the single components, i.e. a higher pressure is needed to achieve the same flow rate. Viscosity of solvent mixtures as a function of composition The graph illustrates how strongly the viscosity of the eluent is influenced by its composition. For example the viscosity (and consequently the back pressure of the column) increases more than double when the eluent is changed from acetonitrile water (70 : 30) to methanol water (70 : 30). See page 38 for some typical back pressures. 30
32 Solvent additives When water is used as a solvent or as a component in gradient mixtures, it is essential that it is completely degassed. Apparent instability can be caused by air bubbles (from not properly degassed solvents) which accumulate at the detector. The best way to remove such air is to first pump degassed solvent through the column and then follow up with degassed water. One can degas solvents with a vacuum pump, by passing helium through or by ultrasonic treatment. Organic solvents commonly used in reversed phase HPLC Solvent Boiling point [ C] Freezing point [ C] UV cut-off [nm] Acetonitrile Butanol 210 Ethanol Methanol Propanol Propanol UV = ultraviolet, 10% transmission Eluent additives Buffers In reversed phase HPLC it is required to add buffers to the mobile phase if ionic or ionisable compounds have to be separated. The ph value of the buffer additives should be at least 2 units distant from the pk a of the analytes to ensure that most of the molecules are either ionised or neutrally charged. It is highly suggested to check if solubility of buffer reagents is in line with eluent composition to avoid precipitation of salts on the stationary phase. Usual buffer concentrations range between 10 and 100 mmol/l. pk a values of common buffers Ion pk a Acetate 4.8 Borate 9.2 Citrate Formate 3.7 Glycinium Perchlorate 9 Phosphate Trifl uoroacetate a) 0.2 Tris b) 8.3 a) Trifl uoroacetic acid is a frequently used volatile additive for the separation of proteins and peptides. It is rapidly decomposed, thus buffers should be prepared fresh daily. TFAA may cause ghost peaks in gradient separations. b) Tris-(hydroxymethyl)aminomethane Ion pairing reagents (PIC) Chromatography of charged compounds on reversed phase columns will normally not lead to viable results due to insufficient nonpolar interactions. Capacity factors of ionic molecules and with it the retention time can be increased by adding a suitable commercially available ion pairing reagent to the mobile phase. Retention behaviour can be controlled via the hydrophobic character of the formed ion pair and also by the concentration of the PIC reagent. For acidic compounds tetraalkylammonium salts (e.g. tetrabutylammonium phosphate) are used while basic compounds can be separated with long-chain alkyl sulfonic acids. We recommend to start with a standard C 18 column, such as NUCLEODUR C 18 ec. It may be noticed that ion pairing reagents could be harmful to silica-based stationary phases and should only be used if all other options to improve the separation failed. 31
33 Particle size, flow rate and efficiency Reversed Phase HPLC Application Gallery Appendices Particle size and efficiency Due to the basic principles of HPLC 4) short diffusion paths in the pores of the stationary phase are required for achieving high column efficiency. According to the van Deemter equation H = A ν B ν + C ν A: band broadening caused by eddy diffusion and stream splitting of the mobile phase B: band broadening caused by longitudinal diffusion C: band broadening caused by equilibrium distribution between stationary and mobile phase ν: velocity of the mobile phase The C term describes the influence of band broadening, which is caused by the equilibrium distribution of the molecules between stationary and mobile phase. In the pores of smaller particles the diffusion paths are shorter. As a result the C term is reduced, which means a favourable effect for gaining low HETP values (height equivalent to one theoretical plate). Provided that the velocity of the mobile phase is in its optimum range, smaller particles exhibit a considerably increased column efficiency. By rule of thumb a well-packed 3 µm HPLC column has about twice the separation efficiency of a 5 µm column. The choice of smaller particle 32
34 Particle size, flow rate and efficiency size packings allows the use of shorter columns for rapid separations without loss of resolution. This is shown in the separation of vanillin compounds on the right. The time of analysis can be reduced to a fifth by the replacement of a conventional 250 mm 5 µm NUCLEOSIL Nautilus column with a 70 mm highspeed column packed with the 3 µm counterparts. The drastic shortening of the analysis time is not only due to reducing column length, but also to increasing the flow rate by 50%. Problems with band broadening do not occur, because of the increased flow rate for smaller-sized packings. An additional benefit in the use of rapid resolution columns is the remarkable reduction of solvent consumption. Vanillin and derivatives MN Appl. No Columns: a) 70 x 4 mm NUCLEOSIL C 18 Nautilus, b) 250 x 4 mm NUCLEOSIL C 18 Nautilus Eluent: ACN water H 3 PO 4, 20:80:0.1 (v/v/v); ambient temperature; fl ow rate: a) 1.5 ml/min, b) 1 ml/min; detection UV, 280 nm Peaks: 1) Isovanillin 2) Vanillin 3) Ethoxyvanillin Flow rate and inner diameter Reducing column size is a continuous trend in HPLC. Therefore HPLC columns with small IDs are needed. Because of the smaller ID and the lower column volume microbore columns allow a much higher detector sensitivity, which is required for small sample amounts. Simultaneously, the consumption of solvent is decreased considerably due to the lower flow rates (see table). Finally, the increasing use of LC/MS instruments is another reason for the rising demand for HPLC columns with IDs < 2 mm. Change of flow rate and solvent saving as a function of the column inner diameter ID [mm] Flow rate [ml/min] Solvent saving Increase in sensitivity ~ 25% ~ ~ 57% ~ ~ 81% ~ ~ 95% ~ 21.7 for a constant length relative to a column with 4.6 mm ID and a flow rate of 1.3 ml/min for isocratic application 33
35 Do s and don ts in RP-HPLC Reversed Phase HPLC Application Gallery Appendices Column care and maintenance HPLC troubleshooting 34
36 Column care and maintenance Column care and maintenance General information HPLC columns from MN are quality products especially developed for qualitative and quantitative analysis of mixtures of substances and single components. To the best of our knowledge all RP HPLC columns based upon NUCLEOSIL and NUCLEODUR can be stored at least for 2 years. Under correct storing conditions there is almost no significant change in selectivity or column performance even after a period of 5 years. Delivery (see column certificate) Solvent: acetonitrile water, 80:20, 70:30, or 60:40 resp. no buffer additive ph value: neutral (about 6 7) Storage conditions: Temperature: ambient (15 30 C) Humidity: ambient, typical lab conditions Initial operation Prior to column installation, the complete HPLC system should be rinsed with a buffer-free, filtered eluent at a low flow rate (0.1 ml/min) for at least 30 min (better several hours or overnight) in order to remove aggressive substances (acids, bases, salts). Especially older HPLC systems tend to release e.g. metal ions and should therefore be cleaned thoroughly. Column installation Choice of the capillary connections including the fittings has an important influence on the dead volume of the system. Dead volume should always be minimised, especially for shorter columns and smaller inner diameters. Recommended capillary diameters Column ID Capillary ID 2 mm 0.13 mm 3 mm 0.18 mm 4 mm mm 4.6 mm 0.25 mm 8 mm 0.25 mm 10 mm 0.5 mm 16 mm 0.5 mm 21 mm 0.75 mm 40 mm 0.75 mm MN recommends either PEEK capillaries or corresponding stainless steel capillaries (1/16 OD x ID). Choice of the material depends on the properties of the mobile phase: PEEK: not compatible with tetrahydrofuran, chlorinated hydrocarbons or strong acids. Stainless steel: not applicable for high salt concentrations and concentrated acids. For installation we recommend either polymer fingertight fittings or original MN connecting nuts incl. ferrules. 35
37 Do s and don ts in RP-HPLC Reversed Phase HPLC Application Gallery Appendices Use of other ferrules could result in dead volume and thus peak broadening or leakage. Connecting systems (inner bores of column heads) vary from manufacturer to manufacturer; we recommend to check the respective product literature for specifications of nuts, ferrules and capillaries. It is also important to check the connections of the injection systems. Special care has to be taken when connecting the capillaries to the column. It is important, that there is no gap between column head and end of capillary always push the capillary into the head as far as it will go, and fix the system thus, that the capillary cannot slip back during tightening. This is especially important for stainless steel capillaries. We recommend to check the column incl. system directly after receipt, e.g. with an RP test mixture. Column equilibration In order to obtain a rapid equilibration, the eluent change should be performed in several steps or with a gradient (4 5 steps, at least two column volumes, increasing fraction of the desired eluent, decreasing fraction of the storage solvent). Prior to operation with eluents containing buffers always precondition with acetonitrile water 25:75 (min. 10 column volumes), then proceed to the buffered eluent. After changing to the desired eluent, always run the column with about 10 column volumes of the eluent, before starting the first injection. For calculation of the column volume (empty tube) use the following formula: with V = π r 2 L V = column volume [ml] r = column radius [cm] L = column length [cm] Some basic rules for changing to other solvents / solvent mixtures: Take care that no demixing or phase separation of the mobile phase occurs this can damage the column packing! When changing from buffered eluents to mobile phases with a high fraction of organic solvent (> 50%) always use an intermediate rinsing step in order to avoid precipitation of the buffer! Never rinse with eluents, which chemically attack the stationary phase, the column fittings or the connecting capillaries. Regeneration and cleaning For all cleaning steps the flow rate should be about 25 50% of the normal working flow. Polar impurities First rinse out buffer solutions with 80% water 20% organic solvent (20 column volumes) Then rinse with a mixture with a high concentration of organic solvent (70:30 to 90:10, methanol water or acetonitrile water), at least 10 column volumes NOTE Most polar impurities are eluted when the buffer is rinsed out. 36
38 Column care and maintenance Nonpolar impurities Rinse column with a high fraction of water (max. 90% water) in order to remove the buffer Rinse column with 100% methanol to remove polar organic compounds Rinse the column with 100% acetonitrile to remove medium polar organic impurities (if necessary increase temperature to 40 C) Rinse column with THF to remove nonpolar organic compounds If necessary rinse column with THF in reversed flow direction (about 1/5 of the initial flow) Proteins / peptides For these impurities we recommend a gradient of water / 0.05% TFA (eluent A) and acetonitrile /0.04% TFA (eluent B) with an increase from 25% B to 100% B in 20 min, 5 min at 100% B, then in 5 min back to 25% B. Repeat this procedure twice. An important indicator for a properly cleaned column is the stability of the base line (not to be mixed up with the signal noise of the UV lamp). At a constant temperature (without thermal drift) acetonitrile water 70:30 should not cause a drift of more than 2-3 mau in a run of 5 min. Column storage Columns should be stored buffer-free and in mixtures of 90:10 to 75:25 volume parts acetonitrile water. Due to possible contamination with metal ions, e.g. iron(iii), methanol is not recommended for long-term storage. The ph value should be between 6 and 7; never use strongly acidic or alkaline component (trifluoroacetic acid, sodium hydroxide solution etc.). Buffer-containing eluents (e.g. phosphate, acetate, hydrogen carbonate, ) have to be rinsed completely from the column. Depending on the concentration of the buffer salt at least column volumes are recommended (i.e. for a standard column of 250 mm length and 4 mm inner diameter about ml of a 25:75 mixture of acetonitrile water or methanol water are required. For 250 mm length and 3 mm ID this corresponds to ml solvent). Please make sure, that the complete HPLC system is rinsed free of buffer! During rinsing, the back pressure should not exceed 250 bar; if necessary, reduce the flow. If not explicitly indicated, the column must not be stored with a high concentration of water (> 95%). This means, that after removal of the buffer the eluent has to be changed to acetonitrile water (90:10 to 75:25). For storage columns should be tightly closed with the respective column end caps. Hand-tighten the caps, because tools may damage the plastic parts. ChromCart columns can be protected from running dry by using the included plastic caps. Storage conditions Temperature: Humidity: ambient (15-30 C) ambient, typical lab conditions ph value: if possible neutral / no buffer 37
39 Do s and don ts in RP-HPLC Reversed Phase HPLC Application Gallery Appendices Resuming operation When resuming operation after column storage, please follow the same steps as described above for initial operation (see page 35) Disposal For the disposal of HPLC columns follow local regulations. All MN HPLC columns leave our production facilities tested and supplied with a certificate which contains important column parameters. Doʼs and Donʼts Column performance should always be checked before running under new conditions. We recommend a simple test mixture (e.g. 2 or 3 neutral compounds like toluene, naphthalene or ethyl benzene) under isocratic conditions. Typical back pressures Mobile phase system Temp. Total back pressure * [ C] [bar (psi)] 250 x 4.6 mm column 5 μm NUCLEODUR 100 C 18 ec acetonitrile/water 25: (2900) 50: (2300) methanol/water 25: (4000) 50: (5000) 50: (2900) 150 x 4.6 mm column 3 μm NUCLEODUR 100 C 18 ec acetonitrile/water 50: (2300) methanol/water 50: (5000) 50: (2800) * may be within ± 5 bar. Includes system back pressure (pump, tubing) of 10 bars. Column back pressure should not exceed 250 bar (3500 psi). Check the viscosity of your mobile phase system, especially in the case of methanol water mixtures and a particle size of 3 μm. The table shows typical back pressures for NUCLEODUR 100 C 18 ec at a flow rate of 1.3 ml/min. For corresponding C 8 systems the back pressure is about 10% lower. Column length and flow rate are in linear relationship to the back pressure, i.e. half of length results in half of back pressure. Either increase the temperature or reduce the flow rate to lower the pressure (also see page 30). 38
40 Column care and maintenance Use precolumns when working with dirty or contaminated samples. Use syringe filters to remove particulate matter. Try to remove hardly soluble or insoluble matrix components (e.g. dirt, plasma, proteins) with a corresponding sample preparation. If you do not use precolumns, make sure to regenerate the main column in regular intervals (see page 36) especially if you observe an increase of more than 20% of the initial back pressure. If the back pressure reaches a critical value please check all connecting capillaries for possible dents or blockages by deinstalling the column and testing the system component by component, beginning with the detector and proceeding to the pump. Donʼts BASIC RULES: Always clean your equipment before running a brand-new column. Keep aggressive or precipitating additives away from your column if possible (salts, acids, polymers). Never use higher buffer concentrations than required. Keep ph values within limits of 2 and 8 except for reasons of selectivity. Donʼt use higher column temperature than necessary. Avoid extreme or sudden pressure changes. Avoid 100% of water (pure aqueous mobile phase) except this is compatible to your columnʼs stationary phase (read the corresponding column guide). Best column lifetime also is correlated to the sample itself. Sometimes critical compounds may be more stable at lower ph levels. Never store columns with buffer salts in the mobile phase. Avoid unnecessary stress (pressure) of the packing. Phosphate or borate buffers are more aggressive and harmful to stationary phases especially at elevated temperatures. Less harmful buffer systems above ph 7 are: ammonia (pk a = 9.2), (acetate, formate) amines (triethylamine: pk a = 10.7, pyrrolidine: pk a = 11.3) hydrogencarbonate (pka = 10.3), NH 4, Na or K salt 39
41 Do s and don ts in RP-HPLC Reversed Phase HPLC Application Gallery Appendices Problem / possible cause Peak deformations Broad peaks (symmetry ~ 1) early eluting analyte due to column overload injection volume too large viscosity of mobile phase is too high retention times too long poor column efficiency peak broadening in the injection valve extra column volume of the LC system too large volume of detector cell too large detector time constant too slow sampling rate of the data system is too low only some peaks broad: late elution of analytes from a previous run Peak fronting (symmetry < 1) column overload formation of channels in the column prevention / remedy dilute sample 1:10 and repeat the separation inject smaller volumes or reduce solvent strength for injection to focus the sample components increase column temperature or use a solvent of lower viscosity use gradient elution or a stronger mobile phase for isocratic elution use mobile phases of lower viscosity, elevated column temperature, lower flow rate or a packing with smaller particle size decrease size of the sample loop, or introduce an air bubble in front and back of the sample in the loop use zero dead volume fittings and connectors; use smallest possible tubing diameter (<0.25 mm) and matched size of fittings use smallest possible cell volume for the sensitivity required; use a detector without heat exchanger in the system adjust the time constant to the peak width increase the sampling rate flush the column with a strong eluent after each run, or end gradient at a higher concentration decrease sample amount; increase column diameter; use a stationary phase with higher capacity buy a new column or have the column repacked 40
42 HPLC troubleshooting Problem / possible cause prevention / remedy Peak tailing (symmetry > 1) basic analytes: interactions with silanol groups sample components which can form chelates: metal traces in the packing silica-based column: silanol interactions silica-based column: degradation at high ph values silica-based column: degradation at high temperatures dead volume at the column head unswept dead volume use silica-based base deactivated RP phases (e.g. NUCLEODUR Gravity, NUCLEOSIL PROTECT I, NUCLEOSIL HD or NUCLEOSIL AB); use a competing base such as triethylamine; use a stronger mobile phase; switch to polymer-based columns (e.g. NUCLEOGEL RP) only use high-purity silica-based packings (e.g. NUCLEODUR, NUCLEOSIL ) with their very low metal contamination; add EDTA or another chelating compound to the mobile phase; switch to polymer columns (e.g. NUCLEOGEL ) decrease the ph value of the mobile phase to suppress ionisation of the silanol groups; increase the buffer concentration; derivatise the sample to avoid polar interactions use RP columns with good surface shielding, polymer columns or sterically protected phases use temperatures below 50 C rotate injection valve quickly; use an injection valve with pressure bypass; avoid pressure pulses replace the deteriorated column, or, if possible, open the upper endfitting and fill the void with the column packing or some silanised glass fibre wadding, for preparative HPLC: with our VarioPrep columns you can compensate dead volumes with the adjustable end fitting. minimise the number of connections; ensure that the rotor seal is tight; check whether all fittings are tight 41
43 Do s and don ts in RP-HPLC Reversed Phase HPLC Application Gallery Appendices Problem / possible cause column overload Double peaks simultaneous elution of an interfering substance simultaneous late elution of a substance from a previous run injection solvent too strong sample volume too large dead volume or formation of channels in the column plugged frit unswept volume in the injector Negative peaks RI detector: refractive index of the analyte lower than that of the mobile phase UV detector: absorption of the analyte lower than absorption of the mobile phase prevention / remedy use sample clean-up or fractionation prior to injection (e. g. SPE with CHROMABOND or CHROMAFIX ); improve selectivity by choice of another mobile or stationary phase flush the column with a strong eluent after each run, or end gradient at a higher concentration see above under peak fronting use a weaker solvent for the sample or a stronger mobile phase if the sample is dissolved in the mobile phase, the injection volume should be smaller than one-sixth of the column volume replace the column or, if possible, open the upper end fitting and fill the void with the same packing; have the column repacked install an in-line filter with 0.5 µm pore size between pump and injector to remove solids from the mobile phase, or between injector and column, to filter particulate matter from the sample if possible, clean or replace the plugged frit replace the rotor of the injection valve reverse detector polarity to obtain positive peaks use a mobile phase with lower UV absorption; if recycling solvent, use fresh HPLC grade eluent when the recycled mobile phase starts to affect detection 42
44 HPLC troubleshooting Problem / possible cause Ghost peaks contamination late elution of an analyte from a previous run unknown interfering substances in the sample in ion pairing chromatography: disturbed equilibrium in peptide mapping: oxidation of trifluoroacetic acid in RP chromatography: contaminated water Spikes air bubbles in the mobile phase column was stored without endcaps prevention / remedy only use HPLC grade solvents; flush the column to remove impurities see above under double peaks use sample clean-up or fractionation prior to injection (e. g. SPE with CHROMABOND or CHROMAFIX ) prepare the sample in the mobile phase; reduce the injection volume prepare fresh trifluoroacetic acid solution daily; add an antioxidant check the suitability of the water by passing different amounts through the column and measure the peak height of the impurity as a function of enrichment time; purify the water by running it through an old RP column or use HPLC grade water degas the mobile phase; install a back pressure restrictor at the detector outlet; ensure that all fittings are tight always store columns tightly capped; flush reversed phase columns with degassed methanol Lack of sensitivity detector attenuation set too high not enough sample injected sample loop of injector underfilled sample loss during sample preparation sample loss on column autosampler line blocked peaks outside the linear range of the detector only during first few injections: sample absorption in sample loop of injector or column reduce detector attenuation increase amount of sample for injection overfill loop with sample use an internal standard for sample preparation and optimise your method see next paragraph: poor sample recovery check the flow and clear any blockages dilute or enrich the sample until the concentration is in the linear range of the detector condition sample loop and column with concentrated sample 43
45 Do s and don ts in RP-HPLC Reversed Phase HPLC Application Gallery Appendices Problem / possible cause Poor sample recovery adsorption on stationary phase acidic substances: <90% yield, irreversible adsorption on active groups basic substances: <90% yield, irreversible adsorption on active groups hydrophobic interactions between stationary phase and biomolecules adsorption of proteins adsorption on tubing and other hardware components Pressure problems High back pressure viscosity of mobile phase too high prevention / remedy increase mobile phase strength to minimise adsorption; for basic compounds add a competing base or use a base deactivated packing like NUCLEODUR Gravity, NUCLEOSIL HD, NUCLEOSIL PROTECT I or NUCLEOSIL AB use endcapped high density stationary phases; acidify the mobile phase use endcapped, base deactivated, sterically protected phases with dense surface coverage (NUCLEOSIL HD, NUCLEODUR Gravity); add a competing base to the mobile phase use short-chain reversed phase packings; as an alternative you may use hydrophilic stationary phases or ion exchangers use another HPLC mode to reduce nonspecific interactions (e.g. gel filtration or ion exchange); use a mobile phase containing reagents which enhance solubility of the proteins, strong acids or bases (only with polymer-based columns) or detergents like SDS use inert tubing and fittings made from e.g. PEEK or titanium use a solvent of lower viscosity or increase the temperature particle size of packing too small use a packing with larger particle size (e. g. 7 µm instead of 5 µm) for polymer-based columns: swelling of the adsorbent caused by eluent changes use only solvents compatible with the column; check proper eluent composition; consult instructions for use for solvent compatibility; use a column with a higher degree of cross-linking 44
46 HPLC troubleshooting Problem / possible cause salt precipitation contamination at the column inlet microbial growth in the column plugged frit in in-line filter or guard column plugged frit at column inlet when the injector is disconnected from the column: plugged injector prevention / remedy especially in reversed phase chromatography with high proportions of organic solvents in the mobile phase; ensure that the solvent composition is compatible with the buffer concentration; reduce the ionic strength and the ratio organic : aqueous in the mobile phase; premix the mobile phase improve sample clean-up; use guard columns; backflush column with a strong solvent in order to dissolve the impurity use a mobile phase with at least 10% organic solvent; prepare fresh buffer daily; add 0.02% sodium azide to aqueous mobile phases; for storage equilibrate the column with at least 25% organic solvent and without buffer replace frit or guard column replace the end fitting or the frit clean the injector or replace the rotor Pressure fluctuations air bubbles in the pump leak in liquid lines between pump and column Increasing pressure accumulation of solids at the column head in aqueous / organic solvent systems: precipitation of buffer components plugged liquid lines degas the solvent; flush the solvent with helium tighten all fittings; replace defective fittings; tighten rotor in the injection valve filter sample and mobile phase; use an 0.5 µm in-line filter; disconnect the contaminated column and clean it by back-flushing; replace plugged inlet frits; replace the guard column ensure that the solvent composition is compatible with the buffer concentration; reduce the ionic strength and the ratio of organic : aqueous in the mobile phase systematically disconnect system components from the detector end to the blockage; clean or replace the plugged component 45
47 Do s and don ts in RP-HPLC Reversed Phase HPLC Application Gallery Appendices Problem / possible cause Decreasing pressure insufficient flow from the pump leak in liquid lines between pump and column leaking pump check valve or seals air bubbles in the pump Baseline problems Undulating baseline prevention / remedy loosen the cap on the mobile phase reservoir tighten all fittings; replace defective fittings; tighten the rotor in the injection valve clean the check valve; replace defective check valves or seals degas all solvents; check for blockage between solvent reservoir and pump; if necessary replace the frit in the inlet line Baseline drifting to lower absorption with gradient elution: UV absorption of mobile phase A use non-uv-absorbing HPLC grade solvents for your mobile phases; if a UV-absorbing solvent is inevitable, use a UV-absorbing additive in mobile phase B Baseline drifting to higher absorption accumulation and elution of impurities with gradient elution: UV absorption of mobile phase B temperature changes in the room use sample clean-up or fractionation prior to injection; use only HPLC grade solvents; clean the contaminated column with a strong solvent use a higher wavelength of the UV detector; use non-uv-absorbing HPLC grade solvents for your mobile phases; if a UV-absorbing solvent is inevitable, use a UV-absorbing additive in mobile phase A monitor or avoid changes in room temperature; isolate the column or use a column oven; cover the RI detector to protect it from air currents 46
48 HPLC troubleshooting Problem / possible cause Baseline noise continuous: detector lamp problem or dirty detector cell periodic: pump pulses random: accumulation of impurities spikes: air bubble in the detector spikes: column temperature higher than the boiling point of the solvent occasional sharp spikes: external electrical interferences prevention / remedy replace the UV lamp or clean the detector cell repair or replace the pulse damper; purge any air from the pump; clean or replace the check valves use sample clean-up or fractionation prior to injection; use only HPLC grade solvents backflush contaminated column with a strong solvent see under Spikes above use lower working temperature use a voltage stabiliser for your LC system or use an independent electrical circuit for your chromatography equipment Baseline noise during gradient elution or isocratic proportioning insufficient solvent mixing malfunctioning proportioning valves mix by hand, or if possible use solvents of lower viscosity; monitor proportioning precision by spiking one solvent with a UV-absorbing substance and measure the resulting detector output clean or replace the proportioning valve; use partially premixed solvents Disturbance at dead time air bubbles in the mobile phase difference in refractive index between injection solvent and mobile phase degas the mobile phase or use premixed eluents normal with many samples; if possible, use the mobile phase as solvent for the sample Leaks column loses stationary phase replace column! serious leaks at column or fittings tighten loose fittings or use new fittings serious leak at the detector serious leak at the injector serious leak at the pump replace defective detector seals or gaskets replace worn or scratched valve rotors replace defective pump seals; check the piston for scratches and replace piston, if necessary 47
49 Do s and don ts in RP-HPLC Reversed Phase HPLC Application Gallery Appendices Problem / possible cause Changing retention times Decreasing retention times column overloaded with sample increasing flow rate active groups at the stationary phase loss of bonded stationary phase Increasing retention times changing mobile phase composition decreasing flow rate loss of bonded stationary phase Fluctuating retention times only during first few injections: active groups insufficient buffer capacity insufficient mixing of the mobile phase selective evaporation of one component from the mobile phase accumulation of impurities fluctuating column temperature Leaks see previous page prevention / remedy reduce the amount of sample or use a column with larger diameter check and if necessary adjust the pump flow rate use a mobile phase containing an organic solvent (modifier) or a competing base, increase the buffer strength; use a packing with higher surface coverage replace column; for silica adsorbents use mobile phases between ph 2 and ph 8 or switch to phases with higher ph stability (NUCLEODUR Gravity) cover the solvent reservoirs; ensure that the gradient system supplies the proper composition; if possible, mix the mobile phase by hand check and if necessary adjust the pump flow rate; check for pump cavitation; check for leaking pump seals and other leaks in the system for silica adsorbents use mobile phases between ph 2 and ph 8 or switch to phases with higher ph stability (NUCLEODUR Gravity) condition the column with concentrated sample use buffer concentrations above 20 mm ensure that the gradient system supplies a mobile phase with constant composition; compare with manually mixed eluents; use partially premixed mobile phases cover the mobile phase reservoirs; avoid vigorous flushing with helium; prepare fresh mobile phase flush the column occasionally with a strong solvent, replace the guard column more frequently ensure that the room temperature is constant; if necessary, thermostat or isolate the column 48
50 HPLC troubleshooting Problem / possible cause prevention / remedy Changing retention times resulting from insufficient equilibration isocratic separation gradient elution reversed phase ion-pairing chromatography pass 10 to 15 column volumes of mobile phase through the column for equilibration increase equilibration time with mobile phase A in order to obtain constant retention times for early peaks, also: pass at least 10 column volumes of eluent A through the column for gradient regeneration increase the equilibration time; in ion-pairing chromatography sometimes 50 column volumes may be required for equilibration; long-chain ion-pairing reagents require more time; if possible, use ion-pairing reagents with shorter alkyl chains 49
51 Preparative HPLC Reversed Phase HPLC Application Gallery Appendices Scale-up for process scale HPLC 50
Packings for HPLC. Packings for HPLC
 Summary of packings for HPLC In analytical HPLC, packings with particle sizes of 3 to 10 µm are preferred. For preparative separation tasks, also particles with diameters larger than 10 µm are applied.
Summary of packings for HPLC In analytical HPLC, packings with particle sizes of 3 to 10 µm are preferred. For preparative separation tasks, also particles with diameters larger than 10 µm are applied.
Overview Applications of NUCLEODUR HPLC Phases
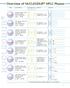 verview Applications of HPLC Phases Phase Specification Characteristics* Stability Structure A B C octadecyl phase, high density coating multi-endcapping 8% C USP L ph stability -, suitable for LC/MS (Si-
verview Applications of HPLC Phases Phase Specification Characteristics* Stability Structure A B C octadecyl phase, high density coating multi-endcapping 8% C USP L ph stability -, suitable for LC/MS (Si-
Hypersil BDS Columns TG 01-05
 TG 0-0 Hypersil BDS Columns Introduction Hypersil BDS columns have gained a reputation over the years as one of the most robust, reproducible and reliable HPLC column brands available. This Technical Guide
TG 0-0 Hypersil BDS Columns Introduction Hypersil BDS columns have gained a reputation over the years as one of the most robust, reproducible and reliable HPLC column brands available. This Technical Guide
LC and LC/MS Column Selection Flow Chart
 LC and LC/MS Column Selection Flow Chart To use the column selection diagram below, simply follow the path for your analyte and mobile phase. At the far right, follow your final column selection to the
LC and LC/MS Column Selection Flow Chart To use the column selection diagram below, simply follow the path for your analyte and mobile phase. At the far right, follow your final column selection to the
Product Bulletin. ACE LC/MS and Rapid Analysis HPLC Columns. HPLC Columns
 Product Bulletin HPLC Columns ACE LC/MS and Rapid Analysis HPLC Columns 0 mm, 30 mm, 35 mm and 50 mm column lengths.0,., 3.0, 4.0 and 4.6 mm column diameters Configured for High Sample Throughput Specially
Product Bulletin HPLC Columns ACE LC/MS and Rapid Analysis HPLC Columns 0 mm, 30 mm, 35 mm and 50 mm column lengths.0,., 3.0, 4.0 and 4.6 mm column diameters Configured for High Sample Throughput Specially
Packed Column for Ultra-Fast Reversed-Phase Liquid Chromatography, TSKgel Super-ODS. Table of Contents
 No. 089 SEPARATION REPORT Packed Column for Ultra-Fast Reversed-Phase Liquid Chromatography, TSKgel Super-ODS Table of Contents 1. Introduction 1 2. Column Specification 1 3. Features of Packing Materials
No. 089 SEPARATION REPORT Packed Column for Ultra-Fast Reversed-Phase Liquid Chromatography, TSKgel Super-ODS Table of Contents 1. Introduction 1 2. Column Specification 1 3. Features of Packing Materials
Shodex TM ODP2 HP series columns
 HPLC Columns Shodex TM ODP2 HP series columns Better retention of highly polar substances Technical notebook No. 6 Contents 1. Introduction 1-1. Specifications 1-2. Eluent Compatibility of ODP2 HP Series
HPLC Columns Shodex TM ODP2 HP series columns Better retention of highly polar substances Technical notebook No. 6 Contents 1. Introduction 1-1. Specifications 1-2. Eluent Compatibility of ODP2 HP Series
Basic principles of HPLC
 Introduction to the theory of HPLC HPLC (High Performance Liquid Chromatography) depends on interaction of sample analytes with the stationary phase (packing) and the mobile phase to effect a separation.
Introduction to the theory of HPLC HPLC (High Performance Liquid Chromatography) depends on interaction of sample analytes with the stationary phase (packing) and the mobile phase to effect a separation.
for Acclaim Mixed-Mode HILIC-1 Column
 for Acclaim Mixed-Mode HILIC-1 Column Product Manual for ACCLAIM Mixed-Mode HILIC-1 Page 1 of 17 Product Manual for ACCLAIM Mixed-Mode HILIC-1 Column 5µm, 4.6 x 250mm, P/N 066844 5µm, 4.6 x 150mm, P/N
for Acclaim Mixed-Mode HILIC-1 Column Product Manual for ACCLAIM Mixed-Mode HILIC-1 Page 1 of 17 Product Manual for ACCLAIM Mixed-Mode HILIC-1 Column 5µm, 4.6 x 250mm, P/N 066844 5µm, 4.6 x 150mm, P/N
High Performance Liquid Chromatography
 Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
High Performance Liquid Chromatography
 Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 1 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 1 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
InertSustainSwift C8
 Physical Properties Silica Particle Size Surface Area Pore Size Pore Volume Bonded Phase End-capping Carbon Loading ph Range USP Code :ES (Evolved Surface) Silica Gel :.9 μm, μm, μm :00 m /g :00 Å (0 nm)
Physical Properties Silica Particle Size Surface Area Pore Size Pore Volume Bonded Phase End-capping Carbon Loading ph Range USP Code :ES (Evolved Surface) Silica Gel :.9 μm, μm, μm :00 m /g :00 Å (0 nm)
ProntoSIL C18-EPS Reversed-Phase HPLC Columns
 ProntoSIL C8-EPS Reversed-Phase HPLC Columns Provides excellent separation of polar compounds Better peak shape for acids and bases Stabilized bonded phase for rugged, robust HPLC methods More retentive
ProntoSIL C8-EPS Reversed-Phase HPLC Columns Provides excellent separation of polar compounds Better peak shape for acids and bases Stabilized bonded phase for rugged, robust HPLC methods More retentive
State-of-the-art C18 HPLC Columns
 An HPLC GL Sciences Newest and Most Advanced ODS Phase-New For 00 State-of-the-art C HPLC s Improved Peak Shapes and Heights Enhancing Sensitivity High Resolution Fast Equilibration Compatible with 00%
An HPLC GL Sciences Newest and Most Advanced ODS Phase-New For 00 State-of-the-art C HPLC s Improved Peak Shapes and Heights Enhancing Sensitivity High Resolution Fast Equilibration Compatible with 00%
Optimisil HPLC & UPLC Column catalogue
 Optimisil HPLC & UPLC Column catalogue Analytical Hub Plot No: 37, P & T Colony, Vikrampuri, Secunderabad 500009 Tel: 040-64621313, Mobile: +91 7799931313 Website: www.analyticalhub.com email: contact@analyticalhub.com
Optimisil HPLC & UPLC Column catalogue Analytical Hub Plot No: 37, P & T Colony, Vikrampuri, Secunderabad 500009 Tel: 040-64621313, Mobile: +91 7799931313 Website: www.analyticalhub.com email: contact@analyticalhub.com
Barry E. Boyes, Ph.D. Consumables and Accessories Business Unit May 10, 2000
 Barry E. Boyes, Ph.D. Consumables and Accessories Business Unit May 0, 000 HPLC Column Troubleshooting What Every HPLC User Should Know :00 a.m. EST Telephone Number: 86-650-06 Chair Person: Tim Spaeder
Barry E. Boyes, Ph.D. Consumables and Accessories Business Unit May 0, 000 HPLC Column Troubleshooting What Every HPLC User Should Know :00 a.m. EST Telephone Number: 86-650-06 Chair Person: Tim Spaeder
ACE Ultra Inert Base Deactivated HPLC Columns
 ACE Ultra Inert Base Deactivated HPLC Columns Contents Page ACE HPLC Columns...- Independent Comparison of HPLC Columns #... Independent Comparison of HPLC Columns #... ACE 00Å HPLC Columns Specifications...-
ACE Ultra Inert Base Deactivated HPLC Columns Contents Page ACE HPLC Columns...- Independent Comparison of HPLC Columns #... Independent Comparison of HPLC Columns #... ACE 00Å HPLC Columns Specifications...-
Guide to Choosing and Using Polymer Reversed Phase Columns!
 Guide to Choosing and Using Polymer Reversed Phase Columns! Choosing the best RP Column to use: Silica is normally used as the packing material for HPLC columns for a number of reasons. It is very strong,
Guide to Choosing and Using Polymer Reversed Phase Columns! Choosing the best RP Column to use: Silica is normally used as the packing material for HPLC columns for a number of reasons. It is very strong,
Inertsil ODS-EP Technical Information
 Technical Information Advantages of The selectivity is completely different from those of conventional columns such as ODS column due to its specific polar group in the stationary phase. Durability can
Technical Information Advantages of The selectivity is completely different from those of conventional columns such as ODS column due to its specific polar group in the stationary phase. Durability can
Reversed Phase Solvents
 Part 1. General Chromatographic Theory Part 2. verview of HPLC Media Part 3. The Role of the Mobile Phase in Selectivity Part 4. Column Care and Use Reversed Phase Solvents 2 Solvents for RP Chromatography
Part 1. General Chromatographic Theory Part 2. verview of HPLC Media Part 3. The Role of the Mobile Phase in Selectivity Part 4. Column Care and Use Reversed Phase Solvents 2 Solvents for RP Chromatography
8. Methods in Developing Mobile Phase Condition for C18 Column
 I. HPLC Columns Technical Information 8. Methods in Developing Mobile Phase Condition for C18 Column Introduction In reversed phase HPLC, octadecyl group bonded silica columns (C18, ODS) are the most widely
I. HPLC Columns Technical Information 8. Methods in Developing Mobile Phase Condition for C18 Column Introduction In reversed phase HPLC, octadecyl group bonded silica columns (C18, ODS) are the most widely
Choosing Columns and Conditions for the Best Peak Shape
 Choosing Columns and Conditions for the Best Peak Shape What is Good Peak Shape and Why is it Important? Good peak shape can be defined as a symmetrical or gaussian peak and poor peak shape can include
Choosing Columns and Conditions for the Best Peak Shape What is Good Peak Shape and Why is it Important? Good peak shape can be defined as a symmetrical or gaussian peak and poor peak shape can include
Orosil HPLC Columns. OroSil HPLC columns are designed for the separation of polar, semi-polar, and nonpolar compounds at low to medium ph.
 Orosil OroSil HPLC columns are designed for the separation of polar, semi-polar, and nonpolar compounds at low to medium ph. Excellent organic base selectivity with very low asymmetry values Compatible
Orosil OroSil HPLC columns are designed for the separation of polar, semi-polar, and nonpolar compounds at low to medium ph. Excellent organic base selectivity with very low asymmetry values Compatible
William E. Barber, Ph.D. Applications Chemist October
 William E. Barber, Ph.D. Applications Chemist ctober 0 00 Secrets of Good Peak Shape in HPLC Time: :00 p.m. CET Telephone Number: + 44 0 76 088 Chairperson: John Vis The Secrets of Good Peak Shape in HPLC
William E. Barber, Ph.D. Applications Chemist ctober 0 00 Secrets of Good Peak Shape in HPLC Time: :00 p.m. CET Telephone Number: + 44 0 76 088 Chairperson: John Vis The Secrets of Good Peak Shape in HPLC
Alltech Alltima HP Introduction
 Alltech Introduction High-Stability, High-Purity, High-Performance, Low-Bleed Columns for Demanding Applications Better Peak Symmetry high-purity silica eliminates peak tailing problems Long Column Life
Alltech Introduction High-Stability, High-Purity, High-Performance, Low-Bleed Columns for Demanding Applications Better Peak Symmetry high-purity silica eliminates peak tailing problems Long Column Life
Comparison of different aqueous mobile phase HPLC techniques
 June 009 ewsletter Pharmaceutical Analysis: What is Your Problem? SIELC Technologies, Inc., Prospect Heights, IL 0070 Pharmaceutical analysis involves liquid chromatography of various compounds, from active
June 009 ewsletter Pharmaceutical Analysis: What is Your Problem? SIELC Technologies, Inc., Prospect Heights, IL 0070 Pharmaceutical analysis involves liquid chromatography of various compounds, from active
penta-hilic UHPLC COLUMNS
 penta-hilic UHPLC COLUMNS penta-hilic Highly retentive, proprietary penta-hydroxy-ligand Excellent peak shape for polar compounds with a variety of functional groups: acids, bases, zwitterions strong and
penta-hilic UHPLC COLUMNS penta-hilic Highly retentive, proprietary penta-hydroxy-ligand Excellent peak shape for polar compounds with a variety of functional groups: acids, bases, zwitterions strong and
LC Technical Information
 LC Technical Information Method Transfer to Accucore.6 μm Columns Containing solid core particles, which are engineered to a diameter of.6μm and a very narrow particle size distribution; Accucore HPLC
LC Technical Information Method Transfer to Accucore.6 μm Columns Containing solid core particles, which are engineered to a diameter of.6μm and a very narrow particle size distribution; Accucore HPLC
Chemistry Instrumental Analysis Lecture 28. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 28 High Performance Liquid Chromatography () Instrumentation Normal Phase Chromatography Normal Phase - a polar stationary phase with a less polar mobile phase.
Chemistry 4631 Instrumental Analysis Lecture 28 High Performance Liquid Chromatography () Instrumentation Normal Phase Chromatography Normal Phase - a polar stationary phase with a less polar mobile phase.
Acclaim Mixed-Mode WCX-1
 Acclaim Mixed-Mode WCX-1 Product Manual for the Acclaim Mixed-Mode WCX-1 Column Page 1 of 28 PRODUCT MANUAL for the Acclaim Mixed-Mode WCX-1 Columns 4.6 x 150 mm, P/N (068353) 4.6 x 250 mm, P/N (068352)
Acclaim Mixed-Mode WCX-1 Product Manual for the Acclaim Mixed-Mode WCX-1 Column Page 1 of 28 PRODUCT MANUAL for the Acclaim Mixed-Mode WCX-1 Columns 4.6 x 150 mm, P/N (068353) 4.6 x 250 mm, P/N (068352)
( )( ) Selectivity Choices in Reversed-Phase Fast LC. Introduction. R s = 1 a 1 k 4 a 1 + k
 Selectivity Choices in Reversed-Phase Fast LC Luisa Pereira, Monica Dolci, Thermo Fisher Scientific, Runcorn, Cheshire, UK Technical Note 20543 Key Words Accucore, Hypersil GOLD, Syncronis, Column Chemistry,
Selectivity Choices in Reversed-Phase Fast LC Luisa Pereira, Monica Dolci, Thermo Fisher Scientific, Runcorn, Cheshire, UK Technical Note 20543 Key Words Accucore, Hypersil GOLD, Syncronis, Column Chemistry,
Sunniest C18 Sunniest RP-AQUA Sunniest C8 Patent pending
 Patent pending Website : www.chromtech.net.au E-mail : info@chromtech.net.au telno : 0 97 0... in AUSTRALIA A Novel Bonding Technique (patent pending) An unique trifunctional silyl-reagent was developed
Patent pending Website : www.chromtech.net.au E-mail : info@chromtech.net.au telno : 0 97 0... in AUSTRALIA A Novel Bonding Technique (patent pending) An unique trifunctional silyl-reagent was developed
Chromatography. Gas Chromatography
 Chromatography Chromatography is essentially the separation of a mixture into its component parts for qualitative and quantitative analysis. The basis of separation is the partitioning of the analyte mixture
Chromatography Chromatography is essentially the separation of a mixture into its component parts for qualitative and quantitative analysis. The basis of separation is the partitioning of the analyte mixture
Open Column Chromatography, GC, TLC, and HPLC
 Open Column Chromatography, GC, TLC, and HPLC Murphy, B. (2017). Introduction to Chromatography: Lecture 1. Lecture presented at PHAR 423 Lecture in UIC College of Pharmacy, Chicago. USES OF CHROMATOGRAPHY
Open Column Chromatography, GC, TLC, and HPLC Murphy, B. (2017). Introduction to Chromatography: Lecture 1. Lecture presented at PHAR 423 Lecture in UIC College of Pharmacy, Chicago. USES OF CHROMATOGRAPHY
High Performance Liquid Chromatography
 STANDARDBASE techniques: High Performance Liquid Chromatography Drenthe College, The Netherlands 1. Introduction HPLC. High Performance Liquid Chromatography High Performance Liquid Chromatography (HPLC)
STANDARDBASE techniques: High Performance Liquid Chromatography Drenthe College, The Netherlands 1. Introduction HPLC. High Performance Liquid Chromatography High Performance Liquid Chromatography (HPLC)
Uptisphere CS Evolution Core Shell columns for fast & highly efficient identification & quantification of small molecules
 Uptisphere CS Evolution Core Shell columns for fast & highly efficient identification & quantification of small molecules www.interchim.com www.blog.interchim.com www.forum.interchim.com www.facebook.com/interchim
Uptisphere CS Evolution Core Shell columns for fast & highly efficient identification & quantification of small molecules www.interchim.com www.blog.interchim.com www.forum.interchim.com www.facebook.com/interchim
 CAPCELL PAK C18 IF2 Type http://hplc.shiseido.co.jp/e/column/html/if2_index.htm Page 1 of 2 List of Sales Representatives Contact Shiseido Technical Materials Catalog List HPLC Columns HPLC Instruments
CAPCELL PAK C18 IF2 Type http://hplc.shiseido.co.jp/e/column/html/if2_index.htm Page 1 of 2 List of Sales Representatives Contact Shiseido Technical Materials Catalog List HPLC Columns HPLC Instruments
The Theory of HPLC. Normal Phase (Absorption) Chromatography
 The Theory of HPLC Normal Phase (Absorption) Chromatography i Wherever you see this symbol, it is important to access the on-line course as there is interactive material that cannot be fully shown in this
The Theory of HPLC Normal Phase (Absorption) Chromatography i Wherever you see this symbol, it is important to access the on-line course as there is interactive material that cannot be fully shown in this
M > ACN > > THF
 Method Development in HPLC Dr. Amitha Hewavitharana School of Pharmacy University of Queensland Method Development in HPLC References: D A Skoog Principles of instrumental analysis, 3 rd Edition Chapters
Method Development in HPLC Dr. Amitha Hewavitharana School of Pharmacy University of Queensland Method Development in HPLC References: D A Skoog Principles of instrumental analysis, 3 rd Edition Chapters
penta-hilic UHPLC COLUMNS
 penta-hilic UHPLC COLUMNS Highly retentive, proprietary penta-hydroxy-ligand penta-hilic Excellent peak shape for polar compounds with a variety of functional groups: acids, bases, zwitterions strong and
penta-hilic UHPLC COLUMNS Highly retentive, proprietary penta-hydroxy-ligand penta-hilic Excellent peak shape for polar compounds with a variety of functional groups: acids, bases, zwitterions strong and
Method Development in Solid Phase Extraction using Non-Polar ISOLUTE SPE Columns for the Extraction of Aqueous Samples
 Technical Note 101 Method Development in Solid Phase Extraction using Non-Polar ISOLUTE SPE Columns for the Extraction of Aqueous Samples This technical note includes by specific information on the extraction
Technical Note 101 Method Development in Solid Phase Extraction using Non-Polar ISOLUTE SPE Columns for the Extraction of Aqueous Samples This technical note includes by specific information on the extraction
High Performance Liquid Chromatography
 High Performance Liquid Chromatography What is HPLC? It is a separation technique that involves: Injection of small volume of liquid sample Into a tube packed with a tiny particles (stationary phase).
High Performance Liquid Chromatography What is HPLC? It is a separation technique that involves: Injection of small volume of liquid sample Into a tube packed with a tiny particles (stationary phase).
LIQUID CHROMATOGRAPHY
 LIQUID CHROMATOGRAPHY RECENT TECHNIQUES HPLC High Performance Liquid Chromatography RRLC Rapid Resolution Liquid Chromatography UPLC Ultra Performance Liquid Chromatography UHPLC Ultra High Pressure Liquid
LIQUID CHROMATOGRAPHY RECENT TECHNIQUES HPLC High Performance Liquid Chromatography RRLC Rapid Resolution Liquid Chromatography UPLC Ultra Performance Liquid Chromatography UHPLC Ultra High Pressure Liquid
P R O D U C T B U L L E T I N. Fused-Core particle technology for hyper-fast and super-rugged HPLC columns
 P R O D U C T B U L L E T I N Fused-Core particle technology for hyper-fast and super-rugged HPLC columns HALO column packings are not made the typical way. Instead, the particles packed into HALO columns
P R O D U C T B U L L E T I N Fused-Core particle technology for hyper-fast and super-rugged HPLC columns HALO column packings are not made the typical way. Instead, the particles packed into HALO columns
Agilent s New Weak Anion Exchange (WAX) Solid Phase Extraction Cartridges: SampliQ WAX
 Agilent s New Weak Anion Exchange (WAX) Solid Phase Extraction Cartridges: SampliQ WAX Technical Note Agilent s SampliQ WAX provides Applications for strongly acidic, acidic and neutral compounds Excellent
Agilent s New Weak Anion Exchange (WAX) Solid Phase Extraction Cartridges: SampliQ WAX Technical Note Agilent s SampliQ WAX provides Applications for strongly acidic, acidic and neutral compounds Excellent
Products for GPC. Products for GPC. Size exclusion chromatography. Basic principles
 Products for GPC Products for GPC Size exclusion chromatography Basic principles injection A B C retention time mobile phase B Size exclusion chromatography (SEC) is a liquid chromatographic separation
Products for GPC Products for GPC Size exclusion chromatography Basic principles injection A B C retention time mobile phase B Size exclusion chromatography (SEC) is a liquid chromatographic separation
Method Development Kits
 Method Development Kits sales representative for ordering information or contact...77 Method Development Kits First column choice for Pharma Development USP L C Method Development Kits Maximize retention
Method Development Kits sales representative for ordering information or contact...77 Method Development Kits First column choice for Pharma Development USP L C Method Development Kits Maximize retention
Inertsil Technical Library
 HPLC COLUMN INERTSIL TECHNICAL LIBRARY H P L C C o l u m n Inertsil Technical Library Inertsil ODS-4, C8-4 Comparison of Performance List of Compared Columns Experimental Explanation, Analytical Conditions
HPLC COLUMN INERTSIL TECHNICAL LIBRARY H P L C C o l u m n Inertsil Technical Library Inertsil ODS-4, C8-4 Comparison of Performance List of Compared Columns Experimental Explanation, Analytical Conditions
Technical Guide Thermo Scientific Syncronis HPLC Columns
 Technical Guide Thermo Scientific Syncronis HPLC Columns Consistent, predictable separations, Column after column, time after time Thermo Scientific Syncronis Columns When developing a new method, one
Technical Guide Thermo Scientific Syncronis HPLC Columns Consistent, predictable separations, Column after column, time after time Thermo Scientific Syncronis Columns When developing a new method, one
InertSustainSwift C8
 HPLC, LC/MS Columns InertSustainSwift TM C8 New! InertSustainSwift C8 is an octyl group (C8) bonded column offering the same extreme inertness to any type of compounds just like InertSustainSwift C8, which
HPLC, LC/MS Columns InertSustainSwift TM C8 New! InertSustainSwift C8 is an octyl group (C8) bonded column offering the same extreme inertness to any type of compounds just like InertSustainSwift C8, which
Advantages of polymerbased. an alternative for ODS
 Advantages of polymerbased HPLC columns an alternative for ODS Yukiko Higai Shodex, 1 Content (1) Comparison between Silica and Polymer-based RP columns (2) Characteristics of Polymer RP columns - an example:
Advantages of polymerbased HPLC columns an alternative for ODS Yukiko Higai Shodex, 1 Content (1) Comparison between Silica and Polymer-based RP columns (2) Characteristics of Polymer RP columns - an example:
FAQ's. 1 - Which column would be the most appropriate for my application?
 1 - Which column would be the most appropriate for my application? Due to the complexity of the chiral recognition mechanism, it is not possible yet to establish rules for the selection of the best chiral
1 - Which column would be the most appropriate for my application? Due to the complexity of the chiral recognition mechanism, it is not possible yet to establish rules for the selection of the best chiral
columns Acclaim Mixed-Mode WCX-1 for Separating Basic Molecules
 columns Acclaim Mixed-Mode WCX- for Separating Basic Molecules The Acclaim Mixed-Mode WCX- is a novel, high-efficiency, silica-based column specially designed for separating various basic analytes. This
columns Acclaim Mixed-Mode WCX- for Separating Basic Molecules The Acclaim Mixed-Mode WCX- is a novel, high-efficiency, silica-based column specially designed for separating various basic analytes. This
COMPARISON GUIDE. to C18 Reversed Phase HPLC Columns. Comparison Data on 60 Commonly Used C18 Phases. Stationary Phase Specifications
 COMPARISON GUIDE to C18 Reversed Phase HPLC Columns Comparison Data on 60 Commonly Used C18 Phases Stationary Phase Specifications Phases Compared According to Relative Hydrophobicity Phases Compared According
COMPARISON GUIDE to C18 Reversed Phase HPLC Columns Comparison Data on 60 Commonly Used C18 Phases Stationary Phase Specifications Phases Compared According to Relative Hydrophobicity Phases Compared According
Kromasil. for your analytical HPLC. Kromasil. The way to peak performance in liquid chromatography
 Kromasil for your analytical HPLC Kromasil The way to peak performance in liquid chromatography Kromasil is known, worldwide, for its high performance and excellent total economy in preparative and industrial
Kromasil for your analytical HPLC Kromasil The way to peak performance in liquid chromatography Kromasil is known, worldwide, for its high performance and excellent total economy in preparative and industrial
Lab.2. Thin layer chromatography
 Key words: Separation techniques, compounds and their physicochemical properties (molecular volume/size, polarity, molecular interactions), mobile phase, stationary phase, liquid chromatography, thin layer
Key words: Separation techniques, compounds and their physicochemical properties (molecular volume/size, polarity, molecular interactions), mobile phase, stationary phase, liquid chromatography, thin layer
C18 Column. Care & Use Sheet
 C18 Column Care & Use Sheet HALO Description HALO C18 is a high-speed, high-performance liquid chromatography column based on a new Fused-CoreTM particle design. The Fused-Core particle provides a thin
C18 Column Care & Use Sheet HALO Description HALO C18 is a high-speed, high-performance liquid chromatography column based on a new Fused-CoreTM particle design. The Fused-Core particle provides a thin
mau 200 ph min
 mau ph - 6 8 TAKE A LOOK INSIDE TWIN Technology TWIN (Two-In-One ) Technology is what gives Gei its superior performance edge. During the final stage of silica manufacturing, a unique silica-organic layer
mau ph - 6 8 TAKE A LOOK INSIDE TWIN Technology TWIN (Two-In-One ) Technology is what gives Gei its superior performance edge. During the final stage of silica manufacturing, a unique silica-organic layer
The Secrets of Rapid HPLC Method Development. Choosing Columns for Rapid Method Development and Short Analysis Times
 The Secrets of Rapid HPLC Method Development Choosing Columns for Rapid Method Development and Short Analysis Times Rapid Analysis Is More Than Run Time It is developing a method to meet a goal and developing
The Secrets of Rapid HPLC Method Development Choosing Columns for Rapid Method Development and Short Analysis Times Rapid Analysis Is More Than Run Time It is developing a method to meet a goal and developing
Instrumental Chemical Analysis
 L2 Page1 Instrumental Chemical Analysis Chromatography (General aspects of chromatography) Dr. Ahmad Najjar Philadelphia University Faculty of Pharmacy Department of Pharmaceutical Sciences 2 nd semester,
L2 Page1 Instrumental Chemical Analysis Chromatography (General aspects of chromatography) Dr. Ahmad Najjar Philadelphia University Faculty of Pharmacy Department of Pharmaceutical Sciences 2 nd semester,
Optimizing GPC Separations
 Optimizing GPC Separations Criteria for Solvent Selection True sample solubility (Polarity and Time dependant) Compatibility with columns Avoid non-size exclusion effects (eg adsorption by reverse phase
Optimizing GPC Separations Criteria for Solvent Selection True sample solubility (Polarity and Time dependant) Compatibility with columns Avoid non-size exclusion effects (eg adsorption by reverse phase
[ CARE AND USE MANUAL ]
![[ CARE AND USE MANUAL ] [ CARE AND USE MANUAL ]](/thumbs/83/87469313.jpg) CONTENTS I. INTRODUCTION II. III. IV. GETTING STARTED a. Column Installation b. Column Equilibration c. Initial Column Efficiency Determination COLUMN USE a. Guard Columns b. Sample Preparation c. ph Range
CONTENTS I. INTRODUCTION II. III. IV. GETTING STARTED a. Column Installation b. Column Equilibration c. Initial Column Efficiency Determination COLUMN USE a. Guard Columns b. Sample Preparation c. ph Range
LC III: HPLC. Originally referred to as High-Pressure Liquid Chromatography. Now more commonly called High Performance Liquid Chromatography
 LC III: HPLC What is HPLC? Originally referred to as High-Pressure Liquid Chromatography Now more commonly called High Performance Liquid Chromatography In general: The instrument controlled version of
LC III: HPLC What is HPLC? Originally referred to as High-Pressure Liquid Chromatography Now more commonly called High Performance Liquid Chromatography In general: The instrument controlled version of
High Pressure/Performance Liquid Chromatography (HPLC)
 High Pressure/Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) is a form of column chromatography that pumps a sample mixture or analyte in a solvent (known as the
High Pressure/Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) is a form of column chromatography that pumps a sample mixture or analyte in a solvent (known as the
zorbax Packed by Phenomenex Material Characteristics High efficiency Guaranteed quality Phenomenex columns packed with Zorbax
 Packed by Phenomenex High efficiency Guaranteed quality Phenomenex columns packed with 7 μm materials offer the advantages of lower cost as well as lower operating backpressures, which result in long column
Packed by Phenomenex High efficiency Guaranteed quality Phenomenex columns packed with 7 μm materials offer the advantages of lower cost as well as lower operating backpressures, which result in long column
An HPLC column offering moderate retentivity with superb peak shape Inertsil ODS Sprint
 An HPLC column offering moderate retentivity with superb peak shape Inertsil ODS Sprint An important new column in the Inertsil series Bonded-Phase Structure Perfect balance of retention for both polar
An HPLC column offering moderate retentivity with superb peak shape Inertsil ODS Sprint An important new column in the Inertsil series Bonded-Phase Structure Perfect balance of retention for both polar
ProPac WCX-10 Columns
 ProPac WCX-10 Columns Guidance for column use Tips to maximize column lifetime ProPac WCX-10 Column Tips and Tricks This guide provides essential information and invaluable guidelines for mobile phases,
ProPac WCX-10 Columns Guidance for column use Tips to maximize column lifetime ProPac WCX-10 Column Tips and Tricks This guide provides essential information and invaluable guidelines for mobile phases,
Pure Chromatography Consumables Pure flexibility. Pure specialization. Pure convenience.
 Pure Chromatography Consumables Pure flexibility. Pure specialization. Pure convenience. Pure Consumables More focus on your application The Pure consumable portfolio offers an unrivaled range of products
Pure Chromatography Consumables Pure flexibility. Pure specialization. Pure convenience. Pure Consumables More focus on your application The Pure consumable portfolio offers an unrivaled range of products
for Acclaim Mixed-Mode WCX-1
 for Acclaim Mixed-Mode WCX- Product Manual for the Acclaim Mixed-Mode WCX- Column Page of 8 Product Manual for Acclaim Mixed-Mode WCX- Columns Acclaim Mixed-Mode WCX-, 3µm, Analytical column, 3.0 x 50mm
for Acclaim Mixed-Mode WCX- Product Manual for the Acclaim Mixed-Mode WCX- Column Page of 8 Product Manual for Acclaim Mixed-Mode WCX- Columns Acclaim Mixed-Mode WCX-, 3µm, Analytical column, 3.0 x 50mm
GUIDELINES FOR THE DESIGN OF CHROMATOGRAPHIC ANALYTICAL METHODS INTENDED FOR CIPAC COLLABORATIVE STUDY
 Page 1 of 13 CIPAC/4105/R GUIDELINES FOR THE DESIGN OF CHROMATOGRAPHIC ANALYTICAL METHODS INTENDED FOR CIPAC COLLABORATIVE STUDY Prepared for CIPAC by Dr M J Tandy*, P M Clarke and B White (UK) The rapid
Page 1 of 13 CIPAC/4105/R GUIDELINES FOR THE DESIGN OF CHROMATOGRAPHIC ANALYTICAL METHODS INTENDED FOR CIPAC COLLABORATIVE STUDY Prepared for CIPAC by Dr M J Tandy*, P M Clarke and B White (UK) The rapid
HICHROM. Chromatography Columns and Supplies. LC COLUMNS Zorbax. Catalogue 9. Hichrom Limited
 HICHROM Chromatography Columns and Supplies LC COLUMNS Zorbax Catalogue 9 1 The Markham Centre, Station Road Theale, Reading, Berks, RG7 4PE, UK Tel: +44 (0)118 930 3660 Fax: +44 (0)118 932 3484 Email:
HICHROM Chromatography Columns and Supplies LC COLUMNS Zorbax Catalogue 9 1 The Markham Centre, Station Road Theale, Reading, Berks, RG7 4PE, UK Tel: +44 (0)118 930 3660 Fax: +44 (0)118 932 3484 Email:
Packed columns for reversed-phase chromatography TSKgel ODS-100V and 100Z
 Packed columns for reversed-phase chromatography TSKgel ODS-100V and 100Z Contents Page 1. Introduction 2 2. Specifications and characteristics 2 3. Properties of packing material 3 4. Chromatographic
Packed columns for reversed-phase chromatography TSKgel ODS-100V and 100Z Contents Page 1. Introduction 2 2. Specifications and characteristics 2 3. Properties of packing material 3 4. Chromatographic
Experiment 1: Thin Layer Chromatography
 Experiment 1: Thin Layer Chromatography Part A: understanding R f values Part B: R f values & solvent polarity Part C: R f values & compound functionality Part D: identification of commercial food dye
Experiment 1: Thin Layer Chromatography Part A: understanding R f values Part B: R f values & solvent polarity Part C: R f values & compound functionality Part D: identification of commercial food dye
An Advanced Base Deactivated Capillary Column for analysis of Volatile amines Ammonia and Alcohols.
 An Advanced Base Deactivated Capillary Column for analysis of Volatile amines Ammonia and Alcohols. Jaap de Zeeuw, Ron Stricek and Gary Stidsen Restek Corp Bellefonte, USA To analyze basic compounds at
An Advanced Base Deactivated Capillary Column for analysis of Volatile amines Ammonia and Alcohols. Jaap de Zeeuw, Ron Stricek and Gary Stidsen Restek Corp Bellefonte, USA To analyze basic compounds at
Small Molecule Selectivity Sampler
 Small Molecule Selectivity Sampler HALO 90 Å C18, AQ-C18, BIPHENYL Fused-Core Particle Technology Why Choose Fused-Core Particle Technology? Fused-Core Out-Performs Totally Porous Particles. HALO particles
Small Molecule Selectivity Sampler HALO 90 Å C18, AQ-C18, BIPHENYL Fused-Core Particle Technology Why Choose Fused-Core Particle Technology? Fused-Core Out-Performs Totally Porous Particles. HALO particles
Chemistry Gas Chromatography: Separation of Volatile Organics
 Chemistry 3200 Gas chromatography (GC) is an instrumental method for separating volatile compounds in a mixture. A small sample of the mixture is injected onto one end of a column housed in an oven. The
Chemistry 3200 Gas chromatography (GC) is an instrumental method for separating volatile compounds in a mixture. A small sample of the mixture is injected onto one end of a column housed in an oven. The
Dilution(*) Chromatography
 WA20264 Poster # 184, HPLC 2002, Montreal, 4-5 June 2002 At-Column Column-Dilution for Preparative Dilution(*) Chromatography Cecilia Mazza, Jie Cavanaugh, Ziling Lu,Tom Sirard,Tom Wheat and Uwe Neue Waters
WA20264 Poster # 184, HPLC 2002, Montreal, 4-5 June 2002 At-Column Column-Dilution for Preparative Dilution(*) Chromatography Cecilia Mazza, Jie Cavanaugh, Ziling Lu,Tom Sirard,Tom Wheat and Uwe Neue Waters
Reversed Phase Chromatography: Mobile phase Considerations
 Reversed Phase: Mobile Phase Considerations Dr. www.forumsci.co.il/hplc Example of a Reversed Phase Method with UV Detection 1 Chromatographic Process B+A Mobile phase Elution through the Column-movie
Reversed Phase: Mobile Phase Considerations Dr. www.forumsci.co.il/hplc Example of a Reversed Phase Method with UV Detection 1 Chromatographic Process B+A Mobile phase Elution through the Column-movie
RP C18 column with feature of a silanol group
 RP C8 column with feature of a silanol group lanol Activity Controlled C8 Column Sunrise ctacosyl C8) Sunrise ctadecyl C8) Sunrise ctadecyl-sac C8-SAC has an interaction of silanol groups ChromaNik Sunrise
RP C8 column with feature of a silanol group lanol Activity Controlled C8 Column Sunrise ctacosyl C8) Sunrise ctadecyl C8) Sunrise ctadecyl-sac C8-SAC has an interaction of silanol groups ChromaNik Sunrise
Welcome to our E-Seminar: Choosing HPLC Columns for Faster Analysis Smaller and Faster
 Welcome to our E-Sear: Choosing HPLC Columns for Faster Analysis Smaller and Faster High Throughput/Fast LC Requires. Short columns 0 mm or shorter Small particle sizes. µm Rapid Resolution or new.8 µm
Welcome to our E-Sear: Choosing HPLC Columns for Faster Analysis Smaller and Faster High Throughput/Fast LC Requires. Short columns 0 mm or shorter Small particle sizes. µm Rapid Resolution or new.8 µm
New 5-micron HALO-5 columns based on Fused- Core particle technology boost the performance of HPLC. Compared to other HPLC columns, HALO-5 columns
 New 5-micron HALO-5 columns based on Fused- Core particle technology boost the performance of HPLC. Compared to other HPLC columns, HALO-5 columns have: the highest plate number versus any other 5-micron
New 5-micron HALO-5 columns based on Fused- Core particle technology boost the performance of HPLC. Compared to other HPLC columns, HALO-5 columns have: the highest plate number versus any other 5-micron
Biochemistry. Biochemical Techniques HPLC
 Description of Module Subject Name Paper Name 12 Module Name/Title 13 1. Objectives 1.1. To understand the basic concept and principle of 1.2. To understand the components and techniques of 1.3. To know
Description of Module Subject Name Paper Name 12 Module Name/Title 13 1. Objectives 1.1. To understand the basic concept and principle of 1.2. To understand the components and techniques of 1.3. To know
Downstream Processing Prof. Mukesh Doble Department Of Biotechnology Indian Institute of Technology, Madras. Lecture - 33 HPLC
 Downstream Processing Prof. Mukesh Doble Department Of Biotechnology Indian Institute of Technology, Madras Lecture - 33 HPLC Today, we are going to talk about the HPLC. HPLC is an analytical tool, which
Downstream Processing Prof. Mukesh Doble Department Of Biotechnology Indian Institute of Technology, Madras Lecture - 33 HPLC Today, we are going to talk about the HPLC. HPLC is an analytical tool, which
HPLC Background Chem 250 F 2008 Page 1 of 24
 HPLC Background Chem 250 F 2008 Page 1 of 24 Outline: General and descriptive aspects of chromatographic retention and separation: phenomenological k, efficiency, selectivity. Quantitative description
HPLC Background Chem 250 F 2008 Page 1 of 24 Outline: General and descriptive aspects of chromatographic retention and separation: phenomenological k, efficiency, selectivity. Quantitative description
Headspace Technology for GC and GC/MS: Features, benefits & applications
 Headspace Technology for GC and GC/MS: Features, benefits & applications Karima Baudin Oct 2015 Why use Headspace? Very Simple no to minimum sample prep Robust enhance uptime Non-detectable carry-over
Headspace Technology for GC and GC/MS: Features, benefits & applications Karima Baudin Oct 2015 Why use Headspace? Very Simple no to minimum sample prep Robust enhance uptime Non-detectable carry-over
Phenogel. GPC/SEC Columns. Sample Elution. Technical Specifications 10 3 Å 10 6 Å
 phenogel Gpc/sec columns HPLC 5 and 10 μm particle sizes Narrow bore (4.6 mm ID) solvent-saver to preparative columns available Very good alternative to Polymer Labs PLgel and Waters Styragel, Ultrastyragel,
phenogel Gpc/sec columns HPLC 5 and 10 μm particle sizes Narrow bore (4.6 mm ID) solvent-saver to preparative columns available Very good alternative to Polymer Labs PLgel and Waters Styragel, Ultrastyragel,
Part 2. Overview of HPLC Media
 Part 1. General Chromatographic Theory Part 2. Overview of HPLC Media Part 3. The Role of the Mobile Phase in Selectivity Part 4. Column Care and Use 1 HPLC Particle Technology Core-Shell Particle Fully
Part 1. General Chromatographic Theory Part 2. Overview of HPLC Media Part 3. The Role of the Mobile Phase in Selectivity Part 4. Column Care and Use 1 HPLC Particle Technology Core-Shell Particle Fully
Colin F. Poole Department of Chemistry Wayne State University USA
 Colin F. Poole Department of Chemistry Wayne State University USA Method Development Process Method Development Process Need to know what to do Before beginning experiments need to decide how to do it
Colin F. Poole Department of Chemistry Wayne State University USA Method Development Process Method Development Process Need to know what to do Before beginning experiments need to decide how to do it
SPE Introduction. general chromatography. solid phase extraction. How to Choose an SPE Product. Solid-Phase Processing Methods
 How to Choose an SPE Product 1. Characterize the Sample Factors such as the analyte s polarity relative to the matrix, the presence of charged functional groups, solubility, molecular weight, etc., determine
How to Choose an SPE Product 1. Characterize the Sample Factors such as the analyte s polarity relative to the matrix, the presence of charged functional groups, solubility, molecular weight, etc., determine
Prelab Reading Assignment: Laboratory Techniques in Organic Chemistry, 4 th Ed. Chapter 19
 CHEM 213 Technique Experiments Experiment 5: Column Chromatography Number of labs - one Reactions performed None Chemicals used: Fluorene-fluorenone mixture, hexanes, methylene chloride, silica gel Supplies
CHEM 213 Technique Experiments Experiment 5: Column Chromatography Number of labs - one Reactions performed None Chemicals used: Fluorene-fluorenone mixture, hexanes, methylene chloride, silica gel Supplies
ImpSilHPLC Columns. The solution for your chromatography IMPTECH SCIENTIFIC
 ImpSilHPLC Columns The solution for your chromatography IMPTECH SCIENTIFIC 7-1-60,203-Veena Dharam Karam Road,Ameerpet,Hyderabad-500 016 Tel:040-23740443/483 Ph:9848051071 imptechscientific@gmail.com www.impscience.com
ImpSilHPLC Columns The solution for your chromatography IMPTECH SCIENTIFIC 7-1-60,203-Veena Dharam Karam Road,Ameerpet,Hyderabad-500 016 Tel:040-23740443/483 Ph:9848051071 imptechscientific@gmail.com www.impscience.com
CHEM340 Tutorial 4: Chromatography
 CHEM340 Tutorial 4: Chromatography 1. The data in the table below was obtained from a chromatogram obtained with a 10 cm liquid chromatography column. Under the conditions used, the compound uracil is
CHEM340 Tutorial 4: Chromatography 1. The data in the table below was obtained from a chromatogram obtained with a 10 cm liquid chromatography column. Under the conditions used, the compound uracil is
Chemistry Instrumental Analysis Lecture 31. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 31 High Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) Solvent Delivery
Chemistry 4631 Instrumental Analysis Lecture 31 High Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) Solvent Delivery
Analysis - HPLC A.136. Primesep 5 µm columns B.136
 Primesep 5 µm columns Primesep columns feature double functionality of the bonding i.e : alkyl chain with anionic or cationic group, chelating group. This feature creates unique selectivities when using
Primesep 5 µm columns Primesep columns feature double functionality of the bonding i.e : alkyl chain with anionic or cationic group, chelating group. This feature creates unique selectivities when using
HPLC Praktikum Skript
 HPLC Praktikum Skript Assistants: Gianluca Bartolomeo HCI D330, 3 46 68, bartolomeo@org.chem.ethz.ch Sahar Ghiasikhou HCI E330, 2 29 29, ghiasikhou@org.chem.ethz.ch 1. Introduction In chromatographic techniques,
HPLC Praktikum Skript Assistants: Gianluca Bartolomeo HCI D330, 3 46 68, bartolomeo@org.chem.ethz.ch Sahar Ghiasikhou HCI E330, 2 29 29, ghiasikhou@org.chem.ethz.ch 1. Introduction In chromatographic techniques,
Thermo Scientific Accucore XL HPLC Columns. Technical Manual
 Thermo Scientific Accucore XL HPLC Columns Technical Manual Thermo Scientific Accucore XL HPLC Columns Based on Core Enhanced Technology using µm solid core particles, Accucore XL HPLC columns allow users
Thermo Scientific Accucore XL HPLC Columns Technical Manual Thermo Scientific Accucore XL HPLC Columns Based on Core Enhanced Technology using µm solid core particles, Accucore XL HPLC columns allow users
Comparison Guide to C18 Reversed Phase HPLC Columns
 Comparison Guide to C18 Reversed Phase HPLC Columns Comparison Data on Commonly Used C18 Phases Stationary Phase Specifications Phases Compared According to Relative Hydrophobicity Phases Compared According
Comparison Guide to C18 Reversed Phase HPLC Columns Comparison Data on Commonly Used C18 Phases Stationary Phase Specifications Phases Compared According to Relative Hydrophobicity Phases Compared According
New Product Update. HPLC Columns
 New Product Update HPLC Columns ZORBAX MicroBore HPLC Columns ZORBAX Extend-C18 Columns ZORBAX Bonus-RP Columns Inertsil ODS-2 Cartridge Columns ZORBAX Carbohydrate Analysis Columns ZORBAX Eclipse dsdna
New Product Update HPLC Columns ZORBAX MicroBore HPLC Columns ZORBAX Extend-C18 Columns ZORBAX Bonus-RP Columns Inertsil ODS-2 Cartridge Columns ZORBAX Carbohydrate Analysis Columns ZORBAX Eclipse dsdna
HICHROM. Chromatography Columns and Supplies. LC COLUMNS SeQuant ZIC-HILIC. Catalogue 9. Hichrom Limited
 HICHROM Chromatography Columns and Supplies LC COLUMNS SeQuant ZIC-HILIC Catalogue 9 The Markham Centre, Station Road Theale, Reading, Berks, RG7 PE, UK Tel: + (0)8 90 660 Fax: + (0)8 9 8 Email: sales@hichrom.co.uk
HICHROM Chromatography Columns and Supplies LC COLUMNS SeQuant ZIC-HILIC Catalogue 9 The Markham Centre, Station Road Theale, Reading, Berks, RG7 PE, UK Tel: + (0)8 90 660 Fax: + (0)8 9 8 Email: sales@hichrom.co.uk
Performance characteristics of the Agilent 1290 Infinity Quaternary Pump
 Performance characteristics of the Agilent 129 Infinity Quaternary Pump Technical Overview Author A.G.Huesgen Agilent Technologies, Inc. Waldbronn, Germany Abstract This Technical Overview presents Proof
Performance characteristics of the Agilent 129 Infinity Quaternary Pump Technical Overview Author A.G.Huesgen Agilent Technologies, Inc. Waldbronn, Germany Abstract This Technical Overview presents Proof
