X-ray diffraction is a non-invasive method for determining many types of
|
|
|
- Norman Davis
- 5 years ago
- Views:
Transcription
1 Chapter X-ray Diffraction.1 Introduction X-ray diffraction is a non-invasive method for determining many types of structural features in both crystalline and amorphous materials. In the case of single crystals, detailed features of the atomic structure, orientation and domain size can be measured. X-ray diffraction is used in a variety of fields from identifying unknown materials in geology to solving the structures of large proteins in biology. It is a well established technique that has been around for most of this century. Recent advances in sources have opened up entirely new areas of research that previously had been unavailable. Synchrotron sources employ a ring of accelerated charged particles which emit intense x-ray radiation that is focused down beamlines into experimental stations. The National Synchrotron Light Source at Brookhaven National Laboratory can produce beams of x-rays with several orders of magnitude more intensity than conventional anode tube sources. In the experiments described in this thesis, the increased intensity coming from the synchrotron radiation and the use of single crystals made it possible to study very small domains in lead magnesium niobate, Pb(Mg 1/3 Nb /3 )O 3. Since synchrotron radiation occurs across a broad spectrum of energies, it was also possible to do energy 7
2 dependent scattering experiments. In contrast, an anode source gives appreciable intensity only at an energy that corresponds to an atomic level in the anode metal. By tuning the wavelength of the x-rays, experiments that exploit the resonant scattering of atoms within a crystal were also performed on PMN. When measuring thin films, x-ray diffraction gives important information about both the film and the substrate. Properties such as strain and other interfacial effects can be measured. Because of the semicrystalline nature of the thin films of lead zirconate in this experiment, synchrotron radiation was needed for accurate structural analysis and to even observe some of the diffraction peaks. The intense synchrotron x-ray beams also allow the measurement of very small domains and the structure of films as thin as 50 Å.. Overview X-ray diffraction involves scattering of incoming x-rays from planes of atoms within a crystal. X-rays are used to study crystalline structure because their wavelengths (~ 1 Å) tend to be on the same order or smaller as atomic spacings in crystals. To better understand the effect of a crystal on a beam of x-rays, it is helpful to start from the basics of electromagnetic radiation scattering from an atom and build up to scattering from an entire crystal. It is assumed initially that the energy of the x-rays is not near to an electronic level of one of the crystal s atoms. When that is the case, the x-ray beam may be absorbed by the atom with the subsequent ejection of electrons, and this case will be covered in Chapter 6. Following classical theory, the incoming radiation has an electric field vector that is oscillating rapidly. Electrons within the atoms in the crystal are subjected to this field 8
3 and experience a corresponding force and acceleration. This acceleration in turn causes those atoms to radiate both at the same frequency (elastic scattering) and at smaller frequencies (Compton modified scattering). The Compton radiation contributes only a diffuse background which is incoherent and can be ignored here. Assuming the dipole approximation, the Thomson formula [1] gives the scattered amplitude emitted from a single electron at position r as a function of the incoming amplitude: Ae e ik f r iki r = Ae 0 e (1) mc R where A e and A o are the emitted and incoming amplitudes, respectively. As is the case for the experiments described here, the assumption of polarization perpendicular to the scattering plane is made so that its effects need not be considered. The emitted and incoming wavenumbers, k f and k i, have magnitudes given by π over the wavelength and describe the direction of each beam. The electron mass and charge are m and e, speed of light is c, and R is the distance from the scattering electron to the detector. Since the factor e /mc is of the order of meters, scattering becomes appreciable only when many electrons are involved. The momentum transfer q is defined as the difference between the incoming and outgoing wavevectors (Figure.1). Requiring elastic scattering gives k i = k f = k. By referring to Figure.1, one sees that Bragg s law is obtained: q = k sin θ () where θ is the angle between the incoming and outgoing beams, and is known as the 9
4 Figure.1. Incoming, k i, and outgoing, k f, wavevectors define the scattering plane []. The momentum transfer, q, is k f - k i and θ is the Bragg angle. Bragg angle. Since the momentum transfer q is k f - k i, equation 1 can be rewritten as: A A e e = 0 exp( iq re) (3) mc R In observing this radiation, only the intensity can be measured which is proportional to the square of the amplitude. Using the kinematical approximation, the scattering amplitude from more than one electron can be found by summing the amplitudes of each electron. Next, the scattering from one atom is considered, following the treatment given by Robinson and Tweet []. Because of the strong interaction between the electromagnetic radiation and the electrons, the effect of the atom s nucleus can be disregarded. The scattering from an atom is then simply the sum of the scattering from 10
5 each electron within the atom. Consider Z electrons at positions r around an atom at r. Then the scattered amplitude is: A atom e = A0 exp [ iq ( r+ r ' ) ] (4) mc R z This can be converted to an integral over electronic density, ψψ * = ρ: A atom e 3 e A i d A mc R mc R f q e iqr = 0 exp[ q ( r+ r' )] ρ ( r' ) r' = 0 ( ) (5) and this expression defines the atomic form factor: f( q) = ρ ( r')exp( i q r 3 ') d r' (6) In practice, atomic form factors are experimentally derived quantities which are either tabulated or fit to polynomials. They are equal to the atomic number when q = 0, and generally decrease as q increases. They are independent of the wavelength except near atomic transitions which will be discussed in Chapter 6. To find the scattering from one unit cell one must sum the scattering from each atom within a unit cell. A unit cell contains all the necessary structural information about a crystal including the number, type and relative position of each atom. The ideal crystal is composed of these space filling unit cells that occupy every point on the crystal lattice. The positions of the unit cells can be described by R n = n 1 a 1 + n a + n 3 a 3 where the a s are the crystal axes. Considering just one unit cell at position R n which has N c atoms at positions r j within the unit cell (Figure.), the sum of the scattering amplitudes is given by: 11
6 ( ) A A e Nc 1 = 0 fj q exp iq R + r (7) mc R 0 j= 1 ( ) ( n j) = A e 1 mc R Fq i 0 ( )exp( q Rn ) 0 which defines the structure factor: N c ( ) ( q rj ) F( q) = f q exp i j= 1 j (8) This complex function contains all the structural information about the crystal. One of the objectives of x-ray diffraction is to measure enough structure factors of a crystal to Figure.. Consider a photon scattering from one unit cell which has its origin at R n and N c atoms located at positions r j within the unit cell []. Detector is much farther than the crystal size at position R 0. 1
7 determine its structure within a certain allowable error. The final step in finding the scattered amplitude from a crystal is to sum the previous expression over every unit cell. Consider an atom with N 1 N N 3 unit cells along the crystal axes a 1,a,a 3. Each unit cell is at a position R n = n 1 a 1 +n a +n 3 a 3, so the net amplitude is: ( ( ) ) A = A e mc R F ( q) exp i q n a + n a + n a (9) xtal 0 N1 N N3 1 0 n 1= 0 F(q) can be pulled out of the sum and contains all the structural information. The number of unit cells is typically of the order N ~ 10 8, and each sum evaluates to: S ( ) exp N q a = exp ( iq Na) ( iq a) 1 1 (10) Since squared amplitudes are measured, this expression must be squared to find the diffracted intensity: S ( q a) = N sin Nq a sin q a (11) which is the well known slit function from optics. Because N is so large, the slit function is very sharply peaked with a height of N when the denominator evaluates to zero (i.e. q.a=π). Everywhere else, it evaluates to nearly zero. Putting together all expressions, the total diffracted amplitude is: A e = A mc R F S S S 0 ( q) ( q a 1 1) ( q a ) ( q a 3 3) (1) tot N N N 0 13
8 In order for there to be any appreciable intensity, each slit function must simultaneously be at or very near a maximum. These three Laue conditions can be written as: q a = πh 1 q a = πk q a = πl 3 (13) The vector q that satisfies all three of these equations is: q= hb1 + kb + lb3 (14) The b s themselves define another lattice--the reciprocal lattice--and they are known as reciprocal lattice vectors. They are derived from the original lattice vectors that described the crystal: b 1 a a3 = π (15) a a a 1 3 and similarly for b and b 3. The reciprocal lattice is a more convenient space to work in when describing and performing diffraction experiments. The diffraction condition is met when the momentum transfer vector, q, connects the origin with one of the points on the reciprocal lattice. In practice, the measured intensities are reduced by the Debye- Waller factor which accounts for the thermal vibrations of each atom about its equilibrium position..3 Size effect and mosaic Considering equation 11 above, the effect of crystalline size can be determined. The slit function S(q) can be approximated as a Gaussian because it is basically a sharp, 14
9 symmetrical peak: sin ( Nq a/ ) N exp[ ( Nq a/ ) π ] (16) sin ( q a/ ) This Gaussian has the same width and total area under the curve as the slit function. The width of the peak at half its maximum is then easily found: 1 = exp[ N q a / 4π ] (17) The solution of this gives the Scherrer formula: 1 / qfwhm = 4π = 094 π = 094 π ln.. N a Na L (18) where L is the crystal size along one direction and q is the width of the diffraction peak measured in reciprocal lattice units. Hence, the inverse of peak width is related to the size of the crystal causing the diffraction. In most diffraction experiments, L is very large and the measured width is due to instrumental effects and is considered resolution limited. However, special regions or domains within the crystal may give rise to additional diffraction peaks, and if their size is small enough, then the width of these peaks will give an estimate of the volume of the special regions. When measuring peak widths, it is important to distinguish between the size effect and mosaic broadening. As discussed above, the size effect causes a peak to be broadened as a consequence of the finite size of the scattering medium. Hence, longrange-order features result in very sharp diffraction peaks, while shorter range order in the crystal result in broad peaks in the diffraction. Mosaic broadening is due to poor crystal quality and occurs when a sample is not single crystalline but is broken up into mosaic blocks that do not align with one another. Then the broadened peak is due to intensity 15
10 coming from the different blocks, or grains, that are at small angles to one another. If the broadening is due to the size effect, then peaks of different orders (e.g. (111) vs. ()) will have roughly constant widths in reciprocal lattice units. In Chapter 4, the widths of superstructure peaks are used to measure the size of the domains that cause those additional peaks. These domains are embedded in the single crystal, but they have a special ordering pattern which allows one to distinguish them from the rest of the crystal. However, if the broadening is mosaic in nature, then the diffraction pattern will fan out as higher orders are measured and the peaks will have fixed widths in the theta angle. A schematic diagram in Figure.3 illustrates this mosaic effect. If the crystal quality is poor, then the mosaic broadening will mask any domain size broadening and the peak widths can not be used to measure domain size. In that case, the peak width is simply a measure of the mosaic blocks angular distribution..4 Experimental details In order to study the diffraction intensity from a given set of planes, the sample must be oriented properly with respect to the incoming beam and the detector. This is accomplished using a diffractometer. In these experiments, a Kappa geometry diffractometer was used (Figure.4). It consists of four motors: one controls the twotheta arm which positions the detector at the proper Bragg angle but does not change the sample position. The theta, kappa and phi motors combine to orient the crystal at the proper Euler angles of theta, chi and phi. This is a variation on the standard four circle diffractometer which would have separate theta, phi and chi motors. The role of the 16
11 I 0 Figure.3. Schematic of a single crystal that is broken up into two mosaic blocks at a small angle to one another. The diffracted radiation is mosaic broadened resulting in a peak that has a width that is constant in theta even as higher order peaks are measured diffractometer is simply to hold the sample at a fixed position in space, while rotating it so that the required planes are meeting the diffraction condition. Since there are four motors, but only three degrees of freedom, there is more than one solution. The convention most commonly used in these experiments was to fix the theta angle to be one half of the two theta angle. This symmetric geometry gives the best access to the bulk of a flat plate sample because the incident and exit beams are kept far away from grazing. The first step in a diffraction experiment is to determine how the sample has been mounted with respect to the diffractometer. One needs to locate a few diffraction peaks, 17
12 Figure.4. Diagram of the Kappa geometry diffractometer. The detector arm analyzer axis sets the θ or Bragg angle. The θ, κ, and φ arms position the sample at the proper orientation in the beam which is coming out of the page. 18
13 and precisely measure the angles where they occur. By knowing the lattice constant of the sample, the two-theta angle is easily determined using Bragg s law. (The lattice constant can be determined by powder diffraction if it is not known.) It is necessary to then hunt around in phi and chi space until a peak is found, the position of which can be used to help find a second peak. By knowing the position of two peaks and the lattice constant and symmetry of the crystal, the orientation is, in theory, determined. However, a more accurate orientation measurement is possible when several Bragg peaks are found, usually around six. With three or more Bragg peaks, the lattice constant, symmetry and orientation of the crystal are uniquely determined. Additional Bragg peaks are then found and a best fit is used to reduce measurement error. These sets of Bragg peaks give the orientation matrix, which is simply a transformation matrix from the known coordinate system of the diffractometer to the unknown coordinate system of the crystal. The results of an accurate orientation matrix are determination of the lattice constants and the face of the crystal that the beam is penetrating. For a crystallographic study, the complex structure factors, F, need to be measured. As seen in equation 1, the intensity of a given peak is proportional to the square modulus of the structure factor, so that the complex phases can not be directly measured. It is thus necessary to have some idea of what structure you are measuring which is then refined to match the measured data. Intensities are measured by scanning the crystal through the diffraction peak and integrating the resulting peak. This must be corrected for absorption affects as described in Chapter 4 before the square root can be taken to give a quantity that is proportional to F. With a measured set of magnitudes for the structure factors within a given range of momentum transfer, it is possible to fit the data to a model. Because of the lack of 19
14 phase information, it is necessary to assume models which provide initial positions for each atom within the unit cell. The overall symmetry of the crystal can restrict the atomic displacements to certain planes. The positions of various atoms as well as Debye-Waller factors can then be varied until a satisfactory fit is obtained. The Debye-Waller factors are tabulated for each element and represent the magnitude of thermal atomic vibrations. It is important to emphasize that because x-ray diffraction collects data from a large region of a crystal, the result is the average crystal structure, hence diffraction is considered a long range order technique. All x-ray diffraction data presented in this thesis were collected at beamline X16C of the National Synchrotron Light Source (NSLS) which is part of Brookhaven National Laboratory. The NSLS is a user facility that supports about 60 x-ray beamlines. The synchrotron source produces a broad spectrum of x-ray energies, from about 0.5 to 50 kev. Typically, the accelerator storage ring is operated at an energy of.5 GeV. X16C is a multi-purpose beamline that can be used for standard diffraction experiments, as well as EXAFS and micro-beam experiments. A vertical slit at the beginning of the beamline helps define the beam and reduce the thermal load on the monochromator. A double crystal Si monochromator is used to define the energy of the beam. The Si single crystals are cut with {111} orientation and can be rotated together to continuously vary the energy. Since the () reflection is forbidden in silicon s diamond structure, second order harmonics are eliminated. The beam diffracts from the first crystal and into the second crystal which also diffracts the beam so that the beam propagation direction is not changed. The second crystal is also curved to help focus the beam at the center of the diffractometer. A set of motors fine tunes the bending of the second crystal to improve the focusing. A typical beam profile at the sample position is 0
15 1.0 mm in the vertical direction and 0.5 mm or less in the horizontal. The focusing greatly reduces the horizontal profile and increases the photon flux by more than an order of magnitude. Third order harmonics can either be discriminated against by the detectors, or can be largely eliminated by de-tuning the second crystal slightly. In the experimental hutch, I 0 slits further define the beam and eliminate any stray beams. On the detector arm, sets of vertical and horizontal slits are present. The first guard slits eliminate stray radiation while the detector slits (positioned immediately before the detector) ensure that only diffracted radiation at the desired Bragg angle reaches the detector. The width of these slits is usually about mm and is set so that each slit just cuts into the actual beam; the beam is then well-defined by the slits but not too much radiation is lost. For the experiments described here, a Kappa geometry four-circle diffractometer was installed inside the experimental hutch, and Bicron scintillators were used to detect the photons..5 Resonant scattering The atomic form factor, f 0, as described above, does not consider the possibility of electronic excitations within the atom. Each atom scatters according to the number and distribution of electrons that it contains as reflected by f 0, which is simply the Fourier transform of the electronic density. For conventional x-ray diffraction experiments, the wavelength is chosen such that no atomic excitations occur. The atomic form factors are 1
16 45 40 Nb 35 Atomic scattering factor, f Mg Momentum transfer (Å -1 ) Figure.5. Atomic form factors, f 0, for Nb and Mg []. At zero momentum transfer, they evaluate to Z. Atomic excitations are included in the anomalous corrections, f and f. then smoothly varying functions with momentum transfer, q, (Figure.5) which can be closely approximated as polynomials as tabulated in the International Tables for Crystallography [3]. The complete expression for atomic form factors, however, includes terms that account for the atomic excitations and is written as: f(q) = f 0 (q) + f (E) + if (E) where f 0 is the smoothly varying form factor, and f (E) and f (E) represent the anomalous scattering corrections. As an example, the f (E) and f (E) of Mg are shown in Figure.6
17 [4]. The K-shell atomic excitation level for Mg is at 1.5 kev, and the effect on f and f are clearly seen as the sharp and narrow dip in f and the jump in f at the absorption edge. When the incoming photon s energy, E, is near an atom s atomic absorption edge, that photon can be absorbed by an electron (usually K or L shell) which is then expelled Figure.6 Anomalous scattering factors, f (E) and f (E), for Mg [4]. 3
18 from the atom with energy E-E binding. The empty shell is then filled by an electron from a higher shell which causes fluorescent radiation at the atom s characteristic energy. The effect of photoelectric absorption is to produce jumps in each atom s scattering power. It is the most probable interaction between photons and matter. As seen in Figure.7 [5], it is about 3 orders of magnitude more likely than Thomson scattering in the x-ray energy range. This process is used in conventional x-ray sources where energetic electrons bombard a metallic target which then radiates x-rays at its characteristic atomic levels. The K lines of copper and molybdenum are the most commonly used sources. The problem with these sources compared with synchrotron radiation (without even Figure.7. Cross sections of the copper atom interacting with photons [5]. The solid line represents the total cross section. Photoelectric absorption is most significant in the x-ray range, but Thomson scattering dominates in the diffraction condition. 4
19 considering their lower intensity) is that one is limited to performing experiments at specific energies pre-determined by nature. With the advent of synchrotron sources, not only has the intensity of x-rays improved by to 4 orders of magnitude, but the white radiation coming from the synchrotron is passed through a monochromator crystal that can be easily tilted to provide any energy within a broad range. For example, at beamline X16C of the NSLS, incoming x-ray energy can be varied from about 5-5 kev with a resolution of a few ev. When detecting radiation that is passed straight through a crystal, one observes primarily the absorption cross section. This is the common procedure for Extended X-ray Absorption Fine Structure (EXAFS) measurements, discussed in Chapter 6. However, for x-ray measurements, the detector is at some angle to the incoming radiation (the Bragg angle). In these cases, the Thomson scattering is predominant in the typical x-ray range. At higher energies (> 50keV), the incoherent Compton scattering actually becomes more likely than Thomson scattering. Although Thomson scattering from an individual electron has a very small cross section, at the proper Bragg angle enough electrons scatter coherently to produce the sharp and intense diffraction peaks. When not situated at a Bragg angle, a certain amount of background radiation from both Thomson and Compton scattering is detected. This background is flat and featureless except when near an absorption edge. As the incoming photon is given enough energy to cause photoemission, the filling of the empty state causes fluorescent radiation which is given off in all directions. This causes a decrease in the amount of the beam that is passed through the sample (at zero degrees) due to the jump in absorption at the edge. This is reflected in the increase of f which is proportional to µ, the absorption coefficient. The 5
20 effect of f is to reduce the scattering of the atom very close to the absorption edge due to resonance effects. In experiments, by tuning the wavelength through an absorption edge, the f can effectively be turned off and on while f is accounted for by a known absorption correction. Thus it is possible to determine the phase and how much of a given atom is contributing to a diffraction peak by measuring intensities as the energy is tuned through the resonance condition. In principle, this is possible even without exploiting the resonance condition because each atom has a unique f 0 as well. However, the f 0 s are generally featureless away from atomic transitions and their smoothly varying shape does not provide enough contrast to perform such an experiment. Measurements of absolute intensity are not precise enough to give reliable information in all cases, so the anomalous scattering signature is used to identify specific atoms and their scattering phases. In Chapter 6, further effects of anomalous scattering will be used to determine short range order. Techniques such as EXAFS and diffraction anomalous fine structure (DAFS) will be described and used to study lead magnesium niobate (PMN). These techniques measure the intensity of photons just above an absorption edge. The ejected photoelectrons interact with the neighboring atoms resulting in an interference pattern of oscillations of intensity. This pattern can be fit to a standard equation to determine the local structural environment out to a few nearest neighbors. Used together with conventional x-ray diffraction, which is a long range order technique, EXAFS and DAFS can give short range complementary information on the detailed structure of a crystal. In addition, resonant scattering will be used to determine the relative Nb composition in different regions of PMN in the second part of Chapter 6. 6
21 .6 References [1] B E Warren, X-ray Diffraction, (Addison-Wesley, Massachusetts, 1969). [] I K Robinson, D J Tweet, Rep. Prog. Phys., (199). [3] International Tables for X-ray Crystallography, nd ed. edited by C H MacGillavry and G D Rieck (Reidel, Dordrecht, 1983), Vol. III. [4] B L Henke, E M Gullickson, J C Davis, Atomic Data and Nuclear Data Tables, 54 [] (1993). [5] A. Fontaine, Neutron and Synchrotron Radiation for Condensed Matter Studies, 1, 33 (1996). 7
An Introduction to Diffraction and Scattering. School of Chemistry The University of Sydney
 An Introduction to Diffraction and Scattering Brendan J. Kennedy School of Chemistry The University of Sydney 1) Strong forces 2) Weak forces Types of Forces 3) Electromagnetic forces 4) Gravity Types
An Introduction to Diffraction and Scattering Brendan J. Kennedy School of Chemistry The University of Sydney 1) Strong forces 2) Weak forces Types of Forces 3) Electromagnetic forces 4) Gravity Types
Swanning about in Reciprocal Space. Kenneth, what is the wavevector?
 Swanning about in Reciprocal Space or, Kenneth, what is the wavevector? Stanford Synchrotron Radiation Laboratory Principles The relationship between the reciprocal lattice vector and the wave vector is
Swanning about in Reciprocal Space or, Kenneth, what is the wavevector? Stanford Synchrotron Radiation Laboratory Principles The relationship between the reciprocal lattice vector and the wave vector is
Setting The motor that rotates the sample about an axis normal to the diffraction plane is called (or ).
 X-Ray Diffraction X-ray diffraction geometry A simple X-ray diffraction (XRD) experiment might be set up as shown below. We need a parallel X-ray source, which is usually an X-ray tube in a fixed position
X-Ray Diffraction X-ray diffraction geometry A simple X-ray diffraction (XRD) experiment might be set up as shown below. We need a parallel X-ray source, which is usually an X-ray tube in a fixed position
General theory of diffraction
 General theory of diffraction X-rays scatter off the charge density (r), neutrons scatter off the spin density. Coherent scattering (diffraction) creates the Fourier transform of (r) from real to reciprocal
General theory of diffraction X-rays scatter off the charge density (r), neutrons scatter off the spin density. Coherent scattering (diffraction) creates the Fourier transform of (r) from real to reciprocal
Structural characterization. Part 1
 Structural characterization Part 1 Experimental methods X-ray diffraction Electron diffraction Neutron diffraction Light diffraction EXAFS-Extended X- ray absorption fine structure XANES-X-ray absorption
Structural characterization Part 1 Experimental methods X-ray diffraction Electron diffraction Neutron diffraction Light diffraction EXAFS-Extended X- ray absorption fine structure XANES-X-ray absorption
4. Other diffraction techniques
 4. Other diffraction techniques 4.1 Reflection High Energy Electron Diffraction (RHEED) Setup: - Grazing-incidence high energy electron beam (3-5 kev: MEED,
4. Other diffraction techniques 4.1 Reflection High Energy Electron Diffraction (RHEED) Setup: - Grazing-incidence high energy electron beam (3-5 kev: MEED,
Part 1: What is XAFS? What does it tell us? The EXAFS equation. Part 2: Basic steps in the analysis Quick overview of typical analysis
 Introduction to XAFS Part 1: What is XAFS? What does it tell us? The EXAFS equation Part 2: Basic steps in the analysis Quick overview of typical analysis Tomorrow Measurement methods and examples The
Introduction to XAFS Part 1: What is XAFS? What does it tell us? The EXAFS equation Part 2: Basic steps in the analysis Quick overview of typical analysis Tomorrow Measurement methods and examples The
Chapter 1 X-ray Absorption Fine Structure (EXAFS)
 1 Chapter 1 X-ray Absorption Fine Structure (EXAFS) 1.1 What is EXAFS? X-ray absorption fine structure (EXAFS, XAFS) is an oscillatory modulation in the X-ray absorption coefficient on the high-energy
1 Chapter 1 X-ray Absorption Fine Structure (EXAFS) 1.1 What is EXAFS? X-ray absorption fine structure (EXAFS, XAFS) is an oscillatory modulation in the X-ray absorption coefficient on the high-energy
Roger Johnson Structure and Dynamics: X-ray Diffraction Lecture 6
 6.1. Summary In this Lecture we cover the theory of x-ray diffraction, which gives direct information about the atomic structure of crystals. In these experiments, the wavelength of the incident beam must
6.1. Summary In this Lecture we cover the theory of x-ray diffraction, which gives direct information about the atomic structure of crystals. In these experiments, the wavelength of the incident beam must
Introduction to XAFS. Grant Bunker Associate Professor, Physics Illinois Institute of Technology. Revised 4/11/97
 Introduction to XAFS Grant Bunker Associate Professor, Physics Illinois Institute of Technology Revised 4/11/97 2 tutorial.nb Outline Overview of Tutorial 1: Overview of XAFS 2: Basic Experimental design
Introduction to XAFS Grant Bunker Associate Professor, Physics Illinois Institute of Technology Revised 4/11/97 2 tutorial.nb Outline Overview of Tutorial 1: Overview of XAFS 2: Basic Experimental design
Good Diffraction Practice Webinar Series
 Good Diffraction Practice Webinar Series High Resolution X-ray Diffractometry (1) Mar 24, 2011 www.bruker-webinars.com Welcome Heiko Ress Global Marketing Manager Bruker AXS Inc. Madison, Wisconsin, USA
Good Diffraction Practice Webinar Series High Resolution X-ray Diffractometry (1) Mar 24, 2011 www.bruker-webinars.com Welcome Heiko Ress Global Marketing Manager Bruker AXS Inc. Madison, Wisconsin, USA
X-Ray Emission and Absorption
 X-Ray Emission and Absorption Author: Mike Nill Alex Bryant February 6, 20 Abstract X-rays were produced by two bench-top diffractometers using a copper target. Various nickel filters were placed in front
X-Ray Emission and Absorption Author: Mike Nill Alex Bryant February 6, 20 Abstract X-rays were produced by two bench-top diffractometers using a copper target. Various nickel filters were placed in front
Scattering Lecture. February 24, 2014
 Scattering Lecture February 24, 2014 Structure Determination by Scattering Waves of radiation scattered by different objects interfere to give rise to an observable pattern! The wavelength needs to close
Scattering Lecture February 24, 2014 Structure Determination by Scattering Waves of radiation scattered by different objects interfere to give rise to an observable pattern! The wavelength needs to close
Surface Sensitive X-ray Scattering
 Surface Sensitive X-ray Scattering Introduction Concepts of surfaces Scattering (Born approximation) Crystal Truncation Rods The basic idea How to calculate Examples Reflectivity In Born approximation
Surface Sensitive X-ray Scattering Introduction Concepts of surfaces Scattering (Born approximation) Crystal Truncation Rods The basic idea How to calculate Examples Reflectivity In Born approximation
Röntgenpraktikum. M. Oehzelt. (based on the diploma thesis of T. Haber [1])
![Röntgenpraktikum. M. Oehzelt. (based on the diploma thesis of T. Haber [1]) Röntgenpraktikum. M. Oehzelt. (based on the diploma thesis of T. Haber [1])](/thumbs/91/106501237.jpg) Röntgenpraktikum M. Oehzelt (based on the diploma thesis of T. Haber [1]) October 21, 2004 Contents 1 Fundamentals 2 1.1 X-Ray Radiation......................... 2 1.1.1 Bremsstrahlung......................
Röntgenpraktikum M. Oehzelt (based on the diploma thesis of T. Haber [1]) October 21, 2004 Contents 1 Fundamentals 2 1.1 X-Ray Radiation......................... 2 1.1.1 Bremsstrahlung......................
Atomic Motion via Inelastic X-Ray Scattering
 Atomic Motion via Inelastic X-Ray Scattering Cheiron School Beamline Practical - Tuesday ONLY at BL43LXU Alfred Q.R. Baron with H. Uchiyama We will introduce students to the use of inelastic x-ray scattering,
Atomic Motion via Inelastic X-Ray Scattering Cheiron School Beamline Practical - Tuesday ONLY at BL43LXU Alfred Q.R. Baron with H. Uchiyama We will introduce students to the use of inelastic x-ray scattering,
Interaction X-rays - Matter
 Interaction X-rays - Matter Pair production hν > M ev Photoelectric absorption hν MATTER hν Transmission X-rays hν' < hν Scattering hν Decay processes hν f Compton Thomson Fluorescence Auger electrons
Interaction X-rays - Matter Pair production hν > M ev Photoelectric absorption hν MATTER hν Transmission X-rays hν' < hν Scattering hν Decay processes hν f Compton Thomson Fluorescence Auger electrons
Chemistry Instrumental Analysis Lecture 19 Chapter 12. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 19 Chapter 12 There are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry X-ray Techniques include:
Chemistry 4631 Instrumental Analysis Lecture 19 Chapter 12 There are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry X-ray Techniques include:
Quantum Condensed Matter Physics Lecture 5
 Quantum Condensed Matter Physics Lecture 5 detector sample X-ray source monochromator David Ritchie http://www.sp.phy.cam.ac.uk/drp2/home QCMP Lent/Easter 2019 5.1 Quantum Condensed Matter Physics 1. Classical
Quantum Condensed Matter Physics Lecture 5 detector sample X-ray source monochromator David Ritchie http://www.sp.phy.cam.ac.uk/drp2/home QCMP Lent/Easter 2019 5.1 Quantum Condensed Matter Physics 1. Classical
X-ray diffraction geometry
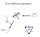 X-ray diffraction geometry Setting controls sample orientation in the diffraction plane. most important for single-crystal diffraction For any poly- (or nano-) crystalline specimen, we usually set: 1 X-ray
X-ray diffraction geometry Setting controls sample orientation in the diffraction plane. most important for single-crystal diffraction For any poly- (or nano-) crystalline specimen, we usually set: 1 X-ray
Surface Sensitivity & Surface Specificity
 Surface Sensitivity & Surface Specificity The problems of sensitivity and detection limits are common to all forms of spectroscopy. In its simplest form, the question of sensitivity boils down to whether
Surface Sensitivity & Surface Specificity The problems of sensitivity and detection limits are common to all forms of spectroscopy. In its simplest form, the question of sensitivity boils down to whether
E. K. A. ADVANCED PHYSICS LABORATORY PHYSICS 3081, 4051
 E. K. A. ADVANCED PHYSICS LABORATORY PHYSICS 38, 45 ELECTRON DIFFRACTION Introduction This experiment, using a cathode ray tube, permits the first hand observation that electrons, which are usually though
E. K. A. ADVANCED PHYSICS LABORATORY PHYSICS 38, 45 ELECTRON DIFFRACTION Introduction This experiment, using a cathode ray tube, permits the first hand observation that electrons, which are usually though
Scattering Techniques and Geometries How to choose a beamline. Christopher J. Tassone
 Scattering Techniques and Geometries How to choose a beamline Christopher J. Tassone Why Care About Geometries? How do you decide which beamline you want to use? Questions you should be asking Do I want
Scattering Techniques and Geometries How to choose a beamline Christopher J. Tassone Why Care About Geometries? How do you decide which beamline you want to use? Questions you should be asking Do I want
Nuclear Physics. (PHY-231) Dr C. M. Cormack. Nuclear Physics This Lecture
 Nuclear Physics (PHY-31) Dr C. M. Cormack 11 Nuclear Physics This Lecture This Lecture We will discuss an important effect in nuclear spectroscopy The Mössbauer Effect and its applications in technology
Nuclear Physics (PHY-31) Dr C. M. Cormack 11 Nuclear Physics This Lecture This Lecture We will discuss an important effect in nuclear spectroscopy The Mössbauer Effect and its applications in technology
Main Notation Used in This Book
 Main Notation Used in This Book z Direction normal to the surface x,y Directions in the plane of the surface Used to describe a component parallel to the interface plane xoz Plane of incidence j Label
Main Notation Used in This Book z Direction normal to the surface x,y Directions in the plane of the surface Used to describe a component parallel to the interface plane xoz Plane of incidence j Label
X-rays. X-ray Radiography - absorption is a function of Z and density. X-ray crystallography. X-ray spectrometry
 X-rays Wilhelm K. Roentgen (1845-1923) NP in Physics 1901 X-ray Radiography - absorption is a function of Z and density X-ray crystallography X-ray spectrometry X-rays Cu K α E = 8.05 kev λ = 1.541 Å Interaction
X-rays Wilhelm K. Roentgen (1845-1923) NP in Physics 1901 X-ray Radiography - absorption is a function of Z and density X-ray crystallography X-ray spectrometry X-rays Cu K α E = 8.05 kev λ = 1.541 Å Interaction
X-ray Absorption Spectroscopy Eric Peterson 9/2/2010
 X-ray Absorption Spectroscopy Eric Peterson 9/2/2010 Outline Generation/Absorption of X-rays History Synchrotron Light Sources Data reduction/analysis Examples Crystallite size from Coordination Number
X-ray Absorption Spectroscopy Eric Peterson 9/2/2010 Outline Generation/Absorption of X-rays History Synchrotron Light Sources Data reduction/analysis Examples Crystallite size from Coordination Number
X-ray Spectroscopy. Danny Bennett and Maeve Madigan. October 12, 2015
 X-ray Spectroscopy Danny Bennett and Maeve Madigan October 12, 2015 Abstract Various X-ray spectra were obtained, and their properties were investigated. The characteristic peaks were identified for a
X-ray Spectroscopy Danny Bennett and Maeve Madigan October 12, 2015 Abstract Various X-ray spectra were obtained, and their properties were investigated. The characteristic peaks were identified for a
Overview of scattering, diffraction & imaging in the TEM
 Overview of scattering, diffraction & imaging in the TEM Eric A. Stach Purdue University Scattering Electrons, photons, neutrons Radiation Elastic Mean Free Path (Å)( Absorption Length (Å)( Minimum Probe
Overview of scattering, diffraction & imaging in the TEM Eric A. Stach Purdue University Scattering Electrons, photons, neutrons Radiation Elastic Mean Free Path (Å)( Absorption Length (Å)( Minimum Probe
Structure of Surfaces
 Structure of Surfaces C Stepped surface Interference of two waves Bragg s law Path difference = AB+BC =2dsin ( =glancing angle) If, n =2dsin, constructive interference Ex) in a cubic lattice of unit cell
Structure of Surfaces C Stepped surface Interference of two waves Bragg s law Path difference = AB+BC =2dsin ( =glancing angle) If, n =2dsin, constructive interference Ex) in a cubic lattice of unit cell
X-ray, Neutron and e-beam scattering
 X-ray, Neutron and e-beam scattering Introduction Why scattering? Diffraction basics Neutrons and x-rays Techniques Direct and reciprocal space Single crystals Powders CaFe 2 As 2 an example What is the
X-ray, Neutron and e-beam scattering Introduction Why scattering? Diffraction basics Neutrons and x-rays Techniques Direct and reciprocal space Single crystals Powders CaFe 2 As 2 an example What is the
MT Electron microscopy Scanning electron microscopy and electron probe microanalysis
 MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
How Does It All Work? A Summary of the IDEAS Beamline at the Canadian Light Source
 How Does It All Work? A Summary of the IDEAS Beamline at the Canadian Light Source What Makes Up The Canadian Light Source? 4. Storage Ring 5. Synchrotron Light 6. Beamline 1. Electron Gun 2. Linear Accelerator
How Does It All Work? A Summary of the IDEAS Beamline at the Canadian Light Source What Makes Up The Canadian Light Source? 4. Storage Ring 5. Synchrotron Light 6. Beamline 1. Electron Gun 2. Linear Accelerator
is the minimum stopping potential for which the current between the plates reduces to zero.
 Module 1 :Quantum Mechanics Chapter 2 : Introduction to Quantum ideas Introduction to Quantum ideas We will now consider some experiments and their implications, which introduce us to quantum ideas. The
Module 1 :Quantum Mechanics Chapter 2 : Introduction to Quantum ideas Introduction to Quantum ideas We will now consider some experiments and their implications, which introduce us to quantum ideas. The
Particles and Waves Particles Waves
 Particles and Waves Particles Discrete and occupy space Exist in only one location at a time Position and velocity can be determined with infinite accuracy Interact by collisions, scattering. Waves Extended,
Particles and Waves Particles Discrete and occupy space Exist in only one location at a time Position and velocity can be determined with infinite accuracy Interact by collisions, scattering. Waves Extended,
DIFFRACTION PHYSICS THIRD REVISED EDITION JOHN M. COWLEY. Regents' Professor enzeritus Arizona State University
 DIFFRACTION PHYSICS THIRD REVISED EDITION JOHN M. COWLEY Regents' Professor enzeritus Arizona State University 1995 ELSEVIER Amsterdam Lausanne New York Oxford Shannon Tokyo CONTENTS Preface to the first
DIFFRACTION PHYSICS THIRD REVISED EDITION JOHN M. COWLEY Regents' Professor enzeritus Arizona State University 1995 ELSEVIER Amsterdam Lausanne New York Oxford Shannon Tokyo CONTENTS Preface to the first
Neutron and x-ray spectroscopy
 Neutron and x-ray spectroscopy B. Keimer Max-Planck-Institute for Solid State Research outline 1. self-contained introduction neutron scattering and spectroscopy x-ray scattering and spectroscopy 2. application
Neutron and x-ray spectroscopy B. Keimer Max-Planck-Institute for Solid State Research outline 1. self-contained introduction neutron scattering and spectroscopy x-ray scattering and spectroscopy 2. application
Interaction of particles with matter - 2. Silvia Masciocchi, GSI and University of Heidelberg SS2017, Heidelberg May 3, 2017
 Interaction of particles with matter - 2 Silvia Masciocchi, GSI and University of Heidelberg SS2017, Heidelberg May 3, 2017 Energy loss by ionization (by heavy particles) Interaction of electrons with
Interaction of particles with matter - 2 Silvia Masciocchi, GSI and University of Heidelberg SS2017, Heidelberg May 3, 2017 Energy loss by ionization (by heavy particles) Interaction of electrons with
PHYS Introduction to Synchrotron Radiation
 C. Segre (IIT) PHYS 570 - Spring 2018 January 09, 2018 1 / 20 PHYS 570 - Introduction to Synchrotron Radiation Term: Spring 2018 Meetings: Tuesday & Thursday 13:50-15:05 Location: 213 Stuart Building Instructor:
C. Segre (IIT) PHYS 570 - Spring 2018 January 09, 2018 1 / 20 PHYS 570 - Introduction to Synchrotron Radiation Term: Spring 2018 Meetings: Tuesday & Thursday 13:50-15:05 Location: 213 Stuart Building Instructor:
Transmission Electron Microscopy
 L. Reimer H. Kohl Transmission Electron Microscopy Physics of Image Formation Fifth Edition el Springer Contents 1 Introduction... 1 1.1 Transmission Electron Microscopy... 1 1.1.1 Conventional Transmission
L. Reimer H. Kohl Transmission Electron Microscopy Physics of Image Formation Fifth Edition el Springer Contents 1 Introduction... 1 1.1 Transmission Electron Microscopy... 1 1.1.1 Conventional Transmission
X-Ray Scattering Studies of Thin Polymer Films
 X-Ray Scattering Studies of Thin Polymer Films Introduction to Neutron and X-Ray Scattering Sunil K. Sinha UCSD/LANL Acknowledgements: Prof. R.Pynn( Indiana U.) Prof. M.Tolan (U. Dortmund) Wilhelm Conrad
X-Ray Scattering Studies of Thin Polymer Films Introduction to Neutron and X-Ray Scattering Sunil K. Sinha UCSD/LANL Acknowledgements: Prof. R.Pynn( Indiana U.) Prof. M.Tolan (U. Dortmund) Wilhelm Conrad
Detecting high energy photons. Interactions of photons with matter Properties of detectors (with examples)
 Detecting high energy photons Interactions of photons with matter Properties of detectors (with examples) Interactions of high energy photons with matter Cross section/attenution length/optical depth Photoelectric
Detecting high energy photons Interactions of photons with matter Properties of detectors (with examples) Interactions of high energy photons with matter Cross section/attenution length/optical depth Photoelectric
Neutron Instruments I & II. Ken Andersen ESS Instruments Division
 Neutron Instruments I & II ESS Instruments Division Neutron Instruments I & II Overview of source characteristics Bragg s Law Elastic scattering: diffractometers Continuous sources Pulsed sources Inelastic
Neutron Instruments I & II ESS Instruments Division Neutron Instruments I & II Overview of source characteristics Bragg s Law Elastic scattering: diffractometers Continuous sources Pulsed sources Inelastic
Strain, Stress and Cracks Klaus Attenkofer PV Reliability Workshop (Orlando) April 7-8, 2015
 Strain, Stress and Cracks Klaus Attenkofer PV Reliability Workshop (Orlando) April 7-8, 2015 1 BROOKHAVEN SCIENCE ASSOCIATES Overview Material s response to applied forces or what to measure Definitions
Strain, Stress and Cracks Klaus Attenkofer PV Reliability Workshop (Orlando) April 7-8, 2015 1 BROOKHAVEN SCIENCE ASSOCIATES Overview Material s response to applied forces or what to measure Definitions
Protein crystallography. Garry Taylor
 Protein crystallography Garry Taylor X-ray Crystallography - the Basics Grow crystals Collect X-ray data Determine phases Calculate ρ-map Interpret map Refine coordinates Do the biology. Nitrogen at -180
Protein crystallography Garry Taylor X-ray Crystallography - the Basics Grow crystals Collect X-ray data Determine phases Calculate ρ-map Interpret map Refine coordinates Do the biology. Nitrogen at -180
NEW CORRECTION PROCEDURE FOR X-RAY SPECTROSCOPIC FLUORESCENCE DATA: SIMULATIONS AND EXPERIMENT
 Copyright JCPDS - International Centre for Diffraction Data 2005, Advances in X-ray Analysis, Volume 48. 266 NEW CORRECTION PROCEDURE FOR X-RAY SPECTROSCOPIC FLUORESCENCE DATA: SIMULATIONS AND EXPERIMENT
Copyright JCPDS - International Centre for Diffraction Data 2005, Advances in X-ray Analysis, Volume 48. 266 NEW CORRECTION PROCEDURE FOR X-RAY SPECTROSCOPIC FLUORESCENCE DATA: SIMULATIONS AND EXPERIMENT
Data Acquisition. What choices need to be made?
 1 Specimen type and preparation Radiation source Wavelength Instrument geometry Detector type Instrument setup Scan parameters 2 Specimen type and preparation Slide mount Front loading cavity Back loading
1 Specimen type and preparation Radiation source Wavelength Instrument geometry Detector type Instrument setup Scan parameters 2 Specimen type and preparation Slide mount Front loading cavity Back loading
and another with a peak frequency ω 2
 Physics Qualifying Examination Part I 7-Minute Questions September 13, 2014 1. A sealed container is divided into two volumes by a moveable piston. There are N A molecules on one side and N B molecules
Physics Qualifying Examination Part I 7-Minute Questions September 13, 2014 1. A sealed container is divided into two volumes by a moveable piston. There are N A molecules on one side and N B molecules
Lecture 10. Transition probabilities and photoelectric cross sections
 Lecture 10 Transition probabilities and photoelectric cross sections TRANSITION PROBABILITIES AND PHOTOELECTRIC CROSS SECTIONS Cross section = = Transition probability per unit time of exciting a single
Lecture 10 Transition probabilities and photoelectric cross sections TRANSITION PROBABILITIES AND PHOTOELECTRIC CROSS SECTIONS Cross section = = Transition probability per unit time of exciting a single
EEE4101F / EEE4103F Radiation Interactions & Detection
 EEE4101F / EEE4103F Radiation Interactions & Detection 1. Interaction of Radiation with Matter Dr. Steve Peterson 5.14 RW James Department of Physics University of Cape Town steve.peterson@uct.ac.za March
EEE4101F / EEE4103F Radiation Interactions & Detection 1. Interaction of Radiation with Matter Dr. Steve Peterson 5.14 RW James Department of Physics University of Cape Town steve.peterson@uct.ac.za March
Lecture 23 X-Ray & UV Techniques
 Lecture 23 X-Ray & UV Techniques Schroder: Chapter 11.3 1/50 Announcements Homework 6/6: Will be online on later today. Due Wednesday June 6th at 10:00am. I will return it at the final exam (14 th June).
Lecture 23 X-Ray & UV Techniques Schroder: Chapter 11.3 1/50 Announcements Homework 6/6: Will be online on later today. Due Wednesday June 6th at 10:00am. I will return it at the final exam (14 th June).
Insertion Devices Lecture 2 Wigglers and Undulators. Jim Clarke ASTeC Daresbury Laboratory
 Insertion Devices Lecture 2 Wigglers and Undulators Jim Clarke ASTeC Daresbury Laboratory Summary from Lecture #1 Synchrotron Radiation is emitted by accelerated charged particles The combination of Lorentz
Insertion Devices Lecture 2 Wigglers and Undulators Jim Clarke ASTeC Daresbury Laboratory Summary from Lecture #1 Synchrotron Radiation is emitted by accelerated charged particles The combination of Lorentz
Crystals, X-rays and Proteins
 Crystals, X-rays and Proteins Comprehensive Protein Crystallography Dennis Sherwood MA (Hons), MPhil, PhD Jon Cooper BA (Hons), PhD OXFORD UNIVERSITY PRESS Contents List of symbols xiv PART I FUNDAMENTALS
Crystals, X-rays and Proteins Comprehensive Protein Crystallography Dennis Sherwood MA (Hons), MPhil, PhD Jon Cooper BA (Hons), PhD OXFORD UNIVERSITY PRESS Contents List of symbols xiv PART I FUNDAMENTALS
X-ray Spectroscopy. Interaction of X-rays with matter XANES and EXAFS XANES analysis Pre-edge analysis EXAFS analysis
 X-ray Spectroscopy Interaction of X-rays with matter XANES and EXAFS XANES analysis Pre-edge analysis EXAFS analysis Element specific Sensitive to low concentrations (0.01-0.1 %) Why XAS? Applicable under
X-ray Spectroscopy Interaction of X-rays with matter XANES and EXAFS XANES analysis Pre-edge analysis EXAFS analysis Element specific Sensitive to low concentrations (0.01-0.1 %) Why XAS? Applicable under
SSRL XAS Beam Lines Soft X-ray
 SSRL SMB Summer School July 20, 2010 SSRL XAS Beam Lines Soft X-ray Thomas Rabedeau SSRL Beam Line Development Objective/Scope Objective - develop a better understanding of the capabilities and limitations
SSRL SMB Summer School July 20, 2010 SSRL XAS Beam Lines Soft X-ray Thomas Rabedeau SSRL Beam Line Development Objective/Scope Objective - develop a better understanding of the capabilities and limitations
X-Ray Photoelectron Spectroscopy (XPS)
 X-Ray Photoelectron Spectroscopy (XPS) Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Electron Spectroscopy for Chemical Analysis (ESCA) The basic principle of the photoelectric effect was enunciated
X-Ray Photoelectron Spectroscopy (XPS) Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Electron Spectroscopy for Chemical Analysis (ESCA) The basic principle of the photoelectric effect was enunciated
Emphasis on what happens to emitted particle (if no nuclear reaction and MEDIUM (i.e., atomic effects)
 LECTURE 5: INTERACTION OF RADIATION WITH MATTER All radiation is detected through its interaction with matter! INTRODUCTION: What happens when radiation passes through matter? Emphasis on what happens
LECTURE 5: INTERACTION OF RADIATION WITH MATTER All radiation is detected through its interaction with matter! INTRODUCTION: What happens when radiation passes through matter? Emphasis on what happens
Chapter 27. Quantum Physics
 Chapter 27 Quantum Physics Need for Quantum Physics Problems remained from classical mechanics that relativity didn t explain Blackbody Radiation The electromagnetic radiation emitted by a heated object
Chapter 27 Quantum Physics Need for Quantum Physics Problems remained from classical mechanics that relativity didn t explain Blackbody Radiation The electromagnetic radiation emitted by a heated object
Revision Guide. Chapter 7 Quantum Behaviour
 Revision Guide Chapter 7 Quantum Behaviour Contents CONTENTS... 2 REVISION CHECKLIST... 3 REVISION NOTES... 4 QUANTUM BEHAVIOUR... 4 Random arrival of photons... 4 Photoelectric effect... 5 PHASE AN PHASORS...
Revision Guide Chapter 7 Quantum Behaviour Contents CONTENTS... 2 REVISION CHECKLIST... 3 REVISION NOTES... 4 QUANTUM BEHAVIOUR... 4 Random arrival of photons... 4 Photoelectric effect... 5 PHASE AN PHASORS...
Solid State Physics Lecture 3 Diffraction and the Reciprocal Lattice (Kittel Ch. 2)
 Solid State Physics 460 - Lecture 3 Diffraction and the Reciprocal Lattice (Kittel Ch. 2) Diffraction (Bragg Scattering) from a powder of crystallites - real example of image at right from http://www.uni-wuerzburg.de/mineralogie/crystal/teaching/pow.html
Solid State Physics 460 - Lecture 3 Diffraction and the Reciprocal Lattice (Kittel Ch. 2) Diffraction (Bragg Scattering) from a powder of crystallites - real example of image at right from http://www.uni-wuerzburg.de/mineralogie/crystal/teaching/pow.html
Neutron Diffraction: a general overview
 RUG1 Neutron Diffraction: a general overview Graeme Blake Zernike Institute for Advanced Materials University of Groningen Outline Elastic scattering of neutrons from matter Comparison of neutron and X-ray
RUG1 Neutron Diffraction: a general overview Graeme Blake Zernike Institute for Advanced Materials University of Groningen Outline Elastic scattering of neutrons from matter Comparison of neutron and X-ray
Particle Interactions in Detectors
 Particle Interactions in Detectors Dr Peter R Hobson C.Phys M.Inst.P. Department of Electronic and Computer Engineering Brunel University, Uxbridge Peter.Hobson@brunel.ac.uk http://www.brunel.ac.uk/~eestprh/
Particle Interactions in Detectors Dr Peter R Hobson C.Phys M.Inst.P. Department of Electronic and Computer Engineering Brunel University, Uxbridge Peter.Hobson@brunel.ac.uk http://www.brunel.ac.uk/~eestprh/
Road map (Where are we headed?)
 Road map (Where are we headed?) oal: Fairly high level understanding of carrier transport and optical transitions in semiconductors Necessary Ingredients Crystal Structure Lattice Vibrations Free Electron
Road map (Where are we headed?) oal: Fairly high level understanding of carrier transport and optical transitions in semiconductors Necessary Ingredients Crystal Structure Lattice Vibrations Free Electron
Physics with Neutrons I, WS 2015/2016. Lecture 11, MLZ is a cooperation between:
 Physics with Neutrons I, WS 2015/2016 Lecture 11, 11.1.2016 MLZ is a cooperation between: Organization Exam (after winter term) Registration: via TUM-Online between 16.11.2015 15.1.2015 Email: sebastian.muehlbauer@frm2.tum.de
Physics with Neutrons I, WS 2015/2016 Lecture 11, 11.1.2016 MLZ is a cooperation between: Organization Exam (after winter term) Registration: via TUM-Online between 16.11.2015 15.1.2015 Email: sebastian.muehlbauer@frm2.tum.de
DR KAZI SAZZAD MANIR
 DR KAZI SAZZAD MANIR PHOTON BEAM MATTER ENERGY TRANSFER IONISATION EXCITATION ATTENUATION removal of photons from the beam by the matter. ABSORPTION SCATTERING TRANSMISSION Taking up the energy from the
DR KAZI SAZZAD MANIR PHOTON BEAM MATTER ENERGY TRANSFER IONISATION EXCITATION ATTENUATION removal of photons from the beam by the matter. ABSORPTION SCATTERING TRANSMISSION Taking up the energy from the
Elastic and Inelastic Scattering in Electron Diffraction and Imaging
 Elastic and Inelastic Scattering in Electron Diffraction and Imaging Contents Introduction Symbols and definitions Part A Diffraction and imaging of elastically scattered electrons Chapter 1. Basic kinematical
Elastic and Inelastic Scattering in Electron Diffraction and Imaging Contents Introduction Symbols and definitions Part A Diffraction and imaging of elastically scattered electrons Chapter 1. Basic kinematical
Geometry of Crystal Lattice
 0 Geometry of Crystal Lattice 0.1 Translational Symmetry The crystalline state of substances is different from other states (gaseous, liquid, amorphous) in that the atoms are in an ordered and symmetrical
0 Geometry of Crystal Lattice 0.1 Translational Symmetry The crystalline state of substances is different from other states (gaseous, liquid, amorphous) in that the atoms are in an ordered and symmetrical
Chapter 1 Introduction to X-Ray Absorption Spectroscopy
 Chapter 1 Introduction to X-Ray Absorption Spectroscopy Claudia S. Schnohr and Mark C. Ridgway X-ray Absorption Spectroscopy (XAS) is a well-established analytical technique used extensively for the characterization
Chapter 1 Introduction to X-Ray Absorption Spectroscopy Claudia S. Schnohr and Mark C. Ridgway X-ray Absorption Spectroscopy (XAS) is a well-established analytical technique used extensively for the characterization
Data collection Strategy. Apurva Mehta
 Data collection Strategy Apurva Mehta Outline Before.. Resolution, Aberrations and detectors During.. What is the scientific question? How will probing the structure help? Is there an alternative method?
Data collection Strategy Apurva Mehta Outline Before.. Resolution, Aberrations and detectors During.. What is the scientific question? How will probing the structure help? Is there an alternative method?
3.012 Structure An Introduction to X-ray Diffraction
 3.012 Structure An Introduction to X-ray Diffraction This handout summarizes some topics that are important for understanding x-ray diffraction. The following references provide a thorough explanation
3.012 Structure An Introduction to X-ray Diffraction This handout summarizes some topics that are important for understanding x-ray diffraction. The following references provide a thorough explanation
Physics 2D Lecture Slides Lecture 11: Jan. 27 th Sunil Sinha UCSD Physics
 Physics 2D Lecture Slides Lecture 11: Jan. 27 th 2010 Sunil Sinha UCSD Physics Einstein s Explanation of PhotoElectric Effect What Maxwell Saw of EM Waves What Einstein Saw of EM Waves Light as bullets
Physics 2D Lecture Slides Lecture 11: Jan. 27 th 2010 Sunil Sinha UCSD Physics Einstein s Explanation of PhotoElectric Effect What Maxwell Saw of EM Waves What Einstein Saw of EM Waves Light as bullets
Ultrafast Electron Crystallography: Principles and Dynamics
 15 Chapter 2 Ultrafast Electron Crystallography: Principles and Dynamics Part of this chapter was adapted from D.-S. Yang, N. Gedik, A. H. Zewail, J. Phys. Chem. C 111, 4889 (2007). 16 Introduction The
15 Chapter 2 Ultrafast Electron Crystallography: Principles and Dynamics Part of this chapter was adapted from D.-S. Yang, N. Gedik, A. H. Zewail, J. Phys. Chem. C 111, 4889 (2007). 16 Introduction The
SOLID STATE 18. Reciprocal Space
 SOLID STATE 8 Reciprocal Space Wave vectors and the concept of K-space can simplify the explanation of several properties of the solid state. They will be introduced to provide more information on diffraction
SOLID STATE 8 Reciprocal Space Wave vectors and the concept of K-space can simplify the explanation of several properties of the solid state. They will be introduced to provide more information on diffraction
Solid State Spectroscopy Problem Set 7
 Solid State Spectroscopy Problem Set 7 Due date: June 29th, 2015 Problem 5.1 EXAFS Study of Mn/Fe substitution in Y(Mn 1-x Fe x ) 2 O 5 From article «EXAFS, XANES, and DFT study of the mixed-valence compound
Solid State Spectroscopy Problem Set 7 Due date: June 29th, 2015 Problem 5.1 EXAFS Study of Mn/Fe substitution in Y(Mn 1-x Fe x ) 2 O 5 From article «EXAFS, XANES, and DFT study of the mixed-valence compound
Crystal Structure SOLID STATE PHYSICS. Lecture 5. A.H. Harker. thelecture thenextlecture. Physics and Astronomy UCL
 Crystal Structure thelecture thenextlecture SOLID STATE PHYSICS Lecture 5 A.H. Harker Physics and Astronomy UCL Structure & Diffraction Crystal Diffraction (continued) 2.4 Experimental Methods Notes: examples
Crystal Structure thelecture thenextlecture SOLID STATE PHYSICS Lecture 5 A.H. Harker Physics and Astronomy UCL Structure & Diffraction Crystal Diffraction (continued) 2.4 Experimental Methods Notes: examples
Lecture 5 Wave and particle beams.
 Lecture 5 Wave and particle beams. 1 What can we learn from scattering experiments The crystal structure, i.e., the position of the atoms in the crystal averaged over a large number of unit cells and over
Lecture 5 Wave and particle beams. 1 What can we learn from scattering experiments The crystal structure, i.e., the position of the atoms in the crystal averaged over a large number of unit cells and over
Quantum Mechanics (made fun and easy)
 Lecture 7 Quantum Mechanics (made fun and easy) Why the world needs quantum mechanics Why the world needs quantum mechanics Why the world needs quantum mechanics Why the world needs quantum mechanics Why
Lecture 7 Quantum Mechanics (made fun and easy) Why the world needs quantum mechanics Why the world needs quantum mechanics Why the world needs quantum mechanics Why the world needs quantum mechanics Why
High-Resolution. Transmission. Electron Microscopy
 Part 4 High-Resolution Transmission Electron Microscopy 186 Significance high-resolution transmission electron microscopy (HRTEM): resolve object details smaller than 1nm (10 9 m) image the interior of
Part 4 High-Resolution Transmission Electron Microscopy 186 Significance high-resolution transmission electron microscopy (HRTEM): resolve object details smaller than 1nm (10 9 m) image the interior of
Electron Microscopy I
 Characterization of Catalysts and Surfaces Characterization Techniques in Heterogeneous Catalysis Electron Microscopy I Introduction Properties of electrons Electron-matter interactions and their applications
Characterization of Catalysts and Surfaces Characterization Techniques in Heterogeneous Catalysis Electron Microscopy I Introduction Properties of electrons Electron-matter interactions and their applications
Notes on x-ray scattering - M. Le Tacon, B. Keimer (06/2015)
 Notes on x-ray scattering - M. Le Tacon, B. Keimer (06/2015) Interaction of x-ray with matter: - Photoelectric absorption - Elastic (coherent) scattering (Thomson Scattering) - Inelastic (incoherent) scattering
Notes on x-ray scattering - M. Le Tacon, B. Keimer (06/2015) Interaction of x-ray with matter: - Photoelectric absorption - Elastic (coherent) scattering (Thomson Scattering) - Inelastic (incoherent) scattering
Structure analysis: Electron diffraction LEED TEM RHEED
 Structure analysis: Electron diffraction LEED: Low Energy Electron Diffraction SPA-LEED: Spot Profile Analysis Low Energy Electron diffraction RHEED: Reflection High Energy Electron Diffraction TEM: Transmission
Structure analysis: Electron diffraction LEED: Low Energy Electron Diffraction SPA-LEED: Spot Profile Analysis Low Energy Electron diffraction RHEED: Reflection High Energy Electron Diffraction TEM: Transmission
Keble College - Hilary 2012 Section VI: Condensed matter physics Tutorial 2 - Lattices and scattering
 Tomi Johnson Keble College - Hilary 2012 Section VI: Condensed matter physics Tutorial 2 - Lattices and scattering Please leave your work in the Clarendon laboratory s J pigeon hole by 5pm on Monday of
Tomi Johnson Keble College - Hilary 2012 Section VI: Condensed matter physics Tutorial 2 - Lattices and scattering Please leave your work in the Clarendon laboratory s J pigeon hole by 5pm on Monday of
Introduction to Triple Axis Neutron Spectroscopy
 Introduction to Triple Axis Neutron Spectroscopy Bruce D Gaulin McMaster University The triple axis spectrometer Constant-Q and constant E Practical concerns Resolution and Spurions Neutron interactions
Introduction to Triple Axis Neutron Spectroscopy Bruce D Gaulin McMaster University The triple axis spectrometer Constant-Q and constant E Practical concerns Resolution and Spurions Neutron interactions
Exercise 1 Atomic line spectra 1/9
 Exercise 1 Atomic line spectra 1/9 The energy-level scheme for the hypothetical one-electron element Juliettium is shown in the figure on the left. The potential energy is taken to be zero for an electron
Exercise 1 Atomic line spectra 1/9 The energy-level scheme for the hypothetical one-electron element Juliettium is shown in the figure on the left. The potential energy is taken to be zero for an electron
3. Synchrotrons. Synchrotron Basics
 1 3. Synchrotrons Synchrotron Basics What you will learn about 2 Overview of a Synchrotron Source Losing & Replenishing Electrons Storage Ring and Magnetic Lattice Synchrotron Radiation Flux, Brilliance
1 3. Synchrotrons Synchrotron Basics What you will learn about 2 Overview of a Synchrotron Source Losing & Replenishing Electrons Storage Ring and Magnetic Lattice Synchrotron Radiation Flux, Brilliance
de Broglie Waves h p de Broglie argued Light exhibits both wave and particle properties
 de Broglie argued de Broglie Waves Light exhibits both wave and particle properties Wave interference, diffraction Particle photoelectric effect, Compton effect Then matter (particles) should exhibit both
de Broglie argued de Broglie Waves Light exhibits both wave and particle properties Wave interference, diffraction Particle photoelectric effect, Compton effect Then matter (particles) should exhibit both
Dept. of Physics, MIT Manipal 1
 Chapter 1: Optics 1. In the phenomenon of interference, there is A Annihilation of light energy B Addition of energy C Redistribution energy D Creation of energy 2. Interference fringes are obtained using
Chapter 1: Optics 1. In the phenomenon of interference, there is A Annihilation of light energy B Addition of energy C Redistribution energy D Creation of energy 2. Interference fringes are obtained using
Basic Crystallography Part 1. Theory and Practice of X-ray Crystal Structure Determination
 Basic Crystallography Part 1 Theory and Practice of X-ray Crystal Structure Determination We have a crystal How do we get there? we want a structure! The Unit Cell Concept Ralph Krätzner Unit Cell Description
Basic Crystallography Part 1 Theory and Practice of X-ray Crystal Structure Determination We have a crystal How do we get there? we want a structure! The Unit Cell Concept Ralph Krätzner Unit Cell Description
Lecture Outline Chapter 30. Physics, 4 th Edition James S. Walker. Copyright 2010 Pearson Education, Inc.
 Lecture Outline Chapter 30 Physics, 4 th Edition James S. Walker Chapter 30 Quantum Physics Units of Chapter 30 Blackbody Radiation and Planck s Hypothesis of Quantized Energy Photons and the Photoelectric
Lecture Outline Chapter 30 Physics, 4 th Edition James S. Walker Chapter 30 Quantum Physics Units of Chapter 30 Blackbody Radiation and Planck s Hypothesis of Quantized Energy Photons and the Photoelectric
X Rays & Crystals. Characterizing Mineral Chemistry & Structure. J.D. Price
 X Rays & Crystals Characterizing Mineral Chemistry & Structure J.D. Price Light - electromagnetic spectrum Wave behavior vs. particle behavior If atoms are on the 10-10 m scale, we need to use sufficiently
X Rays & Crystals Characterizing Mineral Chemistry & Structure J.D. Price Light - electromagnetic spectrum Wave behavior vs. particle behavior If atoms are on the 10-10 m scale, we need to use sufficiently
Semiconductor Physics and Devices
 Introduction to Quantum Mechanics In order to understand the current-voltage characteristics, we need some knowledge of electron behavior in semiconductor when the electron is subjected to various potential
Introduction to Quantum Mechanics In order to understand the current-voltage characteristics, we need some knowledge of electron behavior in semiconductor when the electron is subjected to various potential
Changing and challenging times for service crystallography. Electronic Supplementary Information
 Changing and challenging times for service crystallography Simon J Coles,* a and Philip A Gale* a Electronic Supplementary Information Instrument descriptions and experimental protocols The following firstly
Changing and challenging times for service crystallography Simon J Coles,* a and Philip A Gale* a Electronic Supplementary Information Instrument descriptions and experimental protocols The following firstly
Determining Protein Structure BIBC 100
 Determining Protein Structure BIBC 100 Determining Protein Structure X-Ray Diffraction Interactions of x-rays with electrons in molecules in a crystal NMR- Nuclear Magnetic Resonance Interactions of magnetic
Determining Protein Structure BIBC 100 Determining Protein Structure X-Ray Diffraction Interactions of x-rays with electrons in molecules in a crystal NMR- Nuclear Magnetic Resonance Interactions of magnetic
X-ray Crystallography. Kalyan Das
 X-ray Crystallography Kalyan Das Electromagnetic Spectrum NMR 10 um - 10 mm 700 to 10 4 nm 400 to 700 nm 10 to 400 nm 10-1 to 10 nm 10-4 to 10-1 nm X-ray radiation was discovered by Roentgen in 1895. X-rays
X-ray Crystallography Kalyan Das Electromagnetic Spectrum NMR 10 um - 10 mm 700 to 10 4 nm 400 to 700 nm 10 to 400 nm 10-1 to 10 nm 10-4 to 10-1 nm X-ray radiation was discovered by Roentgen in 1895. X-rays
X-Ray Photoelectron Spectroscopy (XPS)
 X-Ray Photoelectron Spectroscopy (XPS) Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The basic principle of the photoelectric effect
X-Ray Photoelectron Spectroscopy (XPS) Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The basic principle of the photoelectric effect
Simulation studies of atomic resolution X-ray holography
 Bull. Mater. Sci., Vol. 27, No. 1, February 2004, pp. 79 84. Indian Academy of Sciences. Simulation studies of atomic resolution X-ray holography YOGESH KASHYAP, P S SARKAR, AMAR SINHA* and B K GODWAL
Bull. Mater. Sci., Vol. 27, No. 1, February 2004, pp. 79 84. Indian Academy of Sciences. Simulation studies of atomic resolution X-ray holography YOGESH KASHYAP, P S SARKAR, AMAR SINHA* and B K GODWAL
Characteristics and Properties of Synchrotron Radiation
 Characteristics and Properties of Synchrotron Radiation Giorgio Margaritondo Vice-président pour les affaires académiques Ecole Polytechnique Fédérale de Lausanne (EPFL) Outline: How to build an excellent
Characteristics and Properties of Synchrotron Radiation Giorgio Margaritondo Vice-président pour les affaires académiques Ecole Polytechnique Fédérale de Lausanne (EPFL) Outline: How to build an excellent
Interaction theory Photons. Eirik Malinen
 Interaction theory Photons Eirik Malinen Introduction Interaction theory Dosimetry Radiation source Ionizing radiation Atoms Ionizing radiation Matter - Photons - Charged particles - Neutrons Ionizing
Interaction theory Photons Eirik Malinen Introduction Interaction theory Dosimetry Radiation source Ionizing radiation Atoms Ionizing radiation Matter - Photons - Charged particles - Neutrons Ionizing
Applied Nuclear Physics (Fall 2006) Lecture 19 (11/22/06) Gamma Interactions: Compton Scattering
 .101 Applied Nuclear Physics (Fall 006) Lecture 19 (11//06) Gamma Interactions: Compton Scattering References: R. D. Evans, Atomic Nucleus (McGraw-Hill New York, 1955), Chaps 3 5.. W. E. Meyerhof, Elements
.101 Applied Nuclear Physics (Fall 006) Lecture 19 (11//06) Gamma Interactions: Compton Scattering References: R. D. Evans, Atomic Nucleus (McGraw-Hill New York, 1955), Chaps 3 5.. W. E. Meyerhof, Elements
X-ray Absorption Spectroscopy
 X-ray Absorption Spectroscopy Nikki Truss November 26, 2012 Abstract In these experiments, some aspects of x-ray absorption spectroscopy were investigated. The x-ray spectrum of molybdenum was recorded
X-ray Absorption Spectroscopy Nikki Truss November 26, 2012 Abstract In these experiments, some aspects of x-ray absorption spectroscopy were investigated. The x-ray spectrum of molybdenum was recorded
