ESCI 341 Atmospheric Thermodynamics Lesson 13 Phase Changes Dr. DeCaria
|
|
|
- Marjorie Daniel
- 5 years ago
- Views:
Transcription
1 ESCI 341 Atmopherc Thermodynamc Leon 13 Phae Change Dr. DeCara Reference: Thermodynamc and an Introducton to Thermotattc, Caen Phyca Chemtry, Lene GENERAL A phae change a change between od, qud, or apor (ga). A ytem may een hae more than one od or qud phae Carbon ha eera od phae, ncudng graphte and damond The phae of a ytem n equbrum can be repreented on a phae dagram. A typca phae dagram hown beow The ne eparatng the phae repreent pont where the two phae can coext. The trpe pont repreent the one and ony pont on the dagram where a three phae can coext. The crtca pont occur at the end of the apor-to-qud tranton ne. At temperature aboe the crtca temperature, or preure aboe the crtca preure there no dtncton between the qud and apor phae.
2 If you go from the apor to qud phae at temperature or preure exceedng the crtca aue, condenaton doe not occur. Intead, there a gradua ncreae n denty and a contnuou tranton from apor to qud. The ope of the od-to-qud tranton ne how how the metng pont change wth preure. The dagram aboe typca for ubtance uch a CO2. In th cae, an ncreae n preure w ncreae the metng pont. If you are near the od-to-qud tranton ne n th cae, queezng the ubtance w caue t to odfy. The trpe-pont temperature and preure for CO2 are 57C and 5.1 atm. Therefore, at room temperature dry ce a ga, and f t cooed beow 57C t w depot nto the od phae, wthout eer gong through the qud phae. Th why od CO2 referred to a dry ce. PHASE DIAGRAM FOR H2O Water one of the few ubtance that can ext n a three phae at the temperature and preure found n the Earth atmophere. The phae dagram for water 2
3 The trpe pont of water at 0.01C and atm (611 Pa). A unque feature of water that the od-to-qud tranton ne ope upward to the eft, ntead of to the rght. GIBBS FREE ENERGY AND MATERIAL EQUILIBRIUM We hae preouy hown the foowng nequate for coed ytem du TdS pdv dh TdS Vdp df SdT pdv dg SdT Vdp (1) Thee nequate are ad for a procee n a coed ytem that n mechanca and therma equbrum, but not matera equbrum 1. For a mut-component ytem the Gbb free energy a functon of T, p, and the mae of each component, m. The dfferenta of G therefore G G G dg dt dp dm T p m (2) p, m T, m T, p We ao know that for a coed ytem wth fxed compoton ( dm 0 ) that ao n equbrum, that dg SdT Vdp, (3) and comparng Eq. (2) and (3) how that G T G p pm, Tm, S V o Eq. (2) can be wrtten a G dg SdT Vdp dm m. (4) T, p We ao know that the tota Gbb free energy of a ubtance equa to the pecfc Gbb free energy of each conttuent mutped by t ma, 1 See Lene, Chapter 4 (partcuary Secton 4.4) f more deta are dered. 3
4 Dfferentatng Eq. (5) how that 2 G g m. (5) G m T, p g. Ung th n Eq. (4) yed Gong back to Eq. (1) we know that Combnng Eq. (6) and (7) ge whch reduce to dg SdT Vdp g dm. (6) dg SdT Vdp. (7) SdT Vdp g dm SdT Vdp gdm 0. (8) Equaton (8) te u that f a mut-component ytem not n matera equbrum, that t w then pontaneouy eoe unt Equaton (9) the condton for matera equbrum. g dm 0. (9) Note that een though the equbrum condton of Eq. (9) n term of the pecfc Gbb free energy, t not mted to contant preure, contant temperature procee. It the matera equbrum condton wthout any retrcton. MATERIAL EQUILIBRIUM IN A TWO COMPONENT SYSTEM 2 Note that the pecfc Gbb free energy, g, of a conttuent doe not depend on the ma of tef or any g m other conttuent, o that 0 for any aue of or j. Howeer, th not true for the other j u G ntene tate arabe,.e., 0. So, athough g, t not neceary true that m m U m u. j 4
5 In a ytem wth two component, Equaton (8) become dm g dt dm g. (10) dt 0 Any ma ot from component 1 mut be ganed by component 2, o we hae and Eq. (10) become dm dt dm dt dm. (11) dt 1 g g 0 Let examne what happen f the two component are not n matera equbrum, o that g g. There are two pobe cenaro: Cae 1: g g Th cae requre dm1 dt 0 o the ma of component 1 w decreae whe the ma of component 2 w ncreae. Cae 1: g1 g2 Th cae requre dm1 dt 0 o the ma of component 1 w ncreae whe the ma of component 2 w decreae. In ether cae, the reut that the component that ha the owet pecfc Gbb free energy w ncreae at the expene of the component wth the hgher Gbb free energy. Another way to tate th that equbrum faor the component wth the owet pecfc Gbb free energy. The ony way the two component can be n matera equbrum f they hae the ame pecfc Gbb free energy, g1 g2. THE CLAPEYRON EQUATION If we hae two phae preent, the condton for equbrum from Eq. (9) g1 g 2. (12) The equbrum condton ay that at equbrum the pecfc Gbb free energe of the two phae w be equa. Dfferentatng Eq. (12) ge 5
6 From the Gbb equaton we hae o we can wrte Eq. (13) a whch can be rearranged to ge dg1 dg 2 dg dt dp. dt dp dt dp From the frt aw of thermodynamc we hae. (13) dp 2 1 dt. (14) dh Td dp. whch, f the temperature and preure are contant, ntegrate to h T L, where L the atent heat of the phae change (unt of energy per ma). Equaton (14) then become dp dt whch known a the Capeyron Equaton. L, (15) T The Capeyron equaton competey genera. It appe to any equbrum phae change. LIQUID VAPOR EQUILIBRIUM If we appy the Capeyron equaton to the phae change between qud and apor, then the preure jut the aturaton apor preure (p = e) and we get de dt. (16) L T Snce the pecfc oume of a apor o much greater than the pecfc oume of a qud, we can approxmate the dfference between the two a. Th make Eq. (16) If the apor an dea ga, then de dt L. (17) T 6
7 and Eq. (17) become 1 de e dt RT e whch known a the Cauu-Capeyron equaton., L, (18) 2 RT Integratng the Cauu-Capeyron equaton w ge an equaton for the nterface of the od-qud phae change on the phae dagram. Integratng between a known reference apor preure and temperature, e0 and T0 (aumng that the atent heat ndependent of temperature and preure) ge e L 1 1 e 0 exp. (19) R T0 T A uazaton a to why aturaton apor preure depend on temperature can be found at th nk: SOLID VAPOR EQUILIBRIUM For the phae change from od to apor we ony need to repace the atent heat of aporzaton wth the atent heat of ubmaton, L n Eq. (19). Thu, oer an ce urface the aturaton apor preure e L 1 1 e 0 exp. (20) R T0 T SOLID LIQUID EQUILIBRIUM For od qud equbrum we mut return to the Capeyron equaton [Eq. (15)] dp dt L. T For mot ubtance the od more dene than the qud, o that >. Thu, for mot ubtance, the od-qud ne on the phae dagram w tt upward to the rght. 7
8 Water unque n that t od phae (ce) e dene than the qud phae, o that <. Th why the od-qud ne on the water phae dagram tt upward to the eft. THE EFFECT OF AIR PRESSURE ON THE SATURATION VAPOR PRESSURE Up to know we e aumed that the ony ubtance we had n our ytem wa water. What happen f we ao hae an nert ga, uch a ar preent? The equbrum condton, Eq. (9), t requre that g dm 0, but now we hae three conttuent: qud water (m), water apor (m), and dry ar (md). The equbrum condton therefore g dm g dm g dm 0. Snce the ar nert, dmd = 0 and dm = dm. So a before, the equbrum condton d d dg dg. (21) At frt gance th ook ke we are gong to get an exacty equaent anwer to what we had wthout ar preent. Howeer, there a dfference. Athough the ar doe not hae any effect on the Gbb free energy of the water apor (nce both are dea gae, and therefore don t nfuence each other), the ar doe ncreae the preure on the qud. So, we hae dt dp dt de (note that f ar were not preent, then p = e and we woud jut get the Capeyron equaton a before). We want to know what affect changng p ha on e, o f we hod the temperature contant we get whch can be wrtten a dp de f temperature hed contant e p T. (22) 8
9 Equaton (22) caed the Poyntng Equaton. The Poyntng Equaton ay that an ncreae n preure w ncreae the aturaton apor preure. Th mpe that t eaer for water moecue to ecape from the qud to the apor phae f the qud under preure. The phyca expanaton for th phenomena not traght-forward, and there are ome popuar though ncorrect expanaton hang to do wth the number of adjacent moecue hodng a water moecue n the qud phae. One eemngy reaonabe phyca expanaton that ometme ued that a the qud put under preure and the moecue are queezed coer together, the attracte force between moecue become weaker and repue force are enhanced. Th make t eaer for a water moecue to ecape or pop out of the qud urface. Une preure are ery hgh or temperature are ery ow, >>. Therefore, for mot atmopherc appcaton e p T 0, and we can gnore the effect of ambent ar preure on the aturaton apor preure. Howeer, we w ee n the next eon that n a cured dropet the nteror preure ery greaty enhanced, and the effect of the Poyntng Equaton cannot be dmed n th tuaton. It mportant to note that the preence of ar doe not gnfcanty ater the aturaton apor preure. We often peak of the ar hodng the water apor, and of warm ar omehow beng abe to hod more water apor. Th noton actuay pretty y, nce the ar dong nothng. There woud be the ame amount of water apor preent regarde of whether or not ar wa preent. THE MYTH OF THE ICE SKATER The anomaou ope of the od-qud equbrum ne for water ha ed many to attrbute the abty to ce kate on preure metng, whereby a a kater 9
10 pae oer the ce, the ncreaed preure on the ce hft the equbrum from the od phae to the qud phae. It turn out that, athough the expanaton ound paube, t ncorrect. The true nature of the pperne of ce much more compex, and noe a proce caed urface metng. The deta are beyond the cope of th coure, but an oerew of the ubject can be found n Roenburg (2005) 3. 3 Roenberg, R, 2005: Why ce ppery?, Phyc Today, December ue, pp
Introduction to Interfacial Segregation. Xiaozhe Zhang 10/02/2015
 Introducton to Interfacal Segregaton Xaozhe Zhang 10/02/2015 Interfacal egregaton Segregaton n materal refer to the enrchment of a materal conttuent at a free urface or an nternal nterface of a materal.
Introducton to Interfacal Segregaton Xaozhe Zhang 10/02/2015 Interfacal egregaton Segregaton n materal refer to the enrchment of a materal conttuent at a free urface or an nternal nterface of a materal.
A A Non-Constructible Equilibrium 1
 A A Non-Contructbe Equbrum 1 The eampe depct a eparabe contet wth three payer and one prze of common vaue 1 (o v ( ) =1 c ( )). I contruct an equbrum (C, G, G) of the contet, n whch payer 1 bet-repone
A A Non-Contructbe Equbrum 1 The eampe depct a eparabe contet wth three payer and one prze of common vaue 1 (o v ( ) =1 c ( )). I contruct an equbrum (C, G, G) of the contet, n whch payer 1 bet-repone
Not at Steady State! Yes! Only if reactions occur! Yes! Ideal Gas, change in temperature or pressure. Yes! Class 15. Is the following possible?
 Chapter 5-6 (where we are gong) Ideal gae and lqud (today) Dente Partal preure Non-deal gae (next tme) Eqn. of tate Reduced preure and temperature Compreblty chart (z) Vapor-lqud ytem (Ch. 6) Vapor preure
Chapter 5-6 (where we are gong) Ideal gae and lqud (today) Dente Partal preure Non-deal gae (next tme) Eqn. of tate Reduced preure and temperature Compreblty chart (z) Vapor-lqud ytem (Ch. 6) Vapor preure
No! Yes! Only if reactions occur! Yes! Ideal Gas, change in temperature or pressure. Survey Results. Class 15. Is the following possible?
 Survey Reult Chapter 5-6 (where we are gong) % of Student 45% 40% 35% 30% 25% 20% 15% 10% 5% 0% Hour Spent on ChE 273 1-2 3-4 5-6 7-8 9-10 11+ Hour/Week 2008 2009 2010 2011 2012 2013 2014 2015 2017 F17
Survey Reult Chapter 5-6 (where we are gong) % of Student 45% 40% 35% 30% 25% 20% 15% 10% 5% 0% Hour Spent on ChE 273 1-2 3-4 5-6 7-8 9-10 11+ Hour/Week 2008 2009 2010 2011 2012 2013 2014 2015 2017 F17
and decompose in cycles of length two
 Permutaton of Proceedng of the Natona Conference On Undergraduate Reearch (NCUR) 006 Domncan Unverty of Caforna San Rafae, Caforna Apr - 4, 007 that are gven by bnoma and decompoe n cyce of ength two Yeena
Permutaton of Proceedng of the Natona Conference On Undergraduate Reearch (NCUR) 006 Domncan Unverty of Caforna San Rafae, Caforna Apr - 4, 007 that are gven by bnoma and decompoe n cyce of ength two Yeena
Problem Free Expansion of Ideal Gas
 Problem 4.3 Free Expanon o Ideal Ga In general: ds ds du P dv P dv NR V dn Snce U o deal ga ndependent on olume (du=), and N = cont n the proce: dv In a ere o nntemal ree expanon, entropy change by: S
Problem 4.3 Free Expanon o Ideal Ga In general: ds ds du P dv P dv NR V dn Snce U o deal ga ndependent on olume (du=), and N = cont n the proce: dv In a ere o nntemal ree expanon, entropy change by: S
CHAPTER 9 LINEAR MOMENTUM, IMPULSE AND COLLISIONS
 CHAPTER 9 LINEAR MOMENTUM, IMPULSE AND COLLISIONS 103 Phy 1 9.1 Lnear Momentum The prncple o energy conervaton can be ued to olve problem that are harder to olve jut ung Newton law. It ued to decrbe moton
CHAPTER 9 LINEAR MOMENTUM, IMPULSE AND COLLISIONS 103 Phy 1 9.1 Lnear Momentum The prncple o energy conervaton can be ued to olve problem that are harder to olve jut ung Newton law. It ued to decrbe moton
SOLUTIONS
 SOLUTIONS Topic-2 RAOULT S LAW, ALICATIONS AND NUMERICALS VERY SHORT ANSWER QUESTIONS 1. Define vapour preure? An: When a liquid i in equilibrium with it own vapour the preure exerted by the vapour on
SOLUTIONS Topic-2 RAOULT S LAW, ALICATIONS AND NUMERICALS VERY SHORT ANSWER QUESTIONS 1. Define vapour preure? An: When a liquid i in equilibrium with it own vapour the preure exerted by the vapour on
APPLICATIONS: CHEMICAL AND PHASE EQUILIBRIA
 5.60 Sprn 2007 Lecture #28 pae PPLICTIOS: CHMICL D PHS QUILIBRI pply tattcal mechanc to develop mcrocopc model for problem you ve treated o far wth macrocopc thermodynamc 0 Product Reactant Separated atom
5.60 Sprn 2007 Lecture #28 pae PPLICTIOS: CHMICL D PHS QUILIBRI pply tattcal mechanc to develop mcrocopc model for problem you ve treated o far wth macrocopc thermodynamc 0 Product Reactant Separated atom
Electric and magnetic field sensor and integrator equations
 Techncal Note - TN12 Electrc and magnetc feld enor and ntegrator uaton Bertrand Da, montena technology, 1728 oen, Swtzerland Table of content 1. Equaton of the derate electrc feld enor... 1 2. Integraton
Techncal Note - TN12 Electrc and magnetc feld enor and ntegrator uaton Bertrand Da, montena technology, 1728 oen, Swtzerland Table of content 1. Equaton of the derate electrc feld enor... 1 2. Integraton
between standard Gibbs free energies of formation for products and reactants, ΔG! R = ν i ΔG f,i, we
 hermodynamcs, Statstcal hermodynamcs, and Knetcs 4 th Edton,. Engel & P. ed Ch. 6 Part Answers to Selected Problems Q6.. Q6.4. If ξ =0. mole at equlbrum, the reacton s not ery far along. hus, there would
hermodynamcs, Statstcal hermodynamcs, and Knetcs 4 th Edton,. Engel & P. ed Ch. 6 Part Answers to Selected Problems Q6.. Q6.4. If ξ =0. mole at equlbrum, the reacton s not ery far along. hus, there would
Problem #1. Known: All required parameters. Schematic: Find: Depth of freezing as function of time. Strategy:
 BEE 3500 013 Prelm Soluton Problem #1 Known: All requred parameter. Schematc: Fnd: Depth of freezng a functon of tme. Strategy: In thee mplfed analy for freezng tme, a wa done n cla for a lab geometry,
BEE 3500 013 Prelm Soluton Problem #1 Known: All requred parameter. Schematc: Fnd: Depth of freezng a functon of tme. Strategy: In thee mplfed analy for freezng tme, a wa done n cla for a lab geometry,
Projectile Motion. Parabolic Motion curved motion in the shape of a parabola. In the y direction, the equation of motion has a t 2.
 Projectle Moton Phc Inentor Parabolc Moton cured oton n the hape of a parabola. In the drecton, the equaton of oton ha a t ter Projectle Moton the parabolc oton of an object, where the horzontal coponent
Projectle Moton Phc Inentor Parabolc Moton cured oton n the hape of a parabola. In the drecton, the equaton of oton ha a t ter Projectle Moton the parabolc oton of an object, where the horzontal coponent
Open Systems: Chemical Potential and Partial Molar Quantities Chemical Potential
 Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Supervised Learning. Neural Networks and Back-Propagation Learning. Credit Assignment Problem. Feedforward Network. Adaptive System.
 Part 7: Neura Networ & earnng /2/05 Superved earnng Neura Networ and Bac-Propagaton earnng Produce dered output for tranng nput Generaze reaonaby & appropratey to other nput Good exampe: pattern recognton
Part 7: Neura Networ & earnng /2/05 Superved earnng Neura Networ and Bac-Propagaton earnng Produce dered output for tranng nput Generaze reaonaby & appropratey to other nput Good exampe: pattern recognton
Additional File 1 - Detailed explanation of the expression level CPD
 Addtonal Fle - Detaled explanaton of the expreon level CPD A mentoned n the man text, the man CPD for the uterng model cont of two ndvdual factor: P( level gen P( level gen P ( level gen 2 (.).. CPD factor
Addtonal Fle - Detaled explanaton of the expreon level CPD A mentoned n the man text, the man CPD for the uterng model cont of two ndvdual factor: P( level gen P( level gen P ( level gen 2 (.).. CPD factor
Chapter 11. Supplemental Text Material. The method of steepest ascent can be derived as follows. Suppose that we have fit a firstorder
 S-. The Method of Steepet cent Chapter. Supplemental Text Materal The method of teepet acent can be derved a follow. Suppoe that we have ft a frtorder model y = β + β x and we wh to ue th model to determne
S-. The Method of Steepet cent Chapter. Supplemental Text Materal The method of teepet acent can be derved a follow. Suppoe that we have ft a frtorder model y = β + β x and we wh to ue th model to determne
Physics 120. Exam #1. April 15, 2011
 Phyc 120 Exam #1 Aprl 15, 2011 Name Multple Choce /16 Problem #1 /28 Problem #2 /28 Problem #3 /28 Total /100 PartI:Multple Choce:Crclethebetanwertoeachqueton.Anyothermark wllnotbegvencredt.eachmultple
Phyc 120 Exam #1 Aprl 15, 2011 Name Multple Choce /16 Problem #1 /28 Problem #2 /28 Problem #3 /28 Total /100 PartI:Multple Choce:Crclethebetanwertoeachqueton.Anyothermark wllnotbegvencredt.eachmultple
University of Washington Department of Chemistry Chemistry 452/456 Summer Quarter 2014
 Lecture 12 7/25/14 ERD: 7.1-7.5 Devoe: 8.1.1-8.1.2, 8.2.1-8.2.3, 8.4.1-8.4.3 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 2014 A. Free Energy and Changes n Composton: The
Lecture 12 7/25/14 ERD: 7.1-7.5 Devoe: 8.1.1-8.1.2, 8.2.1-8.2.3, 8.4.1-8.4.3 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 2014 A. Free Energy and Changes n Composton: The
Small signal analysis
 Small gnal analy. ntroducton Let u conder the crcut hown n Fg., where the nonlnear retor decrbed by the equaton g v havng graphcal repreentaton hown n Fg.. ( G (t G v(t v Fg. Fg. a D current ource wherea
Small gnal analy. ntroducton Let u conder the crcut hown n Fg., where the nonlnear retor decrbed by the equaton g v havng graphcal repreentaton hown n Fg.. ( G (t G v(t v Fg. Fg. a D current ource wherea
SOLUTION MANUAL ENGLISH UNIT PROBLEMS CHAPTER 9 SONNTAG BORGNAKKE VAN WYLEN. FUNDAMENTALS of. Thermodynamics. Sixth Edition
 SOLUTION MANUAL ENGLISH UNIT PROBLEMS CHAPTER 9 SONNTAG BORGNAKKE VAN WYLEN FUNDAMENTALS of Thermodynamc Sxth Edton CONTENT SUBSECTION PROB NO. Concept-Study Gude Problem 134-141 Steady Sngle Flow Devce
SOLUTION MANUAL ENGLISH UNIT PROBLEMS CHAPTER 9 SONNTAG BORGNAKKE VAN WYLEN FUNDAMENTALS of Thermodynamc Sxth Edton CONTENT SUBSECTION PROB NO. Concept-Study Gude Problem 134-141 Steady Sngle Flow Devce
VIII. Addition of Angular Momenta
 VIII Addition of Anguar Momenta a Couped and Uncouped Bae When deaing with two different ource of anguar momentum, Ĵ and Ĵ, there are two obviou bae that one might chooe to work in The firt i caed the
VIII Addition of Anguar Momenta a Couped and Uncouped Bae When deaing with two different ource of anguar momentum, Ĵ and Ĵ, there are two obviou bae that one might chooe to work in The firt i caed the
Solutions to exercises week 45 FYS2160
 Solution to exercie week 45 FYS2160 Kritian Bjørke, Knut Oddvar Høie Vadla November 29, 2017 Schroeder 5.23 a) Writing Φ = U T S µn in term of infiniteimal change of the quantitie involved: dφ = du T ds
Solution to exercie week 45 FYS2160 Kritian Bjørke, Knut Oddvar Høie Vadla November 29, 2017 Schroeder 5.23 a) Writing Φ = U T S µn in term of infiniteimal change of the quantitie involved: dφ = du T ds
...Thermodynamics. If Clausius Clapeyron fails. l T (v 2 v 1 ) = 0/0 Second order phase transition ( S, v = 0)
 If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
Harmonic oscillator approximation
 armonc ocllator approxmaton armonc ocllator approxmaton Euaton to be olved We are fndng a mnmum of the functon under the retrcton where W P, P,..., P, Q, Q,..., Q P, P,..., P, Q, Q,..., Q lnwgner functon
armonc ocllator approxmaton armonc ocllator approxmaton Euaton to be olved We are fndng a mnmum of the functon under the retrcton where W P, P,..., P, Q, Q,..., Q P, P,..., P, Q, Q,..., Q lnwgner functon
1 cos. where v v sin. Range Equations: for an object that lands at the same height at which it starts. v sin 2 i. t g. and. sin g
 SPH3UW Unt.5 Projectle Moton Pae 1 of 10 Note Phc Inventor Parabolc Moton curved oton n the hape of a parabola. In the drecton, the equaton of oton ha a t ter Projectle Moton the parabolc oton of an object,
SPH3UW Unt.5 Projectle Moton Pae 1 of 10 Note Phc Inventor Parabolc Moton curved oton n the hape of a parabola. In the drecton, the equaton of oton ha a t ter Projectle Moton the parabolc oton of an object,
Lecture 11. Transport in Membranes (1)
 ecture 11. Transport n embranes (1) ass Transfer n embranes uk Fow qud Dffuson through ores Gas Dffuson through orous embranes Transport through onporous embranes - Souton-dffuson for qud mxtures - Souton-dffuson
ecture 11. Transport n embranes (1) ass Transfer n embranes uk Fow qud Dffuson through ores Gas Dffuson through orous embranes Transport through onporous embranes - Souton-dffuson for qud mxtures - Souton-dffuson
You must not circulate this book in any other binding or cover and you must impose this same condition on any acquirer.
 6 Interfacal thermodynamc: Gbb equaton Luuk K. Koopal Chapter 6, Interfacal thermodynamc: Gbb equaton n Interface Scence, Second edton, 008, Wagenngen Unverty, Wagenngen, The Netherland. Avalable va: http://www.reearchgate.net/profle/luuk_koopal
6 Interfacal thermodynamc: Gbb equaton Luuk K. Koopal Chapter 6, Interfacal thermodynamc: Gbb equaton n Interface Scence, Second edton, 008, Wagenngen Unverty, Wagenngen, The Netherland. Avalable va: http://www.reearchgate.net/profle/luuk_koopal
NAME and Section No. it is found that 0.6 mol of O
 NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
1. Intensity of Periodic Sound Waves 2. The Doppler Effect
 1. Intenity o Periodic Sound Wae. The Doppler Eect 1-4-018 1 Objectie: The tudent will be able to Deine the intenity o the ound wae. Deine the Doppler Eect. Undertand ome application on ound 1-4-018 3.3
1. Intenity o Periodic Sound Wae. The Doppler Eect 1-4-018 1 Objectie: The tudent will be able to Deine the intenity o the ound wae. Deine the Doppler Eect. Undertand ome application on ound 1-4-018 3.3
Module 5. Cables and Arches. Version 2 CE IIT, Kharagpur
 odule 5 Cable and Arche Veron CE IIT, Kharagpur Leon 33 Two-nged Arch Veron CE IIT, Kharagpur Intructonal Objectve: After readng th chapter the tudent wll be able to 1. Compute horzontal reacton n two-hnged
odule 5 Cable and Arche Veron CE IIT, Kharagpur Leon 33 Two-nged Arch Veron CE IIT, Kharagpur Intructonal Objectve: After readng th chapter the tudent wll be able to 1. Compute horzontal reacton n two-hnged
Appendix II Summary of Important Equations
 W. M. Whte Geochemstry Equatons of State: Ideal GasLaw: Coeffcent of Thermal Expanson: Compressblty: Van der Waals Equaton: The Laws of Thermdynamcs: Frst Law: Appendx II Summary of Important Equatons
W. M. Whte Geochemstry Equatons of State: Ideal GasLaw: Coeffcent of Thermal Expanson: Compressblty: Van der Waals Equaton: The Laws of Thermdynamcs: Frst Law: Appendx II Summary of Important Equatons
CHAPTER X PHASE-CHANGE PROBLEMS
 Chapter X Phae-Change Problem December 3, 18 917 CHAPER X PHASE-CHANGE PROBLEMS X.1 Introducton Clacal Stefan Problem Geometry of Phae Change Problem Interface Condton X. Analytcal Soluton for Soldfcaton
Chapter X Phae-Change Problem December 3, 18 917 CHAPER X PHASE-CHANGE PROBLEMS X.1 Introducton Clacal Stefan Problem Geometry of Phae Change Problem Interface Condton X. Analytcal Soluton for Soldfcaton
Lecture 13. Thermodynamic Potentials (Ch. 5)
 Lecture 13. hermodynamic Potential (Ch. 5) So far we have been uing the total internal energy U and ometime the enthalpy H to characterize variou macrocopic ytem. hee function are called the thermodynamic
Lecture 13. hermodynamic Potential (Ch. 5) So far we have been uing the total internal energy U and ometime the enthalpy H to characterize variou macrocopic ytem. hee function are called the thermodynamic
Principles of Food and Bioprocess Engineering (FS 231) Solutions to Example Problems on Heat Transfer
 Prncples of Food and Boprocess Engneerng (FS 31) Solutons to Example Problems on Heat Transfer 1. We start wth Fourer s law of heat conducton: Q = k A ( T/ x) Rearrangng, we get: Q/A = k ( T/ x) Here,
Prncples of Food and Boprocess Engneerng (FS 31) Solutons to Example Problems on Heat Transfer 1. We start wth Fourer s law of heat conducton: Q = k A ( T/ x) Rearrangng, we get: Q/A = k ( T/ x) Here,
MAE 101A. Homework 3 Solutions 2/5/2018
 MAE 101A Homework 3 Solution /5/018 Munon 3.6: What preure gradient along the treamline, /d, i required to accelerate water upward in a vertical pipe at a rate of 30 ft/? What i the anwer if the flow i
MAE 101A Homework 3 Solution /5/018 Munon 3.6: What preure gradient along the treamline, /d, i required to accelerate water upward in a vertical pipe at a rate of 30 ft/? What i the anwer if the flow i
Two-Layered Model of Blood Flow through Composite Stenosed Artery
 Avalable at http://pvamu.edu/aam Appl. Appl. Math. ISSN: 93-9466 Vol. 4, Iue (December 9), pp. 343 354 (Prevouly, Vol. 4, No.) Applcaton Appled Mathematc: An Internatonal Journal (AAM) Two-ayered Model
Avalable at http://pvamu.edu/aam Appl. Appl. Math. ISSN: 93-9466 Vol. 4, Iue (December 9), pp. 343 354 (Prevouly, Vol. 4, No.) Applcaton Appled Mathematc: An Internatonal Journal (AAM) Two-ayered Model
NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor
 NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor T o T T o T F o, Q o F T m,q m T m T m T mo Aumption: 1. Homogeneou Sytem 2. Single Reaction 3. Steady State Two type of problem: 1. Given deired
NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor T o T T o T F o, Q o F T m,q m T m T m T mo Aumption: 1. Homogeneou Sytem 2. Single Reaction 3. Steady State Two type of problem: 1. Given deired
Note 2. Ling fong Li. 1 Klein Gordon Equation Probablity interpretation Solutions to Klein-Gordon Equation... 2
 Note 2 Lng fong L Contents Ken Gordon Equaton. Probabty nterpretaton......................................2 Soutons to Ken-Gordon Equaton............................... 2 2 Drac Equaton 3 2. Probabty nterpretaton.....................................
Note 2 Lng fong L Contents Ken Gordon Equaton. Probabty nterpretaton......................................2 Soutons to Ken-Gordon Equaton............................... 2 2 Drac Equaton 3 2. Probabty nterpretaton.....................................
FEEDBACK AMPLIFIERS. v i or v s v 0
 FEEDBCK MPLIFIERS Feedback n mplers FEEDBCK IS THE PROCESS OF FEEDING FRCTION OF OUTPUT ENERGY (VOLTGE OR CURRENT) BCK TO THE INPUT CIRCUIT. THE CIRCUIT EMPLOYED FOR THIS PURPOSE IS CLLED FEEDBCK NETWORK.
FEEDBCK MPLIFIERS Feedback n mplers FEEDBCK IS THE PROCESS OF FEEDING FRCTION OF OUTPUT ENERGY (VOLTGE OR CURRENT) BCK TO THE INPUT CIRCUIT. THE CIRCUIT EMPLOYED FOR THIS PURPOSE IS CLLED FEEDBCK NETWORK.
Specification -- Assumptions of the Simple Classical Linear Regression Model (CLRM) 1. Introduction
 ECONOMICS 35* -- NOTE ECON 35* -- NOTE Specfcaton -- Aumpton of the Smple Clacal Lnear Regreon Model (CLRM). Introducton CLRM tand for the Clacal Lnear Regreon Model. The CLRM alo known a the tandard lnear
ECONOMICS 35* -- NOTE ECON 35* -- NOTE Specfcaton -- Aumpton of the Smple Clacal Lnear Regreon Model (CLRM). Introducton CLRM tand for the Clacal Lnear Regreon Model. The CLRM alo known a the tandard lnear
HO 40 Solutions ( ) ˆ. j, and B v. F m x 10-3 kg = i + ( 4.19 x 10 4 m/s)ˆ. (( )ˆ i + ( 4.19 x 10 4 m/s )ˆ j ) ( 1.40 T )ˆ k.
 .) m.8 x -3 g, q. x -8 C, ( 3. x 5 m/)ˆ, and (.85 T)ˆ The magnetc force : F q (. x -8 C) ( 3. x 5 m/)ˆ (.85 T)ˆ F.98 x -3 N F ma ( ˆ ˆ ) (.98 x -3 N) ˆ o a HO 4 Soluton F m (.98 x -3 N)ˆ.8 x -3 g.65 m.98
.) m.8 x -3 g, q. x -8 C, ( 3. x 5 m/)ˆ, and (.85 T)ˆ The magnetc force : F q (. x -8 C) ( 3. x 5 m/)ˆ (.85 T)ˆ F.98 x -3 N F ma ( ˆ ˆ ) (.98 x -3 N) ˆ o a HO 4 Soluton F m (.98 x -3 N)ˆ.8 x -3 g.65 m.98
LECTURE 21 Mohr s Method for Calculation of General Displacements. 1 The Reciprocal Theorem
 V. DEMENKO MECHANICS OF MATERIALS 05 LECTURE Mohr s Method for Cacuaton of Genera Dspacements The Recproca Theorem The recproca theorem s one of the genera theorems of strength of materas. It foows drect
V. DEMENKO MECHANICS OF MATERIALS 05 LECTURE Mohr s Method for Cacuaton of Genera Dspacements The Recproca Theorem The recproca theorem s one of the genera theorems of strength of materas. It foows drect
( ) 1/ 2. ( P SO2 )( P O2 ) 1/ 2.
 Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
Scattering of two identical particles in the center-of. of-mass frame. (b)
 Lecture # November 5 Scatterng of two dentcal partcle Relatvtc Quantum Mechanc: The Klen-Gordon equaton Interpretaton of the Klen-Gordon equaton The Drac equaton Drac repreentaton for the matrce α and
Lecture # November 5 Scatterng of two dentcal partcle Relatvtc Quantum Mechanc: The Klen-Gordon equaton Interpretaton of the Klen-Gordon equaton The Drac equaton Drac repreentaton for the matrce α and
ESCI 341 Atmospheric Thermodynamics Lesson 6 Thermodynamic Processes
 ESCI 341 Atmosherc Thermodynamcs Lesson 6 Thermodynamc Processes Reerences: An Introducton to Atmosherc Thermodynamcs, Tsons Introducton to Theoretcal Meteorology, Hess Physcal Chemstry (4 th edton), Lene
ESCI 341 Atmosherc Thermodynamcs Lesson 6 Thermodynamc Processes Reerences: An Introducton to Atmosherc Thermodynamcs, Tsons Introducton to Theoretcal Meteorology, Hess Physcal Chemstry (4 th edton), Lene
Chapter.4 MAGNETIC CIRCUIT OF A D.C. MACHINE
 Chapter.4 MAGNETIC CIRCUIT OF A D.C. MACHINE The dfferent part of the dc machne manetc crcut / pole are yoke, pole, ar ap, armature teeth and armature core. Therefore, the ampere-turn /pole to etablh the
Chapter.4 MAGNETIC CIRCUIT OF A D.C. MACHINE The dfferent part of the dc machne manetc crcut / pole are yoke, pole, ar ap, armature teeth and armature core. Therefore, the ampere-turn /pole to etablh the
Graphical Analysis of a BJT Amplifier
 4/6/2011 A Graphcal Analyss of a BJT Amplfer lecture 1/18 Graphcal Analyss of a BJT Amplfer onsder agan ths smple BJT amplfer: ( t) = + ( t) O O o B + We note that for ths amplfer, the output oltage s
4/6/2011 A Graphcal Analyss of a BJT Amplfer lecture 1/18 Graphcal Analyss of a BJT Amplfer onsder agan ths smple BJT amplfer: ( t) = + ( t) O O o B + We note that for ths amplfer, the output oltage s
2.3 Least-Square regressions
 .3 Leat-Square regreon Eample.10 How do chldren grow? The pattern of growth vare from chld to chld, o we can bet undertandng the general pattern b followng the average heght of a number of chldren. Here
.3 Leat-Square regreon Eample.10 How do chldren grow? The pattern of growth vare from chld to chld, o we can bet undertandng the general pattern b followng the average heght of a number of chldren. Here
ESTIMATION OF HENRY S CONSTANTS FROM HIGH PRESSURE GAS SOLUBILITIES. THE SYSTEMS CO 2 + n-
 ESTIMATION OF HENRY S CONSTANTS FROM HIGH RESSURE GAS SOLUBILITIES. THE SYSTEMS CO + n- HEXANE AND N O + n-hexane I.Găinar*, Daniea Baa abtract: Eperimenta determination o a oubiitie o CO and N O in n-heane
ESTIMATION OF HENRY S CONSTANTS FROM HIGH RESSURE GAS SOLUBILITIES. THE SYSTEMS CO + n- HEXANE AND N O + n-hexane I.Găinar*, Daniea Baa abtract: Eperimenta determination o a oubiitie o CO and N O in n-heane
3.2 Terminal Characteristics of Junction Diodes (pp )
 /9/008 secton3_termnal_characterstcs_of_juncton_odes.doc /6 3. Termnal Characterstcs of Juncton odes (pp.47-53) A Juncton ode I.E., A real dode! Smlar to an deal dode, ts crcut symbol s: HO: The Juncton
/9/008 secton3_termnal_characterstcs_of_juncton_odes.doc /6 3. Termnal Characterstcs of Juncton odes (pp.47-53) A Juncton ode I.E., A real dode! Smlar to an deal dode, ts crcut symbol s: HO: The Juncton
Thermodynamics Lecture Series
 Therodynac Lecture Sere Dynac Enery Traner Heat, ork and Ma ppled Scence Educaton Reearch Group (SERG) Faculty o ppled Scence Unvert Teknolo MR Pure utance Properte o Pure Sutance- Revew CHPTER eal: drjjlanta@hotal.co
Therodynac Lecture Sere Dynac Enery Traner Heat, ork and Ma ppled Scence Educaton Reearch Group (SERG) Faculty o ppled Scence Unvert Teknolo MR Pure utance Properte o Pure Sutance- Revew CHPTER eal: drjjlanta@hotal.co
s much time does it take for the dog to run a distance of 10.0m
 ATTENTION: All Diviion I tudent, START HERE. All Diviion II tudent kip the firt 0 quetion, begin on #.. Of the following, which quantity i a vector? Energy (B) Ma Average peed (D) Temperature (E) Linear
ATTENTION: All Diviion I tudent, START HERE. All Diviion II tudent kip the firt 0 quetion, begin on #.. Of the following, which quantity i a vector? Energy (B) Ma Average peed (D) Temperature (E) Linear
Estimation of a proportion under a certain two-stage sampling design
 Etmaton of a roorton under a certan two-tage amng degn Danutė Kraavcatė nttute of athematc and nformatc Lthuana Stattc Lthuana Lthuana e-ma: raav@tmt Abtract The am of th aer to demontrate wth exame that
Etmaton of a roorton under a certan two-tage amng degn Danutė Kraavcatė nttute of athematc and nformatc Lthuana Stattc Lthuana Lthuana e-ma: raav@tmt Abtract The am of th aer to demontrate wth exame that
The Second Law of Thermodynamics (Chapter 4)
 The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
Revision: December 13, E Main Suite D Pullman, WA (509) Voice and Fax
 .9.1: AC power analyss Reson: Deceber 13, 010 15 E Man Sute D Pullan, WA 99163 (509 334 6306 Voce and Fax Oerew n chapter.9.0, we ntroduced soe basc quanttes relate to delery of power usng snusodal sgnals.
.9.1: AC power analyss Reson: Deceber 13, 010 15 E Man Sute D Pullan, WA 99163 (509 334 6306 Voce and Fax Oerew n chapter.9.0, we ntroduced soe basc quanttes relate to delery of power usng snusodal sgnals.
CE 374 K Hydrology. Evaporation. Daene C. McKinney
 C 374 Hydrology aporation Daene C. Mcinney aporation Terminology aporation: liquid ater pae directly to the apor phae Tranpiration: liquid ater pae from liquid to apor through plant metabolim Sublimation:
C 374 Hydrology aporation Daene C. Mcinney aporation Terminology aporation: liquid ater pae directly to the apor phae Tranpiration: liquid ater pae from liquid to apor through plant metabolim Sublimation:
Lecture 2 Thermodynamics and stability October 30, 2009
 The tablty of thn (oft) flm Lecture 2 Thermodynamc and tablty October 30, 2009 Ian Morron 2009 Revew of Lecture 1 The djonng preure a jump n preure at the boundary. It doe not vary between the plate. 1
The tablty of thn (oft) flm Lecture 2 Thermodynamc and tablty October 30, 2009 Ian Morron 2009 Revew of Lecture 1 The djonng preure a jump n preure at the boundary. It doe not vary between the plate. 1
PHYS 100 Worked Examples Week 05: Newton s 2 nd Law
 PHYS 00 Worked Eaple Week 05: ewton nd Law Poor Man Acceleroeter A drver hang an ar frehener fro ther rearvew rror wth a trng. When acceleratng onto the hghwa, the drver notce that the ar frehener ake
PHYS 00 Worked Eaple Week 05: ewton nd Law Poor Man Acceleroeter A drver hang an ar frehener fro ther rearvew rror wth a trng. When acceleratng onto the hghwa, the drver notce that the ar frehener ake
NUMERICAL DIFFERENTIATION
 NUMERICAL DIFFERENTIATION 1 Introducton Dfferentaton s a method to compute the rate at whch a dependent output y changes wth respect to the change n the ndependent nput x. Ths rate of change s called the
NUMERICAL DIFFERENTIATION 1 Introducton Dfferentaton s a method to compute the rate at whch a dependent output y changes wth respect to the change n the ndependent nput x. Ths rate of change s called the
Jacobians: Velocities and Static Force.
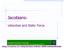 Jaoban: Veote and Stat Fore mrkabr Unerty o ehnoogy Computer Engneerng Inormaton ehnoogy Department http://e.aut.a.r/~hry/eture/robot-4/robot4.htm Derentaton o poton etor Derate o a etor: V Q d dt Q m
Jaoban: Veote and Stat Fore mrkabr Unerty o ehnoogy Computer Engneerng Inormaton ehnoogy Department http://e.aut.a.r/~hry/eture/robot-4/robot4.htm Derentaton o poton etor Derate o a etor: V Q d dt Q m
UNIT 15 RELIABILITY EVALUATION OF k-out-of-n AND STANDBY SYSTEMS
 UNIT 1 RELIABILITY EVALUATION OF k-out-of-n AND STANDBY SYSTEMS Structure 1.1 Introduction Objective 1.2 Redundancy 1.3 Reliability of k-out-of-n Sytem 1.4 Reliability of Standby Sytem 1. Summary 1.6 Solution/Anwer
UNIT 1 RELIABILITY EVALUATION OF k-out-of-n AND STANDBY SYSTEMS Structure 1.1 Introduction Objective 1.2 Redundancy 1.3 Reliability of k-out-of-n Sytem 1.4 Reliability of Standby Sytem 1. Summary 1.6 Solution/Anwer
Cyclic Codes BCH Codes
 Cycc Codes BCH Codes Gaos Feds GF m A Gaos fed of m eements can be obtaned usng the symbos 0,, á, and the eements beng 0,, á, á, á 3 m,... so that fed F* s cosed under mutpcaton wth m eements. The operator
Cycc Codes BCH Codes Gaos Feds GF m A Gaos fed of m eements can be obtaned usng the symbos 0,, á, and the eements beng 0,, á, á, á 3 m,... so that fed F* s cosed under mutpcaton wth m eements. The operator
PCI-697: DISTILLATION CONTROL. Department of Chemical Engineering KFUPM
 PCI-697: DISTILLATION CONTROL Department of Chemcal Engneerng KFUPM Topc: Dtllaton Prncple and Dynamc Dr. Houam Bnou Objectve of dtllaton control Operate n Safe, Stable Manner Operate Wthn Equpment Contrant
PCI-697: DISTILLATION CONTROL Department of Chemcal Engneerng KFUPM Topc: Dtllaton Prncple and Dynamc Dr. Houam Bnou Objectve of dtllaton control Operate n Safe, Stable Manner Operate Wthn Equpment Contrant
E-Companion: Mathematical Proofs
 E-omnon: Mthemtcl Poo Poo o emm : Pt DS Sytem y denton o t ey to vey tht t ncee n wth d ncee n We dene } ] : [ { M whee / We let the ttegy et o ech etle n DS e ]} [ ] [ : { M w whee M lge otve nume oth
E-omnon: Mthemtcl Poo Poo o emm : Pt DS Sytem y denton o t ey to vey tht t ncee n wth d ncee n We dene } ] : [ { M whee / We let the ttegy et o ech etle n DS e ]} [ ] [ : { M w whee M lge otve nume oth
a = f s,max /m = s g. 4. We first analyze the forces on the pig of mass m. The incline angle is.
 Chapter 6 1. The greatet deceleration (of magnitude a) i provided by the maximum friction force (Eq. 6-1, with = mg in thi cae). Uing ewton econd law, we find a = f,max /m = g. Eq. -16 then give the hortet
Chapter 6 1. The greatet deceleration (of magnitude a) i provided by the maximum friction force (Eq. 6-1, with = mg in thi cae). Uing ewton econd law, we find a = f,max /m = g. Eq. -16 then give the hortet
1. A 500-kilogram car is driving at 15 meters/second. What's its kinetic energy? How much does the car weigh?
 9. Solution Work & Energy Homework - KINETIC ENERGY. A 500-kilogram car i driing at 5 meter/econd. What' it kinetic energy? How much doe the car weigh? m= 500 kg 5 m/ Write Equation: Kinetic Energy = ½
9. Solution Work & Energy Homework - KINETIC ENERGY. A 500-kilogram car i driing at 5 meter/econd. What' it kinetic energy? How much doe the car weigh? m= 500 kg 5 m/ Write Equation: Kinetic Energy = ½
Section Induction motor drives
 Section 5.1 - nduction motor drive Electric Drive Sytem 5.1.1. ntroduction he AC induction motor i by far the mot widely ued motor in the indutry. raditionally, it ha been ued in contant and lowly variable-peed
Section 5.1 - nduction motor drive Electric Drive Sytem 5.1.1. ntroduction he AC induction motor i by far the mot widely ued motor in the indutry. raditionally, it ha been ued in contant and lowly variable-peed
III.9. THE HYSTERESIS CYCLE OF FERROELECTRIC SUBSTANCES
 III.9. THE HYSTERESIS CYCLE OF FERROELECTRIC SBSTANCES. Work purpoe The analyi of the behaviour of a ferroelectric ubtance placed in an eternal electric field; the dependence of the electrical polariation
III.9. THE HYSTERESIS CYCLE OF FERROELECTRIC SBSTANCES. Work purpoe The analyi of the behaviour of a ferroelectric ubtance placed in an eternal electric field; the dependence of the electrical polariation
CHAPTER 13. Exercises. E13.1 The emitter current is given by the Shockley equation:
 HPT 3 xercses 3. The emtter current s gen by the Shockley equaton: S exp VT For operaton wth, we hae exp >> S >>, and we can wrte VT S exp VT Solng for, we hae 3. 0 6ln 78.4 mv 0 0.784 5 4.86 V VT ln 4
HPT 3 xercses 3. The emtter current s gen by the Shockley equaton: S exp VT For operaton wth, we hae exp >> S >>, and we can wrte VT S exp VT Solng for, we hae 3. 0 6ln 78.4 mv 0 0.784 5 4.86 V VT ln 4
On the SO 2 Problem in Thermal Power Plants. 2.Two-steps chemical absorption modeling
 Internatonal Journal of Engneerng Reearch ISSN:39-689)(onlne),347-53(prnt) Volume No4, Iue No, pp : 557-56 Oct 5 On the SO Problem n Thermal Power Plant Two-tep chemcal aborpton modelng hr Boyadjev, P
Internatonal Journal of Engneerng Reearch ISSN:39-689)(onlne),347-53(prnt) Volume No4, Iue No, pp : 557-56 Oct 5 On the SO Problem n Thermal Power Plant Two-tep chemcal aborpton modelng hr Boyadjev, P
Quick Visit to Bernoulli Land
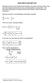 Although we have een the Bernoull equaton and een t derved before, th next note how t dervaton for an uncopreble & nvcd flow. The dervaton follow that of Kuethe &Chow ot cloely (I lke t better than Anderon).
Although we have een the Bernoull equaton and een t derved before, th next note how t dervaton for an uncopreble & nvcd flow. The dervaton follow that of Kuethe &Chow ot cloely (I lke t better than Anderon).
and Statistical Mechanics Material Properties
 Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
= 25 ohms, and line #2 has R c2
 Soution for Aignment #3.A tranmiion-ine circuit i driven by a tep-function generator with V = 0 vot and RS =0 ohm. ine # ha ength of = cm. Both ine have the ame peed of trave, u = 0 cm/n. ine # ha characteritic
Soution for Aignment #3.A tranmiion-ine circuit i driven by a tep-function generator with V = 0 vot and RS =0 ohm. ine # ha ength of = cm. Both ine have the ame peed of trave, u = 0 cm/n. ine # ha characteritic
Magnetism. 1 Paramagnetism. 2 Magnetic order. Introduction. Technology. Our first contact with magnetism. Plasma spectroscopy, Zeeman effect
 agnetm Introducton magnetm nothng new... Paramagnetm agnetc order Our frt contact wth magnetm Technoogy agnetc Reonance Imagng Pama pectrocopy, Zeeman effect Qu. What are the three ource of atomc magnetm
agnetm Introducton magnetm nothng new... Paramagnetm agnetc order Our frt contact wth magnetm Technoogy agnetc Reonance Imagng Pama pectrocopy, Zeeman effect Qu. What are the three ource of atomc magnetm
A Result on a Cyclic Polynomials
 Gen. Math. Note, Vol. 6, No., Feruary 05, pp. 59-65 ISSN 9-78 Copyrght ICSRS Pulcaton, 05.-cr.org Avalale free onlne at http:.geman.n A Reult on a Cyclc Polynomal S.A. Wahd Department of Mathematc & Stattc
Gen. Math. Note, Vol. 6, No., Feruary 05, pp. 59-65 ISSN 9-78 Copyrght ICSRS Pulcaton, 05.-cr.org Avalale free onlne at http:.geman.n A Reult on a Cyclc Polynomal S.A. Wahd Department of Mathematc & Stattc
One-sided finite-difference approximations suitable for use with Richardson extrapolation
 Journal of Computatonal Physcs 219 (2006) 13 20 Short note One-sded fnte-dfference approxmatons sutable for use wth Rchardson extrapolaton Kumar Rahul, S.N. Bhattacharyya * Department of Mechancal Engneerng,
Journal of Computatonal Physcs 219 (2006) 13 20 Short note One-sded fnte-dfference approxmatons sutable for use wth Rchardson extrapolaton Kumar Rahul, S.N. Bhattacharyya * Department of Mechancal Engneerng,
Chapter 5: Root Locus
 Chater 5: Root Locu ey condton for Plottng Root Locu g n G Gven oen-loo tranfer functon G Charactertc equaton n g,,.., n Magntude Condton and Arguent Condton 5-3 Rule for Plottng Root Locu 5.3. Rule Rule
Chater 5: Root Locu ey condton for Plottng Root Locu g n G Gven oen-loo tranfer functon G Charactertc equaton n g,,.., n Magntude Condton and Arguent Condton 5-3 Rule for Plottng Root Locu 5.3. Rule Rule
3. Be able to derive the chemical equilibrium constants from statistical mechanics.
 Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
376 CHAPTER 6. THE FREQUENCY-RESPONSE DESIGN METHOD. D(s) = we get the compensated system with :
 376 CHAPTER 6. THE FREQUENCY-RESPONSE DESIGN METHOD Therefore by applying the lead compenator with ome gain adjutment : D() =.12 4.5 +1 9 +1 we get the compenated ytem with : PM =65, ω c = 22 rad/ec, o
376 CHAPTER 6. THE FREQUENCY-RESPONSE DESIGN METHOD Therefore by applying the lead compenator with ome gain adjutment : D() =.12 4.5 +1 9 +1 we get the compenated ytem with : PM =65, ω c = 22 rad/ec, o
This appendix presents the derivations and proofs omitted from the main text.
 Onlne Appendx A Appendx: Omtted Dervaton and Proof Th appendx preent the dervaton and proof omtted from the man text A Omtted dervaton n Secton Mot of the analy provded n the man text Here, we formally
Onlne Appendx A Appendx: Omtted Dervaton and Proof Th appendx preent the dervaton and proof omtted from the man text A Omtted dervaton n Secton Mot of the analy provded n the man text Here, we formally
6.302 Feedback Systems Recitation 6: Steady-State Errors Prof. Joel L. Dawson S -
 6302 Feedback ytem Recitation 6: teadytate Error Prof Joel L Dawon A valid performance metric for any control ytem center around the final error when the ytem reache teadytate That i, after all initial
6302 Feedback ytem Recitation 6: teadytate Error Prof Joel L Dawon A valid performance metric for any control ytem center around the final error when the ytem reache teadytate That i, after all initial
Physics 5153 Classical Mechanics. Principle of Virtual Work-1
 P. Guterrez 1 Introducton Physcs 5153 Classcal Mechancs Prncple of Vrtual Work The frst varatonal prncple we encounter n mechancs s the prncple of vrtual work. It establshes the equlbrum condton of a mechancal
P. Guterrez 1 Introducton Physcs 5153 Classcal Mechancs Prncple of Vrtual Work The frst varatonal prncple we encounter n mechancs s the prncple of vrtual work. It establshes the equlbrum condton of a mechancal
Numerical Study of a Thermosyphon Cooling System: film condensation
 Numerica Study of a ermoypon Cooing Sytem: fim condenation Fiian Arbiyani epartment of Mecanica Engineering, Unierita Katoik Indoneia Atma Jaya, Jakarta, Indoneia farbiyani@atmajayaacid Abtract Studie
Numerica Study of a ermoypon Cooing Sytem: fim condenation Fiian Arbiyani epartment of Mecanica Engineering, Unierita Katoik Indoneia Atma Jaya, Jakarta, Indoneia farbiyani@atmajayaacid Abtract Studie
Chapter 3. Property Relations The essence of macroscopic thermodynamics Dependence of U, H, S, G, and F on T, P, V, etc.
 Chapter 3 Property Relations The essence of macroscopic thermodynamics Dependence of U, H, S, G, and F on T, P, V, etc. Concepts Energy functions F and G Chemical potential, µ Partial Molar properties
Chapter 3 Property Relations The essence of macroscopic thermodynamics Dependence of U, H, S, G, and F on T, P, V, etc. Concepts Energy functions F and G Chemical potential, µ Partial Molar properties
Prof. Dr. Ibraheem Nasser Examples_6 October 13, Review (Chapter 6)
 Prof. Dr. Ibraheem Naer Example_6 October 13, 017 Review (Chapter 6) cceleration of a loc againt Friction (1) cceleration of a bloc on horizontal urface When body i moving under application of force P,
Prof. Dr. Ibraheem Naer Example_6 October 13, 017 Review (Chapter 6) cceleration of a loc againt Friction (1) cceleration of a bloc on horizontal urface When body i moving under application of force P,
APPENDIX A Some Linear Algebra
 APPENDIX A Some Lnear Algebra The collecton of m, n matrces A.1 Matrces a 1,1,..., a 1,n A = a m,1,..., a m,n wth real elements a,j s denoted by R m,n. If n = 1 then A s called a column vector. Smlarly,
APPENDIX A Some Lnear Algebra The collecton of m, n matrces A.1 Matrces a 1,1,..., a 1,n A = a m,1,..., a m,n wth real elements a,j s denoted by R m,n. If n = 1 then A s called a column vector. Smlarly,
Thermodynamics II. Department of Chemical Engineering. Prof. Kim, Jong Hak
 Thermodynamcs II Department o Chemca ngneerng ro. Km, Jong Hak .5 Fugacty & Fugacty Coecent : ure Speces µ > provdes undamenta crteron or phase equbrum not easy to appy to sove probem Lmtaton o gn (.9
Thermodynamcs II Department o Chemca ngneerng ro. Km, Jong Hak .5 Fugacty & Fugacty Coecent : ure Speces µ > provdes undamenta crteron or phase equbrum not easy to appy to sove probem Lmtaton o gn (.9
Team. Outline. Statistics and Art: Sampling, Response Error, Mixed Models, Missing Data, and Inference
 Team Stattc and Art: Samplng, Repone Error, Mxed Model, Mng Data, and nference Ed Stanek Unverty of Maachuett- Amhert, USA 9/5/8 9/5/8 Outlne. Example: Doe-repone Model n Toxcology. ow to Predct Realzed
Team Stattc and Art: Samplng, Repone Error, Mxed Model, Mng Data, and nference Ed Stanek Unverty of Maachuett- Amhert, USA 9/5/8 9/5/8 Outlne. Example: Doe-repone Model n Toxcology. ow to Predct Realzed
1. The number of significant figures in the number is a. 4 b. 5 c. 6 d. 7
 Name: ID: Anwer Key There a heet o ueul ormulae and ome converon actor at the end. Crcle your anwer clearly. All problem are pont ecept a ew marked wth ther own core. Mamum core 100. There are a total
Name: ID: Anwer Key There a heet o ueul ormulae and ome converon actor at the end. Crcle your anwer clearly. All problem are pont ecept a ew marked wth ther own core. Mamum core 100. There are a total
Assignment 2. Tyler Shendruk February 19, 2010
 Assgnment yler Shendruk February 9, 00 Kadar Ch. Problem 8 We have an N N symmetrc matrx, M. he symmetry means M M and we ll say the elements of the matrx are m j. he elements are pulled from a probablty
Assgnment yler Shendruk February 9, 00 Kadar Ch. Problem 8 We have an N N symmetrc matrx, M. he symmetry means M M and we ll say the elements of the matrx are m j. he elements are pulled from a probablty
Massachusetts Institute of Technology Dynamics and Control II
 I E Maachuett Intitute of Technology Department of Mechanical Engineering 2.004 Dynamic and Control II Laboratory Seion 5: Elimination of Steady-State Error Uing Integral Control Action 1 Laboratory Objective:
I E Maachuett Intitute of Technology Department of Mechanical Engineering 2.004 Dynamic and Control II Laboratory Seion 5: Elimination of Steady-State Error Uing Integral Control Action 1 Laboratory Objective:
Thermodynamic Variables and Relations
 MME 231: Lecture 10 Thermodynamic Variables and Relations A. K. M. B. Rashid Professor, Department of MME BUET, Dhaka Today s Topics Thermodynamic relations derived from the Laws of Thermodynamics Definitions
MME 231: Lecture 10 Thermodynamic Variables and Relations A. K. M. B. Rashid Professor, Department of MME BUET, Dhaka Today s Topics Thermodynamic relations derived from the Laws of Thermodynamics Definitions
Social Studies 201 Notes for March 18, 2005
 1 Social Studie 201 Note for March 18, 2005 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
1 Social Studie 201 Note for March 18, 2005 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
Spring Force and Power
 Lecture 13 Chapter 9 Sprng Force and Power Yeah, energy s better than orces. What s net? Course webste: http://aculty.uml.edu/andry_danylov/teachng/physcsi IN THIS CHAPTER, you wll learn how to solve problems
Lecture 13 Chapter 9 Sprng Force and Power Yeah, energy s better than orces. What s net? Course webste: http://aculty.uml.edu/andry_danylov/teachng/physcsi IN THIS CHAPTER, you wll learn how to solve problems
NCAAPMT Calculus Challenge Challenge #3 Due: October 26, 2011
 NCAAPMT Calculu Challenge 011 01 Challenge #3 Due: October 6, 011 A Model of Traffic Flow Everyone ha at ome time been on a multi-lane highway and encountered road contruction that required the traffic
NCAAPMT Calculu Challenge 011 01 Challenge #3 Due: October 6, 011 A Model of Traffic Flow Everyone ha at ome time been on a multi-lane highway and encountered road contruction that required the traffic
Transfer Functions. Convenient representation of a linear, dynamic model. A transfer function (TF) relates one input and one output: ( ) system
 Transfer Functons Convenent representaton of a lnear, dynamc model. A transfer functon (TF) relates one nput and one output: x t X s y t system Y s The followng termnology s used: x y nput output forcng
Transfer Functons Convenent representaton of a lnear, dynamc model. A transfer functon (TF) relates one nput and one output: x t X s y t system Y s The followng termnology s used: x y nput output forcng
TMA4125 Matematikk 4N Spring 2016
 Norwegian Univerity of Science and Technology Department of Mathematical Science TMA45 Matematikk 4N Spring 6 Solution to problem et 6 In general, unle ele i noted, if f i a function, then F = L(f denote
Norwegian Univerity of Science and Technology Department of Mathematical Science TMA45 Matematikk 4N Spring 6 Solution to problem et 6 In general, unle ele i noted, if f i a function, then F = L(f denote
 Physical Chemistry Physical chemistry is the branch of chemistry that establishes and develops the principles of Chemistry in terms of the underlying concepts of Physics Physical Chemistry Main book: Atkins
Physical Chemistry Physical chemistry is the branch of chemistry that establishes and develops the principles of Chemistry in terms of the underlying concepts of Physics Physical Chemistry Main book: Atkins
UNITS FOR THERMOMECHANICS
 UNITS FOR THERMOMECHANICS 1. Conitent Unit. Every calculation require a conitent et of unit. Hitorically, one et of unit wa ued for mechanic and an apparently unrelated et of unit wa ued for heat. For
UNITS FOR THERMOMECHANICS 1. Conitent Unit. Every calculation require a conitent et of unit. Hitorically, one et of unit wa ued for mechanic and an apparently unrelated et of unit wa ued for heat. For
