Lecture 13. Thermodynamic Potentials (Ch. 5)
|
|
|
- Valentine Mitchell
- 6 years ago
- Views:
Transcription
1 Lecture 13. hermodynamic Potential (Ch. 5) So far we have been uing the total internal energy U and ometime the enthalpy H to characterize variou macrocopic ytem. hee function are called the thermodynamic potential: all the thermodynamic propertie of the ytem can be found by taking partial derivative of the P. For each P a et of o-called natural variable exit: d U d S P d + μ d d H d S + dp + μ d oday we ll introduce the other two thermodynamic potential: thehelmhotzfree energy F and Gibb free energy G. Depending on the type of a proce one of thee four thermodynamic potential provide the mot convenient decription (and i tabulated). All four function have unit of energy. Potential U (S) H (SP) F () G (P) ariable S S P P When conidering different type of procee we will be intereted in two main iue: what determine the tability of a ytem and how the ytem evolve toward an equilibrium; how much work can be extracted from a ytem.
2 Diffuive Equilibrium and Chemical Potential For completene let recall what we ve learned about the chemical potential. d U U A A S A For ub-ytem in diffuive equilibrium: P μ d S P d + μ d d S 1 d U + d d U S S U A A A A he meaning of the partial derivative (S/) U : let fix A and (the membrane poition i fixed) but aume that the membrane become permeable for ga molecule (exchange of both U and between the ubytem the molecule in A and are the ame ). 0 S A A U A A 0 S A A S S U μ S μ S U U S - the chemical potential A In equilibrium A μ A μ P A P U A Sign - : out of equilibrium the ytem with the larger S/ will get more particle. In other word particle will flow from from a high μ/ to a low μ/.
3 Chemical Potential of an Ideal ga S μ U U μ ha unit of energy: it an amount of energy we need to (uually) remove from the ytem after adding one particle in order to keep it total energy fixed. S Monatomic ideal ga: S( U ) k 4π m ln U 3h ln 3/ 5/ + 5 μ S k π m ln k h ln U 3/ k h 3 P ( ) ( ) 3/ 5/ πm k At normal and P μ for an ideal ga i negative (e.g. for He μ ~ J ~ e). Sign - : by adding particle to thi ytem we increae it entropy. o keep ds 0 we need to ubtract ome energy thu ΔU i negative. he chemical potential increae with with it preure. hu the molecule will flow from region of high denity to region of lower denity or from region of high preure to thoe of low preure. μ 0 μ when P increae ote that μ in thi cae i negative becaue S increae with n. hi i not alway the cae. For example for a ytem of fermion at 0 the entropy i zero (all the lowet tate are occupied) but adding one fermion to the ytem cot ome energy (the Fermi energy). hu μ 0 E > ( ) 0 F
4 he Quantum Concentration At 300K P10 5 Pa n << n Q. When n n Q the quantum tatitic come into play. 3/ k h m n Q π - the o-called quantum concentration (one particle per cube of ide equal to the thermal de roglie wavelength). When n << n Q (In the limit of low denitie) the ga i in the claical regime and μ<0. When n n Q μ 0 3/ 3 1 h mk n mk h p h d Q d λ λ Q n n k mk h n k k h m k ln ln ln 3/ 3/ π π μ where n/ i the concentration of particle
5 Iolated Sytem independent variable S and Advantage of U : it i conerved for an iolated ytem (it alo ha a imple phyical meaning the um of all the kin. and pot. energie of all the particle). In particular for an iolated ytem δq0 and du δw. Earlier by conidering the total differential of S a a function of variable U and we arrived at the thermodynamic identity for quaitatic procee : du ( S ) ds Pd + μd he combination of parameter on the right ide i equal to the exact differential of U. hi implie that the natural variable of U are S Conidering S and a independent variable: U U U du ( S ) ds d S + + S S d Since thee two equation for du mut yield the ame reult for any ds and d the correponding coefficient mut be the ame: U S U S P U S μ Again thi how that among everal macrocopic variable that characterize the ytem (P μ etc.) only three are independent the other variable can be found by taking partial derivative of the P with repect to it natural variable.
6 Iolated Sytem independent variable S and (cont.) Work i the tranfer of energy to a ytem by a change in the external parameter uch a volume magnetic and electric field gravitational potential etc. We can repreent δw a the um of two term a mechanical work on changing the volume of a ytem (an expanion work) - Pd and all other kind of work δw other (electrical work work on creating the urface area etc.): If the ytem comprie only olid and liquid we can uually aume d 0 and the difference between δw and δw other vanihe. For gae the difference may be very ignificant. initially the ytem i not necearily in equilibrium he energy balance for an iolated ytem : du ds Pd + δ Wother 0 (for fixed ) δ W other Pd ds δ W Pd + δ W other If we conider a quai-tatic proce (the ytem evolve from one equilibrium tate to the other) than ince for an iolated ytem δqds0 δ W other Pd
7 U A A S A U S Suppoe that the ytem i characterized by a parameter x which i free to vary (e.g. the ytem might conit of ice and water and x i the relative concentration of ice). y pontaneou procee the ytem will approach the table equilibrium (x x eq ) where S attain it abolute maximum. Equilibrium in Iolated Sytem For a thermally iolated ytem δq 0. If the volume i fixed then no work get done (δw 0) and the internal energy i conerved: While thi contraint i alway in place the ytem might be out of equilibrium (e.g. we move a piton that eparate two ub-ytem ee Figure). If the ytem i initially out of equilibrium then ome pontaneou procee will drive the ytem toward equilibrium. In a tate of table equilibrium no further pontaneou procee (other than ever-preent random fluctuation) can take place. he equilibrium tate correpond to the maximum multiplicity and maximum entropy. All microtate in equilibrium are equally acceible (the ytem i in one of thee microtate with equal probability). hi implie that in any of thee pontaneou procee the entropy tend to increae and the change of entropy atifie the condition ds 0 ( S ) eq max S U cont x eq x
8 Enthalpy (independent variable S and P) he volume i not the mot convenient independent variable. In the lab it i uually much eaier to control P than it i to control. o change the natural variable we can ue the following trick: ( S ) U ( S ) P U + ( U + P ) du + Pd dp dh d + du ds Pd dh ds + dp dh ( S P ) ds + dp + μd H (the enthalpy) i alo a thermodynamic potential with it natural variable S P and. - the internal energy of a ytem plu the work needed to make room for it at Pcont. he total differential of H in term of it independent variable : dh H H H S P ( S P ) ds + dp + d P S S P Comparion yield the relation: H S P H P S H S P μ In general if we conider procee with other work: dh ds + dp + δ W other
9 Procee at P cont δw other 0 For what kind of procee i H the mot convenient thermodynamic potential? At thi point we have to conider a ytem which i not iolated: it i in a thermal contact with a thermal reervoir. dh ds + dp + δ W δ Q + dp + δ other W other Let conider the P cont procee with purely expanion work (δw other 0) ( dh ) δ Q P δ W 0 For uch procee the change of enthalpy i equal to the thermal energy ( heat ) received by a ytem. other C P Q P H P For the procee with P cont and δ W other 0 the enthalpy play the ame part a the internal energy for the procee with cont and δw other 0. Example: the evaporation of liquid from an open veel i uch a proce becaue no effective work i done. he heat of vaporization i the enthalpy difference between the vapor phae and the liquid phae.
10 Sytem in Contact with a hermal Reervoir When we conider ytem in contact with a large thermal reervoir (a thermal bath there are two complication: (a) the energy in the ytem i no longer fixed (it may flow between the ytem and reervoir) and (b) in order to invetigate the tability of an equilibrium we need to conider the entropy of the combined ytem ( the ytem of interet+the reervoir) according to the nd Law thi total entropy hould be maximized. What hould be the ytem behavior in order to maximize the total entropy? For the ytem in contact with a eat bath we need to invent a better more ueful approach. he entropy along with and determine the ytem energy U U (S). Among the three variable the entropy i the mot difficult to control (the entropy-meter do not exit!). For an iolated ytem we have to work with the entropy it cannot be replaced with ome other function. And we did not want to do thi o far after all our approach to thermodynamic wa baed on thi concept. However for ytem in thermal contact with a reervoir we can replace the entropy with another more-convenient-to-work-with function. hi of coure doe not mean that we can get rid of entropy. We will be able to work with a different energy-like thermodynamic potential for which entropy i not one of the natural variable.
11 Helmholtz Free Energy (independ. variable and ) ( ) Pd Sd ds Sd Pd ds S U d S U F he natural variable for F are : ( ) d F d F d F df + + Comparion yield the relation: μ F P F S F Let do the trick (Legendre tranformation) again now to exclude S : ( ) ( ) S S U S U Helmholtz free energy can be rewritten a: S U F P + he firt term the energy preure i dominant in mot olid the econd term the entropy preure i dominant in gae. (For an ideal ga U doe not depend on and only the econd term urvive). P F F i the total energy needed to create the ytem minu the heat we can get for free from the environment at temperature. If we annihilate the ytem we can t recover all it U a work becaue we have to dipoe it entropy at a nonzero by dumping ome heat into the environment. ( ) d Pd Sd df μ +
12 S R+ he Minimum Free Energy Principle ( cont) he total energy of the combined ytem ( the ytem of interet+the reervoir) i U U R +U thi energy i to be hared between the reervoir and the ytem (we aume that and for all the ytem are fixed). Sharing i controlled by the maximum entropy principle: S ( U U ) S ( U U ) + S ( U ) max Since U ~ U R >> U S S U R+ R R U R ( U U ) S ( U ) + ( U ) + S ( U ) S ( U ) S S ( U ) R+ R reervoir +ytem R lo in S R due to tranferring U to the ytem ytem parameter only R F gain in S due to tranferring U to the ytem F table equilibrium U ytem U ds R+ du ( U U ) ds [ du ds ] df hu we can enforce the maximum entropy principle by imply minimizing the Helmholtz free energy of the ytem without having to know anything about the reervoir except that it maintain a fixed! Under thee condition (fixed and ) the maximum entropy principle of an iolated ytem i tranformed into a minimum Helmholtz free energy principle for a ytem in thermal contact with the thermal bath. 1
13 Procee at cont In general if we conider procee with other work: For the procee at cont (in thermal equilibrium with a large reervoir): df Sd Pd + δ ( df ) ( Pd + δ W other ) W other he total work performed on a ytem at cont in a reverible proce i equal to the change in the Helmholtz free energy of the ytem. In other word for the cont procee the Helmholtz free energy give all the reverible work. Problem: Conider a cylinder eparated into two part by an adiabatic piton. Compartment a and b each contain one mole of a monatomic ideal ga and their initial volume are ai 10l and bi 1l repectively. he cylinder whoe wall allow heat tranfer only i immered in a large bath at 0 0 C. he piton i now moving reveribly o that the final volume are af 6l and bi 5l. How much work i delivered by (or to) the ytem? U A A S A U S For one mole of monatomic ideal ga: df Pd he proce i iothermal : ( ) ( ) he work delivered by the ytem: F U S δ W ai δ W a + δ W bi b af dfa R R ln R ln + f ( m) 0 0 af bf 3 δ W R ln + R ln.6 10 J ai bf bi df b
14 Gibb Free Energy (independent variable and P) Let do the trick of Legendre tranformation again now to exclude both S and : ( S ) U ( P) S P U + G U S + P - the thermodynamic potential G i called the Gibb free energy. Let rewrite du in term of independent variable and P : ( P ) + dp d( U S + P ) Sd dp du ds Pd d( S) Sd d + dg ( P ) Sd + dp + μd Conidering P and a independent variable: dg G G G P ( P ) d + dp + d P P Comparion yield the relation: G P S G P G P μ
15 Gibb Free Energy and Chemical Potential Combining U S P + μ with G U S + P G μ - thi give u a new interpretation of the chemical potential: at leat for the ytem with only one type of particle the chemical potential i jut the Gibb free energy per particle. he chemical potential μ G P If we add one particle to a ytem holding and P fixed the Gibb free energy of the ytem will increae by μ. y adding more particle we do not change the value of μ ince we do not change the denity: μ μ(). ote that U H and F whoe differential alo have the term μd depend on non-linearly becaue in the procee with the independent variable (S) (SP) and () μ μ() might vary with.
16 Example: Pr.5.9. Sketch a qualitatively accurate graph of G v. for a pure ubtance a it change from olid to liquid to ga at fixed preure. G G P S - the lope of the graph G( ) at fixed P hould be S. hu the lope i alway negative and become teeper a and S increae. When a ubtance undergoe a phae tranformation it entropy increae abruptly o the lope of G( ) i dicontinuou at the tranition. S olid liquid ga G P S ΔG SΔ - thee equation allow computing Gibb free energie at non-tandard (if G i tabulated at a tandard ) olid liquid ga
17 S R+ G he Minimum Free Energy Principle (P cont) he total energy of the combined ytem (the ytem of interet+the reervoir) i U U R +U thi energy i to be hared between the reervoir and the ytem (we aume that P and for all the ytem are fixed). Sharing i controlled by the maximum entropy principle: ds table equilibrium R+ reervoir +ytem U ytem U du S P d ( U U ) S ( U U ) + S ( U ) max R+ R R ( U U ) ds [ du ds + Pd ] 1 dg hu we can enforce the maximum entropy principle by imply minimizing the Gibb free energy of the ytem without having to know anything about the reervoir except that it maintain a fixed! Under thee condition (fixed P and ) the maximum entropy principle of an iolated ytem i tranformed into a minimum Gibb free energy principle for a ytem in the thermal contact + mechanical equilibrium with the reervoir. ( ) 0 dg hu if a ytem whoe parameter P and are fixed i in thermal contact with a heat reervoir the table equilibrium i characterized by the condition: G/ i the net entropy cot that the reervoir pay for allowing the ytem to have volume and energy U which i why minimizing it maximize the total entropy of the whole combined ytem. P G min
18 Procee at P cont and cont Let conider the procee at P cont and cont in general including the procee with other work: hen dg δ W Pd + δ W other d( U S + P ) P ( δq Pd + δ Wother ) ( δ Q) P + ( δ Wother ) ds ( δ Wother ) P P P ds + Pd he other work performed on a ytem at cont and P cont in a reverible proce i equal to the change in the Gibb free energy of the ytem. In other word the Gibb free energy give all the reverible work except the P work. If the mechanical work i the only kind of work performed by a ytem the Gibb free energy i conerved: dg 0. Gibb Free Energy and the Spontaneity of Chemical Reaction he Gibb free energy i particularly ueful when we conider the chemical reaction at contant P and but the volume change a the reaction proceed. ΔG aociated with a chemical reaction i a ueful indicator of weather the reaction will proceed pontaneouly. Since the change in G i equal to the maximum ueful work which can be accomplihed by the reaction then a negative ΔG indicate that the reaction can happen pontaneouly. On the other hand if ΔG i poitive we need to upply the minimum other work δ W other ΔG to make the reaction go.
19 Electrolyi of Water y providing energy from a battery water can be diociated into the diatomic molecule of hydrogen and oxygen. Electrolyi i a (low) proce that i both iothermal and iobaric (P cont). he tank i filled with an electrolyte e.g. dilute ulfuric acid (we need ome ion to provide a current path) platinum electrode do not react with the acid. Diociation: H When I i paed through the cell H + move to the - electrode: he ulfate ion move to the + electrode: he um of the above tep: he electrical work required to decompoe 1 mole of water: (neglect the Joule heating of electrolyte) + SO4 H + SO4 + - H + e H SO4 + HO H SO4 + O + e 1 H O H + O 1 ΔW other ΔG G H + G O G HO ( ) ( ) ( ) In the able (p. 404) the Gibb free energy ΔG repreent the change in G upon forming 1 mole of the material tarting with element in their mot table pure tate: ΔG ( ) 0 ΔG( O ) 0 ΔG( H O) kj/mole H 37 Δ W other ΔG 37 kj/mole - + I I H O
20 Electrolyi of Water (cont.) ΔG 86 kj - ΔH 49 kj - ΔS 37 kj H + O H O Convenience of G: let conider the ame reaction but treat it in term of ΔU Δ and ΔS: Δ W other ΔG ΔU + PΔ ΔS PΔ: we will neglect the initial volume of water in comparion with the final volume of ga. y diociating 1 mole of water we ll get 1.5 mole of ga. he work by ga: ΔW P nr ( 1.5 mol)( 8.3 J/mol K)( 300 K) 3.7 kj -ΔS: the entropy of a mole of ubtance (from the ame able p.404) S(H )130.7 J/K S(O )05.1 J/K S(H O)69.9 J/K ΔS ΔU:???? not in the able... ( 300 K)( J/K J/K J/K) 49 kj Well we got ΔH in the able - ΔH(H ) 0 ΔH(O ) 0 ΔH(H O) kj (ΔH upon forming 1 mol of the material tarting with element in their mot table pure tate). 1
21 Electrolyi of Water (cont.) he proce mut provide the energy for the diociation plu the energy to expand the produced gae. oth of thoe are included in ΔH. Since the enthalpy H U+P the change in internal energy ΔU i then: ΔU ΔH - PΔ 86 kj - 4 kj 8 kj However it i not neceary to put in the whole amount in the form of electrical energy. Since the entropy increae in the proce of diociation the amount ΔS can be provided from the environment. Since the electrolyi reult in an increae in entropy the environment help the proce by contributing ΔS. ΔW he min. voltage required for electrolyi: * 37 kj I Δt Q ΔG e J/mole C/mole A (Pr. 5.4) 1.3 ( e) A I H + O H O Fuel cell Electrolyi 1 If < 0 the reaction will proceed from right to left provided gaeou hydrogen i available at the + electrode and gaeou oxygen at the - electrode. 0
22 Fuel Cell Hydrogen and oxygen can be combined in a fuel cell to produce electrical energy. FC differ from a battery in that the fuel (H and O ) i continuouly upplied. y running the proce of electrolyi in revere (controllable reaction between H and O ) one can extract 37 kj of electrical work for 1 mole of H conumed. he efficiency of an ideal fuel cell : (37 kj / 86 kj)x100% 83%! hi efficiency i far greater than the ideal efficiency of a heat engine that burn the hydrogen and ue the heat to power a generator. he entropy of the gae decreae by 49 kj/mol ince the number of water molecule i le than the number of H and O molecule combining. Since the total entropy cannot decreae in the reaction the exce entropy mut be expelled to the environment a heat.
23 G Fuel Cell at High Fuel cell operate at elevated temperature (from ~70 0 C to ~600 0 C). Our etimate ignored thi fact the value of ΔG in the able are given at room temperature. Pr which require an etimate of the maximum electric work done by the cell operating at 75 0 C how how one can etimate ΔG at different by uing partial derivative of G. G P At 75 0 C (348K): G G S ΔG SΔ - thee equation allow computing Gibb free energie at non-tandard and P: Δ Subtance ΔG(1bar 98K) kj/mol S(1bar 98K) J/K mol H O 0 05 ( H ) 0 ( 130 J/K)( 50 K) 6.5 kj ( O ) 0 ( 05 J/K)( 50 K) 10.5 kj ( H O) 37 kj ( 70 J/K)( 50 K) 40.5 kj 1 G G( O) G( H ) G( O ) 40.5 kj kj + 5.1kJ -8.9 kj H O H hu the maximum electrical work done by the cell i 9 kj about 3.5% le than the room-temperature value of 37 kj. Why the difference? he reacting gae have more entropy at higher temperature and we mut get rid of it by dumping wate heat into the environment.
24 Potential U (S) H (SP) F () G (P) ariable S S P P Concluion: du dh df dg ( S ) ds Pd + μd ( S P ) ds + dp + μd ( ) S d Pd + μd ( P ) S d + dp + μd
Bogoliubov Transformation in Classical Mechanics
 Bogoliubov Tranformation in Claical Mechanic Canonical Tranformation Suppoe we have a et of complex canonical variable, {a j }, and would like to conider another et of variable, {b }, b b ({a j }). How
Bogoliubov Tranformation in Claical Mechanic Canonical Tranformation Suppoe we have a et of complex canonical variable, {a j }, and would like to conider another et of variable, {b }, b b ({a j }). How
SOLUTIONS
 SOLUTIONS Topic-2 RAOULT S LAW, ALICATIONS AND NUMERICALS VERY SHORT ANSWER QUESTIONS 1. Define vapour preure? An: When a liquid i in equilibrium with it own vapour the preure exerted by the vapour on
SOLUTIONS Topic-2 RAOULT S LAW, ALICATIONS AND NUMERICALS VERY SHORT ANSWER QUESTIONS 1. Define vapour preure? An: When a liquid i in equilibrium with it own vapour the preure exerted by the vapour on
NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor
 NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor T o T T o T F o, Q o F T m,q m T m T m T mo Aumption: 1. Homogeneou Sytem 2. Single Reaction 3. Steady State Two type of problem: 1. Given deired
NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor T o T T o T F o, Q o F T m,q m T m T m T mo Aumption: 1. Homogeneou Sytem 2. Single Reaction 3. Steady State Two type of problem: 1. Given deired
THE THERMOELASTIC SQUARE
 HE HERMOELASIC SQUARE A mnemonic for remembering thermodynamic identitie he tate of a material i the collection of variable uch a tre, train, temperature, entropy. A variable i a tate variable if it integral
HE HERMOELASIC SQUARE A mnemonic for remembering thermodynamic identitie he tate of a material i the collection of variable uch a tre, train, temperature, entropy. A variable i a tate variable if it integral
Fermi Distribution Function. n(e) T = 0 T > 0 E F
 LECTURE 3 Maxwell{Boltzmann, Fermi, and Boe Statitic Suppoe we have a ga of N identical point particle in a box ofvolume V. When we ay \ga", we mean that the particle are not interacting with one another.
LECTURE 3 Maxwell{Boltzmann, Fermi, and Boe Statitic Suppoe we have a ga of N identical point particle in a box ofvolume V. When we ay \ga", we mean that the particle are not interacting with one another.
Correction for Simple System Example and Notes on Laplace Transforms / Deviation Variables ECHE 550 Fall 2002
 Correction for Simple Sytem Example and Note on Laplace Tranform / Deviation Variable ECHE 55 Fall 22 Conider a tank draining from an initial height of h o at time t =. With no flow into the tank (F in
Correction for Simple Sytem Example and Note on Laplace Tranform / Deviation Variable ECHE 55 Fall 22 Conider a tank draining from an initial height of h o at time t =. With no flow into the tank (F in
Green-Kubo formulas with symmetrized correlation functions for quantum systems in steady states: the shear viscosity of a fluid in a steady shear flow
 Green-Kubo formula with ymmetrized correlation function for quantum ytem in teady tate: the hear vicoity of a fluid in a teady hear flow Hirohi Matuoa Department of Phyic, Illinoi State Univerity, Normal,
Green-Kubo formula with ymmetrized correlation function for quantum ytem in teady tate: the hear vicoity of a fluid in a teady hear flow Hirohi Matuoa Department of Phyic, Illinoi State Univerity, Normal,
Chemistry You Need to Know and Ohio Science Standards. Antacids. Chpt 2-- Chpt 3-- Chpt 5-- Soap. Chpt 4-- Light. Airbags. Section 4-2.
 Ohio Standard Batterie 9th grade 1. Recognize that all atom of the ame element contain the ame number of proton, and element with the ame number of proton may or may not have the ame ma. Thoe with different
Ohio Standard Batterie 9th grade 1. Recognize that all atom of the ame element contain the ame number of proton, and element with the ame number of proton may or may not have the ame ma. Thoe with different
Lecture 8: Period Finding: Simon s Problem over Z N
 Quantum Computation (CMU 8-859BB, Fall 205) Lecture 8: Period Finding: Simon Problem over Z October 5, 205 Lecturer: John Wright Scribe: icola Rech Problem A mentioned previouly, period finding i a rephraing
Quantum Computation (CMU 8-859BB, Fall 205) Lecture 8: Period Finding: Simon Problem over Z October 5, 205 Lecturer: John Wright Scribe: icola Rech Problem A mentioned previouly, period finding i a rephraing
1 year n0tes chemistry new st CHAPTER 7 THERMOCHEMISTRY MCQs Q.1 Which of the following statements is contrary to the first law of thermodynamics?
 year n0te chemitry new t CHAPTER 7 THERMOCHEMISTRY MCQ Q.1 Which of the following tatement i contrary to the firt law of thermodynamic? (a) energy can neither be created nor detroyed (b) one form of energy
year n0te chemitry new t CHAPTER 7 THERMOCHEMISTRY MCQ Q.1 Which of the following tatement i contrary to the firt law of thermodynamic? (a) energy can neither be created nor detroyed (b) one form of energy
Singular perturbation theory
 Singular perturbation theory Marc R. Rouel June 21, 2004 1 Introduction When we apply the teady-tate approximation (SSA) in chemical kinetic, we typically argue that ome of the intermediate are highly
Singular perturbation theory Marc R. Rouel June 21, 2004 1 Introduction When we apply the teady-tate approximation (SSA) in chemical kinetic, we typically argue that ome of the intermediate are highly
MAE320-HW7A. 1b). The entropy of an isolated system increases during a process. A). sometimes B). always C). never D).
 MAE0-W7A The homework i due Monday, November 4, 06. Each problem i worth the point indicated. Copying o the olution rom another i not acceptable. (). Multiple choice (0 point) a). Which tatement i invalid
MAE0-W7A The homework i due Monday, November 4, 06. Each problem i worth the point indicated. Copying o the olution rom another i not acceptable. (). Multiple choice (0 point) a). Which tatement i invalid
Molecular Dynamics Simulations of Nonequilibrium Effects Associated with Thermally Activated Exothermic Reactions
 Original Paper orma, 5, 9 7, Molecular Dynamic Simulation of Nonequilibrium Effect ociated with Thermally ctivated Exothermic Reaction Jerzy GORECKI and Joanna Natalia GORECK Intitute of Phyical Chemitry,
Original Paper orma, 5, 9 7, Molecular Dynamic Simulation of Nonequilibrium Effect ociated with Thermally ctivated Exothermic Reaction Jerzy GORECKI and Joanna Natalia GORECK Intitute of Phyical Chemitry,
FUNDAMENTALS OF POWER SYSTEMS
 1 FUNDAMENTALS OF POWER SYSTEMS 1 Chapter FUNDAMENTALS OF POWER SYSTEMS INTRODUCTION The three baic element of electrical engineering are reitor, inductor and capacitor. The reitor conume ohmic or diipative
1 FUNDAMENTALS OF POWER SYSTEMS 1 Chapter FUNDAMENTALS OF POWER SYSTEMS INTRODUCTION The three baic element of electrical engineering are reitor, inductor and capacitor. The reitor conume ohmic or diipative
Lecture 21. The Lovasz splitting-off lemma Topics in Combinatorial Optimization April 29th, 2004
 18.997 Topic in Combinatorial Optimization April 29th, 2004 Lecture 21 Lecturer: Michel X. Goeman Scribe: Mohammad Mahdian 1 The Lovaz plitting-off lemma Lovaz plitting-off lemma tate the following. Theorem
18.997 Topic in Combinatorial Optimization April 29th, 2004 Lecture 21 Lecturer: Michel X. Goeman Scribe: Mohammad Mahdian 1 The Lovaz plitting-off lemma Lovaz plitting-off lemma tate the following. Theorem
Chapter 3- Answers to selected exercises
 Chater 3- Anwer to elected exercie. he chemical otential of a imle uid of a ingle comonent i gien by the exreion o ( ) + k B ln o ( ) ; where i the temerature, i the reure, k B i the Boltzmann contant,
Chater 3- Anwer to elected exercie. he chemical otential of a imle uid of a ingle comonent i gien by the exreion o ( ) + k B ln o ( ) ; where i the temerature, i the reure, k B i the Boltzmann contant,
Solutions to exercises week 45 FYS2160
 Solution to exercie week 45 FYS2160 Kritian Bjørke, Knut Oddvar Høie Vadla November 29, 2017 Schroeder 5.23 a) Writing Φ = U T S µn in term of infiniteimal change of the quantitie involved: dφ = du T ds
Solution to exercie week 45 FYS2160 Kritian Bjørke, Knut Oddvar Høie Vadla November 29, 2017 Schroeder 5.23 a) Writing Φ = U T S µn in term of infiniteimal change of the quantitie involved: dφ = du T ds
Solving Differential Equations by the Laplace Transform and by Numerical Methods
 36CH_PHCalter_TechMath_95099 3//007 :8 PM Page Solving Differential Equation by the Laplace Tranform and by Numerical Method OBJECTIVES When you have completed thi chapter, you hould be able to: Find the
36CH_PHCalter_TechMath_95099 3//007 :8 PM Page Solving Differential Equation by the Laplace Tranform and by Numerical Method OBJECTIVES When you have completed thi chapter, you hould be able to: Find the
A Single Particle Thermal Model for Lithium Ion Batteries
 A Single Particle Thermal Model for Lithium Ion Batterie R. Painter* 1, B. Berryhill 1, L. Sharpe 2 and S. Keith Hargrove 2 1 Civil Engineering, Tenneee State Univerity, Nahville, TN, USA 2 Mechanical
A Single Particle Thermal Model for Lithium Ion Batterie R. Painter* 1, B. Berryhill 1, L. Sharpe 2 and S. Keith Hargrove 2 1 Civil Engineering, Tenneee State Univerity, Nahville, TN, USA 2 Mechanical
III.9. THE HYSTERESIS CYCLE OF FERROELECTRIC SUBSTANCES
 III.9. THE HYSTERESIS CYCLE OF FERROELECTRIC SBSTANCES. Work purpoe The analyi of the behaviour of a ferroelectric ubtance placed in an eternal electric field; the dependence of the electrical polariation
III.9. THE HYSTERESIS CYCLE OF FERROELECTRIC SBSTANCES. Work purpoe The analyi of the behaviour of a ferroelectric ubtance placed in an eternal electric field; the dependence of the electrical polariation
V = 4 3 πr3. d dt V = d ( 4 dv dt. = 4 3 π d dt r3 dv π 3r2 dv. dt = 4πr 2 dr
 0.1 Related Rate In many phyical ituation we have a relationhip between multiple quantitie, and we know the rate at which one of the quantitie i changing. Oftentime we can ue thi relationhip a a convenient
0.1 Related Rate In many phyical ituation we have a relationhip between multiple quantitie, and we know the rate at which one of the quantitie i changing. Oftentime we can ue thi relationhip a a convenient
Entropy Minimization in Design of Extractive Distillation System with Internal Heat Exchangers
 Entropy Minimization in Deign of Extractive Ditillation Sytem with Internal Heat Exchanger Diego F. Mendoza, Carlo.M. Riaco * Group of Proce Sytem Engineering, Department of Chemical and Environmental
Entropy Minimization in Deign of Extractive Ditillation Sytem with Internal Heat Exchanger Diego F. Mendoza, Carlo.M. Riaco * Group of Proce Sytem Engineering, Department of Chemical and Environmental
4. The following graphs were prepared from experimental data for a reactant, A. What is the correct order of A?
 Diebolt Summer 010 CHM 15 HOUR EXAM I KEY (15 pt) Part One: Multiple Choice. 1. Conider the reaction IO 3 + 5I + 6H + 3I + 3H O. If the rate of diappearance of I i 9.010-3 M/, what i the rate of appearance
Diebolt Summer 010 CHM 15 HOUR EXAM I KEY (15 pt) Part One: Multiple Choice. 1. Conider the reaction IO 3 + 5I + 6H + 3I + 3H O. If the rate of diappearance of I i 9.010-3 M/, what i the rate of appearance
2 States of a System. 2.1 States / Configurations 2.2 Probabilities of States. 2.3 Counting States 2.4 Entropy of an ideal gas
 2 State of a Sytem Motly chap 1 and 2 of Kittel &Kroemer 2.1 State / Configuration 2.2 Probabilitie of State Fundamental aumption Entropy 2.3 Counting State 2.4 Entropy of an ideal ga Phyic 112 (S2012)
2 State of a Sytem Motly chap 1 and 2 of Kittel &Kroemer 2.1 State / Configuration 2.2 Probabilitie of State Fundamental aumption Entropy 2.3 Counting State 2.4 Entropy of an ideal ga Phyic 112 (S2012)
Chapter 2 Sampling and Quantization. In order to investigate sampling and quantization, the difference between analog
 Chapter Sampling and Quantization.1 Analog and Digital Signal In order to invetigate ampling and quantization, the difference between analog and digital ignal mut be undertood. Analog ignal conit of continuou
Chapter Sampling and Quantization.1 Analog and Digital Signal In order to invetigate ampling and quantization, the difference between analog and digital ignal mut be undertood. Analog ignal conit of continuou
7.2 INVERSE TRANSFORMS AND TRANSFORMS OF DERIVATIVES 281
 72 INVERSE TRANSFORMS AND TRANSFORMS OF DERIVATIVES 28 and i 2 Show how Euler formula (page 33) can then be ued to deduce the reult a ( a) 2 b 2 {e at co bt} {e at in bt} b ( a) 2 b 2 5 Under what condition
72 INVERSE TRANSFORMS AND TRANSFORMS OF DERIVATIVES 28 and i 2 Show how Euler formula (page 33) can then be ued to deduce the reult a ( a) 2 b 2 {e at co bt} {e at in bt} b ( a) 2 b 2 5 Under what condition
Chapter 13. Root Locus Introduction
 Chapter 13 Root Locu 13.1 Introduction In the previou chapter we had a glimpe of controller deign iue through ome imple example. Obviouly when we have higher order ytem, uch imple deign technique will
Chapter 13 Root Locu 13.1 Introduction In the previou chapter we had a glimpe of controller deign iue through ome imple example. Obviouly when we have higher order ytem, uch imple deign technique will
Homework Problem Set 8 Solutions
 Chemistry 360 Dr. Jean M. Standard Homework roblem Set 8 Solutions. Starting from G = H S, derive the fundamental equation for G. o begin, we take the differential of G, dg = dh d( S) = dh ds Sd. Next,
Chemistry 360 Dr. Jean M. Standard Homework roblem Set 8 Solutions. Starting from G = H S, derive the fundamental equation for G. o begin, we take the differential of G, dg = dh d( S) = dh ds Sd. Next,
From what we know now (i.e, ΔH and ΔS) How do we determine whether a reaction is spontaneous?
 pontaneous Rxns A&G-1 From what we know now (i.e, Δ and Δ) ow do we determine whether a reaction is spontaneous? But Δ and Δ are not enough... here is competition between lowering energy and raising entropy!
pontaneous Rxns A&G-1 From what we know now (i.e, Δ and Δ) ow do we determine whether a reaction is spontaneous? But Δ and Δ are not enough... here is competition between lowering energy and raising entropy!
Source slideplayer.com/fundamentals of Analytical Chemistry, F.J. Holler, S.R.Crouch. Chapter 6: Random Errors in Chemical Analysis
 Source lideplayer.com/fundamental of Analytical Chemitry, F.J. Holler, S.R.Crouch Chapter 6: Random Error in Chemical Analyi Random error are preent in every meaurement no matter how careful the experimenter.
Source lideplayer.com/fundamental of Analytical Chemitry, F.J. Holler, S.R.Crouch Chapter 6: Random Error in Chemical Analyi Random error are preent in every meaurement no matter how careful the experimenter.
Chapter 4. The Laplace Transform Method
 Chapter 4. The Laplace Tranform Method The Laplace Tranform i a tranformation, meaning that it change a function into a new function. Actually, it i a linear tranformation, becaue it convert a linear combination
Chapter 4. The Laplace Tranform Method The Laplace Tranform i a tranformation, meaning that it change a function into a new function. Actually, it i a linear tranformation, becaue it convert a linear combination
arxiv:cond-mat/ v1 [cond-mat.stat-mech] 2 Jul 2005
![arxiv:cond-mat/ v1 [cond-mat.stat-mech] 2 Jul 2005 arxiv:cond-mat/ v1 [cond-mat.stat-mech] 2 Jul 2005](/thumbs/74/70856830.jpg) hermodynamic of n Ideal Generalized Ga: I hermodynamic Law B. H. Lavenda Univeritá degli Studi, Camerino 603 (MC) Italy (Dated: February, 008) arxiv:cond-mat/0507047v1 [cond-mat.tat-mech] Jul 005 he equation
hermodynamic of n Ideal Generalized Ga: I hermodynamic Law B. H. Lavenda Univeritá degli Studi, Camerino 603 (MC) Italy (Dated: February, 008) arxiv:cond-mat/0507047v1 [cond-mat.tat-mech] Jul 005 he equation
Digital Control System
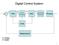 Digital Control Sytem - A D D A Micro ADC DAC Proceor Correction Element Proce Clock Meaurement A: Analog D: Digital Continuou Controller and Digital Control Rt - c Plant yt Continuou Controller Digital
Digital Control Sytem - A D D A Micro ADC DAC Proceor Correction Element Proce Clock Meaurement A: Analog D: Digital Continuou Controller and Digital Control Rt - c Plant yt Continuou Controller Digital
EE Control Systems LECTURE 14
 Updated: Tueday, March 3, 999 EE 434 - Control Sytem LECTURE 4 Copyright FL Lewi 999 All right reerved ROOT LOCUS DESIGN TECHNIQUE Suppoe the cloed-loop tranfer function depend on a deign parameter k We
Updated: Tueday, March 3, 999 EE 434 - Control Sytem LECTURE 4 Copyright FL Lewi 999 All right reerved ROOT LOCUS DESIGN TECHNIQUE Suppoe the cloed-loop tranfer function depend on a deign parameter k We
1. The F-test for Equality of Two Variances
 . The F-tet for Equality of Two Variance Previouly we've learned how to tet whether two population mean are equal, uing data from two independent ample. We can alo tet whether two population variance are
. The F-tet for Equality of Two Variance Previouly we've learned how to tet whether two population mean are equal, uing data from two independent ample. We can alo tet whether two population variance are
The Outline. Fermi degenerate gas, repetition and specific heat calculations. ideal gas with Gibbs sum (grand sum) Gibbs free energy, definition
 The Outline ermi degenerate ga, repetition and pecific heat calculation ideal ga with Gibb um (grand um) Gibb free energy, definition Gibb free energy and chemical potential chemical reaction, concentration
The Outline ermi degenerate ga, repetition and pecific heat calculation ideal ga with Gibb um (grand um) Gibb free energy, definition Gibb free energy and chemical potential chemical reaction, concentration
Physics 2212 G Quiz #2 Solutions Spring 2018
 Phyic 2212 G Quiz #2 Solution Spring 2018 I. (16 point) A hollow inulating phere ha uniform volume charge denity ρ, inner radiu R, and outer radiu 3R. Find the magnitude of the electric field at a ditance
Phyic 2212 G Quiz #2 Solution Spring 2018 I. (16 point) A hollow inulating phere ha uniform volume charge denity ρ, inner radiu R, and outer radiu 3R. Find the magnitude of the electric field at a ditance
two equations that govern the motion of the fluid through some medium, like a pipe. These two equations are the
 Fluid and Fluid Mechanic Fluid in motion Dynamic Equation of Continuity After having worked on fluid at ret we turn to a moving fluid To decribe a moving fluid we develop two equation that govern the motion
Fluid and Fluid Mechanic Fluid in motion Dynamic Equation of Continuity After having worked on fluid at ret we turn to a moving fluid To decribe a moving fluid we develop two equation that govern the motion
KNOWN: Air undergoes a polytropic process in a piston-cylinder assembly. The work is known.
 PROBLEM.7 A hown in Fig. P.7, 0 ft of air at T = 00 o R, 00 lbf/in. undergoe a polytropic expanion to a final preure of 5.4 lbf/in. The proce follow pv. = contant. The work i W = 94.4 Btu. Auming ideal
PROBLEM.7 A hown in Fig. P.7, 0 ft of air at T = 00 o R, 00 lbf/in. undergoe a polytropic expanion to a final preure of 5.4 lbf/in. The proce follow pv. = contant. The work i W = 94.4 Btu. Auming ideal
EP225 Note No. 5 Mechanical Waves
 EP5 Note No. 5 Mechanical Wave 5. Introduction Cacade connection of many ma-pring unit conitute a medium for mechanical wave which require that medium tore both kinetic energy aociated with inertia (ma)
EP5 Note No. 5 Mechanical Wave 5. Introduction Cacade connection of many ma-pring unit conitute a medium for mechanical wave which require that medium tore both kinetic energy aociated with inertia (ma)
Laplace Transformation
 Univerity of Technology Electromechanical Department Energy Branch Advance Mathematic Laplace Tranformation nd Cla Lecture 6 Page of 7 Laplace Tranformation Definition Suppoe that f(t) i a piecewie continuou
Univerity of Technology Electromechanical Department Energy Branch Advance Mathematic Laplace Tranformation nd Cla Lecture 6 Page of 7 Laplace Tranformation Definition Suppoe that f(t) i a piecewie continuou
Problem Set 8 Solutions
 Deign and Analyi of Algorithm April 29, 2015 Maachuett Intitute of Technology 6.046J/18.410J Prof. Erik Demaine, Srini Devada, and Nancy Lynch Problem Set 8 Solution Problem Set 8 Solution Thi problem
Deign and Analyi of Algorithm April 29, 2015 Maachuett Intitute of Technology 6.046J/18.410J Prof. Erik Demaine, Srini Devada, and Nancy Lynch Problem Set 8 Solution Problem Set 8 Solution Thi problem
Linear Motion, Speed & Velocity
 Add Important Linear Motion, Speed & Velocity Page: 136 Linear Motion, Speed & Velocity NGSS Standard: N/A MA Curriculum Framework (006): 1.1, 1. AP Phyic 1 Learning Objective: 3.A.1.1, 3.A.1.3 Knowledge/Undertanding
Add Important Linear Motion, Speed & Velocity Page: 136 Linear Motion, Speed & Velocity NGSS Standard: N/A MA Curriculum Framework (006): 1.1, 1. AP Phyic 1 Learning Objective: 3.A.1.1, 3.A.1.3 Knowledge/Undertanding
1 Routh Array: 15 points
 EE C28 / ME34 Problem Set 3 Solution Fall 2 Routh Array: 5 point Conider the ytem below, with D() k(+), w(t), G() +2, and H y() 2 ++2 2(+). Find the cloed loop tranfer function Y () R(), and range of k
EE C28 / ME34 Problem Set 3 Solution Fall 2 Routh Array: 5 point Conider the ytem below, with D() k(+), w(t), G() +2, and H y() 2 ++2 2(+). Find the cloed loop tranfer function Y () R(), and range of k
Given the following circuit with unknown initial capacitor voltage v(0): X(s) Immediately, we know that the transfer function H(s) is
 EE 4G Note: Chapter 6 Intructor: Cheung More about ZSR and ZIR. Finding unknown initial condition: Given the following circuit with unknown initial capacitor voltage v0: F v0/ / Input xt 0Ω Output yt -
EE 4G Note: Chapter 6 Intructor: Cheung More about ZSR and ZIR. Finding unknown initial condition: Given the following circuit with unknown initial capacitor voltage v0: F v0/ / Input xt 0Ω Output yt -
Control Systems Analysis and Design by the Root-Locus Method
 6 Control Sytem Analyi and Deign by the Root-Locu Method 6 1 INTRODUCTION The baic characteritic of the tranient repone of a cloed-loop ytem i cloely related to the location of the cloed-loop pole. If
6 Control Sytem Analyi and Deign by the Root-Locu Method 6 1 INTRODUCTION The baic characteritic of the tranient repone of a cloed-loop ytem i cloely related to the location of the cloed-loop pole. If
Design spacecraft external surfaces to ensure 95 percent probability of no mission-critical failures from particle impact.
 PREFERRED RELIABILITY PAGE 1 OF 6 PRACTICES METEOROIDS & SPACE DEBRIS Practice: Deign pacecraft external urface to enure 95 percent probability of no miion-critical failure from particle impact. Benefit:
PREFERRED RELIABILITY PAGE 1 OF 6 PRACTICES METEOROIDS & SPACE DEBRIS Practice: Deign pacecraft external urface to enure 95 percent probability of no miion-critical failure from particle impact. Benefit:
Feedback Control Systems (FCS)
 Feedback Control Sytem (FCS) Lecture19-20 Routh-Herwitz Stability Criterion Dr. Imtiaz Huain email: imtiaz.huain@faculty.muet.edu.pk URL :http://imtiazhuainkalwar.weebly.com/ Stability of Higher Order
Feedback Control Sytem (FCS) Lecture19-20 Routh-Herwitz Stability Criterion Dr. Imtiaz Huain email: imtiaz.huain@faculty.muet.edu.pk URL :http://imtiazhuainkalwar.weebly.com/ Stability of Higher Order
Math 273 Solutions to Review Problems for Exam 1
 Math 7 Solution to Review Problem for Exam True or Fale? Circle ONE anwer for each Hint: For effective tudy, explain why if true and give a counterexample if fale (a) T or F : If a b and b c, then a c
Math 7 Solution to Review Problem for Exam True or Fale? Circle ONE anwer for each Hint: For effective tudy, explain why if true and give a counterexample if fale (a) T or F : If a b and b c, then a c
MODERN CONTROL SYSTEMS
 MODERN CONTROL SYSTEMS Lecture 1 Root Locu Emam Fathy Department of Electrical and Control Engineering email: emfmz@aat.edu http://www.aat.edu/cv.php?dip_unit=346&er=68525 1 Introduction What i root locu?
MODERN CONTROL SYSTEMS Lecture 1 Root Locu Emam Fathy Department of Electrical and Control Engineering email: emfmz@aat.edu http://www.aat.edu/cv.php?dip_unit=346&er=68525 1 Introduction What i root locu?
Comparing Means: t-tests for Two Independent Samples
 Comparing ean: t-tet for Two Independent Sample Independent-eaure Deign t-tet for Two Independent Sample Allow reearcher to evaluate the mean difference between two population uing data from two eparate
Comparing ean: t-tet for Two Independent Sample Independent-eaure Deign t-tet for Two Independent Sample Allow reearcher to evaluate the mean difference between two population uing data from two eparate
SOLUTION MANUAL CHAPTER 12
 SOLUION MANUAL CHAPER CONEN SUBSECION PROB NO. In-ext Concept Quetion a-g Concept problem - Brayton cycle, ga turbine - Regenerator, Intercooler, nonideal cycle 5-9 Ericon cycle 0- Jet engine cycle -5
SOLUION MANUAL CHAPER CONEN SUBSECION PROB NO. In-ext Concept Quetion a-g Concept problem - Brayton cycle, ga turbine - Regenerator, Intercooler, nonideal cycle 5-9 Ericon cycle 0- Jet engine cycle -5
Automatic Control Systems. Part III: Root Locus Technique
 www.pdhcenter.com PDH Coure E40 www.pdhonline.org Automatic Control Sytem Part III: Root Locu Technique By Shih-Min Hu, Ph.D., P.E. Page of 30 www.pdhcenter.com PDH Coure E40 www.pdhonline.org VI. Root
www.pdhcenter.com PDH Coure E40 www.pdhonline.org Automatic Control Sytem Part III: Root Locu Technique By Shih-Min Hu, Ph.D., P.E. Page of 30 www.pdhcenter.com PDH Coure E40 www.pdhonline.org VI. Root
NCAAPMT Calculus Challenge Challenge #3 Due: October 26, 2011
 NCAAPMT Calculu Challenge 011 01 Challenge #3 Due: October 6, 011 A Model of Traffic Flow Everyone ha at ome time been on a multi-lane highway and encountered road contruction that required the traffic
NCAAPMT Calculu Challenge 011 01 Challenge #3 Due: October 6, 011 A Model of Traffic Flow Everyone ha at ome time been on a multi-lane highway and encountered road contruction that required the traffic
CHAPTER 8 OBSERVER BASED REDUCED ORDER CONTROLLER DESIGN FOR LARGE SCALE LINEAR DISCRETE-TIME CONTROL SYSTEMS
 CHAPTER 8 OBSERVER BASED REDUCED ORDER CONTROLLER DESIGN FOR LARGE SCALE LINEAR DISCRETE-TIME CONTROL SYSTEMS 8.1 INTRODUCTION 8.2 REDUCED ORDER MODEL DESIGN FOR LINEAR DISCRETE-TIME CONTROL SYSTEMS 8.3
CHAPTER 8 OBSERVER BASED REDUCED ORDER CONTROLLER DESIGN FOR LARGE SCALE LINEAR DISCRETE-TIME CONTROL SYSTEMS 8.1 INTRODUCTION 8.2 REDUCED ORDER MODEL DESIGN FOR LINEAR DISCRETE-TIME CONTROL SYSTEMS 8.3
Non-linearity parameter B=A of binary liquid mixtures at elevated pressures
 PRAMANA cfl Indian Academy of Science Vol. 55, No. 3 journal of September 2000 phyic pp. 433 439 Non-linearity parameter B=A of binary liquid mixture at elevated preure J D PANDEY, J CHHABRA, R DEY, V
PRAMANA cfl Indian Academy of Science Vol. 55, No. 3 journal of September 2000 phyic pp. 433 439 Non-linearity parameter B=A of binary liquid mixture at elevated preure J D PANDEY, J CHHABRA, R DEY, V
μ + = σ = D 4 σ = D 3 σ = σ = All units in parts (a) and (b) are in V. (1) x chart: Center = μ = 0.75 UCL =
 Our online Tutor are available 4*7 to provide Help with Proce control ytem Homework/Aignment or a long term Graduate/Undergraduate Proce control ytem Project. Our Tutor being experienced and proficient
Our online Tutor are available 4*7 to provide Help with Proce control ytem Homework/Aignment or a long term Graduate/Undergraduate Proce control ytem Project. Our Tutor being experienced and proficient
March 18, 2014 Academic Year 2013/14
 POLITONG - SHANGHAI BASIC AUTOMATIC CONTROL Exam grade March 8, 4 Academic Year 3/4 NAME (Pinyin/Italian)... STUDENT ID Ue only thee page (including the back) for anwer. Do not ue additional heet. Ue of
POLITONG - SHANGHAI BASIC AUTOMATIC CONTROL Exam grade March 8, 4 Academic Year 3/4 NAME (Pinyin/Italian)... STUDENT ID Ue only thee page (including the back) for anwer. Do not ue additional heet. Ue of
Blackbody radiation. Main radiation laws. Sun as an energy source. Solar spectrum and solar constant.
 Lecture 3. lackbody radiation. Main radiation law. Sun a an energy ource. Solar pectrum and olar contant. Objective:. Concept of a blackbody, thermodynamical equilibrium, and local thermodynamical equilibrium..
Lecture 3. lackbody radiation. Main radiation law. Sun a an energy ource. Solar pectrum and olar contant. Objective:. Concept of a blackbody, thermodynamical equilibrium, and local thermodynamical equilibrium..
Social Studies 201 Notes for November 14, 2003
 1 Social Studie 201 Note for November 14, 2003 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
1 Social Studie 201 Note for November 14, 2003 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
into a discrete time function. Recall that the table of Laplace/z-transforms is constructed by (i) selecting to get
 Lecture 25 Introduction to Some Matlab c2d Code in Relation to Sampled Sytem here are many way to convert a continuou time function, { h( t) ; t [0, )} into a dicrete time function { h ( k) ; k {0,,, }}
Lecture 25 Introduction to Some Matlab c2d Code in Relation to Sampled Sytem here are many way to convert a continuou time function, { h( t) ; t [0, )} into a dicrete time function { h ( k) ; k {0,,, }}
EC381/MN308 Probability and Some Statistics. Lecture 7 - Outline. Chapter Cumulative Distribution Function (CDF) Continuous Random Variables
 EC38/MN38 Probability and Some Statitic Yanni Pachalidi yannip@bu.edu, http://ionia.bu.edu/ Lecture 7 - Outline. Continuou Random Variable Dept. of Manufacturing Engineering Dept. of Electrical and Computer
EC38/MN38 Probability and Some Statitic Yanni Pachalidi yannip@bu.edu, http://ionia.bu.edu/ Lecture 7 - Outline. Continuou Random Variable Dept. of Manufacturing Engineering Dept. of Electrical and Computer
Social Studies 201 Notes for March 18, 2005
 1 Social Studie 201 Note for March 18, 2005 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
1 Social Studie 201 Note for March 18, 2005 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
UNITS FOR THERMOMECHANICS
 UNITS FOR THERMOMECHANICS 1. Conitent Unit. Every calculation require a conitent et of unit. Hitorically, one et of unit wa ued for mechanic and an apparently unrelated et of unit wa ued for heat. For
UNITS FOR THERMOMECHANICS 1. Conitent Unit. Every calculation require a conitent et of unit. Hitorically, one et of unit wa ued for mechanic and an apparently unrelated et of unit wa ued for heat. For
Chapter 4 Total Entropy Cannot Decrease 2012/5/6
 Chapter 4 Total Entropy Cannot Decreae "Ice melting" - a claic example of entropy increaing It happen all the time! Nothing you can do About it. Spontaneou and irreverible. It i approaching to an equilibrium
Chapter 4 Total Entropy Cannot Decreae "Ice melting" - a claic example of entropy increaing It happen all the time! Nothing you can do About it. Spontaneou and irreverible. It i approaching to an equilibrium
Root Locus Diagram. Root loci: The portion of root locus when k assume positive values: that is 0
 Objective Root Locu Diagram Upon completion of thi chapter you will be able to: Plot the Root Locu for a given Tranfer Function by varying gain of the ytem, Analye the tability of the ytem from the root
Objective Root Locu Diagram Upon completion of thi chapter you will be able to: Plot the Root Locu for a given Tranfer Function by varying gain of the ytem, Analye the tability of the ytem from the root
Chemistry 6A F2007. Dr. J.A. Mack. Titration Curves: 11/30/07. What do I need to bring? Exam 3: Friday 12/7/07 (here in lecture)
 Chemitry 6A F2007 Dr. J.A. Mack Eam : Friday 12/7/07 (here in lecture) What will be covered on the eam? Chapter 6: 6.9-6.15 Chapter 7: All Chapter 8: All Chapter 9: 9.1-9.9 Any thing from lab a well What
Chemitry 6A F2007 Dr. J.A. Mack Eam : Friday 12/7/07 (here in lecture) What will be covered on the eam? Chapter 6: 6.9-6.15 Chapter 7: All Chapter 8: All Chapter 9: 9.1-9.9 Any thing from lab a well What
Physics 741 Graduate Quantum Mechanics 1 Solutions to Final Exam, Fall 2014
 Phyic 7 Graduate Quantum Mechanic Solution to inal Eam all 0 Each quetion i worth 5 point with point for each part marked eparately Some poibly ueful formula appear at the end of the tet In four dimenion
Phyic 7 Graduate Quantum Mechanic Solution to inal Eam all 0 Each quetion i worth 5 point with point for each part marked eparately Some poibly ueful formula appear at the end of the tet In four dimenion
Sample Question Solutions for the Chemistry of Environment Topic Exam
 Name (Lat, Firt): ID Number: Sample Quetion Solution for the Chemitry of Environment Topic Exam 1. The earth tratophere contain a region of high ozone (O3) concentration called the ozone layer. The ozone
Name (Lat, Firt): ID Number: Sample Quetion Solution for the Chemitry of Environment Topic Exam 1. The earth tratophere contain a region of high ozone (O3) concentration called the ozone layer. The ozone
Reading assignment: In this chapter we will cover Sections Definition and the Laplace transform of simple functions
 Chapter 4 Laplace Tranform 4 Introduction Reading aignment: In thi chapter we will cover Section 4 45 4 Definition and the Laplace tranform of imple function Given f, a function of time, with value f(t
Chapter 4 Laplace Tranform 4 Introduction Reading aignment: In thi chapter we will cover Section 4 45 4 Definition and the Laplace tranform of imple function Given f, a function of time, with value f(t
Thermal Resistance Measurements and Thermal Transient Analysis of Power Chip Slug-Up and Slug-Down Mounted on HDI Substrate
 Intl Journal of Microcircuit and Electronic Packaging Thermal Reitance Meaurement and Thermal Tranient Analyi of Power Chip Slug-Up and Slug-Down Mounted on HDI Subtrate Claudio Sartori Magneti Marelli
Intl Journal of Microcircuit and Electronic Packaging Thermal Reitance Meaurement and Thermal Tranient Analyi of Power Chip Slug-Up and Slug-Down Mounted on HDI Subtrate Claudio Sartori Magneti Marelli
One Class of Splitting Iterative Schemes
 One Cla of Splitting Iterative Scheme v Ciegi and V. Pakalnytė Vilniu Gedimina Technical Univerity Saulėtekio al. 11, 2054, Vilniu, Lithuania rc@fm.vtu.lt Abtract. Thi paper deal with the tability analyi
One Cla of Splitting Iterative Scheme v Ciegi and V. Pakalnytė Vilniu Gedimina Technical Univerity Saulėtekio al. 11, 2054, Vilniu, Lithuania rc@fm.vtu.lt Abtract. Thi paper deal with the tability analyi
Annex-A: RTTOV9 Cloud validation
 RTTOV-91 Science and Validation Plan Annex-A: RTTOV9 Cloud validation Author O Embury C J Merchant The Univerity of Edinburgh Intitute for Atmo. & Environ. Science Crew Building King Building Edinburgh
RTTOV-91 Science and Validation Plan Annex-A: RTTOV9 Cloud validation Author O Embury C J Merchant The Univerity of Edinburgh Intitute for Atmo. & Environ. Science Crew Building King Building Edinburgh
/University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2009
 Lecture 0 /6/09 /Univerity of Wahington Department of Chemitry Chemitry 453 Winter Quarter 009. Wave Function and Molecule Can quantum mechanic explain the tructure of molecule by determining wave function
Lecture 0 /6/09 /Univerity of Wahington Department of Chemitry Chemitry 453 Winter Quarter 009. Wave Function and Molecule Can quantum mechanic explain the tructure of molecule by determining wave function
MATEMATIK Datum: Tid: eftermiddag. A.Heintz Telefonvakt: Anders Martinsson Tel.:
 MATEMATIK Datum: 20-08-25 Tid: eftermiddag GU, Chalmer Hjälpmedel: inga A.Heintz Telefonvakt: Ander Martinon Tel.: 073-07926. Löningar till tenta i ODE och matematik modellering, MMG5, MVE6. Define what
MATEMATIK Datum: 20-08-25 Tid: eftermiddag GU, Chalmer Hjälpmedel: inga A.Heintz Telefonvakt: Ander Martinon Tel.: 073-07926. Löningar till tenta i ODE och matematik modellering, MMG5, MVE6. Define what
Lecture 9: Shor s Algorithm
 Quantum Computation (CMU 8-859BB, Fall 05) Lecture 9: Shor Algorithm October 7, 05 Lecturer: Ryan O Donnell Scribe: Sidhanth Mohanty Overview Let u recall the period finding problem that wa et up a a function
Quantum Computation (CMU 8-859BB, Fall 05) Lecture 9: Shor Algorithm October 7, 05 Lecturer: Ryan O Donnell Scribe: Sidhanth Mohanty Overview Let u recall the period finding problem that wa et up a a function
Reading assignment: In this chapter we will cover Sections Definition and the Laplace transform of simple functions
 Chapter 4 Laplace Tranform 4 Introduction Reading aignment: In thi chapter we will cover Section 4 45 4 Definition and the Laplace tranform of imple function Given f, a function of time, with value f(t
Chapter 4 Laplace Tranform 4 Introduction Reading aignment: In thi chapter we will cover Section 4 45 4 Definition and the Laplace tranform of imple function Given f, a function of time, with value f(t
New bounds for Morse clusters
 New bound for More cluter Tamá Vinkó Advanced Concept Team, European Space Agency, ESTEC Keplerlaan 1, 2201 AZ Noordwijk, The Netherland Tama.Vinko@ea.int and Arnold Neumaier Fakultät für Mathematik, Univerität
New bound for More cluter Tamá Vinkó Advanced Concept Team, European Space Agency, ESTEC Keplerlaan 1, 2201 AZ Noordwijk, The Netherland Tama.Vinko@ea.int and Arnold Neumaier Fakultät für Mathematik, Univerität
Moment of Inertia of an Equilateral Triangle with Pivot at one Vertex
 oment of nertia of an Equilateral Triangle with Pivot at one Vertex There are two wa (at leat) to derive the expreion f an equilateral triangle that i rotated about one vertex, and ll how ou both here.
oment of nertia of an Equilateral Triangle with Pivot at one Vertex There are two wa (at leat) to derive the expreion f an equilateral triangle that i rotated about one vertex, and ll how ou both here.
A novel protocol for linearization of the Poisson-Boltzmann equation
 Ann. Univ. Sofia, Fac. Chem. Pharm. 16 (14) 59-64 [arxiv 141.118] A novel protocol for linearization of the Poion-Boltzmann equation Roumen Tekov Department of Phyical Chemitry, Univerity of Sofia, 1164
Ann. Univ. Sofia, Fac. Chem. Pharm. 16 (14) 59-64 [arxiv 141.118] A novel protocol for linearization of the Poion-Boltzmann equation Roumen Tekov Department of Phyical Chemitry, Univerity of Sofia, 1164
ON THE APPROXIMATION ERROR IN HIGH DIMENSIONAL MODEL REPRESENTATION. Xiaoqun Wang
 Proceeding of the 2008 Winter Simulation Conference S. J. Maon, R. R. Hill, L. Mönch, O. Roe, T. Jefferon, J. W. Fowler ed. ON THE APPROXIMATION ERROR IN HIGH DIMENSIONAL MODEL REPRESENTATION Xiaoqun Wang
Proceeding of the 2008 Winter Simulation Conference S. J. Maon, R. R. Hill, L. Mönch, O. Roe, T. Jefferon, J. W. Fowler ed. ON THE APPROXIMATION ERROR IN HIGH DIMENSIONAL MODEL REPRESENTATION Xiaoqun Wang
Dimensional Analysis A Tool for Guiding Mathematical Calculations
 Dimenional Analyi A Tool for Guiding Mathematical Calculation Dougla A. Kerr Iue 1 February 6, 2010 ABSTRACT AND INTRODUCTION In converting quantitie from one unit to another, we may know the applicable
Dimenional Analyi A Tool for Guiding Mathematical Calculation Dougla A. Kerr Iue 1 February 6, 2010 ABSTRACT AND INTRODUCTION In converting quantitie from one unit to another, we may know the applicable
Pulsed Magnet Crimping
 Puled Magnet Crimping Fred Niell 4/5/00 1 Magnetic Crimping Magnetoforming i a metal fabrication technique that ha been in ue for everal decade. A large capacitor bank i ued to tore energy that i ued to
Puled Magnet Crimping Fred Niell 4/5/00 1 Magnetic Crimping Magnetoforming i a metal fabrication technique that ha been in ue for everal decade. A large capacitor bank i ued to tore energy that i ued to
Observing Condensations in Atomic Fermi Gases
 Oberving Condenation in Atomic Fermi Gae (Term Eay for 498ESM, Spring 2004) Ruqing Xu Department of Phyic, UIUC (May 6, 2004) Abtract Oberving condenation in a ga of fermion ha been another intereting
Oberving Condenation in Atomic Fermi Gae (Term Eay for 498ESM, Spring 2004) Ruqing Xu Department of Phyic, UIUC (May 6, 2004) Abtract Oberving condenation in a ga of fermion ha been another intereting
Convective Heat Transfer
 Convective Heat Tranfer Example 1. Melt Spinning of Polymer fiber 2. Heat tranfer in a Condener 3. Temperature control of a Re-entry vehicle Fiber pinning The fiber pinning proce preent a unique engineering
Convective Heat Tranfer Example 1. Melt Spinning of Polymer fiber 2. Heat tranfer in a Condener 3. Temperature control of a Re-entry vehicle Fiber pinning The fiber pinning proce preent a unique engineering
s much time does it take for the dog to run a distance of 10.0m
 ATTENTION: All Diviion I tudent, START HERE. All Diviion II tudent kip the firt 0 quetion, begin on #.. Of the following, which quantity i a vector? Energy (B) Ma Average peed (D) Temperature (E) Linear
ATTENTION: All Diviion I tudent, START HERE. All Diviion II tudent kip the firt 0 quetion, begin on #.. Of the following, which quantity i a vector? Energy (B) Ma Average peed (D) Temperature (E) Linear
1. The reaction rate is defined as the change in concentration of a reactant or product per unit time. Consider the general reaction:
 CHAPTER TWELVE CHEMICAL KINETICS For Review. The reaction rate i defined a the change in concentration of a reactant or product per unit time. Conider the general reaction: A Product where rate = Δ Δt
CHAPTER TWELVE CHEMICAL KINETICS For Review. The reaction rate i defined a the change in concentration of a reactant or product per unit time. Conider the general reaction: A Product where rate = Δ Δt
WELL-POSEDNESS OF A ONE-DIMENSIONAL PLASMA MODEL WITH A HYPERBOLIC FIELD
 WELL-POSEDNESS OF A ONE-DIMENSIONAL PLASMA MODEL WITH A HYPERBOLIC FIELD JENNIFER RAE ANDERSON 1. Introduction A plama i a partially or completely ionized ga. Nearly all (approximately 99.9%) of the matter
WELL-POSEDNESS OF A ONE-DIMENSIONAL PLASMA MODEL WITH A HYPERBOLIC FIELD JENNIFER RAE ANDERSON 1. Introduction A plama i a partially or completely ionized ga. Nearly all (approximately 99.9%) of the matter
Chapter 10. Closed-Loop Control Systems
 hapter 0 loed-loop ontrol Sytem ontrol Diagram of a Typical ontrol Loop Actuator Sytem F F 2 T T 2 ontroller T Senor Sytem T TT omponent and Signal of a Typical ontrol Loop F F 2 T Air 3-5 pig 4-20 ma
hapter 0 loed-loop ontrol Sytem ontrol Diagram of a Typical ontrol Loop Actuator Sytem F F 2 T T 2 ontroller T Senor Sytem T TT omponent and Signal of a Typical ontrol Loop F F 2 T Air 3-5 pig 4-20 ma
Exam 2 Solutions. for a gas obeying the equation of state. Z = PV m RT = 1 + BP + CP 2,
 Chemistry 360 Dr. Jean M. Standard Fall 016 Name KEY 1.) (14 points) Determine # H & % ( $ ' Exam Solutions for a gas obeying the equation of state Z = V m R = 1 + B + C, where B and C are constants. Since
Chemistry 360 Dr. Jean M. Standard Fall 016 Name KEY 1.) (14 points) Determine # H & % ( $ ' Exam Solutions for a gas obeying the equation of state Z = V m R = 1 + B + C, where B and C are constants. Since
SIMON FRASER UNIVERSITY School of Engineering Science ENSC 320 Electric Circuits II. Solutions to Assignment 3 February 2005.
 SIMON FRASER UNIVERSITY School of Engineering Science ENSC 320 Electric Circuit II Solution to Aignment 3 February 2005. Initial Condition Source 0 V battery witch flip at t 0 find i 3 (t) Component value:
SIMON FRASER UNIVERSITY School of Engineering Science ENSC 320 Electric Circuit II Solution to Aignment 3 February 2005. Initial Condition Source 0 V battery witch flip at t 0 find i 3 (t) Component value:
ME 375 FINAL EXAM SOLUTIONS Friday December 17, 2004
 ME 375 FINAL EXAM SOLUTIONS Friday December 7, 004 Diviion Adam 0:30 / Yao :30 (circle one) Name Intruction () Thi i a cloed book eamination, but you are allowed three 8.5 crib heet. () You have two hour
ME 375 FINAL EXAM SOLUTIONS Friday December 7, 004 Diviion Adam 0:30 / Yao :30 (circle one) Name Intruction () Thi i a cloed book eamination, but you are allowed three 8.5 crib heet. () You have two hour
Suggested Answers To Exercises. estimates variability in a sampling distribution of random means. About 68% of means fall
 Beyond Significance Teting ( nd Edition), Rex B. Kline Suggeted Anwer To Exercie Chapter. The tatitic meaure variability among core at the cae level. In a normal ditribution, about 68% of the core fall
Beyond Significance Teting ( nd Edition), Rex B. Kline Suggeted Anwer To Exercie Chapter. The tatitic meaure variability among core at the cae level. In a normal ditribution, about 68% of the core fall
Design By Emulation (Indirect Method)
 Deign By Emulation (Indirect Method he baic trategy here i, that Given a continuou tranfer function, it i required to find the bet dicrete equivalent uch that the ignal produced by paing an input ignal
Deign By Emulation (Indirect Method he baic trategy here i, that Given a continuou tranfer function, it i required to find the bet dicrete equivalent uch that the ignal produced by paing an input ignal
Chapter Landscape of an Optimization Problem. Local Search. Coping With NP-Hardness. Gradient Descent: Vertex Cover
 Coping With NP-Hardne Chapter 12 Local Search Q Suppoe I need to olve an NP-hard problem What hould I do? A Theory ay you're unlikely to find poly-time algorithm Mut acrifice one of three deired feature
Coping With NP-Hardne Chapter 12 Local Search Q Suppoe I need to olve an NP-hard problem What hould I do? A Theory ay you're unlikely to find poly-time algorithm Mut acrifice one of three deired feature
5.5 Application of Frequency Response: Signal Filters
 44 Dynamic Sytem Second order lowpa filter having tranfer function H()=H ()H () u H () H () y Firt order lowpa filter Figure 5.5: Contruction of a econd order low-pa filter by combining two firt order
44 Dynamic Sytem Second order lowpa filter having tranfer function H()=H ()H () u H () H () y Firt order lowpa filter Figure 5.5: Contruction of a econd order low-pa filter by combining two firt order
Conduction Heat transfer: Unsteady state
 Conduction Heat tranfer: Unteady tate Chapter Objective For olving the ituation that Where temperature do not change with poition. In a imple lab geometry where temperature vary alo with poition. Near
Conduction Heat tranfer: Unteady tate Chapter Objective For olving the ituation that Where temperature do not change with poition. In a imple lab geometry where temperature vary alo with poition. Near
Chapter 5 Consistency, Zero Stability, and the Dahlquist Equivalence Theorem
 Chapter 5 Conitency, Zero Stability, and the Dahlquit Equivalence Theorem In Chapter 2 we dicued convergence of numerical method and gave an experimental method for finding the rate of convergence (aka,
Chapter 5 Conitency, Zero Stability, and the Dahlquit Equivalence Theorem In Chapter 2 we dicued convergence of numerical method and gave an experimental method for finding the rate of convergence (aka,
CHAPTER 4 DESIGN OF STATE FEEDBACK CONTROLLERS AND STATE OBSERVERS USING REDUCED ORDER MODEL
 98 CHAPTER DESIGN OF STATE FEEDBACK CONTROLLERS AND STATE OBSERVERS USING REDUCED ORDER MODEL INTRODUCTION The deign of ytem uing tate pace model for the deign i called a modern control deign and it i
98 CHAPTER DESIGN OF STATE FEEDBACK CONTROLLERS AND STATE OBSERVERS USING REDUCED ORDER MODEL INTRODUCTION The deign of ytem uing tate pace model for the deign i called a modern control deign and it i
Massachusetts Institute of Technology Dynamics and Control II
 I E Maachuett Intitute of Technology Department of Mechanical Engineering 2.004 Dynamic and Control II Laboratory Seion 5: Elimination of Steady-State Error Uing Integral Control Action 1 Laboratory Objective:
I E Maachuett Intitute of Technology Department of Mechanical Engineering 2.004 Dynamic and Control II Laboratory Seion 5: Elimination of Steady-State Error Uing Integral Control Action 1 Laboratory Objective:
