1. The reaction rate is defined as the change in concentration of a reactant or product per unit time. Consider the general reaction:
|
|
|
- Philomena Flowers
- 5 years ago
- Views:
Transcription
1 CHAPTER TWELVE CHEMICAL KINETICS For Review. The reaction rate i defined a the change in concentration of a reactant or product per unit time. Conider the general reaction: A Product where rate = Δ Δt If we graph v. t, it would uually loo lie the dar line in the following plot. a b c t t time An intantaneou rate i the lope of a tangent line to the graph of v. t. We can determine the intantaneou rate at any time during the reaction. On the plot, tangent line at t and t = t are drawn. The lope of thee tangent line would be the intantaneou rate at t and t = t. We call the intantaneou rate at t the initial rate. The average rate i meaured over a period of time. For example, the lope of the line connecting point a and c i the average rate of the reaction over the entire length of time to t (average rate = Δ/Δt). An average rate i determined over ome time period while an intantaneou rate i determined at one pecific time. The rate which i larget i generally the initial rate. At t, the lope of the tangent line i greatet, which mean the rate i larget at t. The initial rate i ued by convention o that the rate of reaction only depend on the forward reaction; at t, the revere reaction i inignificant becaue no product are preent yet. 8
2 CHAPTER CHEMICAL KINETICS 9. The differential rate law decribe the dependence of the rate on the concentration of reactant. The integrated rate law expree reactant concentration a a function of time. The differential rate law i generally jut called the rate law. The rate contant i a contant that allow one to equate the rate of a reaction to the concentration of reactant. The order i the exponent that the reactant concentration are raied to in the rate equation.. The method of initial rate ue the reult from everal experiment where each experiment i carried out at a different et of initial reactant concentration and the initial rate i determined. The reult of the experiment are compared to ee how the initial rate depend on the initial concentration. If poible, two experiment are compared where only one reactant concentration change. For thee two experiment, any change in the initial rate mut be due to the change in that one reactant concentration. The reult of the experiment are compared until all of the order are determined. After the order are determined, then one can go bac to any (or all) of the experiment and et the initial rate equal to the rate law uing the concentration in that experiment. The only unnown i which i then olved for. The unit on depend on the reactant and order in the rate law. Becaue there are many different rate law, there are many different unit for. Rate = n ; For a firt-order rate law, n =. If i tripled, then the rate i tripled. When i quadrupled (increaed by a factor of four), and the rate increae by a factor of 6, then A mut be econd order ( = 6). For a third order reaction, a i doubled, the rate will increae by a factor of = 8. For a zero order reaction, the rate i independent of the concentration of A. The only tipulation for zero order reaction i that the reactant or reactant mut be preent; if they are, then the rate i a contant value (rate = ).. Zero order: d dt, d dt t t t t [ A] t, t t, t = t + Firt order: d dt, t d t dt t ln[ A] t, ln t ln t, ln t = t + ln Second order: d dt, t t d dt t t, t, t [A [ A] t t ]
3 CHAPTER CHEMICAL KINETICS 5. The integrated rate law can be put into the equation for a traight line, y = mx + b where x and y are the x and y axe, m i the lope of the line and b i the y-intercept. Zero order: = t + y = mx + b A plot of v. time will be linear with a negative lope equal to and a y-intercept equal to. Firt order: ln = t + ln y = mx + b A plot of ln v. time will be linear with a negative lope equal to and a y-intercept equal to ln. Second order: = t + y = mx + b A plot of / v. time will be linear with a poitive lope equal to and a y-intercept equal to /. When two or more reactant are tudied, only one of the reactant i allowed to change during any one experiment. Thi i accomplihed by having a large exce of the other reactant() a compared to the reactant tudied; o large that the concentration of the other reactant() tay effectively contant during the experiment. The lope of the traight-line plot equal (or ) time the other reactant concentration raied to the correct order. Once all the order are nown for a reaction, then any (or all) of the lope can be ued to determine. 6. At t = t /, = / ; Plugging thee term into the integrated rate law yield the following half-life expreion: zero order firt order econd order [ A] ln t / = t / = t / = The firt order half-life i independent of concentration, the zero order half-life i directly related to the concentration, and the econd order half-life i inverely related to concentration. For a firt order reaction, if the firt half-life equal., the econd half-life will alo be. becaue the half-life for a firt order reaction i concentration independent. The econd half-life for a zero order reaction will be /(.) =.. Thi i becaue the half-life for a zero order reaction ha a direct relationhip with concentration (a the concentration decreae by a factor of, the half-life decreae by a factor of ). For a econd order reaction which ha an invere relationhip between t / and, the econd half-life will be. (twice the firt half-life value). 7. a. An elementary tep (reaction) i one for which the rate law can be written from the molecularity, i.e., from coefficient in the balanced equation.
4 CHAPTER CHEMICAL KINETICS b. The molecularity i the number of pecie that mut collide to produce the reaction repreented by an elementary tep in a reaction mechanim. c. The mechanim of a reaction i the erie of propoed elementary reaction that may occur to give the overall reaction. The um of all the tep in the mechanim give the balanced chemical reaction. d. An intermediate i a pecie that i neither a reactant nor a product but that i formed and conumed in the reaction equence. e. The rate-determining tep i the lowet elementary reaction in any given mechanim. For a mechanim to be acceptable, the um of the elementary tep mut give the overall balanced equation for the reaction and the mechanim mut give a rate law that agree with the experimentally determined rate law. A mechanim can never be proven abolutely. We can only ay it i poibly correct if it follow the two requirement decribed above. Mot reaction occur by a erie of tep. If mot reaction were unimolecular, then mot reaction would have a firt order overall rate law, which i not the cae. 8. The premie of the colliion model i that molecule mut collide to react, but not all colliion between reactant molecule reult in product formation. a. The larger the activation energy, the lower the rate. b. The higher the temperature, the more molecular colliion with ufficient energy to convert to product and the fater the rate. c. The greater the frequency of colliion, the greater the opportunitie for molecule to react, and, hence, the greater the rate. d. For a reaction to occur, it i the reactive portion of each molecule that mut be involved in a colliion. Only ome of all the poible colliion have the correct orientation to convert reactant to product. endothermic, E > exothermic, E < E a E a E R P E R P Reaction Progre Reaction Progre
5 CHAPTER CHEMICAL KINETICS The activation energy for the revere reaction will be the energy difference between the product and the tranition tate at the top of the potential energy hill. For an exothermic reaction, the activation energy for the revere reaction (E a,r ) i larger than the activation energy for the forward reaction (E a ), o the rate of the forward reaction will be greater than the rate of the revere reaction. For an endothermic reaction, E a,r < E a o the rate of the forward reaction will be le than the rate of the revere reaction (with other factor being equal). 9. Arrheniu equation: = E a + lna R T y = m x + b E a / RT Ae ; ln() = The data needed i the value of the rate contant a a function of temperature. One would plot ln veru/t to get a traight line. The lope of the line i equal to E a /R and the y-intercept i equal to ln(a). The R value i 8.5 J/Kmol. If one now the rate contant at two different temperature, then the following equation allow determination of E a : ln Ea R T T. A catalyt increae the rate of a reaction by providing reactant with an alternate pathway (mechanim) to convert to product. Thi alternate pathway ha a lower activation energy, thu increaing the rate of the reaction. Quetion A homogeneou catalyt i one that i in the ame phae a the reacting molecule, and a heterogeneou catalyt i in a different phae than the reactant. The heterogeneou catalyt i uually a olid, although a catalyt in a liquid phae can act a a heterogeneou catalyt for ome ga phae reaction. Since the catalyzed reaction ha a different mechanim than the uncatalyzed reaction, the catalyzed reaction mot liely will have a different rate law. 9. In a unimolecular reaction, a ingle reactant molecule decompoe to product. In a bimolecular reaction, two molecule collide to give product. The probability of the imultaneou colliion of three molecule with enough energy and proper orientation i very mall, maing termolecular tep very unliely.. Some energy mut be added to get the reaction tarted, that i, to overcome the activation energy barrier. Chemically what happen i: Energy + H H The hydrogen atom initiate a chain reaction that proceed very rapidly. Colliion of H and O molecule at room temperature do not have ufficient inetic energy to form hydrogen atom and initiate the reaction.. All of thee choice would affect the rate of the reaction, but only b and c affect the rate by affecting the value of the rate contant. The value of the rate contant i dependent on temperature. The value of the rate contant alo depend on the activation energy. A catalyt
6 CHAPTER CHEMICAL KINETICS will change the value of becaue the activation energy change. Increaing the concentration (partial preure) of either H or NO doe not affect the value of, but it doe increae the rate of the reaction becaue both concentration appear in the rate law.. One experimental method to determine rate law i the method of initial rate. Several experiment are carried out uing different initial concentration of reactant, and the initial rate i determined for each run. The reult are then compared to ee how the initial rate depend on the initial concentration. Thi allow the order in the rate law to be determined. The value of the rate contant i determined from the experiment once the order are nown. The econd experimental method utilize the fact that the integrated rate law can be put in the form of a traight line equation. Concentration v. time data i collected for a reactant a a reaction i run. Thi data i then manipulated and plotted to ee which manipulation give a traight line. From the traight line plot, we get the order of the reactant and the lope of the line i mathematically related to, the rate contant.. The average rate decreae with time becaue the revere reaction occur more frequently a the concentration of product increae. Initially, with no product preent, the rate of the forward reaction i at it fatet; but a time goe on, the rate get lower and lower ince product are converting bac into reactant. The intantaneou rate will alo decreae with time. The only rate that i contant i the initial rate. Thi i the intantaneou rate taen at t. At thi time, the amount of product i inignificant and the rate of the reaction only depend on the rate of the forward reaction.. The mot common method to experimentally determine the differential rate law i the method of initial rate. Once the differential rate law i determined experimentally, the integrated rate law can be derived. However, ometime it i more convenient and more accurate to collect concentration veru time data for a reactant. When thi i the cae, then we do proof plot to determine the integrated rate law. Once the integrated rate law i determined, the differential rate law can be determined. Either experimental procedure allow determination of both the integrated and the differential rate; which rate law i determined by experiment and which i derived i uually decided by which data i eaiet and mot accurately collected. 5. rate rate x x x The rate double a the concentration quadruple: = () x, x = / The order i / (the quare root of the concentration of reactant). For a reactant that ha an order of and the reactant concentration i doubled: rate () rate
7 CHAPTER CHEMICAL KINETICS The rate will decreae by a factor of / when the reactant concentration i doubled for a order reaction. 6. A metal catalyzed reaction i dependent on the number of adorption ite on the metal urface. Once the metal urface i aturated with reactant, the rate of reaction become independent of concentration. 7. Two reaon are: a. The colliion mut involve enough energy to produce the reaction, i.e., the colliion energy mut be equal to or exceed the activation energy. b. The relative orientation of the reactant when they collide mut allow formation of any new bond neceary to produce product. 8. The lope of the ln v. /T (K) plot i equal to E a /R. Becaue E a for the catalyzed reaction will be maller than E a for the uncatalyzed reaction, the lope of the catalyzed plot will be le negative. Exercie Reaction Rate 9. The coefficient in the balanced reaction relate the rate of diappearance of reactant to the rate of production of product. From the balanced reaction, the rate of production of P will be / the rate of diappearance of PH, and the rate of production of H will be 6/ the rate of diappearance of PH. By convention, all rate are given a poitive value. Rate = Δ[PH] (.8mol/. L) =. mol/l Δt [P ] Δt Δ[PH ] =. Δt Δ [H Δt / = 6. mol/l ] 6 Δ[PH ] = 6(. )/ =.6 mol/l Δt Δ. [H Δt ] Δ[N ] Δ[NH] Δ[N ] Δ[H ] and ; So, Δt Δt Δt Δt Δ[NH ] Δt Δ or: Δ [NH ] Δt Δ[H ] Δt Ammonia i produced at a rate equal to / of the rate of conumption of hydrogen.
8 CHAPTER CHEMICAL KINETICS 5. a. average rate = Δ[H O] Δt (.5M.M) (.6 ) =. 5 mol/l From the coefficient in the balanced equation: Δ [O Δt ] Δ[H O ] 5 =.6 mol/l Δt b. Δ[H O] Δt (.5.5) M (..6 ) =.6 5 mol/l Δ[O ] = / ( ) = 5.8 mol/l Δt Notice that a time goe on in a reaction, the average rate decreae.../.8 =.5; Reactant B i ued up.5 time fater than reactant A. Thi correpond to a to mol ratio between B and A in the balanced equation..6/.8 = ; Product C i produced twice a fat a reactant A i ued up. So the coefficient for C i twice the coefficient for A. A poible balanced equation i: A + B C. a. The unit for rate are alway mol/l. b. Rate = ; mut have unit of mol/l. mol mol c. Rate =, L L mut have unit of d. Rate =, mol L mol L. mut have unit of L/mol. e. L /mol /. Rate = [Cl] / mol mol mol [CHCl ], L L L, mut have unit of L / /mol /. Rate Law from Experimental Data: Initial Rate Method 5. a. In the firt two experiment, [NO] i held contant and [Cl ] i doubled. The rate alo doubled. Thu, the reaction i firt order with repect to Cl. Or mathematically: Rate = [NO] x [Cl ] y.6.8 x y (.) (.) (.),. =. y, y = x y y (.) (.) (.) y We can get the dependence on NO from the econd and third experiment. Here, a the NO concentration double (Cl concentration i contant), the rate increae by a factor of four. Thu, the reaction i econd order with repect to NO. Or mathematically:
9 6 CHAPTER CHEMICAL KINETICS.5.6 x x (.) (.) (.),. =. x, x = ; So, Rate = [NO] [Cl x x ] (.) (.) (.) Try to examine experiment where only one concentration change at a time. The more variable that change, the harder it i to determine the order. Alo, thee type of problem can uually be olved by inpection. In general, we will olve uing a mathematical approach, but eep in mind you probably can olve for the order by imple inpection of the data. b. The rate contant can be determined from the experiment. From experiment :.8mol L min.mol L From the other experiment:.mol, = 8 L /mol min L = 8 L /mol min (nd exp.); = 8 L /mol min (rd exp.) The average rate contant i mean =.8 L /mol min a. Rate = [I ] x [S O 8 ] y.5 (.8) (.) ;,. =. x, x = 6 x y 6.5 (.) (.) b. For the firt experiment: 6 (.8)(.) (.8)(.) y y, x y. =. y, y = ; Rate = [I ][S O 8 ].5 mol.8mol.mol, =.9 L/mol L L L Each of the other experiment alo give =.9 o mean =.9 L/mol. 7. a. Rate = [NOCl] n ; Uing experiment two and three: L/mol, n (. ),. =. n, n = ; Rate = [NOCl] 6 n (. ) b cm molecule 6. molecule 9 cm, = 6.6 cm /molecule The other three experiment give (6.7, 6.6 and 6.6) repectively. The mean value for i cm /molecule. 9 cm /molecule,
10 CHAPTER CHEMICAL KINETICS 7 c. 6.6 cm molecule 9 8 L 6. molecule. L cm mol mol 8. Rate = [N O 5 ] x ; The rate law for the firt two experiment are:.6 = (.9) x and 8.9 = (.75) x Dividing:.5 = (.9) (.75) x x = (.5) x, x = ; Rate = [N O 5 ] = Rate 8.9 mol/ L =.9 [N O ].75mol/ L 5 ; mean =.9 9. a. Rate = [Hb] x [CO] y ; Comparing the firt two experiment, [CO] i unchanged, [Hb] double, and the rate double. Therefore, x = and the reaction i firt order in Hb. Comparing the econd and third experiment, [Hb] i unchanged, [CO] triple. and the rate triple. Therefore, y = and the reaction i firt order in CO. b. Rate = [Hb][CO] c. From the firt experiment:.69 µmol/l = (. µmol/l)(. µmol/l), =.8 L/µmol The econd and third experiment give imilar value, o mean =.8 L/µmol. d. Rate = [Hb][CO] =.8L μmol.6μmol.μmol =.6 µmol/l L L. a. Rate = [ClO ] x [OH - ] y ; From the firt two experiment:. = (.) x (.) y and 5.75 = (.5) x (.) y Dividing the two rate law:. = (.) x (.5) x =. x, x = Comparing the econd and third experiment:. = (.)(.) y and.5 = (.)(.5) y (.) Dividing:. = y (.5) y =. y, y = The rate law i: Rate = [ClO ] [OH ]. mol/l = (. mol/l) (. mol/l), =. L /mol = mean
11 8 CHAPTER CHEMICAL KINETICS b. Rate = [ClO ] [OH ] =. mol L.75mol L.8mol L =.59 mol/l Integrated Rate Law. The firt aumption to mae i that the reaction i firt order becaue firt-order reaction are mot common. For a firt-order reaction, a graph of ln [H O ] v time will yield a traight line. If thi plot i not linear, then the reaction i not firt order and we mae another aumption. The data and plot for the firt-order aumption follow. Time [H O ] ln H O ] () (mol/l) Note: We carried extra ignificant figure in ome of the ln value in order to reduce roundoff error. For the plot, we will do thi mot of the time when the ln function i involved. The plot of ln [H O ] v. time i linear. Thu, the reaction i firt order. The rate law and integrated rate law are: Rate = [H O ] and ln [H O ] = t + ln [H O ] o. We determine the rate contant by determining the lope of the ln [H O ] v time plot (lope = ). Uing two point on the curve give: lope = = Δy (.) = 8. Δx 6., = 8. To determine [H O ] at., ue the integrated rate law where at t =, [H O ] o =. M. [H ln [H O ] = t + ln [H O ] o or O] ln = t [HO] o [H O] ln = 8..., ln [H O ] =., [H O ] =. e =.7 M
12 CHAPTER CHEMICAL KINETICS 9. a. Becaue the ln v time plot wa linear, the reaction i firt order in A. The lope of the ln v. time plot equal. Therefore, the rate law, the integrated rate law and the rate contant value are: Rate = ; ln = t + ln o ; =.97 min b. The half-life expreion for a firt-order rate law i: ln t / =, t / = =. min.97 min c..5 M i /8 of the original amount of A preent, o the reaction i 87.5% complete. When a firt-order reaction i 87.5% complete (or.5% remain), the reaction ha gone through half-live: % 5.% 5%.5%; t = t / =. min = 69.9 min t / t / t / Or we can ue the integrated rate law:.5 M ln t, ln = (.97 min o. M ln(.5) t =.97 min = 7. min. Aume the reaction i firt order and ee if the plot of ln [NO ] v. time i linear. If thi in t linear, try the econd-order plot of /[NO ] v. time becaue econd-order reaction are the next mot common after firt-order reaction. The data and plot follow. Time () [NO ] (M) ln [NO ] /[NO ] ( M ) ) t
13 CHAPTER CHEMICAL KINETICS The plot of /[NO ] v. time i linear. The reaction i econd order in NO. The rate law and integrated rate law are: Rate = [NO ] and [NO t. ] [NO] o The lope of the plot /[NO ] v. t give the value of. Uing a couple of point on the plot: lope = = Δy (5.75.) M =.8 L/mol Δx (.8 ) To determine [NO ] at.7, ue the integrated rate law where /[NO ] o = /.5 M =. M. [NO.8 L t,.7 +. ] [NO ] [NO ] mol o M [NO ] = 7.6, [NO ] =. M. a. Becaue the / v. time plot wa linear, the reaction i econd order in A. The lope of the / v. time plot equal the rate contant. Therefore, the rate law, the integrated rate law and the rate contant value are: Rate = ; t ; =.6 o L/mol b. The half-life expreion for a econd-order reaction i: t / = For thi reaction: t / =.6 L/ mol.8 o mol/ L Note: We could have ued the integrated rate law to olve for t / where = (.8 /) mol/l. = 9.9 c. Since the half-life for a econd-order reaction depend on concentration, we will ue the integrated rate law to olve.
14 CHAPTER CHEMICAL KINETICS t, o 7. M.6 mol L t.8 M. 57 =.6 t, t = a. Becaue the [C H 5 OH] v. time plot wa linear, the reaction i zero order in C H 5 OH. The lope of the [C H 5 OH] v. time plot equal -. Therefore, the rate law, the integrated rate law and the rate contant value are: Rate = [C H 5 OH] = ; [C H 5 OH] = t + 5 [C H 5 OH] o ; =. mol/l. b. The half-life expreion for a zero-order reaction i: t / = o /. t / = [CH5OH] o.5. 5 mol/ L mol/ L = 56 Note: we could have ued the integrated rate law to olve for t / where [C H 5 OH] = (.5 /) mol/l. c. [C H 5 OH] = t + [C H 5 OH] o, mol/l = (. mol/l) t +.5 mol/l 5 t =.5. 5 mol/ L mol/ L = 6. From the data, the preure of C H 5 OH decreae at a contant rate of torr for every.. Since the rate of diappearance of C H 5 OH i not dependent on concentration, the reaction i zero order in C H 5 OH. = torr. atm =.7 76torr atm/ The rate law and integrated rate law are: Rate = =.7 atm/; P C H 5 OH = t + 5. torr atm = t +.9 atm 76torr At 9. : P = -.7 C 5 H OH atm/ atm =.76 atm =.8 atm = torr 7. The firt aumption to mae i that the reaction i firt order. For a firt-order reaction, a graph of ln [C H 6 ] v. t hould yield a traight line. If thi in't linear, then try the econdorder plot of /[C H 6 ] v. t. The data and the plot follow. Time [C H 6 ] M ln [C H 6 ] /[C H 6 ] Note: To reduce round-off error, we carried extra ig. fig. in the data point. M
15 CHAPTER CHEMICAL KINETICS The ln plot i not linear, o the reaction i not firt order. Since the econd-order plot of /[C H 6 ] v. t i linear, we can conclude that the reaction i econd order in butadiene. The rate law i: Rate = [C H 6 ] For a econd order reaction, the integrated rate law i: [C t H6] [CH6] o The lope of the traight line equal the value of the rate contant. Uing the point on the line at. and 6. : = lope = L / mol 6. 7L / mol =.. L/mol 8. a. Firt, aume the reaction to be firt order with repect to O. A graph of ln [O] v. t hould be linear if the reaction i firt order in O. t() [O] (atom/cm ) ln[o]
16 CHAPTER CHEMICAL KINETICS The graph i linear, o we can conclude that the reaction i firt order with repect to O. Note: by eeping the NO concentration o large we made thi reaction into a peudofirt-order reaction in O. b. The overall rate law i: Rate = [NO ][O] Becaue NO wa in exce, it concentration i contant. So for thi experiment, the rate law i: Rate = [O] where = [NO ]. In a typical firt-order plot, the lope equal. For thi experiment, the lope equal = [NO ]. From the graph: lope = 9.. (. ) =., = -lope =. To determine, the actual rate contant: = [NO ],. = (. molecule/cm ) =. cm /molecule 9. Becaue the / v. time plot i linear with a poitive lope, the reaction i econd order with repect to A. The y-intercept in the plot will equal / o. Extending the plot, the y- intercept will be about, o / =. M = o.. The lope of the / v time plot in Exercie.9 with equal. lope = = (6 ) L / mol = L/mol (5 ) a. t o L mol 9. M =, =. M b. For a econd-order reaction, the half-life doe depend on concentration: t / = Firt half-life: t / = L mol =. mol L o Second half-life ( o i now.5 M): t / = /(.5) = Third half-life ( o i now.5 M): t / = /(.5) =. a. = t + o, = (5. mol/l) t +. mol/l b. The half-life expreion for a zero-order reaction i: t / = t / =. 5. c. = 5. mol/ L =. mol/ L mol/l [ A] o mol/l = 7.5 mol/l
17 CHAPTER CHEMICAL KINETICS Becaue 7.5 M A remain,.5 B ha been produced. M A reacted, which mean that.5 M. ln o t; ln t /.69 =.85. d d If o =., then after 95.% completion, = ln =.85 d t, t = 6 day.. a. When a reaction i 75.% complete (5.% of reactant remain), thi repreent two half live (% 5% 5%). The firt-order half-life expreion i: t / = (ln )/. Becaue there i no concentration dependence for a firt order half-life:. = two halflive, t / =./ = 6.. Thi i both the firt half-life, the econd half-life, etc. ln ln ln b. t / =, =. t 6. / At 9.% complete,.% of the original amount of the reactant remain, o =.. ln. t, ln = (. o ln. )t, t = = 5.. For a firt-order reaction, the integrated rate law i: ln(/ o ) = t. Solving for :.5mol/ L ln =., =.6.mol/ L.5mol/ L ln = -.6.mol/ L t, t = Comparing experiment and, a the concentration of AB i doubled, the initial rate increae by a factor of. The reaction i econd order in AB. Rate = [AB],. mol/ L = (. M) = 8. L/ mol = mean For a econd order reaction: t / = [AB] 8. o L/ mol.mol/ L =.5 6. a. The integrated rate law for a econd-order reaction i: / = t + / o, and the half-
18 CHAPTER CHEMICAL KINETICS 5 life expreion i: t / = / o. We could ue either to olve for t /. Uing the integrated rate law: b. (.9/ ) mol/ L.mol/ L =. + =.555 L/mol t +,.9mol/ L,.9mol/ L.L/ mol =.555 L/mol. 8.9 L/ mol t = 6.555L/ mol 7. Succeive half-live double a concentration i decreaed by one-half. Thi i conitent with econd-order reaction o aume the reaction i econd order in A. t / = o, t / o =. L/molmin. min(. M ). L a. t 8. min + = 9. M, =. M o mol min. M b.. min = half-live, o 5% of original A i remaining. =.5(. M) =.5 M 8. Becaue [B] o >> o, the B concentration i eentially contant during thi experiment, o rate = where = [B]. For thi experiment, the reaction i a peudo-firt-order reaction in A. a. ln o = t,.8 M ln = 8., =.. M For the reaction: = [B], =. ln.69 b. t / = = 5.8 '. /(. mol/l) =. L /mol c. ln =.. M., = e.(.) =.. =. M d. reacted =. M. M =.8 M [C] reacted =.8 M molc =.6 M. M mola [C] remaining =. M -. M =. M; A expected, the concentration of C baically
19 6 CHAPTER CHEMICAL KINETICS remain contant during thi experiment ince [C] o >> o. Reaction Mechanim 9. For elementary reaction, the rate law can be written uing the coefficient in the balanced equation to determine order. a. Rate = [CH NC] b. Rate = [O ][NO] c. Rate = [O ] d. Rate = [O ][O] 5. The oberved rate law for thi reaction i: Rate = [NO] [H ]. For a mechanim to be plauible, the um of all the tep mut give the overall balanced equation (true for all of the propoed mechanim in thi problem), and the rate law derived from the mechanim mut agree with the oberved mechanim. In each mechanim (I - III), the firt elementary tep i the rate-determining tep (the low tep), o the derived rate law for each mechanim will be the rate of the firt tep. The derived rate law follow: Mechanim I: Rate = [H ] [NO] Mechanim II: Rate = [H ][NO] Mechanim III: Rate = [H ][NO] Only in Mechanim III doe the derived rate law agree with the oberved rate law. Thu, only Mechanim III i a plauible mechanim for thi reaction. 5. A mechanim conit of a erie of elementary reaction where the rate law for each tep can be determined uing the coefficient in the balanced equation. For a plauible mechanim, the rate law derived from a mechanim mut agree with the rate law determined from experiment. To derive the rate law from the mechanim, the rate of the reaction i aumed to equal the rate of the lowet tep in the mechanim. Becaue tep i the rate-determining tep, the rate law for thi mechanim i: Rate = [C H 9 Br]. To get the overall reaction, we um all the individual tep of the mechanim. Summing all tep give: C H 9 Br C H Br C H H O C H 9 OH + C H 9 OH + + H O C H 9 OH + H O + C H 9 Br + H O C H 9 OH + Br + H O + Intermediate in a mechanim are pecie that are neither reactant nor product, but that are formed and conumed during the reaction equence. The intermediate for thi mechanim are C H 9 + and C H 9 OH Becaue the rate of the lowet elementary tep equal the rate of a reaction:
20 CHAPTER CHEMICAL KINETICS 7 Rate = rate of tep = [NO ] The um of all tep in a plauible mechanim mut give the overall balanced reaction. Summing all tep give: NO + NO NO + NO NO + CO NO + CO NO + CO NO + CO Temperature Dependence of Rate Contant and the Colliion Model 5. In the following plot, R = reactant, P = product, E a = activation energy and RC = reaction coordinate which i the ame a reaction progre. Note for thi reaction that ΔE i poitive ince the product are at a higher energy than the reactant. E Ea P R E RC 5. When ΔE i poitive, the product are at a higher energy relative to reactant and, when ΔE i negative, the product are at a lower energy relative to reactant.
21 8 CHAPTER CHEMICAL KINETICS 55. E R 5 J/mol 6 J/mol RC The activation energy for the revere reaction i: P Ea, revere E a, revere = 6 J/mol + 5 J/mol = J/mol 56. When ΔE i negative, then E a, revere > E a, forward (ee energy profile in Exercie.55). When ΔE i poitive (the product have higher energy than the reactant a repreented in the energy profile for Exercie.5), then E a, forward > E a, revere. Therefore, thi reaction ha a poitive ΔE value. 57. The Arrheniu equation i: = A exp (-E a /RT) or in logarithmic form, ln = -E a /RT + ln A. Hence, a graph of ln v. /T hould yield a traight line with a lope equal to -E a /R ince the logarithmic form of the Arrheniu equation i in the form of a traight line equation, y = mx + b. Note: We carried extra ignificant figure in the following ln value in order to reduce round off error. T (K) /T ( K ) ( ) ln
22 CHAPTER CHEMICAL KINETICS ( 5.85) Slope = =. K = E a /R (.. ) K E a = lope R =. K 8.5J, E a =. 5 J/mol =. J/mol K mol 58. From the Arrheniu equation in logarithmic form (ln = -E a /RT + ln A), a graph of ln v. /T hould yield a traight line with a lope equal to E a /R and a y-intercept equal to ln A. a. lope = E a /R, E a =. K 8.5J K mol b. The unit for A are the ame a the unit for ( ). y-intercept = ln A, A = e.5 =.5 c. ln = E a /RT + ln A or = A exp(e a /RT) =.5 exp = 9.5 J/mol = 9.5 J/mol 9.5 J / mol =. 8.5J / K mol 98K 59. = A exp(e a /RT) or ln = E a RT + ln A (the Arrheniu equation) For two condition: E a ln (Auming A i temperature independent.) R T T Let =.5 7 L/mol, T = 555 K; =?, T = 65 K; E a = 86 J/mol 5.86 J / mol ln = J / mol K 555K 65K
23 CHAPTER CHEMICAL KINETICS.5 7 = e 5.6 = 7, = 7( ) = 9.5 L/mol 6. For two condition: 8. ln.6 E a ln (Auming A factor i T independent.) R T T E a 8.5J / mol K 7K 9K E.57 = a (.5 ), E a =.9 J/mol = 9 J/mol ln Ea R T T ; = 7., T = 95 K, E a = 5. J/mol ln(7.) = 5. J / mol 8.5J / mol K 95K, T =. 95K T =.9, T = K = 5 C T Ea 6. ln ; R T T Since the rate double, then =. E ln (.) = a, 8.5J / mol K 98K 8K E a = 5. J/mol = 5 J/mol 6. H O + (aq) + OH (aq) H O(l) hould have the fater rate. H O + and OH will be electrotatically attracted to each other; Ce + and Hg + will repel each other (o E a i much larger). 6. Carbon cannot form the fifth bond neceary for the tranition tate becaue of the mall atomic ize of carbon and becaue carbon doen t have low energy d orbital available to expand the octet. Catalyt 65. a. NO i the catalyt. NO i preent in the firt tep of the mechanim on the reactant ide, but it i not a reactant ince it i regenerated in the econd tep. b. NO i an intermediate. Intermediate alo never appear in the overall balanced equation. In a mechanim, intermediate alway appear firt on the product ide while catalyt alway appear firt on the reactant ide. cat A exp[ Ea (cat)/ RT] Ea (un) Ea (cat) c. = A exp(e a /RT); exp A exp[ E (un)/ RT] RT un
24 CHAPTER CHEMICAL KINETICS cat un J / mol exp = e.85 =. 8.5J / mol K 98K The catalyzed reaction i. time fater than the uncatalyzed reaction at 5 C. 66. The mechanim for the chlorine catalyzed detruction of ozone i: O + Cl O + ClO (low) ClO + O O + Cl (fat) O + O O Becaue the chlorine atom-catalyzed reaction ha a lower activation energy, then the Cl catalyzed rate i fater. Hence, Cl i a more effective catalyt. Uing the activation energy, we can etimate the efficiency with which Cl atom detroy ozone a compared to NO molecule (ee Exercie.65c). Cl Ea (Cl) Ea (NO) (,9) J / mol At 5 C: exp exp = e.96 = 5 NO RT RT (8.5 98) J / mol At 5 C, the Cl catalyzed reaction i roughly 5 time fater than the NO catalyzed reaction, auming the frequency factor A i the ame for each reaction. 67. The reaction at the urface of the catalyt i aumed to follow the tep: H H DCH D D H C C H D D D CH D D CH DCH D(g) metal urface Thu, CH D CH D hould be the product. If the mechanim i poible, then the reaction mut be: C H + D CH DCH D If we got thi product, then we could conclude that thi i a poible mechanim. If we got ome other product, e.g., CH CHD, then we would conclude that the mechanim i wrong. Even though thi mechanim correctly predict the product of the reaction, we cannot ay concluively that thi i the correct mechanim; we might be able to conceive of other mechanim that would give the ame product a our propoed one. 68. a. W, becaue it ha a lower activation energy than the O catalyt. b. w = A w exp[e a (W)/RT]; uncat = A uncat exp[e a (uncat)/rt]; Aume A w = A uncat w uncat Ea (W) E exp RT a (uncat) RT
25 CHAPTER CHEMICAL KINETICS w 6,J / mol 5,J / mol exp =. uncat 8.5J / mol K 98K The W-catalyzed reaction i approximately time fater than the uncatalyzed reaction. c. Becaue [H ] i in the denominator of the rate law, the preence of H decreae the rate of the reaction. For the decompoition to occur, NH molecule mut be adorbed on the urface of the catalyt. If H i alo adorbed on the catalyt urface, then there are fewer ite for NH molecule to be adorbed and the rate decreae. 69. Auming the catalyzed and uncatalyzed reaction have the ame form and order and becaue concentration are aumed equal, the rate will be equal when the value are equal. = A exp(e a /RT), cat = un when E a,cat /RT cat = E a,un /RT un. J / mol 8.5J / mol K 9K 7. J / mol, T un = 88 K = 5C 8.5J / mol K T un 7. Rate = Δ Δt x Auming the catalyzed and uncatalyzed reaction have the ame form and order and becaue concentration are aumed equal, rate. Δt rate rate rate rate cat un cat un cat un Δt Δt un cat and cat cat un yr Δt cat 5.9 exp rate rate un A exp[ E (cat)/ RT] a A exp[ E (un) / RT] a J / mol 8.5J / mol = 5.8 K 6. K cat un exp[ Ea (cat) Ea (un)] RT J / mol = 7.6 Δt Δt un cat rate rate cat cat, = 7.6, t cat =.5 un un yr Δt cat 8 yr ec Additional Exercie 7. Rate = [NO] x [O ] y ; comparing the firt two experiment, [O ] i unchanged, [NO] i tripled, and the rate increae by a factor of nine. Therefore, the reaction i econd order in NO ( = 9). The order of O i more difficult to determine. Comparing the econd and third experiment;
26 CHAPTER CHEMICAL KINETICS (.5 ( ) (.5 8 ) (. ) ) y y,.7 =.69 (.5) y,.5 =.5 y, y = Rate = [NO] [O ]; From experiment :. 6 molecule/cm = (. 8 molecule/cm ) (. 8 molecule/cm ) =. 8 cm 6 /molecule = mean cm 6. molecule 7.6 molecule Rate = molecule cm cm = molecule/cm 7. The preure of a ga i directly proportional to concentration. Therefore, we can ue the preure data to olve the problem becaue Rate = Δ[SO Cl ]/Δt ΔP / Δt. Auming a firt order equation, the data and plot follow. Time (hour) P SO Cl (atm) ln P SO Cl SO Cl
27 CHAPTER CHEMICAL KINETICS Becaue the ln PSO Cl v. time plot i linear, the reaction i firt order in SO Cl. a. Slope of ln(p) v. t plot i.68 hour =, =.68 hour =.67 5 Since concentration unit don t appear in firt-order rate contant, thi value of determined from the preure data will be the ame a if concentration data in molarity unit were ued. ln b. t / = =. hour.68h c. PSO Cl ln = t =.68 Po Fraction left =.7 =.7% h (. h) =.6, PSO P o Cl =.6 e =.7 7. From 8 K data, a plot of ln[n O 5 ] v. t i linear and the lope = -.86 (plot not included). Thi tell u the reaction i firt order in N O 5 with =.86 at 8 K. From 8 K data, the lope of ln[n O 5 ] v t plot i equal to.98, o =.98 at 8 K. We now have two value of at two temperature, o we can olve for E a. ln Ea.86 E, R T T a ln J / mol K 8K 8K E a =. 5 J/mol =. J/mol 7. The Arrheniu equation i: = A exp (-E a /RT) or in logarithmic form, ln = -E a /RT + ln A. Hence, a graph of ln v. /T hould yield a traight line with a lope equal to -E a /R ince the logarithmic form of the Arrheniu equation i in the form of a traight line equation, y = mx + b. Note: We carried one extra ignificant figure in the following ln value in order to reduce round off error. T (K) /T (K ) (L/mol) ln
28 CHAPTER CHEMICAL KINETICS From "eyeballing" the line on the graph: lope = Ea.5 K (5.. ) K. R E a =.5 K 8.5J =. J/mol =. J/mol K mol From a graphing calculator: lope =. K and E a =.9 J/mol 75. At high [S], the enzyme i completely aturated with ubtrate. Once the enzyme i completely aturated, the rate of decompoition of ES can no longer increae, and the overall rate remain contant. 76. = A exp (E a /RT); cat uncat A A cat uncat exp( E exp( E a,cat a,uncat / RT) E exp / RT) a,cat E RT a, uncat.5 E cat = a,cat 5. J / mol exp uncat 8.5J / mol K. K ln (.5 ).58 J/mol = E a,cat + 5. J/mol E a, cat = 5. J/mol. J/mol =.98 J/mol = 9.8 J/mol 77. a. Becaue o < < [B] o or [C] o, the B and C concentration remain contant at. M for thi experiment. So: rate = [B][C] = where = [B][C] For thi peudo-econd-order reaction:
29 6 CHAPTER CHEMICAL KINETICS = t +, 5.6 M = 689 L/molmin = 5 L/mol = (. min) +. M = [B][C], = ' [B][C] 5L/ mol = 5 L / mol (. M )(. M ) b. For thi peudo-econd-order reaction: rate =, t / = ' = 87. 5L/ mol (. mol/ L) c. = t + = 5 L/mol mol/ L = 7.9 L/mol, = 7.9 L/ mol =.7 5 mol/l From the toichiometry in the balanced reaction, mol of B react with every mol of A. amount A reacted =. M.7 5 M = M amount B reacted = mol/l mol B / mol A =.9 5 M [B] =. M.9 5 M =. M A we mentioned in part a, the concentration of B (and C) remain contant ince the A concentration i o mall. Challenge Problem 78. d dt t = d, dt t n n x t x dx ; So: t, t n t o For the half-life equation, t = / : t /, t / t/, t / =
30 CHAPTER CHEMICAL KINETICS 7 The firt half-life i t / =. and correpond to going from to /. The econd half-life correpond to going from / to /. Firt half-life = ; Second half-life = Firt half life = / = / Second half life 6 = 6 Becaue the firt half-life i., the econd half-life will be one-fourth of thi or Rate = [I ] x [OCl ] y [OH ] z ; Comparing the firt and econd experiment: (.6) (.) x x (.) (.) y y (.) (.) z z,. =. x, x = Comparing the firt and third experiment: 9..7 (.)(.) (.) x y (.6) (.) y z (.) z,. =. y, y = Comparing the firt and ixth experiment:.8 9. (.)(.)(.) (.)(.)(.) z z, / =. z, z = Rate = [I ][OCl ] ; The preence of OH decreae the rate of the reaction. [OH ] For the firt experiment: 9. L mol (.mol/ L)(.mol/ L), = 6. (.mol/ L) For all experiment, mean = 6.. = For econd order inetic: t and t [A / o ] o
31 8 CHAPTER CHEMICAL KINETICS a. = (.5 L/mol)t +, = o. M = 5 M, = 6.9 M Amount of A that reacted =..69 =. M [A ] = (. M) =.6 M b. After. minute (8. ): =. [B], 6.9 [B] =. M M =. [B] [B] t, (8.), =.9 L/mol [B]. M.5 M o c. t / = o =..5L/ mol. mol/ L 8. a. We chec for firt-order dependence by graphing ln [concentration] v. time for each et of data. The rate dependence on NO i determined from the firt et of data becaue the ozone concentration i relatively large compared to the NO concentration, o [O ] i effectively contant. Time (m) [NO] (molecule/cm ) ln [NO]
32 CHAPTER CHEMICAL KINETICS 9 Becaue ln [NO] v. t i linear, the reaction i firt order with repect to NO. We follow the ame procedure for ozone uing the econd et of data. The data and plot are: Time (m) [O ] (molecule/cm ) ln [O ] The plot of ln [O ] v. t i linear. Hence, the reaction i firt order with repect to ozone. b. Rate = [NO][O ] i the overall rate law. c. For NO experiment, Rate = [NO] and = (lope from graph of ln [NO] v. t). = -lope = 8.. =.8 (. ) For ozone experiment, Rate = [O ] and = (lope from ln [O ] v. t plot). (.95.) = lope = =.6 (. ) d. From NO experiment, Rate = [NO][O ] = [NO] where = [O ]. =.8 = (. molecule/cm ), =.8 cm /molecule We can chec thi from the ozone data. Rate = [O ] = [NO][O ] where = [NO]. =.6 = (. molecule/cm ), =.8 cm /molecule Both value of agree.
33 CHAPTER CHEMICAL KINETICS 8. On the energy profile to the right, R = reactant, P = product, E a = activation energy, ΔE = overall energy change for the reaction and I = intermediate. a-d. See plot to the right. e. Thi i a two-tep reaction ince an intermediate plateau appear between the reactant and the product. Thi plateau repreent the energy of the intermediate. The general reaction mechanim for thi reaction i: R I I P R P In a mechanim, the rate of the lowet tep determine the rate of the reaction. The activation energy for the lowet tep will be the larget energy barrier that the reaction mut over-come. Since the econd hump in the diagram i at the highet energy, the econd tep ha the larget activation energy and will be the rate-determining tep (the low tep). 8. a. If the interval between flahe i 6. ec, then the rate i: flah/6. = 6. = Interval T C (9. K) 7.8 C (. K) ln E a R T T ; Solving: E a =.5 J/mol = 5 J/mol b. ln 6..5 J / mol 8.5J / mol K 9. K. K =. = e. 6. = 8. ; Interval = / = econd c. T Interval 5-(Interval). C 6. C 7.8 C. 8 C. C. C
34 CHAPTER CHEMICAL KINETICS Thi rule of thumb give excellent agreement to two ignificant figure. 8. We need the value of at 5. K. ln E a R T T 5. J / mol ln. L/ mol 8.5J / mol K 7K 5. K =.. = e., =. L/mol Becaue the decompoition reaction i an elementary reaction, then the rate law can be written uing the coefficient in the balanced equation. For thi reaction: Rate = [NO ]. To olve for the time, we mut ue the integrated rate law for econd-order inetic. The major problem now i converting unit o they match. Rearranging the ideal ga law give n/v = P/RT. Subtituting P/RT for concentration unit in the econd-order integrated rate law equation: [NO t ] [NO, t P / RT P RT P RT P t, RT Po P t P o ] o o / RT o P, t = (.86L atm/ K mol)(5. K).5 atm.5 atm =.. L/ mol.5 atm.5 atm 85. a. [B] >> o that [B] can be conidered contant over the experiment. (Thi give u a peudo-order rate law equation.) b. Note in each data et that ucceive half-live double (in expt. the firt half-life i. ec, the econd i 8. ec; in expt. the firt half-life i. ec, the econd i. ec). Thu, the reaction i econd order in ince t / for econd order reaction i inverely proportional to concentration. Between expt. and expt., we double [B] and the reaction rate double, thu it i firt order in [B]. The overall rate law equation i rate = [B]. Uing t / =, we get = (.)(. actually where rate = and = [B]. mol/ L) =.5 L/mol. But thi i = ' [B].5L/ mol =.5 L / mol 5. mol/ L 86. a. Rate = [H ] x [NO] y ; Looing at the data in experiment, notice that the concentration of
35 CHAPTER CHEMICAL KINETICS H i cut in half every. ec. Only firt-order reaction have a half-life that i independent of concentration. The reaction i firt order in H. In the data for experiment, notice that the half-life i.. Thi indicate that in going from experiment to experiment where the NO concentration doubled, the rate of reaction increaed by a factor of four. Thi tell u that the reaction i econd order in NO. Rate = [H ][NO] b. Thi reaction i peudo-firt-order in [H ] a the concentration of NO i o large, it i baically contant. Rate = [NO] [H ] = [H ] where = [NO] For a firt-order reaction, the integrated rate law i: [H ] ln = t [H] Ue any et of data you want to calculate. For example, in experiment from to. the concentration of H decreaed from. M to.7 M..7 ln = (. ), =.7. = [NO],.7 = (. mol/l) =.7 L / mol We get imilar value for uing other data from either experiment or experiment. [H] c. ln = t = (.7 L / mol )., [H ] = 6. M.M 87. Rate = x [B] y [C] z ; During the coure of experiment, and [C] are eentially contant, and Rate = [B] y x z where = [C. ] [B] (M) time () ln[b] /[B] (M - )
36 CHAPTER CHEMICAL KINETICS A plot of /[B] v. t i linear (plot not included). So the reaction i econd order in B and the integrated rate equation from the lope and y-intercept of the traight line in the plot i: /[B] = (.7 L/ mol ) t +. L/mol; =.7 L/ mol For experiment, [B] and [C] are eentially contant and Rate = x where = y z z [B] [C = [B] [C. ] ] (M) time () ln / ( M ) A plot of ln v. t i linear. So the reaction i firt order in A and the integrated rate law from the lope and y-intercept of the traight line in the plot i: ln = (. ) t.6; =. Note: We will carry an extra ignificant figure in. Experiment : and [B] are contant; Rate = [C] z The plot of [C] v. t i linear. Thu, z =. The overall rate law i: Rate = [B] From Experiment (to determine ): =.7 L/ mol = x z [C] = o = (. M), =. L / mol From Experiment : =. =. [B], = Thu, Rate = [B] and =. - L /mol. 88. a. Rate = ( + [H + ])[I ] m [H O ] n (. M ) =. L / mol In all the experiment, the concentration of H O i mall compared to the concentration of I and H +. Therefore, the concentration of I and H + are effectively contant and the rate law reduce to: Rate = ob [H O ] n where ob = ( + [H + ])[I ] m Since all plot of ln[h O ] v. time are linear, the reaction i firt order with repect to H O (n = ). The lope of the ln[h O ] v. time plot equal ob which equal ( +
37 CHAPTER CHEMICAL KINETICS [H + ])[I ] m. To determine the order of I, compare the lope of two experiment where I change and H + i contant. Comparing the firt two experiment: lope(exp. ) lope(exp.) m.6 [ (.M )][.M ), m. [ (.M )][.M ). =.. m = (.) m, m = The reaction i alo firt order with repect to I. b. The lope equation ha two unnown, and. To olve for and, we mut have two equation. We need to tae one of the firt et of three experiment and one of the nd et of three experiment to generate the two equation in and. Experiment : lope = ( + [H + ])[I ]. Experiment :.76 Subtracting from : min = [ + (. M)]. M or. = + (.) min = [ + (. M)].75 M or. = + (.). = + (.). = (.).9 = (.), = 9.5 L /mol min. = + 9.5(.), =.8 L/ molmin c. There are two pathway, one involving H + with rate = [H + ][I ][H O ] and another pathway not involving H + with rate = [I ][H O ]. The overall rate of reaction depend on which of thee two pathway dominate, and thi depend on the H + concentration. Integrative Problem h =.5 ln ln ; = =. 5 h t/.5 The partial preure of a ga i directly related to the concentration in mol/l. So intead of uing mol/l a the concentration unit in the integrated firt order rate law, we can ue partial preure of SO Cl.
38 CHAPTER CHEMICAL KINETICS 5 ln P P = t, P ln = (. 79torr atm P SO Cl = 9 torr =.87 atm 76torr n = PV RT 5.87atm.5L = L atm 59K molk 6 )(.5 h ) h mol SO Cl 9.9 mol 6. molecule mol = 5.99 molecule SO Cl 9. = ln t/ [In + ] = ln =. 667 molincl.8g InCl 5. g.5l molin mol InCl =.7 mol/l [In ] ln [In ] t, [In ] ln.7m = (. ).5 h 6 h [In + ] =.9 mol/l The balanced redox reaction i: In + (aq) In() + In + (aq) mol In + reacted =.5 L.57 mol In +.7mol.9 mol.5l =.57 mol In + L L molin.8 g In =. g In molin molin 9. ln Ea R T T ;.7 E a ln J / K mol 66. K 7. K E a =. 5 J/mol For at 5C (598 K):.7 ln J / K mol J / mol 598K 7. K, =. 5 For three half-live, we go from % 5% 5%.5%. After three half-live,.5% of the original amount of C H 5 I remain. Partial preure are directly related to ga concentration in mol/l:
39 6 CHAPTER CHEMICAL KINETICS P = 89 torr.5 = torr after half-live C H 5 I Marathon Problem 9. a. Rate = [CH X] x [Y] y ; For experiment, [Y] i contant o Rate = [CH X] x where = (. M) y. A plot (not included) of ln[ch X] v t i linear (x = ). The integrated rate law i: ln[ch X] =.9 t.99; =.9 h For Experiment, [Y] i again contant with Rate = [CH X] x where = (.5 M) y. The ln plot i linear again with an integrated rate law: ln[ch X] =.9 t 5.; =.9 y '.9 (.) Dividing the rate contant value:,. = (.67) y, y = ''.9 y (.5) The reaction i firt order in CH X and zero order in Y. The overall rate law i: Rate = [CH X] where =.9 h h at 5 C. b. t / = (ln )/ =.69/( h ) = 8.8 h c. ln Ea R T T, 7.88 ln.9 8 E a 8.5J / K mol 98K 58K E a =. 5 J/mol =. J/mol d. From part a, the reaction i firt order in CH X and zero order in Y. From part c, the activation energy i cloe to the C-X bond energy. A plauible mechanim that explain the reult in part a and c i: CH X CH + X CH + Y CH Y (low) (fat) Note: Thi i a poible mechanim ince the derived rate law i the ame a the experimental rate law and the um of the tep give the overall balanced equation.
b. The rate law can only be determined from experiment, not from the overall balanced reaction.
 CHAPTER CHEMICAL KINETICS Quetion. a. Activation energ and ΔE are independent of each other. Activation energ depend on the path reactant to tae to convert to product. The overall energ change ΔE onl depend
CHAPTER CHEMICAL KINETICS Quetion. a. Activation energ and ΔE are independent of each other. Activation energ depend on the path reactant to tae to convert to product. The overall energ change ΔE onl depend
AP Chemistry: Kinetics Practice Problems
 AP Chemitr: Kinetic Practice Problem Direction: Write our anwer to the following quetion in the pace provided. For problem olving, how all of our work. Make ure that our anwer how proper unit, notation,
AP Chemitr: Kinetic Practice Problem Direction: Write our anwer to the following quetion in the pace provided. For problem olving, how all of our work. Make ure that our anwer how proper unit, notation,
4. The following graphs were prepared from experimental data for a reactant, A. What is the correct order of A?
 Diebolt Summer 010 CHM 15 HOUR EXAM I KEY (15 pt) Part One: Multiple Choice. 1. Conider the reaction IO 3 + 5I + 6H + 3I + 3H O. If the rate of diappearance of I i 9.010-3 M/, what i the rate of appearance
Diebolt Summer 010 CHM 15 HOUR EXAM I KEY (15 pt) Part One: Multiple Choice. 1. Conider the reaction IO 3 + 5I + 6H + 3I + 3H O. If the rate of diappearance of I i 9.010-3 M/, what i the rate of appearance
 1 t year chemitry n0te new CHAPTER 11 REACTION KINETICS MCQS Q.1 In zero order reaction, the rate i independent of (a) temperature of reaction (b) concentration of reactant (c) concentration of product
1 t year chemitry n0te new CHAPTER 11 REACTION KINETICS MCQS Q.1 In zero order reaction, the rate i independent of (a) temperature of reaction (b) concentration of reactant (c) concentration of product
Source slideplayer.com/fundamentals of Analytical Chemistry, F.J. Holler, S.R.Crouch. Chapter 6: Random Errors in Chemical Analysis
 Source lideplayer.com/fundamental of Analytical Chemitry, F.J. Holler, S.R.Crouch Chapter 6: Random Error in Chemical Analyi Random error are preent in every meaurement no matter how careful the experimenter.
Source lideplayer.com/fundamental of Analytical Chemitry, F.J. Holler, S.R.Crouch Chapter 6: Random Error in Chemical Analyi Random error are preent in every meaurement no matter how careful the experimenter.
Ch 13 Rates of Reaction (Chemical Kinetics)
 Ch 13 Rates of Reaction (Chemical Kinetics) Reaction Rates and Kinetics - The reaction rate is how fast reactants are converted to products. - Chemical kinetics is the study of reaction rates. Kinetics
Ch 13 Rates of Reaction (Chemical Kinetics) Reaction Rates and Kinetics - The reaction rate is how fast reactants are converted to products. - Chemical kinetics is the study of reaction rates. Kinetics
Factors That Affect Rates. Factors That Affect Rates. Factors That Affect Rates. Factors That Affect Rates
 KINETICS Kinetics Study of the speed or rate of a reaction under various conditions Thermodynamically favorable reactions DO NOT mean fast reactions Some reactions take fraction of a second (explosion)
KINETICS Kinetics Study of the speed or rate of a reaction under various conditions Thermodynamically favorable reactions DO NOT mean fast reactions Some reactions take fraction of a second (explosion)
3: Chemical Kinetics Name: HW 6: Review for Unit Test KEY Class: Date: A Products
 3: Chemical Kinetics Name: HW 6: Review for Unit Test KEY Class: Date: Page 1 of 9 AP Multiple Choice Review Questions 1 16 1. The reaction rate is defined as the change in concentration of a reactant
3: Chemical Kinetics Name: HW 6: Review for Unit Test KEY Class: Date: Page 1 of 9 AP Multiple Choice Review Questions 1 16 1. The reaction rate is defined as the change in concentration of a reactant
AP CHEMISTRY CHAPTER 12 KINETICS
 AP CHEMISTRY CHAPTER 12 KINETICS Thermodynamics tells us if a reaction can occur. Kinetics tells us how quickly the reaction occurs. Some reactions that are thermodynamically feasible are kinetically so
AP CHEMISTRY CHAPTER 12 KINETICS Thermodynamics tells us if a reaction can occur. Kinetics tells us how quickly the reaction occurs. Some reactions that are thermodynamically feasible are kinetically so
Chapter 14. Chemical Kinetics
 Chapter 14. Chemical Kinetics Common Student Misconceptions It is possible for mathematics to get in the way of some students understanding of the chemistry of this chapter. Students often assume that
Chapter 14. Chemical Kinetics Common Student Misconceptions It is possible for mathematics to get in the way of some students understanding of the chemistry of this chapter. Students often assume that
Chapter 14 Chemical Kinetics
 Chapter 14 Chemical Kinetics Learning goals and key skills: Understand the factors that affect the rate of chemical reactions Determine the rate of reaction given time and concentration Relate the rate
Chapter 14 Chemical Kinetics Learning goals and key skills: Understand the factors that affect the rate of chemical reactions Determine the rate of reaction given time and concentration Relate the rate
Chapter 12. Chemical Kinetics
 Chapter 12 Chemical Kinetics Chapter 12 Table of Contents 12.1 Reaction Rates 12.2 Rate Laws: An Introduction 12.3 Determining the Form of the Rate Law 12.4 The Integrated Rate Law 12.5 Reaction Mechanisms
Chapter 12 Chemical Kinetics Chapter 12 Table of Contents 12.1 Reaction Rates 12.2 Rate Laws: An Introduction 12.3 Determining the Form of the Rate Law 12.4 The Integrated Rate Law 12.5 Reaction Mechanisms
Reaction Rate. Rate = Conc. of A at t 2 -Conc. of A at t 1. t 2 -t 1. Rate = Δ[A] Δt
![Reaction Rate. Rate = Conc. of A at t 2 -Conc. of A at t 1. t 2 -t 1. Rate = Δ[A] Δt Reaction Rate. Rate = Conc. of A at t 2 -Conc. of A at t 1. t 2 -t 1. Rate = Δ[A] Δt](/thumbs/95/122523995.jpg) Kinetics The study of reaction rates. Spontaneous reactions are reactions that will happen - but we can t tell how fast. Diamond will spontaneously turn to graphite eventually. Reaction mechanism- the
Kinetics The study of reaction rates. Spontaneous reactions are reactions that will happen - but we can t tell how fast. Diamond will spontaneously turn to graphite eventually. Reaction mechanism- the
Solving Differential Equations by the Laplace Transform and by Numerical Methods
 36CH_PHCalter_TechMath_95099 3//007 :8 PM Page Solving Differential Equation by the Laplace Tranform and by Numerical Method OBJECTIVES When you have completed thi chapter, you hould be able to: Find the
36CH_PHCalter_TechMath_95099 3//007 :8 PM Page Solving Differential Equation by the Laplace Tranform and by Numerical Method OBJECTIVES When you have completed thi chapter, you hould be able to: Find the
Social Studies 201 Notes for November 14, 2003
 1 Social Studie 201 Note for November 14, 2003 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
1 Social Studie 201 Note for November 14, 2003 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
How fast reactants turn into products. Usually measured in Molarity per second units. Kinetics
 How fast reactants turn into products. Usually measured in Molarity per second units. Kinetics Reaction rated are fractions of a second for fireworks to explode. Reaction Rates takes years for a metal
How fast reactants turn into products. Usually measured in Molarity per second units. Kinetics Reaction rated are fractions of a second for fireworks to explode. Reaction Rates takes years for a metal
Chapter 14 Chemical Kinetics
 How fast do chemical processes occur? There is an enormous range of time scales. Chapter 14 Chemical Kinetics Kinetics also sheds light on the reaction mechanism (exactly how the reaction occurs). Why
How fast do chemical processes occur? There is an enormous range of time scales. Chapter 14 Chemical Kinetics Kinetics also sheds light on the reaction mechanism (exactly how the reaction occurs). Why
Molecular Dynamics Simulations of Nonequilibrium Effects Associated with Thermally Activated Exothermic Reactions
 Original Paper orma, 5, 9 7, Molecular Dynamic Simulation of Nonequilibrium Effect ociated with Thermally ctivated Exothermic Reaction Jerzy GORECKI and Joanna Natalia GORECK Intitute of Phyical Chemitry,
Original Paper orma, 5, 9 7, Molecular Dynamic Simulation of Nonequilibrium Effect ociated with Thermally ctivated Exothermic Reaction Jerzy GORECKI and Joanna Natalia GORECK Intitute of Phyical Chemitry,
Linear Motion, Speed & Velocity
 Add Important Linear Motion, Speed & Velocity Page: 136 Linear Motion, Speed & Velocity NGSS Standard: N/A MA Curriculum Framework (006): 1.1, 1. AP Phyic 1 Learning Objective: 3.A.1.1, 3.A.1.3 Knowledge/Undertanding
Add Important Linear Motion, Speed & Velocity Page: 136 Linear Motion, Speed & Velocity NGSS Standard: N/A MA Curriculum Framework (006): 1.1, 1. AP Phyic 1 Learning Objective: 3.A.1.1, 3.A.1.3 Knowledge/Undertanding
Singular perturbation theory
 Singular perturbation theory Marc R. Rouel June 21, 2004 1 Introduction When we apply the teady-tate approximation (SSA) in chemical kinetic, we typically argue that ome of the intermediate are highly
Singular perturbation theory Marc R. Rouel June 21, 2004 1 Introduction When we apply the teady-tate approximation (SSA) in chemical kinetic, we typically argue that ome of the intermediate are highly
Chapter 13 Kinetics: Rates and Mechanisms of Chemical Reactions
 Chapter 13 Kinetics: Rates and Mechanisms of Chemical Reactions 14.1 Focusing on Reaction Rate 14.2 Expressing the Reaction Rate 14.3 The Rate Law and Its Components 14.4 Integrated Rate Laws: Concentration
Chapter 13 Kinetics: Rates and Mechanisms of Chemical Reactions 14.1 Focusing on Reaction Rate 14.2 Expressing the Reaction Rate 14.3 The Rate Law and Its Components 14.4 Integrated Rate Laws: Concentration
FUNDAMENTALS OF POWER SYSTEMS
 1 FUNDAMENTALS OF POWER SYSTEMS 1 Chapter FUNDAMENTALS OF POWER SYSTEMS INTRODUCTION The three baic element of electrical engineering are reitor, inductor and capacitor. The reitor conume ohmic or diipative
1 FUNDAMENTALS OF POWER SYSTEMS 1 Chapter FUNDAMENTALS OF POWER SYSTEMS INTRODUCTION The three baic element of electrical engineering are reitor, inductor and capacitor. The reitor conume ohmic or diipative
Chemical Kinetics. Chapter 13. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Chemical Kinetics Chapter 13 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chemical Kinetics Thermodynamics does a reaction take place? Kinetics how fast does
Chemical Kinetics Chapter 13 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chemical Kinetics Thermodynamics does a reaction take place? Kinetics how fast does
Chemical Kinetics. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Chemical Kinetics Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chemical Kinetics Thermodynamics does a reaction take place? Kinetics how fast does a reaction
Chemical Kinetics Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chemical Kinetics Thermodynamics does a reaction take place? Kinetics how fast does a reaction
AP Chemistry - Notes - Chapter 12 - Kinetics Page 1 of 7 Chapter 12 outline : Chemical kinetics
 AP Chemistry - Notes - Chapter 12 - Kinetics Page 1 of 7 Chapter 12 outline : Chemical kinetics A. Chemical Kinetics - chemistry of reaction rates 1. Reaction Rates a. Reaction rate- the change in concentration
AP Chemistry - Notes - Chapter 12 - Kinetics Page 1 of 7 Chapter 12 outline : Chemical kinetics A. Chemical Kinetics - chemistry of reaction rates 1. Reaction Rates a. Reaction rate- the change in concentration
Social Studies 201 Notes for March 18, 2005
 1 Social Studie 201 Note for March 18, 2005 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
1 Social Studie 201 Note for March 18, 2005 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
Kinetics - Chapter 14. reactions are reactions that will happen - but we can t tell how fast. - the steps by which a reaction takes place.
 The study of. Kinetics - Chapter 14 reactions are reactions that will happen - but we can t tell how fast. - the steps by which a reaction takes place. Factors that Affect Rx Rates 1. The more readily
The study of. Kinetics - Chapter 14 reactions are reactions that will happen - but we can t tell how fast. - the steps by which a reaction takes place. Factors that Affect Rx Rates 1. The more readily
Chemical Kinetics. Kinetics is the study of how fast chemical reactions occur. There are 4 important factors which affect rates of reactions:
 Chemical Kinetics Kinetics is the study of how fast chemical reactions occur. There are 4 important factors which affect rates of reactions: reactant concentration temperature action of catalysts surface
Chemical Kinetics Kinetics is the study of how fast chemical reactions occur. There are 4 important factors which affect rates of reactions: reactant concentration temperature action of catalysts surface
Chapter 14 Chemical Kinetics
 Chapter 14 14.1 Factors that Affect Reaction Rates 14.2 Reaction Rates 14.3 Concentration and Rate Laws 14.4 The Change of Concentration with Time 14.5 Temperature and Rate 14.6 Reaction Mechanisms 14.7
Chapter 14 14.1 Factors that Affect Reaction Rates 14.2 Reaction Rates 14.3 Concentration and Rate Laws 14.4 The Change of Concentration with Time 14.5 Temperature and Rate 14.6 Reaction Mechanisms 14.7
Math Skills. Scientific Notation. Uncertainty in Measurements. Appendix A5 SKILLS HANDBOOK
 ppendix 5 Scientific Notation It i difficult to work with very large or very mall number when they are written in common decimal notation. Uually it i poible to accommodate uch number by changing the SI
ppendix 5 Scientific Notation It i difficult to work with very large or very mall number when they are written in common decimal notation. Uually it i poible to accommodate uch number by changing the SI
CHAPTER 12 CHEMICAL KINETICS
 5/9/202 CHAPTER 2 CHEMICAL KINETICS CHM52 GCC Kinetics Some chemical reactions occur almost instantaneously, while others are very slow. Chemical Kinetics - study of factors that affect how fast a reaction
5/9/202 CHAPTER 2 CHEMICAL KINETICS CHM52 GCC Kinetics Some chemical reactions occur almost instantaneously, while others are very slow. Chemical Kinetics - study of factors that affect how fast a reaction
11/2/ and the not so familiar. Chemical kinetics is the study of how fast reactions take place.
 Familiar Kinetics...and the not so familiar Reaction Rates Chemical kinetics is the study of how fast reactions take place. Some happen almost instantaneously, while others can take millions of years.
Familiar Kinetics...and the not so familiar Reaction Rates Chemical kinetics is the study of how fast reactions take place. Some happen almost instantaneously, while others can take millions of years.
Reaction Mechanisms Dependence of rate on temperature Activation Energy E a Activated Complex Arrhenius Equation
 Kinetics Dependence of rate on Concentration (RATE LAW) Reaction Mechanisms Dependence of rate on temperature Activation Energy E a Activated Complex Arrhenius Equation Mary J. Bojan Chem 112 1 A MECHANISM
Kinetics Dependence of rate on Concentration (RATE LAW) Reaction Mechanisms Dependence of rate on temperature Activation Energy E a Activated Complex Arrhenius Equation Mary J. Bojan Chem 112 1 A MECHANISM
NCAAPMT Calculus Challenge Challenge #3 Due: October 26, 2011
 NCAAPMT Calculu Challenge 011 01 Challenge #3 Due: October 6, 011 A Model of Traffic Flow Everyone ha at ome time been on a multi-lane highway and encountered road contruction that required the traffic
NCAAPMT Calculu Challenge 011 01 Challenge #3 Due: October 6, 011 A Model of Traffic Flow Everyone ha at ome time been on a multi-lane highway and encountered road contruction that required the traffic
Halliday/Resnick/Walker 7e Chapter 6
 HRW 7e Chapter 6 Page of Halliday/Renick/Walker 7e Chapter 6 3. We do not conider the poibility that the bureau might tip, and treat thi a a purely horizontal motion problem (with the peron puh F in the
HRW 7e Chapter 6 Page of Halliday/Renick/Walker 7e Chapter 6 3. We do not conider the poibility that the bureau might tip, and treat thi a a purely horizontal motion problem (with the peron puh F in the
Laplace Transformation
 Univerity of Technology Electromechanical Department Energy Branch Advance Mathematic Laplace Tranformation nd Cla Lecture 6 Page of 7 Laplace Tranformation Definition Suppoe that f(t) i a piecewie continuou
Univerity of Technology Electromechanical Department Energy Branch Advance Mathematic Laplace Tranformation nd Cla Lecture 6 Page of 7 Laplace Tranformation Definition Suppoe that f(t) i a piecewie continuou
Suggested Answers To Exercises. estimates variability in a sampling distribution of random means. About 68% of means fall
 Beyond Significance Teting ( nd Edition), Rex B. Kline Suggeted Anwer To Exercie Chapter. The tatitic meaure variability among core at the cae level. In a normal ditribution, about 68% of the core fall
Beyond Significance Teting ( nd Edition), Rex B. Kline Suggeted Anwer To Exercie Chapter. The tatitic meaure variability among core at the cae level. In a normal ditribution, about 68% of the core fall
Lecture Presentation. Chapter 14. James F. Kirby Quinnipiac University Hamden, CT. Chemical Kinetics Pearson Education, Inc.
 Lecture Presentation Chapter 14 James F. Kirby Quinnipiac University Hamden, CT In chemical kinetics we study the rate (or speed) at which a chemical process occurs. Besides information about the speed
Lecture Presentation Chapter 14 James F. Kirby Quinnipiac University Hamden, CT In chemical kinetics we study the rate (or speed) at which a chemical process occurs. Besides information about the speed
Homework #4 Chapter 15 Chemical Kinetics. Therefore, k depends only on temperature. The rate of the reaction depends on all of these items (a d).
 Homework #4 Chapter 5 Chemical Kinetics 8. Arrhenius Equation Therefore, k depends only on temperature. The rate of the reaction depends on all of these items (a d). 4. a) d) b) c) e) 5. Rate has units
Homework #4 Chapter 5 Chemical Kinetics 8. Arrhenius Equation Therefore, k depends only on temperature. The rate of the reaction depends on all of these items (a d). 4. a) d) b) c) e) 5. Rate has units
Digital Control System
 Digital Control Sytem Summary # he -tranform play an important role in digital control and dicrete ignal proceing. he -tranform i defined a F () f(k) k () A. Example Conider the following equence: f(k)
Digital Control Sytem Summary # he -tranform play an important role in digital control and dicrete ignal proceing. he -tranform i defined a F () f(k) k () A. Example Conider the following equence: f(k)
Chapter 12. Chemical Kinetics
 Chapter 12 Chemical Kinetics Section 12.1 Reaction Rates Reaction Rate Change in concentration of a reactant or product per unit time. Rate = concentration of A at time t t 2 1 2 1 concentration of A at
Chapter 12 Chemical Kinetics Section 12.1 Reaction Rates Reaction Rate Change in concentration of a reactant or product per unit time. Rate = concentration of A at time t t 2 1 2 1 concentration of A at
Sample Question Solutions for the Chemistry of Environment Topic Exam
 Name (Lat, Firt): ID Number: Sample Quetion Solution for the Chemitry of Environment Topic Exam 1. The earth tratophere contain a region of high ozone (O3) concentration called the ozone layer. The ozone
Name (Lat, Firt): ID Number: Sample Quetion Solution for the Chemitry of Environment Topic Exam 1. The earth tratophere contain a region of high ozone (O3) concentration called the ozone layer. The ozone
1 year n0tes chemistry new st CHAPTER 7 THERMOCHEMISTRY MCQs Q.1 Which of the following statements is contrary to the first law of thermodynamics?
 year n0te chemitry new t CHAPTER 7 THERMOCHEMISTRY MCQ Q.1 Which of the following tatement i contrary to the firt law of thermodynamic? (a) energy can neither be created nor detroyed (b) one form of energy
year n0te chemitry new t CHAPTER 7 THERMOCHEMISTRY MCQ Q.1 Which of the following tatement i contrary to the firt law of thermodynamic? (a) energy can neither be created nor detroyed (b) one form of energy
CHEM Chapter 14. Chemical Kinetics (Homework) Ky40
 CHEM 1412. Chapter 14. Chemical Kinetics (Homework) Ky40 1. Chlorine dioxide reacts in basic water to form chlorite and chlorate according to the following chemical equation: 2ClO 2 (aq) + 2OH (aq) ClO
CHEM 1412. Chapter 14. Chemical Kinetics (Homework) Ky40 1. Chlorine dioxide reacts in basic water to form chlorite and chlorate according to the following chemical equation: 2ClO 2 (aq) + 2OH (aq) ClO
Chemical Kinetics Ch t ap 1 er
 Chemical Kinetics Chapter 13 1 Chemical Kinetics Thermodynamics does a reaction take place? Kinetics how fast does a reaction proceed? Reaction rate is the change in the concentration of a reactant or
Chemical Kinetics Chapter 13 1 Chemical Kinetics Thermodynamics does a reaction take place? Kinetics how fast does a reaction proceed? Reaction rate is the change in the concentration of a reactant or
Bogoliubov Transformation in Classical Mechanics
 Bogoliubov Tranformation in Claical Mechanic Canonical Tranformation Suppoe we have a et of complex canonical variable, {a j }, and would like to conider another et of variable, {b }, b b ({a j }). How
Bogoliubov Tranformation in Claical Mechanic Canonical Tranformation Suppoe we have a et of complex canonical variable, {a j }, and would like to conider another et of variable, {b }, b b ({a j }). How
Chapter 12. Kinetics. Factors That Affect Reaction Rates. Factors That Affect Reaction Rates. Chemical. Kinetics
 PowerPoint to accompany Kinetics Chapter 12 Chemical Kinetics Studies the rate at which a chemical process occurs. Besides information about the speed at which reactions occur, kinetics also sheds light
PowerPoint to accompany Kinetics Chapter 12 Chemical Kinetics Studies the rate at which a chemical process occurs. Besides information about the speed at which reactions occur, kinetics also sheds light
Name AP CHEM / / Chapter 12 Outline Chemical Kinetics
 Name AP CHEM / / Chapter 12 Outline Chemical Kinetics The area of chemistry that deals with the rate at which reactions occur is called chemical kinetics. One of the goals of chemical kinetics is to understand
Name AP CHEM / / Chapter 12 Outline Chemical Kinetics The area of chemistry that deals with the rate at which reactions occur is called chemical kinetics. One of the goals of chemical kinetics is to understand
Kinetics. Chapter 14. Chemical Kinetics
 Lecture Presentation Chapter 14 Yonsei University In kinetics we study the rate at which a chemical process occurs. Besides information about the speed at which reactions occur, kinetics also sheds light
Lecture Presentation Chapter 14 Yonsei University In kinetics we study the rate at which a chemical process occurs. Besides information about the speed at which reactions occur, kinetics also sheds light
Midterm Review - Part 1
 Honor Phyic Fall, 2016 Midterm Review - Part 1 Name: Mr. Leonard Intruction: Complete the following workheet. SHOW ALL OF YOUR WORK. 1. Determine whether each tatement i True or Fale. If the tatement i
Honor Phyic Fall, 2016 Midterm Review - Part 1 Name: Mr. Leonard Intruction: Complete the following workheet. SHOW ALL OF YOUR WORK. 1. Determine whether each tatement i True or Fale. If the tatement i
Convex Hulls of Curves Sam Burton
 Convex Hull of Curve Sam Burton 1 Introduction Thi paper will primarily be concerned with determining the face of convex hull of curve of the form C = {(t, t a, t b ) t [ 1, 1]}, a < b N in R 3. We hall
Convex Hull of Curve Sam Burton 1 Introduction Thi paper will primarily be concerned with determining the face of convex hull of curve of the form C = {(t, t a, t b ) t [ 1, 1]}, a < b N in R 3. We hall
Chapter 12. Chemical Kinetics
 Chapter 12 Chemical Kinetics Section 12.1 Reaction Rates Section 12.1 Reaction Rates Section 12.1 Reaction Rates Section 12.1 Reaction Rates Section 12.1 Reaction Rates Section 12.1 Reaction Rates Section
Chapter 12 Chemical Kinetics Section 12.1 Reaction Rates Section 12.1 Reaction Rates Section 12.1 Reaction Rates Section 12.1 Reaction Rates Section 12.1 Reaction Rates Section 12.1 Reaction Rates Section
Chapter 12 - Chemical Kinetics
 Chapter 1 - Chemical Kinetics 1.1 Reaction Rates A. Chemical kinetics 1. Study of the speed with which reactants are converted to products B. Reaction Rate 1. The change in concentration of a reactant
Chapter 1 - Chemical Kinetics 1.1 Reaction Rates A. Chemical kinetics 1. Study of the speed with which reactants are converted to products B. Reaction Rate 1. The change in concentration of a reactant
SOLUTIONS
 SOLUTIONS Topic-2 RAOULT S LAW, ALICATIONS AND NUMERICALS VERY SHORT ANSWER QUESTIONS 1. Define vapour preure? An: When a liquid i in equilibrium with it own vapour the preure exerted by the vapour on
SOLUTIONS Topic-2 RAOULT S LAW, ALICATIONS AND NUMERICALS VERY SHORT ANSWER QUESTIONS 1. Define vapour preure? An: When a liquid i in equilibrium with it own vapour the preure exerted by the vapour on
Math 273 Solutions to Review Problems for Exam 1
 Math 7 Solution to Review Problem for Exam True or Fale? Circle ONE anwer for each Hint: For effective tudy, explain why if true and give a counterexample if fale (a) T or F : If a b and b c, then a c
Math 7 Solution to Review Problem for Exam True or Fale? Circle ONE anwer for each Hint: For effective tudy, explain why if true and give a counterexample if fale (a) T or F : If a b and b c, then a c
2/23/2018. Familiar Kinetics. ...and the not so familiar. Chemical kinetics is the study of how fast reactions take place.
 CHEMICAL KINETICS & REACTION MECHANISMS Readings, Examples & Problems Petrucci, et al., th ed. Chapter 20 Petrucci, et al., 0 th ed. Chapter 4 Familiar Kinetics...and the not so familiar Reaction Rates
CHEMICAL KINETICS & REACTION MECHANISMS Readings, Examples & Problems Petrucci, et al., th ed. Chapter 20 Petrucci, et al., 0 th ed. Chapter 4 Familiar Kinetics...and the not so familiar Reaction Rates
Chapter 14. Chemical Kinetics
 Chapter 14. Chemical Kinetics 14.1 Factors that Affect Reaction Rates The speed at which a chemical reaction occurs is the reaction rate. Chemical kinetics is the study of how fast chemical reactions occur.
Chapter 14. Chemical Kinetics 14.1 Factors that Affect Reaction Rates The speed at which a chemical reaction occurs is the reaction rate. Chemical kinetics is the study of how fast chemical reactions occur.
Ch 13 Chemical Kinetics. Modified by Dr. Cheng-Yu Lai
 Ch 13 Chemical Kinetics Modified by Dr. Cheng-Yu Lai Outline 1. Meaning of reaction rate 2. Reaction rate and concentration 3. Writing a Rate Law 4. Reactant concentration and time 5. Reaction rate and
Ch 13 Chemical Kinetics Modified by Dr. Cheng-Yu Lai Outline 1. Meaning of reaction rate 2. Reaction rate and concentration 3. Writing a Rate Law 4. Reactant concentration and time 5. Reaction rate and
Chemical Kinetics and Equilibrium
 Chemical Kinetics and Equilibrium Part 1: Kinetics David A. Katz Department of Chemistry Pima Community College Tucson, AZ USA Chemical Kinetics The study of the rates of chemical reactions and how they
Chemical Kinetics and Equilibrium Part 1: Kinetics David A. Katz Department of Chemistry Pima Community College Tucson, AZ USA Chemical Kinetics The study of the rates of chemical reactions and how they
Correction for Simple System Example and Notes on Laplace Transforms / Deviation Variables ECHE 550 Fall 2002
 Correction for Simple Sytem Example and Note on Laplace Tranform / Deviation Variable ECHE 55 Fall 22 Conider a tank draining from an initial height of h o at time t =. With no flow into the tank (F in
Correction for Simple Sytem Example and Note on Laplace Tranform / Deviation Variable ECHE 55 Fall 22 Conider a tank draining from an initial height of h o at time t =. With no flow into the tank (F in
CHEMISTRY. Chapter 14 Chemical Kinetics
 CHEMISTRY The Central Science 8 th Edition Chapter 14 Kozet YAPSAKLI kinetics is the study of how rapidly chemical reactions occur. rate at which a chemical process occurs. Reaction rates depends on The
CHEMISTRY The Central Science 8 th Edition Chapter 14 Kozet YAPSAKLI kinetics is the study of how rapidly chemical reactions occur. rate at which a chemical process occurs. Reaction rates depends on The
Lecture 13. Thermodynamic Potentials (Ch. 5)
 Lecture 13. hermodynamic Potential (Ch. 5) So far we have been uing the total internal energy U and ometime the enthalpy H to characterize variou macrocopic ytem. hee function are called the thermodynamic
Lecture 13. hermodynamic Potential (Ch. 5) So far we have been uing the total internal energy U and ometime the enthalpy H to characterize variou macrocopic ytem. hee function are called the thermodynamic
Overflow from last lecture: Ewald construction and Brillouin zones Structure factor
 Lecture 5: Overflow from lat lecture: Ewald contruction and Brillouin zone Structure factor Review Conider direct lattice defined by vector R = u 1 a 1 + u 2 a 2 + u 3 a 3 where u 1, u 2, u 3 are integer
Lecture 5: Overflow from lat lecture: Ewald contruction and Brillouin zone Structure factor Review Conider direct lattice defined by vector R = u 1 a 1 + u 2 a 2 + u 3 a 3 where u 1, u 2, u 3 are integer
Cumulative Review of Calculus
 Cumulative Review of Calculu. Uing the limit definition of the lope of a tangent, determine the lope of the tangent to each curve at the given point. a. f 5,, 5 f,, f, f 5,,,. The poition, in metre, of
Cumulative Review of Calculu. Uing the limit definition of the lope of a tangent, determine the lope of the tangent to each curve at the given point. a. f 5,, 5 f,, f, f 5,,,. The poition, in metre, of
Chapter 14, Chemical Kinetics
 Last wee we covered the following material: Review Vapor Pressure with two volatile components Chapter 14, Chemical Kinetics (continued) Quizzes next wee will be on Chap 14 through section 14.5. 13.6 Colloids
Last wee we covered the following material: Review Vapor Pressure with two volatile components Chapter 14, Chemical Kinetics (continued) Quizzes next wee will be on Chap 14 through section 14.5. 13.6 Colloids
Clustering Methods without Given Number of Clusters
 Clutering Method without Given Number of Cluter Peng Xu, Fei Liu Introduction A we now, mean method i a very effective algorithm of clutering. It mot powerful feature i the calability and implicity. However,
Clutering Method without Given Number of Cluter Peng Xu, Fei Liu Introduction A we now, mean method i a very effective algorithm of clutering. It mot powerful feature i the calability and implicity. However,
AP CHEMISTRY NOTES 7-1 KINETICS AND RATE LAW AN INTRODUCTION
 AP CHEMISTRY NOTES 7-1 KINETICS AND RATE LAW AN INTRODUCTION CHEMICAL KINETICS the study of rates of chemical reactions and the mechanisms by which they occur FACTORS WHICH AFFECT REACTION RATES 1. Nature
AP CHEMISTRY NOTES 7-1 KINETICS AND RATE LAW AN INTRODUCTION CHEMICAL KINETICS the study of rates of chemical reactions and the mechanisms by which they occur FACTORS WHICH AFFECT REACTION RATES 1. Nature
Kinetics CHAPTER IN THIS CHAPTER
 CHAPTER 14 Kinetics IN THIS CHAPTER Summary: Thermodynamics often can be used to predict whether a reaction will occur spontaneously, but it gives very little information about the speed at which a reaction
CHAPTER 14 Kinetics IN THIS CHAPTER Summary: Thermodynamics often can be used to predict whether a reaction will occur spontaneously, but it gives very little information about the speed at which a reaction
Lecture (3) 1. Reaction Rates. 2 NO 2 (g) 2 NO(g) + O 2 (g) Summary:
 Summary: Lecture (3) The expressions of rate of reaction and types of rates; Stoichiometric relationships between the rates of appearance or disappearance of components in a given reaction; Determination
Summary: Lecture (3) The expressions of rate of reaction and types of rates; Stoichiometric relationships between the rates of appearance or disappearance of components in a given reaction; Determination
Chapter 2 Sampling and Quantization. In order to investigate sampling and quantization, the difference between analog
 Chapter Sampling and Quantization.1 Analog and Digital Signal In order to invetigate ampling and quantization, the difference between analog and digital ignal mut be undertood. Analog ignal conit of continuou
Chapter Sampling and Quantization.1 Analog and Digital Signal In order to invetigate ampling and quantization, the difference between analog and digital ignal mut be undertood. Analog ignal conit of continuou
Physics 741 Graduate Quantum Mechanics 1 Solutions to Final Exam, Fall 2014
 Phyic 7 Graduate Quantum Mechanic Solution to inal Eam all 0 Each quetion i worth 5 point with point for each part marked eparately Some poibly ueful formula appear at the end of the tet In four dimenion
Phyic 7 Graduate Quantum Mechanic Solution to inal Eam all 0 Each quetion i worth 5 point with point for each part marked eparately Some poibly ueful formula appear at the end of the tet In four dimenion
Chapter 14: Chemical Kinetics
 Chapter 14: Chemical Kinetics NOTE THIS CHAPTER IS #2 TOP TOPICS ON AP EXAM!!! NOT ONLY DO YOU NEED TO FOCUS ON THEORY (and lots of MATH) BUT YOU MUST READ THE FIGURES TOO!!! Ch 14.1 ~ Factors that Affect
Chapter 14: Chemical Kinetics NOTE THIS CHAPTER IS #2 TOP TOPICS ON AP EXAM!!! NOT ONLY DO YOU NEED TO FOCUS ON THEORY (and lots of MATH) BUT YOU MUST READ THE FIGURES TOO!!! Ch 14.1 ~ Factors that Affect
a = f s,max /m = s g. 4. We first analyze the forces on the pig of mass m. The incline angle is.
 Chapter 6 1. The greatet deceleration (of magnitude a) i provided by the maximum friction force (Eq. 6-1, with = mg in thi cae). Uing ewton econd law, we find a = f,max /m = g. Eq. -16 then give the hortet
Chapter 6 1. The greatet deceleration (of magnitude a) i provided by the maximum friction force (Eq. 6-1, with = mg in thi cae). Uing ewton econd law, we find a = f,max /m = g. Eq. -16 then give the hortet
11/9/2012 CHEMICAL REACTIONS. 1. Will the reaction occur? 2. How far will the reaction proceed? 3. How fast will the reaction occur?
 CHEMICAL REACTIONS LECTURE 11: CHEMICAL KINETICS 1. Will the reaction occur? 2. How far will the reaction proceed? 3. How fast will the reaction occur? CHEMICAL REACTIONS C(s, diamond) C(s, graphite) G
CHEMICAL REACTIONS LECTURE 11: CHEMICAL KINETICS 1. Will the reaction occur? 2. How far will the reaction proceed? 3. How fast will the reaction occur? CHEMICAL REACTIONS C(s, diamond) C(s, graphite) G
Calculating Rates of Substances. Rates of Substances. Ch. 12: Kinetics 12/14/2017. Creative Commons License
 Ch. 2: Kinetics An agama lizard basks in the sun. As its body warms, the chemical reactions of its metabolism speed up. Chemistry: OpenStax Creative Commons License Images and tables in this file have
Ch. 2: Kinetics An agama lizard basks in the sun. As its body warms, the chemical reactions of its metabolism speed up. Chemistry: OpenStax Creative Commons License Images and tables in this file have
7.2 INVERSE TRANSFORMS AND TRANSFORMS OF DERIVATIVES 281
 72 INVERSE TRANSFORMS AND TRANSFORMS OF DERIVATIVES 28 and i 2 Show how Euler formula (page 33) can then be ued to deduce the reult a ( a) 2 b 2 {e at co bt} {e at in bt} b ( a) 2 b 2 5 Under what condition
72 INVERSE TRANSFORMS AND TRANSFORMS OF DERIVATIVES 28 and i 2 Show how Euler formula (page 33) can then be ued to deduce the reult a ( a) 2 b 2 {e at co bt} {e at in bt} b ( a) 2 b 2 5 Under what condition
Chapter 14 Chemical Kinetics
 7/10/003 Chapter 14 Chemical Kinetics 14-1 Rates of Chemical Reactions 14- Reaction Rates and Concentrations 14-3 The Dependence of Concentrations on Time 14-4 Reaction Mechanisms 14-5 Reaction Mechanism
7/10/003 Chapter 14 Chemical Kinetics 14-1 Rates of Chemical Reactions 14- Reaction Rates and Concentrations 14-3 The Dependence of Concentrations on Time 14-4 Reaction Mechanisms 14-5 Reaction Mechanism
Q.4 Which of the following of will have the same number of
 year n0te chemitry new t Chapter 3rd GASES MCQ Q.1 The order of the rate of diffuion of gae NH3, SO2, Cl2 and CO2 i: (a) NH3 > SO2 > Cl2 > CO2 (b) NH3 > CO2 > SO2 > Cl2 Cl2> SO2 > CO2 > NH3 None of thee
year n0te chemitry new t Chapter 3rd GASES MCQ Q.1 The order of the rate of diffuion of gae NH3, SO2, Cl2 and CO2 i: (a) NH3 > SO2 > Cl2 > CO2 (b) NH3 > CO2 > SO2 > Cl2 Cl2> SO2 > CO2 > NH3 None of thee
Chapter 14 Chemical Kinetics
 4//004 Chapter 4 Chemical Kinetics 4- Rates of Chemical Reactions 4- Reaction Rates and Concentrations 4-3 The Dependence of Concentrations on Time 4-4 Reaction Mechanisms 4-5 Reaction Mechanism and Rate
4//004 Chapter 4 Chemical Kinetics 4- Rates of Chemical Reactions 4- Reaction Rates and Concentrations 4-3 The Dependence of Concentrations on Time 4-4 Reaction Mechanisms 4-5 Reaction Mechanism and Rate
Digital Control System
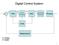 Digital Control Sytem - A D D A Micro ADC DAC Proceor Correction Element Proce Clock Meaurement A: Analog D: Digital Continuou Controller and Digital Control Rt - c Plant yt Continuou Controller Digital
Digital Control Sytem - A D D A Micro ADC DAC Proceor Correction Element Proce Clock Meaurement A: Analog D: Digital Continuou Controller and Digital Control Rt - c Plant yt Continuou Controller Digital
Solutions to exercises week 45 FYS2160
 Solution to exercie week 45 FYS2160 Kritian Bjørke, Knut Oddvar Høie Vadla November 29, 2017 Schroeder 5.23 a) Writing Φ = U T S µn in term of infiniteimal change of the quantitie involved: dφ = du T ds
Solution to exercie week 45 FYS2160 Kritian Bjørke, Knut Oddvar Høie Vadla November 29, 2017 Schroeder 5.23 a) Writing Φ = U T S µn in term of infiniteimal change of the quantitie involved: dφ = du T ds
Q.1. x A =0.8, ε A =δ A *y A = 0.8*5=4 (because feed contains 80 mol% A, y A = 0.8, δ A =((6-1)/1)=5) k= 0.3 hr -1. So, θ = hr Q.
 Q.1 k [ 1 ln(1 x)] x x =.8, ε =δ *y =.8*5=4 (becaue feed contain 8 mol%, y =.8, δ =((6-1)/1)=5) k=. hr -1 So, θ = 16.157 hr Q.2 Q.2 Continue (c) V PFR
Q.1 k [ 1 ln(1 x)] x x =.8, ε =δ *y =.8*5=4 (becaue feed contain 8 mol%, y =.8, δ =((6-1)/1)=5) k=. hr -1 So, θ = 16.157 hr Q.2 Q.2 Continue (c) V PFR
V = 4 3 πr3. d dt V = d ( 4 dv dt. = 4 3 π d dt r3 dv π 3r2 dv. dt = 4πr 2 dr
 0.1 Related Rate In many phyical ituation we have a relationhip between multiple quantitie, and we know the rate at which one of the quantitie i changing. Oftentime we can ue thi relationhip a a convenient
0.1 Related Rate In many phyical ituation we have a relationhip between multiple quantitie, and we know the rate at which one of the quantitie i changing. Oftentime we can ue thi relationhip a a convenient
Unit I Review Worksheet Key
 Unit I Review Workheet Key 1. Which of the following tatement about vector and calar are TRUE? Anwer: CD a. Fale - Thi would never be the cae. Vector imply are direction-conciou, path-independent quantitie
Unit I Review Workheet Key 1. Which of the following tatement about vector and calar are TRUE? Anwer: CD a. Fale - Thi would never be the cae. Vector imply are direction-conciou, path-independent quantitie
Chemical. Chapter 14. Kinetics. Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E.
 Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 14 1 PDF Created with deskpdf PDF www.farq.xyz Writer - Trial :: http://www.docudesk.com
Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 14 1 PDF Created with deskpdf PDF www.farq.xyz Writer - Trial :: http://www.docudesk.com
REACTION KINETICS. Catalysts substances that increase the rates of chemical reactions without being used up. e.g. enzymes.
 REACTION KINETICS Study of reaction rates Why? Rates of chemical reactions are primarily controlled by 5 factors: the chemical nature of the reactants 2 the ability of the reactants to come in contact
REACTION KINETICS Study of reaction rates Why? Rates of chemical reactions are primarily controlled by 5 factors: the chemical nature of the reactants 2 the ability of the reactants to come in contact
Dimensional Analysis A Tool for Guiding Mathematical Calculations
 Dimenional Analyi A Tool for Guiding Mathematical Calculation Dougla A. Kerr Iue 1 February 6, 2010 ABSTRACT AND INTRODUCTION In converting quantitie from one unit to another, we may know the applicable
Dimenional Analyi A Tool for Guiding Mathematical Calculation Dougla A. Kerr Iue 1 February 6, 2010 ABSTRACT AND INTRODUCTION In converting quantitie from one unit to another, we may know the applicable
1 inhibit5.mcd. Product Inhibition. Instructor: Nam Sun Wang
 Product Inhibition. Intructor: Nam Sun Wang inhibit5.mcd Mechanim. nzyme combine with a ubtrate molecule for form a complex, which lead to product. The product can alo combine with an enzyme in a reerible
Product Inhibition. Intructor: Nam Sun Wang inhibit5.mcd Mechanim. nzyme combine with a ubtrate molecule for form a complex, which lead to product. The product can alo combine with an enzyme in a reerible
Take home Exam due Wednesday, Aug 26. In class Exam will be the that morning class multiple choice questions.
 Announcements Take home Exam due Wednesday, Aug 26. In class Exam will be the that morning class. 15-20 multiple choice questions. Updated projects Aug 28: answer what lab chemistry needs to get done to
Announcements Take home Exam due Wednesday, Aug 26. In class Exam will be the that morning class. 15-20 multiple choice questions. Updated projects Aug 28: answer what lab chemistry needs to get done to
Solutions to homework #10
 Solution to homework #0 Problem 7..3 Compute 6 e 3 t t t 8. The firt tep i to ue the linearity of the Laplace tranform to ditribute the tranform over the um and pull the contant factor outide the tranform.
Solution to homework #0 Problem 7..3 Compute 6 e 3 t t t 8. The firt tep i to ue the linearity of the Laplace tranform to ditribute the tranform over the um and pull the contant factor outide the tranform.
Department of Mechanical Engineering Massachusetts Institute of Technology Modeling, Dynamics and Control III Spring 2002
 Department of Mechanical Engineering Maachuett Intitute of Technology 2.010 Modeling, Dynamic and Control III Spring 2002 SOLUTIONS: Problem Set # 10 Problem 1 Etimating tranfer function from Bode Plot.
Department of Mechanical Engineering Maachuett Intitute of Technology 2.010 Modeling, Dynamic and Control III Spring 2002 SOLUTIONS: Problem Set # 10 Problem 1 Etimating tranfer function from Bode Plot.
Question 1 Equivalent Circuits
 MAE 40 inear ircuit Fall 2007 Final Intruction ) Thi exam i open book You may ue whatever written material you chooe, including your cla note and textbook You may ue a hand calculator with no communication
MAE 40 inear ircuit Fall 2007 Final Intruction ) Thi exam i open book You may ue whatever written material you chooe, including your cla note and textbook You may ue a hand calculator with no communication
CHAPTER 13 (MOORE) CHEMICAL KINETICS: RATES AND MECHANISMS OF CHEMICAL REACTIONS
 CHAPTER 13 (MOORE) CHEMICAL KINETICS: RATES AND MECHANISMS OF CHEMICAL REACTIONS This chapter deals with reaction rates, or how fast chemical reactions occur. Reaction rates vary greatly some are very
CHAPTER 13 (MOORE) CHEMICAL KINETICS: RATES AND MECHANISMS OF CHEMICAL REACTIONS This chapter deals with reaction rates, or how fast chemical reactions occur. Reaction rates vary greatly some are very
Chemical Kinetics. What quantities do we study regarding chemical reactions? 15 Chemical Kinetics
 Chemical Kinetics Chemical kinetics: the study of reaction rate, a quantity conditions affecting it, the molecular events during a chemical reaction (mechanism), and presence of other components (catalysis).
Chemical Kinetics Chemical kinetics: the study of reaction rate, a quantity conditions affecting it, the molecular events during a chemical reaction (mechanism), and presence of other components (catalysis).
Chapter 13 Lecture Lecture Presentation. Chapter 13. Chemical Kinetics. Sherril Soman Grand Valley State University Pearson Education, Inc.
 Chapter 13 Lecture Lecture Presentation Chapter 13 Chemical Kinetics Sherril Soman Grand Valley State University Ectotherms Lizards, and other cold-blooded creatures, are ectotherms animals whose body
Chapter 13 Lecture Lecture Presentation Chapter 13 Chemical Kinetics Sherril Soman Grand Valley State University Ectotherms Lizards, and other cold-blooded creatures, are ectotherms animals whose body
concentrations (molarity) rate constant, (k), depends on size, speed, kind of molecule, temperature, etc.
 #73 Notes Unit 9: Kinetics and Equilibrium Ch. Kinetics and Equilibriums I. Reaction Rates NO 2(g) + CO (g) NO (g) + CO 2(g) Rate is defined in terms of the rate of disappearance of one of the reactants,
#73 Notes Unit 9: Kinetics and Equilibrium Ch. Kinetics and Equilibriums I. Reaction Rates NO 2(g) + CO (g) NO (g) + CO 2(g) Rate is defined in terms of the rate of disappearance of one of the reactants,
Sampling and the Discrete Fourier Transform
 Sampling and the Dicrete Fourier Tranform Sampling Method Sampling i mot commonly done with two device, the ample-and-hold (S/H) and the analog-to-digital-converter (ADC) The S/H acquire a CT ignal at
Sampling and the Dicrete Fourier Tranform Sampling Method Sampling i mot commonly done with two device, the ample-and-hold (S/H) and the analog-to-digital-converter (ADC) The S/H acquire a CT ignal at
Chemistry 102 Chapter 14 CHEMICAL KINETICS. The study of the Rates of Chemical Reactions: how fast do chemical reactions proceed to form products
 CHEMICAL KINETICS Chemical Kinetics: The study of the Rates of Chemical Reactions: how fast do chemical reactions proceed to form products The study of Reaction Mechanisms: the steps involved in the change
CHEMICAL KINETICS Chemical Kinetics: The study of the Rates of Chemical Reactions: how fast do chemical reactions proceed to form products The study of Reaction Mechanisms: the steps involved in the change
Unit 12: Chemical Kinetics
 Unit 12: Chemical Kinetics Author: S. Michalek Introductory Resources: Zumdahl v. 5 Chapter 12 Main Ideas: Integrated rate laws Half life reactions Reaction Mechanisms Model for chemical kinetics Catalysis
Unit 12: Chemical Kinetics Author: S. Michalek Introductory Resources: Zumdahl v. 5 Chapter 12 Main Ideas: Integrated rate laws Half life reactions Reaction Mechanisms Model for chemical kinetics Catalysis
Reaction Mechanisms. Chemical Kinetics. Reaction Mechanisms. Reaction Mechanisms. Reaction Mechanisms. Reaction Mechanisms
 Chemical Kinetics Kinetics is a study of the rate at which a chemical reaction occurs. The study of kinetics may be done in steps: Determination of reaction mechanism Prediction of rate law Measurement
Chemical Kinetics Kinetics is a study of the rate at which a chemical reaction occurs. The study of kinetics may be done in steps: Determination of reaction mechanism Prediction of rate law Measurement
