|
|
|
- Mercy Morton
- 6 years ago
- Views:
Transcription
1 1 t year chemitry n0te new CHAPTER 11 REACTION KINETICS MCQS Q.1 In zero order reaction, the rate i independent of (a) temperature of reaction (b) concentration of reactant (c) concentration of product Q.2 If the rate equation of a reaction 2A + B Product, Rate = k [A]2 [B] and A i preent in large exce then order of reaction i: (a) 1 (b) 2 (c) 3 (d) none of thee Q.3 The rate of reaction (a) increae a the reaction proceed (b) decreae a the reaction proceed (c) remain the ame a the reaction proceed (d) may decreae or increae a the reaction proceed Q.4 With increae of 10 oc temperature the rate of reaction double. Thi increae in the rate of reaction i due to (a) decreae in activation energy of reaction (b) decreae in the number of colliion b/w reactant molecule (c) increae in activation energy of reactant (d) increae in number of effective colliion Q.5 The unit of the rate contant i the ame a that of the rate of reaction in (a) firt order reaction (b) econd order reaction (c) zero order reaction (d) third order reaction 1
2 1 t year chemitry n0te new Q.6 The unit of reaction i (a) mole/dm3 (b) mole/pound (c) mole/dm3 ec (d) mole/cm3 Q.7 In the rate equation, when the conc. of reactant i unity then rate i equal to (a) pecific rate contant (b) average rate contant (c) intantaneou rate contant Q.8 The rate of reaction between two pecific time interval i called (a) intantaneou rate (b) average rate (c) pecific rate (d) ordinary rate Q.9 Intantaneou rate of a chemical reaction i (a) rate of reaction in the beginning (b) rate of reaction at the end (c) rate of reaction at a given intant (d) rate of reaction b/w two pecific time interval Q.10 At the beginning the decreae in the conc. of reactant i (a) low (b) moderate (c) rapid Q.11 The um of exponent of the conc. term in the rate equation i called (a) rate of reaction (b) order of reaction (c) pecific rate contant (d) average rate Q.12 The average rate and intantaneou rate of a reaction are equal (a) at the tart (b) at the end (c) in the middle (d) when two rate have time interval equal to zero Q.13 The equation 2N2O5 2N2 ha order (a) firt order (b) econd order (c) negative order (d) fractional order Q.14 The hydrolyi of tertiary butyl ha order (a) firt order (b) peudo firt order (c) fractional order (d) zero order 2
3 1 t year chemitry n0te new Q.15 Photochemical reaction uually have order (a) one (b) zero (c) two (d) three Q.16 The experimental relationhip between a reaction rate and the concentration of reactant i called (a) order of reaction (b) pecific rate (c) law of ma action (d) rate law Q.17 When the rate of reaction i entirely independent of the conc. of reactant molecule then order of reaction i (a) zero (b) firt (c) econd (d) third Q.18 Half life of U i (a) 7.1 x 108 year (b) 6.1 x 108 year (c) 8.1 x 107 year (d) 7.1 x 1010 year Q.19 Half life period for decompoition of N2O5 at 45 oc i (a) 24 minute (b) 34 minute (c) 44 minute (d) 54 minute Q.20 The decompoition of ozone ha order (a) firt (b) negative (c) econd (d) peudo firt order Q.21 The equation CHCl3 + Cl2 CCl4 + HCl ha order (a) firt (b) negative (c) fractional (d) econd Q.22 When a reaction occur in many tep then the lowet tep i the (a) main tep (b) enthalpy determining tep (c) mechanim determining tep (d) rate determining tep Q.23 Spectrometry applied for rate determination when (a) reactant or product aborb U.V., I.R. light (b) reaction involve ion (c) reaction involve change in volume 3
4 1 t year chemitry n0te new Q.24 Electrical conductivity method i applied for rate determination when (a) reactant and product involve aborption of U.V. or I.R. radiation (b) reaction involving ion (c) reaction which involve change in refractive indice (d) reaction which involve mall volume change Q.25 Dilatometric method i ued for rate determination when (a) reaction involving ion (b) reaction involving change of optical activity (c) reaction involving mall volume change Q.26 Refractrometric method i ued when (a) reaction involving aborption of I.R. or U.V. (b) reaction involving change of refractive index (c) reaction involving ion (d) change of optical activity 4
5 1 t year chemitry n0te new Q.27 Optical rotation method i ued when (a) reaction involve ion (b) change of refractive indice (c) reaction involving change of optical activity Q.28 The ubtance which retard the rate of chemical reaction (a) catalyt (b) inhibitor (c) auto catalyt (d) enzyme Q.29 The enzyme ued in the hydrolyi of urea i (a) ureae (b) amylae (c) oxidae (d) reductae Q.30 In the hydrolyi of CH3COO2H5 the acid produce act a (a) inhibitor (b) catalyt (c) auto catalyt Q.31 The order of reaction can be determined by (a) graphical method (b) method of hit and trial (c) differential method (d) all of above Q.32 The factor which affect rate of reaction (a) nature of reactant (b) urface area (c) light (d) all of above Q.33 When temp of reacting gae i raied to 10 K, the reaction rate become (a) remain ame (b) double (c) triple (d) increae four time Q.34 Arrheniu equation decribe the effect of (a) temp on rate of reaction (b) volume on rate of reaction (c) preure on rate of reaction (d) all the above Q.35 A ubtance which alter the rate of reaction (a) inhibitor (b) catalyt (c) promoter (d) auto catalyt Q.36 Homogeneou catalyi when 5
6 1 t year chemitry n0te new (a) reactant and catalyt have ame phae (b) product and catalyt have ame phae (c) reactant and product have ame phae Q.37 The heterogenou catalyi (a) reactant and product have different phae (b) reactant and catalyt have different phae (c) product and catalyt have different phae (d) all the above Q.38 Tetra ethyl lead when added to petrol, act a (a) negative catalyt (b) auto catalyt (c) promoter (d) catalyt Q.39 Concentrated ugar olution undergoe hydrolyi by an enzyme (a) invertae (b) ureae (c) zymae (d) glucae Q.40 Glucoe i converted into ethanol by an enzyme (a) ureae (b) invertae (c) zymae (d) glucoe ANSWERS Quetion Anwer b a b d c Quetion Anwer c a b c c Quetion
7 1 t year chemitry n0te new Anwer b d a b b Quetion Anwer d a a a b Quetion Anwer c d a b c Quetion Anwer b c b a c Quetion Anwer d d b a b Quetion Anwer a b a a c 7
1 year n0tes chemistry new st CHAPTER 7 THERMOCHEMISTRY MCQs Q.1 Which of the following statements is contrary to the first law of thermodynamics?
 year n0te chemitry new t CHAPTER 7 THERMOCHEMISTRY MCQ Q.1 Which of the following tatement i contrary to the firt law of thermodynamic? (a) energy can neither be created nor detroyed (b) one form of energy
year n0te chemitry new t CHAPTER 7 THERMOCHEMISTRY MCQ Q.1 Which of the following tatement i contrary to the firt law of thermodynamic? (a) energy can neither be created nor detroyed (b) one form of energy
 CHAPTER 11 REACTION KINETICS SHORT QUESTION WITH ANSWERS Q.1 What is meant by chemical kinetics? It is that branch of chemistry which helps to study the followings. (i) Rates of chemical reactions. (ii)
CHAPTER 11 REACTION KINETICS SHORT QUESTION WITH ANSWERS Q.1 What is meant by chemical kinetics? It is that branch of chemistry which helps to study the followings. (i) Rates of chemical reactions. (ii)
 1 t year chemitry n0te new CHAPTER 9 SOLUTIONS MCQ Q.1 Which of the following olution ha the highet boiling point? (a) 5.85% olution of NaCl (b) 18.0% olution of glucoe (c) 6.0% olution of urea (d) all
1 t year chemitry n0te new CHAPTER 9 SOLUTIONS MCQ Q.1 Which of the following olution ha the highet boiling point? (a) 5.85% olution of NaCl (b) 18.0% olution of glucoe (c) 6.0% olution of urea (d) all
4. The following graphs were prepared from experimental data for a reactant, A. What is the correct order of A?
 Diebolt Summer 010 CHM 15 HOUR EXAM I KEY (15 pt) Part One: Multiple Choice. 1. Conider the reaction IO 3 + 5I + 6H + 3I + 3H O. If the rate of diappearance of I i 9.010-3 M/, what i the rate of appearance
Diebolt Summer 010 CHM 15 HOUR EXAM I KEY (15 pt) Part One: Multiple Choice. 1. Conider the reaction IO 3 + 5I + 6H + 3I + 3H O. If the rate of diappearance of I i 9.010-3 M/, what i the rate of appearance
Q.4 Which of the following of will have the same number of
 year n0te chemitry new t Chapter 3rd GASES MCQ Q.1 The order of the rate of diffuion of gae NH3, SO2, Cl2 and CO2 i: (a) NH3 > SO2 > Cl2 > CO2 (b) NH3 > CO2 > SO2 > Cl2 Cl2> SO2 > CO2 > NH3 None of thee
year n0te chemitry new t Chapter 3rd GASES MCQ Q.1 The order of the rate of diffuion of gae NH3, SO2, Cl2 and CO2 i: (a) NH3 > SO2 > Cl2 > CO2 (b) NH3 > CO2 > SO2 > Cl2 Cl2> SO2 > CO2 > NH3 None of thee
1. The reaction rate is defined as the change in concentration of a reactant or product per unit time. Consider the general reaction:
 CHAPTER TWELVE CHEMICAL KINETICS For Review. The reaction rate i defined a the change in concentration of a reactant or product per unit time. Conider the general reaction: A Product where rate = Δ Δt
CHAPTER TWELVE CHEMICAL KINETICS For Review. The reaction rate i defined a the change in concentration of a reactant or product per unit time. Conider the general reaction: A Product where rate = Δ Δt
AP Chemistry: Kinetics Practice Problems
 AP Chemitr: Kinetic Practice Problem Direction: Write our anwer to the following quetion in the pace provided. For problem olving, how all of our work. Make ure that our anwer how proper unit, notation,
AP Chemitr: Kinetic Practice Problem Direction: Write our anwer to the following quetion in the pace provided. For problem olving, how all of our work. Make ure that our anwer how proper unit, notation,
1 year chemistry n0tes new st CHAPTER 8 CHEMICAL EQUILIBRIUM MCQs Q.1 A reaction is reversible because (a) reactants are reactive (b) products are
 year chemitry n0te new t CHAPTER 8 CHEMICAL EQUILIBRIUM MCQ Q.1 A reaction i reverible becaue reactant are reactive (b) product are reactive (c) product are table (d) reactant are table Q.2 A large value
year chemitry n0te new t CHAPTER 8 CHEMICAL EQUILIBRIUM MCQ Q.1 A reaction i reverible becaue reactant are reactive (b) product are reactive (c) product are table (d) reactant are table Q.2 A large value
Chemistry 6A F2007. Dr. J.A. Mack. Titration Curves: 11/30/07. What do I need to bring? Exam 3: Friday 12/7/07 (here in lecture)
 Chemitry 6A F2007 Dr. J.A. Mack Eam : Friday 12/7/07 (here in lecture) What will be covered on the eam? Chapter 6: 6.9-6.15 Chapter 7: All Chapter 8: All Chapter 9: 9.1-9.9 Any thing from lab a well What
Chemitry 6A F2007 Dr. J.A. Mack Eam : Friday 12/7/07 (here in lecture) What will be covered on the eam? Chapter 6: 6.9-6.15 Chapter 7: All Chapter 8: All Chapter 9: 9.1-9.9 Any thing from lab a well What
Sample Question Solutions for the Chemistry of Environment Topic Exam
 Name (Lat, Firt): ID Number: Sample Quetion Solution for the Chemitry of Environment Topic Exam 1. The earth tratophere contain a region of high ozone (O3) concentration called the ozone layer. The ozone
Name (Lat, Firt): ID Number: Sample Quetion Solution for the Chemitry of Environment Topic Exam 1. The earth tratophere contain a region of high ozone (O3) concentration called the ozone layer. The ozone
 1 t year n0te chemitry new CHAPTER 5 ATOMIC STRUCTURE MCQ Q.1 Splitting of pectral line when atom are ubjected to trong electric field i called (a) Zeeman effect (b) Stark effect (c) Photoelectric effect
1 t year n0te chemitry new CHAPTER 5 ATOMIC STRUCTURE MCQ Q.1 Splitting of pectral line when atom are ubjected to trong electric field i called (a) Zeeman effect (b) Stark effect (c) Photoelectric effect
Unit 2 Linear Motion
 Unit Linear Motion Linear Motion Key Term - How to calculate Speed & Ditance 1) Motion Term: a. Symbol for time = (t) b. Diplacement (X) How far omething travel in a given direction. c. Rate How much omething
Unit Linear Motion Linear Motion Key Term - How to calculate Speed & Ditance 1) Motion Term: a. Symbol for time = (t) b. Diplacement (X) How far omething travel in a given direction. c. Rate How much omething
 1 t year n0te chemitry new CHAPTER 6 CHEMICAL BONDING MCQ Q.1 An ionic compound A+ B i mot likely to be formed when (a) The ionization energy of A i high and electron affinity of B i low (b) The ionization
1 t year n0te chemitry new CHAPTER 6 CHEMICAL BONDING MCQ Q.1 An ionic compound A+ B i mot likely to be formed when (a) The ionization energy of A i high and electron affinity of B i low (b) The ionization
Radiation Heat Transfer
 CM30 ranport I Part II: Heat ranfer Radiation Heat ranfer Profeor Faith Morrion Department of Chemical Engineering Michigan echnological Univerity CM30 ranport Procee and Unit Operation I Part : Heat ranfer
CM30 ranport I Part II: Heat ranfer Radiation Heat ranfer Profeor Faith Morrion Department of Chemical Engineering Michigan echnological Univerity CM30 ranport Procee and Unit Operation I Part : Heat ranfer
SOLUTIONS
 SOLUTIONS Topic-2 RAOULT S LAW, ALICATIONS AND NUMERICALS VERY SHORT ANSWER QUESTIONS 1. Define vapour preure? An: When a liquid i in equilibrium with it own vapour the preure exerted by the vapour on
SOLUTIONS Topic-2 RAOULT S LAW, ALICATIONS AND NUMERICALS VERY SHORT ANSWER QUESTIONS 1. Define vapour preure? An: When a liquid i in equilibrium with it own vapour the preure exerted by the vapour on
ME 375 FINAL EXAM Wednesday, May 6, 2009
 ME 375 FINAL EXAM Wedneday, May 6, 9 Diviion Meckl :3 / Adam :3 (circle one) Name_ Intruction () Thi i a cloed book examination, but you are allowed three ingle-ided 8.5 crib heet. A calculator i NOT allowed.
ME 375 FINAL EXAM Wedneday, May 6, 9 Diviion Meckl :3 / Adam :3 (circle one) Name_ Intruction () Thi i a cloed book examination, but you are allowed three ingle-ided 8.5 crib heet. A calculator i NOT allowed.
b. The rate law can only be determined from experiment, not from the overall balanced reaction.
 CHAPTER CHEMICAL KINETICS Quetion. a. Activation energ and ΔE are independent of each other. Activation energ depend on the path reactant to tae to convert to product. The overall energ change ΔE onl depend
CHAPTER CHEMICAL KINETICS Quetion. a. Activation energ and ΔE are independent of each other. Activation energ depend on the path reactant to tae to convert to product. The overall energ change ΔE onl depend
Unit #10. Chemical Kinetics
 Unit #10 Chemical Kinetics Zumdahl Chapter 12 College Board Performance Objectives: Express the rate of a reaction in terms of changes in the concentration of a reactant or a product per time. Understand
Unit #10 Chemical Kinetics Zumdahl Chapter 12 College Board Performance Objectives: Express the rate of a reaction in terms of changes in the concentration of a reactant or a product per time. Understand
Chemical Kinetics -- Chapter 14
 Chemical Kinetics -- Chapter 14 1. Factors that Affect Reaction Rate (a) Nature of the reactants: molecular structure, bond polarity, physical state, etc. heterogeneous reaction: homogeneous reaction:
Chemical Kinetics -- Chapter 14 1. Factors that Affect Reaction Rate (a) Nature of the reactants: molecular structure, bond polarity, physical state, etc. heterogeneous reaction: homogeneous reaction:
Digital Control System
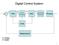 Digital Control Sytem - A D D A Micro ADC DAC Proceor Correction Element Proce Clock Meaurement A: Analog D: Digital Continuou Controller and Digital Control Rt - c Plant yt Continuou Controller Digital
Digital Control Sytem - A D D A Micro ADC DAC Proceor Correction Element Proce Clock Meaurement A: Analog D: Digital Continuou Controller and Digital Control Rt - c Plant yt Continuou Controller Digital
Factors That Affect Rates. Factors That Affect Rates. Factors That Affect Rates. Factors That Affect Rates
 KINETICS Kinetics Study of the speed or rate of a reaction under various conditions Thermodynamically favorable reactions DO NOT mean fast reactions Some reactions take fraction of a second (explosion)
KINETICS Kinetics Study of the speed or rate of a reaction under various conditions Thermodynamically favorable reactions DO NOT mean fast reactions Some reactions take fraction of a second (explosion)
Chemistry Notes for class 12 Chapter 4 Chemical Kinetics
 1 P a g e Chemistry Notes for class 12 Chapter 4 Chemical Kinetics The branch of chemistry, which deals with the rate of chemical reactions. the factors affecting the rate of reactions and the mechanism
1 P a g e Chemistry Notes for class 12 Chapter 4 Chemical Kinetics The branch of chemistry, which deals with the rate of chemical reactions. the factors affecting the rate of reactions and the mechanism
AP CHEMISTRY CHAPTER 12 KINETICS
 AP CHEMISTRY CHAPTER 12 KINETICS Thermodynamics tells us if a reaction can occur. Kinetics tells us how quickly the reaction occurs. Some reactions that are thermodynamically feasible are kinetically so
AP CHEMISTRY CHAPTER 12 KINETICS Thermodynamics tells us if a reaction can occur. Kinetics tells us how quickly the reaction occurs. Some reactions that are thermodynamically feasible are kinetically so
1. Basic introduction to electromagnetic field. wave properties and particulate properties.
 Lecture Baic Radiometric Quantitie. The Beer-Bouguer-Lambert law. Concept of extinction cattering plu aborption and emiion. Schwarzchild equation. Objective:. Baic introduction to electromagnetic field:
Lecture Baic Radiometric Quantitie. The Beer-Bouguer-Lambert law. Concept of extinction cattering plu aborption and emiion. Schwarzchild equation. Objective:. Baic introduction to electromagnetic field:
MCB4UW Handout 4.11 Related Rates of Change
 MCB4UW Handout 4. Related Rate of Change. Water flow into a rectangular pool whoe dimenion are m long, 8 m wide, and 0 m deep. If water i entering the pool at the rate of cubic metre per econd (hint: thi
MCB4UW Handout 4. Related Rate of Change. Water flow into a rectangular pool whoe dimenion are m long, 8 m wide, and 0 m deep. If water i entering the pool at the rate of cubic metre per econd (hint: thi
Linear Motion, Speed & Velocity
 Add Important Linear Motion, Speed & Velocity Page: 136 Linear Motion, Speed & Velocity NGSS Standard: N/A MA Curriculum Framework (006): 1.1, 1. AP Phyic 1 Learning Objective: 3.A.1.1, 3.A.1.3 Knowledge/Undertanding
Add Important Linear Motion, Speed & Velocity Page: 136 Linear Motion, Speed & Velocity NGSS Standard: N/A MA Curriculum Framework (006): 1.1, 1. AP Phyic 1 Learning Objective: 3.A.1.1, 3.A.1.3 Knowledge/Undertanding
Lecture 3 Basic radiometric quantities.
 Lecture 3 Baic radiometric quantitie. The Beer-Bouguer-Lambert law. Concept of extinction cattering plu aborption and emiion. Schwarzchild equation.. Baic introduction to electromagnetic field: Definition,
Lecture 3 Baic radiometric quantitie. The Beer-Bouguer-Lambert law. Concept of extinction cattering plu aborption and emiion. Schwarzchild equation.. Baic introduction to electromagnetic field: Definition,
ELECTROMAGNETIC WAVES AND PHOTONS
 CHAPTER ELECTROMAGNETIC WAVES AND PHOTONS Problem.1 Find the magnitude and direction of the induced electric field of Example.1 at r = 5.00 cm if the magnetic field change at a contant rate from 0.500
CHAPTER ELECTROMAGNETIC WAVES AND PHOTONS Problem.1 Find the magnitude and direction of the induced electric field of Example.1 at r = 5.00 cm if the magnetic field change at a contant rate from 0.500
On the Isentropic Forchheimer s Sound Waves Propagation in a Cylindrical Tube Filled with a Porous Media
 5th WSEAS Int. Conf. on FLUID MECHANICS (FLUIDS') Acapulco, Mexico, January 5-7, On the Ientropic Forchheimer Sound Wave Propagation in a Cylindrical Tube Filled with a Porou Media H. M. Dwairi Civil Engineering
5th WSEAS Int. Conf. on FLUID MECHANICS (FLUIDS') Acapulco, Mexico, January 5-7, On the Ientropic Forchheimer Sound Wave Propagation in a Cylindrical Tube Filled with a Porou Media H. M. Dwairi Civil Engineering
( ) y = Properties of Gaussian curves: Can also be written as: where
 Propertie of Gauian curve: Can alo be written a: e ( x μ ) σ σ π e z σ π where z ( ) x μ σ z repreent the deviation of a reult from the population mean relative to the population tandard deviation. z i
Propertie of Gauian curve: Can alo be written a: e ( x μ ) σ σ π e z σ π where z ( ) x μ σ z repreent the deviation of a reult from the population mean relative to the population tandard deviation. z i
2.7 Aerosols and coagulation
 1 Note on 1.63 Advanced Environmental Fluid Mechanic Intructor: C. C. Mei, 1 ccmei@mit.edu, 1 617 53 994 December 1,.7 Aerool and coagulation [Ref]: Preent, Kinetic Theory of Gae Fuch, Mechanic of Aerool
1 Note on 1.63 Advanced Environmental Fluid Mechanic Intructor: C. C. Mei, 1 ccmei@mit.edu, 1 617 53 994 December 1,.7 Aerool and coagulation [Ref]: Preent, Kinetic Theory of Gae Fuch, Mechanic of Aerool
Saber y Hacer Revista de Ingeniería de la USIL Vol. 1, Nº 1. Primer semestre pp
 Saber y Hacer Revita de Ingeniería de la USIL Vol. 1, Nº 1. Primer emetre 2014. pp. 27-40 Hydrodynamic interaction between upended ediment and the tranport of contaminant in open channel flow Interacción
Saber y Hacer Revita de Ingeniería de la USIL Vol. 1, Nº 1. Primer emetre 2014. pp. 27-40 Hydrodynamic interaction between upended ediment and the tranport of contaminant in open channel flow Interacción
V = 4 3 πr3. d dt V = d ( 4 dv dt. = 4 3 π d dt r3 dv π 3r2 dv. dt = 4πr 2 dr
 0.1 Related Rate In many phyical ituation we have a relationhip between multiple quantitie, and we know the rate at which one of the quantitie i changing. Oftentime we can ue thi relationhip a a convenient
0.1 Related Rate In many phyical ituation we have a relationhip between multiple quantitie, and we know the rate at which one of the quantitie i changing. Oftentime we can ue thi relationhip a a convenient
Isentropic Sound Waves Propagation in a Tube Filled with a Porous Media
 INTERNATIONAL JOURNAL OF ECHANICS Ientropic Sound Wave Propagation in a Tube Filled with a Porou edia H.. Duwairi Abtract A rigid frame, cylindrical capillary theory of ound propagation in porou media
INTERNATIONAL JOURNAL OF ECHANICS Ientropic Sound Wave Propagation in a Tube Filled with a Porou edia H.. Duwairi Abtract A rigid frame, cylindrical capillary theory of ound propagation in porou media
At the end of this lesson, the students should be able to understand:
 Intructional Objective: At the end of thi leon, the tudent hould be able to undertand: Baic failure mechanim of riveted joint. Concept of deign of a riveted joint. 1. Strength of riveted joint: Strength
Intructional Objective: At the end of thi leon, the tudent hould be able to undertand: Baic failure mechanim of riveted joint. Concept of deign of a riveted joint. 1. Strength of riveted joint: Strength
THERMODYNAMICS OF REACTING SYSTEMS
 ChE 505 Chapter N THERMODYNAMICS OF REACTING SYSTEMS. Introduction Thi chapter explain the baic of thermodynamic calculation for reacting ytem. Reaction toichiometry, heat and entropy of reaction a well
ChE 505 Chapter N THERMODYNAMICS OF REACTING SYSTEMS. Introduction Thi chapter explain the baic of thermodynamic calculation for reacting ytem. Reaction toichiometry, heat and entropy of reaction a well
Lecture 13. Thermodynamic Potentials (Ch. 5)
 Lecture 13. hermodynamic Potential (Ch. 5) So far we have been uing the total internal energy U and ometime the enthalpy H to characterize variou macrocopic ytem. hee function are called the thermodynamic
Lecture 13. hermodynamic Potential (Ch. 5) So far we have been uing the total internal energy U and ometime the enthalpy H to characterize variou macrocopic ytem. hee function are called the thermodynamic
CHEMICAL KINETICS (RATES OF REACTION)
 Kinetics F322 1 CHEMICAL KINETICS (RATES OF REACTION) Introduction Chemical kinetics is concerned with the dynamics of chemical reactions such as the way reactions take place and the rate (speed) of the
Kinetics F322 1 CHEMICAL KINETICS (RATES OF REACTION) Introduction Chemical kinetics is concerned with the dynamics of chemical reactions such as the way reactions take place and the rate (speed) of the
Lecture #9 Continuous time filter
 Lecture #9 Continuou time filter Oliver Faut December 5, 2006 Content Review. Motivation......................................... 2 2 Filter pecification 2 2. Low pa..........................................
Lecture #9 Continuou time filter Oliver Faut December 5, 2006 Content Review. Motivation......................................... 2 2 Filter pecification 2 2. Low pa..........................................
Question 1 Equivalent Circuits
 MAE 40 inear ircuit Fall 2007 Final Intruction ) Thi exam i open book You may ue whatever written material you chooe, including your cla note and textbook You may ue a hand calculator with no communication
MAE 40 inear ircuit Fall 2007 Final Intruction ) Thi exam i open book You may ue whatever written material you chooe, including your cla note and textbook You may ue a hand calculator with no communication
Factors that Affect Reaction Rates
 Factors that Affect Reaction Rates Preface: There are 2 kinds of reactions: Homogeneous reactions - all reactants are in the same phase (don't consider products) eg.) 3H 2(g) + N 2(g) 2NH 3(g) Ag + (aq)
Factors that Affect Reaction Rates Preface: There are 2 kinds of reactions: Homogeneous reactions - all reactants are in the same phase (don't consider products) eg.) 3H 2(g) + N 2(g) 2NH 3(g) Ag + (aq)
NCAAPMT Calculus Challenge Challenge #3 Due: October 26, 2011
 NCAAPMT Calculu Challenge 011 01 Challenge #3 Due: October 6, 011 A Model of Traffic Flow Everyone ha at ome time been on a multi-lane highway and encountered road contruction that required the traffic
NCAAPMT Calculu Challenge 011 01 Challenge #3 Due: October 6, 011 A Model of Traffic Flow Everyone ha at ome time been on a multi-lane highway and encountered road contruction that required the traffic
SKAA 1213 Engineering Mechanics
 SKAA 113 Engineering Mechanic TOPIC 8 KINEMATIC OF PARTICLES Lecturer: Roli Anang Dr. Mohd Yunu Ihak Dr. Tan Cher Siang Outline Introduction Rectilinear Motion Curilinear Motion Problem Introduction General
SKAA 113 Engineering Mechanic TOPIC 8 KINEMATIC OF PARTICLES Lecturer: Roli Anang Dr. Mohd Yunu Ihak Dr. Tan Cher Siang Outline Introduction Rectilinear Motion Curilinear Motion Problem Introduction General
AP Physics Quantum Wrap Up
 AP Phyic Quantum Wrap Up Not too many equation in thi unit. Jut a few. Here they be: E hf pc Kmax hf Thi i the equation for the energy of a photon. The hf part ha to do with Planck contant and frequency.
AP Phyic Quantum Wrap Up Not too many equation in thi unit. Jut a few. Here they be: E hf pc Kmax hf Thi i the equation for the energy of a photon. The hf part ha to do with Planck contant and frequency.
s much time does it take for the dog to run a distance of 10.0m
 ATTENTION: All Diviion I tudent, START HERE. All Diviion II tudent kip the firt 0 quetion, begin on #.. Of the following, which quantity i a vector? Energy (B) Ma Average peed (D) Temperature (E) Linear
ATTENTION: All Diviion I tudent, START HERE. All Diviion II tudent kip the firt 0 quetion, begin on #.. Of the following, which quantity i a vector? Energy (B) Ma Average peed (D) Temperature (E) Linear
1.1 Speed and Velocity in One and Two Dimensions
 1.1 Speed and Velocity in One and Two Dienion The tudy of otion i called kineatic. Phyic Tool box Scalar quantity ha agnitude but no direction,. Vector ha both agnitude and direction,. Aerage peed i total
1.1 Speed and Velocity in One and Two Dienion The tudy of otion i called kineatic. Phyic Tool box Scalar quantity ha agnitude but no direction,. Vector ha both agnitude and direction,. Aerage peed i total
PHYSICSBOWL March 29 April 14, 2017
 PHYSICSBOWL 2017 March 29 April 14, 2017 40 QUESTIONS 45 MINUTES The ponor of the 2017 PhyicBowl, including the American Aociation of Phyic Teacher, are providing ome of the prize to recognize outtanding
PHYSICSBOWL 2017 March 29 April 14, 2017 40 QUESTIONS 45 MINUTES The ponor of the 2017 PhyicBowl, including the American Aociation of Phyic Teacher, are providing ome of the prize to recognize outtanding
12th International Congress on the Deterioration and Conservation of Stone Columbia University, New York, 2012
 THE INFLUENCE OF OSMOTIC PRESSURE ON POULTICING TREATMENTS Leo Pel, 1 Victoria Voronina 1 and Alion Heritage 2 1 Tranport in Permeable Media, Department of Applied Phyic, Eindhoven Univerity of Technology,
THE INFLUENCE OF OSMOTIC PRESSURE ON POULTICING TREATMENTS Leo Pel, 1 Victoria Voronina 1 and Alion Heritage 2 1 Tranport in Permeable Media, Department of Applied Phyic, Eindhoven Univerity of Technology,
Handout 4.11 Solutions. Let x represent the depth of the water at any time Let V represent the volume of the pool at any time
 MCBUW Handout 4. Solution. Label 0m m 8m Let repreent the depth of the water at any time Let V repreent the volume of the pool at any time V 96 96 96 The level of the water i riing at the rate of m. a).
MCBUW Handout 4. Solution. Label 0m m 8m Let repreent the depth of the water at any time Let V repreent the volume of the pool at any time V 96 96 96 The level of the water i riing at the rate of m. a).
1. Calculate H for the reaction: 3 Fe 2 O 3 (s) 2 Fe 3 O 4 (s) + ½ O 2 (g) H =? (6 points)
 Chemitry 141 Name Exam 3 SHOW WORK for All Problem No electronic device allowed except a non-programmable cientific calculator. Data for problem below: 2 FeO() + ½ O 2 (g) Fe 2 O 3 () H = 281.5 kj 3 FeO()
Chemitry 141 Name Exam 3 SHOW WORK for All Problem No electronic device allowed except a non-programmable cientific calculator. Data for problem below: 2 FeO() + ½ O 2 (g) Fe 2 O 3 () H = 281.5 kj 3 FeO()
Chapter 13 Rates of Reactions
 Chapter 13 Rates of Reactions Chemical reactions require varying lengths of time for completion, depending on the characteristics of the reactants and products. The study of the rate, or speed, of a reaction
Chapter 13 Rates of Reactions Chemical reactions require varying lengths of time for completion, depending on the characteristics of the reactants and products. The study of the rate, or speed, of a reaction
Chemical Kinetics. What Influences Kinetics?
 Chemical Kinetics Predictions of likelihood for a reaction to occur have been based on difference in energy between products and reactants: Thermodynamics only compares reactants to products, says nothing
Chemical Kinetics Predictions of likelihood for a reaction to occur have been based on difference in energy between products and reactants: Thermodynamics only compares reactants to products, says nothing
Chapter 14. Chemical Kinetics
 Chapter 14. Chemical Kinetics Common Student Misconceptions It is possible for mathematics to get in the way of some students understanding of the chemistry of this chapter. Students often assume that
Chapter 14. Chemical Kinetics Common Student Misconceptions It is possible for mathematics to get in the way of some students understanding of the chemistry of this chapter. Students often assume that
Assessment Schedule 2017 Scholarship Physics (93103)
 Scholarhip Phyic (93103) 201 page 1 of 5 Aement Schedule 201 Scholarhip Phyic (93103) Evidence Statement Q Evidence 1-4 mark 5-6 mark -8 mark ONE (a)(i) Due to the motion of the ource, there are compreion
Scholarhip Phyic (93103) 201 page 1 of 5 Aement Schedule 201 Scholarhip Phyic (93103) Evidence Statement Q Evidence 1-4 mark 5-6 mark -8 mark ONE (a)(i) Due to the motion of the ource, there are compreion
Ch 13 Rates of Reaction (Chemical Kinetics)
 Ch 13 Rates of Reaction (Chemical Kinetics) Reaction Rates and Kinetics - The reaction rate is how fast reactants are converted to products. - Chemical kinetics is the study of reaction rates. Kinetics
Ch 13 Rates of Reaction (Chemical Kinetics) Reaction Rates and Kinetics - The reaction rate is how fast reactants are converted to products. - Chemical kinetics is the study of reaction rates. Kinetics
Conservation of Energy
 Add Iportant Conervation of Energy Page: 340 Note/Cue Here NGSS Standard: HS-PS3- Conervation of Energy MA Curriculu Fraework (006):.,.,.3 AP Phyic Learning Objective: 3.E.., 3.E.., 3.E..3, 3.E..4, 4.C..,
Add Iportant Conervation of Energy Page: 340 Note/Cue Here NGSS Standard: HS-PS3- Conervation of Energy MA Curriculu Fraework (006):.,.,.3 AP Phyic Learning Objective: 3.E.., 3.E.., 3.E..3, 3.E..4, 4.C..,
Cumulative Review of Calculus
 Cumulative Review of Calculu. Uing the limit definition of the lope of a tangent, determine the lope of the tangent to each curve at the given point. a. f 5,, 5 f,, f, f 5,,,. The poition, in metre, of
Cumulative Review of Calculu. Uing the limit definition of the lope of a tangent, determine the lope of the tangent to each curve at the given point. a. f 5,, 5 f,, f, f 5,,,. The poition, in metre, of
Downloaded from
 CHAPTER --4:- --.CHEMICAL KINETICS (1 MARK QUESTIONS) 1. What do you understand by the rate determining step of a reaction? 2. Find the molecularity of following reaction. RCOOR + H 2O -------- H+ RCOOH
CHAPTER --4:- --.CHEMICAL KINETICS (1 MARK QUESTIONS) 1. What do you understand by the rate determining step of a reaction? 2. Find the molecularity of following reaction. RCOOR + H 2O -------- H+ RCOOH
5.5 Application of Frequency Response: Signal Filters
 44 Dynamic Sytem Second order lowpa filter having tranfer function H()=H ()H () u H () H () y Firt order lowpa filter Figure 5.5: Contruction of a econd order low-pa filter by combining two firt order
44 Dynamic Sytem Second order lowpa filter having tranfer function H()=H ()H () u H () H () y Firt order lowpa filter Figure 5.5: Contruction of a econd order low-pa filter by combining two firt order
Chemical Kinetics AP Chemistry Lecture Outline
 Chemical Kinetics AP Chemistry Lecture Outline Name: Factors that govern rates of reactions. Generally... (1)...as the concentration of reactants increases, rate (2)...as temperature increases, rate (3)...with
Chemical Kinetics AP Chemistry Lecture Outline Name: Factors that govern rates of reactions. Generally... (1)...as the concentration of reactants increases, rate (2)...as temperature increases, rate (3)...with
ME 375 FINAL EXAM SOLUTIONS Friday December 17, 2004
 ME 375 FINAL EXAM SOLUTIONS Friday December 7, 004 Diviion Adam 0:30 / Yao :30 (circle one) Name Intruction () Thi i a cloed book eamination, but you are allowed three 8.5 crib heet. () You have two hour
ME 375 FINAL EXAM SOLUTIONS Friday December 7, 004 Diviion Adam 0:30 / Yao :30 (circle one) Name Intruction () Thi i a cloed book eamination, but you are allowed three 8.5 crib heet. () You have two hour
Chemistry I Unit 3 Review Guide: Energy and Electrons
 Cheitry I Unit 3 Review Guide: Energy and Electron Practice Quetion and Proble 1. Energy i the capacity to do work. With reference to thi definition, decribe how you would deontrate that each of the following
Cheitry I Unit 3 Review Guide: Energy and Electron Practice Quetion and Proble 1. Energy i the capacity to do work. With reference to thi definition, decribe how you would deontrate that each of the following
NAME (pinyin/italian)... MATRICULATION NUMBER... SIGNATURE
 POLITONG SHANGHAI BASIC AUTOMATIC CONTROL June Academic Year / Exam grade NAME (pinyin/italian)... MATRICULATION NUMBER... SIGNATURE Ue only thee page (including the bac) for anwer. Do not ue additional
POLITONG SHANGHAI BASIC AUTOMATIC CONTROL June Academic Year / Exam grade NAME (pinyin/italian)... MATRICULATION NUMBER... SIGNATURE Ue only thee page (including the bac) for anwer. Do not ue additional
Blackbody radiation. Main radiation laws. Sun as an energy source. Solar spectrum and solar constant.
 Lecture 3. lackbody radiation. Main radiation law. Sun a an energy ource. Solar pectrum and olar contant. Objective:. Concept of a blackbody, thermodynamical equilibrium, and local thermodynamical equilibrium..
Lecture 3. lackbody radiation. Main radiation law. Sun a an energy ource. Solar pectrum and olar contant. Objective:. Concept of a blackbody, thermodynamical equilibrium, and local thermodynamical equilibrium..
CHEMICAL KINETICS Order and molecularity of reactions with examples, zero and first order reaction with examples
 CHEMICAL KINETICS Topic-2 Order and molecularity of reactions with examples, zero and first der reaction with examples VERY SHORT ANSWER QUESTIONS 1. What is der of as reaction? Ans: The sum of the powers
CHEMICAL KINETICS Topic-2 Order and molecularity of reactions with examples, zero and first der reaction with examples VERY SHORT ANSWER QUESTIONS 1. What is der of as reaction? Ans: The sum of the powers
Chapter 7. Root Locus Analysis
 Chapter 7 Root Locu Analyi jw + KGH ( ) GH ( ) - K 0 z O 4 p 2 p 3 p Root Locu Analyi The root of the cloed-loop characteritic equation define the ytem characteritic repone. Their location in the complex
Chapter 7 Root Locu Analyi jw + KGH ( ) GH ( ) - K 0 z O 4 p 2 p 3 p Root Locu Analyi The root of the cloed-loop characteritic equation define the ytem characteritic repone. Their location in the complex
Sample Problems. Lecture Notes Related Rates page 1
 Lecture Note Related Rate page 1 Sample Problem 1. A city i of a circular hape. The area of the city i growing at a contant rate of mi y year). How fat i the radiu growing when it i exactly 15 mi? (quare
Lecture Note Related Rate page 1 Sample Problem 1. A city i of a circular hape. The area of the city i growing at a contant rate of mi y year). How fat i the radiu growing when it i exactly 15 mi? (quare
Types of Heat Transfer
 ype of Heat ranfer * Dvz Dt x k d dx v S * * v Gr z HH vap lat uject in the coure conduction (Fourier Law) forced convection (due to flow) ource term free convection (fluid motion due to denity variation
ype of Heat ranfer * Dvz Dt x k d dx v S * * v Gr z HH vap lat uject in the coure conduction (Fourier Law) forced convection (due to flow) ource term free convection (fluid motion due to denity variation
Solvation excesses in the water solutions of D-glucose, saturated by humic acid
 Solvation ecee in the ater olution of D-glucoe, aturated by humic acid A. Pendin 1, S. Karabaev, I. Gainullina, A. Kharcheno, S. Lugovoy 3, N. Gridaova 1 St.Peterburg tate univerity, Ruia, pendina@mail.ru
Solvation ecee in the ater olution of D-glucoe, aturated by humic acid A. Pendin 1, S. Karabaev, I. Gainullina, A. Kharcheno, S. Lugovoy 3, N. Gridaova 1 St.Peterburg tate univerity, Ruia, pendina@mail.ru
3.3. The Derivative as a Rate of Change. Instantaneous Rates of Change. DEFINITION Instantaneous Rate of Change
 3.3 The Derivative a a Rate of Change 171 3.3 The Derivative a a Rate of Change In Section 2.1, we initiated the tudy of average and intantaneou rate of change. In thi ection, we continue our invetigation
3.3 The Derivative a a Rate of Change 171 3.3 The Derivative a a Rate of Change In Section 2.1, we initiated the tudy of average and intantaneou rate of change. In thi ection, we continue our invetigation
EP2200 Queueing theory and teletraffic systems
 P00 Queueing theory and teletraffic ytem Lecture 9 M/G/ ytem Vitoria Fodor KTH S/LCN The M/G/ queue Arrival proce memoryle (Poion( Service time general, identical, independent, f(x Single erver M/ r /
P00 Queueing theory and teletraffic ytem Lecture 9 M/G/ ytem Vitoria Fodor KTH S/LCN The M/G/ queue Arrival proce memoryle (Poion( Service time general, identical, independent, f(x Single erver M/ r /
CHAPTER 13 (MOORE) CHEMICAL KINETICS: RATES AND MECHANISMS OF CHEMICAL REACTIONS
 CHAPTER 13 (MOORE) CHEMICAL KINETICS: RATES AND MECHANISMS OF CHEMICAL REACTIONS This chapter deals with reaction rates, or how fast chemical reactions occur. Reaction rates vary greatly some are very
CHAPTER 13 (MOORE) CHEMICAL KINETICS: RATES AND MECHANISMS OF CHEMICAL REACTIONS This chapter deals with reaction rates, or how fast chemical reactions occur. Reaction rates vary greatly some are very
C H E M I C N E S C I
 C H E M I C A L K I N E T S C I 4. Chemical Kinetics Introduction Average and instantaneous Rate of a reaction Express the rate of a reaction in terms of change in concentration Elementary and Complex
C H E M I C A L K I N E T S C I 4. Chemical Kinetics Introduction Average and instantaneous Rate of a reaction Express the rate of a reaction in terms of change in concentration Elementary and Complex
Phy 213: General Physics III 6/14/2007 Chapter 30 Worksheet 1
 Phy 13: General Phyic III 6/14/007 Chapter 30 Workheet 1 Faraday Law of Electromagnetic Induction and Lenz Law 1. For the following cenario, determine whether the magnetic flux change or tay the ame. If
Phy 13: General Phyic III 6/14/007 Chapter 30 Workheet 1 Faraday Law of Electromagnetic Induction and Lenz Law 1. For the following cenario, determine whether the magnetic flux change or tay the ame. If
1 inhibit5.mcd. Product Inhibition. Instructor: Nam Sun Wang
 Product Inhibition. Intructor: Nam Sun Wang inhibit5.mcd Mechanim. nzyme combine with a ubtrate molecule for form a complex, which lead to product. The product can alo combine with an enzyme in a reerible
Product Inhibition. Intructor: Nam Sun Wang inhibit5.mcd Mechanim. nzyme combine with a ubtrate molecule for form a complex, which lead to product. The product can alo combine with an enzyme in a reerible
e st t u(t 2) dt = lim t dt = T 2 2 e st = T e st lim + e st
 Math 46, Profeor David Levermore Anwer to Quetion for Dicuion Friday, 7 October 7 Firt Set of Quetion ( Ue the definition of the Laplace tranform to compute Lf]( for the function f(t = u(t t, where u i
Math 46, Profeor David Levermore Anwer to Quetion for Dicuion Friday, 7 October 7 Firt Set of Quetion ( Ue the definition of the Laplace tranform to compute Lf]( for the function f(t = u(t t, where u i
s s 1 s = m s 2 = 0; Δt = 1.75s; a =? mi hr
 Flipping Phyic Lecture Note: Introduction to Acceleration with Priu Brake Slaing Exaple Proble a Δv a Δv v f v i & a t f t i Acceleration: & flip the guy and ultiply! Acceleration, jut like Diplaceent
Flipping Phyic Lecture Note: Introduction to Acceleration with Priu Brake Slaing Exaple Proble a Δv a Δv v f v i & a t f t i Acceleration: & flip the guy and ultiply! Acceleration, jut like Diplaceent
Chapter 4. The Laplace Transform Method
 Chapter 4. The Laplace Tranform Method The Laplace Tranform i a tranformation, meaning that it change a function into a new function. Actually, it i a linear tranformation, becaue it convert a linear combination
Chapter 4. The Laplace Tranform Method The Laplace Tranform i a tranformation, meaning that it change a function into a new function. Actually, it i a linear tranformation, becaue it convert a linear combination
HOMEWORK ASSIGNMENT #2
 Texa A&M Univerity Electrical Engineering Department ELEN Integrated Active Filter Deign Methodologie Alberto Valde-Garcia TAMU ID# 000 17 September 0, 001 HOMEWORK ASSIGNMENT # PROBLEM 1 Obtain at leat
Texa A&M Univerity Electrical Engineering Department ELEN Integrated Active Filter Deign Methodologie Alberto Valde-Garcia TAMU ID# 000 17 September 0, 001 HOMEWORK ASSIGNMENT # PROBLEM 1 Obtain at leat
Kinetics. Chapter 14. Chemical Kinetics
 Lecture Presentation Chapter 14 Yonsei University In kinetics we study the rate at which a chemical process occurs. Besides information about the speed at which reactions occur, kinetics also sheds light
Lecture Presentation Chapter 14 Yonsei University In kinetics we study the rate at which a chemical process occurs. Besides information about the speed at which reactions occur, kinetics also sheds light
Lecture 4. Chapter 11 Nise. Controller Design via Frequency Response. G. Hovland 2004
 METR4200 Advanced Control Lecture 4 Chapter Nie Controller Deign via Frequency Repone G. Hovland 2004 Deign Goal Tranient repone via imple gain adjutment Cacade compenator to improve teady-tate error Cacade
METR4200 Advanced Control Lecture 4 Chapter Nie Controller Deign via Frequency Repone G. Hovland 2004 Deign Goal Tranient repone via imple gain adjutment Cacade compenator to improve teady-tate error Cacade
Chapter 14 Chemical Kinetics
 Chapter 14 Chemical Kinetics Thermodynamics tells us what can happen and how far towards completion a reaction will proceed. Kinetics tells us how fast the reaction will go. Study of rates of reactions
Chapter 14 Chemical Kinetics Thermodynamics tells us what can happen and how far towards completion a reaction will proceed. Kinetics tells us how fast the reaction will go. Study of rates of reactions
1. Opp. Khuda Baksh Library, Ashok Rajpath, Patna House no. 5A/65, Opp. Mahual Kothi, Alpana Market, Patna
 1. Which of the following expressions does NOT represent a proper expression for the rate of this reaction? 2A + 3B F + 2G a) (- [A)/( t) b) (- [B)/(3 t) c) ( [F)/( t) d) ( [G)/(2 t) e) (- [A)/(2 t) 2.
1. Which of the following expressions does NOT represent a proper expression for the rate of this reaction? 2A + 3B F + 2G a) (- [A)/( t) b) (- [B)/(3 t) c) ( [F)/( t) d) ( [G)/(2 t) e) (- [A)/(2 t) 2.
SOLUTION MANUAL CHAPTER 12
 SOLUION MANUAL CHAPER CONEN SUBSECION PROB NO. In-ext Concept Quetion a-g Concept problem - Brayton cycle, ga turbine - Regenerator, Intercooler, nonideal cycle 5-9 Ericon cycle 0- Jet engine cycle -5
SOLUION MANUAL CHAPER CONEN SUBSECION PROB NO. In-ext Concept Quetion a-g Concept problem - Brayton cycle, ga turbine - Regenerator, Intercooler, nonideal cycle 5-9 Ericon cycle 0- Jet engine cycle -5
AP Chem Chapter 14 Study Questions
 Class: Date: AP Chem Chapter 14 Study Questions 1. A burning splint will burn more vigorously in pure oxygen than in air because a. oxygen is a reactant in combustion and concentration of oxygen is higher
Class: Date: AP Chem Chapter 14 Study Questions 1. A burning splint will burn more vigorously in pure oxygen than in air because a. oxygen is a reactant in combustion and concentration of oxygen is higher
PHYSICSBOWL APRIL 1 APRIL 15, 2010
 PHYSICSBOWL APRIL 1 APRIL 15, 010 40 QUESTIONS 45 MINUTES The ponor of the 010 PhyicBowl, including the American Aociation of Phyic Teacher and Texa Intrument, are proiding ome of the prize to recognize
PHYSICSBOWL APRIL 1 APRIL 15, 010 40 QUESTIONS 45 MINUTES The ponor of the 010 PhyicBowl, including the American Aociation of Phyic Teacher and Texa Intrument, are proiding ome of the prize to recognize
Module 1: Learning objectives
 Heat and Ma Tranfer Module 1: Learning objective Overview: Although much of the material of thi module will be dicued in greater detail, the objective of thi module i to give you a reaonable overview of
Heat and Ma Tranfer Module 1: Learning objective Overview: Although much of the material of thi module will be dicued in greater detail, the objective of thi module i to give you a reaonable overview of
Chapter 7. Principles of Unsteady - State and Convective Mass Transfer
 Suppleental Material for Tranport Proce and Separation Proce Principle hapter 7 Principle of Unteady - State and onvective Ma Tranfer Thi chapter cover different ituation where a tranfer i taking place,
Suppleental Material for Tranport Proce and Separation Proce Principle hapter 7 Principle of Unteady - State and onvective Ma Tranfer Thi chapter cover different ituation where a tranfer i taking place,
= 16.7 m. Using constant acceleration kinematics then yields a = v v E The expression for the resistance of a resistor is given as R = ρl 4 )
 016 PhyicBowl Solution # An # An # An # An # An 1 C 11 C 1 B 31 E 41 D A 1 B E 3 D 4 B 3 D 13 A 3 C 33 B 43 C 4 D 14 E 4 B 34 C 44 E 5 B 15 B 5 A 35 A 45 D 6 D 16 C 6 C 36 B 46 A 7 E 17 A 7 D 37 E 47 C
016 PhyicBowl Solution # An # An # An # An # An 1 C 11 C 1 B 31 E 41 D A 1 B E 3 D 4 B 3 D 13 A 3 C 33 B 43 C 4 D 14 E 4 B 34 C 44 E 5 B 15 B 5 A 35 A 45 D 6 D 16 C 6 C 36 B 46 A 7 E 17 A 7 D 37 E 47 C
into a discrete time function. Recall that the table of Laplace/z-transforms is constructed by (i) selecting to get
 Lecture 25 Introduction to Some Matlab c2d Code in Relation to Sampled Sytem here are many way to convert a continuou time function, { h( t) ; t [0, )} into a dicrete time function { h ( k) ; k {0,,, }}
Lecture 25 Introduction to Some Matlab c2d Code in Relation to Sampled Sytem here are many way to convert a continuou time function, { h( t) ; t [0, )} into a dicrete time function { h ( k) ; k {0,,, }}
376 CHAPTER 6. THE FREQUENCY-RESPONSE DESIGN METHOD. D(s) = we get the compensated system with :
 376 CHAPTER 6. THE FREQUENCY-RESPONSE DESIGN METHOD Therefore by applying the lead compenator with ome gain adjutment : D() =.12 4.5 +1 9 +1 we get the compenated ytem with : PM =65, ω c = 22 rad/ec, o
376 CHAPTER 6. THE FREQUENCY-RESPONSE DESIGN METHOD Therefore by applying the lead compenator with ome gain adjutment : D() =.12 4.5 +1 9 +1 we get the compenated ytem with : PM =65, ω c = 22 rad/ec, o
Chapter 2 Sampling and Quantization. In order to investigate sampling and quantization, the difference between analog
 Chapter Sampling and Quantization.1 Analog and Digital Signal In order to invetigate ampling and quantization, the difference between analog and digital ignal mut be undertood. Analog ignal conit of continuou
Chapter Sampling and Quantization.1 Analog and Digital Signal In order to invetigate ampling and quantization, the difference between analog and digital ignal mut be undertood. Analog ignal conit of continuou
Fundamental Physics of Force and Energy/Work:
 Fundamental Phyic of Force and Energy/Work: Energy and Work: o In general: o The work i given by: dw = F dr (5) (One can argue that Eqn. 4 and 5 are really one in the ame.) o Work or Energy are calar potential
Fundamental Phyic of Force and Energy/Work: Energy and Work: o In general: o The work i given by: dw = F dr (5) (One can argue that Eqn. 4 and 5 are really one in the ame.) o Work or Energy are calar potential
Chemical. Chapter 14. Kinetics. Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E.
 Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 14 1 PDF Created with deskpdf PDF www.farq.xyz Writer - Trial :: http://www.docudesk.com
Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 14 1 PDF Created with deskpdf PDF www.farq.xyz Writer - Trial :: http://www.docudesk.com
To describe a queuing system, an input process and an output process has to be specified.
 5. Queue (aiting Line) Queuing terminology Input Service Output To decribe a ueuing ytem, an input proce and an output proce ha to be pecified. For example ituation input proce output proce Bank Cutomer
5. Queue (aiting Line) Queuing terminology Input Service Output To decribe a ueuing ytem, an input proce and an output proce ha to be pecified. For example ituation input proce output proce Bank Cutomer
Chapter 5 Consistency, Zero Stability, and the Dahlquist Equivalence Theorem
 Chapter 5 Conitency, Zero Stability, and the Dahlquit Equivalence Theorem In Chapter 2 we dicued convergence of numerical method and gave an experimental method for finding the rate of convergence (aka,
Chapter 5 Conitency, Zero Stability, and the Dahlquit Equivalence Theorem In Chapter 2 we dicued convergence of numerical method and gave an experimental method for finding the rate of convergence (aka,
( 7) ( 9) ( 8) Applying Thermo: an Example of Kinetics - Diffusion. Applying Thermo: an Example of Kinetics - Diffusion. dw = F dr = dr (6) r
 Fundamental Phyic of Force and Energy/Work: Energy and Work: o In general: o The work i given by: dw = F dr (5) (One can argue that Eqn. 4 and 5 are really one in the ame.) o Work or Energy are calar potential
Fundamental Phyic of Force and Energy/Work: Energy and Work: o In general: o The work i given by: dw = F dr (5) (One can argue that Eqn. 4 and 5 are really one in the ame.) o Work or Energy are calar potential
time? How will changes in vertical drop of the course affect race time? How will changes in the distance between turns affect race time?
 Unit 1 Leon 1 Invetigation 1 Think About Thi Situation Name: Conider variou port that involve downhill racing. Think about the factor that decreae or increae the time it take to travel from top to bottom.
Unit 1 Leon 1 Invetigation 1 Think About Thi Situation Name: Conider variou port that involve downhill racing. Think about the factor that decreae or increae the time it take to travel from top to bottom.
Midterm Test Nov 10, 2010 Student Number:
 Mathematic 265 Section: 03 Verion A Full Name: Midterm Tet Nov 0, 200 Student Number: Intruction: There are 6 page in thi tet (including thi cover page).. Caution: There may (or may not) be more than one
Mathematic 265 Section: 03 Verion A Full Name: Midterm Tet Nov 0, 200 Student Number: Intruction: There are 6 page in thi tet (including thi cover page).. Caution: There may (or may not) be more than one
Chapter 17 Amplifier Frequency Response
 hapter 7 Amplifier Frequency epone Microelectronic ircuit Deign ichard. Jaeger Travi N. Blalock 8/0/0 hap 7- hapter Goal eview tranfer function analyi and dominant-pole approximation of amplifier tranfer
hapter 7 Amplifier Frequency epone Microelectronic ircuit Deign ichard. Jaeger Travi N. Blalock 8/0/0 hap 7- hapter Goal eview tranfer function analyi and dominant-pole approximation of amplifier tranfer
