THERMODYNAMICS OF REACTING SYSTEMS
|
|
|
- Ernest Martin
- 6 years ago
- Views:
Transcription
1 ChE 505 Chapter N THERMODYNAMICS OF REACTING SYSTEMS. Introduction Thi chapter explain the baic of thermodynamic calculation for reacting ytem. Reaction toichiometry, heat and entropy of reaction a well a free energy are conidered here. Only ingle phae ytem are conidered here. Phae equilibrium will be addreed in the next chapter. Example and MATLAB calculation of the chemical equilibria are provided at the end of the chapter. 5. Reaction Stoichiometry The principle of conervation of each atomic pecie applied to every well defined chemical reaction lead to reaction toichiometry. Imagine that we have placed an inviible envelope around a finite ma of reactant and the content of that envelope are our ytem in it initial tate. We can count the atom of each atomic pecie preent in each reactant pecie. A chemical reaction take place in the ytem. Upon reaction completion, we recount the number of each atomic pecie. The total number of atom of each of the element preent in remaining reaction and product formed mut remain contant. The principle of conervation of ma applied to each atomic pecie yield the ratio in which molecule of product are formed and molecule of reactant are reacted. The repreentation of chemical pecie by a chemical formula indicate how many atom of each pecie are there in a molecule of the pecie under conideration. Hence, in a molecule of carbon dioxide (CO), there are: one atom of carbon, C, and two atom of oxygen, O. In a molecule of methane, CH 4, here i one atom of carbon and 4 of hydrogen, etc. In engineering application, a mole of the pecie under conideration i ued rather than a chemical formula (e.g., CH 4, O, etc.) repreenting an individual molecule of a particular chemical pecie. One hould recall that a mole i a baic unit of the amount of ubtance. The SI definition of a mole i: The mole i the amount of ubtance of a ytem that contain a many elementary entitie a there are carbon atom in 0.0 kg of carbon. The elementary entity (unit) may be an atom, a molecule, an ion, an electron, a photon, etc. The Avogadro contant i L = 6.03 x 0 3 (mol -) ). To obtain the number of mole of pecie, n (mol) in our ytem, we mut divide the ma of in the ytem m (kg), with the molecular weight of, M (g/mol), and multiply the reult by 000. m ( kg) m g n ( mol) x000 () M M
2 ChE 505 Chapter N Thi i equivalent to dividing the ma of the pecie expreed in gram with the molecular weight, a indicated by the econd equality in equation (). So the SI mole i the ame amount of ubtance a the old CGS gram mole that appear in old chemitry and phyic text. In the US we frequently ue a pound-mole (lb mol) a the meaure of the amount of ubtance. n * (lb mol) m (lb) M (a) It i important to note that the molecular weight of a pecie alway ha the ame numerical value independent of the ytem of unit. For example, the molecular weight of carbon i M c = (g/mol) = (lb /lb mol) = (kg/kmol). Therefore, (kmol) i thouand time larger than a mole (e.g. (kmol) = 0 3 mol)) and lbmol i time larger than a mole, i.e. (lb / mol) = mole. Accordingly, the Avogadro contant for a lb mole i L =.7308 x 0 6 (lbmole ) and for a kmole i L = 6.03 x 0 6 (kmol - ). To illutrate how reaction toichiometry i developed, conider the complete combution of methane (CH4) to carbon dioxide, CO. Thi i a reaction between methane, CH 4, and oxygen, O, that create carbon dioxide, CO, and water H O by complete combution. So we have at tart at end CH 4 + O CO + H O To develop a toichiometric equation we aume that we tart with one mole of methane. Thi implie that one mole of carbon mut be found both on the left hand ide and on the right hand ide of the toichiometric equation. So, one mole of CH 4 reacted mut produce one mole of CO. Since hydrogen i only contained in methane on the reactant ide, and there are H (two mole of hydrogen) in a mole of methane on the reactant ide of the toichiometric equation, there mut be two mole of water formed on the product ide in order to balance the amount of hydrogen. Now we have one mole of oxygen (O ) in the mole of carbon dioxide (CO ) on the product ide and another mole of oxygen in two mole of water. Therefore, we mut ue two mole of oxygen on the reactant ide to balance the amount of oxygen. methane: Thi lead to the following toichiometric equation for complete combution of CH 4 O CO H O () Hence, the requirement to balance out the atomic pecie, i.e. the application of the principle of conervation of ma of atomic pecie lead to the etablihment of tiochiometric coefficient (multiplier that multiply the mole of variou reactant and product pecie). The above toichiometric
3 ChE 505 Chapter N equation remain unchanged if multiplied with a common multiplier ay /: CH 4 O CO H O (a) or ay by: where CH 4 4O CO 4H O (b) Reaction toichiometry, for a ingle reaction, uch a that of equation () can now be repreented S S A A 0 (3) total number of peciein the ytem (e.g.4in caeof reaction ) chemicalformula for the - th pecie(e.g.ch toichiome tric coefficient for the - th pecie defined a 0 for product, 0 for reactant 4, O, CO,etc.) The toichiometric equation atifie the overall ma balance for the ytem S M 0 where M = molecular weight of pecie. (4) For example, for reaction () of methane combution we have CH 4 ; 0 ; CO ; H O If a reaction ytem can be decribed by a ingle reaction, then it generalized toichiometry i given by eq (3). Alternatively, for a ingle reaction between two reactant, A and B, and two product, P and S, the toichiometry can alo be repreented by: aa + bb = pp + where A, B, P, S are chemical pecie and a,b,p, are their toichiometry coefficient, repectively. A CH 4, B O, P CO and S H O in our example Naturally, thi implie that molecular weight of pecie A, B, P, S are uch that equation (4) i atified with v, v, v, v. 3 A (3a) B p Thi impler form i convenient for ingle reaction and alo in repreenting kinetic a dicued later. Single reaction implie that moleof Areacted a moleof B reacted b moleof P reacted p moleof S reacted Hence, a ingle reaction implie that the ratio of product produced and reactant conumed, or, a (3b)
4 ChE 505 Chapter N ratio of one reactant conumed to the other reactant conumed, are contant e.g. (mole of P produced) (moleof A reacted) p a ; (moleof A reacted) (mole of B reacted) a b (3c) If that i not the cae, then multiple reaction mut be ued to decribe the ytem. In a generalized form thi can be done a: S i A 0; i,...r (5) where R i toichiometriccoefficientof pecie in reactioni total numberof independentreaction For example, if in combution of carbon, there i alo carbon monoxide preent, then two reaction are needed to decribe the ytem. They can be a given below: C O CO (5a) CO O CO (5b) Here, we have a total of S = 4 pecie ( =,,3,4 for C, O, CO, CO, repectively), which are involved in two (R = ) independent reaction (i =,). If the above combution reaction involve air intead of oxygen, then nitrogen i the fifth pecie and hence S = 5, but it toichiometric coefficient in each reaction i zero ( i 5 0) for i=,) ince nitrogen doe not participate in thee reaction. Note that the matrix of toichiometric coefficient (for a ytem without nitrogen) 0 0 ha rank two (recall that the rank of a matrix i defined a the ize of the larget nonzero determinant). Adding a third reaction C O CO (5c) would add a third row to the above matrix of toichiometric coefficient, namely (- - 0 ) but the rank of the matrix would remain unchanged at. Clearly, eq (5c) i a linear combination (to be precie the exact um) of (5a) and (5b). Hence, we do not have a third independent reaction, and the toichiometry of the ytem can be decribed by any choice of two reaction of the above three reaction given by eq. (5a), (5b) and (5c). Finding the rank of the matrix of toichiometric coefficient will alway tell how many independent reaction are needed to characterize the toichiometry of the ytem. At the end of the chapter we will how how to find the rank of a matrix uing MATLAB. 4
5 ChE 505 Chapter N In ummary, in any reaction ytem we hould trive to etablih the reaction toichiometry by uing the principle of conervation of element. Then, if more than one reaction i preent, the number of independent reaction can be etablihed by determining the rank of the matrix of toichiometric coefficient..3 Meaure of reaction progre Let u conider firt a ingle reaction S A 0 occurring either in a batch ytem (i.e no material flow croe the boundarie of the ytem during reaction) or in a continuou flow ytem at teady tate (e.g. no variation in time). If n denote the mole of pecie in the batch at ome time t, and no i the initial number of mole of at time to, then reaction toichiometry dictate that mole of all pecie can be related to their initial mole via (molar) extent of reaction X. n n o X (mole of preent) = (mole of originally preent) + (mole of produced by reaction) Mole of produced by reaction are given by the product of the toichiometric coefficient,, and molar extent of reaction, X, which repreent "mole equivalent that participated in reaction". For reactant, 0, and mole produced are a negative quantity, hence, they are mole reacted. For product v X i clearly a poitive quantity. For a flow ytem at teady tate,. F Fo X (6b) where F, F o (mol / ) are molar flow rate of at exit and entrance, repectively, and X (mol/ ) i the molar extent of reaction. Equation (6) indicate that in a ingle reaction if we can determine the change in mole of one component (ay = A), then the molar extent of reaction can be calculated X (n A n Ao ) / A. Mole of all other pecie n can now be found provided their initial mole no were given. Equation (6) alo indicate that reaction progre, i.e. it extent, i limited by the limiting reactant. The limiting reactant i the one preent in amount le than required by toichiometry and limit the reaction extent to Xmax where (3) (6a) 5
6 ChE 505 Chapter N X max n o malletvalue over all (7) Uually, the limiting reactant i denoted by A o that X max = n A o / A For multiple reaction S i A 0 ; i,...r (5) molar extent are defined for each independent reaction i o that the mole of are given by n n o R i v i X i (8) (moleof preent) (mole of initially preent) (mole of producedby reaction i) all reaction Now, the change in mole of R pecie mut be determined to evaluate the R extent. Thi involve the olution of R linear algebraic equation. Then the mole of other pecie can be calculated by eq (8). Maximum extent are now thoe that yield zero mole of one or more reactant. Dealing with reaction toichiometry and reaction progre reult in linear algebraic equation to which all rule of linear (matrix) algebra apply. We are intereted only in non-negative olution. of the ytem Sometime other meaure of reaction progre are ued, uch a (molar) extent per unit volume mol L, which ha unit of concentration, or molar extent per mole which i dimenionle, or extent per unit ma. We will define them a we go along and when we need them. reaction or Often converion of a limiting reactant i ued in ingle reaction ytem to meaure progre of x A n Ao n A n Ao (9a) F F Ao A x A (9b) FAo The relationhip between converion and extent i readily etablihed n Ao x A v A X or F x X (0) Ao A A Hence, in a ingle reaction mole of all pecie can be given in term of converion, x A. Note that converion i unitle while extent ha unit of mole, i.e. of amount of ubtance. 6
7 ChE 505 Chapter N.4 Heat of reaction Heat of reaction i calculated a the difference between the heat of formation of product and the reactant. H r,i = H f(product) - H f(reactant) () where H r,i i the heat of the reaction i. The product and reactant react proportionally to their toichiometric coefficient. Therefore, Eqn.() can be written a H r,i = i H f, (a) The heat of formation data i uually available of tandard condition of 98 K and atm. Correponding tandard heat of reaction denoted a H r o,i can be calculated a H r,i = i H f, () where H f, i the heat of formation of the pecie at tandard condition. Tabulated value for ome pecie are hown in Table. The extenive databae chemkin available in the public domain i another ource. We will explain later how to ue it. Table : Heat of formation, entropy of formation, and Gibb free energy of formation at tandard condition for elected pecie. State = ga, temperature, T=98K and preure atm. Ga H J mol S J mol K G H T S J mol K Oxygen Water 4, ,600 Methane -74, ,83 Nitric oxide NO 90, ,686 Nitrogen dioxide NO 33, ,96 Carbon monoxide -0, Carbon dioxide ,355 Sulfur dioxide 96, ,39 Sulfur trioxide -395, ,34 Ozone 4, ,789 7
8 ChE 505 Chapter N Group contribution method are alo available to predict thee for new compound. Uing the value of tandard condition, the value at any other condition can be calculated uing He Law. Thi tate that the enthalpy change i indifferent of the path taken a long a one land up in the ame pot. A reaction carried out at any temperature T i equivalent in term of energy change to the um of following three path (i) Cool reactant form T to T ref. (ii) Carry out reaction at T ref with an aociated enthalpy change of H r, i (iii) Heat the product back form T ref to T. The tep are chematically hown in the Figure. T (Reactant) Reaction at T T (Product) Reactant Cool reactant T ref Same a T Heat product Reaction at T ref Product of T ref Figure. Schematic of He Law: Enthalpy change i indifferent of the path taken a long a one land up in the ame pot. Hence, the enthalpy change due to reaction (i.e. the heat of reaction) at temperature different from the tandard temperature can be calculated by equation (3) which add a temperature correction term to the tandard heat of reaction. Thi temperature correction then i the um over all pecie of the algebraic product of the toichiometric coefficient with the integral of the molar pecific heat for each pecie from the reference (tandard!) temperature to the temperature of interet. H r,i = H r,i + [ T T ref i, C p d T ] (3) The variation of C p i uually expreed a a polynomial function of temperature C pi = max 0 A T (4a) where A are the coefficient for pecie. 8
9 ChE 505 Chapter N or C p = A + BT + CT + DT - (4b) Thi i another approximate form of C p a a function of temperature. Up to even contant are ued in chemkin databae ( max = 7) while 4 contant are ued a an approximation in many book a hown in (4b). Subtituting we find H i,r = H i,r + i max A [ T T ] (5) ref The above expreion provide a method for calculation of heat of reaction at any given temperature T in an exact manner. The information needed i the heat of formation of all pecie at T ref and the coefficient A in eq (5a) for temperature variation of molar pecific heat (e.g. heat change) C p for each pecie..5 Entropy change in reaction S r i the denoted a the entropy change of reaction at tandard condition (T ref ) and can be calculate from the entropy of formation S f, for each pecie participating in the reaction. S r = S f, (6) The entropy change at any other temperature T can be calculated a S r = S r (T ref ) + T T ref Cp dt (7) T Uing the polynomial expreion for C p a a function of temperature and integrating we obtain S r = S r + A 0 ln T T ref + max A [ T - T ref ] (8) Knowing the entropy and enthalpy change, at any given temperature, the free energy change of reaction i calculated a : G r = H r - T S r (9) Knowledge of how G r change with temperature i important in determining the direction that the reaction will favor. If G r < 0 reaction to the right or forward reaction i highly favorable, while if G r > 0 the revere reaction i more favorable. The temperature at which G r = 0 i where the tranition occur. For an exothermic reaction, G r typically increae with temperature. 9
10 ChE 505 Chapter N G r G r T T Exothermic Endothermic Figure : Variation of free energy a a function of temperature for exothermic and endothermic reaction: Example: i) Thermodynamic calculation for CO oxidation to CO. Thi i an exothermic reaction. CO + O CO T(K) H r (J/mole) S r (J/mole K) G r (J/mole) , , , , , , , ,659 Reaction become le favorable at elevated temperature a the reaction i exothermic. ii) Steam reforming of methane CH 4 + H O CO + 3H Thi i an endothermic reaction T(K) H r (J/mole) S r (J/mole K) G r (J/mole) 500 5, , , , , , , ,960 Reaction become favorable only at higher temperature. Thi i alway true for an endothermic proce. Note that G r decreae with increaing the temperature which i conitent with trend hown in Figure. 0
11 ChE 505 Chapter N.6 Chemical Thermodynamic: Brief Review of Chemical Equilibria For implicity conider an iothermal, ingle phae ytem ubect to a ingle reaction. A 0 () ~ The equilibrium tate i then defined by the minimum in Gibb free energy of the ytem (min n G ) which can be expreed by i) ~ G 0 (0) where ii) n n o X e for all () iii) appropriate equation of tate ~ G ( T, P, ) i the partial molal Gibb free energy of and i the function of temperature, preure and x compoition. (It i often called chemical potential and denoted by ) In general ~ G G ~ RT ln a () where 0 G i the Gibb free energy of pure pecie (function of temperature only). a i the activity of pecie ; n i mole of ; no i initial mole of ; i toichiometric coefficient of (poitive for product, negative for reactant); T i temperature of the ytem; Xe i the equilibrium reaction molar extent; Subtitution of the econd equality of equation (4) for into equation () yield G RT ~ ln a 0 (3) Recognizing that na i na and that the um of logarithm S n a i the logarithm of the product n S a we get the following equation
12 ChE 505 Chapter N S S n a G (3a) RT ~ We define the tandard Gibb free energy of reaction at temperature T by G r G 0 (4) with all G ~ being evaluated at the temperature T of interet. Then the thermodynamic equilibrium contant, K, which i a function of temperature only, i obtained by taking the anti-logarithm of equation (3a) and i given by: K a e G r RT where a i the activity of pecie need to: In order to calculate the equilibrium reaction extent, Xe, and the equilibrium compoition we a) calculate K at the temperature of interet, b) relate the activity of each pecie,, a, to a meaure of compoition (e.g. mole fraction) by an appropriate model for the mixture. c) relate the choen meaure of compoition to reaction extent uing toichiometric relation indicated by ii) above. Since the Gibb free energy of formation i tabulated for all chemical pecie at T ref = 98K (5 C) (or can be found from H and S data a hown in previou ection) it i convenient to calculate the Gibb free energy change due to reaction G rt0 o G r at thee tandard condition a: G f (6) (5) temperature: The equilibrium contant at tandard temperature i then obtained from equation (5). Van Hoff' equation etablihe the rate of change of the equilibrium contant K with d nk dt H r RT (7) T To ( 98 K); K K98 exp( GrT / RT0 ) (8) 0 where H r i the tandard heat of reaction at temperature T (calculated a hown in previou ection),
13 ChE 505 Chapter N K98 i the equilibrium contant at the tandard tate temperature of T0 (mot often 98K) and G rro i the tandard Gibb free energy of reaction at T0 which i obtained from tabulated Gibb free energie of formation G f For gae (tandard tate pure ga at atm) we ue y for mole fraction of, a i the activity of, p i partial preure of, P i total preure of the ytem, partial molal fugacity of. i the fugacity coefficient of while f i the The needed relation are included below: a y P f y P / atm p f y P /atm (30) a y P /atm p /atm (30a) K a P atm y (3) K P atm K y K P P 0 K y K where P0= or atm. (3a) K a p / atm (3) K a K P K / atm (3a) The generalized fugacity coefficient, f y P, would have to be evaluated from an appropriate equation of tate. If Lewi-Randall rule i ued For gae at low preure K f P. K P atm K y K P / atm (3b) For liquid (auming tandard tate of unit activity, i.e. the tandard tate of each component i the pure component tate) the following relation hold: a x (33) where i the activity coefficient 3
14 ChE 505 Chapter N K a x K x K (34) Since x = C / C K C K c K (35) For an ideal mixture K Above C i the molar concentration of pecie and C Example: Reaction Sytem: SO + O = SO 3 Converion of 99% i deired C i the total molar concentration. Condition: T = 600 o C = 873 o K P = atm Stoichiometric Feed of Pure Reactant No Nitrogen Calculate equilibrium converion of SO and ee if 99% can be reached. G f and H f for variou pecie are given in ( kcal/mol) below. Bai: mole of SO. Total number of pecie S = 3 3 ntot n o 3ntot n 3 0 X Specie Name No. Stoich. Coeff. G f H f n o n n o X SO X O 0 0 X SO X Aume ideal ga mixture and write the equilibrium contant in term of K y : K K y P P o K y P P o K y P o P where (A) 4
15 ChE 505 Chapter N 3 y K y y y y 3 y3 y y y S03 y S0 y0 Evaluate the mole fraction of each pecie in term of reaction extent X n y X n to t 3 X ; y X 3 X ; y 3 X 3 X Subtitute thee mole fraction in the expreion for K y K y 4 X 3 X 4 X X 3 X 3 X X 3 X X 3 where X = Xe = equilibrium extent From (A) K X 3 X X 3 P 0 P (B) Calculate K98 at 98 K Calculate G r K 98 e x 7.7 x 0 x kcal mol G r o RT o e H r 33, x98 e x0 4 x 70.9 x 0 x kcal mol Aume for implicity (in order to find a firt etimate for equilibrium condition) that H r cont H r Then d nk dt n K T K T K 98 H r RT ; T T o 98 K K 98 K 98 e H r R H r R 98 47, K 873 e K T 98 T 873 e
16 ChE 505 Chapter N Note the dramatic drop in K with temperature due to the exothermicity of the reaction H r 0. While equilibrium would have been all the way to the right at 98 K we cannot operate at uch condition becaue the rate i too low. Let u ee what are the equilibrium limitation at 873 K. Solve equation (B) for Ky expreed in term of extent (ee equation (C)) Solve by trial and error equation (C) for equilibrium extent Xe = X. X 3 X X 3 K 873 P P 0 (C) where K ; P P o. Note that elevated preure, a predicted by L Chatelier principle, would help move the equilibrium to the right..rearrange equation (C) to ue Newton-Raphon procedure X 0 X 3 X.78 X 3 D X 3X X X X n X n X n D X n (D) Uing a tarting gue of Xo = 0.5 ( mol) the Newton Raphon algorithm (D) yield: Iteration No. n Extent (mol) X = Xe = (mol) Converion of SO i: n SO, 0 n SO X x SO n SO, 0 x SO eq X X (mol)/ (mol) Equilibrium converion of 0.99 required by emiion control cannot be reached under thee condition. Equilibrium extent could be increaed by - lowering the temperature (but rate are lower) - increaing the preure (ome of it can be recovered by running a turbine at the end of reactor a done in the former USSR) 6
17 ChE 505 Chapter N.7 Solution Thermodynamic The equilibrium ditribution of inorganic compound in aqueou ytem i of great importance in environmental engineering. Thee calculation are more complex than the ga phae cae becaue of two additional conideration. (i) charge neutrality ha to be maintained. (ii) ytem i often non-ideal. The importance of thi in environmental application i illutrated by a number of example below. Example : Heavy metal have toxicological impact and often get incorporated in biota uch a fih. The free ion form M ++ for example, i often the toxic form while the complexe uch a MOH +, M(OH) or ligand with SO 4 or other negative ion are conidered to be le harmful. The ditribution of the metal in variou form i a function of the total concentration and the ph of the olution. Solution thermodynamic provide u with a mean of doing uch calculation and help in making udiciou policy deciion. Example : Conider a oil ytem that ha ome lead contamination or naturally occurring lead. The quetion i whether the lead i going to remain there or diolve in ground water and enter into drinking water. The lead may remain in the olid phae depending on the ph or the preence of other ion (common ion effect decribed later). Example 3: Conider SO generated in a pollutant plume in the ga phae. Thi i a highly oluble ga and enter the aqueou phae with rain water. It then react to form variou ionic pecie uch a HSO 3, SO -- 3, SO -- 4 etc. The equilibrium ditribution i important in many application. Example 4: Conider mercury, a highly volatile toxic metal, that i highly poionou. Depending on the ph of the aqueou environment, mercury can be found in variou form uch a HgH, Hg, Hg ++, Hg ++, HgO, HHgO and Hg(OH) form. When converted to the organic form, monomethyl mercury (CH 3 Hg) can be inerted by microorganim, it become lipophyllic and get accumulated in body fat of fih. Conumption of contaminated fih reult in damage to the central nervou ytem, liver and kidney and caue impaired child development. In 950, 00 people in Minamata Bay in Japan died from mercury poioned fih. 7
18 ChE 505 Chapter N.8 Rank and checking linear independence of reaction uing MATLAB To appreciate fully what the MATLAB program doe for you look firt at Appendix A. In that appendix we decribe the procedure involved in obtaining the rank of the matrix and identifying independent reaction. Conider the carbon dioxide formation conidered earlier. The reaction cheme i C O CO (5a) CO O CO (5b) C O CO (5c) Step : type in the toichiometric matrix a follow: Each row i reaction index and each column i the pecie index. Thu each row repreent one reaction in the ytem. We take carbon, C, to be pecie, oxygen to be, carbon monoxide i 3, carbon dioxide i 4. coefficient i: v = [- - 0 ; ; ] Step : find the rank by typing the following rank(v) Then the matrix of toichiometric For the above cae the anwer will be o that there are only two independent reaction. In other word the third reaction i a linear combination of the firt two reaction. To find thi combination go to tep a. Step a; do thi if the rank i le than the number of reaction. Type rref(v') Note that v' i the tranpoe of the toichiometric matrix and the above command reduce the tranpoed matrix to the Echelon form (in thi form the main diagonal of the ha one on it). The reult will be
19 ChE 505 Chapter N The lat column give the linear combination for the third reaction. Thu reaction (5c) i equal to 0.5 time the firt reaction plu 0.5 time the econd reaction. In thi imple example it i obviou by inpection a well. Step 3: To find the ytem invariant (thee will be relation between mole produce or conumed among variou pecie), type rref(v) Thi reduce the toichiometric matrix to the echelon form. The reult for our example i: The lat two column here provide the invariant. Thu F 3 = - * F + * F and F 4 = * F - * F Here F repreent the change in molar flow rate between the exit and entrance for pecie I. Note that the lat row i all zero indicating that the third reaction i redundant and doe not contribute to the invariant. (recall that the rank wa two). Let u ee what the implication of all thi i; the above equation tell u that only two value F and F are independent. For example, in an experiment uppoe we find that 5 mole/ec of carbon ha reacted. Then F 5. We alo find that 4 mole/ec of oxygen i conumed. F 4. Then uing the equation above we find Alo F 5 4 = mole/ec i the amount of CO formed. 3 F = 3 mole/ec of CO i formed. 9
20 ChE 505 Chapter N If the meaurement how otherwie, then either the meaurement are in error or ome other C bearing pecie i being formed (not likely here). Hence in thi cae the meaurement would be in error. Thu, the invariant of a reaction are ueful in proper bookkeeping of the variou pecie..9 MATLAB PROGRAM FOR CALCULATION OF EQUILIBRIUM COMPOSITION OF A REACTING GAS MIXTURE The following program calculate the equilibrium compoition of a ga mixture. The program i written for the SO example and can be modified eaily for other cae. The bet way to learn thi i for the tudent to TYPE out the program a it i and execute it on matlab. Thi way they get familiar with the programming a well. The program alo illutrate the ue of the olver FSOLVE to olve a et of non-linear algebraic equation. The program i interpaced with ome explanation and thee tatement need to be omitted in the actual program. PREAMBLE SECTION % filename gaeq; created on an 3-03by P.A.Ramachandran. % compute the equlibirum compoiton of a reacting mixture. % preamble global ng n nr tempin prin ctot xg nu... global keq98 keq delhr global rga global n ntot At thi part of the program the number of ga phae pecie ng and number of reaction nr are to be entered. %number of pecie and number of reaction ng = 3 ; nr = ; DIMENSIONING THE VECTORS Thi i required but no action by uer i needed. % intialize the vector. keq98 = zero(nr, ); keq = zero(nr, ); 0
21 ChE 505 Chapter N delhr = zero(nr, ); xg = zero(ng,); nu = zero ( nr, ng); zeta = zero(nr,) ; USER DATA SECTION A: REACTIONS Here the reaction pecific variable are entered Thee are the toichiometric matrice and the equilibrium contant for each reaction at 98K and the heat of reaction at 98K. The program aume that the heat of reaction i independent of temperature. Note that the ga contant rga mut have conitent unit with the heat of reaction. % provide value for toichimetry and eq contant. nu(,) = -. ; nu(,) = -. nu(,3) =. ; keq98() = 6.593E+4 ; delhr() = ; %aumed contant (not a function of T) rga =.97 ; % ga contant; ue conitent unit B: PROCESS CONDITIONS Here the temperature, preure and the mole of each pecie preent in the initial mixture are pecified. The uer mut provide a gue value for the extent of reaction (zeta vector). Try to provide realitic value, for example the converion of a key component can not be greater than one. Thi will fix the maximum value for zeta % provide the feed condition. tempin = 873.0; prin =.0e+05; % initial mole. xg() =.0; xg() =.0; xg(3) = 0.0;
22 ChE 505 Chapter N % provide initial value for extent of reaction zeta() = 0.4 CALCULATION SECTION At thi tage the matalb take care of the ret of the calulation % mtlab function folve olver i called to find the root % the required equation are programmed in a file fun_eq.m zeta = folve ( 'fun_eq', zeta) % pot proce the reult. % molar fow rate at exit = TYPE n % total mole = TYPE ntot Pot proceing can be done by typing n which give the mole of pecie at Equilibrium,. The converion and other required information can be calculated eaily on the matlab command window. FUNCTION SUBROUTINE The program require a function ubroutine which calculate the function to be olved. The ubroutine i in a file fun_eq.m and the liting i hown below. No change in thi by uer i needed. Student may want to tudy how thi i written by following thi with the text earlier function fvec = fun_eq( zeta) % filename fun_eq; created on an 3-03by P.A.Ramachandran. % Define the (nr) function to be olved. % preamble global ng n nr tempin prin ctot xg nu... global keq98 keq delhr global n ntot global rga fvec = zero ( nr,) ; ptotatm = prin /.0e+05 ;
23 ChE 505 Chapter N % find K at the deired condition for i = : nr keq(i)=keq98(i)*exp( delhr(i) /rga *(./98-./tempin)) ; end %number of mole of ecah pecie for the given extent % n0 = for thee calculation for = : ng n() = xg(); for i=:nr n() = n() + nu(i,) * zeta(i); end end % find ntot ntot = 0.0; for = : ng ntot = ntot + n(); end % find the mole fraction for each pecie pp = n /ntot * ptotatm; % et up dicrepancy function for each reaction for i = : nr prod =.0; for = : ng prod = prod * pp()^nu(i,); end fvec(i) = keq(i) - prod end _ The tudent hould run the program for other cae for example to tudy the effect of inert, like nitrogen, preure or other ytem involving multiple reaction. We will now illutrate ue of thi program for a combution application. 3
24 ChE 505 Chapter N Example: Chemical equilibrium et an upper limit on the compoition of combution gae. Given a mixture of CO, CO and O we need to find the CO concentration a a function of exhaut ga temperature. Once CO i formed, it i unlikely that CO will be formed a the ga cool ince the reaction rate are lowed down. Hence the maximum CO in the exhaut ga can be found from the equilibrium calculation. A an example, conider an exhaut ga mixture with 8%CO. 3.3%O and the ret N. Temperature of thi ga i raied to 600K. Find the equilibrium compoition of thi ga auming the reaction CO CO / O We run the program with 4 pecie the fourth being inert (N ). The ample reult are a follow: ***** COMPUTED RESULTS ********* Equilibrium contant of reaction i e-05 Heat of reaction i (J/mol) Free energy change of reaction i (J/mol) Program converged The extent of reaction i e-03 (mole) We ue 00 mole total a the input condition. Mole of CO in the exhaut ga i therefore.955e-03% of the total mole or 9.5 ppm (by mole or volume). Problem for dicuion Predict the effect of temperature (range of 000 to 000K) on the CO content of exhaut ga and plot the reult. If CO reduction wa the only goal, would you operate at high temperature or low temperature Example: Conider a ga to be 3.3%O and ret N. Thi preumably imulate and exhaut ga from an internal combution engine. The following reaction take place N O NO NO / O NO Find the concentration of NO and NO at800k and atm. 4
25 ChE 505 Chapter N Again we ue the fortran program ince all thee gae are in the chmkin databae. We modify the datafile and run the program to find the following reult. Equilibrium contant of reaction i.98983e-04 Heat of reaction i (J/mole) Free energy change of reaction i Equilibrium contant of reaction i E-03 Heat of reaction i (J/mole) Free energy change of reaction i (J/mole) program converged The extent of reaction i E-0 (J/mole) The extent of reaction i.77360e-04 (J/mole) NO formed = x x N O Formed = x The above are percentage ince the total tarting mole are aumed a00. Hence the ga ha 7ppm of NO and.77 ppm of NO.0 STUDY QUESTIONS What i the ignificance of the rank of the toichiometric matrix? State the meaure of progre of reaction for multiple reaction. How doe the equilibrium contant vary with temperature? How i Keq and K y related to each other? Show one example? 5
26 ChE 505 Chapter N. EXERCISES. The principle of conervation of element mut be applied in order to obtain the toichiometric coefficient in a ingle reaction. Aume that there are S chemical pecie that are either product or reactant in the reaction and there are N element preent a contituent. Let the number of atom of element i in pecie be i. Let the yet unknown toichiometric coefficient of pecie be S. Show i that the conervation principle above require 0 for all i =,,.N. Apply thi to the reaction of complete combution of methane and how how to determine the unknown '.. In a reaction ytem coniting of methane, oxygen, carbon monoxide, water, hydrogen and carbon monoxide, the following 6 reaction (R = 6) may occur: CH 4 + 3/ O = CO + H O () CO + / O = CO () CH 4 + H O = 3H + CO (3) H + / O = H O (4) CO + H O = CO + H (5) CH 4 + O = CO + H O (6) Find the number of independent reaction R..3 Carbon (0 mole initially) i burned with oxygen. At the end of reaction 4 mole of carbon are left and 4 mole of CO are produced. The produced amount of CO i unknown. (i) Write the toichiometric matrix with carbon and CO a component () and () (ii) (iii) Find the matrix in echelon form. Find the mole of O conumed and CO produced in the proce..4 The equilibrium contant for CO CO + O at 000 K i 6.046*0 -. Find the value at 600 K if the heat of reaction i 8 k/mole..5 Find the thermodynamic data for the reaction NO + O NO 6
27 ChE 505 Chapter N Uing a uitable databae (report which one you ued) over a temperature range of 500 to 500 K, determine at what temperature would you expect a larger NO concentration. Low or high? What i the equilibrium compoition..6 What i the effect of preure on CO formation in a combution ytem? At 600 K, find the CO content of the exhaut ga if the preure were atm. Initial compoition i the ame 8 % CO, 3.3 % O,78.7 % N. A in the Matlab program example..7 Propane i burned in air with.5 time the toichiometric air. Find the compoition of exhaut gae auming that only CO and H O are formed..8 For the compoition in Quetion (.7) find the equilibrium compoition of CO if the combution take place at 800 K and atm preure..9 Find the equilibrium compoition for the above cae uing the net program. 7
ChE 505 Chapter 2N APPENDIX A
 ChE 55 Chapter N APPENDIX A A.1 Rank and checking linear indepence of reactions using MATLAB In this appix we will describe the procedures involved in obtaining the rank of the matrix and identifying indepent
ChE 55 Chapter N APPENDIX A A.1 Rank and checking linear indepence of reactions using MATLAB In this appix we will describe the procedures involved in obtaining the rank of the matrix and identifying indepent
NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor
 NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor T o T T o T F o, Q o F T m,q m T m T m T mo Aumption: 1. Homogeneou Sytem 2. Single Reaction 3. Steady State Two type of problem: 1. Given deired
NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor T o T T o T F o, Q o F T m,q m T m T m T mo Aumption: 1. Homogeneou Sytem 2. Single Reaction 3. Steady State Two type of problem: 1. Given deired
Source slideplayer.com/fundamentals of Analytical Chemistry, F.J. Holler, S.R.Crouch. Chapter 6: Random Errors in Chemical Analysis
 Source lideplayer.com/fundamental of Analytical Chemitry, F.J. Holler, S.R.Crouch Chapter 6: Random Error in Chemical Analyi Random error are preent in every meaurement no matter how careful the experimenter.
Source lideplayer.com/fundamental of Analytical Chemitry, F.J. Holler, S.R.Crouch Chapter 6: Random Error in Chemical Analyi Random error are preent in every meaurement no matter how careful the experimenter.
1 year n0tes chemistry new st CHAPTER 7 THERMOCHEMISTRY MCQs Q.1 Which of the following statements is contrary to the first law of thermodynamics?
 year n0te chemitry new t CHAPTER 7 THERMOCHEMISTRY MCQ Q.1 Which of the following tatement i contrary to the firt law of thermodynamic? (a) energy can neither be created nor detroyed (b) one form of energy
year n0te chemitry new t CHAPTER 7 THERMOCHEMISTRY MCQ Q.1 Which of the following tatement i contrary to the firt law of thermodynamic? (a) energy can neither be created nor detroyed (b) one form of energy
Molecular Dynamics Simulations of Nonequilibrium Effects Associated with Thermally Activated Exothermic Reactions
 Original Paper orma, 5, 9 7, Molecular Dynamic Simulation of Nonequilibrium Effect ociated with Thermally ctivated Exothermic Reaction Jerzy GORECKI and Joanna Natalia GORECK Intitute of Phyical Chemitry,
Original Paper orma, 5, 9 7, Molecular Dynamic Simulation of Nonequilibrium Effect ociated with Thermally ctivated Exothermic Reaction Jerzy GORECKI and Joanna Natalia GORECK Intitute of Phyical Chemitry,
Bogoliubov Transformation in Classical Mechanics
 Bogoliubov Tranformation in Claical Mechanic Canonical Tranformation Suppoe we have a et of complex canonical variable, {a j }, and would like to conider another et of variable, {b }, b b ({a j }). How
Bogoliubov Tranformation in Claical Mechanic Canonical Tranformation Suppoe we have a et of complex canonical variable, {a j }, and would like to conider another et of variable, {b }, b b ({a j }). How
7.2 INVERSE TRANSFORMS AND TRANSFORMS OF DERIVATIVES 281
 72 INVERSE TRANSFORMS AND TRANSFORMS OF DERIVATIVES 28 and i 2 Show how Euler formula (page 33) can then be ued to deduce the reult a ( a) 2 b 2 {e at co bt} {e at in bt} b ( a) 2 b 2 5 Under what condition
72 INVERSE TRANSFORMS AND TRANSFORMS OF DERIVATIVES 28 and i 2 Show how Euler formula (page 33) can then be ued to deduce the reult a ( a) 2 b 2 {e at co bt} {e at in bt} b ( a) 2 b 2 5 Under what condition
Lecture 21. The Lovasz splitting-off lemma Topics in Combinatorial Optimization April 29th, 2004
 18.997 Topic in Combinatorial Optimization April 29th, 2004 Lecture 21 Lecturer: Michel X. Goeman Scribe: Mohammad Mahdian 1 The Lovaz plitting-off lemma Lovaz plitting-off lemma tate the following. Theorem
18.997 Topic in Combinatorial Optimization April 29th, 2004 Lecture 21 Lecturer: Michel X. Goeman Scribe: Mohammad Mahdian 1 The Lovaz plitting-off lemma Lovaz plitting-off lemma tate the following. Theorem
CHAPTER 8 OBSERVER BASED REDUCED ORDER CONTROLLER DESIGN FOR LARGE SCALE LINEAR DISCRETE-TIME CONTROL SYSTEMS
 CHAPTER 8 OBSERVER BASED REDUCED ORDER CONTROLLER DESIGN FOR LARGE SCALE LINEAR DISCRETE-TIME CONTROL SYSTEMS 8.1 INTRODUCTION 8.2 REDUCED ORDER MODEL DESIGN FOR LINEAR DISCRETE-TIME CONTROL SYSTEMS 8.3
CHAPTER 8 OBSERVER BASED REDUCED ORDER CONTROLLER DESIGN FOR LARGE SCALE LINEAR DISCRETE-TIME CONTROL SYSTEMS 8.1 INTRODUCTION 8.2 REDUCED ORDER MODEL DESIGN FOR LINEAR DISCRETE-TIME CONTROL SYSTEMS 8.3
Social Studies 201 Notes for November 14, 2003
 1 Social Studie 201 Note for November 14, 2003 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
1 Social Studie 201 Note for November 14, 2003 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
Social Studies 201 Notes for March 18, 2005
 1 Social Studie 201 Note for March 18, 2005 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
1 Social Studie 201 Note for March 18, 2005 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
Dimensional Analysis A Tool for Guiding Mathematical Calculations
 Dimenional Analyi A Tool for Guiding Mathematical Calculation Dougla A. Kerr Iue 1 February 6, 2010 ABSTRACT AND INTRODUCTION In converting quantitie from one unit to another, we may know the applicable
Dimenional Analyi A Tool for Guiding Mathematical Calculation Dougla A. Kerr Iue 1 February 6, 2010 ABSTRACT AND INTRODUCTION In converting quantitie from one unit to another, we may know the applicable
μ + = σ = D 4 σ = D 3 σ = σ = All units in parts (a) and (b) are in V. (1) x chart: Center = μ = 0.75 UCL =
 Our online Tutor are available 4*7 to provide Help with Proce control ytem Homework/Aignment or a long term Graduate/Undergraduate Proce control ytem Project. Our Tutor being experienced and proficient
Our online Tutor are available 4*7 to provide Help with Proce control ytem Homework/Aignment or a long term Graduate/Undergraduate Proce control ytem Project. Our Tutor being experienced and proficient
Sample Question Solutions for the Chemistry of Environment Topic Exam
 Name (Lat, Firt): ID Number: Sample Quetion Solution for the Chemitry of Environment Topic Exam 1. The earth tratophere contain a region of high ozone (O3) concentration called the ozone layer. The ozone
Name (Lat, Firt): ID Number: Sample Quetion Solution for the Chemitry of Environment Topic Exam 1. The earth tratophere contain a region of high ozone (O3) concentration called the ozone layer. The ozone
Lecture 13. Thermodynamic Potentials (Ch. 5)
 Lecture 13. hermodynamic Potential (Ch. 5) So far we have been uing the total internal energy U and ometime the enthalpy H to characterize variou macrocopic ytem. hee function are called the thermodynamic
Lecture 13. hermodynamic Potential (Ch. 5) So far we have been uing the total internal energy U and ometime the enthalpy H to characterize variou macrocopic ytem. hee function are called the thermodynamic
AOS 104 Fundamentals of Air and Water Pollution
 AOS 104 Fundamental of Air and Water Pollution Dr. Jeffrey Lew lew@atmo.ucla.edu AIM: jklew888 MS1961 310-825-3023 1 Grade Homework 150 pt 2 Mierm 300 pt Final Exam Total 550 pt 1000 pt 2 Homework There
AOS 104 Fundamental of Air and Water Pollution Dr. Jeffrey Lew lew@atmo.ucla.edu AIM: jklew888 MS1961 310-825-3023 1 Grade Homework 150 pt 2 Mierm 300 pt Final Exam Total 550 pt 1000 pt 2 Homework There
Chapter 4. The Laplace Transform Method
 Chapter 4. The Laplace Tranform Method The Laplace Tranform i a tranformation, meaning that it change a function into a new function. Actually, it i a linear tranformation, becaue it convert a linear combination
Chapter 4. The Laplace Tranform Method The Laplace Tranform i a tranformation, meaning that it change a function into a new function. Actually, it i a linear tranformation, becaue it convert a linear combination
FUNDAMENTALS OF POWER SYSTEMS
 1 FUNDAMENTALS OF POWER SYSTEMS 1 Chapter FUNDAMENTALS OF POWER SYSTEMS INTRODUCTION The three baic element of electrical engineering are reitor, inductor and capacitor. The reitor conume ohmic or diipative
1 FUNDAMENTALS OF POWER SYSTEMS 1 Chapter FUNDAMENTALS OF POWER SYSTEMS INTRODUCTION The three baic element of electrical engineering are reitor, inductor and capacitor. The reitor conume ohmic or diipative
Linear Motion, Speed & Velocity
 Add Important Linear Motion, Speed & Velocity Page: 136 Linear Motion, Speed & Velocity NGSS Standard: N/A MA Curriculum Framework (006): 1.1, 1. AP Phyic 1 Learning Objective: 3.A.1.1, 3.A.1.3 Knowledge/Undertanding
Add Important Linear Motion, Speed & Velocity Page: 136 Linear Motion, Speed & Velocity NGSS Standard: N/A MA Curriculum Framework (006): 1.1, 1. AP Phyic 1 Learning Objective: 3.A.1.1, 3.A.1.3 Knowledge/Undertanding
Gain and Phase Margins Based Delay Dependent Stability Analysis of Two- Area LFC System with Communication Delays
 Gain and Phae Margin Baed Delay Dependent Stability Analyi of Two- Area LFC Sytem with Communication Delay Şahin Sönmez and Saffet Ayaun Department of Electrical Engineering, Niğde Ömer Halidemir Univerity,
Gain and Phae Margin Baed Delay Dependent Stability Analyi of Two- Area LFC Sytem with Communication Delay Şahin Sönmez and Saffet Ayaun Department of Electrical Engineering, Niğde Ömer Halidemir Univerity,
Correction for Simple System Example and Notes on Laplace Transforms / Deviation Variables ECHE 550 Fall 2002
 Correction for Simple Sytem Example and Note on Laplace Tranform / Deviation Variable ECHE 55 Fall 22 Conider a tank draining from an initial height of h o at time t =. With no flow into the tank (F in
Correction for Simple Sytem Example and Note on Laplace Tranform / Deviation Variable ECHE 55 Fall 22 Conider a tank draining from an initial height of h o at time t =. With no flow into the tank (F in
Modeling of Transport and Reaction in a Catalytic Bed Using a Catalyst Particle Model.
 Excerpt from the Proceeding of the COMSOL Conference 2010 Boton Modeling of Tranport and Reaction in a Catalytic Bed Uing a Catalyt Particle Model. F. Allain *,1, A.G. Dixon 1 1 Worceter Polytechnic Intitute
Excerpt from the Proceeding of the COMSOL Conference 2010 Boton Modeling of Tranport and Reaction in a Catalytic Bed Uing a Catalyt Particle Model. F. Allain *,1, A.G. Dixon 1 1 Worceter Polytechnic Intitute
Singular perturbation theory
 Singular perturbation theory Marc R. Rouel June 21, 2004 1 Introduction When we apply the teady-tate approximation (SSA) in chemical kinetic, we typically argue that ome of the intermediate are highly
Singular perturbation theory Marc R. Rouel June 21, 2004 1 Introduction When we apply the teady-tate approximation (SSA) in chemical kinetic, we typically argue that ome of the intermediate are highly
4. The following graphs were prepared from experimental data for a reactant, A. What is the correct order of A?
 Diebolt Summer 010 CHM 15 HOUR EXAM I KEY (15 pt) Part One: Multiple Choice. 1. Conider the reaction IO 3 + 5I + 6H + 3I + 3H O. If the rate of diappearance of I i 9.010-3 M/, what i the rate of appearance
Diebolt Summer 010 CHM 15 HOUR EXAM I KEY (15 pt) Part One: Multiple Choice. 1. Conider the reaction IO 3 + 5I + 6H + 3I + 3H O. If the rate of diappearance of I i 9.010-3 M/, what i the rate of appearance
1 Routh Array: 15 points
 EE C28 / ME34 Problem Set 3 Solution Fall 2 Routh Array: 5 point Conider the ytem below, with D() k(+), w(t), G() +2, and H y() 2 ++2 2(+). Find the cloed loop tranfer function Y () R(), and range of k
EE C28 / ME34 Problem Set 3 Solution Fall 2 Routh Array: 5 point Conider the ytem below, with D() k(+), w(t), G() +2, and H y() 2 ++2 2(+). Find the cloed loop tranfer function Y () R(), and range of k
Control Systems Analysis and Design by the Root-Locus Method
 6 Control Sytem Analyi and Deign by the Root-Locu Method 6 1 INTRODUCTION The baic characteritic of the tranient repone of a cloed-loop ytem i cloely related to the location of the cloed-loop pole. If
6 Control Sytem Analyi and Deign by the Root-Locu Method 6 1 INTRODUCTION The baic characteritic of the tranient repone of a cloed-loop ytem i cloely related to the location of the cloed-loop pole. If
Lecture 8: Period Finding: Simon s Problem over Z N
 Quantum Computation (CMU 8-859BB, Fall 205) Lecture 8: Period Finding: Simon Problem over Z October 5, 205 Lecturer: John Wright Scribe: icola Rech Problem A mentioned previouly, period finding i a rephraing
Quantum Computation (CMU 8-859BB, Fall 205) Lecture 8: Period Finding: Simon Problem over Z October 5, 205 Lecturer: John Wright Scribe: icola Rech Problem A mentioned previouly, period finding i a rephraing
into a discrete time function. Recall that the table of Laplace/z-transforms is constructed by (i) selecting to get
 Lecture 25 Introduction to Some Matlab c2d Code in Relation to Sampled Sytem here are many way to convert a continuou time function, { h( t) ; t [0, )} into a dicrete time function { h ( k) ; k {0,,, }}
Lecture 25 Introduction to Some Matlab c2d Code in Relation to Sampled Sytem here are many way to convert a continuou time function, { h( t) ; t [0, )} into a dicrete time function { h ( k) ; k {0,,, }}
THE THERMOELASTIC SQUARE
 HE HERMOELASIC SQUARE A mnemonic for remembering thermodynamic identitie he tate of a material i the collection of variable uch a tre, train, temperature, entropy. A variable i a tate variable if it integral
HE HERMOELASIC SQUARE A mnemonic for remembering thermodynamic identitie he tate of a material i the collection of variable uch a tre, train, temperature, entropy. A variable i a tate variable if it integral
IEOR 3106: Fall 2013, Professor Whitt Topics for Discussion: Tuesday, November 19 Alternating Renewal Processes and The Renewal Equation
 IEOR 316: Fall 213, Profeor Whitt Topic for Dicuion: Tueday, November 19 Alternating Renewal Procee and The Renewal Equation 1 Alternating Renewal Procee An alternating renewal proce alternate between
IEOR 316: Fall 213, Profeor Whitt Topic for Dicuion: Tueday, November 19 Alternating Renewal Procee and The Renewal Equation 1 Alternating Renewal Procee An alternating renewal proce alternate between
What lies between Δx E, which represents the steam valve, and ΔP M, which is the mechanical power into the synchronous machine?
 A 2.0 Introduction In the lat et of note, we developed a model of the peed governing mechanim, which i given below: xˆ K ( Pˆ ˆ) E () In thee note, we want to extend thi model o that it relate the actual
A 2.0 Introduction In the lat et of note, we developed a model of the peed governing mechanim, which i given below: xˆ K ( Pˆ ˆ) E () In thee note, we want to extend thi model o that it relate the actual
The Hassenpflug Matrix Tensor Notation
 The Haenpflug Matrix Tenor Notation D.N.J. El Dept of Mech Mechatron Eng Univ of Stellenboch, South Africa e-mail: dnjel@un.ac.za 2009/09/01 Abtract Thi i a ample document to illutrate the typeetting of
The Haenpflug Matrix Tenor Notation D.N.J. El Dept of Mech Mechatron Eng Univ of Stellenboch, South Africa e-mail: dnjel@un.ac.za 2009/09/01 Abtract Thi i a ample document to illutrate the typeetting of
SOLUTIONS
 SOLUTIONS Topic-2 RAOULT S LAW, ALICATIONS AND NUMERICALS VERY SHORT ANSWER QUESTIONS 1. Define vapour preure? An: When a liquid i in equilibrium with it own vapour the preure exerted by the vapour on
SOLUTIONS Topic-2 RAOULT S LAW, ALICATIONS AND NUMERICALS VERY SHORT ANSWER QUESTIONS 1. Define vapour preure? An: When a liquid i in equilibrium with it own vapour the preure exerted by the vapour on
( ) Given: In a constant pressure combustor. C4H10 and theoretical air burns at P1 = 0.2 MPa, T1 = 600K. Products exit at P2 = 0.
 (SP 9) N-butane (C4H1) i burned with 85 percent theoretical air, and the product of combution, an equilibrium mixture containing only O, CO, CO, H, HO, N, and NO, exit from a combution chamber at K,. MPa.
(SP 9) N-butane (C4H1) i burned with 85 percent theoretical air, and the product of combution, an equilibrium mixture containing only O, CO, CO, H, HO, N, and NO, exit from a combution chamber at K,. MPa.
Problem Set 8 Solutions
 Deign and Analyi of Algorithm April 29, 2015 Maachuett Intitute of Technology 6.046J/18.410J Prof. Erik Demaine, Srini Devada, and Nancy Lynch Problem Set 8 Solution Problem Set 8 Solution Thi problem
Deign and Analyi of Algorithm April 29, 2015 Maachuett Intitute of Technology 6.046J/18.410J Prof. Erik Demaine, Srini Devada, and Nancy Lynch Problem Set 8 Solution Problem Set 8 Solution Thi problem
EE Control Systems LECTURE 14
 Updated: Tueday, March 3, 999 EE 434 - Control Sytem LECTURE 4 Copyright FL Lewi 999 All right reerved ROOT LOCUS DESIGN TECHNIQUE Suppoe the cloed-loop tranfer function depend on a deign parameter k We
Updated: Tueday, March 3, 999 EE 434 - Control Sytem LECTURE 4 Copyright FL Lewi 999 All right reerved ROOT LOCUS DESIGN TECHNIQUE Suppoe the cloed-loop tranfer function depend on a deign parameter k We
Suggested Answers To Exercises. estimates variability in a sampling distribution of random means. About 68% of means fall
 Beyond Significance Teting ( nd Edition), Rex B. Kline Suggeted Anwer To Exercie Chapter. The tatitic meaure variability among core at the cae level. In a normal ditribution, about 68% of the core fall
Beyond Significance Teting ( nd Edition), Rex B. Kline Suggeted Anwer To Exercie Chapter. The tatitic meaure variability among core at the cae level. In a normal ditribution, about 68% of the core fall
1. The reaction rate is defined as the change in concentration of a reactant or product per unit time. Consider the general reaction:
 CHAPTER TWELVE CHEMICAL KINETICS For Review. The reaction rate i defined a the change in concentration of a reactant or product per unit time. Conider the general reaction: A Product where rate = Δ Δt
CHAPTER TWELVE CHEMICAL KINETICS For Review. The reaction rate i defined a the change in concentration of a reactant or product per unit time. Conider the general reaction: A Product where rate = Δ Δt
March 18, 2014 Academic Year 2013/14
 POLITONG - SHANGHAI BASIC AUTOMATIC CONTROL Exam grade March 8, 4 Academic Year 3/4 NAME (Pinyin/Italian)... STUDENT ID Ue only thee page (including the back) for anwer. Do not ue additional heet. Ue of
POLITONG - SHANGHAI BASIC AUTOMATIC CONTROL Exam grade March 8, 4 Academic Year 3/4 NAME (Pinyin/Italian)... STUDENT ID Ue only thee page (including the back) for anwer. Do not ue additional heet. Ue of
UNIT 15 RELIABILITY EVALUATION OF k-out-of-n AND STANDBY SYSTEMS
 UNIT 1 RELIABILITY EVALUATION OF k-out-of-n AND STANDBY SYSTEMS Structure 1.1 Introduction Objective 1.2 Redundancy 1.3 Reliability of k-out-of-n Sytem 1.4 Reliability of Standby Sytem 1. Summary 1.6 Solution/Anwer
UNIT 1 RELIABILITY EVALUATION OF k-out-of-n AND STANDBY SYSTEMS Structure 1.1 Introduction Objective 1.2 Redundancy 1.3 Reliability of k-out-of-n Sytem 1.4 Reliability of Standby Sytem 1. Summary 1.6 Solution/Anwer
AP Chemistry: Kinetics Practice Problems
 AP Chemitr: Kinetic Practice Problem Direction: Write our anwer to the following quetion in the pace provided. For problem olving, how all of our work. Make ure that our anwer how proper unit, notation,
AP Chemitr: Kinetic Practice Problem Direction: Write our anwer to the following quetion in the pace provided. For problem olving, how all of our work. Make ure that our anwer how proper unit, notation,
Convex Hulls of Curves Sam Burton
 Convex Hull of Curve Sam Burton 1 Introduction Thi paper will primarily be concerned with determining the face of convex hull of curve of the form C = {(t, t a, t b ) t [ 1, 1]}, a < b N in R 3. We hall
Convex Hull of Curve Sam Burton 1 Introduction Thi paper will primarily be concerned with determining the face of convex hull of curve of the form C = {(t, t a, t b ) t [ 1, 1]}, a < b N in R 3. We hall
Introduction to Laplace Transform Techniques in Circuit Analysis
 Unit 6 Introduction to Laplace Tranform Technique in Circuit Analyi In thi unit we conider the application of Laplace Tranform to circuit analyi. A relevant dicuion of the one-ided Laplace tranform i found
Unit 6 Introduction to Laplace Tranform Technique in Circuit Analyi In thi unit we conider the application of Laplace Tranform to circuit analyi. A relevant dicuion of the one-ided Laplace tranform i found
Lecture 4 Topic 3: General linear models (GLMs), the fundamentals of the analysis of variance (ANOVA), and completely randomized designs (CRDs)
 Lecture 4 Topic 3: General linear model (GLM), the fundamental of the analyi of variance (ANOVA), and completely randomized deign (CRD) The general linear model One population: An obervation i explained
Lecture 4 Topic 3: General linear model (GLM), the fundamental of the analyi of variance (ANOVA), and completely randomized deign (CRD) The general linear model One population: An obervation i explained
A novel protocol for linearization of the Poisson-Boltzmann equation
 Ann. Univ. Sofia, Fac. Chem. Pharm. 16 (14) 59-64 [arxiv 141.118] A novel protocol for linearization of the Poion-Boltzmann equation Roumen Tekov Department of Phyical Chemitry, Univerity of Sofia, 1164
Ann. Univ. Sofia, Fac. Chem. Pharm. 16 (14) 59-64 [arxiv 141.118] A novel protocol for linearization of the Poion-Boltzmann equation Roumen Tekov Department of Phyical Chemitry, Univerity of Sofia, 1164
Vector-Space Methods and Kirchhoff Graphs for Reaction Networks
 Vector-Space Method and Kirchhoff Graph for Reaction Network Joeph D. Fehribach Fuel Cell Center WPI Mathematical Science and Chemical Engineering 00 Intitute Rd. Worceter, MA 0609-2247 Thi article preent
Vector-Space Method and Kirchhoff Graph for Reaction Network Joeph D. Fehribach Fuel Cell Center WPI Mathematical Science and Chemical Engineering 00 Intitute Rd. Worceter, MA 0609-2247 Thi article preent
MAE 101A. Homework 3 Solutions 2/5/2018
 MAE 101A Homework 3 Solution /5/018 Munon 3.6: What preure gradient along the treamline, /d, i required to accelerate water upward in a vertical pipe at a rate of 30 ft/? What i the anwer if the flow i
MAE 101A Homework 3 Solution /5/018 Munon 3.6: What preure gradient along the treamline, /d, i required to accelerate water upward in a vertical pipe at a rate of 30 ft/? What i the anwer if the flow i
At the end of this lesson, the students should be able to understand:
 Intructional Objective: At the end of thi leon, the tudent hould be able to undertand: Baic failure mechanim of riveted joint. Concept of deign of a riveted joint. 1. Strength of riveted joint: Strength
Intructional Objective: At the end of thi leon, the tudent hould be able to undertand: Baic failure mechanim of riveted joint. Concept of deign of a riveted joint. 1. Strength of riveted joint: Strength
Entropy Minimization in Design of Extractive Distillation System with Internal Heat Exchangers
 Entropy Minimization in Deign of Extractive Ditillation Sytem with Internal Heat Exchanger Diego F. Mendoza, Carlo.M. Riaco * Group of Proce Sytem Engineering, Department of Chemical and Environmental
Entropy Minimization in Deign of Extractive Ditillation Sytem with Internal Heat Exchanger Diego F. Mendoza, Carlo.M. Riaco * Group of Proce Sytem Engineering, Department of Chemical and Environmental
SERIES COMPENSATION: VOLTAGE COMPENSATION USING DVR (Lectures 41-48)
 Chapter 5 SERIES COMPENSATION: VOLTAGE COMPENSATION USING DVR (Lecture 41-48) 5.1 Introduction Power ytem hould enure good quality of electric power upply, which mean voltage and current waveform hould
Chapter 5 SERIES COMPENSATION: VOLTAGE COMPENSATION USING DVR (Lecture 41-48) 5.1 Introduction Power ytem hould enure good quality of electric power upply, which mean voltage and current waveform hould
Massachusetts Institute of Technology Dynamics and Control II
 I E Maachuett Intitute of Technology Department of Mechanical Engineering 2.004 Dynamic and Control II Laboratory Seion 5: Elimination of Steady-State Error Uing Integral Control Action 1 Laboratory Objective:
I E Maachuett Intitute of Technology Department of Mechanical Engineering 2.004 Dynamic and Control II Laboratory Seion 5: Elimination of Steady-State Error Uing Integral Control Action 1 Laboratory Objective:
Q.4 Which of the following of will have the same number of
 year n0te chemitry new t Chapter 3rd GASES MCQ Q.1 The order of the rate of diffuion of gae NH3, SO2, Cl2 and CO2 i: (a) NH3 > SO2 > Cl2 > CO2 (b) NH3 > CO2 > SO2 > Cl2 Cl2> SO2 > CO2 > NH3 None of thee
year n0te chemitry new t Chapter 3rd GASES MCQ Q.1 The order of the rate of diffuion of gae NH3, SO2, Cl2 and CO2 i: (a) NH3 > SO2 > Cl2 > CO2 (b) NH3 > CO2 > SO2 > Cl2 Cl2> SO2 > CO2 > NH3 None of thee
DYNAMIC MODELS FOR CONTROLLER DESIGN
 DYNAMIC MODELS FOR CONTROLLER DESIGN M.T. Tham (996,999) Dept. of Chemical and Proce Engineering Newcatle upon Tyne, NE 7RU, UK.. INTRODUCTION The problem of deigning a good control ytem i baically that
DYNAMIC MODELS FOR CONTROLLER DESIGN M.T. Tham (996,999) Dept. of Chemical and Proce Engineering Newcatle upon Tyne, NE 7RU, UK.. INTRODUCTION The problem of deigning a good control ytem i baically that
Green-Kubo formulas with symmetrized correlation functions for quantum systems in steady states: the shear viscosity of a fluid in a steady shear flow
 Green-Kubo formula with ymmetrized correlation function for quantum ytem in teady tate: the hear vicoity of a fluid in a teady hear flow Hirohi Matuoa Department of Phyic, Illinoi State Univerity, Normal,
Green-Kubo formula with ymmetrized correlation function for quantum ytem in teady tate: the hear vicoity of a fluid in a teady hear flow Hirohi Matuoa Department of Phyic, Illinoi State Univerity, Normal,
CHAPTER 4 DESIGN OF STATE FEEDBACK CONTROLLERS AND STATE OBSERVERS USING REDUCED ORDER MODEL
 98 CHAPTER DESIGN OF STATE FEEDBACK CONTROLLERS AND STATE OBSERVERS USING REDUCED ORDER MODEL INTRODUCTION The deign of ytem uing tate pace model for the deign i called a modern control deign and it i
98 CHAPTER DESIGN OF STATE FEEDBACK CONTROLLERS AND STATE OBSERVERS USING REDUCED ORDER MODEL INTRODUCTION The deign of ytem uing tate pace model for the deign i called a modern control deign and it i
Preemptive scheduling on a small number of hierarchical machines
 Available online at www.ciencedirect.com Information and Computation 06 (008) 60 619 www.elevier.com/locate/ic Preemptive cheduling on a mall number of hierarchical machine György Dóa a, Leah Eptein b,
Available online at www.ciencedirect.com Information and Computation 06 (008) 60 619 www.elevier.com/locate/ic Preemptive cheduling on a mall number of hierarchical machine György Dóa a, Leah Eptein b,
MAE320-HW7A. 1b). The entropy of an isolated system increases during a process. A). sometimes B). always C). never D).
 MAE0-W7A The homework i due Monday, November 4, 06. Each problem i worth the point indicated. Copying o the olution rom another i not acceptable. (). Multiple choice (0 point) a). Which tatement i invalid
MAE0-W7A The homework i due Monday, November 4, 06. Each problem i worth the point indicated. Copying o the olution rom another i not acceptable. (). Multiple choice (0 point) a). Which tatement i invalid
SOLUTION MANUAL CHAPTER 12
 SOLUION MANUAL CHAPER CONEN SUBSECION PROB NO. In-ext Concept Quetion a-g Concept problem - Brayton cycle, ga turbine - Regenerator, Intercooler, nonideal cycle 5-9 Ericon cycle 0- Jet engine cycle -5
SOLUION MANUAL CHAPER CONEN SUBSECION PROB NO. In-ext Concept Quetion a-g Concept problem - Brayton cycle, ga turbine - Regenerator, Intercooler, nonideal cycle 5-9 Ericon cycle 0- Jet engine cycle -5
Lecture 10 Filtering: Applied Concepts
 Lecture Filtering: Applied Concept In the previou two lecture, you have learned about finite-impule-repone (FIR) and infinite-impule-repone (IIR) filter. In thee lecture, we introduced the concept of filtering
Lecture Filtering: Applied Concept In the previou two lecture, you have learned about finite-impule-repone (FIR) and infinite-impule-repone (IIR) filter. In thee lecture, we introduced the concept of filtering
MAE 113, Summer Session 1, 2009
 HW #1 1., 1.7, 1.14,.3,.6 MAE 113, Summer Seion 1, 9 1. Develop the following analytical expreion for a turbojet engine: a) When m f
HW #1 1., 1.7, 1.14,.3,.6 MAE 113, Summer Seion 1, 9 1. Develop the following analytical expreion for a turbojet engine: a) When m f
Midterm Review - Part 1
 Honor Phyic Fall, 2016 Midterm Review - Part 1 Name: Mr. Leonard Intruction: Complete the following workheet. SHOW ALL OF YOUR WORK. 1. Determine whether each tatement i True or Fale. If the tatement i
Honor Phyic Fall, 2016 Midterm Review - Part 1 Name: Mr. Leonard Intruction: Complete the following workheet. SHOW ALL OF YOUR WORK. 1. Determine whether each tatement i True or Fale. If the tatement i
Digital Control System
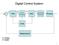 Digital Control Sytem - A D D A Micro ADC DAC Proceor Correction Element Proce Clock Meaurement A: Analog D: Digital Continuou Controller and Digital Control Rt - c Plant yt Continuou Controller Digital
Digital Control Sytem - A D D A Micro ADC DAC Proceor Correction Element Proce Clock Meaurement A: Analog D: Digital Continuou Controller and Digital Control Rt - c Plant yt Continuou Controller Digital
Nonlinear Single-Particle Dynamics in High Energy Accelerators
 Nonlinear Single-Particle Dynamic in High Energy Accelerator Part 6: Canonical Perturbation Theory Nonlinear Single-Particle Dynamic in High Energy Accelerator Thi coure conit of eight lecture: 1. Introduction
Nonlinear Single-Particle Dynamic in High Energy Accelerator Part 6: Canonical Perturbation Theory Nonlinear Single-Particle Dynamic in High Energy Accelerator Thi coure conit of eight lecture: 1. Introduction
Clustering Methods without Given Number of Clusters
 Clutering Method without Given Number of Cluter Peng Xu, Fei Liu Introduction A we now, mean method i a very effective algorithm of clutering. It mot powerful feature i the calability and implicity. However,
Clutering Method without Given Number of Cluter Peng Xu, Fei Liu Introduction A we now, mean method i a very effective algorithm of clutering. It mot powerful feature i the calability and implicity. However,
Design By Emulation (Indirect Method)
 Deign By Emulation (Indirect Method he baic trategy here i, that Given a continuou tranfer function, it i required to find the bet dicrete equivalent uch that the ignal produced by paing an input ignal
Deign By Emulation (Indirect Method he baic trategy here i, that Given a continuou tranfer function, it i required to find the bet dicrete equivalent uch that the ignal produced by paing an input ignal
S_LOOP: SINGLE-LOOP FEEDBACK CONTROL SYSTEM ANALYSIS
 S_LOOP: SINGLE-LOOP FEEDBACK CONTROL SYSTEM ANALYSIS by Michelle Gretzinger, Daniel Zyngier and Thoma Marlin INTRODUCTION One of the challenge to the engineer learning proce control i relating theoretical
S_LOOP: SINGLE-LOOP FEEDBACK CONTROL SYSTEM ANALYSIS by Michelle Gretzinger, Daniel Zyngier and Thoma Marlin INTRODUCTION One of the challenge to the engineer learning proce control i relating theoretical
Codes Correcting Two Deletions
 1 Code Correcting Two Deletion Ryan Gabry and Frederic Sala Spawar Sytem Center Univerity of California, Lo Angele ryan.gabry@navy.mil fredala@ucla.edu Abtract In thi work, we invetigate the problem of
1 Code Correcting Two Deletion Ryan Gabry and Frederic Sala Spawar Sytem Center Univerity of California, Lo Angele ryan.gabry@navy.mil fredala@ucla.edu Abtract In thi work, we invetigate the problem of
1 year chemistry n0tes new st CHAPTER 8 CHEMICAL EQUILIBRIUM MCQs Q.1 A reaction is reversible because (a) reactants are reactive (b) products are
 year chemitry n0te new t CHAPTER 8 CHEMICAL EQUILIBRIUM MCQ Q.1 A reaction i reverible becaue reactant are reactive (b) product are reactive (c) product are table (d) reactant are table Q.2 A large value
year chemitry n0te new t CHAPTER 8 CHEMICAL EQUILIBRIUM MCQ Q.1 A reaction i reverible becaue reactant are reactive (b) product are reactive (c) product are table (d) reactant are table Q.2 A large value
V = 4 3 πr3. d dt V = d ( 4 dv dt. = 4 3 π d dt r3 dv π 3r2 dv. dt = 4πr 2 dr
 0.1 Related Rate In many phyical ituation we have a relationhip between multiple quantitie, and we know the rate at which one of the quantitie i changing. Oftentime we can ue thi relationhip a a convenient
0.1 Related Rate In many phyical ituation we have a relationhip between multiple quantitie, and we know the rate at which one of the quantitie i changing. Oftentime we can ue thi relationhip a a convenient
Reaction rate. reaction rate describes change in concentration of reactants and products with time -> r = dc j
 Reaction rate ChE 400 - Reactive Process Engineering reaction rate describes change in concentration of reactants and products with time -> r = dc j /dt r is proportional to the reactant concentrations
Reaction rate ChE 400 - Reactive Process Engineering reaction rate describes change in concentration of reactants and products with time -> r = dc j /dt r is proportional to the reactant concentrations
Factor Analysis with Poisson Output
 Factor Analyi with Poion Output Gopal Santhanam Byron Yu Krihna V. Shenoy, Department of Electrical Engineering, Neurocience Program Stanford Univerity Stanford, CA 94305, USA {gopal,byronyu,henoy}@tanford.edu
Factor Analyi with Poion Output Gopal Santhanam Byron Yu Krihna V. Shenoy, Department of Electrical Engineering, Neurocience Program Stanford Univerity Stanford, CA 94305, USA {gopal,byronyu,henoy}@tanford.edu
Automatic Control Systems. Part III: Root Locus Technique
 www.pdhcenter.com PDH Coure E40 www.pdhonline.org Automatic Control Sytem Part III: Root Locu Technique By Shih-Min Hu, Ph.D., P.E. Page of 30 www.pdhcenter.com PDH Coure E40 www.pdhonline.org VI. Root
www.pdhcenter.com PDH Coure E40 www.pdhonline.org Automatic Control Sytem Part III: Root Locu Technique By Shih-Min Hu, Ph.D., P.E. Page of 30 www.pdhcenter.com PDH Coure E40 www.pdhonline.org VI. Root
GNSS Solutions: What is the carrier phase measurement? How is it generated in GNSS receivers? Simply put, the carrier phase
 GNSS Solution: Carrier phae and it meaurement for GNSS GNSS Solution i a regular column featuring quetion and anwer about technical apect of GNSS. Reader are invited to end their quetion to the columnit,
GNSS Solution: Carrier phae and it meaurement for GNSS GNSS Solution i a regular column featuring quetion and anwer about technical apect of GNSS. Reader are invited to end their quetion to the columnit,
The Secret Life of the ax + b Group
 The Secret Life of the ax + b Group Linear function x ax + b are prominent if not ubiquitou in high chool mathematic, beginning in, or now before, Algebra I. In particular, they are prime exhibit in any
The Secret Life of the ax + b Group Linear function x ax + b are prominent if not ubiquitou in high chool mathematic, beginning in, or now before, Algebra I. In particular, they are prime exhibit in any
Comparing Means: t-tests for Two Independent Samples
 Comparing ean: t-tet for Two Independent Sample Independent-eaure Deign t-tet for Two Independent Sample Allow reearcher to evaluate the mean difference between two population uing data from two eparate
Comparing ean: t-tet for Two Independent Sample Independent-eaure Deign t-tet for Two Independent Sample Allow reearcher to evaluate the mean difference between two population uing data from two eparate
Math Skills. Scientific Notation. Uncertainty in Measurements. Appendix A5 SKILLS HANDBOOK
 ppendix 5 Scientific Notation It i difficult to work with very large or very mall number when they are written in common decimal notation. Uually it i poible to accommodate uch number by changing the SI
ppendix 5 Scientific Notation It i difficult to work with very large or very mall number when they are written in common decimal notation. Uually it i poible to accommodate uch number by changing the SI
Overflow from last lecture: Ewald construction and Brillouin zones Structure factor
 Lecture 5: Overflow from lat lecture: Ewald contruction and Brillouin zone Structure factor Review Conider direct lattice defined by vector R = u 1 a 1 + u 2 a 2 + u 3 a 3 where u 1, u 2, u 3 are integer
Lecture 5: Overflow from lat lecture: Ewald contruction and Brillouin zone Structure factor Review Conider direct lattice defined by vector R = u 1 a 1 + u 2 a 2 + u 3 a 3 where u 1, u 2, u 3 are integer
A BATCH-ARRIVAL QUEUE WITH MULTIPLE SERVERS AND FUZZY PARAMETERS: PARAMETRIC PROGRAMMING APPROACH
 Mathematical and Computational Application Vol. 11 No. pp. 181-191 006. Aociation for Scientific Reearch A BATCH-ARRIVA QEE WITH MTIPE SERVERS AND FZZY PARAMETERS: PARAMETRIC PROGRAMMING APPROACH Jau-Chuan
Mathematical and Computational Application Vol. 11 No. pp. 181-191 006. Aociation for Scientific Reearch A BATCH-ARRIVA QEE WITH MTIPE SERVERS AND FZZY PARAMETERS: PARAMETRIC PROGRAMMING APPROACH Jau-Chuan
EP225 Note No. 5 Mechanical Waves
 EP5 Note No. 5 Mechanical Wave 5. Introduction Cacade connection of many ma-pring unit conitute a medium for mechanical wave which require that medium tore both kinetic energy aociated with inertia (ma)
EP5 Note No. 5 Mechanical Wave 5. Introduction Cacade connection of many ma-pring unit conitute a medium for mechanical wave which require that medium tore both kinetic energy aociated with inertia (ma)
NCAAPMT Calculus Challenge Challenge #3 Due: October 26, 2011
 NCAAPMT Calculu Challenge 011 01 Challenge #3 Due: October 6, 011 A Model of Traffic Flow Everyone ha at ome time been on a multi-lane highway and encountered road contruction that required the traffic
NCAAPMT Calculu Challenge 011 01 Challenge #3 Due: October 6, 011 A Model of Traffic Flow Everyone ha at ome time been on a multi-lane highway and encountered road contruction that required the traffic
Question 1 Equivalent Circuits
 MAE 40 inear ircuit Fall 2007 Final Intruction ) Thi exam i open book You may ue whatever written material you chooe, including your cla note and textbook You may ue a hand calculator with no communication
MAE 40 inear ircuit Fall 2007 Final Intruction ) Thi exam i open book You may ue whatever written material you chooe, including your cla note and textbook You may ue a hand calculator with no communication
Standard Guide for Conducting Ruggedness Tests 1
 Deignation: E 69 89 (Reapproved 996) Standard Guide for Conducting Ruggedne Tet AMERICA SOCIETY FOR TESTIG AD MATERIALS 00 Barr Harbor Dr., Wet Conhohocken, PA 948 Reprinted from the Annual Book of ASTM
Deignation: E 69 89 (Reapproved 996) Standard Guide for Conducting Ruggedne Tet AMERICA SOCIETY FOR TESTIG AD MATERIALS 00 Barr Harbor Dr., Wet Conhohocken, PA 948 Reprinted from the Annual Book of ASTM
SOME RESULTS ON INFINITE POWER TOWERS
 NNTDM 16 2010) 3, 18-24 SOME RESULTS ON INFINITE POWER TOWERS Mladen Vailev - Miana 5, V. Hugo Str., Sofia 1124, Bulgaria E-mail:miana@abv.bg Abtract To my friend Kratyu Gumnerov In the paper the infinite
NNTDM 16 2010) 3, 18-24 SOME RESULTS ON INFINITE POWER TOWERS Mladen Vailev - Miana 5, V. Hugo Str., Sofia 1124, Bulgaria E-mail:miana@abv.bg Abtract To my friend Kratyu Gumnerov In the paper the infinite
Solving Differential Equations by the Laplace Transform and by Numerical Methods
 36CH_PHCalter_TechMath_95099 3//007 :8 PM Page Solving Differential Equation by the Laplace Tranform and by Numerical Method OBJECTIVES When you have completed thi chapter, you hould be able to: Find the
36CH_PHCalter_TechMath_95099 3//007 :8 PM Page Solving Differential Equation by the Laplace Tranform and by Numerical Method OBJECTIVES When you have completed thi chapter, you hould be able to: Find the
Z a>2 s 1n = X L - m. X L = m + Z a>2 s 1n X L = The decision rule for this one-tail test is
 M09_BERE8380_12_OM_C09.QD 2/21/11 3:44 PM Page 1 9.6 The Power of a Tet 9.6 The Power of a Tet 1 Section 9.1 defined Type I and Type II error and their aociated rik. Recall that a repreent the probability
M09_BERE8380_12_OM_C09.QD 2/21/11 3:44 PM Page 1 9.6 The Power of a Tet 9.6 The Power of a Tet 1 Section 9.1 defined Type I and Type II error and their aociated rik. Recall that a repreent the probability
Math 273 Solutions to Review Problems for Exam 1
 Math 7 Solution to Review Problem for Exam True or Fale? Circle ONE anwer for each Hint: For effective tudy, explain why if true and give a counterexample if fale (a) T or F : If a b and b c, then a c
Math 7 Solution to Review Problem for Exam True or Fale? Circle ONE anwer for each Hint: For effective tudy, explain why if true and give a counterexample if fale (a) T or F : If a b and b c, then a c
NAME (pinyin/italian)... MATRICULATION NUMBER... SIGNATURE
 POLITONG SHANGHAI BASIC AUTOMATIC CONTROL June Academic Year / Exam grade NAME (pinyin/italian)... MATRICULATION NUMBER... SIGNATURE Ue only thee page (including the bac) for anwer. Do not ue additional
POLITONG SHANGHAI BASIC AUTOMATIC CONTROL June Academic Year / Exam grade NAME (pinyin/italian)... MATRICULATION NUMBER... SIGNATURE Ue only thee page (including the bac) for anwer. Do not ue additional
Extending MFM Function Ontology for Representing Separation and Conversion in Process Plant
 Downloaded from orbit.dtu.dk on: Oct 05, 2018 Extending MFM Function Ontology for Repreenting Separation and Converion in Proce Plant Zhang, Xinxin; Lind, Morten; Jørgenen, Sten Bay; Wu, Jing; Karnati,
Downloaded from orbit.dtu.dk on: Oct 05, 2018 Extending MFM Function Ontology for Repreenting Separation and Converion in Proce Plant Zhang, Xinxin; Lind, Morten; Jørgenen, Sten Bay; Wu, Jing; Karnati,
Chapter 13. Root Locus Introduction
 Chapter 13 Root Locu 13.1 Introduction In the previou chapter we had a glimpe of controller deign iue through ome imple example. Obviouly when we have higher order ytem, uch imple deign technique will
Chapter 13 Root Locu 13.1 Introduction In the previou chapter we had a glimpe of controller deign iue through ome imple example. Obviouly when we have higher order ytem, uch imple deign technique will
Non-linearity parameter B=A of binary liquid mixtures at elevated pressures
 PRAMANA cfl Indian Academy of Science Vol. 55, No. 3 journal of September 2000 phyic pp. 433 439 Non-linearity parameter B=A of binary liquid mixture at elevated preure J D PANDEY, J CHHABRA, R DEY, V
PRAMANA cfl Indian Academy of Science Vol. 55, No. 3 journal of September 2000 phyic pp. 433 439 Non-linearity parameter B=A of binary liquid mixture at elevated preure J D PANDEY, J CHHABRA, R DEY, V
Online Appendix for Managerial Attention and Worker Performance by Marina Halac and Andrea Prat
 Online Appendix for Managerial Attention and Worker Performance by Marina Halac and Andrea Prat Thi Online Appendix contain the proof of our reult for the undicounted limit dicued in Section 2 of the paper,
Online Appendix for Managerial Attention and Worker Performance by Marina Halac and Andrea Prat Thi Online Appendix contain the proof of our reult for the undicounted limit dicued in Section 2 of the paper,
Solutions to exercises week 45 FYS2160
 Solution to exercie week 45 FYS2160 Kritian Bjørke, Knut Oddvar Høie Vadla November 29, 2017 Schroeder 5.23 a) Writing Φ = U T S µn in term of infiniteimal change of the quantitie involved: dφ = du T ds
Solution to exercie week 45 FYS2160 Kritian Bjørke, Knut Oddvar Høie Vadla November 29, 2017 Schroeder 5.23 a) Writing Φ = U T S µn in term of infiniteimal change of the quantitie involved: dφ = du T ds
Annex-A: RTTOV9 Cloud validation
 RTTOV-91 Science and Validation Plan Annex-A: RTTOV9 Cloud validation Author O Embury C J Merchant The Univerity of Edinburgh Intitute for Atmo. & Environ. Science Crew Building King Building Edinburgh
RTTOV-91 Science and Validation Plan Annex-A: RTTOV9 Cloud validation Author O Embury C J Merchant The Univerity of Edinburgh Intitute for Atmo. & Environ. Science Crew Building King Building Edinburgh
PhysicsAndMathsTutor.com
 1. A teacher wihe to tet whether playing background muic enable tudent to complete a tak more quickly. The ame tak wa completed by 15 tudent, divided at random into two group. The firt group had background
1. A teacher wihe to tet whether playing background muic enable tudent to complete a tak more quickly. The ame tak wa completed by 15 tudent, divided at random into two group. The firt group had background
A Single Particle Thermal Model for Lithium Ion Batteries
 A Single Particle Thermal Model for Lithium Ion Batterie R. Painter* 1, B. Berryhill 1, L. Sharpe 2 and S. Keith Hargrove 2 1 Civil Engineering, Tenneee State Univerity, Nahville, TN, USA 2 Mechanical
A Single Particle Thermal Model for Lithium Ion Batterie R. Painter* 1, B. Berryhill 1, L. Sharpe 2 and S. Keith Hargrove 2 1 Civil Engineering, Tenneee State Univerity, Nahville, TN, USA 2 Mechanical
two equations that govern the motion of the fluid through some medium, like a pipe. These two equations are the
 Fluid and Fluid Mechanic Fluid in motion Dynamic Equation of Continuity After having worked on fluid at ret we turn to a moving fluid To decribe a moving fluid we develop two equation that govern the motion
Fluid and Fluid Mechanic Fluid in motion Dynamic Equation of Continuity After having worked on fluid at ret we turn to a moving fluid To decribe a moving fluid we develop two equation that govern the motion
Jul 4, 2005 turbo_code_primer Revision 0.0. Turbo Code Primer
 Jul 4, 5 turbo_code_primer Reviion. Turbo Code Primer. Introduction Thi document give a quick tutorial on MAP baed turbo coder. Section develop the background theory. Section work through a imple numerical
Jul 4, 5 turbo_code_primer Reviion. Turbo Code Primer. Introduction Thi document give a quick tutorial on MAP baed turbo coder. Section develop the background theory. Section work through a imple numerical
Laplace Transformation
 Univerity of Technology Electromechanical Department Energy Branch Advance Mathematic Laplace Tranformation nd Cla Lecture 6 Page of 7 Laplace Tranformation Definition Suppoe that f(t) i a piecewie continuou
Univerity of Technology Electromechanical Department Energy Branch Advance Mathematic Laplace Tranformation nd Cla Lecture 6 Page of 7 Laplace Tranformation Definition Suppoe that f(t) i a piecewie continuou
MATEMATIK Datum: Tid: eftermiddag. A.Heintz Telefonvakt: Anders Martinsson Tel.:
 MATEMATIK Datum: 20-08-25 Tid: eftermiddag GU, Chalmer Hjälpmedel: inga A.Heintz Telefonvakt: Ander Martinon Tel.: 073-07926. Löningar till tenta i ODE och matematik modellering, MMG5, MVE6. Define what
MATEMATIK Datum: 20-08-25 Tid: eftermiddag GU, Chalmer Hjälpmedel: inga A.Heintz Telefonvakt: Ander Martinon Tel.: 073-07926. Löningar till tenta i ODE och matematik modellering, MMG5, MVE6. Define what
1. Basic introduction to electromagnetic field. wave properties and particulate properties.
 Lecture Baic Radiometric Quantitie. The Beer-Bouguer-Lambert law. Concept of extinction cattering plu aborption and emiion. Schwarzchild equation. Objective:. Baic introduction to electromagnetic field:
Lecture Baic Radiometric Quantitie. The Beer-Bouguer-Lambert law. Concept of extinction cattering plu aborption and emiion. Schwarzchild equation. Objective:. Baic introduction to electromagnetic field:
