Isotope geochemistry of non-redox active elements
|
|
|
- Aron Heath
- 5 years ago
- Views:
Transcription
1 Isotope geochemistry of non-redox active elements Reading: White s last chapter has short sections on B and Li. - Resources: See Chapters in Johnson, Beard and Albaréde, 2004, Geochemistry of Non- Traditional Stable Isotopes Reviews in Mineralogy and Geochemistry, Vol. 55. Includes Li, Mg, Ca, Zn, Cl - Also see excellent chapter on B isotopes in Reviews in Mineralogy and Geochemistry Vol. 33. Guide Questions: How does diffusion fractionate isotopes? What happens to this effect with increasing T? What is the major driver of Li isotope fractionation at low T? What is the major driver of B isotope fractionation at low T? How does it gives us information about the ocean s ph in the past? How can Ca isotopes be used as a trophic level indicator? How do Si isotopes in marine diatoms change as nutrient levels change in a given area? General issues: Elements with only one valence in nature tend to have smaller isotopic variations. Example: Calcium (Ca): We measure 44/40; 10% mass difference. But Ca is almost always bonded ionically to oxygens, both in solution and in minerals. Little contrast in the bonding environment of Ca between dissolved Ca and Ca in minerals. Observed fractionation is only a few per mil (see DePaolo Chapter in RiMG volume 55) Compare to Chromium (Cr): 53/52. This is only a 2% mass difference, BUT we see 3-4 fractionation for reduction of Cr(VI) to Cr(III). Exceptions: Some elements have major bonding changes without any redox change. o B and Li switch between tetrahedral and octahedral coordination When an element is bonded to organic molecules, the local bonding environment may be altered relative to that in the free aqueous phase Diffusion-related fractionation of isotopes In general, we assume that both equilibrium and kinetic fractionation decrease with increasing T. There is one major exception to this: Diffusion. Recall our very simple equation that describes why, in a gas phase, light isotopes diffuse faster than heavier isotopes: 1 2 m 1v 1 2 = 1 2 m 2v 2 2 This says that at a given T, the kinetic energies of light and heavy isotopes are the same. It demands that the lighter isotopes have greater velocities to compensate for their lesser masses. Note that this is NOT T-dependent, and the isotopic fractionation related to this type of diffusion does not disappear even at very high T. Diffusion in liquids is more complex and the above equation does not directly apply. However, experiments have revealed that diffusion of Mg, Ca, and Li between a basaltic liquid and a rhyolitic liquid caused up to 7 shift in 26 Mg/ 24 Mg, 6 shift in 44 Ca/ 40 Ca, and 40 shift in 7 Li/ 6 Li at temperatures of roughly Lighter isotopes diffuse faster. See series of papers in Geochimica et Cosmochimica Acta by Frank Richter and Creaig Lundstrom s group. Oddly, diffusion in water at room T leads to less isotopic fractionation! In a 2006 paper, Richter and others found no measurable isotopic fractionation when Mg diffuses through water. The Li isotope
2 fractionation was surprisingly small, whereas the 35 Cl diffused about 1.4 faster than 37 Cl. These results are explained somewhat by the fact that small cations like Mg 2+ are bound to a larger sphere of perhaps dozens of polar H 2 O s, the solvation sphere. This makes the effective mass of the diffusing species quite large, and the mass difference between, say, 26 Mg and 24 Mg in the center matters little compared to the entire mass. Equilibrium fractionation of isotopes in a thermal gradient When hot molten silicate materials (i.e., in magmas) come into contact with cooler material, the strong temperature gradients cause isotopic fractionation (and elemental fractionation too). Lighter isotopes migrate toward the hot side; heavy isotopes toward the cool side Eventually, a stable equilibrium fractionation is attained Elements migrate too; mafic elements toward the hot end Temperature contrasts of a few hundred degrees are needed; this effect is probably very weak in aqueous environments Important: This mechanism and rapid diffusion are the ways to get isotopic fractionation at magmatic temperatures. Use of various isotope ratios in igneous systems We expect that equilibrium isotope fractionation between mineral phases, or between minerals and molten silicate material, are very small at magmatic T s (recall our general rules about how isotopic fractionation changes with T). But, there are two types of fractionation that persist to higher T: 1) Diffusion, as described above. So if a magmatic system has strong gradients that move elements rapidly via diffusion, we expect to see isotopic shifts resulting from this. a. Craig Lundstrom has observed shifts in Li and other isotope ratios close to rock structures that look like cracks where magma rose toward a mid-ocean ridge 2) Thermal gradient fractionation. For reasons that are not very well understood so far, when a silicate liquid is subjected to a temperature gradient, lighter isotopes tend to migrate toward the hotter region. This seems to be a thermodynamic effect (i.e., putting the lighter isotopes in hot region and heavier isotopes in cooler region leads to an overall lower free energy). The Lundstrom group is currently exploring isotope ratio difference in igneous systems, and finding trends that suggest elements are being moved around in thermal gradients. Non-redox fractionation of light elements (Li through Cl) Lithium ( 7 Li/ 6 Li): See Tomascak chapter in RiMG Geochemistry of Non-Traditional Stable Isotopes book. Standard is NIST SRM-8545, also known as L-SVEC. Probably close to bulk earth? Bonding contrasts: - 4-fold coordination (tetrahedral) in aqueous solutions - 4- or 6-fold (or irregular, > 4) in minerals In general, lower coordination number means more strength for each bond; stronger bonds means a tendency to take heavier isotopes. So a simple coordination change is enough to induce an equilibrium isotope fractionation; valence change is not needed. We thus expect that minerals with 6-fold coordination of Li + will tend to take the lighter isotope preferentially. This is observed in both experiments and natural settings. In rivers, dissolved Li + is isotopically heavy by about 30 relative to clay minerals in suspension (which are close to 0 ). This presumably reflects partial or complete equilibration of waters and clays in soil environments, with possible exchange later.
3 Ocean water is roughly +30 relative to bulk earth, carbonate rock inherits this somewhat. Clayrich marine sediments tend to be close to 0. Considerable variation has been found in igneous rocks. Subducted crust is heavier than normal mantle, and it is thought that isotopically heavy Li that shows up in subduction-related magmas may be from the subducted slab. Granites have a relatively wide range, positive and negative. Perhaps this reflects diffusive fractionation. Craig Lundstrom found that Li isotopes are fractionated near fissures through which magma migrates upward at mid-ocean ridges. This suggests strong diffusion of Li from the magma into or out of the host rock, which further implies that magmas evolve continuously as they ascend- contrary to traditional models. Boron (B) ( 11 B/ 10 B) -- See Palmer and Swihart chapter in Reviews in Mineralogy, Volume 33, Boron: Mineralogy, Petrology and Geochemistry (Grew and Anovitz, eds.) Standard is SRM 951- synthetic, not related to bulk earth Bonding environment contrast: B(OH) 3 is trigonal, 3-fold coordination B(OH) 4 - is tetrahedral, 4-fold Equilbrium between these two is very quickly attained B(OH) 3 is enriched in the heavier isotope Equilibrium fractionation at ocean temperatures: α 1.020, Δ 20 Application: Possible paleo-ph indicator: Very important, because oceanic ph is critical in determining the CO 2 in the atmosphere via exchange with oceans!!! Speciation (B(OH) 3 vs. B(OH) 4 - ) in waters changes with ph (pk = 8.8) o Total B in seawater is +40 o B(OH) 3 trigonal complex takes heavy isotopes o B(OH) 4- tetrahedral complex takes lighter isotopes! At ph < 7, B(OH) 3 dominates, B(OH) 4 - is +20! At ph > 10.2, B(OH) 4 - dominates, B(OH) 4 - is +40! At ph between 7 and 10.2: δ 11 B in B(OH) 4- is sensitive to ph! This is another linear mixing model: f 4 δ 4 + f 3 δ 3 = δ mix o Carbonate-producing organisms take in B(OH) 4 - preferentially, so! Lower δ 11 B in forams means lower ph in the past! Higher δ 11 B in forams means higher ph Seawater B and B from human use (borax) are very different isotopically Calcium (Ca) Measure: 44/40 via TIMS (best option) or 44/42 for plasma-based instruments (difficult) Low-Temperature environments: Little contrast in bonding environments, but still enough to cause isotopic shifts of a few per mil- much greater than analytical precision. General relationship: Ca put into mineral matter (bone or shell) by organisms is isotopically light relative to the fluids in the organism. Counterintuitive; could be a kinetic effect. Bone formation causes fractionation. Bone is about 1.5 lower in 44/40 relative to blood and/or soft tissues So, if Ca in the diet is well in excess of that needed to make bone, then the soft tissue is little affected by bone formation (preferential removal of light Ca) and is close to the avg. diet. So in this case (common) bone should be 1.5 less than diet.
4 BUT, if Ca in diet is mostly taken up by bone growth, then the soft tissue and blood will become isotopically heavy and the bone will be closer to the avg. diet isotope ratio. FURTHERMORE, if a person has bones that are 1.5 less than the avg. diet 44 Ca/ 40 Ca, AND they begin to lose calcium from their bones (astronauts do this- weightless) this bone loss can be detected via 44 Ca/ 40 Ca measurements of their blood. Trophic level effects. Consistent trends seen in animals. Herbivores are 1 lighter than plants they eat Carnivores are 1 lighter than their diet, roughly Increase in 44/40 observed with increasing trophic level- terrestrial and oceans Paleotemperatures? People would like to use this as a paleotemperature indicator. Studies show the fractionation factor is T-dependent, but I think there are important complications. High-T: Diffusion effects observed in lab experiments with magmatic diffusion. Possible application as a diffusion/disequilibrium indicator in igneous rocks Strontium ( 88 Sr/ 86 Sr mass dependent shifts) Similar to Ca, but Sr is heavier, so less fractionation. Has been used in several studies. Magnesium (Mg) ( 26 Mg/ 24 Mg; plasma mass specs only) Little bonding contrast, like Ca Possible paleoceanographic and other applications Responds to diffusion in high-t studies. Silicon (Si) ( 30 Si/ 28 Si; gas source MS or plasma source MS) see articles by De La Rocha et al. Diatom tests are1.0 to 1.3 LIGHT relative to ocean water the diatoms grew in Surface layer of ocean shifter to greater values This effect more intense when diatoms are more Si-limited- high levels of other nutrients, and high productivity Germanium (Ge) Plasma mass specs. Measureable to high precisision. May tell us about weathering? See articles by Rouxel et al. Chlorine (Cl) ( 37 Cl/ 35 Cl; gas source MS or PIMMS) Standard is SMOC- ocean chloride See Michael Stewart s chapter: RiMG Non-traditional book We dealt with redox-related effect with perchlorate reduction earlier But perchlorate is rare; almost all Cl is found as Cl - Natural δ 37 Cl does vary, probably because of diffusion effects and/or bonding contrasts between Cl - in various matrices (aqueous vs. clays vs. amphiboles, etc.) o Igneous rocks: -1 to +8! Mantle seems heterogeneous! Evidence for recycling of subducted crust in e.g. Hawaii?? o Evaporites and brines- small contrasts used to learn about Cl sources and behavior Important environmental contaminants (TCE, DCE, PCE, etc.)
5 - Chlorinated organics compounds can be very toxic and are a major current challenge - δ 37 Cl varies strongly between different batches of the chemicals- can be used to identify source of contamination - δ 37 Cl is also fractionated during biodegradation of these compounds Zinc (Zn 2+ ) ( 66 Zn/ 64 Zn; Plasma mass specs) preferential uptake of lighter isotopes by algal production in oceans 0.2 changes observed (measurement precision claimed was 0.04 ) Use as an indicator of paleoproductivity in the oceans (maybe lakes)? Used to distinguish different sources of particulate pollution (tires?) Cadmium (Cd 2+ ) Important contaminant Small differences observed in contaminated areas suggest contaminant Cd may be isotopically distinct from the background- little work done yet, see papers by Bullen et al. Summary Although redox-active elements tend to have larger isotopic fractionations than other elements, sometimes large isotopic variations are observed in elements without valence changes. - Contrasts in bonding (e.g., coordination number changes or binding to organic molecules) can cause large equilibrium isotope fractionation - Diffusion or thermal gradients can cause fractionation as well, and this fractionation does not disappear at high T - Even without much bonding contrast, small fractionations can occur and provide ways to track transport and chemical processes
Lecture 5. Introduction to Stable Isotopes
 Lecture 5 Introduction to Stable Isotopes Stable Isotope Geochemistry Primarily concerned with the isotope ratios of H, C, N, O, and S Si and B often included and new instrumentation has opened up others
Lecture 5 Introduction to Stable Isotopes Stable Isotope Geochemistry Primarily concerned with the isotope ratios of H, C, N, O, and S Si and B often included and new instrumentation has opened up others
Mineral Stability and Phase Diagrams Introduction
 1 of 10 10/10/2002 2:50 PM Prof. Stephen A. Nelson Geology 211 Tulane University Mineralogy and Phase Diagrams Introduction This document last updated on 10-Oct-2002 As we discussed previously, there are
1 of 10 10/10/2002 2:50 PM Prof. Stephen A. Nelson Geology 211 Tulane University Mineralogy and Phase Diagrams Introduction This document last updated on 10-Oct-2002 As we discussed previously, there are
Solid Earth materials:
 Solid Earth materials: Elements minerals rocks Nonuniform distribution of matter Molten core Contains most heavy elements Iron, nickel Thin surface crust Mostly lighter elements 8 elements make up 98.6%
Solid Earth materials: Elements minerals rocks Nonuniform distribution of matter Molten core Contains most heavy elements Iron, nickel Thin surface crust Mostly lighter elements 8 elements make up 98.6%
REFERENCE: The Blue Planet An Introduction to Earth System Science. Brian J. Skinner and Barbara W. Murck (2011) Third Edition. John Wiley and Sons
 REFERENCE: The Blue Planet An Introduction to Earth System Science. Brian J. Skinner and Barbara W. Murck (2011) Third Edition. John Wiley and Sons Inc. is substance It has mass and occupies space. The
REFERENCE: The Blue Planet An Introduction to Earth System Science. Brian J. Skinner and Barbara W. Murck (2011) Third Edition. John Wiley and Sons Inc. is substance It has mass and occupies space. The
Differentiation 2: mantle, crust OUTLINE
 Differentiation 2: mantle, crust OUTLINE Reading this week: Should have been White Ch 10 and 11!! 7- Nov Differentiation of the Earth, Core formation W 10.6.6, 11.4 9- Nov Moon, crust, mantle, atmosphere
Differentiation 2: mantle, crust OUTLINE Reading this week: Should have been White Ch 10 and 11!! 7- Nov Differentiation of the Earth, Core formation W 10.6.6, 11.4 9- Nov Moon, crust, mantle, atmosphere
Name Class Date. In your textbook, read about the nature of igneous rocks. Use each of the terms below just once to complete the following statements.
 CHAPTER 5 Igneous Rocks SECTION 5.1 What are igneous rocks? In your textbook, read about the nature of igneous rocks. Use each of the terms below just once to complete the following statements. basaltic
CHAPTER 5 Igneous Rocks SECTION 5.1 What are igneous rocks? In your textbook, read about the nature of igneous rocks. Use each of the terms below just once to complete the following statements. basaltic
Stable Isotopes OUTLINE
 Stable Isotopes OUTLINE Reading: White Ch 9.1 to 9.7.1 (or digital p370-400) Exercise answer? What does the salt do? Today 1. 2 leftovers 2. Stable Isotopes for hydrologic and climate applications 1 CaCO
Stable Isotopes OUTLINE Reading: White Ch 9.1 to 9.7.1 (or digital p370-400) Exercise answer? What does the salt do? Today 1. 2 leftovers 2. Stable Isotopes for hydrologic and climate applications 1 CaCO
Most mafic magmas come from the upper mantle and lower crust. This handout will address five questions:
 Geology 101 Origin of Magma From our discussions of the structure of the interior of the Earth, it is clear that the upper parts of the Earth (crust and mantle) are mostly solid because s-waves penetrate
Geology 101 Origin of Magma From our discussions of the structure of the interior of the Earth, it is clear that the upper parts of the Earth (crust and mantle) are mostly solid because s-waves penetrate
Lecture 6 - Determinants of Seawater Composition. Sets up electric dipole because O is more electronegative A o. Figure 3.
 12.742 - Marine Chemistry Fall 2004 Lecture 6 - Determinants of Seawater Composition Prof. Scott Doney What is seawater? Water Dissolved inorganic salts (major ions) Trace species, organics, colloids,
12.742 - Marine Chemistry Fall 2004 Lecture 6 - Determinants of Seawater Composition Prof. Scott Doney What is seawater? Water Dissolved inorganic salts (major ions) Trace species, organics, colloids,
Engineering Geology. Earth Structure. Hussien aldeeky
 Earth Structure Hussien aldeeky 1 Earth major spheres 1. Hydrosphere Ocean is the most prominent feature of the hydrosphere. - Is nearly 71% of Earth's surface - Holds about 97% of Earth's water Fresh
Earth Structure Hussien aldeeky 1 Earth major spheres 1. Hydrosphere Ocean is the most prominent feature of the hydrosphere. - Is nearly 71% of Earth's surface - Holds about 97% of Earth's water Fresh
1. are most likely to study the images sent back from Mars. A. Astronomers B. Geologists C. Doctors D. Engineers
 1. are most likely to study the images sent back from Mars. A. Astronomers B. Geologists C. Doctors D. Engineers 2. When did the Earth form? A. About 540 million years ago B. About 2.5 billion years ago
1. are most likely to study the images sent back from Mars. A. Astronomers B. Geologists C. Doctors D. Engineers 2. When did the Earth form? A. About 540 million years ago B. About 2.5 billion years ago
2 Igneous Rock. How do igneous rocks form? What factors affect the texture of igneous rock? BEFORE YOU READ. Rocks: Mineral Mixtures
 CHAPTER 2 2 Igneous Rock SECTION Rocks: Mineral Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: How do igneous rocks form? What factors affect the texture
CHAPTER 2 2 Igneous Rock SECTION Rocks: Mineral Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: How do igneous rocks form? What factors affect the texture
Magma Formation and Behavior
 Magma Formation and Behavior Questions What causes mantle rock to melt, resulting in magma formation? Why is magma formation restricted to specific plate tectonic settings? Why are mafic (basaltic) magmas
Magma Formation and Behavior Questions What causes mantle rock to melt, resulting in magma formation? Why is magma formation restricted to specific plate tectonic settings? Why are mafic (basaltic) magmas
Lecture 4 What Controls the Composition of Seawater
 Lecture 4 What Controls the Composition of Seawater Seawater is salty! Why? What controls the composition of seawater? Do Chemical Equilibrium reactions control the composition of the Ocean? What is meant
Lecture 4 What Controls the Composition of Seawater Seawater is salty! Why? What controls the composition of seawater? Do Chemical Equilibrium reactions control the composition of the Ocean? What is meant
Radiogenic Isotopes as Tracers of Sources and Mixing in the Solid Earth, Hydrosphere, Biosphere
 Radiogenic Isotopes as Tracers of Sources and Mixing in the Solid Earth, Hydrosphere, Biosphere Reading: White, Lecture 22, part on the mathematics of binary mixing. Also: Faure, 1986, Chs. 9, 11 OR...
Radiogenic Isotopes as Tracers of Sources and Mixing in the Solid Earth, Hydrosphere, Biosphere Reading: White, Lecture 22, part on the mathematics of binary mixing. Also: Faure, 1986, Chs. 9, 11 OR...
MODULE-21 TRENDS IN THE MODERN PERIODIC TABLE
 MODULE-21 TRENDS IN THE MODERN PERIODIC TABLE Valency is defined as the number of electrons an atom requires to lose, gain, or share in order to complete its valence shell to attain the stable noble gas
MODULE-21 TRENDS IN THE MODERN PERIODIC TABLE Valency is defined as the number of electrons an atom requires to lose, gain, or share in order to complete its valence shell to attain the stable noble gas
Thursday, October 4 th
 Thursday, October 4 th Objective: We will use and define the different ways to classify igneous rocks. Warm-up: 1. Which type of lava is most viscous? 2. Which type of lava has the least amount of silicate?
Thursday, October 4 th Objective: We will use and define the different ways to classify igneous rocks. Warm-up: 1. Which type of lava is most viscous? 2. Which type of lava has the least amount of silicate?
Name PRACTICE Unit 3: Periodic Table
 1. Compared to the atoms of nonmetals in Period 3, the atoms of metals in Period 3 have (1) fewer valence electrons (2) more valence electrons (3) fewer electron shells (4) more electron shells 2. On the
1. Compared to the atoms of nonmetals in Period 3, the atoms of metals in Period 3 have (1) fewer valence electrons (2) more valence electrons (3) fewer electron shells (4) more electron shells 2. On the
Standard 2, Objective 1: Evaluate the source of Earth s internal heat and the evidence of Earth s internal structure.
 Standard 2: Students will understand Earth s internal structure and the dynamic nature of the tectonic plates that form its surface. Standard 2, Objective 1: Evaluate the source of Earth s internal heat
Standard 2: Students will understand Earth s internal structure and the dynamic nature of the tectonic plates that form its surface. Standard 2, Objective 1: Evaluate the source of Earth s internal heat
Earth Science 11: Minerals
 lname: Date: Earth Science 11: Minerals Purpose: Text Pages: I can identify and classify minerals using their physical and chemical properties 90-111 *This is recommended reading! Matter and Atoms (5.1)
lname: Date: Earth Science 11: Minerals Purpose: Text Pages: I can identify and classify minerals using their physical and chemical properties 90-111 *This is recommended reading! Matter and Atoms (5.1)
Lecture 16 - Stable isotopes
 Lecture 16 - Stable isotopes 1. The fractionation of different isotopes of oxygen and their measurement in sediment cores has shown scientists that: (a) ice ages are common and lasted for hundreds of millions
Lecture 16 - Stable isotopes 1. The fractionation of different isotopes of oxygen and their measurement in sediment cores has shown scientists that: (a) ice ages are common and lasted for hundreds of millions
4 th Grade PSI. Slide 1 / 107 Slide 2 / 107. Slide 3 / 107. Slide 4 / 107. Slide 5 / 107. Slide 6 / 107. The History of Planet Earth
 Slide 1 / 107 Slide 2 / 107 4 th Grade PSI The History of Planet Earth 2015-11-10 www.njctl.org Slide 3 / 107 Slide 4 / 107 The History of Planet Earth The Structure of Earth Rock Layers Fossils and Relative
Slide 1 / 107 Slide 2 / 107 4 th Grade PSI The History of Planet Earth 2015-11-10 www.njctl.org Slide 3 / 107 Slide 4 / 107 The History of Planet Earth The Structure of Earth Rock Layers Fossils and Relative
Imagine the first rock and the cycles that it has been through.
 A rock is a naturally formed, consolidated material usually composed of grains of one or more minerals The rock cycle shows how one type of rocky material gets transformed into another The Rock Cycle Representation
A rock is a naturally formed, consolidated material usually composed of grains of one or more minerals The rock cycle shows how one type of rocky material gets transformed into another The Rock Cycle Representation
Campbell's Biology, 9e (Reece et al.) Chapter 2 The Chemical Context of Life
 Campbell's Biology, 9e (Reece et al.) Chapter 2 The Chemical Context of Life This chapter presents basic chemical principles for understanding the chemical context of living organisms, from atomic structure
Campbell's Biology, 9e (Reece et al.) Chapter 2 The Chemical Context of Life This chapter presents basic chemical principles for understanding the chemical context of living organisms, from atomic structure
A Rock is a solid aggregate of minerals.
 Quartz A Rock is a solid aggregate of minerals. Orthoclase Feldspar Plagioclase Feldspar Biotite Four different minerals are obvious in this piece of Granite. The average automobile contains: Minerals
Quartz A Rock is a solid aggregate of minerals. Orthoclase Feldspar Plagioclase Feldspar Biotite Four different minerals are obvious in this piece of Granite. The average automobile contains: Minerals
Formation of the Earth and Solar System
 Formation of the Earth and Solar System a. Supernova and formation of primordial dust cloud. NEBULAR HYPOTHESIS b. Condensation of primordial dust. Forms disk-shaped nubular cloud rotating counterclockwise.
Formation of the Earth and Solar System a. Supernova and formation of primordial dust cloud. NEBULAR HYPOTHESIS b. Condensation of primordial dust. Forms disk-shaped nubular cloud rotating counterclockwise.
About Earth Materials
 Grotzinger Jordan Understanding Earth Sixth Edition Chapter 3: EARTH MATERIALS Minerals and Rocks 2011 by W. H. Freeman and Company About Earth Materials All Earth materials are composed of atoms bound
Grotzinger Jordan Understanding Earth Sixth Edition Chapter 3: EARTH MATERIALS Minerals and Rocks 2011 by W. H. Freeman and Company About Earth Materials All Earth materials are composed of atoms bound
Chapter 1: Introduction...1 Chapter 2: Energy, Entropy and Fundamental Thermodynamic Concepts...24
 Table of Contents Part I: The Geochemical Toolbox Chapter 1: Introduction...1 Geochemistry...1 This Book...2 The Philosophy of Science...4 Building Scientific Understanding...4 The Scientist as Skeptic...5
Table of Contents Part I: The Geochemical Toolbox Chapter 1: Introduction...1 Geochemistry...1 This Book...2 The Philosophy of Science...4 Building Scientific Understanding...4 The Scientist as Skeptic...5
Earth Science 11: Earth Materials: Rock Cycle
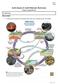 Name: Date: Earth Science 11: Earth Materials: Rock Cycle Chapter 2, pages 44 to 46 2.1: Rock Cycle What is a Rock? A solid mass of mineral or mineral-like matter that occurs naturally as part of our planet
Name: Date: Earth Science 11: Earth Materials: Rock Cycle Chapter 2, pages 44 to 46 2.1: Rock Cycle What is a Rock? A solid mass of mineral or mineral-like matter that occurs naturally as part of our planet
Lecture 6 - Igneous Rocks and Volcanoes
 Lecture 6 - Igneous Rocks and Volcanoes Learning objectives Understand and be able to predict where and why magma will be forming at different tectonic settings Understand the factors controlling magma
Lecture 6 - Igneous Rocks and Volcanoes Learning objectives Understand and be able to predict where and why magma will be forming at different tectonic settings Understand the factors controlling magma
Magma Formation and Behavior
 Magma Formation and Behavior Introduction: The study of body waves as they pass through Earth's interior provides strong evidence that the Earth's mantle is composed almost entirely of solid ultramafic
Magma Formation and Behavior Introduction: The study of body waves as they pass through Earth's interior provides strong evidence that the Earth's mantle is composed almost entirely of solid ultramafic
5 Stable and radioactive isotopes
 5 Stable and radioactive isotopes Outline 1 Stable isotopes Measuring stable isotopic abundances Equilibrium isotope effects Kinetic isotope effects Rayleigh distillation Isotopes: a mainstay of chemical
5 Stable and radioactive isotopes Outline 1 Stable isotopes Measuring stable isotopic abundances Equilibrium isotope effects Kinetic isotope effects Rayleigh distillation Isotopes: a mainstay of chemical
PLATE TECTONICS, VOLCANISM AND IGNEOUS ROCKS
 PLATE TECTONICS, VOLCANISM AND IGNEOUS ROCKS PLATE TECTONICS TO IGNEOUS ROCKS Internal Heat Seafloor Spreading/Plate Tectonics Volcanism Plate Boundary Intra-plate (hot spot) Divergent Convergent Igneous
PLATE TECTONICS, VOLCANISM AND IGNEOUS ROCKS PLATE TECTONICS TO IGNEOUS ROCKS Internal Heat Seafloor Spreading/Plate Tectonics Volcanism Plate Boundary Intra-plate (hot spot) Divergent Convergent Igneous
Stable Isotope Tracers
 Stable Isotope Tracers OCN 623 Chemical Oceanography 5 March 2015 Reading: Emerson and Hedges, Chapter 5, p.134-153 (c) 2015 David Ho and Frank Sansone Outline Stable Isotopes - Introduction & Notation
Stable Isotope Tracers OCN 623 Chemical Oceanography 5 March 2015 Reading: Emerson and Hedges, Chapter 5, p.134-153 (c) 2015 David Ho and Frank Sansone Outline Stable Isotopes - Introduction & Notation
Questions Q1. Describe, in detail, how you would carry out this experiment. (6) ...
 Questions Q1. * An experiment was carried out to compare the rates of reaction between calcium carbonate and two different concentrations of hydrochloric acid. Describe, in detail, how you would carry
Questions Q1. * An experiment was carried out to compare the rates of reaction between calcium carbonate and two different concentrations of hydrochloric acid. Describe, in detail, how you would carry
Most mafic magmas come from the upper mantle and lower crust. This handout will address five questions:
 IDS 102 Origin of Magma From our discussions of the structure of the interior of the Earth, it is clear that the upper parts of the Earth (crust and mantle) are mostly solid because s-waves penetrate those
IDS 102 Origin of Magma From our discussions of the structure of the interior of the Earth, it is clear that the upper parts of the Earth (crust and mantle) are mostly solid because s-waves penetrate those
The Lithosphere. Definition
 10/14/2014 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2014 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
10/14/2014 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2014 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
Rocks: Materials of the Solid Earth
 1 Rocks: Materials of the Solid Earth Presentation modified from: Instructor Resource Center on CD-ROM, Foundations of Earth Science,, 4 th Edition, Lutgens/Tarbuck, Rock Cycle Igneous Rocks Today 2 Rock
1 Rocks: Materials of the Solid Earth Presentation modified from: Instructor Resource Center on CD-ROM, Foundations of Earth Science,, 4 th Edition, Lutgens/Tarbuck, Rock Cycle Igneous Rocks Today 2 Rock
Chapter 18 - Volcanic Activity. Aka Volcano Under the City
 Chapter 18 - Volcanic Activity Aka Volcano Under the City 18.1 Magma Describe factors that affect the formation of magma. Compare and contrast the different types of magma. Temperature and pressure increase
Chapter 18 - Volcanic Activity Aka Volcano Under the City 18.1 Magma Describe factors that affect the formation of magma. Compare and contrast the different types of magma. Temperature and pressure increase
Chapter 7 Chemical Bonding and Molecular Geometry
 Chapter 7 Chemical Bonding and Molecular Geometry 347 Chapter 7 Chemical Bonding and Molecular Geometry Figure 7.1 Nicknamed buckyballs, buckminsterfullerene molecules (C60) contain only carbon atoms.
Chapter 7 Chemical Bonding and Molecular Geometry 347 Chapter 7 Chemical Bonding and Molecular Geometry Figure 7.1 Nicknamed buckyballs, buckminsterfullerene molecules (C60) contain only carbon atoms.
12. Data from Ito et al. (1987) Chemical Geology, 62, ; Figure ; and LeRoex et al. (1983) J. Petrol., 24,
 Announcements Reading for Wed: p.363-399!!! p.362-366; p.373-378; p.383-386; p.392-394; p.395-399 Last lecture on Wednesday Bring food for pizza party Bring class notes, labs, book N-MORBs: 87 Sr/ 86 Sr
Announcements Reading for Wed: p.363-399!!! p.362-366; p.373-378; p.383-386; p.392-394; p.395-399 Last lecture on Wednesday Bring food for pizza party Bring class notes, labs, book N-MORBs: 87 Sr/ 86 Sr
NSS Chemistry Part 2 The Microscopic World I HKCEE Past Paper Questions Structural Questions
 NSS Chemistry Part 2 The Microscopic World I HKCEE Past Paper Questions Structural Questions 1. HKCEE 1994 Q7b The table below lists some physical properties of lead, bromine and lead(ii) bromide. Lead
NSS Chemistry Part 2 The Microscopic World I HKCEE Past Paper Questions Structural Questions 1. HKCEE 1994 Q7b The table below lists some physical properties of lead, bromine and lead(ii) bromide. Lead
The Geochemistry of Natural Waters
 The Geochemistry of Natural Waters Surface and Groundwater Environments Third Edition James I. Drever University of Wyoming Prentice Hall Upper Saddle River. NJ 07458 Contents 3 Preface xi 1 The Hydrologie
The Geochemistry of Natural Waters Surface and Groundwater Environments Third Edition James I. Drever University of Wyoming Prentice Hall Upper Saddle River. NJ 07458 Contents 3 Preface xi 1 The Hydrologie
Evolution of the Earth
 Evolution of the Earth http://static.newworldencyclopedia.org/f/fe/geologic_clock.jpg Evolution of the Earth Solar system, 4.6 byr Collapse of a nebula Star forms as gravity concentrates material at center
Evolution of the Earth http://static.newworldencyclopedia.org/f/fe/geologic_clock.jpg Evolution of the Earth Solar system, 4.6 byr Collapse of a nebula Star forms as gravity concentrates material at center
Lecture 3 Rocks and the Rock Cycle Dr. Shwan Omar
 Rocks A naturally occurring aggregate of one or more minerals (e.g., granite), or a body of non-crystalline material (e.g., obsidian glass), or of solid organic material (e.g., coal). Rock Cycle A sequence
Rocks A naturally occurring aggregate of one or more minerals (e.g., granite), or a body of non-crystalline material (e.g., obsidian glass), or of solid organic material (e.g., coal). Rock Cycle A sequence
Petrology. Petrology: the study of rocks, especially aspects such as physical, chemical, spatial and chronoligic. Associated fields include:
 Petrology Petrology: the study of rocks, especially aspects such as physical, chemical, spatial and chronoligic. Associated fields include: Petrography: study of description and classification of rocks
Petrology Petrology: the study of rocks, especially aspects such as physical, chemical, spatial and chronoligic. Associated fields include: Petrography: study of description and classification of rocks
The Earth System. The Geosphere
 The Earth System The Geosphere Key Concepts How do materials in the geosphere differ? Why does the geosphere have a layered structure? What do you think? Read the three statements below and decide whether
The Earth System The Geosphere Key Concepts How do materials in the geosphere differ? Why does the geosphere have a layered structure? What do you think? Read the three statements below and decide whether
Minerals and Rocks Chapter 20
 Minerals and Rocks Chapter 20 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Minerals and Rocks Chapter 20 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Atoms, the basic building block of matter, are composed of three basic particles. Nucleus: Proton (+) Neutron (-) Outside the nucleus: Electron (-)
 Atoms, the basic building block of matter, are composed of three basic particles Nucleus: Proton (+) Neutron (-) Outside the nucleus: Electron (-) Atomic number = # protons Atomic mass number = # protons
Atoms, the basic building block of matter, are composed of three basic particles Nucleus: Proton (+) Neutron (-) Outside the nucleus: Electron (-) Atomic number = # protons Atomic mass number = # protons
Chapter 4 Up from the Inferno: Magma and Igneous Rocks
 Chapter 4 Up from the Inferno: Magma and Igneous Rocks Up from the Inferno: Magma and Igneous Rocks Updated by: Rick Oches, Professor of Geology & Environmental Sciences Bentley University Waltham, Massachusetts
Chapter 4 Up from the Inferno: Magma and Igneous Rocks Up from the Inferno: Magma and Igneous Rocks Updated by: Rick Oches, Professor of Geology & Environmental Sciences Bentley University Waltham, Massachusetts
Q. How do we know about the Earth s history? A. The ROCKS tell us stories
 Q. How do we know about the Earth s history? A. The ROCKS tell us stories Q. What happened here? Q. What happened here? Q. What happened here? Vocabulary word: Uniformitarianism the scientific rule that
Q. How do we know about the Earth s history? A. The ROCKS tell us stories Q. What happened here? Q. What happened here? Q. What happened here? Vocabulary word: Uniformitarianism the scientific rule that
The Nature of Igneous Rocks
 The Nature of Igneous Rocks Form from Magma Hot, partially molten mixture of solid liquid and gas Mineral crystals form in the magma making a crystal slush Gases - H 2 O, CO 2, etc. - are dissolved in
The Nature of Igneous Rocks Form from Magma Hot, partially molten mixture of solid liquid and gas Mineral crystals form in the magma making a crystal slush Gases - H 2 O, CO 2, etc. - are dissolved in
I. Earth spheres A. Three major spheres 1. atmosphere, thin envelope 2. hydrosphere covers more than 71% of surface 3. geosphere from hydrosphere to
 I. Earth spheres A. Three major spheres 1. atmosphere, thin envelope 2. hydrosphere covers more than 71% of surface 3. geosphere from hydrosphere to center 4. Biosphere penetrates all three, a. only thin
I. Earth spheres A. Three major spheres 1. atmosphere, thin envelope 2. hydrosphere covers more than 71% of surface 3. geosphere from hydrosphere to center 4. Biosphere penetrates all three, a. only thin
Layers of Earth Write us-
 Layers of Earth Three Layers of Earth Crust, Mantle and Core Layers of Earth Layers of Earth : Our Planet, the Earth is made up of different layers. Each layer of the Earth has unique properties. In 1692,
Layers of Earth Three Layers of Earth Crust, Mantle and Core Layers of Earth Layers of Earth : Our Planet, the Earth is made up of different layers. Each layer of the Earth has unique properties. In 1692,
Emily and Megan. Earth System Science. Elements of Earth by weight. Crust Elements, by weight. Minerals. Made of atoms Earth is mostly iron, by weight
 Emily and Megan Chapter 20 MINERALS AND ROCKS Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Emily and Megan Chapter 20 MINERALS AND ROCKS Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
A covalent bond is a shared pair of electrons between atoms of two non-metal elements.
 Bonding, Structure and properties Atoms can be held together by chemical bonds. When atoms form bonds, they can achieve a stable electron arrangement (full outer electron shell). To achieve a stable electron
Bonding, Structure and properties Atoms can be held together by chemical bonds. When atoms form bonds, they can achieve a stable electron arrangement (full outer electron shell). To achieve a stable electron
EARTH SCIENCE. Geology, the Environment and the Universe. Chapter 5: Igneous Rocks
 EARTH SCIENCE Geology, the Environment and the Universe Chapter 5: Igneous Rocks CHAPTER 5 Igneous Rocks Section 5.1 What are igneous rocks? Section 5.2 Classification of Igneous Rocks Click a hyperlink
EARTH SCIENCE Geology, the Environment and the Universe Chapter 5: Igneous Rocks CHAPTER 5 Igneous Rocks Section 5.1 What are igneous rocks? Section 5.2 Classification of Igneous Rocks Click a hyperlink
2 Igneous Rock. How do igneous rocks form? What factors affect the texture of igneous rock? BEFORE YOU READ. Rocks: Mineral Mixtures
 CHAPTER 4 2 Igneous Rock SECTION Rocks: Mineral Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: How do igneous rocks form? What factors affect the texture
CHAPTER 4 2 Igneous Rock SECTION Rocks: Mineral Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: How do igneous rocks form? What factors affect the texture
Earth Science 232 Petrography
 Earth Science 232 Petrography Course notes by Shaun Frape and Alec Blyth Winter 2002 1 Petrology - Introduction Some Definitions Petra Greek for rock Logos Greek for disclosure or explanation Petrology
Earth Science 232 Petrography Course notes by Shaun Frape and Alec Blyth Winter 2002 1 Petrology - Introduction Some Definitions Petra Greek for rock Logos Greek for disclosure or explanation Petrology
Crust Elements. Elements of Earth. Minerals. Crystals. Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air
 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Made of atoms Earth is mostly iron, by weight Elements of Earth Made of atoms
Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Made of atoms Earth is mostly iron, by weight Elements of Earth Made of atoms
Section 1: Earth s Interior and Plate Tectonics Section 2: Earthquakes and Volcanoes Section 3: Minerals and Rocks Section 4: Weathering and Erosion
 Section 1: Earth s Interior and Plate Tectonics Section 2: Earthquakes and Volcanoes Section 3: Minerals and Rocks Section 4: Weathering and Erosion Key Terms Crust Mantle Core Lithosphere Plate Tectonics
Section 1: Earth s Interior and Plate Tectonics Section 2: Earthquakes and Volcanoes Section 3: Minerals and Rocks Section 4: Weathering and Erosion Key Terms Crust Mantle Core Lithosphere Plate Tectonics
The Cycling of Matter. Day 1
 The Cycling of Matter Day 1 Objective I will learn the rock cycle is the series of processes in which rock changes from one form to another. I will learn in the water cycle, water condenses, precipitates
The Cycling of Matter Day 1 Objective I will learn the rock cycle is the series of processes in which rock changes from one form to another. I will learn in the water cycle, water condenses, precipitates
CLASS EXERCISE 5.1 List processes occurring in soils that cause changes in the levels of ions.
 5 SIL CHEMISTRY 5.1 Introduction A knowledge of the chemical composition of a soil is less useful than a knowledge of its component minerals and organic materials. These dictate the reactions that occur
5 SIL CHEMISTRY 5.1 Introduction A knowledge of the chemical composition of a soil is less useful than a knowledge of its component minerals and organic materials. These dictate the reactions that occur
WHAT ARE ROCKS? ROCKS are a naturally occurring SOLID MIXTURE of one or more minerals and organic matter. Rocks are ALWAYS changing.
 WHAT ARE ROCKS? ROCKS are a naturally occurring SOLID MIXTURE of one or more minerals and organic matter. Rocks are ALWAYS changing. How do we classify Rocks? Formation (where and how the rock was formed)
WHAT ARE ROCKS? ROCKS are a naturally occurring SOLID MIXTURE of one or more minerals and organic matter. Rocks are ALWAYS changing. How do we classify Rocks? Formation (where and how the rock was formed)
Plate tectonics, rock cycle
 Dikes, Antarctica Rock Cycle Plate tectonics, rock cycle The Rock Cycle A rock is a naturally formed, consolidated material usually composed of grains of one or more minerals The rock cycle shows how one
Dikes, Antarctica Rock Cycle Plate tectonics, rock cycle The Rock Cycle A rock is a naturally formed, consolidated material usually composed of grains of one or more minerals The rock cycle shows how one
Work hard. Be nice. Name: Period: Date: UNIT 3: Electrons Lesson 5: Atomic Radius and Ionic Radius
 UNIT 3: Electrons Lesson 5: Atomic Radius and Ionic Radius Do Now: By the end of today, you will have an answer to: How the does the radius change when an atom becomes an ion? 1. How many protons, neutrons,
UNIT 3: Electrons Lesson 5: Atomic Radius and Ionic Radius Do Now: By the end of today, you will have an answer to: How the does the radius change when an atom becomes an ion? 1. How many protons, neutrons,
THE ROCK CYCLE & ROCKS. Subtitle
 THE ROCK CYCLE & ROCKS Subtitle 3. Three rocks that do not have minerals or are composed of nonmineral matter. Coal Pumuce Obsidian THE ROCK CYCLE Why do scientists study rocks? Rocks contain clues about
THE ROCK CYCLE & ROCKS Subtitle 3. Three rocks that do not have minerals or are composed of nonmineral matter. Coal Pumuce Obsidian THE ROCK CYCLE Why do scientists study rocks? Rocks contain clues about
Essentials of Geology, 11e
 Essentials of Geology, 11e Igneous Rocks and Intrusive Activity Chapter 3 Instructor Jennifer Barson Spokane Falls Community College Geology 101 Stanley Hatfield Southwestern Illinois College Characteristics
Essentials of Geology, 11e Igneous Rocks and Intrusive Activity Chapter 3 Instructor Jennifer Barson Spokane Falls Community College Geology 101 Stanley Hatfield Southwestern Illinois College Characteristics
GCSE Additional Science
 GCSE Additional Science Module C5 Chemicals of the Natural Environment: What you should know Name: Science Group: Teacher: each of the statements to help focus your revision: R = Red: I don t know this
GCSE Additional Science Module C5 Chemicals of the Natural Environment: What you should know Name: Science Group: Teacher: each of the statements to help focus your revision: R = Red: I don t know this
Chapter 8: The Dynamic Planet
 Chapter 8: The Dynamic Planet I. The Pace of Change A. The Geologic Time Scale II. Earth s Structure and Internal Energy A. The Earth s Core B. The Earth s Mantle C. The Earth s Crust III. The Geologic
Chapter 8: The Dynamic Planet I. The Pace of Change A. The Geologic Time Scale II. Earth s Structure and Internal Energy A. The Earth s Core B. The Earth s Mantle C. The Earth s Crust III. The Geologic
https://www.youtube.com/watch?v=2nebe_brjaq&feature =youtu.be https://www.youtube.com/watch?v=- DSzlxeNCBk
 What is a mineral? H.E.3A.5 Analyze and interpret data to describe the physical and chemical properties of minerals and rocks and classify each based on the properties and environment in which they were
What is a mineral? H.E.3A.5 Analyze and interpret data to describe the physical and chemical properties of minerals and rocks and classify each based on the properties and environment in which they were
Carbon Cycling Internal
 Carbon Cycling Internal The 4 subcycles Atmosphere The Earth s Atmosphere The Earth has a radius of some 6400 km. Ninety-nine percent of the earth's atmosphere is contained within a layer approximately
Carbon Cycling Internal The 4 subcycles Atmosphere The Earth s Atmosphere The Earth has a radius of some 6400 km. Ninety-nine percent of the earth's atmosphere is contained within a layer approximately
Note that the protons and neutrons are each almost 2,000 times more massive than an electron; What is the approximate diameter of an atom?
 Atomic Structure and the Periodic Table Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
Atomic Structure and the Periodic Table Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
At the beginning. Matter + antimatter. Matter has the advantage. baryons quarks, leptons, electrons, photons (no protons or neutrons)
 At the beginning Matter + antimatter Matter has the advantage baryons quarks, leptons, electrons, photons (no protons or neutrons) Hadrons protons, neutrons Hydrogen, helium (:0 H:He) Origin of the Universe
At the beginning Matter + antimatter Matter has the advantage baryons quarks, leptons, electrons, photons (no protons or neutrons) Hadrons protons, neutrons Hydrogen, helium (:0 H:He) Origin of the Universe
Weathering of Rocks. Weathering - Breakdown of rocks into pieces (sediment) 2 main types of weathering to rocks
 Weathering of Rocks Weathering - Breakdown of rocks into pieces (sediment) 2 main types of weathering to rocks Mechanical weathering requires physical forces to break rocks into smaller pieces. Chemical
Weathering of Rocks Weathering - Breakdown of rocks into pieces (sediment) 2 main types of weathering to rocks Mechanical weathering requires physical forces to break rocks into smaller pieces. Chemical
The most common elements that make up minerals are oxygen, silicon, aluminum, iron, calcium, potassium, and magnesium
 Mineralogy: The Study of Minerals and their Properties A Mineral! Occurs! Is a! Is a substance (element or compound)! Has atoms arrange in an orderly pattern ( )! Is (not formed by any process involving
Mineralogy: The Study of Minerals and their Properties A Mineral! Occurs! Is a! Is a substance (element or compound)! Has atoms arrange in an orderly pattern ( )! Is (not formed by any process involving
What monitoring techniques are appropriate and effective for detecting CO2 migration in groundwater: isotope-based monitoring Philippe Négrel
 What monitoring techniques are appropriate and effective for detecting CO2 migration in groundwater: isotope-based monitoring Philippe Négrel Acting in complicity with Pauline Humez. Results from Pauline
What monitoring techniques are appropriate and effective for detecting CO2 migration in groundwater: isotope-based monitoring Philippe Négrel Acting in complicity with Pauline Humez. Results from Pauline
Stable Isotope Tracers OCN 623 Chemical Oceanography
 Stable Isotope Tracers OCN 623 Chemical Oceanography 21 March 2017 Reading: Emerson and Hedges, Chapter 5, p.134-153 2017 Frank Sansone and David Ho Student Learning Outcomes At the completion of this
Stable Isotope Tracers OCN 623 Chemical Oceanography 21 March 2017 Reading: Emerson and Hedges, Chapter 5, p.134-153 2017 Frank Sansone and David Ho Student Learning Outcomes At the completion of this
MARIYA INTERNATIONAL SCHOOL. Work sheet II. Term I. Level 8 Chemistry [Paper IV] Name: ATOMIC STRUCTURE & BONDING
![MARIYA INTERNATIONAL SCHOOL. Work sheet II. Term I. Level 8 Chemistry [Paper IV] Name: ATOMIC STRUCTURE & BONDING MARIYA INTERNATIONAL SCHOOL. Work sheet II. Term I. Level 8 Chemistry [Paper IV] Name: ATOMIC STRUCTURE & BONDING](/thumbs/93/111597617.jpg) MARIYA INTERNATIONAL SCHOOL Work sheet II Term I Level 8 Chemistry [Paper IV] Name: ATOMIC STRUCTURE & BONDING 1. Complete the following table. 2. Draw a diagram showing the arrangement of the valence
MARIYA INTERNATIONAL SCHOOL Work sheet II Term I Level 8 Chemistry [Paper IV] Name: ATOMIC STRUCTURE & BONDING 1. Complete the following table. 2. Draw a diagram showing the arrangement of the valence
Regan & Johnston Chemistry Unit 3 Exam: The Periodic Table Class Period
 Regan & Johnston Name Chemistry Unit 3 Exam: The Periodic Table Class Period 1. An atom of which element has the largest atomic radius? (1) Si (2) Fe (3) Zn (4) Mg 2. Which characteristics both generally
Regan & Johnston Name Chemistry Unit 3 Exam: The Periodic Table Class Period 1. An atom of which element has the largest atomic radius? (1) Si (2) Fe (3) Zn (4) Mg 2. Which characteristics both generally
Ionic Bond Proton. Cation Electron. Valence Electrons Atomic mass. Octet Rule Isotope
 Atoms and Ions Test Study Guide Physical Science Ms. Rowlen 2017 Know these definitions. Atom Ionic Bond Proton Ion Neutron Cation Electron Anion Atomic number Valence Electrons Atomic mass Octet Rule
Atoms and Ions Test Study Guide Physical Science Ms. Rowlen 2017 Know these definitions. Atom Ionic Bond Proton Ion Neutron Cation Electron Anion Atomic number Valence Electrons Atomic mass Octet Rule
8 th Grade Science Tutoring. Earth Space, Ms. Winkle
 8 th Grade Science Tutoring Earth Space, Ms. Winkle List of Topics PART ONE Atoms, molecules, elements, mixtures, compounds Density Physical vs chemical changes Weathering, Erosion, Deposition (include
8 th Grade Science Tutoring Earth Space, Ms. Winkle List of Topics PART ONE Atoms, molecules, elements, mixtures, compounds Density Physical vs chemical changes Weathering, Erosion, Deposition (include
Periodic Table Workbook
 Key Ideas: The placement or location of elements on the Periodic Table gives an indication of physical and chemical properties of that element. The elements on the Periodic Table are arranged in order
Key Ideas: The placement or location of elements on the Periodic Table gives an indication of physical and chemical properties of that element. The elements on the Periodic Table are arranged in order
What is the smallest particle of the element gold (Au) that can still be classified as gold? A. atom B. molecule C. neutron D.
 Use the Periodic Table of Elements to answer the following question(s). Which sentence about the periodic table of elements is true? A. All elements in period 2 are metals. B. All elements in group 18
Use the Periodic Table of Elements to answer the following question(s). Which sentence about the periodic table of elements is true? A. All elements in period 2 are metals. B. All elements in group 18
A non-traditional stable isotope perspective
 The origins of the Moon: A non-traditional stable isotope perspective Fang-Zhen Teng Department of Earth and Space Sciences From the beginning: The Universe: 13.8 Ga The Milky Way Galaxy The Solar System
The origins of the Moon: A non-traditional stable isotope perspective Fang-Zhen Teng Department of Earth and Space Sciences From the beginning: The Universe: 13.8 Ga The Milky Way Galaxy The Solar System
TODAY S FOCUS LAYERS OF THE EARTH
 TODAY S FOCUS LAYERS OF THE EARTH 8.6C investigate and describe applications of Newton s law of inertia, law of force and acceleration, and law of action-reaction such as in vehicle restraints, sports
TODAY S FOCUS LAYERS OF THE EARTH 8.6C investigate and describe applications of Newton s law of inertia, law of force and acceleration, and law of action-reaction such as in vehicle restraints, sports
Petrology. Petrology: the study of rocks, especially aspects such as physical, chemical, spatial and chronoligic. Classification:
 Petrology Petrology: the study of rocks, especially aspects such as physical, chemical, spatial and chronoligic. Associated fields include: Petrography: study of description and classification of rocks
Petrology Petrology: the study of rocks, especially aspects such as physical, chemical, spatial and chronoligic. Associated fields include: Petrography: study of description and classification of rocks
Wednesday week 12. These ions move through the soil to streams and eventually to the ocean. In the ocean; CaCO 3 + H 2 O + CO 2 H 2 O + H 2 O
 Wednesday week 12 I. Control of CO 2 content of atmosphere by the ocean H 4 SiO 4 A. Consider a hypothetical planet with a crust made of single mineral (Wallastonite) CaSiO3. We could use the composition
Wednesday week 12 I. Control of CO 2 content of atmosphere by the ocean H 4 SiO 4 A. Consider a hypothetical planet with a crust made of single mineral (Wallastonite) CaSiO3. We could use the composition
The Lithosphere. Definition
 10/12/2015 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2015 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
10/12/2015 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2015 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
Chapter 3-1. proton positive nucleus 1 amu neutron zero nucleus 1 amu electron negative on energy levels around the nucleus very small
 Chapter 3-1 Sub-atomic Charge Location Mass Particle proton positive nucleus 1 amu neutron zero nucleus 1 amu electron negative on energy levels around the nucleus very small The most mass of the atom
Chapter 3-1 Sub-atomic Charge Location Mass Particle proton positive nucleus 1 amu neutron zero nucleus 1 amu electron negative on energy levels around the nucleus very small The most mass of the atom
BIOGEOCHEMICAL CYCLES
 BIOGEOCHEMICAL CYCLES BASICS Biogeochemical Cycle: The complete path a chemical takes through the four major components, or reservoirs, of Earth s system (atmosphere, lithosphere, hydrosphere and biosphere)
BIOGEOCHEMICAL CYCLES BASICS Biogeochemical Cycle: The complete path a chemical takes through the four major components, or reservoirs, of Earth s system (atmosphere, lithosphere, hydrosphere and biosphere)
Why Does the Mantle Move the Way it Does?
 Why Does the Mantle Move the Way it Does? In the demonstration, you observed warm water rising through cool water. You also observed cool water sinking to replace the warm water. The movement of a fluid
Why Does the Mantle Move the Way it Does? In the demonstration, you observed warm water rising through cool water. You also observed cool water sinking to replace the warm water. The movement of a fluid
Chapter 6: Chemical Bonding
 Chapter 6: Chemical Bonding Learning Objectives Describe the formation of ions by electron loss/gain to obtain the electronic configuration of a noble gas. Describe the formation of ionic bonds between
Chapter 6: Chemical Bonding Learning Objectives Describe the formation of ions by electron loss/gain to obtain the electronic configuration of a noble gas. Describe the formation of ionic bonds between
Lecture # 4a- Stable Isotopes Part I
 Lecture # 4a- Stable Isotopes Part I 1) Stable Isotopes in Geochemistry: Background, etc 2) Intro to Carbon & N Isotopes I. Introduction A. What are stable isotopes? Recall: Stable isotopes vary in mass,
Lecture # 4a- Stable Isotopes Part I 1) Stable Isotopes in Geochemistry: Background, etc 2) Intro to Carbon & N Isotopes I. Introduction A. What are stable isotopes? Recall: Stable isotopes vary in mass,
ESA Study Guide Year 10 Science
 Then and now Questions from pages 26, 27 of ESA Study Guide Year 10 Science 1. Which early scientist thought atoms would combine to form new substances? 2. Which New Zealand scientist found that most of
Then and now Questions from pages 26, 27 of ESA Study Guide Year 10 Science 1. Which early scientist thought atoms would combine to form new substances? 2. Which New Zealand scientist found that most of
PERIODIC CLASSIFICATION
 5 PERIODIC CLASSIFICATION OF ELEMENTS TEXTBOOK, QUESTIONS AND THEIR ANSWERS Q.1. Do Dobereiner s triads also exist in the columns of Newland s octaves? Compare and find out. Ans. Triad of Li, Na and K
5 PERIODIC CLASSIFICATION OF ELEMENTS TEXTBOOK, QUESTIONS AND THEIR ANSWERS Q.1. Do Dobereiner s triads also exist in the columns of Newland s octaves? Compare and find out. Ans. Triad of Li, Na and K
The Rock Cycle CSBLM7-I
 The Rock Cycle neous Rock ock begins as a molten mass of magma in the mantle of the Earth. Magma can ooze Into already formed rock In the Earth's crust and cool to create intrusive igneous rock, if there
The Rock Cycle neous Rock ock begins as a molten mass of magma in the mantle of the Earth. Magma can ooze Into already formed rock In the Earth's crust and cool to create intrusive igneous rock, if there
Minerals Give Clues To Their Environment Of Formation. Also. Rocks: Mixtures of Minerals
 Minerals Give Clues To Their Environment Of Formation!!Can be a unique set of conditions to form a particular mineral or rock!!temperature and pressure determine conditions to form diamond or graphite
Minerals Give Clues To Their Environment Of Formation!!Can be a unique set of conditions to form a particular mineral or rock!!temperature and pressure determine conditions to form diamond or graphite
S= 95.02% S= 4.21% 35. S=radioactive 36 S=0.02% S= 0.75% 34 VI V IV III II I 0 -I -II SO 4 S 2 O 6 H 2 SO 3 HS 2 O 4- S 2 O 3
 SULFUR ISOTOPES 32 S= 95.02% 33 S= 0.75% 34 S= 4.21% 35 S=radioactive 36 S=0.02% S-H S-C S=C S-O S=O S-F S-Cl S-S VI V IV III II I 0 -I -II SO 4 2- S 2 O 6 2- H 2 SO 3 HS 2 O 4- S 2 O 3 2- S 2 F 2 S H
SULFUR ISOTOPES 32 S= 95.02% 33 S= 0.75% 34 S= 4.21% 35 S=radioactive 36 S=0.02% S-H S-C S=C S-O S=O S-F S-Cl S-S VI V IV III II I 0 -I -II SO 4 2- S 2 O 6 2- H 2 SO 3 HS 2 O 4- S 2 O 3 2- S 2 F 2 S H
8/24/2018. Bio 1101 Lecture 2 (guided) Chapters 2: Essential Chemistry. Chapter 2: Essential Chemistry for Biology
 1 2 3 4 5 Bio 1101 Lecture 2 (guided) Chapters 2: Essential Chemistry Chapter 2: Essential Chemistry for Biology Levels of biological organization Ecosystem (e.g. savanna) Community (e.g. the organisms
1 2 3 4 5 Bio 1101 Lecture 2 (guided) Chapters 2: Essential Chemistry Chapter 2: Essential Chemistry for Biology Levels of biological organization Ecosystem (e.g. savanna) Community (e.g. the organisms
