The Composition of Hydrous Partial Melts of Garnet Peridotite at 6 GPa: Implications for the Origin of Group II Kimberlites
|
|
|
- Trevor Preston
- 5 years ago
- Views:
Transcription
1 JOURNAL OF PETROLOGY VOLUME 55 NUMBER11 PAGES 2097^ doi: /petrology/egu051 The Composition of Hydrous Partial Melts of Garnet Peridotite at 6 GPa: Implications for the Origin of Group II Kimberlites DAVIDE NOVELLA* AND DANIEL J. FROST BAYERISCHES GEOINSTITUT, UNIVERSITA«T BAYREUTH, UNIVERSITA«TSSTRASSE 30, D BAYREUTH, GERMANY RECEIVED JUNE 13, 2013; ACCEPTED SEPTEMBER 4, 2014 ADVANCE ACCESS PUBLICATION OCTOBER 29, 2014 Chemical compositions of hydrous melts, compatible with those that would form by incipient melting of upper mantle peridotite at 180 km depth, have been determined using a series of iterative crystallization experiments. Experiments were performed in a multianvil apparatus at 6 GPa and 14008C and a melt was ultimately produced that was saturated in a residual peridotite assemblage (olivine þ clinopyroxene þ garnet orthopyroxene). The multiply saturated hydrous melts have higher (Mg þ Fe)/Si and Al/Ca compared with hydrous melts produced at lower pressures.the melt compositions are similar to those determined near the dry peridotite solidus at 17008C, when compared on an H 2 O-free basis. Melt H 2 O contents were determined to be 11wt % using mass balance, and these estimates were made more accurate by maintaining a large proportion of melt (470 wt %) in each experiment. If the geophysically inferred seismic low-velocity zone is caused by the presence of H 2 O-rich melt then at the base of this zone, at 220 km, these results imply that the melt must contain 15^16 wt % H 2 O. The hydrous melt compositions, when compared on a volatile-free basis, are found to be similar to those of group II kimberlites (orangeites). The low FeO and Na 2 O but enriched K 2 O concentrations in group II magmas imply their derivation from melt-depleted cratonic lithosphere enriched by the metasomatic addition principally of K 2 O and H 2 O. A simple model is proposed in which this enrichment occurs by the addition of phlogopite to the source peridotite. Using determined K 2 OandH 2 O partition coefficients and assuming that the ratio of both components in the source is controlled by their ratio in phlogopite, group II kimberlite magmas can be constrained as being the product of 02 wt % melting of a garnet peridotite source rock enriched with 17 wt % phlogopite, undergoing melting at near-adiabatic temperatures. KEY WORDS: garnet peridotite; volatiles; hydrous melting; group II kimberlites INTRODUCTION Along an average mantle adiabat, with potential temperatures between 1300 and14008c (Lee et al., 2009), dry peridotite melts at depths shallower than 70 km (e.g. Asimow et al., 2004). However, there are many clear examples in nature of melts being formed at significantly greater depths, such as kimberlites and lamproites (e.g. Moore & Gurney, 1989; Moore et al.,1991) and melts responsible for the metasomatism apparent in mantle xenoliths from cratonic lithosphere (Erlank et al.,1987). Evidence for deep melting is also apparent in diamond inclusions (Walter et al., 2008), and has in some instances been inferred from geophysical observations, both from seismic data (Song et al., 2004; Bagley & Revenaugh, 2008; Schmandt et al., 2014) and from measurements of electrical conductivity (Evans et al., 2005). It has, for example, been proposed that the seismic low-velocity zone (LVZ) at the top of the asthenosphere may, in part, be explained by the presence of small-degree volatile-bearing partial melts (Lambert & Wyllie,1968; Green,1971; Mierdel et al.,2007; Green et al.,2010,2011; Hirschmann,2010; Ni et al., 2011; Dasgupta et al.,2013). H 2 O and CO 2 have the potential to lower the peridotite solidus to stabilize partial melts at nominal mantle temperatures at great depths in the mantle (e.g. Kushiro et al., 1968; Mysen & Boettcher, 1975). Beneath mid-ocean ridges such volatiles may extend the onset of partial melting to *Corresponding author. Present address: Laboratoire Magmas et Volcans, Universite Blaise Pascal, 5 Rue Kessler, Clermont-Ferrand, France. Telephone: +33- (0) Fax: +33- (0) d.novella@opgc.univ-bpclermont.fr ß The Author Published by Oxford University Press. All rights reserved. For Permissions, please journals.permissions@ oup.com
2 JOURNAL OF PETROLOGY VOLUME 55 NUMBER 11 NOVEMBER 2014 greater depths than expected for volatile-free magma genesis (Green, 1973; Falloon & Green, 1989, 1990; Plank & Langmuir,1992; Asimow et al.,2004). Initial volatile-bearing, small-degree melts are particularly important as they may strip trace elements and volatiles from larger volumes of mantle compared with the region from which the majority of basaltic melts are extracted (Galer & O Nions, 1986; Asimow et al., 2004; Dasgupta & Hirschmann, 2006). They may also influence estimates for the H 2 O content of the mantle based on the H 2 O contents of basalts (e.g. Saal et al., 2002).To model the onset of melting in such a scenario and to investigate if melts could be responsible for the LVZ, specific information is required concerning the effects of volatiles on melting, which can be determined only by using an experimental approach. For CO 2 the redox relationship with silicate-hosted ferric iron may be a crucial factor affecting carbon participation in the initial production of melts. It has been proposed that at depths 4100 km carbon may be predominantly hosted in graphite and diamond and that oxidation to form carbonatite or a silicate melt CO 2 component is controlled by ferrous^ferric reduction, which may occur onlyat shallowdepths (Stagnoetal.,2013).Tomodel H 2 Oparticipation in deep melting requires information on the compositions of small-degree partial melts in addition to the partitioncoefficientforh 2 O(D min=melt H 2 O )betweenthecoexisting nominally anhydrous minerals and such melts (Hirschmann et al., 2009). Melt compositions, and in particular H 2 O contents, are not only crucial for calculating D min=melt H 2O but melt and residue compositions will probably also have a direct influence on D min=melt H 2O. For this reason it is importantthat D min=melt H 2 O is determined for realistic meltcompositions, which for deep melting scenarios requires that the melts are in equilibriumwith a nominally sub-solidus peridotite assemblage (e.g. Green et al., 2010; Kova cs et al., 2012). The probably low bulk H 2 O content of the mantle (e.g. Palme & O Neill, 2003) means that the degree of melting causedbyh 2 Oatpressuresgreaterthanthe onsetofdrymelting will be insufficient to significantly perturb the major element concentration of the mantle residue. Realistic smalldegree melts are probably enriched in incompatible elements such as Na, K and Ti, which may have an influence on D min=melt H 2O. Many experimental determinations of D min=melt H 2O have been made for melts that are not in equilibrium with a residual peridotite assemblage (e.g. Aubaud et al., 2004) and may therefore not be strictly applicable to modeling the onset of melting. Shifts in mineral compositions near the solidus, particularly for clinopyroxene (see, e.g. Hauri et al., 2006; O Leary et al., 2010), may also have an important influence on D min=melt H 2 O. Whereas many reliable methods exist for determining mineral H 2 O (hydroxyl) contents, the determination of melt H 2 O contents in experimental products is more challenging. To date no studies exist by which to reasonably estimate the chemical composition of melts saturated with a peridotite assemblage at pressures above 35GPa.This is a major barrier to our understanding of the effect of pressure on hydrous melting and it is mainly caused by the difficulties in performing such experiments. One of the main problems is that at pressures above 3 GPa melts of the appropriate composition do not form glasses on quenching but instead form an assemblage of quench crystals (Zhang & Herzberg, 1994; Walter et al., 1998; Tenner et al., 2011). These crystal mats are difficult to analyze in terms of major elements owing to their heterogeneity, large grain size and porosity, and are totally impossible to analyze in terms of H 2 O content using spectroscopic or ion probe techniques. Most of the H 2 O from the original liquid is unlikely to be locked into the prepared surface of the quenched sample. Some studies have attempted to provide estimates of quenched crystallized melt H 2 O contents using the deficit in electron microprobe analysis totals (e.g. Ohtani et al., 2000) but any number of reasons might be the cause of the resulting low analysis totals, such that this cannot be considered an acceptable method. The only reliable technique for determining melt H 2 O contents in the absence of quenched glasses is therefore to perform a mass balance based on analysis of major elements in the run products compared with the original starting material. However, such a treatment also raises further problems. The uncertainties on the concentrations determined through mass balance for a small-degree melt would be extreme, and variable loss of H 2 O from the experimental capsules during heating would significantly bias the result. Furthermore, the infiltration of carbon through metal capsule walls that has been observed in many experiments (Brooker et al., 1998; Liu et al., 2006; Balta et al., 2011) would invalidate the entire mass-balance method as it can produce an unquantifiable amount of CO 2 in the melt phase. One approach to estimating solidus depression as a function of melt H 2 O content at high pressures and temperatures is to develop a generalized model for the effect of H 2 O in suppressing the peridotite solidus (Hirschmann et al., 2009; Hirschmann, 2010). Although this is a very promising method it ignores the likely effects that H 2 O has on influencing the composition of silicate melts in equilibrium with mantle residues. In addition, very few reliable data exist by which to calibrate such models at pressures above 3 GPa. A number of studies have been performed to examine the effect of H 2 O on peridotite partial melting at pressures 35GPa (e.g. Hirose & Kawamoto, 1995; Hirose, 1997; Gaetani & Grove, 1998; Hall, 1999; Balta et al., 2011; Tenner et al., 2012). In the spinel peridotite field the overriding effect of adding H 2 O seems to be an apparent increase in the melt SiO 2 /(MgO þfeo) ratio (Hirose & Kawamoto, 1995; Hirose, 1997; Gaetani & Grove, 1998). This is a consequence of the expansion of the olivine 2098
3 NOVELLA & FROST PERIDOTITE PARTIAL MELTS liquidus field at the expense of pyroxenes in the presence of H 2 O. A similar effect was recently reported for smalldegree melts of garnet peridotite at 3 GPa (Balta et al., 2011), although at 35 GPa Tenner et al. (2012) reported that melt SiO 2 /(MgO þfeo) ratios are shifted to lower values in the presence of H 2 O. At pressures of 5^11GPa Kawamoto & Holloway (1997) reported hydrous garnet peridotite melts to be MgO-rich and low in SiO 2 and Al 2 O 3. Volatile-bearing melts experimentally produced at high pressure and high temperature have been found to be similar to kimberlites (e.g. Kawamoto & Holloway, 1997). Kimberlites are volatile-rich, ultrabasic silicate magmas that as a result of their highly enriched incompatible element contents must be produced by low-degree partial melting of sources that have most probably been previously metasomatized (e.g. Eggler, 1978, 1989; Wyllie, 1980; Tainton & McKenzie, 1994; Mitchell, 2004; Becker & Le Roex, 2006). The presence in kimberlites of diamonds and mantle xenoliths that record high equilibration pressures implies that these magmas must originate from depths greater than 150 km and are therefore the deepest sourced magmas on Earth (e.g. Boyd, 1973; Carswell & Gibb, 1987; Yaxley et al., 2012). Kimberlites have been subdivided into two types. Group I kimberlites appear to be more CO 2 -rich and have been argued to have sub-lithospheric sources (Smith, 1983). Group II rocks, referred to as orangeites after Mitchell (1995), generally contain phlogopite mica (i.e. are more H 2 O-rich) and have been argued to originate within the subcontinental lithosphere (Smith, 1983). Experimental studies conducted to investigate the genesis of both types of kimberlites can also be divided into two types. Experiments of the first type have been performed to determine the conditions under which kimberlite melt compositions become multiply saturated in mantle minerals (e.g. Edgar et al., 1988; Edgar & Charbonneau, 1993; Girnis et al., 1995, 2011; Ulmer & Sweeney, 2002; Sokol et al., 2013). Such experiments must make assumptions about the likely pre-eruptive volatile contents of kimberlites, however, and often come to the conclusion that kimberlites are not saturated in all garnet lherzolite minerals. The melts produced are naturally close to those of the kimberlite melt starting compositions because they are near liquidus. The second type of experiments are those performed to assess if kimberlite melts can be produced from typical mantle garnet lherzolite rocks in the presence of different proportions of volatiles (e.g. Canil & Scarfe, 1990; Ringwood et al., 1992; Kawamoto & Holloway, 1997; Dalton & Presnall, 1998; Gudfinnsson & Presnall, 2005; Brey et al., 2008; Keshav & Gudfinnsson, 2014). In this study, constraints have been placed on the chemical composition of low-degree hydrous melts in equilibrium with a garnet peridotite assemblage at 6 GPa and 14008C, a temperature that corresponds to a mid-ocean ridge adiabat at the lower end of recent estimates (Lee et al., 2009). The principal goal was to determine the H 2 O content of a melt coexisting with a residual mineral assemblage compatible with that expected for the mantle at these conditions. An iterative crystallization technique was used to identify melt compositions that are in equilibrium with peridotite, and large melt to crystal ratios were employed to reduce mass-balance uncertainties, because this is the only way to determine the melt H 2 O content. All the experiments were conducted at melt H 2 O undersaturated conditions (see Dixon et al., 1995), precluding the formation of a fluid phase. To minimize H 2 O loss from the experiments double capsules were used, and, more importantly, heating times were kept to 51h, which should have aided the minimization of carbon contamination from the furnace. A number of tests were used to examine the approach to equilibrium in these experiments. Ultimately, the data collected here are used to model the effect of H 2 O on mantle melting at depths corresponding to the LVZ and to address the origin of group II kimberlites. METHODS Experimental procedure The experimental determination of the composition of small-degree melts (where F, the melt fraction, is 501) in natural peridotite systems is a challenging task, even in the absence of volatiles and quench crystallization. The volume of small-degree melts makes them hard to chemically analyze and promotes melt modification through reaction with solid phases during quenching. Two main methods have in the past been used to overcome these problems. The first employs an initially porous diamond aggregate assemblage to extract and separate the melt from the residue (Johnson & Kushiro, 1992; Hirose & Kushiro, 1993), whereas in the second so-called sandwich technique (Stolper,1980; Falloon & Green,1987) large fractions of proposed small-degree melt compositions are equilibrated with a peridotite residue in a series of iterative experiments (Robinson et al., 1998; Dasgupta & Hirschmann, 2007). The first technique is not useful for H 2 O-bearing experiments owing to the risk of contaminating the melts with CO 2 or CH 4, although Green et al. (2010) addressed this issue to some extent by employing an olivine melt trap. The sandwich technique is potentially more suitable, however, and was recently employed for this purpose at 3GPa by Balta et al. (2011). A small-degree hydrous melt would not significantly affect the major element composition of the solid assemblage; therefore, the correct melt composition should be multiply saturated with a peridotite mineral assemblage. The first step in a sandwich experiment study is to perform a sub-solidus experiment to 2099
4 JOURNAL OF PETROLOGY VOLUME 55 NUMBER 11 NOVEMBER 2014 determine the mineral compositions of a peridotite assemblage at a given pressure and temperature. Then an initial guess melt composition is equilibrated with the peridotite in sub-equal proportions. If the melt is not in equilibrium with the peridotite, solid^melt reactions will occur and the resulting melt, which should still comprise a large and easily analyzed portion of the experimental charge, should shift in composition to be closer to equilibrium with the peridotite. The residual mineral phases will also interact with the melt and change in composition or react out if the melt composition is far from equilibrium. By repeating this procedure and using the resulting melt as the initial melt in successive experiments, a stage is ultimately reached where minimal reaction occurs between the peridotite and the multiply saturated melt. Such a method is potentially very suitable for experiments performed above 3 GPa in which quench crystallization means that mass-balance estimates of melt H 2 O contents must be made. Melt H 2 O contents are determined by assuming all H 2 O from the bulk composition is in the melt and determining the melt fraction from a mass balance based on analyses of all other oxides in the melt and mineral phases. Such a determination, however, becomes highly inaccurate if the proportion of melt is small. Hirschmann & Dasgupta (2007) outlined a modification of the iterative sandwich technique in which, instead of using the product melt composition at the end of each experiment to determine the next melt composition, the partition coefficients between minerals and melts are used to derive a model for the next melt composition based on the chemical composition of the initial melt-free assemblage. Such an approach has the advantage that the iterated melt compositions converge more rapidly on the final equilibrium melt. In the current study it was found that the accuracy of mass-balance results improved if small peridotite assemblage fractions were employed (i.e. 520%). In these circumstances the method of Hirschmann & Dasgupta (2007) is more effective because melt compositions would otherwise shift only gradually upon each iteration. To provide credible estimates of melt H 2 O contents using mass balance requires that experiments do not lose significant H 2 O during heating. Hall et al. (2004) demonstrated that over the course of 24 h heating at 13008C 32% H 2 O can be lost from a basaltic melt in Au 80 Pd 20 capsules. In such circumstances it would seem that only very short run durations can hope to preserve H 2 O contents that are close to the original, and in the current study run times were of the order of 30 min to ensure this. In addition, several studies have shown that carbon can diffuse into experimental capsules (e.g. Brooker et al., 1998), which should also be minimized by short run durations. In this study a double capsule arrangement was also employed with an outer Pt^Rh capsule and inner Au 80 Pd 20, similar to that employed by Balta et al. (2011) for the same purpose. Whereas peridotite and melt compositions were placed in the inner capsule, the outer capsule contained only the melt composition, in the hope of minimizing H 2 O loss in the inner capsule by maintaining a high external H 2 Oactivity and similar fo 2. However, as will be shown, H 2 O loss from both inner and outer capsules was minimal during the course of the experiments and assemblages observed in both inner and outer capsules could be used to determine multiply saturated melt compositions. Initial experiments indicated that the necessary short run durations prevented suitable chemical equilibrium between sub-equal proportions of melt and solid peridotite. Short run times also prevented the conventional use of a melt layer sandwiched between layers of peridotite, because chemical equilibrium cannot be achieved upon the short run times. Instead, it was found that equilibrium could be obtained only by using 520% peridotite and by intimately mixing the melt and peridotite compositions. In addition, the temperature of each experiment was initially raised by 508C above the set point. The run temperature was then approached by cooling over 5 min. In this way the entire assemblage was initially melted and all solid phases crystallized from the melt during the cooling phase, which can be described as an inverse melting experiment. This is therefore a crystallization rather than a sandwich experiment. As will be seen, this also ensured the separation of melt from crystals required to allow the melts to be analyzed properly and to avoid quench modification of the solid assemblage. The chemical analysis of the quenched silicate melt assemblage by electron microprobe is challenging. The formation of large quench crystals and chemical heterogeneity on a scale larger than 10 mm means that large microprobe beam diameters are required despite the fact that the analyzed surface is generally uneven and contains voids. In initial experiments melt analyses performed with the electron microprobe resulted in high massbalance residuals, probably resulting from erroneously low SiO 2 analyses. It was found that melt analyses made using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) resulted in much smaller mass-balance residuals. However, LA-ICP-MS also required large analyzable melt pools. Starting materials The peridotite bulk composition of Jagoutz et al. (1979)was selected for the near-solidus assemblage in the experiments. This composition (PD-1, Table 1) is based on that of primitive ultramafic mantle xenoliths and is similar to pyrolite (Ringwood, 1966, 1979). In Table 1, the compositions of the melts that were employed in the experiments are also reported. The initial melt composition (BASD-1, Table 1) was prepared by examining mineral^melt partition coefficients from experiments performed between 2100
5 NOVELLA & FROST PERIDOTITE PARTIAL MELTS Table 1: Chemical composition of starting mixtures (wt %) PD-1 BASD-1 BASD-2 BASD-3 BASD-4 BASD-5 BASD-6 BASD-7 BASD-8 BASD-9 BASD-10 BASD-11 BASD-12 BASD-13 BASD-14 SiO Al 2 O CaO FeO NiO MgO Na 2 O Cr 2 O TiO MnO K 2 O H 2 O Sum and 3 GPa (Gaetani & Grove,1998; Balta et al., 2011). The subsequent melts were prepared as a result of analyzing and mass balancing the resulting quenched melts, but in some instances other considerations were adopted in their fabrication, as discussed below. All the starting mixtures were prepared by grinding previously weighed proportions of oxides and carbonates for 1h under ethanol in an agate mortar. Mixtures were then dried, placed in an Fe-saturated Pt crucible, and decarbonated at 10008C for 4^6 h. The powders were then glassed by melting in air at 16008C for 20 min and then rapidly quenching in icy water. This step was repeated four times, grinding the quenched glass for 1h under ethanol and drying it at every cycle to ensure higher homogeneity of the mixture. Based on analyses of the peridotite starting mixture, losses of alkalis during sample preparation and analyses were insignificant. The glass was recovered from the crucible and then reduced in a1atm furnace, at10248c and an oxygen fugacity (fo 2 ) of 2 logs units below the quartz^fayalite^magnetite oxygen buffer, for 12 h. This process was performed twice to ensure complete reduction of the mixture. Mg(OH) 2 and Al(OH) 3 werethenaddedtotheglassesto obtainthehydrous melt compositions that had been previously weighed with an intentional deficit of MgOand Al 2 O 3. High-pressure and high-temperature experiments High-pressure (HP) and high-temperature (HT) experiments were performed at 6 GPa and a nominal temperature of 14008C using a 1200 tonne Kawai type multianvil apparatus at the Bayerisches Geoinstitut (BGI). Tungsten carbide anvils of 15 mm truncation edge length were employed with a 25 mm edge length Cr 2 O 3 -doped octahedron. A stepped graphite furnace was employed for the experiments, which was surrounded inside the pressure medium by a ZrO 2 sleeve. A 4 mm diameter outer Pt^Rh capsule was placed at the center of the assembly, separated by an MgO sleeve. MgO spacers were also placed above and below the capsule. The temperature was measured by means of a W 97 Re 3^W 75 Re 25 (D type) thermocouple inserted within an alumina sleeve; the effect of pressure on the thermocouple e.m.f. was ignored. The pressure was calibrated using the CaGeO 3 garnet to perovskite phase transition (Ross et al., 1986). In a separate experiment the temperature variation inside the Pt^Rh capsules was determined to be within 408C of the thermocouple temperature using the two-pyroxene thermometer calibration of Brey et al. (1990). An initial dry sub-solidus experiment was performed with the PD-1 peridotite composition in a graphite sleeve inserted in the Pt^Rh capsule. This experiment was equilibrated for 24 h. Melt crystallization experiments employed double capsules as described by Kagi et al. (2005) and Balta et al. (2011). An inner Au^Pd capsule containing known proportions of mantle peridotite and hydrous melt was enclosed by an outer Pt^Rh capsule containing only hydrous melt. The inner Au^Pd capsules were pre-soaked with Fe by equilibrating with an iron-bearing silicate assemblage in a gas mixing furnace at 11008C and 2 log fo 2 units below the FMQ buffer, for 1 day. After being equilibrated in the gas mixing furnace the capsules were recovered and cleaned with HF overnight at 808C. After packing the peridotite^melt mixtures in the capsule, the top of the Au^Pd capsule was welded. H 2 O loss during welding was checked by weighing the capsule before and 2101
6 JOURNAL OF PETROLOGY VOLUME 55 NUMBER 11 NOVEMBER 2014 after. Once prepared, the Au^Pd capsule was inserted in the outer Pt^Rh capsule along with the outer hydrous melt composition separating the two capsules. The Pt^Rh outer capsule was then welded closed, with the capsule weight being checked again for H 2 Oloss. Crystallization experiments were performed at 6 GPa and a nominal temperature of 14008C. This is the adiabatic temperature at this pressure along the mantle adiabat determined by Katsura et al. (2010) based on the depth of the 410 km seismic discontinuity. Target pressure was reached in 4 h with the target temperature initially reached at a heating rate of 1008Cmin 1. Once at target temperature, the experiment was further heated by 508C over a period of 5 min, then kept at this temperature for 5 min, and then cooled to target temperature over 5 min. Then each experiment was kept at temperature for 30 min. This technique was found to produce wellequilibrated assemblages composed of large crystals (4100 mm) amenable to spectroscopic H 2 O determinations (Novella et al., 2014). The overstep of 508C in the temperature is of similar magnitude to the gradient in temperature over the capsule, which is, therefore, a similar magnitude to the temperature uncertainty. Analytical techniques After recovery to room pressure the capsules were removed from the ceramic assembly, mounted in epoxy resin, sectioned and polished in the absence of water. Chemical compositions of crystalline phases were determined using a JEOL JXA-8200 electron microprobe at the BGI. The operating conditions for chemical analyses were 15 kv acceleration voltage and a current of 15 na, with a counting time of 20 s on the peak position and 10 s on each side for the background. Alkalis were measured first in the procedure, to limit potential losses during the analyses. Natural and synthetic minerals were used as standards for most elements, and metal standards were employed for Ni, Cr, Fe and Ir. A spot beam of 1 mm diameter was used in obtaining the chemical compositions of the mineral phases. Analyses were reduced using the PRZ correction routine. Chemical compositions of quenched melts were obtained by LA-ICP-MS at the BGI, with a 193 nm ArF Excimer laser combined with a quadrupole mass spectrometer. Because large pools of melt can be produced with the crystallization approach described here, up to 10 pits of 80 mm diameter and depth could be ablated on a single charge, providing accurate analyses of the quenched melt. Counts during the analyses were reasonably flat with time, indicating that the encountered melt region was of relatively homogeneous composition at the scale of the analysis. The NIST SRM 610 glass (Spandler et al., 2011) was used as an external standard. Internal standardization was performed by normalizing the sum of the oxides to 100 wt %. RESULTS Dry sub-solidus experiment The mineral phase analyses from the sub-solidus peridotite experiment (PD-1, Table 1) are reported intable 2. The assemblage comprised 14 wt % garnet, 32 wt % clinopyroxene (cpx) and 54 wt % olivine. The absence of orthopyroxene (opx), although not inconsistent with some previous observations, is possibly due to the formation of a metastable cpx composition. Whereas Balta et al. (2011) found an opx-free assemblage at 3 GPa and 13508C, Davis et al. (2011) demonstrated that opx will form from a dry peridotite composition if equilibrated at 3 GPa and 14408C for 1 week. Similar observations were reported by Dasgupta & Hirschmann (2007) and Mallik & Dasgupta (2012). Both Walter (1998) and Herzberg & Zhang (1996) produced opx-free assemblages at the dry peridotite solidus at 6 GPa, but this probably resulted from the higher temperatures (17008C) where the cpx^opx solvus narrows. In the present study the cpx CaO content in the sub-solidus run was 10 wt %, which, based on the phase relations of Brey & Ko«hler (1990) determined at the same conditions, would place the cpx within the cpx^opx solvus. It is possible, therefore, that a metastable cpx composition crystallizes with low CaO, which does not reach equilibrium by exsolving opx even after 1 day at 14008C. As will be seen below, cpx formed from hydrous experiments at the same conditions contains 13^14 wt % CaO, which is similar to the cpx composition found by Brey & Ko«hler (1990) at these conditions. Although garnet 3þ cations sum to slightly less than 2 (19) per 12 oxygen formula units this is not matched by any Si or 2þ cation excess to imply that the sample contains a significant majorite component. The olivine Mg-number [¼ molar Mg/(Mg þ Fe) 100] is 896, but as discussed below the application of several thermometers implies imperfect Fe^Mg equilibration in the experiment. Hydrous melt experiments The products of typical hydrous melt experiments are shown in Fig. 1. In general, crystals were formed in both inner and outer capsules coexisting with large regions of finer, fibrous, quenched melt crystals. In some experiments in which the peridotite portion of the initial starting mixture was 420 wt % or the bulk H 2 O content was 59wt %, incomplete separation of melt and minerals occurred (Fig. 1f). The interstitial melt creates an extremely difficult texture to interpret on quenching because the melt crystals are impossible to discriminate from those of the solid assemblage. Significant quench modification of crystals also occurs. In these experiments the interstitial melts were impossible to meaningfully analyze and the experiments were generally discarded. Only when significantly large pools of melt separated from the solid assemblage was the quenched texture easy to discriminate (Fig. 1b^e). Such 2102
7 NOVELLA & FROST PERIDOTITE PARTIAL MELTS Table 2: Chemical composition (in wt %) and modal abundances of mineral phases in the subsolidus run ol cpx gt SiO (33) 5424(93) 4291(75) Al 2 O 3 012(5) 268(65) 2133(105) CaO 017(2) 963(48) 477(37) FeO 960(15) 518(11) 656(15) NiO 037(6) 016(7) 002(2) MgO 4884(24) 2480(63) 2219(24) Na 2 O 003(2) 103(7) 006(4) Cr 2 O 3 010(2) 044(3) 153(10) TiO 2 005(3) 031(4) 090(5) MnO 013(4) 019(5) 026(4) K 2 O 0004(3) 008(2) 0001(1) Total 9999(48) 9933(100) 10059(23) Mode Numbers in parentheses are standard deviation. Analyses on 20 crystals were used for each phase. separation occurred only when melt fractions were large, but the only way found to ensure that separation always occurred was to initially overstep the set point temperature by 508C. LA-ICP-MS analyses of separated melt pools showed small standard deviations in melt composition and the melts appear well homogenized at the 80 mm scale of the analyses (Fig. 1e). After a melt iteration process described in the next section, at least six experiments produced assemblages of olivine, clinopyroxene and garnet in equilibrium with large pools of hydrous melt. One experiment (S5281) was also additionally saturated in orthopyroxene. Therefore, we conclude that these final melt compositions are close to the lherzolite peritectic, if not exactly upon it. In a number of further experiments, however, orthopyroxene was found to replace olivine in the assemblage and in several experiments clinopyroxene was absent. The compositions of quenched melt and mineral phases produced in the experiments are reported intable 3. Mass balance was performed using mineral and melt analyses of 11 elements. An additional phase composed of 100% FeO was added to account for Fe loss (following Tenner et al., 2012). The system of equations was minimized to obtain the melt H 2 O content and the phase modes. The melt was assumed to be the only H 2 O-bearing phase. The melt composition was normalized to 100% during the minimization. The sum of the squared residuals was generally in the range 01^06, with Na 2 O being by far the main contributor to the residual. Melts with residuals above unity were discarded. In the majority of experiments the melt mode was above 80% (Table 4) such that the difference between the starting composition and the resulting melt composition was relatively small. Refined cpx abundances are generally less than 1%. As melt SiO 2 contents, in particular, are always close to the starting composition the refined cpx modes fall within the mass-balance uncertainties propagated from the melt analyses. Although some experimental charges shown in Fig. 1 appear crystal-rich there is a strong bias in most images because samples were ground down through the quench melt during sample preparation until suitable crystal-rich regions were reached. Fe loss in inner capsules was in the range 2^3% of the initial Fe content, whereas in the outer capsules it was up to 7%. Similar losses of Ni were noted, which may have also alloyed with the capsule. Large losses of K from the quenched melt occurred in all hydrous experiments and Na losses were observed in most of the experiments. Melt compositions and the iteration procedure The melt compositions determined in this study were derived in some instances iteratively by equilibrating peridotite and melt and using the resulting refined melt composition in further experiments. However, in experiments where a three-phase assemblage was observed the predictive procedure of Hirschmann & Dasgupta (2007) was employed, in which mineral^melt partition coefficients are used to derive the next melt composition based on the dry peridotite mineral compositions. This procedure was complicated, however, by a number of difficulties, which are not at all problematic in lower pressure, dry experiments. As previously described, these were related to difficulties in obtaining large pools of analyzable melt in equilibrium with a peridotite rock assemblage and obtaining chemical equilibrium among phases within a time scale that ensured minimal H 2 O loss. The only solution found to this second issue was to keep the proportion of the solid phases small so that they could be totally crystallized out of the melt during cooling after initially overstepping the run temperature by 508. This, however, seems to create a further problem in that crystallization during this 508 temperature drop shifts the melt composition and once the run temperature is reached the melt may no longer be in equilibrium with the crystallized assemblage. Whereas an iterative melt procedure was followed to derive melts up to composition BASD-8 (Table 1) further experiments to address this final issue were performed using a more ad hoc procedure. The first two melt compositions (BASD-1 and BASD-2, Table 1) were designed based on literature data on hydrous melt compositions at lower pressure (Gaetani & Grove, 1998; Balta et al., 2011). BASD-1 failed to segregate a large pool of analyzable melt, indicating that the H 2 O content was too low to produce a suitable amount of melt. A 2103
8 JOURNAL OF PETROLOGY VOLUME 55 NUMBER 11 NOVEMBER 2014 (a) Au/Pd capsule (b) Au/Pd capsule gt opx ol gt cpx gt 100 µm Pt/Rh capsule ol cpx 200 µm 100 µm ( c) (d) (e) 100 µm gt 100 µm (f) 100 µm Fig. 1. Backscattered electron images from selected experimental runs. (a) An overview of the double capsule used in this study (experiment S5252, Table 3), where an outer Pt^Rh capsule contained an inner Au^Pd capsule surrounded by melt. Crystals and large pools of quenched melt with dendritic structure can be recognized in both inner and outer capsule. (b) The phase assemblage of the inner capsule of experiment S5213 (Table 3) in which opx (medium grey crystals) and garnet (grey crystals) formed in equilibrium with a large amount of melt. (c^e) Phase assemblages from experiments S5200, S5581 and S5129 (Table 3), respectively. In these experiments, large crystals of olivine (dark), clinopyroxene (light grey), and garnet (medium grey) up to 200 mm across are in equilibrium with quenched crystals resembling the melt produced at high pressure and high temperature. The black circle in (e) indicates a typical pit produced after LA-ICP-MS analysis. (f) Phase assemblage of an experiment in which the melt failed to segregate from the crystalline matrix. This is an example of an experiment in which incomplete melt segregation meant that it was impossible to identify quench melt from equilibrium crystals. ol cpx second guess melt composition was prepared (BASD-2) with almost double the H 2 O content and slightly more normative olivine owing to the lack of olivine in the previous experiment. This composition was similar to that obtained by Balta et al. (2011) at 3 GPa. In an experiment in which BASD-2 was mixed with 50% peridotite, a melt in equilibrium with cpx þ opx þ garnet was obtained. This separation of melt was unusual and was probably due to the experiment not being thermally stable and probably running at a higher temperature than 14008C. However, analysis of the melt composition and mass balance for H 2 O was used to estimate a new bulk composition BASD-3. When BASD-3 was mixed with only 10% peridotite insufficient melt was produced to adequately separate 2104
9 NOVELLA & FROST PERIDOTITE PARTIAL MELTS Table 3: Experimental run products and chemical composition (in wt %) of mineral phases (microprobe analyses) and melts (LA-ICP-MS analyses) Run (start. mix) SiO 2 Al 2 O 3 CaO FeO NiO MgO Na 2 O Cr 2 O 3 TiO 2 MnO K 2 O H 2 O Total S5129 (BASD-7) ol 41.39(15)* 0.07(2) 0.13(1) 6.13(15) 0.05(2) 53.22(19) 0.08(8) 0.03(2) 0.01(1) 0.08(4) 0.03(2) (29) cpx 55.63(23) 3.06(36) 14.48(51) 2.91(13) 0.01(2) 22.05(65) 1.67(13) 0.16(2) 0.08(4) 0.08(4) 0.06(3) 99.76(28) gt 43.34(29) 22.56(23) 5.27(35) 4.70(23) 0.01(2) 23.78(33) 0.10(4) 0.62(9) 0.24(6) 0.19(6) 0.03(2) (32) melt 40.12(0) y 6.00(0) 8.72(0) 8.51(0) 0.01(2) 21.42(0) 2.32(24) 0.05(4) 1.05(2) 0.18(0) 0.28(0) 11.34(0) S5200 (BASD-8) ol 40.99(15) 0.07(1) 0.14(1) 9.03(28) 0.05(3) 50.78(35) 0.04(1) 0.02(1) 0.03(2) 0.08(4) 0.00(1) (33) cpx 55.33(21) 2.97(31) 14.85(1.1) 4.03(27) 0.02(2) 20.95(96) 1.55(14) 0.20(5) 0.10(1) 0.09(3) 0.02(3) (22) gt 43.03(31) 22.05(70) 5.54(49) 6.94(56) 0.02(3) 21.89(70) 0.07(3) 0.73(10) 0.42(10) 0.16(4) 0.01(1) (32) melt 38.39(2) 4.96(0) 8.62(2) 11.62(0) 0.01(0) 19.90(0) 2.45(14) 0.09(1) 1.54(0) 0.15(0) 0.20(27) 12.09(2) S5213-O (BASD-9) opx 55.80(73) 1.61(14) 0.85(6) 5.97(8) 0.04(2) 34.16(37) 0.19(2) 0.25(3) 0.11(3) 0.13(4) 0.01(1) 99.23(58) melt 40.79(2) 5.14(0) 5.74(0) 11.23(0) 0.03(9) 18.00(3) 1.70(2) 0.19(0) 3.60(3) 0.24(0) 0.66(0) 12.70(0) S5213-I x (BASD-9 þ PD-1) opx 56.35(26) 1.55(12) 0.71(7) 6.13(37) 0.05(3) 34.09(48) 0.19(2) 0.25(2) 0.12(3) 0.12(3) 0.01(1) gt 42.48(25) 20.96(32) 2.82(12) 7.96(16) 0.01(2) 22.82(12) 0.04(1) 1.69(12) 0.74(14) 0.26(3) 0.01(1) 99.89(50) melt 39.25(4) 4.85(2) 6.30(2) 11.85(1) 0.03(21) 18.44(6) 1.37(3) 0.18(0) 3.25(15) 0.23(0) 0.74(0) 13.51(1) S5218 (BASD-9) opx 56.41(44) 1.63(17) 0.96(4) 6.50(25) 0.05(4) 33.95(34) 0.20(2) 0.23(4) 0.11(2) 0.16(3) 0.01(1) (65) gt 42.07(42) 20.77(68) 3.77(39) 8.23(26) 0.03(4) 22.69(35) 0.04(1) 1.27(14) 0.98(24) 0.33(6) 0.00(1) (58) melt 39.93(2) 4.95(2) 6.34(0) 11.54(0) 0.03(9) 17.09(2) 1.03(32) 0.18(0) 3.80(7) 0.25(0) 0.57(9) 14.30(0) S5252 (BASD-10) opx 57.09(61) 1.66(12) 1.80(13) 4.37(12) 0.03(2) 35.18(75) 0.29(3) 0.15(2) 0.03(2) 0.08(5) 0.01(1) (26) cpx 55.82(23) 2.71(8) 11.73(59) 3.74(15) 0.03(3) 24.52(37) 1.22(4) 0.30(3) 0.04(1) 0.11(2) 0.02(1) (30) gt 43.10(36) 21.21(19) 5.57(12) 5.93(14) 0.02(3) 23.25(20) 0.04(2) 1.15(6) 0.33(4) 0.20(2) 0.01(1) (48) melt 38.23(0) 5.49(0) 8.24(1) 8.57(0) 0.02(13) 19.61(0) 3.42(11) 0.12(0) 0.77(17) 0.15(0) 0.20(29) 15.17(0) S5270 (BASD-11) opx 56.37(85) 1.17(14) 1.84(24) 5.46(36) 0.06(3) 34.17(31) 0.17(4) 0.18(5) 0.04(2) 0.14(3) 0.01(2) 99.63(98) cpx 54.98(36) 1.81(15) 14.44(44) 4.25(13) 0.04(3) 22.86(26) 0.75(3) 0.37(8) 0.08(2) 0.14(4) 0.03(1) 99.73(23) gt 42.52(41) 21.16(40) 5.35(23) 6.84(24) 0.02(3) 22.19(21) 0.02(1) 1.70(21) 0.36(4) 0.28(5) 0.00(0) (38) melt 38.43(0) 4.49(0) 9.08(0) 10.57(0) 0.03(5) 19.92(0) 0.51(58) 0.18(2) 1.50(0) 0.22(0) 1.17(0) 13.88(0) S5278 (BASD-11) opx 57.23(46) 1.47(18) 2.24(16) 5.12(15) 0.07(3) 33.70(33) 0.17(2) 0.26(4) 0.03(3) 0.14(3) 0.01(1) (51) cpx 55.10(65) 1.85(17) 13.53(1.10) 4.23(21) 0.03(3) 23.58(92) 0.67(6) 0.37(6) 0.06(2) 0.15(32) 0.03(2) 99.60(49) gt 41.89(1.35) 20.57(53) 5.29(8) 6.30(10) 0.02(3) 23.00(83) 0.02(0) 1.94(24) 0.30(3) 0.28(5) 0.00(0) 99.59(1.42) melt 37.41(0) 4.55(0) 9.14(0) 10.26(0) 0.04(4) 19.68(0) 1.63(6) 0.21(0) 1.24(5) 0.22(0) 0.63(32) 15.01(0) S5281 (BASD-12) ol 40.43(31) 0.07(5) 0.24(30) 7.23(15) 0.12(5) 52.17(68) 0.03(3) 0.03(2) 0.03(2) 0.10(5) 0.01(1) (44) opx 56.46(23) 1.46(15) 2.03(23) 4.33(11) 0.04(3) 34.88(41) 0.28(3) 0.14(4) 0.03(2) 0.09(4) 0.01(1) 99.75(38) cpx 54.62(93) 2.14(19) 13.20(1.17) 3.75(25) 0.03(3) 24.10(1.82) 1.09(10) 0.22(3) 0.06(4) 0.14(3) 0.03(3) 99.37(47) gt 42.71(46) 21.63(39) 4.80(32) 5.64(70) 0.02(2) 23.22(58) 0.04(2) 1.13(19) 0.31(8) 0.21(5) 0.00(1) 99.71(43) melt 40.19(0) 4.66(0) 9.13(1) 9.60(0) 0.02(2) 21.02(0) 1.13(88) 0.11(1) 1.29(2) 0.20(0) 0.30(34) 12.34(0) S5291 (BASD-13) ol 40.63(33) 0.07(2) 0.17(3) 7.27(13) 0.08(3) 51.79(80) 0.04(2) 0.02(2) 0.02(2) 0.10(4) 0.00(0) (1.00) cpx 55.04(59) 2.38(11) 14.25(77) 3.64(11) 0.02(3) 23.02(1.65) 1.35(9) 0.16(3) 0.08(2) 0.10(4) 0.02(1) (67) gt 43.03(54) 22.13(22) 4.50(35) 5.56(1.07) 0.02(3) 23.97(96) 0.06(2) 0.75(12) 0.25(12) 0.19(8) 0.01(0) (28) melt 40.50(0) 4.72(0) 8.83(1) 9.29(0) 0.02(2) 21.96(0) 1.21(159) 0.08(2) 1.23(4) 0.19(0) 0.07(187) 11.91(0) S5577 (BASD-14) ol 40.86(33) 0.06(2) 0.15(2) 6.79(14) 0.08(5) 52.15(47) 0.03(2) 0.02(1) 0.03(3) 0.09(4) 0.01(1) (33) cpx 55.14(62) 2.29(5) 14.13(86) 3.22(9) 0.02(2) 22.55(90) 1.32(7) 0.16(2) 0.06(3) 0.11(4) 0.02(1) 99.01(82) gt 43.24(20) 22.27(26) 4.35(19) 5.40(44) 0.03(3) 23.45(47) 0.06((3) 0.63(7) 0.28(5) 0.19(4) 0.01(1) 99.90(32) melt 41.06(1) 4.90(0) 8.74(0) 8.06(2) 0.01(2) 22.05(0) 1.08(251) 0.08(0) 0.99(0) 0.17(0) 0.09(139) 12.78(2) (continued) 2105
10 JOURNAL OF PETROLOGY VOLUME 55 NUMBER 11 NOVEMBER 2014 Table 3: Continued Run (start. mix) SiO 2 Al 2 O 3 CaO FeO NiO MgO Na 2 O Cr 2 O 3 TiO 2 MnO K 2 O H 2 O Total S5581 (BASD-14) ol 41.11(22) 0.06(2) 0.13(1) 6.20(15) 0.07(4) 52.88(33) 0.05(7) 0.02(1) 0.01(1) 0.09(2) 0.01(1) (37) cpx 55.18(54) 2.49(24) 12.97(92) 3.10(16) 0.03(3) 23.78(88) 1.24(10) 0.17(3) 0.06(2) 0.10(5) 0.01(1) 99.13(39) gt 43.25(19) 21.87(31) 4.76(43) 4.80(33) 0.02(3) 23.70(42) 0.05(2) 0.69(7) 0.32(6) 0.16(4) 0.01(1) 99.62(39) melt 40.63(0) 5.06(0) 8.37(0) 7.43(0) 0.01(2) 22.28(0) 2.76(0) 0.08(1) 0.98(0) 0.16(0) 0.21(24) 12.02(0) The number in parentheses close to the mineral components is one standard deviation. y The number in parentheses close to the melt components is the percent residual given by the mass balance calculations. Values below 1% are indicated as zero. Experiment from outer capsule where the starting composition was melt mixture. x Experiment from inner capsule where the starting composition was 80% in weight of the melt mixture plus 20% in weight of the peridotite mixture. Table 4: Modal abundances (in wt %) of phases by massbalance calculations Run no. ol opx cpx gt melt FeO* S S S5213-O S5213-I S S S S S S S S *Phase added to account for the loss of FeO (see text for details). from the solid assemblage, although the residual assemblage appeared to crystallize ol þ cpx þ garnet. For this reason BASD-4 was assembled with a 1% higher H 2 O content. When BASD-4 was mixed with 10% peridotite, the melt did not sufficiently separate from the crystals, but the melt on its own crystallized an assemblage of opx þcpx þ garnet with significant amounts of melt. The BASD-5 composition was obtained using the analysis of the melt from the previous experiment. Several experiments, in which between 10 and 20% peridotite was equilibrated with BASD-5, produced assemblages of ol þ cpx þ garnet in equilibrium with analyzable melt. Based on these melt analyses and partitioning relations, BASD-6 was derived. BASD-6 mixed with 20% peridotite crystallized dominantly olivine with minor cpx and garnet. Mass balance and partitioning relations gave melt BASD-7. In experiment S5129, BASD-7 crystallized ol þ cpx þ garnet with sufficient melt for an accurate mass balance to be performed. The results were used to derive BASD-8 using melt partitioning relations. BASD-8 crystallized ol þ cpx þ garnet in experiment S5200. Minor differences still existed, however, between cpx and garnet compositions in S5200 when compared with the sub-solidus experiment. The wet cpx CaO content, for example, was much higher than that found in sub-solidus cpx and the TiO 2 content of the wet garnet was a factor of two lower than that of the dry garnet. In the subsequent experiments melt partitioning relations were used based on the S5200 melt composition and the mineral compositions of the sub-solidus experiment in an attempt to crystallize an assemblage with mineral compositions that were as close as possible to the sub-solidus peridotite minerals (Table 2). The last five melt compositions were therefore an attempt to more closely approach a very small-degree melt composition. BASD-9 was derived using the partition coefficients from S5200 and the subsolidus mineral assemblage, but crystallized opx þ garnet. There are probably two reasons why BASD-9 was shifted so significantly in composition compared with the previous melt. First, the higher CaO content of the wet clinopyroxene is probably correct compared with the lower values observed in the dry sub-solidus cpx composition, which, as discussed above, are probably metastable. Second, the partitioning approach based on the sub-solidus experiment naturally resulted in very high TiO 2 contents in the melt, which were approaching those of a very low-degree melt fraction. Raising the TiO 2 content upset concentrations of other major elements and caused olivine to disappear from the assemblage. After this we gave up trying to reproduce such a low melt fraction, as the melt fraction based on the TiO 2 content is simply a function of the bulk TiO 2 content of the rock, which can vary significantly in mantle peridotites. The results of BASD-9 were therefore disregarded and BASD-10 was based on the mass balance of BASD-8 and using partitioning relations for most 2106
11 NOVELLA & FROST PERIDOTITE PARTIAL MELTS elements, except CaO or TiO 2 which were kept close to the results of experiment S5200. BASD-10 also crystallized opx þ garnet even when mixed with 20% peridotite. BASD-11 was a test of the near-solidus melt composition of Walter (1998), which was identified owing to its proximity between BASD-9 and BASD-10. H 2 O was added in a similar proportion to previous experiments. It also crystallized opx þ cpx þ garnet. BASD-12 was an attempt to delimit the compositional range of opx stability. The composition was designed from a mixture of BASD-11 and BASD-7. It crystallized ol þ opx þ cpx þ gt þ melt (run S5281, Table 3). BASD-13 and BASD-14 were based on mixtures of melts that had all previously crystallized ol þ cpx þ gt (BASD-13 from a 50:50 mixture of BASD-12 and BASD-7, and BASD-14 from BASD-13 and the melt S5291). When both compositions were mixed with 20% peridotite opx þ garnet were formed, however, although both compositions crystallized ol þ cpx þ garnet in the absence of peridotite. The melt starting mixtures and those produced in the experiments are displayed on a volatile-free basis in the normative CIPW scheme in Fig. 2, following the procedure of Falloon & Green (1988). For comparison, hydrous (Gaetani & Grove, 1998; Balta et al., 2011; Tenner et al., 2012) and dry (Walter, 1998) melts from the literature are also plotted for a range of conditions. The melt compositions in equilibrium with olivine-bearing three- and four-phase assemblages are nepheline normative, with the exception of S5577, which is olivine^hypersthene normative. The described starting compositions detailed above are shown in Fig. 2a and the iterative evolution of the melts is highlighted by the black lines in Fig. 2b. The melt compositions are spread out on the normative diagrams, but a significant part of this range can be explained by variations in melt alkalis, as discussed below. Attainment of mineral^mineral and mineral^melt chemical equilibrium The experimental run times were short to limit the loss of H 2 O and it is particularly important to test that equilibrium between phases was still achieved. The crystalline phases that could be unambiguously identified as solid before quenching were in general homogeneous in composition (Table 3) and displayed no significant chemical zoning (Fig. 1), except within 5 mm of the crystal edges, where some quench modification occurred in some instances. Many crystals formed, particularly in the outer capsules, had sizes up to 200 mm (Fig. 1d and e). A number of thermometers can be used to test whether equilibrium between mineral phases was probably achieved. The Fe^Mg exchange thermometers between olivine and garnet (O Neill & Wood, 1979) and garnet and clinopyroxene (Ellis & Green, 1979) were employed for this purpose in addition to the enstatite-in-clinopyroxene thermometer of Nimis & Taylor (2000). In experiments containing orthopyroxene the thermometers of Harley (1984) and Brey & Ko«hler (1990) were also applied. In the best instances temperatures using these thermometers showed maximum differences of 5408C. These results are shown in Table 5. In experiments in which the thermometers indicated small ranges of temperature the average temperature was 308C higher than the set point of 14008C, which possibly reflects the crystallization temperature during cooling from the 508 initial overstep or a difference from the thermocouple temperature owing to the thermal gradient. The consistency of many experiments is a good indication that equilibrium was reached, as the O Neill & Wood (1979) thermometer, for example, is particularly sensitive. These tests also indicate that the thermometers operate extremely well at these conditions, which are close to the limit in pressure for which they are generally employed on natural samples. Ulmer & Sweeney (2002) found similar evidence for equilibrium being reached in experiments with large volatile-bearing melt fractions run for even shorter periods of time at similar conditions. Experiments in which differences between temperatures determined using the three main thermometers exceeded 1008C were discarded. In many instances these were experiments where the melt fraction was relatively small (570 wt %) and it is likely that the entire assemblage was not completely molten during the overstep in heating by 508C. This implies that on the timescale of the experiments it is not possible to equilibrate the hydrous melt with a solid residue, but instead equilibrium is achieved as a result of almost simultaneous crystallization of phases from a single homogeneous melt composition. It has been postulated that when sufficient melting occurs in multianvil experiments, the thermal gradient generally leads to rapid dissolution and recrystallization of mineral grains, even without an initial overstep of the temperature (Lesher & Walker, 1988; Zhang & Herzberg, 1994). Such crystallization is probably the only way of approaching chemical equilibrium in short experiments because extremely long run times are probably required for equilibrium to be achieved through mineral cation diffusion. Long run times would lead to gradual H 2 O loss from the capsule. Fe^Mg equilibrium was apparently not reached, for example, in the sub-solidus experiment (Table 2), for which the O Neill & Wood (1979) thermometer, the most sensitive of the three, predicts a temperature 2398C above the run temperature (Table 5). Under dry conditions even run times of 1day are, therefore, insufficient to yield equilibrium. The olivine^melt Fe^Mg exchange coefficient, K D ¼ Xol Fe X melt Fe X melt Mg X ol can be used to assess mineral^melt equilibrium in the experiments. For experiments in which the solid assemblage Mg ð1þ 2107
12 JOURNAL OF PETROLOGY VOLUME 55 NUMBER 11 NOVEMBER 2014 (a) JCL Plg Qz (b) I Ol Pd Hy I starting compositions melt with ol, cpx, gt melt with opx, cpx, gt Walter 98 (3-7 GPa) Balta et al.11 (3 GPa) G&G 98 (1.6-2 GPa) Tenner et al.12 (3.5 GPa) Ol Pd1 Fig. 2. (a) CIPW normative tetrahedron projected from diopside showing starting mixtures (grey circles, labelled only by their number from 1 to 14) and experimentally produced melts. Melt compositions in the experiments are shown by black filled circles when found in equilibrium with ol þ cpx þ gt and open circles when in equilibrium with opx þ cpx þ gt. The black circle with the white cross indicates run S5281, in which ol, opx, cpx and gt were produced in equilibrium with melt. The mantle peridotite composition (PD-1) is also plotted (black cross). Data from the literature are also shown. The projections were conducted following the approach described by Falloon & Green (1988). (b) Close-up of the olivine-rich portion of (a) in which only starting mixtures and resulting melt compositions are plotted. Black lines show the evolution from the starting mixture to the resultant melt composition in a particular experiment. 2108
13 NOVELLA & FROST PERIDOTITE PARTIAL MELTS Table 5: Calculated temperatures (8C) of the experiments performed at a nominal temperature (thermocouple reading) of 14008C Run no. O&W 79 E&G 79 was deemed to be in equilibrium, melt K D values vary from 029 to 035, with the higher values corresponding to charges with greater Fe loss. The range is shifted to slightly lower values compared with that observed by Walter (1998) at 6 GPa (034^036) at the dry solidus, with the small shift being in excellent agreement with the effect of temperature predicted by the model of Toplis (2004). Several experiments were excluded on the grounds that melt K D values were significantly above 04. Oxygen fugacity in the experimental capsules In two experiments, 3 wt % of Ir metal powder was added to the inner capsule to estimate the oxygen fugacity based on the equilibrium Fe 2 SiO 4 þ Mg 2 SiO 4 olivine N&T 00 ¼ 2MgSiO 3 þ 2Fe þo 2 : orthopyroxene alloy Fe from the silicates alloys with the Ir to produce an Fe^Ir alloy, the composition of which is sensitive to fo 2 (Woodland & O Neill, 1997; Stagno & Frost, 2010). The fo 2 can be measured using chemical analyses of the silicate and alloy phases. In the experiments in question olivine failed to crystallize; however, a reasonable estimate can be made of the fictive olivine composition based on orthopyroxene and garnet compositions. The resulting H 84 B&K 90 Average S S S5213-I 1391 S S S S S S S S Subsolidus* *The high subsolidus temperatures are a consequence of chemical disequilibrium (see text for explanation). Geothermometers used: O&W 79, O Neill & Wood (1979); E&G 79, Ellis & Green (1979); N&T 00, Nimis & Taylor (2000); H 84, Harley (1984); B&K 90, Brey & Köhler (1990). s ð2þ oxygen fugacities are 18 and 22 log units relative to the quartz^fayalite^magnetite (QFM) oxygen buffer, which is within error (05 log units) identical to the fo 2 at which the experiment starting powders were initially reduced at 1atm. This range of fo 2 is consistent with that expected for the mantle at 6 GPa (Frost & McCammon, 2008; Stagno et al., 2013) and also indicates that little oxidization of silicates has occurred during the experiment. Significant oxidation of silicates by H 2 O would probably lead to H 2 being lost from the capsule. Loss of H 2 or H 2 O during experiments An important question is whether it is likely that H 2 Owas lost from the capsules during the experiments either as H 2 OorH 2. This can be assessed by examining the extent of crystallization for a given initial bulk H 2 O content. Whether melt successfully separates to form a pool depends on the melt fraction, which in turn depends on the proportion of H 2 O in the experimental charge. The formation of interstitial melts, where the melt did not separate from the residue and owing to quench modification becomes impossible to analyze (Fig. 1f), occurs reproducibly when initial bulk H 2 O contents are below 8 wt % and the melt fraction falls below 70 wt %. In contrast, bulk water contents of 11wt % resulted in refined melt fractions that were between 86 and 90 wt % for six experiments; that is, a reproducible and narrow range. If significant loss of H 2 O occurred during the experiments we would expect to see variable amounts of crystallization and the formation of interstitial melts. DISCUSSION Phase relations Using the experimental procedure highlighted above it was possible to obtain melt compositions that crystallized olivine, clinopyroxene and garnet; that is, the assemblage observed in the sub-solidus peridotite experiment at the same conditions (Table 2). In one experiment (S5281) additional orthopyroxene was also formed. Fe loss to the capsule, however, meant that in most instances the solid residues contained less Fe than expected from the sub-solidus peridotite. The most successful, well-equilibrated experiments to crystallize the three phases were those where only the melt composition was employed and 10% crystallization occurred from this melt. Further melt compositions were then fabricated from the successful melt analyses to check that the achieved melt composition was indeed in equilibrium with peridotite (BASD-9 to BASD-14). This resulted, however, in a number of problems. One problem, as noted above, arose because equilibrium between melt and a solid residue could not be obtained in the short run times, as indicated through the application of various thermometers. However, in several experiments where either small 2109
14 JOURNAL OF PETROLOGY VOLUME 55 NUMBER 11 NOVEMBER 2014 portions of peridotite were mixed with the proposed melt composition or the melt composition alone was crystallized, orthopyroxene replaced olivine in the crystallizing assemblage. As shown in Fig. 3, melts coexisting with ol, cpx and gt were found to have almost identical ranges of composition compared with those coexisting with opx, cpx and gt. There appears to be no chemical component in the melt that can discriminate between these two assemblages. Loss of H 2 O from the capsule does not seem to provide a suitable explanation, as the observation is consistently reproducible; that is, all melt compositions fabricated from melts originally coexisting with the ol-bearing assemblage crystallize opx. The only difference between melts that crystallized olivine and those crystallizing opx appears to be the initial starting melt composition. As shown in Fig. 3a, starting compositions from which olivine-bearing assemblages formed have higher (Mg þ Fe)/Si ratios than the final melts, reflecting the olivine crystallization. In contrast, melts in which opx forms change very little during crystallization. It is the 10% crystallization of the ol-bearing assemblage that causes a shift in the melt (Mgþ Fe)/Si ratio to lower values. A plausible explanation, therefore, is that the melts produced by crystallization of olivine during cooling over the 508C interval have moved towards the opx stability field. However, once the run temperature of 14008C is reached there is insufficient driving force or time for olivine to react with the melt and transform to opx. When the melt compositions are reproduced and equilibrated at the same conditions, however, they crystallize opx. Experiment S5281 indicates that the melts are very close to compositions where all four mineral phases crystallize. As a result, it can be concluded that the melt composition that is saturated in the three mineral phases lies somewhere between the starting compositions and the final mass-balanced melt compositions. In addition, the temperature at which the melt is multiply saturated is also bracketed between 1400 and 14508C, the range in which the crystallization occurs. The differences in melt composition between the initial starting melt and the residual melt, in equilibrium with olivine, are small. The main shifts are a decrease in MgO content by 08^ 18 wt %, coupled with a similar magnitude increase in H 2 O content, and CaO also increases by 05^12wt %. As such the bracket on the three-phase saturated composition is relatively narrow. In fact, the saturating melt composition must be close to the average of the initial and final melts, which is plotted in Fig. 3b. Owing to thermal gradients and the necessity for short run times it is probably difficult to achieve a greater level of precision in the composition of multiply saturated melt compositions in the multianvil. Melt compositions As shown in Fig. 3b the hydrous melt compositions coexisting with ol, cpx and gt have very similar (Mg þ Fe)/Si ratios to those reported by Walter (1998) close to the dry peridotite solidus at 17008C, and have only slightly higher Al/Ca ratios. Na 2 O concentrations are also higher in the hydrous melts but this reflects differences in the effective melt fraction between experiments. Surprisingly, it would seem, therefore, that other than lowering the peridotite solidus temperature, H 2 O has very little discernible effect on the major element melt chemistry. If data are compared at various pressures, then hydrous melts at 6 GPa are more Mg-rich and have higher Al/Ca ratios than those at 16 and 3 GPa (Fig. 3b). There are, however, only small differences with the four-phase saturated melt reported by Tenner et al. (2012) from experiments at 35 GPa and 13508C. It seems slightly surprising that melts shift so dramatically in MgO content between 3 and 35 GPa and that melts at 35GPahavemuchstronger affinities with those at 6 GPa compared with 3 GPa. One possibility is that this arises from the presence of CO 2 in these experiments, which was discussed by Tenner et al. (2012). In Fig. 4 the melt compositions are plotted on a volatilefree basis in two projections using the garnet lherzolite normative scheme described by Kelemen et al. (1992). The first is a projection from cpx onto olivine^opx^garnet, and the second is from opx onto olivine^cpx^garnet. The near-solidus melts of dry mantle peridotite between 3 and 7 GPa reported by Walter (1998) are shown in addition to hydrous near-solidus melts reported between 16 and 35 GPa by Gaetani & Grove (1998), Balta et al. (2011) and Tenner et al. (2012). Hydrous melts from the present study are dominantly olivine- and cpx-normative. Again, it can be seen that there is very little difference between where the olivine- and opx-bearing melts from the present study plot, although results from three of the five opx-bearing samples are opx-normative. The hydrous melts from this study plot close to the anhydrous, near-solidus melts of Walter (1998) at 6^7 GPa. It can be seen that there is a consistent evolution with pressure for both hydrous and anhydrous melts from cpx- and garnet-normative melts at low pressure to dominantly olivine- and cpx-normative melts at high pressure. If we consider melts crystallizing at depth in the lithospheric mantle, for example, this would imply a shift from eclogitic vein rocks at low pressure (53 GPa) to wehrlite assemblages at high pressure (6 GPa). Wehrlite xenoliths that have been reported with equilibration pressures of 6 GPa (e.g. Kopylova & Caro, 2004) could therefore have formed from the crystallization of small-degree hydrous melts. Walter (1998) provided a series of regressions in terms of melt fraction (F) for major elements in melts produced in his nominally dry peridotite melting experiments, which can be extrapolated to zero F. There is good agreement between these estimated zero melt fraction values at 6 GPa compared with the compositions of the hydrous 2110
15 NOVELLA & FROST PERIDOTITE PARTIAL MELTS (a) (b) G&G 98 Balta et al. 11 Walter 98 Tenner et al. 12 Fig. 3. Major element molar ratios, Al/Ca vs (Mg þ Fe)/Si, of hydrous melts produced at 6 GPa and 14008C. (a) Melts and starting compositions from this study. The grey filled circles show the starting compositions BASD-7, -8, -12, -13 and -14 (see Table 1). Black filled circles are melts produced in equilibrium with olivine, cpx and gt (Table 3); open circles are melts in equilibrium with opx, cpx and gt (Table 3). The black circle with the white cross indicates run S5281, in which ol, opx, cpx and gt were produced in equilibrium with melt. (b) Averaged initial and final melt compositions from olivine þ cpx þ gt (þ opx in S5281; white cross) saturated experiments are shown by black filled circles, and are compared with data from the literature (grey). Grey squares are hydrous melts from Gaetani & Grove (1998) produced at 16^2 GPa; grey triangles are low-degree hydrous melts produced by Balta et al. (2011) at 3 GPa; the grey asterisk is a hydrous melt produced by Tenner et al. (2012) at 35 GPa; grey diamonds are melts produced near the dry peridotite solidus at 6 GPa by Walter (1998). The black arrow indicates the change in the melt composition of Walter (1998) with increasing temperature from 1710 to 17558C. 2111
16 JOURNAL OF PETROLOGY VOLUME 55 NUMBER 11 NOVEMBER 2014 starting composition G&G 98 (1.6-2 GPa) Balta et al. 11 (3 GPa) Tenner et al. 12 (3.5 GPa) Walter 98 (6 GPa) x 7 7 x melts in equilibrium with olivine, cpx and garnet from the present study when compared on a volatile-free basis. Walter (1998) predicted melt contents of 10 wt % CaO at zero F, whereas hydrous melts from the present study are in the range 94^105 wt % on a volatile-free basis. The range of MgO and Al 2 O 3 contents of the hydrous melts are similarly within 1wt % of the zero F values of Walter (1998), approximately 24 and 6 wt % respectively. SiO 2 melt contents from the present study cover a relatively wide range from 44 to 47 wt % but the values are correlated with FeO, which varies owing to Fe loss to the metal capsule. The melt with the least Fe loss, however, contains 132 wt % FeO and 437wt % SiO 2, which is in excellent agreement with the zero F values of 134 and441wt % respectively of Walter (1998). Major element hydrous melt Fig. 4. Normative diagram projected from cpx onto the olivine^opx^garnet surface (top) and from opx onto the olivine^cpx^garnet surface (bottom), following the method of Kelemen et al. (1992). Numbers indicate the pressure in GPa of near-solidus melts from the study of Walter (1998). 3 3 compositions are, therefore, in excellent agreement with estimates for small-degree anhydrous melt compositions. The hydrous melt TiO 2 contents vary from 1 to 15wt%. The PD-1 bulk peridotite composition employed in the present study (Jagoutz et al., 1979) contains 024 wt % TiO 2. Using mineral^melt partition coefficients from the present study we can estimate from the melt TiO 2 content the effective melt fraction relative to the proposed bulk composition, which is between 7 and 16%. This range is fairly high, but it is extremely sensitive to the TiO 2 content in the assumed bulk composition. If we were to assume the depleted mid-ocean ridge basalt (MORB) mantle composition of Workman & Hart (2005) that contains only 013 wt % TiO 2 then the melt compositions would be equivalent to melt fractions between 0 and 4%. 2112
17 NOVELLA & FROST PERIDOTITE PARTIAL MELTS There is a wide variation in the Na 2 O and K 2 O contents of the hydrous melts, which vary in the range 1^27 and 007^029 wt % respectively. These variations couple strongly with the mass-balance residuals for these elements, implying that alkalis were variably lost from the melts. If the missing alkalis inferred from the mass balance are added back to the melt then the Na 2 O melt compositions fall in the range 21^37wt % and K 2 O values are in the range 02^04 wt %. Although is it possible that the alkalis were lost during chemical analysis, this does not fit with the observed large variations in the residuals between experiments. Within single experiments standard deviations for melt alkali analyses were small. As similar LA- ICP-MS analysis conditions were used it seems hard to explain why different experiments lost such varying proportions during the analyses. Another possible explanation is that Na 2 O and K 2 O dissolved into an H 2 O-rich fluid that exsolved from the melt during quench crystallization. There is no evidence for hydrous minerals in the quench crystal assemblage, so as crystallization occurred a free fluid, which cannot partition into the structure of the quenched crystals, must have exsolved and separated. In experiments where the outer capsule was pierced on recovery, water was observed to bubble out of the capsules. This was not done for all experiments, so as not to damage the crystalline assemblage in the charge. Loss of alkalis to H 2 O-rich fluids that separate on quenching may explain the variable depletion of the melts. After exsolving the fluid may have migrated to an unsampled region of the capsule or the quenched fluid phase may not have survived polishing. Alkali (Na and K) peridotite^melt partition coefficients for hydrous melts obtained in the present study and from Tenner et al. (2012) at lower pressures are shown in Fig. 5. The partition coefficients were calculated from the data reported in Table 3 and are plotted as a function of the residual of the particular component in the melt obtained from the mass-balance calculations (i.e. the per cent loss of K and Na from the charge compared with the bulk composition). The wide variations of Na and K partition coefficients from the present study are coupled to losses apparent in the mass-balance calculations. As explained above, Na and K may have been mobilized in the charge in a hydrous fluid during quenching. The alkali mineral^ melt partition coefficients are, therefore, maximum values. It can be observed that for both Na and K D pdt/ melt decreases as the melt residual decreases; this is especially so for Na, where it decreases sharply at low residuals. K follows a less steep trend with one point at low melt residuals being the only outlier. This point corresponds to run S5129, which has the highest K contents in the equilibrium crystals. However, it can be observed that the K partition coefficients in Fig. 5 are in good agreement at low melt residuals with data from Tenner et al. (2012). Melt H 2 O concentrations and the depression of melting To estimate the proportion of hydrous melt that may exist in the mantle for a given set of conditions requires information on the depression of the silicate solidus as a function of melt H 2 O content. Such a relationship is necessary, for example, to determine the H 2 O contents required for melts to form along a mantle adiabat at a specific depth. A particularly important aspect in this respect is that the effect of H 2 O on melting point depression is also likely to be a function of silicate melt composition; the melt compositions of specific importance at depths greater than 90 km are those in equilibrium with a residual three- to four-phase peridotite assemblage because the degree of melting will be small. The chemical compositions of the melts produced in this study are very similar, when normalized to H 2 O-free conditions, to those analyzed close to the dry solidus at 17008Cby Walter (1998). The depression of the melting temperature owing to H 2 O in the experiments from the current study can, therefore, be estimated to be 3008C.The corresponding melt H 2 O content is bracketed between the H 2 O content of the three-phase saturated starting melts (105%) and the resulting mass-balanced melt H 2 Ocontents(113^128%).The resulting melting point depression as a function of melt H 2 O content is shown in Fig. 6 along with data at 35GPafrom the previous study of Tenner et al. (2012).Tenner et al. (2012) proposeda simplemodelto describethe depressionof melting owing to H 2 O. Their model was based on the cryoscopic equation T hyd P T dry P ¼ 1 R DS lnð1 X melt fus OH Þ ð3þ where R is the gas constant, T hyd P and T dry P are the hydrous and dry silicate melting temperatures at given pressures, DS fus is the molar entropy of fusion of the silicate and XOH melt is the mole fraction of dissolved OH in the melt. Such a model is similar to that proposed by Silver & Stolper (1985) in which dissolved H 2 O reacts with oxygen in the melt to establish the equilibrium H 2 Omelt ð ÞþOmelt ð Þ¼2OHðmeltÞ: ð4þ Ten ner et al. (2012) assumed that full dissociation occurs to produce 2 OH and calculated the mole fraction of OH in the melt from the H 2 O content (H 2 O wt %) by X melt OH ¼ ð2h 2 Owt%=18:015Þ ð2h 2 Owt%=18:015Þþð100 H 2 Owt%Þ=mol:wt silicate : The molecular weight selected for the silicate phase is potentially an adjustable term in the model. Silver & ð5þ 2113
18 JOURNAL OF PETROLOGY VOLUME 55 NUMBER 11 NOVEMBER 2014 Fig. 5. Peridotite^melt partition coefficients of alkali components Na 2 O and K 2 O as a function of their percentage residual in the melt obtained by mass-balance calculations (see Table 3). Data from Tenner et al. (2012) for a peridotite-saturated assemblage at lower pressure than investigated in this study are also shown. The dashed line connects data for Na 2 O and serves only as a visual guide. The dotted line is a linear fit to the K 2 O data reported in this study. Stolper (1985) assumed a molecular weight of the melt on a one oxygen basis. In contrast, Tenner et al. (2012) fitted their experimental data assuming a three oxygen melt formula, which gives a molar weight of 111g mol 1. Combining this value with literature data they determined a DS fus of 444JK 1 mol 1. A comparison between this model calculated at 35 GPa and at 6 GPa is shown in Fig. 6. The model at 6 GPa is found to be consistent with the experimental bracket provided by this study. The curve within the shaded area indicates the melting point depression required for hydrous melts to exist along a mantle adiabat in the region of the seismic LVZ. The deepest extent of the LVZ is estimated at 220 km, which corresponds approximately to 75 GPa, and data in this study are therefore consistent with melts at this depth containing 15^16 wt % H 2 O. Based on mineral^melt H 2 O partitioning at 6 GPa (Novella et al., 2014) the bulk mantle at this depth would require approximately 750 and 1700 ppm wt H 2 O to produce 01 and 1% melt, respectively. Low-degree hydrous melts and the origin of group II kimberlites In Fig. 7 ranges in the composition of group I and II kimberlites are plotted on a volatile-free basis from the compilation assembled by Becker & le Roex (2006). The melt compositions from our study can be used to investigate the genesis of such rocks because, as outlined below, their similarity to group II orangeites implies a possible origin for these kimberlites from H 2 O-induced partial melting of garnet lherzolite or garnet wehrlite at 6 GPa. Kawamoto & Holloway (1997) performed experiments on H 2 O-saturated garnet lherzolite between 5 and 11GPa and proposed that group II kimberlites might originate from partial melting of hydrated peridotite mantle at relatively low temperatures of 10008C. The four-phase saturated melt compositions at 5 and 75 GPa and 10008C are shown in Fig. 7, on a volatile-free basis, and are indeed remarkably similar to those of group II kimberlites, although FeO contents are too high and K 2 O and other minor components were not considered. The compositions of hydrous melts produced by Kawamoto & Holloway (1997) at higher pressures, 10^11GPa, are too high in MgO (435 wt %) and FeO (15^17 wt %) and low in Al 2 O 3 and SiO 2 compared with natural kimberlites and are therefore not shown. In Fig. 7, the compositions of hydrous melts produced in this study in equilibrium with olivine, cpx and garnet, and in one instance with additional opx, at 14008C and 6 GPa are plotted on a volatile-free basis. Melt concentrations of MgO, CaO, Al 2 O 3, FeO, TiO 2 and MnO fall within the group II field, with SiO 2 contents being only slightly higher. By comparing these compositions with those of melts produced close to the dry peridotite solidus 2114
19 NOVELLA & FROST PERIDOTITE PARTIAL MELTS Fig. 6. The melting temperature depression (deltat) plotted as a function of melt H 2 O content (wt %) for low-degree hydrous peridotite melts. Data at 35 GPa are from Tenner et al. (2012). Data at 6 GPa are from the present study: black filled circles indicate the H 2 O content of the melt starting mixtures (Table 1); open circles indicate the water content of the multiply saturated (olivine-bearing) melts given by the mass-balance calculations. The actual melt H 2 O content should be bracketed by the open and filled symbols. Curves calculated from the cryoscopic equation [equation (3)] are shown calculated at 35 and 6 GPa. The grey field indicates the temperature depression required for hydrous peridotite melts to form over the depth interval of the seismically observed mantle low-velocity zone, as demarcated by Hirschmann et al. (2009). at 6 GPa and 17008C (Walter, 1998), also shown in Fig. 7, and with H 2 O-saturated melts produced at 10008C (Kawamoto & Holloway, 1997), it is apparent that the major element compositions, excluding for the moment FeO and alkalis, do not change significantly with temperature. Over this large range in temperature, the experimentally determined melt compositions at 6 GPa are consistent with the compositional range of group II kimberlites. Melts produced in studies in which peridotite has been melted in the presence of CO 2 and CO 2^H 2 O are also shown in Fig. 7, normalized to volatile-free compositions, to assess how melt compositions are likely to shift in the presence of CO 2 in this pressure range. Brey et al. (2008) investigated carbonated garnet lherzolite melting between 6 and 10 GPa. The four-phase saturated CO 2 -rich melts of Brey et al. (2008), produced between 1400 and 17008C (Fig. 7), are SiO 2 and Al 2 O 3 poor and CaO rich compared with natural kimberlites. Brey et al. (2008) noted that higher temperature melts approach group I kimberlites in SiO 2 and CaO contents, but these melts are still too low in Al 2 O 3 and are no longer in equilibrium with cpx. Brey et al. (2009) performed similar experiments on melting of peridotite in the presence of H 2 O and CO 2 in a 3:2 molar ratio. The multiply saturated melt compositions produced in the range 6^10 GPa and 1300^14008C (Fig. 7) are slightly less CaO rich, but have slightly more Al 2 O 3 and SiO 2 than melts produced from CO 2^peridotite systems at similar conditions. Multiply saturated melts produced by Foley et al. (2009) from K 2 O-enriched peridotite in the presence of a 3:7 ratio H 2 O^CO 2 mixture in the range 5^6GPa and 1180^12408C, however, show the same general trend, but in addition have lower average MgO contents. These melts plot very close to and in some cases within the field of group II kimberlites for the major elements. The effect of H 2 O on carbonated peridotite melts can be rationalized because H 2 O lowers the silicate solidus and therefore increases the proportion of silicate melt components (i.e. SiO 2 and Al 2 O 3 ) in the carbonate melts, which are otherwise dominated by CaO and MgO. Adding H 2 O, therefore, has a similar effect to increasing temperature for a carbonate melt in equilibrium with peridotite. In respect of these four components, much of the field of group II magmas can be produced by melting of garnet wehrlite or garnet lherzolite at 6 GPa and near-adiabatic temperatures in the presence of H 2 O^CO 2.GroupIkimberlites can most probably also be produced from peridotites fluxed by an even narrower range of H 2 O and CO 2 compositions, as proposed by Sokol et al. (2013). Although the compositions of group II kimberlites are consistent with H 2 O-rich CO 2 fluxed melting of garnet 2115
20 JOURNAL OF PETROLOGY VOLUME 55 NUMBER 11 NOVEMBER this study (pdt+h2o) Brey et al. 08 (pdt+co 2 ) K&H 97 (pdt+h2o) Brey et al. 09 (pdt+co 2+H2O) Foley et al. 09 (pdt+co 2 +H 2 O) Walter 98 (pdt) CaO SiO 2 Al 2 O MnO MgO MgO TiO 2 KO 2 Fig. 7. Chemical compositions of hydrous multiply saturated melts compared with melt compositions produced in previous volatile-bearing melting studies and the range of compositions estimated for natural group I and II kimberlites. Melts produced in this study in equilibrium with ol þ cpx þ garnet are indicated by filled circles with the white cross indicating an experiment that was in addition saturated in opx. Black squares indicate the H 2 O-saturated, near-solidus melts of Kawamoto & Holloway (1997). Open triangles indicate multiply saturated, CO 2 -rich melts from Brey et al. (2008). Inverted grey triangles and grey diamonds are the compositions of CO 2 - and H 2 O-bearing melts from Brey et al. (2009) and Foley et al. (2009), respectively. The cross indicates the estimated dry peridotite zero melt fraction composition at 6 GPa and 17008C from the study of Walter (1998). Natural kimberlite ranges are from Becker & le Roex (2006). The darker grey areas are for group I kimberlites; the lighter grey areas are group II. All compositions are plotted on a volatile-free basis. 2116
21 NOVELLA & FROST PERIDOTITE PARTIAL MELTS FeO Cr O Fig. 7. Continued Na 2 O this study (pdt+h2o) NiO MgO Brey et al. 08 (pdt+co ) K&H 97 (pdt+h2o) Brey et al. 09 (pdt+co 2+H2O) Foley et al. 09 (pdt+co +H O) Walter 98 (pdt) lherzolite or wehrlite, FeO, alkalis and several other minor elements are in poor agreement. The hydrous melt FeO contents in this study vary between 85 and 135 wt % as a result of losses of Fe owing to alloying with the noble metal capsules during the experiments. Ni was similarly affected. However, it is the FeO-poor melts, in equilibrium with Fo93 olivine compositions, that show the best agreement with the natural samples. This is consistent with an origin of group II kimberlites in cratonic lithosphere, where such olivine compositions are typical (e.g. Boyd, 1989). Low FeO contents are attributed to an early phase of extensive melting that created the cratonic lithosphere and also raised Cr 2 O 3 contents in the residue. Group I kimberlite FeO contents, on the other hand, are more consistent with an origin in the asthenospheric mantle, where Fo89 olivine probably dominates (e.g. Boyd, 1989). Hydrous melt Na 2 O contents range from 15 to4wt%, and do not match group II values, which are 504wt %. This most probably implies a melt-depleted source, which was not re-enriched in Na 2 O. High K 2 O contents, on the other hand, are a prominent characteristic of group II MgO kimberlites, which average 4 wt % on a volatile-free basis (Becker & le Roex, 2006); K 2 O concentrations in the experimental melts are significantly lower, apart from the study of Foley et al. (2009), in which a K 2 O-enriched (174 wt %) starting material was employed. The high K 2 O concentration of group II kimberlites is a source characteristic (Mitchell, 1995; Becker & le Roex, 2006). Using partition coefficients for K 2 Oitisnotpossibleto obtain these high concentrations from typical garnet lherzolite compositions (i.e. Jagoutz et al., 1979), regardless of the melting fraction, as shown in Fig. 8. For this calculation we employed a K partition coefficient that was determined from the experiments presented in this work (see Fig. 5). The value of D pdt=melt K 2O we employed is and was calculated from the data of experiment S5581 (Table 3), which was the last iteration. This value is lower than the value expected at F ¼ 0% from the trend shown in Fig. 5, but is in good agreement with previous studies (Chamorro et al., 2002; Suzuki et al., 2012; Tenner et al., 2012). Uncertainty bands were calculated by propagating an error of 25% in the melt and 50% in the D pdt=melt K 2O, and 2117
GSA DATA REPOSITORY
 GSA DATA REPOSITORY 2013019 Supplemental information for The Solidus of Alkaline Carbonatite in the Deep Mantle Konstantin D. Litasov, Anton Shatskiy, Eiji Ohtani, and Gregory M. Yaxley EXPERIMENTAL METHODS
GSA DATA REPOSITORY 2013019 Supplemental information for The Solidus of Alkaline Carbonatite in the Deep Mantle Konstantin D. Litasov, Anton Shatskiy, Eiji Ohtani, and Gregory M. Yaxley EXPERIMENTAL METHODS
doi: /nature09369
 doi:10.1038/nature09369 Supplementary Figure S1 Scanning electron microscope images of experimental charges with vapour and vapour phase quench. Experimental runs are in the order of added water concentration
doi:10.1038/nature09369 Supplementary Figure S1 Scanning electron microscope images of experimental charges with vapour and vapour phase quench. Experimental runs are in the order of added water concentration
II-1 HYDROUS, LOW-CARBON MELTING OF GARNET PERIDOTITE. J. Brian Balta. Paul D. Asimow. Jed L. Mosenfelder
 II-1 HYDROUS, LOW-CARBON MELTING OF GARNET PERIDOTITE By J. Brian Balta Paul D. Asimow Jed L. Mosenfelder II-2 ABSTRACT The presence of volatile species in Earth s upper mantle drives the formation of
II-1 HYDROUS, LOW-CARBON MELTING OF GARNET PERIDOTITE By J. Brian Balta Paul D. Asimow Jed L. Mosenfelder II-2 ABSTRACT The presence of volatile species in Earth s upper mantle drives the formation of
Metal saturation in the upper mantle
 Vol 449 27 September 2007 Metal saturation in the upper mantle A. Rohrbach, C. Ballhaus, U. Golla Schindler, P. Ulmer,V.S. Kamenetsky, D.V. Kuzmin NS seminar 2007.10.25 The uppermost mantle is oxidized.
Vol 449 27 September 2007 Metal saturation in the upper mantle A. Rohrbach, C. Ballhaus, U. Golla Schindler, P. Ulmer,V.S. Kamenetsky, D.V. Kuzmin NS seminar 2007.10.25 The uppermost mantle is oxidized.
Harzburgite melting with and without H 2 O: Experimental data and predictive modeling
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 109,, doi:10.1029/2003jb002566, 2004 Harzburgite ing with and without H 2 O: Experimental data and predictive modeling Stephen W. Parman and Timothy L. Grove Department
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 109,, doi:10.1029/2003jb002566, 2004 Harzburgite ing with and without H 2 O: Experimental data and predictive modeling Stephen W. Parman and Timothy L. Grove Department
SCHOOL OF EARTH SCIENCES, UNIVERSITY OF TASMANIA, GPO BOX , HOBART, TAS. 7001, AUSTRALIA
 JOURNAL OF PETROLOGY VOLUME 40 NUMBER 9 PAGES 1343 1375 1999 Peridotite Melting at 1 0 and 1 5 GPa: an Experimental Evaluation of Techniques using Diamond Aggregates and Mineral Mixes for Determination
JOURNAL OF PETROLOGY VOLUME 40 NUMBER 9 PAGES 1343 1375 1999 Peridotite Melting at 1 0 and 1 5 GPa: an Experimental Evaluation of Techniques using Diamond Aggregates and Mineral Mixes for Determination
III-1 MANGANESE PARTITIONING DURING HYDROUS MELTING OF PERIDOTITE. J. Brian Balta. Paul D. Asimow. Jed L. Mosenfelder
 III-1 MANGANESE PARTITIONING DURING HYDROUS MELTING OF PERIDOTITE By J. Brian Balta Paul D. Asimow Jed L. Mosenfelder III-2 ABSTRACT Manganese contents and the iron/manganese ratio of igneous rocks have
III-1 MANGANESE PARTITIONING DURING HYDROUS MELTING OF PERIDOTITE By J. Brian Balta Paul D. Asimow Jed L. Mosenfelder III-2 ABSTRACT Manganese contents and the iron/manganese ratio of igneous rocks have
Effect of tectonic setting on chemistry of mantle-derived melts
 Effect of tectonic setting on chemistry of mantle-derived melts Lherzolite Basalt Factors controlling magma composition Composition of the source Partial melting process Fractional crystallization Crustal
Effect of tectonic setting on chemistry of mantle-derived melts Lherzolite Basalt Factors controlling magma composition Composition of the source Partial melting process Fractional crystallization Crustal
Melts of garnet lherzolite: experiments, models and comparison to melts of pyroxenite and carbonated lherzolite
 Melts of garnet lherzolite: experiments, models and comparison to melts of pyroxenite and carbonated lherzolite The MIT Faculty has made this article openly available. Please share how this access benefits
Melts of garnet lherzolite: experiments, models and comparison to melts of pyroxenite and carbonated lherzolite The MIT Faculty has made this article openly available. Please share how this access benefits
Near-solidus melting of the shallow upper mantle: Partial melting experiments on depleted peridotite
 Near-solidus melting of the shallow upper mantle: Partial melting experiments on depleted peridotite Wasylenki, Laura E. 1, Baker, Michael B., Kent, Adam J.R. 2, and Stolper, Edward M. Division of Geological
Near-solidus melting of the shallow upper mantle: Partial melting experiments on depleted peridotite Wasylenki, Laura E. 1, Baker, Michael B., Kent, Adam J.R. 2, and Stolper, Edward M. Division of Geological
SHANTANU KESHAV 1 *, GUDMUNDUR H. GUDFINNSSON 2 AND DEAN C. PRESNALL 1,3,4 RECEIVED SEPTEMBER 7, 2009; ACCEPTED SEPTEMBER 16, 2011
 JOURNAL OF PETROLOGY VOLUME 52 NUMBER11 PAGES 2265^2291 2011 doi:10.1093/petrology/egr048 Melting Phase Relations of Simplified Carbonated Peridotite at 12^26 GPa in the Systems CaO^MgO^SiO 2^CO 2 and
JOURNAL OF PETROLOGY VOLUME 52 NUMBER11 PAGES 2265^2291 2011 doi:10.1093/petrology/egr048 Melting Phase Relations of Simplified Carbonated Peridotite at 12^26 GPa in the Systems CaO^MgO^SiO 2^CO 2 and
Partial melting of mantle peridotite
 Partial melting of mantle peridotite 1100 1200 1300 1400 1500 (TºC) Depth (km) 50 100 150 Plag lherzolite (ol-opx-cpx-pl) Spinel lherzolite (Ol-opx-cpx-sp) Garnet lherzolite (Ol-opx-cpx-gar) Graphite Diamond
Partial melting of mantle peridotite 1100 1200 1300 1400 1500 (TºC) Depth (km) 50 100 150 Plag lherzolite (ol-opx-cpx-pl) Spinel lherzolite (Ol-opx-cpx-sp) Garnet lherzolite (Ol-opx-cpx-gar) Graphite Diamond
Melts of garnet lherzolite: experiments, models and comparison to melts of pyroxenite and carbonated lherzolite
 Contrib Mineral Petrol (2013) 166:887 910 DOI 10.1007/s00410-013-0899-9 ORIGINAL PAPER Melts of garnet lherzolite: experiments, models and comparison to melts of pyroxenite and carbonated lherzolite Timothy
Contrib Mineral Petrol (2013) 166:887 910 DOI 10.1007/s00410-013-0899-9 ORIGINAL PAPER Melts of garnet lherzolite: experiments, models and comparison to melts of pyroxenite and carbonated lherzolite Timothy
Shantanu Keshav 1,2 and Gudmundur H. Gudfinnsson Introduction
 JOURNAL OF GEOPHYSICAL RESEARCH: SOLID EARTH, VOL. 118, 3341 3353, doi:10.1002/jgrb.50249, 2013 Silicate liquid-carbonatite liquid transition along the melting curve of model, vapor-saturated peridotite
JOURNAL OF GEOPHYSICAL RESEARCH: SOLID EARTH, VOL. 118, 3341 3353, doi:10.1002/jgrb.50249, 2013 Silicate liquid-carbonatite liquid transition along the melting curve of model, vapor-saturated peridotite
Differentiation 1: core formation OUTLINE
 Differentiation 1: core formation Reading this week: White Ch 12 OUTLINE Today 1.Finish some slides 2.Layers 3.Core formation 1 Goldschmidt Classification/Geochemical Periodic Chart Elements can be assigned
Differentiation 1: core formation Reading this week: White Ch 12 OUTLINE Today 1.Finish some slides 2.Layers 3.Core formation 1 Goldschmidt Classification/Geochemical Periodic Chart Elements can be assigned
Deep and persistent melt layer in the Archaean mantle
 SUPPLEMENTARY INFORMATION Articles https://doi.org/10.1038/s41561-017-0053-9 In the format provided by the authors and unedited. Deep and persistent melt layer in the Archaean mantle Denis Andrault 1 *,
SUPPLEMENTARY INFORMATION Articles https://doi.org/10.1038/s41561-017-0053-9 In the format provided by the authors and unedited. Deep and persistent melt layer in the Archaean mantle Denis Andrault 1 *,
WORKING WITH ELECTRON MICROPROBE DATA FROM A HIGH PRESSURE EXPERIMENT CALCULATING MINERAL FORMULAS, UNIT CELL CONTENT, AND GEOTHERMOMETRY
 WORKING WITH ELECTRON MICROPROBE DATA FROM A HIGH PRESSURE EXPERIMENT CALCULATING MINERAL FORMULAS, UNIT CELL CONTENT, AND GEOTHERMOMETRY Brandon E. Schwab Department of Geology Humboldt State University
WORKING WITH ELECTRON MICROPROBE DATA FROM A HIGH PRESSURE EXPERIMENT CALCULATING MINERAL FORMULAS, UNIT CELL CONTENT, AND GEOTHERMOMETRY Brandon E. Schwab Department of Geology Humboldt State University
GEOL 2312 Igneous and Metamorphic Petrology Spring 2016 Score / 58. Midterm 1 Chapters 1-10
 GEOL 2312 Igneous and Metamorphic Petrology Name KEY Spring 2016 Score / 58 Midterm 1 Chapters 1-10 1) Name two things that petrologists want to know about magmas (1 pt) Formation, source, composition,
GEOL 2312 Igneous and Metamorphic Petrology Name KEY Spring 2016 Score / 58 Midterm 1 Chapters 1-10 1) Name two things that petrologists want to know about magmas (1 pt) Formation, source, composition,
PUBLICATIONS. Journal of Geophysical Research: Solid Earth
 PUBLICATIONS Journal of Geophysical Research: Solid Earth RESEARCH ARTICLE Key Points: Phase equilibria of carbonated peridotite solidus ledge Carbonatites along the solidus ledge Possible liquid immiscibility
PUBLICATIONS Journal of Geophysical Research: Solid Earth RESEARCH ARTICLE Key Points: Phase equilibria of carbonated peridotite solidus ledge Carbonatites along the solidus ledge Possible liquid immiscibility
XI LIU*, HUGH ST. C. O NEILLy AND ANDREW J. BERRY
 JOURNAL OF PETROLOGY VOLUME 47 NUMBER 2 PAGES 409 434 2006 doi:10.1093/petrology/egi081 The Effects of Small Amounts of H 2 O, CO 2 and Na 2 O on the Partial Melting of Spinel Lherzolite in the System
JOURNAL OF PETROLOGY VOLUME 47 NUMBER 2 PAGES 409 434 2006 doi:10.1093/petrology/egi081 The Effects of Small Amounts of H 2 O, CO 2 and Na 2 O on the Partial Melting of Spinel Lherzolite in the System
High-T T heating stage: : application for igneous petrogenesis and mantle processes - melt inclusions as key tools -
 High-T T heating stage: : application for igneous petrogenesis and mantle processes - melt inclusions as key tools - SZABÓ, Csaba Lithosphere Fluid Research Lab (LRG), Department of Petrology and Geochemistry,
High-T T heating stage: : application for igneous petrogenesis and mantle processes - melt inclusions as key tools - SZABÓ, Csaba Lithosphere Fluid Research Lab (LRG), Department of Petrology and Geochemistry,
Earth and Planetary Science Letters
 Earth and Planetary Science Letters 288 (2009) 335 347 Contents lists available at ScienceDirect Earth and Planetary Science Letters journal homepage: www.elsevier.com/locate/epsl An experimental study
Earth and Planetary Science Letters 288 (2009) 335 347 Contents lists available at ScienceDirect Earth and Planetary Science Letters journal homepage: www.elsevier.com/locate/epsl An experimental study
An Experimental Study of Liquid Compositions in Equilibrium with Plagioclase þ Spinel Lherzolite at Low Pressures (0 75 GPa)
 JOURNAL OF PETROLOGY VOLUME 51 NUMBER11 PAGES 2349^2376 2010 doi:10.1093/petrology/egq060 An Experimental Study of Liquid Compositions in Equilibrium with Plagioclase þ Spinel Lherzolite at Low Pressures
JOURNAL OF PETROLOGY VOLUME 51 NUMBER11 PAGES 2349^2376 2010 doi:10.1093/petrology/egq060 An Experimental Study of Liquid Compositions in Equilibrium with Plagioclase þ Spinel Lherzolite at Low Pressures
High-T heating stage: application for igneous petrogenesis and mantle processes - melt inclusions as key tools -
 High-T heating stage: application for igneous petrogenesis and mantle processes - melt inclusions as key tools - SZABÓ, Csaba Lithosphere Fluid Research Lab (LRG), Department of Petrology and Geochemistry,
High-T heating stage: application for igneous petrogenesis and mantle processes - melt inclusions as key tools - SZABÓ, Csaba Lithosphere Fluid Research Lab (LRG), Department of Petrology and Geochemistry,
BONINITIC MELT INCLUSIONS IN CHROME SPINEL FROM THE OGASAWARA ARCHIPELAGO
 GSA DATA REPOSITORY 2015057 BONINITIC MELT INCLUSIONS IN CHROME SPINEL FROM THE OGASAWARA ARCHIPELAGO DATA REPOSITORY for Thermal and chemical evolution of the subarc mantle revealed by spinel-hosted melt
GSA DATA REPOSITORY 2015057 BONINITIC MELT INCLUSIONS IN CHROME SPINEL FROM THE OGASAWARA ARCHIPELAGO DATA REPOSITORY for Thermal and chemical evolution of the subarc mantle revealed by spinel-hosted melt
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11294 Review of previous works on deep-liquid properties The major parameters controlling the buoyancy of deep-mantle melts are (i) the changes in atomic packing
SUPPLEMENTARY INFORMATION doi:10.1038/nature11294 Review of previous works on deep-liquid properties The major parameters controlling the buoyancy of deep-mantle melts are (i) the changes in atomic packing
The Effect of Cr 2 O 3 on the Partial Melting of Spinel Lherzolite in the System CaO MgO Al 2 O 3 SiO 2 Cr 2 O 3 at 11 GPa
 JOURNAL OF PETROLOGY VOLUME 45 NUMBER 11 PAGES 2261 2286 2004 doi:10.1093/petrology/egh055 The Effect of Cr 2 O 3 on the Partial Melting of Spinel Lherzolite in the System CaO MgO Al 2 O 3 SiO 2 Cr 2 O
JOURNAL OF PETROLOGY VOLUME 45 NUMBER 11 PAGES 2261 2286 2004 doi:10.1093/petrology/egh055 The Effect of Cr 2 O 3 on the Partial Melting of Spinel Lherzolite in the System CaO MgO Al 2 O 3 SiO 2 Cr 2 O
Plume-Associated Ultramafic Magmas of Phanerozoic Age
 JOURNAL OF PETROLOGY VOLUME 43 NUMBER 10 PAGES 1857 1883 2002 Plume-Associated Ultramafic Magmas of Phanerozoic Age C. HERZBERG 1 AND M. J. O HARA 2 1 DEPARTMENT OF GEOLOGICAL SCIENCES, RUTGERS UNIVERSITY,
JOURNAL OF PETROLOGY VOLUME 43 NUMBER 10 PAGES 1857 1883 2002 Plume-Associated Ultramafic Magmas of Phanerozoic Age C. HERZBERG 1 AND M. J. O HARA 2 1 DEPARTMENT OF GEOLOGICAL SCIENCES, RUTGERS UNIVERSITY,
Composition of primary kimberlite magma: constraints from melting and diamond dissolution experiments
 Contrib Mineral Petrol (2015) 170:26 DOI 10.1007/s00410-015-1182-z ORIGINAL PAPER Composition of primary kimberlite magma: constraints from melting and diamond dissolution experiments A. G. Sokol 1,2 A.
Contrib Mineral Petrol (2015) 170:26 DOI 10.1007/s00410-015-1182-z ORIGINAL PAPER Composition of primary kimberlite magma: constraints from melting and diamond dissolution experiments A. G. Sokol 1,2 A.
The effect of liquid composition on the partitioning of Ni between olivine and silicate melt
 Contrib Mineral Petrol (2017) 172:3 DOI 10.1007/s00410-016-1319-8 ORIGINAL PAPER The effect of liquid composition on the partitioning of between olivine and silicate melt Andrew K. Matzen 1,2,3 Michael
Contrib Mineral Petrol (2017) 172:3 DOI 10.1007/s00410-016-1319-8 ORIGINAL PAPER The effect of liquid composition on the partitioning of between olivine and silicate melt Andrew K. Matzen 1,2,3 Michael
Notes for Use of the Cpx-Plag-Ol Thermobar Workbook Last Updated:
 Notes for Use of the Cpx-Plag-Ol Thermobar Workbook Last Updated: 7-22-05 Cpx-Plag-Ol Thermobar is an Excel workbook that can be used to calculate crystallization pressures and temperatures for clinopyroxene-
Notes for Use of the Cpx-Plag-Ol Thermobar Workbook Last Updated: 7-22-05 Cpx-Plag-Ol Thermobar is an Excel workbook that can be used to calculate crystallization pressures and temperatures for clinopyroxene-
An Experimental Study of Water in Nominally AnhydrousMineralsintheUpperMantlenear the Water-saturated Solidus
 JOURNAL OF PETROLOGY VOLUME 53 NUMBER10 PAGES 2067^2093 2012 doi:10.1093/petrology/egs044 An Experimental Study of Water in Nominally AnhydrousMineralsintheUpperMantlenear the Water-saturated Solidus ISTVA
JOURNAL OF PETROLOGY VOLUME 53 NUMBER10 PAGES 2067^2093 2012 doi:10.1093/petrology/egs044 An Experimental Study of Water in Nominally AnhydrousMineralsintheUpperMantlenear the Water-saturated Solidus ISTVA
Subsolidus and melting experiments of a K-rich basaltic composition to 27 GPa: Implication for the behavior of potassium in the mantle
 American Mineralogist, Volume 84, pages 357 361, 1999 Subsolidus and melting experiments of a K-rich basaltic composition to 27 GPa: Implication for the behavior of potassium in the mantle WUYI WANG* AND
American Mineralogist, Volume 84, pages 357 361, 1999 Subsolidus and melting experiments of a K-rich basaltic composition to 27 GPa: Implication for the behavior of potassium in the mantle WUYI WANG* AND
TWO COMPONENT (BINARY) PHASE DIAGRAMS. Experimental Determination of 2-Component Phase Diagrams
 Page 1 of 12 EENS 211 Earth Materials Tulane University Prof. Stephen A. Nelson TWO COMPONENT (BINARY) PHASE DIAGRAMS This document last updated on 08-Oct-2003 Experimental Determination of 2-Component
Page 1 of 12 EENS 211 Earth Materials Tulane University Prof. Stephen A. Nelson TWO COMPONENT (BINARY) PHASE DIAGRAMS This document last updated on 08-Oct-2003 Experimental Determination of 2-Component
Laboratory Electrical Conductivity Measurement of Mantle Minerals
 Laboratory Electrical Conductivity Measurement of Mantle Minerals Takashi Yoshino Institute for Study of the Earth s Interior, Okayama University Outline 1. Brief introduction 2. Conduction mechanisms
Laboratory Electrical Conductivity Measurement of Mantle Minerals Takashi Yoshino Institute for Study of the Earth s Interior, Okayama University Outline 1. Brief introduction 2. Conduction mechanisms
LAB 9: ULTRAMAFIC ROCKS, CUMULATES AND MELT SOURCES
 Geology 316 (Petrology) (03/26/2012) Name LAB 9: ULTRAMAFIC ROCKS, CUMULATES AND MELT SOURCES INTRODUCTION Ultramafic rocks are igneous rocks containing less than 10% felsic minerals (quartz + feldspars
Geology 316 (Petrology) (03/26/2012) Name LAB 9: ULTRAMAFIC ROCKS, CUMULATES AND MELT SOURCES INTRODUCTION Ultramafic rocks are igneous rocks containing less than 10% felsic minerals (quartz + feldspars
EMMR25 Mineralogy: Ol + opx + chlorite + cpx + amphibole + serpentine + opaque
 GSA Data Repository 2017365 Marshall et al., 2017, The role of serpentinite derived fluids in metasomatism of the Colorado Plateau (USA) lithospheric mantle: Geology, https://doi.org/10.1130/g39444.1 Appendix
GSA Data Repository 2017365 Marshall et al., 2017, The role of serpentinite derived fluids in metasomatism of the Colorado Plateau (USA) lithospheric mantle: Geology, https://doi.org/10.1130/g39444.1 Appendix
Description of Supplementary Files
 Description of Supplementary Files File Name: Supplementary Information Description: Supplementary Figures, Supplementary Tables and Supplementary References File Name: Peer Review File Supplementary figure
Description of Supplementary Files File Name: Supplementary Information Description: Supplementary Figures, Supplementary Tables and Supplementary References File Name: Peer Review File Supplementary figure
Anderson RN, Uyeda S, Miyashiro A. (1976) Geophysical and geochemical constraints
 REFERENCES RELEVANT TO PERIDOTITE PARTIAL MELTING IN THE MANTLE WEDGE Anderson RN, Uyeda S, Miyashiro A. (1976) Geophysical and geochemical constraints at converging plate boundaries - I: Dehydration in
REFERENCES RELEVANT TO PERIDOTITE PARTIAL MELTING IN THE MANTLE WEDGE Anderson RN, Uyeda S, Miyashiro A. (1976) Geophysical and geochemical constraints at converging plate boundaries - I: Dehydration in
WATER IN THE EARTH S MANTLE:
 WATER IN THE EARTH S MANTLE: NATHALIE BOLFAN-CASANOVA LABORATOIRE MAGMAS ET VOLCANS => Heterogeneous distribution of water in the mantle > 10000 pmm wt H2O ~ 10000 pmm wt H2O ~ 1000 pmm wt H2O On what
WATER IN THE EARTH S MANTLE: NATHALIE BOLFAN-CASANOVA LABORATOIRE MAGMAS ET VOLCANS => Heterogeneous distribution of water in the mantle > 10000 pmm wt H2O ~ 10000 pmm wt H2O ~ 1000 pmm wt H2O On what
(Received 26 May 1988; revised typescript accepted 10 March 1989)
 22-3530/90 $3. Reaction Between Ultramafic Rock and Fractionating Basaltic Magma II. Experimental Investigation of Reaction Between Olivine Tholeiite and Harzburgite at 1150-1050 Cand5kb by PETER B. KELEMEN
22-3530/90 $3. Reaction Between Ultramafic Rock and Fractionating Basaltic Magma II. Experimental Investigation of Reaction Between Olivine Tholeiite and Harzburgite at 1150-1050 Cand5kb by PETER B. KELEMEN
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115, B05205, doi: /2009jb006457, 2010
 Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115,, doi:10.1029/2009jb006457, 2010 Experimentally dictated stability of carbonated oceanic crust to moderately great depths in the Earth:
Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115,, doi:10.1029/2009jb006457, 2010 Experimentally dictated stability of carbonated oceanic crust to moderately great depths in the Earth:
Temperature and pressure effects on the partitioning of V and Sc between clinopyroxene and silicate melt: Implications for mantle oxygen fugacity
 1 1 2 3 Temperature and pressure effects on the partitioning of V and Sc between clinopyroxene and silicate melt: Implications for mantle oxygen fugacity 4 5 6 7 8 YUAN LI 1,2* 1 State Key Laboratory of
1 1 2 3 Temperature and pressure effects on the partitioning of V and Sc between clinopyroxene and silicate melt: Implications for mantle oxygen fugacity 4 5 6 7 8 YUAN LI 1,2* 1 State Key Laboratory of
Continental Alkaline Magmatism. The East African Rift
 Announcements No lecture on Friday Lab final begins at 1 PM Today s agenda: Lecture/Demo Go through field trip pics Call for pizza Lab review Lecture Review Make poster Continental Alkaline Magmatism.
Announcements No lecture on Friday Lab final begins at 1 PM Today s agenda: Lecture/Demo Go through field trip pics Call for pizza Lab review Lecture Review Make poster Continental Alkaline Magmatism.
Worked Example of Batch Melting: Rb and Sr
 Worked Example of Batch Melting: Rb and Sr Basalt with the mode: Table 9.2. Conversion from mode to weight percent Mineral Mode Density Wt prop Wt% ol 15 3.6 54 0.18 cpx 33 3.4 112.2 0.37 plag 51 2.7 137.7
Worked Example of Batch Melting: Rb and Sr Basalt with the mode: Table 9.2. Conversion from mode to weight percent Mineral Mode Density Wt prop Wt% ol 15 3.6 54 0.18 cpx 33 3.4 112.2 0.37 plag 51 2.7 137.7
Conditions of core formation in the Earth: Constraints from Nickel and Cobalt partitioning
 doi:10.1016/j.gca.2004.10.019 Geochimica et Cosmochimica Acta, Vol. 69, No. 8, pp. 2141 2151, 2005 Copyright 2005 Elsevier Ltd Printed in the USA. All rights reserved 0016-7037/05 $30.00.00 Conditions
doi:10.1016/j.gca.2004.10.019 Geochimica et Cosmochimica Acta, Vol. 69, No. 8, pp. 2141 2151, 2005 Copyright 2005 Elsevier Ltd Printed in the USA. All rights reserved 0016-7037/05 $30.00.00 Conditions
Partial melting experiments of bimineralic eclogite and the origin of ocean island basalts
 Partial melting experiments of bimineralic eclogite and the origin of ocean island basalts Tetsu Kogiso 1 and Marc M. Hirschmann 2 1 Research Program for Geochemical Evolution, Institute for Frontier Research
Partial melting experiments of bimineralic eclogite and the origin of ocean island basalts Tetsu Kogiso 1 and Marc M. Hirschmann 2 1 Research Program for Geochemical Evolution, Institute for Frontier Research
The mantle metasomatism: diversity and impact What the mantle xenoliths tell us?
 The mantle metasomatism: diversity and impact What the mantle xenoliths tell us? Mantle metasomatism Physical and chemical processes that are implemented during the flow of magmas and / or fluids within
The mantle metasomatism: diversity and impact What the mantle xenoliths tell us? Mantle metasomatism Physical and chemical processes that are implemented during the flow of magmas and / or fluids within
12 Chemistry (Mg,Fe) 2 SiO 4 Olivine is forms what is called an isomorphous solid solution series that ranges between two end members: Forsterite Mg
 11 Olivine Structure Olivine is a common green or brown rock forming minerals which consists of a solid-solution series between Forsterite (Fo) and Fayalite (Fa). It is an orthorhombic orthosilicate with
11 Olivine Structure Olivine is a common green or brown rock forming minerals which consists of a solid-solution series between Forsterite (Fo) and Fayalite (Fa). It is an orthorhombic orthosilicate with
Geochemical and mineralogical technics to investigate the lithosphere and the asthenosphere. 07/11/2017 GEO-DEEP 9300 Claire Aupart
 Geochemical and mineralogical technics to investigate the lithosphere and the asthenosphere 07/11/2017 GEO-DEEP 9300 Claire Aupart Introduction Introduction Getting samples Cores: Maximum depth reach in
Geochemical and mineralogical technics to investigate the lithosphere and the asthenosphere 07/11/2017 GEO-DEEP 9300 Claire Aupart Introduction Introduction Getting samples Cores: Maximum depth reach in
Origin of lunar ultramafic green glasses: Constraints from phase equilibrium studies
 Pergamon PII S0016-7037(00)00365-3 Geochimica et Cosmochimica Acta, Vol. 64, No. 13, pp. 2339 2350, 2000 Copyright 2000 Elsevier Science Ltd Printed in the USA. All rights reserved 0016-7037/00 $20.00.00
Pergamon PII S0016-7037(00)00365-3 Geochimica et Cosmochimica Acta, Vol. 64, No. 13, pp. 2339 2350, 2000 Copyright 2000 Elsevier Science Ltd Printed in the USA. All rights reserved 0016-7037/00 $20.00.00
Lecture 36. Igneous geochemistry
 Lecture 36 Igneous geochemistry Reading - White Chapter 7 Today 1. Overview 2. solid-melt distribution coefficients Igneous geochemistry The chemistry of igneous systems provides clues to a number of important
Lecture 36 Igneous geochemistry Reading - White Chapter 7 Today 1. Overview 2. solid-melt distribution coefficients Igneous geochemistry The chemistry of igneous systems provides clues to a number of important
Fluids, melts, and supercriticality in the MSH system and element transport in subduction zones
 cosmic rays Fluids, s, and supercriticality in the MSH system and element transport in subduction zones 10 Be volcanic front N, O 10 Be ocean water + CO 2 tracing petrologic and geotectonic processes (trace)
cosmic rays Fluids, s, and supercriticality in the MSH system and element transport in subduction zones 10 Be volcanic front N, O 10 Be ocean water + CO 2 tracing petrologic and geotectonic processes (trace)
Near-solidus Melting of the Shallow Upper Mantle: Partial Melting Experiments on Depleted Peridotite
 JOURNAL OF PETROLOGY VOLUME 44 NUMBER 7 PAGES 1163±1191 2003 Near-solidus Melting of the Shallow Upper Mantle: Partial Melting Experiments on Depleted Peridotite LAURA E. WASYLENKI*, MICHAEL B. BAKER,
JOURNAL OF PETROLOGY VOLUME 44 NUMBER 7 PAGES 1163±1191 2003 Near-solidus Melting of the Shallow Upper Mantle: Partial Melting Experiments on Depleted Peridotite LAURA E. WASYLENKI*, MICHAEL B. BAKER,
The Effect of H 2 O on the 410-km Seismic Discontinuity
 The Effect of H 2 O on the 410-km Seismic Discontinuity B.J. Wood Science Paper (1995) Presented by HuajianYao Background Seismic discontinuities at 410 km and 660 km ------ important jumps in mantle density
The Effect of H 2 O on the 410-km Seismic Discontinuity B.J. Wood Science Paper (1995) Presented by HuajianYao Background Seismic discontinuities at 410 km and 660 km ------ important jumps in mantle density
Fate of Pyroxenite-derived Melts in the Peridotitic Mantle: Thermodynamic and Experimental Constraints
 JOURNAL OF PETROLOGY VOLUME 53 NUMBER 3 PAGES451^476 2012 doi:10.1093/petrology/egr068 Fate of Pyroxenite-derived Melts in the Peridotitic Mantle: Thermodynamic and Experimental Constraints S. LAMBART
JOURNAL OF PETROLOGY VOLUME 53 NUMBER 3 PAGES451^476 2012 doi:10.1093/petrology/egr068 Fate of Pyroxenite-derived Melts in the Peridotitic Mantle: Thermodynamic and Experimental Constraints S. LAMBART
TRACE ELEMENT ANALYSIS OF DIAMOND BY LAM ICPMS: STANDARDISATION, RESULTS AND DIRECTIONS
 TRACE ELEMENT ANALYSIS OF DIAMOND BY LAM ICPMS: STANDARDISATION, RESULTS AND DIRECTIONS W.L. Griffin 1, 3, Sonal Rege 1, Rondi M. Davies 1, 2, Simon Jackson 1, Suzanne Y. O Reilly 1 1.ARC National Key
TRACE ELEMENT ANALYSIS OF DIAMOND BY LAM ICPMS: STANDARDISATION, RESULTS AND DIRECTIONS W.L. Griffin 1, 3, Sonal Rege 1, Rondi M. Davies 1, 2, Simon Jackson 1, Suzanne Y. O Reilly 1 1.ARC National Key
A DISSERTATION SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL OF THE UNIVERSITY OF MINNESOTA BY. Frederick Arthur Davis
 The role of partial melts of peridotite in the formation of oceanic island basalts A DISSERTATION SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL OF THE UNIVERSITY OF MINNESOTA BY Frederick Arthur Davis
The role of partial melts of peridotite in the formation of oceanic island basalts A DISSERTATION SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL OF THE UNIVERSITY OF MINNESOTA BY Frederick Arthur Davis
Silica enrichment in the continental upper mantle via melt=rock reaction
 ELSEVIER Earth and Planetary Science Letters 164 (1998) 387 406 Silica enrichment in the continental upper mantle via melt=rock reaction Peter B. Kelemen a,ł,stanleyr.hart a, Stefan Bernstein b a Woods
ELSEVIER Earth and Planetary Science Letters 164 (1998) 387 406 Silica enrichment in the continental upper mantle via melt=rock reaction Peter B. Kelemen a,ł,stanleyr.hart a, Stefan Bernstein b a Woods
Ch 6: Internal Constitution of the Earth
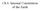 Ch 6: Internal Constitution of the Earth Mantle composition Geological background 88 elements found in the Earth's crust -- of these, only 8 make up 98%: oxygen, silicon, aluminum, iron, calcium, magnesium,
Ch 6: Internal Constitution of the Earth Mantle composition Geological background 88 elements found in the Earth's crust -- of these, only 8 make up 98%: oxygen, silicon, aluminum, iron, calcium, magnesium,
Petrology. Petrology: the study of rocks, especially aspects such as physical, chemical, spatial and chronoligic. Associated fields include:
 Petrology Petrology: the study of rocks, especially aspects such as physical, chemical, spatial and chronoligic. Associated fields include: Petrography: study of description and classification of rocks
Petrology Petrology: the study of rocks, especially aspects such as physical, chemical, spatial and chronoligic. Associated fields include: Petrography: study of description and classification of rocks
Origin of Basaltic Magma. Geology 346- Petrology
 Origin of Basaltic Magma Geology 346- Petrology 2 principal types of basalt in the ocean basins Tholeiitic Basalt and Alkaline Basalt Table 10-1 Common petrographic differences between tholeiitic and alkaline
Origin of Basaltic Magma Geology 346- Petrology 2 principal types of basalt in the ocean basins Tholeiitic Basalt and Alkaline Basalt Table 10-1 Common petrographic differences between tholeiitic and alkaline
Melting Behavior of Simplified Lherzolite in the System CaO-MgO-Al 2 O 3 -SiO 2 -Na 2 O from 7 to 35 kbar
 Melting Behavior of Simplified Lherzolite in the System --Al 2 O 3 - -Na 2 O from 7 to 35 kbar by MICHAEL J. WALTER* AND DEAN C. PRESNALL Center for Lithospheric Studies, University of Texas at Dallas,
Melting Behavior of Simplified Lherzolite in the System --Al 2 O 3 - -Na 2 O from 7 to 35 kbar by MICHAEL J. WALTER* AND DEAN C. PRESNALL Center for Lithospheric Studies, University of Texas at Dallas,
Q. WANG, Q-K. XIA, S. Y. O REILLY, W. L. GRIFFIN, E. E. BEYER AND H. K. BRUECKNER
 Pressure- and stress-induced fabric transition in olivine from peridotites in the Western Gneiss Region (Norway): implications for mantle seismic anisotropy Q. WANG, Q-K. XIA, S. Y. O REILLY, W. L. GRIFFIN,
Pressure- and stress-induced fabric transition in olivine from peridotites in the Western Gneiss Region (Norway): implications for mantle seismic anisotropy Q. WANG, Q-K. XIA, S. Y. O REILLY, W. L. GRIFFIN,
- low FeO*/MgO is controlled by the reaction relation oliv + liq! opx.
 Mantle melting trend to high-sio 2 - low FeO*/MgO is controlled by the reaction relation oliv + liq! opx. 4 3 2 1 Ol+Opx +Cpx+Sp +liq 79-35g 82-66 87S35 85-44 85-41c -ol +opx Mantle melting/reaction 0
Mantle melting trend to high-sio 2 - low FeO*/MgO is controlled by the reaction relation oliv + liq! opx. 4 3 2 1 Ol+Opx +Cpx+Sp +liq 79-35g 82-66 87S35 85-44 85-41c -ol +opx Mantle melting/reaction 0
Chapter 6: The Phase Rule and One and Two-Component Systems aka Phase Equilibria
 Chapter 6: The Phase Rule and One and Two-Component Systems aka Phase Equilibria Makaopuhi Lava Lake Magma samples recovered from various depths beneath solid crust From Wright and Okamura, (1977) USGS
Chapter 6: The Phase Rule and One and Two-Component Systems aka Phase Equilibria Makaopuhi Lava Lake Magma samples recovered from various depths beneath solid crust From Wright and Okamura, (1977) USGS
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/332/6025/88/dc1 Supporting Online Material for Microtomography of Partially Molten Rocks: Three-Dimensional Melt Distribution in Mantle Peridotite Wenlu Zhu, * Glenn
www.sciencemag.org/cgi/content/full/332/6025/88/dc1 Supporting Online Material for Microtomography of Partially Molten Rocks: Three-Dimensional Melt Distribution in Mantle Peridotite Wenlu Zhu, * Glenn
Experimental petrology of peridotites, including effects of water and carbon on melting in the Earth s upper mantle
 DOI 10.1007/s00269-014-0729-2 ORIGINAL PAPER Experimental petrology of peridotites, including effects of water and carbon on melting in the Earth s upper mantle David H. Green Received: 8 October 2014
DOI 10.1007/s00269-014-0729-2 ORIGINAL PAPER Experimental petrology of peridotites, including effects of water and carbon on melting in the Earth s upper mantle David H. Green Received: 8 October 2014
Why cold slabs stagnate in the transition zone
 GSA Data Repository 2015085 Model 1 Model 2 Model 3 Model 4 Model 5 Model 6 Why cold slabs stagnate in the transition zone Scott D. King 1,2, Daniel J. Frost 2, and David C. Rubie 2 1 Department of Geosciences,
GSA Data Repository 2015085 Model 1 Model 2 Model 3 Model 4 Model 5 Model 6 Why cold slabs stagnate in the transition zone Scott D. King 1,2, Daniel J. Frost 2, and David C. Rubie 2 1 Department of Geosciences,
Trace Elements. Today s lecture
 Trace Elements 300 Ni 200 ppm 100 0 300 Zr 200 100 0 40 50 60 70 80 SiO 2 wt. % Updates: M&M due date: Tuesday Today s lecture Topics: Trace element compositions Trace element behavior Partitioning Spider(
Trace Elements 300 Ni 200 ppm 100 0 300 Zr 200 100 0 40 50 60 70 80 SiO 2 wt. % Updates: M&M due date: Tuesday Today s lecture Topics: Trace element compositions Trace element behavior Partitioning Spider(
The influence of H 2 O on mantle wedge melting
 Earth and Planetary Science Letters 249 (2006) 74 89 www.elsevier.com/locate/epsl The influence of H 2 O on mantle wedge melting Timothy L. Grove, Nilanjan Chatterjee, Stephen W. Parman, Etienne Médard
Earth and Planetary Science Letters 249 (2006) 74 89 www.elsevier.com/locate/epsl The influence of H 2 O on mantle wedge melting Timothy L. Grove, Nilanjan Chatterjee, Stephen W. Parman, Etienne Médard
Interpreting Phase Diagrams
 Interpreting Phase Diagrams Understanding chemical reactions requires that we know something about how materials behave as the temperature and pressure change. For a single component (like quartz or ice)
Interpreting Phase Diagrams Understanding chemical reactions requires that we know something about how materials behave as the temperature and pressure change. For a single component (like quartz or ice)
doi: /nature09940
 LETTER doi:10.1038/nature09940 Spin crossover and iron-rich silicate melt in the Earth s deep mantle Ryuichi Nomura 1,2, Haruka Ozawa 1,3, Shigehiko Tateno 1, Kei Hirose 1,3, John Hernlund 4, Shunsuke
LETTER doi:10.1038/nature09940 Spin crossover and iron-rich silicate melt in the Earth s deep mantle Ryuichi Nomura 1,2, Haruka Ozawa 1,3, Shigehiko Tateno 1, Kei Hirose 1,3, John Hernlund 4, Shunsuke
Available online at
 Available online at www.sciencedirect.com Geochimica et osmochimica Acta 102 (2013) 191 212 www.elsevier.com/locate/gca arbon solution and partitioning between metallic and silicate melts in a shallow
Available online at www.sciencedirect.com Geochimica et osmochimica Acta 102 (2013) 191 212 www.elsevier.com/locate/gca arbon solution and partitioning between metallic and silicate melts in a shallow
Diamonds and the Geology of Mantle Carbon
 Diamonds and the Geology of Mantle Carbon Steven B. Shirey 1 Pierre Cartigny 2 Daniel J. Frost 3 Shantanu Keshav 4 Fabrizio Nestola 5 Paolo Nimis 5 D. Graham Pearson 6 Nikolai V. Sobolev 7 Michael J. Walter
Diamonds and the Geology of Mantle Carbon Steven B. Shirey 1 Pierre Cartigny 2 Daniel J. Frost 3 Shantanu Keshav 4 Fabrizio Nestola 5 Paolo Nimis 5 D. Graham Pearson 6 Nikolai V. Sobolev 7 Michael J. Walter
INTRODUCTION PART 1: EVALUATION OF SINGLE-CLINOPYROXENE GEOBAROMETRY
 WHAT GARNET, CLINOPYROXENE AND DIAMOND POTENTIAL CAN TELL US ABOUT THE EVOLUTION OF SUB-CRATONIC MANTLE SECTIONS: A CASE STUDY OF THE ZAGADOCHNAYA KIMBERLITE (YAKUTIA) LUCA ZIBERNA Dipartimento di Geoscienze,
WHAT GARNET, CLINOPYROXENE AND DIAMOND POTENTIAL CAN TELL US ABOUT THE EVOLUTION OF SUB-CRATONIC MANTLE SECTIONS: A CASE STUDY OF THE ZAGADOCHNAYA KIMBERLITE (YAKUTIA) LUCA ZIBERNA Dipartimento di Geoscienze,
Partitioning of U and Th during garnet pyroxenite partial melting: Constraints on the source of alkaline ocean island basalts
 Available online at www.sciencedirect.com Earth and Planetary Science Letters 265 (2008) 270 286 www.elsevier.com/locate/epsl Partitioning of U and Th during garnet pyroxenite partial melting: Constraints
Available online at www.sciencedirect.com Earth and Planetary Science Letters 265 (2008) 270 286 www.elsevier.com/locate/epsl Partitioning of U and Th during garnet pyroxenite partial melting: Constraints
Ultramafic rocks. Types of Ultramafic Rocks. Spinel lherzolite xenolith
 Ultramafic rocks Definition: Color Index > 90, i.e., less than 10% felsic minerals. Not to be confused with Ultrabasic Rocks which are rocks with
Ultramafic rocks Definition: Color Index > 90, i.e., less than 10% felsic minerals. Not to be confused with Ultrabasic Rocks which are rocks with
The application of olivine geothermometry to infer crystallization temperatures of parental liquids: Implications for the temperature of MORB magmas
 Chemical Geology 241 (2007) 207 233 www.elsevier.com/locate/chemgeo The application of olivine geothermometry to infer crystallization temperatures of parental liquids: Implications for the temperature
Chemical Geology 241 (2007) 207 233 www.elsevier.com/locate/chemgeo The application of olivine geothermometry to infer crystallization temperatures of parental liquids: Implications for the temperature
Lecture 12 COMPLEX MELTING MODELS. (see books by Shaw, Trace Elements in Magmas (2006) and Zou, Quantitative Geochemistry (2007))
 Lecture 12 COMPLEX MELTING MODELS (see books by Shaw, Trace Elements in Magmas (2006) and Zou, Quantitative Geochemistry (2007)) Thus far we have considered two end-member melting models, batch melting
Lecture 12 COMPLEX MELTING MODELS (see books by Shaw, Trace Elements in Magmas (2006) and Zou, Quantitative Geochemistry (2007)) Thus far we have considered two end-member melting models, batch melting
Compositional relationships between meteorites and planets I. Kevin Righter NASA Johnson Space Center
 Compositional relationships between meteorites and planets I Kevin Righter NASA Johnson Space Center Accretion models for terrestrial planets Can we make planets from meteorites? What are the outstanding
Compositional relationships between meteorites and planets I Kevin Righter NASA Johnson Space Center Accretion models for terrestrial planets Can we make planets from meteorites? What are the outstanding
SUPPLEMENTARY INFORMATION
 GSA Data Repository 080 Schorn et al., 08, Thermal buffering in the orogenic crust: Geology, https://doi.org/0.30/g4046.. SUPPLEMENTARY INFORMATION 3 PHASE DIAGRAM MODELING 4 5 6 7 8 9 0 3 4 Phase diagrams
GSA Data Repository 080 Schorn et al., 08, Thermal buffering in the orogenic crust: Geology, https://doi.org/0.30/g4046.. SUPPLEMENTARY INFORMATION 3 PHASE DIAGRAM MODELING 4 5 6 7 8 9 0 3 4 Phase diagrams
GEOL 2312 Igneous and Metamorphic Petrology Spring 2009 Sc ore / 40
 GEOL 2312 Igneous and Metamorphic Petrology Name Spring 2009 Sc ore / 40 QUIZ 3 1) Name two geologic features that provide physical evidence for the mineralogy of the earth s mantle (2 pts) Ophiolites,
GEOL 2312 Igneous and Metamorphic Petrology Name Spring 2009 Sc ore / 40 QUIZ 3 1) Name two geologic features that provide physical evidence for the mineralogy of the earth s mantle (2 pts) Ophiolites,
A Reappraisal of Redox Melting in the Earth s Mantle as a Function of Tectonic Setting and Time
 JOURNAL OF PETROLOGY VOLUME 52 NUMBERS 7 & 8 PAGES1363^1391 2011 doi:10.1093/petrology/egq061 A Reappraisal of Redox Melting in the Earth s Mantle as a Function of Tectonic Setting and Time STEPHEN F.
JOURNAL OF PETROLOGY VOLUME 52 NUMBERS 7 & 8 PAGES1363^1391 2011 doi:10.1093/petrology/egq061 A Reappraisal of Redox Melting in the Earth s Mantle as a Function of Tectonic Setting and Time STEPHEN F.
Phase Equilibrium. Phase Rule. Phase Diagram
 Phase Equilibrium Phase Rule Phase Diagram Makaopuhi Lava Lake Magma samples recovered from various depths beneath solid crust From Wright and Okamura, (1977) USGS Prof. Paper, 1004. Makaopuhi Lava Lake
Phase Equilibrium Phase Rule Phase Diagram Makaopuhi Lava Lake Magma samples recovered from various depths beneath solid crust From Wright and Okamura, (1977) USGS Prof. Paper, 1004. Makaopuhi Lava Lake
Geochemical constraints on the core formation and composition
 Geochemical constraints on the core formation and composition Bernard Bourdon ENS Lyon with: Mathieu Touboul, Caroline Fitoussi, John Rudge and Thorsten Kleine Collège de France November 25 th Core formation
Geochemical constraints on the core formation and composition Bernard Bourdon ENS Lyon with: Mathieu Touboul, Caroline Fitoussi, John Rudge and Thorsten Kleine Collège de France November 25 th Core formation
Supporting Information
 Supporting Information Bindi et al. 10.1073/pnas.1111115109 Fig. S1. Electron microprobe X-ray elemental maps for the grain reported in Fig. 1B. Experimental details are given in Experimental Methods.
Supporting Information Bindi et al. 10.1073/pnas.1111115109 Fig. S1. Electron microprobe X-ray elemental maps for the grain reported in Fig. 1B. Experimental details are given in Experimental Methods.
Pyroxenes (Mg, Fe 2+ ) 2 Si 2 O 6 (monoclinic) and. MgSiO 3 FeSiO 3 (orthorhombic) Structure (Figure 2 of handout)
 Pyroxenes (Mg, Fe 2+ ) 2 Si 2 O 6 (monoclinic) and 20 MgSiO 3 FeSiO 3 (orthorhombic) Structure (Figure 2 of handout) Chain silicate eg Diopside Mg and Fe ions link SiO 3 chains The chain runs up and down
Pyroxenes (Mg, Fe 2+ ) 2 Si 2 O 6 (monoclinic) and 20 MgSiO 3 FeSiO 3 (orthorhombic) Structure (Figure 2 of handout) Chain silicate eg Diopside Mg and Fe ions link SiO 3 chains The chain runs up and down
RECEIVED SEPTEMBER 1, 2003; ACCEPTED APRIL 8, 2004 ADVANCE ACCESS PUBLICATION AUGUST 19, 2004
 JOURNAL OF PETROLOGY VOLUME 45 NUMBER 12 PAGES 2369 2388 2004 doi:10.1093/petrology/egh042 The Liquid Line of Descent of Anhydrous, Mantle-Derived, Tholeiitic Liquids by Fractional and Equilibrium Crystallization
JOURNAL OF PETROLOGY VOLUME 45 NUMBER 12 PAGES 2369 2388 2004 doi:10.1093/petrology/egh042 The Liquid Line of Descent of Anhydrous, Mantle-Derived, Tholeiitic Liquids by Fractional and Equilibrium Crystallization
Structure of the Earth and the Origin of Magmas
 Page 1 of 12 EENS 2120 Petrology Tulane University Prof. Stephen A. Nelson Structure of the Earth and the Origin of Magmas This document last updated on 23-Jan-2015 Magmas do not form everywhere beneath
Page 1 of 12 EENS 2120 Petrology Tulane University Prof. Stephen A. Nelson Structure of the Earth and the Origin of Magmas This document last updated on 23-Jan-2015 Magmas do not form everywhere beneath
Depths of Partial Crystallization of H 2 O-bearing MORB: Phase Equilibria Simulations of Basalts at the MAR near Ascension Island (7^118S)
 JOURNAL OF PETROLOGY VOLUME 49 NUMBER1 PAGES 25^45 2008 doi:10.1093/petrology/egm068 Depths of Partial Crystallization of H 2 O-bearing MORB: Phase Equilibria Simulations of Basalts at the MAR near Ascension
JOURNAL OF PETROLOGY VOLUME 49 NUMBER1 PAGES 25^45 2008 doi:10.1093/petrology/egm068 Depths of Partial Crystallization of H 2 O-bearing MORB: Phase Equilibria Simulations of Basalts at the MAR near Ascension
Differentiation 2: mantle, crust OUTLINE
 Differentiation 2: mantle, crust OUTLINE Reading this week: Should have been White Ch 10 and 11!! 7- Nov Differentiation of the Earth, Core formation W 10.6.6, 11.4 9- Nov Moon, crust, mantle, atmosphere
Differentiation 2: mantle, crust OUTLINE Reading this week: Should have been White Ch 10 and 11!! 7- Nov Differentiation of the Earth, Core formation W 10.6.6, 11.4 9- Nov Moon, crust, mantle, atmosphere
G 3. AN ELECTRONIC JOURNAL OF THE EARTH SCIENCES Published by AGU and the Geochemical Society
 Geosystems G 3 AN ELECTRONIC JOURNAL OF THE EARTH SCIENCES Published by AGU and the Geochemical Society Article Volume 5, Number 1 28 January 2004 Q01E16, doi:10.1029/2003gc000568 ISSN: 1525-2027 A hydrous
Geosystems G 3 AN ELECTRONIC JOURNAL OF THE EARTH SCIENCES Published by AGU and the Geochemical Society Article Volume 5, Number 1 28 January 2004 Q01E16, doi:10.1029/2003gc000568 ISSN: 1525-2027 A hydrous
Petrology. Petrology: the study of rocks, especially aspects such as physical, chemical, spatial and chronoligic. Classification:
 Petrology Petrology: the study of rocks, especially aspects such as physical, chemical, spatial and chronoligic. Associated fields include: Petrography: study of description and classification of rocks
Petrology Petrology: the study of rocks, especially aspects such as physical, chemical, spatial and chronoligic. Associated fields include: Petrography: study of description and classification of rocks
Lecture 13. Constraints on Melt Models Arising From Disequilibrium in the Th-U Decay System
 Lecture 13 Constraints on Melt Models Arising From Disequilibrium in the Th-U Decay System (for reference: see Uranium-Series Geochemistry, volume 52 of Reviews in Mineralogy and Geochemistry (Bourdon,
Lecture 13 Constraints on Melt Models Arising From Disequilibrium in the Th-U Decay System (for reference: see Uranium-Series Geochemistry, volume 52 of Reviews in Mineralogy and Geochemistry (Bourdon,
C = 3: Ternary Systems: Example 1: Ternary Eutectic
 Phase Equilibrium C = 3: Ternary Systems: Example 1: Ternary Eutectic Adding components, becomes increasingly difficult to depict 1-C: P - T diagrams easy 2-C: isobaric T-X, isothermal P-X 3-C:?? Still
Phase Equilibrium C = 3: Ternary Systems: Example 1: Ternary Eutectic Adding components, becomes increasingly difficult to depict 1-C: P - T diagrams easy 2-C: isobaric T-X, isothermal P-X 3-C:?? Still
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/2/3/e1501725/dc1 Supplementary Materials for Discovery of natural MgSiO3 tetragonal garnet in a shocked chondritic meteorite The PDF file includes: Naotaka Tomioka,
advances.sciencemag.org/cgi/content/full/2/3/e1501725/dc1 Supplementary Materials for Discovery of natural MgSiO3 tetragonal garnet in a shocked chondritic meteorite The PDF file includes: Naotaka Tomioka,
N = N 0 e -λt D* = N 0 -N D* = N 0 (1-e -λt ) or N(e λt -1) where N is number of parent atoms at time t, N 0
 N = N 0 e -λt D* = N 0 -N D* = N 0 (1-e -λt ) or N(e λt -1) where N is number of parent atoms at time t, N 0 is initial number of parents, D* is number of radiogenic daughter atoms, and λ is the decay
N = N 0 e -λt D* = N 0 -N D* = N 0 (1-e -λt ) or N(e λt -1) where N is number of parent atoms at time t, N 0 is initial number of parents, D* is number of radiogenic daughter atoms, and λ is the decay
Constitution of Magmas. Magmas. Gas Law. Composition. Atomic Structure of Magma. Structural Model. PV = nrt H 2 O + O -2 = 2(OH) -
 Constitution of Magmas Magmas Best, Ch. 8 Hot molten rock T = 700-1200 degrees C Composed of ions or complexes Phase Homogeneous Separable part of the system With an interface Composition Most components
Constitution of Magmas Magmas Best, Ch. 8 Hot molten rock T = 700-1200 degrees C Composed of ions or complexes Phase Homogeneous Separable part of the system With an interface Composition Most components
EXPERIMENTAL DERIVATION OF NEPHELINE SYENITE AND PHONOLITE LIQUIDS BY PARTIAL MELTING OF UPPER MANTLE PERIDOTITES
 EXPERIMENTAL DERIVATION OF NEPHELINE SYENITE AND PHONOLITE LIQUIDS BY PARTIAL MELTING OF UPPER MANTLE PERIDOTITES Didier LAPORTE, Sarah LAMBART, Pierre SCHIANO, and Luisa OTTOLINI SUPPLEMENTARY MATERIAL
EXPERIMENTAL DERIVATION OF NEPHELINE SYENITE AND PHONOLITE LIQUIDS BY PARTIAL MELTING OF UPPER MANTLE PERIDOTITES Didier LAPORTE, Sarah LAMBART, Pierre SCHIANO, and Luisa OTTOLINI SUPPLEMENTARY MATERIAL
