Temperature and pressure effects on the partitioning of V and Sc between clinopyroxene and silicate melt: Implications for mantle oxygen fugacity
|
|
|
- Alaina Horn
- 5 years ago
- Views:
Transcription
1 Temperature and pressure effects on the partitioning of V and Sc between clinopyroxene and silicate melt: Implications for mantle oxygen fugacity YUAN LI 1,2* 1 State Key Laboratory of Isotope Geochemistry, Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, Guangzhou , China 2 Bayerisches Geoinstitut, University Bayreuth, Bayreuth, Germany ABSTRACT The partition coefficients of V and Sc between clinopyroxene and silicate melt (D Cpx/SM V and D Cpx/SM Sc ) have been determined experimentally at C and GPa, using a hornblende- and clinopyroxene-rich mantle rock in graphite-lined Pt 95 Rh 05 capsules. The results show that the D Cpx/SM V and D Cpx/SM Sc values decrease from 3.8 to 2.3 and from 2.6 to 1.1, respectively, as the experimental temperature and pressure vary from 1200 C and 0.8 GPa to 1400 C and 2.3 GPa. The presence of water in silicate melts may also reduce D Cpx/SM V and D Cpx/SM Sc. These results imply that the effects of temperature, pressure, and melt water content on D V Cpx/SM should be considered when using V systematics in cratonic mantle peridotites to constrain cratonic mantle oxygen fugacity (fo 2 ). However, although the dominant V in the present silicate melt is mixed * address: Yuan.Li@gig.ac.cn
2 V 3+ and V 4+, the D Cpx/SM V /D Cpx/SM Sc together with literature data obtained at similar fo 2 shows a nearly constant value of 1.68±0.26, regardless of temperature, pressure, melt composition, and melt water content, indicating that these factors cannot cause fractionation of Sc 3+ from mixed V 3+ and V 4+ in mantle melts through clinopyroxene/silicate melt partitioning. Therefore, in combination with V/Sc systematics in primitive MORBs and arc basalts, using D Cpx/SM V and D Cpx/SM Sc obtained at 1 bar and dry conditions should be valid to constrain mantle fo 2, except for the case that the D Cpx/SM for Sc 3+ can be demonstrated to be fractionated from the D Cpx/SM for mixed V 4+ and V 5+ which are present in oxidized basalts Keywords: oxygen fugacity, upper mantle, Vanadium, Scandium, partitioning, clinopyroxene, silicate melt INTRODUCTION Oxygen fugacity (fo 2 ) is an important parameter that can significantly affect the geochemical and geophysical properties of Earth s mantle material. The fo 2 of MORBs is mainly between FMQ-1 and FMQ, and the fo 2 of arc basalts is higher than that of MORBs, as evidenced by the higher Fe 3+ / Fe in arc basalts (e.g., Frost and McCammon 2008; Kelley and Cottrell 2009, 2012; Brounce et al. 2014, 2015). However, whether the oxidized nature of arc basalts is inherited from the subarc mantle or is derived from magmatic differentiation processes occurring in the crust remains debated (e.g., Evans et al. 2012; Grocke et al. 2016; Lee et al. 2005, 2010; Brounce et al. 2015). The clear
3 correlation between Fe 3+ / Fe and water content in undegassed, olivine-hosted basaltic melt inclusions was used to argue for a high fo 2 in the subarc mantle, which could be caused by fluid fluxing of the subducted slab (Kelley and Cottrell 2012; Brounce et al. 2014, 2015). This argument is supported by measurements of spinel compositions in primitive arc lavas, which imply the subarc mantle 1-4 log units more oxidized than the oceanic mantle (Evans et al. 2012). Nevertheless, based on the partitioning of V and Sc between mantle minerals and silicate melts (D Mineral/SM ) and the similarity of V/Sc in primitive MORBs and arc basalts, Lee et al. (2005) and Mallmann and O Neill (2009) concluded that the fo 2 of the subarc mantle and the oceanic mantle is similar. However, the D Mineral/SM V and D Mineral/SM Sc data used in these two studies were calibrated for one atmosphere pressure (1 bar) and dry conditions (Canil 1997, 1999; Canil and Fedortchouk 2000; Mallmann and O Neill 2009), and fo 2 was assumed to be the only factor causing fractionation of D Mineral/SM V from D Mineral/SM Sc and thus the variation of V/Sc in primitive basalts. The effects of pressure and temperature (P-T) on D V Mineral/SM 56 and D Mineral/SM Sc remain indeed uninvestigated, but which could be important to 57 constrain mantle fo 2. For example, if P-T cause fractionation of D V Mineral/SM from D Mineral/SM Sc at a given fo 2, and if the melting P-T for the genesis of MORBs and arc basalts are not always the same, opposed to what assumed in Lee et al. (2005) and Mallmann and O Neill (2009), then the similar V/Sc in primitive MORBs and arc basalts does not necessarily imply a similar fo 2 of the subarc mantle and the oceanic mantle. Therefore, to ensure that the variation of V/Sc in primitive MORBs and arc basalts are only caused by the heterogeneity in mantle fo 2, and the similar V/Sc reflects a similar fo 2
4 of the subarc mantle and the oceanic mantle, the P-T effects on D V Mineral/SM D Mineral/SM Sc should be determined simultaneously. and In the Earth s upper mantle V and Sc are mainly stored in clinopyroxene, and the D Cpx/SM V and D Cpx/SM Sc as a function of fo 2 have been well calibrated at 1bar (Canil and Fedortchouk 2000; Mallmann and O'Neill 2009). I here therefore determine the effects of P-T on D Cpx/SM V and D Cpx/SM Sc, so as to see if variation of the mantle melting P-T can cause fractionation of V from Sc in primitive MORBs and arc basalts. Determining the P- T effects on D V Cpx/SM would also have important implications for the cratonic mantle fo 2 constrained previously using V systematics alone and D V Cpx/SM obtained at 1 bar (Canil 2002; Lee et al. 2003) EXPERIMENTAL AND ANALYTICAL MTHODS The starting material is a natural mantle rock, which stems from a hornblende- and clinopyroxene-rich, metasomatic vein in orogenic peridotite of the French Pyrenees and has a chemical composition of wt.% SiO 2, wt.% Al 2 O 3, wt.% CaO, wt.% MgO, 7.65 wt.% FeO, 3.59 wt.% TiO 2, 1.60 wt.% Na 2 O, 0.81 wt.% K 2 O, 0.05 wt.% P 2 O 5, 0.12 wt.% MnO, 0.76 wt.% H 2 O, and 0.26 wt.% CO 2 (Fabries et al. 2001; Pilet et al. 2008). The same starting silicate was used in the experiments of Pilet et al. (2008) to study the origin of alkaline magmas, and the adoption of this silicate here is to attempt to grow relatively big clinopyroxene crystals. In all experiments the starting silicate was placed into a graphite capsule (4.5 mm O.D.; 3.6 mm I.D.; 7 mm length),
5 which was then loaded into a Pt 95 Rh 05 capsule (5.0 mm O.D.; 4.6 mm I.D.; 10 mm length). All the experiments were conducted at C and GPa in an endloaded, solid media piston cylinder apparatus, using 0.5/0.75-inch diameter Talc-Pyrex assemblies with tapered graphite heaters. In order to grow clinopyroxene crystals, the experimental P-T were covaried (Table 1). The hot piston-in method was used to pressurize the assembly, and a friction correction of ~18% was applied based on calibration of the quartz-coesite and kyanite-sillimanite transitions. The pressure uncertainty is about 0.1 GPa. The temperature was monitored by Pt-Pt 90 Rh 10 (S-type) thermocouples with an uncertainty of ~10 C. All the experiments were run for 24 hours, except for run Y-4 for 48 hours (Table 1), and were quenched by switching off the electricity to the graphite heaters The major element compositions of quenched silicate melts and minerals were measured with a JEOL JXA-8200 microprobe. The analyses were performed in wavelength-dispersive mode, and a PAP matrix correction was applied to the raw data. The quenched silicate melts and minerals were analyzed with 15 kv / 10 na, with defocused beams of 30 and 10 µm diameter used, respectively, for all the standardizations and sample measurements. Both natural and synthetic standards were used to calibrate the instrument, as described in Li and Audétat (2015) Trace element analyses of V, Sc, Zr and Hf in silicate melts and minerals were carried out on an Agilent 7900 Quadrupole ICP-MS coupled to a Photon Machines Analyte HE 193-nm ArF Excimer Laser Ablation system. A squid signal smoothing device is included in this laser ablation system. Helium was applied as the carrier gas, and nitrogen gas was used as the makeup gas and mixed with the carrier gas via a T-
6 connector before entering the ICP. After measuring the gas blank for 20s, each analysis was performed by a laser beam of µm diameter for silicate minerals, and of 50 µm diameter for silicate melts, at 8 Hz with energy of ~2 J/cm 2 for 40s. NIST SRM 610 glass was used as external standard for all analyses, whereas Si determined by electron microprobe was used as internal standard. Overall analytical uncertainties arising from the internal and external standardization procedure are better than 10% for trace elements RESULTS AND DISCUSSION Experimental conditions and run products are summarized in Table 1. Representative run products are shown in Figure 1. Clinopyroxene crystals are present in all runs, with a few olivine crystals present in the runs at pressures below 1.4 GPa. The volume fraction of silicate melts in the sample capsules ranges between 40% and 90%, increasing with increasing temperature and decreasing pressure. The measured major and trace element compositions of clinopyroxene crystals and silicate melts are given in Table 1. Clinopyroxene crystals contain about wt.% Na 2 O, wt.% MgO, wt.% Al 2 O 3, wt.% SiO 2, wt.% CaO, wt.% FeO, and wt.% TiO 2. The V, Sc, Zr, and Hf contents in clinopyroxene are about ppm, ppm, ppm, and ppm, respectively. The silicate melts contain wt.% Na 2 O, wt.% MgO, wt.% Al 2 O 3, wt.% SiO 2, wt.% K 2 O, wt.% CaO, wt.% FeO, and wt.% TiO 2. The melt water content should be about 1-2 wt.%, according to
7 the degree of melting and the water content in the starting silicate. Consistent with Pilet et al. (2008), the silicate melts are typical basanitic melts. The V, Sc, Zr, and Hf contents in silicate melts are about ppm, ppm, ppm, and ppm, respectively The calculated D Cpx/SM values for V, Sc, Zr, and Hf are summarized in Table 1 and illustrated in Figure 2. The nearly identical D Cpx/SM for V and Sc in runs Y-1 and Y-4 with run duration of 24 and 48 hours, respectively, and the clear temperature dependence 137 of D Cpx/SM (see below) imply that 24 hours is sufficient to achieve equilibrium partitioning. All D Cpx/SM values, for V, for Sc, for Zr, and for Hf, decrease with increasing temperature (Fig. 2; Table 1), although the pressure varies from 0.8 to 2.3 GPa. This indicates that increasing pressure may also result in a decrease in D Cpx/SM, or that pressure does not considerably affect D Cpx/SM. Moreover, the slopes of regression of the D Cpx/SM data for V and Sc are very close to each other (Fig. 2a), as in the case for Zr and Hf (not shown), which suggests that V and Sc share very similar partitioning behavior between clinopyroxene and silicate melt, and hence that P-T do not cause fractionation of V from Sc in mantle melts through clinopyroxene/silicate melt partitioning. Previous experimental studies show that the D Cpx/SM values for Sc, Zr, and Hf all decrease with increasing temperature and/or pressure (Bédard 2014; Hill et al. 2010); thus the D Cpx/SM V should also decrease with increasing pressure The D Cpx/SM V /D Cpx/SM Sc and D Cpx/SM Zr /D Cpx/SM Hf, together with literature data, are plotted as a function of temperature in Figure 3. It can be seen from Figure 3 that the
8 8 152 D Cpx/SM Zr /D Cpx/SM Hf is nearly a constant of 0.52 ± 0.10, which is consistent with the consensus that Zr and Hf are rarely fractionated from each other during mantle melting. The D Cpx/SM V /D Cpx/SM Sc is between 1.4 and 2, and is also a constant within error (1.68± ), except for one literature value up to 3 but with a large uncertainty. It should be noted that the D Cpx/SM values for V, Sc, Zr, and Hf plotted in Figure 3 were obtained at a large range of P-T conditions ( GPa and C). Accordingly, the nearly 158 constant D Cpx/SM V /D Cpx/SM Sc (1.68 ± 0.26) again suggests that P-T may not cause fractionation of V from Sc in mantle melts through clinopyroxene/silicate melt partitioning The D Cpx/SM for trace elements is a multiple function of not only P-T but also crystal chemistry, melt composition, melt water content, and fo 2 (Bédard 2014; Canil 1997; Hill et al. 2010; Lundstrom et al. 1998; Mallmann and O Neill 2013; Michely et al. 2017; Wood and Blundy 2002). Sc is an element with a valence state of 3+ at the Earth s upper mantle conditions, and D Cpx/SM Sc is thus insensitive to the variation of mantle fo 2 (Mallmann and O'Neill 2009). However, V can be present as V 2+, V 3+, V 4+, and V 5+ in the Earth s upper mantle, and D V Cpx/SM is thus very sensitive to the variation of mantle fo 2 (Canil and Fedortchouk 2000; Mallmann and O'Neill 2009). In this study, the fo 2 was not controlled but should be around the C-CO 2 buffer because of the used graphite-lined Pt 95 Rh 05 capsule. Many studies show that the fo 2 prevailing in the experiments performed in graphite-lined Pt or Pt 95 Rh 05 capsules is ~FMQ-2 (Canil and Fedortchouk 2000; Li and Audétat 2012, 2015; Médard et al. 2008). For example, the fo 2 of an experiment
9 9 173 performed at 1200 C and 1.5 GPa in a graphite-lined Pt 95 Rh 05 capsule to study element partitioning between sulfide phases and basanitic melt was determined to be FMQ-2.1 based on Mössbauer-Spectrometry measured Fe 3+ / Fe in the basanitic melt (Li and Audétat 2012). At fo 2 of ~FMQ-2, V could be present mainly as mixed V 3+ and V 4+ in the silicate melt (Sutton et al. 2005; Righter et al. 2006). For example, the valence state of V in a Hawaiian ankaramitic basalt at 1300 C and fo 2 of ~FMQ-2 is ~3.5, determined by vanadium K-edge X-ray absorption near edge structure (XANES) spectroscopy (Righter et al. 2006). Therefore, the present study indeed demonstrates that P-T cannot fractionate mixed V 3+ and V 4+ from Sc 3+ in mantle melts through clinopyroxene/silicate melt partitioning. The presence of a considerable amount of water in silicate melt may reduce the D Cpx/SM for trace elements (Wood and Blundy 2002), which may partly explain the relatively low D Cpx/SM V and D Cpx/SM Sc values obtained in Adam and Green (2006) (Fig. 2b), in which study 6-16 wt.% water was present in the silicate melts. The effect of melt composition on D Cpx/SM cannot be readily assessed in this study, because available experiments were performed not only using different starting silicates but also at different P-T conditions (Fig. 2b). However, the location of the D Cpx/SM V and D Cpx/SM Sc data points obtained in Davis et al. (2013) on the data trend obtained in this study indicates that the effect of melt composition difference in these two studies on D Cpx/SM may just be negligible compared to that of temperature (see Fig. 2b). More importantly, the nearly 192 constant D Cpx/SM V /D Cpx/SM Sc (Fig. 3) implies that neither the variation of melt composition nor the presence of a significant amount of water in silicate melt can fractionate the D Cpx/SM for mixed V 3+ and V 4+ from the D Cpx/SM for Sc 3+.
10 The present study demonstrates that the variation of P-T, melt composition, and melt water content cannot cause fractionation of the D Cpx/SM for mixed V 3+ and V 4+ from the D Cpx/SM for Sc 3+. However, according to the calibrated V valence state as a function of fo 2 in basaltic melts (Sutton et al. 2005; Righter et al. 2006), mixed V 4+ and V 5+ can also be present in the Earth s natural basalts if the fo 2 is above FMQ. Therefore, whether the D Cpx/SM for mixed V 4+ +V 5+ can be fractionated from the D Cpx/SM for Sc 3+ due to variation of the above-mentioned factors remains to be constrained IMPLICATIONS FOR MANTLE OXYGEN FUGACITY The present study shows that the D Cpx/SM for both V and Sc is a function of temperature, pressure, and probably melt water content. Therefore, to constrain mantle fo 2 using V systematics in mantle peridotites, the effects of temperature, pressure, and melt water content on D Cpx/SM V should be considered. Canil (2002) and Lee et al. (2003) used the D Cpx/SM V data obtained at 1 bar and dry conditions to constrain the cratonic mantle fo 2 by modelling V contents in cratonic mantle peridotites. This study thus implies that the error for the cratonic mantle fo 2 constrained in these previous studies can be significant. The error for the cratonic mantle fo 2 constrained previously, introduced by using D Cpx/SM V data obtained at 1 bar and dry conditions, can be illustrated below by performing a simple calculation of V contents in cratonic mantle peridotites. Firstly, I assume partial melting of mantle peridotite occurs at 1700 C and 7 GPa and at fo 2 of FMQ-2, which corresponds to ~20% melting and which is highly possible for the genesis Cpx/SM of some cratonic mantle peridotites (Walter 1998; Canil 2002). Secondly, I use D V
11 of 1.5 and D Olivine/SM V of 0.09 obtained at ~1300 C and 1 bar (Canil 1997, Canil and Fedortchouk 2000) and D Garnet/SM V of 1 (Canil 2002), as have been used in Canil (2002) and Lee et al. (2003) at fo 2 of FMQ-2. Using the calculation method in Lee et al. (2003), the calculated V content in the cratonic mantle peridotite after melt extraction is ~64 ppm. Simply, if we now only consider the P-T effect on D Cpx/SM V and assume that the P-T variation from 1300 C and 1 bar to 1700 C and 7 GPa only decreases D Cpx/SM V by a factor of 2, then the calculated V content in the cratonic mantle peridotite is ~42 ppm. This value can only be achieved at ~FMQ-0.5 if the P-T effect on D Cpx/SM V is not taken into account (Lee et al. 2003). Note that the variation of P-T from 1200 C and 0.8 GPa to 1400 C and 2.3 GPa in this study already decreases D Cpx/SM V by factor of Therefore, these calculations demonstrate that significant error can be introduced for the estimated cratonic mantle fo 2 if the D Cpx/SM V obtained at 1300 C and 1 bar is used and if the P-T effect on D Cpx/SM V is ignored. Also, the previously estimated fo 2 for the cratonic mantles generated by high P-T melt extraction (Canil 2002; Lee et al. 2003), such as the Siberian cratonic mantle, should be considerably higher than the actual fo The present study also shows that the D Cpx/SM V /D Cpx/SM Sc does not vary as the variation of P-T, melt composition, and melt water content; in this case only the variation of fo 2 can cause fractionation of D Cpx/SM V from D Cpx/SM Sc. Therefore, V/Sc should be more robust than V alone in recording fo 2 of the mantle source regions of basalts. Consequently, the use of D Cpx/SM V and D Cpx/SM Sc obtained at 1 bar and dry conditions and the use of V/Sc in the Archean basalts and MORBs (Li and Lee 2004) should be valid to
12 constrain their mantle fo 2 (Fig. 3), considering that V in these basalts should be present mainly as mixed V 3+ and V 4+ (Sutton et al. 2005). The invariance of D Cpx/SM Cpx/SM V /D Sc also supports, at least in part, the studies of Lee et al. (2005) and Mallmann and O Neill (2009), in which the similar V/Sc in primitive MORBs and arc basalts was used to argue for a similar fo 2 in their mantle source regions, by assuming fo 2 being the only factor causing fractionation of D Cpx/SM V from D Cpx/SM Sc. The reason for not fully supporting the studies of Lee et al. (2005) and Mallmann and O Neill is due to the fact that the present study does not demonstrate non-fractionation of the D Cpx/SM for Sc 3+ from the D Cpx/SM for mixed V 4+ and V 5+ which are present in oxidized basalts with fo 2 > FMQ. Whether P-T, melt composition, and melt water content can cause fractionation of the D Cpx/SM for Sc 3+ from the D Cpx/SM for mixed V 4+ and V 5+ actually needs further investigation. In order to fully understand the link between mantle fo 2 and V/Sc systematics in primitive basalts, the D Mineral/SM for both V and Sc as a function of P-T, melt composition, and melt water content should also be determined at largely variable fo 2 s in the future. In addition, the difference of the sub-arc mantle and the oceanic mantle in modal composition or peridotite fertility should also be considered when using V/Sc systematics to constrain mantle fo 2, this is because different peridotite fertilities must result in different bulk partition coefficients for both V and Sc even if mantle partial melting takes place at the same fo ACKNOWLEGEMENTS
13 Support from the Recruitment Program of Global Young Experts (PR China) is appreciated. Constructive reviews by Maryjo Brounce, Ian Swainson, and two anonymous reviewers significantly improved this paper. 262
14 REFERENCES CITED Adam, J., and Green, T. (2006) Trace element partitioning between mica-and amphibolebearing garnet lherzolite and hydrous basanitic melt: 1. Experimental results and the investigation of controls on partitioning behaviour. Contributions to Mineralogy and Petrology, 152, Bédard, J.H. (2014) Parameterizations of calcic clinopyroxene-melt trace element partition coefficients. Geochemistry, Geophysics, Geosystems, 15, Brounce, M., Kelley, K. A., Cottrell, E. (2014) Variations in Fe 3+ / Fe of Mariana Arc Basalts and Mantle Wedge fo2. Jounral of Petrology, 55, Brounce, M., Kelley, K. A., Cottrell, E., and Reagan, M. K. (2015) Temporal evolution of mantle wedge oxygen fugacity during subduction initiation. Geology, 43, Canil, D. (1997) Vanadium partitioning and the oxidation state of Archaean komatiite magmas. Nature 389, Canil, D. (1999) Vanadium partitioning between orthopyroxene, spinel and silicate melt and the redox states of mantle source regions for primary magmas. Geochimica et Cosmochimica Acta, 63, Canil, D. (2002) Vanadium in peridotites, mantle redox and tectonic environments: Archean to present. Earth and Planetary Science Letters, 195, Canil, D., and Fedortchouk, Y. (2000) Clinopyroxene-liquid partitioning for vanadium and the oxygen fugacity during formation of cratonic and oceanic mantle lithosphere. Journal of Geophysical Research: Solid Earth, 105,
15 Davis, F. A., Humayun, M., Hirschmann, M. M., and Cooper, R. S. (2013) Experimentally determined mineral/melt partitioning of first-row transition elements (FRTE) during partial melting of peridotite at 3GPa. Geochimica et Cosmochimica Acta, 104, Evans, K.A., Elburg, M.A., and Kamenetsky, V.S. (2012) Oxidation state of subarc mantle. Geology 40, Fabries, J., Lorand, J.-P., and Guiraud, M. (2001) Petrogenesis of the amphibole-rich veins from the Lherz orogenic lherzolite massif (Eastern Pyrenees, France): a case study for the origin of orthopyroxene-bearing amphibole pyroxenites in the lithospheric mantle. Contributions to Mineralogy and Petrology, 140, Frost, D.J., and McCammon, C.A. (2008) The Redox State of Earth's Mantle. Annual Review of Earth and Planetary Sciences, 36, Grocke, S.B., Cottrell, E., de Silva, S., and Kelley, K.A. (2016) The role of crustal and eruptive processes versus source variations in controlling the oxidation state of iron in Central Andean magmas. Earth and Planetary Science Letters, 440, Hill, E., Blundy, J.D., and Wood, B.J. (2010) Clinopyroxene melt trace element partitioning and the development of a predictive model for HFSE and Sc. Contributions to Mineralogy and Petrology, 161, Kelley, K.A., and Cottrell, E. (2009) Water and the oxidation state of subduction zone magmas. Science 325,
16 Kelley, K.A., and Cottrell, E. (2012) The influence of magmatic differentiation on the oxidation state of Fe in a basaltic arc magma. Earth and Planetary Science Letters, , Lee, C.T., Leeman, W.P., Canil, D., and Li, Z. (2005) Similar V/Sc Systematics in MORB and Arc Basalts: Implications for the Oxygen Fugacities of their Mantle Source Regions. Journal of Petrology, 46, Lee, C.T., Luffi, P., Le Roux, V., Dasgupta, R., Albarede, F., and Leeman, W.P. (2010) The redox state of arc mantle using Zn/Fe systematics. Nature 468, Lee, C.-T.A., Brandon, A.D., and Norman, M. (2003) Vanadium in peridotites as a proxy for paleo-fo2 during partial melting. Geochimica et Cosmochimica Acta, 67, Li, Z.-X., and Lee, C.-T. (2004) The constancy of upper mantle fo2 through time inferred from V/Sc ratios in basalts. Earth and Planetary Science Letters, 228, Li, Y., and Audétat, A. (2015) Effects of temperature, silicate melt composition, and oxygen fugacity on the partitioning of V, Mn, Co, Ni, Cu, Zn, As, Mo, Ag, Sn, Sb, W, Au, Pb, and Bi between sulfide phases and silicate melt. Geochimica et Cosmochimica Acta, 162, Li, Y., and Audétat, A., Partitioning of V, Mn, Co, Ni, Cu, Zn, As, Mo, Ag, Sn, Sb, W, Au, Pb, and Bi between sulfide phases and hydrous basanite melt at upper mantle conditions. Earth and Planetary Science Letters, 355,
17 Lundstrom, C.C., Shaw, H.F., Ryerson, F.J., Williams, Q., and Gill, J. (1998) Crystal chemical control of clinopyroxene-melt partitioning in the Di-Ab-An system: implications for elemental fractionations in the depleted mantle. Geochimica et Cosmochimica Acta, 62, Mallmann, G., and O'Neill, H.S.C. (2009) The Crystal/Melt Partitioning of V during Mantle Melting as a Function of Oxygen Fugacity Compared with some other Elements (Al, P, Ca, Sc, Ti, Cr, Fe, Ga, Y, Zr and Nb). Journal of Petrology, 50, Médard, E., McCammon, C.A., Barr, J.A., and Grove, T.L. (2008) Oxygen fugacity, temperature reproducibility, and H 2 O contents of nominally anhydrous piston- cylinder experiments using graphite capsules. American Mineralogist, 93, Michely, L.T., Leitzke, F.P., Speelmanns, I.M., and Fonseca, R.O.C. (2017) Competing effects of crystal chemistry and silicate melt composition on trace element behavior in magmatic systems: insights from crystal/silicate melt partitioning of the REE, HFSE, Sn, In, Ga, Ba, Pt and Rh. Contributions to Mineralogy and Petrology, Pilet, S., Baker, M.B., and Stolper, E.M. (2008) Metasomatized lithosphere and the origin of alkaline lavas. Science 320, Righter, K., Sutton, S. R., Newville, M., Le, L., Schwandt, C. S., Uchida, H., Lavina, B., and Downs, R. T. (2006) An experimental study of the oxidation state of vanadium in spinel and basaltic melt with implications for the origin of planetary basalt. American Mineralogist, 91,
18 Sutton, S. R., Karner, J., Papike, J., Delaney, J. S., Shearer, C., Newville, M., Eng, P., Rivers, M., and Dyar, M. D. (2005) Vanadium K edge XANES of synthetic and natural basaltic glasses and application to microscale oxygen barometry. Geochimica et Cosmochimica Acta, 69, Walter, M.J. (1998) Melting of garnet peridotite and the origin of komatiite and depleted lithosphere. Journal of Petrology, 39, Wood, B.J., and Blundy, J.D. (2002) The effect of H 2 O on crystal-melt partitioning of trace elements. Geochimica et Cosmochimica Acta, 66,
19 FIGURE 1. Backscattered electron image of typical run products, showing coexisting silicate melt and clinopyroxene (Cpx) synthesized at 1300 C and 1.4 GPa (run W-5). Note that the dendritic material in the boundary between the clinopyroxene crystal and silicate melt was fine crystals produced from silicate melt during quench. 361
20 FIGURE 2. The variation of D V Cpx/SM and D Cpx/SM Sc as a function of experimental temperature and pressure. Note that the regression of D Cpx/SM V and D Cpx/SM Sc data in panel (a) yields similar slopes for V and Sc, indicating similar partitioning behavior of V and Sc. The relatively low D Cpx/SM V and D Cpx/SM Sc from Adam and Green (2006) in panel (b) could be partly due to the high melt water contents (see text for details). Note that all the D Cpx/SM values plotted were simultaneously determined in experiments performed in graphite-lined Pt or Pt 95 Rh 05 capsules (Adam and Green 2006; Davis et al. 2013). The silicate melt in Adam and Green (2006) is basanitic melt with 6-16 wt.% water, whereas in Davis et al. (2013) it is nominally dry basaltic melt which is in equilibrium with KLB- 1 peridotite at ~1460 C and 3 GPa.
21 FIGURE 3. The variation of D Cpx/SM V /D Cpx/SM Sc and D Cpx/SM Zr /D Cpx/SM Hf as a function of experimental temperature and pressure. The D Cpx/SM V /D Cpx/SM Sc and D Cpx/SM Zr /D Cpx/SM Hf are nearly a constant of 1.68±0.26 (excluding the data point in the dash box) and 0.52±0.1, respectively, regardless of experimental temperature, pressure, melt composition, and melt water content, indicating that these factors cannot cause fractionation of V from Sc in mantle melts, as in the case for Zr and Hf, through clinopyroxene/silicate melt partitioning. Note that the D Cpx/SM V /D Cpx/SM Sc of ~1.5 used in Li and Lee (2004), Lee et al. (2005), and Mallmann and O Neill (2009) for fo 2 at ~FMQ-2 is very close to the D Cpx/SM V /D Cpx/SM Sc obtained in this study, implying the valid use of D Cpx/SM Cpx/SM V and D Sc obtained at 1 bar and dry conditions to constrain mantle fo 2. For melt composition and water content, see Figure 2.
22 TABLE 1. Summary of experimental conditions, major element (in wt.%) and trace element (in ppm) compositions of silicate melts and clinopyroxene, and the calculated partition coefficients of trace elements between clinopyroxene and silicate melt (D Cpx/SM ). Run No T ( C) P (GPa) DV Cpx/SM DSc Cpx/SM DZr Cpx/SM DHf Cpx/SM Phase SiO 2 TiO 2 Al 2 O 3 FeO Na 2 O K 2 O CaO MgO P 2 O 5 Total V Sc Zr Hf W (0.6) 2.6(0.3) 0.58(0.17) 0.71(0.08) melt 41.01(0.39) 4.48(0.05) 14.18(0.08) 8.74(0.12) 2.35(0.19) 1.21(0.11) 16.55(0.10) 8.42(0.28) 0.09(0.01) 96.76(0.74) 192(11) 39(2) 120(6) 4.1(0.3) Cpx 48.25(0.67) 2.34(0.31) 7.11(0.52) 4.27(0.49) 0.61(0.05) <d.l 23.00(0.36) 13.83(0.64) 0.01(0.01) 99.59(0.66) 722(102) 99(12) 69(21) 2.9(0.2) Y (0.1) 2.0(0.1) n.d n.d melt 42.18(0.36) 3.90(0.05) 11.99(0.11) 7.35(0.08) 1.71(0.05) 0.90(0.05) 17.26(0.20) 11.36(0.14) 0.07(0.01) 96.71(0.74) 318(6) 63(1) n.d n.d Cpx 49.66(1.10) 1.77(0.05) 6.44(0.09) 2.14(0.02) 0.29(0.01) <d.l 23.70(0.50) 15.78(0.22) 0.01(0.00) 99.78(1.67) 1014(32) 129(4) n.d n.d Y (0.3) 2.2(0.2) n.d n.d melt 42.52(0.39) 4.01(0.04) 12.33(0.14) 6.94(0.22) 1.72(0.10) 0.95(0.03) 17.18(0.17) 10.81(0.16) 0.07(0.01) 96.54(0.37) 327(2) 57(1) n.d n.d Cpx 51.48(0.80) 1.26(0.16) 4.77(0.28) 2.14(0.09) 0.21(0.02) <d.l 24.24(0.32) 16.98(0.51) 0.01(0.00) (0.41) 1007(97) 124(11) n.d n.d Y (0.4) 2.3(0.1) 0.32(0.05) 0.60(0.10) melt 40.75(0.38) 4.35(0.14) 13.36(0.26) 7.94(0.17) 1.30(0.24) 1.07(0.14) 16.26(0.55) 10.9(0.67) 0.09(0.01) 96.01(0.96) 227(23) 49(2) 138(2) 3.5(0.3) Cpx 49.96(0.96) 1.59(0.25) 6.96(0.32) 2.39(0.13) 0.32(0.03) <d.l 23.68(0.22) 15.92(0.52) 0.01(0.00) (0.23) 845(37) 110(4) 44(7) 2.1(0.3) W (0.3) 2.3(0.2) 0.22(0.03) 0.44(0.07) melt 41.94(0.43) 4.50(0.12) 13.92(0.20) 5.16(0.11) 2.08(0.08) 1.18(0.03) 15.99(0.29) 11.47(0.11) 0.08(0.01) 96.32(1.11) 259(9) 50(1) 147(3) 4.0(0.2) Cpx 49.41(1.33) 1.86(0.26) 7.55(0.84) 2.38(0.29) 0.34(0.07) <d.l 23.24(0.45) 16.31(0.72) 0.01(0.00) (0.55) 865(73) 112(8) 32(5) 1.8(0.3) W (0.1) 1.5(0.1) 0.13(0.02) 0.23(0.04) melt 39.66(0.31) 4.51(0.04) 12.66(0.15) 8.24(0.03) 1.91(0.03) 1.11(0.04) 15.2(0.13) 11.34(0.12) 0.08(0.01) 94.71(0.32) 295(4) 57(1) 147(2) 4.0(0.1) Cpx 49.74(0.33) 1.30(0.14) 8.53(0.27) 2.69(0.03) 0.60(0.05) <d.l 22.69(0.41) 14.96(0.33) 0.02(0.01) (0.41) 704(40) 83(3) 18(3) 0.9(0.2) W (0.2) 1.1(0) 0.08(0.01) 0.18(0.04) melt 41.89(0.47) 3.98(0.11) 11.9(0.17) 7.97(0.16) 1.54(0.19) 0.91(0.12) 16.66(0.52) 11.87(0.26) 0.07(0.01) 96.79(1.13) 323(11) 66(2) 125(1) 4.5(0.2) Cpx 50.65(0.44) 1.04(0.23) 7.49(0.87) 2.39(0.13) 0.54(0.04) <d.l 22.69(0.55) 15.35(0.66) 0.01(0.00) (0.93) 743(48) 74(2) 10(2) 0.8(0.2) Notes: For each sample,typically spots and 5-15 spots were analyzed on each silicate phase using electron microprobe and LA-ICP-MS, respectively; all the experiments were run for 24 hours, except that run Y-4 was run for 48 hours; Cpx=clinopyroxene; SM=silicate melt. n.d=not determined; <d.l=below detection limit.
GSA DATA REPOSITORY
 GSA DATA REPOSITORY 2013019 Supplemental information for The Solidus of Alkaline Carbonatite in the Deep Mantle Konstantin D. Litasov, Anton Shatskiy, Eiji Ohtani, and Gregory M. Yaxley EXPERIMENTAL METHODS
GSA DATA REPOSITORY 2013019 Supplemental information for The Solidus of Alkaline Carbonatite in the Deep Mantle Konstantin D. Litasov, Anton Shatskiy, Eiji Ohtani, and Gregory M. Yaxley EXPERIMENTAL METHODS
BONINITIC MELT INCLUSIONS IN CHROME SPINEL FROM THE OGASAWARA ARCHIPELAGO
 GSA DATA REPOSITORY 2015057 BONINITIC MELT INCLUSIONS IN CHROME SPINEL FROM THE OGASAWARA ARCHIPELAGO DATA REPOSITORY for Thermal and chemical evolution of the subarc mantle revealed by spinel-hosted melt
GSA DATA REPOSITORY 2015057 BONINITIC MELT INCLUSIONS IN CHROME SPINEL FROM THE OGASAWARA ARCHIPELAGO DATA REPOSITORY for Thermal and chemical evolution of the subarc mantle revealed by spinel-hosted melt
EMMR25 Mineralogy: Ol + opx + chlorite + cpx + amphibole + serpentine + opaque
 GSA Data Repository 2017365 Marshall et al., 2017, The role of serpentinite derived fluids in metasomatism of the Colorado Plateau (USA) lithospheric mantle: Geology, https://doi.org/10.1130/g39444.1 Appendix
GSA Data Repository 2017365 Marshall et al., 2017, The role of serpentinite derived fluids in metasomatism of the Colorado Plateau (USA) lithospheric mantle: Geology, https://doi.org/10.1130/g39444.1 Appendix
Metal saturation in the upper mantle
 Vol 449 27 September 2007 Metal saturation in the upper mantle A. Rohrbach, C. Ballhaus, U. Golla Schindler, P. Ulmer,V.S. Kamenetsky, D.V. Kuzmin NS seminar 2007.10.25 The uppermost mantle is oxidized.
Vol 449 27 September 2007 Metal saturation in the upper mantle A. Rohrbach, C. Ballhaus, U. Golla Schindler, P. Ulmer,V.S. Kamenetsky, D.V. Kuzmin NS seminar 2007.10.25 The uppermost mantle is oxidized.
Composition of the Earth and its reservoirs: Geochemical observables
 Composition of the Earth and its reservoirs: Geochemical observables Cin-Ty A. Lee Rice University MYRES-I 2004 The Earth is dynamic and heterogeneous Atmosphere Midocean Ridge Plume Ocean Crust Oceanic
Composition of the Earth and its reservoirs: Geochemical observables Cin-Ty A. Lee Rice University MYRES-I 2004 The Earth is dynamic and heterogeneous Atmosphere Midocean Ridge Plume Ocean Crust Oceanic
Effect of tectonic setting on chemistry of mantle-derived melts
 Effect of tectonic setting on chemistry of mantle-derived melts Lherzolite Basalt Factors controlling magma composition Composition of the source Partial melting process Fractional crystallization Crustal
Effect of tectonic setting on chemistry of mantle-derived melts Lherzolite Basalt Factors controlling magma composition Composition of the source Partial melting process Fractional crystallization Crustal
GSA Data Repository
 GSA Data Repository 218145 Parolari et al., 218, A balancing act of crust creation and destruction along the western Mexican convergent margin: Geology, https://doi.org/1.113/g39972.1. 218145_Tables DR1-DR4.xls
GSA Data Repository 218145 Parolari et al., 218, A balancing act of crust creation and destruction along the western Mexican convergent margin: Geology, https://doi.org/1.113/g39972.1. 218145_Tables DR1-DR4.xls
High-T heating stage: application for igneous petrogenesis and mantle processes - melt inclusions as key tools -
 High-T heating stage: application for igneous petrogenesis and mantle processes - melt inclusions as key tools - SZABÓ, Csaba Lithosphere Fluid Research Lab (LRG), Department of Petrology and Geochemistry,
High-T heating stage: application for igneous petrogenesis and mantle processes - melt inclusions as key tools - SZABÓ, Csaba Lithosphere Fluid Research Lab (LRG), Department of Petrology and Geochemistry,
Mantle Wedge Oxygen Fugacity Katherine A. Kelley Graduate School of Oceanography, Univ. of Rhode Island
 Mantle Wedge Oxygen Fugacity Katherine A. Kelley Graduate School of Oceanography, Univ. of Rhode Island With contributions from: M. Brounce GSO/URI E. Cottrell & O. Lopez Smithsonian Inst. J. Warren &
Mantle Wedge Oxygen Fugacity Katherine A. Kelley Graduate School of Oceanography, Univ. of Rhode Island With contributions from: M. Brounce GSO/URI E. Cottrell & O. Lopez Smithsonian Inst. J. Warren &
Supplementary Figure 1 Map of the study area Sample locations and main physiographic features of the study area. Contour interval is 200m (a) and 40m
 Supplementary Figure 1 Map of the study area Sample locations and main physiographic features of the study area. Contour interval is 200m (a) and 40m (b). Dashed lines represent the two successive ridge
Supplementary Figure 1 Map of the study area Sample locations and main physiographic features of the study area. Contour interval is 200m (a) and 40m (b). Dashed lines represent the two successive ridge
III-1 MANGANESE PARTITIONING DURING HYDROUS MELTING OF PERIDOTITE. J. Brian Balta. Paul D. Asimow. Jed L. Mosenfelder
 III-1 MANGANESE PARTITIONING DURING HYDROUS MELTING OF PERIDOTITE By J. Brian Balta Paul D. Asimow Jed L. Mosenfelder III-2 ABSTRACT Manganese contents and the iron/manganese ratio of igneous rocks have
III-1 MANGANESE PARTITIONING DURING HYDROUS MELTING OF PERIDOTITE By J. Brian Balta Paul D. Asimow Jed L. Mosenfelder III-2 ABSTRACT Manganese contents and the iron/manganese ratio of igneous rocks have
Lecture 36. Igneous geochemistry
 Lecture 36 Igneous geochemistry Reading - White Chapter 7 Today 1. Overview 2. solid-melt distribution coefficients Igneous geochemistry The chemistry of igneous systems provides clues to a number of important
Lecture 36 Igneous geochemistry Reading - White Chapter 7 Today 1. Overview 2. solid-melt distribution coefficients Igneous geochemistry The chemistry of igneous systems provides clues to a number of important
Electronic Appendix A: Supplementary material to accompany the manuscript, Fe 3+ / Fe in Mariana Arc basalts and primary fo 2.
 Electronic Appendix A: Supplementary material to accompany the manuscript, Fe 3+ / Fe in Mariana Arc basalts and primary fo 2. Screening for olivine interference in Fe-µ-XANES spectra When collecting Fe-µ-XANES
Electronic Appendix A: Supplementary material to accompany the manuscript, Fe 3+ / Fe in Mariana Arc basalts and primary fo 2. Screening for olivine interference in Fe-µ-XANES spectra When collecting Fe-µ-XANES
Notes for Use of the Cpx-Plag-Ol Thermobar Workbook Last Updated:
 Notes for Use of the Cpx-Plag-Ol Thermobar Workbook Last Updated: 7-22-05 Cpx-Plag-Ol Thermobar is an Excel workbook that can be used to calculate crystallization pressures and temperatures for clinopyroxene-
Notes for Use of the Cpx-Plag-Ol Thermobar Workbook Last Updated: 7-22-05 Cpx-Plag-Ol Thermobar is an Excel workbook that can be used to calculate crystallization pressures and temperatures for clinopyroxene-
Worked Example of Batch Melting: Rb and Sr
 Worked Example of Batch Melting: Rb and Sr Basalt with the mode: Table 9.2. Conversion from mode to weight percent Mineral Mode Density Wt prop Wt% ol 15 3.6 54 0.18 cpx 33 3.4 112.2 0.37 plag 51 2.7 137.7
Worked Example of Batch Melting: Rb and Sr Basalt with the mode: Table 9.2. Conversion from mode to weight percent Mineral Mode Density Wt prop Wt% ol 15 3.6 54 0.18 cpx 33 3.4 112.2 0.37 plag 51 2.7 137.7
Trace Elements. Today s lecture
 Trace Elements 300 Ni 200 ppm 100 0 300 Zr 200 100 0 40 50 60 70 80 SiO 2 wt. % Updates: M&M due date: Tuesday Today s lecture Topics: Trace element compositions Trace element behavior Partitioning Spider(
Trace Elements 300 Ni 200 ppm 100 0 300 Zr 200 100 0 40 50 60 70 80 SiO 2 wt. % Updates: M&M due date: Tuesday Today s lecture Topics: Trace element compositions Trace element behavior Partitioning Spider(
TRACE ELEMENT ANALYSIS OF DIAMOND BY LAM ICPMS: STANDARDISATION, RESULTS AND DIRECTIONS
 TRACE ELEMENT ANALYSIS OF DIAMOND BY LAM ICPMS: STANDARDISATION, RESULTS AND DIRECTIONS W.L. Griffin 1, 3, Sonal Rege 1, Rondi M. Davies 1, 2, Simon Jackson 1, Suzanne Y. O Reilly 1 1.ARC National Key
TRACE ELEMENT ANALYSIS OF DIAMOND BY LAM ICPMS: STANDARDISATION, RESULTS AND DIRECTIONS W.L. Griffin 1, 3, Sonal Rege 1, Rondi M. Davies 1, 2, Simon Jackson 1, Suzanne Y. O Reilly 1 1.ARC National Key
WORKING WITH ELECTRON MICROPROBE DATA FROM A HIGH PRESSURE EXPERIMENT CALCULATING MINERAL FORMULAS, UNIT CELL CONTENT, AND GEOTHERMOMETRY
 WORKING WITH ELECTRON MICROPROBE DATA FROM A HIGH PRESSURE EXPERIMENT CALCULATING MINERAL FORMULAS, UNIT CELL CONTENT, AND GEOTHERMOMETRY Brandon E. Schwab Department of Geology Humboldt State University
WORKING WITH ELECTRON MICROPROBE DATA FROM A HIGH PRESSURE EXPERIMENT CALCULATING MINERAL FORMULAS, UNIT CELL CONTENT, AND GEOTHERMOMETRY Brandon E. Schwab Department of Geology Humboldt State University
Breeding et al., Data Repository Material Figure DR1. Athens. Study Area
 Breeding, Ague, and Brocker 1 Figure DR1 21 o 24 Greece o A 38 o Athens Tinos 37 o Syros Attic-Cycladic Blueschist Belt Syros Kampos B Study Area Ermoupoli N Vari Unit Cycladic HP-LT Unit Marble horizons
Breeding, Ague, and Brocker 1 Figure DR1 21 o 24 Greece o A 38 o Athens Tinos 37 o Syros Attic-Cycladic Blueschist Belt Syros Kampos B Study Area Ermoupoli N Vari Unit Cycladic HP-LT Unit Marble horizons
High-T T heating stage: : application for igneous petrogenesis and mantle processes - melt inclusions as key tools -
 High-T T heating stage: : application for igneous petrogenesis and mantle processes - melt inclusions as key tools - SZABÓ, Csaba Lithosphere Fluid Research Lab (LRG), Department of Petrology and Geochemistry,
High-T T heating stage: : application for igneous petrogenesis and mantle processes - melt inclusions as key tools - SZABÓ, Csaba Lithosphere Fluid Research Lab (LRG), Department of Petrology and Geochemistry,
GSA DATA REPOSITORY
 GSA DATA REPOSITORY 2012161 Allan et al. SUPPLEMENTARY INFORMATION Summary of Magma Types Table DR1 summarizes some of the key petrologic, geochemical and physical characteristics of the three magma types
GSA DATA REPOSITORY 2012161 Allan et al. SUPPLEMENTARY INFORMATION Summary of Magma Types Table DR1 summarizes some of the key petrologic, geochemical and physical characteristics of the three magma types
Eq. 1a. Eq. 1b. peridotite/melt exchange coefficient. Eq. 2
 doi:10.1038/nature09617 Online Methods Zn/Fe partitioning The most abundant minerals in otitic upper mantle, olivine and orthopyroxene, dominate the Zn and Fe budget of such rocks. In a previous study
doi:10.1038/nature09617 Online Methods Zn/Fe partitioning The most abundant minerals in otitic upper mantle, olivine and orthopyroxene, dominate the Zn and Fe budget of such rocks. In a previous study
The mantle metasomatism: diversity and impact What the mantle xenoliths tell us?
 The mantle metasomatism: diversity and impact What the mantle xenoliths tell us? Mantle metasomatism Physical and chemical processes that are implemented during the flow of magmas and / or fluids within
The mantle metasomatism: diversity and impact What the mantle xenoliths tell us? Mantle metasomatism Physical and chemical processes that are implemented during the flow of magmas and / or fluids within
Fluids, melts, and supercriticality in the MSH system and element transport in subduction zones
 cosmic rays Fluids, s, and supercriticality in the MSH system and element transport in subduction zones 10 Be volcanic front N, O 10 Be ocean water + CO 2 tracing petrologic and geotectonic processes (trace)
cosmic rays Fluids, s, and supercriticality in the MSH system and element transport in subduction zones 10 Be volcanic front N, O 10 Be ocean water + CO 2 tracing petrologic and geotectonic processes (trace)
Rare Earth Elements in some representative arc lavas
 Rare Earth Elements in some representative arc lavas Low-K (tholeiitic), Medium-K (calc-alkaline), and High-K basaltic andesites and andesites. A typical N-MORB pattern is included for reference Notes:
Rare Earth Elements in some representative arc lavas Low-K (tholeiitic), Medium-K (calc-alkaline), and High-K basaltic andesites and andesites. A typical N-MORB pattern is included for reference Notes:
Olivine-hosted melt inclusions in Hawaiian picrites: equilibration, melting, and plume source characteristics
 Chemical Geology 183 (2002) 143 168 www.elsevier.com/locate/chemgeo Olivine-hosted melt inclusions in Hawaiian picrites: equilibration, melting, and plume source characteristics Marc D. Norman a, *, Michael
Chemical Geology 183 (2002) 143 168 www.elsevier.com/locate/chemgeo Olivine-hosted melt inclusions in Hawaiian picrites: equilibration, melting, and plume source characteristics Marc D. Norman a, *, Michael
A trapdoor mechanism for slab tearing and melt generation in the northern Andes
 GSA Data Repository 201418 Geology DOI:10.1130/G45429.1 (Rosenbaum et al.) A trapdoor mechanism for slab tearing and melt generation in the northern Andes Gideon Rosenbaum, Mike Sandiford, John Caulfield
GSA Data Repository 201418 Geology DOI:10.1130/G45429.1 (Rosenbaum et al.) A trapdoor mechanism for slab tearing and melt generation in the northern Andes Gideon Rosenbaum, Mike Sandiford, John Caulfield
LATE ARCHAEAN FELSIC ALKALINE MAGMATISM: GEOLOGY, GEOCHEMISTRY, AND TECTONIC SETTING
 LATE ARCHAEAN FELSIC ALKALINE MAGMATISM: GEOLOGY, GEOCHEMISTRY, AND TECTONIC SETTING ZOZULYA DMITRY 1, EBY NELSON 2 1 - Geological Institute Kola Science Centre RAS, Apatity, Russia 2 - Department of Environmental,
LATE ARCHAEAN FELSIC ALKALINE MAGMATISM: GEOLOGY, GEOCHEMISTRY, AND TECTONIC SETTING ZOZULYA DMITRY 1, EBY NELSON 2 1 - Geological Institute Kola Science Centre RAS, Apatity, Russia 2 - Department of Environmental,
Summary of test results for Daya Bay rock samples. by Patrick Dobson Celia Tiemi Onishi Seiji Nakagawa
 Summary of test results for Daya Bay rock samples by Patrick Dobson Celia Tiemi Onishi Seiji Nakagawa October 2004 Summary A series of analytical tests were conducted on a suite of granitic rock samples
Summary of test results for Daya Bay rock samples by Patrick Dobson Celia Tiemi Onishi Seiji Nakagawa October 2004 Summary A series of analytical tests were conducted on a suite of granitic rock samples
Chapter 9: Trace Elements
 Chapter 9: Trace Elements Note magnitude of major element changes Figure 8.2. Harker variation diagram for 310 analyzed volcanic rocks from Crater Lake (Mt. Mazama), Oregon Cascades. Data compiled by Rick
Chapter 9: Trace Elements Note magnitude of major element changes Figure 8.2. Harker variation diagram for 310 analyzed volcanic rocks from Crater Lake (Mt. Mazama), Oregon Cascades. Data compiled by Rick
Similar V/Sc Systematics in MORB and Arc Basalts: Implications for the Oxygen Fugacities of their Mantle Source Regions
 JOURNAL OF PETROLOGY PAGE 1 of 24 doi:10.1093/petrology/egi056 Journal of Petrology Advance Access published June 13, 2005 Similar V/Sc Systematics in MORB and Arc Basalts: Implications for the Oxygen
JOURNAL OF PETROLOGY PAGE 1 of 24 doi:10.1093/petrology/egi056 Journal of Petrology Advance Access published June 13, 2005 Similar V/Sc Systematics in MORB and Arc Basalts: Implications for the Oxygen
HP and UHP garnet peridotites and pyroxenites
 HP and UHP garnet peridotites and pyroxenites Mantle wedge The least known piece of the subduction factory Mantle-wedge peridotites emplace within subducting continental crust (Brueckner, 998; van Roermund
HP and UHP garnet peridotites and pyroxenites Mantle wedge The least known piece of the subduction factory Mantle-wedge peridotites emplace within subducting continental crust (Brueckner, 998; van Roermund
Supplementary material to accompany the manuscript, Redox variations in HSDP2
 Supplementary material to accompany the manuscript, Redox variations in HSDP2 Mauna Kea lavas, the oxygen fugacity of the Hawaiian plume, and the role of volcanic gases in Earth s oxygenation by M. Brounce,
Supplementary material to accompany the manuscript, Redox variations in HSDP2 Mauna Kea lavas, the oxygen fugacity of the Hawaiian plume, and the role of volcanic gases in Earth s oxygenation by M. Brounce,
Lecture 38. Igneous geochemistry. Read White Chapter 7 if you haven t already
 Lecture 38 Igneous geochemistry Read White Chapter 7 if you haven t already Today. Magma mixing/afc 2. Spot light on using the Rare Earth Elements (REE) to constrain mantle sources and conditions of petrogenesis
Lecture 38 Igneous geochemistry Read White Chapter 7 if you haven t already Today. Magma mixing/afc 2. Spot light on using the Rare Earth Elements (REE) to constrain mantle sources and conditions of petrogenesis
Recommended mineral-melt partition coefficients for FRTEs (Cu), Ga, and Ge during mantle melting
 American Mineralogist, Volume, pages 2533 2544, 25 Recommended mineral-melt partition coefficients for FRTEs (Cu), Ga, and Ge during mantle melting Véronique Le Roux, *, Rajdeep Dasgupta 2 and Cin-Ty A.
American Mineralogist, Volume, pages 2533 2544, 25 Recommended mineral-melt partition coefficients for FRTEs (Cu), Ga, and Ge during mantle melting Véronique Le Roux, *, Rajdeep Dasgupta 2 and Cin-Ty A.
Discrimination between Archean A-type granitoids and sanukitoid suites using tectonic setting, geochemistry, and fertility type
 Discrimination between Archean A-type granitoids and sanukitoid suites using tectonic setting, geochemistry, and fertility type ZOZULYA DMITRY 1, EBY NELSON 2 1 - Geological Institute Kola Science Centre
Discrimination between Archean A-type granitoids and sanukitoid suites using tectonic setting, geochemistry, and fertility type ZOZULYA DMITRY 1, EBY NELSON 2 1 - Geological Institute Kola Science Centre
Chapter 9: Trace Elements
 Lecture 13 Introduction to Trace Elements Wednesday, March 9, 2005 Chapter 9: Trace Elements Note magnitude of major element changes Figure 8-2. Harker variation diagram for 310 analyzed volcanic rocks
Lecture 13 Introduction to Trace Elements Wednesday, March 9, 2005 Chapter 9: Trace Elements Note magnitude of major element changes Figure 8-2. Harker variation diagram for 310 analyzed volcanic rocks
XM1/331 XM1/331 BLFX-3 XM1/331
 a b AkC AkC strontian fluoro-apatite clinopyroxene phlogopite K-richterite XM1/331 clinopyroxene XM1/331 Fe-Ti ox c d clinopyroxene kric AkC ilmenite Sr-barite AkC XM1/331 BLFX-3 Supplementary Figure 1.
a b AkC AkC strontian fluoro-apatite clinopyroxene phlogopite K-richterite XM1/331 clinopyroxene XM1/331 Fe-Ti ox c d clinopyroxene kric AkC ilmenite Sr-barite AkC XM1/331 BLFX-3 Supplementary Figure 1.
Volatile rich Fe-oxide melts: base and precious metals enrichment trends. Is there an IOCG connection?
 Volatile rich Fe-oxide melts: base and precious metals enrichment trends. Is there an IOCG connection? Gregory W Lester M.A., MSc., PhD Curtin University Background Nelsonites and Low Ti, FeO deposits
Volatile rich Fe-oxide melts: base and precious metals enrichment trends. Is there an IOCG connection? Gregory W Lester M.A., MSc., PhD Curtin University Background Nelsonites and Low Ti, FeO deposits
doi: /nature09369
 doi:10.1038/nature09369 Supplementary Figure S1 Scanning electron microscope images of experimental charges with vapour and vapour phase quench. Experimental runs are in the order of added water concentration
doi:10.1038/nature09369 Supplementary Figure S1 Scanning electron microscope images of experimental charges with vapour and vapour phase quench. Experimental runs are in the order of added water concentration
Supplementary Information
 Supplementary Information Supplementary Tables Major element compositions of host and daughter crystals: Table 1 reports composition for host-olivine. No daughter olivine has been found. The number for
Supplementary Information Supplementary Tables Major element compositions of host and daughter crystals: Table 1 reports composition for host-olivine. No daughter olivine has been found. The number for
- low FeO*/MgO is controlled by the reaction relation oliv + liq! opx.
 Mantle melting trend to high-sio 2 - low FeO*/MgO is controlled by the reaction relation oliv + liq! opx. 4 3 2 1 Ol+Opx +Cpx+Sp +liq 79-35g 82-66 87S35 85-44 85-41c -ol +opx Mantle melting/reaction 0
Mantle melting trend to high-sio 2 - low FeO*/MgO is controlled by the reaction relation oliv + liq! opx. 4 3 2 1 Ol+Opx +Cpx+Sp +liq 79-35g 82-66 87S35 85-44 85-41c -ol +opx Mantle melting/reaction 0
Melts of garnet lherzolite: experiments, models and comparison to melts of pyroxenite and carbonated lherzolite
 Contrib Mineral Petrol (2013) 166:887 910 DOI 10.1007/s00410-013-0899-9 ORIGINAL PAPER Melts of garnet lherzolite: experiments, models and comparison to melts of pyroxenite and carbonated lherzolite Timothy
Contrib Mineral Petrol (2013) 166:887 910 DOI 10.1007/s00410-013-0899-9 ORIGINAL PAPER Melts of garnet lherzolite: experiments, models and comparison to melts of pyroxenite and carbonated lherzolite Timothy
Melts of garnet lherzolite: experiments, models and comparison to melts of pyroxenite and carbonated lherzolite
 Melts of garnet lherzolite: experiments, models and comparison to melts of pyroxenite and carbonated lherzolite The MIT Faculty has made this article openly available. Please share how this access benefits
Melts of garnet lherzolite: experiments, models and comparison to melts of pyroxenite and carbonated lherzolite The MIT Faculty has made this article openly available. Please share how this access benefits
LETTER. The redox state of arc mantle using Zn/Fe systematics
 LETTER doi:1.138/nature9617 The redox state of arc mantle using Zn/Fe systematics Cin-Ty A. Lee 1, Peter Luffi 1,Véronique Le Roux 1, Rajdeep Dasgupta 1, Francis Albaréde 1,2 & William P. Leeman 1,3 Many
LETTER doi:1.138/nature9617 The redox state of arc mantle using Zn/Fe systematics Cin-Ty A. Lee 1, Peter Luffi 1,Véronique Le Roux 1, Rajdeep Dasgupta 1, Francis Albaréde 1,2 & William P. Leeman 1,3 Many
Zn/Fe systematics in mafic and ultramafic systems: Implications for detecting major element heterogeneities in the Earth s mantle
 Available online at www.sciencedirect.com Geochimica et Cosmochimica Acta 74 (21) 2779 2796 www.elsevier.com/locate/gca Zn/Fe systematics in mafic and ultramafic systems: Implications for detecting major
Available online at www.sciencedirect.com Geochimica et Cosmochimica Acta 74 (21) 2779 2796 www.elsevier.com/locate/gca Zn/Fe systematics in mafic and ultramafic systems: Implications for detecting major
Petrogenetic Constraints at Mount Rainier Volcano, Washington
 Petrogenetic Constraints at Mount Rainier Volcano, Washington S. C. Kuehn and P. R. Hooper, Department of Geology, Washington State University, Pullman, WA A. E. Eggers and C. Kerrick, Department of Geology,
Petrogenetic Constraints at Mount Rainier Volcano, Washington S. C. Kuehn and P. R. Hooper, Department of Geology, Washington State University, Pullman, WA A. E. Eggers and C. Kerrick, Department of Geology,
GEOL 2312 Igneous and Metamorphic Petrology Spring 2016 Score / 58. Midterm 1 Chapters 1-10
 GEOL 2312 Igneous and Metamorphic Petrology Name KEY Spring 2016 Score / 58 Midterm 1 Chapters 1-10 1) Name two things that petrologists want to know about magmas (1 pt) Formation, source, composition,
GEOL 2312 Igneous and Metamorphic Petrology Name KEY Spring 2016 Score / 58 Midterm 1 Chapters 1-10 1) Name two things that petrologists want to know about magmas (1 pt) Formation, source, composition,
GY303 Igneous & Metamorphic Petrology. Lecture 7: Magma Sources and Tectonic Environments
 GY303 Igneous & Metamorphic Petrology Lecture 7: Magma Sources and Tectonic Environments Factors controlling Magma production Source rock composition Amount of fluids, especially H 2 O Pressure (Depth)
GY303 Igneous & Metamorphic Petrology Lecture 7: Magma Sources and Tectonic Environments Factors controlling Magma production Source rock composition Amount of fluids, especially H 2 O Pressure (Depth)
Trace element partitioning into sulfide: How lithophile elements become chalcophile and vice versa k
 American Mineralogist, Volume 100, pages 2371 2379, 2015 Roebling Medal Lecture Trace element partitioning into sulfide: How lithophile elements become chalcophile and vice versa k Bernard J. Wood 1, *
American Mineralogist, Volume 100, pages 2371 2379, 2015 Roebling Medal Lecture Trace element partitioning into sulfide: How lithophile elements become chalcophile and vice versa k Bernard J. Wood 1, *
GSA DATA REPOSITORY
 GSA DATA REPOSITORY 2011130 Diversity of melt conduits in the Izu-Bonin-Mariana forearc mantle: implications for the earliest stage of arc magmatism Tomoaki Morishita, Kenichiro Tani, Hiroshi Shukuno,
GSA DATA REPOSITORY 2011130 Diversity of melt conduits in the Izu-Bonin-Mariana forearc mantle: implications for the earliest stage of arc magmatism Tomoaki Morishita, Kenichiro Tani, Hiroshi Shukuno,
GSA DATA REPOSITORY
 GSA DATA REPOSITORY 2013011 Chen et al. ANALITICAL METHODS Microprobe analysis Microprobe analyses of minerals were done on a JEOL Superprobe JXA 8100 at the Key Laboratory of Orogenic Belts and Crustal
GSA DATA REPOSITORY 2013011 Chen et al. ANALITICAL METHODS Microprobe analysis Microprobe analyses of minerals were done on a JEOL Superprobe JXA 8100 at the Key Laboratory of Orogenic Belts and Crustal
Revision 1. Transition metals in komatiitic olivine: Proxies for mantle composition, redox. conditions, and sulfide mineralization potential
 1 2 3 Revision 1 Transition metals in komatiitic olivine: Proxies for mantle composition, redox conditions, and sulfide mineralization potential 4 5 Marek Locmelis 1 *, Ricardo D. Arevalo Jr 2. Igor S.
1 2 3 Revision 1 Transition metals in komatiitic olivine: Proxies for mantle composition, redox conditions, and sulfide mineralization potential 4 5 Marek Locmelis 1 *, Ricardo D. Arevalo Jr 2. Igor S.
SUPPLEMENTARY INFORMATION
 GSA Data Repository 080 Schorn et al., 08, Thermal buffering in the orogenic crust: Geology, https://doi.org/0.30/g4046.. SUPPLEMENTARY INFORMATION 3 PHASE DIAGRAM MODELING 4 5 6 7 8 9 0 3 4 Phase diagrams
GSA Data Repository 080 Schorn et al., 08, Thermal buffering in the orogenic crust: Geology, https://doi.org/0.30/g4046.. SUPPLEMENTARY INFORMATION 3 PHASE DIAGRAM MODELING 4 5 6 7 8 9 0 3 4 Phase diagrams
The Lead 206/207 Dating Method
 The Lead 206/207 Dating Method 1 U Pb Zircon Ages, Chemical Geology, Volume 211 (2004) Pages 87 109 2 Lead Isotope Planetary Profiling, Chemical Geology, Volume 233 (2006) Pages 1 45 3 U Pb Step-Leaching
The Lead 206/207 Dating Method 1 U Pb Zircon Ages, Chemical Geology, Volume 211 (2004) Pages 87 109 2 Lead Isotope Planetary Profiling, Chemical Geology, Volume 233 (2006) Pages 1 45 3 U Pb Step-Leaching
Geochemical analysis unveils frictional melting process in a
 GSA Data Repository 219116 1 2 3 Geochemical analysis unveils frictional melting process in a subduction zone fault Tsuyoshi Ishikawa and Kohtaro Ujiie 4 Supplemental Material 6 7 8 9 METHOD TABLES (Tables
GSA Data Repository 219116 1 2 3 Geochemical analysis unveils frictional melting process in a subduction zone fault Tsuyoshi Ishikawa and Kohtaro Ujiie 4 Supplemental Material 6 7 8 9 METHOD TABLES (Tables
*Revision 2 for Manuscript 5783* A Review and Update of Mantle Thermobarometry for Primitive Arc Magmas
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 *Revision 2 for Manuscript 5783* A Review and Update of Mantle Thermobarometry for Primitive Arc Magmas Christy B. Till School of Earth &
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 *Revision 2 for Manuscript 5783* A Review and Update of Mantle Thermobarometry for Primitive Arc Magmas Christy B. Till School of Earth &
LAB 9: ULTRAMAFIC ROCKS, CUMULATES AND MELT SOURCES
 Geology 316 (Petrology) (03/26/2012) Name LAB 9: ULTRAMAFIC ROCKS, CUMULATES AND MELT SOURCES INTRODUCTION Ultramafic rocks are igneous rocks containing less than 10% felsic minerals (quartz + feldspars
Geology 316 (Petrology) (03/26/2012) Name LAB 9: ULTRAMAFIC ROCKS, CUMULATES AND MELT SOURCES INTRODUCTION Ultramafic rocks are igneous rocks containing less than 10% felsic minerals (quartz + feldspars
Harzburgite melting with and without H 2 O: Experimental data and predictive modeling
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 109,, doi:10.1029/2003jb002566, 2004 Harzburgite ing with and without H 2 O: Experimental data and predictive modeling Stephen W. Parman and Timothy L. Grove Department
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 109,, doi:10.1029/2003jb002566, 2004 Harzburgite ing with and without H 2 O: Experimental data and predictive modeling Stephen W. Parman and Timothy L. Grove Department
Revision 1. Parameterized lattice strain models for REE. partitioning between amphibole and silicate melt
 1 2 3 Revision 1 Parameterized lattice strain models for REE partitioning between amphibole and silicate melt 4 5 Kei Shimizu 1*, Yan Liang 1, Chenguang Sun 2, Colin R. M. Jackson 3, and Alberto E. Saal
1 2 3 Revision 1 Parameterized lattice strain models for REE partitioning between amphibole and silicate melt 4 5 Kei Shimizu 1*, Yan Liang 1, Chenguang Sun 2, Colin R. M. Jackson 3, and Alberto E. Saal
GRADUATE THESIS PROPOSAL EARTH SCIENCES 6300
 GRADUATE THESIS PROPOSAL EARTH SCIENCES 6300 LAST NAME: KELTIE FIRST NAME: ERIN STUDENT NUMBER: B00597303 DEGREE PROGRAMME: M.SC SUPERVISOR(S): DR. JAMES BRENAN TITLE OF PROPOSAL: AN EXPERIMENTAL INVESTIGATION
GRADUATE THESIS PROPOSAL EARTH SCIENCES 6300 LAST NAME: KELTIE FIRST NAME: ERIN STUDENT NUMBER: B00597303 DEGREE PROGRAMME: M.SC SUPERVISOR(S): DR. JAMES BRENAN TITLE OF PROPOSAL: AN EXPERIMENTAL INVESTIGATION
Metal silicate partitioning and constraints on core composition and oxygen fugacity during Earth accretion
 Available online at www.sciencedirect.com Geochimica et Cosmochimica Acta 72 (2008) 574 589 www.elsevier.com/locate/gca etal silicate partitioning and constraints on core composition and oxygen fugacity
Available online at www.sciencedirect.com Geochimica et Cosmochimica Acta 72 (2008) 574 589 www.elsevier.com/locate/gca etal silicate partitioning and constraints on core composition and oxygen fugacity
SR Allen, RS Fiske, Y Tamura Sumisu methods
 SR Allen, RS Fiske, Y Tamura Sumisu methods FTIR water contents from melt inclusions Quartz-hosted melt inclusions were analyzed for H 2 O and CO 2 by Fourier Transform Infrared Spectroscopy (FTIR) at
SR Allen, RS Fiske, Y Tamura Sumisu methods FTIR water contents from melt inclusions Quartz-hosted melt inclusions were analyzed for H 2 O and CO 2 by Fourier Transform Infrared Spectroscopy (FTIR) at
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11294 Review of previous works on deep-liquid properties The major parameters controlling the buoyancy of deep-mantle melts are (i) the changes in atomic packing
SUPPLEMENTARY INFORMATION doi:10.1038/nature11294 Review of previous works on deep-liquid properties The major parameters controlling the buoyancy of deep-mantle melts are (i) the changes in atomic packing
Ancient depletion and mantle heterogeneity: Revisiting the Jurassic-Permian paradox of Alpine peridotites
 GSA Data Repository 2015092 Ancient depletion and mantle heterogeneity: Revisiting the Jurassic-Permian paradox of Alpine peridotites McCarthy Anders 1 & Müntener Othmar 1 1 Institute of Earth Sciences
GSA Data Repository 2015092 Ancient depletion and mantle heterogeneity: Revisiting the Jurassic-Permian paradox of Alpine peridotites McCarthy Anders 1 & Müntener Othmar 1 1 Institute of Earth Sciences
N = N 0 e -λt D* = N 0 -N D* = N 0 (1-e -λt ) or N(e λt -1) where N is number of parent atoms at time t, N 0
 N = N 0 e -λt D* = N 0 -N D* = N 0 (1-e -λt ) or N(e λt -1) where N is number of parent atoms at time t, N 0 is initial number of parents, D* is number of radiogenic daughter atoms, and λ is the decay
N = N 0 e -λt D* = N 0 -N D* = N 0 (1-e -λt ) or N(e λt -1) where N is number of parent atoms at time t, N 0 is initial number of parents, D* is number of radiogenic daughter atoms, and λ is the decay
Earth and Planetary Science Letters
 Earth and Planetary Science Letters 329 330 (2012) 109 121 Contents lists available at SciVerse ScienceDirect Earth and Planetary Science Letters journal homepage: www.elsevier.com/locate/epsl The influence
Earth and Planetary Science Letters 329 330 (2012) 109 121 Contents lists available at SciVerse ScienceDirect Earth and Planetary Science Letters journal homepage: www.elsevier.com/locate/epsl The influence
Where are these melts generated in the mantle wedge?
 Melt generation processes in subduction zones T.L. Grove, C.B. Till, N. Chatterjee, E. Medard, S.W. Parman New experiments on H2O-saturated melting of mantle peridotite - The role of H2O in mantle wedge
Melt generation processes in subduction zones T.L. Grove, C.B. Till, N. Chatterjee, E. Medard, S.W. Parman New experiments on H2O-saturated melting of mantle peridotite - The role of H2O in mantle wedge
SCHOOL OF EARTH SCIENCES, UNIVERSITY OF TASMANIA, GPO BOX , HOBART, TAS. 7001, AUSTRALIA
 JOURNAL OF PETROLOGY VOLUME 40 NUMBER 9 PAGES 1343 1375 1999 Peridotite Melting at 1 0 and 1 5 GPa: an Experimental Evaluation of Techniques using Diamond Aggregates and Mineral Mixes for Determination
JOURNAL OF PETROLOGY VOLUME 40 NUMBER 9 PAGES 1343 1375 1999 Peridotite Melting at 1 0 and 1 5 GPa: an Experimental Evaluation of Techniques using Diamond Aggregates and Mineral Mixes for Determination
Day et al. (2009) Pyroxenite-rich mantle formed by recycled oceanic lithosphere: oxygen-osmium isotope evidence from Canary Island lavas
 GSA Data Repository DR2009128 Day et al. (2009) Pyroxenite-rich mantle formed by recycled oceanic lithosphere: oxygen-osmium isotope evidence from Canary Island lavas Supplementary Methods Oxygen isotope
GSA Data Repository DR2009128 Day et al. (2009) Pyroxenite-rich mantle formed by recycled oceanic lithosphere: oxygen-osmium isotope evidence from Canary Island lavas Supplementary Methods Oxygen isotope
Minor- and Trace Element Zoning in Plagioclase From Kizimen Volcano, Kamchatka: Insights on the Magma Chamber Processes
 Minor- and Trace Element Zoning in Plagioclase From Kizimen Volcano, Kamchatka: Insights on the Magma Chamber Processes Tatiana Churikova 1,2, Gerhard Wörner 2, John Eichelberger 3, and Boris Ivanov 1
Minor- and Trace Element Zoning in Plagioclase From Kizimen Volcano, Kamchatka: Insights on the Magma Chamber Processes Tatiana Churikova 1,2, Gerhard Wörner 2, John Eichelberger 3, and Boris Ivanov 1
APPENDIX TABLES. Table A2. XRF analytical results for samples from drill hole AP5 (Areachap)
 APPENDIX TABLES Table A2. XRF analytical results for samples from drill hole AP5 (Areachap) Sample No. AP5/19 AP5/20 AP5/21 AP5/22 AP5/23 AP5/24 AP5/25AP5/26AP5/27AP5/28AP5/29AP5/30AP5/31AP5/32 AP5/33
APPENDIX TABLES Table A2. XRF analytical results for samples from drill hole AP5 (Areachap) Sample No. AP5/19 AP5/20 AP5/21 AP5/22 AP5/23 AP5/24 AP5/25AP5/26AP5/27AP5/28AP5/29AP5/30AP5/31AP5/32 AP5/33
Geochemical and mineralogical technics to investigate the lithosphere and the asthenosphere. 07/11/2017 GEO-DEEP 9300 Claire Aupart
 Geochemical and mineralogical technics to investigate the lithosphere and the asthenosphere 07/11/2017 GEO-DEEP 9300 Claire Aupart Introduction Introduction Getting samples Cores: Maximum depth reach in
Geochemical and mineralogical technics to investigate the lithosphere and the asthenosphere 07/11/2017 GEO-DEEP 9300 Claire Aupart Introduction Introduction Getting samples Cores: Maximum depth reach in
NADIA MALASPINA. PLINIUS n. 32, 2006
 ULTRAHIGH-PRESSURE METAMORPHISM AND METASOMATISM IN MAFIC-ULTRAMAFIC ROCKS FROM EASTERN CHINA: IMPLICATIONS FOR FLUID RELEASE AND VOLATILE TRANSFER AT SUBDUCTION ZONES NADIA MALASPINA Dipartimento per
ULTRAHIGH-PRESSURE METAMORPHISM AND METASOMATISM IN MAFIC-ULTRAMAFIC ROCKS FROM EASTERN CHINA: IMPLICATIONS FOR FLUID RELEASE AND VOLATILE TRANSFER AT SUBDUCTION ZONES NADIA MALASPINA Dipartimento per
Continental Alkaline Magmatism. The East African Rift
 Announcements No lecture on Friday Lab final begins at 1 PM Today s agenda: Lecture/Demo Go through field trip pics Call for pizza Lab review Lecture Review Make poster Continental Alkaline Magmatism.
Announcements No lecture on Friday Lab final begins at 1 PM Today s agenda: Lecture/Demo Go through field trip pics Call for pizza Lab review Lecture Review Make poster Continental Alkaline Magmatism.
Supporting Information
 Supporting Information Bindi et al. 10.1073/pnas.1111115109 Fig. S1. Electron microprobe X-ray elemental maps for the grain reported in Fig. 1B. Experimental details are given in Experimental Methods.
Supporting Information Bindi et al. 10.1073/pnas.1111115109 Fig. S1. Electron microprobe X-ray elemental maps for the grain reported in Fig. 1B. Experimental details are given in Experimental Methods.
Ultramafic rocks. Types of Ultramafic Rocks. Spinel lherzolite xenolith
 Ultramafic rocks Definition: Color Index > 90, i.e., less than 10% felsic minerals. Not to be confused with Ultrabasic Rocks which are rocks with
Ultramafic rocks Definition: Color Index > 90, i.e., less than 10% felsic minerals. Not to be confused with Ultrabasic Rocks which are rocks with
Partitioning of U and Th during garnet pyroxenite partial melting: Constraints on the source of alkaline ocean island basalts
 Available online at www.sciencedirect.com Earth and Planetary Science Letters 265 (2008) 270 286 www.elsevier.com/locate/epsl Partitioning of U and Th during garnet pyroxenite partial melting: Constraints
Available online at www.sciencedirect.com Earth and Planetary Science Letters 265 (2008) 270 286 www.elsevier.com/locate/epsl Partitioning of U and Th during garnet pyroxenite partial melting: Constraints
Lecture 13. Constraints on Melt Models Arising From Disequilibrium in the Th-U Decay System
 Lecture 13 Constraints on Melt Models Arising From Disequilibrium in the Th-U Decay System (for reference: see Uranium-Series Geochemistry, volume 52 of Reviews in Mineralogy and Geochemistry (Bourdon,
Lecture 13 Constraints on Melt Models Arising From Disequilibrium in the Th-U Decay System (for reference: see Uranium-Series Geochemistry, volume 52 of Reviews in Mineralogy and Geochemistry (Bourdon,
A DISSERTATION SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL OF THE UNIVERSITY OF MINNESOTA BY. Frederick Arthur Davis
 The role of partial melts of peridotite in the formation of oceanic island basalts A DISSERTATION SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL OF THE UNIVERSITY OF MINNESOTA BY Frederick Arthur Davis
The role of partial melts of peridotite in the formation of oceanic island basalts A DISSERTATION SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL OF THE UNIVERSITY OF MINNESOTA BY Frederick Arthur Davis
The Redox State of Earth s Mantle
 ANNUAL REVIEWS Further Click here for quick links to Annual Reviews content online, including: Other articles in this volume Top cited articles Top downloaded articles Our comprehensive search Annu. Rev.
ANNUAL REVIEWS Further Click here for quick links to Annual Reviews content online, including: Other articles in this volume Top cited articles Top downloaded articles Our comprehensive search Annu. Rev.
Igneous Rocks of the Convergent Margins
 Page 1 of 10 EENS 2120 Petrology Prof. Stephen A. Nelson Tulane University Igneous Rocks of the This document last updated on 08-Feb-2011 The convergent plate margins are the most intense areas of active
Page 1 of 10 EENS 2120 Petrology Prof. Stephen A. Nelson Tulane University Igneous Rocks of the This document last updated on 08-Feb-2011 The convergent plate margins are the most intense areas of active
The Effect of H 2 O on the 410-km Seismic Discontinuity
 The Effect of H 2 O on the 410-km Seismic Discontinuity B.J. Wood Science Paper (1995) Presented by HuajianYao Background Seismic discontinuities at 410 km and 660 km ------ important jumps in mantle density
The Effect of H 2 O on the 410-km Seismic Discontinuity B.J. Wood Science Paper (1995) Presented by HuajianYao Background Seismic discontinuities at 410 km and 660 km ------ important jumps in mantle density
The effect of liquid composition on the partitioning of Ni between olivine and silicate melt
 Contrib Mineral Petrol (2017) 172:3 DOI 10.1007/s00410-016-1319-8 ORIGINAL PAPER The effect of liquid composition on the partitioning of between olivine and silicate melt Andrew K. Matzen 1,2,3 Michael
Contrib Mineral Petrol (2017) 172:3 DOI 10.1007/s00410-016-1319-8 ORIGINAL PAPER The effect of liquid composition on the partitioning of between olivine and silicate melt Andrew K. Matzen 1,2,3 Michael
APPENDICES. Appendix 1
 Corthouts, T.L., Lageson, D.R., and Shaw, C.A., 2016, Polyphase deformation, dynamic metamorphism and metasomatism of Mount Everest s summit limestone, east central Himalaya, Nepal/Tibet: Lithosphere,
Corthouts, T.L., Lageson, D.R., and Shaw, C.A., 2016, Polyphase deformation, dynamic metamorphism and metasomatism of Mount Everest s summit limestone, east central Himalaya, Nepal/Tibet: Lithosphere,
Revision 2. Key Laboratory of Mineral Resources, Institute of Geology and Geophysics,
 1 2 3 4 Tectonic controls on Ni and Cu contents of primary mantle-derived magmas for the formation of magmatic sulfide deposits Revision 2 Zhuosen Yao a,b,d, Kezhang Qin a,b,c*, James E. Mungall d 5 a
1 2 3 4 Tectonic controls on Ni and Cu contents of primary mantle-derived magmas for the formation of magmatic sulfide deposits Revision 2 Zhuosen Yao a,b,d, Kezhang Qin a,b,c*, James E. Mungall d 5 a
The constancy of upper mantle fo 2 through time inferred from V/Sc ratios in basalts
 Earth and Planetary Science Letters 228 (2004) 483 493 www.elsevier.com/locate/epsl The constancy of upper mantle fo 2 through time inferred from V/Sc ratios in basalts Zheng-Xue Anser Li, Cin-Ty Aeolus
Earth and Planetary Science Letters 228 (2004) 483 493 www.elsevier.com/locate/epsl The constancy of upper mantle fo 2 through time inferred from V/Sc ratios in basalts Zheng-Xue Anser Li, Cin-Ty Aeolus
Lecture 25 Subduction Related Magmatism
 Lecture 25 Subduction Related Magmatism Monday, May 2 nd 2005 Subduction Related Magmatism Activity along arcuate volcanic chains along subduction zones Distinctly different from the mainly basaltic provinces
Lecture 25 Subduction Related Magmatism Monday, May 2 nd 2005 Subduction Related Magmatism Activity along arcuate volcanic chains along subduction zones Distinctly different from the mainly basaltic provinces
Education. UNIVERSITY OF MISSOURI, Columbia, MO B.S., with honors in Geological Sciences, Cum Laude. Positions Held
 Fred A Davis University of Minnesota Duluth Department of Earth and Environmental Sciences 229 Heller Hall, 1114 Kirby Dr., Duluth, MN 55812 email: fdavis@d.umn.edu phone: (218) 726-8331 Education UNIVERSITY
Fred A Davis University of Minnesota Duluth Department of Earth and Environmental Sciences 229 Heller Hall, 1114 Kirby Dr., Duluth, MN 55812 email: fdavis@d.umn.edu phone: (218) 726-8331 Education UNIVERSITY
PUBLICATIONS. Geochemistry, Geophysics, Geosystems
 PUBLICATIONS Geochemistry, Geophysics, Geosystems RESEARCH ARTICLE Key Points: Source and magma mixing processes are evident in continental subduction factory The heterogeneous mantle source is generated
PUBLICATIONS Geochemistry, Geophysics, Geosystems RESEARCH ARTICLE Key Points: Source and magma mixing processes are evident in continental subduction factory The heterogeneous mantle source is generated
Differentiation 2: mantle, crust OUTLINE
 Differentiation 2: mantle, crust OUTLINE Reading this week: Should have been White Ch 10 and 11!! 7- Nov Differentiation of the Earth, Core formation W 10.6.6, 11.4 9- Nov Moon, crust, mantle, atmosphere
Differentiation 2: mantle, crust OUTLINE Reading this week: Should have been White Ch 10 and 11!! 7- Nov Differentiation of the Earth, Core formation W 10.6.6, 11.4 9- Nov Moon, crust, mantle, atmosphere
Supplementary information
 Supplementary information Sample details Samples used were from the Natural History Museum, London, UK: collections BM1968 P37 and BM1957 1056, and are listed in Supplementary Table1 and Table 2. Supplementary
Supplementary information Sample details Samples used were from the Natural History Museum, London, UK: collections BM1968 P37 and BM1957 1056, and are listed in Supplementary Table1 and Table 2. Supplementary
PUBLICATIONS. Geochemistry, Geophysics, Geosystems
 PUBLICATIONS RESEARCH ARTICLE Key Points: Adiabatic mantle melting is modeled using incompatible trace elements Effects of hydrous and pyroxenitebearing sources are examined The model explores source conditions
PUBLICATIONS RESEARCH ARTICLE Key Points: Adiabatic mantle melting is modeled using incompatible trace elements Effects of hydrous and pyroxenitebearing sources are examined The model explores source conditions
Magmatic Processes at Subduction Zones
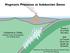 Magmatic Processes at Subduction Zones Katherine A. Kelley Graduate School of Oceanography Univ. of Rhode Island Thanks to Terry Plank Erik Hauri GVP: Liz Cottrell Simon Carn Jennifer Jay Ed Venzke Subduction
Magmatic Processes at Subduction Zones Katherine A. Kelley Graduate School of Oceanography Univ. of Rhode Island Thanks to Terry Plank Erik Hauri GVP: Liz Cottrell Simon Carn Jennifer Jay Ed Venzke Subduction
Conditions of core formation in the Earth: Constraints from Nickel and Cobalt partitioning
 doi:10.1016/j.gca.2004.10.019 Geochimica et Cosmochimica Acta, Vol. 69, No. 8, pp. 2141 2151, 2005 Copyright 2005 Elsevier Ltd Printed in the USA. All rights reserved 0016-7037/05 $30.00.00 Conditions
doi:10.1016/j.gca.2004.10.019 Geochimica et Cosmochimica Acta, Vol. 69, No. 8, pp. 2141 2151, 2005 Copyright 2005 Elsevier Ltd Printed in the USA. All rights reserved 0016-7037/05 $30.00.00 Conditions
Supplemental Material
 GSA Data Repository 2018013 Cruz-Uribe et al., 2018, Generation of alkaline magmas in subduction zones by partial melting of mélange diapirs An experimental study: Geology, https://doi.org/10.1130/g39956.1.
GSA Data Repository 2018013 Cruz-Uribe et al., 2018, Generation of alkaline magmas in subduction zones by partial melting of mélange diapirs An experimental study: Geology, https://doi.org/10.1130/g39956.1.
J. Mangas and F.J. Perez-Torrado. Departamento de Física. Universidad de Las Palmas de Gran Canaria Las Palmas de Gran Canaria.
 Magmatic processes in the oceanic lithosphere: characterization of the ultramafic and mafic materials from the Holocene volcanic centers of Bandama and La Caldera de Pinos de Gáldar (Gran Canaria, Canary
Magmatic processes in the oceanic lithosphere: characterization of the ultramafic and mafic materials from the Holocene volcanic centers of Bandama and La Caldera de Pinos de Gáldar (Gran Canaria, Canary
New evidence for lunar basalt metasomatism by underlying regolith.
 1 2 3 4 5 New evidence for lunar basalt metasomatism by underlying regolith. John F. Pernet-Fisher* School of Earth, Atmospheric, and Environmental Sciences, University of Manchester, Manchester, M13 2PL,
1 2 3 4 5 New evidence for lunar basalt metasomatism by underlying regolith. John F. Pernet-Fisher* School of Earth, Atmospheric, and Environmental Sciences, University of Manchester, Manchester, M13 2PL,
A Reappraisal of Redox Melting in the Earth s Mantle as a Function of Tectonic Setting and Time
 JOURNAL OF PETROLOGY VOLUME 52 NUMBERS 7 & 8 PAGES1363^1391 2011 doi:10.1093/petrology/egq061 A Reappraisal of Redox Melting in the Earth s Mantle as a Function of Tectonic Setting and Time STEPHEN F.
JOURNAL OF PETROLOGY VOLUME 52 NUMBERS 7 & 8 PAGES1363^1391 2011 doi:10.1093/petrology/egq061 A Reappraisal of Redox Melting in the Earth s Mantle as a Function of Tectonic Setting and Time STEPHEN F.
Diamonds and the Geology of Mantle Carbon
 Diamonds and the Geology of Mantle Carbon Steven B. Shirey 1 Pierre Cartigny 2 Daniel J. Frost 3 Shantanu Keshav 4 Fabrizio Nestola 5 Paolo Nimis 5 D. Graham Pearson 6 Nikolai V. Sobolev 7 Michael J. Walter
Diamonds and the Geology of Mantle Carbon Steven B. Shirey 1 Pierre Cartigny 2 Daniel J. Frost 3 Shantanu Keshav 4 Fabrizio Nestola 5 Paolo Nimis 5 D. Graham Pearson 6 Nikolai V. Sobolev 7 Michael J. Walter
