CHAPTER 1: MINERALS: DEFINITION, PROPERTIES AND OCCURRENCES. Sarah Lambart
|
|
|
- Arron Park
- 6 years ago
- Views:
Transcription
1 CHAPTER 1: MINERALS: DEFINITION, PROPERTIES AND OCCURRENCES Sarah Lambart
2 CONTENT OF CHAPTER 1 Goal: learn how to describe and classify minerals 3 elements of classification: chemistry, structure and environment Outline: Occurrences of minerals Classification of minerals Physical properties of minerals
3 Definitions: A mineral is always (1) inorganic, (2) naturally occurring, (3) with a structure and a composition that give it defined macroscopic properties. A mineral is most of the time a (1) crystal and (2) a solid. A crystal is a homogeneous chemical compound with a regular and periodic arrangement of atoms. These arrangements present symmetries. A crystal is not always a mineral. It can be synthetic or organic (e.g., proteins)
4 MINERAL OCCURRENCES AND ENVIRONMENTS Igneous Half dome, Yosemite (granite) Sedimentary Montaña de Oro State Beach (shales) Metamorphic Sequoia National Forest California (marble, schist and gneiss)
5 COMMON MINERALS & MINERAL ASSOCIATIONS Metamorphic Omphacite (amphibole) Jadeite (pyroxene) Epidote, chlorite Sedimentary Carbonates (calcite, dolomite, ) Salts (halite) Gypsum Igneous: Olivine + pyroxenes Plagioclases + pyroxenes
6 ADDITIONAL SLIDE Minerals in igneous rock Acid (SiO 2 >63 wt. %) Rock names Rhyolite, granite (intrusive, extrusive) Common minerals Quartz, alkali feldspar (plagioclase, hornblende, micas) Intermediate (52<SiO 2 <63 wt. %) Andesite, dacite, diorite, tonalite, Sodic plagioclase (hornblende, biotite, quartz, pyroxene). Basic (45<SiO 2 <52 wt. %) Basalt, gabbro, pyroxenites pyroxene, plagioclase, (Olivine, iron oxide, titanium oxide, quartz, amphibole, micas) Ultrabasic (SiO 2 <45 wt. %) Peridotite, kimberlite Olivine, pyroxene (plagioclase garnet, amphibole, spinel, micas)
7 ADDITIONAL SLIDE Minerals in sedimentary rocks Clastic sedimentary rocks Conglomerates and breccias (>2mm) Sandstones (0.06-2mm) Mudrocks (<0.06mm) quartz, gold, diamond, apatite, calcite, and clays Chemical sedimentary rocks Siliceous rocks (silex) Evaporite Limestone, dolostone Organic sedimentary rocks Carbonate rocks (guano, coal) calcite, gypsum, anhydrite, halite and sylvite, borate minerals phosphates, graphite, calcite, fossils
8 ADDITIONAL SLIDE Minerals in metamorphic rocks Basic rock (basalt, gabbro) Clay-rich sedimentary rock (shale, mudstone) Limestone Metabasite Metapelite Marble Amphiboles +epidote for LMG +plagioclase for HMG Muscovite + Quartz +chlorite, biotite garnet for LMG +sillimanite, opx, cordierite for HMG Calcite or dolomite +micas, quartz, clay, pyrite
9 CLASSIFICATION AND NAMING >4000 mineral species Classification: based on the dominant anion
10 Sulfates: with SO 4 2- Phosphates: with PO 4 3-, (or AsO 4 3-,VO 4 3- ) Borates: with BO 3 2- (or BO 4 5- ) Gypsum (CaSO 4 2H 2 O). Apatite Ca 5 (PO 4 ) 3 (OH) Borax Na 2 B III 2 BIV 2 O 5 (OH) 4 8H 2 O Oxide: Cation + oxygen Hydroxides: with OH - Carbonates & nitrates : with CO 3 2- (or NO 3- ) hematite (Fe 2 O 3 ) Ex.: magnetite (Fe 3 O 4 ) Brucite Mg(OH) 2 Ex.: gibbsite Al(OH) 3 Calcite CaCO 3 Ex.: Nitratite NaNO 3
11 Native: no anion Sulfide: with S 2-, (or As, Te) Halides: contained halogens (F, Cl, Br, I) Gold (Au) Ex.: Pt, Ag, Cu, C(graphite or diamond) pyrite(fes 2 ) Ex.: galena (PbS 2 ), sphalerite (ZnS) table-salt (NaCl) Ex.: Fluorite (CaF 2 ) Silicates: with SiO 4 4-
12 SILICATES Orthosilicates Olivine (Fe,Mg)2SiO4 Ex.: pyrope Mg3Al2Si3O12 Chain silicates Amphibole Sorosilicates Epidote Ca2Al2FeO(OH)SiO4 Si2O7 Sheet silicates Muscovite Cyclosilicates Tourmaline Framework silicates (or tectosilicates) Quartz SiO2 Ex.: Feldspar NaAlSi3O8
13 DIAGNOSTIC PROPERTIES - Habit - Morphology - Transparency - Luster - Color - Streak - Tenacity - Cleavage and fractures - Density - Hardness - Others
14 PHYSICAL PROPERTIES Habit Euhedral Subhedral Anhedral Tabular/platy Prismatic, acidular, fibrous Morphologies: Granular
15 Transparency Transparent (quartz) Luster Translucent (garnet) opaque (lapis-lazuli) Metallic (pyrite) Submetallic (rutile) Adamantine (diamond) Resinous (amber) Vitreous (amethyst quartz) Earthy (desert flower)
16 ADDITIONAL SLIDE Luster examples
17 Color Streak Smithsonian collection (D.C.) Tenacity Credits: depthome.brooklyn.cuny.edu/ geology/core332/minerals.htm Definition: the ability of a mineral to deform plastically under stress: Brittle > Sectile > Ductile
18 Cleavage Definition: Cleavage = plane of weakness. Fracture Conchoidal fractures (obsidian) Density (or specific gravity) Most silicates have a density between 2.6 and 3.5 g/cm 3
19 Hardness Mohs scale Moh s scale 1 Talc 2 Gypsum 3 Calcite 4 Fluorite 5 Apatite 6 Orthoclase 7 Quartz 8 Topaz 9 Corundum 10 Diamond Fingernail: 2.5 Copper penny: 3 Window glass: 5.5 Steel nail: 6.5
20 Other properties Taste Magnetism: magnetite (Fe 3 O 4 )> ilmenite (FeTiO 3 ) > pyrolusite (MnO 2 ) Acid-test: Calcite Fluorescence (Fluorite (CaF 2 ), calcite (CaCO 3 ), nepheline) Radioactivity (uraninite (UO 2 ), thorite (ThSiO 4 ), carnotite) Pleochroism Electrical conductivity
ESS Minerals. Lee. 1. The table below shows some properties of four different minerals.
 Name: ESS Minerals Pd. 1. The table below shows some properties of four different minerals. The minerals listed in the table are varieties of which mineral? (A) garnet (B) magnetite (C) olivine (D) quartz
Name: ESS Minerals Pd. 1. The table below shows some properties of four different minerals. The minerals listed in the table are varieties of which mineral? (A) garnet (B) magnetite (C) olivine (D) quartz
Matter and Minerals. Earth 9 th edition Chapter 3 Minerals: summary in haiku form "Mineral" defined: natural, inorganic, solid (and two more).
 1 2 Matter and Minerals Earth 9 th edition Chapter 3 Minerals: summary in haiku form "Mineral" defined: natural, inorganic, solid (and two more). continued... 3 4 5 6 7 8 9 10 11 12 13 14 Also crystalline,
1 2 Matter and Minerals Earth 9 th edition Chapter 3 Minerals: summary in haiku form "Mineral" defined: natural, inorganic, solid (and two more). continued... 3 4 5 6 7 8 9 10 11 12 13 14 Also crystalline,
Chapter 4. Rocks and Minerals: Documents that Record Earth's History
 Chapter 4 Rocks and Minerals: Documents that Record Earth's History What can Minerals Tell Us? 1. Minerals may contain radioactive elements that can be used for radiometric age dating. 2. Minerals that
Chapter 4 Rocks and Minerals: Documents that Record Earth's History What can Minerals Tell Us? 1. Minerals may contain radioactive elements that can be used for radiometric age dating. 2. Minerals that
Atoms>>>Elements>>>Minerals>>>Rocks>>>Continents>>>Planet
 Introduction to Minerals It s all about scale: Atoms>>>Elements>>>Minerals>>>Rocks>>>Continents>>>Planet Basic Chem: Atomic Structure Atom: smallest unit of an element that possesses the properties of
Introduction to Minerals It s all about scale: Atoms>>>Elements>>>Minerals>>>Rocks>>>Continents>>>Planet Basic Chem: Atomic Structure Atom: smallest unit of an element that possesses the properties of
Mineral Identification
 Mineral Identification! Mineral identification is a skill. " Requires learning diagnostic properties #Some properties are easily seen. $Color $Crystal shape #Some properties require handling or testing.
Mineral Identification! Mineral identification is a skill. " Requires learning diagnostic properties #Some properties are easily seen. $Color $Crystal shape #Some properties require handling or testing.
Chapter 1 Lecture Outline. Matter and Minerals
 Chapter 1 Lecture Outline Matter and Minerals Minerals: Building Blocks of Rocks Minerals are the building blocks of rocks Minerals important in human history Flint and chert for weapons and tools Gold,
Chapter 1 Lecture Outline Matter and Minerals Minerals: Building Blocks of Rocks Minerals are the building blocks of rocks Minerals important in human history Flint and chert for weapons and tools Gold,
Unit 2 Exam: Rocks & Minerals
 Name: Date: 1. Base your answer(s) to the following question(s) on the 2001 edition of the Earth Science Reference Tables, the map and cross section below, and your knowledge of Earth science. The shaded
Name: Date: 1. Base your answer(s) to the following question(s) on the 2001 edition of the Earth Science Reference Tables, the map and cross section below, and your knowledge of Earth science. The shaded
2. What is sample 1B? a. chalcopyrite b. plagioclase feldspar c. muscovite d. copper e. magnetite f. galena g. pyrite
 HSAG Mineral and Rock Exam 2014 Note: Most sample numbers do NOT match question numbers so be careful. Team: 1. What is sample 1A? a. magnetite b. galena c. pyrite d. chalcopyrite e. copper f. graphite
HSAG Mineral and Rock Exam 2014 Note: Most sample numbers do NOT match question numbers so be careful. Team: 1. What is sample 1A? a. magnetite b. galena c. pyrite d. chalcopyrite e. copper f. graphite
10/8/15. Earth Materials Minerals and Rocks. I) Minerals. Minerals. (A) Definition: Topics: -- naturally occurring What are minerals?
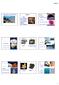 minerals Earth Materials Minerals and Rocks I) Minerals Minerals Topics: What are minerals? Basic Chemistry Amethysts in geode: minerals Characteristics of Minerals Types of Minerals -- orderly arrangement
minerals Earth Materials Minerals and Rocks I) Minerals Minerals Topics: What are minerals? Basic Chemistry Amethysts in geode: minerals Characteristics of Minerals Types of Minerals -- orderly arrangement
Name: Minerals and more minerals
 1. The diagram below shows how a sample of the mineral mica breaks when hit with a rock hammer. 6. The diagrams below show the crystal shapes of two minerals. This mineral breaks in smooth, flat surfaces
1. The diagram below shows how a sample of the mineral mica breaks when hit with a rock hammer. 6. The diagrams below show the crystal shapes of two minerals. This mineral breaks in smooth, flat surfaces
Lab 4: Mineral Identification April 14, 2009
 Name: Lab 4: Mineral Identification April 14, 2009 While about 3000 minerals have been recognized as valid species, very few of these are commonly seen. Comprehensive mineralogy texts typically deal with
Name: Lab 4: Mineral Identification April 14, 2009 While about 3000 minerals have been recognized as valid species, very few of these are commonly seen. Comprehensive mineralogy texts typically deal with
Matter and Minerals Earth: Chapter Pearson Education, Inc.
 Matter and Minerals Earth: Chapter 3 Minerals: Building Blocks of Rocks By definition a mineral is: Naturally occurring An inorganic solid Ordered internal molecular structure Definite chemical composition
Matter and Minerals Earth: Chapter 3 Minerals: Building Blocks of Rocks By definition a mineral is: Naturally occurring An inorganic solid Ordered internal molecular structure Definite chemical composition
4. The diagram of Bowen's Reaction Series below indicates the relative temperatures at which specific minerals crystallize as magma cools.
 Base your answers to questions 1 and 2 on the diagram below, which shows the results of three different physical tests, A, B, and C, that were performed on a mineral. 1. The luster of this mineral could
Base your answers to questions 1 and 2 on the diagram below, which shows the results of three different physical tests, A, B, and C, that were performed on a mineral. 1. The luster of this mineral could
This is how we classify minerals! Silicates and Non-Silicates
 Why are some minerals harder than others? Their atomic structure and chemical formula. This is how we classify minerals! Silicates and Non-Silicates Part #1 - Silicates: Silicon and Oxygen make up 70%
Why are some minerals harder than others? Their atomic structure and chemical formula. This is how we classify minerals! Silicates and Non-Silicates Part #1 - Silicates: Silicon and Oxygen make up 70%
Chemical Classification of Minerals Learning goals: Classification of Minerals by Anionic Species. Periodic Table. Anions are Negative Ions
 Classification of Minerals by Anionic Species (Anions are negative ions) Chemical Classification of Minerals Learning goals: How are minerals classified by chemistry? Why is this useful? Anions are Negative
Classification of Minerals by Anionic Species (Anions are negative ions) Chemical Classification of Minerals Learning goals: How are minerals classified by chemistry? Why is this useful? Anions are Negative
Atoms: Building Blocks of Minerals. Why Atoms Bond. Why Atoms Bond. Halite (NaCl) An Example of Ionic Bonding. Composition of Minerals.
 Matter and Minerals Earth Chapter 3 Minerals: summary in haiku form "Mineral" defined: natural, inorganic, solid (and two more). continued... Also crystalline, chemically specific. There! I fit it in!
Matter and Minerals Earth Chapter 3 Minerals: summary in haiku form "Mineral" defined: natural, inorganic, solid (and two more). continued... Also crystalline, chemically specific. There! I fit it in!
CHAPTER 2 MINERALS. Group Presentation Notes
 CHAPTER 2 MINERALS Group Presentation Notes DEFINITION OF A MINERAL A mineral is naturally occurring, inorganic solid with an orderly crystalline structure and a definite chemical composition. CHARACTERISTICS
CHAPTER 2 MINERALS Group Presentation Notes DEFINITION OF A MINERAL A mineral is naturally occurring, inorganic solid with an orderly crystalline structure and a definite chemical composition. CHARACTERISTICS
Introduction to Prospecting. Session Three Minerals
 Introduction to Prospecting Session Three Minerals Mineral: Solid inorganic substance of natural occurrence with a specific elemental composition and crystal structure. Rock: An aggregate of minerals.
Introduction to Prospecting Session Three Minerals Mineral: Solid inorganic substance of natural occurrence with a specific elemental composition and crystal structure. Rock: An aggregate of minerals.
Minerals: Building Blocks of Rocks Chapter 2. Based on: Earth Science, 10e
 Minerals: Building Blocks of Rocks Chapter 2 Based on: Earth Science, 10e Minerals: the building blocks of rocks Definition of a mineral Solid Inorganic Natural Crystalline Structure - Possess an orderly
Minerals: Building Blocks of Rocks Chapter 2 Based on: Earth Science, 10e Minerals: the building blocks of rocks Definition of a mineral Solid Inorganic Natural Crystalline Structure - Possess an orderly
Review - Unit 2 - Rocks and Minerals
 Review - Unit 2 - Rocks and Minerals Base your answers to questions 1 and 2 on the diagram below, which shows the results of three different physical tests, A, B, and C, that were performed on a mineral.
Review - Unit 2 - Rocks and Minerals Base your answers to questions 1 and 2 on the diagram below, which shows the results of three different physical tests, A, B, and C, that were performed on a mineral.
ALLEGHENY COLLEGE DEPARTMENT OF GEOLOGY STUDENT HANDBOOK
 ALLEGHENY COLLEGE DEPARTMENT OF GEOLOGY STUDENT HANDBOOK This handbook is designed as a resource for all geology majors and minors. The material contained in the handbook includes foundation principles
ALLEGHENY COLLEGE DEPARTMENT OF GEOLOGY STUDENT HANDBOOK This handbook is designed as a resource for all geology majors and minors. The material contained in the handbook includes foundation principles
1 st shell holds 2 electrons. 2 nd shell holds 8 electrons
 ATOM INDIVISIBLE ELEMENTS - Nucleus = protons (+ charge) & neutrons (no charge ) - Electrons (- charge) orbit the nucleus in shells of 2, 8, 8 electrons (inner orbit outward) - Atomic number = number of
ATOM INDIVISIBLE ELEMENTS - Nucleus = protons (+ charge) & neutrons (no charge ) - Electrons (- charge) orbit the nucleus in shells of 2, 8, 8 electrons (inner orbit outward) - Atomic number = number of
Minerals Please do not write on this test packet.
 Please do not write on this test packet. 1. The diagram below shows the index minerals of Mohs hardness scale compared with the hardness of some common objects. 2. Base your answer to the following question
Please do not write on this test packet. 1. The diagram below shows the index minerals of Mohs hardness scale compared with the hardness of some common objects. 2. Base your answer to the following question
1. Which mineral is mined for its iron content? A) hematite B) fluorite C) galena D) talc
 1. Which mineral is mined for its iron content? A) hematite B) fluorite C) galena D) talc 2. Which material is made mostly of the mineral quartz? A) sulfuric acid B) pencil lead C) plaster of paris D)
1. Which mineral is mined for its iron content? A) hematite B) fluorite C) galena D) talc 2. Which material is made mostly of the mineral quartz? A) sulfuric acid B) pencil lead C) plaster of paris D)
Which sample best shows the physical properties normally associated with regional metamorphism? (1) A (3) C (2) B (4) D
 1 Compared to felsic igneous rocks, mafic igneous rocks contain greater amounts of (1) white quartz (3) pink feldspar (2) aluminum (4) iron 2 The diagram below shows how a sample of the mineral mica breaks
1 Compared to felsic igneous rocks, mafic igneous rocks contain greater amounts of (1) white quartz (3) pink feldspar (2) aluminum (4) iron 2 The diagram below shows how a sample of the mineral mica breaks
Unit 2: Minerals and Rocks Practice Questions
 Name: Date: 1. Which mineral is white or colorless, has a hardness of 2.5, and splits with cubic cleavage? 6. Base your answer(s) to the following question(s) on the photograph of a sample of gneiss below.
Name: Date: 1. Which mineral is white or colorless, has a hardness of 2.5, and splits with cubic cleavage? 6. Base your answer(s) to the following question(s) on the photograph of a sample of gneiss below.
Applications and Investigations in Earth Science Seventh Edition
 INSTRUCTOR MANUAL Stanley C. Hatfield Southwestern Illinois College Applications and Investigations in Earth Science Seventh Edition Tarbuck Lutgens Pinzke Exercise One The Study of Minerals MATERIALS
INSTRUCTOR MANUAL Stanley C. Hatfield Southwestern Illinois College Applications and Investigations in Earth Science Seventh Edition Tarbuck Lutgens Pinzke Exercise One The Study of Minerals MATERIALS
How would you organize some 5000 species of minerals? Classification of Minerals by Anionic Species
 Classification Minerals by Anionic Species (Anions are negative ions) How would you organize some 5000 species minerals? Color? Hardness? Occurrence environment? Chemistry? Positive ions? (cations) Negative
Classification Minerals by Anionic Species (Anions are negative ions) How would you organize some 5000 species minerals? Color? Hardness? Occurrence environment? Chemistry? Positive ions? (cations) Negative
Practice Test Rocks and Minerals. Name. Page 1
 Name Practice Test Rocks and Minerals 1. Which rock would be the best source of the mineral garnet? A) basalt B) limestone C) schist D) slate 2. Which mineral is mined for its iron content? A) hematite
Name Practice Test Rocks and Minerals 1. Which rock would be the best source of the mineral garnet? A) basalt B) limestone C) schist D) slate 2. Which mineral is mined for its iron content? A) hematite
Minerals: Minerals: Building blocks of rocks. Atomic Structure of Matter. Building Blocks of Rocks Chapter 3 Outline
 Minerals: Building Blocks of Rocks Chapter 3 Outline Does not contain complete lecture notes. To be used to help organize lecture notes and home/test studies. Minerals: Building blocks of rocks Definition
Minerals: Building Blocks of Rocks Chapter 3 Outline Does not contain complete lecture notes. To be used to help organize lecture notes and home/test studies. Minerals: Building blocks of rocks Definition
Minerals. Gypsum Crystals - Mexico
 Minerals Gypsum Crystals - Mexico Rocks Rocks are Earth materials made from minerals. Most rocks have more than one kind of mineral. Example: Granite Potassium feldspar. Plagioclase Feldspar. Quartz. Hornblende.
Minerals Gypsum Crystals - Mexico Rocks Rocks are Earth materials made from minerals. Most rocks have more than one kind of mineral. Example: Granite Potassium feldspar. Plagioclase Feldspar. Quartz. Hornblende.
Minerals II: Physical Properties and Crystal Forms. From:
 Minerals II: Physical Properties and Crystal Forms From: http://webmineral.com/data/rhodochrosite.shtml The Physical Properties of Minerals Color Streak Luster Hardness External Crystal Form Cleavage The
Minerals II: Physical Properties and Crystal Forms From: http://webmineral.com/data/rhodochrosite.shtml The Physical Properties of Minerals Color Streak Luster Hardness External Crystal Form Cleavage The
5/24/2018. Matter and Minerals
 1 2 3 4 5 6 7 8 9 10 11 Matter and Minerals Earth Chapter 3 Chapter 3 Matter & Minerals Figure 3.1 Minerals: Building Blocks of Rocks Geologic Definition of a Mineral: Naturally occurring Generally inorganic
1 2 3 4 5 6 7 8 9 10 11 Matter and Minerals Earth Chapter 3 Chapter 3 Matter & Minerals Figure 3.1 Minerals: Building Blocks of Rocks Geologic Definition of a Mineral: Naturally occurring Generally inorganic
Lecture Outlines PowerPoint. Chapter 2 Earth Science 11e Tarbuck/Lutgens
 Lecture Outlines PowerPoint Chapter 2 Earth Science 11e Tarbuck/Lutgens 2006 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the use of instructors
Lecture Outlines PowerPoint Chapter 2 Earth Science 11e Tarbuck/Lutgens 2006 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the use of instructors
Page 1. Name:
 Name: 1) What is the approximate density of a mineral with a mass of 262.2 grams that displaces 46 cubic centimeters of water? A) 6.1 g/cm 3 C) 1.8 g/cm 3 B) 5.7 g/cm 3 D) 12.2 g/cm 3 2) In which two Earth
Name: 1) What is the approximate density of a mineral with a mass of 262.2 grams that displaces 46 cubic centimeters of water? A) 6.1 g/cm 3 C) 1.8 g/cm 3 B) 5.7 g/cm 3 D) 12.2 g/cm 3 2) In which two Earth
5. The table below indicates the presence of various minerals in different rock samples.
 1. Which mineral is composed of Calcium and Fluorine? A) Amphiboles B) Calcite C) Hematite D) Fluorite 2. The photograph below shows a broken piece of the mineral calcite. The calcite breaks in smooth,
1. Which mineral is composed of Calcium and Fluorine? A) Amphiboles B) Calcite C) Hematite D) Fluorite 2. The photograph below shows a broken piece of the mineral calcite. The calcite breaks in smooth,
About Earth Materials
 Grotzinger Jordan Understanding Earth Sixth Edition Chapter 3: EARTH MATERIALS Minerals and Rocks 2011 by W. H. Freeman and Company About Earth Materials All Earth materials are composed of atoms bound
Grotzinger Jordan Understanding Earth Sixth Edition Chapter 3: EARTH MATERIALS Minerals and Rocks 2011 by W. H. Freeman and Company About Earth Materials All Earth materials are composed of atoms bound
MINERALS TAKE HOME QUIZ
 NAME 1. Which is an accurate statement about rocks? A) Rocks are located only in continental areas of the Earth. B) Rocks seldom undergo change. C) Most rocks contain fossils. D) Most rocks have several
NAME 1. Which is an accurate statement about rocks? A) Rocks are located only in continental areas of the Earth. B) Rocks seldom undergo change. C) Most rocks contain fossils. D) Most rocks have several
Introduction to Geology
 Introduction to Geology Why the heck would you want to take a geology class? 1) Geology is responsible for supplying many of the things we need. 2) Geology is closely related to the environment, which
Introduction to Geology Why the heck would you want to take a geology class? 1) Geology is responsible for supplying many of the things we need. 2) Geology is closely related to the environment, which
Examining Minerals and Rocks
 Examining Minerals and Rocks What is a mineral? A mineral is homogenous, naturally occurring substance formed through geological processes that has a characteristic chemical composition, a highly ordered
Examining Minerals and Rocks What is a mineral? A mineral is homogenous, naturally occurring substance formed through geological processes that has a characteristic chemical composition, a highly ordered
Sedimentary Rocks. Materials
 Sedimentary Rocks Overview: Sedimentary rocks are broken into three different types: organic, chemical, and clastic. The Acid Test determines which rocks are clastic because they don t react with the acid.
Sedimentary Rocks Overview: Sedimentary rocks are broken into three different types: organic, chemical, and clastic. The Acid Test determines which rocks are clastic because they don t react with the acid.
REVIEW: CHAPTERS 1 TO 5. Sarah Lambart
 REVIEW: CHAPTERS 1 TO 5 Sarah Lambart CHAPTER 1: MINERAL PROPERTIES AND CLASSIFICATION CHAP. 1: MINERAL PROPERTIES AND CLASSIFICATION Mineral: naturally occurring (always) a structure and a composition
REVIEW: CHAPTERS 1 TO 5 Sarah Lambart CHAPTER 1: MINERAL PROPERTIES AND CLASSIFICATION CHAP. 1: MINERAL PROPERTIES AND CLASSIFICATION Mineral: naturally occurring (always) a structure and a composition
Physical Geology 101 Laboratory MINERALS II Silicate and Carbonate Rock-Forming Minerals
 Student Name: College: Grade: Physical Geology 101 Laboratory MINERALS II Silicate and Carbonate Rock-Forming Minerals I. INTRODUCTION: The purpose of this lab is you will improve your mineral identification
Student Name: College: Grade: Physical Geology 101 Laboratory MINERALS II Silicate and Carbonate Rock-Forming Minerals I. INTRODUCTION: The purpose of this lab is you will improve your mineral identification
MINERALS MEGA PACKET
 1. Which rock would be the best source of the mineral garnet? A) basalt B) limestone C) schist D) slate 2. Which rock is composed of the mineral halite that formed when seawater evaporated? A) limestone
1. Which rock would be the best source of the mineral garnet? A) basalt B) limestone C) schist D) slate 2. Which rock is composed of the mineral halite that formed when seawater evaporated? A) limestone
Chapter 3. Atoms and Minerals. Earth Materials
 Chapter 3 Atoms and Minerals Earth Materials Atoms and Elements: Isotopes and Ions A Review of Chemistry Atoms Atoms are composed of Protons, Neutrons and Electrons A proton has an electric charge of +1
Chapter 3 Atoms and Minerals Earth Materials Atoms and Elements: Isotopes and Ions A Review of Chemistry Atoms Atoms are composed of Protons, Neutrons and Electrons A proton has an electric charge of +1
RR#7 - Multiple Choice
 1. Which mineral is mined for its iron content? 1) hematite 2) fluorite 3) galena 4) talc 2. Which rock is composed of the mineral halite that formed when seawater evaporated? 1) limestone 2) dolostone
1. Which mineral is mined for its iron content? 1) hematite 2) fluorite 3) galena 4) talc 2. Which rock is composed of the mineral halite that formed when seawater evaporated? 1) limestone 2) dolostone
Periods on the Periodic Table
 Minerals Chapter 2 Matter Matter includes anything that has mass and takes up space (volume). It exists in 3 main states on Earth solid, liquid, and gas. Matter can be classified based on its physical
Minerals Chapter 2 Matter Matter includes anything that has mass and takes up space (volume). It exists in 3 main states on Earth solid, liquid, and gas. Matter can be classified based on its physical
Chapter 4. Rocks and Minerals: Documents that Record Earth's History
 Chapter 4 Rocks and Minerals: Documents that Record Earth's History What can Minerals Tell Us? 1. Minerals may contain radioactive elements that can be used for radiometric age dating. 2. Minerals that
Chapter 4 Rocks and Minerals: Documents that Record Earth's History What can Minerals Tell Us? 1. Minerals may contain radioactive elements that can be used for radiometric age dating. 2. Minerals that
Rocks Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3
 Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification VI. Additional sub-classes
Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification VI. Additional sub-classes
Rocks Environmental Significance. Rocks Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3. Rocks Definition of a rock
 Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 Environmental Significance I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification
Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 Environmental Significance I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification
Geology 103 Planet Earth (QR II), Laboratory Exercises 1. Minerals
 Geology 103 Planet Earth (QR II), Laboratory Exercises 1 Student Name: Section: Minerals Minerals are naturally occurring, inorganic, crystalline solids with a characteristic chemical composition. Most
Geology 103 Planet Earth (QR II), Laboratory Exercises 1 Student Name: Section: Minerals Minerals are naturally occurring, inorganic, crystalline solids with a characteristic chemical composition. Most
The most common elements that make up minerals are oxygen, silicon, aluminum, iron, calcium, potassium, and magnesium
 Mineralogy: The Study of Minerals and their Properties A Mineral! Occurs! Is a! Is a substance (element or compound)! Has atoms arrange in an orderly pattern ( )! Is (not formed by any process involving
Mineralogy: The Study of Minerals and their Properties A Mineral! Occurs! Is a! Is a substance (element or compound)! Has atoms arrange in an orderly pattern ( )! Is (not formed by any process involving
Mineral List : Rock List:
 Team Name Mineral List : A. Lepidolite B. Calcite C. Dolomite D. Feldspar E. Halite AB. Quartz AC. Apatite AD.Hematite AE. Magnetite BC. Galena BD. Pyrite BE. Gypsum CD. Chalcopyrite CE. Staurolite DE.
Team Name Mineral List : A. Lepidolite B. Calcite C. Dolomite D. Feldspar E. Halite AB. Quartz AC. Apatite AD.Hematite AE. Magnetite BC. Galena BD. Pyrite BE. Gypsum CD. Chalcopyrite CE. Staurolite DE.
Physical Geology 101 Laboratory MINERALS I Properties, Classification and Identification
 Student Name: College: Grade: Physical Geology 101 Laboratory MINERALS I Properties, Classification and Identification INTRODUCTION: The purpose of this lab is to learn the characteristics of minerals,
Student Name: College: Grade: Physical Geology 101 Laboratory MINERALS I Properties, Classification and Identification INTRODUCTION: The purpose of this lab is to learn the characteristics of minerals,
(1) naturally occurring (2) inorganic (3) solid that has a (4) definite chemical composition and (5) crystal structure
 Page 1 (1) naturally occurring (2) inorganic (3) solid that has a (4) definite chemical composition and (5) crystal structure quartz, pyrite cement, steel not formed from living things or the remains of
Page 1 (1) naturally occurring (2) inorganic (3) solid that has a (4) definite chemical composition and (5) crystal structure quartz, pyrite cement, steel not formed from living things or the remains of
1 What Is a Mineral? Critical Thinking 2. Apply Concepts Glass is made up of silicon and oxygen atoms in a 1:2 ratio. The SiO 2
 CHAPTER 5 1 What Is a Mineral? SECTION Minerals of Earth s Crust KEY IDEAS As you read this section, keep these questions in mind: What is a mineral? What are the two main groups of minerals? What are
CHAPTER 5 1 What Is a Mineral? SECTION Minerals of Earth s Crust KEY IDEAS As you read this section, keep these questions in mind: What is a mineral? What are the two main groups of minerals? What are
Name Regents Review #7 Date
 Name Regents Review #7 Date Base your answers to questions 1 and 2 on the pictures of four rocks shown below. Magnified views of the rocks are shown in the circles. 5. The diagrams below show the crystal
Name Regents Review #7 Date Base your answers to questions 1 and 2 on the pictures of four rocks shown below. Magnified views of the rocks are shown in the circles. 5. The diagrams below show the crystal
ENVI.2030L - Minerals
 ENVI.2030L - Minerals Name I. Minerals Minerals are crystalline solids - the particles (atoms) that make-up the solid have a regular arrangement. In glasses, on the other hand, the atoms are not arranged
ENVI.2030L - Minerals Name I. Minerals Minerals are crystalline solids - the particles (atoms) that make-up the solid have a regular arrangement. In glasses, on the other hand, the atoms are not arranged
NAME: Log onto YouTube and search for jocrisci channel.
 NAME: Log onto YouTube and search for jocrisci channel. MINERALS (Video 3.1 ESRT 16) 1. A student claimed that an object in his hand was a rock. The teacher said it was a mineral. What tests would have
NAME: Log onto YouTube and search for jocrisci channel. MINERALS (Video 3.1 ESRT 16) 1. A student claimed that an object in his hand was a rock. The teacher said it was a mineral. What tests would have
Station A. 3. The amount of time it takes molten rock to cool and harden mainly affects the rock s. A. Color B. Mass C. Crystals D.
 Station A 1. Specimen AA is. A. Limestone B. Quartzite C. Basalt D. Slate 2. Specimen AA is. A. Metamorphic B. Igneous C. Sedimentary D. None of the above 3. The amount of time it takes molten rock to
Station A 1. Specimen AA is. A. Limestone B. Quartzite C. Basalt D. Slate 2. Specimen AA is. A. Metamorphic B. Igneous C. Sedimentary D. None of the above 3. The amount of time it takes molten rock to
Minerals. What are minerals and how do we classify them?
 Minerals What are minerals and how do we classify them? 1 Minerals! Minerals are the ingredients needed to form the different types of rocks! Rock - is any naturally formed solid that is part of Earth
Minerals What are minerals and how do we classify them? 1 Minerals! Minerals are the ingredients needed to form the different types of rocks! Rock - is any naturally formed solid that is part of Earth
1. Base your answer to the following question on on the photographs and news article below. Old Man s Loss Felt in New Hampshire
 UNIT 3 EXAM ROCKS AND MINERALS NAME: BLOCK: DATE: 1. Base your answer to the following question on on the photographs and news article below. Old Man s Loss Felt in New Hampshire FRANCONIA, N.H. Crowds
UNIT 3 EXAM ROCKS AND MINERALS NAME: BLOCK: DATE: 1. Base your answer to the following question on on the photographs and news article below. Old Man s Loss Felt in New Hampshire FRANCONIA, N.H. Crowds
The Nucleus. Protons. Positive electrical charge The number of protons in the nucleus determines the atomic number
 Matter Atoms The smallest unit of an element that retain its properties Small nucleus surrounded by a cloud of electrons The nucleus contains protons and neutrons The Nucleus Protons Positive electrical
Matter Atoms The smallest unit of an element that retain its properties Small nucleus surrounded by a cloud of electrons The nucleus contains protons and neutrons The Nucleus Protons Positive electrical
EESC 4701: Igneous and Metamorphic Petrology IGNEOUS MINERALS LAB 1 HANDOUT
 EESC 4701: Igneous and Metamorphic Petrology IGNEOUS MINERALS LAB 1 HANDOUT Sources: Cornell EAS302 lab, UMass Lowell 89.301 Mineralogy, LHRIC.org The Petrographic Microscope As you know, light is an electromagnetic
EESC 4701: Igneous and Metamorphic Petrology IGNEOUS MINERALS LAB 1 HANDOUT Sources: Cornell EAS302 lab, UMass Lowell 89.301 Mineralogy, LHRIC.org The Petrographic Microscope As you know, light is an electromagnetic
1. Which mineral shows no cleavage, has a hardness of 7, and a composition of SiO2? A) Graphite B) Garnet C) Halite D) Quartz 2. Which mineral leaves
 1. Which mineral shows no cleavage, has a hardness of 7, and a composition of SiO2? A) Graphite B) Garnet C) Halite D) Quartz 2. Which mineral leaves a green-black powder when rubbed against an unglazed
1. Which mineral shows no cleavage, has a hardness of 7, and a composition of SiO2? A) Graphite B) Garnet C) Halite D) Quartz 2. Which mineral leaves a green-black powder when rubbed against an unglazed
PHILADELPHIA UNIVERSITY
 PHILADELPHIA UNIVERSITY FACULTY OF ENGINEERING AND TECHNOLOGY DEPARTMENT OF CIVIL ENGINEERING. Engineering Geology Part one 1 2nd semester 2018/2019 Eng. Amany Assouli 1 INTRODUCTION: What is the engineering
PHILADELPHIA UNIVERSITY FACULTY OF ENGINEERING AND TECHNOLOGY DEPARTMENT OF CIVIL ENGINEERING. Engineering Geology Part one 1 2nd semester 2018/2019 Eng. Amany Assouli 1 INTRODUCTION: What is the engineering
Minerals. Atoms, Elements, and Chemical Bonding. Definition of a Mineral 2-1
 Minerals In order to define a what we mean by a mineral we must first make some definitions: 2-1 Most of the Earth s surface is composed of rocky material. An element is a substance which cannot be broken
Minerals In order to define a what we mean by a mineral we must first make some definitions: 2-1 Most of the Earth s surface is composed of rocky material. An element is a substance which cannot be broken
INTRODUCTION. From the earliest time, man has found important uses of minerals.
 CHAPTER 2: MINERALS INTRODUCTION From the earliest time, man has found important uses of minerals. E.g. clay for bricks and pottery; quartz and jade for weapons, garnet, amethyst and other coloured stones
CHAPTER 2: MINERALS INTRODUCTION From the earliest time, man has found important uses of minerals. E.g. clay for bricks and pottery; quartz and jade for weapons, garnet, amethyst and other coloured stones
Time to see your. Registration November
 Time to see your advisor Registration November 16-20 http://www.myspace.com/over60agelesslady 5 points Extra Credit Seminar this week in NS 103 Wednesday November 11, noon to 1 Attend, write a ½ to 1 page
Time to see your advisor Registration November 16-20 http://www.myspace.com/over60agelesslady 5 points Extra Credit Seminar this week in NS 103 Wednesday November 11, noon to 1 Attend, write a ½ to 1 page
Atoms, Molecules and Minerals
 Atoms, Molecules and Minerals Atoms Matter The smallest unit of an element that retain its properties Molecules - a small orderly group of atoms that possess specific properties - H 2 O Small nucleus surrounded
Atoms, Molecules and Minerals Atoms Matter The smallest unit of an element that retain its properties Molecules - a small orderly group of atoms that possess specific properties - H 2 O Small nucleus surrounded
*Theory= If all available testing support a hypothesis. *Law= Theory that continually passes all tests over long periods of time.
 Rodrigo Rivera-Reyes GEOL 1104/1114 Updated October 2012 GEOL LAB Midterm 1 Study Guide 1. Scientific Method. O Observation.- Something is noticed and causes a question to be asked. H Hypothesis.- Proposed
Rodrigo Rivera-Reyes GEOL 1104/1114 Updated October 2012 GEOL LAB Midterm 1 Study Guide 1. Scientific Method. O Observation.- Something is noticed and causes a question to be asked. H Hypothesis.- Proposed
OFFICIAL MID-HUDSON VALLEY GEM & MINERAL SOCIETY (MHVG&MS) 2017 EARTH SCIENCE SCAVENGER HUNT QUESTIONNAIRE. New York s Gemstone
 OFFICIAL MID-HUDSON VALLEY GEM & MINERAL SOCIETY (MHVG&MS) 2017 EARTH SCIENCE SCAVENGER HUNT QUESTIONNAIRE 2017 SHOW THEME Garnet -variety: ALMANDINE New York s Gemstone Please sign in at the Earth Science
OFFICIAL MID-HUDSON VALLEY GEM & MINERAL SOCIETY (MHVG&MS) 2017 EARTH SCIENCE SCAVENGER HUNT QUESTIONNAIRE 2017 SHOW THEME Garnet -variety: ALMANDINE New York s Gemstone Please sign in at the Earth Science
Rocks and Minerals C Key. Science Olympiad North Regional Tournament at the University of Florida
 Rocks and Minerals C Key Science Olympiad North Regional Tournament at the University of Florida Station 1 Answer: Azurite 2. What is the chemical formula Answer: Cu 3 (CO 3 ) 2 (OH) 2 3. What element
Rocks and Minerals C Key Science Olympiad North Regional Tournament at the University of Florida Station 1 Answer: Azurite 2. What is the chemical formula Answer: Cu 3 (CO 3 ) 2 (OH) 2 3. What element
Science Olympiad Captains Tryouts 2018 dupont Manual High School
 Science Olympiad Captains Tryouts 2018 dupont Manual High School Score: / 78 Rocks & Minerals Date: Part I: (25) points Based on the image and information provided, fill in the table. Each answer is worth
Science Olympiad Captains Tryouts 2018 dupont Manual High School Score: / 78 Rocks & Minerals Date: Part I: (25) points Based on the image and information provided, fill in the table. Each answer is worth
ROCKS & MINERALS UNIT. 8 th Grade Earth & Space Science
 ROCKS & MINERALS UNIT 8 th Grade Earth & Space Science Characteristics of Minerals 8 th Grade Earth & Space Science Class Notes Mineral Characteristics Naturally occurring formed by natural processes Inorganic
ROCKS & MINERALS UNIT 8 th Grade Earth & Space Science Characteristics of Minerals 8 th Grade Earth & Space Science Class Notes Mineral Characteristics Naturally occurring formed by natural processes Inorganic
Station A. 1. Specimen AA is. A. Granite B. Basalt C. Garnet Schist D. Gneiss
 Station A 1. Specimen AA is. A. Granite B. Basalt C. Garnet Schist D. Gneiss 2. Specimen AA is. A. Metamorphic B. Igneous C. Sedimentary D. None of the above 3. Specimen AA reacts with hydrochloric acid
Station A 1. Specimen AA is. A. Granite B. Basalt C. Garnet Schist D. Gneiss 2. Specimen AA is. A. Metamorphic B. Igneous C. Sedimentary D. None of the above 3. Specimen AA reacts with hydrochloric acid
Atoms Elements Minerals
 Atoms Elements Minerals Atoms The building blocks of all matter. Atoms The building blocks of all matter. Atoms The building blocks of all matter. 1 Atoms consist of a positively charged nucleus of protons
Atoms Elements Minerals Atoms The building blocks of all matter. Atoms The building blocks of all matter. Atoms The building blocks of all matter. 1 Atoms consist of a positively charged nucleus of protons
Minerals and Rocks. Environmental Learning Community CORC 1332 Sept 21, 2010
 Minerals and Rocks Environmental Learning Community CORC 1332 Sept 21, 2010 Outline Quiz More on minerals Twinkies Rocks How can you identify one mineral from another? Distinguishing One Mineral from Another
Minerals and Rocks Environmental Learning Community CORC 1332 Sept 21, 2010 Outline Quiz More on minerals Twinkies Rocks How can you identify one mineral from another? Distinguishing One Mineral from Another
40-50 Minutes, 3 minutes per station, 13 Stations, samples provided by UWM and Pierre Couture
 Event: Judge: Rocks & Minerals Pierre couture 40-50 Minutes, 3 minutes per station, 13 Stations, samples provided by UWM and Pierre Couture 1-4 Minerals (50 points total) 5-7 Igneous Rocks (50 points total)
Event: Judge: Rocks & Minerals Pierre couture 40-50 Minutes, 3 minutes per station, 13 Stations, samples provided by UWM and Pierre Couture 1-4 Minerals (50 points total) 5-7 Igneous Rocks (50 points total)
ROCKS AND MINERALS E J C H O N O U R S D A Y
 ROCKS AND MINERALS E J C H O N O U R S D A Y 2 0 1 3 DIFFERENCES BETWEEN ROCKS AND MINERALS MINERALS Solid formations that occur naturally in the earth Have a unique chemical composition Defined by its
ROCKS AND MINERALS E J C H O N O U R S D A Y 2 0 1 3 DIFFERENCES BETWEEN ROCKS AND MINERALS MINERALS Solid formations that occur naturally in the earth Have a unique chemical composition Defined by its
Engineering Geology Laboratory Manual
 Engineering Geology Laboratory Manual Civil Engineering Department BRCM College of Engg. & Tech. Bahal-127 028, Bhiwani Haryana Prepared by: Urmil Yadav 2015 Civil Engineering Department Sr. No. LIST OF
Engineering Geology Laboratory Manual Civil Engineering Department BRCM College of Engg. & Tech. Bahal-127 028, Bhiwani Haryana Prepared by: Urmil Yadav 2015 Civil Engineering Department Sr. No. LIST OF
Minerals and Rocks Chapter 20
 Minerals and Rocks Chapter 20 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Minerals and Rocks Chapter 20 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
The Use of Minerals. Chapter 3
 Section 3 The Formation, Mining, and Use of Minerals The Use of Minerals Metallic Minerals are good conductors of heat and electricity. They can be processed for various uses, including building aircraft,
Section 3 The Formation, Mining, and Use of Minerals The Use of Minerals Metallic Minerals are good conductors of heat and electricity. They can be processed for various uses, including building aircraft,
Physical Geology 101 Laboratory MINERALS I Properties, Classification and Identification
 Student Name: College: Physical Geology 101 Laboratory MINERALS I Properties, Classification and Identification Grade: INTRODUCTION: The purpose of this lab is to learn the characteristics of minerals,
Student Name: College: Physical Geology 101 Laboratory MINERALS I Properties, Classification and Identification Grade: INTRODUCTION: The purpose of this lab is to learn the characteristics of minerals,
Earth Science Minerals. Moh s Scale of Hardness In which New York State landscape region was most of the garnet mined?
 Name: ate: 1. Which mineral is white or colorless, has a hardness of 2.5, and splits with cubic cleavage? A. calcite. halite C. pyrite. mica 2. ase your answer(s) to the following question(s) on the map
Name: ate: 1. Which mineral is white or colorless, has a hardness of 2.5, and splits with cubic cleavage? A. calcite. halite C. pyrite. mica 2. ase your answer(s) to the following question(s) on the map
Emily and Megan. Earth System Science. Elements of Earth by weight. Crust Elements, by weight. Minerals. Made of atoms Earth is mostly iron, by weight
 Emily and Megan Chapter 20 MINERALS AND ROCKS Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Emily and Megan Chapter 20 MINERALS AND ROCKS Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
muscovite PART 4 SHEET SILICATES
 muscovite PART 4 SHEET SILICATES SHEET SILICATES = PHYLLOSILICATES Phyllon = leaf Large group of mineral including many common minerals: muscovite, biotite, serpentine, chlorite, talc, clay minerals Structure:
muscovite PART 4 SHEET SILICATES SHEET SILICATES = PHYLLOSILICATES Phyllon = leaf Large group of mineral including many common minerals: muscovite, biotite, serpentine, chlorite, talc, clay minerals Structure:
Rocks. Rocks are composed of 1 or more minerals. Rocks are classified based on how they formed (origin). 3 classes of rocks:
 ROCKS Rocks If a mineral is a naturally occurring homogeneous solid, inorganically formed, with a definite chemical composi:on and a crystalline structure then what is a rock? Rocks Rocks are composed
ROCKS Rocks If a mineral is a naturally occurring homogeneous solid, inorganically formed, with a definite chemical composi:on and a crystalline structure then what is a rock? Rocks Rocks are composed
OFFICIAL MID-HUDSON VALLEY GEM & MINERAL SOCIETY (MHVG&MS) 2018 EARTH SCIENCE SCAVENGER HUNT QUESTIONNAIRE
 OFFICIAL MID-HUDSON VALLEY GEM & MINERAL SOCIETY (MHVG&MS) 2018 EARTH SCIENCE SCAVENGER HUNT QUESTIONNAIRE SHOW THEM Fossils of New York and more! Please sign in at the Earth Science Table before you start
OFFICIAL MID-HUDSON VALLEY GEM & MINERAL SOCIETY (MHVG&MS) 2018 EARTH SCIENCE SCAVENGER HUNT QUESTIONNAIRE SHOW THEM Fossils of New York and more! Please sign in at the Earth Science Table before you start
Full file at
 Minerals: The Building Blocks of Rocks 2 Learning Objectives After reading, studying, and discussing the chapter, students should be able to: List the definitive characteristics that qualify certain Earth
Minerals: The Building Blocks of Rocks 2 Learning Objectives After reading, studying, and discussing the chapter, students should be able to: List the definitive characteristics that qualify certain Earth
Rocks and the Rock Cycle notes from the textbook, integrated with original contributions
 Rocks and the Rock Cycle notes from the textbook, integrated with original contributions Alessandro Grippo, Ph.D. Gneiss (a metamorphic rock) from Catalina Island, California Alessandro Grippo review Rocks
Rocks and the Rock Cycle notes from the textbook, integrated with original contributions Alessandro Grippo, Ph.D. Gneiss (a metamorphic rock) from Catalina Island, California Alessandro Grippo review Rocks
A Coaches Introduction to the 2017 R & M Event of the Science Olympiad. Presentation B
 A Coaches Introduction to the 2017 R & M Event of the Science Olympiad Presentation B Minerals Characteristics Naturally occurring Inorganic Solid Definite chemical composition Orderly internal crystal
A Coaches Introduction to the 2017 R & M Event of the Science Olympiad Presentation B Minerals Characteristics Naturally occurring Inorganic Solid Definite chemical composition Orderly internal crystal
Version 1 Page 1 Barnard/George/Ward
 The Great Mineral & Rock Test 1. Base your answer to the following question on the table below which provides information about the crystal sizes and the mineral compositions of four igneous rocks, A,
The Great Mineral & Rock Test 1. Base your answer to the following question on the table below which provides information about the crystal sizes and the mineral compositions of four igneous rocks, A,
Minerals. Elements and Minerals
 Minerals Gypsum Crystals (actual size) Elements and Minerals 87 naturally occurring elements 12 are found in the earth s crust in amounts >1% These twelve make up 99% of the mass of the crust. 70% of the
Minerals Gypsum Crystals (actual size) Elements and Minerals 87 naturally occurring elements 12 are found in the earth s crust in amounts >1% These twelve make up 99% of the mass of the crust. 70% of the
Minerals. [Most] rocks are [mostly] made of minerals, so identification and interpretation depends on recognizing
![Minerals. [Most] rocks are [mostly] made of minerals, so identification and interpretation depends on recognizing Minerals. [Most] rocks are [mostly] made of minerals, so identification and interpretation depends on recognizing](/thumbs/85/92759959.jpg) Minerals [Most] rocks are [mostly] made of minerals, so identification and interpretation depends on recognizing Over mineral types have been described, but only about account for the bulk of most rocks.
Minerals [Most] rocks are [mostly] made of minerals, so identification and interpretation depends on recognizing Over mineral types have been described, but only about account for the bulk of most rocks.
Lab #4: Minerals: Building Blocks of Rocks
 Lab #4: Minerals: Building Blocks of Rocks Minerals: Building Blocks of Rocks By definition a mineral is/has Naturally occurring Inorganic solid Ordered internal molecular structure Definite chemical composition
Lab #4: Minerals: Building Blocks of Rocks Minerals: Building Blocks of Rocks By definition a mineral is/has Naturally occurring Inorganic solid Ordered internal molecular structure Definite chemical composition
Crust Elements. Elements of Earth. Minerals. Crystals. Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air
 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Made of atoms Earth is mostly iron, by weight Elements of Earth Made of atoms
Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Made of atoms Earth is mostly iron, by weight Elements of Earth Made of atoms
Name: NAME PROPERTY 1 PROPERTY 2. Specimen #41: Specimen #42: (ASK!) Specimen #43: Specimen #44: Tuesday Wednesday (circle lab day)
 Name: Tuesday Wednesday (circle lab day) CEEES/SC 10110-20110 Planet Earth Laboratory Laboratory #3: Identification of Minerals (99 points total) Readings: Chapters 1 & 2, Laboratory Manual (from the web),
Name: Tuesday Wednesday (circle lab day) CEEES/SC 10110-20110 Planet Earth Laboratory Laboratory #3: Identification of Minerals (99 points total) Readings: Chapters 1 & 2, Laboratory Manual (from the web),
MINERALS Smith and Pun Chapter 2 ATOMIC STRUCTURE
 MINERALS Smith and Pun Chapter 2 1 ATOMIC STRUCTURE 2 1 ATOMIC STRUCTURE (2) (See Smith and Pun, pages 29-35) ELEMENT: Substance that cannot be broken down into other substances by ordinary chemical methods
MINERALS Smith and Pun Chapter 2 1 ATOMIC STRUCTURE 2 1 ATOMIC STRUCTURE (2) (See Smith and Pun, pages 29-35) ELEMENT: Substance that cannot be broken down into other substances by ordinary chemical methods
A Study Guide for Learning. Rock Identification. Geology Department Green River Community College
 A Study Guide for Learning Rock Identification Geology Department Green River Community College FORMAT: This Lab Study Guide consists of the following parts: PART I PART II PART III PART IV PART V (A-F)
A Study Guide for Learning Rock Identification Geology Department Green River Community College FORMAT: This Lab Study Guide consists of the following parts: PART I PART II PART III PART IV PART V (A-F)
