#89 Notes Unit 11: Acids & Bases and Radiochemistry Ch. Acids, Bases, and Radioactivity
|
|
|
- Philippa Rice
- 6 years ago
- Views:
Transcription
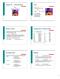
1 #89 Notes Unit 11: Acids & Bases and Radiochemistry Ch. Acids, Bases, and Radioactivity Common Strong Acids Common Strong Bases HCl hydrochloric acid Group #1 + OH HNO 3 nitric acid NaOH, KOH etc. H 2 SO 4 sulfuric acid Ca(OH) 2 HClO 4 perchloric acid Ba(OH) 2 If #O - #H 2, then strong acid. Sr(OH) 2 Common Weak Acids Common Weak Bases H 3 PO 4 phosphoric acid NH 3 ammonia HNO 2 nitrous acid CH 3 NH 2 methyl amine H 2 CO 3 carbonic acid C 2 H 5 NH 2 ethyl amine HC 2 H 3 O 2 = CH 3 CO 2 H ** H 2 NOH hydroxyl amine (acetic acid) ** H 2 NNH 2 hydrazine ** Acids: start with H or end in CO 2 H (-COOH) and have a sour taste. ** Bases: end in OH (positive ion in front) or end in NH, -NH 2, NH 3 They have a bitter taste and feel slippery. * 3 Acid/Base Theories I. Arrhenius Theory a) Acids produce H + in a water solution H 2 O + HCl (g) H + (aq) + Cl - (aq) (+ H 2 O) b) Bases produce OH - in a water solution H 2 O + NaOH (s) Na + (aq) + OH - (aq) (+ H 2 O) Acids, Bases and Salts are Electrolytes (Ion forming in water).
2 II. Brønsted-Lowry Theory a) Acids donate protons (H + ) lose 1e -, 1 p left, 0 neutrons HCl (g) + H 2 O H 3 O + (aq) + Cl - (aq) H 3 O + = hydronium ion conjugate acid conjugate base (acid always has H + ) - deprotonated acid -protonated base b) Bases accept protons + NH 3(g) + H 2 O NH 4 (aq) + OH - (aq) conj. acid conj. base (has H + ) III. Lewis Theory a) Acids are e - pair acceptors. b) Bases are e - pair donors. :NH 3 + H 2 O H:NH OH - * Bases have extra electrons. * Strong acids/bases completely ionize (fall apart). * Weak acids/bases only partially ionize (equilibriums). HF (g) + H 2 O (l) H 3 O + (aq) + F - (aq) K a = 7.2 X10-4 K a = [H 3 O + ] [F - ] [HF] The larger the K a, the more H 3 O +, the stronger the acid. (The larger the K b, the more OH -, the stronger the base.) Ex. 1) List the following in order of increasing acid strength: H 2 O, HCl, & HF. **K w = 1 X10-14 H 2 O (K = 1 X10-14 ) < HF (K = 7.2 X10-4 ) < HCl (no K, so strong acid) smallest K, so weakest
3 #90 Notes IV. ph scale - is a measure of the acidity(h 3 O + or H + ) acid base * ph = -log[h 3 O + ] * poh = -log[oh - ] ** H 3 O + = H + H 2 O (l) + H 2 O (l) H 3 O + (aq)+ OH - (aq) * K w = 1 x = [H 3 O + ][OH - ] -log ( ) * 14 = ph + poh Ex. 1) Find [H 3 O + ], [OH - ], ph, & poh for a strong acid: a) M HNO 3 (no K so strong acid) HNO 3 + H 2 O H 3 O + + NO M 0 0 (Initial) M M M (Change) M M (Final) H 3 O + = M ph = -log(h 3 O + ) = -log(0.213 M) = -(-0.672) = K w = [H 3 O + ][OH - ] 1 x = (0.213 M)(OH - ) 4.69 x M = [OH - ] poh = -log(oh - ) = -log(4.69 x M) = -( ) = **The whole number is the power and is not significant! OR From ph = ph + poh = poh = 14 poh = poh = -log(oh - ) = -log(oh - ) = log (OH - ) move (-) before 10 x 4.70 x = [OH - ]
4 Ex. 2) Calculate [H 3 O + ] and [OH - ] from: a) ph = 4.90 ph < 7 so acidic ph = - log [H 3 O + ] 4.90 = - log [H 3 O + ] = log [H 3 O + ] **move (-) before 10x 10 x (-4.90 = log [H 3 O + ] ) 1.3 X10-5 M = [H 3 O + ] K w = 1 X10-14 = (1.3 X10-5 M) (OH - ) 7.7 X10-10 M = [OH - ] b) poh = 5.80 poh = - log [OH - ] 5.80 = - log [OH - ] 10 x ( = log [OH - ] ) 1.6 X10-6 M = [OH - ] K w = 1 X10-14 = (H 3 O + ) ( 1.6 X10-6 M) 6.3 X10-9 M = [H 3 O + ] Is this acidic or basic? ph = - log [H 3 O + ] = - log 6.3 X10-9 M = 8.20 (ph > 7 so, basic) Or look at [H 3 O + ] and [OH - ]: if [H 3 O + ] is larger, it is acidic, if [OH - ] is larger, it is basic. ** H 3 O + = H +
5 #91 Notes V. Weak Acids/ Bases -only partially ionize (equilibrium) Ex. 1) What is the ph of a M solution of acetic acid (CH 3 COOH = HC 2 H 3 O 2 )? (weak acid) What are the major species present? K = 1.8 X 10-5 HC 2 H 3 O 2 + H 2 O (l) H 3 O C 2 H 3 O M (Initial) - x + x + x (Change) M - x x x (Equilibrium) K a = [H 3 O + ][ C 2 H 3 O - 2 ] [HC 2 H 3 O 2 ] no liquids: H 2 O (l) 1.8 X 10-5 = (x)(x) d If K a is smaller than the Concentration by 10 3 or more, M x assume x is small. (0.200 M x would equal just M) (2.00 X10-1 ) 1.8 X 10-5 = x 2 d M 1.9 X 10-3 M = x = [H 3 O + ] = [C 2 H 3 O 2 - ] [HC 2 H 3 O 2 ] = M x = M ph = -log(1.9 X 10-3 ) = 2.72 Ex. 2) What is the ph of a M solution of ethyl amine, C 2 H 5 NH 2? (weak base) K = 5.6 X10-4 C 2 H 5 NH 2 + H 2 O C 2 H 5 NH OH M -x +x +x x x x K b = [C 2 H 5 NH 3 + ] [OH - ] [C 2 H 5 NH 2 ] 5.6 X10-4 = (x)(x) (0.520 x) x is small (5.20 X10-1 ) 5.6 X10-4 = x 2 (0.520) 1.71 X10-2 = x = OH - poh = - log [OH - ] or K w = [H 3 O + ] [OH - ] poh = = ph + poh = = ph
6 Ex. 3) What is the ph of a M solution of HClO 2 (chlorous acid) K of HClO 2 = 1.2 X10-2 HClO 2 + H 2 O H 3 O ClO M -x +x +x x x x K a = [H 3 O + ] [ClO 2 - ] [HClO 2 ] 1.2 X10-2 = (x) (x) (0.340 x) x is not small (3.40 X10-1 ) (0.340 x) = x x x 2 = 0 a = -1 b = c = b ± b 2-4ac 2a -(-0.012) ± (-0.012) 2 4(-1) ( ) 2 (-1) ± ± ± = or x = or negative concentrations are impossible! [H 3 O + ] = x = M, ph = -log [H 3 O + ] = 1.24 check: K a = (0.058) (0.058) = 1.19 X X10-2 ( )
7 Ex.4) Find ph of 0.10 M Sr(OH) 2. **Strong Bases, like strong acids completely Strong Base: Sr(OH) 2 + H 2 O Sr 2+ (aq) + 2 OH - (aq) fall apart M M M + 2(0.10M) M 0.20 M [OH - ] = 0.20 M poh = - log [OH - ] = poh = ph = 13.30
8 #92 Notes Ch. Aqueous Equilibria I. Buffered Solutions -resist changes in ph. A buffered solution may contain a weak acid with its salt (CB) or a weak base with its salt (CA). Acids: HNO 2 + H 2 O H 3 O NO 2 CA CB + (Na + or K + ) NaNO 2 or KNO 2 = salts of HNO 2 + Bases: C 6 H 5 NH 2 + H 2 O C 6 H 5 NH 3 + OH - CA + (Cl - or Br - ) CB C 6 H 5 NH 3 Cl or C 6 H 5 NH 3 Br = salts of C 6 H 5 NH 2 Ex.1) What is the ph of a M solution of HClO buffered with M NaClO? K of HClO = 3.5 X10-8 HClO + H 2 O H 3 O + + ClO M M from M Na + ClO - -x +x +x x x x 3.5 X10-8 = (x) ( x) (0.300 x) x is small 3.5 X10-8 = (x) (0.0400) (0.300) 2.6 X10-7 = x = [H 3 O + ] ph = - log [H 3 O + ] = 6.58
9 Ex. 2) What is the ph of a solution containing M H 2 NNH 2 and M H 2 NNH 3 Cl. K of H 2 NNH 2 = 3.0 X H 2 NNH 2 + H 2 O H 2 NNH 3 + OH M M from M H 2 NNH + 3 Cl - -x +x +x x x x 3.0 X10-6 = ( x) (x) (0.450 x) x is small 3.0 X10-6 = (0.250) (x) (0.450) 5.4 X10-6 = x = [OH - ] poh = - log [OH - ] = 5.27 ph = 14 - poh = 8.73
10 #93 Notes II. Solubility Equilibria - describes the amount of solid that dissolves in a saturated solution (no more solid will dissolve). Ex. 1) Calculate the solubility of Ag 2 CO 3 in mols per liter. K sp = 8.1 X10-12 Ag 2 CO 3(s) 2 Ag + (aq) + CO 3 2- (aq) -x +2x +x K sp = [Ag + ] 2 [CO 3 2- ] 1 K sp = (2x) 2 (x) 1 K sp = 4x X10-12 = 4x X10-4 M = x 1.3 X10-4 mol/l Ex. 2) Calculate the K sp of CaF 2, if its solubility is 2.15 X10-4 mol/l. CaF 2(s) Ca 2+ (aq) + 2 F - (aq) -x x 2 x x = 2.15 X10-4 mol/l K sp = [Ca 2+ ] 1 [F - ] 2 K sp = (x) 1 (2x) 2 K sp = 4x 3 K sp = 4 (2.15 X10-4 mol/l) 3 = 3.97 X10-11 Ex. 3) Calculate the solubility of Cu 3 (PO 4 ) 2 in mol/l. K sp = 8.4 X10-15 Cu 3 (PO 4 ) 2(s) 3 Cu 2+ (aq) + 2 PO 4 3- (aq) -x +3x +2x K sp = [Cu 2+ ] 3 [PO 4 3- ] 2 K sp = (3x) 3 (2x) 2 K sp = (27 x 3 ) (4 x 2 ) K sp = 108 x X10-15 = 108 x X10-17 = x X10-4 mol/l = x
11 #94 Notes III. Precipitation Reactions Ex.1) Will a precipitate form, if 25 ml of M AgNO 3 is added to 105 ml of M Na 2 CO 3? AgNO 3(aq) + Na 2 CO 3(aq) Ag 2 CO 3 + NaNO 3 Ag + - NO 3 Na + 2- CO 3 K sp = 8.1 X10-12 no K sp a) Find concentration of ions in new volume: 25 ml ml = 130 ml (new solution) ML = ML (0.100 M AgNO 3 ) (25 ml) = M ( 130 ml) (0.200 M Na 2 CO 3 ) (105 ml) = M (130 ml) mol/l = M AgNO mol/l = M Na2CO3 X1Ag + - X1 NO 3 X2Na + 2- X1CO M Ag M NO M Na M CO 3 b) Put concentrations into K sp equation: Ag 2 CO 3(s) 2 Ag + (aq) + CO 3 2- (aq) K sp = [Ag + ] 2 [CO 3 2- ] 1 = ( M Ag + ) 2 (0.162 M CO 3 2- ) 1 = 5.97 X10-5 K sp calculated = 5.97 X10-5 >>> Real K sp from book = 8.1 X10-12 for a saturated solution since Ksp is more than saturated, solid forms (precipitate) If the K sp calculated is less than the real K sp, it is less than saturated (not full), so no solid forms.
12 #95 Notes Ch. Radiochemistry The attractive nuclear force between protons and neutrons is usually stronger than the repulsion energy of the protons. But some isotopes have too many protons and not enough neutrons, so there is too much repulsion. These atoms are unstable (radioactive). Atoms with more than 83 protons are always unstable (atomic # s > 83). I. Nuclear Decay Reactions (to become stable) alpha (α) particle 2 4 He beta (β) particle -1 0 e -barely passes through paper - stopped by 3 mm Al or 10 mm wood gamma (γ) is electromagnetic energy - stopped by 60 cm Al or 7cm Pb positron particle +1 0 e emission (given off), so put particle on product side capture (taken in), so put particle on the reactant side Ex. 1) 265 Bh decays by α-emission Bh 2 4 He Db Ex. 2) 99 Mo decays by β-emission (β-) Mo -1 0 e Tc Ex. 3) 207 Bi decays by positron emission (β+) Bi +1 0 e Pb Ex. 4) 102 Rh decays by electron capture (e.c. or ε) Rh e Ru II. Bombardments -linear or cyclotron particle accelerators can be used to combine smaller atoms into larger atoms or to break up large atoms into smaller atoms. Ex. 1) 249 Bk is bombarded by 22 Ne to make a new heavier element and 4 neutrons ( 0 1 n). Write the reaction Bk Ne n Bh
13 #96 Notes III. Half-lives A half-life is the time it takes for ½ of a sample of a radioactive material to decay into something else. The rate of radioactive decay reactions is 1 st order, so Rate = k [A] 1 decays/sec t 1/2 = / k {t 1/2 is the half-life (not t 2)} ln [A] = -kt + ln [A] o {order in textbook equation may be ln[a] o = kt + ln[a]} amount left after initial time = t amount Ex. 1) 28 g of 87 Sr has a ½ life of 2.8 hrs. a) Find the decays/sec (decays = atoms). Rate = k [A] 1 t 1/2 = / k 2.8 hr 3600 sec = sec 1 hr sec = / k (10080) k = k = X10-5 sec -1 Rate = k [A] 1 = (6.875 X10-5 sec -1 ) (28 g 87 Sr 1 mol X10 23 atoms ) 87g 1 mol Rate = 1.3 X10 19 decays/sec b) How much is left after 4.0 hrs? t 1/2 = 2.8 hr t 1/2 = / k 2.8 hr = / k (2.8) k = k = hr -1 ln A = -kt + ln A o ln A = -( hr -1 ) (4.0 hr) + ln (28 g) ln A = e x (ln A = 2.34) A = 10. g is left
14 c) How much decayed in 4.0 hrs? 28 g {initially} 10. g {left} = 18 g decayed Ex.2) How long does it take for 62.3% of 133 Xe to decay? (1/2 life = 5.3 days) t 1/2 = 0.693/k 5.3 d = 0.693/k (5.3 d) k = k = d % initially = [A] o % decays 37.7% is left = [A] ln [A] = -kt + ln [A] o ln (37.7) = -(0.131 d -1 ) t + ln (100) 3.63 = t = t 7.4 d = t
15 #97 Notes IV. Nuclear Fission / Fusion (splitting/ breaking apart) (combining) Fission: 0 1 n U Te Zr n (nuclear power plants) 0 1 n Pu Ba Sr n (nuclear bombs) Subcritical mass: the reaction dies out. Critical mass: for each neutron that reacts, one neutron that is produced, causes a reaction. Supercritical mass: exponential increase, explosion. Fusion on the sun: 1 1 H H 1 2 H e 1 1 H H 2 3 He or 2 3 He He 4 2 He H 2 3 He H 4 2 He e In Fission and Fusion reactions some mass is lost and converted to energy! E = mc 2 V. Nuclear Power Plants 1) Fuel: U & Pu (produced in the reaction) 2) Moderator: H 2 O, heavy H 2 O, graphite, Be -slows down neutrons, but does not absorb them. 3) Control Rods: B, Cd, or Ga alloyed with Al or steel -absorbs excess neutrons not needed to keep the chain reaction going. 4) Coolant: He, H 2 O, CO 2, liquid metals (Na) -removes heat from the reactor. 5) Heat Exchanger: -transfers heat from the hot coolant, to the cool water, that will make steam to turn the turbines. 6) Safety Shields: Pb or thick concrete -radiation shield around the reactors. *End of Notes* (Assignments #98-99 are Review Assignments. There are no notes for these assignments.)
Acids and bases, as we use them in the lab, are usually aqueous solutions. Ex: when we talk about hydrochloric acid, it is actually hydrogen chloride
 Acids and Bases Acids and bases, as we use them in the lab, are usually aqueous solutions. Ex: when we talk about hydrochloric acid, it is actually hydrogen chloride gas dissolved in water HCl (aq) Concentrated
Acids and Bases Acids and bases, as we use them in the lab, are usually aqueous solutions. Ex: when we talk about hydrochloric acid, it is actually hydrogen chloride gas dissolved in water HCl (aq) Concentrated
Chapter 7 Acids and Bases
 Chapter 7 Acids and Bases 7.1 The Nature of Acids and Bases 7.2 Acid Strength 7.3 The ph Scale 7.4 Calculating the ph of Strong Acid Solutions 7.5 Calculating the ph of Weak Acid Solutions 7.6 Bases 7.7
Chapter 7 Acids and Bases 7.1 The Nature of Acids and Bases 7.2 Acid Strength 7.3 The ph Scale 7.4 Calculating the ph of Strong Acid Solutions 7.5 Calculating the ph of Weak Acid Solutions 7.6 Bases 7.7
Acid/Base Definitions
 Acids and Bases Acid/Base Definitions Arrhenius Model Acids produce hydrogen ions in aqueous solutions Bases produce hydroxide ions in aqueous solutions Bronsted-Lowry Model Acids are proton donors Bases
Acids and Bases Acid/Base Definitions Arrhenius Model Acids produce hydrogen ions in aqueous solutions Bases produce hydroxide ions in aqueous solutions Bronsted-Lowry Model Acids are proton donors Bases
Grace King High School Chemistry Test Review
 CHAPTER 19 Acids, Bases & Salts 1. ACIDS Grace King High School Chemistry Test Review UNITS 7 SOLUTIONS &ACIDS & BASES Arrhenius definition of Acid: Contain Hydrogen and produce Hydrogen ion (aka proton),
CHAPTER 19 Acids, Bases & Salts 1. ACIDS Grace King High School Chemistry Test Review UNITS 7 SOLUTIONS &ACIDS & BASES Arrhenius definition of Acid: Contain Hydrogen and produce Hydrogen ion (aka proton),
Properties of Acids and Bases
 Chapter 15 Aqueous Equilibria: Acids and Bases Properties of Acids and Bases Generally, an acid is a compound that releases hydrogen ions, H +, into water. Blue litmus is used to test for acids. Blue litmus
Chapter 15 Aqueous Equilibria: Acids and Bases Properties of Acids and Bases Generally, an acid is a compound that releases hydrogen ions, H +, into water. Blue litmus is used to test for acids. Blue litmus
Unit 10: Acids and Bases
 Unit 10: Acids and Bases PROPERTIES OF ACIDS & BASES Properties of an Acid: a Tastes sour substance which dissociates (ionizes, breaks apart in solution) in water to form hydrogen ions Turns blue litmus
Unit 10: Acids and Bases PROPERTIES OF ACIDS & BASES Properties of an Acid: a Tastes sour substance which dissociates (ionizes, breaks apart in solution) in water to form hydrogen ions Turns blue litmus
Chapter 14. Objectives
 Section 1 Properties of Acids and Bases Objectives List five general properties of aqueous acids and bases. Name common binary acids and oxyacids, given their chemical formulas. List five acids commonly
Section 1 Properties of Acids and Bases Objectives List five general properties of aqueous acids and bases. Name common binary acids and oxyacids, given their chemical formulas. List five acids commonly
Chapter 10. Acids and Bases
 Chapter 10 Acids and Bases 1 Properties of Aqueous Solutions of Acids and Bases Aqueous acidic solutions have the following properties: 1. They have a sour taste.. They change the colors of many indicators.
Chapter 10 Acids and Bases 1 Properties of Aqueous Solutions of Acids and Bases Aqueous acidic solutions have the following properties: 1. They have a sour taste.. They change the colors of many indicators.
Acids and Bases. Feb 28 4:40 PM
 Acids and Bases H O s O Cl H O O H H N H Na O H H Feb 28 4:40 PM Properties of Acids 1. Taste sour 2. Conduct electrical current 3. Liberate H 2 gas when reacted with a metal. 4. Cause certain dyes to
Acids and Bases H O s O Cl H O O H H N H Na O H H Feb 28 4:40 PM Properties of Acids 1. Taste sour 2. Conduct electrical current 3. Liberate H 2 gas when reacted with a metal. 4. Cause certain dyes to
Unit 9. Acids, Bases, & Salts Acid/Base Equilibrium
 Unit 9 Acids, Bases, & Salts Acid/Base Equilibrium Properties of Acids sour or tart taste strong acids burn; weak acids feel similar to H 2 O acid solutions are electrolytes acids react with most metals
Unit 9 Acids, Bases, & Salts Acid/Base Equilibrium Properties of Acids sour or tart taste strong acids burn; weak acids feel similar to H 2 O acid solutions are electrolytes acids react with most metals
Acids, Bases and ph Preliminary Course. Steffi Thomas 14/09/2017
 Acids, Bases and ph Preliminary Course Steffi Thomas ssthomas@tcd.ie 14/09/2017 Outline What are acids and bases? Can we provide a general definition of acid and base? How can we quantify acidity and basicity?
Acids, Bases and ph Preliminary Course Steffi Thomas ssthomas@tcd.ie 14/09/2017 Outline What are acids and bases? Can we provide a general definition of acid and base? How can we quantify acidity and basicity?
Chapter 10. Acids, Bases, and Salts
 Chapter 10 Acids, Bases, and Salts Topics we ll be looking at in this chapter Arrhenius theory of acids and bases Bronsted-Lowry acid-base theory Mono-, di- and tri-protic acids Strengths of acids and
Chapter 10 Acids, Bases, and Salts Topics we ll be looking at in this chapter Arrhenius theory of acids and bases Bronsted-Lowry acid-base theory Mono-, di- and tri-protic acids Strengths of acids and
Acid and Bases. Physical Properties. Chemical Properties. Indicators. Corrosive when concentrated. Corrosive when concentrated.
 Physical Properties Acid and Bases Chemistry 30 Acids Corrosive when concentrated Have a sour taste Bases Corrosive when concentrated Have a bitter taste Often have a sharp odour Chemical Properties Indicators
Physical Properties Acid and Bases Chemistry 30 Acids Corrosive when concentrated Have a sour taste Bases Corrosive when concentrated Have a bitter taste Often have a sharp odour Chemical Properties Indicators
Chemical Equilibrium Chapter 6
 Chemical Equilibrium Chapter 6 "When a system is in chemical equilibrium, a change in one of the parameters of the equilibrium produces a shift in such a direction that, were no other factors involved
Chemical Equilibrium Chapter 6 "When a system is in chemical equilibrium, a change in one of the parameters of the equilibrium produces a shift in such a direction that, were no other factors involved
INTRODUCTION TO ACIDS AND BASES
 INTRODUCTION TO ACIDS AND BASES ALIGNED STANDARDS S.C. 912.P.8.11 Relate acidity and basicity to hydronium and hydroxide concentration and ph. S.C.912.N.1.2 Describe and explain what characterizes science
INTRODUCTION TO ACIDS AND BASES ALIGNED STANDARDS S.C. 912.P.8.11 Relate acidity and basicity to hydronium and hydroxide concentration and ph. S.C.912.N.1.2 Describe and explain what characterizes science
Advanced Placement Chemistry Chapters Syllabus
 As you work through the chapter, you should be able to: Advanced Placement Chemistry Chapters 14 16 Syllabus Chapter 14 Acids and Bases 1. Describe acid and bases using the Bronsted-Lowry, Arrhenius, and
As you work through the chapter, you should be able to: Advanced Placement Chemistry Chapters 14 16 Syllabus Chapter 14 Acids and Bases 1. Describe acid and bases using the Bronsted-Lowry, Arrhenius, and
ACID BASE EQUILIBRIUM
 ACID BASE EQUILIBRIUM Part one: Acid/Base Theories Learning Goals: to identify acids and bases and their conjugates according to Arrhenius and Bronstead Lowry Theories. to be able to identify amphoteric
ACID BASE EQUILIBRIUM Part one: Acid/Base Theories Learning Goals: to identify acids and bases and their conjugates according to Arrhenius and Bronstead Lowry Theories. to be able to identify amphoteric
11/15/11. Chapter 16. HA(aq) + H 2 O(l) H 3 O + (aq) + A (aq) acid base conjugate conjugate
 Chapter 16 Table of Contents Chapter 16 16.1 16.2 16.3 16.4 16.5 16.6 Buffered Solutions Copyright Cengage Learning. All rights reserved 2 Models of Arrhenius: Acids produce H + ions in solution, bases
Chapter 16 Table of Contents Chapter 16 16.1 16.2 16.3 16.4 16.5 16.6 Buffered Solutions Copyright Cengage Learning. All rights reserved 2 Models of Arrhenius: Acids produce H + ions in solution, bases
What is an acid? What is a base?
 What is an acid? What is a base? Properties of an acid Sour taste Turns litmus paper red Conducts electric current Some acids are strong and some are weak Properties of a base Bitter taste Slippery to
What is an acid? What is a base? Properties of an acid Sour taste Turns litmus paper red Conducts electric current Some acids are strong and some are weak Properties of a base Bitter taste Slippery to
Reactions in Aqueous Solutions I: Acids, Bases & Salts
 10 Reactions in Aqueous Solutions I: Acids, Bases & Salts CHAPTER GOALS 1. Properties of Aqueous Solutions of Acids and Bases 2. The Arrhenius Theory 3. The Hydronium Ion (Hydrated Hydrogen Ion) 4. The
10 Reactions in Aqueous Solutions I: Acids, Bases & Salts CHAPTER GOALS 1. Properties of Aqueous Solutions of Acids and Bases 2. The Arrhenius Theory 3. The Hydronium Ion (Hydrated Hydrogen Ion) 4. The
ADVANCED PLACEMENT CHEMISTRY ACIDS, BASES, AND AQUEOUS EQUILIBRIA
 ADVANCED PLACEMENT CHEMISTRY ACIDS, BASES, AND AQUEOUS EQUILIBRIA Acids- taste sour Bases(alkali)- taste bitter and feel slippery Arrhenius concept- acids produce hydrogen ions in aqueous solution while
ADVANCED PLACEMENT CHEMISTRY ACIDS, BASES, AND AQUEOUS EQUILIBRIA Acids- taste sour Bases(alkali)- taste bitter and feel slippery Arrhenius concept- acids produce hydrogen ions in aqueous solution while
Lesson Five: Acids, Bases, ph, and Buffers
 Lesson Five: Acids, Bases, ph, and Buffers Arrhenius Acids and Bases Acids and bases can be defined a number of ways. One of the oldest and most common ways is the definition according to Arrhenius, named
Lesson Five: Acids, Bases, ph, and Buffers Arrhenius Acids and Bases Acids and bases can be defined a number of ways. One of the oldest and most common ways is the definition according to Arrhenius, named
Name Date Class ACID-BASE THEORIES
 19.1 ACID-BASE THEORIES Section Review Objectives Define the properties of acids and bases Compare and contrast acids and bases as defined by the theories of Arrhenius, Brønsted-Lowry, and Lewis Vocabulary
19.1 ACID-BASE THEORIES Section Review Objectives Define the properties of acids and bases Compare and contrast acids and bases as defined by the theories of Arrhenius, Brønsted-Lowry, and Lewis Vocabulary
Chapter 16. Acids and Bases. Copyright Cengage Learning. All rights reserved 1
 Chapter 16 Acids and Bases Copyright Cengage Learning. All rights reserved 1 Section 16.1 Acids and Bases Models of Acids and Bases Arrhenius: Acids produce H + ions in solution, bases produce OH ions.
Chapter 16 Acids and Bases Copyright Cengage Learning. All rights reserved 1 Section 16.1 Acids and Bases Models of Acids and Bases Arrhenius: Acids produce H + ions in solution, bases produce OH ions.
Chapter Menu Chapter Menu
 Chapter Menu Chapter Menu Section 18.1 Section 18.3 Section 18.4 Introduction to Acids and Bases Hydrogen Ions and ph Neutralization Section 18.1 Intro to Acids and Bases Objectives: Compare the Arrhenius,
Chapter Menu Chapter Menu Section 18.1 Section 18.3 Section 18.4 Introduction to Acids and Bases Hydrogen Ions and ph Neutralization Section 18.1 Intro to Acids and Bases Objectives: Compare the Arrhenius,
Chem 30A. Ch 14. Acids and Bases
 Chem 30A Ch 14. Acids and Bases Acids and Bases Acids and Bases Acids Sour taste Dissolve many metals Turn litmus paper red. Egs. Ace9c acid (vinegar), citric acid (lemons) Bases Bi>er taste, slippery
Chem 30A Ch 14. Acids and Bases Acids and Bases Acids and Bases Acids Sour taste Dissolve many metals Turn litmus paper red. Egs. Ace9c acid (vinegar), citric acid (lemons) Bases Bi>er taste, slippery
Unit 4: Acid/Base I. abinotes. I) Introduction to Acids and Bases What is an acid?
 Unit 4: Acid/Base I I) Introduction to Acids and Bases What is an acid? http://www.kidsknowit.com/flash/animations/acidsbases.swf What are properties of acids? 1) Acids react with. 2) Acids create when
Unit 4: Acid/Base I I) Introduction to Acids and Bases What is an acid? http://www.kidsknowit.com/flash/animations/acidsbases.swf What are properties of acids? 1) Acids react with. 2) Acids create when
What are the properties of acids and bases?
 Text CH. 14 and 15 What are the properties of acids and bases? identify acids and bases based on general observable properties explain how an indicator is used to determine whether a solution is acidic,
Text CH. 14 and 15 What are the properties of acids and bases? identify acids and bases based on general observable properties explain how an indicator is used to determine whether a solution is acidic,
Acids - Bases in Water
 more equilibrium Dr. Fred Omega Garces Chemistry, Miramar College 1 Acids-Bases Characteristics Acids (Properties) Taste Sour Dehydrate Substances Neutralizes bases Dissolves metals Examples: Juices: TJ,
more equilibrium Dr. Fred Omega Garces Chemistry, Miramar College 1 Acids-Bases Characteristics Acids (Properties) Taste Sour Dehydrate Substances Neutralizes bases Dissolves metals Examples: Juices: TJ,
Acids and Bases. Chapter 15. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Acids and Bases Chapter 15 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Acids Have a sour taste. Vinegar owes its taste to acetic acid. Citrus fruits contain
Acids and Bases Chapter 15 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Acids Have a sour taste. Vinegar owes its taste to acetic acid. Citrus fruits contain
What is an acid? What is a base?
 What is an acid? What is a base? Properties of an acid Sour taste Turns litmus paper red Conducts electric current Some acids are strong and some are weak Properties of a base Bitter taste Slippery to
What is an acid? What is a base? Properties of an acid Sour taste Turns litmus paper red Conducts electric current Some acids are strong and some are weak Properties of a base Bitter taste Slippery to
The Chemistry of Acids and Bases
 The Chemistry of Acids and Bases 1 Acid and Bases 4 Acid and Bases 2 Acids Have a sour taste. Vinegar is a solution of acetic acid. Citrus fruits contain citric acid. React with certain metals to produce
The Chemistry of Acids and Bases 1 Acid and Bases 4 Acid and Bases 2 Acids Have a sour taste. Vinegar is a solution of acetic acid. Citrus fruits contain citric acid. React with certain metals to produce
Acids and Bases. Properties, Reactions, ph, and Titration
 Acids and Bases Properties, Reactions, ph, and Titration C-19 2017 Properties of acids 1. Taste Sour (don t try this except with foods). 2. Are electrolytes (conduct electricity). Some are strong, some
Acids and Bases Properties, Reactions, ph, and Titration C-19 2017 Properties of acids 1. Taste Sour (don t try this except with foods). 2. Are electrolytes (conduct electricity). Some are strong, some
Amount of substance dissolved in 1 L of water
 Chapter 7: Phenomena Phenomena: Scientists dissolved different substances in water and then measured the [H + ] and [OH - ] concentrations in each solution. What patterns do you notice about the substances?
Chapter 7: Phenomena Phenomena: Scientists dissolved different substances in water and then measured the [H + ] and [OH - ] concentrations in each solution. What patterns do you notice about the substances?
Chapters 15 & 16 ACIDS & BASES ph & Titrations
 PROPERTIES OF ACIDS Chapters 15 & 16 ACIDS & BASES ph & Titrations There are 5 main properties of acids: 1. sour taste 2. change the color of acidbase indicators 3. react with metals to produce H2 gas
PROPERTIES OF ACIDS Chapters 15 & 16 ACIDS & BASES ph & Titrations There are 5 main properties of acids: 1. sour taste 2. change the color of acidbase indicators 3. react with metals to produce H2 gas
What is an acid? What is a base?
 What is an acid? What is a base? Properties of an acid Sour taste Turns litmus paper red Conducts electric current Some acids are strong and some are weak Properties of a base Bitter taste Slippery to
What is an acid? What is a base? Properties of an acid Sour taste Turns litmus paper red Conducts electric current Some acids are strong and some are weak Properties of a base Bitter taste Slippery to
Chapter 9 Aqueous Solutions and Chemical Equilibria
 Chapter 9 Aqueous Solutions and Chemical Equilibria At equilibrium, the rate of a forward process or reaction and that of the reverse process are equal. 9A The chemical composition of aqueous solutions
Chapter 9 Aqueous Solutions and Chemical Equilibria At equilibrium, the rate of a forward process or reaction and that of the reverse process are equal. 9A The chemical composition of aqueous solutions
Acids, Bases and ph Chapter 19
 Acids, Bases and ph Chapter 19 Compounds That Become Acids When Dissolved in Water General Formula: HX H + X - monatomic or polyatomic anion Naming Acids (p. 250) Binary acids Hydro ic Acid HCl: Hydrochloric
Acids, Bases and ph Chapter 19 Compounds That Become Acids When Dissolved in Water General Formula: HX H + X - monatomic or polyatomic anion Naming Acids (p. 250) Binary acids Hydro ic Acid HCl: Hydrochloric
Aqueous Reactions and Solution Stoichiometry (continuation)
 Aqueous Reactions and Solution Stoichiometry (continuation) 1. Electrolytes and non-electrolytes 2. Determining Moles of Ions in Aqueous Solutions of Ionic Compounds 3. Acids and Bases 4. Acid Strength
Aqueous Reactions and Solution Stoichiometry (continuation) 1. Electrolytes and non-electrolytes 2. Determining Moles of Ions in Aqueous Solutions of Ionic Compounds 3. Acids and Bases 4. Acid Strength
Chapter 16 - Acids and Bases
 Chapter 16 - Acids and Bases 16.1 Acids and Bases: The Brønsted Lowry Model 16.2 ph and the Autoionization of Water 16.3 Calculations Involving ph, K a and K b 16.4 Polyprotic Acids 16.1 Acids and Bases:
Chapter 16 - Acids and Bases 16.1 Acids and Bases: The Brønsted Lowry Model 16.2 ph and the Autoionization of Water 16.3 Calculations Involving ph, K a and K b 16.4 Polyprotic Acids 16.1 Acids and Bases:
A) Arrhenius Acids produce H+ and bases produce OH not always used because it only IDs X OH as basic species
 3 ACID AND BASE THEORIES: A) Arrhenius Acids produce H+ and bases produce OH not always used because it only IDs X OH as basic species B) Bronsted and Lowry Acid = H + donor > CB = formed after H + dissociates
3 ACID AND BASE THEORIES: A) Arrhenius Acids produce H+ and bases produce OH not always used because it only IDs X OH as basic species B) Bronsted and Lowry Acid = H + donor > CB = formed after H + dissociates
Unit 4a Acids, Bases, and Salts Theory
 Unit 4a Acids, Bases, and Salts Theory Chemistry 12 Arrhenius Theory of Acids and Bases The first theory that was proposed to explain the actions of acids and bases was by Svante Arrhenius. It is still
Unit 4a Acids, Bases, and Salts Theory Chemistry 12 Arrhenius Theory of Acids and Bases The first theory that was proposed to explain the actions of acids and bases was by Svante Arrhenius. It is still
Acids and Bases. Unit 10
 Acids and Bases Unit 10 1 Properties of Acids and Bases Acids Bases Taste Sour Turns Litmus Dye Red Reacts with Metals to give H 2 (g) Taste Bitter Turns Litmus Dye Blue Do Not React with Metals Reacts
Acids and Bases Unit 10 1 Properties of Acids and Bases Acids Bases Taste Sour Turns Litmus Dye Red Reacts with Metals to give H 2 (g) Taste Bitter Turns Litmus Dye Blue Do Not React with Metals Reacts
Chemistry I Notes Unit 10: Acids and Bases
 Chemistry I Notes Unit 10: Acids and Bases Acids 1. Sour taste. 2. Acids change the color of acid- base indicators (turn blue litmus red). 3. Some acids react with active metals and release hydrogen gas,
Chemistry I Notes Unit 10: Acids and Bases Acids 1. Sour taste. 2. Acids change the color of acid- base indicators (turn blue litmus red). 3. Some acids react with active metals and release hydrogen gas,
Chapter 15 - Acids and Bases Fundamental Concepts
 Chapter 15 - Acids and Bases Fundamental Concepts Acids and Bases: Basic Definitions Properties of Acids Sour Taste React with active metals (Al, Zn, Fe) to yield H 2 gas: Corrosive React with carbonates
Chapter 15 - Acids and Bases Fundamental Concepts Acids and Bases: Basic Definitions Properties of Acids Sour Taste React with active metals (Al, Zn, Fe) to yield H 2 gas: Corrosive React with carbonates
The Chemistry of Acids and Bases
 The Chemistry of Acids and Bases 1 Acid and Bases 2 Acid and Bases 3 Acid and Bases 4 Acids 5 Have a sour taste. Vinegar is a solution of acetic acid. Citrus fruits contain citric acid. React with certain
The Chemistry of Acids and Bases 1 Acid and Bases 2 Acid and Bases 3 Acid and Bases 4 Acids 5 Have a sour taste. Vinegar is a solution of acetic acid. Citrus fruits contain citric acid. React with certain
CHAPTER 19. Acids, Bases, and Salts Acid Base Theories
 CHAPTER 19 Acids, Bases, and Salts 19.1 Acid Base Theories ACIDS tart or sour taste Electrolytes Strong acids are corrosive Acid Facts... indicators will change color Blue litmus paper turns pink react
CHAPTER 19 Acids, Bases, and Salts 19.1 Acid Base Theories ACIDS tart or sour taste Electrolytes Strong acids are corrosive Acid Facts... indicators will change color Blue litmus paper turns pink react
Chemical Equilibrium. What is the standard state for solutes? a) 1.00 b) 1 M c) 100% What is the standard state for gases? a) 1 bar b) 1.
 Chemical Equilibrium Equilibrium constant for the reaction: aa + bb + cc + dd + [C ] c [D ] d... equilibrium constant K = [ A] a [B ] b... [] = concentration relative to standard state molarity (M): for
Chemical Equilibrium Equilibrium constant for the reaction: aa + bb + cc + dd + [C ] c [D ] d... equilibrium constant K = [ A] a [B ] b... [] = concentration relative to standard state molarity (M): for
Student Review Packet Answer Key
 Student Review Packet Answer Key 1. Label each of the following substances as either acid or base. a. NaOH base b. H 2 SO 4 acid c. H 3 PO 3 acid d. KOH base e. NH 3 base f. HCl acid g. LiOH base h. C
Student Review Packet Answer Key 1. Label each of the following substances as either acid or base. a. NaOH base b. H 2 SO 4 acid c. H 3 PO 3 acid d. KOH base e. NH 3 base f. HCl acid g. LiOH base h. C
CH19 Bronsted-Lowry Definitions
 CH19 Bronsted-Lowry Definitions 1 BRONSTED-LOWRY DEFINITIONS [Acids] An acid is a substance that can donate H + ions HCl hydrochloric acid HNO 3 nitric acid HOAc acetic acid H 3 0 + hydronium ion NH +
CH19 Bronsted-Lowry Definitions 1 BRONSTED-LOWRY DEFINITIONS [Acids] An acid is a substance that can donate H + ions HCl hydrochloric acid HNO 3 nitric acid HOAc acetic acid H 3 0 + hydronium ion NH +
Chapter 7: Phenomena. Chapter 7 Acids and Bases. Acids and Bases. Acids and Bases. Acids and Bases
 Chapter 7: Phenomena Phenomena: Scientists dissolved different substances in water and then measured the [H + ] and [OH - ] concentrations in each solution. What patterns do you notice about the substances?
Chapter 7: Phenomena Phenomena: Scientists dissolved different substances in water and then measured the [H + ] and [OH - ] concentrations in each solution. What patterns do you notice about the substances?
Chapter 17 Acids and Bases
 Chapter 17 Acids and Bases - we are all familiar with 'acids' - depicted on television as burning liquids - from foods (i.e. vinegar) - taste "sour" or "tart' - less familiar with 'bases' - taste "bitter"
Chapter 17 Acids and Bases - we are all familiar with 'acids' - depicted on television as burning liquids - from foods (i.e. vinegar) - taste "sour" or "tart' - less familiar with 'bases' - taste "bitter"
Acids and Bases Unit 11
 Mr. B s Chemistry Acids and Bases Unit 11 Name Block Let s start our discussion of acids and bases by defining some terms that are essential to the topics that follow. Arrhenius acids and bases are: acid
Mr. B s Chemistry Acids and Bases Unit 11 Name Block Let s start our discussion of acids and bases by defining some terms that are essential to the topics that follow. Arrhenius acids and bases are: acid
SCHOOL YEAR CH- 13 IONS IN AQUEOUS SOLUTIONS AND COLLIGATIVE PROPERTIES SUBJECT: CHEMISTRY GRADE : 11 TEST A
 SCHOOL YEAR 2017-18 NAME: CH- 13 IONS IN AQUEOUS SOLUTIONS AND COLLIGATIVE PROPERTIES SUBJECT: CHEMISTRY GRADE : 11 TEST A Choose the best answer from the options that follow each question. 1. A solute
SCHOOL YEAR 2017-18 NAME: CH- 13 IONS IN AQUEOUS SOLUTIONS AND COLLIGATIVE PROPERTIES SUBJECT: CHEMISTRY GRADE : 11 TEST A Choose the best answer from the options that follow each question. 1. A solute
Name. Academic Chemistry. Acid Base. Notes. Unit #14 Test Date: cincochem.pbworks.com
 Periodic Table Name Academic Chemistry Acids & Bases Notes Unit #14 Test Date: 20 cincochem.pbworks.com Acid Base cincochem.pbworks.com Notes Find ph To go from [H 3 O + ] to ph EXAMPLE: [H 3 O + ] = 3.23
Periodic Table Name Academic Chemistry Acids & Bases Notes Unit #14 Test Date: 20 cincochem.pbworks.com Acid Base cincochem.pbworks.com Notes Find ph To go from [H 3 O + ] to ph EXAMPLE: [H 3 O + ] = 3.23
Aqueous solutions of acids have a sour Aqueous solutions of bases taste bitter
 Acid and Bases Exam Review Honors Chemistry 3 April 2012 Chapter 14- Acids and Bases Section 14.1- Acid and Base Properties List five general properties of aqueous acids and bases Properties of Acids Properties
Acid and Bases Exam Review Honors Chemistry 3 April 2012 Chapter 14- Acids and Bases Section 14.1- Acid and Base Properties List five general properties of aqueous acids and bases Properties of Acids Properties
Chapter 14: Acids and Bases
 Chapter 14: Acids and Bases Properties of Acids and Bases What is an acid? Some examples of common items containing acids: Vinegar contains acetic acid; lemons and citrus fruits contain citric acid; many
Chapter 14: Acids and Bases Properties of Acids and Bases What is an acid? Some examples of common items containing acids: Vinegar contains acetic acid; lemons and citrus fruits contain citric acid; many
Notes: Acids and Bases
 Name Chemistry Pre-AP Notes: Acids and Bases Period I. Describing Acids and Bases A. Properties of Acids taste ph 7 Acids change color of an (e.g. blue litmus paper turns in the presence of an acid) React
Name Chemistry Pre-AP Notes: Acids and Bases Period I. Describing Acids and Bases A. Properties of Acids taste ph 7 Acids change color of an (e.g. blue litmus paper turns in the presence of an acid) React
Section 32 Acids and Bases. Copyright (c) 2011 by Michael A. Janusa, PhD. All rights reserved.
 Section 32 Acids and Bases 1 Copyright (c) 2011 by Michael A. Janusa, PhD. All rights reserved. Acid-Base Concepts Acids and bases are among the most familiar and important of all chemical compounds. You
Section 32 Acids and Bases 1 Copyright (c) 2011 by Michael A. Janusa, PhD. All rights reserved. Acid-Base Concepts Acids and bases are among the most familiar and important of all chemical compounds. You
Acids and Bases. Reading Assignments: Acids. Bases. Chapter 15 in R. Chang, Chemistry, 8th Ed., McGraw-Hill, 2005
 Reading Assignments: Chapter 15 in R. Chang, Chemistry, 8th Ed., McGraw-Hill, 2005 Or Related topics in other textbooks. Acids and Bases Consultation outside lecture room: Office Hours: Tuesday & Thursday
Reading Assignments: Chapter 15 in R. Chang, Chemistry, 8th Ed., McGraw-Hill, 2005 Or Related topics in other textbooks. Acids and Bases Consultation outside lecture room: Office Hours: Tuesday & Thursday
Unit 2 Acids and Bases
 Unit 2 Acids and Bases 1 Topics Properties / Operational Definitions Acid-Base Theories ph & poh calculations Equilibria (Kw, K a, K b ) Indicators Titrations STSE: Acids Around Us 2 Operational Definitions
Unit 2 Acids and Bases 1 Topics Properties / Operational Definitions Acid-Base Theories ph & poh calculations Equilibria (Kw, K a, K b ) Indicators Titrations STSE: Acids Around Us 2 Operational Definitions
Chapter 16. Acid-Base Equilibria
 Chapter 16. Acid-Base Equilibria 16.1 Acids and Bases: A Brief Review Acids taste sour and cause certain dyes to change color. Bases taste bitter and feel soapy. Arrhenius concept of acids and bases: An
Chapter 16. Acid-Base Equilibria 16.1 Acids and Bases: A Brief Review Acids taste sour and cause certain dyes to change color. Bases taste bitter and feel soapy. Arrhenius concept of acids and bases: An
Chap 16 Chemical Equilibrium HSU FUYIN
 Chap 16 Chemical Equilibrium HSU FUYIN 1 Definitions: Arrhenius & Brønsted Lowry acid and base Arrhenius theory: An acid is a substance that, when dissolved in water, increases the concentration of hydrogen
Chap 16 Chemical Equilibrium HSU FUYIN 1 Definitions: Arrhenius & Brønsted Lowry acid and base Arrhenius theory: An acid is a substance that, when dissolved in water, increases the concentration of hydrogen
U N I T T E S T P R A C T I C E
 South Pasadena Honors Chemistry Name 4 Salts and Solutions Period Date U N I T T E S T P R A C T I C E You may use a pencil, eraser, and scientific calculator to complete the test. You will be given a
South Pasadena Honors Chemistry Name 4 Salts and Solutions Period Date U N I T T E S T P R A C T I C E You may use a pencil, eraser, and scientific calculator to complete the test. You will be given a
ACIDS & BASES PROPERTIES OF ACIDS ACIDS PROPERTIES OF ACIDS PROPERTIES OF ACIDS 11/1/2016
 SC STANDARD COVERED ACIDS & BASES Standard PS-3.7 Classify various solutions as acids or bases according to their physical properties, chemical properties (including neutralization and reaction with metals),
SC STANDARD COVERED ACIDS & BASES Standard PS-3.7 Classify various solutions as acids or bases according to their physical properties, chemical properties (including neutralization and reaction with metals),
Dynamic equilibrium: rate of evaporation = rate of condensation II. In a closed system a solid obtains a dynamic equilibrium with its dissolved state
 CHEMISTRY 111 LECTURE EXAM III Material PART 1 CHEMICAL EQUILIBRIUM Chapter 14 I Dynamic Equilibrium I. In a closed system a liquid obtains a dynamic equilibrium with its vapor state Dynamic equilibrium:
CHEMISTRY 111 LECTURE EXAM III Material PART 1 CHEMICAL EQUILIBRIUM Chapter 14 I Dynamic Equilibrium I. In a closed system a liquid obtains a dynamic equilibrium with its vapor state Dynamic equilibrium:
11/14/10. Properties of Acids! CHAPTER 15 Acids and Bases. Table 18.1
 11/14/10 CHAPTER 15 Acids and Bases 15-1 Properties of Acids! Sour taste React with active metals i.e., Al, Zn, Fe, but not Cu, Ag, or Au 2 Al + 6 HCl 2 AlCl3 + 3 H2 corrosive React with carbonates, producing
11/14/10 CHAPTER 15 Acids and Bases 15-1 Properties of Acids! Sour taste React with active metals i.e., Al, Zn, Fe, but not Cu, Ag, or Au 2 Al + 6 HCl 2 AlCl3 + 3 H2 corrosive React with carbonates, producing
Ionic Equilibria. weak acids and bases. salts of weak acids and bases. buffer solutions. solubility of slightly soluble salts
 Ionic Equilibria weak acids and bases salts of weak acids and bases buffer solutions solubility of slightly soluble salts Arrhenius Definitions produce H + ions in the solution strong acids ionize completely
Ionic Equilibria weak acids and bases salts of weak acids and bases buffer solutions solubility of slightly soluble salts Arrhenius Definitions produce H + ions in the solution strong acids ionize completely
Acid-base Chemistry. Unit 11.1: Into to acid base chemistry. Unit 11. Name:
 Name: Acid-base Chemistry Unit 11 ( F i ve cla s s peri o ds) Unit 11.1: Into to acid base chemistry 1) Self-ionization of water a) Water molecules collide and the extremely electronegative oxygen can
Name: Acid-base Chemistry Unit 11 ( F i ve cla s s peri o ds) Unit 11.1: Into to acid base chemistry 1) Self-ionization of water a) Water molecules collide and the extremely electronegative oxygen can
THE BIG IDEA: REACTIONS. 1. Review nomenclature rules for acids and bases and the formation of acids and bases from anhydrides. (19.
 HONORS CHEMISTRY - CHAPTER 19 ACIDS, BASES, AND SALTS OBJECTIVES AND NOTES - V14 NAME: DATE: PAGE: THE BIG IDEA: REACTIONS Essential Questions 1. What are the different ways chemists define acids and bases?
HONORS CHEMISTRY - CHAPTER 19 ACIDS, BASES, AND SALTS OBJECTIVES AND NOTES - V14 NAME: DATE: PAGE: THE BIG IDEA: REACTIONS Essential Questions 1. What are the different ways chemists define acids and bases?
Chem 105 Tuesday March 8, Chapter 17. Acids and Bases
 Chem 105 Tuesday March 8, 2011 Chapter 17. Acids and Bases 1) Define Brønsted Acid and Brønsted Base 2) Proton (H + ) transfer reactions: conjugate acid-base pairs 3) Water and other amphiprotic substances
Chem 105 Tuesday March 8, 2011 Chapter 17. Acids and Bases 1) Define Brønsted Acid and Brønsted Base 2) Proton (H + ) transfer reactions: conjugate acid-base pairs 3) Water and other amphiprotic substances
Duncan. UNIT 14 - Acids & Bases. COMMON ACIDS NOTES lactic acetic phosphoric NAMING ACIDS NOTES
 COMMON ACIDS NOTES lactic acetic phosphoric citric malic PROPERTIES OF ACIDS 1. 1. PROPERTIES OF BASES 2. 2. 3. 3. 4. 4. 5. 5. NAMING ACIDS NOTES Binary acids (H + one element) 1. hydro- - HF 2. root of
COMMON ACIDS NOTES lactic acetic phosphoric citric malic PROPERTIES OF ACIDS 1. 1. PROPERTIES OF BASES 2. 2. 3. 3. 4. 4. 5. 5. NAMING ACIDS NOTES Binary acids (H + one element) 1. hydro- - HF 2. root of
Chem12 Acids : Exam Questions M.C.-100
 Chem12 Acids : Exam Questions M.C.-100 1) Given : HPO 4 2- (aq) + NH 4 + (aq) H 2 PO 4 - (aq) + NH 3 (aq), the strongest acid in the above equation is : a) NH 4 + b) HPO 4 2- c) NH 3 d) H 2 PO 4-2)
Chem12 Acids : Exam Questions M.C.-100 1) Given : HPO 4 2- (aq) + NH 4 + (aq) H 2 PO 4 - (aq) + NH 3 (aq), the strongest acid in the above equation is : a) NH 4 + b) HPO 4 2- c) NH 3 d) H 2 PO 4-2)
Acids Bases and Salts Acid
 Acids Bases and Salts Acid ph less than 7.0 Sour taste Electrolyte Names of Acids Binary acids Contain only 2 elements Begin with hydro; end with ic Ternary acids Ex: H 2 S = hydrosulfuric Contain a polyatomic
Acids Bases and Salts Acid ph less than 7.0 Sour taste Electrolyte Names of Acids Binary acids Contain only 2 elements Begin with hydro; end with ic Ternary acids Ex: H 2 S = hydrosulfuric Contain a polyatomic
AP Chemistry: Acid-Base Chemistry Practice Problems
 Name AP Chemistry: Acid-Base Chemistry Practice Problems Date Due Directions: Write your answers to the following questions in the space provided. For problem solving, show all of your work. Make sure
Name AP Chemistry: Acid-Base Chemistry Practice Problems Date Due Directions: Write your answers to the following questions in the space provided. For problem solving, show all of your work. Make sure
Chapter 10 - Acids & Bases
 Chapter 10 - Acids & Bases 10.1-Acids & Bases: Definitions Arrhenius Definitions Acids: substances that produce hydrogen ions when dissolved in H 2 O Common Strong Acids: Common Weak acids: Organic carboxylic
Chapter 10 - Acids & Bases 10.1-Acids & Bases: Definitions Arrhenius Definitions Acids: substances that produce hydrogen ions when dissolved in H 2 O Common Strong Acids: Common Weak acids: Organic carboxylic
Unit 4 Toxins, Section IV, L17-22
 Unit 4 Toxins, Section IV, L17-22 Lesson 17 Heartburn Lesson 18 Pass the Proton Lesson 19 phooey! Lesson 20 Watered Down Lesson 21 Neutral Territory Lesson 22 Drip Drop Acids and Bases What are the properties
Unit 4 Toxins, Section IV, L17-22 Lesson 17 Heartburn Lesson 18 Pass the Proton Lesson 19 phooey! Lesson 20 Watered Down Lesson 21 Neutral Territory Lesson 22 Drip Drop Acids and Bases What are the properties
CHEMISTRY Matter and Change
 CHEMISTRY Matter and Change UNIT 18 Table Of Contents Section 18.1 Introduction to Acids and Bases Unit 18: Acids and Bases Section 18.2 Section 18.3 Section 18.4 Strengths of Acids and Bases Hydrogen
CHEMISTRY Matter and Change UNIT 18 Table Of Contents Section 18.1 Introduction to Acids and Bases Unit 18: Acids and Bases Section 18.2 Section 18.3 Section 18.4 Strengths of Acids and Bases Hydrogen
Acids, Bases, & Neutralization Chapter 20 & 21 Assignment & Problem Set
 Acids, Bases, & Neutralization Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Acids, Bases, & Neutralization 2 Study Guide: Things You Must Know
Acids, Bases, & Neutralization Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Acids, Bases, & Neutralization 2 Study Guide: Things You Must Know
Chapter 14 Acid- Base Equilibria Study Guide
 Chapter 14 Acid- Base Equilibria Study Guide This chapter will illustrate the chemistry of acid- base reactions and equilibria, and provide you with tools for quantifying the concentrations of acids and
Chapter 14 Acid- Base Equilibria Study Guide This chapter will illustrate the chemistry of acid- base reactions and equilibria, and provide you with tools for quantifying the concentrations of acids and
Strong and Weak. Acids and Bases
 Strong and Weak Acids and Bases Strength of Acids H2SO4 HSO4 - + H + HNO3 NO3 - + H + Strong Acids HCl Cl - + H + H3PO4 H2PO4 - + H + Phosphoric acid Moderate Acid CH3COOH CH3COO - + H + Acetic acid HF
Strong and Weak Acids and Bases Strength of Acids H2SO4 HSO4 - + H + HNO3 NO3 - + H + Strong Acids HCl Cl - + H + H3PO4 H2PO4 - + H + Phosphoric acid Moderate Acid CH3COOH CH3COO - + H + Acetic acid HF
Chemistry 400 Homework #3, Chapter 16: Acid-Base Equilibria
 Chemistry 400 Homework #3, Chapter 16: Acid-Base Equilibria I. Multiple Choice (for those with an asterisk, you must show work) These multiple choice (MC) are not "Google-proof", but they were so good
Chemistry 400 Homework #3, Chapter 16: Acid-Base Equilibria I. Multiple Choice (for those with an asterisk, you must show work) These multiple choice (MC) are not "Google-proof", but they were so good
Homework #7 Chapter 8 Applications of Aqueous Equilibrium
 Homework #7 Chapter 8 Applications of Aqueous Equilibrium 15. solution: A solution that resists change in ph when a small amount of acid or base is added. solutions contain a weak acid and its conjugate
Homework #7 Chapter 8 Applications of Aqueous Equilibrium 15. solution: A solution that resists change in ph when a small amount of acid or base is added. solutions contain a weak acid and its conjugate
Worksheet 4.1 Conjugate Acid-Base Pairs
 Worksheet 4.1 Conjugate AcidBase Pairs 1. List five properties of acids that are in your textbook. Acids conduct electricity, taste sour, neutralize bases, change the color of indicators, and react with
Worksheet 4.1 Conjugate AcidBase Pairs 1. List five properties of acids that are in your textbook. Acids conduct electricity, taste sour, neutralize bases, change the color of indicators, and react with
8.1 Explaining the Properties of Acids & Bases. SCH4U - Chemistry, Gr. 12, University Prep
 8.1 Explaining the Properties of Acids & Bases SCH4U - Chemistry, Gr. 12, University Prep Equilibrium & Acids & Bases 2 So far, we have looked at equilibrium of general chemical systems: We learned about
8.1 Explaining the Properties of Acids & Bases SCH4U - Chemistry, Gr. 12, University Prep Equilibrium & Acids & Bases 2 So far, we have looked at equilibrium of general chemical systems: We learned about
UNIT 14 - Acids & Bases
 COMMON ACIDS NOTES lactic sour milk, sore muscles acetic vinegar phosphoric soft drinks citric citrus fruits malic apples PROPERTIES OF ACIDS PROPERTIES OF BASES 1. Taste sour 1. Taste bitter 2. react
COMMON ACIDS NOTES lactic sour milk, sore muscles acetic vinegar phosphoric soft drinks citric citrus fruits malic apples PROPERTIES OF ACIDS PROPERTIES OF BASES 1. Taste sour 1. Taste bitter 2. react
AP Chemistry CHAPTER 16 STUDY GUIDE Acid-Base Equilibrium
 AP Chemistry CHAPTER 16 STUDY GUIDE AcidBase Equilibrium 16.1 Acids and Bases: A Brief Review Acids taste sour and cause certain dyes to change color. Bases taste bitter and feel soapy. Arrhenius concept
AP Chemistry CHAPTER 16 STUDY GUIDE AcidBase Equilibrium 16.1 Acids and Bases: A Brief Review Acids taste sour and cause certain dyes to change color. Bases taste bitter and feel soapy. Arrhenius concept
Acids. Names of Acids. Naming Some Common Acids. Solution. Learning Check Acids and Bases. Arrhenius acids Produce H + ions in water.
 Chapter 10 Acids and Bases Acids 10.1 Acids and Bases Arrhenius acids Produce H + ions in water. H 2 O HCl(g) H + (aq) + Cl (aq) Are electrolytes. Have a sour taste. Turn litmus red. Neutralize bases.
Chapter 10 Acids and Bases Acids 10.1 Acids and Bases Arrhenius acids Produce H + ions in water. H 2 O HCl(g) H + (aq) + Cl (aq) Are electrolytes. Have a sour taste. Turn litmus red. Neutralize bases.
Contents and Concepts
 Chapter 16 1 Learning Objectives Acid Base Concepts Arrhenius Concept of Acids and Base a. Define acid and base according to the Arrhenius concept. Brønsted Lowry Concept of Acids and Bases a. Define acid
Chapter 16 1 Learning Objectives Acid Base Concepts Arrhenius Concept of Acids and Base a. Define acid and base according to the Arrhenius concept. Brønsted Lowry Concept of Acids and Bases a. Define acid
Chapter 16 Acid-Base Equilibria
 Chapter 16 Acid-Base Equilibria Learning goals and key skills: Understand the nature of the hydrated proton, represented as either H + (aq) or H 3 O + (aq) Define and identify Arrhenuis acids and bases.
Chapter 16 Acid-Base Equilibria Learning goals and key skills: Understand the nature of the hydrated proton, represented as either H + (aq) or H 3 O + (aq) Define and identify Arrhenuis acids and bases.
UNIT #11: Acids and Bases ph and poh Neutralization Reactions Oxidation and Reduction
 NAME: UNIT #11: Acids and Bases ph and poh Neutralization Reactions Oxidation and Reduction 1. SELF-IONIZATION OF WATER a) Water molecules collide, causing a very small number to ionize in a reversible
NAME: UNIT #11: Acids and Bases ph and poh Neutralization Reactions Oxidation and Reduction 1. SELF-IONIZATION OF WATER a) Water molecules collide, causing a very small number to ionize in a reversible
You may remove this page. ph + poh = 14. ph = -log[h+], [H+] = 10-pH qlost = -qgained
![You may remove this page. ph + poh = 14. ph = -log[h+], [H+] = 10-pH qlost = -qgained You may remove this page. ph + poh = 14. ph = -log[h+], [H+] = 10-pH qlost = -qgained](/thumbs/88/115139826.jpg) You may remove this page. ph = -log[h+], [H+] = 10-pH 1 2 / ph + poh = 14 0.693 q = mc T q = nlr HLR qlost = -qgained JBA 2018 Chemistry Exam 3 Name: Score: /100 = /80 Multiple choice questions are worth
You may remove this page. ph = -log[h+], [H+] = 10-pH 1 2 / ph + poh = 14 0.693 q = mc T q = nlr HLR qlost = -qgained JBA 2018 Chemistry Exam 3 Name: Score: /100 = /80 Multiple choice questions are worth
Announcements. There are 3-classes of chemical reactions that occur in aqueous solution.
 Announcements Exam 1 Results: Mean: 71% Range: 39.5%-93.5% Median: 72% Other Bio-LS Class Mean 72% Please read Chapter 4 and complete problems. Please see me for help. There are 3-classes of chemical reactions
Announcements Exam 1 Results: Mean: 71% Range: 39.5%-93.5% Median: 72% Other Bio-LS Class Mean 72% Please read Chapter 4 and complete problems. Please see me for help. There are 3-classes of chemical reactions
Unit 9: Acid and Base Multiple Choice Practice
 Unit 9: Acid and Base Multiple Choice Practice Name June 14, 2017 1. Consider the following acidbase equilibrium: HCO3 H2O H2CO3 OH In the reaction above, the BrönstedLowry acids are: A. H2O and OH B.
Unit 9: Acid and Base Multiple Choice Practice Name June 14, 2017 1. Consider the following acidbase equilibrium: HCO3 H2O H2CO3 OH In the reaction above, the BrönstedLowry acids are: A. H2O and OH B.
The Chemistry of Acids and Bases
 The Chemistry of 1 Acids and Bases 2 Acid and Bases 3 Acid and Bases 4 Acid and Bases 5 Strong and Weak Acids/Bases Generally divide acids and bases into STRONG or WEAK ones. STRONG ACID: HNO 3 (aq) +
The Chemistry of 1 Acids and Bases 2 Acid and Bases 3 Acid and Bases 4 Acid and Bases 5 Strong and Weak Acids/Bases Generally divide acids and bases into STRONG or WEAK ones. STRONG ACID: HNO 3 (aq) +
Acids And Bases. H + (aq) + Cl (aq) ARRHENIUS THEORY
 Acids And Bases A. Characteristics of Acids and Bases 1. Acids and bases are both ionic compounds that are dissolved in water. Since acids and bases both form ionic solutions, their solutions conduct electricity
Acids And Bases A. Characteristics of Acids and Bases 1. Acids and bases are both ionic compounds that are dissolved in water. Since acids and bases both form ionic solutions, their solutions conduct electricity
Acid/Base Theories The common characteristics of acids
 Acid/Base Theories The common characteristics of acids describe them as: Acids aving a sour taste Being electrolytes (some weak) Reacting with metals to produce gas (usually 2 ) Reacting with bases to
Acid/Base Theories The common characteristics of acids describe them as: Acids aving a sour taste Being electrolytes (some weak) Reacting with metals to produce gas (usually 2 ) Reacting with bases to
Acid-Base Equilibria
 Acid-Base Equilibria 1. Classify each of the following species as an acid, a base, or amphoteric in aqueous solution: (a) H 2 O; (b) CH 3 CH 2 ; (c) PO 4 3 ; (d) C 6 H 5 NH 3 2. Write the proton transfer
Acid-Base Equilibria 1. Classify each of the following species as an acid, a base, or amphoteric in aqueous solution: (a) H 2 O; (b) CH 3 CH 2 ; (c) PO 4 3 ; (d) C 6 H 5 NH 3 2. Write the proton transfer
Part 01 - Assignment: Introduction to Acids &Bases
 Part 01 - Assignment: Introduction to Acids &Bases Classify the following acids are monoprotic, diprotic, or triprotic by writing M, D, or T, respectively. 1. HCl 2. HClO4 3. H3As 4. H2SO4 5. H2S 6. H3PO4
Part 01 - Assignment: Introduction to Acids &Bases Classify the following acids are monoprotic, diprotic, or triprotic by writing M, D, or T, respectively. 1. HCl 2. HClO4 3. H3As 4. H2SO4 5. H2S 6. H3PO4
15 Acids, Bases, and Salts. Lemons and limes are examples of foods that contain acidic solutions.
 15 Acids, Bases, and Salts Lemons and limes are examples of foods that contain acidic solutions. Chapter Outline 15.1 Acids and Bases 15.2 Reactions of Acids and Bases 15.3 Salts 15.4 Electrolytes and
15 Acids, Bases, and Salts Lemons and limes are examples of foods that contain acidic solutions. Chapter Outline 15.1 Acids and Bases 15.2 Reactions of Acids and Bases 15.3 Salts 15.4 Electrolytes and
