Plant and Cell Physiology Advance Access published April 17, Running title: ravity-regulated release from apical dominance
|
|
|
- Joleen Casey
- 5 years ago
- Views:
Transcription
1 Plant and Cell Physiology Advance Access published April 17, 2008 Running title: ravity-regulated release from apical dominance Corresponding author: Prof. Dr. Hideyuki Takahashi Graduate School of Life Sciences, Tohoku University Katahira, Aoba-ku, Sendai , Japan Phone: Fax: Subject area: (1) Growth and development Number of black and white figures: 5 Number of color figures: 1 Number of tables: 0 Number of supplementary figures: 4 Number of supplementary tables: 1 The Author Published by Oxford University Press on behalf of Japanese Society of Plant Physiologists. All rights reserved. For Permissions, please journals.permissions@oxfordjournals.org 1
2 The Gravity-Regulated Growth of Axillary Buds is Mediated by a Mechanism Different from Decapitation-Induced Release Daisuke Kitazawa 1, Yutaka Miyazawa 1, Nobuharu Fujii 1, Atsushi Hoshino 2, Shigeru Iida 2, Eiji Nitasaka 3, and Hideyuki Takahashi 1, * 1 Graduate School of Life Sciences, Tohoku University, Katahira, Aoba-ku, Sendai , Japan 2 National Institute for Basic Biology, Nishigonaka 38, Myodaiji, Okazaki , Japan 3 Department of Biological Science, Graduate School of Science, Kyushu University, Hakozaki, Fukuoka , Japan Footnotes: * Corresponding author: , hideyuki@ige.tohoku.ac.jp; Fax,
3 When the upper part of the main shoot of the Japanese morning glory (Pharbitis nil or Ipomoea nil) is bent down, the axillary bud situated on the uppermost node of the bending region is released from apical dominance and elongates. Here, we demonstrate that this release of axillary buds from apical dominance is gravity-regulated. We utilized two agravitropic mutants of morning glory defective in gravisensing cell differentiation, weeping (we) and weeping2 (we2). Bending the main shoots of either we or we2 plants resulted in minimal elongation of their axillary buds. This aberration was genetically linked to the agravitropism phenotype of the weeping mutants, which implied that shoot bending-induced release from apical dominance required gravisensing cells. Previous studies have shown that basipetal translocation of auxin from the apical bud inhibits axillary bud growth, whereas cytokinin promotes axillary bud outgrowth. We therefore compared the roles of auxin and cytokinin in bending- or decapitation-induced axillary bud growth. In the wild-type and we plants, decapitation increased cytokinin levels and reduced auxin response. In contrast, shoot bending did not cause significant changes in either cytokinin level or auxin response, suggesting that the mechanisms underlying gravity- and decapitation-regulated release from apical dominance are distinct and unique. Keywords: Apical dominance Gravitropism Auxin Cytokinin Morning glory weeping. Abbreviations: IAA, indole-3-acetic acid; IPT, isopentenyltransferase; ORF, open reading frame; RT-PCR, reverse transcription-pcr; t-zr, trans-zeatin riboside; we, weeping; WT, wild type. The nucleotide sequence reported in this paper has been submitted to GenBank data libraries under accession numbers: PnIAA1 (AB371299), PnIPT1 (AB371300), and PnIPT2 (AB371301). 3
4 Introduction Apical dominance is the central regulatory system for maintaining plant shoot architecture, wherein the growing apical shoot suppresses the growth of axillary buds on the axils of leaves below it (reviewed in Cline 1991). Apical dominance is best demonstrated via shoot tip removal, also known as decapitation, which releases axillary buds from apical dominance and initiates their outgrowth. Although the concept of apical dominance has been known for many centuries, the mechanisms underlying this phenomenon are not well understood and have been the center of debate for over 100 years. The plant hormones auxin and cytokinin are thought to have major roles in controlling apical dominance, with axillary bud growth being inhibited by basipetal translocation of auxin but promoted by cytokinin (reviewed in Shimizu-Sato and Mori 2001, reviewed in Ongaro and Leyser 2008). Following decapitation of shoot apex, auxin levels in the stem decrease, cytokinin levels increase, and the axillary buds elongate. Furthermore, direct application of cytokinin to axillary buds of intact plants is sufficient to promote their outgrowth, suggesting a preferential role for cytokinin in apical dominance (Pillay and Railton 1983, Cline et al. 1997). Apical dominance in plants, although regulated by intrinsic signals such as the developmental stage of plant, is plastic in its response to various environmental cues such as light and nutrient conditions (Snowden and Napoli 2003), and may also be affected by the influence of gravity (Cline 1983, Prasad and Cline 1987). For example, horizontal placement of the Japanese morning glory results in the outgrowth of several 4
5 axillary buds in random locations on the main shoot, while inversion of these plants results in elongation of the buds near the shoot base (Prasad and Cline 1985). Furthermore, bending the upper part of main shoot releases axillary bud at the bending-region from apical dominance and initiates elongation of the bud. This release is prevented by clinorotation of the bent plants (Prasad and Cline 1987). These results suggest that release from apical dominance in morning glory is due, at least in part, to gravistimulation. Similar phenomena have also been observed in agriculture and horticulture. For instance, it is known that shoot bending induces lateral branching and flower bud development in Japanese pear, cherry and plum trees (Wareing and Nasr 1958, Ito et al. 2001). Despite these myriad observations, there has been no direct evidence for the relationship between graviresponse and apical dominance. It has been proposed that perhaps the plant hormone auxin may mediate the influence of gravity on apical dominance. One proposed mechanism is that gravity affects auxin transport and distribution (Wright et al. 1978, Wright 1981). However, no change in auxin distribution during shoot bending was observed when the dynamics of endogenous auxin were examined via mass spectrometry (Prasad et al. 1993). In addition, there have been no reports delineating a role for cytokinin in axillary bud growth induced by shoot bending. Therefore, it is unclear whether shoot bending-induced release from apical dominance is governed by the same mechanisms as decapitation-induced release. The Japanese morning glory (Pharbitis nil and Ipomoea nil) is characterized as an absolute short-day plant, which is advantageous for the study of apical dominance by 5
6 enabling researchers to carry out experiments in the vegetative stage without floral transition of meristems. To elucidate the mechanisms of the gravity-regulated apical dominance, we utilized two agravitropic mutants of the Japanese morning glory, weeping (we) and weeping2 (we2). These mutants display agravitropism due to mutations in the SCARECROW (PnSCR) and SHORT-ROOT (PnSHR1) genes of the we and we2 plants, respectively, which confer defects in the normal differentiation of shoot-gravisensing endodermal cells in morning glory (Hatakeda et al. 2003, Kitazawa et al. 2005, Kitazawa et al. 2008). The shoots of these mutants lack gravisensing cells, allowing extraction of plant graviresponse phenotypes and genetic analyses through comparisons between we, we2, and wild-type plants. For example, we recently demonstrated that gravisensing cells are required for shoot circumnutation and for the winding movements of morning glory, since both movements are impaired in we and we2 shoots (Kitazawa et al. 2005, Kitazawa et al. 2008). We believe that these mutants may also be useful for verifying the role of gravity in the release of axillary bud from apical dominance. In this study, we first verified gravity-regulated release from apical dominance in the Japanese morning glory by examining whether bending the main shoots of weeping mutants caused an outgrowth of the axillary buds. We then investigated the involvement of the plant hormones auxin and cytokinin in two modes of axillary bud outgrowth, bending- and decapitation-induced release from apical dominance. 6
7 Results Effect of shoot bending on axillary bud growth Upon bending an upright morning glory shoot downward, the axillary bud situated on the uppermost node is released from apical dominance and begins to elongate, a response that is obliterated with clinorotation (Prasad and Cline 1987). These results suggest that the bending-induced outgrowth of the axillary bud depends on graviresponse. We verified this hypothesis in our system, using the agravitropic weeping mutants of morning glory. The axillary buds located at nodes 1 through 3 of four-week-old WT, we, and we2 plants grown under conventional conditions were approximately 2 mm in length. There was no statistical difference between the lengths of these axillary buds. Using WT, we, and we2 mutant shoots, we performed shoot bending treatment, according to the procedure described by Cline (1983). When the main shoots of four-week-old plants were bent down above node 6, the axillary bud on node 6 (the uppermost node) began to elongate in WT plants, whereas no outgrowth of the axillary bud was observed in either the we or we2 mutants (Fig. 1A, B). To investigate whether this aberrant response of axillary bud was genetically linked to the agravitropism of these mutants, we performed a linkage analysis of F 2 generations. As shown in Fig. 2, all gravitropic F 2 generations responded to shoot bending by an outgrowth of the axillary buds on their uppermost nodes. In contrast, elongation was not observed in any of the agravitropic F 2 generation plants. These results clearly demonstrated the idea that graviresponse was involved in apical dominance. 7
8 Effect of decapitation on axillary bud growth Classical studies have shown that pinching off the shoot tip (decapitation) induces the outgrowth of the axillary buds in many plant species, a process that is often used for the study of apical dominance (reviewed in Cline 1991). To examine whether endodermis-mediated graviresponse also affects the regulatory mechanism of decapitation-induced axillary bud outgrowth, we decapitated the shoots above node 1 of two-week-old WT, we, and we2 plants. We also investigated the response of these decapitated plants to the application of exogenous auxin to the stump, and determined whether the presence or absence of an endodermis affected the apical dominance-associated basipetal auxin translocation. Decapitation of WT plants caused a rapid release of the axillary buds from apical dominance (Fig. 3, upper panel). Likewise, decapitation induced outgrowth of the axillary buds in both we and we2 plants (Fig. 3, middle and lower panel). Exogenous indole-3-acetic acid (IAA) applied to the cut surface of the decapitated stem inhibited axillary bud outgrowth in WT, we, and we2 plants (Fig. 3), suggesting that abnormal development of the endodermis does not affect the apical dominance-related auxin translocation. These results further suggest that endodermis-mediated gravisensing does not influence decapitation-induced axillary bud growth. Molecular identification of auxin and cytokinin reporter genes The molecular mechanisms underlying shoot bending-induced release from apical dominance remain unclear. Auxin and cytokinin are known to play a role in 8
9 decapitation-induced release, and it is therefore important to clarify whether the dynamics of these hormones are also involved in bending-induced release. In this study, we examined the involvement of auxin and cytokinin in bending- and decapitation-induced axillary bud growth. The expression of an auxin-inducible gene and a biosynthetic gene of cytokinin were adopted as molecular markers. Aux/IAA is a well-characterized family of auxin-inducible genes (Tiwari et al. 2004). We isolated Aux/IAA homologous genes from the Japanese morning glory. Using a PCR-based strategy (see Materials and Methods), we isolated a cdna clone homologous to Aux/IAA from the Japanese morning glory cv. Violet, based on the sequence information of the expressed sequence tag (EST) of the Japanese morning glory cv. TKS (Morita et al. 2006). The predicted polypeptide of the obtained cdna sequence contains domains I, II, III and IV, which are highly conserved among Aux/IAA protein families (Tiwari et al. 2004). We then verified whether the corresponding gene was responsive to auxin using a stem section of the morning glory, according to a method previously described (Fujii et al. 2000). Expression of the gene in the excised sections of the WT plant was reduced by auxin starvation, while subsequent treatment of the sections with exogenous IAA elevated its expression in dose-dependent manner (Supplementary Fig. S1). These data indicate that cdna isolated from the stem of the Japanese morning glory plant is transcribed from a gene belonging to the auxin-responsive Aux/IAA family. We named this gene PnIAA1. It has been reported that one of the cytokinin biosynthetic gene isopentenyltransferase (IPT) contributes to decapitation-induced axillary bud growth in 9
10 the pea plant (Pisum sativum L.) (Tanaka et al. 2006). Following decapitation, levels of cytokinin and PsIPT1 and PsIPT2 mrna were markedly increased in the nodal stem. Expression of these genes was repressed by application of IAA. We searched IPT homologous genes in the morning glory EST database. In Arabidopsis, the IPT family is known for its diverse function (Miyawaki et al. 2006). ATP/ADP IPT and their homologs, AtIPT1 and AtIPT3 AtIPT8 are Arabidopsis enzymes that catalyze the first step of cytokinin biosynthesis (Kakimoto 2003, Miyawaki et al. 2004). AtIPT2 and AtIPT9 are thought to catalyze the isopentenylation of trna, and are probably not involved in cytokinin biosynthesis (Golovko et al. 2002). We performed a phylogenetic analysis of members of the IPT family in various plants based upon the information in the EST database (Supplementary Fig. S2). Since two EST sequences were likely to be transcribed from genes that belong to the cytokinin biosynthetic IPT family, we used a PCR-based strategy to isolate the corresponding cdnas from the WT morning glory (see Materials and Methods). The obtained cdnas of these two IPT-homologous genes, PnIPT1 and PnIPT2, encode putative proteins with sequence identities of 50% and 52%, respectively, to PsIPT1; and 44% and 49%, respectively, to PsIPT2. We therefore designated these IPT-homologous genes PnIPT1 and PnIPT2, respectively. Effect of decapitation on the expression of PnIAA1 and PnIPT1 genes To examine whether PnIAA1 and PnIPTs respond to decapitation, we studied their expression patterns in the first node of the morning glory shoots before and after 10
11 decapitation. The mrna level of PnIAA1 began to decrease 1 h after decapitation, and reached a minimum 3 h after decapitation (Fig. 4A). In contrast, mrna level of PnIPT1 began to increase 1 h, reached a maximum level 3 h, and then began to decrease 6 h after decapitation (Fig. 4B). This expression pattern was similar to that of PsIPT2 reported previously (Tanaka et al. 2006). In the current study, mrna level of PnIPT2 was low, and did not show an explicit expression pattern in response to the decapitation (data not shown). To examine the correlation between the expression of PnIPT1 and the endogenous cytokinin level, we used an ELISA method to quantify trans-zeatin riboside (t-zr) before and after decapitation (Fig. 4C) (Weiler 1980, Weiler 1984). Decapitation induced a marked increase in the level of t-zr in the node situated below the decapitated stump in the WT plant, implying a correlation between PnIPT1 expression and the endogenous cytokinin level. We therefore concluded that the PnIPT1 gene could be used as a reporter for analysis of cytokinin dynamics in the regulation of apical dominance. Effect of auxin application on the expression of PnIAA1 and PnIPT1 genes To elucidate the relationship between basipetal auxin transport and the expression of PnIPT1, we investigated both PnIAA1 and PnIPT1 expressions in the node by applying IAA to the stump after decapitation. Furthermore, to examine whether decapitation-induced release from apical dominance is regulated by the same mechanisms in WT and we plants, we analyzed the expression of the marker genes in 11
12 we plants. Immediately following decapitation of WT or we plants, lanolin with or without IAA was applied to their stumps. When measured at 3 h after application, in either the WT or we plants the levels of the PnIAA1 transcript were decreased in plants treated with lanolin alone, and were increased in plants treated with IAA (Fig. 5A). This suggests that the removal of shoot tip (auxin source) causes a deprivation of the available endogenous auxin in the node and that the IAA applied to the stump basipetally translocates from the stump to the node. In contrast, levels of the PnIPT1 transcript were increased by lanolin alone, but not by IAA, in WT plants (Fig. 5B), strongly suggesting that PnIPT1 transcription is negatively controlled by the basipetal transport of auxin in the stem. In we mutant, PnIAA1 and PnIPT1 expressions were similar to those observed in WT plants (Fig. 5A, B). This result demonstrates that decapitation induces the release from apical dominance in both WT and we plant and that this mechanism of release mediates auxin and cytokinin dynamics. Effect of shoot bending on the expression of PnIAA1 and PnIPT1 genes We next examined the involvement of auxin and cytokinin in the gravity-regulated growth of the axillary bud. We analyzed PnIAA1 and PnIPT1 expression in node 6 at bending region (uppermost node) or in the corresponding node (node 6) of control upright plants of WT and we plants. Surprisingly, in WT plants shoot bending resulted in minimally significant changes in the mrna levels of PnIAA1 and PnIPT1 at 72 h after bending treatment (Fig. 6A). Likewise, levels of PnIAA1 transcripts in we mutant did not display significant fluctuations, nor were there 12
13 significant differences between the WT and we PnIAA1 transcript levels (Fig. 6A). The level of PnIPT1 in we did not differ from that of WT by 24 h after shoot bending, although its level became lower in WT thereafter (Fig. 6A). To confirm these results, we used an ELISA method to measure the endogenous cytokinin content in the uppermost node of the bent plants. In contrast with the results of the decapitation experiments, in both WT and we, endogenous t-zr levels did not significantly increase in the uppermost node within 24 h of bending (Fig. 6B). This result was consistent with the expression pattern of PnIPT1. These observations imply that a dramatic change of neither basipetal auxin transport nor cytokinin biosynthesis in the uppermost node is involved in the bending-induced release from apical dominance. Consequently, we conclude from these data that the mechanism of gravity-regulated release from apical dominance is unique from that of decapitation-induced release, and possibly involves a novel pathway that requires neither a reduction of basipetal auxin transport nor cytokinin biosynthesis. Discussion Our results in this study with agravitropic shoots of the Japanese morning glory demonstrate that endodermis-mediated gravisensing plays an important role in the bending-induced release of axillary buds from apical dominance. Bending shoot down stimulated the outgrowth of the axillary bud situated on the uppermost node in WT but not in weeping plants (Figs. 1, 2). Interestingly, these weeping mutants showed normal response to decapitation, stimulating outgrowth of axillary buds just below the cut 13
14 stump (Fig. 3). Auxin applied to the stump suppressed the decapitation-induced release of axillary buds from apical dominance in both WT and weeping plants (Fig. 3). These results suggest that graviresponse mediates the bending-induced release from apical dominance but not the decapitation-induced response. Thus, the mechanisms for the two modes of axillary bud growth may differ from one another. The mechanism for gravity sensing has been well studied in Arabidopsis; that is, gravisensing apparatus for shoot gravitropism resides in the endodermal cells (Morita and Tasaka 2004). Shoots of we and we2 morning glory do not show gravitropic response because of their defects in normal differentiation of endodermal cells (Kitazawa et al. 2005, 2008). Also, aberrant response of we and we2 shoots in the bending-induced outgrowth of axillary buds is genetically linked to their agravitropic response (Fig. 2). It is therefore assumed that the same gravisensing mechanism is shared by both bending-induced release from apical dominance and shoot gravitropism. At present, however, we cannot rule out the possibility that endodermal cells exert gravisensing mechanisms different for the two graviresponses. Auxin and cytokinin are thought to be key regulators of apical dominance. Basipetal translocation of auxin from shoot apex inhibits axillary bud growth, while cytokinin promotes this process. In many plant species, the direct application of cytokinin to axillary buds promotes their outgrowth, and endogenous cytokinin levels rise in and around axillary buds during growth initiation (Li et al. 1995, Turnbull et al. 1997, Emery et al. 1998). There is unequivocal evidence for cytokinin biosynthesis in shoots (Nordström et al. 2004). To date, cytokinin is the only plant hormone known to 14
15 induce the outgrowth of axillary buds. Tanaka et al. (2006) proposed that one role of auxin is to repress IPT gene expression; that is, local biosynthesis of cytokinin in the nodal stem is negatively regulated by auxin through the control of IPT expression in apical dominance. In agreement with this hypothesis, PnIPT1 gene expression was up-regulated by removal of the auxin source, and was repressed by exogenous IAA application to the cut stump in the morning glory. This suggests that the mechanism underlying the relationship between auxin and cytokinin in the regulation of apical dominance is universal among various plant species. When the upper shoot of the WT plant is bent down, the axillary bud on the uppermost node begins to grow out within h (Cline and Riley 1984). The kinetics of the axillary bud growth induced by shoot bending are similar to those induced by decapitation (Figs. 1, 3). It would be thus possible that bending-induced release from apical dominance was also mediated by auxin and cytokinin. However, surprisingly, shoot bending (gravistimulation) did not affect both auxin and cytokinin dynamics, despite being accompanied by axillary bud outgrowth. Prasad et al. (1993) reported that shoot bending treatment did not significantly cause the reduction of basipetal auxin transport from shoot apex. The expression of an auxin-inducible gene (PnIAA1) hardly changed during bending-induced release from apical dominance (Fig. 6), supporting this observation. In addition, we measured the basipetal auxin transport activity using 3 H-labeled IAA in WT and we plants. Our results showed that basipetal transport activity of exogenously applied 3 H-IAA in we did not differ from that of WT (Supplementary Table S1). This observation also suggests that the gravity-regulated 15
16 release of axillary buds from apical dominance is not related to basipetal auxin transport. Our present study was the first report investigating cytokinin dynamics during bending-induced release from apical dominance. The expression of PnIPT1 and the level of endogenous t-zr did not change significantly during shoot bending, in a sharp contrast to those in decapitation-induced (Fig. 6A, B). In the time-course study, the expression level of PnIPT1 declined h after bending treatment in WT but not in weeping plants. However, this change in WT may not be a cause for the bending-induced release from apical dominance because axillary bud outgrowth commences within 24 h after bending and because the outgrowth is expected to accompany an increase in cytokinin level. In addition, we isolated cdnas of genes (designated as PnRRs) homologous to type-a Arabidopsis Response Regulator (type-a ARR) from morning glory and analyzed their expressions by the real-time PCR in both decapitated and bent shoots of morning glory (Figs. S3, S4). The results was similar to that of t-zr; that is, decapitation unequivocally increased the expression level of PnRR1 in both WT and weeping morning glory, but no significant change in PnRR1 expression was observed following bending treatment in both WT and weeping plants. Type-A ARRs are rapidly and specifically induced by cytokinin and characterized as primary cytokinin response regulator genes (D Agostino et al. 2000). Taking all of this information into consideration, we conclude that a change in neither basipetal auxin transport nor de novo cytokinin biosynthesis in the uppermost node is necessarily required for the gravity-regulated axillary bud growth, which implies the existence of a novel system in the regulation of axillary bud growth. 16
17 A key to elucidating the mechanism of bending-induced release from apical dominance may be hidden in the cascade mediated by the gravisensing endodermal cells. The agravitropic weeping mutants used here lack endodermal cells (Kitazawa et al. 2005, Kitazawa et al. 2008). Since weeping mutants do not respond to shoot bending and respond normally to decapitation, it is clear that endodermal cells are required specifically for the bending-induced release from apical dominance. The present study utilizes agravitropic mutants of the morning glory as a useful tool for the study of the gravity-regulated release from apical dominance. One of the most intriguing questions in the shoot bending-induced release from apical dominance is how plants recognize the highest position of the axillary meristem, and how they direct the buds located in these nodes to initiate growth of a new apical meristem. Gravisensing is a quite novel mechanism that plants have acquired to address such concepts. Materials and Methods Plant materials and growth conditions Pharbitis nil Choisy cv. Violet was used as the wild-type (WT) strain throughout these studies. Seeds of the WT plants were purchased from Marutane Seed Co., Kyoto, Japan. Seeds of the weeping (we) and weeping2 (we2) mutants were propagated as described previously (Kitazawa et al. 2005, Kitazawa et al. 2008). For all experiments, we used weeping mutants that had been crossed with WT plants more than twice to obtain uniform genetic backgrounds. The morning glory plants were grown in a greenhouse as described by Kitazawa et al. (2005). 17
18 Shoot bending experiment For shoot-bending experiments, the internode above node 6 was gently bent down so that the upper part of the shoot was inverted when plants were four weeks old. The length of the axillary bud on node 6 (uppermost node) was monitored every day. Twelve individuals were examined on each morning glory strain. Linkage analysis of F 2 plants between gravitropic response and axillary bud growth due to shoot bending was performed as follows: the we and we2 mutants were crossed with Violet, respectively, and the resulting F 1 plants were self-pollinated to generate F 2 population. Twenty-five individuals of F 2 plants showing gravitropic or agravitropic response were cultivated, respectively, and subjected to shoot bending treatment when plants were four weeks old. Ten days after the start of bending treatment, we measured the lengths of the axillary buds on the uppermost node. Decapitation For decapitation studies, two week-old plants were decapitated, and lanolin paste (lanolin-dehydrate; Wako, Osaka, Japan) containing 0.1% (w/v) IAA (indole-3-acetic acid; Wako) was applied to the cut surface of the decapitated stem, and the plants were grown for six days. Plants without decapitation and decapitated plants to which lanolin paste without IAA was applied were also grown. The length of axillary bud was monitored every day, and lanolin paste was renewed every other day. Twenty-four individuals were examined in each experiment. 18
19 RNA isolation and cloning of full-length PnIAA1 and PnIPT1 cdnas Total RNA was extracted from the stems of two-week-old WT plants grown in greenhouse, by using an RNeasy Plant Mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer s instructions. First-strand cdna was synthesized by using a ReverTra Ace α kit (TOYOBO, Osaka, Japan) following the supplier s instructions. The EST database of the Japanese morning glory contained a sequence that was likely to be transcribed from a gene homologous to Aux/IAA and contained two sequences that were likely to be transcribed from genes homologous to ATP/ADP IPT. These EST sequences contained full-length cdna sequences of the corresponding genes. These were amplified using gene specific primers: 5 -AATGACGGCGAAGCGGTGAA-3 (forward) and 5 -GGATCAGAAGCCATTGGACT-3 (reverse) for PnIAA1, and 5 -TAACAAGGTCACCGACGAGG-3 (forward) and 5 -AATGGCGTCTTCCAGTAGCC-3 (reverse) for PnIPT1. Amplified cdna fragments were then cloned into the pgem-t Easy vector (Promega, Madison, WI, USA). DNA sequences were determined by using an ABI 310 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) according to the standard procedures. Quantitative reverse-transcription (RT)-PCR analysis First-strand cdna was synthesized as described above. Quantitative real-time PCR was performed on a MyiQ single-color real-time PCR detection system using an iq SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) according to 19
20 the manufacturer s instructions. Target gene expressions were normalized to mitochondrial F 1 F 0 ATP synthase γ-subunit expression, which is commonly used as an internal standard in morning glory (GenBank accession no. AB194067). PCR was carried out using the following program: once 120 s at 95 C, 45 cycles of 10 s at 95 C, 30 s at 55 C (PnIAA1 and ATP synthase) or 30s at 57 C (PnIPT1), 45 s at 72 C, followed by melting curve analysis. Calibration was performed using a cdna fragment cloned in a plasmid as a template. The following primers used for amplification were set at the specific region for each gene: PnIAA1 F1 (5 -AATGACGGCGAAGCGGTGAA-3 ) and PnIAA1 R1 (5 -GGATCAGAAGCCATTGGACT-3 ) for PnIAA1, PnIPT1 F2 (5 -TAACAAGGTCACCGACGAGG-3 ) and PnIPT1 R2 (5 -AATGGCGTCTTCCAGTAGCC-3 ) for PnIPT1, ATPS F (5 -CAGTGGATCCTGATGACATCCTTAAAAATG-3 ) and ATPS R (5 -CTTCCTCGAGTTTTATTTCATCACCATCAG-3 ) for ATP synthase γ-subunit. Measurement of trans-zeatin riboside (t-zr) content The t-zr standard was purchased from Sigma Chemical Co. (St. Louis, MO, USA), and prepared via a dilution series with absolute methanol for making a standard curve. Collected samples of morning glory were frozen once with liquid nitrogen, and then homogenized in absolute methanol. After centrifugation of the homogenate, the supernatant was collected as a crude extract. We used a commercial competitive ELISA kit (trans-zeatin riboside immunoassay detection kit; Sigma). The crude extracts were 20
21 preincubated in wells for 3 h at 4 C and then placed in an incubator at 37 C for 1 h. Resultant products of the enzymatic reactions were quantified by a spectrophotometer at 405 nm. The detailed procedure was as per the manufacturer s instructions. Acknowledgments This work was financially supported by Grant-in-Aid for Scientific Research (B) (No ) from the Japan Society for the Promotion of Science; Grant-in-Aid for Scientific Research on Priority Areas (No and ) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to H.T.); and Japan Society for the Promotion of Science Research Fellowships for Young Scientists (to D.K.). This study was also carried out as part of the Ground-Based Research Announcement for Space Utilization promoted by the Japan Space Forum. References Cline, M.G. (1983) Apical dominance in Pharbitis nil: effects induced by inverting the apex of the main shoot. Ann. Bot. 52: Cline, M.G. and Riley, L. (1984) The presentation time for shoot inversion release of apical dominance in Pharbitis nil. Ann. Bot. 53: Cline, M.G. (1991) Apical dominance. Bot. Rev. 57: Cline, M., Wessel, T. and Iwamura, H. (1997) Cytokinin/auxin control of apical dominance in Ipomoea nil. Plant Cell Physiol. 37:
22 D Agostino, I.B., Deruère, J. and Kieber, J.J. (2000) Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiol. 124: Emery, R.J.N., Longnecker, N.E. and Atkins, C.A. (1998) Branch development in Lupinus angustifolius L. II. Relationship with endogenous ABA, IAA and cytokinins in axillary and main stem buds. J. Exp. Bot. 49: Fujii, N., Kamada, M., Yamasaki, S. and Takahashi, H. (2000) Differential accumulation of Aux/IAA mrna during seedling development and gravity response in cucumber (Cucumis sativus L.). Plant Mol. Biol. 42: Golovko, A., Sitbon, F., Tillberg, E. and Nicander, B. (2002) Identification of a trna isopentenyltransferase gene from Arabidopsis thaliana. Plant Mol. Biol. 49: Hatakeda, Y., Kamada, M., Goto, N., Fukaki, H., Tasaka, M., Suge, H. and Takahashi, H. (2003) Gravitropic response plays an important role in the nutational movements of the shoots of Pharbitis nil and Arabidopsis thaliana. Physiol. Plant. 118: Ito, A., Hayama, H. and Yoshida, H. (2001) The effect of shoot-bending on the amount of diffusible indole-3-acetic acid and its transport in shoots of Japanese pear. Plant Growth Regul. 34: Kakimoto, T. (2003) Biosynthesis of cytokinins. J. Plant Res. 116:
23 Kitazawa, D., Hatakeda, Y., Kamada, M., Fujii, N., Miyazawa, Y. et al. (2005) Shoot circumnutation and winding movements require gravisensing cells. Proc. Natl. Acad. Sci. USA 102: Kitazawa, D., Miyazawa, Y., Fujii, N., Nitasaka, E. and Takahashi, H. (2008) Characterization of a novel gravitropic mutant of morning glory, weeping2. Adv. Space Res. in press (doi: /j.asr ). Li, C.J., Guevara, E., Gerrera, J. and Bangerth, F. (1995) Effect of apex excision and replacement by 1-naphtylacetic acid and cytokinin concentration and apical dominance in pea plants. Physiol. Plant. 94: Miyawaki, K., Matsumoto-Kitano, M. and Kakimoto, T. (2004) Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J. 37: Miyawaki, K., Tarkowski, P., Matsumoto-Kitano, M., Kato, T., Sato, S., Tarkowska, D., Tabata, S., Sandberg, G. and Kakimoto, T. (2006) Roles of Arabidopsis ATP/ADP isopentenyltransferases and trna isopentenyltransferases in cytokinin biosynthesis. Proc. Natl. Acad. Sci. USA 103: Morita, M.T. and Tasaka, M. (2004) Gravity sensing and signaling. Curr. Opin. Plant Biol. 7: Morita, Y., Saitoh, M., Hoshino, A., Nitasaka, E. and Iida, S. (2006) Isolation of cdnas for R2R3-MYB, bhlh and WDR transcriptional regulators and identification of c and ca mutations conferring white flowers in the Japanese morning glory. Plant Cell Physiol. 47:
24 Nordström, A., Tarkowski, P., Tarkowska, D., Norbaek, R., Åstot, C., Dolezal, K. and Sandberg, G. (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin-cytokinin-regulated development. Proc. Natl. Acad. Sci. USA 101: Ongaro, V. and Leyser, O. (2008) Hormonal control of shoot branching. J. Exp. Bot. 59: Pillay, I. and Railton, I.D. (1983) Complete release of axillary buds from apical dominance in intact, light-grown seedlings of Pisum sativum L. following a single application of cytokinin. Plant Physiol. 71: Prasad, T.K. and Cline, M.G. (1985) Shoot inversion-induced ethylene in Pharbitis nil induces the release of apical dominance by restricting shoot elongation. Plant Sci. 38: Prasad, T.K. and Cline, M.G. (1987) The role of gravity in apical dominance. Plant Physiol. 83: Prasad, T.K., Li, X., Abdel-Rahman, A.M., Hosokawa, Z., Cloud, N.P., LaMotte, C.E. and Cline, M.G. (1993) Does auxin play a role in the release of apical dominance by shoot inversion in Ipomoea nil? Ann. Bot. 71: Shimizu-Sato, S. and Mori, H. (2001) Control of outgrowth and dormancy in axillary buds. Plant Physiol. 127: Snowden, K.C. and Napoli, C.A. (2003) A quantitative study of lateral branching in petunia. Funct. Plant Biol. 30:
25 Tanaka, M., Takei, K., Kojima, M., Sakakibara, H. and Mori, H. (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J. 45: Tiwari, S.B., Hagen, G. and Guilfoyle, T.J. (2004) Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell 16: Turnbull, C.G.N., Raymond, M.A.A., Dodd, I.C. and Morris, S.E. (1997) Rapid increases in cytokinin concentration in lateral buds of chickpea (Cicer arietinum L.) during release of apical dominance. Planta 202: Wareing, P.F. and Nasr, T.A.A. (1958) Gravimorphism in trees: effects of gravity on growth, apical dominance and flowering in fruit trees. Nature 182: Weiler, E.W. (1980) Radioimmunoassays for trans-zeatin and related cytokinins. Planta 149: Weiler, E.W. (1984) Immunoassay of plant growth regulators. Ann. Rev. Plant Physiol. 35: Wright, M., Mousdale, D.M. and Osborne, D.J. (1978) Evidence for a gravity-regulated level of endogenous auxin controlling cell elongation and ethylene production during geotropic bending in grass nodes. Biochem. Physiol. Pflanzen. 172: Wright, M. (1981) Reversal of the polarity of IAA transport in the leaf sheath base of Echinochloa colonum. J. Exp. Bot. 32:
26 Legends to Figures Fig. 1 Shoot-bending treatment on Violet and weeping mutants. (A) Photographs of the bent plants (upper panel) and magnification of one of their axillary buds on the uppermost node in the bending region (lower panel). Arrowheads indicate the axillary bud on the uppermost node. Arrow (g) indicates the orientation of gravity. Bars = 5 cm. (B) Measurement of the axillary bud length on the uppermost node. After bending treatment, the length of the axillary bud on the uppermost node was measured everyday. Data represent the means of twelve individuals. Vertical bars indicate ± SD. Open circles, Violet without bending treatment; closed circles, Violet with bending treatment; open squares, weeping without bending treatment; closed squares, weeping with bending treatment; open triangles, weeping2 without bending treatment; closed triangles, weeping2 with bending treatment. Fig. 2 F 2 linkage analysis between gravitropic response and axillary bud growth. Ten days after shoot-bending treatment, the axillary buds on the uppermost node were measured. Distributions of the length of the axillary buds on the uppermost node of F 2 plants are shown: we mutant F 2 segregants (upper panel), we2 mutant F 2 segregants (lower panel). Open column, F 2 generation showing proper gravitropism; closed column, F 2 generation showing abnormal gravitropism. Twenty-five individuals of F 2 plants showing gravitropic or agravitropic response were tested. 26
27 Fig. 3 Effects of decapitation and auxin application to the stump on axillary bud growth in Violet and weeping mutants. Two-week-old plants were decapitated and lanolin paste containing 0.1% (w/v) IAA was applied to the cut surface of the decapitated stem. Plants without decapitation and decapitated plants to which lanolin paste without IAA was applied (mock treatment) were also grown as controls. Axillary bud growth was monitored everyday, and lanolin paste was renewed every other day. Lengths of axillary buds of twenty-four individuals were examined in each experiment. Data are the means ± SD. Closed circles, plants without decapitation; open circles, decapitated plants with mock treatment; open triangles, decapitated plants with IAA application to the stump. Fig. 4 Expression patterns of auxin and cytokinin marker genes and level of endogenous t-zr in the nodal stem after decapitation. Quantitative real-time PCR analysis of the expression of PnIAA1 (A) and PnIPT1 (B) was done. The shoot apex was removed 1 cm above the first node, then the first nodal stems were collected at the indicated times. The expression levels of these genes were normalized to F 1 F 0 ATP synthase γ-subunit gene expression levels. Relative expression levels are shown, where those at time zero was set as 1. Data are the means ± SD. Experiments were performed in triplicate using independent RNA samples. (C) Endogenous t-zr contents of the nodal stem after decapitation. The shoot apex was removed 1 cm above the first node, and then the first nodal stems were collected at the indicated times. The t-zr contents were analyzed by ELISA as described in Materials and Methods. Data are the means ± SE. 27
28 Fig. 5 Effects of IAA applied to the decapitated stump on PnIAA1 and PnIPT1 expressions in WT and we plants. Quantitative real-time PCR analysis of the expression of PnIAA1 (A) and PnIPT1 (B) was done. The shoot apex was removed 1 cm above the first node, and lanolin paste with 0.1% IAA and without IAA was immediately applied to the stump, then the first nodal stems were collected at 3 h after treatment. The expression levels of these genes were normalized to F 1 F 0 ATP synthase γ-subunit gene expression levels. Relative expression levels are shown, where that of Violet intact plants was set as 1. Data are the means ± SD. Open column, Violet; shaded column, weeping. Fig. 6 The effects of shoot bending treatment on the expression of PnIAA1 and PnIPT1, and endogenous t-zr contents at the uppermost nodal stems of Violet and we plants. Bending treatment was performed, and then sampling of the uppermost nodes (the sixth node) was done at indicated times after treatment, respectively. For non-treated plants, the node corresponding to the uppermost node (the sixth node) of bent plants was sampled. (A) Quantitative real-time PCR analysis of the expression of PnIAA1 (upper panel) and PnIPT1 (lower panel) was performed. The expression levels of these genes were normalized to F 1 F 0 ATP synthase γ-subunit gene expression levels. Relative expression levels are shown, where that of Violet plants at time zero (0 h) was set as value 1. Data represent means ± SD. (B) Endogenous t-zr contents of the nodal stem on the uppermost node during shoot bending treatment. The nodal stems on the uppermost node (the sixth node) were collected at the indicated times. The t-zr contents were analyzed by ELISA as described in Materials and Methods. Data are the means ± SE. Open circles, Violet; closed circles, weeping. 28
29 Fig. 1 29
30 Fig. 2 30
31 Fig. 3 31
32 Fig. 4 32
33 Fig. 5 33
34 Fig. 6 34
Apical dominance models can generate basipetal patterns of bud activation
 Apical dominance models can generate basipetal patterns of bud activation Przemyslaw Prusinkiewicz 1, Richard S. Smith 1 and Ottoline Leyser 2 1 Department of Computer Science, University of Calgary 2
Apical dominance models can generate basipetal patterns of bud activation Przemyslaw Prusinkiewicz 1, Richard S. Smith 1 and Ottoline Leyser 2 1 Department of Computer Science, University of Calgary 2
Codonopsis pilosula twines either to the left or to the right
 1 2 BRIEF COMMUNICATION Codonopsis pilosula twines either to the left or to the right 3 Gonghua Lin 1*, Fang Zhao 2, Eviatar Nevo 3, Tongzuo Zhang 1, Jianping Su 1 Nature Precedings : doi:10.1038/npre.2012.7026.1
1 2 BRIEF COMMUNICATION Codonopsis pilosula twines either to the left or to the right 3 Gonghua Lin 1*, Fang Zhao 2, Eviatar Nevo 3, Tongzuo Zhang 1, Jianping Su 1 Nature Precedings : doi:10.1038/npre.2012.7026.1
23-. Shoot and root development depend on ratio of IAA/CK
 Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
BIO1PS 2012 Plant Science Lecture 4 Hormones Pt. I
 BIO1PS 2012 Plant Science Lecture 4 Hormones Pt. I Dr. Michael Emmerling Department of Botany Room 410 m.emmerling@latrobe.edu.au Hormones and Ghost gum Eucalyptus papuana Coordination ~3 Lectures Leaves
BIO1PS 2012 Plant Science Lecture 4 Hormones Pt. I Dr. Michael Emmerling Department of Botany Room 410 m.emmerling@latrobe.edu.au Hormones and Ghost gum Eucalyptus papuana Coordination ~3 Lectures Leaves
10/4/2017. Chapter 39
 Chapter 39 1 Reception 1 Reception 2 Transduction CYTOPLASM CYTOPLASM Cell wall Plasma membrane Phytochrome activated by light Cell wall Plasma membrane Phytochrome activated by light cgmp Second messenger
Chapter 39 1 Reception 1 Reception 2 Transduction CYTOPLASM CYTOPLASM Cell wall Plasma membrane Phytochrome activated by light Cell wall Plasma membrane Phytochrome activated by light cgmp Second messenger
CONTROL SYSTEMS IN PLANTS
 AP BIOLOGY PLANTS FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROL SYSTEMS IN PLANTS HORMONES MECHANISM FOR HORMONE ACTION Plant Form and Function Activity #5 page 1 CONTROL OF CELL ELONGATION Plant
AP BIOLOGY PLANTS FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROL SYSTEMS IN PLANTS HORMONES MECHANISM FOR HORMONE ACTION Plant Form and Function Activity #5 page 1 CONTROL OF CELL ELONGATION Plant
COMPETITIVE CANALIZATION OF AUXIN IN PEA CAN BE INVOLVED IN INITIATION OF AXILLARY BUD OUTGROWTH
 COMPETITIVE CANALIZATION OF AUXIN IN PEA CAN BE INVOLVED IN INITIATION OF AXILLARY BUD OUTGROWTH Medveďová Z. 1, Balla J. 1, 2, Procházka S. 1 1 CEITEC - Central European Institute of Technology, Mendel
COMPETITIVE CANALIZATION OF AUXIN IN PEA CAN BE INVOLVED IN INITIATION OF AXILLARY BUD OUTGROWTH Medveďová Z. 1, Balla J. 1, 2, Procházka S. 1 1 CEITEC - Central European Institute of Technology, Mendel
Supplemental Data. Wang et al. (2014). Plant Cell /tpc
 Supplemental Figure1: Mock and NPA-treated tomato plants. (A) NPA treated tomato (cv. Moneymaker) developed a pin-like inflorescence (arrowhead). (B) Comparison of first and second leaves from mock and
Supplemental Figure1: Mock and NPA-treated tomato plants. (A) NPA treated tomato (cv. Moneymaker) developed a pin-like inflorescence (arrowhead). (B) Comparison of first and second leaves from mock and
Useful Propagation Terms. Propagation The application of specific biological principles and concepts in the multiplication of plants.
 Useful Propagation Terms Propagation The application of specific biological principles and concepts in the multiplication of plants. Adventitious Typically describes new organs such as roots that develop
Useful Propagation Terms Propagation The application of specific biological principles and concepts in the multiplication of plants. Adventitious Typically describes new organs such as roots that develop
EFFECTS OF DIFFERENT MORPHOREGULATORS ON GROWTH AND DEVELOPMENT OF CANNABIS SATIVA L.
 EFFECTS OF DIFFERENT MORPHOREGULATORS ON GROWTH AND DEVELOPMENT OF CANNABIS SATIVA L. AJINKYA BHARAT LALGE, PETER MENDEL, TOMAS VYHNANEK, VACLAV TROJAN, PETR KALOUSEK, LADISLAV HAVEL Department of Plant
EFFECTS OF DIFFERENT MORPHOREGULATORS ON GROWTH AND DEVELOPMENT OF CANNABIS SATIVA L. AJINKYA BHARAT LALGE, PETER MENDEL, TOMAS VYHNANEK, VACLAV TROJAN, PETR KALOUSEK, LADISLAV HAVEL Department of Plant
Plant Growth and Development
 Plant Growth and Development Concept 26.1 Plants Develop in Response to the Environment Factors involved in regulating plant growth and development: 1. Environmental cues (e.g., day length) 2. Receptors
Plant Growth and Development Concept 26.1 Plants Develop in Response to the Environment Factors involved in regulating plant growth and development: 1. Environmental cues (e.g., day length) 2. Receptors
GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL
 GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL Kelsey Hoth 1 Dr. Maria Ivanchenko 2 Bioresourse Research 1, Department of Botany and Plant Physiology 2, Oregon State University, Corvallis,
GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL Kelsey Hoth 1 Dr. Maria Ivanchenko 2 Bioresourse Research 1, Department of Botany and Plant Physiology 2, Oregon State University, Corvallis,
Biological Roles of Cytokinins
 Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme By Kurakawa et. Al. Published in Nature Presented by Boyana Grigorova Biological Roles of Cytokinins Cytokinins are positive regulators
Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme By Kurakawa et. Al. Published in Nature Presented by Boyana Grigorova Biological Roles of Cytokinins Cytokinins are positive regulators
Major Plant Hormones 1.Auxins 2.Cytokinins 3.Gibberelins 4.Ethylene 5.Abscisic acid
 Plant Hormones Lecture 9: Control Systems in Plants What is a Plant Hormone? Compound produced by one part of an organism that is translocated to other parts where it triggers a response in target cells
Plant Hormones Lecture 9: Control Systems in Plants What is a Plant Hormone? Compound produced by one part of an organism that is translocated to other parts where it triggers a response in target cells
Reproduction, Seeds and Propagation
 Reproduction, Seeds and Propagation Diploid (2n) somatic cell Two diploid (2n) somatic cells Telophase Anaphase Metaphase Prophase I One pair of homologous chromosomes (homologues) II Homologues condense
Reproduction, Seeds and Propagation Diploid (2n) somatic cell Two diploid (2n) somatic cells Telophase Anaphase Metaphase Prophase I One pair of homologous chromosomes (homologues) II Homologues condense
PLANT HORMONES-Introduction
 PLANT HORMONES-Introduction By convention hormone are said to be a substances whose site of synthesis and site of action are different; the two events are separated by space and time. Hormones are known
PLANT HORMONES-Introduction By convention hormone are said to be a substances whose site of synthesis and site of action are different; the two events are separated by space and time. Hormones are known
The stationary nature of plants distinguishes them from other
 Shoot circumnutation and winding movements require gravisensing cells Daisuke Kitazawa*, Yasuko Hatakeda*, Motoshi Kamada*, Nobuharu Fujii*, Yutaka Miyazawa*, Atsushi Hoshino, Shigeru Iida, Hidehiro Fukaki,
Shoot circumnutation and winding movements require gravisensing cells Daisuke Kitazawa*, Yasuko Hatakeda*, Motoshi Kamada*, Nobuharu Fujii*, Yutaka Miyazawa*, Atsushi Hoshino, Shigeru Iida, Hidehiro Fukaki,
CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E
 CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E The development of a plant the series of progressive changes that take place throughout its life is regulated in complex ways. Factors take part
CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E The development of a plant the series of progressive changes that take place throughout its life is regulated in complex ways. Factors take part
Bio 100 Guide 27.
 Bio 100 Guide 27 http://www.offthemarkcartoons.com/cartoons/1994-11-09.gif http://www.cneccc.edu.hk/subjects/bio/album/chapter20/images/plant_growth.jpg http://pgjennielove.files.wordpress.com/2008/06/apical_meristem.png
Bio 100 Guide 27 http://www.offthemarkcartoons.com/cartoons/1994-11-09.gif http://www.cneccc.edu.hk/subjects/bio/album/chapter20/images/plant_growth.jpg http://pgjennielove.files.wordpress.com/2008/06/apical_meristem.png
Maria V. Yamburenko, Yan O. Zubo, Radomíra Vanková, Victor V. Kusnetsov, Olga N. Kulaeva, Thomas Börner
 ABA represses the transcription of chloroplast genes Maria V. Yamburenko, Yan O. Zubo, Radomíra Vanková, Victor V. Kusnetsov, Olga N. Kulaeva, Thomas Börner Supplementary data Supplementary tables Table
ABA represses the transcription of chloroplast genes Maria V. Yamburenko, Yan O. Zubo, Radomíra Vanková, Victor V. Kusnetsov, Olga N. Kulaeva, Thomas Börner Supplementary data Supplementary tables Table
Endogenous Hormones Levels and Csexpansin 10 Gene Expression in the Fruit Set and Early Development of Cucumber
 Yongdong Sun et al., J.Chem.Soc.Pak., Vol. 39, No. 1, 217 59 Endogenous Hormones Levels and Csexpansin Gene Expression in the Fruit Set and Early Development of Cucumber Yongdong Sun*, Weirong Luo, Zhenxia
Yongdong Sun et al., J.Chem.Soc.Pak., Vol. 39, No. 1, 217 59 Endogenous Hormones Levels and Csexpansin Gene Expression in the Fruit Set and Early Development of Cucumber Yongdong Sun*, Weirong Luo, Zhenxia
Chapter 33 Plant Responses
 Chapter 33 Plant Responses R. Cummins 1 Chapter 33 Plant Responses External Factors Light, Day Length, Gravity, Temperature Internal Factors Hormones R. Cummins 2 Tropisms R. Cummins 3 Phototropism and
Chapter 33 Plant Responses R. Cummins 1 Chapter 33 Plant Responses External Factors Light, Day Length, Gravity, Temperature Internal Factors Hormones R. Cummins 2 Tropisms R. Cummins 3 Phototropism and
Topic 14. The Root System. II. Anatomy of an Actively Growing Root Tip
 Topic 14. The Root System Introduction. This is the first of two lab topics that focus on the three plant organs (root, stem, leaf). In these labs we want you to recognize how tissues are organized in
Topic 14. The Root System Introduction. This is the first of two lab topics that focus on the three plant organs (root, stem, leaf). In these labs we want you to recognize how tissues are organized in
NATURAL VARIATION IN THE CYTOKININ METABOLIC NETWORK IN ARABIDOPSIS THALIANA
 NATURAL VARIATION IN THE CYTOKININ METABOLIC NETWORK IN ARABIDOPSIS THALIANA PŘÍRODNÍ VARIACE METABOLISMU CYTOKININŮ U ARABIDOPSIS THALIANA Samsonová Z. 1, 2, 3, Kuklová A. 1, 2, Mazura P. 1, 2, Rotková
NATURAL VARIATION IN THE CYTOKININ METABOLIC NETWORK IN ARABIDOPSIS THALIANA PŘÍRODNÍ VARIACE METABOLISMU CYTOKININŮ U ARABIDOPSIS THALIANA Samsonová Z. 1, 2, 3, Kuklová A. 1, 2, Mazura P. 1, 2, Rotková
Cytokinin. Fig Cytokinin needed for growth of shoot apical meristem. F Cytokinin stimulates chloroplast development in the dark
 Cytokinin Abundant in young, dividing cells Shoot apical meristem Root apical meristem Synthesized in root tip, developing embryos, young leaves, fruits Transported passively via xylem into shoots from
Cytokinin Abundant in young, dividing cells Shoot apical meristem Root apical meristem Synthesized in root tip, developing embryos, young leaves, fruits Transported passively via xylem into shoots from
CBSE Quick Revision Notes (Class-11 Biology) CHAPTER-15 PLANT GROWTH AND DEVELOPMENT
 CBSE Quick Revision Notes (Class-11 Biology) CHAPTER-15 PLANT GROWTH AND DEVELOPMENT Root, stem leaves, flower, fruits and seeds arise in orderly manner in plants. The sequence of growth is as follows-
CBSE Quick Revision Notes (Class-11 Biology) CHAPTER-15 PLANT GROWTH AND DEVELOPMENT Root, stem leaves, flower, fruits and seeds arise in orderly manner in plants. The sequence of growth is as follows-
Actions of auxin. Hormones: communicating with chemicals History: Discovery of a growth substance (hormone- auxin)
 Hormones: communicating with chemicals History- discovery of plant hormone. Auxin Concepts of hormones Auxin levels are regulated by synthesis/degradation, transport, compartmentation, conjugation. Polar
Hormones: communicating with chemicals History- discovery of plant hormone. Auxin Concepts of hormones Auxin levels are regulated by synthesis/degradation, transport, compartmentation, conjugation. Polar
CONTROL OF GROWTH BY HORMONES
 CONTROL OF GROWTH BY HORMONES Growth and organogenesis are controlled......by genes (independent of environment): e.g., number of primary vascular bundles, general shape of a leaf or flower...by genes
CONTROL OF GROWTH BY HORMONES Growth and organogenesis are controlled......by genes (independent of environment): e.g., number of primary vascular bundles, general shape of a leaf or flower...by genes
can affect division, elongation, & differentiation of cells to another region of plant where they have an effect
 Note that the following is a rudimentary outline of the class lecture; it does not contain everything discussed in class. Plant Hormones Plant Hormones compounds regulators growth or can affect division,
Note that the following is a rudimentary outline of the class lecture; it does not contain everything discussed in class. Plant Hormones Plant Hormones compounds regulators growth or can affect division,
Other funding Sources Agency Name: MSU Agricultural Experiment Station /Project GREEEN Amount requested or awarded: 30,000
 FINAL PROJECT REPORT Project Title: Functional genomics of flowering in apple PI: Herb Aldwinckle Co-PI(2): Steve VanNocker Organization: Cornell University Organization: Michigan State University Telephone/email:
FINAL PROJECT REPORT Project Title: Functional genomics of flowering in apple PI: Herb Aldwinckle Co-PI(2): Steve VanNocker Organization: Cornell University Organization: Michigan State University Telephone/email:
Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated
 Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated form by recombinant UGT74E2. The naturally occurring auxin
Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated form by recombinant UGT74E2. The naturally occurring auxin
Plant Responses. NOTE: plant responses involve growth and changes in growth. Their movement is much slower than that of animals.
 Plant Responses A stimulus is anything that causes a reaction in an organism. Examples: light, gravity and temperature A response is the activity of an organism as a result of a stimulus. Examples: Growth,
Plant Responses A stimulus is anything that causes a reaction in an organism. Examples: light, gravity and temperature A response is the activity of an organism as a result of a stimulus. Examples: Growth,
TOPIC 9.3 GROWTH IN PLANTS
 TOPIC 9.3 GROWTH IN PLANTS 9.3 A Growth INTRO http://cdn2.hubspot.net/hubfs/18130/social-suggested-images/plant_growing.jpeg IB BIO 9.3 3 In general, plants are able to grow indeterminately. This means
TOPIC 9.3 GROWTH IN PLANTS 9.3 A Growth INTRO http://cdn2.hubspot.net/hubfs/18130/social-suggested-images/plant_growing.jpeg IB BIO 9.3 3 In general, plants are able to grow indeterminately. This means
The Branching Gene RAMOSUS1 Mediates Interactions among Two Novel Signals and Auxin in Pea
 The Plant Cell, Vol. 17, 464 474, February 2005, www.plantcell.org ª 2005 American Society of Plant Biologists The Branching Gene RAMOSUS1 Mediates Interactions among Two Novel Signals and Auxin in Pea
The Plant Cell, Vol. 17, 464 474, February 2005, www.plantcell.org ª 2005 American Society of Plant Biologists The Branching Gene RAMOSUS1 Mediates Interactions among Two Novel Signals and Auxin in Pea
The basal-branching pea mutant rms7-1
 Morris, S.E., Beveridge, C.A., Murfet, I.C, Prioul, S. and Rameau, C. The basal-branching pea mutant rms7-1 Australian Res. Council Cent, of Excellence for Integrative Legume Res., and School of Life Sci.
Morris, S.E., Beveridge, C.A., Murfet, I.C, Prioul, S. and Rameau, C. The basal-branching pea mutant rms7-1 Australian Res. Council Cent, of Excellence for Integrative Legume Res., and School of Life Sci.
Analysis of regulatory function of circadian clock. on photoreceptor gene expression
 Thesis of Ph.D. dissertation Analysis of regulatory function of circadian clock on photoreceptor gene expression Tóth Réka Supervisor: Dr. Ferenc Nagy Biological Research Center of the Hungarian Academy
Thesis of Ph.D. dissertation Analysis of regulatory function of circadian clock on photoreceptor gene expression Tóth Réka Supervisor: Dr. Ferenc Nagy Biological Research Center of the Hungarian Academy
Gibberellins (GA) are involved in cell elongation, particularly in the stem.
 Plant Hormone Lab Plant hormones influence many aspects of plant growth, particularly cell proliferation and elongation. Different hormones are synthesized in different parts of the plant, and have complex
Plant Hormone Lab Plant hormones influence many aspects of plant growth, particularly cell proliferation and elongation. Different hormones are synthesized in different parts of the plant, and have complex
Title Allantoin by Inosine in Nutrient So. Author(s) Toshihiro; Yokoi, Daisuke; Osaki, M
 Title Rice Root Growth with Increasing in Allantoin by Inosine in Nutrient So Author(s) Tokuhisa, Dai; Okazaki, Keiki; Shin Toshihiro; Yokoi, Daisuke; Osaki, M Citation The Proceedings of the Internationa
Title Rice Root Growth with Increasing in Allantoin by Inosine in Nutrient So Author(s) Tokuhisa, Dai; Okazaki, Keiki; Shin Toshihiro; Yokoi, Daisuke; Osaki, M Citation The Proceedings of the Internationa
Nature Genetics: doi: /ng Supplementary Figure 1. The phenotypes of PI , BR121, and Harosoy under short-day conditions.
 Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Plant. Responses and Adaptations. Plant Hormones. Plant Hormones. Auxins. Auxins. Hormones tell plants:
 Plant Responses and Adaptations Plant Hormones Hormone - a substance that is produced in 1 part of an organism & affects another part of the same individual (a chemical messenger) Plant hormones are chemical
Plant Responses and Adaptations Plant Hormones Hormone - a substance that is produced in 1 part of an organism & affects another part of the same individual (a chemical messenger) Plant hormones are chemical
Plant Growth Regulators
 Plant Growth Regulators Dr.H.B.Mahesha, Yuvaraja s College, University of Mysore, India. Growth is an important factor of living organism defined as a permanent and irreversible change in size or volume
Plant Growth Regulators Dr.H.B.Mahesha, Yuvaraja s College, University of Mysore, India. Growth is an important factor of living organism defined as a permanent and irreversible change in size or volume
What is Growth? Increment in biomass Increase in volume Increase in length or area Cell division, expansion and differentiation. Fig. 35.
 What is Growth? Increment in biomass Increase in volume Increase in length or area Cell division, expansion and differentiation Fig. 35.18 Copyright 2002 Pearson Education, Inc., publishing as Benjamin
What is Growth? Increment in biomass Increase in volume Increase in length or area Cell division, expansion and differentiation Fig. 35.18 Copyright 2002 Pearson Education, Inc., publishing as Benjamin
Plant hormones. Characteristics
 Plant hormones Plant hormones (also known as phytohormones) are chemicals that regulate plant growth, which, in the UK, are termed 'plant growth substances'. Plant hormones are signal molecules produced
Plant hormones Plant hormones (also known as phytohormones) are chemicals that regulate plant growth, which, in the UK, are termed 'plant growth substances'. Plant hormones are signal molecules produced
Plant hormones: a. produced in many parts of the plant b. have many functions
 Plant hormones: a. produced in many parts of the plant b. have many functions Illustrated with 4 plant hormones: Gibberellins Auxin Cytokinins Ethylene Gibberellins Gibberellins illustrate how plant hormones
Plant hormones: a. produced in many parts of the plant b. have many functions Illustrated with 4 plant hormones: Gibberellins Auxin Cytokinins Ethylene Gibberellins Gibberellins illustrate how plant hormones
Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family
 Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family GENES & DEVELOPMENT (2000) 14: 108 117 INTRODUCTION Flower Diagram INTRODUCTION Abscission In plant, the process by which a plant
Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family GENES & DEVELOPMENT (2000) 14: 108 117 INTRODUCTION Flower Diagram INTRODUCTION Abscission In plant, the process by which a plant
Horticulture 201H Spring, 2002 Exam 2 Name:
 Horticulture 201H Spring, 2002 Exam 2 Name: Section 1. In the space to the left of the statements below, write the word(s) that best fit the definition or description. (20 pts) Vegetative reproduction
Horticulture 201H Spring, 2002 Exam 2 Name: Section 1. In the space to the left of the statements below, write the word(s) that best fit the definition or description. (20 pts) Vegetative reproduction
Plants are sessile. 10d-17/giraffe-grazing.jpg
 Plants are sessile www.mccullagh.org/db9/ 10d-17/giraffe-grazing.jpg Plants have distinct requirements because of their sessile nature Organism-level requirements Must adjust to environment at given location
Plants are sessile www.mccullagh.org/db9/ 10d-17/giraffe-grazing.jpg Plants have distinct requirements because of their sessile nature Organism-level requirements Must adjust to environment at given location
Electromagenetic spectrum
 Light Controls of Plant Development 1 Electromagenetic spectrum 2 Light It is vital for photosynthesis and is also necessary to direct plant growth and development. It acts as a signal to initiate and
Light Controls of Plant Development 1 Electromagenetic spectrum 2 Light It is vital for photosynthesis and is also necessary to direct plant growth and development. It acts as a signal to initiate and
A. Stimulus Response:
 Plant Hormones A. Stimulus Response: A house plant on a windowsill grows light. If you rotate the plant, it reorients its growth until its leaves face the window again. The growth of a shoot towards light
Plant Hormones A. Stimulus Response: A house plant on a windowsill grows light. If you rotate the plant, it reorients its growth until its leaves face the window again. The growth of a shoot towards light
The Plant Cell, November. 2017, American Society of Plant Biologists. All rights reserved
 The Genetics of Floral Development Teaching Guide Overview The development of flowers in angiosperm plants provided a critical evolutionary advantage, allowing more options for pollen dispersal and seed
The Genetics of Floral Development Teaching Guide Overview The development of flowers in angiosperm plants provided a critical evolutionary advantage, allowing more options for pollen dispersal and seed
Pea Has Its Tendrils in Branching Discoveries Spanning a Century from Auxin to Strigolactones 1
 Update on Pea Shoot Branching Pea Has Its Tendrils in Branching Discoveries Spanning a Century from Auxin to Strigolactones 1 Christine A. Beveridge*, Elizabeth A. Dun, and Catherine Rameau School of Biological
Update on Pea Shoot Branching Pea Has Its Tendrils in Branching Discoveries Spanning a Century from Auxin to Strigolactones 1 Christine A. Beveridge*, Elizabeth A. Dun, and Catherine Rameau School of Biological
Hormonal and genetic regulation of axillary bud outgrowth in Chrysanthemum morifolium during floral initiation
 Hormonal and genetic regulation of axillary bud outgrowth in Chrysanthemum morifolium during floral initiation Robrecht Dierck 1, Ellen De Keyser 1, Jan De Riek 1, Emmy Dhooghe 1, Johan Van Huylenbroeck
Hormonal and genetic regulation of axillary bud outgrowth in Chrysanthemum morifolium during floral initiation Robrecht Dierck 1, Ellen De Keyser 1, Jan De Riek 1, Emmy Dhooghe 1, Johan Van Huylenbroeck
Name Class Date. In the space provided, write the letter of the description that best matches the term or phrase.
 Assessment Chapter Test B Plant Responses In the space provided, write the letter of the description that best matches the term or phrase. 1. thigmonasty 2. auxin 3. ethylene 4. phytochrome 5. abscisic
Assessment Chapter Test B Plant Responses In the space provided, write the letter of the description that best matches the term or phrase. 1. thigmonasty 2. auxin 3. ethylene 4. phytochrome 5. abscisic
Botany. Mechanisms by which basal branches suppress axillary bud outgrowth in pea (Pisum sativum)
 Mechanisms by which basal branches suppress axillary bud outgrowth in pea (Pisum sativum) Journal: Botany Manuscript ID cjb-2017-0198.r1 Manuscript Type: Article Date Submitted by the Author: 02-Dec-2017
Mechanisms by which basal branches suppress axillary bud outgrowth in pea (Pisum sativum) Journal: Botany Manuscript ID cjb-2017-0198.r1 Manuscript Type: Article Date Submitted by the Author: 02-Dec-2017
TREES. Functions, structure, physiology
 TREES Functions, structure, physiology Trees in Agroecosystems - 1 Microclimate effects lower soil temperature alter soil moisture reduce temperature fluctuations Maintain or increase soil fertility biological
TREES Functions, structure, physiology Trees in Agroecosystems - 1 Microclimate effects lower soil temperature alter soil moisture reduce temperature fluctuations Maintain or increase soil fertility biological
APICAL DOMINANCE IN TUBERS OF POTATO (SOLANUM TUBEROSUM L. )
 MAURI ORA, 1976, 4: 53-59 53 APICAL DOMINANCE IN TUBERS OF POTATO (SOLANUM TUBEROSUM L. ) N. LALLU and J.A. McWHA Department of Botany, University of Canterbury, Christchurch, New Zealand. ABSTRACT Apical
MAURI ORA, 1976, 4: 53-59 53 APICAL DOMINANCE IN TUBERS OF POTATO (SOLANUM TUBEROSUM L. ) N. LALLU and J.A. McWHA Department of Botany, University of Canterbury, Christchurch, New Zealand. ABSTRACT Apical
Chapter 25 Plant Processes. Biology II
 Chapter 25 Plant Processes Biology II 25.1 Nutrients and Transport Plants grow by adding new cells through cell division Must have steady supply of raw materials to build new cells Nutrients (most) Plants
Chapter 25 Plant Processes Biology II 25.1 Nutrients and Transport Plants grow by adding new cells through cell division Must have steady supply of raw materials to build new cells Nutrients (most) Plants
Regulatory Systems in Plants (Ch 39)
 Regulatory Systems in Plants (Ch 39) Plants show complex responses to environmental stimuli Problem: no nervous system (detection) & no muscular system (response) Various mechanisms for detecting stimuli
Regulatory Systems in Plants (Ch 39) Plants show complex responses to environmental stimuli Problem: no nervous system (detection) & no muscular system (response) Various mechanisms for detecting stimuli
LECTURE 4: PHOTOTROPISM
 http://smtom.lecture.ub.ac.id/ Password: https://syukur16tom.wordpress.com/ LECTURE 4: PHOTOTROPISM LECTURE FLOW 1. 2. 3. 4. 5. INTRODUCTION DEFINITION INITIAL STUDY PHOTROPISM MECHANISM PHOTORECEPTORS
http://smtom.lecture.ub.ac.id/ Password: https://syukur16tom.wordpress.com/ LECTURE 4: PHOTOTROPISM LECTURE FLOW 1. 2. 3. 4. 5. INTRODUCTION DEFINITION INITIAL STUDY PHOTROPISM MECHANISM PHOTORECEPTORS
Lecture-6. The physiological basis of adventitious root formation in cutting and layering. Learning objective
 Lecture-6 The physiological basis of adventitious root formation in cutting and layering Learning objective Introduction To know about the physiological, anatomical and biochemical basis of root formation
Lecture-6 The physiological basis of adventitious root formation in cutting and layering Learning objective Introduction To know about the physiological, anatomical and biochemical basis of root formation
Reflexions, le site de vulgarisation de l'université de Liège
 When tomatoes flower 3/13/12 Understanding the mechanisms responsible for tomato plant flowering will enable new selection procedures to be developed in order to obtain even more productive varieties.
When tomatoes flower 3/13/12 Understanding the mechanisms responsible for tomato plant flowering will enable new selection procedures to be developed in order to obtain even more productive varieties.
PLANT GROWTH AND DEVELOPMENT
 84 BIOLOGY, EXEMPLAR PROBLEMS CHAPTER 15 PLANT GROWTH AND DEVELOPMENT MULTIPLE CHOICE QUESTIONS 1. Ethylene is used for a. Retarding ripening of tomatoes b. Hastening of ripening of fruits c. Slowing down
84 BIOLOGY, EXEMPLAR PROBLEMS CHAPTER 15 PLANT GROWTH AND DEVELOPMENT MULTIPLE CHOICE QUESTIONS 1. Ethylene is used for a. Retarding ripening of tomatoes b. Hastening of ripening of fruits c. Slowing down
Chapter 4. Biology of Flowering Plants. Regulation of Plant Growth by Plant Hormones
 BOT 3015L (Sherdan/Outlaw/Aghoram); Page 1 of 8 Chapter 4 Biology of Flowering Plants Regulation of Plant Growth by Plant Hormones Objectives Plant Growth Regulators. Know the names of the plant growth
BOT 3015L (Sherdan/Outlaw/Aghoram); Page 1 of 8 Chapter 4 Biology of Flowering Plants Regulation of Plant Growth by Plant Hormones Objectives Plant Growth Regulators. Know the names of the plant growth
Chapter 17. From Gene to Protein. Biology Kevin Dees
 Chapter 17 From Gene to Protein DNA The information molecule Sequences of bases is a code DNA organized in to chromosomes Chromosomes are organized into genes What do the genes actually say??? Reflecting
Chapter 17 From Gene to Protein DNA The information molecule Sequences of bases is a code DNA organized in to chromosomes Chromosomes are organized into genes What do the genes actually say??? Reflecting
Chapter 6. General discussion
 Chapter 6 General discussion 67 Chapter 6 General discussion Plants react in various ways on environmental stress conditions. The riverplain species Rumex palustris responds to submergence with an upward
Chapter 6 General discussion 67 Chapter 6 General discussion Plants react in various ways on environmental stress conditions. The riverplain species Rumex palustris responds to submergence with an upward
Hormonal and other chemical effects on plant growth and functioning. Bill Davies Lancaster Environment Centre, UK
 Hormonal and other chemical effects on plant growth and functioning Bill Davies Lancaster Environment Centre, UK Integrating the impacts of soil drought and atmospheric stress High radiant load Reduced
Hormonal and other chemical effects on plant growth and functioning Bill Davies Lancaster Environment Centre, UK Integrating the impacts of soil drought and atmospheric stress High radiant load Reduced
Plant Development. Chapter 31 Part 1
 Plant Development Chapter 31 Part 1 Impacts, Issues Foolish Seedlings, Gorgeous Grapes Gibberellin and other plant hormones control the growth and development of plants environmental cues influence hormone
Plant Development Chapter 31 Part 1 Impacts, Issues Foolish Seedlings, Gorgeous Grapes Gibberellin and other plant hormones control the growth and development of plants environmental cues influence hormone
Simon Scofield, Walter Dewitte, and James AH Murray* School of Biosciences; Cardiff University; Cardiff, UK
 Short Communication Plant Signaling & Behavior 9, e28934; April; 2014 Landes Bioscience Short Communication STM sustains stem cell function in the Arabidopsis shoot apical meristem and controls KNOX gene
Short Communication Plant Signaling & Behavior 9, e28934; April; 2014 Landes Bioscience Short Communication STM sustains stem cell function in the Arabidopsis shoot apical meristem and controls KNOX gene
Plant Growth Regulators(NCERT)
 Plant Growth Regulators(NCERT) Promoters: 1. Auxins: -first isolated from urine, contains Zinc. -Natural: Indole Acetic Acid (IAA) Indole Butyric Acid (IBA) -Synthetic: Naphthalene Acetic Acid (NAA) 2-4
Plant Growth Regulators(NCERT) Promoters: 1. Auxins: -first isolated from urine, contains Zinc. -Natural: Indole Acetic Acid (IAA) Indole Butyric Acid (IBA) -Synthetic: Naphthalene Acetic Acid (NAA) 2-4
Interaction between strigolactone and other plant hormones
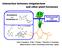 Interaction between strigolactone and other plant hormones Biosynthesis and Metabolism of Normal growth and development Kaori Yoneyama, X Xie, T Kisugi, T Nomura, K Yoneyama (Weed Science Center, Utsunomiya
Interaction between strigolactone and other plant hormones Biosynthesis and Metabolism of Normal growth and development Kaori Yoneyama, X Xie, T Kisugi, T Nomura, K Yoneyama (Weed Science Center, Utsunomiya
Plant Responses and Adaptations Video
 Plant Responses and Adaptations Video Hormone -a substance that is produced in one part of an organism & affects another part of the same individual Plant hormones are chemical substances Control a plant
Plant Responses and Adaptations Video Hormone -a substance that is produced in one part of an organism & affects another part of the same individual Plant hormones are chemical substances Control a plant
Chapter 33 Control Systems in Plants
 Chapter 33 Control Systems in Plants PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Copyright 2009 Pearson Education, Inc. Lecture by
Chapter 33 Control Systems in Plants PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Copyright 2009 Pearson Education, Inc. Lecture by
HRS1 Acts as a Negative Regulator of Abscisic Acid Signaling to Promote Timely Germination of Arabidopsis Seeds
 HRS1 Acts as a Negative Regulator of Abscisic Acid Signaling to Promote Timely Germination of Arabidopsis Seeds Chongming Wu 1,2., Juanjuan Feng 1,2., Ran Wang 1,2, Hong Liu 1,2, Huixia Yang 1,2, Pedro
HRS1 Acts as a Negative Regulator of Abscisic Acid Signaling to Promote Timely Germination of Arabidopsis Seeds Chongming Wu 1,2., Juanjuan Feng 1,2., Ran Wang 1,2, Hong Liu 1,2, Huixia Yang 1,2, Pedro
Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach
 Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach ABSTRACT SUBMITTED TO JAMIA MILLIA ISLAMIA NEW DELHI IN PARTIAL FULFILMENT OF
Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach ABSTRACT SUBMITTED TO JAMIA MILLIA ISLAMIA NEW DELHI IN PARTIAL FULFILMENT OF
Plant transformation
 Plant transformation Objectives: 1. What is plant transformation? 2. What is Agrobacterium? How and why does it transform plant cells? 3. How is Agrobacterium used as a tool in molecular genetics? References:
Plant transformation Objectives: 1. What is plant transformation? 2. What is Agrobacterium? How and why does it transform plant cells? 3. How is Agrobacterium used as a tool in molecular genetics? References:
Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering
 Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering by Valverde et. Al Published in Science 2004 Presented by Boyana Grigorova CBMG 688R Feb. 12, 2007 Circadian Rhythms: The Clock Within
Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering by Valverde et. Al Published in Science 2004 Presented by Boyana Grigorova CBMG 688R Feb. 12, 2007 Circadian Rhythms: The Clock Within
EFFECTS OF CROP LOAD ON VEGETATIVE GROWTH OF CITRUS
 EFFECTS OF CROP LOAD ON VEGETATIVE GROWTH OF CITRUS HOS 6545 ADVANCED CITRICULTURE I Regulation of Vegetative Growth L. GENE ALBRIGO Smith, P.F. 1976. Collapse of Murcott tangerine trees. J. Amer. Soc.
EFFECTS OF CROP LOAD ON VEGETATIVE GROWTH OF CITRUS HOS 6545 ADVANCED CITRICULTURE I Regulation of Vegetative Growth L. GENE ALBRIGO Smith, P.F. 1976. Collapse of Murcott tangerine trees. J. Amer. Soc.
Unit Two: Chemical Control
 Unit Two: Chemical Control 3.1 Plant growth and development are regulated by hormones Tropism is a biological phenomenon in which plants grow toward or away from an environmental stimulus, such as light,
Unit Two: Chemical Control 3.1 Plant growth and development are regulated by hormones Tropism is a biological phenomenon in which plants grow toward or away from an environmental stimulus, such as light,
FURTHER EXPERIMENTS ON THE INHIBITION OF THE DE-
 480 PHYSIOLOG Y: SKOOG A ND THIMA NN PROC. N. A. S. FURTHER EXPERIMENTS ON THE INHIBITION OF THE DE- VELOPMENT OF LATERAL BUDS BY GROWTH HORMONE By FOLKE SKOOG AND KENNETH V. THIMANN WILLIAM G. KERCKHOFF
480 PHYSIOLOG Y: SKOOG A ND THIMA NN PROC. N. A. S. FURTHER EXPERIMENTS ON THE INHIBITION OF THE DE- VELOPMENT OF LATERAL BUDS BY GROWTH HORMONE By FOLKE SKOOG AND KENNETH V. THIMANN WILLIAM G. KERCKHOFF
Chapter 39. Plant Response. AP Biology
 Chapter 39. Plant Response 1 Plant Reactions Stimuli & a Stationary Life u animals respond to stimuli by changing behavior move toward positive stimuli move away from negative stimuli u plants respond
Chapter 39. Plant Response 1 Plant Reactions Stimuli & a Stationary Life u animals respond to stimuli by changing behavior move toward positive stimuli move away from negative stimuli u plants respond
Trees are: woody complex, large, long-lived self-feeding shedding generating systems compartmented, self optimizing
 BASIC TREE BIOLOGY Trees are: woody complex, large, long-lived self-feeding shedding generating systems compartmented, self optimizing Roots: absorb water and minerals store energy support and anchor
BASIC TREE BIOLOGY Trees are: woody complex, large, long-lived self-feeding shedding generating systems compartmented, self optimizing Roots: absorb water and minerals store energy support and anchor
Plant Growth and Development
 1. Define plasticity. Give an example? A: Plant Growth and Development The ability of the plants to follow different pathways in response to the environment or phases of life to form different kinds of
1. Define plasticity. Give an example? A: Plant Growth and Development The ability of the plants to follow different pathways in response to the environment or phases of life to form different kinds of
THE ROLE OF THE PHYTOCHROME B PHOTORECEPTOR IN THE REGULATION OF PHOTOPERIODIC FLOWERING. AnitaHajdu. Thesis of the Ph.D.
 THE ROLE OF THE PHYTOCHROME B PHOTORECEPTOR IN THE REGULATION OF PHOTOPERIODIC FLOWERING AnitaHajdu Thesis of the Ph.D. dissertation Supervisor: Dr. LászlóKozma-Bognár - senior research associate Doctoral
THE ROLE OF THE PHYTOCHROME B PHOTORECEPTOR IN THE REGULATION OF PHOTOPERIODIC FLOWERING AnitaHajdu Thesis of the Ph.D. dissertation Supervisor: Dr. LászlóKozma-Bognár - senior research associate Doctoral
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
Topic 15. The Shoot System
 Topic 15. The Shoot System Introduction. This is the second of two lab topics that focus on the three plant organs (root, stem, leaf). In these labs we want you to recognize how tissues are organized in
Topic 15. The Shoot System Introduction. This is the second of two lab topics that focus on the three plant organs (root, stem, leaf). In these labs we want you to recognize how tissues are organized in
Class XI Chapter 15 Plant Growth and Development Biology
 Question 1: Define growth, differentiation, development, dedifferentiation, redifferentiation, determinate growth, meristem and growth rate. (a) Growth It is an irreversible and permanent process, accomplished
Question 1: Define growth, differentiation, development, dedifferentiation, redifferentiation, determinate growth, meristem and growth rate. (a) Growth It is an irreversible and permanent process, accomplished
Class XI Chapter 15 Plant Growth and Development Biology
 Question 1: Define growth, differentiation, development, dedifferentiation, redifferentiation, determinate growth, meristem and growth rate. (a) Growth It is an irreversible and permanent process, accomplished
Question 1: Define growth, differentiation, development, dedifferentiation, redifferentiation, determinate growth, meristem and growth rate. (a) Growth It is an irreversible and permanent process, accomplished
Interactions between Axillary Branches of Arabidopsis
 Molecular Plant Volume 1 Number 2 Pages 388 400 March 2008 RESEARCH ARTICLE Interactions between Axillary Branches of Arabidopsis Veronica Ongaro a, Katherine Bainbridge a,b, Lisa Williamson a and Ottoline
Molecular Plant Volume 1 Number 2 Pages 388 400 March 2008 RESEARCH ARTICLE Interactions between Axillary Branches of Arabidopsis Veronica Ongaro a, Katherine Bainbridge a,b, Lisa Williamson a and Ottoline
(17) CYCLANILIDE: MECHANISM OF ACTION AND USES AS A PLANT GROWTH REGULATOR IN COTTON
 (17) CYCLANILIDE: MECHANISM OF ACTION AND USES AS A PLANT GROWTH REGULATOR IN COTTON Jim Burton 1 and Marianne Pedersen Abstract. Cyclanilide [1-(2,4-dichlorophenylaminocarbonyl)-cyclopropane carboxylic
(17) CYCLANILIDE: MECHANISM OF ACTION AND USES AS A PLANT GROWTH REGULATOR IN COTTON Jim Burton 1 and Marianne Pedersen Abstract. Cyclanilide [1-(2,4-dichlorophenylaminocarbonyl)-cyclopropane carboxylic
Ch Plant Hormones
 Ch. 39 Plant Hormones I. Plant Hormones Chemical signals that coordinate the parts of an organism. Only minute amounts are needed to get the desired response. Control plant growth and development by affecting
Ch. 39 Plant Hormones I. Plant Hormones Chemical signals that coordinate the parts of an organism. Only minute amounts are needed to get the desired response. Control plant growth and development by affecting
Is that artificial turf or real grass? Its thicker than Bermuda!
 Is that artificial turf or real grass? Its thicker than Bermuda! 1 Using Plant Growth Regulators Growth regulators DO NOT interfere with plant respiration, photosynthesis, or other internal plant functions
Is that artificial turf or real grass? Its thicker than Bermuda! 1 Using Plant Growth Regulators Growth regulators DO NOT interfere with plant respiration, photosynthesis, or other internal plant functions
(A) Ethylene (B) Absisic acid (C) Auxin (D) Gibberellin (E) Cytokinin
 College Biology - Problem Drill 17: Plant Function Question No. 1 of 10 1. Which of the following plant hormones is responsible for phototropism? Question #01 (A) Ethylene (B) Absisic acid (C) Auxin (D)
College Biology - Problem Drill 17: Plant Function Question No. 1 of 10 1. Which of the following plant hormones is responsible for phototropism? Question #01 (A) Ethylene (B) Absisic acid (C) Auxin (D)
Phytochrome Regulation of Branching in Arabidopsis 1[W][OA]
![Phytochrome Regulation of Branching in Arabidopsis 1[W][OA] Phytochrome Regulation of Branching in Arabidopsis 1[W][OA]](/thumbs/76/73891908.jpg) Phytochrome Regulation of Branching in Arabidopsis 1[W][OA] Scott A. Finlayson*, Srirama R. Krishnareddy, Tesfamichael H. Kebrom, and Jorge J. Casal Department of Soil and Crop Sciences, Texas A&M University,
Phytochrome Regulation of Branching in Arabidopsis 1[W][OA] Scott A. Finlayson*, Srirama R. Krishnareddy, Tesfamichael H. Kebrom, and Jorge J. Casal Department of Soil and Crop Sciences, Texas A&M University,
Chapter 39. Plant Reactions. Plant Hormones 2/25/2013. Plants Response. What mechanisms causes this response? Signal Transduction Pathway model
 Chapter 39 Plants Response Plant Reactions Stimuli & a Stationary life Animals respond to stimuli by changing behavior Move toward positive stimuli Move away from negative stimuli Plants respond to stimuli
Chapter 39 Plants Response Plant Reactions Stimuli & a Stationary life Animals respond to stimuli by changing behavior Move toward positive stimuli Move away from negative stimuli Plants respond to stimuli
Last time: Obtaining information from a cloned gene
 Last time: Obtaining information from a cloned gene Objectives: 1. What is the biochemical role of the gene? 2. Where and when is the gene expressed (transcribed)? 3. Where and when is the protein made?
Last time: Obtaining information from a cloned gene Objectives: 1. What is the biochemical role of the gene? 2. Where and when is the gene expressed (transcribed)? 3. Where and when is the protein made?
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles
 Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
The possible roles of nutrient deprivation and auxin repression in apical control
 Trees (2009) 23:489-500 DOI 10.1007/s00468-008-0294-8 The possible roles of nutrient deprivation and auxin repression in apical control Morris G. Cline - Neela Bhave - Constance A. Harrington Received:
Trees (2009) 23:489-500 DOI 10.1007/s00468-008-0294-8 The possible roles of nutrient deprivation and auxin repression in apical control Morris G. Cline - Neela Bhave - Constance A. Harrington Received:
Ph.D. thesis. Study of proline accumulation and transcriptional regulation of genes involved in this process in Arabidopsis thaliana
 Ph.D. thesis Study of proline accumulation and transcriptional regulation of genes involved in this process in Arabidopsis thaliana Written by: Edit Ábrahám Temesváriné Supervisors: Dr. László Szabados
Ph.D. thesis Study of proline accumulation and transcriptional regulation of genes involved in this process in Arabidopsis thaliana Written by: Edit Ábrahám Temesváriné Supervisors: Dr. László Szabados
WORKSHEET-8 BIOLOGY (PLANT GROWTH &
 DATE : / / 2018. TOTAL MARKS: 304 M DURATION: 6 HR General Instruction: - All questions are compulsory. The question paper consists of 88 questions divided into five sections. Section -I comprises of 60
DATE : / / 2018. TOTAL MARKS: 304 M DURATION: 6 HR General Instruction: - All questions are compulsory. The question paper consists of 88 questions divided into five sections. Section -I comprises of 60
Regulation of Phosphate Homeostasis by microrna in Plants
 Regulation of Phosphate Homeostasis by microrna in Plants Tzyy-Jen Chiou 1 *, Kyaw Aung 1,2, Shu-I Lin 1,3, Chia-Chune Wu 1, Su-Fen Chiang 1, and Chun-Lin Su 1 Abstract Upon phosphate (Pi) starvation,
Regulation of Phosphate Homeostasis by microrna in Plants Tzyy-Jen Chiou 1 *, Kyaw Aung 1,2, Shu-I Lin 1,3, Chia-Chune Wu 1, Su-Fen Chiang 1, and Chun-Lin Su 1 Abstract Upon phosphate (Pi) starvation,
