Role of the Cytoplasmic Domain in Anabaena Sensory Rhodopsin Photocycling: Vectoriality of Schiff Base Deprotonation
|
|
|
- Gilbert Phillips
- 5 years ago
- Views:
Transcription
1 Biophysical Journal Volume 91 December Role of the Cytoplasmic Domain in Anabaena Sensory Rhodopsin Photocycling: Vectoriality of Schiff Base Deprotonation Oleg A. Sineshchekov,* y Elena N. Spudich,* Vishwa D. Trivedi,* and John L. Spudich* *Center for Membrane Biology, Department of Biochemistry and Molecular Biology, University of Texas Medical School, Houston, Texas; and y Biology Department, Moscow State University, Vorobievy Gory, Moscow, Russia ABSTRACT Light-induced electric signals in intact Escherichia coli cells generated by heterologously expressed full-length and C-terminally truncated versions of Anabaena sensory rhodopsin (ASR) demonstrate that the charge movements within the membrane-embedded part of the molecule are stringently controlled by the cytoplasmic domain. In particular, truncation inverts the direction of proton movement during Schiff base deprotonation from outward to cytoplasmic. Truncation also alters faster charge movements that occur before Schiff base deprotonation. Asp 217 as previously shown by FTIR serves as a proton acceptor in the truncated ASR but not in the full-length version, and its mutation to Asn restores the natural outward direction of proton movement. Introduction of a potential negative charge (Ser 86 to Asp) on the cytoplasmic side favors a cytoplasmic direction of proton release from the Schiff base. In contrast, mutation of the counterion Asp 75 to Glu reverses the photocurrent to the outward direction in the truncated pigment, and in both truncated and full-length versions accelerates Schiff base deprotonation more than 10-fold. The communication between the cytoplasmic domain and the membrane-embedded photoactive site of ASR demonstrated here is likely to derive from the receptor s use of a cytoplasmic protein for signal transduction, as has been suggested previously from binding studies. INTRODUCTION Rhodopsins constitute a large diverse group of membraneembedded retinylidene proteins with seven transmembrane segments found throughout organisms ranging from bacteria to humans (1 4). Based on their primary structures, spatial arrangements of their helices, and configurations of their retinal chromophores, rhodopsins fall into two classes. Type 1 rhodopsins function as ion pumps to capture light energy (5,6) or as light receptors in sensory transduction pathways in prokaryotic and eukaryotic microorganisms (7). Type 2 rhodopsins are light sensors in visual systems and function as G-protein coupled receptors (8). In both types, seven transmembrane a-helices form a pocket for the retinal chromophore buried in the membrane-embedded domain of the protein. The retinal is attached in a protonated Schiff base linkage to a lysine residue in the seventh helix. In proton-pumping rhodopsins, microbial sensory rhodopsins, and visual pigments, photoisomerization of retinal causes transfer of the Schiff base proton to proton acceptors on the protein. The deprotonation of the retinylidene Schiff base causes a large blueshift in the pigment, thereby generating a spectrally distinct intermediate called the M intermediate in type 1 rhodopsins and Meta-II in visual pigments. The M and Meta-II intermediates are signaling states of sensory rhodopsins, and M is also a critical intermediate in the proton-pumping rhodopsins. Type 1 microbial rhodopsins so far characterized differ from type 2 visual pigments in the importance of the C-terminal extension of the proteins, which protrudes from the cytoplasmic end of the seventh helix into the cytoplasm. In the well-characterized mammalian rod visual pigment, the C-terminal extension is an active participant in the signal transduction process, and its modification alters the photochemical properties and biochemical functions of the protein (8,9). In contrast, early studies of the first discovered type 1 proteins, the haloarchaeal proton pump bacteriorhodopsin and phototaxis receptors sensory rhodopsins I and II (SRI and SRII), established that their flexible cytoplasmic C-terminal domains could be truncated or modified without loss of function (10 12). Removal of this flexible tail has been advantageous. Truncation of the C-terminus greatly increased expression levels of SRI (13). Furthermore, truncation of the dispensable C-terminal extensions favors crystallization by eliminating a flexible domain. A large number of new type 1 rhodopsins have been identified by microbial genomics during the last few years (14), which are predicted to be in some cases light-driven ion transporters and in others, photosensors. A striking diversity in photochemistry and mechanisms of signal transduction has been observed among the photosensors (7), for which the sensory function has been proven (15 17) or predicted (18,19). Anabaena sensory rhodopsin (ASR) is one of the new type 1 rhodopsins. Its gene was identified in the genome of the freshwater cyanobacterium Anabaena (Nostoc) sp. PCC7120 (18). A 14-kDa soluble protein is encoded under the same promoter with the opsin and is believed to be a cytoplasmic messenger in the sensory transduction chain initiated by photoexcitation of ASR (7). ASR signaling to a soluble cytoplasmic transducer is more analogous to visual pigment function than to that of haloarchaeal SRI and SRII, which relay signals to integral membrane transducers. Submitted July 19, 2006, and accepted for publication September 8, Address reprint requests to John L. Spudich, Center for Membrane Biology, University of Texas, Medical School, Houston, TX Tel.: ; Fax: ; john.l.spudich@uth.tmc.edu. Ó 2006 by the Biophysical Society /06/12/4519/09 $2.00 doi: /biophysj
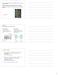
2 4520 Sineshchekov et al. Here we report that the C-terminal extension plays an important role in the photochemical processes of the ASR protein, as in visual pigments. Truncation of this part of ASR causes dramatic changes in charge movements. In particular, the vectoriality of proton transfer from the Schiff base during M formation is inverted from outwardly directed to toward the cytoplasm. Also charge movements occurring during L-intermediate formation (which precedes Schiff base deprotonation) are altered by truncation. These effects are difficult to detect with conventional UV-Vis kinetic absorption spectroscopy, which is insensitive to charge vectoriality. METHODS Plasmid construction Full-length Anabaena opsin (ASR ) was cloned into pet15b modified using NdeI and NotI restriction sites. Polymerase chain reaction (PCR) was used to obtain a truncated sequence encoding 229 residues (ASR ). PCR was used to introduce a His 6 tag at the carboxy terminus of the two opsin proteins for protein purification. The constructs were expressed under the plac1 promoter of pms107 in UT5600 Escherichia coli cells. The opsin fragment from pet vector (XbaI/NotI insert) was ligated into the expression vector pms107 (18). Site-directed mutagenesis The mutations D75E, S86D, and D217N were introduced either by QuickChange site-directed mutagenesis (Stratagene, La Jolla, CA) or by the two-step PCR method (20). All mutagenesis was conducted on full-length and truncated Anabaena opsin genes with and without His 6 tags and confirmed by DNA sequencing. Protein expression and purification All constructs were transformed into E.coli UT5600 for expression. The cells were grown at 37 C toa 600 ¼ and induced with 1 mm IPTG added with 5 mm all-trans retinal. Cells were harvested by centrifugation at 4000 g. For membrane preparation, cells were resuspended in 50 mm Tris, 300 mm NaCl, ph 7.6 buffer (buffer A) containing 10% glycerol. Resuspended cells were lysed using a microfluidizer, and unlysed cells were removed by centrifugation at g. The membranes were prepared by centrifugation of lysed cells at 150,0003 g for 1 h. For purification, the protein was extracted using 1% dodecyl maltoside (DDM) in buffer A overnight, followed by centrifugation at 20,0003 g to remove unextracted material. The protein was loaded on a preequilibrated Ni-NTA agarose column, washed with 20 mm imidazole in buffer B (buffer A with 0.05% DDM), and finally eluted with 250 mm imidazole in buffer B. Eluted protein was concentrated and dialyzed to remove imidazole. All steps were performed at 4 C. Photoinduced current measurements E. coli cells, cultured as described above, were washed with distilled water and resuspended in measuring buffer containing 10 mm Tris-HCl, 2 mm NaCl, 0.1 mm MgCl 2, and 0.1 mm CaCl 2 (ph ; 7.7). Charge movements within ASR molecules were detected by measurements of laser flash-induced currents in suspensions of E. coli cells expressing the pigment or its mutated versions. This approach is based on a light-focusing effect through the virtually transparent cell body (see Sineshchekov and Spudich (21) for details). Briefly, suspensions of cells were flashed along the line between platinum electrodes by Nd:YAG Minilite laser (532 nm, 6-ns pulse; Continuum, Santa Clara, CA). Essentially equal quanta of light should be absorbed on both halves of the cell membrane because of the low absorption of light within the cell. Nevertheless, the projection of elementary currents through the uniformly excited molecules on the illuminated side of the cell on the line between the measuring electrodes is smaller than the projection on the opposite side, where light beams are concentrated at the center. The difference in the oppositely directed currents caused by the excitation light was detected by a low-noise current amplifier 428 (Keithley Instruments, Cleveland, OH) with 5-ms rise time. Signals were digitized by a DIGIDATA 1320A at 2 or 4 ms/ point and stored in a PC using the Clampex 9.0 program (both from Axon Instruments, Foster City, CA), and transients obtained with a 10-s flash interval were averaged. Small flash artifacts were recorded using a suspension of E. coli cells not expressing pigment and were subtracted from the current signals. To measure voltage signals reflecting the overall charge movement, current traces were time-integrated using the same program. Data were reduced with logarithmically increasing numbers of averaging points along the time axis and analyzed by the Origin 7.0 program (OriginLab, Northampton, MA). Absorption and flash photolysis Flash-induced absorption changes were acquired in parallel with a laboratory-constructed cross-beam flash-photolysis system under conditions identical to those of the current measurements except for a higher intensity of the Nd-YAG pulse laser (Continuum, Surelite I, Santa Clara, CA) as described (22). Absorption spectra were measured with a Cary 4000 UV-Vis Spectrophotometer (Varian, Palo Alto, CA) using an integrating sphere. RESULTS C-terminal truncation has only minor effects on ASR absorption and photochemistry. The full-length (ASR ) and C-terminally truncated (ASR ) versions of ASR used in this study are schematically shown in Fig. 1. The expression level of ASR in E. coli cells was ;40% of that of ASR A similar reduced level of expression of full-length versus truncated versions of all tested mutants was observed (see below). The full-length ASR revealed the same unusual light-dark adaptation as was reported earlier in the truncated pigment (22). The light-adapted form of ASR has mostly 13-cis/15- syn retinal, whereas dark adaptation leads to its thermal conversion to the all-trans/15-anti form (22,23). The two isomeric forms of ASR have different absorption spectra and undergo mutual light-induced photoconversion. Thus, their ratio depends on the wavelength of illumination, providing a potential mechanism for single-pigment color sensing (19,22). Nearly identical dark-adapted minus light-adapted difference absorption spectra of freshly purified ASR and ASR (Fig. 2 A) showed that the truncation does not affect absorption properties of either the all-trans or the 13- cis form of pigment, but the rate of dark adaptation was ;1.5-fold slower in the full-length compared to the truncated pigment (Fig. 2 B). The photocycle rate of the purified fulllength ASR was ;20% faster than that of the truncated pigment (Fig. 2 C). As we reported earlier, the photocycle of truncated ASR significantly slows down in intact E. coli cells, as compared to that of purified pigment in detergent
3 ASR C-Domain/Photoactive Site Coupling 4521 FIGURE 1 Representation of the full-length ASR. Transmembrane helices (white), retinal (yellow), and key residues and water molecules (cyan) are shown according to the crystal structure of the truncated protein (19). The structure of the cytoplasmic 35 residues beyond residue 226 is unknown and shown arbitrary as an a-helical extension. (22). The full-length ASR showed a much stronger deceleration of the photocycle in intact cells. Consequently, in intact E. coli cells, the peak and decay times of the flash-induced absorption changes were about twofold longer in the fulllength ASR than in the truncated pigment (Fig. 2 D). From the above UV-Vis spectroscopy and flash photolysis results, we conclude that the full-length and C-terminally truncated versions of ASR show only quantitative differences in their optical characteristics, which were more pronounced in intact cells than in detergent. C-terminal truncation has major effects on charge movements in the ASR photocycle. In contrast, we observed a dramatic qualitative effect of the C-terminal truncation on the photoinduced electrical charge movement in E. coli cells. Experimental current signals are better suited for analysis of fast electrical events (Fig. 3 A), whereas computed photovoltaic curves (time integrals of the currents, Fig. 3 B) reflect the overall charge movement within the rhodopsin molecule and are more informative than current signals over an extended timescale (24,25). The charge movement in the truncated ASR comprises several kinetic components (Fig. 3 B). The first component (t of several microseconds) reflects charge movement related to the formation of the K intermediate, unresolved in our measurements (26). We used the amplitude of this component as a point for normalization of current and charge signals to compensate for differences in the expression levels of the full-length and the truncated ASR. A component with t ; ms correlates with the time constant of L intermediate formation in purified ASR (22). A slow (t of several milliseconds) component corresponds to the time window of M intermediate formation, which includes Schiff base deprotonation. In many cases, it can be decomposed into a minor submillisecond and a major several-millisecond phase, which fits well to the biphasic formation of the M intermediate reported earlier (22). As we have shown in a previous study (21), in the truncated version of ASR, all phases of the charge movement up to the peak time of the M formation are directed toward the cytoplasmic side of the membrane (Fig. 3 B). The charge movement detected in the native full-length pigment reveals similar kinetic components as found in the truncated ASR. However, only the fastest unresolved component, related to the formation of the K intermediate, has a negative (inwardly directed) sign in the full-length pigment (Fig. 3 B). The later components have the outward direction, in contrast to the respective signal components in the truncated ASR. The effect of truncation is most evident in the difference curve between charge movement in full-length and truncated versions of the pigment (Fig. 3 C, solid line). The difference signal comprises two main components with peaks ;100 ms and.10 ms time ranges. The close kinetic match of the millisecond charge difference signal with the kinetics of M formation and decay (monitored by absorbance changes at 400 nm) shows that truncation inverts the direction of proton movement associated with the Schiff base deprotonation. A fast component of the difference signal peaking at ;100 ms precedes Schiff base deprotonation and fits well to the kinetics of L intermediate formation (monitored by absorbance changes at 460 nm on Fig. 3 C). Charge motion associated with rearrangements of several residues, retinal, and bound water contributes to photocurrent during L formation in bacteriorhodopsin (BR) (27,28). The dependence of the fast charge movement in ASR on the presence of the fulllength C-terminus shows that the structures of either the unphotolyzed state, the L-intermediate, or both are different in full-length and truncated pigments. Although the kinetics of the millisecond charge movement correlates well with M formation in both the full-length and the truncated ASR, additional slower components are superimposed on the Schiff base deprotonation signals in the later part of the photocycle (Fig. 4). Their chemical origin is not clear from the available data. The inversion of charge movement vectoriality by truncation is not influenced by C-terminal His 6 tags. It has been reported that His 6 tags modify the photochemistry of visual rhodopsins (9). To test the possibility that this sequence,
4 4522 Sineshchekov et al. FIGURE 2 Comparison of spectroscopic and photochemical properties of full-length and truncated ASR. (A) Difference absorption spectra of darkadapted minus light-adapted purified pigments in detergent. (B) Kinetics of dark adaptation in purified pigments. Absorption changes were normalized to the absorption of dark-adapted samples. (C) Laser flash induced absorption changes of purified pigments at different wavelengths, monitoring the unphotolyzed state (550 nm) and M intermediate (400 nm). (D) Formation and decay of M intermediate pigments in intact E. coli cells. located in the truncated version close to the opening of the cytoplasmic channel, contributes to the inversion of charge movements, we expressed His 6 -tag-free full-length and truncated ASR. The inverting effect of truncation on charge movements was observed both in the presence and absence of the His 6 tags (cf. Fig. 3 and Fig. 4). Thus, the inversion of the direction of charge and proton movements is caused specifically by the truncation of the C terminus and is independent of the His 6 tag. Mutation of Asp 217 to Asn inverts the charge movement in truncated ASR A recent time-resolved FTIR study of the truncated ASR showed that Asp 217, ;15 Å to the cytoplasmic side of the membrane from the Schiff base, acts as a proton acceptor (29). These data are in accordance with the cytoplasmic direction of charge movement in the truncated ASR reported earlier (21). Here we used site-directed mutagenesis to probe for the role of this residue in the photoinduced charge movement within ASR. In the truncated ASR, neutralization of this residue by the D217N mutation inverted the direction of charge movement from negative (inwardly directed) to positive, so that the mutated truncated ASR behaved similarly to the full-length wild-type pigment (Fig. 5 A). At least three kinetic components of difference signals between the truncated and full-length versions of the wild-type ASR and between the truncated wild-type pigments and truncated D217N mutant pigment are very similar, although the millisecond component is more pronounced in the case of the D217N mutation (Fig. 5 B). In the full-length version of the D217N mutant pigment, the outwardly directed charge movement is further increased in amplitude compared to the truncated version of the mutant. However, the effect of the truncation in the D217N mutant is relatively larger on the fast (t, 100 ms) component, which corresponds to the L intermediate formation in the purified protein and precedes deprotonation of the Schiff base. The absence of significant effects of the D217N mutation on the charge movement on the millisecond time scale in the full-length ASR, as opposed to dramatic changes induced by this mutation in the truncated version, indicates that Asp 217 acts as a proton acceptor only in the truncated pigment. This result is in accord with the observation from an FTIR study that no aspartate protonation signal occurs in the full-length protein, ASR (23), unlike the case of ASR (29). Flash-induced absorption changes of D217N mutants also differed between the full-length and truncated versions of ASR. As noted above, in intact E. coli cells a slower decay of the M intermediate was detected in the wild-type full-length version of ASR than in the truncated version (Fig. 2 D). The D217N mutation increased the lifetime of the M intermediate of the truncated pigment nearly threefold in intact E. coli cells, in accordance with a similar effect in purified truncated pigment (29). In contrast to the wild type, the full-length version of this mutant showed a significantly faster decay of the M intermediate than the truncated version (Fig. 5 C). As a result, the lifetimes of the M intermediates were very similar (;200 ms) in the full-length versions of the wild type and the D217N mutant, which further confirms that Asp 217 does not play an important role in photochemistry of the full-length (native) ASR.
5 ASR C-Domain/Photoactive Site Coupling 4523 FIGURE 4 Charge movement (solid lines) and M intermediate kinetics (dashed lines) in full-length (A) and truncated (B) ASR without C-terminal His 6 tags. FIGURE 3 (A) Laser flash induced electric currents generated by fulllength and truncated ASR in suspension of intact E. coli cells (for details of the method, see Sineshchekov and Spudich (21)). (B) Time integrals of current signals reflecting overall charge movement in full-length and truncated ASR. Outwardly directed positive charge movement corresponds to an upward slope of the curves. (C) Correlation of difference charge signal between full-length and truncated versions of ASR (solid line) with absorption changes (dashed lines) at wavelength characteristic for L- and M-like intermediates (460 nm and 400 nm, respectively). Because of the high light scattering of cell suspensions, L formation was measured in purified pigment. Mutation of Asp 75 to Glu reverses the inverted vectoriality of charge movement in truncated ASR Asp 75 in the primary sequence of ASR corresponds to the Schiff base counterion and proton acceptor Asp 85 in BR. The position of Asp 75 on the extracellular side of the membrane with respect to the Schiff base has been confirmed by x-ray crystallography, and its proximity argues that it serves as the Schiff base counterion as in BR and SRII (19). The positive sign of the voltage signal in full-length ASR indicates that the proton moves in the direction of this residue, as occurs in protonpumping rhodopsins and SRII (21,27). In ASR the rate of this charge movement, as well as the rate of M intermediate formation, is very slow compared to that of other microbial rhodopsins. This indicates that Asp 75 does not serve as a strong proton acceptor even in the full-length native ASR in which the charge moves outward. The mutation of Asp 75 to Glu in the truncated ASR leads to the appearance of a fast outwardly directed current of large amplitude, typical for proton transfers to the counterion carboxylate in other rhodopsins (Fig. 6 A). The full-length version of this D75E mutant also demonstrates a similar acceleration of the outward photocurrent and charge movement (Fig. 6, A and B). In both full-length and truncated pigments, the D75E mutation
6 4524 Sineshchekov et al. FIGURE 5 (A) Charge movement in full-length (solid line) and truncated (dashed line) D217N mutant. Charge movement in truncated wild-type ASR is given for comparison (dotted line). (B) Normalized difference signals reflecting the effect of D217N mutation of truncated ASR (truncated D217N minus truncated ASR, black line), and the effect of truncation in wild-type ASR (full-length ASR minus truncated ASR, dashed line). (C) Effect of truncation on the M intermediate kinetics in the D217N mutant. FIGURE 6 Effect of mutation of Asp 75 in ASR (a homolog of the proton acceptor Asp 85 in BR) to glutamate. Photocurrents (A) and charge movement (B) in the full-length (solid lines) and the truncated (dashed lines) D75E mutant (signals in wild-type ASR are given for comparison by dotted and dashed-dotted lines). (C) M intermediate kinetics in truncated wild-type ASR and truncated D75E mutants in intact E. coli cells. brings about a corresponding acceleration of M intermediate formation (Fig. 6 C). Mutation of Ser 86 to Asp inverts the direction of late charge movement in full-length and truncated D75E mutants. The lack of proton-pumping activity in ASR may be caused by the lack of a strong Schiff base proton acceptor and/or the absence of a Schiff base proton donor because a nonprotonatable residue, Ser 86,inASR is at the position in helix C homologous to that of the Schiff base proton donor,
7 ASR C-Domain/Photoactive Site Coupling 4525 Asp 96, in BR. To test whether the Schiff base would be reprotonated from this position when the strong proton acceptor Glu 75 is present in the BR Asp 85 position, we constructed full-length and truncated versions of the double mutant D75E/S86D. Not only does the double mutant not show an increase in outwardly directed charge transfer but, unexpectedly, the S86D mutation inverted the late phases of charge movement to the cytoplasmic direction (Fig. 7 A). When this mutation was introduced into full-length versions of wildtype ASR, the outwardly directed proton movement was greatly suppressed, or even inverted to the cytoplasmic direction. Only the millisecond component of charge movement is affected by this mutation (Fig. 7 B). These results indicate that the introduction of potential negative charge in the ASR cytoplasmic channel is sufficient to influence the efficiency and even the direction of proton movement. FIGURE 7 Effect of substituting a protonatable residue at the ASR Ser 86 position (homologous to the Schiff base proton donor in BR, Asp 96 ). (A) Inversion of the late phases of proton movement in the full-length (solid line) and truncated (dashed line) double mutant D75E/S86D. Charge movements in single D75E mutants are given for comparison (dashed-dotted and dotted lines for full-length and truncated, respectively). (B) Comparison of the effect of truncation in the wild-type ASR (dashed line) with the effect of S86D mutation in the full-length ASR (solid line). DISCUSSION The above results show that the direction of charge movement in the photochemical reaction cycle of ASR leading to formation of the L and M intermediates is regulated by the hydrophilic C-terminal portion of the protein. In particular, the proton transfer from the retinylidene Schiff base located in the membrane-embedded interior of the protein is inverted from outwardly directed to inwardly directed when the cytoplasmic C-terminal portion is truncated. Apparently the proton transfer direction from the Schiff base is variable in the ASR protein and able to be changed by distant modifications. Confirming this, site-specific mutations distant from the photoactive site residues on the cytoplasmic side of the protein that introduce (S96D) or remove (D217N) a potential negative charge favor or disfavor, respectively, a cytoplasmic direction of proton release from the Schiff base. Our interpretation is that the photoactive site and the cytoplasmic domain of the protein are in communication, likely through a network of hydrogen bonds connecting the cytoplasmic residues and the retinylidene Schiff base, which may be modified by minor and/or distant changes in the molecule. A crystal structure of ASR (truncated) shows numerous hydrophilic residues on the cytoplasmic side networked by water molecules, providing such a connection from the photoactive site to the cytoplasmic surface (19). The existence of such strong cytoplasmic/photoactive site coupling in ASR is not evident in haloarchaeal phototaxis receptors SRI and SRII. Supporting our interpretation, the cytoplasmic half-channel in SRII is highly hydrophobic according to its crystal structure (30). The difference between ASR and haloarchaeal sensory rhodopsins may be correlated with their different signal relay mechanisms. SRI and SRII signal through integral membrane transducers (31) and have a membrane-embedded receptor-transducer interface (32), whereas ASR most probably initiates a signal transduction pathway by interaction with the 14-kDa cytoplasmic transducer. The transducer is a water-soluble protein that presumably interacts with cytoplasmic extensions of ASR, which are comprised of its cytoplasmic loops and the C-terminal tail. In support of a contribution of the C-terminal tail, microcalorimetric measurements show an about fourfold greater affinity of the 14- kda protein for the full-length version of ASR than for the truncated form (V. D. Trivedi and J. L. Spudich, unpublished data). C-terminal truncation affects at least two kinetically separated processes in ;100 ms and several-millisecond timescales. Inversion of the charge movement in the millisecond timescale and the matching kinetics of the slower difference charge signal with the kinetics of the M intermediate show that the direction of proton movement following the Schiff base deprotonation is opposite in truncated and full-length pigments (Figs. 3 and 4). The inwardly directed movement
8 4526 Sineshchekov et al. in the truncated ASR is reversed by the D217N mutation (Fig. 5 A), consistent with it serving as a proton acceptor in the truncated pigment (29). In the full-length ASR, the direction of proton movement is toward the extracellular side of the membrane (Figs. 3 B and 4 B), which means that Asp 217, located to the cytoplasmic side of the membrane with respect to the Schiff base, does not serve as a proton acceptor in the native pigment, in accord with the lack of a carboxylate protonation signal in FTIR spectra (23). This is confirmed by the very small effect of D217N mutation on the charge movement and photocycle rate of the full-length pigment. Consequently, one does not expect a signaling role of the cytoplasmic proton shuttling identified recently in the truncated protein (29), at least during M intermediate formation, because such shuttling does not take place in the native fulllength ASR at this stage. However, the accessibility of the released Schiff base proton to a cytoplasmic channel may appear in the later part of the cycle, similar to a switch of the Schiff base accessibility from the extracellular to a cytoplasmic channel, which occurs in late M in BR (5). The proton transfer ;15 Å from the Schiff base to Asp 217 in truncated ASR correlates with a high-amplitude inwardly directed charge movement in this time window. We attribute this long-distance movement to the extensive hydrogenbonded network of water molecules in the hydrophilic cytoplasmic channel evident in the crystal structure of truncated ASR (19). According to our data, Asp 217 becomes inaccessible to the Schiff base proton in the full-length pigment. This may be because of the disruption of the hydrogenbonded network and, hence, a different structure of the cytoplasmic channel in the native pigment. Truncation not only makes Asp 217 an artificial proton acceptor but also modifies the vicinity of the retinal involved in L intermediate formation in the hundred-microsecond time range. Although the direction of proton movement in the D217N mutant does not depend on truncation, differences affecting the microsecond component are still observed between full-length and truncated versions (Fig. 5). Hydrogen-bonded water molecules are involved in structural rearrangements and charge movement during L formation in BR (27,28). Therefore, our finding may be relevant to analysis of FTIR data on the state of hydrogen-bonded water molecules (33,34), since there is expected to be a structural difference between full-length and truncated ASR. The M formation and associated charge movement are slow in the full-length as well as in truncated versions of ASR compared to known proton-pumping rhodopsins. Therefore, Asp 75 does not serve as a strong proton acceptor even in the full-length ASR, although we cannot fully exclude its participation in Schiff base deprotonation. This is consistent with the lack of an Asp 75 protonation signal during M formation from FTIR of photoactivated ASR (23). The Schiff base proton may instead be accepted by networked water molecules at the extracellular side of the Schiff base. Creation of an active primary carboxylate acceptor in ASR is achieved by the mutation D75E, as indicated by the appearance of fast outward charge movement of large amplitude, characteristic of proton transfer to the nearby Schiff base counterion in other microbial rhodopsins, in both the full-length and the truncated versions of the pigment (Fig. 6). Similar acceleration of M formation caused by Asp-to-Glu mutation of the counterion has been reported earlier for BR (35). Two possibilities for ASR signaling were suggested earlier (22). The first is that the ratio of the unphotolyzed forms with trans- and cis-retinals, which not only changes greatly on light and dark adaptation but also depends on light quality, may modulate a photobiological function. Alternatively, the long-lived M intermediate may serve as a signaling form. The strong coupling between the C-terminal region and processes involved in M formation reported here favors the second possibility because it suggests that M formation in turn can cause conformational changes in the cytoplasmic C-terminal extension and modulate its interaction with the water-soluble transducer. The importance of extramembranous regions of rhodopsin molecules to their photochemistry and function may also be expected in other newly found microbial rhodopsins. Recently, we reported strong deceleration of the photocycle by truncation of the extracellular N-terminal of rhodopsin from the cryptophyte alga Guillardia (36). In Chlamydomonas sensory rhodopsins (CSRA and CSRB), the length of the C-terminal parts of the molecules exceeds that of the seven transmembrane helices (17,37 39). The functional roles of the C-terminal domains have not been elucidated. It has been reported that they are not necessary for the channel activity of these pigments when heterologously expressed (37,38). However, the signal transduction pathway for Chlamydomonas phototaxis involves a biochemical amplification cascade (40,41), and the C-terminal rhodopsin domains may relay the signal to this cascade. Alternatively, the C-terminal domain may control the photochemical activity of the molecule. Therefore, in this case also, one might expect coupling between the photoactive site and C-terminal domains, as reported here for ASR. The authors are grateful to Kevin Ridge for discussion and comments on the manuscript and to Elena Govorunova for help in manuscript preparation. This work was supported by the Russian Foundation for Basic Research grant (to O.A.S.), and by National Institutes of Health grant R37GM27750 and the Robert A. Welch Foundation (to J.L.S.). REFERENCES 1. Spudich, J. L., C. S. Yang, K. H. Jung, and E. N. Spudich Retinylidene proteins: structures and functions from archaea to humans. Annu. Rev. Cell Dev. Biol. 16: Ebrey, T., and Y. Koutalos Vertebrate photoreceptors. Prog. Retin. Eye Res. 20: Spudich, J. L., and K. H. Jung Microbial rhodopsins: phylogenetic and functional diversity. In Handbook of Photosensory Receptors. W. R. Briggs and J. L. Spudich, editors. Wiley-VCH, Weinheim, Germany
9 ASR C-Domain/Photoactive Site Coupling Terakita, A The opsins. Genome Biol. 6: Luecke, H., and J. K. Lanyi Structural clues to the mechanism of ion pumping in bacteriorhodopsin. Adv. Protein Chem. 63: Brown, L. S., and K. H. Jung Bacteriorhodopsin-like proteins of eubacteria and fungi: the extent of conservation of the haloarchaeal proton-pumping mechanism. Photochem. Photobiol. Sci. 5: Spudich, J. L The multitalented microbial sensory rhodopsins. Trends Microbiol. 14: Ridge, K. D., N. G. Abdulaev, M. Sousa, and K. Palczewski Phototransduction: crystal clear. Trends Biochem. Sci. 28: Janssen, J. J., P. H. Bovee-Geurts, M. Merkx, and W. J. DeGrip Histidine tagging both allows convenient single-step purification of bovine rhodopsin and exerts ionic strength-dependent effects on its photochemistry. J. Biol. Chem. 270: Ovchinnikov, Y. A., N. G. Abdulaev, A. V. Kiselev, L. A. Drachev, A. D. Kaulen, and V. P. Skulachev The water-exposed C-terminal sequence of bacteriorhodopsin does not affect H 1 pumping. FEBS Lett. 194: Olson, K. D., and J. L. Spudich Removal of the transducer protein from sensory rhodopsin I exposes sites of proton release and uptake during the receptor photocycle. Biophys. J. 65: Yang, C. S., and J. L. Spudich Light-induced structural changes occur in the transmembrane helices of the Natronobacterium pharaonis HtrII transducer. Biochemistry. 40: Ferrando-May, E., B. Brustmann, and D. A. Oesterhelt A C-terminal truncation results in high-level expression of the functional photoreceptor sensory rhodopsin I in the archaeon Halobacterium salinarium. Mol. Microbiol. 9: Sharma, A. K., J. L. Spudich, and W. F. Doolittle Microbial rhodopsins: functional versatility and genetic mobility. Trends Microbiol. 14: Spudich, J. L., and R. A. Bogomolni Mechanism of color discrimination by a bacterial sensory rhodopsin. Nature. 312: Hoff, W. D., K. H. Jung, and J. L. Spudich Molecular mechanism of photosignaling by archaeal sensory rhodopsins. Annu. Rev. Biophys. Biomol. Struct. 26: Sineshchekov, O. A., K. H. Jung, and J. L. Spudich Two rhodopsins medicate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA. 99: Jung, K. H., V. D. Trivedi, and J. L. Spudich Demonstration of a sensory rhodopsin in eubacteria. Mol. Microbiol. 47: Vogeley, L., O. A. Sineshchekov, V. D. Trivedi, J. Sasaki, J. L. Spudich, and H. Luecke Anabaena sensory rhodopsin: a photochromic color sensor at 2.0 A o. Science. 306: Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 77: Sineshchekov, O. A., and J. L. Spudich Light-induced intramolecular charge movements in microbial rhodopsins in intact E. coli cells. Photochem. Photobiol. Sci. 3: Sineshchekov, O. A., V. D. Trivedi, J. Sasaki, and J. L. Spudich Photochromicity of Anabaena sensory rhodopsin, an atypical microbial receptor with a cis-retinal light-adapted form. J. Biol. Chem. 280: Bergo, V. B., M. Ntefidou, V. D. Trivedi, J. J. Amsden, J. M. Kralj, K. J. Rothschild, and J. L. Spudich Conformational changes in the photocycle of Anabaena sensory rhodopsin: Absence of the Schiff base counterion protonation signal. J. Biol. Chem. 281: Ludmann, K., C. Gergely, A. Dér, and G. Váró Electric signals during the bacteriorhodopsin photocycle, determined over a wide ph range. Biophys. J. 75: Oroszi, L., A. Dér, and P. Ormos Theory of electric signals of membrane proteins in three dimensions. Eur. Biophys. J. 31: Dér, A., and L. Keszthelyi Charge motion during the photocycle of bacteriorhodopsin. Biochemistry (Mosc.). 66: Lanyi, J. K What is the real crystallographic structure of the L photointermediate of bacteriorhodopsin? Biochim. Biophys. Acta. 1658: Tóth-Boconádi, R., A. Dér, S. G. Taneva, and L. Keszthelyi Excitation of the L intermediate of bacteriorhodopsin: electric responses to test x-ray structures. Biophys. J. 90: Shi, L., S. R. Yoon, A. G. Bezerra, Jr., K. H. Jung, and L. S. Brown Cytoplasmic shuttling of protons in Anabaena sensory rhodopsin: implications for signaling mechanism. J. Mol. Biol. 358: Luecke, H., B. Schobert, J. K. Lanyi, E. N. Spudich, and J. L. Spudich Crystal structure of sensory rhodopsin II at 2.4 angstroms: insights into color tuning and transducer interaction. Science. 293: Zhang, X. N., J. Zhu, and J. L. Spudich The specificity of interaction of archaeal transducers with their cognate sensory rhodopsins is determined by their transmembrane helices. Proc. Natl. Acad. Sci. USA. 96: Gordeliy, V. I., J. Labahn, R. Moukhametzianov, R. Efremov, J. Granzin, R. Schlesinger, G. Büldt, T. Savopol, A. J. Scheidig, J. P. Klare, and M. Engelhard Molecular basis of transmembrane signalling by sensory rhodopsin II-transducer complex. Nature. 419: Furutani, Y., A. Kawanabe, K. H. Jung, and H. Kandori FTIR spectroscopy of the all-trans form of Anabaena sensory rhodopsin at 77 K: hydrogen bond of a water between the Schiff base and Asp75. Biochemistry. 44: Kawanabe, A., Y. Furutani, K. H. Jung, and H. Kandori FTIR study of the photoisomerization processes in the 13-cis and all-trans forms of Anabaena sensory rhodopsin at 77 K. Biochemistry. 45: Heberle, J., D. Oesterhelt, and N. A. Dencher Decoupling of photo- and proton cycle in the Asp85 Glu mutant of bacteriorhodopsin. EMBO J. 12: Sineshchekov, O. A., E. G. Govorunova, K. H. Jung, S. Zauner, U.-G. Maier, and J. L. Spudich Rhodopsin-mediated photoreception in Cryptophyte flagellates. Biophys. J. 89: Nagel, G., D. Ollig, M. Fuhrmann, S. Kateriya, A. M. Musti, E. Bamberg, and P. Hegemann Channelrhodopsin-1: a light-gated proton channel in green algae. Science. 296: Nagel, G., T. Szellas, W. Huhn, S. Kateriya, N. Adeishvili, P. Berthold, D. Ollig, P. Hegemann, and E. Bamberg Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA. 100: Suzuki, T., K. Yamasaki, S. Fujita, K. Oda, M. Iseki, K. Yoshida, M. Watanabe, H. Daiyasu, H. Toh, E. Asamizu, S. Tabata, K. Miura, et al Archaeal-type rhodopsins in Chlamydomonas: model structure and intracellular localization. Biochem. Biophys. Res. Commun. 301: Sineshchekov, O. A Electrophysiology of photomovements in flagellated algae. In Biophysics of Photoreceptors and Photomovements in Microorganisms. F. Lenci, F. Ghetti, G. Colombetti, D. P. Häder, and P.-S. Song, editors. Plenum Press, New York Sineshchekov, O. A., and J. L. Spudich Sensory rhodopsin signaling in green flagellate algae. In Handbook of Photosensory Receptors. W. R. Briggs and J. L. Spudich, editors. Wiley-VCH, Weinheim, Germany
Proteorhodopsin phototrophy in the ocean
 Nature 411, 786-789 (14 June 2001) doi:10.1038/35081051; Received 3 January 2001; Accepted 26 March 2001 Proteorhodopsin phototrophy in the ocean Oded Béjà, Elena N. Spudich, John L. Spudich, Marion Leclerc
Nature 411, 786-789 (14 June 2001) doi:10.1038/35081051; Received 3 January 2001; Accepted 26 March 2001 Proteorhodopsin phototrophy in the ocean Oded Béjà, Elena N. Spudich, John L. Spudich, Marion Leclerc
sensors ISSN
 Sensors 2009, 9, 9741-9804; doi:10.3390/s91209741 OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Review Photoreactions and Structural Changes of Anabaena Sensory Rhodopsin Akira Kawanabe
Sensors 2009, 9, 9741-9804; doi:10.3390/s91209741 OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Review Photoreactions and Structural Changes of Anabaena Sensory Rhodopsin Akira Kawanabe
ABSORPTION KINETICS AND ELECTRIC SIGNALS MEASURED ON LEPTOSPHAERIA RHODOPSIN
 Digest Journal of Nanomaterials and Biostructures Vol. 2, No. 3, September 2007, p. 265 269 ABSORPTION KINETICS AND ELECTRIC SIGNALS MEASURED ON LEPTOSPHAERIA RHODOPSIN K. Magyari *, V. Simon, Y. Fan a,
Digest Journal of Nanomaterials and Biostructures Vol. 2, No. 3, September 2007, p. 265 269 ABSORPTION KINETICS AND ELECTRIC SIGNALS MEASURED ON LEPTOSPHAERIA RHODOPSIN K. Magyari *, V. Simon, Y. Fan a,
Tyr-199 and Charged Residues of pharaonis Phoborhodopsin Are Important for the Interaction with its Transducer
 Biophysical Journal Volume 83 July 2002 427 432 427 Tyr-199 and Charged Residues of pharaonis Phoborhodopsin Are Important for the Interaction with its Transducer Yuki Sudo, Masayuki Iwamoto, Kazumi Shimono,
Biophysical Journal Volume 83 July 2002 427 432 427 Tyr-199 and Charged Residues of pharaonis Phoborhodopsin Are Important for the Interaction with its Transducer Yuki Sudo, Masayuki Iwamoto, Kazumi Shimono,
Title. Author(s)Sudo, Yuki; Iwamoto, Masayuki; Shimono, Kazumi; Kamo. CitationBiochimica et Biophysica Acta (BBA) - Biomembranes,
 Title Association of pharaonis phoborhodopsin with its cog water-soluble reagents of azide and hydroxylamine. Author(s)Sudo, Yuki; Iwamoto, Masayuki; Shimono, Kazumi; Kamo CitationBiochimica et Biophysica
Title Association of pharaonis phoborhodopsin with its cog water-soluble reagents of azide and hydroxylamine. Author(s)Sudo, Yuki; Iwamoto, Masayuki; Shimono, Kazumi; Kamo CitationBiochimica et Biophysica
Retinal Proteins (Rhodopsins) Vision, Bioenergetics, Phototaxis. Bacteriorhodopsin s Photocycle. Bacteriorhodopsin s Photocycle
 Molecular chanisms of Photoactivation and Spectral Tuning in Retinal Proteins Emad Tajkhorshid Theoretical and Computational Biophysics Group Beckman Institute University of Illinois at Urbana-Champaign
Molecular chanisms of Photoactivation and Spectral Tuning in Retinal Proteins Emad Tajkhorshid Theoretical and Computational Biophysics Group Beckman Institute University of Illinois at Urbana-Champaign
Comparison between Bacteriorhodopsin and Halorhodopsin. Halorhodopsin (HR) and Bacteriorhodopsin (BR) belong to a subfamily of
 Comparison between Bacteriorhodopsin and Halorhodopsin Halorhodopsin (HR) and Bacteriorhodopsin (BR) belong to a subfamily of heptahelical membrane proteins, the archaeal rhodopsins. They are found in
Comparison between Bacteriorhodopsin and Halorhodopsin Halorhodopsin (HR) and Bacteriorhodopsin (BR) belong to a subfamily of heptahelical membrane proteins, the archaeal rhodopsins. They are found in
Characterization of the Azide-Dependent Bacteriorhodopsin-Like Photocycle of Salinarum Halorhodopsin
 Biophysical Journal Volume 82 April 2002 1687 1695 1687 Characterization of the Azide-Dependent Bacteriorhodopsin-Like Photocycle of Salinarum Halorhodopsin Melinda Lakatos,* Géza I. Groma,* Constanta
Biophysical Journal Volume 82 April 2002 1687 1695 1687 Characterization of the Azide-Dependent Bacteriorhodopsin-Like Photocycle of Salinarum Halorhodopsin Melinda Lakatos,* Géza I. Groma,* Constanta
Ion Translocation Across Biological Membranes. Janos K. Lanyi University of California, Irvine
 Ion Translocation Across Biological Membranes Janos K. Lanyi University of California, Irvine Examples of transmembrane ion pumps Protein Cofactor, substrate, etc. MW Subunits Mitoch. cytochrome oxidase
Ion Translocation Across Biological Membranes Janos K. Lanyi University of California, Irvine Examples of transmembrane ion pumps Protein Cofactor, substrate, etc. MW Subunits Mitoch. cytochrome oxidase
Two Groups Control Light-Induced Schiff Base Deprotonation and the Proton Affinity of Asp 85 in the Arg 82 His Mutant of Bacteriorhodopsin
 2750 Biophysical Journal Volume 77 November 1999 2750 2763 Two Groups Control Light-Induced Schiff Base Deprotonation and the Proton Affinity of Asp 85 in the Arg 82 His Mutant of Bacteriorhodopsin Eleonora
2750 Biophysical Journal Volume 77 November 1999 2750 2763 Two Groups Control Light-Induced Schiff Base Deprotonation and the Proton Affinity of Asp 85 in the Arg 82 His Mutant of Bacteriorhodopsin Eleonora
Microbial rhodopsins are widespread photoactive proteins
 This is an open access article published under an ACS AuthorChoice License, which permits copying and redistribution of the article or any adaptations for non-commercial purposes. pubs.acs.org/biochemistry
This is an open access article published under an ACS AuthorChoice License, which permits copying and redistribution of the article or any adaptations for non-commercial purposes. pubs.acs.org/biochemistry
Five Residues in the HtrI Transducer Membrane-Proximal Domain Close the Cytoplasmic Proton-Conducting Channel of Sensory Rhodopsin I
 JBC Papers in Press. Published on July 13, 2004 as Manuscript M406503200 JUNE 10, 2004 Five Residues in the HtrI Transducer Membrane-Proximal Domain Close the Cytoplasmic Proton-Conducting Channel of Sensory
JBC Papers in Press. Published on July 13, 2004 as Manuscript M406503200 JUNE 10, 2004 Five Residues in the HtrI Transducer Membrane-Proximal Domain Close the Cytoplasmic Proton-Conducting Channel of Sensory
Hydration switch model for the proton transfer in the Schiff base region of bacteriorhodopsin
 Biochimica et Biophysica Acta 1658 (2004) 72 79 Review Hydration switch model for the proton transfer in the Schiff base region of bacteriorhodopsin Hideki Kandori* Department of Materials Science and
Biochimica et Biophysica Acta 1658 (2004) 72 79 Review Hydration switch model for the proton transfer in the Schiff base region of bacteriorhodopsin Hideki Kandori* Department of Materials Science and
Structural Changes of Salinibacter Sensory Rhodopsin I upon Formation of the K and M Photointermediates
 12750 Biochemistry 2008, 47, 12750 12759 Structural Changes of Salinibacter Sensory Rhodopsin I upon Formation of the K and M Photointermediates Daisuke Suzuki, Yuki Sudo, Yuji Furutani, Hazuki Takahashi,
12750 Biochemistry 2008, 47, 12750 12759 Structural Changes of Salinibacter Sensory Rhodopsin I upon Formation of the K and M Photointermediates Daisuke Suzuki, Yuki Sudo, Yuji Furutani, Hazuki Takahashi,
Title. Author(s)Sudo, Yuki; Iwamoto, Masayuki; Shimono, Kazumi; Kamo. CitationJournal of Photochemistry and Photobiology B Biology. Issue Date
 Title Association between a photo-intermediate of a M-lack transducer. Author(s)Sudo, Yuki; Iwamoto, Masayuki; Shimono, Kazumi; Kamo CitationJournal of Photochemistry and Photobiology B Biology Issue Date
Title Association between a photo-intermediate of a M-lack transducer. Author(s)Sudo, Yuki; Iwamoto, Masayuki; Shimono, Kazumi; Kamo CitationJournal of Photochemistry and Photobiology B Biology Issue Date
MINIREVIEW. into SR-I-HtrI interaction. The aim of this minireview is to describe recent progress concerning SR-I activation and signal
 JOURNAL OF BACTERIOLOGY, Dec. 1993, P. 7755-7761 0021-9193/93/247755-07$02.00/0 Copyright 1993, American Society for Microbiology Vol. 175, No. 24 MINIREVIEW Color Sensing in the Archaea: a Eukaryotic-Like
JOURNAL OF BACTERIOLOGY, Dec. 1993, P. 7755-7761 0021-9193/93/247755-07$02.00/0 Copyright 1993, American Society for Microbiology Vol. 175, No. 24 MINIREVIEW Color Sensing in the Archaea: a Eukaryotic-Like
Three strategically placed hydrogen-bonding residues convert a proton pump into a sensory receptor
 Three strategically placed hydrogen-bonding residues convert a proton pump into a sensory receptor Yuki Sudo and John L. Spudich* Center for Membrane Biology, Department of Biochemistry and Molecular Biology,
Three strategically placed hydrogen-bonding residues convert a proton pump into a sensory receptor Yuki Sudo and John L. Spudich* Center for Membrane Biology, Department of Biochemistry and Molecular Biology,
Laser-Induced Transient Grating Analysis of Dynamics of Interaction between Sensory Rhodopsin II D75N and the HtrII Transducer
 2028 Biophysical Journal Volume 92 March 2007 2028 2040 Laser-Induced Transient Grating Analysis of Dynamics of Interaction between Sensory Rhodopsin II D75N and the HtrII Transducer Keiichi Inoue,* Jun
2028 Biophysical Journal Volume 92 March 2007 2028 2040 Laser-Induced Transient Grating Analysis of Dynamics of Interaction between Sensory Rhodopsin II D75N and the HtrII Transducer Keiichi Inoue,* Jun
MOLECULAR MECHANISM OF PHOTOSIGNALING BY ARCHAEAL SENSORY RHODOPSINS
 Annu. Rev. Biophys. Biomol. Struct. 1997. 26:223 58 Copyright c 1997 by Annual Reviews Inc. All rights reserved MOLECULAR MECHANISM OF PHOTOSIGNALING BY ARCHAEAL SENSORY RHODOPSINS Wouter D. Hoff, Kwang-Hwan
Annu. Rev. Biophys. Biomol. Struct. 1997. 26:223 58 Copyright c 1997 by Annual Reviews Inc. All rights reserved MOLECULAR MECHANISM OF PHOTOSIGNALING BY ARCHAEAL SENSORY RHODOPSINS Wouter D. Hoff, Kwang-Hwan
Intermediate spectra and photocycle kinetics of the Asp96 3 Asn mutant bacteriorhodopsin determined by singular value decomposition with self-modeling
 Proc. Natl. Acad. Sci. USA Vol. 96, pp. 4414 4419, April 1999 Biophysics Intermediate spectra and photocycle kinetics of the Asp96 3 Asn mutant bacteriorhodopsin determined by singular value decomposition
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 4414 4419, April 1999 Biophysics Intermediate spectra and photocycle kinetics of the Asp96 3 Asn mutant bacteriorhodopsin determined by singular value decomposition
RETINYLIDENE PROTEINS: Structures and Functions from Archaea to Humans
 Annu. Rev. Cell Dev. Biol. 2000. 16:365 92 Copyright c 2000 by Annual Reviews. All rights reserved RETINYLIDENE PROTEINS: Structures and Functions from Archaea to Humans John L. Spudich, Chii-Shen Yang,
Annu. Rev. Cell Dev. Biol. 2000. 16:365 92 Copyright c 2000 by Annual Reviews. All rights reserved RETINYLIDENE PROTEINS: Structures and Functions from Archaea to Humans John L. Spudich, Chii-Shen Yang,
Biophysics 490M Project
 Biophysics 490M Project Dan Han Department of Biochemistry Structure Exploration of aa 3 -type Cytochrome c Oxidase from Rhodobacter sphaeroides I. Introduction: All organisms need energy to live. They
Biophysics 490M Project Dan Han Department of Biochemistry Structure Exploration of aa 3 -type Cytochrome c Oxidase from Rhodobacter sphaeroides I. Introduction: All organisms need energy to live. They
asparagine, initiated in the unprotonated Schiff base state (photovoltage/photocycle/purple membrane)
 Proc. Natl. Acad. Sci. USA Vol. 92, pp. 11519-11523, December 1995 Biophysics Proton transport by a bacteriorhodopsin mutant, aspartic acid-85 asparagine, initiated in the unprotonated Schiff base state
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 11519-11523, December 1995 Biophysics Proton transport by a bacteriorhodopsin mutant, aspartic acid-85 asparagine, initiated in the unprotonated Schiff base state
Resonance Raman Investigation of the Chromophore Structure of Heliorhodopsins
 Supporting information Resonance Raman Investigation of the Chromophore Structure of Heliorhodopsins Akihiro Otomo, Misao Mizuno, Manish Singh, Wataru Shihoya, Keiichi Inoue, % Osamu Nureki, Oded Béjà,
Supporting information Resonance Raman Investigation of the Chromophore Structure of Heliorhodopsins Akihiro Otomo, Misao Mizuno, Manish Singh, Wataru Shihoya, Keiichi Inoue, % Osamu Nureki, Oded Béjà,
Title. Author(s)Iwamoto, Masayuki; Sudo, Yuki; Shimono, Kazumi; Kamo. CitationBiochimica et Biophysica Acta (BBA) - Biomembranes,
 Title Selective reaction of hydroxylamine with chromophore Author(s)Iwamoto, Masayuki; Sudo, Yuki; Shimono, Kazumi; Kamo CitationBiochimica et Biophysica Acta (BBA) - Biomembranes, Issue Date 2001-09-03
Title Selective reaction of hydroxylamine with chromophore Author(s)Iwamoto, Masayuki; Sudo, Yuki; Shimono, Kazumi; Kamo CitationBiochimica et Biophysica Acta (BBA) - Biomembranes, Issue Date 2001-09-03
Title. Author(s)Sudo, Yuki; Iwamoto, Masayuki; Shimono, Kazumi; Kamo. CitationBiophysical Journal, 83(1): Issue Date Doc URL.
 Title Tyr-199 and charged residues of pharaonis Phoborhodo Author(s)Sudo, Yuki; Iwamoto, Masayuki; Shimono, Kazumi; Kamo CitationBiophysical Journal, 83(1): 427-432 Issue Date 2002-07 Doc URL http://hdl.handle.net/2115/16002
Title Tyr-199 and charged residues of pharaonis Phoborhodo Author(s)Sudo, Yuki; Iwamoto, Masayuki; Shimono, Kazumi; Kamo CitationBiophysical Journal, 83(1): 427-432 Issue Date 2002-07 Doc URL http://hdl.handle.net/2115/16002
6 Discussion. 6.1 Bacterial reaction center X-Ray crystallography of RC mutants
 6.1 Bacterial reaction center Proton and electron transfer in bacterial reaction centers were investigated using time-resolved FTIR spectroscopy and X-ray crystallography. The effect of sitespecific mutations
6.1 Bacterial reaction center Proton and electron transfer in bacterial reaction centers were investigated using time-resolved FTIR spectroscopy and X-ray crystallography. The effect of sitespecific mutations
Visual pigments. Neuroscience, Biochemistry Dr. Mamoun Ahram Third year, 2019
 Visual pigments Neuroscience, Biochemistry Dr. Mamoun Ahram Third year, 2019 References Webvision: The Organization of the Retina and Visual System (http://www.ncbi.nlm.nih.gov/books/nbk11522/#a 127) The
Visual pigments Neuroscience, Biochemistry Dr. Mamoun Ahram Third year, 2019 References Webvision: The Organization of the Retina and Visual System (http://www.ncbi.nlm.nih.gov/books/nbk11522/#a 127) The
Supplementary Figure 1 Crystal packing of ClR and electron density maps. Crystal packing of type A crystal (a) and type B crystal (b).
 Supplementary Figure 1 Crystal packing of ClR and electron density maps. Crystal packing of type A crystal (a) and type B crystal (b). Crystal contacts at B-C loop are magnified and stereo view of A-weighted
Supplementary Figure 1 Crystal packing of ClR and electron density maps. Crystal packing of type A crystal (a) and type B crystal (b). Crystal contacts at B-C loop are magnified and stereo view of A-weighted
3 Time-resolved spectroscopy of protein function
 3 Time-resolved spectroscopy of protein function Milian Wolff and David Ehrenberg Freie Universität Berlin (Supervised by Bernd Schultz and Victor Lorenz Fonfria) (Dated: June 25, 213) In this experiment
3 Time-resolved spectroscopy of protein function Milian Wolff and David Ehrenberg Freie Universität Berlin (Supervised by Bernd Schultz and Victor Lorenz Fonfria) (Dated: June 25, 213) In this experiment
Structural basis for sensory rhodopsin function
 Biochimica et Biophysica Acta 1565 (2002) 196 205 Review Structural basis for sensory rhodopsin function www.bba-direct.com Eva Pebay-Peyroula a, Antoine Royant a,b, Ehud M. Landau c, Javier Navarro c,
Biochimica et Biophysica Acta 1565 (2002) 196 205 Review Structural basis for sensory rhodopsin function www.bba-direct.com Eva Pebay-Peyroula a, Antoine Royant a,b, Ehud M. Landau c, Javier Navarro c,
Visible and IR Absorption Spectroscopy. Andrew Rouff and Kyle Chau
 Visible and IR Absorption Spectroscopy Andrew Rouff and Kyle Chau The Basics wavelength= (λ) original intensity= Ι o sample slab thickness= dl Final intensity= I f ε = molar extinction coefficient -di=
Visible and IR Absorption Spectroscopy Andrew Rouff and Kyle Chau The Basics wavelength= (λ) original intensity= Ι o sample slab thickness= dl Final intensity= I f ε = molar extinction coefficient -di=
Title. Author(s)Ikeura, Yukako; Shimono, Kazumi; Iwamoto, Masayuki; CitationBiophysical Journal, 86(5): Issue Date Doc URL.
 Title Role of Arg-72 of pharaonis Phoborhodopsin (sensory Author(s)Ikeura, Yukako; Shimono, Kazumi; Iwamoto, Masayuki; CitationBiophysical Journal, 86(5): 3112-3120 Issue Date 2004-05 Doc URL http://hdl.handle.net/2115/16000
Title Role of Arg-72 of pharaonis Phoborhodopsin (sensory Author(s)Ikeura, Yukako; Shimono, Kazumi; Iwamoto, Masayuki; CitationBiophysical Journal, 86(5): 3112-3120 Issue Date 2004-05 Doc URL http://hdl.handle.net/2115/16000
Secondary Structure. Bioch/BIMS 503 Lecture 2. Structure and Function of Proteins. Further Reading. Φ, Ψ angles alone determine protein structure
 Bioch/BIMS 503 Lecture 2 Structure and Function of Proteins August 28, 2008 Robert Nakamoto rkn3c@virginia.edu 2-0279 Secondary Structure Φ Ψ angles determine protein structure Φ Ψ angles are restricted
Bioch/BIMS 503 Lecture 2 Structure and Function of Proteins August 28, 2008 Robert Nakamoto rkn3c@virginia.edu 2-0279 Secondary Structure Φ Ψ angles determine protein structure Φ Ψ angles are restricted
Reorientations in the Bacteriorhodopsin Photocycle Are ph Dependent
 2352 Biophysical Journal Volume 70 May 1996 2352-2357 Reorientations in the Bacteriorhodopsin Photocycle Are ph Dependent Greg S. Harms, Qin Song, and Carey K. Johnson Department of Chemistry, University
2352 Biophysical Journal Volume 70 May 1996 2352-2357 Reorientations in the Bacteriorhodopsin Photocycle Are ph Dependent Greg S. Harms, Qin Song, and Carey K. Johnson Department of Chemistry, University
Computational Studies of the Photoreceptor Rhodopsin. Scott E. Feller Wabash College
 Computational Studies of the Photoreceptor Rhodopsin Scott E. Feller Wabash College Rhodopsin Photocycle Dark-adapted Rhodopsin hn Isomerize retinal Photorhodopsin ~200 fs Bathorhodopsin Meta-II ms timescale
Computational Studies of the Photoreceptor Rhodopsin Scott E. Feller Wabash College Rhodopsin Photocycle Dark-adapted Rhodopsin hn Isomerize retinal Photorhodopsin ~200 fs Bathorhodopsin Meta-II ms timescale
Molecular Dynamics Study of the. Proton Pump Cycle of. Bacteriorhodopsin
 Molecular Dynamics Study of the Proton Pump Cycle of Bacteriorhodopsin Feng Zhou, Andreas Windemuth, Klaus Schulten Beckman Institute and Departments of Biophysics and Physics, University of Illinois at
Molecular Dynamics Study of the Proton Pump Cycle of Bacteriorhodopsin Feng Zhou, Andreas Windemuth, Klaus Schulten Beckman Institute and Departments of Biophysics and Physics, University of Illinois at
A molecular-dynamics study of the rhodopsin chromophore using ultrasoft pseudopotentials
 Progress of Theoretical Physics Supplement No. 138, 2000 107 A molecular-dynamics study of the rhodopsin chromophore using ultrasoft pseudopotentials Minoru Sugihara, 1 ) Peter Entel, 1 Hendrik Meyer,
Progress of Theoretical Physics Supplement No. 138, 2000 107 A molecular-dynamics study of the rhodopsin chromophore using ultrasoft pseudopotentials Minoru Sugihara, 1 ) Peter Entel, 1 Hendrik Meyer,
The Hydroxylamine Reaction of Sensory Rhodopsin II: Light-Induced Conformational Alterations with C 13 5C 14 Nonisomerizable Pigment
 2610 Biophysical Journal Volume 89 October 2005 2610 2617 The Hydroxylamine Reaction of Sensory Rhodopsin II: Light-Induced Conformational Alterations with C 13 5C 14 Nonisomerizable Pigment U.Zadok,*J.P.Klare,
2610 Biophysical Journal Volume 89 October 2005 2610 2617 The Hydroxylamine Reaction of Sensory Rhodopsin II: Light-Induced Conformational Alterations with C 13 5C 14 Nonisomerizable Pigment U.Zadok,*J.P.Klare,
CURRICULUM VITAE JOHN LEE SPUDICH
 CURRICULUM VITAE JOHN LEE SPUDICH Updated: Sep 21, 2017 CURRENT TITLE: ADDRESS: Robert A. Welch Distinguished Chair in Chemistry Director, Center for Membrane Biology Professor, Department of Biochemistry
CURRICULUM VITAE JOHN LEE SPUDICH Updated: Sep 21, 2017 CURRENT TITLE: ADDRESS: Robert A. Welch Distinguished Chair in Chemistry Director, Center for Membrane Biology Professor, Department of Biochemistry
AUGUST 29, 2008 VOLUME 283 NUMBER 35 JOURNAL OF BIOLOGICAL CHEMISTRY 23533
 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 283, NO. 35, pp. 23533 23541, August 29, 2008 2008 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Salinibacter Sensory
THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 283, NO. 35, pp. 23533 23541, August 29, 2008 2008 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Salinibacter Sensory
Chapter 1. Topic: Overview of basic principles
 Chapter 1 Topic: Overview of basic principles Four major themes of biochemistry I. What are living organism made from? II. How do organism acquire and use energy? III. How does an organism maintain its
Chapter 1 Topic: Overview of basic principles Four major themes of biochemistry I. What are living organism made from? II. How do organism acquire and use energy? III. How does an organism maintain its
sensors ISSN
 Sensors 2010, 10, 4010-4039; doi:10.3390/s100404010 OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Review Phototactic and Chemotactic Signal Transduction by Transmembrane Receptors and
Sensors 2010, 10, 4010-4039; doi:10.3390/s100404010 OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Review Phototactic and Chemotactic Signal Transduction by Transmembrane Receptors and
Large Deformation of Helix F during the Photoreaction Cycle of Pharaonis Halorhodopsin in Complex with Azide
 Biophysical Journal Volume 104 January 2013 377 385 377 Large Deformation of Helix F during the Photoreaction Cycle of Pharaonis Halorhodopsin in Complex with Azide Taichi Nakanishi, Soun Kanada, Midori
Biophysical Journal Volume 104 January 2013 377 385 377 Large Deformation of Helix F during the Photoreaction Cycle of Pharaonis Halorhodopsin in Complex with Azide Taichi Nakanishi, Soun Kanada, Midori
CD Basis Set of Spectra that is used is that derived from comparing the spectra of globular proteins whose secondary structures are known from X-ray
 CD Basis Set of Spectra that is used is that derived from comparing the spectra of globular proteins whose secondary structures are known from X-ray crystallography An example of the use of CD Modeling
CD Basis Set of Spectra that is used is that derived from comparing the spectra of globular proteins whose secondary structures are known from X-ray crystallography An example of the use of CD Modeling
Supporting online material
 Supporting online material Materials and Methods Target proteins All predicted ORFs in the E. coli genome (1) were downloaded from the Colibri data base (2) (http://genolist.pasteur.fr/colibri/). 737 proteins
Supporting online material Materials and Methods Target proteins All predicted ORFs in the E. coli genome (1) were downloaded from the Colibri data base (2) (http://genolist.pasteur.fr/colibri/). 737 proteins
Selective reaction of hydroxylamine with chromophore during the photocycle of pharaonis phoborhodopsin
 Biochimica et Biophysica Acta 1514 (2001) 152^158 www.bba-direct.com Selective reaction of hydroxylamine with chromophore during the photocycle of pharaonis phoborhodopsin Masayuki Iwamoto, Yuki Sudo,
Biochimica et Biophysica Acta 1514 (2001) 152^158 www.bba-direct.com Selective reaction of hydroxylamine with chromophore during the photocycle of pharaonis phoborhodopsin Masayuki Iwamoto, Yuki Sudo,
Vibrational Spectroscopy of Bacteriorhodopsin Mutants
 THE JOURNAL OF BIOLOGICAL CHEMISTRY IC' 1991 by The American Society for Biochemistry and Molecular Biology, Inc. Vol. 266, No. 17, Issue of dune 15, pp. 11063-11067,1991 Printed in U.S.A. Vibrational
THE JOURNAL OF BIOLOGICAL CHEMISTRY IC' 1991 by The American Society for Biochemistry and Molecular Biology, Inc. Vol. 266, No. 17, Issue of dune 15, pp. 11063-11067,1991 Printed in U.S.A. Vibrational
A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility
 (P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
(P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
Salt bridge in the conserved His Asp cluster in Gloeobacter rhodopsin contributes to trimer formation
 FEBS Letters 587 (2013) 322 327 journal homepage: www.febsletters.org Salt bridge in the conserved His Asp cluster in Gloeobacter rhodopsin contributes to trimer formation Takashi Tsukamoto a, Takashi
FEBS Letters 587 (2013) 322 327 journal homepage: www.febsletters.org Salt bridge in the conserved His Asp cluster in Gloeobacter rhodopsin contributes to trimer formation Takashi Tsukamoto a, Takashi
Steric Constraint in the Primary Photoproduct of Sensory Rhodopsin II Is a Prerequisite for Light-Signal Transfer to HtrII
 6208 Biochemistry 2008, 47, 6208 6215 Steric Constraint in the Primary Photoproduct of Sensory Rhodopsin II Is a Prerequisite for Light-Signal Transfer to HtrII Motohiro Ito, Yuki Sudo,, Yuji Furutani,
6208 Biochemistry 2008, 47, 6208 6215 Steric Constraint in the Primary Photoproduct of Sensory Rhodopsin II Is a Prerequisite for Light-Signal Transfer to HtrII Motohiro Ito, Yuki Sudo,, Yuji Furutani,
Analysis of a hybrid TATA box binding protein originating from mesophilic and thermophilic donor organisms
 Spectroscopy 24 (2010) 233 237 233 DOI 10.3233/SPE-2010-0434 IOS Press Analysis of a hybrid TATA box binding protein originating from mesophilic and thermophilic donor organisms Annette Kopitz a,b, Jörg
Spectroscopy 24 (2010) 233 237 233 DOI 10.3233/SPE-2010-0434 IOS Press Analysis of a hybrid TATA box binding protein originating from mesophilic and thermophilic donor organisms Annette Kopitz a,b, Jörg
MOLECULAR DYNAMICS SIMULATIONS ON STRUCTURAL CONFORMATIONS OF RHODOPSIN AND PRION PROTEINS
 Ó³ Ÿ. 2005.. 2, º 6(129).. 87Ä97 Š [51-72:530.145]+[51-72:541.1] MOLECULAR DYNAMICS SIMULATIONS ON STRUCTURAL CONFORMATIONS OF RHODOPSIN AND PRION PROTEINS Kh. T. Kholmurodov 1 Joint Institute for Nuclear
Ó³ Ÿ. 2005.. 2, º 6(129).. 87Ä97 Š [51-72:530.145]+[51-72:541.1] MOLECULAR DYNAMICS SIMULATIONS ON STRUCTURAL CONFORMATIONS OF RHODOPSIN AND PRION PROTEINS Kh. T. Kholmurodov 1 Joint Institute for Nuclear
MINIREVIEW Mechanism of Light-Dependent Proton Translocation by Bacteriorhodopsin
 JOURNAL OF BACTERIOLOGY, Mar. 1993, p. 1555-1560 0021-9193/93/061555-06$02.00/0 Copyright 1993, American Society for Microbiology Vol. 175, No. 6 MINIREVIEW Mechanism of Light-Dependent Proton Translocation
JOURNAL OF BACTERIOLOGY, Mar. 1993, p. 1555-1560 0021-9193/93/061555-06$02.00/0 Copyright 1993, American Society for Microbiology Vol. 175, No. 6 MINIREVIEW Mechanism of Light-Dependent Proton Translocation
Post Print. Anabaena sensory rhodopsin is a light-driven unidirectional rotor
 Post Print Anabaena sensory rhodopsin is a light-driven unidirectional rotor Angela Strambi, Bo Durbeej, Nicolas Ferre and Massimo Olivucci N.B.: When citing this work, cite the original article. Original
Post Print Anabaena sensory rhodopsin is a light-driven unidirectional rotor Angela Strambi, Bo Durbeej, Nicolas Ferre and Massimo Olivucci N.B.: When citing this work, cite the original article. Original
T H E J O U R N A L O F G E N E R A L P H Y S I O L O G Y. jgp
 S u p p l e m e n ta l m at e r i a l jgp Lee et al., http://www.jgp.org/cgi/content/full/jgp.201411219/dc1 T H E J O U R N A L O F G E N E R A L P H Y S I O L O G Y S u p p l e m e n ta l D I S C U S
S u p p l e m e n ta l m at e r i a l jgp Lee et al., http://www.jgp.org/cgi/content/full/jgp.201411219/dc1 T H E J O U R N A L O F G E N E R A L P H Y S I O L O G Y S u p p l e m e n ta l D I S C U S
Time-Resolved X-Ray Diffraction Reveals Movement of F Helix of D96N Bacteriorhodopsin during M-MN Transition at Neutral ph
 2610 Biophysical Journal Volume 82 May 2002 2610 2616 Time-Resolved X-Ray Diffraction Reveals Movement of F Helix of D96N Bacteriorhodopsin during M-MN Transition at Neutral ph Toshihiko Oka,* Naoto Yagi,
2610 Biophysical Journal Volume 82 May 2002 2610 2616 Time-Resolved X-Ray Diffraction Reveals Movement of F Helix of D96N Bacteriorhodopsin during M-MN Transition at Neutral ph Toshihiko Oka,* Naoto Yagi,
RHODOPSIN: Insights from Recent Structural Studies
 Annu. Rev. Biophys. Biomol. Struct. 2002. 31:443 84 DOI: 10.1146/annurev.biophys.31.082901.134348 Copyright c 2002 by Annual Reviews. All rights reserved RHODOPSIN: Insights from Recent Structural Studies
Annu. Rev. Biophys. Biomol. Struct. 2002. 31:443 84 DOI: 10.1146/annurev.biophys.31.082901.134348 Copyright c 2002 by Annual Reviews. All rights reserved RHODOPSIN: Insights from Recent Structural Studies
Table 1. Kinetic data obtained from SPR analysis of domain 11 mutants interacting with IGF-II. Kinetic parameters K D 1.
 Kinetics and Thermodynamics of the Insulin-like Growth Factor II (IGF-II) Interaction with IGF-II/Mannose 6-phosphate Receptor and the function of CD and AB Loop Solvent-exposed Residues. Research Team:
Kinetics and Thermodynamics of the Insulin-like Growth Factor II (IGF-II) Interaction with IGF-II/Mannose 6-phosphate Receptor and the function of CD and AB Loop Solvent-exposed Residues. Research Team:
Proton-Pump Mechanism in Retinal Schiff Base: On the molecular structure of the M-state
 Proton-Pump Mechanism in Retinal Schiff Base: On the molecular structure of the M-state Ayan Datta and Swapan K. Pati * Theoretical Sciences Unit and Chemistry and Physics of Materials Unit, Jawaharlal
Proton-Pump Mechanism in Retinal Schiff Base: On the molecular structure of the M-state Ayan Datta and Swapan K. Pati * Theoretical Sciences Unit and Chemistry and Physics of Materials Unit, Jawaharlal
BACTERIORHODOPSIN. Janos K. Lanyi
 1 BACTERIORHODOPSIN Janos K. Lanyi Department of Physiology & Biophysics, University of California, Irvine, CA 92697-4560 1. INTRODUCTION Bacteriorhodopsin (BR), a retinal protein in the cell membranes
1 BACTERIORHODOPSIN Janos K. Lanyi Department of Physiology & Biophysics, University of California, Irvine, CA 92697-4560 1. INTRODUCTION Bacteriorhodopsin (BR), a retinal protein in the cell membranes
Full-length GlpG sequence was generated by PCR from E. coli genomic DNA. (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
 Supplementary Methods Protein expression and purification Full-length GlpG sequence was generated by PCR from E. coli genomic DNA (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
Supplementary Methods Protein expression and purification Full-length GlpG sequence was generated by PCR from E. coli genomic DNA (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
The role of the retinylidene Schiff base counterion in rhodopsin in determining wavelength absorbance and Schiff base pkã
 Proc. Nail. Acad. Sci. USA Vol. 88, pp. 3079-3083, April 1991 Biochemistry The role of the retinylidene Schiff base counterion in rhodopsin in determining wavelength absorbance and Schiff base pkã (transmembrane
Proc. Nail. Acad. Sci. USA Vol. 88, pp. 3079-3083, April 1991 Biochemistry The role of the retinylidene Schiff base counterion in rhodopsin in determining wavelength absorbance and Schiff base pkã (transmembrane
Protein Structure. W. M. Grogan, Ph.D. OBJECTIVES
 Protein Structure W. M. Grogan, Ph.D. OBJECTIVES 1. Describe the structure and characteristic properties of typical proteins. 2. List and describe the four levels of structure found in proteins. 3. Relate
Protein Structure W. M. Grogan, Ph.D. OBJECTIVES 1. Describe the structure and characteristic properties of typical proteins. 2. List and describe the four levels of structure found in proteins. 3. Relate
Infrared Spectra of Protonated Networks of Water Molecules in the Interior of Bacteriorhodopsin: A QM/MM Molecular Dynamics Study. Volker Kleinschmidt
 Infrared Spectra of Protonated Networks of Water Molecules in the Interior of Bacteriorhodopsin: A QM/MM Molecular Dynamics Study Volker Kleinschmidt Bochum, 2006 Infrared Spectra of Protonated Networks
Infrared Spectra of Protonated Networks of Water Molecules in the Interior of Bacteriorhodopsin: A QM/MM Molecular Dynamics Study Volker Kleinschmidt Bochum, 2006 Infrared Spectra of Protonated Networks
Effects of Chloride Ion Binding on the Photochemical Properties of Salinibacter Sensory Rhodopsin I
 doi:10.1016/j.jmb.2009.06.050 J. Mol. Biol. (2009) 392, 48 62 Available online at www.sciencedirect.com Effects of Chloride Ion Binding on the Photochemical Properties of Salinibacter Sensory Rhodopsin
doi:10.1016/j.jmb.2009.06.050 J. Mol. Biol. (2009) 392, 48 62 Available online at www.sciencedirect.com Effects of Chloride Ion Binding on the Photochemical Properties of Salinibacter Sensory Rhodopsin
Copyright Mark Brandt, Ph.D A third method, cryogenic electron microscopy has seen increasing use over the past few years.
 Structure Determination and Sequence Analysis The vast majority of the experimentally determined three-dimensional protein structures have been solved by one of two methods: X-ray diffraction and Nuclear
Structure Determination and Sequence Analysis The vast majority of the experimentally determined three-dimensional protein structures have been solved by one of two methods: X-ray diffraction and Nuclear
Hydrogen bonding changes of internal water molecules in rhodopsin during metarhodopsin I and metarhodopsin II formation
 Biochem. J. (1998) 329, 713 717 (Printed in Great Britain) 713 Hydrogen bonding changes of internal water molecules in rhodopsin during metarhodopsin I and metarhodopsin II formation Parshuram RATH* 1,
Biochem. J. (1998) 329, 713 717 (Printed in Great Britain) 713 Hydrogen bonding changes of internal water molecules in rhodopsin during metarhodopsin I and metarhodopsin II formation Parshuram RATH* 1,
FUNCTIONAL DIVERSITY AND OPTOGENETIC POTENTIALS OF MICROBIAL RHODOPSINS
 J P P JOURNAL OF PROTEINS AND PROTEOMICS 2(2), July-December 2011, pp. 115-123 FUNCTIONAL DIVERSITY AND OPTOGENETIC POTENTIALS OF MICROBIAL RHODOPSINS Mayanka Awasthi and Suneel Kateriya Department of
J P P JOURNAL OF PROTEINS AND PROTEOMICS 2(2), July-December 2011, pp. 115-123 FUNCTIONAL DIVERSITY AND OPTOGENETIC POTENTIALS OF MICROBIAL RHODOPSINS Mayanka Awasthi and Suneel Kateriya Department of
Homology models of the tetramerization domain of six eukaryotic voltage-gated potassium channels Kv1.1-Kv1.6
 Homology models of the tetramerization domain of six eukaryotic voltage-gated potassium channels Kv1.1-Kv1.6 Hsuan-Liang Liu* and Chin-Wen Chen Department of Chemical Engineering and Graduate Institute
Homology models of the tetramerization domain of six eukaryotic voltage-gated potassium channels Kv1.1-Kv1.6 Hsuan-Liang Liu* and Chin-Wen Chen Department of Chemical Engineering and Graduate Institute
Gene regulation I Biochemistry 302. Bob Kelm February 25, 2005
 Gene regulation I Biochemistry 302 Bob Kelm February 25, 2005 Principles of gene regulation (cellular versus molecular level) Extracellular signals Chemical (e.g. hormones, growth factors) Environmental
Gene regulation I Biochemistry 302 Bob Kelm February 25, 2005 Principles of gene regulation (cellular versus molecular level) Extracellular signals Chemical (e.g. hormones, growth factors) Environmental
Membrane Protein Pumps
 Membrane Protein Pumps Learning objectives You should be able to understand & discuss: Active transport-na + /K + ATPase ABC transporters Metabolite transport by lactose permease 1. Ion pumps: ATP-driven
Membrane Protein Pumps Learning objectives You should be able to understand & discuss: Active transport-na + /K + ATPase ABC transporters Metabolite transport by lactose permease 1. Ion pumps: ATP-driven
Visual pigments. Neuroscience, Biochemistry Dr. Mamoun Ahram Third year, 2015
 Visual pigments Neuroscience, Biochemistry Dr. Mamoun Ahram Third year, 2015 References Photoreceptors and visual pigments Webvision: The Organization of the Retina and Visual System (http://www.ncbi.nlm.nih.gov/books/nbk11522/#a127)
Visual pigments Neuroscience, Biochemistry Dr. Mamoun Ahram Third year, 2015 References Photoreceptors and visual pigments Webvision: The Organization of the Retina and Visual System (http://www.ncbi.nlm.nih.gov/books/nbk11522/#a127)
Interpreting and evaluating biological NMR in the literature. Worksheet 1
 Interpreting and evaluating biological NMR in the literature Worksheet 1 1D NMR spectra Application of RF pulses of specified lengths and frequencies can make certain nuclei detectable We can selectively
Interpreting and evaluating biological NMR in the literature Worksheet 1 1D NMR spectra Application of RF pulses of specified lengths and frequencies can make certain nuclei detectable We can selectively
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11524 Supplementary discussion Functional analysis of the sugar porter family (SP) signature motifs. As seen in Fig. 5c, single point mutation of the conserved
SUPPLEMENTARY INFORMATION doi:10.1038/nature11524 Supplementary discussion Functional analysis of the sugar porter family (SP) signature motifs. As seen in Fig. 5c, single point mutation of the conserved
Newly isolated archaerhodopsin from a strain of Chinese halobacteria and its proton pumping behavior
 Biochimica et Biophysica Acta 1466 (2000) 260^266 www.elsevier.com/locate/bba Newly isolated archaerhodopsin from a strain of Chinese halobacteria and its proton pumping behavior Qingguo Li *, Qingan Sun,
Biochimica et Biophysica Acta 1466 (2000) 260^266 www.elsevier.com/locate/bba Newly isolated archaerhodopsin from a strain of Chinese halobacteria and its proton pumping behavior Qingguo Li *, Qingan Sun,
Calculation of Proton Transfers in Bacteriorhodopsin br and M Intermediates
 Biochemistry 2003, 42, 9875-9888 9875 Calculation of Proton Transfers in Bacteriorhodopsin br and M Intermediates Yifan Song, Junjun Mao, and M. R. Gunner* Physics Department J-419, City College of New
Biochemistry 2003, 42, 9875-9888 9875 Calculation of Proton Transfers in Bacteriorhodopsin br and M Intermediates Yifan Song, Junjun Mao, and M. R. Gunner* Physics Department J-419, City College of New
Solid-state NMR studies of membrane protein structure and dynamics in synthetic lipids and cell membranes
 Solid-state NMR studies of membrane protein structure and dynamics in synthetic lipids and cell membranes Vlad Ladizhansky University of Guelph, Ontario Canada Winter School on Biomolecular NMR January
Solid-state NMR studies of membrane protein structure and dynamics in synthetic lipids and cell membranes Vlad Ladizhansky University of Guelph, Ontario Canada Winter School on Biomolecular NMR January
A Eukaryotic Protein, NOP-1, Binds Retinal To Form an Archaeal Rhodopsin-like Photochemically Reactive Pigment
 14138 Biochemistry 1999, 38, 14138-14145 A Eukaryotic Protein, NOP-1, Binds Retinal To Form an Archaeal Rhodopsin-like Photochemically Reactive Pigment Jennifer A. Bieszke, Elena N. Spudich, Kenneth L.
14138 Biochemistry 1999, 38, 14138-14145 A Eukaryotic Protein, NOP-1, Binds Retinal To Form an Archaeal Rhodopsin-like Photochemically Reactive Pigment Jennifer A. Bieszke, Elena N. Spudich, Kenneth L.
Channelrhodopsins (ChRs) found in green flagellate algae
 pubs.acs.org/biochemistry Open Access on 05/28/2015 Retinal Chromophore Structure and Schiff Base Interactions in Red- Shifted Channelrhodopsin 1 from Chlamydomonas augustae John I. Ogren, Sergey Mamaev,
pubs.acs.org/biochemistry Open Access on 05/28/2015 Retinal Chromophore Structure and Schiff Base Interactions in Red- Shifted Channelrhodopsin 1 from Chlamydomonas augustae John I. Ogren, Sergey Mamaev,
SUPPLEMENTARY INFORMATION
 Supplementary Table 1: Data collection, phasing and refinement statistics ChbC/Ta 6 Br 12 Native ChbC Data collection Space group P4 3 2 1 2 P4 3 2 1 2 Cell dimensions a, c (Å) 132.75, 453.57 132.81, 452.95
Supplementary Table 1: Data collection, phasing and refinement statistics ChbC/Ta 6 Br 12 Native ChbC Data collection Space group P4 3 2 1 2 P4 3 2 1 2 Cell dimensions a, c (Å) 132.75, 453.57 132.81, 452.95
Review. Membrane proteins. Membrane transport
 Quiz 1 For problem set 11 Q1, you need the equation for the average lateral distance transversed (s) of a molecule in the membrane with respect to the diffusion constant (D) and time (t). s = (4 D t) 1/2
Quiz 1 For problem set 11 Q1, you need the equation for the average lateral distance transversed (s) of a molecule in the membrane with respect to the diffusion constant (D) and time (t). s = (4 D t) 1/2
Active State of Sensory Rhodopsin II: Structural determinants for signal transfer and proton pumping
 Active State of Sensory Rhodopsin II: Structural determinants for signal transfer and proton pumping Ivan Yu. Gushchin, Anastasia Reshetnyak, Valentin Borshchevskiy, Andrii Ishchenko, Ekaterina Round,
Active State of Sensory Rhodopsin II: Structural determinants for signal transfer and proton pumping Ivan Yu. Gushchin, Anastasia Reshetnyak, Valentin Borshchevskiy, Andrii Ishchenko, Ekaterina Round,
Supplementary materials. Crystal structure of the carboxyltransferase domain. of acetyl coenzyme A carboxylase. Department of Biological Sciences
 Supplementary materials Crystal structure of the carboxyltransferase domain of acetyl coenzyme A carboxylase Hailong Zhang, Zhiru Yang, 1 Yang Shen, 1 Liang Tong Department of Biological Sciences Columbia
Supplementary materials Crystal structure of the carboxyltransferase domain of acetyl coenzyme A carboxylase Hailong Zhang, Zhiru Yang, 1 Yang Shen, 1 Liang Tong Department of Biological Sciences Columbia
Microbial and Animal Rhodopsins: Structures, Functions and Molecular Mechanisms
 Microbial and Animal Rhodopsins: Structures, Functions and Molecular Mechanisms Oliver P. Ernst 1, David T. Lodowski 2, Marcus Elstner 3, Peter Hegemann 4, Leonid S. Brown 5, and Hideki Kandori 6 1 Departments
Microbial and Animal Rhodopsins: Structures, Functions and Molecular Mechanisms Oliver P. Ernst 1, David T. Lodowski 2, Marcus Elstner 3, Peter Hegemann 4, Leonid S. Brown 5, and Hideki Kandori 6 1 Departments
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature17991 Supplementary Discussion Structural comparison with E. coli EmrE The DMT superfamily includes a wide variety of transporters with 4-10 TM segments 1. Since the subfamilies of the
doi:10.1038/nature17991 Supplementary Discussion Structural comparison with E. coli EmrE The DMT superfamily includes a wide variety of transporters with 4-10 TM segments 1. Since the subfamilies of the
4 Examples of enzymes
 Catalysis 1 4 Examples of enzymes Adding water to a substrate: Serine proteases. Carbonic anhydrase. Restrictions Endonuclease. Transfer of a Phosphoryl group from ATP to a nucleotide. Nucleoside monophosphate
Catalysis 1 4 Examples of enzymes Adding water to a substrate: Serine proteases. Carbonic anhydrase. Restrictions Endonuclease. Transfer of a Phosphoryl group from ATP to a nucleotide. Nucleoside monophosphate
Three-layer ONIOM studies of the dark state of Rhodopsin: the protonation state of Glu181
 Accepted Manuscript Three-layer ONIOM studies of the dark state of Rhodopsin: the protonation state of Glu181 Katherine. F. Hall, Thom Vreven, Michael J. Frisch, Michael J. Bearpark PII: S0022-2836(08)00987-X
Accepted Manuscript Three-layer ONIOM studies of the dark state of Rhodopsin: the protonation state of Glu181 Katherine. F. Hall, Thom Vreven, Michael J. Frisch, Michael J. Bearpark PII: S0022-2836(08)00987-X
Titration of Aspartate-85 in Bacteriorhodopsin: What It Says About Chromophore Isomerization and Proton Release
 Biophysical Journal Volume 7 January 1996 473-481 473 Titration of Aspartate-85 in Bacteriorhodopsin: What It Says About Chromophore Isomerization and Proton Release Sergei P. Balashov,'I Eleonora S. lmasheva,*`
Biophysical Journal Volume 7 January 1996 473-481 473 Titration of Aspartate-85 in Bacteriorhodopsin: What It Says About Chromophore Isomerization and Proton Release Sergei P. Balashov,'I Eleonora S. lmasheva,*`
The Potassium Ion Channel: Rahmat Muhammad
 The Potassium Ion Channel: 1952-1998 1998 Rahmat Muhammad Ions: Cell volume regulation Electrical impulse formation (e.g. sodium, potassium) Lipid membrane: the dielectric barrier Pro: compartmentalization
The Potassium Ion Channel: 1952-1998 1998 Rahmat Muhammad Ions: Cell volume regulation Electrical impulse formation (e.g. sodium, potassium) Lipid membrane: the dielectric barrier Pro: compartmentalization
Bahnson Biochemistry Cume, April 8, 2006 The Structural Biology of Signal Transduction
 Name page 1 of 6 Bahnson Biochemistry Cume, April 8, 2006 The Structural Biology of Signal Transduction Part I. The ion Ca 2+ can function as a 2 nd messenger. Pick a specific signal transduction pathway
Name page 1 of 6 Bahnson Biochemistry Cume, April 8, 2006 The Structural Biology of Signal Transduction Part I. The ion Ca 2+ can function as a 2 nd messenger. Pick a specific signal transduction pathway
Effects of individual genetic substitutions of arginine residues on. during the bacteriorhodopsin photocycle
 Effects of individual genetic substitutions of arginine residues on the deprotonation and reprotonation kinetics of the Schiff base during the bacteriorhodopsin photocycle Gloria C. Lin,* Mostafa A. El-Sayed,*
Effects of individual genetic substitutions of arginine residues on the deprotonation and reprotonation kinetics of the Schiff base during the bacteriorhodopsin photocycle Gloria C. Lin,* Mostafa A. El-Sayed,*
Hydrogen Bond Switching among Flavin and. Amino Acids Determines the Nature of Proton- Coupled Electron Transfer in BLUF.
 Hydrogen Bond Switching among Flavin and Amino Acids Determines the Nature of Proton- Coupled Electron Transfer in BLUF Photoreceptors Tilo Mathes 1,2, Jingyi Zhu 1, Ivo H.M. van Stokkum 1, M.L. Groot
Hydrogen Bond Switching among Flavin and Amino Acids Determines the Nature of Proton- Coupled Electron Transfer in BLUF Photoreceptors Tilo Mathes 1,2, Jingyi Zhu 1, Ivo H.M. van Stokkum 1, M.L. Groot
Neurons and the membrane potential. N500 John Beggs 23 Aug, 2016
 Neurons and the membrane potential N500 John Beggs 23 Aug, 2016 My background, briefly Neurons Structural elements of a typical neuron Figure 1.2 Some nerve cell morphologies found in the human
Neurons and the membrane potential N500 John Beggs 23 Aug, 2016 My background, briefly Neurons Structural elements of a typical neuron Figure 1.2 Some nerve cell morphologies found in the human
Location of a Cation-Binding Site in the Loop between Helices F and G of Bacteriorhodopsin as Studied by 13 C NMR
 Biophysical Journal Volume 76 March 1999 1523 1531 1523 Location of a Cation-Binding Site in the Loop between Helices F and G of Bacteriorhodopsin as Studied by 13 C NMR Satoru Tuzi,* Satoru Yamaguchi,*
Biophysical Journal Volume 76 March 1999 1523 1531 1523 Location of a Cation-Binding Site in the Loop between Helices F and G of Bacteriorhodopsin as Studied by 13 C NMR Satoru Tuzi,* Satoru Yamaguchi,*
time-resolved stroboscopic-ftir-spectroscopy
 Proton uptake mechanism of bacteriorhodopsin as determined by time-resolved stroboscopic-ftir-spectroscopy Georg Souvignier and Klaus Gerwert Max-Planck-Institut for Ernahrungsphysiologie, Rheinlanddamm
Proton uptake mechanism of bacteriorhodopsin as determined by time-resolved stroboscopic-ftir-spectroscopy Georg Souvignier and Klaus Gerwert Max-Planck-Institut for Ernahrungsphysiologie, Rheinlanddamm
It s really this simple.
 Background Light harvesting complexes exist to facilitate and maximize the absorption capacity of the reaction centers (RC) as well as PSI and PSII Purple bacteria utilize these functions by having an
Background Light harvesting complexes exist to facilitate and maximize the absorption capacity of the reaction centers (RC) as well as PSI and PSII Purple bacteria utilize these functions by having an
Chapter 6. The interaction of Src SH2 with the focal adhesion kinase catalytic domain studied by NMR
 The interaction of Src SH2 with the focal adhesion kinase catalytic domain studied by NMR 103 Abstract The interaction of the Src SH2 domain with the catalytic domain of FAK, including the Y397 SH2 domain
The interaction of Src SH2 with the focal adhesion kinase catalytic domain studied by NMR 103 Abstract The interaction of the Src SH2 domain with the catalytic domain of FAK, including the Y397 SH2 domain
Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015,
 Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015, Course,Informa5on, BIOC%530% GraduateAlevel,discussion,of,the,structure,,func5on,,and,chemistry,of,proteins,and, nucleic,acids,,control,of,enzyma5c,reac5ons.,please,see,the,course,syllabus,and,
Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015, Course,Informa5on, BIOC%530% GraduateAlevel,discussion,of,the,structure,,func5on,,and,chemistry,of,proteins,and, nucleic,acids,,control,of,enzyma5c,reac5ons.,please,see,the,course,syllabus,and,
Regulació electrostàtica de canals microfluídics i porus biològics. Jordi Faraudo Institut de Ciència de Materials de Barcelona
 Regulació electrostàtica de canals microfluídics i porus biològics Jordi Faraudo Institut de Ciència de Materials de Barcelona A few (interesting?) examples of nanofluidic devices Electrostatic regulation
Regulació electrostàtica de canals microfluídics i porus biològics Jordi Faraudo Institut de Ciència de Materials de Barcelona A few (interesting?) examples of nanofluidic devices Electrostatic regulation
